LICENSE AND COLLABORATION AGREEMENT
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Exhibit 10.4
LICENSE AND COLLABORATION AGREEMENT
This License and Collaboration Agreement (this “Agreement”) is made as of April 21, 2017 (the “Effective Date”), by and between Paratek Bermuda Ltd. a corporation organized and existing under the laws of Bermuda, located at ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇ 11, Bermuda, (“Paratek”), and Zai Lab (Shanghai) Co., Ltd., an exempted company organized and existing under the laws of P.R. of China, located at ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇, Zhangjiang ▇▇-▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ (“Zai”). Paratek and Zai are referred to in this Agreement individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Paratek is a pharmaceutical company specializing in anti-infective drug development, and Paratek and its Affiliates own or control rights to the Compound and Licensed Product (as defined herein);
WHEREAS, Zai is a pharmaceutical company having experience in the development, manufacture and commercialization of pharmaceutical products in the Territory;
WHEREAS, Zai is prepared to develop and commercialize the Licensed Product in the Territory, providing it receives supporting materials such as clinical trial data, regulatory submissions, and starting materials that may allow for earlier market entry and market exclusivity of the Licensed Product compared to competitors;
WHEREAS, Paratek wishes to have Licensed Product developed and commercialized in the Territory, and is prepared to provide supporting materials such as clinical trial data, regulatory submissions, and starting materials to Zai, which may allow for earlier market entry and market exclusivity for the Licensed Product compared to competitors.
WHEREAS, Paratek wishes to grant to Zai, and Zai wishes to be granted, an exclusive license under Paratek’s rights to Develop, Manufacture and Commercialize (each as defined herein) the Licensed Product in the Field in the Territory (each as defined herein) in accordance with the terms and conditions set forth below.
AGREEMENT
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants contained herein, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
EXECUTION COPY
CONFIDENTIAL
ARTICLE 1
DEFINITIONS
Unless specifically set forth to the contrary herein, the following terms, whether used in the singular or plural, will have the respective meanings set forth below:
1.1. “Activity Target” will have the meaning set forth in Section 5.3.
1.2. “Activity Target Deadline” will have the meaning set forth in Section 5.3.
1.3. “Adverse Event” means any unwanted or harmful medical occurrence in a patient or subject who is administered a Licensed Product, whether or not considered related to such Licensed Product, including any undesirable sign (including abnormal laboratory findings of clinical concern), symptom or disease temporally associated with the use of such Licensed Product.
1.4. “Affiliate” means, with respect to a Party, any entity that directly or indirectly controls, is controlled by or is under common control with such Party. As used in this Section 1.4, “Control” (and, with correlative meanings, the terms “controlled by” and “under common control with”) means, in the case of a corporation, the ownership of 50% or more of the outstanding voting securities thereof or, in the case of any other type of entity, an interest that results in the ability to direct or cause the direction of the management and policies of such party or the power to appoint 50% or more of the members of the governing body of the party or, where ownership of 50% or more of such securities or interest is prohibited by law, ownership of the maximum amount legally permitted.
1.5. “Agreement” will have the meaning set forth in the introduction to this agreement.
1.6. “Alliance Manager” will have the meaning set forth in Section 3.1.
1.7. “Anti-Corruption Laws” will have the meaning set forth in Section 11.6(a)(i).
1.8. “Applicable Laws” means all statutes, ordinances, regulations, rules or orders of any kind whatsoever of any Governmental Authority that may be in effect from time to time and applicable to the activities contemplated by this Agreement.
1.9. “Biodefense” means a use related to the defense from Biothreat Agents.
1.10. “Biothreat Agent” means (a) pathogens that cause a high rate of illness in people exposed, result in a high rate of mortality, have a short incubation period, and have a limited number of persons with immunity, or (b) a bacterium, virus, protozoan, parasite, or fungus that can be used as a weapon in biological warfare.
1.11. “Business Day” means a day other than Saturday, Sunday or any day on which banks located in the United States or the PRC are authorized or obligated to close. Whenever this Agreement refers to a number of days, such number will refer to calendar days unless Business Days are specified.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
2
1.12. “Calendar Quarter” means the respective periods of three consecutive calendar months ending on March 31, June 30, September 30 and December 31.
1.13. “Calendar Year” means each 12 month period commencing on January 1.
1.14. “CFDA” means the China Food and Drug Administration, and local counterparts thereto, and any successor agency(ies) or authority thereto having substantially the same function.
1.15. “cGMP” means all applicable current Good Manufacturing Practices including, as applicable, (a) the principles detailed in the U.S. Current Good Manufacturing Practices, 21 C.F.R. Parts 4, 210, 211, 601, 610 and 820, (b) European Directive 2003/94/EC and Eudralex 4, (c) the principles detailed in the ICH Q7 guidelines, and (d) the equivalent Applicable Laws in any relevant country or region, each as may be amended and applicable from time to time.
1.16. “Clinical Trial” means any clinical testing of Licensed Product in human subjects.
1.17. “Clinical Trial Material” means Licensed Product and placebo for administration to humans in a Clinical Trial.
1.18. “CMC” means data, information, or procedures (as applicable) relating to the composition, Manufacture, or control of the Compound or Licensed Product, which may be requested or required by a Regulatory Authority for Regulatory Approval, including but not limited to data, information, and procedures relating to structure, Manufacturing process, validation, characterization, container closure systems, stability, quality, and purity.
1.19. “Combination Product” mean (a) any single product comprising both (i) a Compound and (ii) one or more other therapies or pharmaceutically active compounds or substances and do not require the use of any Paratek Technology; (b) any sale of a Licensed Product with another therapy(ies) or product(s) for a single invoice price; or (c) any sale of a Licensed Product as part of a bundle with other therapy(ies), product(s) or service(s) (i.e., where a Licensed Product and such other therapy(ies), product(s) or service(s) are sold for a single invoice price or where a discount, rebate or other amount that reduces the price of a Licensed Product is provided in exchange for (or otherwise conditioned upon) the purchase of such other therapy(ies), product(s) or services), to the extent not described in clause (a) or (b). The Compound portion of any Combination Product shall be deemed the “Licensed Component” and the other portion of such Combination Product the “Other Component”, and each Combination Product shall be deemed a Licensed Product hereunder.
1.20. “Commercialization” or “Commercialize” means all activities directed to marketing, distribution, detailing or selling of pharmaceutical products (including manufacturing, importing and exporting activities in connection therewith).
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
3
1.21. “Commercialization Plan” means the written plan for the Commercialization of the Licensed Product.
1.22. “Commercially Reasonable Efforts” means the use of diligent, good faith efforts and resources, in an active and ongoing program, as normally used by a similarly situated company for a product discovered or identified internally that is important to such company’s overall strategy or objectives, which product is at a similar stage in its development or product life and is of similar market potential and intellectual property protection, [*]; and in no event will such efforts and resources be less than the applicable Party would apply to achieve its own high priority goals. Commercially Reasonable Efforts requires that a Party, at a minimum, assign responsibility for such obligations to qualified employees, set annual goals and objectives for carrying out such obligations, and allocate adequate resources designed to meet such goals and objectives, in each case, in order to develop the Licensed Product as an active and ongoing program, and obtain Regulatory Approval for the Licensed Product in the Territory in an expeditious manner. Additionally, Commercially Reasonable Efforts requires [*] such efforts and resources as described above [*] for the Licensed Product, which includes [*] for the Licensed Product [*].
1.23. “Compound” means (i) omadacycline having the chemical structure set forth in Schedule 1.23, (ii) a prodrug or metabolite of the compound specified in (i), and (iii) any salt or polymorph of the compound specified in (i).
1.24. “Confidential Information” means all confidential information of the Disclosing Party or its Affiliates, regardless of its form or medium as provided to the Receiving Party or its Affiliates in connection with this Agreement; provided that, Confidential Information will not include any information that the Receiving Party can show by competent evidence: (a) is already known to the Receiving Party at the time it is disclosed to the Receiving Party by the Disclosing Party without an obligation of confidentiality and not through a prior disclosure by the Disclosing Party, (b) is or becomes generally known to the public through no act or omission of the Receiving Party in violation of the terms of this Agreement, (c) has been lawfully received by the Receiving Party from a Third Party without restriction on its disclosure and without, to the knowledge of the Receiving Party, a breach by such Third Party of an obligation of confidentiality to the Disclosing Party, or (d) has been independently developed by the Receiving Party without use of or reference to the Confidential Information of the Disclosing Party. The terms of this Agreement shall be the Confidential Information of both Parties.
1.25. “Continuing Technology Transfer” will have the meaning set forth in Section 4.1.
1.26. “Control” or “Controlled” means, with respect to any Know-How, Patents or other intellectual property rights, that a party has the legal authority or right (whether by ownership, license or otherwise) to grant a license, sublicense, access or right to use (as applicable) under such Know-How, Patents, or other intellectual property rights, on the terms and conditions set forth herein, in each case without breaching the terms of any agreement with a Third Party.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
4
1.27. “Develop” or “Development” or “Developing” means research, discovery, and preclinical and clinical drug or biological development activities, including test method development and stability testing, toxicology, formulation, quality assurance/quality control development, statistical analysis, preclinical and clinical studies and regulatory affairs, approval and registration.
1.28. “Development Plan” will have the meaning set forth in Section 5.2.
1.29. “Disclosing Party” will have the meaning set forth in Section 10.1(a).
1.30. “Dispute” will have the meaning set forth in Section 15.1.
1.31. “Effective Date” will have the meaning set forth in the introduction in this Agreement.
1.32. “Executive Officers” will have the meaning set forth in Section 3.2(f).
1.33. “Exploit” or “Exploitation” or “Exploiting” means to use, Develop and Commercialize, including to have Developed and have Commercialized, and to Manufacture and to have Manufactured to support the foregoing.
1.34. “Field” means, except for Biodefense, all human therapeutic and preventative uses.
1.35. “First Commercial Sale” means, with respect to any Licensed Product, the first arm’s length sale of such Licensed Product to a Third Party in a region of the Territory by Zai, its Affiliate(s) or Sublicensee(s) for use or consumption in such region following Regulatory Approval. Sales prior to receipt of marketing and pricing approvals, such as so-called “treatment IND sales,” “named patient sales” and “compassionate use sales” and any sales to any government, foreign or domestic, including purchases for immediate sale and/or stockpiling purposes, are not a First Commercial Sale in that region.
1.36. “FTE” means the equivalent of the work of a full-time individual for a 12 month period.
1.37. “FTE Rate” means a rate of [*] per FTE per year, to be pro-rated on a hourly basis of [*] per FTE per hour, assuming [*] hours per year for an FTE.
1.38. “GAAP” means United States generally accepted accounting principles, consistently applied.
1.39. “GCP” means all applicable Good Clinical Practice standards for the design, conduct, performance, monitoring, auditing, recording, analyses and reporting of clinical trials, including, as applicable (a) as set forth in the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Harmonized Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) and any other guidelines for good clinical practice for trials on medicinal products in the Territory, (b) the Declaration of
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
5
Helsinki (2004) as last amended at the 52nd World Medical Association in October 2000 and any further amendments or clarifications thereto, (c) U.S. Code of Federal Regulations Title 21, Parts 50 (Protection of Human Subjects), 56 (Institutional Review Boards) and 312 (Investigational New Drug Application), as may be amended from time to time, and (d) the equivalent Applicable Laws in the region in the Territory, each as may be amended and applicable from time to time and in each case, that provide for, among other things, assurance that the clinical data and reported results are credible and accurate and protect the rights, integrity, and confidentiality of trial subjects.
1.40. “GLP” means all applicable Good Laboratory Practice standards, including, as applicable, as set forth in the then current good laboratory practice standards promulgated or endorsed by the U.S. Food and Drug Administration as defined in 21 C.F.R. Part 58, or the equivalent Applicable Laws in the region in the Territory, each as may be amended and applicable from time to time.
1.41. “Governmental Authority” means any court, commission, authority, department, ministry, official or other instrumentality of, or being vested with public authority under any law of, any country, region, state or local authority or any political subdivision thereof, or any association of countries.
1.42. “GSP” means all applicable Good Supply Practice standards, including, as applicable, as set forth in the then current good supply practice standards promulgated or endorsed by the CFDA as defined in Good Supply Practice for Pharmaceutical Products or the equivalent Applicable Laws in the region in the Territory, each as may be amended and applicable from time to time.
1.43. “[*]” will have the meaning set forth in Section [*].
1.44. “Imported Product Agreement” will have the meaning set forth in Section 7.1.
1.45. “IND” means an investigational new drug application or equivalent application filed with the applicable Regulatory Authority, which application is required to commence Clinical Trials in the applicable country.
1.46. “Indemnifying Party” will have the meaning set forth in Section 12.3.
1.47. “Indemnitee” will have the meaning set forth in Section 12.3.
1.48. “Initial Development Plan” will have the meaning set forth in Section 5.2.
1.49. “Initial Technology Transfer” will have the meaning set forth in Section 4.1.
1.50. “Invention” will mean any process, method, composition of matter, article of manufacture, discovery or finding, patentable or otherwise, that is invented as a result of a Party exercising its rights or carrying out its obligations under this Agreement, including all rights, title and interest in and to the intellectual property rights therein.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
6
1.51. “IP Transfer Agreement” means the Intellectual Property Transfer Agreement between Paratek Pharmaceuticals, Inc. and Paratek Bermuda Ltd. dated June 6, 2016, as amended by the First Amendment dated February 27, 2017 and as may be further amended from time to time.
1.52. “Joint Development Committee” or “JDC” will have the meaning set forth in Section 3.3(b)(i).
1.53. “Joint Inventions” will have the meaning set forth in Section 13.1(b).
1.54. “Joint Patents” will have the meaning set forth in Section 13.1(b).
1.55. “Joint Steering Committee” or “JSC” will have the meaning set forth in Section 3.2(a).
1.56. “Know-How” means any proprietary scientific or technical information, results and data of any type whatsoever, in any tangible or intangible form whatsoever, including databases, safety information, practices, methods, techniques, specifications, formulations, formulae, knowledge, know-how, skill, experience, test data including pharmacological, medicinal chemistry, biological, chemical, biochemical, toxicological and clinical test data, analytical and quality control data, stability data, studies and procedures, and manufacturing process and development information, results and data.
1.57. “Licensed Product” means any pharmaceutical product containing the Compound, either alone or in combination with other active ingredients.
1.58. “Losses” will have the meaning set forth in Section 12.1.
1.59. “Manufacture” or “Manufacturing” or “Manufactured” means all operations involved in the manufacturing, filling and finishing, quality control testing (including in-process, release and stability testing, if applicable), storage, releasing and packaging.
1.60. “Material Sublicense” means a sublicense granted, or desired to be granted, by Zai to (a) [*], but not [*], or (b) [*], and/or [*].
