LICENSE AGREEMENT BY AND AMONG COWEN HEALTHCARE ROYALTY PARTNERS, L.P. AND SHORE THERAPEUTICS, INC. AND SANTARUS, INC. DATED DECEMBER 21, 2011
Exhibit 10.1
CERTAIN MATERIAL (INDICATED BY AN ASTERISK) HAS BEEN OMITTED FROM THIS DOCUMENT PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
Execution Copy
BY AND AMONG
XXXXX HEALTHCARE ROYALTY PARTNERS, L.P.
AND
SHORE THERAPEUTICS, INC.
AND
SANTARUS, INC.
DATED
DECEMBER 21, 2011
TABLE OF CONTENTS
| Page | ||||
| ARTICLE 1. Definitions |
1 | |||
| 1.1 |
“Adverse Event” | 1 | ||
| 1.2 |
“Additional Product” | 1 | ||
| 1.3 |
“Affiliate” | 2 | ||
| 1.4 |
“API Supply Agreement” | 2 | ||
| 1.5 |
“Applicable Law” | 2 | ||
| 1.6 |
“Assigned Sections” | 2 | ||
| 1.7 |
“Assumed Product Agreements” | 2 | ||
| 1.8 |
“Authorized Generic” | 2 | ||
| 1.9 |
“Authorized Generic Revenues” | 3 | ||
| 1.10 |
“Calendar Quarter” | 3 | ||
| 1.11 |
“Calendar Year” | 3 | ||
| 1.12 |
“CHRP Shore Agreement” | 3 | ||
| 1.13 |
“Commercial Supply Agreement” | 3 | ||
| 1.14 |
“Commercialize” | 3 | ||
| 1.15 |
“Commercially Reasonable Efforts” | 3 | ||
| 1.16 |
“Compound” | 3 | ||
| 1.17 |
“Confidential Information” | 4 | ||
| 1.18 |
“Confidentiality Agreement” | 4 | ||
| 1.19 |
“Control” | 4 | ||
| 1.20 |
“Corporate Marks” | 5 | ||
| 1.21 |
“Detail” or “Detailing” | 5 | ||
| 1.22 |
“Development” | 5 | ||
| 1.23 |
“Dollar” or “$” | 5 | ||
| 1.24 |
“Domain Names” | 5 | ||
| 1.25 |
“Excluded Liabilities and Obligations” | 5 | ||
| 1.26 |
“Excluded Product Agreements” | 5 | ||
| 1.27 |
“FDA” | 5 | ||
| 1.28 |
“FD&C Act” | 6 | ||
| 1.29 |
“Field” | 6 | ||
| 1.30 |
“First Commercial Sale” | 6 | ||
| 1.31 |
“GAAP” | 6 | ||
| 1.32 |
“Generic Equivalent” | 6 | ||
| 1.33 |
“Impax” | 6 | ||
| 1.34 |
“Impax Litigation” | 6 | ||
| 1.35 |
“Impax Settlement Agreement” | 6 | ||
| 1.36 |
“Impax Sublicense Agreement” | 6 | ||
| 1.37 |
“Indemnification/Payment Fund” | 7 | ||
| 1.38 |
“Indemnitee” | 7 | ||
| 1.39 |
“Invention” | 7 | ||
| 1.40 |
“Inventory” | 7 | ||
| 1.41 |
“Joint Inventions” | 7 | ||
| 1.42 |
“Joint Patents” | 7 | ||
| 1.43 |
“Know-How” | 7 | ||
| 1.44 |
“Launch Date” | 7 | ||
| 1.45 |
“LCP Core Technology” | 8 | ||
| 1.46 |
“LCP Core Technology Improvements” | 8 | ||
| 1.47 |
“Licensed Know-How” | 8 | ||
| 1.48 |
“Licensed LCP Xxxx” | 8 | ||
| 1.49 |
“Licensed Patents” | 8 | ||
| 1.50 |
“Licensed Product” | 8 | ||
| 1.51 |
“Licensed Technology” | 8 | ||
| 1.52 |
“LifeCycle Agreement” | 8 | ||
| 1.53 |
“LifeCycle Know-How” | 9 | ||
| 1.54 |
“LifeCycle Marks” | 9 | ||
| 1.55 |
“LifeCycle Patents” | 9 | ||
| 1.56 |
“LifeCycle Technology” | 9 | ||
| 1.57 |
“Manufacture” or “Manufacturing” | 9 | ||
| 1.58 |
“Net Sales” | 9 | ||
| 1.59 |
“Patents” | 10 | ||
| 1.60 |
“Product” | 10 | ||
| 1.61 |
“Product Agreements” | 10 | ||
| 1.62 |
“Product Data” | 11 | ||
| 1.63 |
“Product Materials” | 11 | ||
| 1.64 |
“Product-Specific Patents” | 11 | ||
| 1.65 |
“Product Trademarks” | 11 | ||
| 1.66 |
“Promotion” or “Promote” | 11 | ||
| 1.67 |
“PROSAR Agreement” | 11 | ||
| 1.68 |
“Prosecution” or “Prosecute” | 11 | ||
| 1.69 |
“Regulatory Approval” | 11 | ||
| 1.70 |
“Regulatory Authority” | 11 | ||
| 1.71 |
“Regulatory Filings” | 12 | ||
| 1.72 |
“Royalty Term” | 12 | ||
| 1.73 |
“Santarus Know-How” | 12 | ||
| 1.74 |
“Santarus Patents” | 12 | ||
| 1.75 |
“Santarus Technology” | 12 | ||
| 1.76 |
“Santarus Sales Representative” | 12 | ||
| 1.77 |
“Shionogi Know-How” | 13 | ||
| 1.78 |
“Shionogi Patents” | 13 | ||
| 1.79 |
“Shionogi Technology” | 13 | ||
| 1.80 |
“Tank Agreement” | 13 | ||
| 1.81 |
“Tank Equipment” | 13 | ||
| 1.82 |
“Term” | 13 | ||
| 1.83 |
“Territory” | 13 | ||
| 1.84 |
“Third Party” | 13 | ||
| 1.85 |
“Third Party Royalty Payments” | 13 | ||
| 1.86 |
“Trademark” | 14 | ||
| 1.87 |
“Trademark Use Guidelines” | 14 |
| 1.88 |
“Transition Services” | 14 | ||||
| 1.89 |
“Upstream Agreements” | 14 | ||||
| 1.90 |
“Valid Claim” | 14 | ||||
| ARTICLE 2. Grant and Assignment of Rights |
14 | |||||
| 2.1 |
License Grants | 14 | ||||
| 2.2 |
Sublicensing | 15 | ||||
| 2.3 |
Upstream Agreements | 15 | ||||
| 2.4 |
No Other Rights | 16 | ||||
| 2.5 |
Assignment and Transfer of Other Product Related Items | 17 | ||||
| 2.6 |
Impax Agreements | 18 | ||||
| ARTICLE 3. Trademark Usage and Maintenance |
19 | |||||
| 3.1 |
Ownership of Trademarks | 19 | ||||
| 3.2 |
Use of Licensed LCP Xxxx | 19 | ||||
| 3.3 |
Corporate Marks | 21 | ||||
| 3.4 |
Infringement of Product Trademarks | 21 | ||||
| 3.5 |
Third-Party Trademark Claims | 22 | ||||
| ARTICLE 4. Development and Regulatory Activities |
22 | |||||
| 4.1 |
Additional Development Activities | 22 | ||||
| 4.2 |
Rights of Reference | 22 | ||||
| 4.3 |
Regulatory Filings and Responsibilities; Consultation | 23 | ||||
| 4.4 |
Drug Safety | 24 | ||||
| ARTICLE 5. Commercialization |
25 | |||||
| 5.1 |
Diligence | 25 | ||||
| 5.2 |
Product Materials | 26 | ||||
| 5.3 |
Complaints and Inquiries | 26 | ||||
| 5.4 |
Pricing | 27 | ||||
| 5.5 |
Exclusivity | 27 | ||||
| ARTICLE 6. Manufacturing and Supply |
27 | |||||
| 6.1 |
Manufacturing | 27 | ||||
| 6.2 |
NDC Numbers, Product Returns, Rebates and Chargebacks | 27 | ||||
| ARTICLE 7. Payments |
28 | |||||
| 7.1 |
Upfront Payment | 28 | ||||
| 7.2 |
Sales Milestones | 29 | ||||
| 7.3 |
Royalty Payments | 29 | ||||
| 7.4 |
Authorized Generic Revenues | 29 | ||||
| 7.5 |
Third Party Royalties; Other Credits Against Royalties | 30 | ||||
| 7.6 |
Payments and Reports | 30 | ||||
| 7.7 |
Wire Transfers | 30 | ||||
| 7.8 |
Taxes | 31 | ||||
| 7.9 |
Records; Audit Rights | 31 | ||||
| 7.10 |
Late Payments | 32 | ||||
| ARTICLE 8. Inventions and Patents |
32 | |||||
| 8.1 |
Inventions | 32 | ||||
| 8.2 |
Patent Prosecution | 34 | ||||
| 8.3 |
Enforcement | 34 | ||||
| 8.4 |
Declaratory Actions and Counterclaims | 36 | ||||
| 8.5 |
Infringement Defense | 36 | ||||
| ARTICLE 9. Representations, Warranties, and Covenants |
37 | |||||
| 9.1 |
Representations, Warranties and Covenants | 37 | ||||
| 9.2 |
Representations, Warranties and Covenants of Shore | 38 | ||||
| 9.3 |
Representations and Warranties of CHRP | 42 | ||||
| 9.4 |
Disclaimer | 42 | ||||
| ARTICLE 10. Confidentiality |
42 | |||||
| 10.1 |
Treatment of Confidential Information | 42 | ||||
| 10.2 |
Authorized Disclosures | 43 | ||||
| 10.3 |
Publicity | 43 | ||||
| 10.4 |
Publication | 44 | ||||
| 10.5 |
Confidentiality Agreement | 44 | ||||
| ARTICLE 11. Indemnification |
44 | |||||
| 11.1 |
Indemnification by Santarus | 44 | ||||
| 11.2 |
Indemnification by Shore | 45 | ||||
| 11.3 |
Indemnification by CHRP | 45 | ||||
| 11.4 |
Procedure | 45 | ||||
| 11.5 |
Insurance | 46 | ||||
| 11.6 |
Limitation of Liability | 47 | ||||
| 11.7 |
Indemnification/Payment Fund | 47 | ||||
| ARTICLE 12. Term and Termination |
47 | |||||
| 12.1 |
Term | 47 | ||||
| 12.2 |
Termination by Santarus | 47 | ||||
| 12.3 |
Termination for Cause | 48 | ||||
| 12.4 |
Termination for Bankruptcy | 48 | ||||
| 12.5 |
Consequences of Expiration or Termination | 48 | ||||
| 12.6 |
Survival | 49 | ||||
| 12.7 |
No Waiver of Remedies | 49 | ||||
| ARTICLE 13. Dispute Resolution |
50 | |||
| 13.1 |
Disputes | 50 | ||
| 13.2 |
Governing Law | 51 | ||
| ARTICLE 14. Miscellaneous |
51 | |||
| 14.1 |
Entire Agreement | 51 | ||
| 14.2 |
Assignment | 51 | ||
| 14.3 |
Amendments | 52 | ||
| 14.4 |
Bankruptcy | 52 | ||
| 14.5 |
Non-Waiver | 52 | ||
| 14.6 |
Severability | 52 | ||
| 14.7 |
Notice | 53 | ||
| 14.8 |
Further Assurances | 54 | ||
| 14.9 |
Force Majeure | 54 | ||
| 14.10 |
Cooperation | 54 | ||
| 14.11 |
Independent Contractors | 55 | ||
| 14.12 |
No Third Party Beneficiaries | 55 | ||
| 14.13 |
Interpretation | 55 | ||
| 14.14 |
Counterparts | 56 | ||
EXHIBITS
| Exhibit 1.16 |
Compound | |
| Exhibit 1.24 |
Domain Names | |
| Exhibit 1.40 |
Inventory Lot Numbers | |
| Exhibit 1.49 |
Licensed Patents | |
| Exhibit 1.61 |
Product Agreements | |
| Exhibit 1.65 |
Product Trademarks | |
| Exhibit 2.5(b) |
FDA Transfer Letter | |
| Exhibit 9.2 |
Exceptions to Warranties and Representations | |
| Exhibit 9.2(w) |
Unfilled Firm Orders | |
| Exhibit 10.3 |
Press Release | |
THIS LICENSE AGREEMENT (the “Agreement”) is made and entered into as of December 21, 2011 (the “Effective Date”), by and among XXXXX HEALTHCARE ROYALTY PARTNERS, L.P., a Delaware limited partnership, having its principal place of business at 000 Xxxxx Xxxxxx, Xxxxx 0000, Xxxxxxxx, XX 00000 (“CHRP”), SHORE THERAPEUTICS, INC., a Delaware corporation, having its principal place of business at 000 Xxxxx Xxxxxx, Xxxxx 0000, Xxxxxxxx, XX 00000 (“Shore”) and SANTARUS, INC., a Delaware corporation, having its principal place of business at 0000 Xxxxxx Xxxxxx Xxxxx, Xxxxx 000, Xxx Xxxxx, Xxxxxxxxxx 00000 (“Santarus”). CHRP and Shore, on the one hand, and Santarus, on the other hand, are referred to individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Veloxis Pharmaceuticals A/S f/k/a LifeCycle Pharma A/S (“LifeCycle”) and CHRP entered into a License Agreement dated August 20, 2008, as amended on July 11, 2011 (the “LifeCycle Agreement”), pursuant to which LifeCycle granted CHRP the exclusive rights to manufacture and commercialize certain fenofibrate products in the Field in the Territory (each as defined below);
WHEREAS, CHRP and Shore entered into a License Agreement dated October 11, 2010 (the “CHRP Shore Agreement”), pursuant to which, among other things, CHRP sublicensed to Shore the exclusive rights to manufacture within and outside the Territory, solely for certain activities within the Territory, and to commercialize the fenofibrate products in the Field in the Territory; and
WHEREAS, Shore desires to grant to Santarus, and Santarus desires to accept, exclusive rights to manufacture and commercialize the fenofibrate products in the Field in the Territory.
NOW, THEREFORE, in consideration of the mutual covenants and agreements contained herein and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Parties agree as follows:
ARTICLE 1.
DEFINITIONS
The following terms shall have the following meanings as used in this Agreement:
1.1 “Adverse Event” means any undesirable medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with the treatment, including any variant of an “adverse drug experience” as those terms are defined at either 21 C.F.R. Section 312.32 or 21 C.F.R. Section 314.80, whether arising in or outside of a clinical study.
1.2 “Additional Product” means a delivery and/or formulation modification to the Product as to which a supplement to NDA No. 22,118 would be required prior to marketing or selling of such product.
1.3 “Affiliate” means (a) an entity that owns directly or indirectly a controlling interest in a Party, by stock ownership or otherwise, (b) any entity in which a Party owns a controlling interest, by stock ownership or otherwise, or (c) any entity under common control with a Party, directly or indirectly. Solely for purposes of the foregoing sentence, “controlling interest” and “control” means the power, whether or not exercised, to direct the management and affairs of a Party, directly or indirectly, whether through the ownership of voting securities, by contract, or otherwise. The direct or indirect ownership of fifty percent (50%) or more of a Party’s outstanding voting securities shall in any case be deemed to confer “control.”
1.4 “API Supply Agreement” means that certain API Supply Agreement, effective as of December 5, 2008, by and between Shore (as successor to Sciele Pharma, Inc.) and Chemagis (“Chemagis”), as amended on June 28, 2011, and as further amended from time to time following the Effective Date, pursuant to which Chemagis agreed to manufacture and supply to Shore the Compound.
1.5 “Applicable Law” means all laws, statutes, ordinances, codes, rules, and regulations that have been enacted by a government authority and which are in force as of the Effective Date or come into force during the Term, in each case to the extent that the same are applicable to the performance by the Parties of their respective obligations under this Agreement, including, with respect to the United States, the Prescription Drug Marketing Act, the FD&C Act, the Health Insurance Portability and Accountability Act, the Federal Anti-Kickback Statute, and any applicable FDA regulations relating to sampling practices.
1.6 [Intentionally Omitted]
1.7 “Assumed Product Agreements” has the meaning set forth in Section 2.5(d).
1.8 “Authorized Generic” means a pharmaceutical product that (i) is sold under the Regulatory Approval for a Product or Additional Product, (ii) is sold under a different Trademark than such Product or Additional Product, and (iii) has a National Drug Code (“NDC”) number that differs from the NDC number for such Product or Additional Product (other than on a temporary basis as may be necessary to launch such Product or Additional Product in the applicable market).
1.9 “Authorized Generic Revenues” means all net revenue Santarus or its Affiliates receive from sublicensees or authorized Third Parties as a royalty payment for their “net sales” or any comparable definition (as defined in the applicable sublicense agreement) of an Authorized Generic in the Territory during the Term; provided that “Authorized Generic Revenues” shall not include any royalties or other revenues received by Santarus or its Affiliates pursuant to the Impax Sublicense Agreement.
1.10 “Calendar Quarter” means each of the three (3) month periods ending on March 31, June 30, September 30, and December 31, provided that the first Calendar Quarter during the Term shall commence on the Effective Date and end on December 31, 2011.
1.11 “Calendar Year” means each twelve (12) month period beginning on January 1 and ending on December 31, provided that the first Calendar Year during the Term shall commence on the Effective Date and end on December 31, 2011.
1.12 “CHRP Shore Agreement” has the meaning set forth in the recitals.
1.13 “Commercial Supply Agreement” means that certain Commercial Supply and Packaging Agreement, dated as of January 15, 2011, by and between Shore and Catalent Pharma Solution, LLC (“Catalent”), as amended from time to time following the Effective Date, pursuant to which Catalent agreed to Manufacture the Licensed Product.
1.14 “Commercialize”, with a correlative meaning for “Commercializing” and “Commercialization”, means any and all activities relating to the commercialization and exploitation of the Licensed Products in the Field in the Territory, including the marketing, Promotion, Detailing, distribution, sale, offer for sale, and importation of the Licensed Products after Regulatory Approval of such Licensed Products, excluding any and all Manufacturing of the Licensed Products.
1.15 “Commercially Reasonable Efforts” means, with respect to the efforts of a Party to complete specific tasks or obligations under this Agreement relating to a Licensed Product, at least the level of efforts and resources that would be applied, consistent with prevailing pharmaceutical industry standards, by such Party with respect to a pharmaceutical product at a similar stage in its product life and of similar market potential to such Licensed Product. It is anticipated that the level of effort will change over time, reflecting changes in the status of the Licensed Product.
