SUPPLY AGREEMENT
CONFIDENTIAL TREATMENT
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED AS TO CERTAIN PORTIONS OF THIS DOCUMENT. EACH SUCH PORTION, WHICH HAS BEEN OMITTED HEREIN AND REPLACED WITH AN ASTERISK [*], HAS BEEN FILED SEPERATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
THIS SUPPLY AGREEMENT (this “Agreement”) is made and entered into as of September_1_, 2017 (the “Effective Date”), by and between Polyzen, Inc., a North Carolina corporation with its principal office located at 0000 Xxxxxxx Xxxx, Xxxx, Xxxxx Xxxxxxxx 00000 (“Polyzen”), and Motus GI Medical Technologies Ltd., an, Israeli company with its principal office located at Keren Hayesod 00, Xxxxx Xxxxxx, Xxxxxx, 3902638 (“Company”).
RECITALS
WHEREAS, Company is in the business of marketing and selling Food and Drug Administration (“FDA”) cleared or approved medical devices;
WHEREAS, Polyzen is in the business of developing, manufacturing and supplying products related to Company’s business; and
WHEREAS, Polyzen desires to develop manufacture and supply to Company, and Company desires to purchase from Polyzen, the Products (as defined below) according to the terms and conditions set forth herein.
NOW THEREFORE, in consideration of the mutual covenants and promises contained herein, and of other good and valuable consideration, the receipt and sufficiency of which hereby are acknowledged, Polyzen and Company hereby agree as follows:
1. Definitions.
1.1 “Affiliate” means with respect to any party, any person/entity which, directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, such party. A person/entity shall be deemed to control a corporation (or other entity) if such person or entity possesses, directly or indirectly, more than fifty percent (50%) of the outstanding voting securities or other equity or voting interest of such corporation (or other entity) or has the power to vote, by contract or otherwise, or to control in fact, the management decisions of such entity.
1.2 “Product” or “Products” shall mean those products consisting of (i) component parts and packaged assemblies developed and manufactured by Polyzen; and (ii) fully assembled Medical Devices (as defined below) assembled by Polyzen, in each case, in accordance with the Specifications, and described in, Exhibit A attached hereto and incorporated herein by reference, which may be modified from time to time to add or remove products, in each case, with the written approval of an authorized representative of each party hereto. The parties shall negotiate in good faith with regard to any appropriate written amendment of, or addendum to, this Agreement or any exhibit attached hereto, to accommodate such additional products, as the case may be, and to modify the Specifications, pricing and delivery requirements, as applicable, therefor.
1.3 “Medical Device” shall mean the FDA cleared device Pure-Vu Product manufactured by Company.
1.4 “Regulatory Authority” shall mean an authorized agent of any federal, state or local or international regulatory agency, department, bureau or other governmental entity, including the FDA, that is responsible for issuing approvals, licenses, registrations or authorizations necessary for the production, use, storage, import, transport or sale of Products in any jurisdiction as part of the Services.
1.5 “Specifications” shall mean the written specifications for the Products set forth in Exhibit A attached hereto.
2. Manufacture and Supply of Products. Subject to the terms and conditions set forth herein, Polyzen shall manufacture and assemble Products in accordance with the Specifications, and supply and deliver (collectively, the “Services”) to Company the Products in such quantities as are required to fulfill Purchase Orders (as defined below) issued by Company or its Affiliates from time to time. Any use of secondary suppliers or other outsourcing of the Services to third parties by Polyzen shall be approved in writing in advance by Company and set forth in Exhibit C attached hereto and incorporated herein by reference. Polyzen shall ensure that any approved secondary suppliers and other third parties are bound by written agreements with substantially similar provisions as contained herein; provided that with respect to suppliers of components that are “off-the-shelf”, Polyzen’s obligation under this section shall be to ensure that there are quality agreements in place with such suppliers that ensure that Polyzen can meet its obligations under this Agreement. In the event that Polyzen subcontracts all or part of the Services hereunder to a third party supplier or other third party, Polyzen shall be responsible for such secondary supplier’s or other third parties’ compliance with the terms of this Agreement and will retain primary liability vis-à-vis Company for the performance of all obligations of such secondary suppliers and other third parties.
2.1 Based on the quality control standards developed mutually by the parties and incorporated herein by reference in the Quality Agreement attached hereto as Exhibit B, which shall meet but in no event exceed the applicable standards set forth in ISO 9001, ISO 13485 and 21 C.F.R. Part 820 for contract manufacturers, Polyzen shall manufacture the Products in accordance with the Specifications and shall perform all quality control and testing of the Products to ensure that they comply with the Specifications. To the extent that any specialized tooling or equipment (as outlined in Exhibit D attached hereto and incorporated herein by reference) is necessary to provide the Services, the parties shall negotiate in good faith the costs of such specialized tooling and/or equipment and agree on the use and ownership of such tooling and/or equipment in writing in advance in accordance with Section 10.8 of this Agreement. At or prior to the purchase of a new tool, Polyzen and Company will discuss in good faith the cost and payment options for such tools. Exhibit D attached hereto lists the owner of, and the purchase price paid for, each tool.
2.2 Purchase Orders. Polyzen’s performance of the Services shall be subject to Polyzen’s receipt from Company or its Affiliates, and Polyzen’s written acceptance (except as provided below), of a written purchase order, each of which will set forth the requested quantity of Product, price per the terms of this Agreement, and the desired delivery dates (each, a “Delivery Date”), for the Products then ordered (each, a “Purchase Order”). Company shall have the right, but not the obligation, to deliver Purchase Orders as provided herein. Each Purchase Order shall cover a period of three (3) months (the “Order Period”) and, except for the Initial Purchase Order, which shall be delivered as set forth in Section 2.4.1 below, shall be delivered by no later than 5:00 p.m. (EST) on the last business day of the second (2nd) month of the then current Order Period. Polyzen will consider in good faith accepting any Purchase Order delivered by Company or its Affiliate at any other point. Each Purchase Order shall include the requested amount of Product to be delivered in each month of the subsequent Order Period (each, a “Monthly Order”). Within five (5) business days after Polyzen’s receipt of each Purchase Order, Polyzen shall notify Company in writing either of its acceptance of such Purchase Order or of its rejection thereof and the reason therefor. Any Purchase Order that reflects Monthly Orders that are within twenty percent (20%) of the amount forecasted for such month in the most recent Forecast (as defined below) for the given period shall be deemed accepted by Polyzen. Except as provided in the previous sentence, no Purchase Order submitted by Company shall be deemed to be accepted by Polyzen unless and until confirmed in writing by an authorized representative of Polyzen. Polyzen shall deliver all Products pursuant to an accepted Purchase Order on the desired Delivery Dates specified in the subject Purchase Order, provided that such date is no less than forty-five (45) days from the date of the Purchase Order is received by Polyzen. Company, at its option, may upon prior written notice to Polyzen, delay the acceptance of any Monthly Order in an Order Period for up to three (3) months (an “Order Delay”). Written notice of an Order Delay must be given to Polyzen at least fifteen (15) calendar days in advance of the subject Delivery Date and must include new Delivery Dates for the delayed delivery. Products subject to an Order Delay will be invoiced as follows: 50% of the total purchase price for the subject Products on the original Delivery Date, as provided in the subject Purchase Order; and the remaining 50% of the purchase price at time of shipment of the Products subject to the Order Delay. By way of example, if a Purchase Order for the third calendar quarter of 2017 specified a Monthly Order of 100 units of Product in July 2017, Company will have the option of having the subject 100 units of Product delivered through October 2017 without incurring any penalty. Further, any subsequent Monthly Orders will be subject to the same delivery standards; the Monthly Orders for August 2017 and September 2017 could then be similarly delayed as requested by Company and delivered through November 2017 and December 2017 respectively.
2.3 Order Interruption. In the event that the Company desires to deliver a Purchase Order subsequent to a calendar quarter in which there have not been any deliveries of Product from Polyzen, Company shall provide Polyzen not less than sixty (60) days’ notice of the next anticipated Delivery Date and a restart fee equal to $50,000 to resume production of the Products.