1.61. “Material Sublicensee” means a Third Party, or Affiliates granted, or for which Zai desires to grant, a Material Sublicense.
1.62. “Materials” means reference and starting materials including the active pharmaceutical ingredient (API) or other materials as may be defined by the Parties.
1.63. “Milestone Event” will have the meaning set forth in Section 9.3.
1.64. “Milestone Payment” will have the meaning set forth in Section 9.3.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
7
1.65. “Net Sales” means the gross price billed or invoiced on sales of the Licensed Product by Zai, its Affiliates, or Sublicensees for sale of the Licensed Product to a Third Party in the Territory, less:
(a) freight expense (actual), including insurance, to the extent it is not charged to or reimbursed by the customer, [*];
(b) cash, trade or quantity discounts actually granted and deducted solely on account of sales of the Licensed Product;
(c) rebates actually paid to individual or group purchasers of the Licensed Product that are solely on account of the purchase of such Licensed Product;
(d) credits issued for the Licensed Product recalled or not accepted by customers or other refunds, allowances and chargebacks related to the Licensed Product;
(e) Taxes (including, but not limited to sales, value added, consumption and similar taxes; but excluding income taxes) actually incurred, paid or collected and remitted to the relevant tax authority for the sale of the Licensed Product; and
(f) other similar or customary deductions taken in the ordinary course of business or in accordance with GAAP;
Each of the amounts set forth above will be determined from the books and records of Zai, its Affiliate or Sublicensee, maintained in accordance with GAAP or in the case of Sublicensees, such similar accounting principles, consistently applied.
The transfer of a Licensed Product to an Affiliate, Sublicensee, or other Third Party (w) in connection with the research, development or testing of a Licensed Product (including, without limitation, the conduct of clinical studies), (x) for purposes of distribution as promotional samples, (y) for indigent or similar public support or compassionate use programs, or (z) by and between Zai and its Affiliates or Sublicensees will not, in any case, be considered a Net Sale of a Licensed Product under this Agreement.
Net Sales will also include and be deemed to have been made with respect to any Licensed Products used by Zai or any Affiliate, for its own commercial purposes, or transferred to any Third Party for less than the transferee is then charging in normal arms-length sales transactions; and Net Sales in all such cases will be deemed to have been made at the prices therefor at which such Licensed Products are then being sold to the customers of such user or transferor (or of Zai, if an Affiliate is a user but not a seller) in arms-length sales transactions. For clarity, in the event the Product is sold in an arms-length transaction to a governmental agency, a group purchase entity and/or any other entity having the bargaining power to negotiate the purchase price below normal retail price in transactions of lesser volume, Net Sales shall be calculated based on the actual price negotiated and agreed to for such agency and/or entity and not be based on the price charged in other arms-length sales transactions.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
8
If Zai or any of its Affiliates, or Sublicensees, sells a Licensed Product as a Licensed Component of a Combination Product in the Territory in any Calendar Quarter, then Net Sales will be calculated by multiplying the Net Sales of the Combination Product during such Calendar Quarter by the fraction A/(A+B), where A is the average Net Sales per unit sold of the Licensed Component when sold separately in the Territory during such Calendar Year (calculated by determining the Net Sales of the Licensed Component during such Calendar Quarter in accordance with the definition of Net Sales set forth herein and dividing such Net Sales by the number of units of the Licensed Component during such Calendar Quarter) and B is the average Net Sales per unit sold of the Other Component(s) included in the Combination Product when sold separately during such Calendar Quarter (calculated by determining the Net Sales of such Other Component(s) sold during such Calendar Quarter by applying the definition of Net Sales set forth herein as if it applied to sales of such Other Component(s) and dividing such Net Sales by the number of units of such Other Component(s) sold during such Calendar Quarter).
For purposes of calculating the average Net Sales per unit sold of a Licensed Component and Other Component(s) of a Combination Product, any of the deductions described herein that apply to such Combination Product will be allocated among sales of the Licensed Component and sales of the Other Component(s) included in such Combination Product as follows: (1) deductions that are attributable solely to the Licensed Component or one of the Other Component(s) will be allocated solely to Net Sales of the Licensed Component or such Other Component, as applicable, and (2) all other deductions will be allocated among sales of the Licensed Component and sales of the Other Component(s) in proportion to Zai’s and Paratek’s mutual agreement of the fair market value of the Licensed Component and the Other Component(s).
In the event that no separate sales of the Licensed Component or any Other Component(s) included in a Combination Product are made by Zai or its Affiliates, or Sublicensees, during a Calendar Quarter in which such Combination Product is sold, the average Net Sales per unit sold in the above described equation will be replaced with Zai’s and Paratek’s mutual agreement of the fair market value of the Licensed Component and each of the Other Component(s) included in such Combination Product.
1.66. “Paratek” will have the meaning set forth in the introduction of this Agreement.
1.67. “Paratek Indemnitee(s)” will have the meaning set forth in Section 12.1.
1.68. “Paratek Know-How” means any and all Know-How Controlled by Paratek, as of the Effective Date or during the Term, that is reasonably necessary or useful in connection with the Exploitation of the Licensed Product in the Field in the Territory.
1.69. “Paratek Patents” means Patents in the Territory Controlled by Paratek as of the Effective Date or during the Term that contain one or more claims that cover the composition of matter or formulation of, or salt of or polymorph forms of, or the method of making or method of using, a Licensed Product, including all Patents which contain a Valid Claim that the Exploitation of a Licensed Product would infringe if unlicensed. The Paratek Patents as of the Effective Date are listed in Schedule 1.69, which shall be updated by the Parties from time to time during the Term.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
9
1.70. “Paratek Prosecution Patents” will have the meaning set forth in Section 13.3(a).
1.71. “Paratek Technology” means the Paratek Know-How, Paratek Patents, Paratek’s interest in Joint Inventions, and Paratek’s interest in Joint Patents.
1.72. “Party” or “Parties” will have the meaning set forth in the introduction to this Agreement.
1.73. “Patent Prosecution” means the responsibility and authority for (a) preparing, filing and prosecuting applications (of all types) for any Patent, (b) managing any interference, opposition, re-issue, reexamination, invalidation proceedings, revocation, nullification, or cancellation proceeding relating to the foregoing, (c) deciding to abandon Patent(s), (d) listing in regulatory publications (as applicable), (e) patent term extension, and (f) settling any interference, opposition, revocation, nullification or cancellation proceeding.
1.74. “Patents” means all national, regional and international patents and patent applications, including divisions, continuations, continuations-in-part, additions, re-issues, renewals, extensions, substitutions, re-examinations or restorations, registrations and revalidations, and supplementary protection certificates and equivalents to any of the foregoing.
1.75. “Phase III Clinical Study” means any pivotal Clinical Trial(s), which Clinical Trial(s) is(are) designed to (a) establish that the Licensed Product is safe and efficacious for its intended use; (b) define warnings, precautions and adverse reactions that are associated with the Licensed Product in the dosage range to be prescribed; (c) be a pivotal study for submission of an Regulatory Approval Application to obtain regulatory approval for such Licensed Product in any region or regulatory jurisdiction, as defined in 21 C.F.R. § 312.21(c), as may be amended from time to time, or any analogous clinical trial described or defined in Applicable Laws.
1.76. “PRC” means the People’s Republic of China, which for the purposes of this Agreement will exclude Hong Kong, Macau, and Taiwan.
1.77. “Prime Rate” means for any day a per annum rate of interest equal to the “prime rate,” as published in the “Money Rates” column of The Wall Street Journal, from time to time, or if for any reason such rate is no longer available, a rate equivalent to the base rate on corporate loans posted by at least 70% of the ten largest U.S. banks.
1.78. “Product Infringement” will have the meaning set forth in Section 13.5(a).
1.79. “Product Marks” will have the meaning set forth in Section 8.4.
1.80. “Product Specifications” means the acceptance criteria agreed by the Parties, including numerical limits, ranges or other criteria for the Licensed Product.
1.81. “Public Official” will have the meaning set forth in Section 11.6(d).
1.82. “Receiving Party” will have the meaning set forth in Section 10.1(a).
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
10
1.83. “Regulatory Approval” means, with respect to a Licensed Product in a region in the Territory, all approvals from the necessary Governmental Authority or Regulatory Authority to manufacture, import, market and sell such Licensed Product in such region in the Territory (excluding pricing and reimbursement approvals).
1.84. “Regulatory Approval Application” means a New Drug Approval Application or Biologics License Application (each, as defined in the U.S. Federal Food, Drug and Cosmetic Act (21 U.S.C. §301 et seq.), as amended from time to time) in the U.S., or any corresponding application for approval to market and/or sell a product in any country, region or jurisdiction in the Territory outside the U.S.
1.85. “Regulatory Authority” means any applicable Government Authority responsible for granting Regulatory Approvals for Licensed Products, including the CFDA, and any corresponding national or regional regulatory authorities.
1.86. “Regulatory Submissions” means any filing, application, or submission with any Regulatory Authority, including authorizations, approvals or clearances arising from the foregoing, including Regulatory Approvals, and all correspondence or communication with or from the relevant Regulatory Authority, as well as minutes of any material meetings, telephone conferences or discussions with the relevant Regulatory Authority, in each case, with respect to a Licensed Product.
1.87. “Remedial Action” will have the meaning set forth in Section 6.8.
1.88. “Retained Rights” will have the meaning set forth in Section 2.3.
1.89. “ROFN Compound” will have the meaning set forth in Section 2.2.
1.90. “ROFN Negotiation Period” will have the meaning set forth in Section 2.2.
1.91. “ROFN Notice Period” will have the meaning set forth in Section 2.2.
1.92. “ROFN Trigger Notice” will have the meaning set forth in Section 2.2.
1.93. “Royalty Payment” will have the meaning set forth in Section 9.4(a).
1.94. “Royalty Term” will have the meaning set forth in Section 9.4(c).
1.95. “Safety Agreement” will have the meaning set forth in Section 6.4(a).
1.96. “Sole Inventions” will have the meaning set forth in Section 13.1(b).
1.97. “Subcommittee” will have the meaning set forth in Section 3.2(b).
1.98. “Sublicensee” means a Third Party, or Zai’s Affiliates granted a sublicense by Zai under the license granted in Section 2.1. For the avoidance of doubt, a Material Sublicensee is a type of Sublicensee.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
11
1.99. “Tax” or “Taxes” means any present or future taxes, levies, imposts, duties, charges, assessments or fees of any nature (including any interest thereon). For the avoidance of doubt, Taxes includes VAT.
1.100. “Technology Transfer” will have the meaning set forth in Section 4.1.
1.101. “Technology Transfer Plan” will have the meaning set forth in Section 4.1.
1.102. “Term” will have the meaning set forth in Section 14.1.
1.103. “Territory” means the PRC, Hong Kong, Macau, and Taiwan (which for purposes of this Agreement will each be deemed a region).
1.104. “Third Party” means an entity other than (a) Zai and its Affiliates or (b) Paratek and its Affiliates.
1.105. “Tufts Agreement” means the Tufts University License Agreement executed between Paratek Pharmaceuticals, Inc. and Tufts University dated February 1, 1997, as amended from time to time.
1.106. “U.S. Dollars” or “$” means United States dollars, the lawful currency of the United States.
1.107. “Upfront Payment” will have the meaning set forth in Section 9.2.
1.108. “Valid Claim” means (a) a claim of an issued and unexpired Patent included within the Paratek Patents with regard to the Licensed Product in the Territory that has not been permanently revoked or held unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, which decision is not appealable or is not appealed within the time allowed for appeal, and has not been abandoned, disclaimed or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise or (b) a bona fide claim of a pending patent application included within the Paratek Patents in the Territory that has not been (i) cancelled, withdrawn or abandoned without being refiled in another application in the applicable jurisdiction or (ii) finally rejected by an administrative agency action from which no appeal can be taken or that has not been appealed within the time allowed for appeal.
1.109. “VAT” means value-added taxes or other similar taxes.
1.110. “Zai” will have the meaning set forth in the introduction of this Agreement.
1.111. “Zai Indemnitee(s)” will have the meaning set forth in Section 12.2.
1.112. “Zai Know-How” means any and all Know-How, to the extent controlled by Zai as of the Effective Date or during the Term, that is reasonably necessary or useful in connection with the Exploitation of a Licensed Product in the Field in the Territory.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
12
1.113. “Zai Patent” means Patents in the Territory controlled by Zai as of the Effective Date or during the Term that contain one or more claims that cover the composition of matter or formulation of, or salt of or polymorph forms of, or the method of making or method of using, a Licensed Product.
1.114. “Zai Prosecution Patents” will have the meaning set forth in Section 13.3(b).
1.115. “Zai Technology” means Zai Know-How and Zai Patents.
ARTICLE 2
LICENSES; NON-COMPETE
2.1. Exclusive License. Subject to the terms and conditions of this Agreement, Paratek hereby grants to Zai, during the Term, an exclusive (subject to the Retained Rights and Section 2.5(c)), royalty-bearing license under the Paratek Technology to Exploit the Licensed Product in the Field in the Territory, including the right to grant sublicenses (subject to Section 2.4). For the avoidance of doubt, the license granted pursuant to this Section 2.1 will extend only to the Paratek Technology Controlled by Paratek during the Term, and to the extent any Paratek Technology is no longer Controlled by Paratek, such Paratek Technology will no longer be licensed to Zai. For clarity, Zai has the right pursuant to this Section 2.1 and subject to Section 3.2(f) to Exploit the Licensed Product in the form of a Combination Product. For further clarity, Paratek will not grant a license after the Effective Date and during the Term that will diminish the Paratek Technology Controlled by Paratek that is exclusively licensed to Zai.
2.2. Right of First Negotiation. During the Term, if Paratek decides to seek a partner to Develop (with the right to Commercialize or the right to obtain or negotiate Commercialization rights) any derivative or modification of omadacycline (a “ROFN Compound”) in the Territory, then Paratek will provide Zai with written notice of its decision to do so (the “ROFN Trigger Notice”). After Zai’s receipt of the ROFN Trigger Notice, Zai will have [*] days (the “ROFN Notice Period”) to provide written notice to Paratek of its desire to negotiate with Paratek regarding the partnership for such ROFN Compound. If Zai provides such written notice during the ROFN Notice Period, the Parties will negotiate exclusively for a period of [*] days following Paratek’s receipt of such notice from Zai (the “ROFN Negotiation Period”) regarding the terms of a definitive agreement. With respect to a ROFN Compound, if (a) Zai does not deliver written notice of its desire to negotiate with Paratek during the ROFN Notice Period or (b) the Parties are unable to reach terms of a definitive agreement during the ROFN Negotiation Period, then in either case (a) or (b), Paratek will have no further obligation to Zai with respect to such ROFN Compound in the Territory. For the avoidance of doubt, a ROFN Compound is a derivative or modification to omadacycline itself, and not other tetracyclines or derivatives or modifications to other tetracyclines.
