EX-10.35
17
b86680a1exv10w35.htm
EX-10.35
Exhibit 10.35
TOLL MANUFACTURING AGREEMENT
TOLL MANUFACTURING AGREEMENT (the “Agreement”) effective as of the 18th day of May, 2009 (the
“Effective Date”) between BioEnergy International, LLC, a Delaware limited liability company
(“CUSTOMER”) and Fermic Sa de CV (“Fermic”). CUSTOMER and Fermic shall be separately referred to
hereafter as a “Party” and collectively referred to as “Parties”.
RECITALS
CUSTOMER owns Patents (as defined below), patents pending and Know-How (as defined below)
concerning microbial and biochemical processes by which a strain of microorganism was developed and
is owned by CUSTOMER (the “Microorganism”). The Microorganism is grown and processed to yield
certain amounts of succinic acid in a “broth-like” mixture, as further described on Schedule
B (the “Fermentation Product”), which will be further processed using CUSTOMER’S Patents,
patents pending and Know How to yield a purer succinic acid as further described on Schedule
C (the “Separation Product”).
CUSTOMER wishes to produce Product in quantities for research and future commercial development
purposes and, in order to do so, requires access to a facility with adequate fermentation capacity,
separation capacity and a trained labor force. Fermic operates a fermentation and separation plant
in Mexico City (the “Facility”) which has the capacity required to produce a sufficient quantity of
Product for research and future commercial development.
NOW, THEREFORE, THE PARTIES AGREE AS FOLLOWS:
ARTICLE I
DEFINITIONS
As used in this Agreement, the following terms shall have the meanings assigned to them below:
“Agreement” shall have the meaning set forth in the introductory paragraph.
“Control” means the possession, directly or indirectly, of the power to direct or cause the
direction of the management or policies of a Person, whether through the ability to exercise
voting power, by contract or otherwise. “Controlling,” “Controls” and “Controlled” have meanings
correlative thereto.
“Convenantors” shall have the meaning set forth in Section 15.1.
“CUSTOMER” shall have the meaning set forth in the introductory paragraph.
“Customer Indemnified Party” and “Customer Indemnified Parties” shall have the meaning set forth in
Section 9.2.
“Customer Supplied Separation Equipment” shall have the meaning set forth in Section 6.6.
“Effective Date” shall have the meaning set forth in the introductory paragraph.
“Equipment” shall collectively mean Fermenting Equipment and Separation Equipment.
“Facility” shall have the meaning set forth in the recitals.
“Fermenting Equipment” shall mean the equipment used in the Developmental Start-Up phase and during
the Fermentation Process.
“Fermentation Product” shall have the meaning set forth in the recitals.
“Fermentation Tolling Fee” shall have the meaning set forth in Section 4.3.
“Fermic” shall have the meaning set forth in the introductory paragraph.
“Fermic Indemnified Party” and “Fermic Indemnified Parties” shall have the meaning set forth in
Section 9.1.
“Initial Termination Date” shall have the meaning set forth in Section 12.1.
“Know-How” shall mean all confidential information, know-how and data not disclosed in a published
patent, whether or not patentable, relating to the Patented Process.
“Mexican Authorities” shall mean any applicable federal, state or local governmental authority
within Mexico.
“Microorganism” shall have the meaning set forth in the recitals.
“MSDS” shall have the meaning set forth in Section 9.3.
“Operations Manual” shall mean the detailed written instructions and specifications prepared by
CUSTOMER describing the process for the manufacture, packaging and storage of Product, and provided
by CUSTOMER for use by Fermic under the terms of this Agreement.
“Party” and “Parties” shall have the meaning set forth in the introductory paragraph.
“Patents” shall mean the patents and patent applications (if any) owned by or licensed to CUSTOMER
and any patent or patents issuing there from or thereon, including foreign patents claiming
priority under patent conventions, the inventions claimed thereby and any continuations or
continuations in part, reissues, substitutions or divisions thereof.
“Patented Process” shall mean the process for manufacturing Product covered by one or more of the
claims of Patents.
“Product” shall collectively mean the Fermentation Product and Separation Product.
“Proprietary Information” shall mean any information, drawings, manuals and other documents
transmitted or communicated directly or indirectly on behalf of the disclosing Party to the
receiving Party and marked confidential or proprietary and any information or data orally
-2-
described as confidential or proprietary provided that the disclosing Party shall confirm the same
in writing to the receiving Party within thirty (30) days from the first oral disclosure.
Proprietary Information may include, without limitation, information relating to the disclosing
Party’s business and activities, product research and development, marketing plans or techniques,
client lists and any scientific or technical information, design, process, procedure, formula, or
Know-How (whether or not patentable). The Know-How, including the Operations Manual and the
Microorganism, are Proprietary Information of CUSTOMER.
“Raw Materials” shall mean the raw materials described in Schedule D meeting the
specifications set forth in Schedule D.
“Separation Equipment” shall mean the equipment used in the Separation Process.
“Separation Product” shall have the meaning set forth in the recitals.
“Separation Tolling Fee” shall have the meaning set forth in Section 5.4.
“Tolling Fees” shall collectively mean the Fermentation Tolling Fee and the Separation Tolling Fee.
ARTICLE II
AGREEMENT TO MANUFACTURE; LICENSE
| 2.1 | | Subject to the terms and conditions of this Agreement, Fermic agrees to manufacture Product
at the Facility as instructed by the Customer during the Term of this Agreement using the
Microorganism, the Patented Process and the Know-How provided by CUSTOMER. |
| |
| 2.1 | | CUSTOMER hereby grants to Fermic a nonexclusive right and license during the Term of this
Agreement, and only during the Term of this Agreement, to use the Patented Process, the
Microorganism and the Know-How for the sole purpose of manufacturing Product for the CUSTOMER.
Except as otherwise agreed upon by the Parties, (a) all Product manufactured by Fermic shall
belong to CUSTOMER, (b) Fermic may not use the Patented Process, the Microorganism or the
Know-How to produce the Product other than for or on behalf of CUSTOMER, and (c) no right,
express or implied, is granted by this Agreement to Fermic to use in any manner any trademark
or any other trade name of CUSTOMER in connection with the performance of this Agreement. Any
Know-How or other proprietary information learned by Fermic or Fermic’s contractors in
connection with the services being performed by Fermic on behalf of the CUSTOMER under this
Agreement shall belong solely to the CUSTOMER and Fermic disclaims any ownership right or
right to use such Know-How or proprietary information (except on behalf of the CUSTOMER).