1.16 “Compound” means the bulk active pharmaceutical ingredient fenofibrate as set forth on Exhibit 1.16.
1.17 “Confidential Information” of a Party means all secret, confidential or proprietary information or data, including any and all information exchanged between the Parties under the Confidentiality Agreement, whether provided in written, oral, graphic, video, computer or other form, provided by such Party (the “Disclosing Party”) to any other Party (the “Receiving Party”) (including information generated by or on behalf of such Party pursuant to this Agreement and disclosed to any other Party), which may include information relating to the Disclosing Party’s existing or proposed research, development efforts, patent applications, business or products and any other materials that have not been made available by the Disclosing Party to the general public. The terms of this Agreement shall also be deemed Confidential Information of each Party, except to the extent disclosed pursuant to ARTICLE 10 herein. Notwithstanding the foregoing sentences, the term “Confidential Information” shall not include any information or materials that the Receiving Party can demonstrate:
(a) were already known to the Receiving Party (other than under an obligation of confidentiality), at the time of disclosure by the Disclosing Party to the extent such Receiving Party has documentary evidence to that effect;
(b) were generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure or development, as the case may be, and other than through any act or omission of the Receiving Party in breach of its confidentiality obligations under this Agreement;
(d) were subsequently lawfully disclosed to the Receiving Party by a Third Party who had no obligation to the Disclosing Party not to disclose such information to others;
(e) were independently discovered or developed by or on behalf of the Receiving Party by persons who had no access to and without the use of the Confidential Information belonging to the other Party and the Receiving Party has documentary evidence to that effect; or
(f) is approved for release by the Disclosing Party in writing.
1.18 “Confidentiality Agreement” means the Mutual Confidentiality Agreement between Shore and Santarus effective January 26, 2011.
1.19 “Control” means, with respect to any information or intellectual property right, possession by a Party of the ability (whether by ownership, license, or otherwise) to grant access, a license, or a
sublicense to such information or intellectual property right without the payment of additional consideration, and without violating the terms of any agreement or other arrangement with, or rights of, any Third Party as of the time such Party would first be required hereunder to grant the other Party such access, license or sublicense.
1.20 “Corporate Marks” shall mean, with respect to each of the Parties, the corporate name of such Party or those of Affiliates of such Party, and its and their trade names, trademarks, service marks, domain names, and associated logos and designs; provided that Corporate Marks shall not include the Product Trademarks or the Licensed LCP Xxxx.
1.21 “Detail” or “Detailing” means an in-person, face-to-face presentation of a Licensed Product by a sales representative with respect to a physician or other individuals or entities with prescribing authority involved or potentially involved in prescribing the Licensed Products, which presentation is for the purpose of Promoting the Licensed Product in the Field in the Territory.
1.22 “Development”, with a correlative meaning for “Develop” and “Developing”, means all non-clinical, pre-clinical and clinical drug development, and regulatory activities with respect to seeking Regulatory Approval of the Licensed Products, as applicable, for any indication in the Field in the Territory, and post-approval studies, including label extensions in support of a Licensed Product in the Field in the Territory and any studies required by a Regulatory Authority, such activities to occur within or outside the Territory for purposes of Commercialization, and excluding any and all Manufacturing of the Licensed Products.
1.23 “Dollar” or “$” means the legal tender of the United States.
1.24 “Domain Names” means those domain names and web sites set forth on Exhibit 1.24.
1.25 “Excluded Liabilities and Obligations” has the meaning set forth in Section 2.6(a).
1.26 “Excluded Product Agreements” has the meaning set forth in Section 2.5(d).
1.27 “FDA” means the United States Food and Drug Administration, or any successor organization thereto.
1.28 “FD&C Act” means the United States Federal Food, Drug and Cosmetic Act, 21 U.S.C. 301, et. seq., as it may be amended from time to time, and the regulations promulgated thereunder.
1.29 “Field” means the prevention, palliation or treatment of any condition, indication or diseases in humans.
1.30 “First Commercial Sale” means the first commercial sale by Santarus or its Affiliates or sublicensees of a Licensed Product to a Third Party for end use or consumption in the Territory.
1.31 “GAAP” means generally accepted accounting principles in the United States, consistently applied.
1.32 “Generic Equivalent” means, with respect to a Licensed Product, a second or subsequent product that (a) is “therapeutically equivalent,” as evaluated by the FDA, applying the definition of “therapeutically equivalent” set forth in the preface to the then-current edition of the FDA publication “Approved Drug Products With Therapeutic Equivalence Evaluations” or any other similar definitions set forth in Applicable Laws, in each case, as is necessary to permit pharmacists or other individuals authorized to dispense pharmaceuticals under Applicable Laws to substitute one product for another product in the absence of specific instruction from a physician or other authorized prescriber under Applicable Laws, and (b) is not an Authorized Generic of such Licensed Product.
1.33 “Impax” means Impax Laboratories, Inc.
1.34 “Impax Litigation” means the existing litigation entitled LifeCycle Pharma A/S v. Impax Laboratories, Inc., Civil Action No. 10-358 (GMS).
1.35 “Impax Settlement Agreement” means that certain Settlement Agreement and Release of Claims, dated December 19, 2011, by and among LifeCycle, Shore and Impax, as amended from time to time.
1.36 “Impax Sublicense Agreement” means that certain Sublicense Agreement, dated December 19, 2011, by and among CHRP, Shore and Impax, as amended from time to time.
1.37 “Indemnification/Payment Fund” has the meaning set forth in Section 11.7.
1.38 “Indemnitee” means, with respect to a Party, such Party and its Affiliates, and their respective directors, officers, managers, members, employees, agents, contractors and licensees.
1.39 “Invention” means any invention or discovery, whether or not patentable, made as a result of the activities of a Party or the Parties pursuant to this Agreement performed after the Effective Date that is necessary or useful in the Development, Manufacture, use, or Commercialization of a Licensed Product.
1.40 “Inventory” means inventory of bulk Compound or finished Product (including samples) that have been demonstrated to meet established release specifications, whether held at a location or facility of Shore or any of its Affiliates (or any Third Party on behalf of Shore or any of its Affiliates) or in transit to or from Shore or any of its Affiliates (or any such Third Party) with the lot numbers as set forth in Exhibit 1.40.
1.41 “Joint Inventions” has the meaning set forth in Section 8.1(d).
1.42 “Joint Patents” means all Patents that cover or claim Joint Inventions.
1.43 “Know-How” means all non-public Inventions, information, results and data of any type whatsoever, in any tangible or intangible form (and whether or not patentable), including databases, practices, methods, techniques, specifications, formulations, formulae, knowledge, skill, experience, data and results (including pharmacological, medicinal chemistry, biological, chemical, biochemical, toxicological and clinical study data and results), analytical and quality control data, stability data, studies and procedures, and manufacturing process and development information, results and data.
1.44 “Launch Date” means the date of First Commercial Sale of any Licensed Product by Santarus.
1.45 “LCP Core Technology” means (a) the inventions and subject matter covered or claimed by the Patents listed in Part B of Exhibit 1.49 of this Agreement and/or in any rights or interests in Patents based on or derived from such applications; and (b) all Know-How that is Controlled by LifeCycle and relates generally to its MeltDose® technology or the manufacture of pharmaceutical products in MeltDose® formulations.
1.46 “LCP Core Technology Improvements” means (a) all Inventions that are enhancements, improvements, modifications or derivatives of LCP Core Technology and are made by or on behalf of Santarus or its Affiliates at any time during the Term, and (b) all Patents claiming, or other intellectual property rights appurtenant to, any of such Inventions set forth in subsection (a).
1.47 “Licensed Know-How” means all Know-How related to Licensed Products (including information not covered by the Licensed Patents) that is Controlled by Shore as of the Effective Date or at any time during the Term and is necessary or useful to Develop, Manufacture, or Commercialize Licensed Products in the Field, including the LifeCycle Know-How, and Know-How relating to Joint Inventions. Notwithstanding anything herein to the contrary, Licensed Know-How shall exclude Licensed Patents and Product Trademarks.
1.48 “Licensed LCP Xxxx” means MeltDose®.
1.49 “Licensed Patents” means all Patents that cover or claim a Licensed Product or its manufacture or use that are Controlled by Shore as of the Effective Date or at any time during the Term, and are necessary or useful to Develop, Manufacture or Commercialize Licensed Products in the Field, including the LifeCycle Patents and Joint Patents. The Licensed Patents in existence as of the Effective Date are as set forth on Exhibit 1.49.
1.50 “Licensed Product” means the Product and any Additional Product.
1.51 “Licensed Technology” means, collectively, the Licensed Patents, Licensed Know-How, and all copyrights owned or otherwise Controlled by Shore that are associated with the Compound or Licensed Products.
1.52 “LifeCycle Agreement” has the meaning set forth in the recitals.
1.53 “LifeCycle Know-How” means all Know-How related to Licensed Products (including information not covered by the Licensed Patents) that is Controlled by LifeCycle as of the Effective Date or at any time during the Term and is necessary or useful to Develop, Manufacture, or Commercialize Licensed Products. Notwithstanding anything herein to the contrary, LifeCycle Know-How shall exclude LifeCycle Patents and the Licensed LCP Xxxx.
1.54 “LifeCycle Marks” has the meaning set forth in Section 3.1(b).
1.55 “LifeCycle Patents” means all Patents that cover or claim any aspect of a Licensed Product or its manufacture or use that are Controlled by LifeCycle as of the Effective Date or at any time during the Term, and are necessary or useful for Development, Manufacture, or Commercialization.
1.56 “LifeCycle Technology” means, collectively, the LifeCycle Patents and the LifeCycle Know-How.
1.57 “Manufacture” or “Manufacturing” means all activities related to the manufacture of Licensed Products, including analytical method development, formulation and process development, quality assurance/quality control procedures, quality control testing (including raw material, in-process, release and stability testing), process scale-up, validation, clinical and commercial manufacturing (including bulk manufacturing and finished pharmaceutical product manufacturing), packaging and release activities, either within or outside the Territory, for purposes of Development or Commercialization.
1.58 “Net Sales” means, for any period of determination, the gross amount invoiced for Licensed Products (but excluding Authorized Generics) by Santarus, its Affiliates and any sublicensees to Third Parties for such period of determination, less the aggregate of the following deductions to the extent actually incurred in connection with such sales:
(a) reasonable and customary cash, trade, and quantity discounts off the invoiced price, promotional allowances actually incurred and fees paid to wholesalers and retailers based on the sale or dispensing of Licensed Products;
(b) excise, sales, value added, good and services and other consumption taxes and import/export and custom duties or other taxes imposed on the importation, use or sale of the Licensed Product to Third Parties, to the extent included in the gross amount invoiced;
(c) freight, insurance and other transportation charges to the extent billed separately;
(d) amounts repaid, credited or accrued, or allowances or adjustments made, by reason of customer returns (consistent with Santarus’ then reasonable applicable return policies), rejections, or recalls;
(e) reasonable and customary rebates and chargebacks; and
(f) rebates associated with any voucher, coupon, loyalty card or other co-pay assistance programs;
solely to the extent the above deductions are in accordance with law and taken in accordance with GAAP.
Products or Additional Products may not be sold as “loss leaders” or together with another product in the practice commonly known as “bundling.
In calculating Net Sales, any transfer from Santarus to an Affiliate or sublicensee shall be disregarded and the calculation shall instead be based on the first transfer to a Third Party.
1.59 “Patents” means (a) patents and patent applications, including provisional patent applications, (b) all divisionals, continuations, continuations in-part thereof or any other patent application, including national, regional and international counterparts, claiming priority, or entitled to claim priority, directly or indirectly to (i) any such patents or patent applications or (ii) any patent or patent application from which such patents or patent applications claim, or is entitled to claim, direct or indirect priority, and (c) all patents issuing on any of the foregoing, together with all registrations, reissues, re-examinations, patents of addition, renewals, supplementary protection certificates, or extensions of any of the foregoing.
1.60 “Product” means the finished pharmaceutical product containing the Compound as the sole active ingredient, formulated using LifeCycle’s MeltDose® technology, which is marketed under the brand name Fenoglide® as of the Effective Date under NDA No. 22,118, approved by the FDA on August 10, 2007 (as such NDA has been amended or supplemented through the Effective Date), including modifications to dosage strength, modifications of excipients, or conversion of dosage form, for instance, to a capsule or caplet, and including any Authorized Generic, but not including any Additional Product or Generic Equivalent.
1.61 “Product Agreements” means (a) the agreements in effect as of the Effective Date by and between Shore and Third Parties relating to the Licensed Products and (b) the purchase orders issued by Shore, in each case, as set forth in Exhibit 1.61.
1.62 “Product Data” means all preclinical, non-clinical, analytical, Manufacturing, regulatory, and clinical data, and books and records, relating to the Licensed Products that are necessary to the Development, Manufacture, use or Commercialization of the Licensed Products, including any Licensed Product safety database.
1.63 “Product Materials” means all materials, including training materials, medical education materials, market research, customer lists, advertisements, leave behinds, and Detail aids used in the Promotion and Commercialization of the Licensed Products.
1.64 “Product-Specific Patents” means the Patents listed in Part A of Exhibit 1.49.
1.65 “Product Trademarks” means (a) Fenoglide® and all other Trademarks used by CHRP, Shore or their Affiliates as of the Effective Date in connection with the marketing or sale of the Licensed Products in the Field in the Territory, as set forth in Exhibit 1.65, excluding the Licensed LCP Xxxx and any Corporate Marks and (b) all related Domain Names and other Trademark related rights, in each case excluding the Corporate Marks of the Parties.
1.66 “Promotion” or “Promote” means all direct and indirect marketing and promotion of the Licensed Products in the Field in the Territory, including all direct and indirect sales force activities and expenses.
1.67 “PROSAR Agreement” has the meaning set forth in Section 2.5(d).
1.68 “Prosecution” or “Prosecute” means, with respect to Patents, the preparation, filing for, prosecuting, filing reissue applications, responding to oppositions, nullity actions, re-examinations, revocation actions, post-grant reviews, inter partes reviews and similar proceedings (including conducting or participating in interference and oppositions) filed by Third Parties against, and maintaining, Patents.
1.69 “Regulatory Approval” means any approvals, licenses, registrations or authorizations of any Regulatory Authority, whether or not conditional, that are necessary for the commercial sale of the Licensed Products in the Field in the Territory.
1.70 “Regulatory Authority” means any and all supranational, national, or regional, state, provincial or other local government, court, governmental agency, authority, board, bureau, instrumentality, regulatory
agency, department, bureau, commission, council or other government entity, whose approval or authorization is necessary for, or to whom notice must be given prior to, the Development, Manufacture, Commercialization or use of a Licensed Product, including the FDA.
1.71 “Regulatory Filings” means the technical, medical and scientific registrations, authorizations and approvals (including approvals of NDAs, supplements and amendments, pre- and post- approvals, and labeling approvals) of any Regulatory Authority necessary for the Development, Manufacture, and for distribution, marketing, Promotion, offer for sale, use, import, reimbursement, export or sale of a subject product in the Territory, together with all related correspondence to or from any Regulatory Authority and all documents referenced in the complete regulatory chronology for each NDA, including the Drug Master File (if any), IND, NDA and supplemental new drug applications (sNDAs).
1.72 “Royalty Term” means, on a Licensed Product-by-Licensed Product basis, the period commencing on the First Commercial Sale of the applicable Licensed Product and continuing until the earlier of: (a) the expiration of all Valid Claims in the Licensed Patents that claim or cover the Licensed Product or its use (excluding, for clarity, method of manufacture claims), or (b) the launch in the Territory of a Generic Equivalent or an Authorized Generic of such Licensed Product by a Third Party.
1.73 “Santarus Know-How” means all Know-How related to Licensed Products (including information not covered by the Santarus Patents) that is Controlled by Santarus as of the Effective Date or developed or acquired by Santarus at any time during the Term and is necessary or useful to Develop, Manufacture, use or Commercialize Licensed Products in the Field in the Territory, but excluding all Shionogi Technology, LCP Core Technology Improvements, Licensed Technology and Joint Inventions.
1.74 “Santarus Patents” means all Patents that cover or claim the composition, manufacture, or method of use of a Licensed Product or any other Santarus Know-How that are Controlled by Santarus as of the Effective Date or acquired by, or developed by and reduced to practice by, Santarus at any time during the Term and are necessary or useful to Develop, Manufacture, use or Commercialize Licensed Products in the Field in the Territory, but excluding all Licensed Technology, Shionogi Technology, LCP Core Technology Improvements, LifeCycle Technology and Joint Inventions.
1.75 “Santarus Technology” means, collectively, the Santarus Patents and Santarus Know-How.
1.76 “Santarus Sales Representative” means a member of Santarus’ sales force engaged in the conduct of Details of the Licensed Products, whether as an employee or contractor.
1.77 “Shionogi Know-How” means all Know-How related to Licensed Products (including information not covered by the Shionogi Patents) that was Controlled by Shionogi as of the date of termination of the Shionogi License Agreement (as defined in Section 1.79), but excluding all LCP Core Technology Improvements and LifeCycle Technology.
1.78 “Shionogi Patents” means all Patents that cover or claim a Licensed Product or its manufacture or use, or any other Shionogi Know-How that was Controlled by Shionogi as of the date of termination of the Shionogi License Agreement, but excluding all LCP Core Technology Improvements and LifeCycle Technology.
1.79 “Shionogi Technology” means, collectively, the Shionogi Patents and Shionogi Know-How. For clarity, the Shionogi Technology was licensed to LifeCycle pursuant to that certain License Agreement, dated April 30, 2007, as amended, between LifeCycle and Shionogi (the “Shionogi License Agreement”).
1.80 “Tank Agreement” has the meaning set forth in Section 2.5(h).
1.81 “Tank Equipment” has the meaning set forth in Section 2.5(h).
1.82 “Term” has the meaning set forth in Section 12.1.
1.83 “Territory” means the United States of America, including its territories and possessions and Puerto Rico.
1.84 “Third Party” means any entity other than a Party or its Affiliates.
1.85 “Third Party Royalty Payment” has the meaning set forth in Section 7.5.
1.86 “Trademark” means any word, name, symbol, color, designation, or device or any combination thereof, whether registered or unregistered, including any trademark, trade dress, service xxxx, service name, brand xxxx, trade name, brand name, logo, or business symbol.
