LETTER AGREEMENT FOR THE COMMERCIAL MANUFACTURING OF IMMU- 132 PRODUCT
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
LETTER AGREEMENT
FOR THE COMMERCIAL MANUFACTURING OF IMMU- 132 PRODUCT
This Letter of Intent (this “LOI”) is entered into between Immunomedics, Inc., a corporation with its principal office located at 000 Xxx Xxxxxxxx Xxxx Xxxxxx Xxxxxx, XX 00000, XXX (“Immunomedics”), and BSP Pharmaceuticals S.p.A., having a place of business at xxx Xxxxx xx 00,000 00000 Xxxxxx Xxxxx (XX), Xxxxx (“BSP”), effective as of February 26, 2018 (the “Effective Date”).
For the purpose of this LOI, Immunomedics and BSP are both referred hereinafter also a “Party” or the “Parties”, as applicable.
The Parties are currently in the process of negotiating in good faith toward a Master Service Agreement (the “Agreement”) for the commercial supply of the product, named IMMU-132 (the “Product”). This LOI confirms the mutual intentions of the Parties with respect to the negotiations of the Agreement and the commitments of the Parties for the manufacturing of the bulk drug substance and the commercial supply of the Product for the calendar year 2018, based on BSP’s proposal dated January 30, 2018 (the “Proposal”) and attached hereto as Exhibit A.
In consideration of the mutual covenants set forth in this LOI, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows.
1. | Agreement. The Parties will use reasonably diligent efforts in good faith to negotiate, execute, and deliver the Agreement on or before [***], unless extended by mutual agreement. The Parties agree to conduct the negotiations on a confidential basis. |
2. | Binding Forecast. The quantities of Product identified in Table 1 in Exhibit B represent the Parties’ current binding commitment (the “Binding Forecast”) for the manufacture and purchase of [***] Batches of bulk drug substance (“DS”) and [***] Batches of drug product (“DP”) distributed during each calendar quarter of 2018 at the prices agreed in the Proposal. The volumes of [***] Batches of DS and [***] Batch of DP specifically set forth in Table 2 of Exhibit 2 correspond to the minimum quantities for 2018 (the “Minimum Quantities”). Upon execution of this LOI, Immunomedics shall issue separate purchase orders (each a “Purchase Order”) for the Minimum Quantities of Product and for the Material Costs (as defined below in Section 5). The Purchase Order shall specify each manufacturing dates within such calendar quarter respectively for DS or DP, as set forth in Table 1. As used herein, “Batch” with respect to DS shall mean theoretical [***] Liters (10mg/mL per vial) and “Batch” with respect to DP shall mean theoretical [***] vials. |
3. | Financial Obligations. Throughout the term of this LOI [***], as distributed according to the manufacturing dates identified by the Parties, then Immunomedics is obligated to pay [***] per cent ([***]%) of the capacity reserved for the Binding Forecast at the price set forth in the Proposal, as amended from time to time, in accordance with the terms set forth in this Section 3. For clarity, the price for capacity reservation for any Batch of DS is €[***] and for any Batch of DP is €[***]. In accordance with the provisions of this LOI, the Parties hereby agree that should Immunomedics not utilize the capacity reserved by BSP for the manufacture of the Minimum Quantities, then Immunomedics shall pay to BSP an amount equal to the product of (i) the difference between (A) the aggregate quantity of Product set forth in the Binding Forecast limited to the Minimum Quantities of Product for such calendar year, less (B) the aggregate quantity of Product ordered by Immunomedics for delivery in such calendar year (such difference, the “Order Shortfall”), multiplied by (ii) the prices applicable to the Order Shortfall for such calendar year (i.e. €[***] for any Batch of DS and €[***] for any Batch of DP). In addition, Immunomedics shall pay to BSP an amount equal to [***] |
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Euro, as a reservation fee (the “Reservation Fee”) to compensate the remainder of the Binding Forecast for 2018, exclusive of the Minimum Quantities, provided that in the event Immunomedics orders an amount of DS or DP in excess of the Minimum Quantities in calendar year 2018, Immunomedics shall be credited the manufacture price for each Batch of DS and DP (as provided in Exhibit C) from the Reservation Fee, provided that Immunomedics shall issue purchase order for additional quantities at least [***] in advance of the specified delivery date for respective DS or DP Batches, such manufacture to be performed within [***], and provided that the intermediates (i.e. payload and mAb) necessary for manufacture of such additional quantities shall be delivered and released by Immunomedics before [***]. Upon receipt of the first Purchase Orders for the Material Costs and the Minimum Annual Quantities, BSP shall issue a separate invoice for the Material Costs, and BSP shall issue separate invoices for the Reservation Fee as provided in Exhibit C. The Minimum Quantities of Product shall be invoiced by BSP to Immunomedics in accordance with Section 5 below. The Reservation Fee shall be paid by Immunomedics in accordance with the payment schedule set forth in Exhibit C and [***] of the receipt of the relevant invoice by BSP and, in any event, within the calendar year 2018.
