CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***] HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED. PURCHASE AND SALE AGREEMENT by and among ALKERMES...
Exhibit 10.5
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
by and among
ALKERMES PHARMA IRELAND LIMITED,
DARAVITA LIMITED,
EAGLE HOLDINGS USA, INC.,
RECRO PHARMA, INC.
and
RECRO PHARMA LLC
Dated as of ▇▇▇▇▇ ▇, ▇▇▇▇
▇▇▇▇▇▇▇ CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
TABLE OF CONTENTS
| Page | ||||||||
| ARTICLE I DEFINITIONS |
2 | |||||||
| 1.1 |
Defined Terms |
2 | ||||||
| ARTICLE II THE SALE |
17 | |||||||
| 2.1 |
The Sale |
17 | ||||||
| 2.2 |
Purchase Price; Allocation |
17 | ||||||
|
|
2.3 |
Closing |
19 | |||||
| 2.4 |
Closing Adjustment |
20 | ||||||
| 2.5 |
Post-Closing Statement |
21 | ||||||
| 2.6 |
Post-Closing Adjustment |
23 | ||||||
| 2.7 |
Calculations |
23 | ||||||
| 2.8 |
Earn-Out Consideration |
23 | ||||||
| ARTICLE III REPRESENTATIONS AND WARRANTIES OF SELLERS |
23 | |||||||
| 3.1 |
Organization; Authorization |
24 | ||||||
| 3.2 |
Title to Shares; Capitalization; Structure |
25 | ||||||
| 3.3 |
No Consents |
26 | ||||||
| 3.4 |
Financial Statements |
26 | ||||||
| 3.5 |
No Undisclosed Liabilities |
26 | ||||||
| 3.6 |
Properties; Sufficiency |
26 | ||||||
| 3.7 |
Absence of Certain Changes |
27 | ||||||
| 3.8 |
Litigation; Orders |
28 | ||||||
| 3.9 |
Intellectual Property |
28 | ||||||
| 3.10 |
Licenses; Authorizations; Reports |
29 | ||||||
| 3.11 |
Labor Matters |
30 | ||||||
| 3.12 |
Taxes |
31 | ||||||
| 3.13 |
Compliance with Laws |
32 | ||||||
| 3.14 |
Insurance |
33 | ||||||
| 3.15 |
Material Contracts |
34 | ||||||
| 3.16 |
Brokers, Finders |
35 | ||||||
| 3.17 |
Board Approval |
35 | ||||||
| 3.18 |
Environmental Health and Safety Matters |
36 | ||||||
| 3.19 |
Employee Benefit Plans |
36 | ||||||
-i-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| 3.20 | Products; Recalls |
37 | ||||||
| 3.21 | Transactions with Affiliates |
38 | ||||||
| 3.22 | Customers and Suppliers |
38 | ||||||
| 3.23 | Accounts Receivable |
38 | ||||||
| 3.24 | Regulatory Matters |
39 | ||||||
| 3.25 | Investor Representations |
39 | ||||||
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF PURCHASERS |
40 | |||||||
| 4.1 | Organization; Authorization; Ownership |
40 | ||||||
| 4.2 | No Consents |
41 | ||||||
| 4.3 | Compliance with Laws |
41 | ||||||
| 4.4 | Brokers; Finders |
41 | ||||||
| 4.5 | Acquisition of Transferred Interests for Investment |
41 | ||||||
| 4.6 | Debt Financing |
42 | ||||||
| 4.7 | Regulatory Matters |
43 | ||||||
| 4.8 | No Other Representations or Warranties |
43 | ||||||
| ARTICLE V COVENANTS |
43 | |||||||
| 5.1 | Access to Books and Records |
43 | ||||||
| 5.2 | Efforts |
44 | ||||||
| 5.3 | Further Assurances |
46 | ||||||
| 5.4 | Conduct of Business |
47 | ||||||
| 5.5 | Consents |
49 | ||||||
| 5.6 | Public Announcements |
49 | ||||||
| 5.7 | Intercompany Accounts |
49 | ||||||
| 5.8 | Termination of Intercompany Agreements |
49 | ||||||
| 5.9 | Insurance |
49 | ||||||
| 5.10 | Litigation Support |
50 | ||||||
| 5.11 | Payments |
50 | ||||||
| 5.12 | Debt Financing |
50 | ||||||
| 5.13 | Directors and Officers Indemnification |
52 | ||||||
| 5.14 | Non-Competition; Non-Solicitation |
52 | ||||||
| 5.15 | Indebtedness/Lien Release |
53 | ||||||
| 5.16 | Additional Financial Statements |
53 | ||||||
| 5.17 | Change of Name of Transferred Entities |
53 | ||||||
| 5.18 | Transition Services Agreement and Supply Agreements |
54 | ||||||
-ii-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| 5.19 | Exclusivity |
54 | ||||||
| 5.20 | Solvency |
54 | ||||||
| 5.21 | Data Room |
54 | ||||||
| ARTICLE VI EMPLOYEE MATTERS COVENANTS |
54 | |||||||
| 6.1 | Benefit Continuation |
54 | ||||||
| 6.2 | Service Credit |
55 | ||||||
| 6.3 | Employment Continuation |
55 | ||||||
| 6.4 | Retention Bonuses |
55 | ||||||
| 6.5 | Accrued Vacation Payment |
56 | ||||||
| ARTICLE VII TAX MATTERS |
56 | |||||||
| 7.1 | Generally |
56 | ||||||
| 7.2 | Tax Indemnification |
56 | ||||||
| 7.3 | Straddle Period |
57 | ||||||
| 7.4 | Tax Proceedings |
57 | ||||||
| 7.5 | Cooperation on Tax Matters |
58 | ||||||
| 7.6 | Certain Taxes and Fees |
58 | ||||||
| 7.7 | Survival |
58 | ||||||
| ARTICLE VIII CONDITIONS TO OBLIGATIONS TO CLOSE |
59 | |||||||
| 8.1 | Conditions to Obligation of Each Party to Close |
59 | ||||||
| 8.2 | Conditions to Purchasers’ Obligation to Close |
59 | ||||||
| 8.3 | Conditions to Sellers’ Obligation to Close |
60 | ||||||
| ARTICLE IX TERMINATION |
60 | |||||||
| 9.1 | Termination |
60 | ||||||
| 9.2 | Notice of Termination |
61 | ||||||
| 9.3 | Effect of Termination |
61 | ||||||
| 9.4 | Reverse Termination Fee |
61 | ||||||
| ARTICLE X SURVIVAL; INDEMNIFICATION; LIQUIDATED DAMAGES |
62 | |||||||
| 10.1 | Survival Periods |
62 | ||||||
| 10.2 | Indemnification by Sellers |
63 | ||||||
| 10.3 | Indemnification by Purchasers |
63 | ||||||
| 10.4 | Indemnification Procedures |
63 | ||||||
| 10.5 | Limitations |
65 | ||||||
| 10.6 | Mitigation; Additional Indemnification Provisions |
66 | ||||||
| 10.7 | Liquidated Damages |
66 | ||||||
-iii-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| 10.8 | Exclusive Remedies |
67 | ||||||
| 10.9 | Subrogation |
67 | ||||||
| 10.10 | Tax Indemnification Matters |
67 | ||||||
| 10.11 | No Duplication |
67 | ||||||
| ARTICLE XI MISCELLANEOUS |
67 | |||||||
| 11.1 | Counterparts |
67 | ||||||
| 11.2 | Governing Law; Jurisdiction and Forum; Waiver of Jury Trial |
68 | ||||||
| 11.3 | Confidentiality |
68 | ||||||
| 11.4 | Entire Agreement |
70 | ||||||
| 11.5 | Expenses |
70 | ||||||
| 11.6 | Notices |
70 | ||||||
| 11.7 | Assignment |
71 | ||||||
| 11.8 | Third-Party Beneficiaries |
72 | ||||||
| 11.9 | Amendments and Waivers |
72 | ||||||
| 11.10 | Specific Performance |
72 | ||||||
| 11.11 | Interpretation; Absence of Presumption |
73 | ||||||
| 11.12 | Headings; Definitions |
74 | ||||||
| 11.13 | Severability |
74 | ||||||
| 11.14 | No Recourse to Debt Financing Sources |
74 | ||||||
Exhibits
| Exhibit A: | Transition Services Agreement | |
| Exhibit B: | Supply Agreements | |
| Exhibit C: | Sample Adjustment Amount Statement | |
| Exhibit D: | Excluded Assets | |
| Exhibit E: | Earn-Out Consideration | |
| Exhibit F: | Warrant | |
| Exhibit G: | Reorganization |
-iv-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
This PURCHASE AND SALE AGREEMENT (this “Agreement”), dated as of March 7, 2015, is by and among Alkermes Pharma Ireland Limited, a private limited company incorporated in Ireland (“APIL”), Daravita Limited, a private limited company incorporated in Ireland (“Daravita”), Eagle Holdings USA, Inc., a Delaware corporation (“Eagle Holdings”, and together with APIL, “Sellers”), Recro Pharma, Inc., a Pennsylvania corporation (“Recro”) and Recro Pharma LLC, a Delaware limited liability company and wholly-owned subsidiary of Recro (“Acquisition Sub,” and together with Recro, “Purchasers”).
RECITALS
WHEREAS, Alkermes Ireland Holdings Limited, a private limited company incorporated in Ireland (“Alkermes Ireland Holdings”), holds all of the issued and outstanding ordinary shares in Daravita, and Eagle Holdings holds all of the issued and outstanding membership units in Alkermes Gainesville LLC, a Massachusetts limited liability company (“Alkermes Gainesville”);
WHEREAS, APIL intends to newly form a Delaware limited liability company (“Newco”);
WHEREAS, prior to the Closing, Newco will, directly or indirectly, acquire substantially all of the assets and liabilities of Daravita from Daravita through effectuation of a reorganization described in Exhibit G (such steps, collectively, the “Reorganization”);
WHEREAS, Sellers desire to sell and transfer, and Purchasers desire to purchase, the Transferred Interests for the consideration set forth below, subject to the terms and conditions of this Agreement;
WHEREAS, Sellers, or their designated Affiliate, and Purchasers shall enter into (i) concurrently with the Closing, the Transition Services Agreement and, (ii) within sixty (60) days following Closing, the Supply Agreements (the agreements specified in clauses (i) and (ii), and the Warrant (as defined herein), collectively, the “Ancillary Agreements”);
WHEREAS, simultaneously with the execution of this Agreement, Purchasers shall enter into the Debt Financing Agreements; and
WHEREAS, the Parties desire to make certain representations, warranties, covenants and agreements as set forth in this Agreement.
NOW, THEREFORE, in consideration of the mutual promises hereinafter set forth and other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, and intending to be legally bound, the Parties hereby agree as follows:
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
ARTICLE I
DEFINITIONS
1.1 Defined Terms. For the purposes of this Agreement, the following terms shall have the following meanings:
“Accounting Methodology” shall have the meaning set forth in the definition of Working Capital.
“Action” shall mean any action, claim, suit, arbitration, litigation, proceeding, or governmental investigation.
“Acquisition Sub” shall have the meaning set forth in the preamble.
“Acquisition Proposal” with respect to the Transferred Entities, means any offer or proposal relating to any transaction or series of related transactions involving: (a) any purchase from such party or acquisition by any Person or “group” (as defined under Section 13(d) of the Exchange Act) of fifteen percent (15%) or more of the total outstanding voting securities of the Transferred Entities, (b) any merger, consolidation, business combination or similar transaction involving the Transferred Entities, (c) any joint venture, sale, lease (other than in the ordinary course of business), exchange, transfer, exclusive license (other than in the ordinary course of business), acquisition or disposition of fifteen percent (15%) or more of the assets of the Transferred Entities or (d) any liquidation or dissolution of the Transferred Entities (provided, however, that the transactions between Purchasers and Sellers contemplated by this Agreement shall not be deemed an Acquisition Proposal).
“Additional Retention Bonus” has the meaning set forth in Section 6.4(b).
“Additional Retention Bonus Date” has the meaning set forth in Section 6.4(b).
“Adjustment Amount” shall equal (i) the Closing Working Capital, plus (ii) the Closing Cash Amount, less (iii) the Target Working Capital, less (iv) the Closing Date Indebtedness, and (v) less the Closing Date Transaction Fees.
“Affiliate” shall mean, with respect to any Person, any other Person that directly, or through one or more intermediaries, controls or is controlled by or is under common control with such Person; provided that, after the Closing, (a) none of the Transferred Entities shall be considered an Affiliate of Parent, Sellers or their respective Affiliates and (b) none of Parent, Sellers or their respective Affiliates shall be considered an Affiliate of any Transferred Entity. For purposes of this Agreement, “control” shall mean, as to any Person, the power to direct or cause the direction of the management and policies of such Person, whether through the ownership of voting securities, by contract or otherwise (and the terms “controlled by” and “under common control with” shall have correlative meanings).
“Agreement” shall have the meaning set forth in the preamble.
“Alkermes Gainesville” shall have the meaning set forth in the recitals.
-2-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Alkermes Ireland Holdings” shall have the meaning set forth in the recitals.
“Allocation Schedule” shall have the meaning set forth in Section 2.2(d).
“Alternative Financing” shall have the meaning set forth in Section 5.12(a).
“Ancillary Agreements” shall have the meaning set forth in the recitals.
“Anti-Bribery Laws” shall have the meaning set forth in Section 3.13(d)(i).
“APIL” shall have the meaning set forth in the preamble.
“Appraised Value” shall have the meaning set forth in Section 2.2(d).
“Balance Sheet Date” shall have the meaning set forth in Section 3.4(a).
“Benefit Plan” shall mean any “employee benefit plan,” as defined in Section 3(3) of ERISA, and any other written or unwritten profit-sharing, bonus, stock option, stock purchase, stock ownership, pension, retirement, severance, deferred compensation, excess benefit, supplemental unemployment, post-retirement medical or life insurance, welfare, incentive, sick leave, long-term disability, medical, hospitalization, life insurance, other insurance or employee benefit plan, policy, agreement or arrangement maintained or contributed to by Parent or its Subsidiaries for the benefit of any Transferred Entity Employees with respect to service as an employee of any Transferred Entity or with respect to which Parent or its Subsidiaries have any current or contingent Liability pertaining to any Transferred Entity Employee for service as an employee of any Transferred Entity.
“BiDil Products” shall mean the Transferred Entities’ BiDil XR™, a fixed dose combination of hydralazine hydrochloride and isosorbide dinitrate, existing as of the date of this Agreement.
“Books and Records” shall mean all of the books, records (including Tax records), information and data of a Person, including (a) corporate minute books, (b) books and records relating to employees, research and development, manufacture and sale of products and services, advertising, packaging, promotional materials and dealings with customers, (c) books of account, ledgers, general, financial, accounting and personnel records, files, customer and counterparty lists, documents, agreements, sales data and information, billing records, manuals, material client, counterparty and supplier correspondence and (d) all other registers or books required to be maintained under applicable Law.
“Business” shall mean the (a) operations of Alkermes Gainesville related to the Products and (b) development, license, manufacture, testing, packaging, storage, sale and shipment of the Products, in each case of (a) and (b) as conducted by the Transferred Entities as of the date of this Agreement; provided, however, that “Business” shall not include any operations, business, assets, rights or obligations solely related to the Excluded Assets.
“Business Balance Sheet” shall have the meaning set forth in Section 3.4(a).
-3-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Business Confidential Information” shall have the meaning set forth in Section 11.3(b).
“Business Day” shall mean any day that is not a Saturday, a Sunday or other day on which commercial banks in the City of New York, New York or Dublin, Ireland are required or authorized by Law to be closed.
“Business Intellectual Property” shall mean all Intellectual Property Rights owned by the Transferred Entities and used or held for use in the Business.
“Business IP Agreements” means any (a) Contract under which any of the Transferred Entities grants a license under or other right to use any Intellectual Property Rights to another Person that are material to the Transferred Entities, (b) Contract under which any of the Transferred Entities is granted a license or other right to use any Intellectual Property Rights of another Person that are material to the Transferred Entities (other than commercially available “off the shelf” software licenses each with annual fees of less than Fifty Thousand Dollars ($50,000)), and (c) consent-to-use agreement, co-existence agreement, settlements agreement or other similar Contract limiting the use, validity or enforceability of the Business Intellectual Property.
“Business Material Contracts” shall have the meaning set forth in Section 3.15.
“Business Real Property” shall have the meaning set forth in Section 3.6(b).
“Business Registered Intellectual Property” shall have the meaning set forth in Section 3.9(a).
“Cap” shall mean an amount equal to (a) Five Million Dollars ($5,000,000) plus (b) ten percent (10.00%) of any Development Milestone Earn-Out Consideration and Commercial Milestone Earn-Out Consideration, if any, that is actually paid to APIL pursuant to Exhibit E.
“Cash” shall mean the amount of cash and cash equivalents (including marketable securities and marketable short term investments) that would be recorded on a consolidated balance sheet for the Transferred Entities which is prepared in accordance with the Accounting Methodology.
“CERCLA” shall mean the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended from time to time, and any rules or regulations promulgated thereunder.
“Certificate of Analysis and Conformity” shall mean the certificate for each batch of Product delivered with such Product listing the tests performed, the specifications for the manufacture of such Product, and the test results and certifying that such batch of Product was manufactured in accordance with applicable Law, including cGMPs, and production standard operating procedures.
“cGMPs” shall mean current good manufacturing practices as defined in the U.S. Code of Federal Regulations, 21 CFR Part 210 et seq., the European Union Guidelines to Good Manufacturing Practices for Medicinal Products for Human and Veterinary Use (Vol. IV Rules Governing Medicinal Products in the European Union 2004), and any successor regulatory schemes, as well as any corresponding requirements in other regulatory jurisdictions.
-4-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Closing” shall have the meaning set forth in Section 2.1.
“Closing Adjustment” shall have the meaning set forth in Section 2.4.
“Closing Cash Amount” means all Cash held in the accounts of the Transferred Entities as of 11:59 p.m. Eastern Time on the Closing Date. The Closing Cash Amount shall be (x) reduced by the amount of any checks issued by a Transferred Entity on or before the Closing whether or not any such checks remain in the possession of a Transferred Entity on or before the Closing (with a corresponding adjustment to current liabilities, if any); (y) increased by the amount of any checks or wire transfers received by a Transferred Entity on or before the Closing whether or not they have been deposited or have cleared any bank holding procedures (with a corresponding adjustment to current assets, if any), provided such amounts have not already been reflected in the accounts of the Transferred Entities; and (z) adjusted to reflect the settlement of all intercompany accounts pursuant to Section 5.7.
“Closing Date” shall have the meaning set forth in Section 2.3(a).
“Closing Date Indebtedness” means the amount of Indebtedness outstanding as of the Closing (without giving effect to the transactions contemplated herein), excluding (i) any Indebtedness that is included in the Working Capital calculations in accordance with Section 2.7 and (ii) any Indebtedness (including guarantees thereof) that will be released upon or immediately following the Closing.
“Closing Date Transaction Fees” means the amount of Transaction Fees on the Closing Date, excluding any Transaction Fees that are included in the Working Capital calculations in accordance with Section 2.7.
“Closing Estimates” shall have the meaning set forth in Section 2.4.
“Closing Working Capital” shall mean the Working Capital of the Transferred Entities as of the Closing.
“Code” shall mean the U.S. Internal Revenue Code of 1986, as amended.
“Commercial Milestone Earn-Out Consideration” shall have the meaning set forth in Exhibit E.
“Confidentiality Agreement” shall mean the confidentiality agreement, dated as of June 6, 2014, as amended as of February 3, 2015, by and between Daravita (f/k/a Alkermes Science One Limited) and Recro Pharma, Inc.
“Confidential Disclosure Agreement” shall mean the confidentiality agreement, dated as of December 19, 2014, by and among Daravita, Recro Pharma, Inc. and ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ LLP (the “Confidential Disclosure Agreement”).
-5-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Consents” shall have the meaning set forth in Section 3.3.
“Contract” shall mean, with respect to any Person, any agreement, contract, obligation or undertaking (whether written or oral and whether express or implied) to which such Person is bound.
“Credit Agreement” shall have the meaning set forth in Section 2.3(b)(i)(F).
“Daravita” shall have the meaning set forth in the preamble.
“Debt Financing” shall have the meaning set forth in Section 4.6(a).
“Debt Financing Agreements” shall have the meaning set forth in Section 4.6(a).
“Deductible” shall have the meaning set forth in Section 10.5(a).
“De Minimis Damages” shall mean any single claim (or series of claims arising from the same or similar facts, events, or circumstances) for Losses in an amount that is less than Twenty-Five Thousand dollars ($25,000).
“Development Milestone Earn-Out Consideration” shall have the meaning set forth in Exhibit E.
“Disputed Items” shall have the meaning set forth in Section 2.5(b).
“Divestiture” shall have the meaning set forth in Exhibit E.
“DOJ” shall have the meaning set forth in Section 5.2(a).
“Eagle Holdings” shall have the meaning set forth in the preamble.
“Earn-Out Consideration” shall have the meaning set forth in Exhibit E.
“Enforceability Exceptions” shall have the meaning set forth in Section 3.1(d).
“Environmental Laws” shall mean any Law relating to pollution of the environment, including those relating to the use, production, generation, handling, transport, treatment, storage, disposal, distribution, labeling, testing, processing, discharge, Release or threatened Release of any Hazardous Material.
“ERISA” shall mean the Employee Retirement Income Security Act of 1974, as amended.
“ERISA Affiliate” shall mean, with respect to any entity, trade or business, any other entity, trade or business that is, or was at the relevant time, a member of a group described in Section 414(b), (c), (m) or (o) of the Code or Section 4001(b)(1) of ERISA that includes or included the first entity, trade or business, or that is, or was at the relevant time, a member of the same “controlled group” as the first entity, trade or business pursuant to Section 4001(a)(14) of ERISA.
-6-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Estimated Closing Cash Amount” shall have the meaning set forth in Section 2.4.
“Estimated Closing Working Capital” shall have the meaning set forth in Section 2.4.
“Estimated Closing Date Indebtedness” shall have the meaning set forth in Section 2.4.
“Estimated Closing Date Transaction Fees” shall have the meaning set forth in Section 2.4.
“Exchange Act” shall mean shall mean the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Excluded Assets” shall mean (a) the assets disclosed on Exhibit D and (b) Books and Records and regulatory filings located at the Alkermes Gainesville facility only to the extent related to any pharmaceutical products, including pharmaceutical product candidates, other than the Products.
“FATCA” means Sections 1471 through 1474 of the Code, any current or future regulations or official interpretations thereof, and any intergovernmental agreements entered into pursuant thereto.
“External Demand” shall have the meaning set forth in Section 11.3(d).
“FCPA” shall mean the Foreign Corrupt Practices Act of 1977.
“FDA” shall have the meaning set forth in Section 3.10.
“FDCA” shall have the meaning set forth in Section 3.10.
“Final Adjustment Amount” shall have the meaning set forth in Section 2.5(e).
“Final Post-Closing Adjustment Statement” shall have the meaning set forth in Section 2.5(e).
“FTC” shall have the meaning set forth in Section 5.2(a).
“Focalin Products” shall mean the Transferred Entities’ Focalin XR®, an extended-release oral formulation of dexmethylphenidate, existing as of the date of this Agreement.
“Fundamental Representations” means the representations and warranties contained in Section 3.1 (Organization; Authorization), Section 3.2 (Title to Shares; Capitalization; Structure), Section 3.3 (No Consents), and Section 3.16 (Brokers, Finders).
“GAAP” shall mean generally accepted accounting principles in the United States.
“Governing Documents” shall mean with respect to any Person, (a) if a corporation, the memorandum and articles of association, articles or certificate of incorporation, the bylaws or similar documents (as applicable); (b) if a general partnership or limited liability partnership, the partnership agreement and any statement of partnership; (c) if a limited partnership, the limited
-7-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
partnership agreement and the certificate of limited partnership; (d) if a limited liability company, the certificate of formation and limited liability company agreement; (c) if another type of Person, any charter or similar document adopted or filed in connection with the creation, formation or organization of the Person; (f) all equity holders’ agreements, voting agreements, voting trust agreements or other similar agreements or documents relating to the organization, management or operation of such entity; and (g) any amendment or supplement to any of the foregoing.
“Governmental Approvals” shall have the meaning set forth in Section 5.2(a).
“Government Official” shall mean any officer, employee, official advisor or agent of a (a) Governmental Entity; (b) public international organization (e.g., the World Bank); (c) political party or official thereof; or (d) candidate for any political office.
“Governmental Entity” shall mean any court, administrative agency, commission or other governmental authority, body or instrumentality, federal, state, local, domestic or foreign governmental or regulatory authority.
“Hazardous Materials” shall mean any pollutant, contaminant, hazardous waste, hazardous substance or hazardous material regulated under any Environmental Law, and includes asbestos-containing materials, polychlorinated biphenyls and petroleum and its derivatives.
“Highly Paid Employees” shall have the meaning set forth in Section 3.11(b).
“Historical Financial Statements” shall have the meaning set forth in Section 3.4(a).
“HSR Act” shall mean the ▇▇▇▇-▇▇▇▇▇-▇▇▇▇▇▇ Antitrust Improvements Act of 1976, as amended.
“Income Tax” shall mean any federal, state, local, or foreign Tax based upon or measured by net income of the relevant Transferred Entity.
“Indebtedness” shall mean with respect to the Transferred Entities, at the time of any determination, without duplication: all obligations, contingent or otherwise that, in accordance with GAAP, would be included on the balance sheet of the Transferred Entities as indebtedness, but in any event includes the outstanding principal amount of, all accrued and unpaid interest on and other payment obligations (including any premiums, termination fees, expenses, breakage costs or penalties due upon prepayment of or payable in connection with this Agreement or the consummation of the transactions contemplated by this Agreement) in respect of, (A) all indebtedness of the Transferred Entities for borrowed money, which shall include borrowing agreements such as notes, bonds, indentures, mortgages, loans and lines of credit or similar instruments, (B) all obligations (including breakage costs) payable by the Transferred Entities under interest rate or currency protection agreements, (C) any reimbursement obligation with respect to letters of credit (including standby letters of credit to the extent drawn upon), bankers’ acceptances or similar facilities issued for the account of the Transferred Entities and for which the Transferred Entities shall be liable, (D) all obligations under capital leases (as determined in accordance with GAAP), and (E) any obligation of the type referred to in clauses (A) through
-8-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(D) of this definition of another Person, the payment of which either of the Transferred Entities has guaranteed, or which is secured by any property or assets of either of the Transferred Entities; with respect to clauses (A) through (E) above, for which either of the Transferred Entities is responsible or liable from and after the Closing.
“Indemnified Party” shall have the meaning set forth in Section 10.4(a).
“Indemnifying Party” shall have the meaning set forth in Section 10.4(a).
“Independent Accounting Firm” shall have the meaning set forth in Section 2.5(d)(i).
“Initial Post-Closing Adjustment Statement” shall have the meaning set forth in Section 2.5(a).
“Initial Purchase Price” shall have the meaning set forth in Section 2.2(a).
“Initial Retention Bonuses” shall have the meaning set forth in Section 6.4(b).
“Intellectual Property Right” shall mean any of the following intellectual property rights arising anywhere in the world: (i) all Registered Intellectual Property; (ii) all inventions (whether or not patentable), invention disclosures, improvements, trade secrets, proprietary or confidential information, know how, technology, business methods, technical data and customer lists, tangible or intangible proprietary information, and all documentation relating to any of the foregoing; (iii) computer software programs, including source code, object code, systems, tools, data, databases, firmware, APIs, interfaces, menus, libraries and related documentation; (iv) original works of authorship and copyrights; (v) all industrial designs; (vi) trademarks, service marks, trade dress, trade names, logos, or other source identifiers, including common law trademarks and service marks, and any goodwill associated with any of the foregoing; (vii) all databases and data collections and all rights therein throughout the world; (viii) all Web addresses, sites and domain names and numbers; and (ix) any similar or equivalent rights to any of the foregoing anywhere in the world.
“IP License Agreement” shall have the meaning set forth in Section 5.3(b).
“Intellectual Property Transfer and License Agreement” shall mean that certain Intellectual Property Transfer and License Agreement by and between APIL and Daravita, dated May 8, 2014, as amended.
“IRS” shall mean the Internal Revenue Service.
“Knowledge of Sellers” shall mean the actual knowledge of ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇ and ▇▇▇▇▇▇ ▇▇▇▇.
“Law” shall mean any United States federal, state or local, or any non-United States law, statute, ordinance, rule, regulation, judgment, order, injunction, decree, arbitration award, agency requirement, license or permit of any Governmental Entity.
“Lender” shall have the meaning set forth in Section 4.6(a).
-9-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Licenses” shall have the meaning set forth in Section 3.10.
“Liability” shall mean with respect to any Person, any indebtedness, liability or obligation of such Person of any kind, character or description, whether known or unknown, absolute or contingent, accrued or unaccrued, disputed or undisputed, liquidated or unliquidated, secured or unsecured, joint or several, due or to become due, vested or unvested, executory, determined, determinable or otherwise, and whether or not the same is required by GAAP to be accrued on the financial statements of such Person.
“Liens” shall mean all liens, pledges, mortgages, charges, claims, security interests, restrictions on transfer, encroachments or encumbrance, but not including any license(s) of Business Intellectual Property.
“Liquidated Damages Event” shall have the meaning set forth in Section 10.7.
“Losses” shall mean all losses, costs, charges, expenses (including reasonable attorneys’ fees and professional fees), obligations, liabilities, settlement payments, awards, judgments, fines, penalties, damages, demands, claims, assessments or deficiencies.
“Material Adverse Effect” shall mean any circumstance, condition, effect, event, change or occurrence that, individually or in the aggregate, has or would reasonably be expected to have a material adverse impact or effect on: (a) the ability of Sellers to perform their obligations hereunder or to consummate the transactions contemplated by this Agreement or (b) the business, financial condition, properties, assets or results of operations of the Transferred Entities taken as a whole; provided, however, that no change or effect shall constitute a Material Adverse Effect to the extent (and only to the extent) it results from:
(i) changes in conditions generally affecting any or all of the industries in which the Transferred Entities operate;
(ii) general political, economic or business conditions or changes therein (including the commencement, continuation or escalation of a war, armed hostilities or other international or national calamity or acts of terrorism);
(iii) general financial or capital market conditions, including interest rates, the availability of debt financing or currency exchange rates; or changes to any of the foregoing;
(iv) any earthquake, hurricane or other natural disaster, weather-related event or act of god;
(v) any changes in applicable Law, rules, regulations, or GAAP, or other accounting standards applicable to the Business, or authoritative interpretations thereof, from and after the date of this Agreement;
(vi) the announcement of the potential sale of the Business or any portion thereof; the negotiation, execution, announcement, existence or performance of this Agreement or the transactions contemplated by this Agreement; the consummation of the
-10-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
transactions contemplated by this Agreement; or changes or actions resulting from any of the foregoing, including impacts on relationships with customers, suppliers, employees, labor organizations, or governmental entities;
(vii) any action or omission required pursuant to the terms of this Agreement, or pursuant to the written request of Purchasers, or any action otherwise taken by Purchasers or any of their Affiliates; and
(viii) any failure of Sellers, the Transferred Entities or the Business to meet financial projections or any estimates of revenues or earnings; provided, that the underlying causes of such failures shall not be excluded under this clause (viii);
provided, further, that, in the cases of clauses (i)-(iv), in each case to the extent that such change or effect does not disproportionately affect the Transferred Entities, taken as a whole, in relation to others in the same business as the Transferred Entities.
“Meloxicam” shall mean the Transferred Entities’ Meloxicam IV/IM, an aqueous extended-release formulation of the selective ▇▇▇-2 inhibitor non-steroidal anti-inflammatory drug meloxicam developed by APIL using nanocrystal technology, in intravenous or intramuscular form existing as of the date of this Agreement.
“Net Sales Earn-Out Consideration” shall have the meaning set forth in Exhibit E.
“Net Sales Report” shall have the meaning set forth in Exhibit E.
“Newco” shall have the meaning set forth in the recitals.
“Non-Competition Period” shall have the meaning set forth in Section 5.14.
“Notice of Disagreement” shall have the meaning set forth in Section 2.5(b).
“OCR IP” shall have the meaning ascribed to such term in the Intellectual Property Transfer and License Agreement.
“Outside Date” shall have the meaning set forth in Section 9.1(b)(i).
“Owned Real Property” shall have the meaning set forth in Section 3.6(b).
“Paladin Products” shall mean the Transferred Entities’ pharmaceutical products licensed to Paladin Labs, Inc. by Daravita as of the date of this Agreement.
“Parent” shall mean Alkermes plc, a public limited company incorporated in Ireland (registered number 498284) whose registered address is ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇.
“Parties” shall mean Purchasers, Daravita and Sellers.
“PBGC” shall mean the Pension Benefit Guaranty Corporation.
-11-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Permitted Liens” shall mean the following Liens: (a) Liens for Taxes, assessments or other governmental charges or levies that are not yet due or payable or that are being contested in good faith by appropriate proceedings or that may thereafter be paid without penalty; (b) statutory Liens of landlords and Liens of carriers, warehousemen, mechanics, materialmen, workmen, repairmen and other Liens imposed by Law and on a basis consistent with past practice; (c) Liens incurred or deposits made in the ordinary course of business and on a basis consistent with past practice in connection with workers’ compensation, unemployment insurance or other types of social security; (d) defects or imperfections of title, easements, covenants, rights-of-way, restrictions and other similar charges or encumbrances not materially interfering with the ordinary conduct of the Business; (e) in the case of the Business Real Property, zoning, building, subdivision, or other similar requirements or restrictions; (f) Liens incurred in the ordinary course of business and on a basis consistent with past practice securing obligations or liabilities that are not material to the Transferred Entities or the Transferred Interests; (g) such other imperfections of title as do not materially detract from the value or otherwise interfere with the present use of the Business Real Property or otherwise impair the operation of the Business as presently conducted; and (h) Liens set forth on Section 1.1 of the Sellers Disclosure Letter.
“Person” shall mean a person, corporation, partnership, limited liability company, joint venture, trust or other entity or organization.
“Post-Closing Adjustment” shall have the meaning set forth in Section 2.6.
“Pre-Closing Tax Period” shall mean all taxable periods ending on or before the Closing Date and the portion through the end of the Closing Date for any Straddle Period.
“Products” shall mean the BiDil Products, the Focalin Products, the Paladin Products, the Ritalin Products, the Verapamil Products, the Zogenix Products and Meloxicam, each of these Products individually, a “Product.”
“Prohibitive Order” shall have the meaning set forth in Section 8.1(b).
“Purchase Price” shall have the meaning set forth in Section 2.2(a).
“Purchasers” shall have the meaning set forth in the preamble.
“Purchasers Disclosure Letter” shall have the meaning set forth in Article IV.
“Purchasers Fundamental Representations” means the representations and warranties contained in Section 4.1 (Organization; Authorization), Section 4.2 (No Consents), Section 4.4 (Brokers; Finders) and Section 4.6(e) (Solvency).
“Purchasers Indemnified Parties” shall have the meaning set forth in Section 10.2.
“Purchaser Plan” shall have the meaning set forth in Section 6.2(a).
“Purchaser Related Parties” shall have the meaning set forth in Section 9.4(c)
-12-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Recro” shall have the meaning set forth in the preamble.
