THE SYMBOL “[****]” DENOTES PLACES WHERE PORTIONS OF THIS DOCUMENT HAVE BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT. SUCH MATERIAL HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. Service Agreement - Y...
Exhibit 4.11
THE SYMBOL “[****]” DENOTES PLACES WHERE PORTIONS OF THIS DOCUMENT HAVE BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT. SUCH MATERIAL HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
THIS SERVICE AGREEMENT Y is made and entered into this ___________ day of October, 2014 (the “Service Agreement”).
|
BETWEEN:
|
[****], a corporation duly incorporated under the laws of Canada and having its principal place of business at [****];
|
|
|
(hereinafter referred to as “[****]”)
|
|
AND
|
RedHill Biopharma Ltd., a company duly incorporated under the laws of Israel, and having its principal place of business at 21 Ha’arba’a Xx., Xxx-Xxxx, 00000 Xxxxxx;
|
|
|
(hereinafter referred to as the “Client”)
|
|
|
([****] and the Client are at times referred to individually as the “Party” and collectively the “Parties”)
|
RECITALS
|
A.
|
The Client and [****] entered into a Master Service Agreement dated on the 7th day of August, 2012 (the “MSA”).
|
|
B.
|
The Parties hereto wish to describe the services to be performed in connection with the MSA, subject to the terms and conditions set forth herein and in the MSA.
|
|
C.
|
Unless the context otherwise requires, all capitalized terms used in this Service Agreement shall have the meanings attributed to them in the MSA.
|
|
1.
|
INTERPRETATION
|
The recitals of this Service Agreement as well as all of its Appendices form an integral part of this Service Agreement.
Page 1 of 13
CONFIDENTIAL
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
2.
|
DESCRIPTION AND DELIVERABLES
|
The objective of this Agreement is to GMP manufacture, the warehousing, the analytical release testing and the stability storage and testing of the RHB-104 CTM resupply.
|
2.1-
|
API, excipients and packaging components reception and storage
|
|
2.1 a)
|
API sourcing
|
It is estimated that sufficient quantities of fully released GMP clarithromycin API and GMP clofazimine API are available at [****] and the Client at its own cost will ship to [****] sufficient quantities of GMP rifabutin API (collectively the clarithromycin API, the clofazimine API and the rifabutin API are called “APIs” and individually “API”). Upon reception of the GMP rifabutin API [****] will execute a full release testing of the material according to GMP requirements. Should additional APIs be required, the sourcing will be the exclusive responsibility of the Client. Upon request by the Client, [****] could characterize and analyze the APIs which will be considered Extra Work.
|
|
Cost:
|
[****]
|
|
*
|
It is estimated that sufficient quantities of each of GMP clarithromycin API and GMP clofazimine API are available at [****] to execute the manufacture of the CTM. However the Client will source and ship to [****] sufficient quantities of GMP rifabutin API for the manufacture of the Drug product included in this Agreement.
|
The cost includes:
|
·
|
the reception of one lot of GMP rifabutin API (i.e., documentation review, material registration in [****] inventory, and material sampling following GMP requirements) (If API lot is in more than one container then the sampling and ID testing of each of the additional containers will be considered Extra Work at 200$/additional container),
|
|
·
|
the full testing for the release of one lot of GMP rifabutin API using validated method. (Client will ship the GMP rifabutin API to [****] with the complete certificate of analysis and related documentation to assure API conformity to the appropriate regulatory authorities),
|
|
·
|
the GMP warehousing of the Client’s Materials (e.g., API, raw material, objects, inactive ingredients) for the period where the Project is active and for a volume not exceeding 1 m3. If the material need to be stored for a longer period of time or the volume of the material is more than 1 m3 then the storage cost will be considered Extra Work or the material shipped at Client.
|
The cost does not include:
|
·
|
The purchase of the analytical reference materials to be used as a standard, if needed, reference impurities (synthesis by-products, Related Substances, metabolites) of known purity, HPLC columns, and any dedicated peripherals (e.g., guard column) and reagents.
|
|
·
|
The release testing of any additional material (i.e., additional API) should the Client change the drug product material specifications or API source or any decision taken by the Client that requires additional analytical testing.
|
|
·
|
The repackaging of material (if required) as well as the shipping cost and custom fees, if any.
|
|
·
|
The destruction fees of any material after being pre-authorized by the Client.
|
|
·
|
Documentation fees for the shipment or reception of GMP API or GMP drug products at 200$/ event (i.e., reception or reception).
