License Agreement
Exhibit 99.4
Between
and
Maruho Co. Ltd.
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| Table of Contents | ||
| Preamble | 3 | |
| 1. | Definitions | 3 |
| 2. | License | 7 |
| 3. | Considerations | 8 |
| 4. | Dossier | 10 |
| 5. | Obtaining and maintaining the Marketing Authorizations | 11 |
| 6. | Further Development and Cooperation | 12 |
| 7. | Manufacture and supply of Product | 13 |
| 8. | Illumination Device with the Product | 14 |
| 9. | Compliance with Regulations | 15 |
| 10. | Commercialization | 15 |
| 11. | Records and Audits | 15 |
| 12. | Confidentiality | 16 |
| 13. | Representations and Undertakings | 17 |
| 14. | Third party infringements; defence against Third Party claims and invalidity attacks | 18 |
| 15. | Liability | 20 |
| 16. | Indemnification | 20 |
| 17. | Force Majeure | 21 |
| 18. | Term and Xxxxxxxxxxx | 00 |
| 00. | Applicable Law and Venue | 23 |
| 20. | Notices and Other Communications | 24 |
| 21. | General Provisions | 24 |
| 2/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
Between
| (1) | Biofrontera AG, with registered business address at Hemmelrather Xxx 000, 00000 Xxxxxxxxxx, Xxxxxxx (“BIOFRONTERA”); |
| and | |
| (2) | Maruho Co., Ltd., a Japanese corporation having its principal office at 0-0-00 Xxxxxxx, Xxxx-xx, Xxxxx, Xxxxx, 000-0000 (“MARUHO”). |
Preamble
| (A) | BIOFRONTERA engages in the development, production and marketing of pharmaceutical products and cosmetics; |
| (B) | MARUHO has the facilities to research, develop, register, make, import, promote, market, sell, resell, distribute and use prescription drugs, medical devices and cosmetics in the Territory; |
| (C) | BIOFRONTERA is the owner of certain proprietary information and certain intellectual property rights relating to a product containing the active ingredient 5-aminolevulinic acid (5-ALA); |
| (D) | BIOFRONTERA wishes to grant, and MARUHO wishes to obtain a license to use certain proprietary information for the purpose of Marketing Authorizations for products containing the active ingredient 5-ALA in the Territory and for the development, registration, import, distribution, promotion, marketing, use, and sale of such products in the Territory; |
Therefore, BIOFRONTERA and MARUHO (together the “Parties”, each a “Party”) agree as follows (the “Agreement”):
| 1. | Definitions |
| In addition to the terms defined above and other terms defined in other Sections of this Agreement the following initially capitalized terms have the meanings set forth below for purposes of this Agreement: | |
| 1.1 | “Active Ingredient” shall mean the active substance(s) listed in ANNEX 1.1. |
| 1.2 | “Additional R&D” shall have the meaning as defined in Section 2.3. |
| 1.3 | “Affiliate” shall mean, with respect to either Party, any person that controls, is controlled by, or is under common control with that Party. For the purpose of this definition, “control” (including, with correlative meaning, the terms “controlled by” and “under the common control”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of such person, whether by the ownership of more than 50 % (fifty per cent) of the voting stocking of such person, by contract or otherwise. |
| 3/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 1.4 | “Annual Net Sales” shall mean Net Sales (as defined in Section 1.28) in the period from October 1st of one calendar year to September 30th of the subsequent calendar year. |
| 1.5 | “BIOFRONTERA Dossiers” shall mean the registration dossiers relating to the Product prepared by BIOFRONTERA, in particular the Common Technical Document (CTD) as specified in Annex 1.5, and all information, data, know-how and documents contained therein or necessary or useful for developing, obtaining and/or maintaining the Marketing Authorizations for the Product in the Territory, and as updated from time to time. |
| 1.6 | “Commercially Reasonable Efforts” shall mean with respect to a Party’s obligations or tasks under this Agreement with respect to the Territory, the performance of such obligations or tasks by a Party in an active and sustained manner, without undue interruption, pause or delay, using a level of effort and resources consistent with the exercise of good faith and prudent scientific and business judgment as commonly practiced in the pharmaceutical industry for the development and commercialization of similarly situated products as the Product at a similar stage of its product life (e.g, development or commercialization), taking into account efficacy, safety, the proprietary position of the Product (e.g., patent and regulatory exclusivity), the regulatory status (e.g., anticipated or approved labeling), the establishment of the Product in the market, present and future market potential, competitive market conditions, the profitability of the Product in the light of pricing and reimbursement issues, including rebates under risk sharing schemes, and all other relevant factors, and corresponding at least to the type (quality and quantity) of channels, methods, investments and staff (including, without limitation, sales force), which are used by reputable pharmaceutical companies in the marketing, promotion and sales of products with a similar potential in the Territory, but without regard to other products then being developed or commercialized by the Party required to apply such level of efforts pursuant to this Agreement. |
| 1.7 | “Competing Product” shall mean a topical product having aminolevulinic acid and/or methyl-aminolevulinic acid as active ingredient. |
| 1.8 | “Compact Device” shall have the meaning defined in Section 8.3. |
| 1.9 | “Confidential Information” shall mean all commercial, financial, technical or other information made accessible to or otherwise disclosed by one Party to the other Party under or in connection with this Agreement. |
| 1.10 | “Control” shall mean with respect to any patents or other intellectual property rights, or know-how, possession of the right to disclose to and grant a license to such items under the terms of this Agreement without violating the terms of any agreement with any third party, or any applicable law or governmental regulation, or incurring any financial or other additional obligation. |
| 4/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 1.11 | “Device” shall have the meaning as defined in Section 8.1. |
| 1.12 | “Device Supply Agreement” shall mean the agreement on the supply of the Device by BIOFRONTERA to the MARUHO to be negotiated by the Parties immediately following the Effective Date. |
| 1.13 | “Effective Date” means the date of the last signature to this Agreement. |
| 1.14 | “Field” means the prevention, diagnosis and treatment for all indications including medical and aesthetic therapy (including, but not limited to, OTCs, quasi-drugs and cosmetics) |
| 1.15 | “Force Majeure” shall have the meaning as defined in clause 17 of this Agreement. |
| 1.16 | “Generic Product” shall mean a product that is introduced in the Territory by an entity other than MARUHO or a Sublicensee or their respective Affiliates, which contains the same active ingredient, has the same indications and is approved in reliance, in whole or in part, on a prior Regulatory Approval of a Product by an applicable Regulatory Authority. |
| 1.17 | “ICH” shall mean the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. |
| 1.18 | “Initial Term” shall have the meaning as defined in Section 18.1 of this Agreement. |
| 1.19 | “JSC” shall have the meaning as defined in Section 6.2. |
| 1.20 | “Licensed Proprietary Information” shall mean all scientific, medical, toxicological, pharmacological, analytical, clinical and technical data and information, on processes, methods and techniques and other know-how in the ownership of, or Controlled by, BIOFRONTERA relating to the Active Ingredient and/or the Product, particularly the BIOFRONTERA Dossiers and, if required for the MARUHO Dossier, other documents provided by BIOFRONTERA, but notwithstanding anything to the contrary herein, under exclusion of the MARUHO Developments. Any Licensed Proprietary Information related to material remains property of BIOFRONTERA. |
| 1.21 | “Licensed Patents” shall mean the Patents that are Controlled by BIOFRONTERA which are used for the marketing, sale or use of the Product in the Territory as exclusively specified in ANNEX 1.21. |
| 1.22 | “Licensed Trademarks” shall mean the trademarks exclusively specified in ANNEX 1.22. MARUHO may use the trademarks exclusively in the form shown in ANNEX 1.22 hereto. |
| 1.23 | “Marketing Authorization” shall mean any approval, license, registration or other regulatory Authorization by the competent Regulatory Authority/ies in the Territory relating to the Product which may be required in the Territory to import, distribute, promote, market, use and/or sell the Product in the Territory, including (without limitation) price and reimbursement approvals. |
