DEVELOPMENT, LICENSE & OPTION AGREEMENT
DEVELOPMENT, LICENSE & OPTION AGREEMENT
This Development, License & Option Agreement (the “Agreement”), dated November 3, 2014 (the “Effective Date”) is made by and among AxoGen, Corporation, a Delaware corporation with an address of 00000 Xxxxxxxx Xxxx., Xxxxx 000, Xxxxxxx, XX 00000 (“AxoGen”), Sensory Management Services LLC (“SMS”), a limited liability company with an address of 00 Xxxx Xxx Xxx, Xxxx 0, Xxxxxxxxx, Xxxxxx 00000, AxoGen and SMS herein referred to each as a “Party” and collectively, the “Parties”.
R E C I T A L S
WHEREAS, SMS has developed a proprietary pressure-specified sensory device, DIGI-Grip and Pinch device (collectively “PSSD”);
WHEREAS, AxoGen and SMS wish to enter into a development license and option agreement for AxoGen to validate and commercialize an updated PSSD (Next Generation PSSD) and SMS to grant AxoGen a license under the Licensed Technology, as defined below, to make, to develop and commercialize such Next Generation PSSD product; and
WHEREAS, SMS wishes to grant to AxoGen, and AxoGen wishes to obtain, an option for AxoGen to purchase assets relating to its existing proprietary PSSD.
NOW, THEREFORE, in consideration of the mutual covenants set forth in this Agreement, AxoGen and SMS, intending to be legally bound, hereby agree as follows:
DEFINITIONS
Unless specifically set forth to the contrary herein, the following terms, whether used in the singular or plural, shall have the respective meanings set forth below:
“Affiliate” shall mean (a) any corporation or business entity of which fifty percent (50%) (or the maximum ownership interest permitted by law) or more of the securities or other ownership interests representing the equity, the voting stock or general partnership or membership interest are owned, controlled or held, directly or indirectly, by AxoGen or SMS, as applicable; (b) any corporation or business entity which, directly or indirectly, owns, controls or holds fifty percent (50%) (or the maximum ownership interest permitted by law) or more of the securities or other ownership interests representing the equity, the voting stock or general partnership or membership interest, of AxoGen or SMS, as applicable; (c) any corporation or business entity of which fifty percent (50%) or more of the securities or other ownership interests representing the equity, the voting stock or general partnership or membership interest are owned, controlled or held, directly or indirectly, by a corporation or business entity described in (a) or (b); and (d) possession, directly or indirectly, of the power to direct or cause the direction of management or policies of the entity in question (whether through ownership of securities or other ownership interests, by contract or otherwise).
“Applicable Laws” shall mean the applicable laws, rules, regulations, guidelines or other requirements of any governmental authority and Regulatory Authority, that may be in effect from time to time in the Territory.
“Assets” shall mean the Existing Product, Licensed Technology, Tangible Assets and Existing Product Regulatory Approvals.
“Calendar Quarter” shall mean any period of three (3) consecutive calendar months ending on March 31, June 30, September 30 or December 31.
“Calendar Year” shall mean a period of twelve consecutive (12) months commencing on January 1 and ending on December 31.
“Control” shall mean, when used in relation to an intellectual or other property right, the right of one Party to license or transfer such intellectual or other property right to the other Party without breaching any agreement or obligations to any other Third Party.
“Development Activities” shall mean any and all processes and activities conducted in researching, optimizing, developing and/or seeking, and obtaining Regulatory Approvals for the Existing Product or Next Generation Product. “Develop” and “Developing” shall have their correlative meanings.
1
“Existing Product” shall mean SMS’ existing proprietary PSSD device, including sensory measurement device, box, DIGIT-grip device, and Pinch device and including touch screen, laptop and PDA versions of such PSSD device, as described on Exhibit B.
“FDA” shall mean the United States Food and Drug Administration or any successor regulatory agency.
“Licensed Technology” shall mean all of the following: (a) the Patent Rights and (b) all SMS Know-How.
“Net Sales” shall mean, for any time period, the total of all invoiced gross sales in that time period by AxoGen or by an Affiliate of AxoGen, for Next Generation Products sold by AxoGen, an Affiliate of AxoGen, or a sublicensee of AxoGen to arm’s length purchasers (but excluding sales by AxoGen to an Affiliate or sublicensee for resale to such purchasers), net of, where applicable:
|
(b) |
price adjustments to customers’ inventories; |
|
(d) |
free goods, including without limitation promotional samples; |
|
(e) |
import and customs duties and taxes including sales, excise, turnover, inventory, value-added, and similar taxes assessed on the sale of the Existing Product (but excluding income taxes) to the extent separately included in the amount billed; |
|
(f) |
other payments required by law to be made under Medicaid, Medicare or other government special medical assistance programs; |
|
(g) |
transportation charges and insurance that are separately itemized; |
|
(h) |
credits or allowances for Existing Product returns and rejected Existing Product; |
|
(i) |
amounts repaid, credited or written off by reason of uncollectible debt, rejections, recalls, billing errors and returns; and |
|
(j) |
other allowances (including third party royalties) actually given by AxoGen or an Affiliate of AxoGen to Third Parties. |
“Next Generation Product” shall mean the PSSD product validated and updated by AxoGen pursuant to this agreement representing changes made to the Existing Product by AxoGen and its suppliers.
“Patent Rights” shall mean the patent and patent applications, if any, including those that have expired (a) Controlled or previously controlled by SMS (or any of its Affiliates) as of the Effective Date or at any time thereafter relating to Existing Product, and (b) necessary or useful for the development, manufacture or commercialization of the Next Generation Product, including: (i) the patents and patent applications set forth on Exhibit C; (ii) any divisions, continuations, continuations-in-part, reissues, re-examinations, renewals, extensions, supplementary protection certificates, and the like of any patent and patent applications set forth in subsection (i), and (c) foreign equivalents of subsections (i) and (ii).
“Regulatory Approval” shall mean the permission or consent granted by any relevant Regulatory Authority for the commercialization of the Existing Product and/or Next Generation Product.
“Regulatory Authority” shall mean any applicable government regulatory authority involved in granting approvals for the development, manufacture and commercialization of the Existing Product and Next Generation Product, including without limitation, in the United States, the FDA, and any successor government authority having substantially the same function, and foreign equivalents thereof.