Fermic will provide all commercially reasonable assistance to the CUSTOMER in order to
properly transfer the ownership of such know-how and proprietary information to the CUSTOMER. |
| |
| 2.2 | | Fermic shall obtain and maintain all required licenses, certifications and approvals from the
Mexican Authorities necessary to authorize and permit (a) the use of the |
-3-
| | | Microorganism and any Customer Supplied Separation Equipment at the Facility, (b) the
manufacture of Product at the Facility and (c) the delivery of Product manufactured under
this Agreement to CUSTOMER. Applications for such licenses, certifications and approvals
will be made in the name of Fermic, and Fermic, upon request of the CUSTOMER, will furnish
to CUSTOMER copies of all such documentation promptly after its creation. |
| 2.3 | | CUSTOMER shall be responsible for obtaining any licenses or permits required for shipping of
the Microorganism from CUSTOMER to Fermic or Fermic’s contractors or for shipping of Product
to CUSTOMER or CUSTOMER’s customers. Fermic shall provide commercially reasonable assistance,
if requested by CUSTOMER, to the CUSTOMER with respect to CUSTOMER’S above-described
obligations. |
ARTICLE III
DEVELOPMENTAL START-UP
| 3.1 | | The Developmental Start-Up phase shall commence in July, 2009. CUSTOMER and Fermic shall
conduct development batch runs at the Facility using the Microorganism provided by CUSTOMER,
starting with a 500 liter fermentation run provided by Fermic, with the objective to reproduce
the fermentation process as described by CUSTOMER. |
| |
| 3.2 | | CUSTOMER will provide if requested by Fermic, at no cost to Fermic, a technical
representative knowledgeable about the Patented Process and the Know-How to train Fermic’s
employees before and during the development batches and to otherwise assist with these
batches. CUSTOMER will provide Fermic with a copy of the Operations Manual and such other
written or oral information and documents concerning the Patented Process and the Know-How as
CUSTOMER considers necessary to permit Fermic to produce Fermentation Product. |
| |
| 3.3 | | CUSTOMER anticipates that the batch runs listed in Schedule A will be the batches
that will need to be conducted during the developmental start-up period. The cost per batch
is set forth in Schedule A. The CUSTOMER, in its sole discretion, shall have the
right to select the number of batch runs that it performs and the size of the batch runs. In
addition, to the cost of the batch runs, the CUSTOMER will reimburse Fermic for the cost of
Raw Materials pursuant to Section 6.1. |
| |
| 3.4 | | Notwithstanding anything else set forth in this Agreement, if CUSTOMER, in CUSTOMER’S sole
discretion, determines that it no longer wants to keep conducting development runs because
CUSTOMER is not satisfied with the results of such developmental batch runs, then CUSTOMER
shall have the right to terminate this Agreement. Upon completion of the batch runs, the
CUSTOMER will notify Fermic that the development start-up period is completed and that the
CUSTOMER is ready to begin production of Fermentation Product pursuant to Article IV of this
Agreement. |
| |
| 3.5 | | CUSTOMER and Fermic have determined that the Fermenting Equipment is sufficient to conduct
the Development Start-Up phase. |
-4-
ARTICLE IV
FERMENTATION PROCESS
| 4.1 | | CUSTOMER and Fermic have determined that the Fermenting Equipment is sufficient to produce
the Fermentation Product. Notwithstanding the above, Fermic will be responsible for obtaining
a CO2 tank and the CUSTOMER will reimburse Fermic for the actual cost of the Co2 tank. In
addition, the CUSTOMER will be responsible for the costs of any structural changes or
additions to the Facility that may be needed for the installation and operation of the
Fermenting Equipment. All such changes or additions must be pre-approved by CUSTOMER in
writing. The cost for changes and additions not pre-approved by CUSTOMER will be the
responsibility of Fermic. Fermic will supply the Raw Materials required to produce the
Fermentation Product in accordance with the terms and conditions of Section 6.1. |
| |
| 4.2 | | Schedule B sets forth the amount of Fermentation Product that should be produced
based upon the amounts of Raw Materials used and Microorganism used and process and product
specifications. |
| |
| 4.3 | | Excluding the cost of Raw Materials, the CUSTOMER shall pay to Fermic according to the fee
structure in Schedule B. To the extent the Fermentation Product does not meet the
specifications set forth in this Agreement, the Fermentation Tolling Fee shall be reduced or
refunded to CUSTOMER, if the CUSTOMER has already paid the Fermentation Tolling Fee to Fermic,
by the pro-rata amount based on the amount of off-spec Fermentation Product produced as
compared to the total amount of Fermentation Product and off-spec Fermentation Product
produced. In addition, Fermic shall be responsible for paying or refunding to the CUSTOMER,
if the CUSTOMER has already paid, the total cost of the Raw Materials used in connection with
the production of the off-spec Fermentation Product. |
| |
| 4.4 | | Fermic will dedicate fermentation capacity at the Facility in the volume specified in
Schedule B in order to manufacture the Fermentation Product. |
ARTICLE V
SEPARATION PROCESS
| 5.1 | | CUSTOMER and Fermic have determined that additional Separation Equipment might be required to
conduct the separation process at the Facility. Fermic will use commercially reasonable
efforts to locate the needed Separation Equipment within the Fermic organization. If Fermic
is able to locate the additional Separation Equipment within the Fermic organization, and such
equipment is available, then Fermic will install such equipment at its Facility, at no cost to
CUSTOMER. If Fermic can not locate the additional Separation Equipment within the Fermic
organization, then CUSTOMER will purchase the additional Separation Equipment (“Customer
Supplied Separation Equipment”) needed by Fermic at the Facility in order to produce
Separation Product at the Facility. The Customer Supplied Separation Equipment shall be used
solely by |
-5-
| | | Fermic to produce Separated Product for the CUSTOMER. Fermic and CUSTOMER will determine
the optimum design for the processing requirements to convert Fermentation Product into
Separated Product, locate vendors for the Customer Supplied Separation Equipment, determine
whether new or used equipment should be purchased, purchase the Customer Supplied Separation
Equipment for installation at the Facility, and work with Fermic’s contractor to install the
Customer Supplied Separation Equipment. Other than equipment items included in the Customer
Supplied Separation Equipment, which shall be paid for by CUSTOMER, CUSTOMER will be
responsible for the costs of any structural changes or additions to the Facility that may be
needed for the installation and operation of the Separating Equipment. All such changes or
additions must be pre-approved by CUSTOMER in writing. The cost for changes and additions
not pre-approved by CUSTOMER will be the responsibility of Fermic. Upon termination of this
Agreement, Fermic will give the CUSTOMER or CUSTOMER’s contractors reasonable access to the
Facility in order to allow for the removal by the CUSTOMER or the CUSTOMER’S contractor of
the Customer Supplied Separation Equipment. |
| 5.2 | | After Fermic has installed the Separation Equipment, including, if necessary, any Customer
Supplied Separation Equipment, the Parties will meet to develop a developmental start up
program for the separation process. Upon the CUSTOMER’s approval of the developmental
start-up process for the separation process, the separation process shall be governed pursuant
to the terms of this Article V. The CUSTOMER shall have the right to use CUSTOMER’S
operators at the Facility to produce the Separation Product and use of the CUSTOMER’s
operators will be a factor in determining the Separation Tolling Fee. |
| |
| 5.3 | | Schedule C shall set forth the amount of Separation Product that should be produced
based upon the amounts of Raw Materials used and Fermentation Product used and the process and
product specifications. |
| |
| 5.4 | | The Parties shall negotiate in good faith a tolling fee for the separation process (the
“Separation Tolling Fee”) which is based upon the methodology used by the Parties in
determining the Fermentation Tolling Fee. In no event should the Separation Tolling Fee be
above 50% of the Fermentation Tolling Fee. To the extent the Separation Product does not meet
the specifications set forth in this Agreement, and in the event that CUSTOMER is not
operating the separation process, the Separation Tolling Fee shall be reduced or refunded to
CUSTOMER, if the CUSTOMER has already paid the Separation Tolling Fee to Fermic, by the
pro-rata amount based on the amount of off spec Separation Product produced by the total
amount of Separation Product and off-spec Separation Product produced by Fermic. In addition,
Fermic shall be responsible for paying or refunding to the CUSTOMER, if the CUSTOMER has
already paid, the total cost of the Raw Materials and Fermentation Product used in connection
with the production of the off-spec Separation Product. |
| |
| 5.5 | | If, prior to the Initial Termination Date, this Agreement terminates for any reason other
than CUSTOMER’s breach of this Agreement, CUSTOMER shall have the right to remove the