“Registered Intellectual Property” means all United States, international and foreign: (i) patents and patent applications (including, but not limited to, reissues, continuations, divisionals, renewals, extensions, continuations-in-part, and all patents and applications claiming priority thereto or serving as a basis for priority thereof, provisional applications and design patents and applications); (ii) registered trademarks and service marks, applications to register trademarks, applications to register service marks, including in either case intent-to-use applications, or other registrations or applications related to trademarks, service marks or other source identifiers; (iii) registered copyrights and applications for copyright registration; (iv) domain name registrations and Internet number assignments; and (v) any other Intellectual Property Right that is the subject of an application, certificate, filing, registration or other document issued, filed with, or recorded by any Governmental Entity.
“Related Persons” shall have the meaning set forth in Section 11.14.
“Release” shall have the meaning set forth in CERCLA.
“Reorganization” shall have the meaning set forth the recitals.
“Reorganization Transfer Agreements” shall have the meaning set forth in Section 5.3(a).
“Representatives” shall mean a Person’s officers, directors, consultants, advisors, employees, stockholders, agents or other advisors or representatives.
“Resolution Period” shall have the meaning set forth in Section 2.5(c).
“Retention Bonuses” shall have the meaning set forth in Section 6.4(b).
“Review Period” shall have the meaning set forth in Section 2.5(b).
“Reverse Termination Fee” shall have the meaning set forth in Section 9.4(a).
“Ritalin Products” shall mean the Transferred Entities’ Ritalin SR®, a sustained-release oral formulation of methylphenidate, existing as of the date of this Agreement.
“Sale” shall have the meaning set forth in Section 2.1.
“Sample Adjustment Amount Statement” shall have the meaning set forth in the definition of Working Capital.
“SEC” shall mean the United States Securities and Exchange Commission.
“Securities Act” shall mean the U.S. Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Securitization” shall have the meaning set forth in Section 11.7(b).
“Seller Financial Advisor” shall mean Lazard Frères & Co. LLC.
-13-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Sellers” shall have the meaning set forth in the preamble.
“Seller Confidential Information” shall have the meaning set forth in Section 11.3(c).
“Sellers Disclosure Letter” shall have the meaning set forth in Article III.
“Significant Customers” shall have the meaning set forth in Section 3.15(n).
“Significant Suppliers” shall have the meaning set forth in Section 3.15(n).
“Similar Law” shall mean any law of a jurisdiction outside the United States that is similar to the applicable U.S. federal, state or local Law.
“Solvent” means, with respect to any Person on a particular date, that on such date (i) the fair value of the property of such Person is greater than the total amount of liabilities, including contingent liabilities, of such Person, (ii) the present fair saleable value of the assets of such Person is not less than the amount that will be required to pay the probable liability of such Person on its debts as they become absolute and matured, (iii) such Person does not intend to, and does not believe that it will, incur debts or liabilities beyond its ability to pay as such debts and liabilities as they mature, (iv) such Person is not engaged in a business or a transaction, and is not about to engage in a business or a transaction, for which the assets of such Person would constitute an unreasonably small capital; (v) such Person has the ability to pay their debts and obligations as they come due in the ordinary course of business; (vi) such Person has sufficient capital to operate the Business in the ordinary course of business (including, without limitation, manufacturing and developing the Products and performing its obligations under the Supply Agreements); and (vii) such Person has not made any transfer or incurred any obligations, with actual intent to hinder, delay or defraud either present or future creditors. The amount of contingent liabilities at any time shall be computed as the amount that, in light of all the facts and circumstances existing at such time, can reasonably be expected to become an actual or matured liability.
“Straddle Period” any taxable period that includes (but does not end on) the Closing Date.
“Subject Assets” shall have the meaning set forth in Section 5.2(c).
“Subsidiary” shall mean, with respect to any Person, any other entity (a) whose securities or other ownership interests, having by their terms the power to elect a majority of the board of directors or other Persons performing similar functions, are beneficially owned or controlled, directly or indirectly, by such Person, (b) whose business and policies such Person has the power, directly or indirectly, to direct, or (c) of which 50% or more of the securities, partnership or other ownership interests are owned, directly or indirectly, by such Person.
“Supply Agreements” shall mean the agreements between Sellers, or their designated Affiliate, and Acquisition Sub to be executed within sixty (60) days following the Closing in forms mutually acceptable to Sellers and Purchasers and to include the terms and conditions specified in Exhibit B.
-14-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Target Working Capital Amount” shall mean Nineteen Million Dollars ($19,000,000).
“Tax” shall mean any tax of any kind, including any federal, state, local and foreign income, profits, license, severance, occupation, windfall profits, capital gains, capital stock, transfer, registration, social security (or similar), production, franchise, gross receipts, payroll, sales, employment, use, property, excise, value added, estimated, stamp, alternative or add-on minimum, environmental, withholding or any other tax, governmental duty or assessment, together with all interest, penalties and additions imposed with respect to such amounts.
“Tax Proceeding” means any audit, examination, investigation, assessment, claim or litigation by a Governmental Entity relating to Taxes of the Transferred Entities for Pre-Closing Tax Periods or Straddle Periods or for which Sellers may have Liability for Taxes under this Agreement or otherwise.
“Tax Returns” means any return, report, information return or other statement (including schedules or any related or supporting information) required to be filed or prepared with respect to any Tax.
“Third Party Claim” shall have the meaning set forth in Section 10.4(a).
“Transaction Fees” means all unpaid fees, expenses and other similar amounts that have been or are expected to be incurred on or prior to the Closing Date on behalf of either of the Transferred Entities in connection with the preparation, negotiation and execution of this Agreement and the consummation of the transactions contemplated hereby, including: (i) the fees and expenses of, or other similar amounts charged by, any external counsel, accountants, financial advisors, consultants and experts engaged by either of the Transferred Entities; and (ii) the amount of the Retention Bonuses and any other sale bonuses, change in control bonuses, retention bonuses or similar bonuses that become payable in connection with the consummation of the transactions contemplated by this Agreement (plus the employer portion of any payroll and employment taxes relating thereto) except, in the case of (i) and (ii) above, (a) to the extent otherwise included in the determination of Working Capital or (b) to the extent such fees have not been paid as of the Closing and will become the liability of the Transferred Entities from and after the Closing.
“Transferred Entities” shall mean (a) prior to consummation and completion of the Reorganization, Daravita and Alkermes Gainesville, or (b) from and after consummation and completion of the Reorganization, Newco and Alkermes Gainesville.
“Transferred Entity Benefit Plan” shall mean any Benefit Plan solely sponsored or maintained by any Transferred Entity.
“Transferred Entity Employee” shall mean any employee employed by any Transferred Entity.
“Transferred Interests” shall mean (a) all of the issued and outstanding membership units of Newco and (b) all of the issued and outstanding membership units of Alkermes Gainesville.
-15-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
“Transition Services Agreement” shall mean the transition services agreement to be executed and delivered on or prior to the Closing Date in a form mutually acceptable to Sellers and Purchasers, which services and other terms and conditions are specified in Exhibit A.
“U.S.-Ireland Treaty” means the Convention Between the Government of the United States of America and the Government of Ireland for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and Capital Gains signed on July 28, 1997, and any existing protocols with respect thereto.
“VAT” means value added tax.
“Verapamil Products” shall mean the Transferred Entities’ sustained release oral formulations of verapamil hydrochloride existing as of the date of this Agreement.
“WARN Act” shall have the meaning set forth in Section 3.11(d).
“Warrant” shall mean the warrant to purchase stock to be executed and delivered at the Closing in the form of Exhibit F. The warrant to purchase stock grants to APIL the right to purchase 350,000 shares (subject to adjustment as provided therein) of common stock of Recro at a price equal to two times the closing price of the common stock of Recro on the day prior to the Closing Date for a period of seven (7) years from the Closing Date.
“Working Capital” shall mean current assets of the Transferred Entities minus current liabilities of the Transferred Entities and excluding (a) Cash, (b) any intercompany accounts and other intercompany obligations required to be settled or eliminated at or prior to the Closing pursuant to Section 5.7, or (c) any assets or liabilities in respect of Income Taxes. For purposes of this Agreement, Working Capital shall be calculated in accordance with this Agreement (including the sample calculation of the Adjustment Amount as of December 31, 2014 set forth on Exhibit C (the “Sample Adjustment Amount Statement”)) and with GAAP applied using the same data sources, policies, procedures and method of calculation, with consistent classifications, judgments and estimation methodology, as were used in preparation of the Business Balance Sheet; provided, however, Working Capital shall not be calculated to include any changes in assets or liabilities as a result of purchase accounting adjustments or other changes arising from or resulting as a consequence of this Agreement or the transactions contemplated hereby other than as expressly set forth in the Sample Adjustment Amount Statement; provided, further, however, that if there is any conflict between the accounting methods, practices, principles, policies and procedures used in preparing the Business Balance Sheet and GAAP, GAAP shall control and no reversal of any reserves reflected in the balance sheet of the Transferred Entities shall be taken into account except that reserved for a litigation matter may be reversed only in connection with the final resolution of such litigation and reserves against an account receivable may be reversed only upon actual collection of such reserved receivable (the methods set forth in this definition of Working Capital, the “Accounting Methodology”).
“Zogenix Products” shall mean the Transferred Entities’ Zohydro™ ER, an extended-release oral formulation of hydrocodone bitartrate, including the modified Zohydro™ ER product containing an abuse deterrent component comprising polyethylene oxide and povidone (and further including the FDA-approved form thereof) existing as of the date of this Agreement.
-16-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
ARTICLE II
THE SALE
2.1 The Sale. Upon the terms and subject to the conditions set forth in this Agreement, at the closing of the transactions contemplated by this Agreement (the “Closing”), Sellers shall transfer, convey, assign and deliver to Acquisition Sub, and Acquisition Sub shall purchase and acquire from Sellers, all of Sellers’ right, title and interest in and to the Transferred Interests (the “Sale”).
2.2 Purchase Price; Allocation.
(a) In consideration for the Transferred Interests, Purchasers shall pay to Sellers an aggregate of (a) Fifty Million Dollars ($50,000,000) in cash (the “Initial Purchase Price”) plus (b) the Warrant (collectively, the “Purchase Price”) at the Closing. The Initial Purchase Price shall be subject to adjustment as provided in Section 2.4 through Section 2.7.
(b) The Parties agree that:
(i) For U.S. federal Income Tax purposes, the sale of (A) the Transferred Interests in Alkermes Gainesville (which is a disregarded entity with respect to Eagle Holdings) shall be treated as a sale of the assets of Alkermes Gainesville and (B) the Transferred Interests in Newco (which is a disregarded entity with respect to APIL) shall be treated as a sale of the assets of Newco;
(ii) An amount of the Initial Purchase Price equal to the lesser of (A) the Appraised Value of Alkermes Gainesville (as determined pursuant to Section 2.2(d)) less any liabilities of Alkermes Gainesville that are required to be treated as part of the purchase price of the assets of Alkermes Gainesville for U.S. federal Income Tax purposes and (B) the Initial Purchase Price shall be allocated to, and paid to Eagle Holdings in full payment for the Transferred Interests in Alkermes Gainesville; and
(iii) The balance of the Initial Purchase Price plus the Warrant shall be allocated to and paid to APIL in full payment for the Transferred Interests in Newco and the Earn-Out Consideration shall be allocated to and paid to APIL in full payment of the amounts due under the terms of the IP License Agreement.
(c) The right of APIL to receive the Earn-Out Consideration: (i) is solely a contractual right and is not a security for purposes of any federal or state securities Laws; (ii) will not be represented by any form of certificate or instrument; and (iii) does not give APIL any dividend rights, voting rights, liquidation rights, preemptive rights or other rights common to holders of the equity securities of Acquisition Sub or any of its Affiliates. The transactions contemplated by this Agreement are intended to be, and shall be treated solely as, a sale of the Transferred Interests by Sellers to Acquisition Sub, and nothing hereunder shall be deemed to create a joint venture or partnership between or among any of the Parties, the Transferred Entities and/or any of their Affiliates.
-17-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(d) Eagle Holdings shall retain Duff & ▇▇▇▇▇▇ Corporation which shall conduct an appraisal and determine the gross fair market value of the assets of Alkermes Gainesville (the “Appraised Value”). Within sixty (60) days after the Closing Date, Eagle Holdings shall deliver to Purchasers a schedule setting forth the Appraised Value and the allocation of the Initial Purchase Price allocable to the Transferred Interests in Alkermes Gainesville (as determined pursuant to Section 2.2(b)(ii)) (plus any liabilities of Alkermes Gainesville that are required to be treated as part of the purchase price of the assets of Alkermes Gainesville for U.S. federal Income Tax purposes) among the asset classes of Alkermes Gainesville (the “Allocation Schedule”), with the asset classes being those set forth in Treas. Reg. Sec. 1.338-6. The Allocation Schedule will not allocate to various assets within the asset class. The Appraised Value and Allocation Schedule shall be subject to such appropriate adjustments, if any, by the appraisers and Eagle Holdings upon the determination of the Post-Closing Adjustment. The Allocation Schedule shall be prepared in accordance with Section 1060 of the Code. The Appraised Value and Allocation Schedule shall be deemed final unless Purchasers notify Eagle Holdings in writing that Purchasers object to the Appraised Value and/or one or more items reflected in the Allocation Schedule within thirty (30) days after delivery of the Allocation Schedule to Purchasers. In the event of any such objection, Sellers and Purchasers shall negotiate in good faith to resolve such dispute; provided, however, that if Sellers and Purchasers are unable to resolve any such dispute within thirty (30) days after the delivery of the Allocation Schedule to Sellers, such dispute shall be resolved by an impartial nationally recognized firm of independent certified public accountants mutually appointed by Sellers and Purchasers whose determination shall be final and binding upon the Parties. The fees and expenses of such accounting firm shall be borne equally by Sellers, on the one hand, and Purchasers, on the other hand; provided, however, that if one such side substantially prevails in such dispute, then the non-prevailing Party(ies) shall bear all such fees and expenses. For the avoidance of doubt a Party shall be deemed to have “substantially prevailed” if the final determination by the accounting firm, in the case of Purchasers, is at least twenty percent (20%) greater than the Appraised Value, and, in the case of Sellers, is not more than twenty percent (20%) greater than the Appraised Value. Sellers and Purchasers agree to file their respective IRS Forms 8594 and all Tax Returns in accordance with the Allocation Schedule. Neither Purchasers nor Sellers shall take any position in a filed Income Tax Return or statement that is inconsistent with such allocations and Purchasers and Sellers will use reasonable efforts to sustain such position in any Tax Proceeding.
(e) Purchasers shall have the right to withhold all Taxes it is required by Law to withhold from all payments made hereunder, and will provide Sellers with proof of deposit or payment of any such Taxes withheld. For the avoidance of doubt, however, in connection with the sale of the Transferred Interests in Newco, APIL shall provide to Purchasers a valid and properly completed W-8BEN-E establishing its status as the beneficial owner for purposes of the U.S.-Ireland Treaty of those payments to APIL of the Purchase Price (including, for the avoidance of doubt, portions of the Initial Purchase Price, the Warrant and the Earn-Out Consideration) made under Section 2.2(b)(ii) and so long as APIL has provided Purchasers with such a W-8BEN-E that has not expired, Purchasers shall treat all such payments to APIL as exempt from U.S. federal Income Tax pursuant to the Code and/or Article 12 or Article 13 of
-18-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
the U.S.-Ireland Income Tax Treaty. In addition, provided that APIL provides a form W-8BEN-E upon which Purchasers may rely to show that the payments made to APIL are not subject to FATCA withholding, Purchasers shall not withhold any amounts under FATCA from payments to be made to APIL
2.3 Closing.
(a) The Closing shall take place at the offices of ▇▇▇▇▇▇▇ Procter LLP, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇, at 10:00 a.m., prevailing Eastern time, on the third (3rd) Business Day following the satisfaction or waiver of the conditions set forth in Article VIII (other than those conditions that by their nature are to be satisfied or waived at the Closing, but subject to the satisfaction or waiver of those conditions) or at such other place, time or date as may be mutually agreed upon in writing by Sellers and Purchasers (the “Closing Date”).
(b) At the Closing:
(i) Sellers shall:
(A) deliver to Purchasers certificates evidencing the Transferred Interests to the extent that such Transferred Interests are in certificate form, duly endorsed in blank or with stock powers duly executed in proper form for transfer, and with any required stock transfer stamps affixed thereto;
(B) deliver to Purchasers the Transition Services Agreement, duly executed by Sellers;
(C) deliver to Purchasers the certificate required to be delivered pursuant to Section 8.2(c);
(D) deliver to Purchasers the resignations, effective as of the Closing Date, of those directors or officers of the Transferred Entities as Purchasers may reasonably request in writing no less than ten (10) days prior to the Closing Date;
(E) deliver to Purchasers the common seal, if applicable, and all registers, minute books, and other statutory books, required to be kept by Law, and, to the extent applicable, all certificates of incorporation and certificates of incorporation on change of name for each Transferred Entity;
(F) deliver to Purchasers (1) copies of all UCC-3 discharge statements to be filed with respect to Alkermes Gainesville and copies of releases or other relevant filings, in each case to be filed after the Closing, and any other security release documentation reasonably requested by Purchasers, including releases under Irish law, for any Lien, including the Liens granted to ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Senior Funding, Inc., as collateral agent, under the Credit Agreement, dated as of September 25, 2012, as amended on February 14, 2013 and May 22, 2013 (as amended, restated, amended and restated, supplemented, replaced or otherwise modified from time to time, the “Credit Agreement”), among Alkermes plc, Alkermes Pharma Ireland Limited, Alkermes, Inc., Alkermes US Holdings, Inc., the several banks and other financial institutions or entities
-19-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
from time to time parties to the Credit Agreement as lenders, ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Senior Funding, Inc., as administrative agent, ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Senior Funding, Inc., Citigroup Global Markets, Inc. and JPMorgan Chase Bank, N.A. as co-syndication agents, and ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Senior Funding, Inc., as collateral agent in favor of the lenders thereunder, on (i) any assets owned by the Transferred Entities, other than the Excluded Assets or (ii) the Transferred Interests; and (2) a release of Alkermes Gainesville from its obligations as a guarantor under the Credit Agreement;
(G) deliver to Purchasers a copy of the resolutions or written consent of the boards of directors of the Transferred Entities evidencing that the boards of directors of the Transferred Entities have, prior to Closing, (i) voted in favor of the transfer of the Transferred Interests to Acquisition Sub (or its nominee(s)) and voted in favor of the registration of the Acquisition Sub (or its nominee(s)) as stockholder(s) or member(s), as applicable, of the Transferred Entities in respect of the Transferred Interests (subject to the production of duly stamped transfers) and (ii) appointed such persons as the Purchasers have nominated as directors and secretary of the Transferred Entities, effective at the Closing; and
(H) deliver to Purchasers certificates dated as of the Closing Date in form and substance reasonably satisfactory to Purchasers, sworn under penalty of perjury and in form and substance required under the Treasury Regulations issued pursuant to Section 1445 of the Code stating, as applicable, that Eagle Holdings is not a “foreign person” as defined in Section 1445 of the Code and the interests in Newco do not constitute U.S. real property interests as defined in Section 897(c) of the Code; and
(I) the Forms W-8BEN-E required to be furnished pursuant to Section 2.2(e).
(ii) Purchasers shall:
(A) pay, by wire transfer, to an account or accounts designated by Sellers, immediately available funds in an amount equal to either: (i) the Initial Purchase Price plus the Closing Adjustment (if the Closing Adjustment is a positive amount) or (ii) the Initial Purchase Price minus the Closing Adjustment (if the Closing Adjustment is a negative amount), in each case as determined pursuant to Section 2.4, to Eagle Holdings in the amount set forth in Section 2.2(b)(ii) and the remaining amount, if any, to APIL;
(B) deliver to APIL the Warrant, duly executed by Recro;
(C) deliver to Seller the Transition Services Agreement, duly executed by Purchasers; and
(D) deliver to Sellers the certificate required to be delivered pursuant to Section 8.3(c).
2.4 Closing Adjustment. Not less than three (3) Business Days prior to the anticipated Closing Date, Sellers shall provide Purchasers with a certificate signed by an officer
-20-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
of each of the Sellers attaching reasonable and good faith estimates (the “Closing Estimates”) of each of (i) the Closing Working Capital (the “Estimated Closing Working Capital”), (ii) the Closing Cash Amount (the “Estimated Closing Cash Amount”); (iii) the Closing Date Indebtedness (the “Estimated Closing Date Indebtedness”); (iv) the Closing Date Transaction Fees (the “Estimated Closing Date Transaction Fees”); and (v) the Closing Adjustment (as defined below). Each of the Closing Estimates shall be determined in accordance with the Accounting Methodology. Purchasers shall be entitled to review, and propose reasonable changes to the Closing Estimates and Sellers shall provide Purchasers and their Representatives with reasonable access, at reasonable times following prior notice, to the officers, employees, agreements and books and records of the Transferred Entities to verify the accuracy of such amounts. The Sellers shall consider the Purchasers’ proposed changes in good faith. If the Parties are unable to reach agreement on any proposed changes, the Closing Estimates (and the components thereof) as proposed by the Sellers shall control solely for purposes of payments to be made at Closing and shall not limit or otherwise effect the Purchasers’ remedies under this Agreement or otherwise constitute an acknowledgment by Purchasers of the accuracy of the Closing Estimates. The “Closing Adjustment” shall equal (i) the Estimated Closing Working Capital, plus (ii) the Estimated Closing Cash Amount, less (iii) the Target Working Capital, less (iv) the Estimated Closing Date Indebtedness, and (v) less the Estimated Closing Date Transaction Fees.
2.5 Post-Closing Statement.
(a) After the Closing Date, Sellers and Purchasers shall cooperate with each other and provide each other with such access to their respective books, records, accountants, audit work papers and relevant employees as they may reasonably request in connection with the matters addressed in this Section 2.5; provided, however, that nothing contained in this Section 2.5 shall require Sellers, Purchasers or any of their respective Affiliates to disclose any attorney-client privileged information to the extent that disclosure thereof might result in the loss of attorney-client privilege. As promptly as practicable but no later than sixty (60) days after the Closing Date, Purchasers shall prepare and deliver to Sellers a statement (the “Initial Post-Closing Adjustment Statement”) of (i) the Closing Working Capital, (ii) the Closing Cash Amount, (iii) the Closing Date Indebtedness, (iv) the Closing Date Transaction Fees; and (v) the Adjustment Amount, setting forth Purchasers’ calculation of the Adjustment Amount as of the Closing together with reasonable supporting calculations and detail. The Initial Post-Closing Adjustment Statement shall be determined in accordance with the Accounting Methodology.
(b) If Sellers disagree in whole or in part with the Initial Post-Closing Adjustment Statement, Seller shall notify Purchasers in writing of such disagreement (the “Notice of Disagreement”) within thirty (30) days following Sellers’ receipt of the Initial Post-Closing Adjustment Statement (the “Review Period”), indicating the specific line items that are in dispute (the “Disputed Items”), describing the basis for such objection and providing Seller’s estimate of such Disputed Items; provided that Sellers and Purchasers shall be deemed to have agreed upon all items and amounts that are not Disputed Items, unless the resolution of a Disputed Item affects an undisputed item, in which case such undisputed item shall remain open and be considered a Disputed Item. If no Notice of Disagreement is received by Purchasers prior to the expiration of the Review Period, then the Initial Post-Closing Adjustment Statement shall be deemed to have been accepted by Sellers and shall become final and binding upon the Parties in accordance with Section 2.5(e).
-21-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(c) During the thirty (30) days (or such longer period as the Parties may mutually agree) immediately following the delivery of a Notice of Disagreement (the “Resolution Period”), Sellers and Purchasers shall seek in good faith to resolve any differences that they may have with respect to Disputed Items. The Parties shall cooperate during such Resolution Period and Sellers and their Representatives shall have access to the books and records, working papers, schedules and calculations of the Purchasers and the Transferred Entities used in the preparation of the Initial Post-Closing Adjustment Statement, the Notice of Disagreement and the determination of the Disputed Items, and to the personnel involved in the preparation and calculation thereof, during normal business hours, upon reasonable notice.
(d) Dispute Resolution.
(i) If, at the end of the Resolution Period, Sellers and Purchasers have been unable to resolve all Disputed Items, Sellers and Purchasers shall submit the remaining Disputed Items with respect to the Notice of Disagreement (along with a copy of the Initial Post-Closing Adjustment Statement marked to indicate those line items that are not in dispute) to PricewaterhouseCoopers LLP or, if that firm declines to act as provided in this Section 2.5(d) or it is determined that PricewaterhouseCoopers LLP would not be considered “independent” under applicable professional standards, another firm of independent public accountants, selected promptly by and mutually reasonably acceptable to Purchasers and Sellers (the “Independent Accounting Firm”). The Independent Accounting Firm shall be instructed to make, within thirty (30) days after the expiration of the Resolution Period or, if applicable, the date of selection of the Independent Accounting Firm pursuant to the preceding sentence, a final determination in accordance with this Agreement, binding on the Parties, of the appropriate amount of each of the Disputed Items which Sellers and Purchasers have submitted to the Independent Accounting Firm, calculated in accordance with the standards set forth in this Agreement, and to promptly notify the Parties in writing of its determination. With respect to each amount in dispute, the Independent Accounting Firm’s determination, if not in accordance with the position of either Sellers or Purchasers, shall not be in excess of the higher, nor less than the lower, of the amounts advocated by Sellers in the Notice of Disagreement or by Purchasers in the Initial Post-Closing Adjustment Statement with respect to such disputed amount.
(ii) During the review by the Independent Accounting Firm, Purchasers and Sellers will each provide the Independent Accounting Firm with such access to their respective books, records, accountants, audit work papers and relevant employees as may be reasonably required by the Independent Accounting Firm to fulfill its obligations under this Section 2.5; provided, however, that nothing contained in this Section 2.5 shall require Sellers, Purchasers or any of their respective Affiliates to disclose any attorney-client privileged information to the extent that disclosure thereof might result in the loss of attorney-client privilege.
(iii) All fees and expenses relating to the work, if any, to be performed by the Independent Accounting Firm shall be split equally between Sellers, on the one hand, and Purchasers on the other hand.
-22-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(e) The version of the Initial Post-Closing Adjustment Statement that is final and binding on the Parties, as determined either through agreement of the Parties pursuant to Section 2.5(b) or Section 2.5(c) or through the action of the Independent Accounting Firm pursuant to Section 2.5(d), is referred to as the “Final Post-Closing Adjustment Statement”, and the Adjustment Amount set forth therein as the “Final Adjustment Amount.”
2.6 Post-Closing Adjustment. The “Post-Closing Adjustment” shall be equal to (i) the Final Adjustment Amount set forth in the Final Post-Closing Adjustment Statement less (ii) the Closing Adjustment. If the Post-Closing Adjustment is a positive amount, then Purchasers shall pay or cause Acquisition Sub to pay in cash to Sellers (or one or more Affiliates designated by Sellers) the amount of the Post-Closing Adjustment via wire transfer of immediately available funds to the account(s) designed in writing by the Sellers. If the Post-Closing Adjustment is a negative amount, then Sellers (or an Affiliate designated by Sellers) shall pay in cash to Acquisition Sub the amount of the Post-Closing Adjustment via wire transfer of immediately available funds to the account(s) designed in writing by the Acquisition Sub. Any such payment shall be made within five (5) Business Days after the Final Post-Closing Adjustment Statement is determined.
2.7 Calculations. Except as otherwise expressly provided in this Agreement, the Parties hereto covenant and agree that no amount shall be (or is intended to be) included, in whole or in part (either as an increase or a reduction), more than once in the calculation of (including any component of) the Closing Estimates, the Initial Post-Closing Adjustment Statement, the Final Post-Closing Adjustment Statement, the Adjustment Amount or any other calculated amount pursuant to this Agreement if the effect of such additional inclusion (either as an increase or a reduction) would be to cause such amount to be over- or under-counted for purposes of such calculation.
2.8 Earn-Out Consideration. Following the Closing, Purchasers shall pay or cause Newco to pay to APIL the Earn-Out Consideration, in accordance with the terms of Exhibit E.
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF SELLERS
Except as set forth in the corresponding sections or subsections of the disclosure letter delivered to Purchasers by the Sellers prior to the date hereof (the “Sellers Disclosure Letter”), Sellers hereby make to Purchasers, as of the date hereof and as of the Closing, each of the representations and warranties contained in this Article III; it being understood that disclosure of any item in any section or subsection of the Sellers Disclosure Letter shall also be deemed disclosure with respect to any other section or subsection to which the relevance of such item is readily apparent on its face. The representations and warranties contained in this Article III and in any other provision of this Agreement and certificate delivered pursuant to this Agreement constitute all of the representations and warranties of Sellers with respect to the transactions contemplated hereby.
-23-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.1 Organization; Authorization.
(a) Each Seller is duly organized, validly existing and, to the extent applicable, in good standing under the Laws of its jurisdiction of organization.
(b) Each Transferred Entity is duly organized and validly existing and, to the extent applicable, in good standing under the Laws of the jurisdiction of its organization and has the requisite corporate or similar power and authority to own its properties and assets and to carry on its business as it is now being conducted. Each Transferred Entity is duly qualified to transact business in each jurisdiction in which the nature of property owned or leased by it or the conduct of its business requires it to be so qualified, except where the failure to be so duly qualified to transact business, or to have such power and authority, would not, individually or in the aggregate, have or reasonably be expected to have a Material Adverse Effect. Section 3.1(b) of the Sellers Disclosure Letter sets forth a complete and accurate list of the jurisdiction of incorporation or organization of each Transferred Entity and all jurisdictions in which each Transferred Entity is duly qualified to transact business.
(c) Except as set forth in Section 3.1(c) of the Sellers Disclosure Letter, each Seller (i) has the requisite corporate or similar right, authority and power to execute and deliver this Agreement and to perform its obligations hereunder and to consummate the transactions contemplated hereby, including the sale, assignment and transfer of the Transferred Interests and (ii) will have, on or before the date of signing each Ancillary Agreement to which it will be a party, the requisite corporate or similar right, authority and power to execute and deliver the Ancillary Agreements to which it will be a party and to perform its obligations thereunder and to consummate the transactions contemplated thereby. The execution, delivery and performance of this Agreement and the consummation of the transactions contemplated herein have been duly and validly authorized by all corporate or similar action in respect thereof on the part of each Seller. The execution, delivery and performance of each Ancillary Agreement to which it is a party and the consummation of the transactions contemplated therein will, at the time of signing of such Ancillary Agreement, have been duly and validly authorized by all corporate or similar action in respect thereof on the part of each Seller.
(d) This Agreement has been, and each of the Ancillary Agreements to which each Seller is a party will be, on or prior to the date of signing such Ancillary Agreement, duly and validly executed and delivered by each Seller, and, assuming the due authorization and execution of this Agreement by Purchasers, this Agreement constitutes, and, assuming the due authorization and execution of the other parties to each Ancillary Agreement, each Ancillary Agreement will constitute, the legal and binding obligation of each Seller, enforceable against each Seller in accordance with its terms: (i) except as enforcement may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws affecting creditors’ rights generally and (ii) except insofar as the availability of equitable remedies may be limited by applicable Law (the preceding clauses (i) and (ii) are referred to herein collectively as the “Enforceability Exceptions”).
-24-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(e) Except as set forth in Section 3.1(e) of the Sellers Disclosure Letter, neither the execution, delivery and performance of this Agreement and the Ancillary Agreements, nor the consummation of the transactions contemplated hereby and thereby, including the Reorganization, will (i) conflict with or violate any provision of any Governing Documents of Sellers or the Transferred Entities, (ii) constitute or result in a default under, violate any provision of, or be an event that is (or with the passage of time will result in) a violation of, or result in the acceleration of or entitle any party to accelerate or exercise (whether after the giving of notice or lapse of time or both) any obligation or right under, or result in the imposition of any Lien upon or the creation of a security interest in any of the Transferred Interests, or any Lien on any asset of the Transferred Entities, any material Contract, instrument, order, arbitration award, judgment or decree to which any Transferred Entity or Seller is a party or by which any of them is bound, or (iii) violate or conflict with any Law or other restriction of any kind or character to which any Seller or Transferred Entity is subject, that, in the case of clauses (ii) or (iii) would, individually or in the aggregate, have or reasonably be expected to have a Material Adverse Effect.
3.2 Title to Shares; Capitalization; Structure.
(a) (i) The authorized capital stock of Alkermes Gainesville consists of 393,075 Membership Units, of which 393,075 Membership Units are outstanding, all of which are owned, beneficially and of record, by Eagle Holdings free and clear of all Liens except Permitted Liens and Liens set forth on Section 3.2 of the Sellers Disclosure Letter; and (ii) the authorized capital stock of Daravita consists of 150,000,000 Ordinary Shares, of which 102,000,001 Ordinary Shares are in issue, which are owned, beneficially and of record, by Alkermes Ireland Holdings free and clear of all Liens except for Permitted Liens and Liens set forth on Section 3.2 of the Sellers Disclosure Letter. At the Closing, all of the authorized capital interests in Newco will be owned, beneficially and of record, by APIL free and clear of all Liens except Permitted Liens and Liens set forth on Section 3.2 of the Sellers Disclosure Letter. All of the outstanding Membership Units of Alkermes Gainesville have been duly authorized and validly issued, are fully paid and nonassessable, and have not been issued in violation of any preemptive or third-party rights. At the Closing, all of the issued and outstanding capital stock of Newco will be validly issued, fully paid and nonassessable, and will not have been issued in violation of any preemptive or third-party rights. At the Closing, Sellers will deliver to Purchasers good and valid title to all of the Transferred Interests free and clear of any Lien, except for Permitted Liens.
(b) Except as set forth in Section 3.2 of the Sellers Disclosure Letter, the Transferred Entities have no other equity interests issued or outstanding and there are no outstanding options, warrants or other rights of any kind to acquire, or obligations to issue, shares of capital stock of any class of, or other equity interests in, the Transferred Entities. No Transferred Entity owns any equity interest, directly or indirectly, in any Person other than a Transferred Entity. There are no outstanding obligations of any Transferred Entity (i) to repurchase, redeem or otherwise acquire any shares of capital stock or other equity interests in any Transferred Entity or (ii) to grant preemptive or anti-dilutive rights with respect to any such shares or interests.
-25-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.3 No Consents. Section 3.3 of the Sellers Disclosure Letter contains a list of all registrations, filings, applications, notices, consents, approvals, orders, qualifications and waivers required to be made, filed, given or obtained by Sellers or the Transferred Entities with, to or from any Persons or Governmental Entities in connection with the consummation of this Agreement or the Ancillary Agreements or the other transactions contemplated hereby or thereby, including the Reorganization, except for those with respect to which the failure to make, file, give or obtain would not, individually or in the aggregate, have or reasonably be expected to have a Material Adverse Effect (the “Consents”).
3.4 Financial Statements.
(a) Section 3.4 of the Sellers Disclosure Letter sets forth true and complete copies of the unaudited consolidated statements of income, balance sheets and statements of cash flows of the Business (i) as of December 31, 2013 and for the nine-month period then ended and (ii) as of December 31, 2014 and for the twelve-month period then ended (collectively, the “Historical Financial Statements”). The Historical Financial Statements, present fairly in all material respects the consolidated financial position and results of operations and cash flows of the Business for the respective periods or as of the respective dates set forth therein, in each case in accordance with GAAP applied on a consistent basis throughout the periods involved. The Historical Financial Statements have been prepared from and in all material respects in accordance with the Books and Records of the Transferred Entities and the Business, which Books and Records fairly reflect in reasonable detail all assets, liabilities and transactions relating to the Transferred Entities and the Business. The balance sheet as of December 31, 2014 (the “Balance Sheet Date”) included in the Historical Financial Statements is referred to herein as the “Business Balance Sheet.”