“SMS Know-How” shall mean all information and materials (including but not limited to, discoveries, improvements, processes, copyrights (whether or not registered), computer software, proprietary reference data bases, customers lists, design history files including all change documentation and graphical user interface designs, data, inventions, know-how
2
and trade secrets, patentable or otherwise) relating to the Licensed Technology and to the Existing Product, which at the Effective Date or during the term of this Agreement: (a) are in the possession or Control of SMS or any of its Affiliates, (b) were not provided by AxoGen or an Affiliate of AxoGen, (c) are not generally known outside SMS and (d) are necessary or useful to AxoGen in connection with the development, manufacture or commercialization of an Existing Product and/or Next Generation Product.
“Tangible Assets” shall mean all tangible assets related to the Existing Product, including, but not limited to the assets on Exhibit A.
“Third Party” shall mean any person or entity that is not a Party or an Affiliate of a Party.
Other Definitions. The following definitions have the meanings ascribed to them in the corresponding Section:
|
Definition |
Section |
|
Agreement |
Introduction |
|
AxoGen |
Introduction |
|
AxoGen Indemnitees |
9.01 |
|
Confidential Information |
8.01 |
|
Deferred Purchase Price |
5.02 |
|
Disclosing Party |
8.01 |
|
Effective Date |
Introduction |
|
Exercise Fee |
5.02 |
|
Inventions |
6.01 |
|
Milestone |
5.01 |
|
Notice Period |
10.04 |
|
Option |
3.02(a) |
|
Option Term |
3.02(b) |
|
Party(ies) |
Introduction |
|
PSSD |
Recitals |
|
Quarterly Payment |
5.02 |
|
Receiving Party |
8.01 |
|
SMS |
Introduction |
|
SMS Indemnitees |
9.03 |
Article II.
DEVELOPMENT & COMMERCIALIZATION
Section 2.01 Development. AxoGen shall conduct the Development Activities to develop a Next Generation Product.
Section 2.02 Development Costs. All costs relating to development shall be the responsibility of AxoGen.
Section 2.03 Product Permits and Licenses. SMS shall obtain and maintain the necessary licenses and permits for sale of the Existing Product. AxoGen shall obtain and maintain the necessary licenses and permits for development, manufacture, testing, storage and commercialization of the Next Generation Product as required under this Agreement.
Article III.
GRANT OF RIGHTS
Section 3.01 License Grant from SMS to AxoGen. Subject to the terms and conditions of this Agreement, during the Term of this Agreement, SMS hereby grants to AxoGen and its Affiliates, subject to the license to be provided in Section 3.02 (c), an exclusive, irrevocable, perpetual, world-wide right and license with the right to grant sublicenses in the Licensed Technology and Existing Product, to develop, make, have made and use the Next Generation Product.
|
(a) |
Grant to AxoGen. Subject to the terms and conditions of this Agreement, during the Option Term of this Agreement, SMS hereby grants to Company an exclusive option to purchase the Assets (the “Option”). |
|
(b) |
Exercise of Option. AxoGen may exercise the Option any time from the Effective Date until April 1, 2016 (“Option Term”) by providing written notice to SMS (“Exercise Notice”). Within thirty (30) days of SMS |
3
receiving such Exercise Notice, SMS shall execute any and all documents to effectuate the transfer of the Assets to AxoGen, including but not limited to, executing, and causing any of its employees or Affiliates to execute assignment and assumption agreements. |
|
(c) |
Warranty on Existing Product. AxoGen will have no responsibility for service and warranty on Existing Products either prior to or after exercise of the Option and is not responsible, and assumes no obligation, for any liabilities of SMS or its Affiliates. |
Section 3.03 Option Payment No Implied License. Each Party acknowledges that the rights granted in this Agreement are limited to the terms and agreements expressly granted and provided in this Agreement. Accordingly, except for the rights expressly granted under this Agreement, no right, title, or interest of any nature whatsoever is granted whether by implication, estoppel, reliance, or otherwise, by either Party to the other Party.
Section 3.04 Claw Back of Assets After Option Exercise. During a period of 5 years from the exercise of the Option, if AxoGen does not continue to diligently pursue the commercialization, or once commercialized, the sale of Next Generation Products, SMS may demand, in its reasonable discretion, the return of the Assets, any Existing Products held by AxoGen and revocation of all licenses provided to AxoGen herein. Upon SMS’s notice of revocation to AxoGen, AxoGen must return the Assets and Existing Products to SMS within 60 days. After an effective revocation neither party will have any obligation to the other party as a result of this Agreement, except the return of Assets and Existing Product as provided in this Section 3.04.
Article IV.
REGULATORY
Section 4.01 SMS Data Technology Transfer. At the reasonable request of AxoGen, anytime after the Effective Date, SMS shall transfer, at no cost to AxoGen, except for costs AxoGen will pay associated with the physical transfer of any items both to receive such items or to send them back in the event the Option is not exercised or a revocation occurs pursuant to Section 3.04, all SMS Know-How that exists in written or electronic form. SMS shall provide to AxoGen all information required for AxoGen to update a design history file or update an existing Regulatory Filing or complete a new Regulatory Filing for the Next Generation Product.
PAYMENTS
Section 5.01 Milestone and Other Payments to SMS.
