EXCLUSIVE LICENSE, DEVELOPMENT, AND COMMERCIALIZATION AGREEMENT
Exhibit 10.4
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
EXCLUSIVE LICENSE, DEVELOPMENT, AND
COMMERCIALIZATION AGREEMENT
COMMERCIALIZATION AGREEMENT
This Exclusive License, Development, and Commercialization Agreement (the
“Agreement”) is entered into as of August 30, 2007 (the “Effective Date”) by and between
Aradigm Corporation, a California corporation having its principal office at 0000 Xxxxx
Xxxx Xxx, Xxxxxxx, Xxxxxxxxxx 00000 (“Aradigm”), and Lung Rx, Inc., a wholly-owned
subsidiary of United Therapeutics Corporation, a Delaware corporation, having an address of 0000
Xxxxxx Xxxxxx, Xxxxxx Xxxxxx, Xxxxxxxx 00000 (“Lung Rx”). Aradigm and Lung Rx may be referred to
individually as a “Party”, and collectively as the “Parties”.
Recitals
Whereas, Aradigm possesses certain proprietary rights related to a pulmonary drug
delivery technology known as AERx® technology, as well as expertise and know-how relating to the
use and manufacture of such technology; and
Whereas, Lung Rx possesses certain proprietary rights related to the compound known
as treprostinil, as well as expertise and know-how relating to the use and manufacture of such
compound;
Whereas, Lung Rx wishes to obtain, and Aradigm is willing to grant to Lung Rx, an
exclusive license throughout the universe to develop and commercialize products comprising
treprostinil incorporated into the AERx pulmonary drug delivery system, and improvements thereto,
including new formulations, subject to the terms and conditions set forth herein.
Now, Therefore, in consideration of the foregoing premises and the mutual covenants
contained herein and other good and valuable consideration, the receipt and sufficiency of which
are hereby acknowledged, the Parties agree as follows:
ARTICLE 1
Definitions
1.1 “Affiliate” means, with respect to a Party, any company or other entity controlled by,
controlling, or under common control with such Party. As used in this definition, the term
“controlling” a particular entity (with correlative meanings for the terms “controlled by” and
“under common control with”) means that the applicable Party owns, directly or indirectly, stock of
the subject entity representing more than 50% of the voting power for electing Board (or similar
management) members, or otherwise has the actual power to direct and control the management and
affairs of such entity, whether by contract or otherwise.
1.2 “Aradigm FTE Rate” means the fully burdened cost (defined as overhead, salaries, bonuses
and related payroll taxes and the cost of direct benefits) to Aradigm of one full-time equivalent
employee performing Development activities hereunder, which for clarity may
vary based on the
duties and experience of such employee. The Aradigm FTE Rate for each position will be fixed for
[*]. Thereafter the Parties will renegotiate the Aradigm FTE Rate for each position; provided
however, the overhead for each position shall be no greater than [*]. Furthermore, if during any
calendar year greater than [*] full-time equivalent employees are required to perform Development
activities and it is anticipated to continue for more than [*] of the following year, the Parties
will renegotiate the Aradigm FTE Rate for each position. If Aradigm reduces its total full-time
equivalent employees such that it would impact the percentage of the total full-time equivalent
employees performing Development activities, then Aradigm shall provide Lung Rx sixty (60) days
advance written notice of such reduction. The methodology to be used in calculating the fully
burdened cost referred to above shall be as elaborated in Exhibit A of this Agreement.
1.3 “Aradigm Improvements” means any and all improvements to the Aradigm Technology for use in
the Field, whether or not patentable, including intellectual property rights, improvements,
modifications, variations, revisions, versions and derivatives, as well as all data, know-how,
methods, Information, processes, machines, or compositions of matter pertaining to the Aradigm
Technology, which Aradigm may conceive, discover, Develop, acquire or Commercialize at any time
before or after the Effective Date of this Agreement and during the term hereof, and which Aradigm
Controls.
1.4 “Aradigm Know-How” means all Information (other than Patents and whether or not
patentable) that (a) is Controlled by Aradigm or any of its Affiliates as of the Effective Date or
during the Term and (b) relates directly to the manufacture, use, sale, or importation of the
Product and is necessary or useful for the research, Development, regulatory approval, manufacture,
or Commercialization of Products, or otherwise useful or necessary for Lung Rx to practice the
Aradigm Technology.
1.5 “Aradigm Patents” means all Patents that (a) include one or more claims that cover a
Product or the manufacture, importation, use, offer for sale or sale of such Product and (b) are
Controlled by Aradigm or any of its Affiliates as of the Effective Date or during the Term. The
Aradigm Patents include those Patents set forth on Exhibit B of this Agreement. To the
extent that any Patent or Improvement Controlled by Aradigm is necessary to practice any Patent as
defined above, then such necessary Patent or Improvement will be included as Aradigm Patents.
1.6 “Aradigm Technology” means, only if it relates to the AERx pulmonary drug delivery system
and collectively, the (i) Aradigm Patents, (ii) Aradigm Know-How, (iii) Aradigm New Intellectual
Property, (iv) all Information relating to the Aradigm Patents, Aradigm Know-How, and Aradigm New
Intellectual Property in the course of any work conducted pursuant to this Agreement, and (v)
Aradigm Improvements.
1.7 “Commercialization” means the marketing, promotion, sale or distribution of the Product.
“Commercialize” has a correlative meaning.
1.8 “Commercially Reasonable Efforts” means, with respect to the efforts of a particular Party
to complete specific tasks or obligations under this Agreement, the efforts and resources that
would be used, consistent with prevailing biopharmaceutical industry standards,
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
2
generally by
companies of similar size and profitability as such Party with respect to products or potential
products at a similar stage in development or product life and of similar market potential.
1.9 “Confidential Information” has the meaning provided in Section 10.1.
1.10 “Control” means, with respect to any Improvements, Information, Patent, know-how or other
intellectual property right, that the applicable Party exclusively owns or has a license to such
Information or intellectual property right and has the ability to grant to the other Party access
to and a license (or sublicense, as applicable) to same without violating the terms of any
agreement with a Third Party.
1.11 “Development” means all activities that relate to (a) obtaining, maintaining or expanding
a Regulatory Approval for the Product or (b) developing the ability to manufacture clinical and
commercial quantities of the Product. “Develop” has a correlative meaning.
1.12 “Development Plan” has the meaning set forth in Section 4.2(a).
1.13 “Development Timeline” has the meaning set forth in Section 4.2(a).
1.14 “FDA” means the U.S. Food and Drug Administration, or any successor agency thereto.
1.15 “Field” means pulmonary delivery of prostacyclin drugs for the treatment of pulmonary
arterial hypertension and any and all other therapeutic indications and uses other than control of
glucose levels in humans.
1.16 “First Commercial Sale” means, with respect to any Product, the first sale for use or
consumption by the general public of such Product following Regulatory Approval on a country by
country basis. A transfer of the Product by Lung Rx, its Affiliates or its sublicensees (i) for
research and Development purposes, or (ii) prior to Lung Rx’s receipt of Regulatory Approval for
use of such Product in humans, shall not be considered a First Commercial Sale.
1.17 “IND” means an Investigational New Drug Application filed with the FDA, or the equivalent
application or filing filed with any equivalent agency or governmental authority outside the U.S.
(including any supra-national agency such as in the European Union) necessary to commence human
clinical trials in such jurisdiction.
1.18 “Information” means all tangible and intangible (a) techniques, technology, practices,
procedures, methods, processes, protocols, formulations, formulae, knowledge, trade secrets,
inventions (whether patentable or not), methods, knowledge, ideas, concepts, know-how, skill,
experience, test data and results of any type whatsoever (including pharmacological, biological,
toxicological, clinical and pre-clinical test data and results), analytical and quality control
data, specifications, results or descriptions, reports, analyses, software and algorithms and the
like, (b) compositions of matter, cells, cell lines, assays, animal models and physical, biological
or chemical material and results of experimentation, (c) the Regulatory Dossier related
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
3
to the
Aradigm Technology, and (d) all intellectual property rights in those items described in (a)
through (c) of this Section 1.18.
1.19 “Licensed Molecule” means the compound known as treprostinil, having the structure set
forth in Exhibit C of the Agreement, or any of its pharmaceutically acceptable salts or
stereoisomers.
1.20 “Lung Rx Know-How” means all Information (other than Patents and whether or not
patentable) that (a) is Controlled by Lung Rx or any of its Affiliates as of the Effective Date or
during the Term and (b) relates directly to the manufacture, use, sale, or importation of the
Licensed Molecule or the Product and is necessary or useful for the research, Development,
regulatory approval, manufacture, or Commercialization of the Product, or otherwise useful or
necessary for Aradigm to fulfill its obligations for the Development of the Product.
1.21 “Lung Rx Patents” means all Patents that (a) include one or more claims that cover the
Licensed Molecule or the Product or the manufacture, importation, use, offer for sale or sale of
Licensed Molecule or the Product and (b) are Controlled by Lung Rx or any of its Affiliates as of
the Effective Date or during the Term. To the extent that any Patent or Improvement Controlled by
Lung Rx is necessary to practice any Patent as defined above, then such necessary Patent or
Improvement will be included as Lung Rx Patents.
1.22 “Lung Rx Technology” means, collectively, the (i) Licensed Molecule, (ii) Lung Rx
Patents, (iii) Lung Rx Know-How, (iv) Lung Rx New Intellectual Property, (v) all Information
relating to the Lung Rx Patents, Lung Rx Know-How, and Lung Rx New Intellectual Property in the
course of any work conducted pursuant to this Agreement, and (vi) Lung Rx Improvements.
1.23 “NDA” means a New Drug Application filed with the FDA, or the equivalent application or
filing filed with any equivalent agency or governmental authority outside the U.S. (including any
supra-national agency such as in the European Union) necessary to market pharmaceutical products in
such jurisdiction.
1.24 “Net Sales” means the gross amounts invoiced by Lung Rx, its Affiliates and their
respective sublicensees for sales of all Products to Third Parties that are not Affiliates or
sublicensees of Lung Rx (unless such Affiliate or sublicensee is the end user of such Products, in
which case the amount billed therefor shall be deemed to be the amount that would be billed to a
Third Party purchaser in an arm’s length transaction) following First Commercial Sale of a Product
in any country in the Territory, less the following items, to the extent actually allowed or
incurred with respect to sales of, and allocable to, such Products: (i) trade, cash, or quantity
discounts off of the invoiced price (including cash, governmental (e.g., Medicare/Medicaid and
other insurance rebates and managed care rebates), and hospitals or other buying group chargebacks;
(ii) credits, allowances discounts, rebates and other price reductions, chargebacks, and
adjustments, granted upon returns, rejections, or recalls; (iii) freight, insurance, exportation,
importation and transportation charges; (iv) taxes, duties or other governmental tariffs or charges
(other than income taxes); and (vi) bad debts or uncollectible accounts.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
4
1.25 “New Intellectual Property” means (i) any and all Information specific to the
Development and/or Commercialization of Aradigm Technology or Lung Rx Technology solely
conceived, reduced to practice, made, Developed, discovered or created by or on behalf of one
Party or jointly developed by or on behalf of the Parties and all intellectual property rights
therein, including any Patents and Improvements to the extent claiming such Information, and (ii)
any and all other Information solely invented by or on behalf of one Party or jointly developed
by or on behalf of the Parties pursuant to this Agreement and all intellectual property rights
therein, including any Patents and Improvements claiming such Information.
1.26 “Patents” means (a) patents, including any and all re-examinations, reissues, renewals,
revalidations, extensions, confirmations, registrations, term restorations, and supplementary
patent certificates, (b) patent applications, including provisional applications, additions,
continuations, continuations-in-part, divisional and substitute applications, and inventors’
certificates, and (c) any international counterparts of the forgoing.
1.27 “POC Study” means the proof-of-concept study described in the Development Plan, all
expenses of conducting the study being the sole responsibility of Aradigm.
1.28 “Product” means any product, use, application, administration, method of administration
or treatment for use in the Field consisting of a Licensed Molecule (including any formulations
thereof) incorporated into the AERx pulmonary drug delivery system and any future improvements to
the AERx pulmonary drug delivery system made during the Term.
1.29 “PSC” means the Product Steering Committee that has the meaning set forth in Section 3.1.
1.30 “Regulatory Approval” means any and all approvals (including price and reimbursement
approvals, if required), licenses, registrations, or authorizations of a Regulatory Authority that
are necessary to market and sell the Product in the Field in the applicable country or regulatory
jurisdiction.
1.31 “Regulatory Authority” means any supra-national, national (e.g., the FDA), state,
provincial or local regulatory agency, department, bureau, commission, council or other
governmental entity involved in or responsible for regulation of the manufacture, promotion and/or
sale of medicinal products in the Field that are intended for human use in the applicable country
or regulatory jurisdiction.
1.32 “Regulatory Dossier” means (a) all INDs, NDAs and equivalent foreign applications or
registrations for authority to conduct clinical trials on the Product or for Regulatory Approval,
anywhere in the Territory; (b) all Regulatory Approvals and any other technical, medical and
scientific registrations, authorizations and approvals (including approvals of NDAs or foreign
equivalents, supplements and amendments, pre- and post- approvals, pricing and Third Party
reimbursement approvals, and labeling approvals) of any Regulatory Authority necessary for or
applicable to the Development (including the conduct of clinical trials), Commercialization,
manufacture, distribution, marketing, promotion, offer for sale, use, import, reimbursement, export
or sale of a Product in any regulatory jurisdiction in the Territory, together with all related
correspondence to or from any Regulatory Authority and all documents
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
5
referenced in the complete
regulatory chronology for each NDA or foreign equivalent, including the drug master file (if any)
or device master file (if any), IND, NDA and supplemental NDA, or foreign equivalents; and (c) all
data and other Information contained in either (a) or (b) above.
