INTERNATIONAL DISTRIBUTION AGREEMENT
EXHIBIT 10.13
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
INTERNATIONAL DISTRIBUTION AGREEMENT
This International Distribution Agreement (this “Agreement”) is entered into as of the 30th March 2012 (the “Effective Date”), by and between INFRAREDX, INC., a Delaware corporation with principal offices at 00 Xxxxx Xxxxxx, Xxxxxxxxxx, XX 00000, X.X.X. (“INFRAREDX”) and NIPRO CORPORATION, a Japan corporation with principal offices at 0-0-00, Xxxxxxxx, Xxxx-xx, Xxxxx 000-0000, Xxxxx (“Distributor”).
In consideration of the mutual promises contained herein, the parties agree as follows:
| 1. | DEFINITIONS |
(a) “Products” shall mean components of and disposables for, TVC Imaging System, including those products listed in Exhibit A attached hereto. The Products shall include any products changed in accordance with Subsection 7(c) even if their catalogue number or product name has been changed from those described in Exhibit A. Products may be changed on, abandoned from or added to the list by mutual written agreement of both parties hereto. INFRAREDX shall be under no obligation to continue the production of any Product, except as provided herein, and no such deletion shall be deemed a termination or partial termination of this Agreement.
(b) “Territory” shall mean that geographic area identified in Exhibit A attached hereto.
(c) “Trademarks” shall mean those trademarks and trade names, whether registered in the Territory or not, labelling, trade dress, packaging and devices which are owned by, licensed or assigned to INFRAREDX and which are applied to or used with the Products by INFRAREDX.
(d) “Products PMDA Approval Date” shall be designated as the calendar date upon which all of the Products specified in Exhibit A at the Effective Date have received regulatory approval for commercial sale by Japan’s Pharmaceutical and Medical Device Agency.
(e) A “Business Day” shall mean a day other than a Saturday, Sunday or a public holiday in the U.S or in Japan.
| 2. | APPOINTMENT AND AUTHORITY OF DISTRIBUTOR |
(a) Appointment. Subject to the terms and conditions set forth herein, INFRAREDX hereby appoints Distributor as INFRAREDX’s exclusive distributor for the promotion, sale, marketing and distribution of Products in the Territory, and Distributor hereby accepts such appointment. During the term of this Agreement, as long as Distributor is performing in compliance with this Agreement and subject to Section 8
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
hereof, INFRAREDX shall not appoint any other distributor with responsibility for sale of the Products in the Territory. Distributor warrants that it will use commercially reasonable efforts to obtain authorization to import the Products into the Territory and to distribute them therein, and that it will exercise its best efforts to maintain these authorizations during the term of this Agreement. Upon request by INFRAREDX, Distributor shall provide INFRAREDX with evidence of these authorizations. Distributor shall immediately notify INFRAREDX if it suffers the loss or impairment of any license, permit or other authorization that it requires in order to import the Products into the Territory or to distribute them therein.
(b) Territorial Responsibility. Distributor shall pursue appropriate sales policies and procedures to realize the maximum sales potential for the Products in the Territory. Subject to applicable laws or regulations, Distributor shall promote and sell the Products to customers only for use or consumption within the Territory, and Distributor agrees not to solicit orders outside the Territory without the prior written approval of INFRAREDX.
(c) Conflict of Interest. Distributor warrants to INFRAREDX that it does not currently represent or promote any lines or products that compete with the Products. Distributor shall not, without INFRAREDX’s prior written consent, represent, promote or otherwise sell within the Territory any lines or products that compete with the Products.
(d) Independent Contractors. The relationship of INFRAREDX and Distributor established by this Agreement is that of independent contractors, and nothing contained in this Agreement shall be construed to (i) give either party the power to direct or control the day-to-day activities of the other, (ii) constitute the parties as partners, joint venturers, co-owners or otherwise as participants in a joint or common undertaking, or (iii) allow Distributor to create or assume any obligation on behalf of INFRAREDX for any purpose whatsoever. The appointment of Distributor does not constitute a grant of any rights or interests other than the rights specifically granted to the Distributor hereunder. All financial obligations associated with Distributor’s business are the sole responsibility of Distributor. All sales and other agreements between Distributor and its customers are Distributor’s exclusive responsibility and shall have no effect on Distributor’s obligations under this Agreement. Distributor shall be solely responsible for, and shall indemnify and hold INFRAREDX free and harmless from, any and all claims, damages or lawsuits (including INFRAREDX’s reasonable attorneys’ fees) arising out of (i) the handling, storage, sale or distribution of the Product by Distributor or its sub-distributors, and their employees or agents, and (ii) the wilful or negligent acts or omissions of Distributor or its sub-distributors, and their employees or—agents, except for any claims, damages or lawsuits to the extent arising from the failure of a Product to conform to the specifications at the time of delivery to Distributor or the wilful or negligent act or omission of INFRAREDX.
(e) Representations. INFRAREDX and Distributor each represent and warrant to the other party that (i) such party is a corporation duly organized, validly
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
existing and in good standing under the laws of its incorporating jurisdiction, and has all requisite corporate power and authority to enter into this Agreement; (ii) the party is duly authorized by all requisite action to execute, deliver and perform this Agreement and to consummate the transactions contemplated hereby in accordance with their terms, and that the same do not conflict with or cause a default with respect to its obligations under any other agreement; and (iii) such party has duly executed and delivered this Agreement.
| 3. | TERMS OF PURCHASE OF PRODUCTS BY DISTRIBUTOR |
(a) Terms and Conditions. All purchases of Products by Distributor from INFRAREDX during the term of this Agreement shall be subject to the terms and conditions of this Agreement.
(b) Prices. All prices are Ex-Works (Incoterms 2010) INFRAREDX or INFRAREDX’s contractor’s manufacturing facility. The purchase price to Distributor for each of the Products (“Purchase Price”) shall be as set forth in Exhibit B attached hereto. The difference between Distributor’s Purchase Price and Distributor’s selling price to its customers shall be Distributor’s sole remuneration for sale of the Products. The prices in Exhibit B may not be revised and shall be held firm and fixed from the Effective Date through the year 2013. Beginning in 2014 and proceeding thereafter until the Term or Termination of this Agreement, the parties agree that the Purchase Price paid for Products may be subject to renegotiation and revision due to any reason affecting the selling price of the Products by Distributor upon the mutual consent of both parties, which includes, but not limited to, change in hospital reimbursement rate; and competitiveness of the Products in the market. In case Purchase Price is subject to renegotiation and revision due to a material change in the hospital reimbursement rate(s) for the Products as published by Japan’s Ministry of Health, Labor and Welfare (MHLW), it is hereby stipulated that in order for a change in hospital reimbursement rates to be considered material and to generate a condition sufficient to warrant entering into negotiations to revise the Purchase Price paid for the Products, this change in hospital reimbursement must exceed [***] percent ([***]%) of the immediately preceding published hospital reimbursement rate in effect for the Products. INFRAREDX acknowledges that the hospital reimbursement rate does not apply to any Products other than TVC Insight catheters. Such revisions shall apply to all orders received after the effective date of revision. Price increases shall not affect unfulfilled purchase orders accepted by INFRAREDX before the effective date of the price increase. Price decreases shall apply to pending purchase orders that are not yet accepted by INFRAREDX before the effective date of the decrease.
(c) Taxes. Distributor is responsible for all sales, excise, value-added taxes, and similar taxes or duties, freight, insurance and other shipping charges that are imposed as a result of the export of the Products, including such taxes if they are assessed against the Products after delivery to the carrier at INFRAREDX’s or INFRAREDX’s U.S. contractor’s manufacturing plant, as well as all taxes imposed by governmental authorities within the Territory, including without limitation import and withholding taxes (“Distributor Taxes”). When INFRAREDX has the legal obligation to collect
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Distributor Taxes, the appropriate amount shall be added to Distributor’s invoice and paid by Distributor unless Distributor provides INFRAREDX with a valid tax exemption certificate authorized by the appropriate taxing authority.
(d) Order and Acceptance. All orders for Products submitted by Distributor shall be initiated by written purchase order delivered in person or by first class mail, facsimile or electronic submission to INFRAREDX during the term of this Agreement. No order shall be binding upon INFRAREDX until accepted by INFRAREDX in writing. INFRAREDX shall use its reasonable best efforts to notify Distributor of the acceptance or rejection of an order and of the assigned delivery date for accepted orders within five (5) business days after receipt of the purchase order. Notwithstanding the foregoing, if Distributor does not receive such notice within five (5) business days after receipt of the purchase order, the orders shall be deemed as having been accepted by INFRAREDX. INFRAREDX may accept a purchase order by electronic transmission, in writing, by shipment of a partial order or the entire order, or other methods of express acceptance. INFRAREDX shall use commercially reasonable efforts to accept purchase orders if such order is issued seventy-five (75) days prior to the requested delivery date. In any event, INFRAREDX shall treat orders of Distributor in the manner that is not less favourable than any orders by other distributors as to the priority for delivery. INFRAREDX shall deliver Products within seventy-five (75) days after acceptance of Distributor’s purchase orders.
(e) Terms of Purchase Orders. Distributor’s purchase orders submitted to INFRAREDX from time to time with respect to Products to be purchased hereunder shall be governed by the terms of this Agreement, and nothing contained in any such purchase order shall in any way modify such terms of purchase or add or delete any additional terms or conditions. All sales of the Products to Distributor under this Agreement shall be at INFRAREDX’s then-current prices, in US dollars, as reflected on Exhibit B. All such purchase orders shall include the purchase order number; billing address; name, list number, quantity of Products being ordered, requested delivery date, detailed shipping instructions and preferred warehouse of Distributor for delivery. In each invoice in respect of any such sale, INFRAREDX shall xxxx Distributor at currently-applicable prices. In the event of a conflict between the terms of this Agreement and the terms of any purchase order form or other document submitted by Distributor to INFRAREDX in connection with any order for Products, this Agreement shall control unless the parties specifically otherwise agree in writing.
(f) Change Orders. Distributor may utilize written change orders without penalty for orders that have not yet been accepted by INFRAREDX. Any other change orders shall be subject to acceptance by INFRAREDX, at its sole discretion.
(g) Payment. Full payment of Distributor’s Purchase Price for the Products (including any freight, taxes or other applicable costs initially paid by INFRAREDX but to be borne by Distributor) shall be made by Distributor to INFRAREDX. Payment shall be in U.S. dollars. INFRAREDX shall extend the Distributor an open account credit in an amount and on terms to be determined by
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
INFRAREDX. Unless otherwise agreed in writing, full payment is due sixty (60) days from the date of xxxx of lading. Any invoiced amount not paid when due shall be subject to a service charge of one and one percent (1%) per month or the highest rate allowed by law, whichever is lower.
(h) Shipping. Prior to shipment of the Products, INFRAREDX shall conduct outgoing inspection over the Products and provide Distributor with the certificate of such inspection. All Products delivered pursuant to the terms of this Agreement shall be suitably packed for air freight shipment in shipping cartons as agreed by Distributor, marked for shipment at Distributor’s address set forth above or as set forth on any applicable purchase order, and shall be delivered to Distributor or its carrier agent Ex-Works INFRAREDX’s or INFRAREDX’s U.S. contractor’s manufacturing plant, at which time title to such Products and risk of loss shall pass to Distributor. Unless otherwise instructed in writing by Distributor, INFRAREDX shall select the carrier. All freight, insurance, and other shipping expenses, as well as any special packing expense, shall be paid by Distributor. Distributor shall also bear all applicable Distributor Taxes, duties, and similar charges. INFRAREDX shall endeavor to ensure prompt delivery to Distributor of all Products ordered by Distributor under this Agreement, but shall not be liable for loss or damage suffered by Distributor as a result of any delay in shipment or delivery due to reasons beyond its control.
(i) Non-Conforming and Undelivered Goods. If any shipment of Products intended by INFRAREDX to be delivered to Distributor in response to any order contains any non-conforming Product, contains fewer than the number of units of Products ordered by Distributor or is not delivered to Distributor on the date agreed upon by the parties, then Distributor shall notify INFRAREDX by facsimile or electronic mail within fifteen (15) days following such date that Distributor finds such non-conformity of Product, that of the number of units, or non-delivery on time, precisely specifying the non-conformity or non-delivery in question. If Distributor fails to provide INFRAREDX on a timely basis with any notice required by this Subsection 3(i) with respect to any shipment of Products ordered by Distributor, then Distributor shall be deemed to have acknowledged the timely delivery and conformity of all Products covered by that order. INFRAREDX acknowledges that Distributor will conduct inspection of the delivered Products only with appearance of unpacked shipping cartons.
| 4. | TRAINING AND SERVICE |
(a) Services by Distributor. Distributor shall train the customers with respect to the Products sold and provide services in accordance with Exhibit C. The services shall be performed only by specially and properly trained personnel of Distributor and shall be prompt and of the highest quality. Distributor shall maintain properly equipped sales and training departments as required and shall keep on hand, at all times, Products sufficient to meet the needs of the Territory (in accordance with Subsection 6(f) below).