|
(a) |
Milestone Payments. In partial consideration for the license to Licensed Technology and Option granted to AxoGen under Article 3, AxoGen shall pay SMS the following amounts after the first achievement by AxoGen, its Affiliates or sublicensees, as the case may be, of each of the following milestones with respect to the Next Generation Product (each, a “Milestone”): |
|
Milestone |
|
Amounts (US dollars) |
|
|
|
Next Generation Product receives a reimbursement code from a Regulatory Authority in the United States |
|
$ |
100,000.00 |
|
|
Issuance of a Next Generation Product Patent that was originally approved by AxoGen for filing. |
|
$ |
25,000.00 |
|
|
Total of Potential Milestones |
|
$ |
125,000.00 |
|
|
(i) |
No Milestone payment shall be paid more than one time irrespective of the number of the Next Generation Products Developed; |
|
(ii) |
Payment shall not be owed for a Milestone that is not achieved; and |
|
(iii) |
Milestone payments shall be payable by AxoGen to SMS within forty-five (45) calendar days after achievement of the Milestone. |
|
(b) |
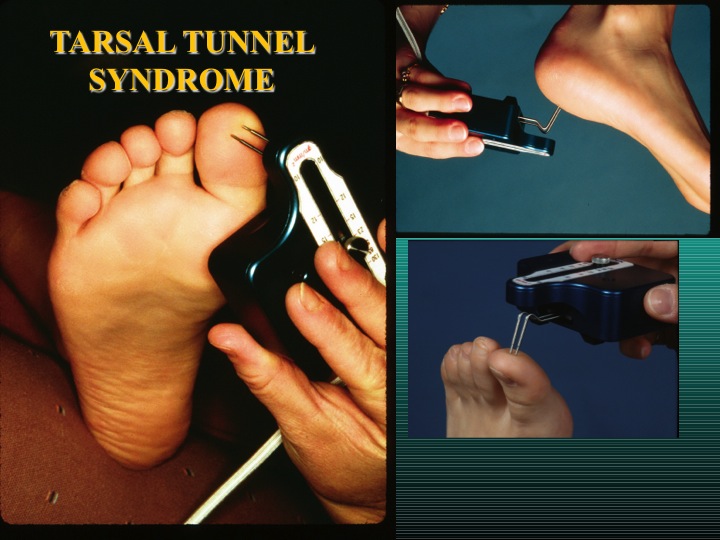
Other Payments. The Next Generation Product Patent as provided in Section 5.01 (a) provides for an instrument for use initially in the areas of acute nerve injury/regeneration in the upper or lower extremity, |
4
chronic nerve compression such as carpal tunnel syndrome and tarsal tunnel syndrome, and neuropathy related to chemotherapy, diabetes and unknown etiology, and its treatment as it relates to individual patients. If SMS or its Affiliates provide to AxoGen in writing inventions or concepts which result in granted patents to AxoGen outside of the Next Generation Product Patent, AxoGen will pay SMS or its Affiliates $25,000 per patent granted without any cap as to the maximum number of payments that may be made pursuant to this Section 5.01 (b). Establishment of such payments hereby is to provide SMS and its Affiliates the incentive to grow AxoGen’s product line and product usage beyond the New Generation Product Patent. Notwithstanding the forgoing, no payment will be owed if AxoGen can provide proof in writing that it conceived of any invention or concepts both (1) independently, without use or reference to information provided by SMS or its Affiliates, and (2) prior to the written disclosure provided by SMS or its Affiliates. |
Section 5.02 Purchase Price Upon Exercise of Option. Upon AxoGen’s exercise of the Option set forth in Section 3.01 and assignment of the Assets, AxoGen shall pay SMS (a) fifteen thousand dollars ($15,000.00) to purchase the Assets (“Exercise Fee”), (b) a fee equal to fifteen percent (15%) of Net Sales on the Existing or Next Generation Product (“Deferred Purchase Price”) for a period of 10 years from the Option exercise date; (c) SMS actual out of pocket costs for up to 5 Existing Products that they may have in inventory, such up to 5 Existing Products being included in the Assets, (d) AxoGen will provide SMS with one Next Generation Product from the first twenty Next Generation Products produced, and provide upgrades so long as the Deferred Purchase Price is being paid, at no cost to SMS ; and (d) AxoGen will assume the responsibility of deferred payments owed to Cybernetic Research Laboratories, Inc. by SMS on the sale of Existing Products to a maximum of $39,500 (it being agreed that such amount prior to exercise of the Option will be reduced by $500 for every Existing Product sold by SMS after the execution of this Agreement).
Payment of the Deferred Purchase Price shall be made sixty (60) days after the end of each Calendar Quarter on all Net Sales in the preceding quarter (“Quarterly Payment”). Each Quarterly Payment shall be accompanied by a report detailing the total Net Sales for the preceding Calendar Quarter.
Section 5.03 Exempt Sales. The Deferred Purchase Price shall not be payable under Section 5.02 above with respect to sales of the Existing Product among AxoGen, its Affiliates and sublicensees for resale to a Third Party. In no event shall AxoGen make payments to SMS hereunder with respect to the sale of any Existing Product to its Affiliates or sublicensees.
Section 5.04 Currency and Manner of Payment. Payments under this Agreement shall be made in United States dollars. All sums due to SMS under this Agreement shall be payable in immediately available fund.
Section 5.05 Tax Withholding. Any tax, duty or other levy paid or required to be withheld by AxoGen or its sublicensees on account of Deferred Purchase Price or other payments payable to SMS under this Agreement shall be deducted from the amount of Deferred Purchase Price or payments otherwise due, provided that AxoGen shall make such deductions only to the minimum extent required by the relevant jurisdiction. AxoGen shall secure and send to SMS proof of any such taxes, duties or other levies withheld and paid by AxoGen or its sublicensees for the benefit of SMS, and cooperate at SMS’s reasonable request and expense to ensure that amounts withheld are reduced to the fullest extent permitted by the relevant jurisdiction.
INTELLECTUAL PROPERTY
Section 6.01 Ownership of Inventions. All inventions, discoveries, data, work product, results and information (“Inventions”) conceived, generated, discovered or made by any Party, its employees, agents and consultants in connection with this Agreement, the Next Generation Product, use of the Licensed Technology and/or Tangible Assets shall be owned by AxoGen.
Section 6.02 Disclosure of Patent Rights. SMS shall ensure that only those of its and its Affiliate’s personnel or permitted sub-contractors who are necessary to perform SMS’ obligations under this Agreement shall perform work under this Agreement and have access to the Confidential Information of AxoGen. SMS shall require and ensure that all such personnel, prior to performing any work under this Agreement, shall have: (a) been advised of SMS’ obligations of confidentiality under this Agreement and have agreed in writing to confidentiality obligations at least as strict as those applicable to SMS under this Agreement; and (b) signed and delivered to SMS written agreements assigning to SMS all Inventions, discoveries and works of authorship arising from or related to work performed under this Agreement; each to the extent permitted by applicable law. For clarity, SMS shall require Affiliates to execute an employee invention disclosure
5
and assignment agreement, and AxoGen shall be a third-party beneficiary to such agreement. SMS shall reasonably disclose to AxoGen in writing any and all Inventions or improvements, whether patentable or not, made or conceived in connection with, and all data and information arising from, this Agreement. Such disclosures of Inventions shall identify all inventors, the date such Inventions were conceived, and shall describe such inventions in sufficient detail to permit the other Party to evaluate the ownership, subject matter and patentability of such Inventions.
Section 6.03 Filing, Prosecution, Maintenance. AxoGen shall have the sole right, using in-house or outside legal counsel selected at AxoGen’s sole discretion, to prepare, file, prosecute, maintain and extend patent applications and patents concerning all Inventions.