| 5/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 1.24 | “MARUHO Dossiers” shall mean the registration dossiers relating to the Product prepared by MARUHO and all information, data and documents contained therein and necessary or useful for developing, obtaining and/or maintaining the Marketing Authorizations for the Product in the Territory, and as updated from time to time. |
| 1.25 | “MARUHO Developments” shall mean the Results of research and development (including, without limitation, the discovery of additional indications and formulation design) concerning the Active Ingredient and/or the Product carried out by MARUHO in the Territory in the Field, irrespective of whether as part of the Additional R&D or otherwise. |
| 1.26 | “Milestone Events” shall have the meaning as specified in clause 3.3 of this Agreement. |
| 1.27 | “Milestone Payments” shall have the meaning as specified in clause 3.3 of this Agreement. |
| 1.28 | “Net Sales” shall mean the amounts invoiced by MARUHO as well as by Sublicensees on all sales of the Product in the Territory to its customers, less the following deductions, in each case related specifically to the Product and actually allowed and taken by such third parties and not otherwise recovered or reimbursed: (A) credits and allowances or adjustments (consistent with generally accepted accounting principles), granted to such customers on account of rejections, recalls or returns of the Product and; (B) any trade and cash discounts, rebates, including government rebates, granted in connection with the sale of the Product to such customers, (C) value added taxes and (D) distribution costs (which shall only include costs for transportation of Products from warehouse of MARUHO as well as warehouse of Sublicensees to its customers). |
| 1.29 | “Party/ies” shall mean either BIOFRONTERA or MARUHO, or both, as the case may be. |
| 1.30 | “Patents” shall mean worldwide (i) all patents, utility models, supplementary protection certificates and all applications and term extensions of all kind for such rights, (ii) all other intellectual protection rights for inventions worldwide, and applications for such rights, including, without limitation, provisionals, substitutions, extensions, reissues, re-exams, renewals, divisions, continuations, continuations-in-part, continued prosecution applications and term extensions, (iii) the right to claim priority from any of the aforementioned rights, and (iv) all other rights and claims resulting worldwide from inventions (under exclusion of inventor’s personal rights), including in particular the right to a patent. |
| 1.31 | “Product” shall mean Ameluz (10% 5-ALA gel using nanoemulsion technology) as well as any other formulation of Active Ingredient and any other concentration of Active Ingredient using nano-emulsion technology. |
| 1.32 | “Quality Agreement” shall mean the agreement on the quality of the Product between BIOFRONTERA and MARUHO to be negotiated by the Parties immediately following the Effective Date. |
| 1.33 | “Regulatory Authority” shall mean any governmental authority or notified body competent for issuing Marketing Authorizations for the Product within the Territory. |
| 6/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 1.34 | “Results” means all materials, data and other information generated pursuant to this Agreement, including without limitation, findings, test results, screening results, discoveries, inventions, know-how, work of authorship, software, processes, methods, techniques, formulae, substances, specifications, studies, designs or improvements whatsoever (whether patentable or not) as well as all raw data obtained as a result of screenings or studies conducted, and all experimental procedures developed that are originated, conceived, derived, produced, discovered, invented or otherwise made by each of the Parties either separately or jointly under this Agreement. |
| 1.35 | “Specifications” shall mean the technical characteristics of the Product as approved by the respective Regulatory Authorities in the Territory and set out in the respective Annex to the Supply Agreement and the Quality Agreement. |
| 1.36 | “Supply Agreement” shall mean the agreement on the supply of the Product by BIOFRONTERA to the MARUHO to be negotiated by the Parties immediately following the Effective Date. |
| 1.37 | “Sublicensee” shall mean any Affiliate or Third Party to whom MARUHO grants sub-licenses according to this Agreement. |
| 1.38 | “Territory” shall mean the countries listed in ANNEX 1.38. |
| 1.39 | “Third Party” shall mean any party other than the Parties and their Affiliates. |
| 1.40 | “Upfront Payment” shall have the meaning as specified in clause 3.2 of this Agreement. |
In this Agreement, unless the context requires otherwise: (i) references to clauses and annexes are to clauses of and annexes to this Agreement; (ii) references to the singular shall include the plural and vice versa; (iii) the annexes will have the same force and effect as if expressly set out in the body of this Agreement; and (iv) headings are inserted for convenience only and shall not affect the construction of the Agreement.
| 2. | License |
| 2.1 | BIOFRONTERA hereby grants to MARUHO, and MARUHO hereby accepts, an exclusive, transferable to Affiliates, and – subject to Section 2.2 – sub-licensable license, to use the Licensed Proprietary Information, the Licensed Patents and the Licensed Trademarks for (i) researching and developing as well as using Product, (ii) obtaining and/or maintaining Marketing Authorizations for Product, and/or (iii) commercializing (i.e. importing, distributing, marketing and selling) Product, in each case in the Territory. |
| 2.2 | MARUHO shall have the right to sublicense, without any further consideration, the Licensed Proprietary Information and the Licensed Patents as well as Licensed Trademark to Affiliates and to any Third Party, it being understood that MARUHO shall notify BIOFRONTERA of any sublicense to Third Parties in writing prior to the grant of such sublicense. The Parties share the common understanding that all sub-licenses granted by MARUHO are subject to an existing and valid main license under this Agreement between BIOFRONTERA and MARUHO and any sub-license is granted by MARUHO under the stipulation that it automatically terminates in the event the license to MARUHO under this Agreement ends. |
| 7/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| MARUHO undertakes vis-à-vis BIOFRONTERA to include a dissolving condition (“auflösende Bedingung”) in the sublicense agreement with any Sublicensee, according to which the sub-license will automatically and ipso jure lapse if this Agreement on which the sub-license is based does not longer exist, i.e. as a result of expiration or termination of the Agreement according to Section 18. The sub-license agreement must also contain a clause stating that all rights granted to any Sublicensee (Affiliate and/or any Third Party) according to the sublicense agreement will be relinquished to BIOFRONTERA in the event of the occurrence of the dissolving condition (“auflösende Bedingung”). MARUHO shall be liable vis-à-vis BIOFRONTERA for all damages resulting from any omission of these obligations. | |
| Except as explicitly stipulated in this Agreement, BIOFRONTERA shall not be entitled to receive any license fees or margin share of payments made to MARUHO by any Sublicensee. | |
| 2.3 | MARUHO is entitled to perform additional research and development with the aim of (i) developing any other formulations, i.e. any formulation design other than Ameluz(10% 5-ALA gel using nanoemulsion technology), in the Territory and in the Field, and/or (ii) discovering additional indications, i.e. any indications other than actinic keratosis, basal cell carcinoma and acne (including acne scar) for the Product (“Additional R&D”), in each case upon prior written consent by BIOFRONTERA, which consent is not to be unreasonably withheld and to be given within [***] weeks of a notice by MARUHO of its intention to start such Additional R&D. MARUHO shall own the entire right, title, and interest, including any and all intellectual property, in and to all Results generated or obtained or otherwise perceived or reduced to practice in the course of the Additional R&D. BIOFRONTERA shall be entitled to participate in, and contribute to, Additional R&D in each case upon prior written consent by MARUHO, which consent is not to be unreasonably withheld and to be given within [***] weeks of a notice by BIOFRONTERA of its intention to participate in, and contribute to such Additional R&D. Irrespective of whether BIOFRONTERA participated in such Additional R&D or did not participate, BIOFRONTERA shall have the right to be granted by MARUHO a royalty-free, non-exclusive licence to make use of and market the results of the Additional R&D by MARUHO outside of the Territory. |
