BISx INTERNATIONAL LICENSE AGREEMENT
Exhibit 10.1
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Asterisks denote omissions.
Securities and Exchange Commission. Asterisks denote omissions.

THIS AGREEMENT is made and entered into as of March 21, 2008 (the “Effective Date”), by and between
ASPECT MEDICAL SYSTEMS, INC. (“Aspect”), a Delaware, U.S.A. corporation having offices at Xxx
Xxxxxx Xxxx, Xxxxxxx, Xxxxxxxxxxxxx 00000, X.X.X. and NIHON KOHDEN CORPORATION (“NK”), a Japanese
company having offices at 00-0 Xxxxxxxxxxx, 0-xxxxx, Xxxxxxxx-xx, Xxxxx 000 Xxxxx.
WHEREAS, Aspect possesses certain intellectual and industrial property rights; and
WHEREAS, Aspect is willing to grant, and NK desires to acquire, non-exclusive worldwide rights
to use such rights in accordance with the terms and conditions hereinafter set forth.
NOW, THEREFORE, in consideration of the premises and mutual promises, terms and conditions
hereinafter set forth, and other good and valuable consideration, the receipt and sufficiency of
which are hereby acknowledged, Aspect and NK (the “Parties”) do hereby agree as follows:
1. DEFINITIONS
As used herein, the following terms shall have the following definitions.
| 1.1 | Affiliates. “Affiliates” of a Party hereto shall mean companies which are controlled by, control or under common control with such Party. A company shall be considered an “Affiliate” for only so long as such control exists. For the purposes of this definition, partnerships or similar entities where a majority-in-interest of its partners or owners are a Party hereto and/or Affiliates of such Party shall also be deemed to be Affiliates of such Party. | ||
| 1.2 | Agreement Term. “Agreement Term” shall mean the period beginning on the Effective Date and ending on the date of termination or expiration of this Agreement, as the case may be. | ||
| 1.3 | BIS. “BIS” shall mean the Bispectral Index™ which is Aspect’s proprietary processed EEG parameter that directly measures the hypnotic effects of anesthetic and sedative agents on the brain. | ||
| 1.4 | BIS Engine. “BIS Engine” shall mean Aspect’s processing unit for deriving the BIS from the raw EEG signal. | ||
| 1.5 | BIS Ready System. “BIS Ready System” shall mean that a BIS port is standard on all monitoring systems ready to accept a BISx System, with BIS software installed on the host monitor. If a dedicated BIS port is not utilized, then at least one multi-connector |
| V.8. 12-5-06 | Initials: |
| port must be available on a patient monitoring system in order for that system to be identified as a “BIS Ready System”. | |||
| 1.6 | BIS Sensor. “BIS Sensor” shall mean a single use disposable sensor manufactured by Aspect for use with the BISx System in the OR, ICU and other sedation locations that is required to generate Aspect’s Bispectral Index. These sensors include the BIS Quatro Sensor, the BIS Extend Sensor and the BIS Pediatric Sensor. | ||
| 1.7 | BISx. “BISx” shall mean a device that acquires up to two channels of EEG and computes BIS and other EEG parameters, uniting the functionality of the existing Aspect’s BIS Engine and DSC-XP. | ||
| 1.8 | BISx System. “BISx System” shall mean the system comprised of a BISx with a Monitor Cable and a Patient Interface Cable (“PIC”) designed for use with the NK Patient Monitor. | ||
| 1.9 | Business Day. “Business Day” shall mean a day on which banks are open for business in Norwood, Massachusetts, U.S.A and Tokyo, Japan. | ||
| 1.10 | Commencement Date. “Commencement Date” shall mean the MHLW Approval Date of the Product. | ||
| 1.11 | Contract Year. “Contract Year” shall mean the 12-month period commencing on the Commencement Date, and then each 12-month period thereafter. | ||
| 1.12 | DSC-XP. “DSC-XP” shall mean Aspect’s digital signal converter using XP technology. The DSC-XP is a small box that is kept close to the patient that converts the analog EEG signals to digital signals for processing the BIS value. | ||
| 1.13 | Licensed Technology. “Licensed Technology” shall mean the Products and the Technical Information. | ||
| 1.14 | License Term. “License Term” shall mean the period beginning on the Effective Date and ending on the date of termination or expiration of this Agreement, as the case may be. | ||
| 1.15 | Monitor Cable. “Monitor Cable” is a cable that connects the BISx to the NK Patient Monitor. The Monitor Cable connector will be supplied by Aspect using a standard Hypertronics connector. | ||
| 1.16 | MHLW Approval Date. “MHLW Approval Date” shall mean the date on which NK receives the approval of the Japanese Ministry of Health, Labor and Welfare to market the Product in Japan. | ||
| 1.17 | NK Patient Monitor. “NK Patient Monitor” shall mean all NK multiparameter patient monitors that include a universal (MULTI) module connector including, but not limited to, the BSM 2000, 4000, 5000, 6000 and BSS 9800 series of patient monitors. | ||
| 1.18 | Product. “Product” shall mean the BISx System. | ||
| 1.19 | Software “Software” shall mean Aspect software programs that are designed for use |
| V.8. 12-5-06 | Initials: |
2/31
| with the BISx Systems. | |||
| 1.20 | Technical Information. “Technical Information” shall mean the trade secrets, know-how, computer programs (including copyrights in said software), knowledge, technology, means, methods, processes, practices, formulas, techniques, procedures, technical assistance, designs, drawings, apparatus, written and oral rectifications of data, specifications, assembly procedures, schematics and other valuable information of whatever nature, whether confidential or not, and whether proprietary or not, which is now in (or hereafter, during the Agreement Term, comes into) the possession of Aspect and which is necessary for the integration, sale, distribution, use, installation, servicing (for which NK is responsible under this Agreement) or testing of the Product. | ||
| 1.20 | U.S. Dollars. “U.S. Dollars” shall mean lawful money of the United States of America, in immediately available funds. |
2. LICENSES
| 2.1 | Licenses. |
| (a) | For the term of this Agreement, subject to the terms and conditions of this Agreement, Aspect agrees to sell and NK agrees to purchase and distribute the Aspect Products listed in Exhibit A (Aspect Products and Purchase Prices) | ||
| The components of the BISx System purchased from Aspect under this Agreement shall only be used as components in, incorporated into, or integrated with, systems and products of NK which NK, its Affiliates, distributors or sub-distributors sell or lease to third-party users in the regular course of business. NK, its Affiliates, distributors or sub-distributors shall only sell Aspect approved accessories including cables and sensors in connection with any BISx System. Aspect agrees to provide NK with all information in its possession that is necessary for the interfacing of the BISx System with NK patient monitoring systems. Aspect agrees to provide NK with information that will allow NK to provide BISx System specific service and support to the end-user. | |||
| (b) | Aspect hereby grants NK, its Affiliates, distributors or sub-distributors a non-exclusive and non-transferable world-wide license, without the right to sublicense (except to purchasers of BISx Systems), during the term of this Agreement to use the Software provided by Aspect solely in connection with the operation of the components of the BISx System. | ||
| (c) | During the Agreement Term, Aspect shall not grant, directly or indirectly, the right and license for distribution of BISx within Japan to any other manufacturer; whose business concerns primarily Patient Monitors and whose ultimate parent Affiliate has a principal place of business in Japan (specifically Fukuda Denshi). | ||
| (d) | Aspect, however, shall have the right to grant, directly or indirectly, the right and license for distribution of BISx outside of Japan to any manufacturer regardless of |
| V.8. 12-5-06 | Initials: |
3/31
| principle place of business. | |||
| (e) | After the termination or expiration of this Agreement, Aspect grants to NK, its Affiliates, distributors or sub-distributors a right to use the Software used in conjunction with the BISx System being sold by NK, its Affiliates, distributors or sub-distributors on the date of termination with respect to service and support of installed BISx Systems, and a right to distribute the Software as part of the BISx Systems in its inventory following termination of this Agreement on grounds other than non-payment without a valid reason by NK. All rights granted to NK’s, its Affiliates’, distributors’ or sub-distributor’s customers to use the Software with the BISx System shall survive any termination of the Agreement as long as customers remain in compliance with the BISx license agreement supplied by Aspect to NK for such BISx System. | ||
| (f) | NK shall not disclose, furnish, transfer, distribute or otherwise make available the Software or any portion thereof in any form to any third party (other than to purchaser of the BISx System and to NK’s distributors, sub-distributors and Affiliates that are distributing the BISx Systems) and shall not duplicate the Software or any part thereof. | ||
| (g) | Title to and ownership of any and all proprietary rights in or related to the Software thereof shall at all times remain with Aspect or its licensor(s). Nothing in this Agreement shall be construed as a sale of any rights in the Software. | ||
| (h) | NK, its distributors, sub-distributors, its Affiliates and purchasers of BISx Systems shall not disassemble, decompile or otherwise reverse engineer the Software or any part thereof. |
| 2.2 | Use of Technical Information. |
| (a) | Aspect grants to NK a non-exclusive worldwide right and license during the Agreement Term to use the Technical Information in connection with NK’s exercise of its rights and licenses granted in Section 2.1, and for no other purpose. | ||