|
|
·
|
Sampling fees of 200$/ containers.
|
|
·
|
The shipping document preparation and shipment of material.
|
Page 2 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
2.1 b)
|
GMP Materials (excipients and packaging components) storage and handling-
|
[****] will handle the reception, the full release testing, the shipping and the storage of GMP Materials (i.e., excipients and packaging materials) in its cGMP warehouse. All material handling operations and storage conditions will respect the ICH GMP requirements.
|
|
Cost:
|
[****]
|
|
*
|
The cost includes:
|
|
·
|
The reception of 10 lots of excipients and packaging components (i.e., documentation review, material registration in [****] inventory, and material sampling following GMP requirements) as well as their full release testing as per the [****] (The cost is based on the hypothesis that all GMP material will be received in one single shipment at 200$/ shipment and the full release testing of 10 GMP materials at an average cost of [****] of GMP material received. Any additional shipment or reception of GMP material will be considered as Extra Work at [****] or reception. Any additional sampling and testing of GMP material received will be considered Extra Work at [****] of GMP material received.).
|
|
·
|
The GMP warehousing of the APIs and the drug product until completion of the study. If the material needs to be stored for a longer period of time then the storage cost will be [****]/ month.
|
|
·
|
All the GMP Materials, APIs and RHB-104 drug products must have a volume of less than 1 m3.
|
The cost does not include:
|
·
|
The cost of APIs.
|
|
·
|
The purchase cost of the reference materials to be used as a standards and reference impurities (synthesis by-products, degradation products, metabolites) of known purity, if required.
|
|
·
|
Additional packaging or repackaging of APIs, Drug Products or any GMP material will be considered Extra Work.
|
|
2.2-
|
Manufacturing of the Clinical Trial Material (CTM)
|
The CTM manufacturing process, manufacturing, packaging, equipment calibration and validation, will be done by [****] GMP laboratory. All the manufacturing, packaging and analytical equipments that will be used for the CTM will be calibrated, validated and released for their cleanliness prior to their utilization.
|
2.2 a)
|
Manufacturing and bulk packaging
|
[****] will manufacture and package the single dose strength of RHB-104 capsule as per the optimized formulation developed in the execution of Service Agreement N (document 004-140225rev4).
Specifically with respect to manufacturing, packaging and bulk labeling, the followings items will be provided by [****]:
|
a)
|
Recommendation and justification of specific finished product release specifications,
|
|
b)
|
Redaction of Master Manufacturing File (MMF)
|
Page 3 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
c)
|
The CTM will be manufactured and packaged in labeled* double lined sealed LDPE bags inserted in hard shell sealed barrels:
|
|
·
|
RHB-104 capsule –[****] units
|
|
Cost:
|
145,000$**
|
|
*
|
The labelling on the bags and barrels will include the basic information for GMP drug products (i.e., Name of sponsor, date of manufacturing, Lot number and storage conditions). This Agreement does not include preparation of special packaging and labelling which would require randomization, patient kits preparation of special shipments of clinical supplies.
|
|
**
|
The cost is based on the assumption that one batch of [****] RHB-104 capsules will be manufactured and packaged within the same manufacturing campaign. The cost includes the GMP excipients, the GMP packaging materials, the manufacturing, the bulk packaging, the labelling, the cleaning verification of the manufacturing suites and the equipments for one manufacturing campaign. The cost does not include the transportation cost of the clinical supplies, the broker and custom fees, the cost of the APIs. No manufacturing engineering batch of the drug product will have been executed using the new RHB-104 capsule formulation developed by [****] in the execution of Service Agreement N prior to the execution of this GMP manufacturing campaign and thus, [****] could not be held responsible for batch failure unless the failure is du to a [****] negligence or wilful misconduct. Furthermore, the manufacturing campaign will last 10 working days. The manufacturing campaign will start when the APIs are brought in the GMP manufacturing suites and will be terminated when the GMP manufacturing suites will have been released for their cleanliness. Should the manufacturing campaign be delayed by the Client, the Client’s suppliers, the Client’s APIs or any other factors outside the control of [****], every additional day to the GMP manufacturing campaign will be considered Extra Work at [****]/ day for GMP suite rental and labour cost may also apply in addition to the suite rental.