| 2.4 | MARUHO may select the names for the Products in the Territory on a country-by-country basis. BIOFRONTERA shall choose, either to (i) register and maintain such trademarks or (ii) allow MARUHO to register and maintain such trademarks in the Territory for the names selected by MARUHO for the Product in the Territory. In the case BIOFRONTERA chooses item (i) above, the trademarks registered and maintained BIOFRONTERA shall be automatically added to the Licensed Trademarks. |
| 3. | Considerations |
| 3.1 | In consideration of the rights granted pursuant to this Agreement, MARUHO shall make the payments to BIOFRONTERA set forth in this Section 3, excluding value added and withholding taxes. |
| 8/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 3.2 | Up-front Payment: MARUHO shall make a non-refundable, one-time payment to BIOFRONTERA of EUR 6,000,000.00 (six million euros) within [***] days after the Effective Date (“Upfront Payment”); |
| 3.3 | Milestone Payments: MARUHO shall make the following non-refundable payments to BIOFRONTERA (the “Milestone Payments”) upon occurrence of the following events (“Milestone Events”): |
| a) | one-time payment of EUR [***] upon the issuance of a Marketing Authorization in Japan for a Product for Acne indication; | |
| b) | one-time payment of EUR [***]upon the first achievement of aggregate Net Sales of ≥ ¥[***] during a year starting on the 1st day of a quarter year and lasting to the last day of the previous quarter in the subsequent calendar year on a rolling quarterly basis; | |
| c) | one-time payment of EUR [***] upon the first achievement of aggregate Net Sales of ≥ ¥[***] during a year starting on the 1st day of a quarter year and lasting to the last day of the previous quarter in the subsequent calendar year on a rolling quarterly basis; |
| MARUHO will notify BIOFRONTERA in writing within [***] days, of the occurrence of a Milestone Event as set out above giving rise to payment of a Milestone Payment. Upon receipt of each such written notification from MARUHO, BIOFRONTERA will issue an invoice to MARUHO in respect of the corresponding Milestone Payment, which will become due and payable within [***] days after the date of the issue of the relevant invoice. | |
| 3.4 | Running Royalty: MARUHO shall pay to BIOFRONTERA, on a country-by-country basis, a royalty which is (i) 6% of Net Sales in case the aggregate Annual Net Sales do not exceed EUR [***], and (ii) in case the aggregate Annual Net Sales exceed EUR [***], [***]% of Net Sales in the amount of EUR [***] plus [***]% of the amount of aggregate Annual Net Sales remaining after deduction of Net Sales in the amount of EUR [***], in each case on a country-by-country basis. provided, however, that irrespective of the aggregate amount of Annual Net Sales the royalty shall be reduced, on a country-by-country basis, to [***]% of Net Sales in case a Generic Product is marketed by a Third Party in the Field in a country of the Territory. The reduction of the royalty rate shall apply on a country-by country basis from the month of launch of the Generic Product. |
| 3.5 | Exchange Rate: When conversion from any currency of country in which MARUHO and Sublicensees invoice to their customers to Japanese Yen or Euro is required, such conversion shall be at an exchange rate equal to the telegraphic transfer middle rates on the last business day of each month of exchange for the currency of the country as published by the MUFG Bank, Ltd. in Japan (or such other source agreed in writing by the Parties). |
| 9/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 3.6 | If any withholding tax is imposed on any payment to be made by MARUHO, MARUHO shall withhold the corresponding amount from the payment due to BIOFRONTERA according to the rates or exemptions provided for under the applicable convention for the avoidance of double taxation with respect to taxes on income, i.e. the treaty between Germany and Japan, in force on the payment date (“Treaty”). For this purpose, Parties will comply with the following process: BIOFRONTERA shall without delay provide MARUHO with all application forms and other documents properly filled in as may be required under the relevant Treaty. If MARUHO does not receive such application forms and other documents by the due date of payment provided for in Section 3.2, 3.3 and 3.4, MARUHO may postpone such date until the date which is in [***] days after the receipt of such documents; and |
| After payment of the withholding tax, MARUHO shall obtain and send to BIOFRONTERA without delay the withholding tax receipt issued by the Japanese tax authority as well as any other document that may be necessary for BIOFRONTERA to get the benefit of a tax credit under the applicable Treaty. | |
| 3.7 | MARUHO shall send quarterly royalty reports to BIOFRONTERA, i.e. within [***] days of the last day of each December, March, June and September MARUHO shall report to BIOFRONTERA the Net Sales in the preceding 3-(three)-months-period (“Quarterly Royalty Report”). The Running Royalty shall become due and payable by MARUHO within [***] days of the date of issuance of the relevant invoice by BIOFRONTERA upon receipt of a Quarterly Royalty Report from MARUHO. |
| 4. | Dossier |
| 4.1 | In no case will BIOFRONTERA be Marketing Approval Holder (“MAH”) in the Territory. MARUHO will be responsible to assume or control or, if applicable, have its Sublicensee’s to assume or control any responsibility that the MAH in the Territory may and shall have. |
| 4.2 | BIOFRONTERA shall provide MARUHO with an electronic copy of the most recent version of the BIOFRONTERA Dossiers within [***] days after the Effective Date. |
| 4.3 | Throughout the term of this Agreement, BIOFRONTERA shall provide MARUHO, free of charge, with any update of the BIOFRONTERA Dossiers that is relevant to the dossiers submitted in the Territory, including but not limited to non-clinical and clinical studies for the development of Product for acne and acne scar in the United States according to the applicable ICH guidelines, as soon as such data is available to BIOFRONTERA. |
| 10/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 4.4 | MARUHO acknowledges BIOFRONTERA’s ownership of the BIOFRONTERA Dossiers. BIOFRONTERA reserves the unrestricted right to use the BIOFRONTERA Dossiers, including the creation and utilization of Marketing Authorizations in the Territory other than those obtained by MARUHO based on this Agreement. MARUHO shall become the owner of copies of the BIOFRONTERA Dossier provided pursuant to Sections 4.2 and 4.3 as well as of all Marketing Authorizations, including without limitation MARUHO Dossiers, obtained by MARUHO or its Affiliates, respectively. In case of sublicensing MARUHO shall be entitled to transfer a further copy of the BIOFRONTERA Dossier to the respective Sublicensee for the relevant country of the Territory. Unless provided for otherwise herein, the mere Marketing Authorizations which are lawfully obtained by MARUHO based on this Agreement shall be and remain with MARUHO during and after the term of this Agreement, except when the Agreement is terminated due to a breach of this Agreement by MARUHO. The forgoing sentence does not impair the general understanding that any sublicense shall automatically terminate in the event the license to MARUHO under this Agreement ends. Obtaining and maintaining the Marketing Authorizations. |
| 5. | Obtaining and maintaining Marketing Authorizations |
| 5.1 | BIOFRONTERA shall use Commercially Reasonable Efforts to assist MARUHO with existing data and information in obtaining and/or maintaining Marketing Authorizations in the Territory. BIOFRONTERA will make Commercially Reasonable Efforts to cause its API and, where applicable, excipient and packaging supplier(s) to submit the Drug Master File (“DMF”) in the Territory and provide a letter of authorization to MARUHO or its Sublicensees within a deadline requested by MARUHO. |
| 5.2 | All costs related to and/or in connection with obtaining and/or maintaining and/or amending the Marketing Authorizations and the DMFs in the Territory as well as any other costs related to the registration procedures, including but not limited to the registration fees, variation fees and potential consultant’s fees, shall be borne by MARUHO. |
| 5.3 | Should a Regulatory Authority in the Territory require additional data, documentation or information relating to the Product, or otherwise information for obtaining and/or maintaining the Marketing Authorizations, then such additional data, information and documentation shall be provided by BIOFRONTERA to MARUHO insofar as such additional data, documentation or information are available to BIOFRONTERA. If a Regulatory Authority requests additional data, documentation or information regarding the Product which are not readily available to BIOFRONTERA, the Parties shall negotiate in good faith the necessary steps. In case that BIOFRONTERA and MARUHO are unable to agree on a mutually acceptable solution within a time frame of [***] calendar days, MARUHO shall be entitled to terminate this Agreement with [***] prior written notice with regard to the country/ies of the Territory concerned. |
