Execution Version FIRST AMENDMENT This first amendment to the License Agreement between Angion Biomedica Corp, 51 Charles Lindbergh Blvd., NY 11553 Uniondale, the United States of America (“Angion”) and Ohr Cosmetic LLC, 7 Essex Street, Suite 4A, New...

Execution Version FIRST AMENDMENT This first amendment to the License Agreement between Angion Biomedica Corp, 00 Xxxxxxx Xxxxxxxxx Xxxx., XX 00000 Xxxxxxxxx, xxx Xxxxxx Xxxxxx xx Xxxxxxx (“Angion”) and Ohr Cosmetic LLC, 0 Xxxxx Xxxxxx, Xxxxx 0X, Xxx Xxxx XX 00000 (“Ohr”), dated November 15, 2013 (the “First Amendment”). Angion and Ohr may hereinafter be referred to individually as a “Party” or jointly as the “Parties”. Capitalized terms used herein without definition shall have the meanings ascribed to them in the Agreement. PREAMBLE WHEREAS, the Parties concluded a License Agreement with an effective date of November 15, 2013 (the “Agreement”); WHEREAS, the Parties would like to amend certain provisions of the Agreement to reflect the current business strategies, operations and priorities of each Party; WHEREAS, Angion no longer desires to prosecute and maintain the patent rights licensed under the Agreement at its expense; and WHEREAS, the Parties agree this first amendment is required for the continuation and preservation of the Agreement. NOW THEREFORE, in consideration of the mutual covenants expressed herein and for good and valuable consideration, the Parties hereto agree as follows: ARTICLE 1 – DEFINITIONS Section 1.7. Schedule 1 setting forth the Licensed Patent Rights defined in Section 1.7 is hereby deleted and replaced with Schedule 1 (2022), attached hereto as Appendix A to First Amendment. Section 1.8. “Licensed Product” is hereby deleted and replaced with the following language: “Section 1.8. "Licensed Product" means any compound covered by or incorporating the Licensed Patent Rights, including but not limited to ANG-3522, for use in conditions of the skin or hair, or whose making, use, manufacture or sale, that but for the licenses granted to Ohr in this Agreement would infringe at least one Valid Patent Claim.” ARTICLE 2 – LICENSES A Section 2.3 is hereby added to the Agreement, the language of which is as set forth below: “2.3. License to Angion. To the extent that Ohr determines to maintain and prosecute Licensed Patent Rights at its own expense, as set forth in Section 6.2, Ohr hereby grants Angion a fully paid, irrevocable, exclusive license for any use or purpose to any and all patent applications, patents and claims resulting from Licensed Patent Rights outside the Licensed Field. ARTICLE 3 – FEES AND