Section 6.04 Enforcement of Patent Rights. In the event that a Party learns that any of the Patent Rights are infringed or misappropriated by activities of a Third Party, such Party shall promptly notify in writing the other Party hereto. AxoGen shall have the initial right (but not the obligation) to enforce such Patent Rights and SMS agrees to join such proceeding, at AxoGen’s expense, if required by law for AxoGen to bring such action. AxoGen agrees to indemnify SMS, its Affiliates and Xxxxxx Xxxxx and A. Xxx Xxxxxx for any expenses actually and reasonably paid or incurred by SMS in connection with any action or proceeding by AxoGen to enforce such Patent Rights, except for such actions or proceedings that are a result of activity by such parties prior to the Effective Date and not disclosed to AxoGen. In the event AxoGen is required to provide indemnification pursuant to the sentence immediately preceding this sentence, SMS, its Affiliates and Xxxxxx Xxxxx and A. Xxx Xxxxxx shall have the right to advancement by AxoGen of any and all expenses actually and reasonably paid or incurred in connection with AxoGen’s enforcement of its Patent Rights.
Section 6.05 Infringement Claims. If the manufacture, sale, use or importation of an Existing Product or Next Generation Product results in any claim, suit or proceeding alleging patent infringement against SMS or AxoGen, such Party shall promptly notify in writing the other Party hereto. If AxoGen is named as a party to such claim, suit or proceeding, or if AxoGen intervenes, at its own expense and through counsel of its own choice, in any such claim, suit or proceeding initiated against SMS (which intervention SMS will not oppose), AxoGen shall have the right to control the defense and settlement of such claim, suit or proceeding, at its own expense, using counsel of its own choice, provided that SMS shall have the right to participate in the defense at its own expense. No Party shall enter into any agreement that makes any admission regarding (a) wrongdoing on the part of the other Party, or (b) the invalidity, unenforceability or infringement of any Patent Rights, without the prior written consent of the other Party. The Parties shall cooperate with each other in connection with any such claim, suit or proceeding and shall keep each other reasonably informed of all material developments in connection with any such claim, suit or proceeding.
REPRESENTATIONS, WARRANTIES & COVENANTS
Section 7.01 Mutual Representations and Warranties. Each Party warrants and represents to the other that:
|
(a) |
as of the Effective Date, it has the full right and authority to enter into this Agreement; |
|
(b) |
as of the Effective Date, there are no existing or threatened actions, suits or claims pending against it with respect to its right to enter into and perform its obligations under this Agreement; |
|
(c) |
there is nothing in any Third Party agreement or understanding, written or oral, entered into or agreed to by such Party as of the Effective Date, that, in any way, will preclude such Party’s ability to perform all of the obligations undertaken by it hereunder, and that it will not enter into any agreement after the Effective Date under which such performance would be precluded; |
|
(d) |
it is not a party to any agreement or arrangement with any Third Party or under any obligation or restriction agreement (including any outstanding order, judgment or decree of any court or administrative agency) which in any way limits or conflicts with its ability to fulfill any of its obligations under this Agreement; |
|
(e) |
this Agreement is a legal and valid obligation binding upon it and enforceable in accordance with its terms. The execution, delivery and performance of this Agreement by such Party does not violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it; and |
|
(f) |
it has never been, is not currently, and, during the term of this Agreement, will not become, a Debarred Entity, Excluded Entity or Convicted Entity. The Parties further warrant and represent that no Debarred Individual, Debarred Entity, Excluded Individual, Excluded Entity, Convicted Individual or Convicted Entity has performed |
6
or rendered, or will perform or render, any services or assistance on its behalf relating to activities taken pursuant to this Agreement. |
|
(iv) |
A “Debarred Individual” is an individual who has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) from providing services in any capacity to a person that has an approved or pending drug product application, or an employer, employee or partner of a Debarred Individual; |
|
(v) |
A “Debarred Entity” is a corporation, partnership or association that has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) from submitting or assisting in the submission of any abbreviated drug application, or an employee, partner, shareholder, member, subsidiary or affiliate of a Debarred Entity; |
|
(vi) |
An “Excluded Individual” or “Excluded Entity” is (x) an individual or entity who has been excluded, debarred, suspended or is otherwise ineligible to participate in federal health care programs such as Medicare or Medicaid by the Office of the Inspector General (OIG/HHS) of the U.S. Department of Health and Human Services, or (y) is an individual or entity who has been excluded debarred, suspended or is otherwise ineligible to participate in federal procurement and non-procurement programs, including those produced by the U.S. General Services Administration (GSA); and |
|
(vii) |
A “Convicted Individual” or “Convicted Entity” is an individual or entity who has been convicted of a criminal offense that falls within the ambit of 42 U.S.C. §1320a – 7(a), but has not yet been excluded, debarred, suspended or otherwise declared ineligible. |
|
(g) |
“Notwithstanding anything to the contrary in this Agreement, as a material part of the consideration for this Agreement, AxoGen and SMS agree that AxoGen is taking the Existing Product "AS IS" with any and all latent and patent defects and that there is no warranty by Seller that the Existing Product is fit for a particular purpose. AxoGen acknowledges that it is not relying upon any representation, statement or other assertion with respect to the Existing Product condition, but is relying upon its own due diligence, including its own examination of the Existing Product. AxoGen takes the Existing Product under the express understanding there are no express or implied warranties (except for limited warranties of title set above.) |
Section 7.02 SMS Representations, Warranties & Covenants. SMS represents, warrants and covenants to AxoGen that:
|
(a) |
SMS owns all right, title and interest in and to the Assets; |
|
(b) |
SMS has not entered into any licensing or other agreement with any Third Party in conflict with the rights granted to AxoGen hereby; |
|
(c) |
SMS has full control of the Licensed Technology and Assets, is entitled to grant the rights, licenses and options granted under Article 3, and is not currently subject to any Third Party agreement or to any outstanding order, judgment or decree of any court or administrative agency that restricts it in any way from using the Licensed Technology or Assets or from licensing, sublicensing or assigning to AxoGen, any know-how or patent rights that would otherwise be necessary or useful for the manufacture, development or commercialization of the Next Generation Product; |
|
(d) |
there are no existing or threatened actions, suits or claims pending against SMS with respect to the Existing Product, Assets and the Licensed Technology; |
|
(e) |
as of the Effective Date, SMS has not granted, and will not grant during the term of this Agreement, any right, license or interest in or to the Licensed Technology that is in conflict with the rights or licenses granted under this Agreement, nor, as of the Effective Date, has it encumbered any Asset, SMS Know-How and/or Patent Rights and SMS will not encumber any Asset, SMS Know-How and/or Patent Rights; |
|
(f) |
the Licensed Technology licensed or sublicensed to AxoGen pursuant to this Agreement has not been obtained by SMS or its Affiliates (or its or their predecessors-in-interest) in violation of any contractual or fiduciary obligation owed by any of them to a Third Party or by misappropriation of the trade secrets of any Third Party; |
|
(g) |
the Patent Rights listed on Exhibit C list all Patent Rights SMS had relating to the Licensed Technology. To the best of SMS’s knowledge, the issued claims under any issued Patent Rights were valid and in full force and effect; |