| 5.4 | At MARUHO’s request, BIOFRONTERA will assist MARUHO in answering deficiency letters and other queries raised by a Regulatory Authority with regard to the MARUHO Dossiers and/or the Marketing Authorizations. BIOFRONTERA will use its best endeavors to provide such answers, provided the information is available to BIOFRONTERA, to such queries within a deadline requested from MARUHO. |
| 11/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 5.5 | In case BIOFRONTERA is obliged to or wishes to amend the BIOFRONTERA Dossier and/or any information or document relevant to the Marketing Authorizations with the Regulatory Authorities in the Territory, BIOFRONTERA shall make Commercially Reasonable Efforts to inform MARUHO at least [***] months or otherwise as soon as reasonably possible prior to the assumed filing date to each Regulatory Authority. Biofrontera shall share drafts of the intended variations with MARUHO as early as possible. BIOFRONTERA shall provide MARUHO with copies of the submitted variations immediately after filing. The notification of amendment of the BIOFRONTERA Dossier regarding quality and safety information of the Product is further specified in the Quality Agreement and pharmacovigilance agreement or Safety Data Exchange Agreement (“SDEA”), respectively. |
| 5.6 | BIOFRONTERA shall assist MARUHO in the preparation and submission of annual reports or any safety reports to the Regulatory Authorities in the Territory. |
| 5.7 | In case MARUHO wishes to cease the commercialization of the Product in the Territory, it shall notify BIOFRONTERA thereof in writing and Section 18.4 shall apply mutatis mutandis. |
| 5.8 | MARUHO or its Sublicensee will be responsible for any post-approval requirements from the Regulatory Authorities within the Territory (including submission and maintenance of regulatory dossier, submission of regular reports including safety reports, and any other local post-marketing requirements). |
| 5.9 | MARUHO exclusively bears the pharmacovigilance responsibilities for the Product in the Territory, Biofrontera will be the holder of the global safety database and responsible for the management of global pharmacovigilance activities at its own expenses. Further details will be described in the Pharmacovigilance Agreement. |
| 6. | Further Development and Cooperation |
| 6.1 | BIOFRONTERA shall conduct POC studies for acne and acne scar in the USA or other appropriate countries at its own responsibility and expense. BIOFRONTERA shall discuss and agree in good faith with MARUHO the design of these studies and any changes of such design in advance prior to conducting or changing such studies. |
| 6.2 | MARUHO and BIOFRONTERA agree to cooperate in the further research and development with respect to acne, acne scar, Basal Cell Carcinoma and/or Actinic Keratosis as well as Ameluz marketing in the world, including, but not limited to, the Territory, USA and EU, through a joint steering committee (“JSC”). The JSC shall consist of two representatives of either Party and shall review (i) progress; (ii) a Party’s performance; (iii) changes or termination of development; (iv) any changes to the development plan; (v) the synopsis of the study designs; and (vi) the marketing efforts the product. Each Party may propose discussion of life cycle management of the Product and of BF-RhodoLED to the JSC. |
| 12/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 6.3 | As regards the Product marketing in East Asia and/or in Oceania, should a Sublicensee in those regions (except Japan) become available who provides the opportunity to launch the Product significantly sooner than anticipated by MARUHO for its own launch, the JSC will discuss and jointly decide in line with the process set forth in Section 6.4 on the opportunity taking into consideration the best commercial interests of either Party, it being understood that the decision of the JSC must neither put BIOFRONTERA at a significant financial disadvantage nor trigger MARUHO losing any prior investment in the respective region. |
| 6.4 | The JSC shall regularly come together or hold conference calls, at least twice per year or as frequently as either Party may reasonably request, provided that, the first JSC shall be held within [***] months following the Effective Date. Unless the members of the JSC agree otherwise, the following procedural rules shall apply: The quorum for a meeting of the JSC shall be two individuals comprising one individual representing each of the Parties. All decisions of the JSC shall be made by majority decision. In case that no majority vote can be obtained with respect to an item voted on, MARUHO shall have a tie-breaking vote in each case within the Territory. The JSC shall keep accurate minutes of its deliberations, which record all proposed decisions and all actions recommended or taken. The JSC’s role outside the Territory serves the mere purpose of information and discussion but includes no voting power. Each Party shall bear all travel and related expenses as well as other costs associated with the activity of its members of the JSC. |
| 7. | Manufacture and supply of Product |
| 7.1 | BIOFRONTERA agrees to supply all of MARUHO’s requirements of Product in accordance with the terms and conditions set forth in the Supply Agreement to be negotiated and the Quality Agreement to be negotiated (e.g., the purchase price which shall be equal to BIOFRONTERA’s external costs plus [***]% of BIOFRONTERA’s external costs). If pricing becomes unreasonable for MARUHO, MARUHO is entitled to discuss the adequacy of the price with BIOFRONTERA. BIOFRONTERA shall make Commercially Reasonable Efforts to minimize waste when supplying Product and will discuss disposal costs and the minimum order quantity at the time of the negotiation of the Supply Agreement. |
| 7.2 | BIOFRONTERA shall manufacture Product for non-clinical studies, clinical trials and commercial sales. BIOFRONTERA shall make Commercially Reasonable Efforts to extend the shelf-life of Product at its own responsibility and expense under the cooperation of MARUHO. If the shelf-life of the Product cannot be maintained for at least [***] months when the Product is received by MARUHO as carton box of the Product, MARUHO is entitled to request BIOFRONTERA to manufacture the Product complying with MARUHO’s specifications which are provided in the Quality Agreement and/or supply it to MARUHO as a product that is not filled in the primary container. |
| 13/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 7.3 | If MARUHO’s procurement of the Product from BIOFRONTERA is from MARUHO’s point of view no longer commercially reasonable for MARUHO with respect to quality, pricing and/or shelf life according to Section 7.2, MARUHO is entitled to establish its own source of supply of the Product. In this case, upon MARUHO’s request, BIOFRONTERA shall (i) provide MARUHO or a mere manufacturing entity (not allowed to develop, obtain/maintain Marketing Authorizations and/or to commercialize the Product) designated by MARUHO with any and all Licensed Proprietary Information as well as other know-how, data and technology reasonably required for the mere manufacture of the Product and (ii) expand the license granted to MARUHO in Section 2 such that MARUHO is also granted the exclusive, not sublicensable right to use the Licensed Proprietary Information, the Licensed Patents and the Licensed Trademarks to make and have made the Product in the Field in the Territory, it being understood that MARUHO is not authorized to use the technology provided by BIOFRONTERA outside the Territory without BIOFRONTERA’s written consent. The actual costs associated with such provision will be exclusively borne by MARUHO. |
| 7.4 | In the event that MARUHO is entitled to establish its own source of supply of the Product according to Section 7.3, MARUHO shall take due and reasonable care throughout the term of this Agreement to ensure that the Product |
| ● | is fit for its purpose and free from defect in workmanship or materials; and | |
| ● | complies with any applicable laws, regulations and/or relevant approvals, including but not limited to any industry health, environmental and safety standards. |
| 8. | Illumination Device with the Product |
| 8.1 | MARUHO may select, at its own discretion, any illumination device for use with the Product in the Field in the Territory (“Device”). |