| -2- |
2.4 Forecasts.
| 2.4.1 | Rolling Forecasts. Company shall provide Polyzen with a quarterly, rolling, written non-binding twelve (12) month forecast of its purchase requirements for the Products (each, a “Forecast”). Company’s initial Forecast shall be provided to Polyzen on the Effective Date. Company’s initial Purchase Order (the “Initial Purchase Order”) shall reflect the initial three months of the Forecast and shall be subject to the terms and conditions as provided in Section 2.2 of this Agreement. Thereafter, Company shall deliver to Polyzen its updated Forecast by no later than 5:00p.m. (EST) of the last business day of the second (2nd) month of the then current Order Period. For example, since the Effective Date of this Agreement is in July 2017, the initial Forecast delivered on the Effective Date would cover August 2017 through July 2018. Polyzen will use commercially reasonable efforts to maintain sufficient production capacity and redundancy to satisfy Company’s then forecasted requirements for the Products, which, in no event, will equal less than three (3) months of orders plus twenty percent (20%) upside flexibility. |
| 2.4.2 | Material Planning Meeting. Within five (5) business days of receipt of a Forecast, Company and Polyzen shall have a materials planning meeting to determine the appropriate volume of raw materials to order. Based on the determined volumes, Polyzen will place purchase orders with vendors to obtain the appropriate level of pricing and lead times. Upon receipt and acceptance of all raw materials, Polyzen will invoice Company for raw materials as set forth in Section 3.1. Polyzen shall ensure that all raw materials are of good quality, free from defects, meet applicable specifications, are sourced in accordance with applicable law and fit for the purpose intended prior to acceptance. In the event of termination or expiration of this Agreement, other than a termination of this Agreement by Company pursuant to Section 8.2 or 8.4 below, all non-cancellable purchase orders to vendors or sources component manufacturers will be binding and paid for by Company with no xxxx-up by Polyzen; provided that in the event of a termination pursuant to Section 8.4 below as contemplated in this sentence, the Company will be obligated to pay for such non-cancelable purchase orders to the extent provided under Section 8.5(a). Such payments will be subject to the invoicing and payment provisions set forth in Section 3 below. |
2.5 Acceptance and Rejection of Products. Upon Company’s receipt of each delivery of Product delivered in accordance with the terms and conditions set forth herein, Company shall inspect such Product for non- conformance, defects and damages as per its Specifications (“Non-conforming Products”) and furnish to Polyzen in a reasonably detailed writing any bona fide claim Company has in connection with such Non-conforming Products within thirty (30) days after its receipt thereof (“Non-conforming Products Notice Period”). Failure to give written notice within the Non-conforming Products Notice Period shall constitute acceptance of Products by Company as delivered, except in the case of latent Non-conforming Products that: (i) would not have been revealed by a timely inspection in accordance with customary and reasonable procedures (“Latent Non-Conforming Products”), and (ii) are the subject of a written notice to Polyzen in reasonable detail within seven (7) business days of Company’s initial knowledge thereof (“Latent Non-conforming Products Notice Period”), provided however that COMPANY shall provide notice of such Latent Non-Conforming Products no later than the shelf-life of the product as defined in the FDA approved Product labeling. If Company submits a claim to Polyzen within the Non-conforming Products Notice Period or the Latent Non-conforming Products Notice Period, as applicable, Polyzen will, promptly upon receipt of such claim, contact Company to discuss and evaluate the validity of such claim. At Polyzen’s sole discretion, it may request that Company deliver to Polyzen a sample of the potentially defective Product for evaluation. If, upon concluding its evaluation of such Product, Polyzen reasonably determines that such Product is Non-conforming Product, Polyzen shall send Company a return material authorization, and at its sole expense, arrange for, and accept from Company, the return of any Non-conforming Product and ship to Company compliant, non-damaged and non-defective Product as replacement for such returned Non-conforming Product. If Polyzen disagrees with Company’s determination that a Product is a defective Non-conforming Product, the parties will first use good faith efforts to settle such dispute within twenty (20) business days of Company’s claim. If the parties are unable to resolve such dispute within such twenty (20)-business day period, such Product shall be submitted to a mutually acceptable third party testing service, which shall determine whether such Product meets the Specifications, and the parties agree that such testing service’s determination shall be final and binding on the parties. The party against whom the testing services rules shall bear all costs of the third party testing service. If the third party testing service determines that the Products are Non-conforming Products, Polyzen shall send Company a return material authorization, and at its sole expense, arrange for, and accept from Company, the return of any non-compliant, damaged or defective Non-conforming Product and ship to Company compliant, non-damaged and non-defective Product as replacement for such returned Non-conforming Product. In the event Polyzen becomes aware that any Product supplied may be Non-conforming Products despite quality assurance activities, Polyzen shall immediately notify Company in writing.
| -3- |
2.6 Change in Specifications. From time to time during the Term (as defined below), either party may propose modifications to the Specifications, including, without limitation, modifications that may enhance the Products’ performance, safety or reliability, or that may make it easier or more economical to manufacture, handle or repair the Products, or that otherwise may be an improvement thereof. All such changes shall be agreed to in accordance with Section 10.8 of this Agreement. Such proposals shall be made in writing describing the modification in reasonable detail. Any such proposal by Polyzen shall also include a written estimate of the resulting change in the price, if any, for the Product affected by such modification. If Polyzen receives a proposal from Company to modify the Specifications, Polyzen shall promptly provide Company with a written estimate of the resulting change in the price, if any, for the Product affected by such modification. The parties shall negotiate in good faith with regard to any appropriate written amendment of, or addendum to, this Agreement or any exhibit attached hereto, to accommodate such agreed to modifications, as the case may be, and to modify the pricing and delivery requirements, as applicable, therefore.
2.7 Technology Transfer. Subject to the terms and conditions set forth herein, Polyzen agrees that it will transfer to Company or its designee such intellectual property or other information as is necessary for Company or its designee to manufacture the Products and provide such other reasonable assistance to be billed at Polyzen’s then current FTE rate, and Company agrees to pay such FTE rates and, in the event such transfer includes Polyzen’s Confidential Information and/or Polyzen’s Background Intellectual Property, the compensation described herein, all in furtherance of an efficient and smooth transfer of the production of the Product (a “Technology Transfer”); provided that, in the event that Polyzen terminates this Agreement under Section 8.2 below, it will have no obligation in connection with the Technology Transfer. Company may request a Technology Transfer at any time during the Term (provided Company is not then in breach of any term or condition set forth herein) or upon the expiration or termination of this Agreement for any reason (except as set forth in the proviso to the previous sentence). Company shall provide Polyzen with not less than thirty (30) days’ prior written notice of a request for a Technology Transfer. In the event that any Polyzen’s Background Intellectual Property is included in the Technology Transfer, the Parties shall negotiate in good faith fair compensation for Polyzen in respect of any licenses granted by Polyzen in connection with such Technology Transfer. Polyzen shall not incorporate any Polyzen’s Background Intellectual Property in the development, manufacture or supply of Products in a manner that would restrict Company’s freedom to develop, manufacture or commercialize a Product without a license from Polyzen without first receiving Company’s written consent. In no event will Polyzen will be responsible for any equipment or tooling replacement costs or material costs associated with such Technology Transfer.
3. Pricing; Payment Terms; Title.
3.1 Pricing and Payment Terms. The price payable by Company or its Affiliates, as the case may be, to Polyzen for each Product purchased during the Term (as defined below) is set forth in Exhibit C attached hereto and incorporated herein by reference. Company will pay Polyzen for the Products purchased according to the prices set forth in Exhibit C attached hereto. Polyzen shall invoice Company for the Product upon shipment. Invoices shall be submitted by e-mail to the following address: xxxxxxx@xxxxxxx.xxx. All payments for undisputed invoices are due thirty (30) days from the date of the e-mail containing the invoice. In the event Company disputes one or more items in an invoice, such dispute must be in good faith, and Company will pay the undisputed portion within thirty (30) days from the date of the e- mail containing the invoice and notify Polyzen in writing within ten (10) days of receipt of an invoice of the items being disputed and the basis therefor. The parties will use good faith efforts to resolve any such disputes within twenty (20) days. Once resolved, payment will be made by Company within twenty (20) days from the date on which resolution was reached by the parties. Any payment not received by Polyzen by the due date may be subject, at Polyzen’s sole discretion, to a late fee equal to one and one half percent (1.5%) (or the maximum rate permitted by law) of the amount then due, for each month overdue. Also, Polyzen may, at its election, discontinue, terminate or suspend the Services without incurring any liability to Company, provided that Polyzen provides written notice to Company at least seven (7) business days in advance of any discontinuation, termination or suspension of Services. For amounts outstanding after sixty (60) days from the date of the e-mail containing the invoice therefor, Company shall be responsible for, and agrees to pay, reasonable costs and expenses of collection, including, but not limited to court and reasonable attorneys’ fees and expenses. Prices do not include any governmental taxes (including, without limitation, sales, use, excise, withholding, consumption or other VAT), or duties imposed by governmental authorities that are applicable to the import or purchase of the Products, and Company shall bear all such taxes and duties.
| -4- |
3.2 Price Adjustments. During the twelve (12)-month period that starts on the Effective Date, the parties shall undertake a review of pricing for the Products once every ninety (90) days (“Quarterly Pricing Review”) beginning on the first business day that is no less than fifteen (15) days prior to the ninety (90)-day anniversary of the Effective Date, considering all relevant costs to Polyzen reflected in determining the pricing including, but not limited to, labor costs, material and supplier component costs (“Pricing Review Criteria”), and to use commercially reasonable efforts to reach agreement on any future pricing adjustments, reflecting both reductions and increases in cost, (“Pricing Adjustment(s)”). After the twelve (12)-month anniversary of the Effective Date, the parties shall undertake an annual pricing review based on the Pricing Review Criteria beginning on the first business day that is no more than thirty (30) days before the end of each subsequent twelve (12)-month anniversary of the Effective Date (“Annual Pricing Review”). Any Pricing Adjustments agreed to by the Parties in a Quarterly Pricing Review or an Annual Pricing Review shall become effective when agreed to in writing in an amendment to Exhibit C in accordance with Section 10.8 of this Agreement. Polyzen shall make available to Company all supporting documentation reasonably necessary to calculate any Pricing Adjustments including labor costs and material and supplier component costs and will reasonably cooperate with Company in negotiating any Pricing Adjustments. For clarity sake, any future Pricing Adjustments resulting in an increase to Company will be limited to situations where the underlying documented costs to Polyzen increased. Polyzen agrees that any price increase associated with Polyzen labor costs shall be capped at two percent (2%); any agreed to reduction in the then current pricing set forth in Exhibit C shall be retained by Company provided Company pre-purchases inventory as outlined in Section 2.4.2 above. If Polyzen purchases inventory, such increases / decreases and xxxx-ups will be reasonably negotiated with Company.
