SUPPLY AGREEMENT
Exhibit 10.1
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
THIS SUPPLY AGREEMENT (this “Agreement”) is entered into as of February 3rd, 2014 (the “Effective Date”), between ChromaDex, Inc., a California corporation (“ChromaDex”), having a place of business at 00000 Xxxxxxxxx Xxxx., Xxxxx X, Xxxxxx, XX 00000, and Elysium Health, LLC, a Florida limited liability corporation (“Elysium Health”), having a place of business at 000 Xxxxxxxx Xxxx Xxxxx, Xxxxx 000, Xxxxxx Xxxxx, XX 00000.
WHEREAS, ChromaDex has rights in, and provides supply of, Niagen (as defined below).
WHEREAS, Elysium Health desires to develop dietary supplements containing Niagen for use in the Field.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, the parties agree as follows:
1. DEFINITIONS
1.1 “Affiliate” shall mean, with respect to any Person, any other Person which directly or indirectly controls, is controlled by, or is under common control with, such Person. A Person shall be regarded as in control of another Person if it owns, or directly or indirectly controls, at least fifty percent (50%) of the voting stock or other ownership interest of the other Person, or if it directly or indirectly possesses the power to direct or cause the direction of the management and policies of the other Person by any means whatsoever.
1.2 “Facility” means any facility where Niagen that is supplied by ChromaDex under this Agreement is Manufactured.
1.3 “cGMPs” mean current good manufacturing practices (i) as described in Parts 210 and 211 of Title 21 of the United States’ Code of Federal Regulations and the latest FDA guidance documents pertaining to manufacturing and quality control practice, and (ii) as applicable in each other country in which Elysium Health advises ChromaDex in writing that Niagen products are intended to be sold; all as updated, amended and revised from time to time.
1.4 “Confidential Information” shall mean, with respect to a party, all information of any kind whatsoever, and all tangible and intangible embodiments thereof of any kind whatsoever, which is disclosed by such party to the other party and is marked, identified as or otherwise acknowledged to be confidential at the time of disclosure to the other party. Notwithstanding the foregoing, Confidential Information of a party shall not include information which the other party can establish by written documentation (a) to have been publicly known prior to disclosure of such information by the disclosing party to the other party, (b) to have become publicly known, without fault on the part of the other party, subsequent to disclosure of such information by the disclosing party to the other party, (c) to have been received by the other party at any time from a source, other than the disclosing party, rightfully having possession of and the right to disclose such information, (d) to have been otherwise known by the other party prior to disclosure of such information by the disclosing party to the other party, or (e) to have been independently developed by employees or agents of the other party without access to or use of such information disclosed by the disclosing party to the other party. For the avoidance of doubt, all Royalty Reports and any information concerning the pricing and sale of Niagen products shall be Elysium Health Confidential Information for purposes of this Agreement.
1.5 “Excluded Products” means topical skincare or cosmetic products and any and all dietary supplements in the form of a melt (melting or dissolvable tablet or delivery system).
1.6 “Excluded Field” means the doctor channel and the Multi-Level Marketing channel.
1.7 “Field” shall mean the sale of dietary supplements in any channel within the Territory, except for those in the Excluded Field so long as Elysium Health provides written notice to ChromaDex thirty (30) days prior to selling in a new channel of distribution.
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
1.8 “FDA” means the United States Food and Drug Administration and any successor agency or entity that may be established thereafter.
1.9 “First Commercial Sale” shall mean, with respect to any finished product containing Niagen, the first sale for use or consumption by the general public of such product.
1.10 “Force Majeure” shall mean a failure or delay in fulfilling or performing any term of this Agreement to the extent such failure or delay is caused by or results from causes beyond the reasonable control of the affected party including fire, floods, embargoes, war, acts of war (whether war be declared or not), acts of terrorism, insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, acts of God or acts, omissions or delays in acting by any governmental authority.
1.11 “Manufacture” means the manufacturing, processing, formulation, supplying, testing, packaging, labeling, storing and preparing for shipment of the Niagen supplied by ChromaDex under this Agreement.