1.33 “Senior Executive” means, with respect to a particular Party, the Chief Executive Officer
of such Party, or the representative designated by such individual (provided that such
representative is a senior executive officer of such Party with authority to settle the applicable
issue or dispute submitted for resolution under Section 13.2).
1.34 “Successful” means, with respect to the POC Study, that the results thereof satisfy the
criteria set forth in Exhibit D.
1.35 “Term” means the term of this Agreement, as provided in Section 11.1.
1.36 “Territory” means the entire universe.
1.37 “Third Party” means any entity other than Aradigm or Lung Rx or an Affiliate of Aradigm
or Lung Rx.
1.38 “U.S.” means the United States of America.
1.39 “Valid Claim” means an unexpired claim of an issued Patent within the Aradigm Patents or
New Intellectual Property which has not been canceled or otherwise found to be unpatentable,
invalid or unenforceable by a court or other authority in the subject country, from which decision
no appeal is taken or can be taken, has been admitted to be invalid or unenforceable through
reissue, disclaimer or otherwise, or been abandoned.
ARTICLE 2
Licenses and Options
2.1 Licenses to Lung Rx.
(a) Subject to the terms and conditions of this Agreement, and solely upon satisfaction of
Lung Rx’s payment and investment obligations set forth in Section 6.2, Aradigm hereby grants to
Lung Rx a royalty-bearing, exclusive (even as to Aradigm) license for the Aradigm Technology in the
Territory to research, Develop, Commercialize, use, import, register, sell, have sold, and
distribute Products in the Field within the Territory. The license granted in this Section 2.1(a)
shall be sublicensable by Lung Rx solely to the extent necessary to exercise Lung Rx’s rights under
this Agreement.
(b) Subject to the terms and conditions of this Agreement, and solely upon satisfaction of
Lung Rx’s payment and investment obligations set forth in Section 6.2, Aradigm hereby grants to
Lung Rx a royalty-bearing, non-exclusive license for the Aradigm Technology in the Territory to
make and have made Products in the Field in the Territory. The license granted in this Section
2.1(b) shall not be sublicensable without Aradigm’s prior written consent,
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
6
which consent may be
withheld in good faith and, if Aradigm does not consent, Aradigm shall identify the basis for its
objection to Lung Rx in writing within 10 days; provided, however that Lung Rx may, without such
consent but with prior written notice to Aradigm, engage one or more bona fide subcontractors to
manufacture Products on Lung Rx’s behalf.
2.2 License to Aradigm. Subject to the terms and conditions of this Agreement, Lung Rx hereby
grants to Aradigm a non-exclusive, royalty-free license for the Lung Rx Technology solely to the
extent necessary to conduct its obligations under this Agreement, including those Development
responsibilities assigned to Aradigm under the Development Plan, and for no other use. The license
granted in this Section 2.2 shall be sublicensable by Aradigm solely to subcontractors approved by
Lung Rx and solely to the extent necessary to fulfill Aradigm’s obligations under this Agreement.
2.3 Aradigm Retained Rights. Notwithstanding anything in this Agreement to the contrary,
Aradigm shall retain the right under the Aradigm Technology to conduct its obligations under this
Agreement, including those Development responsibilities assigned to Aradigm under the Development
Plan.
2.4 Third Party Licenses.
(a) Lung Rx shall be solely responsible for obtaining, at its sole expense (subject to Section
6.5), any licenses from Third Parties that Lung Rx determines, in its sole discretion, are required
in order to lawfully research, Develop, manufacture, and Commercialize Products in the Field in the
Territory.
(b) Aradigm shall promptly notify Lung Rx in writing of any license agreement entered into by
Aradigm after the Effective Date pursuant to which Aradigm obtains a license to enhance Aradigm
Technology from a Third Party, which license was not obtained in order to avoid infringing such
Third Party Patent or other intellectual property, as more fully discussed in Section 6.5(b).
Notwithstanding anything to the contrary herein other than Section 6.5(b), such subsequently
in-licensed Aradigm Technology shall be excluded from the licenses granted to Lung Rx in Section
2.1. Lung Rx shall have a right of first option to negotiate for any enhancement to the Aradigm
Technology in the Field that Aradigm obtains from a Third Party . At Aradigm’s sole discretion and
to the extent practical, Lung Rx shall have the opportunity to participate in the negotiation of
such license agreements in conjunction with Aradigm.
2.5 No Non-Permitted Use.
(a) Lung Rx hereby covenants that it shall not, nor shall it cause or permit any Affiliate or
sublicensee to, use or practice any Aradigm Technology for any purposes other than those expressly
permitted in Section 2.1; provided, however, Lung Rx does not have a duty to enforce any breach.
(b) Aradigm hereby covenants that it shall not, nor shall it cause or permit any Affiliate or
sublicensee to, use or practice any Lung Rx Technology for any purposes other than
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
7
those expressly
permitted in Section 2.2; provided, however, Aradigm does not have a duty to enforce any breach.
2.6 No Other Licenses. Nothing in this Agreement shall be interpreted to grant either Party
any rights under any Patents or Information of the other Party that are not expressly granted
herein.
2.7 Covenant Not to Xxx.
(a) Aradigm agrees that it will not assert nor cause to be asserted against Lung Rx, its
Affiliates or its sublicensees any existing invention, Information, patent or know-how not
included in the Aradigm Technology that is or might be infringed by reason of Lung Rx, its
Affiliates or its sublicensees’ exercise of rights granted to Lung Rx under Section 2.1.
(b) Lung Rx agrees that it will not assert nor cause to be asserted against Aradigm, its
Affiliates or its sublicensees any existing invention, Information, patent or know-how not
included in the Lung Rx Technology that is or might be infringed by reason of Lung Rx, its
Affiliates or its sublicensees’ exercise of rights granted to Aradigm under Section 2.2.
2.8 Right of First Option to Alternate Technology Subject to the terms and conditions of
this Agreement, and solely upon satisfaction of Lung Rx’s payment and investment obligations set
forth in Section 6.2, Aradigm hereby grants, subject to any limitations it may have to Third
Parties, to Lung Rx an exclusive right of first option to negotiate a license agreement for any
alternate superior technology in the Field to the AERx pulmonary drug delivery system (“Alternate
Technology”) Controlled by Aradigm on or after the Effective Date. If the Parties do not reach
an agreement for an Alternate Technology within sixty (60) days after Aradigm gives Lung Rx
detailed written notice of an Alternate Technology, the right of first option shall expire for
such Alternate Technology and Aradigm may exploit each such Alternate Technology as described in
the notice to Lung Rx in any manner whatsoever.
ARTICLE 3
Governance
3.1 PSC; Meetings. Aradigm and Lung Rx shall form a product steering committee (the “PSC”)
consisting of two (2) representatives from Aradigm and two (2) representatives from Lung Rx, all
such representatives having an appropriate level of skill, experience and familiarity with the
Development Plan.
(a) The initial representatives for Aradigm shall be Xxxx Xxxxx and Xxxxx Xxxxxxx. The
initial representatives for Lung Rx shall be [*] and [*]. Each Party may replace its PSC
representatives at any time upon prior written notice to the other Party.
(b) Unless otherwise agreed to by the Parties, the PSC shall meet not less than twice every
calendar year, on such dates and at such times as agreed to by Lung Rx and
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
8
Aradigm, with all
scheduled in-person meetings to alternate between the offices of the Parties, or at such other
locations as mutually agreed upon by the Parties. Meetings may be held by audio or video
conference at the request of either Party, provided that at least one (1) meeting per calendar year
shall be held in person. Visitors may attend meetings of the PSC upon the consent of the Parties.
Each Party shall be responsible for its own expenses for participating in the PSC, including the
fully burdened cost of its personnel.
(c) Meetings of the PSC shall be effective only if at least one representative of each Party
is present or participating. The members of the PSC will designate one (1) representative at each
meeting to serve as secretary for such meeting, who will promptly prepare and distribute to the
Parties written minutes summarizing the matters discussed and actions taken, if any, at such
meeting. The meeting minutes will be approved by each of Lung Rx and Aradigm promptly following
the applicable PSC meeting and will reflect any agreement or disagreement of the Parties with
regard to the matters therein.
3.2 Responsibilities of the PSC. The PSC shall have the responsibility and authority to:
(a) review each of the Parties’ Development activities conducted in connection with the
Development Plan;
(b) propose amendments or updates to the Development Plan to the Parties;
(c) review and discuss draft amendments or updates to the Development Plan;
(d) monitor progress toward achieving goals set forth in the Development Plan;
(e) address and attempt to resolve conflicts or disputes between the Parties that may arise
during the course of performing the Development Plan;
(f) serve as a forum for communication between the Parties for the activities performed
pursuant to the Development Plan;
(g) monitor all regulatory, manufacturing, and commercial activities involving the Product
that are conducted by a Party, its Affiliates, or their respective licensees; and
(h) perform such other functions as the Parties may agree in writing.
3.3 Limitation on PSC’s Authority. The PSC shall have no authority other than that expressly
set forth in Section 3.2 and, specifically, shall have no authority to amend this Agreement,
determine whether a Party has complied with its obligations under this Agreement, or enter into
subsequent agreements on behalf of either Party.
3.4 PSC Decisions.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
9
(a) Consensus; Good Faith; Action Without Meeting. Subject to Section 3.4(b), the PSC shall
decide all matters by consensus, with each Party having one collective vote. The members of the
PSC shall act reasonably and in good faith to cooperate with one another and to reach agreement
with respect to issues to be decided by the PSC. Action that may be taken at a meeting of the PSC
also may be taken without a meeting if a written consent setting forth the action so taken is
signed by all members of the PSC.
(b) Failure to Reach Consensus. If the members of the PSC cannot reach consensus with respect
to any matter over which the PSC has authority and responsibility, then the Senior Executive of
Lung Rx shall have the right to decide such matter in his or her sole discretion, provided that (i)
such Senior Executive takes into consideration the reasonable commercial interests of Aradigm among
all other factors bearing on the decision, and (ii) such decision is consistent with Lung Rx’s
obligations under Section 4.11. Notwithstanding the foregoing, Aradigm’s approval shall be
necessary prior to the amendment of the Development Plan in a manner that materially increases
Aradigm’s obligations under such Development Plan or narrows the patient population as specified
in such Development Plan. If Aradigm does not agree to a material increase in its obligations, but
Lung Rx nevertheless deems such activity necessary, Lung Rx may arrange for the services or
activities to be provided otherwise.
ARTICLE 4
Development and Commercialization
4.1 Development — Overview.
(a) Promptly following the Effective Date, Aradigm shall use Commercially Reasonable Efforts
to perform the POC Study at its sole cost and expense in close consultation with Lung Rx, with the
complete final report describing the results of the POC Study to be delivered to Lung Rx within a
commercially reasonable period of time.
(b) Following Lung Rx’s satisfaction of its payment and investment obligations set forth in
Section 6.2, Lung Rx shall be primarily responsible for the Development of the Product in the Field
in the Territory and shall bear all costs associated with Development of the Product in the Field
in the Territory, as set forth in more detail in Section 4.6 below.
(c) With respect to those Development activities assigned to a Party, such Party shall perform
such Development activities using its Commercially Reasonable Efforts in accordance with the
Development Plan. In addition, the Parties shall closely cooperate on all matters involved in and
arising from the Development and Commercialization process of Products and the AERx pulmonary drug
delivery system (and its future improvements, successors and alternatives) that may materially
affect the other Party’s efforts to obtain Regulatory Approvals.
4.2 Development Plan.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
10
(a) Scope. The Development of each Product under this Agreement shall be governed by a
worldwide development plan (each, a “Development Plan”). Each Development Plan shall be created
(and updated from time to time) in good faith with the overall objective of reasonably optimizing
the commercial potential of the Product. The Development Plan shall describe the proposed overall
program of Development for the Product in the Territory, including preclinical studies, toxicology,
formulation, process development, clinical studies, regulatory plans and other elements of
obtaining Regulatory Approval(s) in major markets, as well as an overall timeline of the estimated
target dates for completing the various development tasks described within the Development Plan
(the “Development Timeline”). A pro forma budget for one year with a non-binding three year
look-ahead shall be created by the Parties no later than [*] days following completion of the POC
study, based on the assumptions and particulars of the Development Plan. Thereafter, the Parties
shall prepare pro forma budgets showing twelve-month projected estimated Development expenses for
each subsequent twelve-month period. In the event of any inconsistency between the Development
Plan and this Agreement, the terms of this Agreement shall prevail.
(b) Initial Development Plan. The Parties have agreed on the form of the initial Development
Plan, a copy of which is attached hereto as Exhibit E.