3.5 No Undisclosed Liabilities. Except for Liabilities (a) which are reflected or reserved against in the Business Balance Sheet, (b) set forth in Section 3.5 of the Sellers Disclosure Letter or (c) incurred in the ordinary course of business since the Balance Sheet Date which are of a category reflected or reserved against and in amounts consistent with those reflected on the Business Balance Sheet the Business has no Liabilities that would be required to be reflected on a balance sheet prepared in accordance with GAAP.
3.6 Properties; Sufficiency.
(a) With the exception of (i) properties disposed of since the Balance Sheet Date in the ordinary course of business, (ii) the Excluded Assets, and (iii) the Liens set forth in Section 3.6(a) of the Sellers Disclosure Letter, which (other than Permitted Liens) will be released prior to or at Closing, the Transferred Entities have good and marketable title to, or a valid and existing lease or license to, free and clear of all Liens other than Permitted Liens, each piece of real and material personal property capitalized on or included in the Business Balance Sheet (or for real and personal property acquired by the Business since the date of the Business Balance Sheet, that would have been, had it been acquired prior to such date, capitalized on or included in the Business Balance Sheet) and each other piece of real and material personal property used or held for use in the Business. All documents necessary to prove such title are in the possession or under the control of the Transferred Entities, copies of which have been made available to Purchasers.
-26-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(b) Section 3.6(b) of the Sellers Disclosure Letter sets forth a list of all the real property owned or leased by the Transferred Entities in connection with the Business (the “Business Real Property”). Sellers have made available correct and complete copies of all material leases and subleases (including all material amendments, modifications and side letters thereto, and all notices of default and other material notices thereunder) relating to the Business Real Property to which the Transferred Entities are a party, all of which are identified in Section 3.6(b) of the Sellers Disclosure Letter and each of which is valid and in full force and effect. With respect to the Business Real Property owned by the Transferred Entities (the “Owned Real Property”), except as set forth in Section 3.6(a) of the Sellers Disclosure Letter, the applicable Transferred Entity has good and marketable title in fee simple to such property subject only to Permitted Liens. There are no pending or, to the Knowledge of Sellers, threatened condemnation proceedings relating to any Business Real Property for which written notice has been received by the Transferred Entities. To the Knowledge of Sellers, except as set forth in Section 3.6(a) of the Sellers Disclosure Letter and except pursuant to this Agreement, no Person has any right, option, lease, license, right of first refusal or any other Contract with respect to the purchase, assignment, possession, use or transfer of all or a portion of the Owned Real Property. To the Knowledge of Sellers, no Owned Real Property encroaches upon adjoining real estate. The ownership, occupancy, use and operation of the Business Real Property has complied and complies in all material respects with all applicable Laws, including but not limited to planning, zoning or use Laws, and Sellers have received no written, or to the Knowledge of Sellers, oral, notice of any material defaults by the Transferred Entities in respect of the Business Real Property in complying with the requirements of any notice received from a Governmental Entity under any such Laws. Except as disclosed in Section 3.6(b) of the Sellers Disclosure Letter, none of the properties owned or leased by the Transferred Entities or otherwise used in the Business is shared by the Business, on the one hand, and the other businesses, divisions or Subsidiaries of Parent, on the other hand.
(c) The buildings, structures and improvements on each Business Real Property are in all material respects in reasonable operating condition and repair, are structurally sound and free of material defects, with no material alterations or repairs required under applicable Law and are suitable in all material respects for their current use, operation and occupancy.
(d) All fixtures and mechanical systems located at the Business Real Property are currently in good working order except for ordinary wear and tear and for fixtures and mechanical systems under repair or out of service in the ordinary course of business.
(e) The assets, properties and rights of the Transferred Entities constitute all of the assets (other than (i) the Excluded Assets, (ii) services to be provided pursuant to the applicable Ancillary Agreements, and (iii) the services excluded under Part I(b) (Excluded IT Services) of Schedule 2 of Exhibit A) necessary to own and operate the Business in the manner being conducted as of the date hereof. The Transferred Entities collectively own or lease, or otherwise have good and valid rights to, all material assets, properties and other rights related to the Business.
3.7 Absence of Certain Changes. Except as set forth in Section 3.7 of the Sellers Disclosure Letter, since the Balance Sheet Date, there has been no (a) change or development in
-27-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
or effect on the Business that has had, or would reasonably be expected to have, a Material Adverse Effect, (b) other than in connection with the transactions contemplated by this Agreement, action or omission by the Transferred Entities that was not in the ordinary course of business or (c) action or omission that, if taken from the date hereof through the Closing, would violate any of the provisions of Sections 5.4(a) or 5.4(b).
3.8 Litigation; Orders. Except as set forth in Section 3.8 of the Sellers Disclosure Letter, there are no Actions pending or, to the Knowledge of Sellers, threatened (i) against any Seller that challenges or would reasonably be expected to have the effect of preventing or making illegal any of the transactions contemplated by this Agreement or (ii) against the Business or the Transferred Entities. There are no judgments or outstanding orders, injunctions, decrees, stipulations or awards (whether rendered by a court or administrative agency, or by arbitration) against or applicable to the Business or the Transferred Entities, or any of their respective properties or businesses.
3.9 Intellectual Property.
(a) The Transferred Entities own, or have a license or right to use, the Intellectual Property Rights necessary for the conduct of the Business; provided, however, that the foregoing is not a representation of non-infringement of the Intellectual Property Rights of another Person, which representation is solely set forth in the first sentence of Section 3.9(c) below. Section 3.9(a)(i) of the Sellers Disclosure Letter sets forth a true and complete list of all Registered Intellectual Property included in the Business Intellectual Property (the “Business Registered Intellectual Property”), setting forth as to each item, if applicable: the owner of record, jurisdiction of application and/or registration, and the date of application and/or registration. The Business Registered Intellectual Property is subsisting, and to the Knowledge of Sellers, valid and enforceable. Except as set forth in Section 3.9(a)(ii) of the Sellers Disclosure Letter, there are no oppositions, cancellations, invalidity proceedings, interference or re-examinations, or any other proceedings challenging the scope, validity, registrability or ownership of any Business Registered Intellectual Property currently pending, or, to the Knowledge of Sellers, threatened in writing, against the Transferred Entities (other than office actions or similar communications issued by any Governmental Entity in the ordinary course of prosecution of any pending applications for registration of any such Business Registered Intellectual Property). Except as set forth in Section 3.9(a)(iii) of the Sellers Disclosure Letter, the Transferred Entities exclusively own the entire right, title and interest in and to the Business Registered Intellectual Property free and clear of all Liens except Permitted Liens.
(b) Except as set forth in Section 3.9(b) of the Sellers Disclosure Letter, no Business Intellectual Property is subject to any outstanding judgment, injunction, order, decree or agreement that restricts the use thereof by the Transferred Entities or that would be reasonably be expected to restrict the use thereof by the Transferred Entities following the Closing.
(c) Except as set forth in Section 3.9(c) of the Sellers Disclosure Letter, to the Knowledge of Sellers, the conduct of the Business as currently conducted has not and does not infringe, misappropriate or violate any Intellectual Property Rights of any other Person. Except as set forth in Section 3.9(c) of the Sellers Disclosure Letter, neither of the Transferred Entities has received, since January 1, 2012, any written claim or demand, nor are there any pending
-28-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Actions: (i) alleging infringement or misappropriation of any Intellectual Property Rights of any other Person or (ii) challenging the use, ownership, enforceability or validity of any of the Business Intellectual Property.
(d) Except as set forth in Section 3.9(d) of the Sellers Disclosure Letter, to the Knowledge of Sellers, no Person is currently infringing, misappropriating or violating any Business Intellectual Property.
(e) The Transferred Entities currently take commercially reasonable security measures to protect the confidentiality of all material Business Intellectual Property, including the secrecy and confidentiality of their trade secrets. Each current employee and consultant of, and any former employee or consultant employed by, a Transferred Entity since January 1, 2014 has executed a confidentiality agreement and invention assignment or an employment or consulting agreement maintaining confidentiality in any material trade secret or material confidential information of the Transferred Entities and assigning to the applicable Transferred Entity any rights in the Business Intellectual Property invented by such employee or consultant and embodied in one of the Products as it exists as of the date of this Agreement.
(f) To the Knowledge of Sellers, there were no material defects of form in the preparation or filing of the patent applications that are part of the Business Registered Intellectual Property. To the Knowledge of Sellers, Sellers and Transferred Entities have complied in all material respects with the United States Patent Office (“USPTO”) duty of candor and disclosure as required under 37 C.F.R. § 1.56 for the each of the U.S. patents and patent applications that are part of the Business Registered Intellectual Property.
3.10 Licenses; Authorizations; Reports. Section 3.10(a) of the Sellers Disclosure Letter contains a complete and accurate list of all material governmental licenses, consents, qualifications, registrations, clearances, permits, franchises, variances, exemptions and other authorizations, including all authorizations under the Federal Food, Drug and Cosmetic Act of 1938, as amended (the “FDCA”), the Public Health Service Act of 1944 and the regulations of the United States Food and Drug Administration and any successor agency thereto (the “FDA”) promulgated under any of the foregoing or any Similar Law or authorization of any other Governmental Entity, and any other Governmental Entity that is concerned with the quality, identity, strength, purity, safety, efficacy, developing or manufacturing of the Products (“Licenses”) necessary for the lawful operating of the Business, issued, granted, given or otherwise made available by or under the authority of, or any required notification to, any Governmental Entity or pursuant to any Law necessary for the conduct of the Business as conducted on the date hereof. Except as set forth on Section 3.10(b) of the Sellers Disclosure Letter, each material License (i) is in the name of a Transferred Entity and is in full force and effect and (ii) is not subject to any pending or, to the Knowledge of Sellers, threatened Action for the purposes of revoking, limiting or amending such License. As of the date hereof, neither of the Transferred Entities has received written notice or, to the Knowledge of Sellers, any other notice from any Governmental Entity that (A) any existing material License will be revoked or (B) any pending application for any material new License or renewal of any existing material License will be denied.
-29-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.11 Labor Matters.
(a) With respect to employees of the Business, the Transferred Entities are not bound by any agreements with labor unions or associations representing any employees, or purporting to represent or attempting to represent any employees, of the Business. Except as set forth in Section 3.11(a) of the Sellers Disclosure Letter, neither of the Transferred Entities is involved in, or, to the Knowledge of Sellers, threatened with any material work stoppage, strike, shutdown, lockout, demand for recognition or other material labor dispute, arbitration, lawsuit or administrative proceeding relating to labor matters involving Transferred Entity Employees, and there have been no such actions or disputes in the past three (3) years. To the Knowledge of Sellers, during the past three (3) years there has not been any attempt by any Transferred Entity Employees or any labor organization or other employee representative to organize or certify a collective bargaining unit or to engage in any other union organization activity with respect to the workforce of any Transferred Entity.
(b) Section 3.11(b) of the Sellers Disclosure Letter sets forth a true and complete list of the employees currently employed by each Transferred Entity, in each case whose annualized aggregate compensation as salary, wages and bonuses during 2014 exceeded One Hundred Thousand Dollars ($100,000) (the “Highly Paid Employees”). The Sellers have delivered to Purchasers a true and correct listing of the compensation amounts paid to each Highly Paid Employee in 2014 and the base salary and target bonus for such personnel in 2015. Except as set forth on Section 3.11(b) of the Sellers Disclosure Letter, to the Knowledge of Sellers, no Highly Paid Employee has plans to terminate employment with the Transferred Entities.
(c) Except as set forth on Section 3.11(c) of the Sellers Disclosure Letter, no Transferred Entity is the subject of any pending Action asserting that it has committed an unfair labor practice (within the meaning of the National Labor Relations Act or comparable state or foreign Law) or other violation of state or federal labor Law or seeking to compel it to bargain with any labor organization as to wages, terms or conditions of employment. Each Transferred Entity is in material compliance with all applicable Laws relating to the employment of labor, including those related to wages, hours, collective bargaining, immigration, equal employment opportunities and retaliation. There is no claim with respect to payment of wages, salary or overtime pay that has been asserted in writing to a Transferred Entity or is pending or, to the Knowledge of Seller, threatened before any Governmental Entity with respect to any Persons currently or formerly employed by a Transferred Entity.
(d) Section 3.11(d) of the Sellers Disclosure Letter lists each employee of a Transferred Entity who was terminated or laid off for any reason other than for cause, or whose hours were reduced by more than 50%, during the ninety (90) days preceding the date of this Agreement, and for each such employee, sets forth: (i) the date of such termination, layoff or reduction in hours; and (ii) the location to which the employee was assigned. Each of the Transferred Entities is in material compliance with its obligations pursuant to the Worker Adjustment and Retraining Notification Act of 1988 (the “WARN Act”) and any similar Law and neither Transferred Entity has ordered or implemented a plant closing or mass layoff within the meaning of the WARN Act or any similar Law in the past three (3) years.
-30-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.12 Taxes. Except as set forth in Section 3.12 of the Sellers Disclosure Letter:
(a) Since September 16, 2011, Alkermes Gainesville has been a disregarded entity within the meaning of Treasury Regulation Section 301.7701-3 for U.S. Federal income tax purposes.
(b) Since the formation of Newco by APIL, Newco has been a disregarded entity within the meaning of Treasury Regulation Section 301.7701-3 for U.S. Federal income tax purposes.
(c) Each of the Transferred Entities has filed all material Tax Returns that they were required to file under applicable Laws and regulations. All such Tax Returns were correct and complete in all material respects and were prepared in substantial compliance with all applicable Laws and regulations. All material Taxes due and owing by the Transferred Entities (whether or not shown on any Tax Return) have been paid. Neither of the Transferred Entities currently is the beneficiary of any extension of time within which to file any such Tax Return. No written claim has ever been made by an authority in a jurisdiction where the Transferred Entities do not file Tax Returns that the Transferred Entities are or may be subject to taxation by that jurisdiction.
(d) There are no material Liens for Taxes (other than Taxes not yet due and payable) upon any of the assets of the Transferred Entities, except for Permitted Liens.
(e) Each of the Transferred Entities have withheld and paid all material Taxes required to have been withheld and paid in connection with any amounts paid or owing to any employee, independent contractor, creditor, stockholder, or other third party, and all IRS Forms W-2 and 1099 with respect thereto have been properly completed and timely filed.
(f) To the Knowledge of Sellers, no federal, state, local, or non-U.S. tax audits, investigations or administrative or judicial Tax proceedings are pending or being conducted with respect to the Transferred Entities. Neither of the Transferred Entities has received from any Governmental Entity (including those in jurisdictions where the Transferred Entities have not filed Tax Returns) any (i) written notice indicating an intent to open an audit or other review, (ii) request for information related to Tax matters, or (iii) notice of deficiency or proposed adjustment for any amount of Tax proposed, asserted, or assessed by any Governmental Entity against the Transferred Entities.
(g) The Transferred Entities have not waived any statute of limitations in respect of Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency. No Transferred Entity has issued any power of attorney (or any such equivalent) with respect to any material Taxes that is still in effect.
(h) No Transferred Entity has any equity interest in any entity taxed as a “partnership” or a “controlled foreign corporation” for U.S. federal income tax purposes.
(i) The Transferred Entities have and, to the extent requested, have provided Purchasers with access to, true, correct and complete copies of all material Tax Returns filed by them for all pre-Closing Tax periods beginning on or after September 16, 2011 and true, correct
-31-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
and complete copies of any examination reports received by the Transferred Entities and statements of deficiencies assessed against or agreed to by the Transferred Entities for all Pre-Closing Tax periods beginning on or after September 16, 2011 with respect to any Tax.
(j) None of Newco or Alkermes Gainesville has a liability for the Taxes of any other Person, including as a result of being a part of a VAT grouping. Neither Transferred Entity is a party to or bound by any Tax allocation or sharing agreement.
(k) The laws of Ireland do not require any withholding tax to be deducted or withheld from any payment of Earn-Out Consideration.
3.13 Compliance with Laws.
(a) The Transferred Entities operate, and since January 1, 2012 have operated, the Business in compliance in all material respects with all Laws applicable thereto. Except as set forth in Section 3.13(a) of the Sellers Disclosure Letter, since January 1, 2012, none of the Transferred Entities has received any communication from a Governmental Entity that (i) alleges that the Business or such Person (in respect of the Business) is in material violation of any applicable Law, (ii) any investigation or review by any Governmental Entity with respect to the Business or such Person is pending or contemplated and, to the Knowledge of Sellers, no such investigation or review is threatened.
(b) All imports, exports, reexports/retransfers, “deemed exports” and “deemed reexports/retransfers” of the Business have been made in all material respects in accordance with all statutory and regulatory requirements under the Export Administration Regulations and associated executive orders, and the Laws implemented by the Office of Foreign Assets Controls, the United States Department of the Treasury and any other applicable import, export control and sanctions Laws.
(c) Since January 1, 2012, all applications, submissions, information and data utilized by the Transferred Entities in respect of the Business as the basis for, or submitted by or, to the Knowledge of Sellers, on behalf of the Transferred Entities in connection with, any and all requests for a License relating to the Business or any Products, when submitted to the FDA or other Governmental Entity, were true and correct in all material respects as of the date of submission, and any updates, changes, corrections or modification to such applications, submissions, information and data required under applicable Laws have been submitted to the FDA or other Governmental Entity.
(d) Since January 1, 2012:
(i) the Transferred Entities have been in compliance with all legal requirements under (A) the FCPA, and (B) the Irish Prevention of Corruption Acts, 1889 to 2010 and the Irish Ethics in Public Office Acts 1995 and 2001 (collectively, the “Anti-Bribery Laws”); and
(ii) none of the Transferred Entities has, in relationship to the Business, taken any act in furtherance of an offer, payment, promise to pay, authorization, or ratification of the payment, directly or indirectly, of any gift, money or
-32-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
anything of value to a Government Official to secure any improper advantage (e.g., to obtain a tax rate lower than allowed by Law) or to obtain or retain business for any Person.
(e) None of the Transferred Entities is aware of (A) any investigation of or request for information from the Transferred Entities relating to the Business by law enforcement officials regarding the Anti-Bribery Laws, or (B) any other allegation, investigation or inquiry regarding any of their or the Business’ actual or possible violation of the Anti-Bribery Laws.
(f) The Transferred Entities and, in relation to the Business, the Sellers have maintained their Books and Records in a manner that, in reasonable detail, accurately and fairly reflects the transactions and disposition of their assets, and maintain a system of internal accounting controls sufficient to provide reasonable assurances that:
(i) transactions are executed and access to assets is given only in accordance with management’s authorization;
(ii) transactions are recorded as necessary to permit preparation of periodic financial statements in accordance with GAAP and to maintain accountability of corporate assets; and
(iii) recorded assets are compared with existing assets at reasonable intervals and appropriate action is taken with respect to any differences between recorded and actual assets.
(g) No director or officer of any of the Transferred Entities has, directly or indirectly, made false or misleading statements to, or attempted to coerce or fraudulently influence, an accountant in connection with any audit, review, or examination of the financial statements of the Business.
3.14 Insurance. All of the assets and properties of the Business are covered by valid and currently effective insurance policies held by Parent or its Affiliates, other than the Transferred Entities. Such insurance policies provide insurance coverage that, in the Sellers’ judgment, insures against such losses and risks and in such amounts as are customary in the businesses in which the Transferred Entities are engaged. All of such insurance policies are valid, enforceable and in full force and effect and all premiums due and payable thereon have been paid. None of the Transferred Entities, Parent or Affiliates of Parent are in breach or default thereunder or has taken an action or failed to take any action which, with notice or the lapse of time, would constitute a material breach or material default or permit termination or modification of any such policy in a manner that would have a material and adverse effect on the Transferred Entities. Except as set forth on Section 3.14 of the Sellers Disclosure Letter, there are no claims, by or with respect to any Transferred Entity, pending under any of such insurance policies, or material disputes with insurers with respect thereto. None of Sellers, Parent, Affiliates of Parent or any Transferred Entity has received any written notice regarding any cancellation or termination of or refusal of any coverage or rejection of any material claim related to the Transferred Entities under any such insurance policy.
-33-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.15 Material Contracts. Section 3.15 of the Sellers Disclosure Letter sets forth all of the following contracts to which any Transferred Entity is a party or bound, or by which any of the assets or properties of any of them, with the exception of the Excluded Assets, or Business is bound:
(a) any employment or consulting agreement with an individual (i) requiring payments of base compensation in excess of One Hundred Thousand Dollars ($100,000) per year or (ii) related to a Retention Bonus;
(b) any note, mortgage, indenture and other obligation and agreement and other instrument for or relating to any lending or borrowing (including assumed or guaranteed debt) effected by any Transferred Entity or to which any properties or assets of any of them, with the exception of the Excluded Assets, or the Business, is subject;
(c) any agreement or commitment for any capital expenditure in excess of One Hundred Thousand Dollars ($100,000) outside the ordinary course of business;
(d) customer sale and purchase agreements with indicated or estimated future payment obligations in excess of One Hundred Thousand Dollars ($100,000) in any 12-month period or Two Hundred Fifty Thousand Dollars ($250,000) in the aggregate;
(e) drug delivery agreements or contract manufacturing agreements;
(f) any joint venture, partnership, technical assistance, research and development and other similar collaborative agreements;
(g) any contract which is terminable by the other party or parties thereto upon an assignment or change of control of any Transferred Entity, other than such contracts the termination of which would not, individually or in the aggregate, have or reasonably be expected to have a Material Adverse Effect;
(h) any contract, agreement or arrangement, entered into other than in the ordinary course of business, with indicated or estimated future payment obligations in excess of One Hundred Thousand Dollars ($100,000) in any 12-month period or Two Hundred Fifty Thousand Dollars ($250,000) in the aggregate;
(i) any service and supply agreements with indicated or estimated future payment obligations in excess of One Hundred Thousand Dollars ($100,000) in any 12-month period or Two Hundred Fifty Thousand Dollars ($250,000) in the aggregate;
(j) any Business IP Agreement;
(k) any agreement or commitment for the disposition of assets or any interest in any business enterprise outside the ordinary course of business;
(l) any agreement or commitment limiting or restraining it from engaging or competing in any lines of business, with any Person or in any geographic area or from soliciting any Person for business, and any agreement or commitment limiting or restraining it from soliciting any individual for employment;
-34-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(m) any agreement or commitment that would become payable upon a change of control of a Transferred Entity;
(n) (i) any agreement with the five (5) largest customers of the Transferred Entities, taken as a whole (determined based on monthly recurring revenue as of the end of the last fiscal year) (such customers, the “Significant Customers”); and (ii) any agreement with the ten (10) largest suppliers (excluding service providers) of the Transferred Entities, taken as a whole (determined based on payments from the Transferred Entities for the last fiscal year) (such suppliers, the “Significant Suppliers”);
(o) any lease or agreement under which a Transferred Entity is lessee of any personal property owned by another party, for which annual rent exceeds Two Hundred and Fifty Thousand Dollars ($250,000);
(p) any stock purchase agreement, asset purchase agreement or other acquisition or divestiture Contract entered into by any Transferred Entity during the past five (5) years; and
(q) any material amendments, modifications, extensions or renewals of any of the foregoing or any exercise of any option in respect of any of the foregoing.
The contracts listed on Section 3.15 of the Sellers Disclosure Letter are referred to herein as “Business Material Contracts.” With respect to all Business Material Contracts, except as set forth in Section 3.15 of the Sellers Disclosure Letter, (i) none of the Transferred Entities, nor, to the Knowledge of Sellers, any other party to any such Business Material Contract is in material breach thereof or default thereunder, and (ii) there does not exist under any provision thereof, any event that, with the giving of notice or the lapse of time or both, would constitute such a material breach or default. Sellers have made available to Purchasers true, correct and complete copies of all Business Material Contracts. Each Business Material Contract is in full force and effect in accordance with the terms thereof and constitutes a legal, valid, and binding agreement of the parties thereto, and is enforceable in accordance with its terms by the applicable Transferred Entity who is a party thereto against each counterparty thereto, except as such enforceability may be limited by the Enforceability Exceptions.
3.16 Brokers, Finders. Except for the services of the Seller Financial Advisor, whose fees with respect to the transactions contemplated by this Agreement will be borne by Sellers, neither the Transferred Entities nor the Sellers has employed, or is subject to any valid claim of, any broker, finder, consultant or other intermediary in connection with the transactions contemplated by this Agreement who might be entitled to a fee or commission in connection with such transactions.
3.17 Board Approval. The board of directors of each Seller, by resolutions duly adopted, has approved this Agreement.
-35-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.18 Environmental Health and Safety Matters. Except as set forth in Section 3.18 of the Sellers Disclosure Letter:
(a) The Transferred Entities are in compliance in all material respects with all applicable Environmental Laws, including holding and complying in all material respects with all permits, certificates, licenses, approvals, registrations and authorizations required under Environmental Laws for their operations.
(b) The Transferred Entities are not subject to any pending Action or written notice from a Governmental Entity alleging that the Transferred Entities are in violation of, or have liability under, any Environmental Law.
(c) To the Knowledge of Sellers, there has been no Release of Hazardous Materials at any Business Real Property in an amount, manner or condition that would reasonably be expected to result in material liability to the Transferred Entities under applicable Environmental Laws.
(d) Sellers have made available to Purchasers copies of all material written environmental assessments, audits, and reports in their possession and relating to the Business or any Business Real Property.
(e) Without limiting the generality of the foregoing, none of the Transferred Entities have any outstanding material indemnification obligation, or any unresolved material enforcement action or liability, pursuant to any Environmental Law, including but not limited to, any investigation, cleanup, removal action, response action, remediation, or corrective action obligation, relating to the Business Real Property or, to the Knowledge of Sellers, to any (i) formerly owned or operated property, or (ii) offsite disposal location.
(f) None of the Transferred Entities has treated, stored, disposed of, arranged for or permitted the disposal of, transported, handled, or released any Hazardous Material in material violation of any Environmental Laws, or in a manner that would reasonably be expected to result in material liability (including, but not limited to, any material obligation to conduct an investigation, cleanup, removal action, response action, remediation or corrective action) to any of the Transferred Entities under applicable Environmental Laws.
(g) To the Knowledge of Sellers, neither this Agreement nor the consummation of the transactions contemplated hereby will result in any obligations for site investigation or cleanup, or notification to or consent of any Governmental Entity or third parties, pursuant to any of the so-called “transaction-triggered” or “responsible property transfer” Environmental Laws.
3.19 Employee Benefit Plans.
(a) Sellers have made available to Purchasers true and complete copies of each Transferred Entity Benefit Plan and all amendments thereto together with the most recent annual report, if required by Law, summary plan description and any material modifications thereto, actuarial valuation report prepared in connection with any such Benefit Plan and all trust agreements, insurance contracts and other funding vehicles relating thereto. Section 3.19(a) of the Sellers Disclosure Letter lists all material Benefit Plans, specifically identifying each Transferred Entity Benefit Plan.
-36-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(b) Each Benefit Plan that is intended to be qualified under Section 401(a) of the Code and each trust created under any such Benefit Plan that is intended to be exempt from tax under Section 501(a) of the Code has received a favorable determination or opinion letter from the IRS. Sellers have made available to Purchasers the most recent determination or opinion letter of the Internal Revenue Service relating to each such Benefit Plan. Any such IRS determination or opinion letter remains in effect and has not been revoked by the IRS. Each Benefit Plan has been maintained in material compliance with its terms and with the requirements prescribed by any and all applicable statutes, orders, rules and regulations, including ERISA and the Code.
(c) There are no pending or, to the Knowledge of Sellers, threatened claims (other than claims for benefits in the ordinary course), investigations, lawsuits or arbitrations which have been asserted or instituted against any Benefit Plan, any fiduciaries thereof with respect to their duties to any Benefit Plan or the assets of any of the trusts under any of such Benefit Plans which would reasonably be expected to result in any liability of Purchasers or any of its Affiliates to the PBGC, the Department of Treasury, the Department of Labor, or any other Governmental Entity, to any of such Benefit Plan or to any participant or beneficiary of any such Benefit Plan.
(d) No Transferred Entity Benefit Plan is (i) a plan subject to Title IV of ERISA, (ii) an arrangement providing post-employment welfare benefits or (iii) a self-insured welfare benefit plan. No Transferred Entity Benefit Plan is a “multiemployer plan” within the meaning of Section 4001(a)(3) of ERISA.
(e) Except as set forth in Section 3.19(e) of the Sellers Disclosure Letter, the execution of and performance of the transactions contemplated by this Agreement will not (either alone or upon the occurrence of any additional or subsequent events) result in any payment to or acceleration, vesting or increase in the rights of any Transferred Entity Employee under any Benefit Plan. No payment or benefit which is or may be made or provided by, from or with respect to any Benefit Plan in connection with the transactions contemplated by this Agreement to any Transferred Entity Employee constitutes an “excess parachute payment” under Section 280G of the Code.
(f) No Transferred Entity Benefit Plan, or Transferred Entity Employee or other service provider of the Transferred Entities is, or would reasonably in the future expect to be, subject to additional Tax or interest imposed under Section 409A or 457(A)(c) of the Code by virtue of the form or operation of any Benefit Plan.
3.20 Products; Recalls. Since January 1, 2012:
(a) except as would not, individually or in the aggregate, have or reasonably be expected to have a Material Adverse Effect, all Products manufactured and supplied by the Transferred Entities in respect of the Business: (i) were manufactured in compliance with applicable Law, including applicable cGMPs or Similar Laws; (ii) conformed to the
-37-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
specifications for the manufacture, storage, and handling of such Product in effect at the time of delivery thereof; (iii) at the time of delivery thereof, were not adulterated or misbranded within the meaning of the FDCA or Similar Laws; and (iv) conformed to the Certificate of Analysis and Conformity supplied with the shipment of such Product; and
(b) to the Knowledge of Sellers, except as set forth in Section 3.20(b) of the Sellers Disclosure Letter, there have been no product recalls, safety alerts, withdrawals, clinical holds, marketing suspensions, removals or the like conducted, undertaken or issued by any Person, whether or not at the request, demand or order of any Governmental Entity or otherwise, related to the manufacture of the Products by the Transferred Entities.
3.21 Transactions with Affiliates. Other than pursuant to any Benefit Plan or other compensatory arrangement, and excluding this Agreement, the Ancillary Agreements and the transactions contemplated hereby and thereby, no executive officer or director of any Seller, Transferred Entity, Parent or their respective Affiliates (i) is party to any Contract with or binding upon a Transferred Entity, (ii) has any interest in property owned by a Transferred Entity or (iii) has engaged in any transaction with any Transferred Entity within the twelve (12) months preceding the date of this Agreement. Since January 1, 2014, except as disclosed in Section 3.21 of the Sellers Disclosure Letter, and excluding this Agreement, the Ancillary Agreements and the transactions contemplated hereby and thereby, there have been no Contracts between either Seller and/or any of its Affiliates (other than any Transferred Entity), on the one hand, and any Transferred Entity, on the other hand (each a “Related Party Agreement”).
3.22 Customers and Suppliers. Except as set forth on Section 3.22 of the Sellers Disclosure Letter, since January 1, 2014, no Significant Customer or Significant Supplier has amended, or proposed in writing, or to the Knowledge of Sellers, proposed orally, to amend, any material terms of, or terminated any Contract with a Transferred Entity in accordance with the terms thereof or has otherwise indicated in writing or, to the Knowledge of Sellers, orally, that they will cease to use or sell to the Transferred Entities, or will substantially reduce the use of services of or sale to the Transferred Entities. Except as set forth on the Section 3.22 of the Sellers Disclosure Letter, the execution, delivery and performance of this Agreement by the Sellers does not, and the consummation of the transactions contemplated hereby, including the Reorganization, by the Sellers will not, constitute or result in a breach or violation of or a default under, or require consent under, any Contract with a Significant Customer or Significant Supplier.
3.23 Accounts Receivable. The accounts receivable reflected on the Business Balance Sheet and the accounts receivable arising after the date thereof (a) have arisen from bona fide transactions entered into by the Transferred Entities involving the sale of goods or the rendering of services in the ordinary course of business consistent with past practice and (b) constitute, to the Knowledge of Sellers, only undisputed claims of the Transferred Entities not subject to claims of set-off or other defenses or counterclaims other than normal cash discounts accrued in the ordinary course of business consistent with past practice. The reserve for bad debts shown on the Business Balance Sheet or, with respect to accounts receivable arising after the Balance Sheet Date, on the accounting records of the Transferred Entities have been determined in accordance with GAAP, consistently applied.
-38-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
3.24 Regulatory Matters.
(a) As set forth in Section 3.10(a) of the Sellers Disclosure Letter, and as limited by Section 3.10(b), Sellers have provided a complete and accurate list of all material Licenses necessary for the lawful operation of the Business as issued, granted, given or otherwise made available by any Governmental Entity, specifically including, but not limited to, those Licenses issued under the FDCA, Public Health Service Act of 1944, and regulations promulgated by the FDA. Subject to Section 3.10(b) of the Sellers Disclosure Letter, each material License (i) is in the name of the Transferred Entity and is in full force and effect and (ii) is not subject to any pending or, to the Knowledge of Sellers, threatened Action or other proceeding for the purposes of revoking, limiting, amending or withdrawing such License.
(b) To the Knowledge of Sellers, since January 1, 2012, the Transferred Entities have complied in all material respects with all Laws as set forth by the FDA or applicable Governmental Entity relating to the Business. To the Knowledge of Sellers, the Transferred Entities have not made any material misrepresentations or fraudulent statements to the FDA or applicable Governmental Entity relating to the Business.
(c) To the Knowledge of Sellers, since January 1, 2012, except as would not, individually or in the aggregate, have or reasonably be expected to have a Material Adverse Effect, Products manufactured by the Transferred Entities in respect of the Business have complied in all material respects with current Good Manufacturing Practices (“cGMPs”) and, at the time of delivery thereof, were not adulterated or misbranded within the meaning of the FDCA. To the Knowledge of Sellers, neither the FDA nor any Governmental Entity is currently alleging non-compliance with cGMPs and no pending clinical trial is subject to termination or suspension relating to the Products.
(d) Except as set forth in Section 3.8 of the Sellers Disclosure Letter, the Transferred Entities are not currently subject to any Action brought by the FDA or other Governmental Entity in respect of the Business.
(e) To the Knowledge of Sellers, since January 1, 2012, neither the Transferred Entities, nor any individual who is an officer, director, employee, stockholder, agent or managing agent of any Transferred Entity, have been subject to an Action that has resulted in exclusion from a governmental or private health care program under which the Products have been reimbursed or have been subject to a debarment proceeding under 21 U.S.C. § 335a; nor is any such proceeding relating to debarment currently pending.
3.25 Investor Representations
(a) Sellers are acquiring the Warrant and the shares issuable upon its exercise for their own account, for investment and not for, with a view to, or in connection with, any sale or distribution thereof within the meaning of the Securities Act, and Sellers will not offer, sell or otherwise dispose of the Warrant and the shares issuable upon its exercise except as permitted by the Securities Act and any applicable state securities law.
(b) Sellers are “accredited investors” as the term is used in Regulation D promulgated under the Securities Act and for the purposes of acquiring the Warrant and the
-39-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
shares issuable upon its exercise. Sellers have sufficient knowledge and experience in business and financial matters and with respect to investments so as to enable Sellers to analyze and evaluate the merits and risks of the investment contemplated hereby with respect to the Warrant.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF PURCHASERS
Except as set forth in the corresponding sections or subsections of the disclosure letter delivered to Sellers prior to the date hereof (the “Purchasers Disclosure Letter”), Purchasers hereby make to Sellers, as of the date hereof and as of the Closing, each of the representations and warranties contained in this Article IV; it being understood that disclosure of any item in any section or subsection of the Purchasers Disclosure Letter shall also be deemed disclosure with respect to any other section or subsection to which the relevance of such item is readily apparent on its face. The representations and warranties contained in this Article IV and in any other provision of this Agreement and certificate delivered pursuant to this Agreement constitute all of the representations and warranties of Purchasers with respect to the transactions contemplated hereby:
4.1 Organization; Authorization; Ownership.
(a) Each Purchaser is duly organized and validly existing and in good standing under the Laws of the jurisdiction of its organization and has the requisite corporate or similar power and authority to own its properties and assets and to carry on its business as it is now being conducted. Each Purchaser is duly qualified to transact business in each jurisdiction in which the nature of property owned or leased by it or the conduct of its business requires it to be so qualified, except where the failure to be so duly qualified to transact business would not, individually or in the aggregate, have or reasonably be expected to have a material adverse effect on the ability of Purchasers to consummate the transactions contemplated by this Agreement, the Ancillary Agreements, the Debt Financing Agreements and the Debt Financing.