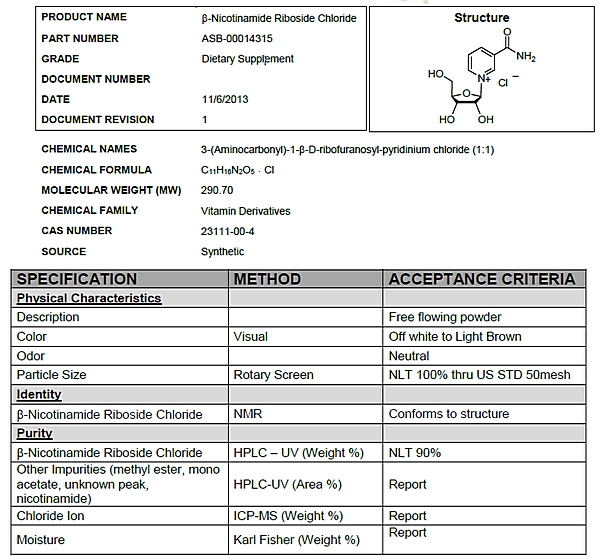
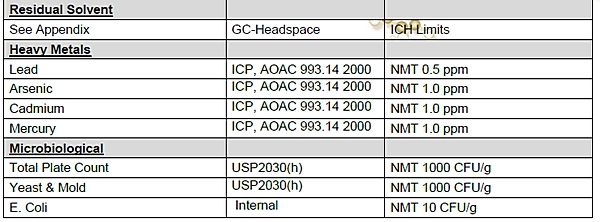
1.12 “Niagen” shall mean the dietary ingredient comprised of nicotinamide riboside (NR) chloride supplied by ChromaDex and conforming to the specifications set forth on Exhibit A.
1.13 “Person” shall mean an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, pool, syndicate, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed herein.
1.14 “Territory” shall mean the United States and Canada and can be expanded by mutual agreement of the parties in writing.
1.15 “Third Party” shall mean any Person other than ChromaDex, Elysium Health and their respective Affiliates.
2. REPRESENTATIONS AND WARRANTIES
2.1 Mutual Representations and Warranties. Each party hereby represents and warrants to the other party as follows:
2.1.1 Corporate Existence. Such party is a corporation duly organized, validly existing and in good standing under the laws of the state in which it is incorporated.
2.1.2 Authorization and Enforcement of Obligations. Such party (a) has the corporate power and authority and the legal right to enter into this Agreement and to perform its obligations hereunder, and (b) has taken all necessary corporate action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder. This Agreement has been duly executed and delivered on behalf of such party, and constitutes a legal, valid, binding obligation, enforceable against such party in accordance with its terms.
2.1.3 No Consents. All necessary consents, approvals and authorizations of all governmental authorities and other Persons required to be obtained by such party in connection with this Agreement have been obtained.
2.1.4 No Conflict. The execution and delivery of this Agreement and the performance of such party’s obligations hereunder (a) do not conflict with or violate any requirement of applicable laws or regulations, and (b) do not conflict with, or constitute a default under, any contractual obligation of such party.
3. SUPPLY
ChromaDex shall sell and deliver, and Elysium Health shall purchase from ChromaDex, such Niagen as Elysium Health orders from time to time on the terms and subject to the conditions set forth below:
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
3.1 Price. With respect to all Niagen provided by ChromaDex to Elysium Health under this Agreement Elysium Health shall pay to ChromaDex a maximum price of [*] US dollars per kilogram ($[*] per kg) (“Maximum Price”); If, at any time during the Term, ChromaDex supplies Niagen (or a substantially similar product) to a Third Party at a price that is lower than that at which Niagen is supplied to Elysium Health under this Agreement, then the price of Niagen supplied under this Agreement shall be revised to such Third Party price with effect from the date of the applicable sale to such Third Party and ChromaDex shall promptly provide Elysium Health with any refund or credits thereby created; provided Elysium Health purchases equal volumes or higher volumes than the Third Party. For the sake of clarity this Section does not apply to inter-Affiliate transfers.
3.2 Delivery. All the Niagen supplied under this Agreement shall be shipped FCA (INCOTERMS 2010) from the ChromaDex dock. Elysium Health shall be responsible for all freight, insurance charges, taxes, import and export duties, inspection fees and other charges applicable to the sale and transport of the Niagen purchased by Elysium Health hereunder. Title and risk of loss and damages to the Niagen purchased by Elysium Health hereunder shall pass to Elysium Health upon delivery of Niagen to a common carrier at ChromaDex dock and Elysium Health shall be fully responsible, and shall hold ChromaDex harmless for and assume all risk of loss, destruction of or damage to the Niagen. Loss or damage to the Niagen after risk of loss has passed to Elysium Health will not release or excuse Elysium Health from its obligations under this Agreement to ChromaDex, including the obligation to make full payment of the purchase price.
