CONTRACT RESEARCH AGREEMENT
This Contract Research Agreement is entered into as of this __ day of _________, 2017 (the “Effective Date”), by and between The Washington University, a corporation established by special act of the Missouri General Assembly approved February 22, 1853 and acts amendatory thereto, having its principal offices at ▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇. ▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ (“University”) and Microbot Medical Ltd., a company formed under the laws of Israel, with offices at ▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇ (the “Company”).
WHEREAS, in the course of their research at University, Drs. ▇▇▇▇▇ ▇. (▇▇▇) ▇▇▇▇▇▇▇▇▇▇ II and ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇, ▇▇ (hereinafter “the Principal Investigators”) have developed a method for making Models (as defined below); and
WHEREAS, the Company wishes to have the Principal Investigators and other members of their research teams at University perform certain services, from time to time, for the Company using the Model (as further defined below, the “Services”); and
WHEREAS, University is willing to perform the Services under the supervision of the Principal Investigators, in accordance with the Workplan (as defined below), all in accordance with the terms and conditions of this Agreement.
NOW, THEREFORE, the parties hereto, intending to be legally bound, hereby agree as follows:
1. Definitions.
Whenever used in this Agreement with an initial capital letter, the terms defined in this Section 1, whether used in the singular or the plural, shall have the meanings specified below.
1.1. “Company Device” shall mean the Company’s proprietary device described in Exhibit A hereto, and any improvements, modifications and derivatives thereof.
1.2. “Company Device Results” shall mean Service Results resulting from experiments using shunts containing the Company Device.
1.3 “Joint Inventions” shall mean any and all patentable inventions, other than Model Improvements, obtained or arrived at in the performance of the Services for which (a) one or more inventor(s) is a member of the University Team and (b) one or more inventor(s) is an employee or consultant of Company.
1.4. “Model” shall mean any animal model in which hydrocephalus has been induced experimentally by the University Team. Most likely these will include pigs, sheep or ferrets at any age.
1.5. “Model Improvements” shall mean any and all improvements or modifications of the Model generated by members of the University Team in the performance of the Services, but specifically excluding any improvements or modifications attributable to the Company Device.
1.6. “Services” shall mean the services to be performed by the University Team in accordance with the Workplan during the time period set forth in the Workplan.
| 1 |
1.7. “Services Results” shall mean any and all data, information and results obtained or arrived at by members of the University Team in the performance of the Services, other than University Inventions.
1.8. “University Inventions” shall mean any and all patentable inventions, other than Model Improvements and Joint Inventions, obtained or arrived at by members of the University Team in the performance of the Services.
1.9. “University Team” shall mean the Principal Investigators and other members of their research team working under the Principal Investigators’ direction in the performance of the Services.
1.10. “Workplan” means the written workplan attached hereto as Exhibit B, as may be amended, expanded or supplemented, from time to time, in accordance with Section 2.1 by the mutual written agreement of the parties.
2. Services.
2.1. Workplans. From time to time during the term of this Agreement, the parties may decide to amend, expand or supplement the Workplan for the performance of Services under this Agreement via written amendment. To the extent the terms in a Workplan shall at any time conflict with the terms of this Agreement, the terms of this Agreement shall control, unless specifically stated otherwise in the Workplan.
2.2. Delivery and Use of Company Device. Company shall deliver units of the Company Device to the Principal Investigators in the amounts and in accordance with the procedures set forth in the Workplan. University shall use the units of the Company Device solely for the purpose of performing the Services under the Workplan. University shall not reverse engineer the Company Device nor undertake any additional analyses of the Company Device, including, without limitation, any attempt to determine the composition, design, structure or properties of the Company Device (except as specifically set forth in the Workplan), without the advance express written permission of Company. Units of the Company Device shall not be used in humans. University shall not sell or transfer units of the Company Device to any person other than members of the University Team without Company’s prior written consent. University shall comply with all applicable laws and regulations in the use of the units of the Company Device. Company’s transfer of the units Company Device to University shall not constitute a sale thereof or a grant, option or license under any patent or other rights owned or controlled by Company. Upon completion of the Services, University shall return to Company all units of the Company Device in its possession or control.
2.3. Performance of Services. University shall cause the University Team to perform the Services in accordance with the Workplan and state-of-the-art scientific standards and laboratory practices. The Services will be directed and supervised by the Principal Investigators, who shall have primary responsibility for the performance of the Services.
3. Fees. In consideration for the performance of the Services, the Company shall pay University the amounts set forth in the Workplan in accordance with the time schedule set forth in the Workplan.
| 2 |
4. Reports. The Principal Investigators shall provide the Company written reports setting forth the Services Results, including raw data and analyses, in accordance with the schedule set forth in the Workplan.
5. Title.
5.1 Service Results and Joint Inventions. All rights, title and interest in and to the Service Results (other than the Model and Model Improvements) and Joint Inventions will be jointly owned by the parties. Subject to Sections 5.4 and 6, Company and University each shall have the full right to practice and to grant licenses under its interest in Joint Inventions (including with respect to patent rights covering Joint Inventions) without any obligation to seek the consent of the other or to account for any profits made as a result of any such license.