ROYALTIES Section 3.1 of the Agreement is deleted and replaced with the following language: “3.1. Upfront and Expense Reimbursement Payments. Prior to November 22, 2022, Ohr shall reimburse Angion for all expenses associated with the ANG-3522 human topical irritation study and expenses associated with patent protection for the Licensed Patent Rights, and all expenses related to the review of any agreements between the Parties. For patent expenses related to Licensed Patent Rights, if a patent application covers only subject matter in the Licensed Field Ohr shall reimburse Angion 100% of the expenses. If a patent application includes, but does not only cover, the Licensed Field, Ohr shall reimburse Angion one-third (33.3%) of the expenses related thereto. DocuSign Envelope ID: 643EFA0F-2014-4FF9-A4D1-603C4EE9C638

Execution Version Reimbursable expenses shall be invoiced by Angion to Ohr on a monthly basis via email and payment of such expenses shall be due to Angion within thirty (30) days of the date of Angion’s invoice. On or after November 22, 2022, Ohr shall reimburse Angion 100% of expenses related to the prosecution and maintenance of all Licensed Patent Rights.” ARTICLE 6 – INTELLECTUAL PROPERTY Section 6.2 of the Agreement is deleted and replaced with the following language: “6.2. Prosecution. As of the effective date of Agreement Amendment No. 1, since Xxxxxx has determined it will no longer prosecute and maintain the Licensed Patent Rights at its expense, Ohr shall have the right to maintain and prosecute such Licensed Patent Rights at its own expense without reimbursement by Xxxxxx (such Licensed Patent Rights hereafter referred to as “Ohr-Controlled Licensed Patent Rights”). Angion shall have the right to review and comment on any proposed strategy to be undertaken by Ohr for the Ohr-Controlled Licensed Patent Rights, and Ohr shall provide any change in strategy or copies of any drafts therefor to Angion at least 30 days prior to any deadline. For purposes of clarity, Angion shall have no obligations to maintain or prosecute at its expense any of the Licensed Patent Rights. To the extent that Xxxxxx incurred or incurs reimbursable prosecution expenses since November 22, 2022 or continues to incur reimbursable expenses related to the prosecution and maintenance of the Licensed Patent Rights or transfer of prosecution responsibility of the Licensed Patent Rights to Ohr, Ohr shall reimburse Angion for those expenses as set forth in Section 3.1 herein.” A Section 6.6 is added to the Agreement, the language of which is set forth below: “6.6. Covenant Not to Xxx for Patent