1.87 “Trademark Use Guidelines” has the meaning set forth in Section 3.2(d).
1.88 “Transition Services” has the meaning set forth in Section 2.5(f).
1.89 “Upstream Agreements” means the LifeCycle Agreement, the CHRP Shore Agreement and the Tank Agreement.
1.90 “Valid Claim” means a claim of an issued and unexpired patent, or a claim of a pending patent application, within the Licensed Patents, which claim has not been held invalid, unpatentable or unenforceable by a court or other government agency of competent jurisdiction from which no appeal can be further taken, and has not been held or admitted to be invalid, unpatentable or unenforceable through abandonment, re-examination or disclaimer, opposition procedure, nullity suit or otherwise, which claim covers or claims a Licensed Product or its manufacture or use; provided, however, that if a claim of a pending patent application shall not have issued as a claim of an issued patent within [***] after the earliest filing date from which such claim takes priority such claim shall not constitute a Valid Claim for the purposes of this Agreement unless and until such claim shall issue as a claim of an issued patent.
ARTICLE 2.
GRANT AND ASSIGNMENT OF RIGHTS
2.1 License Grants.
(a) Subject to the terms and conditions of this Agreement, the LifeCycle Agreement, and the Impax Sublicense Agreement, Shore hereby grants to Santarus:
(i) an exclusive (even as to Shore), royalty-bearing, license, with the right to grant sublicenses (subject to Section 2.2), under the Licensed Technology, to market, import, use, sell, offer for sale and otherwise Commercialize Licensed Products in the Field in the Territory;
(ii) a worldwide, non-exclusive, royalty-bearing, license, with the right to grant sublicenses (subject to Section 2.2), under the Licensed Technology, to Develop and Manufacture the Licensed Products solely as necessary for obtaining Regulatory Approval in, or for Commercialization in, the Field in the Territory;
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(iii) an exclusive (even as to Shore), royalty-bearing, license, with the right to grant sublicenses (subject to Section 2.2), under the Shionogi Technology, to market, import, use, sell, offer for sale and otherwise Commercialize Licensed Products in the Field in the Territory; and
(iv) a worldwide, non-exclusive, royalty-bearing, license, with the right to grant sublicenses (subject to Section 2.2), under the Shionogi Technology, to Develop and Manufacture the Licensed Products solely as necessary for obtaining Regulatory Approval in, or for Commercialization in, the Field in the Territory.
For clarity, rights to Manufacture or Develop Licensed Products outside the Territory are granted to Santarus to the extent such rights are granted to CHRP and/or Shore.
(b) Santarus acknowledges that Shore has entered into the Impax Sublicense Agreement prior to the date hereof, which agreement grants to Impax certain rights under the Licensed Patents in the Territory. Shore has delivered to Santarus true, correct and complete copies of the Impax Sublicense Agreement and the Impax Settlement Agreement.
(c) Subject to the terms and conditions of this Agreement, the LifeCycle Agreement and the Impax Sublicense Agreement, CHRP hereby grants to Santarus an exclusive (even as to CHRP with respect to Commercialization), royalty-free, license, with the right to grant sublicenses (subject to Section 2.2), under any Joint Inventions and Joint Patents, to make, have made, market, import, use, sell, offer for sale and otherwise Develop, Manufacture and Commercialize Licensed Products.
2.2 Sublicensing. Santarus shall have the right to grant sublicenses under the rights granted to Santarus in Section 2.1 to its Affiliates or Third Parties without the prior written consent of Shore or CHRP; provided, however, that any such sublicense shall be consistent with and subject to the terms and conditions of this Agreement, and, to the extent applicable to the LifeCycle Technology licensed under the LifeCycle Agreement and sublicensed to Santarus hereunder, each sublicense agreement entered into by Santarus shall be in compliance and not inconsistent with the terms and conditions of the LifeCycle Agreement; provided further, however, that no such sublicense shall relieve Santarus of any of its obligations under this Agreement.
2.3 Upstream Agreements; Impax Settlement Agreement and Impax Sublicense Agreement. Subject to this Section 2.3 and Section 2.6, Shore and CHRP shall have the sole responsibility for exercising their respective rights and discharging their respective obligations under the Upstream Agreements, Impax Settlement Agreement and Impax Sublicense Agreement.
(a) Copy. On or before the Effective Date, CHRP has delivered to Santarus true, correct and complete copies of the Upstream Agreements.
(b) Covenants.
(i) Neither Shore nor CHRP will modify, amend or waive any provision of an Upstream Agreement, the Impax Settlement Agreement or the Impax Sublicense Agreement in such a manner that could have an adverse impact on Manufacture or Commercialization of the Licensed Products hereunder [***], without the prior written consent of Santarus, such consent not to be unreasonably withheld, conditioned or delayed.
(ii) Shore or CHRP, as the case may be, will immediately (but in no event later than [***]) notify Santarus if Shore or CHRP, as the case may be, fails to meet any of their respective obligations, including any payment obligations, under an Upstream Agreement, the Impax Settlement Agreement or the Impax Sublicense Agreement or receives notice from LifeCycle or Impax, as the case may be, alleging any such failure which failure could have a material adverse effect on the rights of Santarus hereunder.
(iii) Each of CHRP and Shore shall (A) maintain in full force and effect each Upstream Agreement, the Impax Settlement Agreement and the Impax Sublicense Agreement to which it is a party and (B) comply in all material respects with the terms and conditions of each Upstream Agreement, the Impax Settlement Agreement and the Impax Sublicense Agreement to which it is a party; and neither CHRP nor Shore shall terminate any Upstream Agreement, the Impax Settlement Agreement or the Impax Sublicense Agreement.
(iv) Each of Shore and CHRP, as the case may be, shall remain responsible for any and all payments due under each Upstream Agreement, the Impax Settlement Agreement or the Impax Sublicense Agreement to which it is a party.
(c) Santarus Step-in Rights. If a failure described in Section 2.3(b)(ii) above occurs or is alleged to have occurred with respect to the LifeCycle Agreement, the Parties shall promptly meet and confer on how best to proceed to effect a cure of such failure. CHRP will keep Santarus timely informed of its efforts to cure or remedy such failure or alleged failure. Santarus will have the right to step-in and meet the failed obligations, including the right to make payment on behalf of CHRP, and CHRP will take all necessary steps such that LifeCycle accepts performance by Santarus on behalf of CHRP for such obligations. Before Santarus exercises its right to step-in under this Section 2.3(c), Santarus shall deliver to CHRP written notice of its intent to exercise such “step-in” right, which exercise shall not occur sooner than ten (10) days after receipt by Santarus of written notice of any such failure. Any amounts owed by CHRP and paid by Santarus pursuant to the immediately preceding sentence will be credited towards any amount due to Shore from Santarus under this Agreement. The step-in rights set forth in this Section 2.3(c) are not Santarus’ sole remedies in the event of a failure described in Section 2.3(b)(ii); nothing in this Section shall be deemed to limit any other legal or equitable recourse or remedies Santarus might have.
2.4 No Other Rights. Except as explicitly set forth in this Agreement, no license or other right is or shall be created or granted hereunder by implication, estoppel, or otherwise.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
2.5 Assignment and Transfer of Other Product Related Items.
(a) Transfer of Product Trademarks. Shore hereby assigns and transfers to Santarus all of its right, title and interest in and to all Product Trademarks.
(b) Transfer of Regulatory Approvals and Filings. Shore hereby assigns and transfers to Santarus all of its right, title and interest in and to all Regulatory Approvals and all Regulatory Filings that primarily relate to the Compound or the Licensed Products in the Territory, including NDA No. 22-118. The Parties acknowledge that, pursuant to the terms hereof and Section 3.1(c) of the LifeCycle Agreement, LifeCycle and its licensees (including, CHRP and Shore) retain the rights to license, to use any and all information contained in the Regulatory Approvals and Regulatory Filings assigned and transferred to Santarus hereunder, and the right of reference to all such Regulatory Approvals, solely for purposes relating to development and commercialization of the Licensed Products outside the Territory, and the manufacture of Licensed Products (within and without the Territory) for sale outside the Territory. A copy of the letter transferring such Regulatory Approvals to be delivered to the FDA on the Effective Date is attached hereto as Exhibit 2.5(b).
(c) Transfer of Domain Names, Product Data and Product Materials. Shore hereby assigns and transfers to Santarus all of its right, title and interest in and to all Domain Names, Product Data and Product Materials related to the Licensed Product. Promptly after the Effective Date, Shore shall provide to Santarus copies of all such Product Data, Product Materials and medical education materials related to the Licensed Product.
(d) Assignment and/or Termination of Product Agreements. Shore hereby assigns to Santarus and Santarus hereby assumes the Product Agreements designated for assignment as set forth in Exhibit 1.61 (the “Assumed Product Agreements”). For the avoidance of doubt, the foregoing assignment and assumption shall include all of Shore’s rights and obligations under the Assumed Product Agreements, other than obligations accrued prior to the Effective Date. Shore shall terminate those Product Agreements designated for termination as set forth in Exhibit 1.61 (the “Excluded Product Agreements”); provided, however, that the Agreement, dated November 1, 2010, between Product Safety Resources, Inc. (“PROSAR”) and Shore (the “PROSAR Agreement”), shall remain in effect until [***] from the Effective Date; and provided further that during such [***] period, Shore shall be responsible, at Santarus’ sole cost and expense (other than with respect to any termination fees), for all obligations under such agreement, including Adverse Event and other pharmacovigilance reporting related to Licensed Products, and shall promptly provide such pharmacovigilance information to Santarus.
(e) Inventory. As of the Effective Date, Shore shall deliver to Santarus all Inventory, [***]. Title to and risk of loss with respect to the Inventory shall pass from Shore to Santarus upon delivery of such Inventory to Santarus. Santarus shall pay Shore for such Inventory within thirty (30) days of the date of invoice delivered to a Third Party in connection with the sale of Product or use of the Compound in Manufacturing operations at the costs set forth in Exhibit 1.40, which costs represent actual out-of-pocket Third Party costs [***]. For clarity, Inventory which is not invoiced by Santarus, its Affiliates or sublicensees to Third Parties
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
or used in the Manufacturing process shall result in no payment due to Shore. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED HEREIN, ALL REPRESENTATIONS AND WARRANTIES REGARDING THE INVENTORY, INCLUDING, WITHOUT LIMITATION, IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS OF PURPOSE FOR INTENDED USE, ARE HEREBY EXPRESSLY DISCLAIMED.
(f) Transition Assistance. Following the Effective Date, for a period of [***], Shore shall provide reasonable assistance, including with respect to preparing Regulatory Filings for the Licensed Products and making its personnel reasonably available for meetings or teleconferences, to support and assist Santarus in the Manufacture and Commercialization of the Licensed Products (the “Transition Services”) at Shore’s sole cost and expense.
(g) Transfer of Call Center Telephone Number. Shore hereby assigns and transfers to Santarus all of its right, title and interest in and to (000) 000-0000 and (000) 000-0000, and shall transfer management of such call center telephone numbers no later than the end of the [***] period described in Section 2.5(d) above.
(h) Hot Melt Tanks.
(i) On or before the Effective Date, CHRP and Shore shall enter into an agreement with LifeCycle, in form and substance reasonably acceptable to Santarus (the “Tank Agreement”). CHRP and Shore shall provide access to, and ensure that Santarus is able to use, (itself or on its behalf) the two (2) skid-mounted hot melt tanks and associated equipment (the “Tank Equipment”) currently situated at Catalent in the manufacture of Licensed Products and Authorized Generic Products (as defined in the Impax Sublicense Agreement). A copy of the Tank Agreement executed by all parties thereto, with only financial terms not applicable to Santarus redacted, has previously been provided to Santarus. CHRP and/or Shore shall purchase the Tank Equipment pursuant to Section 1 of the Tank Agreement. CHRP and/or Shore shall be solely responsible for all costs and expenses associated with or incurred pursuant to the Tank Agreement, including, without limitation, any costs or expenses associated with the purchase of the Tank Equipment pursuant to Section 1 of the Tank Agreement. For clarity, Santarus shall be responsible for maintenance costs associated with the Tank Equipment as provided for in the Commercial Supply Agreement and the unredacted sections of the Tank Agreement previously provided to Santarus and Santarus shall comply with Sections 4(a)-(f), 4(h) and 5 of the Tank Agreement.
(ii) [***]
(i) Additional Assurances. The Parties shall execute such documents and take such actions as are reasonably necessary to effectuate the foregoing transfers and transition matters.
2.6 Impax Agreements.
(a) Upon the Dismissal Effective Date (as defined in Section 4 of the Impax Settlement Agreement), Shore shall assign and transfer to Santarus the Impax Sublicense
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Agreement in its entirety. For clarity, prior to the Dismissal Effective Date, Santarus shall not be responsible for any rights or obligations of Shore or any of its Affiliates of whatever nature, whether presently in existence or arising or asserted hereafter, whether known or unknown, and whether under the Impax Sublicense Agreement, the Impax Settlement Agreement or otherwise. Shore and its Affiliates shall retain responsibility for all such rights and obligations (the “Excluded Liabilities and Obligations”) until such time, if ever, that the Dismissal Effective Date occurs. Santarus shall only assume responsibility for the Excluded Liabilities and Obligations that arise after such time that the entire Impax Settlement Agreement is assigned to and assumed by Santarus pursuant to this Section 2.6(a). Notwithstanding the foregoing, prior to the Dismissal Effective Date, Santarus and Shore shall reasonably cooperate to ensure compliance with the terms of the Impax Sublicense Agreement.
(b) Throughout the Term, each Party shall, as soon as practicable, but in any event, within [***], provide each other Party with copies of all [***] in the Impax Litigation to the extent related to the Impax Settlement Agreement or the Impax Sublicense Agreement [***].
(c) [***]
ARTICLE 3.
TRADEMARK USAGE AND MAINTENANCE
3.1 Ownership of Trademarks.
(a) From and after the Effective Date, Santarus shall be responsible for filing, registering and maintaining, and shall use commercially reasonable efforts to file, register and maintain, the Product Trademarks in the Territory, and will be responsible for the payment of any costs relating to filing, prosecution, and maintenance of all Product Trademarks in the Territory.
(b) LifeCycle shall retain ownership of the Licensed LCP Xxxx, and will be responsible for filing, prosecution, maintenance and defense of all registrations of the Licensed LCP Xxxx. In addition, the Parties acknowledge that LifeCycle, or its Affiliate or Licensee (as defined in the LifeCycle Agreement), shall retain the exclusive rights to market Licensed Products in the Field in all countries and jurisdictions outside the Territory, under such Trademarks as LifeCycle (or its Affiliate or other licensee) shall select in its sole discretion (the “LifeCycle Marks”), and that neither Santarus nor Shore or CHRP shall be responsible for filing, registering or maintaining the LifeCycle Marks.
3.2 Use of Licensed LCP Xxxx.
(a) Notwithstanding the foregoing, Santarus may use the Licensed LCP Xxxx, solely to identify the MeltDose® technology in its Product Materials and marketing messages used in Promoting the Licensed Products in the Territory, and provided that Santarus and its Affiliates comply strictly with all obligations of, and subject to the terms and conditions set forth in this Section 3.2.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(b) Santarus acknowledges that LifeCycle is the owner of the Licensed LCP Xxxx, and that all use of the Licensed LCP Xxxx by Santarus (or its Affiliates) inures to the benefit of LifeCycle. Santarus further acknowledges that the Licensed LCP Xxxx embodies substantial goodwill and enjoys favorable public recognition, and that LifeCycle’s rights therein constitute valuable assets of LifeCycle. Santarus agrees that it will not do anything inconsistent with such LifeCycle ownership thereof, and that nothing in this Agreement shall give Santarus any right, title or interest in the Trademark other than the right to use the Licensed LCP Xxxx as permitted in this Section 3.2. Santarus agrees it will not attack the rights or title of LifeCycle to the Licensed LCP Xxxx.
(c) In all publicly disseminated packaging, labeling or Product Materials of Santarus (or its Affiliates) referencing or using the Licensed LCP Xxxx, Santarus (and its Affiliates) shall use the Licensed LCP Xxxx in the manner set forth in this Section 3.2. Without limiting any other provision of this Agreement, the Licensed Products, packaging, labeling or Product Materials therefor, and the Manufacture and Commercialization thereof shall comply with all Applicable Laws. Santarus shall not use the Licensed LCP Xxxx on any materials or goods other than in connection with Licensed Products sold or otherwise Promoted or distributed in the Territory.
(d) In order to protect the goodwill and reputation associated with the Licensed LCP Xxxx, Santarus covenants and agrees that it shall comply with the Trademark Use Guidelines (as defined below) and shall ensure that the use of the Licensed LCP Xxxx by Santarus hereunder, including in any promotional materials or Licensed Product labels and inserts, in whatever form or medium, shall be in accordance with the Trademark Use Guidelines. For purposes of this Agreement, “Trademark Use Guidelines” means those guidelines with respect to use by Santarus of the Licensed LCP Xxxx in connection with the Commercialization, to be established and mutually agreed upon by CHRP, Shore, LifeCycle and Santarus within [***] of the Effective Date, as such guidelines may be amended by agreement between Shore, LifeCycle and Santarus from time to time during the Term.
(i) Quality of Products. Prior to the establishment of the Trademark Use Guidelines pursuant to this Section 3.2(d), before selling, using or distributing any goods or materials bearing the Licensed LCP Xxxx which have not previously been approved for use by CHRP and/or Shore: (A) Santarus shall furnish and shall require its sublicensees to furnish, free of cost for LifeCycle’s express written approval, one prototype sample of each article, packaging, label, advertisement, sign or other materials bearing the Licensed LCP Xxxx; (B) if LifeCycle has not responded within [***] after the office of the Chief Executive Officer or Chief Financial Officer of LifeCycle has received the particular prototype sample, then LifeCycle’s approval shall be deemed to have been granted as provided in the LifeCycle Agreement; (C) pursuant to the LifeCycle Agreement, if LifeCycle does not approve any such sample, LifeCycle is obligated to provide Santarus or its sublicensee, as applicable, with a written explanation of why it was not approved; and (D) after samples have been provided and approved, Santarus and its sublicensees shall not depart from the approved form in any material respect without the express prior written consent of LifeCycle.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(ii) Inspection of Products. In the event Santarus desires to use the Licensed LCP Xxxx other than as provided in the Trademark Use Guidelines, Santarus agrees that at LifeCycle’s reasonable request, Santarus or its sublicensee, as applicable, shall provide LifeCycle with representative specimens of the Licensed Product bearing the Licensed LCP Xxxx and of any packaging, labeling, advertising, and promotional material bearing the Licensed LCP Xxxx. The nature, quality, construction, workmanship, styling, and materials of all of the Licensed Products sold bearing the Licensed LCP Xxxx as permitted under this Agreement and all of the advertising, packaging, publicity material, and promotional material therefor shall be of a high standard and quality and shall in no manner reflect adversely upon the Licensed LCP Xxxx or the LifeCycle, and shall be consistent with the specimens provided pursuant to this Section.