4. | Intermediates. Should the intermediates (i.e. toxin and mAb) not be available to be released at BSP at least four (4) weeks before the manufacturing date then Immunomedics shall immediately notify BSP thereof. Promptly thereafter, the Parties shall meet to discuss in good faith if BSP can comply with the original schedule using its best efforts to do so, and if BSP cannot comply with the original schedule, BSP shall use best efforts to adjust the manufacturing in accordance with the terms of this LOI and with BSP’s production plan. Should the adjustment of manufacturing not be possible, the relevant production shall be cancelled and Immunomedics shall remain obligated to pay such part of unused capacity; provided that BSP is unable to fill the unused capacity with work from a third party after using best efforts to do so. |
5. | Payment. BSP will submit invoices upon completion of the Services at the issuance of the Certificate of Analysis by BSP. The material costs for an amount estimated (in good faith) to be equal to Euro [***] (€ [***]) (the “Material Costs”) shall be paid separately [***] by Immunomedics immediately upon receipt of the invoice by BSP. Unless otherwise agreed by the Parties, BSP shall promptly return., at Immunomedics’ costs any material paid in advance by Immunomedics and not used by BSP in connection with the manufacture of the quantity of Product set forth in the Binding Forecast for the calendar year 2018 and which cannot be used for the manufacturing of Product under any other agreement between the Parties. |
BSP will submit invoices upon completion the manufacturing of each Batch of DS and each Batch of DP at the issuance of the respective Certificate of Analysis by BSP. Immunomedics shall pay undisputed (in good faith) invoices within forty-five (45) calendar days of the date of receipt of BSP’s invoice. All invoices and copies of receipts will be submitted electronically to:
[***]
BSP Pharmaceuticals S.p.A.
[***]
[***]
[***]
For clarity, the payment schedule for Services to be performed by BSP under this LOI is attached as Exhibit C.
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
6. | Compliance. BSP represents and warrants that it will use its best efforts and exercise due care and sound business judgment in performing the services and it will carry out the Services hereunder in compliance with the current GMP, applicable laws, BSP’s standard operating procedures and any Immunomedics specifications and/or procedures agreed upon in writing by the Parties. BSP shall reserve appropriate resources required for the scope of work as set forth herein. Any non-conformance in Product supplied hereunder shall be governed by that certain Master Clinical Supply and Services Agreement between the parties dated as of April 12, 2018, unless and until the Agreement is executed, in which event the Agreement shall govern. |