2.3. Paratek Retained Rights. Notwithstanding anything to the contrary in this Agreement, Paratek hereby expressly retains, on behalf of itself (and its Affiliates, licensees, and sublicensees) the non-exclusive rights under the Paratek Technology to Manufacture the Compound and Licensed Product in the Territory in compliance with Applicable Laws and to support the Development and Commercialization of the Compound and Licensed Product
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
13
outside of the Territory (the “Retained Rights”). Zai acknowledges and agrees that the Retained Rights includes the right for Paratek to grant licenses under the Retained Rights to its Affiliates and Third Parties in the Field in the Territory, provided that Paratek shall not, and shall obligate its Affiliates, licensees, and sublicensees to not, sell or offer for sale in the Territory any Licensed Product manufactured under the Retained Rights. In addition, Paratek shall obligate, and obligate that its Affiliates, licensees, and sublicensees obligate, any contract manufacturing organization in the Territory to comply with all Applicable Laws, including GMP, and ensure that any such contract manufacturing organization is not, and has not been, debarred or disqualified by any Regulatory Authority. For the avoidance of doubt, the Retained Rights exclude the right under the Paratek Technology to Develop or Commercialize the Compound or Licensed Product in the Territory, and Paratek will not undertake such Development or Commercialization without Zai’s express prior written consent. Zai hereby grants to Paratek a non-exclusive, royalty-free, fully paid-up, sublicensable license under the Zai Technology, solely to exercise the rights set forth in the Retained Rights.
2.4. Right to Sublicense.
(a) General. Zai will have the right to grant sublicenses under the license granted in Section 2.1 to Sublicensees, solely for such Sublicensees to perform Zai’s obligations under this Agreement; provided that if such sublicense is (i) a sublicense of [*] under this Agreement, [*] such sublicense [*], and (ii) a Material Sublicense, then the additional provisions of Section 2.4(b) will also apply. Zai will be liable for Sublicensee conduct that is prohibited under this Agreement, and Sublicensee conduct that would have constituted a breach of this Agreement will be deemed a breach of this Agreement as if it had been engaged in by Zai.
(b) Material Sublicenses. [*] Material Sublicenses to a Material Sublicensee [*]. Notwithstanding the foregoing, the Parties agree that the Material Sublicensees set forth in Schedule 2.4(b) [*].
(c) Restrictions. Zai will not grant a sublicense to any Sublicensee that has been debarred or disqualified by a Regulatory Authority. Zai will ensure that, prior to engaging any Sublicensee that such Sublicensee is subject to written agreements containing the following terms and conditions: (i) requiring each such Sublicensee to protect and keep confidential any Confidential Information of the Parties, including in accordance with ARTICLE 10; (ii) providing that Paratek will have the right to audit (either by itself or through Zai or Zai’s designee) the books and records of each such Sublicensee in accordance with this Agreement (including pursuant to Sections 8.6, 9.6(d), and 11.6(a)(iv)); (iii) that does not impose any payment obligations or liability on Paratek; and (iv) that is otherwise consistent with the terms of this Agreement. Zai will provide a copy of the complete executed agreement with each Sublicensee to Paratek, [*]. Zai will remain directly responsible for all of its obligations under this Agreement that have been delegated or sublicensed to any Sublicensee.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
14
2.5. Tufts Agreement.
(a) Zai will, and will cause its Affiliates and Sublicensees to, be bound by and comply with all obligations that the Tufts Agreement states would apply to sublicenses or sublicensees of the Tufts Agreement, [*]. Zai’s obligations in relation to the Tufts Agreement and the Sections of the Tufts Agreement stated above will be owed by Zai to Paratek and Tufts University and enforceable by both Paratek and Tufts University. Zai expressly permits Paratek to disclose to Tufts University (i) complete copies of agreements Zai enters into with Sublicensees and amendments thereto and (ii) any other information under this Agreement as needed to comply with the provisions of the Tufts Agreement.
(b) During the Term, Paratek will promptly furnish Zai with a copy of (i) the Tufts Agreement (with certain terms that do not apply to Zai redacted) and any relevant ancillary agreements, exhibits, schedules, or other documents which set forth and are sufficient to fully describe all the terms and conditions with which Zai must comply in relation to the Tufts Agreement, (ii) all amendments of the Tufts Agreement, and (iii) all correspondence (or in the case of oral discussions, a summary of such discussions) with or from and reports received from or provided to licensors under the Tufts Agreement to the extent material to Zai or the rights granted or to be granted to Zai under this Agreement. In addition, during the Term, Paratek will provide copies of all notices received by Paratek relating to any alleged breach or default by Paratek under the Tufts Agreement within five Business Days after Paratek’s receipt thereof. Paratek will be solely responsible for all payment obligations set forth in the Tufts Agreement.
(c) Zai acknowledges and agrees that (i) Tufts University has the right to convert the License (as defined in the Tufts Agreement) from an exclusive license to a non-exclusive license and (ii) if Tufts University converts the License from an exclusive license to a non-exclusive license pursuant to Article VI of the Tufts Agreement, any rights with respect to the License sublicensed by Paratek to Zai (including any such rights sublicensed under Section 2.1) will become non-exclusive. For clarity, in such event the foregoing shall only affect Paratek Technology Controlled by Paratek pursuant to the Tufts Agreement, and the license granted by Paratek to Zai with respect to all other Paratek Technology shall in such an event remain exclusive.
2.6. No Implied Licenses; Negative Covenant. Except as set forth herein, neither Party will acquire any license or other intellectual property interest, by implication or otherwise, under any trademarks, patents or patent applications of the other Party. Each Party will not, and will not permit any of its Affiliates or sublicensees to, practice any Patent or Know-How licensed to it by the other Party outside the scope of the license granted to it under this Agreement.
2.7. Non-Compete. During the Term, Zai will not, and will cause its Affiliates and Sublicensees to not, engage in (independently or for or with any Third Party) any Commercialization in the Territory of (a) [*] or (b) [*]. Notwithstanding the foregoing clause (a), if [*], and [*], then the restriction set forth in clause (a) above shall not apply with respect to [*].
ARTICLE 3
GOVERNANCE
3.1. Alliance Managers. Within 30 days following the Effective Date, each Party will appoint (and notify the other Party of the identity of) a representative having the appropriate qualifications (including a general understanding of pharmaceutical Development,
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
15
Manufacturing, and Commercialization issues) to act as its alliance manager under this Agreement (“Alliance Manager”). The Alliance Managers will serve as the primary contact points between the Parties regarding the activities contemplated by this Agreement. The Alliance Managers will facilitate the flow of information and otherwise promote communication, coordination and collaboration between the Parties, providing single point communication for seeking consensus both internally within each Party’s respective organization, including facilitating review of external corporate communications, and raising cross-Party and/or cross-functional disputes in a timely manner. Each Party may replace its Alliance Manager by written notice to the other Party.
3.2. Joint Steering Committee.
(a) Formation. Within 30 days after the Effective Date, the Parties will establish a joint steering committee (the “Joint Steering Committee” or the “JSC”) to oversee the Development, Manufacture, and Commercialization of the Licensed Products in the Field in the Territory under this Agreement. Each Party will appoint three representatives to the JSC, each of whom will be an officer or employee of the applicable Party having sufficient seniority within such Party to make decisions arising within the scope of the JSC’s responsibilities. Each Party may replace its JSC representatives upon written notice to the other Party. Each Party will appoint one of its JSC representatives to act as a co-chairperson of the JSC.
(b) Role. The JSC will (i) provide a forum for the discussion of the Parties’ activities under this Agreement; (ii) review, discuss and approve the overall strategy for the Development, Manufacture, and Commercialization of the Licensed Product in the Field in the Territory; (iii) review, discuss and approve the Development Plan and amendments thereto; (iv) review and discuss the Commercialization Plan and amendments thereto; (v) review, discuss and approve the Product Specifications; (vi) review and discuss Manufacturing activities, and approve such Manufacturing activities that could affect Paratek’s global clinical and/or regulatory program outside the Territory and outside the Field; (vii) establish joint subcommittees (each, a “Subcommittee”) as necessary or advisable to further the purpose of this Agreement; and (viii) perform such other functions as expressly set forth in this Agreement or allocated to it by the Parties’ written agreement.
(c) Limitation of Authority. The JSC will only have the powers expressly assigned to it in this ARTICLE 3 and elsewhere in this Agreement and will not have the authority to: (i) modify or amend the terms and conditions of this Agreement; (ii) waive either Party’s compliance with the terms and conditions of this Agreement; or (iii) determine any such issue in a manner that would conflict with the express terms and conditions of this Agreement.
(d) Meetings. The JSC will hold meetings at such times as it elects to do so, but in no event will such meetings be held less frequently than once every Calendar Quarter until the earlier of (i) three years after the Effective Date, or (ii) Zai’s submission of a Regulatory Submission for Regulatory Approval for the Licensed Product in the Territory. Thereafter, the JSC will hold meeting no less frequently than once every six months. Each Party may call additional ad hoc JSC meetings as the needs arise with reasonable advance notice to the other Party. Meetings of the JSC may be held in person, by audio or video teleconference; provided
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
16
that at least one meeting per Calendar Year of the JSC will be held in person. In-person JSC meetings will be held at locations selected alternately by the Parties. The co-chairpersons of the JSC will jointly prepare the agenda and minutes for each JSC meeting. Each Party will be responsible for all of its own expenses of participating in the JSC meetings. No action taken at any JSC meeting will be effective unless at least one representative of each Party is participating in such JSC meeting.
(e) Non-Member Attendance. Each Party may from time to time invite a reasonable number of participants, in addition to its representatives, to attend the JSC meetings in a non-voting capacity; provided that if either Party intends to have any Third Party (including any consultant) attend such a meeting, such Party will provide prior written notice to the other Party. Such Party will also ensure that such Third Party is bound by confidentiality and non-use obligations consistent with the terms of this Agreement.
(f) Decision-Making. All decisions of the JSC will be made by unanimous vote, with each Party’s representatives having one vote. If after reasonable discussion and good faith consideration of each Party’s view on a particular matter before the JSC, the JSC cannot reach a decision as to such matter within 30 days after such matter was brought to the JSC for resolution, such matter will be referred to the President of Paratek and the Chief Executive Officer of Zai (the “Executive Officers”) for resolution. If the Executive Officers cannot resolve such matter within 10 Business Days after such matter has been referred to them, then the Parties will be deemed to be deadlocked and [*] final decision making authority over [*]; provided that [*] final decision making authority over [*]; provided further that [*] such final decision making authority in a manner that [*]. If [*] that [*] did not have a good faith basis to conclude that such matter [*], then [*] may submit the matter to arbitration pursuant to Section 15.4; provided that the expedited procedure rules of the [*] will apply. For clarity, [*] would have the right to [*] with respect to the [*].
(g) Exchange of Information. The Parties will cooperate to exchange information with respect to Development activities conducted by Paratek outside the Territory that could affect Zai’s activities in the Territory, and Development activities conducted by Zai that could affect Paratek’s global clinical and regulatory program outside the Territory and outside the Field (such as new indications, dosing, and formulations).
3.3. Subcommittees.
(a) General. Pursuant to Section 3.2(b), the JSC will have the authority to establish Subcommittees. Each Subcommittee (including the Joint Development Committee) will be composed of an equal number of representatives from each Party. Each Party may replace its Subcommittee representatives upon written notice to the other Party. All decisions of a Subcommittee will be made by unanimous vote, with each Party’s representatives having one vote. In the event the Parties are unable to reach a unanimous vote with respect to a matter, such matter will be referred to the JSC for resolution.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
17
(b) Joint Development Committee.
(i) General. Within 30 days of the Effective Date, the Parties will establish a joint development committee (the “Joint Development Committee” or the “JDC”) to oversee (1) the day-to-day Development of the Licensed Product and the execution of the Development Plans, and (2) the progress of the Regulatory Approvals and Regulatory Submissions for the Licensed Product. Each Party will appoint three representatives to the JDC, each of whom will be an officer or employee of the applicable Party having sufficient knowledge regarding Development and Commercialization of the Licensed Product.
(ii) Meetings. While the Parties are developing and conducting Clinical Trials for Licensed Product in the Territory, the JDC will meet at least once per Calendar Quarter. The Parties will endeavor to schedule meetings of the JDC at least two months in advance.
ARTICLE 4
TECHNOLOGY TRANSFERS
4.1. Technology Transfer. Within 30 days of the Effective Date, the Parties will coordinate and agree to a technology transfer plan for Paratek to provide and transfer to Zai the Paratek Know-How that exists on the Effective Date and was not previously provided to Zai, and a timeline for such technology transfer, which may be updated or amended by the JSC from time to time as needed (such schedule and timeline, the “Technology Transfer Plan”). Paratek will transfer the Paratek Know-How to Zai in accordance with the Technology Transfer Plan, and Zai will cooperate to facilitate the receipt of such transfer of Paratek Know-How (the “Initial Technology Transfer”). Thereafter, upon Zai’s reasonable request, Paratek will provide Zai with reasonable assistance in the Development and Manufacture of the Licensed Products in the Field in the Territory (the “Continuing Technology Transfer,” and together with the Initial Technology Transfer, the “Technology Transfer”). The Continuing Technology Transfer will include the transfer of additional Paratek Know-How to Zai and reasonable access to Paratek personnel involved in the research and Development of the Compound and Licensed Products, either in-person at Paratek’s facility or by teleconference, but will not include an obligation for Paratek personnel to travel.
4.2. Transfer of Materials. Paratek will provide a one-time transfer of reasonable quantities of Materials for Zai to conduct its Development activities under this Agreement; provided that the Parties discuss in good faith and enter into a separate materials transfer agreement containing reasonable and customary terms for such transfer of Materials. Zai will [*] provide assistance to Zai for the transfer of Materials pursuant this Section 4.2.
4.3. Technology Transfer Costs. [*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
18
ARTICLE 5
DEVELOPMENT PROGRAM
5.1. Diligence and Responsibilities.
(a) Zai will be responsible for, and use Commercially Reasonable Efforts to Develop the Licensed Product in the Field in the Territory in accordance with the Development Plan, at its sole cost and expense.
(b) Zai will use Commercially Reasonable Efforts to conduct its tasks pursuant to the Development Plan and to attempt to achieve the objectives of the Development Plan. Zai will perform such obligations under the Development Plan in a professional manner, and in compliance in all material respects with the Development Plan and the requirements of Applicable Law, GCP and cGMP. Changes in the scope or direction of the Development work under this Agreement that would require a material deviation from the Development Plan must be approved by the JSC as set forth in Section 3.2(b).
