Agreement for Delivery and Licensed Use of Data Generated from OT-101 U.S. Expanded Access (Data License 1 Agreement) between WideTrial and Oncotelic
Agreement
for Delivery and Licensed Use of Data Generated from OT-101 U.S. Expanded Access
(Data License 1 Agreement)
between WideTrial and Oncotelic
This Agreement is made and entered into on September 5, 2019 (the “Effective Date”), by and between WideTrial Inc., a corporation headquartered at 0 Xxx Xxxxx, Xxxxx 0000, Xxxxx, XX 00000 (“WideTrial) (the “Licensor”), and Oncotelic Inc., headquartered at 00000 Xxxxxx Xx. Xxx 000, Xxxxxx Xxxxx, XX 00000 (“Oncotelic”) (the “Licensee”) (each a “Party”, collectively “Parties” to this bilateral Agreement).
WHEREAS, Licensor and Licensee intend to collaborate as Sponsor and Manufacturer, respectively, in a U.S. Expanded Access Program for treatment use of OT-101 for 25 or more patients in pancreatic cancer and other cancers known to overexpress TGF-B2 (“USEAPOT101”, “The Expanded Access Program”, “The EAP”), as defined and agreed to under the Parties’ Investigational Product Supply and Use Authorization Agreement for OT-101 U.S. Expanded Access (“IPSUA”), and
WHEREAS, Licensor endeavors to capture information and clinical data relating to patients treated in the Expanded Access Program (“USEAPOT101 Dataset” plus Safety Addendums, collectively for this Agreement known as the “Data”) and to format and store the Data in Licensor’s proprietary database, and
WHEREAS, Licensee desires to receive access to the Data from Licensor and receive license to use the Data in support of its commercial activities, subject to the terms and conditions of this Agreement, and
NOW, THEREFORE, in consideration of the mutual covenants, terms, and conditions set forth herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
| 1. | Definitions |
| 1.1 | “Applicable Law” means all laws, statutes, ordinances, codes, rules, and regulations that have been enacted by a Regulatory Authority in any jurisdiction relevant to the collection and management of clinical trial data and which are in force as of the Effective Date or come into force during the Term, in each case to the extent that the same are applicable to the performance by the Parties of their respective obligations under this Agreement, including, with respect to the United States, the Prescription Drug Marketing Act, the Federal Food, Drug and Cosmetics Act of 1938, as amended, The Federal Policy for the Protection of Human Subjects (“Common Rule”), and the Health Insurance Portability and Accountability Act (“HIPAA”). | |
| 1.2 | “Course of Treatment” means patients treatment with OT-101 consisting of 1-8 cycles, as described in the Protocol. | |
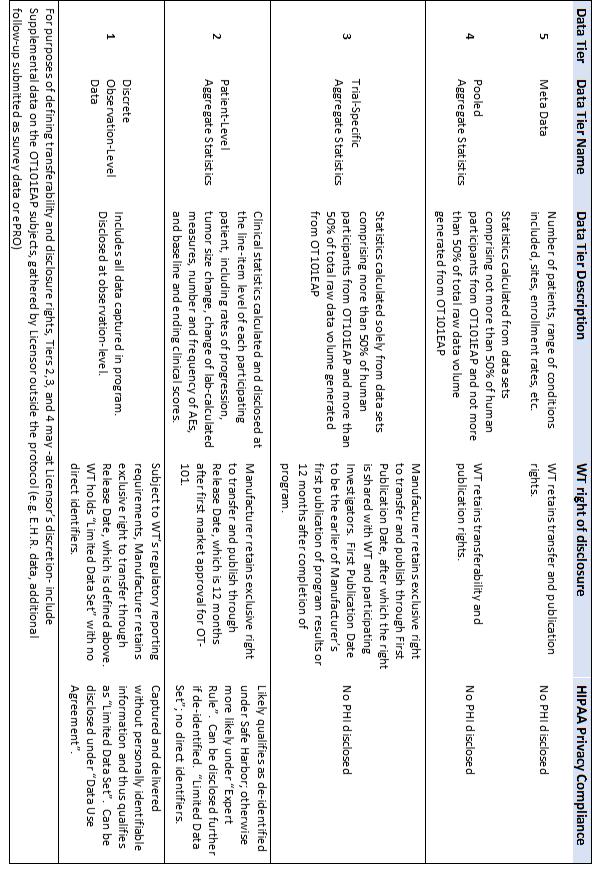
| 1.3 | “Data Tier” means the level of granularity in which the referenced Dataset is to be transferred or disclosed, as defined in Sections 1.3 – 1.7 and summarized in Exhibit 1 |
| Page 1 of 12 |
| 1.4 | “Data Tier 1” shall be defined as Discrete Observation-Level Data, which includes all information captured from the Expanded Access Program, including Protected Health Information (PHI) and treatment outcomes of individual patients. | |
| 1.5 | “Data Tier 2” shall be defined as Patient-Level Aggregated Statistics, which are clinical statistics calculated and disclosed at the line-item level of each participating patient, including rates of progression, tumor size change, change of lab-calculated measures, number and frequency of AEs, and baseline and ending clinical scores. | |
| 1.6 | “Data Tier 3” shall be defined as Trial-Specific Aggregate Statistics, calculated from data sets for which more than 50% of human subjects are from USEAPOT101 or more than 50% of total raw data volume is generated from USEAPOT101. | |
| 1.7 | “Data Tier 4” shall be defined as Pooled Aggregate Statistics, which are calculated solely from data sets for which no more than 50% of human subjects are represented by USEAPOT101 and no more than 50% of total raw data volume is generated from USEAPOT101. | |
| 1.8 | “Data Tier 5” shall be defined as Meta Data to include measures such as number of patients enrolled, number of sites, ranges of health conditions presented, and overall enrollment rate. | |