3.3 Corporate Marks. In connection with Commercialization and (a) to the extent included on Inventory, Product labeling, Product Materials or packaging transferred hereunder, (b) to the extent required by Applicable Law, or (c) as otherwise reasonably required following the Effective Date for any relabeling of the Inventory, Product labeling, Product Materials or packaging transferred hereunder, Santarus shall have the non-exclusive right to use and display the Corporate Marks of Shore. Except as otherwise provided herein, no right, express or implied, is granted under this Agreement to any Party to use in any manner the Corporate Marks of the other Parties in connection with the performance of this Agreement. Notwithstanding the foregoing, Santarus agrees that it will not manufacture new Licensed Products labeled with Shore’s Corporate Marks after the date that is [***] following the Effective Date.
3.4 Infringement of Product Trademarks. In the event that Shore, CHRP or Santarus becomes aware of (a) actual infringement of a Product Trademark in the Territory; (b) a xxxx or name confusingly similar to a Product Trademark in the Territory; or (c) any unfair trade practices, trade dress imitation, passing off, or like offenses in the Territory that relate to a Product Trademark in the Territory, such Party shall promptly so notify the other Party in writing. Santarus shall have the right, but not the obligation, at its sole cost and expense, to initiate, prosecute, and control an infringement action or file any other appropriate action or claim related to infringement of such Product Trademark against any Third Party in the Territory. If Santarus fails to bring such infringement action within a period of [***] after delivery of the notice set forth above, then Shore shall have the right, but not the obligation, at its cost and expense, to initiate, prosecute, and control an infringement action or file any other appropriate action or claim related to infringement of the Product Trademark against any Third Party. In either event, as between Shore and Santarus, the Party not bringing any such action (i) shall have the right (at its own expense) to participate in such action and to be represented by counsel of its own choice, and (ii) agrees, at the request and expense of the Party bringing such action, to be joined as a party to the suit and to provide reasonable assistance in any such action (at the controlling Party’s cost). The Party controlling such action shall take all reasonable and appropriate steps to protect, defend, and maintain the Product Trademarks for use by the Parties and shall have the right to control settlement of such action; provided, however, that no settlement shall be entered into without the written consent of the other Party (Shore or Santarus, as the case may be), which consent shall not be unreasonably
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
withheld, conditioned or delayed. Any damages or monetary award recovered shall be retained by the Party bringing such action in connection with such litigation; provided, however, that any net damages or monetary award recovered by Santarus and attributable to lost sales of Licensed Products shall be included in Net Sales for which royalties shall be paid under ARTICLE 7.
3.5 Third-Party Trademark Claims. If a claim is brought by a Third Party that use of any Product Trademark infringes such Third Party’s trademarks, the Party against which the action is brought will give prompt written notice to the other Parties of such claim. Santarus shall have the right, but not the obligation, to defend or settle such claim with the consent of Shore, which consent shall not be unreasonably withheld, and defend or settle any resulting suit at its expense and Shore, at Santarus’ reasonable request and expense, shall cooperate with Santarus with respect to any such defense. In the event Santarus does not elect to defend against or settle such claim, Shore shall have the right, but not the obligation, to defend or settle such claim, and shall have the right to require Santarus to discontinue use of such Product Trademark and adopt a new Product Trademark.
ARTICLE 4.
DEVELOPMENT AND REGULATORY ACTIVITIES
4.1 Additional Development Activities.
(a) Santarus shall have the sole right and responsibility to conduct (or have conducted) all Development, including post-marketing Phase IV studies and any label enhancement studies for the Product, and any Development in support of an Additional Product.
(b) Shore shall bear any and all costs and expenses incurred in connection with Development prior to the Effective Date, and Santarus shall bear any and all costs and expenses incurred by it in connection with Development from or after the Effective Date.
4.2 Rights of Reference
(a) To the extent any Regulatory Filings are Controlled by LifeCycle, and CHRP has been granted such rights by LifeCycle, or to the extent any Regulatory Filings are Controlled by CHRP, and Shore has been granted any such rights by CHRP, CHRP and Shore hereby grant to Santarus and its sublicensees, a “Right of Reference or Use” as that term is defined in 21 C.F.R. § 314.3(b), to any and all Regulatory Filings and Product Data within the LifeCycle Know-How relating directly to Licensed Products, which includes that related to pharmacology, toxicology, preclinical testing, clinical testing, chemistry, manufacturing and controls data, batch records, trials and studies, safety and efficacy, manufacturing information, analytical and quality control, and agrees to sign, and cause their respective Affiliates to sign, any instruments reasonably requested by Santarus in order to effect such grant, solely for the purposes of conducting such activities as are contemplated by this Agreement.
(b) Each of CHRP and Shore hereby grants to Santarus the full right to use and refer to any Drug Master File for the Licensed Product to which it has access and will
provide a copy thereof to Santarus upon Santarus’ request solely for the purposes of Developing and seeking Regulatory Approval of the Licensed Products and conducting such other activities as are contemplated by this Agreement.
(c) Shore hereby grants to Santarus and its sublicensees, a “Right of Reference or Use” as that term is defined in 21 C.F.R. § 314.3(b), to any and all Regulatory Filings and Product Data within the Licensed Know-How relating directly to Licensed Products to which it has access, which includes that related to pharmacology, toxicology, preclinical testing, clinical testing, chemistry, manufacturing and controls data, batch records, trials and studies, safety and efficacy, manufacturing information, analytical and quality control, and agrees to sign, and cause its Affiliates to sign, any instruments reasonably requested by Santarus in order to effect such grant, solely for the purposes of conducting such activities as are contemplated by this Agreement.
(d) Santarus hereby grants to Shore (and at Shore’s written request, shall grant to LifeCycle) the foreign equivalents, in any country or jurisdiction outside the Territory, to a “Right of Reference or Use” as that term is defined in 21 C.F.R. § 314.3(b), to any and all Regulatory Filings and Product Data relating directly to Licensed Products, which includes that related to pharmacology, toxicology, preclinical testing, clinical testing, chemistry, manufacturing and controls data, batch records, trials and studies, safety and efficacy, manufacturing information, analytical and quality control, and agrees to sign, and cause its Affiliates to sign, any instruments reasonably requested by Shore in order to effect any such grant, solely for the purposes of Developing and seeking Regulatory Approval outside the Territory of the Licensed Products. Upon Shore’s reasonable request made from time to time, Santarus shall, or shall cause its Affiliates or sublicensees to, transfer to Shore or LifeCycle a copy of all such Regulatory Filings with respect to the Licensed Products.
4.3 Regulatory Filings and Responsibilities; Consultation.
(a) Shore has provided to Santarus a true, correct and complete electronic copy of all Regulatory Filings and Regulatory Approvals in its possession as of the Effective Date and will provide to Santarus such true and complete copies of such information which hereafter comes into its possession.
(b) From and after the Effective Date, Santarus shall own all Regulatory Filings and Regulatory Approvals. Santarus shall be solely responsible for preparing any and all Regulatory Filings at its sole expense, and shall use Commercially Reasonable Efforts to maintain all necessary Regulatory Approvals.
(c) Santarus shall be solely responsible for any discussions with any Regulatory Authority related to Development, Manufacture and Commercialization.
(d) Each of Shore and CHRP shall consult and cooperate with Santarus, as Santarus may reasonably request, in connection with the preparation and filing of all Regulatory Filings or Regulatory Approvals. In addition, each of Shore and CHRP shall consult and cooperate with Santarus, as Santarus may reasonably request, in connection with any interactions or discussions with any Regulatory Authority.
4.4 Drug Safety.
(a) Adverse Event Reporting. Subject to Section 2.5(d) and the Impax Sublicense Agreement, Santarus shall be responsible for all activities related to the processing, evaluation, and reporting of Adverse Events to appropriate Regulatory Authorities, in accordance with Applicable Laws, for the Licensed Products in the Field in the Territory. Each of CHRP and Shore shall notify Santarus of all serious Adverse Event reports within twenty-four (24) hours of the time such Adverse Event becomes known to CHRP or Shore, as the case may be, and all other Adverse Event reports within one (1) Business Day of the time such Adverse Event becomes known to CHRP or Shore, as the case may be, including any such Adverse Event reports received from LifeCycle pursuant to the Upstream Agreement or Impax pursuant to the Impax Sublicense Agreement.
(b) Product Withdrawals and Recalls. In the event that (a) an event, incident, or circumstance has occurred which may result in the need for a recall or other removal of any Licensed Product or any lot or lots thereof from the market in the Field in the Territory; (b) any Regulatory Authority in the Territory threatens or initiates any action to remove the Licensed Product from the market in the Field in the Territory; or (c) any Regulatory Authority in the Territory requires distribution of a “Dear Doctor” letter or its equivalent, regarding use of the Licensed Product in the Field in the Territory, Santarus shall promptly advise Shore in writing with respect thereto. Unless otherwise agreed by the Parties and subject to the Impax Sublicense Agreement, Santarus shall be responsible for conducting the recall or other removal from the market and neither CHRP nor Shore shall consent to the conduct of a recall or other removal of any Licensed Product from the market in the Territory without obtaining the prior written consent of Santarus, not to be unreasonably withheld, conditioned or delayed. Shore (and CHRP to the extent required by Applicable Law) shall, upon reasonable request by Santarus, assist Santarus in the conduct of any such recall or removal from the market in the Territory, which recall or removal shall be controlled by Santarus in Santarus’ sole discretion. Subject to any obligations under ARTICLE 11, to the extent any recall or removal of a Licensed Product is implemented as a result of lots Manufactured prior to the Effective Date, Shore shall bear all reasonable costs incurred in connection with such recall or removal to the extent it is responsible for such lots under Section 6.2 with respect to Licensed Product returns (including all of Santarus’ reasonable out-of-pocket costs); otherwise (subject to the Impax Sublicense Agreement), Santarus shall bear all of Santarus’ costs, all of Shore’s reasonable out-of-pocket costs, and all other reasonable costs incurred in connection with such recall.
(c) Global Safety Database. In the event that Shore commercializes a Licensed Product outside of the Territory, Santarus and Shore shall enter into a written agreement providing for the establishment of a global safety database by Santarus or its designee to permit the Parties to fulfill local and international regulatory reporting obligations to Regulatory Authorities. The agreement will set forth mutually agreeable guidelines and
procedures for the receipt, investigation, recordation, communication and exchange (as between the Parties) and regulatory submission of Adverse Event reports (subject to Section 4.4(a) with respect to the Territory). Santarus shall maintain the global safety database, and, as between the Parties, shall bear all costs with regard to establishing or maintaining such database.
ARTICLE 5.
COMMERCIALIZATION
5.1 Diligence.
(a) Commercially Reasonable Efforts. Santarus shall have the sole right to undertake, and shall use, and shall require its sublicensees to use, Commercially Reasonable Efforts to undertake Commercialization of the Product in the Territory.
(b) Additional Diligence Requirements. In addition, Santarus, either directly or through a sublicensee, shall:
(i) provide a minimum of [***] Details per Calendar Quarter for the Licensed Products during the [***] Calendar Quarters beginning [***] following the Effective Date, pro-rated for any partial Calendar Quarters;
(ii) during [***] Calendar Quarters beginning on [***], provide a minimum spend on Promotion of the Licensed Products meeting a threshold of [***] calculated on a rolling basis with respect to [***] Calendar Quarters (providing for a minimum of [***] per Calendar Quarter), pro-rated for any partial Calendar Quarters;
(iii) following [***] and thereafter during the Term provide a minimum spend on Promotion of the Licensed Products meeting a threshold of [***] calculated on a rolling basis with respect to [***] Calendar Quarters (providing for a minimum of [***] per Calendar Quarter), pro-rated for any partial Calendar Quarters;
(iv) provide an incentive compensation plan associated with sales of the Licensed Products to the Santarus Sales Representatives amounting to a target bonus of at least [***] of the Santarus Sales Representatives’ incentive compensation plan during [***]; and
(v) use Commercially Reasonable Efforts to evaluate and modify, as appropriate, its prescriber called-on list by [***] which will include fenofibrate and Fenoglide prescribers.
Notwithstanding anything to the contrary, each of the diligence requirements set forth in this Section 5.1(b) shall terminate upon the first commercial sale of an Authorized Generic or Generic Equivalent.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(c) Records and Reports.
(i) Every [***] following the Effective Date, Santarus shall provide Shore with a written report summarizing the efforts and accomplishments of Santarus, its Affiliates and its sublicensees during the preceding [***] period in Developing and Commercializing Licensed Products. Such reports shall include, summaries of scientific and clinical data obtained in furtherance of or based on Santarus’ (or its Affiliate’s or sublicensee’s) efforts to Develop or Commercialize Licensed Products.
(ii) Shore shall have the right to arrange for its employees involved in the activities contemplated hereunder to visit the offices of Santarus and any of its Affiliates, no more than [***] per calendar year during normal business hours and upon reasonable prior written notice, to discuss the Development and Commercialization work and results in detail with a technical representative of Santarus.
(iii) Santarus shall provide to Shore, not later than [***] following the end of each Calendar Quarter (commencing with the second Calendar Quarter following the Effective Date), a report detailing the Promotional expenditures incurred by Santarus, its Affiliates and sublicensees in such Calendar Quarter. In addition, Santarus shall provide to CHRP, prior to the end of each Calendar Year, a summary of Promotion activities contemplated for the upcoming Calendar Year.
(iv) For clarity, all reports and other information provided or made available by Santarus under this Section 5.1(c) shall constitute the Confidential Information of Santarus and shall be subject to the provisions of Article 7. Santarus acknowledges and agrees that the quarterly marketing expenditure reports provided to Shore pursuant to Section 5.1(c)(iii) above, may be shared by Shore with Impax, provided Impax agrees to keep such information confidential.
5.2 Product Materials.
(a) Santarus will be responsible for the development of all Product Materials related to the use of the Licensed Products in the Field in the Territory and shall own all right, title, and interest in and to any intellectual property in the Product Materials, excluding any Corporate Marks of Shore marked thereon.
(b) Santarus shall timely file with the relevant Regulatory Authority, in accordance with all Applicable Law, all Product Materials required to be filed with such Regulatory Authority with respect to use of the Licensed Products in the Field in the Territory.
5.3 Complaints and Inquiries. Santarus shall be responsible for responding to complaints, medical questions, or other inquiries relating to the Licensed Products; provided, however, that Shore and CHRP shall promptly (but in any event within [***]) inform Santarus of any complaints, medical questions, or other inquiries (or issues related thereto) it receives. Each of Shore and CHRP shall notify Santarus of, and provide all pertinent information in its possession or of which it is aware relating to, any and all suspected or actual tampering, counterfeiting, or contamination or other similar problems with respect to the Licensed Products or Generic Equivalents in the Field in the Territory.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
5.4 Pricing. Santarus shall book all sales of the Licensed Products in the Territory and shall be solely responsible for establishing pricing and for entering into any contracts and other arrangements regarding the distribution and sale of the Licensed Products in the Territory. Santarus shall be responsible for administering its own discount and rebate systems.
5.5 Exclusivity.
(a) Shore hereby covenants and agrees (and shall cause its Affiliates (other than CHRP and any related or successor fund) to agree) not to, in whole or in part, develop, manufacture, or commercialize any pharmaceutical product containing the Compound in the Field in the Territory during the Term, directly for themselves or by a Third Party licensee or sublicensee.
(b) CHRP hereby covenants and agrees not to, in whole or in part, Develop, Manufacture or Commercialize the Licensed Product in the Field in the Territory during the Term directly for itself or by a Third Party licensee or sublicensee.
ARTICLE 6.
MANUFACTURING AND SUPPLY
6.1 Manufacturing. Santarus shall be solely responsible, at its own cost and expense, for the Manufacture and supply of the Compound and Licensed Products for use in Development or Commercialization in the Territory after the Effective Date.
6.2 NDC Numbers, Product Returns, Rebates and Chargebacks.
(a) NDC Numbers. Santarus shall have the right to sell and distribute Licensed Product bearing Shore’s NDC Number and to increase the invoice price of such Licensed Product. Santarus shall use Commercially Reasonable Efforts to obtain its own NDC numbers for the Licensed Products within a reasonable period of time following the Effective Date. As soon as reasonably practicable following receipt of new NDC numbers, Santarus shall use such NDC numbers on all invoices, orders and other communications with customers and Regulatory Authorities.
(b) Product Returns. Santarus shall be responsible for all returned Licensed Products sold or distributed by Santarus after the Effective Date, and Shore shall be responsible for all other returned Licensed Product. The Parties shall track responsibility for such returns
based on lot number. In the case of split lots (e.g., where each Party has sold some portion of a lot), the Parties shall be responsible for a portion of each return based on the proportion of the total lot sold by each Party. In the event that any returns for which a Party is responsible are delivered to the other Party, such returns shall be processed by the other Party and the responsible Party shall reimburse the other Party for such returns within [***] of receipt of an invoice that describes the requested payments in reasonable detail and includes reasonable supporting documentation.
(c) Rebates. Shore shall be financially responsible for all government or commercial rebates related to Licensed Products pursuant to any Product Agreement or other similar agreement in effect prior to the Effective Date. Santarus shall be responsible for all other government and commercial rebates relating to Licensed Products.
(d) Chargeback Claims. Shore shall be financially responsible for all chargebacks related to Licensed Products pursuant to any Product Agreement or other similar agreement in effect prior to the Effective Date. Santarus shall be responsible for all other chargebacks relating to Licensed Products.
(e) Discount Card Program. Shore shall be financially responsible for all discount or savings card redemptions or fees related to Licensed Products pursuant to any Product Agreement or other similar agreement in effect prior to the Effective Date. Santarus shall be responsible for all other discount or savings card redemptions or fees relating to Licensed Products.
