1) LIPOXEN TECHNOLOGIES LTD - and - (2) PHARMASYNTHEZ ZAO COLLABORATION, LICENCE AND DEVELOPMENT AGREEMENT
Exhibit 10.20
| Final Version (11 November 2009) | Private and Confidential |
| DATED | [ ] |
(1) LIPOXEN TECHNOLOGIES LTD
- and -
(2) PHARMASYNTHEZ ZAO
COLLABORATION, LICENCE AND
DEVELOPMENT AGREEMENT
| Final Version (11 November 2009) | Private and Confidential |
THIS AGREEMENT is made the [ ] day of [ ] 2009
BETWEEN:
| (1) | Lipoxen Technologies Ltd, a Company registered under the laws of England whose registered office is at Suite 000 Xxxxxxxx Xxxxx, Xxxxxxxx Xxxxx, Xxxxxx XXXX 0XX, Xxxxxxx (“Lipoxen”); and |
| (2) | Pharmasynthez Zao, a limited liability company incorporated under the laws of Russian Federation, registration number P-15450.16, having its Registered Office at s 188663, Leningradskaya oblast, Vsevologsky district, Capitolovo, Experimental Factory RNZ “Applied Chemistry” (“Pharms”). |
RECITALS:
| (1) | Lipoxen is a drug and vaccine delivery company and is dedicated to innovative methods for the optimal delivery of therapeutics in the treatment and prevention of disease. |
| (2) | Lipoxen has two proprietary technologies, ImuXen and PolyXen, and has a number of drug candidates in development. |
| (3) | ImuXen is an advanced enabling technology that uses liposome-based constructs to boost the effectiveness of DNA, protein and polysaccharide vaccines. |
| (4) | PolyXen involves the use of polysialic acid conjugation as a means to improve the pharmacokinetics and pharmacodynamics of protein drugs. |
| (5) | Pharms is engaged in the manufacture of pharmaceuticals and biotechnology products and has developed certain protein and vaccine drug candidates. Pharms owns or will exclusive rights to certain active compounds that may benefit from the application of Lipoxen’s technology. |
| (6) | Lipoxen and Pharms now wish to enter into a collaboration to develop certain products combining Lipoxen’s technology and Pharms’ technology which, if successful, will lead to clinical development of product candidates by Pharms in the Pharms Territory (defined below) and by the parties jointly in the Joint Territory (defined below), subject to and in accordance with the terms of this Agreement. |
IT IS AGREED as follows:
Definitions
In this Agreement, the following words shall have the following meanings:
| “Actives” | means DNAse, Doxorubicin, Oxyntomodulin, MBP Epitope, HIV Antigen and H1; |
2
| Final Version (11 November 2009) | Private and Confidential |
| “Affiliate” | in relation to a party, means any entity or person which controls, is controlled by, or is under common control with that party. For the purposes of this definition, “control” shall mean direct or indirect beneficial ownership of 50% (or, outside a party’s home territory, such lesser percentage as is the maximum, permitted level of foreign investment) or more of the share capital, stock or other participating interest carrying the right to vote or to distribution of profits of that entity or person, as the case may be; | |
| “Appointed CRO” | means any contract research organisation appointed by either of the parties to carry out the clinical trials in relation to the Products; | |
| “Appointed CMO” | means the CMO appointed in accordance with clause 7.6 of this Agreement; | |
| “Arising IPR” | means any and all Intellectual Property Rights arising from or in relation to the work carried out by or on behalf of Pharms and/or Lipoxen in relation to this Agreement, including any and all Intellectual Property Rights relating to the Results and any and all data and results arising from the Pharms Trials and the Clinical Trials; | |
| “Clinical Trials” | means the clinical trials to be carried out by the parties in relation to the Products in the Joint Territory in Stage 3; | |
| “Commencement Date” | means the date of this Agreement; | |
| “Confidential Information” | means any and all data, results, know-how, show-how, software, algorithms, trade secrets, plans, forecasts, analyses, evaluations, research, technical information, business information, financial information, business plans, strategies, customer lists, marketing plans, or other information whether oral, in writing, in electronic form or in any other form, and any physical items, compounds, components or other materials disclosed before, on or after the date of this Agreement by one party (and/or its Affiliates) to the other party (and/or its Affiliates) including, but not limited to, the Lipoxen Know How and the Pharms Know How; | |
| “Development Programme” | means the detailed programme for the collaboration for each Product set out in Schedule 1 of this Agreement as modified from time to time by the Programme Committee in accordance with clause 7.9.2 and otherwise in accordance with the terms of this Agreement; | |
3
| Final Version (11 November 2009) | Private and Confidential |
| “DNAse” | means deoxyribonuclease-1 protein as further described in Part 1 of Schedule 2 of this Agreement; | |
| “Doxorubicin” | means doxorubicin as further described in Part 2 of Schedule 2 of this Agreement; | |
| “EMEA” | means the European Medicines Agency (formerly known as the European Agency for the Evaluation of Medicinal Products) and/or any successor to it; | |
| “FDA” | means the US Food and Drug Administration and/or any successor to it; | |
| “GMP” | means current Good Manufacturing Practice as defined by regulations issued from time to time by regulatory authorities, including EMEA and FDA; | |
| “H1” | means human recombinant histone H1.3 as further described in Part 6 of Schedule 2 of this Agreement; | |
| “HIV Antigen” | means the HIV GP120 based recombinant fusion protein which is further described in Part 5 of Schedule 2 of this Agreement; | |
| “ImuXen Know How” | means the any and all know how which is disclosed to Pharms pursuant to this Agreement that relates to the inventions disclosed in the ImuXen Patents; | |
| “ImuXen Patents” | means the patents and patent applications set out in Schedule 3 of this Agreement, including any continuations, continuations in part, extensions, reissues, divisions, and any patents, supplementary protection certificates and similar rights that are based on or derive priority from the foregoing; | |
| “ImuXen Products” | means Product D, Product E and Product F; | |
| “ImuXen Technology” | means the advanced platform vaccine delivery technology that employs novel liposome constructs to boost the effectiveness of DNA, protein and polysaccharide vaccines that is described in detail in the ImuXen Patents; | |
| “Intellectual Property Rights” | means inventions, patents, any extensions of the exclusivity granted in connection with patents, xxxxx patents, utility models, applications for any of the foregoing (including, but not limited to, continuations, continuations-in-part and divisional applications), the right to apply for any of the foregoing, database rights, rights in data and know-how, trade secrets and confidential information and all other forms of intellectual property rights having equivalent or similar effect to any of the foregoing which may exist anywhere in the world; | |
| “Joint Arising IPR” | means the Arising IPR which is owned jointly by Lipoxen and Pharms pursuant to clause 8.3; | |
4
| Final Version (11 November 2009) | Private and Confidential |
| “Joint Territory” | means the world, excluding the Pharms Territory; | |||
| “Joint Products” | means Products that are not Lipoxen Products; | |||
| “Know How Transfer Time” | means the time of two scientist each working for ten (10) working days; | |||
| “Licensee” | means a third party to which Lipoxen has granted a licence to exploit a Product in the Joint Territory; | |||
| “Liposomal HIV Antigen” | means liposomal vehicles containing HIV Antigen; | |||
| “Liposomal H1” | means liposomal vehicles containing H1; | |||
| “Liposomal MBP Epitopes” | means liposomal vehicles containing MBP Epitopes; | |||
| “Lipoxen Arising IPR” | means any and all Arising IPR which is owned by Lipoxen pursuant to clause 8.2 of this Agreement; | |||
| “Lipoxen Know How” | means the ImuXen Know How and the PolyXen Know How; | |||
| “Lipoxen Patents” | means the ImuXen Patents and PolyXen Patents; | |||
| “Lipoxen Products” | means any of the Products which fall within the scope of clause 5.3; | |||
| “Lipoxen Technology” | means the ImuXen Technology, the PolyXen Technology and the PSA IP; | |||
| “MBP Epitopes” | means the oligopeptides representing immunogenic epitopes of myelin basic protein which are described in Part 4 of Schedule 2 of this Agreement; | |||
| “Oxyntomodulin” | means human recombinant Oxyntomodulin as further described in Part 3 of Schedule 2 of this Agreement; | |||
| “Pharms Active Components” | means the aspects of the Products which are owned by or licensed to Pharms, as set out in Schedule 4 of this Agreement; | |||
| “Pharms Arising IPR” | means any and all Arising IPR which is owned by Pharms pursuant to clause 8.1 of this Agreement; | |||
| “Pharms Background IP” | means and any all Intellectual Property Rights owned by or licensed to Pharms that relate to the Products including (to the extent they do not form part of the Joint Arising IPR), but not limited to, any and all Intellectual Property Rights relating to: | |||
