Contract
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit 10.1
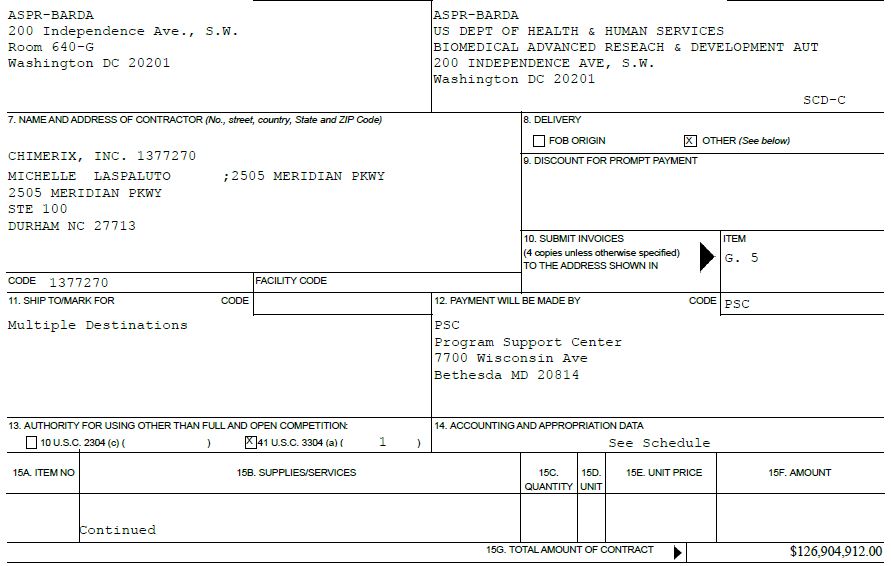
| AWARD/CONTACT | 1. THIS CONTRACT IS RATED ORDER UNDER DPAS (15 CFR 700) | RATING | PAGE OF PAGES | ||||||||||||||
| 1 | 51 | ||||||||||||||||
2. CONTRACT (Proc. Inst. Ident.) NO. | 3. EFFECTIVE DATE | 4. REQUISITION/PURCHASE REQUEST/PROJECT NO. | |||||||||||||||
| 75A50122C00047 | 08/29/2022 | See Schedule | |||||||||||||||
5. ISSUED BY | CODE | ASPR-BARDA | 6. ADMINISTERED BY (If other than Item 5) | CODE | ASPR-BARDA | ||||||||||||
16. TABLE OF CONTENTS
(X) | SEC. | DESCRIPTION | PAGE(S) | (X) | SEC. | DESCRIPTION | PAGE(S) | ||||||||||||||||
PART I - THE SCHEDULE | PART II - CONTRACT CLAUSES | ||||||||||||||||||||||
X | A | SOLICITATION/CONTRACT FORM | 1 | X | I | CONTRACT CLAUSES | 45 | ||||||||||||||||
X | B | SUPPLIES OR SERVICES AND PRICES/COSTS | 3 | PART III - LIST OF DOCUMENTS, EXHIBITS AND OTHER ATTACH. | |||||||||||||||||||
X | C | DESCRIPTION/SPECS./WORK STATEMENT | 11 | X | J | LIST OF ATTACHMENTS | 51 | ||||||||||||||||
X | D | PACKAGING AND MARKING | 12 | PART IV - REPRESENTATIONS AND INSTRUCTIONS | |||||||||||||||||||
X | E | INSPECTION AND ACCEPTANCE | 13 | K | REPRESENTATIONS, CERTIFICATIONS AND OTHER STATEMENTS OF OFFERORS | ||||||||||||||||||
X | F | DELIVERIES OR PERFORMANCE | 14 | ||||||||||||||||||||
X | G | CONTRACT ADMINISTRATION DATA | 26 | L | INSTRS., CONDS., AND NOTICES TO OFFERORS | ||||||||||||||||||
X | H | SPECIAL CONTRACT REQUIREMENTS | 32 | M | EVALUATION FACTORS FOR AWARD | ||||||||||||||||||
CONTRACTING OFFICER WILL COMPLETE ITEM 17 (SEALED-BID OR NEGOTIATED PROCUREMENT) OR 18 (SEALED-BID PROCUREMENT) AS APPLICABLE
document and return 1 copies to issuing office.) Contractor agrees to furnish and deliver all items or perform all the services set forth or otherwise identified above and on any continuation sheets for the consideration stated herein. The rights and obligations of the parties to this contract shall be subject to and governed by the following documents: (a) this award/contract, (b) the solicitation, if any, and (c) such provisions, representations, certifications, and specifications, as are attached or incorporated by reference herein. (Attachments are listed herein.) | 18. SEALED-BID AWARD (Contractor is not required to sign this document.) Your bid on Solicitation Number , including the additions or changes made by you which additions or changes are set forth in full above, is hereby accepted as to the items listed above and on any continuation sheets. This award consummates the contract which consists of the following documents: (a) the Government's solicitation and your bid, and (b) this award/contract. No further contractual document is necessary. (Block 18 should be checked only when awarding a sealed-bid contract.) | ||||||||||
19A. NAME AND TITLE OF SIGNER (Type or print) | 20A. NAME OF CONTRACTING OFFICER XXXX X. XXXXXXX | ||||||||||
19B. NAME OF CONTRACTOR CHIMERIX, INC. 1377270 BY s/ Xxxxxxxx Xxxxxxxxx (Signature of person authorized to sign) | 19C. DATE SIGNED 08/26/2022 | 20B. UNITED STATES OF AMERICA BY /s/ Xxxx X. Xxxxxxx (Signature of the Contracting Officer) | 20C. DATE SIGNED 08/26/2022 | ||||||||
AUTHORIZED FOR LOCAL REPRODUCTION
Previous edition is NOT usable
STANDARD FORM 26 (Rev. 3/2013)
Prescribed by GSA - FAR (48 CFR) 53.214(a)
| CONTINUATION SHEET | REFERENCE NO. OF DOCUMENT BEING CONTINUED 75A50122C00047 | PAGE OF | ||||||||||||||||||||||||
| 2 | 51 | |||||||||||||||||||||||||
NAME OF OFFEROR OR CONTRACTOR CHIMERIX, INC. 1377270 | ||||||||||||||||||||||||||
ITEM NO. (A) | SUPPLIES/SERVICES (B) | QUANTITY (C) | UNIT (D) | UNIT PRICE (E) | AMOUNT (F) | |||||||||||||||||||||
1 2 3 4 5 6 7 | Tax ID Number:33-0903395 DUNS Number: 121785997 Smallpox Antiviral ASPR-22-01970 CLIN 0001 CPFF Base: Post Marketing Commitments, Suspension Manufacturing Scale-up and Other Stability Extension Support Obligated Amount: $[*] Requisition No: OS300891 Accounting Info: 2022.0000000.25103 Appr. Yr.: 2022 CAN: 1992126 Object Class: 25103 Funded: $[*] ASPR-22-01970 CLIN 0002 FFP Base period: Delivery of FDP to the SNS Requisition No: OS300891 Accounting Info: 2022.0000000.25103 Appr. Yr.: 2022 CAN: 1990178 Object Class: 25103 Funded: $[*] Accounting Info: 2022.0000000.25103 Appr. Yr.: 2022 CAN: 1992126 Object Class: 25103 Funded: $[*] CLIN 0003 Conduct of Ph 4 PMR Field Study Amount: $[*] (Option Line Item) CLIN 0004 Delivery of FDP to the SNS Amount: $[*] (Option Line Item) CLIN 0005 Delivery of FDP to the SNS Amount: $[*] (Option Line Item) CLIN 0006 Delivery of FDP to the SNS Amount: $[*] (Option Line Item) CLIN 0007 Delivery of FDP to the SNS Amount: $[*] (Option Line Item) | [*] [*] 0.00 0.00 0.00 0.00 0.00 | ||||||||||||||||||||||||
AUTHORIZED FOR LOCAL REPR OPTIONAL FORM 336 (4-86)
Sponsored by GSA FAR (48 CFR) 53.110
PART I – THE SCHEDULE
SECTION B – SUPPLIES OR SERVICE AND PRICE / COST
B.1.BRIEF DESCRIPTION OF SUPPLIES OR SERVICES
In 2004, the Department of Homeland Security (DHS) issued a Material Threat Determination for smallpox, establishing it as a threat to national security. Periodic smallpox outbreaks were common prior to the twentieth century with fatality rates of greater than thirty percent. Smallpox was eliminated as an endemic disease by 1980 following an intensive focused international ring vaccination campaign. The eradication of the disease led the scientific community to reduce the number of laboratories holding live variola virus so that at present the only known repositories of live variola virus are the World Health Organization (WHO) Collaborating Centers at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia and the State Center of Virology and Technology (VECTOR) in Novosibirsk, Russian Federation. While variola virus no longer exists in nature, the possibility of preserved, unrecognized samples or clandestine stocks, the potential re-emergence from natural sources, and recent advances in synthetic biology describing the construction of poxvirus genomes de novo, mean the threat of the reemergence of smallpox, although small, still exists.
Although the primary response to smallpox is the development and procurement of vaccines to break the chain of transmission, the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) quickly recognized that in a smallpox attack many of those exposed to the virus would develop symptoms and become refractory to vaccination. A Department of Health and Human Services (HHS)-led collaboration between epidemiological modelers and subject matter experts resulted in the development of a public health consequence assessment for the number of primary and secondary infections after a deliberate large-scale release of smallpox in an urban setting. After substantial discussion over the refinement of the model, the scenario-based Requirements for Smallpox Antivirals were presented to the PHEMCE Enterprise Executive Committee (EEC) for approval describing a scenario requiring 1.7 million treatment courses of antivirals to mitigate the consequences of a large smallpox attack.
In March 2009, the Biomedical Advanced Research and Development Authority (BARDA) issued a Request for Proposals (RFP) under Project BioShield (PBS) to support the advanced development and procurement of 1.7 million treatment courses of a smallpox antiviral. An award under this solicitation resulted in the FDA approval of the first antiviral for the treatment of smallpox in 2018.
The development of antivirals against smallpox was deemed important and essential in the Institute of Medicine (IoM) report Live Variola Virus, Considerations for Continuing Research with the panel emphasizing the need for the development of multiple countermeasures with distinct mechanisms of action to obviate the potential for the evolution of viral resistance through selective pressure.
Additionally, developments in molecular biology since the Institute of Medicine report have increased the possibility of de novo synthesis of poxviruses genomes, leading to the possible creation of resistant virus and further emphasizing the danger of relying on a single therapeutic countermeasure. PBS funding will support the development and procurement of a second smallpox therapeutic with an alternative mechanism of action to address the potential for the development of resistance to the current stockpile drug.
Contract No. 75A50122C00047
Page 3 of 51
B.1.PRICES / COSTS
The Base Period includes both a Cost-Plus Fixed Fee (CPFF) Contract Line Item Number (CLIN) to support completion of post marketing commitments, suspension manufacturing scale-up and stability extension support and a Firm Fixed Price (FFP) CLIN to support an initial procurement of final drug product (FDP) for delivery to the Strategic National Stockpile (SNS).
B.2.1.BASE PERIOD
a.The estimated cost of CLIN 0001 is $[*].
b.The fixed fee for CLIN 0001 is $[*]. The fixed fee shall be subject to the withholding provisions of the clauses ALLOWABLE COST AND PAYMENT and FIXED FEE referenced in Section I of this contract.
c.The total estimated amount of CLIN 0001, represented by the sum of the estimated cost plus the fixed fee, is $11,878,478.
d.The firm fixed price amount of CLIN 0002 is $115,026,434.
e.The total contract value of CPFF and FFP CLINs 0001 and 0002 is $126,904,912.
Table 1. Base Period Cost Reimbursement CLIN | |||||||||||||||||
| CLIN | Period of Performance | Supplies/Services | Estimated Cost | Fixed Fee | Cost + Fixed Fee (CPFF) | ||||||||||||
0001 (Base) | 08/29/2022 – 08/28/2027 | Post Marketing Commitments, Suspension Manufacturing | $[*] | $[*] | $11,878,478 | ||||||||||||
Scale-up and Other Stability | |||||||||||||||||
Extension Support | |||||||||||||||||
Table 2. Base Period Firm Fixed Price CLIN | |||||||||||||||||
| CLIN | Period of Performance | Supplies/Services | Units (Treatment Courses) | Unit Price ($) | Total ($) | ||||||||||||
0002 (Base) | 08/29/2022 – 08/28/2027 | Delivery of FDP to the SNS -Oral Solid Dosage Formulation -Liquid Formulation (Rebate-Prior Contract Costs)** Total | [*] [*] | [*] [*] | $ [*] $ [*] | ||||||||||||
| $ [*] | |||||||||||||||||
| 319,000 | $115,026,434 | ||||||||||||||||
**See Rebates under Advanced Understandings in Section B.3 below.
B.2.2.OPTIONS
a.The contract includes optional cost reimbursement CLIN 0003 and optional Firm Fixed Price CLINs 0004, 0005, 0006 and 0007. The Government may exercise Options in accordance with 52.217-7 Option for Increased Quantity – Separately Priced Line Item (March 1989), as set forth
Contract No. 75A50122C00047
Page 4 of 51
in Section I of the contract.
b.Unless the government exercises its option pursuant to the option clause contained in SECTION I, as well as successful completion of the “go criteria,” the contract consists only of the Base Work segment specified in the Statement of Work as defined in SECTIONS C and F, for the price set forth in SECTION B.2 of the contract.
c.The Government may modify the contract bilaterally and require the contractor to provide supplies and services for Option Periods listed below, in accordance with 52.217-7.
d.If the Government decides to exercise an option(s), the Government will provide the Contractor a preliminary written notice of its intent as referenced in Section B.3.j below. Specific information regarding the time frame for this notice is set forth in Section B.3.j . The tentative time frame for period of performance is set forth below.
e.As the government reserves the right to activate the option CLINs over a range of years, the pricing of the Option CLINs will vary to take inflation into consideration, as reflected in the Table 3. The final price per treatment course for the option CLINs will be the price identified for the fiscal year in which the CLIN is exercised, as laid out in Table 3.
Table 3. Option Period Pricing Based on Year of CLIN Activation | |||||||||||||||||||||||||||||
FY23 | FY24 | FY25 | FY26 | FY27 | FY28 | FY29 | FY30 | FY31 | |||||||||||||||||||||
Tablet | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||||||||||||
Suspension | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||||||||||||
Table 4. Option Period Cost Reimbursement CLIN | |||||||||||||||||
| CLIN | Period of Performance | Supplies/Services | Estimated Cost | Fixed Fee | Cost + Fixed Fee (CPFF) | ||||||||||||
| 0003 | estimated [*] | Conduct of Ph 4 PMR Field Study | $[*] | $[*] | $[*] | ||||||||||||
Table 5. Option Period Firm Fixed Price CLINs | |||||||||||||||||
| CLIN | Period of Performance | Supplies/Services | Units (Treatment Courses) | Unit Price ($)* | Total ($) | ||||||||||||
| 0004 | estimated [*] | Delivery of FDP to the SNS -Oral Solid Dosage Formulation -Liquid Formulation (Rebate-CLIN 0001 Costs)** Total | [*] [*] | [*] [*] | $ [*] $ [*] $ [*] | ||||||||||||
| [*] | $ [*] | ||||||||||||||||
Contract No. 75A50122C00047
Page 5 of 51
| 0005 | estimated [*] | Delivery of FDP to the SNS -Oral Solid Dosage Formulation -Liquid Formulation Total | [*] [*] [*] | [*] [*] | $ [*] $ [*] $ [*] | ||||||||||||
| 0006 | estimated [*] | Delivery of FDP to the SNS -Oral Solid Dosage Formulation -Liquid Formulation Total | [*] [*] [*] | [*] [*] | $ [*] $ [*] $ [*] | ||||||||||||
| 0007 | estimated [*] | Delivery of FDP to the SNS -Oral Solid Dosage Formulation -Liquid Formulation Total | [*] [*] [*] | [*] [*] | $ [*] $ [*] $ [*] | ||||||||||||
*Pricing will be based on the year of the CLIN activation in accordance with Table 3.
**See Rebates under Advanced Understandings in Section B.3 below.
B.2.3. PAYMENT FOR FIRM FIXED PRICE CLINs
The Contractor will be entitled to receive payment under firm fixed price CLINs upon delivery and acceptance by the USG of any material or product delivered to the SNS.
B.3.ADVANCE UNDERSTANDINGS
a.Subcontracts and Consultants
Award of any FFP subcontract or FFP consulting agreement in excess of $250,000 or any cost reimbursement subcontract or consulting agreement under CPFF CLINs under this contract shall not proceed without the prior written consent of the Contracting Officer via a Contracting Officer Authorization (COA) Letter. COA letters will only be issued upon review of the supporting documentation required by FAR Clause 52.244-2, Subcontracts. After receiving written consent of the subcontract by the Contracting Officer, a copy of the signed, executed subcontract or consulting agreement shall be provided to the Contracting Officer within ten (10) calendar days of full execution.
b.Site Visits, Inspections and General Audits
At the discretion of the USG, with two (2) business days’ notice to the Contractor, the USG reserves the right to conduct site visits and inspections of activities under CPFF CLINs under this contract on an as needed basis, including collection of product samples and intermediates held by the Contractor, or subcontractor. All costs reasonably incurred by the Contractor and subcontractor for such visit and/or inspection shall be allowable costs. The Contractor shall coordinate these visits and shall have the opportunity to accompany the USG on any such visits. Under time-sensitive or critical situations, the USG reserves the right to suspend the two-business days’ notice to the Contractor.
If the Government, Contractor, or other party identifies any issues during an audit, the Contractor shall capture the issues, identify potential solutions, and provide a report to the Government for review and acceptance.
Contract No. 75A50122C00047
Page 6 of 51
•If issues are identified during the audit, Contractor shall submit an issue report to the CO and COR within 15 business days detailing the finding and corrective action(s) of the audit.
•COR and CO will review the issues report and provide a response to the Contractor within ten (10) business days.
•Once corrective action is completed, the Contractor will provide a final report to the CO and COR within a time frame negotiated with the COR in writing after review of the issues report.
c.Man-in-Plant
At the discretion of the Government and with seven calendar (7) days advance notice to the Contractor in writing from the Contracting Officer, the Government may place a man-in-plant in the Contractor’s facility who shall be subject to the Contractor’s policies and procedures regarding security and facility access at all times while in the Contractor’s facility. As determined by federal law, no Government representative shall publish, divulge, disclose, or make known in any manner, or to any extent not authorized by law, any information coming to him in the course of employment or official duties, while stationed in a contractor plant.
d.Emergency Use Authorization (EUA)
It is anticipated that the licensed therapeutic could be administered under an Expanded Access Investigational New Drug (EA IND) or under an “Emergency Use Authorization” (EUA) sponsored by BARDA and/or the Centers for Disease Control and Prevention (CDC) for an indication other than that for which it is licensed by the FDA. The Contractor will provide necessary supporting information and data per USG request to support USG regulatory filing for emergency preparedness, distribution, and use of the product. This may include but is not limited to the following: clinical and non-clinical data, the manufacturing facility, chemistry, manufacturing, and controls information, pharmacology and toxicology information, cross- reference authorization letter, including the right of reference to the information contained in Contractor’s regulatory applications filed with the FDA. The Contractor shall support USG to address any FDA comments on the EA IND, pre-EUA, or EUA package, as applicable. If additional work is required, the parties will negotiate in good faith a modification to the Statement of Work to cover generation of any data required to support the USG’s filing of an EA IND, pre-EUA, or EUA. USG will be responsible for submission and will work directly with FDA to seek authorization or licensure.
USG shall provide the Contractor with relevant product usage and distribution data or relevant patient treatment data collected under an EA IND or EUA, as such information is available and as legally able. Contractor understands that such data sharing may require an additional agreement with the sponsor of the EA IND or EUA. The Contractor shall maintain and update, as required by FDA, all required regulatory documentation (investigator brochure, regulatory binder, etc.) that will be used to support use under EA IND, pre-EUA, or EUA.
For information concerning EUA, please consult xxxxx://xxx.xxx.xxx/XxxxxxxxxxXxxxxxxxxxx/Xxxxxxxxx/xxx000000 and
xxxx://xxx.xxx.xxx/XxxxxxxxxXxxxxxxxxxxx/Xxxxxxxxxxxxxxxx/XxxxxxxXxxxxxxxxxxxxxx/XXXXxxxxXxxxxxxxxxxxxXxxxxxXxxxxxxxx/xxx000000.xxx
e.Sharing of contract deliverables within United States Government (USG)
Contract No. 75A50122C00047
Page 7 of 51
In an effort to build a robust medical countermeasure pipeline through increased collaboration, BARDA may share technical deliverables with USG entities responsible for Medical Countermeasure Development. In accordance with recommendations from the Public Health Emergency Medical Countermeasure Enterprise (PHEMCE) Review, agreements established in the Integrated Portfolio’s Portfolio Advisory Committee (PAC) Charter, and agreements between BARDA and members of the PHEMCE, BARDA may share technical deliverables and data created in the performance of this contract with colleagues within the Integrated Portfolio. This advance understanding does not authorize BARDA to share financial information outside HHS. The Contractor is advised to review the terms of FAR 52.227-14, Rights in Data – General, regarding the Government’s rights to deliverables submitted during performance as well as the Government’s rights to data contained within those deliverables.
f.Overtime Compensation
No overtime (premium) compensation is authorized under the CPFF CLINs of the subject contract.
g.Contract Number Designation
On all correspondence submitted under this contract, the Contractor agrees to clearly identify the contract number that appears on the face page of the contract as follows:
75A50122C00047
h.Quality Agreement
Within 30 (thirty) days of contract award, the Contractor, BARDA and SNS will develop a Quality Agreement that shall establish, define and document the responsibilities of both the Contractor and the USG (i.e., SNS and BARDA) for product shipping, receiving, and acceptance into the SNS. This document shall be drafted, approved, and signed by all parties prior to the commencement of product shipment and acceptance by the USG. The Contractor shall provide documentation and resolution for all concerns raised by USG and commits to cooperation in execution of this agreement.
i.Confidential Treatment of Sensitive Information
The Contractor shall, to the extent permitted by law, guarantee strict confidentiality of sensitive/confidential information/test results that are provided by the USG during the performance of the contract. The USG has determined that certain information/test results that the Contractor will be provided during the performance of the contract are of a sensitive nature.
Disclosure of confidential/sensitive information/test results, in whole or in part, by the Contractor can only be made after the Contractor receives prior written approval from the CO. Whenever the Contractor is uncertain with regard to the proper handling of information/ test results under the contract, the Contractor shall obtain a written determination from the CO.
Notwithstanding the foregoing, such information/test results shall not be deemed of a sensitive or confidential nature with respect to the Contractor for purposes of this Contract if such information/test results: (a) was already known to the Contractor other than by prior disclosure by the USG or discovered through work under a prior USG contract; (b) was generally available or known, or was otherwise part of the public domain, at the time of its disclosure to the Contractor; (c) became generally available or known, or otherwise became part of the public domain, after its disclosure to, or, with respect to the information/test results by, the Contractor through no fault of the Contractor; (d) was disclosed to the Contractor, other than under an obligation of confidentiality or non-use, by a third party who had no obligation to the USG that controls such
Contract No. 75A50122C00047
Page 8 of 51
information/test results not to disclose such information/test results to others; or (e) was independently discovered or developed by the Contractor, as evidenced by its written records, without the use of information/test results belonging to the USG.