(c) Updates to the Development Plans and Additional Development Plans. The Parties will work
together to evaluate from time to time in light of then-current circumstances the status of
implementing a Development Plan and progress compared to the Development Timeline. The PSC, Lung
Rx or Aradigm may propose modifications to the Development Plan and new Development Plans, which
will be discussed in good faith by the Parties or the PSC and shall become effective solely upon
written approval of Lung Rx in its sole discretion. The PSC also will review any modifications to
any Development Plan suggested or required by any Regulatory Authority, and Lung Rx will be
responsible for amending such Development Plan as necessary to incorporate feedback from any
Regulatory Authority about Development of such Product and to comply with any legal requirement or
formal action imposed or suggested by any Regulatory Authority. In addition, and without limiting
the generality of the foregoing, Lung Rx shall, as early as necessary in each calendar year,
prepare a revision of each Development Plan for the following calendar year to take into account
completion, commencement or cessation of Development activities not contemplated by each
then-current Development Plan, and submit such proposed revised Development Plan to the PSC no
later than October 15 of such year for review. Notwithstanding anything to the contrary herein,
written approval of both Lung Rx and Aradigm shall only be necessary prior to the amendment of the
Development Plan in a manner that materially increases Aradigm’s financial obligations under such
Development Plan or narrows the patient population as specified in such Development Plan.
(d) Diligence. In addition to the specific diligence obligations of Lung Rx pursuant to
Section 4.11, each of Aradigm and Lung Rx will use Commercially Reasonable Efforts to carry out the
activities set forth in a Development Plan for which such Party is responsible in accordance with
the Development Timeline.
4.3 Development Reports and Records.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
11
(a) Each Party will keep the PSC fully informed regarding the progress and results of such
Party’s Development activities and those of its Affiliates, licensees, and Third Party contractors.
Within thirty (30) days after the end of each calendar quarter, each Party shall provide the PSC
with a written report that summarizes, in reasonable detail, all Development activities performed
by such Party and its Affiliates, licensees, and Third Party contractors during such quarter, and
compares such performance with the goals and Development Timeline set forth in the Development
Plan. Each Party shall also promptly provide the PSC or the other Party with any additional
information regarding its Development of the Product reasonably requested thereby. Subject to its
confidentiality obligations to Third Parties and to the degree it is able, Aradigm shall provide
the PSC with semi-annual written progress reports, summarizing in detail Aradigm’s Development and
Commercialization activities relating to the AERx pulmonary drug delivery system (and its future
improvements, successors and alternatives) other than in connection with the Licensed Molecule, and
shall provide such other information reasonably requested by Lung Rx. Each party shall give the
other prompt written notice with respect to information that would materially affect the Product.
The Parties shall maintain such reports and the information disclosed therein in confidence in
accordance with Article 10.
(b) Records. Each Party shall maintain records, in sufficient detail and in good scientific
manner appropriate for patent and regulatory purposes, which shall accurately reflect all work done
and results achieved in the performance of the Development Plan by such Party. Each Party shall
have the right, during normal business hours and upon reasonable prior written notice, to inspect
all such records of the other Party, and to obtain copies of such records to the extent reasonably
needed by such Party in exercising its rights under this Agreement. Each Party shall maintain such
records and the information disclosed therein in confidence in accordance with Article 10. Each
Party shall have the right to arrange for its employees involved in the activities contemplated
hereunder to visit the offices and laboratories of the other Party and any of its Affiliates as may
reasonably be desirable during normal business hours and upon reasonable prior written notice, to
discuss the Development work and its results in detail with the technical personnel and consultants
of the other Party.
(c) Disclosure of Inventions and Development Results. Each Party shall provide to the other
Party a complete written disclosure for each and every invention or other discovery, whether or
not patentable, first conceived or reduced to practice in the performance of a Development Plan
including Improvements and New Intellectual Property, promptly after each such invention is
made.
4.4 Standards of Conduct. Each Party shall perform, and shall ensure that its Affiliates,
licensees, and Third Party contractors perform, the Development activities for which it is
responsible under the Development Plan in good scientific manner and in compliance with applicable
laws, rules and regulations.
4.5 Development Limitations. Neither Party may conduct or have conducted on its behalf, or
enable any Third Party to conduct, any activities with respect to the Product that are not approved
under the Development Plan or by the PSC, without the prior written consent of the other Party,
such consent not to be unreasonably withheld or delayed.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
12
4.6 Development Expenses.
(a) To the extent that the Development Plan in Exhibit E provides that Aradigm will
perform Development activities after satisfaction of Lung Rx’s payment and investment obligations
set forth in Section 6.2, Lung Rx shall be responsible for all reasonable costs and expenses
incurred by or on behalf of Aradigm in connection with such authorized Development activities in
accordance with the Development Plan and corresponding budget that have been pre-approved by Lung
Rx in writing.
(i) In particular, Lung Rx shall pay for such costs and expenses as follows: (a) any Aradigm
personnel time will be paid for at the Aradigm FTE Rate; and (b) any out-of-pocket expenses
incurred by Aradigm will be reimbursed in full, at Aradigm’s reasonable and direct cost without
markup.
(ii) Unless the Parties agree in writing otherwise, payments for Development work to be
performed by Aradigm in a particular calendar quarter shall be made in advance prior to the first
day of such calendar quarter. Such payments shall be based on estimated costs and expenses for the
relevant Development work, as specified in the most recent written forecast for the applicable
quarter submitted by Aradigm to Lung Rx in accordance with the Development Plan and corresponding
budget. Any advance payments made by Lung Rx under this Section 4.6 will be reconciled on a
quarterly basis against actual costs and expenses incurred by Aradigm for the relevant Development
work as reported by Aradigm in a detailed monthly statement of costs incurred, and any payments
made by Lung Rx under this Section 4.6 that are not applied to actual costs and expenses during a
particular quarter may be credited by Lung Rx against payments owed by Lung Rx in a subsequent
quarter. In the event that the actual costs and expenses incurred by Aradigm in a particular
quarter in connection with Development work exceed the advance payment made by Lung Rx for such
quarter, Lung Rx shall reimburse any such undisputed excess costs and expenses no later than thirty
(30) days following Lung Rx’s receipt of written accounting of such costs and expenses from
Aradigm.
(iii) Aradigm shall provide Lung Rx with a detailed monthly invoice within 20 days of the end
of each month listing of all costs and expenses incurred by category of the Development Plan
budget. Aradigm shall provide Lung Rx with copies of all vendor invoices being passed through on
the monthly invoice. The Parties agree that Aradigm shall be solely responsible for payment of any
cost or expense incurred by or on behalf of Aradigm following the Effective Date that is not (i) in
accordance with a Development Plan and corresponding budget approved by Lung Rx, or (ii) with the
prior written approval of Lung Rx.
(b) As between the Parties, Lung Rx shall be responsible for all costs and expenses incurred
by or on behalf of Lung Rx, its Affiliates, or their respective licensees in connection with the
Development of Products with the exception of all costs and expenses incurred by or on behalf of
Aradigm relating to the POC Study. If the POC Study is materially changed from that described in
the Development Plan and Lung Rx agrees to such changes in writing, Lung Rx shall be responsible
for all the specified additional costs and expenses incurred by or on behalf of Aradigm because of
such changes.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
13
4.7 Regulatory Coordination.
(a) Lung Rx shall be responsible for preparing, filing and managing all regulatory filings and
efforts seeking all Regulatory Approvals in the Territory. All regulatory filings for the Product
in the Territory shall be filed in the name of Lung Rx, and Lung Rx alone shall be responsible for
all communications and other dealings with Regulatory Authorities relating to the Product in the
Territory. As between the Parties, Lung Rx shall be the sole and exclusive legal and beneficial
owner of all regulatory applications, clinical data, Regulatory Dossiers and Regulatory Approvals
in the Territory. Lung Rx shall have the final authority to make all clinical and regulatory
decisions with respect to the registration of Products within the Territory in its sole discretion.
Lung Rx shall have the right to reference any of Aradigm’s regulatory files Controlled by Aradigm
relating to the AERx pulmonary drug delivery system, in connection with regulatory filings made by
or on behalf of Lung Rx and Aradigm shall reasonably cooperate with Regulatory Authorities in
connection therewith.
(b) The PSC shall develop and implement procedures for drafting and review of any applications
for Regulatory Approval for the Product in the Territory, which shall provide sufficient time for
Aradigm to provide substantive comments. Unless otherwise agreed upon by the Parties, Aradigm
shall provide any comments it may have to Lung Rx within 20 days of its receipt of each
application. Lung Rx shall consider Aradigm’s comments on any such applications in good faith;
provided, however, that Lung Rx shall have the right to make all final decisions relating to the
content of each such application.
(c) Lung Rx shall promptly notify Aradigm of all regulatory filings that it submits and all
communications from Regulatory Authorities that it receives relating to Products, and shall
promptly provide Aradigm with a copy (which may be wholly or partly in electronic form) of such
regulatory filings or communications. Lung Rx will provide Aradigm with reasonable advance notice
of any scheduled meetings with any regulatory agencies relating to Development and/or any
application for Regulatory Approval of the Product in the Territory, and Aradigm shall have the
right to participate in any such meeting, to the extent permitted by law. Lung Rx also shall
promptly furnish Aradigm with summaries of all material correspondence or material meetings with
any regulatory agency relating to Development, regulatory filings and/or a Regulatory Approval in
the Territory, and Lung Rx shall promptly furnish Aradigm with copies of such correspondence or
copies of minutes of such meetings.
(d) Following receipt of Regulatory Approval of the Product, Lung Rx shall retain primary
responsibility for dealings with the applicable regulatory agency with respect to the Product,
including filing all supplements and other documents with such agency with respect to such
Regulatory Approval.
(e) Aradigm shall promptly notify Lung Rx of all regulatory filings that it submits and all
communications from Regulatory Authorities that it receives relating to Products and, shall
promptly provide Lung Rx with a copy (which may be wholly or partly in electronic form) of such
regulatory filings. Aradigm will provide Lung Rx with reasonable advance notice of any scheduled
meetings with any regulatory agencies relating to Development and/or any application for Regulatory
Approval of the Product in the Territory, and Lung Rx shall have the
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
14
right to participate in any
such meeting, to the extent permitted by law. Aradigm also shall promptly furnish Lung Rx with
summaries of all material correspondence or material meetings with any regulatory agency relating
to Development, regulatory filings and/or a Regulatory Approval in the Territory, and Aradigm shall
promptly furnish Lung Rx with copies of such correspondence or copies of minutes of such meetings.
(f) Subject to its confidentiality obligations to Third Parties and to the degree it is able,
Aradigm shall promptly notify Lung Rx of all regulatory filings that it submits and all
communications from Regulatory Authorities that it receives relating to the AERx pulmonary drug
delivery system (and its future improvements, successors and alternatives) other than Products,
and, at Lung Rx’s request, shall promptly provide Lung Rx with a copy (which may be wholly or
partly in electronic form) of such regulatory filings or communications. Aradigm will provide Lung
Rx with reasonable advance notice of any scheduled meetings with any regulatory agencies relating
to Development and/or any application for Regulatory Approval of any product incorporating the AERx
pulmonary drug delivery system (and its future improvements, successors and alternatives) in the
Territory. Subject to its confidentiality obligations to Third Parties and to the degree it is
able, Aradigm also shall promptly furnish Lung Rx with summaries of all material correspondence or
material meetings with any regulatory agency relating to Development, regulatory filings and/or a
Regulatory Approval in the Territory of any product incorporating the AERx pulmonary drug delivery
system (and its future improvements, successors and alternatives), and Aradigm shall, at Lung Rx’s
request, promptly furnish Lung Rx with copies of such correspondence or copies of minutes of such
meetings.
(g) Lung Rx may, at its expense, register the exclusive license granted under this Agreement
in any country, or community or association of countries within the Territory, where the use, sale
or manufacture of a Product in such country would be covered by a Valid Claim. Upon request of
Lung Rx, Aradigm agrees, after Aradigm reviews for accuracy, promptly to execute any “short form”
licenses in a form submitted to it by Lung Rx from time to time in order to effect the foregoing
registration in such country.
4.8 Product Withdrawals and Recalls. In the event that any regulatory agency (a) threatens or
initiates any action to remove the Product from the market in any country in the Territory or (b)
requires Lung Rx, its Affiliates, or its sublicensees to distribute a “Dear Doctor” letter or its
equivalent regarding use of the Product in the Field, Lung Rx shall notify Aradigm of such event
within two (2) business days after Lung Rx becomes aware of the action, threat, or requirement (as
applicable). Lung Rx shall consult with Aradigm prior to initiating a recall or withdrawal of the
Product; provided, however, that the final decision as to whether to recall or withdraw the Product
in the Territory shall be made by Lung Rx in its sole discretion. Lung Rx shall be responsible, at
its sole expense, for conducting any recalls or taking such other necessary remedial action, unless
the remedial action relates to a medical device manufactured by or on behalf of Aradigm, in which
event Aradigm shall be solely responsible for all expenses in conducting any recalls or taking such
other necessary remedial action.
4.9 Adverse Event Reporting.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
15
(a) Each Party shall, and shall require its respective Affiliates to:
(i) notify the other Party promptly of all information coming into its possession concerning
any adverse events associated with clinical or commercial uses of the Product, the Licensed
Molecule, or an AERx pulmonary drug delivery system device (and its future improvements,
successors and alternatives) (an “Adverse Event”), subject to any applicable confidentiality
obligations to Third Parties;
(ii) provide to the other Party a copy of any written submission made by such Party to a
Regulatory Authority regarding Adverse Events no later than five (5) days following finalization of
such written submission (and, to the extent permissible under time constraints and reporting
requirements, in advance of submission to the applicable Regulatory Authority); and
(iii) adhere to all requirements of applicable laws, rules and regulations that relate to the
reporting and investigation of Adverse Events.
(b) If a Party contracts with a Third Party for research to be performed by such Third Party
on the Product, that Party shall require such Third Party to report to the contracting Party the
information set forth above.
4.10 Commercialization of Product. Subject to the terms and conditions of this Agreement
(including Section 4.11 and Section 4.12), Lung Rx shall control, and be solely responsible for the
costs and expenses associated with, the Commercialization of the Product in the Territory.