Infringement: Xxx agrees and covenants not to xxx Xxxxxx for patent infringement for Xxxxxx’x practice of the Licensed Patent Rights, outside the Licensed Field, consistent with the Agreement as amended.” ARTICLE 8 – FURTHER DEVELOPMENT ACTIVITIES The first sentence of Section 8.2 of the Agreement shall be deleted and replaced with the following language: “8.2. Further development by Ohr. Ohr shall own all intellectual property not resulting from the Licensed Patent Rights and clinical trial data it develops independently. …” ARTICLE 9 – GENERAL The contact information in Section 9.2 of the Agreement for Ohr is amended as follows: “If to Ohr: Ohr Cosmetics LLC 0 Xxxxx Xxxxxx, Xxxxx 0X Xxx Xxxx XX 00000 If to Angion: Angion Biomedica Corp. 00 Xxxxxxx Xxxxxxxxx Xxxxxxxxx Xxxxxxxxx, XX 00000 With copy for Notice purposes only to: On behalf of Angion Biomedica Corp. DocuSign Envelope ID: 643EFA0F-2014-4FF9-A4D1-603C4EE9C638

Execution Version Xxxxxx, Xxxxxxx & Xxxx Two International Place Boston, MA 02110 Other than modifications and changes specifically included in this First Amendment, the Agreement shall remain unchanged. IN WITNESS WHEREOF, the Parties have caused this First Amendment to be executed. Angion Biomedica Corp. Ohr Cosmetics LLC ________________________________ _______________________________ Print name: Print name: Title: Title: Date: Date: DocuSign Envelope ID: 643EFA0F-2014-4FF9-A4D1-603C4EE9C638 Xxxxxx Xxxxxxxx 2/5/2023 Director Xxxxxxxx Xxxxxx 2/5/2023 EVP General Counsel
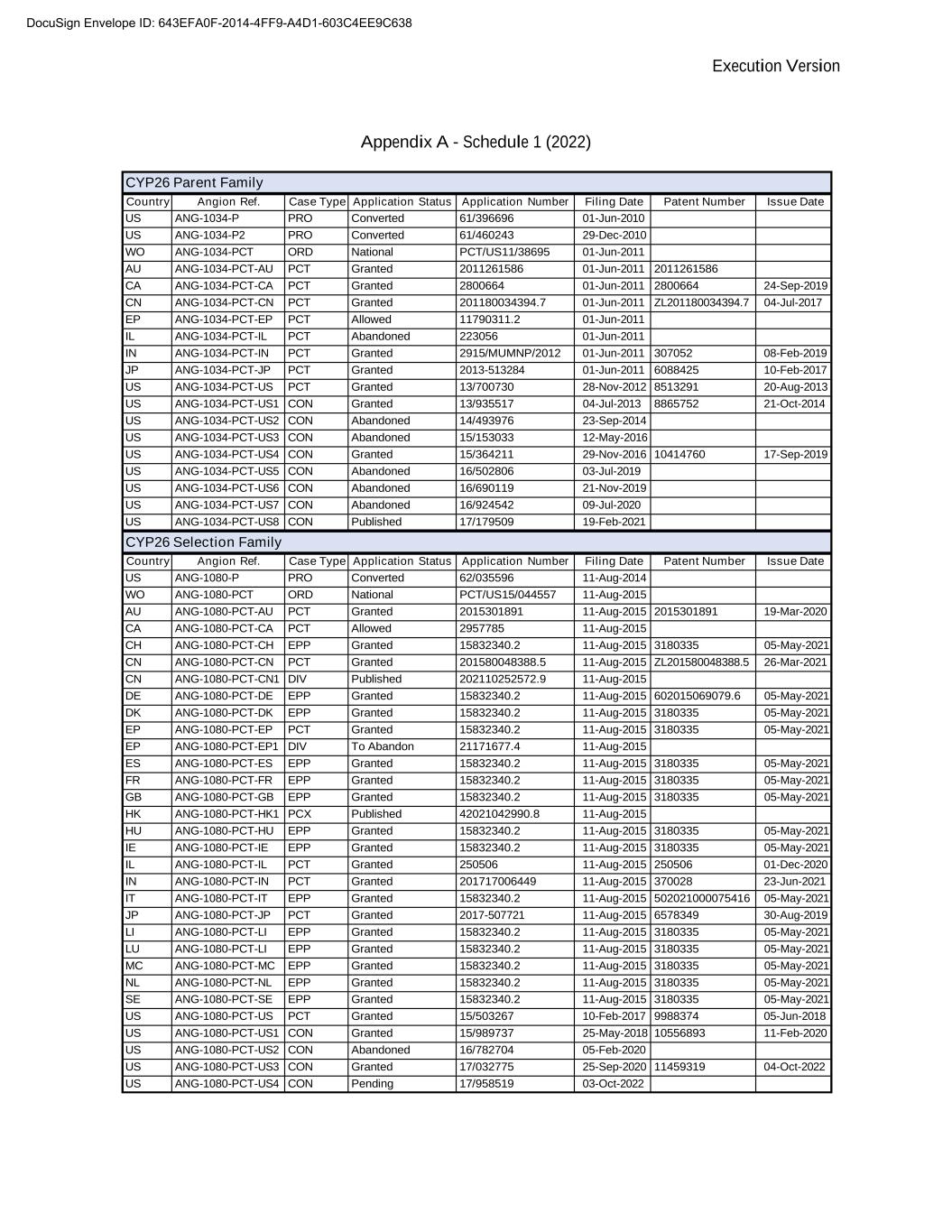
Execution Version Appendix A - Schedule 1 (2022) CYP26 Parent Family Country Angion Ref. Case Type Application Status Application Number Filing Date Patent Number Issue Date US ANG-1034-P PRO Converted 61/396696 01-Jun-2010 US ANG-1034-P2 PRO Converted 61/460243 29-Dec-2010 WO ANG-1034-PCT ORD National PCT/US11/38695 01-Jun-2011 AU ANG-1034-PCT-AU PCT Granted 2011261586 01-Jun-2011 2011261586 CA ANG-1034-PCT-CA PCT Granted 2800664 01-Jun-2011 2800664 24-Sep-2019 CN ANG-1034-PCT-CN PCT Granted 201180034394.7 01-Jun-2011 ZL201180034394.7 04-Jul-2017 EP ANG-1034-PCT-EP PCT Allowed 11790311.2 01-Jun-2011 IL ANG-1034-PCT-IL PCT Abandoned 223056 01-Jun-2011 IN ANG-1034-PCT-IN PCT Granted 2915/MUMNP/2012 01-Jun-2011 307052 08-Feb-2019 JP ANG-1034-PCT-JP PCT Granted 2013-513284 01-Jun-2011 6088425 10-Feb-2017 US ANG-1034-PCT-US PCT Granted 13/700730 28-Nov-2012 8513291 20-Aug-2013 US ANG-1034-PCT-US1 CON Granted 13/935517 04-Jul-2013 8865752 21-Oct-2014 US ANG-1034-PCT-US2 CON Abandoned 14/493976 23-Sep-2014 US ANG-1034-PCT-US3 CON Abandoned 15/153033 12-May-2016 