ASSET PURCHASE AGREEMENT by and among ADITXT, INC. and CELLVERA GLOBAL HOLDINGS LLC, CELLVERA DEVELOPMENT, LLC, CELLVERA HOLDINGS LTD, AIPHARMA GROUP LTD, CELLVERA LTD. and Alex Gadotti, in his capacity as Sellers’ Representative April 19, 2023
Exhibit 2.1
by and among
and
CELLVERA GLOBAL HOLDINGS LLC,
CELLVERA DEVELOPMENT, LLC,
CELLVERA HOLDINGS LTD,
AIPHARMA GROUP LTD,
CELLVERA LTD.
and
Xxxx Xxxxxxx, in his capacity as Sellers’ Representative
April 19, 2023
TABLE OF CONTENTS
| ARTICLE I PURCHASE AND SALE | 1 | |
| 1.1 | Acquired Assets. | 1 |
| 1.2 | Excluded Assets. | 2 |
| 1.3 | Assumed Liabilities. | 2 |
| 1.4 | Retained Liabilities. | 3 |
| 1.5 | Procedures for Assets Not Transferable. | 4 |
| 1.6 | Waiver of Bulk Sales Compliance. | 4 |
| ARTICLE II PURCHASE PRICE | 5 | |
| 2.1. | Purchase Price. | 5 |
| 2.2. | Payments at Closing. | 5 |
| 2.3. | Revenue Sharing. | 6 |
| 2.4. | Allocation of Purchase Price. | 6 |
| 2.5. | Accounts and Notes Receivable. | 6 |
| 2.6. | Taxes. | 7 |
| ARTICLE III REPRESENTATIONS AND WARRANTIES OF EACH SELLER AND SELLER OWNER | 7 | |
| 3.1. | Organization and Good Standing. | 7 |
| 3.2. | Authorization of Agreement. | 7 |
| 3.3. | Conflicts; Consents of Third Parties. | 8 |
| 3.4. | Capitalization. | 8 |
| 3.5. | Subsidiaries. | 8 |
| 3.6. | Financial Statements; Books and Records; Accounts Receivable. | 8 |
| 3.7. | No Undisclosed Liabilities. | 9 |
| 3.8. | Absence of Certain Developments. | 9 |
| 3.9. | Taxes. | 11 |
| 3.10. | Reserved. | 12 |
| 3.11. | Tangible Personal Property; Sufficiency. | 12 |
i
| 3.12. | Intellectual Property. | 12 |
| 3.13. | Contracts and Agreements. | 13 |
| 3.14. | Reserved. | 14 |
| 3.15. | Reserved. | 14 |
| 3.16. | Reserved. | 14 |
| 3.17. | Litigation. | 15 |
| 3.18. | Compliance with Laws; Permits. | 15 |
| 3.19. | Reserved. | 15 |
| 3.20. | Insurance. | 15 |
| 3.21. | Significant Customers and Suppliers. | 16 |
| 3.22. | Certain Payments. | 16 |
| 3.23. | Affiliate Transactions. | 17 |
| 3.24. | Products Liability. | 17 |
| 3.25. | Regulatory Compliance; Permits. | 17 |
| 3.26. | Solvency. | 19 |
| 3.27. | International Trade. | 19 |
| 3.28. | Disclosure. | 21 |
| 3.29. | No Limitation. | 21 |
| ARTICLE IV | 21 | |
| 4.1. | Organization, Authority and Qualification. | 21 |
| 4.2. | No Conflicts; Consents. | 22 |
| 4.3. | Legal Proceedings. | 22 |
| 4.4. | Financial Statements. | 22 |
| 4.5. | Undisclosed Liabilities. | 22 |
| 4.6. | Ownership of GRA Membership Interest. | 22 |
| 4.7. | Compliance with Laws; Permits. | 23 |
| 4.8. | Taxes. | 23 |
ii
| 4.9. | Additional Provisions. | 23 |
| ARTICLE V REPRESENTATIONS AND WARRANTIES OF PURCHASER | 34 | |
| 5.1. | Organization and Good Standing. | 34 |
| 5.2. | Authorization of Agreement. | 34 |
| 5.3. | Conflicts; Consents of Third Parties. | 34 |
| 5.4. | Litigation. | 34 |
| ARTICLE VI COVENANTS | 34 | |
| 6.1. | Consents; Failure to Obtain Consents. | 34 |
| 6.2. | Post-Closing Cooperation; Mail Received After Closing. | 35 |
| 6.3. | Other Post-Closing Expenses. | 36 |
| 6.4. | Payment of Retained Liabilities. | 36 |
| 6.5. | Further Assurances. | 36 |
| 6.6. | Press Releases and Announcements. | 36 |
| 6.7. | Access. | 36 |
| 6.8. | Operation of the Business. | 37 |
| 6.9. | Conduct Prior to Closing. | 37 |
| 6.10. | No Negotiation. | 38 |
| 6.11. | Taxes. | 39 |
| 6.12. | Reserved. | 39 |
| 6.13. | Diligence Materials. | 39 |
| 6.14. | Release. | 39 |
| 6.15. | Regulatory Filings. | 40 |
| ARTICLE VII CLOSING | 41 | |
| 7.1. | Closing. | 41 |
| 7.2. | Conditions to Obligation of Purchaser. | 41 |
| 7.3. | Conditions to Obligation of Seller. | 44 |
iii
| ARTICLE VIII TERMINATION | 44 | |
| 8.1. | Termination of Agreement. | 44 |
| 8.2. | Procedure Upon Termination. | 45 |
| 8.3. | Effect of Termination. | 45 |
| ARTICLE IX SURVIVAL AND INDEMNIFICATION | 45 | |
| 9.1. | Survival of Representations and Warranties. | 45 |
| 9.2. | Indemnification of Purchaser Indemnified Parties. | 45 |
| 9.3. | Reserved. | 47 |
| 9.4. | Limitations on Indemnification. | 47 |
| 9.5. | Indemnification Procedures. | 47 |
| 9.6. | Fundamental Representation Damages. | 48 |
| 9.7. | General Limitation; Right of Set-Off. | 48 |
| 9.8. | Tax Treatment of Indemnity Payments. | 48 |
| ARTICLE X NON-COMPETITION AND CONFIDENTIALITY; SELLERS’ REPRESENTATIVE | 49 | |
| 10.1. | Definition. | 49 |
| 10.2. | Acknowledgments and Agreements by each Seller and Seller Owner. | 49 |
| 10.3. | Limited Activities. | 50 |
| 10.4. | Severability; Reformation. | 50 |
| 10.5. | Independent Covenant. | 50 |
| 10.6. | Materiality. | 50 |
| 10.7. | Sellers’ Representative. | 51 |
| ARTICLE XI MISCELLANEOUS | 52 | |
| 11.1. | Expenses. | 52 |
| 11.2. | Submission to Jurisdiction; Consent to Service of Process; Specific Performance. | 52 |
| 11.3. | Entire Agreement; Amendments and Waivers. | 52 |
| 11.4. | Governing Law. | 52 |
iv
| 11.5. | Notices. | 53 |
| 11.6. | Severability. | 54 |
| 11.7. | Binding Effect; Assignment. | 54 |
| 11.8. | Counterparts. | 54 |
| 11.9. | Prevailing Parties. | 54 |
| 11.10. | Joint Participation. | 54 |
| 11.11. | Other Definitional and Interpretive Matters. | 55 |
| 11.12. | Seller Dissolution. | 56 |
| Exhibits: | |
| Exhibit A – Favipiravir Compound Description | A-1 |
v
This Asset Purchase Agreement (this “Agreement”) is made as of the commencement of business on the 19th day of April, 2023, by and among Aditxt, Inc., a Delaware corporation (the “Purchaser”), Cellvera Global Holdings LLC, a Delaware limited liability company (“DE Topco”), Cellvera Holdings Ltd., a company incorporated under the laws of the British Virgin Islands f/k/a AiPharma Holdings Limited (“BVI Holdco”), Cellvera Ltd, a company incorporated under the laws of the British Virgin Islands (“Cellvera Ltd”), Cellvera Development LLC, a Delaware limited liability company (“Cellvera Development” and together with DE Topco, BVI Holdco, Cellvera Ltd and Cellvera Development each a “Seller” and collectively, the “Sellers”), AiPharma Group Ltd., a Cayman Island corporation (the “Seller Owner”; and collectively with the Sellers “Cellvera”), Xxxx Xxxxxxx, as the representative of Cellvera (in such capacity, the “Sellers’ Representative”). Capitalized terms not otherwise defined shall have the meaning ascribed to such terms in Annex I.
WHEREAS, Cellvera is a biopharmaceutical company focused on discovering, developing and commercializing oral therapies and monitoring tools to address the unmet needs of patients with life-threatening viral diseases and ownership of fifty percent (50%) of the outstanding equity interests of GRA (the “Business”);
WHEREAS, Seller Owner owns, directly or indirectly, all of the issued and outstanding equity interest of each Seller on the date hereof (the “Seller Equity”), and will continue to own such Seller Equity at the time of Closing; and
WHEREAS, Purchaser wishes to purchase from Seller and Seller is willing to sell to Purchaser, certain of the Assets constituting, used, or held for use in, or necessary for the operation of the Business, all on and subject to the terms and conditions set forth in this Agreement (the “Acquisition”).
NOW, THEREFORE, in consideration of the premises and mutual representations, warranties, covenants and agreements hereinafter set forth, the sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
ARTICLE
I
PURCHASE AND SALE
1.1 Acquired Assets. Subject to the terms and conditions set forth in this Agreement, at the Closing, each Seller and Seller Owner shall sell, convey, assign, transfer and deliver to Purchaser and Purchaser shall purchase, accept, acquire and take assignment and delivery of, all right, title and interest in, to and under the assets of such Seller used or held for use in the Business (wherever located and whether tangible or intangible), except for the Excluded Assets (collectively, the “Acquired Assets”) free and clear of all Liens, including the following:
(a) all of Cellvera Ltd’s membership interest in GRA;
(b) all Intellectual Property listed on Schedule 1.1(d);
1
(c) all copyrights on all printed materials, brochures and other materials listed on Schedule 1.1(e);
(d) all goodwill related to, arising from or used in connection with the Acquired Assets; and
1.2 Excluded Assets. Notwithstanding anything contained herein to the contrary, Purchaser is not purchasing, and neither Seller nor Seller Owner is selling, assigning, transferring, or conveying any assets, other than the Acquired Assets (collectively referred to as the “Excluded Assets”), including, without limitation:
(a) all taxpayer and other identification numbers and minute books, stock transfer books and other documents relating to the organization, maintenance, and existence of any Seller;
(b) any Real Property Lease;
(c) civic or charitable sponsorship Contracts;
(d) each Seller’s rights under this Agreement and the agreements to be executed by each Seller and Seller Owner in connection herewith;
(e) the bank account of any Seller set forth on Schedule 1.2(e) and all Cash contained therein;
(f) all securities owned by Cellvera (other than Cellvera Ltd’s interest in GRA);
(g) all employee-related or employee benefit-related files or records;
(h) all of Cellvera’s benefit plans, and all insurance Contracts, policies and/or administrative service arrangements related thereto;
(i) all of Cellvera’s insurance policies;
(j) any tax credits or refunds or claims for refunds of credits of Taxes or other amounts paid to Taxing Authorities in connection with the Business or the Acquired Assets and relating to any Pre-Closing Tax Periods and pre-Closing portion of the Straddle Period;
(k) all Tax Returns of any Seller or Seller Owner;
(l) all rights and claims to the extent related to any of the foregoing or any Retained Liability; and
(m) all Contracts of Cellvera except as specifically included in Section 1.1.
1.3 Assumed Liabilities. As part of the consideration for the Acquired Assets, subject to Section 1.4, at the Closing, Purchaser shall assume only the following Liabilities of Seller (the “Assumed Liabilities”):
(a) the non-payment obligations to be performed after the Closing under the Acquired Assets, in each case solely to the extent legally assigned to Purchaser but excluding any obligations or Liabilities arising from or related to any activities or events occurring on or prior to the Closing.
2
Notwithstanding anything herein to the contrary and for the avoidance of doubt, the fact that a Liability may fall under the definition of “Assumed Liabilities” and may have been assumed by the Purchaser hereunder, shall not in any respect prevent Purchaser from seeking or receiving indemnification hereunder with respect to such Liability to the extent the Purchaser is entitled to indemnity with respect to such liability or obligation pursuant to the terms of Article VIII. Furthermore, Purchaser shall not have any obligation to indemnify Seller with respect to a Liability constituting an Assumed Liability to the extent Purchaser is entitled to indemnification with respect to such Liability pursuant to the terms of Article VIII. For explanation purposes, if a Liability is an Assumed Liability but also constitutes a breach of a representation and warranty made by Seller or any Seller Owner, Purchaser shall be entitled to be indemnified with respect to such Liability, and Seller shall not be entitled to be indemnified by Purchaser for any Losses it incurs with respect to such Liability (pursuant to the indemnification of Purchaser or otherwise).
1.4 Retained Liabilities. Notwithstanding anything in this Agreement to the contrary, neither Purchaser nor any of its Affiliates shall assume and in no event shall be deemed to have assumed, any Liability of Seller or any of its Affiliates whatsoever (collectively, the “Retained Liabilities”), other than as specifically set forth in Section 1.3. Without limiting the generality of the foregoing, the Retained Liabilities shall include the following:
(a) all Liabilities for Taxes;
(b) any Liability arising from or related to the operations of Seller or the Business, whenever arising or incurred, or the ownership, development, or distribution of the Business products or the Acquired Assets by Sellers prior to and through the Closing Date, including any Warranty claims;
(c) all Liabilities with respect to any Seller’s employees (including Liabilities with respect to employment compensation, benefits or severance), including any accrued sales commissions or other accrued payroll obligations of any Seller which are unpaid at Closing (including the employer portion of any employment or payroll Taxes with respect to any such accruals);
(d) any Debt or other operating Liabilities of any Seller (including accounts payable or other obligations under any Contract (other than as specifically set forth in Section 1.3)). Purchaser shall be under no obligation to hire any of such Seller’s or Seller Owner’s employees and shall not assume any Liabilities with respect to such employees. In furtherance of the foregoing, Purchaser is not assuming any obligations or Liabilities with respect to any employees that Purchaser chooses not to hire;
3
(e) all Liabilities relating to or arising out of charges or assessments of any Governmental Body;
(f) all Liabilities or obligations owed or owing to any shareholders, members, Persons or any Affiliate of the foregoing or any Affiliate of any Seller;
(g) all Liabilities with respect to any Seller's violation or alleged violation of any law, including laws relating to civil rights, health, safety, labor, discrimination, intellectual property, export controls and Environmental Laws;
(h) all Liabilities for Warranty claims relating to or arising from operations of the Business prior to the Closing Date;
(i) any obligation of any Seller or Seller Owner to indemnify any Person;
(j) indebtedness or guaranty of any indebtedness;
(k) past, pending, future suits, investigations, administrative proceedings or any other proceedings or claims based on violations of any Laws relating to any Seller or Seller Owner operation of the Business prior to the Closing;
(l) any accrued sales commissions or other accrued payroll obligations of any Seller or Seller Owner which are unpaid at Closing (including the employer portion of any employment or payroll Taxes with respect to any such accruals);
(m) all Contracts with, and obligations and liabilities to, any Person or legal entity that is subject to U.S. economic sanctions as administered by the Office of Foreign Assets Control of the U.S. Department of Treasury, or any person or legal entity that is located in any of the following countries or regions: the People’s Republic of China, the Russian Federation, and the Crimea, Donbas and Luhansk regions of Ukraine; and
1.5 Procedures for Assets Not Transferable. Unless expressly waived by the Purchaser, if any Contract, Permit, or any other property or right included in the Assumed Liabilities or the Acquired Assets is not assignable or transferable without the Consent of any Person, and such Consent has not been obtained prior to the Closing: (a) this Agreement and the related instruments of transfer shall not constitute an assignment or transfer thereof; (b) Cellvera’s obligations with respect to such Contract, Permit or other property or right shall be governed by Section 6.1; and (c) Purchaser shall not assume Cellvera’s obligations with respect thereto until such time as the applicable Consent has been obtained (at which point Purchaser shall only assume Cellvera’s obligations with respect thereto arising after such Consent has been obtained).
1.6 Waiver of Bulk Sales Compliance. Purchaser and Cellvera hereby waive compliance with the bulk sales Laws of any applicable jurisdiction, and Cellvera agrees to indemnify, defend and hold harmless Purchaser and its Affiliates from and against any claims arising out of or due to the failure to comply with such bulk sales Laws.
4
ARTICLE
II
PURCHASE PRICE
2.1. Purchase Price. The aggregate consideration for the Acquired Assets and the covenants of each Seller and the Seller Owner contained herein shall be Twenty-Four Million Five Hundred Thousand Dollars, subject to adjustment in accordance with Section 2.2, minus (i) Closing Date Debt amount, minus (ii) the Loan Forgiveness Amount, minus (iii) Seller Expenses plus (iv) the Revenue Sharing Payment, when and if earned, (such total being referred to herein as the “Purchase Price”). Seller and Seller Owner each acknowledge and represent that the Purchase Price is fair market value for the Acquisition.
2.2. Payments at Closing. At Closing, Purchaser shall make (or cause one or more of its Affiliates to make) the following payments with respect to the Purchase Price:
(a) To the holders of Closing Date Debt set forth on Schedule 2.2(a) (which such schedule shall be updated two (2) days prior to Closing and also contain the wire instructions for each such Person), the Payoff Amounts, as specified in each Payoff Letter and Release Agreement;
(b) to the payees of Seller Expenses set forth on Schedule 2.2(b) (which such schedule shall be updated two (2) days prior to Closing and also contain wire instructions for each such Person), the amount specified in an invoice provided to Purchaser;
(c) to an account or accounts designated by the Sellers prior to the Closing Date, the following amount (such total, the “Closing Payment”), which shall be paid by wire transfer of immediately available United States funds:
(i) the Purchase Price; minus
(ii) the Closing Date Debt set forth on Schedule 2.2(a); minus
(iii) the Loan Forgiveness Amount set forth on Schedule 2.2(a); minus
(iv) the Seller Expenses set forth on Schedule 2.2(b).
Upon delivery of any payment by Purchaser in accordance with this Section 2.2, Purchaser’s obligations to pay such amounts to Seller shall be deemed satisfied and discharged to the extent of such payment.
5
2.3. Revenue Sharing.
(a) Revenue Sharing Payments. From the Closing Date until the seven (7) year anniversary of the Closing Date, Purchaser shall pay to Seller Owner an amount equal to 30% of Net Sales of Cellvera Products sold by or on behalf of Purchaser (the “Revenue Sharing Payment”). Revenue Sharing Payments will be made on a quarterly basis beginning after the Closing Date (the “Revenue Sharing Payment Date”).
(b) Reports and Timing of Payments. Revenue Sharing Payments will be made within ninety (90) calendar days following each Revenue Sharing Payment Date. Purchaser shall provide Seller Owner with a report of Net Sales of Cellvera Products in sufficient detail to permit confirmation of the accuracy of the applicable Revenue Sharing Payment, including, on a product-by-product basis: the number of Cellvera Products sold; the gross sales and the Net Sales of each Cellvera Product and the method used (including the exchange rate) to calculate the Revenue Sharing Payment (the “Payments Report”). Revenue Sharing Payments shall be made on the date of delivery of such Payments Report, but in any event no later than the ninetieth (90th) calendar day following the end of each applicable Revenue Sharing Date.
(c) Right of Set-Off. Notwithstanding anything to the contrary in this Section 2.3 or elsewhere in this Agreement, and without prejudice to any other right or remedy it has or may have, Purchaser shall have the absolute right to offset and reduce all or any portion of the Revenue Sharing Payment in order to satisfy any indemnification obligations pursuant to ARTICLE IX.
2.4. Allocation of Purchase Price. The parties hereto shall allocate the Purchase Price among the Acquired Assets and the covenants contained in Article IX as set forth on Schedule 2.5. Such allocation is intended to comply with the requirements of Section 1060 of the Code. Seller and Purchaser shall file Form 8594 with their respective Tax Returns consistent with such allocation. The parties shall treat and report the transaction contemplated by this Agreement in all respects consistently for purposes of any federal, state or local Tax, including the calculation of gain, loss and basis with reference to the Purchase Price allocation made pursuant to this Section 2.4. The parties hereto shall not take any action or position inconsistent with the obligations set forth in this Section 2.4, except as may otherwise be required by applicable Law. Purchaser and Seller shall, within thirty (30) days of the date of any post-Closing payment made pursuant to or in connection with this Agreement, revise Schedule 2.4 to the extent necessary to reflect any such post-Closing payment.
2.5. Accounts and Notes Receivable. Each Seller and Seller Owner will deliver to Purchaser a schedule of all accounts and notes receivable (and the face amounts thereof) of such Seller or Seller Owner which are outstanding on the Closing Date. The parties hereto agree that Purchaser may assign to any Seller any accounts and notes receivable which are outstanding on the Closing Date and which are uncollected as of the date six (6) months after the Closing Date, and concurrently with such assignment, Seller Owner shall pay to Purchaser in cash an amount equal to the aggregate value of such accounts and notes receivable to the extent the same exceeds the reserve for doubtful accounts on the Reference Balance Sheet. All amounts which are collected by Purchaser from an account or note debtor after the Closing Date shall be first applied to reduce the oldest outstanding balance on such account or with such note debtor. The amounts payable by Seller pursuant to this Section 2.5 shall not be considered Losses subject to the provisions of Article VII.
6
2.6. Taxes. Each Seller and Seller Owner shall timely pay all Taxes and fees imposed by Governmental Bodies and required to be paid in connection with or arising from the sale, transfer, or assignment of the Acquired Assets, including all sales, use, transfer, intangible, recordation, documentary stamp or similar Taxes, and shall timely submit all related Tax Returns to the appropriate Governmental Bodies.
ARTICLE
III
REPRESENTATIONS AND WARRANTIES OF EACH SELLER AND SELLER OWNER
Each Seller and Seller Owner, jointly and severally, represent and warrant to Purchaser as of the date hereof and as of the Closing Date as follows:
3.1. Organization and Good Standing. Each Seller and Seller Owner is duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is organized, that it is not in penalty, and has all requisite power and authority to conduct its business as now conducted and to own and operate its assets as now owned and operated by it. Each Seller and Seller Owner is duly qualified or authorized to do business as a foreign corporation and is in good standing under the laws of each jurisdiction in which it owns or leases real property and each other jurisdiction in which the conduct of its business or the ownership of its assets requires such qualification or authorization (which jurisdictions are set forth on Schedule 3.1). True, correct and complete copies of the Organizational Documents of each Seller and Seller Owner, as currently in effect, have been delivered to Purchaser.