(b) Each Purchaser (i) has the requisite corporate or similar right, authority and power to execute and deliver this Agreement and to perform its obligations hereunder and to consummate the transactions contemplated hereby and (ii) will have, on or before the date of signing each Ancillary Agreement and the Debt Financing Agreements to which it will be a party, the requisite corporate or similar right, authority and power to execute and deliver the Ancillary Agreements and Debt Financing Agreements to which it will be a party and to perform its obligations thereunder and to consummate the transactions contemplated thereby. The execution, delivery and performance of this Agreement and the consummation of the transactions contemplated herein have been duly and validly authorized by all corporate or similar action in respect thereof on the part of each Purchaser. The execution, delivery and performance of each Ancillary Agreement and Debt Financing Agreement to which each Purchaser is a party and the consummation of the transactions contemplated therein will, at the time of signing of such Ancillary Agreement or Debt Financing Agreement, have been duly and validly authorized by all corporate or similar action in respect thereof on the part of each Purchaser.
-40-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(c) This Agreement has been, and each of the Ancillary Agreements to which each Purchaser is a party will be, on or prior to the date of signing such Ancillary Agreement, duly and validly executed and delivered by each Purchaser, and, assuming the due authorization and execution of this Agreement by Sellers, this Agreement constitutes, and, assuming the due authorization and execution of the other parties to each Ancillary Agreement, each Ancillary Agreement will constitute, the legal and binding obligation of Purchasers, enforceable against each Purchaser in accordance with its terms, except as limited by the Enforceability Exceptions.
(d) Neither the execution, delivery and performance of this Agreement and the Ancillary Agreements, nor the consummation of the transactions contemplated hereby and thereby, will (i) conflict with or violate any provision of any Governing Documents of Purchasers, (ii) constitute or result in a default under, violate any provision of, or be an event that is (or with the passage of time will result in) a violation of, or result in the acceleration of or entitle any party to accelerate or exercise (whether after the giving of notice or lapse of time or both) any obligation or right under, or result in the imposition of any Lien, any material Contract, instrument, order, arbitration award, judgment or decree to which any Purchaser is a party or by it is bound, or (iii) violate or conflict with any Law or other restriction of any kind or character to which any Purchaser is subject, that, in the case of clauses (ii) or (iii) would, individually or in the aggregate, have or reasonably be expected to have a material adverse effect on the ability of Purchasers to consummate the transactions contemplated by this Agreement, the Ancillary Agreements, and the Debt Financing Agreements.
(e) Recro is the sole beneficial and record owner of all of the outstanding membership interests of Acquisition Sub.
4.2 No Consents. Section 4.2 of the Purchasers Disclosure Letter contains a list of all registrations, filings, applications, notices, consents, approvals, orders, qualifications and waivers required to be made, filed, given or obtained by Purchasers or any of their Subsidiaries with, to or from any Persons or Governmental Entities in connection with the consummation of this Agreement or the Ancillary Agreements or the other transactions contemplated hereby or thereby, except for those with respect to which the failure to make, file, give or obtain would not, individually or in the aggregate, have a material adverse effect on the ability of Purchasers to consummate the transactions contemplated by this Agreement, the Ancillary Agreements and the Debt Financing Agreements.
4.3 Compliance with Laws. The conduct of the business of Purchasers and their Subsidiaries complies in all material respects with all Laws which would affect their ability to perform their obligations hereunder and under the Ancillary Agreements and the Debt Financing Agreements.
4.4 Brokers; Finders. No broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the transactions contemplated by this Agreement based upon arrangements made by or on behalf of Purchasers.
4.5 Acquisition of Transferred Interests for Investment. Purchasers have such knowledge and experience in financial and business matters, and are capable of evaluating the merits and risks of its purchase of the Transferred Interests. Purchasers confirms that Sellers
-41-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
have made available to Purchasers and Purchasers’ agents the opportunity to ask questions of the officers and management employees of Sellers and of the Transferred Entities as well as access to the documents, information and records of Sellers and the Transferred Entities and to acquire additional information about the business and financial condition of Sellers and the Transferred Entities, and each Purchaser confirms that it has made an independent investigation, analysis and evaluation of the Transferred Entities and their properties, assets, business, financial condition, prospects, documents, information and records. Purchasers are acquiring the Transferred Interests for investment and not with a view toward or for sale in connection with any distribution thereof, or with any present intention of distributing or selling the Transferred Interests. Purchasers acknowledge that the Transferred Interests have not been registered under the Securities Act or any state securities Laws, and agrees that the Transferred Interests may not be sold, transferred, offered for sale, pledged, hypothecated or otherwise disposed of without registration under the Securities Act, except pursuant to an exemption from such registration available under the Securities Act, and without compliance with foreign securities Laws, in each case, to the extent applicable.
4.6 Debt Financing.
(a) Purchasers have delivered to Sellers true and complete copies of the executed definitive agreements dated as of the date hereof (as they may be amended, restated or modified from time to time in accordance with the terms hereof, collectively, the “Debt Financing Agreements”) entered into with the lender party to the Debt Financing Agreements (the “Lender”) relating to the commitment of the Lender to provide the full amount of the Initial Purchase Price and all related fees and expenses, collectively referred to in this Agreement as the “Debt Financing”. At Closing, Purchasers will fully pay or cause to be fully paid any and all commitment fees and other fees required to be paid pursuant to the terms of the Debt Financing Agreements.
(b) Except as set forth in the Debt Financing Agreements, there are no conditions precedent or other contingencies to the obligations of the Lender to provide the Debt Financing or any contingencies that would permit the Lender to reduce the total amount of the Debt Financing.
(c) The Debt Financing, when funded in accordance with the terms of the Debt Financing Agreements, shall provide Purchasers with acquisition financing on the Closing Date sufficient to pay the Initial Purchase Price and to pay related fees and expenses.
(d) The Debt Financing Agreements are valid, binding and in full force and effect and no event has occurred that, with or without notice, lapse of time, or both, would reasonably be expected to constitute a default or breach or a failure to satisfy a condition precedent on the part of Purchasers under the terms and conditions of the Debt Financing Agreements, other than any such default, breach or failure that has been waived by the Lender or otherwise cured in a timely manner by Purchasers to the satisfaction of the Lender and Purchasers do not have any reason to believe that they will be unable to satisfy on a timely basis any term or condition to closing to be satisfied by it in the Debt Financing Agreements on or prior to the Closing Date.
(e) As of the Closing, and after giving effect to all of the transactions contemplated by this Agreement, Purchasers will be Solvent.
-42-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
4.7 Regulatory Matters. Purchasers have not, nor has any Affiliate or representative of Purchasers, been convicted of any crime or engaged in any conduct for which debarment is mandated by 21 U.S.C. §335a(a) or any similar applicable Laws or authorized by 21 U.S.C. §335a(b) or any similar applicable Laws. The Purchasers have not, nor has any Affiliate or representative of Purchasers, been convicted of any crime or engaged in any conduct for which such Person could be excluded from participating in any U.S. federal health care programs and each of the Purchasers has appropriate policies and restrictions in its agreements with third parties precluding the use of any individuals convicted of any crimes or engaged in any conduct for which such Person could be excluded from participating in any U.S. federal health care programs.
4.8 No Other Representations or Warranties. Except for the representations and warranties contained in Article III (including the Schedules and Exhibits to this Agreement), Purchasers acknowledge that (i) none of Sellers, any Transferred Entity nor any of their respective Affiliates and Representatives, nor any other Person, made or shall be deemed to have made any representation or warranty to Purchasers, express or implied, at law or in equity, on behalf of Sellers or any Transferred Entity or any Affiliate of Sellers or any Transferred Entity and (ii) Purchasers have not relied on any information, representations, warranties or financial projections contained in any document, confidential memorandum or agreement delivered to it or made available by the Sellers or their Representatives, including any information, document or materials made available or provided to Purchasers in the data room.
ARTICLE V
COVENANTS
5.1 Access to Books and Records.
(a) After the date of this Agreement until the earlier of the Closing or termination of this Agreement, Sellers shall afford to Representatives of Purchasers reasonable access to the Books and Records of the Transferred Entities’ Businesses during normal business hours consistent with applicable Law and in accordance with the procedures established by Sellers; provided, however, that (i) no Seller or Transferred Entity shall be required to violate any obligation of confidentiality to which a Seller or a Transferred Entity or any of their respective Affiliates may be subject in discharging their obligations pursuant to this Section 5.1(a), and (ii) Sellers shall make available, or cause the Transferred Entities to make available, Transferred Entity Employee personnel files only after the Closing Date. Any information provided to Purchasers or their Representatives in accordance with this Section 5.1 or otherwise pursuant to this Agreement shall be held by Purchasers and their Representatives in accordance with, shall be considered under, and shall be subject to the terms of, the Confidentiality Agreement.
(b) Purchasers agree that any permitted investigation undertaken by Purchasers pursuant to the access granted under Section 5.1(a) shall be conducted in such a
-43-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
manner as not to interfere unreasonably with the operation of the Business by Sellers or the Transferred Entities, and Purchasers and their representatives shall not communicate with any of the employees of Sellers or the Transferred Entities without the prior written consent of Sellers. Notwithstanding anything to the contrary in this Agreement, neither Sellers nor the Transferred Entities shall be required to provide access to or disclose information where, upon the advice of counsel, such access or disclosure would jeopardize the attorney-client privilege of such Party or any of its Affiliates or contravene any Laws.
(c) At and after the Closing Date, Purchasers shall, and shall cause their Affiliates to, afford Sellers and their representatives, during normal business hours, upon reasonable notice, full access to the books, records, properties and employees of each Transferred Entity to the extent that such access may be reasonably requested by Sellers, including in connection with financial statements or a proceeding before the Independent Accounting Firm under Section 2.5(d).
(d) Purchasers agree to hold all the Books and Records of each Transferred Entity’s Business existing on the Closing Date and not to destroy or dispose of any thereof for a period of seven (7) years from the Closing Date or such longer time as may be required by Law, and thereafter, if they desire to destroy or dispose of such Books and Records, to offer first in writing at least sixty (60) days prior to such destruction or disposition to surrender them to Sellers.
5.2 Efforts.
(a) Subject to the terms and conditions herein provided, each of Purchasers and Sellers shall use reasonable best efforts to promptly take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable under this Agreement and applicable Laws to consummate and make effective as promptly as practicable after the date hereof the transactions contemplated by this Agreement, including (i) preparing as promptly as practicable all necessary applications, notices, petitions, filings, ruling requests, and other documents and to obtain as promptly as practicable all consents, waivers, licenses, orders, registrations, approvals, permits, rulings, authorizations and clearances necessary or advisable to be obtained from any Governmental Entity in order to consummate the transactions contemplated by this Agreement (collectively, the “Governmental Approvals”) and (ii) as promptly as practicable taking all steps as may be necessary to obtain all such Governmental Approvals. In furtherance and not in limitation of the foregoing, each Party agrees to (A) within ten (10) Business Days of the date of this Agreement, make all necessary filings and submissions under the HSR Act, (B) make all other required filings pursuant to other antitrust or competition Laws with respect to the transactions contemplated hereby as promptly as practicable, and (C) not extend any waiting period under the HSR Act or any other antitrust Law, nor enter into any agreement with the United States Federal Trade Commission (the “FTC”) or the United States Department of Justice (the “DOJ”) or any other Governmental Entity not to consummate the transactions contemplated by this Agreement, except with the prior written consent of the other Parties (which shall not be unreasonably withheld, conditioned or delayed). Each Party shall supply as promptly as practicable any additional information or documentation that may be requested pursuant to the HSR Act or any other antitrust or competition Law and use its reasonable best efforts to take all other actions necessary, proper or advisable to cause the
-44-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
expiration or termination of the applicable waiting periods under the HSR Act and any other antitrust Law as soon as possible. The Parties agree to request early termination with respect to the waiting period prescribed by the HSR Act together with the initial filings and submissions under the HSR Act.
(b) Each of Purchasers and Sellers shall, in connection with the actions referenced in Section 5.2(a) to obtain all Governmental Approvals for the transactions contemplated by this Agreement under the HSR Act or any other antitrust or competition Law, (i) cooperate in all respects with each other in connection with any communication, filing or submission and in connection with any investigation or other inquiry, including any proceeding initiated by a private party; (ii) keep the other Party and/or its counsel informed of any communication received by such Party from, or given by such Party to, the FTC, the DOJ or any other U.S. or other Governmental Entity and of any communication received or given in connection with any proceeding by a private party, in each case regarding any of the transactions contemplated hereby; (iii) consult with each other in advance of any meeting or conference with the FTC, the DOJ or any other Governmental Entity or, in connection with any proceeding by a private party, with any other Person, and to the extent permitted by the FTC, the DOJ or such other Governmental Entity or other Person, give the other Parties and/or their counsel the opportunity to attend and participate in such meetings and conferences; and (iv) permit the other Parties and/or their counsel to review in advance any submission, filing or communication (and documents submitted therewith) intended to be given by it to the FTC, the DOJ or any other Governmental Entity; provided, that materials may be redacted to remove references concerning the valuation of the businesses of Sellers, to the extent permitted by Law. Purchasers and Sellers, as each deems advisable and necessary, may reasonably designate any competitively sensitive material to be provided to the other under this Section 5.2(b) as “Antitrust Counsel Only Material.” Such materials and the information contained therein shall be given only to the outside antitrust counsel of the recipient and will not be disclosed by such outside counsel to employees, officers or directors of the recipient unless express permission is obtained in advance from the source of the materials (Purchasers or Sellers, as the case may be) or its legal counsel.
(c) In furtherance and not in limitation of the covenants of the Parties contained in Section 5.2(a) and Section 5.2(b), each of Purchasers and Sellers shall use their reasonable best efforts to avoid the entry of, or to have vacated, lifted, reversed or overturned, any decree, judgment, injunction or other order, whether temporary, preliminary or permanent, that would restrain, prevent or delay the Closing on or before the Outside Date. It shall not be deemed a failure to satisfy the conditions specified in Section 8.1(a) if, as a result of any suit brought by any Person or Governmental Entity challenging the transactions contemplated by this Agreement as violating any antitrust Law, a court enters or the applicable Governmental Entity makes an order or decree permitting the transactions contemplated by this Agreement, but requiring that any of the businesses, product lines or assets of Purchasers or their Affiliates (collectively, the “Subject Assets”) be divested or held separate by Purchasers, or that would otherwise limit Purchasers’ freedom of action with respect to, or their ability to operate and retain, the Subject Assets.
(d) Without limiting any other obligation under this Agreement, during the period from the date of this Agreement until the Closing Date, each of Purchasers and Sellers shall not, and shall cause its Subsidiaries not to, take or agree to take any action that would
-45-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
reasonably be expected to prevent or delay the Parties from obtaining any Governmental Approval in connection with the transactions contemplated by this Agreement, or to prevent or materially delay or impede the consummation of the transactions contemplated herein.
(e) Purchasers agree to provide such security and assurances as to financial capability, resources and creditworthiness as may be reasonably requested by any Governmental Entity or other third party whose consent or approval is sought in connection with the transactions contemplated hereby. Whether or not the Sale is consummated, Purchasers and Sellers shall each be responsible for 50% of all filing fees and payments to any Governmental Entity in order to obtain any consents, approvals or waivers pursuant to this Section 5.2.
5.3 Further Assurances. Sellers and Purchasers agree that, from time to time, whether before, at or after the Closing Date, each of them will execute and deliver such further instruments of conveyance and transfer and take such other action as may be necessary to carry out the purposes and intents of this Agreement. Without limiting the generality of the foregoing:
(a) Between the date hereof and the Closing, Sellers shall, and shall cause their Affiliates to, (A) effect the Reorganization as described in Exhibit G, including transferring all of the assets and liabilities of Daravita, other than those listed on Section 5.3 of the Sellers Disclosure Letter, to Newco, or as otherwise mutually and reasonably agreed upon by Sellers and Purchasers, and (B) use commercially reasonable efforts to ensure that, as of the Closing, Newco and Alkermes Gainesville hold no other assets or liabilities (including assets or liabilities relating to Excluded Assets) other than those of the Business. Sellers shall make available to Purchasers in a timely manner for review and comment all drafts of the agreements relating to the Reorganization (collectively, the “Reorganization Transfer Agreements”) or other instruments or documentation relating to the Reorganization, and Sellers shall not, and shall cause its Affiliates not to, execute any such Reorganization Transfer Agreements or other instruments or documentation, or to take any actions or consummate any steps or transactions contemplated thereby, in each case, that is not reasonably satisfactory to Purchasers.
(b) In connection with the Reorganization, APIL and Newco will enter into a license agreement (the “IP License Agreement”), in form reasonably acceptable to the Parties, granting to Newco licenses in substantially similar form and scope as those granted to Daravita pursuant to the Intellectual Property Transfer and License Agreement. The Parties hereto agree to amend the provisions regarding Earn-Out Consideration set forth on Exhibit E hereto as may be reasonably necessary in order to effect the intent of the Parties upon entry into the IP License Agreement.
(c) After the consummation of the Reorganization, to the extent that Daravita receives any payments for accounts receivable owned by Newco, Daravita will transfer such payments to an account designated by Newco.
(d) After the Closing, Purchasers will, and will cause their Affiliates to, upon the discovery of any Excluded Assets in the Transferred Entities’ properties, offices, plants, storage spaces or similar locations, use reasonable efforts to return such Excluded Assets to Sellers at Sellers’ expense.
-46-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
5.4 Conduct of Business.
(a) From the date of this Agreement through the earlier of the Closing or the termination of this Agreement, except as otherwise contemplated by this Agreement (including transactions required by the Reorganization), as required by Law, as disclosed in Section 5.4 of the Sellers Disclosure Letter, or with Purchasers’ written consent (which shall not be unreasonably withheld, conditioned or delayed; provided that Purchasers shall be deemed to have consented in writing to any written request by Sellers to which Purchasers fail to respond within five (5) Business Days following receipt), Sellers shall cause each Transferred Entity to:
(i) conduct the Business in the ordinary course of business; and
(ii) use reasonable best efforts to preserve intact their respective business organizations and goodwill, keep available the services of their respective present senior officers and key employees, and preserve the goodwill and business relationships with customers and others having business relationships with them.
(b) Without limiting the generality of Section 5.4(a), from the date of this Agreement through the earlier of the Closing or the termination of this Agreement, except as otherwise contemplated by this Agreement (including transactions required by the Reorganization, including without limitation the accession of Newco to the Credit Agreement), as required by Law, as disclosed in Section 5.4 of the Sellers Disclosure Letter or with Purchasers’ consent (which shall not be unreasonably withheld, conditioned or delayed (provided that Purchasers shall be deemed to have consented in writing to any written request by Sellers to which Purchasers fail to respond within five (5) Business Days following receipt)), Sellers shall cause the Transferred Entities not to take any of the following actions with respect to the Business:
(i) (A) amend or propose to amend their respective certificates of incorporation or by-laws or equivalent organizational documents, (B) adjust, split, combine or reclassify their outstanding capital stock, or (C) declare, set aside or pay any non-cash dividend or non-cash distribution;
(ii) merge or consolidate itself with any other Person, or restructure, reorganize or completely or partially liquidate itself;
(iii) issue, sell, pledge, encumber or dispose of, or agree to issue, sell, pledge, encumber or dispose of, any additional shares of, or any options, warrants or rights of any kind to acquire any shares of their capital stock of any class or any debt or equity securities which are convertible into or exchangeable for such capital stock;
(iv) except for transactions among the Transferred Entities in the ordinary course, (A) make any acquisition of any assets other than acquisitions made in response to a force majeure event or emergency or (B) sell, pledge, dispose of or encumber any material assets or businesses other than sales or dispositions of assets in the ordinary course of business;
-47-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(v) enter into or amend any employment, severance, special pay arrangement or other similar arrangements or agreements with any directors, officers or other employees of the Transferred Entities, so as to increase or accelerate benefits, except (A) pursuant to applicable Law, (B) pursuant to contractual arrangements or policies in effect as of the date of this Agreement, (C) 401(k) plan loans in the ordinary course of business, or (D) the Retention Bonuses;
(vi) increase the cash compensation of any senior officer or key employee of the Transferred Entities, except for increases in the ordinary course of business or except pursuant to contractual or incentive compensation arrangements in effect as of the date of this Agreement;
(vii) enter into, modify, amend or terminate any Business Material Contract, or waive, release, compromise or assign any material rights or claims under any Business Material Contract or any material lease (other than (A) in the ordinary course of business and (B) terminations of contracts and leases as a result of the expiration of the term of such contracts or leases);
(viii) purchase all or substantially all of the assets of, any securities of or make any investment in, either by purchase of stock or other securities or by contributions to capital, any Person, or acquire direct or indirect control over any Person;
(ix) make any new Tax election or modify or revoke any existing Tax election, change any Tax or accounting methods or systems of internal accounting controls of Newco or Alkermes Gainesville (except as may be required to conform to Laws relating to Taxes or regulatory accounting requirements or GAAP), file any amended Tax Return, enter into any Tax indemnity, sharing or allocation agreement, surrender any right to claim a refund, offset or other reduction of Taxes, consent to any extension or waiver of the limitations period applicable to any Tax claim or assessment relating to Newco or Alkermes Gainesville, or settle or compromise any Tax claim;
(x) commence any Action other than in accordance with past practice;
(xi) settle any Action involving any Liability of any Transferred Entity for money damages where the settlement amount exceeds Two Hundred Fifty Thousand Dollars ($250,000) or where the settlement would include any restriction upon the operations of any Transferred Entity;
(xii) enter into any line of business in which the Transferred Entities do not participate or engage as of the date hereof;
(xiii) divest, sell, lease, license, transfer or otherwise dispose or permit the cancellation, abandonment, or dedication to the public domain of any Business Intellectual Property, other than: (i) in the ordinary course of business and, in the case of patents included in the Business Intellectual Property, pursuant to the expiration of their statutory term; and (ii) transfers of Intellectual Property Rights from one Transferred Entity to another Transferred Entity;
-48-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(xiv) sell, assign, transfer, convey, pledge or otherwise dispose of or encumber any accounts receivable, except in the ordinary course of business;
(xv) fail to pay its accounts payable and similar debts in the ordinary course of business;
(xvi) fail to maintain the assets of the Transferred Entities in substantially their current state of repair, excepting normal wear and tear; or
(xvii) authorize or enter into any agreement or otherwise make any commitment to do any of the foregoing.
Notwithstanding anything to the contrary in this Agreement, including this Section 5.4, the Sellers and the Transferred Entities may divest any and all Excluded Assets prior to Closing.
5.5 Consents. Seller shall use commercially reasonably efforts to obtain any consents required from third parties in connection with the consummation of the transactions contemplated by this Agreement pursuant to the Business Material Contracts.
5.6 Public Announcements. Except as otherwise required by Law, each of Sellers and Purchasers will consult with the other and obtain the consent of the other (which consent shall not be unreasonably withheld, conditioned or delayed) before issuing any press releases or any public statements with respect to this Agreement and the transactions contemplated by this Agreement, except as may be required by Law or stock exchange rules, in which case the Party required to publish such press release or public announcement or make such other communication shall use reasonable efforts to provide the other Parties a reasonable opportunity to comment on such press release or public announcement in advance of the time of disclosure of such publication or such other communication.
5.7 Intercompany Accounts. At or prior to the Closing, all intercompany accounts between each Seller and/or any of its Affiliates (other than any Transferred Entity), on the one hand, and each Transferred Entity, on the other hand, shall be settled or otherwise eliminated. This provision shall not apply to intercompany accounts between and among the Transferred Entities.
5.8 Termination of Intercompany Agreements. Effective at the Closing, all Related Party Agreements shall be terminated, except for (i) this Agreement, (ii) the Transition Services Agreement, (iii) the Supply Agreements and (iv) the Contracts listed in Section 5.8 of the Sellers Disclosure Letter.
5.9 Insurance. From and after the Closing Date, the Transferred Entities shall cease to be insured by Parent or its respective Affiliates’ (other than the Transferred Entities’) insurance policies or by any of their self-insured programs, and shall have no access to any such insurance policies other than as set forth in this Agreement and the Transition Services Agreement. Each Seller or any of its respective Affiliates may, to be effective as of the Closing, amend any insurance policies in the manner it deems appropriate to give effect to this Section 5.9. From and after the Closing, Purchasers shall be responsible for securing all insurance it considers appropriate for its operation of the Transferred Entities and the Business.
-49-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
At the Closing Date, Sellers agree to take over and assume all the known and incurred claims of the Transferred Entities and the Business set forth on Section 5.9 of the Sellers Disclosure Letter, which have been incurred as of the Closing Date, and Sellers agrees to be responsible to pay such claims until they are finally settled. Purchasers further covenant and agree not to seek to assert or to exercise any rights or claims of the Transferred Entities or the Business under or in respect of any past or current insurance policy under which any member of the Transferred Entities or any Affiliate thereof or the Business is a named insured.
5.10 Litigation Support. In the event and for so long as Sellers actively are prosecuting, contesting or defending any Action by a Person in connection with (a) any transactions contemplated under this Agreement or (b) any fact, situation, circumstance, status, condition, activity, practice, plan, occurrence, event, incident, action, failure to act, or transaction involving the Business or the Transferred Entities, Purchasers shall, and shall cause their Affiliates to, cooperate with Sellers and their respective counsel in the prosecution, contest or defense, make available its personnel, and provide such testimony and access to its Books and Records and such other files, documents and records as shall be reasonably necessary in connection with the prosecution, contest or defense; provided that, such access and cooperation shall not unreasonably interfere with the ongoing operations of Purchasers, the Transferred Entities and their Affiliates; and provided, further, that Seller shall promptly, upon request by Purchasers, the Transferred Entities or their Affiliates, reimburse Purchasers, the Transferred Entities or their Affiliates for all reasonable and documented out-of-pocket costs incurred by them in connection with such cooperation and access.
5.11 Payments.
(a) Sellers shall promptly pay or deliver to Purchasers any monies or checks which have been sent to Sellers or their respective Affiliates after the Closing Date by customers, suppliers or other contracting parties of the Transferred Entities and the Business and which should have been sent to Purchasers.
(b) Purchasers shall promptly pay or deliver to Sellers any monies or checks which have been sent after the Closing Date to Purchasers to the extent they are not due to the Business or the Transferred Entities or should have otherwise been sent to any Seller or any of their respective Affiliates, other than the Transferred Entities (including promptly forwarding invoices or similar documentation to Sellers).
5.12 Debt Financing.
(a) Without limiting the generality of Section 5.2, Purchasers shall use their reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable to obtain the proceeds of the Debt Financing on the terms and conditions described in the Debt Financing Agreements. Purchasers shall use their reasonable best efforts to comply with its obligations under the Debt Financing Agreements, and shall use its reasonable best efforts to cause the Debt Financing to be fully funded on the Closing Date, including by enforcing its rights under the Debt Financing Agreements and drawing on any interim or bridge financing in the event that other elements of the Debt Financing are not available. Purchasers shall give Sellers prompt notice of any material breach by any party to the
-50-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Debt Financing Agreements of which Purchasers have become aware or any termination of the Debt Financing Agreements. In the event that any portion of the Debt Financing becomes unavailable, regardless of the reason therefor, Purchasers will (x) use their reasonable best efforts to obtain alternative debt financing (in an amount sufficient to pay the Initial Purchase Price and Closing Adjustment) on terms not materially less favorable, taken as a whole, to Purchasers from other sources and which do not include any conditions to the consummation of such alternative debt financing that are materially more onerous than the conditions set forth in the Debt Financing (such financing, “Alternative Financing”), and (y) promptly notify Sellers of such unavailability and the reason therefor.
(b) Notwithstanding anything to the contrary in this Agreement, Purchasers shall not, without the prior written consent of Sellers, (i) amend, modify, supplement or waive any of the conditions to funding contained in the Debt Financing Agreements or any other provision thereof or remedies thereunder, in each case to the extent such amendment, modification, supplement or waiver would reasonably be expected to adversely affect the ability of Purchasers to timely consummate the transactions contemplated by this Agreement (including by making the conditions herein less likely to be satisfied or unreasonably delaying the Closing); (ii) undertake any merger, acquisition, joint venture, disposition, lease, contract or debt or equity financing that would reasonably be expected to impair, delay or prevent consummation of the Debt Financing contemplated by the Debt Financing Agreements or any Alternative Financing contemplated by any new debt commitment letter; or (iii) amend or alter, or agree to amend or alter, the Debt Financing Agreements in any manner that would reasonably be expected to prevent, impair or delay the consummation of the Debt Financing or the transactions contemplated by this Agreement.
(c) Prior to the Closing, Sellers shall use commercially reasonable efforts to, and to cause the Transferred Entities and their respective officers, employees and advisors, including legal, financial and accounting advisors, of Sellers and the Transferred Entities to, provide to Purchasers such cooperation as is reasonably requested by Purchasers and the Lenders in connection with the Debt Financing (provided that such requested cooperation does not unreasonably interfere with the ongoing operations of Sellers and their Affiliates), including (i) furnishing Purchasers and the Lender with financial and other pertinent information; (ii) in each case, upon reasonable notice, making management of the Transferred Entities (including some members of the financial staff) available to participate in a reasonable number of meetings (including customary one-on-one meetings with parties acting as lead arrangers or agents for, and prospective lenders and purchasers of, any such financing), presentations and due diligence sessions in connection with such financing; (iii) assisting Purchasers and the Lender in the preparation of (A) a customary offering document, private placement memorandum and/or bank information memorandum and similar marketing documents for any of the Debt Financing; and (B) materials for rating agency presentations; (iv) using commercially reasonable efforts to cause its independent auditors to cooperate with the Debt Financing; (v) taking all actions reasonably necessary that are consistent with the terms of this Agreement or otherwise facilitating the pledging of collateral of the Transferred Entities in respect of the Business from and after the Closing as may be reasonably requested by Purchasers; (vi) promptly furnishing all documentation and other information about the Transferred Entities required by Governmental Entities with respect to the financing under applicable “know your customer” and anti-money laundering rules and regulations including without limitation the USA Patriot Act; and (vii)
-51-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
taking all corporate or limited liability company actions, subject to the occurrence of the Closing, reasonably requested to permit the consummation of any such financing and to permit the proceeds thereof to be made available to the Transferred Entities, including entering into one or more credit agreements, indentures or other instruments on terms reasonably satisfactory to Purchasers in connection therewith; provided that neither Sellers nor any of their Affiliates shall be required to pay any commitment or other similar fee, provide any security or incur any other liability in connection with the Debt Financing; provided, further, that the effectiveness of any documentation executed by any Seller with respect thereto shall be subject to the consummation of the Closing; and provided, further, that Purchasers shall promptly, upon request by Sellers, reimburse Sellers for all reasonable and documented out-of-pocket costs incurred by Sellers or any of their Affiliates in connection with such cooperation. Any information provided to Purchasers, or on behalf of or at the request of Purchasers, pursuant to this Section 5.12(c) shall be subject to the Confidentiality Agreement and Section 5.2.
5.13 Directors and Officers Indemnification. The Parties agree that all rights to exculpation, indemnification and advancement of expenses for acts or omissions occurring at or prior to the Closing, whether asserted or claimed prior to, at or after the Closing, now existing in favor of the current or former directors, officers or employees, as the case may be, of the Transferred Entities (whether provided in the respective Governing Documents of such entity or in any agreement as in effect on the date of this Agreement) shall survive the Closing and remain in full force and effect.
5.14 Non-Competition; Non-Solicitation.
(a) Until the second (2nd) anniversary of the Closing, Sellers shall not, directly or indirectly, (i) solicit for employment or any similar arrangement any Transferred Entity Employee or (ii) hire any Transferred Entity Employee; provided, that this Section 5.14(a) shall (a) not apply to Transferred Entity Employees whose employment has been terminated by Purchasers, (b) not prohibit general solicitations for employment through advertisements or other means not targeted specifically to Transferred Entity Employees; and (c) shall not prevent the hiring of Transferred Entity Employees that respond to general solicitations such as those specified in (b) above.
(b) During the period beginning on the Closing Date and ending on the third (3rd) anniversary of the Closing Date (the “Non-Compete Period”), except for ownership of the equity in Recro issued pursuant to the Warrant, Sellers and their Affiliates agree not to directly or indirectly engage in, or have an ownership interest in, any business or enterprise (or subsidiary or division thereof) that engages in the development, license, manufacture, testing, packaging, storage, sale and shipment of the Products (other than Meloxicam) or the underlying molecules or salts thereof in combination with the OCR IP covering such Products (a “Competing Business”). If Sellers and/or their Affiliates are directly or indirectly acquired by (whether by merger, acquisition of assets or equity, or otherwise), or directly or indirectly acquire (whether by merger, acquisition of assets or equity, or otherwise), a third party which engages in a Competing Business, such third party and its Affiliates (other than Sellers and/or their Affiliates existing prior to the date of such acquisition) shall not be restricted from continuing to engage in such Competing Business pursuant to this Section 5.14(b), provided that the rights of such third party and its Affiliates to utilize the OCR IP in such Competing Business existed prior to the date of such acquisition.
-52-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(c) Each Party acknowledges and agrees that the provisions of this Section 5.14 are reasonable and necessary to protect the legitimate business interests of the other Party, including without limitation such Party’s confidential information and goodwill. Each Party agrees, and shall not contest, that the other Party’s remedies at law for any breach or threat of breach by such Party or its Affiliates of the provisions of this Section 5.14 will be inadequate, and that the other Party shall be entitled to an injunction or injunctions to prevent breaches of the provisions of this Section 5.14 and to enforce specifically such terms and provisions, in addition to any other remedy to which the other Party may be entitled at law or in equity. The restrictive covenants contained in this Section 5.14 are covenants independent of any other provision of this Agreement or other agreement between the Parties and the existence of any claim which a Party may allege against another Party under any provision of this Agreement, any other Agreement, or otherwise will not prevent the enforcement of the covenants in this Section 5.14. If any of the provisions contained in this Section 5.14 shall for any reason be held to be excessively broad as to duration, scope, activity or subject, then such provision shall be construed by limited and reducing it, so as to be valid and enforceable to the extent compatible with applicable Law or the determination by a court of competent jurisdiction. The Parties agree and intent that a Party’s obligations under this Section 5.14 will be tolled during any period that such party is found to be in breach of any of the obligations under this Section 5.14, so that the other Party is provided with the full benefit of the restrictive periods set forth herein.
5.15 Indebtedness/Lien Release. Sellers shall cause the Transferred Entities to have no Indebtedness. Simultaneously with Closing, Sellers shall cause any lender or representative thereof and any lienholder to release any and all Liens on the assets and properties of either of the Transferred Entities, other than Permitted Liens, it being understood that filings (including mortgage filings and UCC terminations) to terminate Liens as a matter of record will be filed after Closing.