| (1) | (a) any methods or processes used by Pharms to manufacture the Products, the Actives and/or the Pharms Active Components; and | |||
| (2) | (b) the components of the Products, including the Actives, the Pharms Active Components; | |||
5
| Final Version (11 November 2009) | Private and Confidential |
| “Pharms Know How” | means any and all know how which is disclosed to Lipoxen pursuant to this Agreement that relates to the Pharms Background IP; | |
| ; | ||
| “Pharms Territory” | means Russian Federation; | |
| “Pharms Trials” | means the clinical trials to be carried out by Pharms in the Pharms Territory in relation to the Products in Stage 2 as set out in Schedule 5; | |
| “PolyXen Know How” | means the any and all know how which is disclosed to Pharms pursuant to this Agreement that relates to the inventions disclosed in the PolyXen Patents; | |
| “PolyXen Patents” | means the patents and patent applications set out in Schedule 6 of this Agreement, including any continuations, continuations in part, extensions, reissues, divisions, and any patents, supplementary protection certificates and similar rights that are based on or derive priority from the foregoing; | |
| “PolyXen Products” | means Product A, Product B and Product C; | |
| “PolyXen Technology” | means the multifaceted platform technology that employs PSA to prolong the active life and improve the pharmacokinetics of therapeutic proteins and peptides, as well as conventional drugs, that is described in detail in the PolyXen Patents; | |
| “Products” | means Product A, Product B, Product C, Product D, Product E and Product F; | |
| “Product A” | means a pharmaceutical preparation for the prevention and/or treatment of cystic fibrosis in humans containing PSA DNAse; | |
| “Product B” | means a pharmaceutical preparation for the prevention and/or treatment of acute myeloid leukemia and/or non-Hodgkin lymphoma in humans containing PSA Doxorubicin; | |
| “Product C” | means a pharmaceutical preparation for the prevention and/or treatment of type 2 diabetes in humans containing PSA Oxyntomodulin; | |
| “Product D” | means a Vaccine for the prevention and/or treatment of secondary progressive multiple sclerosis in humans which is comprised of Liposomal MBP Epitopes; | |
6
| Final Version (11 November 2009) | Private and Confidential |
| “Product E” | means a Vaccine for the prevention and/or treatment of HIV in humans which is comprised of Liposomal HIV Antigen; | |
| “Product F” | means a Vaccine for the prevention and/or treatment of non-hodgkin lymphoma in humans which is comprised of Liposomal H1; | |
| “Programme Committee” | means a committee formed and operating in accordance with clause 8 of this Agreement; | |
| “PSA” | means any polymer containing two or more sialic acid residues, including the natural polymer polysialic acid, the chemical formula for which is set out in Schedule 7; | |
| “PSA Doxorubicin” | means a conjugate of PSA and Doxorubicin forming a mono-PSA/multi-Doxorubicin conjugate; | |
| “PSA DNAse” | means a conjugate of PSA and DNAse; | |
| “PSA IP” | means any and all Intellectual Property Rights owned by or licensed to Lipoxen relating to the manufacture of PSA; | |
| “PSA Oxyntomodulin” | means a conjugate of PSA and Oxyntomodulin; | |
| “Quarter” | means the quarterly periods ending 31 March, 30 June, 30 September and 31 December; | |
| “Results” | means the results of the Development Programme; | |
| “Specifications” | means the specifications for the Products to be determined by the Programme Committee in accordance with clause 7.9.1 of this Agreement; | |
| “Stage 1” | means stage 1 of the collaboration which will involve optimisation of the Products through application of the PolyXen Technology and the ImuXen Technology as further described in Part 1 of the Development Programme for each Product; | |
| “Stage 2” | means stage 2 of the collaboration which will involve testing of the Products in the Pharms Trials in the Territory to achieve clinical proof of principal for the Products, as further described in Part 2 of the Development Programme for each Product; | |
| “Stage 2 Expiry Date” | means in relation to a Product the date upon which the Pharms Trial relating to the relevant Product has been completed; | |
7
| Final Version (11 November 2009) | Private and Confidential |
| “Stage 3” | means full-scale pharmaceutical and clinical development of the Products under EMEA/FDA regulations, to be determined by the Programme Committee in accordance with clause 7.9.2 in relation to the Joint Products or by Lipoxen in relation to the Lipoxen Products; | |
| “Stage 1 Costs” | means any and all costs and expenses incurred by Lipoxen and/or Pharms in relation to Stage 1; | |
| “Stage 2 Costs” | means any and all costs and expenses incurred by Lipoxen and/or Pharms in relation to Stage 2; | |
| “Stage 3 Costs” | means any and all costs and expenses properly and reasonably incurred by Lipoxen and/or Pharms in relation to Stage 3; | |
| “Success Criteria” | means the criteria to be determined by the Programme Committee for each of the Products which the relevant Product must meet prior to entering Stage 2 and/or Stage 3, as described in clause 7.9.1 of this Agreement; | |
| “Third Party IP Rights” | means Third Party IP Rights as defined in clause 8.12; | |
| “Timetable” | means the timetable for the Development Programme set out in Schedule 1 of this Agreement; | |
| “Vaccine” | means preparations of antigenic substances that are administered for the purpose of inducing in the recipient a specific and active immunity against the infective agent or toxin produced by it; and. | |
| “Valid Claim” | means a claim of a patent or patent application that has not expired or been held invalid or unenforceable by a decision of a patent office or court of competent jurisdiction, which decision (a) it is not possible to appeal or, (b) is not the subject of an appeal within the prescribed time limits. | |
| 2. | Doxorubicin |
| 2.1 | Subject to clause 2.1, the parties agree that Doxorubicin and Product B shall be excluded entirely from the scope of this Agreement until such time that Lipoxen notifies Pharms in writing that Lipoxen is free and able to grant rights to Pharms in relation to Doxorubicin and Product B. |
| 2.2 | Clause 8.3 of this Agreement shall be binding on Pharms from the Commencement Date in so far as it relates to Doxorubicin, Product B and/or Active Pharms Components relating to Doxorubicin and/or Product B. |
| 2.3 | On receipt of the notice referred to in clause 2.1 by Pharms, Doxorubicin and Product B shall automatically be deemed to fall under the scope of this Agreement without any further action by either of the parties. |
8
| Final Version (11 November 2009) | Private and Confidential |
| 3 | Stage 1: Candidate Optimisation |
| 3.1 | Lipoxen and Pharms shall collaborate to fulfill the objectives of Stage 1. |
| 3.2 | Each party shall use its reasonable endeavours to fulfill the obligations allocated to it in Stage 1 in accordance with the Timetable. |
| 3.3 | The parties acknowledge that in Xxxxx 0, Xxxxxxx’s obligations are limited to a transfer of know how from Lipoxen to Pharms to enable Pharms to carry out its obligations under Stage 1. In order to fulfill the transfer of know how, unless Lipoxen agrees otherwise in writing, Lipoxen shall not be obliged to provide more than the Know How Transfer Time. The transfer of know how shall take place, unless the parties agree otherwise in writing, by telephone calls and/or at Lipoxen’s premises in England. |
| 3.4 | Pharms shall promptly provide Lipoxen with any and all Actives reasonably required by Lipoxen to carry out its obligations under Stage 1. |
| 3.5 | Unless Pharms and Lipoxen agree otherwise, a Product shall not become part of Stage 2 unless it meets the Success Criteria. The Success Criteria, and whether a Product meets the Success Criteria, shall be determined by the Programme Committee in accordance with clause 7.9.1, together with a specification for each of the Products to enter Stage 2. |
| 3.6 | The parties agree that during Stage 1 Pharms shall prepare and submit applications in relation to each of the Products in the EU and US for orphan drug status. The parties agree that the applications shall be made in the name of Lipoxen. |
| 4. | Stage 2: Clinical Trials in Pharms Territory |
| 4.1 | Pharms shall conduct the Pharms Trials in the Pharms Territory in accordance with the Timetable, the Development Programme and the Specification. Pharms shall be entitled to manage the Pharms Trials through its in-house regulatory department or via an Appointed CRO. |
9
| Final Version (11 November 2009) | Private and Confidential |
| 4.2 | Without prejudice to the generality of clause 4.1, Pharms shall:- |
4.2.1 submit the CTA (Clinical Trials Application) to the regulatory authorities in the Pharms Territory for permission to conduct the Pharms Trials in relation to each of the Products on or before the dates set out in Schedule 8 of this Agreement; and
4.2.2 commence the Pharms Trials within 6 (six) calendar months of receiving permission form the regulatory authorities in the Pharms Territory to conduct the relevant Pharms Trial.