(f) Invoicing; Offset. On a monthly basis, each Party shall report to the other in writing any amounts processed and paid by it, or short-paid by any Third Party to it, for which the other Party is financially responsible pursuant to Sections 6.2(c), (d) or (e) above (the “Financially Responsible Party”). Any amounts shall be invoiced on a monthly basis and, at the election of the Party to whom payment is due, either paid by the Financially Responsible Party within [***] after receipt thereof or offset against amounts otherwise due to the Financially Responsible Party.
ARTICLE 7.
PAYMENTS
7.1 Upfront Payment. In consideration for the rights granted to Santarus under this Agreement, Santarus, within [***] days after the Effective Date, shall pay to Shore a one-time-only non-refundable payment of Eleven Million Dollars ($11,000,000).
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
7.2 Sales Milestones. In consideration for the rights granted to Santarus under this Agreement, Santarus shall pay to Shore the following non-refundable milestone payments, within [***] after the end of the first Calendar Year in which a sales milestone event described below is first achieved during the Royalty Term:
| Sales Milestone Event |
Payment | |||
| Calendar Year Net Sales equal or exceed $20,000,000 |
$ | 2,000,000 | ||
| Calendar Year Net Sales equal or exceed $30,000,000 |
$ | 3,000,000 | ||
For clarity, if both sales milestone events are triggered during any particular Calendar Year during the Royalty Term and have not been previously paid, then both sales milestones shall become due at the end of such Calendar Year.
7.3 Royalty Payments. In consideration for the rights granted to Santarus under this Agreement, Santarus shall pay to Shore a royalty at the following royalty rates, on Net Sales in the Territory in a Calendar Year during the Royalty Term; provided, however, that no royalty shall be due on that portion of Calendar Year Net Sales that are less than or equal to Ten Million Dollars during the 2011 and 2012 Calendar Years:
| Calendar Year Net Sales in the Territory |
Royalty Rate Applicable to Such Net Sales |
|||
| Portion of Calendar Year Net Sales that are less than or equal to $10,000,000 |
5 | % | ||
| Portion of Calendar Year Net Sales that are greater than $10,000,000 but are less than or equal to $20,000,000 |
20 | % | ||
| Portion of Calendar Year Net Sales that are greater than $20,000,000 |
25 | % | ||
By way of example, if the Calendar Year Net Sales to which the royalty obligations in this Section 7.3 apply were $25,000,000 prior to 2013, no royalty rate would apply to the first $10,000,000 of such Net Sales, the 20% royalty rate would apply to the next $10,000,000 of such Net Sales, and the 25% royalty rate would apply to the final $5,000,000 of such Net Sales, resulting in a payment of $3,250,000; and if the Calendar Year Net Sales to which the royalty obligations in this Section 7.3 apply were $25,000,000 during or after 2013, the 5% royalty rate would apply to the first $10,000,000 of such Net Sales, the 20% royalty rate would apply to the next $10,000,000 of such Net Sales, and the 25% royalty rate would apply to the final $5,000,000 of such Net Sales, resulting in a payment of $3,750,000.
7.4 Authorized Generic Revenues. Santarus and its Affiliates shall pay to Shore [***] of the Authorized Generic Revenues received by Santarus and its Affiliates during the Term
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
7.5 Third Party Royalties; Other Credits Against Royalties.
(a) If, during the Term, Santarus, its Affiliates or its sublicensees are obligated to pay royalties to a Third Party under an agreement with respect to the sales of a Licensed Product in the Territory which is, in the reasonable discretion of Santarus after consultation with Shore, required to resolve a claim of infringement of a Third Party’s Patent Rights, such agreement to be entered into after consulting with Shore (a “Third Party Royalty Payment”), the royalties payable under Section 7.3, in each case with respect to such Licensed Product in the Territory, shall be decreased by [***] of the amount of such Third Party Royalty Payment attributed to sales of the applicable Licensed Product; provided, however, that in no case shall such reduction under this Section 7.5(a) lower the amount of royalties otherwise payable under Section 7.3 by more than [***]; and provided further that, on a Licensed Product-by-Licensed Product basis, any Third Party Royalty Payments that are not credited against royalties paid in the Calendar Quarter in which such royalties were accrued shall be carried forward and credited against royalties payable in a subsequent Calendar Quarter(s) hereunder, subject to the limitations set forth in this Section 7.5, at Santarus’ option, until such royalty credits are completely expended.
(b) For a period of [***] following the Effective Date, in addition to any reductions under Section 7.5(a), Santarus may credit against royalties due to Shore under Section 7.3 [***] of any amounts paid by Santarus in connection with returns, rebates, chargebacks, redemptions or fees for which Shore is responsible pursuant to Section 6.2.
(c) In the event that royalties are not due to Shore in a given period, or are not due in a sufficient amount to cover the credits contemplated by Sections 7.5(a) and/or (b) above, Santarus shall have the right to elect either (i) a credit for any remaining amounts against the royalties due with respect to following periods, or (ii) reimbursement by Shore for such amounts within [***] of receipt of an invoice from Santarus that describes the requested credits/payments in reasonable detail and includes reasonable supporting documentation.
7.6 Payments and Reports. Santarus shall deliver to Shore, within [***] after the end of the first three (3) Calendar Quarters in a Calendar Year and within [***] after the end of the fourth Calendar Quarter in a Calendar Year, a report setting forth for such Calendar Quarter the following information for each Licensed Product: (i) total invoiced gross sales and Net Sales of such Licensed Product by Santarus and its Affiliates and sublicensees in the Territory; and (ii) the royalties due in respect of such Net Sales. The total royalties due in respect of Net Sales of the Licensed Products during such Calendar Quarter shall be paid no later than [***].
7.7 Wire Transfers. All payments hereunder shall be made by Santarus or Shore, as the case may be, in Dollars by bank wire transfer in immediately available funds to the other Party, to such bank account as shall be designated in writing by such other Party.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
7.8 Taxes. Any payments made under this Agreement by Santarus may be reduced by the amount of any withholding taxes required to be paid or withheld with respect to such payments pursuant to any Applicable Law, including United States federal, state or local tax law. Santarus shall submit to Shore reasonable proof of payment of such withholding taxes, together with an accounting of the calculations of such taxes, within [***] after such withholding taxes are remitted to the proper authority. The Parties will reasonably cooperate in completing and filing documents required under the provisions of any Applicable Law in connection with the making of any required tax payment or withholding payment, or in connection with any claim to a refund of or credit for any such payment.
7.9 Records; Audit Rights. Each of Shore and Santarus shall keep (and shall cause its Affiliates and shall require its sublicensees to keep) complete and accurate books and records that are necessary for the other Party to ascertain and verify the payments owed hereunder and Santarus’ expenditures to Promote the Licensed Product required under Section 5.1(b). During the Term and for a period [***] thereafter, each of Shore and Santarus shall permit an independent, certified public accountant of nationally recognized standing appointed by the other Party, at reasonable times and upon reasonable notice, but in no case more than once per Calendar Year, to examine (but not copy) such records as may be necessary for the sole purpose of verifying the calculation and reporting of Net Sales and the correctness of any payment made under this Agreement for any period within the preceding [***] and of the amount of Santarus’ expenditures to Promote the Licensed Product during each Calendar Quarter as required under Section 5.1(b) hereof and compliance with any other terms and provisions of this Agreement; provided that each Party shall only be entitled to one audit following expiration or termination of this Agreement; and provided further that neither Party shall be permitted to audit the same period of time more than once. The independent, certified public accountant will prepare and provide to each of Shore and Santarus a written report stating whether the royalty reports submitted and royalties paid, the expenditures to Promote the Licensed Product reported to Shore, or other payments made by either Party, as the case may be, are correct or incorrect and the details concerning any discrepancies. Such accountant shall disclose to Shore or Santarus, as the case may be, only the amounts that the independent auditor believes to be due and payable hereunder to such Party, details concerning any discrepancy from the amount paid and the amount due, and shall disclose no other information revealed in such audit. Any and all records of a Party examined by such independent accountant shall be deemed such Party’s Confidential Information which may not be disclosed by said independent, certified public accountant to any Third Party, and such Party may require such accountant to enter into an appropriate written agreement obligating it to be bound by obligations of confidentiality and restrictions on use of such Confidential Information that are no less restrictive than the obligations set forth in ARTICLE 10. In the event there was an underpayment by either Party of amounts owed under this Agreement, such Party shall promptly (but in no event later than [***] after its receipt of the independent auditor’s report so concluding) make payment to the other Party of any such shortfall. In the event that there was an overpayment by either Party hereunder, the other Party shall promptly (but in no event later than [***] after the other Party’s receipt of the independent auditor’s report so concluding) refund to Shore or Santarus, as the case may be, or credit to or against future royalties, at Santarus’ election, the excess amount. The expense of such audit shall be borne by the auditing Party; provided, however, that, if such audit establishes that the audited Party underpaid the auditing
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Party by more than [***] of the aggregate amount due hereunder for the period covered by such audit, or that expenditures to Promote the Licensed Product for a Calendar Quarter covered by such audit were less than the minimum spend required by Section 5.1(b), then the reasonable out-of-pocket expense of such audit shall be paid by the audited Party.
7.10 Late Payments. Subject to the terms of this Agreement, payments not made within the time period set forth in this ARTICLE 7 shall bear interest at a rate of [***] per annum effective for the date that payment was due, as published by The Wall Street Journal, U.S. Edition, available online at xxxxxx.xxx.xxx on the date such payment was due or the maximum rate permitted by Applicable Law, whichever is lower, calculated on the number of days such payment is delinquent until paid in full; provided, however, that credits given to Santarus against royalties shall not bear interest.
ARTICLE 8.
INVENTIONS AND PATENTS
8.1 Inventions.
(a) Inventorship of any Inventions that, in the case of patentable Inventions, were conceived and reduced to practice, and in the case of non-patentable Inventions, were made or developed, in the course of performing activities under this Agreement, together with all any Patents claiming such Inventions, will be determined in accordance with the rules of inventorship under United States patent laws with respect to patentable Inventions, and in accordance with applicable United States federal or state law with respect to non-patentable Inventions, and ownership of such Inventions shall be as set forth in further detail in Sections 8.1(b) through (d).
(b) Shore will own all right, title and interest in and to all Inventions, other than LCP Core Technology Improvements, that are conceived, reduced to practice, made or developed solely by or on behalf of Shore or CHRP from the Effective Date until the expiration or termination of the Term (whether or not patentable), and all intellectual property rights appurtenant thereto, subject only to the license rights granted by Shore to Santarus under this Agreement.
(c) Santarus will own all right title and interest in and to all Inventions, other than LCP Core Technology Improvements, that are conceived, reduced to practice, made or developed solely by or on behalf of Santarus or its sublicensees from the Effective Date until the expiration or termination of the Term (whether or not patentable), and all intellectual property rights appurtenant thereto.
(d) All right, title and interest in and to Inventions, other than LCP Core Technology Improvements, that are conceived, reduced to practice, made or developed jointly by employees or contractors of more than one Party from the Effective Date until the expiration or termination of the Term (whether or not patentable) (“Joint Inventions”), and all intellectual property rights appurtenant thereto, will be owned jointly by such Parties. Each such Party shall have the right to use and license such Joint Inventions freely and without any consent of or
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
payment obligations towards the other Party(ies), subject to the terms and conditions of this Agreement, including Section 5.5. Except to the extent each such Party is restricted by the foregoing or by the licenses granted to the other Parties and covenants contained herein, each Party will be entitled to practice and license Joint Inventions without restriction or consent of the other Parties. Each Party shall give written notice to the other Parties of any license to or rights granted under the Joint Inventions to a Third Party. The applicable Parties will confer and cooperate in good faith with respect to the Prosecution of Joint Patents. Each Party hereby agrees to take all actions and execute and deliver all documents reasonably necessary to Prosecute such Joint Patents.
(e) The Parties acknowledge that LifeCycle will own all right, title and interest in and to all LCP Core Technology and LCP Core Technology Improvements that are conceived, reduced to practice, made or developed solely by or on behalf of any Party and/or its Affiliate or sublicensees, or by the Parties jointly, from the Effective Date until the expiration or termination of the Term (whether or not patentable), and all intellectual property rights appurtenant thereto, subject only to the sublicense rights under the LifeCycle Technology granted by Shore to Santarus under this Agreement. Santarus agrees to assign and hereby assigns to LifeCycle all its right, title and interest in and to any LCP Core Technology Improvements that are conceived, reduced to practice, made or developed solely by or on behalf of Santarus and/or its Affiliate or sublicensees, or by the Parties jointly, and all intellectual property rights appurtenant thereto. For purposes of this Agreement, all LCP Core Technology and LCP Core Technology Improvements shall be the Confidential Information of Shore.
(f) Disclosure of Inventions.
(i) Subject to Section 8.1(f)(ii) below, each Party shall, at its own expense, promptly disclose to the other Parties all Inventions owned or Controlled by such Party relating to Licensed Products that arise during the Term.
(ii) Santarus shall, or shall cause its Affiliates or sublicensees to promptly disclose to CHRP or LifeCycle any and all Inventions relating to LifeCycle’s MeltDose® technology that arise during the Term, and shall disclose all LCP Core Technology Improvements conceived or made by or on behalf of Santarus or its Affiliates, and any Santarus Technology of which Santarus becomes aware is needed or reasonably useful to the development or commercialization of the Licensed Products outside the Territory, at no additional cost, and in each case promptly after Santarus’ patent counsel is made aware of such Inventions, LCP Core Technology Improvements or Santarus Technology.
(iii) Information provided by the Parties under this Section 8.1(f) with respect to Inventions will be in reasonable detail but in no circumstance less than would be sufficient to permit an understanding of the nature of the Inventions by a practitioner reasonably skilled in the relevant technical or scientific area.
8.2 Patent Prosecution.
(a) Prosecution. Shore shall, or shall collaborate with LifeCycle in accordance with the Upstream Agreements to, use Commercially Reasonable Efforts to Prosecute the Licensed Patents in the Territory, at its sole expense, except as set forth below; provided that Shore shall provide Santarus with a reasonable opportunity to review and comment on such Prosecution, and shall consider such comments in good faith. If Shore is required to assume Prosecution of the Product-Specific Patents in the Territory under the LifeCycle Agreement, Shore may elect by [***] prior written notification to Santarus, to have Santarus assume such Prosecution. Thereafter, Santarus shall use Commercially Reasonable Efforts to Prosecute the Product-Specific Patents in the Territory, at Santarus’ expense and through patent counsel selected by Santarus; provided, however, that Santarus’ out-of-pocket expenses under this Section 8.2(a) shall not exceed [***] over the Term. If Shore elects to have Santarus conduct Prosecution of particular Product-Specific Patents in the Territory, then Santarus shall provide Shore and LifeCycle reasonable opportunities to consult with Santarus regarding such Prosecution by Santarus and Shore shall cooperate with and assist Santarus reasonably in such Prosecution of the Product-Specific Patents.
(b) Patent Term Extensions. Upon request by Santarus, CHRP shall request that LifeCycle file all applications and take actions necessary to obtain patent extension pursuant to 35 U.S.C. § 156 for the Licensed Patents in the Territory, which extensions shall be owned by LifeCycle. If LifeCycle declines to pursue such patent extensions, then Santarus shall have the right (at Santarus’ cost and expense) on behalf of LifeCycle, CHRP and Shore to file all such applications and take all such actions necessary to obtain such patent extensions.
8.3 Enforcement.
(a) Notice. If Shore or Santarus becomes aware of infringement of any Licensed Patent by a Third Party anywhere in the world, such Party shall promptly notify the other Party in writing to that effect and provide a summary of the relevant facts and circumstances known to such Party relating to such infringement (“Infringement Notice”). Promptly after receipt of an Infringement Notice, Shore and Santarus shall discuss in good faith the infringement and appropriate actions that could be taken to cause it to cease.
(b) Product-Specific Patents. Santarus shall have the right, at its sole discretion, on its own behalf, to institute, prosecute and control any action or proceeding to restrain infringement of any Product-Specific Patent in the Territory by a Third Party, other than the Impax Litigation or any such action or proceeding for which Santarus does not have standing to so institute or prosecute. In any instance in which Santarus does not have such standing, or to the extent the Impax Litigation continues following the Effective Date, Shore shall, under Santarus’ direction and control and expense (except with respect to the Impax Litigation, the cost and expense for which CHRP and/or Shore shall be solely responsible), institute or prosecute such action or proceeding to restrain infringement. The Parties which are not party to such an action or proceeding agree to be joined, and shall request that LifeCycle join, as a party plaintiff if necessary or appropriate, and shall provide all reasonable cooperation, at Santarus’ expense, required to prosecute such action. Santarus shall have sole control of any such action and all negotiations for its settlement or compromise and shall have the sole right to settle or compromise any such action (or direct such settlement or compromise), provided that no party shall settle or
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
compromise any such action or enter into any consent order for the settlement or compromise of such action that may materially negatively impact the rights or interests of LifeCycle and/or Shore without the prior written consent of LifeCycle and/or Shore, as applicable, such consent by Shore not to be unreasonably withheld, conditioned or delayed; and provided further, however, that Santarus shall keep Shore reasonably informed of the status of any such action and provide Shore and LifeCycle reasonable opportunities to consult with Santarus regarding such action. If, prior to the expiration of [***] from an Infringement Notice regarding infringement of any Product-Specific Patent, Santarus has not obtained a discontinuance of the alleged infringement or brought an infringement action or proceeding or otherwise taken appropriate action to xxxxx such infringement, then Shore and LifeCycle shall each have the right, but not the obligation, to institute, prosecute and control any action or proceeding to restrain such infringement. In such event, Shore or LifeCycle, as applicable, shall have control of any such action and all negotiations for its settlement or compromise and shall have the sole right to settle or compromise any such action (or direct such settlement or compromise), provided that Shore shall not settle or compromise any such action or enter into any consent order for the settlement or compromise of such action that may materially negatively impact the rights or interests of Santarus without the prior written consent of Santarus, such consent not to be unreasonably withheld, conditioned or delayed; and provided further, however, that Shore shall (if a party to such action), or shall request LifeCycle, if applicable, to keep Santarus reasonably informed of the status of any such action and provide Santarus reasonable opportunities to consult with Shore or LifeCycle regarding such action.
