Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Asterisks denote omissions. Amendment 2 OEM Development and Purchase Agreement
Exhibit 10.2
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Asterisks denote omissions.
Securities and Exchange Commission. Asterisks denote omissions.
Amendment 2
OEM Development and Purchase Agreement
OEM Development and Purchase Agreement
This Amendment 2 to the Purchase Agreement dated the 30th day of August, 2005, as
amended (the “Agreement”), by and between Aspect Medical Systems, Inc. (“Aspect” or “Seller”) and
General Electric Company, acting by and through its GE Healthcare division (“GE Healthcare” or
“Buyer”) is made as of this 15th day of April 2009 (“Amendment Effective Date”)
Now, therefore, for good and valuable consideration, the receipt and sufficiency of which are
hereby acknowledged, the undersigned agree as follows:
| 1. | Capitalized terms used herein and otherwise not defined have the meanings ascribed to such terms in the Agreement. | ||
| 2. | All attachments to the Agreement are hereby superseded, deleted, and replaced by the attachments to this Amendment. | ||
| 3. | Section 20 of the Agreement, currently reserved, is replaced by the following: |
| 20. | Distribution of BIS Sensors. |
| a. | In addition to Buyer’s limited right to sell BIS Sensors as set forth in Section 21(a), Buyer and Seller may agree during the Term to select Buyer or one of its Affiliates as a distributor of the BIS Sensor in a specified territory or country. In the event Seller and one of Buyer’s Affiliates agrees to such distribution rights, Buyer’s Affiliate and Seller shall negotiate and execute a Distribution Agreement in a form which shall be approved by both the Buyer’s and Seller’s corporate headquarters. Within five (5) days of execution of such a distribution agreement, Seller shall notify Buyer of the final execution and the Buyer Affiliate shall be listed as a new distributor of the BIS Sensor for the identified region or country . Each distribution agreement executed by Seller and Buyer Affiliate shall then be attached hereto and incorporated herein as part of a new Exhibit to the Agreement. |
| 4. | Section 1 part (f) “Definitions.” will be replaced by the following: |
Bispectral Index or BIS
|
Seller’s proprietary processed EEG parameter that may be used as an aid in monitoring the effects of certain anesthetic agents. | |
Buyer Patient Monitoring System(s) or Buyer Patient Monitors or Buyer’s Patient Monitors |
Buyer-designed multi-parameter patient monitoring systems, which may include monitors and modules. |
| Addendum 1 v 1.0 | Initials: |
1/16
BISx or BISx4 Device
|
The integrated solution of Seller’s BIS processing technology and digital signal conversion technology, which can process either 2 or 4 channels of EEG. | |
BISx Kit or BISx4 Kit
|
Consists of either a 2 channel BISx Device or 4 channel BISx4 Device designed for use with the Buyer Patient Monitoring Systems, a Host Monitor Cable, and a PIC. | |
PIC
|
A patient interface cable designed for use with the Buyer Patient Monitoring Systems | |
Host Monitor Cable
|
The cable designed or to be designed by Seller with Buyer’s assistance, which will connect a BISx Device or BISx4 Device to the BISx Module using an integrated host cable connector. | |
BISx Module
|
That portion of the Buyer Patient Monitoring System that provides power, communication and control to the BISx Kit. | |
BIS Sensor
|
All single-use disposable or semi-reusable sensors manufactured by Seller for use with the BIS/EEG Module Kit or a BISx Kit that is required to generate the Bispectral Index. | |
Aspect Products or
Products
|
BISx Kit, BIS Sensors and any other product that can be ordered by Buyer as listed in Attachment D. | |
Party(ies)
|
Buyer and Seller each individually or jointly. | |
Buyer BIS/EEG Engine
|
is the processing unit for deriving the BIS and EEG data from the raw EEG signal and consists of Seller’s “BIS Engine” board modified for Buyer. | |
BIS/EEG Module Kit
|
means the bundle of all components of the Buyer BIS/EEG Module or the BIS Module that are developed and manufactured by or for Seller and licensed/sold to Buyer under this Agreement: | |
| DSC-XP, DSC Cable, Buyer BIS/EEG Engine, and module cable. “Digital Signal Converter” or |
| Addendum 1 v 1.0 | Initials: |
2/16
| “DSC” means the processing unit that amplifies the analog EEG signals as acquired by the BIS sensors and converts them from analog to digital signals. The DSC-XP is used by Buyer BIS/EEG Module customers to obtain the BIS. | ||
Custom Sensor
|
means a single use sensor that incorporates level-of-consciousness monitoring technology developed by Buyer, as more fully described in the attached product specifications. | |
Smart Sensor Technology
or “SST”
|
means the Seller technology which may be used by Buyer’s Patient Monitors to interface with the Custom Sensors. | |
Smart Chip Module
|
means the component integrated into the Custom Sensor to enable use of the SST. | |
Sensor Connector
|
means the sensor connector currently being used with the Custom Sensor, or any replacement sensor connector where the change was made in accordance with the terms of this Agreement. | |
Entropy module
|
is the processing unit for deriving Buyer’s proprietary processed EEG parameter that measures the hypnotic effect of certain anesthetics on the brain during general anesthesia. | |
Cable Connector
|
means the mating connector to the Sensor Connector currently being used with the Custom Sensor, or any replacement mating connector where the change was made in accordance with the terms of this Agreement. | |
Purchase Order
|
means a purchase order released by Buyer for Products. | |
Distribution Agreement
|
means an agreement that can be or has been, as applicable, executed between the Seller and a Buyer’s wholly owned affiliate to govern the distribution of the BIS Sensor in a specific region or country, the negotiation of which shall, to the extent applicable, include the provisions set forth in Attachment P. |
| Addendum 1 v 1.0 | Initials: |
3/16
Authorized Representative
|
means Seller’s territory-specific and third-party channel of distribution for BIS sensors and other Seller products. | |
Multiparameter Monitoring Sales Opportunity |
means commercial sales opportunities in which the end user customer is making an initial choice between or among multiparameter monitoring vendors, is interested in level of consciousness monitoring, and is therefore selecting among level of consciousness monitoring providers for general anesthesia, such as BIS and Entropy. | |
Other Sales Opportunity
|
Means all commercial sales opportunities other than a Multiparameter Monitoring Sales Opportunity | |
BISx System
|
means the integrated solution of Seller’s BIS Engine processing technology and the DSC-XP. The BISx System includes a patient interface cable and a host monitor cable. | |
Buyer BIS/EEG Module
|
means all components involved in integrating the BISx and/or BIS/EEG with Buyer Patient Monitoring Systems. | |
Buyer Socket
|
means either a BISx Kit or a BIS/EEG Module Kit. | |
Term
|
The term of this Agreement, as described in Section 2, unless earlier terminated in accordance with this Agreement. | |
Central Procurement
Facility(ies)
|
means Buyer’s principle location(s) for ordering and receiving Product meant for further integration into Buyer’s product or distribution through Buyer’s respective channels. |
| 5. | Section 1 (b) of the Agreement is hereby replaced by the following: |
| (b) | Parties. Seller expressly acknowledges that this Agreement is not intended to govern or obligate General Electric Company itself or |
| Addendum 1 v 1.0 | Initials: |
4/16
| any business, division or Affiliate of General Electric Company other than General Electric Company’s GE Healthcare division. Seller agrees that General Electric Company’s GE Healthcare division’s Central Procurement Facilities may place a Purchase Order under this Agreement. If any transaction-specific or country-specific modifications to this Agreement are required to facilitate the sale of the Products to any market where Buyer sells or intends to sell Products, both parties agree to negotiate such modifications in good faith, and to make only such modifications as are required by local law or as are required for logistics purposes. An “Affiliate” shall mean, with respect to any specified party, any other legal entity that directly or indirectly controls, is controlled by or is under common control with, such specified party. |
| 6. | Section 2 of the Agreement is hereby replaced by the following: |
| Term |
| A) | Initial Term. The initial term of this Agreement shall commence on the Effective Date and continue through December 31st, 2011, unless earlier terminated as provided herein. | ||
| B) | Extensions. The term of this Agreement shall thereafter be renewed automatically for successive twelve (12) month periods, unless either Party provides written notice of termination to the other Party at least ninety (90) days prior to expiration of the Agreement. |
| 7. | Section 4, part (g) of the Agreement is hereby replaced by the following: |
(g) BIS Sensor Commission. For each Aspect BIS Sensor sold by Seller to
Buyer’s customers for use with Buyer’s BIS/EEG Module or BISx, Seller shall pay
Buyer [**] received by Seller for such Aspect BIS Sensors less the revenue received
from Distributors (as identified in Attachment P or the relevant Distribution
Agreement). Sensor commission payments will be made quarterly and shall be
provided to Buyer no later than 60 days following the end of each calendar quarter.
Sensor commission will be paid for a period of [**] full years for each Buyer
BIS/EEG Module or BISx from the date of installation at the customer site.
The sensor commission in the US will be calculated [**]. Outside the US,
commission will be calculated by [**].
| Addendum 1 v 1.0 | Initials: |
5/16
In the event that Aspect’s standalone BIS monitors, the BIS modules and/or the BISx
Systems of other manufacturers have also been installed at such sites/regions in
addition to Buyer Socket, Buyer will be entitled to the commission [**]. A pro rata
determination will be based on the total number of BIS units of different types
installed at such locations during the period according to Seller’s installed base
records. Together with such quarterly payments, Seller shall provide to Buyer a
list of accounts or regions for which Buyer is entitled to such commission, the
related total number of BIS units sold to such account or region, and the
percentage of Buyer Sockets used for the pro rata calculation to back up the
quarterly payment.