| 4.3 | PHARMS shall be responsible for all costs and expenses for conducting the Pharms Trials, including the costs and expenses of any Appointed CRO which Pharms may appoint. |
| 4.4 | Pharms shall keep Lipoxen fully informed of all decisions it makes and all plans it has to conduct the Pharms Trials. Pharms shall comply with all instructions provided by Lipoxen in relation to conduct of the Pharms Trials which are reasonably required to ensure that the Pharms Trials are conducted in accordance with all applicable US and European Union laws, regulations, codes of practice, principles and guidelines, including EMEA and FDA requirements. |
| 4.5 | PHARMS shall enter into a written agreement with any Appointed CRO which shall contain all the terms normally found in such an agreement and which shall:- |
| 4.5.1 | provide that all Intellectual Property Rights generated pursuant to the Pharms Trials shall be owned either by Lipoxen and/or Pharms and/or jointly by the parties in accordance with the terms of this Agreement; |
| 4.5.2 | enable Pharms to comply with its obligations under this Agreement; and |
| 4.5.3 | be capable of assignment to Lipoxen, without the prior consent of the Appointed CRO, if this Agreement expires or is terminated by either of the parties. |
| 4.6 | Pharms undertakes that:- |
4.6.1 the conduct of the Pharms Trials for the Products shall at all times comply with all the advice and instructions received from Lipoxen;
| 4.6.2 | all relevant data obtained from the Pharms Trials shall be made available to Lipoxen for the purposes of conducting further clinical trials and/or seeking marketing authorisations in the Joint Territory; and |
| 4.6.3 | it will not knowingly conduct, or permit the Appointed CRO to conduct, a Pharms Trial in a manner that is inconsistent with |
10
| Final Version (11 November 2009) | Private and Confidential |
| US and European Union laws, regulations, codes of practice, principles and guidelines, including EMEA and FDA requirements. |
| 4.7 | Pharms shall obtain the prior written approval of the Programme Committee of any and all protocols to be used in the Pharms Trials and shall comply with all reasonable instructions of the Programme Committee in relation to such protocols. |
| 5 | Stage 3: Clinical Development |
| 5.1 | The Programme Committee shall promptly review the results of the Pharms Trials and shall decide which, if any, Products have met the Success Criteria and which shall therefore move into Stage 3. |
| 5.2 | Subject to clause 5.3, the Programme Committee shall decide the strategy and responsibilities of the parties for full-scale pharmaceutical and clinical development of the Products in the Joint Territory in Stage 3 but the parties agree that the principles set out in this clause 5 shall be adopted. |
| 5.3 | Lipoxen shall be entitled to serve written notice on Pharms at any time after the Stage 2 Expiry Date in relation to a Product, specifying that Lipoxen intends, subject to the revenue sharing provisions set out in Schedule 10, to develop the relevant Product alone in the Joint Territories. Such notice shall only be effective in relation to a Product if at the time the notice is served, Pharms does not own or have licensed exclusively to it any material Intellectual Property Right relating to the Active of the relevant Product. If the notice referred to in this clause is effective, Lipoxen shall have the exclusive right, entirely at its own cost, to develop, distribute, manufacture, supply and sell the relevant Product in the Joint Territory without reference to Pharms and/or the Development Committee and the Product shall be deemed to be a Lipoxen Product. |
| 5.4 | Pharms will have exclusive rights and responsibility entirely at its own cost to develop, distribute and sell the Products in the Pharms Territory in accordance with the licence granted in clause 9 of this Agreement. Lipoxen shall not have any responsibility to carry out any research and/or development in the Pharms Territory. |
| 5.5 | Subject to clause 5.6, Lipoxen shall be responsible pursuant to instructions from the Programme Committee for:- |
| 5.5.1 | any and all applications for marketing authorisations to be made to the regulatory authorities, including EMEA and |
11
| Final Version (11 November 2009) | Private and Confidential |
| FDA, in the Joint Territory in respect of the Products, which applications for the avoidance of doubt, shall be made in the name of Lipoxen; |
| 5.5.2 | any and all exploitation of the Products in the Joint Territory including, without limitation, negotiations with third parties and the determination of licensing arrangements with third parties for exploitation of the Products. |
| 5.6 | Lipoxen shall keep PHARMS fully informed on all developments relating to the exploitation of the Products and shall promptly provide a copy to PHARMS of any agreement entered into between Lipoxen and/or its Affiliates and a Licensee. |
| 6. | Manufacture |
| 6.1 | Pharms shall manufacture sufficient quantities of the Products meeting the Specifications for use in Stage 1 and Stage 2, at all times in accordance with the Timetable and the Development Programme. |
| 6.2 | Pharms shall be responsible for sourcing any and all PSA required by Pharms to manufacture the Products for use in Stage 2. If Pharms is unable to obtain a supply of PSA on reasonable commercial terms from a third party manufacturer it shall notify Lipoxen in writing and Lipoxen shall xxxxx Xxxxxx a right to use any PSA IP in the possession and control of Lipoxen at the date of the notice on reasonable commercial terms to be agreed between the parties. |
| 6.3 | Pharms warrants that it shall at all times comply with all laws regulations, codes of practice, principles and guidelines applicable to the manufacturing of the Actives and/or the Products in the Pharms Territory, including all relevant regulatory requirements in the Pharms relating to the manufacture of chemical and biological medicines and the administration of such medicines to humans. Prior to commencing any Pharms Trials in relation to the Products, Pharms shall provide evidence to Lipoxen that it has complied with this clause 6.3. |
| 6.4 | During Stage 1 and Stage 2, Pharms shall from time to time at the request of Lipoxen provide samples of the Products free of charge to Lipoxen for use by Lipoxen in research and development for commercial purposes. |
| 6.5 | Prior to commencing the Pharms Trials, PHARMS shall demonstrate to the satisfaction of Lipoxen that it is able to manufacture samples of the Products meeting the Specifications. |
12
| Final Version (11 November 2009) | Private and Confidential |
| 6.6 | On or before the commencement of Stage 3 the parties shall jointly seek and appoint a contract manufacturing organisation to manufacture the Products to GMP to be used in the Joint Territory in Stage 3 (the “Appointed CMO”). The parties agree that the costs of the Appointed CMO shall be a Stage 3 Cost. |
| 6.7 | At the request of Lipoxen, Pharms shall transfer the Pharms Background IP to the Appointed CMO in accordance with clause 8.11. |
| 7 | Conduct, Reporting and Decision Making |
Conduct
| 7.1 | Each of Pharms and Lipoxen shall perform its obligations under this Agreement:- |
| 7.1.1 | in accordance with the Development Programme; |
| 7.1.2 | to the best of its ability in a professional manner consistent with industry standards; |
| 7.1.3 | in accordance with the standard of care customarily observed with regard to such activities; |
| 7.1.4 | in a timely manner and in accordance with the Timetable; |
| 7.1.5 | in accordance with all reasonable instructions received from the other party; |
| 7.1.6 | in compliance with all applicable laws, rules and regulations, including without limitation, where applicable, GMP, current good clinical or laboratory practices and good clinical practice. |
Reporting
| 7.2 | Pharms and Lipoxen shall, and Pharms shall procure that the Appointed CRO shall, during the term of this Agreement :- |
| 7.2.1 | keep detailed written records of its progress with the Development Programme and, at the request of the other party, promptly provide the other party with access to and/or copies of such records; |
| 7.2.2 | supply to the other party at least once every six weeks with an interim report describing the progress of the Development Programme including, without limitation, details of all material Arising IPR which has been made or which has come to its attention and containing recommendations regarding the future progress of the Development Programme; |
13
| Final Version (11 November 2009) | Private and Confidential |
| 7.2.3 | notwithstanding clause 7.2.2 above, keep the other parties fully informed of the progress of the Development Programme and of all Arising IPR; |
| 7.2.4 | immediately notify the other parties in writing if there is an unexpected technical or scientific problem which may make it difficult or impossible to achieve or is likely to cause a material delay to the Development Programme, including any adverse events arising pursuant to the Pharms Trials. |
| 7.3 | Pharms will allow, and/or will procure that the Appointed CRO will allow, Lipoxen and/or its employees to:- |
| 7.3.1 | visit Pharms’ facilities and/or the Appointed CRO’s facilities; and |
| 7.3.2 | review Pharms’ and/or the Appointed CRO’s records at reasonable times and with reasonable frequency during normal business hours to:- |
| (a) | verify compliance by Pharms and/or the Appointed CRO with the terms of this Agreement; and/or |
| (b) | observe the progress of the Development Programme. |
| 7.4 | Pharms shall, or shall procure that the Appointed CRO shall, update the Programme Committee on the progress of the Pharms Trials on a monthly basis via a telephone conference call with the Programme Committee. |
Programme Committee
| 7.5 | The parties shall establish a Programme Committee consisting of four individuals, comprising two representatives of Pharms and two representatives of Lipoxen. The initial representatives of each of Lipoxen and Pharms are identified in Schedule 9. The expenses of the Pharms representatives shall be borne by Pharms and the expenses of the Lipoxen representatives shall be borne by Lipoxen. |
| 7.6 | Lipoxen and Pharms may from time to time change its representatives on the Programme Committee by notifying the other parties in writing in advance. The replacement shall be suitably qualified and capable of fulfilling the responsibilities of a member of the Programme Committee under this agreement. |
| 7.7 | Lipoxen shall be entitled to appoint one of its representatives on the Programme Committee as the chair person of the Programme Committee. |
| 7.8 | The Programme Committee will be responsible for the overall management of the Development Programme and shall meet at |
14
| Final Version (11 November 2009) | Private and Confidential |
| least once every month either in person or through teleconference or in any other mode to discuss the progress of the Development Programme. |
| 7.9 | The Programme Committee shall:- |
| 7.9.1 | on or promptly after the Commencement Date, meet and agree the Specifications and Success Criteria for the Products; |
| 7.9.2 | during Stage 2 meet and agree an extension to the Development Programme to address the development of the Products which are not Lipoxen Products in Stage 3; and |
| 7.9.3 | at the relevant time during the Development Programme determine whether the Products meet the Success Criteria. |
| 7.10 | All material decisions of the Programme Committee shall be recorded in writing. |
| 7.11 | The parties shall agree mutually when to conduct the monthly meetings of the Programme Committee. In addition and/or if the parties cannot agree a date for the monthly meetings, each party shall be entitled to convene a meeting of the Programme Committee on giving not less than one calendar months’ written notice to the other party. |
| 7.12 | The parties agree that:- |
| 7.12.1 | meetings of the Programme Committee may occur by telephone conference call; |
| 7.12.2 | the quorum for a meeting of the Programme Committee shall be two representatives of each party; |
| 7.12.3 | no valid meeting of the Programme Committee may be held unless a quorum is present and the parties have agreed the date of the meeting in writing or all parties have received not less than one calendar months written notice of the meeting (or such shorter notice period as the parties shall previously agree in writing); |
| 7.12.4 | each person present at a meeting of the Programme Committee shall have a single vote; and |
| 7.12.5 | the chair person of the Programme Committee shall have the casting vote in relation to any decisions to be made by the Programme Committee. |
| 7.13 | For the avoidance of doubt, other than as set out in clause 7.9, the Programme Committee shall not have the authority to amend the Development Programme, the Timetable or the terms of this Agreement. |
15
| Final Version (11 November 2009) | Private and Confidential |