(c) Other Licensed Patents. LifeCycle or CHRP, as the case may be in accordance with the Upstream Agreements, shall have the right, at its sole discretion, to institute, prosecute and control any action or proceeding to restrain infringement of any Licensed Patent other than the Product-Specific Patents in the Territory by a Third Party. If, prior to the expiration of [***] from an Infringement Notice regarding infringement of any such Licensed Patent, LifeCycle or CHRP, as the case may be, has not obtained a discontinuance of the alleged infringement or brought an infringement action or proceeding or otherwise taken appropriate action to xxxxx such infringement, then, unless LifeCycle has a reasonable business justification for not taking action against such alleged infringement, Santarus shall have the right, but not the obligation, to institute, prosecute and control any action or proceeding to restrain such infringement unless Santarus does not have standing to so institute or prosecute. In any instance in which Santarus does not have such standing, Shore shall, under Santarus’ direction and control and expense, institute or prosecute such action or proceeding to restrain infringement. The Parties which are not party to such an action, or proceeding to agree to be joined, and shall request that LifeCycle join, as a party plaintiff if necessary or appropriate, and shall provide all reasonable cooperation, at Santarus’ expense, required to prosecute such action, and provided however, that Santarus shall keep Shore reasonably informed of the status of any such action and provide Shore and LifeCycle reasonable opportunities to consult with Santarus regarding such action.
(d) Orange Book Listings. Santarus shall have the sole right, but not the obligation, to submit to all applicable Regulatory Authorities in the Territory Patent information pertaining to each Licensed Product pursuant to 21 U.S.C. §355(b)(1)(G) (or any amendment or successor statute thereto).
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
(e) Costs and Recoveries. Except as otherwise expressly set forth herein, each Party shall assume and pay all of its own out-of-pocket costs incurred in connection with any litigation or proceedings described in this Section 8.3 including the fees and expenses of such Party’s counsel. Any recovery obtained by any Party as a result of any proceeding described in this Section 8.3, by settlement or otherwise, shall be applied in the following order of priority: (i) first, to reimburse each Party for all reasonable costs in connection with such proceeding paid by that Party and not otherwise recovered (on a pro rata basis based on each Party’s respective costs, to the extent the recovery was less than all such litigation costs); and (ii) second, the remainder of the recovery shall be retained by the Party that brought and prosecuted the suit, provided that any net recovery from an action by Santarus to the extent attributable to lost sales of Licensed Products. shall be deemed Net Sales of Santarus for which royalties shall be paid under ARTICLE 7 and Santarus shall retain the balance.
8.4 Declaratory Actions and Counterclaims. In the event that an action alleging invalidity or non-infringement of any of the Licensed Patents is brought against LifeCycle, Shore or Santarus, LifeCycle or CHRP, at its sole discretion, shall have the right, within [***] after the commencement of such action, to take or regain control of the action at its own expense; provided, however, that CHRP shall, if it assumes control of such an action, diligently defend or assert such Licensed Rights or, if LifeCycle assumes control of such action, CHRP shall use Commercially Reasonable Efforts to cause LifeCycle to diligently defend or assert such Licensed Rights in accordance with Section 5.08 of the Purchase Agreement (as that term is defined in the LifeCycle Agreement), and CHRP shall not settle or compromise any such action or enter into any consent order for the settlement or compromise thereof, and shall use Commercially Reasonable Efforts to cause LifeCycle not to settle or compromise any such action or enter into any consent order for the settlement or compromise thereof, as the case may be, without the prior written consent of Santarus, which consent shall not be unreasonably withheld, conditioned or delayed. If LifeCycle and CHRP determine not to exercise this right, Santarus may take over or remain as lead counsel for the action at Santarus’ sole discretion and expense; provided however, that Santarus shall keep Shore reasonably informed of the status of any such action and provide Shore and LifeCycle reasonable opportunities to consult with Santarus regarding such action. Any recovery obtained from such litigation, proceeding or settlement shall be shared in accordance with Section 8.3(d).
8.5 Infringement Defense.
(a) Defense of Third Party Claims. If a Third Party asserts that a Patent owned or otherwise controlled by it is infringed by the Development, Manufacture, use or Commercialization of a Licensed Product in the Territory, the Party first obtaining knowledge of such a claim shall immediately provide the other Parties notice of such claim, along with the related facts in reasonable detail. Santarus shall have the first right, but not the obligation, to control and defend any action or proceeding with respect to such claim, and Shore and CHRP, at Santarus’ reasonable request and expense (including reasonable attorney’s fees), shall cooperate with Santarus with respect to any such action. Santarus shall have sole control of any such suit and all negotiations for its settlement or compromise, provided that Santarus shall not settle or
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
compromise any such action or enter into any consent order for the settlement or compromise of such action that would adversely impact (i) LifeCycle, or its rights or interests in or to any LifeCycle Technology without the prior written consent of LifeCycle, such consent not to be unreasonably withheld, condition, or delayed; or (ii) Shore, or its rights or interests in or to any Licensed Technology, without the prior written consent of Shore; not to be unreasonably withheld, conditioned or delayed; and provided further, however, that Santarus shall keep Shore reasonably informed of the status of any such action and provide Shore reasonable opportunities to consult with Santarus regarding such action.
(b) Step-In Right. If Santarus has not undertaken to defend any Third Party action that is subject to Section 8.5(a) above, or if Santarus shall at any time fail to continue to defend such Third Party action, then LifeCycle, CHRP and Shore shall have the right, as provided in the applicable Upstream Agreement, but not the obligation, to defend and control such action. Santarus shall provide all reasonable cooperation, at the requesting party’s expense, required to defend such litigation.
ARTICLE 9.
REPRESENTATIONS, WARRANTIES, AND COVENANTS
9.1 Representations, Warranties and Covenants. Each Party represents, warrants and covenants to the other Parties the following:
(a) it is duly organized, validly existing and in good standing under the laws of its jurisdiction of incorporation or formation, and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof;
(b) it is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder, and the person or persons executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate action;
(c) all necessary consents, approvals and authorizations of all Regulatory Authorities and other Third Parties required to be obtained by such it in connection with the execution and delivery of this Agreement and the performance of its obligations hereunder have been obtained, other than the delivery of written assignment of the NDA for the Product to the FDA;
(d) this Agreement is a legal and valid obligation binding upon it and enforceable in accordance with its terms;
(e) the execution, delivery, and performance of this Agreement by it does not conflict with or result in a breach of any of the terms or provisions of any agreement, instrument, or understanding, oral or written, to which it is a Party or by which it is bound, nor violate any material law or regulation of any court, governmental body, or administrative or other agency having jurisdiction over it;
(f) it has not granted and will not during the Term grant any right to any Third Party that would conflict with the rights granted to the other Parties hereunder;
(g) it shall comply and cause its employees and consultants who will be undertaking any activities related to this Agreement or the Licensed Products to comply, with all Applicable Laws respecting such activities; and
(h) neither its name nor the name of any of its employees or consultants who will be undertaking any activities related to this Agreement or the Licensed Products are listed on the debarment list maintained by the FDA pursuant to 21 U.S.C. Sections 335(a) and Section 335(b) and published on the internet at the following address (or any successor address): xxxx://xxx.xxx.xxx/xxx/xxxxxxxxxx_xxx/xxxxx/xxxxxxx.xxx. In the course of the Development, Manufacture and Commercialization of the Licensed Products prior to or pursuant to this Agreement, it has not used, and during the Term will not use, any employee or consultant that is debarred by any Regulatory Authority or, to the best of its knowledge, is the subject of debarment proceedings by any Regulatory Authority. If it learns that its employee or consultant performing on its behalf under this Agreement has been debarred by any Regulatory Authority, or has become the subject of debarment proceedings by any Regulatory Authority, it shall so promptly notify the other Party and shall prohibit such employee or consultant from performing on its behalf under this Agreement.
9.2 Representations, Warranties and Covenants of Shore.
Shore hereby represents and warrants to Santarus the following:
(a) it was, at all times following transfer of the Regulatory Approvals for the Licensed Product by Shionogi to Shore prior to the Effective Date, the lawful holder of all rights under the Regulatory Approvals and the Regulatory Filings for the Licensed Products in the Field in the Territory, and that it has provided to Santarus true, correct and complete copies of the Regulatory Approvals, Regulatory Filings and any material correspondence with Regulatory Authorities received by it, including 483s, warning letters, field alerts or other notices for the Licensed Products in the Field in the Territory. Shore is not aware of any Regulatory Filing that is not in its possession, which could have a material adverse effect on Santarus’ ability to Manufacture or Commercialize the Licensed Product;
(b) Shore has not committed fraud in relation to the filing or acquisition of the Regulatory Approvals for the Licensed Products in the Field in the Territory or used unfair methods of competition in connection with such filing or acquisition or maintenance, including, in either case, in connection with any data supplied by Shore to the FDA. To Shore’s knowledge, the data regarding the efficacy, safety, chemistry, manufacturing and control of the Licensed Products contained in the Regulatory Approvals for the Licensed Products in the Field in the Territory are complete and accurate in all material respects and do not contain any material misstatement of a material fact related to safety or efficacy nor omit to state any material fact in Shore’s possession related to safety or efficacy of the Licensed Products. Shore has not received any written communication from FDA stating that any development activities are required by the FDA as a condition to maintenance of the Regulatory Approvals for the Licensed Products in the Field in the Territory;
(c) except as set forth in Exhibit 9.2, all activities prior to the Effective Date following transfer of the Regulatory Approvals for the Licensed Product by Shionogi to Shore,
and, to its knowledge, all such activities prior to transfer of the Regulatory Approvals for the Licensed Product by Shionogi to Shore, in connection with (i) Manufacture of the Licensed Product, (ii) Commercialization and (iii) preparation and submission to the relevant Regulatory Authorities of the Regulatory Approvals and the Regulatory Filings for the Licensed Products in the Field in the Territory in existence as of the Effective Date, were in compliance in all material respects with all Applicable Law;
(d) Shore has not received any written notice or communication which has, or reasonably should have, led it to believe that either the Regulatory Approvals or the Regulatory Filings for the Licensed Products in the Field in the Territory in existence as of the Effective Date are not in good standing with relevant Regulatory Authorities;
(e) as of the Effective Date, there is no pending action by relevant Regulatory Authorities in respect of the Regulatory Approvals or the Regulatory Filings for the Licensed Products in the Field in the Territory, and, except as set forth in Exhibit 9.2, prior to the Effective Date following transfer of the Regulatory Approvals for the Licensed Product by Shionogi to Shore, and, to its knowledge, prior to transfer of the Regulatory Approvals for the Licensed Product by Shionogi to Shore, there has been no action by relevant Regulatory Authorities in respect of Regulatory Approvals or the Regulatory Filings for the Licensed Products in the Field in the Territory;
(f) it has the full legal right, power and authority to grant the license rights set forth in ARTICLE 2;
(g) it has granted Santarus a license to all intellectual property rights it owns or controls that are necessary to Develop, Manufacture, use or Commercialize the Licensed Products in the Field in the Territory, each subject to and in accordance with the terms and conditions of this Agreement;
(h) except as set forth in Exhibit 9.2, there are no liens or security interests currently existing on or to, and following the Effective Date, it shall not, and shall not grant any Third Party the right to, place any liens or security interests on or to, the intellectual property rights, Regulatory Approvals, Regulatory Filings, Upstream Agreements, Product Agreements or Product Materials Licensed or transferred by it to Santarus hereunder that could reasonably be expected to adversely affect Santarus’ Commercialization of the Licensed Product under this Agreement;
(i) there is no claim, action, suit, or proceeding, pending or, to its knowledge, threatened by a Third Party alleging that the Development or Commercialization of the Licensed Products infringes or misappropriates any patents or other intellectual property rights of any Third Party;
(j) except as set forth in Exhibit 9.2, there is no litigation, arbitration proceeding, governmental investigation, action or claims of any kind, pending or, to the knowledge of Shore, threatened, by or against Shore or any of its Affiliates relating to the Licensed Products or, except for the Impax Litigation, Santarus’ ability to exercise its rights hereunder, nor, to Shore’s knowledge, is any litigation, arbitration proceeding, governmental
investigation, action or claims of any kind, pending or, to the knowledge of Shore, threatened, by or against Chemagis or Catalent or their respective Affiliates relating to the Licensed Products. Shore is not a party to any litigation regarding any claim of product liability or damage to person (including death) or property resulting from the use or consumption of a Licensed Product in the Territory, nor has Shore received any written communication threatening any such litigation. Shore represents and warrants that any action it may bring against Shionogi for breach of its obligations under the Shionogi License Agreement will not have a material adverse impact on Santarus’ ability to Manufacture or Commercialize the Licensed Products and Shore reserves the right to commence such action against Shionogi, at its sole cost and expense;
(k) to its knowledge, the Manufacture of the Licensed Products in the Territory and the Development, Commercialization and use of the Licensed Products in the Field in the Territory does not infringe or misappropriate any Patents or other intellectual property rights of any Third Party;
(l) it has not given any notice to any Third Party asserting infringement by such Third Party with respect to the Licensed Products in the Field in the Territory of any of the intellectual property rights licensed hereunder, and it is not aware of any such infringements other than as alleged in the Impax Litigation;
(m) to its knowledge, no issued patent or patent application within the Licensed Patents is the subject of any pending interference, opposition, cancellation, protest, or other challenge or adversarial proceeding in the Territory;
(n) it is not aware of (i) any facts that it believes would result in invalidity or unenforceability of any of the Licensed Patents or Product Trademarks, (ii) any claim, action, suit or proceeding pending or threatened that any of the Licensed Patents are invalid or unenforceable (other than the Impax Litigation and any claims made in writing in connection therewith), and (iii) the abandonment of any of the Licensed Patents (except for expiring provisional applications, expiring PCT applications, and other Patents expiring by operation of law), disclaimer of any of the Licensed Patents (other than with respect to terminal disclaimers) or expiration of any of the Licensed Patents due to failure to timely pay applicable maintenance and renewal fees;
(o) each Upstream Agreement is in full force and effect, and no event has occurred which, after the giving of notice or the lapse of time or both, would constitute a material breach by it or Shore under such Upstream Agreement. Shore has not received from a Third Party any written notice to the effect that Shore is in breach of any Upstream Agreement and is not otherwise aware of any such breach;
(p) neither it nor any of its Affiliates has granted any licenses to, agreed not to xxx, or otherwise authorized, any person or entity, under the Licensed Technology or Shionogi Technology to Develop, Manufacture, use or Commercialize the Licensed Products in the Field in the Territory, other than the grant of rights by CHRP to Shore under the Shore Agreement and the grant of rights by Shore to Impax under the Impax Sublicense Agreement;
(q) prior to the Effective Date, to its knowledge, there have been no FDA “field alerts” (or the equivalent in countries outside the United States) with respect to the Licensed Products, and as of the Effective Date no such “field alerts” are pending. Shore has no plans to initiate any recall, market withdrawal or other corrective action with respect to Licensed Product and, except as set forth on Exhibit 9.2, is not aware of any investigation or inquiry by any governmental authority or Regulatory Authority related to Development, Manufacturing, or Commercialization of Licensed Products;
(r) there are no written understandings or agreements relating to the Development, Manufacture or Commercialization of the Licensed Products between Shore and any Third Party, other than the Product Agreements and the Impax Sublicense Agreement;
(s) it has provided true, correct and complete copies of each Product Agreement, as in effect on the Effective Date, to Santarus. Shore has provided to Santarus all excerpts from any existing agreements providing for Rights of Reference with respect to the Licensed Products or the right of access to data described in Section 4.2, and all such excerpts are true and accurate;
(t) each of the Impax Settlement Agreement, the Impax Sublicense Agreement, and each Product Agreement is in full force and effect, and no event has occurred which, after the giving of notice or the lapse of time or both, would constitute a material breach by it or the Shore under such agreements. Shore has not received from a Third Party any written notice to the effect that Shore is in breach of the Impax Settlement Agreement, the Impax Sublicense Agreement or any Product Agreement;
(u) since January 1, 2011, Shore has not (A) (i) materially altered its distribution practices with respect to the Licensed Products, (ii) materially altered its activities and practices with respect to inventory levels of the Licensed Products maintained at the wholesale, chain, institutional or retail levels in any material respect, or (iii) experienced abnormally high levels of returns of the Licensed Products; in each such case, which would have a material adverse effect on Santarus or the Commercialization of the Licensed Products, or (B) sold any Licensed Product dispensed under a Medicare contract;
(v) all of the Inventory (a) is good, issuable and merchantable in the ordinary course of business, and is free of any material defect or deficiency, (b) fully conforms to the specifications for the Licensed Products in the Regulatory Approvals in the Territory, (c) was manufactured, packaged, labeled, held, tested and shipped in accordance with the specifications for the Licensed Products as set forth in the Regulatory Approvals in the Territory, cGMPs, all other applicable laws, regulations and requirements of applicable Regulatory Authorities, (d) is not adulterated or misbranded and is of suitable quality; and (e) may be introduced into interstate commerce in the Territory pursuant to the FD&C Act;
(w) Exhibit 9.2(w) sets forth a true, complete and accurate list of all unfilled firm orders placed or deemed to have been placed for Product or Compound under the Product Agreements as of December 11, 2011 and the most recent forecasts provided by Shore under the API Supply Agreement and/or the Commercial Supply Agreement;
(x) there was no Shionogi Technology identified or transferred by Shionogi when the rights to the product were transferred by Shionogi to Shore; and
(y) the LifeCycle Technology and Shionogi Technology existing as of the Effective Date constitute all of the Patents and Know-How Controlled by Shore as of such date that are necessary or useful to Develop, Commercialize, or Manufacture Licensed Products.
9.3 Representations and Warranties of CHRP.
CHRP hereby represents and warrants to Santarus the following:
(a) The LifeCycle Agreement is in full force and effect, and no event has occurred which, after the giving of notice or the lapse of time or both, would constitute a material breach by it or LifeCycle. CHRP has not received from LifeCycle any written notice to the effect that CHRP is in breach of the LifeCycle Agreement and is not otherwise aware of any such breach;
(b) neither it nor any of its Affiliates has granted any licenses to, agreed not to xxx, or otherwise authorized, any person or entity, under the Licensed Technology or Shionogi Technology to Develop, Manufacture, use or Commercialize the Licensed Products in the Field in the Territory, other than the grant of rights by it to Shore under the Shore Agreement and the grant of rights by Shore to Impax under the Impax Sublicense Agreement; and
(c) all rights Controlled by it in the Licensed Technology or Shionogi Technology have been exclusively granted to Shore.
9.4 Disclaimer. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, NO PARTY MAKES ANY, AND EACH OF THE PARTIES HEREBY DISCLAIM ALL, REPRESENTATIONS AND WARRANTIES, WHETHER EXPRESS, IMPLIED OR STATUTORY, WRITTEN OR ORAL, INCLUDING THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND TITLE.