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(b) Training by INFRAREDX. INFRAREDX shall provide sales and technical training to Distributor’s personnel prior to initial Product shipment and additional training will be provided on an as needed basis as mutually agreed by the parties during the term of the agreement. The dates, location and content of the training shall be determined by mutual agreement by the parties. Such training may be given at a location in or near the Territory. In either case, INFRAREDX and Distributor shall each pay their own costs for travel, food, and lodging during the training period.
| 5. | WARRANTY |
(a) Limited Warranty. INFRAREDX warrants that the Products shall be free from defects in material and workmanship for fourteen (14) months from installation date for equipment and twelve (12) months for disposables from the date of shipment of the Products from INFRAREDX’s or INFRAREDX’s United States contractor’s manufacturing facility. Further, INFRAREDX warrants that disposables remaining shelf live on date of delivery to Distributor shall be a minimum of ten (10) months unless otherwise agreed by the parties on a case by case basis. If the approved shelf life period is extended by the U.S. health authorities or the Japanese health authorities, the warranty period in this Section 5(a) for the disposables shall be automatically extended to a term equal to such extended term, and the approved shelf life period shall also be automatically extended to a term equal to such extended shelf life period which is less two (2) months. During the warranty period, INFRAREDX will provide spare parts and technical assistance phone support during regular business hours (UTC -5:00 or US Eastern Time) excluding INFRAREDX holidays. Further, INFRAREDX warrants the software, when used as permitted under this Agreement, will operate as described in the product documentation for fourteen (14) months from the date of installation date of such software. Software upgrades, when and if available, will be provided at no charge during the warranty. Software upgrades consist of changes, bug fixes, improvements, or enhancements that enable the software to perform in accordance with the product specifications as defined at the time of original equipment purchase. New hardware, if required to run the software upgrade that relates to increased performance and not a repair, will be made available at an additional cost. Hardware related to repairs will be made available free of charge. For spare parts, add-ons, hardware upgrade packages, factory-rebuilt subassemblies (not under original equipment warranty), the warranty is for thirty (30) months from the date of shipment from INFRAREDX’s or INFRAREDX’s United States contractor’s manufacturing facility or fourteen (14) months from the date of installation of such spare parts, add-ons, hardware upgrade package, factory-rebuilt subassemblies, whichever the last day comes first, unless otherwise agreed in writing by INFRAREDX. All warranties are conditioned upon INFRAREDX’s receipt of notice of any defect prior to the end of the applicable warranty period. The remedy of Distributor for INFRAREDX’s breach of the foregoing warranty shall include, in INFRAREDX’s option, the replacement of a confirmed defective Product or the refund of the purchase price paid by Distributor for such defective Product, provided that Distributor may have recourse to any other remedies available under applicable laws. This warranty is contingent upon proper use of Products in the application for which they were intended as indicated in the instructions for use
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
therefore, and the foregoing warranty shall not apply to Products that were modified, or otherwise altered or changed or used in conjunction with other products, materials or substances, without INFRAREDX’s approval or that were subjected by the customer to unusual physical, electrical or environment stress. Distributor shall pass on to its customers the foregoing standard limited warranty and, except as required by law, Distributor shall not pass on to its customers a warranty or limitation of liability which is more protective of such customers than the warranty (including the limited remedy and exclusions) set forth in this Section 5 and the limitation of liability set forth in Section 9.
(b) No Other Warranty. EXCEPT FOR THE LIMITED WARRANTY PROVIDED IN THIS SECTION 5, INFRAREDX MAKES NO OTHER WARRANTIES OR CONDITIONS, EXPRESS OR IMPLIED, BY STATUTE OR OTHERWISE, REGARDING THE PRODUCTS, AND INFRAREDX SPECIFICALLY DISCLAIMS THE IMPLIED WARRANTIES OF FITNESS FOR A PARTICULAR PURPOSE, MERCHANTABILITY, SATISFACTORY QUALITY AND THOSE ARISING OUT OF COURSE OF DEALINGS OR USAGE IN TRADE. INFRAREDX NEITHER ASSUMES NOR AUTHORIZES ANY OTHER PERSON TO ASSUME ANY OTHER LIABILITIES ARISING OUT OF OR IN CONNECTION WITH THE SALE OR USE OF ANY PRODUCT. NOTWITHSTANDING THE FOREGOING, INFRAREDX DOES NOT EXCLUDE LIABILITY TO THE EXTENT THAT SUCH LIABILITY MAY NOT BE EXCLUDED OR LIMITED BY LAW.
| 6. | ADDITIONAL OBLIGATIONS OF DISTRIBUTOR |
(a) Payment of Distribution Franchise Fee. Distributor shall pay to INFRAREDX a one-time Distribution Franchise Fee in the amount of [***] US dollars ($[***]) according to the payment schedule specified in the following sub-section, 6(a)(i). Distributor agrees to remit these Distribution Franchise Fee payments according to the terms specified in Subsection 3(g), Payment.
(i) Schedule of Payments for Distribution Franchise Fee. Distributor shall remit payment to INFRAREDX according to the following schedule of events:
| Event |
Percent of Distribution Franchise Fee |
Payment Due | ||||||
| Execution of Distribution Agreement |
[***] Percent ([***] | %) | $ | 2,500,000.00 USD | ||||
| Products (Exhibit A) PMDA Approval Date |
[***] Percent ([***] | %) | $ | [***] USD | ||||
(b) Minimum Purchase Commitment. Distributor and INFRAREDX have mutually agreed on the annual quantity of Products to be purchased by Distributor from INFRAREDX as set forth in Exhibit D (“Minimum Purchase Commitment”).
(c) Forecasts. Within the first five (5) business days of every calendar quarter, Distributor shall provide INFRAREDX with a twelve (12) month rolling forecast (“Forecast”) showing prospective orders by Product model, intended submittal date, and then-current inventory levels of Products. The Forecast shall not be binding and shall be for planning purposes only.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(d) Promotion of the Products. Distributor shall, at its own expense, use its commercially reasonable efforts to vigorously promote the sale of the Products within the Territory; provided, that such sales shall be made for use only by qualified individuals as appropriate in the Territory, in compliance with local laws and regulations and good commercial practices and for uses and applications reasonably approved by INFRAREDX for the Products. Such promotion shall include but not be limited to all registration, translation and labelling requirements, preparing the promotional material in appropriate languages for the Territory, advertising the Products in trade publications within the Territory, participating in appropriate trade shows, and directly soliciting orders from customers for the Products. National and local meetings within the Territory shall be sponsored by Distributor while international congresses shall be sponsored by INFRAREDX. For international congresses, INFRAREDX shall use its reasonable best efforts to support the faculty participation of any Distributor clients but is under no obligation to underwrite any associated expenses incurred by Distributor or Distributor’s client. In the event that Distributor’s promotion of the Products requires it to use material that differs from routine promotional material, including in connection with the use of translations, Distributor shall make commercially reasonable efforts to provide copies of such material to INFRAREDX for approval and Distributor shall not use such material until receipt of written approval of such material by INFRAREDX, provided that in the event that INFRAREDX did not give notice on its approval in a timely manner, Distributor may use such material without approval of INFRAREDX subject to Distributor’s indemnity obligations in this Agreement. Distributor shall not use promotional materials contrary to the best interest of INFRAREDX. INFRAREDX shall make marketing materials in English available to Distributor free of charge. Such marketing material may be in printed or electronic format as appropriate.
(e) Representations. Distributor shall not make any false or misleading representations to customers or others regarding INFRAREDX or the Products. Distributor shall not make any representations, warranties or guarantees with respect to the specifications, features or capabilities of the Products that are not consistent with INFRAREDX’s documentation accompanying the Products or INFRAREDX’s literature describing the Products, including the limited warranty and disclaimers.
(f) Finances, Facilities and Personnel. Distributor shall (i) maintain a net worth and working capital and devote financial resources, (ii) maintain, lease or contract with such offices, warehouses and sales facilities, and (iii) maintain such competent and technically qualified sales and service personnel for the Products, in each case as shall be reasonably sufficient to allow Distributor to perform fully and faithfully its obligations under this Agreement.
(g) Inventory. Distributor shall, at its own expense, maintain a sufficient inventory of the Products at all times during the term of this Agreement as necessary to meet the reasonable requirements of any customer or potential customer within the Territory.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(h) Customer and Sales Reporting. Distributor shall, at its own expense and consistent with the sales policies of INFRAREDX:
(i) Place the Products in Distributor’s catalogues, both printed and electronic, including websites, as soon as possible and feature the Products in any applicable trade show that it finds appropriate;
(ii) Maintain adequate contact with existing and potential customers within the Territory on a regular basis, consistent with good business practice, which shall include but shall not be limited to maintaining a list of its Product customers including the customer name and address, the customer point of contact, the customer telephone and facsimile numbers, Product serial number and Product installation date and location, software revision level or upgrade level;
(iii) Assist INFRAREDX in assessing customer requirements for the Products, including modifications and improvements thereto, in terms of quality, design, functional capability, and other features; and
(iv) Prepare accurate and orderly business and accounting records concerning its inventories and sales of the Products. Distributor shall maintain these records for a period of at least two years after any calendar quarter to which they apply. Distributor shall provide INFRAREDX with copies of such records within fifteen (15) days of any written request by INFRAREDX; and
(v) On the Effective Date and thereafter at the beginning of each calendar year, provide INFRAREDX with a report containing information concerning the following: (A) Distributor’s quarterly sales volumes for each of the Products during the period covered by the report; (B) Distributor’s promotional activities regarding the Products, both during the period covered by the report and for the upcoming year; (C) a summary of current market conditions for the Products, including information concerning introductions, promotional activities and sales levels of products competitive with the Products; and (D) additional market research information, as reasonably requested by INFRAREDX, regarding competition and changes in the market within the Territory, including without limitation identification of competing products, market prices and market trends.
(i) Customer Complaints. Distributor shall report to INFRAREDX no later than within two (2) Business Days of receipt all customer complaints of any nature concerning the Products and all notices of serious or adverse reaction associated with the use of the Products, or if Distributor shall otherwise become aware of any adverse experience with any of the Products. Distributor shall cooperate fully with INFRAREDX in the resolution of such complaints, and shall take such action to resolve such complaints as may be reasonably requested by INFRAREDX. Distributor shall maintain records of
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
such complaints for at least seven (7) years after their receipt and shall make such records available to INFRAREDX for inspection and copying upon INFRAREDX’s request at any time during Distributor’s normal business hours. In the event of termination of this Agreement for any reason, a copy of such records immediately shall be transferred to INFRAREDX. As to quality control of the Products including handling of customer complaints, the parties shall enter into a good quality practice agreement attached as EXHIBIT E on the Effective Date. If there is any discrepancy between this Agreement and such good quality practice agreement, such good quality practice agreement shall prevail.
(j) Import and Export Requirements. Distributor shall, at its own expense, pay all import and export licenses and permits, pay customs charges and duty fees, and take all other actions required to accomplish the export and import of the Products purchased by Distributor, including without limitation paying all applicable Distributor Taxes. Distributor understands that INFRAREDX is subject to regulation by agencies of various governments, including the U.S. Department of Commerce, which prohibits export or diversion of certain technical products to certain countries. Distributor warrants that it will comply in all respects with the export and re-export restrictions set forth in any required export license for every Product shipped to Distributor.
(k) Government Regulations.