|
(h) |
SMS has no knowledge of any infringement by any Third Party of any of the Licensed Technology as of the Effective Date; |
7
|
(i) |
upon AxoGen’s exercise of the Option, SMS shall not, directly or indirectly, make, use, or sell any Existing Product or any Next Generation Product to any Third Party; |
|
(j) |
Except for the sale of Existing Product as provided herein prior to exercise of the Option and allowance to distribute pursuant to Section 3.02(c), during the Term, SMS and its Affiliates will not distribute, sell, develop or advise others regarding instruments, devices or products that could compete with the Existing Product, Next Generation Product or any similar diagnostic product or device developed by the Company. |
|
(k) |
Approval necessary from all Regulatory Authorities and are legally adequate to allow for its sales and distribution and is exactly as described in applicable 510(k) clearance letters, except as to any and all changes that have been made to the device since any 510(k) was first released to market as provided in Exhibit A; |
|
(l) |
any 510(k) applicable to the Existing Product has not been previously transferred to another party; |
|
(m) |
has provide complete copies of applicable 510(k)s for the Existing Products including correspondence between SMS and any of its Affiliates and FDA, all letter to files concerning changes to labeling, components or design and the design history file including all change documentation; and |
|
(n) |
has provided complaint files, reports of adverse events, removals and device master records for the Existing Product. |
CONFIDENTIALITY AND EXCHANGE OF INFORMATION
Section 8.01 Confidential Information. As used in this Agreement, the term “Confidential Information” means, except as set forth in this Section 8.01, all information that is secret, confidential or proprietary, whether provided in written, oral, graphic, video, electronic or other form, provided pursuant to this Agreement by a Party (the “Disclosing Party”) to the other Party (the “Receiving Party”); provided that the Disclosing Party shall prominently xxxx any such tangible or electronic documents as “Confidential” and shall identify any such orally or visually disclosed information as “Confidential” at the time of first disclosure to the Receiving Party and shall summarize such Confidential Information in a writing delivered to Receiving Party within thirty (30) days after such first disclosure. Notwithstanding the foregoing sentence, Confidential Information shall exclude any information or materials that:
|
(a) |
was already known to the Receiving Party before receipt of such information under this Agreement; |
|
(c) |
is hereafter disclosed to the Receiving Party without restriction by a Third Party having a legal right to make such disclosure; or |
|
(d) |
is or becomes part of the public domain through no breach of this Agreement by the Receiving Party. |
Section 8.02 Confidentiality Obligations. During the term of this Agreement and for seven (7) years thereafter, the Receiving Party shall exercise all reasonable care to prevent the disclosure of Confidential Information received from the Disclosing Party and shall not use or permit to be used such Confidential Information for any purpose other than that expressly permitted under this Agreement without the Disclosing Party’s prior written approval. The Receiving Party may disclose Confidential Information of the Disclosing Party to the Receiving Party’s employees, consultants, contractors and Affiliates so long as such persons agree in writing, before such disclosure, to abide by non–disclosure obligations at least as strict as with those set forth in this Article 8.
Section 8.03 Required Disclosures. Nothing in this Agreement shall be construed to restrict the Parties from disclosing Confidential Information as required by law or court order or other governmental order or request, provided in each case the Party requested to make such disclosure shall timely inform the other Party and use all reasonable efforts to limit the disclosure and maintain the confidentiality of such Confidential Information to the extent possible. In addition, the Party requested to make such disclosure will allow the other Party to prevent or limit such disclosure by appropriate legal means.
Section 8.04 Public Announcements. No public announcement or other disclosure to any Third Party concerning the existence of, terms, or subject matter of this Agreement shall be made, either directly or indirectly, by any Party to this Agreement, except as may be legally required or as may be required for recording purposes, without first obtaining the approval of the other Party (not to be unreasonably withheld) and agreement upon the nature and text of such announcement or disclosure; provided, however, that each Party shall be entitled to disclose this Agreement or its terms (a) as required by law, including without limitation any disclosure requirements imposed by a stock exchange or securities regulatory agency
8
or CMS, and (b) to such Party’s financial, tax and legal advisors, and to potential investors, corporate partners or acquirers, in each case provided that such persons or entities have confidentiality obligations at least as stringent as set forth in this Article 8. The Party desiring to make any legally-required public announcement or other disclosure (including those which may be required for recording purposes) shall inform the other Party of the proposed announcement or disclosure in reasonably sufficient time prior to public release, which shall be at least ten (10) business days prior to release of such proposed announcement or disclosure, and shall provide the other Party with a written copy thereof, in order to allow such other Party to comment upon such announcement or disclosure. Each Party agrees that it shall cooperate fully with the other with respect to all disclosures regarding this Agreement to the Securities Exchange Commission and any other governmental or regulatory agencies, including requests for confidential treatment of Confidential Information of either Party included in any such disclosure.
Section 8.05 Bankruptcy. All Confidential Information disclosed by one Party to the other Party shall remain the intellectual property of the Disclosing Party. In the event that a court or other legal or administrative tribunal, directly or through an appointed master, trustee or receiver, assumes partial or complete control over the assets of a Party to this Agreement based on the insolvency or bankruptcy of such Party, the bankrupt or insolvent Party shall promptly notify the court or other tribunal (a) that Confidential Information received from the other Party under this Agreement remains the property of the other Party, and (b) of the confidentiality obligations under this Agreement. In addition, the bankrupt or insolvent Party shall, to the extent permitted by law, take all steps necessary or desirable to maintain the confidentiality of the other Party’s Confidential Information and to ensure that the court, other tribunal or appointee maintains such information in confidence in accordance with the terms of this Agreement.
Article IX.
INDEMNIFICATION
Section 9.01 Indemnification of AxoGen. SMS shall defend, indemnify and hold harmless AxoGen and its Affiliates, and their respective directors, officers, employees, agents and counsel, and the successors and assigns of the foregoing (the “AxoGen Indemnitees”), from and against any and all liabilities, damages, losses, costs or expenses (including reasonable attorneys’ and professional fees and other expenses of litigation and/or arbitration) incurred in connection with a claim, suit or proceeding brought by a Third Party against an AxoGen Indemnitee, arising from or occurring as a result of: (a) the failure by SMS to take reasonable measures to obtain or maintain rights under the Licensed Technology sufficient to grant AxoGen a license under Section 3.01 and an Option under Section 3.02(a) of this Agreement; (b) SMS’ development, manufacture and commercialization of the Existing Product, including all warranties and service costs for the Existing Product prior to exercise of the Option, or (c) SMS’s material breach of any representation or warranty set forth in Section 7.01 or 7.02, except, in each case, to the extent caused by the negligence or willful misconduct of a AxoGen Indemnitee.