Customer Supplied Separation Equipment (at CUSTOMER’s expense) from |
-6-
| | | the Facility and Fermic will grant reasonable access rights to CUSTOMER or CUSTOMER’s
contractors to remove the Customer Supplied Separation Equipment. |
ARTICLE VI
GENERAL PRODUCTION; RAW MATERIALS; EQUIPMENT
| 6.1 | | Fermic shall provide laboratory facilities and labor at the Facility necessary to manufacture
Product, and shall be responsible for purchasing Raw Materials meeting the specifications set
forth in Schedule D (other than the Microorganism, which shall be supplied by
CUSTOMER). CUSTOMER will reimburse Fermic for the Raw Materials, within 30 days of receipt
from Fermic of an invoice for such Raw Materials. CUSTOMER shall reimburse Fermic only for
the actual costs paid for the Raw Materials by Fermic. The invoice shall include a copy of
all invoices from third parties reflecting the cost paid by Fermic for such Raw Materials. |
| |
| 6.2 | | The Equipment and laboratory facilities to be used by Fermic must at all times be reasonably
acceptable to CUSTOMER and no changes shall be made to the Equipment and facilities used in
the manufacture of Product unless approved in advance by CUSTOMER, which approval shall not be
unreasonably withheld. |
| |
| 6.3 | | Fermic warrants that the Equipment, the laboratory facilities and the Facility will be
maintained in good condition at all times and Fermic will retain a trained workforce adequate
to manufacture Product under this Agreement. |
| |
| 6.4 | | Fermic shall have sole responsibility for compliance with all applicable laws, including,
without limitation, environmental laws and regulations applicable to the Facility and the
manufacture of Product and for disposal of wastes. CUSTOMER shall have sole responsibility
for compliance with all laws and regulations applicable to the Microorganism and the
marketing, sale and distribution of Product. |
| |
| 6.5 | | Fermic agrees to package and ship Product in such packaging and volumes as CUSTOMER shall
instruct. The CUSTOMER shall provide to Fermic the packaging specifications. All shipping and
any other directly related costs actually incurred by Fermic in connection with Product
packaging and shipping shall be invoiced separately to CUSTOMER, which shall pay the amounts
due within thirty (30) days of receipt of the invoice. |
| |
| 6.6 | | The Tolling Fee under this Agreement shall include storage of reasonable quantifies of
Product (excluding Fermentation Product being stored for production of Separation Product) for
up to thirty (30) days from the date of manufacture. After that date, Fermic may, at its
option, ship the Product to a third-party storage facility. CUSTOMER will pay the reasonable
costs of such shipping and storage. |
| |
| 6.7 | | During the term of the Agreement, the CUSTOMER and CUSTOMER’S consultants and advisors shall
have access to the Facility, during normal business hours and upon advance notice to Fermic,
to assist Fermic and monitor the fermentation and separation processes. |
-7-
ARTICLE VII
TOLLING FEES/BILLING
| 7.1 | | CUSTOMER shall pay Fermic the Fermentation Tolling Fee and the Separation Tolling Fee,
subject to offset as provided in Section 4.3 and Section 5.4. CUSTOMER will separately pay
for all Raw Materials purchased by Fermic for use under this Agreement as set forth in Section
6.1. |
| |
| 7.2 | | Payment of the Tolling Fees and all other charges under this Agreement shall be made within
thirty (30) days from receipt by the CUSTOMER of the invoice by wire transfer to the account
designated by Fermic. CUSTOMER will pay a 1-1/2% monthly charge on all overdue payments under
this Agreement. |
| |
| 7.3 | | The Tolling Fees will be adjusted annually commencing with the end of each calendar year,
according to changes in the US CPI (Consumer Price Index), using January 1, 2009 as the
baseline date for measuring such changes. |
ARTICLE VIII
CONFIDENTIALITY
| 8.1 | | Except as specifically provided by this Agreement, (a) Fermic shall not acquire any right,
title or interest in the CUSTOMER Patents, the Microorganism or the Know-How, (b) Fermic shall
use the Patents, the Know-How and any Microorganism in its possession solely in accordance
with its rights and licenses hereunder and not for any other purpose, and (c) CUSTOMER shall
not acquire any right, title or interest in the Proprietary Information of Fermic. |
| |
| 8.2 | | The receiving Party undertakes not to divulge, or to make accessible, directly or indirectly,
any part of the Proprietary Information to any third party for any reason whatsoever, or use
the same for any purpose other than for the purposes of performing this Agreement. The
receiving Party shall do everything reasonably necessary to prevent the disclosure of any
Proprietary Information to anyone who is not entitled thereto according to this Agreement. |
| |
| 8.3 | | The receiving Party undertakes to limit disclosure of the Proprietary Information only to
those of its employees, officers, consultants and/or affiliates who have to be informed for
the purposes of performing this Agreement. The obligation of confidentiality as contained
herein shall be imposed on the said employees, officers, consultants and/or affiliates. The
receiving Party hereby assumes full responsibility for all such employees, officers,
consultants and/or affiliates for the preservation of the Proprietary Information. |
| |
| 8.4 | | Nothing contained herein shall in any way restrict or impair the receiving Party’s right to
use information which the receiving Party can demonstrate (a) is in the public domain at the
time of disclosure hereunder; or (b) becomes part of the public domain through no fault of the
receiving Party; or (c) is received by the receiving Party from a third party, provided that
such information was not obtained by said third party directly or indirectly |
-8-
| | | from the disclosing Party pursuant to obligations of confidentiality; or (d) is shown by the
receiving Party to be in its possession at the time of disclosure and which was not acquired
directly or indirectly under obligations of confidentiality to the disclosing Party. |
| | | In the event that only part of the Proprietary Information is demonstrated to be within the
foregoing exceptions, the receiving Party may disclose only those parts and the remainder
will be deemed to be Proprietary Information for the purposes of this Agreement. |
| |
| | | CUSTOMER understands that Fermic participates in the development, manufacture, promotion,
sale and commercialization of products for itself and for third parties, including products
that may be competitive with Products. Notwithstanding anything contained in this Agreement
to the contrary, nothing in this Agreement shall be construed as precluding Fermic at any
time from engaging in such activities, subject, however, to Fermic’s compliance with the
provisions of this Agreement with respect to disclosure and use of Proprietary Information. |
| |
| 8.5 | | The receiving Party shall be obligated to return forthwith, any Proprietary Information which
is in its possession, including all copies and abstracts thereof, to the disclosing Party upon
its written request or, at the receiving Party’s option, to destroy such Proprietary
Information, provided that such destruction is confirmed in writing to the disclosing Party. |
| |
| 8.6 | | The obligations of confidentiality contained herein shall bind the parties for a period of
five (5) years from the date of expiration or termination of this Agreement, but the
expiration or termination of such confidentiality obligations shall not permit Fermic to use
the Patents if the Patents have not expired. |
ARTICLE IX
INFRINGEMENT INDEMNITY
| 9.1 | | CUSTOMER shall, at its own expense, defend, indemnify and hold harmless Fermic, its
affiliates and their respective officers, directors and employees (each a “Fermic Indemnified
Party” and collectively the “Fermic Indemnified Parties”) against and from any and all
liabilities, damages, judgments, civil penalties, and expenses (including reasonable
attorneys’ fees) which are incurred by or asserted against a Fermic Indemnified Party,
directly or indirectly, in any claim, litigation or other proceeding brought or made by any
party based upon or arising out of any actual or alleged infringement, misappropriation or
violation of any patents, trade secrets or other intellectual property rights owned by others
relating to the manufacture by Fermic of Product for CUSTOMER under this Agreement. The
defense, settlement, adjustment or compromise of any claim or suit for which CUSTOMER becomes
obligated under this indemnity shall be in the sole control of CUSTOMER. Fermic may, if it
desires, employ counsel at its own expense. |
| |
| 9.2 | | Fermic shall, at its own expense, defend, indemnify and hold harmless the CUSTOMER, its
affiliates and their respective officers, directors and employees (each a “Customer |
-9-
| | | Indemnified Party” and collectively the “Customer Indemnified Parties”) against and from any
and all liabilities, damages, judgments, civil penalties, and expenses (including reasonable
attorneys’ fees) which are incurred by or asserted against an Indemnified Party, directly or
indirectly, in any claim, litigation or other proceeding brought or made by any party based
upon or arising out of (a) any bodily injuries (including death) to any person, including,