The Contractor may disclose information/test results of a sensitive nature provided by the USG to the extent that such disclosure is: (a) made in response to a valid order of a court of competent jurisdiction (b) otherwise required by law or regulation, or (c) made by the Contractor to the Regulatory Authorities as required in connection with any filing, application or request for Regulatory Approval; provided, however, that reasonable measures shall be taken to assure confidential treatment of such information/test results.
j.Understanding of Exercising of Option Period FFP CLINs
The schedule of delivery of the option period FFP CLINs will be determined during the modifications providing for the exercise of these options. If the USG does not intend to exercise an option in a fiscal year, USG will make a good faith effort to alert the Contractor in writing by December 30 of that fiscal year. USG will alert the Contractor in writing of the USG’s intent to exercise an option at least 30 days prior to intended exercise of the option. The delivery schedules for the exercised option will be agreed to by the USG and Contractor. If the delivery schedule is greater than 30 months from the exercise of the option, USG reserves the right to renegotiate the price of the option period FFP CLIN to a lower cost per treatment course.
k.Rebates
In its invoicing under CLIN 0002, the Contractor will credit the USG for a fixed rebate amount of
$[*] (stated in B.2.1 above). This is the amount that the USG reimbursed the Contractor under contract no. HHSO100201100013C, Development of CMX001 For the Treatment of Smallpox, for drug substance validation material and a post validation batch. In its invoicing under CLIN [*]. The amount stated in B.2.2 above is the minimum estimated rebate amount.
l.Order of Precedence for Timeline of Deliverables
Any inconsistency among the Contract and the incorporated attachments in Section J regarding the timeline of deliverables shall be resolved by giving precedence to the contract.
B.4.PROVISIONS CONCERNING ALLOWABLE COSTS
Notwithstanding the clause FAR 52.216-7, Allowable Cost and Payment, incorporated in this contract, the costs of the following items or activities shall be unallowable as direct costs under the CPFF CLINS of this contract unless authorized in writing in advance by the Contracting Officer:
a.Acquisition, by purchase or lease, of any interest in real property;
b.Rearrangement or alteration of facilities;
c.Purchase or lease of any item of general purpose office furniture or office equipment regardless of dollar value;
d.Accountable Government Property, property (defined as both real and personal property with an acquisition cost of $1,000 or more and a life expectancy of more than two years) and “sensitive
Contract No. 75A50122C00047
Page 9 of 51
items” (defined as items of personal property, supplies and equipment that are highly desirable and easily converted to personal use), regardless of acquisition value;
e.Overtime;
f.Travel to attend general scientific meetings/conferences;
g.Foreign travel;
h.Costs incurred in the performance of any cost-reimbursement type subcontract (including consulting agreements);
i.Costs to be paid for the performance of a fixed-price subcontract that exceeds $250,000;
j.Refreshments and meal expenditures;
k.Promotional items;
l.Printing;
m.Clinical trial insurance.
n.Patient care costs.
Contract No. 75A50122C00047
Page 10 of 51
SECTION C – STATEMENT OF WORK
C.1.BACKGROUND AND PURPOSE
This contract is to procure a second approved smallpox antiviral for delivery to the Strategic National Stockpile (SNS) to achieve the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) smallpox preparedness requirement of having two readily available smallpox antivirals with distinct mechanisms of action. The scope of work for this contract (across the Base and Option periods) includes the manufacture and delivery of up to 1.7 Million Treatment Courses (TCs) of the smallpox antiviral TEMBEXA, comprising oral tablets and oral suspension formulation to the SNS and the associated activities to support these deliveries: Post Marketing Commitments, Suspension Manufacturing Scale-up, Stability Extension Support, and Conduct of a Phase 4 Field Study. The Procurement and R&D efforts for the TEMBEXA program will progress in specific stages that cover Base Periods and Option Periods as specified in this contract.
C.2.STATEMENT OF WORK
Independently and not as an agent of the Government, the Contractor shall furnish all the necessary services, qualified personnel, material, equipment, and facilities, not otherwise provided by the Government as needed to perform the Statement of Work, set forth in SECTION J – LIST OF ATTACHMENTS, attached hereto and made a part of this contract.
Contract No. 75A50122C00047
Page 11 of 51
SECTION D – PACKAGING, MARKING AND SHIPPING
D.1.METHOD OF DELIVERY
All deliverables required under this contract shall be packaged, marked and shipped in accordance with Government specifications. At a minimum, all deliverables shall be marked with the contract number and Contractor’s name. The Contractor shall guarantee that all required materials shall be delivered in immediate usable and acceptable condition.
D.2.FOB DESTINATION DELIVERIES
The Contractor shall describe the storage conditions for each product, specifically noting the acceptable temperature range required to maintain product quality. The Contractor shall be responsible for maintaining product temperature control until the product(s) arrives at the Strategic National Stockpile (SNS) and has completed product acceptance by the USG. The Contractor shall provide the Government with an ambient exposure letter that covers the time the product(s) leaves the Contractor’s validated storage facility until arrival at the SNS. Upon Government acceptance of the product(s), the responsibility for temperature control shall transfer to the Government as well as the responsibility for logging ambient exposure time (temperatures between 8-25°C). The Contractor will provide and place TempTale(s) on each pallet of product while the product is inside the Contractor’s validated storage facility prior to placing the product(s) onto the truck(s) of the designated carrier. The Government’s acceptance of the aforementioned responsibility applies only to temperature control and does not indicate its acceptance of the lot(s).
Contract No. 75A50122C00047
Page 12 of 51
SECTION E – INSPECTION AND ACCEPTANCE
E.1.INSPECTION AND ACCEPTANCE
The Contracting Officer or the duly authorized representative will perform inspection and acceptance of materials and services to be provided under this contract.
For the purpose of this SECTION E, the designated Contracting Officer’s Representative (COR) is the authorized representative of the Contracting Officer. The COR will assist in resolving technical issues that arise during performance. The COR however is not authorized to change any contract terms, authorize any changes in the Statement of Work, modify or extend the period of performance, or authorize reimbursement of any costs incurred during performance. The Contractor is advised to review FAR 52.243-1 Changes – Fixed Price Contracts Alternate V and FAR 52.243-2 Changes-Cost reimbursement contracts Alternative V, which are incorporated by reference into this contract in ARTICLE I.1.
Inspection and acceptance of reports will be performed at:
Office of Contract Management and Acquisition (CMA) Administration for Strategic Preparedness and Response (ASPR)
U.S. Department of Health and Human Services X’Xxxxx House Office Building
Washington, DC 20515
Acceptance may be presumed unless otherwise indicated in writing by the Contracting Officer or the duly authorized representative within 30 days of receipt.
E.2.FEDERAL ACQUISITION REGULATION CLAUSES INCORPORATED BY REFERENCE
This contract incorporates the following clause by reference, with the same force and effect as if it were given in full text. Upon request, the Contracting Officer will make its full text available.
•FAR 52.246-2, Inspection of Supplies-Fixed-Price (Aug 1996) – applicable to FFP CLINs
•FAR 52.246-7, Inspection of Research and Development – Fixed-Price (Aug 1996) – applicable to FFP CLINs
•FAR 52.246-9, Inspection of Research and Development (Short Form) (April 1984) – applicable to CPFF CLINs
•FAR 52.246-16, Responsibility for Supplies (April 1984)
Contract No. 75A50122C00047
Page 13 of 51
SECTION F – DELIVERIES OR PERFORMANCE
F.1.PERIOD OF PERFORMANCE
The contract will consist of a base period of five (5) years from the contract effective date. The period of performance may be extended with the exercise of option(s), structured as CLINs, as set forth in SECTION B, to a maximum of ten (10) years.
F.2.DELIVERIES
Successful performance of the final contract shall be deemed to occur upon performance of the work described in the Statement of Work (see SECTION C and SECTION J) and upon delivery and acceptance of the items described in SECTION F.3 by the Contracting Officer or their duly authorized representative.
All product deliveries under FFP CLINs will adhere to the following. Upon delivery of the product at the agreed upon SNS location, the following procedures shall apply. The Government shall:
a.Inspect the shipment for accuracy against the bill of lading, inspect product containers for damage and retrieve data logging devices and data upon receipt;
b.Notify Contractor within 24 hours if there is any damage to packaging or if temperature as recorded on the data logging device(s) during Product shipment to the designated storage location exceeds shipping temperature specifications.
c.Within one business day notice of excursions or damage, the Contractor shall provide the government with further instructions on how to proceed or a certificate of compliance.
d.The government will complete all incoming inspections within three (3) business days of delivery or receipt of the aforementioned documents. If BARDA does not reject the Product within such three (3) day period, the Product shall be deemed accepted, subject to the government’s rights under paragraph (k) of FAR 52.246-2, Inspection for supplies-Fixed Price.
F.3.CONTRACT DELIVERABLES AND REPORTING REQUIREMENTS
F.3.1.Submission of Contract Deliverables
Documents shall be delivered electronically via email to the Contracting Officer (CO) and the Contracting Officer’s Representative (COR). Additionally, the Contractor shall upload documents to the appropriate Government designated file sharing system. The Government will provide authorized log in access to the file share program to two Contractor representatives. Each representative must complete a mandatory training provided by the Government prior to gaining user access. A notification email shall be sent to the CO and COR upon electronic delivery of any documents.
F.3.2.Reporting Requirements and Other Deliverables
In addition to those reports required by other terms of this contract, the Contractor shall submit to the CO and the COR technical progress reports. These reports shall be subject to the technical inspection and requests for clarification by the COR. These reports shall be brief, factual, and prepared in accordance with the following formats:
1.Kick-off Meeting
Contract No. 75A50122C00047
Page 14 of 51
The Contractor and Government shall conduct a kickoff meeting within 30 calendar days after contract award to review HHS procedures, processes, and expectations. Contractor shall provide an itinerary/agenda no later than two (2) business days before meeting. Contractor shall provide minutes from the kickoff meeting within seven (7) business days after the meeting.
2.Project Meeting Conference Calls, Agendas, and Meeting Minutes
A conference call between the Contract Officer (CO), the Contracting Officer’s Representative (COR) and designees and the Contractor’s Project Leader/delegate and designees shall occur biweekly or at a frequency determined by the CO and/or COR. During this call the Contractor’s Project Leader/delegate and designees will discuss the activities since the last call, any problems that have arisen and the activities planned until the next call takes place. The Contractor’s Project Leader/delegate may choose to include other key personnel on the conference call to give detailed updates on specific projects or this may be requested by the COR. Electronic copy of conference call meeting agendas shall be provided via e-mail to the CO, COR, and uploaded into an agreed digital sharesite by the Contractor no later than two (2) business days before the conference call is held. Electronic copy of conference call meeting minutes/summaries shall be provided via e-mail to the CO, COR, and uploaded into an agreed digital sharesite by the Contractor within seven (7) business days after the conference call is held.
3.Monthly Progress Report
This report shall include a description of the activities during the reporting period and the activities planned for the ensuing reporting period. The first reporting period consists of the first full month of performance plus any fractional part of the initial month. Thereafter, the reporting period shall consist of each calendar month.
The Contractor shall submit a Monthly Progress Report on or before the 15th of each month following the end of each reporting period and shall include the following:
Title Page: The title page for this report shall include the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission.
Distribution List: A list of individuals receiving the Technical Progress report. Progress:
SECTION I - An introduction covering the purpose and scope of the contract effort. SECTION II Part A: SUMMARY - A description or table summarizing ongoing activities.
SECTION II Part B: MANAGEMENT AND ADMINISTRATIVE UPDATE – This section shall
include a description of all meetings, conference calls, etc. that have taken place during the reporting period. Include progress on administration and management issues (e.g. evaluating and managing subcontractor performance and personnel changes). Include all Quality Management System, Quality Control, and Quality Assurance Plans as part of this report or as requested by the COR.
SECTION II Part C: TECHNICAL PROGRESS – This section shall document the results of work completed and costs incurred during the period covered in relation to the proposed progress,
Contract No. 75A50122C00047
Page 15 of 51
effort, and budget. The report shall be in sufficient detail to explain comprehensively the results achieved.
SECTION II Part D: ISSUES – This section shall include a description of problems encountered and proposed corrective action; differences between planned and actual progress; why the differences have occurred and what corrective actions are planned; and if a project activity is delinquent, then what corrective action steps are planned. Revised timelines shall be provided.
SECTION II Part E: PROPOSED WORK – This section shall include a summary of work proposed as a rolling three (3) month forecast for the next reporting period, by a certain date, and by whom.
SECTION II Part F: MANUFACTURING AND SUPPLY CHAIN MANAGEMENT – This
section shall include a summary of the manufacturing and supply-chain related activities. Also include in this section updates to the production plan, capacity projections, stability results, inventory and shipment/distribution information.
Invoices: Summary of any invoices submitted during the reporting period.
A Monthly Progress Report will not be required in the same month Annual Progress Reports or a Final Report are due.
4.Annual Progress Report
This report shall include a summation of the activities during the reporting period, and the activities planned for the ensuing reporting period. The first reporting period consists of the first full year of performance plus any fractional part of the initial year. Thereafter, the reporting period shall consist of each calendar year.
The Contractor shall submit an Annual Progress Report on or before the 30th calendar day following the end of each reporting period and shall include the following:
Title Page: The title page for this report shall include the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission.
Distribution List: A list of individuals receiving the Technical Progress report. Progress:
SECTION I - An introduction covering the purpose and scope of the contract effort. SECTION II Part A: SUMMARY - A description or table summarizing ongoing activities.
SECTION II Part B: MANAGEMENT AND ADMINISTRATIVE UPDATE – This section shall
include a description of all meetings, conference calls, etc. that have taken place during the reporting period. Include progress on administration and management issues (e.g. evaluating and managing subcontractor performance and personnel changes). Include all Quality Management System, Quality Control, and Quality Assurance Plans as part of this report or as requested by the COR.
Contract No. 75A50122C00047
Page 16 of 51
SECTION II Part C: TECHNICAL PROGRESS – This section shall document the results of work completed and costs incurred during the period covered in relation to proposed progress, effort, and budget. The report shall be in sufficient detail to explain comprehensively the results achieved.
SECTION II Part D: ISSUES – This section shall include a description of problems encountered and proposed corrective action; differences between planned and actual progress; why the differences have occurred and what corrective actions are planned; and if a project activity is delinquent, then what corrective action steps are planned. Revised timelines shall be provided.
SECTION II Part E: PROPOSED WORK – This section shall include a summary of work proposed as a rolling three (3) month forecast for the next reporting period, by a certain date, and by whom.
SECTION II Part F: MANUFACTURING AND SUPPLY CHAIN MANAGEMENT – This
section shall include a summary of the manufacturing and supply-chain related activities. Also include in this section updates to the production plan, capacity projections, stability results, inventory and shipment/distribution information.
Invoices: Summary of any invoices submitted during the reporting period.
An Annual Progress Report will not be required for the period when the Final Technical Report is due.
5.Draft Final Report and Final Report
These reports are to include a summation of the work performed and results obtained for execution of various studies or technical work packages during the entire contract period of performance. This report shall be in sufficient detail to describe comprehensively the results achieved. The Draft Final Report shall be due no later than forty-five (45) calendar days prior to the expiration date of the contract and the Final Report is due before the expiration date of the contract. The report shall be draft only in that XXXXX comments have not been received/adjudicated and the report shall conform to the following format:
Title Page: The title for these reports shall include the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e- mail address; and the date of submission.
Distribution List: A list of individuals receiving the Technical Progress report. Progress:
SECTION I: EXECUTIVE SUMMARY - Summarize the purpose and scope of the contract effort including a summary of the major accomplishments relative to the specific activities set forth in the Statement of Work.
SECTION II: RESULTS - A detailed description of the work performed and the results obtained including all expenses for the entire contract period of performance.
6.FDA/Regulatory Agency Correspondence, Meeting Summaries, and Submissions.
a.The Contractor shall inform BARDA of all communications with the FDA for the orthopox
Contract No. 75A50122C00047
Page 17 of 51
indications for this specific product, provide all communications and submissions related to the product, and provide previous correspondences with FDA related to the development of the therapeutic within five (5) business days of submission to or receipt from FDA.
b.The Contractor shall maintain and update, as required by the FDA, all required regulatory documentation (investigator brochure, regulatory binder, etc.), that will be used to support use under EUA and/or licensure.
c.The Contractor shall obtain FDA concurrence on the regulatory pathway to licensure for orthopox indications (i.e. traditional approval, accelerated approval, or Animal Rule), if applicable.
d.The Contractor shall conduct all necessary meetings with the FDA to support submission of an sNDA for orthopox indications to the FDA and endeavor to include BARDA staff, as silent observers, in all scheduled phone calls or face to face meetings with regulatory authorities, if applicable.
e.Within five business days of any informal meeting with the FDA, the Contractor shall forward the initial draft minutes to BARDA. The Contractor shall forward the final minutes when available and if applicable.
f.The Contractor shall provide BARDA the opportunity to review and comment upon any documents to be submitted to the FDA. The Contractor shall provide BARDA with ten (10) business days, or as soon as possible in which to review and provide comments back to the Contractor prior to the Contractor’s submission to the FDA. The Contractor shall address in writing all concerns raised by BARDA before FDA submission.
g.The Contractor shall re-label investigational product (upon licensure) to be consistent with the licensed product, in accordance with regulatory requirements. Within five (5) business days of any formal meeting with the FDA, the Contractor shall forward the initial draft minutes to BARDA. The Contractor shall forward the final minutes when available.
h.The Contractor shall notify the Contracting Officer’s Representative and Contracting Officer within one (1) business day of all FDA arrivals to conduct site visits/audits by any regulatory agency. The Contractor shall provide the USG with an exact copy (non-redacted) of the FDA Form 483 and the Establishment Inspection Report (EIR). The Contractor shall provide the Contracting Officer’s Representative and Contracting Officer copies of the plan for addressing areas of non-conformance to FDA regulations for GLP guidelines as identified in the audit report, status updates during the plans execution, and a copy of all final responses to the FDA. The Contractor shall also provide redacted copies of any FDA audits received from subcontractors that occur as a result of this contract or for this product. The Contractor shall make arrangements with the COR for the appropriate BARDA representative(s) to be present during the final debrief by the regulatory inspector.
i.The Contractor shall forward Standard Operating Procedures (SOPs) upon request from COR.
j.The Contractor shall provide raw data and/or specific analysis of data first produced in the performance of this contract with USG funds upon request from the COR.
7.Integrated Master Project Plan
The Contractor shall provide an Integrated Master Project Plan (including tabular and Xxxxx forms) to BARDA that clearly indicates the critical path to annual deliverables and Work Breakdown Structure (WBS) elements. Attention shall be placed on providing sufficient turnaround time for the USG (BARDA, FDA, and CDC) for review of critical documentation. The Contractor shall integrate to demonstrate interdependencies among all CLINs. The Integrated Master Project Plan shall be incorporated into any potential contract and will be used to monitor performance of the contract. A Draft Plan shall be provided within 30 calendar days of contract
Contract No. 75A50122C00047
Page 18 of 51
award and the Final Plan shall be due within 90 days of contract award. Updates shall be due as requested by the COR.
•Critical Path Milestones
The Integrated Master Project Plan shall outline key, critical path milestones, with “Go/No Go” decision criteria (entrance and exit criteria for each phase of the project). This report shall be due within 90 days of contract award. Updates shall be due as requested by the COR.
•Work Breakdown Structure
The Work Breakdown Structure (WBS) shall be discernable and consistent. BARDA may require the Contractor to furnish WBS data at the work package level or at a lower level if there is significant complexity and risk associated with the task. This report shall be due within 90 days of contract award. Updates shall be due as requested by the COR.
•Risk Mitigation Plan/Matrix
The Contractor shall develop and maintain a risk management plan that highlights potential problems and/or issues that may arise during the life of the contract, their impact on cost, schedule and performance, and appropriate remediation plans. This plan shall reference relevant WBS/SOW elements where appropriate. The USG has provided a Risk Mitigation Matrix template to be completed by any prospective Contractor. This report shall be due within 90 days of contract award. Updates shall be due as requested by the COR.
8.Technology Packages
Technology packages developed under the contract including complete protocols must be submitted within 10 business days of request by the COR. See FAR clauses 52.227-11, Patent Rights-Ownership by the Contractor, and 52.227-14, Rights in Data.
9.Clinical and Nonclinical Study Protocols and Reports
The Contractor shall submit to the COR Draft Protocols and associated study/experiment/test plans 30 days prior to study initiation prior to submission to regulatory agencies (IACUC, FDA, etc.). The Contractor shall submit to the COR Final Protocols 10 business days prior to the execution of the study or within 10 business days of request by the COR. Approval must be provided in writing by the COR prior to the execution of the study. Draft Reports are due within 45 calendar days after completion of analysis and at least 15 business days prior to submission to the FDA. Final Reports are due 30 calendar days after receiving comments on the Draft Report. Final FDA submissions shall be provided to BARDA concurrently or no later than one (1) business day after submission to the FDA.
10.Raw Data and Data Analysis
Contractor shall provide data or data analysis to the CO and COR within 20 business days of request, amend reports if required, and adjudicate all comments.
11.Annual/Final Invention Report
All reports and documentation required by FAR Clause 52.227-11, Patent Rights-Ownership by the Contractor, including, but not limited to, the invention disclosure report, the confirmatory license, and the Government support certification. An Annual Invention Report shall be due on or before the 30th calendar day after the completion of each reporting period. A Final Invention Report (see FAR 27.303 (b)(2)(ii)) shall be due on or before the expiration date of the contract. If
Contract No. 75A50122C00047
Page 19 of 51
no invention is disclosed or no activity has occurred on a previously disclosed invention during the applicable reporting period, a negative report shall be submitted to the Contracting Officer.
12.Publications
Any manuscript or scientific meeting abstract containing data generated under this contract must be submitted to the COR for review prior to submission. Reports shall be submitted to the COR for review 30 calendar days for manuscripts and fifteen (15) calendar days for abstracts prior to intended publication or external submission.
13.Press Releases
The Contractor agrees to accurately and factually represent the work conducted under this contract in all press releases. The Contractor shall ensure the Contracting Officer has received and approved an advanced copy of any press release not less than five (5) business days prior to the issuance of any potential press release.
14.Security Report
The Contractor shall report to the government any activity; or incident that is in violation of established security standards; or indicates the loss or theft of government products. Reports shall be due within 24 hours of an activity or incident.
15.Security Plan
The Contractor shall provide a Security Plan within 90 days of contract award. The requirements for the Security Plan and general instructions are outlined in SECTION J, Attachment 6.
16.Manufacturing Plan
The Contractor shall submit to the COR a comprehensive manufacturing plan for review and approval within 90 days of contract award.
17.Quality Management Systems Plan
The Contractor shall submit to the COR a Quality Management System Plan for approval within 90 days of contract award.
F.3.3.NOTIFICATION OF CRITICAL PROGRAMMATIC CONCERNS, RISKS, OR POTENTIAL RISKS
The Contractor shall provide an Incident Report for any major developments or deviations that significantly impact the Contractor’s ability to successfully meet objectives, deliverables or schedule (e.g., significant manufacturing deviations or loss, BSL-4 laboratory incidents, etc.). If any incident occurs that creates a cause for critical programmatic concern, risk, or potential risk to BARDA or the Contractor, the Contractor shall:
•Within one (1) business day of notification of an incident, notify the CO and COR.
•Provide updates to the CO and COR within two (2) business days of additional developments.
•Submit within fifteen (15) business days a Corrective Action Plan (if deemed necessary by either party) to address any potential issues. If critical observations are found, submit a Corrective Action Plan within five (5) business days.
•Provide an Incident Report with critical observations within forty five (45) days of the incident.
Contract No. 75A50122C00047
Page 20 of 51
F.3.4.QUALITY ASSURANCE (QA) AUDIT REPORTS
BARDA reserves the right to participate in QA audits as related to activities funded under the CPFF CLINs of this contract. Upon completion of the audit/site visit the Contractor shall provide a report capturing the findings, results and next steps in proceeding with the subcontractor. If action is requested of the subcontractor, detailed concerns for addressing areas of non-conformance to FDA regulations for GLP, GMP, or GCP guidelines, as identified in the audit report, must be provided to BARDA within 60 days of the audit or site visit. The Contractor shall provide responses from the subcontractors to address these concerns and plans for corrective action execution.