5.16 Additional Financial Statements. Sellers shall use commercially reasonable efforts to, within 60 days of Closing, provide to Purchasers audited consolidated statements of income, balance sheets and statements of cash flows of the Business (i) as of December 31, 2013 and for the nine-month period then ended and (ii) as of December 31, 2014 and for the twelve-month period then ended. If the Closing Date is after March 31, 2015, Sellers shall use commercially reasonable efforts to also provide to Purchasers unaudited consolidated statements of income, balance sheets and statements of cash flows of the Business (i) as of March 31, 2015 and for the three-month period then ended and (ii) as of March 31, 2014 and for the three-month period then ended.
5.17 Change of Name of Transferred Entities. As soon as possible following the Closing Date, Purchasers shall eliminate the use of all of the trademarks, tradenames, service marks and service names related to “ALKERMES”, in any of their forms or spellings, in the names of the Transferred Entities and on all advertising, purchase orders and acknowledgements, customer agreements and other Contracts retained by Purchasers following the Closing.
-53-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
5.18 Transition Services Agreement and Supply Agreements. The Parties shall, and shall cause their Affiliates to, negotiate in good faith to finalize (i) the Transition Services Agreement, on terms and conditions consistent with Exhibit A and (ii) the Supply Agreements, on terms and conditions consistent with Exhibit B.
5.19 Exclusivity. The Sellers agree that between the date of this Agreement and the earlier of the Closing and the termination of this Agreement pursuant to its terms, the Sellers shall not, and shall take all action necessary to ensure that none of the Sellers’ Affiliates or any of their respective Representatives shall, (a) directly or indirectly, solicit, initiate, encourage or accept any proposal or offer that constitutes an Acquisition Proposal, (b) participate in any discussions, conversations, negotiations or other communications regarding, or otherwise cooperate in any way, assist or participate in, or facilitate or knowingly encourage the submission of, any proposal that constitutes, or would reasonably be expected to lead to, an Acquisition Proposal or (c) furnish to any Person other than Purchasers any information regarding the Transferred Entities in response to an Acquisition Proposal or an inquiry or indication of interest for the purpose of making or pursuing an Acquisition Proposal. The Sellers immediately shall cease and cause to be terminated all existing discussions, conversations, negotiations and other communications with any Persons conducted heretofore with respect to any Acquisition Proposal.
5.20 Solvency. For a period of two years following the Closing Date, Recro and its Subsidiaries, taken as a whole, will maintain sufficient capital to operate the Business in the ordinary course of business and to pay its debts and perform obligations (including under the Agreement and Ancillary Agreements) as they come due.
5.21 Data Room. Within ten (10) days of the date hereof, Sellers shall deliver to Purchasers a disk containing copies of all documents contained in the virtual data room maintained by Sellers as of the date hereof in connection with the sale of the Business.
ARTICLE VI
EMPLOYEE MATTERS COVENANTS
6.1 Benefit Continuation. For a period of one (1) year from the Closing Date (or for such longer period as and to the extent required by applicable Law), Purchasers shall, or shall cause its Affiliates to, provide to each Transferred Entity Employee, (a) base salary or wages and target bonus opportunities (excluding, without limitation, any retention, change in control or equity incentive compensation) that are, in each case, no less favorable than those provided to such Transferred Entity Employee immediately prior to the Closing Date, (b) equity or long-term incentive compensation opportunities that are substantially similar to those provided to similarly-situated employees of entities similarly-situated to Purchasers, and (c) other employee benefits and terms and conditions of employment that are no less favorable than those provided to similarly situated employees of Purchasers or their applicable Affiliate. No later than the Closing Date, Purchasers and/or their applicable Affiliates shall establish Purchaser Plans (as defined below) to the extent necessary to comply with their obligations under this Article VI.
-54-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
6.2 Service Credit. From and after the Closing Date, Purchasers and their Affiliates shall (a) recognize, for all purposes under all plans, programs and arrangements established or maintained by Purchasers or any of their Affiliates for the benefit of each Transferred Entity Employee (each, a “Purchaser Plan”) service with Sellers and their Affiliates and predecessors prior to the Closing Date to the extent such service was recognized under the corresponding Benefit Plan of Sellers or their Affiliates covering such Transferred Entity Employee as of immediately prior to the Closing Date, including, for purposes of eligibility, vesting and benefit levels and accruals, including, without limitation, for purposes of vacation accruals, (b) waive any pre-existing condition exclusion, actively-at-work requirement or waiting period under all applicable Purchaser Plans except to the extent such pre-existing condition, exclusion, requirement or waiting period would have applied to such Transferred Entity Employee under the corresponding Benefit Plan of Sellers or their Affiliates covering such Transferred Entity Employee as of immediately prior to the Closing Date, and (c) provide full credit for any co-payments, deductibles or similar payments made or incurred with respect to each Transferred Entity Employee prior to the date of this Agreement for the plan year in which the Closing Date occurs.
6.3 Employment Continuation. Notwithstanding the covenants of Purchasers and their Affiliates set forth in Sections 6.1 and 6.2, nothing contained in this Article VI: (i) shall limit or condition the ability of Purchasers, the Transferred Entities, or any of their respective Affiliates to terminate, either with or without “cause,” the employment of any Transferred Entity Employees at any time; (ii) shall alter or limit the ability of Purchasers, the Transferred Entities, or any of their respective Affiliates to amend, modify or terminate any benefit plan, program, agreement or arrangement at any time assumed, established, sponsored or maintained by any of them; or (iii) is intended to confer upon any current or former employee or any Person any right to employment or continued employment for any period of time by reason of this Agreement, or any right to a particular term or condition of employment.
6.4 Retention Bonuses.
(a) On or prior to the Closing Date, Sellers shall pay to each Transferred Entity Employee listed on Schedule 6.4(a) of the Sellers Disclosure Letter the bonus amounts listed opposite such Transferred Entity Employee’s name (collectively, the “Initial Retention Bonuses”). The Retention Bonuses, once paid, will not be included in the calculation of Working Capital.
(b) Sellers shall pay directly to each Transferred Entity Employee listed on Schedule 6.4(b) of the Sellers Disclosure Letter, and be responsible for the employer portion of any payroll and employment taxes relating thereto and all related withholding (and Purchasers shall provide to Sellers such information and documentation as Sellers shall reasonably request related thereto), so long as such Transferred Entity Employee (i) is employed by a Transferred Entity or an Affiliate of Purchasers as of the Additional Retention Bonus Date (as defined below) and (ii) waives and releases any and all claims against Sellers and their Affiliates (not including Newco and Alkermes Gainesville), the bonus amounts listed opposite such Transferred Entity Employee’s name (collectively, the “Additional Retention Bonuses” and together with the Initial Retention Bonuses, the “Retention Bonuses”), which Additional Retention Bonuses shall be paid on December 15, 2015 or such other date prior to December 25, 2015 as Sellers may determine
-55-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(the “Additional Retention Bonus Date”). Purchasers shall provide Sellers a list of Transferred Entity Employees employed by either a Transferred Entity or an Affiliate of Purchasers as of December 1, 2015 and shall be obligated to notify Sellers of any resignation or expected resignation of a Transferred Entity Employee prior to December 15, 2015. The Additional Retention Bonuses will not be included in the calculation of Working Capital.
6.5 Accrued Vacation Payment. Upon and subject to the Closing, Seller shall pay to each Transferred Entity Employee a cash amount in respect of any unused vacation accrued through December 31, 2014 under the applicable plan of Seller. Such amount, once paid, will not be included in the calculation of Working Capital.
ARTICLE VII
TAX MATTERS
7.1 Generally. The following provisions shall govern the allocation of responsibility as between Purchasers and Sellers for certain Tax matters following the Closing Date.
7.2 Tax Indemnification.
(a) Each Seller shall jointly and severally indemnify the Transferred Entities, Purchasers and each of Purchasers’ Affiliates and hold them harmless from and against any loss, claim, liability, expense or other damage attributable to: (i) all Taxes (or the non-payment thereof) of the Transferred Entities that relate to all Pre-Closing Tax Periods; (ii) all Taxes of any member of an affiliated, consolidated, combined, VAT or unitary group of which the Transferred Entities (or any predecessor of any of the foregoing) is or was a member on or prior to the Closing Date, including pursuant to Treasury Regulation §1.1502-6 or any analogous or similar state, local, or non-U.S. Law or regulation; (iii) any and all Taxes of any person (other than the Transferred Entities) imposed on the Transferred Entities as a transferee or successor, by contract or pursuant to any law, rule, or regulation, which Taxes relate to an event or transaction occurring before the Closing (including any Transferred Entity being a member of a VAT grouping); (iv) any Taxes of another party that a Transferred Entity was required to withhold in any Pre-Closing Tax Period, (v) a breach of Section 3.12(k) of this Agreement, and (vi) any Irish stamp duty arising under Section 79(7)(b) of the Stamp Duties Consolidation ▇▇▇ ▇▇▇▇ or Irish corporation tax on chargeable gains arising under Section 623(4) of the Taxes Consolidation ▇▇▇ ▇▇▇▇ arising or imposed on a Transferred Entity as a direct consequence of the Transferred Entity ceasing on Closing to be associated for stamp duty purposes or a member of a group for Irish corporation tax on chargeable gains; provided, however, that Sellers shall (x) have no obligation to indemnify Purchasers against any Taxes or other expenses incurred by the Transferred Entities resulting from, consisting of, or related to any transaction entered into or action taken by Purchasers occurring on or after the Closing Date after the Closing (including, without limitation, any Divestiture by Purchasers) and (y) be liable only to the extent that such Taxes exceed the amount, if any, reserved specifically for such Taxes in Working Capital and taken into account in Sections 2.4 and 2.5 of this Agreement.
(b) Purchasers and their Affiliates shall pay or cause to be paid and shall indemnify Sellers and their Affiliates and protect, save and hold them harmless from and against
-56-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
any loss, claim, liability, expense or other damage attributable to any Taxes imposed on Sellers and their Affiliates (including, prior to Closing, the Transferred Entities) caused by, resulting from, consisting of, or related to (i) Purchasers’ failure to comply with their obligations under Section 2.2(b) with respect to the allocation and reporting of the Purchase Price for the Transferred Interests or failure to treat any portion of the Initial Purchase Price, the Warrant or the Earn-Out Consideration paid to APIL (other than by reason of APIL failing to meet its obligations under Section 2.2(e) to provide Purchasers with valid withholding certificates as set forth therein) as exempt from United States federal Income Tax under the Code and/or Article 12 or Article 13 of the U.S.-Ireland Tax Treaty or for exemption from FATCA Taxes, and (ii) any transaction entered into or action taken by Purchasers occurring on or after the Closing Date after the Closing (including, without limitation, any Divestiture by Purchasers).
(c) The provisions of Section 10.6(c) shall apply with respect to Tax indemnity payments required to be made pursuant to this Section 7.2. Payment of any Tax indemnity payment required to be made under this Section 7.2 shall be made to the party entitled to indemnification at least two (2) Business Days before the date payment of the Taxes to which such payment relates is due by such indemnitee and, if payment has not been made at such time, within two (2) Business Days after receipt of a written demand from the indemnitee.
7.3 Straddle Period. In the case of a Straddle Period, the amount of any Taxes based on or measured by income, receipts, or payroll of the Transferred Entities for the Pre-Closing Tax Period shall be determined based on a deemed interim closing of the books as of the close of business on the Closing Date, and the amount of other Taxes of the Transferred Entities for a Straddle Period that relates to the Pre-Closing Tax Period shall be deemed to be the amount of such Tax for the entire taxable period multiplied by a fraction the numerator of which is the number of days in the taxable period ending on the Closing Date and the denominator of which is the number of days in such Straddle Period.
7.4 Tax Proceedings.
(a) Notices. Purchasers shall promptly notify Sellers in writing upon receipt by Purchasers, the Transferred Entities or any of their Affiliates of notice of any Tax Proceeding in respect of the Transferred Entities relating to any Pre-Closing Tax Period or Straddle Period for which Sellers could be liable for Taxes under Law or this Agreement. Such notification shall specify in reasonable detail the basis for such Tax Proceeding and shall include a copy of the relevant portion of any correspondence received from the Governmental Entity.
(b) Pre-Closing Tax Periods. Sellers shall have the right, at their expense, to control any Tax Proceeding in respect of the Transferred Entities for any Pre-Closing Tax Period; provided, however, that Sellers shall provide Purchasers with a timely and reasonably detailed account of each stage of such Tax Proceeding and shall permit Purchasers to participate in the Tax Proceeding at its expense, through counsel or accountants reasonably acceptable to Sellers, and Sellers shall not settle, compromise, or conclude any such Tax Proceeding without the prior written consent of the applicable Purchaser, which consent shall not be unreasonably withheld or conditioned. Purchasers, at Sellers’ expense, may control and contest any Tax Proceeding which Sellers would otherwise have the right to control under this Section 7.4(b) if Sellers (i) decline or fail to contest such Tax Proceeding or (ii) do not substantially comply with
-57-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
the provisions of the preceding sentence; provided, however, that if the applicable Purchaser exercises its right to control and contest any Tax Proceeding under the preceding clause, such Purchaser shall (i) provide Sellers with a timely and reasonably detailed account of each stage of such Tax Proceeding, (ii) not settle, compromise or abandon any such Tax Proceeding without obtaining the prior written consent of Sellers, which consent shall not be unreasonably withheld or delayed, and (iii) consult with Sellers concerning the appropriate strategy for contesting such Tax Proceeding.
(c) Straddle Periods. Purchasers shall control, at their own expense, any Tax Proceeding in respect of the Transferred Entities for any Straddle Period; provided, however, that Purchasers shall provide Sellers with a timely and reasonably detailed account of each stage of such Tax Proceeding.
7.5 Cooperation on Tax Matters.
(a) Purchasers, the Transferred Entities, and Sellers shall cooperate fully, as and to the extent reasonably requested by the other Party, in connection with the filing of Tax Returns pursuant to this Section 7.5 and any Tax Proceeding. Such cooperation shall include the retention and (upon the other Party’s request) the provision of records and information that are reasonably relevant to any such Tax Proceeding and making employees available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder. The Transferred Entities and Sellers agree (A) to retain all books and records with respect to Tax matters pertinent to the Transferred Entities relating to any taxable period beginning before the Closing Date until the expiration of the statute of limitations (and, to the extent notified by Purchasers or Sellers, any extensions thereof) of the respective taxable periods, and to abide by all record retention agreements entered into with any taxing authority, and (B) to give the other Party reasonable written notice prior to transferring, destroying or discarding any such books and records and, if the other Party so requests, the Transferred Entities or Sellers, as the case may be, shall allow the other Party to take possession of such books and records.
(b) Purchasers and Sellers further agree, upon request, to use their best efforts to obtain any certificate or other document from any Governmental Entity or any other Person as may be necessary to mitigate, reduce or eliminate any Tax that could be imposed (including, but not limited to, with respect to the transactions contemplated hereby).
7.6 Certain Taxes and Fees. Except as otherwise provided in this Agreement, and in particular Section 7.2(a) of this Agreement, all transfer, documentary, sales, use, stamp, registration and other such Taxes (including any penalties and interest) incurred in connection with the purchase of the Transferred Interests shall be borne by the Party that bears liability therefor under applicable Law.
7.7 Survival. The representations and warranties in Section 3.12 shall survive the Closing Date until sixty (60) days following the expiration of the applicable statute of limitations.
-58-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
ARTICLE VIII
CONDITIONS TO OBLIGATIONS TO CLOSE
8.1 Conditions to Obligation of Each Party to Close. The respective obligations of each Party to effect the transactions contemplated by this Agreement shall be subject to the satisfaction or waiver at or prior to the Closing of the following conditions:
(a) HSR Act. Any waiting period (and any extension thereof) applicable to the consummation of the Sale under the HSR Act shall have expired or been terminated.
(b) No Injunctions or Illegality. No court or other Governmental Entity of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any Law (whether temporary, preliminary or permanent) that is in effect and prevents, restrains, enjoins or otherwise prohibits the consummation of the Sale (a “Prohibitive Order”).
(c) Governmental and Regulatory Approvals. Other than the filings pursuant to the HSR Act and any other required antitrust Laws identified after the date hereof, all consents, approvals and actions of, filings with and notices to any Governmental Entity that are material to the Business and required of Purchasers or Sellers to consummate the transactions contemplated hereby shall have been obtained or made.
(d) Consents. All Consents listed on Section 3.3(a) of the Sellers Disclosure Letter except for those Consents listed on Section 8.1(d) of the Sellers Disclosure Letter shall have been obtained.
8.2 Conditions to Purchasers’ Obligation to Close. Purchasers’ obligation to effect the transactions contemplated by this Agreement shall be subject to the satisfaction or waiver on or prior to the Closing of all of the following conditions:
(a) Representations and Warranties. (i) The representations and warranties made by Sellers in this Agreement that are qualified by Material Adverse Effect shall be true and correct, and (ii) the representations and warranties made by the Sellers in this Agreement that are not so qualified shall be true and correct in all material respects (determined without regard to any “materiality” qualifications); in the case of each of clauses (i) and (ii) above, as of the Closing Date as if made on and as of the Closing Date (except to the extent that any such representation or warranty, by its terms, is expressly limited to a specific date, in which case, as of such specific date).
(b) Covenants and Agreements. The covenants and agreements of Sellers to be performed on or before the Closing Date in accordance with this Agreement shall have been duly performed in all material respects.
(c) Officer’s Certificate. Purchasers shall have received a certificate, dated as of the Closing Date and signed on behalf of each Seller by an executive officer of each Seller, stating that the conditions specified in Section 8.2(a) and Section 8.2(b) have been satisfied.
-59-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(d) Closing Deliverables. Purchasers shall have received from Sellers the deliverables listed in Sections 2.3(b)(i)(A)-(I).
(e) Financing. The Debt Financing (or Alternative Financing in accordance with Section 2) has been funded or will be available to be funded at Closing.
8.3 Conditions to Sellers’ Obligation to Close. The obligations of Sellers to effect the transactions contemplated by this Agreement shall be subject to the satisfaction or waiver on or prior to the Closing of all of the following conditions:
(a) Representations and Warranties. The representations and warranties made by Purchasers in this Agreement and the Warrant shall be true and correct in all material respects (determined without regard to any “materiality” or “material adverse effect” qualifications), as of the Closing Date as if made on and as of the Closing Date (except to the extent that any such representation or warranty, by its terms, is expressly limited to a specific date, in which case, as of such specific date).
(b) Covenants and Agreements. The covenants and agreements of Purchasers to be performed on or before the Closing Date in accordance with this Agreement shall have been duly performed in all material respects.
(c) Officer’s Certificate. Sellers shall have received a certificate, dated as of the Closing Date and signed on behalf of Purchasers by an executive officer of each Purchaser, stating that the conditions specified in Section 8.3(a) and Section 8.3(b) have been satisfied.
(d) Closing Deliverables. Sellers shall have received from Purchasers the deliverables listed in Sections 2.3(b)(ii)(A)-(D).
ARTICLE IX
TERMINATION
9.1 Termination. This Agreement may be terminated at any time prior to the Closing:
(a) by mutual written consent of Sellers and Purchasers;
(b) by either Sellers or Purchasers, if:
(i) the Closing shall not have occurred on or before May 8, 2015 (the “Outside Date”); provided, however, that the right to terminate this Agreement under this Section 9.1(b)(i) shall not be available to any Party to this Agreement whose failure or whose Affiliate’s failure to perform any material covenant or obligation under this Agreement has been the primary cause of the failure of the transactions contemplated by this Agreement to occur on or before such date;
(ii) if the other Party shall have breached or failed to perform in any material respect any of its respective representations, warranties, covenants or other agreements contained in this Agreement, and such breach or failure to perform (A) would
-60-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
give rise to the failure of a condition set forth in Section 8.2(a) or Section 8.2(b) (in the case of a breach by Sellers) or Section 8.3(a) or Section 8.3(b) (in the case of a breach by Purchasers), and (B) cannot be or has not been cured prior to the earlier of (1) the Business Day prior to the Outside Date or (2) the date that is thirty (30) days from the date that Purchasers or Sellers, as applicable, is notified by the other in writing of such breach or failure to perform; or
(iii) if any Prohibitive Order permanently prevents, restrains, enjoins or otherwise prohibits the consummation of the Sale, and such Prohibitive Order becomes effective (and final and nonappealable);
(c) by Sellers if Closing has not occurred by the third (3rd) Business Day following the satisfaction or waiver of the conditions set forth in Article VIII excluding the conditions set forth in Section 8.2(e) (other than those conditions that by their nature are to be satisfied or waived at the Closing, but subject to the satisfaction or waiver of those conditions) or by such other time and date as mutually agreed by the Parties pursuant to Section 2.3, provided that such failure to close is not the result of any action or inaction of Sellers; and
(d) by Purchasers if Closing has not occurred by the third (3rd) Business Day following the satisfaction or waiver of the conditions set forth in Article VIII (other than those conditions that by their nature are to be satisfied or waived at the Closing, but subject to the satisfaction or waiver of those conditions) or by such other time and date as mutually agreed by the Parties pursuant to Section 2.3, provided that such failure to close is not the result of any action or inaction of Purchasers.
9.2 Notice of Termination. In the event of termination of this Agreement by either or both of Sellers and Purchasers pursuant to Section 9.1, written notice of such termination shall be given by the terminating Party to the other Parties to this Agreement.
9.3 Effect of Termination. In the event of termination of this Agreement by either or both of Sellers and Purchasers pursuant to Section 9.1, this Agreement shall terminate and become void and have no effect, and the transactions contemplated by this Agreement shall be abandoned without further action by the Parties to this Agreement, except that the provisions of Section 5.1(a), Section 9.4, Section 11.2 and Section 11.5 shall survive the termination of this Agreement; provided, however, that such termination shall not relieve any Party to this Agreement of liability for any fraud or willful breach of this Agreement.
9.4 Reverse Termination Fee.
(a) If this Agreement is validly terminated by Sellers pursuant to Section 9.1(b)(ii) or Section 9.1(c), then Purchasers shall pay by wire transfer of immediately available funds, to an account designated by Sellers, within two (2) Business Days after the date on which this Agreement is so terminated, the amount of Five Million Dollars ($5,000,000) (the “Reverse Termination Fee”); provided, however, that Purchasers shall not be liable to Sellers for the Reverse Termination Fee solely due to a failure to satisfy the conditions of Section 8.2(e), provided that Purchasers have complied with its obligations under Section 5.12.
-61-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(b) Each Party acknowledges that the agreements contained in this Section 9.4 are an integral part of the transactions contemplated by this Agreement and that, without these agreements, the other Parties would not enter into this Agreement. Accordingly, if Purchasers fail promptly to pay the amounts due pursuant to this Section 9.4, and, in order to obtain such payments, Sellers commence a suit that results in a judgment against Purchasers for the amounts set forth in this Section 9.4, Purchasers will pay to Sellers, Sellers’ costs and expenses (including reasonable attorney’s fees and disbursements) in connection with such suit. The Parties acknowledge that the Reverse Termination Fee shall not constitute a penalty but rather is liquidated damages, in a reasonable amount that will compensate Sellers in the circumstances in which the Reverse Termination Fee is payable for the efforts and resources expended and opportunities foregone while negotiating this Agreement and in reliance on this Agreement and on the expectation of the consummation of the Sale, which amount would otherwise be impossible to calculate with precision.
(c) Except as set forth in Section 9.4(b), in any circumstance in which Sellers have the right to receive the Reverse Termination Fee pursuant to Section 9.4(a), Sellers’ termination of this Agreement and receipt of the Reverse Termination Fee shall be the sole and exclusive remedy of Sellers and their Affiliates against Purchasers, the financing sources of the Debt Financing and any of their respective, direct or indirect, former, current or future general or limited partners, managers, members, stockholders, officers, directors, Affiliates, employees, representatives, agents, successors and assigns (collectively, the “Purchaser Related Parties”) for any loss suffered as a result of any breach of any representation, warranty, covenant or agreement in this Agreement, the transactions contemplated hereby, or the Debt Financing Agreements, and upon such termination by Sellers and receipt of the Reverse Termination Fee, none of the Purchasers, the financing sources of the Debt Financing, or any of their respective Purchaser Related Parties shall have any further liability or obligation relating to or arising out of this Agreement, the transactions contemplated hereby, or the Debt Financing Agreements (except that the applicable Purchaser Related Parties of the Purchasers (and not the Purchaser Related Parties of the financing sources of the Debt Financing) shall remain obligated for, and Sellers and their Subsidiaries may be entitled to remedies with respect to, any breach of the Confidentiality Agreement or the provisions of Section 11.3, whether in equity or at law, in contract, in tort or otherwise).
ARTICLE X
SURVIVAL; INDEMNIFICATION; LIQUIDATED DAMAGES
10.1 Survival Periods. All representations and warranties contained in this Agreement, and the right to commence any claim with respect thereto under Section 10.2 and Section 10.3, shall survive until the fifteen (15) month anniversary of the Closing Date; provided that (i) the Fundamental Representations and the Purchasers Fundamental Representations shall survive indefinitely; and (ii) the representations and warranties set forth in Section 3.9 (Intellectual Property), Section 3.18 (Environmental Health and Safety Matters) and Section 3.19 (Employee Benefits Plans) shall survive until the three (3) year anniversary of the Closing Date. Those covenants that contemplate or may involve actions to be taken or obligations in effect after the Closing shall survive in accordance with their terms. Written notice of a claim under this Article X must be given to the Indemnifying Party in accordance with the provisions hereof prior to the expiration of the survival period set forth in this Section 10.1.
-62-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
10.2 Indemnification by Sellers. Subject to the provisions of this Article X, from and after the Closing Date, Sellers shall indemnify and hold harmless Purchasers and its Affiliates, each of their respective directors, officers, employees and agents, and each of the heirs, executors, successors and assigns of any of the foregoing (collectively, the “Purchasers Indemnified Parties”) from and against any and all Losses to the extent resulting from or arising out of:
(a) any breach of any representation or warranty of Sellers contained in Article III of this Agreement as of the Closing Date (or with respect to representations and warranties that are made as of a specific date, as of such date);
(b) any breach of any covenant or agreement contained in this Agreement to be performed by Sellers after the Closing; and
(c) the Initial Retention Bonuses.
10.3 Indemnification by Purchasers. Subject to the provisions of this Article X, from and after the Closing Date, Purchasers shall indemnify and hold harmless Sellers and their Affiliates, each of their respective directors, officers, employees and agents, and each of the heirs, executors, successors and assigns of any of the foregoing from and against any and all Losses resulting from or arising out of:
(a) any breach of any representation or warranty of Purchasers contained in Article IV of this Agreement as of the Closing Date (or with respect to representations and warranties that are made as of a specific date, as of such date);
(b) any breach of any covenant or agreement contained in this Agreement to be performed by Purchasers after the Closing; and
(c) actions taken on behalf of, or at the request of, Purchasers in connection with the Debt Financing or any Alternative Financing.
10.4 Indemnification Procedures.
(a) A Person that may be entitled to be indemnified under this Agreement (the “Indemnified Party”), shall promptly notify the Party or Parties liable for such indemnification (the “Indemnifying Party”) in writing of any pending or threatened claim or demand other than a Tax Proceeding that the Indemnified Party has determined gives or would reasonably be expected to give rise to a right of indemnification under this Agreement (including a pending or threatened claim or demand asserted by a third party against the Indemnified Party, such claim being a “Third Party Claim”), describing in reasonable detail the facts and circumstances with respect to the subject matter of such claim or demand (including copies of any summons, complaint or other pleading which may have been served on it and any written claim, demand, invoice, billing or other document evidencing or asserting the same); provided, however, that the failure to provide such notice shall not release the Indemnifying Party from any of its obligations
-63-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
under this Article X except to the extent the Indemnifying Party is materially prejudiced by such failure, it being agreed that notices for claims in respect of a breach of a representation, warranty, covenant or agreement must be delivered prior to the expiration of any applicable survival period specified in Section 10.1 for such representation, warranty, covenant or agreement.
(b) Upon receipt of a notice of a Third Party Claim for indemnity from an Indemnified Party pursuant to Section 10.2 or Section 10.3, other than a Tax Proceeding, the Indemnifying Party will be entitled, by notice to the Indemnified Party delivered within twenty (20) Business Days of the receipt of notice of such Third Party Claim, to undertake, conduct and control the settlement or defense of such Third Party Claim (at the expense of such Indemnifying Party); provided that the Indemnifying Party shall only be entitled to undertake, conduct and control such settlement or defense if it acknowledges, in writing, to the Indemnified Party, its obligation to indemnify the Indemnified Party pursuant to the terms and subject to the limitations of this Article X. The Indemnifying Party shall allow the Indemnified Party a reasonable opportunity to participate in the defense of such Third Party Claim with its own counsel and at its own expense. If the Indemnifying Party does not assume the defense and control of any Third Party Claim pursuant to this Section 10.4(b), the Indemnified Party shall be entitled to assume and control such defense through counsel of its own choice, and the reasonable fees and expenses incurred in connection with such defense shall be considered Losses hereunder with respect to the subject matter of such claim, indemnifiable to the extent provided in this Article X, but the Indemnifying Party may nonetheless participate in the defense of such Third Party Claim with its own counsel and at its own expense. Notwithstanding the foregoing, the Indemnifying Party shall not be entitled to assume the settlement or defense of a Third Party Claim under this Section 10.4(b) unless: (A) the Third Party Claim involves solely monetary damages, (B) the Indemnifying Party demonstrates to the Indemnified Party’s reasonable satisfaction that the Indemnifying Party has sufficient financial resources in order to indemnify for the full amount of such potential Losses for which the Indemnifying Party is reasonably likely to be liable pursuant to this Article X, and (C) the amount of such potential Losses for which the Indemnifying Party is reasonably likely to be liable does not exceed the Cap. If either the Indemnifying Party or the Indemnified Party assumes the defense and control of a Third Party Claim, the Indemnifying Party or the Indemnified Party, as applicable, shall select counsel and shall use commercially reasonable efforts in the defense or settlement of such Third Party Claim. Purchasers or Sellers, as the case may be, shall, and shall cause each of their Affiliates and Representatives to, reasonably cooperate with the Indemnifying Party or Indemnified Party, as applicable, in the defense of any Third Party Claim, including by furnishing Books and Records, personnel and witnesses, as appropriate for any defense of such Third Party Claim. If the Indemnifying Party has assumed the defense and control of a Third Party Claim, it shall be authorized to consent to a settlement of, or the entry of any judgment arising from, any Third Party Claim, in its sole discretion and without the consent of the Indemnified Party; provided that such settlement or judgment shall consist solely of a recovery of monetary damages for which the Indemnifying Party shall be liable pursuant to this Article X, and the Indemnifying Party shall (i) pay or cause to be paid all amounts in such settlement or judgment (other than solely with respect to the Deductible would be applicable in accordance with the applicable provisions of Section 10.5) and (ii) obtain, as a condition of any settlement or other resolution, a complete and unconditional release of any Indemnified Party affected by such Third Party Claim. Except as set forth in the previous sentence, no Party shall settle or compromise any Third Party Claim without the prior written consent of the other Party, which consent shall not be unreasonably withheld,
-64-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
conditioned or delayed; provided, that is shall not be unreasonable for an Indemnified Party to withhold its consent to any settlement that involves any injunctive relief binding on any of the Indemnified Parties or a finding or admission of any violation of Law or admission of any wrongdoing by any Indemnified Party. No Indemnified Party will consent to the entry of any judgment or enter into any settlement or compromise with respect to a Third Party Claim without the prior written consent of the Indemnifying Party. Notwithstanding the foregoing, this Section 10.4 shall be subject to the provisions of any Contract providing for the defense or prosecution of any Action.
10.5 Limitations.
(a) Sellers shall have no liability for indemnification pursuant to Section 10.2(a) with respect to Losses for which indemnification is provided thereunder, (i) to the extent such Losses were included in the calculation of Working Capital on the Final Post-Closing Adjustment Statement, as finally determined pursuant to Sections 2.4 through 2.7, (ii) to the extent that Purchasers received a benefit from the reflection of such matter in the calculation of the adjustment of the Initial Purchase Price, if any, as finally determined pursuant to Sections 2.4 through 2.7, (iii) that are De Minimis Damages or (iv) unless the aggregate of all Losses (other than De Minimis Damages) exceeds Five Hundred Thousand Dollars ($500,000) (the “Deductible”) in which case Sellers shall be liable for all such Losses (other than De Minimis Damages) in excess of the amount of the Deductible. Notwithstanding the foregoing, Sellers shall have no liability for indemnification pursuant to Section 10.2(a) with respect to Losses of any aggregate amount that exceeds the Cap, it being understood that in the event any Purchasers Indemnified Party seeks indemnification for Losses in excess of the Cap and the Cap subsequently increases to a greater value as a result of the payment of Development Milestone Earn-Out Consideration and/or Commercial Milestone Earn-Out Consideration to APIL, such Purchasers Indemnified Party shall be entitled to seek indemnification in accordance with such increased Cap and the terms of this Agreement.
(b) The Deductible and the Cap shall not apply to any Losses arising out of or resulting from (1) the breach of any Fundamental Representation, (2) the breach of any representation or warranty set forth in Section 3.12 (Taxes); (3) the breach of any Purchasers Fundamental Representation; (4) the breach of any covenant set forth in this Agreement; or (5) fraud.
(c) Purchasers shall have no liability for indemnification pursuant to Section 10.3(a) with respect to Losses for which indemnification is provided thereunder, (i) that are De Minimis Damages or (ii) unless the aggregate of all Losses (other than De Minimis Damages) exceeds the Deductible, in which case Purchasers shall be liable for all such Losses (other than De Minimis Damages) in excess of the amount of the Deductible.
(d) For purposes of calculating the amount of any Losses arising out of or resulting from any breach of any representation or warranty of set forth in this Agreement, any reference to “Material Adverse Effect” or “materiality” or other correlative terms in such representations or warranties shall be disregarded.
-65-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(e) Sellers shall not be liable under this Article X for any Losses based upon or arising out of any inaccuracy in or breach of any of the representations or warranties of Sellers contained in this Agreement if Purchasers had knowledge, based solely upon written documentation included in the data room, of such inaccuracy or breach prior to the Closing. For purposes of this Section 10.5(e), Purchasers shall be deemed to have knowledge of all written documentation included in the data room as of the date hereof and included on the disk delivered pursuant to Section 5.21.
10.6 Mitigation; Additional Indemnification Provisions.
(a) Each Indemnified Party shall use, and cause its Affiliates to use, commercially reasonable efforts to mitigate any claim or liability that an Indemnified Party asserts under this Article X (including by taking reasonable best efforts to seek full recovery under all insurance and indemnity provisions covering any Losses for which it is seeking indemnification hereunder, to the same extent as it would if such Loss were not subject to indemnification hereunder).
(b) For purposes of this Agreement, Losses shall be calculated after giving effect to amounts actually received under any insurance policy, (net of any costs to recover such amounts and increases in premiums resulting from such claim).
(c) The amount of any Losses for which indemnification is provided shall be adjusted to take into account the amount of any net Tax benefit actually realized by the Indemnified Party as a result of the incurrence or payment of any such Losses in the form of a refund or reduction in Taxes otherwise payable within the tax year in which the Losses were incurred or paid, or the next two immediately succeeding tax years, in each case, calculated by comparing Taxes that would have been payable without taking into account any deduction or credit resulting from such Losses and Taxes actually payable by taking into account such deductions or credits (but only after all other items of income, gain, loss and deduction have been taken into account). If the Indemnified Party actually realizes a Tax benefit after an indemnification payment is made to it that was not taken into account at the time the indemnification payment was made, the Indemnified Party shall pay to the Indemnifying Party the amount that the indemnification payment would have been reduced by if such Tax benefit had been actually realized prior to the time such indemnification payment was made.