without limitation, the injury or death of Fermic’s employees and contractors, (b) damage to
any property, including, without limitation, CUSTOMER’s Property, the Microorganism, and the
Facility, (c) contamination of or adverse effects on the environment, including, without
limitation, contamination of the Facility, or (d) any violation or alleged violation of
statues, ordinances, orders, rules or regulations of any governmental entity or agency, and
which is directly or indirectly, and to the extent that it is caused by, or arises out of
any breach of any warranties by Fermic, or any negligent or willful act or omission of
Fermic, its employees or contractors in the performance of this Agreement. The defense
settlement or compromise of any claim or suit for which Fermic becomes obligated under this
indemnity shall be in the sole control of Fermic. The CUSTOMER may, if it desires, employ
counsel at its own expense. |
| 9.3 | | Fermic acknowledges that it has been adequately warned by the CUSTOMER of the risks
associated with handling, using, transporting, storing and disposing of the Raw Material, the
Microorganism and the Product delivered under this Agreement, including, without limitation,
those set forth in the CUSTOMER’s Material Safety Data Sheet for Product (“MSDS”) delivered by
the CUSTOMER to Fermic, and that Fermic is familiar with the Raw Material, the Microorganism
and the Product. Fermic further acknowledges its separate and independent knowledge of such
risks, which are known in Fermic’s industry. Fermic shall maintain compliance with
appropriate safe handling and use procedures and all safety and health-related governmental
requirements concerning the Raw Material, the Microorganism and the Product and shall take
such steps as are reasonable and practicable to inform its employees, agents, customers and
other third parties associated with the Product, including handling, shipment, storage, use
and disposal thereof. Such steps include, but are not limited to, dissemination of pertinent
information contained in the MSDS, as appropriate. |
ARTICLE X
WARRANTY; WARRANTY DISCLAIMER
| 10.1 | | Fermic warrants that all Raw Materials and Product (Fermentation Product and Separation
Product) shall meet the applicable specifications attached to this Agreement. Fermic warrants
that Raw Materials and Product (Fermentation Product and Separation Product) will at all
times be free and clear of any liens. |
| |
| 10.2 | | EXCEPT AS OTHERWISE EXPRESSLY STATED IN THIS AGREEMENT, FERMIC EXPRESSLY DISCLAIMS ALL
WARRANTIES, STATUTORY, EXPRESSED OR IMPLIED, WITH RESPECT TO THE PRODUCTS, INCLUDING THE
WARRANTY OF MERCHANTABILITY AND THE WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE OR WHETHER
PRODUCTION OF PRODUCT AS CONTEMPLATED HEREIN WILL BE COMMERCIALLY VIABLE. EXCEPT AS |
-10-
| | | OTHERWISE EXPRESSLY STATED IN THIS AGREEMENT, NEITHER PARTY WILL BE LIABLE TO THE OTHER FOR
ANY INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES OF ANY NATURE OR LOSS OF BUSINESS, PROFITS,
OR ANY OTHER COMMERCIAL OR ECONOMIC LOSS, EVEN IF THE PARTY HAS BEEN ADVISED OF THE
POSSIBILITY OR LIKELIHOOD OF SUCH DAMAGES. LIABILITY FOR DEFECTIVE PRODUCT OR FOR FAILURE TO
DELIVER PRODUCT, UNLESS ATTRIBUTABLE TO ACTS OR OMISSIONS OF CUSTOMER, WILL BE LIMITED TO
RETURN OF PRODUCT (FOR A FULL REFUND WHICH SHALL INCLUDE A REFUND OF A PORTION OF THE
TOLLING FEE AS SET FORTH IN SECTION 4.3 AND SECTION 5.4) OR REPLACEMENT OF THE DEFECTIVE
PRODUCT AT FERMIC’S EXPENSE. |
ARTICLE XI
TAXES
| 11.1 | | All prices stated under this Agreement are exclusive of applicable sales, value added, use
and similar governmental taxes and surcharges, which shall be the responsibility of CUSTOMER. |
| |
| 11.1 | | The CUSTOMER reserves its rights to claim U.S. Customs duty drawback, and Fermic acknowledges
and consents to such reservation. Fermic will cooperate with the CUSTOMER in providing any
information required by the CUSTOMER in order to obtain any tax refund or duty drawback with
respect to any Raw Materials, Product or the Microorganism. |
ARTICLE XII
TERM AND TERMINATION
| 12.1 | | The Term of this Agreement shall commence on the Effective Date and, unless earlier
terminated as set forth in this Agreement, shall terminate six (6) months after the separation
process is operating in the amounts set forth in Schedule C (the “Initial Termination
Date”) and will be extended automatically for successive one-year periods unless CUSTOMER or
Fermic gives three (3) month prior written notice of termination to the other Party. |
| |
| 12.2 | | If Fermic is unable to manufacture Product at the Facility for any reason, including force
majeure, for a period of more than three (3) months, and Fermic has not thereafter resumed
manufacture of Product under this Agreement, CUSTOMER may terminate this Agreement, whereupon
neither Party shall have any further obligation to the other except payment for amounts due
the other as of the date of termination and obligations under those Articles and Sections of
the Agreement that specifically survive its termination or expiration. |
| |
| 12.3 | | Either Party shall have the right (but not the obligation), by giving written notice to the
other, to terminate this Agreement upon the happening of any of the following events: |
-11-
| | 12.3.1 | | The other Party defaults in the performance or observance of any agreement contained
in this Agreement and such default is not cured within 60 days of notice thereof from
the non-defaulting Party. |
| |
| | 12.3.2 | | The other Party admits in writing its inability to pay its debts generally as they
become due; files or consents to the filing against it of a petition under bankruptcy
or any insolvency or similar law; appoints or consents to the appointment of a receiver
of itself of all or a substantial part of its property; becomes subject to a court
order under which all or a substantial part of its property is under the control and
custody of a court; is subject to an involuntary filing against it of a petition under
bankruptcy or other insolvency law; or is in a circumstance substantially similar in
character to any of the above. |
| 12.4 | | Notwithstanding anything else herein, the CUSTOMER shall have the right to immediately
terminate this Agreement, without incurring any liability to Fermic, if, at CUSTOMER’S sole
discretion, the CUSTOMER feels that the health and/or safety of its employees or contractors
could be jeopardized in connection with travelling to or visiting the Facility. |
| |
| 12.5 | | Upon termination of this Agreement for any reason, each Party shall, upon request, promptly
return or destroy all Proprietary Information of the other. Without limiting the foregoing,
Fermic, at the Customer’s sole option, shall return or destroy any Microorganism. Each Party
shall certify in writing to the other that it has fully complied with this Section 12.5. |
| |
| 12.6 | | The following Articles and Sections shall survive termination or expiration of this
Agreement: Article VIII (Confidentiality), Article IX (Infringement Indemnity; Article X
(Warranty; Warranty Disclaimer); Article XI (Taxes); Section 12.5 (return or destruction of
Proprietary Information); Section 16.3 (independent contractor relationship); Section 16.6
(governing law); and Section 16.7 (dispute resolution). |
ARTICLE XIII
RISK OF LOSS; INSURANCE
| 13.1 | | Fermic shall bear the risk of loss of Raw Materials (other than CUSTOMER supplied
Microorganism), work in process and Product while the foregoing are stored at the Facility. As
between Fermic and CUSTOMER, the risk of loss shall pass to CUSTOMER upon shipment of Product
from the Facility. |
| |
| 13.2 | | Fermic shall maintain in continuous force insurance against loss of or damage to the
Equipment (including any Customer Supplied Separation Equipment), the Facility, and all Raw
Materials (including the Microorganism), work in process, and Product, in each case while
stored at the Facility, from all casualty risks and all other risks usually insured against by
persons operating a similar business. Without limiting the above, Fermic shall maintain the
insurance coverages in the amounts set forth below from insurance carriers which are
reasonably acceptable to the CUSTOMER. |
-12-
| | 13.2.1 | | Employer’s Liability: |
| | | |
|
| Bodily Injury by Accident
| | $1,000,000 per accident |
| Bodily Injury by Disease
| | $1,000,000 policy limit |
| Bodily Injury by Disease
| | $1,000,000 each employee |
| | 13.2.2 | | Commercial General Liability insurance including coverage for Products/Completed
Operations, and Contractual Liability: |
| | | |
|
| Bodily Injury and Property Damage
| | $1,000,000 per occurrence |
|
| | $2,000,000 annual aggregate |
| | 13.2.3 | | Umbrella Liability Insurance: |
| | | |
|
| Umbrella Liability
| | $10,000,000 per occurrence |
|
| | $10,000,000 annual aggregate |
| 13.3 | | All policies described above shall be endorsed to provide that CUSTOMER is named as an
additional insured. For the avoidance of doubt, with respect to claims with respect to the
personal property of the CUSTOMER, the CUSTOMER shall not be able to recover a dollar amount
under the above insurance policies that exceeds the replacement cost of such personal
property. “Personal property: shall include, without limitation, raw materials paid for by the
CUSTOMER, Customer Supplied Separation Equipment and Microorganism supplied by the CUSTOMER.
