Execution Version CONCORDIA INTERNATIONAL CORP. $350,000,000 9.000% First Lien Senior Secured Notes due 2022 Purchase Agreement October 6, 2016 Goldman, Sachs & Co., 200 West Street New York, New York 10282-2198 Ladies and Gentlemen: Concordia...

Execution Version
CONCORDIA INTERNATIONAL CORP.
$350,000,000 9.000% First Lien Senior Secured Notes due 2022
Purchase Agreement
October 6, 2016
Xxxxxxx, Xxxxx & Co.,
000 Xxxx Xxxxxx
Xxx Xxxx, Xxx Xxxx 00000-0000
Ladies and Gentlemen:
Concordia International Corp., a corporation organized under the laws of the province of
Ontario (the “Company”), proposes to issue and sell to Xxxxxxx, Sachs & Co. (the “Initial Pur-
chaser”), $350,000,000 principal amount of its 9.000% First Lien Senior Secured Notes due
2022 (the “Notes”). The Notes will be issued pursuant to an indenture to be dated as of October
13, 2016 (the “Indenture”) among the Company, the Guarantors (as defined herein, and, together
with the Company, the “Issuers”), U.S. Bank National Association, as trustee (in such capacity,
the “Trustee”) and as collateral agent (in such capacity, the “Collateral Agent”).
The payment of principal, premium, if any, and interest on the Notes will be fully and
unconditionally guaranteed on a senior secured basis, jointly and severally, at the Closing Date
(as defined below) or on or before the Specified Guarantor Accession Deadline (as defined be-
low), as applicable, by the entities listed on Schedule 2 hereto (the “Guarantors”), pursuant to
their guarantees (the “Guarantees”). The Notes and the Guarantees attached thereto are herein
collectively referred to as the “Securities.”
From and after the Closing Date, the Securities will be secured by liens (subject to certain
exceptions and Permitted Liens (as defined in the Pricing Disclosure Package and the Offering
Memorandum, both as defined below)) on substantially all of the tangible and intangible assets
of the Company and the Identified Guarantors, now owned or hereafter acquired by the Compa-
ny or any such Identified Guarantor, that secure borrowings under the Credit Agreement (as de-
fined below) on a first-priority basis (the “Closing Date Collateral”), in each case as more partic-
ularly described in the Pricing Disclosure Package and the Offering Memorandum, and docu-
mented by certain security agreements dated as of the Closing Date (the “Closing Date Collateral
Agreements”) each in favor of the Collateral Agent, for its benefit and the benefit of the Trustee
and the holders of the Securities. After the Time of Delivery and in any event within 30 days
thereafter (as such period may be extended by the Collateral Agent in its sole discretion) (the
“Post-Closing Security Date”), the Company shall cause the remaining Guarantors to enter into
security documents (the “Post-Closing Collateral Agreements”, and, together with the Closing
Date Collateral Agreements, the “Collateral Documents”) each in favor of the Collateral Agent,
for its benefit and the benefit of the Trustee and the holders of the Securities to secure the Securi-
ties with liens (subject to certain exceptions and Permitted Liens) on substantially all of the tan-

2
gible and intangible assets of such Guarantors, now owned or hereafter acquired by such Guaran-
tors, that secure borrowings under the Credit Agreement on a first-priority basis (the “Post-
Closing Collateral” and together with the Closing Date Collateral, the “Collateral”), in each case
as more particularly described in the Pricing Disclosure Package and the Offering Memorandum.
The rights of the holders of the Securities with respect to the Collateral will be further
governed by (i) that certain intercreditor agreement (the “Intercreditor Agreement”) to be dated
as of the Time of Delivery, by and among, among others, Xxxxxxx Xxxxx Bank USA, as Collat-
eral Agent under the Credit Agreement (the “Existing First Lien Agent”), the Collateral Agent,
the Company and the Guarantors and (ii) that certain intra-group subordination agreement (the
“Subordination Agreement”) to be dated as of the Time of Delivery, by and among, among oth-
ers, the Collateral Agent, the Trustee, the Company and the Identified Guarantors.
On or prior to the Closing Date, each Identified Guarantor will enter into a joinder
agreement to this Agreement, the form of which is attached hereto as Exhibit B (the “Joinder
Agreement”), pursuant to which such Identified Guarantor will become party to this Agreement.
On or prior to the Specified Guarantor Accession Deadline, each Specified Guarantor will (i) en-
ter into a Joinder Agreement, pursuant to which such Specified Guarantor will become party to
this Agreement, (ii) enter into the Supplemental Indenture, a form of which will be attached to
the Indenture, pursuant to which such Specified Guarantor will become a party to the Indenture,
(iii) enter into a supplement to the Intercreditor Agreement, a form of which will be attached to
the Intercreditor Agreement, pursuant to which such Specified Guarantor will become a party to
the Intercreditor Agreement and (iv) enter into a supplement to the Subordination Agreement, a
form of which will be attached to the Subordination Agreement, pursuant to which such Speci-
fied Guarantor will become a party to the Subordination Agreement.
The Securities will be sold to the Initial Purchaser without being registered under the Se-
curities Act of 1933, as amended (the “Securities Act”), in reliance upon an exemption therefrom
and to non-U.S. persons in the Canadian Offering Jurisdictions (as defined below) in accordance
with NI 45-106 (as defined below) pursuant to the exemption available under Section 2.3 of NI
45-106 or Section 73.3 of the Securities Act (Ontario) from the prospectus requirements under
Canadian Securities Laws (as defined below) (the “Offering”). The Issuers have prepared a pre-
liminary offering memorandum dated October 5, 2016 (together with any documents incorpo-
rated or deemed to be incorporated by reference therein, the “Preliminary Offering Memoran-
dum”), and will prepare an offering memorandum dated the date hereof (together with any doc-
uments incorporated or deemed to be incorporated by reference therein, the “Offering Memoran-
dum”) setting forth information concerning the Company and the Securities. For the purposes
hereof, any reference to the Preliminary Offering Memorandum includes the Canadian offering
memorandum dated October 5, 2016 of the Company regarding the offer for sale of the Notes
being made in Canada (together with any documents incorporated or deemed to be incorporated
by reference therein, the “Canadian Preliminary Offering Memorandum”), to the extent applica-
ble, and any reference to the Offering Memorandum includes the Canadian offering memoran-
dum dated the date hereof of the Company regarding the offer for sale of the Notes being made
in Canada (together with any documents incorporated or deemed to be incorporated by reference
therein, the “Canadian Offering Memorandum”), to the extent applicable.

3
Copies of the Preliminary Offering Memorandum have been, and copies of the Offering
Memorandum will be, delivered by the Company to the Initial Purchaser pursuant to the terms of
this Agreement. The Company hereby confirms that it has authorized the use of the Preliminary
Offering Memorandum, the other Time of Sale Information and the Offering Memorandum in
connection with the offering and resale of the Securities by the Initial Purchaser in the manner
contemplated by this Agreement. Capitalized terms used but not defined herein shall have the
meanings given to such terms in the Preliminary Offering Memorandum.
At or prior to the time when sales of the Securities were first made (the “Time of Sale”),
the Preliminary Offering Memorandum, as supplemented and amended by the written communi-
cations listed on Annex A hereto (collectively, the “Time of Sale Information”), shall have been
prepared.
The issuance and sale of the Notes (including the execution and delivery of the Guaran-
tees) on the Closing Date and the payment of transaction costs are referred to herein, collective-
ly, as the “Transactions.”
Each of the Company and the Guarantors hereby confirms its agreement with the Initial
Purchaser concerning the purchase and resale of the Securities, as follows:
Definitions. Unless otherwise defined herein, the following words and terms shall have
the meanings ascribed below:
“Accession Documents” means the Joinder Agreement, the Supplemental Indenture, the
supplement to the Intercreditor Agreement and the supplement to the Subordination Agreement.
“AMCo” means Concordia International (Jersey) Limited (formerly known as Amdi-
pharm Mercury Limited), a company incorporated in Jersey.
“AMCo Auditors” means KPMG LLP, the auditors for AMCo.
“Ancillary Documents” means the Notes, the Indenture (including each Guarantee set
forth therein), the Collateral Documents, the Accession Documents, the Subordination Agree-
ment and the Intercreditor Agreement.
“Canadian Offering Jurisdictions” means each of the provinces of Alberta, British Co-
lumbia, Ontario and Quebec.
“Canadian Securities Laws” means, collectively, securities laws in each of the Canadian
Offering Jurisdictions applicable in connection with the offer for sale of the Notes being made in
the Canadian Offering Jurisdictions and the respective rules and regulations made thereunder,
together with applicable multilateral or national instruments, and published orders and rulings
issued or adopted by each of the Securities Regulators.
“CMS” means The Centers for Medicare and Medicaid Services, a division of the United
States Department of Health and Human Services, or any successor agency thereto.

4
“Company Option Plan” means the stock option plan of the Company, as amended from
time to time.
“Concordia Auditors” means the Concordia Current Auditors and the Concordia Former
Auditors.
“Concordia Current Auditors” means PricewaterhouseCoopers LLP, the Company’s au-
ditors.
“Concordia Former Auditors” means Xxxxxxx Xxxxxx Toronto LLP, the auditors for the
Company from January 20, 2010 to June 25, 2015.
“Covis” means, collectively, Covis Pharma S.à.x.x., Zug Branch and Covis Injectables
S.à.x.x., Zug Branch.
“Covis Acquisition” means the acquisition described in the Company’s business acquisi-
tion report dated July 3, 2015.
“Covis Auditors” means Xxxxx Xxxxxxx LLP.
“Credit Agreement” means the Credit and Guaranty Agreement dated as of October 21,
2015, between the Corporation, as borrower, the Guarantors, as guarantors, certain lenders there-
to from time to time, and Xxxxxxx Sachs Bank USA, as administrative agent and collateral agent
(as amended, restated, supplemented or modified from time to time).
“DOJ” means the United States Department of Justice.
“FDA” means the U.S. Food and Drug Administration of the U.S., Department of Health
& Human Services.
“Federal Health Care Program” has the meaning specified in Section 1128B(f) of the So-
cial Security Act and includes the Medicare, Medicaid and TRICARE programs.
“Governmental Authority” means any federal, provincial, state, municipal, local or other
governmental or public department, commission, board, bureau, agency, instrumentality or body,
domestic or foreign (including, without limitation, any stock exchange, securities regulatory au-
thority, central bank, fiscal or monetary authority or authority regulating banks), any subdivision
or authority of any of the foregoing or any quasi-governmental, self-regulatory organization or
private body exercising any regulatory, expropriation or taxing authority under or for the account
of its members or any of the foregoing.
“Health Care Laws” means the following statutes, regulations, guidelines, ordinances, or-
ders, standards, requirements, approvals, or consents, to the extent applicable to the business of
the Company: Title XVIII of the Social Security Act, 42 U.S.C. §§ 1395-1395, including specif-
ically and without limitation, the DMEPOS standards and conditions for Medicare payment, 42
C.F.R. § 424.57 and the Ethics in Patient Referrals Act, as amended, 42 U.S.C. § 1395nn; Ti-
tle XIX of the Social Security Act, 42 U.S.C. §§ 1396 – 1396w-5v; the Federal Xxxx-Xxxxxxxx
Xxxxxxx, 00 X.X.X. § 0000x-0x(x); the False Claims Act, 31 U.S.C. §§ 3729-3733 (as amended);

5
the Program Fraud Civil Remedies Act, 31 U.S.C. §§ 3801-3812; the Anti-Kickback Act, 41
U.S.C. §§ 8701 – 8707; the Civil Monetary Xxxxxxxxx Xxx, 00 X.X.X. § 0000x-0x; the Criminal
Penalties for Acts Involving Federal Health Care Programs, 42 U.S.C. § 1320a-7b; Mail and
Wire Fraud, 18 U.S.C. §§ 1341-1343; False Statements Relating to Health Care Matters, 18
U.S.C. § 1035; Health Care Xxxxx, 00 X.X.X. § 0000; the Exclusion Laws, 42 U.S.C. § 1320a-7;
Title II, Subtitle F of the Health Insurance Portability and Accountability Act of 1996, 42 U.S.C.
§§ 1320d-1329d-9, as amended by Subtitle D of the Health Information Technology for Eco-
nomic and Clinical Health (HITECH) Act as incorporated in the American Recovery and Rein-
vestment Act of 2009, 42 U.S.C. §§ 17921-17953; Supplemental Medical Insurance Benefit reg-
ulations, 42 C.F.R. Part 410; the Transparency Reports and Reporting of Physician Ownership or
Investment Interests Law (the “Sunshine Act”), 42 U.S.C. § 1320a-7h; The Food, Drug, and
Cosmetic Act, 21 U.S.C. §§ 301-399f (including the Orphan Drug Act), and any implementing
regulations pursuant to any of the foregoing and any similar statutes, regulations, guidelines, or-
dinances, orders, standards, requirements, approvals, or consents which may be applicable in any
other jurisdiction in which the Products are sold.
“Identified Guarantors” means the Guarantors identified as such under the heading “Iden-
tified Guarantors (Closing Date)” on Schedule 2.
“IFRS” means International Financial Reporting Standards as issued by the International
Accounting Standards Board, which were adopted by the Canadian Accounting Standards Board
as Canadian generally accepted accounting principles applicable to publicly accountable enter-
prises.
“LTIP” means the long-term incentive plan of the Company, as amended from time to
time.
“NI 45-106” means National Instrument 45-106 – Prospectus Exemptions of the Canadi-
an Securities Administrators.
“OIG” means the United States Department of Health and Human Services Office of In-
xxxxxxx General.
“Permitted Encumbrances” means (i) any validly perfected security interest given by the
Company in respect of any indebtedness; (ii) any other security given by the Company in con-
nection with the operation of the business of the Company; (iii) liens against the Company or its
assets for taxes, assessments or governmental charges or levies not due and delinquent; (iv) un-
determined or inchoate liens and charges incidental to the current operations of the Company
which have not been filed pursuant to law or which relate to obligations not due or delinquent;
and (v) those otherwise disclosed in the Transaction Documents or the Offering Memorandum,
including in connection with the Credit Agreement.
“Person” means any individual, partnership, limited partnership, limited liability partner-
ship, limited liability corporation, limited liability company, joint venture, syndicate, sole propri-
etorship, company or corporation with or without share capital, unincorporated association, trust,
trustee, executor, administrator or other legal personal representative or other entity however
designated or constituted.

6
“Products” means the pharmaceutical products currently sold by the Company or any of
the Subsidiaries.
“Purchaser” means any Person who shall purchase Notes pursuant to the Offering.
“Regulatory Authority” means any Governmental Authority or other body authorized by
applicable law, exercising regulatory authority for the purpose of protecting or promoting public
health and safety, over the testing, development, marketing, manufacturing, or distribution of any
drug or medical device intended for use in human beings, including without limitation, the FDA.
“Securities Regulators” means the applicable securities commissions or regulatory au-
thorities in each of the Canadian Offering Jurisdictions.
“Specified Guarantors” means the Guarantors identified as such under the heading “Spec-
ified Guarantors” on Schedule 2 hereto.
“Specified Guarantor Accession Date” means the date on or before the Specified Guaran-
tor Accession Deadline upon which the Specified Guarantors execute their respective Accession
Documents.
“Specified Guarantor Accession Deadline” means the date that is fifteen (15) days after
the Closing Date.
“Supplemental Indenture” means a supplemental indenture to the Indenture.
"Swiss Withholding Tax" means the tax imposed based on the Swiss Federal Act on
Withholding Tax of 13 October 1965 (Bundesgesetz über die Verrechnungssteuer).
“Transaction Documents” means this Agreement, the Notes, the Indenture (including
each Guarantee set forth therein), the Collateral Documents, the Accession Documents, the Sub-
ordination Agreement and the Intercreditor Agreement.
1. Purchase and Resale of the Notes.
(a) The Issuers agree to issue and sell the Securities to the Initial Purchaser as provid-
ed in this Agreement, and the Initial Purchaser, on the basis of the representations, warranties
and agreements set forth herein and subject to the conditions set forth herein, agrees to purchase
from the Company the principal amount of Notes (including the Guarantees) set forth opposite
the Initial Purchaser’s name in Schedule 1 hereto at a price equal to [REDACTED -
commercially sensitive information] of the principal amount thereof plus accrued interest, if
any, from October 13, 2016 to the Closing Date. The Issuers will not be obligated to deliver any
of the Notes (including the Guarantees) except upon payment for all the Notes (including the
Guarantees) to be purchased as provided herein.
(b) The Issuers understand that the Initial Purchaser intends to offer the Securities for
resale on the terms set forth in the Time of Sale Information and this Agreement. The Initial
Purchaser represents, warrants and agrees that:

7
(i) it is a qualified institutional buyer within the meaning of Rule 144A under
the Securities Act (a “QIB”) and an accredited investor within the meaning of
Rule 501(a) under the Securities Act;
(ii) it has not solicited offers for, or offered or sold, and will not solicit offers
for, or offer or sell, the Securities by means of any form of general solicitation or general
advertising within the meaning of Rule 502(c) of Regulation D under the Securities Act
(“Regulation D”) or in any manner involving a public offering within the meaning of
Section 4(a)(2) of the Securities Act; and
(iii) it has not solicited offers for, or offered or sold, and will not solicit offers
for, or offer or sell, the Securities as part of their initial offering except:
(A) within the United States to persons whom it reasonably believes to
be QIBs or, if any such person is buying for one or more institutional accounts for
which such person is acting as fiduciary or agent, only when such person has rep-
resented to the Initial Purchaser that such account is a QIB, to whom notice has
been given that such sale or delivery is being made in reliance on Rule 144A un-
der the Securities Act (“Rule 144A”), and in each case, in transactions under
Rule 144A; or
(B) in accordance with the restrictions set forth in Annex C hereto.
(c) The Initial Purchaser acknowledges and agrees that the Issuers and, for purposes
of the opinions to be delivered to the Initial Purchaser pursuant to Sections 6(f) and 6(g), counsel
for the Issuers and counsel for the Initial Purchaser, respectively, may rely upon the accuracy of
the representations and warranties of the Initial Purchaser, and compliance by the Initial Pur-
chaser with its agreements, contained in paragraph (b) above (including Annex C hereto), and the
Initial Purchaser hereby consents to such reliance.
(d) The Issuers acknowledge and agree that the Initial Purchaser may offer and sell
Securities to or through any affiliate of the Initial Purchaser in the manner contemplated by the
Time of Sale Information and the Offering Memorandum and that any such affiliate may offer
and sell Securities purchased by it to or through the Initial Purchaser.
(e) The Issuers acknowledge and agree that the Initial Purchaser is acting solely in
the capacity of an arm’s length contractual counterparty to the Issuers with respect to the offering
of Securities contemplated hereby (including in connection with determining the terms of the
Offering) and not as financial advisors or fiduciaries to, or agents of, the Company, the Guaran-
tors or any other person. Additionally, the Initial Purchaser is not advising the Company, the
Guarantors or any other person as to any legal, tax, investment, accounting or regulatory matters
in any jurisdiction in connection with the offering of Securities. The Company and the Guaran-
tors shall consult with their own advisors concerning such matters and shall be responsible for
making their own independent investigation and appraisal of the transactions contemplated here-
by, and the Initial Purchaser shall have no responsibility or liability to the Issuers with respect
thereto. Any review by the Initial Purchaser of the Company, the Guarantors, and the transac-
tions contemplated hereby or other matters relating to such transactions will be performed solely