•Contractor shall notify CO and COR of upcoming, ongoing, or recent audits/site visits of subcontractors as part of weekly communications.
•Contractor shall notify the CO and COR within five (5) business days of completion of activities identified in any report.
F.3.5.BARDA AUDITS
Contractor shall accommodate periodic or reasonable ad hoc site visits during normal business hours by the Government related to activities funded under the CPFF CLINs of this contract with two (2) business days’ advance notice. If the Government, the Contractor, or other parties identifies any issues during an audit, the Contractor shall capture the issues, identify potential solutions, and provide a report to the Government.
•If issues are identified during the audit, Contractor shall submit a report to the CO and COR detailing the finding and corrective action(s) within 15 business days of the audit.
•CO and COR will review the report and provide a response to the Contractor with ten (10) business days.
•Once corrective action is completed, the Contractor will provide a final report to the CO and COR.
F.3.6.IN-PROCESS REVIEW
In Process Reviews (IPR) will be conducted at the discretion of the Government to discuss the progression of the milestones. The Government reserves the right to revise the milestones and budget pending the development of the project. Deliverables such as an overall project summary report and/or slides will be required when the IPRs are conducted. The Contractor’s success in completing the required tasks under each work segment must be demonstrated through the Deliverables and Milestones specified under SECTION F. Those deliverables will constitute the basis for the Government’s decision, at its sole discretion, to proceed with the work segment, or institute changes to the work segment, or terminate the work segment
IPRs may be scheduled at the discretion of the Government to discuss progression of the contract. The Contractor shall provide a presentation following a prescribed template which will be provided by the Government at least 30 business days prior to the IPR. Subsequently, the contractor will be requested to provide a revised/final presentation to the Contracting Officer at least 10 business days prior to the IPR. Contractor shall submit written justification of progress towards satisfying Go/No-Go criteria. CO/COR will provide a written response within 10 days.
Contract No. 75A50122C00047
Page 21 of 51
F.3.7.DELIVERABLE SCHEDULE
Item No. | Description | Addresses | Deliverable Schedule | ||||||||
| 1 | Kick-off Meeting | CO: (1) electronic copy COR: (1) electronic copy | Due within 30 days of contract award. Meeting minutes are due no later than 7 (seven) business days following meeting. | ||||||||
| 2 | Project Meeting Conference Call, Agendas and Meeting Minutes | CO: (1) electronic copy COR: (1) electronic copy | Meeting agendas are due no later than 2 (two) business days before each meeting. Meeting minutes are due no later than 7 (seven) business days following each meeting. | ||||||||
| 3 | Monthly Progress Report | CO: (1) electronic copy COR: (1) electronic copy | Reports are due on or before the 15th of each month following the end of each reporting period. | ||||||||
| 4 | Annual Progress Report | CO: (1) electronic copy COR: (1) electronic copy | Reports are due on or before the 30th calendar day following the end of each reporting period. | ||||||||
| 5 | Draft Final Report | CO: (1) electronic copy COR: (1) electronic copy | Report is due no later than 45 calendar days prior to the expiration date of the contract. | ||||||||
| 6 | Final Report | CO: (1) electronic copy COR: (1) electronic copy | Report is due before the expiration date of the contract. | ||||||||
| 7 | FDA/Regulatory Agency Correspondence and Meeting Summaries | CO: (1) electronic copy COR: (1) electronic copy | Correspondence, meeting summaries, and reports are due within 5 (five) business days of each meeting or submission to or receipt from FDA/regulatory agency. | ||||||||
| 8 | Integrated Master Project Plan - Critical Path Milestones | CO: (1) electronic copy | Draft Plan due within 30 days of contract award. | ||||||||
Contract No. 75A50122C00047
Page 22 of 51
-Work Breakdown Structure -Risk Mitigation Plan/Matrix | COR: (1) electronic copy | Final Plan due within 90 days of contract award. Updates due as requested by the COR. | |||||||||
| 9 | Technology Packages | CO: (1) electronic copy COR: (1) electronic copy | Due within 10 business days of request by the COR. | ||||||||
| 10 | Clinical and Nonclinical Study Protocols and Reports | CO: (1) electronic copy COR: (1) electronic copy | Draft Protocols due 30 days prior to study initiation. Final Protocols due 10 business days prior to execution of the study or within 10 business days of request by the COR. Draft Report due within 45 calendar days after completion of analysis and at least 15 business days prior to submission to FDA. Final Report due 30 calendar days after receiving comments on the Draft Report. Final FDA submissions shall be provided to BARDA concurrently or no later than one (1) business day after submission to the FDA. | ||||||||
| 11 | Raw Data and Data Analysis | CO: (1) electronic copy COR: (1) electronic copy | Contractor shall provide data or data analysis to the CO and COR within 20 business days of request, amend reports if required, and adjudicate all comments. | ||||||||
| 12 | Annual/Final Invention Report | CO: (1) electronic copy COR: (1) electronic copy | An Annual Invention Report is due on or before the 30th calendar day after the completion of each reporting period. A Final Invention Report is due on or before the | ||||||||
Contract No. 75A50122C00047
Page 23 of 51
expiration date of the contract. | |||||||||||
| 13 | Publications | CO: (1) electronic copy COR: (1) electronic copy | Reports are due within 30 calendar days for manuscripts and 15 calendar days for abstracts. | ||||||||
| 14 | Press Releases | CO: (1) electronic copy COR: (1) electronic copy | Reports/Notices discussing data obtained under this contract are due for approval to the CO not less than five (5) business days prior to the issuance of any potential press release. | ||||||||
| 15 | Security Report | CO: (1) electronic copy COR: (1) electronic copy | Reports are due within 24 hours of an activity or incident. | ||||||||
| 16 | Security Plan | CO: (1) electronic copy COR: (1) electronic copy | Final plan due within 90 days of contract award. | ||||||||
| 17 | Manufacturing Plan | CO: (1) electronic copy COR: (1) electronic copy | Due within 90 days of contract award. | ||||||||
| 18 | Quality Management System Plan | CO: (1) electronic copy COR: (1) electronic copy | Due within 90 days of contract award. | ||||||||
| 19 | Notification of Critical Programmatic Concerns, Risks, or Potential Risks (i.e. Incident Report) | CO: (1) electronic copy COR: (1) electronic copy | Contractor shall alert the CO and COR within one (1) business day after notification of an incident, provide updates within two (2) business days of additional developments, submit corrective action plans as required in Section F.3.3 above and provide an incident report | ||||||||
Contract No. 75A50122C00047
Page 24 of 51
within 45 days of the incident. | |||||||||||
| 20 | QA Audit | CO: (1) electronic copy COR: (1) electronic copy | Contractor shall submit a report to the CO and COR detailing the finding(s) and corrective action(s) within 60 days of the audit or site visit. | ||||||||
| 21 | BARDA Audit | CO: (1) electronic copy COR: (1) electronic copy | If issues are identified during the audit, Contractor shall submit a report to the CO and COR detailing the finding(s) and corrective action(s) within 15 business days of the audit. | ||||||||
| 22 | Go/No-Go In-Process Review (IPR) | CO: (1) electronic copy COR: (1) electronic copy | Contractor shall provide presentation materials to CO and COR 10 business days prior to the IPR. Submit written justification of progress towards satisfying Go/No- Go criteria. CO/COR will provide a written response within 10 days. | ||||||||
| 23 | Contractor’s Data Sharing Plan | CO: (1) electronic copy COR: (1) electronic copy | Contractor shall submit plan within 90 days of the effective date of the contract. | ||||||||
F.2.FEDERAL ACQUISITION REGULATION CLAUSES INCORPORATED BY REFERENCE, FAR 52.252-2 (FEB 1998)
This contract incorporates the following clause(s) by reference, with the same force and effect as if it were given in full text. Upon request, the Contracting Officer will make its full text available. The full text of each clause may be accessed electronically at this address: xxxx://xxx.xxxxxxxxxxx.xxx/xxxxxx/xxxxx/xxx.
•FAR 52.242-15, Stop Work Order (August 1989) – applicable to FFP CLINs
•FAR 52.242-15, Stop Work Order (August 1989), Alternate 1 (April 1984) – applicable to CPFF CLIN
Contract No. 75A50122C00047
Page 25 of 51
SECTION G – CONTRACT ADMINISTRATION
G.1.CONTRACTING OFFICER (CO)
The Contracting Officer is the only individual who can legally commit and bind the Government to the expenditure of public funds. No person other than the Contracting Officer can make any changes to the terms, conditions, general provisions or other stipulations of this contract. Any other commitment, either explicit or implied, is invalid.
The CO is the only person with authority to act as agent of the Government under this contract. Only the Contracting Officer has authority to: (1) direct or negotiate any changes in the statement of objectives; (2) modify or extend the period of performance; (3) change the delivery schedule; (4) authorize reimbursement to the Contractor for any costs incurred during the performance of this contract; (5) obligate or de-obligate funds into the contract; (6) sign written licensing agreements; or (7) otherwise change any terms and conditions of this contract.
No information, other than that which may be contained in an authorized modification to this contract duly issued by the Contracting Officer, which may be received from any person employed by the United States Government, or otherwise, shall be considered grounds for deviation from any stipulation of this contract.
The Contracting Officer (CO) is:
Xxxx Xxxxxxx (xxxx.xxxxxxx@xxx.xxx)
G.2.CONTRACTING OFFICER’S REPRESENTATIVE (COR)
As delegated by the CO, the Contracting Officer’s Representative (COR) is responsible for: (1) monitoring the Contractor's technical progress, including the surveillance and assessment of performance and recommending to the Contracting Officer changes in requirements; (2) assisting the CO in interpreting the statement of work and any other technical performance requirements; (3) performing technical evaluation as required; (4) performing technical inspections required by this contract; and (5) assisting in the resolution of technical problems encountered during performance.
The Contracting Officer’s Representative (COR) is:
Xxxxx Xxxxxxxxx, PhD (xxxxx.xxxxxxxxx@xxx.xxx)
The Alternate Contracting Officer’s Representative (Alt-COR) is: Xx Xx, PhD (xx.xx@xxx.xxx)
G.3.CONTRACTOR’S POINTS OF CONTACT
The Contractor shall provide primary and secondary points of contact that will be available 24 hours per day, 7 days per week, to be notified in case of a public health emergency, as follows:
Primary Point of Contact:
[*]
Contract No. 75A50122C00047
Page 26 of 51
[*]
cell: [*]
email: [*]
Secondary Point of Contact:
[*]
[*]
cell: [*]
email: [*]
G.4.KEY PERSONNEL, HHSAR 352.237-75 (DEC 2015)
The key personnel specified in this contract are considered to be essential to work performance. At least 30 calendar days prior to the Contractor voluntarily diverting any of the specified individuals to other programs or contracts (or as soon as possible, if an individual must be replaced, for example, as a result of leaving the employ of the Contractor), the Contractor shall notify the Contracting Officer and shall submit comprehensive justification for the diversion or replacement request (including proposed substitutions for key personnel) to permit evaluation by the Government of the impact on performance under this contract. The Contractor shall not divert or otherwise replace any key personnel without the written consent of the Contracting Officer. The Government may modify the contract to add or delete key personnel at the request of the Contractor or Government.
The following individual(s) is/are considered to be essential to the work being performed hereunder:
| Name | Title | ||||
| [*] | [*] | ||||
| [*] | [*] | ||||
G.5.Electronic Invoicing and Payment Requirements – Invoice Processing Platform (IPP)
•All Invoice submissions for goods and or services delivered to facilitate payments must be made electronically through the U.S. Department of Treasury’s Invoice Processing Platform System (IPP).
•Invoice Submission for Payment means any request for contract financing payment or invoice payment by the Contractor. To constitute a proper invoice, the payment request must comply with the requirements identified in the applicable Prompt Payment clause included in the contract, or the clause 52.212-4 Contract Terms and Conditions – Commercial Items included in commercial items contracts. The IPP website address is: xxxxx://xxx.xxx.xxx.
•The Agency will enroll the Contractors new to IPP. The Contractor must follow the IPP registration email instructions for enrollment to register the Collector Account for submitting invoice requests for payment. The Contractor Government Business Point of Contact (as listed in XXX) will receive Registration email from the Federal Reserve Bank of St. Louis (FRBSTL) within 3 – 5 business days of the contract award for new contracts or date of modification for existing contracts.
oRegistration emails are sent via email from xxx.xxxxxxx@xxxx.xxxx.xxxx.xxx. Contractor assistance with enrollment can be obtained by contacting the IPP Production Helpdesk via
Contract No. 75A50122C00047
Page 27 of 51
email to XXXXxxxxxxxXxxxxxx@xxxxxx.xxxxxxxx.xxx or phone (000) 000-0000.
oThe Contractor POC will receive two emails from IPP Customer Support, the first email contains the initial administrative IPP User ID. The second email, sent within 24 hours of receipt of the first email, contains a temporary password. You must log in with the temporary password within 30 days.
•If your company is already registered to use IPP, you will not be required to re-register.
•If the Contractor is unable to comply with the requirement to use IPP for submitting invoices for payment as authorized by HHSAR 332.7002, a written request must be submitted to the Contracting Officer to explain the circumstances that require the authorization of alternate payment procedures.
G.5.1.Additional Administration for Strategic Preparedness and Response (ASPR) requirements:
•The contractor shall submit monthly invoices under this contract unless otherwise agreed upon by all parties. For indefinite delivery and blanket purchase agreement vehicles, separate invoices must be submitted for each order.
•Invoices must break-out price/cost by contract line item number (CLIN) as specified in the pricing section of the contract.
•Invoices must include the Dun & Bradstreet Number (DUNS) of the Contractor.
•Invoices that include time and materials or labor hours CLINs must include supporting documentation to (1) substantiate the number of labor hours invoiced for each labor category, and
(2) substantiate material costs incurred (when applicable).
•Invoices that include cost-reimbursement CLINs must be submitted in a format showing expenditures for that month, as well as contract cumulative amounts.
At a minimum the following cost information shall be included, in addition to supporting documentation to substantiate costs incurred.
•Direct Labor - include all persons, listing the person's name, title, scope of work, number of hours worked, hourly rate, the total cost per person and a total amount for this category;
•Indirect Costs (i.e., Fringe Benefits, Overhead, General and Administrative, Other Indirects)- show rate, base and total amount;
•Consultants (if applicable) - include the name, number of days or hours worked, daily or hourly rate, and a total amount per consultant;
•Travel - include for each airplane or train trip taken the name of the traveler, date of travel, destination, the transportation costs including ground transportation shown separately and the per diem costs. Other travel costs shall also be listed;
•Subcontractors (if applicable) - include, for each subcontractor, the same data as required for the prime Contractor;
•Other Direct Costs - include a listing of all other direct charges to the contract, i.e., office supplies, telephone, duplication, postage; and
•Fee – amount as allowable in accordance with the Schedule and FAR 52.216-8 if applicable.
Contract No. 75A50122C00047
Page 28 of 51
G.6.REIMBURSEMENT OF COST
The Government shall reimburse the Contractor under the CPFF CLINs for the cost determined by the Contracting Officer to be allowable (hereinafter referred to as allowable cost) in accordance with FAR Clause 52.216-7, Allowable Cost and Payment incorporated by reference in SECTION I – CONTRACT CLAUSES, of this contract, and FAR Subpart 31.2. Examples of allowable costs include, but are not limited to, the following:
a.All direct materials and supplies that are used in performing the work provided for under the contract, including those purchased for subcontracts and purchase orders.
b.All direct labor, including supervisory, that is properly chargeable directly to the contract, plus fringe benefits.
c.All other items of cost budgeted for and accepted in the negotiation of this basic contract or modifications thereto.
d.Travel costs including per diem or actual subsistence for personnel while in an actual travel status in direct performance of the work and services required under this contract subject to the following:
i.Air travel shall be by the most direct route using “air coach” or “air tourist” (less than first class) unless it is clearly unreasonable or impractical (e.g., not available for reasons other than avoidable delay in making reservations, would require circuitous routing or entail additional expense offsetting the savings on fare, or would not make necessary connections).
ii.Rail travel shall be by the most direct route, first class with lower berth or nearest equivalent.
iii.Costs incurred for lodging, meals, and incidental expenses shall be considered reasonable and allowable to the extent that they do not exceed on a daily basis the per diem rates set forth in the Federal Travel Regulation (FTR).
iv.Travel via privately owned automobile shall be reimbursed at not more than the current General Services Administration (GSA) FTR established mileage rate.
G.7.NEGOTIATED INDIRECT COST RATES (Applied to CPFF CLINs)
a.The following provisional billing rates are incorporated into the contract and will be utilized for billing purposes during the Base Period pending the establishment of final indirect cost rates for each fiscal year or until revised by the CO in accordance with the provisions of FAR 42.705-1.
Rate Type | Rate Ceiling | Allocation Base | ||||||
Fringe Benefits | [*] % | Total Salaries | ||||||
General and Administrative | [*] % | Total Direct Costs Plus Applied Fringe | ||||||
b.Notwithstanding the provisions of FAR 42.704, ceilings are hereby established on indirect costs reimbursable under this contract. Therefore, the Government will not be obligated to pay any additional amounts if the final indirect cost rates developed by the cognizant audit
Contract No. 75A50122C00047
Page 29 of 51
activity based on actual allowable costs exceed the ceiling rates set forth above. In the event the final indirect cost rates are less than the above-established ceiling rates, the negotiated final rates shall be reduced to conform to the lower rates
c.In accordance with FAR Part 5.216-7(d), the contractor shall submit an adequate final indirect cost rate proposal to the contracting officer and the cognizant auditor within the six- month period following the end of each of its fiscal years during the period of contract performance. The contracting officer xxx xxxxx, in writing, reasonable extensions, for exceptional circumstances only, when requested in writing by the contractor. The Government shall not be obligated to (1) pay any additional amount should the final indirect cost rates exceed the established ceiling rates, and (2) in the event the final rates are less than the established ceiling rates, the negotiated rates will be reduced to conform with the lower rates.
G.8.CONTRACT FINANCIAL REPORT
a.Financial reports on the attached Financial Report of Individual Project/Contract shall be submitted by the Contractor for the CPFF CLINs to the CO with a copy to the COR in accordance with the instructions for completing this form, which accompany the form, in an original and one electronic copy, not later than the 30th business day after the close of the reporting period. The line entries for subdivisions of work and elements of cost (expenditure categories), which shall be reported within the total contract, are discussed in paragraph e., below. Subsequent changes and/or additions in the line entries shall be made in writing.
b.Unless otherwise stated in the instructions for completing this form, all columns A through J, shall be completed for each report submitted.
c.The first financial report shall cover the period consisting of the first full three calendar months following the date of the contract, in addition to any fractional part of the initial month. Thereafter, reports will be on a quarterly basis.
d.The Contracting Officer may require the Contractor to submit detailed support for costs contained in one or more interim financial reports. This clause does not supersede the record retention requirements in FAR Part 4.7.
e.The listing of expenditure categories to be reported is incorporated as a part of this contract and can be found under SECTION J entitled, “Financial Report of Individual Project/Contract.”
G.9.GOVERNMENT PROPERTY
In addition to the requirements of the Government Property clause incorporated in SECTION I of this contract, the Contractor shall comply with the provisions of HHS Publication, "HHS
Contracting Guide for Control of Government Property," which is incorporated into this contract by reference. This document can be accessed at: xxxxx://xxxxxxx.xxx/xxxxxxx/xxxxxxxxxxxxxxxx00xxxx.
Among other issues, this publication provides a summary of the Contractor's responsibilities regarding purchasing authorizations and inventory and reporting requirements under the contract.
Notwithstanding the provisions outlined in the HHS Publication, "HHS Contracting Guide for Control of Government Property," which is incorporated in this contract in paragraph 1 above, the Contractor shall use the form entitled, "Report of Government Owned, Contractor Held Property" for submitting summary
Contract No. 75A50122C00047
Page 30 of 51
reports required under this contract, as directed by the Contracting Officer or his/her designee. This form is attached to this contract (see SECTION J – LIST OF ATTACHMENTS). Title will vest in the Government for equipment purchased as a direct cost under the CPFF CLINs.
G.10.CONTRACT COMMUNICATIONS/CORRESPONDENCE
The Contractor shall identify all correspondence, reports, and other data pertinent to this contract by imprinting thereon the contract number from Page 1 of the contract.
G.11.POST AWARD EVALUATION OF CONTRACTOR PERFORMANCE
a.Purpose: In accordance with FAR Subpart 42.15, the Contractor’s performance will be periodically evaluated by the government in order to provide current information for source selection purposes. These evaluations will therefore be marked “Source Selection Information.”
b.Performance Evaluation Period: The Contractor’s performance will be evaluated at least annually.
c.Evaluators: The performance evaluation will be completed jointly by the Contracting Officer’s Representative and the Contracting Officer.
d.Performance Evaluation Factors: The Contractor's performance will be evaluated in accordance with FAR Subpart 42.15 and Attachment #5, Contract Performance Evaluation Report.
e.Contractor Review: A copy of the evaluation will be provided to the Contractor as soon as practicable after completion of the evaluation. The Contractor shall submit comments, rebutting statements, or additional information to the Contracting Officer within 14 calendar days after receipt of the evaluation.
f.Resolving Disagreements between the Government and the Contractor: Disagreements between the parties regarding the evaluation will be reviewed at a level above the Contracting Officer. The ultimate conclusion on the performance evaluation is a decision of the contracting agency. Copies of the evaluation, Contractor's response, and review comments, if any, will be retained as part of the evaluation.
g.Release of Contractor Performance Evaluation Information: The completed evaluation will not be released to other than Government personnel and the Contractor whose performance is being evaluated. Disclosure of such information could cause harm both to the commercial interest of the Government and to the competitive position of the Contractor being evaluated, as well as impede the efficiency of Government operations.
h.Source Selection Information: Departments and agencies may share past performance information with other Government departments and agencies when requested to support future award decisions. The information may be provided through interview and/or by sending the evaluation and comment document to the requesting source selection official.
i.Retention Period: The agency will retain past performance information for a maximum period of 3 years after completion of contract performance for the purpose of providing source selection information for future contract awards.
Contract No. 75A50122C00047
Page 31 of 51
SECTION H – SPECIAL CONTRACT REQUIREMENTS
H.1.PROTECTION OF HUMAN SUBJECTS
a.The Contractor agrees that the rights and welfare of human subjects involved in research under this contract shall be protected in accordance with 45 CFR Part 46 and with the Contractor's current Assurance of Compliance on file with the Office for Human Research Protections (OHRP), Department of Health and Human Services. The Contractor further agrees to provide certification at least annually that the Institutional Review Board has reviewed and approved the procedures, which involve human subjects in accordance with 45 CFR Part 46 and the Assurance of Compliance.
b.The Contractor shall bear full responsibility for the performance of all work and services involving the use of human subjects under this contract and shall ensure that work is conducted in a proper manner and as safely as is feasible. The parties hereto agree that the Contractor retains the right to control and direct the performance of all work under this contract. The Contractor shall not deem anything in this contract to constitute the Contractor or any subcontractor, agent or employee of the Contractor, or any other person, organization, institution, or group of any kind whatsoever, as the agent or employee of the Government. The Contractor agrees that it has entered into this contract and will discharge its obligations, duties, and undertakings and the work pursuant thereto, whether requiring professional judgment or otherwise, as an independent Contractor without imputing liability on the part of the Government for the acts of the Contractor or its employees.
c.Contractors involving other agencies or institutions in activities considered to be engaged in research involving human subjects must ensure that such other agencies or institutions obtain their own FWA if they are routinely engaged in research involving human subjects or ensure that such agencies or institutions are covered by the Contractors’ FWA via designation as agents of the institution or via individual investigator agreements (see OHRP website at: xxxx://xxx.xxx.xxx/xxxx/xxxxxx/xxxxxxxxxxxxxxxxxxxxxxxxxx.xxx).
d.If at any time during the performance of this contract, the Contracting Officer determines, in consultation with OHRP that the Contractor is not in compliance with any of the requirements and/or standards stated in paragraphs (a) and (b) above, the Contracting Officer may immediately suspend, in whole or in part, work and further payments under this contract until the Contractor corrects the noncompliance. The Contracting Officer may communicate the notice of suspension by telephone with confirmation in writing. If the Contractor fails to complete corrective action within the period of time designated in the Contracting Officer's written notice of suspension, the Contracting Officer may, after consultation with OHRP, terminate this contract in whole or in part, and the Contractor's name may be removed from the list of those Contractors with approved Human Subject Assurances.
