INTELLECTUAL PROPERTY LICENSE AND CONVEYANCE AGREEMENT AMONG NEOTOPE BIOSCIENCES LIMITED AND ELAN PHARMA INTERNATIONAL LIMITED AND ELAN PHARMACEUTICALS, INC. Dated as of December , 2012
Exhibit 2.3
INTELLECTUAL PROPERTY LICENSE AND CONVEYANCE
AGREEMENT
AMONG
NEOTOPE BIOSCIENCES LIMITED
AND
ELAN PHARMA INTERNATIONAL LIMITED
AND
ELAN PHARMACEUTICALS, INC.
Dated as of December , 2012
1
INTELLECTUAL PROPERTY LICENSE AND CONVEYANCE AGREEMENT
This INTELLECTUAL PROPERTY LICENSE AND CONVEYANCE AGREEMENT (the “Agreement”) is made this day of December 2012 (the “Effective Date”) among NEOTOPE BIOSCIENCES LIMITED, a private limited company incorporated under the laws of Ireland with offices at Xxxxxxxx Xxxxxxxx, Xxxxx Xxxxx Xxxxx Xxxxxx, Xxxxxx 0, Xxxxxxx (“NBL”) on the one hand, and ELAN PHARMA INTERNATIONAL LIMITED, a private limited company incorporated under the laws of Ireland with offices at Xxxxxxxx Xxxxxxxx, Xxxxx Xxxxx Xxxxx Xxxxxx, Xxxxxx 0, Xxxxxxx (“EPIL”) and ELAN PHARMACEUTICALS, INC., a Delaware corporation having an address at 000 Xxxxxx Xxxxx Xxxxxxxxx, Xxxxx Xxx Xxxxxxxxx, XX 00000 (“EPI”) on the other hand (collectively, “Elan”).
WHEREAS:
| A. | Elan Corporation, plc (“PLC”) and Prothena Corporation plc (“Prothena”) have entered into that Certain Demerger Agreement dated as of November 8, 2012 (the “Demerger Agreement”). |
| B. | NBL, an Affiliate of EPIL and EPI as of the Effective Date, will be wholly owned by Prothena upon the consummation of the transactions contemplated by the Demerger Agreement (the “Demerger”). |
| C. | The Demerger Agreement contemplates that, prior to the Demerger, the Pre-Demerger Restructuring (as defined in the Demerger Agreement) shall have been consummated, which Pre-Demerger Restructuring is intended to allocate, assign, and transfer to entities that will be owned by Prothena from and after the Demerger (including NBL), assets and liabilities that comprise the Prothena Business (as defined in the Demerger Agreement). |
| D. | As part of the Pre-Demerger Restructuring, EPI, EPIL and NBL wish to allocate, assign and transfer to NBL the assets and liabilities relating to the Projects (as defined below) to the extent set forth herein. |
NOW IT IS HEREBY AGREED AS FOLLOWS:
ARTICLE I
DEFINITIONS
In this Agreement, the following definitions shall apply:
| “Acquired Assets” | Assigned IP, Project Contracts, Project Materials other than Elan Materials, and Project Records that are owned by Elan as of the Effective Date. | |
| “Acquired Liabilities” | Debts, liabilities, losses, guarantees, commitments and obligations relating to or associated with the Acquired Assets. | |
| “Active Immunotherapeutic Approaches” |
Direct immunization with a target, or a fragment derived from a target (“Immunogen”), either with adjuvant alone or coupled to a carrier molecule designed to elicit an immune response of humoral or cellular nature in the host. Included are Immunogens in complex with another protein, such as, for example, lipid stabilized Immunogens and protein conjugated Immunogens, as well as multivalent vaccines incorporating multiple Immunogens. | |
2
| “Affiliate” | A corporation or other entity that controls, is controlled by or is under common control with such corporation or entity. A person or entity shall be regarded as in control of another entity if it owns or controls more than fifty percent (50%) of the voting securities or other ownership interest of the other corporation or entity. | |
| “AGE” | Advanced glycation end products. | |
| “Ancillary Intellectual Property” | The Intellectual Property licensed by Elan to NBL pursuant to Article III hereof and set forth on Schedule E. | |
| “Assigned Intellectual Property” or “Assigned IP” | Neotope Patent Rights, Project Know-How and Neotope Trademark Rights. | |
| “ELND2 Materials” | Antibodies 6F10, 5E10, 5D8 and 8G9, which specifically bind ELND-002. | |
| “Elan Materials” | The materials listed in Schedule D. | |
| “Exclusive License” | A fully paid, perpetual, irrevocable (except as otherwise expressly provided in Article III) and royalty-free license including the right to sublicense, whereby licensee’s rights are sole and entire and operate to exclude all others including licensor and its Affiliates, except as otherwise expressly provided herein. | |