| 1.9 | “Expanded Access Program” or “EAP” generally means an FDA-authorized clinical trial for the treatment use of an Investigational Product in patients who are not candidates to participate in any research trial for the particular Investigational Product. For purposes of this Agreement, “The Expanded Access Program” or “The EAP” means the particular Expanded Access Program contemplated by this Agreement for the treatment use of OT-101 in patients with pancreatic cancer and other cancers characterized by overexpression of TGF-B2. | |
| 1.10 | “Health Insurance Portability and Accountability Act of 1996” or “HIPAA” is the U.S. regulation of healthcare-related data privacy described in the Code of Federal Regulations Title 45, Parts 160 and 164. | |
| 1.11 | “Investigational New Drug Filing”, or “IND” is a required written claim of exemption from the Food, Drug, and Cosmetic Act prohibition on interstate delivery of investigational therapeutics for pre-market use in humans, for authorized research programs or treatment-use programs. For this Agreement, “The IND” or “The INDs” shall refer to the specific filing(s) submitted by the Sponsor to FDA for 30-day authorization of The Expanded Access Program. | |
| 1.12 | “IND Authorization” means the passage of 30 days after submission of properly assembled EAP IND to the appropriate FDA review division with no response from FDA review division, OR an affirmative statement of authorization from the FDA review division. |
| Page 2 of 12 |
| 1.13 | “Personally Identifiable Information” or “PII” means data that directly or indirectly could be used to identity individuals in association with sensitive personal information. E.g. Name, social security number, address, medical record number. | |
| 1.14 | “Protected Health Information” or “PHI” is individually identifiable health information created by health care providers, as defined in the HIPAA Privacy Rule. | |
| 1.15 | “Privacy Rule” is the set of requirements under HIPAA defining the permitted capture and electronic transfer of Protected Health Information in clinical programs including Expanded Access Programs, codified in 45CFR160 and 45CFR164 Subparts A and E. | |
| 1.16 | “Protocol” is the clinical plan for Expanded Access, including eligibility criteria, treatment regimen, and data capture, included in The IND(s) for the particular study population and authorized by FDA and IRB(s). | |
| 1.17 | “Publication Date” is the earlier of (a) the date of Licensee’s first publication of clinical results of the EAP or (b) 12 months following the completion of the program. | |
| 1.18 | “Release Date” is 12 months after the first market approval for OT-101. |
| 2 | License. |
| 2.1 | License Grant. Subject to Licensee’s compliance with all other terms of this Agreement and conditioned on Licensee’s payment of Fees, Licensor hereby grants Licensee a perpetual, transferable license to the full USEAPOT101 Dataset with partial exclusivity as defined in Section 2.2 | |
| 2.2 | Exclusivity. Licensee’s right to disclose or transfer The Data shall be exclusive to Licensee for periods of time according to the levels of data disclosure or transfer contemplated, as defined below: |
| 2.2.1 | Data Tier 1: the right to disclose or transfer Discrete Observation-Level Data shall remain exclusively with the Licensee until the Release Date, as defined in Section 1.18. This right of Licensee to transfer Tier 1 Data to third parties is subject to and may be limited by HIPAA Privacy Rule. | |
| 2.2.2 | Data Tier 2: the right to disclose or transfer Patient-Level Aggregate Statistics shall remain exclusively with the Licensee until the Release Date, as defined in Section 1.18. |
| Page 3 of 12 |
| 2.2.3 | Data Tier 3: the right to disclose or transfer Trial-Specific Aggregated Statistics, including inventions or derivations based primarily on the Data, shall remain exclusively with the Licensee until the Release Date, as defined in Section 1.18. | |
| 2.2.4 | Data Tier 3: Notwithstanding the exclusivity provided generally in 2.2.3, certain Trial-Specific Aggregated Statistics that are publicly released by the Licensee may subsequently be released by the Licensor upon the Publication Date, as defined in section 1.17. |