US ANG-1034-PCT-US4 CON Granted 15/364211 29-Nov-2016 10414760 17-Sep-2019 US ANG-1034-PCT-US5 CON Abandoned 16/502806 03-Jul-2019 US ANG-1034-PCT-US6 CON Abandoned 16/690119 21-Nov-2019 US ANG-1034-PCT-US7 CON Abandoned 16/924542 09-Jul-2020 US ANG-1034-PCT-US8 CON Published 17/179509 19-Feb-2021 CYP26 Selection Family Country Angion Ref. Case Type Application Status Application Number Filing Date Patent Number Issue Date US ANG-1080-P PRO Converted 62/035596 11-Aug-2014 WO ANG-1080-PCT ORD National PCT/US15/044557 11-Aug-2015 AU ANG-1080-PCT-AU PCT Granted 2015301891 11-Aug-2015 2015301891 19-Mar-2020 CA ANG-1080-PCT-CA PCT Allowed 2957785 11-Aug-2015 CH ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 CN ANG-1080-PCT-CN PCT Granted 201580048388.5 11-Aug-2015 ZL201580048388.5 26-Mar-2021 CN ANG-1080-PCT-CN1 DIV Published 202110252572.9 11-Aug-2015 DE ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 602015069079.6 05-May-2021 DK ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 EP ANG-1080-PCT-EP PCT Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 EP ANG-1080-PCT-EP1 DIV To Abandon 21171677.4 11-Aug-2015 ES ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 FR ANG-1080-PCT-FR EPP Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 GB ANG-1080-PCT-GB EPP Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 HK ANG-1080-PCT-HK1 PCX Published 42021042990.8 11-Aug-2015 HU ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 IE ANG-1080-PCT-IE EPP Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 IL ANG-1080-PCT-IL PCT Granted 250506 11-Aug-2015 250506 01-Dec-2020 IN ANG-1080-PCT-IN PCT Granted 201717006449 11-Aug-2015 370028 23-Jun-2021 IT ANG-1080-PCT-IT EPP Granted 15832340.2 11-Aug-2015 502021000075416 05-May-2021 JP ANG-1080-PCT-JP PCT Granted 2017-507721 11-Aug-2015 6578349 30-Aug-2019 XX XXX-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 LU ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 MC ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 NL ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 SE ANG-1080-PCT-XX XXX Granted 15832340.2 11-Aug-2015 3180335 05-May-2021 US ANG-1080-PCT-US PCT Granted 15/503267 10-Feb-2017 9988374 05-Jun-2018 US ANG-1080-PCT-US1 CON Granted 15/989737 25-May-2018 10556893 11-Feb-2020 US ANG-1080-PCT-US2 CON Abandoned 16/782704 05-Feb-2020 US ANG-1080-PCT-US3 CON Granted 17/032775 25-Sep-2020 11459319 04-Oct-2022 US ANG-1080-PCT-US4 CON Pending 17/958519 03-Oct-2022 DocuSign Envelope ID: 643EFA0F-2014-4FF9-A4D1-603C4EE9C638

Execution Version DocuSign Envelope ID: 643EFA0F-2014-4FF9-A4D1-603C4EE9C638