8
for the benefit of the Initial Purchaser and shall not be on behalf of the Company, the Guarantors
or any other person.
(f) The Initial Purchaser will use all commercially reasonable efforts to complete the
distribution of the Notes as soon as possible and the Initial Purchaser shall promptly notify the
Company when, in its good faith opinion, the Initial Purchaser has ceased distribution of the
Notes.
(g) The Company will file or cause to be filed all documents required to be filed by
the Company in connection with the transactions contemplated by this Agreement so that the of-
fer for sale of the Notes being made in the Canadian Offering Jurisdictions may be effected in a
manner exempt from the prospectus requirements of Canadian Securities Laws, including filing
within 10 days after the Closing Date a Form 45-106F1 pursuant to NI 45-106. The Initial Pur-
chaser shall deliver to the Company, as soon as practicable and, in any event, in sufficient time to
allow the Company to comply with all Canadian Securities Laws and other regulatory require-
ments applicable in any Canadian Offering Jurisdiction (including in order to enable the Compa-
ny to make the filings referenced in the prior sentence), all necessary information to allow the
Company to file all required forms with the relevant Securities Regulators in Canada including
the full name, residential address, telephone number, e-mail address, prospectus exemption relied
upon, corporate account number, and the name and registered representative number of the Ini-
tial Purchaser responsible for the purchase of Notes by, and the aggregate principal amount of
Notes purchased by, each Purchaser in a Canadian Offering Jurisdiction to whom the Initial Pur-
chaser has sold Notes and the name and telephone number of a contact person for each Purchas-
er.
(h) None of the Company, the Initial Purchaser nor any of their respective affiliates
shall provide to prospective Purchasers in any Canadian Offering Jurisdiction any document or
other material that would constitute an offering memorandum within the meaning of Canadian
Securities Laws other than the Canadian Preliminary Offering Memorandum or the Canadian
Offering Memorandum, or other documents agreed upon in writing by the Company and the Ini-
tial Purchaser, and the offer for sale of the Notes being made in the Canadian Offering Jurisdic-
tions will not be advertised in any newspaper, magazine, printed media or similar medium of
general and regular paid circulation, broadcast over radio or television or by means of the inter-
net and no seminar or meeting relating to the offer for sale of the Notes being made in the Cana-
dian Offering Jurisdictions whose attendees have been invited by general solicitation or advertis-
ing will be conducted.
(i) Each of the Company and the Guarantors acknowledges and agrees that the Initial
Purchaser may offer and sell Securities to or through any affiliate of the Initial Purchaser (includ-
ing, without limitation, Xxxxxxx Sachs International and Xxxxxxx Xxxxx Canada Inc.) and that
any such affiliate may offer and sell Securities purchased by it from the Initial Purchaser in ac-
cordance with applicable laws.
2. Payment and Delivery.
(a) Payment for and delivery of the Securities will be made at the offices of Xxxxxxxx
& Xxxxxxxx LLP, 000 Xxxxx Xxxxxx, Xxx Xxxx, Xxx Xxxx 00000 at 10:00 A.M., New York City

9
time, on October 13, 2016 or at such other time or place on the same or such other date, as the
Initial Purchaser and the Company may agree upon in writing. The time and date of such pay-
ment and delivery is referred to herein as the “Closing Date.”
(b) Payment for the Securities shall be made by wire transfer in immediately availa-
ble funds to the account(s) specified by the Company to the Initial Purchaser against delivery to
the nominee of The Depository Trust Company, for the account of the Initial Purchaser, of one or
more global notes representing the Notes (collectively, the “Global Note”), with any transfer tax-
es payable in connection with the sale of the Securities duly paid by the Company. The Global
Note will be made available for inspection by the Initial Purchaser not later than 1:00 P.M., New
York City time, on the business day prior to the Closing Date.
3. Representations and Warranties of the Company and the Guarantors. The Com-
pany and each Guarantor jointly and severally represent and warrant to the Initial Purchaser, as
of the date hereof and as of the Closing Date that:
(a) Preliminary Offering Memorandum, Time of Sale Information and Offer-
ing Memorandum. The Preliminary Offering Memorandum, as of its date, did not, the
Time of Sale Information, at the Time of Sale, did not, and at the Closing Date, will not,
and the Offering Memorandum, as of its date (as amended or supplemented in accordance
with Section 4(b), as applicable) and as of the Closing Date, will not, contain any untrue
statement of a material fact or omit to state a material fact necessary in order to make the
statements therein, in the light of the circumstances under which they were made, not
misleading, and the Canadian Preliminary Offering Memorandum, as of its date, did not,
and the Canadian Offering Memorandum, as of the Time of Sale and as of the Closing
Date, will not, contain a “misrepresentation” (as defined under Canadian Securities
Laws); provided that the Company and the Guarantors make no representation or xxxxxx-
xx with respect to any statements or omissions made solely in reliance upon and in con-
formity with information relating to the Initial Purchaser furnished to the Company in
writing by the Initial Purchaser expressly for use in the Preliminary Offering Memoran-
dum, the Canadian Preliminary Offering Memorandum, the Time of Sale Information, the
Offering Memorandum or the Canadian Offering Memorandum.
(b) Additional Written Communications. The Issuers (including their agents
and representatives, other than the Initial Purchaser in its capacity as such) have not pre-
pared, made, used, authorized, approved or referred to and will not prepare, make, use,
authorize, approve or refer to any written communication that constitutes an offer to sell
or solicitation of an offer to buy the Securities (each such communication by the Issuers
or their agents and representatives (other than a communication referred to in clauses (i),
(ii), (iii) and (iv) below) an “Issuer Written Communication”) other than (i) the Prelimi-
nary Offering Memorandum, (ii) the Offering Memorandum, (iii) the documents listed on
Annex A hereto, including a term sheet substantially in the form of Annex B hereto,
which constitute part of the Time of Sale Information, and (iv) any electronic road show
or other written communications, in each case used in accordance with Section 4(c).
Each such Issuer Written Communication, when taken together with the Time of Sale In-
formation, did not, and at the Closing Date will not, contain (i) any untrue statement of a
material fact or omit to state a material fact necessary in order to make the statements

10
therein, in light of the circumstances under which they were made, not misleading and
(ii) any information that conflicted, conflicts or will conflict with the information con-
tained in this Agreement; provided that the Company makes no representation and war-
ranty with respect to any statements or omissions made in each such Issuer Written
Communication solely in reliance upon and in conformity with information relating to the
Initial Purchaser furnished to the Company in writing by the Initial Purchaser expressly
for use in any Issuer Written Communication.
(c) Eligible for Resale. The Securities are eligible for resale pursuant to
Rule 144A and there are no securities of the Issuers that are listed on a national securities
exchange registered under Section 6 of the Securities Exchange Act of 1934, as amended
(the “Exchange Act”) or that are quoted in a United States automated interdealer quota-
tion system of the same class within the meaning of Rule 144A as the Securities. In Can-
ada, the Company is a reporting issuer in each of the provinces of Canada and the Securi-
ties may be sold either by a person not required to register as a dealer under applicable
Canadian Securities Laws or through registrants who are registered in the appropriate
category (or have an appropriate exemption therefrom) to sell the Securities on a private
placement basis subject to compliance with applicable Canadian Securities Laws and in
compliance with Section 5 hereof.
(d) Financial Statements.
(i) The financial statements of the Company and the related notes thereto in-
cluded or incorporated by reference in each of the Preliminary Offering Memorandum,
the Time of Sale Information and the Offering Memorandum present fairly, in all materi-
al respects, the consolidated financial position of the Company as of the dates indicated
and its results of operations and cash flows for the periods specified, and there has been
no change in accounting policies or practices of the Company since January 1, 2016, ex-
cept as disclosed in the Company’s financial statements, Management’s Discussion and
Analysis or the Preliminary Offering Memorandum, the Time of Sale Information or the
Offering Memorandum; such financial statements have been prepared in accordance with
IFRS, applied on a consistent basis throughout the periods covered; the other financial in-
formation of the Company included or incorporated by reference in each of the Prelimi-
nary Offering Memorandum, the Time of Sale Information and the Offering Memoran-
dum has been derived from the accounting or other records of the Company and its Sub-
sidiaries and presents fairly, in all material respects, the information shown thereby. Ex-
cept as set out in the Company’s financial statements or as not otherwise required to be
reported in accordance with IFRS, the Company does not have any outstanding indebted-
ness or any liabilities or obligations including any unfunded obligation under any em-
ployee plan, whether accrued, absolute, contingent or otherwise as of the date of such fi-
nancial statements.
(ii) To the Company’s knowledge, the financial statements relating to AMCo
included or incorporated by reference in each of the Preliminary Offering Memorandum,
the Time of Sale Information and the Offering Memorandum have been prepared in ac-
cordance with IFRS and present fairly in all material respects the consolidated financial
position of AMCo, as of the dates indicated and for the periods specified.

11
(iii) To the Company’s knowledge, the carve-out financial statements in re-
spect of the Covis Acquisition and the related notes thereto included or incorporated by
reference in each of the Preliminary Offering Memorandum, the Time of Sale Infor-
mation and the Offering Memorandum present fairly the financial position of the Covis
Acquisition as of the dates indicated and for the periods specified, and such financial
statements have been prepared in conformity with U.S. generally accepted accounting
principles (“GAAP”) applied on a consistent basis throughout the periods covered.
(iv) The unaudited pro forma financial statements (including the notes thereto)
or other pro forma financial information included or incorporated by reference in each of
the Preliminary Offering Memorandum, the Time of Sale Information and the Offering
Memorandum have been properly computed and presented based on the assumptions de-
scribed therein. The assumptions used in the preparation of the unaudited pro forma fi-
nancial statements and the other pro forma and adjusted financial information included in
the Offering Memorandum (including Adjusted EBITDA) are reasonable, and the ad-
justments used therein are appropriate to give effect to the transactions or circumstances
referred to therein.
(e) No Material Adverse Change.
Since the date of the most recent statement of financial position of the Company
included in each of the Time of Sale Information and the Offering Memorandum, and ex-
cept as otherwise disclosed therein, (w) there has not been any material change in the
capital stock or long-term debt of the Company and any of its respective Subsidiaries tak-
en as a whole, or any dividend or distribution of any kind declared, set aside for payment,
paid or made by the Company on any class of capital stock, other than in accordance with
the Company’s current dividend policy, or any material adverse change, or any develop-
ment involving a prospective material adverse change, in or affecting the business, prop-
erties, rights, assets, financial position or results of operations of the Company and its re-
spective Subsidiaries taken as a whole; (x) neither the Company nor any of its Subsidiar-
ies has entered into any transaction or agreement that is material to the Company and its
respective Subsidiaries taken as a whole, or other than in the ordinary course of business,
incurred any liability or obligation, direct or contingent, that is material to the Company
and its Subsidiaries taken as a whole, (y) neither the Company nor any of its respective
Subsidiaries has sustained any material loss or interference with its business from fire,
explosion, flood or other calamity, whether or not covered by insurance, or from any la-
bor disturbance or dispute or any action, order or decree of any court or arbitrator or gov-
ernmental or regulatory authority and (z) there has not been any material adverse change
in the ability of the Company or any of its respective Subsidiaries to consummate any of
the Transactions on a timely basis, except in each case as otherwise disclosed in the Time
of Sale Information.
(f) Organization and Good Standing. Attached as Exhibit A is a true and
complete list of each entity in which the Company has a direct or indirect majority equity
or voting interest (each, a “Subsidiary” and, together, the “Subsidiaries”), their jurisdic-
tions of organization or incorporation, name(s) of their equityholder(s) and percentage
held by each equityholder. The Company does not own or control, directly or indirectly,

12
any corporation, association or other entity other than the Subsidiaries. The Guarantors
have been duly organized or incorporated and are validly existing and in good standing
(to the extent the concept of “good standing” exists in such jurisdiction) under the laws of
their respective jurisdictions of organization or incorporation, are duly qualified to do
business and, to the extent applicable, are in good standing in each jurisdiction in which
their respective ownership or lease of property or the conduct of their respective busi-
nesses requires such qualification, and have all power and authority necessary to own or
hold their respective properties and to conduct the businesses in which they are engaged,
except where the failure to be so qualified, in good standing or have such power or au-
thority would not, individually or in the aggregate, reasonably be expected to have a ma-
terial adverse effect on the business, properties, rights, assets, financial position, results
of operations of the Company and its respective Subsidiaries taken as a whole or on the
performance by the Company and the Guarantors of their obligations under the Securities
(a “Material Adverse Effect”).
(g) Capitalization. The Company has an authorized capitalization as set forth
in each of the Time of Sale Information and the Offering Memorandum under the head-
ing “Capitalization.” Other than as set forth in Exhibit A attached hereto, the Company
legally and beneficially owns, directly or indirectly, all of the issued and outstanding
shares in the capital of each of its Subsidiaries and all shares of such Subsidiaries are held
free and clear of all liens, claims, charges, encumbrances or demands of any kind what-
soever other than Permitted Encumbrances. All of the issued and outstanding equity in-
terests of each Guarantor have been duly and validly authorized and issued; are fully paid
and nonassessable (except as such nonassessability may be affected by provisions of such
jurisdiction’s statutory rules); have been issued in compliance with the applicable U.S.
federal or state securities laws, Canadian federal or provincial securities laws or, in the
case of an interest in an entity formed under the laws of another foreign jurisdiction, simi-
lar laws of such jurisdiction; were not issued in violation of any preemptive, right of first
refusal, or similar right and, except as set forth in the Offering Memorandum; are owned,
directly or indirectly through Subsidiaries, by the Company free and clear of all liens oth-
er than Permitted Encumbrances. Except (i) as set forth in the Offering Memorandum
,the Company’s annual information form dated Xxxxx 00, 0000, (xx) any options and re-
stricted share units issued pursuant to the Company Option Plan and the LTIP, respec-
tively, and (iii) the estimated 603,774 common shares of the Company issuable as part of
the consideration for the Company’s acquisition of its former specialty healthcare distri-
bution business, there are no outstanding options, warrants or other rights to acquire or
purchase, or instruments convertible into or exchangeable for, any equity interests of the
Company or any of the Guarantors.
(h) Due Authorization.
(i) The Company has all requisite corporate power, capacity and authority to
execute and deliver this Agreement and to perform its respective obligations hereunder;
and upon execution of this Agreement, all action required to be taken for the due and
proper authorization, execution and delivery of this Agreement and the consummation of
the transactions contemplated hereby will have been duly and validly taken.

13
(ii) On or prior to the Closing Date, each of the Company and the Identified
Guarantor shall have, and on or prior to the Specified Guarantor Accession Date, each of
the Specified Guarantors shall have, all requisite corporate power, capacity and authority
to execute and deliver the Ancillary Documents to which it is a party and to perform its
respective obligations thereunder; and upon execution of the Ancillary Documents to
which it is a party, all action required to be taken for the due and proper authorization,
execution and delivery of each of the Ancillary Documents to which it is a party and the
consummation of the transactions contemplated thereby will have been duly and validly
taken.
(i) The Indenture. On or prior to the Closing Date, the Indenture shall have
been duly authorized by each of the Company and the Identified Guarantors and, upon
execution of the Supplemental Indenture by the Specified Guarantors, will be duly au-
thorized by such Specified Guarantors, and, when duly executed and delivered in accord-
ance with its terms by each of the parties thereto, the Indenture (as supplemented by the
Supplemental Indenture with respect to the Specified Guarantors) will constitute a valid
and legally binding agreement of the Company and each of the Guarantors enforceable
against the Company and each of the Guarantors in accordance with its terms by the other
parties thereto, except as enforceability may be limited by applicable bankruptcy, insol-
vency or similar laws affecting the enforcement of creditors’ rights generally or by equi-
table principles relating to enforceability (collectively, the “Enforceability Exceptions”).
The Indenture, when executed and delivered, will conform in all material respects to the
description thereof in the Offering Memorandum.
(j) The Notes. On or prior to the Closing Date, the Notes shall have been du-
ly and validly authorized for issuance and sale to the Initial Purchaser by the Company,
and when issued, authenticated and delivered by the Company against payment therefor
by the Initial Purchaser in accordance with the terms of this Agreement and the Inden-
ture, the Notes will be in the form contemplated by the Indenture and will be legally
binding and valid obligations of the Company, entitled to the benefits of the Indenture
and enforceable against the Company in accordance with their terms, except as the en-
forcement thereof may be limited by the Enforceability Exceptions. The Notes, when is-
sued, authenticated and delivered, will conform in all material respects to the description
thereof in the Offering Memorandum.
(k) The Guarantees. On or prior to the Closing Date, the Guarantees shall
have been duly and validly authorized by each of the Identified Guarantors and, upon ex-
ecution of the Supplemental Indenture by the Specified Guarantors, by each such Speci-
fied Guarantor and, when the Notes are issued, authenticated by the Trustee and delivered
by the Company against payment by the Initial Purchaser in accordance with the terms of
this Agreement and the Indenture, will be in the form contemplated by the Indenture and
will be legally binding and valid obligations of the Identified Guarantors, and upon exe-
cution of the Supplemental Indenture by the Specified Guarantors, such Specified Guar-
antors, entitled to the benefits of the Indenture, enforceable against each of them in ac-
cordance with their terms, except that enforceability thereof may be limited by the En-
forceability Exceptions. The Guarantees, when issued, authenticated and delivered, will
conform in all material respects to the description thereof in the Offering Memorandum.

14
(l) Purchase Agreement; Joinders. This Agreement has been duly author-
ized, executed and delivered by the Company. With respect to the Identified Guarantors,
on or prior to the Closing Date, the Joinder Agreement shall have been duly authorized,
executed and delivered in accordance with its terms by each of the Identified Guarantors.
With respect to the Specified Guarantors, on or prior to the Specified Guarantor Acces-
sion Deadline, the Joinder Agreement will have been duly authorized, executed and de-
livered in accordance with its terms by each of the Specified Guarantors.
(m) Collateral Documents. Each of the Collateral Documents, on or prior to
the Closing Date, shall have been duly authorized by the Company and the Identified
Guarantors, and, on or prior to the Specified Guarantor Accession Date, shall have been
duly authorized by the Specified Guarantors, as appropriate, and when executed and de-
livered by the Company and/or the applicable Guarantors, and assuming the execution
and delivery thereof by the other parties thereto, will constitute a legal, valid and binding
agreement of the Company and/or the applicable Guarantors, as appropriate, in accord-
ance with its terms, except as the enforcement thereof may be limited by the Enforceabil-
ity Exceptions. The Collateral Documents, when executed and delivered in connection
with the sale of the Securities, will create in favor of the Collateral Agent for the benefit
of itself, the Trustee and the holders of the Securities, valid and enforceable security in-
terests in and liens on the Collateral and, as applicable, upon the filing of appropriate Per-
xxxxx Property Security Act filings in the applicable Canadian jurisdictions and Uniform
Commercial Code financing statements in the applicable United States jurisdictions and
the taking of the other actions, in each case as further described in the respective Collat-
eral Documents, the security interests in and liens on the rights of the Company or the
applicable Guarantor in such Collateral will be perfected security interests and liens (to
the extent that such security interests and liens can be perfected by the filing of Personal
Property Security Act and Uniform Commercial Code financing statements or the taking
of such other actions), superior to and prior to the liens of all third persons other than the
liens securing the Credit Agreement and Permitted Liens.
(n) Descriptions of Certain Documents. Each Transaction Document con-
forms in all material respects to the description thereof contained in each of the Time of
Sale Information and the Offering Memorandum.
(o) No Violation or Default. None of the Company or any of the Guarantors
is (i) in violation of its charter or by-laws or similar organizational documents; (ii) in de-
fault, and no event has occurred that, with notice or lapse of time or both, would consti-
tute such a default, in the due performance or observance of any term, covenant or condi-
tion contained in any indenture, mortgage, deed of trust, loan agreement or other agree-
ment or instrument to which the Company or any of the Guarantors is a party or by which
the Company or any of the Guarantors is bound or to which any of the properties, rights
or assets of the Company or any of the Guarantors is subject; or (iii) in violation of any
law or statute or any judgment, order, proceeding, rule or regulation of any court or arbi-
trator or governmental or regulatory authority that is binding on the Company or any of
the Guarantors, except, in the case of clauses (ii) and (iii) above, for any such default or
violation that would not, individually or in the aggregate, reasonably be expected to have
a Material Adverse Effect.

