EX-10.5 14 d226593dex105.htm SUPPLY AGREEMENT Portions of this exhibit marked [*] are requested to be treated confidentially. SUPPLY AGREEMENT
Exhibit 10.5
Portions of this exhibit marked [*] are requested to be treated confidentially.
This Supply Agreement (hereinafter referred to as this “Agreement”), effective as March 15, 2011 (the “Effective Date”), is entered into by and between Ercros S.A, a Spanish corporation having a place of business at Xxxxx xxx Xxxxxxx x/x, 00000 Xxxxxxxx-Xxxxxx (Xxxxx) (“Ercros” or “Supplier”), Gyma Laboratories of America, Inc., a New York corporation having a place of business at 000 Xxxxxxxxx Xxxx Xxxx, Xxxxxxxx, Xxx Xxxx 00000 XXX (“Gyma” or “Supplier Agent”), and CEM-102 Pharmaceuticals, Inc., a Delaware corporation having a place of business at Building Four Quadrangle, 0000 Xxxxxxxxxx Xxxxx, Xxxxx 000, Xxxxxx Xxxx, Xxxxx Xxxxxxxx 00000 XXX (“Cempra”).
WITNESSETH
WHEREAS, Cempra wishes to develop and commercialize certain pharmaceutical products incorporating Sodium Fusidate (as defined below) and Fusidic Acid (as defined below, and together with Sodium Fusidate, the “Supplied API”) as an API (as defined below);
WHEREAS, Supplier has the expertise and the facilities suitable for the manufacture and supply of Supplied API for use as an active pharmaceutical ingredient;
WHEREAS, Cempra wishes to have Supplier manufacture and supply clinical and commercial batches of Supplied API pursuant to the terms and conditions of this Agreement;
WHEREAS, Supplier Agent is currently the agent for Supplier with respect to the distribution of Supplied API in the Exclusive Territory (as defined below).
NOW, THEREFORE, for and in consideration of the covenants, conditions, and undertakings hereinafter set forth, it is agreed by and between the parties as follows.
1. Definitions.
1.1 “Act” means the United States’ Federal Food, Drug & Cosmetic Act (21 U.S.C. §§301 et seq.), as amended, and the regulations promulgated thereunder.
1.2 “Affiliate” means a person, corporation, partnership, or other entity that controls, is controlled by or is under common control with a party to this Agreement. For the purposes of this Section 1.2, the word “control” (including, with correlative meaning, the terms “controlled by” or “under the common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct the management and policies of such entity, whether by the ownership of at least fifty percent (50%) of the voting stock of such entity, or by contract or otherwise
1.3 “API” means active pharmaceutical ingredient.
1.4 “Applicable Adjustment Period” shall have the meaning set forth in Section 7.1.
1.5 “Applicable Laws” means all applicable provisions of all statutes, laws, rules, regulations, administrative codes, ordinances, decrees, orders, decisions, guidance documents (including FDA guidance documents), injunctions, awards judgments, and permits and licenses of or from governmental authorities relating to the manufacture, use, sale, distribution, marketing, or regulation of the subject item.
1.6 “Cempra Intellectual Property” means all intellectual property rights owned, licensed, or controlled by Cempra relating to the use, dosing, manufacture, or composition of Supplied API or Products.
1.7 “Cempra Products” means Products developed or sold by Cempra, its Affiliates, or their licensees or distributors pursuant to contracts regarding the same executed between Cempra or its Affiliates and such third parties.
1.8 “Cempra Specifications” shall mean those specifications set forth on Exhibit A, as it may be amended or supplemented from time to time.
1.9 “Disclosing Party” shall have the meaning set forth in Section 13.1.
1.10 “Exclusive Territory” means the United States of America and its territories and protectorates.
1.11 “FDA” means the United States Food and Drug Administration or any successor agency having the administrative authority to regulate the approval for testing or marketing of human pharmaceutical or biological therapeutic products in the United States.
1.12 “Forecast” means the written forecast describing Cempra’s anticipated requirements with respect to Supplied API for a given time period, including the anticipated delivery schedule with respect to such Supplied API.
1.13 “Forecast Period” has the meaning set forth at Section 5.3.
1.14 “Fusidic Acid” means ent-(17z)-16a-(acetoxy)-3b, 11b-dihydroxy-4b, 8, 14-trimethyl-19-nor-5b, 10a-cholesta-17(20), 24-dien-21-oic acid.
1.15 “GMP” means the applicable current good manufacturing practices promulgated from time to time by the FDA in accordance with the Act, including those set forth in 21 C.F.R. Parts 210 and 211, and consistent with ICH guidelines.
1.16 “HICP” shall have the meaning set forth in Section 7.1.
1.17 “ICH” means the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
1.18 “IFRS” means International Financial Reporting Standards, as adopted and amended from time-to-time by the International Accounting Standards Board.
2
1.19 “Manufacturing Costs” means, with respect to the Supplier’s manufacturing and supply of Supplied API to Cempra hereunder, the reasonable, documented direct costs of all raw materials and direct labor used or consumed in such manufacture, as calculated in accordance with IFRS.
1.20 “Order” means a written purchase order for Supplied API, which order shall include a delivery schedule specifying the requested delivery date(s) and quantity(ies) for Supplied API ordered, and the location to which shipment of Supplied API is to be delivered. Orders shall include, but not be limited to, the Initial Order.
1.21 “Order Period” has the meaning set forth at Section 5.3.
1.22 “Price” means the applicable prices for Supplied API established in accordance with Section 7.
1.23 “Product” means any human or animal pharmaceutical product incorporating Supplied API as API.
1.24 “Product Approvals” means any approvals, licenses, registrations or authorizations granted by any national, federal, state or local regulatory agency, department, bureau or other government entity, including but not limited to the FDA, necessary for the development, clinical testing, marketing, manufacture, use, storage, import, transport, or sale of Products in any jurisdiction, any and all regulatory filings or submissions necessary to procure any of the foregoing, including, but not limited to, an investigational new drug application (“IND”), an abbreviated new drug application (“ANDA”), a new drug application (“NDA”), or any other application acceptable to the FDA or equivalent foreign regulatory authority for the development, clinical testing, and/or marketing approval of a pharmaceutical or biological product, including any supplements or amendments thereto and any and all correspondence, filings, and documents related to any of the foregoing.
1.25 “Receiving Party” shall have the meaning set forth in Section 13.1.