In addition, all policies described above shall be endorsed to provide that the underwriters
and insurance companies of Fermic shall not have any right of subrogation against CUSTOMER,
its subsidiaries or affiliates, agents, employees, directors, officers, underwriters or
insurers. |
| |
| 13.4 | | Fermic shall furnish certificates of insurance to CUSTOMER evidencing the insurance required
hereunder. Each certificate shall provide that thirty (30) days’ prior written notice shall
be given to CUSTOMER in the event of any cancellation, reduction in coverage under, or
material change in the policy. In order to avoid delays in commencing work, certificates
shall be provided to CUSTOMER promptly upon the execution of this Agreement. CUSTOMER shall
have no responsibility for payment of deductibles or for payment of any premium. |
| |
| 13.5 | | In the event of any casualty loss to Customer Supplied Separation Equipment, Fermic hereby
assigns to CUSTOMER the right to collect insurance proceeds up to the amount still owed by
Fermic to CUSTOMER and CUSTOMER shall be the sole loss payee with respect to the proceeds for
the Customer Supplied Separation Equipment. |
ARTICLE XIV
FORCE MAJEURE
| | 14.1 | | Except as specifically provided in Section 12.2, no failure or omission to carry out or to
observe any of the terms, provisions or conditions of this Agreement shall give rise to any |
-13-
| | | | claim by one Party against the other, or be deemed to be a breach of this Agreement, if such
failure or omission is caused by one or more of the following: war (whether or not declared
and whether or not United States is a participant) or hostilities; acts of the public enemy
or belligerents; sabotage; blockade, revolution, insurrection, riot or disorder;
expropriation, requisition, confiscation or nationalization; embargoes; export or import
restrictions or rationing or allocation, whether imposed by law, decree or regulation or by
voluntary cooperation of industry at the instance or request of any governmental authority
or organization owned or controlled by any government or person purporting to act therefore
interference by, or restriction or onerous regulation imposed by, any governmental
authority, to whose jurisdiction the Party is subject; act of God; fire; earthquake; storm;
epidemics; quarantine; strikes, lockouts or other labor disturbances; explosion; breakage or
accidents by fire or otherwise to transportation or distribution facilities or equipment;
unavailability of Raw Materials; failure of the Facility to operate for any reason beyond
the control of Fermic; shutdown of the Facility for prudent maintenance; or any other event,
matter or thing wherever occurring, and whether or not of the same class or kind as those
set forth above which, by the exercise of due diligence, the Party concerned is unable to
overcome. |
| 14.2 | | A Party affected by an actual or potential force majeure situation shall promptly notify the
other Party of such situation. |
ARTICLE XV
NON-COMPETITION
| 15.1 | | Fermic on its own behalf and on behalf of the affiliates it Controls (collectively,
“Covenantors”) agrees that from the date of this Agreement until five (5) years after
termination of this Agreement the Covenantors shall (a) not engage in any capacity whatsoever
in the succinic acid business (whether as promoter, owner, officer, director, employee,
partner, shareholder, member, lessee, lessor, lender, agent, consultant, broker, commission
salesman or otherwise) and (b) not have an interest in any corporation, partnership, joint
venture or limited liability company (other than less than a ten (10%) percent interest in a
publicly traded company) engaged in the succinic acid business. |
| |
| 15.2 | | If any Covenantor fails to keep and perform its covenants under Section 15.1, CUSTOMER shall
be entitled to specifically enforce the same by injunction in equity in addition to any other
remedies which CUSTOMER may have. If any portion of Section 15.1 above shall be invalid or
unenforceable, such invalidity or unenforceability shall in no way be deemed or construed to
affect in any way the enforceability of any other portion of Section 15.1 hereof. If any
court in which CUSTOMER seeks to have the provisions of this Non-Competition provision
specifically enforced determines that the activities, time or geographical area hereinabove
specified are too broad, such court may determine a reasonable activity, time or geographical
area and shall specifically enforce Section 15.1 for such reasonable activity, time and
geographical area. |
-14-
ARTICLE XVI
MISCELLANEOUS
| 16.1 | | Any notice to be given under this Agreement shall be in writing and delivered by a recognized
overnight courier service, addressed as follows: |
If to Fermic to it at:
Reforma No. 873
Col — San Xxxxxxx Xxxxxxxxx
C.P. 00000 Xxxxxx D.F.
Attention: Xxxxxxxxxx Xxxxxxx
Telephone: 5255-5037-0004
Facsimile: 5255-5656-1542
If to CUSTOMER to it at:
BioEnergy International, LLC
0 Xxxxxxxxxxxx Xxxx, Xxxxx 000
Xxxxxx, XX 00000-0000 XXX
Attention: Alif Saleh, Director of Special Chemicals Business Unit
Telephone: x0 000-000-0000
Facsimile: x0 000-000-0000
| 16.2 | | Failure of either Party to insist upon strict observance of or compliance with all of the
terms of this Agreement in one or more instances shall not be deemed to be a waiver of its
rights to insist upon such observance in the future or compliance with the other terms hereof. |
| |
| 16.3 | | Except for the limited agency established in Section 5.1, this Agreement shall not be deemed
to establish the relationship of principal and agent, master and servant or a partnership or
joint venture of any kind between Fermic and CUSTOMER, and neither Party shall be liable for
any act of or failure to act by the other Party except as expressly provided in this
Agreement. Without in any way limiting the foregoing, Fermic will be responsible for any
liability derived from the labor relationship with its employees and in no case nor under any
circumstances shall CUSTOMER be considered a direct or substitute employer of Fermic or any of
Fermic’s employees. |
| |
| 16.4 | | This Agreement constitutes the entire understanding and supersedes all prior agreements
between the parties hereto with respect to the subject matter hereof. The provisions herein
shall not be extended or modified except by written agreement between Fermic and CUSTOMER. |
| |
| 16.5 | | In the event that any provision of this Agreement shall be held to be unenforceable, invalid
or otherwise indefinite, the balance of this Agreement shall continue in full force and
effect, unless the severance of the portions held unenforceable would reasonably |
-15-
| | | frustrate the commercial purposes of this Agreement, in which case, reasonable efforts will
be made to reform this Agreement to achieve such commercial purposes. |
| 16.6 | | This Agreement is written in the English language and shall be construed accordingly. This
Agreement shall be performed, interpreted and enforced under the laws of the State of New
York, regardless of the laws that might otherwise govern under any conflict of laws rules or
provisions of the State of New York. |
| |
| 16.7 | | The parties will use their best efforts to resolve by negotiation any dispute, controversy or
claim which may arise in connection with this Agreement. In the event the parties cannot
directly resolve such dispute, controversy or claim, the parties agree to be bound by
arbitration to occur in New York, New York. The arbitration is to be conducted in English by a
single arbitrator acceptable to both parties in accordance with the Rules of Conciliation and
Arbitration of the International Chamber of Commerce. The arbitration decision shall be
binding and final and the local courts, except to enforce such arbitration award, shall have
no jurisdiction over this matter. |
| |
| 16.8 | | This Agreement may not be assigned by either Party without the prior written consent of the
other Party. This Agreement shall inure to the benefit of and be binding on the successors and
permitted assigns of the parties. |
| |
| 16.9 | | Each Party represents to the other that the person signing below on its behalf is legally
authorized and empowered to do so under applicable law and that, upon signature by such
individual on behalf of such Party, this Agreement and any amendments hereto shall be binding
upon and enforceable against it in accordance with its terms. |
| |
| 16.10 | | This Agreement may be executed and/or delivered by facsimile and in counterparts, each of
which shall constitute an original and all of which taken together shall constitute one and
the same instrument. |
[Signature page follows]
-16-
IN WITNESS WHEREOF the parties have caused this instrument to be executed in duplicate as of
the year and day first above written.
| | | | | | | |
|
| BIOENERGY INTERNATIONAL, LLC | | FERMIC SA DE CV |
|
| | | | | | |
| Signature:
| | /s/ Xxxxx Xxxxxxx
| | Signature:
| | /s/ Xxxxxxxxxx Xxxxxxx |
|
| |
| | | | |
| Name:
| | Xxxxx Xxxxxxx
| | Name:
| | Xxxxxxxxxx Xxxxxxx |
| Title:
| | General Manager & Senior Vice President
| | Title:
| | Chief Executive Officer |
SCHEDULE A
Developmental Start-Up Phase
During the developmental start-up phase, the CUSTOMER anticipates performing eight (8) 500 liter
batch runs, eight (8) 5,000 liter batch runs and one (1) 50,000 liter batch run.
Cost Per Batch Run
| | | | | |
|
| 500 liters
| | =
| | US$6,000 |
| 5,000 liters
| | =
| | US$12,500 |
| 50,000 liters
| | =
| | US$25,000 |
The cost per batch run includes all inoculation, seeding, sterilization, cleaning, sampling
and process control needed to produce the Fermentation Product.
A-1
SCHEDULE B
Fermentation Product
At the end of the Development Start-Up, CUSTOMER and Fermic will meet to set production
specifications, amounts of Raw Materials and Microorganism needed to produce the Fermentation
Product, yield, fermentation time and final Fermentation Product concentration in fermentor After
having set the specification, Fermic will be responsible to maintain such specification, which will
be calculated after each fermentation run. Notwithstanding the above, the CUSTOMER will be
responsible for demonstrating the parameters described above during the Developmental Start-Up
phase and Fermic will not be required during the Fermentation Process to produce amounts which
exceed the parameters produced by Customer during the Developmental Start-Up phase. Upon
development of the above parameters, such parameters shall be incorporated by reference into this
Agreement.