5.2. Development Plan. The Parties will undertake the Development of the Licensed Product in a collaborative and efficient manner in accordance with this ARTICLE 5. The Development of the Licensed Product in the Territory under this Agreement will be governed by a written development plan (the “Development Plan”), as such Development Plan may be revised from time to time in accordance with this Section 5.2. The Development Plan will contain in reasonable detail the major Development activities and the timelines for achieving such activities. As of the Effective Date, the Parties have agreed to the initial Development Plan, which is attached hereto as Schedule 5.2 (the “Initial Development Plan”). From time to time, but at least every 12 months, Zai will propose updates or amendments, if any, to the Development Plan in consultation with Paratek and submit such proposed updated or amended plan to the JSC for review, discussion, and approval. In accordance with Section 3.2(b), the JSC will review and approve any updates or amendments to the Development Plan.
5.3. Activity Target. Prior to [*], Zai will file an IND with the CFDA for the Licensed Product (the “Activity Target,” and the date, the “Activity Target Deadline”); provided that (a) if Zai is unable to achieve the Activity Target by the Activity Target Deadline and demonstrates to Paratek that Zai utilized Commercially Reasonable Efforts in Zai’s attempt to satisfy the obligations of this Section 5.3, or (b) if Zai is unable to achieve the Activity Target by the Activity Target Deadline as a direct result of Paratek [*], the Activity Target Deadline will be extended [*]. For the avoidance of doubt, with respect to subsection (a) the Activity Target Deadline is [*], and with respect to subsection (b), the Activity Target Deadline is [*]. [*]
5.4. Development Reports. The status, progress and results of Zai’s Development activities under this Agreement will be discussed at meetings of the JSC. At least five Business Days before each regularly scheduled JSC meeting, Zai will provide the JSC with a written report detailing its Development activities and the results thereof, covering subject matter at a level of detail reasonably required by Paratek and sufficient to enable Paratek to determine Zai’s compliance with its diligence obligations pursuant to Section 5.1. In addition, Zai will make available to Paratek such additional information about its Development activities as may be reasonably requested by Paratek from time to time. All updates and reports generated pursuant to this Section 5.4 shall be the Confidential Information of Zai.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
19
5.5. Records. Zai will maintain appropriate records in either tangible or electronic form of (a) all significant Development, Manufacturing, and Commercialization events and activities conducted by it or on its behalf related to a Licensed Product; and (b) all significant information generated by it or on its behalf in connection with Development, Manufacturing, or Commercialization of a Licensed Product under this Agreement, in each case in accordance with Zai’s usual documentation and cGMP record retention practices. Such records will be in sufficient detail to properly reflect, in a good scientific manner, all significant work done and the results of studies and trials undertaken and, further, will be at a level of detail appropriate for patent and regulatory purposes. Zai will document all non-clinical studies and Clinical Trials in formal written study reports according to Applicable Laws and national and international guidelines. Upon Paratek’s request, Zai will, and will cause its Affiliates and Sublicensees, to provide to Paratek copies of such records (including access to relevant databases, if any) of Development, Manufacturing, and Commercialization activities to the extent necessary or useful for the Development, Manufacturing, and Commercialization of the Compound or Licensed Product outside the Territory, including for regulatory and patent purposes. All such records, reports, information and data provided will be subject to the confidentiality provisions of ARTICLE 10.
ARTICLE 6
REGULATORY
6.1. Zai’s Responsibilities. Zai will be responsible for all regulatory activities leading up to and including the obtaining of the Regulatory Approvals for a Licensed Product from the Regulatory Authority on a region-by-region basis, at its sole cost and expense. Zai or its designee will own and hold all Regulatory Approvals for a Licensed Product in the Territory. Zai will keep Paratek informed of regulatory developments related to the Licensed Products in the Territory and will promptly notify Paratek in writing of any decision by any Regulatory Authority in the Territory regarding the Licensed Product. Zai will notify Paratek of any Regulatory Submissions submitted to or received from any Regulatory Authority in the Territory and will provide Paratek with copies thereof within five days after submission or receipt. If any material Regulatory Submission is not in the English language, Zai will also provide Paratek with a summary thereof in English as soon as practicable.
6.2. Paratek’s Responsibilities. [*] Paratek will reasonably cooperate with Zai in obtaining any Regulatory Approvals for a Licensed Product in the Territory by providing, to the extent reasonably required by and reasonably useful to Zai, access to regulatory approvals, Regulatory Submissions, clinical data, and other data, information, and documentation for the Licensed Product outside of the Territory. In addition, upon Zai’s reasonable request, Paratek will, and will cause its Affiliates and sublicensees (to the extent permitted in such sublicensees’ agreement with Paratek), to provide to Zai copies of such records of Development, Manufacturing, and Commercialization activities to the extent necessary or reasonably useful to obtain Regulatory Approval of the Licensed Product in the Territory. [*] provide assistance to Zai for such cooperation.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
20
6.3. Right of Reference. Each Party hereby grants to the other Party the right of reference to all Regulatory Submissions pertaining to the Licensed Product in the Field submitted by or on behalf of such Party. Zai may use such right of reference to Paratek’s Regulatory Submissions in the Field solely for the purpose of seeking, obtaining and maintaining Regulatory Approval of the Licensed Products in Field in the Territory. Paratek may use the right of reference to Zai’s Regulatory Submissions in the Field solely for the purpose of seeking, obtaining and maintaining regulatory approval of the Licensed Products outside the Territory.
6.4. Adverse Events Reporting.
(a) Promptly following the Effective Date, but in no event later than 60 days thereafter, Zai and Paratek will develop and agree to the worldwide safety and pharmacovigilance procedures for the Parties with respect to the Licensed Products, such as safety data sharing and exchange, Adverse Events reporting and prescription events monitoring in a written agreement (the “Safety Agreement”). Such agreement will describe the coordination of collection, investigation, reporting, and exchange of information concerning Adverse Events or any other safety problem of any significance, and product quality and product complaints involving Adverse Events, sufficient to permit each Party, its Affiliates, licensees or sublicensees to comply with its legal obligations. The Safety Agreement will be promptly updated if required by changes in legal requirements. Each Party hereby agrees to comply with its respective obligations under the Safety Agreement and to cause its Affiliates, licensees and sublicensees to comply with such obligations. To the extent there is any disagreement between this Section 6.4, Section 6.5, or any related definitions and the Safety Agreement, the Safety Agreement shall control with respect to safety matters and this Agreement shall control with respect to all other matters.
(b) Zai will maintain an Adverse Event database for the Licensed Products in the Territory, at its sole cost and expense, and will be responsible for reporting quality complaints, Adverse Events and safety data related to the Licensed Products to the applicable Regulatory Authorities in the Territory, as well as responding to safety issues and to all requests of Regulatory Authorities related to the Licensed Products in the Territory. Zai will provide to Paratek access to, and the information contained in, Zai’s Adverse Event database for the Territory, and Paratek will maintain a global Adverse Event database at its sole cost and expense.
(c) Zai will be responsible for complying with all Applicable Law governing Adverse Events in the Territory that occur after the Effective Date. Zai will notify Paratek on a timely basis of any Adverse Events occurring at or reported by any Clinical Trial location at which Zai is responsible for performing Clinical Trials. Zai will submit copies of reports of Adverse Events to Paratek simultaneously with submission to the applicable Regulatory Authorities. Each Party will notify the other in a timely manner and in any event within 24 hours of receiving any serious Adverse Event reports from Clinical Trials that each Party is monitoring, notice from a Regulatory Authority, independent review committee, data safety monitoring board or another similar clinical trial or post-marketing monitoring body alleging significant concern regarding a patient safety issue or other material information relevant to the safety or efficacy of Licensed Product.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
21
6.5. Safety and Regulatory Audits. Upon reasonable notification, and no more frequently than [*] (provided that the foregoing frequency limit shall not apply if Paratek has cause), Paratek will be entitled to conduct an audit of safety and regulatory systems, procedures and practices of Zai, including on-site evaluations to the extent permitting such on-site evaluations is in the control of Zai. Further details including notification, timing, response and scope of such audits will be included in the Safety Agreement.
6.6. No Harmful Actions. If Paratek believes that Zai is taking or intends to take any action with respect to the Licensed Product that could have a material adverse impact upon the regulatory status of the Licensed Product outside the Territory, Paratek will have the right to bring the matter to the attention of the JSC and the Parties will discuss in good faith to resolve such concern. Without limiting the foregoing, unless the Parties otherwise agree: (a) Zai will not communicate with any Regulatory Authority having jurisdiction outside the Territory, unless so ordered by such Regulatory Authority, in which case Zai will immediately notify Paratek of such order; and (b) Zai will not submit any Regulatory Submissions or seek regulatory approvals for the Licensed Product outside the Territory. To the extent practicable, Paratek will provide Zai with any information that reasonably could affect the Development or Commercialization of the Licensed Product in the Territory, prior to making such information public.
6.7. Notification of Threatened Action. Each Party will immediately notify the other Party of any information it receives regarding any threatened or pending action, inspection or communication by any Regulatory Authority, which may affect the safety or efficacy claims of any Licensed Product or the continued marketing of any Licensed Product. Upon receipt of such information, the Parties will consult with each other in an effort to arrive at a mutually acceptable procedure for taking appropriate action.
6.8. Remedial Actions. Each Party will notify the other immediately, and promptly confirm such notice in writing, if it obtains information indicating that any Licensed Product may be subject to any recall, corrective action or other regulatory action by any Governmental Authority or Regulatory Authority (a “Remedial Action”). The Parties will assist each other in gathering and evaluating such information as is necessary to determine the necessity of conducting a Remedial Action. Zai will have sole discretion with respect to any matters relating to any Remedial Action in the Territory, including the decision to commence such Remedial Action and the control over such Remedial Action. The cost and expenses of any Remedial Action in the Territory will be borne solely by Zai. Zai will, and will ensure that its Affiliates and Sublicensees will, maintain adequate records to permit the Parties to trace the manufacture, distribution and use of the Licensed Product in the Territory.
ARTICLE 7
MANUFACTURING
7.1. Manufacture and Supply. Zai will be responsible for, and use Commercially Reasonable Efforts to Manufacture, or have Manufactured (pursuant to Section 2.4), Licensed Products, sufficient and solely to meet the Development and Commercialization requirements of a Licensed Product in the Territory, at its sole cost and expense. Zai will undertake such Manufacturing activities of the Licensed Products in accordance with the Product Specifications.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
22
If [*], Paratek will permit Paratek’s suppliers to provide such supply to Zai and shall reasonably assist Zai to obtain a supply of Licensed Products for the Development and Commercialization activities contemplated hereunder by introducing Zai to suppliers that Paratek utilizes at that time. Zai will ensure that any arrangement between Zai and such suppliers (a) will not alter or affect Paratek’s supply related to the Licensed Product, and (b) Paratek will not have any liability or obligation related to such arrangements. If Zai is required by the CFDA to Commercialize the Licensed Product as an imported product, the Parties will negotiate in good faith the terms of an agreement to address this event (an “Imported Product Agreement”), and such agreement will include, but not be limited to, provisions whereby Zai will indemnify Paratek for any liability (including product liability) related to Paratek’s involvement in the Development, Manufacture or Commercialization of the Licensed Product as an imported product, and Zai will maintain appropriate minimum liability insurance (to be determined in the Imported Product Agreement) levels. For the avoidance of doubt, (y) Paratek will be adequately protected from any liability based on Zai’s activities in the Territory including Zai’s sourcing of the Compound or Licensed Product, and (z) absent the Parties agreement to terms pursuant to an Imported Product Agreement, Paratek will not have any obligation to (i) accommodate the supply (directly or indirectly) of the Compound or Licensed Product to Zai, or (ii) be an applicant on a regulatory application or holder of a regulatory approval related to Zai’s Exploitation of the Licensed Product as an imported product.
7.2. Transfer of Manufacturing Know-How. As part of the Initial Technology Transfer, in accordance with the Technology Transfer Plan, Paratek will make available to Zai the Paratek Know-How that constitutes the then-current process used by Paratek or its Third Party manufacturer in the manufacture of Licensed Products. In addition, as per the Continuing Technology Transfer, Paratek will provide reasonable technical assistance regarding such manufacturing related Paratek Know-How as requested by Zai in accordance with Section 4.1. Zai will be responsible for the costs and expenses incurred by Paratek in performing such part of the Technology Transfer in accordance with Section 4.3. After the completion of such part of the Initial Technology Transfer, each Party will promptly notify the other Party of any changes in its manufacturing process for the Licensed Products and upon such other Party’s request, will provide reasonable assistance to enable such other Party to implement such changes, with each Party bearing its own costs.
7.3. Agreement with Contract Manufacturer. To the extent that Zai enters into an agreement with any contract manufacturing organization to manufacture Licensed Product for and on behalf of Zai, such agreement shall set forth the respective responsibilities of the parties with regards to quality assurance for the Licensed Product, and Zai shall obligate such contract manufacturing organization in the Territory to comply with all Applicable Laws, including GMP, and ensure that any such contract manufacturing organization is not, and has not been, debarred or disqualified by any Regulatory Authority.
ARTICLE 8
COMMERCIALIZATION
8.1. Commercialization Diligence. Zai will be responsible for, and use Commercially Reasonable Efforts to Commercialize the Licensed Products in the Field in the Territory in accordance with the Commercialization Plan, at its sole cost and expense. Upon Zai’s reasonable request, Paratek will reasonably assist Zai in such Commercialization of the Licensed Product.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
23
8.2. Commercialization Plan. The Commercialization Plan will contain in reasonable detail the major Commercialization activities and the timelines for achieving such activities. Zai will deliver an initial Commercialization Plan to the JSC for review and discussion no later than 12 months prior to the anticipated date of the first filing of the first Regulatory Approval for a Licensed Product in the Territory. Thereafter, from time to time, but at least every 12 months, Zai will propose updates or amendments to the Commercialization Plan in consultation with Paratek to reflect changes in such plans, including those in response to changes in the marketplace, relative success of the Licensed Product, and other relevant factors influencing such plan and activities, and submit such proposed updated or amended plan to the JSC for review, discussion, and approval. In accordance with Section 3.2(b), the JSC will review and discuss any updates or amendments to the Commercialization Plan.
8.3. Commercialization Reports. Zai will update the JSC at each regularly scheduled JSC meeting regarding Zai’s Commercialization activities with respect to the Licensed Products in the Territory. Each such update will be in a form to be agreed by the JSC and will summarize Zai’s, its Affiliates’ and Sublicensees’ significant Commercialization activities with respect to the Licensed Products in the Territory, covering subject matter at a level of detail reasonably required by Paratek and sufficient to enable Paratek to determine Zai’s compliance with its diligence obligations pursuant to Section 8.1. In addition, Zai will make available to Paratek such additional information about its Commercialization activities as may be reasonably requested by Paratek from time to time. For clarity, Zai will not be required to include information in its updates and reports under this Section 8.3 that it does not otherwise create for its own internal purposes. All updates and reports generated pursuant to this Section 8.3 shall be the Confidential Information of Zai.