1.26 “Regulatory Authority” means any federal, national, supranational, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity with authority over the testing, manufacture, use, storage, import, promotion, marketing and sale of a pharmaceutical product in a country, including but not limited to the FDA and EMEA.
1.27 “Sodium Fusidate” means sodium (Z)-ent-16a-(acetoxy)-3b,11b-dihydroxy-4b,8,14-trimethyl-18-nor-5b,10a-cholesta-17(20),24-dien-21-oate, as further described in the Specifications.
1.28 “Specifications” means the description of and specifications for Supplied API described on Exhibit A, attached hereto, and shall include the Cempra Specifications, all of which may be further amended by the parties according to the terms hereof.
1.29 “Supplied API” means Sodium Fusidate and Fusidic Acid.
2. Effectiveness; Term. This Agreement shall be effective from the execution of this Agreement until the longer of (i) eighteen (18) years from the date of execution of this Agreement, (ii) the last to expire issued patent owned, controlled or licensed by Cempra related to the Product, (iii) any
3
period of regulatory exclusivity for a Cempra Product in the Exclusive Territory; subject to earlier termination pursuant to Sections 15 or 16 below.
3. Product Approval.
3.1 Supplier will reasonably assist Cempra in compilation of information for the chemistry, manufacturing and control documentation which Cempra determines in good faith is needed for completion of Product Approvals or filings or submissions with respect thereto, regulatory or otherwise. Supplier shall (i) provide Cempra with the publicly available portion of any drug master file established, maintained, or referenced by Supplier with respect to Supplied API supplied hereunder or otherwise related to its performance under this Agreement, (ii) maintain and update all such drug master files in compliance with all Applicable Laws (including but not limited to GMP and all FDA regulations and guidelines and those requirements included in the Product Approvals), and (iii) grant Cempra a right of reference (including rights of sublicense and assignment of such right of reference) to any such drug master file or related supporting information. Supplier Agent may perform certain of the Supplier’s foregoing obligations at the request of Supplier.
3.2 Supplier or Supplier Agent shall notify Cempra in writing as soon as possible of any notification received by either Supplier or Supplier Agent from FDA or any other applicable Regulatory Authority to conduct an inspection of Supplier’s manufacturing, development or other facilities directly related to the manufacture or supply of Supplied API under this Agreement. Within five (5) business days of receipt thereof, Supplier or Supplier Agent shall provide to Cempra a copy of any report and other written communications (including a detailed summary, in English, of any oral comments made by an agent of the FDA or applicable Regulatory Authority) received by them from the FDA or applicable Regulatory Authority to the extent that such report or communication relates to Supplier’s performance under this Agreement or the manufacture of Supplied API. Supplier or Supplier Agent shall provide Cempra with frequent, prompt status updates with regard to any audit or inspection conducted by FDA (or other applicable Regulatory Authority) of Supplier which relates directly to Supplied API supplied under this Agreement or which could impact on the ability to make or to continue to make and supply Supplied API under this Agreement.
3A. Diligence; Responsibility. Cempra shall use commercially reasonable efforts to develop and commercialize a Cempra Product in the Exclusive Territory, provided that the actions of Cempra’s Affiliates and their sublicensees, distributors and other commercial partners shall be deemed the acts of Cempra for purposes of satisfying Cempra’s obligations under this Section 3A.
4. Manufacturing Change Control. No changes to any methods, processes, procedures or testing protocols and/or methods governing the manufacture, storage, testing (in process, release, stability) of Supplied API to be supplied hereunder or in any manufacturing equipment, facilities, or site(s) related to the manufacture of Supplied API for Cempra shall be made (i) unless in accordance with GMP and (ii) without the appropriate notification and, if applicable, approval, of FDA. Supplier shall notify Cempra in the event of any such change but shall not be required to inform Cempra of the particulars of such change if such information is not part of the publicly available portion of the applicable drug master file. In addition, Supplier shall provide written notice to Cempra of any proposed
4
change in manufacturing site as soon as reasonably possible, but in any event at least [*] ([*]) [*] in advance of any such proposed change, provided that any such change shall not affect or limit Supplier’s obligation to comply with the Cempra Specifications or any Specifications or process requirements resulting from Required Manufacturing Changes.
5. Supply.
5.1 Supplier shall supply Supplied API to Cempra as further described in this Agreement.
5.2 Cempra may from time to time place Orders for Supplied API as more fully described below. No terms and conditions contained in any Order, acknowledgment, invoice, xxxx of lading, acceptance or other preprinted form issued by either party shall be effective to the extent they are inconsistent with or modify the terms and conditions contained herein.
5.3 Cempra shall, not less than [*] ([*]) days before the beginning of each Order Period, submit to Supplier Agent (i) its Order for the Supplied API to be delivered by Supplier to Cempra during that Order Period (if any) and (ii) a Forecast for the Forecast Period (including the Order Period covered by the Order (if any)), provided that, notwithstanding the foregoing, (a) Cempra shall not be required to order any Supplied API for any Order Period under this Agreement and (b) Forecasts shall not be binding obligations of Cempra (except to the extent Supplied API is ordered for the first Order Period thereof pursuant to an Order). Supplier Agent shall submit any Order or Forecast to Supplier within [*] ([*]) [*] of receipt and Supplier shall confirm to Cempra in writing receipt of such Order or Forecast within [*] ([*]) [*] of receipt from Supplier Agent. Supplier shall accept any amendment to an Order made by Cempra within [*] ([*]) [*] after such Order is given, provided, however, Supplier shall not be obligated to accept such amendment if quantities are increased to amounts that exceed the capacity required by Section 5.6 hereof. For any Order, Supplier shall deliver Supplied API no later than the end of the Order Period. An “Order Period” shall mean a [*] until such time as Cempra elects to make the Order Period a [*], which shall be no later than the date upon which [*]. A Forecast Period shall be the [*] ([*]) Order Periods following the Order Period covered by the applicable Order.
5.4 Cempra shall be entitled at its option to reject the whole or part of any delivery of Supplied API which does not comply with the Specifications, GMP, or applicable regulatory requirements of the United States and country of manufacture (including but not limited to those contained in any applicable Product Approvals). Supplier shall, as elected by Cempra in its reasonable discretion, immediately replace (without additional cost) or refund to Cempra the Price of any Supplied API which does not comply with the Specifications, the requirements of this Agreement, GMP, or applicable regulatory requirements of the United States and country of manufacture (including but not limited to those contained in Product Approvals). Cempra shall be deemed to have accepted any delivery of Supplied API unless it gives Supplier or Supplier Agent notice of its rejection within [*] ([*]) [*] of delivery. If and as requested by Supplier, Cempra shall return to Supplier Agent, at [*] cost, any Supplied API rejected properly in accordance with this Section 5, in which case [*] shall pay to [*] the actual cost incurred by [*] in effecting the return of such Supplied API.