15
(p) No Conflicts. None of the execution, delivery and performance by the
Company and each of the Guarantors of each of the Transaction Documents to which
each is a party, the issuance and sale of the Securities (including the Guarantees), compli-
ance by the Company and each of the Guarantors with the terms thereof and the con-
summation of each of the transactions contemplated by the Transaction Documents will
(i) result in a breach or violation of any of the terms or provisions of, or constitute a de-
fault under any indenture, mortgage, deed of trust, loan agreement or other agreement or
instrument to which the Company or any of the Guarantors is a party or by which the
Company or any of the Guarantors is bound or to which any of the properties, rights or
assets of the Company or any of the Guarantors is subject, (ii) result in any violation of
the provisions of the charter or by-laws or similar organizational documents of the Com-
pany or any of the Guarantors, which are in effect as of the date hereof, in any material
respect or (iii) result in the violation of any law, statute or regulation applicable to the
Company or any of the Guarantors or any judgment, order or rule of any court or arbitra-
tor or governmental or regulatory authority that is binding on the Company or any of the
Guarantors, except, in the case of clauses (i) and (iii) above, for any such breach, xxxxx-
tion or default that would not, individually or in the aggregate, reasonably be expected to
have a Material Adverse Effect.
(q) No Consents Required. Assuming the accuracy of the representations and
warranties of the Initial Purchaser in Section 1(b) of this Agreement, no consent, approv-
al, authorization, order, registration or qualification of or with any court or arbitrator or
governmental or regulatory authority is required for the execution, delivery and perfor-
xxxxx by the Company and each of the Guarantors of each of the Transaction Documents
to which each is a party, the issuance and sale of the Notes (including the Guarantees)
and each of the transactions contemplated by the Transaction Documents, except for such
consents, approvals, authorizations, orders and registrations or qualifications (i) as may
be required under applicable state securities laws in connection with the purchase and re-
sale of the Securities by the Initial Purchaser and the filing of certain notices and payment
of filing fees, if any, required by Canadian Securities Laws, including filing a report or
reports of exempt distribution under NI 45-106 with payment of applicable filing fees and
the delivery or filing of the Canadian Offering Memorandum, if applicable, (ii) that have
been obtained or (iii) the absence of which would not, individually or in the aggregate,
reasonably be expected to have a Material Adverse Effect.
(r) Legal Proceedings. Other than a regulatory investigation disclosed to the
Initial Purchaser, or as otherwise disclosed in each of the Time of Sale Information and
the Offering Memorandum or otherwise set forth herein, or except as would not reasona-
xxx be expected to have a Material Adverse Effect, there are no legal, governmental or
regulatory investigations, actions, suits or proceedings pending to which the Company or
any of its Subsidiaries is or may be a party or, to the knowledge of the Company, to
which any property of the Company or any of its Subsidiaries is or may be the subject
that, individually or in the aggregate, if determined adversely to the Company or any of
its Subsidiaries, would reasonably be expected to have a Material Adverse Effect; and no
such investigations, actions, suits or proceedings are threatened or, to the knowledge of
the Company, contemplated by any governmental or regulatory authority or by others.

16
(s) Independent Accountant.
(i) To the knowledge of the Company, PricewaterhouseCoopers LLP, the cur-
rent auditors of the Company, is independent with respect to the Company within the
meaning of Section 152 of the Business Corporations Act (Ontario) and there has never
been a reportable event (within the meaning of National Instrument 51-102 - Continuous
Disclosure Obligations) between the Company and PricewaterhouseCoopers LLP, and
the Company’s audit committee’s composition complies and the Company’s audit com-
mittee’s responsibilities comply in all material respects with National Instrument 52-110
- Audit Committees.
(ii) To the knowledge of the Company, KPMG LLP, who has certified certain
financial statements of AMCo and its subsidiaries, was an independent public accounting
firm with respect to the Company at the time of their audits within the meaning of the au-
dit regulations and guidance issued by the Institute of Chartered Accountants in England
and Wales.
(iii) To the knowledge of the Company, Xxxxxxx Xxxxxx Toronto LLP, who has
certified certain historical financial statements of the Company and its Subsidiaries and
was independent with respect to the Company at the time of their audits within the mean-
ing of Section 152 of the Business Corporations Act (Ontario) and there has never been a
reportable event (within the meaning of National Instrument 51-102 - Continuous Disclo-
sure Obligations) between the Company and Xxxxxxx Xxxxxx Toronto LLP.
(iv) To the knowledge of the Company, Xxxxx Xxxxxxx LLP, who has certi-
fied certain financial statements relating to the Covis Acquisition, was an independent
public accountant with respect to the Company at the time of their audits within the
meaning of Rule 101 of the Code of Professional Conduct of the American Institute of
Certified Public Accountants and its interpretations and rulings thereunder.
(t) Title to Real and Personal Property. Neither the Company nor any of the
Guarantors owns real property (excluding, for certainty, leaseholds) and each of them has
good and marketable title to all personal property free and clear of all liens, encumbranc-
es and claims other than Permitted Encumbrances. With respect to each premises of the
Company or any of the Guarantors, which is material to the business of the Company and
the Guarantors taken as a whole and which the Company or a Guarantor occupies as ten-
ant (the “Leased Premises”), the Company or any of the Guarantors occupies the Leased
Premises and has the exclusive right to occupy and use the Leased Premises and each of
the leases pursuant to which the Company or a Guarantor occupies the Leased Premises
is in good standing and in full force and effect in all material respects.
(u) Title to Intellectual Property. (i) The Company and the Guarantors own
or possess adequate rights or licenses to use, and have taken all commercially reasonable
steps to protect and maintain (other than in the case of third party Intellectual Property)
all material patents, patent applications, trademarks, service marks, trade names, trade
name, and service xxxx registrations and applications thereof, copyrights, domain names,
licenses and know-how (including trade secrets and other unpatented and/or unpatentable

17
proprietary or confidential information, systems or procedures) and all other U.S. and
foreign intellectual property rights (collectively, “Intellectual Property”) which are mate-
rial to their respective businesses; (ii) to the knowledge of the Company, the conduct of
its business does not infringe, misappropriate, or otherwise violate in any material respect
any Intellectual Property rights of others; (iii) the Company and the Guarantors have not
received any written notice alleging or threatening any claim of infringement, misappro-
priation, or other violation of any Intellectual Property rights of others; and (iv) to the
knowledge of the Company, the Intellectual Property owned by the Company and the
Guarantors that is material to their respective businesses is not being infringed, misap-
propriated or otherwise violated by any third party, except where such infringement, mis-
appropriation or violation would not reasonably be expected to have a Material Adverse
Effect.
(v) Investment Company Act. None of the Company or any of the Guarantors
is, and after giving effect to the offering and sale of the Securities and the application of
the proceeds thereof as described in each of the Time of Sale Information and the Offer-
ing Memorandum none of them will be, an “investment company” or an entity “con-
trolled” by an “investment company” within the meaning of the Investment Company Act
of 1940, as amended, and the rules and regulations of the Commission thereunder (collec-
tively, the “Investment Company Act”).
(w) No Event of Default. None of the Company or any Guarantor is in default
or breach of any material contract to which the Company or a Guarantor is a party or by
which the Company or any Guarantor is bound, except to the extent such default or
breach would not reasonably be expected to have a Material Adverse Effect, and except
as set forth in the Preliminary Offering Memorandum, the Time of Sale Information and
the Offering Memorandum, to the knowledge of the Company, there exists no condition,
event or act which with the giving of notice or the lapse of time or both would constitute
a default or breach under any such contract which would give a right of termination on
the part of any other party to such contract; except in any case as would not, individually
or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(x) Taxes. Each of the Company and the Guarantors has paid all U.S. federal,
state and local, Canadian federal, provincial and local, and other non-U.S. taxes and any
related or similar assessment, fine, interest or penalty required to be paid by it, and filed
all tax returns required to be filed by it; and there is no tax deficiency that has been, or, to
the knowledge of the Company, would reasonably be expected to be, asserted against the
Company or any of the Guarantors or any of their respective properties or assets, except
such failure to pay or file or deficiencies that would not, individually or in the aggregate,
reasonably be expected to result in a Material Adverse Effect. There are no audits or in-
vestigations in progress or, to the knowledge of the Company, pending or threatened,
against the Company or the Guarantors, and there are no outstanding assessments or reas-
sessments of the Company or the Guarantors, in respect of taxes that would be reasonably
expected to have a Material Adverse Effect. The Company has made adequate charges,
accruals and reserves in accordance with applicable accounting standards in its xxxxxxx-
dated financial statements for the year ended December 31, 2015 in respect of all U.S.

18
federal, state and local, Canadian federal, provincial and local, and other non-U.S. taxes,
including such taxes that are not yet required to be paid.
(y) Stamp Taxes. To the knowledge of the Company, as at the Closing Date,
there are no stamp or other issuance or transfer taxes or duties or other similar taxes, fees
or charges required to be paid by or on behalf of the Initial Purchaser in Canada or to a
taxing authority thereof in connection with the execution and delivery of the Transaction
Documents or the creation, issuance, sale or delivery to the Initial Purchaser of the Secu-
rities or the resale of the Securities by the Initial Purchaser.
(z) No Withholding Tax. On the date hereof, no payments to be made by the
Company or the Guarantors to holders or beneficial owners of Notes who are non-
residents of Canada for purposes of the Income Tax Act (Canada) on or under the Notes
or the Guarantees provided by the Guarantors in respect of interest, principal or premium
will be subject to withholding or deduction in Canada or any political subdivision or tax-
ing authority thereof or therein (except where (i) such holder or beneficial owner does not
deal at arm’s length with the Company or a Guarantor for purposes of the Income Tax Act
(Canada), (ii) such holder or beneficial owner is a “specified shareholder” of the Compa-
ny or does not deal at arm’s length with a “specified shareholder” of the Company, in
each case, within the meaning of and for purposes of the Income Tax Act (Canada), or
(iii) interest is paid or payable in respect of a debt or other obligation to pay an amount to
a person with whom the Company or a Guarantor is not dealing at arm’s length).
(aa) Licenses and Permits.
The Company and the Guarantors possess all licenses, sub-licenses, certificates,
permits and other authorizations issued by, and have made all declarations and filings
with, the appropriate federal, state, local or foreign governmental or regulatory authorities
that are necessary or required to conduct their respective businesses as described in each
of the Time of Sale Information and the Offering Memorandum, except where the failure
to possess or make the same would not, individually or in the aggregate, reasonably be
expected to have a Material Adverse Effect; and to the knowledge of the Company, nei-
ther the Company nor any of the Guarantors has received written notice of any revocation
or modification of any such license, sublicense, certificate, permit or authorization or has
any reason to believe that any such license, certificate, permit or authorization will not be
renewed in the ordinary course, including with respect to any license, certificate, permit
or authorization issued by FDA, and to the knowledge of the Company, neither the FDA
nor any Governmental Authority or Regulatory Authority is considering such action.
(bb) Other Regulatory and Related Items.
(i) There is no untrue or misleading information or significant omission in
any Product application or other submission to the FDA or any comparable Regulatory
Authority; provided, however that such representation is limited to the actual knowledge
of the Company with respect to a Product application or other submission that was made
to the FDA or any comparable Regulatory Authority prior to the time period when a
Product was purchased or in-licensed by the Company or a Subsidiary, as applicable.

19
(ii) All Products developed, tested, investigated, manufactured, stored, dis-
tributed, marketed, or sold by or on behalf of the Company or the Guarantors that are
subject to the jurisdiction of the FDA or any comparable Regulatory Authority have been
and are being developed, tested, investigated, manufactured, stored, distributed, market-
ed, and sold in material compliance with FDA legal requirements or any other applicable
legal requirement, including those regarding non-clinical testing, clinical research, estab-
lishment registration, device listing, pre-market notification, good manufacturing practic-
es, labeling, advertising, record-keeping, adverse event reporting and reporting of correc-
tions and removals; provided, however, that such representation is limited to the actual
knowledge of the Company with respect to the period prior to the time a Product was
purchased or in-licensed by the Company or a Subsidiary, as applicable.
(iii) The clinical, pre-clinical and other studies and tests conducted by or on
behalf of or sponsored by the Company or the Guarantors that are described or referred to
in the Preliminary Offering Memorandum, the Time of Sale Information or the Offering
Memorandum (collectively, the “Clinical Trials”) were and, if still pending, are being
conducted, in all material respects, in accordance with all experimental protocols, proce-
dures and controls, accepted professional scientific standards, and applicable laws, in-
cluding applicable laws administered by Regulatory Authorities; provided, however, that
such representation is limited to the actual knowledge of the Company with respect to the
period prior to the time a Product was purchased or in-licensed by the Company or a Sub-
sidiary, as applicable. Except for ordinary course correspondence, neither the Company
nor any of the Guarantors has received any written notices or written correspondence
from any Governmental Authority or Regulatory Authority with respect to any Clinical
Trial requesting information about or requiring the termination or suspension of such
Clinical Trial.
(iv) Each of the Company and the Guarantors has filed with the applicable
Regulatory Authority all material filings, declarations, listings, registrations, reports, up-
dates and submissions that are required to be so filed. All such filings were in compli-
ance in all material respects with applicable laws when filed and no material deficiencies
have been asserted by any Regulatory Authority with respect to any such filings, declara-
tions, listings, registrations, reports, updates or submissions.
(v) Except as would not reasonably be expected to have a Material Adverse
Effect or except as disclosed in the Preliminary Offering Memorandum, the Time of Sale
Information or the Offering Memorandum, neither the Company nor the Guarantors have
received any Form FDA-483, notice of adverse finding, FDA warning letters, notice of
violation or “untitled letters,” or notice of FDA action for import detentions or refusals or
any other written correspondence from any Regulatory Authority alleging or asserting
noncompliance with any applicable legal requirements or registrations. Except as would
not reasonably be expected to have a Material Adverse Effect or as disclosed in the Pre-
liminary Offering Memorandum, the Time of Sale Information or the Offering Memo-
randum, neither the Company nor the Guarantors are subject to any obligation arising un-
der an administrative or regulatory action, inspection, warning letter, notice of violation
letter, or other written notice, response or commitment made to or with any Regulatory
Authority, and, to the knowledge of the Company, no such proceedings have been threat-

20
ened. The Company and the Guarantors have made all material notifications, submis-
sions and reports required by FDA legal requirements.
(vi) Other than in respect of certain Intellectual Property licence agreements
entered into in the ordinary course, certain flow through arrangements with respect to cer-
tain products identified in writing to the Initial Purchaser which were acquired from
Covis, certain intellectual property license agreements entered into in connection with the
acquisition of the Products and certain distribution agreements relating to the Products,
copies of which distribution agreements and such material Intellectual Property license
agreements have been made available to the Initial Purchaser, the Company and the
Guarantors are the sole legal and beneficial owners of, have good and marketable title to,
and own all right, title and interest in the Products or otherwise acquired and own the ex-
clusive rights to and in the Products, except to the extent that the failure to hold such title,
right or interest would not reasonably be expected to have a Material Adverse Effect.
(vii) Other than with respect to the Company’s orphan drug product Photofrin®
and the product Donnatal® and except for such matters as would not reasonably be ex-
pected to have a Material Adverse Effect, there has not been, nor, to the knowledge of the
Company, is there currently under consideration by the Company, any of the Guarantors,
or any Governmental Authority or Regulatory Authority, any material seizure, withdraw-
al, recall, field, notification, detention, field correction, safety alert, or suspension of
manufacturing relating to any Product; a material change in safety-related labeling of any
such Product; or a termination, seizure, or suspension of marketing of any such Product;
provided, however, that such representation is limited to the actual knowledge of the
Company with respect to the period prior to the time a Product was purchased or in-
licensed by the Company or any of the Guarantors, as applicable.
(viii) Other than with respect to the FDA warning letter in 2014 relating to the
marketing of Kapvay®, the FDA warning letter in 2016 relating to Ulesfia®, the inad-
vertent disclosure of protected health information by the Company’s former specialty
healthcare distribution division and other than disclosed in the Preliminary Offering
Memorandum, the Time of Sale Information or the Offering Memorandum, the Compa-
ny, the Guarantors and their respective officers, directors, and employees, and to the
knowledge of the Company, their representatives, contractors, and agents, have been and
are currently in compliance in all material respects with all applicable Health Care Laws
in relation to the Products including, without limitation, the Company’s and all of the
Guarantors’ actions regarding coupon programs, discount programs, co-pay assistance
programs, lead generation programs, marketing efforts, and requirements pertaining to
mail-order pharmacies such as change of ownership requirements, licensing require-
ments, inspection requirements, and generic substitution laws, and, to the knowledge of
the Company, neither the Company or any of the Guarantors nor their officers, directors,
or employees, have engaged in any act or omission that violates or would violate any
Health Care Laws in any material respect including, without limitation, misbranding,
adulteration, or off-label promotion.
(ix) Other than as disclosed in the Preliminary Offering Memorandum, the
Time of Sale Information or the Offering Memorandum and other than a regulatory in-

21
vestigation disclosed to the Initial Purchaser and the Settlement Agreement dated October
17, 2013 with the United States of America, acting through the DOJ and the OIG, pursu-
ant to which the Company is obligated to make certain payments to the United States, the
Company, the Guarantors and their respective officers, directors, and employees (a) are
not and have not been a party to, or bound by, any order, individual integrity agreement,
corporate integrity agreement or other formal agreement with any Governmental Authori-
ty concerning compliance with Health Care Laws; (b) have not made any filings pursuant
to the OIG or CMS self-disclosure protocol; (c) have not been a defendant in any action,
or received a threat of any action, brought under a federal or state whistleblower statute,
including without limitation the False Claims Act of 1863 (31 U.S.C. § 3729 et seq.); and
(d) have not been served with or received any written search warrant, subpoena (other
than those related to actions against third parties), civil investigative demand or contact
letter from a Governmental Authority or Regulatory Authority.
(x) Other than with respect to the claims relating to the investigation of the
DOJ and the United States Attorney’s Office for the Districts of Louisiana and Kansas
with respect to the Company’s former specialty healthcare distribution division as dis-
closed in the Preliminary Offering Memorandum, the Time of Sale Information and the
Offering Memorandum, neither the Company nor any of the Guarantors have presented
or caused to be presented to any government or any other Person any claim for payment
for an item or service in violation of, or that would be the basis for liability under, the
False Claims Act, 31 U.S.C. § 3729 — 3733, any similar state false claims act, the Civil
Monetary Penalties Law, 42 U.S.C. §§ 1320a-7a and 1320a-7b, the Program Fraud Civil
Remedies Act, 31 U.S.C. §§ 3801-3812, any other Health Care Laws, or the common law
or administrative theories of recoupment, payment by mistake, unjust enrichment, dis-
gorgement, conversion, breach of contract, or fraud. Other than with respect to the
claims relating to the investigation of the DOJ and the United States Attorney’s Office for
the Districts of Louisiana and Kansas with respect to the Company’s former specialty
healthcare distribution division as disclosed in the Preliminary Offering Memorandum,
the Time of Sale Information and the Offering Memorandum, the Company and each of
the Guarantors have submitted all claims for reimbursement to Federal Health Care Pro-
grams in accordance with all applicable Health Care Laws.
(xi) Other than as disclosed in the Preliminary Offering Memorandum, the
Time of Sale Information or the Offering Memorandum, neither the Company nor any of
the Guarantors has received notice of any action by any Governmental Authority or Reg-
ulatory Authority to terminate, suspend, limit, withdraw, or forfeit the participation of the
Company or any of the Guarantors in any Federal Health Care Program.
(xii) Other than as disclosed in the Preliminary Offering Memorandum, the
Time of Sale Information or the Offering Memorandum and other than the regulatory in-
vestigation disclosed to the Initial Purchaser, the Company has no knowledge of any cur-
rent inquiry or investigation relating to any Federal Health Care Program-related offense
of which the Company or any of the Guarantors is a target or subject, or any other actual
or threatened enforcement actions by any Governmental Authority or Regulatory Author-
ity.