5.2. Model and all Model Improvements. All rights, title and interest in and to the Model and all Model Improvements shall be owned solely and exclusively by University.
5.3. University Inventions. University grants to Company: (a) a non-exclusive, worldwide, royalty-free, fully paid-up, perpetual and irrevocable, license (with the right to sublicense through multiple tiers of sublicenses in conjunction with the license of Company intellectual property or sale of Company products) to use and practice University Inventions (including under any and all patent rights claiming University Inventions) to develop, have developed, make, use, have made, market, sell, have sold and import the Company Device or products for the prevention of occlusion and/or reduction of debris or tissue accumulation in shunts and/or shunts incorporating such prevention devices that rely upon, make use of or are based on the Company Device; and (b) an exclusive option (the “Option”) to obtain an exclusive, worldwide license, with the right to grant sublicenses, to make, use, sell, have made, have sold, offer to sell, and import under University’s rights in University Inventions on terms to be negotiated in good faith between the parties. Company may exercise the Option by sending written notice to University at any time within ninety (90) days following the receipt of a written disclosure from University describing in detail such University Invention (the “Option Period”). If, at the end of the Option Period, Company has not exercised the Option, or in the event the Parties fail to reach a mutually acceptable licensing arrangement within six (6) months after the Option Period, University shall be entitled to negotiate with a third party for a license to University’s rights in University Inventions.
6. Confidential Information.
6.1. Unless agreed otherwise by the Company in writing, University shall not, during the term of this Agreement and for five (5) years thereafter, disclose Company Confidential Information (as defined below) other than to members of the University Team or use Company Confidential Information other than for the purpose of performing the Services. University shall ensure that all members of the University Team are legally bound by obligations that impose confidentiality and non-use obligations comparable to those set forth in this Section 6. University shall treat the Company Confidential Information with the same degree of confidentiality as it keeps its own confidential information, but in all events no less than a reasonable degree of confidentiality. University shall safeguard any and all copies of the Company Confidential Information against unauthorized disclosure, shall not tamper with, bypass or alter its security features or attempt to do so, and shall take all reasonable steps to ensure that the provisions of this Agreement are not violated by any person under University’s control or in University’s service.
| 3 |
6.2. For purposes of this Agreement, “Company Confidential Information” means proprietary or confidential information relating to the Company’s scientific, technical, trade or business information relating to the subject matter of this Agreement (including, without limitation, the technical attributes of Company Device which are not known to the public (“Company Device Information”)) disclosed by or on behalf of the Company to members of the University Team in connection with the Services. Both Parties agree that in order for written information to be Confidential Information, it must be delivered in written form clearly marked as “Confidential.” All information, other than Company Device Information, disclosed in oral or some other non-written form must be declared at the time of delivery to be confidential and must be confirmed and summarized in writing and clearly marked as “Confidential” within thirty (30) days of disclosure to be Company Confidential Information, provided , however, information that unintentionally or inadvertently lacks such a legend, or that is disclosed orally or visually which is not subsequently documented, but, by its nature, is reasonably understood to be Company Confidential Information shall be treated as such by University. Notwithstanding the foregoing, information disclosed by Company to the University Team as set forth above shall not be deemed Company Confidential Information to the extent such information: (i) was known to any member of the University Team at the time it was disclosed, as evidenced by written records at the time of disclosure; (ii) is at the time of disclosure or later becomes publicly known under circumstances involving no breach of this Agreement; (iii) is lawfully and in good faith made available to a member of the University Team by a third party who is not subject to obligations of confidentiality to the Company with respect to such information; (iv) is independently developed by a member of the University Team without the use of or reference to Company Confidential Information, as demonstrated by documentary evidence; or (v) is required to be disclosed pursuant to a legal order or mandate. University agrees to keep confidential all Company Device Results until such results are published in accordance with Section 9.
7. Indemnity.
7.1 Notwithstanding the rest of this agreement, Company shall indemnify, defend and hold harmless University, University personnel and representatives, from and against any liability, cost, expense, damage, deficiency, loss or obligation or any kind or nature (including, without limitation, reasonable attorney’s fees and other costs and expenses of litigation) resulting from a claim, suit or proceeding brought by a third party against University to the extent resulting from the use or commercialization of the Services Results or University Inventions by Company or a Company’s sublicensee (a “Claim”), except (in each case) to the extent caused by the negligence or willful misconduct of University or anyone on its behalf.
7.2 If University receives notice of any Claim, University shall, as promptly as is reasonably possible, give the Company notice of such Claim; provided, however, that failure to give such notice promptly shall only relieve the Company of any indemnification obligation it may have hereunder to the extent such failure diminishes the ability of the Company to respond to or to defend University against such Claim. University and the Company shall consult and cooperate with each other regarding the response to and the defense of any such Claim and the Company shall be entitled to assume sole control of the defense and/or represent the interests of University in respect of such Claim, that shall include the right to select and direct legal counsel and other consultants to appear in proceedings on behalf of University and propose, accept or reject offers of settlement, all at its sole cost provided that Company shall not settle a claim which admits fault on behalf of University without University’s prior written consent. Nothing herein shall prevent University from retaining its own counsel and participating in its own defense at its own cost and expense.
| 4 |
8. Term and Termination.
8.1. The term of this Agreement shall commence on the date first written above and, unless terminated earlier in accordance with this Section 8, shall continue for a period of two (2) years, unless extended by the mutual written agreement of the parties.