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
5
5.5 If, with respect to any Supplied API which has been replaced and/or for which the Price therefor has been refunded is, following investigation, found by reasonable, independent, neutral, mutually agreeable third party analysis pursuant to generally-accepted scientific methods, to have complied with the Specifications, GMP, and all Applicable Laws (including but not limited to requirements described in the Product Approvals), Cempra shall:
(a) accept such Supplied API as part of the next order and, if no Order will be placed before the termination of this Agreement, pay Supplier Agent the applicable Price therefor, and
(b) refund any additional amount paid by [*] to [*] with respect thereto.
5.6 Supplier shall use commercially reasonable efforts to ensure that they have sufficient capacity to enable them to supply [*] percent ([*]%) of the quantity of Supplied API specified in each Forecast, and if Orders are placed for such increased quantities the Supplier will use best efforts to meet and fulfill Orders.
5.7 If Supplier determines that it will not be able to supply the respective Supplied API to Cempra in material satisfaction of the most recent Orders and/or Forecast, Supplier shall, within [*] ([*]) [*], notify Cempra in writing of such determination, which notice shall provide Cempra with the details on the extent of the expected shortfall of supply, the causes of such inability to supply, and a proposed solution to the problem. Upon such notice of a supply problem, without limiting any of the remedies available to Supplier and Cempra set forth in this Agreement, Cempra and the Supplier (and Supplier Agent) will immediately meet and work together, in good faith, to identify an appropriate resolution to the supply problem. Any agreed resolution to the supply problem will be set forth in a writing executed by all parties.
5.8 Neither Supplier nor Supplier Agent shall (i) engage any third party to perform any portion of its obligations under this Agreement, (ii) directly or indirectly market, sell, or distribute Supplied API, any Product, or any product incorporating Supplied API, including, without limitation, any other salt forms of Supplied API, to any Affiliate of either Supplier, Supplier Agent or any third party (including but not limited to consumers, end users, or commercial customers) in the Exclusive Territory, (iii) directly or indirectly supply Supplied API, any Product, or any product incorporating Supplied API including, without limitation, any other salt forms of Supplied API, to any Affiliate of either Supplier or Supplier Agent or any third party (including but not limited to consumers, end users, or commercial customers) for use, sale, or distribution in the Exclusive Territory, nor (iv) otherwise assist, directly or indirectly, any Affiliate of either Supplier or Supplier Agent or any third party in the manufacture, development, marketing, distribution, or sale of Products or products incorporating Supplied API in the Exclusive Territory, and neither Supplier nor Supplier Agent shall use commercially reasonable efforts to impose upon either Supplier’s or Supplier Agent’s Affiliates, distributors, customers, and other contractors, to the extent such relationships involve Supplied API, contractual obligations prohibiting the activities prohibited by (ii), (iii), and (iv) above, provided that Supplier shall be entitled to engage Supplier Agent and/or sell Supplied API to Supplier Agent as necessary to satisfy Supplier’s obligations under this Agreement. Cempra shall not purchase any Supplied API from any third party for any purposes of commercially selling Products in the Exclusive Territory, except as permitted by Section 5.9 of this Agreement. The restrictions set forth in clauses (ii), (iii) and (iv) above shall terminate in the event
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
6
that Cempra does not file with the FDA an NDA for the sale of the initial Cempra Product prior to [*]; provided that such delay was not caused in part or in whole by Supplier or Supplier Agent in which case clauses (ii), (iii) and (iv) shall remain in effect; provided, further, that if such delay was not caused in part or in whole by either Cempra, Supplier or Supplier Agent, Cempra and Supplier shall negotiate in good faith to extend the [*] to a reasonable future date.
5.9 Cempra shall have the right to secure a second source of Supplied API from a third party, and to purchase from such second source an amount Cempra reasonably determines is sufficient to sustain such third party as a viable second source, such amount not to exceed [*] percent ([*]%) of Cempra’s requirements for the respective Supplied API for the Cempra Product in any given calendar year. The foregoing restrictions and quantity limitation shall not apply to the extent Supplier is not able to adequately supply Cempra on a timely basis with Supplied API in conformance with GMP and the Specifications under the terms set forth herein.
5.10 Supplier Agent is acting solely as an agent to Supplier with respect to (i) the ordering and payment for the Product, (ii) for certain communications with the FDA and other Regulatory Authorities, and (iii) the receipt, handling and storage of Product received from Supplier prior to delivery to Cempra. Supplier Agent shall not be responsible for any obligation of Supplier except as specifically provided herein. Supplier Agent may be replaced or removed as Supplier Agent pursuant to this Agreement by Supplier only with the consent of Cempra.
6. Quality; Regulatory.
6.1 Supplier warrants that all Supplied API supplied pursuant to this Agreement shall (i) be manufactured in accordance with current GMP and (ii) comply with the Specifications and all Applicable Laws (including but not limited to FDA regulations and requirements included in the Product Approvals), provided that Specifications may be amended as (a) reasonably requested by Cempra and agreed upon by Supplier, such agreement not to be unreasonably withheld, or (b) necessary to conform such Specifications to the regulatory requirements necessary to obtain and maintain Product Approvals with respect to Cempra Products, including but not limited to the approval of alternative dosages or indications of Cempra Products. In the event any change in the Specifications materially increases or decreases the Manufacturing Costs, then the parties will meet and negotiate in good faith an increase or decrease in the Price.