| 8 | Intellectual Property Rights |
| 8.1 | Provided Pharms is not in breach of clause 8.6 in relation to the relevant Pharms Active Component, any and all Arising IPR that relates specifically to the Pharms Active Components shall belong to Pharms. |
| 8.2 | Any and all Arising IPR that relates specifically to the Lipoxen Technology shall belong to Lipoxen. |
| 8.3 | Any Arising IPR that is not owned by Pharms or Lipoxen pursuant to clauses 8.1 and 8.2 shall be owned jointly by the Lipoxen and Pharms. Subject to clauses 8.4 and 8.5, and the parties’ respective rights to use the Joint Arising IPR pursuant to clauses 8.6 and 8.7, the parties shall collaborate to agree the appropriate method for the protection, development and exploitation of the Joint Arising IPR. |
| 8.4 | Lipoxen shall have sole conduct and control of any and all patent applications made in respect of the Joint Arising IPR. The cost of any such patent applications (and the cost of maintaining any patents granted in respect thereof) shall be shared jointly by Lipoxen and Pharms. |
| 8.5 | Lipoxen shall consult regularly with Pharms in relation to the patents and patent applications referred to in clause 8.4 and shall comply with all reasonable suggestions made by Pharms in relation to the prosecution of such patent applications. PHARMS shall provide Lipoxen with all assistance reasonably required by Lipoxen in relation to the prosecution and maintenance of the patents and patent applications referred to in clause 8.4. |
Pharms Active Components
| 8.6 | Pharms undertakes to Lipoxen that Pharms: |
8.6.1 owns or has the exclusive, world wide right to use (with the right to grant sub-licenses) the Pharms Active Components; and/or
| 8.6.2 | it will acquire the rights referred to in clause 8.6.1 prior to the expiry of Stage 2 or by 31 December 2010 (whichever is earlier) on terms that are reasonably acceptable to Lipoxen. |
16
| Final Version (11 November 2009) | Private and Confidential |
| 8.7 | As and when requested to do so by Lipoxen, Pharms shall provide written evidence to Lipoxen that Pharms is not in breach of the terms of clause 8.6. |
| 8.8 | Lipoxen shall have the right at any time to terminate this Agreement on a Product by Product basis with immediate effect on written notice to Pharms if Pharms is in breach of clause 8.6 and/or 8.7 of this Agreement in relation to any Pharms Active Component that relates to the relevant Product. |
Licence to Lipoxen
| 8.9 | Pharms grants to Lipoxen and its Affiliates an exclusive licence, with the right to grant sub-licences, in the Joint Territory to research, develop, make, have made, market, supply, sell and distribute Products using:- |
| 8.9.1 | the Pharms Background IPR; |
| 8.9.2 | the Pharms Know How; |
| 8.9.3 | the Joint Arising IPR; and |
| 8.9.4 | the Pharms Arising IPR. |
| 8.10 | Pharms shall, at the request of Lipoxen, supply to Lipoxen any cell lines used by Pharms in the development and/or manufacture of the Products and the licence set out in clause 8.9 shall, for the avoidance of doubt, include the right to use any such cell lines. |
| 8.11 | At Lipoxen’s request, Pharms will disclose and/or transfer to Lipoxen, its Licensee and/or the Appointed CRO, using a method of know how transfer reasonably acceptable to Lipoxen, all information and materials (including samples of the cell lines referred to in clause 8.10) that are reasonably required to enable Lipoxen to exploit the licence granted under clause 8.9. |
Third Party Intellectual Property Rights
| 8.12 | Each party shall immediately notify the other party in writing if it becomes aware of any third party Intellectual Property Rights relating to any of the Products (“Third Party IP Rights”). |
| 8.13 | The parties shall co-operate to evaluate the strength and validity of any Third Party IP Rights and the Programme Committee shall decide how to address the Third Party IP Rights. |
| 8.14 | If the Programme Committee decides to challenge or take a licence of the Third Party IP Rights, Lipoxen shall be responsible, at the joint cost of the parties, for any action recommended by the Programme Committee. |
17
| Final Version (11 November 2009) | Private and Confidential |
| 8.15 | Either party may terminate this Agreement on 30 (thirty) days written notice to the other party in relation to a particular Product if, in its reasonable opinion, a Third Party IP Right exists which would have a material effect on the research and/or development of the relevant Product. |
| 8.16 | For the avoidance of doubt, any and all costs and/or expenses reasonably and properly incurred by the parties in relation to a Third Party IP Right, including any licence fees and/or costs of evaluating and challenging a Third Party IP Right, shall be deemed to be a Stage 3 Cost. |
| 9. | Grant of Rights to Pharms |
PolyXen Licence
| 9.1 | Subject to clause 2, Lipoxen hereby grants to Pharms, subject to the provisions of this Agreement, an exclusive licence to use the PolyXen Patents and the PolyXen Know How in the Pharms Territory to research, develop, manufacture, have manufactured, use, sell, supply and otherwise exploit the PolyXen Products. This licence shall include any and all Lipoxen Arising IPR and Joint Arising IPR to the extent it relates to the PolyXen Technology. |
| 9.2 | The licence granted pursuant to Clause 9.1 shall expire on the later of the following dates: |
| 9.2.1 | the date upon which no Valid Claim of the PolyXen Patents exists in the Pharms Territory; or |
| 9.2.2 | fifteen (15) years from the Commencement Date. |
ImuXen Licence
| 9.3 | Subject to clause 2, Lipoxen hereby grants to Pharms, subject to the provisions of this Agreement, an exclusive licence to use the ImuXen Patents and the ImuXen Know How in the Pharms Territory to research, develop, manufacture, have manufactured, use, sell, supply and otherwise exploit ImuXen Products. This licence shall include any and all Lipoxen Arising IPR and Joint Arising IPR to the extent it relates to the ImuXen Technology. |
| 9.4 | The licence granted pursuant to Clause 9.3 shall expire on the later of the following dates: |
| 9.4.1 | the date upon which no Valid Claim of the ImuXen Patents exists in the Pharms Territory; or |
| 9.4.2 | fifteen (15) years from the Commencement Date. |
18
| Final Version (11 November 2009) | Private and Confidential |
Sub-licensing
| 9.5 | Pharms shall not be entitled to sub-licence and/or sub-contract its granted rights under this Agreement to any person without the prior written consent of Lipoxen. |
No Other License
| 9.6 | It is acknowledged and agreed that no licence is granted by Lipoxen to Pharms other than the licences expressly granted by the provisions of this Clause 9. Without prejudice to the generality of the foregoing, Lipoxen reserves all rights under the Lipoxen Patents and the Lipoxen Know How:- |
| 9.6.1 | in relation to any products which are not Products; and |
| 9.6.2 | outside the Pharms Territory. |
Quality
| 9.7 | Pharms shall ensure that all of the Products sold of supplied by it are of satisfactory quality and comply with all applicable laws and regulations in each part of the Pharms Territory. |
| 10. | Costs |
| 10.1 | Subject to Clause 10.2, Lipoxen and Pharms shall each be entirely responsible for their own Stage 1 Costs which they incur. |
| 10.2 | If Lipoxen agrees to provide more than the Know How Transfer Time, Lipoxen shall be entitled to charge Pharms for any additional time provided by Lipoxen at a rate of US$1000 (one thousand US dollars) per working day per scientist. |
| 10.3 | Pharms shall be entirely responsible for all of the Stage 2 Costs. |
| 10.4 | Subject to Clause 10.5, Lipoxen and Pharms shall share equally the Stage 3 Costs. |
| 10.5 | A cost and/or expense shall not be deemed to be properly incurred by a party if it exceeds £5,000 (five thousand pounds sterling) and a party has not obtained the prior written consent of the Program Committee to the relevant cost or expense. |
19
| Final Version (11 November 2009) | Private and Confidential |
| 10.6 | In relation to the costs that are to be shared equally, Lipoxen and Pharms shall carry out a reconciliation at the end of each Quarter as follows:- |
| 10.6.1 | within 10 working days of the end of the Quarter, Pharms and Lipoxen will submit an invoice to the other party setting out details of the costs it incurred in the previous Quarter in relation to this Agreement which if incurred in a currency other than US dollars shall be converted to US dollars using the open middle market spot rate of exchange in London as published in the Financial Times on the last day of the relevant Quarter; |
| 10.6.2 | provided the costs shown on the relevant invoice are reasonable and have been properly incurred, the party with the lower invoice shall pay half of the balance of the other party’s invoice within 30 working days of the date of the other party’s invoice. |
| 11. | Records and Auditing |
| 11.1 | Lipoxen and Pharms shall during the term of this Agreement and for a period of five (5) years thereafter, keep at their normal place of business detailed and up-to-date records and accounts showing:- |
| 11.1.1 | any and all costs and expenses it has incurred in relation to the Development Programme; and |
| 11.1.2 | the quantity, description, and value of Products sold by it, on a country-by-country basis, and being sufficient to ascertain the payments due under this Agreement. |
| 11.2 | Each of the parties shall make its records and accounts available, on reasonable notice, for inspection during business hours by an independent chartered accountant nominated by the other party for the purpose of verifying the accuracy of any statement or report provided under this Agreement and any payments due under this Agreement. The accountant shall be required to keep confidential all information learnt during any such inspection, and to disclose to the inspecting party only such details as may be necessary to report on the accuracy of the statement, report or payment. The inspecting party shall be responsible for the accountant’s charges unless the accountant certifies that there is an inaccuracy of more than 5% (five per cent) in any statement or payment, in which case the party being inspected shall pay the accountant’s charges in respect of that inspection. |
| 12 | Revenue Sharing |
| 12.1 | The parties agree that the revenues from the Products shall be shared by the parties as set out in Schedule 10. |
20
| Final Version (11 November 2009) | Private and Confidential |
| 13 | Payment Terms |
| 13.1 | All sums due under this Agreement: |
| 13.1.1 | are exclusive of Value Added Tax or any other sales tax or duties, which if and where applicable will be paid by the payor to the payee in addition to any sum in respect of which they are calculated; |
| 13.1.2 | shall be paid in US dollars to the credit of the payee’s bank account, details of which shall be notified to the payor as and when necessary; |
| 13.1.3 | shall be made without deduction of income tax or other taxes charges or duties that may be imposed, except insofar as the payor is required to deduct the same to comply with applicable laws. The parties shall co-operate and take all steps reasonably and lawfully available to them, at the expense of the payee, to avoid deducting such taxes and to obtain double taxation relief. If the payor is required to make any such deduction it shall provide the payee with such certificates or other documents as it can reasonably obtain to enable the payee to obtain appropriate relief from double taxation of the payment in question; and |
| 13.1.4 | shall be made by the due date, failing which the payee may charge interest on any outstanding amount calculated on a monthly basis at a rate equivalent to 5% above the London Inter-Bank Offer Rate (6 months). |
| 13.2 | If either party is obliged pursuant to a government order or otherwise to withhold payment of any sum due under this Agreement to the other party, the payor shall use its best endeavours to release the payment to the other party. If the payment has not been released within 30 (thirty) days of its due date for payment, the payee shall be entitled to deduct the payment from any sums to the payor from the payee pursuant to this Agreement. |
| 13.3 | The parties agree that each party shall be responsible for paying any taxes arising pursuant to or in relation to this Agreement for which the party is primarily liable. |
| 13.4 | The parties agree that they will use their best endeavours to collaborate to establish a corporate structure for the licensing of the Products and for the receipt of any revenues that is tax efficient for the parties. |
21
| Final Version (11 November 2009) | Private and Confidential |
| 14 | Liability |
14.1 Pharms shall be responsible for all risks and liability arising from or in relation to the Pharms Trials and/or Pharms’ development, sale and/or supply of Products in the Pharms Territory. Pharms shall maintain appropriate insurance to cover any such liability.
