Contract

CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED FIRST AMENDMENT TO THE STATEMENT OF WORK NO. 2-PRODUCT This First Amendment to the Statement of Work No. 2 - Product (this “Amendment”) dated as of October 19, 2023 (the “Amendment Effective Date”), is entered into by and between Xeris Pharmaceuticals, Inc., a company existing under the laws of Delaware, with an office at 0000 Xxxx Xxxxxx Xxxxxx, Xxxxx 0000, Xxxxxxx, XX 00000, Xxxxxx Xxxxxx (hereinafter “Customer”), and SHL Pharma LLC, a company existing under the laws of Florida, with an office at 000 Xxx Xxxxx Xxxxxxxxx, Xxxxxxxxx Xxxxx, XX 00000, Xxxxxx Xxxxxx (hereinafter “SHL”). Customer and SHL are referred to herein individually as a “Party” and collectively as the “Parties”. RECITALS WHEREAS, SHL and Customer are parties to the Amended and Restated Product Supply Agreement (the “Amended and Restated Supply Agreement”), Statement of Work No. 1 – Device (“SOW No. 1”), and Statement of Work No. 2 – Product (“SOW No. 2” and, together with the Amended and Restated Supply Agreement and SOW No. 1, the “Agreement”) effective as of January 30, 2023 which amended and restated, in its entirety, the Product Supply Agreement effective as of August 1, 2018, as amended by the First Amendment to the Product Supply Agreement effective as of June 24, 2020, between the Parties; and WHEREAS, Customer and SHL wish to enter into this Amendment for the purpose of amending the Fees set forth in SOW No. 2 as of the Amendment Effective Date. NOW THEREFORE, in consideration of the mutual covenants and conditions set forth below, the Parties agree as follows: TERMS AND CONDITIONS Exhibit 10.39
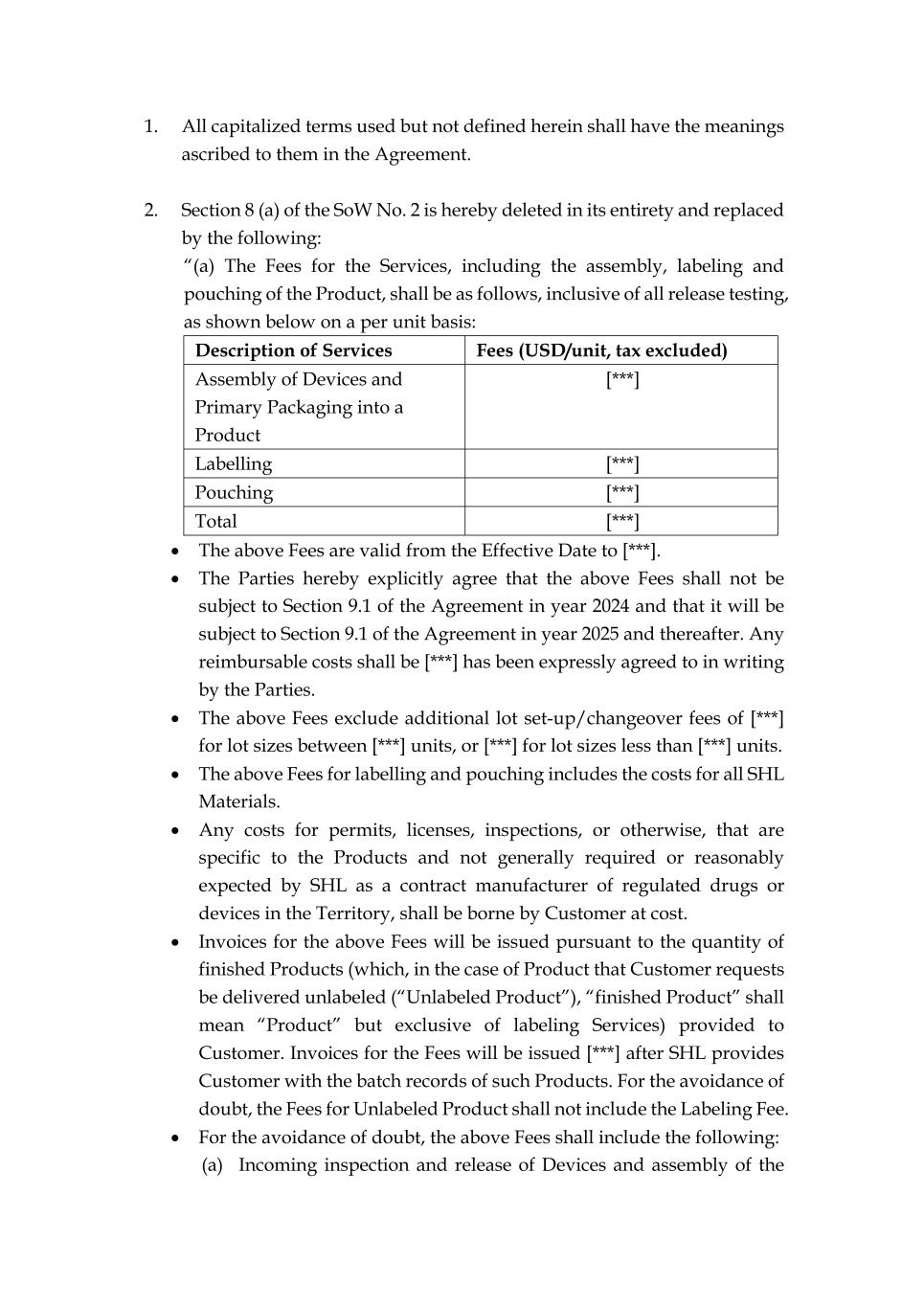
1. All capitalized terms used but not defined herein shall have the meanings ascribed to them in the Agreement. 2. Section 8 (a) of the SoW No. 2 is hereby deleted in its entirety and replaced by the following: “(a) The Fees for the Services, including the assembly, labeling and pouching of the Product, shall be as follows, inclusive of all release testing, as shown below on a per unit basis: Description of Services Fees (USD/unit, tax excluded) Assembly of Devices and Primary Packaging into a Product [***] Labelling [***] Pouching [***] Total [***] • The above Fees are valid from the Effective Date to [***]. • The Parties hereby explicitly agree that the above Fees shall not be subject to Section 9.1 of the Agreement in year 2024 and that it will be subject to Section 9.1 of the Agreement in year 2025 and thereafter. Any reimbursable costs shall be [***] has been expressly agreed to in writing by the Parties. • The above Fees exclude additional lot set-up/changeover fees of [***] for lot sizes between [***] units, or [***] for lot sizes less than [***] units. • The above Fees for labelling and pouching includes the costs for all SHL Materials. • Any costs for permits, licenses, inspections, or otherwise, that are specific to the Products and not generally required or reasonably expected by SHL as a contract manufacturer of regulated drugs or devices in the Territory, shall be borne by Customer at cost. • Invoices for the above Fees will be issued pursuant to the quantity of finished Products (which, in the case of Product that Customer requests be delivered unlabeled (“Unlabeled Product”), “finished Product” shall mean “Product” but exclusive of labeling Services) provided to Customer. Invoices for the Fees will be issued [***] after SHL provides Customer with the batch records of such Products. For the avoidance of doubt, the Fees for Unlabeled Product shall not include the Labeling Fee. • For the avoidance of doubt, the above Fees shall include the following: (a) Incoming inspection and release of Devices and assembly of the