8.4. Product Trademarks. Zai will have the right to brand the Licensed Products in the Territory using trademarks, logos, and trade names it determines appropriate for the Licensed Products, which may vary by region or within a region (the “Product Marks”). Zai will own all rights in the Product Marks in the Territory and will register and maintain the Product Marks in the Territory that it determines reasonably necessary, at Zai’s cost and expense. Upon Zai’s request, Paratek will reasonably assist Zai in the selection and design of the Product Marks. Zai will also have the right (pursuant to this Section 8.4) to use certain trademarks in the Territory as set forth in Schedule 8.4 (the “Paratek Product Marks”). If Zai elects to use the Paratek Product Marks in connection with the Commercialization of the Licensed Products in the Territory, Paratek will and hereby does grant to Zai, during the Term and subject to the terms and conditions of this Agreement, a royalty-free, exclusive license under Paratek’s rights to use such Paratek Product Marks in connection with the Commercialization of the Licensed Products in the Field in the Territory in compliance with Applicable Laws. Zai will comply with Paratek’s brand usage guidelines provided to Zai in its use of the Paratek Product Marks. For the avoidance of doubt, Paratek (a) has sole discretion regarding prosecution and maintenance of the Paratek Product Marks, provided that, after Zai has initiated launch efforts to Commercialize the
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
24
Product under any particular Paratek Product ▇▇▇▇, Paratek shall notify Zai in writing of any decision to modify and/or discontinue the application or registration of such Paratek Product ▇▇▇▇ in the Territory, and shall not carry out such modification or discontinuation without Zai’s prior written consent (not to be unreasonably withheld), further provided that Paratek shall not be required to obtain Zai’s consent if such modification and/or discontinuation is required by the applicable Regulatory Authority in the Territory or is necessary to avoid any potential infringement of the rights of any Third Party, and (b) has no obligation to ensure that, and provides no guarantee that, any applications included in the Paratek Products Marks issues to a registered trademark in the Territory.
8.5. Commercialization Assistance. Zai will reimburse Paratek’s actual internal expenses and costs at the FTE Rate for FTEs engaged to, and out-of-pocket expenses and costs incurred by Paratek to, provide assistance to Zai Commercialization activities, including assistance pursuant to Sections 8.1 and 8.4.
8.6. Compliance. Zai will (a) comply, and will cause its Affiliates and Sublicensees to comply, with all Applicable Laws and all applicable cGMP, GCP, GLP and GSP (or similar standards) in their conduct of the Development, Manufacturing, and Commercialization activities under this Agreement and (b) ensure that its Affiliates and Sublicensees do not transfer or divert the Compound or Licensed Product to an entity other than Zai, or an entity approved by Zai, in each case in a manner that would cause the sale of such Compound or Licensed Product in the chain of distribution (from Zai or its Affiliates or Sublicensees to the end user) to be excluded (except as an exception provided in the Net Sales definition) in the calculation of Net Sales, provided that for each unit of the Compound and/or Licensed Product, the inclusion of such sales in the calculation of Net Sales shall occur only once. Upon reasonable notification, but no more than [*] (provided that the foregoing frequency limit shall not apply if Paratek has cause), Paratek will have the right to conduct audits of Zai, and Zai will procure such right for Paratek to audit Zai’s Affiliates and Sublicensees (either directly or through Zai and its designee), to ensure (y) compliance with applicable cGMP, GCP, GLP, and GSP standards, including on-site evaluations (to the extent permitting such evaluations is under the control of the audited Party), and (z) compliance with Section 8.6(b).
ARTICLE 9
PAYMENTS AND MILESTONES
9.1. Tufts Agreement and IP Transfer Agreement Payments.
(a) Paratek will be responsible, at its costs, for all payments, royalties or milestones under the Tufts Agreement.
(b) Paratek will be responsible, at its costs, for all payments under the IP Transfer Agreement.
9.2. Upfront Payment. In partial consideration of the rights granted by Paratek to Zai hereunder, Zai will pay to Paratek US$7,500,000 (the “Upfront Payment”) within [*] days of the Effective Date.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
25
9.3. Milestones Payments to Paratek.
(a) In partial consideration of the rights granted herein, Zai will pay to Paratek the following milestone payments (each such payment, a “Milestone Payment”) within [*] days of the achievement of the corresponding milestone events set forth below (each such event, a “Milestone Event”), or in the case of Net Sales Milestone Events, within [*] days after the end of the Calendar Quarter in which the Net Sales Milestone Event occurs.
| Milestone Event |
Milestone Payment | |
| First regulatory approval for a Licensed Product in the U.S. for the CABP indication | US$5,000,000 | |
| [*] | US$[*] | |
| [*] | US$[*] | |
| First time that Net Sales of Licensed Products in a Calendar Year exceeds US$[*] | US$[*] | |
| First time that Net Sales of Licensed Products in a Calendar Year exceeds US$[*] | US$[*] | |
| First time that Net Sales of Licensed Products in a Calendar Year exceeds US$[*] | US$[*] | |
(b) For the avoidance of doubt (i) each Milestone Payment will be payable on the first occurrence of the corresponding Milestone Event, and (ii) none of the Milestone Payments will be payable more than once.
9.4. Royalties.
(a) Royalty Payment. During the Royalty Term, Zai will pay to Paratek tiered royalties based on annual Net Sales of Licensed Product in the Territory in a Calendar Year (a “Royalty Payment”). The royalty rates will be as set forth below (subject to Section 9.4(d)):
| Tier |
Royalty % | |
| ³ US$[*] and £ US$[*] | [*]% | |
| > US$[*] and £ US$[*] | [*]% | |
| > US$[*] and £ US$[*] | [*]% | |
| > US$[*] | [*]% | |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
26
(b) Example. By way of example, if the Net Sales in a Calendar Year of Licensed Product within the Territory equals $[*], the royalty amount owed by Zai to Paratek would be US$[*].
(c) Royalty Term. The Royalty Payments payable under this Section 9.4 will be payable on a region-by-region basis from the First Commercial Sale of the Licensed Product in such region until the later of: (i) the abandonment, expiry or final determination of invalidity of the last Valid Claim within the Paratek Patents that covers the Exploitation of the Licensed Products in the region in the Territory in the manner that Zai or its Affiliates or Sublicensees Exploit the Licensed Product or intend for the Licensed Product to be Exploited; or (ii) the close of business of the day that is exactly 11 years after the date of the First Commercial Sale of such Licensed Product in such region (the “Royalty Term”).
(d) Royalty Rate Reduction for Generic Product Market Effect. If there is no longer a Valid Claim within the Paratek Patents covering a Licensed Product in a region in the Territory, then Zai may reduce the Royalty Payments for Net Sales in such region by (i) [*]% in any Calendar Quarter that Zai can demonstrate that one or more generic equivalent products are on the market in such region and sales of such generic equivalent product(s) in the region constitute [*]% or more of the total sales of such generic equivalent product(s) and Licensed Product in such region or (ii) [*]% in any Calendar Quarter that Zai can demonstrate that one or more generic equivalent products are on the market in such region and sales of such generic equivalent product(s) in the region constitute [*]% or more of the total sales of such generic equivalent product(s) and Licensed Product in such region.
(e) Royalty Estimates and Royalty Reports. Following the First Commercial Sale of any Licensed Product for which royalties are due pursuant to this Section 9.4, and continuing for so long as royalties are due hereunder:
(i) Zai will, within [*] days after the end of each Calendar Quarter, provide Paratek a good faith estimate of the royalties due for such Calendar Quarter; and
(ii) Zai will, within [*] days after the end of each Calendar Quarter, provide a royalty report showing, on a region-by-region basis:
(1) the Net Sales of each Licensed Product sold by Zai, its Affiliates and Sublicensees during such Calendar Quarter reporting period;
(2) the Royalty Payments in United States dollars which will have accrued hereunder with respect to such Net Sales, with supporting calculations showing the applicable royalty rate applied;
(3) the rate of exchange with supporting calculations, determined in accordance with Section 9.5(b), used by Zai in determining the amount of United States dollars payable hereunder.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
27
(f) Royalty Payment. Zai will pay to Paratek the royalties for each Calendar Quarter within [*] days after the end of such Calendar Quarter. If no royalty is due for any Calendar Quarter following commencement of the reporting obligation, Zai will so report.
9.5. Payment.
(a) Mode of Payment. All payments to be made under this Agreement will be made in U.S. Dollars and will be paid by electronic transfer in immediately available funds to such bank account in the United States as is designated in writing by a Party. All payments will be free and clear of any transfer fees or charges.
(b) Currency Exchange Rate. All payments under this Agreement will be payable in U.S. Dollars. All expense amounts will be calculated in the foreign currency for the country or region in which expenses are incurred, and will then be converted into U.S. Dollars by applying the rate of exchange used by a Party for its own financial reporting purposes in connection with its other products or accounts, consistently applied, which will be consistent with US GAAP. The rate of exchange to be used in computing the amount of currency equivalent in U.S. Dollars for calculating Net Sales in a Calendar Quarter (for purposes of both the royalty calculation and whether a Net Sales milestone has been achieved) shall be made at the exchange rate as published by the Wall Street Journal on the last Business Day of such Calendar Quarter, or such other source as the Parties may agree in writing.
9.6. Audits.
(a) Zai will keep, and will require its Affiliates and Sublicensees to keep (all in accordance with US GAAP, consistently applied), for a period not less than [*] complete and accurate records in sufficient detail to properly reflect Net Sales and to enable any Milestone Payment payable hereunder to be determined.
(b) Upon the written request of Paratek, Zai will permit, and will cause its Affiliates and Sublicensees to permit, an independent certified public accounting firm of nationally recognized standing selected by Paratek and reasonably acceptable to Zai, at Paratek’s expense, to have access during normal business hours to such records of Zai and/or its Affiliates as may be reasonably necessary to verify the accuracy of the payments hereunder for any Calendar Year ending not more than [*] prior to the date of such request. These rights with respect to any Calendar Year will terminate [*] after the end of any such Calendar Year and shall be limited to (i) [*] and (ii) [*] with respect to records covering any specific period of time (provided that the foregoing frequency limits ((i) and (ii)) shall not apply if Paratek has cause). Paratek will provide Zai with a copy of the accounting firm’s written report within [*] days of completion of such report. If such accounting firm correctly concludes that an underpayment was made, then Zai will pay the amount due within [*] days of the date Paratek delivers to Zai such accounting firm’s written report so correctly concluding. Paratek will bear the full cost of such audit unless such audit correctly discloses that the additional payment payable by Zai for the audited period is more than [*]% of the amount otherwise paid for that audited period, in which case Zai will pay the reasonable fees and expenses charged by the accounting firm.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
28
(c) Paratek will treat all financial information, subject to review under this Section 9.6 in accordance with the confidentiality provisions of ARTICLE 10, and, prior to commencing such audit, will cause its accounting firm to enter into a confidentiality agreement with Zai obligating it to treat all such financial information in confidence pursuant to such confidentiality provisions. Such accounting firm shall not disclose Zai’s Confidential Information to Paratek, except to the extent such disclosure is necessary to verify the accuracy of the financial reports furnished by Zai or the amount of payments to or by Zai under this Agreement.
(d) Zai will include in each relevant sublicense granted by it a provision requiring any Sublicensee to maintain records of sales of Licensed Products made pursuant to such sublicense, and to grant access to such records by an accounting firm to the same extent and under the same obligations as required of Zai under this Agreement. Paratek will advise Zai in advance of each audit of any such Sublicensee with respect to Licensed Product sales either by Paratek or its designated auditor under the terms of such Sublicensee agreement. Paratek will provide Zai with a summary of the results received from the audit and, if Zai so requests, a copy of the audit report. Paratek will pay the full costs charged by the accounting firm, unless the audit discloses that the additional payments payable to Paratek for the audited period is more than [*]% from the amounts otherwise paid for that audited period, in which case Zai will pay the reasonable fees and expenses charged by the accounting firm.
9.7. Interest. Each Party will pay interest on any amounts overdue under this Agreement at a per annum rate of [*] point above the Prime Rate assessed from the day payment was initially due; provided, however, that in no case will such interest rate exceed the highest rate permitted by Applicable Law. The payment of such interest will not foreclose a Party from exercising any other rights it may have because any payment is overdue.
9.8. Taxes.
(a) [*] any VAT required to be deducted or withheld by Zai under Applicable Law on payments payable by Zai under this Agreement, and will [*] the deduction or withholding for VAT. If Zai is required to deduct or withhold Taxes (including VAT) on any payments payable by Zai under this Agreement, Zai will (i) pay such Tax on behalf of Paratek to the appropriate Governmental Authority, (ii) furnish Paratek with proof of payment of such Tax, and (iii) [*] required to be deducted or withheld [*] as set forth in the Agreement. For example, if Paratek is due US[*] under this Agreement, and Zai is required by Applicable Law to withhold [*], [*] and [*].
(b) Zai and Paratek will cooperate with respect to all documentation required by any taxing authority or reasonably requested by Zai to secure a reduction in the rate of applicable Taxes.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
29
ARTICLE 10
CONFIDENTIALITY; PUBLICATION
10.1. Nondisclosure Obligation.
(a) For the Term of this Agreement and [*] thereafter, the Party receiving the Confidential Information of the other Party (such receiving Party, the “Receiving Party”) will keep confidential and not publish, make available or otherwise disclose any Confidential Information to any Third Party, without the express prior written consent of the Party that disclosed such Confidential Information (the “Disclosing Party”); provided however, the Receiving Party may disclose the Confidential Information to those of its Affiliates, officers, directors, employees, agents, consultants and/or independent contractors (including sublicensees) of such Receiving Party who need to know the Confidential Information in connection with this Agreement and are bound by confidentiality obligations with respect to such Confidential Information. The Receiving Party will exercise at a minimum the same degree of care it would exercise to protect its own confidential information (and in no event less than a reasonable standard of care) to keep confidential the Confidential Information. The Receiving Party will use the Confidential Information solely in connection with the purposes of this Agreement.
(b) It will not be considered a breach of this Agreement if the Receiving Party discloses Confidential Information in order to comply with a lawfully issued court or governmental order or with a requirement of Applicable Law or the rules of any internationally recognized stock exchange; provided that: (i) the Receiving Party gives prompt written notice of such disclosure requirement to the Disclosing Party and cooperates with the Disclosing Party’s efforts to oppose such disclosure or obtain a protective order for such Confidential Information, and (ii) if such disclosure requirement is not quashed or a protective order is not obtained, the Receiving Party will only disclose those portions of the Confidential Information that it is legally required to disclose and will make a reasonable effort to obtain confidential treatment for the disclosed Confidential Information. To the extent there is any conflict between this ARTICLE 10 and any other agreement related to Confidential Information entered into between the Parties, the terms of this ARTICLE 10 will control to the extent of such conflict.