| (b) | As soon as practical after the Effective Date, Aspect shall provide to NK, at no additional cost to NK, the Technical Information. |
| 2.3 | Trademarks, Service Marks and Trade Names. |
| (a) | NK shall be required to xxxx the Products with Aspect’s trademarks, service marks and trade names listed in Exhibit E hereto (the “Trademarks”). Aspect hereby grants NK the right to use the Trademarks on a non-exclusive basis only for the License Term and solely for display or advertising purposes in connection with the Products purchased from Aspect and sold in accordance with this Agreement. During the License Term, NK may use, without Aspect’s prior written consent, trademarks, service marks and trade names in connection with the Products other than the Trademarks; provided, however that the Trademarks are always used in a manner which makes them at least as large and at least as prominent as any other such trademarks, service marks or trade names used in |
| V.8. 12-5-06 | Initials: |
4/31
| connection with the Products including but not limited to any such trademarks, service marks or trade names appearing on any such label, display or advertisement. Any use by NK of the Trademarks shall be deemed to be a use of the same by Aspect. NK shall not at any time do or permit any act to be done (including without limitation registering any of the Trademarks in its own name or the name of any entity other than Aspect) which may in any way impair the rights of Aspect in the Trademarks. Except as provided above, NK has no rights in the Trademarks or of any goodwill associated therewith and NK agrees that, except as expressly provided in this Agreement, it shall not acquire any rights in respect thereof and that all such rights and goodwill are, and shall remain, vested in, and inure to the benefit of, Aspect. | |||
| (b) | NK shall be required to xxxx all NK Patient Monitoring Systems that are BIS Ready with a label that says “BIS Ready”. The label will be of a mutually agreeable size and will be placed in a mutually agreeable location on the patient monitoring system. | ||
| (c) | NK shall |
| (i) | whenever it uses the Trademarks, include a statement that the Trademarks are trademarks of Aspect; | ||
| (ii) | use the Trademarks in compliance with all relevant laws and regulations; | ||
| (iii) | not modify any of the Trademarks in any way and not use any of the Trademarks on or in connection with any goods or services other than the Products. |
| 2.4 | Right to Sublicense. | ||
| NK shall not have the right to sublicense any of the rights or licenses granted hereunder without Aspect’s prior written consent, which consent shall be withheld in Aspect’s absolute discretion; provided, however it is understood that NK shall have the right to grant sublicenses to NK’s Affiliates and distributors without Aspect’s prior written consent. Sublicenses except for NK’s Affiliates and distributors shall not become effective until the sublicensee confirms in writing to Aspect that it agrees to be bound by all of the terms and conditions contained in this Agreement. | |||
| 2.5 | No Rights by Implication. | ||
| No rights or licenses with respect to Licensed Technology are granted or deemed granted hereunder or in connection herewith, other than those rights or licenses expressly granted in this Agreement. | |||
| 2.6 | Licensed Technology. NK shall not disclose, furnish, transfer or otherwise make available in any form to any third party (other than to purchasers of the Product, NK’s Affiliates and distributors which become sublicensees pursuant to Section 2.4 above) and shall not duplicate the Licensed Technology or any part thereof. Title to and ownership of and all proprietary rights in or related to the Licensed Technology |
| V.8. 12-5-06 | Initials: |
5/31
| and all partial or complete copies thereof shall at all times remain with Aspect or its licensor(s). This Agreement shall not be construed as a sale of any rights in the Licensed Technology any copies thereof or any part thereof. All references in this Agreement to sale, resale or purchase of the Products, or references of like effect, shall, with respect to the Licensed Technology mean licenses or sublicenses of the Licensed Technology pursuant to this Section 2.6. NK shall not disassemble, decompile or reverse engineer the Licensed Technology or any part thereof. NK shall retain and shall not alter or obscure any notices, markings or other insignia which are affixed to the Licensed Technology or any part thereof at the time of delivery of such Licensed Technology. | |||
| 2.7 | Non-Competition. | ||
| NK confirms that it has not previously, directly or indirectly, marketed or manufactured monitoring equipment, either as stand-alone monitors, modules for monitors, or external devices that attach to a NK Patient Monitor through a multi-connector which were designed to monitor the depth of anesthesia or sedation other than that allowed under the International License Agreement and International Distribution Agreement, both dated January 21, 1998, by and between NK and Aspect (the “1998 Agreements”). NK confirms that it has not previously, directly or indirectly, developed monitoring equipment, either as stand-alone monitors, modules for monitors, or external devices that attach to a NK Patient Monitor through a multi-connector which was designed to: (i) monitor the depth or effects of anesthesia or sedation being administered to patients; and (ii) indicate any index of the depth or effects of anesthesia or sedation to assist anesthesiologists and other clinicians to evaluate the depth or effects of anesthesia using EEG (electroencephalogram) except for those allowed under the 1998 Agreements. Until the first (1st) anniversary of the later of the termination or expiration of this Agreement and the 1998 Agreements, as the case may be, NK shall not, directly or indirectly, develop, market or manufacture monitoring equipment, either as stand-alone monitors, modules for monitors, or external devices that attach to a NK Patient Monitor through a multi-connector which is designed to: (x) monitor the depth or effects of anesthesia or sedation being administered to patients; and (y) indicate any index of the depth or effects of anesthesia or sedation to assist anesthesiologists and other clinicians to evaluate the depth or effects of anesthesia; provided, however that: (I) NK shall be permitted, at any time, to develop monitoring equipment designed to monitor the depth or effects of anesthesia using physiological measures other than EEG; (II) NK shall be permitted, at any time, to market and manufacture monitoring equipment designed to monitor the depth or effects of anesthesia using physiological measures other than EEG, so long as such equipment has been developed and manufactured by NK; (III) NK shall be permitted after the expiration or termination of this Agreement to manufacture and market monitoring equipment developed and manufactured by NK or by any third party that is designed to monitor the depth or effects of anesthesia using physiological measures other than EEG; and (IV) NK shall be permitted to continue to market and improve its existing EEG monitors and modules so long as |
| V.8. 12-5-06 | Initials: |
6/31
| they are not designed to measure the depth of consciousness. Specifically, it will not be considered a violation of this Section 2.7 for NK to market and improve its existing EEG monitors or modules that display any of the following parameters of brain function: EEG, EEG trends, CSA, DSA, EP, EMG, or NCV, subject to the terms and conditions of this Section 2.7. | |||
| 2.8 | Changes to Products. |
| (a) | Aspect shall have the right, at any time and from time to time, to make substitutions and modifications to Products, provided that such substitutions or modifications shall not materially affect form, fit, function, functional interchangeability or interface capability or materially negatively affect the reliability, serviceability or performance of Products. In the event that any proposed substitution or modification affects such form, fit, function, reliability, serviceability, performance, functional interchangeability or interface capability of an Product, Aspect shall give NK written notice of such proposed substitution or modification at least six (6) months prior to its taking effect and if such changes need a new or additional approval by the MHLW, NK shall have the right to order Aspect Products without such substitution or modification for delivery up to one year after the date of such notice or until NK obtains such a new or additional approval by the MHLW, (whichever is less) after such substitution or modification takes effect. Aspect shall provide the appropriate verification and validation information for evaluating the effect of the change on the BISx System. | ||
| (b) | Notwithstanding anything contained in this Agreement to the contrary, Aspect reserves the right from time to time during the License Term to require NK, after consulting with NK, to modify or improve the Product (including without limitation the software programs used in connection with the Product) if the modification or improvement reasonably relates to efficacy or patient safety. NK shall implement those changes to the Products and associated NK Patient Monitors being manufactured or to be manufactured and to modify and improve Products previously manufactured and shipped to customers in order to incorporate such changes. In that event the change or modification relates to patient safety, Aspect agrees to repair or replace Products previously provided to NK, or collected by NK from its customers, free of charge, whether or not such repair or replacement occurs during the relevant Warranty Period, provided such repair or replacement is necessary to implement the modification or improvement. | ||