(i) Distributor agrees that it will secure any and all required approvals by any government other than the United States of America and all required product and public health registrations for the implementation, execution and performance of this Agreement. In particular, and without limitation of the foregoing, Distributor shall exercise due diligence to promptly obtain and maintain government approvals to import, register and market the Products in each jurisdiction in the Territory and to diligently secure and maintain, as may be required from time to time, government importing, registration and marketing approvals, import and export licenses, customs clearances and currency authorizations and any permits necessary in each jurisdiction in the Territory. Distributor shall use only materials approved in advance by INFRAREDX in applying for and maintaining such approvals. INFRAREDX shall supply all documentation necessary for such registration to Distributor free of charge. Distributor shall keep INFRAREDX generally informed of the regulatory requirements in each jurisdiction in the Territory and shall submit to the government health authorities in each jurisdiction in the Territory where the sale of the Products is planned a complete application for registration and marketing plan, as required. Distributor shall promptly advise INFRAREDX of all developments relating to these registrations, authorizations, licenses and approvals. Distributor will apply for such governmental approvals and product registration under its name and will bear all expenses for obtaining any such government approvals and product registrations. Upon any expiration, cancellation, or termination of this Agreement, such approvals shall be transferred and delivered to, and shall inure to the benefit of INFRAREDX or its designee, at reimbursement of cost to Distributor upon presentation of invoice. Following the initial five (5) year term of this
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Agreement, should the transfer of such approvals come to be required as a result of INFRAREDX or its successors terminating this Agreement for any reason not specified in Sections 8(a),(b),(c),(c), (e), (f)(i), or (f)(ii), then subsequent to the Distributor’s transfer of such approvals, INFRAREDX or its successor shall enter into good faith negotiation with the Distributor to assign a fair market value to the intrinsic INFRAREDX brand and product franchise within the Territory that has been created directly by Distributor’s efforts under this Agreement for the express purpose of compensating Distributor for its efforts in this regard. Both parties agree that this intrinsic market value shall be based on the share of the total intravascular imaging market held by INFRAREDX Products for the twelve (12) months immediately preceding the effective date of such termination and that such negotiations shall be carried out in good faith for a period not to exceed thirty (30) days. Following the conclusion of these negotiations, INFRAREDX or its successor shall remit payment to Distributor for the amount agreed upon by the parties. This payment shall not exceed the documented marketing costs—excluding direct sales costs—which were actually incurred by DISTRIBUTOR to develop the market in the TERRITORY. No such payment shall be paid for any value generated following the non-renewal or expiration of this Agreement. In no event shall the transfer of such approvals be delayed or withheld if the parties are unable to agree on a reasonable payment amount. If the parties cannot agree on the amount of such reasonable payment within the 30-day negotiation period, then the determination of such amount will be arbitrated, according to the terms and conditions specified in Section 13(b). Distributor shall obtain all necessary documents or licenses and shall comply with all applicable laws, including, if required, registration of this Agreement. Distributor shall notify INFRAREDX of all permits, approvals and registrations obtained by it and shall provide INFRAREDX with copies of all material documents related thereto. If Distributor is unable to obtain all necessary documents or licenses or to comply with all applicable laws in order to perform this Agreement within a reasonable time, not to exceed twenty-four (24) months after INFRAREDX has supplied all documentation (including documentation that is additionally required by the authorities) necessary for such licenses or government approvals, then INFRAREDX may terminate this Agreement upon giving thirty (30) days written notice to Distributor or may amend Exhibit A by deleting any product for which Distributor has been unable to obtain the necessary government approvals; provided, however, that the twenty four (24) month period shall be extended by the extent of any delay directly caused by the requirement imposed by a governmental authority.
(ii) Distributor agrees that it shall not allow the Products supplied to it by INFRAREDX, INFRAREDX’s Trademarks, any proprietary data of INFRAREDX, or any direct product of such data, to be knowingly made available, either directly or indirectly, or in any way to be knowingly given, transferred, sold or re-exported to any country in violation of its laws and export control regulations or applicable laws of any country (or the European Union). United States laws and export control regulations governing the exportability of technical data and Products to nations are subject to change. If any country included within the Territory shall, at the time of execution of this Agreement, be placed in an excluded category by the United States government for the receipt of either technical data or the manufacture or sale of products
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
of the type supplied by INFRAREDX, Distributor agrees that it shall take all actions necessary to cease business activity in Products in the excluded country. Distributor shall comply with all laws, statutes, decrees, regulations and policies which have the effect of law in the Territory, including but not limited to anti-bribery laws, and which apply to its activities under this Agreement.
(l) Compliance with Laws. Distributor acknowledges and expressly agrees that certain laws of the Territory and the US, including, but not limited to, the Export Control Regulations, the Food and Drug Act and the Foreign Corrupt Practices Act, may result in the imposition of sanctions on INFRAREDX or its affiliates in the event that, directly or indirectly, (i) any Product is exported to various countries, such as Cuba, Libya and North Korea, or any country embargoed by executive order, (ii) any Product is delivered to a customer that does not have authorization to use such Product under the provisions of the Food and Drug Act and the regulations promulgated thereunder, or (iii) Distributor offers to pay, promises to pay or payments are made to government officials or others for the purpose of influencing decisions favorable to INFRAREDX. Distributor expressly agrees, therefore, that in performing its obligations under this Agreement it shall comply at all times with such laws or regulations of the Territory and the US, and shall furnish to INFRAREDX, by affidavit or other reasonable means from time to time at INFRAREDX’s request, and to INFRAREDX’s reasonable satisfaction, assurances that the appointment of Distributor and Distributor’s activities under this Agreement are proper and lawful under the laws and regulations in the Territory. Distributor further agrees that no person employed by it shall be an official of any government agency or a corporation owned by a governmental unit within the Territory and that no proceeds from the sale of the Products in the Territory shall accrue for the benefit of any such official.
(m) Traceability Programs. Distributor agrees to maintain records to ensure the traceability of the Products in accordance with applicable regulatory requirements, if any. In particular, and without limiting the generality of the foregoing, Distributor agrees to maintain a complete and current list of all customers who have purchased Products from Distributor, the date of such purchases and the serial and/or lot numbers of the units purchased. Such records shall be kept for such period of time and under the same terms mentioned in Subsection 6(h) above.
(n) Product Recalls. In the event that INFRAREDX deems it necessary to recall any Product because such Product fails to comply with the warranties set forth in this Agreement, or if any governmental authority shall request recall of any Product for any reason, Distributor shall promptly affect such recall in accordance with its standard procedures then in effect. Distributor shall keep INFRAREDX fully informed as to the status of such recall. Distributor shall initiate no communications regarding any such recall with the news media, customers, governmental or regulatory authorities, except if and to the extent required by applicable law, without the prior approval of INFRAREDX, which approval will not be unreasonably withheld or delayed; provided, that Distributor shall notify all affected customers within two (2) Business Days of INFRAREDX’s request (unless a shorter period is required under applicable
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
laws or regulations), using materials and documentation prepared and approved by INFRAREDX, and shall promptly provide INFRAREDX with a written status report of all units subject to the recall. All costs and expenses associated with implementation of a recall shall be borne by (a) INFRAREDX, if the recall results from fault attributable to INFRAREDX, or (b) Distributor, if the recall results from fault attributable to Distributor; provided, that each party will provide, at its expense, any assistance reasonably requested by the other party in connection with the implementation of any recall pursuant to this Subsection 6(m).
(o) Standard of Conduct. Distributor agrees to use good judgment, high ethical standards and honesty in Distributor’s dealings with INFRAREDX, customers, end users, employees and government officials, recognizing that even the appearance of unethical actions is not acceptable.
(p) ISO Compliance. In the event the Territory includes any country adopting the International Standards Organization 9002 standards (or any successor thereto), Distributor shall be in compliance with the standards applicable to the Distributor’s activities under this Agreement at the date the standards become effective, and shall furnish proof to INFRAREDX that the Distributor is in compliance. The Distributor’s failure to comply with the provisions of this section shall give INFRAREDX the right to sell and service the Products in the Territory directly or through a third party, and the right to terminate this Agreement upon notice to the Distributor.
(q) Sub Distributors. Distributor shall provide INFRAREDX with written notice of any sub-distributor appointed by Distributor for the Products. Prior to allowing any such sub-distributor to distribute Products, Distributor shall enter into a written agreement with such sub-distributor that obligates such sub-distributor to be bound by the terms and conditions of this Agreement in the same manner as such terms and conditions apply to Distributor. Distributor shall be obligated and responsible for the performance of the obligations under this Agreement, regardless of whether any portion of such obligations is delegated to a sub-distributor.
| 7. | ADDITIONAL OBLIGATIONS OF INFRAREDX |
(a) Materials. INFRAREDX shall promptly provide Distributor with marketing and technical information concerning the Products as well as reasonable quantities of brochures, instructional material., advertising literature and other Product data, with all such material printed in the English language or in electronic form. In addition, INFRAREDX shall provide Distributor with materials reasonably necessary to obtain health registrations, to the extent practicable, as well as any other documents which Distributor may reasonably require for registration purposes, free of charge. INFRAREDX shall use commercially reasonable efforts to provide Distributor with academic papers and articles concerning the Products.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(b) Quotations to Exporters. INFRAREDX shall refrain from giving quotations to exporters for Products to be shipped to the Territory. Notwithstanding the above, the parties agree that it may be in their mutual best interests to participate with other third parties in consolidated or other bulk purchases of Products for the Territory and agree to consult in advance and agree on mutually acceptable terms so as to participate in these transactions.
(c) Changes in Product. INFRAREDX may make such changes in the Products or in any of the parts thereof or in any specifications as INFRAREDX may deem necessary. INFRAREDX agrees to give Distributor a minimum of twelve (12) months prior written notice of any changes it intends to make in the Products or the parts thereof. INFRAREDX expressly acknowledges that the Japanese health authorities will require approval on any change in specifications of the Products or the parts thereof, even if such change is minor or such changes in no way affect the function of the Products. INFRAREDX reserves the right to discontinue at any time or from time to time the sale of any of the Products, test equipment or parts covered by this Agreement. INFRAREDX agrees to give Distributor twelve (12) months prior written notice of any discontinued product and will, upon agreement with Distributor, agree to continue supply of discontinued product to satisfy existing tenders or contracts entered into by Distributor. Notwithstanding the foregoing, INFRAREDX shall continue to sell the existing Products and the existing parts (i) until Distributor completes government approval or registration for such change in the Products or in parts thereof or in specification in case such approval or registration is required, or (ii) as long as the existing spare parts are necessary for operation of the Products sold to the customer from Distributor.
(d) The First Refusal Right of Distributor. If INFRAREDX develops any new products that are based on the technology used in the Products but will require a completely new application for marketing approval, INFRAREDX shall promptly notify Distributor and give it the first refusal right to obtain exclusive right to distribute such new products in the Territory in accordance with this Section 7(d). Distributor shall notify INFRAREDX whether it wants to obtain such exclusive distribution right within sixty (60) days of receipt of such notice from INFRAREDX. If Distributor so requests this right in writing within such time period, then the Parties shall use commercially reasonable efforts to negotiate the terms and conditions in good faith, applicable to such new product for a period not to exceed sixty (60) days from INFRAREDX’s receipt of Distributor’s notice of its desire to obtain rights to such new product. If the parties are unable to agree on the terms and conditions for such new products within such 60-day period, INFRAREDX shall have no further obligations to Distributor with respect to such new products under this Section 7(d).
(e) Return of Distribution Franchise Fee. INFRAREDX shall be obligated to return to Distributor the full amount of the Distribution Franchise Fee the Distributor has remitted to INFRAREDX upon any of the following circumstances:
(i) Prior to the five (5) year anniversary of the Products PMDA Approval Date, a Change of Control of INFRAREDX results in the termination of this Agreement by INFRAREDX or the acquiror of INFRAREDX or the Distributor’s loss of its appointment to distribute the Products in the Territory;
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(ii) Prior to the five (5) year anniversary of the Products PMDA Approval Date, (i) the institution by or against INFRAREDX of insolvency, receivership or bankruptcy proceedings, appointment of an administrator or any other proceedings for the settlement of INFRAREDX’s debts, (ii) INFRAREDX’s making an assignment for the benefit of creditors, (iii) INFRAREDX’s dissolution or ceasing to do business or (iv) otherwise INFRAREDX’s becoming incapable to supply the Products to Distributor;
(iii) Prior to the five (5) year anniversary of the Products PMDA Approval Date, INFRAREDX’s voluntary withdrawal of the Products from the Japanese market;
(iv) Prior to the five (5) year anniversary of the Products PMDA Approval Date, the forced withdrawal or recall of Products from the Japanese market by Japan’s PMDA, if said recall and prohibition against sale in Japan cannot be cured by the parties within six (6) months;
(v) Failure by Distributor and INFRAREDX to receive regulatory approval for the Products from Japan PMDA in accordance with Subsection 6(k)(i); provided that such failure is not otherwise due to Distributor.