| 2.3 | Licensor Regulatory Compliance. Notwithstanding the above exclusivity of Licensee’s data transfer rights for commercial purposes, Licensor reserves the right to disclose any part of the USEAPOT101 Dataset to participating Investigators, IRBs, FDA, or any other authority as needed for safety reporting and any other purpose required by Applicable Law. | |
| 2.4 | Reservation of Rights. Licensor reserves all rights not expressly granted to Licensee in this Agreement. |
| 3 | Delivery and Content of Data |
| 3.1 | Delivery. Licensee shall have full view and full download access to the USEAPOT101 Dataset within Licensor’s clinical trial management system, beginning with first patient enrolled. | |
| 3.2 | Content. USEAPOT101 Dataset shall include the following, as defined by the Protocol: |
| 3.2.1 | Meta Data relating to overall enrollment and conduct of the program | |
| 3.2.2 | Clinical Trial Data including patient screening information, concomitant medications, co-morbidities, baseline clinical assessment, interim and ending clinical assessment, adverse event log, and -as available- imagery and lab tests captured throughout treatment period under patient’s standard of care | |
| 3.2.3 | Patient-specific identification code mapped to key held by health care provider |
| 3.3 | Safety Addendums. All event-specific safety information shall be made available to Licensee for the purpose of enabling Licensee’s compliance with regulatory reporting requirements. These addendums shall comprise safety event processing forms and relevant correspondence, SAE narratives, SUSAR reports, regulatory correspondence with IRB(s) and FDA, and transcripts of MedWatch filings. These addendums shall be considered separate from the USEAPOT101 Dataset. |
| Page 4 of 12 |
| 3.4 | Compliance with Privacy Rule. Licensee warrants that it understands HIPAA definition for Protected Health Information (PHI) and that permissible transfer of PHI to other parties for research purposes is legally permissible only under certain conditions as defined in Section 4 of this Agreement and subject to Code of Federal Regulations Title 45 Sections 160 and 164. | |
| 3.5 | Covenant to Secure Data. Licensee covenants to maintain all parts of the USEAPOT101 Dataset in a secure manner. |
| 4 | HIPAA Compliance and Data Use Agreement under Privacy Rule |
| 4.1 | PHI in Data Tier 1. The USEAPOT101 Dataset shall not include personally identifiable information (PII). But it shall include specific dates of treatment and safety events, and therefore Tier 1 may not qualify as de-identified data under the HIPAA Privacy Rule and therefore must be treated as Protected Health Information (PHI). | |
| 4.2 | Permissible Use of PHI that excludes PII. Use and disclosure of Protected Health Information (PHI) is permissible without written authorization from the patient, provided there is no Personally Identifiable Information (PII) in the dataset and provided the resulting “Limited Data Set” is transferred under a “Data Use Agreement” as composed in Section 4.3. | |
| 4.3 | Data Use Agreement. As required by HIPAA Privacy Rule, Licensee hereby agrees to the following covenants of data privacy: |
| 4.3.1 | Licensee is not permitted to use or further disclose the Limited Data Set in a way that, if done by the Health Care Provider, would violate the Privacy Rule. | |
| 4.3.2 | Licensee will use appropriate safeguards to prevent the use or disclosure of the information, except as provided for in the Agreement, and report to the Licensor any uses or disclosures in violation of the Agreement of which the Licensee becomes aware. | |
| 4.3.3 | Licensee will hold any agent or subcontractor to the standards, restrictions, and conditions stated in this Data Use Agreement with respect to the information. | |
| 4.3.4 | Licensee will not use the Data to support attempts to identify or contact any of the patients involved in the EAP. |
| 4.4 | Use of Safety Addendum Information. The Licensee may also receive safety event documentation, defined in 3.3, that reveals Personally Identifiable Information (PII) and therefore constitutes Protected Health Information (PHI). Licensor agrees to not use or disclose this information for research purposes without (a) redacting all PII and (b) implementing a Data Use Agreement with any subsequent recipient of the information. |