| (c) | Aspect agrees to notify NK in writing not less than twelve (12) months in advance of the discontinuance of any Aspect Products. NK shall be able to place orders for at least six (6) months after receipt of the written notice in any case. |
| V.8. 12-5-06 | Initials: |
7/31
| In addition, NK shall be entitled to determine its lifetime-buy quantities and place a corresponding last purchase order within the first six (6) months after receipt of written notice. Aspect shall continue to provide NK with repair service or service parts (or at Aspect’s sole discretion, comparable exchange units for the Aspect Products) necessary for after-sales service for such Products for eight (8) years after discontinuance. | |||
| (d) | Aspect shall immediately provide NK with a written notice upon Aspect becoming aware of the occurrence of any of the following events: (i) Aspect recalls any BISx System, or ceases or suspends the sale of any BISx System due to any problem which relates to such BISx System’s efficacy or patient safety in any country outside Japan; (ii) any defect of any BISx System or the Licensed Technology, which relates to such BISx System’s efficacy or patient safety, is published, reported or made known to the public by any third party, or found by Aspect; or (iii) any BISx System or the Licensed Technology contributed to or caused a death or serious injury, or any BISx System or the Licensed Technology malfunctioned and if that malfunction occurred again, it would be likely to contribute or cause a death or serious injury. |
| 2.9 | No Knowledge of Third Party Claims. | ||
| Aspect represents and warrants to NK that Aspect knows of no claim by any third party of infringement by Aspect on such party’s patent, trademark, copyright, trade secret or other intellectual property rights. | |||
| 2.10 | Disclaimer of Liability. | ||
| ASPECT MAKES NO EXPRESS OR IMPLIED WARRANTY, STATUTORY OR OTHERWISE, CONCERNING THE LICENSED TECHNOLOGY OR ANY OTHER INFORMATION COMMUNICATED TO NK, INCLUDING WITHOUT LIMITATION NO WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, OR NO WARRANTIES AS TO QUALITY OR THE USEFULNESS OF THE LICENSED TECHNOLOGY FOR ITS INTENDED PURPOSE; PROVIDED, HOWEVER IF ASPECT OR NK SHALL DISCOVER ANY ERRORS IN THE LICENSED SOFTWARE DURING THE LICENSE TERM, ASPECT, AT ITS SOLE DISCRETION, SHALL CORRECT SUCH ERRORS IN THE LICENSED SOFTWARE WITHOUT COST TO NK. | |||
| IN NO EVENT, HOWEVER, SHALL ASPECT BE LIABLE TO NK FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, EXEMPLARY OR OTHER INDIRECT LOSSES OR DAMAGES RESULTING FROM SUCH ERRORS IN THE LICENSED TECHNOLOGY. | |||
| ASPECT’S OBLIGATIONS SET FORTH IN THIS AGREEMENT, INCLUDING, BUT NOT LIMITED TO, SECTION 4.5 (LIMITED WARRANTY) AND SECTION 7 (INDEMNIFICATIONS), SHALL BE EXCLUED FROM THIS SECTION 2.10 |
3. DEVELOPMENT OF BISx AND COMMUNICATION (HOST) SOFTWARE
| V.8. 12-5-06 | Initials: |
8/31
| 3.1 | Project Management. |
| (a) | Each Party shall appoint a “Project Manager” who shall oversee and manage the joint project on a day-to-day basis. | ||
| (b) | The Project Managers shall meet regularly based on the project needs to assess the project status and discuss and resolve any issues or problems. These meetings may be held face-to-face or as telephone or videoconferences. | ||
| (c) | Each Party shall bear its own communication and travel costs | ||
| (d) | All communication in conjunction with this Agreement shall be directed to the appropriate person and address listed in Exhibit C (Contact Persons / Addresses). |
| 3.2 | Nihon Kohden Responsibilities. |
| (a) | NK will develop the necessary software for the NK Patient Monitors to communicate with the BISx System, allow the user to configure BIS parameters, and display the BIS related information on the NK Patient Monitor display. | ||
| (b) | NK shall integrate and test the integration of the components of the BISx System with the NK Patient Monitors. | ||
| (c) | NK will report the number of BISx Systems installed in the United States, dates of installation and overall installed base of BISx Systems by hospital on a quarterly basis. Outside of the United States, NK will report on a calendar quarter basis the total number of BISx Systems installed, and overall installed base of NK BISx Systems by country. NK will report these data within 20 business days after the end of each quarter. |
| 3.3 | Aspect Responsibilities. |
| (a) | Aspect shall provide NK with the necessary Technical Information to facilitate development of appropriate communications software to interface to the NK Patient Monitors. | ||
| (b) | Aspect shall design, develop and test the BISx System according to the mutually agreed upon specifications. | ||
| (c) | Upon NK completing the integration of BIS into the NK Patient Monitoring Systems (or any subsequent new NK Patient Monitoring System which is BIS Ready), Aspect will “certify” this integration. This entails Aspect verifying the accurate display on the NK Patient Monitor. Once successfully verified, Aspect will provide NK with a Certification Letter so indicating. To facilitate the certification process, NK will lend Aspect a NK Patient Monitor for testing purposes. Said NK Patient Monitor (and any new NK BIS Ready Patient Monitor) will be provided to Aspect, at NK’s cost and expense, within thirty (30) days after execution of this Agreement, and it will be returned to NK, at Aspect’s cost and expense, |
| V.8. 12-5-06 | Initials: |
9/31
| within thirty (30) days after the certification process is completed. |
4. SALES BY ASPECT TO NK
| 4.1 | Offer and Acceptance; Pricing. | |
| Aspect shall sell and NK shall purchase the Aspect Products during the calendar year at the prices set forth in Exhibit A (Aspect Products and Purchase Prices), except as noted in 4.1(a). The parties have agreed to initiate at least once a year discussions on market conditions and trends as well as pricing of the Products. Additional pricing programs will be discussed as new NK Patient Monitors are introduced into the Japanese markets; no sooner than one year after the Commencement Date. |
| (a) | Additional costs which have been agreed upon in writing (both NRE and incremental unit cost) for customization of the product (mold color, labeling, changes to or customization of the BISx Monitor Cable) will be borne by NK. NK shall be responsible for any charges associated with scrap of inventory due to any subsequent changes made to customized product. | ||
| (b) | For each proposed purchase by NK from Aspect, NK shall present a purchase order to Aspect (a “Purchase Order”). Each Purchase Order shall be deemed an offer to purchase and, unless NK is notified in writing to the contrary within five (5) Business Days after Aspect receives it, such Purchase Order shall be deemed accepted by Aspect. | ||
| (c) | Aspect’s transfer prices shall be EXW (EXWORKS) Norwood, Massachusetts, U.S.A. Starting with the second (2nd) Contract Year, Aspect may change those transfer prices; provided, however that: (i) such change may be made only once a year effective as of the first day of April with the prior written notice to be given by Aspect no later than the last day of December of the preceding year, after consulting with NK; (ii) the annual increase shall be no more than [**] percent ([**]%) or actual cost increases, whichever is greater; and (iii) no price change shall affect Purchase Orders offered by NK and accepted by Aspect prior to the date such price change becomes effective. In the event actual cost increases exceed 5%, Aspect shall provide documentation of actual cost increases to NK. |
| 4.2 | Delivery. | |
| Unless NK requests otherwise, all BISx Systems ordered by NK shall be packed for shipment and storage in accordance with Aspect’s standard commercial practices. It is NK’s obligation to notify Aspect of any special packaging requirements (which shall be at NK’s expense if such requirement is in excess of the scope of normal and necessary packaging for export). Aspect shall deliver BISx Systems into the possession of a common carrier designated by NK in Norwood, Massachusetts, U.S.A. no later than the date that would be required to meet the date specified for such delivery on the relevant Purchase Order. Risk of loss and damage to a BISx System shall pass to NK upon the delivery |
| V.8. 12-5-06 | Initials: |
10/31
BISx INTERNATIONAL LICENSE AGREEMENT
| thereof to the common carrier designated by NK. If NK does not designate a common carrier by the specified delivery date, then Aspect may do so on NK’s behalf. All claims for non-conforming shipments must be made in writing to Aspect within thirty (30) days of the passing of risk of loss and damage. |
| 4.3 | Forecast | |
| NK shall furnish to Aspect a non-binding quarterly forecast during the term of this Agreement with the number and type of Products for which NK expects to submit orders for the following 12 months. |
| 4.4 | Method of Payment. |
| (a) | All amounts due and payable with respect to BISx Systems delivered by Aspect in accordance with this Section 4 shall be paid in full within 30 days after the date of Aspect’s invoice. All such amounts shall be paid in U.S. Dollars by wire transfer, to such bank or account as Aspect may from time to time designate in writing. All costs incurred in connection with such wire transfer shall be the responsibility of NK. Whenever any amount hereunder is due on a day which is not a day on which banks in Norwood, Massachusetts, U.S.A. are open for business (a “Business Day”), such amount shall be paid on the next such Business Day. Amounts hereunder shall be considered to be paid as of the day on which funds are received by Aspect’s bank. No part of any amount payable to Aspect hereunder may be reduced due to any counterclaim, set-off, adjustment or other right which NK might have or assert against Aspect, any other party or otherwise. | ||