3.3 Shipping Terms and Title to Product. All standard shipments of Product shall be shipped (via air or water) F.O.B. Origin using the Company’s Shipping Account Number. All shipments shall be accompanied by a packing slip which describes the Products and states the Purchase Order number. Title and risk of loss with respect to any shipment of Products shall pass to the Company or its Affiliates after delivery of the shipment by Polyzen to the agreed upon carrier. Polyzen shall assist Company, at Company’s risk and expense, in obtaining any required export or import license or other official authorization necessary for the export from the United States of Products imported to Israel or such other location designated in writing by Company If there is any conflict or inconsistency between this Agreement and any Purchase Order, Purchase Order release, confirmation, acceptance or any similar document, the terms of this Agreement shall govern.
4. Confidentiality; Intellectual Property.
4.1 Restrictions on Use and Disclosure of Confidential Information. Any Confidential Information (as defined below) of a party shall: (i) be maintained by the receiving party in strict confidence using the same degree of care such party would use to protect its own Confidential Information (but in any event, using no less than a reasonable degree of care); (ii) not be disclosed, directly or indirectly, to any third party; and (iii) not be used for any purpose not expressly set forth in this Agreement; provided, however, that the parties may disclose Confidential Information to their respective employees and agents requiring access to such information for purposes of this Agreement, so long as, prior to such disclosure, each such person: (a) is advised of his/her obligation under this Section 4.1; and (b) shall have entered into a written agreement with confidentiality and non-use restrictions, which are at least as restrictive as those restrictions contained in this Section 4.
4.2 Definition of Confidential Information. “Confidential Information,” means all confidential, non-public or proprietary information that is disclosed or made available by one party to the other party in connection with this Agreement, that is labeled as “confidential” or with a similar designation, or that the receiving party should reasonably know if confidential or proprietary under the circumstances of disclosure, including, without limitation, all inventions, discoveries, improvements, developments, ideas, know-how, trade secrets, technical and non-technical data, specifications, formulae, compounds, formulations, assays, methods, processes, techniques, practices, procedures, manufacturing techniques, designs, works of authorship, trade names, logos and other intellectual property, whether or not patentable or protectable by copyright or trademark, business and product plans, research and development plans or results, and sales, marketing, financial and pricing information, in each case, whether disclosed or made available in visual, oral, written, electronic, graphic or any other form. Confidential Information includes all copies, reproductions, notes and repositories thereof or based thereon, whether in written, electronic, graphic or any other form, including in the form of samples. Confidential Information shall not include any information that:
| -5- |
(a) at the time of disclosure is/was generally available to the public; or
(b) after disclosure becomes generally available to the public, except through breach of this Agreement by the receiving party; or
(c) is/was already possessed by the receiving party, as evidenced by its written records, predating receipt thereof from the disclosing party, so long as the receiving party did not receive such information directly or indirectly from a third party under an obligation of confidentiality to the disclosing party; or
(d) is/was independently developed by or on behalf of the receiving party, as evidenced by written records, without direct or indirect use of any Confidential Information of the disclosing party and without access to or knowledge of any Confidential Information of the disclosing party; or
(e) is required by law to be disclosed; provided, however, that receiving party shall promptly provide the disclosing party with written notice of such legal requirement and shall cooperate with the disclosing party to seek and obtain a protective order or other appropriate remedy prior to the disclosure of such Confidential Information.
4.3 Return of Confidential Information. All Confidential Information and copies and reproductions thereof (in whatever form, including information stored on readable media) shall be promptly returned to the disclosing party upon the expiration or termination of this Agreement for any reason, or at any time at the disclosing party’s request, except one copy of it can be retained for archival purposes. All information related to the production of Products that is not Polyzen’s Background Intellectual Property shall be deemed Confidential Information of Company regardless of the party that discloses such Confidential Information.
4.4 Injunctive Relief. Each party acknowledges and agrees that any breach by the other party of any provision of this Section 4 would result in irreparable harm to the non-breaching party for which money damages would be an inadequate remedy and, therefore, agrees that the non-breaching party shall be entitled to injunctive relief to prevent or restrain any breach or threatened breach of the provisions of this Section 4, in addition to any other remedies available to the non-breaching party at law or in equity.
4.5 Intellectual Property. Except to the extent set forth herein, all right and title to any inventions, improvements or discoveries (“Inventions”), whether patentable or not, developed, discovered, designed, produced or manufactured by Polyzen, any of its agents, employees or subcontractors or by Company in connection with its obligations under this Agreement or using Confidential Information of Company, shall be and remain the exclusive property of the Company, upon Polyzen’s receipt of payment for Services performed in connection therewith; provided, however, that inventions, ideas, know how, data, intellectual property, improvements and discoveries that are developed, discovered, designed, conceived of, produced or manufactured by Polyzen: (i) before the Term; (ii) during the Term and outside of the scope of this Agreement; and (iii) during the Term in connection with the performance of its obligations hereunder but, in each case that are not unique to the Products, which includes, for illustrative purposes, certain intellectual properties, processes, and improvements relating to Polyzen’s business developed before or during the Term without the use of Company’s Confidential Information or Company Intellectual Property (as defined below), in each case, as evidenced by Polyzen’s records, shall be and remain the property of Polyzen, including such intellectual property used in connection with the Services as of the Effective Date as is outlined in Exhibit E attached hereto (collectively, “Polyzen’s Background Intellectual Property”). In connection with Polyzen’s Background Intellectual Property, and upon Company’s payment to Polyzen of all then outstanding amounts due in accordance herewith for Products delivered by Polyzen, Polyzen grants to Company a limited, non-exclusive, non-sub licensable, fully-paid, royalty-free license to use Polyzen’s Background Intellectual Property solely to the extent comprising, and only in connection with, Product delivered to Company hereunder. Except for such license or as Polyzen may agree otherwise in writing, no right, title or interest in or to the Polyzen’s Background Intellectual Property is granted by Polyzen to Company, whether expressly, by implication, estoppel, or otherwise. Company hereby agrees not to derive or attempt to derive by reverse engineering, disassembling, decompiling or otherwise, any portion of Polyzen’s Background Intellectual Property. If Company requests, and at Company’s expense, Polyzen will provide Company with all reasonable assistance to obtain patents, and other intellectual property protection on such Inventions, except to the extent comprising Polyzen’s Background Intellectual Property, including procurement of written assignments and title commitments, in forms acceptable to Company, from all affiliates, personnel and agents of Company. Polyzen acknowledges and agrees that Company has the exclusive right to file patent applications and own patents in connection with any Inventions to the extent not comprised of Polyzen’s Background Intellectual Property. Polyzen shall be free to use any Invention that it develops, discovers, designs, produces or manufacturers for the sole purpose of fulfilling its obligation under this Agreement. Polyzen shall promptly disclose all Inventions pertinent to the Products to Company.
| -6- |
4.6 Company Intellectual Property. Intellectual property, processes and improvements relating to Company’s business and/or the Products or production of the Products, including all Inventions, in each case developed before or during the Term by Company and/or Polyzen or its agents, employees or subcontractors and that are not Polyzen’s Background Intellectual Property, shall be and remain the property of Company (“Company Intellectual Property”). Company hereby grants to Polyzen a non-exclusive, non-sub licensable, fully-paid, royalty-free license to use Company Intellectual Property solely to the extent necessary to provide the Services. Except as provided in the previous sentence, no right, title or interest in or to the Company Intellectual Property is granted by Company to Polyzen, whether expressly, by implication, estoppel, or otherwise.
5. Representations and Warranties. Each party represents and warrants to the other party that: (i) it is a company duly organized and validly existing in good standing under the laws of the state of its formation or incorporation; (ii) it has all requisite right, power and authority to enter into and execute this Agreement, and to perform and consummate the transactions contemplated hereby; (iii) this Agreement, when executed by it, constitutes a legal, valid and binding obligation enforceable against it in accordance with the terms hereof; (iv) its execution, delivery and performance of this Agreement will not result in any violation of any other contract or agreement; and (v) it shall comply with and shall not take any action which would violate or cause the other party to violate the provisions of: (a) the United States Foreign Corrupt Practices Act of 1977; or (b) The Xxxxxxx Xxx 0000 (c.23) of the United Kingdom; or (c) the Convention on Combating Bribery of Foreign Public Officials in International Business Transactions of the Organization for Economic Co-operation and Development. Neither party nor any of its affiliates or their respective directors, officers, shareholders, employees or agents shall make or offer, in respect of the performance of its obligations hereunder, any loan, gift or other payment, directly or indirectly, whether in cash or in kind, for the use or benefit of a Foreign Official (as defined herein) for the purposes of influencing any act or decision of such Foreign Official in its official capacity, or inducing such Foreign Official to do or omit to do any act in order to obtain or retain business or otherwise to secure any improper advantage. The term “Foreign Official” shall mean (A) any officer or employee of a foreign government, department (whether executive, legislative, judicial or administrative), agency or instrumentality of such foreign government, including a regional governmental body or a government-owned business, or of a public international organization; (B) any person acting in an official capacity for or on behalf of such foreign government, department, agency, instrumentality, or public international organization; (C) any candidate for a foreign political office; or (D) any foreign political party. Polyzen further represents and warrants to the Company that: (1) it shall provide the Services in compliance with all applicable international, federal and state laws and regulations (“Applicable Laws”); (2) except to the extent arising in connection with Company’s payment obligations hereunder, Products, at the time received by Company at its end destination, will be free and clear from all liens, encumbrances, and defects of title; (3) neither it nor any of its officers or directors or employees or agents providing Services hereunder has been debarred pursuant to the Federal Food Drug and Cosmetic Act (“FDCA”) or excluded from participating in a federal health care program, including without limitation the Medicare or Medicaid programs and shall notify the Company promptly in writing in the event Polyzen or its officers, directors or employees or agents providing Services hereunder subsequently becomes debarred under the FDCA or excluded from a federal healthcare program; and (4) it possesses all required licenses, permits and registration of any relevant governmental authority required to provide the Services hereunder. Company further represents and warrants to Polyzen: (aa) neither it nor any of its officers or directors or employees or agents providing Services hereunder has been debarred pursuant to the FDCA or excluded from participating in a federal health care program, including without limitation the Medicare or Medicaid programs; (bb) it controls all rights to Products, the Specifications and the intellectual property rights associated therewith and has the right to grant to Polyzen the rights and licenses granted herein; and (cc) the Products, the Specifications, the intellectual property rights associated therewith, and any trademarks, logos and trade dress provided to Polyzen hereunder do not, and will not, infringe, violate or misappropriate the intellectual property or other rights of any third party.