For example: [**].
To facilitate such sensor commission calculations, Buyer will provide Seller [**]
for Buyer’s Sockets by region at the individual account level.
All such information shall be treated as Seller / Buyer Confidential Information
(depending on which Party disclosed such information) in accordance with the terms
of this Agreement.
| 8. | Section 21(a) and (b) are hereby replaced by the following: |
| 21. | MARKETING AUTHORITY | ||
| a. | Seller hereby grants to Buyer and its distributors, sub-distributors, field organization and channel partners: (1) the exclusive, perpetual, irrevocable, royalty-free right to promote, sell, resell, license, sub-license, distribute and service the Products listed as “Exclusive Products” on Attachment D and purchased from Seller on a world-wide basis; (2) the limited, non-exclusive, perpetual, irrevocable, royalty-free right to sell, resell, license, sub-license, distribute and service limited quantities of the Products listed as “BIS Sensors” on Attachment D and purchased from Seller on a world-wide basis solely to [**]; (3) the limited, non-exclusive, perpetual, irrevocable, world-wide, royalty-free right to sell, resell, license, sub-license, distribute and service [**] (as listed on Attachment D) solely to Buyer customers who have purchased a BIS/EEG Module Kit or BISx Kit provided that this single box of sensors is sold and delivered to the customer at the time of the purchase of the BIS module or BISx technology and (4) the non-exclusive, perpetual, irrevocable, royalty-free right to promote, sell, resell, license, sub-license, distribute and service all other Products purchased from Seller on a world-wide basis. The Products may be promoted, sold, resold, licensed, sublicensed and distributed by Buyer directly and/or indirectly through its distributors, sub-distributors, field organization and channel partners, and may be used |
| Addendum 1 v 1.0 | Initials: |
6/16
| as components in, or be incorporated into, or integrated with, systems and products of Buyer, which Buyer, its distributors, sub-distributors, field organization and/or channel partners sell or lease to third party users in the regular course of business. The provisions of this Section 21(a) will survive any change in control of Seller and Seller agrees that, if it sells all or substantially all of the assets relating to the business that produces the Products, it will require that the purchaser of the assets agrees to assume this Agreement as well. | |||
| b. | General. The components of the Buyer BIS/EEG Module or BISx Systems shall only be resold, leased rented, licensed or otherwise transferred to third parties for use as a part of a Buyer BIS/EEG Module or BISx System or as replacement parts used in Buyer BIS/EEG Modules or BISx Systems and Buyer shall only sell Seller approved accessories including cables and sensor products in connection with any Buyer BIS/EEG Module or BISx System. During the Term of this Agreement, Buyer agrees that it may offer complimentary but not directly competitive products to the Buyer BIS/EEG Module and BISx System, with the exception of Buyer’s Entropy module. Buyer products other than the Buyer BIS/EEG Module, BISx System, and the Entropy module that display a parameter claiming to be a measure of the hypnotic effects of certain anesthetic agents on the brain during general anesthesia are considered to be directly competitive products for purposes of this Section, provided that such obligations of Buyer shall terminate in the event Seller’s Products do not have material competitive features for measuring the hypnotic effects of anesthetic agents on the brain and Seller does not incorporate such features into the Products within six (6) months of receipt of notice of such deficiency. |
| 9. | Section 21 part (f) shall hereby be replaced by the following: |
| f. | Recognizing that the Buyer has a product that is competitive to BIS, Buyer agrees to devote “reasonable sales and marketing efforts” to support the Buyer BIS/EEG Module and the BISx Kits but solely in connection with Multiparameter Monitoring Sales Opportunities. Seller acknowledges and agrees that Buyer has no obligation to provide reasonable sales and marketing efforts, or otherwise promote, discuss, or mention, Seller’s BIS technology in Other Sales Opportunities. “Reasonable sales and marketing efforts” shall mean: | ||
| i. | Buyer communicating to Buyer’s Monitoring Solutions GMs in the respective pole organizations the fact that Buyer has alternative product offerings for patient consciousness monitoring, with a |
| Addendum 1 v 1.0 | Initials: |
7/16
| reasonably detailed description of the features, benefits and customer support available for each product; | |||
| ii. | Buyer communicating to Buyer’s Monitoring Solutions GMs in the respective pole organizations responsible for selling Buyer’s multiparameter monitoring product line: (1) that customers interested in consciousness monitoring should be informed that Buyer has two technologies (BIS and Entropy modules) that are available to meet their needs, and (2) that sales representatives, distributors, and dealers should permit customers to choose freely between these alternative solutions; (3) that the BIS technology shall be fairly represented offering marketing materials and literature provided by Aspect, and (4) that customer requests for quotes and orders will be processed in normal turnaround time. | ||
| iii. | In the event that Buyer elects to display (e.g. at medical congresses, tradeshows or seminars) or otherwise advertise (e.g. website, journals, etc.) the availability of the Entropy module at the annual meetings of the American Society of Anesthesiologists, the European Society of Anesthesiologists, or the World Congress, Buyer agrees to utilize commercially reasonable efforts within the context of a complex worldwide organization to display or otherwise advertise the availability of Buyer’s BIS monitoring solutions for Buyer’s customers; | ||
| iv. | In the event that Buyer elects to include the Entropy technology in its multi-parameter monitoring advertisement(s) (e.g. website, brochures, etc.), Buyer agrees to use commercially reasonable efforts within the context of a complex worldwide organization to also include the BIS technology; | ||
| v. | Buyer BIS/EEG Modules and BISx Systems will be available to Buyer’s sales representatives, dealers and distributors that sell the full Buyer monitoring portfolio on the same basis that Buyer provides demonstration equipment of its Entropy module for use in connection with Multiparameter Monitoring Sales Opportunities; | ||
| vi. | Buyer will allow Seller to directly demonstrate Buyer BIS/EEG Module or BISx Kit directly to end-users. Buyer will sell to Seller a reasonable quantity of Buyer BIS/EEG Module or BISx Kits which Seller may use for demonstration purposes only. In the case of BISx Kits, Buyer will sell Seller a complete BISx Kit for no more than 10% more than the original transfer price between the Seller and Buyer. In the case of the BIS/EEG Module, Buyer will sell Seller a complete BIS/EEG Kit for no more than 20% more |
| Addendum 1 v 1.0 | Initials: |
8/16
| than the original transfer price between the Seller and Buyer. A reasonable quantity shall be defined as no more than two (2) Buyer BIS/EEG Module or BISx Systems per sales person or regional manager in Seller’s Sales organization. | |||
| Buyer and Seller will develop a process to ensure the safe and effective demonstration process of Buyer BIS/EEG Module or BISx Kits (including adequate training of Seller’s Sales force). It is anticipated by Buyer and Seller that such training will take no more than one day per calendar year and that training will take place at a mutually agreed time, date and location. |
Seller agrees that its direct and indirect customers shall not be provided unfair
representations of the features, benefits and customer support available for
Entropy.
For the avoidance of doubt, nothing in this Agreement or this section prevents or
limits Buyer from developing marketing materials, clinical papers, sales materials,
advertisements or promotions for its Entropy technology, including, without
limitation, comparisons to Seller’s BIS technology. Buyer has no obligation to
ensure that such Entropy materials reference Aspect or its BIS technology.
Any failure of either party to comply with these provisions will be escalated to
the agreement managers and discussed at the next quarterly meeting.
| 10. | Section 21 part (h) shall be added to the Agreement as follows: |
(h). If there is a reasonable belief that Buyer or Buyer’s Affiliate may have
violated Section 21(a) of the Agreement by selling “BIS Sensors” to customers other
than those identified specifically in Section 21(a), the Parties agree to conduct a
good faith negotiation to identify a reasonable resolution to the potential
violation. If the Parties cannot agree to a resolution, Seller has the right to
conduct an independent audit, at the Seller’s expense, of BIS Sensor sales only in
the specific market where the violation may have occurred solely to determine
whether unauthorized BIS Sensor sales in fact occurred. If such audit reveals that
such unauthorized sales occurred in violation of Section 21(a), then (i) the Buyer
shall reimburse the Seller for the direct costs for conducting such audit, and (ii)
if the Parties cannot reach a negotiated resolution, Seller has the option to
immediately cease selling BIS Sensors to the specific market country in which the
violation occurred, or to the Buyer’s Affiliate distributor responsible for such
violation.
| 11. | Section 23 is amended by adding the following subsection (e): |
| Addendum 1 v 1.0 | Initials: |
9/16
(e) Buyer grants to Seller a non-exclusive, revocable upon termination or
expiration of this Agreement, royalty-free right to purchase the Smart Chip Module
directly from the manufacturer solely for the purpose of allowing Seller to
incorporate the Smart Chip Module into the Custom Sensor; provided, however, that
Seller may only purchase the Smart Chip Sensor in quantities necessary to
manufacture Custom Sensors. Seller represents and warrants that it will not
purchase Smart Chip Modules in quantities that exceed the number required to
manufacture the Customer Sensor, accounting for additional quantities due to scrap,
testing, and safety stock. Buyer has the right to conduct a reasonable audit of
Seller’s records to ensure compliance with this Section, including, without
limitation, communications with the manufacturer of the Smart Chip Module. The
price of the Custom Sensor to the Buyer includes the actual cost of the Smart Chip
Module plus 10%. For the purposes of establishing the initial price for the
Entropy Sensor as shown in Amendment 2, Attachment D, Section D, Customer Sensor,
the Smart Chip Module cost as of the Effective Date is $0.35. Any increase in cost
of the Smart Chip Module cost by more than 10% will result in an increase in Custom
Sensor cost by the actual increase in cost of the Smart Chip Module plus 10%.
