FIFTH ADDENDUM AGREEMENT (“this Addendum”) Dated: February 22, 2018 (“Effective Date”) BY AND BETWEEN YEDA RESEARCH AND DEVELOPMENT COMPANY LTD. of P.O. Box 95, Rehovot 76100, Israel (hereinafter “Yeda”) and BRAINSWAY, INC. a company duly registered...
FIFTH ADDENDUM AGREEMENT
(“this Addendum”)
Dated: February 22, 2018 (“Effective Date”)
BY AND BETWEEN
YEDA RESEARCH AND DEVELOPMENT COMPANY LTD.
of ▇.▇. ▇▇▇ ▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇
(hereinafter “Yeda”)
and
BRAINSWAY, INC.
a company duly registered under the laws of the state of Delaware, U.S.A
(hereinafter “the Company”)
WHEREAS Yeda and the Company (collectively, “the Parties”) are parties to a Research and License Agreement dated June 2, 2005, as amended by the First Addendum Agreement effective from June 1, 2007; the Second Addendum Agreement effective from August 20, 2008; the Third Addendum effective from March 23, 2010 (“Third Addendum”); the Fourth Addendum Agreement effective from August 1, 2009 (as amended by the First Amendment to the Fourth Addendum dated May 11, 2010) (all of the above, together, “R&L Agreement”); and
WHEREAS in the course of research conducted at the Institute, ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇ of the Department of Physics of Complex Systems, together with other scientists of the Institute (“rfTMS Inventors”) arrived at an invention (“rfTMS Invention”) relating to Rotating Field Transcranial Magnetic Stimulation (rfTMS), as more fully described in PCT patent application number PCT/IL2010/000171 entitled “MAGNETIC CONFIGURATION AND TIMING SCHEME
|
Ref: 09-2595-17-66 |
No.: 007_200098 |
FOR TRANSCRANIAL MAGNETIC STIMULATION” and corresponding patent applications and patents (Yeda reference number 2009-014), all as listed in the patent card attached as Annex A(1) hereto (“rfTMS Patent”), and created and/or generated the know-how and/or materials and other information relating to the rfTMS Invention as described in Annex A(2) hereto (the said know-how, materials and information, together with the rfTMS Invention, “rfTMS IP”); and
WHEREAS the Company wishes to conduct an internal evaluation as detailed below, following which the Company may elect, in the manner set forth below, to obtain a license to the rfTMS IP and rfTMS Patent, for the research, development, manufacture or sale of any therapeutic product, apparatus, or device within the field of transcranial magnetic stimulation, under the terms and conditions set out below; and
WHEREAS in connection with this evaluation, the Company wishes to provide funding, in the manner set forth below, which will include “Magneton” funding, with respect to a clinical study to be performed at Geha Mental Health Center, intended, inter alia, to gauge the efficacy of the rfTMS Invention,
NOW THEREFORE THE PARTIES HERETO AGREE AS FOLLOWS:
1. Terms and phrases included in this Addendum which are defined in the R&L Agreement shall have the same meaning attributed to them in the R&L Agreement, unless otherwise defined in this Addendum.
2. This Addendum and the R&L Agreement shall be read as one, and shall represent the complete, current understanding between the Parties with respect to the subject matter hereof. Subject to the modifications contained herein, all provisions of the R&L Agreement shall remain unaltered and in full force and effect.
3. The preamble and annexes hereto form an integral part of this
Addendum, and are incorporated herein by reference.
Clinical Study and Funding
4. Yeda will procure the conduct of a clinical study at Geha Mental Health Center in accordance with the work plan attached hereto as Annex B (“Clinical Study” and “Work Plan”).
5. The Parties shall fund the Clinical Study as follows:
5.1 The Company submitted a Magneton application to the Israel Innovation Authority (“IIA”) in October 2017, to conduct the Clinical Study and Evaluation (as described in Section 10, below), a copy of which is attached hereto as Annex C, which was subsequently granted in December 2017. The Company shall remit to Yeda such portion of the Magneton Funding intended for Yeda, constituting 66% of the full budget for Yeda’s activities, and the Company shall remit to Yeda the remaining 34% of the budget for Yeda’s activities. The Company shall remit to Yeda the portion of the budget, as described above, for each respective year of the twenty-four (24) month Magneton period, on a quarterly basis in equal installments, within thirty (30) days of receipt of each quarterly installment; however in the event of the remittance by Magneton to the Company of advances of amounts greater than equal quarterly payments, then the Company shall remit amounts earlier to Yeda accordingly.
5.2 The Company shall promptly provide Yeda with copies of all notices, reports, and other correspondence exchanged between Company and IIA with respect to the Magneton application and funded research.
6. Brainsway will provide assistance for the Clinical Study as detailed in the Work Plan.
7. If ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇ of the Department of Physics of Complex Systems
shall cease to be available for the performance of the Clinical Study, such cessation shall not constitute a breach of this Agreement by Yeda. In such event, Yeda shall use its reasonable efforts to find, from amongst the scientists of the Institute, a replacement scientist acceptable to the Company, but gives no undertaking to find such a replacement. If no such acceptable replacement scientist can be found within sixty (60) days of the scientist becoming unavailable as aforesaid, then the Company shall be entitled, by written notice to Yeda, to terminate this Agreement, and in such case, termination shall become effective at the end of a further period of thirty (30) days from the date of receipt by Yeda of such written notice. In the event of such termination, Yeda shall be released from any obligation to procure the performance of the Clinical Study during the period after such effective date of termination, and the Company shall be released from any obligation to finance the Clinical Study, other than any related amounts due at the time of termination or any other non-cancellable costs and expenses.
8. It is hereby specifically agreed and acknowledged that Brainsway will not serve as a sponsor of the Clinical Study. The Company shall not be obligated to indemnify Yeda for liabilities resulting from injury to participants in the Clinical Study caused solely by the negligence or willful misconduct of Yeda and/or the rftMS inventors, in performance of the Clinical Study.
Nothing in this Agreement shall constitute a representation or warranty by Yeda, express or implied, that the Clinical Study shall be performed or that any results will be achieved by the Clinical Study, and Yeda furthermore makes no warranties or representations, express or implied, whatsoever as to the Clinical Study.