(f) Provision of certain Products. Upon receipt of Distributor’s full Distribution Franchise Fee payment(s), INFRAREDX shall, at its own expense, transfer ownership to Distributor of ten (10) sets of TVC Imaging System consoles (NIRC-MC7) and its necessary components and disposables as listed in Exhibit A. Provided Distributor’s [***]% of Distribution Franchise Fee payment is made, INFRAREDX shall agree to, at its own expense, transfer ownership to Distributor of at most five (5) TVC Imaging System consoles (NIRC-MC7) prior to the approval as needed basis. Transportation and delivery terms for these particular Products shall be in accordance with Subsection 3(h), Shipping above.
| 8. | TERM AND TERMINATION |
(a) Term. This Agreement shall continue in force until the fifth (5th) anniversary of the Products PMDA Approval Date, unless terminated earlier under the provisions of this Section 8. At the end of the initial 5-year term, this Agreement shall automatically renew for subsequent one year periods provided that the parties agree on minimum purchases per Exhibit D for each subsequent period. If Distributor wants to renew the Agreement but the parties fail to agree on such minimum purchase for any renewed year, the minimum purchases shall be [***] percent of the average number of the relevant Products which were purchased during the last [***] contract years immediately preceding each end of the term.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(b) Termination for Failure to Meet Minimums. If Distributor fails to meet the Minimum Purchase Commitments in any given period, INFRAREDX may either (i) provide Distributor the opportunity to purchase Products to satisfy such Minimum Purchase Commitment, (ii) terminate Distributor’s exclusivity in the Territory and appoint other distributors to distribute Products in the Territory, or (iii) terminate this Agreement upon ninety (90) days’ written notice.
(c) Termination for Cause. If either party materially defaults in the performance of any provision of this Agreement, then the non-defaulting party may give written notice to the defaulting party that if the default is not cured within thirty (30) days this Agreement will be terminated. If the non-defaulting party gives such notice and the default is not cured during the thirty (30) day period, then this Agreement shall automatically terminate at the end of that period.
(d) Termination for Insolvency. This Agreement shall terminate automatically, with no action required to be taken by or on behalf of INFRAREDX, (i) upon the institution by or against Distributor of insolvency, receivership or bankruptcy proceedings, appointment of an administrator or any other proceedings for the settlement of Distributor’s debts, (ii) upon Distributor’s making an assignment for the benefit of creditors, or (iii) upon Distributor’s dissolution or ceasing to do business. Distributor immediately shall notify INFRAREDX of the occurrence of any of the above.
(e) Termination for Distributor Disqualification. INFRAREDX may terminate this Agreement immediately by providing written notice to Distributor if Distributor becomes legally disqualified for any reason from importing or distributing the Products in the Territory; if all necessary registration, licenses and permits required to sell and distribute the Products in the Territory are not obtained in accordance with Subsection 6(k)(i); or if Distributor loses any license or authorization that is required under the laws of the Territory for the importation, promotion or sale of the Products. If (i) Distributor or any of its directors, officers or significant consultants shall be indicted for a criminal offense in connection of the importation, promotion or sales of the Products, or (ii) Distributor or any of its directors, officers or significant consultants shall violate the applicable laws, including the U.S. Foreign Corrupt Practices Act, then INFRAREDX shall, at its option, have the right to immediately terminate this Agreement upon giving written notice to Distributor.
(f) Manufacturer Termination. INFRAREDX will have the right to terminate this Agreement (i) immediately in the event of a Change of Control of Distributor; (ii) within ninety (90) days written notice to Distributor in the event of a Change of Control of INFRAREDX, subject to Section 7(e)(i); and (iii) upon its determination that the continued use or sale of the Product creates the risk of a product liability claim or presents a safety concern.
(g) Termination for Change of Control of INFRAREDX. Within thirty (30) days following a Change of Control of Distributor, Distributor may terminate this agreement with ninety (90) days written notice to INFRAREDX in the event INFRAREDX undergoes a Change of Control with a third party that is a competitor of Distributor in the Territory.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(h) Effect of Termination.
(i) Termination shall not relieve either party of obligations incurred before the termination, and the rights of each party against the other which may have accrued up to the date of such termination or expiration shall remain in force. All payments outstanding shall become immediately due upon any termination of this Agreement.
(ii) Upon termination or expiration of this Agreement for other than Distributor’s breach, non-performance or insolvency, INFRAREDX shall continue to fulfill, subject to the terms of Section 3 above, all orders accepted by INFRAREDX before the date of termination. In addition, INFRAREDX may continue to accept orders from Distributor after termination; however, such acceptance shall not constitute a renewal of this Agreement or a waiver of the right of INFRAREDX to treat this Agreement as terminated. INFRAREDX shall be entitled, before shipment of any pending or new orders, to require advance payment, or other security for payment, of all previously outstanding balances (whether or not otherwise then due) plus the amount of any new order.
(iii) All Trademarks, patents, copyrights, designs, drawings, formulas or other data, photographs, samples, literature, Standard Promo Material, promotional and other material developed by Distributor, and all sales aids of every kind shall remain the property of INFRAREDX. Within thirty (30) days after the termination of this Agreement, INFRAREDX may repurchase all or any portion of its Products from Distributor at the original sales price, provided said Products are in good condition and have available shelf life remaining. INFRAREDX reserves the right to charge a 7.5 (seven and a half) percent restocking fee for any Products repurchased. INFRAREDX agrees to repurchase any Distributor-owned non-disposable Products which the Distributor has placed into service within the Territory at a price depreciated from the original sales price, according to the following schedule:
| Months Since Original Distributor Purchase from Infraredx |
Repurchase Price as a Percentage of Original Purchase Price |
|||
| New, Distributor’s inventory |
100 | % | ||
| 0 months to 12 months of customer use |
67 | % | ||
| 13 months to 24 months of customer use |
33 | % | ||
| 25 months onward of customer use |
0 | % | ||
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
If INFRAREDX decides to repurchase the Products, Distributor shall prepare all such items in its possession for shipment, as INFRAREDX may direct, at INFRAREDX’s expense. Distributor shall not make, use, dispose of or retain any copies of any confidential items or information which may have been entrusted to it. Effective upon the termination of this Agreement, Distributor shall cease to use all Trademarks of INFRAREDX and shall cease to engage in any act or omission that would indicate or suggest a relationship with INFRAREDX except to the extent that Distributor continues to sell existing inventory as provided in Subsection 8(h)(v) below. Distributor shall for a period of five (5) years after termination continue to hold in strict confidence all such information.
(iv) Upon request by INFRAREDX, Distributor shall take all necessary or appropriate actions to transfer to INFRAREDX or its designee any Product registrations, licenses, permits, consents or other approvals by government agencies obtained by Distributor in order to import, distribute or sell the Products in the Territory; provided, however, that INFRAREDX will grant to Distributor a non-exclusive right under such registrations, licenses, permits, consents or other approvals as far as necessary for Distributor to sell existing inventory permitted by Subsection 8(h)(v) below.
(v) Except for any termination by INFRAREDX under Subsections 8(c) or 8(f), Distributor may continue to sell existing inventory of the Products that are not repurchased by INFRAREDX within one (1) year of the termination date of this Agreement subject to the terms of this Agreement.
(i) Limitation on Liability. It is expressly understood and agreed that the rights of termination and non-renewal set forth in this Agreement are absolute, and that the parties have considered the possibility of such termination or non-renewal and the possibility of loss and damage resulting therefrom, in making expenditures pursuant to the performance of this Agreement. It is the express intent and agreement of the parties that neither shall be liable to the other for damages or otherwise by reason of the termination of this Agreement as provided in this Section 8, provided that such termination shall not operate to discharge or release either party of obligations assumed by it prior to such termination. The parties expressly agree that the notice periods in this Agreement are reasonable under the contemplated circumstances and that the parties have considered the possibility of the making of expenditures by one or both of the parties hereto in preparing for and in the actual performance of this Agreement, and have considered the possibility of loss and damage resulting from the termination hereof. In the event of termination by either party in accordance with the provisions of this Agreement, neither party shall be liable to the other, because of such termination itself; for compensation, reimbursement or damages on account of the loss of prospective profits or anticipated sales or on account of expenditures, inventory, investments, leases or commitments in connection with the business or goodwill of INFRAREDX or Distributor.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 9. | LIMITATION ON LIABILITY |
(a) Product Liability. INFRAREDX shall defend or settle third party claims brought against Distributor, or its directors, officers or employees alleging personal injury liability or any other product liability claim alleging defective design applicable law in the Territory to the extent proximately and solely caused by defects in Products supplied to Distributor by INFRAREDX, and shall pay any damages finally awarded in connection therewith, provided that (i) such defect existed at the time the Product was shipped by INFRAREDX, (ii) no modifications in design have been made by or with the approval of Distributor or the end user, (iii) the Product has not been subject to misuse, negligence or accident, (iv) the Product has not had its serial or lot number altered, effaced or removed, (v) the Product has been used in accordance with its Instructions for Use, and (v) the Distributor promptly notifies INFRAREDX of such claim, gives INFRAREDX sole control over the defense or settlement of such claims, and provides INFRAREDX with full information and reasonable assistance in the defense of such claim.
(b) Limitation on Liability. INFRAREDX’S LIABILITY ARISING OUT OF THIS AGREEMENT AND/OR THE SALE OF THE PRODUCTS SHALL BE LIMITED TO THE AMOUNT PAID BY DISTRIBUTOR FOR THE PRODUCTS. IN NO EVENT SHALL INFRAREDX BE LIABLE FOR COSTS OF PROCUREMENT OF SUBSTITUTE GOODS OR SERVICES, OR FOR ANY LOST PROFITS OR LOSS OF ANTICIPATED PROFITS. IN NO EVENT SHALL INFRAREDX BE LIABLE TO DISTRIBUTOR FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, PUNITIVE OR INDIRECT DAMAGES, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY, ARISING OUT OF THIS AGREEMENT. THESE LIMITATIONS SHALL APPLY NOTWITHSTANDING ANY FAILURE OF ESSENTIAL PURPOSE OF ANY LIMITED REMEDY. HOWEVER, THIS SECTION 9 WILL NOT LIMIT INFRAREDX’S LIABILITY FOR CONTRIBUTION OR INDEMNITY, IF ANY, WITH RESPECT TO THIRD PARTY CLAIMS FOR PERSONAL INJURY, DEATH OR PHYSICAL DAMAGE TO PROPERTY.
| 10. | PROPERTY RIGHTS AND CONFIDENTIALITY |
(a) Property Rights. Distributor agrees that INFRAREDX owns all right, title, and interest in the product lines that include the Products and in all of INFRAREDX’s patents, Trademarks, inventions, copyrights, know-how, trade secrets and other intellectual property rights relating to the design, manufacture, operation or service of the Products. The use by Distributor of any of these property rights is authorized only for the purposes herein set forth, and upon termination of this Agreement for any reason such authorization shall cease.
(b) Confidentiality. Both parties acknowledge that by reason of its relationship hereunder it will have access to certain information and materials concerning the other parties’ business, plans, customers, technology and products that are confidential and of substantial value, which value would be impaired if such information were disclosed to third parties. The receiving party agrees that it will not use in any way for its own account or the account of any third party, nor disclose to any third party, any
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
such confidential information revealed to it by the disclosing party. The receiving party shall take every reasonable precaution to protect the confidentiality of such information. Upon request by receiving party, the disclosing party shall advise whether or not it considers any particular information or materials to be confidential; provided, that information shall not be deemed confidential if it (i) is known to the receiving party, as evidenced by the receiving party’s written records, before receipt thereof under this Agreement; (ii) is disclosed to the receiving party by a third person who is under no obligation of confidentiality to the disclosing party with respect to such information and who otherwise has a right to make such disclosure; (iii) is or becomes generally known in the trade through no fault of the receiving party; or (iv) is independently developed by the receiving party, as evidenced by the receiving party’s written records, without access to such information. The receiving party shall not publish any technical description of the Products beyond the description published by the disclosing party (except to translate that description to appropriate languages for the Territory). In the event of termination of this Agreement, there shall be no use or disclosure by the receiving party of any confidential information of the disclosing party, and the receiving party shall not manufacture or have manufactured any devices, components or assemblies utilizing any of the disclosing party’s confidential information.
| 11. | TRADEMARKS |
(a) Use. During the term of this Agreement, Distributor shall have the right to indicate to the public that it is INFRAREDX’s exclusive distributor of the Products in the Territory, provided that Distributor remains in compliance with its obligations under this Agreement, and to advertise (within the Territory) such Products under the Trademarks that INFRAREDX may adopt from time to time. Distributor shall not alter or remove any INFRAREDX’s Trademarks applied to the Products at the factory, and shall promote and sell the Products in the Territory only under the Trademarks. Except as set forth in this Section 11 nothing contained in this Agreement shall grant to Distributor any right, title or interest in INFRAREDX’s Trademarks, and Distributor shall not assert any right, title or interest in or to any of the Trademarks. Distributor acknowledges the validity of the Trademarks and at no time during or after the term of this Agreement shall Distributor challenge or assist others to challenge INFRAREDX’s Trademarks or the registration thereof or attempt to register any Trademarks confusingly similar to those of INFRAREDX.