| “Inactive” | Funded at an average annual rate of less than seventy-five thousand dollars ($75,000) over a period of two calendar years, including both internal and external expenditures in the aggregate. | |
| “Intellectual Property” | All (a) inventions (whether or not patentable and whether or not reduced to practice), records of inventions, test information, developments, applications, improvements, formulae, concepts, ideas, methods or processes, research property rights, all improvements to any of the foregoing, and all Patents, (b) copyrights, and all applications, registrations and renewals in connection therewith, (c) trade secrets, Know-How and confidential information, (d) domain names, computer software, firmware and applications (including source code, executable code, data, databases, programming and notes and documents and other related documentation), other than commercial off-the-shelf software, (e) works and designs embodied in advertising and promotional materials, (f) other proprietary rights and (g) copies and tangible embodiments of the foregoing in whatever form or medium. | |
3
| “Know-How” | Confidential scientific, technical, medical and marketing data, trade secrets and information, including all ideas, concepts, research and development, know-how, composition information and embodiments, manufacturing and production processes, techniques and information, specifications, technical and business data, designs, drawings, supplier lists, pricing and cost information, and data and know-how embodied in business and marketing plans and proposals, inventions (whether or not patentable and whether or not reduced to practice), records of inventions, test information, developments, applications, improvements, formulae, concepts, ideas, methods or processes, research property rights and all improvements to any of the foregoing. | |
| “Laminin” | Laminin 411 (Xxxxxxxx et al., Laminin-411 Is a Vascular Ligand for MCAM and Facilitates TH17 Cell Entry into the CNS, Plos One, July 2012, Vol. 7, Issue 7) and other laminin molecules. | |
| “MCAM” | Melanoma cell adhesion molecule (Xxxxxxxx et al., Laminin-411 Is a Vascular Ligand for MCAM and Facilitates TH17 Cell Entry into the CNS, Plos One, July 2012, Vol. 7, Issue 7). | |
| “Neotope Patent Rights” | The Patents listed on Schedule A-I and any Patents claiming priority thereto. | |
| “Neotope Trademark Rights” | The Trademark Rights listed on Schedule A-II. | |
| “OTL” | Onclave Therapeutics Limited. | |
| “Passive Immunotherapeutic Approaches” | Treatment of a host with either a whole antibody, or a fragment of an antibody which recognizes a target (or fragment or epitope in the target). | |
| “Patents” | All patents and patent applications, whether foreign or domestic, all patents arising from such applications, and all patents and patent applications based on, or claiming or corresponding to the priority dates, of any of the foregoing and any renewals, reissues, extensions (or other governmental actions that provide exclusive rights to the owner thereof in the patented subject matter beyond the original expiration date), substitutions, confirmations, registrations, revalidations, reexaminations, additions, continuations, continued prosecutions, continuations-in-part or divisions of or to any of the foregoing, including without limitation, supplementary protection certificates or the equivalent thereof. | |
| “Person” | Any individual, firm, partnership, company, corporation, government authority or other entity. | |
| “Project Contracts” | The contracts listed in Schedule B. | |
4
| “Project Know-How” | All Know-How relating solely to the Projects. | |
| “Project Materials” | The materials listed in Schedule C. | |
| “Project Records” | Copies of all files, documents and correspondence relating solely to the Projects, including data, reports, certificates, laboratory notebooks, written notes, standard operating procedures, logs, studies, databases, raw or experimental data, research records, assay protocols, meeting minutes, certificates of analysis, and vendor and supplier lists necessary for furthering the Projects and products arising therefrom. | |
| “Projects” | Research, development and commercialization activities directed to the use, in the diagnosis, prevention and treatment of diseases, of (a) Active Immunotherapeutic Approaches and Passive Immunotherapeutic Approaches, in each case directly targeting one or more Targets and/or (b) any and all Syn103 Program Compounds. | |