8.2. In the event that either party commits a material breach of its obligations under this Agreement and fails to cure that breach within thirty (30) days after receiving written notice thereof, the other party may terminate this Agreement immediately upon written notice to the party in breach
8.3. If either Principal Investigator ceases to supervise the Services, the Company or University may terminate this Agreement upon written notice to the other party.
8.4. Upon termination, the parties sole obligations to the other shall be to return all Company Confidential Information and pay any monies due and owing up to the time of termination for work actually performed and all costs reasonably and properly incurred by the parties as of the date that termination is effective, including all non-cancelable obligations reasonably and properly entered into for the purposes of the Services, which may include any non-cancelable University Team salaries, fellowships or post-doctoral stipends, and other non-cancelable executory obligations reasonably and properly incurred by the parties in furtherance of Services, subject to the parties taking reasonable steps to mitigate and minimize such costs.
8.5. The parties’ respective rights, obligations and duties under Sections 4, 5, 6, 7, 8, 9, 10, 11, and 12 , as well as any rights, obligations and duties which by their nature extend beyond the expiration or termination of this Agreement, shall survive any expiration or termination of this Agreement.
9. Publications.
9.1 Company acknowledges that the Principal Investigators and University Team have the right and academic duty to publish the Service Results, and agrees that the University Team will be permitted to present at symposia or professional meetings and to publish in books, journals, and other media of their choosing, any and all Service Results, University Inventions, Models, and Model Improvements; provided however, that University shall not disclose Company Confidential Information without the prior written consent of Company. The University Team will at all times have the first opportunity to publish or present the Service Results, Models, and Model Improvements subject to Section 9.2; provided however that Company shall be entitled to disclose Services Results and regulatory and/or patent filings prior to any such publication.
9.2 If University chooses to publish Service Results on its own (i.e. not publish jointly with Company), Company will be furnished a copy of any proposed publication or a summary of a presentation containing Service Results in advance of submission in the case of publication and rendering in the case of presentation. Company will have thirty (30) days after receipt to review the copy or summary for specific matter which is Company Confidential Information and provide University with a written request for removal or revision. If such a request is received within the thirty days, the Parties will have an additional thirty (30) days (a total of sixty (60) days) to agree upon removal or revisions to protect the Company Confidential Information. Upon completion of this publication process or, if applicable, confidentiality is specifically waived under Section 6, University may proceed with publication, provided that University may not publish or otherwise disclose Company Confidential Information without Company’s express prior written approval. Company shall not encumber publication by University other than to remove Company Confidential Information.
| 5 |
9.3 All papers and presentations reporting Service Results will contain a dignified statement in a form that is customary and appropriate in scholarly journals or presentations for acknowledging that financial support for such research was provided by Company.
9.4 Company will not have an opportunity to change, alter or redact the contents of any student thesis, dissertation, or presentation thereof, provided that no such thesis, dissertation or presentation may contain Company Confidential Information without Company’s prior written consent.
10. Disclaimer and Limitation. NOTWITHSTANDING ANYTHING HEREIN TO THE CONTRARY, EVERYTHING PROVIDED BY EITHER PARTY UNDER THIS AGREEMENT IS UNDERSTOOD TO BE EXPERIMENTAL IN NATURE, MAY HAVE HAZARDOUS PROPERTIES, AND IS PROVIDED WITHOUT ANY WARRANTY OF ANY KIND, EXPRESSED OR IMPLIED, INCLUDING WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE, OR NON-INFRINGEMENT OF ANY THIRD-PARTY PATENT, TRADEMARK, COPYRIGHT OR ANY OTHER THIRD-PARTY RIGHT. NEITHER PARTY MAKES ANY WARRANTIES REGARDING THE QUALITY, ACCURACY, COMMERCIAL VIABILITY OR ANY OTHER ASPECT OF ITS PERFORMANCE PURSUANT TO THIS AGREEMENT OR REGARDING THE PERFORMANCE, VALIDITY, SAFETY, EFFICACY OR COMMERCIAL VIABILITY OF ANYTHING PROVIDED BY IT UNDER THIS AGREEMENT. IN NO EVENT SHALL UNIVERSITY OR COMPANY BE LIABLE FOR ANY INDIRECT, SPECIAL OR CONSEQUENTIAL DAMAGES ARISING OUT OF OR IN ANY WAY CONNECTED WITH THIS AGREEMENT, WHETHER IN BREACH OF CONTRACT, TORT OR OTHERWISE, EVEN IF THE PARTY IS ADVISED OF THE POSSIBLITY OF SUCH DAMAGES. EXCEPT FOR THEIR RESPECTIVE INDEMNITY OBLIGATIONS AND BREACH OF CONFIDENTIALITY OBLIGATIONS, EACH OF UNIVERSITY’S AND COMPANY’S AGGREGATE LIABILITY TO THE OTHER UNDER THIS AGREEMENT SHALL NOT EXCEED THE PAYMENTS MADE OR PAYMENTS DUE UNDER THIS AGREEMENT, RESPECTIVELY.