(b) Approval of Representations. All representations of INFRAREDX’s Trademarks that Distributor intends to use shall be submitted to INFRAREDX and shall be exact copies of those used by INFRAREDX. If any of INFRAREDX’s Trademarks are to be used in conjunction with another trademark on or in relation to the Products, then INFRAREDX’s Trademarks shall be presented equally legibly, equally prominently, and of greater size than the other but nevertheless separated from the other so that each appears to be a xxxx in its own right, distinct from the other xxxx.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(c) Quality Control. Distributor shall maintain the quality of its distribution services at a level consistent with the historical quality of services associated with INFRAREDX. Distributor undertakes not to use the Trademarks in a manner that will impair the name or jeopardize the goodwill or reputation of the Products or INFRAREDX. Distributor shall not use any Trademark except in connection with the Products. In the event that Distributor makes any permissible modifications to the Products or their packaging pursuant to the terms of this Agreement, the quality of such modified Products and their packaging shall be at least equal to that of the Products and their packaging.
(d) Notice of Infringement of Trademarks. Distributor shall promptly notify INFRAREDX of any apparent infringement or threatened infringement of any Trademark, and shall, upon request by INFRAREDX and at INFRAREDX’s expense, use its best efforts to assist INFRAREDX to restrain any such infringement or threatened infringement.
| 12. | PATENT, COPYRIGHT AND TRADEMARK INDEMNITY |
(a) Indemnification by INFRAREDX. INFRAREDX shall defend, or at its option shall settle, any claim, suit or proceeding brought against Distributor, or its directors, officers or employees or its customer by a third party on the issue of infringement of any U.S. or Japanese patent existing as of the Effective Date, copyright or trademark by the Products sold hereunder or the use thereof, subject to the limitations hereinafter set forth. INFRAREDX shall have sole control of any such action or settlement negotiations, and INFRAREDX agrees to pay, subject to the limitations hereinafter set forth, any final judgment entered against Distributor or its customer on such issue in any such suit or proceeding defended by INFRAREDX. Distributor agrees that INFRAREDX at its sole option shall be relieved of the foregoing obligations unless Distributor notifies INFRAREDX promptly in writing of such claim, suit or proceeding and gives INFRAREDX authority to proceed as contemplated herein and, at INFRAREDX’s expense, gives INFRAREDX proper and full information and assistance to settle and/or defend any such claim suit or proceeding. If the Products, or any part thereof, are, or in the opinion of INFRAREDX may become, the subject of any claim, suit or proceeding for infringement of any patent, copyright or trademark, or if it is adjudicatively determined that the Products, or any part thereof, infringe any patent, copyright or trademark, or if the sale or use of the Products, or any part thereof: is, as a result, enjoined, then INFRAREDX may, at its option and expense either: (i) procure for Distributor and its customers the right under such patent, copyright or trademark to sell or use, as appropriate, the Products or such part thereof; or (ii) replace the Products, or part thereof, with other suitable Products or parts; or (iii) suitably modify the Products, or part thereof; or (iv) if the use of the Products, or part thereof, is prevented by injunction, remove the Products, or part thereof, and refund the aggregate payments paid therefore by Distributor, less a reasonable sum for use and damage. INFRAREDX shall not be liable for any costs or expenses incurred without its prior written authorization.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(b) Limitation. Notwithstanding the provisions of Subsection 12(a) above, INFRAREDX assumes no liability for (i) infringements covering completed equipment or any assembly, combination, method or process in which any of the Products may be used but not covering the Products when used alone; (ii) trademark infringements involving any marking or branding not applied by INFRAREDX or involving any marking or branding applied at the request of Distributor; or (iii) infringements involving the modification or servicing of the Products, or any part thereof unless such modification or servicing was done by INFRAREDX.
(c) Entire Liability. The foregoing provisions of this Section 12 state the entire liability and obligations of INFRAREDX and the exclusive remedy of Distributor and its customers, with respect to any alleged infringement of patents, copyrights, trademarks or other intellectual property rights by the Products or any part thereof.
(d) Indemnification by Distributor. Distributor agrees to save and hold INFRAREDX harmless from any and all claims, costs, liabilities and responsibilities, regardless of the claimant or his place of filing a claim, resulting from or in any way associated solely with the functioning or performance of Distributor or its sub-distributors as a distributor, supplier and seller, or other related descriptive classifications, for Products supplied to Distributor by INFRAREDX. The foregoing indemnity shall not extend to claims for which INFRAREDX is responsible under Section 9(a).
(e) No Joinder. Distributor agrees not to join INFRAREDX or any INFRAREDX shareholder, director, officer, employee or consultant as a party defendant or plaintiff, or any interest thereof, in any action at law or in equity or in any other proceeding, regardless of the descriptive classification, arising out of the above described liabilities, duties and responsibilities which Distributor assumes or performs. Distributor shall promptly notify INFRAREDX of any and all actions at law or equity or claims or governmental administrative proceeding arising out of the operation or performance of this Agreement.
| 13. | GENERAL PROVISIONS |
(a) Governing Law. This Agreement, and INFRAREDX’s and Distributor’s relationship, and any matter arising in respect or in connection therewith, shall be solely and exclusively governed by and construed under the laws of the State of New York without giving effect to the conflict of laws provisions thereof. The parties expressly disclaim the application of the United Nations Convention on the International Sale of Goods to this Agreement.
(b) Arbitration. The parties hereto agree that any dispute or controversy arising out of, in relation to, or in connection with this Agreement, or the validity, enforceability, construction, performance or breach thereof, shall be finally settled by binding arbitration conducted under the then-current Rules of Arbitration of the International Chamber of Commerce by three (3) arbitrators appointed in accordance
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
with such Rules. Any discovery proceeding shall not be adopted by the arbitrator. The place of the arbitration shall be Honolulu, Hawaii, USA. Distributor hereby expressly consents to (i) the conduct of the arbitration in Honolulu, Hawaii; (ii) service of process being effected upon it by registered mail sent to the address set forth at the beginning of this Agreement, and (iii) the uncontested enforcement of a final judgment from such court in another jurisdiction wherein Distributor or any of its assets are present or the personal jurisdiction of the federal and state courts within Hawaii, USA. The parties agree that judgment upon the decision and/or award rendered by the decision may be entered in any court of competent jurisdiction. The costs of the arbitration, including administrative fees and fees of the arbitrator and reasonable costs to be incurred by a party for necessary translation and interpretation of evidences, shall be shared equally by the parties, unless otherwise determined by the arbitrator. Each party shall bear its own cost including its own attorneys’ fees and expert fees. In addition to the right under the Rules to petition the court for provisional relief, the parties agree that any party may also petition the court for provisional injunctive relief where either party alleges or claims a violation of any of Sections 6, 10, 11, and 12 of this Agreement. In the event either party seeks injunctive relief, the prevailing party will be entitled to recover reasonable costs and attorneys’ fees.
(c) Entire Agreement; Amendments. This Agreement and the Exhibits attached hereto sets forth the entire agreement and understanding of the parties relating to the subject matter herein and merges all prior discussions between them. No representations, inducements, promises or agreements, whether oral or otherwise, between the parties not contained herein or incorporated herein by reference shall be of any force or effect. Except as set forth herein, no modification of or amendment to this Agreement, nor any waiver of any rights under this Agreement, nor any agreement or understanding extending this Agreement or varying its terms (including any inconsistent terms in any purchase order, acknowledgement or similar form) shall be effective unless in writing signed by both parties to this Agreement.
(d) Notices. Any notice required or permitted by this Agreement shall be in writing and shall be (i) delivered in person, (ii) sent by prepaid registered or certified mail, return receipt requested, (iii) sent by facsimile, or (iv) sent by email, addressed to the other party at the addresses or numbers as follows, or to such addresses or numbers changed by a party upon written notice to the other party:
| For Infraredx: Address: INFRAREDX, INC., 00 Xxxxx Xxxxxx, Xxxxxxxxxx, XX 00000, X.X.X. Attention: Vice President of Finance Fax: 000-000-0000 Email: xxxxxx@xxxxxxxxx.xxx |
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| For Distributor: Address: |
|
|
| Attention: Fax: Email: |
All notice sent by one party to the other party in accordance with this Agreement shall be deemed to have been received by the other party:
| (i) | Three (3) days after deposit in the mail if sent by registered or certified mail. |
| (ii) | On the date when usual evidence of confirmation of the transmission is received by the sender if sent by facsimile. |
| (iii) | On the date when the email is sent unless the sender does not received a response that the email cannot be sent to the other party if sent by email. |
If the relevant day of receipt is a non Business Day, all notices shall be deemed to have been received by the other party on the following Business Day.
(e) Force Majeure and Inability to Deliver.
(i) Non-performance of either party shall be excused to the extent that performance is rendered impossible due to industrial conflicts, mobilization, requisition, embargo, currency restriction, insurrection, general shortage of transport, material or power supply, fire, explosion, terrorism, stroke of lightning, force majeure and similar casualties or other events beyond Distributor’s or INFRAREDX’s control, as well as default in deliveries from subcontractors due to such circumstances as defined in this clause.
(ii) If the performance of this Agreement by either party is made commercially impracticable (i) by the occurrence of an economic contingency the non-occurrence of which was a basic assumption on which this Agreement was made or (ii) by compliance in good faith with any applicable foreign or domestic governmental law, regulation, or order, then this Agreement shall terminate immediately. For purposes of this Agreement, currency devaluation, currency restrictions, currency and exchange controls, and other monetary controls, restrictions, and restraints shall not be considered to render the performance of this Agreement by the party commercially impracticable, or otherwise be considered force majeure with respect to the party.
(f) Nonassignability and Binding Effect. A mutually agreed consideration for INFRAREDX’s entering into this Agreement is the reputation, business standing, and goodwill already honored and enjoyed by Distributor under its present ownership, and, accordingly, Distributor agrees that its rights and obligations under this
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Agreement may not be transferred or assigned directly or indirectly without the prior written consent of INFRAREDX. INFRAREDX’s rights and obligations under this Agreement may be transferred or assigned to (i) an affiliate or (ii) successor in interest by way of merger, consolidation, sale of all or substantially all of its assets or similar transaction (a “Change of Control”). Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the parties hereto and their successors and assigns.
(g) Legal Expenses. The prevailing party in any legal action brought by one party against the other and arising out of this Agreement shall be entitled, in addition to any other rights and remedies it may have, to reimbursement for its expenses, including court costs and reasonable attorneys’ fees.
(h) Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original and all of which together shall constitute one instrument
(i) Language. This Agreement is in the English language only, which language shall be controlling in all respects, and all versions hereof in any other language shall not be binding on the parties. All communications and notices to be made or given pursuant to this Agreement shall be in the English language.
(j) Conflict or Inconsistency. In the event of any conflict or inconsistency between the terms and conditions of this Agreement and any terms or conditions set forth in any purchase order or other document relating to the transactions contemplated by this Agreement, the terms and conditions set forth in this Agreement shall prevail.
(k) Severability. If one or more of the provisions in this Agreement are deemed void by law, then the remaining provisions will continue in full force and effect.
(l) No Waiver of Rights. No failure or delay by either party in exercising any right or remedy under this Agreement shall be construed as a waiver of such right or remedy, nor shall any single or partial exercise of any right or remedy preclude any further or other exercise of such right or remedy. All rights and remedies under this Agreement are cumulative and shall not be deemed exclusive of any other rights or remedies provided by law.
(m) Captions. Captions of the sections and subsections of this Agreement are for reference purposes only and do not constitute terms or conditions of this Agreement and shall not limit or affect the meaning or construction of the terms and conditions hereof.
(n) Word Meanings. Words such as herein, hereinafter, hereof and hereunder refer to this Agreement as a whole and not merely to a section or paragraph in which such words appear, unless the context otherwise requires. The singular shall include the plural, and each masculine, feminine and neuter reference shall include and refer also to the others, unless the context otherwise requires.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
(o) Rules of Construction. The parties agree that they have participated equally in the formation of this Agreement and that the language and terms of this Agreement shall not be preemptively construed against either of them.