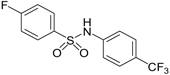
| “Syn 103 Program Compounds” | ELN484103 (4-fluoro-N-(4-(trifluoromethyl)phenyl)benzenesulfonamide) having the structure:
And related compounds ELN584092, ELN584105, ELN584164, ELN584095, ELN584090. | |
| “Synuclein Patent Rights” | The Patents listed in Schedule A-III and Patents claiming priority thereto. | |
| “Target” | Any and all of (i) MCAM; (ii) Laminin; (iii) AGE; (iv) damaged myelin; (v) fragments of any and all of (i)-(iv); and/or (vi) epitopes presented by any and all of (i)-(v) complexed with other molecular entities, including without limitation proteins, lipids, polymers, nucleic acids, compounds; provided, however, that in the case of (vi), such epitope shall include at least two amino acids of any of (i)-(iv) as determined by standard epitope mapping techniques. | |
| “Territory” | The world. | |
| “Trademark Rights” | All trademarks, trademark rights, service marks, service xxxx rights, trade dress, logos, slogans, trade names, trade name rights, Internet domain names and subdomains, together with all translations, adaptations, derivations, and combinations thereof and all goodwill associated therewith, and all applications, registrations, and renewals in connection therewith. | |
5
ARTICLE II
PURCHASE AND SALE OF ACQUIRED ASSETS
| 1. | Elan hereby transfers, sells, conveys, assigns and delivers to NBL all right, title and interest in the Territory to (i) the Acquired Assets and (ii) subject to the Demerger Agreement, the Acquired Liabilities. Subject to Article VI hereof and notwithstanding anything in this Agreement to the contrary, this Agreement shall not constitute an agreement to transfer, sell, convey, or assign any Acquired Asset if an attempted transfer, sale, conveyance, or assignment thereof, without the consent of a third party, would constitute a breach or other contravention of the rights of such Acquired Asset, or would in any way adversely affect the rights of EPI or EPIL, or upon transfer, sale, conveyance or assignment, NBL under such Acquired Asset. |
| 2. | Subject to Article VI hereof, Elan shall use commercially reasonable efforts to conclude as soon as reasonably practicable after the Effective Date the perfected assignments of, and to consummate the transfer of all of Elan’s rights, title, and interest in the Acquired Assets to NBL. |
| 3. | Elan shall use commercially reasonable efforts to transfer and deliver all Project Materials and Project Records when and in the manner requested by NBL. |
ARTICLE III
GRANT OF EXCLUSIVE LICENSE OF PATENT RIGHTS AND MATERIALS
| 1. | Elan hereby grants to NBL an Exclusive License in the Territory solely for the Projects to (1) conduct research and development activities, and (2) make, have made, use, offer for sale, sell and import products within the Projects, under: |
| a. | Synuclein Patent Rights; and |
| b. | Elan Materials. |
| 2. | For the avoidance of doubt, Elan and its Affiliates, other than NBL and OTL, shall use certain Target antibodies, i.e., 11A5, 12C6, 6H7, 8A5, 5C12 and the lipid/synuclein antibodies (collectively, “Synuclein Antibodies”) solely for research purposes, excluding use in studies relating to the Projects, and for no other purpose (“Elan’s Permitted Use”). |
| 3. | Except as provided in this Section 3, Elan and its Affiliates, other than NBL and OTL, shall not distribute to third parties or make a public disclosure of any Synuclein Antibodies without NBL’s prior written consent. Elan may, without NBL’s prior written consent: |
| a. | Distribute to collaborators other than academic institutions under a written agreement prohibiting publication or further distribution of the Elan Materials and expressly limiting use to Elan’s Permitted Use; or |
6
| b. | Distribute no more than 1 mg of any of 11A5, 6H7, and 8A5 under a written agreement prohibiting identification of the antibody structure and further distribution and expressly limiting use to Elan’s Permitted Use; or |
| c. | Publish results obtained with, but not the structure of, any of 11A5, 6H7, and 8A5; or |
| d. | Disclose and publish in a patent application and any patent issuing therefrom any results obtained with the Synuclein Antibodies in Elan’s Permitted Use. |