9. The Company acknowledges that Yeda has no contract with Geha Mental Health Center with respect to any intellectual property rights which may arise from the Clinical Study, and further acknowledges that it is unlikely that new intellectual property or inventions will arise from the Clinical
Study. In the event that intellectual property or inventions will arise in connection with the Clinical Study, Yeda shall make commercially reasonable efforts to negotiate with Geha Mental Health Center as needed, in order to secure the rights to such intellectual property and inventions, to the extent necessary or desirable for the inclusion of the rfTMS IP and rfTMS Patent in the License, if the Company’s Option (as defined in Section 13, below) is exercised, and Yeda makes no representation or guarantee in this regard. Furthermore, to the extent there are any intellectual property claims or claims for royalties asserted or raised by Geha Mental Health Center which relate to or arise from the Clinical Study, Yeda shall cover any and all such claims up to the total aggregate amount of royalties received by Yeda from Brainsway under this Fifth Addendum.
Evaluation of rfTMS IP
10. Yeda hereby grants the Company the right to conduct a limited evaluation (“Evaluation”) of the rfTMS IP and the rfTMS Patent and any related know how generated or created in connection with the performance of the Clinical Study (“Know How”) to determine the desirability of exercising the Company’s Option. The Company’s right to conduct the Evaluation shall expire on the earlier of (a) December 31, 2018, and (b) thirty (30) days following the achievement of all milestones described in Table 2 of Annex B (“Evaluation Deadline”). If actions described in Table 1 of Annex B are not fully performed by December 31, 2018, for reasons other than the Company’s non-compliance or delay in performance of its obligations under the Work Plan, then the Evaluation Deadline shall be extended until the earlier of (y) the full performance of the actions described in Table 1 of Annex B, and (z) June 30, 2019; however the Parties may, subject to the discretion of each party in good faith, agree to further extend the Evaluation Deadline until those actions are fully performed (the “rfTMS Extension Period”). All dates are subject to requirements for Magneton funding.
Yeda grants the Company an exclusive, non-assignable, royalty-free license to all data, results, reports and other work product that may be obtained or generated in the course of performance of the Clinical Study (collectively: the “Clinical Results”), and will ensure that access to such Clinical Results is provided to Brainsway during the course of the Clinical Study, for the sole purpose of conducting the Evaluation, during the period commencing from the Effective Date of this Agreement and expiring sixty (60) days after the Evaluation Deadline or rfTMS Extension Period as applicable (the “Option Period”). Upon request, and with the context of such license, the Company will be provided with a copy of all Clinical Results and Know How, in confidence and upon becoming available.
Title: Option
11. All right and title to the rfTMS IP, rftMS Invention, rfTMS Patent, Know How, Clinical Results, and all patent applications and patents derived from the Clinical Results, if any, shall vest exclusively in Yeda.
12. Yeda grants the Company the exclusive right and option, exercisable by written notice during the Option Period, to amend the R&L Agreement in the manner set forth below (the “Company’s Option”):
The rfTMS IP, rftMS Invention, rfTMS Patent, Know How, Clinical Results, and all patent applications and patents derived from the Clinical Results (“Additional Licensed Information”) shall be deemed included as Licensed Information and Patents, as the case may be, under the R&L Agreement. For the avoidance of doubt, during the Option Period, and thereafter if the Company’s Option is indeed exercised, Brainsway shall be granted exclusive licensing rights with respect to the Additional Licensed Information. Yeda shall not be entitled to grant any licensing rights with respect thereto to any third parties, nor take any other steps for the commercialization thereof.
a. any Clinical Results which constitute inventions or know-how,
which neither constitute nor refer to the results of the Evaluation of the rftMS Invention, shall not be licensed to the Company hereunder;
b. The License with respect to the Additional Licensed Information shall be limited to the use thereof for the research, development, manufacture or sale of any therapeutic, diagnostic, research or any other product, apparatus, or device solely within the field of transcranial magnetic stimulation;
c. The rfTMS Patent shall be subject to all provisions of the R&L Agreement, including, but not limited to, the provisions of clause 6 (PATENTS; PATENT INFRINGMENT) thereof; in addition, within thirty (30) days following the exercise by the Company of the Company’s Option the Company shall reimburse Yeda the sum of one hundred thirty-four thousand seven hundred and fifty-five (US $134,755) US Dollars + VAT, constituting the aggregate costs and fees paid or incurred by Yeda prior to the Effective Date in connection with the rfTMS Patent, and as a condition for the inclusion of the Additional Licensed Information in the License under the R&L Agreement as aforesaid. For the avoidance of doubt, should the Company’s Option not be exercised, Company will not be required to reimburse Yeda any of the amounts set forth in this paragraph whatsoever.
d. Any product based on, the development, manufacture or sale of which is based, in whole or in part, on, or involves the use of, the Additional Licensed Information or any part thereof, or is otherwise covered (in whole or in part) by, or falls within the scope of, or which are produced or manufactured using a process or method covered by, or falling within the scope of, any claim under the rfTMS Patent (including under any patent application falling within the definition of Patents) (“rfTMS Product”), shall be deemed a Product under the R&L Agreement, provided that the royalty rate applicable to Net Sales of rfTMS Products shall be subject to the
following:
i. for a rfTMS Product which, in addition to being a rfTMS Product as defined above, falls within the scope of, or which is produced or manufactured using a process or method covered by, or falling within the scope of, any claim under a Patent other than a rfTMS Patent (a “Combined Product”) and which is not a U.S. DHHS Patent Protected Product, the royalty rate applicable under Section 9.1.2 of the Agreement and Section 6 of the Third Addendum shall be increased by an additional two (2%) percent;
ii. for a Combined Product which is also a U.S. DHHS Patent Protected Product, the royalty rate applicable under Section 9.1.2 and Section 6 of the Third Addendum of the Agreement shall be increased by an additional one point six (1.6%) percent; and
iii. for a rfTMS Product which is not a Combined Product, the royalty rate under Section 9.1.2 of the Agreement and Section 6 of the Third Addendum shall be the fixed amount of five (5%) percent.