10.2. Scientific Publication. The JDC will discuss the publication strategy for the publication of scientific papers, abstracts, meeting presentations and other disclosure of the results of the studies carried out under this Agreement, taking into consideration the Parties’ interest in publishing the results of the Development work in order to obtain recognition within the scientific community and to advance the state of scientific knowledge, and the need to protect Confidential Information, intellectual property rights and other business interests of the Parties. Zai will provide Paratek with the opportunity to review and comment on any proposed publication that pertains to the Compound or Licensed Products at least [*] days prior to its intended submission for publication. Paratek will provide Zai with its comments, if any, within [*] days after the receipt of such proposed publication. Zai will consider in good faith the comments provided by Paratek and will comply with Paratek’s request to: (a) remove any and all Confidential Information of Paratek from such proposed publication; and (b) delay the submission for a period up to [*] days as may be reasonably necessary to seek patent protection for the information disclosed in the proposed publication. Zai agrees to acknowledge the contribution of Paratek and Paratek’s employees in all publication as scientifically appropriate.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
30
10.3. Publicity; Use of Names.
(a) Each of the Parties agrees not to disclose to any Third Party the terms and conditions of this Agreement without the prior approval of the other Party, except to (i) advisors (including consultants, financial advisors, attorneys and accountants), (ii) bona fide potential and existing investors and acquirers on a need to know basis, in each case under circumstances that reasonably protect the confidentiality thereof, (iii) to the extent necessary to comply with the terms of agreements with Third Parties, or (iv) to the extent required by Applicable Laws, including securities laws and regulations. Notwithstanding the foregoing, the Parties must agree upon the initial press release(s) to announce the execution of this Agreement; thereafter, Paratek and Zai may each disclose to Third Parties the information contained in such press release(s) without the need for further approval by the other.
(b) The Parties acknowledge the importance of supporting each other’s efforts to publicly disclose results and significant developments regarding a Licensed Product for use in the Field in the Territory and other activities in connection with this Agreement, beyond what may be strictly required by Applicable Laws and the rules of a recognized stock exchange, and Zai may make such disclosures from time to time with respect to the Licensed Product with the approval of Paratek, which approval will not be unreasonably withheld, conditioned or delayed. Such disclosures may include achievement of significant events in the Development (including regulatory process) or Commercialization of a Licensed Product for use in the Field in the Territory. Unless otherwise requested by the applicable Party, each Party will indicate that Paratek is the licensor of a Licensed Product, Paratek Patents, and Paratek Know-How, as applicable, in each public disclosure issued by such Party regarding a Licensed Product. When Zai elects to make any public disclosure under this Section 10.3(b), it will give Paratek reasonable notice to review and comment on such statement, it being understood that (i) if Paratek does not notify Zai in writing within [*] days or such shorter period if required by Applicable Laws of any reasonable objections, as contemplated in this Section 10.3(b), such disclosure will be deemed approved, and (ii) if Paratek does notify Zai in writing within the time period set forth in clause (i) above, and reasonably determines that such public disclosure would entail the public disclosure of Paratek’s Confidential Information or of patentable inventions upon which patent applications should be filed prior to such public disclosure, such public disclosure will be delayed for such period as may be reasonably necessary for deleting any such Confidential Information of Paratek, or the drafting and filing of a patent application covering such inventions, provided such additional period will not exceed [*] days from the proposed date of the public disclosure, and, in any event, Paratek will work diligently and reasonably to agree on the text of any proposed disclosure in an expeditious manner. The principles to be observed in such disclosures will be accuracy, compliance with Applicable Laws and regulatory guidance documents, and reasonable sensitivity to potential negative reactions of applicable Regulatory Authorities.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
31
(c) The Parties acknowledge the need to keep investors and others informed regarding such Party’s business under this Agreement, including as required by the rules of a recognized stock exchange. To the extent a Party is publicly listed or becomes publicly listed, and subject to Sections 10.3(a) and 10.3(b), such Party may issue press releases or make disclosures to the SEC or other applicable agency as it determines, based on advice of counsel, as reasonably necessary to comply with laws or regulations or for appropriate market disclosure; provided that each Party shall provide the other Party with advance notice of legally required disclosures to the extent practicable. The Parties will consult with each other on the provisions of this Agreement to be redacted in any filings made by a Party with the SEC or as otherwise required by Applicable Laws; provided that each Party shall have the right to make any such filing as it reasonably determines necessary under Applicable Laws.
ARTICLE 11
REPRESENTATIONS, WARRANTIES, AND COVENANTS
11.1. Representations, Warranties, and Covenants of Each Party. Each Party represents and warrants, and covenants to the other Party as of the Effective Date that:
(a) it is a company or corporation duly organized, validly existing, and in good standing under the laws of the jurisdiction in which it is incorporated, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement, including, without limitation, the right to grant the licenses granted by it hereunder; and
(b) (i) it has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; (ii) it has taken all necessary corporate action on its part required to authorize the execution and delivery of the Agreement and the performance of its obligations hereunder; and (iii) the Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms;
(c) it is not a party to any agreement that would prevent it from granting the rights granted to the other Party under this Agreement or performing its obligations under the Agreement;
(d) in the course of performing its obligations or exercising its rights under this Agreement, it will comply with all Applicable Laws, including as applicable, cGMP, GCP, GLP, and GSP standards, and will not employ or engage any party who has been debarred by any Regulatory Authority, or, to such Party’s knowledge, is the subject of debarment proceedings by a Regulatory Authority.
11.2. Additional Representations and Warranties of Paratek. Paratek represents and warrants to Zai that as of the Effective Date:
(a) it has the right under the Paratek Technology to grant the licenses to Zai as purported to be granted pursuant to this Agreement;
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
32
(b) to Paratek’s actual knowledge, the Manufacture, use or sale of the Licensed Product in the Territory for the purposes set forth in the Development Plan will not infringe any issued claim of an issued Patent of any Third Party (except Patents for which Paratek has a license);
(c) Schedule 1.69 lists all Patents in the Territory Controlled by Paratek that cover the composition of matter or formulation of, or salt of or polymorph forms of, or the method of making or method of using, a Licensed Product;
(d) it has not granted any liens or security interests on the Paratek Technology;
(e) Paratek has not as of the Effective Date, and will not during the Term, grant any right to any Third Party under the Paratek Technology that would conflict with the rights granted to Zai hereunder;
(f) Paratek and its Affiliates is not, and has not been, debarred or disqualified by any Regulatory Authority;
(g) no claim or action has been brought against Paratek or, to Paratek’s knowledge, threatened in writing to Paratek, by any Third Party alleging that the Paratek Patents are invalid or unenforceable, and no interference, opposition, cancellation or other protest proceeding has been filed against a Paratek Patent owned by Paratek; and
(h) Paratek has made available to Zai, via the virtual data room, copies of all patient safety and efficacy data tables, in all material respects, that are in Paratek’s possession as of the Effective Date, in connection with the global Phase III Clinical Study conducted by Paratek for acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP).
11.3. Covenants of Paratek.
(a) Paratek will not modify, amend, or terminate the Tufts Agreement in a manner that is materially adverse to Zai without Zai’s prior written consent.
(b) Paratek will not modify, amend, or terminate, or cause to modify, amend or terminate, the IP Transfer Agreement in a manner that is materially adverse to Zai without Zai’s prior written consent.
11.4. Representations, Warranties, and Covenants of Zai. Zai represents, warrants, and covenants to Paratek that as of the Effective Date:
(a) there are no legal claims, judgments or settlements against or owed by Zai, or pending or, to Zai’s actual knowledge, threatened, legal claims or litigation, in each case, relating to antitrust, anti-competition, anti-bribery or corruption violations;
(b) Zai and its Affiliates is not, and has not been, debarred or disqualified by any Regulatory Authority;
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
33
(c) Zai has sufficient financial wherewithal to (i) perform all of its obligations pursuant to this Agreement, and (ii) meet all of its obligations that come due in the ordinary course of business;
(d) Zai has, or will obtain, sufficient technical, clinical, and regulatory expertise to perform all of its obligations pursuant to this Agreement, including its obligations relating to Development, Manufacturing, Commercialization, and obtaining Regulatory Approvals; and
(e) Zai will, and will cause its Affiliates and Sublicensees to, be bound by and comply with all obligations that the Tufts Agreement states would apply to sublicenses or sublicensees of the Tufts Agreement.
11.5. NO OTHER REPRESENTATIONS OR WARRANTIES. EXCEPT AS EXPRESSLY STATED IN THIS AGREEMENT, NO REPRESENTATIONS OR WARRANTIES WHATSOEVER, WHETHER EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT, OR NON-MISAPPROPRIATION OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS, ARE MADE OR GIVEN BY OR ON BEHALF OF A PARTY. ALL SUCH REPRESENTATIONS AND WARRANTIES, WHETHER ARISING BY OPERATION OF LAW OR OTHERWISE, ARE HEREBY EXPRESSLY EXCLUDED.
11.6. Compliance with Anti-Corruption Laws.
(a) Notwithstanding anything to the contrary in the Agreement, Zai hereby agrees that:
(i) it will not, in the performance of this Agreement, perform any actions that are prohibited by local and other anti-corruption laws (including the provisions of the U.S. Foreign Corrupt Practices Act, collectively “Anti-Corruption Laws”) that may be applicable to one or both Parties to the Agreement;
(ii) it will not, in the performance of this Agreement, directly or indirectly, make any payment, or offer or transfer anything of value, or agree or promise to make any payment or offer or transfer anything of value, to a government official or government employee, to any political party or any candidate for political office or to any other Third Party with the purpose of influencing decisions related to either Party and/or its business in a manner that would violate Anti-Corruption Laws;
(iii) it will, on an annual basis upon request by the other Party, verify in writing that to the best of such Party’s knowledge, there have been no violations of Anti-Corruption Laws by such Party or persons employed by or subcontractors used by such Party in the performance of the Agreement, or will provide details of any exception to the foregoing; and
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
34
(iv) it will maintain records (financial and otherwise) and supporting documentation related to the subject matter of the Agreement in order to document or verify compliance with the provisions of this Section 11.6, and upon request of the other Party, up to once per year and upon reasonable advance notice, will provide a Third Party auditor mutually acceptable to the Parties with access to such records for purposes of verifying compliance with the provisions of this Section 11.6. Acceptance of a proposed Third Party auditor may not be unreasonably withheld by either Party. It is expressly agreed that the costs related to the Third Party auditor will be fully paid by the Party requesting the audit, and that any auditing activities may not unduly interfere with the normal business operations of Party subject to such auditing activities. The audited Party may require the Third Party auditor to enter into a reasonable confidentiality agreement in connection with such an audit.
(b) To its knowledge as of the Effective Date, neither Zai nor any of its subsidiaries nor any of their Affiliates, directors, officers, employees, distributors, agents, representatives, sales intermediaries or other Third Parties acting on behalf of Zai or any of its subsidiaries or any of their Affiliates:
(i) has taken any action in violation of any applicable anticorruption law, including the U.S. Foreign Corrupt Practices Act (15 U.S.C. § 78 dd-1 et seq.); or
(ii) has corruptly, offered, paid, given, promised to pay or give, or authorized the payment or gift of anything of value, directly or indirectly, to any Public Official (as defined in Section 11.6(d) below), for the purposes of:
(iii) influencing any act or decision of any Public Official in his official capacity;
(iv) inducing such Public Official to do or omit to do any act in violation of his lawful duty;
(v) securing any improper advantage; or
(vi) inducing such Public Official to use his or her influence with a government, governmental entity, or commercial enterprise owned or controlled by any government (including state-owned or controlled veterinary or medical facilities) in obtaining or retaining any business whatsoever.
(c) As of the Effective Date, none of the officers, directors, employees, of Zai or of any of its Affiliates or agents acting on behalf of Zai or any of its Affiliates, in each case that are employed or reside outside the United States, are themselves Public Officials.
(d) For purposes of this Section 11.6, “Public Official” means (i) any officer, employee or representative of any regional, federal, state, provincial, county or municipal government or government department, agency or other division; (ii) any officer, employee or representative of any commercial enterprise that is owned or controlled by a government, including any state-owned or controlled veterinary or medical facility; (iii) any officer, employee or representative of any public international organization, such as the African Union, the International Monetary Fund, the United Nations or the World Bank; and (iv) any person acting in an official capacity for any government or government entity, enterprise or organization identified above.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
35
ARTICLE 12
INDEMNIFICATION
12.1. By Zai. Zai will indemnify and hold harmless Paratek, its Affiliates, and their directors, officers, employees and agents (individually and collectively, the “Paratek Indemnitee(s)”) from and against all losses, liabilities, damages and expenses (including reasonable attorneys’ fees and costs) incurred in connection with any claims, demands, actions or other proceedings by any Third Party (individually and collectively, “Losses”) first arising after the Effective Date to the extent arising from (a) Manufacturing, Development, and Commercialization activities, including the promotion of a Licensed Product and product liability claims relating to the Licensed Product, by Zai or any of its Affiliates or Sublicensees, (b) the [*], illegal conduct or willful misconduct of Zai, or (c) Zai’s breach of any of its representations or warranties made in or pursuant to this Agreement or any covenants or obligations set forth in or entered into pursuant to this Agreement, in each case of clauses (a) through (c) above except to the extent such Losses arise out of an Paratek Indemnitee’s gross negligence, illegal conduct or willful misconduct, or breach of this Agreement.
12.2. By Paratek. Paratek will indemnify and hold harmless Zai, its Affiliates, and their directors, officers, employees and agents (individually and collectively, the “Zai Indemnitee(s)”) from and against all Losses to the extent arising from (a) to the extent any of the following occur, Manufacturing, Development and Commercialization activities in the Territory, including the promotion of a Licensed Product and product liability claims relating to the Licensed Product in the Territory, by Paratek or any of its Affiliates or licensees (other than Zai), (b) the [*], illegal conduct or willful misconduct of Paratek, or (c) Paratek’s breach of any of its representations or warranties made in or pursuant to this Agreement or any covenants or obligations set forth in or entered into pursuant to this Agreement, in each case of clauses (a) through (c) above, except to the extent such Losses arise out of any of a Zai Indemnitee’s gross negligence, illegal conduct or willful misconduct, or breach of this Agreement.
12.3. Defined Indemnification Terms. Either of the Zai Indemnitee or the Paratek Indemnitee will be an “Indemnitee” for the purpose of this ARTICLE 12, and the Party that is obligated to indemnify the Indemnitee under Section 12.1 or Section 12.2 will be the “Indemnifying Party.”