3.3 Sales and Use Taxes. Elysium Health shall pay any federal, state, county or municipal sales or use tax, excise or similar charge, or other tax assessment (other than that assessed against income), assessed or charged on the sale of the Niagen sold to it pursuant to this Agreement.
3.4 Payments. Elysium Health shall pay ChromaDex within thirty (30) days from the date of the applicable invoice by ChromaDex to Elysium Health for all Niagen purchased hereunder. Elysium Health shall make all payments under the Agreement to ChromaDex in United States dollars to ChromaDex’s account in a financial institution located in the United States.
3.5 Orders. Elysium Health shall make all purchases hereunder by submitting firm purchase orders to ChromaDex. Each such purchase order shall be in writing in a form reasonably acceptable to ChromaDex, and shall specify the quantity ordered, the transfer price therefor under Section 3.1 above, the place of delivery and the required delivery date therefor, which shall not be less than thirty (30) days after the date of such purchase order. In the event of a conflict between the terms and conditions of any purchase order or invoice or other purchasing document and this Agreement, the terms and conditions of this Agreement shall prevail.
3.6 Returned Niagen. If any Niagen does not conform to the specifications set forth on Exhibit A, any rejection or revocation of acceptance by Elysium Health must be made within thirty (30) days of delivery and any attempted rejection or revocation of acceptance of the Niagen made thereafter shall be null and void unless agreed to in writing by ChromaDex, except that, notwithstanding the foregoing, in the event that a defect in the Niagen could not reasonably be discovered within such thirty (30) day period (“Latent Defect”), Elysium Health shall have the right to reject such Niagen within fifteen (15) days after discovering the Latent Defect. Failure to make a claim within such period shall be conclusive evidence that the Niagen was supplied in accordance with the specifications set forth on Exhibit A. Subject to the foregoing, Elysium Health shall return the nonconforming Niagen to ChromaDex in accordance with the reasonable instructions of ChromaDex or, on ChromaDex’s request, dispose of such nonconforming Niagen. In both cases all costs shall be borne by ChromaDex. Should any Niagen be returned as provided above, ChromaDex shall replace the returned Niagen as soon as reasonably practicable. Such replacement Niagen shall be at no additional cost to Elysium Health if Elysium Health had previously paid ChromaDex for the returned Niagen.
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
3.7 Limited Warranty and Disclaimer of all other Warranties. (a) CHROMADEX WARRANTS THAT THE NIAGEN SOLD HEREUNDER SHALL BE (i) MANUFACTURED IN ACCORDANCE WITH cGMP AND APPLICABLE LAWS AND REGULATIONS IN THE UNITED STATES AND (ii) SHALL CONFORM TO THE SPECIFICATIONS SET FORTH ON EXHIBIT A; (b) EXCEPT AS OTHERWISE PROVIDED IN SECTION 3.7(a) HEREOF, CHROMADEX HEREBY EXPRESSLY DISCLAIMS ANY AND ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, WITH RESPECT TO THE NIAGEN, INCLUDING BUT NOT LIMITED TO THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NON-INFRINGEMENT. CHROMADEX HAS NOT MADE ANY RECOMMENDATION TO ELYSIUM HEALTH REGARDING THE USE OR SUBSEQUENT SALE OF THE NIAGEN. ELYSIUM HEALTH ASSUMES ALL RISKS AND LIBAILITIES FOR ANY LOSS, DAMAGE OR INJURY TO PERSONS OR PROPERTY RESULTING FROM THE USE OR SUBSEQUENT SALE OF THE NIAGEN, EITHER ALONE OR IN COMBINATION WITH OTHER INGREDIENTS. ELYSIUM HEALTH HAS SATISFIED ITSELF THAT THE NIAGEN AND THE PURPOSE FOR WHICH IT WILL BE USED AND/OR SOLD IS IN COMPLIANCE WITH THE LAWS OF THE RELEVANT COUNTRIES; (c) ELYSIUM HEALTH’S EXCLUSIVE REMEDY AND CHROMADEX’S EXCLUSIVE LIABILITY FOR SHIPMENT OF NON-CONFORMING NIAGEN SHALL BE LIMITED TO, AT CHROMADEX’S SOLE OPTION, EITHER REPLACEMENT OF THE NON-CONFORMING NIAGEN OR A REFUND OF THE PURCHASE PRICE PAID. EXCEPT AS SET FORTH IN SECTION 3.6: (x) ALL CLAIMS MADE WITH RESPECT TO THE PRODUCT SHALL BE DEEMED WAIVED BY ELYSIUM HEALTH UNLESS MADE IN WRITING AND RECEIVED BY CHROMADEX WITHIN THIRTY (30) DAYS OF DELIVERY; (y) ELYSIUM HEALTH MUST MAKE ANY CLAIM FOR NON-COMFORMING NIAGEN, BREACH OF WARRANTY WITH RESPECT TO THE NIAGEN SOLD, OR ANY CLAIM OF ANY NATURE WHATSOEVER WITH RESPECT TO THE NIAGEN SOLD HEREUNDER IN WRITING WITHIN THIRTY (30) DAYS AFTER ELYSIUM HEALTH’S RECEIPT OF NIAGEN; AND (z) ELYSIUM HEALTH IRREVOCABLY WAIVES AND RELEASES ALL CLAIMS THAT ARE NOT PROPERLY MADE WITHIN SAID PERIOD.