22
(xiii) Other than with respect to the claims relating to the investigation of the
DOJ and the United States Attorney’s Office for the Districts of Louisiana and Kansas
with respect to the Company’s former specialty healthcare distribution division as dis-
closed in the Preliminary Offering Memorandum, the Time of Sale Information and the
Offering Memorandum, to the knowledge of the Company, neither the Company nor any
Guarantor has presented to any Federal Health Care Program or any other Person any
claim for payment for an item or service provided or performed pursuant to: (A) the fed-
eral anti-kickback statute, 42 U.S.C. § 1320a-7b(b) or similar state anti-kickback statute;
or (B) a prohibited referral under 42 U.S.C., § 1395nn or the regulations promulgated
thereunder at 42 C.F.R. § 411.351 — 411.389.
(xiv) To the knowledge of the Company, neither the Company nor any Guaran-
tor has (A) offered, authorized, promised, made or agreed to make gifts of money, other
property, other value or similar benefits or contributions to, or entered into any fee-
splitting arrangement with, any actual or potential patient, health care provider, actual or
potential business partner, governmental employee, or other Person in a position to assist
or hinder the Company or any Guarantor in connection with any actual or proposed trans-
action, or to any political party, political party official or candidate for federal, state or
local public office in violation of any applicable law or (B) maintained any unrecorded
fund or asset of the Company or any Guarantor for any improper purpose or made any
false entries on its books and records for any reason.
(xv) Neither the Company nor any Guarantor has any material liability for any
refund, overpayment, discount, or adjustment under any Federal Health Care Program,
other than adjustments made lawfully in the normal course of business.
(xvi) To the knowledge of the Company, no Persons who have engaged in any
activity that is in violation of, or have been convicted of, charged with, or investigated
for, a felony or a criminal offense under any Health Care Law, or who are excluded, sus-
pended, debarred, prohibited from providing services under, or otherwise ineligible to
participate in any government program, or who have committed any act or have engaged
in any activity that is permissive or mandatory grounds for exclusion, debarment, suspen-
sion, or other ineligibility to participate, or to the actual knowledge of the Company, who
have been threatened with exclusion, debarment or being or otherwise ineligible to partic-
ipate, in any government program, are either employed by, under a consulting contract
with, or agents of the Company or any Guarantor or provide items or services on behalf
of the Company or any Guarantor.
(xvii) Except in respect of certain materials inadvertently delivered to a business
partner of the Company’s former specialty healthcare distribution division, neither the
Company nor any Guarantor has ever notified, either voluntarily or as required by a
Health Care Law or other applicable law, any affected individual, any Governmental Au-
thority, or the media about, or is otherwise aware of, any breach or unauthorized acquisi-
tion, access, use, or disclosure of protected health information or any other breach in vio-
lation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), 42
U.S.C. §§ 1320d-1329d-8; Health Information Technology for Economic and Clinical
Health Act (HITECH); or implementing regulations; including a breach of any business

23
associate agreement (within the meaning of HIPAA); nor, to the knowledge of the Com-
pany, is the Company aware of any investigation by any Governmental Authority for a
violation of HIPAA, HITECH, or any other legal requirement applicable to the Company
or any Guarantor’s use and dissemination of any personally-identifiable information con-
cerning individuals, including receiving any written notices from the United States De-
partment of Health and Human Services Office of Civil Rights relating to any such xxxxx-
tions.
(xviii) The Company and the Guarantors have recorded all of the information re-
quired to be recorded under the Sunshine Act.
(xix) The Company and the Guarantors, in conducting the business of the Com-
pany, do not participate in, directly or, to the knowledge of the Company, through any
broker, distributor or similar party, any arrangement pursuant to which customers of the
business receive, directly or indirectly, a reduction in the purchase price or other benefits,
monetary or otherwise, other than normal and legal customary trade practices, discounts
and incidental business-related entertainment.
(xx) The Company and the Guarantors’ billing practices to all third party
payors, including private insurance companies, have been true, current, and complete in
all material respects and in compliance in all material respects with all applicable laws
and the policies of those third party payors.
(xxi) To the extent the Company or any Guarantor provides to customers or
others reimbursement coding or billing advice regarding products offered for sale by the
Company or any Guarantor and procedures related thereto, such advice is: (a) true, accu-
rate and complete in all material respects, and (b) in compliance in all material respects
applicable all Health Care Laws.
(xxii) Except as set forth in (x), (ix), (xiii) and (xvii) above, the Company and
the Guarantors maintain and operate in material compliance with a compliance program
in accordance with the criteria established by the OIG; the current U.S. Federal Sentenc-
ing Guidelines standards for effective compliance programs; the code of the Pharmaceu-
tical Research and Manufacturers of America on Interactions with Health Care Profes-
sionals; and other similar laws.
(cc) No Labor Disputes. No labor disturbance by or dispute with employees of
the Company or any of the Guarantors exists or, to the knowledge of the Company, is
contemplated or threatened, and the Company is not aware of any existing or imminent
labor disturbance by, or dispute with, the employees of any of the Company’s or any of
the Guarantors’ principal suppliers, contractors or customers, except as would not rea-
sonably be expected to have a Material Adverse Effect; other than settlement agreements
entered into with former employees or consultants of the Company and its Subsidiaries,
which settlement amounts are not material, there has not been in the last two years and
there is not currently any material labor disruption or dispute with the employees of the
Company or any of the Guarantors, and no material labor disruption or dispute, to the
knowledge of the Company, is imminent.

24
(dd) Compliance With Environmental Laws. (i) Except as referenced in each of
the Time of Sale Information and the Offering Memorandum: the Company and the
Guarantors (x) to the knowledge of the Company, are, and at all prior times were, in
compliance, in all material respects, with any and all federal, state, local and foreign
laws, rules, regulations, requirements, decisions and orders relating to the protection of
human health or safety, the environment, natural resources, hazardous or toxic substances
or wastes, pollutants or contaminants (collectively, “Environmental Laws”), (y) have re-
ceived and are in compliance, in all material respects, with all material permits, licenses,
certificates or other authorizations or approvals required of them under applicable Envi-
ronmental Laws to conduct their respective businesses, and (z) have not received written
notice of any actual or potential liability under or relating to any Environmental Laws,
including for the investigation or remediation of any disposal or release of hazardous or
toxic substances or wastes, pollutants or contaminants, and the Company has no
knowledge of any event or condition that would reasonably be expected to result in any
such notice, and (ii) there are no costs or liabilities associated with Environmental Laws
of or relating to the Company or the Guarantors, except in the case of each of (i) and (ii)
above, for any such notice, failure to comply, or failure to receive required permits, li-
censes, certificates, authorizations or approvals, or cost or liability, as would not, individ-
ually or in the aggregate, reasonably be expected to have a Material Adverse Effect; and
(iii) except as referenced in each of the Time of Sale Information and the Offering Mem-
orandum, there are no proceedings that are pending, or to the knowledge of the Company,
contemplated, against the Company under any Environmental Laws in which a govern-
mental entity is also a party. The Initial Purchaser acknowledge that this representation is
limited to the actual knowledge of the Company with respect to the period prior to the
time a Product or business was purchased or in-licensed, as applicable, by the Company
or a Subsidiary, as applicable.
(ee) Compliance With Laws Governing Employment And Employee Plans.
(i) Neither the Company nor any Guarantor is a party to or bound by any col-
lective agreement and is not currently conducting negotiations with any labor union or
employee association.
(ii) The Company and each Guarantor is in compliance in all material respects
with all laws respecting employment and employment practices, terms and conditions of
employment, pay equity and wages and has not and is not engaged in any unfair labor
practice.
(iii) The Company and certain of the Guarantors have agreements, plans or
practices relating to the payment of management, consulting, service or other fees or bo-
nuses, pensions, share of profits or retirement allowance, insurance, health or other em-
ployee benefits or for retirement, stock purchase, profit sharing, stock option (including
the Company Option Plan), equity plans (including the LTIP), deferred compensation,
severance or termination pay, insurance, medical, hospital, dental, vision care, drug, sick
leave, disability, salary continuation, legal benefits, unemployment benefits, vacation, in-
centive or otherwise contributed to, or required to be contributed to, by the Company or
any of the Guarantors for the benefit of any current or former director, officer, employee

25
or consultant of the Company or any of the Guarantors, as applicable, (each an “Employ-
ee Plan”). The Company has made available to the Initial Purchaser the opportunity to
review true and complete copies of documents, contracts and arrangements relating to
each such Employee Plan, other than standard medical and dental benefit plans. Each
Employee Plan has been established, operated in the ordinary course and administered in
all material respects in accordance with their terms and applicable laws. Neither the
Company nor any Guarantor sponsors, maintains, contributes to or otherwise has, or rea-
sonably expects to incur, any liability, direct or indirect, contingent or otherwise, with re-
spect to any employee benefit plan that is subject to Title IV of the Employee Retirement
Income Security Act of 1974, as amended.
(iv) Except as disclosed in the Preliminary Offering Memorandum, the Time
of Sale Information or the Offering Memorandum, since January 1, 2016, none of the di-
rectors, officers or employees of the Company, the Guarantors or any associate or affili-
ate of any of the foregoing has any interest, direct or indirect, in any transaction with the
Company or any Guarantor that materially affects, is material to or will materially affect
the Company and the Guarantors taken as a whole.
(v) Except for wages, salaries and other compensation-related payments in the
ordinary course and except for such other amounts as would not be material, neither the
Company nor any Guarantor is indebted to: (i) any director or officer of the Company or
a Guarantor; (ii) any individual related to any of the foregoing by blood, marriage or
adoption; or (iii) any corporation controlled, directly or indirectly, by any one or more of
those individuals referred to in this Subsection (v). None of those Persons referred to in
this Subsection (v) is indebted to the Company or a Guarantor in a material amount. Nei-
ther the Company nor a Guarantor is currently a party to any material contract, agreement
or understanding with any officer, director, employee, or any other Person not dealing at
arm’s length with the Company or the Guarantors other than employment and consulting
agreements.
(ff) Insurance. Except as described in each of the Preliminary Offering Mem-
orandum, Time of Sale Information and the Offering Memorandum, the Company and
the Guarantors have adequate insurance covering their respective properties, operations,
personnel and businesses, including business interruption insurance, liability and product
liability insurance, which insurance is in amounts and insures against such material losses
and risks as are adequate to protect the Company and the Guarantors and their respective
businesses; and none of the Company or any of the Guarantors has (i) received written
notice from any insurer or agent of such insurer that capital improvements or other ex-
penditures are required or necessary to be made in order to continue such insurance or (ii)
any reason to believe that it will not be able to renew its existing insurance coverage as
and when such coverage expires or to obtain similar coverage at reasonable cost from
similar insurers as may be necessary to continue its business.
(gg) [Reserved].
(hh) No Unlawful Payments. None of the Company or any of its Subsidiaries
nor, to the knowledge of the Company, any director, officer, agent, employee or other

26
person acting on behalf of the Company or any of its Subsidiaries has (i) used any corpo-
rate funds for any unlawful contribution, gift, entertainment or other unlawful expense re-
lating to political activity; (ii) made any direct or indirect unlawful payment to any for-
eign or domestic government official or employee from corporate funds; (iii) violated or
is in violation of any provision of the Corruption of Foreign Public Officials Act (Cana-
da), the Foreign Corrupt Practices Act of 1977, the Xxxxxxx Xxx 0000 of the United King-
dom or similar law of a jurisdiction in which the Company or its Subsidiaries conduct
their business and to which they are lawfully subject; or (iv) other than with respect to the
claims relating to the investigation of the DOJ and the United States Attorney’s Office for
the Districts of Louisiana and Kansas with respect to the Company’s former specialty
healthcare distribution division as disclosed in the Preliminary Offering Memorandum,
the Time of Sale Information and the Offering Memorandum, made any bribe, rebate,
payoff, influence payment, kickback or other unlawful payment.
(ii) Compliance with Money Laundering Laws. The operations of the Compa-
ny and its Subsidiaries are and have been conducted at all times in material compliance
with (i) the applicable financial recordkeeping and reporting requirements of the Curren-
cy and Foreign Transactions Reporting Act of 1970, as amended, as well as (ii) the mon-
ey laundering statutes and the rules and regulations thereunder and any related or similar
rules, regulations or guidelines, issued, administered or enforced by any governmental or
regulatory agency in a jurisdiction in which the Company or any of its Subsidiaries con-
duct their business and to which they are lawfully subject (collectively, the “Money
Laundering Laws”), and no action, suit or proceeding by or before any court or govern-
mental or regulatory agency, authority or body or any arbitrator involving the Company
or any of its Subsidiaries with respect to the Money Laundering Laws is pending or, to
the knowledge of the Company, threatened.
(jj) Sanctions. Neither the Company nor any of its Subsidiaries, nor, to the
knowledge of the Company, none of the Company or any director, officer, agent, em-
ployee or affiliate of the Company or any of its Subsidiaries is currently the subject or the
target of any sanctions administered or enforced by the U.S. government, (including,
without limitation, the Office of Foreign Assets Control of the U.S. Department of the
Treasury (“OFAC”) or the U.S. Department of State and including, without limitation,
the designation as a “specially designated national” or “blocked person”), the United Na-
tions Security Council (“UNSC”), the European Union, or other sanctions authority of a
jurisdiction where the Company or any Subsidiary conducts business (collectively,
“Sanctions”), nor is the Company, nor any of its Subsidiaries located, organized or resi-
dent in a country or territory that is the subject or the target of Sanctions, including, Xx-
xx, xxx Xxxxxx xxxxxx xx Xxxxxxx, Iran, North Korea, Sudan and Syria (each, a “Sanc-
tioned Country”); and the Company will not directly or indirectly use the proceeds of the
offering of the Securities hereunder, or lend, contribute or otherwise make available such
proceeds to any Subsidiary, joint venture partner or other person or entity (i) to fund or
facilitate any activities of or business with any person that, at the time of such funding or
facilitation, is the subject or the target of Sanctions, (ii) to fund or facilitate any activities
of or business in any Sanctioned Country in violation of Sanctions or (iii) in any other
manner that will result in a violation by any person (including any person participating in
the transaction, whether as an underwriter, initial purchaser, advisor, investor or other-

27
wise) of Sanctions. For the past 5 years, neither the Company nor the Subsidiaries have
knowingly engaged in and are not now knowingly engaged in any dealings or transac-
tions with any person that at the time of the dealing or transaction is or was the subject or
the target of Sanctions or with any Sanctioned Country in violation of Sanctions. Not-
withstanding the foregoing, the provisions of this Section 3(jj) shall not be interpreted to
contravene, or require any notification to the Attorney General of Canada under, the For-
eign Extraterritorial Measures (United States) Order, 1992, by the Company or any of its
Subsidiaries located in Canada.
(kk) Solvency. On and immediately after the Closing Date, the Company and
the Guarantors (after giving effect to the Transactions and the other transactions related
thereto as described in each of the Time of Sale Information and the Offering Memoran-
dum) will be Solvent. As used in this paragraph, the term “Solvent” means, with respect
to a particular date, that on such date (i) the present fair market value (or present fair
saleable value) of the assets of the Company and the Guarantors is not less than the total
amount required to pay the liabilities of the Company and the Guarantors on their total
existing debts and liabilities (including contingent liabilities) as they become absolute
and matured; and (ii) assuming consummation of the issuance of the Securities as con-
templated by this Agreement, the Time of Sale Information and the Offering Memoran-
dum, the Company and the Guarantors is not incurring debts or liabilities beyond its abil-
ity to pay as such debts and liabilities mature.
(ll) No Restrictions on Guarantors. No Guarantor is currently prohibited, di-
rectly or indirectly, under any agreement or other instrument to which it is a party or is
subject, from paying any dividends to its shareholders, from making any other distribu-
tion on such Guarantor’s capital stock, from repaying to the Company any loans or ad-
vances to such Guarantor from the Company or from transferring any of such Guaran-
tor’s properties or assets to the Company or any other Subsidiary of the Company, except
for any such restrictions that will be permitted by the Indenture or except for restrictions
imposed by applicable laws.
(mm) No Broker’s Fees. Other than payments to Xxxxxxxxx & Co. LLC and PJT
Partners, neither the Company or the Guarantors is a party to any contract, agreement or
understanding with any person (other than this Agreement) that would give rise to a valid
claim against any of them or the Initial Purchaser for a brokerage commission, finder’s
fee or like payment in connection with the Offering.
(nn) Rule 144A Eligibility. The Securities are eligible for resale pursuant to
Rule 144A and on the Closing Date, the Securities will not be of the same class as securi-
ties listed on a national securities exchange registered under Section 6 of the Exchange
Act or quoted in an automated inter-dealer quotation system; and each of the Preliminary
Offering Memorandum, the Time of Sale Information and the Offering Memorandum, as
of its respective date, contains or will contain all the information in all material respects
that, if requested by a prospective purchaser of the Securities, would be required to be
provided to such prospective purchaser pursuant to Rule 144A(d)(4) under the Securities
Act.

28
(oo) No Integration. None of the Company or any of the Guarantors (as de-
fined in Rule 501(b) of Regulation D) has, directly or through any agent, sold, offered for
sale, solicited offers to buy or otherwise negotiated in respect of, any security (as defined
in the Securities Act), that is or will be integrated with the sale of the Securities in a man-
ner that would require registration of the Securities under the Securities Act.
(pp) No General Solicitation or Directed Selling Efforts. None of the Compa-
ny, the Guarantors or any of their affiliates or any other Person acting on its or their be-
half (other than the Initial Purchaser, as to which no representation is made) has (i) solic-
ited offers for, or offered or sold, the Securities by means of any form of general solicita-
tion or general advertising within the meaning of Rule 502(c) of Regulation D or in any
manner involving a public offering within the meaning of Section 4(a)(2) of the Securi-
ties Act or (ii) engaged in any directed selling efforts within the meaning of Regulation S
under the Securities Act (“Regulation S”), and all such Persons have complied with the
offering restrictions requirement of Regulation S. Neither the Company nor any of its af-
filiates has entered into, or will enter into, any contractual arrangement with respect to the
distribution of the Securities except for this Agreement.
(qq) Securities Law Exemptions. Assuming the accuracy of the representations
and warranties of the Initial Purchaser contained in Section 1(b) and Section 5 (including
Annex C hereto) and their compliance with their agreements set forth therein, it is not
necessary, in connection with the issuance and sale of the Securities to the Initial Pur-
chaser and the offer, resale and delivery of the Securities by the Initial Purchaser in the
manner contemplated by this Agreement, the Time of Sale Information and the Offering
Memorandum, to register the Securities under the Securities Act or to qualify the Inden-
ture under the Trust Indenture Act or to file a prospectus in Canada with the Canadian
Securities Regulators in connection with the Securities.
(rr) No Stabilization. Neither the Company nor any of the Guarantors has tak-
en, directly or indirectly, any action designed to or that could reasonably be expected to
cause or result in any stabilization or manipulation of the price of the Securities.
(ss) Margin Rules. Neither the issuance, sale and delivery of the Securities nor
the application of the proceeds thereof by the Company as described in each of the Time
of Sale Information and the Offering Memorandum will violate Regulation T, U or X of
the Board of Governors of the Federal Reserve System or any other regulation of such
Board of Governors.
(tt) Forward-Looking Statements. All forward-looking statements of the
Company described under the caption “Cautionary Note Regarding Forward-Looking
Statements” in the Offering Memorandum and the assumptions underlying such infor-
mation and statements, subject to any qualifications contained therein, including any
forecasts and estimates, expressions of opinion, intention and expectation, as at the time
they were or will be made, were or will be made based on assumptions that are reasona-
ble.