4.11 Diligence Obligations.
(a) Lung Rx shall use Commercially Reasonable Efforts to Develop, and if in Lung Rx’s opinion
the results of the Development Plan so justify, to seek Regulatory Approval for, and Commercialize
Products in major markets throughout the Territory as Lung Rx determines are commercially feasible
in its sole discretion. The Development Plan shall include such Product Development work as Lung
Rx may, in its sole discretion, consider necessary to support Regulatory Approval.
(b) In the event that Lung Rx fails to materially comply with the diligence obligations set
forth in Section 4.11(a), and Lung Rx fails to cure its material non-compliance within thirty (30)
days following written notice from Aradigm providing a detailed explanation of such material
non-compliance, Aradigm may, in its sole discretion, elect to convert the exclusive license granted
to Lung Rx pursuant to Section 2.1(a) to a non-exclusive license with respect to a given Product,
with all other terms and conditions of this Agreement remaining unchanged. Any such conversion to
a non-exclusive license for a Product shall be effective upon Lung Rx’s receipt of written notice
from Aradigm, but shall not affect other Products which are being Developed and/or Commercialized
under this Agreement.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
16
(c) Notwithstanding the non-exclusivity conversion provision in Section 4.11(b), the diligence
obligations set forth in this Section 4.11 shall not apply to the extent (i) Aradigm’s failure to
perform its obligations under this Agreement in a timely manner causes a delay in Lung Rx’s
Development, efforts to seek Regulatory Approval for, or Commercialization of such Product; or (ii)
there exists any material regulatory action affecting such Product; or (iii) there exists any
material issue relating to the toxicology, safety, bioavailability or efficacy of such Product and
the absence of any impediments caused by formulation, material sourcing, manufacturing, or other
technical issues related to Product; in each case that leads Lung Rx to conclude, in exercising
reasonable business judgment, that Development or Commercialization of such Product should be
suspended or stopped altogether. Upon the occurrence of circumstances described in either
subsection (i), (ii) or (iii), the following terms shall apply: (A) Lung Rx shall notify Aradigm
in writing of the existence of such circumstances, specifying the details thereof and (in the case
of circumstances described in subsection (ii) or (iii) the reasons why Lung Rx concluded that
Development or Commercialization of such Product should be suspended or stopped, (B) Lung Rx’s
obligations to Develop or Commercialize such Product shall be suspended so long as any such
circumstances exists, and (C) the obligations set forth in Section 4.11(a) shall be extended by the
period of any such suspension with respect to such Product.
4.12 Other Inhalation Devices.
(a) If the AERx pulmonary drug delivery system is commercially viable in the reasonable
opinion of the PSC, Lung Rx and its Affiliates shall not spend more in cash and/or in kind
cumulatively on Developing the Licensed Molecule for a product involving an inhalation device that
is handheld and able to deliver a therapeutic dose of the Licensed Molecule in less than 10
breaths, than is spends on Development activities for the product. The restriction in this Section
4.12(a) shall end upon the date of the last patient visit in the Phase III registration trial of
the Product as defined in the Development Plan. The restrictions in this section 4.12(a) shall not
apply to nebulizers, or their improvements, used in current clinical trials by Lung Rx, or similar
devices and their improvements.
(b) In the event that a Product has received Regulatory Approval in a given country in the
Territory and Lung Rx performs sales and marketing activities in such country with respect to an
inhalation device for the Licensed Molecule other than the Product, [*].
4.13 Additional Obligations of Aradigm.
(a) Information. Aradigm, at its sole expense (excluding those expenses to be paid by Lung
Rx under this Agreement), shall be responsible for the timely delivery to Lung Rx of all
Information that Aradigm Controls regarding the Aradigm Technology and reasonably required by
Lung Rx to fulfill its obligations under this Agreement.
(b) Cooperation. Aradigm shall, upon the request by Lung Rx, provide Lung Rx with reasonable
assistance and consultation regarding the Aradigm Technology, including reasonable access to
sample materials and data and the execution of necessary and appropriate instruments and
documents.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
17
(c) AERx Expenses. Notwithstanding any other provision of this Agreement to the contrary,
Aradigm shall be solely responsible for all costs and expenses related to meeting and maintaining
regulatory requirements with respect to the design and operation of the AERx pulmonary drug
delivery system as distinguished from those costs incurred and paid by Lung Rx in connection with
meeting and maintaining regulatory requirements with respect to the Product.
ARTICLE 5
Manufacturing
5.1 Manufacturing Responsibility.
(a) Following Lung Rx’s satisfaction of its payment and investment obligations set forth in
Section 6.2, Lung Rx will begin preparations to manufacture the Product in bulk and finished form
for use by Lung Rx, its Affiliates, and its sublicensees in the Field in the Territory and for
secondary conditional use by Aradigm pursuant to Section 5.3 and the Development Plan. Lung Rx
will not make any material changes to the specifications for Products or components of Products
without the prior written approval of Aradigm, such approval not to be unreasonably withheld or
delayed. Lung Rx will not make any material changes to the specifications for the Aradigm
Technology without the prior written approval of Aradigm. Following Lung Rx’s satisfaction of its
payment and investment obligations set forth in Section 6.2, all supplier relationships will be
managed solely by Lung Rx, with the participation of Aradigm in its discretion.
(b) Following the date that Lung Rx reasonably agrees that the POC Study was Successful until
the (i) full and complete validation of a manufacturing plant operated by Lung Lx or a Third Party
and (ii) full and complete validation of the manufacturing process used at any such plant, Aradigm
shall, to the degree it is able individually and/or through a Third Party manufacturer, supply the
necessary Products or components thereof for clinical trials in such quantities as Lung Rx shall
from time to time require in accordance with the Development Plan and corresponding budgets. If
Aradigm is not able to supply the necessary Products or components thereof for clinical trials,
then Lung Rx shall be responsible for obtaining such Products or components. If Aradigm supplies
the Products for clinical trials, the cost of such clinical Products shall be at Lung Rx’s expense
and determined in accordance with Section 4.6. If Aradigm is able to supply the Products for
clinical trials, it shall supply the Product to Lung Rx in accordance with the agreed-upon
specifications and any relevant additional requirements specified in the Development Plan, and
shall comply with all applicable laws, rules, and regulations in the manufacture of Product.
5.2 Transfer of Manufacturing Technology.
(a) As soon as reasonably possible after satisfaction of Lung Rx’s payment and investment
obligations set forth in Section 6.2, Aradigm shall transfer to Lung Rx and/or Third Party
manufacturers designated by Lung Rx and reasonably acceptable to Aradigm all
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
18
Aradigm Technology
that is reasonably related to, or otherwise desirable to support, Lung Rx’s establishment of
clinical and commercial manufacturing capabilities with respect to Products in the Field that
comply with the cGMP regulations set forth in 21 C.F.R. Parts 210 – 211, 820 and 21 C.F.R.
Subchapter C (Drugs), Quality System Regulations and the requirements thereunder imposed by the
FDA, and, as applicable, the equivalent regulations and requirements in jurisdictions outside the
United States. The costs and expenses incurred by Aradigm in carrying out such transfer and
subsequent assistance in accordance with the Development Plan and corresponding budget that have
been pre-approved by Lung Rx in writing shall be reimbursed by Lung Rx in accordance with Section
4.6, with such transfer being deemed to be a Development activity for the purpose of such Section.
(b) Lung Rx and/or its Third Party manufacturers shall use any Aradigm Technology transferred
pursuant to Section 5.2(a) in accordance with the license granted in Section 2.1 solely for the
purpose of manufacturing Products for uses permitted under this Agreement, and for no other
purpose.
(c) Lung Rx acknowledges and agrees that Aradigm may reasonably condition its agreement to
transfer any Aradigm Technology and any other manufacturing technology to a Third Party
manufacturer on the execution of a confidentiality agreement between such Third Party manufacturer
and Aradigm that contains terms substantially equivalent to those of Article 10 of this Agreement.
5.3 Lung Rx Supply Obligations. In the event that Lung Rx reasonably agrees that the POC
Study was Successful, Lung Rx shall thereafter begin preparations and, at its sole expense, fund
the construction of a manufacturing plant that it will operate to manufacture AERx pulmonary drug
delivery system devices (and any future improvements, successors and alternatives) for use as
Product components. At Aradigm’s reasonable written request from time to time in accordance with
rolling-forecast procedures to be mutually developed, during the three (3) years following both (i)
full and complete validation of such plant and (ii) full and complete validation of the
manufacturing process, Lung Rx will supply Aradigm with AERx pulmonary drug delivery system devices
and/or AERx Strip® dosage forms (for use in products other than the Product) at mutually agreed
volumes and pursuant to a mutually agreed price schedule. Notwithstanding the foregoing sentence,
Lung Rx shall only be obligated to provide such supply to the extent that it has excess capacity
available which does not jeopardize in any manner the cost, quality and timely delivery of
Products, such excess capacity, if any, to be determined after Lung Rx’s projected requirements
have been met and subject to the mutually agreeable successful completion of a cleaning
validation/QA protocol.
ARTICLE 6
Fees and Payments
6.1 Upfront Fee.
(a) No later than five (5) business days after the Effective Date, Lung Rx shall pay to
Aradigm the non-creditable, non-refundable sum of $440,000.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
19
(b) No later than One Hundred and Twenty (120) days after the Effective Date, Lung Rx shall
pay to Aradigm the additional non-creditable and non-refundable sum of $440,000.
6.2 Additional Consideration.
(a) License Fee. No later than fifteen (15) days after Aradigm’s delivery of a complete final
report to Lung Rx describing the results of the POC Study, and Lung Rx reasonably agrees that the
POC Study was Successful, Lung Rx shall pay to Aradigm the non-creditable, non-refundable sum of
$[*]. Lung Rx shall have no more than thirty (30) days from delivery of the complete final report
describing the results of the POC Study to determine whether the POC Study was Successful.
(b) Purchase of Shares. No later than fifteen (15) days after Aradigm’s delivery of a
complete final report to Lung Rx describing the results of the POC Study, and Lung Rx reasonably
agrees that the POC Study was Successful, Aradigm shall issue to Lung Rx, and Lung Rx shall
purchase, shares of Aradigm common stock for an aggregate purchase price of $3,470,000 and at a per
share price equal to the average closing price as quoted on the OTC Bulletin Board for the thirty
(30) days prior to either (i) the date of entry into such stock purchase agreement, or (ii) the
45th day following delivery to Lung Rx of the complete final POC Study report, whichever first
occurs. Lung Rx shall have no more than thirty (30) days from delivery of the complete final
report describing the results of the POC Study to determine whether the POC Study was Successful.
The issuance and purchase of such shares shall be subject to the terms of a separate stock purchase
agreement (and other agreements and related documents executed pursuant thereto) which contains the
usual and customary terms for such stock purchase agreements and also provides for customary
piggyback registration rights.
6.3 Milestone Payments. Within thirty (30) days following the first occurrence of each of the
events set forth below, Lung Rx shall pay to Aradigm the applicable milestone payment set forth
below (whether such milestone is achieved by Lung Rx, its Affiliate or any of their respective
licensees):
| Milestone Event | Milestone Payment | |
First to occur of (i) the issuance of purchasing
requisitions for a [*]; and (ii) the first anniversary
of the Effective Date;
|
[*] | |
First to occur of (i) the completion of the [*]; and
(ii) the second anniversary of the Effective Date;
|
[*] | |
First to occur of (i) [*], and (ii) the third
anniversary of the Effective Date.
|
[*] |
Each of the milestone payments described in this Section 6.3 shall be payable only one time (i.e.,
upon the first achievement of the applicable milestone event by a Product). All payments made to
Aradigm pursuant to this Section 6.3 are non-refundable and non-creditable. In the event of a
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
20
delay in Development or Commercialization that occurs prior to achievement of a particular
milestone event that is the result of the action of a Regulatory Agency, the date set forth in
clause (ii) of the description of each such milestone event in the chart above shall be
automatically extended by an amount equal to the duration of such delay. Following the occurrence
of such a delay, (a) Lung Rx shall promptly notify Aradigm in writing of the existence of such
delay, specifying the details thereof, (b) Lung Rx shall describe its plan to overcome such delay
and the anticipated duration of such delay, and (c) upon the cessation of such delay, Lung Rx shall
notify Aradigm in writing of such cessation and shall state the actual duration of such delay.
6.4 Royalties. Subject to Section 6.5 below and the other terms and conditions of this
Agreement, in consideration for the grant of the licenses to the Aradigm Technology under Section
2.1, Lung Rx shall pay to Aradigm following First Commercial Sale of a Product in any country of
the Territory royalties on Net Sales of all Products in the Territory by Lung Rx, its Affiliates
and their respective licensees, with the royalty rate determined by the amount of aggregate Net
Sales of Products during the applicable calendar year according to the following schedule:
(a) 10% of the first [*] million of Net Sales of Products occurring in the calendar year;
(b) [*] of that portion of total annual Net Sales of Products in excess of [*] million and up
to and including [*] million;
(c) [*] of that portion of total annual Net Sales of Products in excess of [*] million and up
to and including [*] million;
(d) [*] of that portion of total annual Net Sales of Products in excess of [*] million and up
to and including [*] million; and
(e) [*] of that portion of total annual Net Sales of Products in excess of [*] million.
Notwithstanding the foregoing, the royalty rates set forth in subsections (a)-(e) above shall be
reduced by fifty percent (50%) with respect to Net Sales of Products accrued prior to the first
regulatory approval of an AERx pulmonary drug delivery system device (other than the Product). In
such event, however, the effective royalty rate due to Aradigm shall not be reduced to below [*]
percent ([*]%) of Net Sales.