6.2 For additions or changes to the Specifications or manufacturing processes that are required by GMP or Applicable Laws of the United States or other applicable Regulatory Authority (including but not limited to those requirements of the FDA and Product Approvals) (collectively, “Required Manufacturing Changes”), the parties shall cooperate in making such changes in a timely fashion and amending the Specifications to reflect such additions or changes. Either Cempra or Supplier shall be free to propose changes to the Specifications or manufacturing processes that are not Required Manufacturing Changes (collectively, “Discretionary Manufacturing Changes”). Any Discretionary Manufacturing Changes proposed by Cempra shall only be effective upon the approval of FDA (or other applicable Regulatory Authority) and Supplier’s written agreement to such changes and, such approval which shall not unreasonably be withheld, and the Supplier shall implement any such Discretionary Manufacturing Changes agreed upon in writing by the parties as soon as reasonably practicable. Any
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
7
Discretionary Manufacturing Changes proposed by Supplier shall only be effective upon the approval of FDA (or other applicable Regulatory Authority), provided that no Discretionary Manufacturing Changes affecting any (i) Specifications or process changes that are the result of Required Manufacturing Changes or (ii) Cempra Specifications may be effected unless approved by Cempra in advance and in writing, which approval shall not be unreasonably withheld. Notwithstanding the foregoing, the commercially reasonable, documented, direct costs, including, without limitation, obsolete raw materials, work-in-process, packaging and labeling materials (i) associated with implementing any Required Manufacturing Changes shall be born by [*] (ii) associated with implementing any Discretionary Manufacturing Changes shall be borne by [*]. In the event any Required Manufacturing Changes or Discretionary Manufacturing Changes materially increase or decrease the ongoing Manufacturing Costs, then the parties will negotiate in good faith an increase or decrease in the Price by an amount equal to the increase or decrease in Manufacturing Costs.
6.3 Supplier shall conduct quality control testing of every batch of Supplied API manufactured hereunder and provide Cempra with certificates of quality assurance and quality control analysis with respect to all deliveries of Supplied API, as customary in the pharmaceutical industry and in compliance with GMP and all applicable regulatory requirements of the United States (including those of the FDA) and other applicable Regulatory Authority, and with the manufacturing and export documents necessary for (i) import of the Supplied API and (ii) compliance with GMP and all Applicable Laws. Supplier shall keep complete, accurate and authentic accounts, notes, data and records of the work performed by it under this Agreement and shall maintain complete and adequate records pertaining to the methods and facilities used by it for the manufacture, processing, testing, packing, labeling, holding and distribution of Supplied API in accordance with the Applicable Laws and GMP. All raw data generated in the manufacturer, testing, and supply of Supplied API under this Agreement shall be maintained by the Supplier in a readily accessible manner for at least the longer of (i) [*] ([*]) [*] following the expiration date of the final Product into which the Supplied API is incorporated or (ii) such time as may be required by GMP or Applicable Law.
6.4 All facilities utilized by the Supplier to manufacture, store, or otherwise handle Supplied API manufactured for Cempra under this Agreement or utilized by Supplier Agent to store or otherwise handle Supplied API manufactured by Supplier for Cempra hereunder, or source intermediates therefore, shall comply with and satisfy all requirements under GMP and all Applicable Laws concerning the manufacture of Supplied API for use in pharmaceutical or biological products for human use (including but not limited to those of the FDA or included in the Product Approvals), including the maintenance of such standards or requirements sufficient to pass any inspection pursuant to such requirements. Without limiting the generality of the foregoing, the Supplier shall obtain and maintain all licenses, registrations, and other authorizations required to operate a GMP facility under Applicable Laws (including but not limited to those of the FDA). Supplier shall be responsible for all costs and fees related to obtaining and maintaining regulatory permits, certificates, approvals, or other authorizations required to manufacture Supplied API, and/or qualify any manufacturing sites for the manufacture of Supplied API, under this Agreement in compliance with all Applicable Laws (including but not limited to GMP and those of the FDA). Supplier shall comply with all Applicable Laws in performing their obligations hereunder, including but not limited to any applicable environmental, toxic or hazardous waste, or similar regulations.
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
8
6.5 Supplier will be responsible for any reporting of matters regarding the general manufacture of Supplied API, as applicable, to the FDA and other applicable Regulatory Authority in accordance with GMP and Applicable Laws and may utilize Supplier Agent to perform such responsibilities. Supplier or Supplier Agent shall notify Cempra of any such matter within [*] ([*]) [*] and furnish copies of such reports to Cempra within [*] ([*]) [*]. Supplier or Supplier Agent also shall advise Cempra of any material occurrence or new information which arises out of the Supplier’s manufacturing, testing (including in process, release or stability), or other activities which may reasonably be expected to have adverse regulatory compliance and/or reporting consequences concerning any Product. Supplier or Supplier Agent shall promptly (and in any event, within the time period reasonably necessary to enable Cempra to comply with GMP and any Applicable Laws with respect to the clinical use or commercial sale of Products for human therapeutic use) report any adverse events, customer complaints, trend analysis, or other circumstances of which either Supplier or Supplier Agent becomes aware that may relate to the safety or efficacy of any Products.
6.6 Supplier shall, subject to this paragraph, be responsible for handling and responding to any appropriate FDA (or other applicable) Regulatory Authority inspections with respect to manufacturing of Supplied API during the term of this Agreement and may utilize Supplier Agent to perform such responsibilities. Supplier or Supplier Agent shall promptly provide to Cempra copies of any (i) request or inquiry made by FDA (or other applicable Regulatory Authority) with respect to Supplied API or its manufacture, testing, or storage for Cempra under this Agreement and (ii) any response thereto or information provided in response with respect to the foregoing by either Supplier or Supplier Agent, provided that, when reasonably practicable, Cempra shall be provided an opportunity to review and comment on any and all such responses reasonably in advance of their submission by either Supplier or Supplier Agent to FDA (or other applicable Regulatory Authority), provided that final discretion with respect to any such response remains with Supplier. Supplier and Supplier Agent shall use best efforts to promptly (but in any event within [*] ([*]) [*]) (i) advise Cempra of any requests by FDA (or other applicable Regulatory Authority) for any inspections with respect to the manufacturing of Supplied API under this Agreement and (ii) provide Cempra with copies of any correspondence related thereto.
6.7 Supplier certifies that they have not and covenant that they will not use in any capacity in connection with the manufacture of Supplied API under this Agreement the services of any person, including any firm or individual, debarred or subject to debarment under Applicable Laws (including but not limited to GMP or those of the FDA). Supplier and Supplier Agent agree to notify Cempra immediately in the event any person providing services to either Supplier or Supplier Agent under this Agreement is debarred or becomes subject to debarment.
6.8 Supplier shall retain a reasonably sufficient quantity of each batch of Supplied API to perform quality control testing and representative batches of Supplied API will be placed on stability testing as described in the current drug master file for Supplied API and as necessary to comply with GMP and FDA requirements and other Applicable Law. Supplier shall maintain samples of each batch in a reasonably suitable storage facility until at least the later of (i) the [*] ([*]) [*] or (iii) such longer period as may be required under the Specifications, GMP, and/or Applicable Laws (including but not limited to those of the FDA and the Product Approvals). Portions of all such samples and representative batches shall be made reasonably available (i) for testing by Cempra upon request and,
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
9
upon Cempra’s prior written consent, such consent not to be unreasonably withheld, (ii) to FDA and other applicable Regulatory Authority. Samples of any samples provided to FDA and other applicable Regulatory Authority shall be retained by the Supplier in quantities reasonably sufficient to allow quadruplicate replicatory testing by Cempra.