11. Insurance. The parties shall obtain and maintain an adequate self-insurance or insurance program to protect against potential liabilities and risk, including coverage for the indemnity obligations herein; provided that Company’s obligations under this Section 11 shall come into effect only if and when the Company commences clinical trials (a) based on Services Results or (b) with a product developed under the license granted to Company pursuant to Section 5.3. Prior to the first clinical study in humans of a product developed under the license granted to Company pursuant to Section 5.3, Company shall obtain and maintain product liability insurance in the amount of $5,000,000 per occurrence and $10,000,000 in the aggregate.
12. Miscellaneous.
12.1. Entire Agreement. This Agreement is the sole agreement with respect to the subject matter hereof and except as expressly set forth herein, supersedes all other agreements and understandings between the parties with respect to same.
| 6 |
12.2. Notices. Unless otherwise specifically provided, all notices required or permitted by this Agreement shall be in writing and may be delivered personally, or may be sent by facsimile or certified mail, return receipt requested, to the following addresses, unless the parties are subsequently notified of any change of address in accordance with this Section 9.2:
If to the Company:
Microbot Ltd.
Attention: ▇▇▇▇ ▇▇▇▇▇▇▇▇▇, COO
▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇▇
▇▇▇▇▇▇
If to University:
Joint Research Office of Contracts
Attention: ▇▇▇▇▇ ▇▇▇▇▇
One Brookings Dr., ▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇
▇▇. ▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Copy to:
▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇
Department of Neurosurgery
BJC Institute of Health
▇▇▇ ▇. ▇▇▇▇▇▇, ▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇
▇▇. ▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Any notice shall be deemed to have been received as follows: (i) by personal delivery, upon receipt; (ii) by facsimile, one business day after transmission or dispatch; (iii) by airmail, seven (7) business days after delivery to the postal authorities by the party serving notice. If notice is sent by facsimile, a confirming copy of the same shall be sent by mail to the same address.
12.3. Governing Law and Jurisdiction. This Agreement will be governed by, and construed in accordance with, the substantive laws of the State of New York, without giving effect to any choice or conflict of law provision, and sole jurisdiction is granted to the competent court in the City of New York, NY.
12.4. Amendment; Waiver. This Agreement may be amended, modified, superseded or canceled, and any of the terms may be waived, only by a written instrument executed by each party or, in the case of waiver, by the party waiving compliance. The delay or failure of any party at any time or times to require performance of any provisions hereof shall in no manner affect the rights at a later time to enforce the same. No waiver by either party of any condition or of the breach of any term contained in this Agreement, whether by conduct, or otherwise, in any one or more instances, shall be deemed to be, or considered as, a further or continuing waiver of any such condition or of the breach of such term or any other term of this Agreement.
| 7 |
12.5. No Agency or Partnership. Nothing contained in this Agreement shall give any party the right to bind another, or be deemed to constitute either parties as agents for each other or as partners with each other or any third party.
12.6. Force Majeure. Neither party will be responsible for delays resulting from causes beyond the reasonable control of such party, including without limitation fire, explosion, flood, war, strike, or riot, provided that the nonperforming party uses commercially reasonable efforts to avoid or remove such causes of nonperformance and continues performance under this Agreement with reasonable dispatch whenever such causes are removed.
12.7. Severability. If any provision of this Agreement is or becomes invalid or is ruled invalid by any court of competent jurisdiction or is deemed unenforceable, it is the intention of the parties that the remainder of this Agreement shall not be affected.
12.8. Names and Marks. Neither Party may use the trademarks or name of the other Party or its employees for any commercial, advertisement, or promotional purposes without the prior written consent of the other.
12.9. Assignment. This Agreement may not be assigned by either party without the consent of the other, which consent shall not be unreasonably withheld, except that each party may, without such consent, assign this Agreement and the rights, obligations and interests of such party to any of its affiliates, to any purchaser of all or substantially all of its assets to which the subject matter of this Agreement relates, or to any successor corporation resulting from any merger or consolidation of such party with or into such corporation; provided, in each case, that the assignee agrees in writing to be bound by the terms of this Agreement.
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized representatives as of the date first written above.
| Microbot Medical Ltd. | The Washington University | ||||
| By: | /s/ Harel Gadot | By: | /s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇ | ||
| Name: | Harel Gadot | Name: | ▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇.▇. | ||
| Title: | Chief Executive Officer | Title: | Assistant Vice Chancellor | ||
I, the undersigned, hereby confirm that I have read the Agreement, that its content is acceptable to me and that I will act in accordance with its terms.
| /s/ ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇ ▇▇ | January 24, 2017 | ||
| ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇ ▇▇, PhD | Date |
| /s/ ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ | January 26, 2017 | ||
| ▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇, ▇▇, MD, PhD | Date |
| 8 |
Exhibit A
Company Device
See following pages
| 9 |
SCS Product Description
Head Set Configuration
Approvals:
| Name | Title | Signature | Date | |||||
| Author | Or Samocha | Project Leader | July 1, 2016 | |||||
| Reviewed | ▇▇▇▇▇ ▇▇▇▇▇ | S/W Engineer | July 1, 2016 | |||||
| Approved By | ▇▇▇▇▇ ▇▇▇▇▇▇ | COO | July 1, 2016 |
| 10 |
The Product
The Microbot SCS device is designed to serve as the ventricular catheter portion of a Cerebrospinal Fluid shunt system. The Microbot ventricular catheter can connect to valves that are currently on the market. The advantage of the Microbot SCS device is its ability to maintain CSF flow through the ventricular catheter.