| (b) | All amounts due and owing to Aspect hereunder but not paid by NK on the due date thereof shall bear interest (in U.S. Dollars) at the rate 18 per cent per annum. Such interest shall accrue on the balance of unpaid amounts from time to time outstanding from the date on which portions of such amounts become due and owing until payment thereof in full. | ||
| (c) | NK is responsible to pay expenses incurred by Aspect in the event that Aspect hires a third party collection agency in order to recover overdue debts owed to Aspect by NK. |
| 4.5 | Limited Warranty. |
| (a) | With respect to the BISx System, Aspect makes the warranties set forth in Exhibit D attached hereto and made a part hereof. Under no circumstances shall the warranties set forth in Exhibit D hereto apply to a BISx System which has been customized, modified, damaged or misused by NK or any third party without Aspect’s authorization. NK’s sole remedy for a non-conforming BISx System is, at Aspect’s election, the repair or replacement thereof. | ||
| (b) | THE PROVISIONS OF THE FOREGOING WARRANTIES ARE IN LIEU OF ANY OTHER WARRANTY, WHETHER EXPRESS OR IMPLIED, WRITTEN OR ORAL (INCLUDING ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NONINFRINGEMENT). |
| V.8. 12-5-06 | Initials: |
11/31
BISx INTERNATIONAL LICENSE AGREEMENT
| (c) | EXCEPT AS OTHERWISE PROVIDED IN THIS AGREEMENT, ASPECT’S LIABILITY ARISING OUT OF THE MANUFACTURE, SALE OR SUPPLYING OF BISx SYSTEMS OR THEIR USE OR DISPOSITION, WHETHER BASED UPON WARRANTY, CONTRACT, TORT OR OTHERWISE, SHALL NOT EXCEED THE ACTUAL PURCHASE PRICE PAID BY NK FOR SUCH BISX SYSTEMS. | ||
| (d) | After expiration of the Warranty Period, Aspect shall undertake repairs of BISx Systems or shall provide parts for repairs by NK, at reasonable cost to NK. Both parties shall agree on the charge for such repairs and parts. |
| 4.6 | Priority of Agreement. | |
| In the event of any discrepancy between any Purchase Order and this Agreement, the terms of this Agreement shall govern. |
| 4.7 | Distribution of Aspect BIS Sensors. |
| 4.7.1 | Outside the United States | ||
| Distribution of BIS Sensors outside the United States will be covered under a separate agreement between Aspect and NK. | |||
| 4.7.2 | Within the United States. | ||
| NK is prohibited from selling BIS Sensors to customers within the United States. |
5. CONFIDENTIAL INFORMATION
| 5.1 | Agreement. | ||
| This Agreement and all documents, drawings, manuals and other materials incidental thereto and transmitted between the Parties shall be treated as confidential by the Parties and their employees and such information shall not be disclosed to any third party. |
| 5.2 | Confidentiality Obligations. | ||
| Each Party (the “disclosing Party”) has a proprietary interest in information which it discloses to the other Party (the “receiving Party”), whether in connection with this Agreement or otherwise, which is (a) a trade secret, confidential or proprietary information, (b) not publicly known, and (c) annotated by a legend, stamp or other written identification as confidential or proprietary information, or if disclosed orally, is identified as confidential or proprietary by a written instrument within 30 days of such disclosure (hereinafter referred to as ‘Proprietary Information”). The receiving Party shall disclose the Proprietary Information of the disclosing Party only to those of its agents and employees to whom it is necessary in order properly to carry out their duties as limited by the terms and conditions hereof. Both during and after the Agreement Term, all disclosures of Proprietary Information of the disclosing Party by the receiving Party to its agents and employees shall be held in strict confidence by such agents and employees. During and after the Agreement Term, the receiving Party, its agents and employees shall not use the Proprietary Information of the disclosing Party for any purpose other than in connection with discharging its duties |
| V.8. 12-5-06 | Initials: |
12/31
BISx INTERNATIONAL LICENSE AGREEMENT
| pursuant to this Agreement. The receiving Party shall, at its expense, return to the disclosing Party the Proprietary Information of the disclosing Party or destroy such Proprietary Information as soon as practicable after the termination or expiration of this Agreement. During the Agreement Term and thereafter, all such Proprietary Information of the disclosing Party shall remain the exclusive property of the disclosing Party. This Section 5 shall also apply to any consultants or subcontractors that the receiving Party may engage in connection with its obligations under this Agreement. | |||
| 5.3 | Exceptions. | ||
| Notwithstanding anything contained in this Agreement to the contrary, the receiving Party shall not be liable for a disclosure of the Proprietary Information of the disclosing Party if the information so disclosed: (a) was in the public domain at the time of disclosure without breach of this Agreement; or (b) was known to or contained in the records of the receiving Party from a source other than the disclosing Party at the time of disclosure by the disclosing Party to the receiving Party and can be so demonstrated; or (c) becomes known to the receiving Party from a source other than the disclosing Party without breach of this Agreement by the receiving Party and can be so demonstrated; or (d) was independently developed by the receiving Party without reference to the Proprietary Information; (e) was disclosed pursuant to court order or as otherwise compelled by law. |
6. Quality Assurance / Regulatory Matters
| 6.1 | Certification Status. | ||
| Both parties agree to maintain, as applicable, ISO900x or ISO13485, European directive 93/42/EEC Annex II (“MDD AX-II”) certification status and compliance with the U.S. Food and Drug Administration’s (“FDA”) Quality System Regulation (“QSR”), the European Medical Device Directive (“MDD”), and other appropriate regulations pertinent to the development, manufacturing and marketing of medical products similar to the Aspect Products. | |||
| 6.2 | Regulatory Responsibility — Aspect. | ||
| In particular, Aspect shall be responsible for generating its own Device Master Record for the BISx System. The BISx shall be labeled as manufactured by Aspect. | |||
| 6.3 | Regulatory Requirements — Nihon Kohden. | ||
| NK shall assume the regulatory responsibility for the BISx System. Aspect shall provide commercially reasonable support to NK as required in the process of obtaining regulatory approvals by making available to NK any required information, data, certificates, or technical files in the requested formats as requested by the regulatory authorities. | |||
| 6.4 | Product Complaints and Incident Reporting. | ||
| NK and Aspect shall inform each other (in writing) quarterly of any and all customer complaints that have come to their attention during the prior quarter regarding the BISx System or BIS Sensors that |
| V.8. 12-5-06 | Initials: |
13/31
BISx INTERNATIONAL LICENSE AGREEMENT
| were used in conjunction with a NK Patient Monitor and relating to Aspect technology. Both parties will cooperate and use reasonable efforts to resolve such customer complaints. Closure of any such customer complaints relating to the BISx System or NK Patient Monitor will occur when the problem is resolved. Closure of any such customer complaint relating solely to Products or BIS Sensors will occur when the problem is resolved. | |||
| NK and Aspect shall inform each other in writing of all known and/or reported incidents relating to the Products, distributed by NK and/or used in conjunction with NK Patient Monitors, within 2 business days of making a determination by NK or Aspect that such event may require reporting under any applicable regulatory or other governmental reporting requirements, including without limitation incidents involving death or serious injury, malfunctions that, if recurrent, may cause or contribute to death or serious injury or other material quality problems or concerns; provided, however, for the purposes of clarity, if such reportable event involves a third party product into which the BISx System or other Aspect product has been incorporated, but the cause of reportable incident was some other aspect or attribute of such third party product, then the notification requirement in this paragraph shall not apply. Aspect will be responsible for reporting such incidents to the appropriate regulatory authority for the BISx System and BIS Sensors in the United States and NK will guide Aspect with any reporting requirements in Japan. NK will be responsible for reporting such incidents to the appropriate regulatory authority for the NK Patient Monitor. Both parties shall fully cooperate with each other as may be necessary to comply with any reporting obligations regarding such incidents or quality concerns. If FDA or other authorities contact either party to inquire about or investigate the Products sold to NK under this Agreement, the contacted party, unless required to maintain confidentiality by such authorities, shall inform the other party immediately thereof. The parties shall cooperate closely to clear any regulatory issues or potential regulatory issues promptly. | |||
| 6.5 | Traceability. | ||
| NK agrees to maintain traceability through Aspect serial number and/or lot code for all Products shipped to NK. | |||
| 6.6 | Recall. | ||
| In the event of any Product recall in Japan, both parties shall fully cooperate on the recall. In principle, NK shall recall the Products in Japan and shall return the recalled Products to Aspect. Aspect shall provide NK with substitute products for the recalled Products. Aspect shall reimburse NK for reasonable expenses incurred by NK to effect the recall. | |||
| 6.7 | Compliance with U.S. Foreign Corrupt Practices Act | ||