6.9 Supplier shall maintain all records as are necessary to comply with all Applicable Laws of the United States, including but not limited to all records reasonably necessary to support all GMP guidelines and requirements. All such records shall be available for inspection, audit and copying by Cempra, its representatives, and FDA upon reasonable request during normal business hours. All such records shall be maintained for a period as required under GMP and the Applicable Laws of the United States, provided that all records relating to the manufacture, stability and quality control of each batch of Supplied API shall be retained at least until the later of (i) the [*] ([*]) [*], or (ii) such longer period as may be required under the Specifications, GMP, and all Applicable Laws (including but not limited to those of the FDA and the Product Approvals).
6.10 Supplier and Supplier Agent agree to notify Cempra forthwith of its knowledge or receipt of notice of the initiation of any inquiries, notices or inspection activity by FDA (or other applicable Regulatory Authority) with respect to Supplied API and shall provide Cempra with a reasonable description of any such inquiries and documentation (including but not limited to any FDA Establishment Inspection Report Form 483 or FDA warning letter or equivalent if a Regulatory Authority other than the FDA) not later than five (5) working days after such visit or inquiry.
6.11 Supplier and Supplier Agent shall reasonably assist Cempra in the finalization of the chemistry, manufacturing, and controls portion of any and all regulatory filings or correspondence (including but not limited to Product Approvals or applications therefor), as requested by Cempra in conjunction with its preparation and submission of such materials with respect to any Products. Supplier shall provide Cempra with all manufacturing procedures, controls for active and inactive ingredients and finished dosage forms, chemistry and stability information, and any other information to the extent or in a manner reasonably necessary for the preparation of Product Approvals.
6.12 The Supplier and Cempra shall negotiate in good faith and execute a quality agreement within ninety days after the date hereof concerning additional detailed logistical and regulatory procedures, and allocating responsibility for specific regulatory requirements, between the parties, which agreement shall be reasonable and consistent with industry standards.
7. Price; Payment.
7.1 The Price of Supplied API supplied hereunder shall be as follows (pro rated for any portions thereof):
| [*] | [*] | |
| [*] | [*] | |
| [*] | [*] |
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
10
For purposes of determining the pricing tier above, purchases of all Supplied API and Fusidic Acid in any [*] shall be combined. On the date that is [*] ([*]) [*] from the date of [*], and on each subsequent [*] of such date during the term of this Agreement, Supplier and Cempra will revisit the pricing structure set forth above to reflect the current market conditions and costs to manufacture Supplied API and to consider the impact of any generic competition to the Product; provided that in no case shall any increase to the then current [*] exceed the [*] of (i) [*]; provided, that if [*], and (ii) [*]. For purposes of the foregoing price adjustment mechanism, the [*] shall mean the [*] ([*]) [*] period prior to the [*]. Except as provided in this Agreement, the Price shall not be increased or decreased without the written consent of Supplier and Cempra.
Cempra shall pay Supplier Agent the applicable Price for all Supplied API delivered to it within [*] ([*]) [*] of Cempra’s receipt of (i) conforming Supplied API delivered in accordance with this Agreement and (ii) a detailed written invoice with respect to such Supplied API. In the event that Cempra rejects any Supplied API pursuant to Section 5 hereof, Cempra shall pay as provided in the previous sentence for all Supplied API in such shipment that was not rejected.
7.2 Any payment by Cempra to Supplier Agent of the full price for any delivered Supplied API shall satisfy any and all payment obligations with respect to Supplier. Supplier shall have no recourse against Cempra with respect to payment for any supply of Supplied API under this Agreement. Cempra shall not be liable for, and Supplier Agent shall indemnify and hold Cempra harmless for, any failure of Supplier to receive payment from Supplier Agent for the supply of Supplied API under this Agreement.
8. Delivery, Title and Risk.
8.1 Delivery of the Supplied API shall be effected CIF (Incoterms) the location specified by Cempra in each Order, at which time all risk of loss and damage to the Supplied API shall pass to Cempra, provided that the Supplier or Supplier Agent shall carry out all customs and export clearances necessary for the shipment, export, and import of Supplied API out of and/or into any jurisdiction and obtain, at their own expense, any export or import license or other governmental authority required for exportation and/or importation into and/or out of any jurisdiction.
8.2 Prior to release or shipment to Cempra, the Supplier shall perform release testing, consistent with industry standards, pursuant to the Specifications, GMP, and all Applicable Laws (including but not limited to those of the FDA and the Product Approvals).
8.3 If Cempra refuses in writing to take delivery of conforming Supplied API, ordered by Cempra and delivered on time, at the time stated for delivery, Supplier or Supplier Agent shall be entitled, at their discretion, to store Supplied API at Cempra’s cost, which shall be commercially reasonable, and include insurance with coverage in amounts and types reasonably sufficient to cover the loss of such Supplied API.
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
11
9. Product Recall. Cempra shall provide prior written notice to Supplier and Supplier Agent of any Recall planned by Cempra with respect to Products incorporating the Supplied API supplied hereunder. Neither Supplier nor Supplier Agent shall be entitled to effect any Recall with respect Cempra Products without Cempra’s prior written consent. Supplier and Supplier Agent shall reasonably cooperate with Cempra in connection with any Recall.
10. Manufacturing Rights and Regulatory Assistance. Supplier and Supplier Agent shall, as reasonably requested by Cempra, at Cempra’s expense, assist Cempra’s efforts to maintain and/or obtain Product Approvals for products incorporating Supplied API, including but not limited to such approvals as may be necessary for marketing and selling products incorporating Supplied API (1) in additional formulations or dosage strengths or (2) for additional indications, and otherwise provide such information, data, materials, documents, and assistance as may be requested by Cempra in order to satisfy any Regulatory Authority’s request, respond to any inquiry, audit, or correspondence from any Regulatory Authority, or otherwise seek, maintain, or support any Product Approval or application, amendment, or supplement with respect thereto.
11. Inspections; Audit. In order for Cempra to determine whether the Supplier is operating in accordance with the provisions of this Agreement and for Cempra to ensure the adequacy of its supply of Supplied API, the Supplier and Supplier Agent agree to allow Cempra or an agent or designee of Cempra, upon reasonable prior notice and at Cempra’s expense, to periodically inspect the Supplier’s or Supplier Agent’s respective facility(ies), technical, quality assurance and quality control records, and associated business functions relating specifically to the supply of Supplied API to be provided pursuant to this Agreement, during normal business hours, subject to Section 13 of this Agreement.