| Page 5 of 12 |
| 4.5 | Other Uses or Disclosures. Under Safe Harbor provision of the Privacy Rule, Data Tier 2 is considered de-identified data because it does not include PII and does not include specific dates of treatment or safety events. Data Tiers 2-5 do not meet the definition of PHI and therefore are not restricted by HIPAA Privacy Rule in their use or disclosure. | |
| 4.6 | Liability. The Licensor shall not be responsible for any Privacy Rule violations made by the Licensee in its use or disclosure of USEAPOT101 Dataset or related Safety Addendums. |
| 5 | Intellectual Property Ownership. |
| 5.1 | Licensee acknowledges that, as between Licensee and Licensor, Licensor owns all right, title, and interest in and to the Data. Licensee further acknowledges that: (a) the Data is an original compilation protected by United States copyright laws and (b) Licensor has dedicated substantial resources to collect, manage, and compile the Data. Licensee agrees that it will be considered a material breach by Licensee under this Agreement if Licensee contests any of Licensor’s right, title, or interest in or to the Data, including without limitation, in a judicial proceeding anywhere throughout the world. | |
| 5.2 | Notwithstanding Licensor’s Data ownership, as defined in 5.1 of this Agreement, any derivation of the Data created by the Licensee, including analyses, discoveries, and inventions shall be regarded as the intellectual property of the Licensee. |
| 6 | Fees and Payment |
| 6.1 | Data Fee. In consideration for delivery and license of the USEAPOT101 Dataset and Safety Addendums, Licensee shall pay Licensor USD 2500 per patient treated, for up to 150 patients. After 150 patients have been registered, the Data Fee shall be USD 1500 per patient. | |
| 6.2 | Count of Patients. For its use in this section of the Agreement, a “Patient” shall mean a patient’s single Course of Treatment, which comprises 1-8 individual cycles. If the same patient returns for a second Course of Treatment, 6 or more months after completing the first Course of Treatment, he or she shall be considered a new additional patient and shall be counted a second time. | |
| 6.3 | Timing of Payments. |
| 6.3.1 | First Payment. Upon FDA IND Authorization of the first IND submitted for UAEAPOT101, Licensee shall complete its payment to the Licensor for the first 12 patients, equaling a fixed sum of USD 30,000. | |
| 6.3.2 | Second Payment. Upon start of treatment of the 6th patient, Licensee shall complete its payment to the Licensor for the 13th through 25th patients, equaling a fixed sum of USD 32,500. |
| Page 6 of 12 |
| 6.3.3 | Subsequent Payment. If the number of patients exceeds 25, Data Fee shall be paid for each additional patient within 30 days of start of treatment. |
| 6.4 | Investigator Allowance. On behalf of Licensee, Licensor may pay certain health care providers (HCPs) up to $1500 per treated patient to cover clinical coordination and data entry costs at the particular site. These costs will be passed through to Licensee on the same billing cycle under which the Licensor agrees to pay the HCP. Licensee hereby agrees to reimburse Licensor for these payments within the billing cycle presented. |
| 7 | Disclaimer of Warranties. The Data is provided “As Is” and Licensor hereby disclaims all warranties, whether express, implied, statutory, or otherwise. Licensor specifically disclaims all implied warranties of merchantability, fitness for a particular purpose, title, and non-infringement, and all warranties arising from course of dealing, usage or trade practice, licensor makes no warranty of any kind that the data, or any products or results of its use, will meet licensee’s or any other person’s requirements, operate without interruption, achieve any intended result, be compatible or work with any software, system, or other services, or be free of harmful code or errors. |