Primary Packaging and Devices into Products; (b) Applying a label, batch number, and expiration date on the outside of the assembled Product (including label and printing inspection/verification); (c) Packaging labelled Products into bulk foil pouches, labeling bulk pouches, packing bulk pouches into corrugated shippers, and palletization; (d) Quality Control sampling and testing of Product via generally accepted statistical methods and SHL release of Product; (e) Certificate of analysis/Certificate of conformance; (f) GMP-required retention samples of the Products; (g) Any and all scrap costs related to the assembly of Product; (h) Routine sampling, analysis and release as part of Product assembly; and (i) Routine maintenance and calibration of equipment and Facility.” 3. All other terms of the SOW No. 2 and the Agreement shall remain in full force and effect. To the extent any provision of the SOW No. 2 or Agreement conflicts with any provision of this Amendment, this Amendment shall control. 4. If a court or other tribunal of competent jurisdiction should hold any term or provision of this Amendment to be excessive, invalid, void, or unenforceable, the offending term or provision shall be deleted, and if possible, replaced by a term or provision which, so far as practicable achieves the legitimate aims of the Parties. Any invalidity or unenforceability of any article or provision of this Amendment shall not affect the remainder of the Amendment. 5. The failure of either Party to require performance by the other Party of any of that other Party’s obligations hereunder shall in no manner affect the right of such Party to enforce the same at a later time. No waiver by any Party hereto of any condition, or of the breach of any provision, term, representation or warranty contained in this Amendment shall be deemed to be or construed as a further or continuing waiver of any such condition or breach, or of any other condition or of the breach of any other provision, term, representation, or warranty hereof.

6. Sections 21, 22 and 24 of the Agreement shall apply to this Amendment directly as if incorporated herein, mutatis mutandis. 7. This Amendment sets forth all intentions, understandings, covenants, promises, warranties, representations, conditions, rights and obligations of the Parties and supersedes all previous and contemporaneous agreements, understandings, negotiations and proposals relating to the subject matter hereof. No subsequent modifications or amendments to this Amendment shall be binding upon the Parties unless reduced in writing and signed by the respective authorized officers of the Parties. 8. This Amendment may be executed in one or more counterparts, each of which when executed and delivered will be deemed an original and all of which together will constitute one and the same agreement. A signed copy of this Amendment delivered by facsimile, e-mail or other means of electronic transmission is deemed to have the same legal effect as delivery of an original signed copy of this Amendment. 9. The Parties agree that this Amendment may be electronically signed and that the electronic signatures appearing on this Amendment are the same as handwritten signatures for the purposes of validity, enforceability, and admissibility. (Signature page follows)

IN WITNESS WHEREOF, the authorized representatives of the Parties hereto have signed this Amendment as of the Amendment Effective Date. Xeris Pharmaceuticals, Inc. SHL Pharma LLC By:_/s/ Xxxx Xxxxxxx By: /s/ Xxxxxxxxx Xxxxxx Name: Xxxx Xxxxxxx Name: Xxxxxxxxx Xxxxxx Title: President and COO Title: Managing Director