12.4. Defense. If any such claims or actions are made, the Indemnitee will be defended at the Indemnifying Party’s sole expense by counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnitee, provided that the Indemnitee may, at its own expense, also be represented by counsel of its own choosing. The Indemnifying Party will have the sole right to control the defense of any such claim or action, subject to the terms of this ARTICLE 12.
12.5. Settlement. The Indemnifying Party may settle any such claim, demand, action or other proceeding or otherwise consent to an adverse judgment (a) with prior written notice to the Indemnitee but without the consent of the Indemnitee where the only liability to the Indemnitee is the payment of money and the Indemnifying Party makes such payment, or (b) in all other cases, only with the prior written consent of the Indemnitee, such consent not to be unreasonably withheld or delayed.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
36
12.6. Notice. The Indemnitee will notify the Indemnifying Party promptly of any claim, demand, action or other proceeding under Sections 12.1 or 12.2 and will reasonably cooperate with all reasonable requests of the Indemnifying Party with respect thereto.
12.7. Permission by Indemnifying Party. The Indemnitee may not settle any such claim, demand, action or other proceeding or otherwise consent to an adverse judgment in any such action or other proceeding or make any admission as to liability or fault without the express written permission of the Indemnifying Party.
12.8. LIMITATION OF LIABILITY. SUBJECT TO AND WITHOUT LIMITING THE INDEMNIFICATION OBLIGATIONS OF EACH PARTY WITH RESPECT TO THIRD PARTY CLAIMS UNDER SECTIONS 12.1 OR 12.2 OR LIABILITY AS A RESULT OF A BREACH OF ARTICLE 10, NO PARTY OR ANY OF ITS AFFILIATES WILL BE LIABLE TO THE OTHER PARTY UNDER ANY CONTRACT, WARRANTY, NEGLIGENCE, TORT, STRICT LIABILITY OR OTHER LEGAL OR EQUITABLE THEORY FOR ANY SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE, MULTIPLIED OR CONSEQUENTIAL DAMAGES OR FOR LOST PROFITS (EVEN IF DEEMED DIRECT DAMAGES) ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT.
ARTICLE 13
INTELLECTUAL PROPERTY
13.1. Ownership of Intellectual Property.
(a) As between the Parties, (i) Paratek will remain the sole and exclusive owner of all Paratek Technology, and (ii) Zai will remain the sole and exclusive owner of all Zai Technology.
(b) Ownership of all Inventions will be assigned based on inventorship, as determined in accordance with the rules of inventorship under United States patent laws. Each Party will own all Inventions, that are made solely by its and its Affiliates’ employees, agents, and independent contractors, that are made during the performance of activities under this Agreement (“Sole Inventions”). The Parties will jointly own all Inventions that are made jointly by the employees, agents, and independent contractors of one Party and its Affiliates together with the employees, agents, and independent contractors of the other Party and its Affiliates (“Joint Inventions”). Patents covering the Joint Inventions will be referred to as “Joint Patents.” Each Party will own an undivided half interest in the Joint Inventions, without a duty of accounting or an obligation to seek consent from the other Party for the exploitation or license of the Joint Inventions (subject to the licenses granted to the other Party under this Agreement). Zai hereby grants to Paratek a non-exclusive, royalty-free, fully paid-up, sublicensable license under Zai’s Sole Inventions, solely for Paratek to Develop, Manufacture, or Commercialize products outside of the Territory and Manufacture products in the Territory.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
37
13.2. Disclosure of Inventions. Each Party will promptly disclose to the other Party all Inventions, including all invention disclosure or other similar documents submitted to such party by its or its Affiliates’ employees, agents, or independent contractors relating to such Inventions, and will also promptly respond to reasonable requests from the other Party for additional information relating to such Inventions.
13.3. Patent Prosecution.
(a) Paratek Responsibilities. Subject to Section 13.5(b), Paratek will have sole decision making authority, at its sole cost and expense, over Patent Prosecution and maintenance of applications and registrations covering (i) Paratek Know-How, Paratek Patents, and Paratek’s Sole Inventions (such applications and registrations, the “Paratek Prosecution Patents”) and (ii) Joint Inventions that are specific to the Licensed Products. Paratek will keep Zai reasonably informed of the status of all actions taken, and will consider in good faith Zai’s recommendations with respect to the Paratek Prosecution Patents in the Territory and Joint Inventions that are specific to the Licensed Products worldwide.
(b) Zai Responsibilities. Zai will have sole decision making authority, at its sole cost and expense, over the Patent Prosecution and maintenance of patent applications and registrations covering (i) Zai Technology and (ii) Zai’s Sole Inventions (such applications and registrations, the “Zai Prosecution Patents”). Zai will keep Paratek reasonably informed of the status of all actions taken, and will consider in good faith Paratek’s recommendations with respect to the Zai Prosecution Patents and Joint Inventions prosecuted by Zai.
(c) The Parties will discuss the appropriate allocation of responsibility with respect to Joint Inventions that are not specific to the Licensed Products.
(d) Abandonment.
(i) Paratek Responsibilities. Paratek will notify Zai of any decision to cease Patent Prosecution or maintenance of any Paratek Prosecution Patents owned by Paratek in the Territory, or Joint Patents prosecuted by Paratek, and will provide such notice at least 60 days prior to any filing or payment due date, or any other due date that requires action, in connection with such Paratek Prosecution Patent in the Territory or such Joint Patent. In such event, Paratek will permit Zai, at its sole cost and expense, to continue Patent Prosecution or maintenance of such Paratek Prosecution Patent in the Territory or such Joint Patent. If Zai decides to take over Patent Prosecution or maintenance of such Paratek Prosecution Patent or such Joint Patent, then Paratek will promptly deliver to Zai copies of all necessary files related to such Paratek Prosecution Patent or such Joint Patent and will take all actions and execute all documents reasonably necessary for Zai to assume such responsibility. For the avoidance of doubt, Zai’s maintenance or Patent Prosecution of such Paratek Prosecution Patent or such Joint Patent will not change the Parties’ respective ownership rights with respect to such Paratek Prosecution Patent or such Joint Patent.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
38
(ii) Zai Responsibilities. Zai will notify Paratek of any decision to cease Patent Prosecution or maintenance of any Zai Prosecution Patents or Joint Patents prosecuted by Zai (if any), and will provide such notice at least 60 days prior to any filing or payment due date, or any other due date that requires action, in connection with such Zai Prosecution Patent (to the extent relating to the Licensed Product) or such Joint Patent. In such event, Zai will permit Paratek, at its sole cost and expense, to continue Patent Prosecution or maintenance of such Zai Prosecution Patent or such Joint Patent. If Paratek decides to take over Patent Prosecution or maintenance for a Zai Prosecution Patent or a Joint Patent, then Zai will promptly deliver to Paratek copies of all necessary files related to such Zai Prosecution Patent or such Joint Patent and will take all actions and execute all documents reasonably necessary for Paratek to assume such responsibility. For the avoidance of doubt, Paratek’s maintenance or Patent Prosecution of such Zai Prosecution Patent or such Joint Patent will not change the Parties’ respective ownership rights with respect to such Zai Prosecution Patent or such Joint Patent.
13.4. Patent and Trademark Prosecution Cooperation. With respect to all Patent Prosecution or trademark prosecution each Party will:
(a) execute any instruments to document their respective ownership consistent with this Agreement as reasonably requested by the other Party;
(b) make its employees, agents and consultants reasonably available to the other Party (or to the other Party’s authorized attorneys, agents or representatives), to the extent reasonably necessary to enable the appropriate Party hereunder to undertake its Patent Prosecution responsibilities;
(c) cooperate, if necessary, with the other Party in gaining Patent term extensions; and
(d) act in good faith to coordinate its efforts under this Agreement with the other Party to minimize or avoid interference with the Patent Prosecution of the other Party’s Patents to a Licensed Product or trademarks.
13.5. Enforcement.
(a) Each Party will notify the other within 30 Business Days of becoming aware of any alleged or threatened infringement by a Third Party of any of the Paratek Patents, Zai Patents, or Joint Patents which infringement adversely affects or is expected to adversely affect any Licensed Product, and any related declaratory judgment, opposition, or similar action alleging the invalidity, unenforceability or non-infringement of any of the Paratek Patents, Zai Patents, or Joint Patents (collectively “Product Infringement”).
(b) Zai will have the first right to bring and control any legal action in connection with such Product Infringement in the Territory at its own expense as it reasonably determines appropriate. If Zai decides not to bring such legal action, it will so inform Paratek promptly and Paratek will have the right to bring and control any legal action in connection with such Product Infringement in the Territory at its own expense as it reasonably determines appropriate.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
39
(c) Paratek will have the exclusive right to bring and control any legal action in connection with Product Infringement outside the Territory at its own expense as it reasonably determines appropriate.
(d) Each Party will have the first right in its territory to enforce the Joint Patents for any infringement that is not a Product Infringement at its own expense as it reasonably determines appropriate. If such Party decides not to bring such legal action, it will so inform the other Party promptly and the other Party will have the right to bring and control any legal action in connection with such infringement at its own expense as it reasonably determines appropriate.
(e) At the request of the Party bringing an action related to Product Infringement, the other Party will provide reasonable assistance in connection therewith, including by executing reasonably appropriate documents, cooperating in discovery and joining as a party to the action if required by Applicable Law to pursue such action, at each such Party’s sole cost and expense. In connection with an action related to Product Infringement, the Party bringing the action will not enter into any settlement admitting the invalidity or non-infringement of, or otherwise impairing the other Party’s rights in the Paratek Patents, Zai Patents or Joint Patents (as applicable) without the prior written consent of the other Party.
(f) Any recoveries resulting from enforcement action relating to a claim of Product Infringement in the Territory will be first applied against payment of each Party’s costs and expenses in connection therewith. Any such recoveries in excess of such costs and expenses will be split as follows: [*]
13.6. Defense.
(a) Each Party will notify the other in writing of any allegations it receives from a Third Party that the Exploitation of any Licensed Product or any embodiment of any technology or intellectual property licensed by a Party under this Agreement infringes the intellectual property rights of such Third Party. Such notice will be provided promptly, but in no event after more than 15 days following receipt of such allegations. Such written notice will include a copy of any summons or complaint (or the equivalent thereof) received regarding the foregoing. Each Party will assert and not waive the joint defense privilege with respect to all communications between the Parties.
(b) In such event, the Parties will agree how best to mitigate or control the defense of any such legal proceeding, agree whether to enter into a joint defense agreement to, among other reasons, preserve the confidentiality of communications or cooperation between the Parties in relation to such defense, and determine which Party is best suited to assume the primary responsibility for the conduct of the defense of any such claim at their expense. The other Party will have the right, but not the obligation, to participate and be separately represented in any such suit at its sole option and at its own expense. Each Party will reasonably cooperate with the Party conducting the defense of the claim. If a Party or any of its Affiliates have been individually named as a defendant in a legal proceeding relating to the alleged infringement of a Third Party’s Patents or other intellectual property right as a result of the Exploitation of a Licensed Product, then that Party will conduct the defense and the other Party will be allowed to join in such action, at its own expense.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
40
(c) The Parties will keep each other informed of the status of and of their respective activities regarding any infringement litigation initiated by a Third Party concerning a Party’s Exploitation of a Licensed Product or settlement thereof; provided, however, that no settlement or consent judgment or other voluntary final disposition of a suit under this Section 13.6 may be undertaken by a Party without the consent of the other Party which consent will not be unreasonably withheld or delayed.
ARTICLE 14
TERMS AND TERMINATION
14.1. Term. This Agreement will be effective as of the Effective Date, and will continue, on a region-by-region basis, in effect until the expiration of and payment by Zai of all Zai’s payment obligations set forth in Section 9.4(c) applicable to such region (the “Term”). On a region-by-region basis, upon the natural expiration of this Agreement as contemplated in this Section 14.1, the licenses granted by Paratek to Zai under this Agreement in such region will become a fully paid-up, non-exclusive, perpetual, and irrevocable license.
14.2. Termination for Convenience. At any time prior to [*], Zai will have the right to terminate this Agreement in its entirety for any or no reason upon [*] written notice to Paratek. Following [*], Zai will have the right to terminate this Agreement in its entirety for any or no reason upon [*] written notice to Paratek. Zai shall terminate this Agreement if it determines that it will permanently discontinue all Development and Commercialization activities with respect to the Licensed Product under this Agreement.
14.3. Termination for Material Breach.
(a) This Agreement may be terminated in its entirety at any time during the Term upon written notice by either Party if the other Party materially breaches a material term of the Agreement and, if such breach is curable, such breach has not been cured within [*] ([*] if such breach is a material breach of any obligation under the Tufts Agreement) after notice requesting cure of such breach; provided that the applicable material breach cure period will not apply to [*], and [*] will have the right to terminate this Agreement, with immediate effect, upon written notice [*].
(b) For the avoidance of doubt, the Parties agree that [*] will be deemed material terms of the Agreement.
14.4. Termination for Patent Challenge. Except to the extent the following is unenforceable under the laws of a particular jurisdiction, Paratek may terminate this Agreement in its entirety, immediately if Zai or its Affiliates or Sublicensees, individually or in association with any other person or entity, commences a legal action challenging the validity, enforceability or scope of any Patents owned or Controlled by Paratek anywhere in the world Notwithstanding the foregoing, if Zai promptly terminates the sublicense agreement of any Sublicensee that commences a legal action challenging the validity, enforceability or scope of any Patents owned or Controlled by Paratek anywhere in the world, Paratek shall not have the right to terminate this Agreement under this Section 14.4.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
41
14.5. Termination for Insolvency. Each Party will have the right to terminate this Agreement upon delivery of written notice to the other Party in the event that (a) such other Party files in any court or agency pursuant to any statute or regulation of any jurisdiction a petition in bankruptcy or insolvency or for reorganization or similar arrangement for the benefit of creditors or for the appointment of a receiver or trustee of such other Party or its assets, (b) such other Party is served with an involuntary petition against it in any insolvency proceeding and such involuntary petition has not been stayed or dismissed within [*] of its filing, or (c) such other Party makes an assignment of substantially all of its assets for the benefit of its creditors.
14.6. Election to Terminate. If either Party has the right to terminate under Sections 14.2 through 14.5, it may at its sole option, elect either to (a) terminate this Agreement and pursue any legal or equitable remedy available to it or (b) maintain this Agreement in effect and pursue any legal or equitable remedy available to it.
14.7. Effect of Termination.
(a) Upon the termination of this Agreement for any reason, all rights and licenses (including the rights and licenses with respect to the Licensed Product) granted to a Party herein will immediately terminate, and all sublicenses of such rights and licenses will also terminate; provided that the licenses granted by Zai to Paratek pursuant to Sections 2.3 and 13.1(b) will become perpetual and irrevocable to Develop, Manufacture and Commercialize Licensed Products worldwide. Termination of this Agreement for any reason will not release either Party of any obligation or liability which, at the time of such termination, has already accrued to the other Party or which is attributable to a period prior to such termination. Notwithstanding anything herein to the contrary, termination of this Agreement by a Party will be without prejudice to other remedies such Party may have at law or equity.