|
2.2 b) Packaging and labeling of the Clinical Trial Material (CTM) (optional)
Specifically with respect to packaging and labeling, the followings items will be provided by [****]:
|
a)
|
Redaction of Master Manufacturing File (MMF); and
|
|
b)
|
The RHB-104 capsules of the [****] units batch will be packaged in induction sealed HDPE bottle containing [****]; and
|
|
c)
|
Labeling of bottles in a ratio of two RHB-104 bottles to one RHB-104 placebo bottle (i.e., [****]) using the labels to be provided by the Client.
|
The followings items will be provided by the Client to [****]:
|
a)
|
About [****]; and
|
|
b)
|
The randomization list; and
|
|
c)
|
The labels.
|
Page 4 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
Cost:
|
[****]
|
|
*
|
This cost is not included in the total cost of the study in section 7 and should Services included in this section required by the Client it will be considered Extra Work and invoiced monthly. The cost is based on the assumption that [****] RHB-104 capsules will be bottled [****] will be labelled with labels to be provided by the Client AND all the bottling and the labelling will be executed within the same manufacturing campaign. The cost includes the GMP packaging materials, the packaging, the labelling, the cleaning verification of the packaging suites and the equipments. The cost does not include the transportation cost of the clinical supplies, the broker and custom fees, the preparation of Patient Kits. If the Services included in this Agreement is executed within the same manufacturing campaign as of the manufacturing of the RHB-104 capsule (Section 2.2 a)) and the bulk packaging is not required then [****]$ will be subtracted from the discounted cost above.
|
2.2 c) Release testing of CTM and cleaning verification
The validation of the analytical methods for the release testing of the CTM and for the cleaning verification has already been validated. All analysis will be performed using the validated methods. Except for the cleaning verification all of the analyses are outsourced to a [****] qualified third Party laboratory.
Analyses to be performed on CTM are:
|
·
|
appearance,
|
|
·
|
Identification,
|
|
·
|
water content (KF),
|
|
·
|
assay* and degradation products*,
|
|
·
|
microbiology,
|
|
·
|
content uniformity,
|
|
·
|
dissolution** and disintegration time.
|
|
Cost:
|
10,965$***
|
|
*
|
Assay and Related Substances for the three APIs for RHB-104.
|
|
**
|
[****].
|
|
***
|
The cost is not discounted as all of the CTM analyses are outsourced to a Third Party qualified laboratory. The cost includes the analysis of [****] (i.e., RHB-104 capsules), the analysis of [****] for cleaning verification for one GMP manufacturing campaign. Except for the cleaning verification all the analytical methods for release testing have been validated by a third Party [****] qualified laboratory.
|
2.2 d) Stability study of CTM and cleaning verification
The validation of the analytical methods for the analysis of the CTM has already been validated by a qualified third Party laboratory. All analysis will be performed using the validated methods.
Page 5 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
The CTM will be stored in cGMP stability xxxxxxxx at [****], and the samples analyzed using the Client’s validated methods. The single strength RHB-104 capsule will be characterized for appearance, assay, related substances, dissolution*, disintegration and water content using the schedule below.
The following stability testing schedule will be used and modified per mutual agreement:
|
Storage Condition
|
Time point (Month)
|
|||||||||
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
|
|
[****]
|
-
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
|
[****]
|
-
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
[****]
|
|
[****]
|
-
|
[****]
|
[****]
|
[****]
|
-
|
-
|
-
|
-
|
-
|
-
|
X: Sample to be analyzed.
Y: Sample removed from chamber and analyzed only at the request of Client.
|
|
Cost:
|
74,120$**
|
|
*
|
A dissolution test is [****].
|
|
**
|
The cost is not discounted as all of the CTM analyses are outsourced to a Third Party qualified laboratory. The cost is based on the analysis of [****].
|
|
2.3-
|
Reports and submission documentation
|
|
·
|
Telephone meetings, [****] facility and quality audit by Client’s or Client’s representative will be held on an as needed basis.
|
|
·
|
Client or Client’s representative meeting in [****] facility will be held at Client’s request.
|
|
·
|
Progress reports will be provided on a Monthly basis or as needed.
|
|
·
|
Item reports will be provided as they are completed.
|
|
·
|
A final manufacturing report will be provided at the end of the study. It will include all the necessary regulatory submission documents related to manufacturing and packaging which include (but not limited to):
|
|
o
|
The finished products release & stability certificate of analysis.
|
|
o
|
In process testing results.
|
|
o
|
QA Reviewed and audited Manufacturing and Packaging/labeling Documents.