EXCEPT AS SET FORTH IN THIS SECTION 5, THE SERVICES AND THE PRODUCTS ARE PROVIDED “AS IS” WITHOUT REPRESENTATION OR WARRANTY OF ANY KIND. EXCEPT AS SET FORTH IN THIS SECTION 5, POLYZEN MAKES NO REPRESENTATION OR WARRANTY UNDER THIS AGREEMENT, ORAL OR WRITTEN, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION, ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, USE OR TITLE OR NONINFRINGEMENT. EXCEPT AS SET FORTH IN THIS SECTION 5, POLYZEN DOES NOT WARRANT THAT THE SERVICES WILL MEET COMPANY’S REQUIREMENTS NOR DOES IT GIVE ANY WARRANTY ABOUT THE RESULTS THAT MAY BE OBTAINED BY USING THE SERVICES.
| -7- |
6. Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR ANY DIRECT, INDIRECT, INCIDENTAL, SPECIAL, LOST PROFITS, PUNITIVE, EXEMPLARY, REMOTE OR CONSEQUENTIAL DAMAGES (INCLUDING BUT NOT LIMITED TO LOSS OF REVENUE OR PROFITS) ARISING FROM OR CAUSED, DIRECTLY OR INDIRECTLY, BY THE PERFORMANCE OR FAILURE TO PERFORM UNDER THIS AGREEMENT, OR BY ANY OTHER ACT OR OMISSION OF THE PARTIES, OR BY ANY OTHER CAUSE. POLYZEN’S TOTAL CUMULATIVE LIABILITY TO COMPANY FOR ANY CLAIM, LOSS OR DAMAGE OF ANY KIND ARISING UNDER THIS AGREEMENT, WHETHER BASED ON CONTRACT, TORT, NEGLIGENCE, OR OTHERWISE WILL NOT EXCEED TWO (2) TIMES THE ACTUAL AMOUNT INVOICED BY POLYZEN FOR SERVICES RENDERED FOR THE COST OF PRODUCT RESPONSIBLE FOR SUCH CLAIMS, PROVIDED THAT SUCH CLAIMS ARE NEITHER AS A RESULT OF POLYZEN’S GROSS NEGLIGENCE, OR WILLFUL OR FRAUDULENT MISCONDUCT IN THE PERFORMANCE OF THE SERVICES NOR ARE SUCH CLAIMS AN INDEMNIFICATION OBLIGATION OF POLYZEN UNDER SECTION 7. IN CONNECTION WITH POLYZEN’S INDEMNIFICATION OBLIGATIONS HEREUNDER, EXCEPT WITH RESPECT TO CLAIMS FOR FRAUD, GROSS NEGLIGENCE OR WILLFUL MISCONDUCT, IN NO EVENT WILL POLYZEN’S TOTAL LIABILITY TO COMPANY OR TO ANY THIRD PARTY EXCEED ONE MILLION DOLLARS ($1,000,000) PER INCIDENT PROVIDED THAT POLYZEN’S TOTAL AGGREGATE LIABILITY HEREUNDER WILL NOT EXCEED THE GREATER OF (A) THREE MILLION DOLLARS ($3,000,000) OR (B) TWELVE MONTHS OF PAYMENTS TO POLYZEN UNDER THIS AGREEMENT (EXCLUDING EXPENSES).
7. Indemnification and Insurance.
7.1 Indemnity Obligations. In addition to its other indemnification obligations set forth herein, at its sole cost, each party (in such capacity, the “Indemnifying Party”) hereby agrees to indemnify, defend and hold harmless the other party and its shareholders, officers, directors, employees, agents, representatives, subcontractors, invitees, successors and assigns (each, an “Indemnitee”) from and against any and all claims, suits, actions, liabilities, losses, costs and expenses (including reasonable attorneys’ fees), judgments and damages (“Claims”) brought against any Indemnitee by a third party which results or arises from, or is attributable to, (i) the Indemnifying Party’s gross negligence, intentional misconduct, or failure to comply with Applicable Laws; or (ii) any breach of this Agreement or any term or condition set forth herein by the Indemnifying Party, or its employees or agents, or any breach of any of such Indemnifying Party’s representations or warranties set forth herein. In addition to its other indemnification obligations hereunder, and except to the extent Polyzen is the Indemnifying Party pursuant to this Section 7.1, Company hereby agrees to indemnify, defend and hold harmless Polyzen and its Indemnitees from and against any and all Claims related to the sale, resale, licensing or registration, distribution or use by Company or any of its end-users of any Product accepted by Company pursuant to Section 2.5 above.
7.2 Procedure for Indemnity. If any claim or action is asserted that would entitle Indemnitee to indemnification pursuant to Section 7.1 (a “Proceeding”), the Indemnitee will give written notice thereof to the Indemnifying Party promptly of any demand, claim, loss, cost or damage or the commencement of any legal proceeding for which indemnification is sought hereunder (but in no event later than 15 days from such event); provided, however, that the failure of the Indemnitee seeking indemnification to give timely notice hereunder will not affect its rights to indemnification hereunder, except to the extent the Indemnifying Party demonstrates actual damage caused by such failure. An Indemnifying Party will not settle or consent to any entry of judgment in connection with any Proceeding without obtaining the prior written consent of the Indemnitee seeking indemnification hereunder, such consent not to be unreasonably withheld. The parties will fully cooperate with each other in any such Proceeding and will make available to each other any books or records useful for the defense of any such Proceeding
7.3 Insurance. Each party shall maintain general liability insurance, including products liability coverage, and professional/ errors & omissions liability insurance each in a minimum amount of $1,000,000 per occurrence or claims and $3,000,000 in the annual aggregate, with deductibles not exceeding $250,000 per occurrence or claim that provides coverage for the Products and the Services, as applicable contemplated by this Agreement. At a minimum, such party shall maintain such insurance coverage required hereunder for the entire Term and for a period of not less than three (3) years following expiration or termination of the Agreement. If any such policy shall provide coverage on a claims made basis, the party holding such policy shall be required to maintain a claims made policy providing such coverage for an additional period of not less than three (3) years following the expiration or termination of this Agreement. Each party shall deliver to the other party a certificate from the insurance carrier or broker evidencing such coverage and noting any exclusions and agreeing to provide no less than thirty (30) days’ prior written notice to such other party in the event of a material change in coverage or policy cancellation.
| -8- |
8. Term and Termination.
8.1 Term. The term of this Agreement shall commence on the Effective Date and shall continue for a period of five (5) years unless earlier terminated in accordance with this Section 8 (the “Term”). At the end of the Term, this Agreement will automatically renew for a period of one (1) year unless terminated in accordance with this Section 8. Prior to the end of the Term, the parties may mutually agree in writing to renew this Agreement for such period as they may mutually agree on the same, or substantially similar, terms and conditions as those terms and conditions set forth herein.
8.2 Termination for Breach. A party may terminate immediately this Agreement upon written notice to the other party, if such other party commits a material breach of this Agreement and fails to cure such material breach to the sole satisfaction of the non-breaching party within thirty (30) days after receiving written notice thereof.
8.3 Termination for Any Reason. Company may terminate this Agreement for any reason, upon providing Polyzen with one hundred eighty (180) days’ prior written notice of its intention to terminate. For purposes of avoiding doubt, in the event of a termination of this Agreement under this Section 8.3, Company agrees to purchase the amount of Products it is required to purchase under Section 2.2 (as modified by Section 2.3) during the one hundred eighty (180)-day notice period based on the most recent Forecast provided by Company.
8.4 Termination for Force Majeure. Company may terminate this Agreement as provided in Section 10.5.
8.5 Effect of Termination.
(a) Expiration or termination of this Agreement for any reason shall not release any party hereto from any liability which, at the time thereof, has already accrued to such party. Except in the event of Company’s termination of this Agreement pursuant to Section 8.2 or Section 8.4 above, Polyzen shall be entitled to payment of all fees incurred up to the date of termination and all non-cancellable obligations, including all purchased inventory, work-in-progress inventory, incurred in connection with any open and non-cancellable purchase order with a third-party vendor and all work-in-progress inventory and finished goods inventory at Polyzen, in each case, that is the subject of a Purchase Order; provided that, in the event of a termination under Section 8.4 by Company, Polyzen shall be eligible for the payment of all purchased inventory and materials that were procured in connection with a binding Purchase Order and that Polyzen actually delivers to the Company.
(b) Within ten (10) days after the effective date of the termination or expiration of this Agreement, each party shall return to the other party such other party’s Confidential Information in accordance with Section 4.3 above, except to the extent such Confidential Information would be required in connection with a Technology Transfer.
(c) Any and all provisions, promises and warranties contained herein which by their nature or effect are required or intended to be observed, kept or performed after termination of this Agreement will survive the termination of this Agreement and remain binding upon and for the benefit of the parties hereto, including, without limitation, the provisions of Sections 1, 2.7, 3, 4, 6, 7, 8, 9 and 10.