29
(uu) Statistical and Market Data. The statistical, industry and market related
data included in the Offering Memorandum are derived from sources which the Company
reasonably believes to be accurate, reasonable and reliable, and such data agrees in all
material respects with the sources from which it was derived.
(vv) Accounting Controls. The Company has established and maintains a sys-
tem of disclosure controls and procedures and internal control over financial reporting,
and has: (i) designed such disclosure controls and procedures, or caused them to be de-
signed under management’s supervision, to provide reasonable assurance that material in-
formation relating to each of the Company and the Guarantors is made known to man-
agement by others, particularly during the period in which the financial statements are be-
ing prepared; and (ii) designed such internal control over financial reporting, or caused it
to be designed under management’s supervision, to provide reasonable assurance regard-
ing the reliability of financial reporting and the preparation of financial statements for ex-
ternal purposes in accordance with IFRS. Since the date of the latest audited financial
statements of the Company included in the Offering Memorandum, there has been no
change in the Company’s internal control over financial reporting that has materially af-
fected, or is reasonably likely to materially affect, the Company’s internal control over
financial reporting.
(ww) Regulation S. The Company, the Guarantors and their respective affiliates
and all persons acting on their behalf (other than the Initial Purchaser, as to whom the
Company and the Guarantors make no such representation) have complied with and will
comply with the offering restrictions requirements of Regulation S in connection with the
offering of the Securities outside the United States and, in connection therewith, the Time
of Sale Information and the Offering Memorandum will contain the disclosures required
by Rule 902. The Company is a “foreign issuer,” as defined in Rule 902 under the Secu-
rities Act.
4. Further Agreements of the Company and the Guarantors. Each of the Company
and Guarantors, jointly and severally, further covenants and agrees with the Initial Purchaser
that:
(a) Delivery of Copies. The Company will provide, without charge, to the Ini-
tial Purchaser and counsel to the Initial Purchaser as many copies of the Preliminary Of-
fering Memorandum, any other Time of Sale Information, any Issuer Written Communi-
cation and the Offering Memorandum (including all amendments and supplements there-
to) as the Initial Purchaser may reasonably request.
(b) Offering Memorandum, Amendments or Supplements. Before finalizing
the Time of Sale Information and the Offering Memorandum or making or distributing
any amendment or supplement to any of the Time of Sale Information or the Offering
Memorandum, the Company will furnish to the Initial Purchaser and counsel for the Ini-
tial Purchaser a copy of the proposed Time of Sale Information and Offering Memoran-
dum or such amendment or supplement for review, and will not distribute any such pro-
posed Time of Sale Information and Offering Memorandum, amendment or supplement
to which the Initial Purchaser reasonably objects.

30
(c) Additional Written Communications. Before making, preparing, using, au-
thorizing, approving or referring to any Issuer Written Communication, the Company
will furnish to the Initial Purchaser and counsel for the Initial Purchaser a copy of such
written communication for review and will not make, prepare, use, authorize, approve or
refer to any such written communication to which the Initial Purchaser reasonably ob-
jects.
(d) Notice to the Initial Purchaser. The Company will advise the Initial Pur-
chaser promptly, and confirm such advice in writing, (i) of the issuance by any Govern-
mental Authority or Regulatory Authority of any order preventing or suspending the use
of any of the Time of Sale Information, any Issuer Written Communication or the Offer-
ing Memorandum or the initiation or threatening of any proceeding for that purpose; (ii)
of the occurrence of any event at any time prior to the completion of the initial offering of
the Securities as a result of which any of the Time of Sale Information, any Issuer Writ-
ten Communication or the Offering Memorandum as then amended or supplemented
would include any untrue statement of a material fact or omit to state a material fact nec-
xxxxxx in order to make the statements therein, in the light of the circumstances existing
when such Time of Sale Information, Issuer Written Communication or the Offering
Memorandum is delivered to a purchaser, not misleading; and (iii) of the receipt by the
Company or any of the Guarantors of any notice with respect to any suspension of the
distribution of the Securities for offer and sale in any jurisdiction or the initiation or
threatening of any proceeding for such purpose; and the Company and the Guarantors
will use its reasonable best efforts to prevent the issuance of any such order preventing or
suspending the use of any of the Time of Sale Information, any Issuer Written Communi-
cation or the Offering Memorandum or suspending any such distribution of the Securities
and, if any such order is issued, will obtain as soon as possible the withdrawal thereof.
(e) Time of Sale Information. If at any time prior to the Closing Date (i) any
event shall occur or condition shall exist as a result of which any of the Time of Sale In-
formation as then amended or supplemented would include any untrue statement of a ma-
terial fact or omit to state any material fact necessary in order to make the statements
therein, in the light of the circumstances under which they were made, not misleading or
(ii) it is necessary to amend or supplement any of the Time of Sale Information to comply
with applicable law, the Company will immediately notify the Initial Purchaser thereof
and forthwith prepare and, subject to paragraph (b) above, furnish to the Initial Purchaser
such amendments or supplements to any of the Time of Sale Information as may be nec-
xxxxxx so that the statements in any of the Time of Sale Information as so amended or
supplemented will not, in light of the circumstances under which they were made, be mis-
leading or so that any of the Time of Sale Information will comply with law.
(f) Ongoing Compliance of the Offering Memorandum. If at any time prior to
the completion of the initial offering of the Securities (i) any event shall occur or condi-
tion shall exist as a result of which the Offering Memorandum as then amended or sup-
plemented would include any untrue statement of a material fact or omit to state any ma-
terial fact necessary in order to make the statements therein, in the light of the circum-
stances existing when the Offering Memorandum is delivered to a purchaser, not mis-
leading or (ii) it is necessary to amend or supplement the Offering Memorandum to com-

31
ply with applicable law, the Company will promptly notify the Initial Purchaser thereof
and forthwith prepare and, subject to paragraph (b) above, furnish to the Initial Purchaser
such amendments or supplements to the Offering Memorandum as may be necessary so
that the statements in the Offering Memorandum as so amended or supplemented will
not, in the light of the circumstances existing when the Offering Memorandum is deliv-
ered to a purchaser, be misleading or so that the Offering Memorandum will comply with
law.
(g) Blue Sky Compliance. The Company will qualify the Securities for offer
and sale under the securities or Blue Sky laws of such jurisdictions as the Initial Purchas-
er shall reasonably request and will continue such qualifications in effect so long as re-
quired for the offering and resale of the Securities; provided that neither the Company nor
any of the Guarantors shall be required to (i) qualify as a foreign corporation or other en-
tity or as a dealer in securities in any such jurisdiction where it would not otherwise be
required to so qualify, (ii) file any general consent to service of process in any such juris-
diction or (iii) subject itself to taxation in any such jurisdiction if it is not otherwise so
subject.
(h) Clear Market. During the period from the date hereof through and includ-
ing the date that is 90 days after the date hereof, the Company and each of the Guarantors
will not, without the prior written consent of the Initial Purchaser, directly or indirectly,
offer, sell, contract to sell, grant any option to purchase, issue any instrument convertible
into or exchangeable for, or otherwise transfer or dispose of (or enter into any transaction
or devise that is designed to, or could be expected to, result in the disposition in the future
of) any debt securities issued or guaranteed by the Company or any of the Guarantors,
other than those contemplated by the Transactions.
(i) Use of Proceeds. The Company will apply the net proceeds from the sale
of the Securities as described in each of the Time of Sale Information and the Offering
Memorandum under the heading “Use of Proceeds.” No proceeds from the sale of the Se-
curities in accordance with this Agreement and as described in the Offering Memoran-
dum and the Time of Sale Information will be used, directly or indirectly, in Switzerland,
unless the Swiss Federal Tax Administration has confirmed in writing that such use of
proceeds in Switzerland does not result in Swiss withholding tax consequences.
(j) Supplying Information. While the Securities remain outstanding and are
“restricted securities” within the meaning of Rule 144(a)(3) under the Securities Act, the
Company and each of the Guarantors will, during any period in which the Company is
not subject to and in compliance with Section 13 or 15(d) of the Exchange Act, furnish to
holders of the Securities and prospective purchasers of the Securities designated by such
holders, upon the request of such holders or such prospective purchasers, the information
required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
(k) DTC. The Company will use its commercially reasonably efforts to assist
the Initial Purchaser in arranging for the Securities to be eligible for clearance and settle-
ment through The Depository Trust Company (“DTC”).

32
(l) No Resales by the Company. The Company will not, and will not permit
any of its affiliates (as defined in Rule 144 under the Securities Act) to, resell any of the
Securities that have been acquired by any of them, except for Securities purchased by the
Company or any of its affiliates and resold in a transaction registered under the Securities
Act.
(m) No Integration. Neither the Company nor any of its affiliates (as defined
in Rule 501(b) of Regulation D) will, directly or through any agent, sell, offer for sale,
solicit offers to buy or otherwise negotiate in respect of, any security (as defined in the
Securities Act), that is or will be integrated with the sale of the Securities in a manner that
would require registration of the Securities under the Securities Act.
(n) No General Solicitation or Directed Selling Efforts. None of the Compa-
ny or any of its affiliates or any other person acting on its or their behalf (other than the
Initial Purchaser, as to which no covenant is given) will (i) solicit offers for, or offer or
sell, the Securities by means of any form of general solicitation or general advertising
within the meaning of Rule 502(c) of Regulation D or in any manner involving a public
offering within the meaning of Section 4(a)(2) of the Securities Act or (ii) engage in any
directed selling efforts within the meaning of Regulation S, and all such persons will
comply with the offering restrictions requirement of Regulation S.
(o) No Stabilization. None of the Company or any of its affiliates or any other
person acting on its or their behalf (other than the Initial Purchaser, as to which no cove-
nant is given) will take, directly or indirectly, any action designed to or that could reason-
ably be expected to cause or result in any stabilization or manipulation of the price of the
Securities.
(p) Collateral. (i) With respect to liens on the assets of the Company pursuant
to the applicable Collateral Documents, if not completed on the Closing Date after the
use of commercially reasonable efforts by the Company, promptly after the Closing Date
(and in any event within 30 days), and (ii) with respect to liens on the assets of the Guar-
antors pursuant to the applicable Collateral Documents, promptly after the Closing Date
or the Post-Closing Security Date, as applicable (and in any event within 120 days after
the Closing Date), each Guarantor (A) shall complete all filings and other similar actions
required in connection with the perfection of first-priority security interests in the Collat-
eral as and to the extent contemplated by the Indenture and the Collateral Documents and
(B) shall take all actions necessary to maintain such security interests after the Closing
Date and to perfect security interests in any Collateral acquired after the Closing Date, in
each case as and to the extent contemplated by the Indenture and the Collateral Docu-
ments; provided that all Personal Property Security Act and Uniform Commercial Code
filings with respect to the Closing Date Collateral shall be completed on the Closing
Date.
(q) Execution of the Accession Documents. On or prior to the Specified Guar-
antor Accession Deadline, each Specified Guarantor shall (i) become a party to the Inden-
ture by executing a Supplemental Indenture, (ii) become a party to the Subordination
Agreement by executing a supplement thereto in accordance with the Subordination

33
Agreement, (iii) become a party to the Intercreditor Agreement by executing a supple-
ment thereto in accordance with the Intercreditor Agreement and (iv) become a party to
this Agreement by executing a Joinder Agreement. Unless the Specified Guarantor Ac-
cession Date occurs on or prior to the Closing Date, on the date of accession by each
Specified Guarantor, such Specified Guarantor shall (i) cause to be furnished to the Initial
Purchaser a written opinion (unless such opinions are being provided by counsel to the
Initial Purchaser), addressed to the Initial Purchaser and dated the date of such Specified
Guarantor’s accession, in form and substance reasonably satisfactory to the Initial Pur-
chaser and (ii) deliver certificates with respect to such Specified Guarantor substantially
consistent with those delivered pursuant to Section 6(d)(ii). On or prior to the Post-
Closing Security Date, each Specified Guarantor shall enter into the Post-Closing Collat-
eral Agreements.
5. Certain Agreements of the Initial Purchaser.
(a) The Initial Purchaser hereby represents and agrees with the Issuers that:
(i) it has not and will not use, authorize use of, refer to, or participate in the
planning for use of, any written communication that constitutes an offer to sell or the so-
licitation of an offer to buy the Securities other than (A) the Time of Sale Information,
the Preliminary Offering Memorandum and the Offering Memorandum, (B) a written
communication that contains no “issuer information” (as defined in Rule 433(h)(2) under
the Securities Act) that was not included (including through incorporation by reference)
in the Time of Sale Information, the Preliminary Offering Memorandum or the Offering
Memorandum, (C) any written communication listed on Annex A or prepared pursuant to
Section 4(c) above (including any electronic road show presentation), (D) any written
communication prepared by the Initial Purchaser and approved by the Company in ad-
xxxxx in writing or (E) any written communication relating to or that contains the terms
of the Securities and/or other information that was included (including through incorpora-
tion by reference) in the Time of Sale Information, the Preliminary Offering Memoran-
dum or the Offering Memorandum;
(ii) it (or its Canadian affiliate(s) selling Notes in the Canadian Offering Juris-
dictions) is duly registered in the Canadian Offering Jurisdictions, or is exempt from the
dealer registration requirements under Canadian Securities Laws, and is legally permitted
to offer and sell the Notes in the Canadian Offering Jurisdictions as contemplated by this
Agreement in compliance with applicable Canadian dealer registration requirements or
exemptions therefrom;
(iii) it will conduct activities in connection with arranging for the sale and dis-
tribution of the Notes upon the terms and conditions set forth in this Agreement, in the
Time of Sale Information and the Offering Memorandum; and
(iv) it will offer and sell the Notes in Canada only in the Canadian Offering Ju-
risdictions and only to such Purchasers as it has determined, based on reasonable investi-
gation, to be “accredited investors” within the meaning of Section 2.3 of NI 45-106 or
Section 73.3 of the Securities Act (Ontario) and otherwise in accordance with the re-

34
quirements set out in the Canadian Offering Memorandum and Canadian Securities
Laws.
(b) The Initial Purchaser acknowledges that (i) the Securities have not been qualified
for distribution by prospectus in any Canadian province, and are not eligible for resale in Canada
for a period ending four (4) months plus one day from the Closing Date other than pursuant to an
exemption from the prospectus requirement under Canadian Securities Laws, or in circumstances
where the prospectus requirements of Canadian Securities Laws do not apply, and (ii) any certif-
icate representing the Securities will bear, or if the Securities are entered into a direct registration
or other electronic book-entry system then the Initial Purchaser acknowledges notice of such Se-
curities being subject to, the legend set forth below:
“IN CANADA, UNLESS PERMITTED UNDER SECURITIES LEGISLA-
TION, THE HOLDER OF THIS SECURITY MUST NOT TRADE THE
SECURITY BEFORE [NOTE: THE DATE THAT IS FOUR MONTHS
AND ONE DAY FOLLOWING THE ISSUE DATE OF THE NOTES WILL
BE INSERTED HERE].”
6. Conditions of Initial Purchaser’s Obligations. The obligation of the Initial Pur-
chaser to purchase Securities on the Closing Date as provided herein is subject to the perfor-
xxxxx by the Company and the Guarantors of their respective covenants and other obligations
hereunder and to the following additional conditions:
(a) Representations and Warranties. The representations and warranties of
the Company and the Guarantors contained herein shall be true and correct (subject to all
materiality and other qualifications contained therein) on the date hereof and on and as of
the Closing Date (other than such representations and warranties that are made as of a
specified date, which shall be true and correct as of such date); and the statements of the
Company, the Guarantors and their respective officers made in any certificates delivered
pursuant to this Agreement shall be true and correct (subject to all materiality and other
qualifications contained therein) on and as of the Closing Date (other than such state-
ments that are made as of a specified date, which shall be true and correct as of such
date).
(b) No Downgrade. Subsequent to the earlier of (A) the Time of Sale and (B)
the execution and delivery of this Agreement, (i) no downgrading shall have occurred in
the rating accorded the Securities by any “nationally recognized statistical rating organi-
zation,” as such term is defined by the Commission for purposes of Section 3(a)(62) of
the Exchange Act; and (ii) no such organization shall have publicly announced that it has
under surveillance or review, or has changed its outlook with respect to, its rating of the
Securities (other than an announcement with positive implications of a possible upgrad-
ing).
(c) No Material Adverse Change. No event or condition of a type described
in Section 3(e) hereof shall have occurred or shall exist, which event or condition is not
described in each of the Time of Sale Information (excluding any amendment or supple-
ment thereto) and the Offering Memorandum (excluding any amendment or supplement

35
thereto) the effect of which in the reasonable judgment of the Initial Purchaser makes it
impracticable or inadvisable to proceed with the offering, sale or delivery of the Securi-
ties on the terms and in the manner contemplated by this Agreement, the Time of Sale In-
formation and the Offering Memorandum.
(d) Officer’s Certificates and Management Comfort Certificate.
(i) The Initial Purchaser shall have received on and as of the Closing Date a
certificate of the chief executive officer and the chief financial officer of the Company
and of an executive officer of each Identified Guarantor who has specific knowledge of
the Company’s or such Identified Guarantor’s financial matters and is satisfactory to the
Initial Purchaser, on behalf of the Company or such Identified Guarantor, without per-
xxxxx liability, (i) confirming that such officer has carefully reviewed the Time of Sale In-
formation and the Offering Memorandum and, to the knowledge of such officer, the rep-
resentations set forth in Sections 3(a) and 3(b) hereof are true and correct, (ii) confirming
that the other representations and warranties of the Company and the Identified Guaran-
tors in this Agreement are true and correct in all material respects and that the Company
and the Identified Guarantors have complied in all material respects with all agreements
and satisfied all conditions on their part to be performed or satisfied hereunder at or prior
to the Closing Date and (iii) to the effect set forth in paragraphs (b) and (c) above.
(ii) The Initial Purchaser shall have received on and as of the Closing Date or
the Specified Guarantor Accession Date, as applicable, a certificate dated the Closing
Date or the Specified Guarantor Accession Date, as applicable of the secretary of the
Company and a certificate of a senior officer of each of the Guarantors, on behalf of the
Company or such Guarantor, without personal liability, each such certificate addressed to
the Initial Purchaser and counsel to the Initial Purchaser, in each case, with respect to:
(A) the constating documents and the by-laws (or equivalent) of the Company or the rel-
evant Guarantor, as applicable; (B) all resolutions of the board of directors and, if appli-
cable, the shareholders of the Company or the relevant Guarantor, as applicable, relating
to the transactions contemplated hereby; and (C) the incumbency and specimen signa-
tures of the signing officers relating to this Agreement and the Indenture, as applicable.
(e) Comfort Letters.
(i) On the date of this Agreement and on the Closing Date, Pricewaterhouse-
Coopers LLP with respect to the Company and its Subsidiaries shall have furnished to the
Initial Purchaser, at the request of the Company, letters, dated the respective dates of de-
livery thereof and addressed to the Initial Purchaser, in form and substance reasonably
satisfactory to the Initial Purchaser, containing statements and information of the type
customarily included in auditors’ “comfort letters” to underwriters with respect to the fi-
nancial statements and certain financial information contained in each of the Time of Sale
Information and the Offering Memorandum; provided that the letter delivered on the
Closing Date shall use a “cut-off” date no more than three business days prior to the
Closing Date.