| 6.7.1 | NK represents that it has not, and agrees that it shall not, in connection with the transactions contemplated by this Agreement, or in connection with any other business transactions involving Aspect or product supplied under this Agreement, |
| V.8. 12-5-06 | Initials: |
14/31
BISx INTERNATIONAL LICENSE AGREEMENT
| make any payment or transfer anything of value, directly or indirectly: | |||
| (a.) to any governmental official or employee (including employees of a government corporation or public international organization) or to any political party or candidate for public office for the purpose of obtaining or retaining business for or with, or directing business to, any person, or for the purpose of securing any improper business advantage; or | |||
| (b.) to any other person or entity if such payments or transfers would violate the laws of the country in which made or the laws of the United States. | |||
| 6.7.2 | It is the intent of the parties that no payments or transfers of value shall be made which have the purpose or effect of public or commercial bribery, or acceptance of or acquiescence in, extortion, kickbacks, or other unlawful or improper means of obtaining business. |
7. INDEMNIFICATIONS
| 7.1 | In Favor of Aspect. | ||
| NK hereby agrees to indemnify, defend and hold harmless Aspect, its Affiliates and all officers, directors, employees and agents thereof from all liabilities, claims, damages, losses, costs, expenses, demands, suits and actions (including without limitation attorneys’ fees, expenses and settlement costs) (collectively, “Damages”) arising out of: (i) NK’s failure to comply with relevant laws and regulations; (ii) personal injuries and/or property damages resulting from the software developed and manufactured by NK or the failure of NK to follow the Technical Information provided by Aspect hereunder in incorporating the Product for use with NK Patient Monitors; or (iii) NK’s making representations or warranties with respect to the BISx Systems which are not authorized by Aspect hereunder. | |||
| 7.2 | In Favor of NK. | ||
| Notwithstanding anything to the contrary contained herein, Aspect hereby agrees to indemnify, defend and hold harmless NK, its Affiliates and all officers, directors, employees and agents thereof from all Damages arising out of: (i) the Products infringing on the intellectual property rights of third parties; (ii) use of the Trademarks in accordance with Section 2.3(a) above which infringes on the trademark, service xxxx or trade name rights of third parties; or (iii) personal injuries and/or property damages resulting from the Product; provided, however that: |
| (a) | Aspect shall have no obligation for any claim of infringement arising from: |
| (i) | any combination by NK of the BISx System with any other product not supplied or approved in writing by Aspect where such infringement would not have occurred but for such combination; | ||
| (ii) | the adaptation or modification of the BISx System not performed or not |
| V.8. 12-5-06 | Initials: |
15/31
BISx INTERNATIONAL LICENSE AGREEMENT
| authorized by Aspect, where such infringement would not have occurred but for such adaptation or modification; | |||
| (iii) | the misuse of the BISx System or the use of the BISx System in an application for which it was not designed by Aspect, where such infringement would not have occurred but for such use or misuse; or | ||
| (iv) | a claim based on intellectual property rights owned by NK or any of its Affiliates. |
| (b) | In the event that the Products are held in a suit or proceeding to infringe any intellectual property rights of a third party, and the use of the BISx System is enjoined or Aspect reasonably believes that it is likely to be found to infringe or likely to be enjoined, Aspect shall, at its sole cost and expense, either (i) procure for NK the right to continue manufacturing, using and selling the BISx System, or (ii) replace the BISx System with a non-infringing BISx System of equivalent functionality. If neither (i) nor (ii) are practicable, either Party may terminate this Agreement, effective immediately, upon giving the other Party written notice. | ||
| (c) | This Section 7.2 constitutes NK’s exclusive remedy in the event that the BISx System and/or the Trademarks infringe on the intellectual property rights of third parties. |
| 7.3 | Indemnification Procedures. |
| (a) | In the event that any person intends to claim indemnification pursuant to this Agreement, (an “Indemnitee”), it shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of such alleged liability, provided that the failure to promptly notify the Indemnitor shall not relieve the Indemnitor of any obligation under this Agreement except to the extent such failure to provide prompt notice adversely impairs the Indemnitor’s ability to defend against the claim, suit or proceeding. | ||
| (b) | The Indemnitor shall have the sole right to control the defense and settlement thereof, provided, that (i) the Indemnitor may not consent to imposition of any obligation or restriction on, or the admission of any wrongdoing on the part of, the Indemnitee in any settlement unless mutually agreed among Aspect and NK (ii) Indemnitor shall keep Indemnitee fully informed and permit the Indemnitee to participate (at Indemnitee’s expense) as the Indemnitee may reasonably request and (iii) Indemnitee may, without affecting its right to indemnity hereunder, defend and settle any such claim, suit or proceeding if Indemnitor declines to defend against such claim, suit or proceeding or Files for Bankruptcy. The Indemnitee shall reasonably cooperate with the Indemnitor and its legal representatives in the investigation of any action, claim or liability covered by this Agreement, at the expense of the Indemnitor. | ||
| (c) | The Indemnitee shall not, except at its own cost, voluntarily make any payment |
| V.8. 12-5-06 | Initials: |
16/31
BISx INTERNATIONAL LICENSE AGREEMENT
| or incur any expense with respect to any claim or suit without the prior written consent of Indemnitor, which Indemnitor shall not be required to give, provided that the Indemnitee may, without affecting its right to indemnity hereunder, defend and settle any such claim, suit or proceeding if the Indemnitor declines to take responsibility or Files for Bankruptcy. |
| 7.4 | Partial Indemnification. | ||
| In the event a claim is based partially on an indemnified claim described in Sections 7.1 and/or 7.2 above and partially on a non-indemnified claim, or is based partially on a claim described in Section 7.1 above and partially on a claim described in Section 7.2 above, any payments and reasonable attorney fees incurred in connection with such claims are to be apportioned between the Parties in accordance with the degree of cause attributable to each Party. |
8. TERMINATION OR EXPIRATION
| 8.1 | Term and Renewal. | ||
| Unless it is terminated earlier pursuant to this Section, the initial term of this Agreement shall commence on the Effective Date and shall continue for a period of five (5) years following the Commencement Date. The term of this Agreement shall be renewed automatically for successive twelve (12) month periods, unless either Party provides written notice of termination to the other Party at least six (6) months prior to the expiration of the Agreement or any renewal term. | |||
| 8.2 | Termination for Cause. | ||
| Upon the occurrence of a material breach or default as to any obligation hereunder by either Party and the failure of the breaching Party to cure within thirty (30) days after receiving written notice thereof from the non-breaching Party a such material breach or default, this Agreement may be terminated by the non-breaching Party by giving written notice of termination to the breaching Party after such thirty (30) day period, such termination being immediately effective upon the giving of such notice of termination. | |||
| 8.3 | After Termination or Expiration. | ||
| The Parties agree that, once this Agreement is terminated or expires, NK shall immediately cease: |
| (a) | any use or practice of the Licensed Technology; and | ||
| (b) | any use or sale of the Product; provided, however that: |
| (i) | NK shall have the right to sell Products (if payment has been made in full by NK to Aspect) until NK’s stock of Products at the time of such termination or expiration is depleted; | ||
| (ii) | Aspect or any third party designated by Aspect shall sell to NK the parts necessary to repair the Products sold by NK and shall grant to NK the |
| V.8. 12-5-06 | Initials: |
17/31
BISx INTERNATIONAL LICENSE AGREEMENT
| right to repair Products sold by NK, for a period reasonably deemed that Products are used by the customers; and | |||
| (iii) | Aspect or any third party designated by Aspect shall continue to supply end-users with BIS Sensors to use with Products. |
| 8.4 | Payment Obligations Continue. | ||
| Upon termination or expiration of this Agreement, nothing shall be construed to release NK from its obligations to pay Aspect any and all amounts accrued but unpaid pursuant to Section 4 above prior to the date of such termination or expiration. | |||
| 8.5 | No Damages for Termination. | ||
| The Parties agree that if either Party terminates the other Party pursuant to this Section 8, then the terminating Party shall not be liable for damages or injuries suffered by the other Party as a result of that termination, unless otherwise expressly provided herein. |
9. MISCELLANEOUS
| 9.1 | No Indirect Damages. | ||
| EXCEPT AS OTHERWISE PROVIDED IN THIS AGREEMENT, IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER PARTY FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL OR OTHER INDIRECT DAMAGES (INCLUDING, BUT NOT LIMITED TO, LOSS OF PROFITS OR LOSS OF USE DAMAGES) ARISING OUT OF THE MANUFACTURE, USE, SALE OR SUPPLYING OF THE PRODUCT, EVEN IF SUCH PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES OR LOSSES. | |||
| 9.2 | Assignments. | ||