9. Recalls. Company shall have sole responsibility for and shall make all decisions with respect to any recall, market withdrawals or any other corrective action related to the Medical Device. If any Medical Device is recalled as a result of the gross negligence or intentionally wrongful acts or omissions of Polyzen or its representatives in the manufacture of the Products, then Polyzen shall bear and reimburse the Company for all of the costs and expenses of such recall, including reasonable attorney fees, expenses related to communications and meetings with Regulatory Authorities, expenses of replacement Products, the cost of notifying users of the Medical Devices, including costs associated with shipment of recalled Product from customers and shipment of an equal amount of replacement Product to those same customers (collectively, “Recall Costs”). If any Product is recalled as a result of the negligent or intentionally wrongful acts or omissions of the Company or its representatives, or is not due to the fault of either party, then the Company shall bear all Recall Costs including any outstanding inventory costs such as raw goods, work in progress and finished goods that cannot not be used by Polyzen in future manufacturing of the Products that may be related to such Recall. To the extent that the reason for any recall of the Medical Device is in part the responsibility of the Company or its agents and in part the responsibility of Polyzen, then the Recall Costs shall be allocated in an equitable manner between the parties. Any liability of Polyzen hereunder shall be subject to the limitation of liability set forth in Section 6 of this Agreement.
| -9- |
10. Miscellaneous.
10.1 Governing Law. This Agreement shall be governed and construed by, and enforced in accordance with, the laws of the State of New York, without reference to its conflicts of laws principles. The U.N Convention on Contracts for the International Sale of Goods shall not apply.
10.2 Financial Audit. During the Term and during the one (1)-year period thereafter, Polyzen agrees to allow the Company and its representatives, including its external auditors, access to its records solely to conduct an invoice reconciliation related to Polyzen’s provision of the Services hereunder, provided that: (i) access will be provided no more than one (1) time per year; (ii) Company provides Polyzen with reasonable prior written notice of its need for access; (iii) access will not disrupt unreasonably Polyzen’s normal business operations and will be provided only during Polyzen’s standard business hours; and (iv) if access is granted to Company’s representatives, such representatives must enter into a confidentiality and non-disclosure agreement reasonably acceptable to Polyzen. If any reconciliation reveals that Polyzen has overcharged Company, Polyzen shall promptly reimburse the Company for such overcharge and in the event that any such overcharge equals an amount equal to or greater than five percent (5%) of the amount that should have been charged under the terms of this Agreement”), then Polyzen shall promptly reimburse Company for fifty percent (50%) of reasonable costs and expenses incurred to third parties in the conduct of the audit, up to a maximum amount of five thousand dollars ($5,000) per audit.
10.3 Relationship of the Parties. The parties agree that they are independent contractors and that neither of them has any fiduciary duty to the other. Neither party is the agent of the other. Neither party may represent to any person that it has the power to bind the other party on any service contract or other agreement, or take any action reasonably likely to lead a third party to believe that it is the agent or representative of the other party.
10.4 Notices. All notices hereunder shall be in writing and delivered: (i) personally; (ii) by registered or certified mail, postage prepaid, return receipt request; or (iii) by overnight courier service; in each case, to the following addresses of the respective parties:
| If to Company: | Motus GI Medical Technologies Ltd. | |
| Keren Hayesod 22, | ||
| Tirat Carmel, Israel, 3902638 | ||
| Attn: General Manager | ||
| with a copy to: | ||
| Motus GI Medical Technologies Ltd. | ||
| 0000 X. Xxxxxxx Xxxx. | ||
| Xxxxx 000 | ||
| Xx. Xxxxxxxxxx, XX 00000 | ||
| Attn: CFO | ||
| If to Polyzen: | Polyzen, Inc. | |
| 0000 Xxxxxxx Xxxx | ||
| Xxxx, Xxxxx Xxxxxxxx 00000 | ||
| Attn: Executive Management |
Notices shall be effective upon receipt if personally delivered, on the fifth (5th) business day following the date of mailing if mailed, and upon receipt if sent by overnight courier service. A party may change its address listed above by notice to the other party.
10.5 Force Majeure. Except with respect to payments of money, neither party shall be liable to the other party for delays or failures in performance resulting from causes beyond its reasonable control, including, without limitation, acts of God; fires, floods or explosions; actions of governing or Regulatory Authorities; judicial orders; strikes or other labor disputes or disturbances; power disruptions or equipment malfunctions; acts of terrorism or war; riots or civil disturbances; or communication, utility or transportation failures (“Force Majeure Event”), provided, that the affected party promptly notifies the other of the cause and its effects on the Services to be performed hereunder and shall resume performance as soon as practicable following the end of the Force Majeure Event causing the delay. In the event that a Force Majeure Event continues for ninety (90) days, Company may terminate this Agreement immediately upon providing notice to Polyzen.
| -10- |
10.6 Severability. In case any one or more of the provisions of this Agreement shall be held by a court with proper jurisdiction to be invalid, illegal, or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained herein shall not in any way be affected or impaired thereby.
10.7 Assignment. This Agreement may not be assigned by either party without first obtaining the prior written consent of other party; provided, however, that no such consent shall be required for assignments to an Affiliate or the successor or the transferee of all or substantially all of a party’s business or assets to which this Agreement relates. This Agreement shall be binding upon and inure to the benefit of the parties hereto and their respective permitted successors and assigns. Any assignment by a party in violation of this section shall be null and void.
10.8 Waiver; Modification of Agreement. No waiver, amendment or modification of any of the terms of this Agreement shall be valid unless in writing and signed by authorized representatives of both parties hereto. No modification to this Agreement shall be affected by the acknowledgment or acceptance of any Purchase Order, invoice or similar documents containing terms or conditions at variance with or in addition to those set forth herein. Failure by either party to enforce any rights under this Agreement shall not be construed as a waiver of such rights nor shall a waiver by either party in one or more instances be construed as constituting a continuing waiver or as a waiver in other instances.
10.9 Counterparts. This Agreement and any exhibit attached hereto may be executed in one or more counterparts, each of which shall for all purposes be deemed to be an original and all of which shall constitute one and the same Agreement and shall become effective when signed by each of the Parties hereto and delivered to the other Party in accordance with the terms of this Agreement. Facsimile or a Portable Document Format (i.e., PDF) data file signatures of any original document shall be considered the same as delivery of an original.
10.10 Entire Agreement. This Agreement is the final, complete and exclusive agreement of the parties with respect to subject matter hereof and supersedes and merges all prior discussions between the parties.
10.11 Export Restrictions. Each party acknowledges that any Product sold under this Agreement is subject to customs and export controls laws and regulations of the United States and other countries. Each party agrees to abide by those laws and regulations. Further, under the laws of the United States, the Product shipped pursuant to this Agreement may not be sold, leased or otherwise transferred to restricted end-users or to restricted countries. Such shipped Product may not be sold, leased or otherwise transferred to, or utilized by, an end-user engaged in activities related to weapons of mass destruction, including without limitation, activities relating to the design, development, production or use of nuclear weapons, materials, or facilities, missiles or the support of missile projects, and chemical or biological weapons.
[Signature page follows]
| -11- |
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized officers as of the Effective Date.