| 4. | For the avoidance of doubt, NBL and its Affiliates shall use certain non-Target antibodies, i.e., ELND2 Materials, TY11/15, 27-1, 2E4, 3G10 and APP antibodies solely for research purposes relating to the Projects, and for no other purpose (“NBL’s Permitted Use”). |
| 5. | Except as provided in this Xxxxxxx 0, XXX and its Affiliates shall not distribute to third parties or make a public disclosure of any ELND2 Materials without Elan’s prior written consent. NBL may, without Elan’s prior written consent: |
| a. | Distribute to collaborators other than academic institutions under a written agreement prohibiting publication or further distribution of the ELND2 Materials and expressly limiting use to NBL’s Permitted Use; or |
| b. | Distribute to academic institutions conducting research in furtherance of the Projects under a written agreement prohibiting further distribution of the ELND2 Materials and expressly limiting use to NBL’s Permitted Use; or |
| c. | Disclose and publish in a patent application and any patent issuing therefrom any results obtained with the ELND2 Materials in NBL’s Permitted Use. |
| 6. | On an annual basis, NBL shall review Projects to identify which have been Inactive. Within sixty (60) days of such identification, NBL shall notify Elan of such Inactive Projects, and the rights granted to NBL under Article III with respect to Ancillary Intellectual Property related solely to such identified Inactive Projects shall terminate and shall revert to Elan. |
ARTICLE IV
CONSIDERATION
In consideration for the transfer of the Acquired Assets to NBL, NBL shall pay EPI and EPIL a total of $375,000 and assume (subject to the terms of the Demerger Agreement) the Acquired Liabilities.
ARTICLE V
PROSECUTION OF PATENT RIGHTS
NBL shall solely control the prosecution and maintenance of all Neotope Patent Rights, and shall pay all costs associated therewith incurred after the Effective Date and shall have no obligation to Elan in respect of such Neotope Patent Rights. EPI shall solely
7
control the prosecution of Synuclein Patent Rights; provided, however, that EPI shall keep NBL reasonably apprised of the status of the Synuclein Patent Rights and reasonably consider the input of NBL with respect to the prosecution of any claims in the Synuclein Patent Rights solely related to the Projects.
ARTICLE VI
RELATIONSHIP TO DEMERGER AGREEMENT
This Agreement is subject in all respects to the terms and conditions of the Demerger Agreement. The consummation of the transactions contemplated by this Agreement shall constitute part of the Pre-Demerger Restructuring (as defined in the Demerger Agreement) under the Demerger Agreement and shall accordingly be consummated prior to the consummation of the transactions contemplated by the Demerger Agreement. The Acquired Assets and Acquired Liabilities conveyed to NBL pursuant to this Agreement shall constitute assets of the Prothena Business and Prothena Business Liabilities, respectively, for all purposes of the Demerger Agreement. Nothing contained in this Agreement shall be deemed to supersede any of the covenants, agreements, representations or warranties of Elan, Seller, Prothena, or Buyer contained in the Demerger Agreement. In the event of a conflict between this Agreement and the Demerger Agreement, the terms of the Demerger Agreement shall control.
ARTICLE VII
TERM AND TERMINATION
This Agreement may be terminated at any time prior to the Demerger by written consent of the parties hereto.
ARTICLE VIII
MISCELLANEOUS
| 1. | Force Majeure |
Neither party to this Agreement shall be liable for delay in the performance of any of its obligations hereunder if such delay results from causes beyond its reasonable control, including, without limitation, acts of God, fires, strikes, acts of war, or intervention of any government authority, but any such delay or failure shall be remedied by such party as soon as practicable.
| 2. | Relationship of the Parties |
Nothing contained in this Agreement is intended or is to be construed to constitute EPI, EPIL and NBL as partners or joint venturers or employees of the other party. Neither party hereto shall have any express or implied right or authority to assume or create any obligations on behalf of or in the name of the other party or to bind the other party to any contract, agreement or undertaking with any third party.