e. The Company shall inform Yeda in writing of the First Commercial Sale in each country of an rfTMS Product, and, without derogating from clause 9.3.2.1 of the R&L Agreement, the Company shall include a breakdown of sales based on the type of each rfTMS Product in its reports under clause 9.3.2 of the R&L Agreement. In the event that rfTMS Products which constitute Products which were initially developed and/or sold as non-rfTMS Products, are thereafter used or converted into rfTMS Products, under the License granted under this Addendum (“Upgraded Product”), the Parties agree that:
i. the License term set out in clause 7.3 of the R&L Agreement shall be measured based on the First Commercial Sale of the Upgraded Product after it has
become an rfTMS Product, and not based on the First Commercial Sale of such Product prior to becoming an Upgraded Product, provided that, in the event the License term set out in clause 7.3 of the R&L Agreement applicable to such Upgraded Product would have otherwise expired had it not become an rfTMS Product as aforesaid (the “First Expiration Date”), then the royalty rate applicable to such Upgraded Product hereunder, beginning from the First Expiration Date until the end of the relevant License term for such Upgraded Product shall be the fixed rate of two (2%) percent; and
f. Upon exercise of the Company’s Option, the Company shall implement the Development Program attached hereto as Annex D for the development of rfTMS Products (such Development Program called “the rfTMS Development Program”). The Company may periodically update the rfTMS Development Program, based on reasonable grounds to be communicated to Yeda in writing, subject to the Company’s discretion and without derogating from the milestones set out therein (“the rfTMS Milestones”). The rfTMS Development Program shall be considered a Development Program under the R&L Agreement for all intents and purposes. The rfTMS Milestones set forth in Annex D hereto, which shall be deemed an integral part hereof, shall apply in connection with the rfTMS Products. Notwithstanding clause 13 of the R&L Agreement, if any of the aforesaid rfTMS Milestones in this clause 6 and Annex D has not been reached by the Company within the applicable time period, the Company shall have an additional period of six (6) months to cure and to reach the applicable rfTMS Milestone, provided that: (i) the aggregate amount of any and all of the above cure periods applied in order to postpone the final date for the achievement of the said rfTMS Milestones shall not exceed twelve (12) months (the “Aggregate Delay”), however for delays relating to the last
three (3) rfTMS Milestones caused by regulatory bodies such as the FDA (including applicable related rules and regulations), the Aggregate Delay will be extended to a total maximum delay of twenty-four (24) months; and (ii) the Company shall use reasonable commercial efforts to prevent and/or mitigate the duration of such delay, and that the Company has, as soon as reasonably practicable following such a delay, submitted an amended rfTMS Development Program to Yeda detailing how it reasonably intends to reach such rfTMS Milestone within the above-referenced cure period. If the Company does not cure and reach the applicable rfTMS Milestone(s) as described above, Yeda may (effective immediately), as a sole remedy available thereto, exclude the rfTMS IP and rfTMS Patent from the License.
13. During the Option Period, the Company shall reimburse Yeda, upon such frequency as determined by Yeda, for ongoing patent expenses incurred during such period in connection with the rfTMS Patent. During the Option Period, the Company shall, subject to the Company’s consent, provided on a case-by-case basis, reimburse Yeda, upon such frequency as determined by Yeda, for patents based on the Clinical Study results, incurred and paid by Yeda at Yeda’s discretion. Reimbursement shall be made against written proof of actual expenses borne by Yeda, and shall be made against invoices. However, Yeda shall be entitled to abandon any patent, or exclude it from the Option, in the event that the Company elects not to pay the costs of such patent.
14. If the Company does not exercise the Company’s Option within the Option Period, or if the Company notifies Yeda in writing, at any time, that it does not intend to exercise the Company’s Option and wishes to terminate this Addendum, this Addendum, and any corresponding right to add the Additional Licensed Information to the License, shall immediately terminate. The provisions of Sections 5.1, 16, and 17 hereof shall survive the termination of this Addendum. Termination of this Addendum shall not affect the validity of the R&L Agreement.
15. Each Party shall maintain confidential information (in writing or otherwise)
received from the other Party in connection with the Clinical Study, in strict confidence, and shall not use such information, except as required for the performance of the Clinical Study or as otherwise explicitly allowed by this Addendum. The provisions of the R&L Agreement relating to confidentiality shall be extended to apply to such confidential information. All publications relating to the Clinical Study shall be subject to the applicable provisions of the R&L Agreement.
16. The Work Plan may be amended only upon written consent of the Company and Yeda.
Additional Amendments; Clarifications
17. Sub clause 9.1.2.2(ii) of the R&L Agreement shall hereby be deleted in its entirety and replaced with the following:
“(ii) in the event that in any calendar year during the term of this Agreement commencing on the first day of January of the first calendar year following the date of expiry of the research (as extended, if extended, pursuant to the last sentence in clause 2.1 above), the total royalties payable by the Company to Yeda in respect of Net Sales of Products shall be less than six thousand (US $6,000) United States Dollars, the Company shall pay to Yeda, within thirty (30) days after the end of such calendar year, in addition to such royalties as aforesaid, the sum being the difference between six thousand (US $6,000) United States Dollars and such total royalties payable in such calendar year; and”
18. The rfTMS Inventors shall be considered “Scientists” under clauses 7.4.3, 10 and 12.1 of the R&L Agreement.
19. Unless set forth expressly herein, this Addendum is not intended to limit the scope of the License granted under the R&L Agreement that is not the subject matter of this Addendum.
20. The Company acknowledges that the rfTMS technology was developed, in whole or in part, utilizing funding from the “▇▇▇▇▇” program of the Israel Innovation Authority, and accordingly may be subject to restrictions on
overseas transfer, licensing and manufacture pursuant to the Encouragement of Research and Development Law of 1984 and the rules of the Israel Innovation Authority.