3.2. Authorization of Agreement. Each Seller and Seller Owner has all requisite corporate power and authority to execute and deliver this Agreement and each other agreement, document, instrument or certificate contemplated by this Agreement or to be executed by each Seller and Seller Owner in connection with the consummation of the transactions contemplated by this Agreement (collectively with this Agreement, the “Seller Documents”), and to consummate the transactions and perform its obligations as contemplated thereby. The execution, delivery and performance of each Seller and Seller Owner with respect to its Seller Documents and the consummation of the transactions contemplated thereby have been duly authorized by all requisite corporate action on the part of each Seller (including the Seller Owner). This Agreement has been, and each of the other Seller Documents will be at or prior to the Closing, duly and validly executed and delivered by each Seller and (assuming the due authorization, execution and delivery by Purchaser) this Agreement constitutes, and the each of the other Seller Documents will constitute, the legal, valid and binding obligation of each Seller and Seller Owner, enforceable against it in accordance with its terms.
7
3.3. Conflicts; Consents of Third Parties.
(a) None of the execution, delivery or performance by any Seller of the Seller Documents, the consummation of the transactions contemplated thereby, or compliance by each Seller with any of the provisions thereof will: (i) cause any Seller to violate or breach any Law or Order; (ii) conflict with or result in a violation of the Organizational Documents of any Seller; or (iii) except as set forth on Schedule 3.3(a), conflict with or result in a breach or termination of any of the terms, conditions or provisions of, or constitute a default under, accelerate any obligations arising under, trigger any payment under, require any Consent under or any notice under, result in the creation of any Lien pursuant to, or otherwise adversely affect, any Contract to which any Seller is a party or by which its assets may be bound.
(b) Except as set forth on Schedule 3.3(b), no waiver, Order, Permit or Consent of any Governmental Body is required on the part of any Seller in connection with the execution and delivery of the Seller Documents or the compliance by any Seller with any of the provisions thereof, or the consummation of the transactions contemplated thereby.
3.4. Capitalization. Schedule 3.4 sets forth the Seller Equity of each Seller that is owned and held, beneficially and of record, by Seller Owner. None of Seller or Seller Owner has granted any other Person any rights with respect to any Seller Equity. The Seller Equity held by such Seller Owner and Seller, as applicable: (i) constitutes all of the equity securities of each Seller (including any securities convertible into or exchangeable for equity securities of each Seller); and (ii) is not subject to any Liens, or any Liabilities, Contracts or other rights or obligations that would require Seller Owner, any Seller, or entitle any other Person (contingent or otherwise), to issue, transfer, sell, repurchase, retire, redeem or otherwise acquire or dispose of, directly or indirectly, the Seller Equity to any Person. There are no voting trusts, proxies, or any other similar Contracts to which such Seller Owner is a party or by which Seller Owner is subject with respect to any Seller Equity.
3.5. Subsidiaries. No Seller owns any equity or debt securities or other ownership interest, directly or indirectly, in any other Person, nor is it party to any Contract to acquire any such securities or other ownership interest.
3.6. Financial Statements; Books and Records; Accounts Receivable.
(a) Schedule 3.6 contains true, correct and complete copies with respect to each Seller and Seller Owner of: (i) the audited balance sheets of such Seller for each of the fiscal years ended December 31, 2020 and 2021 and the related unaudited consolidated statements of income and of cash flows (the “Annual Financial Statements”); (ii) the audited balance sheet of such Seller at December 31, 2022 and the related consolidated statements of income and cash flows of such Seller for the two-month period then ended (the “Interim Financial Statements”, collectively with the Annual Financial Statements, the “Financial Statements”). Except in the case of unaudited financial statements, for the absence of notes, the Financial Statements: (i) have been prepared in accordance with GAAP and fairly present the financial position, results of operations and cash flows of such Seller as at the dates and for the periods indicated therein, and (ii) are consistent with the books and records of such Seller maintained in the ordinary course of business.
8
(b) The books of account and other business records of each Seller, all of which have been previously delivered to Purchaser and its representatives, are accurate and complete in all material respects. The books and records of each Seller accurately reflect the assets, liabilities, financial condition and results of operations of the Business and have been maintained in accordance with good business and bookkeeping practices.
(c) Each Seller maintains systems of internal accounting controls sufficient to provide reasonable assurances that transactions are recorded as necessary to present accurately and fairly in all material respects the financial condition of such Seller.
(d) All receivables reflected in the balance sheet in the Interim Financial Statements (the “Reference Balance Sheet”), or which have arisen from the conduct of the Business since the date of the Reference Balance Sheet (the “Reference Balance Sheet Date”), are valid and have arisen only from bona fide, arms-length transactions entered into in the ordinary course of business consistent with past practices, and are not subject to defeasance, offset or any counterclaim and are good and collectible within ninety (90) days from the Closing Date. Such receivables, net of adequate reserves, are fairly presented in accordance with GAAP, consistently applied in the Financial Statements. No Seller has accepted any prepayment or other payment for products to be delivered or services to be performed on or after the Closing Date. No Seller has any issued and outstanding invoices for payments due in consideration for services not yet rendered or goods not yet delivered as of the Closing Date.
3.7. No Undisclosed Liabilities. Except as set forth on Schedule 3.7 or to the extent reflected or provided for in the Reference Balance Sheet, no Seller has Liabilities other than: (i) accounts payable and accrued expenses incurred after the Reference Balance Sheet Date in the ordinary course of business consistent with past practices, none of which is, individually or in the aggregate, material, (ii) executory obligations or liabilities under Contracts listed on Schedule 3.13 (but not including any Liabilities or obligations arising out of any breach of Contract), and (iii) Liabilities incurred in connection with the Acquisition.
3.8. Absence of Certain Developments. Since the Reference Balance Sheet Date: (i) each Seller has conducted the Business only in the ordinary course of business consistent with past practices; and (ii) there has not been any event, change, occurrence or circumstance that has had, or is reasonably likely to have, individually or in the aggregate, a Material Adverse Effect. Except as contemplated by this Agreement or as set forth on Schedule 3.8, since the Reference Balance Sheet Date, no Seller has:
(a) incurred any Debt, other than trade accounts payable in the ordinary course of business consistent with past practices;
9
(b) changed any accounting principles, methods or practices, or the manner each such Seller keeps its books and records, or its practices with regard to the booking of sales, receivables, payables or accrued expenses or materially altered its payment or collection practices;
(c) (i) granted any severance, continuation or termination pay to any director, officer, shareholder or employee; (ii) entered into any employment, deferred compensation or other similar agreement (or any amendment to any such existing agreement) with any director, officer, shareholder or employee; (iii) increased, amended, or changed compensation, bonus or other benefits payable or potentially payable to current or former directors, officers, shareholders or employees, other than that as may be required by Law; (iv) adopted any new or changed the terms of any existing bonus, pension, insurance, health or other benefit plan; or (v) represented to any employee or former employee that Seller, Purchaser or any other Person would continue to maintain or implement any benefit or would continue to employ such employee after the Closing Date;
(d) suffered any damage, destruction or loss (whether or not covered by insurance) to any of its material properties or assets or disposed of any assets other than inventory in the ordinary course of business consistent with past practices;
(e) except in the ordinary course of business consistent with past practices and levels, granted customers of the Business any rebates, price concessions, discounts or allowances, materially altered its pricing or payment terms or agreed to any material reduction in discounts received from suppliers or any material increase in the price of raw materials;
(f) made any declaration, setting aside or payment of any non-cash dividend or other distribution with respect to, or any repurchase, redemption or other acquisition of, any of its capital stock or other equity interests;
(g) purchased, leased or otherwise acquired (whether by merger, consolidation or other business combination, purchase of securities, purchase of assets or otherwise) any material portion of the business or assets of any other Person;
(h) made, changed or revoked any material Tax election, elected or changed any material method of accounting for Tax purposes, settled any Legal Proceeding in respect of Taxes or entered into any Contract in respect of Taxes with any Governmental Body;
(i) terminated or closed any facility, business or operation;
(j) written up or down any of its material properties or assets or materially revalued any of its inventory or altered its inventory management or valuation policies or practices;
(k) cancelled, waived or compromised any Debt, right or claim having a value of more than $10,000 (individually) or an aggregate value in excess of $25,000;
10
(l) sold, assigned, transferred or granted any Intellectual Property, entered into any settlement regarding the breach or infringement of any Intellectual Property, or taken any action (or failed to take any action) that has resulted in, or would reasonably be likely to result in, the loss, lapse, abandonment, invalidity or enforceability of any of its Intellectual Property;
(m) made any capital expenditures or capital additions or betterments in excess of an aggregate of $25,000;
(n) made any purchase commitment outside the ordinary course of business consistent with past practice, or made any advances to any Person, other than to employees in the ordinary course of business consistent with past practice; or
(o) committed or agreed to do any of the foregoing.
3.9. Taxes. Except as set forth on the applicable subpart of Schedule 3.9(a) – (d):
(a) Each Seller has timely filed all Tax Returns and reports required to be filed by it, all of which were true, correct and complete in all material respects. Each Seller has provided Purchaser with copies of such Tax Returns filed in each of the immediately preceding three (3) calendar years. All Taxes required to be paid by each Seller have been fully and timely paid, whether or not shown on any such Tax Returns. There are no Liens as a result of any unpaid Taxes upon any of the assets of any Seller and no Seller has Knowledge of any information that indicates any such Lien is currently threatened or contemplated to be filed by any Taxing Authority with respect to Seller or any Acquired Asset. Seller has set aside adequate reserves for all accrued but unpaid Taxes.
(b) All Taxes required to be withheld or collected by each Seller have been withheld or collected and have been (or will be) duly and timely paid to the proper Taxing Authority. All persons performing services on behalf of each Seller have been properly classified by such Seller for purposes of Tax reporting and Tax withholding as required by applicable Law. Each Seller has complied in all material respects with Tax recordkeeping requirements. No deficiencies for any Taxes have been proposed, asserted or assessed by any Taxing Authority against any Seller that are still pending, and no Tax Return of such Seller is under current examination by any Taxing Authority. No requests for waivers of the time to assess any Taxes have been granted by any Seller that are still pending.
(c) There is no pending claim by any Taxing Authority of a jurisdiction where a Seller has not filed Tax Returns that such Seller is subject to Taxation in that jurisdiction.
(d) No Seller is a party to any Tax sharing agreement and has no Liability for the Taxes of any other Person as successor, by Contract or otherwise.
(e) Schedule 3.9(e) sets forth the withholding taxes paid by any Seller or Seller Owner for each calendar year since December 31, 2019, and provides: (i) the amount, (ii) the recipient, (iii) the nation imposing the tax and (iv) the type of Tax with respect to which the Tax was imposed.
11
3.10. Reserved.
3.11. Tangible Personal Property; Sufficiency.
(a) Except as noted on Schedule 3.11(a): (i) all tangible Acquired Assets owned or leased by each Seller are in the possession of such Seller at one of the Seller Properties; (ii) such tangible Acquired Assets are in good operating condition and repair (ordinary wear and tear excepted) and are suitable for the use to which they are put; and (iii) with respect to any tangible Acquired Assets leased by a Seller, such assets are in such condition as to permit the surrender thereof on the date hereof without any cost or expense for repair or restoration if the related leases were terminated on the date hereof in the ordinary course of business,
(b) Sellers and Seller Owner do not own inventory.
(c) Each Seller owns all right, title and interest in and to all of its properties and assets reflected in the Reference Balance Sheet or acquired since the Reference Balance Sheet Date, free and clear of any and all Liens.
(d) The Acquired Assets constitute all of the properties and assets necessary to conduct the Business.
3.12. Intellectual Property.
(a) Set forth on the applicable subpart of Schedule 3.l2(a) is a list of all: (i) patents and applications therefor; (ii) trademarks, service marks, service names or trade names, or registrations or applications for registration thereof, (iii) material unregistered trademarks, service marks, service names or trade names, (iv) copyright registrations, applications for copyright registration and unregistered copyrights material to the operation of the Business; and (v) domain names, in each case owned or held by each Seller (the “Listed Intellectual Property”). Each item of Listed Intellectual Property is in full force and effect. All necessary registration, maintenance and renewal fees in connection with such Listed Intellectual Property have been paid and all necessary documents and certificates in connection with such Listed Intellectual Property have been filed with the relevant Governmental Body for the purpose of perfecting or maintaining such Listed Intellectual Property, including any filings or payments due within one hundred twenty (120) days of the Closing. None of the Listed Intellectual Property is the subject of any challenge pending with any patent or trademark office, and no Seller has received notice of any such challenge in writing.
(b) Except as set forth on Schedule 3.12(b), the current use of any Intellectual Property by each Seller does not conflict with, infringe upon or violate any rights of any third party. No Seller has received in the past five (5) years any notice, charge, claim or other assertion of any present, impending or threatened infringement by, or misappropriation of, or conflict with any Intellectual Property of any other Person. No third party is infringing any Listed Intellectual Property of a Seller.
12
(c) Each Seller owns or has the right to use all Intellectual Property used in the Business. Except as set forth on Schedule 3.12(c), no Seller is required to pay any royalties, honoraria, fees or other payments to any Person by reason of the use of any Intellectual Property.
(d) All personnel, including employees, agents, consultants and contractors, who have contributed to or participated in the conception and development of any Intellectual Property on behalf of the Business either: (i) have been party to a “work-for-hire” arrangement or agreement with each Seller that has accorded such Seller full, effective, exclusive and original ownership of all Intellectual Property and tangible and intangible embodiments arising therefrom; or (ii) have executed appropriate instruments of assignment in favor of each Seller as assignee that have conveyed to such Seller full, effective and exclusive ownership of all Intellectual Property and tangible embodiments arising therefrom.
3.13. Contracts and Agreements.
(a) The applicable subpart of Schedule 3.13(a) sets forth all of the Contracts to which any Seller or Seller Owner is a party or by which it or any of its assets is bound, including (the “Material Contracts”):
(i) Contracts entered into within the last three (3) years or otherwise having executor obligations on the part of each Seller and relating to the acquisition or disposition by each Seller of: (A) any business, real property or business segment (whether by merger, consolidation or other business combination, sale of assets or otherwise) or the capital stock of any Person, (B) any of the assets of such Seller (other than sales of inventory or the disposition of obsolete equipment, in each case in the ordinary course of business) for consideration in excess of $10,000;
(ii) Contracts relating to the incurrence, assumption or guarantee of any Debt;
(iii) any other Contracts (or groups of related Contracts) which involve the expenditure or receipt of more than $10,000 annually or more than $25,000 over the remaining term thereof (other than purchases of inventory in the ordinary course of business), or require performance by any party more than one year from the date hereof, that, in each case, are not terminable by Seller without penalty or notice of sixty (60) days or less;
(iv) Contracts restricting the ability of any Seller to operate any business;
(v) Contracts that require any Seller to purchase minimum quantities (or pay any amount for failure to purchase any specific quantities) of goods or services, or to deal with any Person on an exclusive basis, or containing “most favored nations” or similar pricing arrangements;
13
(vi) Contracts that require any Seller to indemnify or hold harmless any other Person;
(vii) Contracts that provide for any partnership, joint venture, revenue sharing, strategic alliance, teaming or similar arrangement;
(viii) Contracts that provide for or relate to any employment or consulting relationship with any Person (other than at-will arrangements);
(ix) Contracts pursuant to which any Seller grants or is granted a license of any Intellectual Property (other than standard, shrink-wrap or commercially available off-the-shelf licenses);
(x) Contracts granting a power of attorney;
(xi) Contracts with any Governmental Body;
(xii) Contracts relating to the sales or distributions of any Seller’s products or services (excluding purchase and sales orders entered into in the ordinary course of business but including master sales contracts with such Seller’s customers); and
(xiii) Contracts that are otherwise material to the business, operations or financial condition of any Seller and is outside such Seller’s ordinary course of business.
(b) Schedule 3.13(b) sets forth the material terms of each Material Contract which is an oral Contract.
(c) True, correct and complete copies of all Material Contracts as currently in effect have previously been delivered to Purchaser. No Seller is in default under any Material Contract. Each Seller has complied with each provision of the Material Contracts which obligates such Seller to include any particular term or provision in any Contract with such Seller’s subcontractors, suppliers or vendors. To the Knowledge of Seller, no other party to a Material Contract has breached, violated or defaulted under any Material Contract and no circumstance exists that, with notice or lapse of time or both (including the Acquisition), would constitute a default by any party thereto.
3.14. Reserved.
3.15. Reserved.
3.16. Reserved.
14
3.17. Litigation.
(a) Except as set forth on Schedule 3.17(a), there are no Legal Proceedings pending or threatened against such Seller, or to which such Seller is otherwise a party, or otherwise affecting any of the Acquired Assets, Assumed Liabilities or the Business. Schedule 3.17(a) also lists all Legal Proceedings threatened against such Seller or to which such Seller was a party during the past five (5) years.
(b) Except as described on Schedule 3.17(b), there are no outstanding Orders that are applicable to, or otherwise affect, any Seller, the Acquired Assets, Assumed Liabilities or the Business. Schedule 3.17(b) lists any settlement agreements to which such Seller is a party or by which it is bound.
(c) Schedule 3.17(c) describes all claims for indemnification or breach asserted by or against each Seller at any time in the past five (5) years arising out of the acquisition of any business or business segment.
(d) There is no Legal Proceeding pending or threatened, that in any manner challenges or seeks, or reasonably could be expected to prevent, enjoin, alter or delay the Acquisition.
3.18. Compliance with Laws; Permits.
(a) Each Seller is, and at all times in the preceding five (5) years has been, in compliance in all material respects with all Laws. No claims or investigations alleging any material violation by any Seller of any Laws are pending or threatened. Schedule 3.18(a) sets forth any claims or investigations resolved or otherwise concluded within the past five (5) years alleging any violation of any Laws.
(b) Each Seller currently has all Permits which are required for the operation of the Business (all of which: (i) are listed on Schedule 3.18(b); (ii) have been previously delivered to Purchaser; and (iii) are in full force and effect). Each Seller has complied at all times in the preceding five (5) years, and is presently in compliance, in all material respects, with the terms and conditions of all Permits. No loss, non-renewal, suspension, modification or expiration of, nor any noncompliance with, any Permit is pending or threatened.
3.19. Reserved.
3.20. Insurance. All insurance policies pertaining to the Business are listed on Schedule 3.20 and are in full force and effect on the date hereof. Each Seller has delivered to Purchaser true, correct and complete copies of such insurance policies. Excluding insurance policies that have expired and replaced in the ordinary course of business, no insurance policy has been cancelled or not renewed within the last two (2) years and no threat has been made to cancel or not renew any insurance policy of any Seller. Each Seller has delivered to Purchaser: (i) a complete insurance claims history during the three (3) years ending December 31, 2022, and the three-month period ended March 31, 2023 (including claims under former policies); and (ii) a list of all pending insurance claims (including such claims made under any former policies). None of the insurers under any such insurance policies has rejected the defense or coverage of any claim purported to be covered by such insurer or has reserved the right to reject the defense or coverage of any claim purported to be covered by such insurer. No Seller has Liability for retrospective premium adjustments under any insurance policies.
15
3.21. Significant Customers and Suppliers. Schedule 3.21 sets forth a complete and accurate list of (a) the ten (10) largest customers of the Business (measured by aggregate xxxxxxxx) during the fiscal year ended December 31, 2022; and (b) the ten (10) largest suppliers of materials, products or services to the Business (measured by the aggregate amount purchased by Seller) during the fiscal year ended December 31, 2022. No such customer or supplier has canceled, terminated or otherwise materially altered its business relationship with any Seller, or notified any Seller of any intent to do so. There exists no condition or state of facts or circumstances that: (a) adversely impacts such customer or suppliers; or (b) otherwise involves customers or suppliers of or to any Seller that could reasonably be expected to have a Material Adverse Effect. Except as set forth on Schedule 3.21, none of the Material Contracts were on the basis of small business, social disadvantage, woman-owned, or other standing under government socio-economic programs (“Socio-Economic Status”), and no approvals, consents, or notifications are required to be made before or after Closing relating to the transactions contemplated hereby for the continued performance of any Contracts awarded partly or wholly on the basis of such Socio-Economic Status.
3.22. Certain Payments. No Seller nor any of its directors, officers, shareholders, agents, employees or any other Person acting for or on behalf of such Seller, has directly or indirectly: (a) made any contribution, gift, bribe, rebate, payoff, influence payment, kickback, or other payment to any Person, private or public, regardless of form, whether in money, property or services, or promised to do any of the foregoing: (i) to obtain favorable treatment in securing business; (ii) to pay for favorable treatment for business secured; (iii) to obtain special concessions or for special concessions already obtained, for or in respect of Seller; (iv) in violation of any Law; or (v) to any foreign official (as such term is defined in the U.S. Foreign Corrupt Practices Act (the “FCPA”)) for the purpose of influencing any official act or decision of such official or inducing him or her to use his or her influence to affect any act or decision of a Governmental Body or to any foreign political party or official thereof or candidate for foreign political office for the purpose of influencing any official act or decision of such party, official or candidate or inducing such party, official or candidate to use his, her or its influence to affect any act or decision of a foreign Governmental Body, in either case (x) in order to assist any Seller or any of such Seller’s Affiliates to obtain or retain business for, or direct business to such Seller or any of such Seller’s Affiliates, as applicable, and (y) that would result in a violation of the FCPA or any other anti-corruption Laws by such Seller; or (b) established or maintained any fund or asset that has not been recorded in the books and records. No Seller nor any of its directors, officers, shareholders, agents, employees or any other Person acting for or on behalf of Seller has received or retained any funds in violation of any Law. Except for marketing-related promotions given to actual customers valued individually at less than $1,000 in each instance, no Seller has given or agreed to give any money, gift or similar benefit to any actual or potential customer, supplier, government employee, insider or any other Person in a position to assist or hinder a Seller in connection with any actual or proposed transaction.
16
3.23. Affiliate Transactions.
(a) Except as disclosed on Schedule 3.23(a): (i) no Seller Owner nor any Affiliate of a Seller or any Seller Owner is an officer, director, employee, consultant, competitor, creditor, debtor, customer, distributor, supplier or vendor of, or is a party to any Contract or transaction with a Seller; (ii) no current officer or director of a Seller (or any person that has served as an officer or director of a Seller in the past three (3) years), or any Affiliate of the foregoing, is a party to any Contract or transaction with a Seller; and (iii) no Affiliate of a Seller has any right, title or interest in any property or asset used in or necessary for the conduct of the Business.
(b) Schedule 3.23(b) sets forth all Contracts entered into with each Seller and an Affiliate of such Seller.
3.24. Products Liability.
(a) Schedule 3.24 sets forth all pending claims, and all claims threatened in writing against Seller or to which Seller was a party during the past five (5) years, for product liability, warranty, material back charge, material additional work, or other claims by any third party (whether based on contract or tort and whether relating to personal injury, including death, property damage or economic loss) arising from: (i) services rendered by the Business; or (ii) the manufacture, sale, distribution, erection or installation of products by the Business. All services rendered and products sold by the Business have been in conformity with all contractual commitments and all express and implied warranties. No services or products provided by the Business are subject to any guaranty, warranty, or other indemnity beyond Seller’s standard written terms and conditions of sale, true, correct and complete copies of which have been delivered to Purchaser.