In addition to the charges outlined in Section 3 (c) should Seller hold excess
inventory of the Smart Chip Module due to order cancelation or other actions, e.g.
design or specification changes, by Buyer which cause the Smart Chip Modules in
Seller’s inventory to become unusable or viewed as excess, Buyer agrees to
reimburse Seller for the cost of such inventory. Buyer will then have the option
to take possession of such excess Smart Chip Module inventory or instruct Seller to
dispose of them in a responsible manner.
| 10. | Section 18. part (b) shall hereby be replaced by the following: |
(b) Addresses. Any notice required under this Agreement shall be sent by fax
(with the original to promptly follow by applicable national mail service or a
nationally recognized overnight courier), by a nationally recognized overnight
courier, or transmitted electronically pursuant to the terms of Section 15.
Notices will be deemed given on the date delivered to the recipient if sent by fax
or overnight courier (it being agreed that the sender shall retain proof of
transmission or delivery, as the case may be), or when accessible electronically if
sent electronically under Section 15. Notices shall be sent to the persons
identified below (or as otherwise directed in writing by a party):
Buyer:
|
GE Healthcare | |
| 0000 X. Xxxxx Xxx. | ||
| Xxxxxxxxx, XX 00000 |
| Addendum 1 v 1.0 | Initials: |
10/16
| Attention: Xxxxxxx Xxx Xxxxx, GM Market Development | ||
| Fax: 000-000-0000 | ||
Copy To:
|
GE Healthcare | |
| 0000 X. Xxxxxxxxxx Xx. | ||
| Xxxxxxxxx, XX 00000 | ||
| Attention: General Counsel |
Seller (for all issues relating to this OEM Development and Purchase Agreement, and
Attachment P, Independent Distribution, Template and Distribution Agreements:
| Aspect Medical Systems, Inc. | ||
| Xxx Xxxxxx Xxxx | ||
| Xxxxxxx, XX 00000 | ||
| Attention: Xxxx Xxxxx | ||
| Fax: 000 000-0000 |
Seller (for issues relating to Attachment P, Independent Distribution of the
OEM Development and Purchase Agreement and Distribution Agreements:
| Aspect Medical Systems, Inc. | ||
| Xxx Xxxxxx Xxxx | ||
| Xxxxxxx, XX 00000 | ||
| Attention: Xxxx Xxxxxxx | ||
| Fax: 000 000-0000 |
| 13. | Section 4 (c) is amended by adding the following paragraph: | ||
| Aspect will “certify” the integration of Products into Buyer’s Patient Monitoring Systems. This entails Seller verifying the accurate display of BIS on the Buyer Patient Monitoring Systems. Once successfully verified, Seller will provide Buyer with a Certification Letter indicating the system that has been certified with the specific Buyer Patient Monitoring System. Certification must be obtained before commercial shipment of the Buyer Patient Monitoring System with the BIS features enabled. To facilitate the certification process, Buyer will lend to Seller Buyer Patient Monitoring Systems for testing purposes only. Said Buyer Patient Monitoring Systems will be provided to Seller, at Buyer’s cost and expense, within thirty (30) days after completion of integration of new BISx Kits, and it will be returned to Buyer, at Seller’s cost and expense, within thirty (30) days after termination of this Agreement or written request from the Seller. | |||
| 14. | Section 25(f) is hereby deleted and replaced by the following: |
| Addendum 1 v 1.0 | Initials: |
11/16
LIMITATION ON LIABILITY. EACH PARTY AND IT’S
AFFILIATES LIABILITY TO THE OTHER PARTY ARISING OUT OF
THE MANUFACTURE, SALE, DISTRIBUTION (INCLUDING ANY DISTRIBUTION OF PRODUCTS PURSUANT TO SECTION
20(A) AND ALL DISTRIBUTION AGREEMENTS) OR SUPPLYING OF PRODUCTS OR THEIR USE OR DISPOSITION OR
THEIR OBLIGATIONS OR RESPONSIBILITIES UNDER THIS AGREEMENT, WHETHER BASED UPON WARRANTY, CONTRACT,
TORT OR OTHERWISE, SHALL NOT EXCEED THE GREATER OF (A) FIVE MILLION DOLLARS ($5,000,000) OR (B) THE
SUM OF (i) THE TOTAL ACTUAL PURCHASE PRICE PAID OR PAYABLE BY BUYER FOR ALL SELLER’S PRODUCTS
PURCHASED HEREUNDER; AND (ii) ALL AMOUNTS PAID OR PAYABLE BY BUYER TO SELLER FOR SERVICE,
DISTRIBUTION AND SUPPORT PROVIDED UNDER THIS AGREEMENT. IN NO EVENT SHALL EITHER PARTY BE LIABLE
TO THE OTHER PARTY FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL, OR OTHER INDIRECT DAMAGES (INCLUDING,
BUT NOT LIMITED TO, LOSS OF PROFITS, LOSS OF DATA OR LOSS OF USE DAMAGES) ARISING OUT OF THE
MANUFACTURE, SALE, DISTRIBUTION OR SUPPLYING OF PRODUCTS OR THE OBLIGATIONS OR RESPONSIBILITIES
UNDER THIS AGREEMENT. THESE LIMITATIONS WILL NOT APPLY TO CLAIMS FOR DAMAGES FOR BODILY INJURY
(INCLUDING DEATH) AND DAMAGE TO REAL PROPERTY AND TANGIBLE PERSONAL PROPERTY FOR WHICH A PARTY IS
LEGALLY LIABLE AND/OR A PARTY’S INDEMNIFICATION OBLIGATIONS CONTAINED IN THIS AGREEMENT OR ANY
ATTACHMENT.
15. General.
Aspect hereby rescinds its termination of the Agreement as of December 31, 2008 pursuant to the
letter to Buyer dated September 8, 2008, as amended by the Parties pursuant to letter agreements
dated December 23, 2008 and January 28, 2009.
All terms and conditions of the Agreement not modified herein shall remain unchanged, in full force
and effect. After the Amendment Effective Date, every reference in the Agreement to the
“Agreement” shall mean the Agreement as amended by this Amendment 2.
Acceptance of this Amendment 2 is indicated by signatures below.
| Aspect Medical Systems, Inc. | General Electric Company | |||||||||
| GE Healthcare Division | ||||||||||
By:
|
/s/ Xxxx Xxxxxxxxx | By: | /s/ Xxxxx Xxxxxx | |||||||
| Name: | Xxxx Xxxxxxxxx | Name: Xxxxx Xxxxxx | ||||||||
| Title: | Chief Financial Officer | Title: General Manager –Monitoring Solutions | ||||||||
| Addendum 1 v 1.0 | Initials: |
12/16
AMENDMENT 1
Attachment D
Revised Product Schedule
Attachment D
Revised Product Schedule
A) BIS/EEG MODULE KIT:
| i) | List price for BIS/EEG Module Kit (through December 31, 2009): |
| Aspect P/N | GE P/N | Description | Price | |||||
186-0138-GE |
2004815-001 | BIS Engine (PCB) | [**] | |||||
186-0155-GE |
2007350-001 | BIS DSC-XP | [**] | |||||
186-0138-DO |
900505 | BIS Engine (PCB) | [**] | |||||
186-0161-DO |
900506 | SW-License for BIS-Engine | [**] | |||||
186-0155-DO |
900510-HEL | BIS DSC-XP | [**] | |||||
| Buyer will be responsible for providing Seller with documentation, on a monthly basis, of the total number of BIS/EEG Module Kits installed, the locations of such BIS/EEG Module Kits and the dates of installation. | |||
| ii) | Lead time for the BIS/EEG Module Kit is 10 weeks. | ||
| iii) | The BIS/EEG Module Kit is a non-exclusive product, available for sale and distribution world-wide. | ||
| iv) | Regulatory clearances for integration into patient monitoring system to be completed by Buyer. Seller has received 510(k) for the BIS/EEG Module Kit (K002837). | ||
| v) | The BIS/EEG Module Kit will become obsolete on December 31, 2009 and Buyer will be afforded a last time buy opportunity as reflected in Section 8 (c) of the Agreement. |
B) BISx Kit:
| i) | List price for the BISx Kit: | ||
| Volume discounts: |
Aspect P/N |
GE P/N | Description | [**] | [**] | [**] | |||||||||||
186-0195-GE |
2026859-001 | BISx Kit | [**] | [**] | [**] | |||||||||||
XXX |
XXX | BISx 4 Kit | [**] | [**] | [**] |
| Buyer will be responsible for providing Seller with documentation, on a monthly basis, of the total number of BISx Kits installed, the locations of such BISx Kits and the dates of installation. | |||
| ii) | Lead time for the BISx Kit is 10 weeks. |
| Addendum 1 v 1.0 | Initials: |
13/16
| ii) | The BISx Kit is a non-exclusive product, available for sale and distribution world-wide. | ||
| iii) | For purposes of volume calculations, BISx Kit and BISx4 Kit sales will be aggregated on a calendar year basis to determine the pricing levels. | ||
| iv) | Regulatory clearances for integration into patient monitoring system to be completed by Buyer. Seller has received 510(k) for the BISx Kit (K040183) and for the BISx4 Kit (K052981). | ||
| v) | FOB Norwood, Massachusetts, USA | ||
| vi) | Price at the Amendment Effective Date will be set at the [**] level. Notwithstanding anything to the contrary in Section 4(a) of the Agreement, Section F of this Attachment D shall be used to determine the pricing for the BISx Kit. |
C) BIS SENSORS
| Unit of | ||||||||||
| Aspect P/N | GE P/N | Product Description | Measure | Price | ||||||
186-0100 |
2011643-001 | BIS Standard Sensor | Box of 25 | [**] | ||||||
186-0106 |