IN WITNESS WHEREOF, THE PARTIES HERETO HAVE SET THEIR SIGNATURES ON THE DATE FIRST MENTIONED ABOVE.
|
/s/ ▇▇▇ ▇▇▇▇▇▇-▇▇▇▇▇ |
|
|
|
/s/ Prof. Mudi Sheves |
|
/s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ |
|
YEDA RESEARCH AND |
|
BRAINSWAY, INC. |
|
DEVELOPMENT COMPANY LTD. |
|
|
Annex A(1) - rfTMS Patent Card
PATENT CARD
2009-014
Title: MAGNETIC CONFIGURATION AND TIMING SCHEME FOR TRANSCRANIAL MAGNETIC STIMULATION
Inventors: ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇
|
Country |
|
Application |
|
Publication |
|
Grant |
|
Status |
|
U.S.A |
|
02/03/2009 - 61/156,835 |
|
— |
|
— |
|
Expired |
|
PCT |
|
02/03/2010 - PCT/IL2010/000171 |
|
10/09/2010 - WO 2010/100643 |
|
— |
|
Expired |
|
European Patent Office |
|
— |
|
— |
|
— |
|
Pre-filing |
|
European Patent Office |
|
02/03/2010 - 10710911.8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Allowed |
|
France |
|
02/03/2010 - 10710911.8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Germany |
|
02/03/2010 - 10710911.8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Italy |
|
02/03/2010 - 10710911.8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Spain |
|
02/03/2010 - 10710911.8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
United Kingdom |
|
02/03/2010 - 10710911.8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Israel |
|
02/03/2010 - 214905 |
|
— |
|
214905 - 30/03/2017 |
|
Granted |
|
Israel |
|
02/03/2010 - 230414 |
|
— |
|
230414 - 30/03/2017 |
|
Granted |
|
Japan |
|
02/03/2010 - 2011-552573 |
|
23/08/2012 - 2012-519050 |
|
5688380 - 30/01/2015 |
|
Granted |
|
U.S.A |
|
02/03/2010 - 13/254,361 |
|
01/03/2012 - 2012-0053449 |
|
9,067,052 - 30/06/2015 |
|
Granted |
|
U.S.A |
|
02/03/2010 - 14/714,368 |
|
03/09/2015 - US-2015-0246238-A1 |
|
— |
|
Allowed |
Annex A(2) - rfTMS Know-How
In general:
The forces applied by magnetic pulses of magnitudes necessary for TMS are stretching the limit of material strength of the coils. This poses limits on achievable fields under safety restrictions. In addition, trial and error with magnetic coils is undesirable as it is often causes irreversible damage to the coil, often resulting in loss of valuable time and funding and in increased safety issues during R&D. Any know-how technology that can relieve this limitation fully or in part will improve the development process and the final performance of a TMS system. We possess such knowledge at multiple phases of system development:
Design:
Correct mutual Inductance between the two coils is crucial for controlling the forces within and between coils, as well as the resulting pulses waveforms. We have computed and measured the mutual inductance of many different configurations of double coils, and therefore have the knowledge and experience to predict a-priori which configuration can be successful and which may fail.
Fabrication:
After having fabricated numerous coils in-house we have the experience and knowledge of material choice and fabrication processes for magnetic coils that can safely withstand high fields (up to 10T) and the resulting forces. The possible angles that can be acceptable between the two coils are limited, and we have some experience with this.
Operation:
The waveforms and the resulting forces of magnetic pulses in the double coil are very sensitive to the triggering configuration used. After experimenting with numerous such temporal configurations we have the experience and knowledge to predict a-priori which configuration can be successful and which may fail. The issue of the third dimension is particularly important, since we rotate the field only in a plane.
In addition, we have experience and knowledge in correct interface between double coils and commercially available power supplies.
Annex B — Work Plan
Rotational field TMS (rfTMS) - Workplan
Brainsway and Yeda want to evaluate the rfTMS technology. The evaluation will be performed according to the current workplan as detailed below:
1. Brainsway submitted a Magneton application, accompanied by supporting documents provided by Yeda, to the Israel Innovation Authority / Rishut Lihadshanut (“IIA”), for the development of the rfTMS technology, in October 2017.
2. Yeda will procure that ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇ will conduct a clinical trial in major depression disorder (MDD) patients in Geha Mental Health Center (“Geha”). ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇ has already obtained Helsinki Committee approval for the study.
3. Yeda and ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇ will procure that the supervisor of the Clinical Trial at Geha shall submit, for approval, an amendment to the Helsinki Committee, in accordance with the following modifications:
a. The study will be performed with a deep TMS dual-coil array, that will be developed by Brainsway, and with a single H10 coil that will be supplied by Brainsway. Brainsway will also provide an EMG system for the performance of the study (all such devices to be used solely in the conduct of the study, and to be returned to Brainsway upon completion or termination of the Study, as applicable).
b. The following procedures will be added to the study protocol, which protocol is attached hereto as part of the work plan and will be performed as part of the clinical trial:
1. Leg motor threshold: Rotational field (RF) with dual deep TMS H10 coil vs. single H10 coil. The H10 coil is designed and was shown to stimulate effectively the leg motor cortex.
Hypothesis: We found in previous studies that the directionality in the leg motor cortex is low, and the motor threshold with postero-anterior and lateral-medial directions is similar. Hence we hypothesize lower threshold with use of RF dual coil due to greater population of relevant neurons with various orientations recruited by the RF dual stimulation.
Procedure: in each subject the leg motor threshold will be determined with the dual deep TMS H10 coil, with a H10 coil oriented along postero-anterior axis, and with a H10 coil oriented along lateral-medial axis.
2. Paired pulse LICI measurement in motor cortex: RF dual deep TMS H10 coil vs. single H10 coil.
Hypothesis: Greater degree of inhibition measured by EMG (motor) between coils due to greater proportion of relevant GABA neurons recruited by RF-dual coil.
Procedure: in each subject a LICI protocol will be applied with the dual deep TMS H10 coil and with a H10 coil, over the motor cortex. The long term intracortical inhibition (LICI) will be measured using EMG.
3. Plasticity measurements: Low (LF) and high frequency (HF) rTMS in motor cortex: RF dual deep TMS H10 coil vs. single H10 coil.