| This Agreement and the rights and obligations hereunder may not be assigned, delegated or transferred by either Party without the prior written consent of the other Party; provided, however that the other Party’s consent shall not be required with respect to any assignment, delegation or transfer by a Party to (i) an Affiliate of such Party; or (ii) the purchaser of all or substantially all of the assets related to this Agreement or stock of such Party, through merger, consolidation or otherwise. To the extent permitted by this Agreement, this Agreement shall be binding upon and inure to the benefit of the permitted successors and assigns of both Parties. | |||
| 9.3 | Governing Law. | ||
| This Agreement shall be construed and governed according to, and any arbitration shall be conducted in accordance with, the laws of the Commonwealth of Massachusetts, U.S.A., excluding its conflicts of laws principles. | |||
| 9.4 | Dispute Resolution. | ||
| Any dispute, controversy or claim arising out of or relating to this Agreement or to a breach hereof, including its interpretation, performance or termination, shall be finally resolved by arbitration. The arbitration shall be conducted by three (3) arbitrators, one to be appointed by Aspect, one to be appointed by NK and a third |
| V.8. 12-5-06 | Initials: |
18/31
BISx INTERNATIONAL LICENSE AGREEMENT
| being nominated by the two arbitrators so selected or, if they cannot agree on a third arbitrator, by the President of the American Arbitration Association. The arbitration shall be conducted in English and in accordance with the commercial arbitration rules of the United Nations Commission on International Trade Law. The arbitration, including the rendering of the award, shall take place in Los Angeles, California, U.S.A. and shall be the exclusive forum for resolving such dispute, controversy or claim. The decision of the arbitrators shall be binding upon the parties hereto, and the expense of the arbitration (including without limitation the award of attorneys’ fees to the prevailing Party) shall be paid as the arbitrators determine. The decision of the arbitrators shall be executory, and judgment thereon may be entered by any court of competent jurisdiction. Notwithstanding anything contained in this Section to the contrary, each Party shall have the right to institute judicial proceedings against the other Party or anyone acting by, through or under such other Party, in order to enforce the instituting Party’s rights hereunder through reformation of contract, specific performance, injunction or similar equitable relief. | |||
| 9.5 | Entire Agreement. | ||
| This Agreement supersedes and cancels the “International License Agreement” between NK and Aspect dated January 21st, 1998 and any previous agreements or understandings, whether oral, written or implied, heretofore in effect, and sets forth the entire agreement between Aspect and NK, with respect to the Products listed in Exhibit A. However, this Agreement does not cancel the “International Distribution Agreement” dated January 21st, 1998. No modification or change may be made in this Agreement except by written instrument duly signed by a duly authorized representative of each Party. | |||
| 9.6 | Notices. | ||
| All notices given under this Agreement shall be in writing and shall be addressed to the Parties at their respective addresses and telecopy numbers, and to the attention of the individuals set forth in Exhibit C. Either Party may change its address, telecopy number and contact person for purposes of this Agreement by giving the other Party written notice of its new address, telecopy number or contact person. Any such notice if given or made by registered or recorded delivery international air mail letter shall be deemed to have been received on the date actually received and if given or made by telecopy transmission shall be deemed to have been received at the time of dispatch, unless such date of deemed receipt is not a day on which banks in the receiving Party’s home city are open for business, in which case the date of deemed receipt shall be the next day on which banks in the receiving Party’s home city are open for business. | |||
| 9.7 | Waivers. | ||
| None of the conditions or provisions of this Agreement shall be held to have been waived by any act or knowledge on the part of either Party, except by an instrument in writing signed by a duly authorized officer or representative of such Party. Further, the waiver by either Party of any right hereunder or the failure to enforce at any time any of the provisions of this |
| V.8. 12-5-06 | Initials: |
19/31
BISx INTERNATIONAL LICENSE AGREEMENT
| Agreement, or any rights with respect thereto, shall not be deemed to be a waiver of any other rights hereunder or any breach or failure of performance of the other Party. | |||
| 9.8 | Responsibility for Taxes. | ||
| Taxes now or hereafter imposed with respect to the transactions contemplated hereunder (with the exception of income taxes or other taxes imposed upon Aspect) shall be the responsibility of NK, and if paid or required to be paid by Aspect, the amount thereof shall be added to and become a part of the amounts payable by NK hereunder. | |||
| 9.9 | Severability. | ||
| If any provision of this Agreement is declared invalid or unenforceable by a court having competent jurisdiction, it is mutually agreed that this Agreement shall endure except for the part declared invalid or unenforceable by order of such court. The Parties shall consult and use their best efforts to agree upon a valid and enforceable provision which shall be a reasonable substitute for such invalid or unenforceable provision in light of the intent of this Agreement. | |||
| 9.10 | Counterparts. | ||
| This Agreement may be executed in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. | |||
| 9.11 | Relationship of the Parties. |
| (a) | The relationship between Aspect and NK shall not be construed to be that of employer and employee, or to constitute a partnership, joint venture or agency of any kind. Neither Party shall have any right to enter into any contracts or commitments in the name of, or on behalf of, the other Party, or to bind the other Party in any respect whatsoever. | ||
| (b) | NK shall not obligate or purport to obligate Aspect by issuing or making any affirmations, representations, warranties or guarantees with respect to BISx Systems to any third party, other than the warranties described in Exhibit D hereto. |
| 9.12 | Language. | ||
| All written material, correspondence, Technical Information, notices and oral assistance supplied by either Party hereunder shall be in the English language. | |||
| 9.13 | Survival of Contents. | ||
| Notwithstanding anything else in this Agreement to the contrary, the parties agree that Sections 2.7 (for one year after the termination or expiration of this Agreement), 2.10, 4.4 and 4.5 and Sections 5, 6, 7, 8 and 9 shall survive the termination or expiration of this Agreement, as the case may be. | |||
| 9.14 | Compliance With Laws. | ||
| NK covenants that all of its activities under or pursuant to this Agreement shall comply with all applicable laws, rules and regulations. NK shall be responsible for |
| V.8. 12-5-06 | Initials: |
20/31
BISx INTERNATIONAL LICENSE AGREEMENT
| obtaining all licenses, permits and approvals which are necessary or advisable for sales of Products in all jurisdictions and for the performance of its duties hereunder. In particular, but without limitation, NK shall be responsible for all submissions to the MHLW which may be required to obtain marketing approval of the Product. NK shall use its best efforts to obtain such MHLW approvals as expeditiously as possible. NK shall promptly give Aspect written notice of the MHLW Approval Date. Aspect shall: (i) fully comply with any applicable law, regulation and rule of government of the United States and agencies or instrumentalities thereof; and (ii) maintain all U.S. governmental approvals and licenses necessary to produce and export the BISx System. | |||
| 9.15 | Headings. | ||
| Any headings contained herein are for directory purposes only, do not constitute a part of this Agreement, and shall not be employed in interpreting this Agreement. |
| 9.16 | Exhibits. | ||
| The following Exhibits shall be part of this Agreement: |
EXHIBIT A
|
Aspect Products and Purchase Prices | ||
EXHIBIT B
|
Specifications: BISx System | ||
EXHIBIT C
|
Contact Persons / Addresses | ||
EXHIBIT D
|
Warranty | ||
EXHIBIT E
|
Aspect and Nihon Kohden Trademarks |
IN WITNESS WHEREOF, the parties hereto have signed this Agreement under seal.
| ASPECT MEDICAL SYSTEMS, INC. | ||||||
| By Name: |
/s/ Xxxxxxx Xxxxxx
|
|||||
| Title: | Chief Financial Officer | |||||
| NIHON KOHDEN CORPORATION | ||||||
| By Name: |
/s/ Xxxxx Xxxxx
|
|||||
| Title: | President and Chief Executive Officer | |||||
| V.8. 12-5-06 | Initials: |
21/31
BISx INTERNATIONAL LICENSE AGREEMENT
EXHIBIT A
ASPECT PRODUCTS AND PURCHASE PRICES
ASPECT PRODUCTS AND PURCHASE PRICES
A) BISx SYSTEM:
List price for the BISx System: $ [**]. Volume discounts are available as follows:
| As of Effective Date | As of Commencement Date | |||||
| Volume | Pricing | Volume | Pricing | |||
| 0 – 250 | $[**] | 0 – 1000 | $[**] | |||
| 251 – 500 | $[**] | 1001 – 2000 | $[**] | |||
| >500 | $[**] | 2001– 3500 | $[**] | |||
| >3500 | $[**] | |||||
| Demo Units | $[**] | |||||
| Japanese Rental Units* | $[**] | |||||
| Three purchase opportunities for demonstration units during introduction of BISx in Japan, Europe and the United States. | ||||||
| Demo volumes to be agreed by Aspect and Nihon Kohden | ||||||
For the purpose of calculating the volume discount for a given calendar year, all BISx Systems,
shipped during that calendar year (excluding Products that are provided free of charge) will be
included in the total volume discount calculation.