2011640-001 | BIS Quatro Sensor | Box of 25 | [**] | ||||||
186-0110-GE |
2007374-002 | BIS Pediatric Sensor | Box of 25 | [**] | ||||||
186-0160 |
TBD | BIS Extend Sensor | Box of 25 | [**] | ||||||
186-0212 |
TBD | BIS Bilateral Sensor | Box of 10 | [**] | ||||||
186-0023 |
2007373-001 | ZIP Prep Electrode | Box of 15 | [**] | ||||||
186-0150 |
2007945-002A1 | BIS Quatro Starter Kit | Box of 5 | [**] | ||||||
186-0154 |
TBD | BIS Pediatric Starter Kit | Box of 5 | [**] | ||||||
| i) | Buyer will be allowed to purchase limited BIS Sensor quantities as described in Section 21(a) and as defined in the Distributor Agreements as executed and attached in Attachment P | ||
| ii) | Further sensor sales will be furnished directly by Seller or Seller’s Authorized Representative. | ||
| iii) | The BIS Sensors are non-exclusive products, available for sale and distribution world-wide subject to the conditions in Sections 20 and 21. | ||
| iv) | Lead time for the BIS Sensor is 4 weeks. |
| Addendum 1 v 1.0 | Initials: |
14/16
D) CUSTOM SENSOR
| i) | List price for the Custom Sensor: | ||
| Volume discounts: |
Aspect P/N |
GE P/N | Description | [**] | [**] | [**] | [**] | ||||||||||||||
| 186-0156 | 8002858 | Custom Sensor (Entropy) – Box of 25 | [**] | [**] | [**] | [**] |
| ii) | Lead time for the Custom Sensor is 4 weeks. | ||
| iii) | The Custom Sensor is an Exclusive product. | ||
| iv) | Regulatory clearances and 510(k) numbers maintained by Buyer. | ||
| v) | Warranty on the Custom Sensor is 12 months from date of manufacture. | ||
| vi) | FOB Norwood, Massachusetts, USA. | ||
| vii) | Price at the Amendment Effective Date will be set at the 250,001 – 500,000 level. Notwithstanding anything to the contrary in Section 4(a) of the Agreement, Section F of this Attachment D shall be used to determine the pricing for the Custom Sensor. |
E) SPARE PARTS/ACCESSORY PRICES
| Orderable | ||||||||
| Aspect P/N | GE P/N | Parts/Products | [**] | |||||
186-00157-DO |
900510 | DSC-XP (DO) | [**] | |||||
186-00139-DO |
900505 | BIS Engine PCB (DO) | [**] | |||||
186-00157-GE |
DSC-XP (GE) | [**] | ||||||
186-00139-GE |
BIS Engine PCB (GE) | [**] | ||||||
186-00142 |
2007694-001 | Sensor Cable | [**] | |||||
| i) | These are non-exclusive products, available world-wide. | ||
| ii) | FOB Norwood, Massachusetts, USA. |
F) CALCULATION OF VOLUME DISCOUNT:
For the purpose of calculating the volume discount for a given calendar year, all BIS/EEG
Module Kits, BISx Kits and Custom Sensors shipped during that calendar year (excluding Products
that are provided free of charge) will be included in the total volume discount calculation.
| Addendum 1 v 1.0 | Initials: |
15/16
The initial pricing for a given calendar year is based on the total volume of BISx Kits and
Custom Sensors purchased in the prior calendar year. Buyer will maintain the previous year
pricing as long as they purchase [**]% of the volumes levels required for that pricing. For
example, [**].
If a higher volume level of BISx Kits and Custom Sensors is achieved during a given calendar
year, the price on purchases made after achieving the higher volume level will reflect the
price associated with the appropriate volume level achieved. All price adjustments are
proactive and no credit will apply retroactively to units purchased prior to achieving the
volume break point. For example, if midway through year 1, [**] will reflect the next volume
break. Achieving this higher volume level will also reduce the initial pricing for the
following year.
G) CURRENCY
United States Dollars
| Addendum 1 v 1.0 | Initials: |
16/16
Attachment A
Product Specifications
Product Specifications
BISx System
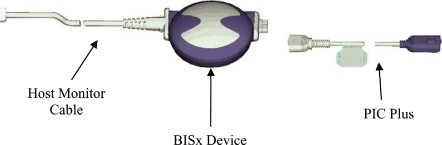
PRODUCT DESCRIPTION
The BISx Kit includes a PIC+, BISx Device, and a Host Monitor Cable. The BISx Kit will here forth
be referred to as BISx. The BISx4 Kit includes a 4 channel PIC, BISx4 Device, and a Host Monitor
Cable. The BISx4 Kit will here forth be referred to as BISx4.
BISx is a device that acquires up to two channels of EEG and computes BIS and other EEG parameters.
BISx4 is a device that acquires up to four channels of EEG and computes BIS and other EEG
parameters. Both the BISx and BISx4 are designed to mate with Aspect’s XP platform 1 or 2 channel
sensors. The BISx4 is designed to additionally interface with Aspect’s Bilateral sensor, which is
required for acquisition of 4 channels of EEG. BISx and BISx4 have no display or user interface.
They plug into a host monitor system for display of EEG and processed parameters. BISx and BISx4
are designed for use wherever sedative drugs are administered, including but not limited to the
following environments: Operating rooms, Intensive Care Units, Procedural Sedation, and Clinical
Research areas.
BISx and BISx4 interface to one or more of the following interfaces: standard RS-232 asynchronous
interface, RS-232 type asynchronous interface but with TTL 3.3V signal levels, or Universal Serial
Bus (USB) interface. All interface versions also support USB interfacing for software upgrade and
download purposes. BISx and BISx4 can be connected and disconnected to an already powered up host
monitor. The host monitor should automatically detect its presence and configure it accordingly.
The BISx connects to a sensor via the Aspect Patient Interface Cable (here forth referred to as
PIC+). The PIC+ is nominally 4.5 ft. long. The PIC+ connection is integral to the enclosure (no
pigtail), and can be detached from the BISx device for service or replacement without the use of
tools. The enclosure is sealed against liquid ingress even when the PIC+ is detached.
The BISx4 connects to a sensor via the Aspect 4 channel Patient Interface Cable (here forth
referred to as CM-PIC). The CM-PIC is nominally 54 inches long. The CM-PIC connection is integral
to the enclosure (no pigtail), and can be detached from the BISx4 device for service or replacement
without the use of tools. The enclosure is sealed against liquid ingress even when the PIC+ is
detached.
The BISx and BISx4 is attached to the host monitoring system via a nominal 9-foot monitor cable.
The wire is narrow and highly flexible multi-conductor cable used to supply power from the host
monitor to the BISx/BISx4 as well as allow communication between the devices. Additionally, the
inability of the host monitor to provide the BISx specified power requirements or communication
requirements may be addressed through the inclusion of a circuit which is integral to the host
monitor cable.
The host monitor cable connection to the BISx/BISx4 device is integral to the enclosure (no
pigtail), and may require the use of tools for detachment from the BISx/BISx4 device enclosure for
the purposes of service or replacement. The enclosure is sealed against liquid ingress only when
the monitor cable is attached. There are no adjustable parts inside the BISx. The cables may be
replaced without opening the enclosure.
The BISx and BISx4 include software which is stored in reprogrammable FLASH memory. Software
upgrades can be accomplished via the serial / USB interface. Each BISx and BISx4 is given a unique
serial identifier, allowing for electronic identification and tracking of every BISx/BISx4.
TECHNICAL SPECIFICATIONS
(Unless otherwise noted specifications apply to both the BISx and BISx4)
(Unless otherwise noted specifications apply to both the BISx and BISx4)
Physical Specifications
Output Parameters
The following information is provided to the patient monitor by the BISx and BISx4:
| • | BIS (Bispectral Index) | ||
| • | EMG (Electromyographic strength) | ||
| • | SQI (Signal Quality Index) | ||
| • | SR (Suppression Ratio) | ||
| • | EEG (Continuous, two channel, real-time EEG waveform) | ||
| • | SEF (Spectral Edge Frequency) |
| • | TP (Total Power) | ||
| • | BC (Burst Count) – Extend Sensor only |
Electrode-to-skin impedances are continuously measured.
Filters
Users can select from a range of low-pass and high-pass filters which are available for artifact
rejection. The low-pass filters allow the rejection of muscle artifacts. The high-pass filters
allow the rejection of ECG signal interference. In addition, a notch filter suppresses line
frequency interference.
The BIS XP technology built into the BISx system suppresses interference from electrocautery, EMG,
and other high frequency noise sources.