As further described below, the Microbot SCS device incorporates an internal cleaning mechanism embedded in the lumen of the ventricular catheter. The internal part is comprised of a central shaft with small protrusions (resembling a stalk of wheat), which prevents cell ingrowth into the catheter perforations using minute vibrational movements. The vibrational action is externally operated by the patient wearing of a specially designed headset for approximately 5 minute per day. When activated, the headset applies a small magnetic field, which causes the internal part to vibrate and thereby mechanically keeps tissue from entering the catheter perforations while maintaining the CSF flow in the ventricular catheter.
The Microbot SCS ventricular catheter consists of a silicone tube with a perforated titanium tip, which connects to a standard shunt valve at its distal end. The internal cleaning mechanism is embedded in the lumen of the titanium tip.
The internal cleaning mechanism vibrates by means of an induced magnetic field that is externally generated by a user friendly headset that transmits a magnetic field with a pre-determined frequency and operating sequence protocol. The magnetic field that is created by the headset externally, is captured by the intermediate flat flexible coil and PCB that are placed just under the patient’s scalp (in the same location where the valve is currently located). The actuated PCB assembly converts this power to an altering current which flows through the wires to an internal coil located in the distal end of the catheter’s titanium tube. The internal coil produces an electromagnetic field which induces small vibrational movement of the internal cleaning mechanism within the proximal part of the ventricular catheter. The vibrational movement maintains CSF flow in the ventricular catheter by preventing surrounding tissue from entering the catheter perforations, without interfering with the cerebrospinal fluid (CSF) drainage. The vibrational movements of the internal part drive out the infiltrating tissue from the catheter perforations.
Global view
The Microbot SCS device is composed of the following main components:
| 1. | Implanted Ventricular Catheter with vibrating internal part | |
| 2. | Implanted Intermediate Flexible Coil and PCB Assembly | |
| 3. | External Head Set Activation Unit |
The Microbot SCS device is shown in Figure 1 below.
| 11 |

Figure 1 - Microbot SCS Device
The ventricular catheter is depicted in Figure 2 and contains:
| ● | Silicone tube with the following components embedded within the silicone: |
| ● | Small coil | ||
| ● | wires |
| ● | Titanium Tip with the vibrating internal part contained within its lumen. |
| 12 |
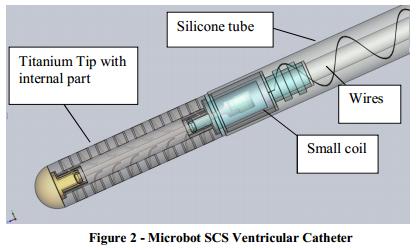
A more detailed description of system’s main components is provided below.
Detailed Component Description
Silicone Tube
The silicone tube is constructed from the same material as standard silicone shunts and has similar inner and outer diameters as standard ventricular catheters. The silicone tube is connected to the titanium tip at the proximal end and to a conventional, commercially available CSF valve at the distal end. Compatibility with different valve systems will be demonstrated as part of the performance data. The internal small coil is embedded inside the silicone tube at the proximal end. Electrical wires are embedded in the wall thickness of the silicone tube (as shown in Figure 2 above) connecting the small coil and the intermediate flat flexible coil assembly that are implanted under the scalp.
Titanium Tip
The titanium tip is a perforated tube, similar to the standard silicone tips of ventricular catheters, only it is manufactured from titanium instead of silicone. The titanium tip houses the vibrating internal cleaning mechanism and is designed to allow mobility while protecting it during implantation of the ventricular catheter. The titanium tip is connected at the distal end to the silicone tube. The tip may be made of other materials as well.
| 13 |

Internal Part and Magnet
The vibrating internal part (Figure 4) is located within the lumen of the titanium tip and maintains CSF flow by using vibrational movements to prevent the tissue or other cells from entering and accumulating within the catheter perforations. The internal part resembles a stalk of wheat with small arms or protrusions along a central shaft and has a small neodymium magnet at the end. The internal part is also manufactured from titanium and the neodymium magnet will either be enclosed in a titanium housing or coated with a biocompatible material (to be determined). The magnet is located in the internal small coil which is located in the silicone tube, as shown in Figure 6.

| 14 |
Figure 5 shows the internal part within the titanium tip.

Internal Actuator
Figure 6 shows the internal part inside the titanium tip, connected to the silicon tube.