(b) No Governmental Body regulating the design, manufacture, production, marketing, distribution, sale or advertising of any of the products currently sold or distributed by, or used in, the Business has requested, within the preceding five (5) years, that any such product be modified, removed from the market, removed from installation of any product or recalled, or that substantial new product testing be undertaken as a condition to the continued manufacturing, production, selling, distribution or use of any such product.
3.25. Regulatory Compliance; Permits.
(a) So far as the Sellers are aware, the Marketing Authorizations are in full force and effect.
17
(b) The Marketing Authorizations specified in Schedule 1.1(h) comprise all of the Cellvera Product-specific material licenses, permissions, authorizations and consents necessary for the commercialization of the Cellvera Products.
(c) All pending variations or amendments to and extensions of the Marketing Authorizations specified in Schedule 1.1(h) that have been initiated by the Sellers have been disclosed in the Data Room and, so far as the Sellers are aware, there are no other pending variations, amendments or extensions thereto.
(d) No Seller nor any contract manufacturer of any Seller has received any written notice, regulatory communication, request for information, demand letter, inspectional observations or other written communication from any Governmental Body (i) contesting the Marketing Authorization of, the uses of or the development, manufacture (including synthesis, formulation, finishing, labeling or packaging), holding, marketing, offer for sale, sale, distribution, export, import and promotion of any Cellvera Product; or (ii) otherwise alleging any violation of any Laws by a Seller or any contract manufacturer of a Seller with respect to any Product. Schedule 3.25(a) sets forth any claims or investigations resolved or otherwise concluded within the past five (5) years alleging any violation of any Laws.
(e) All development, manufacturing and commercialization activities conducted or sponsored by any Seller regarding the Business were and continue to be conducted in compliance in all material respects with all Marketing Authorizations and applicable Laws, including those related to good clinical practice, good laboratory practice, good manufacturing practice, the protection of human study subjects and safety reporting.
(f) No Product is under consideration by any Seller or any of its contract manufacturers, its customers or by any Governmental Body for a recall, withdrawal, suspension, seizure or discontinuation, or has been recalled, withdrawn, suspended, seized or discontinued by any Seller, its contract manufacturers, its customers or any Governmental Body in any part of the world (whether voluntarily or otherwise).
(g) All data, information and representations contained in any submission to, or communications with, any Governmental Body regarding any Cellvera Product were accurate, complete, truthful and non-misleading in all material respects when submitted or communicated to any Governmental Body (or were corrected in or supplemented by a subsequent filing) and remain so currently. No Seller is the subject of any pending or threatened investigation regarding any Product by the U.S. Food and Drug Administration (“FDA”) pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto (“FDA Fraud Policy”), or similar policy enforced by any other Governmental Body. With regard to any Cellvera Product, no Seller has nor has any officer, employee or agent of any Seller made an untrue statement of material fact to any Governmental Body, failed to disclose a material fact required to be disclosed to any Governmental Body, or committed an act, made a statement or failed to make a statement that, at the time such disclosure was made, would reasonably be expected to provide a basis for the FDA or any other Governmental Body to invoke the FDA Fraud Policy or any similar policy.
18
(h) Each Seller has obtained and has in force all material regulatory Permits, including Marketing Authorizations, necessary to operate the Business and own and operate its assets in the places and in the manner in which the Business is now operating. Schedule 3.18(b) contains a complete and accurate list of all such Permits. Each Seller is, and has been, in compliance in all material respects with all such Permits. No Seller has received any notice that it is in default (or with the giving of notice or lapse of time or both, would be in default) under any such Permit. Prior to the Closing Date, each Seller provided to Purchaser true and complete copies of all such Permits.
3.26. Solvency. Upon payment of the Purchase Price, no Seller will be insolvent as that word is defined under applicable law, and no Seller will have any unsatisfied liabilities. Upon the Acquisition of the Acquired Assets, the assets of each Seller will exceed its liabilities. As used in this section, “insolvent” means that the sum of the debts and other probable Liabilities of a Seller exceeds the present fair saleable value of Seller’s assets. Immediately after giving effect to the Acquisition: (a) Seller will be able to pay its Liabilities (including the Retained Liabilities) as they become due in the usual course of business; (b) Seller will not have unreasonably small capital with which to conduct its proposed business; (c) Seller will have assets (calculated at fair market value) that exceed its Liabilities; and (d) taking into account all pending and threatened litigation, no final judgments against Seller in actions for money damages are reasonably anticipated to be rendered at a time when, or in amounts such that, Seller will be unable to satisfy any such judgments promptly in accordance with their terms and all other obligations of Seller. None of the transactions contemplated by this Agreement have the effect of placing any of the holders of Closing Date Debt into a position which, in the event of any of the Sellers, including BVI Holdco, going into insolvent liquidation, will be better than the position a holder of the Closing Date Debt would have been in if the transactions had not been entered into.
3.27. International Trade.
(a) Except as set forth in Schedule 3.27(a), at all times during the five years prior to Closing, (a) each Seller has fully complied with all Ex-Im Laws and conducted no business that would have been illegal for a Seller if it was subject to U.S. jurisdiction, and (b) no Seller, director, member, manager, officer, agent, employee, representative, consultant or other Person acting for or on behalf of any Seller has, with respect to the Acquired Assets, violated any Ex-Im Law, and (c) no Seller has received any written notice alleging any violation of any Ex-Im Law.
(b) Without limiting the generality of the foregoing, except as set forth on Schedule 3.5(b), in the five years preceding Closing, no Seller has done any business or entered into any transaction with any individual, organization, government, association, or other entity who is subject to any U.S. sanctions or embargo Laws.
19
(c) Except as set forth on Schedule 3.27(c), neither any Seller or its personnel have been investigated, charged civilly or criminally, convicted of any crime or civil offense, subpoenaed, assessed any penalties, called as a witness, participated in or been the subject of any voluntary self-disclosure relating to, or otherwise been involved in any enforcement matter pertaining to Ex-Im Laws (collectively “International Trade Enforcement Matters”) during the five years prior to Closing, and there is no reason to think Purchaser or any Seller will become involved in such International Trade Enforcement Matters before or after Closing.
(d) Schedule 3.27(d) hereto includes a complete list of all policies, procedures, manuals, handbooks, or other programs or materials developed or used by each Seller Entity to aid with international trade compliance matters (e.g. export control compliance, Customs compliance, sanctions compliance, FCPA compliance, and other Ex-Im Laws, etc.) and Each Seller has complied fully with such requirements during the five years prior to Closing.
(e) Except as set forth in Schedule 3.27(e), no Seller has served during the five years prior to Closing as the importer of record or agent for the importation of any products into the Customs territory of the United States or any other country. Schedule 3.27(e) includes (a) a complete list of all such imported products, and (b) a complete list of all of the products, services, and technologies of each Seller that are part of the Acquired Assets together with their country of origin, tariff classification, tariff rate, export control status, including but not limited to any tariffs applicable to products from specific countries (e.g. tariffs imposed by the U.S. on certain Chinese goods).
(f) Except to the extent set forth on Schedule 3.27(f), none of each Seller’s products, technologies, materials, ingredients, equipment, or services are subject to any restrictions relating to importation into or exportation from the United States either physically or in the form of data or information or services provided to a non-U.S. organization or individual, nor would such products, technologies, materials, ingredients, equipment, or services be subject to any such restrictions if they were made in the United States, present in the United states, or were derived from U.S. technology or technical data. Schedule 3.27(f) includes a complete list of all products, technologies, materials, equipment or services of each Seller that are subject to restrictions on exportation from the United States, listed by product or service name, description, part or family number (if applicable), and export control jurisdiction and classification status.
(g) Except as set forth on Schedule 3.27(g), no Seller has applied for or received or been named in any license, authorization, commodity jurisdiction ruling under the International Traffic in Arms Regulation, classification decision under the Export Administration Regulation, advisory opinion, or other request for guidance or confirmation under any United States Government export control, sanctions, FCPA, or other international trade-related laws (including but not limited to regulations and executive orders) during the five years prior to Closing.
20
(h) Except as set forth on Schedule 3.27(h), each Seller has fully complied during the six years prior to Closing with any and all product origin requirements, Laws and contract obligations.
(i) Except as set forth on Schedule 3.27(i), No Seller has any marketing, lobbying, sales agent, or other agreements relating to the marketing, distribution, or sale of its goods or services in the United States (“US Sales Agreements”), and all such US Sales Agreements comply fully with applicable law and are on arms-length and market terms.
3.28. Debt and Seller Expenses. Schedule 3.28 sets forth a true, correct, and complete list of the individual components (indicating the amount and the Person to whom such amount is owed) of all (a) the Closing Date Debt and (b) Seller Expenses.
3.29. Disclosure. Neither this Agreement, or any of the Schedules or Exhibits hereto, contains any untrue statement of a material fact or omits a material fact necessary to make the statements contained herein or therein, in light of the circumstances in which they were made, not misleading.
3.30. No Limitation. No investigation or due diligence conducted by, or knowledge obtained by, Purchaser shall limit, modify or negate any of the foregoing representations and warranties.
3.30 No Financial Advisors. No broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the transactions contemplated hereby.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES WITH RESPECT TO GRA
Each Seller and Seller Owner represent and warrant to Purchaser as of the date hereof and as of the Closing Date as follows:
4.1. Organization, Authority and Qualification. GRA is duly formed, validly existing and in good standing under the laws of Jebel Ali Free Zone Authority, UAE, with license number 224370 and whose registered office is at Workstation No. FZJOA 12 WS37, Jebel Ali Free Zone, Dubai, UAE and is qualified to do business in every jurisdiction in which its ownership of property or conduct of business requires it to qualify. Schedule 4.1 sets forth each jurisdiction in which GRA is so qualified or licensed and in good standing. GRA possesses all requisite corporate or limited liability company power and authority, as applicable, necessary to own, lease and operate its properties, to carry on its businesses as now conducted. The copies of GRA’s respective Organizational Documents, which have been made available to Purchaser, reflect all amendments made thereto at any time prior to the date of this Agreement and are correct and complete in all material respects. GRA is not in default under or in violation of any provision of its Organizational Documents. Schedule 4.1 sets forth (i) a list of the officers and directors of GRA as of immediately prior to the Closing and (ii) a list of each jurisdiction in which GRA has conducted any business under or otherwise used, for any purpose, in any jurisdiction, any fictitious names, assumed names, trade names or other name.
21
4.2. Capitalization. Schedule 4.2 sets forth the authorized Shares and issued and outstanding Shares of GRA, which constitutes the only Shares of GRA. All of the Shares of GRA are held beneficially and of record as set forth on Schedule 4.2, free and clear of all Liens. No Shares of GRA were issued in violation of any preemptive rights. Except as set forth on Schedule 4.2, GRA does not have any outstanding securities (including options, warrants or similar rights) convertible or exchangeable for any of its Shares or containing any profit participation features, nor any rights or options to subscribe for or to purchase its Shares or any stock or securities convertible into or exchangeable for its Shares or any stock appreciation rights or phantom stock plan. No Shares of GRA are subjected to, or issued in violation of, any purchase option, call option, right of first refusal, subscription right or any similar right, and no such rights arise or become exercisable by virtue of or in connection with the transactions contemplated by this Agreement. GRA is not subject to any option or obligation (contingent or otherwise) to repurchase or otherwise acquire or retire any of its Shares. GRA has not violated any foreign, federal or state securities Laws in connection with the offer, sale or issuance of its Shares. GRA has not registered any of its Shares with any stock exchange. All of the outstanding Shares of GRA have been duly authorized and validly issued and are fully paid. There are no voting agreements, proxies or other similar agreements or arrangements between GRA’s shareholders with respect to the voting or transfer of any GRA Share. The share certificate books, register of shareholders, register of transfers, register of directors and similar corporate records of GRA are complete, accurate and current. Except as set forth on Schedule 4.2, there are no bonds, debentures, notes or other Debt of GRA outstanding having the right to vote (or convertible into, or exchangeable for, securities having the right to vote) on any matters on which the shareholders of GRA may vote.
4.3. No Conflicts; Consents.
(a) No act (corporate or otherwise) or other proceeding on the part of GRA, or any other Person is necessary to authorize the execution, delivery or performance of the Seller Documents and the consummation of the transactions contemplated hereby or thereby.
(b) Except as set forth on Schedule 4.3(b), none of the execution, delivery or performance by any Seller of the Seller Documents, the consummation of the transactions contemplated thereby, or compliance by each Seller with any of the provisions thereof will: (i) violate the Organizational Documents of GRA, (ii) conflict with or result in a breach or termination of any of the terms, conditions or provisions of, or constitute a default under, accelerate any obligations arising under, trigger any payment under, require any Consent under or any notice under, result in the creation of any Lien pursuant to, or otherwise adversely affect, any Contract to which GRA is a party or by which its assets may be bound, (iii) result in the creation of any Lien upon the Shares or any assets of GRA, (iv) violate any Law applicable to or binding upon GRA, or (v) require any authorization, consent or approval of, or notice or declaration to, or filing with, any third party or any Governmental Body.
(c) Except as set forth on Schedule 4.3(c), no waiver, Order, Permit or Consent of any Governmental Body is required on the part of any Seller in connection with the execution and delivery of the Seller Documents or the compliance by any Seller with any of the provisions thereof, or the consummation of the transactions contemplated thereby.
4.4. Subsidiaries. GRA does not own, nor has it ever owned, any equity or debt securities or other ownership interest, directly or indirectly, in any other Person, nor is it party to any Contract to acquire any such securities or other ownership interest.
4.5. Legal Proceedings. There is no claim, action, suit, proceeding, or governmental investigation of any nature pending or threatened: (a) against or by GRA; or (b) against or by any Seller, Seller Owner or GRA that challenges or seeks to prevent, enjoin, or otherwise delay the transaction contemplated by this Agreement. There is no Legal Proceeding against any current or former shareholder, manager, or employee of GRA with respect to which GRA has, or is reasonably likely to have, an indemnification obligation. No event has occurred, or circumstances exist that may give rise to, or serve as a basis for, any such Legal Proceeding.
4.6. Financial Statements; Books and Records.
(a) Except for the GRA Latest Balance Sheet (as defined below), attached hereto as Schedule 4.6 are true, correct and complete copies of the (i) audited balance sheet of GRA as of December 31, 2021 and the related audited statement of income, shareholders’ equity for the fiscal year then ended, (ii) audited balance sheet of GRA as of December 31, 2022 and the related audited statement of income, shareholders’ equity for the fiscal year then ended and (iii) the unaudited balance sheet of GRA as of February 28, 2023 (the “GRA Latest Balance Sheet”) and the related unaudited statements of income for the two (2) month period then ended (the “GRA Interim Financial Statements”, together with the items listed in clauses (i) and (ii) in this Section 4.6, the “GRA Financial Statements”). The GRA Financial Statements: (a) have been prepared in accordance with GAAP, applied on a consistent basis throughout the period involved; (b) were prepared from and are consistent in all respects with the books and records of GRA; and (c) present fairly in all respects the financial position of GRA as of the date thereof and the results of its operations for the period then ended.
(b) The books of account and other business records of GRA, all of which have been previously delivered to Purchaser and its representatives, are accurate and complete in all material respects. The books and records of GRA accurately reflect the assets, liabilities, financial condition and results of operations of GRA and have been maintained in accordance with good business and bookkeeping practices. The minute books and other similar records of GRA contain accurate and complete records, in all material respects, of actions taken at any meeting of the board of managers, equityholders or other governing body of GRA or any committee thereof and of written consents executed in lieu of the holding of any such meeting.
22
(c) GRA has established and adheres to a system of internal accounting controls appropriate for its size and the industry in which they operate which are designed to provide assurance regarding the reliability of financial reporting. There has not been (i) any significant deficiency or weakness in the system of internal accounting controls used by GRA, (ii) any fraud or other wrongdoing that involves any management or other employees of GRA who have a role in the preparation of financial statements or the internal accounting controls used by GRA or (iii) any claim or allegation regarding any of the foregoing.
4.7. Undisclosed Liabilities. GRA does not or will not have any obligation or liability (whether accrued, absolute, contingent, unliquidated or otherwise, whether known or unknown, whether due or to become due, regardless of when or by whom asserted and whether or not the magnitude of such obligation or liability can be reasonably estimated as of the date hereof) arising out of any transaction entered at or prior to the date hereof, or any action or inaction at or prior to the date hereof, or any state of facts existing at or prior to the date hereof, other than (a) liabilities to the extent adequately reflected on or reserved against the GRA Latest Balance Sheet and (b) liabilities and obligations which have arisen after the date of the GRA Latest Balance Sheet (the “GRA Latest Balance Sheet Date”) in the ordinary course of business (none of which is a liability for breach of contract, breach of warranty, tort, infringement, violation of Law, claim or lawsuit) which, individually or in the aggregate, are not material in amount.
4.8. Absence of Certain Developments. Since the GRA Latest Balance Sheet Date: (i) GRA has conducted its business and operations only in the ordinary course of business; and (ii) there has not been any event, change, occurrence or circumstance that has had, or is reasonably likely to have, individually or in the aggregate, a Material Adverse Effect. Except as contemplated by this Agreement or as set forth on Schedule 4.8, since the GRA Latest Balance Sheet Date, no state of facts exist that would be required to be disclosed on Schedule 3.8 if GRA was any Seller.
4.9. Real Property; Assets and Inventory.
(a) GRA does not own any real property or lease or sublease any real property.
(b) Schedule 4.9 contains a complete list of all tangible assets (the “GRA Tangible Assets”) owned or leased by GRA along with each assets’ physical location. Such tangible assets are (i) in the possession of GRA; (ii) in good operating condition and repair (ordinary wear and tear excepted) and are suitable for the use to which they are put; and (iii) with respect to any tangible assets leased by GRA, are in such condition as to permit the surrender thereof on the date hereof without any cost or expense for repair or restoration if the related leases were terminated on the date hereof in the ordinary course of business.
(c) GRA does not own inventory.
(d) GRA owns all right, title and interest in and to all of its properties and assets reflected in the GRA Latest Balance Sheet or acquired since the GRA Latest Balance Sheet Date, free and clear of any and all Liens.
23
(e) The GRA Tangible Assets constitute all of the properties and assets necessary to conduct GRA’s business.
4.10. Contracts and Agreements.
(a) The applicable subpart of Schedule 4.10(a) sets forth all of the Contracts to which GRA is a party or by which it or any of its assets is bound, including (the “GRA Material Contracts”) any contract required to be disclosed on Schedule 3.13(a) if GRA was any Seller or Seller Owner.
(b) True, correct and complete copies of all GRA Material Contracts as currently in effect have previously been delivered to Purchaser. GRA is not in default under any GRA Material Contract. GRA has complied with each provision of the GRA Material Contracts which obligates GRA to include any particular term or provision in any Contract with such GRA’s subcontractors, suppliers or vendors. No other party to a GRA Material Contract has breached, violated or defaulted under any GRA Material Contract and no circumstance exists that, with notice or lapse of time or both (including the Acquisition), would constitute a default by any party thereto.
4.11. Intellectual Property.
(a) Set forth on the applicable subpart of Schedule 4.11(a) is a list of all: (i) patents and applications therefor; (ii) trademarks, service marks, service names or trade names, or registrations or applications for registration thereof, (iii) material unregistered trademarks, service marks, service names or trade names, (iv) copyright registrations, applications for copyright registration and unregistered copyrights material to the operation of the Business; and (v) domain names, in each case owned or held by GRA (the “GRA Listed Intellectual Property”). Each item of GRA Listed Intellectual Property is in full force and effect. All necessary registration, maintenance and renewal fees in connection with such GRA Listed Intellectual Property have been paid and all necessary documents and certificates in connection with such GRA Listed Intellectual Property have been filed with the relevant Governmental Body for the purpose of perfecting or maintaining such GRA Listed Intellectual Property, including any filings or payments due within one hundred twenty (120) days of the Closing. None of the GRA Listed Intellectual Property is the subject of any challenge pending with any patent or trademark office, and neither GRA nor any Seller has received notice of any such challenge in writing.
(b) Except as set forth on Schedule 4.11(b), the current use of any GRA Intellectual Property does not conflict with, infringe upon or violate any rights of any third party. Neither GRA nor any Seller has received in the past five (5) years any notice, charge, claim or other assertion of any present, impending or threatened infringement by, or misappropriation of, or conflict with any Intellectual Property of any other Person. No third party is infringing any Intellectual Property of GRA.
24
(c) GRA owns or has the right to use all Intellectual Property used by GRA. Except as set forth on Schedule 4.11(c), neither GRA nor any Seller is required to pay any royalties, honoraria, fees or other payments to any Person by reason of the use of any Intellectual Property.
(d) All personnel, including employees, agents, consultants and contractors, who have contributed to or participated in the conception and development of any Intellectual Property on behalf of GRA either: (i) have been party to a “work-for-hire” arrangement or agreement with GRA that has accorded GRA full, effective, exclusive and original ownership of all Intellectual Property and tangible and intangible embodiments arising therefrom; or (ii) have executed appropriate instruments of assignment in favor of GRA as assignee that have conveyed to GRA full, effective and exclusive ownership of all Intellectual Property and tangible embodiments arising therefrom.
4.12. Compliance with Laws; Permits.
(a) GRA is and has been in compliance in all material respects with all applicable Laws of, or issued or enforced by, any Governmental Body relating to the operation of its business and the maintenance and operation of its properties and assets. Since its incorporation, (i) no written notices have been sent by an individual, entity, Governmental Body, regulator or other regulatory body alleging a material violation of any such Laws and (ii) GRA has not been subject to any adverse inspection, finding, investigation, penalty assessment, audit or other compliance or enforcement action by any Governmental Body which is material and remains unresolved.
(b) GRA holds and is in compliance in all material respects with all material permits, licenses, bonds, certificates, registrations, accreditations and other authorizations of all Governmental Bodies required for the conduct of its business and the ownership and use of its properties and the attached Schedule 4.12(b) sets forth a list of all such permits, licenses, bonds, approvals, certificates, registrations, accreditations and other authorizations as currently conducted. Since its incorporation, there have been no written notices nor oral notices alleging the failure to hold any of the foregoing. No event has occurred that, with or without notice or lapse of time or both, would be expected to result in the revocation, suspension, lapse or limitation of any material permits, licenses, bonds, accreditations, certificates, registrations or authorizations from a Governmental Body. All of such permits, licenses, bonds, approvals, accreditations, certificates, registrations and authorizations will be available for use by GRA immediately after the Closing.