| 8.2 | If MARUHO selects to develop and commercialize BF-RhodoLED as the Device, BIOFRONTERA shall be obliged to provide BF-RhodoLED at BIOFRONTERA’s actual cost plus [***]% of BIOFRONTERA’s actual cost to MARUHO upon MARUHO’s request. Actual cost will include all of BIOFRONTERA’s internal and external cost exclusively to manufacture BF-RhodoLED. |
| 8.3 | If MARUHO selects to develop and commercialize a portable compact Device having a performance based on the BF-RhodoLED for use with the Product in the Field in the Territory, MARUHO shall own the entire right, title, and interest, including any and all intellectual property, in and to all Results generated or obtained or otherwise perceived or reduced to practice in the course of the development of the Compact Device. BIOFRONTERA shall reasonably cooperate with MARUHO upon MARUHO’s request in the development of such portable compact Device (“Compact Device”). The actual cost to develop such Compact Device will be borne by MARUHO. In this case, for all regions outside the Territory, MARUHO grants an exclusive, royalty-bearing license to this intellectual property to BIOFRONTERA. |
| 8.4 | If MARUHO selects to develop the Device independently from BIOFRONTERA, MARUHO shall own the entire right, title, and interest, including any and all intellectual property, in and to all Results generated or obtained or otherwise perceived or reduced to practice in the course of the development of the Device and BIOFRONTERA shall have no right to obtain any license to any Results generated by MARUHO related to the Device. |
| 14/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 9. | Compliance with Regulations |
| 9.1 | MARUHO shall use the Marketing Authorizations in strict compliance with all applicable governmental regulations in the Territory. |
| 9.2 | The Parties shall enter into a suitable pharmacovigilance agreement or SDEA prior to the initiation of the first clinical study or the first commercial launch of the Product in the Territory, whichever comes earlier. |
| 9.3 | Throughout the term of this Agreement, the Parties shall give each other such assistance as either Party may reasonably require for the purposes of compliance with the pharmacovigilance agreement. |
| 10. | Commercialization |
| 10.1 | MARUHO shall use its Commercially Reasonable Efforts |
| a) | to make the Product available in the market in each country of the Territory after receipt of the Marketing Authorization in such country, provided that BIOFRONTERA has timely delivered to MARUHO the Product meeting the agreed quality (as defined in the Supply Agreement and the Quality Agreement both to be negotiated), and | |
| b) | to exploit the Marketing Authorization by marketing and selling the Product in the Territory during the term of this Agreement. |
| 10.2 | If MARUHO has not applied for a Marketing Authorization for the Product (i) in the East Asia countries of the Territory within [***] years after regulatory approval of the commercialization of the Product for the Acne indication in the USA by BIOFRONTERA, and/or (ii) in the Oceania countries of the Territory within [***] years after the Effective Date, BIOFRONTERA may request MARUHO, on a country-by-country basis, to return any license granted to MARUHO by BIOFRONTERA hereunder, in each case unless MARUHO can invoke reasonable regulatory or commercial reasons for not having applied for a Marketing Authorization in the respective country within the applicable period of time. |
| 10.3 | In case of BIOFRONTERA’s request in line with Section 10.2 to return a license, MARUHO shall, at MARUHO’s discretion, either (i) pay to BIOFRONTERA an annual amount of EUR [***] per country instead of returning the license, or (ii) return the license in such country to BIOFRONTERA, i.e. will provide to BIOFRONTERA, free of charge, any data or documentation related to the registration and/or reimbursement of the Product in the respective country that MARUHO may have. |
| 11. | Records and Audits |
| 11.1 | MARUHO shall keep complete and accurate books and records of the sales of and payments made for the Product, and shall permit an independent auditor access to such books and records in order to confirm the accuracy of any reports and payments made to BIOFRONTERA pursuant to this Agreement. BIOFRONTERA may appoint such independent auditor to check MARUHO’s relevant books and records once a year upon prior written notice to MARUHO. The auditor’s report shall only state whether the relevant calculations have been accurate or reveal an underpayment. The charges for such an audit shall be borne by BIOFRONTERA, unless the audit reveals an underpayment of royalties in excess of [***]% [***] of the royalties actually due, in which case MARUHO shall bear the cost of such audit and shall remit any amounts due to BIOFRONTERA within [***] days of receiving an invoice from BIOFRONTERA. BIOFRONTERA’s audit right pursuant to this clause shall expire [***] after the Quarterly Royalty Report has been received by BIOFRONTERA. |
| 15/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 11.2 | BIOFRONTERA shall keep complete and accurate books and records (i) of the manufacturing and supply of the Product and of the Device to MARUHO as well as (ii) of all payments of BIOFRONTERA’s external costs for the Product and of the actual costs for the Device and shall permit MARUHO and/or an independent auditor access to such books and records in order to confirm the accuracy of any invoices issued to MARUHO pursuant to this Agreement for the supply of Product and/or Devices. Section 11.1 shall apply mutatis mutandis with respect to a check of BIOFRONTERA’s books and records by MARUHO. |
| 12. | Confidentiality |
| 12.1 | Each Party shall keep, during the Term and thereafter for an unlimited period of time, as confidential and shall not publish or otherwise disclose and shall not use for any purpose other than as provided for in this Agreement (which includes the exercise of any rights or the performance of any obligations hereunder or thereunder) the contents of this Agreement, of the Supply Agreement to be negotiated and of the Quality Agreement to be negotiated as well as the Confidential Information furnished to it or its Affiliates by the other Party or its Affiliates pursuant to this Agreement except to the extent expressly authorized by this Agreement or as otherwise agreed to in writing by the Parties. Either Party may disclose Confidential Information of the other Party to its employees, directors, personnel and Affiliates, potential Sublicensees, as well as Regulatory Authorities, service providers, consultants, non-clinical and clinical investigators, manufacturers and distributors only to the extent required to exercise its rights and/or to fulfil its obligations under this Agreement, the Supply Agreement to be negotiated or the Quality Agreement to be negotiated, provided that same shall be bound by confidentiality and non-use obligations no less stringent than those set forth under this Agreement. The duty of confidentiality as per this clause 12.1 does not apply to: |
| a) | information, which was already in the public domain at the time of signing of this Agreement; |
| 16/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| b) | information, which becomes a part of the public domain by publication or otherwise during the term of this Agreement, except by breach of this Agreement; | |
| c) | information, which the receiving Party can establish was in its possession before signing of this Agreement and which was not acquired directly or indirectly from the disclosing Party; | |
| d) | information, which the receiving Party can establish was acquired from a third party, such third party having acquired the information neither directly nor indirectly from the disclosing Party; | |
| e) | information which has to be disclosed by law, a court decision or an administrative order; |
| 12.2 | In the event that information is required to be disclosed under clause 12.1e) above, the receiving Party shall notify the disclosing Party in advance and allow the disclosing Party to assert whatever exclusions or exemptions may be available to it under such law or regulation. |
| 12.3 | In the event this Agreement will be terminated for any reason, the receiving Party and/or its Affiliates shall at the discretion of the disclosing Party either destroy or return all Confidential Information to the disclosing Party and shall destroy all copies thereof, except for 1 (one) copy which the receiving Party shall have the right to retain in its confidential files in order to monitor its compliance with the terms and conditions of this Agreement. |
| 12.4 | The duty of confidentiality as per this clause 12 shall survive the termination of this Agreement for the duration of [***] years. |
| 13. | Representations and Undertakings |