| 14.2 | Pharms shall, if requested to do so by Lipoxen, provide evidence to Lipoxen that it has complied with the terms of this clause 14.1. Pharms shall indemnify and shall keep Lipoxen indemnified against any and all liability, damages, claims, proceedings and expenses (including, but not limited to, legal expenses and expert’s fees) arising out of or in connection with the Pharms Trials and/or Pharms’ development, sale and/or supply of Products in the Pharms Territory provided that Pharms shall not be liable under this clause 14.2 for any and all liability, damages, claims, proceedings and expenses (including but not limited to, legal expenses and expert’s fees) that arise directly as a result of express instructions received from Lipoxen in relation to conduct of the Pharms Trials. |
| 14.3 | The parties shall be jointly responsible for all risks and liability arising from or in relation to the Clinical Trials and/or the development, sale and/or supply of the Joint Products in the Joint Territory. The parties shall maintain appropriate insurance to cover any such liability. |
| 14.4 | Each party shall indemnify the other and keep the other indemnified against half of any and all liability, damages, claims, proceedings and expenses (including, but not limited to, legal expenses and expert’s fees) arising out of or in connection with the Clinical Trials and/or the development, sale and/or supply of Joint Products in the Joint Territory provided that neither party shall be liable under this clause 14.4 for any and all liability, damages, claims, proceedings and expenses (including but not limited to, legal expenses and expert’s fees) that arise as a result of a breach of this Agreement by the other party |
| 15 | Confidentiality and Publication |
| 15.1 | Each party (the “Receiving Party”) undertakes:- |
| 15.1.1 | to maintain as secret and confidential all Confidential Information obtained directly or indirectly from the other party (“Disclosing Party”) in the course of or in anticipation of this Agreement; |
| 15.1.2 | to use and disclose the Confidential Information of the other party only for the purposes of this Agreement and/or in so far as such use and/or disclosure is reasonably required to enable the party to exploit its rights under this Agreement; |
22
| Final Version (11 November 2009) | Private and Confidential |
| 15.1.3 | to disclose the Confidential Information of the other party only to those of its employees, contractors, and sub-licensees to whom and to the extent that such disclosure is reasonably necessary for the purposes of exploiting its rights and complying with its obligations under this Agreement; |
| 15.1.4 | to comply with the obligations of this clause 15 for so long as it has knowledge of any Confidential Information received or derived from the other party which period shall, for the avoidance of doubt, survive termination or expiry of this Agreement. |
| 15.2 | The provisions of clause 15.1 shall not apply to Confidential Information which the Receiving Party can prove:- |
| 15.2.1 | was, prior to its receipt by the Receiving Party from the Disclosing Party, in the possession of the Receiving Party and at its free disposal; |
| 15.2.2 | is subsequently disclosed to the Receiving Party without any obligations of confidence by a third party who has not derived it directly or indirectly from the Disclosing Party; |
| 15.2.3 | is or becomes generally available to the public through no act or default of the Receiving Party or its agents, employees, Affiliates or sub-licensees; |
| 15.2.4 | the Receiving Party is required to disclose to the courts of any competent jurisdiction, or to any government regulatory agency or financial authority, provided that the Receiving Party shall:- |
| (a) | inform the Disclosing Party as soon as is reasonably practicable of its obligation to disclose such information; and |
| (b) | at the Disclosing Party’s request seek to persuade the court, agency or authority to have such information treated in a confidential manner, where this is possible under the court, agency or authority’s procedures. |
| 15.3 | The Receiving Party shall procure that all of its employees, contractors who have access to any of the Disclosing Party’s Confidential Information, shall be made aware of and subject to these obligations and shall have entered into written undertakings of confidentiality at least as restrictive as those set out in this Clause 15. |
| 15.4 | The parties agree that any publications relating to the Results shall be approved in advance by the Development Committee. Any publications shall acknowledge both parties appropriately, and Lipoxen shall have the first right to submit any paper for publication. |
23
| Final Version (11 November 2009) | Private and Confidential |
| 16. | Duration and Termination |
| 16.1 | This Agreement shall commence on the Commencement Date and shall continue until terminated in accordance with its terms. |
| 16.2 | Without prejudice to any other right or remedy any party may terminate this Agreement by notice in writing to the other Party (“Other Party”), such notice to take effect as specified in the notice:- |
| 16.2.1 | if the Other Party is in material breach of this Agreement and, in the case of a breach capable of remedy, the breach is not remedied within 90 (ninety) days of the Other Party receiving notice specifying the breach and requiring its remedy; and/or |
| 16.2.2 | if (A) the Other Party becomes insolvent or unable to pay its debts as and when they become due, or (B) an order is made or a resolution is passed for the winding up of Other Party (other than voluntarily for the purpose of solvent amalgamation or reconstruction), or (C) a liquidator, administrator, administrative receiver, receiver, or trustee is appointed in respect of the whole or any part of the Other Party’s assets or business, or (D) the Other Party makes any composition with its creditors, or (E) the Other Party ceases to continue its business, or (F) as a result of debt and/or maladministration the Other Party takes or suffers any similar or analogous action in any jurisdiction. |
| 16.3 | If Pharms is in breach of clauses 4.2.1 or 4.2.2 of this Agreement in relation to one or more Products then Lipoxen shall be entitled to terminate this Agreement in relation just to the Product or Products to which the breach relates with immediate effect by notice in writing to Pharms. |
| 16.4 | Lipoxen may terminate this Agreement in accordance with clause 8.8 in relation to the specific Product. |
| 16.5 | Either party may terminate this Agreement in relation to a specific Product if the relevant Product does not meet the relevant Success Criteria for the Product. |
| 16.6 | Either party may terminate this Agreement on a Product by Product basis in accordance with clause 8.15. |
24
| Final Version (11 November 2009) | Private and Confidential |
| 17 | Consequences of Termination |
| 17.1 | Upon termination or expiry of this Agreement for any reason: |
| 17.1.1 | Pharms shall provide to Lipoxen a detailed report setting out the progress it has made with the Development Programme; |
| 17.1.2 | Pharms shall provide to Lipoxen all data (including without limitation clinical trials data), know-how and materials generated by Pharms in connection with the Development Programme; |
| 17.1.3 | to the extent that title has not previously passed to Lipoxen pursuant to this Agreement, Pharms shall assign to Lipoxen all of the Arising IPR; |
| 17.1.4 | at Lipoxen’s option Pharms shall return to Lipoxen or destroy all other data, know-how and materials provided to Pharms by Lipoxen, or generated by Pharms in connection with the Development Programme; |
| 17.1.5 | any rights or remedies of any of the parties arising from any breach of this Agreement shall continue to be enforceable; |
| 17.1.6 | Pharms shall no longer be licensed to use or otherwise exploit in any way, either directly or indirectly, the Lipoxen Technology, the Lipoxen Arising IPR or the Joint Arising IPR in the Pharms Territory or the Joint Territory and Pharms shall, and shall procure that its Appointed CRO shall, forthwith cease all activities requiring a licence from Lipoxen; |
| 17.1.7 | at the request of Lipoxen, Pharms shall assign to Lipoxen any one or all of the CRO Agreements; |
| 17.1.8 | the following clauses shall continue in full force and effect: 1, 2, 6.7, 8.9 to 8.11, 11, 14 (in so far as it relates to liability arising prior to termination), 15.1 to 15.3, 17 and 18; and |
| 17.1.9 | each party shall return to the other within a reasonable period of time all Confidential Information and any copies thereof disclosed to it by the other party. |
| 17.2 | Upon expiry or termination of this Agreement in relation to a one or more Products, the consequences set out in clause 17.1 shall apply but only in so far as they relate to the relevant Product. |
25
| Final Version (11 November 2009) | Private and Confidential |
| 18 | General |
Amendment
| 18.1 | This Agreement, the Development Programme and the Timetable may only be amended in writing signed by duly authorised representatives of the parties or by the Development Committee as is expressly set out in this agreement. |