H.2.CLINICAL RESEARCH
These Clinical Terms apply to all grants and contracts that involve clinical research.
The Government shall have rights to all protocols, data generated from the execution of these protocols, and final reports, funded by the Government under this contract, as defined in Rights in Data Clause in FAR 52.227-14. The Government reserves the right to request that the Contractor provide any contract deliverable in a non-proprietary form, to ensure the Government has the ability to review and distribute the deliverables, as the Government deems necessary.
Contract No. 75A50122C00047
Page 32 of 51
H.2.1.Safety and Monitoring Issues
Institutional Review Board (IRB) or Independent Ethics Committee (IEC) Approval
Before award or upon regulatory acceptance and then with Annual Progress Reports, the Contractor shall submit to the Government a copy of the current IRB or IEC approved informed consent document, documentation of continuing review and approval and the Office of Human Research Protections (OHRP) FWA number for the institution or site.
If other institutions are involved in the research (e.g., a multicenter clinical trial or study), each institution's IRB or IEC must review and approve the protocol. They must also provide the Government initial and annual documentation of continuing review and approval, including the current approved informed consent document and FWA number.
The grantee institution must ensure that the applications as well as all protocols are reviewed by their IRB or IEC.
To help ensure the safety of participants enrolled in BARDA-funded studies, the Contractor must provide the Government a summary explanation and copies of documents related to all major changes in the status of ongoing protocols, including the following:
a.All amendments or changes to the protocol, identified by protocol version number, date, or both and date it is valid.
b.All changes in informed consent documents, identified by version number, date, or both and dates it is valid.
c.Termination or temporary suspension of patient accrual.
d.Termination or temporary suspension of the protocol.
e.Any change in IRB approval.
f.Any other problems or issues that could affect the participants in the studies.
H.2.2.Data and Safety Monitoring Requirements
The Contractor may be required to conduct independent safety monitoring for clinical trials of investigational drugs, devices, or biologics; clinical trials of licensed products; and clinical research of any type involving more than minimal risk to volunteers. Independent monitoring can take a variety of forms. Phase III clinical trials must have an assigned independent data and safety monitoring board (DSMB); other trials may require DSMB oversight as well. The Contractor shall inform the Government of any upcoming site visits and/or audits of Contractor facilities funded under the CPFF CLINs of this contract. BARDA reserves the right to accompany the Contractor on site visits and/or audits of Contractors and Subcontractors as the Government deems necessary.
The type of monitoring to be used shall be mutually agreed upon between the Contractor and the Government before enrollment starts. Discussions with the responsible BARDA COR regarding appropriate safety monitoring and approval of the final monitoring plan by BARDA must occur before patient enrollment begins and may include discussions about the appointment of one of the following:
a.Independent Safety Monitor – a physician or other appropriate expert who is independent of the study and available in real time to review and recommend appropriate action regarding adverse events and other safety issues.
Contract No. 75A50122C00047
Page 33 of 51
b.Independent Monitoring Committee (IMC) or Safety Monitoring Committee (SMC) – a small group of independent investigators and biostatisticians who review data from a particular study.
c.Data and Safety Monitoring Board – an independent committee charged with reviewing safety and trial progress and providing advice with respect to study continuation, modification, and termination. The Contractor may be required to use an established BARDA DSMB or to organize an independent DSMB. All phase III clinical trials must be reviewed by a DSMB; other trials may require DSMB oversight as well. Refer to: NIAID Principles for Use of a Data and Safety Monitoring Board (DSMB) for Oversight of Clinical Trials Policy. The Government retains the right to place a nonvoting member on the DSMB.
When a monitor or monitoring board is organized, a description of it, its charter or operating procedures (including a proposed meeting schedule and plan for review of adverse events), and roster and curriculum vitae from all members must be submitted to and approved by the Government before enrollment starts.
Additionally, the Contractor must submit written summaries of all reviews conducted by the monitoring group to the Government within 30 days of reviews or meetings.
H.2.3. BARDA Protocol Review Process Before Patient Enrollment Begins
BARDA has a responsibility to ensure that mechanisms and procedures are in place to protect the safety of participants in BARDA-supported clinical trials. Therefore, before patient accrual or participant enrollment, the Contractor must provide the following (as applicable) for review and approval by the Government:
b.IRB or IEC approved clinical research protocol identified by version number, date, or both, including details of study design, proposed interventions, patient eligibility, and exclusion criteria;
c.Documentation of IRB or IEC approval, including OHRP FWA number, IRB or IEC registration number, and IRB or IEC name;
d.IRB or IEC approved informed consent document, identified by version number, date, or both and date it is valid;
e.Plans for the management of side effects;
f.Procedures for assessing and reporting adverse events;
g.Plans for data and safety monitoring (see B above) and monitoring of the clinical study site, pharmacy, and laboratory;
h.Documentation that the Contractor and all study staff responsible for the design or conduct of the research have received Good Clinical Practice (GCP) training in the protection of human subjects.
XXXXX comments will be forwarded to the Contractor within ten (10) business days of receipt of the above information. The Contractor must address in writing all study design, safety, regulatory, ethical, and conflict of interest concerns raised by the BARDA COR to the satisfaction of the Government before patient accrual or participant enrollment can begin. After the Government receives the corrected documentation, a written Contracting Officer Authorization (COA) letter may be provided to the Contractor. This COA provides authorization to the Contractor to execute the specific clinical study funded in part or in whole by the Government.
Contract No. 75A50122C00047
Page 34 of 51
H.2.4. Required Time-Sensitive Notification
Under an IND or IDE, the sponsor must provide FDA safety reports of serious adverse events. Under these Clinical Terms of Award, the Contractor must submit copies to the responsible BARDA Contracting Officer's representative (COR) as follows:
a.Expedited safety report of unexpected or life-threatening experience or death – A copy of any report of unexpected or life-threatening experience or death associated with the use of an IND drug, which must be reported to FDA by telephone or fax as soon as possible but no later than seven days after the IND sponsor’s receipt of the information, must be submitted to the BARDA program officer or the Contracting Officer's Representative within 24 hours of FDA notification.
b.Expedited safety reports of serious and unexpected adverse experiences – A copy of any report of unexpected and serious adverse experience associated with use of an IND drug or any finding from tests in laboratory animals that suggests a significant risk for human subjects, which must be reported in writing to FDA as soon as possible but no later than 15 calendar days after the IND sponsor’s receipt of the information, must be submitted to the BARDA Contracting Officer’s Representative within 24 hours of FDA notification.
c.IDE reports of unanticipated adverse device effect – A copy of any reports of unanticipated adverse device effect submitted to FDA must be submitted to the BARDA Contracting Officer’s Representative within 24 hours of FDA notification.
d.Expedited safety reports – shall be sent to the BARDA COR concurrently with the report to FDA.
e.Other adverse events documented during the course of the trial shall be included in the annual IND or IDE report and reported to the BARDA annually.
In case of problems or issues, the BARDA COR will contact the Contractor within 10 working days by email, followed within 7 calendar days by an official letter to the Contractor. The Contractor shall forward the official letter to the principal investigator listing issues and appropriate actions to be discussed.
Safety reporting for research not performed under an IND or IDE.
Ongoing safety reporting requirements for research not performed under an IND or IDE shall be mutually agreed upon by the BARDA Contracting Officer’s Representative and the Contractor.
H.3.HUMAN MATERIALS
The acquisition and supply of all human specimen material (including fetal material) used under this contract shall be obtained by the Contractor in full compliance with applicable State and Local laws and the provisions of the Uniform Anatomical Gift Act in the United States, and no undue inducements, monetary or otherwise, will be offered to any person to influence their donation of human material.
H.4.HHSAR 352.270-12 NEEDLE EXCHANGE (DEC 2015)
The Contractor shall not use any funds obligated under this contract to carry out any program of distributing sterile needles or syringes for the hypodermic injection of any illegal drug.
H.5.GUN CONTROL
The Contractor shall not use contract funds in whole or in part, to advocate or promote gun control.
Contract No. 75A50122C00047
Page 35 of 51
H.6.DISSEMINATION OF FALSE OR DELIBERATELY MISLEADING INFORMATION
The Contractor shall not use contract funds to disseminate information that is deliberately false or misleading.
H.7.CARE OF LIVE VERTEBRATE ANIMALS, HHSAR 352.270-5(b) (DEC 2015)
a.Before undertaking performance of any contract involving animal-related activities where the species is regulated by the United Sates Department of Agriculture (USDA), the Contractor shall register with the Secretary of Agriculture of the United States in accordance with 7 U.S.C. 2136 and 9 CFR 2.25 through 2.28. The Contractor shall furnish evidence of the registration to the Contracting Officer.
b.The Contractor shall acquire vertebrate animals used in research from a dealer licensed by the Secretary of Agriculture under 7 U.S.C. 2133 and 9 CFR 2.1 2.11, or from a source that is exempt from licensing under those sections.
c.The Contractor agrees that the care, use, and intended use of any live vertebrate animals in the performance of this contract shall conform with the Public Health Service (PHS) Policy on Humane Care of Use of Laboratory Animals (PHS Policy), the current Animal Welfare Assurance (Assurance), the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC) and the pertinent laws and regulations of the United States Department of Agriculture (see 7 U.S.C. 2131 et seq. and 9 CFR subchapter A, Parts 1-4). In case of conflict between standards, the more stringent standard shall govern.
d.If at any time during performance of this contract, the Contracting Officer determines, in consultation with the Office of Laboratory Animal Welfare (OLAW), National Institutes of Health (NIH), that the Contractor is not in compliance with any of the requirements and standards stated in paragraphs (a) through (c) above, the Contracting Officer may immediately suspend, in whole or in part, work and further payments under this contract until the Contractor corrects the noncompliance. Notice of the suspension may be communicated by telephone and confirmed in writing. If the Contractor fails to complete corrective action within the period of time designated in the Contracting Officer's written notice of suspension, the Contracting Officer may, in consultation with OLAW, NIH, terminate this contract in whole or in part, and the Contractor's name may be removed from the list of those contractors with Animal Welfare Assurances.
Note: The Contractor may request registration of its facility and a current listing of licensed dealers from the Regional Office of the Animal and Plant Health Inspection Service (APHIS), USDA, for the region in which its research facility is located. The location of the appropriate APHIS Regional Office, as well as information concerning this program may be obtained by contacting the Animal Care Staff, USDA/APHIS, 0000 Xxxxx Xxxx, Xxxxxxxxx, Xxxxxxxx 00000 (Email: xxx@xxxxx.xxxx.xxx; Web site: (xxxx://xxx.xxxxx.xxxx.xxx/xxx/xxxxxx/xxxxx/xxxxxxxx/xxxxxxxxxxxxx).
H.8.ANIMAL WELFARE
All research involving live, vertebrate animals shall be conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy). The PHS Policy can be accessed at: xxxx://xxxxxx0.xxx.xxx/xxxxxx/xxxx/xxxxxxxxxx/xxxxxx.xxx
H.9.HHSAR 352.211-3 PAPERWORK REDUCTION ACT (DEC 2015)
Contract No. 75A50122C00047
Page 36 of 51
a.This contract involves a requirement to collect or record information calling either for answers to identical questions from 10 or more persons other than Federal employees, or information from Federal employees which is outside the scope of their employment, for use by the Federal government or disclosure to third parties; therefore, the Paperwork Reduction Act of 1995 (44 U.S.C. 3501 et seq.) shall apply to this contract. No plan, questionnaire, interview guide or other similar device for collecting information (whether repetitive or single time) may be used without the Office of Management and Budget (OMB) first providing clearance. Contractors and the Contracting Officer's Representative shall be guided by the provisions of 5 CFR part 1320, Controlling Paperwork Burdens on the Public, and seek the advice of the HHS operating division or Office of the Secretary Reports Clearance Officer to determine the procedures for acquiring OMB clearance.
b.The Contractor shall not expend any funds or begin any data collection until the Contracting Officer provides the Contractor with written notification authorizing the expenditure of funds and the collection of data. The Contractor shall allow at least 120 days for OMB clearance. The Contracting Officer will consider excessive delays caused by the Government which arise out of causes beyond the control and without the fault or negligence of the Contractor in accordance with the Excusable Delays or Default clause of this contract.
H.10.RESTRICTION ON PORNOGRAPHY ON COMPUTER NETWORKS
The Contractor shall not use contract funds to maintain or establish a computer network unless such network blocks the viewing, downloading, and exchanging of pornography.
H.11.352.239-74 ELECTRONIC AND INFORMATION TECHNOLOGY ACCESSIBILITY (DEC 2015)
a.Pursuant to Section 508 of the Rehabilitation Act of 1973 (29 U.S.C. 794d), as amended by the Workforce Investment Act of 1998, all electronic and information technology (EIT) supplies and services developed, acquired, or maintained under this contract or order must comply with the “Architectural and Transportation Barriers Compliance Board Electronic and Information Technology (EIT) Accessibility Standards” set forth by the Architectural and Transportation Barriers Compliance Board (also referred to as the “Access Board”) in 36 CFR part 1194. Information about Section 508 is available at xxxx://xxx.xxx.xxx/xxx/000. The complete text of Section 508 Final Provisions can be accessed at xxxx://xxx.xxxxxx-xxxxx.xxx/xxxxxxxxxx-xxx- standards/communications-and-it/about-the-section-508-standards.
b.The Section 508 accessibility standards applicable to this contract or order are identified in the Statement of Work or Specification or Performance Work Statement. The contractor must provide any necessary updates to the submitted HHS Product Assessment Template(s) at the end of each contract or order exceeding the simplified acquisition threshold (see FAR 2.101) when the contract or order duration is one year or less. If it is determined by the Government that EIT supplies and services provided by the Contractor do not conform to the described accessibility standards in the contract, remediation of the supplies or services to the level of conformance specified in the contract will be the responsibility of the Contractor at its own expense.
c.The Section 508 accessibility standards applicable to this contract are: E205 Electronic Content Standards. Note: Items provided incidental to contract administration are not subject to this section.
d.In the event of a modification(s) to this contract or order, which adds new EIT supplies or services or revises the type of, or specifications for, supplies or services, the Contracting Officer
Contract No. 75A50122C00047
Page 37 of 51
may require that the contractor submit a completed HHS Section 508 Product Assessment Template and any other additional information necessary to assist the Government in determining that the EIT supplies or services conform to Section 508 accessibility standards. Instructions for documenting accessibility via the HHS Section 508 Product Assessment Template may be found under Section 508 policy on the HHS website: (xxxx://xxx.xxx.xxx/xxx/000). If it is determined by the Government that EIT supplies and services provided by the Contractor do not conform to the described accessibility standards in the contract, remediation of the supplies or services to the level of conformance specified in the contract will be the responsibility of the Contractor at its own expense.
e.If this is an Indefinite Delivery contract, a Blanket Purchase Agreement or a Basic Ordering Agreement, the task/delivery order requests that include EIT supplies or services will define the specifications and accessibility standards for the order. In those cases, the Contractor may be required to provide a completed HHS Section 508 Product Assessment Template and any other additional information necessary to assist the Government in determining that the EIT supplies or services conform to Section 508 accessibility standards. Instructions for documenting accessibility via the HHS Section 508 Product Assessment Template may be found
at xxxx://xxx.xxx.xxx/xxx/000. If it is determined by the Government that EIT supplies and services provided by the Contractor do not conform to the described accessibility standards in the provided documentation, remediation of the supplies or services to the level of conformance specified in the contract will be the responsibility of the Contractor at its own expense.
H.12.CONFIDENTIALITY OF INFORMATION
a.Confidential information, as used in this article, means information or data of a personal nature about an individual, or proprietary information or data submitted by or pertaining to an institution or organization.
b.The Contracting Officer and the Contractor may, by mutual consent, identify elsewhere in this contract specific information and/or categories of information which the Government will furnish to the Contractor or that the Contractor is expected to generate which is confidential. Similarly, the Contracting Officer and the Contractor may, by mutual consent, identify such confidential information from time to time during the performance of the contract. Failure to agree will be settled pursuant to the "Disputes" clause.
c.If it is established elsewhere in this contract that information to be utilized under this contract, or a portion thereof, is subject to the Privacy Act, the Contractor will follow the rules and procedures of disclosure set forth in the Privacy Act of 1974, 5 U.S.C. 552a, and implementing regulations and policies, with respect to systems of records determined to be subject to the Privacy Act.
d.Confidential information, as defined in paragraph (a) of this article, shall not be disclosed without the prior written consent of the individual, institution, or organization.
e.Whenever the Contractor is uncertain with regard to the proper handling of material under the contract, or if the material in question is subject to the Privacy Act or is confidential information subject to the provisions of this article, the Contractor shall obtain a written determination from the Contracting Officer prior to any release, disclosure, dissemination, or publication.
f.Contracting Officer determinations will reflect the result of internal coordination with appropriate program and legal officials.
g.The provisions of paragraph (d) of this article shall not apply to conflicting or overlapping
Contract No. 75A50122C00047
Page 38 of 51
provisions in other Federal, State or local laws.
H.13.INSTITUTIONAL RESPONSIBILITY REGARDING INVESTIGATOR CONFLICTS OF INTERESTS
The Institution (includes any Contractor, public or private, excluding a Federal agency) shall comply with the requirements of 45 CFR Part 94, Responsible Prospective Contractors, which promotes objectivity in research by establishing standards to ensure that Investigators (defined as the project director or principal Investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research funded under BARDA contracts, or proposed for such funding, which may include, for example, collaborators or consultants) will not be biased by any Investigator financial conflicts of interest. 45 CFR Part 94 is available at the following Web site: xxxxx://xxx.xxxx.xxx/xxxxxxx/xxxxx-00/xxxx-00
As required by 45 CFR Part 94, the Institution shall, at a minimum:
a.Maintain an up-to-date, written, enforceable policy on financial conflicts of interest that complies with 45 CFR Part 94, inform each Investigator of the policy, the Investigator's reporting responsibilities regarding disclosure of significant financial interests, and the applicable regulation, and make such policy available via a publicly accessible Web site, or if none currently exist, available to any requestor within five business days of a request. A significant financial interest means a financial interest consisting of one or more of the following interests of the Investigator (and those of the Investigator's spouse and dependent children) that reasonably appears to be related to the Investigator's institutional responsibilities:
1.With regard to any publicly traded entity, a significant financial interest exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure and the value of any equity interest in the entity as of the date of disclosure, when aggregated, exceeds $5,000. Included are payments and equity interests;
2.With regard to any non-publicly traded entity, a significant financial interest exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure, when aggregated, exceeds $5,000, or when the Investigator (or the Investigator's spouse or dependent children) holds any equity interest; or
3.Intellectual property rights and interests, upon receipt of income related to such rights and interest.
Significant financial interests do not include the following:
1.Income from seminars, lectures, or teaching, and service on advisory or review panels for government agencies, Institutions of higher education, academic teaching hospitals, medical centers, or research institutes with an Institution of higher learning; and
2.Income from investment vehicles, such as mutual funds and retirement accounts, as long as the Investigator does not directly control the investment decisions made in these vehicles.
b.Require each Investigator to complete training regarding the Institution's financial conflicts of interest policy prior to engaging in research related to any BARDA funded contract and at least every four years. The Institution must take reasonable steps [see Part 94.4(c)] to ensure that investigators working as collaborators, consultants or subcontractors comply with the regulations.
Contract No. 75A50122C00047
Page 39 of 51
c.Designate an official(s) to solicit and review disclosures of significant financial interests from each Investigator who is planning to participate in, or is participating in, the BARDA funded research.
d.Require that each Investigator who is planning to participate in the BARDA funded research disclose to the Institution's designated official(s) the Investigator's significant financial interest (and those of the Investigator's spouse and dependent children) no later than the date of submission of the Institution's proposal for XXXXX funded research. Require that each Investigator who is participating in the BARDA funded research to submit an updated disclosure of significant financial interests at least annually, in accordance with the specific time period prescribed by the Institution during the period of the award as well as within thirty days of discovering or acquiring a new significant financial interest.
e.Provide guidelines consistent with the regulations for the designated official(s) to determine whether an Investigator's significant financial interest is related to BARDA funded research and, if so related, whether the significant financial interest is a financial conflict of interest. An Investigator's significant financial interest is related to BARDA funded research when the Institution, thorough its designated official(s), reasonably determines that the significant financial interest: Could be affected by the BARDA funded research; or is in an entity whose financial interest could be affected by the research. A financial conflict of interest exists when the Institution, through its designated official(s), reasonably determines that the significant financial interest could directly and significantly affect the design, conduct, or reporting of the BARDA funded research.
f.Take such actions as necessary to manage financial conflicts of interest, including any financial conflicts of a subcontractor Investigator. Management of an identified financial conflict of interest requires development and implementation of a management plan and, if necessary, a retrospective review and mitigation report pursuant to Part 94.5(a).
g.Provide initial and ongoing FCOI reports to the Contracting Officer pursuant to Part 94.5(b).
h.Maintain records relating to all Investigator disclosures of financial interests and the Institution's review of, and response to, such disclosures, and all actions under the Institution's policy or retrospective review, if applicable, for at least 3 years from the date of final payment or, where applicable, for the other time periods specified in 48 CFR Part 4, subpart 4.7, Contract Records Retention.
i.Establish adequate enforcement mechanisms and provide for employee sanctions or other administrative actions to ensure Investigator compliance as appropriate.
j.Complete the certification titled "Certification of Institutional Policy on Financial Conflicts of Interest".
If the failure of an Institution to comply with an Institution's financial conflicts of interest policy or a financial conflict of interest management plan appears to have biased the design, conduct, or reporting of the BARDA funded research, the Institution must promptly notify the Contracting Officer of the corrective action taken or to be taken. The Contracting Officer will consider the situation and, as necessary, take appropriate action or refer the matter to the Institution for further action, which may include directions to the Institution on how to maintain appropriate objectivity in the BARDA funded research project.
Contract No. 75A50122C00047
Page 40 of 51
The Contracting Officer and/or HHS may inquire at any time before, during, or after award into any Investigator disclosure of financial interests, and the Institution's review of, and response to, such disclosure, regardless of whether the disclosure resulted in the Institution's determination of a financial conflict of interests. The Contracting Officer may require submission of the records or review them on site. On the basis of this review of records or other information that may be available, the Contracting Officer may decide that a particular financial conflict of interest will bias the objectivity of the BARDA funded research to such an extent that further corrective action is needed or that the Institution has not managed the financial conflict of interest in accordance with Part 94.6(b). The issuance of a Stop Work Order by the Contracting Officer may be necessary until the matter is resolved.
If the Contracting Officer determines that XXXXX funded clinical research, whose purpose is to evaluate the safety or effectiveness of a drug, medical device, or treatment, has been designed, conducted, or reported by an Investigator with a financial conflict of interest that was not managed or reported by the Institution, the Institution shall require the Investigator involved to disclose the financial conflict of interest in each public presentation of the results of the research and to request an addendum to previously published presentations.
H.14.PUBLICATION AND PUBLICITY
The Contractor agrees to accurately and factually represent the work conducted under this contract in all press releases. The Contractor shall provide and the Contracting Officer shall approve an advance copy of any such press release not less than five (5) business days prior to the issuance of any potential press release.