7. | Authority. The Parties further represent and warrant that they have the corporate power, authority and legal right to enter into this LOI and to perform its obligations. |
8. | Other provisions. This LOI, together with the Mutual Confidential Disclosure Agreement dated October 13, 2014 (“MCDA”) represent the entire agreement among the Parties with respect to the subject matter hereof. The Parties agree that all information provided by the Parties regarding the transactions outlined herein, as well as each Party’s business information, processes and standard operating procedures, shall be proprietary of such Party, held in strict confidence by the other Party, shall not be used by the receiving Party except as strictly required for the purposes of this LOI and shall not be disclosed to any third party without the prior written consent of the other Party. In the event that either Party determines that applicable securities laws require disclosure of Confidential Information (including this LOI), such Party shall promptly notify the other and the Parties shall cooperate in making a disclosure (joint disclosure if necessary) which shall meet the requirements of the applicable securities laws. |
9. | Term. This LOI shall take effect as of the Effective Date and shall continue until the earliest of (i) the date of the completion of the activities specified in the Proposal, or (ii) the effective date of the Agreement, unless otherwise agreed in writing by the Parties. |
10. | Prevalence. Upon its execution, the Agreement will supersede this LOI and shall govern the terms of the project initiated by virtue of this LOI. The Agreement shall include such other terms as are customary for such agreements, including without limitation, provisions relating to forecasting, acceptance and rejection, representation and warranties, indemnification, insurance, limitation of liability, termination, publicity, and allocation of intellectual property rights. |
11. | Liability. EXCEPT FOR BREACHES OF CONFIDENTIALITY UNDER SECTION 8, IT IS UNDERSTOOD AND AGREED THAT NEITHER OF THE PARTIES SHALL BE LIABLE TOWARDS THE OTHER FOR INDIRECT, SPECIAL, PUNITIVE, EXEMPLARY, INCIDENTAL OR CONSEQUENTIAL DAMAGES, INCLUDING WITHOUT LIMITATION LOSS OF PROFITS OR REVENUES, REGARDLESS OF WHETHER SUCH DAMAGES WERE FORESEEABLE OR NOT. |
12. | Taxes. Each Party shall comply with its applicable taxation guidelines regarding filing and reporting for income tax purposes. Neither Party shall treat their relationship under this Agreement as a partnership or as a pass through entity for tax purposes. |