The vibration of the internal part is achieved by an altering magnetic field which is generated by an electromagnet (internal small coil up’on actuating by the external headset). The magnetic field creates a moment on the magnet and causes it to rotate around its axis. The minute magnet movement causes the internal part to vibrate slightly and mechanically drives out the tissue or cells from the catheter perforations and thus prevents infiltration and lodging of the tissue in the catheter perforations. The small coil is manufactured from gold/copper coated with biocompatible material and it is encapsulated inside the silicone tube.
| 15 |

Intermediate Flexible Coil and PCB Assembly
The intermediate flat flexible coil and PCB assembly is implanted under the scalp near the implanted valve. This is a flexible printed circuit board (PCB) that contains an intermediate coil made of thin gold\copper wires or other material. The entire flexible coil assembly is encapsulated in silicone such that no internal component comes in contact with human tissue or fluids.
The electromagnetic power that is generated by the external head set unit is induced on the intermediate flexible coil and pcb assembly. The PCB circuitry converts the power from the headset and generates the power that controls the vibrating internal part. The PCB also sends and receives sensory data and from, and to, the headset. The flexible coil, PCB assembly and the silicone section that leads to the PCB are connected to the silicone tube using a miniature connector.
External Head Set Activation Unit
The external head set activation unit is the headset configuration schematically depicted in Figure 7. The headset is worm by the patient once a day for a predetermined period of time. The head set contains an electromagnet that, when properly placed, is automatically activated and transforms electromagnetic power on the intermediate flexible coil assembly to create vibration of the internal part and initiates communication with the implant.
| 16 |

Figure 7 - External Head Set Activation Unit
The head set is pre-adjusted for each patient such that when it is worn, the active area of the head set is positioned proximal to the implanted flexible coil assembly and the electromagnetic energy is induced on the implanted coil.
The head set contains a rechargeable battery. A charged battery will suffice for several days of operation. A microprocessor with dedicated embedded software also resides in the headset. The vibration of the internal part is automatically initiated when the patient places the external head set activation unit on his/her head. The head set activation unit contains a set of LED indicators and auditory feedback, controlled by the embedded software which provides the patient and physician with treatment information. The communication with the implant provides the means to receive sensory data from the implant regarding its state including malfunctions it can experience. The headset has also the ability to upload SW updates to the implant.

Figure 8 – Communication and power scheme
The following indications are provided by the headset:
| ● | Device readiness, including battery state, charging indication, error indication (e.g. hardware failures). |
| ● | Treatment start - this indicates proper placement of the headset over the flexible coil. |
| ● | Device operation - “treatment in progress” indicates proper functioning of the vibrational movement of the internal part, while the headset is placed over the flexible coil. |
| ● | Device compliance – indicates user compliance with the treatment regimen. |
| The following functions are enabled using the Headset via a USB port: | |
| ● | Charging the battery. |
| ● | Retrieving treatment history by the physician. |
| 17 |
Mode of Operation

Upon activation by the user, the external head set activation unit generates an alternating magnetic field, which is induced on the intermediate implanted flat flexible coil. The magnetic field is induced on the intermediate flexible coil assembly which generates power for the PCB circuitry within the intermediate flexible coil assembly. The PCB circuitry generates an alternating current that flows through the electromagnet (small coil) in the proximal ventricular catheter, generating an alternating magnetic field which in turn generates the moment of the neodymium (Nd) magnet and vibrates the internal part. The vibrations maintain the perforations in the proximal ventricular catheter clear of cell ingrowth and enable the CSF to flow freely, thus preventing the buildup of tissue that causes occlusion. The communication between the headset and the implant is based on amplitude modulation..
| 18 |
Exhibit B
Workplan
Scope of Work
IN VIVO TESTS OF THE MICROBOT MEDICAL SCS DEVICE
Washington University School of Medicine and the Saint Louis Children’s Hospital Investigators:
▇▇▇▇▇ ▇. (▇▇▇) ▇▇▇▇▇▇▇▇▇▇, PhD; ▇▇▇▇▇▇▇▇▇
▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇, ▇▇., MD, PhD: Professor and Chief of Pediatric Neurosurgery
▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇, PhD: Visiting Researcher and Postdoctoral Fellow
The Microbot device seeks to prevent tissue obstructions from occluding cerebrospinal fluid (CSF) drainage catheters implanted into the lateral ventricle. Therefore, the experiments will include implantation of these catheters into the lateral ventricle as part of a CSF drainage system, or shunt. In animals that have developed hydrocephalus a ventricular catheter will be inserted into the lateral ventricle and attached to a subcutaneous reservoir whose distal catheter will be subcutaneously placed into the peritoneal cavity. This is the customary design for clinical shunts.
The main objective of this study is to determine the effectiveness of the Microbot Medical SCS Device to prevent obstruction in cerebrospinal fluid (CSF) catheters in a clinically relevant in vivo model of hydrocephalus. To meet this objective one initial Specific Aim will be addressed:
Aim 1: Determine the effectiveness of a porcine model of hydrocephalus to test the Microbot Medical SCS Device.
This short-term pilot study on 4-10 infant pigs is intended to solve any logistical issues and collect preliminary data before the long-term efficacy testing begins in Aim 2. In particular, we will determine the extent of ventriculomegaly needed for implantation of the SCS Device, the time needed prior to implantation, the time required for typical catheter obstruction to occur, the most effective placement of the extracranial components subcutaneously and externally on the body of the pig, and how catheter obstruction will be detected. This initial study will lead to a second Specific Aim that will be formulated once Aim 1 is complete; in other words, once we have optimized the mechanics of catheter positioning (presumably in about 3 animals) we will begin testing different device activation regimes.