BISx Kit
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
| Weight: | approx 8.0 oz without cables approx 10.0 oz including PIC+ approx 1 lb. including host cable |
BISx Integral Cables
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
BISx4 Kit
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
Weight: 227g (8.0 oz) without cables
Weight: 227g (8.0 oz) without cables
BISx4 Integral Cables
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (CM-PIC): 1.4m (4.5 ft)
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (CM-PIC): 1.4m (4.5 ft)
Safety Specifications
| • | The BISx and BISx4 comply with the essential requirements of the Medical Device Directive 93/42/EEC, as well as IEC 60601-1 and IEC 00000-0-00. | |
| • | EMC RF Emissions: CISPR-11 Class A | |
| • | They are both Type BF applied parts. Both the BISx and BISx4 have internal optical coupling and an isolation transformer for patient isolation. | |
| • | Both are protected against damage from defibrillation as long as the sensor is not located between the defibrillator pads and are resistant to artifact from electrosurgery. | |
| • | United States federal law restricts these devices to sale by or on the order of a physician. | |
| • | BISx and cables are latex free. |
Environmental Specifications
Liquid Ingress: IEC 529 IPX4
Temperature
Liquid Ingress: IEC 529 IPX4
Temperature
0 to 40 degrees C operating
-40 to 70 degrees C storage
-40 to 70 degrees C storage
Humidity
10% to 95% non-condensing operating
10% to 95% non-condensing storage
10% to 95% non-condensing storage
Pressure
480 to 1067 hPa operating (Approx -1250 to 20,000 feet)
116 to 1067 hPa storage (Approx -1250 to 50,000 feet)
116 to 1067 hPa storage (Approx -1250 to 50,000 feet)
Performance Specifications
Measurement Ranges
Bispectral Index (BIS): 0 to 100 unitless scale
Electromyographic Strength (EMG): 30 to 80 dB where 1µV2=40dB
Signal Quality Index (SQI): 0 to 100%
Suppression Ratio (SR): 0 to 100%
Spectral Edge Frequency (SEF): 0.5 to 30.0 Hz
Total Power: 40 to 100 dB where 1µV RMS=40dB
Burst Count: 0 to 30
Measurement Ranges
Bispectral Index (BIS): 0 to 100 unitless scale
Electromyographic Strength (EMG): 30 to 80 dB where 1µV2=40dB
Signal Quality Index (SQI): 0 to 100%
Suppression Ratio (SR): 0 to 100%
Spectral Edge Frequency (SEF): 0.5 to 30.0 Hz
Total Power: 40 to 100 dB where 1µV RMS=40dB
Burst Count: 0 to 30
Noise (EEG Waveform) < 0.3 µVRMS (2.0µV peak-to-peak)
BIS Numeric Update Frequency Once per second
EEG Bandwidth 0.25 to 100 Hz (- 3dB)
Patient Leakage <10uA
BIS Numeric Update Frequency Once per second
EEG Bandwidth 0.25 to 100 Hz (- 3dB)
Patient Leakage <10uA
BIS/EEG Module Kit
The BIS/EEG Module Kit is designed specifically for OEM applications and allows the integration of
Aspect’s BIS monitoring technology into OEM equipment. The BIS/EEG Module Kit will interface to
the patient via the Aspect BIS Sensor and to the OEM equipment utilizing a serial (RS-232) 3-wire
interface and the necessary power connections.
The BIS/EEG Module Kit consists of a Digital Signal Converter (DSC-XP) that is placed in proximity
to the patient and a small circuit board that resides in the OEM equipment. The DSC-XP is a small
(palm sized) front-end to the BIS Engine circuit board that provides the patient interface and
performs the high performance analog to digital conversion of the EEG signals. The EEG signals are
transmitted in digital format from the DSC-XP to the BIS Engine circuit board via a 12 foot cable
that is hard wired at the DSC-XP.
The small BIS Engine circuit board performs digital signal processing on the digitized EEG signal
and outputs the Bispectral Index to the OEM system via the RS-232 serial connection. The board is
constructed using double-sided surface mount techniques. The connections to the BIS Engine circuit
board are a serial interface (RS-232), power, and DSC connections. Two means of connecting to the
BIS Engine are provided: board-to-board connectors on one side and keyed cable connectors on the
opposite side.
Detailed Technical Specifications:
Digital Output:
|
RS-232 Serial Output | |||
| (8 data, 1 stop, no parity, 57,600 Baud) | ||||
Main Parameters:
|
BIS, Suppression Ratio, EMG, Raw EEG | |||
Electrical Safety:
|
Conforms to IEC-601-1 | |||
Power:
|
4 Xxxxx Maximum (2 Xxxxx Typical) | |||
| +5V (450 mA) | ||||
| +12V (150 mA) |
EMC RF Emissions: CISPR-11 Class A
Note: Most of the 12V supply power is dissipated by the DSC-XP that is located near the patient and is therefore external to the module housing. Maximum calculated power dissipated within the module housing is 2.5 Xxxxx.
Note: Most of the 12V supply power is dissipated by the DSC-XP that is located near the patient and is therefore external to the module housing. Maximum calculated power dissipated within the module housing is 2.5 Xxxxx.
Environmental Requirements:
| IEC601-1 | BIS Engine | |||||
Operation
|
Temperature Range | +10ºC to +40ºC | 0ºC to +55ºC (BIS Module) | |||
| Humidity (non-condensing) | 25% to 95% | 10% to 95% | ||||
| Atmospheric Pressure | 700 hPa to 1060 hPa | 465 hPa to 1067 hPa (up to 20,000 ft.) |
Artifact Rejection: Automatic
Bispectral Index: 0-100 unitless scale
Bispectral Index: 0-100 unitless scale
Digital Signal Converter (DSC-XP)
Description:
|
The DSC amplifies and digitizes the signal close to the patient to minimize electrical interference. | |
Weight:
|
4.7 oz (0.13 kg) (not including cables) | |
Dimensions:
|
2.6 x 1.0 x 4.3 inches
(6.6 x 2.5x10.8 cm) |
|
Cable Length:
|
12 ft (3.7 m) integral DSC cable, 4 ft (1.2 m) patient interface cable. | |
BIS Engine PCB |
||
Physical:
|
3x4 inch SMT PCB | |
Processing Power:
|
T.I. TMS320VC33-120 (~120 MFLOPS) |
Software Upgrades
The BIS engine software is stored in reprogrammable FLASH memory. Software upgrades can be
accomplished via the serial interface.
Serial Identifier
Each BIS engine is given a unique serial identifier. This allows for electronic
identification/tracking of every BIS Engine.
BIS XP Sensor
Overview:
The Sensor XP (also called Smart Sensor) is a single patient use disposable pre-gelled electrode
array containing 4 electrodes. It is applied directly to the patient’s forehead and temple to be
used in conjunction with Bispectral Index monitors, BIS/EEG Module Kits and BISx Systems. The XP
Sensor is designed to provide ease of use and electrode placement accuracy. It collects EEG
signals from the forehead and the temple areas.
The XP Sensor contains Aspect’s ZipPrep technology, a polymer material embedded in the electrolyte
that displaces the upper layer of skin and allows the electrolyte to quickly hydrate the top layer
of skin. This patented technology lowers the electrical impedance at the epidermis-gel interface.
The XP Sensor connects to the monitor, module or BISx system via a single connector that is low
profile and easy to insert and remove. The XP Sensor contains an electronic Smart Card memory
device (SST) that stores information concerning the sensor including:
Lot #
Expiration Date
BIS Configuration (Sensor Type)
Sensor Integrity / Validation
Expiration Date
BIS Configuration (Sensor Type)
Sensor Integrity / Validation
XP Sensors:
The following sensors are considered to be part of the XP Sensor family.
| • | Quatro Sensor | ||
| • | Extend Sensor | ||
| • | Pediatric Sensor |
Intended Use:
| • | To be used in conjunction with the BIS Monitoring System | ||
| • | Single patient use device. | ||
| • | Disposable | ||
| • | Adult patient use (Quatro and Extend) | ||
| • | Pediatric patient use (Pediatric) | ||
| • | Rx Only |
Length of Use (Duration):
Up to 24 hours from initial application
Smart Card Module (SST)
The Smart Card module is a self-contained integrated circuit that contains digital information
about the sensor. It utilizes smart card technology that prevents tampering.
Shelf Life:
|
12 months at 24C storage | |
Shipping Temperature:
|
-10C to 50C max | |
Operating Temperature:
|
10C to 40C max |
Custom Sensor
Overview:
The Custom Sensor is a single patient use disposable pre-gelled electrode array with three
electrodes. It is applied directly to the patient’s forehead and temple. The Custom Sensor is
designed to provide ease of use and electrode placement accuracy. It collects EEG signals from the
forehead and the temple areas.
The Custom Sensor contains Aspect’s ZipPrep technology, a polymer material embedded in the
electrolyte that displaces the upper layer of skin and allows the electrolyte to quickly hydrate
the top layer of skin. This patented technology lowers the electrical impedance at the
epidermis-gel interface.
The Custom Sensor connects to the monitor via a single connector that is low profile and easy to
insert and remove. The Custom Sensor contains an electronic Smart Card memory device (SST) that
stores information concerning the sensor including:
Lot #
Expiration Date
BIS Configuration (Sensor Type)
Sensor Integrity / Validation
Expiration Date
BIS Configuration (Sensor Type)
Sensor Integrity / Validation
Length of Use (Duration):
Up to 24 hours from initial application
Up to 24 hours from initial application
Smart Card Module (SST)
The Smart Card module is a self-contained integrated circuit that contains digital information
about the sensor. It utilizes smart card technology that prevents tampering.
Shelf Life:
|
12 months at 24C storage | |
Shipping Temperature:
|
-10C to 50C max | |
Operating Temperature:
|
10C to 40C max |
Medical Products Distribution Agreement
AMENDMENT 2
Attachment P
Independent Distribution Template
Attachment P
Independent Distribution Template
Between
Aspect Medical Systems, Inc.
And
General
This Distribution Agreement between Aspect Medical Systems, Inc. (referred to herein as
“Aspect” or “AMS”), with its principal offices at Xxx Xxxxxx Xxxx, Xxxxxxx, XX,
00000, XXX, and (referred to herein as “Distributor”), with its principal
offices at , (each, a “Party,” and collectively, the
“Parties”) is effective , 20___(“Effective Date”).