The Contractor shall acknowledge the support of the Department of Health and Human Services, Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority whenever publicizing the work under this contract in any media by including an acknowledgment substantially as follows:
"This project has been funded in whole or in part with federal funds from the Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority, under Contract No. Insert Contract Number ".
H.15.REPORTING MATTERS INVOLVING FRAUD, WASTE, AND ABUSE
Anyone who becomes aware of the existence or apparent existence of fraud, waste and abuse in BARDA funded programs is encouraged to report such matters to the HHS Inspector General's Office in writing or on the Inspector General's Hotline. The toll-free number is 0-000-XXX-XXXX (0-000-000-0000). All telephone calls will be handled confidentially. The website to file a complaint on-line is: xxxxx://xxx.xxx.xxx/xxxxx/xxxxxx-xxxxx/ and the mailing address is:
US Department of Health and Human Services Office of Inspector General
ATTN: OIG HOTLINE OPERATIONS
P.O. Box 23489
Washington, D.C. 20026
Contract No. 75A50122C00047
Page 41 of 51
H.16.PROHIBITION ON CONTRACTOR INVOLVEMENT WITH TERRORIST ACTIVITIES
The Contractor acknowledges that U.S. Executive Orders and Laws, including but not limited to E.O. 13224 and Pub. L. 107-56, prohibit transactions with, and the provision of resources and support to, individuals and organizations associated with terrorism. It is the legal responsibility of the Contractor to ensure compliance with these Executive Orders and Laws. This clause must be included in all subcontracts issued under this contract.
H.17.ACCESS TO DOCUMENTATION/DATA
The Government shall have physical and electronic access to all documentation and data generated under this contract, including: all data documenting Contractor performance, all data generated, all communications and correspondence with regulatory agencies and bodies to include all audit observations, inspection reports, milestone completion documents, and all Contractor commitments and responses. The Contractor shall provide the Government with an electronic copy of all correspondence with the FDA within five (5) business days of submission to or receipt from FDA. The Government shall acquire rights to all data funded under any contract awarded in response to this RFP in accordance with FAR Subpart 27.4 and FAR Clause 52.227-14.
H.18.IDENTIFICATION AND DISPOSITION OF DATA
The Contractor will be required to provide certain data generated under the CPFF CLINs of this contract to the Department of Health and Human Services (HHS). HHS reserves the right to review any other such data determined by HHS to be relevant to this contract. The Contractor shall keep copies of all data required by the Food and Drug Administration (FDA) relevant to this contract for the time specified by the FDA.
H.19.DISSEMINATION OF INFORMATION
Except for necessary releases to subcontractors and advisors and in connection with regulatory reporting requirements, no information related to data obtained under the CPFF CLINs of this contract shall be released or publicized without the prior written consent of the COR, whose approval shall not be unreasonably withheld, conditioned, or delayed, provided that no such consent is required to comply with any law, rule, regulation, court ruling or similar order; for submission to any government entity’ for submission to any securities exchange on which the Contractor’s (or its parent corporation’s) securities may be listed for trading; or to third parties relating to securing, seeking, establishing or maintaining regulatory or other legal approvals or compliance, financing and capital raising activities, or mergers, acquisitions, or other business transactions.
H.20.PUBLIC ACCESS TO ARCHIVED PUBLICATIONS RESULTING FROM ASPR FUNDED RESEARCH
All ASPR-funded investigators shall submit to the NIH National Library of Medicine's (NLM) PubMed Central (PMC) an electronic version of the author's final manuscript, upon acceptance for publication, of any peer-reviewed scientific publications resulting from research supported in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response. XXXX defines the author's final manuscript as the final version accepted for journal publication, and includes all modifications from the publishing peer review process. The PMC archive will preserve permanently these manuscripts for use by the public, health care providers,
Contract No. 75A50122C00047
Page 42 of 51
educators, scientists, and ASPR. The Policy directs electronic submissions to the NIH/NLM/PMC: xxxx://xxx.xxxxxxxxxxxxx.xxx.xxx.
Additional information is available at: xxxx://xxx.xxx.xxx/Xxxxxxxxxxxx/xxxxxxxx/xxxxxxx/Xxxxx/XxxxxxXxxx.xxxx
H.21.REGISTRATION WITH THE SELECT AGENT PROGRAM FOR WORK INVOLVING THE POSSESSION, USE, AND/OR TRANSFER OF SELECT BIOLOGICAL AGENTS OR TOXINS
Work involving select biological agents or toxins shall not be conducted under this contract until the Contractor and any affected subcontractor(s) are granted a certificate of registration or are authorized to work with the applicable select agents.
For prime or subcontract awards to domestic institutions who possess, use, and/or transfer Select Agents under this contract, the institution must complete registration with the Centers for Disease Control and Prevention (CDC), Department of Health and Human Services (DHHS) or the Animal and Plant Health Inspection Services (APHIS), U.S. Department of Agriculture (USDA), as applicable, before performing work involving Select Agents, in accordance with 42 CFR 73. No Government funds can be used for work involving Select Agents, as defined in 42 CFR 73, if the final registration certificate is denied.
For prime or subcontract awards to foreign institutions who possess, use, and/or transfer Select Agents under this contract, the institution must provide information satisfactory to the Government that a process equivalent to that described in 42 CFR 73 (xxxxx://xxx.xxxx.xxx/xxxxxxx/xxxxx-00/xxxx-00) for U.S. institutions is in place and will be administered on behalf of all Select Agent work sponsored by these funds before using these funds for any work directly involving the Select Agents. The Contractor must provide information addressing the following key elements appropriate for the foreign institution: safety, security, training, procedures for ensuring that only approved/appropriate individuals have access to the Select Agents, and any applicable laws, regulations and policies equivalent to 42 CFR 73. The Government will assess the policies and procedures for comparability to the U.S. requirements described in 42 CFR Part 73. When requested by the contracting officer, the Contractor shall provide key information delineating any laws, regulations, policies, and procedures applicable to the foreign institution for the safe and secure possession, use, and transfer of Select Agents. This includes summaries of safety, security, and training plans, and applicable laws, regulations, and policies. For the purpose of security risk assessments, the Contractor must provide the names of all individuals at the foreign institution who will have access to the Select Agents and procedures for ensuring that only approved and appropriate individuals have access to Select Agents under the contract.
Listings of HHS select agents and toxins, biologic agents and toxins, and overlap agents or toxins as well as information about the registration process, can be obtained on the Select Agent Program Web site at xxxxx://xxx.xxxxxxxxxxxx.xxx/
H.22.SHARING RESEARCH DATA
The Contractor’s data sharing plan, due within 90 days of the effective date of the contract, is hereby incorporated by reference. The Contractor agrees to adhere to its plan and shall request prior approval of the Contracting Officer for any changes in its plan.
BARDA endorses the sharing of final research data to serve health. This contract is expected to generate research data that must be shared with the public and other researchers.
Contract No. 75A50122C00047
Page 43 of 51
XXXXX recognizes that data sharing may be complicated or limited, in some cases, by institutional policies, local IRB rules, as well as local, state and Federal laws and regulations, including the Privacy Rule (see HHS-published documentation on the Health Information Privacy at xxxx://xxx.xxx.xxx/xxx/xxxxxxx/xxxxx.xxxx). The rights and privacy of people who participate in BARDA- funded research must be protected at all times; thus, data intended for broader use should be free of identifiers that would permit linkages to individual research participants and variables that could lead to deductive disclosure of the identity of individual subjects.
H.23.DISCLOSURE OF FINANCIAL PERFORMANCE AND TRANSFER OF TECHNOLOGY
This clause shall remain in effect during the term of the Contract.
a.Contractor Financial Performance
The Contractor shall provide quarterly a summary or collection of financial statements, such as balance sheets, income statements, and cash flow statements. These may include SEC filings or other documents that highlight Contractor revenues and expenditures to be agreed upon with consultation from the Contracting Officer and COR. Additionally, the company must notify the Contracting Officer of financial issues leading to potential liquidation of assets as soon as feasible after notifying the SEC.
b.Post-award Transfer of Ownership of Technology
The Contractor shall provide notice to the Contracting Officer and COR upon entering into any new term sheet, between the Contractor and an external party, that outlines the transfer of ownership, or operational, corporate, or economic control of technology or establishment of a licensing agreement of any technology funded under this Contract from the Contractor to another institution. This clause excludes subcontracts.
H.24.HHSAR 352.270-13 CONTINUED BAN ON FUNDING ABORTION AND CONTINUED BAN ON FUNDING OF HUMAN EMBRYO RESEARCH (DEC 2015)
a.The Contractor shall not use any funds obligated under this contract for any abortion.
b.The Contractor shall not use any funds obligated under this contract for the following:
(1)The creation of a human embryo or embryos for research purposes; or
(2)Research in which a human embryo or embryos are destroyed, discarded, or knowingly subjected to risk of injury of death greater than that allowed for research on fetuses in utero under 45 CFR Part 46 and Section 498(b) of the Public Health Service Act (42 U.S.C. 289g(b)).
c.The term “human embryo or embryos” includes any organism, not protected as a human subject under 45 CFR Part 46 as of the date of the enactment of this Act, that is derived by fertilization, parthenogenesis, cloning, or any other means from one or more human gametes of human diploid cells.
d.The Contractor shall not use any Federal funds for the cloning of human beings.
Contract No. 75A50122C00047
Page 44 of 51
PART II – CONTRACT CLAUSES
SECTION I – CONTRACT CLAUSES
FAR 52.252-2 Clauses Incorporated by Reference (Feb 1998)
This contract incorporates one or more clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available.
I.1.FEDERAL ACQUISITION REGULATION (FAR) (48 CFR Chapter 1) FFP SUPPLIES CLAUSES
Full text of the FAR clauses may be accessed electronically at: xxxxx://xxx.xxxxxxxxxxx.xxx/xxx/xxxxx.xxxx
| Reg | Clause | Date | Clause Title | ||||||||
| FAR | 52.202-1 | Jun 2020 | Definitions | ||||||||
| FAR | 52.203-3 | Apr 1984 | Gratuities | ||||||||
| FAR | 52.203-5 | May 2014 | Covenant Against Contingent Fees | ||||||||
| FAR | 52.203-6 | Jun 2020 | Restrictions on Subcontractor Sales to the Government | ||||||||
| FAR | 52.203-7 | Jun 2020 | Anti-Kickback Procedures | ||||||||
| FAR | 52.203-8 | May 2014 | Cancellation, Rescission, and Recovery of Funds for Illegal or Improper Activity | ||||||||
| FAR | 52.203-10 | May 2014 | Price or Fee Adjustment for Illegal or Improper Activity | ||||||||
| FAR | 52.203-12 | Jun 2020 | Limitation on Payments to Influence Certain Federal Transactions | ||||||||
| FAR | 52.203-13 | Nov 2021 | Contractor Code of Business Ethics and Conduct | ||||||||
| FAR | 52.203-14 | Nov 2021 | Display of Hotline Poster(s) | ||||||||
| FAR | 52.203-17 | Jun 2020 | Contractor Employee Whistleblower Rights and Requirement To Inform Employees of Whistleblower Rights | ||||||||
| FAR | 52.203-19 | Jan 2017 | Prohibition on Requiring Certain Internal Confidentiality Agreements or Statements | ||||||||
| FAR | 52.204-4 | May 2011 | Printed or Copied Double-Sided on Postconsumer Fiber Content Paper | ||||||||
| FAR | 52.204-10 | Jun 2020 | Reporting Executive Compensation and First-Tier Subcontract Awards | ||||||||
| FAR | 52.204-13 | Oct 2018 | System for Award Management Maintenance | ||||||||
| FAR | 52.204-18 | Aug 2020 | Commercial and Government Entity Code Maintenance | ||||||||
| FAR | 52.204-19 | Dec 2014 | Incorporation by Reference of Representations and Certifications | ||||||||
| FAR | 52.204-21 | Nov 2021 | Basic Safeguarding of Covered Contractor Information Systems | ||||||||
| FAR | 52.204-23 | Nov 2021 | Prohibition on Contracting for Hardware, Software, and Services Developed or Provided by Kaspersky Lab and Other Covered Entities | ||||||||
| FAR | 52.204-25 | Nov 2021 | Prohibition on Contracting for Certain Telecommunications and Video Surveillance Services or Equipment | ||||||||
| FAR | 52.209-6 | Nov 2021 | Protecting the Government's Interests When Subcontracting With Offerors Debarred, Suspended, or Proposed for Debarment | ||||||||
| FAR | 52.209-9 | Oct 2018 | Updates of Publicly Available Information Regarding Responsibility Matters | ||||||||
| FAR | 52.209-10 | Nov 2015 | Prohibition on Contracting with Inverted Domestic Corporations | ||||||||
| FAR | 52.210-1 | Nov 2021 | Market Research | ||||||||
| FAR | 52.211-5 | Aug 2000 | Material Requirements | ||||||||
| FAR | 52.215-2 | Jun 2020 | Audit and Records – Negotiation | ||||||||
| FAR | 52.215-8 | Oct 1997 | Order of Precedence - Uniform Contract Format | ||||||||
| FAR | 52.215-10 | Aug 2011 | Price Reduction for Defective Certified Cost or Pricing Data | ||||||||
| FAR | 52.215-11 | Jun 2020 | Price Reduction for Defective Certified Cost or Pricing Data—Modifications. | ||||||||
| FAR | 52.215-12 | Jun 2020 | Subcontractor Certified Cost or Pricing Data | ||||||||
| FAR | 52.215-13 | Jun 2020 | Subcontractor Certified Cost or Pricing Data—Modifications | ||||||||
| FAR | 52.215-14 | Nov 2021 | Integrity of Unit Prices | ||||||||
| FAR | 52.215-15 | Oct 2010 | Pension Adjustments and Asset Reversions | ||||||||
| FAR | 52.215-18 | Jul 2005 | Reversion or Adjustment of Plans for Postretirement Benefits (PRB) other than Pensions | ||||||||
Contract No. 75A50122C00047
Page 45 of 51
| FAR | 52.215-19 | Oct 1997 | Notification of Ownership Changes | ||||||||
| FAR | 52.215-21 | Nov 2021 | Requirements for Certified Cost or Pricing Data and Data Other Than Certified Cost or Pricing Data -Modifications | ||||||||
| FAR | 52.215-23 | Jun 2020 | Limitations on Pass-Through Charges | ||||||||
| FAR | 52.219-8 | Oct 2018 | Utilization of Small Business Concerns | ||||||||
| FAR | 52.219-28 | Sep 2021 | Post-Award Small Business Program Representation | ||||||||
| FAR | 52.222-1 | Feb 1997 | Notice to the Government of Labor Disputes | ||||||||
| FAR | 52.222-3 | Jun2003 | Convict Labor | ||||||||
| FAR | 52.222-21 | Apr 2015 | Prohibition of Segregated Facilities | ||||||||
| FAR | 52.222-26 | Sept 2016 | Equal Opportunity | ||||||||
| FAR | 52.222-35 | Jun 2020 | Equal Opportunity for Veterans | ||||||||
| FAR | 52.222-36 | Jun 2020 | Equal Opportunity for Workers with Disabilities | ||||||||
| FAR | 52.222-37 | Jun 2020 | Employment Reports on Veterans | ||||||||
| FAR | 52.222-40 | Dec 2010 | Notification of Employee Rights Under the National Labor Relations Act | ||||||||
| FAR | 52.222-50 | Nov 2021 | Combating Trafficking in Persons | ||||||||
| FAR | 52.222-54 | May 2022 | Employment Eligibility Verification | ||||||||
| FAR | 52.223-6 | May 2001 | Drug-Free Workplace | ||||||||
| FAR | 52.223-18 | Jun 2020 | Encouraging Contractor Policies to Ban Text Messaging While Driving | ||||||||
| FAR | 52.224-1 | Apr 1984 | Privacy Act Notification | ||||||||
| FAR | 52.224-2 | Apr 1984 | Privacy Act | ||||||||
| FAR | 52.225-13 | Feb 2021 | Restrictions on Certain Foreign Purchases | ||||||||
| FAR | 52.226-1 | Jun 2000 | Utilization of Indian Organizations and Indian-Owned Economic Enterprises. | ||||||||
| FAR | 52.227-1 | June 2020 | Authorization and Consent, Alternate 1 (APR 1984) | ||||||||
| FAR | 52.227-2 | Jun 2020 | Notice and Assistance Regarding Patent and Copyright Infringement | ||||||||
| FAR | 52.227-11 | May 2014 | Patent Rights – Ownership by the Contractor | ||||||||
| FAR | 52.227-14 | May 2014 | Rights in Data - General, Alternate II | ||||||||
| FAR | 52.227-16 | June 1987 | Additional Data Requirements | ||||||||
| FAR | 52.228-7 | Mar 1996 | Insurance – Liability to Third Persons | ||||||||
| FAR | 52.229-3 | Feb 2013 | Federal, State and Local Taxes | ||||||||
| FAR | 52.232-1 | Apr 1984 | Payments | ||||||||
| FAR | 52.232-2 | Apr 1984 | Payments under Fixed-Price Research and Development Contracts | ||||||||
| FAR | 52.232-8 | Feb 2002 | Discounts for Prompt Payment | ||||||||
| FAR | 52.232-9 | Apr 1984 | Limitation on Withholding of Payments | ||||||||
| FAR | 52.232-11 | Apr 1984 | Extras | ||||||||
| FAR | 52.232-17 | May 2014 | Interest | ||||||||
| FAR | 52.232-23 | May 2014 | Assignment of Claims | ||||||||
| FAR | 52.232-25 | Jan 2017 | Prompt Payment | ||||||||
| FAR | 52.232-33 | Oct 2018 | Payment by Electronic Funds Transfer--System for Award Management | ||||||||
| FAR | 52.232-39 | Jun 2013 | Unenforceability of Unauthorized Obligations | ||||||||
| FAR | 52.232-40 | Nov 2021 | Providing Accelerated Payments to Small Business SubOfferors | ||||||||
| FAR | 52.233-1 | May 2014 | Disputes | ||||||||
| FAR | 52.233-3 | Aug 1996 | Protest After Award | ||||||||
| FAR | 52.233-4 | Oct 2004 | Applicable Law for Breach of Contract Claim | ||||||||
| FAR | 52.242-2 | Apr 1991 | Production Progress Reports | ||||||||
| FAR | 52.242-13 | Jul 1995 | Bankruptcy | ||||||||
| FAR | 52.243-1 | Aug 1987 | Changes - Fixed-Price Alternate V (Apr 1984). | ||||||||
| FAR | 52.243-6 | Apr 1984 | Change Order Accounting | ||||||||
| FAR | 52.243-7 | Jan 2017 | Notification of Changes | ||||||||
| FAR | 52.244-6 | Jan 2022 | Subcontracts for Commercial Products and Commercial Services | ||||||||
| FAR | 52.246-23 | Feb 1997 | Limitation of Liability. | ||||||||
| FAR | 52.246-25 | Feb 1997 | Limitation of Liability—Services | ||||||||
Contract No. 75A50122C00047
Page 46 of 51
| FAR | 52.247-34 | Nov 1991 | F.o.b Destination | ||||||||
| FAR | 52.247-67 | Feb 2006 | Submission of Transportation Documents for Audit | ||||||||
| FAR | 52.249-2 | Apr 2012 | Termination for the Convenience of the Government (Fixed-Price) | ||||||||
| FAR | 52.249-8 | Apr 1984 | Default (Fixed-Price Supply and Service) | ||||||||
| FAR | 52.249-9 | Apr 1984 | Default (Fixed-Price Research and Development) | ||||||||
| FAR | 52.253-1 | Jan 1991 | Computer Generated Forms | ||||||||
I.2.FEDERAL ACQUISITION REGULATION (FAR) (48 CFR Chapter 1) CPFF RESEARCH AND DEVELOPMENT CLAUSES
Full text of the FAR clauses may be accessed electronically at: xxxxx://xxx.xxxxxxxxxxx.xxx/xxx/xxxxx.xxxx
| Reg | Clause | Date | Clause Title | ||||||||
| FAR | 52.202-1 | Jun 2020 | Definitions | ||||||||
| FAR | 52.203-3 | Apr 1984 | Gratuities | ||||||||
| FAR | 52.203-5 | May 2014 | Covenant Against Contingent Fees | ||||||||
| FAR | 52.203-6 | Jun 2020 | Restrictions on Subcontractor Sales to the Government | ||||||||
| FAR | 52.203-7 | Jun 2020 | Anti-Kickback Procedures | ||||||||
| FAR | 52.203-8 | May 2014 | Cancellation, Rescission, and Recovery of Funds for Illegal or Improper Activity | ||||||||
| FAR | 52.203-10 | May 2014 | Price or Fee Adjustment for Illegal or Improper Activity | ||||||||
| FAR | 52.203-12 | Jun 2020 | Limitation on Payments to Influence Certain Federal Transactions | ||||||||
| FAR | 52.203-13 | Nov 2021 | Contractor Code of Business Ethics and Conduct | ||||||||
| FAR | 52.203-14 | Nov 2021 | Display of Hotline Poster(s) | ||||||||
| FAR | 52.203-17 | Jun 2020 | Contractor Employee Whistleblower Rights and Requirement To Inform Employees of Whistleblower Rights | ||||||||
| FAR | 52.203-19 | Jan 2017 | Prohibition on Requiring Certain Internal Confidentiality Agreements or Statements | ||||||||
| FAR | 52.204-4 | May 2011 | Printed or Copied Double-Sided on Postconsumer Fiber Content Paper | ||||||||
| FAR | 52.204-10 | Jun 2020 | Reporting Executive Compensation and First-Tier Subcontract Awards | ||||||||
| FAR | 52.204-13 | Oct 2018 | System for Award Management Maintenance | ||||||||
| FAR | 52.204-18 | Aug 2020 | Commercial and Government Entity Code Maintenance | ||||||||
| FAR | 52.204-19 | Dec 2014 | Incorporation by Reference of Representations and Certifications | ||||||||
| FAR | 52.204-21 | Nov 2021 | Basic Safeguarding of Covered Contractor Information Systems | ||||||||
| FAR | 52.204-23 | Nov 2021 | Prohibition on Contracting for Hardware, Software, and Services Developed or Provided by Kaspersky Lab and Other Covered Entities | ||||||||
| FAR | 52.204-25 | Nov 2021 | Prohibition on Contracting for Certain Telecommunications and Video Surveillance Services or Equipment | ||||||||
| FAR | 52.209-6 | Nov 2021 | Protecting the Government's Interests When Subcontracting With Offerors Debarred, Suspended, or Proposed for Debarment | ||||||||
| FAR | 52.209-9 | Oct 2018 | Updates of Publicly Available Information Regarding Responsibility Matters | ||||||||
| FAR | 52.209-10 | Nov 2015 | Prohibition on Contracting with Inverted Domestic Corporations | ||||||||
| FAR | 52.210-1 | Nov 2021 | Market Research | ||||||||
| FAR | 52.215-2 | Jun 2020 | Audit and Records – Negotiation | ||||||||
| FAR | 52.215-8 | Oct 1997 | Order of Precedence - Uniform Contract Format | ||||||||
| FAR | 52.215-10 | Aug 2011 | Price Reduction for Defective Certified Cost or Pricing Data | ||||||||
| FAR | 52.215-11 | Jun 2020 | Price Reduction for Defective Certified Cost or Pricing Data—Modifications. | ||||||||
| FAR | 52.215-12 | Jun 2020 | Subcontractor Certified Cost or Pricing Data | ||||||||
| FAR | 52.215-13 | Jun 2020 | Subcontractor Certified Cost or Pricing Data—Modifications | ||||||||
| FAR | 52.215-14 | Nov 2021 | Integrity of Unit Prices | ||||||||
| FAR | 52.215-15 | Oct 2010 | Pension Adjustments and Asset Reversions | ||||||||
| FAR | 52.215-18 | Jul 2005 | Reversion or Adjustment of Plans for Postretirement Benefits (PRB) other than Pensions | ||||||||
| FAR | 52.215-19 | Oct 1997 | Notification of Ownership Changes | ||||||||
Contract No. 75A50122C00047
Page 47 of 51
| FAR | 52.215-21 | Nov 2021 | Requirements for Certified Cost or Pricing Data and Data Other Than Certified Cost or Pricing Data -Modifications | ||||||||
| FAR | 52.215-23 | Jun 2020 | Limitations on Pass-Through Charges | ||||||||
| FAR | 52.216-7 | Aug 2018 | Allowable Cost and Payment | ||||||||
| FAR | 52.216-8 | Jun 2011 | Fixed Fee | ||||||||
| FAR | 52.219-8 | Oct 2018 | Utilization of Small Business Concerns | ||||||||
| FAR | 52.219-28 | Sep 2021 | Post-Award Small Business Program Representation | ||||||||
| FAR | 52.222-1 | Feb 1997 | Notice to the Government of Labor Disputes | ||||||||