Section 9.02 Procedure. An AxoGen Indemnitee that intends to claim indemnification under this Article 9 shall promptly notify SMS in writing of any loss, claim, damage, liability or action in respect of which the AxoGen Indemnitee or any of its Affiliates, sublicensees or their directors, officers, employees, agents or counsel intend to claim such indemnification, and SMS shall have the right to participate in, and, to the extent SMS so desires, to assume the defense thereof with counsel of its own choice, subject to AxoGen’s approval of such counsel, which approval may not be unreasonably withheld. The indemnity agreement in this Article 9 shall not apply to amounts paid in settlement of any loss, claim, damage, liability or action if such settlement is made without the consent of SMS, which consent shall not be withheld unreasonably. The failure to deliver written notice to SMS within a reasonable time after the commencement of any such action, if materially prejudicial to its ability to defend such action, shall relieve SMS of any liability to the AxoGen Indemnitee under this Article 9. At SMS’ request, the AxoGen Indemnitee under this Article 9, and its employees and agents, shall cooperate fully with SMS and its legal representatives in the investigation and defense of any action, claim or liability covered by this indemnification and provide full information with respect thereto.
Section 9.03 Indemnification of SMS. AxoGen shall defend, indemnify and hold harmless SMS and its Affiliates, and their respective directors, officers, employees, agents and counsel, Xxxxxx Xxxxx and A. Xxx Xxxxxx, and the successors and assigns of the foregoing (the “SMS Indemnitees”), from and against any and all liabilities, damages, losses, costs or expenses (including reasonable attorneys’ and professional fees and other expenses of litigation and/or arbitration) incurred in connection with a claim, suit or proceeding brought by a Third Party against a SMS Indemnitee, arising from or occurring as a result of: (a) AxoGen’s development, manufacture and commercialization of the Next Generation Product, including all warranties and service costs for the Next Generation Product, or (b) AxoGen’s material breach of any representation or warranty set forth in this Agreement, except, in each case, to the extent caused by the negligence or willful misconduct of a SMS Indemnitee. The SMS Indemnitees shall have the right to the advance of reasonable attorneys’ fees and expenses in
9
connection with defending against any Third party claim, suit or proceeding arising from or in connection with the Development Activities or AxoGen’s negligence, misconduct or fraud.
Section 9.04 Procedure. An SMS Indemnitee that intends to claim indemnification under this Article 9 shall promptly notify AxoGen in writing of any loss, claim, damage, liability or action in respect of which the SMS Indemnitee or any of its Affiliates, sublicensees or their directors, officers, employees, agents or counsel intend to claim such indemnification, and AxoGen shall have the right to participate in, and, to the extent AxoGen so desires, to assume the defense thereof with counsel of its own choice, subject to SMS Indemnitee’s approval of such counsel, which approval may not be unreasonably withheld. The indemnity agreement in this Article 9 shall not apply to amounts paid in settlement of any loss, claim, damage, liability or action if such settlement is made without the consent of AxoGen, which consent shall not be withheld unreasonably. The failure to deliver written notice to AxoGen within a reasonable time after the commencement of any such action, if materially prejudicial to its ability to defend such action, shall relieve AxoGen of any liability to the SMS Indemnitee under this Article 9. At AxoGen’s request, the SMS Indemnitee under this Article 9, and its employees and agents, shall cooperate fully with AxoGen and its legal representatives in the investigation and defense of any action, claim or liability covered by this indemnification and provide full information with respect thereto.
Limitation of Liability.
|
(a) |
EXCEPT FOR A PARTY’S INDEMNIFICATION OBLIGATIONS, NEITHER PARTY WILL BE LIABLE TO THE OTHER PARTY FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, INDIRECT OR PUNITIVE DAMAGES, INCLUDING WITHOUT LIMITATION, LOST PROFITS OR LOST REVENUES, ARISING OUT OF THIS AGREEMENT, HOWEVER CAUSED, UNDER ANY THEORY OF LIABILITY, even if SUCH PARTY has been advised of the possibility of such damages. |
|
(b) |
AXOGEN SHALL NOT BE LIABLE TO SMS OR ANY THIRD PARTY FOR ANY DAMAGES ARISING OUT OF the DEVELOPMENT, MANUFACTURE OR COMMERCIALIZATION OF THE EXISTING PRODUCT BY SMS, ITS AFFILIATES, SUBLICENSEES OR ANY THIRD PARTY. |
TERM AND TERMINATION
Section 10.01 Term. This Agreement shall commence on the Effective Date and shall continue in full force and effect until terminated pursuant to this Article 10, provided, however, this Agreement will expire at the end of the Option Term if AxoGen has not exercised the Option and if the Option is exercised it will terminate 10 years from the date of such Option exercise.
Section 10.02 Termination for Material Breach. Either Party shall have the right to terminate this Agreement in the event the other Party has materially breached or defaulted in the performance of any of its obligations hereunder, and if such breach or default is not corrected within sixty (60) days after the breaching Party receives written notice identifying such breach.
Section 10.03 Termination for Insolvency; Retention of License. If voluntary or involuntary proceedings by or against a Party are instituted in bankruptcy under any insolvency law, or a receiver or custodian is appointed for such Party, or proceedings are instituted by or against such Party for corporate reorganization or the dissolution of such Party, which proceedings, if involuntary, shall not have been dismissed within ninety (90) days after the date of filing, or if such Party makes an assignment for the benefit of creditors, or substantially all of the assets of such Party are seized or attached and not released within ninety (90) days thereafter, the other Party may immediately terminate this Agreement effective upon notice of such termination. Notwithstanding the bankruptcy of a Party, or the impairment of performance by a Party of its obligations under this Agreement as a result of bankruptcy or insolvency of such Party, and subject to such other Party’s rights to terminate this Agreement for reasons other than bankruptcy or insolvency as expressly provided in this Agreement, the other Party shall be entitled to retain the licenses under the terms and conditions granted herein.
Section 10.04 Termination by AxoGen. Prior to exercise of the Option, if any, AxoGen shall have the right to terminate this Agreement in its sole discretion in its entirety by providing thirty (30) days written notice thereof of the termination of this Agreement (the “Notice Period”).