(c) Neither GRA, nor any of their respective equityholders, directors, managers, officers, employees, agents, or Affiliates, or customers is: (i) a Person that is listed in the Annex to, or is otherwise subject to, the provisions of the U.S. Executive Order No. 13224 on Terrorist Financing, effective September 24, 2001 and relating to Blocking Property and Prohibiting Transactions With Persons Who Commit, Threaten to Commit, or Support Terrorism (the “Executive Order”) and the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (the “USA Patriot Act”); (ii) a Person owned or controlled by, or acting for or on behalf of, any person or entity that is listed in the Annex to, or is otherwise subject to the provisions of, the Executive Order and the USA Patriot Act; (iii) a Person who commits, threatens or conspires to commit or support “terrorism” as defined in Section 3(d) of the Executive Order; (iv) a Person that is named as a “specially designated national and blocked person” on the current list published by the U.S. Treasury Department Office of Foreign Assets Control at its official website, or at any replacement website or other replacement official publication of such list; and (v) a Person who is affiliated with a person or entity listed in items (i) through (iv) above.
25
4.13. Healthcare Matters.
(a) GRA has complied, and is in compliance in all material respects, with all applicable Healthcare Laws of, or issued or enforced by, any Governmental Body relating to the operation of its business and the maintenance and operation of its properties and assets. Since its incorporation, no written or oral notices have been received from an individual, entity, Governmental Body, regulator or other regulatory body alleging a violation of any such Healthcare Laws and GRA has not been subject to any adverse inspection, survey, finding, investigation, penalty assessment, audit or other compliance or enforcement action.
(b) GRA and each other Person involved in the marketing, sale, provision, performance, reporting or delivery of any of GRA’s healthcare products or services, has obtained all permits, licenses, certifications, approvals, clearances, certifications, registrations, accreditations and other authorizations from, and has made all appropriate applications and other submissions to, and has prepared and maintained all records, reports, procedures, studies, testing data and other documentation needed to satisfy and demonstrate compliance with all applicable requirements of all applicable Governmental Bodies necessary for operation of their past and present business activities relating to an GRA’s healthcare products or services in compliance with all applicable Healthcare Laws. All required permits, licenses, certifications, approvals, clearances, certifications, licenses, registrations, accreditations, and other authorizations are in full force and effect and GRA has not, since its incorporation, received notice of any violation of any of them.
(c) Neither GRA nor any individual with an “ownership or control interest” (as defined at 42 C.F.R. § 1001.2) GRA, including its shareholders or members with an ownership interest of five percent (5%) or more in GRA, or holders of any mortgage, deed of trust, note or other obligation secured by GRA with an interest equal to or exceeding five percent (5%) of the total property and assets of GRA, nor any of GRA’s officers, directors, managers, employees or agents: (i) has been charged with, convicted of, or pled nolo contendere to any criminal offense relating to the delivery of any item or service under any Healthcare Law, including under 42 U.S.C. Sections 1320a-7(a), 1320a-7(b)(1), (2), or (3); (ii) has had civil money penalties or assessments imposed under 42 U.S.C. Section 1320a-7a; (iii) has been excluded, suspended or debarred from participation, or otherwise determined to be or identified as ineligible to participate, in Medicare or any other governmental healthcare program; (iv) is currently listed on the Office of Inspector General’s List of Excluded Individuals and Entities or the General Services Administration published list of parties excluded from federal procurement programs and non-procurement programs; or (v) is the target or subject of any current or potential proceeding relating to any Healthcare Law related offense.
26
(d) Except to the extent not prohibited by Law, GRA, nor any of its directors, officers, members, managers, agents, employees or any other Person acting for or on behalf of GRA, has directly or indirectly: (i) made or received any contribution, gift, bribe, rebate, payoff, influence payment, kickback, or payment of any other consideration to any Person, private or public, regardless of form, whether in money, property or services: (A) to obtain favorable treatment in securing business; (B) to pay for favorable treatment for business secured; (C) to obtain special concessions or for special concessions already obtained, for or in respect of GRA; (D) to obtain, or in return for obtaining, a referral of business; or (E) in violation of any Law; or (ii) established or maintained any fund or asset that has not been recorded in the books and records of GRA.
4.14. Environmental and Safety Matters.
(a) (i) There is no written notice that GRA is not in material compliance with, or has any liabilities relating to GRA, or its facilities arising under, any Environmental and Safety Requirements, and (ii) there is no written or oral notice of potential material liability or formal request for information pursuant to any Environmental and Safety Requirements from a Governmental Body.
(b) There has been no Release by GRA, threatened Release by GRA or other activities engaged in by GRA (with respect to the business or ownership of the property of GRA) that would reasonably result in GRA incurring Liability under any Environmental and Safety Requirements for conditions on, at or under the property of GRA or any other real property, whether now owned or leased or owned or leased in the past.
(c) GRA has not treated, stored, disposed of, arranged for the disposal of, transported, handled, released, or exposed any Person to, any Hazardous Material, so as to give rise to liabilities pursuant to any Environmental and Safety Requirements.
(d) GRA has not entered into any Contract for the primary purpose of retaining, assuming, undertaking, or providing an indemnity with respect to any material liability of any other Person relating to any matter arising under Environmental and Safety Requirements.
(e) GRA has made available to Purchaser true and complete copies of all environmental audits, reports, and studies conducted by or on behalf of GRA that are in its possession or obtainable.
4.15. Employee Benefit Plans.
(a) GRA has not, nor does it propose to introduce, any share incentive scheme, share option scheme or profit-sharing bonus or other incentive scheme for any employee.
27
(b) Other than as required by applicable Law, GRA has not set up nor participates in any pension fund nor has it incurred or will incur any liability for pensions, allowances, lump sum gratuities or like benefits in favour of its employees, and there are no obligations whatsoever to make any such payments or contributions.
(c) GRA is and has been fully compliant with all applicable Law regarding pensions regarding its employees and has paid all required pension contributions and related amounts as required by applicable Law.
4.16. Labor.
(a) All employees of GRA are lawfully engaged by XXX and all necessary steps have been taken by GRA to ensure that all such employees are legally permitted to carry out the duties for which they are engaged for. GRA has complied with the requirements of all relevant immigration legislation.
(b) No present or former employee of GRA or any applicant for any role in GRA has any: (i) accrued rights to end of service gratuity which has not been provided for in full in the GRA Financial Statements; (ii) outstanding claim against GRA; or right to be indemnified by GRA.
(c) There are no facts that might suggest that there may be grounds for any dispute with any present or former employee of GRA (in relation to end of service benefits or otherwise).
(d) GRA has paid to the appropriate Governmental Body and each other human resources and social development, labour and social insurance authority all taxes and other levies and charges in respect of the GRA employees and accruing in the period up to and including the Closing Date.
(e) No employee of GRA is represented by a labor union and there are no Contracts with any labor union or association representing any employees of GRA. No petition has been filed or other proceedings instituted by an employee or group of employees with any labor relations board seeking recognition of a bargaining representative and there is no organizational effort currently being made or threatened by, or on behalf of, any labor union to organize any employees, and no demand for recognition of employees has been made by, or on behalf of, any labor union.
(f) Neither GRA nor any of its officers, directors or employees is or has been, since GRA’s incorporation, the subject of any charge, grievance or complaint of any unfair labor practice, grievance, arbitration, negotiation, suit, or action by any employee or employee representative, and no complaint or charge is pending against GRA before any Governmental Body.
28
(g) GRA has previously delivered to Purchaser true, correct and complete summaries of all: (i) workers’ compensation claims filed against GRA; and (ii) charges, grievances, complaints or notices of violation filed with, or otherwise made by, the any Governmental Body against such Seller
4.17. Insurance. All insurance policies pertaining to the Business are listed on Schedule 4.17 and are in full force and effect on the date hereof. GRA has delivered to Purchaser true, correct and complete copies of such insurance policies. Excluding insurance policies that have expired and replaced in the ordinary course of business, no insurance policy has been cancelled or not renewed since GRA’s incorporation and no threat has been made to cancel or not renew any insurance policy of GRA. GRA has delivered to Purchaser: (i) a complete insurance claims history since GRA’s incorporation, and the three-month period ended March 31, 2023 (including claims under former policies); and (ii) a list of all pending insurance claims (including such claims made under any former policies). None of the insurers under any such insurance policies has rejected the defense or coverage of any claim purported to be covered by such insurer or has reserved the right to reject the defense or coverage of any claim purported to be covered by such insurer. GRA does not have Liability for retrospective premium adjustments under any insurance policies.
4.18. Significant Customers and Suppliers. Schedule 4.18 sets forth a complete and accurate list of (a) the ten (10) largest customers of GRA (measured by aggregate xxxxxxxx) during the fiscal year ended December 31, 2022; (b) the ten (10) largest suppliers of materials, products or services to GRA (measured by the aggregate amount purchased by GRA) during the fiscal year ended December 31, 2022; and (c) the ten (10) largest distributors of materials, products or services of GRA (measured by the by the dollar value transactions between such distributor and GRA) during the fiscal year ended December 31, 2022. No such customer or supplier has canceled, terminated or otherwise materially altered its business relationship with GRA, or notified GRA of any intent to do so. There exists no condition or state of facts or circumstances that: (a) adversely impacts such customer or suppliers; or (b) otherwise involves customers or suppliers of or to any Seller that could reasonably be expected to have a Material Adverse Effect. Except as set forth on Schedule 4.18, none of the GRA Material Contracts were on the basis of Socio-Economic Status, and no approvals, consents, or notifications are required to be made before or after Closing relating to the transactions contemplated hereby for the continued performance of any Contracts awarded partly or wholly on the basis of such Socio-Economic Status.
4.19. Banks; Powers of Attorney, Books and Records. Schedule 4.19 lists as of the date hereof (i) the names and locations of all banks, trust companies and other financial institutions in which GRA maintains accounts of any nature or safe deposit boxes, (ii) the names of all persons authorized to draw thereon or to have access thereto, and (iii) the account number for each bank account of GRA or other identifying descriptions of such accounts or safe deposit boxes. Except as set forth on Schedule 4.19, no person holds a power of attorney to act on behalf of GRA.
29
4.20. Affiliate Transactions.
(a) Except as disclosed on Schedule 4.20(a): (i) no Seller Owner nor any Affiliate of a Seller or any Seller is an officer, director, employee, consultant, competitor, creditor, debtor, customer, distributor, supplier or vendor of, or is a party to any Contract or transaction with GRA; (ii) no current officer or director of GRA (or any person that has served as an officer or director of GRA), or any Affiliate of the foregoing, is a party to any Contract or transaction with GRA; and (iii) no Affiliate of GRA has any right, title or interest in any property or asset used in or necessary for the conduct of GRA’s business.
(b) Schedule 4.20(b) sets forth all Contracts entered into with GRA and an Affiliate of GRA.
4.21. Products Liability.
(a) Schedule 4.21, sets forth all pending claims, and all claims threatened in writing against Seller or to which Seller was a party, for product liability, warranty, material back charge, material additional work, or other claims by any third party (whether based on contract or tort and whether relating to personal injury, including death, property damage or economic loss) arising from: (i) services rendered by GRA; or (ii) the manufacture, sale, distribution, erection or installation of products by GRA. All services rendered and products sold by GRA have been in conformity with all contractual commitments and all express and implied warranties. No services or products provided by GRA are subject to any guaranty, warranty, or other indemnity beyond GRA’s standard written terms and conditions of sale, true, correct and complete copies of which have been delivered to Purchaser.
(b) No Governmental Body regulating the design, manufacture, production, marketing, distribution, sale or advertising of any of the products currently sold or distributed by (or on behalf of), or used by, GRA has requested that any such product be modified, removed from the market, removed from installation of any product or recalled, or that substantial new product testing be undertaken as a condition to the continued manufacturing, production, selling, distribution or use of any such product.
4.22. Regulatory Compliance; Permits.
(a) The Marketing Authorizations are in full force and effect.
(b) The Marketing Authorizations specified in Schedule 1.1(h) comprise all of the GRA product-specific material licenses, permissions, authorizations and consents necessary for the commercialization of the GRA products.
(c) All pending variations or amendments to and extensions of the Marketing Authorizations specified in Schedule 1.1(h) that have been initiated by GRA have been disclosed in the Data Room and there are no other pending variations, amendments or extensions thereto.
30
(d) Neither GRA nor any contract manufacturer of GRA has received any written notice, regulatory communication, request for information, demand letter, inspectional observations or other written communication from any Governmental Body (i) contesting the Marketing Authorization of, the uses of or the development, manufacture (including synthesis, formulation, finishing, labeling or packaging), holding, marketing, offer for sale, sale, distribution, export, import and promotion of any GRA product; or (ii) otherwise alleging any violation of any Laws by GRA or any contract manufacturer of GRA with respect to any GRA product. There are no claims or investigations resolved or otherwise concluded alleging any violation of any Laws.
(e) All development, manufacturing and commercialization activities conducted or sponsored by GRA were and continue to be conducted in compliance in all material respects with all Marketing Authorizations and applicable Laws, including those related to good clinical practice, good laboratory practice, good manufacturing practice, the protection of human study subjects and safety reporting.
(f) No Product is under consideration by GRA or any of its contract manufacturers, its customers or by any Governmental Body for a recall, withdrawal, suspension, seizure or discontinuation, or has been recalled, withdrawn, suspended, seized or discontinued by GRA, its contract manufacturers, its customers or any Governmental Body in any part of the world (whether voluntarily or otherwise).
(g) All data, information and representations contained in any submission to, or communications with, any Governmental Body regarding any GRA product were accurate, complete, truthful and non-misleading in all material respects when submitted or communicated to any Governmental Body (or were corrected in or supplemented by a subsequent filing) and remain so currently. GRA is not the subject of any pending or threatened investigation regarding any Product by the FDA pursuant to FDA Fraud Policy, or similar policy enforced by any other Governmental Body. With regard to any GRA Product, GRA has not nor has any officer, employee or agent of GRA made an untrue statement of material fact to any Governmental Body, failed to disclose a material fact required to be disclosed to any Governmental Body, or committed an act, made a statement or failed to make a statement that, at the time such disclosure was made, would reasonably be expected to provide a basis for the FDA or any other Governmental Body to invoke the FDA Fraud Policy or any similar policy.
(h) GRA has obtained and has in force all material regulatory Permits, including Marketing Authorizations, necessary to operate GRA’s business and own and operate its assets in the places and in the manner in which GRA is now operating. Schedule 4.22(h) contains a complete and accurate list of all such Permits. GRA is, and has been, in compliance in all material respects with all such Permits. GRA has not received any notice that it is in default (or with the giving of notice or lapse of time or both, would be in default) under any such Permit. Prior to the Closing Date, GRA provided to Purchaser true and complete copies of all such Permits.
31
4.23. Solvency. GRA is not now insolvent and will not be rendered insolvent by the Acquisition. As used in this section, “insolvent” means that the sum of the debts and other probable Liabilities of GRA exceeds the present fair saleable value of GRA’s assets. Immediately after giving effect to the Acquisition: (a) GRA will be able to pay its Liabilities (including the Retained Liabilities) as they become due in the usual course of business; (b) GRA will not have unreasonably small capital with which to conduct its proposed business; (c) GRA will have assets (calculated at fair market value) that exceed its Liabilities; and (d) taking into account all pending and threatened litigation, no final judgments against GRA in actions for money damages are reasonably anticipated to be rendered at a time when, or in amounts such that, GRA will be unable to satisfy any such judgments promptly in accordance with their terms and all other obligations of GRA.
4.24. International Trade.
(a) Except as set forth in Schedule 4.24(a), (a) GRA has fully complied with all Ex-Im Laws and conducted no business that would have been illegal for GRA if it was subject to U.S. jurisdiction, and (b) neither GRA nor any director, member, manager, officer, agent, employee, representative, consultant or other Person acting for or on behalf of GRA has violated any Ex-Im Law, and (c) there is no written notice alleging any violation of any Ex-Im Law.
(b) Without limiting the generality of the foregoing, except as set forth on Schedule 4.24(b), GRA has not done any business or entered into any transaction with any individual, organization, government, association, or other entity who is subject to any U.S. sanctions or embargo Laws.
(c) Neither GRA nor its personnel have been investigated, charged civilly or criminally, convicted of any crime or civil offense, subpoenaed, assessed any penalties, called as a witness, participated in or been the subject of any voluntary self-disclosure relating to, or otherwise been involved in any International Trade Enforcement Matters at any time prior to Closing, and there is no reason to think Purchaser or GRA will become involved in such International Trade Enforcement Matters before or after Closing.
(d) Schedule 4.24(d) hereto includes a complete list of all policies, procedures, manuals, handbooks, or other programs or materials developed or used by GRA to aid with international trade compliance matters (e.g. export control compliance, Customs compliance, sanctions compliance, FCPA compliance, and other Ex-Im Laws, etc.) and GRA has at all times fully complied fully with such requirements.
(e) GRA has not served during the five years prior to Closing as the importer of record or agent for the importation of any products into the Customs territory of the United States or any other country. Schedule 4.24(e) includes (a) a complete list of all such imported products, and (b) a complete list of all of the products, services, and technologies of GRA together with their country of origin, tariff classification, tariff rate, export control status, including but not limited to any tariffs applicable to products from specific countries (e.g. tariffs imposed by the U.S. on certain Chinese goods).
32
(f) Except to the extent set forth on Schedule 4.24 (f), none of GRA’s products, technologies, materials, ingredients, equipment, or services are subject to any restrictions relating to importation into or exportation from the United States either physically or in the form of data or information or services provided to a non-U.S. organization or individual, nor would such products, technologies, materials, ingredients, equipment, or services be subject to any such restrictions if they were made in the United States, present in the United states, or were derived from U.S. technology or technical data. Schedule 4.24(f) includes a complete list of all products, technologies, materials, equipment or services of GRA that are subject to restrictions on exportation from the United States, listed by product or service name, description, part or family number (if applicable), and export control jurisdiction and classification status.
(g) Except as set forth on Schedule 4.24(g), GRA has not applied for or received or been named in any license, authorization, commodity jurisdiction ruling under the International Traffic in Arms Regulation, classification decision under the Export Administration Regulation, advisory opinion, or other request for guidance or confirmation under any United States Government export control, sanctions, FCPA, or other international trade-related laws (including but not limited to regulations and executive orders) during the five years prior to Closing.
(h) Except as set forth on Schedule 4.24(h), GRA has at all times fully complied during the six years prior to Closing with any and all product origin requirements, Laws and contract obligations.
(i) Except as set forth on Schedule 4.24(i), GRA does not have any US Sales Agreements, and all such US Sales Agreements comply fully with applicable law and are on arms-length and market terms.
4.25. Disclosure. Neither this Agreement, or any of the Schedules or Exhibits hereto, contains any untrue statement of a material fact or omits a material fact necessary to make the statements contained herein or therein, in light of the circumstances in which they were made, not misleading.
4.26. No Limitation. No investigation or due diligence conducted by, or knowledge obtained by, Purchaser shall limit, modify or negate any of the foregoing representations and warranties.
4.28. Taxes. (a) All Tax returns (including information returns) required to be filed on or before the Closing Date by GRA have ben timely filed; (b) all such Tax Returns are true, complete, and correct in all respect; (c) all Taxes due and owing by GRA (whether or not shown on any Tax Return) have been timely paid; (d) all deficiencies asserted, or assessments made, against GRA as a result of any examinations by any taxing authority have been fully paid; and (e) there are no pending or threatened actions by any Taxing Authority. GRA is registered in the UAE for the purposes of the legislation related to VAT and is not registered, and is not required to register, in any other jurisdiction in respect of VAT or any similar tax.
33
ARTICLE
V
REPRESENTATIONS AND WARRANTIES OF PURCHASER
Purchaser represents and warrants to Seller as of the date hereof and the Closing Date as follows:
5.1. Organization and Good Standing. Purchaser is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware and has all requisite corporate power and authority to conduct its business as heretofore conducted.
5.2. Authorization of Agreement. Purchaser has all requisite corporate power and authority to execute and deliver this Agreement and each other agreement, document, instrument or certificate contemplated by this Agreement or to be executed by Purchaser in connection with the consummation of the Acquisition (together with this Agreement, the “Purchaser Documents”), and to consummate the transactions and perform its obligations contemplated thereby. The execution, delivery and performance by Purchaser of each Purchaser Document and the consummation of the transactions contemplated thereby have been duly authorized by all requisite corporate action on behalf of Purchaser. This Agreement has been, and each other Purchaser Document will be at or prior to the Closing, duly executed and delivered by Xxxxxxxxx and (assuming the due authorization, execution and delivery by the other parties hereto and thereto) this Agreement constitutes, and each other Purchaser Document when so executed and delivered will constitute, the legal, valid and binding obligation of Purchaser, enforceable against Purchaser in accordance with its terms.
5.3. Conflicts; Consents of Third Parties. Other than those which will have been made or obtained, as applicable, as of the Closing, no waiver, Order or Permit of, or declaration or filing with, or notification to, any Person or Governmental Body is required on the part of Purchaser in connection with the execution and delivery of Purchaser Documents or the compliance by Purchaser with any of the provisions thereof.
5.4. Litigation. There are no Legal Proceedings pending that are reasonably likely to prohibit or restrain the ability of Purchaser to enter into this Agreement or consummate the transactions contemplated hereby.
ARTICLE
VI
COVENANTS
6.1. Consents; Failure to Obtain Consents.
(a) Prior to the Closing Date, each Seller and Seller Owner will use its commercially reasonable efforts to obtain or cause to be obtained any Consents required to be obtained by each Seller or Seller owner, as applicable, in connection with the transactions contemplated by this Agreement that are requested by Purchaser and that have not been previously obtained prior to or at the Closing, and Purchaser shall provide its cooperation in such regard if reasonably requested by such Seller or Seller Owner. Each Seller and Seller Owner shall cooperate in any reasonable arrangement which is designed to provide Purchaser with the benefits of such Consent until such time the Consent is actually obtained by such Seller or Seller Owner.
(b) If, following the Closing, authorization, approval, consent or waiver for the sale, assignment, transfer, conveyance or delivery of any such asset not sold, assigned, transferred, conveyed or delivered at the Closing is obtained, then each Seller or Seller Owner, as applicable, shall assign, transfer, convey and deliver such asset to the Purchaser at no additional cost.
34
(c) To the extent that any such asset cannot be sold, assigned, transferred, conveyed or delivered to the Purchaser within 90 days following the Closing Date pursuant to this Section 6.1, then the Purchaser and each Seller or Seller Owner, as applicable, shall enter into such arrangements (including subleasing, sublicensing or subcontracting arrangements) to provide to the parties the economic (taking into account Tax costs and benefits) and operational equivalent, to the extent permitted, of obtaining such authorization, approval, consent or waiver and the performance by the Purchaser of the obligations thereunder. Each Seller and Seller Owner shall hold in trust for and pay to the Purchaser promptly upon receipt thereof all income, proceeds and other monies received by such Seller in connection with such Seller's operation thereof (net of any Taxes and any other costs imposed upon the Seller) in connection with the arrangements under this Section 6.1.