8
| 3. | Counterparts |
This Agreement may be executed in any number of counterparts, each of which when so executed shall be deemed to be an original and all of which when taken together shall constitute this Agreement. This Agreement may be executed by facsimile (including electronically by PDF). The parties agree that facsimile copies of signatures have the same effect as original signatures.
| 4. | Notices |
Any notice or other communication required or permitted to be given to either party under this Agreement shall be given in writing and shall be delivered by hand or by facsimile (and promptly confirmed by registered mail, postage prepaid and return receipt requested, or by reputable overnight delivery service or courier), addressed to each party at the following addresses or such other address as may be designated by notice pursuant to this Article VII Section 4:
| If to NBL: | Neotope Biosciences Limited | |||
| Xxxxxxxx Xxxxxxxx | ||||
| Xxxxx Xxxxx Xxxxx Xxxxxx | ||||
| Xxxxxx 0, Xxxxxxx | ||||
| Attention: Director | ||||
| If to EPI: | Elan Pharmaceuticals, Inc. | |||
| 000 Xxxxxx Xxxxx Xxxx. | ||||
| Xxxxx Xxx Xxxxxxxxx, XX 00000 | ||||
| Attention: Secretary | ||||
| If to EPIL: | Elan Pharma International Limited | |||
| Xxxxxxxx Xxxxxxxx | ||||
| Xxxxx Xxxxx Xxxxx Xxxxxx | ||||
| Xxxxxx 0, Xxxxxxx | ||||
| Attention: Director | ||||
Any notice or communication given in conformity with this Article VIII Section 4 shall be deemed to be effective when received by the addressee, if delivered by facsimile, hand or delivery service or courier, and four days after mailing, if mailed.
| 5. | Governing Law |
This Agreement shall be governed by and construed in accordance with the laws of Ireland.
| 6. | Severability |
If any provision in this Agreement is deemed to be or becomes invalid, illegal or unenforceable, (i) such provision will be deemed amended to conform to applicable laws so as to be valid and enforceable or, if it cannot be so amended without materially altering the intention of the parties, it will be deleted, and (ii) the validity, legality and enforceability of the remaining provisions of this Agreement shall not be impaired or affected in any way.
9
| 7. | Amendments |
No amendment, modification or addition hereto shall be effective or binding on either party unless set forth in writing and executed by a duly authorized representative of both parties.
| 8. | Waiver |
No waiver of any right under this Agreement shall be deemed effective unless contained in a writing signed by the party charged with such waiver, and no waiver of any breach or failure to perform shall be deemed to be a waiver of any future breach or failure to perform or of any other right arising under this Agreement.
| 9. | Headings |
The section headings contained in this Agreement are included for convenience only and form no part of the agreement between the parties.
| 10. | Assignment, Etc. |
Neither party may assign its rights and obligations hereunder without the prior written consent of the other party; provided, however, that either party shall have the right to assign such rights and obligations hereunder to an Affiliate or to any Person with which such party is merged or consolidated or which purchases all or substantially all of the assets of such party.
| 11. | No Effect on Other Agreements |
No provision of this Agreement shall be construed so as to negate, modify or affect in any way the provisions of any other agreement between the parties unless specifically referred to, and solely to the extent provided in any such other agreement.
| 12. | Successors |
This Agreement will inure to the benefit of and be binding upon the successors of the parties hereto.
IN WITNESS WHEREOF, the parties hereto have executed this Amended and Restated Agreement on the date last written below, effective as of the Effective Date.
| NEOTOPE BIOSCIENCES LIMITED | ||
| By: |
| |
| Name: | ||
| Title: | ||
| Date: | ||
00
| XXXX XXXXXXXXXXXXXXX, INC. | ELAN PHARMA INTERNATIONAL LIMITED | |||||
| By: |
|
By: |
| |||
| NAME: | Name: | |||||
| Title: | Title: | |||||
| Date: | Date: | |||||
11