12. Technical Representatives. Each party shall designate a suitably skilled technical representative, who shall be available for consultation and discussion with respect to regulatory compliance, QA/QC processes and procedures, manufacturing issues, and technology transfer, including but not limited to as needed to enable and assist (i) in enabling and qualifying additional sites/suppliers for the manufacture of Supplied API in accordance with all legal and regulatory requirements, as may be required pursuant to Section 10.1, or (ii) Cempra’s efforts to maintain and/or obtain Product Approvals for Products, including but not limited to such approvals as may be necessary for marketing and selling Products (1) in additional formulations or dosage strengths or (2) for additional indications.
13. Confidential Information.
13.1 All technical, business, regulatory, and marketing information of either Supplier, Supplier Agent or Cempra provided by one party (in such a case the “Disclosing Party”) to another party (the “Receiving Party”), including but not limited to that information of Cempra concerning Supplied API, Products, Cempra Intellectual Property, and all information generated in the course of the Supplier’s and Supplier Agent’s performance under this Agreement or which otherwise concerns Cempra Intellectual Property or Cempra Products, shall be deemed to be “Confidential Information”. Any Receiving Party agrees to treat any Confidential Information as such, according the Confidential Information the same protections as their own proprietary and confidential information of a similar nature, which shall be no less than reasonable level of such protection. Any Receiving Party agrees that they shall not disclose Confidential Information to any third party or use any such Confidential Information for any purpose other than for the purposes of fulfilling its obligations under this Agreement.
12
13.2 Nothing in this Section 13 shall be construed to prevent a Receiving Party from:
(a) disclosing Confidential Information to a Regulatory Authority as necessary in connection with its obligations under this Agreement; or
(b) disclosing such information as is required by law or judicial order to be disclosed;
provided that, in either case such Receiving Party (i) provides advance written notice of such disclosure as soon as reasonably practicable, (ii) assists Disclosing Party, as reasonably requested by Disclosing Party, in seeking or obtaining confidential or protective treatment of such information, and (iii) minimizes the extent of such disclosure to the extent legally permissible.
13.3 Each party’s confidentiality obligations of this Section 13 shall not extend to information which:
(a) is or becomes known to the public through no fault or action by any Receiving Party; or
(b) is disclosed to any Receiving Party without restriction on disclosure by a third party not under an obligation of secrecy to Disclosing Party.
13.4 Each party acknowledges and agrees that (i) their obligations under this Section 13 are necessary and reasonable to protect Disclosing Party and their business, (ii) any violation of these provisions could cause irreparable injury to Disclosing Party for which money damages would be inadequate, and (iii) Disclosing Party shall be entitled to injunctive relief against the threatened breach of the provisions of this Section 13 without the necessity of proving actual damages. The parties agree to cooperate with respect to requests for confidential treatment to be submitted to any securities exchange with respect to certain portions of this Agreement.
14. Intellectual Property. All materials, documents, information, and deliverables of any kind supplied to Cempra from either Supplier or Supplier Agent—other than any drug master file, which shall remain the property of Supplier—or generated by either Supplier or Supplier Agent as a result of the services performed hereunder or access to or knowledge of Cempra Confidential Information shall be the sole and exclusive property of Cempra.
15. Termination by Supplier.
15.1 If Cempra should materially breach this Agreement, Supplier shall have the right to terminate this Agreement by written notice to Cempra (given within [*] ([*]) [*] of the initial notice of breach) if Cempra has failed to cure any such breach within [*] ([*]) [*] of Cempra’s receipt of written notice from the Supplier describing such breach.
15.2 Supplier shall have the right to cancel and terminate this Agreement immediately by written notice to the Cempra in the event Cempra is generally unable to meet its debts when due, or makes a general assignment for the benefit of its creditors, or there shall have been appointed a receiver,
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
13
trustee or other custodian for Cempra for or a substantial part of its assets, or any case or proceeding shall have been commenced or other action taken by or against Cempra in bankruptcy or seeking the reorganization, liquidation, dissolution or winding-up of Cempra or any other relief under any bankruptcy, insolvency, reorganization or other similar act or law, and any such event shall have continued for [*] ([*]) [*] undismissed, unstayed, unbonded and undischarged.
16. Termination by Cempra.
16.1 If either Supplier or Supplier Agent should materially breach this Agreement, Cempra shall have the right to terminate this Agreement by written notice to Supplier and Supplier Agent (given within [*] ([*]) [*] of the initial notice of breach) if the breaching Supplier has failed to cure any such breach within [*] ([*]) [*] of Supplier’s receipt of written notice from Cempra describing such breach.
16.2 In the event (i) Cempra determines, in its discretion, to cease development of Cempra Products prior to FDA approval thereof (e.g. due to financial, health, safety, or other business reasons) or (ii) FDA approval of the marketing and sale of the initial Cempra Product is not obtained by [*].
16.3 Cempra shall have the right to cancel and terminate this Agreement immediately by written notice to the Supplier and Supplier Agent in the event either Supplier or Supplier Agent is generally unable to meet its debts when due, or makes a general assignment for the benefit of its creditors, or there shall have been appointed a receiver, trustee or other custodian for either Supplier or Supplier Agent for or a substantial part of their assets, or any case or proceeding shall have been commenced or other action taken by or against either Supplier or Supplier Agent in bankruptcy or seeking the reorganization, liquidation, dissolution or winding-up of such Supplier or Supplier Agent or any other relief under any bankruptcy, insolvency, reorganization or other similar act or law, and any such event shall have continued for [*] ([*]) [*] undismissed, unstayed, unbonded and undischarged.
17. Effects of Termination.
17.1 In the event of termination of this Agreement, Cempra shall have no further obligation to purchase Supplied API from the Supplier and Supplier Agent, provided that (i) Cempra shall, except in the event of a termination pursuant to Section 16.1, (a) be obligated to purchase, and Supplier shall be obligated to deliver, any Supplied API ordered by Cempra prior to the date of termination and (b) Cempra shall reimburse Supplier Agent for the reasonable, documented, noncancelable cost of any raw materials reasonably purchased by Supplier for purposes of fulfilling Cempra’s reasonably anticipated future Orders of Supplied API to the extent expressed in the most recent Forecast, except to the extent such raw materials may reasonably be used for Supplier’s business activities beyond the performance of this Agreement, (ii) Cempra shall, in the event of a termination pursuant to Section 16.1, have the option, upon written notice to Supplier and Supplier Agent given in Cempra’s sole discretion, to cause the Supplier to satisfy any Orders to the extent outstanding and unfulfilled as of the effective date of such termination (provided that Cempra’s payment obligations—and Supplier’s obligations with respect to nonconforming or defective Supplied API—shall apply with respect to such Orders).