Assignment and third party rights
| 18.2 | Other than as is expressly set out in this Agreement, none of the parties shall assign, mortgage, charge or otherwise transfer any rights or obligations under this Agreement without the prior written consent of the other Party. |
| 18.3 | Any of the parties may assign all its rights and obligations under this Agreement to any company to which it transfers all of its assets or business, PROVIDED that the assignee undertakes to the other parties to be bound by and perform the obligations of the assignor under this Agreement. |
Waiver
| 18.4 | No failure or delay on the part of any party to exercise any right or remedy under this Agreement shall be construed or operate as a waiver thereof, nor shall any single or partial exercise of any right or remedy preclude the further exercise of such right or remedy. |
Invalid clause
| 18.5 | If any provision or part of this Agreement is held to be void or invalid, amendments to this Agreement may be made by the addition or deletion of wording as appropriate to remove the void or invalid part or provision but otherwise retain the provision and the other provisions of this Agreement to the maximum extent permissible under applicable law. The parties shall endeavour to agree amendments to such void or invalid provisions in a reasonable manner so as to achieve the original intention of the parties. |
Change of Control
| 18.6 | Any substantial change in the management and control of either of the parties and/or any merger of either of the parties with another entity shall not result in termination of this Agreement and it shall be the responsibility of the then existing management of the party to see that the continuity of this Agreement is maintained in all respects and the agreement shall continue to be in force. |
26
| Final Version (11 November 2009) | Private and Confidential |
Formal licences
| 18.7 | The parties shall execute such formal licences, documents as may be necessary or appropriate for registration of the rights granted under this Agreement with Patent Offices and other relevant authorities. The parties shall use reasonable endeavours to ensure that, to the extent permitted by relevant authorities and unless required to submit this Agreement by any order of law, this Agreement shall not form part of any public record. |
Role of Parties
| 18.8 | The parties hereto expressly understand and agree that Lipoxen and Pharms are independent contractors in the performance of each and every part of this Agreement. Subject to the provisions of clause 8.3 relating to joint ownership of the Joint Foreground, nothing contained herein shall be construed as creating any agency, partnership or other form of joint enterprise between the parties. |
Interpretation
| 18.9 | In this Agreement: |
| 18.9.1 | the headings are used for convenience only and shall not affect its interpretation; |
| 18.9.2 | references to persons shall include incorporated and unincorporated persons; references to the singular include the plural and vice versa; and references to the masculine include the feminine; |
| 18.9.3 | references to Parties or parties means Lipoxen, Pharms and FDS; |
| 18.9.4 | references to clauses and Schedules mean clauses of, and schedules to, this Agreement; and |
| 18.9.5 | references to the grant of “exclusive” rights shall mean that the person granting the rights shall neither grant the same rights (in the same field and territory) to any other person, nor exercise those rights itself. |
Notices
| 18.10 | Any notice to be given under this Agreement shall be in writing and shall be sent by first class mail or air mail, or by fax (confirmed by first class mail or air mail) to the address of the relevant party set out at the head of this Agreement, or to the relevant fax number set out below, or such other address or fax number as that party may from time to time notify to the other |
27
| Final Version (11 November 2009) | Private and Confidential |
| parties in accordance with this clause. The fax numbers of the parties are as follows: Lipoxen x00 00 0000 0000; Pharms x0 000 000 0000. |
| 18.11 | Notices sent as specified in clause 18.10 shall be deemed to have been received three working days after the day of posting (in the case of inland first class mail), or ten working days after the date of posting (in the case of air mail), or on the next working day after transmission (in the case of fax messages, but only if a transmission report is generated by the sender’s fax machine recording a message from the recipient’s fax machine, confirming that the fax was sent to the number indicated above and confirming that all pages were successfully transmitted). |
Law and Jurisdiction
| 18.2 | The validity, construction and performance of this Agreement shall be governed by the laws of England and Wales and shall be subject to the exclusive jurisdiction of the courts of England and Wales to which the parties hereby irrevocably submit, except that a party may seek an interim injunction in any court of competent jurisdiction. |
Further action
| 18.3 | Each party agrees to execute, acknowledge and deliver such further instruments, and do all further similar acts, as may be necessary or appropriate to carry out the purposes and intent of this Agreement. |
Announcements
| 18.4 | Neither party shall make any press or other public announcement concerning any aspect of this Agreement, or make any use of the name of the other party in connection with or in consequence of this Agreement, without the prior written consent of the other party. The parties agree that any agreed announcements will first be made in the name of Lipoxen. |
Entire agreement
| 18.15 | This Agreement, including its Schedules, sets out the entire agreement between the parties relating to its subject matter and supersedes all prior oral or written agreements, arrangements or understandings between them relating to such subject matter. |
| 18.16 | The parties acknowledge that they are not relying on any representation, agreement, term or condition which is not set out in this Agreement. |
| 18.17 | Nothing in this Agreement shall exclude any of the parties’ liability for fraudulent misrepresentation. |
28
| Final Version (11 November 2009) | Private and Confidential |
Third parties
| 18.18 | With the exception of any rights expressly created in this Agreement in favour of Affiliates of Lipoxen , this Agreement does not create any right enforceable by any person who is not a party to it. |
29
| Final Version (11 November 2009) | Private and Confidential |
AGREED by the parties through their authorised signatories on the date written above:
| For and on behalf of Lipoxen Technologies Limited |
For and on behalf of Pharmasynthez | |||
|
|
| |||
| Signed | Signed | |||
|
|
| |||
| Print name | Print name | |||
|
|
| |||
| Title | Title | |||
Schedule 1
30
| Final Version (11 November 2009) | Private and Confidential |
Development Programme
Schedule 2
Components of the Products
Part 1 – [***] (Product A)
E.coli expressed human recombinant deoxyribonuclease-1 protein having following amino acid structure:-
Part 2 – [***] (Product B)
[***]
| [***] |
31
| Final Version (11 November 2009) | Private and Confidential |
Part 3 – (Product C)
[***]
[***]
Part 4 – [***] (Product D)
[***]
[***]
[***]
[***]
[***]
[***]
[***]
Part 5 – [***] (Product E)
[***]
[***]
| Final Version (11 November 2009) | Private and Confidential |
Part 6 - [***] (Product F)
[***]
[***]
Schedule 3
ImuXen Patents
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority Country |
1st Priority Appln No. |
1st Priority Date |
|||||||||||||||
| Gene Vaccine | Australia | Granted. | 42154/97 | 15/09/1997 | 26/04/2001 | Xxxxxxxxxxx, Xxxxxxx | GB | 9619172.1 | 13/09/1996 | |||||||||||||||
| Gene Vaccine | Canada | Granted. | 2271388 | 15/09/1997 | 06/11/2007 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | China | Granted. | 97199674.1 | 15/09/1997 | 18/02/2004 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | France | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Ireland | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Italy | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Belgium | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | United Kingdom | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Spain | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Germany | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Switzerland | Granted. | 97940250.0 | 15/09/1997 | 04/12/2002 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine | Japan | Filed. | 1998-513398 | 15/09/1997 | GB | 9619172.1 | 13/09/1996 | |||||||||||||||||
| Gene Vaccine | Korea (Republic of) | Granted. | 00-0000000 | 15/09/1997 | 02/08/2005 | GB | 9619172.1 | 13/09/1996 | ||||||||||||||||
| Gene Vaccine div | European Patent Office | Allowed | 02016936.3 | 15/09/1997 | GB | 9619172.1 | 00/00/0000 | |||||||||||||||||
| Xxxx Xxxxxxx Xxx. | Xxxxxx Xxxxxx xx Xxxxxxx | Allowed | 10/617734 | 15/09/1997 | GB | 9619172.1 | 13/09/1996 | |||||||||||||||||
33
| Final Version (11 November 2009) | Private and Confidential |