(b) Upon termination of this Agreement for any reason (other than termination by Zai pursuant to Section 14.3), the following additional provisions will apply:
(i) Reversion of Rights to Paratek. Any rights and licenses with respect to the Licensed Product granted to Zai under this Agreement will immediately terminate, and all such rights will revert back to Paratek.
(ii) Regulatory Materials; Data. Zai will, and will cause its Affiliates and Sublicensees to, at no cost to Paratek, (1) assign all Regulatory Materials and Regulatory Approvals of Licensed Products to Paratek to the maximum extent permitted by Applicable Law at the time of any such termination, and (2) assign all data generated by or on behalf of Zai while conducting Development, Manufacturing, or Commercialization activities under the Agreement to Paratek, including non-clinical and clinical studies conducted by or on behalf of Zai on Licensed Products and all pharmacovigilance data (including all Adverse Event database information) on Licensed Products.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
42
(iii) Trademarks. Zai will, and will cause its Affiliates and Sublicensees, to promptly transfer and assign to Paratek, at no cost to Paratek, all Product Marks (excluding any such ▇▇▇▇ that include, in whole or in part, any corporate name or logos of Zai or its Affiliates).
(iv) Transition Assistance. Zai will, and will cause its Affiliates and Sublicensees, to provide assistance, [*], as may be reasonably necessary or useful for Paratek to commence or continue Developing, Manufacturing or Commercializing Licensed Products in the Territory, to the extent Zai is then performing or having performed such activities, including without limitation transferring or amending as appropriate, upon request of Paratek, any agreements or arrangements with Third Party to Develop, Manufacture, and Commercialize the Licensed Products in the Territory. To the extent that any such contract between Zai and a Third Party is not assignable to Paratek, then Zai will reasonably cooperate with Paratek to arrange to continue to and provide such services from such entity.
(v) Ongoing Clinical Trial. If at the time of such termination, any Clinical Trials for the Licensed Products are being conducted by or on behalf of Zai, then, at Paratek’s election on a trial-by-trial basis: (1) Zai will, and will cause its Affiliates and Sublicensees to, fully cooperate with Paratek to transfer the conduct of all such Clinical Trials to Paratek and Paratek will assume any and all liability and costs for such Clinical Trials after the effective date of such termination; or (2) Zai will, and will cause its Affiliates and Sublicensees to, [*], orderly wind down the conduct of any such Clinical Trial which is not assumed by Paratek under clause (1).
(c) Termination by Zai Due to Material Breach. Upon termination of this Agreement by Zai pursuant to Section 14.3, [*] to the extent [*], including [*].
(d) Royalty after Termination. If (i) [*] terminates this Agreement pursuant to [*] or (ii) this Agreement is terminated [*], and if Paratek, itself or through an Affiliate or a Third Party, Commercializes any Licensed Product in the Territory, Paratek shall pay Zai a commercially reasonable royalty on the Net Sales of all such Licensed Products in the Territory at a royalty rate and duration to be determined by the Parties by good faith negotiations. If the Parties are unable to agree to terms within [*] of commencing such negotiations, the disputed terms will be resolved by arbitration as set forth in Section 15.4.
14.8. Survival. Termination or expiration of this Agreement shall not affect any rights or obligations of the Parties under this Agreement that have accrued prior to the date of termination or expiration. The following provisions will survive the termination or expiration of this Agreement for any reason: [*].
ARTICLE 15
DISPUTE RESOLUTION
15.1. General. The Parties recognize that a dispute may arise relating to this Agreement (a “Dispute”). Any Dispute, including Disputes that may involve the Affiliates of any Party, will be resolved in accordance with this ARTICLE 15.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
43
15.2. Continuance of Rights and Obligations During Pendency of Dispute Resolution. If there are any Disputes in connection with this Agreement, including Disputes related to termination of this Agreement under ARTICLE 14, all rights and obligations of the Parties will continue until such time as any Dispute has been resolved in accordance with the provisions of this ARTICLE 15.
15.3. Escalation. Any claim, Dispute, or controversy as to the breach, enforcement, interpretation or validity of this Agreement will be referred to the Executive Officers set forth in Section 3.2(f) for attempted resolution. In the event the Executive Officers are unable to resolve such Dispute within 30 days of such Dispute being referred to them, then, upon the written request of either Party to the other Party, the Dispute will be subject to arbitration in accordance with Section 15.4.
15.4. Arbitration.
(a) If the Parties fail to resolve the Dispute through escalation to the Executive Officers under Section 15.3, and a Party desires to pursue resolution of the Dispute, the Dispute will be submitted by either Party for resolution in arbitration under the [*].
(b) There will be three arbitrators, the chairperson of whom will be appointed by the two party arbitrators. If, however, the aggregate award sought by the Parties is less than [*] and equitable relief is not sought, a single arbitrator will be chosen in accordance with the [*].
(c) The seat of arbitration will be [*] and the language of the proceedings will be English.
(d) The Parties agree that any award or decision made by the arbitral tribunal will be final and binding upon them and may be enforced in the same manner as a judgment or order of a court of competent jurisdiction. The arbitral tribunal will render its final award within nine months from the date on which the Request for Arbitration by one of the Parties wishing to have recourse to arbitration is received by the [*]. The arbitral tribunal will determine the dispute by applying the provisions of this Agreement and the governing law set forth in Section 16.5.
(e) By agreeing to arbitration, the Parties do not intend to deprive any court of its jurisdiction to issue, at the request of a Party, a pre-arbitral injunction, pre-arbitral attachment or other order to avoid irreparable harm, maintain the status quo, preserve the subject matter of the Dispute, or aid the arbitration proceedings and the enforcement of any award. Without prejudice to such provisional or interim remedies in aid of arbitration as may be available under the jurisdiction of a competent court, the arbitral tribunal will have full authority to grant provisional or interim remedies and to award damages for the failure of any Party to the dispute to respect the arbitral tribunal’s order to that effect.
(f) EACH PARTY HERETO WAIVES: (I) ITS RIGHT TO TRIAL OF ANY ISSUE BY JURY, AND (II) ANY CLAIM FOR ATTORNEY FEES, COSTS AND PREJUDGMENT INTEREST.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
44
(g) Each Party will bear its own attorney’s fees, costs, and disbursements arising out of the arbitration, and will pay an equal share of the fees and costs of the administrator and the arbitrator; provided, however, that the arbitrator will be authorized to determine whether a Party is the prevailing party, and if so, to award to that prevailing party reimbursement for any or all of its reasonable attorneys’ fees, costs and disbursements (including, for example, expert witness fees and expenses, photocopy charges, travel expenses, etc.), and/or the fees and costs of the administrator and the arbitrator.
(h) Notwithstanding anything in this Section 15.4, in the event of a Dispute with respect to the validity, scope, enforceability or ownership of any Patent or other intellectual property rights, and such Dispute is not resolved in accordance with Section 15.3, such Dispute will not be submitted to an arbitration proceeding in accordance with this Section 15.4, unless otherwise agreed by the Parties in writing, and instead, either Party may initiate litigation in a court of competent jurisdiction in any country in which such rights apply.
ARTICLE 16
MISCELLANEOUS
16.1. Force Majeure. Neither Party will be held liable to the other Party nor be deemed to have defaulted under or breached this Agreement for failure or delay in performing any obligation under this Agreement to the extent such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party including embargoes, war, acts of war (whether war be declared or not), insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, fire, floods, or other acts of God or any other deity, or acts, omissions or delays in acting by any Governmental Authority. The affected Party will notify the other Party of such force majeure circumstances as soon as reasonably practical, and will promptly undertake all reasonable efforts necessary to cure such force majeure circumstances.
16.2. Assignment. Neither Party may assign this Agreement to a Third Party without the other Party’s prior written consent (such consent not to be unreasonably withheld); except that (a) Paratek may make such an assignment without Zai’s consent to a successor to substantially all of the business of Paratek to which this Agreement relates (whether by merger, sale of stock, sale of assets or other transaction), (b) Zai may make such an assignment without Paratek’s consent to a successor to substantially all of the business of Zai (whether by merger, sale of stock, sale of assets or other transaction), and (c) either Party may assign this Agreement to an Affiliate without the other Party’s consent. This Agreement will inure to the benefit of and be binding on the Parties’ successors and permitted assigns. Any assignment or transfer in violation of this Section 16.2 will be null and void and wholly invalid, the assignee or transferee in any such assignment or transfer will acquire no rights whatsoever, and the non-assigning non-transferring Party will not recognize, nor will it be required to recognize, such assignment or transfer.
16.3. Severability. If any one or more of the provisions contained in this Agreement is held invalid, illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained herein will not in any way be affected or impaired thereby, unless the absence of the invalidated provision(s) adversely affects the substantive rights of the Parties. The Parties will in such an instance use their best efforts to replace the invalid, illegal or unenforceable provision(s) with valid, legal and enforceable provision(s) which, insofar as practical, implement the purposes of this Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
45
16.4. Notices. All notices which are required or permitted hereunder will be in writing and sufficient if delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), sent by nationally-recognized overnight courier or sent by registered or certified mail, postage prepaid, return receipt requested, addressed as follows:
If to Paratek:
Paratek Bermuda Ltd.
C/O Paratek Pharmaceuticals, Inc.
Address: ▇▇ ▇▇▇▇ ▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
[*]
with a copy to:
Ropes & ▇▇▇▇, LLP
Address: 36/F, ▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇
[*]
If to Zai:
Zai Lab (Shanghai) Co., Ltd.
Address: ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇, P.R. China, 201203
[*]
with a copy to:
▇▇▇▇▇▇ LLP
Address: ▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇ ▇▇▇
[*]
or to such other address as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such notice will be deemed to have been given: (a) when delivered if personally delivered or sent by facsimile on a Business Day; (b) on the Business Day after dispatch if sent by nationally-recognized overnight courier; or (c) on the fifth Business Day following the date of mailing if sent by mail.
16.5. Governing Law. This Agreement will be governed by and construed in accordance with the laws of the State of New York, U.S. without reference to any rules of conflict of laws.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
46
16.6. Entire Agreement; Amendments. The Agreement contains the entire understanding of the Parties with respect to the subject matter hereof. All express or implied agreements and understandings, either oral or written, with regard to the subject matter hereof (including the licenses granted hereunder) are superseded by the terms of this Agreement. Neither Party is relying on any representation, promise, nor warranty not expressly set forth in this Agreement. This Agreement may be amended, or any term hereof modified, only by a written instrument duly executed by authorized representatives of both Parties hereto.
16.7. Headings. The captions to the several Sections hereof are not a part of this Agreement, but are merely for convenience to assist in locating and reading the Sections of this Agreement.
16.8. Independent Contractors. It is expressly agreed that Paratek and Zai will be independent contractors and that the relationship between the two Parties will not constitute a partnership, joint venture or agency. Neither Paratek nor Zai will have the authority to make any statements, representations or commitments of any kind, or to take any action, which will be binding on the other Party, without the prior written consent of the other Party.
16.9. Waiver. The waiver by either Party of any right hereunder, or the failure of the other Party to perform, or a breach by the other Party, will not be deemed a waiver of any other right hereunder or of any other breach or failure by such other Party whether of a similar nature or otherwise.
16.10. Waiver of Rule of Construction. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement will be construed against the drafting Party will not apply.
16.11. Construction. Except where the context expressly requires otherwise, (a) the use of any gender herein will be deemed to encompass references to either or both genders, and the use of the singular will be deemed to include the plural (and vice versa), (b) the words “include”, “includes” and “including” will be deemed to be followed by the phrase “without limitation”, (c) the word “will” will be construed to have the same meaning and effect as the word “shall”, (d) any definition of or reference to any agreement, instrument or other document herein will be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any person will be construed to include the person’s successors and assigns, (f) the words “herein”, “hereof” and “hereunder”, and words of similar import, will be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to Sections, Schedules, or Exhibits will be construed to refer to Sections, Schedules or Exhibits of this Agreement, and references to this Agreement include all Schedules and Exhibits hereto, (h) the word “notice” means notice in writing (whether or not specifically stated) and will include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder “agree”, “consent” or “approve” or the like will require that such agreement, consent or approval
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
47
be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (j) references to any specific law, rule or regulation, or Section, section or other division thereof, will be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof, and (k) the term “or” will be interpreted in the inclusive sense commonly associated with the term “and/or” where applicable.
16.12. Counterparts. This Agreement may be executed in two or more counterparts, each of which will be deemed an original, but all of which together will constitute one and the same instrument. Each Party will be entitled to rely on the delivery of executed facsimile copies of counterpart execution pages of this Agreement and such facsimile copies will be legally effective to create a valid and binding agreement among the Parties.
16.13. Language. This Agreement is in the English language only, which language will be controlling in all respects, and all versions hereof in any other language will be for accommodation only and will not be binding upon the Parties. All communications and notices to be made or given pursuant to this Agreement, and any dispute proceeding related to or arising hereunder, will be in the English language. If there is a discrepancy between any translation of this Agreement and this Agreement, this Agreement will prevail.
{Signature Page Follows}
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
48
IN WITNESS WHEREOF, the Parties intending to be bound have caused this License and Collaboration Agreement to be executed by their duly authorized representatives as of the Effective Date.
| Paratek Bermuda Ltd. | Zai Lab (Shanghai) Co., Ltd. | |||||||
| By: | /s/ ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ |
By: | /s/ Samantha Du | |||||
| Name: | ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | Name: | Samantha Du | |||||
| Title: | Director | Title: | CEO | |||||
| Date: | April 21, 2017 | Date: | April 21, 2017 | |||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Schedule 1.23
Chemical Structure of the Compound
Omadacycline (OMC, PTK-796)
(4S,4aS,5aR,12aS)-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-9-
((neopentylamino)methyl)-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-
carboxamide
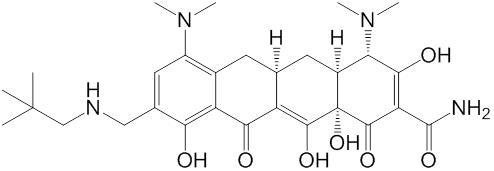
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Schedule 1.69
Paratek Patents as of the Effective Date
| Country |
M&E Ref. |
Paratek |
Type |
Application No. Publication No. |
Title |
Filing Date |
Patent No. |
Issue Date |
Expiration Date |
Status | ||||||||||
| [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | [*] | ||||||||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Schedule 2.4(b)
Material Sublicensees [*]
[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Schedule 5.2
Initial Development Plan
[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Schedule 8.4
Paratek Product Marks
| Cntry |
Trademark |
Status |
App. No. |
Reg. No. | ||||
| [*] | [*] | [*] | [*] | [*] | ||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.