Section 10.05 General Effect of Termination.
|
(a) |
Accrued Obligations. Termination of this Agreement for any reason shall not release any Party hereto from any liability which, at the time of such termination, has already accrued to the other Party or which is attributable to a |
10
|
(b) |
Return of Materials. Upon any termination of this Agreement, each Party shall promptly return to the other Party all materials and tangible Confidential Information received from the other Party (except one copy of which may be retained by legal counsel for archival purposes). |
Section 10.06 Bankruptcy Provisions. All rights and distribution rights granted under or pursuant to this Agreement by SMS to AxoGen are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of rights to “intellectual property” as defined under Section 101(52) of the U.S. Bankruptcy Code. The Parties agree that AxoGen, as licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code or any comparably rights under other applicable bankruptcy law, subject to performance by AxoGen of its preexisting obligations under this Agreement. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against SMS, AxoGen shall be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, and same, if not already in its possession, shall be promptly delivered to AxoGen (a) upon any commencement of any bankruptcy proceeding upon written request therefore by AxoGen, unless SMS elects to continue to perform all of its obligations under this Agreement, or (b) if not delivered under (a) above, upon the rejection of this Agreement by or on behalf of SMS upon written request therefore by AxoGen, provided, however, that upon SMS’s (or its successor’s) written notification to AxoGen that it is again willing and able to perform all of its obligations under this Agreement, AxoGen shall promptly return all such tangible materials to SMS, but only to the extent that AxoGen does not require continued access to such materials to enable AxoGen to perform its obligations under this Agreement.
Section 10.07 Survival. Articles 7, 8 and 9 and Sections 11.02 and 11.04, shall survive the expiration or termination of this Agreement for any reason. In addition, any other provision required to interpret and enforce the Parties’ rights and obligations under this Agreement shall also survive, but only to the extent required for the observation and performance of the aforementioned surviving portions of this Agreement.
MISCELLANEOUS
Section 11.01 Governing Law; Equitable Relief.
|
(a) |
This Agreement shall be governed by and interpreted in accordance with the laws of the State of Maryland without giving effect to any conflict of laws provisions, except matters of intellectual property that will be determined in accordance the intellectual property laws relevant to the intellectual property in question. |
|
(b) |
No provision herein shall be construed as precluding a Party from bringing an action for injunctive relief or other equitable relief prior to the initiation or completion of the above procedure. |
Section 11.02 Waiver. Neither Party may waive or release any of its rights or interests in this Agreement except in writing. The failure of either Party to assert a right hereunder or to insist upon compliance with any term or condition of this Agreement shall not constitute a waiver of that right or excuse a similar subsequent failure to perform any such term or condition. No waiver by either Party of any condition or term in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term.
Section 11.03 Assignment. This Agreement shall not be assignable by SMS without the written consent of AxoGen. The terms and conditions of this Agreement shall be binding on and inure to the benefit of the permitted successors and assigns of the Parties.
Section 11.04 Notices. Any notices, requests and other communications hereunder shall be in writing and shall be personally delivered or sent by international express delivery service, registered or certified air mail, return receipt requested, postage prepaid, or by facsimile (confirmed by prepaid registered or certified air mail letter or by international express delivery mail) (e.g., FedEx)), and shall be deemed to have been properly served to the addressee upon receipt of such written communication, to the following addresses of the Parties, or such other address as may be specified in writing to the other Parties hereto:
11
|
if to AxoGen: |
AxoGen Corporation |
|
|
13630 Xxxxxxxx Xxxx. |
|
|
Xxxxx 000 |
|
|
Xxxxxxx, XX 00000 |
|
|
Attention: Xxxxx Xxxxxxx |
|
|
Fax: 000-000-0000 |
|
|
|
|
if to SMS: |
Sensory Management Services LLC |
|
|
|
|
|
10 Lxxx Xxx Xxx |
|
|
Xxxx 0 |
|
|
Xxxxxxxxx, Xxxxxx 00000 |
|
|
Attention: Xxx Xxxxxx |
|
|
|
|
|
And |
|
|
|
|
|
1122 Xxxxxxxxxxx Xxxxx |
|
|
Xxxxx 00 |
|
|
Xxxxxx, Xxxxxxxx 00000 |
|
|
Attention: Xxx Xxxxxx |
|
|
|
|
|
Attention: Xxx Xxxxxx |
|
Fax: 000-000-0000 |
Section 11.05 Force Majeure. Neither Party shall be liable to the other for failure or delay in the performance of any of its obligations under this Agreement for the time and to the extent such failure or delay is caused by earthquake, riot, civil commotion, terrorism, war, hostilities between nations, governmental law, order or regulation, embargo, action by the government or any agency thereof, act of God, storm, fire, accident, labor dispute or strike, sabotage, explosion or other similar or different contingencies, in each case, beyond the reasonable control of the respective Party. The Party affected by force majeure shall provide the other Party with full particulars thereof as soon as it becomes aware of the same (including its best estimate of the likely extent and duration of the interference with its activities), and will use its best endeavors to overcome the difficulties created thereby and to resume performance of its obligations as soon as practicable. If the performance of any obligation under this Agreement is delayed owing to a force majeure for any continuous period of more than six (6) months, the Parties hereto shall consult with respect to an equitable solution including the possible termination of this Agreement.
Section 11.06 Independent Contractors. Nothing contained in this Agreement is intended implicitly, or is to be construed, to constitute AxoGen or SMS as partners or joint venturers in the legal sense. No Party hereto shall have any express or implied right or authority to assume or create any obligations on behalf of or in the name of any other Party or to bind any other Party to any contract, agreement or undertaking with any Third Party.
Section 11.07 Severability. If any of the terms or provisions of this Agreement are in conflict with any applicable statute or rule of law, then such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith and shall be deemed to be modified to conform with such statute or rule of law. In the event that the terms and conditions of this Agreement are materially altered as a result of the above, the Parties will renegotiate the terms and conditions of this Agreement to resolve any inequities
Section 11.08 Further Assurances. At any time or from time to time on and after the date of this Agreement, either Party shall at the request of the other Party (i) deliver to the requesting Party such records, data or other documents consistent with the provisions of this Agreement, (ii) execute, and deliver or cause to be delivered, all such consents, documents or further instruments of assignment, transfer or license, and (iii) take or cause to be taken all such actions, as the requesting Party may reasonably deem necessary or desirable in order for the requesting Party to obtain the full benefits of this Agreement and the transactions contemplated hereby.