The initial pricing for a given calendar year is based on the total volume of BISx Systems,
purchased in the prior calendar year. Buyer will maintain the previous year pricing as long as
they purchase the volumes levels required for that pricing. For example, if 1,200 BISx Systems
were purchased in year 1, the initial volume pricing level for year 2 will be the 1,001–2,000
unit level. If in year 2, 1,001 BISx Systems were purchased, Buyer would again qualify for the
1,001 – 2,000 volume pricing level in year 3.
If a higher volume level of BISx Systems is achieved during a given calendar year, the price on
purchases made after achieving the higher volume level will reflect the price associated with
the appropriate volume level achieved. All price adjustments are proactive and no credit will
apply retroactively to units purchased prior to achieving the volume break point. For example,
if midway through year 1, 1,000 BISx Systems are purchased, the price on 1,001st Aspect BISx
System will reflect the next volume break. Achieving this higher volume level will also reduce
the initial pricing for the following year.
*Japanese Rental Units reflect BISx units which are placed at no charge to the end-user by NK
within the Japanese market. Aspect
| V.8. 12-5-06 | Initials: |
22/31
BISx INTERNATIONAL LICENSE AGREEMENT
will have the right to request documentation from NK indicating the unit was placed at no charge.
B) ASPECT BIS SENSORS
| • | Distribution of BIS Sensors outside the United States will be covered under a separate agreement. Sensor sales by NK are prohibited in the United States. |
C) SERVICE PARTS:
| Part # | Description | Aspect List Price | NK Price | ||||
186-0195-NK
|
BISx System (replacement) | $[**] | [**] | ||||
186-0126
|
PIC+ Cable (replacement) | $[**] | $[**] | ||||
175-0061-DC
|
Hypertronics Monitor Cable | $[**] | $[**] | ||||
| ** | Price shall be the same as the current BISx System price (Exhibit A, Section A) |
| V.8. 12-5-06 | Initials: |
23/31
BISx INTERNATIONAL LICENSE AGREEMENT
EXHIBIT B
SPECIFICATIONS: BISx SYSTEM
SPECIFICATIONS: BISx SYSTEM
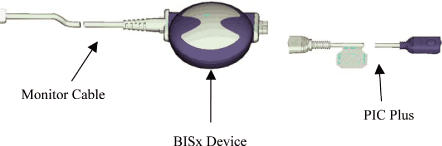
BISx is a device that acquires up to two channels of EEG and computes BIS and other EEG parameters,
uniting the functionality of the existing Aspect BIS Engine and DSC-XP. BISx is designed to mate
with Aspect’s XP platform 1 or 2 channel sensors. BISx has no display or user interface. It plugs
into a host monitor system for display of EEG and processed parameters. BISx is designed for use
wherever sedative drugs are administered, including but not limited to the following environments:
Operating rooms, Intensive Care Units, Procedural Sedation, and Clinical Research areas.
The standard BISx acquires EEG via single channel or two channel referential XP platform sensors
and XP compatible sensors. The inputs are protected against damage from electro-static discharge
(ESD), a direct hit from an electrocautery device, and defibrillation of the patient to which it is
attached. The BISx recovers from large signal saturation quickly. The BISx is resistant to
electro-surgical (ESU) interference.
The BISx is designed to be placed near the patient’s head. It is unobtrusive and conveniently
handled. It is sealed so as not to allow liquids to enter when splashed. The enclosure is not
painted, but rather is of materials that are a solid color throughout. The BISx includes a
convenient method for attaching and de-attaching the enclosure to surgical draperies, sheets, or an
IV pole.
The BISx is attached to the host monitoring system via a 9-foot monitor cable. The wire is narrow
and highly flexible. The monitor connection is integral to the enclosure (no pigtail), and may
require the use of tools for detachment from the device for service or replacement. The enclosure
is sealed against liquid ingress only when the monitor cable is attached. The connector on the host
monitor end is a standard Hypertronics (or an equivalent brand as determined by
| V.8. 12-5-06 | Initials: |
24/31
BISx INTERNATIONAL LICENSE AGREEMENT
Aspect) connector.
There are no adjustable parts inside the BISx. The cables may be replaced without opening the
enclosure.
The BISx connects to a sensor via the Aspect PIC Plus (Aspect part number 186-0107). The PIC Plus
is approximately 48 inches long. The PIC Plus connection is integral to the enclosure (no
pigtail), and can be detached from the device for service or replacement without the use of tools.
The enclosure is sealed against liquid ingress even when the PIC is detached.
Each BISx is given a unique serial identifier, allowing for electronic identification and tracking
of every device.
The BISx software is stored in reprogrammable FLASH memory. Software upgrades and downloads can be
accomplished on-site or remotely via the USB interface. BISx can be connected and disconnected to
an already
powered up host monitor. The host monitor should automatically detect its presence and configure
it accordingly.
The BISx is a High Power, USB Powered Device which adheres to the power requirements of the USB 2.0
specification. The BISx requires 5 Vdc +/- 5% to operate and may draw up to 500 mA.
PHYSICAL SPECIFICATIONS:
BISx
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
Weight: 227g (8.0 oz) without cables
456g (1.0 lb) with cables (Estimated)
Weight: 227g (8.0 oz) without cables
456g (1.0 lb) with cables (Estimated)
BISx Integral Cables
Monitor Cable: 2.74m (9ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
SAFETY SPECIFICATIONS:
| • | The BISx complies with the essential requirements of the Medical Device Directive 93/42/EEC, as well as IEC 60601-1 and IEC 00000-0-00. |
| • | It is a Type BF applied part. It has internal optical coupling and an isolation transformer for patient isolation. |
| • | It is protected against damage from defibrillation as long as the sensor is not located between the defibrillator pads and is resistant to artifact from electrosurgery. |
| • | United States federal law restricts this device to sale by or on the order of a physician. |
| • | BISx and cables are latex free. |
ENVIRONMENTAL SPECIFICATIONS:
Liquid Ingress Rating
| V.8. 12-5-06 | Initials: |
25/31
BISx INTERNATIONAL LICENSE AGREEMENT
IEC 529 IPX4
Temperature Range
Operating: 0 to 40°C (32 to 104°F)
Storage: -40 to 70°C (-40 to 158°F)
Storage: -40 to 70°C (-40 to 158°F)
Humidity
Operating: 10 to 95% RH at 40°C (104°F), non-condensing
Storage: 10 to 95% RH at 60°C (140°F), non-condensing
Storage: 10 to 95% RH at 60°C (140°F), non-condensing
Altitude Range
Operating: Up to 6,130 m (20,000 ft)
Storage: Up to 15,300 m (50,000 ft)
Storage: Up to 15,300 m (50,000 ft)
PERFORMANCE SPECIFICATIONS:
Noise (EEG Waveform) |
< 0.3µVRMS (2.0µV peak-to-peak) | |
BIS Numeric Update Frequency |
Once per second | |
Bandwidth |
0.25 to 100 Hz (- 3dB) | |
Impedance Measurement Range |
0 to 999 kOhm | |
Input Impedance |
>50 Mohm | |
Patient Leakage |
<10µA | |
Measurement Ranges |
||
Bispectral Index (BIS): |
0 to 100 | |
Electromyographic Strength (EMG): |
25 to 100 dB, where 1µV2=40dB | |
Signal Quality Index (SQI): |
0 to 100% | |
Suppression Ratio (SR): |
0 to 100% | |
Spectral Edge Frequency (SEF): |
0.5 to 30.0 Hz | |
Total Power: |
40 to 100 dB, where 1µV RMS=40dB | |
Burst Count: |
0 to 30 |
INTERFACE SPECIFICATIONS:
Communication |
||
USB Interface: |