|
|
o
|
Certificate of cGMP compliance.
|
|
o
|
Certificate of analysis of raw materials and packaging components.
|
|
o
|
Any atypical report or Out-of-Specification reports.
|
Cost: 0$*
|
*
|
Included in the costs of the previous sections.
|
Page 6 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
3.
|
GENERAL PROJECT TIMELINES
|
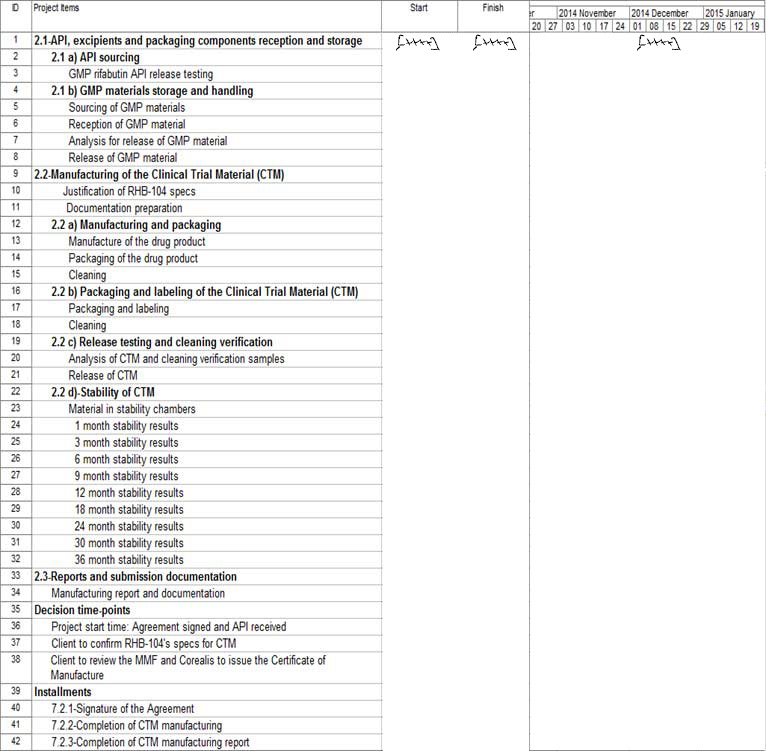
Page 7 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
4.
|
STARTING DATE AND COMPLETION
|
|
4.1
|
Notwithstanding the date of signature of this Service Agreement, [****] shall start the performance of the Services within ten (10) business days after [****] satisfaction of the following:
|
|
4.1.1
|
signature by the Client of this Service Agreement; and
|
|
4.1.2
|
complete delivery by the Client of all of the items mentioned at sub-section 6.1 of section 6 hereof entitled “REQUIREMENTS”.
|
|
4.2
|
This Service Agreement shall be deemed completed upon full delivery of the Services by [****] and receipt by [****] of the final and last payment for the Services.
|
|
5.
|
ASSUMPTIONS
|
|
5.1
|
The RHB-104 optimized formulation containing 95 mg Clarithromycin, 45 mg Rifabutin, and 10 mg Clofazimine will be manufactured for clinical supplies.
|
|
5.2
|
Any subcontractor that will used within this project will need to satisfy the [****] quality audit. Otherwise Extra Work may be required to support subcontractors and/or take actions not to delay the project (e.g., [****] to purchase and release material after approval by the Client). Use of subcontractors must be approved in advance by the Client.
|
|
5.3
|
[****] is not responsible for the qualification of any API manufacturer, any delays in the manufacturing of the APIs, the delivery of the APIs, and for the quality of the APIs purchased by the Client.
|
|
5.4
|
If different lots of APIs are utilized in the execution of the Project and the physical and chemical properties of the different lots are different or, if the physical and chemical properties of the API intended to be utilized in the execution of this Projects differs from the expected API properties when this Agreement was signed by both Parties, then additional formulation development and/or manufacturing process adjustments and/or additional manufacturing time and/or additional sample analysis may be needed and if needed, they will be considered Extra Work and the Project’s time lines adjusted accordingly.
|
|
5.5
|
When a decision is required to move the project forward, the Client will provide its decision in writing to [****] within a period of 5 days, or the project may be delayed. [****] will develop a final timeline for this project and all deviations will be immediately reported to the Client. [****] will make its best efforts to correct all deviations in order to maintain the project timeline.