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
14
17.2 Any termination or cancellation under any provision of this Agreement shall not relieve Cempra of its obligation to pay any amounts due or owing at the time of such cancellation, expiration, or termination.
17.3 In the event of termination of this Agreement by Cempra pursuant to Section 16.1 or 16.3 in connection with Supplier’s inability or refusal to manufacture or supply Supplied API to Cempra, including, but not limited to, in the case of a sale or assignment of the assets related to the production of Supplied API to a third party, Supplier or such third party assignee shall provide to Cempra reasonable technical assistance and documentation to enable a third party to manufacture and supply Supplied API to Cempra, including for such purpose, but not limited to, the transfer of the techniques, methods, materials and processes required to produce Fusidic Acid.
18. Waiver. It is agreed that no waiver by either party hereto of any breach or default of any of the covenants or agreements herein set forth shall be deemed a waiver as to any subsequent and/or similar breach or default.
19. Assignments. No party may without written approval of the other parties, such approval not to be unreasonably withheld, assign this Agreement or transfer its interest or any part thereof under this Agreement to any third party, provided that Cempra shall be entitled, without the Supplier’s or Supplier Agent’s prior written consent, to assign this Agreement to any affiliate of Cempra or to any other third party in the event of Cempra’s merger, sale, consolidation, reorganization, or sale or transfer of the portion of its business or assets relating to Products.
20. Insurance.
20.1 Cempra. Prior to administering any Product incorporating any Supplied API supplied hereunder to human subjects, Cempra shall obtain comprehensive general liability insurance and clinical trials insurance coverage with reputable and financially secure insurance carrier(s) covering, in a commercially reasonable fashion, such risks as are reasonably appropriate to sound business judgment and Cempra’s obligations and activities contemplated by this Agreement. At a Supplier’s written request, Cempra shall furnish a Certificate of Insurance evidencing such coverage.
20.2 Suppliers. Each of Supplier and Supplier Agent shall maintain comprehensive general liability insurance and products liability insurance coverage with reputable and financially secure insurance carrier(s) covering, in a commercially reasonable fashion, such risks as are reasonably appropriate to sound business judgment and their obligations and activities contemplated by this Agreement. At Cempra’s written request, each of Supplier and Supplier Agent shall furnish a Certificate of Insurance evidencing such coverage.
21. Independent Contractors. It is understood that the parties hereto are independent contractors and engage in the operation of their own respective businesses and no party is to be considered the agent of the other party for any purpose whatsoever and no party has any authority to enter into any contract or assume any obligation for the other parties or to make any warranty or representation on behalf of the other party(ies). Each party shall be fully responsible for its own employees and consultants, and the employees of one party shall not be deemed to be employees of either other party for any purpose whatsoever.
15
22. Representations and Warranties. Each party (the “Representing Party”) hereby represents and warrants to the other parties that as of the Effective Date and any period otherwise indicated below, the following statements are true and correct:
(a) It is duly organized and validly existing under the laws of the jurisdiction of its formation noted in the preamble, and has the power and authority to enter into and perform this Agreement.
(b) All corporate action on the part of the Representing Party necessary for the authorization, execution and delivery of this Agreement and for the performance of all of its obligations hereunder has been taken, and this Agreement, when fully executed and delivered, shall constitute a valid, legally binding and enforceable obligation of the Representing Party.
(c) No consent, authorization, license, permit, registration or approval of, or exemption or other action by, any governmental authority, or any other third party, is required in connection with the Representing Party’s execution, delivery and performance of this Agreement or, if any such consent is required, the Representing Party has satisfied any applicable requirements.
(d) There are no outstanding written or oral agreements binding on the Representing Party or its assets that conflict with this Agreement or restrict it from entering into this Agreement. This Agreement is enforceable against such party in accordance with the terms of this Agreement, subject to the effect of bankruptcy, insolvency, or similar laws affecting the rights and remedies of creditors generally and the effects of general principles of equity (whether applied by a court of law or equity) and the effect of public policy. There are no actions, suits or proceedings pending or, to the Representing Party’s knowledge, threatened, against the Representing Party before any governmental authority which question its right to enter into or perform this Agreement, or which question the validity of this Agreement.
23. Miscellaneous.
23.1 Governing Law; Dispute Resolution. The validity, construction and enforceability of this Agreement and the resolution of disputes arising out of and relating to this Agreement and all related agreements, collectively or separately, shall be governed by and construed in accordance with the laws of the State of New York without regard to conflicts of laws or choice of law provisions thereof and without regard to the United Nations Convention on Contracts for the International Sale of Goods. The parties shall attempt to resolve all disputes between the parties arising out of or relating to this Agreement amicably through good faith discussions upon the written request of any party. In the event that any such dispute cannot be resolved thereby within a period of [*] ([*]) [*] after such notice has been given (the last day of such [*] ([*]) [*] period being herein referred to as the “Arbitration Date”), such dispute shall be finally settled by binding arbitration in New York, New York, and using the English language in accordance with the JAMS International Arbitration Rules then in effect (the “Rules”). Arbitration shall be commenced by filing a Request for Arbitration in accordance with the Rules within [*] ([*]) [*] of the Arbitration Date. A party’s failure to timely file a Request for Arbitration in accordance with this Section 23.1 shall constitute a waiver and release of the claim or dispute at issue. Following the filing of the Request for Arbitration, the parties shall attempt to mutually agree on one or
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
16
more commercial arbitrator(s) with substantial experience in resolving complex commercial contract disputes in the pharmaceutical industry, who may or may not be selected from the appropriate list of JAMS arbitrators. If the parties cannot agree upon the number and identity of the arbitrators within [*] ([*]) [*] following the filing of the Request for Arbitration, then a single arbitrator shall be selected on an expedited basis in accordance with the Rules, provided that any arbitrator so selected shall have substantial experience in resolving complex commercial contract disputes in the pharmaceutical industry. The arbitrator(s) shall have the authority to grant specific performance and to allocate between the parties the costs of arbitration (including service fees, arbitrator fees and all other fees related to the arbitration) in such equitable manner as the arbitrator(s) may determine. The prevailing party in the arbitration shall be entitled to receive reimbursement of its reasonable expenses (including reasonable attorneys’ fees, expert witness fees and all other expenses) incurred in connection therewith. Judgment upon the award so rendered may be entered in a court having jurisdiction or application may be made to such court for judicial acceptance of any award and an order of enforcement, as the case may be. Notwithstanding the foregoing, each party shall have the right to institute an action in a court of proper jurisdiction for preliminary injunctive relief pending a final decision by the arbitrator(s), provided that a permanent injunction and damages shall only be awarded by the arbitrator(s). In any action or proceeding to enforce rights under this Agreement, the prevailing party shall be entitled to recover costs and attorneys’ fees. For all purposes of this Section 23.1, the parties consent to exclusive jurisdiction and venue in the United States federal courts located in New York, New York. For the avoidance of doubt, the validity, construction, and enforceability of this Agreement and the resolution of disputes arising out of and relating to this Agreement and any related agreements, collectively or separately, shall be governed solely by this Section 23.1.