Upon the expiration of the last-to-expire Valid Claim on a country-by-country basis (“Patent
Expiration”), the royalty rates set forth in subsections (a) — (e) above shall be reduced by fifty
percent (50%) with respect to Net Sales of Products. Additionally, upon Patent Expiration and the
approval of a generic version of the Product in each country, at the discretion of Lung Rx, the
Agreement may be terminated as to such country in accordance with section 11.1 below and the
royalty rates set forth in subsections (a) – (e) above shall be reduced to 0%. In the alternative,
upon Patent Expiration and the approval of a generic version of the Product in each country, Lung
Rx may elect not to terminate the Agreement as to such country in which case the
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
21
royalty rates set forth in subsections (a) — (e) above shall be reduced by fifty percent (50%)
with respect to Net Sales of Products.
6.5 Royalty Reduction.
(a) If (i) it is necessary for Lung Rx, its Affiliate, or one of their sublicensees to obtain
a royalty-bearing license from a Third Party because the Development, manufacture or
Commercialization of the Product infringes a Patent or other intellectual property of such Third
Party in a particular country and (ii) despite Commercially Reasonable Efforts of Lung Rx it is not
possible to practice the Aradigm Technology licensed to Lung Rx hereunder in a commercially
reasonable manner without infringing such Third Party Patent or other intellectual property, then
Lung Rx shall be entitled to a credit, against the royalty payments due to Aradigm upon sales of
the Product in the applicable country, equal to [*] percent ([*]%) of the royalties actually paid
to such Third Party by Lung Rx, its Affiliate, or one of their sublicensees based upon the sales of
the Product in such country; provided that such credit shall in no event cause the effective
royalty rate due to Aradigm to be reduced to below [*] percent ([*]%) of Net Sales, except in the
case of Patent Expiration in which event the effective royalty rate due to Aradigm shall not be
reduced to below [*] percent ([*]%), or in the case of a Patent Expiration and the approval of a
generic version of the product in which event the effective royalty rate due to Aradigm shall be
reduced to [*] percent ([*]%).
(b) If Aradigm obtains a royalty-bearing license from a Third Party because the Development,
manufacture or Commercialization of the Product infringes a Patent or other intellectual property
of such Third Party in a particular country, and (i) despite Commercially Reasonable Efforts of
Aradigm it is not possible to practice the Aradigm Technology licensed to Lung Rx hereunder in a
commercially reasonable manner without infringing such Third Party Patent or other intellectual
property, and (ii) Lung Rx is required to pay a royalty to such Third Party, then upon the
occurrence of such events, Lung Rx shall be entitled to a credit, against the royalty payments due
to Aradigm upon sales of the Product in the applicable country, equal to [*] percent ([*]%) of the
royalties actually paid to such Third Party by Lung Rx, its Affiliate, or one of their sublicensees
based upon the sales of the Product in such country; provided that such credit shall in no event
cause the effective royalty rate due to Aradigm to be reduced to below [*] percent ([*]%) of Net
Sales, except in the case of Patent Expiration in which event the effective royalty rate due to
Aradigm shall not be reduced to below [*] percent ([*]%), or in the case of a Patent Expiration and
the approval of a generic version of the product in which event the effective royalty rate due to
Aradigm shall be reduced to [*] percent ([*]%).
(c) If Lung Rx, its Affiliate, or one of their sublicensees adds to the Product a component or
feature, and Lung Rx, its Affiliate, or one of their sublicensees must pay royalties for Third
Party patent rights covering such component or feature, such royalties shall not be offset against
royalties owed to Aradigm, unless, at the time added, it is not possible to make the Product
approvable or commercially viable without the component or feature. As to any license that Lung Rx
may believe is desirable to enter into with respect to a Product, other than those for which
royalties may be offset in accordance with this Section 6.5, if Lung Rx requests, the Parties will
discuss such license and the possibility of Aradigm sharing some part of the costs of such license.
In no event, however, shall royalty payments due to Aradigm be reduced in
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
22
accordance with this Section 6.5 for any royalties paid by Lung Rx for the Licensed Molecule or its
formulations.
6.6 Accrual of Royalties. No royalties shall be payable on a Product distributed to Third
Parties solely as a sample for testing or evaluation purposes. No multiple royalty shall be
payable on the sale of any one Product. No multiple royalty shall be payable on a Product
because the manufacture, use or sale of such Product is covered by more than one Patent or is
subject to both Know-How and a Valid Claim.
6.7 Compulsory Licenses. Should a compulsory license be granted to a Third Party under the
applicable laws of any country in the Territory under the Aradigm Technology, the Parties will
consult and cooperate to position Lung Rx, its Affiliates or its sublicensees to competitively
market such Product in such country.
6.8 Commercial Hardship. If in any country Lung Rx can demonstrate that for any reason
beyond its, its Affiliates’ or its sublicensees’ control the royalty payable hereunder by Lung Rx
causes or may cause Lung Rx, its Affiliate or its sublicensee a significant reduction in its or
their sales of Product in that country, or otherwise causes or may cause hardship in the
promotion or sale of Product in a country, the Parties will consult and cooperate to position
Lung Rx, its Affiliates or its sublicensees to competitively market such Product in such country.
ARTICLE 7
Payments; Records; Audits
7.1 Payment; Reports. Royalty obligations under Section 6.4 shall accrue upon the First
Commercial Sale of a Product by Lung Rx, its Affiliates and their sublicensees for the Term of this
Agreement. Royalties shall be calculated and reported and paid on a calendar quarter basis for all
royalties accruing in each calendar quarter, although they may be made in whole or in part in
advance of such due date in Lung Rx’s discretion. All royalty payments by Lung Rx to Aradigm under
this Agreement shall be paid within forty-five (45) days after the end of each calendar quarter.
Each payment shall be accompanied by a report of Net Sales of Products by Lung Rx, its Affiliates
and their respective sublicensees, each in reasonably sufficient detail to permit confirmation of
the accuracy of the payment made, including, on a regional basis, including the U.S. separately,
the number of Products sold, the gross sales and Net Sales of such Products, the royalties payable,
the withholding taxes, if any, required by law to be deducted in respect of such sales, the method
used to calculate the royalties, and the exchange rates used, if any.
7.2 Exchange Rate; Manner and Place of Payment. All payments under this Agreement shall be
made in U.S. dollars. Conversion of payments to U.S. dollars from a currency in a country from
which royalties are payable shall be at an exchange rate equal to the rates of exchange for the
country’s currency as published by The Wall Street Journal. All payments owed under this Agreement
shall be made by wire transfer in immediately available
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
23
funds to a bank and account designated in writing by Aradigm, unless otherwise agreed in writing by
Aradigm.
7.3 Tax Withholding. Lung Rx will be responsible for and pay any and all taxes levied on
account of any payments made by it under this Agreement (other than taxes based on Aradigm’s
income) without deduction from any payment due to Aradigm hereunder, except for any required
withholding or comparable taxes.
7.4 Records; Audits. During the Term and for a period of three (3) years thereafter, Lung Rx
shall keep, and shall cause its Affiliates and their respective licensees to keep, complete and
accurate records pertaining to the sale or other disposition of Products, in sufficient detail to
permit Aradigm to confirm the accuracy of all payments due hereunder. Aradigm, at its own expense,
shall have the right once each calendar year to cause an independent, certified public accountant
of a nationally recognized firm reasonably acceptable to Lung Rx to audit such records as are
reasonably necessary to confirm such Net Sales, royalties and other payments for any year ending
not more than twenty-four months prior to the date of such request. Such audits may be exercised
during normal business hours upon reasonable prior written notice to Lung Rx, and subject to an
appropriate confidentiality agreement. The accountant shall disclose to Aradigm only whether the
records are correct or not and the specific details concerning any discrepancies. No other
information shall be shared. Prompt adjustments shall be made by the Parties to reflect the
results of such audit. Aradigm shall bear the full cost of such audit unless such audit discloses
an underpayment by Lung Rx of more than [*] percent ([*]%) of the amount of royalties or other
payments due under this Agreement for the period audited, in which case Lung Rx shall pay the
reasonable and direct cost of such audit.
7.5 Late Payments. In the event that any payment due under this Agreement is not made when
due, the payment shall accrue interest from the date due at the rate of [*] percent ([*]%) per
month; provided, however, that in no event shall such rate exceed the maximum legal annual interest
rate. The payment of such interest shall not limit Aradigm from exercising any other rights it may
have as a consequence of the lateness of any payment.
ARTICLE 8
Intellectual Property
8.1 Filing Prosecution and Maintenance of Patents. Aradigm at its sole expense shall be
responsible for prosecuting and maintaining the Aradigm Patents in the Territory, and Lung Rx
shall be responsible at its sole expense for prosecuting and maintaining the Lung Rx Patents in
the Territory.
8.2 Ownership of New Intellectual Property.
(a) Title to all New Intellectual Property that relates to the core intellectual property,
know-how and proprietary technology in the field of prostacyclin drugs (“Prostacyclin Field”) owned
by Lung Rx, whether patentable or unpatentable, made directly or indirectly by
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
24
either Party as a result of the collaboration under this Agreement or further collaboration between
the Parties on the subject matter of this Agreement, shall be owned solely and exclusively by Lung
Rx.
(b) Title to all New Intellectual Property that relates to the core intellectual property,
know how, and proprietary technology in the field of pulmonary delivery technologies (“Pulmonary
Delivery Field”) owned by Aradigm, whether patentable or unpatentable, made directly or indirectly
by either Party as a result of the collaboration under this Agreement or further collaboration
between the Parties on the subject matter of this Agreement, shall be owned solely and exclusively
by Aradigm.
(c) Title to all New Intellectual Property shall be held by the Party(ies) employing the
respective author(s) and/or inventor(s) or to which said author(s) and/or inventor(s) has/have an
obligation to assign the subject intellectual property in accordance with U.S. copyright, patent
and any other laws addressing intellectual property. Any such New Intellectual Property, to the
extent owned by Aradigm, shall be licensed exclusively to Lung Rx within the Prostacyclin Field,
and to the extent owned by Lung Rx, shall be licensed exclusively to Aradigm within the Pulmonary
Delivery Field, in both cases any such license shall be royalty free and shall subsist for so long
as any such right subsists.
(d) Each Party agrees (i) to disclose promptly to the other any and all Patent applications
prepared or filed by that Party which applications are directed to either of the stated core
technologies and are made directly or indirectly as a result of the collaboration under this
Agreement or further collaboration between the Parties on the subject matter of this Agreement,
and (ii) promptly upon request by the other Party to execute assignments of intellectual property
rights in accordance with this Section.
8.3 Filing, Prosecution and Maintenance of New Intellectual Property.
8.3.1 Each Party shall, using Commercially Reasonable Efforts, control the preparation,
filing, prosecution, grant and maintenance of Patent rights regarding its New Intellectual
Property in the Territory and shall select all patent counsel or other professionals to advise,
represent or act for it in all matters relating to the prosecution and maintenance of such Patent
rights regarding its New Intellectual Property in the Territory.
8.3.2 In the event that a Party elects not to file a Patent application or decides to
abandon any pending application or granted Patent under its New Intellectual Property in any
country of the Territory, it shall provide adequate advance notice to the other Party and give
the other Party the opportunity to file or maintain such application or Patent at its own
expense.
8.4 Cooperation.
(a) Each Party shall make available to the other Party or its authorized attorneys, agents,
consultants or representatives, if available, such information necessary or appropriate to enable
the appropriate Party (at the appropriate Party’s cost and expense) to prepare, file, prosecute
and maintain Patent applications and resulting Patents with respect to its
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
25
New Intellectual Property as set forth in Section 8.2 above, for a period of time reasonably
sufficient for such Party to obtain the assistance it needs from such personnel. Where
appropriate, each Party shall sign or cause to have signed all documents relating to said Patent
applications or Patents at no charge to the other.
(b) The Parties’ patent counsel shall provide comments to the Parties in sufficient time for
the Parties to reflect such comments in any response. Any comments made by a Party shall be made
in good faith and shall be directed to maximizing the claims covered by such Patents. If a Party
agrees with the comments of the other Party’s patent counsel, it shall reflect such comments in
its response. If a Party disagrees with such comments, it shall provide a detailed explanation
of its position and, in the event the Parties do not thereafter arrive at a consensus on how to
proceed with respect to the comments, either Party may then submit such dispute (a “Patent
Comment Dispute”) for resolution by an intellectual property lawyer (the “Neutral Lawyer”) with
at least ten years of experience and a background in biotechnology or pharmaceutical patent
matters. The Neutral Lawyer shall be selected by mutual agreement of the Parties; provided,
however, that if the Parties cannot agree on a Neutral Lawyer within five days of a Party’s
request for a Neutral Lawyer under this provision, the Neutral Lawyer shall be selected by the
American Arbitration Association in Washington, D.C. Each Party shall submit its position as to
the Patent Comment Dispute to the Neutral Lawyer, who shall resolve the dispute by agreeing to
one of the submitted positions of the Parties without any changes to such position. The Parties
agree that the position agreed to by the Neutral Lawyer shall be reflected in the action or
response being prepared and that the costs of the Neutral Lawyer shall be paid by the Party whose
position is not agreed to by the Neutral Lawyer. The decision of the Neutral Lawyer shall be
final and binding on the Parties. The Parties shall cooperate in all respects to resolve any
Patent Comment Dispute in sufficient time to avoid any loss of rights, including jointly
instructing the Neutral Lawyer to make a decision in sufficient time to avoid any loss of rights.