Whereas AMS manufactures medical products and seeks to establish a distribution channel in a
certain Territory as defined below; and,
Whereas Distributor distributes medical products and seeks to distribute additional products
to its customers; and,
Whereas AMS desires to appoint Distributor as an authorized distributor in a certain Territory
of certain medical products, accessories and related goods to be supplied by AMS and Distributor
desires to accept such appointment; and,
Whereas, AMS and Distributor’s affiliate, General Electric Company, acting by and through its
GE Healthcare division (“GE”), have executed a purchase agreement dated ___, 2005 (as amended) for
the licensing and sale of specified AMS Products (“Purchase Agreement”) under which the parties
have negotiated and agreed upon certain terms and conditions for the delivery of AMS Products
Therefore Distributor agrees to purchase and AMS agrees to sell the Products upon the
following terms and conditions:
Article 1
Definitions
Definitions
Any capitalized terms not identified in this Article have the meaning set forth in the
Purchase Agreement. The following terms have the meaning indicated here when used in this
Agreement:
“Customers”: Those persons and entities who purchase Products from, or to whom Products are
marketed by, Distributor in accordance with the provisions of this Agreement.
“Equipment”: The hardware products set forth on Exhibit 1.
“Prices”: Net prices at which AMS shall sell Products to Distributor as described in Article
8.
“Products”: The Equipment and the Software.
“Purchase Targets”: The purchase targets set forth in Exhibit 4 for the Initial Term,
and as mutually agreed in a Change Order Form signed by the Parties for any Renewal Terms.
“Software”: The computer programs, in object code form only, and ancillary related
documentation for the operation of the Equipment.
“Software License Agreement”: AMS’s standard end user license agreement for the Software, as
in effect from time to time.
“Territory”: Locations as set forth in Exhibit 2.
“Written Consent”: Consent as evidenced in a written Addendum to this Agreement or in a
Change Order Form signed by an authorized representative of both Parties. For clarity it is
understood that Written Consent does not include consent as evidenced in a letter sent by a Party
unless countersigned by the other Party.
Article 2
Appointment
Appointment
2.1 AMS hereby appoints Distributor as a non-exclusive distributor for the Products in the
Territory. Distributor may not appoint a secondary or sub-distributor to sell, lease or license
Products without AMS’s prior Written Consent.
2.2 If the Territory does not include any country in the European Economic Area or Switzerland
(collectively, the “EEA”), Distributor may not distribute the Products outside the
Territory without AMS’s prior Written Consent, which consent may be withheld in the sole discretion
of AMS. If the Territory includes any country in the EEA, (a) Distributor may not, without AMS’s
prior Written Consent, which consent may be withheld in the sole discretion of AMS, distribute the
Products outside the EEA; and (b) Distributor may not actively sell the Products outside the
Territory but inside the EEA, if such sale is into a territory or to a customer group which is
either reserved to AMS or allocated by AMS to another distributor, but Distributor may passively
sell the Products into such territory or to such customer group. “Actively sell” mean
actively approaching individual customers inside another distributor’s exclusive territory or
exclusive customer group by, for instance, direct mail or visits; or actively approaching a
specific customer group or customers in a specific territory allocated exclusively to another
distributor through advertisement in media or other promotions specifically targeted at that
customer group or targeted at customers in that territory; or establishing a warehouse or
distribution outlet in another distributor’s exclusive territory. “Passively sell” mean
responding to unsolicited requests from individual customers including delivery of goods or
services to such customers. General advertising or promotion in media or on the Internet that
reaches customers in other distributors’ exclusive territories or customer groups but which is a
reasonable way to reach customers outside those territories or customer groups, for instance to
reach customers in nonexclusive territories or in one’s own territory, are passive sales.
2.3 Distributor hereby acknowledges and agrees that this Agreement confers only non-exclusive
rights upon Distributor. Without limiting the generality of the foregoing, AMS expressly reserves
the right (a) to market, sell, lease or license on its own behalf, either directly or through one
or more subsidiaries or divisions, the Products in the Territory, and (b) to appoint any person
other than Distributor to act as a distributor, original equipment manufacturer, value
added reseller, agent or representative with respect to the marketing, sale or licensing of
Products in the Territory.
-2-
2.4 Except as expressly set forth in this Agreement or the Purchase Agreement, AMS and/or its
licensors and suppliers retain all rights with respect to the Products, any intellectual property
rights therein or the Trademarks (as defined below) and no rights or licenses with respect thereto
are granted to Distributor.
2.5 Unless specifically addressed in the terms of this Agreement, the parties’ distribution
relationship shall be governed by any applicable terms and conditions of the Purchase Agreement.
2.6 [Insert terms to be negotiated at time of entering distribution relationship]
Article 3
Relationship
Relationship
3.1 [In Purchase Agreement] [In Purchase Agreement]Neither Party shall hold itself out as the
agent of the other, nor shall it incur any indebtedness or obligations in the name of, or which
shall be binding on, the other Party, without the prior Written Consent of the other Party. Each
Party assumes full responsibility for its own personnel under laws and regulations of the
governmental authorities of the competent jurisdiction.
3.2 In addition to other requirements set forth in the Agreement, the Distributor shall:
(a) [Insert terms to be negotiated at time of entering distribution
relationship]
In the event that AMS believes in its reasonable discretion that Distributor has not satisfied
the obligations specified in this Article 3.4, AMS may terminate the Agreement in accordance with
the terms of Article 6.2.
Article 4
Term of Agreement
Unless earlier terminated pursuant to the terms hereof, this Agreement shall remain in effect
until (“Initial Term”). Thereafter, unless terminated by either Party
with at least 45 days’ notice prior to said expiration date, this Agreement shall be automatically
renewed for successive one year terms (each a “Renewal Term”). At the commencement of the
Renewal Term and continuing for a period of thirty (30) days thereafter, the Parties shall
negotiate in good faith for Purchase Targets for the then-current Renewal Term. If, by the end of
the thirty (30) day period, the Parties have not confirmed revised Purchase Targets with Written
Consent, the Agreement will terminate immediately.
Article 5
Termination
Termination
5.1 [Following terms to be negotiated at time of distribution contracting.]
-3-
5.2 Termination of this agreement will have no affect on the Purchase Agreement or the rights
of GE to distribute products granted under the Purchase Agreement.
5.3 If a Party materially breaches this Agreement (the “Breaching Party”), the other
Party (the “Aggrieved Party”) may provide written notice of such breach to the Breaching
Party. If the Breaching Party does not cure such breach within forty-five (45) of its receipt of
such notice, the Aggrieved Party may terminate this Agreement immediately upon written notice to
the other Party delivered after such forty-five (45) cure period. Such termination shall be
effective immediately upon the Breaching Party’s receipt of such termination notice.
5.4 Notwithstanding any other termination provision, AMS shall have the right to terminate
this Agreement by providing written notice to Distributor in the event that Distributor does not
meet the Purchase Targets for two (2) consecutive calendar quarters. Such termination shall be
effective immediately upon receipt by Distributor of such notice of termination.
5.5 Upon termination or expiration of this Agreement Distributor shall immediately (a) cease
from acting as a distributor of Products and abstain from making further distribution of Products
except with the written approval of AMS; (b) pay to AMS, in full within thirty (30) days of such
termination, all amounts owed to AMS; (c) promptly return to AMS all AMS-owned Products, or other
equipment, materials, documentation or data, including without limitation all Software, in the
possession of Distributor; (d) cooperate with AMS in completing all outstanding obligations to AMS;
and (e) refrain from representing itself as an authorized AMS distributor and from using any AMS
trademark or trade name. Distributor agrees that neither it nor its employees shall be entitled to
any compensation or severance payment resulting from the fact of the termination of this Agreement
or relating to any goodwill created by Distributor, and whether relating to loss of prospective
sales, investments, compensation or goodwill. Distributor, for itself and on behalf of its
employees, hereby waives any right it may have under any applicable laws with respect to any such
payments, including but not limited to applicable termination, labor or other similar laws or
regulations.
5.6 During the Repurchase Period (as defined herein), AMS shall have the right, but not the
obligation to repurchase any or all of the Products which Distributor may have in its possession at
the time of termination, or to arrange the purchase of such Products by successor distributors or
customers in the Territory. Any purchase pursuant to this Section 5.6 shall be at the Price paid
by the Distributor to Aspect for such Products, unless another price is mutually agreed by the
parties. Any Products repurchased by AMS pursuant to this Section 5.6 shall be shipped by
Distributor freight prepaid, according to AMS’s instructions, and AMS shall pay Distributor for
such repurchased Products within thirty (30) days after AMS receives such Products at its
designated facility. As used herein the term “Repurchase Period” means the period
beginning on the date of the termination or expiration of this Agreement and expiring on the
earlier of (a) ninety (90) days from the date of termination or expiration of this Agreement, or
(b) the date on which AMS given notice to Distributor or its intention not to repurchase any
Products. In the event AMS fails to purchase, or to arrange the purchase of, such Products prior
to the expiration of the Repurchase Period, Distributor shall be permitted to sell its existing
inventory of Products in the Territory for a period of ninety (90) days following the expiration of
the Repurchase Period, provided that Distributor is otherwise in compliance with the terms of this
Agreement.
-4-
Article 6
Modification of Agreement
Modification of Agreement
6.1 Distributor may not delete any Products from Exhibit 1 without the prior written
approval of AMS, which may be withheld in its sole discretion.
Article 7
Prices and Payments
Prices and Payments
7.1 Distributor has sole discretion over the prices it charges the Customers.
7.2 For all Products ordered hereunder, Distributor shall pay AMS the Price specified in
Exhibit 1.
7.3 The Prices set forth in Exhibit 1 are fixed until December 31 of the first
calendar year of this Agreement. [Further terms to be negotiated at time of contracting]
Thereafter, AMS shall have the right to revise the prices annually, effective January 1 of each
year, by giving Distributor thirty (30) days prior notice of such change.