6.2. Post-Closing Cooperation; Mail Received After Closing.
(a) Following the Closing Date, Purchaser may receive and open all mail addressed to any Seller or Seller Owner that Purchaser reasonably believes relates to the Business, the Acquired Assets or the Assumed Liabilities, and, to the extent that such mail and the contents thereof relate to the Business, the Acquired Assets or the Assumed Liabilities, deal with the contents thereof at its reasonable discretion. From and after the Closing Date, each Seller and Seller Owner shall promptly forward or cause to be forwarded to Purchaser any mail received by such Seller or Seller Owner that relates to the Business, the Acquired Assets or the Assumed Liabilities, and Purchaser shall promptly forward or cause to be forwarded to Seller Owner any mail received by Purchaser that relates to the Excluded Assets or the Retained Liabilities.
(b) Following the Closing Date, each Seller and Seller Owner hereby grants to Purchaser the power, right and authority, coupled with an interest, to receive, endorse, cash, deposit, and otherwise deal with, in the name of such Seller, any checks, drafts, documents and instruments constituting payment of any notes or accounts receivable included in the Acquired Assets and that are payable to, payable to the order of, or endorsed in favor of a Seller or any agent of a Seller. Each Seller and Seller Owner agrees promptly to endorse and pay over or cause to be endorsed and paid over to Purchaser, without deduction or offset, the full amount of any payment received by such Seller after the Closing Date in respect of goods sold or services rendered as part of the Business.
(c) Following the Closing, no Seller or Seller Owner shall retain or use any copy of any Technology (i) constituting, used in, held for use in, or necessary for the operation of, the Business, or (ii) that is owned by, held in the name of, or transferable by, a Seller or Seller Owner.
(d) Effective on the Closing Date, each Seller and Seller Owner hereby constitutes and appoints Purchaser the true and lawful attorney of such Seller, with full power of substitution, in the name of such Seller or Purchaser, but on behalf of and for the benefit of Purchaser: (i) to demand and receive from time to time any and all of the Acquired Assets and to make endorsements and give receipts and releases for and in respect of the same and any part thereof; (ii) to institute, prosecute, compromise and settle any and all Legal Proceedings that Purchaser may deem proper in order to collect, assert or enforce any claim, right or title of any kind in or to the Acquired Assets; (iii) to defend or compromise any or all Legal Proceedings in respect of any of the Acquired Assets; and (iv) to do all such acts and things in relation to the matters set forth in the preceding clauses (i) through (iii) as Purchaser shall deem desirable. Each Seller and Seller Owner hereby acknowledge that the appointment hereby made, and the powers hereby granted are coupled with an interest and are not and shall not be revocable by it in any manner or for any reason.
(e) Following the Closing, each Seller and Seller Owner will afford Purchaser, its counsel and its accountants, during normal business hours, reasonable access to the books, records and other data in such Seller's or Seller Owner’s possession relating to the Business or Acquired Assets with respect to periods prior to the Closing and the right to make copies and extracts therefrom, to the extent that such access may be reasonably required by Purchaser in connection with: (i) the preparation of Tax Returns; (ii) the determination or enforcement of rights and obligations under this Agreement, including by any Indemnified Party; (iii) compliance with the requirements of any Governmental Body; (iv) any actual or threatened Legal Proceeding or (v) proper accounting of the Acquisition or a request from Purchaser's auditors.
35
6.3. Other Post-Closing Expenses. Each Seller and Seller Owner is responsible for all expenses (other than Assumed Liabilities) related to the Business incurred prior to and on the Closing Date, and Purchaser will forward to Seller Owner invoices for expenses relating solely to the period on and before the Closing Date (other than the Assumed Liabilities) and Seller Owner shall pay such invoices directly to the payee. In order to assure Purchaser of no disruption in services, Purchaser may (but is not obligated to) pay any invoices which reflect expenses relating to both the period before and after the Closing Date; provided, however, that Seller Owner shall remain obligated for its portion of such expenses in accordance with the terms of this Agreement.
6.4. Payment of Retained Liabilities. Each Seller and Seller Owner shall pay, or make adequate provision for the payment, in full all of the Retained Liabilities and other Liabilities of such Seller or Seller Owner under this Agreement. If any such Liabilities are not so paid or provided for, or if Purchaser reasonably determines that failure to make any payments will impair Purchaser’s use or enjoyment of the Acquired Assets or conduct of the Business, Purchaser may, at any time after the Closing Date, elect to make all such payments directly (but shall have no obligation to do so) and the full amount of all such payments made by Purchaser shall be promptly reimbursed by Seller Owner following Purchaser’s written notice to Seller Owner thereof.
6.5. Further Assurances. All deliveries, payments and other transactions and documents relating to the transactions contemplated herein shall be interdependent and none shall be effective unless and until all are effective (except to the extent that the party entitled to the benefit thereof has expressly waived in writing satisfaction or performance thereof as a condition precedent to Closing). Each party hereto shall, at the request of another party hereto, from time to time and at any time, whether on or after the Closing Date, and without further consideration, execute and deliver such deeds, assignments, transfers, assumptions, conveyances, powers of attorney, receipts, acknowledgments, acceptances and assurances as may be reasonably necessary to procure for the party so requesting, and its successors and assigns, or for aiding and assisting in collecting and reducing to possession, any and all of the Acquired Assets, or for the assumption of the Assumed Liabilities, or to otherwise satisfy and perform the obligations of the parties hereunder or to otherwise give effect to the transactions contemplated hereby. Without limiting the generality of the foregoing, Seller shall, upon the request of Purchaser and without further consideration, in a timely manner on and after the Closing Date execute and deliver to Purchaser such other documents, releases, assignments and other instruments as may be reasonably required to effectuate completely the transfer and assignment to Purchaser of, and to vest fully in Purchaser all of Seller’s rights to, the Acquired Assets.
6.6. Press Releases and Announcements. None of Seller or any Seller Owner shall issue any press release or public announcement concerning this Agreement or the transactions contemplated hereby without obtaining the prior written approval of Purchaser.
6.7. Access. During the period from the date hereof through and including the Closing, each Seller and Seller Owner shall give to Purchaser, its officers, agents, employees, counsel, accountants and other representatives, full and complete access, during normal business hours, to the books, records, personnel and properties of the Business as Purchaser may reasonably request. Each Seller and Seller Owner shall cause its officers, employees, consultants, agents, accountants and other representatives to cooperate in all respects with Purchaser and Purchaser’s representatives in connection with any and all due diligence investigations and examinations.
36
6.8. Operation of the Business. Between the date of this Agreement and the Closing Date (or earlier termination of this Agreement), unless otherwise agreed in writing by Purchaser (which agreement shall not be unreasonably withheld, conditioned or delayed), Sellers and Seller Owner will:
(a) except as otherwise allowed or required pursuant to the terms of this Agreement, conduct the Business in the ordinary course in a manner consistent with past practice and in accordance with all applicable Laws;
(b) use commercially reasonable efforts to preserve intact the current business operation of Sellers and Seller Owner, keep available the services of the current officers, employees and agents of Sellers and Seller Owner, and use commercially reasonable efforts to maintain the relations and goodwill with its beta testers, customers, suppliers, consultants, contractors, licensors, employees, distributors and others having business relationships with Sellers and Seller Owner;
(c) maintain all of the Acquired Assets in their current condition, ordinary wear and tear excepted and, in the event of any damage to or destruction of any of the Acquired Assets prior to the Closing Date, promptly replace, repair or restore such Acquired Assets;
(d) pay the debts and Taxes of each Seller and Seller Owner when due and pay and perform other obligations of each Seller and Seller Owner when due;
(e) maintain the books and records in the usual, regular and ordinary manner, on a basis consistent with prior years;
(f) notify Purchaser of any bugs and defects in the Seller’s Technology which become known;
(g) maintain the Seller’s Intellectual Property (including paying all necessary registration, maintenance and renewal fees and filing all necessary documents and certificates with the relevant Governmental Body);
(h) maintain in full force all insurance policies currently in effect; and
(i) report periodically to Purchaser concerning the status and operation of the Business and the Acquired Assets.
6.9. Conduct Prior to Closing. Except as otherwise expressly permitted by this Agreement, without the prior written consent of Purchaser between the date of this Agreement and the Closing Date (or earlier termination of this Agreement), Sellers and Seller Owner will not:
(a) enter into any (i) inbound license agreement with respect to the Intellectual Property rights of any third party, other than shrink-wrap code or (ii) outbound license agreement with respect to any of the Seller’s Intellectual Property or Seller’s Technology;
(b) settle any pending Legal Proceedings or obtain any releases of threatened Legal Proceedings involving or related to the Business;
37
(c) undertake any expenditure, transaction or commitment exceeding $5,000 individually or $10,000 in the aggregate (other than capital expenditures for which payment is fully made prior to the Closing);
(d) enter into (i) any Contract of the type described in Section 3.13 or Section 4.10, or (ii) any new employment agreement or modify any existing employment agreement (other than to remove any change of control or severance provisions) with respect to GRA;
(e) incur or guarantee any Debt involving or related to the Business;
(f) file a petition in bankruptcy, make an assignment for the benefit of creditors or file a petition seeking reorganization or arrangement or other action under federal or state bankruptcy laws;
(g) enter into any agency, partnership, joint venture or trust;
(h) acquire or agree to acquire by merging or consolidating with, or by purchasing any assets or equity securities of, or by any other manner, any business or any corporation, partnership, association or other business organization or division thereof, or otherwise acquire or agree to acquire any assets or any equity securities;
(i) terminate, renew or amend or modify any agreements with respect to the Purchased Assets;
(j) sell, assign, license, lease, transfer, convey or pledge the Acquired Assets or commit itself to sell, assign, license, lease, transfer, convey or pledge the Acquired Assets or subject any of the Acquired Assets to a security interest;
(k) enter into any agreement to purchase or sell any interest in real property, grant any security interest in any real property, enter into any lease, sublease, license or other occupancy agreement with respect to any real property;
(l) revalue any of the Acquired Assets including writing down the value of inventory;
(m) approve or waive any rights with respect to the issuance of any GRA equity or sell any equity interests in GRA;
(n) cause or approve GRA to do any of the foregoing; or
(o) take, or agree in writing or otherwise to take, any of the actions described in Sections 6.9(a)-(n) above.
6.10. No Negotiation. Until the earlier of the Closing or the termination of this Agreement, no Seller or Seller Owner, or any of their respective agents, Affiliates or representatives, shall, directly or indirectly, solicit, initiate, encourage or enter into any discussions or negotiations with, or provide any assistance or information to, or enter into any agreement with, any Person or group of Persons (other than Purchaser) concerning any acquisition, directly or indirectly, of the Shares of Seller in, or any merger, consolidation, recapitalization, liquidation, dissolution or similar transaction involving, or any purchase of all or a substantial portion of the assets of any Seller or Seller Owner. In furtherance of the foregoing, Seller Owner shall terminate, or cause the termination of, any current discussions or negotiations with any Person or group of Persons (other than Purchaser) concerning any acquisition, directly or indirectly, of the Shares of any Seller or Seller Owner in, or any merger, consolidation, recapitalization, liquidation, dissolution or similar transaction involving, or any purchase of all or a substantial portion of the assets of, Sellers or Seller Owner.
38
6.11. Taxes. Each Seller and Seller Owner shall timely file all applicable Tax Returns and timely pay all Taxes required to be paid by it under applicable Law that, if left unpaid, could result in the imposition of a Lien on any of the Acquired Assets or subject Purchaser to a claim by any Taxing Authority for unpaid Taxes of any Seller or Seller Owner. Each Seller and Seller Owner will retain copies of all Tax Returns and Tax records as required by applicable Law and will give Purchaser reasonable access to inspect such materials if such inspection is reasonably necessary for Purchaser to respond to any claim by any Taxing Authority relating to the Acquired Assets, Assumed Liabilities or Business.
6.12. Pre-Closing Cooperation. From the date hereof through the Closing Date, Seller Owner and each Seller will use their best efforts to take, or cause to be taken, all actions and do, or cause to be done, all things necessary, proper, or advisable to consummate and effectuate the transactions contemplated by this Agreement and the Transaction Documents in an expeditious manner. Seller Owner and each Seller shall cooperate fully with Purchaser to effect a smooth and orderly transition of the Acquired Assets and the Business to Purchaser, including by furnishing information, evidence, testimony, and other assistance in connection with any actions, proceedings or disputes of any nature with respect to matters pertaining to all periods prior to the Closing.
6.13. Diligence Materials. At the Closing Date, Seller Owner shall deliver, or cause to be delivered, to Purchaser a USB flash drive containing a copy of all documents contained on the final, updated data room on behalf of Seller or Seller Owner.
6.14. Release. Effective as of the Closing, each Seller and Seller Owner releases and discharges each other Seller’s past, present and/or future Affiliates, directors, managers, officers, employees, agents, attorneys, equity holders, members, insurers, successors and/or assigns (collectively “Released Parties”), from any and all claims, demands and causes of action, whether known or unknown, liquidated or contingent, relating to, arising out of or in any way connected with the dealings of the Acquired Assets and any Released Party from the beginning of time through the Closing Date, it being understood, however, that such release shall not operate to release such Released Party from any indemnity obligations under Article VIII. Each Seller acknowledges that the Laws of many states provide substantially the following: “A GENERAL
RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.” Each Seller acknowledges that such provisions are designed to protect a party from waiving claims which it does not know exist or may exist. Nonetheless, each Seller agrees that, effective as of the Closing Date, each Seller shall waive any such provision. Each Seller further agrees that none of each such Seller or its Affiliates, shall: (a) institute a Legal Proceeding based upon, arising out of, or relating to any of the released claims; (b) participate, assist or cooperate in any such Legal Proceeding; or (c) encourage, assist and/or solicit any other Person to institute any such Legal Proceeding.
39
6.15. Regulatory Filings.
(a) From and after the date of this Agreement, each party shall, as promptly as possible, (i) make, or cause to be made, all filings and submissions required under any Law applicable to such party or any of its Affiliates; and (ii) use commercially reasonable efforts to obtain, or cause to be obtained, all Consents, authorizations, orders and approvals from all Governmental Bodies that may be or become necessary for its execution and delivery of this Agreement, the performance of its obligations pursuant to this Agreement and the transfer of all Acquired Assets to Purchaser; provided, however, that Purchaser shall be required to pay any fees or other payments to any such Governmental Bodies in order to obtain any such Consent, authorization, order or approval, including any filing fees associated with any filing, notification or notice required to be made hereunder with any Governmental Body, including all fees in connection with transfers and changes to Marketing Authorizations. Each party shall cooperate fully with the other parties and their Affiliates in promptly seeking to obtain all such Consents, authorizations, orders and approvals. The parties shall not willfully take any action that will have the effect of delaying, impairing or impeding the receipt of any required Consents, authorizations, orders and approvals.
(b) As soon as practicable but no more than sixty (60) days after Closing, the Sellers will transfer to Purchaser the Marketing Authorization(s) for the Cellvera Products. If the transfer of the Marketing Authorization(s) for the Cellvera Products is not possible within such sixty (60) day period, then the Sellers and Purchaser will use commercially reasonable efforts to complete the transfer as promptly as possible thereafter. For the purpose of such transfer, the Sellers shall transfer or assign the Marketing Authorization(s) for the Cellvera Products to Purchaser and/or cause for the Marketing Authorization(s) for the Cellvera Products to issue in the name of Purchaser and until such transfer or assignment has been effected the Purchaser shall have the right to continue to use, market and sell the Cellvera Products under the Sellers’ existing “Cellvera” artwork, packaging (including patient inserts/leaflets) labelling and any other trade dress relating to the Cellvera Products. The Sellers shall execute and deliver all documents reasonably necessary to effect that transfer, assignment or issuance, and take all actions reasonably necessary to effect that transfer, assignment or issuance. The Sellers hereby appoint Purchaser as the Sellers’ attorney-in-fact, with full power of substitution, to execute, deliver and file in the Sellers’ name any documents and take any actions reasonably necessary or desirable to transfer or assign any Marketing Authorization(s) for the Cellvera Product to Purchaser or cause any Marketing Authorization(s) for the Cellvera Product to issue in the name of Purchaser. It is expressly agreed that any communication with Governmental Authorities (including but not limited to the EMA) after Closing by Sellers or any other entity acting in the name or on behalf of the Sellers requires the prior written approval of Purchaser. After the transfer, Purchaser will be the Marketing Authorization holder and will maintain such Marketing Authorizations. Should there be a variation in any approval by any Governmental Body that would necessitate a variation in Marketing Authorization(s), the Sellers must inform Xxxxxxxxx as soon as practicable, but not more than fifteen (15) days after such variation has become effective.
40
ARTICLE
VII
CLOSING
7.1. Closing. The consummation of the transactions contemplated in this Agreement (the “Closing”) is taking place by simultaneous transmission of signature pages via facsimile or electronic mail, within two (2) Business Days of the satisfaction or waiver in writing (to the extent waivable) of the conditions set forth in Sections 7.2 and 7.3 or at such other place and time as the parties have mutually agreed in writing. The date on which the Closing actually occurs is referred to herein as the “Closing Date.”
7.2. Conditions to Obligation of Purchaser. The obligations of Purchaser to purchase the Acquired Assets and assume the Assumed Liabilities at the Closing are subject to the satisfaction of each of the following conditions:
(a) Each Seller and Seller Owner shall have performed and satisfied each of its obligations hereunder required to be performed and satisfied at or prior to the Closing;
(b) (i) Each of the representations and warranties of each Seller and Seller Owner contained herein shall have been true and correct in all material respects as of the date hereof and shall be true and correct in all material respects at and as of the Closing Date (disregarding for the purposes of the condition set forth in this Section 7.2(b) any “material adverse effect” or other “materiality” qualifier contained in any such representations or warranties), except for Fundamental Representations, which shall have been true and correct in all respects as of the date hereof and shall be true and correct in all respects at and as of the Closing Date; and (ii) there shall not have been any material adverse change in the Acquired Assets, Assumed Liabilities or the business, prospects, condition (financial or otherwise) or results of operations of the Business;
(c) Purchaser shall have received from each Seller and Seller Owner a certificate signed by a duly authorized executive officer of each Seller and Seller Owner certifying the satisfaction of the conditions set forth in Sections 7.2(a) and 7.2(b);
(d) All required Consents from Governmental Bodies for the Acquisition, and all Consents set forth or required to be set forth on Schedule 3.3 shall have been obtained in form and substance reasonably satisfactory to Purchaser and its counsel and shall remain in full force and effect as of the Closing Date;
(e) No temporary restraining order, preliminary or permanent injunction, cease and desist order or other order issued by any court of competent jurisdiction or Governmental Body preventing any transfer contemplated hereby or the consummation of the Closing, or imposing damages in respect thereto, shall be in effect, there shall be no pending or threatened Legal Proceedings by any Governmental Body or by any other Person challenging or in any manner seeking to restrict or prohibit the Acquisition or the consummation of any other transactions contemplated hereby and there shall be no currently effective Law that has the effect of making the transactions contemplated hereby illegal or which has the effect of otherwise preventing or prohibiting the consummation hereof;
(f) Purchaser shall have received a certificate from the Secretary, a director or comparable official of each Seller and Seller Owner, dated as of the Closing Date, attesting to the resolutions of the Seller Owner and each Seller’s Board of Directors (or other governing body) authorizing the execution, delivery and performance of the Seller Documents, and to the incumbency of the officer(s) executing any Seller Document;
(g) Purchaser shall have received a Bill of Sale, in form and substance satisfactory to Purchaser in its absolute and sole discretion, duly executed by each Seller (the “Bill of Sale”), and such other instruments as may be reasonably requested by Purchaser to transfer full legal and beneficial ownership of the Acquired Assets to Purchaser, free and clear of Liens;
41
(h) Purchaser shall have received a counterpart to the Assignment and Assumption Agreement, in the form and substance satisfactory to Purchaser in its absolute and sole discretion, duly executed by each Seller (the “Assignment and Assumption Agreement”);
(i) Purchaser shall have received an Intellectual Property Assignment, in form and substance satisfactory to Purchaser in its absolute and sole discretion, pursuant to which each Seller shall transfer title to all Intellectual Property to Purchaser, duly executed by each such Seller (the “Intellectual Property Assignment”);
(j) Purchaser shall have received good standing certificates (including tax good standing) with respect to each Seller and Seller Owner from the applicable authority(ies) in Delaware and any other jurisdiction in which such Seller and Seller Owner is incorporated or qualified to do business, dated within ten (10) days of Closing (and in the case of Cellvera Ltd and BVI Holdco, within 1 day of Closing);
(k) Purchaser shall have received executed payoff letters, satisfactory to Purchaser and its counsel, reflecting the amount required to fully pay and satisfy all of each Seller’s and Seller Owner’s Debt to be paid at Closing pursuant to Section 2.2 (including the Seller Expenses), which payoff letters shall confirm that all Liens on any of the Acquired Assets shall be released, discharged and terminated in full upon payment of the amount set forth therein and each Seller and Seller Owner is released from any liability (collectively, the “Payoff Letter and Release Agreements”);
(l) Each Seller and Seller Owner shall have delivered possession of the Acquired Assets to Purchaser, free and clear of all Liens;
(m) Purchaser shall have received a written acknowledgment, satisfactory to Purchaser and its counsel, in each case executed by each Person to whom any Seller Expenses are owed: (i) of the total amount of fees, costs and expenses of any nature that is owing to such Person; and (ii) that, upon payment of the amounts described in clause “(i)” above, it is not (and will not be) owed any other amount by any Seller, any Seller Owner, Purchaser, or their respective Affiliates;
(n) Purchaser shall have completed its due diligence to its full satisfaction;
(o) Purchaser shall have obtained immediately available funds in respect of a financing sufficient to consummate the transactions contemplated by this Agreement, on terms acceptable to Purchase in its sole discretion;
(p) A release from Agility in form and substance reasonably acceptable by Purchaser;
(q) An assignment of membership interest by and between Purchaser and Cellvera Ltd in form and substance acceptable to Purchaser assigning Cellvera Ltd’s membership interest in GRA to Purchaser and Agility’s consent thereto (the “GRA Assignment”);
(r) The completion of all requirements under and pursuant to the GRA Term Sheet and any definitive agreements executed in connection therewith;
(s) Purchaser shall have been unconditionally released from the Spirit Complaint and received a release from Spirit Global Investments, Inc., a California corporation, in form and substance acceptable to Purchaser, in its sole discretion, releasing Purchaser from any and all Losses related thereto;
42
(t) A release from each Person identified by Purchaser in Purchaser’s sole and absolute discretion, in form and substance reasonably acceptable to Purchaser;
(u) An agreement acceptable to Purchaser, in its sole discretion, by and between Purchaser and Feynman Labs in form and substance acceptable to Purchaser with respect to certain intellectual property owned by Xxxxxxx Xxxx;
(v) A fourth amendment to the Fuji APA in form and substance acceptable to Purchaser, in its sole discretion, duly executed by Xxxx and Cellvera Ltd;
(w) The matters set forth on Schedule 7.2(w) shall have been completed to Purchaser’s satisfaction;
(x) Purchaser shall have received all other documents, instruments and certificates in connection with the transactions contemplated by this Agreement as Purchaser may reasonably request in form and substance reasonably satisfactory to Purchaser and its counsel; and
(y) Purchaser shall have received a fairness opinion satisfactory to Purchaser in its sole and absolute discretion with respect to the transactions contemplated herein;
(z) The Purchaser’s board of directors shall have approved of the transactions contemplated herein;
(aa) Between the date hereof and the Closing Date, there shall not have occurred any Material Adverse Effect, nor shall any event or events have occurred that, individually or in the aggregate, with or without the lapse of time, could reasonably be expected to result in a Material Adverse Effect;
(bb) Purchaser shall have received a certificate of the secretary (or equivalent officer) of Seller Owner certifying that attached thereto are true and complete copies of all resolutions adopted by the board of directors (or equivalent governing body) of Seller Owner authorizing the execution, delivery and performance of this Agreement and the and Transaction Documents and the consummation of the transactions contemplated hereby and thereby, and that all such resolutions are in full force and effect and are all the resolutions adopted in connection with the transactions contemplated hereby and thereby;
(cc) Purchaser shall have received a certificate of the secretary (or equivalent officer) of Seller Owner certifying the names and signatures of the officers of Seller Owner authorized to sign this Agreement, the Transaction Documents and the other documents to be delivered hereunder and thereunder;
(dd) Each Seller and Seller Owner shall have delivered the disclosures required under Schedule 3.6 and the GRA Latest Balance Sheet to Purchaser;
(ee) Purchaser shall have received form the Seller Owner, whether through its official liquidators (if appointed by the Cayman Islands Grand Court in Proceedings FSD 72 of 2023 (DDJ)) or the board of directors of the Seller Owner (if the Cayman Islands Grand Court, in those proceedings, declines to appoint official liquidators to the Seller Owners), written consent to the sale and purchase of the Acquired Assets and Assumed Liabilities pursuant to this Agreement; and
(ff)At any time before Closing, Purchaser may request any additional documentation or agreements in form and substance reasonably acceptable to Purchaser in all respects.