Experimental Design
All of the animal procedures will be reviewed and approved by the Washington University Animal Studies Committee in compliance with federal regulations.
| 19 |
Aim 1: Prior to testing the Microbot device, obstructive hydrocephalus will be induced in 4-10 infant pigs by intracisternal injection of kaolin. These procedures have been conducted many times by the principal investigators (PIs) and have been shown to be reliable and cost-effective methods for creating hydrocephalus in experimental animals. We will begin with the following age-based timeline, with modifications applied as we learn more about the model and the mechanics of implanting the Microbot device:
| 1. | 2-weeks of age: Kaolin induction of hydrocephalus | |
| 2. | 2-4 weeks of age: |
| a. | Neuroimaging – ultrasound, CT or MRI – to determine the size of the cerebral ventricles. | ||
| b. | Implantation of a non-activated Microbot device, which includes |
| i. | a 1.5 cm ventricular catheter. | |||
| ii. | a drainage catheter 11-20 ▇▇ ▇▇▇▇ implanted subcutaneously and inserted into the peritoneal cavity. | |||
| iii. | a 10 cm silicone rod attached to a 2cm diameter coil both of which are implanted subcutaneously. |
| c. | Attachment externally to the body wall of a 3-4 cm receiver coil connected by wires to a 5x5 cm data collection box. A jacket will be customized to fit snugly around the animal and hold the equipment in pockets. |
| 3. | 4-10 weeks of age: Practical testing of the data collection abilities of the Microbot device and the general effects of CSF drainage and obstruction on ventricular size and animal behavior. Most likely these tests will be conducted once a day or as needed to determine the feasibility of the animal model. The longer time points will be tested on animals that survived the 10 weeks period in order to identify any chronic complications that might affect Aim 2 experiments. | |
| 4. | At the termination of the experiment each animal will undergo neuroimaging and intracranial pressure (ICP) measurements |
Each animal will be monitored daily for neurological status and general health by ▇▇. ▇▇▇▇▇▇▇▇▇▇ and his trained personnel, as well as the veterinary and animal care staff.
Aim 2: Following the pilot study to determine the optimal implantation parameters, the ability of the Microbot device to prevent catheter obstruction will be tested in the groups described above. Initially each group will consist of 5-8 pigs; 5 animals will be needed for routine statistical analyses so the higher number allows for complications that would remove an animal from the study. The same estimated timeline and experimental design described in Aim 1 will be used with the exception that:
| 1. | The time following implantation for all experimental groups will be extended to at least 12 weeks. | |
| 2. | In the Activated Group, the Microbot device will be turned on for the intervals that were determined in Aim 1. This frequency will also be determined initially by the in vitro experiments conducted in ▇▇. ▇▇▇▇▇▇’ lab at ▇▇▇▇▇ State University in Detroit. | |
| 3. | Quantitative assessments of neurological outcome (balance, motor coordination, irritability, lethargy and other signs that change with increased ICP) will be performed on a regular (at least weekly) basis. | |
| 4. | Quantitative assessments of catheter patency will be performed on a weekly basis. | |
| 5. | Ventricular catheters will be harvested at the end of the experiments and analyzed with routine histology for tissue obstruction. |
Note that future work may include autologous blood injections into the lateral ventricles to model post-hemorrhagic hydrocephalus and test the efficacy of the Microbot device under these clinical conditions.
| 20 |
Study Timeline: We will conduct these experiments as expeditiously as possible, but we anticipate that Aim 1 will be completed in 3-6 months and the comprehensive study will follow.
Roles and Responsibilities:
▇▇. ▇▇▇▇▇▇▇▇▇▇, as Principal Investigator, will oversee and supervise all aspects of this study. In addition, he will perform all of the surgical procedures, monitor animals frequently, communicate frequently with the veterinary and support staff, supervise the Research Associate/Postdoctoral Fellow, maintain protocols, and manage funding. He will participate in semi-weekly conference calls with members of Microbot Medical. This effort will require 50% of his time for the 6-month study period.
▇▇. ▇▇▇▇▇▇▇▇, as a pediatric neurosurgeon who specializes in the surgical management of hydrocephalus and current Chief of Pediatric Neurosurgery at the Washington University School of Medicine and the St. Louis Children’s Hospital, will be a clinical consultant and co-investigator on this study. He will provide advice on all aspects of the study and occasionally participate in surgery. This will constitute 5% effort but his salary and benefits will be cost shared by the Washington University School of Medicine.