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority Country |
1st Priority Appln No. |
1st Priority Date |
|||||||||||||||
| Taxol in DRV | Germany | Granted. | 01948934.3 | 31/01/2001 | 04/10/2006 | Xxxx, Xxxxxx | EP | 00300904.0 | 04/02/2000 | |||||||||||||||
| Taxol in DRV | United Kingdom | Granted. | 01948934.3 | 31/01/2001 | 04/10/2006 | EP | 00300904.0 | 04/02/2000 | ||||||||||||||||
| Taxol in DRV | France | Granted. | 01948934.3 | 31/01/2001 | 04/10/2006 | EP | 00300904.0 | 04/02/2000 | ||||||||||||||||
| Taxol in DRV | Spain | Granted. | 01948934.3 | 31/01/2001 | 04/10/2006 | EP | 00300904.0 | 04/02/2000 | ||||||||||||||||
| Taxol in DRV | Italy | Granted. | 01948934.3 | 31/01/2001 | 04/10/2006 | EP | 00300904.0 | 04/02/2000 | ||||||||||||||||
| Taxol in DRV | Switzerland | Granted. | 01948934.3 | 31/01/2001 | 04/10/2006 | EP | 00300904.0 | 04/02/2000 | ||||||||||||||||
| Taxol in DRV | Japan | Filed. | 2001-556240 | 31/01/2001 | EP | 00300904.0 | 04/02/2000 | |||||||||||||||||
| Taxol in DRV | United States of America | Granted. | 10/182921 | 31/01/2001 | 11/04/2006 | EP | 00300904.0 | 04/02/2000 | ||||||||||||||||
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority |
1st Priority |
1st Priority |
|||||||||||||||
| Oral Delivery | Canada | Filed. | 2386024 | 02/10/2000 | Gregoriades, G. and Xxxxxx, Y. | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | China | Granted. | 00813476.6 | 02/10/2000 | 13/04/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | Italy | Granted. | 00964471.7 | 02/10/2000 | 14/12/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | United Kingdom | Granted. | 00964471.7 | 02/10/2000 | 14/12/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | Germany | Granted. | 00964471.7 | 02/10/2000 | 14/12/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | France | Granted. | 00964471.7 | 02/10/2000 | 14/12/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | Spain | Granted. | 00964471.7 | 02/10/2000 | 14/12/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | Switzerland | Granted. | 00964471.7 | 02/10/2000 | 14/12/2005 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | Japan | Filed. | 2001-527772 | 02/10/2000 | EP | 99307786.6 | 01/10/1999 | |||||||||||||||||
| Oral Delivery | Korea (Republic of) | Granted. | 2002-7003922 | 02/10/2000 | 24/07/2007 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Oral Delivery | United States of America | Granted. | 10/089312 | 02/10/2000 | 07/03/2006 | EP | 99307786.6 | 01/10/1999 | ||||||||||||||||
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority |
1st Priority |
1st Priority |
|||||||||||||||
| Capisomes | Switzerland | Granted. | 00981480.7 | 12/12/2000 | 01/09/2004 | Gregoriadis, G | EP | 99310032.0 | 13/12/1999 | |||||||||||||||
| Capisomes | United Kingdom | Granted. | 00981480.7 | 12/12/2000 | 01/09/2004 | EP | 99310032.0 | 13/12/1999 | ||||||||||||||||
| Capisomes | Belgium | Granted. | 00981480.7 | 12/12/2000 | 01/09/2004 | EP | 99310032.0 | 13/12/1999 | ||||||||||||||||
| Capisomes | Italy | Granted. | 00981480.7 | 12/12/2000 | 01/09/2004 | EP | 99310032.0 | 13/12/1999 | ||||||||||||||||
| Capisomes | France | Granted. | 00901480.7 | 12/12/2000 | 01/09/2004 | EP | 99310032.0 | 13/12/1999 | ||||||||||||||||
| Capisomes | Germany | Granted. | 00981480.7 | 12/12/2000 | 01/09/2004 | EP | 99310032.0 | 13/12/1999 | ||||||||||||||||
| Capisomes | United States of America | Filed. | 10/149670 | 12/12/2000 | EP | 99310032.0 | 13/12/1999 | |||||||||||||||||
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority |
1st Priority |
1st Priority |
|||||||||||||||
| Co-Delivery | China | Granted. | 03815952.X | 07/07/2003 | 03/10/2007 | Bacon. et. al. | EP | 02254733.5 | 05/07/2002 | |||||||||||||||
| Co-Delivery | Switzerland | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | Italy | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | Ireland | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | United Kingdom | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | France | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | Spain | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | Germany | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | Belgium | Granted. | 03738331.2 | 07/07/2003 | 20/12/2006 | EP | 02254733.5 | 05/07/2002 | ||||||||||||||||
| Co-Delivery | India | Filed. | 376/DELNP/2005 | 07/07/2003 | EP | 02254733.5 | 05/07/2002 | |||||||||||||||||
| Co-Delivery | Japan | Filed. | 2004-518995 | 07/07/2003 | EP | 02254733.5 | 05/07/2002 | |||||||||||||||||
| Co-Delivery | Russian Federation | Filed. | 2004137791 | 07/07/2003 | EP | 02254733.5 | 00/00/0000 | |||||||||||||||||
| Xx-Xxxxxxxx | Xxxxxx Xxxxxx xx Xxxxxxx | Filed. | 10/520169 | 07/07/2003 | EP | 02254733.5 | 05/07/2002 | |||||||||||||||||
34
| Final Version (11 November 2009) | Private and Confidential |
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority Country |
1st Priority Appln No. |
1st Priority Date |
|||||||||||||
| PS Vaccines TT/DT C |
W.I.P.O. | Filed. | PCT/EP 06/66935 | 29/09/2006 | Bacon, et al. | EP | 05256160.2 | 30/09/2005 | ||||||||||||||
| Multivalent Vaccines | W.I.P.O. | Filed. | PCT/EP 06/66938 | 29/09/2006 | Bacon, et al. | EP | 05256160.2 | 30/09/2005 | ||||||||||||||
35
| Final Version (11 November 2009) | Private and Confidential |
Schedule 4
Pharms Active Components
| Product |
Active |
Pharms Active Component | ||
| [***] | [***] | [***] | ||
| [***] | [***] | [***] | ||
| [***] | [***] | [***] | ||
| [***] | [***] | [***] | ||
| [***] | [***] | [***] | ||
| [***] | [***] | [***] | ||
36
| Final Version (11 November 2009) | Private and Confidential |
Schedule 5
Pharms Trials
| Product |
Active |
Trial to be conducted by Pharms in Pharms Territory for Stage 2 | ||
| Product A | [***] | Phase I-IIA | ||
| Product B | [***] | Phase I-IIA | ||
| Product C | [***] | Phase I | ||
| Product D | [***] | Phase I-IIA | ||
| Product E | [***] | Phase I-IIA | ||
| Product F | [***] | Xxxxx X-XXX | ||
00
| Final Version (11 November 2009) | Private and Confidential |
Schedule 6
PolyXen Patents
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
Patent No. |
1st Priority Date |
||||||||||||||
| Polysaccharide B in DDS | Germany | Granted. | 92911095.5 | 08/06/1992 | 16/08/2001 | Xxxxxxxxxxx, Xxxxxxx | EP0587639 | 06/06/1991 | ||||||||||||||
| Polysaccharide B in DDS | United Kingdom | Granted. | 92911095.5 | 08/06/1992 | 16/08/2001 | EP0587639 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | France | Granted. | 92911095.5 | 08/06/1992 | 16/08/2001 | EP0587639 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | Italy | Granted. | 92911095.5 | 08/06/1992 | 16/08/2001 | EP0587639 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | Spain | Granted. | 92911095.5 | 08/06/1992 | 16/08/2001 | EP0587639 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | USA | Granted. | 08/431474 | 01/05/1995 | 08/12/1998 | 5846951 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | Japan | Granted. | 510527/92 | 08/06/1992 | 22/04/2005 | 3671054 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | Canada | Granted. | 2109952 | 08/06/1992 | 18/11/2003 | 2109952 | 06/06/1991 | |||||||||||||||
| Polysaccharide B in DDS | Korea (Republic of) |
Granted. | 93-703716 | 08/06/1992 | 18/09/2002 | 354944 | 06/06/1991 | |||||||||||||||
| PSB in DDS Div | Japan | Granted. | 2005-42054 | 08/06/1992 | 26/06/2009 | 4332507 | 06/06/1991 | |||||||||||||||
| Patent Name |
Country Of Filing |
Case Status |
Application |
Application |
Grant Date |
Inventors |
Patent No. |
1st Priority |
||||||||||||||
| Polysialylation in SDS | Spain | Granted. | 1931843.5 | 14/05/2001 | 21/12/2005 | Xxxxxxxxxxx, Xxxxxxx | EP1335931 | 16/05/2000 | ||||||||||||||
| Polysialylation in SDS | Germany | Granted. | 1931843.5 | 14/05/2001 | 21/12/2005 | EP1335931 | 16/05/2000 | |||||||||||||||
| Polysialylation in SDS | France | Granted. | 1931843.5 | 14/05/2001 | 21/12/2005 | EP1335931 | 16/05/2000 | |||||||||||||||
| Polysialylation in SDS | Switzerland | Granted. | 1931843.5 | 14/05/2001 | 21/12/2005 | EP1335931 | 16/05/2000 | |||||||||||||||
| Polysialylation in SDS | Italy | Granted. | 1931843.5 | 14/05/2001 | 21/12/2005 | EP1335931 | 16/05/2000 | |||||||||||||||
| Polysialylation in SDS | United Kingdom | Granted. | 1931843.5 | 14/05/2001 | 21/12/2005 | EP1335931 | 16/05/2000 | |||||||||||||||
| Polysialylation in SDS | Japan | Filed. | 2001-585141 | 14/05/2001 | 16/05/2000 | |||||||||||||||||
| Polysialylation in SDS | USA | Granted. | 10/276552 | 14/05/2001 | 08/11/2005 | 6962972 | 16/05/2000 | |||||||||||||||
| Patent Name |
Country Of Filing |
Case Status |
Application |
Application |
Grant Date |
Inventors |
Patent No. |
1st Priority |
||||||||||||||
| Monofunctional PSA | Eur. Patent Office | Filed. | 4768074.9 | 12/08/2004 | Jain, et al. | 12/08/2003 | ||||||||||||||||
| Monofunctional PSA | India | Filed. | 985/DELNP/2006 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Monofunctional PSA | Japan | Filed. | 2006-523058 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Monofunctional PSA | Korea (Republic of) |