| 13.1 | The Parties represent and undertake that they have obtained all necessary corporate approvals to enter into this Agreement. |
| 13.2 | MARUHO represents and undertakes that: |
| a) | it is a company duly organized and validly existing under the laws of Japan; | |
| b) | it has all corporate power, authority and approvals required to enter into, execute and deliver this Agreement and to perform its obligations hereunder; | |
| c) | it has obtained and will maintain throughout the term of this Agreement all consents, approvals, licenses, permits, Authorizations, certifications and registrations with any governmental authority required for the performance of its obligations hereunder; and | |
| d) | it is not and will not offer Competing Products categorized in photodynamic therapy in the Field and the Territory. |
| 17/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 13.3 | BIOFRONTERA represents and undertakes that: |
| a) | it is a company duly organized and validly existing under the laws of Germany; | |
| b) | it has all corporate power, authority and approvals required to enter into, execute and deliver this Agreement and to perform its obligations hereunder; | |
| c) | it has the full right and power to grant MARUHO the rights specified herein and is not aware of any rights of Third Parties that could limit the use of the Licensed Proprietary Information, the Licensed Patents and the Licensed Trademarks; | |
| d) | all studies conducted by or on behalf of BIOFRONTERA have been performed in compliance with current regulatory requirements and applicable guidance and industry standards applicable at that time; | |
| e) | the BIOFRONTERA Dossiers contain all data held by BIOFRONTERA on the Product and all corresponding reports, analysis records and other scientific and technical documents which are necessary for obtaining or maintaining the Marketing Authorization for the Product in the EU and/or USA according to BIOFRONTERA’S assessment, in such format that may be issued and delivered at any time to MARUHO or to any Regulatory Authority upon MARUHO’s request; | |
| f) | all development work in relation to the BIOFRONTERA Dossiers has been carried out in accordance with the applicable rules and regulations relating to clinical products in force at the time in the EU and the US; and | |
| g) | to the best of its knowledge BIOFRONTERA owns all right, title and interest in the BIOFRONTERA Dossiers, the Licensed Proprietary Information and the Product. |
| 14. | Third party infringements; defence against Third Party claims and invalidity attacks |
| 14.1 | Either Party shall notify the other Party immediately about any information it might become aware of regarding |
| a) | any intellectual property rights of a Third Party which are critical to the use of the Product and/or the Licensed Proprietary Information and/or the Licensed Patents in the Field in the Territory; | |
| b) | any actual or anticipated infringement of the Licensed Proprietary Information and/or Licensed Patents and/or Licensed Trademarks by a Third party; or | |
| c) | any actual or anticipated claim by a Third party alleging that the Product, its commercialization and/or use infringes any Third party’s intellectual property rights. |
| 18/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 14.2 | In case a Third party infringes the Licensed Proprietary Information and/or the Licensed Patents and/or Licensed Trademarks the Parties shall negotiate in good faith about the necessity of taking action against such infringement. For the purposes of the relationship between the Parties, BIOFRONTERA alone is entitled, but not obligated, to take action in response to such infringements. Without prior written authorization by BIOFRONTERA, MARUHO is not entitled to take any action against Third Parties for infringements of the Licensed Proprietary Information and/or the Licensed Patents and/or Licensed Trademarks. |
| 14.3 | In case a Third Party asserts or threatens to assert any claim of alleged infringement of intellectual property rights by the Product or its use in the Territory (“Third Party Claim”), the Parties shall negotiate in good faith whether a joint defence is in either Party’s interest in which case the Parties shall jointly defend themselves against such claims whereas BIOFRONTERA shall have the ultimate right to decide on any measure to be taken. The costs in connection with the joint defence shall be shared equally between the Parties, it being understood that each Party bears its own internal costs as well as the costs of its own legal counsel. Should BIOFRONTERA not be interested in joining the defence against a Third Party Claim, MARUHO shall be entitled to autonomously defend itself against a Third Party Claim at its own costs. Any payments to be made according to (i) a settlement agreement or (ii) a legally binding court decision, in each case in order to settle the Third Party Claim shall be shared equally by the Parties provided that (i) in case of a settlement agreement BIOFRONTERA agreed prior to the conclusion of the settlement to the terms stipulated in the settlement agreement, or (ii) in case of a legally binding court decision MARUHO informed BIOFRONTERA faithfully and comprehensively on the course and progress of the case. If the settlement of the Third Party Claims include future payments (such as running royalties) to be paid by MARUHO, BIOFRONTERA and MARUHO shall negotiate in good faith a reasonable reduction of the royalties due in the countries affected by such future payments. |
| 14.4 | In any case, either Party shall reasonably support the other Party in enforcing the Licensed Proprietary Information and/or the Licensed Patents and/or the Licensed Trademarks or defending the use of the Products against Third Party claims and provide the other Party with all information, data and documentation it has available and which is needed by the other Party for the enforcement of or, respectively, the defence against such claims. |
| 14.5 | Neither Party shall enter into any settlement of any suit or controversy related to intellectual property rights, which might adversely and materially affect the other Party’s rights and privileges granted hereunder without the consent of the other Party, such consent not to be unreasonably withheld by the Party. |
| 14.6 | BIOFRONTERA shall (i) maintain, and (ii) to the extent commercially reasonable, defend the Licensed Proprietary Information, the Licensed Patents and the Licensed Trademarks against challenges to validity (“Invalidity Challenge”) at its own costs. BIOFRONTERA shall promptly inform MARUHO of any Invalidity Challenge. In case BIOFRONTERA, in its reasonable discretion exercised in good faith, determines that the defence against an Invalidity Challenge is not commercially reasonable, BIOFRONTERA shall (i) be free to refrain from such defence, and (ii) notify MARUHO of its determination accordingly in good time to enable MARUHO to conduct the defence, if so desired by MARUHO (“Defence Notice”). |
| 19/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 14.7 | In case of a Defence Notice, the following shall apply: |
| a) | MARUHO shall be entitled to conduct the defence against the Invalidity Challenge at its own costs and BIOFRONTERA shall provide MARUHO with full power of attorney and all documents reasonably required by MARUHO to conduct and terminate the defense proceeding in the manner deemed appropriate by MARUHO. | |
| b) | In case the defence conducted by MARUHO against the Invalidity Challenge is successful, i.e. the Invalidity Challenge is either fully or partly dismissed or settled, MAURHO shall be entitled to offset any costs incurred directly by the invalidity defence against the royalties due in the countries affected by the Invalidity Challenge and the Milestone Payments, respectively. |
| 14.8 | In case the Invalidity Challenge is partly or fully successful, BIOFRONTERA and MARUHO shall negotiate in good faith a reasonable reduction of the royalties due in the countries affected by the Invalidity Challenge. |
| 15. | Liability |
| 15.1 | Either Party shall be liable vis-à-vis the other Party for the latter’s damages, whether based on contract or any other legal theory, only if such damage has been caused by gross negligence or by intent or in case of material breach of this Agreement. |
| 15.2 | In no event shall either Party be liable for or have any obligation to compensate or indemnify the other Party for any indirect or consequential damages, claimed by the other Party, including, but not limited to the loss of opportunity, loss of use, or loss of revenue or profit in connection with or arising out of this Agreement or breach thereof. |