3.8 Regulatory Requirements. ChromaDex shall keep Elysium Health reasonably and timely informed of regulatory developments related to Niagen throughout the Territory. Without limiting the foregoing, ChromaDex represents and warrants that to the best of its knowledge Niagen may be lawfully sold under the Federal Food, Drug, and Cosmetic Act (as amended, including by the Dietary Supplement Nonprescription Drug Consumer Protection Act).
3.9 Product Safety. ChromaDex shall promptly inform Elysium Health in writing of any information concerning or that can potentially impact the safety, identity, strength, quality or purity of any Niagen of which it becomes aware, and shall provide supporting documentation. Without limiting the foregoing, ChromaDex further agrees to notify Elysium Health within five (5) days if it receives notice of a serious adverse event, as defined in the Dietary Supplement Nonprescription Drug Consumer Protection Act, associated with an Elysium Health product containing Niagen, or to the extent legally obligated to do so.
3.10 Niagen Control. Each shipment of Niagen by ChromaDex shall contain a Certificate of Analysis providing an identifying lot number, expiration date, and lot-specific quality control report.
3.11 Minimum Purchase Commitments. Elysium Health shall order and pay for at least the minimum quantities of Niagen for each of the periods specified below.
|
Period
|
Length of Period
|
Minimum Purchase Commitment for the Applicable Period
|
|
1
|
12 months commencing upon date of First Commercial Sale
|
[*] kilograms ([*] kgs)
|
|
2
|
12 months commencing on the expiration of period 1 above
|
[*] kilograms ([*] kgs)
|
|
3
|
12 months commencing on the expiration of period 2 above
|
[*] kilograms ([*] kgs)
|
|
Each subsequent successive 12 month period, the first such period commencing on the expiration of period 3 above
|
To be negotiated in good faith within 90 days prior to the expiration of such period 3.
|
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
If Elysium Health fails to make First Commercial Sale of Niagen six (6) months from the Effective Date, or if Elysium Health fails to meet the minimum purchase requirements set forth herein, ChromaDex, at its sole option and discretion, and upon written notice to Elysium Health, has the right to terminate this Agreement.
3.12 Patent Marking. During the Term, Elysium Health will ensure proper patent marking for all uses of Niagen, all Niagen product shall be marked as follows:
“Patent: See xxx.xxxxxxxxx.xxx/xxxxxxxxxxx/xxxxxxx;”
xxxxx://xxxxxxxxx.xxx/Xxxxxxxxxxx/Xxxxxxx.xxxx;
or as mutually agreed to in writing by the Parties.
4. CONFIDENTIALITY
4.1 Confidential Information. During the Term, and for a period of five (5) years following the termination hereof, each party shall maintain in confidence all Confidential Information disclosed by the other party, and shall not use, disclose or grant the use of the Confidential Information except on a need-to-know basis to those Affiliates, directors, officers, employees, consultants, clinical investigators, contractors, agents, or permitted assignees, to the extent such disclosure is reasonably necessary in connection with such party’s activities as expressly authorized by this Agreement. To the extent that disclosure is authorized by this Agreement, prior to disclosure, each party hereto shall obtain agreement of any such person or entity to hold in confidence and not make use of the Confidential Information for any purpose other than those permitted by this Agreement. Each party shall notify the other promptly upon discovery of any unauthorized use or disclosure of the other party’s Confidential Information.