(d) No Indemnified Party will, in any event, be entitled to any incidental, indirect, consequential, special, exemplary or punitive damages, including actual or potential lost profits, diminution in value or measures of damages based on a multiple.
10.7 Liquidated Damages. In the event of the occurrence of an event described on Section 10.7 of the Sellers Disclosure Letter (a “Liquidated Damages Event”), monetary damages would be difficult, if not impossible, to measure. The Parties therefore agree that liquidated damages shall be payable by Sellers to Purchasers in the manner and amount described on Schedule 10.7 of the Sellers Disclosure Letter. Such payments shall begin to be made to Purchasers within sixty (60) days of receipt by Sellers from Purchasers of written notice of the existence of a Liquidated Damages Event, and thereafter shall be made within thirty (30) days of the end of any month in which a Liquidated Damages Event exists. The Parties
-66-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
acknowledge and agree that this liquidated damages provision is reasonable and does not constitute a penalty. Notwithstanding anything contained in this Agreement, the Parties further agree that this is the sole and exclusive remedy for any Losses arising from or in connection with the events or circumstances set forth on Section 10.7 of the Sellers Disclosure Letter.
10.8 Exclusive Remedies. Except with respect to the matters covered by Section 2.5, Section 2.6, Section 5.14, Section 10.7, Section 11.10, and, with respect to indemnification for Tax matters, Article VII, Sellers and Purchasers acknowledge and agree that, following the Closing, the indemnification provisions of Section 10.2 and Section 10.3 shall be the sole and exclusive remedies of Sellers and Purchasers, respectively, for any Losses (whether predicated on common law, statute, strict liability, Environmental Law (including CERCLA or any similar state law) or otherwise) that each Party may at any time suffer or incur, or become subject to, as a result of, or in connection with, any breach of any representation or warranty in this Agreement by the other Parties or any failure by the other Parties to perform or comply with any covenant or agreement that, by its terms, was to have been performed, or complied with, by such other Parties prior to the Closing.
10.9 Subrogation. In the event of payment by or on behalf of any Indemnifying Party to any Indemnified Party (including pursuant to this Agreement) in connection with any claim or demand by any Person other than the Parties hereto or their respective Affiliates, such Indemnifying Party shall be subrogated to and shall stand in the place of such Indemnified Party as to any events or circumstances in respect of which such Indemnified Party may have any right, defense or claim relating to such claim or demand against any claimant or plaintiff asserting such claim or demand. Such Indemnified Party shall cooperate with such Indemnifying Party in a reasonable manner, and at the cost of such Indemnifying Party, in presenting any subrogated right, defense or claim.
10.10 Tax Indemnification Matters. Notwithstanding anything to the contrary in this Article X, the above provisions of this Article X shall not apply to Tax indemnification matters, which shall instead be governed by Article VII.
10.11 No Duplication. Any liability for indemnification under this Agreement shall be determined without duplication of recovery due to the facts giving rise to such liability constituting a breach of more than one representation, warranty, covenant or agreement.
ARTICLE XI
MISCELLANEOUS
11.1 Counterparts. This Agreement may be executed in two (2) or more counterparts, all of which shall be considered one and the same agreement, and shall become effective when one or more counterparts have been signed by each of the Parties and delivered to the other Parties. This Agreement may be executed and delivered by facsimile or as an attachment to an e-mail and upon such delivery the signature will be deemed to have the same effect as if the original signature had been delivered to the other Parties.
-67-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
11.2 Governing Law; Jurisdiction and Forum; Waiver of Jury Trial.
(a) This Agreement, and all claims or causes of action (whether based on contract, tort or any other theory) that may be based upon, arise out of or related to this Agreement or the negotiation, execution or performance of this Agreement shall be governed by and construed in accordance with the laws of the State of Delaware applicable to contracts negotiated, made and performed in such State without giving effect to the choice of law principles of such State or other jurisdiction that would require or permit the application of the laws of another jurisdiction.
(b) Each of the Parties hereto irrevocably consents to the exclusive jurisdiction and venue of any court within the State of Delaware in connection with any matter based upon or arising out of this Agreement or the matters contemplated herein, agrees that process may be served upon them in any manner authorized by the laws of the State of Delaware for such Persons and waives and covenants not to assert or plead any objection which they might otherwise have to such jurisdiction, venue and such process.
(c) Each Party to this Agreement knowingly, intentionally and voluntarily waives to the fullest extent permitted by applicable Law trial by jury in any Action brought by any of them against any other arising out of or in any way connected with this Agreement, or any other agreements executed in connection herewith or the administration thereof or any of the transactions contemplated herein or therein. No Party to this Agreement shall seek a jury trial in any Action based upon, or arising out of, this Agreement or any related instruments or the relationship between the Parties. No Party will seek to consolidate any such Action in which a jury trial has been waived with any other Action in which a jury trial cannot be or has not been waived. Each Party to this Agreement certifies that it has been induced to enter into this Agreement or instrument by, among other things, the mutual waivers and certifications set forth above in this Section 11.2. No Party has in any way agreed with or represented to any other Party that the provisions of this Section 11.2 will not be fully enforced in all instances.
11.3 Confidentiality.
(a) The Confidentiality Agreement and the Confidential Disclosure Agreement shall continue in full force and effect until the Closing Date, at which time such Confidentiality Agreement and Confidential Disclosure Agreement shall terminate, except for the provisions which expressly survive the termination thereof.
(b) Except as expressly permitted pursuant to this Agreement, the Ancillary Agreements, the Intellectual Property Transfer and License Agreement and the IP License Agreement, from and after the Closing Date, Sellers will refrain from, either alone or in conjunction with any other Person, or directly or indirectly through their Affiliates or Representatives, disclosing to any other Person, or using in any manner, any confidential, proprietary or secret information to the extent relating solely to the Transferred Entities or the Business (“Business Confidential Information”); provided that the foregoing obligations of confidentiality and non-use will not apply to any portion of the Business Confidential Information that (A) is or becomes generally available to the public or otherwise part of the public domain after the Closing Date and other than through any act or omission of the foregoing Persons or their Affiliates in breach of this Agreement, the Ancillary Agreements, the Intellectual Property Transfer and License Agreement or the IP License Agreement, (B) is
-68-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
disclosed after the Closing Date to the foregoing Persons on a non-confidential basis by a third party that is not subject to an obligation of confidentiality with respect to such Business Confidential Information, and (C) is independently discovered or developed by the foregoing Persons or their Affiliates after the Closing Date without the aid, application, or use of such Business Confidential Information.
(c) Except as expressly permitted pursuant to this Agreement, the Ancillary Agreements, the Intellectual Property Transfer and License Agreement and the IP License Agreement, from and after the Closing Date, Purchasers will refrain from, either alone or in conjunction with any other Person, or directly or indirectly through its Affiliates or Representatives, disclosing to any other Person, or using in any manner, any confidential, proprietary or secret information relating to the Sellers and their businesses other than the Transferred Entities and the Business (“Seller Confidential Information”); provided that the foregoing obligations of confidentiality and non-use will not apply to any portion of the Seller Confidential Information that (A) is or becomes generally available to the public or otherwise part of the public domain after the Closing Date and other than through any act or omission of the foregoing Persons or their Affiliates in breach of this Agreement, the Ancillary Agreements, the Intellectual Property Transfer and License Agreement or the IP License Agreement, (B) is disclosed after the Closing Date to the foregoing Persons on a non-confidential basis by a third party that is not subject to an obligation of confidentiality with respect to such Seller Confidential Information, and (C) is independently discovered or developed by the foregoing Persons or their Affiliates after the Closing Date without the aid, application, or use of such Seller Confidential Information.
(d) Notwithstanding Section 11.3(b) and Section 11.3(c), Sellers may disclose the Business Confidential Information and Purchasers may disclose the Seller Confidential Information in order to comply with (i) applicable non-patent Law (including any securities law or regulation or the rules of a securities exchange) and (ii) a request or requirement by deposition, interrogatory, request for documents, subpoena, civil investigation demand or similar process or a formal request from a regulatory examiner, if in the reasonable opinion of counsel, such disclosure is necessary for such compliance (an “External Demand”); and (iii) to its Affiliates, and potential and actual acquirers, merger partners, investors, investment bankers or lenders and their respective counsels and advisors; and; provided that, (A) with regard to disclosure under clause (ii), prior to making such disclosure, the Party subject to such demand or request shall (x) immediately notify the other Party of the existence, terms and circumstances surrounding such External Demand, (y) consult with the other Party on the availability of taking legally available steps to resist or narrow such request or disclosure, and (z) assist the other Party, at the other Party’s expense, in seeking a protective order or other appropriate remedy to the extent available under the circumstances and (B) with regard to disclosure under clause (iii), prior to making such disclosure, such entities are bound by commercially reasonable obligations of confidentiality with respect to the use and disclosure of such Business Confidential Information or Seller Confidential Information, as applicable.
(e) The Parties acknowledge that either or both Parties may be obligated to make filings (including, but not limited to, the filing of a copy of this Agreement or the Ancillary Agreements) with the SEC or other Governmental Entity. Each Party shall be entitled to make such required filings, provided that it requests confidential treatment of at least the financial
-69-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
terms and sensitive technical terms of this Agreement or the Ancillary Agreements to the extent such confidential treatment is reasonably available to such Party. In the event of any such filing of this Agreement or the Ancillary Agreements, the Party making such filing shall provide notice to the other Party with a copy of such disclosure and, if applicable, a copy of this Agreement or the Ancillary Agreements marked to show provisions for which such Party intends to seek confidential treatment not less than five (5) Business Days prior to such filing (and any revisions to such portions of the proposed filing a reasonable time prior to the filing thereof), and shall give good faith consideration to the other Party’s comments thereon to the extent consistent with the legal requirements. No such notice shall be required under this Section 11.3(e) if the substance of the description of or reference to this Agreement contained in the proposed filing has been included in any previous filing made by either Party hereunder or otherwise approved by the other Party.
11.4 Entire Agreement. This Agreement (including the Schedules and Exhibits to this Agreement) together with the Confidentiality Agreement, the Confidential Disclosure Agreement and the Ancillary Agreements contain the entire agreement and understanding among the Parties with respect to the subject matter hereof and thereof and supersede any prior discussion, correspondence, negotiation, proposed term sheet, agreement, understanding or arrangement, and there are no agreements, understandings, representations or warranties among the Parties other than those set forth or referred to in these documents. None of the Parties shall be liable or bound to any other Party in any manner by any representations, warranties or covenants relating to such subject matter except as specifically set forth in this Agreement (including the Schedules and Exhibits to this Agreement), the Ancillary Agreements or the Confidentiality Agreement.
11.5 Expenses. Except as otherwise set forth in this Agreement, whether the transactions contemplated by this Agreement are consummated or not, all legal and other costs and expenses incurred in connection with this Agreement and the transactions contemplated by this Agreement shall be paid by the Party incurring such costs and expenses and any such costs of Sellers shall be the obligation of Parent; provided, however, that all filing fees paid in connection with the antitrust filings made pursuant to Section 5.2(a) shall be borne equally by Purchasers and Sellers.
11.6 Notices. All notices and other communications to be given to any Party hereunder shall be sufficiently given for all purposes hereunder if in writing and delivered by hand, courier or overnight delivery service or three (3) days after being mailed by certified or registered mail, return receipt requested, with appropriate postage prepaid, or when received in the form of telegram or facsimile and shall be directed to the address set forth below (or at such other address or facsimile number as such Party shall designate by like notice):
| (a) | If to Sellers or Daravita Limited: | |
| Alkermes plc | ||
| ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ | ||
| ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇ | ||
| ▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇ | ||
| Attn.: Company Secretary | ||
| Fax No.: ▇(▇▇▇) ▇ ▇▇▇ ▇▇▇▇ |
-70-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| with a copy (which shall not constitute notice) to: | ||
| ▇▇▇▇▇▇▇ Procter LLP | ||
| ▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇ | ||
| ▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | ||
| Attn.: ▇▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇, Esq. | ||
| ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇, Esq. | ||
| Fax No.: (▇▇▇) ▇▇▇-▇▇▇▇ | ||
| And with a copy (which shall not constitute notice) to: | ||
| ▇▇▇▇▇▇ ▇▇▇ | ||
| ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ | ||
| ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ | ||
| ▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇ | ||
| Attn.: ▇▇▇▇▇▇▇▇▇▇▇ ▇.▇. ▇▇▇▇▇▇▇▇▇▇ | ||
| Fax No.: + ▇▇▇ ▇ ▇▇▇ ▇▇▇▇ | ||
| (b) | If to Purchasers: | |
| Recro Pharma, Inc. | ||
| ▇▇▇ ▇▇▇▇ ▇▇▇▇ | ||
| ▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | ||
| Attention: ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇ | ||
| Email: ▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ | ||
| with a copy (which shall not constitute notice) to: | ||
| ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ LLP | ||
| Two ▇▇▇▇▇ Square | ||
| Eighteenth and Arch Streets | ||
| Philadelphia, PA 19103 | ||
| Attention: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇, Esq. | ||
| Fax No.: (▇▇▇) ▇▇▇-▇▇▇▇ | ||
11.7 Assignment.
(a) This Agreement shall be binding upon and inure to the benefit of the Parties to this Agreement and their respective successors and assigns; provided, however, that no Party to this Agreement will assign its rights or delegate any or all of its obligations under this Agreement without the express prior written consent of each other Party to this Agreement, except that either Party may assign its benefits under this Agreement to an Affiliate of that Party. Any attempted assignment in violation of this Section 11.7 shall be void.
(b) Notwithstanding anything to the contrary in Section 11.7(a) or elsewhere in this Agreement, APIL may assign its rights to any third party to (a) receive the Net Sales Earn-Out Consideration, (b) receive the Net Sales Report, (c) audit the records of Purchasers,
-71-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
their Affiliates, licensees and sublicensees as described in Section 3.3 of Exhibit E, and (d) make indemnification claims against Purchasers, in connection with any securitization or monetization of the Net Sales Earn-Out Consideration (a “Securitization”), and APIL may disclose Business Confidential Information to a third party in connection with a Securitization to the extent reasonably necessary to enable the third party to evaluate the Securitization opportunity and to allow APIL to exercise its rights under this Section 11.7(b).
11.8 Third-Party Beneficiaries. This Agreement is not intended to confer upon any Person not a party to this Agreement (and their successors and assigns) any rights or remedies hereunder; provided, that the rights of the financing sources of the Debt Financing provided in this Section 11.8 and Section 9.4(c), Section 11.9 and Section 11.14 shall be enforceable by the financing sources of the Debt Financing, their Affiliates and their respective successors and assigns.
11.9 Amendments and Waivers. This Agreement may not be modified or amended except by an instrument or instruments in writing signed by the Party against whom enforcement of any such modification or amendment is sought. Each Party to this Agreement may, only by an instrument in writing, waive compliance by the other Parties to this Agreement with any term or provision of this Agreement on the part of such other Parties to this Agreement to be performed or complied with. The waiver by any Party to this Agreement of a breach of any term or provision of this Agreement shall not be construed as a waiver of any subsequent breach. Notwithstanding the foregoing, any amendment or waiver of this sentence of Section 11.9 or Section 9.4(c), Section 11.8 or Section 11.14 shall require the prior written consent of Orbimed Royalty Opportunities II, LP, but only to the extent that such Sections relate to the financing sources of the Debt Financing, their Affiliates or their respective successors or assigns.
11.10 Specific Performance. The Parties agree that irreparable damage, for which monetary damages (even if available) would not be an adequate remedy, would occur in the event that the Parties do not perform any provision of this Agreement in accordance with its specified terms or otherwise breach such provisions. Accordingly, the Parties acknowledge and agree that, to prevent breaches or threatened breaches by the Parties of any of their respective covenants or obligations set forth in this Agreement and to enforce specifically the terms and provisions of this Agreement, the Parties shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, in addition to any other remedy to which they are entitled in law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief on the basis that any of the other Parties has an adequate remedy at law or that any award of specific performance is not an appropriate remedy for any reason at law or in equity. Any Party against whom an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement is sought hereby waives any requirement for the Party seeking an injunction or injunctions to provide any bond or other security in connection with such order or injunction. The foregoing is in addition to any other remedy to which any Party is entitled at law, in equity or otherwise. The Parties further agree that nothing set forth in this Section 11.10 shall require any Party hereto to institute any Action for (or limit any Party’s right to institute any Action for) specific performance under this Section 11.10 prior or as a condition to exercising any termination right under Article IX (and pursuing damages after such termination). The Parties
-72-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
hereto agree that, notwithstanding anything herein to the contrary, Sellers shall be entitled to specific performance (or any other equitable relief) to cause Purchasers to consummate the transactions contemplated hereby, including to draw down the Debt Financing under the Debt Financing Agreements (including any bridge financing or “flex” provisions thereunder) or Alternative Financing commitments obtained under Section 5.12, and to effect the Closing on the terms and subject to the conditions in this Agreement, if, and only if: (i) all conditions in Section 8.1, Section 8.2 and Section 8.3 have been satisfied as of the date on which the Closing would otherwise be required to occur (other than those conditions that, by their nature, are to be satisfied at the Closing (provided such conditions would reasonably be expected to have been satisfied as of such date)), (ii) Purchasers fail to complete the Closing by the date the Closing would otherwise be required to have occurred pursuant to Section 2.3, (iii) the Debt Financing (or Alternative Financing in accordance with Section 5.12) has been funded or will be available to be funded to Purchasers at the Closing, and (iv) the Closing would reasonably be expected to occur substantially simultaneously with the draw down of Debt Financing (or Alternative Financing in accordance with Section 5.12)).
11.11 Interpretation; Absence of Presumption.
(a) It is understood and agreed that the specification of any dollar amount in the representations and warranties contained in this Agreement or the inclusion of any specific item in the Sellers Disclosure Letter is not intended to imply that such amounts or higher or lower amounts, or such items so included or other items, are or are not material, and no Party shall use the fact of the setting of any amount or the fact of the inclusion of any item in the Sellers Disclosure Letter in any dispute or controversy between the Parties as to whether any obligation, item or matter not described in this Agreement or included in the Sellers Disclosure Letter is or is not material for purposes of this Agreement.
(b) For the purposes of this Agreement, (i) words in the singular shall be held to include the plural and vice versa, and words of one gender shall be held to include the other gender as the context requires; (ii) references to the terms Article, Section, paragraph, Exhibit and Schedule are references to the Articles, Sections, paragraphs, Exhibits and Schedules to this Agreement unless otherwise specified; (iii) the terms “hereof,” “herein,” “hereby,” “hereto,” and derivative or similar words refer to this entire Agreement, including the Schedules and Exhibits hereto; (iv) references to “$” or cash shall mean U.S. dollars; (v) the word “including” and words of similar import when used in this Agreement shall mean “including without limitation,” unless otherwise specified; (vi) the word “or” shall not be exclusive; (vii) references to “written” or “in writing” include in electronic form; (viii) provisions shall apply, when appropriate, to successive events and transactions; (ix) Sellers and Purchasers have each participated in the negotiation and drafting of this Agreement and if an ambiguity or question of interpretation should arise, this Agreement shall be construed as if drafted jointly by the Parties hereto and no presumption or burden of proof shall arise favoring or burdening any Party by virtue of the authorship of any of the provisions in this Agreement; (x) a reference to any Person includes such Person’s successors and permitted assigns; (xi) any reference to “days” shall mean calendar days unless Business Days are expressly specified; and (xii) when calculating the period of time before which, within which or following which any act is to be done or step taken pursuant to this Agreement, the date that is the reference date in calculating such period shall be excluded and if the last day of such period is not a Business Day, the period shall end at the close of business on the next succeeding Business Day.
(c) If the Closing shall occur, notwithstanding anything in this Agreement to the contrary, any payment obligation of Purchasers hereunder shall be a joint and several obligation of Purchasers and the Transferred Entities.
-73-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
11.12 Headings; Definitions. The Section and Article headings contained in this Agreement are inserted for convenience of reference only and will not affect the meaning or interpretation of this Agreement.
11.13 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction or other authority to be invalid, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions of this Agreement (or portions thereof) shall remain in full force and effect and shall in no way be affected, impaired or invalidated so long as the economic or legal substance of the transactions contemplated hereby is not affected in any manner materially adverse to any Party hereto. If any provision of this Agreement (or any portion thereof) shall be held to be so broad as to be unenforceable, such provision shall be interpreted to be only so broad as is enforceable. Upon a determination that any term, provision, covenant or restriction of this Agreement is invalid, void or unenforceable, the Parties shall negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in a mutually acceptable manner in order that the transactions contemplated hereby be consummated as originally contemplated to the fullest extent possible.
11.14 No Recourse to Debt Financing Sources. Subject to the rights of the parties to the Debt Financing Agreements under the terms thereof, none of the Parties hereto, nor any of their respective, direct or indirect, former, current or future general or limited partners, managers, members, stockholders, officers, directors, Affiliates, employees, representatives, agents, successors or assigns (collectively, the “Related Persons”), shall have any rights or claims against the financing sources of the Debt Financing or any of their Affiliates in connection with this Agreement, the Debt Financing, or the transactions contemplated hereby or thereby, whether at law or equity, in contract, in tort or otherwise, nor shall any of the financing sources of the Debt Financing or any of their Affiliates have any obligations or liabilities to the Parties hereto or their respective Related Persons, all of which are hereby waived (provided that nothing in this Section 11.14 shall in any way limit or modify any of the obligations owed under the Debt Financing Agreements by the financing sources of the Debt Financing to the Purchasers and their Affiliates), and the financing sources of the Debt Financing and their Affiliates and their respective Related Persons shall not have any rights or claims against any Party hereto or any Related Person thereof, in connection with this Agreement or the Debt Financing, whether at law or equity, in contract, in tort or otherwise
[Remainder of page left intentionally blank]
-74-
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
IN WITNESS WHEREOF, this Agreement has been signed by or on behalf of each of the parties set forth below as of the day first above written.
| ALKERMES PHARMA IRELAND LIMITED | ||||
| By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇ | |||
| Name: | ▇▇▇▇▇ ▇▇▇▇▇ | |||
| Title: | Director | |||
| DARAVITA LIMITED | ||||
| By: | /s/ ▇▇▇ ▇▇▇▇▇▇▇ | |||
| Name: | ▇▇▇ ▇▇▇▇▇▇▇ | |||
| Title: | Director | |||
| EAGLE HOLDINGS USA, INC. | ||||
| By: | /s/ ▇▇▇▇▇ Frutes | |||
| Name: | ▇▇▇▇▇ Frutes | |||
| Title: | VP, CFO and Treasurer | |||
| RECRO PHARMA, INC. | ||||
| By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇▇ | |||
| Name: | ▇▇▇▇▇ ▇▇▇▇▇▇▇ | |||
| Title: | President and Chief Executive Officer | |||
| RECRO PHARMA LLC | ||||
| By: | /s/ ▇▇▇▇▇▇▇ ▇▇▇▇ | |||
| Name: | ▇▇▇▇▇▇▇ ▇▇▇▇ | |||
| Title: | President | |||
[Signature Page to Purchase and Sale Agreement]
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT A
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT A
Transitional Services Agreement Terms
| 1. Parties |
• “Supplier”: Alkermes Pharma Ireland Limited
• “Recipient Representative”: Recro Pharma, Inc.
• “Recipients”: Recro Pharma, Inc., Recro Pharma LLC (the “Company”) and Alkermes Gainesville | |
| 2. Defined Terms |
Capitalized terms used but not defined in this Exhibit A shall have the meanings set forth in the remainder of the Agreement. | |
| 3. Services |
Supplier shall provide, or cause its Subsidiaries to provide, the Services to the Recipients.
“Services” shall mean the following:
• The services listed on Schedule 1 of this Exhibit A (“Migration Services”)
• The services listed on Schedule 2 of this Exhibit A (“Transition Services”)
• Consulting Services (defined below in Section 8 hereof)
Alkermes Gainesville shall provide the Reverse Transition Services to Supplier or to one or more of its Subsidiaries designated by Supplier.
“Reverse Transition Services” shall mean the provision of (i) the packaging services for Ampyra, Zanaflex and Tizanidine, (ii) stability testing to support the last commercial batch of Avinza manufactured at Alkermes Gainesville’s facility and (iii) the clinical supply of ALKS 5461, in each case by Alkermes Gainesville to Supplier or its Subsidiaries from the Closing Date until June 30, 2016 or such earlier date on which Supplier notifies the Recipient Representative in writing that such packaging services and such clinical supply are no longer required, provided, however, that Supplier shall provide at least 90 days’ written notice of such termination. Supplier and Recipient Representative shall negotiate in good faith between the date of the Agreement and Closing terms and conditions for the provision of such packaging services and such clinical supply that are satisfactory in form and substance to the parties and their legal advisors and that are customarily found in agreements of this nature. | |
| 4. Cost |
• Migration Services: Actual out-of-pocket costs (including third party consent costs) incurred by Supplier and its Subsidiaries to provide the Services, as evidenced by Supplier’s or its Subsidiaries’ records.
• Transition Services: Supplier and its Subsidiaries’ standard full time equivalent (FTE) rates plus actual out-of-pocket costs (including third party consent costs) incurred by Supplier and its Subsidiaries to provide the Services, as evidenced by Supplier’s or its Subsidiaries’ records.
• Consulting Services: Supplier and its Subsidiaries’ standard fully burdened cost plus actual out-of-pocket costs incurred by Supplier and its Subsidiaries to provide the Services, as evidenced by Supplier’s or its Subsidiaries’ records.
• Reverse Transition Services:
• Fully burdened costs of Recipients
• Supplier to purchase all raw materials and finished goods upon termination of the Reverse Transition Services. | |
| 5. Payment Procedures |
• Invoices to be provided by the applicable of Supplier and Recipient Representative to the other monthly in arrears and to be paid by the applicable of Supplier and Recipient Representative within 45 days after receipt. Invoices shall be itemized and contain reasonable detail on the underlying cost constituents of the invoiced amount. | |
1
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| If Supplier (in the case of the Reverse Transition Services) or Recipient Representative (in the case of the Services) reasonably requests, such party shall have the right to access the books, records and employees of the other to the extent reasonably necessary to complete such audit of an invoice as is reasonably necessary to (i) determine whether costs have been stated correctly in the invoice or (ii) assist in the determination of any payment dispute.
• In the event of a payment dispute, the party with the payment obligation shall pay to the other of Supplier and Recipient Representative the undisputed portion and the disputed portion shall be subject to 15 days discussion between the parties, followed (to the extent necessary) by 15 days’ discussion between the CFOs of Supplier and Recipient Representative, or their designees, before any party may initiate any formal proceeding with respect to such dispute. | ||
| 6. IT Compliance |
• Customary provisions regarding compliance with Supplier’s and Recipient’s IT policies, practices and procedures. | |
| 7. Third Party Consents |
• Supplier shall use commercially reasonable efforts to procure all third party consents and licenses/sub-licenses required in connection with the provision and receipt of the Services. Costs incurred in connection therewith will be borne by the Recipient Representative.
• Supplier shall be responsible for and procure all third party consents and licenses/sub-licenses required in connection with the provision and receipt of the Reverse Transition Services. Costs incurred in connection therewith will be borne by Supplier. | |
| 8. Consulting Services |
• Until the three month anniversary of the Closing Date, [***] shall serve as acting General Manager of the Gainesville facility and an advisor to the management of Recipient Representative.
• Following such three month anniversary of the Closing Date, [***] will be reasonably available to act as an advisor to the management of Recipient Representative until the earlier of (i) October 1, 2015 or (ii) the Recipient Representative’s notice to Supplier that [***] Consulting Services are not required.
• In the performance of the Consulting Services, [***] shall remain employed by Supplier, and all Consulting Services shall be performed subject to the terms of his employment with Supplier. | |
| 9. Indemnification |
• To parallel the indemnity in the Agreement, except with no Deductible or De Minimis Damages. To cover, among other things, breach of representations and breach of covenants.
• In addition, Recipient Representative shall indemnify, defend and hold harmless Supplier and its Affiliates and [***], in his personal capacity, for all Losses to the extent resulting from or arising out of the Consulting Services. | |
| 10. Records |
• Records related to the Services and the Reverse Transition Services to be retained in accordance with the record retention provisions of the Agreement or in accordance with EU, US and International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) records retention requirements, whichever is longer. | |
| 11. Term |
• Migration Services shall be completed by and terminate within 45 calendar days following Closing; provided, that Recipient shall have the right to reasonably request additional information or documents up to an additional 45 days and Supplier shall make a good faith effort to cooperate with such requests.
• Transition Services shall be completed by and terminate by June 30, 2016.
• Reverse Transition Services shall have the duration described in this Exhibit A. | |
| 12. Termination |
• By Recipients at any time (solely with respect to the Services) upon 5 business days prior written notice (or with respect to such Services the cessation of which requires the cooperation of a third party, as promptly as practicable). | |
2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| • By Supplier upon 90 days’ written notice (solely with respect to Reverse Transition Services).
• By Recipients with respect to Reverse Transition Services upon 30 days’ written notice due to failure by Supplier to pay material amounts not in dispute that are greater than 90 days past due.
• By Supplier with respect to any of the Services upon 30 days’ written notice due to failure by Recipient Representative to pay material amounts not in dispute that are greater than 90 days past due.
• By mutual consent of the parties.
• Changes to Services or Reverse Transition Services shall require the written consent of Recipient Representative and Supplier. | ||
| 13. Governing Law/Jurisdiction |
As per Agreement. | |
| 14. Other |
Other customary terms and conditions satisfactory in form and substance to the parties and their legal advisors to be negotiated in good faith by the parties between the date of the Agreement and Closing. | |
| 15. Contact Information |
• Supplier Contact:
▇▇▇▇▇▇▇ ▇▇▇▇▇ Vice President Alliance Management Alkermes Pharma Ireland Limited ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇ T: ▇▇▇▇ ▇ ▇▇▇▇▇▇▇ F: ▇▇▇▇ ▇ ▇▇▇▇▇▇▇ E: ▇▇▇▇▇▇▇.▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇
• Recipients Contact:
▇▇▇▇▇ ▇▇▇▇▇ Head of Clinical Manufacturing Recro Pharma, Inc. ▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ O: ▇▇▇-▇▇▇-▇▇▇▇ | |
3
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Schedule 1
Migration Services
In each case, subject to the terms of the Agreement:
| • | Transfer of emails and other electronic data (including electronic documents and employee data) related primarily to the Business to the extent not fully transferred to Recipients at Closing; |
| • | Transfer of any hard copy records related primarily to the Business to the extent not fully transferred to Recipients at Closing; |
| • | Transfer of the existing safety database for Verelan/Verapamil (Argus/Quintiles) to Recipients or their nominated third party provider to the extent not fully transferred to Recipients at Closing; |
| • | Transfer of ownership for any applications/INDs currently owned by Supplier or its Subsidiaries (other than the Company or Alkermes Gainesville) (e.g. DMF, IND, NDA’s, etc.) that relate solely to the Products to the extent not fully transferred to Recipients at Closing, provided that Supplier and Recipient shall collaborate with respect to the letters/correspondence to be provided to Governmental Entities regarding transfer of regulatory responsibilities before such applications/INDs are transferred; |
| • | Transfer of information with respect to the Business and in the possession and control of Supplier or its Subsidiaries (other than the Company or Alkermes Gainesville) relating to: IP/patent litigation and employee litigation, provided that Supplier shall provide any necessary consents or letters reasonably requested by the Recipient Representative’s legal counsel to transfer such information relating to such litigation; R&D related primarily to the Products, manufacturing and facilities; CMC files (only to the extent not included in the Meloxicam DMF), Clinical Trial Masterfiles and case report forms and nonclinical data relating to the Products; in each case, to the extent not fully transferred to Recipients at Closing; |
| • | Assistance with the support and transfer to Recipients of (i) business relationships, (ii) audit, tax, accounting, financial, insurance, claims handling and treasury functions, (iii) employee files and (iv) ongoing regulatory activities (CMC, documentation, facilities), in each case of (i) through (iv) primarily related to the Business, to the extent not fully transferred to Recipients at Closing, provided that Supplier will collaborate with Recipient to transfer such items as soon as reasonably practicable after Closing; and |
| • | Assistance to Alkermes Gainesville employees who hold stock or stock options in Alkermes plc from Supplier and its Subsidiaries’ captive broker in exercising options, determining tax basis of equity grants, retrieving tax/transaction reports, and transferring shares to their personal accounts. |
4
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Schedule 2
Transition Services
Part I(a): IT SERVICES
| Services: | The services described below are those that are currently in use by Supplier and its Subsidiaries with respect to the Business. Supplier and Recipient Representative will agree upon which of these Services should be put in place as part of the Transition Services:
• SAP Services (including maintenance; GL account creation, cost center creation, reporting hierarchies)
• Maximo (Plant Maintenance)
• ComplianceWire Learning Management System
• LIMS (Thermo Scientific Laboratory Information Management System)
• SDMS/ELN (Waters)
• SLIM (H&A Scientific Stability Laboratory Information System)
• Veeva Vault (QA Doc Mgt)
• Octagon and SafeBio Pharma (Regulatory Submissions and eSignature)
• External Collaboration (SharePoint, Extranets)
• Intranet Support Services
• E-mail (SmartPhone, Tablet, Exchange, External gateways, Mobile Device Management, Spam Filtering, PGP)
• IT Help Desk Application (ServiceNow)
• Data separation / parsing for each application above
• Modifications to existing system configuration, reports and interfaces to support application and source data changes
• Development of new reports and interfaces necessary to support Alkermes Gainesville
• End-user Computing Infrastructure (Internet, SEP/PGP Wireless, Remote PC Desktop Control, Self-Service P/W Management, PC Helps/Vitalyst desktop support)
• Wide Area Network
• Active Directory Domain Services Support
• Network Infrastructure Services and Support (Backup/Replication, Monitoring, Anti-virus, VMware, Web Filtering, Firewalls, etc.)
• Remote Access System Support / VPN
• IT Contracts Management and Support
• IT Security Services and Support
• Pharmacovigilance / Adverse Event Reporting Services |
5
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Part I(b): EXCLUDED IT SERVICES
| Services: | The services described below are those that are currently in use by Supplier and its Subsidiaries with respect to the Business. Supplier and Recipient Representative have agreed that the Services listed below will not be put in place as part of the Transition Services:
• Oracle eBusiness Suite HRIS Services
• Oracle Fusion (Employee Performance and Comp Planning)
• Concur Travel & Expense System
• ADP Payroll/ADP Connect
• NextDocs (Change Control)
• Taleo (HR Recruiting and Applicant Tracking)
• Okta (Single-Signon)
• Third-Party Personnel Benefit Providers
• Backup Support Services (NetBackup, Data Domain)
• IT Quality Management Services and Support
• IT Management System (ITMS) Framework, Audit and Control Testing Support
Supplier shall provide, as part of the Migration Services, to Recipient flat files, data and/or an alternate solution (where appropriate) from the services described above in this Part I(b) of Schedule 2 as of the Closing Date, along with a file description. |
Part II: OTHER
| Services: | Supplier will provide the following agreed Services to Recipients:
• Transition Services with respect to ongoing prosecution of the patent applications owned by the Company
• Transition Services with respect to SOX controls for Alkermes Gainesville, i.e., documentation that identifies key processes and key controls within those processes |
6
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Exhibit B
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT B
Terms for a Development, Manufacturing and Supply Agreement for BC Parenteral Meloxicam
| Concept: | This Exhibit B sets forth certain terms agreed upon by the Parties relating to (A) the clinical supply of Finished Meloxicam and BC Parenteral Meloxicam; (B) the provision of development services with respect to the Chemistry, Manufacturing and Controls (CMC) section for bulk nanocrystals (in support of clinical trials related to BC Parenteral Meloxicam) of a New Drug Application (“NDA”) for BC Parenteral Meloxicam; and (C) the commercial supply of BC Parenteral Meloxicam (the “Commercial Supply”). The full terms and conditions for each of the foregoing, described in more detail below, will be set forth in a definitive Development, Manufacturing and Supply Agreement (the “Development and Supply Agreement”) by and between Alkermes Pharma Ireland Limited (“APIL”) and Recro Pharma LLC (“Recro Sub”) to be executed in accordance with the terms of the Agreement. Capitalized terms used but not defined in this Exhibit B shall have the meanings set forth in the remainder of the Agreement. | |
| Defined Terms: | ||
| “Allocable Overhead” means costs incurred by APIL or for its account which are attributable to APIL’s costs of supervisory services, general and administrative activities, occupancy (including utilities and property taxes), registrations, permits and licenses, insurance, depreciation, payroll, non-cash compensation, information systems, human resources and purchasing, as allocated to company departments based on space occupied, headcount or activity-based methods, in all cases as applied by APIL in accordance with GAAP and APIL’s accounting standards on a consistent basis.