| 8 | Exclusion of Liability. Subject to the indemnification obligations set forth in Section 9, in no event shall either Party be liable (including without limitation, contract, and tort liability) for any indirect, incidental, punitive, exemplary, special or consequential damages, loss of profit, or costs of substitute services suffered by the other Party or any third party, however caused, regardless of the theory of liability, whether in contract, tort, product liability or otherwise |
| 9 | Indemnification |
| 9.1 | Licensee Indemnification: Licensee shall hold harmless, indemnify, and defend Licensor from and against any claim, liability, loss, damage or expense, including, without limitation, reasonable attorneys’ fees (collectively, “Loss”), arising out of any third party claim resulting from the use of the misuse of the Data, provided that Licensee’s obligation to hold harmless, indemnify and defend as aforesaid shall be proportionately reduced and shall not apply to the extent that such Loss is the result of Licensor’s act of fraud, negligence or willful misconduct or breach of Applicable Law, and further provided that Licensee is notified in writing as soon as practicable under the circumstances of any complaint or claim potentially subject to indemnification and has full control of any disposition or settlement of such claim, and Licensor and everyone on its behalf has fully cooperated with Licensee regarding such disposition or settlement; provided however that Licensee shall not dispose or settle any claim admitting liability on the part of Licensor without its prior consent, which consent shall not be unreasonably withheld. |
| Page 7 of 12 |
| 9.2 | Licensor Indemnification. The Licensor shall hold harmless, indemnify, and defend Licensee for any Loss arising out of: (i) Licensor’s own or any of its representatives’ or agents’ act of fraud, negligence or willful misconduct (ii) breach of this Agreement (iii) failure to manage the data according to HIPAA Privacy Rule; (iv) breach of Applicable Law or regulation, provided that Licensor’s obligation to hold harmless, indemnify and defend as aforesaid shall be proportionately reduced and shall not apply to the extent that such Loss is the result of Licensee’s act of fraud, negligence or willful misconduct or breach of Applicable Law, and further provided that the Licensor is notified in writing as soon as practicable under the circumstances of any complaint or claim and has full control of any disposition or settlement of such claim, and Licensee and everyone on its behalf has fully cooperated with the Licensor regarding such disposition or settlement; provided however that the Licensor shall not dispose or settle any claim admitting liability on the part of Licensee without its prior consent, which consent shall not be unreasonably withheld. |
| 10 | Term & Termination |
| 10.1 | Term. The Term of this Agreement shall commence on the Effective Date of this Agreement and shall continue through the completion of The Expanded Access Program as defined in Section 16.1 of the “Investigational Product Supply and Use Authorization Agreement for OT-101 U.S. Expanded Access (IPSUA)” | |
| 10.2 | Privacy Breach by Licensee. Notwithstanding provision of Section 10.1, Licensor may terminate this Agreement, with possible revocation of License -if required by law- in the event of Licensee’s material breach of the data privacy provisions herein, including Sections 3.4, 4.3, and 4.4. | |
| 10.3 | Early Termination of EAP. Notwithstanding provision of Section 10.1, Licensor may terminate this Agreement, without refund of any paid data fees as described in Sections 6.3.1 and 6.3.2, in the event of any of the following: |
| 10.3.1 | Non-Sponsor-caused early termination of The Expanded Access Program due to causes cited in Section 16.2 of the IPSUA | |
| 10.3.2 | In the capacity of Manufacturer, Licensee’s material breach of the IPSUA | |
| 10.3.3 | Licensee’s material breach of this Agreement | |
| 10.3.4 | Licensee’s material breach of Data License 2 Agreement |
| 10.4 | Material Breach by Licensor. Notwithstanding the provision of Section 10.1, the Licensee may terminate this Agreement in the event of any of the following: |
| 10.4.1 | Licensor’s material breach of this Agreement | |
| 10.4.2 | Licensor’s material breach of Data License 2 Agreement | |