36
(ii) On the date of this Agreement and on the Closing Date, Xxxxxxx Xxxxxx
Toronto LLP with respect to the Company and its Subsidiaries and shall have furnished
to the Initial Purchaser, at the request of the Company, letters, dated the respective dates
of delivery thereof and addressed to the Initial Purchaser, in form and substance reasona-
xxx satisfactory to the Initial Purchaser, containing statements and information of the type
customarily included in auditors’ “comfort letters” to underwriters with respect to the fi-
nancial statements and certain financial information contained in each of the Time of Sale
Information and the Offering Memorandum; provided that the letter delivered on the
Closing Date shall use a “cut-off” date no more than three business days prior to the
Closing Date.
(iii) On the date of this Agreement and on the Closing Date, KPMG LLP, with
respect to AMCo and its subsidiaries shall have furnished to the Initial Purchaser, at the
request of the Company, letters, dated the respective dates of delivery thereof and ad-
dressed to the Initial Purchaser, in form and substance reasonably satisfactory to the Ini-
tial Purchaser, containing statements and information of the type customarily included in
auditors’ “comfort letters” to underwriters with respect to the financial statements and
certain financial information contained in each of the Time of Sale Information and the
Offering Memorandum; provided that the letter delivered on the Closing Date shall use a
“cut-off” date no more than three business days prior to the Closing Date.
(iv) On the date of this Agreement and on the Closing Date, Xxxxx Xxxxxxx
LLP with respect to the Covis Acquisition shall have furnished to the Initial Purchaser, at
the request of the Company, letters, dated the respective dates of delivery thereof and ad-
dressed to the Initial Purchaser, in form and substance reasonably satisfactory to the Ini-
tial Purchaser, containing statements and information of the type customarily included in
auditors’ “comfort letters” to underwriters with respect to the financial statements and
certain financial information contained in each of the Time of Sale Information and the
Offering Memorandum; provided that the letter delivered on the Closing Date shall use a
“cut-off” date no more than three business days prior to the Closing Date.
(f) Opinion and 10b-5 Statement of Counsel for the Company and the Guar-
antors.
(i) Xxxxxxxx & Xxxxxxxx LLP, United States counsel for the Company, shall
have furnished to the Initial Purchaser, at the request of the Company, their written opin-
ion and disclosure letter, dated the Closing Date and addressed to the Initial Purchaser, in
form and substance reasonably satisfactory to the Initial Purchaser.
(ii) Fasken Xxxxxxxxx DuMoulin LLP, Canadian counsel for the Company,
and local counsel (only in respect of matters governed by laws of the provinces of Cana-
da where Fasken Xxxxxxxxx XxXxxxxx LLP is not qualified to practice and only in any
such province where the Notes are sold) shall have furnished to the Initial Purchaser, at
the request of the Company, their written opinions, dated the Closing Date and addressed
to the Initial Purchaser, in form and substance reasonably satisfactory to the Initial Pur-
chaser.

37
(iii) AMMC Law S.A., Luxembourg counsel for the Company, shall have fur-
nished to the Initial Purchaser, at the request of the Company, their written opinion, dated
the Closing Date and addressed to the Initial Purchaser, in form and substance reasonably
satisfactory to the Initial Purchaser.
(iv) Xxxxxxx Global, Jersey counsel for the Company, shall have furnished to
the Initial Purchaser, at the request of the Company, their written opinion, dated the Clos-
ing Date and addressed to the Initial Purchaser, in form and substance reasonably satis-
factory to the Initial Purchaser.
(g) Opinion and 10b-5 Statement of Counsel for the Initial Purchaser.
(i) The Initial Purchaser shall have received on and as of the Closing Date an
opinion and 10b-5 statement of Xxxxxx Xxxxxx & Xxxxxxx XXX, Xxxxxx Xxxxxx counsel for
the Initial Purchaser, with respect to such matters as the Initial Purchaser may reasonably
request, and such counsel shall have received such documents and information as they
may reasonably request to enable them to pass upon such matters.
(ii) The Initial Purchaser shall have received on and as of the Closing Date an
opinion of Osler, Xxxxxx & Harcourt LLP, Canadian counsel for the Initial Purchaser,
with respect to such matters as the Initial Purchaser may reasonably request, and such
counsel shall have received such documents and information as they may reasonably re-
quest to enable them to pass upon such matters.
(iii) The Initial Purchaser shall have received on and as of the Closing Date an
opinion of Xxxxx & Overy LLP, England and Wales counsel for the Initial Purchaser, with
respect to such matters as the Initial Purchaser may reasonably request, and such counsel
shall have received such documents and information as they may reasonably request to
enable them to pass upon such matters.
(iv) The Initial Purchaser shall have received on and as of the Closing Date an
opinion of Ogier, Jersey counsel for the Initial Purchaser, with respect to such matters as
the Initial Purchaser may reasonably request, and such counsel shall have received such
documents and information as they may reasonably request to enable them to pass upon
such matters.
(h) No Legal Impediment to Issuance. No action shall have been taken and no
statute, rule, regulation or order shall have been enacted, adopted or issued by any xxxxx-
al, state or foreign governmental or regulatory authority that would, as of the Closing
Date, prevent the issuance or sale of the Notes or the issuance of the Guarantees to be
provided by the Guarantors; and no injunction or order of any federal, state or foreign
court shall have been issued that would, as of the Closing Date, prevent the issuance or
sale of the Notes or the issuance of the Guarantees to be provided by the Guarantors.
(i) Good Standing. The Initial Purchaser shall have received on and as of the
Closing Date a certificate of status or similar certificate, as applicable, with respect to the
jurisdictions in which the Company and the Guarantors are established or incorporated

38
(to the extent the concept of “good standing” exists in such jurisdiction), as the case may
be.
(j) DTC. The Securities shall be eligible for clearance and settlement through
DTC.
(k) Indenture. The Company, the Identified Guarantors, the Collateral Agent
and the Trustee shall have executed and delivered the Indenture in form and substance
satisfactory to the Initial Purchaser and the Initial Purchaser shall have received copies
thereof.
(l) Collateral. The Company and the Identified Guarantors shall have exe-
cuted and delivered the Intercreditor Agreement, the Subordination Agreement and the
Closing Date Collateral Agreements to which it is a party, each dated as of the Closing
Date. Except as otherwise provided for in the Closing Date Collateral Agreements, the
Intercreditor Agreement, the Subordination Agreement, the Indenture or the other docu-
ments entered into related to the issuance and sale of the Securities, the Initial Purchaser
and the Collateral Agent shall have received each of the Closing Date Collateral Docu-
ments, in form and substance reasonably satisfactory to the Initial Purchaser, and all other
certificates, agreements or instruments and all Uniform Commercial Code and Personal
Property Security Act filings necessary to perfect the Collateral Agent’s security interest
in all of the Closing Date Collateral as and to the extent contemplated by the Indenture
and the Closing Date Collateral Documents; each such document executed by the Com-
pany and/or Guarantor, as applicable, shall be in full force and effect.
(m) Joinder Agreements. Each Identified Guarantor shall have executed and
delivered a Joinder Agreement, in form and substance reasonably satisfactory to the Ini-
tial Purchaser, and the Initial Purchaser shall have received such executed counterparts.
All opinions, letters, certificates and evidence mentioned above or elsewhere in this
Agreement shall be deemed to be in compliance with the provisions hereof only if they are in
form and substance reasonably satisfactory to counsel for the Initial Purchaser.
7. Indemnification and Contribution.
(a) Indemnification of the Initial Purchaser. Each of the Company and the Guaran-
tors jointly and severally agree to indemnify and hold harmless the Initial Purchaser, its affiliates,
directors, officers and employees and each person, if any, who controls the Initial Purchaser
within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act, from
and against any and all losses (other than loss of profits), claims, damages, liabilities and expens-
es (including, without limitation, reasonable and documented fees and disbursements of counsel
and other expenses incurred in connection with investigating, defending, settling, compromising
or paying any such loss, claim, damage, liability, expense or action), joint or several, that arise
out of, or are based upon: any untrue statement or alleged untrue statement of a material fact
contained in the Preliminary Offering Memorandum, any of the other Time of Sale Information,
any Issuer Written Communication or the Offering Memorandum (or any amendment or supple-
ment thereto) or any omission or alleged omission to state therein a material fact necessary in

39
order to make the statements therein, in the light of the circumstances under which they were
made, not misleading; provided, however, that the foregoing indemnity agreement shall not ap-
ply, with respect to the Initial Purchaser, to any loss, claim, damage, liability or expense to the
extent, but only to the extent, arising out of or based upon any untrue statement or alleged untrue
statement or omission or alleged omission made in reliance upon and in conformity with written
information furnished to the Company by the Initial Purchaser expressly for use in the Prelimi-
nary Offering Memorandum, the Time of Sale Information, any Issuer Written Communication
or the Offering Memorandum (or any amendment or supplement thereto); and to reimburse the
Initial Purchaser and each such affiliate, director, officer, employee or controlling person for any
and all expenses (including the reasonable and documented fees and disbursements of counsel
chosen by Xxxxxxx, Xxxxx & Co.) as such expenses are reasonably incurred by the Initial Pur-
chaser or such affiliate, director, officer, employee or controlling person in connection with in-
vestigating, defending, settling, compromising or paying any such loss, claim, damage, liability,
expense or action.
(b) Indemnification of the Company and the Guarantors. The Initial Purchaser agrees
to indemnify and hold harmless, the Company and Guarantors, each of their respective affiliates,
directors, officers and employees and each person, if any, who controls, as of the date hereof, the
Company or any of the Guarantors within the meaning of Section 15 of the Securities Act or
Section 20 of the Exchange Act to the same extent as the indemnity set forth in paragraph (a)
above, but only with respect to any losses, claims, damages or liabilities and expenses (including,
without limitation, reasonable and documented fees and disbursements of counsel and other ex-
penses incurred in connection with investigating, defending, settling, compromising or paying
any such loss, claim, damage, liability, expense or action) that arise out of, or are based upon,
any untrue statement or omission or alleged untrue statement or omission made in reliance upon
and in conformity with any information relating to the Initial Purchaser furnished to the Compa-
ny in writing by the Initial Purchaser expressly for use in the Preliminary Offering Memoran-
dum, any of the other Time of Sale Information, any Issuer Written Communication or the Offer-
ing Memorandum (or any amendment or supplement thereto), it being understood and agreed
that the only such information consists of the information set forth under (a) the first sentence of
the third paragraph, (b) the third sentence of the ninth paragraph, and (c) the third and fourth sen-
tences of the twelfth paragraph under the heading “Plan of Distribution” and the second sentence
of third paragraph under the heading “Canadian Offering Memorandum” in the Preliminary Of-
fering Memorandum and the Offering Memorandum.
(c) Notice and Procedures. If any suit, action, proceeding (including any govern-
mental or regulatory investigation), claim or demand shall be brought or asserted against any
person in respect of which indemnification may be sought pursuant to either paragraph (a) or (b)
above, such person (the “Indemnified Person”) shall promptly notify the person against whom
such indemnification may be sought (the “Indemnifying Person”) in writing; provided that the
failure to notify the Indemnifying Person shall not relieve it from any liability that it may have
under this Section 7 except to the extent that it has been prejudiced (through the forfeiture of
substantive rights or defenses) by such failure; and provided, further, that the failure to notify the
Indemnifying Person shall not relieve it from any liability that it may have to an Indemnified
Person otherwise than under this Section 7. If any such proceeding shall be brought or asserted
against an Indemnified Person and it shall have notified the Indemnifying Person thereof, the In-
demnifying Person shall retain counsel reasonably satisfactory to the Indemnified Person (who

40
shall not, without the consent of the Indemnified Person, be counsel to the Indemnifying Person)
to represent the Indemnified Person and any others entitled to indemnification pursuant to this
Section 7 that the Indemnifying Person may designate in such proceeding and shall pay the rea-
sonable and documented fees and expenses of such proceeding and shall pay the reasonable and
documented fees and expenses of such counsel related to such proceeding, as incurred. In any
such proceeding, any Indemnified Person shall have the right to retain its own counsel, but the
fees and expenses of such counsel shall be at the expense of such Indemnified Person unless (i)
the Indemnifying Person and the Indemnified Person shall have mutually agreed to the contrary;
(ii) the Indemnifying Person has failed within a reasonable time to retain counsel reasonably sat-
isfactory to the Indemnified Person; (iii) the Indemnified Person shall have reasonably concluded
that there may be legal defenses available to it that are different from or in addition to those
available to the Indemnifying Person; or (iv) the named parties in any such proceeding (including
any impleaded parties) include both the Indemnifying Person and the Indemnified Person and
representation of both parties by the same counsel would be inappropriate due to actual or poten-
tial differing interests between them. It is understood and agreed that the Indemnifying Person
shall not, in connection with any proceeding or related proceeding in the same jurisdiction, be
liable for the fees and expenses of more than one separate firm (in addition to any local counsel)
for all Indemnified Persons, and that all such fees and expenses shall be reimbursed as they are
incurred. Any such separate firm for the Initial Purchaser, its affiliates, directors and officers and
any control persons of the Initial Purchaser shall be designated in writing by Xxxxxxx, Xxxxx &
Co., and any such separate firm for the Company, the Guarantors, their respective affiliates, di-
rectors and officers and any control persons of the Company and the Guarantors shall be desig-
nated in writing by the Company. The Indemnifying Person shall not be liable for any settlement
of any proceeding effected without its written consent, but if settled with such consent or if there
be a final judgment for the plaintiff, the Indemnifying Person agrees to indemnify each Indemni-
fied Person from and against any loss or liability by reason of such settlement or judgment.
Notwithstanding the foregoing sentence, if at any time an Indemnified Person shall have request-
ed that an Indemnifying Person reimburse the Indemnified Person for the reasonable and docu-
mented fees and expenses of counsel as contemplated by this paragraph, the Indemnifying Per-
son shall be liable for any settlement of any proceeding effected without its written consent if (i)
such settlement is entered into more than 60 days after receipt by the Indemnifying Person of
such request and (ii) the Indemnifying Person shall not have reimbursed the Indemnified Person
in accordance with such request or contested such reimbursement in good faith prior to the date
of such settlement. No Indemnifying Person shall, without the written consent of the Indemni-
fied Person, effect any settlement of any pending or threatened proceeding in respect of which
any Indemnified Person is or could have been a party and indemnification could have been
sought hereunder by such Indemnified Person, unless such settlement (x) includes an uncondi-
tional release of such Indemnified Person, in form and substance reasonably satisfactory to such
Indemnified Person, from all liability on claims that are the subject matter of such proceeding
and (y) does not include any statement as to or any admission of fault, culpability or a failure to
act by or on behalf of any Indemnified Person.
(d) Contribution. If the indemnification provided for in paragraphs (a) and (b) above
is unavailable to an Indemnified Person or insufficient in respect of any losses, claims, damages,
liabilities or expenses referred to therein, then each Indemnifying Person under such paragraph,
in lieu of indemnifying such Indemnified Person thereunder, shall contribute to the amount paid
or payable by such Indemnified Person as a result of such losses, claims, damages, liabilities or

41
expenses (i) in such proportion as is appropriate to reflect the relative benefits received by the
Indemnifying Person on the one hand and the Indemnified Person on the other from the offering
of the Securities or (ii) if the allocation provided by clause (i) is not permitted by applicable law,
in such proportion as is appropriate to reflect not only the relative benefits referred to in clause
(i) but also the relative fault of the Indemnifying Person on the one hand and the Indemnified
Person on the other in connection with the statements or omissions that resulted in such losses,
claims, damages, liabilities or expenses, as well as any other relevant equitable considerations.
The relative benefits received by the Company and the Guarantors on the one hand and the Initial
Purchaser on the other shall be deemed to be in the same respective proportions as the net pro-
ceeds (before deducting expenses) received by the Company from the sale of the Securities and
the total discounts and commissions received by the Initial Purchaser in connection therewith, as
provided in this Agreement, bear to the aggregate offering price of the Securities. The relative
fault of the parties shall be determined by reference to, among other things, whether the untrue or
alleged untrue statement of a material fact or the omission or alleged omission to state a material
fact relates to information supplied by the Company or by the Initial Purchaser and the parties’
relative intent, knowledge, access to information and opportunity to correct or prevent such
statement or omission.
(e) Limitation on Liability. Each of the Company, the Guarantors and the Initial Pur-
chaser agree that it would not be just and equitable if contribution pursuant to this Section 7 were
determined by pro rata allocation or by any other method of allocation that does not take account
of the equitable considerations referred to in paragraph (d) above. The amount paid or payable
by an Indemnified Person as a result of the losses, claims, damages, liabilities or expenses re-
ferred to in paragraph (d) above shall be deemed to include, subject to the limitations set forth
above, any legal or other expenses incurred by such Indemnified Person in connection with any
such action or claim. Notwithstanding the provisions of this Section 7, in no event shall the Ini-
tial Purchaser be required to contribute any amount in excess of the amount by which the total
discounts and commissions received by the Initial Purchaser with respect to the offering of the
Securities exceeds the amount of any damages that the Initial Purchaser has otherwise been re-
quired to pay by reason of an untrue or alleged untrue statement or omission or alleged omission.
No person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Se-
curities Act) shall be entitled to contribution from any person who was not guilty of such fraudu-
lent misrepresentation.
(f) Non-Exclusive Remedies. The remedies provided for in this Section 7 are not ex-
clusive and shall not limit any rights or remedies that may otherwise be available to any Indem-
nified Person at law or in equity.
8. Termination. This Agreement may be terminated in the absolute discretion of the
Initial Purchaser, by notice to the Company, if after the execution and delivery of this Agreement
and on or prior to the Closing Date (i) the Company shall have failed, refused or been unable to
perform any agreement on its part to be performed under this Agreement when and as required;
(ii) any other condition to the obligations of the Initial Purchaser under this Agreement to be ful-
filled by the Company and each of the Guarantors pursuant to Section 6 is not fulfilled when and
as required in any material respect; (iii) trading in any securities of the Company shall be sus-
pended or limited by the Ontario Securities Commission, any other Canadian securities regulator
whose jurisdiction the Company is subject to, the Toronto Stock Exchange, NASDAQ, or the