▇▇. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇, who is currently a postdoctoral fellow in the ▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇ lab, will assist in all surgical procedures and animal monitoring. In addition, he will perform all of the histology conducted on the ventricular catheters and animal brains. This effort will require 75% of his time.
| 21 |
BUDGET FOR AIM 1
January 1 to June 30, ▇▇▇▇
▇▇▇▇▇▇▇▇▇
▇▇. ▇▇▇▇▇▇▇▇▇▇ 50%
$24,384
▇▇. ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇ 75%
$20,063
▇▇. ▇▇▇▇▇▇▇▇ 5% cost-shared
$0
Total Personnel for 6 months
$44,447
Animals
| 1. | Purchase infant pigs @ $180 each x 10 |
| $1,800 |
| 2. | Administrative fee $25 x 10 |
| $250 |
| 3. | Shipping - $205/2-3 pigs |
| $750 |
| 4. | Per diem - $15.50/d for 10-days postop single housing x 10 = $930; |
| 11-70 days = $18.19/d double housing x 5 x 60d = $5,457 | |
| $6,387 |
| 5. | Disposal fee = $85/pig x 10 |
| $850 |
| 6. | Veterinary consultation & support |
| $1,000 |
Total Animals for 6 months
$11,037
Surgery
| 1. | 1st surgery (1 hr + 2 hr tech) = $550/pig x 10 |
| $5,500 |
| 2. | 2nd surgery (3 hr + tech) = $870/pig x 10 |
| $8,700 |
| 22 |
Total Surgery for 6 months
$14,200
Neuroimaging
| 1. | Transport $250/2 pigs x 5 x 2 studies |
| $2,500 |
| 2. | CT scans $180/h x 20 studies (2 per pig) |
| $3,600 |
| 3. | Veterinary/animal care technical support $150/study x 20 |
| $3,000 |
| 4. | Ultrasonography $50/study x 20 |
| $1,000 |
Total Neuroimaging for 6 months
$10,100
Histology and Immunohistochemistry
| 1. | Reagents, fixatives, buffers |
| $750 |
| 2. | Stains and antibodies |
| $3,000 |
| 3. | Glass slides and coverslips |
| $750 |
| 4. | Digital microscopy @ $20/h |
| $1,000 |
Total Histology and Immunohistochemistry for 6 months
$5,500
| Total Direct Costs | ||
| $85,284 | ||
| Total Indirect Costs @ 50% | ||
| $42,642 | ||
| Grand Total for 6 months | ||
| $127,926 |
BUDGET JUSTIFICATION for AIM 1
Personnel – described above in the Scope of Work, Roles and Responsibilities
Animals
Detailed expenses are listed in the Budget Aim 1. A maximum of 10 animals has been requested but fewer pigs may be; every effort will be made to use the minimal number of animals needed to satisfy the objectives.
Surgery
Two surgeries will be needed on each animal: the first to induce hydrocephalus and the second to implant the Microbot device. Charges are based on the current rate to use the operating room and the institutional (IACUC) requirement for a surgical assistant from the Division of Comparative Medicine) to perform anesthesia and monitor each animal following surgery.
| 23 |
Neuroimaging
Two assessments will be needed for each animal: the first just prior to implantation of the Microbot device to determine the extent of ventriculomegaly and the second just prior to termination of the experiment to determine the effectiveness of the device. As described under “Animals”, fewer studies may be conducted if the objectives have been met. Furthermore, if ultrasonography can be shown to accurately determine the extent of ventriculomegaly then the CT scans will not be needed. However, if CT scans are needed, then transportation and veterinary care expenses will be incurred as required by the IACUC protocol.
Histology and Immunohistochemistry
Post-mortem analyses of the brain will include routine histology and targeted immunohistochemistry to determine (1) the effects of the Microbot device on the brain as well as (2) the effectiveness of the Microbot device to maintain patency in the catheter. As described previously, these costs will be reduced if fewer animals are needed.
Indirect Costs
These costs (often termed “F&A”) are determined by the Washington University Joint Research Office for Contracts and administered through the Office of Sponsored Research Services and Sponsored Projects Accounting. After extensive discussions between ▇▇. ▇▇▇▇▇▇▇▇▇▇ and these offices, the rate of 50% of Direct Costs is non-negotiable. Therefore, $42,642 has been added to the Direct Costs to provide a total budget of $127,926.
| 24 |
ACCOUNTING AND PAYMENT
Microbot will pay WU a total of $127,926 US (inclusive of all and any taxes. Such amount shall be paid on a fixed-price basis and CORPORATION agrees that WU will retain residual funds, if any, upon completion of the Project. These payments shall be made as set out below:
Within fifteen (15) days of the Effective Date, CORPORATION will pay to WU the sum of $63,963US.
Within fifteen (15) days of after induction of Hydrocephalus in animal, CORPORATION will pay to WU the sum of $51,170.40US.
Microbot will pay the remainder, $12,792.60US, within thirty (30) days of receipt of the Aim 1 Progress Report: Pilot Studies on the Porcine Model of Hydrocephalus to Test the Microbot Medical SCS Device.
Invoices:
Invoices, will be sent to:
Microbot Medical
▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇▇, ▇▇▇▇▇
▇▇▇▇▇▇
Checks shall reference WU Contract Number OTM10394 and will be made payable to Washington University in St. Louis and sent to:
Washington University
Sponsored Projects Accounting
Campus Box 1034
▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇. ▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
FAX ▇▇▇-▇▇▇-▇▇▇▇
Where payments are made via wire transfer, CORPORATION agrees to be responsible for fees related to the wire transfer. The wire transfer information is:
| 25 |