Hypothesis: Influence of greater degree of plasticity assessed in the following manner:
· for stimulation of the primary motor cortex, as a larger change in EMG signal (motor target)
Procedure: in each subject a session of LF rTMS (1 Hz) and a session of HF rTMS (10 Hz) will be applied with the dual deep TMS H10 coil and with a H10 coil, over the motor cortex. The motor cortex excitability will be measured using EMG following each session Each session will be applied on a separate day.
4. Brainsway will train the study operators in Geha in the performance of the procedures listed in #3.
Table 1: Timeline for Clinical Trial
|
Action |
|
Date |
|
Responsibility |
|
Submission of Magneton |
|
Oct ▇▇▇▇ |
|
▇▇▇▇▇▇▇▇▇ & Yeda |
|
File amendment to Geha Helsinki committee |
|
Feb 2018 |
|
Supervisor of clinical trial at Geha, procured by Yeda and ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇, and subject to Helsinki committee’s approval |
|
Complete development of a H10 dual-coil array, and install a H10 dual-coil array and a H10 coil at Geha |
|
Apr ▇▇▇▇ |
|
▇▇▇▇▇▇▇▇▇ |
|
Train operators in Geha to perform procedures added to the modified protocol |
|
Apr 2018 |
|
Brainsway |
|
|
|
|
|
|
|
Complete Clinical Trial as described herein |
|
Dec 2018 |
|
Yeda |
Table 2: Milestones
|
Milestone |
|
Date |
|
Demonstration of advantage of rfTMS over single coil TMS in leg motor threshold |
|
Dec 2018 |
|
Demonstration of advantage of rfTMS over single coil TMS in the degree of inhibition in LICI protocols |
|
Dec 2018 |
|
Demonstration of advantage of rfTMS over single coil TMS in neuroplastic changes following HF and LF rTMS sessions |
|
Dec 2018 |
The Geha Mental Health Center
Affiliated with Tel Aviv University
The Sackler Medical Faculty
Treatment of Depression with Repetitive Transcranial Magnetic Stimulation
Using a Rotating Magnetic Field
▇▇. ▇▇▇▇▇▇ ▇▇▇▇, Prof. Avi Weizman, Prof. Avi Valevski, Dr. Yuri Burnishev, ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇
— Research Proposal —
Version 3
Dated: December 3, 2017
1. Scientific background
Clinical depression is one of the most common mental disorders. The condition is characterized mostly by a combination of a number of symptoms such as pessimism, loss of interest or pleasure, major weight change or change in appetite, fatigue or loss of energy, sleep disorder, loss of concentration, feelings of guilt, thoughts of death, etc. that last at least two weeks. In various studies, the average incidence of this disorder worldwide is between 5% and 15% annually. This situation adversely affects many areas of a person’s life, such as family, work and studies, quality of life and general health. It can be a considerable burden on the health services and at the
same time can cause major functional damage, including loss of ability to work and alcoholism.
The most accepted treatments in situations of depression are currently therapeutic treatment and psychotherapeutic treatment (on their own or in combination). However, there are cases in which these treatments do not lead to the desired results and to the patient’s remission (1-3). Between 20% and 40% of patients do not respond to the existing therapeutic treatment or to a combination of therapeutic treatment and psychotherapeutic treatment (4).
In cases of persistent depression, patients who do not respond to the standard treatment occasionally switch to treatment with electro convulsive therapy. This treatment is considered the most effective (5) but is administered under general anesthetic, and apart from the risks of the anesthesia, it involves side effects, including a risk of developing cognitive disorders and permanent damage (6).
Transcranial Magnetic Stimulation (TMS) is a non-invasive method in which the nerve cells in specific regions of the brain can be stimulated. This method, which has been in use for 20 years, works by means of a coil into which flows a pulsating electrical current. During pulsation, the current in the coil causes electromagnetic induction that permeates into the brain and creates an electrical field that arouses the nerve cells in the brain in the region at which the coil is directed.
In the last few years, use has been made of Repetitive Transcranial Magentic Stimulation (rTMS) in the treatment of depression, whereby the TMS device is operated at a number of consecutive pulses at a certain frequency. This treatment is non-invasive, is administered when the patient is fully conscious, and has few side effects. In depressive patients with no psychotic component it is even as effective as the electro convulsive therapy (7). TMS has been approved by the FDA for major depressive disorder in patients who are not responsive to the standard treatment (8).
The assumption is that in a state of depression there is an asymmetry in brain activity in the frontal lobe with resultant hypoactivity in the left Dorsolateral Prefrontal Cortex (DLPFC) and hyperactivity in the right DLPFC. The DLPFC is TMS sensitive and synaptically connected to the limbic system associated with the regulation of mood.
The current most common protocols for treating depression using rTMS are: application of TMS to the left DLPFC at a high frequency of between 5-20Hz, a treatment that is thought to stimulate activity and on which the FDA (10-20Hz) protocol is based, or applying it at a lower frequency of 1Hz to the right DLPFC, a treatment that is thought to reduce activity. Two types of protocols have been found to be effective in the treatment of depression (9-10), however no advantage has been found in combining both options (11). The highest frequency protocol has a broader research base and has a better chance of being effective and was therefore chosen for this study. Meta-analyses have shown that the treatment with the highest frequency directed towards the left DLPFC is effective in the treatment of depression
in comparison with sham stimulation (12-14). The medical center that administers TMS treatments has examined the first hundred subjects who received the treatment after it was approved by the FDA and showed that TMS was indeed effective in the treatment of depression (15). Another study also showed an improvement with the more intensive treatment of twice a day for a total duration of only two weeks (16).
Another method for treating persistent depression is deep TMS in which the magnetic field created by the coil permeates deeper into the brain in comparison with the field created by the standard coils. The Israeli company Brainsway, which has developed a coil of this type, has shown that it is effective in the treatment of depression (17) and has also received FDA approval for the treatment of depression using this device (18).
Another location for the treatment of depression is the medial prefrontal cortex (MPFC). The Brainsway H7 coil is designed to stimulate this region and has shown good results in depressive patients (19).