| POLYZEN, INC. | MOTUS GI MEDICAL TECHNOLOGIES, LTD | |||
| By: | /s/ Xxxxx Xxxx | By: | /s/ Xxxx Xxxxxxxx | |
| Name: | Xxxxx Xxxx | Name: | Xxxx Xxxxxxxx | |
| Title: | CFO / COO | Title: | CEO | |
| Date: | August 31, 2017 | Date: | August 31, 2017 | |
| -12- |
Exhibit A
Products and Specifications
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510254
Rev A
| Part Description: | Motus GI Add-On Assembly - Packaged | |
| Polyzen P/N: | 510254 | Customer P/N: PV-OSK-001 Rev: A |
| Customer Drawing: PV-OSK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
Polyzen P/N 410015 correlates to Motus GI Drawing PV-OS-001
Inspection Tools:
Reverse Vacuum Tube Fixture MF00046 (TL0154) 800476
Sleeve Clamp (TL0188) 800477
Inflation Fixture MF00046 (TL0045)
Inflation Hub Tester (TL0151) PZ0977
Rigid Head Clamping Jig (TL0183) 800485
Microscope PZ
USON (TL0153) PZ0955 / PZ1013
Specifications:
| ● | * | |
| ● | * | |
| ● | * | |
| ● | * | |
| ● | * |
Packaging and Labeling Requirements Add-On
| ● | The IFU shall be included in the final packaging | |
| ● | The Add-on outer and primary packaging shall be labeled with the following information: product name, lot number, product code, expiry date, company name & contact information, latex or phthalate content, international symbols can be used as appropriate. | |
| ● | All package labels must be legible, with no obvious wear or smudging | |
| ● | Each single disposable shall be primary packaged in a pouch or a lidded box within a secondary shipper box | |
| ● | Each shipper box shall contain up to 5 single disposable units. |
Regulatory & Safety Requirements
| ● | The Add-on shall be manufactured in a clean room | |
| ● | Bioburden requirement: as per Motus GI’s specifications (< * CFU) |
| 1 of 5 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510254
Rev A
| Part Description: | Motus GI Add-On Assembly - Packaged | |
| Polyzen P/N: | 510254 | Customer P/N: PV-OSK-001 Rev: A |
| Customer Drawing: PV-OSK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
Labeling
| ● | Labels printed through Kodit system, content per spec XX-XX-000, XX-XXX-000 | ||
| ● | Label will contain at a minimum | ||
| o | Product Description | ||
| o | Reference Number (Customer PN) | ||
| o | Polyzen Lot Number | ||
| o | Manufacture Date | ||
| o | Expiration Date | ||
Certificate of Conformance Requirements:
| ● | Date | variable | |
| ● | Supplier Name: | Polyzen, Inc. | |
| ● | Customer PO#: | variable | |
| ● | Quantity Shipped: | variable | |
| ● | Product Description: | Motus GI Add-On Assembly – Packaged | |
| ● | Customer Part No.: | PV-OSK-001 Rev. A | |
| ● | Polyzen Part No.: | 510254 | |
| ● | Polyzen Lot No.: | variable | |
| ● | The COC shall be approved by Quality Assurance. | ||
Packaging (single part per PV-OS-001, Box of 5 per PV-OSK-001)
| ● | Assemblies to be packaged in five (5) individual In-patient Add-On boxes | |
| ● | One (1) IFU – Add-On Assembly | |
| ● | One (1 set) In-Patient Shipper Box Die Cut Foam Pad | |
| ● | One (1) Pure Vu Add-On Label on Shipper Box |
Note: parts to be sold will always be as a box of 5 (PV-OSK-001)
| 2 of 5 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510254
Rev A
| Part Description: | Motus GI Add-On Assembly - Packaged | |
| Polyzen P/N: | 510254 | Customer P/N: PV-OSK-001 Rev: A |
| Customer Drawing: PV-OSK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
In process inspection type and level:
| ● | Visual 100% QC Inspection | |||
| o | 410016 | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410017 | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410010 | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410011 | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410012 | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410013 | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410014 | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 410015 | |||
| ▪ | * | |||
| ▪ | * | |||
| 3 of 5 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510254
Rev A
| Part Description: | Motus GI Add-On Assembly - Packaged | |
| Polyzen P/N: | 510254 | Customer P/N: PV-OSK-001 Rev: A |
| Customer Drawing: PV-OSK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
| ▪ | 100% visually inspect Add-On assembly as per table below: | |||||
| ● | External Sensor Line Red | |||||
| o | Red luer | |||||
| o | Filter | |||||
| ● | Internal Sensor Line Blue | |||||
| o | Blue Luer | |||||
| o | Filter | |||||
| ● | Irrigation line | |||||
| o | Clear Luer | |||||
| ● | Pumping Line | |||||
| o | Cone x2 | |||||
| o | Strain Relief x2 | |||||
| o | Silicone tubes according to length x2 | |||||
| ● | Head | |||||
| o | * | |||||
| o | * | |||||
| o | * | |||||
| ● | Complete Seal. | |||||
| ● | No folds in seal area. | |||||
| ● | Anchoring points are closed in trays. | |||||
Final Release Testing:
| ● | 410015 | |||||
| o | Sampling Plan: | |||||
| ▪ | * | |||||
| ▪ | * | |||||
| ▪ | * | |||||
| o | 100% Visual Inspection | |||||
| ▪ | Ensure the seal seam is: | |||||
| ● | Complete | |||||
| ● | Clear | |||||
| ● | No Folds | |||||
| ● | Ensure the tubes inside the package are not kinked | |||||
Sterilization:
| ● | This product is not sterile | |
| ● | Sterilization is not required for this product |
Contract agreements
| ● | Customer Contract: FQA00006p |
| 4 of 5 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT - CONFIDENTIAL
CS 510254
Rev A
| Part Description: | Motus GI Cartridge Assembly - Packaged | |
| Polyzen P/N: | 510254 | Customer P/N: PV-WSCK-001 Rev: A |
| Customer Drawing: PV-WSCK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
Revision History
| Date | DCO # | Rev | Initiator | Changes |
| 19Jan2018 | 18-023 | A | * | Initial release of document |
| 5 of 5 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510255
Rev A
| Part Description: | Motus GI Cartridge Assembly - Packaged | |
| Polyzen P/N: | 510255 | Customer P/N: PV-WSCK-001 Rev: A |
| Customer Drawing: PV-WSCK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
Polyzen P/N 410018 correlates to Motus GI Drawing PV-WSC-001
Inspection Tools:
Cartridge testing Device TL0152 PZ0978
USON TL0153 PZ0955 / PZ1013
Specifications:
Packaging and Labeling Requirements Add-On
| ● | All package labels must be legible, with no obvious wear or smudging | |
| ● | Each single disposable shall be primary packaged in a pouch or a lidded box within a secondary shipper box | |
| ● | Each shipper box shall contain up to 5 single disposable units. |
Regulatory & Safety Requirements
| ● | WS Connector shall be manufactured in a Cleanroom | |
| ● | Bioburden requirement: as per Motus GI specifications (< * CFU) |
Labeling
| ● | Labels printed through the Kodit system per spec PV-WSC-001, and PV-WSCK-001 | ||
| ● | Label will contain at a minimum | ||
| o | Product Description | ||
| o | Reference Number (Customer PN) | ||
| o | Polyzen Lot Number | ||
| o | Manufacture Date | ||
| o | Expiration Date | ||
Certificate of Conformance Requirements:
| ● | Date | variable | |
| ● | Supplier Name: | Polyzen, Inc. | |
| ● | Customer PO#: | variable | |
| ● | Quantity Shipped: | variable | |
| ● | Product Description: | Motus GI Cartridge Assembly – Packaged | |
| ● | Customer Part No.: | PV-WSCK-001 Rev. A | |
| ● | Polyzen Part No.: | 510255 | |
| ● | Polyzen Lot No.: | variable | |
| ● | The COC shall be approved by Quality Assurance. | ||
| 1 of 3 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510255
Rev A
| Part Description: | Motus GI Cartridge Assembly - Packaged | |
| Polyzen P/N: | 510255 | Customer P/N: PV-WSCK-001 Rev: A |
| Customer Drawing: PV-WSCK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
Packaging (single part per PV-WSC-001, Box of 5 per PV-WSCK-001)
| ● | Packaged Five (5) WS Cartridge Assemblies in Cartridge Boxes | |
| ● | One (1 set) Cartridge Shipper Box Die Cut Foam Pad | |
| ● | One (1) Pure Xx XX Cartridge Label | |
| ● | One (1) Cartridge Shipper Box |
Note: Parts to be sold will always be as a box of 5 (PV-WSCK-001)
In process inspection type and level:
| ● | Visual 100% QC Inspection | |||
| o | 410018 | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
Final Release Testing:
| ● | 410018 | |||
| o | Sampling Plan: | |||
| ▪ | * | |||
| ▪ | * | |||
| ▪ | * | |||
| o | 100% Visual Inspection | |||
| ▪ | Complete Seal | |||
| ▪ | No folds in seal area | |||
| ▪ | Anchoring points are closed in trays | |||
Sterilization:
| ● | This product is not sterile |
| ● | Sterilization is not required for this product |
Contract agreements
| ● | Customer Contract: FQA00006p |
| 2 of 3 |
QAF 403a Customer Specification Template Rev C
POLYZEN, INC
CONTROLLED DOCUMENT – CONFIDENTIAL
CS 510255
Rev A
| Part Description: | Motus GI Cartridge Assembly - Packaged | |
| Polyzen P/N: | 510255 | Customer P/N: PV-WSCK-001 Rev: A |
| Customer Drawing: PV-WSCK-001 Rev: A | ||
| Customer Contracts: FQA00006p |
Revision History
| Date | DCO # | Rev | Initiator | Changes |
| 19Jan2018 | 18-023 | A | * | Initial release of document |
| 3 of 3 |
Exhibit B
Quality Agreement
[Attached]
 |
Title: Quality Agreement Form |
Document No: FQA00006p |
Rev: 3.0 |
Page: 2 of 4 |
Quality Agreement – Suppliers of Materials
MOTUS GI Medical Technologies Ltd. has entered into a technical supply agreement with Polyzen, (the “Supplier”), dated 6/30/17 (the “Agreement”), for the provision of Services. Capitalized terms used but not defined herein shall have the respective meanings given to such terms in the Agreement.
| 1.1 | The supplier will establish and maintain a quality system in accordance with the relevant standards and regulations. A copy of any quality system certification will be sent to MOTUS GI Medical Technologies Ltd (i.e., ISO: 9001, ISO: 13485, etc.). |
| 1.2 | The supplier agrees to supply only products complying with the purchasing specification developed and maintained by MOTUS GI Medical Technologies Ltd for the specific material. |
| 1.3 | The Company will provide Supplier with copies of all material filings, submissions and correspondence with and to Regulatory Authorities with respect to issues reasonably related to the performance of the Services by Supplier. Supplier will maintain pertinent Products documents, as applicable, to support the Company’s ongoing regulatory activities required for the manufacture of the Medical Devices all in accordance with applicable laws and industry standards, including 21 C.F.R. Part 820 and ISO 9001, ISO 13485. |
| 1.4 | During the term of the Agreement and for a period of time equivalent to the design and expected life of the Medical Device, but in no case, less than five years after the last product has been manufactured, Supplier shall keep complete records related to the manufacture of the Products at the Facility |
| 1.5 | The supplier agrees not to make any design changes, including, but not limited to changes to the material, such as changes to manufacturing process, testing methods, facility, site of manufacture etc., that may have impact on the quality system before the change is implemented for the materials sourced without the prior approval of MOTUS GI Medical Technologies Ltd. Requests for changes shall be submitted by supplier on FQA00006q - Supplier Change Request (SCR) form. |
| 1.6 | The supplier agrees to inform MOTUS GI Medical Technologies Ltd immediately of any errors or deviations to manufacture of the material that may have impact on the quality of the materials supplied. |
| 1.7 | The supplier agrees not to pass any information regarding the supply of materials to a 3rd party without the prior approval of MOTUS GI Medical Technologies Ltd. |
| 1.8 | Supplier will promptly advise the Company if a Regulatory Authority visits the Facility and requests or requires information or changes that directly pertain to the Product(s). Supplier shall supply the Company with copies of any correspondence provide by the Regulatory Authority, as well as any other documents related thereto requested by the Company. Supplier agrees to permit access to its Facility and records to any Regulatory Authority and to cooperate with such Regulatory Authority. |
This document is property of MOTUS Gl Medical Technologies LTD, its contents are CONFIDENTIAL and shall not be disclosed,
disseminated, copied or used, without a written permission.