8.5 Infringement by Third Parties.
(a) Notice. If either Party becomes aware that a Third Party is infringing any rights in the
Aradigm Technology or the Lung Rx Technology, such Party shall give written notice to the other
Party describing in detail the nature of such infringement.
(b) Right to Bring Suit. Lung Rx, in the case of intellectual property owned by Lung Rx and
Aradigm, in the case of intellectual property owned by Aradigm (the “Enforcing Party”), shall have
the sole right to enforce such intellectual property rights against infringers at their own
expense. In the case of New Intellectual Property, both Parties shall have the right to enforce
such intellectual property rights and to obtain the joinder of the non-enforcing Party, where
required by law, at the expense of the Enforcing Party.
(c) Cooperation; Settlement. The Party controlling the action may not settle the action or
otherwise consent to an adverse judgment in such action that diminishes the rights or interests of
the non-controlling Party without first consulting the non-controlling Party and considering any
objections thereto raised by the non-controlling Party. Aradigm and Lung Rx
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
26
shall provide all reasonable assistance to the other Party in any action to enforce such
intellectual property rights.
(d) Recoveries. Except as otherwise agreed to by the Parties in writing as part of a
cost-sharing arrangement, all in kind settlements or monies recovered upon the final judgment or
settlement of any such suit shall be shared, after reimbursement of the costs of participating in
such action, by Aradigm and Lung Rx pro rata according to the respective percentages of
costs borne by each Party in such action pursuant to this Section 8.5.
8.6 Infringement of Third Party Rights. In the event that a Third Party files an action
against a Party, its Affiliates, distributors or sublicensees alleging that such Party’s or its
Affiliates’, distributors’ or sublicensees’ activities under this Agreement infringe such Third
Party’s patent or other intellectual property rights, such Party shall give written notice to the
other Party, and the Parties will consult and cooperate on the best course of action. If the
alleged infringing process, method or composition is claimed under the Aradigm Technology and the
Lung Rx Technology, in the Territory, the Parties shall consult and cooperate to decide which Party
shall control the defense of such suit. If the alleged infringing process, method or composition
is claimed under the Lung Rx Technology, in the Territory, Lung Rx shall have the right to control
the defense of such suit. If the alleged infringing process, method or composition is claimed
under the Aradigm Technology, in the Territory, Aradigm shall have the right to control the defense
of such suit. Each Party shall pay its own expenses, have the right to be represented by advisory
counsel of its own selection, and shall cooperate fully in the defense of such suit and furnish to
the other all evidence and assistance in its control. If one Party does not elect within thirty
(30) days after such notice to control the defense of a suit, the other may undertake such control
at its own expense, and the non-controlling Party shall then have the right to be represented by
advisory counsel of its own selection, at its own expense, and shall cooperate fully in the defense
of such suit and furnish to the controlling Party all evidence and assistance in its control. The
Party controlling the suit may not settle the suit or otherwise consent to an adverse judgment in
such suit that diminishes the rights or interests of the non-controlling Party without the express
written consent of the non-controlling Party. Any judgments, settlements or damages payable with
respect to legal proceedings covered by this Section 8.6 shall be paid by the Party which controls
the litigation, subject to the other Party’s indemnification obligations in accordance with Article
12 below, if any.
8.7 Product Trademark; License to Aradigm Trademark.
(a) The Product, Product packaging, promotional materials, package inserts, and labeling shall
bear a trademark chosen and owned by Lung Rx. The Product, Product packaging, promotional
materials, package inserts, and labeling shall also bear the Aradigm®, Aradigm Logo™, AERx®, AERx
Essence®, and/or AERx Strip® trademarks (collectively, the “Licensed Marks”). The Product, Product
packaging, promotional materials, package inserts, and labeling shall also bear an acknowledgment
line that specifies, in part, that the Licensed Marks are registered trademarks of Aradigm and
lists the key Aradigm Patents.
(b) Subject to and conditioned upon Lung Rx’s full compliance with this Agreement, Aradigm
grants to Lung Rx, and Lung Rx accepts, during the term of this Agreement a limited,
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
27
non-exclusive,
royalty-free, revocable license to use the Licensed Marks in the Territory in connection with the
Commercialization, use, import, sale and distribution of Products in the Field. No other right,
title or license to the Licensed Marks is granted hereunder.
ARTICLE
9
Representations and Warranties
9.1 Mutual Representations, Warranties and Covenants.
(a) Each Party represents and warrants to the other that, as of the Effective Date: (a) it is
duly organized and validly existing under the laws of its jurisdiction of incorporation or
formation, and has full corporate power and authority to enter into this Agreement and to carry out
the provisions hereof; (b) it is duly authorized to execute and deliver this Agreement and to
perform its obligations hereunder, and the person executing this Agreement on its behalf has been
duly authorized to do so by all requisite corporate action; (c) this Agreement is legally binding
upon it, enforceable in accordance with its terms, and does not conflict with any agreement,
instrument or understanding, oral or written, to which it is a Party or by which it may be bound,
nor violate any material law or regulation of any court, governmental body or administrative or
other agency having jurisdiction over it; (d) to the best of its knowledge, there are no Third
Party intellectual property rights which could prevent it from performing all of its obligations
hereunder.
(b) Each Party represents and warrants that it will use its best efforts to obtain and
maintain in full force and effect all necessary licenses, permits and other authorizations required
by law to carry out its duties and obligations under this Agreement. Each Party shall cooperate
with the other to provide such letters, documentation and other Information on a timely basis as
the other Party may reasonably require to fulfill its reporting and other obligations to applicable
Regulatory Authorities. Except for such amounts as are expressly required to be paid by a Party to
the other under this Agreement, each Party shall be solely responsible for any costs incurred by it
to comply with its legal obligations. Each Party shall conduct its activities hereunder in an
ethical and professional manner.
(c) Each Party hereby covenants that each of its employees and other entities performing any
work under this Agreement shall have entered into a written invention assignment agreement
requiring that each such person or entity assign to such Party all right, title and interest in and
to any Information conceived of and/or reduced to practice by such person or entity and its
employees, consultants or agents in connection with any such activities.
(d) Each Party shall cooperate with the other and provide such assistance and resources as the
other Party may reasonably request in connection with performance of the obligations under this
Agreement.
9.2 Aradigm Representations and Warranties. Aradigm represents and warrants to Lung Rx that,
as of the Effective Date: (a) Aradigm Controls the Aradigm Technology in the
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
28
Territory with the
full right and authority to grant the rights and licenses granted to Lung Rx under this Agreement;
(b) Aradigm has not granted any right or license under the Aradigm Technology that is in conflict
with the rights and licenses granted to Lung Rx under this Agreement; (c) to the best of Aradigm’s
knowledge, there is no basis upon which the use of the Aradigm Technology in accordance with this
Agreement would infringe upon any Third Party’s know-how, patent or other intellectual property
rights or constitute misuse of confidential information by Lung Rx; (d) to the best of Aradigm’s
knowledge, no claim, whether or not embodied in an action past or present, of any infringement, of
any conflict with, or of any violation of any patent, trade secret or other intellectual property
right or similar right of any Third Party has been made or is pending, and Aradigm has no knowledge
of any potential claim with respect to the Aradigm Technology; (e) a complete list of (i) all
Patents as of the Effective Date and (ii) all Patents owned by Third Parties and validly and
exclusively licensed to Aradigm, with the unrestricted right to exclusively sublicense to Lung Rx,
is provided in Exhibit B attached to this Agreement; (f) to the best of Aradigm’s
knowledge, no Patent and/or other intellectual property rights Controlled by a Third Party are
required to be in-licensed by Aradigm because the Development, manufacture or Commercialization of
the Product infringes such Third Party Patent or other intellectual property rights in the
Territory, (g) other than as contained in Exhibit B, Aradigm has not prior to the Effective
Date applied for Patents involving the Licensed Molecule or other prostacyclin drugs; and (h)
Aradigm has made or will make available to Lung Rx all material technical Information in its
possession or control that is reasonably necessary to Develop, manufacture and Commercialize
Products in accordance with this Agreement.
9.3 Lung Rx Representations and Warranties. Lung Rx represents and warrants to Aradigm that,
as of the Effective Date: (a) Lung Rx Controls the Lung Rx Technology in the Territory with the
full right and authority to grant the rights and licenses granted to Aradigm under this Agreement;
(b) Lung Rx has not granted any right or license under the Lung Rx Technology that is in conflict
with the rights and licenses granted to Aradigm under this Agreement; (c) to the best of Lung Rx’s
knowledge, there is no basis upon which the use of the Licensed Molecule in accordance with this
Agreement would infringe upon any Third Party’s know-how, patent or other intellectual property
rights or constitute misuse of confidential information by Aradigm; (d) to the best of Lung Rx’s
knowledge, no claim, whether or not embodied in an action past or present, of any infringement, of
any conflict with, or of any violation of any patent, trade secret or other intellectual property
right or similar right of any Third Party has been made or is pending, and Lung Rx has no knowledge
of any potential claim with respect to the Lung Rx Technology; (e) to the best of Lung Rx’s
knowledge, no Patent and/or other intellectual property rights Controlled by a Third Party are
required to be in-licensed by Lung Rx because the Development, manufacture or Commercialization of
the Product infringes such Third Party Patent or other intellectual property rights in the
Territory; (f) Lung Rx has not prior to the Effective Date applied for Patents involving Aradigm
Technology; and (g) Lung Rx has made or will make available to Aradigm all material technical
Information in its possession or control that is reasonably necessary to Develop, manufacture and
Commercialize Products in accordance with this Agreement.
9.4 Disclaimer. EXCEPT AS EXPRESSLY SET FORTH IN SECTIONS 9.1 THROUGH 9.3, THE TECHNOLOGY AND
INTELLECTUAL PROPERTY RIGHTS
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
29
PROVIDED BY EACH PARTY UNDER THIS AGREEMENT ARE PROVIDED “AS IS”, AND
EACH PARTY MAKES NO, AND EXPRESSLY DISCLAIMS ANY AND ALL, WARRANTIES OF ANY KIND, EXPRESS OR
IMPLIED, INCLUDING THE WARRANTIES OF DESIGN, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE,
NONINFRINGEMENT OF THE INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES, OR ARISING FROM A COURSE OF
DEALING, USAGE OR TRADE PRACTICES.
ARTICLE 10
Confidentiality
10.1 Confidential Information. Except to the extent expressly authorized by this Agreement or
otherwise agreed in writing by the Parties, each Party agrees that, during the Term and for seven
(7) years thereafter, such Party shall keep confidential and shall not publish or otherwise
disclose and shall not use for any purpose other than as expressly provided for in this Agreement
any Information furnished to it by the other Party pursuant to this Agreement (the “Confidential
Information” of the disclosing Party). Each Party may use the Confidential Information of the
other Party only to the extent required to accomplish the purposes of this Agreement. Each Party
will use at least the same standard of care (but in any case not less than reasonable care) as it
uses to protect proprietary or confidential information of its own to ensure that its employees,
agents, consultants and other representatives do not disclose or make any unauthorized use of the
Confidential Information of the other Party. Each Party will promptly notify the other Party upon
discovery of any unauthorized use or disclosure of the Confidential Information of the other Party.
10.2 Exceptions. The obligations of the receiving Party under this Article 10 with respect to
specific Confidential Information of the other Party shall not apply to any specific Information
that the receiving Party can demonstrate by competent evidence: (a) is, at the time of disclosure
by the disclosing Party to the receiving Party, or thereafter becomes, through no act or failure to
act on the part of the receiving Party, generally known or available; (b) is known by the receiving
Party at the time of receiving such Information, as evidenced by its records; (c) is, after the
time of disclosure by the disclosing Party to the receiving Party, furnished to the receiving Party
by a Third Party, as a matter of right and without restriction on disclosure; or (d) is
independently discovered or developed by the receiving Party without access to or the use of any
Confidential Information of the disclosing Party.
10.3 Authorized Disclosure. Notwithstanding the other provisions of this Article 10, a Party
may disclose Confidential Information of the other Party to the extent such disclosure is
reasonably necessary in the following instances, but solely for the limited purpose of such
necessity:
(a) on a need-to-know basis and in connection with a Party’s performance or its obligations
and/or exercise of its rights under this Agreement, to its Affiliates, licensees, employees,
consultants, or agents provided that such individuals or entities are bound by
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
30
confidentiality and
non-use obligations at least equivalent in scope to those set forth in this Article 10;
(b) as expressly permitted by this Agreement;
(c) to comply with applicable laws, court orders, or governmental regulations, including
NASDAQ, and SEC disclosure requirements;
(d) to the extent that such disclosure is required by a court order, or in order to comply
with applicable laws or regulations, but provided that such Party required to make the disclosure
will, except where impracticable, shall give reasonable advance notice to the other Party of such
required disclosure and use efforts to secure, or to assist the other Party in securing, a
protective order relating to, or confidential treatment of, such information; and
(e) general information of a non-material nature regarding the general status of the
Development Plan.
10.4 Terms of Agreement. The terms of this Agreement shall be deemed to be the Confidential
Information of each Party; provided, however, that each Party may disclose the terms of this
Agreement in confidence to its investors, directors and professional advisors, and to its
prospective investors, acquirers and merger partners and their respective professional advisors.
The Parties will consult with each other reasonably on the provisions of this Agreement to be
reasonably redacted in any filings made by either Party with the Securities and Exchange Commission
or as otherwise required by law.