However, the current application of TMS for research and treatment of brain disorders is still restricted by great differentiation, and it is difficult to obtain uniform brain reactions among the subjects. Recently conducted studies have shown that one of the reasons for this is the angular sensitivity of TMS.
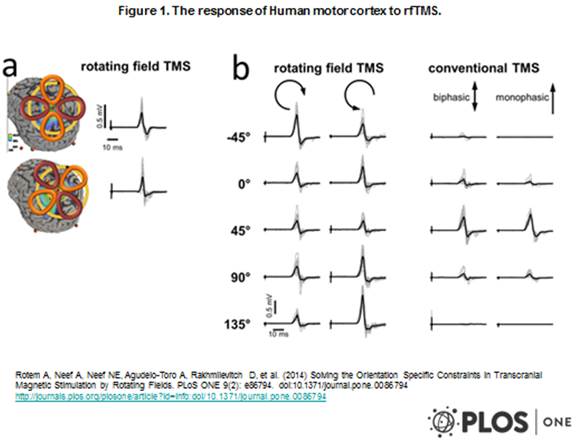
A new coil, the cloverleaf coil, has been developed by the Weizmann Institute and is specially adapted for the existing TMS system (20). This coil more effectively activates the region at which it is directed and thus improves the angular sensitivity of TMS. This improvement is made by rotating the magnetic field achieved by superposition of the fields of two coils in the common figure-of-eight configuration with a rotating field and a diameter of 79 mm located perpendicular to each other and operating with a phase time difference. Rotating field TMS facilitates the optimal location of the regions in the brain for which it is impossible to know the correct direction of the stimulation. The figure-of-eight coil used in most rTMS studies for the treatment of depression activates neurons with axons in only one specific direction parallel to the magnetic field, whereas the cloverleaf activates neurons with axons that go in all directions in a plane on which the magnetic field is exerted. A comparison can be seen in figure 1 from (20) between the angular sensitivity of both coils, expressed in a measurement of the thumb’s response after a pulse that activates it in the human motor cortex, which demonstrates the advantage the cloverleaf coil’s angular sensitivity has over the figure-of-eight coil (20). Unlike the deep TMS treatment that requires an original system, the cloverleaf coil can connect to standard TMS systems.
As previously explained, in the standard TMS treatments of depression, patients vary in their response to the treatment which may be due to the angular sensitivity. In this study we will therefore examine if use of this new technology which has been found
to be effective, can lead to a greater improvement in the treatment of depressive patients in comparison with treatment using the commonly used coil.
In order to test this, the study will be double blind, so that only at the end of the follow up will it be possible to make a connection between the patient’s details and their serial number used in the trial. The cloverleaf coil may also be operated in the single configuration of figure-of-eight, so that both the subject and the person conducting the trial cannot know in which trial conditions they are.
In addition and in parallel, the increased effect of rotating the magnetic field using a deep TMS device will be examined by expanding the configuration of the Brainsway H7 coil. The expansion of deep TMS into a rotating field is achieved in the identical fashion to the figure-of-eight coil, in other words a configuration of two coils perpendicular to each other and operated by a quarter turn of the power supply of one coil with respect to the H7 and Dual H7 devices independently.
The purpose of the study
Improvement in the symptoms of depression after treatment with rTMS using the cloverleaf coil (or Dual H7).
The study assumption
The treatment with the cloverleaf coil (or Dual H7) will be more effective than the treatment with the most commonly used coil, the figure-of-eight coil (or H7).
2. The study methodology
Subjects
Number of participants: 64. The number was determined on the basis of the results in (21), in order to reach a statistical power of 90% and one-tailed test alpha of 5% in favor of the cloverleaf coil or Dual H7. Based on a standard t test, the difference in effectiveness between the coils independent of frequency is expressed by a drop of two points more than for the figure-of-eight or H7 coil, respectively, indicating the HAM-D/17 after 20 treatments.
Recruitment
The subjects in the trial will be recruited from patients in one of the Geha’s hospitalization departments (inpatients or patients being monitored), from patients in the Geha outpatient clinics or from those referred to the ▇▇▇▇ ▇.▇. All the subjects will be interviewed and examined on their medical history so that only suitable subjects will be recruited.
Criteria
Inclusion: Every patient, male or female, in an age range of 18-75 who comes to be examined or monitored or for treatment in one of the Geha units, diagnosed as suffering from persistent depression according to DSM-5 (24), who failed in at least one other therapeutic treatment with antidepressants according to the criteria in B(22), with a current episode of at least three years, with a score of 18 or more in HAM-D/17, and capable of providing written informed consent to being included in the study.
Exclusion: Inability to sign an informed consent, subjects suffering from intellectual disorders or clear cognitive deterioration. Subjects who have had a guardian appointed, subjects who have been diagnosed as suffering from a psychotic condition; subjects diagnosed as suffering from epilepsy or who have a first-degree family member with known epilepsy; subjects with a pacemaker or with a metal/magnetic component in the head or near it; subjects who are taking medications that lower the threshold for an epileptic attack (23); subjects diagnosed as suffering from PTSD or an eating disorder in the last year; or pregnant women.
Withdrawal from the trial: At any stage during the trial, if the subject feels unwell during the treatment and asks to stop the trial; if the subject’s condition deteriorates between the tests; if in parallel to participation in the study, there is a significant change in the relevant permanent treatment for the disorder; or a subject who has not completed five treatments a week (at the discretion of the principal investigator).
Trial design
Under all trial conditions, the cloverleaf (or Dual H7) coil will be used and operated by two Magstim Rapid1 (Magstim Company Ltd., Wales, UK) devices specially adapted with an external control box. In this format it is possible to choose to operate the coil in a figure-of-eight (or H7) configuration by operating only one device, or in the form of a rotating field by operating both devices with a fixed time difference.
The TMS parameter
A standard calibration test will be conducted at the start of every treatment in order to determine the strength of the pulse. The strength will be determined according to the resting motor threshold which will be ascertained by delivering single pules to the left motor cortex, and gradually increasing the strength until a response of at least 50µv is measured by an electromyogram device (EMG) in the activity of the right thumb muscle, and by visual feedback. The coil will be placed over the cortex using a mechanical arm with the subject’s head kept in place with a chin and forehead rest. In any event, the strength will remain within the safety limits (23).