Production of Fermentation Product will be conducted in a 50,000 L fermentor. CUSTOMER anticipates
5 batch fermentation runs to produce the target amount of Fermentation Product.
Cost per batch run:
50,000L=$25,000
The cost per batch run includes all inoculation, seeding, sterilization, cleaning, sampling and
process control needed to produce the Fermentation Product.
B-1
SCHEDULE C
Separation Product
At the end of the development start-up process for the separation process, CUSTOMER and Fermic will
meet to set production specifications, amounts of Raw Materials and Fermentation Product, yield,
separation time and final product specification. After having set the production and product
parameters, Fermic will be responsible to maintain such parameters, which will be calculated after
each separation run.
Upon development of the above parameters, such parameters shall be incorporated by reference into
this Agreement.
C-1
SCHEDULE D
Raw Materials
Before the development start up phase is initiated, CUSTOMER will specify the type of Raw Materials
are required for producing the Fermentation Product and the specification and quality required.
Fermic will be responsible for maintaining such Raw Material specifications and quality.
Before the fermentation process phase is initiated, CUSTOMER will specify the type of Raw Materials
are required for producing the Fermentation Product and the specification and quality required.
Fermic will be responsible for maintaining such Raw Material specifications and quality.
Before the separation process phase is initiated, CUSTOMER will specify the type of Raw Materials
are required for producing the Fermentation Product and the specification and quality required.
Fermic will be responsible for maintaining such Raw Material specifications and quality.
D-1
EXECUTION VERSION (Effective December 18, 2009)
FIRST AMENDMENT TO
TOLL MANUFACTURING AGREEMENT
This First Amendment to
Toll Manufacturing Agreement dated effective as of December 18, 2009
(this “Amendment”), is by and among Myriant Technologies, LLC, a Delaware limited liability company
(“CUSTOMER”), and Fermic SA de CV (“Fermic”). CUSTOMER and Fermic shall be separately referred to
hereafter as a “Party” and collectively referred to as “Parties.”
RECITALS
WHEREAS, BioEnergy International, LLC (“BioEnergy”) and Fermic entered into a
Toll
Manufacturing Agreement dated as of May 18, 2009 (the “Agreement”);
WHEREAS, BioEnergy assigned the Agreement, with Fermic’s prior consent, to the CUSTOMER
pursuant to an Assignment Agreement dated July 16, 2009; and
WHEREAS, the Parties desire to amend certain terms of the Agreement and therefore enter into
this Amendment in order to amend such terms.
NOW THEREFORE, BE IT RESOLVED that the Parties agree that the Agreement shall be amended as
follows, with effect as of the signature of this Amendment by both Parties:
AGREEMENT
| I. | | Definitions. Capitalized terms used but not otherwise defined in this Amendment have
the respective meanings set forth in the Agreement. |
| |
| II. | | Amendment and Agreement. |
| | 1. | | CUSTOMER and Fermic acknowledge that, except as set forth in the remainder of
this Section 1, all equipment costs, costs of structural changes, labor costs and other
costs, fees or expenses (including, without limitation, the costs, fees and expenses
incurred by Fermic in connection with Fermic’s performance of its responsibilities set
forth in Section 6 and Section 8 of this Amendment and the payment of all utility costs
required to produce the Fermentation Product and/or Separation Product) which the
CUSTOMER could potentially owe to Fermic in the Agreement and this Amendment are
included in the Payment (as defined below). The only amounts that CUSTOMER will pay to
Fermic under the Agreement or this Amendment in excess of the Payment will be (a)
amounts required to reimburse Fermic for the Raw Materials in accordance with the terms
set forth in Section 6.1 of the Agreement, (b) amounts required to reimburse Fermic (if
not paid directly by CUSTOMER) for the actual, reasonable, documented cost of any
electrical or insulation materials required by |
2
| | | | CUSTOMER to be installed by Fermic with the ECC Unit (as described in Section 8
below); (c) amounts required to reimburse Fermic (if not paid directly by CUSTOMER)
for the actual, reasonable, documented costs (including applicable import duties)
reasonably required to be incurred in order to effect the importation into Mexico of
any materials or equipment required by CUSTOMER under the Agreement; and (d) amounts
required to reimburse Fermic (if not paid directly by CUSTOMER) for the actual,
reasonable, documented cost of shipping (including the cost of any packaging
materials or totes) any materials or equipment required by CUSTOMER under the
Agreement. |
| | 2. | | Schedules B, C and D of the Agreement shall be deleted in their
entirety and replaced with Schedules B, C and D attached to this Amendment. In
addition, Schedules E and F attached to, and referenced in Section 6 of, this
Amendment shall be added to and made a part of the Agreement. |
| |
| | 3. | | The first sentence of Section 4.3 of the Agreement shall be deleted. All
references to “Fermentation Tolling Fee” in such section shall also be changed to
“Payment.” |
| |
| | 4. | | The text of Section 5.3 of the Agreement shall be deleted and replaced with the
following: “Intentionally omitted.” |
| |
| | 5. | | The first two sentences in Section 5.4 of the Agreement shall be deleted. All
references to “Separation Tolling Fee” in such section shall also be changed to
“Payment.” |
| |
| | 6. | | Following the completion of sufficient runs during the Developmental Startup
Phase (defined in Schedule A of the original Agreement) to give the CUSTOMER,
in its reasonable discretion, sufficient confidence to have batches run effectively at
a larger scale, the Parties agree that Fermic will run a number of batches that would
be sufficient to manufacture an aggregate volume of 500,000 liters of Fermentation
Product accounted for in a manner consistent with the Fermenting Equipment operating at
its name-plate volumetric capacity for each such batch (“Nominal Volume”). Of the
500,000l total volume: |
| | (a) | | Fermic will produce and further process a minimum of 250,000
liters of the Fermentation Product into an intermediate liquid Separation
Product and then into a dry, crystalline Final Product (as defined in
Schedule C) in Customer Supplied Equipment (as defined in Section
II.8). |
| |
| | (b) | | For the remainder of the 250,000 liters of Fermentation Product
that will be produced (out of the 500,000l total), it will be shipped offsite
for processing after standard solids removal processing has occurred. |
| |
| | (c) | | These volumes of materials will be generated during the
fermentation process and the above described work shall be collectively
referred to as the “Work”. If, after the Developmental Start-Up phase has
ended and the regular fermentation process has started, the CUSTOMER identifies |
3
| | | | changes to the operating protocols (including changes to substrate or media
composition) that are substantial enough that the CUSTOMER requests further
Development Startup Phase runs, these shall be conducted by Fermic as long
as they fall within the parameters defined in Schedule A as to the
number of runs, and the fee and shall be scheduled as per the protocol for
run scheduling defined in Schedule E. |
| | 7. | | The protocol for scheduling production of Fermentation Product and its
subsequent processing is attached hereto as Schedule E. Notwithstanding
anything set forth in Schedule E, the CUSTOMER shall have the right to make
alterations to the schedule and such alterations shall not permit Fermic to increase
the payments owed by the CUSTOMER to Fermic or for Fermic to make any other claim under
the Agreement against the CUSTOMER. In the event that the CUSTOMER, in its sole
discretion, decides to cancel the production of any or all of the Fermentation Product,
then the Payment for the Work will be reduced (prorated) or refunded to the CUSTOMER
based upon the amount of US$20,000 for each 50,000 liters of Fermentation Product
cancelled by CUSTOMER. Notwithstanding the above, as a result of cancelling the
production of Fermentation Product as described above, the Payment shall not be reduced
by more than US$100,000. |
| |
| | 8. | | Two GEA filtration units (GEA units), one skid mounted Simulated Moving Bed
Chromatography product separation unit (SMB Unit) and an Evaporation and
Crystallization System (ECC Unit) (each as defined below)) shall be provided by or on
behalf of the CUSTOMER and installed by Fermic in accordance with Section 10. For
purposes of this Amendment, the GEA units (2), SMB Unit, and ECC Unit will be
considered “Customer Supplied Equipment.” The GEA filtration units (2), SMB Unit and
ECC Unit will be promptly returned to the CUSTOMER or CUSTOMER’s representative upon
completion of the Work. The cost and responsibility to disconnect, clean and prepare
the GEA units (2), SMB Unit, and ECC Unit for transportation shall be the sole
responsibility of Fermic. |
| |
| | 9. | | If during the fermentation process the CUSTOMER identifies further additional
equipment that may be required as a result of process developments or process changes,
the CUSTOMER may with the agreement of Fermic bring such equipment to the site as an
additional component of the Customer Supplied Equipment used to produce Final Product.