| 15.3 | Each Party shall procure and maintain insurance, including product liability insurance, with a reputable insurer or shall self-insure, in each case in a manner adequate to cover its obligations hereunder and consistent with normal business practices of prudent companies similarly situated at all times during which any Product is being clinically tested or commercially distributed or sold by such Party. Each Party shall procure insurance or self-insure at its own expense. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Section 15. Each Party shall provide the other Party with written evidence of such insurance or self-insurance upon request. |
| 16. | Indemnification |
| 16.1 | Subject to Sections 15.2 and 16.3, BIOFRONTERA agrees to indemnify and hold harmless MARUHO from and against all damages, losses and expenses (including reasonable attorney’s fees) claimed by a third party from MARUHO for any personal injury or death or property damage to the extent such damages, losses and expenses have not been caused by MARUHO itself, provided that such damages, losses and expenses (i) arise from BIOFRONTERA’s willful or criminal wrongdoing or omission, respectively, or negligent breach of this Agreement or BIOFRONTERA’s activities under this Agreement, and (ii) have been established by final and legally binding court ruling. |
| 20/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 16.2 | Subject to Sections 15.2 and 16.3, MARUHO agrees to indemnify and hold harmless BIOFRONTERA from and against all damages, losses and expenses (including reasonable attorney’s fees) claimed by a third party from BIOFRONTERA for any personal injury or death or property damage to the extent such damages, losses and expenses have not been caused by BIOFRONTERA, provided that such damages, losses and expenses (i) arise from MARUHO’s willful or criminal wrongdoing or omission, respectively, or negligent breach of this Agreement or MARUHO’s activities relating to the Marketing Authorizations or the Product, and (ii) have been established by final and legally binding court ruling. |
| 16.3 | The indemnification obligations set forth in this Section 16 shall only be applicable, if the Party claiming indemnity is in full compliance with all of the following requirements: |
| a) | the indemnified Party shall notify the indemnifying Party promptly in writing of any claim which may give rise to an obligation on the part of the indemnifying Party hereunder; | |
| b) | the indemnifying Party shall be allowed to timely undertake the sole control of the defence of any such action and claim, including all negotiations for the settlement, or compromise of such claim or action, at its sole expense; and | |
| c) | the indemnified Party shall at the expense of the indemnifying Party render reasonable assistance, information, co-operation and authority to permit the indemnifying Party to defend such action. |
| 17. | Force Majeure |
| 17.1 | Neither Party shall be liable for its failure to perform its obligations under this Agreement due to any contingency beyond its reasonable control, including but not limited to strikes, riots, wars, fire, flood, accident, labour disputes, embargoes, epidemics, pandemics, governmental restrictions, inability to obtain export or import licenses, acts of God, or acts in compliance with any governmental or state law, regulation or order (hereinafter referred to as “Force Majeure”). Upon the occurrence of a Force Majeure event, the Party affected thereby shall be excused from performance during its continuance. Such Party shall promptly notify the other in writing of the occurrence of this event and the time during which it is anticipated such event shall affect the notifying Party’s ability to perform its obligations under this Agreement. The Party affected by a Force Majeure event shall use its Commercially Reasonable Efforts to correct or mitigate the conditions giving rise to any delay or failure of performance. |
| 21/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 17.2 | If Force Majeure prevents one of the Parties from fulfilling its obligations hereunder for a period of more than [***] months, the Parties shall meet and negotiate in good faith to find a mutually acceptable solution. In case no agreement can be found, the Party not affected by the Force Majeure event shall be entitled to terminate this Agreement in accordance with clause 18.3.3. |
| 18. | Term and Termination |
| 18.1 | This Agreement shall commence on the Effective Date and, unless terminated earlier pursuant to this Agreement, shall continue thereafter, on a country-by-country basis, for a period of [***] years from the launch date of the Product in a country of the Territory (“Initial Term”). The Initial Term shall be extended automatically by consecutive [***] periods unless terminated by either Party giving written notice to the other Party pursuant to this Section 18 on a country-by country basis. |
| 18.2 | BIOFRONTERA shall have the right to terminate on a country-by country basis during (i) the Initial Term only for cause, and (ii) any of the consecutive [***] year periods after the Initial Term without cause by giving written notice at least [***] days prior to the expiration of the then-current term. MARUHO shall have the right to terminate on a country-by country basis during (i) the Initial Term without cause by written notice upon a notice period of [***] days and (ii) any of the consecutive [***] periods after the Initial Term without cause by giving written notice at least [***] days prior to the expiration of the then-current term. |
| 18.3 | A cause for termination shall in particular, but without limitation, be deemed to be given upon the occurrence of one or several of the following events |
| 18.3.1 | By either Party if the respective other Party materially breaches or defaults on any of its obligations under this Agreement and does not cure such material breach or default within [***] calendar days from the receipt date of such written notice. | |
| 18.3.2 | In the event either Party receives or is made aware of: |
| (i) | a valid claim of infringement of a third party intellectual property right relating to the Product or the manufacture thereof, or | |
| (ii) | a valid product liability claim relating to the Product, | |
| (iii) | suspected unforeseen side effects related to the Product. |
and the continued activities relating to the Product constitute, in the reasonable opinion of either Party, an unacceptable risk of infringement or liability, then either Party shall have the right to require that BIOFRONTERA suspends further deliveries of the Product to MARUHO (thereby subjecting neither Party to the liability for non-performance of their respective delivery and purchase obligations) until such matter has been resolved to the reasonable satisfaction of both Parties.
| 22/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 18.3.3 | By either Party if Force Majeure prevents the other Party from fulfilling its obligations hereunder for a period of more than [***] months, and thereafter, the Parties are not able to find a mutually acceptable solution within [***] days. | |
| 18.3.4 | Each Party retains the right to terminate this Agreement for cause by giving written notice to the other Party, effective immediately and without prejudice to any other remedy available to it under the governing law and this Agreement, if the respective other Party becomes involved in litigation or proceedings relating to its own bankruptcy or insolvency or if such proceedings are refused due to lack of sufficient assets. This includes in particular but is not limited to declared insolvency, adjudged bankruptcy, filing of a petition for bankruptcy, reorganization under any bankruptcy act, is sequestered or expropriated, or submits or has to submit any other administrative or judicial matters of control. | |
| 18.3.5 | MARUHO shall also have the right to terminate this Agreement with regard to the country of the Territory concerned upon [***] days prior written notice to the other Party in the event that (i) Product is not launched due to reasons set forth in clause 18.3.2 and/or related injunction, (ii) Product has to be definitely withdrawn from the market due to reasons as set forth in clause 18.3.2 and/or related injunction, or (iii) infringement of third party rights with a negative final decision and/or settlement requiring the withdrawal. |
| 18.4 | Upon expiration or termination of this Agreement pursuant to any provision in this Agreement, MARUHO shall have the right to sell-off its remaining stock of the Product pursuant to the terms and conditions of this Agreement, of the Supply Agreement to be negotiated and of the Quality Agreement to be negotiated. |
| 18.5 | The following provisions shall survive termination of this Agreement: Sections 11 (Records and Audits), 12 (Confidentiality), 14 (Third Party infringements/defence), 15 (Liability), 16 (Indemnification), 18 (Term and Termination), 19 (Applicable Law and Venue), and 20. (Notices). |