1
In high frequency conditions, the pulse power is set to 120% of the motor threshold power measured at the start of the meeting. The subject will receive 55 series of treatment at a frequency of 18Hz, at intervals of 2 seconds pulses and 20 seconds rest, directed at the left DLPFC or at the MPFC. The subject will receive a total of 1980 pulses during each meeting (21).
In low frequency conditions, the pulse power is set to 110% of the motor threshold power measured at the start of the meeting. The subject will receive four series of treatment at a frequency of 1Hz, at intervals of 180 second pulses and 30 seconds rest, directed at the right DLPFC or at the MPFC. The subject will receive a total of 720 pulses during each meeting (21).
The parameters in the FDA-approved protocol are: a pulse power of 120% of the motor threshold power, 75 series of treatment at a frequency of 10Hz at intervals of 4 seconds pulses and 26 seconds rest, directed at the left DLPFC. A total of 3000 pulses. The parameters in this research proposal can be seen to be less intensive than the parameters of the FDA protocol, in order to keep within the safety margin with the new coil.
3. The study protocol
The study will be conducted in the Geha Mental Health Center.
The subjects will come for four weeks of treatments, one treatment every day (Sunday thru Thursday) — a total of 20 meeting over a period of 4 weeks.
Before the trial begins, the subjects will sign an informed consent and will complete a standard demographic questionnaire.
At the start of every meeting, the patient’s motor threshold will be tested and then headphones will be put on them.
The responses to standard and correct operation of the rotating field in the motor regions will be checked in relation to the standard coil. Every Sunday in the first three weeks of treatment, one of the following tests will be conducted in combination with the EMG system.
1. A motor stimulation threshold will be established for the region operating the leg with a double coil as against two perpendicular states of directionality of the standard single coil — posterior-anterior and lateral-medial.
2. A double-pulse stimulation to create long-interval intracortical inhibition (LICI) in the motor region. A first pulse with a rotating field is expected to be more effective at stimulating inhibition due to multiple excitations of the GABA cells.
3. Measuring plasticity in the response to stimulation at frequencies of 1Hz and 10Hz in a series of stimuli with a double coil rotating field, the excitability of the motor cortex will be measured with an EMG, and will also be measured after the series.
In accordance with the trial conditions, the TMS coil will be placed on the subject over the left or right DLPFC for the entire treatment. The DLPFC will be located by: measuring 6 centimeters anterior from the motor point at which the hand is operated or by using an EEG in a 20/10 configuration, and locating the coil above the location of the 3F or 4F electrode depending on the side to be stimulated.
The subjects will be divided randomly into two equal groups. One trial group will receive treatment with the figure-of-eight (or H7) coil and the other trial group will receive treatment with the cloverleaf (or Dual H7) coil. Both groups will receive treatment at frequency of 18Hz directed at the left DLPFC or the MPFC. After ten treatments, the subjects who did not respond to treatment (a reduction of <25% or a score of at least 18 in HAM-D/17) will transfer to the ten additional treatments in the parallel group. Those who began treatment with the figure-of-eight-coil will transfer to treatments with the cloverleaf coil and those who began treatment with the cloverleaf coil will transfer to treatment with the figure-of-eight coil.
Subjects who experience the treatment with 10Hz as too deep or intensive, will be offered a transfer to a low frequency of 1Hz directed at the right DLPFC and considered more tolerant.
Division of the groups
The subjects will be randomly and equally divided between the two trial groups using computer software. The investigator who conducts the trial will be blind to the coil’s status, since, as previously explained, this coil can operate both in the figure-of-eight (or H7) configuration and in the cloverleaf (or Dual H7) configuration without it being possible to distinguish between them.
After ten meetings the blind will be removed from anyone who has not responded to the treatment and he will be transferred for treatment with the other coil.
Clinical questionnaires
The questionnaires will be completed before the first meeting and after the fifth, tenth, fifteenth, and last meeting and two weeks after the end of the trial.
· ▇▇▇▇▇▇▇▇ Depression Rating Scale-17 items (HAM-D/17)) — to rate the intensity of the depression.
· Quick Inventory of Depressive Symptomatology (QIDS) — self-report questionnaire to rate the intensity of the depression.
· Clinical Global Impression (CGI)
· ▇▇▇▇▇▇▇▇ Anxiety Rating Scale (HAM-A) — to rate the intensity of the anxiety
· ▇▇▇▇▇▇▇▇▇▇ Test for Nicotine Dependence (FTND) — to test for nicotine dependence among smokers
The main study variable: the intensity of the depression
The other study variables: the intensity of the anxiety and the characteristics of smoking
Statistical analysis
The data will be analyzed using Excel and Statistica software. The T, F and x2 tests to determine if the TMS treatment reduced the intensity of the depression, if there are significant differences between the coils, if there is an advantage to one paradigm over the other (18Hz compared with 1Hz), and if there has been an improvement after the transfer from one treatment to the other. Subjects who experience a reduction of more than 50% in the median in HAM-D/17 will be considered as responding to the treatments and a reduction to under a rating of 10 in HAM-D/17 will be considered as remission in the illness.
4. Safety
The new coil in effect comprises two 70 mm diameter figure-of-eight coils perpendicular to each other (used by Dual H7) with the new coil installed in both H7 coils. These coils are used in their single configuration for most of the trials in the treatment of depression, until now with no technical malfunctions. The new coil has been meticulously tested and has a mechanism to prevent malfunctions such as overheating or electrocution in operation, precisely as for the single coil. The time intervals between the subjects will be sufficiently long (at least an hour between patients) to prevent the coil overheating.
All the parameters in the trial are protected by safety lines in (23). These lines describe the maximum intensity and frequency during the treatment, and these are kept within the study’s safety range. The subjects will be able to stop or leave the trial at any time, as they decide and as they wish, without any threat or concern of any kind being applied. If a significant deterioration in the subject’s condition between meetings is diagnosed, he will be removed from the study. The subjects will arrive on the recommendation of the medical team, so that only subjects suitable for the trial will be recruited. The trial will be conducted in the hospital so that the medical team will be nearby at all times in the trial. The investigator will also be present beside the patient at all times during the trial and can stop the treatment and remove the coil from the subject immediately, if so required.