(p) Survival of Certain Terms. The provisions of Sections 1, 3(g), 3(i), 5, 6(i), 6(j), 6(k), 6(l), 6(m), 6(n), 7(e), 8(e), 8(g), 8(h), 8(i), 9, 10, 11(a), 12 and 13 shall survive the termination of this Agreement for any reason. All other rights and obligations of the parties shall cease upon termination of this Agreement.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
IN WITNESS WHEREOF, the parties have executed this Agreement as of the date written above.
| INFRAREDX INC. | NIPRO CORPORATION | |||||||
| By: | /s/ Xxx Xxxxxxxx |
By: | /s/ Xxxxxxxxx Xxxx | |||||
| Name: | Xxx Xxxxxxxx | Name: | Xxxxxxxxx XXXX | |||||
| Title: | President & CEO | Title: | Managing Director | |||||
| Date: | 3- 30- 2012 | Date: | 30 March, 2012 | |||||
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
EXHIBIT A
PRODUCT DESCRIPTION AND TERRITORY
Product:
| Product |
Catalog Number | |
| TVC Imaging™ System Console | NIRS-MC7 | |
| TVC Insight™ Catheter | NIRC-MC7-70-3.2F | |
| TVC Imaging™ Sterile Accessory Kit | NIRS-MC7-SA | |
| TVC Imaging™ System Extended End User Software Support License Agreement, 4 years extended coverage |
NIRS-MC7-SSLA-4YR |
Territory:
Japan
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
EXHIBIT B
PRICING
| Product |
Catalog Number |
Price | ||
| TVC Imaging™ System Console | NIRS-MC7 | $[***] each | ||
| TVC Insight™ Catheter | NIRC-MC7-70-3.2F | $[***] each | ||
| TVC Imaging™ Sterile Accessory Kit | NIRS-MC7-SA | N/A | ||
| TVC Imaging™ System Extended End User Software Support License Agreement, 4 years extended coverage |
NIRS-MC7-SSLA-4YR | $[***] each, per covered system |
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
EXHIBIT C
SERVICE PROCEDURES AND STANDARDS
Distributor will serve as the primary point of contact for the delivery of service for all INFRAREDX products in the Territory,
Product Repairs
Distributor will provide customers with product repair service as necessary. Distributor may fulfill this obligation in one of three ways:
| • | Distributor may, after the successful completion of a INFRAREDX service training course and designation as a INFRAREDX Authorized Service Center, perform repairs itself in accordance with servicing procedures defined by INFRAREDX; or |
| • | Distributor may return product to INFRAREDX for repair at INFRAREDX’s service facility; or |
| • | Distributor may purchase repair services from any INFRAREDX Authorized Service Center. |
Records
Distributor will maintain records of any servicing activity it performs for a period of 5 years. Such records shall at a minimum, include the following information:
Installation:
| 1. | customer name |
| 2. | address |
| 3. | contact name |
| 4. | telephone number |
| 5. | install date |
| 6. | product description |
| 7. | model number |
| 8. | serial number |
| 9. | software revision level or upgrade level |
Repair:
| 1. | customer name |
| 2. | address |
| 3. | contact name |
| 4. | telephone number |
| 5. | repair date |
| 6. | product description |
| 7. | product reference number |
| 8. | software revision level or upgrade level |
| 9. | description of repair |
| 10. | part number and reference number of parts replaced |
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 11. | part number and reference number of new parts |
| 12. | name of technician performing repair |
Product Returns
Distributor will make all product returns to INFRAREDX in accordance with INFRAREDX Material Return Procedures.
Complaint and Incident Reporting
As stated in Section 6(h), Distributor shall regularly report to INFRAREDX any customer complaints received by the Distributor related to the Products. Distributor will report such information in accordance with INFRAREDX Complaint Procedures.
INFRAREDX Service Contact
All service inquiries should be directed to INFRAREDX Technical Support at 0 (000) 000-0000. After the warranty period, INFRAREDX will make available to Distributor a Basic Service Agreement which will cover replacement parts, technical phone support, and software upgrades. Additionally, INFRAREDX will make available replacement parts and equipment upgrades for purchase as needed. Contact INFRAREDX Technical Support for spare parts, upgrades, or service agreement pricing.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
EXHIBIT D
MINIMUM PURCHASES & DEMO PRODUCTS
Minimum Purchases:
During the initial term of this Agreement, the Distributor shall make the following minimum purchases by the end of each of the following anniversaries of the date of the first delivery of the Products from INFRAREDX to Distributor for commercial sale after PMDA Approval Date:
| Minimum Annual Purchases, by year, for each year that follows the delivery date of the first Products shipped to Distributor for commercial sale after PMDA approval | ||||||||||
| During Year 1 |
During Year 2 |
During Year 3 |
During Year 4 |
During Year 5 | ||||||
| TVC Imaging™ Systems |
[***] | [***] | [***] | [***] | [***] | |||||
| TVC Insight™ Catheters |
[***] | [***] | [***] | [***] | [***] | |||||
During the term of this Agreement the Distributor shall maintain at least the following Demo Product quantities:
| TVC Imaging™ Systems (NIRS-MC7) | 10 each |
INFRAREDX warrants that all disposable Products shall possess a rated shelf life of at least eighteen (18) months on or before the third anniversary of the date the Products were first delivered to the Distributor as part of this Agreement.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
EXHIBIT E
GOOD QUALITY PRACTICE AGREEMENT
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
GOOD QUALITY PRACTICE AGREEMENT
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
CONTRACT FOR SECURING OF MANUFACTURING CONTROL AND QUALITY CONTROL
Marketing Authorization Holders; Nipro Corporation (Located at 0-0-0 Xxxxx-Xxxxx, Xxxx-xx, Xxxxx, Xxxxx hereinafter called NIPRO) and manufacturer; Infraredx, Inc. (Located at 00 Xxxxx Xxxxxx, Xxxxxxxxxx, XX 00000, X.X.X. hereinafter called (INFRAREDX) hereby conclude a contract as follows to secure appropriate execution of manufacturing control and quality control of medical device in accordance with the revised Pharmaceutical Affairs Law to be effected on April 1, 2005 in regard to products to be manufactured at the plant of INFRAREDX by INFRAREDX on assignment from NIPRO.
| 1. | Assigned products and the scope of assigned work |
The products for which manufacture is to be assigned by NIPRO to INFRAREDX shall be as set in Annexed Document 1.
INFRAREDX shall not re-assign the entire or part of the manufacturing of the products, assigned from NIPRO to a third party without the written approval of NIPRO in advance.
| 2. | Manufacturing control, quality control, and procedures on shipment |
| 1) | INFRAREDX shall execute manufacturing control and quality control of the products basing on ministerial ordinance No. 169 of the Ministry of Health, Labor and Welfare, dated December 17, 2004 regarding standards of manufacturing control and quality control of medical device and external diagnostic medicines (hereinafter called QMS) /IS013485:2003. |
| 2) | The QMS/IS013485:2003 system in the plant of INFRAREDX shall be specified in Annexed Document 2. |
| 3) | Procedures relating to shipment of the products shall be specified in Annexed Document 3. |
| 3. | Technical conditions relating to manufacturing method, test methods, etc. |
| 1) | Technical conditions relating to manufacturing methods and testing and inspection methods of the products shall be items specified in the quality specifications. |
| 2) | INFRAREDX shall specify the above technical conditions in The Device Master Record /Technical Documentation. |
| 3) | INFRAREDX shall manufacture the products in accordance with the manufacturing method specified in The Device Master Record /Technical Documentation and shall supply NIPRO with the quality specifications. |
| 4) | INFRAREDX shall furnish NIPRO with the process chart, the QC process chart, and the sterilizing method and sterilizing conditions specified in The Device Master Record /Technical Documentation. |
| 4. | Confirmation by NIPRO in regard to manufacturing control and quality control NIPRO shall, in accordance with Annexed Document 4, confirm that the products are manufactured under appropriate manufacturing control and quality control |
.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| according to the Contract and QMS/IS013485:2003 at the Accreditation of Foreign Manufactures of INFRAREDX (Accreditation #BG30401929) and shipped. INFRAREDX shall cooperate in this confirmation. |
| 5. | Improvement instructions |
In case NIPRO recognizes that improvements are necessary in regard to manufacturing control and quality control of the product, NIPRO may give instructions to INFRAREDX to take required improvement measures by the form specified in Annexed Document 3. INFRAREDX shall promptly and appropriately execute the improvement instructions and report the results to NIPRO in the form specified in Annexed Document 3. NIPRO shall confirm the results as required at the actual site.
| 6. | Quality control method in transportation and at the time of delivery The transportation method of the product from INFRAREDX to the place designated by NIPRO and the method relating to quality control at the time of delivery shall be as specified in Annexed Document 5. |
| 7. | Procedures on reporting of changes |
In case INFRAREDX is to make changes specified in Annexed Document 6, INFRAREDX shall report the change in advance to NIPRO in accordance with the procedures specified in Annexed Document 6 and shall execute the said change upon receiving permission of NIPRO.
| 8. | Report relating to quality, effectiveness and safety |
NIPRO shall furnish to INFRAREDX, information relating to quality which is necessary in executing appropriate and smooth manufacturing control and quality control of the product in the form specified in Annexed Document 3.
| 9. | Disposition of complaints, disposition of quality information and defects of quality, etc. |
NIPRO and INFRAREDX shall execute work relating to disposition of complaints and work relating to quality information and defects of quality, etc. in accordance with the procedures stated in Annexed Document 7.
| 10. | Recovery disposition |
NIPRO and INFRAREDX shall conduct work relating to recovery disposition in accordance with procedures stated in Annexed Document 8.
| 11. | Keeping of documents and such |
INFRAREDX shall keep procedure manuals on manufacturing control and quality control of the products established by INFRAREDX and records relating to manufacturing, testing and inspecting at least 10 years from the date of preparation (for procedure manuals, etc. from the date of suspension of use). However in case the law stipulates the maintaining of documents for periods longer than mentioned above, such periods shall be observed.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 12. | Contacting party and person in charge |
The contacting party for agreed items in this contract and the person in charge shall be as specified in Annexed Document 9. In case no particular contacting method is specified, contact shall be made promptly by telephone, electronic mails, etc. and subsequently confirmed in detail in writing.
| 13. | Other necessary items |
| 1) | Reports on administrative examination items |
| (1) | In case of receiving communication of administrative examination, INFRAREDX shall promptly notify to NIPRO of that. |
| (2) | In case a particular item relating to manufacturing of the product is pointed out by administrative examinations, INFRAREDX shall promptly inform NIPRO to that effect in writing. |
| (3) | In case of indicating improvement for administrative examinations NIPRO instruct INFRAREDX by documents. |
| 2) | Method on affixing lot numbers |
Lot numbers for this product shall be affixed by the method specified in the quality specifications.
| 14. | Items for discussions |
In case of items which are not specified in this contract are seen or in case a doubt arises in the contents of an item, both NIPRO and INFRAREDX shall work to settle the matter through discussions.
| 15. | Changes in Annexed Documents and forms |
In case changes are to be made in the Annexed Documents or Forms, the concerned Annexed Documents or Forms may be changed without amending this contract by agreeing to exchange “Inquiry on changes of Annexed Documents and forms/agreement on changes of Annexed Document and forms,” mentioned in Annexed Document 10. NIPRO shall keep custody of the Annexed Document 10. Furthermore, NIPRO shall enter the outline of the changed items in the amendment history list (Annexed Document 11) and report this to INFRAREDX.
| 16. | Effective period of this contract |
The agreement by this contract shall become effective as of May 11, 2012 and shall continue to be effective until six years have elapsed after the product is launched on the market. However in case neither of the parties informs the other party to the contrary in writing six months before the expiry of the contract, the contract shall be automatically renewed for another year and this procedure shall apply thereafter. However the provisions of Item 11 shall continue to remain effective for the period specified by the Item, and Article 9 and Article 10 shall continue to remain effective as long their necessity exists.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
IN WITNESS WHEREOF, this contract shall be executed in duplicate and upon affixing the signatures and seals of both parties, each party shall retain one copy.
Date: , 2012
| NIPRO CORPORATION 0-0-0 XXXXX-XXXXX XXXX-XX XXXXX 000-0000 XXXXX |
| [Sign Here] |
| /s/ Xxxxxxx Xxxxxxx |
| Xxxxxxx Xxxxxxx |
| Quality Assurance & Regulatory Compliance Division Director |
| 1NFRAREDX, INC. |
| 00 Xxxxx Xxxxxx, Xxxxxxxxxx, XX, 00000, X.X.X. |
| [Sign Here] |
| /s/ Xxxxxx Xxxxxxxx |
| Xxxxxx Xxxxxxxx |
| CEO |
.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Contract for Securing of Manufacturing
Control and Quality Control
Annexed Document
NIPRO CORPORATION
AND
INFRAREDX, INC.
1.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 8, 2012
Revisions, none
[Annexed Document 1. Medical devices assigned for manufacture by NIPRO to INFRAREDX and the assigned work]
List 1 Medical device assigned for manufacture by NIPRO to INFRAREDX
| Sales name |
Medical device approval (certification, notification) No. | |
| TVC ImagingTM System Console | Not determined yet | |
| TVC InsightTM Catheter | Not determined yet | |
| TVC ImagingTM Sterile Accessory Kit | Not determined yet |
2.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
NIPRO shall assign the scope of work specified in List 2 in regard to medical device specified in List 1.