7.4 Prices are exclusive of insurance and any federal, state, municipal, excise, sales, use or
other similar taxes now in force or enacted in the future, all of which shall be paid by
Distributor, except for such taxes as are imposed on AMS’s net income, which shall be paid by AMS.
AMS may invoice Distributor for any such insurance and taxes, and remit any tax payments made on
any such invoice directly to the appropriate taxing authorities. Distributor is responsible for
obtaining and providing to AMS any certificate of exemption or similar document required to exempt
any sale or license from sales, use or similar tax liability.
7.5 Payment terms are governed by the terms of the Purchase Agreement.
Article 8
Order, Shipment and Delivery
Order, Shipment and Delivery
8.1 [To be negotiated at time of contracting]
Article 9
Advertising, Promotions, Trademarks and Copyrighted Material
Advertising, Promotions, Trademarks and Copyrighted Material
9.1 Distributor shall use commercially reasonable efforts to promote, sell and license the
Products to Customers in the Territory, as specifically defined in the Purchase Agreement or as
otherwise specified below:
9.2 [Insert terms at time of contracting]
9.3 AMS agrees to provide reasonable quantities of current or new sales literature, artwork,
advertising materials, promotional plans and other information or programs reasonably related to
this Agreement in relevant languages as mutually agreed by the Parties. Distributor-
-5-
specific literature and advertising will be the responsibility of Distributor, provided
however that such literature shall be consistent in all respects with the documentation supplied by
AMS and shall not include any claims, warranties or information concerning any Products that are
not included in the documentation supplied by AMS. All Distributor-specific literature and
advertising shall be subject to AMS’s prior written approval, which shall not be unreasonably
withheld.
9.4 Use of promotional materials shall be governed by the terms of the Purchase Agreement.
Article 10
Sales and Support
Sales and Support
10.1 Distributor agrees to purchase a reasonable quantity of demonstration Products and to
maintain a reasonable number of trained staff capable of demonstrating and selling the Products.
Distributor agrees to have its sales personnel attend, at Distributor’s expense, AMS’s sales and
marketing meetings, Product and competitive training courses and Product launch meetings, as AMS
may reasonably request.
10.2 AMS agrees to provide technical, service and educational support to the Distributor at
the commencement of this Agreement to show Distributor how the Products work; how to service the
Products; and how to educate clinicians in order to have clinicians integrate the Products into
their practice.
Distributor agrees to work with clinicians, both before they acquire Products and after, to
teach them how integrate the Products into their practice.
10.3 Distributor agrees, at its own expense, to stock a reasonable quantity of necessary spare
parts and test equipment according to AMS’s recommendation to support Products installed in the
Territory. Out-of-warranty spare parts are at Distributor’s cost, as set forth in Article 17.
10.4 Distributor shall use its reasonable efforts to handle and resolve feedback from the
Customers. Problems that cannot be resolved locally will be escalated in accordance with Article
26. Except as provided in Section 4.3, in no event shall Distributor have the right to any source
code for the Software.
Article 11
Quality Assurance, Regulatory Compliance
Quality Assurance, Regulatory Compliance
11.1 The parties’ obligations with respect to regulatory compliance and quality assurance
shall be governed by the terms of the Purchase Agreement.
-6-
Article 12
Modification of Products
Modification of Products
12.1 Packaging and marketing of the Products, including requests for modifications, shall be
governed by the terms of the Purchase Agreement.
Article 13
Import Licenses, Product Localization
Import Licenses, Product Localization
13.1 Aspect’s rights and obligations with respect to compliance with laws and regulations,
including but not limited to international trade controls, shall be governed by the terms of the
Purchase Agreement.
13.2 Distributor shall comply with all laws which are applicable to Distributor in connection
with the performance of its obligations under this Agreement, including, without limitation, the
Foreign Corrupt Practices Act.
Article 14
Warranty and Limitation of Remedies
Warranty and Limitation of Remedies
14.1 AMS product warranties and limitations of remedies with respect thereto are set forth in
and governed by the terms of the Purchase Agreement.
Article 15
Complaints, Quality Records and Recalls
Complaints, Quality Records and Recalls
All regulatory compliance obligations of the parties are set forth in the Purchase Agreement.
Article 16
Limitation of Liability
Limitation of Liability
16.1 The limitation of liability for the parties set forth in Section 25(f) of the Purchase
Agreement shall apply to all claims or liabilities arising in connection with the performance of
the parties’ respective obligations hereunder.
Article 17
APPLICABLE LAW AND DISPUTE RESOLUTION
APPLICABLE LAW AND DISPUTE RESOLUTION
Any dispute, controversy or claim arising from or relating to this Agreement shall be
resolved in accordance with the dispute resolution procedures set forth in Attachment F of the
Purchase Agreement. The parties expressly acknowledge that the laws of the State of New York,
except its conflict of law rules, will govern the relationship of the parties and any dispute under
this Agreement.
-7-
Article 18
Entire Agreement
Entire Agreement
This Agreement, which includes the Exhibits attached hereto, together with the Purchase
Agreement, contains the entire agreement of the Parties regarding the subject matter hereof and
sets forth the entire understanding between the Parties with respect to its subject matter and
supersedes all prior and contemporaneous agreements, promises, purchase orders, proposals,
representations, understandings and negotiations, whether written or oral, between the Parties
concerning the subject matter hereof. The Parties acknowledge that there are no oral or implied
agreements or other modifications not specifically set forth herein. No modification of this
Agreement may be made except by Written Consent. Any terms and conditions in any purchase order or
other instrument issued by Distributor, AMS or any Customer in connection with this Agreement which
are in addition to or inconsistent with the terms and conditions of this Agreement shall not be
binding on the other party. Distributor and AMS each acknowledge that it is not entering into this
Agreement on the basis of any representations not expressly contained herein.
Article 19
Captions and Headings
Captions and Headings
The captions and headings appearing in this Agreement are for reference purposes only and
shall not be considered a part of this Agreement. Such captions and headings shall not modify,
amend or affect the provision hereof. This Agreement has been jointly prepared and shall not be
strictly construed against either Party hereto.
Article 20
Waiver and Severability
Waiver and Severability
Either Party may, at its option, choose to delay enforcing or waive any of its rights under
this Agreement in certain circumstances. Waiver of a particular right does not thereby waive the
same right in other circumstances. Either Party may delay enforcing or waive any of its rights
under this Agreement without affecting any of its other rights.
If, under applicable law or regulation, any provision of this Agreement is invalid or
unenforceable, or otherwise directly or indirectly affects the validity of any other material
provision(s) of this Agreement (“Severed Clause”), it is mutually agreed that this
Agreement shall endure except for the Severed Clause. The Parties shall consult and use their best
efforts to agree upon a valid and enforceable provision which shall be a reasonable substitute for
such Severed Clause in light of the intent of this Agreement.
Article 21
Registration
Registration
In the event that this Agreement is required to be registered with any governmental authority,
Distributor shall cause such registration to be made and shall bear any expense or tax payable in
respect thereof.
-8-
Article 22
Counterparts
Counterparts
This Agreement may be executed in one or more counterparts, each of which shall be deemed an
original, but all of which together shall constitute one and the same instrument.
By signing this document, the Parties below indicate their agreement with and acceptance of
this Agreement, including all Exhibits.
[Remainder of Page Intentionally Left Blank]
-9-
SIGNATURES
| Aspect Medical Systems, Inc. | ||||||||||||
| (“AMS” or “Aspect”) | (“Distributor”) | |||||||||||
| Authorized Representative Signature | Authorized Representative Signature | |||||||||||
Name:
|
Name: | |||||||||||
Title:
|
Title: | |||||||||||
Date:
|
Date: | |||||||||||
-10-
Exhibit 1 — Products and Prices
The following Exhibit is attached to and forms part of the Distribution Agreement between
AMS and
AMS and
All Prices shown in this Exhibit 1 are stated in [US Dollars].
[Product and Price list to be reviewed and updated by AMS]
| PART | DESCRIPTION | PRICE | ||
-11-
Exhibit 2 — Territory
The following Exhibit is attached to and forms part of the Distribution Agreement between
AMS and
AMS and
The following Territory is established for the above referenced Agreement.
| Countries/Regions | ||||
-12-
Exhibit 3 — General Provisions
The following Exhibit is attached to and forms part of the Distribution Agreement between
AMS and
AMS and
Any notice pursuant to this Agreement should be sent to the Address(s) below in accordance with
Article 27 of the Agreement:
If to Distributor:
[ ]
If to Aspect Medical Systems, Inc.:
Xxxx Xxxxxxxxx
Vice President & CFO
Aspect Medical Systems
Xxx Xxxxxx Xxxx
Xxxxxxx, XX 00000
XXX
Xxxx Xxxxxxxxx
Vice President & CFO
Aspect Medical Systems
Xxx Xxxxxx Xxxx
Xxxxxxx, XX 00000
XXX
Ship-to address for Products from AMS to [ ]
Invoice-to address for Products from AMS to [ ]
-13-
Exhibit 4 — Purchase Targets
The following Exhibit is attached to and forms part of the Distribution Agreement between
AMS and
AMS and
[To be negotiated at time of contracting]
Purchase Targets/Units 200X
| Q1 | Q2 | Q3 | X0 | |||||||||||||||
X-0000 BIS monitor
|
1 | 1 | 0 | 1 | Total Calendar Year 200X = | |||||||||||||
Sensors
|
Total Calendar Year 200X = | |||||||||||||||||
SRS
|
Total Calendar Year 200X = | |||||||||||||||||
-14-
| GE Healthcare Global Quality Form GEHC_GQP_11.01.003_F001 Purchased Material Quality Requirements |
 |
Supplier
Name: |
||
Applicable To All Suppliers:
Section 1
— Quality Systems: The Supplier shall maintain a documented quality system. The
ISO9000 system is an example of an acceptable quality standard. Key components of a robust quality
system are properly implemented quality procedures for collection and processing defects (parts or
services), appropriate statistical techniques to analyze defects and identifying opportunities for
corrective and preventive actions, along with evidence of validation that actions are effective in
eliminating and preventing further defects. Upon request from GE Healthcare, the Supplier shall
provide documented corrective action plans to prevent future deviations from the specification
within thirty (30) days from receiving a corrective action request from GE Healthcare.