13. | Law and Jurisdiction. This LOI shall be governed by and construed in accordance with the laws of New York, United States of America, without regard to any conflicts-of-law provisions that directs the application to another jurisdiction’s law, and the United Nations Convention on Agreements for the International Sale of Goods is hereby excluded. Both Parties hereby submit to the exclusive jurisdiction of the federal and state courts located in the State of New York, U.S.A. |
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
14. | General. This LOI may be executed in one or more counterparts (including electronic counterparts), each of which shall be deemed to be an original document, but all such separate counterparts shall constitute only this LOI. The Parties shall perform their obligations under this LOI as independent contractors and nothing contained in this LOI shall be construed to be inconsistent with such relationship or status. The Parties shall neither amend this LOI, except by mutual written agreement nor assign the rights and/or obligations under this LOI to any third party in any manner without the prior written consent of the other Party. |
[Signature page follows]
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
BSP Pharmaceuticals S.p.A | Immunomedics, Inc. | |||
By: | /s/ Xxxx Xxxxx | By: | /s/ Xxxxxxx Xxxx | |
Xxxx Xxxxx | Xxxxxxx Xxxx | |||
Title: | President & CEO | Title: | Chief Executive Officer | |
Date: | July 5, 2018 | Date | July 3, 2018 | |
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
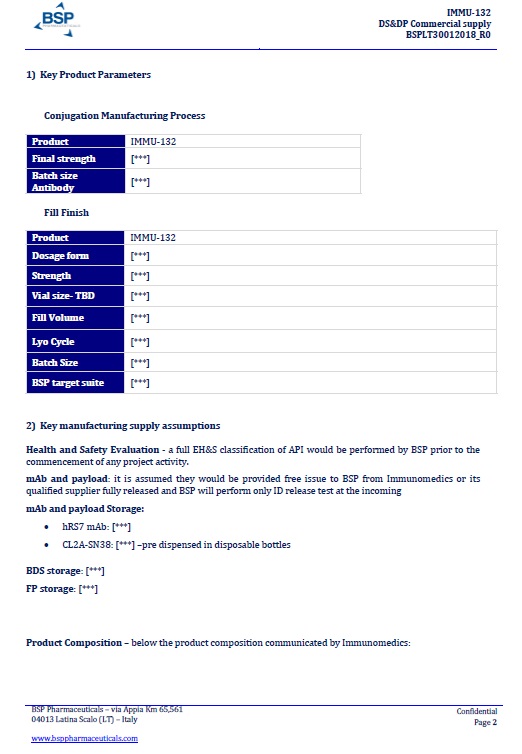
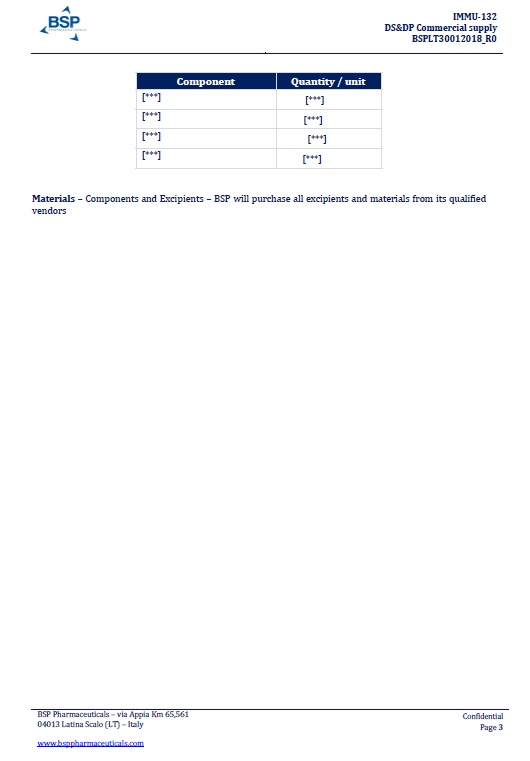
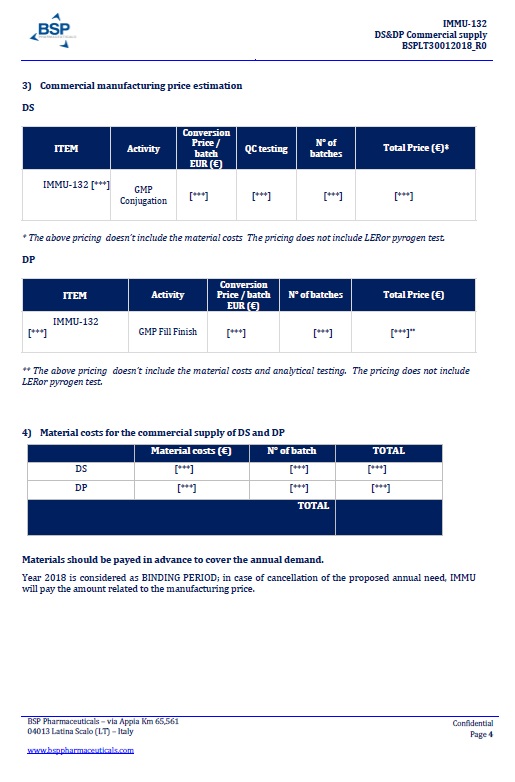
EXHIBIT A
BSP PROPOSAL “DS&DP Commercial supply n. BSPLT30012018_R0”dated January 30, 2018

Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.

Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
EXHIBIT B
BINDING FORECAST AND MANUFACTURING DATES
Table 1 - Binding Forecast delivered on October 2017
Year 2018 | |
Conjugation Batches | Fill-Finish Batches |
[***] | [***] |
` | |
[***] | [***] |
[***] | |
[***] | [***] |
[***] | |
[***] | [***] |
[***] | |
[***] | |
[***] | |
Table 2 - Manufacturing Dates for [***] of DS and [***] of DP
relevant for the purpose of Section 3
Year 2018 | |
Conjugation Batches | Fill-Finish Batches |
[***] | [***] |
[***] | |
[***] | |
[***] | |
[***] | |
Confidential treatment has been requested with respect to portions of this agreement as indicated by “[***]” and such confidential portions have been deleted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
EXHIBIT C
PAYMENT SCHEDULE
Item | PO Issuance | Invoice Issuance | Payment | |
[***] | [***] | LOI Execution | LOI approval | [***] |
[***] | [***] | September 30, 2018: [***] November 30, 2018: [***] | [***] | |
[***] DS batches | LOI Execution | CoA issuance | [***] | |
[***] DP batch | [***] /batch | LOI Execution | CoA issuance | [***] |