| 18.6 | Termination or expiration of this Agreement, for whatever reason, shall be without prejudice to any rights, claims or obligations of either Party which may have accrued prior to, or become due at the date of such termination. |
| 19. | Applicable Law and Venue |
| 19.1 | The validity, construction, interpretation, and effect of this Agreement and the respective rights and obligations of the Parties hereunder shall be governed and determined by and in accordance with the substantive laws of Germany, to the exclusion of the provisions on conflicts of laws. |
| 23/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 19.2 | The Parties shall attempt to amicably settle any dispute, controversy or claim arising out of or relating to this contract, including the validity, invalidity, breach or termination thereof. In case any such dispute, controversy or claim cannot be amicably settled during a period of [***] days after one Party has notified the other Party in writing of such matter, the respective dispute, controversy or claim shall be finally settled under the Rules of Arbitration of the International Chamber of Commerce by 3 (three) arbitrators appointed in accordance with said Rules provided, however, that only arbitrators shall be eligible who have at least ten (10) years of professional experience with licensing in the pharmaceutical industry, either as a company representative or as a member of the legal profession. The arbitration proceedings shall be held in Düsseldorf, Germany. The arbitration proceedings shall be conducted in English language; however, the parties are entitled (but not obliged) to also present in these proceedings documentary evidence originally drafted and written in German. This only relates to documentary evidence and does not pertain to memorials and briefs which shall be filed pursuant to the Rules of Arbitration of the International Chamber of Commerce. |
| 20. | Notices and Other Communications |
Any and all notices, requests or other communication required or permitted to be made or given under or in connection with this Agreement or the subject matter hereof by either of the Parties hereto shall be in writing and in English and shall be deemed to be sufficiently served for all purposes hereof at the date of certified receipt by the receiving Party, if sent by registered mail or by courier addressed to the Party to be notified at the following addresses or such other address as a Party may relocate its headquarters to:
If to BIOFRONTERA:
[***]
If to MARUHO:
[***]
| 21. | General Provisions |
| 21.1 | Relationship of the Parties. It is expressly agreed that the relationship between BIOFRONTERA and MARUHO shall not constitute a partnership, joint venture, or agency. BIOFRONTERA and MARUHO are independent contractors. Neither BIOFRONTERA nor MARUHO shall have the authority to make any statements, representations, or commitments of any kind, or to take any action, which shall be binding on the other, without the prior consent of the other Party to do so. |
| 21.2 | Expenses. Whether or not the transactions contemplated hereby are consummated, and except as otherwise specified herein, each Party will bear its own costs and expenses in connection with this Agreement and with respect to the transactions contemplated by this Agreement. |
| 24/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
| 21.3 | Assignment. Unless otherwise agreed in this Agreement, neither Party shall assign any of its rights and/or interest to this Agreement, or any part thereof, or any benefit or interest therein, without the prior written consent of the other Party. However, each Party shall have the right to assign this Agreement without the other Party’s consent to its Affiliate. |
| 21.4 | Entire Understanding: This Agreement and the annexes attached hereto, the Supply Agreement to be negotiated and the Quality Agreement to be negotiated embody the entire understanding of the Parties and override or supersede all or any prior representations, understandings and implications made by either Party at any time prior, whether orally or in writing related to the subject matter hereof. |
| 21.5 | Amendments: The Parties may, during the term of this Agreement, amend any of the provisions of this Agreement (including its annexes) by mutual agreement. Any such amendment to this Agreement shall be deemed invalid unless made in writing and signed by both Parties; the same applies to any amendment of this requirement of written form. |
| 21.6 | Modification and Waiver: None of the terms of this Agreement (including its annexes) shall be deemed to be waived or modified except by a written document drawn expressly for such purpose and executed by the Party against whom enforcement of such waiver or modification is sought. Failure or delay of either Party hereto to enforce any of its rights under this Agreement shall not be deemed a modification or a continuing waiver by such Party of any of its rights under this Agreement. |
| 21.7 | Severability: If any provision of this Agreement should be or become invalid or unenforceable, the validity of the remaining provisions hereof shall not be affected thereby. Such invalid or unenforceable provision shall be converted by mutual consent of the Parties, to the extent possible, to a valid and enforceable provision which comes as close as possible to the business objective of the original provision. This provision shall also apply if this Agreement should be incomplete. |
As WITNESS the Parties have caused this Agreement to be entered into by their duly authorised representatives on behalf of the Parties as of the date first above written.
| 25/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
Signatures
| Biofrontera AG: | ||||
| Date: | 20 April 2020 | Date: | 20 April 2020 | |
| /s/ Xxxx. Xxxxxxx Xxxxxxx | /s/ Xxxxxx Xxxxxxxx | |||
| Name: | Xxxx. Xxxxxxx Xxxxxxx | Name: | Xxxxxx Xxxxxxxx | |
| Position: | Chief Executive Officer | Position: | Chief Financial Officer | |
| Maruho Co., Ltd.: | ||||
| Date: | 20 April 2020 | |||
| [***] | ||||
| Name: | [***] | |||
| Position: | [***] | |||
| 26/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
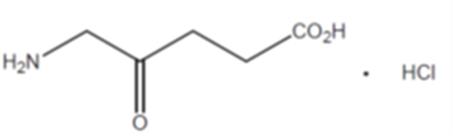
Annex 1.1 (Active Substance)
5-aminolevulinic acid
| 27/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
Annex 1.5 (BIOFRONTERA Dossiers)
[***]
[***]
| 28/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
Annex 1.21 (Licensed Patents)
Nanoemulsion
The following granted patents with the title “Nanoemulsion” whose subject matter is the specific nanoemulsion used in Ameluz (BF-200) and also in other formulations currently in development with MARUHO having an expiration date of December 21, 2027:
| ● | AU (Australia): patent no. 2007338323 |
| ● | CN (China): patent no. ZL200780047469.9 |
| ● | HK (Hongkong): patent no. 0000000 |
| ● | JP (Japan): patent no. 5 558 827 |
| ● | NZ (New Zealand): patent no. 577061 |
| ● | SG (Singapore): patent no. 153157 |
Kombi-PDT
Unpublished international patent application PCT/EP2018/072823 with the title “Improved photodynamic therapy” filed with the EPO on August 23, 2018 whose subject matter is painless PDT through the combination of daylight and red light PDT
PDT-Lamp
Unpublished international patent application PCT/EP2019/064642 with the title “Illumination for photodynamic therapy” filed with the EPO on June 5, 2019 whose subject matter are certain software features of the PDT lamp
| 29/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
Annex 1.22 (Licensed Trademarks)
The country codes are as follows:
AU = Australia
CN = China
JP = Japan
KR = Republic of Korea
SG = Singapore
Ameluz
(word xxxx)
granted: AU, CN, KR, SG
BF-RhodoLED
(word xxxx)
granted: AU, CN, JP, KR, SG
RHODOLED
(word xxxx)
granted: AU, CN, JP, KR, SG
| 30/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |
Annex 1.38 (Territory)
The Territory shall comprise the following countries:
| I. | East Asia |
|
| “East Asia” refers the territories of the following countries: | ||
| – | Japan | |
| – | Bangladesh | |
| – | Bhutan | |
| – | Brunei | |
| – | Cambodia | |
| – | China | |
| – | India | |
| – | Indonesia | |
| – | Republic of Korea | |
| – | Laos | |
| – | Malaysia | |
| – | Maldives | |
| – | Mongolia | |
| – | Myanmar | |
| – | Nepal | |
| – | Pakistan | |
| – | Philippines | |
| – | Singapore | |
| – | Sri Lanka | |
| – | Thailand | |
| – | Timor-Leste | |
| – | Viet Nam | |
| – | North Korea | |
| – | Taiwan | |
| II. | Oceania | |
| “Oceania” refers to the territories of the following countries: | ||
| – | Australia |  |
| – | Xxxx | |
| – | Fiji | |
| – | Kiribati | |
| – | Xxxxxxxx | |
| – | Micronesia | |
| – | Nauru | |
| – | New Zealand | |
| – | Niue | |
| – | Palau | |
| – | Papua New Guinea | |
| – | Samoa | |
| – | Solomon | |
| – | Tonga | |
| – | Tuvalu | |
| – | Vanuatu |
| 31/31 |
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. |