10.5 Publicity. Any news release or other public announcement relating to this Agreement or
to the performance hereunder, shall first be reviewed in good faith and approved by both Parties,
such approval not to be unreasonably withheld or delayed; provided, however, that any disclosure
which is required by law as advised by the disclosing Party’s counsel may be made without the prior
consent of the other Party, although the other Party shall be given prompt notice of any such
legally required disclosure and to the extent practicable shall provide the other Party with an
opportunity to comment on the proposed disclosure. In addition, either Party shall be free to
disclose, without the other Party’s prior written consent, the existence of this Agreement, the
identity of the other Party, and the terms of this Agreement, in each case to the extent such
information has already been publicly disclosed in accordance herewith.
10.6 Publication. From time to time it may be to the mutual interest of the Parties to
publish articles relating to data generated or analyzed as a part of this Agreement. Neither Party
shall submit for written or oral publication or presentation any manuscript, abstract, writing,
printed material or the like which includes data or any other information generated and provided
solely by the other Party without first obtaining the prior written consent of the other Party,
which consent shall not be unreasonably withheld or delayed, provided however, that valid
commercial reasons may exist for withholding such consent. Nothing contained herein shall be
construed as precluding either Party from making, in its discretion, any disclosures of information
of any type which relate to the safety, efficacy, toxicology, or pharmacokinetic
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
31
characteristics of
the Products to the extent that either Party may be required by law to make disclosures of such
information.
ARTICLE 11
Term and Termination
11.1 Term. The term of this Agreement (the “Term”) shall commence on the Effective Date and,
unless earlier terminated in accordance with the terms of this Article 11 or extended by mutual
agreement of the Parties, will expire, on a country-by-country basis upon the approval of a generic
Product and Patent Expiration in such country.
11.2 Termination for Breach. Each Party shall have the right to terminate this Agreement upon
forty-five (45) days’ prior written notice to the other Party if such other Party materially
breaches any provision or obligation of this Agreement and the breaching Party has not cured such
breach within the forty-five (45) days following detailed written notice describing the breach.
11.3 Termination by Lung Rx.
(a) Lung Rx shall have the right to terminate this Agreement by written notice to Aradigm in
the event that the POC Study is not Successful in the sole reasonable opinion of Lung Rx.
(b) Lung Rx shall have the right to terminate this Agreement at any time following
consultation with the PSC, upon thirty (30) days’ prior written notice to Aradigm solely for
technical, toxicology, safety, bioavailability, efficacy, regulatory, or market-related issues
(including competition, patient-related issues, prohibitive Development costs, emergence of new
technologies, impediments caused by formulation, material sourcing, manufacturing, or other
technical issues related to Product, etc.) that, in Lung Rx’s sole, reasonable and good faith
opinion, renders further Development and Commercialization of the Product unjustified.
(c) Lung Rx shall have the right to terminate this Agreement upon written notice to Aradigm if
the Parties learn that a Third Party has rights to Patents or other intellectual property that
would prevent Development and/or Commercialization of such Product and negotiations to obtain a
necessary license from such Third Party are unsuccessful.
11.4 Effect of Termination; Surviving Obligations.
(a) In the event of an expiration or termination of this Agreement for any reason, then:
(i) Each Party shall promptly return to the other Party all relevant Information, records and
materials in its possession or control containing or comprising the other Party’s Confidential
Information and to which the Party does not retain rights hereunder;
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
32
provided however, that each
Party may retain one copy of each document of the other Party’s Confidential Information to enable
such Party to determine its surviving obligations of confidentiality and non-use with respect to
the other Party’s Confidential Information.
(ii) Subject to Section 11.4(c), all rights and licenses granted by each Party to the other
shall terminate and revert.
(iii) Following a six-month sell-off period if Product was being marketed at the time of
expiration or termination of this Agreement, (A) Lung Rx and its Affiliates shall discontinue
making any representation regarding their status as licensees of Aradigm or a distributor of the
Product, and shall cause any sublicensees to do the same, and (B) Lung Rx and its Affiliates shall
cease conducting any activities with respect to the marketing, promotion, sale or distribution of
the Product, and shall cause any sublicensees to do the same.
(b) In the event of any termination of this Agreement (but not its expiration) other than
termination by Lung Rx pursuant to Section 11.2 for Aradigm’s uncured material breach or pursuant
to Section 11.3(a) in the event that the POC Study is not Successful, the following shall also
apply (i.e., in addition to the provisions of Section 11.4(d)):
(i) As soon as reasonably practicable, Lung Rx shall ship to a location designated by Aradigm
all of the AERx technology-related manufacturing equipment, portable infrastructure, and materials
(except the Licensed Molecule) in Lung Rx’s possession or on order as of the effective date of
termination or expiration of this Agreement. All costs associated with such shipment or the
preparation of such shipment shall be borne solely by Lung Rx.
(ii) Lung Rx shall immediately assign and transfer to Aradigm the entire Regulatory Dossier,
except for those documents relating solely to Lung Rx Technology.
(iii) Each Party shall promptly execute any and all other instruments, forms of assignment or
other documents and take such further actions as the other Party may reasonably request in order to
give effect to or evidence the assignments and grants made to a Party in accordance with this
Agreement.
(c) In the event of a termination of this Agreement by Lung Rx pursuant to Section 11.2 for
Aradigm’s uncured material breach, then:
(i) all licenses granted to Lung Rx with respect to the Product set forth in Section 2.1 shall
remain in full force and effect;
(ii) all licenses granted to Aradigm pursuant to Section 2.2 shall terminate, and all of
Aradigm’s Development obligations under this Agreement (including any outstanding obligations with
respect to the POC Study) shall cease;
(iii) Lung Rx’s obligations under Article 6 “Fees and Payments” shall survive so long as
Aradigm has fully transferred the Aradigm Technology to Lung Rx in accordance with section 5.2(a)
above, or the full and complete approval of the manufacturing
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
33
plant and full and complete
validation of the manufacturing process has occurred in accordance with section 5.1(b)(i) and (ii)
above;
(iv) the PSC shall be abolished, and Lung Rx shall have the right to make any decisions that,
absent termination of this Agreement, would have been the right and responsibility of the PSC; and
(v) in addition to the provisions set forth in Section 11.4(d), the following provisions shall
also survive: Articles 7 and 8, and Sections 4.3(b) and (c), 4.7, 4.8, 4.9 and 4.13.
(d) Expiration or termination of this Agreement shall not relieve the Parties of any
obligation accruing prior to such expiration or termination, including any payments owed by Lung Rx
pursuant to Article 6 or Section 4.6, nor preclude either Party from pursuing all rights and
remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement,
nor prejudice either Party’s right to obtain performance of any obligation. Except as set forth
below or elsewhere in this Agreement, the obligations and rights of the Parties under the following
provisions shall survive expiration or termination of this Agreement: Articles 1, 9, 10, 12, 13
and 14, and Sections 7.4, 8.2, 8.3, 8.4, 8.5, 11.4(a), 11.5 and 11.6.
11.5 Damages; Relief. Termination of this Agreement shall not preclude either Party from
claiming any other damages, compensation or relief that it may be entitled to upon such
termination.
11.6 Rights in Bankruptcy.
(a) In the event of the institution by or against either Party of insolvency, receivership,
bankruptcy proceedings, or any other proceedings for the settlement of a Party’s debts which are
not dismissed within sixty (60) days, or upon a Party’s making an assignment for the benefit of
creditors, or upon a Party’s dissolution or ceasing to do business, the other Party may terminate
this Agreement upon written notice in its sole discretion.
(b) All rights and licenses granted under or pursuant to this Agreement by each Party (the
“Licensor”) are, and will otherwise be deemed to be, for purposes of Section 365(n) of the U.S.
Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the
U.S. Bankruptcy Code. The Parties agree that the other Party (the “Licensee”), as licensee of such
rights under this Agreement, will retain and may fully exercise all of its rights and elections
under the U.S. Bankruptcy Code; provided however, nothing herein shall be deemed to constitute a
present exercise of such rights and elections. In the event of the commencement of a bankruptcy
proceeding by or against the Licensor under the U.S. Bankruptcy Code, the Licensee will be entitled
to a complete duplicate of (or complete access to, as appropriate) any such intellectual property
and all embodiments of such intellectual property, and same, if not already in its possession, will
be promptly delivered to it (i) upon any such commencement of a bankruptcy proceeding upon its
written request therefor, unless the
Licensor elects to continue to perform all of its obligations under this Agreement, or (ii) if not
delivered under (i) above, following the rejection of this Agreement by or on behalf of the
Licensor upon written request therefor by the Licensee.
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
34
ARTICLE 12
Indemnification
12.1 Indemnification by Aradigm. Subject to Lung Rx’s timely notice obligation in Section
12.3, Aradigm hereby agrees to indemnify, defend and hold Lung Rx and its Affiliates and their
respective directors, officers, employees and agents (each, a “Lung Rx Indemnitee”) harmless from
and against any and all claims, suits, actions, demands, liabilities, expenses and/or loss,
including reasonable legal expense and attorneys’ fees and amounts paid in settlement of any action
(collectively, “Losses”), to which any Lung Rx Indemnitee may become subject as a result of any
claim, demand, action or other proceeding by any Third Party to the extent based on or resulting
from (a) the practice by Lung Rx of any license granted by Aradigm under this Agreement, (b) the
negligence or willful misconduct of Aradigm, its Affiliates, or their respective directors,
officers, employees, or agents in the performance of Aradigm’s obligations under this Agreement,
including product liability claims relating to an item or technology manufactured or supplied by or
on behalf of Aradigm; and (c) the breach by Aradigm of any warranty, representation, covenant or
agreement made by Aradigm in this Agreement; except, in each case, to the extent such Losses result
from the negligence or willful misconduct of any Lung Rx Indemnitee or the breach by Lung Rx of any
warranty, representation, covenant or agreement made by Lung Rx in this Agreement.
12.2 Indemnification by Lung Rx. Subject to Aradigm’s timely notice obligation in Section
12.3, Lung Rx hereby agrees to indemnify, defend and hold Aradigm and its Affiliates and their
respective directors, officers, employees and agents (each, an “Aradigm Indemnitee”) harmless from
and against any and all Losses to which any Aradigm Indemnitee may become subject as a result of
any claim, demand, action or other proceeding by any Third Party to the extent based on or
resulting from: (a) the practice by Aradigm of any license granted by Lung Rx under this
Agreement, (b) the negligence or willful misconduct of Lung Rx, its Affiliates, or their respective
directors, officers, employees, or agents in the performance of Lung Rx’s obligations under this
Agreement, including product liability claims relating to an item or technology manufactured or
supplied by or on behalf of Lung Rx; (c) the Development, manufacture, use, handling, storage, sale
or other disposition of any Product by Lung Rx, its Affiliates, or sublicensees, or by any of their
respective suppliers or distributors, or (d) the breach by Lung Rx of any warranty, representation,
covenant or agreement made by Lung Rx in this Agreement; except, in each case, to the extent such
Losses result from the negligence or willful misconduct of any Aradigm Indemnitee or the breach by
Aradigm of any warranty, representation, covenant or agreement made by Aradigm in this Agreement.
12.3 Control of Defense. Any entity entitled to indemnification under this Article 12 shall
(i) give written notice to the indemnifying Party of any Losses that may be subject to
indemnification, promptly after learning of such Losses, and (ii) cooperate fully with the
indemnifying Party in connection with the investigation and defense of such claim or lawsuit, and
the indemnifying Party shall assume the defense of such Losses with counsel reasonably satisfactory
to the indemnified Party. If such defense is assumed by the indemnifying Party with
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
35
counsel so
selected, the indemnifying Party will not be subject to any liability for any settlement of such
Losses made by the indemnified Party without its consent (but such consent will not be unreasonably
withheld or delayed), and will not be obligated to pay the fees and expenses of any separate
counsel retained by the indemnified Party with respect to such Losses.
12.4 Insurance. Lung Rx, at its own expense, shall maintain product liability insurance in an
amount of not less than [*] per occurrence and [*] in the aggregate during the Term of the
Agreement and shall name Aradigm as an additional insured with respect to such insurance or self
insure for the same or higher coverage limits. Lung Rx shall provide a certificate of insurance
evidencing such coverage to Aradigm upon request.
ARTICLE 13
Dispute Resolution
13.1 Disputes. The Parties recognize that issues or disputes as to certain matters may arise
from time-to-time during the Term relating to or under this Agreement. It is the objective of the
Parties to seek to resolve any issues or disputes arising under this Agreement in good faith in an
expedient manner and, if at all possible, without resort to litigation, and to that end the Parties
agree to abide by the following procedures set forth in this Article 13 to resolve any such issues
or disputes arising under or relating to this Agreement, including any Party’s rights or
obligations or performance under this Agreement (each, a “Dispute”). The Parties initially shall
attempt to settle any such Dispute through good faith negotiations in the spirit of mutual
cooperation between business executives with authority to resolve the Dispute. Notwithstanding
anything to the contrary set forth herein, any issue or dispute falling within the PSC’s authority
will be handled in accordance with Section 3.4, not this Article 13.