43
7.3. Conditions to Obligation of Seller. The obligations of each Seller to sell the Acquired Assets and assign the Assumed Liabilities at the Closing are subject to the satisfaction of each of the following conditions:
(a) Purchaser shall have performed and satisfied each of its obligations hereunder required to be performed and satisfied by it at or prior to the Closing.
(b) Each of the representations and warranties of Purchaser contained herein shall have been true and correct in all material respects as of the date hereof and shall be true and correct in all material respects at and as of the Closing Date (disregarding for the purposes of the condition set forth in this Section 7.3(b) any “material adverse effect” or other “materiality” qualifier contained in any such representations or warranties).
(c) No temporary restraining order, preliminary or permanent injunction, cease and desist order or other order issued by any court of competent jurisdiction or any Governmental Body preventing any transfer contemplated hereby or the consummation of the Closing, or imposing damages in respect thereto, shall be in effect, and there shall be no pending or threatened actions or Legal Proceedings by any Governmental Body (or determinations by any Governmental Body) or by any other Person challenging or in any manner seeking to restrict or prohibit the sale of the Acquired Assets or the consummation of any other transactions contemplated hereby.
(d) Each Seller shall have received a counterpart of the Assignment and Assumption Agreement, duly executed by Purchaser.
(e) Each Seller shall have received a counterpart of the Purchase and Sale Agreement, duly executed by Purchaser.
(f) Purchaser shall have tendered payment of the amounts to be tendered at Closing, as described in Section 2.2.
(g) Each Seller shall have received all other documents, instruments and certificates in connection with the transactions contemplated by this Agreement as each such Seller may reasonably request in form and substance reasonably satisfactory to each such Seller.
ARTICLE
VIII
TERMINATION
8.1. Termination of Agreement. This Agreement may be terminated prior to the Closing as follows:
(a) by mutual written consent of Xxxxxxxxx, on the one hand, and Seller Owner, on the other hand;
(b) Purchaser (in its sole discretion) may terminate this Agreement by giving written notice to Seller Owner at any time prior to the Closing: (i) if Seller Owner or any Seller has breached any covenant, representation or warranty in any material respect contained in this Agreement; (ii) if the Closing shall not have occurred on or before May 31, 2023 (the “Expiration Date”); (iii) if the Purchaser is unsatisfied (in its sole discretion) with the contents of the disclosure schedules; or (iv) Purchase is not satisfied (in its sole discretion) with its diligence review which shall be ongoing through and including the Expiration Date; or
(c) by any Seller or Purchaser if there shall be in effect a final nonappealable judgment or order of a Governmental Body of competent jurisdiction restraining, enjoining or otherwise prohibiting the consummation of the transactions contemplated hereby; it being agreed that the parties hereto shall use commercially reasonable efforts to appeal any adverse determination which is not nonappealable (and pursue such appeal with reasonable diligence).
44
8.2. Procedure Upon Termination. In the event of termination and abandonment by Purchaser or Seller Owner, or both, in accordance with Section 8.1, written notice thereof shall forthwith be given to the other party, and this Agreement shall terminate, and the Acquisition shall be abandoned, without further action by Purchaser or Seller Owner or any Seller.
8.3. Effect of Termination. In the event that this Agreement is validly terminated in accordance with Section 8.2, the parties hereto shall be relieved of their duties and obligations arising under this Agreement after the date of such termination and such termination shall be without Liability to the parties; provided, that no such termination shall relieve any party hereto from Liability for any willful breach of this Agreement; provided further, that the obligations of the parties set forth in Section 6.6 (Press Releases and Announcements) and Article XI (Miscellaneous) shall survive any such termination and shall be enforceable hereunder.
ARTICLE
IX
SURVIVAL AND INDEMNIFICATION
9.1. Survival of Representations and Warranties. With the exception of the representations and warranties contained in: (a) Sections 3.1, (Organization and Good Standing) 3.2, (Authorization of Agreement) 3.3, (Conflicts; Consents of Third Parties) 3.4, (Capitalization) 3.11(c) and (d), (Tangible Personal Property; Sufficiency) 3.12, (Intellectual Property) 3.17, (Litigation) 3.25, (Regulatory Compliance; Permits) 3.26, (Solvency) 3.27, (International Trade), 3.28 (Debt and Seller Expenses) 4.1, (Organization, Authority and Qualification) 4.2, (Capitalization) 4.3, (No Conflicts; Consents) 4.4, (Subsidiaries) 4.5, (Legal Proceedings) 4.6, (Financial Statements; Books and Records) 4.11, (Intellectual Property) 4.12, (Compliance with Laws; Permits) 4.22, (Regulatory Compliance; Permits) 4.23 (Solvency) and 5.2 (Authorization of Agreement), which shall survive the Closing indefinitely; (b) Sections 3.9, (Taxes) 3.24 (Products Liability), 4.7 (Undisclosed Liabilities), and 4.8 (Absence of Certain Developments) which shall survive the Closing until the 30th day following the expiration of the respective statutes of limitations for such representations (after giving effect to any extension or tolling thereof) (the representations and warranties described in clauses (a) and (b) above, collectively, the “Fundamental Representations”); and (c) any representations and warranties intentionally misrepresented or fraudulently made, the representations and warranties of the parties to this Agreement shall survive Closing for a period of three (3) years (the “General Survival Period”), after which the indemnification obligation of a party contained herein shall terminate (unless a party has, prior to such expiration date, provided notice of facts that may reasonably lead to a written claim for indemnification by such party (in which case the relevant survival period of the representations and warranties applicable to such set of facts shall be extended automatically to include any time period necessary until all claims with respect to such facts shall have been finally settled, decided or adjudicated)). The covenants contained in this Agreement shall survive Closing according to their terms.
9.2. Indemnification of Purchaser Indemnified Parties.
(a) Each Seller and Seller Owner agrees to, jointly and severally, indemnify, defend and hold Purchaser, its Affiliates and each of their respective directors, officers, employees, Affiliates, agents, representatives, successors and assigns (collectively, the “Purchaser Indemnified Parties”) harmless from and against any and all Losses that any of Purchaser Indemnified Parties may sustain (whether or not instituted by a third party), or to which any of Purchaser Indemnified Parties may be subjected, arising out of or in connection with:
(i) any inaccuracy or misrepresentation in or breach of (or an alleged breach arising from an Indemnification Claim arising from an allegation by a third party that, if true, would be a breach of) the representations or warranties made in any Seller Document by any Seller or Seller Owner;
45
(ii) any breach of any covenant or agreement set forth in any Seller Document by any Seller or any Seller Owner;
(iii) any pending or threatened Legal Proceedings;
(iv) any and all Taxes (or the nonpayment thereof) of Seller, including those related to the ownership of the Acquired Assets and operation of the business on or prior to the Closing Date;
(v) Seller Expenses, solely to the extent not paid out of the Purchase Price at Closing;
(vi) any amounts in respect of any Debt, solely to the extent not paid out of the Purchase Price at Closing;
(vii) any Retained Liabilities and any Liabilities that are not directly related to the Business;
(viii) the ownership of the Acquired Assets and the operation of the Business on or before the Closing Date;
(ix) the enforcement of this indemnification by Xxxxxxxxx; and
(x) any amounts in respect of those matters set forth on Schedule 9.2(a)1.
(b) The right to indemnification, payment of Losses or other remedy based on such representations, warranties, covenants and obligations will not be affected by any investigation conducted or any knowledge acquired (or capable of being acquired) at any time, with respect to the accuracy or inaccuracy of any representation or warranty, or the compliance with any covenant or obligation. The waiver of any closing condition based on the accuracy of any representation or warranty, or on the performance of or compliance with any covenant or obligation, will not affect the right to indemnification, payment of Losses, or other remedy based on such representations, warranties, covenants and obligations.
(c) In addition, each Seller and Seller Owner acknowledges and agrees that the Purchaser Indemnified Parties shall have an express right of set-off against any post-Closing payments required of Purchaser under this Agreement or any Purchaser Document for any Losses for which the Purchaser Indemnified Parties are entitled to indemnification pursuant to Article IX.
(d) Seller and each Seller Owner hereby knowingly and irrevocably waive, release and relinquish any right to seek indemnification, contribution or other payment from, or otherwise bring a claim against, the current or former directors, officers, managers, employees, agents and other representatives (and any of them) of Seller as a result of Losses for which Seller or any Seller Owner is required to indemnify any Purchaser Indemnified Party under this Agreement.
| 1 | To be determined based on the results of Purchaser’s due diligence. |
46
9.3. Reserved.
9.4. Limitations on Indemnification. Notwithstanding any provisions of this Article VIII to the contrary:
(a) except for claims arising out of fraud, intentional misrepresentations or a breach of a Fundamental Representation, no party subject to indemnification obligations hereunder (the “Indemnifying Party”) shall have any liability for any Losses arising out of Section 9.2(a) until all such Losses exceed an aggregate of One Thousand Dollars ($1,000.00) at which time such Person shall indemnify and hold the Person with the right to indemnification hereunder (the “Indemnified Party”) harmless from and against all such Losses from the first dollar thereof;
(b) for purposes of determining any inaccuracy in or breach of any representation or warranty and determining the amount of Loss resulting from any inaccuracy in or breach of any representation or warranty, the parties hereto shall disregard any “material,”
“Material Adverse Effect” or similar qualifiers set forth therein or otherwise applicable to such representation and warranty.
9.5. Indemnification Procedures.
(a) In the event an Indemnified Party seeks or believes it may be entitled to indemnification under Sections 9.2 (an “Indemnification Claim”), the Indemnified Party shall promptly provide written notice of such Indemnification Claim to the Indemnifying Party; provided, that no delay by the Indemnified Party will relieve the Indemnifying Party from any obligation hereunder, unless, and then solely to the extent that, the Indemnifying Party is actually and materially prejudiced thereby. The Indemnifying Party may, with counsel of its choice (as long as such counsel is reasonably satisfactory to the Indemnified Party), defend against, negotiate, settle or otherwise deal with such Indemnification Claim, subject to the provisions hereof, and shall within thirty (30) days (or sooner, if the nature of the Indemnification Claim so requires), provide affirmative notice to the Indemnifying Party of its intent to comply with its obligations under this Section 9.5; provided, however, that the Indemnifying Party shall not (and shall not be entitled to) assume the control in any such defense (and the Indemnified Party shall control such defense at the expense of the Indemnifying Party) if: (i) the Indemnification Claim relates to or arises in connection with any criminal Legal Proceeding; (ii) the Indemnified Party reasonably believes that an adverse determination with respect to the Indemnification Claim would be materially detrimental to or materially injure the Indemnified Party’s reputation or business; (iii) the Indemnification Claim seeks an equitable relief; (iv) in the opinion of the Indemnified Party, a conflict or potential conflict exists between the Indemnified Party and the Indemnifying Party that would make such separate representation advisable; (v) the Indemnified Party has additional defenses to the Indemnification Claim not available to the Indemnifying Party; or (vi) the Indemnifying Party failed or is failing to actively prosecute or defend such Indemnification Claim. If the Indemnifying Party fails to provide the Indemnified Party timely notice of its intent to comply with its obligations under this Section 9.5, or is otherwise prohibited from defending against, negotiating, settling or otherwise dealing with any Indemnification Claim pursuant to the provisions of this Section 9.5, the Indemnified Party may defend against, negotiate, settle or otherwise deal with such Indemnification Claim without prejudice to its rights to be indemnified hereunder. If the Indemnifying Party shall assume the defense of any Indemnification Claim, the Indemnified Party may participate, at its own expense, in the defense of such Indemnification Claim; provided, however, that the fees and expenses of the Indemnified Party’s counsel that are incurred prior to the Indemnifying Party’s effective assumption of any Indemnification Claim shall be the responsibility of the Indemnifying Party. If the Indemnified Party desires to consent to the entry of judgment with respect to or to settle an Indemnification Claim but the Indemnifying Party refuses, then the Indemnifying Party will be responsible for all Losses with respect to such Indemnification Claim, without regard to the limitations in Section 8.4. The parties hereto agree to cooperate fully with each other in connection with the defense, negotiation or settlement of any such Indemnification Claim.
47
(b) Notwithstanding anything in Section 9.5(a) to the contrary, the Indemnifying Party shall not, without the written consent of the Indemnified Party, settle or compromise any Indemnification Claim or permit a default or consent to entry of any judgment unless: (i) the sole relief is monetary damages that will be solely satisfied by the Indemnifying Party; and (ii) the claimant provides the Indemnified Party an unqualified release from all Liability in respect of the Indemnification Claim. Notwithstanding the foregoing, if a settlement offer solely for money damages, which includes a complete release of the Indemnified Party from all other liability in respect of the Indemnification Claim, is made by the applicable third party claimant, and the Indemnifying Party notifies the Indemnified Party in writing of the Indemnifying Party’s willingness to accept the settlement offer and pay the amount called for by such offer (and offers evidence reasonable acceptable to the Indemnified Party of the Indemnifying Party’s ability to pay such amount), and the Indemnified Party declines to accept such offer, the Indemnified Party may continue to contest such Indemnification Claim, free of any participation by the Indemnifying Party, and the amount of any ultimate liability with respect to such Indemnification Claim that the Indemnifying Party has an obligation to pay hereunder shall be limited to the lesser of: (A) the amount of the settlement offer that the Indemnified Party declined to accept plus the Losses of the Indemnified Party relating to such Indemnification Claim through the date of its rejection of the settlement offer; or (B) the aggregate Losses of the Indemnified Party with respect to such Indemnification Claim.
(c) In the event an Indemnified Party notifies the Indemnifying Party of any claim for indemnification hereunder that does not involve a third party claim, the Indemnifying Party shall, within thirty (30) days after the date of such notice, pay to the Indemnified Party the amount of all Losses payable pursuant to this Article VIII and shall thereafter pay any other Losses payable pursuant to this Article VIII arising out of the same matter on demand, unless the Indemnifying Party disputes in writing the Indemnifying Party’s liability for, or the amount of, any such Losses within such 30-day period, in which case such payment shall be made as provided above in respect of any matters not so disputed and any Losses in respect of the matters so disputed shall be paid within five (5) Business Days after any determination (by agreement of the Indemnified Party and Indemnifying Party or otherwise) that the Indemnifying Party is liable therefor, pursuant to this Article VIII.
9.6. Fundamental Representation Damages. If a Purchaser Indemnified Party is entitled to indemnification under a state of facts or circumstances enumerated Section 9.2(a) that also constitute a breach of a Fundamental Representation, the indemnification procedures set forth herein with respect to breaches of Fundamental Representations shall apply. For the avoidance of doubt, in such event, the Seller Owner and Sellers, as the Indemnifying Parties, shall be responsible for and reimburse Purchaser Indemnified Party as such damages are incurred until the expiration of the latest expiring statute of limitations applicable to the subject matter underlying such Indemnification Claim (after giving effect to any extension or tolling thereof) and any indemnification payments made by the Seller Owner and Sellers with respect to such Purchaser Indemnification Claim shall not be considered in determining whether the limitation contained in Section 9.4(a) has been met.
9.7. General Limitation; Right of Set-Off.
(a) No limitation set forth in this Article VIII (including the survival period set forth in Section 9.1) shall apply to claims arising out of fraud, intentional misrepresentations or a breach of a Fundamental Representation.
(b) Purchaser may, in its sole and absolute discretion, offset against any payment of any amount due from any Purchaser Indemnified Party to any Seller Party hereunder, including the Revenue Sharing Payment, or under any other agreement to which any Purchaser Indemnified Party and a Selling Party are parties, (i) the amount of any Loss for which the Purchaser Indemnified Parties are entitled to be indemnified under this Agreement, and (ii) any amount due to the Purchaser Indemnified Parties from any Selling Party as damages for the breach of any provision of any agreement to which any Purchaser Indemnified Party and any of the Selling Parties are parties.
9.8. Tax Treatment of Indemnity Payments. Each Seller, the Seller Owner and Purchaser agree to treat any indemnity payment made pursuant to this Article VIII as an adjustment to the Purchase Price for all Tax purposes.
48
ARTICLE
X
COVENANTS
10.1. Definition. “Confidential Information” shall mean any and all information concerning the business affairs of the Purchaser or Sellers and Seller Owner and shall include such information as it relates to any Affiliate of the Purchaser, Sellers or Seller Owner. Without limiting the generality of the foregoing, Confidential Information includes information:
(a) which constitutes proprietary information of the Purchaser, Sellers and Seller Owner;
(b) which contains financial statements, financial projections and budgets, historical and projected sales, capital spending budgets and plans, business plans, the names and backgrounds of personnel, customer lists and customer information, personnel training and techniques and materials, marketing plans or market expansion proposals and sales techniques and materials of the Purchaser, Sellers or Seller Owner, however documented;
(c) from which it could be reasonably inferred that would confer a competitive advantage on the Purchaser, Sellers or Seller Owner;
(d) from which it could be reasonably inferred that disclosure thereof would be detrimental to the Purchaser, Sellers or Seller Owner;
(e) product specifications, discoveries, improvements, processes, marketing and service methods or techniques, formulae, designs, styles, specifications, data bases, computer programs (whether in source code or object code), know-how, strategies, current and anticipated customer requirements, price lists, market studies, and any other information, however documented, that is a trade secret of Purchaser, Sellers or Seller Owner under applicable Law; and
(f) notes, analyses, compilations, studies, summaries, and other material prepared by or for the Purchaser, Sellers or Seller Owner containing or based, in whole or in part, on any information included in the foregoing.
Notwithstanding anything to the contrary above, the term “Confidential Information” does not include information that: (x) is or becomes generally available to the public other than as a result of a disclosure by the receiving party or its representatives; (y) was within the receiving party’s possession prior to its being furnished to the receiving party by or on behalf of the disclosing party pursuant hereto; or (z) becomes available to the receiving party on a non-confidential basis from a source other than the disclosing party or any of its representatives; provided, however, that: (1) with respect to clause (y) above, information concerning the Business shall be deemed “Confidential Information” of Purchaser notwithstanding Seller Owner having possession thereof prior to disclosure thereof by Purchaser; and (2) with respect to clauses (y) and (z) above, the source of such information was not bound by a confidentiality agreement with, or other contractual, legal or fiduciary obligation of confidentiality to, the disclosing party or any other party with respect to such information.
10.2. Acknowledgments and Agreements by each Seller and Seller Owner. Each Seller and Seller Owner hereby acknowledges, agrees and covenants that on and after the Closing Date, each Seller, Seller Owner and each of their respective Affiliates will keep confidential, will hold for the sole benefit of the Purchaser, and will not use except on behalf of the Purchaser, all Confidential Information, which such Person acknowledges is, or shall be, proprietary to the Purchaser; provided, however, that any Confidential Information that is also considered a trade secret under applicable Law, shall not be disclosed by such Person as long as such information remains a trade secret and is not generally known or available to the public other than as a result of unauthorized or unlawful disclosure directly or indirectly by such Person. Each Seller and Seller Owner agrees that upon request they shall forthwith return to the Purchaser, or destroy to the satisfaction of the Purchaser, all Confidential Information in whatever form such information is in the possession of such Person or under such Person’s control. Notwithstanding the foregoing, the obligations of confidentiality, nondisclosure and non-use with respect to Confidential Information required by this Section 9.2 shall not apply to any Confidential Information required to be disclosed in a judicial or administrative Legal Proceeding, or is otherwise required to be disclosed by Law, in any such case only after giving Purchaser as much advance notice of the possibility of such disclosure as practical so that Purchaser may attempt to stop such disclosure or obtain a protective order concerning such disclosure (in which case each Seller and Seller Owner, as applicable, shall cooperate with Purchaser at Purchaser’s expense in such attempts).
49
10.3. Limited Activities. Until the date that is five (5) years after the Closing Date, for the purposes of protecting the goodwill of the Business which Purchaser is acquiring, neither Seller Owner nor any Seller will, directly or indirectly, for any reason, for its own benefit, or for the benefit of or together with any other Person, directly or indirectly:
(a) solicit or conspire with, or attempt to solicit or conspire with, any employee, manager, director or advisor of Purchaser or its Affiliates to terminate that Person’s engagement or relationship with Purchaser or its Affiliates;
(b) solicit or attempt to solicit, any of the Customers or Suppliers of Purchaser or GRA or their Affiliates (as defined below) with whom a Seller has had material contact during the two years prior to the date hereof to terminate their business relationship with Purchaser or its Affiliates (as used herein, “Customers” and “Suppliers” shall be defined as any Person that has been an active customer or supplier of a Seller or Seller Owner or a targeted prospective customer or supplier of a Seller or Seller Owner or one of its respective Affiliates during the two years prior to the date hereof );
(c) divert or attempt to divert any or all of such Customers’ or Suppliers’ business from Purchaser or GRA or their Affiliates in violation of any unfair competition Laws or other applicable Laws; or
(d) except for the benefit of the Purchaser, be engaged as an executive officer, limited liability entity manager or director or in any other managerial or sales capacity or as an owner, co-owner, or other investor of or in, or lender to, whether as an employee, independent contractor, consultant or advisor, or sales representative or distributor of any kind, in any business marketing, manufacturing, selling or distributing biopharmaceutical products (or any business substitutable therefore) (the “Restricted Business”) anywhere in the Prohibited Territory, with the parties acknowledging that Purchaser, GRA and their Affiliates are actively engaged in such business throughout and beyond all parts of the Prohibited Territory; provided, however, the foregoing prohibition on ownership shall not apply to ownership of less than one percent (1%) of the outstanding capital stock of any such Restricted Business that is publicly traded.