Fermic shall invoice CUSTOMER for such installation at Fermic’s actual cost (without
any overhead charges or other administrative or similar charges) via normal invoicing
practices. |
| |
| | 10. | | Fermic shall be responsible for obtaining all equipment to achieve the
functionality as shown on the agreed upon two (2) Process Flow Diagrams included as
Schedule F, with the exception of the Customer Supplied Equipment (the GEA
units (2), SMB Unit, and ECC Unit). Fermic shall be responsible for installing all
equipment not within the Customer Supplied Equipment skids, connecting the Customer
Supplied Equipment within the skids to the remainder of |
4
| | | | the facility, and maintaining all the equipment necessary to produce Fermentation
Product, Separation Product, and Final Product. |
| | 11. | | Notwithstanding anything to the contrary in the original text of the Agreement
but subject to the last sentence of Section 1 of this Amendment, CUSTOMER has agreed,
as sole payment for the Work and the return of the Customer Supplied Equipment, to pay
Fermic One Million Dollars (US$1,000,000)(the “Payment”). Invoices shall state that
Payment is to be made to Fermic International Ltd., and Fermic agrees that the Payment
shall be made by CUSTOMER to such entity and not Fermic. The Payment shall be made as
follows: |
| | (a) | | Upon execution of this Amendment, the CUSTOMER shall pay,
within three (3) business days, Fermic One Hundred Twenty Five Thousand Dollars
(US$125,000). |
| |
| | (b) | | Upon completion of the first successful 20,000 liters batch of
Fermentation Product, the CUSTOMER shall pay, within thirty (30) days, Fermic
One Hundred Twenty Five Thousand Dollars (US$125,000). |
| |
| | (c) | | Upon completion of the first 1000 liters of Fermentation
Product produced in Fermic’s fermentation equipment and processed through the
SMB Unit (commissioning step), the CUSTOMER shall pay, within thirty (30) days,
Fermic One Hundred Fifty Thousand Dollars (US$150,000). |
| |
| | (d) | | Upon completion of processing 1000 liters of Separation Product
in the ECC Unit (evaporation and drying unit commissioning), the CUSTOMER shall
pay, within thirty (30) days, Fermic One Hundred Thousand Dollars (US$100,000). |
| |
| | (e) | | Upon completion of the first 1000kg of on specification Final
Product in crystal form from the evaporator and crystal skid, the CUSTOMER
shall pay, within thirty (30) days, Fermic Two Hundred Thousand Dollars
(US$200,000). |
| |
| | (f) | | Upon completion of 50% of the contract production quantity
(“contract production quantity” defined as 250,000 liters of Fermentation
Product processed through the Customer Supplied Equipment to Final Product on a
Nominal Volume basis (as defined in Section II.6), the CUSTOMER shall pay,
within thirty (30) days, Fermic One Hundred Fifty Thousand Dollars
(US$150,000). |
| |
| | (g) | | Upon (1) production of 500,000 total liters of Fermentation
Product, (2) completion of processing of 250,000 of such Fermentation Product
through the Customer Supplied Equipment to Final Product on a Nominal Volume
basis as defined in Section II.6, and (3) the return to CUSTOMER of all
Customer Supplied Equipment, the CUSTOMER shall pay, within thirty (30) days,
Fermic One Hundred Fifty Thousand Dollars (US$150,000). |
5
| | 12. | | The phrase “in the amounts set forth in Schedule C” shall be deleted
from Section 12.1 of the Agreement. |
| III. | | Agreement Otherwise Unchanged. Except as provided in this Amendment, the Agreement
remains unchanged and in full force and effect, and each reference to the “Agreement” and
words of similar import in the Agreement, refer to the “Agreement” as amended by this
Amendment. |
| |
| IV. | | Miscellaneous. The Parties may execute this Amendment in counterparts and by
electronic transmission. THIS AMENDMENT SHALL BE GOVERNED BY, AND CONSTRUED IN ACCORDANCE
WITH, THE LAWS OF THE STATE OF NEW YORK, UNITED STATES OF AMERICA, REGARDLESS OF THE LAWS THAT
MIGHT OTHERWISE GOVERN UNDER ANY CONFLICT OF LAWS RULES OR PROVISIONS OF THE STATE OF NEW
YORK. |
The remainder of this page has intentionally been left blank.
6
IN WITNESS WHEREOF, this First Amendment to the
Toll Manufacturing Agreement is to be effective as
of the date first written above.
| | | |
|
| MYRIANT TECHNOLOGIES, LLC
| | FERMIC SA DE CV |
|
| | |
| /s/ Xxxxx Xxxxxxx
| | /s/ Xxxxxxxxxx Xxxxxxx |
|
| | |
| Name: Xxxxx Xxxxxxx
| | Name: Xxxxxxxxxx Xxxxxxx |
| Title: Senior Vice President
| | Title: Chief Executive Officer |
Signature Page to First Amendment to
Toll Manufacturing Agreement
SCHEDULE B
Fermentation Product Production Operations
Within a reasonable period of time following CUSTOMER’s notification to Fermic in accordance with
Section 3.4 that CUSTOMER is ready to begin production of Fermentation Product, CUSTOMER and Fermic
will meet to set production specifications, amounts of Raw Materials and Microorganism needed to
produce the Fermentation Product, yield, fermentation time and final Fermentation Product
concentration in the fermentor. These specifications will be communicated by the transmittal of
fermentation protocols, and may be adjusted by CUSTOMER prior to any specific runs including
variations in feed materials, process conditions, or final processing steps. Runs subsequent to the
Development Start-Up period will be considered part of and tracked as part of the fermentation
process for purposes of determining whether the contract production quantity (as defined in Section
II.11 of this Amendment) and other production requirements set forth in the Agreement, as amended,
have been achieved.
After having received the specification and agreed to the operating protocol, Fermic will be
responsible to maintain such process operations specifications, which will be confirmed in writing
by Fermic after each fermentation run. Notwithstanding the above, the CUSTOMER will be responsible
for demonstrating the parameters described above during any Developmental Start-Up phase run and
Fermic will not be required following the Developmental Start-Up phase to produce amounts of
succinic acid product concentration which exceed the parameters produced by CUSTOMER during the
Developmental Start-Up phase.
As noted in Section II.6 of this Amendment, the CUSTOMER may run additional Development Start-Up
phase runs at the sizes and number of runs identified in Schedule A if significant changes are
identified in the operating protocols as a result of process development work, feedstock or media
modifications, or other CUSTOMER or Fermic identified reasons.
The Parties agree that Fermic will run a number of batches that would be sufficient to manufacture
an aggregate volume of 500,000 liters of Fermentation Product if the Fermenting Equipment were
operating at name-plate capacity for each such batch. Production of Fermentation Product will be
conducted in either 50,000 L or 20,000 L batches, as agreed by CUSTOMER and Fermic, but CUSTOMER
reserves the right to require at least 3 successful 50,000 L batches at the CUSTOMER’s discretion.
Tracking of volumes of Fermentation Product, and the processing of Fermentation Product into
Separation Product and Final Product will be done after each run in a summary manner as per the
Table below.
B-1
Summary Table for Tracking of Fermentation Runs, and Disposition of Fermentation Product
| | | | | | | | | | | |
| | | | | | | | | Cumulative | | |
| | | | | | | | | Production | | |
| | | | | | | | | Fermentation | | |
| | | | | Fermentation | | | | Product | | Fermentation |
| | | | | Batch / Run | | | | produced (for | | Product |
| | | | | Nominal | | | | purposes of | | processed to |
| Run ID | | Run Date | | Volume | | Run Type | | agreement) | | Final Product |
| 500D-1
| | Sept, 2009 | | 500 | | Developmental | | — | | — |
| 500D-1
| | Sept, 2009 | | 500 | | Developmental | | — | | — |
| 50,000D-1
| | November, 2009 | | 50,000l | | Developmental | | — | | — |
| 20,000A-1
| | 12/3/2009 | | 20,000l | | Production | | 20,000 | | — |
| Contractually Agree Upon Value
| | | | 20,000l or 50,000l | | Production | | 500,000l | | 250,000l |
This table has been populated, purely for example purposes, with a representation of the runs that
have been completed as of December 9, 2009.