ARTICLE 10.
CONFIDENTIALITY
10.1 Treatment of Confidential Information. Except as provided below, the Parties agree that during the Term, and for a period of [***] after the Term, a Party receiving Confidential Information (the “Receiving Party”) of another Party (the “Disclosing Party”), whether such receipt occurs before or during the Term, shall (a) maintain Confidential Information of the Disclosing Party in confidence to the same extent and with the same degree of care as the Receiving Party maintains its own proprietary industrial information of similar kind and value (but at a minimum each Party shall use Commercially Reasonable Efforts), (b) not disclose such Confidential Information to any Third Party without prior written consent of the Disclosing Party, except for disclosures made in
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
confidence to sublicensees and commercial partners for Licensed Products who agree to be bound by obligations of nondisclosure and non-use at least as stringent as those contained in this ARTICLE 10, and (c) not use such Confidential Information for any purpose except those permitted by this Agreement. If any Party becomes aware or has knowledge of any unauthorized use or disclosure of another Party’s Confidential Information, it shall promptly notify the other Party of such unauthorized use or disclosure.
10.2 Authorized Disclosures. Nothing in this Agreement shall prohibit the Receiving Party from disclosing Confidential Information of a Disclosing Party, as well as the terms and conditions of this Agreement, to the extent:
(a) such disclosure (i) is reasonably necessary for filing or prosecuting Patent rights as contemplated by this Agreement; or (ii) is reasonably necessary for prosecuting or defending litigation as contemplated by this Agreement;
(b) such disclosure is reasonably necessary: (i) to the Receiving Party’s and its Affiliates directors, attorneys, independent accountants, or financial advisors for the sole purpose of enabling such directors, attorneys, independent accountants or financial advisors to provide advice to the Receiving Party, provided that in each such case on the condition that such directors, attorneys, independent accountants and financial advisors are bound by confidentiality and non-use obligations consistent with those contained in this Agreement; or (ii) to actual or potential investors or acquirors solely for the purpose of evaluating an actual or potential investment or acquisition; provided that in each such case on the condition that such actual or potential investors or acquirors are bound by confidentiality and non-use obligations consistent with those contained in the Agreement; and
(c) such disclosure is required by Applicable Law (including the rules and regulations of the Securities and Exchange Commission or any national securities exchange), or judicial or administrative process, provided that in such event such Receiving Party shall promptly inform the Disclosing Party of such required disclosure and provide the Disclosing Party an opportunity to challenge or limit the disclosure obligations. Confidential Information that is disclosed by Applicable Law, or judicial or administrative process shall remain otherwise subject to the confidentiality and non-use provisions of this ARTICLE 10, and the Party disclosing Confidential Information pursuant to law or court order shall take all steps reasonably necessary, including seeking of confidential treatment or a protective order, to ensure the continued confidential treatment of such Confidential Information.
10.3 Publicity. The Parties agree that any public announcement of the execution of this Agreement shall be substantially in the form attached as Exhibit 10.3 and shall cooperate in the issuance thereof as soon as practicable after the execution of this Agreement unless agreed otherwise In addition, each of CHRP and Shore recognizes that Santarus may from time to time desire to issue additional press releases and make other public statements or disclosures regarding the subject matter of this Agreement. Santarus shall use reasonable efforts to provide Shore with an
opportunity to review and comment on any such disclosures and shall consider Shore’s comments in good faith. Notwithstanding anything else in this ARTICLE 10, any disclosure which is required by law or the rules of a securities exchange, or as advised by the disclosing Party’s counsel, may be made without the prior consent or review of the other Parties hereto. Except as set forth herein, neither CHRP nor Shore shall issue press releases or make other public statements or disclosures regarding the subject matter of this Agreement without Santarus’ prior written consent, not to be unreasonably withheld, conditioned or delayed.
10.4 Publication. Shore agrees that it shall not publish or present to the public the results of any non-clinical scientific studies or clinical trials related to Licensed Products in the Field without the opportunity for prior review by Santarus. If Shore wishes to publish or to present to the public such results, then it shall provide Santarus the opportunity to review any of Shore’s proposed abstracts, manuscripts or presentations (including verbal presentations) regarding Licensed Products at least [***] prior to the intended date of submission for publication. Shore understands that a reasonable commercial or regulatory strategy may require delay of publication of information. For the avoidance of doubt, except as provided in this ARTICLE 10, no Party shall publicly disclose any Confidential Information of another Party without such other Party’s prior written consent.
10.5 Confidentiality Agreement. Disclosures of information made by the Parties pursuant to the Confidentiality Agreement are deemed to have been made pursuant to this Agreement and subject to this ARTICLE 10. The Confidentiality Agreement is hereby terminated as of the Effective Date and of no further force or effect, except with respect to any breach of the Confidentiality Agreement prior to the Effective Date.
ARTICLE 11.
INDEMNIFICATION
11.1 Indemnification by Santarus. Subject to Section 11.4, Santarus agrees to indemnify, defend and hold harmless the LifeCycle Indemnitees, CHRP Indemnitees and Shore Indemnitees from and against any liabilities, losses, costs, damages, fees, or expenses (including reasonable legal expenses and attorneys’ fees) payable to a Third Party (collectively, “Losses”) arising out of any claim, action, lawsuit, or other proceeding (collectively, “Claims”) brought against any LifeCycle Indemnitees, CHRP Indemnitees and/or Shore Indemnitees by a Third Party to the extent resulting directly or indirectly from (a) the negligence or willful misconduct of the Santarus Indemnitees; (b) any material breach by Santarus of any of its representations, warranties, covenants or obligations pursuant to this Agreement, including, without limitation, the Assigned Rights; or (c) the Development, Manufacture, use, or Commercialization of the Licensed Products by any and all Santarus Indemnitees (including products liability claims) on or after the Effective Date; except to the extent such Losses result from activities for which Shore or CHRP must indemnify the Santarus Indemnitees pursuant to Sections 11.2. or 11.3.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
11.2 Indemnification by Shore. Subject to Section 11.4, Shore agrees to indemnify, defend and hold harmless the Santarus Indemnitees from and against any Losses arising out of any Claims brought against any Santarus Indemnitee by a Third Party to the extent resulting directly or indirectly from (a) any Product Agreement assumed by Santarus pursuant to Section 2.1(d) to the extent arising from activities prior to the Effective Date, (b) any Product Agreement not assumed by Santarus pursuant to Section 2.1(d), (c) the Development, Manufacturing, use or Commercialization of Licensed Products prior to the Effective Date; (c) any material breach by Shore or CHRP of any of their respective representations, warranties, covenants or obligations under any agreement related to the Licensed Technology, Shore Technology or Shionogi Technology to which CHRP or Shore are party, including the Upstream Agreements, other than the Assigned Rights; (d) any product liability claim made with respect to the Licensed Products (including the Inventory) Manufactured or Commercialized prior to the Effective Date; (e) the negligence or willful misconduct of the CHRP Indemnitees or the Shore Indemnitees; (f) any material breach by Shore of any of its representations, warranties, covenants or obligations pursuant to this Agreement, the Impax Settlement Agreement, or the Impax Sublicense Agreement (other than the Assigned Rights), including any failure to satisfy any Excluded Liabilities or Obligations; or (g) the performance of obligations by Shore or CHRP under any agreement between CHRP and Shore, except to the extent such Losses result from activities for which Santarus must indemnify the LifeCycle Indemnitees, CHRP Indemnitees and/or Shore Indemnitees pursuant to Section 11.1. For clarity, Santarus shall be solely responsible for its legal costs and expenses related to the Impax Litigation, if any.
11.3 Indemnification by CHRP. Subject to Section 11.4, CHRP agrees to indemnify, defend and hold harmless the Santarus Indemnitees from and against any Losses arising out of any Claims brought against any Santarus Indemnitee by a Third Party to the extent resulting directly or indirectly from (a) the negligence or willful misconduct of CHRP; (b) any material breach by CHRP of any of its representations, warranties, covenants or obligations pursuant to this Agreement; or (c) the performance of obligations by Shore or CHRP under any agreement between CHRP and Shore, except to the extent such Losses result from activities for which Santarus must indemnify the LifeCycle Indemnitees, CHRP Indemnitees and/or Shore Indemnitees pursuant to Section 11.1.
11.4 Procedure. The indemnified Party shall provide the indemnifying Party with prompt notice of the Claim which might give rise to an indemnification obligation pursuant to this ARTICLE 11 indicating the nature of the Claim and the basis therefore. The indemnifying Party shall have the right, at its option, to assume the defense of, at its own cost and by its own counsel, any such Claim involving the asserted liability of the indemnified Party. The indemnified Party shall cooperate fully with the indemnifying Party and its counsel in the defense against any such Claim, including making available to the indemnifying Party any books, records or other documents within its control that are necessary for such defense. All reasonable costs incurred in
connection with the indemnified Party’s cooperation will be borne by the indemnifying Party. The indemnifying Party shall keep the other Party advised of the status of such Claim and the defense thereof and shall consider recommendations made by the indemnified Party with respect thereto. The indemnified Party shall not agree to any settlement of such Claim without the prior written consent of the indemnifying Party, which consent shall not be unreasonably withheld, conditioned or delayed. The indemnifying Party shall not agree to any settlement of such Claim or consent to any judgment in respect thereof that does not include a complete and unconditional release of the indemnified Party from all liability with respect thereto or that imposes any liability or obligation on the indemnified Party or adversely affects the indemnified Party without the prior written consent of the indemnified Party, which consent shall not be unreasonably withheld, conditioned or delayed. Notwithstanding an election by the indemnifying Party to assume the defense of any Claim as set forth above, such indemnified Party shall have the right (at its own cost if the indemnifying Party has elected to assume such defense) to employ separate counsel and to participate in the defense of any Claim. In the event that Shore possesses insufficient funds to indemnify Santarus pursuant to Section 11.2 for any Claim, Santarus shall have the right to offset all Losses arising out of any such Claim against the sales milestones and royalties otherwise payable by Santarus under Sections 7.2 and 7.3; provided, however, that such offset right shall not be Santarus’ sole remedy in the event of an indemnification claim hereunder.
11.5 Insurance. Each Party shall procure and maintain insurance, including product liability insurance, adequate to cover its obligations hereunder and which are consistent with normal business practices of prudent companies similarly situated for so long as Licensed Products in the Territory continue to be Developed, Manufactured or sold and thereafter. It is understood that such insurance shall not be construed to create a limit of any Party’s liability with respect to its indemnification obligations under this ARTICLE 11. Each Party shall provide the other Party with written evidence of such insurance upon request. Each Party shall provide the other Party with written notice at least [***] prior to the cancellation, non-renewal or material change in such insurance or self-insurance which materially adversely affects the rights of the other Party hereunder.
Without limiting the foregoing, Santarus shall obtain and maintain commercial general liability insurance, including clinical trials and products liability insurance, with reputable and financially secure insurance carriers, in such amounts and subject to such deductibles as are reasonable and customary in the pharmaceutical industry for companies of comparable size and activities (but in no event less than the amounts required under the API Supply Agreement and Commercial Supply Agreement or any other supply agreement for the Licensed Products), which insurance shall name LifeCycle, CHRP and Shore as additional insureds. Santarus shall maintain such insurance for so long as Licensed Products in the Territory continue to be Developed, Manufactured or sold and thereafter for [***] to cover any and all Claims which may arise during such time from the Development, Manufacture or sale of a Licensed Product in the Territory. Upon reasonable request by CHRP, Santarus shall deliver to CHRP evidence satisfactory to CHRP that such insurance policies are valid, kept up to date and in full force and effect.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
11.6 Limitation of Liability. NO PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL, INCIDENTAL, PUNITIVE, INDIRECT, OR CONSEQUENTIAL DAMAGES (INCLUDING DAMAGES FOR LOST PROFITS), ARISING FROM OR RELATING TO THIS AGREEMENT (EVEN IF SUCH DAMAGES MAY HAVE BEEN FORESEEABLE), REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. NOTHING IN THIS SECTION 11.6 IS INTENDED TO LIMIT OR RESTRICT THE OBLIGATIONS OF CHRP OR SHORE UNDER SECTION 2.6(c), THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY WITH RESPECT TO THIRD PARTY CLAIMS UNDER SECTIONS 11.1, 11.2, OR 11.3, OR DAMAGES AVAILABLE FOR ANY BREACH OF CONFIDENTIALITY OBLIGATIONS SET FORTH IN ARTICLE 10.
11.7 Indemnification/Payment Fund. Following the Effective Date for a period of [***], Shore shall retain [***] of the Upfront Fee to serve as (a) a source of payment and remedy for any indemnification claim for which Santarus is entitled to recovery pursuant to this ARTICLE 11, (b) a fund by which to make any other payments required of Shore pursuant to this Agreement or (c) a fund by which to make other payments reasonably required in connection with the operation of Shore’s business (the “Indemnification/Payment Fund”). This obligation shall not in any way affect Shore’s obligations under Delaware corporate law to maintain sufficient assets to discharge its liabilities.
ARTICLE 12.
TERM AND TERMINATION
12.1 Term. Unless earlier terminated in accordance with the terms of this ARTICLE 12, the term of this Agreement shall begin on the Effective Date and shall continue in effect until the cessation of all Commercialization of Licensed Products by Santarus in the Territory (the “Term”).
12.2 Termination by Santarus. Santarus shall have the right to terminate this Agreement in its entirety at any time during the Term:
(a) upon one hundred eighty (180) days written notice to Shore, which right may be exercised in Santarus’ discretion. Notwithstanding such termination, all payments made hereunder by Santarus shall be non-refundable but subject to offset and credit as set forth in Section 7.5; and
(b) upon ninety (90) calendar days’ written notice to Shore in the event that Santarus elects to cease sales of Licensed Products in the Territory and desires to transfer and assign to Impax or its designee the Product NDA (as defined in the Impax Sublicense Agreement) and all non-terminable without penalty manufacturing and supply agreements for the Manufacture, distribution and storage of raw materials, Compound and Licensed Products, as set forth in Section 2.4 of the Impax Sublicense Agreement.
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
12.3 Termination for Cause. Either Shore or Santarus may terminate this Agreement, effective at any time after providing ninety (90) days written notice and an opportunity to cure during such ninety (90) day period, in the event of a material breach by Santarus, on the one hand, or CHRP or Shore, on the other hand, of their respective obligations under this Agreement; provided that, if such breach is not susceptible to cure within ninety (90) days, then, the non-breaching Party’s right to termination shall be suspended for so long as the breaching Party is diligently proceeding to cure such default, but in no event longer than one hundred-eighty (180) days (or such longer period as mutually agreed by Shore and Santarus).
12.4 Termination for Bankruptcy. To the extent permitted by Applicable Law, either Shore or Santarus may terminate this Agreement immediately upon notice to each other Party, in the event of any of the following: (a) the entry of an order for relief under the United States Bankruptcy Code (or any corresponding remedy under successor laws) against Santarus, on the one hand, or CHRP or Shore, on the other hand; (b) the filing of a petition by or against Santarus, on the one hand, or CHRP or Shore, on the other hand, under any bankruptcy, insolvency or similar law (which petition is not dismissed within sixty (60) days after filing), except Chapter 11 of the United States Bankruptcy Code or any successor statute that permits a corporation to continue its operation while protecting it from creditors; (c) the appointment of a receiver for Santarus’, on the one hand, or CHRP’s or Shore’s, on the other hand, business or property; or (d) Santarus’, on the one hand, or CHRP’s or Shore’s, on the other hand, making of a general assignment for the benefit of its creditors.
12.5 Consequences of Expiration or Termination.
(a) Upon expiration or early termination of this Agreement by either Santarus or Shore (and except as set forth in Section 2.4 of the Impax Sublicense Agreement, if Santarus has terminated this Agreement pursuant to Section 12.2(b)):
(i) the licenses granted to Santarus under this Agreement shall terminate, and, after a wind-down period to be mutually agreed by the Parties (or one hundred eighty (180) days if no agreement is reached), Santarus shall cease all Commercialization activities;
(ii) Santarus shall assign, transfer and deliver to Shore all right, title and interest in and to the Product Trademarks, Product Data, Product Materials, Regulatory Approvals and Regulatory Filings (or, to the extent transfer is not permitted by Applicable Laws, a right of reference) for the Licensed Products then-Controlled by Santarus and/or its Affiliates, and Santarus shall promptly execute any and all other instruments, forms of assignment or other documents and take such further actions as Shore may reasonably request in order to give effect to or evidence the foregoing assignments and grants;
(iii) Shore shall have an exclusive, sublicenseable, royalty-free license under the Santarus Technology solely to make, have made, market, import, use, sell, offer for sale and otherwise Develop and Commercialize the Licensed Products in the Field in the Territory;
(iv) if Santarus is then a party to any agreements with Third Party independent contractors for the Licensed Product, it shall cooperate with Shore and use Commercially Reasonable Efforts to enable Shore to obtain the benefit of such agreements as necessary to enable Shore to exercise its rights under this ARTICLE 12, including by assigning such agreements to Shore where reasonably practicable;
(v) Santarus shall transfer and assign to Shore the Assigned Rights, or the Impax Sublicense Agreement, as applicable; and
(vi) all Shore Confidential Information shall be subject to Section 12.5(b).
(b) Return of Confidential Information. Upon the early termination of this Agreement, upon the request of the non-defaulting Party, the other Party(ies) will promptly return to the terminating Party or destroy all material embodying the non-defaulting Party’s Confidential Information in its possession or under its Control, including all copies thereof, except for a single copy retained solely for the purpose of ensuring compliance with the terms of this Agreement.
12.6 Survival. The rights and obligations of the Parties under the following provisions of this Agreement shall survive any expiration or termination of this Agreement: ARTICLE 1, Sections 2.4, 2.5(e) (to the extent that any amounts payable remain unpaid), 4.4(b), 5.3, 6.2(b), 6.2(c), ARTICLE 7 (to the extent that any amounts payable remain unpaid, provided that Section 7.9 shall survive only for the period set forth therein, but not including Section 7.1), Sections 8.1, 8.3(e) (with respect to actions commenced prior to the expiration or termination of this Agreement) and 9.4, ARTICLE 10 (for the period set forth in Section 10.1), Sections 11.1, 11.2, 11.3, 11.4, 11.5 (for the period set forth in Section 11.5), 11.6, 12.5 (as applicable), 12.6 and 12.7, ARTICLE 13 and ARTICLE 14.