To ensure products and services provided to GE Healthcare shall meet or exceed GE Healthcare
requirements, GE Healthcare may audit the Supplier’s quality system at periodic intervals upon
written advance notification. GE Healthcare may also request periodic, joint quality assurance
meetings at the Supplier’s facility to update the status of product quality and reliability.
Section 2
— Quality Record Retention: If the Supplier is required to perform acceptance
activities per GE Healthcare written agreement or purchase specification, the Supplier shall
maintain records of the acceptance activities for the services performed and/or products and
services delivered to GE Healthcare. These records may include as appropriate test/inspection
criteria, revision level of documents/equipment/software used, operating procedures (planning,
routing or traveler sheets), dates of test/inspection, and the results. The records required shall
be retained until GE Healthcare notifies the Supplier that the product life has ended or for a
minimum of fifteen (15) years, whichever is longer, unless the required records are submitted to GE
Healthcare by written agreement or purchase specification.
Section 3
— Compliance: The Supplier shall comply with the terms of the Purchase Order or
purchase agreement with GE Healthcare (“Agreement”). The Supplier shall maintain compliance with
the laws and government regulations that apply in the manufacturing and delivery of its products or
services. Such laws may include, but are not limited to, regulations and directives, labor laws,
environmental laws, Custom Trade Partnership Against Terrorism (CTPAT) and product safety laws. The
Supplier shall provide GE Healthcare all information requested that is necessary to enable GE
Healthcare to comply with the laws and regulations applicable to the GE Healthcare sale and use of
GE Healthcare products. As per GE Healthcare purchase specifications or for OEM (Original Equipment
Manufacturer) items, the Supplier shall maintain compliance to industry standards and product
listings for all Products delivered to GE Healthcare.
Section 4
— Change Notification: Changes proposed by Supplier, both material and process or
software changes, which may affect form, fit, function, reliability, serviceability, performance,
functional interchangeability, regulatory compliance, safety, options or spare parts
interchangeability or interface capability with GE
Healthcare Product must be submitted along with a written change notice, for GE Healthcare
approval. This includes, but is not limited to, changes of sources of material and parts, changes
in manufacturing processes, test procedures, manufacturing locations, relocation or replacement of
equipment and any similar changes that are anticipated by Sub-Suppliers. Items
Before using this document, make sure it is the latest revision. Access My Workshop to verify the current revision. If you
do not have access to, or are unfamiliar with, the My Workshop system, consult your quality representative.
do not have access to, or are unfamiliar with, the My Workshop system, consult your quality representative.
DOC0288647
Page 1 of 3
| GE Healthcare Global Quality Form GEHC_GQP_11.01.003_F001 Purchased Material Quality Requirements |
 |
affected by such changes may not be delivered to GE Healthcare until the Supplier has received
written approval for the changes from GE Healthcare. At minimum, the change notice must include the
Supplier’s affected part number or software revision, date of implementation, serial number
effectivity of the assembly that is changed, reason for the change, specific details of the change
and, if available, supporting data that demonstrates that part reliability has not been impacted
negatively. The change must not be implemented without prior written consent from GE Healthcare,
which shall not be unreasonably withheld. In addition, GE Healthcare has the right to request
samples for evaluation prior to approval by GE Healthcare of such changes.
Section 5 — Engineering Specifications: The Supplier is responsible to meet or exceed
the part requirements and specifications as referenced in the Agreement. The Supplier is
accountable to ensure that delivered items meet the requirements of the revisions and/or versions
specified on the applicable Agreement.
The Supplier shall ensure that GE Healthcare documentation is controlled and distributed with the
correct revision level to the appropriate personnel that produce the product for GE Healthcare. The
Supplier shall also ensure that all GE Healthcare documentation is treated as proprietary and
confidential.
For GE Healthcare designed Products, the Supplier is responsible for ensuring that all applicable
GE Healthcare documentation is provided to all of the Supplier’s Sub-Suppliers involved in the
supply of product for GE Healthcare. The Supplier shall ensure that both they and their
Sub-Suppliers that use GE Healthcare engineering documentation are maintained in compliance with
all accepted Engineering Change Requests/Engineering Change Orders issued by GE Healthcare.
Upon request from GE Healthcare, the Supplier shall ensure that part qualification is conducted and
documents are submitted as required. The part qualification requirements shall be determined by GE
Healthcare and shall consist of at a minimum a part layout plan, capability study, and a process
control plan.
Section 6 –– Electrostatic Discharge: If Electrostatic Discharge (ESD) sensitive devices
are supplied to GE Healthcare, the Supplier must have an active ESD program and use proper ESD
handling and packaging procedures. Applicable components include circuit boards, electronic
assemblies with exposed components or connectors, semi-conductors and any other devices that may
require ESD protection. Suppliers must maintain records of the testing done and training provided.
Section 7 — Packaging and Shipping Methods: The Supplier shall provide packaging and
shipping methods to prevent cosmetic, mechanical and electrical damage to the Product. The Supplier
shall meet or exceed the detailed specifications of the GE Healthcare packaging requirements found
in the GE Healthcare purchase specification or per the Global Packaging Requirements, document
number 2100268PRE.
Section 8 –– Order of Precedence: This document shall be an addendum to any Purchase
Agreement or any Purchase Orders between Supplier and GE Healthcare. Any conflict between this
document and a Purchase Agreement regarding the minimum material quality requirements shall be
resolved pursuant to the terms of the Purchase Agreement. Any conflict between this document and
the Purchase Order regarding the minimum material quality requirements shall be resolved pursuant
to the terms of this document.
Before using this document, make sure it is the latest revision. Access My Workshop to verify the current revision. If you
do not have access to, or are unfamiliar with, the My Workshop system, consult your quality representative.
do not have access to, or are unfamiliar with, the My Workshop system, consult your quality representative.
DOC0288647
Page 2 of 3
| GE Healthcare Global Quality Form GEHC_GQP_11.01.003_F001 Purchased Material Quality Requirements |
 |
For Finished Medical Device Suppliers:
ADDENDUM TO Section 3 — Compliance: The Supplier shall maintain compliance with any and
all laws and government regulations that apply in the manufacturing and delivery of its products,
including reporting, record keeping and production testing applicable to the manufacture of medical
devices, radiation emitting devices and electromagnetic compatibility for all products delivered to
GE
Healthcare. Such laws may include, but are not limited to, United States and other country medical
device laws, regulations and directives.
Section 9 — Quality and Safety Reporting: The Supplier shall maintain a documented
reporting system to GE Healthcare when the Supplier has knowledge of any product issue related to
safety or quality that results in stopping shipment or requires a recall. Any actions taken by the
Supplier to report a recall to a regulated agency must be communicated to GE Healthcare immediately
(within 3 business days). GE Healthcare has the right to request Supplier to provide all documents
regarding the specific issue including the analysis, root cause and corrective action taken to
minimize any risk to GE Healthcare customers.
By signing below, you agree to the terms hereof and consent to meet all requirements that apply for
any and all products and services provided to GE Healthcare.
Agreed to and Accepted by Supplier
| Signature: | ||||
| Printed Name: | ||||
Title: |
||||
Date: |
||||
| Supplier Name: | ||||
Agreed to and Accepted by the General Electric Company on Behalf of its Division, GE
Healthcare
| Signature: | ||||
| Printed Name: | ||||
Title: |
||||
Date: |
||||
Before using this document, make sure it is the latest revision. Access My Workshop to verify the current revision. If you
do not have access to, or are unfamiliar with, the My Workshop system, consult your quality representative.
do not have access to, or are unfamiliar with, the My Workshop system, consult your quality representative.
DOC0288647
Page 3 of 3
Dear GE Healthcare BIS Sensor Customer
For 10 years GE Healthcare and Aspect Medical Systems have worked together to provide anesthesia
and critical care providers BIS technology through GE BIS modules, and Aspect BIS Sensors.
Effective April 1st, 2009, Aspect will take over worldwide distribution of BIS sensors.
GE and Aspect are making every effort to provide a seamless transition of your BIS sensor needs.
If you have a BIS Sensor contract with GE, both companies will work together to ensure that
contractual obligations continue to be met through the contract’s term. GE will supply BIS sensors
to customers with long-term sensor commitments until such contracts.
GE Healthcare will continue marketing and supporting BIS modules as they have done since their
integration into GE monitors. Educational, technical and customer support for BIS sensors will be
provided by Aspect Medical, or its authorized representative, while GE Healthcare will continue to
provide direct support for all GE branded equipment, including the BIS module/BISx.
Both GE and Aspect are committed to continue to serve you in the best way.
For further questions kindly contact your GE or Aspect representative. For your reference, a list
of local Aspect representatives can be found at the following website:
xxxx://xxx.xxxxxxxx.xxx/xxxxxxxxxxxxx/xxxxxxxxxxx.xxxx
Alternatively you may contact Aspect directly at