12
Section 11.09 Entire Agreement. This Agreement constitutes the entire agreement, both written and oral, with respect to the subject matter hereof, and supersedes and terminates all prior or contemporaneous understandings or agreements, whether written or oral, between AxoGen and SMS with respect to such subject matter. No terms or provisions of this Agreement shall be varied or modified by any prior or subsequent statement, conduct or act of either of the Parties, except that the Parties may amend this Agreement by written instruments specifically referring to and executed by authorized representatives of each Party in the same manner as this Agreement.
Section 11.10 Headings. The captions to the Articles and Sections hereof are not a part of this Agreement, but are included merely for convenience of reference only and shall not affect its meaning or interpretation.
Section 11.11 Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original and all of which together shall constitute one and the same instrument.
Section 11.12 Venue. Any dispute arising under or in connection with the agreement or related to any matter which is the subject of the agreement shall be subject to the exclusive jurisdiction of the state and/or federal courts located in Baltimore, Maryland.
Section 11.13 Arbitration. Any controversy or claim arising out of or relating to this contract, or the breach thereof, shall be settled by arbitration administered by the American Arbitration Association in the State of Maryland and City of Baltimore in accordance with its Commercial or other Arbitration Rules, and judgment on the award rendered by the arbitrator[s] may be entered in any court having jurisdiction thereof.
Section 11.14 Assignment. This Agreement or the right to receive royalties hereunder may be assigned by SMS without the consent of AxoGen. AxoGen may assign this Agreement without consent in the event of a merger or acquisition of the Company, sale of substantially all of the Company’s assets or a change of greater than 50% of the ownership of the Company or its parent Company AxoGen Inc.
13
IN WITNESS WHEREOF, the Parties hereto have caused this Development, License and Option Agreement to be duly executed by their authorized representatives effective as of Effective Date.
|
Sensory Management Services LLC |
AxoGen Corporation |
||
|
By: |
/s/ A Xxx Xxxxxx |
By: |
/s/Xxxxx Xxxxxxx |
|
Name: |
A Xxx Xxxxxx |
Name: |
Xxxxx Xxxxxxx |
|
Title: |
President |
Title: |
CEO |
|
Date: |
11/5/14 |
Date: |
11/3/2014 |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/Xxxxxx X. Xxxxx |
|
|
|
Name: |
Xxxxxx X. Xxxxx |
|
|
|
Title: |
CEO |
|
|
|
Date: |
11/5/14 |
|
|
14
EXHIBIT A
ASSETS
1.Regulatory Approvals (including filings and changes since filings)
2.Design history files
3.Quality systems
4.Customer files
5.Product molds
6.CAD drawings
7.Existing inventory of the Existing Product
8.Customer Lists
15
EXHIBIT B
EXISTING PRODUCT
(From: SMS Assets List June 2013)
Copyright to Instructional Manuals
1.Xxxxxx D, Xxxxxxx XX, Xxxxxx AL:
Interpretation Guide to Neurosensory and Motor Testing
Sensory Management Services, LLC, publisher Baltimore, MD, 2002.
2.Xxxxxx, D, Motwani, L, Dellon, AL,
Interpretation Guide to Neurosensory and Motor Testing: The PDA Platform
Sensory Management Services, LLC, publisher, Nevada, 2007.
3.Xxxxxx, D, Xxxxxxx, L, Xxxxxx, AL,
Interpretation Guide to Neurosensory and Motor Testing: The Touch-Screen Platform,
Sensory Management Services, LLC, publisher, Nevada, 2013.
Copyright to Instructional Video
1.Dellon, A.L., Computer-Assisted Sensorimotor Testing, 1995.
Artwork
1.The Pressure-Specified Sensory: Framed Schematic
Trademarks
1.Pressure-Specified Sensory Device™
2.Disk-Criminator™
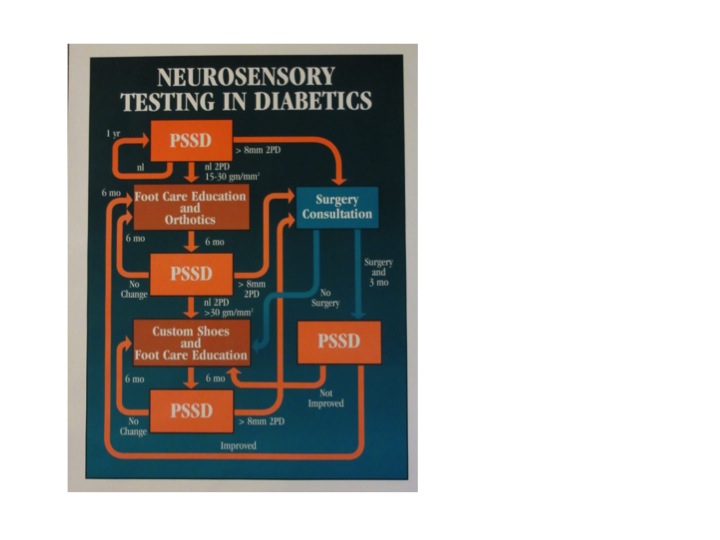
Neurosensory Testing Algorithm
16
Administrative Law Judge Proceedings:
1.Medicare copies of medicare procedings, ALJ DECISIONS, ETC related to the PSSD, AND THE SURGERY
Neurosensory Testing Lecture
1.Powerpoint, (153 MB)
Collection of PSSD Reprints
1.Binder with 180 different reprints related to the PSSD
17
EXHIBIT C
PATENT RIGHTS
1.Disk-Criminatortm, Canadian Patent #1,282,660, 1991;
Xxxxxxxxx, X.X., and Xxxxxx, A.L.,
Registration Numbers: Canadian TMA336,211; United States 1,511,664
2.Pressure Specifying Sensory Device, Patent #5,027,828, 1991;
Dellon, A.L. and Xxxxxxxxx, N.
3.Digit-Grip, Patent #5, 317, 916, 1992;
Dellon, A.L., Xxxxxxxxx, N.
Sensory Management Services, LLC
4.Force-Defined Vibrometer, Patent pending, 1991;
Dellon, A.L. and Xxxxxxxxx, N.
5.Skin Compliance Device, Patent #5, 373, 730, 1992;
Dellon, A.L. and Xxxxxxxxx, N.
6.Breast Compliance Device, Patent #346, 124, 1993;
Dellon, A.L., Xxxxxxxxx, N
Patent Applications:
1.11/643,281 System and method for managing neurosensory test information.
2.11/643,398 Apparatus and method for testing of neurosensory sensitivity.
3.11/643,205 Apparatus and method for testing of neurosensory response.
4.29/270,609 Portable Medical Device.
18