12.7 No Waiver of Remedies. Expiration or termination of this Agreement shall not preclude any Party from (a) claiming any other damages, compensation or relief that it may be entitled to upon such expiration or termination, (b) any right to receive any amounts accrued under this Agreement prior to the expiration or termination date but which are unpaid or become payable thereafter and (c) any right to obtain performance of any obligation provided for in this Agreement which shall survive expiration or termination.
ARTICLE 13.
DISPUTE RESOLUTION
13.1 Disputes.
(a) The Parties recognize that disputes as to certain matters may from time to time arise during the Term that relate to a Party’s rights and/or obligations hereunder. It is the desire of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation and, to the extent possible, without resort to arbitration or litigation. To accomplish this objective, the Parties agree to follow the procedures set forth in this ARTICLE 13 if and when a dispute arises under this Agreement. Each Party may refer a dispute under this Agreement to the Parties’ officers having responsibility for the subject matter of the dispute, or their designees. If such officers are unable to resolve any such dispute within thirty (30) days after such dispute is submitted to them, any Party may, by written notice to the other Parties, have such dispute referred to their respective executive officers designated below or their successors, for attempted resolution by good faith negotiations within thirty (30) days after such notice is received. Such designated officers are as follows:
For Shore: Xxxxxx Xxxxx
For Santarus: Chief Executive Officer
In the event the designated officers are not able to resolve such dispute within such thirty (30) day period after receipt of written notice, then each party shall be entitled to pursue other available remedies at law or in equity.
(b) To the extent a dispute exists pursuant to Section 2.6 (c) (an “Arbitrable Dispute”), and the Parties are unable resolve such Arbitrable Dispute pursuant to Section 13.1(a), any Party may have the given dispute settled by binding arbitration in the manner described below.
(i) If a Party intends to begin an arbitration to resolve an Arbitrable Dispute arising under this Agreement, such Party shall provide written notice (the “Arbitration Request”) to the other Parties of such intention and the issues for resolution. From the date of the Arbitration Request and until such time as the dispute has become finally settled, the running of the time periods as to which a Party must cure a breach of this Agreement becomes suspended as to the subject matter of the dispute.
(ii) Discovery shall be under the U.S. Federal Rules of Civil Procedure then in effect in the District Court for the Southern District of New York. The Arbitration shall be held in the City of New York, under the rules of the American Arbitration Association (“AAA”). The arbitration shall be conducted by three (3) arbitrators who are knowledgeable in the subject matter at issue in the dispute. One (1) arbitrator will be selected by Santarus, one (1) arbitrator will be selected by CHRP and Shore, and the third arbitrator will be selected by mutual agreement of the two (2) arbitrators selected by the Parties. The arbitrators may proceed to an award, notwithstanding the failure of either Party to participate in the proceedings. The arbitrators shall, within fifteen (15) days after the conclusion of the arbitration hearing, issue a written award and statement of decision describing the essential findings and conclusions on which the award is based, including the calculation of any damages awarded. The arbitrators
shall be authorized to grant any temporary, preliminary or permanent equitable remedy or relief the arbitrators deem just and equitable and within the scope of this Agreement, including an injunction or order for specific performance. The award of the arbitrators shall be the sole and exclusive remedy of the Parties. Judgment on the award rendered by the arbitrators may be enforced in any court having competent jurisdiction thereof, subject only to revocation on grounds of fraud or clear bias on the part of the arbitrators. Notwithstanding anything contained in this Section 13.1(b) to the contrary, each Party shall have the right to institute judicial proceedings against the another Party or anyone acting by, through or under such other Party, in order to enforce the instituting Party’s rights hereunder through specific performance, injunction or similar equitable relief.
(iii) Each Party shall bear its own attorneys’ fees, costs, and disbursements arising out of the arbitration, and shall pay an equal share of the fees and costs of the arbitrators; provided, however, that the arbitrators shall be authorized to determine whether a Party is the prevailing Party, and if so, to award to that prevailing Party reimbursement for its reasonable attorneys’ fees, costs and disbursements (including, for example, expert witness fees and expenses, photocopy charges and travel expenses), and/or the fees and costs of the arbitrators. Absent the filing of an application to correct or vacate the arbitration award as permitted by applicable law, each Party shall fully perform and satisfy the arbitration award within fifteen (15) days of the service of the award.
(iv) By agreeing to this binding arbitration provision, the Parties understand that they are waiving certain rights and protections which may otherwise be available if a dispute between the Parties were determined by litigation in court, including the right to seek or obtain certain types of damages precluded by this provision, the right to a jury trial, certain rights of appeal, and a right to invoke formal rules of procedure and evidence.
13.2 Governing Law. This Agreement shall be construed and interpreted in accordance with the laws of the State of New York, without regard to any conflicts of law principles that would provide for the application of the laws of another jurisdiction.
ARTICLE 14.
MISCELLANEOUS
14.1 Entire Agreement. This Agreement and the exhibits hereto, constitute the entire understanding between the Parties with respect to the subject matter contained herein and supersedes any and all prior and contemporaneous agreements, understandings and arrangements whether oral or written between the Parties relating to the subject matter hereof.
14.2 Assignment. Shore and CHRP may assign their rights and obligations under this Agreement at any time without the consent of Santarus; provided that the assigning Party shall provide written notice to Santarus of any such assignment. Santarus may not assign this Agreement or any rights
or obligations hereunder without the prior written consent of the non-assigning Parties, not to be unreasonably withheld, conditioned or delayed, and any attempted assignment without such consent shall be null and void. Notwithstanding the foregoing, Santarus may assign this Agreement in conjunction with the simultaneous assignment of the then-effective Product Agreements to which it is a party without the other Parties’ consent to any of its Affiliates, or in connection with a merger or acquisition of or by Santarus, or a sale of all or substantially all of Santarus’ assets to which this Agreement relates. This Agreement shall be binding upon and, subject to the terms of this Section 14.2, inure to the benefit of a Party’s successors and permitted assigns.
Notwithstanding the foregoing, CHRP and Shore agree that no assignment of rights or obligations hereunder by either of them shall have a material adverse effect on the ability of Santarus to Manufacture or Commercialize the Licensed Products as contemplated hereunder.
14.3 Amendments. No amendment, change, modification or alteration of the terms and conditions of this Agreement shall be binding upon any Party unless in writing and signed by the Party to be charged.
14.4 Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by CHRP, Shore or Santarus are, and will otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Parties, as licensees of such rights under this Agreement, will retain and may fully exercise all of their rights and elections under the U.S. Bankruptcy Code. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against either Party under the U.S. Bankruptcy Code, the Party hereto that is not a party to such proceeding will be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, and same, if not already in their possession, will be promptly delivered to them (a) upon any such commencement of a bankruptcy proceeding upon their written request therefor, unless the Party subject to such proceeding elects to continue to perform all of its obligations under this Agreement, or (b) if not delivered under (a) above, following the rejection of this Agreement by or on behalf of the Party subject to such proceeding upon written request therefor by the non-subject Party.
14.5 Non-Waiver. The waiver by any of the Parties of any breach of any provision hereof by any of the other Parties shall not be construed to be a waiver of any succeeding breach of such provision or a waiver of the provision itself.
14.6 Severability. If and to the extent that any court or tribunal of competent jurisdiction holds any of the terms or provisions of this Agreement, or the application thereof to any circumstances, to be
invalid or unenforceable in a final nonappealable order, the Parties shall use their best efforts to reform the portions of this Agreement declared invalid to realize the intent of the Parties as fully as practical, and the remainder of this Agreement and the application of such invalid term or provision to circumstances other than those as to which it is held invalid or unenforceable shall not be affected thereby, and each of the remaining terms and provisions of this Agreement shall remain valid and enforceable to the fullest extent of the law.
14.7 Notice. Any notice to be given under this Agreement must be in writing and delivered either in person, by any method of mail (postage prepaid) requiring return receipt, or by international courier or facsimile confirmed thereafter by any of the foregoing, to the Party to be notified at its address(es) given below, or at any address such Party has previously designated by prior written notice to the other. Notice shall be deemed sufficiently given for all purposes upon the earlier of: (a) the date of actual receipt; (b) if mailed, five (5) calendar days after the date of postmark; or (c) if delivered by international courier, the next business day the overnight courier regularly makes deliveries in the country of the recipient:
If to CHRP, as follows:
Xxxxx Healthcare Royalty Partners,
X.X.000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Facsimile No.: (000) 000-0000
Attention: Xxxxxx X. Xxxxx
With a copy to (which shall not constitute notice):
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx, P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxx, XX 00000
Facsimile No.: (000) 000-0000
Attention: Y. Xxxxx Xxxxx, Esq.
If to Shore, as follows:
Shore Therapeutics, Inc.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Facsimile No.: (000) 000-0000
Attn: Xxxxxx X. Xxxxx
With a copy to (which shall not constitute notice):
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx, P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxx, XX 00000
Facsimile No.: (000) 000-0000
Attention: Y. Xxxxx Xxxxx, Esq.
If to Santarus, as follows:
Santarus, Inc.
0000 Xxxxxx Xxxxxx Xxxxx
Xxxxx 000
Xxx Xxxxx, Xxxxxxxxxx 00000
Attn: Legal Affairs Department
Facsimile: (000) 000-0000
With a copy to (which shall not constitute notice):
Xxxxxx & Xxxxxxx LLP
00000 Xxxx Xxxxx Xxxxx, Xxxxx 000
Xxx Xxxxx, Xxxxxxxxxx 00000
Attention: Xxxx X. Xxxxxxx, Esq.
Fax No: (000) 000-0000
or to such other address as to which the Party has given written notice thereof. Such notices shall be deemed given upon receipt.
14.8 Further Assurances. Each Party shall, at its own expense, furnish, execute, and deliver all documents and take all actions as may reasonably be required to effect the terms and purposes of this Agreement.
14.9 Force Majeure. Except with respect to Santarus’ obligation to make payments to CHRP, no failure or omission by the Parties in the performance of any obligation of this Agreement shall be deemed a breach of this Agreement nor shall it create any liability if the same shall arise from any cause or causes beyond the reasonable control of the affected Party, including the following, which for purposes of this Agreement shall be regarded as beyond the control of the Party in question: acts of nature; acts or omissions of any government; any rules, regulations, or orders issued by any governmental authority or by any officer, department, agency or instrumentality thereof; fire; storm; flood; earthquake; accident; war; rebellion; insurrection; riot; invasion; strikes; and lockouts or the like; provided that the Party so affected shall use its best efforts to avoid or remove such causes or nonperformance and shall continue performance hereunder with the utmost dispatch whenever such causes are removed, and provided further, however, that any such failure or omission to perform does not continue for a period in excess of one hundred eighty (180) days.
14.10 Cooperation. Shore shall (a) prepare and cause its independent auditors to audit the financial statements of the business related to the manufacturing and sale of the Products for the two (2)-year period ended December 31, 2010 (or such shorter period required by the U.S. Securities and Exchange Commission (“SEC”)) and (b) prepare interim financial statements, in each case as required to be included in Santarus’ reports and filings with the SEC in connection with the
acquisition of a “significant business” pursuant to Regulation S-X of the SEC. Shore shall complete and deliver such financial statements and cause to be delivered a report of its auditors (such report to be unqualified) with respect to the audited financial statements on or prior to the sixtieth (60th) calendar day after the Effective Date. All such financial statements shall be prepared in accordance with GAAP and Regulation S-X of the SEC and in a manner reasonably satisfactory to Santarus’ independent auditors. Shore shall request, and take all reasonable steps necessary to encourage, its auditors to cooperate with Santarus and provide all necessary consents required by the SEC and customary “comfort letters” in connection with securities offerings of Santarus and with its preparation of any financial statements or other reports pursuant to Applicable Laws.
14.11 Independent Contractors. It is understood that the Parties are independent contractors and engage in the operation of their own respective businesses, and no Party is to be considered the agent or partner of any other Party for any purpose whatsoever, except as otherwise expressly provided in this Agreement. No Party has any authority to enter into any contracts or assume any obligations for any other Party or make any warranties or representations on behalf of any other Party. Furthermore, nothing in this Agreement shall be construed as creating a partnership or joint venture among the Parties.
14.12 No Third Party Beneficiaries. This Agreement is neither expressly nor impliedly made for the benefit of any Party other than those executing it.
14.13 Interpretation.
(a) Captions & Headings. The captions and headings of clauses contained in this Agreement preceding the text of the articles, sections, subsections and paragraphs hereof are inserted solely for convenience and ease of reference only and shall not constitute any part of this Agreement, or have any effect on its interpretation or construction.
(b) Singular & Plural. All references in this Agreement to the singular shall include the plural where applicable, and all references to gender shall include both genders and the neuter.
(c) Articles, Sections & Subsections. Unless otherwise specified, references in this Agreement to any article shall include all sections, subsections, and paragraphs in such article; references in this Agreement to any section shall include all subsections and paragraphs in such sections; and references in this Agreement to any subsection shall include all paragraphs in such subsection.
(d) Days. All references to days in this Agreement mean calendar days, unless otherwise specified.
(e) Clarification. The word “including” shall be deemed to mean “including without limitation” and “including, but not limited to”. A consent that is identified in this Agreement as not “to be unreasonably withheld” shall not be unreasonably withheld, delayed or conditioned.
(f) Ambiguities. Ambiguities and uncertainties in this Agreement, if any, shall not be interpreted against either Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to exist.
(g) Priority. In the event of any inconsistency between the provisions of this Agreement and the Manufacturing and Supply Agreement, the provisions of this Agreement shall control.
14.14 Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original and both of which together shall constitute one and the same instrument.
[Signature Page Follows]
IN WITNESS WHEREOF, the Parties, intending to be bound hereby, have executed this License Agreement by their duly authorized representatives as of the Effective Date.
| SHORE: |
| SHORE THERAPEUTICS, INC. |
| By: /s/ Xxxx Xxxxxx |
| Name: Xxxx Xxxxxx |
| Title: General Manager |
| CHRP: |
| XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. |
| By: /s/ Xxxxxx X. Xxxxx |
| Name: Xxxxxx X. Xxxxx |
| Title: Managing Director |
| SANTARUS: |
| SANTARUS, INC. |
| By: /s/ Xxxxxx X. Xxxxxx |
| Name: Xxxxxx X. Xxxxxx |
| Title: President and CEO |
[Signature Page to License Agreement]
Exhibit 1.16
Compound
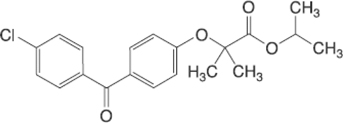
Chemical Name: 24-(4-chlorobenzoyl) phenoxy 2-methyl-propanoic acid, 1-methyl ethyl ester
Exhibit 1.24
Domain Names
Xxxxxxxxx.xxx
Exhibit 1.40
Inventory Lot Numbers
Product
120mg / 90-count
| Lot # |
Cost per Bottle | |
| 1100149 |
[***] | |
| 1100150 |
[***] | |
| 1100742 |
[***] | |
| 1100743 |
[***] | |
| 1100744 |
[***] |
40mg / 90-count
| Lot # |
Cost per Bottle | |
| 1101080 |
[***] | |
| 1100741 |
[***] |
120mg / samples
| Lot # |
Cost per Bottle | |
| 1100746 |
[***] |
Compound
| Lot # |
Cost per Kilogram | |
| 2445115 |
[***] | |
| 2986098 |
[***] | |
| 3333605 |
[***] |
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Exhibit 1.49
Licensed Patents
Part A:
| Country |
Issue Date/ Filing Date |
Patent Number/ |
Title | |||
| USA | 09FEB2010 | 7658944 | Solid Dosage Form Comprising a Fibrate | |||
| USA | 18DEC2009 | 12/642,563 | Solid Dosage Form Comprising a Fibrate | |||
| USA | 9DEC2011 | 13/315,030 | Solid Dosage Form Comprising a Fibrate | |||
| Canada | 08FEB2011 | 2,540,984 | Solid Dosage Form Comprising a Fibrate | |||
| USA | 00XXX0000 | 11/449,918 | Tablet Comprising a Fibrate | |||
| Canada | 00XXX0000 (PCT) | 2,604,970 | Tablet Comprising a Fibrate |
Part B:
| Country |
Issue Date/ Filing Date |
Patent Number/ |
Title | |||
| USA | 15MAY2007 | 7217431 | Controlled Agglomeration | |||
| USA | 27FEB2007 | 11/711,965 | Controlled Agglomeration | |||
| Canada | 22MAR2011 | 2,452,330 | Controlled Agglomeration | |||
| USA | 00XXX0000 | 7252247 | Self-Cleaning Spray Nozzle | |||
| Canada | 06JUL2010 | 2,511,150 | Self-Cleaning Spray Nozzle |
Exhibit 1.61
Product Agreements
| Product Agreement |
Action | |
| API Supply Agreement |
Assign | |
| Commercial Supply Agreement | Assign | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] |
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Exhibit 1.65
Product Trademarks
Fenoglide®
Exhibit 2.5(b)
FDA Transfer Letter
December , 2011
Xxxx Xxxxx, M.D., Director
Division of Metabolism and Endocrinology Products
Office of Drug Evaluation II
Food and Drug Administration
0000-X Xxxxxxxxx Xxxx
Xxxxxxxxxx, XX 00000-0000
(1) RE: NDA 22-118: FENOGLIDE® (fenofibrate) Tablets, 40 mg and 120 mg
Transfer of ownership
Dear Xx. Xxxxx:
Reference is made to NDA 22-118 for FENOGLIDE® (fenofibrate) Tablets, 40 mg and 120 mg. This letter is to inform you that Shore Therapeutics, Inc. transferred ownership of NDA 22-118 to Santarus, Inc., effective December , 2011.
The contact information at Santarus, Inc. is:
Xxxx Xxxxx
Senior Director, Regulatory Affairs
Santarus, Inc.
0000 Xxxxxx Xxxxxx Xxxxx, Xxxxx 000
Xxx Xxxxx, XX 00000
Phone: (000) 000-0000
Email: xxxxxx@xxxxxxxx.xxx.
Please contact me should you have any questions or require additional information. I may be reached by phone at (000) 000-0000, facsimile at (000) 000-0000, or by email at xxxxxxx@xxxxxxxxxxxxxxxxx.xxx.
Sincerely,
Xxxx Xxxxxx
General Manager
Exhibit 9.2
Exceptions to Warranties and Representations
[***]
[***]
[***]
[***]
[***]
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Exhibit 9.2(w)
Unfilled Firm Orders
[***]
[***]
[***]
[***]
[***]
[***]
[***]
[***]
[***]
***Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.