 |
Title: Quality Agreement Form |
Document No: FQA00006p |
Rev: 3.0 |
Page: 3 of 4 |
| Supplier will, to the extent possible, allow a representative of the Company to be present during any such inspection, investigation or inquiry. | |
| 1.9 | Each party shall promptly (and in any event, within three (3) business days of the date of receipt of notice unless otherwise set forth herein) notify the other party in writing of, and shall provide the other party with copies of any correspondence and other documentation received or prepared by such party in connection with any of the following events: receipt of a letter from a Regulatory Authority including a Warning Letter or Untitled Letter related to the Product(s), FDA Form 483 (list of inspectional observations) or similar item, from the FDA or any other Regulatory Authority directed to the Product(s), or in connection with any general inspection applicable to the Facility that is impactful upon the Services or the Product(s) (“Regulatory Notices”). The parties shall cooperate with each other in responding to any such Regulatory Notices and shall provide copies to the other party of any documentation submitted to the Regulatory Authority in connection therewith. |
| 1.10 | The Company or its representatives, including its external auditors, may perform on site quality assurance audits and audit any records of Supplier related to the performance of the Services at any time during the Term of this Agreement and for the one (1) year period following the expiration or termination of this Agreement during normal business hours and without notice to Service Provider (unannounced audits). Supplier shall make any records readily available for such audit, and the Company or its designees may copy any and all such records in connection with any such audit. |
| 1.11 | In case non-conformance are found during the audit, the supplier undertakes to correct them within a reasonable time frame and acceptable by MOTUS GI Medical Technologies Ltd. If deficiencies are such that hinder safety, performance or compliance with regulatory requirements, for the supplier to stop production of the MOTUS GI Medical Technologies Ltd products and correct the deficiencies immediately. Beginning of remanufacturing is subject to approval in writing from the MOTUS GI Medical Technologies Ltd. |
| 1.12 | All parts supplied should be procured only from official distributors. |
| 1.13 | All the processes performed in the supplier facilities that are not verifiable by audits, are required to pass validation. MOTUS GI Medical Technologies Ltd will receive a copy of the final validation report concerning its products. |
| 1.14 | Electronic parts will not exceed the date code of 24 month. Older part will be provided only after coordination and written approval of MOTUS GI Medical Technologies Ltd quality management. |
| 1.15 | The supplier agrees to supply with each shipment a Certificate of Assurance or Certificate of Compliance or Certificate of Tests as applicable for the material. |
| 1.16 | The supplier agrees to investigate complaints regarding the purchased materials and issue a written report to MOTUS GI Medical Technologies Ltd detailing the findings and applicable corrective actions. |
This document is property of MOTUS Gl Medical Technologies LTD, its contents are CONFIDENTIAL and shall not be disclosed,
disseminated, copied or used, without a written permission.
 |
Title: Quality Agreement Form |
Document No: FQA00006p |
Rev: 3.0 |
Page: 4 of 4 |
| 1.17 | The Company will be solely responsible for interacting with the public or third parties with respect to complaints regarding the Medical Devices. Supplier will cooperate with the Company in investigating any such complaints to the extent that such complaint involves Products manufactured by Supplier for the Company pursuant to this Agreement. |
| 1.18 | The Company will be solely responsible for all medical device reporting required under applicable laws for the Medical Device. To the extent, Supplier receives a report of any adverse experience related to the Medical Device, Supplier will immediately, and in no event later than two (2) calendar days of receipt, forward the report to the Company. |
Records and Traceability
| 1.19 | If the validity of the agreement with MOTUS GI Medical Technologies Ltd expires, the supplier agrees to transfer to MOTUS GI Medical Technologies Ltd all records related to the company orders at least the last seven years. |
The agreement scope is listed bellow:
Material / service description: Manufacturing of finished disposable Oversleevcs, Work station Connectors, including all packaging, labeling and shipping. Finished good lot packages to be provided & approved to MOTUS GI prior to release I shipping.
| This supplier Quality agreement has been signed by: | ||||
| for MOTUS GI Medical Technologies Ltd | for Polyzen (supplier) | |||
| By: | Mado Otzri | By: | Xxxx Xxxxxxx | |
| Job Description: | QA Director | Job Description: | Director of Quality | |
| Date: | 4-Jul-2017 | Date: | 3-Jul-2017 | |
| Signature: | /s/ Mado Otzri | Signature: | /s/ Xxxx Xxxxxxx | |
This document is property of MOTUS Gl Medical Technologies LTD, its contents are CONFIDENTIAL and shall not be disclosed,
disseminated, copied or used, without a written permission.
Exhibit C
Price and Suppliers
Polyzen
Sleeve Assembly
Component: Sleeve Assembly
Supplier: Polyzen
| Motus Part Number | Volume / Capacity | Price | ||
| ASM100016 | * sleeve assemblies per week | $ * / sleeve Pricing as of October 2016 |
Other
Polyzen Components
Component: Leaf Seals
Supplier: Polyzen
Motus Part Number | Description | Quantity Per Device | Volume / Capacity | Price | ||||
| Leaf Seals – * | * | |||||||
| Leaf Seals – * | * |
Polyzen Sourced Components
Pricing Methodology for Sourced Components: Polyzen will provide open-book pricing on all sourced components, provided that Polyzen charge a xxxx-up all sourced components by * % to account for purchasing, incoming inspection / quality, and supplier management costs.
| ● | Component: Injection Molded Parts |
| Supplier: Medacys | |
| Address: C6, Mingzhuo Industrial Park, Guangming New District, Shenzhen, Guangdong, China 518107 |
Note: Pricing is based on validations being complete. Thus, any additional inspections will be charged as a separate line item.
| Price Breaks | ||||||||||||
| Motus Part Number | Rev | Part Description | Quantity Per Device | MOQ = 1,000 | 2,000 | 5,000 | ||||||
| ASM100003 | A | * | 2 | $ * | $ * | $ * | ||||||
| ASM100043 | N/A | * | 1 | $ * | $ * | $ * | ||||||
| XXX000000 | A | * | 1 | $ * | $ * | $ * | ||||||
| 10,000 | 50,000 | 100,000 | ||||||||||
| ASM100003 | A | * | 2 | $ * | $ * | $ * | ||||||
| ASM100043 | N/A | * | 1 | $ * | $ * | $ * | ||||||
| XXX000000 | A | * | 1 | $ * | $ * | $ * | ||||||
| Price Breaks | ||||||||||||
| Motus Part Number | Rev | Part Description | Quantity Per Device | MOQ = 1,000 | 2,000 | 5,000 | ||||||
| MFR000388 | A | * | 1 | $ * | $ * | $ * | ||||||
| MFR000212 | A | * | 1 | $ * | $ * | $ * | ||||||
| MFR000213 | N/A | * | 1 | $ * | $ * | $ * | ||||||
| MFR000334 | A | * | 2 | $ * | $ * | $ * | ||||||
| 10,000 | 50,000 | 100,000 | ||||||||||
| MFR000388 | A | * | 1 | $ * | $ * | $ * | ||||||
| MFR000212 | A | * | 1 | $ * | $ * | $ * | ||||||
| MFR000213 | N/A | * | 1 | $ * | $ * | $ * | ||||||
| MFR000334 | A | * | 2 | $ * | $ * | $ * |
| ● | Component: Tubing |
| Supplier: Natvar | |
| Address: 0000 X.X. 00, Xxxxxxx, XX 00000 |
| Motus Part Number | Description | Price (per unit) | Quantity per Device | |||
| MFR000214 | * | $ * | 2 | |||
| XXX000000 | * | $ * | 1 | |||
| TUB000052-01 | * | $ * | 1 | |||
| TUB000052-02 | * | $ * | 1 | |||
| TUB000052-03 | * | $ * | 1 | |||
| TUB000051-07 | * | $ * | 1 | |||
| TUB000051-04 | * | $ * | 1 | |||
| TUB000051-05 | * | $ * | 1 | |||
| TUB000051-06 | * | $ * | 1 |
Supplier: Vesta
Address: 000 XXX Xxx, Xxxxxx, XX 00000
| Motus Part Number | Description | Price (per unit) | Quantity per Device | |||
| TBD | * | TBD |
Other Sourced Components
| Vendor | Motus Part Number | Description | Price (per unit) | Quantity per Device | ||||
| Borla | STP000195 | * | $ * | 0 | ||||
| Xxxxx | XXX000000 | * | $ * | 0 | ||||
| Xxxxx | XXX000000 | * | $ * | 0 | ||||
| Xxxx | XXX000000 | * | $ * | 1 |
Full Medical Device Assembly
Supplier: Polyzen
Pricing will be determined and agreed to by the parties once full assembly transfer process has been determined, setup and confirmed. Initial estimates can be provided based on current time-studies and process in Motus Israel.