23.2 Notices. Any and all notices given under this Agreement shall be in writing and to the respective parties at the following addresses by confirmed facsimile or express courier:
If to Ercros:
Ercros S.A.
Xxxxx xxx Xxxxxxx, x/x
00000 Xxxxxxxx (Xxxxxx)
Xxxxx
If to Gyma:
Gyma Laboratories of America, Inc.
000 Xxxxxxxxx Xxxx Xxxx
Xxxxxxxx, Xxx Xxxx 00000
XXX
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |
17
If to Cempra:
CEM-102 Pharmaceuticals, Inc.
Building Four Quadrangle
0000 Xxxxxxxxxx Xxxxx, Xxxxx 000
Xxxxxx Xxxx, Xxxxx Xxxxxxxx 00000
XXX
Attn: Chief Executive Officer
Fax: (000) 000-0000
or to such other addresses as may be subsequently furnished by one party to the other in writing. Any such notice if given by facsimile or express courier shall be deemed to have been given on the day following the dispatch.
23.3 Severability. In the event one or more terms of this Agreement are declared by any individual or competent authority to be void, voidable, illegal or otherwise unenforceable, the remaining provisions of this Agreement shall remain in full force and effect. The invalid or unenforceable part or provision shall be replaced with a provision which accomplishes, to the extent possible, the original business purpose of such invalid or unenforceable part or provision in a commercially reasonable, valid and enforceable manner, and the remainder of this Agreement shall remain binding upon the parties hereto. The parties hereto shall negotiate in good faith to modify this Agreement, but only to the extent necessary to make the terms of this Agreement valid and enforceable, having full regard for all applicable laws and the intent and purposes of the parties entering into this Agreement.
23.4 Complete Agreement. This Agreement, including the Exhibits, whether appended at the time of execution of this Agreement or later, as provided herein constitutes the entire Agreement between parties hereto relating to the subject matter hereof, and this Agreement may not be varied except by an instrument in writing signed by each party hereto by a duly authorized officer or representative; provided, that, Cempra and Supplier may amend or waive any portion of the Agreement without the consent of Supplier Agent so long as such amendment or waiver does not modify the obligations and responsibilities of Supplier Agent.
23.5 Force Majeure. Neither party shall be liable in damages for, nor shall this Agreement be terminable or cancelable by reason of, any delay or default in such party’s performance hereunder if such default or delay is caused by events beyond such party’s reasonable control including, but not limited to, acts of God, any regulation or law imposed following the Effective Date or other action or failure to act of any government or agency thereof, war or insurrection, civil commotion, destruction of production facilities or materials by earthquake, fire, flood or storm, labor strikes, epidemic, or failure of public utilities or common carriers; provided however, that the party seeking relief hereunder shall immediately notify the other party of such cause(s) beyond such party’s reasonable control. The party which may invoke this Section 23.5 shall use all reasonable endeavors to reinstate its ongoing obligations to the other. If the cause(s) shall continue unabated for sixty (60) days then both parties shall meet to discuss and negotiate in good faith what modifications to this Agreement, if any, should result from this force majeure.
18
23.6 Counterparts. This Agreement may be executed in one or more counterparts, all of which shall comprise the original instrument
23.7 Survival. The provisions of Sections 1, 3, 5.4, 5.5, 5.6, 5.7, 6.1, 6.3, 6.4, 6.5, 6.6, 6.8, 6.9, 6.10, 7.2, 9, 11, 12, 13, 14, 17, 18, 19, 21, and 23 shall survive the expiration or termination of this Agreement.
23.8 Publicity. Save as required by law, no public announcement or circular in connection with the subject matter of this Agreement shall be made by or on behalf of any party thereto without the prior written approval of the other parties, such approval not to be unreasonably withheld.
[Signature page to follow.]
19
IN WITNESS WHEREOF, Ercros, Gyma, and Cempra have executed this Manufacturing Agreement by their respective officers hereunto duly authorized, the day and year first above written.
| ERCROS S.A. | CEM-102 PHARMACEUTICALS, INC. | |||||||
| By: | /s/ M. Xxxxxx Xxxxxxx | By: | /s/ Xxxxxxxxxxx Xxxxxxxxx PhD | |||||
| Name: | M. Xxxxxx Xxxxxxx | Name: | Xxxxxxxxxxx Xxxxxxxxx PhD | |||||
| Title: | Manager | Title: | President & CEO |
| GYMA LABORATORIES OF AMERICA, INC. | ||||
| By: | /s/ Xxx Xxxxxx | |||
| Name: | Xxx Xxxxxx | |||
| Title: | President |
Signature page to Supply Agreement
Exhibit A
Cempra Specifications
[NOTE: To include particle size to be determined following signing.]
Sodium Fusidate
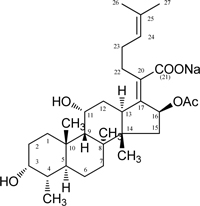
Chemical Formula: C31H47 NaO6
Molecular Weight: 538.69
[NOTE: Additional specifications to be attached.]
Fusidic Acid
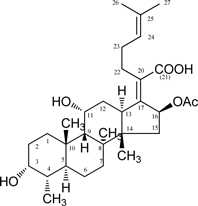
Chemical Formula: C31H48 O6
Molecular Weight: 516.71
[*]
[NOTE: Additional specifications to be attached.]
| [*] | Confidential treatment requested; certain information omitted and filed separately with the SEC. |