13.2 Escalation. Prior to taking action as provided in Section 13.3 or 13.4 below, and at the
request of any Party if there is a Dispute, the Parties shall first submit such Dispute to the
Parties’ respective Senior Executives for good faith discussion and attempted resolution. The
Senior Executives to whom any Dispute is submitted shall attempt to resolve the dispute through
good faith negotiations over a reasonable period, not to exceed forty-five (45) days, unless the
Senior Executives mutually agree in writing to extend such period of negotiation. Such forty-five
(45) day period shall be deemed to commence on the date the dispute was submitted by a Party to the
Senior Executives. The Senior Executives shall, if mutually agreed by the Senior Executives,
submit the dispute to voluntary mediation at such place and following such procedures as the
Parties shall reasonably agree. All negotiations and discussions pursuant to this Section 13.2
shall be confidential, and the Parties agree that all information concerning or disclosed as part
of such negotiations and discussions are and such shall be treated as compromise and settlement
negotiations for purposes of applicable rules of evidence.
13.3 Arbitration. Any Dispute that is not resolved by the Parties by negotiation and/or
mediation pursuant to Sections 13.1 and/or 13.2 above shall, upon the submission of a written
request of either Party to the other Party within forty-five (45) days of initiating such
negotiations or mediation, be submitted for non-binding arbitration before a three-person panel
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
36
of arbitrators (the “Panel”), conducted in accordance with the rules of arbitration of the American
Arbitration Association for commercial disputes (the “Rules”), except to the extent that such Rules
are inconsistent with this Agreement. Each Party shall select one independent, neutral arbitrator
with experience and expertise in pharmaceutical licensing and in the particular area involved in
the Dispute (a “Party Arbitrator”), and shall notify the other Party of its selection of such Party
Arbitrator within twenty (20) days after receipt by one Party of the other Party’s written request
for binding arbitration. The two (2) Party Arbitrators shall then mutually select a third
arbitrator (a “Neutral Arbitrator”) in accordance with the Rules. The Panel shall resolve the
Dispute in accordance with this Agreement and the substantive rules of law (but not the rules of
procedure or conflicts of laws) that would be applied by a federal court sitting in New York. The
final decision of the Panel shall be appealable. The arbitration shall take place in New York, New
York and shall be conducted in the English language. The Parties agree that they shall share
equally the cost of the arbitration filing and hearing fees, and the cost of the arbitrators that
constitute the Panel. Each Party shall bear its own attorneys’ and expert fees and all associated
costs and expenses. Notwithstanding the foregoing, the arbitrators may in their discretion award
to the prevailing Party reimbursement of its reasonable attorneys’ fees, expert witness fees and
out-of-pocket costs incurred in connection with such proceeding, in addition to any other relief
such Party may be awarded.
13.4 Court Actions. Notwithstanding the foregoing provisions of this Article 13, to the full
extent allowed by law, either Party may bring an action in any court of competent jurisdiction for
injunctive relief (or any other provisional remedy) to protect the Parties’ rights or enforce the
Parties’ obligations under this Agreement pending final resolution of any claims related thereto in
an arbitration proceeding as provided above. In addition, either Party may bring an action in any
court of competent jurisdiction to resolve disputes pertaining to the validity, construction,
scope, enforceability, infringement or other violations of patents or other proprietary or
intellectual property rights. The Parties shall use Commercially Reasonable Efforts to conduct all
dispute resolution procedures under this Agreement as expeditiously, efficiently and
cost-effectively as possible.
ARTICLE 14
General Provisions
14.1 Governing Law. This Agreement shall be governed by, and construed and enforced in
accordance with, the laws of the State of New York, excluding its conflicts of laws principles. No
lawsuit pertaining to any matter arising under or growing out of this Agreement shall be instituted
in any jurisdiction other than in the courts located in the State of New York, and the Parties
consent to exclusive jurisdiction before the federal or state courts of the State of New York.
14.2 Entire Agreement; Modification. This Agreement is both a final expression of the
Parties’ agreement and a complete and exclusive statement with respect to all of its terms. This
Agreement supersedes all prior and contemporaneous agreements and communications, whether oral,
written or otherwise, concerning any and all matters contained herein. No rights or
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
37
licenses with
respect to any intellectual property of either Party are granted or deemed granted hereunder or in
connection herewith, other than those rights expressly granted in this Agreement. This Agreement
may only be modified or supplemented in a writing expressly stated for such purpose and signed by
the Parties to this Agreement.
14.3 Relationship Between the Parties. The Parties’ relationship, as established by this
Agreement, is solely that of independent contractors. This Agreement does not create any
partnership, joint venture or similar business relationship between the Parties. Neither Party is
a legal representative or agent of the other Party, and neither Party can assume or create any
obligation, representation, warranty or guarantee, express or implied, on behalf of the other Party
for any purpose whatsoever.
14.4 Performance by Affiliates, Sublicensees and Subcontractors. The Parties recognize that
each may perform some or all of its obligations or exercise some or all of its rights under this
Agreement (to the extent expressly permitted hereby) through one or more Affiliates, subcontractors
or sublicensees; provided, however, that each Party shall remain responsible and be guarantor of
the performance by its Affiliates, subcontractors and sublicensees and shall cause its Affiliates,
subcontractors and sublicensees to comply with the provisions of this Agreement in connection with
such performance. In particular, if any Affiliate, subcontractor or sublicensee of a Party
participates in research, Development or Commercialization activities under this Agreement or with
respect to Products, the restrictions of this Agreement which apply to the activities of such Party
with respect to Products shall apply equally to the activities of such Affiliate, subcontractor or
sublicensee.
14.5 Non-Waiver. The failure of a Party to insist upon strict performance of any provision of
this Agreement or to exercise any right arising out of this Agreement shall neither impair that
provision or right nor constitute a waiver of that provision or right, in whole or in part, in that
instance or in any other instance. Any waiver by a Party of a particular provision or right shall
be in writing, shall be as to a particular matter and, if applicable, for a particular period of
time and shall be signed by such Party.
14.6 Assignment. Except as expressly provided hereunder, neither this Agreement nor any
rights or obligations hereunder may be assigned or otherwise transferred by either Party without
the prior written consent of the other Party (which consent shall not be unreasonably withheld or
delayed); provided, however, that either Party may assign this Agreement and its rights and
obligations hereunder without the other Party’s consent:
(a) to its successor in interest in connection with the transfer or sale of all or
substantially all of the business of such Party, whether by acquisition, merger, sale of stock,
sale of assets or other similar transaction; or
(b) to an Affiliate, provided that the assigning Party shall remain liable and responsible to
the non-assigning Party hereto for the performance and observance of all such duties and
obligations by such Affiliate.
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
38
The rights and obligations of the Parties under this Agreement shall be binding upon and inure to
the benefit of the successors and permitted assigns of the Parties. Any assignment not in
accordance with this Agreement shall be void. In the event of a transaction in which this
Agreement is actually assigned or is assumed by the acquiring party by operation of law (e.g., in
the context of a reverse triangular merger), intellectual property rights of the acquiring party to
such transaction (if other than one of the Parties to this Agreement) shall not be included in the
technology licensed to the other Party (i.e., the Party that is not participating in such
transaction) under this Agreement.
14.7 No Third Party Beneficiaries. This Agreement is neither expressly nor impliedly made for
the benefit of any Party other than those executing it.
14.8 Severability. If, for any reason, any part of this Agreement is adjudicated invalid,
unenforceable or illegal by a court of competent jurisdiction, such adjudication shall not affect
or impair, in whole or in part, the validity, enforceability or legality of any remaining portions
of this Agreement. All remaining portions shall remain in full force and effect as if the original
Agreement had been executed without the invalidated, unenforceable or illegal part. The Parties
shall make a good faith effort to replace any invalid or unenforceable provision with a valid and
enforceable one such that the objectives contemplated by the Parties when entering this Agreement
may be realized
14.9 Notices. Any notice, consent or report required or permitted to be given under this
Agreement shall be in writing, shall specifically refer to this Agreement and shall be delivered
either in person or by overnight courier or facsimile confirmed thereafter by any of the foregoing,
to the Party to be notified at its address(es) given below, or at any address such Party has
previously designated by prior written notice to the other. Notice shall be deemed sufficiently
given for all purposes upon the earliest of: (a) the date of actual receipt or (b) if delivered by
overnight courier, the next business day the overnight courier regularly makes deliveries.
If to Lung Rx, notices must be addressed to:
Lung Rx, Inc.
0000 Xxxxxx Xxxxxx
Xxxxxx Xxxxxx, Xxxxxxxx 00000
Attention: [*]
Facsimile: [*]
0000 Xxxxxx Xxxxxx
Xxxxxx Xxxxxx, Xxxxxxxx 00000
Attention: [*]
Facsimile: [*]
With a copy to:
Lung Rx, Inc.
0000 Xxxxxxxxxxx Xxxxxx, X.X.
Xxxxxxxxxx, X.X. 00000
Attention: [*]
Fax Number: [*]
0000 Xxxxxxxxxxx Xxxxxx, X.X.
Xxxxxxxxxx, X.X. 00000
Attention: [*]
Fax Number: [*]
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
39
If to Aradigm, notices must be addressed to:
Aradigm Corporation
0000 Xxxxx Xxxx Xxx
Xxxxxxx, Xxxxxxxxxx 00000
Attention: Xxxx Xxxxx, Ph.D
Facsimile: [*]
0000 Xxxxx Xxxx Xxx
Xxxxxxx, Xxxxxxxxxx 00000
Attention: Xxxx Xxxxx, Ph.D
Facsimile: [*]
With a copy to:
Attention: [*]
Facsimile: [*]
Facsimile: [*]
14.10 Force Majeure. Except for the obligation to make payment when due, each Party shall be
excused from liability for the failure or delay in performance of any obligation under this
Agreement by reason of any event beyond such Party’s reasonable control including but not limited
to Acts of God, fire, flood, explosion, earthquake, or other natural forces, war, civil unrest,
accident, destruction or other casualty, any lack or failure of transportation facilities, any lack
or failure of supply of raw materials, any strike or labor disturbance, or any other event similar
to those enumerated above. Such excuse from liability shall be effective only to the extent and
duration of the event(s) causing the failure or delay in performance and provided that the Party
has not caused such event(s) to occur. Notice of a Party’s failure or delay in performance due to
force majeure must be given to the other Party within ten (10) days after its occurrence. All
delivery dates under this Agreement that have been affected by force majeure shall be tolled for
the duration of such force majeure. In no event shall any Party be required to prevent or settle
any labor disturbance or dispute. Notwithstanding the foregoing, should the event(s) of force
majeure suffered by a Party extend beyond a 30-day period, the other Party may then terminate this
Agreement in its sole discretion by written notice to the non-performing Party, with the
consequences of such termination as set forth in Section 11.4.
14.11 Limitation of Liability. NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER
PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THIS AGREEMENT
OR ANY LICENSE GRANTED HEREUNDER; PROVIDED, HOWEVER, THAT THIS SECTION 14.11 SHALL NOT BE CONSTRUED
TO LIMIT EITHER PARTY’S INDEMNIFICATION OBLIGATIONS UNDER ARTICLE 12.
14.12 Further Actions. Each Party agrees to execute, acknowledge and deliver such further
instruments, and to do all such other acts, as may be necessary or appropriate in order to carry
out the purposes and intent of this Agreement.
14.13 Interpretation.
(a) Captions and Headings. The captions and headings preceding the text of the articles,
sections, subsections and paragraphs hereof are inserted solely for convenience and ease of
reference only and shall not constitute any part of this Agreement, or have any effect on its
interpretation or construction.
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
40
(b) Singular and Plural. All references in this Agreement to the singular shall include the
plural where applicable, and all references to gender shall include both genders and the neuter.
(c) Articles, Sections & Subsections. Unless otherwise specified, references in this
Agreement to any article shall include all sections, subsections, and paragraphs in such article;
references in this Agreement to any section shall include all subsections and paragraphs in such
sections; and references in this Agreement to any subsection shall include all paragraphs in such
subsection.
(d) Days. All references to days in this Agreement shall mean calendar days, unless otherwise
specified.
(e) Ambiguities. Ambiguities and uncertainties in this Agreement, if any, shall not be
interpreted against either Party, irrespective of which Party may be deemed to have caused the
ambiguity or uncertainty to exist.
(f) English Language. This Agreement has been prepared in the English language and the
English language shall control its interpretation. In addition, all notices required or permitted
to be given hereunder, and all written, electronic, oral or other communications between the
Parties regarding this Agreement shall be in the English language.
(g) Exemplary Term. The term “including” shall be deemed to mean “including without
limitation”, and the matters referenced directly after the term “including” shall be deemed to be
examples only and not to limit the subject matter coming before the term.
14.14 Counterparts. This Agreement may be executed in two or more counterparts, each of which
shall be deemed an original document, and all of which, together with this writing, shall be deemed
one instrument.
[Remainder of this page intentionally left blank.]
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
41
In Witness Whereof, the Parties hereto have duly executed and entered into this
License, Development, and Commercialization Agreement as of the Effective Date.
| Aradigm Corporation | Lung Rx, Inc. | |||||
By:
|
/s/ Xxxx Xxxxx | By: | /s/ [*] | |||
Name:
|
Xxxx Xxxxx | Name: | [*] | |||
Title:
|
President and Chief Executive Officer | Title: | [*] | |||
[ * ] = Certain confidential information contained in this document, marked by brackets, has
been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule
24b-2 of the Securities Exchange Act of 1934, as amended.
42
Exhibit A
Aradigm FTE Calculation Methodology
[*]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
43
Exhibit B
Certain Aradigm Patents
[*]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
44
Exhibit C
Licensed Molecule
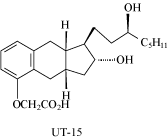
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
45
Exhibit D
In vivo and In vitro Bridging POC Study and Success Criteria for AERx EssenceÒ
technology with Treprostinil
[*]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
46
Exhibit E
Initial Development Plan
[*]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
47