10.4. Severability; Reformation. The covenants in this Article X are severable and separate, and the unenforceability of any specific covenant in this Article X is not intended by either party to, and shall not, affect the provisions of any other covenant in this Article X. If any court of competent jurisdiction shall determine that the scope, time, or territorial restrictions set forth in Section 9.3 are unreasonable as applied to Seller or any Seller Owner, the parties acknowledge their mutual intention and agreement that those restrictions be enforced to the fullest extent the court deems reasonable, and thereby shall be reformed to that extent.
10.5. Independent Covenant. All of the covenants in this Article X are intended by each party hereto to be, and shall be construed as, an agreement independent of any other provision in this Agreement, and the existence of any claim or cause of action of Seller or any Seller Owner against Purchaser or its Affiliates, whether predicated on this Agreement or otherwise, shall not constitute a defense to the enforcement by Purchaser or its Affiliates of any covenant in this Article X. It is specifically agreed that the period specified in Section 10.3 shall be computed by excluding from that computation any time during which Seller or any Seller Owner is in violation of any provision of Section 10.3.
10.6. Materiality. Purchaser, each Seller and Seller Owner hereby agree that this Article X is a material and substantial part of this Agreement, and absent each Seller and Seller Owner entering into the restrictions of this Article X, Purchaser would not have entered into this Agreement and consummated the Acquisition.
50
10.7. Sellers’ Representative.
(a) Each Seller and Seller Owner hereby irrevocably appoints the Sellers’ Representative with power of designation and assignment as such Seller’s true and lawful attorney-in-fact and agent with full power of substitution, to act on behalf of, and in the name of, such Seller with full power, without the consent of the other Sellers, to exercise the powers which such Seller could exercise under the provisions of this Agreement and to take all actions necessary or appropriate as the Sellers’ Representative, in the Sellers’ Representative’s reasonable discretion, may deem necessary or desirable on behalf of the Sellers in connection with this Agreement and the Transactions, including (i) the power and authority to amend, modify or waive any provision of this Agreement, (ii) to execute, deliver and accept such waivers and consents and any and all notices, documents, certificates or other papers to be delivered in connection with this Agreement and the Transactions, and (iii) to act on behalf of the Sellers in connection with matters arising under Article IX (Survival and Indemnification); provided, however, that it is acknowledged and agreed that the Sellers’ Representative shall not be authorized to execute any amendment, modification, or waiver to this Agreement on behalf of any other Seller unless such amendment, modification, waiver, or action applies to all Sellers in the same fashion. Purchaser and any Purchaser Indemnified Party will be entitled to rely exclusively upon any notices and other acts of the Sellers’ Representative as being legally binding acts of all of the Sellers.
(b) Each Seller and Seller Owner acknowledges and agrees that the Sellers’ Representative will not be liable to such Seller for any act done or omitted hereunder as the Sellers’ Representative while acting in good faith and in the exercise of reasonable judgment, and any act done or omitted pursuant to the advice of counsel will be conclusive evidence of such good faith. Each Seller will indemnify and hold the Sellers’ Representative harmless against any Losses incurred without gross negligence or bad faith on the part of the Sellers’ Representative and arising out of or in connection with the acceptance or administration of the Sellers’ Representative’s duties under this Agreement and in connection with the Transactions.
(c) Each Seller and Seller Owner will reimburse the Sellers’ Representative for any out-of-pocket, independent, third-party fees and expenses (including reasonable fees and expenses of counsel, accountants and other advisors) incurred by the Sellers’ Representative that arise out of or are in connection with the acceptance or administration of the Sellers’ Representative’s duties under this Agreement and in connection with the Transactions.
10.8. Supplements to Disclosure Schedules. From time to time prior to the Closing, each of Seller and Seller shall have the right (but not the obligation, unless required to do so by Purchaser) to supplement or amend the Disclosure Schedules hereto with respect to any matter hereafter arising or of which it or Purchaser becomes aware after the date hereof (each a “Schedule Supplement”). Any disclosure in any such Schedule Supplement shall not be deemed to have cured any inaccuracy in or breach of any representation or warranty contained in this Agreement, including for purposes of the indemnification or termination rights contained in this Agreement or of determining whether or not the conditions set forth in Section 7.2 have been satisfied.
51
ARTICLE
XI
MISCELLANEOUS
11.1. Expenses. Except as otherwise provided in this Agreement, each Seller, the Seller Owner and Purchaser shall bear its own expenses incurred in connection with the negotiation and execution of this Agreement and each other agreement, document and instrument contemplated by this Agreement and the consummation of the transactions contemplated hereby and thereby.
11.2. Submission to Jurisdiction; Consent to Service of Process; Specific Performance.
(a) The parties hereto hereby irrevocably submit to the exclusive jurisdiction of any federal or state court located within the State of Delaware over any dispute arising out of or relating to this Agreement or any of the transactions contemplated hereby, and all claims relating to or arising out of this Agreement, or the breach thereof, whether sounding in contract, tort or otherwise, and each party hereby irrevocably agrees that all claims in respect of such dispute or any suit, action or proceeding related thereto shall be heard and determined solely in such courts. The parties hereby irrevocably waive, to the fullest extent permitted by applicable Law, any objection which they may now or hereafter have to the laying of venue of any such dispute brought in such court or any defense of inconvenient forum for the maintenance of such dispute. Each of the parties hereto agrees that a judgment in any such dispute may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by Xxx.
(b) Each of the parties hereto hereby consents to process being served by any party to this Agreement in any suit, action or proceeding by delivery of a copy thereof in accordance with the provisions of Section 11.5.
(c) The parties agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. Accordingly, the parties shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement, in addition to any other remedy to which they are entitled at law or in equity.
11.3. Entire Agreement; Amendments and Waivers. This Agreement (including the schedules and exhibits hereto), the other Seller Documents and Purchaser Documents represent the entire understanding and agreement between the parties hereto with respect to the subject matter hereof. This Agreement can be amended, supplemented or changed, and any provision hereof can be waived, only by written instrument making specific reference to this Agreement signed by each Seller, the Seller Owner and Purchaser. No action taken pursuant to this Agreement, including without limitation, any investigation by or on behalf of any party, shall be deemed to constitute a waiver by the party taking such action of compliance with any representation, warranty, covenant or agreement contained herein. The waiver by any party hereto of a breach of any provision of this Agreement shall not operate or be construed as a further or continuing waiver of such breach or as a waiver of any other or subsequent breach. No failure on the part of any party to exercise, and no delay in exercising, any right, power or remedy hereunder shall operate as a waiver thereof, nor shall any single or partial exercise of such right, power or remedy by such party preclude any other or further exercise thereof or the exercise of any other right, power or remedy.
11.4. Governing Law. This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware applicable to contracts made and performed in such state (excluding all other choice of law and conflicts of law rules), and all claims relating to or arising out of this Agreement, or the breach thereof, whether sounding in contract, tort or otherwise, shall likewise be governed by the laws of the State of Delaware applicable to contracts made and performed in such state (excluding all other choice of law and conflicts of law rules).
52
11.5. Notices. All notices, requests, demands, claims and other communications hereunder shall be in writing. Any notice, request, demand, claim or other communication hereunder shall be deemed duly given: (a) if personally delivered, when so delivered; (b) if mailed, five (5) Business Days after having been sent by first class, registered or certified U.S. mail, return receipt requested, postage prepaid and addressed to the intended recipient as set forth below; (c) if given by facsimile, once such notice or other communication is transmitted to the facsimile number specified below, provided, that: (i) the sending facsimile generates a transmission report showing successful completion of such transaction; and (ii) if such telecopy is sent after 5:00 p.m. local time at the location of the receiving facsimile, or is sent on a day other than a Business Day, such notice or communication shall be deemed given as of 9:00 a.m. local time at such location on the next succeeding Business Day; or (d) if sent through a nationally-recognized overnight delivery service that guarantees next day delivery, the Business Day following its delivery to such service in time for next day delivery:
If to Seller to:
Cellvera Global Holdings, LLC
14th Floor, ONE JLT Building
Jumeriah Lake Towers, Dubai 103805
Attn: Xxxxxxxxxxx Xxxxxxx
Email: xxxxxxxxxxx.xxxxxxx@xxxxxxxx.xxx
If to the Seller Owner, to:
AiPharma Group Ltd.
14th Floor, ONE JLT Building
Jumeriah Lake Towers, Dubai 103805
Attn: Xxxxxxxxxxx Xxxxxxx
Email: xxxxxxxxxxx.xxxxxxx@xxxxxxxx.xxx
If to Purchaser, to:
737 n. Fifth St., Suite 200
Richmand, VA 23219
Attention: Xxxx Xxxxxxx
Attention: xxxxxxxx@xxxxxx.xxx
53
with a copy to:
Xxxxxx Xxxxxxx Xxxxx & Xxxxxxxxxxx LLP
000 00xx Xxxxxx, Xxxxx 0000
Atlanta, GA 30363
Facsimile: (000) 000-0000
Attention: Xxxxxxx X. Xxxxxxxxxxxxx XX
Attention: Xxxxxxx X. Xxxxxxxxxx
Email: Xxxxxxx.xxxxxxxxxxxxx@xxxxxxxxxxxxx.xxx
Email: Xxxxxxx.xxxxxxxxxx@xxxxxxxxxxxxx.xxx
Any party entitled to notice hereunder may change the address or facsimile number to which notices, requests, demands, claims and other communications hereunder are to be delivered by giving the other parties notice in the manner herein set forth.
11.6. Severability. If any term or other provision of this Agreement is invalid, illegal, or incapable of being enforced by any Law or public policy, all other terms or provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated hereby is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal, or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in an acceptable manner in order that the transactions contemplated hereby are consummated as originally contemplated to the greatest extent possible.
11.7. Binding Effect; Assignment. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and permitted assigns. Except for rights and protections provided under Article IX, nothing in this Agreement shall create or be deemed to create any third-party beneficiary rights in any Person not a party to this Agreement. No assignment of this Agreement or of any rights or obligations hereunder may be made by Seller or any Seller Owner, directly or indirectly (by operation of law or otherwise), without the prior written consent of Purchaser. Upon any such permitted assignment, the references in this Agreement to the assignor shall also apply to any such assignee unless the context otherwise requires.
11.8. Counterparts. This Agreement may be executed in one or more counterparts, including by way of electronic transmission, each of which will be deemed to be an original copy of this Agreement and all of which, when taken together, will be deemed to constitute one and the same agreement.
11.9. Prevailing Parties. Notwithstanding anything to the contrary herein, in the event of any lawsuit in connection with this Agreement or any ancillary document, the prevailing party in any such lawsuit shall be entitled to recover from the other party its costs and expenses incurred in connection with the lawsuit, including reasonable legal fees and expenses as determined by the court.
11.10. Joint Participation. All parties have participated in the drafting of this entire Agreement and expressly acknowledge such joint participation to avoid application of any rule construing contractual language against the party which drafted the language.
54
11.11. Other Definitional and Interpretive Matters.
(a) Unless otherwise expressly provided, for purposes of this Agreement, the following rules of interpretation shall apply:
(i) Exhibits/Schedules. The Exhibits and Schedules to this Agreement are hereby incorporated and made a part hereof and are an integral part of this Agreement. All Exhibits and Schedules annexed hereto or referred to herein are hereby incorporated in and made a part of this Agreement as if set forth in full herein. Any capitalized terms used in any Schedule or Exhibit but not otherwise defined therein shall be defined as set forth in this Agreement. Any matter set forth on any of the Schedules hereto shall be deemed set forth in all other Schedules to the extent there is a specific cross-reference or to the extent the relevance of such matter to another Schedule is readily apparent from the face of the disclosure. The information contained in this Agreement, the Schedules and Exhibits is disclosed solely for purposes of this Agreement, and no information contained herein or therein shall be deemed to be an admission by any party hereto to any third party of any matter whatsoever (including any violation of applicable Law or breach of contract).
(ii) Gender and Number. Any reference in this Agreement to gender shall include all genders, and words imparting the singular number only shall include the plural and vice versa.
(iii) Headings. The provision of a Table of Contents, the division of this Agreement into Articles, Sections and other subdivisions and the insertion of headings are for convenience of reference only and shall not affect or be utilized in construing or interpreting this Agreement. All references in this Agreement to any “Section” are to the corresponding Section of this Agreement unless otherwise specified.
(iv) Herein. The words such as “herein,” “hereinafter,” “hereof;” and “hereunder” refer to this Agreement as a whole and not merely to a subdivision in which such words appear unless the context otherwise requires.
(v) Including. The word “including” or any variation thereof means “including, without limitation” and shall not be construed to limit any general statement that it follows to the specific or similar items or matters immediately following it.
(vi) Reflected On or Set Forth In. An item arising with respect to a specific representation, or warranty or other provision of this Agreement shall be deemed to be “reflected on” or “set forth in” a balance sheet or financial statements, to the extent any such phrase appears in such provision, if (a) there is a reserve, accrual or other similar item underlying a number on such referenced balance sheet or financial statements that related to the subject matter of such representation, (b) such item is otherwise specifically set forth on the referenced balance sheet or financial statements or (c) such item is reflected on the referenced balance sheet or financial statements and is specifically set forth in the notes thereto.
55
(vii) Or. The term “or” is not exclusive and has the meaning represented by the phrase “and/or.”
(viii) To the extent. The phrase “to the extent” means the degree to which a subject or other thing extends, and such phrase shall not mean simply “if.”
(ix) Default Under. Reference herein to “default under”, “violation of” or other expression of similar import shall be deemed to be followed by the phrase “with or without notice or lapse of time, or both”, whether or not so specified.
(b) The parties hereto have participated jointly in the negotiation and drafting of this Agreement and, in the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as jointly drafted by the parties hereto and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement. Any references to any federal, state, local or foreign statute or law will be deemed also to refer to all rules and regulations promulgated thereunder, unless the context requires otherwise. Unless specifically stated otherwise, all references to “$” or dollar amounts are to lawful currency of the United States of America without accounting for any currency exchange rates. The phrases “delivered”, “made available”, “furnished” and phrases of similar import mean that the information or document referred to has been made physically or electronically delivered to the relevant Parties; except, that, in the case of “delivered”, “made available”, “furnished” and phrases of similar import to Purchaser, such information or document must have been delivered to Purchaser no later than three business days prior to the Closing Date.
11.12. Seller Dissolution. No Seller shall, and Seller Owner shall not cause, any Seller to be dissolved until the end of the General Survival Period. In the event of the dissolution of any Seller at or after the General Survival Period, all obligations of such Seller pursuant to this Agreement shall become the joint and several obligations of the Seller Owner without any action on the part of a party hereto and all of the rights that run to such Seller pursuant to this Agreement shall become rights of the Seller Owner without any action.
[Signature Pages Follow]
56
IN WITNESS WHEREOF, the parties have executed and caused this Asset Purchase Agreement to be executed and delivered on the date first above written.
| PURCHASER: | ADITXT, INC. | |
| By: | /s/ Xxxx Xxxxxxx | |
| Name: | Xxxx Xxxxxxx | |
| Title: | CEO | |
Signature Page to Asset Purchase Agreement
IN WITNESS WHEREOF, the parties have executed and caused this Asset Purchase Agreement to be executed and delivered on the date first above written.
| THE SELLER: | CELLVERA GLOBAL HOLDINGS LLC | |
| By: | /s/ Xxxx Xxxxxxx | |
| Name: | Xxxx Xxxxxxx | |
| Title: | Legal Representative | |
| CELLVERA DEVELOPMENT LLC | ||
| By: | /s/ Xxxx Xxxxxxx | |
| Name: | Legal Representative | |
| CELLVERA HOLDINGS LTD | ||
| By: | /s/ Xxxx Xxxxxxx | |
| Name: | Xxxx Xxxxxxx | |
| Title: | Legal Representative | |
| CELLVERA LTD | ||
| By: | /s/ Xxxx Xxxxxxx | |
| Name: | Xxxx Xxxxxxx | |
| Title: | Legal Representative | |
| THE SELLER OWNER: | AIPHARMA GROUP LTD | |
| By: | /s/ Xxxx Xxxxxxx | |
| Name: | Xxxx Xxxxxxx | |
| Title: | Legal Representative | |
Signature Page to Asset Purchase Agreement
ANNEX I
CERTAIN DEFINITIONS
Definitions. The following terms, as used in this Agreement, have the following meanings:
“Accounting Referee” has the meaning set forth in Section 2.4.
“Acquired Asset” has the meaning set forth in Section 1.1.
“Acquisition” has the meaning set forth in the Recitals.
“Affiliate” means, with respect to any Person, any other Person that, directly or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, such Person, and the term “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Person, whether through ownership of voting securities, by contract or otherwise. With respect to any natural person, “Affiliate” will include such person’s grandparents, any descendants of such person’s grandparents, such person’s spouse, the grandparents of such person’s spouse, and any descendants of the grandparents of such person’s spouse (in each case, whether by blood, adoption or marriage).
“Agility” means Agility The Public Warehousing KSCP and its affiliates.
“Agreement” has the meaning set forth in the Recitals.
“Annual Financial Statements” has the meaning set forth in Section 3.6.
“Assumed Liability” has the meaning set forth in Section 1.3.
“Business” has the meaning set forth in the Recitals.
“Business Day” means any day of the year on which national banking institutions in New York and the British Virgin Islands are open to the public for conducting business and are not required or authorized to close.
“Cash” means the amount of cash and cash equivalents determined in accordance with GAAP.
“Cellvera” has the meaning set forth in the in the introductory paragraph.
“Cellvera Products” means all derivatives, formulations or combinations that utilize any components of the anti-viral Favipiravir.
“Closing” has the meaning set forth in Section 7.1.
“Closing Date” has the meaning set forth in Section 7.1.
A-1
“Closing Date Debt” means the Debt of the Seller Owner and each Seller as of immediately prior to Closing.
“Closing Payment” has the meaning set forth in Section 2.2(c).
“Code” shall mean the Internal Revenue Code of 1986, as amended.
“Confidential Information” has the meaning set forth in Section 10.1.
“Consent” means any approval, consent, ratification, waiver or other authorization of any Person.
“Contract” means any contract, indenture, note, bond, lease, commitment, plan, arrangement, instrument or other agreement, in each case whether written or oral.
“Customer” has the meaning set forth in Section 10.3.
“Data Room” means the information and the documents referred to in the index of data room documents.
“Debt” means any indebtedness of a Person, (i) in respect of borrowed money or evidenced by bonds, notes, debentures, revolving credit facilities or other similar instruments or letters of credit (or reimbursement agreements in respect thereof) or banker’s acceptances or (ii) representing capitalized or synthetic lease obligations or the unpaid balance of the purchase price of any property or assets (including any earn-out, whether or not contingent) or (iii) any outstanding checks, drafts or overdrafts, (iv) the amount of all indebtedness of others secured by a Lien on any asset of such Person (whether or not such indebtedness is assumed by such Person), (v) amounts owed to creditors of Cellvera set forth on Schedule 3.28 (which Schedule may be updated prior to Closing and include any additional amounts that may arise to any creditor on or after the date hereof), (vi) any and all amounts owed by any Seller or Seller Owner to any of their Affiliates, including any shareholders of Seller Owner or any of their Affiliates, (vii) any obligations to shareholders or other equity holders, (viii) any debt that may be discoverable or appear on any lien or search of the Uniform Commercial Code (or other applicable Law) and (ix) to the extent not otherwise included, the amount of any indebtedness of any other Person guaranteed by such Person, and interest expense accrued but unpaid, income Tax obligations, investment banking fees, intercompany debt, and other expenses and any other Liabilities that are not directly related to the continuing operation of the Business, and all penalties, premiums, termination fees or breakage costs due upon or relating to the prepayment of any of such indebtedness, but the term “Debt” does not include Seller’s ordinary course accounts payable of the Business that are not yet overdue.
“Disclosure Schedules” means the disclosure schedules prepared by Seller and Seller Owner and attached to this Agreement, supplementing the representations and warranties regarding Seller, Seller Owner and GRA set forth in this Agreement.
“Environmental and Safety Requirements” means all federal, state, local and foreign statutes, Laws, rules, regulations, ordinances and other provisions having the force or effect of Law, all judicial and administrative orders and determinations, in each case concerning public health and safety, worker health and safety, any emission, discharge, generation, processing, storage, holding, abatement, existence, Release, threatened Release or transportation of any Hazardous Materials, pollution (or cleanup thereof) or protection of the environment (including ambient air, soil, surface water or groundwater, or subsurface strata), endangered or threatened species, or the protection of natural resources, including without limitation (i) CERCLA, the Solid Waste Disposal Act, as amended by the Resource Conservation and Recovery Act of 1976, as amended by the Hazardous and Solid Waste Amendments of 1984, 42 U.S.C. §§ 6901 et seq.; the Federal Water Pollution Control Act of 1972, as amended by the Clean Water Act of 1977, 33 U.S.C. §§ 1251 et seq.; the Toxic Substances Control Act of 1976, as amended, 15 U.S.C. §§ 2601 et seq.; the Emergency Planning and Community Right-to-Know Act of 1986, 42 U.S.C. §§ 11001 et seq.; the Clean Air Act of 1966, as amended by the Clean Air Act Amendments of 1990, 42 U.S.C. §§ 7401 et seq.; the Occupational Safety and Health Act of 1970, as amended, 29 U.S.C. §§ 651 et seq.; the Hazardous Materials Transportation Act, 49 U.S.C. § 5101 et seq.; the Federal Insecticide, Fungicide, and Rodenticide Act, 7 U.S.C. § 136 et seq.; the Safe Drinking Water Act, 41 U.S.C. §§ 300f et seq.; any foreign, state, county, municipal or local statues, laws or ordinances similar or analogous to the federal statutes listed above; any amendments to the statutes, laws or ordinances listed above, regardless of whether in existence on the date hereof; any rules, regulations, guidelines, directives, orders or the like adopted pursuant to or implementing the statutes, laws, ordinances and amendments above; and any other law, statute, ordinance, amendment, rule, regulation, guideline, directive, order or the like in effect now relating to environmental, health or safety matters, and (ii) all those relating to the presence, use, production, manufacture, generation, handling, holding, transport, treatment, storage, receipt, disposal, distribution, sale, labeling, testing, registration, recycling, processing, emission, discharge, treatment, existence, Release, threatened Release, control or cleanup of, or exposure to, any Hazardous Materials or relating to reporting, licensing, permitting, investigating and remediating emissions, discharges, or Releases of Hazardous Materials into the air, surface water, sediments, groundwater or land or human or worker health and safety (as they may be affected by the release of or exposure to Hazardous Materials).
A-2
“Excluded Asset” has the meaning set forth in Section 1.2.
“Ex-Im Laws” shall mean all Laws relating to export, reexport, transfer, and import of goods, services, information, and other items, including but not limited to the Export Administration Regulations, the International Traffic in Arms Regulations, trade sanctions and embargoes, Customers and tariffs, trade agreements, corruption, or other international trade issues, the Arms Export Control Act, Export Administration Act, the International Emergency Economic Powers Act, the regulations administered by the U.S. Treasury Department’s Office of Foreign Asset Controls, regulations administered by the Drug Enforcement Administration, regulations administered by the Bureau of Alcohol, Tobacco, and Fire Arms, regulations administered by the Food and Drug Administration, executive orders relating to international trade matters, and the customs and import Laws administered by U.S. Customs and Border Protection, as well as any and all other U.S. Laws, executive agency guidance, the Foreign Corrupt Practices Act, the U.K. Anti-Bribery Act, and other laws relating to importation and exportation, and trade sanctions.
“Expiration Date” has the meaning set forth in Section 8.1.
“Favipiravir” means the compound description more particularly described on Exhibit A.
“Feynman Labs” means Feynman Labs, LLC, Dubai (AE).
“Financial Statements” has the meaning set forth in Section 3.6.
“Fundamental Representation” has the meaning set forth in Section 9.1.
“Fuji” means Fujifilm Toyama Chemicals Co. Ltd.
“Fuji APA” means that certain Asset Purchase Agreement dated February 2, 2022, by and between Fuji and Seller Owner.
“GAAP” means generally accepted accounting principles in the United States as of the date hereof.
“Governmental Body” means any government or governmental or regulatory body thereof, or political subdivision thereof, whether federal, state, local or foreign, or any agency, commission, instrumentality or authority thereof; or any court or arbitrator (public or private).
“GRA” means G Response Aid FZCO, a Free Zone Company incorporated in the Jebel Ali Free Zone.
“GRA Assignment” has the meaning set forth in Section 7.2(r).
“GRA Financial Statements” has the meaning set forth in Section 4.4.
“GRA Interim Financial Statement” has the meaning set forth in Section 4.6.
“GRA Latest Balance Sheet” has the meaning set forth in Section 4.6.
“GRA Listed Intellectual Property” has the meaning set forth in Section 4.11.
“GRA Tangible Assets” has the meaning set forth in Section 4.9.
“GRA Membership Interest” means Cellvera Ltd’s membership interest in GRA, which represents 50% of the outstanding ownership interests in GRA.
“GRA Shareholders’ Agreement” means that certain Shareholders’ Agreement dated July 13, 2020, by and between SHIPA Investment LLC, AiNanolab FZ LLC and GRA, and all amendments, transfers and integrations thereto.
“GRA Term Sheet” means that certain legally binding term sheet dated March 3, 2023, by and among, Agility, Purchaser, GRA and Cellvera Ltd.
A-3
“Governing Documents” means, with respect to GRA, GRA’s certificate of formation, charter, bylaws, shareholders’ agreement, or other certificates, instruments, documents, or agreements adopted to govern the formation or internal affairs of the entity, as applicable, including any and all amendments or restatements to such documents.
“Hazardous Material” means any waste, substance, chemical, or material that (i) is defined, listed, identified, or regulated as “hazardous,” “acutely hazardous,” “toxic,” “dangerous,” a “pollutant,” a “contaminant” or words of similar import or regulatory effect which may give rise to liability or standards of conduct pursuant to, Environmental and Safety Requirements; (ii) requires investigation, removal or remediation under any Environmental and Safety Requirements; (iii) is toxic, explosive, corrosive, flammable, infectious, radioactive, carcinogenic, mutagenic, or otherwise hazardous and is regulated by any Governmental Body or Environmental and Safety Requirements; and (iv) is or contains pesticides, pollutants, contaminants, toxic chemicals, petroleum products or byproducts (including natural gas, gasoline, diesel fuel, oil, and other fuels and petroleum products or fractions thereof), asbestos or asbestos containing materials, urea formaldehyde foam insulation, polychlorinated biphenyls, poly- and perfluoroalkyl substances, gas or related materials, noise, odor, mold, radiation, radioactive materials, or radon.
“Healthcare Laws” means any domestic or foreign, federal, state, municipality or local law relating to the regulation, provision, performance, reporting, marketing, selling or administration of, or payment for, healthcare products or services, such as laboratory tests, including, without limitation (i) the federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.) (“FDCA”), the Public Health Service Act (42 U.S.C. § 201 et seq.) (“PHSA”), the Clinical Laboratory Improvement Amendments of 1988 (42 U.S.C. § 263a) (“CLIA”), the federal Anti-Kickback Statute (42 U.S.C. §1320a-7b(b)), Sections 1320a-7 and 1320a-7a of Title 42 of the United States Code, the Physician Self-Referral Law, commonly known as the “Xxxxx Law” (42 U.S.C. §§1395nn and 1396b), the False Statements Relating to Health Care Matters law (18 U.S.C. § 1035), Health Care Fraud (18 U.S.C. § 1347), the False Claims Act (31 U.S.C. § 3729 et seq.), Section 6032 of the Deficit Reduction Act of 2005 (codified at 41 U.S.C. § 1396(a)(68)), or any regulations and guidance documents promulgated pursuant to such statutes, and all similar state or local healthcare-related statutes, regulations and guidance documents, (ii) HIPAA, (iii) the 21st Century Cures Act, Public Law 114-255 (2016), its implementing regulations codified at 45 C.F.R. Parts 170 and 171, and all implementation guidance issued thereon by the Office of the National Coordinator for Health IT (“ONC”), (iv) Medicare (Title XVIII of the Social Security Act) and the regulations promulgated thereunder, (v) Medicaid (Title XIX of the Social Security Act) and the regulations promulgated thereunder as well as related state Medicaid statutes and regulations, (vi) TRICARE (10 U.S.C. Section 1071 et seq.) and the regulations promulgated thereunder, (vii) quality, facility, personnel, public health reporting and safety Laws, rules, regulations or guidance documents relating to the regulation, storage, provision, performance, reporting, marketing, selling or administration of, or payment for, healthcare products or services, (viii) Laws governing the provision of services to employees with workers compensation coverage or licensure or certification as a healthcare organization to provide such services, and (ix) licensure Laws relating to the regulation, provision, performance, reporting, marketing, selling or administration of, or payment for, healthcare items, products, services or goods and the ownership, operation or distribution of medical equipment, supplies or accessories, each of (i) through (ix) as amended from time to time.
“Indemnification Claim” has the meaning set forth in Section 9.5.
“Intellectual Property” means all intellectual property rights in respect of the following: (i) patents and applications therefor, including continuations, divisionals, continuations-in-part or reissues of patent applications and patents issuing thereon, docketed inventions, (ii) trademarks, service marks, trade names, service names, brand names, trade dress rights, logos, domain names, corporate names and all IP addresses and URL/Internet domain names, together with the goodwill associated with any of the foregoing, and all applications, registrations and renewals thereof; (iii) copyrights and registrations and applications therefor, works of authorship and mask work rights; and (iv) Software and Technology.
“Intellectual Property Assignment” has the meaning set forth in Section 6.2.
“Interim Financial Statements” has the meaning set forth in Section 3.6.
“Irish Release” has the meaning set forth in Section 7.2(v).
“IRS” means the Internal Revenue Service.
A-4
“Knowledge” means the knowledge of a particular fact or other matter that a Person will be deemed to have if: (a) that Person is actually aware of that fact or matter; (b) a reasonable Person could be expected to become aware of that fact or matter in the ordinary course of performing his relevant duties; or (c) a reasonable Person could be expected to become aware by performing a reasonable investigation (including asking those individuals responsible for the matters in question). The words “know,” “knowing” and “known” shall be construed accordingly. In the case of each Seller, “knowledge” means the knowledge of Xxxx Xxxxxxx xnd Xxxx X’Xxxxx.
“Law” means any domestic, foreign, federal, state, local law, statute, code, ordinance, rule, regulation, code, act, treaty or order of general applicability, including any rules and regulations promulgated thereunder.
“Leased Real Property” has the meaning set forth in Section 3.10.
“Legal Proceeding” means any judicial, administrative or arbitral actions, audits, claims, complaints, notices of violation, citations, notices of potential responsible party liability, grievances, suits, litigation, arbitrations, investigations, suits, inquiries, hearings or proceedings (public or private), in each case, whether civil, criminal or administrative, by or before, or otherwise involving, a Governmental Body.
“Liability” means any debt, liability or obligation (whether direct or indirect, absolute or contingent, accrued or unaccrued, liquidated or unliquidated, known or unknown or due or to become due) and including all costs and expenses relating thereto.
“Lien” means any lien, encumbrance, pledge, mortgage, deed of trust, security interest, claim, lease, charge, option, right of first refusal, easement, servitude or transfer restriction.
“Listed Intellectual Property” has the meaning set forth in Section 3.12.
“Loan Forgiveness Amount” means the amount set forth on Schedule 2.2(a), including any accrued and unpaid interest, fees or expenses, to forgive the loans Purchaser made to each Seller and Seller Owner pursuant to the Secured Credit Agreement.
“Losses” means losses, liabilities, obligations and damages (including consequential damages, lost profits, diminution in value and punitive damages) and any and all notices, actions, suits, proceedings, claims, demands, assessments, judgments, costs, penalties and expenses, including defense costs, e-discovery costs, amounts paid in settlement and reasonable attorneys’ and other professionals’ fees and disbursements incident to any and all Losses with respect to which indemnification is provided hereunder, including costs of enforcing rights to indemnification provided herein and expenses of Legal Proceedings.
“Marketing Authorizations” means:
(a) any and all licenses, registrations, authorizations, permits, exemptions, clearances, certificates, consents and approvals (including Marketing Authorizations, supplements and amendments and revisions, pre- and post- approvals, pricing and Third Party reimbursement approvals, and labelling approvals) of any Governmental Body, necessary or useful for the study, development (including the conduct of and the applications for clinical trials), distribution, marketing, promotion, offer for sale, use, import, reimbursement, export or sale of the Cellvera Products and GRA products;
(b) any pending application to the applicable Governmental Body for any of the items listed in paragraph (a) above, and all supplements and amendments filed with respect thereto that have been submitted to, but not yet approved by, the applicable Governmental Body; and
(c) product notifications not requiring pre-clearance by regulatory authorities and other clearance procedures required to lawfully market the any Cellvera Product or GRA product in the relevant jurisdictions.
A-5
“Material Adverse Effect” means any change or effect that, individually or in the aggregate (i) is or is like to be materially adverse to the business, condition (financial or otherwise), results of operations, prospects, properties or assets or Liabilities of Seller (including, specifically, the termination or cancellation, or other materially adverse alteration of its business relationship with the Business, of any customer or group of customers, of the Business representing revenues equal to or greater than 10% of the revenues of the Business (determined for the Business’ 2022 fiscal year)), in any case as over the five (5) year period following the Closing, except for any change or effect that arises out of (unless the following exceptions were the result of a change or effect that is not included in the following exceptions): (A) any change in conditions in the global economy, or (B) any change in the economic or business conditions affecting the industries in which Seller and the Subsidiaries conduct business (in each case that do not disproportionately affect Seller or the Subsidiaries); or (ii) materially and adversely affects the ability of the Seller Owner or Seller to consummate the transactions contemplated by this Agreement.
“Material Contracts” has the meaning set forth in Section 3.13.
“Net Sales” means the actual gross amounts invoiced and collected by Purchaser on all of its sales of Cellvera Products to third parties less only the following documented costs actually and reasonably paid or delivered to unaffiliated third parties and not refunded, credited back to, exchanged for other products or services:
(a) cost of raw materials for products and services sold (including cost of freight carriers for exporting products outside of the country or origin), but excluding general administrative costs);
(b) sales and excise taxes or other applicable taxes, value added taxes, and duties which fall due and are paid by purchase as a direct consequence of such sales and other governmental charges imposed upon the importation, use or sale of the product or service, but only to the extent that such taxes and duties are: (i) actually included and itemized in the gross amounts invoiced to and specifically paid by the purchaser over and above the usual selling price of the products, and (ii) customarily included and itemized in gross amounts invoiced to and specifically paid by the purchase over and above the usual selling price of all comparable products in the relevant market;
(c) trade, quantity and cash discounts that are of amounts customary in the pharmaceutical industry for companies of a similar size, revenue, geography and product line and that are actually allowed or accrued on the product or service;
(d) allowances or credits to customers on account of off-invoice promotional discounts, price and shelf adjustments, volume reimbursements, failure to supply, rejection, withdrawal, recall, or return of the product or on account of retroactive price reductions affecting the product or service, to the extent that such allowances or credits are of amounts customary in the pharmaceutical industry for companies of a similar size, revenue, geography, product line and service line and are actually allowed or accrued on the product or service;
(e) rebates, discounts and charge backs specifically related to the product or service on an actual credited, paid or accrued basis that are of amounts customary in the pharmaceutical industry for companies of a similar size, revenue, geography and product line, including, but not limited to, those granted to government agencies;
A-6
(f) services allowances (including, but not limited to, wholesalers’ fees for services) and independent broker or agents’ commissions, if any, allowed or paid that are of amounts customary in the pharmaceutical industry for compliance of a similar size, revenue, geography, product line and service line;
(g) any bona fide and regular credit note issued by Purchaser to customers (provided that upon payment or hypothecation of any amount of principal outstanding of such credit note, and/or interest thereon, such amount so received from such payment or hypothecation shall be included in Net Sales);
(h) royalties required to be paid to Fuji or any other owner or enforcer of intellectual property rights under any license agreement in respect of the product, excluding any future royalties to be paid to Purchaser as a result of its acquisition of certain intellectual property from Fuji pursuant to the Fuji APA;
(i) fees, commissions and costs required to be paid to distributors or sales agents in relation to the sale of Cellvera Products that are of amounts customary in the pharmaceutical industry for companies of a similar size, revenue, geography and product line of Purchaser; and
(j) fees, commissions and costs to be paid in relation to the factoring of any invoices in respect of the product that are amounts customary in the pharmaceutical industry for companies of a similar size, revenue, geography and product line of Purchaser.
Net Sales with respect to sales of Cellvera Products that are not made on an arm’s length basis or that are made for consideration other than cash shall be calculated based on the average per-unit Net Sales of the applicable Cellvera Product without regard to such non-arm’s length or non-cash sales and without regard to Cellvera Products given pro xxxx or for charitable or clinical use. If the Cellvera Product is bundled with other products, any discounts or other adjustments with respect to the products shall be allocated proportionally to the Cellvera Product at no greater than the non-bundled discount rate for the product. Cellvera Products given to third parties or for charitable use or given to third parties for the execution of clinical trials are excluded from Net Sales.
“OFAC” has the meaning set forth in Section 3.27.
“Order” means any order, injunction, judgment, decree, consent decree, ruling, writ, assessment or award of a Governmental Body.
“Organizational Documents” means, as applicable, the certificate or articles of incorporation, certificate or articles of formation or organization, as applicable, and bylaws, memorandum of association, articles of association, shareholder agreements, operating agreements, partnership agreements and any similar governing or constitutive documents or agreements of any Person, each as currently in effect.
“Payoff Letter and Release Agreements” has the meaning set forth in Section 7.2.
“Permits” means any Marketing Authorizations, approvals, authorizations, Consents, licenses, permits or certificates of, or registrations with, a Governmental Body necessary for the ownership or operation of the Acquired Assets, or the conduct of the Business.
“Person” means any individual, corporation, partnership, firm, joint venture, association, joint-stock company, trust, unincorporated organization, Governmental Body or other entity.
A-7
“Prohibited Territory” means the United States of America, Canada, Mexico, the UAE and any other country or territory in which an Acquired Asset is located or a sale of any Cellvera product.
“Purchase and Sale Agreement” has the meaning set forth in Section 7.2.
“Purchase Price” has the meaning set forth in Section 2.1.
“Purchaser” has the meaning set forth in the Recitals.
“Purchaser Documents” has the meaning set forth in Section 4.2.
“Purchaser Indemnified Parties” has the meaning set forth in Section 8.2.
“Reference Balance Sheet” has the meaning set forth in Section 3.6.
“Reference Balance Sheet Date” has the meaning set forth in Section 3.6.
“Restricted Business” has the meaning set forth in Section 10.3.
“Release” means any actual or threatened release, spill, emission, seep, leaking, pumping, pouring, injection, escaping, deposit, disposal, discharge, dispersal, dumping, leaching in or allowing to escape or migrate, into or through the environment (including ambient air (indoor or outdoor), surface water, groundwater, land surface or subsurface strata or within any building, structure, facility or fixture).
“Retained Liabilities” has the meaning set forth in Section 1.4.
“Schedule Supplement” has the meaning set forth in Section 10.8.
“Secured Credit Agreement” means that certain Secured Credit Agreement dated as of August 27, 2021, as amended by the First Amendment to Secured Credit Agreement, dated as of October 18, 2021, the Second Amendment to Secured Credit Agreement, dated as of October 27, 2021, the Third Amendment to Secured Credit Agreement, dated as of December 1, 2021, the Fourth Amendment to Secured Credit Agreement dated as of December 17, 2021, the Fifth Amendment to Secured Credit Agreement dated as of December 22, 2021, and the Sixth Amendment to Secured Credit Agreement dated as of December 28, 2021, the Forbearance Agreement and Seventh Amendment to Secured Credit Agreement dated as of February 13, 2022, and Forbearance Agreement and Eighth Amendment to Secured Credit Agreement dated as of March 31, 2022, by and between Purchaser, each Seller and Seller Owner.
“Seller” has the meaning set forth in the Recitals.
“Seller Documents” has the meaning set forth in Section 3.2.
“Seller Expenses” means the sum of the aggregate amount of all out-of-pocket fees and expenses, incurred by or on behalf of, or paid or to be paid by, any Seller or Seller Owner in connection with the negotiation, preparation or execution of this Agreement or any documents or agreements contemplated hereby or the performance or consummation of the transactions contemplated hereby, including (i) all brokers’ or finders’ fees, (ii) costs, fees, disbursements and expenses of counsel, advisors, consultants, investment bankers, accountants, auditors and experts, (iii) any fees and expenses associated with obtaining necessary or appropriate Consents of third parties (including any Governmental Body) on behalf of any Seller or Seller Owner, and (iv) any fees or expenses associated with obtaining the release and termination of any Liens.
“Seller Equity” has the meaning set forth in the recitals.
“Seller Owner” has the meaning set forth in the Recitals.
“Shares” means any and all shares, equity interests, equity participations or other equity equivalents (however designated) of capital stock of a corporation or of a company and any and all equity ownership interests in a Person other than a corporation, including units, membership interests, partnership interests, beneficial interests, and any and all warrants, options or other rights to purchase or acquire any of the foregoing.
A-8
“Socio-Economic Status” has the meaning set forth in Section 3.21.
“Software” means any and all (i) computer programs, including any and all software implementations of algorithms, models and methodologies, whether in source code or object code, (ii) databases and compilations, including any and all data and collections of data, whether machine readable or otherwise, (iii) descriptions, flowcharts and other work product used to design, plan, organize and develop any of the foregoing, screens, user interfaces, report formats, firmware, development tools, templates, menus, buttons and icons and (iv) all documentation including user manuals and other training documentation related to any of the foregoing.
“Spirit Complaint” means that certain complaint for breach of contract, breach of fiduciary duty, and breach of the implied covenant of good faith and fair dealing filed in the Superior Court of California on January 17, 2023, by Spirit Global Investments, Inc., a California corporation, against Cellvera and Purchaser.
“Supplier” has the meaning set forth in Section 10.3.
“Taxes” means (i) all federal, state, local or foreign taxes, charges, fees, imposts, levies or other assessments in the nature of taxes, including all income, alternative or add-on minimum tax, gross receipts, capital, sales, use, ad valorem, value added, transfer, franchise, profits, excess profits or windfall profits, inventory, capital stock, escheat, license, business organization, withholding, payroll, employment (including employee withholding or employer payroll tax, FICA or FUTA), social security, unemployment, excise, severance, stamp, healthcare or health insurance, occupation, real or personal property and estimated taxes, prohibited transactions, premiums and occupation taxes, customs, duties, fees, assessments and charges of any kind whatsoever in the nature of taxes, whether or not disputed; (ii) all interest, penalties, fines, additions to tax or additional amounts imposed by any Taxing Authority in connection with, resulting from or attributed to any item described in clause (i) whether or not disputed; and (iii) any Liability with respect to any items described in clauses (i) or (ii) payable by reason of Contract, assumption, transferee liability, operation of law, Treasury Regulation 1.1502-6 (or any predecessor or successor thereof or any analogous or similar provision of Law) or otherwise.
“Tax Return” means any return, report or statement required to be filed with respect to any Tax (including any elections, declarations, schedules or attachments thereto and any amendment thereof), including any information return, claim for refund, amended return or declaration of estimated Tax, and including, where permitted or required, combined, consolidated or unitary returns for any group of entities that includes Seller or any of its Affiliates.
“Taxing Authority” means the IRS and any other Governmental Body responsible for the imposition, collection or administration of any Tax or matters relating to Taxes.
“Technology” means, collectively, all designs, formulae, algorithms, procedures, methods, techniques, ideas, know-how, research and development, technical data, programs, subroutines, tools, materials, specifications, processes, engineering, product specifications, inventions (whether patentable or unpatentable and whether or not reduced to practice), apparatus, creations, improvements, works of authorship and other similar materials, and all recordings, graphs, drawings, reports, analyses, and other writings, and other tangible embodiments of the foregoing, in any form whether or not specifically listed herein, and all related technology.
“Transaction Documents” means, collectively, this Agreement, the Bill of Sale, and all other agreements or certificates delivered by any party hereto pursuant to the terms of this Agreement.
“Transactions” means the transactions contemplated by this Agreement and the other Transaction Documents.
A-9
Exhibit A
Compound Description
Name:
Favipiravir, T-705, and AVIGAN®
IUPAC Name (per EPA website):
5-flouro-2-oxo-1H-pyrazine-3-carboxamide
Chemical Name:
6-flouro-3-hydroxypyrazine-2-carboxamide
Chemical Abstracts number (CAS) Registry Number:
259793-96-9
Patent Reference:
Worldwide patent WO00/10569 dated March 2, 2000 (expired on August 18, 2019, except in Japan)
Molecular Formula:
C5H4FN3O2
Chemical Structure:
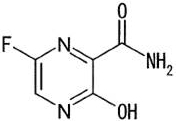
Exhibit A to Asset Purchase Agreement