4.2 Terms of this Agreement. Except as otherwise provided in Section 7.1 above, neither party shall disclose any terms or conditions of this Agreement to any Third Party without the prior consent of the other party. Notwithstanding the foregoing, prior to execution of this Agreement, the parties have agreed upon the substance of information that can be used to describe the terms of this transaction, and each party may disclose such information, as modified by mutual agreement from time to time, without the other party’s consent.
4.3 Permitted Disclosures. The confidentiality obligations contained in this Section 7 shall not apply to the extent that the receiving party (the “Recipient”) is required (a) to disclose information by law, order or regulation of a governmental agency or a court of competent jurisdiction, or (b) to disclose information to any governmental agency for purposes of obtaining approval to test or market a Niagen product, provided in either case that the Recipient shall provide written notice thereof to the other party and sufficient opportunity to object to any such disclosure or to request confidential treatment thereof.
5. TERM; TERMINATION
5.1 Term. This Agreement shall be effective as of the Effective Date and shall continue for an initial term of three (3) years (the “Initial Term”). At the end of the Initial Term, this Agreement shall continue automatically for successive additional one (1) year periods (each a “Renewal Term,” together with the Initial Term, the “Term”) under the same terms and conditions hereunder until terminated in accordance with the terms of Section 5.2.
5.2 Termination. This Agreement may be terminated by:
(i) Any Party upon ninety (90) days written notice prior to the end of the Initial Term or any subsequent Renewal Term.
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
(ii) Any Party in the event that the other Party breaches any material term of this agreement and fails to cure such breach within ninety (90) days following notice thereof from the non-breaching party in writing.
(iii) a Party immediately upon the giving of notice if the other Party files a petition for bankruptcy, is adjudicated bankrupt, takes advantage of the insolvency laws of any state, territory or country, or has a receiver, trustee, or other court officer appointed for its property.
(iv) a Party if an event of Force Majeure (as described in Section 1.9 of this Agreement) with respect to the other Party shall have continued for ninety (90) days or is reasonably expected to continue for more than one hundred eighty (180) days.
5.3 Nonexclusive Rights and Remedies. Termination is not an election of remedies. Except as otherwise specifically provided herein, all rights and remedies of the Parties provided under this Agreement are not exclusive and are in addition to any other rights and remedies provided by law or under this Agreement. Termination of this Agreement shall not relieve either Party of any liability which has accrued prior to the effective date of such termination, or prejudice either Party’s right to obtain performance of any obligation provided for in this Agreement, which by its express terms or context survives termination. Without limiting the foregoing, Sections 4, 5.3, 6 and 7 shall survive the termination of this Agreement.
6. INDEMNIFICATION
6.1 Indemnification. Each party shall defend, indemnify and hold the other party harmless from all losses, liabilities, damages and expenses (including reasonable attorneys’ fees and costs) (“Losses”) resulting from any claims, demands, actions and other proceedings by any Third Party (a “Claim”) to the extent resulting from such party’s breach of a representation, warranty or covenant under this Agreement. In addition, (i) ChromaDex shall defend, indemnify and hold Elysium Health harmless from all Losses resulting from any Claims to the extent resulting from ChromaDex’s research, development or commercialization of Niagen; and (ii) Elysium Health shall defend, indemnify and hold ChromaDex harmless from all Losses resulting from any Claims to the extent resulting from Elysium Health’s research, development or commercialization of Niagen products.
6.2 Procedure. In the event of a Claim, a party (the “Indemnitee”) that intends to claim indemnification under this Section shall promptly notify the other party (the “Indemnitor”) of such Claim. The Indemnitor shall have the right to assume the defense thereof with counsel selected by the Indemnitor. The indemnity obligations under this Section shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior express written consent of the Indemnitor, which consent shall not be unreasonably withheld or delayed. The failure to deliver notice to the Indemnitor within a reasonable time after notice of any such Claim, if prejudicial to its ability to defend such Claim, shall relieve such Indemnitor of any liability to the Indemnitee under this Section with respect thereto. The Indemnitee, its employees and agents, shall reasonably cooperate with the Indemnitor and its legal representatives in the investigation of any Claim.