42
Commission; (iv) trading in securities generally on either the Nasdaq Stock Market or the
New York Stock Exchange shall have been suspended or materially limited, or minimum prices
shall have been established thereon by the Commission, FINRA, or by such exchange or other
regulatory body or governmental authority having jurisdiction; (v) a general moratorium shall
have been declared by either federal or New York State authorities or a material disruption in
commercial banking or securities settlement or clearance services in the United States shall have
occurred; (vi) there is an outbreak or escalation of hostilities or national or international calamity
or crisis in any case involving the United States, on or after the date of this Agreement, or if
there has been a declaration by the United States of a national emergency or war or other nation-
al or international calamity or crisis (economic, political, financial or otherwise) which affects
the U.S. and international financial markets, that, in the judgment of the Initial Purchaser (acting
reasonably), is material and adverse and makes it impracticable or inadvisable to proceed with
the offering, sale or delivery of the Securities on the terms and in the manner contemplated by
this Agreement, the Time of Sale Information and the Offering Memorandum; or (vii) there shall
have been such a material adverse change in general economic, political or financial conditions
or the effect (or potential effect if the financial markets in the United States or Canada have not
yet opened) of international conditions on the financial markets in the United States or Canada
shall be such that, in the judgment of the Initial Purchaser (acting reasonably), makes it impracti-
cable or inadvisable to proceed with the offering, sale or delivery of the Securities on the terms
and in the manner contemplated by this Agreement, the Time of Sale Information and the Offer-
ing Memorandum.
9. [Reserved].
10. Payment of Expenses.
(a) Whether or not the transactions contemplated by this Agreement are consummat-
ed or this Agreement is terminated, the Company and each of the Guarantors jointly and several-
ly agree to pay or cause to be paid all reasonable costs and expenses incident to the performance
of their respective obligations hereunder, including without limitation, (i) the costs incident to
the authorization, issuance, sale, preparation and delivery of the Securities and, subject to Sec-
tion 12, any taxes payable in that connection; (ii) the costs incident to the preparation and print-
ing of the Preliminary Offering Memorandum, any other Time of Sale Information, any Issuer
Written Communication and the Offering Memorandum (including any amendment or supple-
ment thereto) and the distribution thereof; (iii) the costs of reproducing and distributing each of
the Transaction Documents; (iv) the reasonable fees and expenses of the Company’s and the
Guarantors’ counsel (including local and special counsel) and independent accountants; (v) the
fees and expenses incurred in connection with the registration or qualification and determination
of eligibility for investment of the Securities under the laws of such jurisdictions as the Initial
Purchaser may designate and the preparation, printing and distribution of a Blue Sky Memoran-
dum (including the related, reasonable and documented fees and expenses of counsel for the Ini-
tial Purchaser); (vi) any fees charged by rating agencies for rating the Securities; (vii) the fees
and expenses of the Trustee and any paying agent (including related fees and expenses of any
counsel to such parties); (viii) all expenses and application fees incurred in connection with the
approval of the Securities for book-entry transfer by DTC; (ix) the reasonable and documented
fees and expenses of the Collateral Agent, including the fees and disbursements of counsel for
the Collateral Agent in connection with the Indenture, the Collateral Documents, the Xxxxxxxxx-

00
tion Agreement, the Intercreditor Agreement and the Securities, (x) all fees and expenses in-
curred with respect to negotiating, disclosing, creating and perfecting the security interests in the
Collateral contemplated by the Collateral Documents, the Subordination Agreement and the In-
tercreditor Agreement (including the related reasonable and documented fees and expenses of
counsel for the Initial Purchaser in each applicable jurisdiction) and (xi) all expenses incurred by
the Company in connection with any “road show” presentation to potential investors including,
without limitation, expenses associated with the preparation or dissemination of any electronic
road show presentation, expenses associated with production of road show slides and graphics,
and, with the prior written approval of the Company, reasonable and documented fees and ex-
penses of any consultants engaged in connection with the road show presentations, and travel
and lodging expenses of the representatives and officers of the Company and any such consult-
ants.
(b) If (i) this Agreement is terminated pursuant to Section 8, (ii) the Company for any
reason fails to tender the Securities for delivery to the Initial Purchaser or (iii) the Initial Pur-
chaser declines to purchase the Securities for any reason permitted under this Agreement, the
Company and each of the Guarantors jointly and severally agrees to reimburse the Initial Pur-
chaser for all reasonable and documented out-of-pocket costs and expenses (including the rea-
sonable and documented fees and expenses of its counsel) reasonably incurred by the Initial Pur-
chaser in connection with this Agreement and the offering contemplated hereby which are re-
quired to be borne by the Company and shall be payable by the Company promptly upon receiv-
ing an invoice therefor from the Initial Purchaser.
11. [Reserved].
12. Taxes.
(a) All amounts payable hereunder to the Initial Purchaser, including indemnification
and contribution payments, and reimbursement payments, shall be paid in U.S. dollars without
deduction for any taxes, levies, imposts, duties, deductions, charges or withholdings imposed by
any national, federal, provincial, state or local taxing authority, except as required by applicable
law.
(b) If any applicable law requires the deduction or withholding of any tax from any
such payment by the Company, a Guarantor, or any other withholding agent, (i) the applicable
withholding agent shall make such deduction or withholding; (ii) the applicable withholding
agent shall timely pay the full amount deducted or withheld to the relevant governmental authori-
ty in accordance with applicable law; and (iii) the sum payable by the Company or Guarantor, as
applicable, shall be increased as necessary so that after such deduction or withholding has been
made (including such deductions and withholdings applicable to additional sums payable under
this Section) the Initial Purchaser receives an amount equal to the sum it would have received
had no such deduction or withholding been made, provided that this clause (iii) shall not apply in
the case of Canadian withholding tax required to be withheld by applicable law (taking into ac-
count taxation authority administrative policies) from payments to the Initial Purchaser hereun-
der that are in respect of services rendered by the Initial Purchaser in Canada.

44
(c) If Canadian tax is required to be withheld by applicable law (taking into account
taxation authority administrative policies) from payments to the Initial Purchaser hereunder that
are in respect of services rendered by the Initial Purchaser in Canada, the applicable Initial Pur-
chaser shall make a good faith determination of the value of any such services rendered in Cana-
da in respect of which withholding is required and shall separately identify this to the Company.
If the Company or a Guarantor is required to make such withholding, the Company shall prompt-
ly notify the relevant Initial Purchaser in advance of making such withholding, and shall, upon
request, provide evidence of remittance of such tax withheld.
(d) The Company shall be responsible for and shall pay, as required by the relevant
tax law, any sales, goods and services, harmonized sales, value-added or similar tax (“Sales
Tax”) in addition to any fees, reimbursement payments and other amounts payable herein.
Where the Initial Purchaser is required by law to directly collect and remit such Sales Taxes, the
Company shall pay such Sales Taxes to the Initial Purchaser, against proper invoice from the Ini-
tial Purchaser, indicating its relevant Sales Tax registration number(s) and the amount of Sales
Taxes payable, and the Initial Purchaser shall remit same to the relevant governmental authority
and shall be responsible for any penalties and interest assessed by a governmental authority for
failure or delay to collect or remit such Sales Tax as and when required by applicable law.
(e) For greater certainty, the Initial Purchaser will not be responsible for the collec-
tion or remittance of any tax in connection with a payment received by the Company or a Guar-
antor, or the receipts or earnings of the Company or a Guarantor, arising from the conduct of the
business of the Company or a Guarantor. Neither the Company nor any Guarantor will be re-
sponsible for any taxes imposed on or measured by net income (however denominated), xxxx-
chise taxes, or branch profits taxes, in each case, imposed on the Initial Purchaser as a result of
the Initial Purchaser being organized under the laws of, or having its principal office in, the ju-
risdiction imposing such tax (or any political subdivision thereof).
13. Persons Entitled to Benefit of Agreement. This Agreement shall inure to the ben-
efit of and be binding upon the parties hereto and their respective successors and any controlling
persons referred to herein, and the affiliates, officers and directors of the Initial Purchaser re-
ferred to in Section 7 hereof. Nothing in this Agreement is intended or shall be construed to give
any other person any legal or equitable right, remedy or claim under or in respect of this Agree-
ment or any provision contained herein. No purchaser of Securities from the Initial Purchaser
shall be deemed to be a successor merely by reason of such purchase.
14. Survival. The respective indemnities, rights of contribution, representations, war-
ranties and agreements of the Company, the Guarantors and the Initial Purchaser contained in
this Agreement or made by or on behalf of the Company, the Guarantors or the Initial Purchaser
pursuant to this Agreement or any certificate delivered pursuant hereto shall survive the delivery
of and payment for the Securities and shall remain in full force and effect, regardless of any ter-
mination of this Agreement or any investigation made by or on behalf of the Company, the
Guarantors or the Initial Purchaser.
15. Certain Defined Terms. For purposes of this Agreement, (a) except where other-
wise expressly provided, the term “affiliate” has the meaning set forth in Rule 405 under the Se-
curities Act; (b) the term “business day” means any day other than a day on which banks are

45
permitted or required to be closed in New York City; (c) the term “subsidiary” has the meaning
set forth in Rule 405 under the Securities Act; and (d) the term “written communication” has the
meaning set forth in Rule 405 under the Securities Act.
16. Miscellaneous.
(a) [Reserved].
(b) Partial Unenforceability. The invalidity or unenforceability of any section, para-
graph or provision of this Agreement shall not affect the validity or enforceability of any other
section, paragraph or provision hereof. If any section, paragraph or provision of this Agreement
is for any reason determined to be invalid or unenforceable, there shall be deemed to be made
such minor changes (and only such minor changes) as are necessary to make it valid and en-
forceable.
(c) Notices. All notices and other communications hereunder shall be in writing and
shall be deemed to have been duly given if mailed or transmitted and confirmed by any standard
form of telecommunication. Notices to the Initial Purchaser shall be given to Xxxxxxx, Xxxxx &
Co., 000 Xxxx Xxxxxx, Xxx Xxxx, Xxx Xxxx 00000-0000 (fax: (000) 000-0000); Attention:
Registration Department (with a copy to Xxxxxx Xxxxxx & Xxxxxxx LLP, 00 Xxxx Xxxxxx, Xxx Xxxx
Xxx Xxxx, 00000, Attention: Xxxxxxx X. Xxxxxx. Notices to the Company and the Guarantors
shall be given to them at Concordia International Corp., 000 Xxxxxxxxx Xxxx Xxxx, Xxxxx 000,
Xxxxxxxx, Xxxxxxx X0X0X0, (fax: 000-000-0000); Attention: Xxxx Xxxxxxxx, Chief Executive
Officer.
In accordance with the requirements of the USA Patriot Act (Title III of Pub. L. 107-56
(signed into law October 26, 2001)), the Initial Purchaser is required to obtain, verify and record
information that identifies their respective clients, including the Company and the Guarantors,
which information may include the name and address of their respective clients, as well as other
information that will allow the Initial Purchaser to properly identify their respective clients.
(d) Governing Law. This Agreement shall be governed by and construed in accord-
ance with the internal laws of the State of New York applicable to agreements made and to be
performed in such state without regard to conflicts of law principles thereof.
(e) Currency. Any reference in this Agreement to “$” or to “dollars” shall refer to
the lawful currency of the United States unless otherwise specified.
(f) Submission to Jurisdiction; Waiver of Jury Trial. The Company agrees that any
suit, action or proceeding against the Company brought by the Initial Purchaser, the directors,
officers, employees and agents of the Initial Purchaser, or by any person who controls the Initial
Purchaser, arising out of or based upon this Agreement or the transactions contemplated hereby
may be instituted in the courts of the State of New York located in the City and County of New
York or in the United States District Court for the Southern District of New York and waives any
objection which it may now or hereafter have to the laying of venue of any such proceeding, and
irrevocably submits to the non-exclusive jurisdiction of such courts in any suit, action or pro-
ceeding. The Issuers hereby waive all right to trial by jury in any proceeding (whether based up-
on contract, tort or otherwise) in any way arising out of or relating to this Agreement. The Issu-

46
ers agree that a final judgment in any such proceeding brought in any such court shall be conclu-
sive and binding upon the Issuers and may be enforced in any other courts in the jurisdiction of
which the Issuers are or may be subject, by suit upon such judgment.
(g) Waiver of Immunity. With respect to any proceeding related to this Agreement or
the transactions contemplated hereby, each party irrevocably waives, to the fullest extent permit-
xxx by applicable law, all immunity (whether on the basis of sovereignty or otherwise) from ju-
risdiction, service of process, attachment (both before and after judgment) and execution to
which it might otherwise be entitled in the courts of the State of New York located in the City
and County of New York or in the United States District Court for the Southern District of New
York, and with respect to any suits, actions, or proceedings instituted in regard to the enforce-
ment of a judgment of any such proceeding brought in any such court, each party waives any
such immunity in any such court or any other court of competent jurisdiction, and will not raise
or claim or cause to be pleaded any such immunity at or in respect of any such proceeding or en-
forcement of a judgment, including, without limitation, any immunity pursuant to the United
States Foreign Sovereign Immunities Act of 1976, as amended. Each Issuer hereby designates,
appoints and empowers CT Corporation System, 000 0xx Xxxxxx, Xxx Xxxx, XX 00000 (and
any successor thereto, the “Agent of Service”) as its designee, appointee and agent to receive,
accept and acknowledge for and on its behalf, and its properties, assets and revenues, service of
any and all legal process, summons, notices and documents that may be served in any action,
suits or proceedings brought against it in any courts of the State of New York located in the City
and County of New York or in the United States District Court for the Southern District of New
York with respect to any proceeding related to this Agreement or the transactions contemplated
hereby. Each Issuer further hereby irrevocably consents and agrees to the service of any and all
legal process, summons, notices and documents that may be served in any action, suits or pro-
ceedings brought against it by serving a copy thereof upon the Agent of Service. The Issuers
agree that the failure of the Agent of Service to give any notice of such service to them shall not
impair or affect in any way the validity of such service or any judgment rendered in any action or
proceeding based thereon.
(h) Judgment Currency. If for the purposes of obtaining judgment in any court it is
necessary to convert a sum due hereunder into any currency other than U.S. dollars, the parties
hereto agree, to the fullest extent that they may effectively do so, that the rate of exchange used
shall be the rate at which in accordance with normal banking procedures the Initial Purchaser
could purchase U.S. dollars with such other currency in the City of New York on the business
day preceding that on which final judgment is given. The obligations of the Issuers in respect of
any sum due from them to the Initial Purchaser shall, notwithstanding any judgment in any cur-
rency other than U.S. dollars, not be discharged until the first business day, following receipt by
the Initial Purchaser of any sum adjudged to be so due in such other currency, on which (and on-
ly to the extent that) the Initial Purchaser may in accordance with normal banking procedures
purchase U.S. dollars with such other currency; if the U.S. dollars so purchased are less than the
sum originally due to the Initial Purchaser hereunder, the Issuers agree, as a separate obligation
and notwithstanding any such judgment, to indemnify the Initial Purchaser against such loss. If
the U.S. dollars so purchased are greater than the sum originally due to the Initial Purchaser
hereunder, the Initial Purchaser agree to pay to the Issuers (but without duplication) an amount
equal to the excess of the U.S. dollars so purchased over the sum originally due to the Initial Pur-
chaser hereunder.

47
(i) Counterparts. This Agreement may be signed in counterparts (which may include
counterparts delivered by any standard form of telecommunication), each of which shall be an
original and all of which together shall constitute one and the same instrument.
(j) Amendments or Waivers. No amendment or waiver of any provision of this
Agreement, nor any consent or approval to any departure therefrom, shall in any event be effec-
tive unless the same shall be in writing and signed by the parties hereto.
(k) Headings. The headings herein are included for convenience of reference only
and are not intended to be part of, or to affect the meaning or interpretation of, this Agreement.

[Signature Page to Purchase Agreement]
If the foregoing is in accordance with your understanding, please indicate your ac-
ceptance of this Agreement by signing in the space provided below.
Very truly yours,
CONCORDIA INTERNATIONAL CORP.,
as Company
By: ______“Xxxxxx Xxxxxxxxx”________
Name: Xxxxxx Xxxxxxxxx
Title: Chief Financial Officer

[Signature Page to Purchase Agreement]
The foregoing Purchase Agreement is
hereby confirmed and accepted as of the
date first written above.
XXXXXXX, XXXXX & CO.
By: “Xxxxxxx Xxxxxx”
Name: Xxxxxxx Xxxxxx
Title: Managing Director

Schedule 1
Initial Purchaser Principal Amount
Xxxxxxx, Sachs & Co............................................................................. $ 350,000,000
Total $ 350,000,000

Schedule 2
Guarantors
Identified Guarantors (Closing Date)
1. Concordia Pharmaceuticals Inc., S.à x.x., a Luxembourg société à responsabilité limitée
(private limited liability company), with a share capital of USD 5,000,000.-, having its
registered office at 0-00, Xxxxxx xx xx Xxxx, X-0000 Xxxxxxxxxx, Xxxxx-Xxxxx of Lux-
embourg and registered with the Registre de Commerce et des Sociétés, Luxembourg
(Trade and Companies Register, Luxembourg) under number B 200.344
2. Concordia Laboratories Inc., S.à x.x., a Luxembourg société à responsabilité limitée (pri-
vate limited liability company), with a share capital of USD 500,000.-, having its regis-
tered office at 0-00, Xxxxxx xx xx Xxxx, X-0000 Xxxxxxxxxx, Xxxxx-Xxxxx of Xxxxx-
xxxxx and registered with the Registre de Commerce et des Sociétés, Luxembourg (Trade
and Companies Register, Luxembourg) under number B 200.376
3. Concordia Financing (Jersey) Limited, a Jersey corporation
4. Concordia Investments (Jersey) Limited, a Jersey corporation
5. Concordia Holdings (Jersey) Limited, a Jersey corporation
6. Concordia Investment Holdings (UK) Limited, an English private limited company
Specified Guarantors
1. Focus Pharmaceuticals Limited, an English private limited company
2. Mercury Pharmaceuticals Limited, an English private limited company
3. Mercury Pharma (Generics) Limited, an English private limited company
4. Amdipharm Limited, an Irish single member private limited company
5. Amdipharm Mercury International Limited, a Jersey limited company
6. Mercury Pharma International Limited, an Irish private limited company
7. Mercury Pharmaceuticals (Ireland) Limited, an Irish private limited company
8. Focus Pharma Holdings Limited, an English private limited company
9. Mercury Pharma Group Limited, an English private limited company
10. Amdipharm B.V., a Dutch private limited liability company

11. Amdipharm AG, a Swiss limited company
12. Amdipharm Holdings S.à x.x., a Luxembourg société à responsabilité limitée (private lim-
ited liability company), with a share capital of USD 127,609,000.-, having its registered
office at 00X, Xxxxxx X.X. Xxxxxxx, X-0000 Xxxxxxxxxx, Grand-Duchy of Luxembourg
and registered with the Registre de Commerce et des Sociétés, Luxembourg (Trade and
Companies Register, Luxembourg) under number B 105.086
13. Abcur AB, a Swedish limited company
14. Concordia International (Jersey) Limited, a Jersey limited company
15. Amdipharm Mercury Holdco Limited, a Jersey limited company
16. Amdipharm Mercury Midco Limited, a Jersey limited company
17. Amdipharm Mercury Debtco Limited, a Jersey limited company
18. Amdipharm Mercury Newco Limited, a Jersey limited company
19. Amdipharm International Holdings Limited, a Jersey limited company
20. Amdipharm Mercury UK Limited, an English private limited company
21. Amdipharm Mercury Holdco UK Limited, an English private limited company
22. Concordia International Rx (UK) Limited, an English private limited company

A-1
ANNEX A
Time of Sale Information
1. Term sheet containing the terms of the Securities, attached hereto as Annex B.