List 2 Scope of work assigned by NIPRO to INFRAREDX
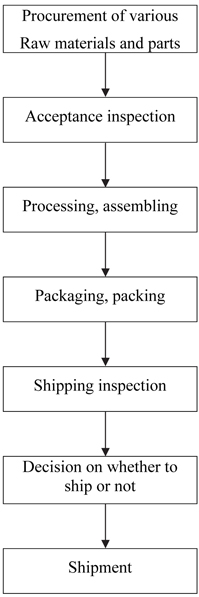
| * | Process inspection shall be performed in accordance with The Device Master Record / Technical Documentation kept by INFRAREDX. |
3.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
[Annexed Document 2 QMS/ISO13485:2003 system]
Medical device QMS organization chart
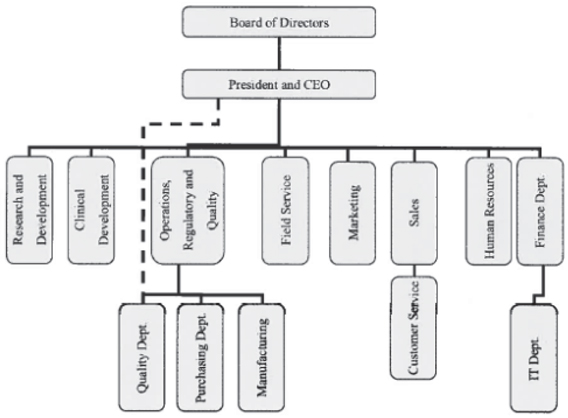
4.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012 Revisions, none
[Annexed Document 3 QMS/ISO13485:2003 system]
| 1. | Procedures relating to shipment |
| 1) | After the decision on whether to make shipment or not is appropriately made by INFRAREDX, INFRAREDX shall ship the products. |
| 2) | INFRAREDX shall decide whether to ship each lot or not by evaluating the following items. |
| (1) | Results of the manufacturing control and quality control (Manufacturing record, printed record, process inspection record, shipping inspection record and sterilizing record (excluding unsterilized products)) |
| (2) | The existence of deviation specified in item 2 |
| (3) | The existence and contents of information from NIPRO relating to quality, effectiveness and safety |
| 3) | Basing on the results of decisions for making of shipments, INFRAREDX shall prepare “Shipment qualified products report/ Shipment qualified products confirmation (Form 3-1)” every day of shipment qualified and report to NIPRO. And INFRAREDX shall report the shipping inspection to NIPRO when report “Shipment qualified products report/ Shipment qualified products confirmation (Form 3-1).” |
| 4) | In case NIPRO receives information relating to quality, effectiveness, or safety which may affect the decision on making of shipment, NIPRO shall prepare a “Report on information relating to quality, effectiveness and safety (Form 3-3)” upon evaluating the effect upon shipping decision and report to INFRAREDX. INFRAREDX shall decide shipment of the concerned product basing on the received report. |
| 5) | In case NIPRO judges that improvement is required in shipping control work by INFRAREDX, NIPRO shall state improvement instruction items on “Work improvement execution plan report (Form 3-4)” and instruct INFRAREDX accordingly. |
In accordance with the instructions from NIPRO, INFRAREDX shall set up an improvement plan for shipping control work, prepare “Work improvement execution plan report (Form 3-5)” and report to NIPRO. After reporting, INFRAREDX shall improve shipping control work in accordance with the improvement plan.
NIPRO shall confirm the improvement results as required on shipping control work when confirming QMS/IS013485:2003 of INFRAREDX.
5.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 2. | Deviation action |
| 1) | Deviations on which reports to NIPRO are required |
Classify the nonconformities which occurred at the plant of INFRAREDX as follows and define nonconformities which apply to (1) and/or (2) as deviations, NIPRO and INFRAREDX shall take deviation action in accordance with Item 2.(2).
| (1) | In case of occurrence of nonconformities on shipping inspection, INFRAREDX shall report to NIPRO in accordance with item 2.(2). |
| (2) | In case of occurrence of serious nonconformities which lead to recall and MDR, INFRAREDX shall report to NIPRO in accordance with item 2.(2). |
| 2) | Procedure of the deviations |
In case a deviation specified in item 2.(1)a, b occurs, INFRAREDX shall prepare a form administered by INFRAREDX (Use of report by form administered by INFRAREDX to be limited to cases where product name, date of occurrence, lot No. of occurrence, deviation description, cause of deviation and measures to cope with deviation can be stated) or “Deviation report/Deviation confirmation (Form 3-2)” and promptly report to NIPRO.
In case inadequacies in confirmation of report from INFRAREDX to NIPRO, NIPRO may instruct INFRAREDX to take investigation of revaluation or unclear matter by documents
INFRAREDX shall take investigation of revaluation or unclear matter in accordance with instructions from NIPRO, and INFRAREDX report to NIPRO by document.
NIPRO shall evaluate the contents of deviation and measures and give instructions to INFRAREDX relating to shipping using “Deviation report/Deviation confirmation (Form 3-2)”
INFRAREDX shall take measures on the deviation item in accordance with instructions from NIPRO.
6.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 3-1 Revisions, none
Date:
Shipment qualified products report
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: Infraredx, Inc. | ||
| Responsible engineer | (signature) |
The following report is made on shipment qualified products at (year, month, date). And the report is attached the shipping inspections.
| product name | : | |
| Lot No. | : | |
| quantity | : | |
(Contents of confirmation)
| (1) |
Results of manufacturing control and quality control |
Appropriate / inappropriate | ||
| (2) |
Presence of deviations |
Yes / no (See below if no) | ||
|
| ||||
| Contents of deviations |
| (3) | Information relating to quality, effectiveness, and safety which could affect the decision on shipping to the market was submitted by NIPRO | Yes / no | ||
| Mention evaluation and contents of information relating to quality, effectiveness, and safety if Yes. | Appropriate / inappropriate (Contents of information: ) | |||
Date:
Shipment qualified product confirmation
To Responsible engineer
Manufacturer:
7.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| Marketing Authorization Holders,: Nipro Corporation Person in charge of quality assurance: (signature) |
| Comments |
8.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 3-2 Revisions, none
Date:
Deviation Report
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Responsible engineer | (signature) |
| Product name | ||
| Date of occurrence | ||
| Occurrence Lot. No | ||
| Description of deviation | ||
| Cause of deviation | ||
| Measures taken on deviation (Planned execution and contents of executed measures) | ||
Date:
Deviation confirmation
To Engineer in charge
Manufacturer:
| Person in charge of quality assurance: (signature) Marketing Authorization Holders, Nipro Corporation |
9.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| Evaluation on report contents from INFRAREDX and instructions to INFRAREDX. (Including instructions relating to shipment) |
10.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 3-3 Revisions, none
Date:
Report on information relating to quality, effectiveness, and safety
To Engineer in charge
Manufacturer:
| Person in charge of quality assurance: |
| (signature) |
| Marketing Authorization Holders, Nipro Corporation |
We are submitting a report to you as follows in regard to received information which relates to quality, effectiveness and safety of products being manufactured by your plant.
| Subject product | ||
| Date information received | ||
| Information relating to quality, effectiveness, safety | ||
| Effect on the decision to allow shipping to the market. | ||
11.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 3-4 Revisions, none
Date:
Work improvement execution plan report
To Engineer in charge
Manufacturer
| Person in charge of quality assurance: |
| (signature) |
| Marketing Authorization Holders, Nipro Corporation |
Since we believe that improvements are required in regard to work being performed by you, we request that you make the following improvements. Please report on the improvement results of the respective improvement instructed items.
Work improvement instruction items
(1)
(2)
(3)
(4)
12.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 3-5 Revisions, none
Date:
Work improvement execution plan report
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Responsible engineer | (signature) |
Basing on the improvement instructions of (date), we report to you on as follows in regard to improvement plan and improvement execution time.
Improvement plan, improvement execution period
(1)
(2)
(3)
(4)
13.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
[Annexed Document 4, Confirmation by NIPRO relating to manufacturing control and quality control]
NIPRO shall audit INFRAREDX periodically in spot or by paper; in order to confirm that INFRAREDX manufactures the product under appropriate manufacturing control and quality control according to this contract and QMS/IS013485:2003. INFRAREDX shall cooperate with NIPRO in conducting the confirmation.
If NIPRO audit in spot, following the undermentioned procedure 3.
| 1. | Frequency of periodic audit execution |
Periodic audit by NIPRO shall be, in principle, once a year.
| 2. | Extraordinary audit |
Besides the periodic audit specified in the above 1. NIPRO may audit the plant of INFRAREDX in case any of the following applies.
| 1) | When INFRAREDX changes the manufacturing facilities of the product |
| 2) | When INFRAREDX changes the manufacturing method, testing methods etc. of the product |
| 3) | When abnormal quality is found or risk of abnormal quality is found in the product delivered to NIPRO by INFRAREDX. |
| 4) | When INFRAREDX manufacture new product. |
| 5) | Other cases in which NIPRO deems it necessary. |
| 3. | Spot audit procedures |
| 1) | In case NIPRO is to conduct an audit, a notice to that effect will be given to INFRAREDX in advance and adjustment of the audit schedule shall be made. After the schedule is confirmed, NIPRO shall prepare an “Audit execution plan (Form 4-1)” and report to INFRAREDX. |
| 2) | NIPRO shall conduct an audit to see that the products are manufactured and shipped under appropriate manufacturing control and quality control in accordance with the contract and with QMS/IS013485:2003. In case inadequacies in manufacturing control and quality control of INFRAREDX are found as the result of the audit, NIPRO shall prepare a “Report on nonconformities and improvement requirements (Form 4-2)” and submit it to INFRAREDX as instructions for improvement. |
| 3) | INFRAREDX shall set up an improvement plan for nonconformities and improvement requirements, and shall prepare an “Improvement plan on nonconformities and improvement requirements (Form 4-3)” and submit it to NIPRO. |
14.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 4) | Upon confirming the submitted improvement plan, NIPRO shall instruct INFRAREDX to execute the improvement measures if the contents are found to be appropriate. In case the contents are found to be inappropriate, NIPRO shall instruct INFRAREDX to re-submit the “Improvement plan on nonconformities and improvement requirements (Form 4-3).” |
| 5) | INFRAREDX shall perform improvement measures in accordance with the improvement plan. INFRAREDX shall report the results of the improvement measures taken on nonconformity items to NIPRO by a “Report on improvement measures on nonconformities (Form 4-4).” |
| 6) | NIPRO shall confirm the submitted improvement measure results and if the improvement measures results are found to be appropriate, the concerned audit shall be terminated. In case NIPRO finds an inadequacy in the improvement measure results, NIPRO shall instruct INFRAREDX to re-study the improvement measure. |
15.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 4-1 Revisions, none
Date:
Audit execution plan
To Person in charge of contacts
Manufacturer:
| Person in charge of quality assurance: |
| (signature) |
| Marketing Authorization Holders, Nipro Corporation |
| Audit date | Approved (Marketing Supervisor-General) | Examination (person in charge of quality assurance) | Prepared by: (Leader) | |||||
| Kind of audit | Regular Extraordinary | |||||||
| Object of the audit | / / | / / | / / | |||||
| Scope of audit | [Auditors] Leader: Members: | |||||||
| Order and time schedule of executing audit |
16.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 4-2 Revisions, none
Report on nonconformities and improvement requirements
| Manufacturer | ||||||
| Date of audit | ||||||
| Auditors | Audit leader: Audit members: |
|||||
| Person interviewed | ||||||
| Nonconformity items Improvement requirements |
Case Case | |||||
| Deadline for submitting improvement plan |
Date | |||||
Please submit improvement plans on the following “1. Nonconformity items” and “2. Improvement requirements” by the above mentioned deadline.
| Audit leader |
(signature) |
I agree to the deadline for submitting of the indicated items and the improvement plan.
Person responsible for the manufacturer
(signature)
1. Nonconformity items
(1)
(2)
2. Improvement requirements
(1)
(2)
17.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 4-3 Revisions, none
Date:
Improvement plan on nonconformities and improvement requirements
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Person in charge of contacts | (signature) |
An improvement plan on nonconformities and improvement requirements indicated in the audit conducted on (date) has been prepared and this is reported as follows:
1. Improvement plan on nonconformity items
(1)
(2)
2. Improvement plan on improvement requirements
(1)
(2)
18.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 4-4 Revisions, none
Report on improvement measures on nonconformities
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Person in charge of contacts | (signature) |
Improvements have been made on nonconformity items indicated in the audit held on (date) and are reported as follows:
(1)
(2)
19.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
[Annexed Document 5 Transporting method and quality control method in delivering ]
Transporting of products by ship and airplane shall form the basic transporting method. Transporting by other means shall be performed after receiving instructions or requests to do so from the other party.