|
Page 8 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
5.6
|
Any analytical reference materials (e.g., standards and impurities) and dedicated materials (e.g., HPLC columns, speciality reagents) purchased by [****] and utilized solely for the execution of the Client’s Project and any other equipments or materials that are damaged by the Client’s API or APIs (e.g., corrosion, unusual xxxx, staining, contamination, loss of operational functions) or becomes dedicated to the Client’s Project or requires unusual cleaning efforts and resources due to the nature of the Client’s APIs will be considered Extra Work and invoiced at cost to the Client.
|
|
5.7
|
The Client decided not to execute a stability study of the CTM.
|
|
5.8
|
All shipments from [****] to the Client or to a designated location specified by the Client will be invoiced at cost as per the EXW ([****]) Incoterms® 2010. All shipments from Clients or from a designated supplier of the Client to [****] will be invoiced at cost as per the DDP (000 Xxxxxx-Xxxxxxxx xxxxxxxxx, Xxxxx, Xxxxxx, Xxxxxx, X0X 0X0) Incoterms® 2010.
|
|
5.9
|
The Client is responsible to verify that the Services and deliverables provided in the execution of this Service Agreement do not violate or infringe any patent, trade secret or other proprietary or intellectual property right of any third Party.
|
|
5.10
|
During an audit, [****] will allow the Client's representatives to examine the batch records, technical reports, methods and protocols pertaining to the Services. The assistance provided by [****] to the Client during an audit will, under no circumstances, give rise to the payment of additional expenses unless the audit last more than three working days (a working day consist of an 8 hour shift). Should additional time is required for the audit it will be considered Extra Work.
|
|
5.11
|
[****] will allow the Client’s representatives to assist to the execution of the Services for a maximum period of one day per manufacturing campaign. Should the Client’s representative need additional time and/or if the normal execution of the Services is disturbed by the presence of the Client’s representative then [****] may consider it Extra Work.
|
|
5.12
|
If different lots of APIs are utilized in the execution of the Project and the physical and chemical properties of the different lots are different or, if the physical and chemical properties of the API intended to be utilized in the execution of this Project differs from the expected API properties when this Agreement was signed by both Parties, then additional formulation development and/or manufacturing process adjustments may be needed and if needed, they will be considered Extra Work and the Project’s time lines adjusted accordingly.
|
Page 9 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
5.13
|
The costs included in this Agreement for GMP manufacturing are based on the premises that all of the GMP manufacturing operations will be executed within the same manufacturing campaign, unless explicitly specified in section 2. Should the GMP manufacturing campaign be delayed or split in several manufacturing campaigns and where the delays or the split is not caused by [****] or by [****] qualified suppliers, the additional cost that may apply will be considered Extra Work.
|
|
5.14
|
All of the Client’s Materials (e.g., API, raw material, objects, inactive ingredients) stored in [****] warehouse will not exceed a volume of 1 m3. Exceeding storage volume will be considered Extra Work
|
|
6.
|
REQUIREMENTS
|
The Client shall provide to [****], at no cost to [****], the following:
|
6.1
|
If the Client send additional APIs to [****] for the manufacture of the RHB-104, the GMP APIs, reference materials to be used as a standard, reference impurities (synthesis by-products, degradation products, metabolites) of known purity, the certificate of analysis, the BSE & TSE statements, the APIs manufacturer GMP certification.
|
|
7.
|
COST AND PAYMENTS
|
|
7.1
|
The cost of the Services is 247,701.00 $USD [****]. Any amount exceeding a total of 247,701$ requires a pre-approval in writing by the Client.
|
|
7.2
|
The Client shall pay to [****] the following installments in US currency ($USD):
|
|
7.2.1
|
[****] upon signature of this Agreement; and
|
|
7.2.2
|
[****] at the completion of the CTM manufacturing; and
|
|
7.2.3
|
[****] at the acceptance of the final CTM manufacturing report; and
|
|
7.2.4
|
Stability study of CTM invoiced monthly.
|
|
7.3
|
Each of the above payments is subject to receipt of a non-disputed invoice from [****] and subject to the payment terms detailed in the Master Service Agreement.
|
Page 10 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
7.4
|
Notwithstanding section 7.1, for any extra work not covered by this Service Agreement and agreed upon in writing between the Parties (the “Extra Work”), the Client shall pay to [****] the relevant sum as agreed in writing. For any such Extra Work [****] will apply the hourly rates and other fees indicated in this Appendix I attached hereto for the performance of the Services (The costs of the Services for the Extra Work and described in section 7.1 are collectively, the “Fees”).