Filed. | 2006-7002900 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Monofunctional PSA | Russian Federation | Granted | 0000000000 | 12/08/2004 | 10/09/2008 | 2333223 | 12/08/2003 | |||||||||||||||
| Monofunctional PSA | USA | Filed. | 10/568043 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Monofunctional PSA | Eur. Patent Office | Filed. | 47680749 | 12/08/2004 | ||||||||||||||||||
| Maleimido-PSA | Switzerland | Granted. | 4768054.1 | 12/08/2004 | 03/10/2007 | Hreczuk-Hirst et al. | EP1654289 | 12/08/2003 | ||||||||||||||
| Maleimido-PSA | Spain | Granted. | 4768054.1 | 12/08/2004 | 03/10/2007 | EP1654289 | 12/08/2003 | |||||||||||||||
| Maleimido-PSA | France | Granted. | 4768054.1 | 12/08/2004 | 03/10/2007 | EP1654289 | 12/08/2003 | |||||||||||||||
| Maleimido-PSA | Italy | Granted. | 4768054.1 | 12/08/2004 | 03/10/2007 | EP1654289 | 12/08/2003 | |||||||||||||||
| Maleimido-PSA | Germany | Granted. | 4768054.1 | 12/08/2004 | 03/10/2007 | EP1654289 | 12/08/2003 | |||||||||||||||
| Maleimido-PSA | United Kingdom | Granted. | 4768054.1 | 12/08/2004 | 03/10/2007 | EP1654289 | 12/08/2003 | |||||||||||||||
| Maleimido-PSA | India | Allowed | 903/DELNP/2006 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Maleimido-PSA | Japan | Filed. | 2006-523054 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Maleimido-PSA | Korea (Republic of) |
Filed. | 2006-7002875 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Maleimido-PSA | Russian Federation | Filed. | 2006107545 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Maleimido-PSA | USA | Filed. | 10/568111 | 12/08/2004 | 12/08/2003 | |||||||||||||||||
| Maleimido-PSA Div | India | Filed. | 812/DELNP/2009 | 12/08/2009 | ||||||||||||||||||
| NHS Functional PSA | China | Filed. | 2.0068E+11 | 16/02/2006 | Jain et al. | 23/02/2005 | ||||||||||||||||
| NHS Functional PSA | European Patent Office | Filed. | 6709777.4 | 16/02/2006 | 23/02/2005 | |||||||||||||||||
| NHS Functional PSA | India | Filed. | 6400/DELNP/2007 | 16/02/2006 | 23/02/2005 | |||||||||||||||||
| NHS Functional PSA | Japan | Filed. | 2007-555696 | 16/02/2006 | 23/02/2005 | |||||||||||||||||
| NHS Functional PSA | United States of America | Filed. | 11/816823 | 16/02/2006 | 23/02/2005 | |||||||||||||||||
| NHS-Amino PSA Reactions | China | Filed. | 2.0058E+11 | 12/08/2005 | Jain et al. | 12/08/2004 | ||||||||||||||||
| NHS-Amino PSA Reactions | European Patent Office | Filed. | 5794259.1 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| NHS-Amino PSA Reactions | India | Filed. | 1100/DELNP/2007 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| NHS-Amino PSA Reactions | Japan | Filed. | 2007-525356 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| NHS-Amino PSA Reactions | United States of America | Filed. | 11/660128 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| Fractionation | China | Filed. | 2.0058E+11 | 12/08/2005 | Jain et al. | 12/08/2004 | ||||||||||||||||
| Fractionation | European Patent Office | Filed. | 5794240.1 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| Fractionation | India | Filed. | 1009/DELNP/2007 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| Fractionation | Japan | Filed. | 2007-525353 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
| Fractionation | United States of America | Filed. | 11/660133 | 12/08/2005 | 12/08/2004 | |||||||||||||||||
38
| Final Version (11 November 2009) | Private and Confidential |
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority |
1st Priority |
1st Priority |
|||||||||||||
| Fractionation | China | Filed. | 2.0058E+11 | 12/08/2005 | Jain et al. | GB | PCT/GB 04/03511 | 12/08/2004 | ||||||||||||||
| Fractionation | Eur. Patent Office | Filed. | 5794240.1 | 12/08/2005 | GB | PCT/GB 04/03511 | 12/08/2004 | |||||||||||||||
| Fractionation | India | Filed. | 1009/DELNP/2007 | 12/08/2005 | GB | PCT/GB 04/03511 | 12/08/2004 | |||||||||||||||
| Fractionation | Japan | Filed. | 2007-525353 | 12/08/2005 | GB | PCT/GB 04/03511 | 12/08/2004 | |||||||||||||||
| Fractionation | USA | Filed. | 11/660133 | 12/08/2005 | Jain et al. | GB | PCT/GB 04/03511 | 12/08/2004 | ||||||||||||||
| Endotoxin Removal | PCT | Filed. | PCT/GB/2008/050138 | 28/02/2007 | Jain et al. | 28/02/2007 | ||||||||||||||||
| Patent Name |
Country Of Filing |
Case Status |
Application No. |
Application Date |
Grant Date |
Inventors |
1st Priority Country |
1st Priority Appln No. |
1st Priority |
|||||||||||
| N-terminal polysialylation | W.I.P.O. | Filed. | PCT/GB 07/02839 | 25/07/2007 | Jain et al. | EP | 06117830.7 | 25/07/2006 | ||||||||||||
| N-terminally-polysialylated GCSF | W.I.P.O. | Filed. | PCT/GB 07/02816 | 25/07/2007 | Jain et al. | EP | 06117830.7 | 25/07/2006 | ||||||||||||
| Polysialylation of EPO | W.I.P.O. | Filed. | PCT/GB 07/02841 | 25/07/2007 | Jain et al. | EP | 06117830.7 | 25/07/2006 | ||||||||||||
| Polysialylated Insulin | W.I.P.O. | Filed. | PCT/GB 07/02821 | 25/07/2007 | Jain et al. | EP | 06117830.7 | 25/07/2006 | ||||||||||||
Schedule 7
PSA
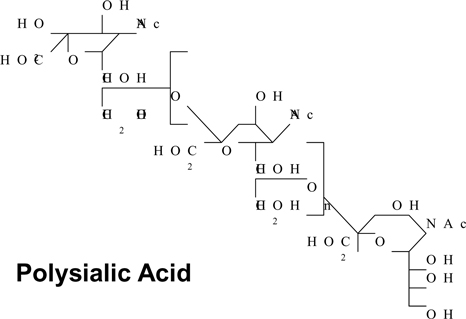
| Final Version (11 November 2009) | Private and Confidential |
Schedule 8
Target Date for Submission of CTA in Pharms Territory
| PRODUCT |
DATE FOR SUBMISSION OF CLINICAL TRIALS APPLICATION IN PHARMS TERRITORY | |
| A |
[***] | |
| B |
[***] | |
| C |
[***] | |
| D |
[***] | |
| E |
[***] | |
| F |
[***] | |
40
| Final Version (11 November 2009) | Private and Confidential |
Schedule 9
Members of the Programme Committee
PHARMS
To be elected prior to committee meetings
LIPOXEN
To be elected prior to committee meetings
41
| Final Version (11 November 2009) | Private and Confidential |
Schedule 10
Revenue Sharing
| 1. | For the purposes of this Schedule 10, the following words shall have the following meaning:- |
| “Lipoxen Net Sales” | the amount received by Lipoxen, its Affiliates and or Licensees from third parties in respect of supplies of Lipoxen Products in arms length transactions (or the amount that would have been received if the transactions had been at arms length) less the following items provided they are shown in writing on the relevant invoice or in other documentary evidence: sales taxes, costs of delivery, customary trade discounts actually granted, amounts actually repaid or credited for defective or returned and, in the case of export orders, any import duties or similar applicable governmental levies and any government rebates charged on the purchase price of the Lipoxen Products; | |
| “Joint Net Sales” | the amount received by Lipoxen and/or its Affiliates from third parties in respect of supplies of Joint Products in arms length transactions (or the amount that would have been received if the transactions had been at arms length) less the following items provided they are shown in writing on the relevant invoice or in other documentary evidence: sales taxes, costs of delivery, customary trade discounts actually granted, amounts actually repaid or credited for defective or returned and, in the case of export orders, any import duties or similar applicable governmental levies and any government rebates charged on the purchase price of the Joint Products; | |
42
| Final Version (11 November 2009) | Private and Confidential |
| “Joint Net Receipts” | all signing fees, milestones, royalties and other licence fees (excluding research and development fees) received by Lipoxen and/or its Affiliates from Licensees in relation to Joint Products, less any less any Value Added Tax or other sales tax and any direct and/or third party costs and/or expenses incurred by Lipoxen in procuring payment of such sums; | |
| “Joint Product Revenue” | the Joint Net Sales and the Joint Net Receipts; and | |
| “Pharms Net Sales” | the amount received by Pharms and/or its Affiliates from third parties in respect of supplies of the Products in arms length transactions (or the amount received if the transactions had been at arms length) less the following items provided they are shown in writing on the relevant invoice or in other documentary evidence: sales taxes, costs of delivery, customary trade discounts actually granted, amounts actually repaid or credited for defective or returned. | |
| 2. | Pharms shall pay to Lipoxen a royalty of [***] of all Pharms Net Sales. The royalty payable to Lipoxen pursuant to this paragraph shall become due 30 (thirty) days after the expiry of the Quarter in which the Products to which the royalty relate were sold and/or supplied by Pharms. |
| 3. | Lipoxen shall pay to Pharms a royalty of [***] of all Lipoxen Net Sales. The royalty payable to Pharms pursuant to this paragraph shall become due 30 (thirty) days after the expiry of the Quarter in which the Lipoxen Products to which the royalty relate were sold and/or supplied by Lipoxen. |
| 4. | The parties agree that the Joint Product Revenue shall be shared equally by the parties. Pharms’ half share of the Joint Product Revenue shall become due 30 (thirty) days after the expiry of the Quarter in which the relevant Joint Product Revenue was received by Lipoxen. |
| 5. | Lipoxen shall be entitled to deduct from the sums due to Pharms under paragraphs 3 and 4 above, any sums due and unpaid to Lipoxen pursuant to this Agreement. |
43