| FAR | 52.222-2 | July 1990 | Payment for Overtime Premiums | ||||||||
| FAR | 52.222-3 | Jun2003 | Convict Labor | ||||||||
| FAR | 52.222-21 | Apr 2015 | Prohibition of Segregated Facilities | ||||||||
| FAR | 52.222-26 | Sept 2016 | Equal Opportunity | ||||||||
| FAR | 52.222-35 | Jun 2020 | Equal Opportunity for Veterans | ||||||||
| FAR | 52.222-36 | Jun 2020 | Equal Opportunity for Workers with Disabilities | ||||||||
| FAR | 52.222-37 | Jun 2020 | Employment Reports on Veterans | ||||||||
| FAR | 52.222-40 | Dec 2010 | Notification of Employee Rights Under the National Labor Relations Act | ||||||||
| FAR | 52.222-50 | Nov 2021 | Combating Trafficking in Persons | ||||||||
| FAR | 52.222-54 | May 2022 | Employment Eligibility Verification | ||||||||
| FAR | 52.223-6 | May 2001 | Drug-Free Workplace | ||||||||
| FAR | 52.223-18 | Jun 2020 | Encouraging Contractor Policies to Ban Text Messaging While Driving | ||||||||
| FAR | 52.224-1 | Apr 1984 | Privacy Act Notification | ||||||||
| FAR | 52.224-2 | Apr 1984 | Privacy Act | ||||||||
| FAR | 52.225-13 | Feb 2021 | Restrictions on Certain Foreign Purchases | ||||||||
| FAR | 52.226-1 | Jun 2000 | Utilization of Indian Organizations and Indian-Owned Economic Enterprises. | ||||||||
| FAR | 52.227-1 | June 2020 | Authorization and Consent, Alternate 1 (APR 1984) | ||||||||
| FAR | 52.227-2 | Jun 2020 | Notice and Assistance Regarding Patent and Copyright Infringement | ||||||||
| FAR | 52.227-11 | May 2014 | Patent Rights – Ownership by the Contractor | ||||||||
| FAR | 52.227-14 | May 2014 | Rights in Data - General, Alternate II | ||||||||
| FAR | 52.227-16 | June 1987 | Additional Data Requirements | ||||||||
| FAR | 52.228-7 | Mar 1996 | Insurance – Liability to Third Persons | ||||||||
| FAR | 52.232-9 | Apr 1984 | Limitation on Withholding of Payments | ||||||||
| FAR | 52.232-17 | May 2014 | Interest | ||||||||
| FAR | 52.232-20 | Apr 1984 | Limitation of Cost | ||||||||
| FAR | 52.232-23 | May 2014 | Assignment of Claims | ||||||||
| FAR | 52.232-25 | Jan 2017 | Prompt Payment | ||||||||
| FAR | 52.232-33 | Oct 2018 | Payment by Electronic Funds Transfer--System for Award Management | ||||||||
| FAR | 52.232-39 | Jun 2013 | Unenforceability of Unauthorized Obligations | ||||||||
| FAR | 52.232-40 | Nov 2021 | Providing Accelerated Payments to Small Business SubOfferors | ||||||||
| FAR | 52.233-1 | May 2014 | Disputes | ||||||||
| FAR | 52.233-3 | Aug 1996 | Protest After Award | ||||||||
| FAR | 52.233-4 | Oct 2004 | Applicable Law for Breach of Contract Claim | ||||||||
| FAR | 52.242-1 | Apr 1984 | Notice of Intent to Disallow Costs | ||||||||
| FAR | 52.242-2 | Apr 1991 | Production Progress Reports | ||||||||
| FAR | 52.242-3 | Sep 2021 | Penalties for Unallowable Costs | ||||||||
| FAR | 52.242-4 | Jan 1997 | Certification of Final Indirect Costs | ||||||||
| FAR | 52.242-13 | Jul 1995 | Bankruptcy | ||||||||
| FAR | 52.243-2 | Aug 1987 | Changes—Cost-Reimbursement Alternate V (Apr 1984). | ||||||||
| FAR | 52.243-6 | Apr 1984 | Change Order Accounting | ||||||||
| FAR | 52.243-7 | Jan 2017 | Notification of Changes | ||||||||
| FAR | 52.244-2 | Jun 2020 | Subcontracts, Alternate 1 (Jun 2020) | ||||||||
| FAR | 52.244-5 | Dec 1996 | Competition in Subcontracting | ||||||||
Contract No. 75A50122C00047
Page 48 of 51
| FAR | 52.244-6 | Jan 2022 | Subcontracts for Commercial Products and Commercial Services | ||||||||
| FAR | 52.245-1 | Sep 2021 | Government Property | ||||||||
| FAR | 52.245-9 | Apr 2012 | Use and Charges | ||||||||
| FAR | 52.246-23 | Feb 1997 | Limitation of Liability. | ||||||||
| FAR | 52.246-25 | Feb 1997 | Limitation of Liability—Services | ||||||||
| FAR | 52.247-67 | Feb 2006 | Submission of Transportation Documents for Audit | ||||||||
| FAR | 52.249-6 | May 2004 | Termination (Cost-Reimbursement) | ||||||||
| FAR | 52.249-14 | Apr 1984 | Excusable Delays | ||||||||
| FAR | 52.253-1 | Jan 1991 | Computer Generated Forms | ||||||||
I.1.DEPARTMENT OF HEALTH AND HUMAN SERVICES ACQUISITION REGULATION (HHSAR) (48 CFR Chapter 3) CLAUSES
Full text of the HHSAR clauses may be accessed electronically at xxxxx://xxx.xxxxxxxxxxx.xxx/xxxxx/xxxx-000- solicitation-provisions-and-contract-clauses
HHSAR | 352.203-70 | Dec 2015 | Anti-Lobbying | ||||||||
HHSAR | 352.208-70 | Dec 2015 | Printing and Duplication | ||||||||
HHSAR | 352.211-2 | Dec 2015 | Conference Sponsorship Requests and Conference Materials Disclaimer | ||||||||
HHSAR | 352.215-70 | Dec 2015 | Late Proposals and Revisions | ||||||||
HHSAR | 352.216-70 | Dec 2015 | Additional Cost Principles | ||||||||
HHSAR | 352.222-70 | Dec 2015 | Offeror Cooperation in Equal Employment Opportunity Investigations | ||||||||
HHSAR | 352.223-70 | Dec 2015 | Safety and Health | ||||||||
HHSAR | 352.224-70 | Dec 2015 | Privacy Act | ||||||||
HHSAR | 352.224-71 | Dec 2015 | Confidential Information | ||||||||
HHSAR | 352.227-70 | Dec 2015 | Publications and Publicity | ||||||||
HHSAR | 352.233-71 | Dec 2015 | Litigation and Claims | ||||||||
HHSAR | 352.237-75 | Dec 2015 | Key Personnel | ||||||||
HHSAR | 352.270-6 | Dec 2015 | Restriction on use of Human Subjects | ||||||||
HHSAR | 352.270-9 | Dec 2015 | Non-Discrimination for Conscience | ||||||||
I.2.ADDITIONAL CONTRACT CLAUSES
I.4.1.Additional HHS Acquisition Regulation (HHSAR) Clauses – In Full Text HHSAR 352.231-70 Salary Rate Limitation (DEC 2015)
f.The Offeror shall not use contract funds to pay the direct salary of an individual at a rate in excess of the Federal Executive Schedule Level II in effect on the date the funding was obligated
g.For purposes of the salary rate limitation, the terms ‘‘direct salary,’’ ‘‘salary,’’ and ‘‘institutional base salary’’ have the same meaning and are collectively referred to as ‘‘direct salary’’ in this clause. An individual’s direct salary is the annual compensation that the Offeror pays for an individual’s direct effort (costs) under the contract. Direct salary excludes any income that an individual may be permitted to earn outside of duties to the Offeror. Direct salary also excludes fringe benefits, overhead, and general and administrative expenses (also referred to as indirect costs or facilities and administrative costs).
Contract No. 75A50122C00047
Page 49 of 51
The salary rate limitation does not restrict the salary that an organization may pay an individual working under an HHS contract or order; it merely limits the portion of that salary that may be paid with federal funds.
d.The salary rate limitation also applies to individuals under subcontracts.
e.If this is a multiple-year contract or order, it may be subject to unilateral modification by the CO to ensure that an individual is not paid at a rate that exceeds the salary rate limitation provision established in the HHS appropriations act used to fund this contract.
f.See the salaries and wages pay tables on the U.S. Office of Personnel Management website for federal Executive Schedule salary levels.
HHSAR 352.232-71 Electronic Submission of Payment Requests (FEB 2022)
a.Definitions. As used in this clause—Payment request means a bill, voucher, invoice, or request for contract financing payment with associated supporting documentation. The payment request must comply with the requirements identified in FAR 32.905(b), “Content of Invoices” and the applicable Payment clause included in this contract.
b.Except as provided in paragraph (c) of this clause, the Contractor shall submit payment requests electronically using the Department of Treasury Invoice Processing Platform (IPP) or successor system. Information regarding IPP, including IPP Customer Support contact information, is available at xxx.xxx.xxx or any successor site.
c.The Contractor may submit payment requests using other than IPP only when the Contracting Officer authorizes alternate procedures in writing in accordance with HHS procedures.
d.If alternate payment procedures are authorized, the Contractor shall include a copy of the Contracting Officer's written authorization with each payment request.
I.4.2. Additional FAR (48 CFR Chapter 1) Clauses – In Full Text
52.217-7 Option for Increased Quantity -- Separately Priced Line Item (MAR 1989)
The Government may require the delivery of the numbered line item, identified in the Schedule as an option item, in the quantity and at the price stated in the Schedule. The CO may exercise the option by written notice to the Contractor. Delivery of added items shall continue at the same rate that like items are called for under the contract, unless the parties otherwise agree.
Contract No. 75A50122C00047
Page 50 of 51
PART III – ATTACHMENTS
SECTION J – LIST OF ATTACHMENTS
The following documents are attached and incorporated in this contract:
1.Statement of Work, dated 08/25/2022, 12 pages
2.Invoice Instructions for Cost Reimbursement Contracts
3.Invoice Instructions for Fixed Price Contracts
4.Sample Invoice Form
5.Protection of Human Subjects - xxxxx://xxx.xxx.xxx/xxxx/xxxxxxxx-xxxx-xxx-xxxxxx- fwas/forms/index.html
6.BARDA Security Requirements
7.Financial Report of Individual Project/Contract
8.Report of Government Owned, Contractor Held Property
Contract No. 75A50122C00047
Page 51 of 51
Attachment 1 - Statement of Work
SMALLPOX ANTIVIRAL
Date 08/25/2022
1.PREAMBLE
Independently and not as an agent of the USG, the Contractor shall furnish all the necessary services, qualified personnel, materials, supplies, equipment, and facilities (except Government property) to develop and deliver FDA-approved smallpox therapeutic with an alternative mechanism of action to address the potential for the development of resistance to the current stockpile drug.
In accordance with FAR 52.243-2, Changes-Cost Reimbursement (Alt. V), the Government reserves the right to modify the milestones, progress, schedule, budget, or to add or delete deliverables, process or schedules if the need arises. Because of the nature of the research and development (R&D) elements of the contract and the complexities inherent in this and prior programs, at designated milestones the Government will evaluate whether work should be redirected, removed, or whether schedule or budget adjustments should be made.
1.0 Overall Objectives and Scope
The overall objective of this contract is to procure a second approved smallpox antiviral for delivery to the Strategic National Stockpile (ASPR/SNS) to achieve the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) smallpox preparedness requirement of having two readily available smallpox antivirals with distinct mechanisms of action. The scope of work for this contract includes the manufacture and delivery of up to 1.7 Million Treatment Courses (TCs) of the smallpox antiviral TEMBEXA®, with an approximate 90/10 split of oral tablets/oral suspension to the SNS site(s) and the associated activities to support these deliveries, and post approval Research and Development (R&D) activities: Post Marketing Commitment (PMC) non-clinical activities; Post Marketing Requirement (PMR) clinical activities; oral suspension formulation post marketing manufacturing scale-up activities; shelf-life extension support clinical activities and all associated regulatory, quality assurance, management, and administrative activities. The Procurement and R&D efforts for the TEMBEXA program will progress in specific stages that cover Base Periods and Option Periods as specified in this contract. The scope of work has been broken into the following seven (7) CLINs which are discrete work segments:
1.Base CLIN 1 Post Marketing Commitments, Suspension Manufacturing Scale- up and Other Stability Extension Support
2.Base CLIN 2 Delivery of FDP to the SNS
3.Option CLIN 3 Conduct of Ph 4 PMR Field Study
4.Option CLIN 4 Delivery of FDP to the SNS
5.Option CLIN 5 Delivery of FDP to the SNS
6.Option CLIN 6 Delivery of FDP to the SNS
7.Option CLIN 7 Delivery of FDP to the SNS
2.Applicable Documents
Approved UNITED STATES PRESCRIBING INFORMATION (USPI) FOR TEMBEXA
Contract No. 75A50122C0047
Attachment 1
Page 1 of 12
3.Statement of Work
Provide a 2nd FDA approved antiviral for the treatment of smallpox, including delivery of product to the SNS, post-approval research and development (R&D), scale-up of suspension formulation, post marketing requirement (PMR), post-marketing commitment (PMC) as set forth below.
3.1CLIN 1 POST MARKETING COMMITMENTS, SUSPENSION MANUFACTURING SCALE-UP AND OTHER STABILITY EXTENSION SUPPORT
Complete all efforts required to fulfill the PMR protocol and PMC study established by the FDA. This includes development of a clinical Phase 4 Field Study protocol in the event of a smallpox outbreak as well as a cell culture study to characterize TEMBEXA antiviral activity against specific mutant viruses. [*]. Additionally, it includes Program Management, post-licensure Regulatory and Safety requirements and Quality Assurance (QA).
3.1.1Program Management
As outlined below and in the contract deliverables the Contractor shall:
3.1.1.1Program Management Deliverables
3.1.1.1.1Provide and maintain a list of individuals to serve as primary and secondary points of contact who will be notified in case of a public health emergency.
3.1.1.1.2Provide Key Milestones as part of the Integrated Master Project Plan, outlining key, critical path milestones, with “Go/No Go” decision criteria (entrance and exit criteria for each phase of the project). This report shall be due within 90 days after contract award. Updates shall be due as requested by the Contract Officer (CO) or Contracting Officer Representative (COR).
3.1.1.1.3Provide an Integrated Master Schedule (IMS), which will include key milestones, as part of the Integrated Master Project Plan. Within 30 calendar days of the effective date of the contract submit a first draft of an updated IMS in a format agreed upon by XXXXX to the CO and COR for review and comment.
3.1.1.1.4Provide a Work Breakdown Structure (WBS) as part of the Integrated Master Project Plan. Utilize a WBS template agreed upon by BARDA for reporting on the contract. BARDA may require WBS data at the work package level or at a lower level if there is significant complexity and risk associated with the task.
3.1.1.1.5Provide a Risk Management Plan/Matrix: Develop and maintain a risk management plan/matrix that highlights potential problems and/or issues that may arise during the life of the contract, their impact on cost, schedule and performance, and appropriate
Contract No. 75A50122C0047
Attachment 1
Page 2 of 12
remediation plans. This plan shall reference relevant WBS/SOW elements where appropriate. This report shall be due within 90 days of contract award. Updates shall be due as requested by the CO or COR.
3.1.1.1.1Provide a Security Plan associated with all aspects of manufacture of product, process, storage and inventory. The Security Plan includes all sites within the supply chain, including proposed shipping carriers. For those sites/carriers that are not defined at the time of award or are added during the period of performance, individualized Security Plans shall be provided to the USG prior to inclusion of sites/carriers into the supply chain. This report shall be due within 90 days of contract award. Updates shall be due as requested by the CO or COR.
3.1.1.1.6.1Provide access to BARDA’s Program Protection Office to review and approve Security Plans and conduct audits / site visits as requested to ensure a reliable product is delivered to the USG.
3.1.1.1.1Provide copies of Manufacturing Plan as approved in NDA(s) upon request of the CO or COR. Updates shall be due as requested by the CO or COR.
3.1.1.1.2Provide a Manufacturing/Delivery Schedule to the ASPR/SNS within 30 days of agreement on SNS delivery location
3.1.1.1.3Provide a Quality Manual describing the Quality Management System (QMS) within 90 Days of contract award. Updates shall be due as requested by the CO or COR.
3.1.1.1.4Complete a Quality Agreement between BARDA, SNS and Chimerix within 30 Days of contract award.
3.1.1.2 Progress Reports
3.1.1.2.1Deliver a Monthly Project Status Report on or before the 15th calendar day following the last day of each reporting period. A Monthly Progress Report will not be required in the same month Annual Progress Reports or a Final Report are due. This report shall include a description of the activities during the reporting period and the activities planned for the ensuing reporting period.
3.1.1.2.2Deliver an Annual Progress Report on or before the 30th calendar day following the last day of each reporting period. An Annual Progress Report will not be required for the period when the Final Technical Progress Report is due. This report shall include a summation of the activities during the reporting period, and the activities planned for the ensuing reporting period.
3.1.1.2.3Submit a Draft Final Report and Deliver a Final Report. These reports are to include a summation of the work performed and results obtained for execution of various studies or technical work packages during the entire contract period of performance. This report shall be in sufficient detail to describe comprehensively the results achieved. The Draft Final Report shall be due forty-five (45) calendar days prior to the expiration date of the contract and the Final Report is due on or before the expiration date of the contract.
Contract No. 75A50122C0047
Attachment 1
Page 3 of 12
3.1.1.1.4Submit a Quarterly Financial Report on or before the 30th calendar day following the last day of each reporting period.
3.1.1.3Meetings/Site Visits
3.1.1.3.1Plan and conduct a kick-off meeting within 30 days of contract award. The purpose of the kickoff meeting will be to orient the Contractor to HHS/BARDA and review contract requirements. The goals of the kick-off meeting are as follows:
•Jointly assess areas such as planning for complete coverage of the SOW, logical scheduling of the work activities, adequate resources, and identification of inherent risks
•Review and approve the project milestones
•Review and approve the Integrated Master Schedule and set a baseline
•Review and approve the Risk Management Matrix
•Review and approve the Quality Agreement
•Present and review the Delivery Schedule to the ASPR/SNS for the Base CLIN and all Optional CLINs
•Draft minutes (for approval at the next meeting) will be due no later than seven
(7) business days following the kick-off Meeting.
3.1.1.3.2Participate in expected bi-weekly or monthly status update meetings/teleconferences to coordinate and oversee the contract effort. Such meetings may include, but are not limited to updates on PMRs, PMCs, scale-up manufacturing development, clinical pharmacology studies, and regulatory issues; meetings with subcontractors/consultants and other HHS officials to discuss the technical, regulatory, and ethical aspects of the program; and meeting with technical consultants to discuss technical data provided by the Contractor. The schedule for these meetings will be established by the CO and COR.
•Meeting agendas are due no later than two business days before each meeting.
•Meeting minutes are due no later than seven (7) business days following each meeting.
3.1.1.3.3Participate in In-Process Review (IPR) as requested by CO and COR (up to 1 per year). Contractor shall provide presentation materials to CO and COR 10 business days prior to the IPR. Submit written justification of progress towards satisfying Go/No-Go criteria. CO/COR will provide a written response within 10 days.
3.1.1.4Audits
3.1.1.4.1Notify the CO and COR within one (1) business day of FDA presence on site to conduct site visits/audits. Provide the USG with an exact copy (non-redacted) of the FDA Form 483 and the Establishment Inspection Report (EIR) for content that is pertinent to the contract. Provide the COR and CO copies of the plan for addressing areas of non-conformance to FDA regulations for GLP guidelines as identified in the audit report, status updates during the plan execution, and a copy of all final
Contract No. 75A50122C0047
Attachment 1
Page 4 of 12
responses to the FDA. Provide redacted copies of any FDA audits received from subcontractors that occur as a result of this contract or for this product. When appropriate, make arrangements with the COR for the appropriate BARDA representative(s) to be present during the final debrief by the regulatory inspector.
3.1.1.4.2 Within thirty (30) calendar days of an FDA audit of Contractor or subcontractor facilities, the Contractor shall provide copies of the audit findings, final report, and a plan for addressing areas of nonconformance to FDA regulations and guidance for GLP, GMP or GCP guidelines as identified in the final audit report.
3.1.1.4.3 Qualify vendors involved in cGMP production and GCP clinical studies using Contractor's existing Quality Management System. Perform initial assessment of all subcontractors in accordance with existing SOPs. Conduct on-site audits as needed where suitable standards of GxP are employed and work is initiated, including dissemination of relative audit report detailing any observations to the vendor. Conduct on-site audits at strategic times once a vendor's work has been initiated as part of the overall management of the project.
3.1.1.4.3.1 Within 30 days of receipt of a final audit report, procure a written response from vendor that describes corrective actions.
3.1.1.4.3.2 Notify CO and COR of upcoming, ongoing, or recent audits/site visits of subcontractors as part of weekly communications.
3.1.1.4.3.3 Notify the COR and CO within five (5) business days of report completion.
3.1.1.4.4 Accommodate periodic or reasonable ad hoc site visits from BARDA during normal business hours by the Government where two (2) business days advance notice has been given. If the Government, the Contractor, or other parties identifies any issues during an audit, capture the issues, identify potential solutions, and provide a report to the Government.
3.1.1.4.4.1 If issues are identified during the audit, submit a report to the CO and COR detailing the finding and corrective action(s) within 15 business days of the audit.
3.1.1.4.4.2 COR and CO will review the report and provide a response within ten (10) business days.
3.1.1.4.4.3 Once corrective action is completed, provide a final report to the CO and COR within a time frame negotiated with the COR in writing after review of the issues report.
3.1.1.5Other Communication Deliverables
3.1.1.5.1Technology packages developed under the contract including complete protocols must be submitted within 10 business days of request by the COR. See FAR clauses 52.227-11, Patent Rights-Ownership by the Contractor, and 52.227-14, Rights in Data.
3.1.1.5.2The Contractor shall submit to the COR Draft Protocols and associated study/experiment/test plans 30 days prior to study initiation prior to submission to
Contract No. 75A50122C0047
Attachment 1
Page 5 of 12
regulatory agencies (IACUC, FDA, etc.). The Contractor shall submit to the COR Final Protocols 10 business days prior to the execution of the study or within 10 business days of request by the COR. Approval must be provided in writing by the COR prior to the execution of the study. Draft Reports are due within 45 calendar days after completion of analysis and at least 15 business days prior to submission to the FDA. Final Reports are due 30 calendar days after receiving comments on the Draft Report. Final FDA submissions shall be provided to BARDA concurrently or no later than one (1) business day after submission to the FDA.