In conducting transporting or making deliveries, the storage conditions, handling methods. etc. described on the transporting box of the respective products to be strictly observed.
In case there are quality controls methods other than those described on the transporting box, this quality control methods shall be specified in quality specifications and INFRAREDX shall observe this quality control methods.
20.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
[Annexed Document 6 Procedures on reporting on changes]
| 1. | Procedures of changes |
| 1) | Procedure for reports |
| (1) | In case INFRAREDX is to make changes on matters subject to advance reports as specified in item 2 mentioned below, INFRAREDX shall make a report in advance to NIPRO by using a form administered by INFRAREDX or by using “Change proposal/Change proposal confirmation (Form 6-1)” . (However a report with use of the form administered by INFRAREDX shall be limited to forms in which the subject product, object of change, contents of change, verification planned items, and planned date for executing of change may be entered.) |
| (2) | NIPRO shall confirm the contents of the change proposal and evaluate the appropriateness of the contents of change and verification items. NIPRO shall enter the evaluated results in “Change proposal/change proposal confirmation (Form 6-1)” and instruct INFRAREDX. |
| 2) | When NIPRO judged to be able to change after verification by INFRAREDX, NIPRO mention the instruction on “Change proposal/change proposal confirmation (Form 6-1)”,and NIPRO shall instruct INFRAREDX. |
| (1) | After the change is verified, INFRAREDX shall prepare a form administered by INFRAREDX (However a report by the use of a form administered by INFRAREDX shall be limited to forms in which the subject product and verification results may be entered) or “Report on change execution results/Change approval (Form 6-2)” , and report the verification results to NIPRO. |
| (2) | After confirming the verification results, NIPRO shall prepare “Report on change execution results/Change approval (Form 6-2)” and report to INFRAREDX on approval of production start. |
| (3) | INFRAREDX shall start production only after receiving the approval to start production. |
| 3) | Others |
| (4) | In regard to the concerned change, NIPRO may confirm control conditions of INFRAREDX at actual site as required. |
| (5) | In case the said change may seriously affect the quality of the product, NIPRO may instruct INFRAREDX to take necessary measures for improvements. |
21.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 2. | Matters subject to report |
In case of carrying out the change that was mentioned in “Separate sheet: Necessary change of report”, INFRAREDX shall report to NIPRO.
22.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Separate sheet
Necessary change of report
Necessary change that INFRAREDX report to NIPRO is from item 1) to 4).
| 1) | Changes requiring change in information disclosed to NIPRO by INFRAREDX in order for NIPRO to prepare Marketing Authorization, Marketing certification, Marketing notification, Application for Accreditation of foreign Manufacture, Application for QMS conformity examination. |
| 2) | Changes requiring changes in entered items in quality specifications |
| 3) | Changes relating to process chart, QC process chart, sterilizing method, and sterilizing conditions (excluding unsterilized items) |
| 4) | Other changes in which the possibility of affecting quality of the product cannot be denied. |
Examples of the change shall be mentioned in this list.
| Changed items |
Contents of change | |||
|
1.1 Raw materials | |||
|
(1) Plastic materials, colorant |
model, Grade | |||
| Manufacturer | ||||
|
(2) Adhesive, silicon oil, etc. |
model | |||
| Manufacturer | ||||
|
1.2 Packing materials |
||||
|
(1) Bag, wrapper packing, blister packing, base board, film |
Form | |||
| Material, film thickness | ||||
| model | ||||
| Printing design | ||||
| Manufacturer | ||||
|
(2) Inner box |
Printing design | |||
|
(3) Outer box |
Printing design | |||
|
(4) Attached documents |
Printing design, contents | |||
|
(5) Labels |
Printing design, contents | |||
|
1.3 Packing form |
Packing form | |||
| Packing material | ||||
|
1.4 Components |
Form, dimensions | |||
| Supplier | ||||
|
1.5 Products specifications |
Production specification specified in the Quality specifications | |||
|
1.6 Tests, inspections |
Tests and inspections specified in the Quality specifications | |||
23.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| Changed items |
Contents of change | |||
| 1.7 Change of applicable/cited specifications | ||||
|
|
(1) Manufacturing, processing process |
|||
|
(a) New manufacturing method |
New manufacturing method | |||
|
(b) Interchanging of process sequence |
Interchanging of process sequence | |||
|
(c) Addition, deletion of process |
Addition, deletion of process | |||
|
(d) Change of process chart and QC chart |
Change of process sequence, change of control item; etc. | |||
|
(2) Facilities |
||||
|
(a) Change (Including metal molds/dies) |
Manufacturing by new facilities | |||
|
3. Structural facilities |
Change of cleanliness class | |||
| Introduction of new structural facilities | ||||
24.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 6-1 Revision: None
Date
Change Proposal
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Person in charge of contacts | (signature) |
| Subject product |
| Object of change |
| Contents of change |
| Planned verification items |
| Planned date for executing change |
Date
Change proposal confirmation
To Person in charge of contacts
Manufacturer:
| Person in charge of quality assurance: |
| (signature) |
| Marketing Authorization Holders, Nipro Corporation |
| Subject product | ||
| Pharmaceutical Affairs application requirement | Required Not required | |
| Designation of verification data | ||
25.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| Instructions to manufacturer | ||
| Approval of change | Approved Rejected | |
26.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 6-2 Revision: None
Date
Report on change execution results
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Person in charge of contacts | (signature) |
| Subject product |
| Verification results |
Date
Change approval
To Person in charge of contacts
Manufacturer:
| Person in charge of quality assurance: |
| (signature) |
| Marketing Authorization Holders, Nipro Corporation |
| Subject product | ||
| Pharmaceutical Affairs application | Completed Not applicable | |
| Change approval | Approved Rejected | |
| Instruction items | ||
27.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
[Annexed Document 7 Quality information and disposition of quality defect]
| 1. | Procedures on complaint disposition |
| 1) | In case NIPRO receives information on a complaint, NIPRO shall promptly inform INFRAREDX of such in writing. |
| 2) | INFRAREDX shall promptly study the cause in regard to the information received from NIPRO. |
| 3) | In case an improvement is necessary in regard to manufacturing control and quality control, INFRAREDX shall take the required measures. |
| 4) | INFRAREDX shall report the results of the investigation on the cause and in case measures are taken for improvement, report such measures to NIPRO in writing in accordance with Annexed Document 6. |
| 5) | In case an inadequacy is seen in the report submitted by INFRAREDX, NIPRO may instruct INFRAREDX to take necessary measures for improvement. |
| 2. | Quality information and procedures on disposing of quality defects |
| 1) | Quality information and procedures on disposing of quality defects |
| (1) | In case quality information specified in Item 2.(2) and information on quality defects are received by INFRAREDX, INFRAREDX shall enter quality information and quality defect information in the “Quality information, quality defect occurrence report/Quality information, quality defect confirmation (Form 7-1)” and promptly report to NIPRO. |
| (2) | NIPRO shall confirm the contents of the information received from INFRAREDX and give instructions to INFRAREDX using “Quality information, quality defect report/Quality information, quality defect confirmation (Form 7-1).” In case it is necessary to obtain a report on occurrence cause and on measure results from INFRAREDX, NIPRO shall state such and report to INFRAREDX. |
| (3) | INFRAREDX shall observe the instructions of NIPRO and take appropriate measures. In case NIPRO requires a report on results of measures taken, INFRAREDX shall prepare “Quality information, quality defect measure report/ Quality information, quality defect measures content confirmation (Form 7-2)” and report to NIPRO. |
| (4) | NIPRO shall confirm the report from INFRAREDX and use “Quality information, quality defect measure report/Quality information, quality defect measures content confirmation (Form 7-2)” to instruct INFRAREDX. INFRAREDX shall take appropriate measures in accordance with instructions from NIPRO. |
28.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
| 2) | Quality information and quality defect which are to be reported |
| (1) | Information relating to quality as well as the safety of the product |
| (2) | Information relating to suspension, recovery, or abolition of manufacturing or sales of the product or other information relating to measures taken to prevent occurrence or spreading of damage to health or hygiene. |
29.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 7-1 Revision: None
Date
Quality information, quality defect occurrence report
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Person in charge of contacts | (signature) |
| Product name | ||||
| Xxx.Xx | Occurrence date | Date | ||
| Quality information, quality defect contents | ||||
Date
Quality information quality defect confirmation
To Person in charge of contacts
Manufacturer:
| Person in charge of quality assurance: (signature) Marketing Authorization Holders, Nipro Corporation |
| Instructions to manufacturer | ||||
| Whether continuation of reports required | Required / Not required | |||
30.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Form 7-2 Revision: None
Date
Quality information, quality defect measure report
To: Person in charge of quality assurance, Marketing Authorization Holders, Nipro Corporation
| Manufacturer: | ||
| Person in charge of contacts | (signature) |
| Product name |
| Lot. No |
| Quality information, quality defect contents |
| Cause of occurrence |
| Contents of measures |
Date
Quality information, quality defect measure content confirmation
To Person in charge of contacts
Manufacturer:
| Person in charge of quality assurance: |
| (signature) |
| Marketing Authorization Holders, Nipro Corporation |
| Instructions to manufacturer |
31.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11. 2012
Revisions, none
[Annexed Document 8 Recovery disposition]
| 1. | In case a possibility of conducting product recovery arises, both NIPRO and INFRAREDX shall promptly inform the other party of such, both parties will jointly gather information, shall mutually discuss and decide whether recovery is required or not. |
| 2. | In regard to the recovered product and its disposition, NIPRO and INFRAREDX shall mutually discuss and cope promptly with the matter. |
32.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012 Revisions, none
[Annexed Document 9 Contracting party and person responsible]
Person of NIPRO in charge of contacts (responsible for quality assurance)
| Full name | Xxxxxxx Xxxxxxxx | |
| Post and title | Quality Assurance & Regulatory Compliance Division, Quality Assurance Section Manager | |
| Address of contacting party | 0-0-0 XXXXX-XXXXX XXXX-XX XXXXX 000-0000 XXXXX | |
| Contacting telephone No. | 0-0000-0000 | |
| Contacting FAX No. | 0-0000-0000 | |
| E-mail address | xxxxxxxx-xxxxxxx@xxxxx.xx.xx |
Responsible engineer / Management representative of INFRAREDX
| Full name | Xxxxx Xxx | |
| Post and title | Director of Quality | |
| Address of contacting party | 00 0xx Xxxxxx Xxxxxxxxxx, XX 00000 XXX | |
| Contacting telephone No. | 000-000-0000 | |
| Contacting FAX No. | 000-000-0000 | |
| E-mail address |
Shipment judgment person of INFRAREDX
| Full name | Xxxxxx Xxxxxxxx | |
| Post and title | VP of Operations, Regulatory and Quality | |
| Address of contacting party | 00 0xx Xxxxxx Xxxxxxxxxx, XX 00000 XXX | |
| Contacting telephone No. | 000-000-0000 | |
| Contacting FAX No. | 000-000-0000 | |
| E-mail address | xxxxxxxxxx@xxxxxxxxx.xxx |
Person of INFRAREDX in charge of contacts
| Full name | Xxxxx Xxxxxxx | |
| Post and title | VP of Marketing | |
| Address of contacting party | 00 0xx Xxxxxx Xxxxxxxxxx, XX 00000 XXX | |
| Contacting telephone No. | 000-000-0000 | |
| Contacting FAX No. | 000-000-0000 | |
| E-mail address | xxxxxxxx@xxxxxxxxx.xxx |
33.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
[Annexed Document 10 Annexed Document, Form revision inquiry/Annexed Document, Form revision agreement]
Date
To:
Annexed Document, Form Revision Inquiry (No. )
A revision of the Annexed Document, Form is proposed as follows:
| Name of the proposing Company |
| |
| (signature) |
| Item to be revised | Annexed Document No.: | |
| Contents of revision | ||
| Reason for revision | ||
| Remarks | No attached paper, With attached paper (... sheets) | |
Annexed Document, Form Revision Agreement
Date
| ¨ | We agree to the above proposal |
| ¨ | We cannot agree to the above proposal |
Reason:
| Name of the proposing Company |
| |
| (signature) |
34.
[***] = Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Section 200.80(b)(4) and Rule 406 of the Securities Act of 1933, as amended.
Effective as of May 11, 2012
Revisions, none
Effective as of May , 2012 May 11, 2012
Revisions, none
[Annexed Document 11 List of revision history]
| Date |
Revision inquiry No. |
Revision Contents, etc. |
Remarks | |||
| Newly established | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
| Annexed Document, Form | ||||||
35.