Approval and inclusion of runs in the required total shall be dependent upon agreement and approval
by CUSTOMER that the run was conducted as per CUSTOMER’s protocol and guidance, in properly
configured, functioning, and adequate process equipment. Disputes as to whether a run meets the
acceptable requirements of a successful run shall be resolved within two weeks (14 calendar days)
of any run completion.
B-2
SCHEDULE C
Separation Product and Final Product
Subsequent to production, Fermentation Product will be processed at the Fermic site in Customer
Supplied Equipment (as well as Fermic equipment) for cell removal and pH adjustment as indicated in
Schedule F, and then processed through the SMB Unit (or any substantively equivalent unit which
separates the succinic acid from the fermentation broth that may be brought to Fermic as Customer
Supplied Equipment at a later date). The succinic acid rich broth stream from the SMB Unit is
considered the Separation Product for the purposes of this Agreement; the second stream from the
SMB Unit is rich in ammonium sulfate and is considered the Ammonium Sulfate coproduct stream. For
purposes of this Agreement, “Final Product” shall mean the crystal produced in the evaporation and
crystallization skid when processed further in Fermic’s dryer.
The CUSTOMER will ensure that Fermic is provided with all training and operating documentation to
effectively operate Customer Supplied Equipment, and will provide direction and operating protocols
for this equipment during the equipment commissioning period. After having commissioned the
equipment to achieve the production and product parameters, Fermic will be responsible to maintain
such parameters as per CUSTOMER’s direction and operating protocols, which may be adjusted by
CUSTOMER for each separation run.
Approval and inclusion of processing runs in the required total of Separation Product and Final
Product shall be dependent upon agreement and approval by CUSTOMER that the run was conducted as
per CUSTOMER’s protocol and guidance, in properly configured, functioning, and adequate process
equipment. CUSTOMER and Fermic shall use commercially reasonable efforts to resolve any disputes as
to whether a run meets the acceptable requirements of a successful run within two weeks (14
calendar days) of any run completion.
The specifications and operating parameters for Separation Product and Final Product will be
established by CUSTOMER prior to each applicable run.
C-1
SCHEDULE D
Raw Materials, Chemicals and Supplies
CUSTOMER will specify and/or approve in writing the type of Raw Materials, chemicals and supplies
required for producing the Fermentation Product and the specification and quality required. Fermic
will be responsible for maintaining such Raw Material, chemicals and supplies specifications and
quality.
Before the fermentation process phase is initiated, CUSTOMER will specify in writing the type of
Raw Materials, chemicals and supplies required for producing the Separation Product and the
specification and quality required. Fermic will be responsible for maintaining the specifications
and quality of such Raw Materials, chemicals and supplies.
Before the product separation process is initiated, CUSTOMER will specify in writing the type of
Raw Materials, chemicals and supplies required for producing the Final Product and the
specification and quality required. Fermic will be responsible for maintaining the specifications
and quality of such Raw Materials, chemicals and supplies.
D-1
SCHEDULE E
Schedule Protocol
At all times during the term of the Agreement, Fermic shall produce and maintain a supply and
inventory of Fermentation Product available for processing in the Customer Supplied Equipment to
produce Separation Product and Final Product, until the total volumes identified in this Agreement
have been achieved. In case there is reasonable cause for exception to this requirement (such as a
shutdown period at the plant), CUSTOMER shall be notified 7 days in advance and shall not
unreasonably withhold its approval for the inventory reduction.
The inventory shall be maintained by notifying the CUSTOMER when the existing site inventory
reaches 10,000l or less and by starting a new fermentation run no later than when the Fermentation
Product available has reached 6000l. These levels are set such that a new Fermentation Product run
can be started in sufficient time such that the inventory is never depleted at the identified
operating rate of the SMB Unit (80 liters per hour) unless approved by CUSTOMER.
Additionally, upon reasonable advance written notice (2 weeks or 14 calendar days) from CUSTOMER,
Fermic shall produce additional runs of at least 20,000 liters of Fermentation Product, for
shipping offsite by the CUSTOMER after processing of the Fermentation Product is completed up to
500,000 liters of Fermentation Product in the aggregate.
The two week advance notice period also applies to any requested developmental runs required during
the fermentation process following the date of CUSTOMER’s delivery of its notice in accordance with
Section 3.4 that it is ready to begin production of Fermentation Product, notwithstanding any
physical modifications required to the developmental equipment that would require a timeframe
exceeding two weeks. All such exceptions are to be identified in writing to the CUSTOMER, and
CUSTOMER and Fermic shall use commercially reasonable efforts to agree upon such physical
modifications and to set a firm schedule date for operations no later than two weeks after any
required components arrive onsite.
A four week forward schedule of planned Fermentation Product runs will be maintained by Fermic, and
CUSTOMER will approve or disapprove (in its reasonable discretion) of such schedule on a weekly
basis during regular Tuesday production meetings (onsite or via teleconference).
E-1
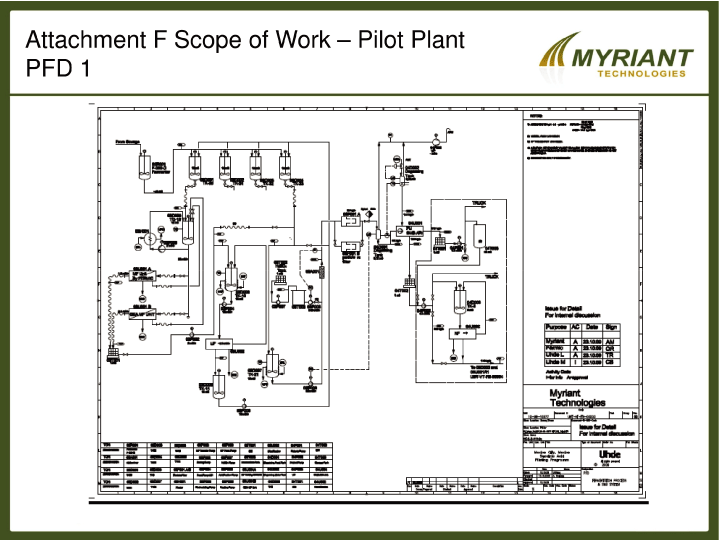
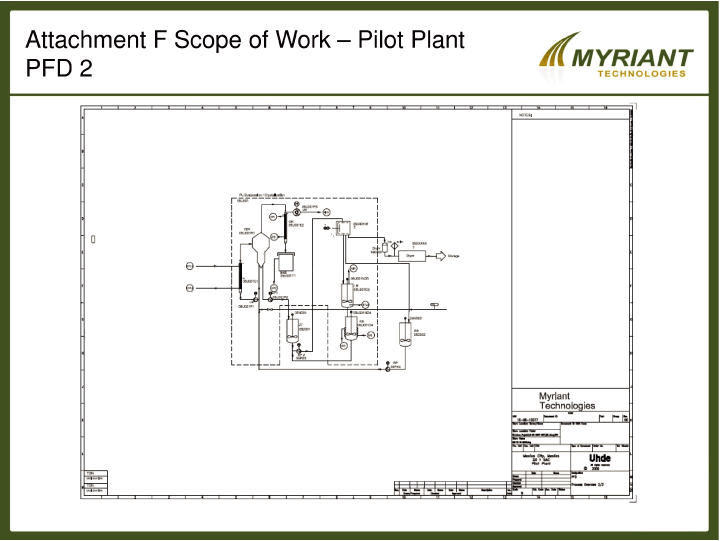
SCHEDULE F
Fermentation Product Separation and Final Product Production Equipment Configuration
As shown in the attached Process Flow Diagrams, Fermic is to install the Customer Supplied
Equipment to achieve the functional piping and process requirements indicated by these drawings. In
the event that process requirements are identified that additional equipment to be brought to the
site, installed and operated by Fermic, updated PFDs will be prepared for CUSTOMER’s approval, and
installation costs with no additional charges will be identified, documented and passed through to
the CUSTOMER for payment in the form of an invoice by FERMIC.
[DIAGRAMS TO BE ATTACHED]
F-1