3.1.1.5.3 Submit an Annual Invention Report on or before the 30th calendar day after the completion of each reporting period. A Final Invention Report is due on or before the expiration date of the contract.
3.1.1.5.4 Submit any manuscript or scientific meeting abstract containing data generated under this contract to COR for review prior to submission. Reports shall be due within 30 calendar days for manuscripts and 15 calendar days for abstracts.
3.1.1.5.5 In any press release where work performed pursuant to this SOW is discussed or presented, Contractor will, in all material respects, accurately represent the work conducted. Ensure the CO or COR has received and approved an advanced copy of any press release not less than five (5) business days prior to the issuance of any potential press release.
3.1.1.5.6 Report to the government any activity; or incident that is in violation of the agreed upon Security Plan; or indicates the loss or theft of government products. Communication shall be due within 24 hours after occurrence of an activity or incident.
3.1.1.5.7 Provide an Incident Report for any major developments or deviations that significantly impact the Contractor's ability to successfully meet objectives, deliverables or schedule (e.g., significant manufacturing deviations or loss, BSL-4 laboratory incidents, etc.).
3.1.1.5.8 Provide raw data and/or specific analysis of data generated with USG funds within 20 business days of request from CO or COR and amend reports if required and adjudicate all comments.
3.1.1.5.9 Submit a Deviation Report upon request of the COR when any needed changes to IMS activities as baselined at the kick-off meeting are required. This report shall request a change in the agreed-upon IMS and timelines. This report shall include: (i) discussion of the justification/rationale for the proposed change; (ii) options for addressing the needed changes from the agreed upon timelines, including a cost- benefit analysis of each option; and (iii) recommendations for the preferred option that includes a full analysis and discussion of the effect of the change on the entire product development program, timelines, and budget.
3.1.1.5.10 Submit Draft report within 45 calendar days after completion of analysis and at least 15 business days prior to submission to FDA. Final report due 30 calendar days after receiving comments on the Draft report for Clinical and Non-Clinical Studies. Final
Contract No. 75A50122C0047
Attachment 1
Page 6 of 12
FDA submissions shall be provided to BARDA concurrently or no later than one (1) business day after submission to the FDA.
3.1.1.6FDA Regulatory Agency Correspondence, Meeting Summaries, and Submissions
3.1.1.6.1Inform BARDA of all communications with the FDA for the orthopox indications for this specific product, provide all communications and submissions related to the product, and provide previous correspondences with FDA related to the development of the therapeutic within five (5) business days of submission to or receipt from FDA.
3.1.1.6.2Maintain and update, as required by the FDA, all required regulatory documentation (including but not limited to; investigator brochure, annual reports, PADERs DSURs), that will be used to support continued licensure.
3.1.1.6.3Provide, within five (5) business days of any informal meeting with the FDA or other regulatory agency, initial draft minutes to BARDA. Forward the final minutes when available and if applicable.
3.1.1.6.4Provide BARDA any documents to be submitted to the FDA or other regulatory agency within five (5) business days prior to submission, without impacting meeting FDA deadlines.
3.1.1.6.5Submit Standard Operating Procedures (SOPs) related to the Product upon reasonable request from COR.
3.1.1.6.6Generate all necessary documentation for the [*]. Provide Regulatory support in accordance with 3.1.1.6.
3.1.1.6.7Provide expertise and advise on [*] regulatory filings. Provide authoring, review, and guidance during FDA submissions. Provide publishing work for [*]
3.1.2Post Marketing Requirements and Commitments
Comply, in all material respects, with Post-Marketing Requirements/Commitments as specified by the FDA. Work scope may be revisited once final FDA guidance on PMR has been provided.
3.1.2.1Collaborate with [*] and other stakeholders to finalize and submit the PMR clinical Ph 4 Field Study Protocol to the FDA to evaluate TEMBEXA [*]when used for the treatment of human smallpox disease due to VARV infection. Support all work to implement required state of readiness for the field study.
3.1.2.2Generate all necessary documentation for the set-up and oversight of pharmacovigilance safety reporting for TEMBEXA.
3.1.2.2.1Support PVG tracking and database updates for studies using TEMBEXA.
Contract No. 75A50122C0047
Attachment 1
Page 7 of 12
3.1.2.2.2Track and prepare all required reporting and assessments for PVG. Provide to BARDA and FDA as required.
3.1.2.3Conduct a study to complete a post-marketing commitment to characterize brincidofovir antiviral activity against recombinant vaccinia viruses encoding specific amino acid substitutions.
3.1.2.3.1Provide final protocols and reports that will be shared with BARDA and the FDA.
3.1.2.3.2Provide Analyzed Data to BARDA at Status Update Meeting
3.1.2.3.3Provide Final Reports in accordance with 3.1.1.5.10
3.1.2.4Store additional samples from past studies until PMC work is complete.
3.1.3[*]
3.1.3.1Conduct [*].
3.1.3.2Provide the necessary biostatistical data and data management services [*].
3.1.3.3Author the Clinical Study Report (CSR) [*].
3.1.3.4Submit all required regulatory documentation (CBE 30) [*].
3.1.4Clinical Pharmacology
3.1.4.1Develop and validate assay [*].
3.1.4.2Provide analytical testing (including pharmacokinetics) of drug in plasma samples [*]
3.1.5[*]
3.1.5.1[*]
Contract No. 75A50122C0047
Attachment 1
Page 8 of 12
3.1.5.2[*].
3.1.5.3Complete an [*]
3.1.5.4Upon completion [*].
3.1.6[*]
3.1.6.1[*].
3.1.6.2Provide annual stability data for shelf-life extension to BARDA.
3.1.6.3Submit annual stability data for shelf-life extension to FDA as required.
3.2CLIN 2 DELIVERY OF FDP TO THE SNS
Under CLIN 2 provide initial shipment of product 319,000 treatment courses of a therapeutic against smallpox, in accordance with all federal, state and local regulations, as well as international regulations if applicable. As outlined below and in the contract deliverables the Contractor shall:
3.2.1Manufacture and deliver smallpox antiviral Final Drug Product (FDP) treatment courses of 100 mg tablets.
3.2.1.1[*].
3.2.1.2[*].
Contract No. 75A50122C0047
Attachment 1
Page 9 of 12
3.2.2Manufacture and deliver smallpox antiviral FDP treatment courses of 10 mg/mL oral suspension.
3.2.2.1[*].
3.2.2.2[*].
3.3CLIN 3 CONDUCT OF PH 4 PMR FIELD STUDY
Under CLIN 3 support all FDA post marketing requirements in the conduct of a Ph 4 Field Study as outlined in 3.1.2.1. Work scope may be revisited once final FDA guidance on PMR has been provided.
As outlined below and in the contract deliverables the Contractor shall:
3.3.1Program Management
3.3.1.1Manage program scope as described CLIN 1.
3.3.1Ph 4 PMR Field Active Study (in the event of an outbreak)
3.3.2.1Support the NIH in the conduct the PMR clinical Ph 4 Field Study to evaluate [*].
3.3.2.2Collaborate with NIH to generate all necessary regulatory documentation for the completion for the TEMBEXA PMR, conduct of a Ph4 Field Study in accordance with 3.1.1.6.
3.3.2.3Where and when possible, set up and calibrate equipment and train staff at sites enrolled in the Ph4 Field Study to collect and process PBMC samples
3.3.2.4Perform analytical testing [*]
3.3.2.5Complete [*]
3.4CLIN 4 DELIVERY OF FDP TO THE SNS
Under CLIN 4 in order to augment the existing inventory of smallpox therapeutic MCMs stored in the SNS and/or replace expiring product, send additional shipment of [*] treatment courses of a therapeutic against smallpox. As outlined below and in the contract deliverables the Contractor shall:
Contract No. 75A50122C0047
Attachment 1
Page 10 of 12
3.4.1Manufacture and deliver smallpox antiviral Final Drug Product (FDP) treatment courses of 100 mg tablets.
3.4.1.1[*].
3.4.1.2[*].
3.4.2Manufacture and deliver smallpox antiviral FDP treatment courses of 10 mg/mL oral suspension.
3.4.2.1[*].
3.4.2.2[*].
3.5CLIN 5 DELIVERY OF FDP TO THE SNS
Under CLIN 5 in order to augment the existing inventory of smallpox therapeutic MCMs stored in the SNS and/or replace expiring product, send additional shipment of [*] treatment courses of a therapeutic against smallpox. As outlined below and in the contract deliverables the Contractor shall:
3.5.1Manufacture and deliver smallpox antiviral Final Drug Product (FDP) treatment courses of 100 mg tablets.
3.5.1.1[*].
3.5.1.2[*].
3.5.2Manufacture and deliver smallpox antiviral FDP treatment courses of 10 mg/mL oral suspension.
3.5.2.1[*].
3.5.2.2[*].
3.6CLIN 6 DELIVERY OF FDP TO THE SNS
Under CLIN 6 in order to augment the existing inventory of smallpox therapeutic MCMs stored in the SNS and/or replace expiring product, send additional shipment of [*] treatment courses of a therapeutic against smallpox. As outlined below and in the contract deliverables the Contractor shall:
Contract No. 75A50122C0047
Attachment 1
Page 11 of 12
3.6.1Manufacture and deliver smallpox antiviral Final Drug Product (FDP) treatment courses of 100 mg tablets.
3.6.1.1[*].
3.6.1.2[*].
3.6.2Manufacture and deliver smallpox antiviral FDP treatment courses of 10 mg/mL oral suspension.
3.6.2.1[*].
3.6.2.2[*].
3.7CLIN 7 DELIVERY OF FDP TO THE SNS
Under CLIN 7 in order to augment the existing inventory of smallpox therapeutic MCMs stored in the SNS and/or replace expiring product, send additional shipment of [*] treatment courses of a therapeutic against smallpox. As outlined below and in the contract deliverables the Contractor shall:
3.7.1Manufacture and deliver smallpox antiviral Final Drug Product (FDP) treatment courses of 100 mg tablets.
3.7.1.1[*].
3.7.1.2[*].
3.7.2Manufacture and deliver smallpox antiviral FDP treatment courses of 10 mg/mL oral suspension.
3.7.2.1[*].
3.7.2.2[*].
Contract No. 75A50122C0047
Attachment 1
Page 12 of 12
ATTACHMENT #2
INVOICE/FINANCING REQUEST INSTRUCTIONS - FOR COST-REIMBURSEMENT TYPE CONTRACTS
Format: Payment requests shall be submitted on the Contractor’s self-generated form in the manner and format prescribed herein and as illustrated in the Sample Invoice/Financing Request. Standard Form 1034, Public Voucher for Purchases and Services Other Than Personal, may be used in lieu of the Contractor’s self-generated form provided it contains all of the information shown on the Sample Invoice/Financing Request. DO NOT include a cover letter with the payment request.
Number of Copies: Payment requests shall be submitted in the quantity specified in the Invoice Submission Instructions in Section G of the Contract Schedule.
Frequency: Payment requests shall not be submitted more frequently than once every two weeks in accordance with the Allowable Cost and Payment Clause incorporated into this contract. Small business concerns may submit invoices/financing requests more frequently than every two weeks when authorized by the Contracting Officer.
Cost Incurrence Period: Costs incurred must be within the contract performance period or covered by pre-contract cost provisions.
Billing of Costs Incurred: If billed costs include (1) costs of a prior billing period, but not previously billed, or (2) costs incurred during the contract period and claimed after the contract period has expired, the Contractor shall site the amount(s) and month(s) in which it incurred such costs.
Contractor’s Fiscal Year: Payment requests shall be prepared in such a manner that the Government can identify costs claimed with the Contractor’s fiscal year.
Currency: All contracts are expressed in United States dollars. When the Government pays in a currency other than United States dollars, billings shall be expressed, and payment by the Government shall be made, in that other currency at amounts coincident with actual costs incurred. Currency fluctuations may not be a basis of gain or loss to the Contractor. Notwithstanding the above, the total of all invoices paid under this contract may not exceed the United States dollars authorized.
Costs Requiring Prior Approval: Costs requiring the Contracting Officer's approval, including those set forth in an Advance Understanding in the contract, shall be identified and reference the Contracting Officer's Authorization (COA) Number. In addition, the Contractor shall show any cost set forth in an Advance Understanding as a separate line item on the payment request.
Invoice/Financing Request Identification: Each payment request shall be identified as either:
(a)Interim Invoice/Contract Financing Request: These are interim payment requests submitted during the contract performance period.
(b)Completion Invoice: The completion invoice shall be submitted promptly upon completion of the work, but no later than one year from the contract completion date, or within 120 days after
Contract No. 75A50122C00047
Attachment 2
Page 1 of 4
settlement of the final indirect cost rates covering the year in which the contract is physically complete (whichever date is later). The Contractor shall submit the completion invoice when all costs have been assigned to the contract and it completes all performance provisions.
(c)Final Invoice: A final invoice may be required after the amounts owed have been settled between the Government and the Contractor (e.g., resolution of all suspensions and audit exceptions).
Preparation and Itemization of the Invoice/Financing Request: The Contractor shall furnish the information set forth in the instructions below. The instructions are keyed to the entries on the Sample Invoice/Financing Request.
(a)Designated Billing Office Name and Address: Enter the designated billing office name and address, as identified in the Invoice Submission Instructions in Section G of the Contract Schedule.
(b)Contractor’s Name, Address, Point of Contact, VIN, and UEI: Show the Contractor’s name and address exactly as they appear in the contract, along with the name, title, phone number, and e-mail address of the person to notify in the event of an improper invoice or, in the case of payment by method other than Electronic Funds Transfer, to whom payment is to be sent. Provide the Contractor’s Vendor Identification Number (VIN), Unique Entity Identifier (UEI). The UEI must identify the Contractor’s name and address exactly as stated on the face page of the contract. When an approved assignment has been made by the Contractor, or a different payee has been designated, provide the same information for the payee as is required for the Contractor (i.e., name, address, point of contact, VIN, and UEI).
(c)Invoice/Financing Request Number: Insert the appropriate serial number of the payment request.
(d)Date Invoice/Financing Request Prepared: Insert the date the payment request is prepared.
(e)Contract Number and Order Number (if applicable): Insert the contract number and order number (if applicable).
(f)Effective Date: Insert the effective date of the contract or if billing under an order, the effective date of the order.
(g)Total Estimated Cost of Contract/Order: Insert the total estimated cost of the contract, exclusive of fixed-fee. If billing under an order, insert the total estimated cost of the order, exclusive of fixed- fee. For incrementally funded contracts/orders, enter the amount currently obligated and available for payment.
(h)Total Fixed-Fee: Insert the total fixed-fee (where applicable) or the portion of the fixed-fee applicable to a particular invoice as defined in the contract.
(i)Two-Way/Three-Way Match: Identify whether payment is to be made using a two-way or three- way match. To determine required payment method, refer to the Invoice Submission Instructions in Section G of the Contract Schedule.
(j)Office of Acquisitions: Insert the name of the Office of Acquisitions, as identified in the Invoice
Contract No. 75A50122C00047
Attachment 2
Page 2 of 4
Submission Instructions in Section G of the Contract Schedule.
(k)Central Point of Distribution: Insert the Central Point of Distribution, as identified in the Invoice Submission Instructions in Section G of the Contract Schedule.
(l)Billing Period: Insert the beginning and ending dates (month, day, and year) of the period in which costs were incurred and for which reimbursement is claimed.
(m)Amount Billed - Current Period: Insert the amount claimed for the current billing period by major cost element, including any adjustments and fixed-fee. If the Contract Schedule contains separately priced line items, identify the contract line item(s) on the payment request and include a separate breakdown (by major cost element) for each line item.
(n)Amount Billed - Cumulative: Insert the cumulative amounts claimed by major cost element, including any adjustments and fixed-fee. If the Contract Schedule contains separately priced line items, identify the contract line item(s) on the payment request and include a separate breakdown (by major cost element) for each line item.
(o)Direct Costs: Insert the major cost elements. For each element, consider the application of the paragraph entitled "Costs Requiring Prior Approval" on page 1 of these instructions.
(1)Direct Labor: Include salaries and wages paid (or accrued) for direct performance of the contract. List individuals by name, title/position, hourly/annual rate, level of effort (actual hours or % of effort), breakdown by task performed by personnel, and amount claimed.
(2)Fringe Benefits: List any fringe benefits applicable to direct labor and billed as a direct cost. Do not include in this category fringe benefits that are included in indirect costs.
(3)Accountable Personal Property: Include any property having a unit acquisition cost of
$5,000 or more, with a life expectancy of more than two years, and sensitive property regardless of cost (see the HHS Contractor's Guide for Control of Government Property)(e.g. personal computers). Note this is not permitted for reimbursement without pre-authorization from the CO.
On a separate sheet of paper attached to the payment request, list each item for which reimbursement is requested. Include reference to the following (as applicable):
-Item number for the specific piece of equipment listed in the Property Schedule, and
-COA number, if the equipment is not covered by the Property Schedule.
The Contracting Officer may require the Contractor to provide further itemization of property having specific limitations set forth in the contract.
(4)Materials and Supplies: Include all consumable material and supplies regardless of amount. Detailed line-item breakdown (e.g. receipts, quotes, etc.) is required.
(5)Premium Pay: List remuneration in excess of the basic hourly rate.
Contract No. 75A50122C00047
Attachment 2
Page 3 of 4
(6)Consultant Fee: List fees paid to consultants. Identify consultant by name or category as set forth in the contract or COA, as well as the effort (i.e., number of hours, days, etc.) and rate billed.
(7)Travel: Include domestic and foreign travel. Identify travelers, dates, destination, purpose of trip, and total breaking out amounts for transportation (plane, car etc), lodging, M&IE. Cite COA, if appropriate. List separately, domestic travel, general scientific meeting travel, and foreign travel. Foreign travel is travel outside of Canada, the United States and its territories and possessions. However, for an organization located outside Canada, the United States and its territories and possessions, foreign travel means travel outside that country. Foreign travel must be billed separately from domestic travel.
(8)Subcontract Costs: List Subcontractors by name and amount billed. Provide subcontract invoices/receipts as backup documentation. If subcontract is of the cost-reimbursement variety, detailed breakdown will be required. Regardless, include backup documentation (e.g. Subcontractor invoices, quotes, etc.).
(9)Other: Include all other direct costs not fitting into an aforementioned category. If over
$1,000, list cost elements and dollar amounts separately. If the contract contains restrictions on any cost element, that cost element must be listed separately.
(p)Cost of Money (COM): Cite the COM factor and base in effect during the time the cost was incurred and for which reimbursement is claimed, if applicable.
(q)Indirect Costs: Identify the indirect cost base (IDC), indirect cost rate, and amount billed for each indirect cost category.
(r)Fixed-Fee: Cite the formula or method of computation for fixed-fee, if applicable. The fixed-fee must be claimed as provided for by the contract.
(s)Total Amounts Claimed: Insert the total amounts claimed for the current and cumulative periods.
(t)Adjustments: Include amounts conceded by the Contractor, outstanding suspensions, and/or disapprovals subject to appeal.
(u)Grand Totals
(v)Certification of Salary Rate Limitation: If required by the contract (see Invoice Submission Instructions in Section G of the Contract Schedule), the Contractor shall include the following certification at the bottom of the payment request:
“I hereby certify that the salaries billed in this payment request are in compliance with the Salary Rate Limitation Provisions in Section H of the contract.”
**Note the Contracting Officer may require the Contractor to submit detailed support for costs claimed on payment requests. Every cost must be determined to be allocable, reasonable, and allowable per FAR Part 31.
Contract No. 75A50122C00047
Attachment 2
Page 4 of 4
ATTACHMENT #3
INVOICE/FINANCING REQUEST INSTRUCTIONS FOR FIXED PRICE TYPE CONTRACTS
General: The Contractor shall submit vouchers or invoices as prescribed herein.
Format: Standard Form l034, Public Voucher for Purchases and Services Other Than Personal, and Standard Form l035, Public Voucher for Purchases and Services Other than Personal--Continuation Sheet, and the payee's letterhead or self-designed form should be used to submit claims for reimbursement.
Number of Copies: As indicated in the contract.
Frequency: Invoices submitted in accordance with the Payment Clause shall be submitted monthly upon delivery of goods or services unless otherwise authorized by the Contracting Officer.
Preparation and Itemization of the Invoice: The invoice shall be prepared as follows:
(a)Designated Billing Office and address:
The Contractor shall submit payment requests electronically using the Department of Treasury Invoice Processing Platform (IPP) or successor system. Information regarding IPP, including IPP Customer Support contact information, is available at xxx.xxx.xxx or any successor site.
(b)Invoice Number
(c)Date of Invoice
(d)Contract number and date
(e)Xxxxx's name and address. Show the Contractor’s name (as it appears in the contract), correct address, and the title and phone number of the responsible official to whom payment is to be sent. When an approved assignment has been made by the Contractor, or a different payee has been designated, then insert the name and address of the payee instead of the Contractor.
(f)Description of goods or services, quantity, unit price, (where appropriate), and total amount.
(g)Charges for freight or express shipments other than F.O.B. destination. (If shipped by freight or express and charges are more than $25, attach prepaid bill.)
(h)Equipment - If there is a contract clause authorizing the purchase of any item of equipment, the final invoice must contain a statement indicating that no item of equipment was purchased or include a completed form
HHS-565, Report of Capitalized Nonexpendable Equipment.
Currency: Where payments are made in a currency other than United States dollars, billings on the contract shall be expressed, and payment by the United States Government shall be made, in that other currency at amounts coincident with actual costs incurred. Currency fluctuations may not be a basis of gain or loss to the Contractor.
Notwithstanding the above, the total of all invoices paid under this contract may not exceed the United States dollars authorized.
Contract No. 75A50122C00047
Attachment 3
ATTACHMENT #4
SAMPLE INVOICE/PAYMENT REQUEST
(a)Designated Billing Office Name and Address: DHHS/OS/ASPR/BARDA/CMA Attn: Xxxx Xxxxxxx, Contracting Officer US DEPT OF HEALTH & HUMAN SERVICES ASST SEC OF PREPAREDNESS & RESPONSE Division of Contract Management & Acquisitions X'XXXXX HOUSE OFFICE BUILDING Washington DC 20515 (b)Contractor’s Name, Address, Point of Contact, VIN, and UEI: ABC CORPORATION 000 Xxxx Xxxxxx Xxxxxxxx, XXX Zip Code Name, Title, Phone Number, and E-mail Address of person to notify in the event of an improper invoice or, in the case of payment by method other than Electronic Funds Transfer, to whom payment is to be sent. VIN: UEI: | (c)Invoice/Financing Request No.: (d)Date Invoice Prepared: (e)Contract No. and Order No. (if applicable): (f)Effective Date: (g)Total Estimated Cost of Contract/Order: (h)Total Fixed-Fee (if applicable): (i)Two-Way Match: Three-Way Match: (j)Office of Acquisitions: (k)Central Point of Distribution: | ||||||||||||||||||||||
(l) This invoice/financing request represents reimbursable costs for the period from to TABLE 1 of 2 | |||||||||||||||||||||||
Expenditure Category* A | Cumulative Percentage of Effort/Hrs. | Amount Billed | Cost at Completi on F | Contrac t Amount G | Variance H | ||||||||||||||||||
Negotiate d B | Actual C | (m) Current D | (n) Cumulativ e E | ||||||||||||||||||||
(o) Direct Costs: | |||||||||||||||||||||||
(1) Direct Labor | |||||||||||||||||||||||
(2) Fringe Benefits | |||||||||||||||||||||||
(3) Accountable Property | |||||||||||||||||||||||
(4) Materials & Supplies | |||||||||||||||||||||||
(5) Premium Pay | |||||||||||||||||||||||
Contract No. 75A50122C00047
Attachment #4
(6) Consultant Fees | |||||||||||||||||||||||
(7) Travel | |||||||||||||||||||||||
(8) Subcontracts | |||||||||||||||||||||||
(9) Other | |||||||||||||||||||||||
Total Direct Costs | |||||||||||||||||||||||
(p) Cost of Money | |||||||||||||||||||||||
(q) Indirect Costs | |||||||||||||||||||||||
(r) Fixed Fee | |||||||||||||||||||||||
(s) Total Amount Claimed | |||||||||||||||||||||||
(t) Adjustments | |||||||||||||||||||||||
(u) Grand Totals | |||||||||||||||||||||||
I certify that all payments are for appropriate purposes and in accordance with the contract. (Name of Official) (Title) * Attach details as specified in the contract | |||||||||||||||||||||||
TABLE 2 of 2
| CLIN | Requisition Number | Mod # | Total Funds Obligated | Cumulative Spend to Date | Remaining Funds | Spend Current Invoice | ||||||||||||||
CLIN XXXX | OS#XXXXXX | # | $ | $ | $ | $ | ||||||||||||||
Contract No. 75A50122C00047
Attachment #4
OMB No. 0990-0263
Approved for use through June 30, 2024
Protection of Human Subjects Assurance Identification/IRB Certification/Declaration of Exemption (Common Rule)
Policy: Research activities involving human subjects may not be conducted or supported by the Departments and Agencies adopting the Common Rule unless the activities are exempt from or approved in accordance with the Common Rule. The “pre-2018 Common Rule (or pre-2018 Requirements)” was originally promulgated in 1991 and amended on June 23, 2005 (70 FR 36325). The “2018 Common Rule (or 2018 Requirements)” was originally published on January 19, 2017 (82 FR 7149) and amended on January 22, 2018 (83 FR 2885) and June 19, 2018 (83 FR 28497). The categories of exempt research are provided in Section 101(b) of the pre-2018 Common Rule and Section 104(d) of the 2018 Common Rule.