Conforms to USB Rev. 1.1 Full-Speed protocol | |
Protocol: |
Aspect proprietary | |
Power |
12 Vdc +/-25%, 400 mA maximum current draw | |
Connector |
3M Inc. 10120-6000E Connector, 20 position, .050” Mini D Ribbon (or equivalent) |
| V.8. 12-5-06 | Initials: |
26/31
BISx INTERNATIONAL LICENSE AGREEMENT
EXHIBIT C
CONTACT PERSONS/ADDRESSES
CONTACT PERSONS/ADDRESSES
Contact Persons and responsibilities at Aspect:
| Phone Number | ||||||
| Person | Title | Responsibility | Fax Number | |||
Xxxx Xxxxxxx
|
Xx. Director, OEM Partnerships | Contract and Marketing | xxxxxxxx@xxxxxxxx.xxx | |||
Xxxxx Xxxxxxxxx
|
Director, OEM Engineering | Project Manager | xxxxxxxxxx@xxxxxxxx.xxx | |||
Xxxxxxxxx Xxxxxxx
|
Xx. Director, RA/QA | Quality and Regulatory Matters | xxxxxxxx@xxxxxxxx.xxx | |||
Xxx Xxxxxxxxxx
|
Xx. Support Representative | Technical Service | ttramontano@aspectms. com | |||
Xxxxx Nilhand
|
Sr. Revenue Analyst | IB Reporting | xxxxxxx@xxxxxxxx.xxx |
Mailing Address:
Contact Persons and responsibilities at Nihon Kohden:
| Phone Number | ||||||
| Person | Title | Responsibility | Fax Number | |||
| Hiroshi Xxxx | General Manager,
Import Business
Operations
|
Contact | Xxxxxxx_Xxxx@xx0.xxx.xx.xx | |||
| Xxxxxxx Xxxxx | Xx. Marketing
Manager, OR/ICU
Group, Marketing
Business Group
|
Project Manager | Xxxxxxx_Xxxxx@xx0.xxx.xx.xx |
| V.8. 12-5-06 | Initials: |
27/31
BISx INTERNATIONAL LICENSE AGREEMENT
| Phone Number | ||||||
| Person | Title | Responsibility | Fax Number | |||
| Xxxxxxxxx Xxxxxxxx | Xx. Manager,
Quality Assurance
Department
|
Quality and Regulatory Matters | Masatoshi_Tamagawa@mb3.nkc. xx.xx | |||
| Yoshikazu Midorikawa | Sr. Manager,
Product Engineering
Department
|
Technical Service | Xxxxxxxxx_Xxxxxxxxxx@xx0.xx x.xx.xx |
Mailing Address:
Nihon Kohden Corporation
00-0 Xxxxxxxxxxx, 0-xxxxx,
Xxxxxxxx-xx, Xxxxx 000
Xxxxx
00-0 Xxxxxxxxxxx, 0-xxxxx,
Xxxxxxxx-xx, Xxxxx 000
Xxxxx
| V.8. 12-5-06 | Initials: |
28/31
BISx INTERNATIONAL LICENSE AGREEMENT
EXHIBIT D
WARRANTY
WARRANTY
Aspect warrants to the initial Purchaser that the BISx System (“Warranted Product”) will be free
from defects in workmanship or materials, when given normal, proper, and intended usage for a
period of 18 months from the date of its initial shipment to NK, or 12 months from the date of
resale by the NK, whichever period first expires. This warranty shall not apply to expendable
components and supply items, such as, but not limited to, cables (except for failures occurring
within 180 days of receipt of shipment to NK) or disposable items such as a BIS Sensor after the
expiration date marked on the BIS Sensor packaging. Aspect’s obligations under this warranty are
to repair or replace any Warranted Product or part thereof that Aspect reasonably determines to be
covered by this warranty and to be defective in workmanship or materials provided that the
Warranted Product is returned to the factory with freight prepaid. Repair or replacement of
Products under this warranty does not extend the Warranty Period.
To request repair or replacement under this warranty, NK should contact the Aspect Technical
Services contact as defined in Exhibit C. Aspect will authorize NK to return the Warranted Product
(or part thereof) to Aspect. Aspect shall determine whether to repair or replace Products and
parts covered by this warranty and all Products or parts replaced shall become Aspect’s property.
In the course of warranty service, Aspect may, but shall not be required, to make engineering
improvements to the Warranted Product or part thereof. If Aspect reasonably determines that a
repair or replacement is covered by the warranty, Aspect shall bear the costs of shipping the
repaired or replacement Product to the NK. All other shipping costs shall be paid by the NK. Risk
of loss or damage during shipments under this warranty shall be borne by the Party shipping the
Product. Products shipped by NK under this warranty shall be packaged in the original shipping
container or equivalent packaging to protect the Product. If NK ships a Product to Aspect in
unsuitable packaging, any physical damage present in the Product upon receipt by Aspect (and not
previously reported) will be presumed to have occurred in transit and will be the responsibility of
the NK.
Unless authorized or instructed by Aspect in advance, this warranty does not extend to any
Warranted Products or part thereof: that have been subject to misuse, neglect or accident; that
have been damaged by causes external to the Warranted Product, including but not limited to failure
of or faulty electrical power; that have been used in violation of Aspect’s instructions; that have
been affixed to any nonstandard accessory attachment; on which the serial number has been removed
or made illegible; that have been modified by anyone other than Aspect; or that have been
disassembled, serviced or reassembled by anyone other than Aspect, unless authorized by Aspect.
Aspect shall have no obligation to make repairs, replacements, or corrections which result, in
whole or in part, from normal wear and tear. Aspect makes no warranty (a) with respect to any
products that are not Warranted Products, (b) with respect to any products purchased from a person
other than Aspect or an Aspect authorized distributor or (c) with respect to any product sold under
a brand name other than Aspect.
| V.8. 12-5-06 | Initials: |
29/31
BISx INTERNATIONAL LICENSE AGREEMENT
THIS WARRANTY IS THE SOLE AND EXCLUSIVE WARRANTY FOR ASPECT’S PRODUCTS, EXTENDS ONLY TO THE NK AND
IS EXPRESSLY IN LIEU OF ANY OTHER EXPRESS OR IMPLIED WARRANTIES INCLUDING WITHOUT LIMITATION ANY
WARRANTY AS TO MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NONINFRINGEMENT. EXCEPT AS
OTHERWISE PROVIDED HEREIN, ASPECT’S MAXIMUM LIABILITY ARISING OUT OF THE SALE OF THE PRODUCTS OR
THEIR USE, WHETHER BASED ON WARRANTY, CONTRACT, TORT OR OTHERWISE, SHALL NOT EXCEED THE ACTUAL
PAYMENTS RECEIVED BY ASPECT IN CONNECTION THEREWITH. EXCEPT FOR THE INDEMNIFICATION SET FORTH IN
SECTION 7, ASPECT SHALL NOT BE LIABLE FOR ANY INCINDENTAL, SPECIAL, CONSEQUENTIAL OR OTHER
INDIRECT LOSS, DAMAGE OR EXPENSE (INCLUDING WITHOUT LIMITATION LOST PROFITS) DIRECTLY OR INDIRECTLY
ARISING FROM THE SALE, INABILITY TO SELL, USE OR LOSS OF USE OF ANY PRODUCT. EXCEPT AS SET FORTH
HEREIN, ALL PRODUCTS ARE SUPPLIED “AS IS” WITHOUT WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED.
| V.8. 12-5-06 | Initials: |
30/31
BISx INTERNATIONAL LICENSE AGREEMENT
EXHIBIT E
ASPECT TRADEMARKS
ASPECT TRADEMARKS
| Aspect Trademarks | Reference | |
Aspect ®
|
Aspect is a registered trademark of Aspect Medical Systems, Inc | |
A-2000 TM
|
A-2000 is a trademark of Aspect Medical Systems, Inc. | |
Bispectral Index ®
|
Bispectral is a registered trademark of Aspect Medical Systems, Inc. | |
BIS ®
|
BIS is a registered trademark of Aspect Medical Systems, Inc. | |

|
BIS logo is a registered trademark of Aspect Medical Systems, Inc. | |
BISx ™
|
BISx is a trademark of Aspect Medical Systems, Inc. | |
BIS Ready™
|
BIS Ready is a trademark of Aspect Medical Systems, Inc. | |
BIS VISTA ™
|
BIS VISTA is a trademark of Aspect Medical Systems, Inc. | |
|
BISx logo is a trademark of Aspect Medical Systems, Inc. | |

|
BIS Ready logo is a registered trademark of Aspect Medical Systems, Inc. |
| V.8. 12-5-06 | Initials: |
31/31