“BC Meloxicam SC” means BC Parenteral Meloxicam that may not be designed for IV or IM administration.
“BC Parenteral Meloxicam” means any parenteral Meloxicam in bulk crystal/formulated nanocrystal form.
“Finished Meloxicam” means BC Parenteral Meloxicam in appropriate sealed injectable filled vials.
“Finished Meloxicam SC” means Finished Meloxicam that may not be designed for IV or IM administration.
“Fully Burdened Manufacturing Cost” means 100% of APIL’s manufacturing cost of BC Parenteral Meloxicam, Finished Meloxicam, and subject to additional agreement between the parties, BC Meloxicam SC and Finished | ||
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| Meloxicam SC, which shall include APIL’s costs of materials, labor, warehousing, quality assurance/control, delivery, storage, and Allocable Overhead. The Fully Burdened Manufacturing Cost will be calculated in a manner consistent with GAAP and APIL’s accounting standards on a consistent basis. | ||
| Commercial Supply: | Prior to the initiation of the first Phase III Clinical Trial for Finished Meloxicam, Recro Sub shall notify APIL as to whether APIL shall be required to supply Recro Sub (and such affiliates, licensees or distributors as Recro Sub may nominate) with 100% of their worldwide commercial requirements for BC Parenteral Meloxicam including BC Meloxicam SC (subject to additional agreement between the parties). If APIL shall be nominated as Recro Sub’s supplier, then in exchange for this supply, Recro Sub shall pay APIL on a cost-plus basis as set forth below. | |
| Clinical Supply: | APIL shall supply Recro Sub (and its affiliates) with 100% of their clinical supply requirements for Finished Meloxicam, Finished Meloxicam SC (subject to additional agreement between the parties), BC Parenteral Meloxicam, and BC Meloxicam SC (subject to additional agreement between the parties), and Recro Sub shall pay APIL for such clinical supply requirements in accordance with the terms set forth below. | |
| Pricing: | Commercial Supply. For APIL’s supply to Recro Sub (and such affiliates, licensees and distributors as Recro Sub may nominate) of BC Parenteral Meloxicam for commercial sale, Recro Sub shall pay APIL the Fully Burdened Manufacturing Cost plus [***]. | |
| Clinical Supply. Recro Sub shall pay APIL [***] for (i) Finished Meloxicam supplied by APIL for clinical use prior to FDA approval and (ii) BC Parenteral Meloxicam supplied by APIL for clinical use prior to FDA approval. The maximum amount of Finished Meloxicam and BC Parenteral Meloxicam that will be supplied by APIL to Recro Sub (or its affiliates) for clinical use shall be agreed by the parties in the Development and Supply Agreement but shall be no less than Recro Sub (or its affiliates) reasonably require to complete any clinical work relating to Finished Meloxicam, provided that, such maximum amount is subject to change as agreed between such parties and based on periodic updates of the development plan, clinical trial results and implications of such results, as well as feedback from regulatory authorities. Notwithstanding the foregoing, currently produced batches and stability work completed prior to the Closing Date shall be provided to Recro Sub at no cost. | ||
| APIL shall be responsible for procuring all components and materials, including active pharmaceutical ingredient (API), required to prepare, | ||
2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| manufacture, test, store and supply BC Parenteral Meloxicam and Finished Meloxicam, prior to final clinical or commercial labeling and packaging, for commercial and clinical use; provided, that, in the case of clinical use of Finished Meloxicam only, APIL shall supply finished product prior to labeling. | ||
| Recro Sub shall be responsible for product labeling and distribution to clinical sites or commercial distribution for BC Parenteral Meloxicam and Finished Meloxicam. | ||
| Expenses: | APIL shall pay all capital expenses required for the commercial manufacture of BC Parenteral Meloxicam at a 5-kilogram scale. | |
| If a NanoMill is required for the scale up of commercial manufacture of BC Parenteral Meloxicam to a 10-kilogram scale, Recro Sub shall be required to pay all capital expenses required for such scale up. | ||
| Upon tech transfer of the commercial manufacture of BC Parenteral Meloxicam to Recro Sub or its designated third party manufacturer, in accordance with the terms of the Development and Supply Agreement, (i) if Recro Sub has paid all capital expenses for such scale up, then APIL shall ship such NanoMill to Recro Sub or its designated third party manufacturer and (ii) Recro Sub shall pay APIL, pursuant to such tech transfer, any of APIL’s capital expenditures that were required for the commercial manufacture of BC Parenteral Meloxicam and have not been recouped through the Fully Burdened Manufacturing Cost for the cost of goods sold. | ||
| Exclusivity: | APIL shall supply BC Parenteral Meloxicam exclusively to Recro Sub (and its affiliates, licensees and/or distributors) and shall not supply BC Parenteral Meloxicam to any other person. | |
| Development: | Recro Sub and APIL shall establish a joint committee to coordinate, review and approve the CMC Development Services (defined below). | |
| Subject to such approval by such joint committee, APIL shall provide to Recro Sub (or such affiliate(s) as Recro Sub shall designate in writing) (i) reasonable support with respect to the CMC section of the NDA(s) for BC Parenteral Meloxicam sufficient to allow Recro Sub to complete and file such CMC sections to the same standard as APIL would apply in submission of an NDA for its own similar products, (ii) information and expertise, including advisory, related to BC Parenteral Meloxicam and (iii) CMC regulatory assistance during the clinical work for BC Parenteral Meloxicam (the services described in clauses (i) through (iii), the “CMC Development Services”). APIL shall provide such CMC Development Services at [***]. The maximum number of full time equivalent (FTE) hours that will be provided by APIL to Recro Sub (or its affiliates) in connection with such CMC Development Services shall be agreed by the parties in the Development and Supply Agreement. | ||
3
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| Term: | The term of the Development and Supply Agreement will commence when executed in accordance with the terms of the Agreement and extend until (i) ten (10) years from the date of the first commercial sale of Finished Meloxicam to a third party (the “First-Sale Term”), if the first commercial sale of Finished Meloxicam to a third party occurs on or before December 31, 2020 or (ii) December 31, 2020, if the first commercial sale of Finished Meloxicam to a third party does not occur on or before December 31, 2020 (the foregoing (i) and (ii), collectively, (the “Initial Period”)). | |
| During the Initial Period, Recro Sub can terminate the Development and Supply Agreement on 180 days’ prior written notice at any time subsequent to the first day on which a product is marketed by a third party pursuant to an abbreviated new drug application referencing Finished Meloxicam (i.e. the date of first generic entry). | ||
| After the First-Sale Term, the Development and Supply Agreement will automatically renew for successive one (1) year periods (the “Extension Periods”). Either party shall have the right to terminate the Development and Supply Agreement on 180 days’ prior written notice during an Extension Period; provided, that if APIL provides notice of its intent to terminate the Development and Supply Agreement, APIL will cooperate with Recro Sub for technology transfer of the process to another supplier, and Recro Sub will reimburse APIL for reasonable expenses incurred in the process. | ||
| At any time following the two year anniversary of the NDA approval for Finished Meloxicam, either party shall have the right to terminate the commercial supply agreement upon 12 months’ written notice; in such event APIL will cooperate with Recro Sub for technology transfer of the process to another supplier, and Recro Sub will reimburse APIL for reasonable expenses incurred in the process. | ||
| In the event that the reversion rights under Exhibit E of the Agreement are exercised, the Development and Supply Agreement shall automatically terminate as of the Reversion Date. | ||
| License: | Recro Sub and its affiliates shall grant a non-exclusive license to APIL of such intellectual property as is required solely for the purposes of APIL’s development, manufacture and supply of BC Parenteral Meloxicam and Finished Meloxicam pursuant to the Development and Supply Agreement. | |
| Other Terms: | The Development and Supply Agreement will also contain such other terms and conditions as may be agreed upon by the parties, including provisions relating to (i) forecasting, purchase orders, quality of the product, shelf life, | |
4
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| rejection of the product, storage, delivery, recalls and seizures, product complaints, facility audits, regulatory compliance, rights to improvements, changes in specifications, representations and warranties, insurance and indemnification and (ii) with respect to the CMC Development Services, the scope of the development services, responsibilities for the conduct of development and the schedule for the conduct of the development. In connection with the Development and Supply Agreement, the parties shall enter into a quality agreement pursuant to which, among other things, Recro Sub will be responsible for reviewing and releasing batches and APIL will be responsible for testing. In addition to the foregoing, the Development and Supply Agreement will contain other terms and conditions satisfactory in form and substance to the parties and their legal advisors as are customarily found in agreements of that nature. APIL shall hold the DMF for BC Parenteral Meloxicam. APIL shall permit a qualified third party on behalf of Recro Sub to review and comment on the DMF and its adequacy to support IND or NDA filings. |
5
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT C
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Exhibit C
Sample Adjustment Amount Statement ($‘000s)*
| $‘000 | ||||||
| Estimated Working Capital: |
||||||
| Accounts receivable |
9,464 | |||||
| Inventory |
11,014 | |||||
| Prepaid expenses and other current assets |
744 | |||||
| Accounts payable and accrued expenses |
(3,700 | ) | ||||
| Retention bonus accrual |
380 | |||||
| Vacation pay accrual |
181 | |||||
|
|
|
|||||
| Estimated working capital |
18,083 | (A) | ||||
|
|
|
|||||
| Estimated Closing Cash Amount |
1,500 | (B) | ||||
|
|
|
|||||
| Estimated Closing Indebtedness |
— | (C) | ||||
|
|
|
|||||
| Estimated Closing Transaction Fees |
— | (D) | ||||
|
|
|
|||||
| Target Working Capital |
19,000 | (E) | ||||
|
|
|
|||||
| Adjustment Amount |
583 | A + B - C - D - E | ||||
|
|
|
|||||
| * | Not included in the Adjustment Statement are amounts in respect of the Retention Bonuses, vacation accruals through December 31, 2014, and the [***] claim, all of which will be paid directly by Sellers. |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT D
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT D
Excluded Assets
The following equipment:
| • | NM60 Media Mill |
| • | NM60 Chamber |
| • | NM60 Agitator |
| • | NM60 Chamber Removal Cart |
| • | NM60 Agitator Removal Cart |
| • | NM-60 WM 840 Pump |
| • | NM60 Chamber w/extra cooling |
| • | NM-60 Mill |
| • | NM-60 Cart |
| • | NM-60 Chamber |
| • | NM Agitator |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Exhibit E
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Exhibit E to Agreement
Earn-Out Consideration
| ARTICLE 1 | Definitions. |
The following terms shall have the following meaning for this Exhibit E; and terms used, but not defined in this Exhibit E, shall have the meanings set forth in the remainder of the Agreement.
(a) “Commercially Reasonable Efforts” shall mean, with respect to the efforts to be expended by Purchaser and its Affiliates, licensees and sublicensees with respect to the Development Milestones and Commercial Milestones, reasonable, diligent, good faith efforts to accomplish any such Development Milestones and Commercial Milestones as is commonly used in the pharmaceutical industry generally to accomplish a similar objective under similar circumstances, it being understood and agreed that with respect to the research, development and commercialization of any Earn-Out Product, such efforts shall be substantially equivalent to those efforts and resources commonly used in the pharmaceutical industry generally by a pharmaceutical company for a product owned by it or to which it has rights, which product is at a similar stage in its development and is of similar market potential taking into account efficacy, safety, approved labeling, the competitiveness of alternative products in the marketplace, the patent and other proprietary position of the product, the likelihood of regulatory approval, the profitability and commercial potential of the product, but without regard to any Earn-Out Consideration payable under this Exhibit E.
(b) “Divestiture” (and other correlative terms) shall mean any transaction in which any Earn-Out Product or any intellectual property assets related to the same are divested or transferred by any means, including by way of merger, consolidation, asset acquisition or sale, license, sublicense, purchase, sale, assignment or other similar transfer.
(c) “Earn-Out Consideration” shall mean, collectively, (i) Development Milestone Earn-Out Consideration, (ii) Commercial Milestone Earn-Out Consideration, and (iii) Net Sales Earn-Out Consideration.
(d) “Earn-Out Product Patents” shall mean (i) the Nanotechnology Patents, (ii) the Meloxicam Transferred Patents, (iii) the OCR Patents and (iv) all Patents of Purchaser and its Affiliates, licensors, licensees or sublicensees that claim an Earn-Out Product or manufacture or use thereof, together with all Patents that claim priority (in whole or in part, directly or indirectly) with any of the foregoing of clauses (i), (ii), (iii) or (iv).
(e) “Earn-Out Products” shall mean (i) Meloxicam, and (ii) any other product discovered or identified using the Nanotechnology IP, the OCR IP or the Meloxicam Transferred Patents, and that contains the same active pharmaceutical ingredient as Meloxicam (including any salts or other versions of such active pharmaceutical ingredient).
(f) “First Commercial Sale” shall mean, on an Earn-Out Product-by-Earn-Out Product and country-by-country basis, the first commercial sale in an arms’ length transaction of an Earn-Out Product to a Third Party by Purchaser or any of its Affiliates, licensees or sublicensees in such country following Regulatory Approval of such Earn-Out Product in such country.
(g) “IND” shall mean an investigational new drug application filed with the FDA, the competent authorities of a European Union member state, or equivalents in other countries or regulatory jurisdictions for authorization to commence clinical studies of a pharmaceutical product.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(h) “Know-How” shall mean all proprietary data, information, knowledge, know-how, inventions, discoveries, trade secrets, processes, techniques, strategies, methods, practices, skills, experience, documents, apparatus, devices, assays, screens, databases (including safety databases), database structures and data analysis methods, compositions, materials, methods, formulas, improvements, clinical and non-clinical study reports, test data including pharmacological, biological, chemical, biochemical, toxicological, and clinical test data, analytical and quality control data, stability data, studies and procedures.
(i) “MAA” shall mean a Marketing Authorization Application as defined in EU Directive 2001/83/EC and EU Regulation (EC) No. 726/2004.
(j) “Meloxicam Transferred Patents” shall have the meaning set forth in Section E of the Sellers Disclosure Letter.
(k) “Nanotechnology IP” shall have the meaning set forth in the Intellectual Property Transfer and License Agreement and, subsequently, in the IP License Agreement.
(l) “Nanotechnology Patents” shall have the meaning set forth in the Intellectual Property Transfer and License Agreement and, subsequently, in the IP License Agreement.
(m) “NDA” shall mean a New Drug Application or Supplemental New Drug Application filed with the FDA.
(n) “Net Sales” shall mean, consistent with GAAP:
(i) Subject to clause (ii) of this definition, the aggregate gross amount invoiced to unrelated Third Parties by Purchaser, its Affiliates, its licensees and its sublicensees for the sale of Earn-Out Products, less to the extent applicable to such sale: (A) trade, cash and quantity discounts, if any, actually accrued or paid; (B) credits, allowances and adjustments actually accrued or paid to customers, including credits for rejected or returned Earn-Out Products previously sold; (C) freight, insurance and other transportation costs actually accrued or paid, to the extent separately identified on the invoice; (D) rebates or reimbursements actually accrued or paid to managed health care organizations, national, federal, state, or local governments (or their agencies), and managed health organizations (including Medicaid rebates), and (E) taxes, including value added taxes (VAT) (other than taxes on Purchaser’s, its Affiliates’, its licensees’ or its sublicensees’ income), customs duties or other governmental charges on sales or use actually paid by Purchaser, its Affiliates, its licensees or its sublicensees with respect to the sale of such Earn-Out Products. No fines, penalties or comparable payments to national, federal, state, or local governments (or their agencies) or to other third parties shall be deductible from Net Sales.
(ii) Sales between Purchaser, its Affiliates, its licensees or its sublicensees shall be disregarded for the purposes of calculating Net Sales as long as the Earn-Out Products are (A) resold to an unrelated Third Party in which case the final sale to such unrelated Third Party shall be included in Net Sales or (B) transferred or disposed of by Purchaser, its Affiliates, its licensees or its sublicensees for a purpose specified in clause (i) of this definition. Transfers or dispositions of Earn-Out Products, where on a non-profit basis and in line with normal industry practice, (1) for charitable purposes; (2) for preclinical, clinical trial, or non-commercial manufacturing purposes; or (3) for regulatory or governmental purposes shall not in each case be deemed “sales” for the purposes of calculating Net Sales. In addition, transfers or dispositions of free promotional samples of Earn-Out Products in line with normal industry practice shall not be deemed “sales” for the purposes of calculating Net Sales. Otherwise, for the purposes of calculating Net Sales, a “sale” shall include any transfer or other disposition of any Earn-Out Product for consideration.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
With respect to sales of Earn-Out Products invoiced in U.S. dollars, Net Sales shall be determined in U.S. dollars. With respect to sales of Earn-Out Products invoiced in a currency other than U.S. dollars, Net Sales shall be determined by converting the currencies in which the sales are made into U.S. dollars, at rates of exchange determined in a manner consistent with Purchaser’s, its Affiliates’, its licensees’ or its sublicensees’, as applicable, method for calculating rates of exchange in the preparation of such entity’s annual financial statements in accordance with GAAP consistently applied. No amount for which deduction is permitted pursuant to this definition shall be deducted more than once.
(o) “Net Sales Earn-Out Consideration Term” shall mean, on an Earn-Out Product-by-Earn-Out Product and country-by-country basis, the period of time commencing upon the First Commercial Sale of an Earn-Out Product and ending upon the later of (i) the fifteenth (15th) anniversary of the First Commercial Sale of such Earn-Out Product in such country, and (ii) the date of the last to expire Valid Claim of an Earn-Out Product Patent covering such Earn-Out Product in such country.
(p) “OCR IP” shall have the meaning set forth in the Intellectual Property Transfer and License Agreement and, subsequently, in the IP License Agreement.
(q) “OCR Patents” shall have the meaning set forth in the Intellectual Property Transfer and License Agreement and, subsequently, in the IP License Agreement.
(r) “Patents” shall mean any and all patents and patent applications (which for purposes of this Agreement shall include certificates of invention and applications for such certificates), including any divisionals, continuations, continuations-in-part, substitutions, reissues, re-examinations, revalidations, extensions (including pediatric exclusivity patent extensions), registrations, supplementary protection certificates, renewals, and foreign equivalents of any such patents or patent applications.
(s) “Regulatory Approval” shall mean, with respect to a country or extra-national territory, all approvals, licenses, registrations or authorizations of any Regulatory Authority necessary in order to commercially distribute, sell or market a drug product in such country or some or all of such extra-national territory.
(t) “Regulatory Authority” shall mean any supra-national, federal, national, regional, state, provincial or local governmental regulatory agencies, departments, bureaus, commissions, councils or other government entities regulating or otherwise exercising authority with respect to the development, manufacture and commercialization of drug products, including the FDA.
(u) “Regulatory Materials” shall mean regulatory applications, submissions, notifications, registrations, or other filings made to or with a Regulatory Authority that are necessary or reasonably desirable in order to develop, manufacture, market, sell or otherwise commercialize a product in a particular country or regulatory jurisdiction. Regulatory Materials include INDs, ▇▇▇▇ and NDAs (as applications, but not the approvals with respect thereto).
(v) “Third Party” shall mean, as of any relevant time, any Person who is not an Affiliate of Purchaser.
(w) “United States” or “U.S.” shall mean the United States of America, including its territories and possessions, the District of Columbia and Puerto Rico.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(x) “Valid Claim” shall mean a claim of an issued or pending Patent which claim (i) in the case of an issued Patent, has not been found to be unpatentable, invalid or unenforceable by a court or other authority of competent jurisdiction, from which decision no appeal is taken or can be taken and which otherwise has not been dedicated to the public or finally disclaimed, and (ii) in the case of a pending Patent, a Valid Claim shall not include a claim in a pending Patent that has a filing date or an earliest claimed priority date that is more than five (5) years prior to the date upon which pendency of the pending Patent is determined.
Definitions for each of the following terms are found in this Exhibit E as indicated below:
| Defined Term |
Location | |
| Assigned Reversion IP Assets | Section 4.2(c) | |
| Assigned Reversion Know-How | Section 4.2(c) | |
| Assigned Reversion Patents | Section 4.2(c) | |
| Challenge Period | Section 4.1(b) | |
| Commercial Milestone Earn-Out Consideration | Section 2.1(b)(i) | |
| Commercial Milestones | Section 2.1(b)(i) | |
| Cure Period | Section 4.1(c) | |
| Development Milestone Earn-Out Consideration | Section 2.1(a)(i) | |
| Development Milestones | Section 2.1(a)(i) | |
| Disagreement Notice | Section 4.1(b) | |
| Divested Assets | Section 2.4 | |
| NDA Requirement | Section 3.1 | |
| Net Sales Earn-Out Consideration | Section 2.1(c)(i) | |
| Net Sales Report | Section 2.2 | |
| Reversion Date | Section 4.1(d) | |
| Reversion Event | Section 4.1(a) | |
| Reversion Material | Section 4.2(j) | |
| Reversion Notice | Section 4.1(b) | |
| Transferee | Section 2.4 |
| ARTICLE 2 | Earn-Out Consideration. |
2.1 Earn-Out Consideration.
(a) Development Milestone Earn-Out Consideration.
(i) The following amounts (“Development Milestone Earn-Out Consideration”) shall be payable in accordance with Section 2.7 of the Agreement and this Exhibit E upon achievement of the following events (“Development Milestones”) by Purchaser and its Affiliates, licensees and sublicensees, and shall be non-refundable and non-creditable and not subject to deduction or set-off:
| Development Milestone |
Amount of Development Milestone Earn-Out Consideration (U.S. Dollars) |
|||
| Submission of an NDA for the first Earn-Out Product |
$ | 10,000,000.00 | ||
| Approval of an NDA for the first Earn-Out Product |
$ | 30,000,000.00 | ||
(ii) Purchaser shall notify and pay to APIL each Development Milestone Earn-Out Consideration within thirty (30) calendar days after the occurrence of the corresponding Development Milestone. Each such payment shall be made by wire transfer of immediately available funds to such account or accounts as are designated in writing by APIL.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
(b) Commercial Milestone Earn-Out Consideration.
(i) The following amounts (“Commercial Milestone Earn-Out Consideration”) shall be payable in accordance with Section 2.7 of the Agreement and this Exhibit E following the first calendar year during which the aggregate annual Net Sales of Earn-Out Products by Purchaser and its Affiliates, licensees and sublicensees first exceed the threshold amounts set forth in the table below (“Commercial Milestones”), and shall be non-refundable and non-creditable and not subject to deduction or set-off:
| Commercial Milestones |
Amount of Commercial Milestone Earn-Out Consideration (U.S. Dollars) |
|||
| $[***] in annual Net Sales |
$ | [ | ***] | |
| $[***] in annual Net Sales |
$ | [ | ***] | |
| $[***] in annual Net Sales |
$ | [ | ***] | |
(ii) Purchaser shall notify and pay to APIL each Commercial Milestone Earn-Out Consideration within thirty (30) calendar days after the end of the calendar quarter in which the corresponding Commercial Milestone is achieved. Each such payment shall be made by wire transfer of immediately available funds to such account or accounts as are designated in writing by APIL.
(c) Net Sales Earn-Out Consideration.
(i) During the Net Sales Earn-Out Consideration Term, Purchaser shall pay in accordance with Section 2.7 of the Agreement and this Exhibit E an amount of [***] percent ([***]%) of the aggregate Net Sales of Earn-Out Products (“Net Sales Earn-Out Consideration”), which amount shall be non-refundable and non-creditable and not subject to deduction or set-off.
(ii) If, pursuant to Section 2.1(c)(i) of this Exhibit E, any Net Sales Earn-Out Consideration is payable on Net Sales of an Earn-Out Product in the U.S. and there is no Valid Claim of an Earn-Out Product Patent in the U.S. covering such Earn-Out Product at the time of such sale, the percentage applicable to calculate such Net Sales Earn-Out Consideration shall be reduced by thirty percent (30%) from the percentage set forth in Section 2.1(c)(i) of this Exhibit E.
2.2 Net Sales Reports. During the Net Sales Earn-Out Consideration Term, (a) within five (5) Business Days after the end of each calendar quarter, Purchaser shall provide an estimate of the Net Sales, on a Earn-Out Product-by-Earn-Out Product and country-by-country basis, to APIL for the preceding calendar quarter and (b) within forty-five (45) calendar days after the end of each calendar quarter, Purchaser shall provide a sales report (“Net Sales Report”), on a Earn-Out Product-by-Earn-Out Product and country-by-country basis, to APIL showing a reconciliation of gross sales to Net Sales of each Earn-Out Product during such calendar quarter reporting period (including the related permitted deductions) and the Net Sales Earn-Out Consideration payable with respect thereto. Purchaser shall pay to APIL the Net Sales Earn-Out Consideration for each calendar quarter at the time of submission of the corresponding Net Sales Report. If no Net Sales Earn-Out Consideration is due for any period hereunder following commencement of the reporting obligation, Purchaser shall so report.
2.3 Late Payments. If APIL does not receive payment of any sum due to them on or before the due date, simple interest shall thereafter accrue on the sum due to APIL until the date of payment at the per annum rate of two percent (2%) over the then-current prime rate quoted by Citibank in New York City or the maximum rate allowable by applicable Law, whichever is lower.
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
2.4 Divestitures. If at any time after the Closing, Purchaser Divests to a Third Party or an Affiliate any Earn-Out Product, Earn-Out Product Patent or any other intellectual property asset related to an Earn-Out Product (collectively, “Divested Assets” and the party receiving any Divested Assets the “Transferee”), Purchaser shall: (a) make provision for the Transferee to assume and succeed to the obligations of Purchaser set forth in this Exhibit E; and (b) prior to or simultaneously with the consummation of any such Divestiture, cause such Transferee to provide to APIL an instrument of assumption in a reasonable form for the benefit of APIL effecting the assumption and succession described in the foregoing Clause (a), and proof satisfactory to APIL of such Transferee’s financial capacity to assume Purchaser’s obligations set forth in this Exhibit E. Purchaser shall remain liable to APIL for all obligations set forth in this Exhibit E following any such Divestiture.
| ARTICLE 3 | Diligence; Reporting; Audit. |
3.1 Diligence. Purchaser, itself or through one or more of its Affiliates, licensees and sublicensees, shall use Commercially Reasonable Efforts to achieve each of the Development Milestones and Commercial Milestones. Without limiting the foregoing, Purchaser, itself or through one or more of its Affiliates, licensees and sublicensees, shall file an NDA for an Earn-Out Product with the FDA on or before [***] (the “NDA Requirement”).
3.2 Reporting.
(a) For so long as any Earn-Out Product is in development, on each anniversary of the Closing Date, Purchaser shall provide a written report to APIL detailing the development activities with respect to the Earn-Out Products completed for the past annual reporting period and anticipated to be undertaken for the next twelve (12) months period. At a minimum, such report shall include a list and general status (i.e., what stage in discovery/development using Purchaser’s internal measures) of each Earn-Out Product then in development, and any ongoing pre-clinical or clinical activities (including initiations and cessations) and results, and submission and approvals to or from Regulatory Authorities (including anticipated date of achievement of the Development Milestones), and any other similar information relating to development activities for the Earn-Out Products. Purchaser shall cause its senior officers from its research and clinical development operations to be reasonably available to APIL to answer questions related to the matters required to be discussed in each report.
(b) For so long as any Earn-Out Product is being marketed, sold, or otherwise commercialized, within sixty (60) days of the end of each calendar quarter, Purchaser shall provide a written report to APIL detailing the commercialization efforts with respect to the Earn-Out Products completed for the past quarterly reporting period and anticipated to be undertaken for the next calendar quarter and for the three (3) calendar quarters thereafter. At a minimum, such report shall include with respect to the commercialized Earn Out Products marketing and sales efforts, forecasted sales, pricing changes, anticipated date of achievement of the Commercial Milestones, and any other similar information relating to commercialization activities for the Earn-Out Products. Purchaser shall cause its senior officers from business operations to be reasonably available to APIL to answer questions related to the matters required to be discussed in each report.
3.3 Audit. Purchaser shall maintain, and shall cause its Affiliates, licensees and sublicensees to maintain, complete and accurate books and records in sufficient detail to permit APIL, at its expense, to confirm the achievement of Development Milestones and Commercial Milestones, and the calculation of Earn-Out Consideration payable under this Agreement and this Exhibit E. Upon reasonable prior notice,
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
such books and records shall be open during regular business hours for a period of three (3) years from the creation of individual books and records for examination, by an independent certified public accountant selected by APIL and reasonably acceptable to Purchaser for the sole purpose of verifying for APIL the accuracy of the financial statements, reports and notices furnished by Purchaser pursuant to this Agreement and this Exhibit E, and of any payments made, or required to be made, by Purchaser to APIL pursuant to this Agreement and this Exhibit E. Any amounts shown to be owed to APIL but unpaid shall be paid by Purchaser within thirty (30) days after the accountant’s report, plus interest (as set forth in Section 2.3 of this Exhibit E) from the original due date. If Purchaser is found to have underpaid amounts owed to APIL by five percent (5%) or more, then Purchaser shall also pay for the conduct of the audit. In the event that Purchaser has overpaid APIL, at APIL’s option, Purchaser shall either credit the amount of any such overpayment to amounts subsequently due by Purchaser to APIL under this Exhibit E or APIL shall reimburse Purchaser the amount of any such overpayment.
| ARTICLE 4 | Reversion. |
4.1 Determination of Reversion Event.
(a) A “Reversion Event” shall exist in the event that Purchaser, itself or through one or more of its Affiliates, licensees and sublicensees, (i) fails to satisfy the NDA Requirement, or (ii) in the reasonable judgment of APIL, fails to comply with its Commercially Reasonable Efforts requirements under Section 3.1 of this Exhibit E; provided that, any such failure is not attributable to the material breach by APIL or any of its Affiliates of any of the Ancillary Agreements, which material breach was noticed by Purchaser prior to its receipt of a Reversion Notice from APIL under this Section 4.1.
(A) In the event the failure to satisfy the NDA Requirement is the result of a change in the FDA’s policies or procedures regarding the approval of the Earn-Out Product or drugs in the same class as the Earn-Out Product, and Purchaser, its Affiliates, licensees or sublicensees have used Commercially Reasonable Efforts to accommodate such change and were still unable to satisfy the NDA Requirement, then the deadline shall be extended for a reasonable period of time, but not more than three-hundred sixty-five (365) calendar days or such longer period of time as determined by APIL in good faith based on the impact of such change in the FDA’s policies or procedures on their ability to accommodate such change in policies or procedures. (B) In the event the failure to satisfy the NDA Requirement is the result of other delays or circumstances that are outside of the reasonable control of Purchaser or its Affiliates, licensees and sublicensees, and Purchaser, its Affiliates, licensees or sublicensees have used Commercially Reasonable Efforts consistent with Section 3.1 of this Exhibit E to overcome such delay or circumstance, then APIL will reasonably consider extending the deadline for a reasonable period of time, but not more than three-hundred sixty-five (365) days, to overcome such failure. In each case of (A) or (B), the compliance with such new deadline shall remain an obligation of Purchaser, its Affiliates, licensees or sublicensees subject to their diligence efforts under Section 3.1 of this Exhibit E and APIL’s rights under this Section 4.1(a).
(b) Upon a Reversion Event, APIL shall provide Purchaser with written notice of such Reversion Event (a “Reversion Notice”). If Purchaser disagrees with APIL regarding the existence of a Reversion Event, it shall notify APIL within ten (10) Business Days of receipt of a Reversion Notice of its disagreement (such period, the “Challenge Period,” and such notice, a “Disagreement Notice”). For a period of thirty (30) Business Days following the delivery of such Disagreement Notice, Purchaser and APIL shall seek in good faith to come to an agreement on the existence of the Reversion Event. If at the end of such thirty (30) Business Day period the Purchaser and APIL have not reached an agreement, they shall jointly select an independent mediator, free of any conflict with either Party, having the requisite licensing and pharmaceutical industry experience to render a decision regarding the existence of a Reversion Event, and selected from a panel of persons experienced in the pharmaceutical and life sciences
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
industries provided by Judicial Administration and Arbitration Services or its successor (“JAMS”). If the Parties do not agree on an independent mediator within five (5) Business Days of initiating mediation under this Section 4.1(b), the independent mediator shall be selected by JAMS in accordance with its rules. Each Party shall prepare and submit a written summary of such Party’s position, which shall not exceed twenty-five (25) pages, and any relevant evidence in support thereof to the independent mediator within ten (10) Business Days of the selection of the independent mediator. Upon receipt of such summaries from both Parties, the independent mediator shall provide copies of the same to the other Party. The independent mediator shall be authorized to solicit briefing or other submissions on particular questions and to set specific page limits for such additional briefing and submissions. Within five (5) Business Days of the delivery of such summaries by the independent mediator, each Party shall submit a written rebuttal of the other Party’s summary and may also amend and re-submit its original summary, with each Party’s response to include a supporting explanation of why its proposed terms are more appropriate than the other Party’s proposed terms and any documentary evidence in support thereof. Oral presentations shall not be permitted unless otherwise requested by the independent mediator. Only if so permitted, a neutral location of any such oral presentations shall be selected by the independent mediator. The independent mediator shall make a final decision with respect to the arbitration matter within ten (10) Business Days following receipt of the last of such rebuttal statements submitted by the Parties, and shall make a determination by selecting the resolution proposed by one of the Parties that as a whole is the most fair and reasonable to the Parties in light of the totality of the circumstances, and shall provide the Parties with a written statement setting forth the basis of the determination in connection therewith. For clarity, the independent mediator shall only have the right to select a resolution proposed by one of the Parties in its entirety and without modification. The decision of the independent mediator shall be controlling regarding the existence of a Reversion Event. The Purchaser and APIL shall bear the costs of the independent mediator equally.
(c) If the existence of a Reversion Event, other than one that involves a failure to satisfy the NDA Requirement, is confirmed, through (i) Purchaser’s non-delivery of a Notice of Disagreement or (ii) through the determination of an independent mediator, Purchaser, itself or through one or more of its Affiliates, licensees or sublicensees shall have the right to cure such Reversion Event for a period of four (4) months from the determination of such Reversion Event (such period, the “Cure Period”) and, if it elects to exercise such right, shall notify APIL in writing of the same. In the case that such Reversion Event is cured in APIL’s reasonable judgment during or by the end of the Cure Period, such Reversion Event shall no longer exist and APIL shall not have the right to invoke any other rights under this Article 4 in connection with such event that would have remained a Reversion Event but for such cure.