The pre-2018 Common Rule requires institutions to certify that each application or proposal for research has been reviewed and approved by an Institutional Review Board (IRB) (Section 103(f)). The 2018 Common Rule requires institutions to certify that each proposed research study has been reviewed and approved by an IRB (Section 103(d)). Institutions must have an assurance of compliance that applies to the research to be conducted and should submit certification of IRB review and approval with each application or proposal, or proposed research study, unless otherwise advised by the Department or Agency.
1. Request Type [ ] ORIGINAL [ CONTINUATION [ ] EXEMPTION | 2. Type of Mechanism [ ] GRANT [ ] CONTRACT [ ] FELLOWSHIP [ ] COOPERATIVE AGREEMENT [ ] OTHER: | 3. Name of Federal Department or Agency and, if known, Application or Proposal Identification No. | ||||||
4. Title of Application or Activity | 5. Name of Principal Investigator, Program Director, Fellow, or Other | |||||||
Contract No. 75A50122C00047
Attachment 5
6.Assurance Status of this Project (Respond to one of the following)
[ ] This Assurance, on file with the Department of Health and Human Services, covers this activity:
Assurance Identification No. , the expiration date IRB Registration No.
[ ] This Assurance, on file with (agency/dept) , covers this activity.
Assurance No. , the expiration date IRB Registration/Identification No. (if applicable)
[ ] No assurance has been filed for this institution. This institution declares that it will provide an Assurance and Certification of IRB review and approval upon request.
[ ] Exemption Status: Human subjects are involved, but this activity qualifies for exemption under the pre-2018 Common Rule, Section 101(b), paragraph .
[ ] Exemption Status: Human subjects are involved, but this activity qualifies for exemption under the 2018 Common Rule, Section 104(d), paragraph
7.Certification of IRB Review (Respond to one of the following IF you have an Assurance on file)
[ ] This activity has been reviewed and approved by the IRB in accordance with the Common Rule and any other governing regulations.
by:
[ ] Full IRB Review on (date of IRB meeting) or [ ] Expedited Review on (date)
[ ] If less than one year approval, provide expiration date
[ ] This activity contains multiple projects, some of which have not been reviewed. The IRB has granted approval on condition that all projects covered by the Common Rule will be reviewed and approved before they are initiated and that appropriate further certification will be submitted.
8.Comments
9. The official signing below certifies that the information provided above is correct | 10. Name and Address of Institution | ||||
Contract No. 75A50122C00047
Attachment 5
and that, as required, future reviews will be performed until study closure and certification will be provided. | |||||
11.Phone No. (with area code) 12.Email: | |||||
13. Name of Official | 14. Title | ||||
15. Signature | 16. Date | ||||
Authorized for local Reproduction Sponsored by HHS
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0990-0263. The time required to complete this information collection is estimated to average 30 minutes per response. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: U.S. Department of Health & Human Services, OS/OCIO/PRA, 000 Xxxxxxxxxxxx Xxx., X.X., Xxxxx 000-X, Xxxxxxxxxx X.X. 00000, Attention: PRA Reports Clearance Officer.
Contract No. 75A50122C00047
Attachment 5
ATTACHMENT #6
BARDA SECURITY REQUIREMENTS
The following table outlines the minimum security requirements for any partner facility receiving a BARDA contract under which the USG purchases products or technologies.
1. Security Administration | |||||
Security Program | The partner facility shall have a comprehensive security program that provides a security plan for the overall protection of personnel, information, data, and facilities associated with fulfilling the BARDA requirement. The proposal submitted shall include a security plan which establishes security practices and procedures that demonstrate how the Offeror will meet and adhere to the security requirements outlined below by time of contract award. The Offeror shall also ensure that other entities (subcontractors, consultants, etc.) performing work on behalf of the Offeror establishes and manages a security program that complies with BARDA security requirements. | ||||
2. Facility Security Plan | |||||
As part of the partner facility’s overall security program, they shall submit a written security plan with their proposal to BARDA for review and approval by the BARDA PPO. Performance of work under the BARDA contract will be in accordance with the approved security plan. The security plan will include the following processes and procedures at a minimum: | |||||
Security Administration | Organization and responsibilities; security risk assessment for site; threat levels identification matrix; security procedures during elevated threats; liaison with law enforcement; security education and training | ||||
Personnel Security Policies and Procedures | Candidate recruitment process; background investigations; employment suitability policy; access determination; rules of behavior/ conduct; termination procedures; non-disclosure agreements. | ||||
Physical Security Policies and Procedures | Internal/external access control; protective services; identification/badging; visitor access controls; parking areas and access control; perimeter fencing/barriers; shipping, receiving and transport; security lighting; restricted areas; signage; intrusion detection systems; alarm monitoring/response; closed circuit television; product storage security; other control measures. | ||||
Information Security | Identification of sensitive information; access control; storage of information; document control; retention/ destruction requirements. | ||||
Information Technology/Cyber Security Policies and Procedures | Intrusion detection and prevention systems; threat identification; employee training; encryption systems; identification of sensitive information/media; password policy; removable media policy; laptop policy; access control and determination; system document control; system backup; system disaster recovery; incident response; | ||||
system audit procedures; property accountability. | |||||
3. Site Security Master Plan | |||||
The partner facility shall provide a site schematic for security systems which includes: main access points; security cameras; electronic access points; bio-containment laboratories | |||||
4. Site Threat / Vulnerability / Risk Assessment | |||||
The partner facility shall provide a written risk assessment for the facility addressing: criminal threat; terrorist threat; industrial espionage; natural disasters; and potential loss of critical infrastructure (power/water/natural gas, etc.) This assessment shall include recent data obtained from local law enforcement agencies. | |||||
5. Physical Security | |||||
Closed Circuit Television (CCTV) Monitoring | Layered (internal/external) CCTV coverage with time-lapse video recording for buildings and areas where critical assets are processed or stored. CCTV coverage should include entry and exits to critical facilities, perimeters, and areas within the facility deemed critical to the execution of the contract. Video recordings must be maintained for a minimum of 30 days. CCTV surveillance system must be on emergency power backup. | ||||
Facility Lighting | Lighting must cover facility perimeter, parking areas, critical infrastructure, and entrances and exits to buildings. Lighting must have emergency power backup. Lighting must be sufficient for the effective operation of the CCTV surveillance system during hours of darkness. | ||||
Shipping and Receiving | Should have CCTV coverage and an electronic access control system. Should have procedures in place to control access and movement of drivers picking up or delivering shipments. Must identify drivers picking up BARDA products by government issued photo identification. | ||||
Access Control | Should have an electronic intrusion detection system with centralized monitoring. Responses to alarms must be immediate and documented in writing. Employ an electronic system (i.e. card key) to control access to areas where assets critical to the contract are located (facilities, laboratories, clean rooms, production | ||||
Attachment No. 6
Page 2 of 6
facilities, warehouses, server rooms, records storage, etc.) The electronic access control should signal an alarm notification of unauthorized attempts to access restricted areas. Should have procedures to prevent employee piggybacking. Access to critical infrastructure (generators, air handlers, fuel storage, etc.) should be controlled and limited to those with a legitimate need for access. Should have a manual key accountability and inventory process. Physical access controls should present a layered approach to critical assets within the facility. | |||||
Employee/Visitor Identification | Should issue company photo identification to all employees. Photo identification should be displayed above the waist anytime the employee is on company property. Visitors should be sponsored by an employee and must present government issued photo identification to enter the property. Visitors should be logged in and out of the facility and should be escorted by an employee while on the premises. | ||||
Security Fencing | Requirements for security fencing will be determined by the criticality of the program and the potential threat environment. | ||||
Protective Security Forces | Requirements for a security force will be determined by the criticality of the program and the potential threat environment. | ||||
6. Security Operations | |||||
Information Sharing | Establish formal liaison with law enforcement and implement procedures for receiving and disseminating threat information. | ||||
| Training | Conduct new employee security awareness training. Conduct and maintain records of annual security awareness training. | ||||
Security Management | Designate a knowledgeable security professional to manage security of the facility. Ensure subOfferor compliance with BARDA security requirements. | ||||
7. Personnel Security | |||||
Records Checks | Verification of date of birth, citizenship, education credentials, five-year previous employment history, five-year previous residence history, FDA disbarment, and local | ||||
Attachment No. 6
Page 3 of 6
/ national criminal history search. | |||||
Hiring and Retention Standards | Policies and procedures concerning hiring, and retention of employees to include employee conduct expectations. | ||||
8. Information Security | |||||
Physical Document Control | Applicable documents shall be identified and marked as procurement sensitive, proprietary or with appropriate government markings. Sensitive, proprietary, and government documents should be maintained in a lockable filing cabinet / desk or other storage device and not be left unattended. Access to sensitive information should be restricted to those with a need to know. | ||||
Document Destruction | Documents shall be destroyed using approved destruction measures (i.e. shredders / approved third party vendors / pulverizing / incinerating). | ||||
9. Information Technology & Cybersecurity | |||||
Access Control | Limit information systems access to authorized users. Identify information system users, processes acting on behalf of users, or devices and authenticate identities before allowing access. Limit physical access to information systems, equipment, and server rooms with electronic access controls. | ||||
| Training | Ensure that personnel are trained and are made aware of the security risks associated with their activities and of the applicable laws, policies, standards, regulations, or procedures related to information technology systems. | ||||
Audit and Accountability | Create, protect, and retain information system audit records to the extent to the extent needed to enable the monitoring, analysis, investigation, and reporting of unlawful, unauthorized, or inappropriate system activity. Ensure the actions of individual information system users can be uniquely traced to those users. | ||||
Configuration Management | Establish and enforce security configuration settings. | ||||
Contingency Planning | Establish, implement, and maintain plans for emergency response, backup operations, and post-disaster recovery for information systems to ensure the availability of critical information resources at all times. | ||||
Attachment No. 6
Page 4 of 6
Incident Response | Establish an operational incident handling capability for information systems that includes adequate preparation, detection, analysis, containment, and recovery of cybersecurity incidents. | ||||
Media and Information Protection | Protect information system media, both paper and digital. Limit access to information on information systems media to authorized users Sanitize and destroy media no longer in use. Control the use of removable media through technology or policy. | ||||
Physical and Environmental Protection | Limit access to information systems, equipment, and the respective operating environments to authorized individuals. Protect the physical and support infrastructure for all information systems. Protect information systems against environmental hazards. | ||||
Network Protection | Employ intrusion prevention and detection technology. | ||||
10. Transportation Security | |||||
Adequate security controls must be implemented to protect materials while in transit from theft, destruction, manipulation, or damage. | |||||
| Drivers | Drivers should be vetted in accordance with BARDA Personnel Security Requirements. Drivers should be trained on specific security and emergency procedures. Drivers should be equipped with backup communications. Driver identity should be 100 percent confirmed before pick-up of any BARDA product. Drivers should never leave BARDA product unattended and two drivers may be required for longer transport routes or critical products during times of emergency. | ||||
Transport Routes | Transport routes should be pre-planned and never deviated from except when approved or in the event of an emergency. Transport routes should be continuously evaluated based upon new threats, large planned events, weather, and other situations that may delay or disrupt transport. | ||||
Product Security | BARDA products should be secured with tamper resistant seals during transport and | ||||
Attachment No. 6
Page 5 of 6
the transport trailer should be locked and sealed. Tamper resistant seals should be verified as “secure” after the product is placed in the transport vehicle. BARDA product should be continually monitored by GPS technology while in transport and any deviations from planned routes should be investigated and documented. Contingency plans should be in place to keep the product secure during emergencies such as accidents and transport vehicle breakdowns. | |||||
11. Security Reporting Requirements | |||||
The partner facility shall immediately report to the government any activity or incident that is in violation of established security standards or indicates the loss or theft of government products. The facts and circumstances associated with these incidents will be documented in writing for government review. | |||||
12. Security Audits | |||||
The partner facility agrees to formal security audits conducted at the discretion of the government. Security audits may include both prime and sub locations. | |||||
Attachment No. 6
Page 6 of 6
FINANCIAL REPORT OF INDIVIDUAL PROJECT/CONTRACT Note: Complete this Form in Accordance with Accompanying Instructions. | Project Task: | Contract No.: | Date of Report: | 0990-0134 0990-0131 | ||||||||||||||||||||||||||||||||||
Reporting Period: | Contractor Name and Address: | |||||||||||||||||||||||||||||||||||||
Expenditure Category | Percentage of Effort/Hours | Cumulative Incurred Cost at End of Prior Period | Incurred Cost-- Current Period | Cumulative Cost to Date (D + E) | Estimated Cost to Complete | Estimated Cost at Completion (F + G) | Negotiated Contract Amount | Variance (Over or Under) (I - H) | ||||||||||||||||||||||||||||||
Negotiated | Actual | |||||||||||||||||||||||||||||||||||||
A | B | C | D | E | F | G | H | I | J | |||||||||||||||||||||||||||||
Contract No. 75A50122C00047 Attachment 7 - Financial Report
INSTRUCTIONS FOR COMPLETING FORM "FINANCIAL REPORT OF INDIVIDUAL PROJECT/CONTRACT"
GENERAL INFORMATION
Purpose. This Quarterly Financial Report is designed to: (1) provide a management tool for use by BARDA in monitoring the application of financial and personnel resources to the BARDA contracts; (2) provide contractors with financial and personnel management data which is usable in their management processes; (3) promptly indicate potential areas of contract underruns or overruns by making possible comparisons of actual performance and projections with prior estimates on individual elements of cost and personnel; and (4) obtain contractor's analysis of cause and effect of significant variations between actual and prior estimates of financial and personnel performance.
REPORTING REQUIREMENTS
Scope. The specific cost and personnel elements to be reported shall be established by mutual agreement prior to award. The Government may require the contractor to provide detailed documentation to support any element(s) on one or more financial reports.
Number of Copies and Mailing Address. An original and two (2) copies of the report(s) shall be sent to the contracting officer at the address shown on the face page of the contract, no later than 30 working days after the end of the period reported. However, the contract may provide for one of the copies to be sent directly to the Contracting Officer's Representative.
REPORTING STATISTICS
A modification which extends the period of performance of an existing contract will not require reporting on a separate form, except where it is determined by the contracting officer that separate reporting is necessary. Furthermore, when incrementally funded contracts are involved, each separate allotment is not considered a separate contract entity (only a funding action). Therefore, the statistics under incrementally funded contracts should be reported cumulatively from the inception of the contract through completion.
Definitions and Instructions for Completing Form. For the purpose of establishing expenditure categories in Column A, the following definitions and instructions will be utilized. Each contract will specify the categories to be reported.
(1)Key Personnel. Include key personnel regardless of annual salary rates. All such individuals should be listed by names and job titles on a separate line including those whose salary is not directly charged to the contract but whose effort is directly associated with the contract. The listing must be kept up to date.
(2)Personnel--Other. List as one amount unless otherwise required by the contract.
(3)Fringe Benefits. Include allowances and services provided by the contractor to employees as compensation in addition to regular salaries and wages. If a fringe benefit rate(s) has been established, identify the base, rate, and amount billed for each category. If a rate has not been established, the various fringe benefit costs may be required to be shown separately. Fringe benefits which are included in the indirect cost rate should not be shown here.
(4)Accountable Personal Property. Include nonexpendable personal property with an acquisition cost of
$1,000 or more and with an expected useful life of two or more years, and sensitive items regardless of cost. Form HHS 565, "Report of Accountable Property," must accompany the contractor's public voucher (SF 1034/SF 1035) or this report if not previously submitted. See "Contractor's Guide for Control of Government Property."
(5)Supplies. Include the cost of supplies and material and equipment charged directly to the contract, but excludes the cost of nonexpendable equipment as defined in (4) above.
Instructions for Completing Form
Attachment 7
Page 2 of 4
(6)Inpatient Care. Include costs associated with a subject while occupying a bed in a patient care setting. It normally includes both routine and ancillary costs.
(7)Outpatient Care. Include costs associated with a subject while not occupying a bed. It normally includes ancillary costs only.
(8)Travel. Include all direct costs of travel, including transportation, subsistence and miscellaneous expenses. Travel for staff and consultants shall be shown separately. Identify foreign and domestic travel separately. If required by the contract, the following information shall be submitted: (i) Name of traveler and purpose of trip; (ii) Place of departure, destination and return, including time and dates; and
(iii) Total cost of trip.
(9)Consultant Fee. Include fees paid to consultant(s). Identify each consultant with effort expended, billing rate, and amount billed.
(10)Premium Pay. Include the amount of salaries and wages over and above the basic rate of pay.
(11)Subcontracts. List each subcontract by name and amount billed.
(12)Other Costs. Include any expenditure categories for which the Government does not require individual line item reporting. It may include some of the above categories.
(13)Overhead/Indirect Costs. Identify the cost base, indirect cost rate, and amount billed for each indirect cost category.
(14)General and Administrative Expense. Cite the rate and the base. In the case of nonprofit organizations, this item will usually be included in the indirect cost.
(15)Fee. Cite the fee earned, if any.
(16)Total Costs to the Government.
PREPARATION INSTRUCTIONS
These instructions are keyed to the Columns on Form NIH 2706.
Column A--Expenditure Category. Enter the expenditure categories required by the contract.
Column B--Percentage of Effort/Hours Negotiated. Enter the percentage of effort or number of hours agreed to during contract negotiations for each labor category listed in Column A.
Column C--Percentage of Effort/Hours-Actual. Enter the cumulative percentage of effort or number of hours worked by each employee or group of employees listed in Column A.
Column D--Cumulative Incurred Cost at End of Prior Period. Enter the cumulative incurred costs up to the end of the prior reporting period. This column will be blank at the time of the submission of the initial report.
Column E--Incurred Cost-Current Period. Enter the costs which were incurred during the current period.
Column F--Cumulative Incurred Cost to Date. Enter the combined total of Columns D and E.
Column G--Estimated Cost to Complete. Make entries only when the contractor estimates that a particular expenditure category will vary from the amount negotiated. Realistic estimates are essential.
Column H--Estimated Costs at Completion. Complete only if an entry is made in Column G.
Instructions for Completing Form
Attachment 7
Page 3 of 4
Column I--Negotiated Contract Amount. Enter in this column the costs agreed to during contract negotiations for all expenditure categories listed in Column A.
Column J--Variance (Over or Under). Complete only if an entry is made in Column H. When entries have been made in Column H, this column should show the difference between the estimated costs at completion (Column H) and negotiated costs (Column I). When a line item varies by plus or minus 10 percent, i.e., the percentage arrived at by dividing Column J by Column I, an explanation of the variance should be submitted. In the case of an overrun (net negative variance), this submission shall not be deemed as notice under the Limitation of Cost (Funds) Clause of the contract.
Modifications. List any modification in the amount negotiated for an item since the preceding report in the appropriate cost category.
Expenditures Not Negotiated. List any expenditure for an item for which no amount was negotiated (e.g., at the discretion of the contractor in performance of its contract) in the appropriate cost category and complete all columns except for I. Column J will of course show a 100 percent variance and will be explained along with those identified under J above.
Instructions for Completing Form
Attachment 7
Page 4 of 4
REPORT OF GOVERNMENT OWNED, CONTRACTOR HELD PROPERTY | |||||||||||||||||||||||||||||||||||||||||
| CONTRACTOR: | CONTRACT NUMBER: | ||||||||||||||||||||||||||||||||||||||||
| ADDRESS: | REPORT DATE: | ||||||||||||||||||||||||||||||||||||||||
| ADDRESS1: | |||||||||||||||||||||||||||||||||||||||||
| ADDRESS2: | FISCAL YEAR: | ||||||||||||||||||||||||||||||||||||||||
| CITY: | |||||||||||||||||||||||||||||||||||||||||
| STATE: | |||||||||||||||||||||||||||||||||||||||||
| ZIP: | |||||||||||||||||||||||||||||||||||||||||
| CLASSIFICATION | BEGINNING OF PERIOD | ADJUSTMENTS | END OF PERIOD | ||||||||||||||||||||||||||||||||||||||
| #ITEMS | VALUE | GFP ADDED | CAP ADDED | DELETIONS | #ITEMS | VALUE | |||||||||||||||||||||||||||||||||||
LAND >=$25K | |||||||||||||||||||||||||||||||||||||||||
LAND <$25K | |||||||||||||||||||||||||||||||||||||||||
OTHER REAL >=$25K | |||||||||||||||||||||||||||||||||||||||||
OTHER REAL <$25K | |||||||||||||||||||||||||||||||||||||||||
PROPERTY UNDER CONST >=$25K | |||||||||||||||||||||||||||||||||||||||||
PROPERTY UNDER CONST <$25K | |||||||||||||||||||||||||||||||||||||||||
PLANT EQUIP >=$25K | |||||||||||||||||||||||||||||||||||||||||
PLANT EQUIP <$25K | |||||||||||||||||||||||||||||||||||||||||
SPECIAL TOOLING >=$25K | |||||||||||||||||||||||||||||||||||||||||
SPECIAL TOOLING <$25K | |||||||||||||||||||||||||||||||||||||||||
SPECIAL TEST EQUIP >=$25K | |||||||||||||||||||||||||||||||||||||||||
SPECIAL TEST EQUIP <$25K | |||||||||||||||||||||||||||||||||||||||||
AGENCY PECULIAR >=$25K | |||||||||||||||||||||||||||||||||||||||||
AGENCY PECULIAR <$25K | |||||||||||||||||||||||||||||||||||||||||
MATERIAL >=$25K (CUMULATIVE) | |||||||||||||||||||||||||||||||||||||||||
PROPERTY UNDER MFR >=$25K | |||||||||||||||||||||||||||||||||||||||||
PROPERTY UNDER MFR <$25K | |||||||||||||||||||||||||||||||||||||||||
SIGNED BY: | |||||||||||||||||||||||||||||||||||||||||
SIGNATURE | DATE SIGNED: | ||||||||||||||||||||||||||||||||||||||||
NAME PRINTED | Email | ||||||||||||||||||||||||||||||||||||||||
TITLE | TELEPHONE | ||||||||||||||||||||||||||||||||||||||||
Report of Government Owned, Contractor Held Property (Rev 10/2014) Attachment 8