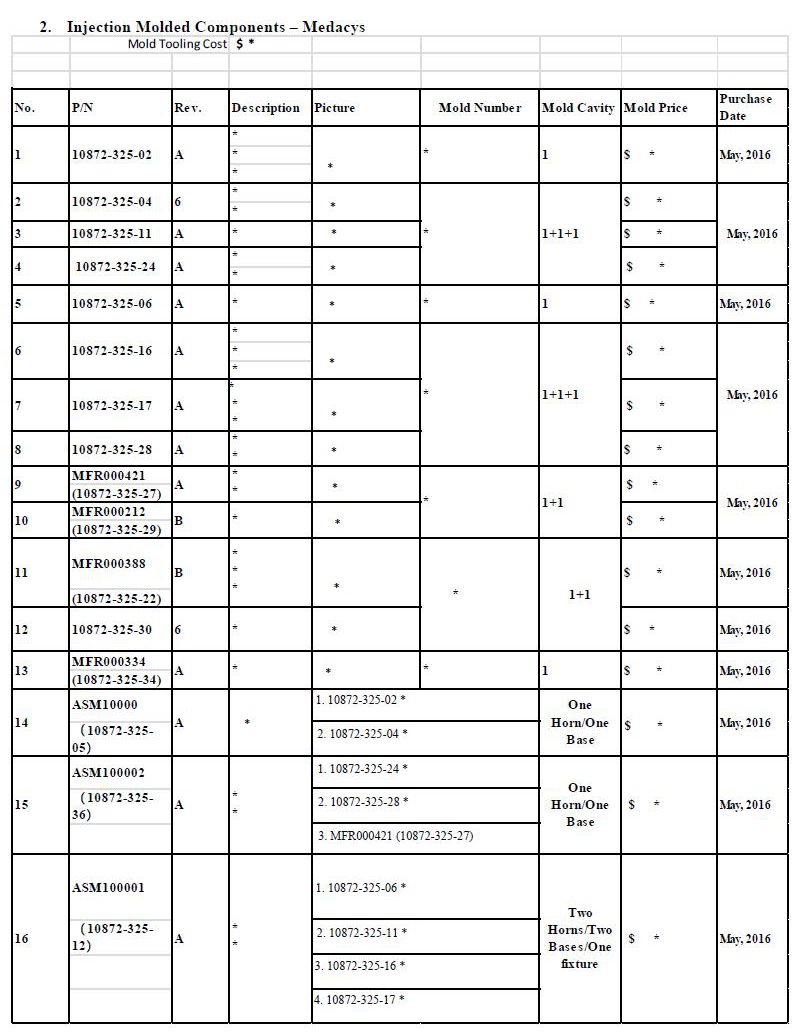
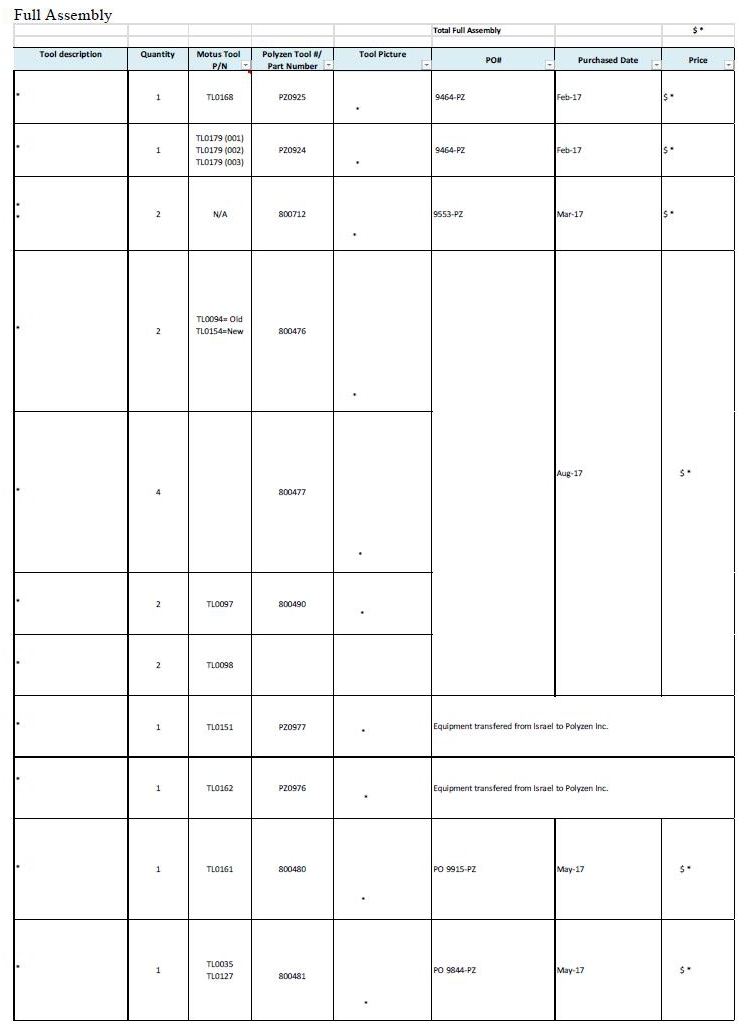
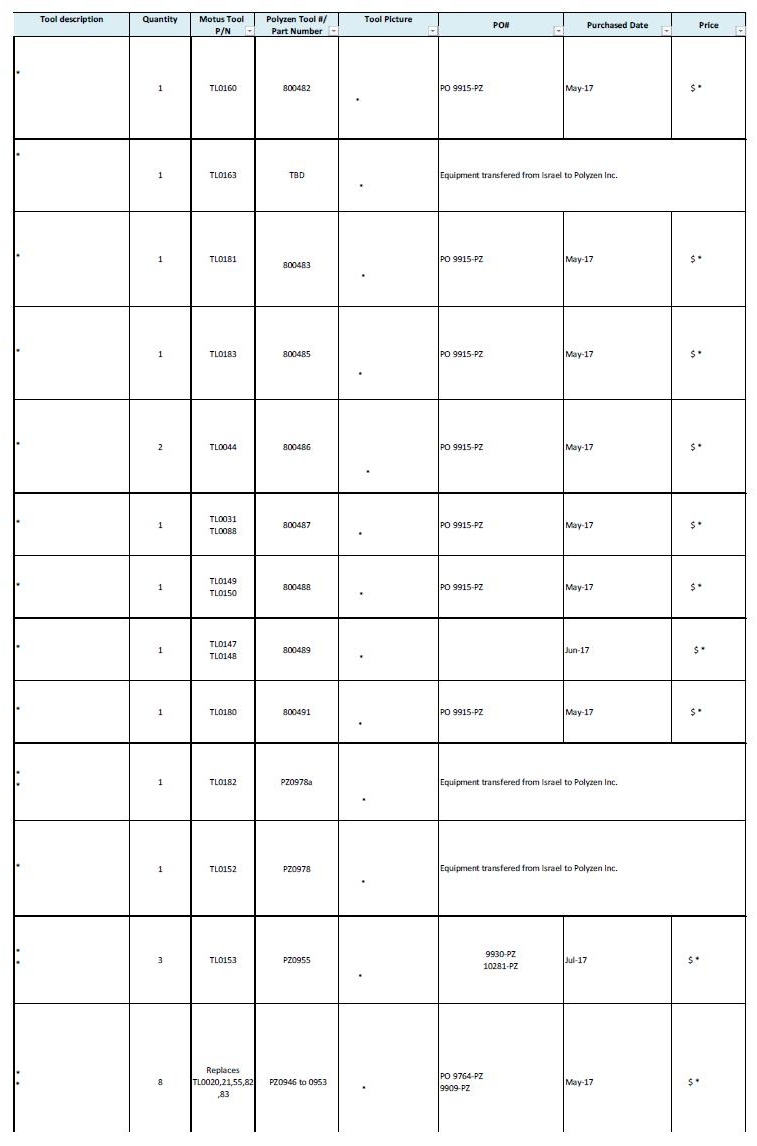
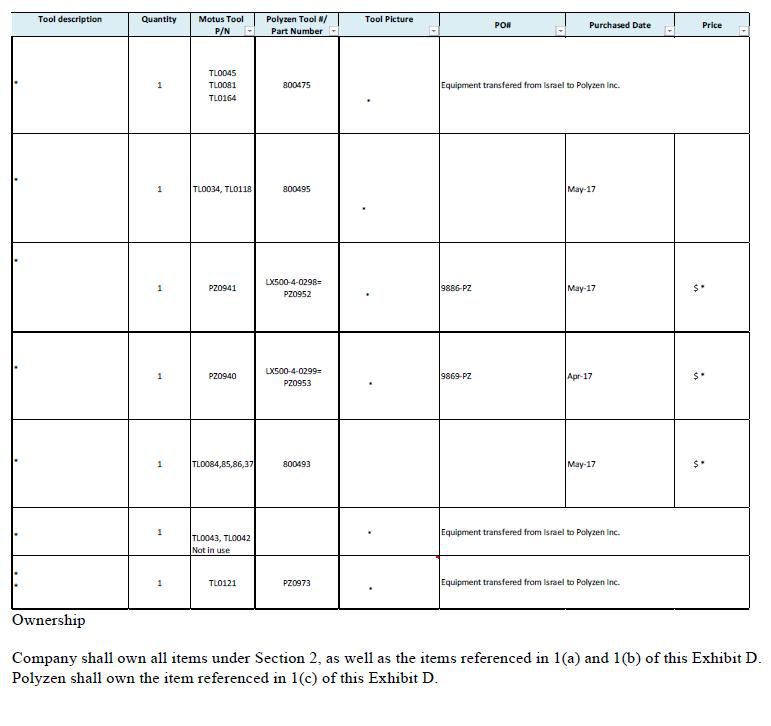
Exhibit E
Polyzen’s Background Intellectual Property as of the Effective Date
| 1. | Polyzen Add-On Sleeve Assembly, Coated | |
| a. | * | |
| b. | * | |
| c. | * | |