(d) For purposes of this Article 4, the “Reversion Date” shall be (i) the date on which the Challenge Period expires without Purchaser having sent a Disagreement Notice to APIL, unless the Reversion Event at issue qualifies for a right to cure pursuant to Section 4.1(c) of this Exhibit E and Purchaser has elected to exercise such right to cure in which case clause (d)(iii) below shall apply, (ii) where the Reversion Event involves a failure to satisfy the NDA Requirement and Purchaser has sent a Disagreement Notice in connection therewith, the date on which the Parties agree, or the independent mediator issues its determination, that such Reversion Event has occurred, or (iii) where the Reversion Event does not involve a failure to satisfy the NDA Requirement, and Purchaser has elected its right to cure pursuant to Section 4.1(c) of this Exhibit E, the date on which the Cure Period expires without Purchaser having cured such Reversion Event to APIL’s reasonable satisfaction.
4.2 Events Upon Determination of a Reversion Event.
(a) On the Reversion Date, all licenses and other rights of Purchaser and its Affiliates, licensees and sublicensees under the Intellectual Property Transfer and License Agreement
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
and, subsequently, the IP License Agreement (i) with respect to the Nanotechnology IP shall automatically terminate in their entirety, and (ii) with respect to the OCR IP shall automatically terminate solely in regards to the Earn-Out Products.
(b) As of the Reversion Date, but subject to Sections 4.2(h), 4.2(i) and 4.2(j), Purchaser, and its Affiliates, licensees and sublicensees shall cease all research, development, manufacture, sales, offers to sell, use, importation and commercialization of the Earn-Out Products.
(c) Promptly following the Reversion Date, Purchaser shall (i) assign to APIL the Meloxicam Transferred Patents and any other Patents owned or controlled by it and its Affiliates, licensees or sublicensees solely relating to the Earn-Out Products (collectively, the “Assigned Reversion Patents”), and (ii) transfer to APIL all Know-How owned or controlled by it and its Affiliates, licensees or sublicensees solely relating to the Earn-Out Products (the “Assigned Reversion Know-How” and together with the Assigned Reversion Patents, the “Assigned Reversion IP Assets”).
(d) As of the Reversion Date, Purchaser grants to APIL a non-exclusive, worldwide license (sublicenseable through multiple tiers) under all Patents and Know-How owned or controlled by Purchaser and its Affiliates, licensees or sublicensees (other than Assigned Reversion Patents and Assigned Reversion Know-How) that are practiced by or on behalf of Purchaser and its Affiliates, licensees and sublicensees as of the Reversion Date that are necessary or useful to research, develop, manufacture, sell, offer to sell, use, import or otherwise commercialize any of the Earn-Out Products.
(e) Promptly following the Reversion Date, Purchaser shall assign to APIL all right, title and interest in and to those trademarks used exclusively with the Earn-Out Products, excluding any such trademarks that include, in whole or part, any corporate name or logo of Purchaser or its Affiliate.
(f) Promptly following the Reversion Date, Purchaser shall (i) transfer and assign to APIL all Regulatory Materials and Regulatory Approvals relating to the Earn-Out Products, or, if not possible, grant to APIL an exclusive right of reference thereunder.
(g) To the extent that any payments would be owed by Purchaser to any Third Party under any agreement with such Third Party that is applicable to the exercise by APIL of any (sub)license, right of reference or other right provided in this Section 4.1, Purchaser shall notify APIL of the existence and anticipated amounts of such payments and APIL shall have the right either to decline such (sub)license, right of reference or other right provided in this Section 4.1 or to take the same, in which case APIL agrees to comply with any obligations under such agreement of Purchaser that apply to APIL and of which APIL was informed by Purchaser and to make such payments.
(h) Promptly following the Reversion Date and as requested by APIL, Purchaser shall, and shall cause its Affiliates, licensees and sublicensees to, (i) wind up the performance of any clinical trials for Earn-Out Products ongoing as of the Reversion Date, or (ii) transfer and assign to APIL, to the extent assignable by Purchaser in accordance with applicable Law, the management and continued performance of any clinical trials for Earn-Out Products ongoing as of the Reversion Date (provided that if the management and continued performance thereof is not assignable, then at the request of APIL, Purchaser shall, and shall cause its Affiliates, licensees and sublicensees to, continue to manage and perform such clinical trial(s) for a limited time period at the direction of APIL) the reasonable and documented out-of-pocket cost of which that is incurred after the Reversion Date shall be borne by APIL.
(i) Promptly following the Reversion Date and as requested by APIL, Purchaser shall assign to APIL any agreements with third party suppliers covering the supply or sale of the Earn-Out Products, or, if such agreements cover other products or do not permit assignment under their terms, then,
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
to enable APIL to qualify an alternate, validated source of supply, Purchaser shall supply finished Earn-Out Products for a reasonable period (not to exceed six (6) months) and at a cost equal to the cost of goods for any such Earn-Out Product calculated in accordance with industry standards (including overhead) plus [***].
(j) As of the Reversion Date, if Purchaser or any of its and its Affiliates, licensees or sublicensees have any inventory of Earn-Out Product, or any components thereof, suitable for use in clinical trials or for commercialization (“Reversion Material”), Purchaser and its Affiliates, licensees and sublicensees shall offer in writing to sell the Reversion Material to APIL at Purchaser’s or the applicable Affiliate’s, licensee’s or sublicensee’s fully-allocated cost of manufacturing, and APIL shall have the option (but no obligation) to purchase the same by responding in writing to such offer within thirty (30) days. If APIL does not exercise such option, Purchaser and its Affiliates, licensees or sublicensees shall be entitled to sell any such Reversion Material, subject to any Earn-Out Consideration applicable to such sale pursuant to the terms and conditions of this Exhibit E.
4.3 Without limiting the generality of this Article 4, any assignment, transfer, license or other right made or granted to APIL pursuant to this Article 4 shall be effected without any consideration payable by APIL.
4.4 Without limiting the generality of this Article 4, following the assignment and transfer under Section 4.2(c), (a) Purchaser shall have no right to use any of the Assigned Reversion IP Assets; (b) APIL shall have at its expense the sole and exclusive right to prosecute, maintain, defend and enforce all Assigned Reversion Patents assigned to APIL pursuant to such Section 4.2(c), and for purposes of all those activities, (i) APIL shall be treated as the owner of such Assigned Reversion Patents, and shall be solely responsible for the costs associated with such activities and shall have the sole right to retain any and all recoveries resulting from such activities, and (ii) to the extent required by applicable Law, at the cost of APIL, Purchaser shall, and shall cause each of its Affiliates, licensees and sublicensees to, join any suit or proceeding regarding any such Assigned Reversion Patents, or designate APIL (or an Affiliate thereof) as such party’s authorized agent for such Assigned Reversion Patents.
4.5 Purchaser shall ensure that Purchaser receives from any of its licensees and sublicensees all rights necessary for Purchaser to effectuate the assignments and transfers to APIL and to grant to APIL the rights and licenses set forth in this Article 4.
4.6 Purchaser shall not, and shall ensure that its Affiliates shall not, grant any Lien in or to any Assigned Reversion IP Assets, or take any action or commit any omission that may adversely affect or in any way impair, interfere with or prevent APIL’s right to receive the benefit of the assignments, transfers and licenses granted under this Article 4.
4.7 The right of reversion hereunder shall be APIL’s sole and exclusive remedy for Purchaser’s failure to satisfy the NDA Requirement or failure to comply with its Commercially Reasonable Efforts requirements under Section 3.1 of this Exhibit E, provided the foregoing shall in no way limit APIL’s right to collect and be paid any Earn-Out Consideration based on Development Milestones or Commercial Milestones achieved as of the Reversion Date, or on sales of the Earn-Out Products prior to the Reversion Date or permitted thereafter pursuant to Section 4.2(j).
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT F
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
THIS WARRANT AND THE SHARES ISSUABLE HEREUNDER HAVE NOT BEEN REGISTERED OR QUALIFIED UNDER THE SECURITIES ACT OF 1933 (THE “SECURITIES ACT”) OR UNDER ANY APPLICABLE STATE SECURITIES LAWS, AND HAVE BEEN ACQUIRED FOR INVESTMENT AND NOT WITH A VIEW TO, OR IN CONNECTION WITH, THE SALE OR DISTRIBUTION THEREOF. NO SUCH SALE OR DISTRIBUTION MAY BE LAWFULLY EFFECTED WITHOUT AN EFFECTIVE REGISTRATION, UNLESS AN EXEMPTION FROM SUCH REGISTRATION IS AVAILABLE.
WARRANT TO PURCHASE STOCK
| Company: | Recro Pharma, Inc., a Pennsylvania corporation | |
| Number of Shares: | 350,000, subject to adjustment | |
| Class of Stock: | Common Stock, $0.01 par value per share | |
| Warrant Price: | $[ ]1, subject to adjustment | |
| Issue Date: | [ ], 20152 | |
| Expiration Date: | The seven (7) year anniversary of the Issue Date. |
THIS WARRANT CERTIFIES THAT, for good and valuable consideration, Alkermes Pharma Ireland Limited, a private limited company incorporated in Ireland (together with any successor or permitted assignee or transferee of this Warrant, “Holder”) is entitled to purchase the number of fully paid and non-assessable shares (the “Shares”) of the above-stated Class of Stock (the “Class”) of the above-named company (the “Company”) at the above-stated Warrant Price per Share, all as set forth above and as adjusted pursuant to Article 2 of this Warrant, subject to the provisions and upon the terms and conditions set forth in this Warrant.
ARTICLE 1
EXERCISE
1.1 Method of Exercise. Holder may exercise this Warrant, in whole or in part, at any time or from time to time on any day on or prior to the Expiration Date, by delivering the original of this Warrant together with a duly executed Notice of Exercise in substantially the form attached as Appendix 1 to the principal office of the Company. Unless Holder is exercising the net exercise right set forth in Article 1.2, Holder shall also deliver to the Company a check, wire transfer (to an account designated by the Company), or other form of payment acceptable to the Company for the aggregate Warrant Price for the Shares being purchased.
1.2 Net Exercise. In lieu of exercising this Warrant as specified in Article 1.1, Holder may from time to time convert this Warrant, in whole or in part, into a number of Shares determined by dividing (a) the aggregate fair market value of the Shares or other securities otherwise issuable upon exercise of this Warrant minus the aggregate Warrant Price of such Shares by (b) the fair market value of one Share. The fair market value of the Shares shall be determined pursuant to Article 1.3. The Company acknowledges that the provisions of this
| 1 | NTD: to equal two times the closing price of Recro stock on the trading day immediately prior to the Closing Date. |
| 2 | NTD: to insert Closing Date. |
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Article 1.2 are intended, in part, to ensure that a full or partial exchange of this Warrant pursuant to this Article 1.2 will qualify as a conversion, within the meaning of paragraph (d)(3)(ii) of Rule 144 under the Securities Act. At the request of Holder, the Company will accept reasonable modifications to the exchange procedures provided for in this Article in order to accomplish such intent. For all purposes of this Warrant (other than this Article 1.2), any reference herein to the exercise of this Warrant shall be deemed to include a reference to the exchange of this Warrant into Shares in accordance with the terms of this Article 1.2.
1.3 Fair Market Value. If the Company’s common stock is traded in a public market, the fair market value of a Share shall be the closing price of a share of common stock reported for the business day immediately before Holder delivers this Warrant together with its Notice of Exercise to the Company. If the Company’s common stock is not traded in a public market, the Board of Directors of the Company shall determine fair market value of a Share (the “Appraised Value”) in its reasonable good faith judgment. If Holder (as defined below) shall, for any reason whatsoever, disagree with such Appraised Value, then such Holder shall, by giving written notice to the Company (an “Appraisal Notice”) within ten (10) days after the Company notifies the Holder of such determination, elect to dispute such determination, and such dispute shall be resolved as set forth in Article 1.3.1 below.
1.3.1 Appraisal Resolution. The Company shall within ten (10) days after an Appraisal Notice shall have been given, engage an independent investment bank of national repute (the “Appraiser”) that is mutually agreeable to the Company and Holder and retained pursuant to an engagement letter between the Company and the Appraiser with respect to such valuation in form and substance reasonably acceptable to Holder, to make an independent determination of the Appraised Value of a Share. The costs of engagement of such investment bank for any such determination of Appraised Value shall be shared equally between the Company and Holder and Holder shall promptly reimburse the Company for fifty percent (50%) of such costs.
1.4 Delivery of Certificate and New Warrant. Promptly after Holder exercises or converts this Warrant and, if applicable, the Company receives payment of the aggregate Warrant Price, the Company shall deliver to Holder certificates for the Shares acquired and, if this Warrant has not been fully exercised or converted and has not expired, a new warrant of like tenor representing the Shares not so acquired.
1.5 Replacement of Warrants. On receipt of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant and, in the case of loss, theft or destruction, on delivery of an indemnity agreement reasonably satisfactory in form and amount to the Company or, in the case of mutilation, on surrender and cancellation of this Warrant, the Company shall execute and deliver, in lieu of this Warrant, a new warrant of like tenor.
2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
1.6 Treatment of Warrant upon Corporate Reorganization.
1.6.1 Corporate Reorganization.
(a) Without limiting any of the provisions hereof, if any: (i) capital reorganization; (ii) reclassification of the capital stock of the Company; (iii) merger, consolidation or reorganization or other similar transaction or series of related transactions which results in the voting securities of the Company outstanding immediately prior thereto representing immediately thereafter (either by remaining outstanding or by being converted into voting securities of the surviving or acquiring entity) less than 50% of the combined voting power of the voting securities of or economic interests in the Company or such surviving or acquiring entity outstanding immediately after such merger, consolidation or reorganization; (iv) sale, lease, license, transfer, conveyance or other disposition of all or substantially all of the assets of the Company; (v) sale of shares of capital stock of the Company, in a single transaction or series of related transactions, representing at least 50% of the voting power of the voting securities of or economic interests in the Company; or (vi) the acquisition by any “person” (together with his, her or its Affiliates) or “group” (within the meaning of Section 13(d) or 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) directly or indirectly, of the beneficial ownership (as such term is defined in Rule 13d-3 promulgated under the Exchange Act) of outstanding shares of capital stock and/or other equity securities of the Company, in a single transaction or series of related transactions (including, without limitation, one or more tender offers or exchange offers), representing at least 50% of the voting power of or economic interests in the then outstanding shares of capital stock of the corporation (each of (i)-(vi) above a “Corporate Reorganization”) shall be effected, then the Company shall use its commercially reasonable best efforts to ensure that lawful and adequate provision shall be made whereby each Holder shall thereafter continue to have the right to purchase and receive upon the basis and upon the terms and conditions herein specified and in lieu of the Shares issuable upon exercise of the Warrants held by such Holder, shares of stock in the surviving or acquiring entity (“Acquirer”), as the case may be, such that the aggregate value of the Holder’s warrants to purchase such number of shares, where the value of each new warrant to purchase one share in the Acquirer is determined in accordance with the Black-Scholes Option Pricing formula set forth in Appendix 2 hereto, is at least equivalent to the aggregate value of the Warrants held by such Holder immediately prior to such Corporate Reorganization, where the value of each Warrant to purchase one share in the Company is determined in accordance with the Black-Scholes Option Pricing formula set forth Appendix 3 hereto. Furthermore, the new warrants to purchase shares in the Acquirer referred to herein shall have the same expiration date as the Warrant, and shall have a strike price, KAcq, that is calculated in accordance with Appendix 2 hereto. For the avoidance of doubt, if the surviving or acquiring entity, as the case may be, is a member of a consolidated group for financial reporting purposes, the “Acquirer” shall be deemed to be the parent of such consolidated group for purposes of this Article 1.6 and Appendix 2 hereto.
(b) Appropriate provision shall be made with respect to the rights and interests of each Holder to the end that the provisions hereof (including, without limitation, provision for adjustment of the Warrant Price) shall thereafter be applicable, as nearly equivalent as may be practicable in relation to any shares of stock thereafter deliverable upon the exercise thereof. The Company shall use its commercially reasonable best efforts to ensure that prior to
3
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
or simultaneously with the consummation of a Corporate Reorganization, the successor corporation resulting from such consolidation or merger, or the corporation purchasing or otherwise acquiring such assets or other appropriate corporation or entity shall assume by written instrument, reasonably deemed by the Board of Directors of the Company and Holder to be satisfactory in form and substance, the obligation to deliver to the holder of the Warrants, at the last address of such holder appearing on the books of the Company, such shares of stock, as, in accordance with the foregoing provisions, such holder may be entitled to purchase, and the other obligations under these Warrants. The provisions of this Article 1.6 shall similarly apply to successive Corporate Reorganizations. If the Company, in spite of using its commercially reasonable best efforts, is unable to cause these Warrants to continue in full force and effect until the Expiration Date in connection with any Corporate Reorganization, then the Company shall pay or cause to be paid to Holder, prior to or contemporaneously with the consummation of any such Corporate Reorganization, an amount per Warrant to purchase one share in the Company that is calculated in accordance with the Black-Scholes Option Pricing formula set forth in Appendix 3 hereto. Such payment shall be made in cash in the event that the Corporate Reorganization results in the shareholders of the Company receiving cash from the Acquirer at the closing of the transaction, and shall be made in shares of the Company (with the value of each share in the Company is determined according to SCorp Appendix 3 hereto) in the event that the Corporate Reorganization results in the shareholders of the Company receiving shares in the Acquirer or other entity at the closing of the transaction, in the event that the shareholders of the Company receive both cash and shares at the closing of the transaction, such payment to Holder shall be also be made in both cash and shares in the same proportion as the consideration received by the shareholders.
ARTICLE 2
ADJUSTMENTS TO THE SHARES
2.1 Stock Dividends, Splits, Etc. If the Company declares or pays a dividend on the outstanding shares of the Class payable in common stock or other securities, then upon exercise of this Warrant, for each Share acquired, Holder shall receive, without cost to Holder, the total number and kind of securities to which Holder would have been entitled had Holder owned the Shares of record as of the date the dividend occurred. If the Company subdivides the outstanding shares of the Class by reclassification or otherwise into a greater number of shares, the number of Shares purchasable hereunder shall be proportionately increased and the Warrant Price shall be proportionately decreased. If the outstanding shares of the Class are combined or consolidated, by reclassification or otherwise, into a lesser number of shares, the Warrant Price shall be proportionately increased and the number of Shares shall be proportionately decreased. The Company or its successor shall promptly issue to the Holder a certificate pursuant to Article 2.6 below setting forth the number and kind of such new securities or other property issuable upon exercise or conversion of this Warrant as a result of such stock dividend, subdivision, combination or consolidation by reclassification or otherwise or other event that results in a change to the number and/or class of securities issuable upon exercise or conversion of this Warrant. The provisions of this Article 2.1 shall similarly apply to successive stock dividends, subdivisions, combinations, consolidations or other events.
4
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
2.2 Reclassification, Exchange, Combinations or Substitution. Upon any reclassification, exchange, substitution, or other event that results in a change of the number and/or class of the securities issuable upon exercise or conversion of this Warrant, Holder shall be entitled to receive, upon exercise or conversion of this Warrant, the number and kind of securities and property that Holder would have received for the Shares if this Warrant had been exercised immediately before such reclassification, exchange, substitution, or other event. Such an event shall include, without limitation, any automatic conversion of the outstanding or issuable securities of the Company of the same class or series as the Shares to common stock pursuant to the terms of the Company’s Articles of Incorporation. The Company or its successor shall promptly issue to Holder a certificate pursuant to Article 2.6 below setting forth the number and kind of such new securities or other property issuable upon exercise or conversion of this Warrant as a result of such reclassification, exchange, substitution or other event that results in a change of the number and/or class of securities issuable upon exercise or conversion of this Warrant. The provisions of this Article 2.2 shall similarly apply to successive reclassifications, exchanges, substitutions, or other events.
2.3 No Impairment. The Company shall not, by amendment of its Articles of Incorporation or bylaws or through a reorganization, transfer of assets, consolidation, merger, dissolution, issue, or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms to be observed or performed under this Warrant by the Company, but shall at all times in good faith assist in carrying out of all the provisions of this Article 2 and in taking such action as may be reasonably necessary or appropriate to protect Holder’s rights under this Article against impairment.
2.4 Fractional Shares. No fractional Shares shall be issuable upon exercise or conversion of the Warrant and the number of Shares to be issued shall be rounded down to the nearest whole Share. If a fractional share interest arises upon any exercise or conversion of the Warrant, the Company shall eliminate such fractional share interest by paying Holder the amount computed by multiplying the fractional interest by the fair market value of a full Share.
2.5 Certificate as to Adjustments. Upon each adjustment of the Warrant Price, Class and/or number of Shares, the Company shall promptly notify Holder in writing, and, at the Company’s expense, promptly compute such adjustment, and furnish Holder with a certificate of its Chief Financial Officer setting forth such adjustment and the facts upon which such adjustment is based. The Company shall, upon written request, furnish Holder a certificate setting forth the Warrant Price, Class and number of Shares in effect upon the date thereof and the series of adjustments leading to such Warrant Price, Class and number of Shares.
2.6 Proceedings Prior to Any Action Requiring Adjustment. As a condition precedent to the taking of any action which would require an adjustment pursuant to this Article 2, the Company shall take any action which may be necessary, including obtaining regulatory approvals or exemptions, in order that the Company may thereafter validly and legally issue as fully paid and nonassessable all Shares which the Holder is entitled to receive upon exercise of the Warrant.
5
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
ARTICLE 3
REPRESENTATIONS AND COVENANTS OF THE COMPANY
3.1 Representations and Warranties. The Company represents and warrants to, and agrees with, the Holder as follows:
3.1.1 This Warrant has been duly authorized, is validly issued and constitutes the valid and binding obligation of the Company.
3.1.2 All Shares which may be issued from time to time upon the exercise of any of the purchase rights represented by this Warrant, and all securities, if any, issuable upon conversion of the Shares, shall, upon issuance, be duly authorized, validly issued, fully paid and non-assessable, and free of any liens and encumbrances except for restrictions on transfer provided for herein or under applicable federal and state securities laws.
3.2 Notice of Certain Events. If the Company proposes at any time (a) to declare any dividend or distribution upon the outstanding shares of the Class, whether in cash, property, stock, or other securities and whether or not a regular cash dividend; (b) to offer for subscription or sale pro rata to the holders of the outstanding shares of the Class any additional shares of any class or series of the Company’s stock (other than pursuant to contractual pre-emptive rights); (c) to effect any reclassification, reorganization or recapitalization of the shares of the Class; or (d) to effect an Acquisition or to liquidate, dissolve or wind up, then, in connection with each such event, the Company shall give Holder: (1) at least 10 days prior written notice of the date on which a record will be taken for such dividend, distribution, or subscription rights (and specifying the date on which the holders of shares of the Class will be entitled thereto) or for determining rights to vote, if any, in respect of the matters referred to in (c) and (d) above; and (2) in the case of the matters referred to in (c) and (d) above at least 10 days prior written notice of the date when the same will take place (and specifying the date on which the holders of shares of the Class will be entitled to exchange their shares for the securities or other property deliverable upon the occurrence of such event).
3.3 No Shareholder Rights. Except as provided in this Warrant, Holder will not have any rights as a shareholder of the Company until the exercise of this Warrant.
3.4 Certain Information. The Company agrees to provide Holder at any time and from time to time with such information as Holder may reasonably request for purposes of Holder’s compliance with regulatory, accounting and reporting requirements applicable to Holder.
ARTICLE 4
REPRESENTATIONS, WARRANTIES OF THE HOLDER
The Holder represents and warrants to the Company as follows:
4.1 Purchase for Investment. Holder is acquiring this Warrant and the Shares issuable upon exercise of this Warrant for its own account, for investment and not for, with a view to, or in connection with, any sale or distribution thereof within the meaning of the Securities Act, and Holder will not offer, sell or otherwise dispose of this Warrant and the Shares issuable upon exercise of this Warrant except as permitted by the Securities Act and any state securities law.
6
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
4.2 Unregistered Securities; Legend. Holder understands that the Warrants and the Shares have not been, and will not be, registered under the Securities Act or any state securities law, by reason of their issuance in a transaction exempt from the registration requirements of the Securities Act and such laws, that the Warrants and the Shares must be held indefinitely unless they are subsequently registered under the Securities Act and such laws or a subsequent disposition thereof is exempt from registration, that the Warrants and the Shares shall bear a legend to such effect, and that appropriate stop transfer instructions may be issued. Holder further understands that such exemption depends upon, among other things, the bona fide nature of Holder’s investment intent expressed herein.
4.3 Status of the Holder. Holder has not been formed for the specific purpose of acquiring the Warrants or the Shares. Holder understands the term “accredited investor” as used in Regulation D promulgated under the Securities Act and represents and warrants to the Company that Holder is an “accredited investor” for purposes of acquiring the Warrants and the Shares. If Holder is not a United States person (as defined by Section 7701(a)(30) of the Internal Revenue Code of 1986), Holder has satisfied itself as to the full observance of the laws of its jurisdiction in connection with this Agreement, including (a) the legal requirements within its jurisdiction for issuance of this Warrant and the Shares issuable upon exercise of this Warrant, (b) any foreign exchange restrictions applicable to such purchase, (c) any governmental or other consents that may need to be obtained, and (d) the income tax and other tax consequences, if any, that may be relevant to the purchase, holding, redemption, sale, or transfer of this Warrant or the Shares issuable upon exercise. Holder’s subscription and payment for and continued beneficial ownership of the Warrant and Shares issuable upon exercise will not violate any applicable securities or other laws of the Holder’s jurisdiction.
4.4 Knowledge and Experience; Economic Risk. Holder has sufficient knowledge and experience in business and financial matters and with respect to investment in securities of privately held companies so as to enable it to analyze and evaluate the merits and risks of the investment contemplated hereby and is capable of protecting its interest in connection with this transaction. Holder is able to bear the economic risk of such investment, including a complete loss of the investment.
4.5 Access to Information. Holder acknowledges that Holder and its representatives have had the opportunity to ask questions and receive answers from officers and representatives of the Company concerning the Company and its business and the transactions contemplated by this Agreement and to obtain any additional information which the Company possesses or can acquire that is necessary to verify the accuracy of the information regarding the Company herein set forth or otherwise desired in connection with its purchase of the Warrants and the Shares.
4.6 Rule 144. Holder understands that the exemption from registration afforded by Rule 144 (the provisions of which are known to such Investor) promulgated by the Securities and Exchange Commission under the Securities Act depends upon the satisfaction of various conditions, and that such exemption is not currently available.
7
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
ARTICLE 5
MISCELLANEOUS
5.1 Term. This Warrant is exercisable in whole or in part at any time and from time to time on or before the Expiration Date.
5.2 Legends. This Warrant and the Shares shall be imprinted with a legend in substantially the following form:
“THIS WARRANT AND THE SHARES ISSUABLE HEREUNDER HAVE NOT BEEN REGISTERED OR QUALIFIED UNDER THE SECURITIES ACT OF 1933 OR UNDER ANY APPLICABLE STATE SECURITIES LAWS, AND HAVE BEEN ACQUIRED FOR INVESTMENT AND NOT WITH A VIEW TO, OR IN CONNECTION WITH, THE SALE OR DISTRIBUTION THEREOF. NO SUCH SALE OR DISTRIBUTION MAY BE LAWFULLY EFFECTED WITHOUT AN EFFECTIVE REGISTRATION STATEMENT, UNLESS AN EXEMPTION FROM SUCH REGISTRATION IS AVAILABLE.”
5.3 Compliance with Securities Laws on Transfer. This Warrant and the Shares may not be transferred or assigned in whole or in part without compliance with applicable federal and state securities laws by the transferor and the transferee.
5.4 Transfer Procedure. Subject to the provisions of Article 5.3, Holder may transfer or pledge all or part of this Warrant or the Shares issuable upon exercise of this Warrant to any transferee, provided, however, in connection with any transfer, Holder will give the Company notice of the portion of the Warrant being transferred with the name, address and taxpayer identification number of the transferee and Holder will surrender this Warrant to the Company for reissuance to the transferee(s) (and Holder if applicable). The Company may refuse to transfer this Warrant or the Shares to any person who directly competes with the Company, unless, in either case, the stock of the Company is publicly traded.
5.5 Notices. All notices and other communications from the Company to the Holder, or vice versa, shall be deemed delivered and effective when given personally or mailed by first-class registered or certified mail, postage prepaid (or on the first business day after transmission by facsimile), at such address as may have been furnished to the Company or Holder, as the case may be, in writing by the Company or such holder from time to time. All notices to Holder shall be addressed as follows until the Company receives notice of a change in address:
Alkermes Pharma Ireland Limited
[c/o Alkermes plc
Attn.: Company Secretary
▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇
Telephone: [●]
Facsimile: ▇▇▇▇ ▇.▇▇▇.▇▇▇▇]
8
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
Notice to the Company shall be addressed as follows until Holder receives notice of a change in address:
Recro Pharma Inc.
Attn: ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇, President and Chief Executive Officer
▇▇▇ ▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Telephone: (▇▇▇) ▇▇▇-▇▇▇▇
Facsimile: (▇▇▇) ▇▇▇-▇▇▇▇
5.6 Waiver. This Warrant and any term hereof may be changed, waived, discharged or terminated only by an instrument in writing signed by Holder and the party against which enforcement of such change, waiver, discharge or termination is sought.
5.7 Automatic Conversion upon Expiration. In the event that, upon the Expiration Date, the fair market value of one Share (or other security issuable upon the exercise hereof) as determined in accordance with Article 1.3 above is greater than the Warrant Price in effect on such date, then this Warrant shall automatically be deemed on and as of such date to be converted pursuant to Article 1.2 above as to all Shares (or such other securities) for which it shall not previously have been exercised or converted, and the Company shall promptly deliver a certificate representing the Shares (or such other securities) issued upon such conversion to Holder.
5.8 Counterparts. This Warrant may be executed in counterparts, all of which together shall constitute one and the same agreement.
5.9 Governing Law. This Warrant shall be governed by and construed in accordance with the laws of the Commonwealth or Pennsylvania, without giving effect to its principles regarding conflicts of law.
[Remainder of page left blank intentionally]
9
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
| “COMPANY” | ||
| RECRO PHARMA, INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
| “HOLDER” | ||
| ALKERMES PHARMA IRELAND LIMITED, INC. | ||
| By: |
| |
| Name: |
| |
| (Print) | ||
| Title: | ||
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
APPENDIX 1
NOTICE OF EXERCISE
1. Holder elects to purchase shares of the Common Stock of pursuant to the terms of the attached Warrant, and tenders payment of the purchase price of the shares in full.
[or]
1. Holder elects to convert the attached Warrant into Shares in the manner specified in the Warrant. This conversion is exercised for of the Shares covered by the Warrant.
[Strike paragraph that does not apply.]
2. Please issue a certificate or certificates representing the Shares in the name specified below:
|
|
| Holder Name |
|
|
|
|
| (Address) |
3. By its execution below and for the benefit of the Company, Holder hereby restates each of the representations and warranties in Article 4 of the Warrant as of the date hereof.
| HOLDER | ||
|
| ||
| By: |
| |
| Name: |
| |
| Title: |
| |
| Date: |
| |
Appendix 1 - 1
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
APPENDIX 2
Black Scholes Option Pricing formula to be used when calculating the value of each new warrant to purchase one share in the Acquirer shall be:
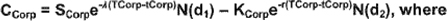
CAcq = value of each warrant to purchase one share in the Acquirer.
SAcq = price of Acquirer’s stock as determined by reference to the average of the closing prices on the securities exchange over the 20-day period ending three trading days prior to the closing of the Corporate Reorganization described in Article 1.6.1 if the Acquirer’s stock is then traded on such exchange or system, or the average of the closing bid or sale prices (whichever is applicable) in the over-the-counter market over the 20-day period ending three trading days prior to the closing of the Corporate Reorganization if the Acquirer’s stock is then actively traded in the over-the-counter market, or the then most recently completed financing if the Acquirer’s stock is not then traded on a securities exchange or system or in the over-the-counter market.
TAcq = expiration date of new warrants to purchase shares in the Acquirer = TCorp.
tAcq = date of issue of new warrants to purchase shares in the Acquirer.
TAcq-tAcq = time until warrant expiration, expressed in years.
s = volatility = annualized standard deviation of daily log-returns (using a 262-day annualization factor) of the Acquirer’s stock price on the securities exchange over a 20-day trading period, determined by Holder, that is within the 100-day trading period ending on the trading day immediately after the public announcement of the Corporate Reorganization described in Article 1.6.1 if the Acquirer’s stock is then traded on such exchange or system, or the annualized standard deviation of daily-log returns (using a 262-day annualization factor) of the closing bid or sale prices (whichever is applicable) in the over-the-counter market over a 20-day trading period, determined by the Holder, that is within the 100-day trading period ending on the trading day immediately after the public announcement of the Corporate Reorganization if the Acquirer’s stock is then actively traded in the over-the-counter market, or 0.6 (or 60%) if the Acquirer’s stock is not then traded on a securities exchange or system or in the over-the-counter market.
N = cumulative normal distribution function.
d1 = (ln(SAcq/KAcq) + (r- + l +s2/2)(TAcq-tAcq )) ÷ (sÖ(TAcq-tAcq)).
In = natural logarithm.
l - dividend rate of the Acquirer for the most recent 12-month period at the time of closing of the Corporate Reorganization.
KAcq = strike price of new warrants to purchase shares in the Acquirer = KCorp* (SAcq/ SCorp).
Appendix 2 - 1
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
r = annual yield, as reported by Bloomberg at time tAcq, of the United States Treasury security measuring the nearest time TAcq.
d2= d1- s Ö(TAcq-tAcq).
Appendix 2 - 2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
APPENDIX 3
Black Scholes Option Pricing formula to be used when calculating the value of each Warrant to purchase one share in the Company shall be:
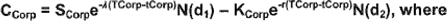
CCorp = value of each Warrant to purchase one share in the Company.
SCorp = price of Company stock as determined by reference to the average of the closing prices on the securities exchange over the 20-day period ending three trading days prior to the closing of the Corporate Reorganization described in Article 1.6.1 if the Company’s stock is then traded on such exchange or system, or the average of the closing bid or sale prices (whichever is applicable) in the over-the-counter market over the 20-day period ending three trading days prior to the closing of the Corporate Reorganization if the Company’s stock is then actively traded in the over-the-counter market, or the then most recently completed financing if the Company’s stock is not then traded on a securities exchange or system or in the over-the-counter market.
TCorp = expiration date of Warrants to purchase shares in the Company.
tCorp - date of public announcement of transaction.
TCorp-tCorp - time until Warrant expiration, expressed in years.
s = volatility ~ the annualized standard deviation of daily log-returns (using a 262-day annualization factor) of the Company’s stock price on the securities exchange over a 20-day trading period, determined by Holder, that is within the 100-day trading period ending on the trading day immediately after the public announcement of the Corporate Reorganization described in Article 5(d) if the Company’s stock is then traded on such exchange or system, or the annualized standard deviation of daily-log returns (using a 262-day annualization factor) of the closing bid or sale prices (whichever is applicable) in the over-the-counter market over a 20-day trading period, determined by the Holder, that is within the 100-day trading period ending on the trading day immediately after the public announcement of the Corporate Reorganization if the Company’s stock is then actively traded in the over-the-counter market, or 0.6 (or 60%) if the Company’s stock is not then traded on a securities exchange or system or in the over-the-counter market.
N = cumulative normal distribution function.
d1 = (ln(SCorp/KCorp) + (r- + l +s2/2)(TCorp-tCorp)) ÷ (sÖ(TCorp-tCorp)).
In = natural logarithm.
l = dividend rate of the Company for the most recent 12-month period at the time of closing of the Corporate Reorganization.
KCorp = strike price of warrant.
Appendix 3 - 1
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
r = annual yield, as reported by Bloomberg at time tCorp, of the United States Treasury security measuring the nearest time TCorp.
d2= d1- s Ö(TCorp-tCorp).
Appendix 3 - 2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT G
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***]
HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE
COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
EXHIBIT G: Reorganisation
[***]