B-1
ANNEX B
Pricing Term Sheet
[See Attached]

C-1
ANNEX C
Restrictions on Offers and Sales Outside the United States
In connection with offers and sales of Securities outside the United States:
(a) The Initial Purchaser acknowledges that the Securities have not been reg-
istered under the Securities Act and may not be offered or sold within the United States
or to, or for the account or benefit of, U.S. persons except pursuant to an exemption from,
or in transactions not subject to, the registration requirements of the Securities Act.
(b) The Initial Purchaser represents, warrants and agrees that:
(i) The Initial Purchaser has offered and sold the Securities, and will
offer and sell the Securities, (A) as part of their distribution at any time and (B)
otherwise until 40 days after the later of the commencement of the offering of the
Securities and the Closing Date, only in accordance with Regulation S under the
Securities Act (“Regulation S”) or Rule 144A or any other available exemption
from registration under the Securities Act.
(ii) None of the Initial Purchaser or any of its affiliates or any other
person acting on its or their behalf has engaged or will engage in any directed
selling efforts with respect to the Securities, and all such persons have complied
and will comply with the offering restrictions requirement of Regulation S.
(iii) At or prior to the confirmation of sale of any Securities sold in re-
liance on Regulation S, the Initial Purchaser will have sent to each distributor,
dealer or other person receiving a selling concession, fee or other remuneration
that purchases Securities from it during the distribution compliance period a con-
firmation or notice to substantially the following effect:
The Securities covered hereby have not been registered under the
U.S. Securities Act of 1933, as amended (the “Securities Act”),
and may not be offered or sold within the United States or to, or
for the account or benefit of, U.S. persons (i) as part of their distri-
bution at any time or (ii) otherwise until 40 days after the later of
the commencement of the offering of the Securities and the date of
original issuance of the Securities, except in accordance with Reg-
ulation S or Rule 144A or any other available exemption from reg-
istration under the Securities Act. Terms used above have the
meanings given to them by Regulation S.
(iv) The Initial Purchaser has not and will not enter into any contractual
arrangement with any distributor with respect to the distribution of the Securities,
except with its affiliates or with the prior written consent of the Company.

2
Terms used in paragraph (a) and this paragraph (b) and not otherwise defined in this
Agreement have the meanings given to them by Regulation S.
(c) The Initial Purchaser acknowledges that no action has been or will be tak-
en by the Company that would permit a public offering of the Securities, or possession or
distribution of any of the Preliminary Offering Memorandum, the Time of Sale Infor-
mation, the Offering Memorandum, any Issuer Written Communication or any other of-
fering or publicity material relating to the Securities, in any country or jurisdiction where
action for that purpose is required.
(d) The Initial Purchaser acknowledges that the Securities may only be sold in
Canada pursuant to an exemption from the prospectus requirements of applicable Cana-
xxxx Securities Laws, and only through an appropriately registered dealer or in accord-
ance with an exemption from the dealer registration requirements of Canadian Securities
Laws.
(e) The Initial Purchaser represents, warrants and agrees that in relation to
each Member State of the European Economic Area which has implemented the Prospec-
tus Directive) (each, a “Relevant Member State”), with effect from and including the date
on which the Prospectus Directive is implemented in that Relevant Member State , it has
not made and will not make an offer to the public of Notes which are the subject of the
offering contemplated by the Preliminary Offering Memorandum and/or the Offering
Memorandum in that Relevant Member State other than:
(i) To any legal entity which is a qualified investor as defined in the
Prospectus Directive;
(ii) To fewer than 150, natural or legal persons (other than qualified
investors as defined in the Prospectus Directive), subject to obtaining the prior
consent of the Initial Purchaser nominated by the Company for any such offer; or
(iii) In any other circumstances falling within Article 3(2) of the Pro-
spectus Directive,
provided that no such offer of Notes shall require the Company, the Guarantors or
the Initial Purchaser to publish a prospectus pursuant to Article 3 of the Prospectus Di-
rective.
For the purposes of this provision, the expression an “offer to the public of Notes”
in relation to any of the Notes in any Relevant Member State means the communication
in any form and by any means of sufficient information on the terms of the offer and the
Notes to be offered so as to enable an investor to decide to purchase or subscribe for the
Notes, as the same may be varied in that Relevant Member State by any measure imple-
xxxxxxx the Prospectus Directive in that Relevant Member State, and the expression
“Prospectus Directive” means Directive 2003/71/EC (as amended, including by Directive
2010/73/EU), and includes any relevant implementing measure in the Relevant Member
State.

3
(f) The Initial Purchaser represents, warrants and agrees that:
(i) It has only communicated or caused to be communicated and will
only communicate or cause to be communicated an invitation or inducement to
engage in investment activity (within the meaning of section 21 of the Financial
Services and Markets Act of 2000 (the “FSMA”)) received by it in connection
with the issue or sale of the Notes in circumstances in which section 21 of the
FSMA does not apply to the Company or the Guarantors; and
(ii) It has complied with, and will comply with, all applicable provi-
sions of the FSMA with respect to anything done by it in relation to the Notes in,
from or otherwise involving the United Kingdom.
(g) The Initial Purchaser represents, warrants and agrees that the Notes (in-
cluding the rights representing an interest in the notes in global form) which are the sub-
ject of the offering contemplated by the Preliminary Offering Memorandum and/or the
Offering Memorandum have not been and shall not be offered, sold, transferred or deliv-
ered in the Netherlands other than to qualified investors (within the meaning of the Pro-
spectus Directive).
For the purposes of this provision, the expression an “offer of notes to the public”
in relation to any notes in the Netherlands means the announcement or communication in
any form and by any means of sufficient information on the terms of the offer and the
notes to be offered so as to enable an investor to decide to purchase or subscribe for the
notes and the expression “Prospectus Directive” means Directive 2003/71/EC (as amend-
ed, including by Directive 2010/73/EU), and includes any relevant implementing measure
in the Netherlands. No approved prospectus within the meaning of the Prospectus Di-
rective is required to be made generally available in connection with the offer.
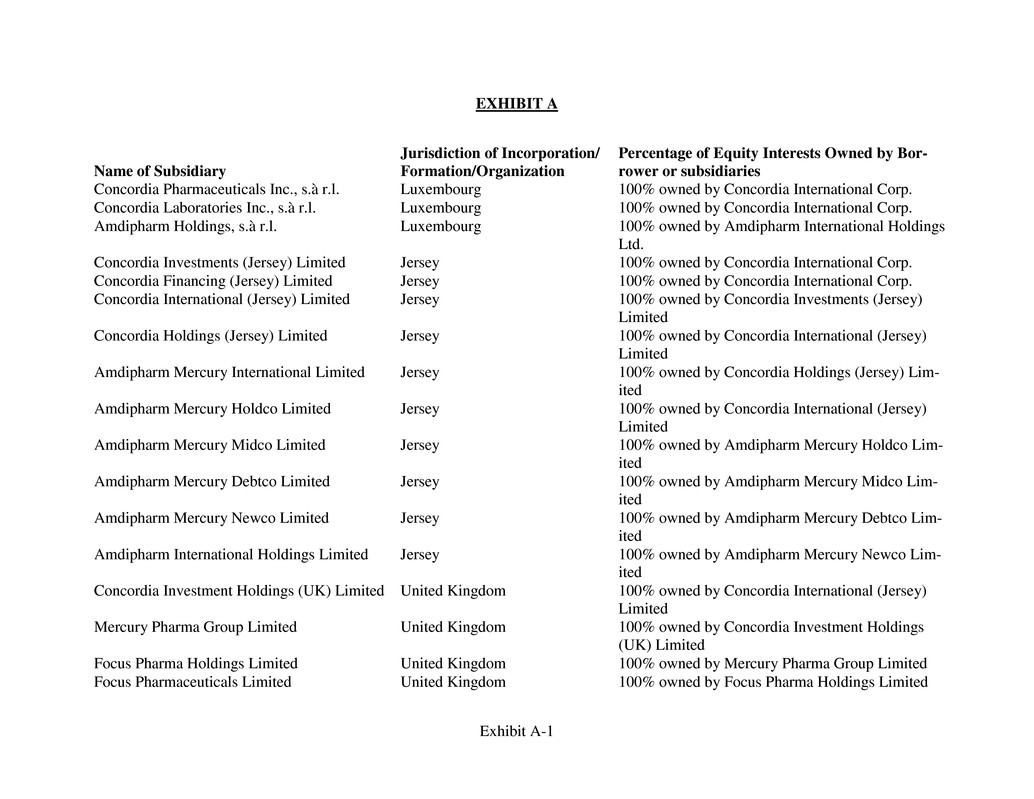
Exhibit A-1
EXHIBIT A
Name of Subsidiary
Jurisdiction of Incorporation/
Formation/Organization
Percentage of Equity Interests Owned by Bor-
rower or subsidiaries
Concordia Pharmaceuticals Inc., s.à x.x. Luxembourg 100% owned by Concordia International Corp.
Concordia Laboratories Inc., s.à x.x. Luxembourg 100% owned by Concordia International Corp.
Amdipharm Holdings, s.à x.x. Luxembourg 100% owned by Amdipharm International Holdings
Ltd.
Concordia Investments (Jersey) Limited Jersey 100% owned by Concordia International Corp.
Concordia Financing (Jersey) Limited Jersey 100% owned by Concordia International Corp.
Concordia International (Jersey) Limited Jersey 100% owned by Concordia Investments (Jersey)
Limited
Concordia Holdings (Jersey) Limited Jersey 100% owned by Concordia International (Jersey)
Limited
Amdipharm Mercury International Limited Jersey 100% owned by Concordia Holdings (Jersey) Lim-
ited
Amdipharm Mercury Holdco Limited Jersey 100% owned by Concordia International (Jersey)
Limited
Amdipharm Mercury Midco Limited Jersey 100% owned by Amdipharm Mercury Holdco Lim-
ited
Amdipharm Mercury Debtco Limited Jersey 100% owned by Amdipharm Mercury Midco Lim-
ited
Amdipharm Mercury Newco Limited Jersey 100% owned by Amdipharm Mercury Debtco Lim-
ited
Amdipharm International Holdings Limited Jersey 100% owned by Amdipharm Mercury Newco Lim-
ited
Concordia Investment Holdings (UK) Limited United Kingdom 100% owned by Concordia International (Jersey)
Limited
Mercury Pharma Group Limited United Kingdom 100% owned by Concordia Investment Holdings
(UK) Limited
Focus Pharma Holdings Limited United Kingdom 100% owned by Mercury Pharma Group Limited
Focus Pharmaceuticals Limited United Kingdom 100% owned by Focus Pharma Holdings Limited

Exhibit A-2
Mercury Pharma (Generics) Limited United Kingdom 100% owned by Mercury Pharma Group Limited
Mercury Pharmaceuticals Limited United Kingdom 100% owned by Mercury Pharma Group Limited
Amdipharm Mercury Holdco UK Limited United Kingdom 100% owned by Amdipharm Mercury Newco Lim-
ited
Amdipharm Mercury UK Limited United Kingdom 100% owned by Amdipharm Mercury Holdco UK
Limited
Amdipharm Limited Ireland 100% owned by Amdipharm B.V.
Mercury Pharma International Limited Ireland 100% owned by Mercury Pharmaceuticals (Ireland)
Limited
Mercury Pharmaceuticals (Ireland) Limited Ireland 100% owned by Mercury Pharma Group Limited
Abcur AB Sweden 100% owned by Mercury Pharma Group Ltd.
Amdipharm AG Switzerland 100% owned by Amdipharm Holdings, s.à x.x.
Amdipharm B.V. Netherlands 100% owned by Amdipharm AG
Concordia Labs Inc. Delaware, USA 100% owned by Concordia International Corp.
Concordia Pharmaceuticals (US) Inc. Delaware, USA 100% owned by Concordia International Corp.
Pinnacle Biologics, Inc. Delaware, USA 100% owned by Concordia Labs Inc.
Hawthorn Drug Group, Inc. Delaware, USA 100% owned by Concordia Pharmaceuticals (US)
Inc.
Complete Medical Homecare, Inc.1 Missouri, USA 100% owned by Concordia Pharmaceuticals (US)
Inc.
Compagnie Biologiques Pinnacle Quebec, Canada 100% owned by Pinnacle Biologics, Inc.
Amdipharm Sales & Marketing Limited Jersey 100% owned by Amdipharm Mercury International
Limited
Amdipharm Group Holdings Limited Jersey 100% owned by Amdipharm Mercury Midco Lim-
ited
Primegen Limited United Kingdom 100% owned by Mercury Pharma Group Limited
Amdipharm Mercury Midco UK Limited United Kingdom 100% owned by Amdipharm Mercury Holdco UK
Limited
Midas Bidco Limited United Kingdom 100% owned by Amdipharm Mercury UK Limited
WIHL Finance Plc United Kingdom 99.99% owned by Amdipharm International Hold-
1 Legally dissolved though not legally terminated as of October 6, 2016.

Exhibit A-3
ings Limited and 0.01% owned by Amdipharm
Group Holdings Limited
Amdipharm UK Limited United Kingdom 100% owned by Amdipharm BV
Amdipharm Marketing Limited United Kingdom 100% owned by Amdipharm UK Limited
RMR Pharmaceuticals Limited United Kingdom 100% owned by Focus Pharmaceuticals Limited
Concordia International Rx (UK) Limited United Kingdom 100% owned by Mercury Pharma Group Limited
Concordia International Rx (Ireland) Limited Ireland 100% owned by Mercury Pharmaceuticals (Ireland)
Limited
Mercurypharm Limited Ireland 100% owned by Mercury Pharmaceuticals (Ireland)
Limited
Mercury Pharmaceuticals (ROI) Limited Ireland 100% owned by Mercury Pharma Group Limited
Amdipharm Coöperatief U.A. Netherlands 99% owned by Amdipharm Sales & Marketing Lim-
ited and 1% owned by Amdipharm Mercury Interna-
tional Limited
Concordia International (Netherlands) B.V. Netherlands 100% owned by Amdipharm Coöperatief U.A.
Pinnacle Biologics B.V. Netherlands 100% owned by Pinnacle Biologics, Inc.
Concordia International (India) Services Pri-
vate Limited
India 99.99% owned by Mercury Pharma International
Ltd and 0.01% owned by Goldshield Healthcare
Private Limited
Goldshield Real Estate Private Limited India 99.9% owned by Mercury Pharma Group Limited
and 0.01% owned by Concordia International Rx
(UK) Limited
Goldshield Healthcare Private Limited India 99.9% owned by Mercury Pharma Group Ltd and
0.01% owned by Mercury Pharmaceuticals Ltd
Pinnacle Biopharma India Private Limited India 99% owned by Mercury Pharma International Lim-
ited and 1% owned by Xxxxx Xxxxxx
AMCo (Germany) GmbH Germany 100% owned by Amdipharm Limited
Concordia International (France), s.à x.x. France 100% owned by Amdipharm Limited
Concordia Pharma (RSA) Pty Limited South Africa 100% owned by Amdipharm Limited
Concordia International (Middle East) FZ-
LLC
United Arab Emirates 100% owned by Amdipharm Limited
Concordia International (Italy), S.R.L. Italy 100% owned by Amdipharm Limited
Amdipharm Mercury (Hong Kong) Limited Hong Kong 99% owned by Mercury Pharma Group Limited and

Exhibit A-4
Mercury Pharma (Australia) Pty Limited Australia
Concordia International (Australia) Pty Lim-
ited
Australia
Xxxxxxx & Xxxx Pty Limited Australia
Emerge Medical Pty Limited Australia
Xxxxxxx & Xxxx (New Zealand) Ltd New Zealand
ABM Pharma Ltd New Zealand
Xxxxxxx & Xxxx (PNG) Pty Limited Papua New Guinea
Southpac Healthcare Ltd. Papua New Guinea
1% owned by Mercury Pharmaceuticals Limited
100% owned by Mercury Pharmaceuticals Limited
100% owned by Mercury Pharma Group Limited
200 Class A & 2,508 Class B owned by Concordia
International (Australia) Pty Limited and 240 Class
A & 608 Class B owned by Mercury Pharma Group
Limited
100% owned by Xxxxxxx & Xxxx Pty Limited
100% owned by Xxxxxxx & Xxxx Pty Limited
100% owned by Xxxxxxx & Xxxx Pty Limited
100% owned by Xxxxxxx & Xxxx Pty Limited
50% owned by Xxxxxxx & Xxxx (PNG) Pty Limited
and 50% owned by an unrelated party [REDACTED
- Third Party Confidentiality Concerns]

Exhibit A-1
EXHIBIT B
Form of Joinder Agreement
[ ], 2016
Xxxxxxx, Sachs & Co.
000 Xxxx Xxxxxx
Xxx Xxxx, Xxx Xxxx 00000
Ladies and Gentlemen:
Reference is made to the Purchase Agreement (the “Purchase Agreement”) dated October
6, 2016, among Concordia International Corp., a corporation organized under the laws of the
province of Ontario (the “Company”) and Xxxxxxx, Xxxxx & Co. (the “Initial Purchaser”), con-
cerning the purchase of the Securities (as defined in the Purchase Agreement) from the Company
by the Initial Purchaser. Capitalized terms used herein but not defined herein shall have the
meanings assigned to such terms in the Purchase Agreement.
[ ] (collectively, “Guarantors”) hereby agree that this Joinder Agreement is
being executed and delivered in connection with the issue and sale of the Securities pursuant to
the Purchase Agreement and is being executed [on the Closing Date][on the Specified Guarantor
Accession Date applicable to the Guarantors party hereto].
1. Joinder. Each of the parties hereto hereby agrees to be bound by the terms, condi-
tions and other provisions of the Purchase Agreement with all attendant rights, duties and obliga-
tions stated therein, with the same force and effect as if originally named as a “Guarantor,” there-
in and as if such party executed the Purchase Agreement on the date thereof.
2. Representations, Warranties and Agreements of each Guarantor. Each Guarantor
represents and warrants to, and agrees with, the Initial Purchaser on and as of the date hereof
that:
(a) Such Guarantor has the corporate or other organizational power and au-
thority to execute, deliver and perform this Joinder Agreement and to consummate the
transactions contemplated hereby and this Joinder Agreement has been duly authorized,
executed and delivered by such Guarantor; and
(b) the representations, warranties and agreements of such Guarantor set forth
in the Purchase Agreement are true and correct on and as of the date hereof subject to all
materiality and other qualifications contained therein and other than such representations
and warranties that are made as of a specified date, which shall be true and correct as of
such date; and
(c) it shall be bound by all covenants, agreements and acknowledgments at-
tributable to a Guarantor in the Purchase Agreement and it shall perform all obligations
and duties required of a Guarantor pursuant to the Purchase Agreement.

Exhibit A-2
3. [SAVINGS CLAUSE. To be inserted]
4. GOVERNING LAW. THIS JOINDER AGREEMENT AND ANY CLAIM,
CONTROVERSY OR DISPUTE UNDER OR RELATED TO THIS JOINDER AGREEMENT
SHALL BE GOVERNED BY, AND CONSTRUED IN ACCORDANCE WITH, THE LAWS
OF THE STATE OF NEW YORK.
5. Counterparts. This Joinder Agreement may be signed in counterparts (which may
include counterparts delivered by any standard form of telecommunication), each of which shall
be an original and all of which together shall constitute one and the same instrument.
6. Amendments. No amendment or waiver of any provision of this Joinder Agree-
ment, nor any consent or approval to any departure therefrom, shall in any event be effective un-
less the same shall be in writing and signed by the parties hereto.
7. Headings. All headings of this Joinder Agreement are included for convenience
of reference only and shall not be deemed a part of this Joinder Agreement.
8. Survival. Except as otherwise provided herein, this Joinder Agreement does not
cancel, extinguish, limit or otherwise adversely affect any right or obligation of the parties under
the Purchase Agreement and each Guarantor party hereto acknowledges and agrees that all of the
provisions of the Purchase Agreement shall remain in full force and effect.
If the foregoing is in accordance with your understanding of our agreement, please indi-
cate your acceptance of this Joinder Agreement by signing in the space provided below, where-
upon this Joinder Agreement and the Purchase Agreement will become binding agreements
among the Guarantors party hereto in accordance with their terms.

Exhibit A
[GUARANTORS]
By:
Name:
Title:

