Intellectual Property License Agreement
Exhibit 10.6
Intellectual Property License Agreement
This Intellectual Property License Agreement (this “Agreement”) is entered into as of June 28, 2021 (“Effective Date”) between INVO Bioscience, Inc., a Nevada corporation, and Bio X Cell, Inc., a Massachusetts corporation (each a “Licensor” and together, “Licensors”), and Bloom INVO LLC, a Delaware limited liability company (“Licensee”). All defined terms used herein appear in Article 9.
Whereas, Licensors are medical device companies focused on developing and creating simplified, lower cost treatments for patients diagnosed with infertility; and
Whereas, Licensors have pioneered, tested, created and developed proprietary intravaginal culture medical devices (“INVOcell”), in vivo methods of vaginal incubation (“INVO Procedures”), and other related treatments and technologies to enhance reproductive success (together with the INVOcell and INVO Procedures, the “INVO Technologies”); and
Whereas, Licensors are, individually or collectively, the owners of certain IP related to the INVO Technologies, including the INVO IP, and desire to grant to Licensee a license to the INVO IP, under the terms and conditions set forth in this Agreement, in connection with the establishment of one or more INVO Clinics operating in the Field and in the Territory;
Now, Therefore, in exchange for good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, and incorporating the foregoing recitals, the parties agree as follows:
1. License
1.1 License Grant and Retention of Rights. Subject to the terms and conditions of this Agreement, Licensors (individually and collectively) hereby grant to Licensee, as of the Effective Date, a non-exclusive, royalty-free, perpetual, sublicensable (through multiple tiers), right and license to make, use, import, sell, offer for sale, have sold, and otherwise Commercialize the INVO IP in the Field in the Territory (collectively, the “License”).
1.2 Trademark Quality Control.
(a) Licensee shall not, and shall ensure that its sublicensee(s) shall not, reproduce or use any Trademarks licensed under Section 1.1 (“Licensed Trademarks”) in any manner whatsoever other than as expressly authorized by this Agreement, and all uses of the Licensed Trademarks will materially comply with any reasonable trademark guidelines that Licensors may provide from time to time.
(b) Licensee shall conduct its business, and shall ensure that its sublicensee(s) shall conduct their businesses, in a manner that will reflect positively on the Licensed Trademarks. Licensee shall use, and shall ensure that its sublicensee(s) shall use, the Licensed Trademarks in a manner that does not derogate Licensors’ respective rights in the Licensed Trademarks or the value of the Licensed Trademarks, and shall take no action that would interfere with, diminish or tarnish those rights or value. All Products that bear the Licensed Trademarks shall be of a quality at least as great as the quality of Licensors’ INVOcell prior to the Effective Date.
(c) Licensee will not use, and shall ensure that its sublicensee(s) shall not use, any Trademark confusingly similar to any of the Licensed Trademarks, without both Licensors’ consent. Licensee agrees, and shall ensure that its sublicensee(s) agree, not to register or attempt to register any Licensed Trademarks or any Trademark confusingly similar to any of the Licensed Trademarks, without both Licensors’ consent.
| 1 |
(d) Licensors will have the right to monitor Licensee’s and Licensee’s sublicensee(s)’ use of the Licensed Trademarks. Upon either Licensors’ reasonable request, but no more frequently than once per year collectively, Licensee shall, and shall ensure that its sublicensee(s) shall, submit to either or both Licensors copies of any materials bearing the Licensed Trademarks. If either or both Licensors discover any improper use of the Licensed Trademarks on any such submission and delivers a writing describing in detail the improper use to Licensee within seven (7) days after receiving such submission, Licensee shall, and shall ensure that its sublicensee(s) shall, promptly remedy such improper use.
1.3 Reservation of Rights. Except for those licenses and rights expressly granted in this Agreement, no license or right is granted by either party to the other party by implication, estoppel or otherwise. Without limiting the generality of the foregoing and for the avoidance of doubt, to the extent either party should wish to extend its rights beyond those rights as set forth in this Article 1, such party shall first seek the express written consent of the other party for such extension, which consent such other party may grant or deny in its sole discretion.
1.4 Ownership of INVO IP. Licensors shall retain their respective exclusive ownership of the INVO IP. Nothing in this Agreement transfers any ownership rights of such INVO IP. All use of any Licensed Trademarks by Licensee and its sublicensee(s), and all goodwill associated with such use, shall inure to the sole benefit of the Licensor that owns the respective Licensed Trademark. Except as expressly set forth in this agreement, Licensors shall not be required to file, maintain or otherwise prosecute or litigate the protection of any INVO IP other than in the sole and absolute discretion of Licensors. Licensee covenants and agrees, and shall ensure that its sublicensee(s) covenant and agree, not to challenge or assist any Person in challenging the validity, enforceability or other viability of any INVO IP.
2. IP Protection
2.1 Enforcement. Licensors shall have the right (but not the obligation), in its sole discretion, to enforce the INVO IP during the Term of this Agreement. In the event that any of the INVO IP are infringed by a third Person in the Field and one or more of Licensors institute an action or proceeding against such third Person to enforce the INVO IP, Licensee, at the sole cost and expense of Licensor, shall cooperate with and provide assistance reasonably requested by such Licensor(s) in furtherance of such action or proceeding.
2.2 Prosecution and Maintenance. Licensors shall take commercially reasonable efforts to protect and maintain the INVO IP. Each Licensor’s decision, which may be made in its sole discretion as to INVO IP for which it is the sole owner, to rely on unregistered forms of protection instead of registered forms of protection shall in all cases be deemed to be commercially reasonable under this Agreement; provided, however, that neither Licensor may abandon any INVO Registered IP for which such Licensor is the sole owner and which was registered as of the Effective Date, without first providing prior written notice to Licensee and offering to transfer and assign to Licensee all right, title and interest in and to such IP, subject to Licensee agreeing to any costs or fees associated with such IP, and subject to a perpetual, royalty-free, sublicensable, unlimited, non-exclusive license from Licensee back to the Licensors under such IP.
| 2 |
3.1 Confidential Information. Each party agrees to maintain all Confidential Information of the other party in confidence to the same extent that it protects its own similar Confidential Information, but not less than reasonable care, and to use such Confidential Information of the other party only as permitted under this Agreement. Each party agrees to take reasonable precautions to prevent any unauthorized disclosure or use of Confidential Information of the other party, including, without limitation, by disclosing such Confidential Information only to its employees, contractors, agents, or Licensee’s sublicensee(s): (a) with a need to know such information, (b) who are parties to appropriate agreements or confidentiality obligations sufficient to comply with this Section 3.1. In each case of a disclosure of Confidential Information from one Party to the other, the receiving Party will take appropriate steps to implement and enforce such non-disclosure/non-use obligations. The parties acknowledge and agree that Licensor may be required to file all or a portion of this Agreement and make filings with respect to it with the SEC, and such disclosure will not be deemed a violation of Licensor’s obligations hereunder.
3.2 Exclusions. The foregoing restrictions on disclosure and use will not apply with respect to any Confidential Information which: (a) was or becomes publicly known through no fault of the receiving party; (b) was rightfully known or becomes rightfully known to the receiving party without confidential or proprietary restriction from a source other than the disclosing party; (c) is documented by the receiving party as having been independently developed by the receiving party without the participation of individuals who have had access to the Confidential Information; (d) is approved by the disclosing party for disclosure without restriction in a written document signed by a duly authorized officer of such disclosing party; and (e) the receiving party is legally compelled to disclose, provided, however, prior to any such compelled disclosure, the receiving party will (i) assert the privileged and confidential nature of the Confidential Information against the third Person seeking disclosure and (ii) reasonably cooperate with the disclosing party in allowing the disclosing party to protect against any such disclosure and/or obtain a protective order narrowing the scope of such disclosure and/or use of the Confidential Information. In the event that such protection against disclosure is not obtained, the receiving party will be entitled to disclose the Confidential Information, but only as and to the extent reasonably necessary to legally comply with such compelled disclosure. Each party agrees that the terms and conditions of this Agreement will be treated as Confidential Information of the other party, provided that each party may disclose the terms and conditions of this Agreement: (1) as required by the applicable securities laws or (2) in confidence, to legal counsel, accountants, banks and financing sources and their advisors, and in connection with the enforcement of this Agreement or any rights hereunder.
4. Licensors’ Representations and Warranties; Disclaimer.
Licensors represent and warrant to Licensee that Licensors have full corporate power and authority to enter into this Agreement and to carry out their respective obligations hereunder. The execution and delivery by Licensors of this Agreement and the performance by Licensors of their obligations hereunder have been duly authorized by all requisite corporate or stockholder action on the part of each Licensor. This Agreement has been duly executed and delivered by Licensors, and constitutes a legal, valid and binding obligation of Licensors enforceable against Licensors in accordance with its terms, except to the extent that enforcement of the rights and remedies created thereby is subject to bankruptcy, insolvency, reorganization, moratorium and other similar laws of general application affecting the rights and remedies of creditors and to general principles of equity (regardless of whether enforceability is considered in a proceeding in equity or at law). EXCEPT FOR THE REPRESENTATIONS AND WARRANTIES EXPRESSLY SET FORTH HEREIN, LICENSORS MAKE NO FURTHER REPRESENTATIONS OR WARRANTIES OF ANY KIND WHATSOEVER TO THE OTHER PARTY OR ANY OTHER ENTITY.
| 3 |
5. Licensee Representations and Warranties; Disclaimers.
Licensee has full corporate power and authority to enter into this Agreement and to carry out its obligations hereunder. The execution and delivery by Licensee of this Agreement and the performance of its obligations hereunder have been duly authorized by all requisite company and member action on the part of such party. This Agreement has been duly executed and delivered by Licensee, and constitutes a legal, valid and binding obligation of Licensee enforceable against it in accordance with its terms, except to the extent that enforcement of the rights and remedies created thereby is subject to bankruptcy, insolvency, reorganization, moratorium and other similar laws of general application affecting the rights and remedies of creditors and to general principles of equity (regardless of whether enforceability is considered in a proceeding in equity or at law). EXCEPT FOR THE REPRESENTATIONS AND WARRANTIES EXPRESSLY SET FORTH IN THIS AGREEMENT, LICENSEE MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND WHATSOEVER TO THE OTHER PARTY OR ANY OTHER ENTITY.
Each party acknowledges and agrees that this Agreement represents the complete allocation of risks between the parties, it has voluntarily accepted all risks assigned to it herein, and the disclaimer of warranties and limitation of remedies herein form an essential basis of the bargain. TO THE EXTENT ALLOWED BY APPLICABLE LAW, IN NO EVENT SHALL A PARTY BE LIABLE TO THE OTHER PARTY OR ANY OTHER PERSON FOR CONSEQUENTIAL, INCIDENTAL, SPECIAL, EXEMPLARY, PUNITIVE, OR INDIRECT DAMAGES ARISING UNDER OR RELATED TO THIS AGREEMENT (INCLUDING LOST PROFITS, LOSS OF BUSINESS OR DATA, BUSINESS INTERRUPTION) EVEN IF SUCH PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES OR LOSSES.
7.1 Term; Termination. This Agreement will commence on the Effective Date and continue in perpetuity, unless terminated by the written agreement of all parties to this Agreement, or unless terminated earlier in accordance with the terms hereof (the “Term”). Either party shall have the right to terminate this Agreement if the other party is in material breach of any material term or condition of this Agreement and fails to cure such breach within one hundred eighty (180) days after receipt of written notice of such breach given by the non-breaching party.
7.2 Obligations Upon Termination. Upon termination of this Agreement, each party shall return to the other party any Confidential Information received hereunder for which the party’s rights do not survive, or destroy or purge its own system and files of any such Confidential Information (to the extent practicable) and deliver to the other party a written certificate signed by an officer of such party that such destruction and purging have been carried out; provided, however, that the receiving party may retain copies that are automatically retained as part of a computer back-up, recovery or similar archival or disaster recovery system, subject to the terms of this Agreement.
7.3 Survival. Articles 3-5, 6-8, and 9 shall survive termination or expiration of the Agreement.
| 4 |
8.1 Notices. Any notice, request, demand or other communication required or permitted hereunder will be in writing and deemed to be properly given upon the earlier of (a) the date of transmission when sent by electronic mail, return receipt requested during normal business hours; or (b) five (5) business days after deposit when mailed by certified mail, return receipt requested, or two (2) business days after being sent via private industry courier to the respective parties at the addresses set forth on the signature page hereto, which may be amended by a party upon five (5) days’ notice to the other party.
8.2 Assignment. Except as otherwise set forth herein with respect to sublicensees, Licensee shall not assign this Agreement, nor any of its rights or obligations hereunder, to any other Person without the prior written consent of both Licensors, which may be withheld for any reason in each Licensor’s sole discretion.
8.3 Governing Law. This Agreement is to be construed in accordance with and governed by the internal laws of the State of Delaware (“State”) without giving effect to any choice or conflict of law provision or rule (whether of the State or any other jurisdiction). Any legal suit, action or proceeding arising out of or relating to this Agreement will be commenced in federal court in the State, and each party hereto irrevocably submits to the exclusive jurisdiction and venue of any such court in any such suit, action or proceeding.
8.4 Waiver. The waiver by any party of a breach of or a default under any provision of this Agreement will be in writing and will not be construed as a waiver of any subsequent breach of or default under the same or any other provision of the Agreement, nor will any delay or omission on the part of a party to exercise or avail itself of any right or remedy that it has or may have hereunder operate as a waiver of any right or remedy.
8.5 Severability. If the application of any provision or provisions of this Agreement to any particular facts or circumstances is held to be invalid or unenforceable by any court of competent jurisdiction, then (a) the validity and enforceability of such provision or provisions as applied to any other particular facts or circumstances and the validity of other provisions of this Agreement will not in any way be affected or impaired thereby, and (b) such provision or provisions will be reformed without further action by the parties hereto and only to the extent necessary to make such provision or provisions valid and enforceable when applied to such particular facts and circumstances.
8.6 Relationship of the Parties. This Agreement, in and of itself, will not be construed as creating an agency, partnership, joint venture or any other form of association, for tax purposes or otherwise, between the parties.
8.7 Construction. This Agreement shall be construed as if jointly drafted by the parties hereto and no rule of construction or strict interpretation shall be applied against either party. The titles to Articles and headings of Sections contained in this Agreement, in any Schedule and in the table of contents to this Agreement have been inserted for convenience of reference only and shall not be deemed to be a part of or to affect the meaning or interpretation of this Agreement.
8.8 Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed to be an original, and which together will constitute one Agreement.
| 5 |
8.9 Entire Agreement. This Agreement constitutes the entire agreement between the parties concerning the subject matter hereof and supersedes all prior negotiations, conditions, agreements, or communications between them relating to the subject matter hereof. No amendment or modification of any provision of this Agreement will be effective unless in writing and signed by the Parties.
9. Definitions
Capitalized terms used in this Agreement will have the following meanings:
9.1 “Affiliate” as to a party means any entity controlled by, controlling or under common control with that party now or in the future (control shall be deemed to mean having a right to 50% of the entity’s profits or ownership of at least 50% of the voting rights in the entity).
9.2 “Clinical Trial” means a human clinical study conducted on sufficient numbers of human subjects that is designed to (a) establish that a pharmaceutical product or medical device is reasonably safe for continued testing; (b) investigate the safety and efficacy of the pharmaceutical product or medical device for its intended use, and to define warnings, precautions and adverse reactions that may be associated with the pharmaceutical product in the dosage range to be prescribed or the medical device; or (c) support regulatory approval of such pharmaceutical product or medical device or Label expansion of such pharmaceutical product or medical device.
9.3 “Confidential Information” means any information and/or materials disclosed by one party to the other in written, oral, graphic, electronic or other form that (a) is marked or identified in writing as confidential, proprietary, or with other marks indicative of a confidential nature, (b) if disclosed orally or in other intangible form or in any form that is not so marked, is identified as confidential (or other terms indicative of a confidential nature) at the time of such disclosure, or (c) includes, comprises or contains financial information, proposals, prospects or customer lists, research, development, testing data, samples, parts, pricing, computer programs (including source code), models, designs, unpublished patent applications, trade secrets, Know-how, formulas, processes, flow charts, techniques, ideas, inventions (whether patentable or not), schematics and other technical, business, financial and product development plans, forecasts, strategies, systems, works of authorship, projects, any other business, marketing, financial, technical, scientific, engineering, and other non-public information, or other information of the disclosing party that is disclosed in circumstances of confidence.
9.4 “Commercialization” means any and all activities of marketing, promoting, distributing, offering for sale or selling the Product in the Field in the Territory, including, for example, marketing, branding, pricing, distribution, sales, obtaining health insurance reimbursement coverage, market research, business analytics, pharmacovigilance and medical affairs activities (including conducting post-marketing clinical studies), pre-commercial launch market development activities conducted in anticipation of regulatory approval to sell or market the Product, seeking pricing and reimbursement approvals for the Product, preparing advertising and promotional materials, sales force training, and all interactions and correspondence with a regulatory authority regarding post-regulatory approval Clinical Trials. When used as a verb, “Commercialize” means to engage in Commercialization.
9.5 “Documentation” means with respect to the INVO Technologies, all documentation, including, but not limited to, user manuals, data sheets, schematics, test and verification plans and reports, application notes, design documents, programming guides, and other documents of either or both Licensors related to the technology useful to Commercialize the same.
| 6 |
9.6 “Ferring Agreement” means that certain Distribution Agreement by and among Ferring International Center S.A. and INVO Bioscience, Inc., and BIO X Cell, Inc., dated November 12, 2018 (together with any current and future amendments thereto).
9.7 “Field” means the Commercialization of the INVO Technologies and the Product through an INVO Clinic in the Territory.
9.8 “Governmental Authority” means any federal, state, local or foreign government or political subdivision thereof, or any agency or instrumentality of such government or political subdivision, or any self-regulated organization or other non-governmental regulatory authority or quasi-governmental authority (but only to the extent that the rules, regulations or orders of such organization or authority have the force of law), or any arbitrator, court or tribunal of competent jurisdiction.
9.9 “Intellectual Property” or “IP” means any and all of the following in any jurisdiction throughout the world: (a) patents and patent applications, (including all related continuations, continuations-in-part, divisionals, reissues, reexaminations, substitutions, renewals, extensions, nationalizations, validations, counterparts (domestic or foreign) or restorations of any of the foregoing (regardless of lapse, expiration or abandonment status), and other Governmental Authority-issued indicia of invention ownership (including certificates of invention, xxxxx patents, and patent utility models) together with industrial designs, registrations, applications for registration, and renewals thereof (“Patents”); (b) copyrights and works of authorship, whether or not copyrightable, and all registrations, applications for registration, and renewals of, any of the foregoing, and mask works, and all registrations, applications for registrations, and renewals thereof, including: computer aided design files including electrical schematics, xxxx of materials, layout files, gerber files, mechanical CAD files, and artwork files (“Copyrights”); (c) trademarks, service marks, brands, certification marks, logos, trade dress, trade names, and other similar indicia of source or origin, together with the goodwill connected with the use of and symbolized by, and all registrations, applications for registration, and renewals of, any of the foregoing (“Trademarks”); and (d) trade secrets, know-how, inventions (whether or not patentable), discoveries, improvements, technology, business and technical information, databases, data compilations and collections, tools, methods, processes, techniques, and other confidential and proprietary information (“Know-How”) Software.
9.10 “INVO Clinic” means any clinic, established in whole or in part by INVO Bioscience, Inc. (or an Affiliate thereof), that exclusively Commercializes the INVO Technologies in the Territory in order to Commercialize the Product.
9.11 “INVO IP” means all IP owned by Licensors, individually or collectively, as of the Effective Date and throughout the Term that relate to the INVO Technologies.
9.12 “INVO Registered IP” means all INVO IP that is subject to any issuance, registration, or application for registration by or with any Governmental Authority or authorized private registrar in any jurisdiction, including those specifically identified in Exhibit A.
9.13 “Label” means the approved display of written, printed or graphic matter either (a) on the immediate container, packaging or wrapper of an article or (b) inside a container, package or wrapper so long as it is easily legible through the outside container or wrapper.
9.14 “Person” means an individual, corporation, partnership, joint venture, limited liability company, Governmental Authority, unincorporated organization, trust, association or other entity.
| 7 |
9.15 “Product” means INVO’s proprietary intravaginal culture device known as INVOcell, together with any applicable accessories, including, without limitation, the retention device (each of which is more specifically described in Exhibit B), together with all current and future formulations, versions, improvements, modifications and presentations of such product by Licensors and their Affiliates from time to time.
9.16 “Software” means any and all of the following: (i) computer programs and other software, including software implementations of algorithms, models, and methodologies, whether in source code, object code, firmware or other form, including libraries, application programming interfaces, subroutines and other components thereof; (ii) computerized databases and other computerized compilations and collections of data or information, including all data and information included in such databases, compilations or collections; (iii) screens, user interfaces, command structures, report formats, templates, menus, buttons and icons; (iv) descriptions, flow-charts, architectures, development tools, specifications, protocols, and other materials used to design, plan, organize and develop any of the foregoing; (v) all Documentation, including development, diagnostic, support, user and training documentation related to any of the foregoing; and (vi) enhancements, updates, releases, upgrades, bug fixes, error corrections, patches, new versions, translations, conversions or other modifications or additions, as applicable, to any of the foregoing.
9.17 “Territory” means the State of Georgia.
[Signature Page Follows]
| 8 |
In Witness Whereof, the parties have caused this Agreement to be executed by duly authorized representatives of the parties as of the Effective Date.
| LICENSORS | LICENSEE | |||
| INVO Bioscience, Inc., a Nevada corporation | Bloom INVO LLC, a Delaware limited liability company | |||
| By: | /s/ Xxxxxx Xxxx | By: | /s/Xxx Xxxxx Xxxxxxxxx | |
| Name: | Xxxxxx Xxxx | Name: | Xxx Xxxxx Xxxxxxxxx, M.D. | |
| Title: | Chief Executive Officer | Title: | Chief Executive Officer | |
Address:
0000 Xxxxxxxxx Xxxxx Xxxxxxxx, Xxxxxxx 00000 Email: xxxxx@xxxxxxx.xxx |
Address:
Bloom INVO, LLC 0000 Xxxxxxxxx Xxxxx Xxxxxxxx, Xxxxxxx 00000 Email: xxxxx@xxxxxxx.xxx |
| Bio X Cell, Inc. | ||
| By: | ||
| Name: | Xxxxxx Xxxx | |
| Title: | Treasurer and Secretary | |
Address:
Bio X Cell, Inc. 0000 Xxxxxxxxx Xxxxx Xxxxxxxx, Xxxxxxx 00000 Email: xxxxx@xxxxxxx.xxx |
| 9 |
Exhibit A
Schedule of INVO Registered IP
Patents and Patent Applications
| No. | Title | Xxx./Pub. No. | App. No. | Status | Jurisdiction | |||||
| 1. | Incubation and/or storage container system and method | 7,759,115 | 10/360,630 | Active | US | |||||
| 2. | Intravaginal culture incubation container and method | US 2021-0145560 | 16/949,960 | Pending | US | |||||
| 3. | Improved Incubation and/or Storage Container System and Method | N/A | 62/938,122 | Expired | US | |||||
| 4. | IVC Container and Method | N/A | 62/938,154 | Expired | US | |||||
| 5. | Improved intravaginal culture incubation container and method | WO 2021/102473 | PCT/US2021/013798 | Pending | PCT |
Registered Trademarks
| No. | Xxxx | Jurisdiction | Status | Holder | App. No. | Reg. No. | App. Date. | |||||||
| 1 | INVO BIOSCIENCE | US | Active | INVO Bioscience, Inc. | 77613704 | 4009828 | 11/13/2008 | |||||||
| 2 | INVOCELL | IS | Pending | INVO Bioscience, Inc. | V0118990 | N/A | 10/11/2020 | |||||||
| 3 | INVOCELL | IL | Pending | INVO Bioscience, Inc. | 332248 | N/A | 8/5/2020 | |||||||
| 4 | INVOCELL | CA | Pending | INVO Bioscience, Inc. | 2056336 | N/A | 8/5/2020 | |||||||
| 5 | INVOCELL | WO | Active | INVO Bioscience, Inc. | 1553631 | 1553631 | 8/5/2020 | |||||||
| 6 | INVOBABY | US | Pending | INVO Bioscience, Inc. | 88804749 | N/A | 2/20/2020 | |||||||
| 7 | INVOCELL | US | Active | INVO Bioscience, Inc. | 88797622 | 6146631 | 2/14/2020 | |||||||
| 8 | INVOCELL | IS | Active | INVO Bioscience, Inc. | V0115473 | V0115473 | 12/1/2019 | |||||||
| 9 | INVO | IS | Pending | INVO Bioscience, Inc. | V0115405 | N/A | 11/24/2019 | |||||||
| 10 | INVOcell | JO | Active | INVO Bioscience, Inc. | JOT1152758 | 169758 | 11/6/2019 | |||||||
| 11 | INVO | JO | Active | INVO Bioscience, Inc. | JOT1152757 | 169820 | 11/6/2019 | |||||||
| 12 | INVO | WO | Active | INVO Bioscience, Inc. | 1499563 | 1499563 | 10/10/2019 | |||||||
| 13 | INVOCELL | WO | Active | INVO Bioscience, Inc. | 1500083 | 1500083 | 10/10/2019 | |||||||
| 14 | INVO | IL | Active | INVO Bioscience, Inc. | 322569 | 322569 | 10/10/2019 | |||||||
| 15 | INVOCELL | IL | Active | INVO Bioscience, Inc. | 322763 | 322763 | 10/10/2019 | |||||||
| 16 | INVO | CA | Pending | INVO Bioscience, Inc. | 1997132 | N/A | 10/10/2019 | |||||||
| 17 | INVOCELL | CA | Pending | INVO Bioscience, Inc. | 1998355 | N/A | 10/10/2019 | |||||||
| 18 | INVO CENTER | US | Pending | INVO Bioscience, Inc. | 88564596 | N/A | 8/2/2019 | |||||||
| 19 | INVO | US | Active | INVO Bioscience, Inc. | 77613693 | 4009827 | 11/13/2008 | |||||||
| 20 | INVOCELL | US | Active | INVO Bioscience, Inc. | 77180109 | 3757982 | 5/14/2007 | |||||||
| 21 | INVO | BR | Active | INVO Bioscience, Inc. | 918229723 | 918229723 | 09/17/2019 | |||||||
| 22 | INVO | AR | Active | INVO Bioscience, Inc. | 3844974 | 3155564 | 10/18/2019 | |||||||
| 23 | INVOCELL | BR | Active | INVO Bioscience, Inc. | 918229790 | 918229790 | 09/17/2019 | |||||||
| 24 | INVOCELL | AR | Pending | INVO Bioscience, Inc. | 3916439 | N/A | 07/22/2020 | |||||||
| 25 | INVOCELL | BR | Pending | INVO Bioscience, Inc. | 501553631 | N/A | 08/05/2020 | |||||||
| 26 | INVOcell | AR | Pending | INVO Bioscience, Inc. | 3844977 | N/A | 10/18/2019 |
Registered Copyrights
None.
Domain Names
None.
| 10 |
Exhibit B
Product Description
The INVOcell Culture Device (FG-003) is a two-part assembly enclosed in two separate packages. The first package contains the first part, including the inner chamber coupled with a cap (see Figures 1-3). The second package contains the second part, including the top and bottom parts of the outer vessel (see Figure 4).
The inner chamber holds culture medium, eggs and sperm, or ICSI fertilized embryos. In an INVOcell procedure, the inner chamber is placed into the outer vessel, which provides additional resistance to contamination. Following the loading of gametes or embryos, the INVOcell Culture Device is assembled and placed in the vaginal cavity for 72 hours (3-days) to allow for embryo development.
FIGURE 1: INNER CHAMBER (contents of the first package)
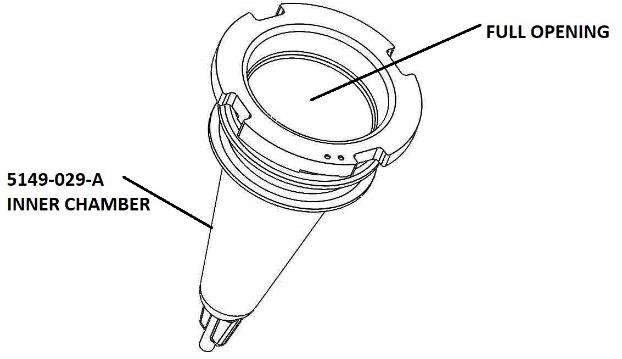
With the Cap off the Inner Chamber (5149-029-A) the entire center section is open to load the contents. See Figure 1, above. After the contents are loaded, line up the tabs in the Cap (5149-030-A) with the slots on the top of the Inner Chamber (5149-029-A) and press the Cap (5149-030-A) on as illustrated in Figure 2, below.
| 11 |
FIGURE 2: CAP AND INNER CHAMBER (contents of the first package)
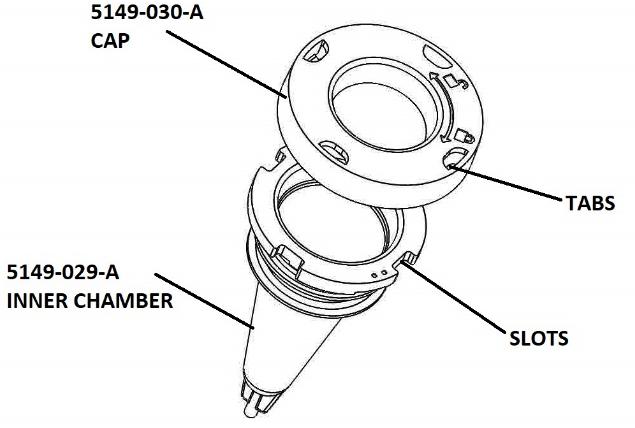
With the Cap (5149-030-A) pressed firmly onto the Inner Chamber (5149-029-A) the tabs will be aligned with the slots and the Cap can then be rotated into a locked position, thereby securing the Cap to the Inner Chamber as shown below in Figure 3.
FIGURE 3: CAP COUPLED WITH INNER CHAMBER (contents of the first package)
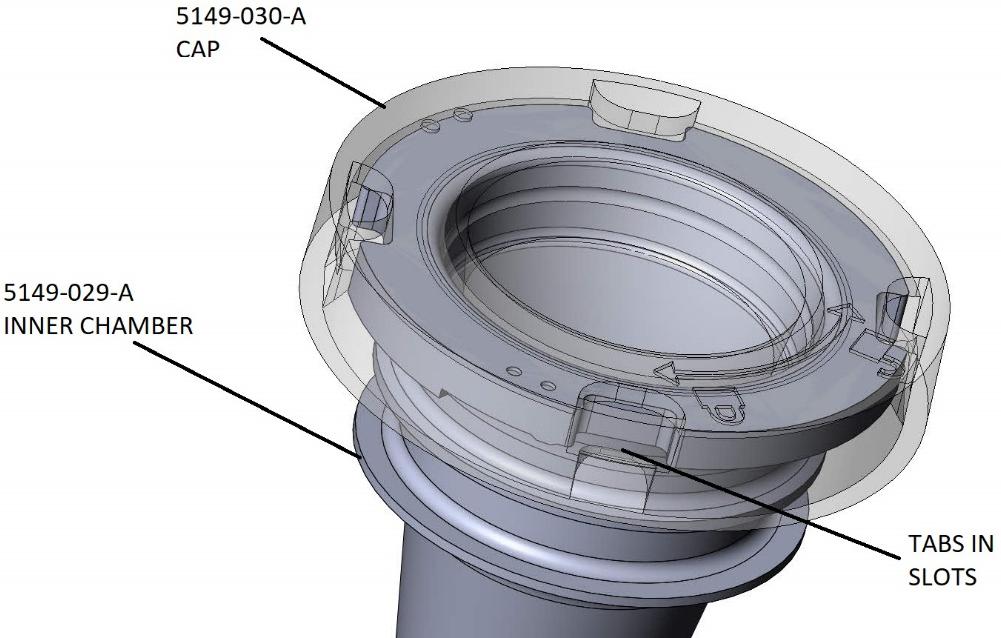
| 12 |
FIGURE 4: OUTER RIGID SHELL FOR CELL CULTURE CONTAINER (contents of the second package)
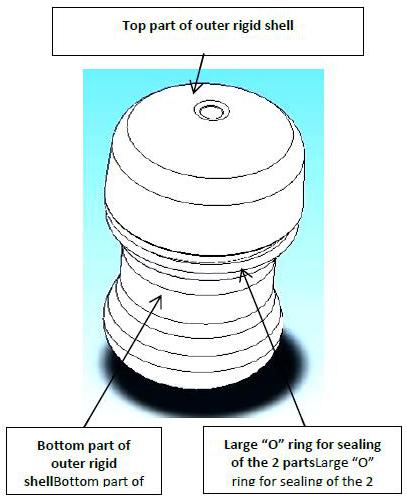
In addition to the outer vessel (referred to in Figure 4 as “outer rigid shell”), the INVOcell Intravaginal Culture System also includes a Retention Device (P-017). The Retention Device is a single-use, medical grade silicone device that is similar in shape and material to a diaphragm or menstrual cup. The Retention Device has holes to allow for natural drainage of vaginal fluids. The Retention Device is placed into the vaginal cavity with the INVOcell Culture Device to ensure that the INVOcell Culture Device is retained in the vaginal cavity for 72 hours (3-days). The retention device comes in 1 size: 70mm.
| 13 |