| 10.4.3 | In the capacity of Sponsor, Licensor’s material breach of the IPSUA |
| Page 8 of 12 |
| 10.5 | Survival of License. With the exception of any legally required revocation of License, as described in Section 10.2, termination of this Agreement shall not invalidate the perpetual License to full use of the data that has already been delivered as described Sections 2 and 3. | |
| 10.6 | The Parties may terminate this Agreement by Mutual Consent. | |
| 10.7 | Obligations after Termination and Wind-Down Activities. Upon notification of termination, the Parties agree to cooperate with each other to ensure an orderly wind-down of the Services and discharge of their respective obligations under this Agreement and applicable Law (“Wind-Down Activities”): |
| 10.7.1 | Subject to Applicable Law, upon the termination of the Agreement as provided above, the Sponsor will discontinue its use of the Investigational Product and will, upon direction from Manufacturer, return or destroy any remaining Investigational Product. |
| 10.8 | Survival. Sections 1, 2, 4, 5, 7, 8, 9, 12, and 13 shall survive the termination of this Agreement howsoever caused. |
| 11 | Force Majeure |
| 11.1 | No Party shall be considered to be in breach of this Agreement if it is prevented from fulfilling its obligations under this Agreement by Force Majeure, if instructed by regulatory or by law, or as a matter of safety. | |
| 11.2 | Each Party will notify the other Party of any Force Majeure without undue delay. |
| 12 | Governing Law and Venue |
This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware, U.S.A without regard for conflict of laws principles. The Parties consent to the exclusive jurisdiction and venue of the competent courts in Delaware, U.S.A.
| 13 | Miscellaneous |
| 13.1 | Assignment. Neither party may assign, delegate or otherwise transfer any of its rights or obligations under this Agreement without the prior written consent of the other party. Any attempt to assign without compliance with this provision shall be void. | |
| 13.2 | Entire Agreement. This Agreement, with all forthcoming Exhibits, in conjunction with the IPSUA and Data License 2, constitute the entire scope of Agreement and understanding between the parties. It supersedes all prior discussions (whether oral or written) between the parties with regards to the subject matter, and neither party will be bound by conditions, definitions, warranties, understandings, or representations concerning such subject matter except as provided in this Agreement. This Agreement can only be modified by written Agreement duly signed by persons authorized to sign Agreements on behalf of both Manufacturer and Sponsor. In the event of any inconsistency between the terms of this Agreement and any attached Exhibits, the terms of this Agreement will prevail. |
| Page 9 of 12 |
| 13.3 | Waiver. The failure of a party in any instance to insist upon the strict performance of the terms of this Agreement will not be construed to be a waiver or relinquishment of any of the terms of this Agreement, either at the time of the party’s failure to insist upon strict performance or at any time in the future, and such terms will continue in full force and effect. All waivers of the terms of this Agreement shall be in writing. | |
| 13.4 | Severability. Each clause of this Agreement is a distinct and severable clause and if any clause is deemed illegal, void or unenforceable, the validity, legality or enforceability of any other clause or portion of this Agreement will not be affected thereby. | |
| 13.5 | Titles. All titles and articles headings contained in this Agreement are inserted only as a matter of convenience and reference. They do not define, limit, extend or describe the scope of this Agreement or the intent of any of its provisions. |
| Page 10 of 12 |
The undersigned have executed this Agreement as of the day and year noted below.
| Oncotelic Inc. | WideTrial Inc. | |||
| Signature: | /s/ Xxxxx Xxxxx | Signature: | /s/ Xxxx Xxxxxxx | |
| Name: | Xxxxx Xxxxx | Name: | Xxxx Xxxxxxx | |
| Title: | CEO | Title: | CEO | |
| Date: | 9/5/2019 | Date: | 9/5/2019 | |
| Page 11 of 12 |
EXHIBIT 1
SUMMARY OF USEAPOT101 DATA TIERS
| Page 12 of 12 |