7. MISCELLANEOUS
7.1 ChromaDex represents, warrants and covenants that ChromaDex has no pre-existing obligations or commitments (and will not assume or otherwise undertake any obligations or commitments) that would be in conflict or inconsistent with or that would hinder ChromaDex’s performance of its obligations under this Agreement. ChromaDex shall notify Elysium Health in writing at least thirty (30) days prior to entering into any agreement with any Third Party with respect to the supply or sale of Niagen in the dietary supplement channel in which Elysium Health has provided written notice to ChromaDex in accordance with Section 1.7 that it would be selling Niagen, and, if Elysium Health notifies ChromaDex within ten (10) days from receipt of such notice that it would like to negotiate an exclusive arrangement relating to the supply or sale of Niagen in such channel, ChromaDex agrees to negotiate in good faith with Elysium Health the terms of such exclusive arrangement; provided that ChromaDex will be entitled to continue to negotiate with such Third Party.
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
7.2 Notices. Any consent, notice or report required or permitted to be given or made under this Agreement by one of the parties to the other shall be in writing and addressed to such other party at its address indicated below, or to such other address as the addressee shall have last furnished in writing to the addressor, and shall be effective upon receipt by the addressee.
If to ChromaDex: ChromaDex, Inc.
00000 Xxxxxxxxx Xxxx., Xxxxx X,
Xxxxxx, XX 00000
Attention: General Counsel
If to Elysium Health: Elysium Health, LLC
000 Xxxxxxxx Xxxx Xxxxx, Xxxxx 000,
Xxxxxx Xxxxx, XX 00000
Attention: CEO
7.3 Assignment. Except as otherwise expressly provided under this Agreement neither this Agreement nor any right or obligation hereunder may be assigned or otherwise transferred (whether voluntarily, by operation of law or otherwise), without the prior express written consent of the other party; provided, however, that either party may, without such consent, assign this Agreement and its rights and obligations hereunder in connection with the transfer or sale of all or substantially all of its business, or in the event of its merger, consolidation, change in control or similar transaction. Any permitted assignee shall assume all obligations of its assignor under this Agreement. Any purported assignment or transfer in violation of this Section 7.3 shall be void.
7.4 Applicable Law. This Agreement shall be governed by and construed in accordance with the laws of the State of California, without regard to the conflicts of law principles thereof.
7.5 Entire Agreement. This Agreement and the Trademark License and Royalty Agreement entered into between the parties as of the Effective Date contains the entire understanding of the parties with respect to the subject matter hereof. All express or implied representations, agreements and understandings, either oral or written, heretofore made are expressly superseded by this Agreement.
7.6 Amendment. No change, modification, extension, termination or waiver of this Agreement, or any of the provisions herein contained, shall be valid unless made in writing and signed by duly authorized representatives of the parties hereto.
7.7 Insurance. Each Party shall carry liability insurance at a sufficient level to meet its obligations and liability under this Agreement.
7.8 Independent Contractors. Each party hereby acknowledges that the parties shall be independent contractors and that the relationship between the parties shall not constitute a partnership, joint venture or agency. Neither party shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other party, without the prior consent of the other party to do so.
7.9 Severability. Any of the provisions of this Agreement which are determined to be invalid or unenforceable in any jurisdiction shall be ineffective to the extent of such invalidity or unenforceability in such jurisdiction, without rendering invalid or unenforceable the remaining provisions hereof and without affecting the validity or enforceability of any of the terms of this Agreement in any other jurisdiction.
7.10 Waiver. The waiver by a party of any right hereunder, or of any failure to perform or breach by the other party hereunder, shall not be deemed a waiver of any other right hereunder or of any other breach or failure by the other party hereunder whether of a similar nature or otherwise.
7.11 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
IN WITNESS WHEREOF, the parties have executed this Agreement as of the Effective Date.
|
CHROMADEX, INC.
By /s/ Xxxxx Xxxxxx
Title CEO
ELYSIUM HEALTH, LLC
By /s/ Xxxx Xxxxxxxxxx
Title CEO
|
[*] INDICATES CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT AND HAS BEEN FILED SEPARATELY WITH THE COMMISSION
EXHIBIT A
Niagen Specifications