|
|
7.5
|
Notwithstanding section 7.2 hereof, [****] will invoice the Client for the Extra Work, on a monthly basis for the Services that (i) have been pre-approved in writing by the Client, and; (ii) that have been delivered or rendered by [****].
|
|
8.
|
CONFIDENTIALITY
|
|
8.1
|
Confidentiality issues are covered per the Non Disclosure Agreement and the MSA.
|
|
9.
|
REPRESENTATIONS AND WARRANTIES
|
|
9.1
|
[****] hereby represents and warrants to the Client that:
|
|
9.1.1
|
it is a duly organized and validly existing corporation under the laws of the jurisdiction in which it is incorporated;
|
|
9.1.2
|
it has the necessary corporate power, authority, skills, and capacity and is properly authorized to enter into this Service Agreement and to perform its obligations as per the terms and conditions of this Service Agreement. The execution and delivery of this Service Agreement and the performance of the transactions contemplated hereby have been duly authorized.
|
|
9.2
|
The Client hereby represents and warrants to [****] that:
|
|
9.2.1
|
it is a duly organized and validly existing corporation under the laws of the jurisdiction in which it is incorporated;
|
|
9.2.2
|
it has the necessary corporate power, authority, skills, and capacity and is properly authorized to enter into this Service Agreement and to perform its obligations as per the terms and conditions of this Service Agreement. The execution and delivery of this Service Agreement and the performance of the transactions contemplated hereby have been duly authorized;
|
|
10.
|
TERMS AND CONDITIONS
|
|
10.1
|
This Service Agreement shall be governed, construed and interpreted according to the laws in force in the [****] and the applicable laws of Canada therein, and the courts of the legal district of [****] (Canada) shall have exclusive jurisdiction to hear any and all disputes arising hereunder.
|
Page 11 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
|
10.2
|
This Service Agreement is subject to the terms and conditions provided in the MSA and bind the parties as well as their respective successors, permitted assigns and legal representatives.
|
|
10.3
|
This Service Agreement may be executed in counterparts, each of which shall be deemed to be an original and which together shall constitute one and the same agreement. This Service Agreement may also be executed between the Parties by exchange of facsimile transmissions or electronic transmissions in legible form, including without limitation in a tagged image format file (TIFF) or portable document format (PDF).
|
|
10.4
|
The Parties hereto have requested that this Service Agreement be drafted in the English language. Les Parties ont exigé que ce contrat de services soit rédigé en anglais.
|
IN WITNESS THEREOF, the Parties have executed this Service Agreement as of the Date written above, by their authorised representatives, who by signing confirm their authority and intention to bind the Parties they represent.
|
[****]
|
RedHill Biopharma Ltd.
|
||||
| Per: |
/s/
|
Per:
|
/s/ Xxxx Xxx-Xxxxx | ||
| Name: |
[****]
|
Name: Xxxx Xxx-Xxxxx
|
|||
| Title: |
President
|
Title: CEO
|
|||
| Per: |
/s/ Ori Shilo
|
||||
|
Name: Ori Shilo
|
|||||
|
Title: VP Finance and Operation
|
|||||
Page 12 of 13
CONFIDENTIAL
Service Agreement - Y
RedHill Biopharma Ltd. – [****]
Manufacture of resupply of Clinical Trial Material of
[****] RHB-104 capsules
Appendix I
Professional Consultation Rates
|
Professional
(Chemist or Engineer)
|
Hourly Rate*, **
($USD)
|
|
Senior scientist
|
[****]
|
|
Scientist
|
[****]
|
|
Technician
|
[****]
|
|
R&D laboratory overhead
( Equipment and supplies)
|
[****]
|
Analytical Services
|
Analyses
|
Cost / Sample *
($USD)
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****] ****
|
[****]
|
|
[****] ****
|
[****]
|
|
[****] ****
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
[****]
|
[****]
|
|
*
|
Prices can be changed by [****] without any prior notice. Prices apply only for non GMP work and analysis. GMP prices will be supplied on demand.
|
|
**
|
All expenses will be charged at cost.
|
|
***
|
Will be invoiced in addition to the professional fees when laboratory work is required.
|
|
****
|
A set-up charge of [****] method will be invoiced in addition to the sample cost.
|
Page 13 of 13