The risks and/or discomfort that may be caused to participants in the study: discomfort from sitting for 20 minutes while receiving the treatment and the time necessary to complete the questionnaire. After the treatment, some patients report tenderness in the region of the stimulus, ringing in the ears, and slight tingling in the face that passes spontaneously. In exceptional circumstances, the treatment may induce an epileptic seizure. An epileptic seizure is a condition defined as passing
symptoms of a disease that is the result of increase electrical activity in the brain nerve cells. The external effect can be severe, such as movements and kicks (tonic clonic seizure) or mild, such as a brief loss of consciousness. However, the induction of an epileptic seizure as a result of the treatment is an extremely rare event among healthy subjects and if it occurs, it will be described mainly among those who have a background of suffering from epilepsy (extremely rare at low frequency, and at high frequency found in 1.4% of subjects with a background of epilepsy, and less than 1% among subjects with no background of epilepsy (23). All the subjects will have an EEG before the trial begins to rule out any concern over epilepsy. If there is an epileptic seizure, there will be a doctor and a nursing team nearby trained to treat this condition and with rapid access to first-aid equipment and advanced treatment, including anti-convulsive medications.
Bibliography
1. Fava, M. (2002). The role of the serotonergic and noradrenergic neurotransmitter systems in the treatment of psychological and physical symptoms of depression. The Journal of clinical psychiatry, 64, 26-29.
2. Berlim, M. T., & Turecki, G. (2007). Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Canadian Journal of Psychiatry, 52(1), 46.
3. ▇▇▇▇▇▇▇▇, ▇. ▇. (2007). The burden of severe depression: a review of diagnostic challenges and treatment alternatives. Journal of psychiatric research, 41(3), 189-206.
4. ▇▇▇▇▇▇, ▇. ▇. (2001). The burden of recurrent depression: Causes, consequences, and future prospects. Journal of Clinical Psychiatry.
5. ▇▇▇▇, ▇. ▇., & ▇▇▇▇▇▇▇, ▇. ▇. (2009). Clinical issues in considering vagus nerve stimulation for treatment-resistant depression. Experimental neurology, 219(1), 36-43.
6. ▇▇▇▇▇▇▇▇▇, A., Bersani, F. S., ▇▇▇▇▇, E., ▇▇▇▇▇▇▇, R., ▇▇▇▇▇▇▇, C., Salviati, M,. ▇▇▇▇▇▇▇ Delle Chiaie, R., & ▇▇▇▇▇▇, M. (2012). ECT,
rTMS, and deepTMS in pharmacoresistant drug-free patients with unipolar depression: a comparative review. Neuropsychiatric disease and treatment, 8, 55-64.
7. Ren, J., Li, H., Palaniyappan, L., Liu, H., Wang, J., Li, C., & Rossini, P. M. (2014). Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 51, 181-189.
8. Melkersson, M. (2008). Special Premarket 510 (k) notification for neuroStar TMS Therapy System for Major depressive disorder. Food and Drug Administration.
9. Lefaucheur, J. P., André-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., ▇▇▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇, M., ▇▇▇▇▇▇▇▇, M. D., ▇▇▇▇▇▇, ▇. ▇., Devanne, H., ▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇▇, S. R., ▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇, G., ▇▇▇▇▇▇▇▇, B., ▇▇▇▇▇▇▇▇, T., Oliviero, A., Padberg, F., Poulet, E., Rossi, S., Rossini, P. M., ▇▇▇▇▇▇▇▇, ▇. ▇., & ▇▇▇▇▇▇▇▇▇▇-▇▇▇▇▇▇▇, C. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS).Clinical Neurophysiology, 125(11), 2150-2206.
10. Dell’Osso, B., ▇▇▇▇▇▇, L., Camuri, G., Dobrea, C., Cremaschi, L., Benatti, B., ... & ▇▇▇▇▇▇▇▇, ▇. ▇. (2015). Augmentative repetitive Transcranial Magnetic Stimulation (rTMS) in the acute treatment of poor responder depressed patients: A comparison study between high and low frequency stimulation.European Psychiatry, 30(2), 271-276.
11. Chen, J., Zhou, C., Wu, B., Wang, Y., Li, Q., Wei, Y., ... & Xie, P. (2013). Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry research, 210(3), 1260-1264.
12. ▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇, C., & ▇▇▇▇▇▇, ▇. ▇. (2006). Recent developments and current controversies in depression. The Lancet, 367(9505), 153-167.
13. ▇▇▇▇▇▇, ▇. ▇., Lloyd, S. W., Lux, L., Gartlehner, G., ▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇, S., ... & ▇▇▇▇, ▇. ▇. (2014). Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. The Journal of clinical psychiatry, 75(5), 477-489.
14. ▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇ ▇▇▇▇, J., ▇▇▇▇, ▇. ▇., & ▇▇▇▇▇▇, I. E. (2010). Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. Journal of Clinical Psychiatry, 71(7), 873.
15. onnolly, K. R., Helmer, A., ▇▇▇▇▇▇▇▇▇▇, M. A., ▇▇▇▇▇▇▇▇▇▇, P., & ▇’▇▇▇▇▇▇▇, ▇. ▇. (2012). Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. The Journal of clinical psychiatry, 73(4), e567-73.
16. ▇▇▇▇▇▇, A., Van den Eynde, F., ▇▇▇▇▇-▇▇▇▇▇▇▇, S., ▇▇▇▇▇, ▇. ▇., & Berlim, M. T. (2015). Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder: An open label trial. Journal of affective disorders, 173, 216-220.
17. Levkovitz, Y., Isserles, M., Padberg, F., ▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇▇▇▇▇▇▇▇, A., Xia, G., ... & Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64-73.
18. FDA 510(k) No. K122288
19. ▇▇▇▇▇▇▇, A., ▇. ▇▇▇▇▇, ▇. ▇▇▇▇▇▇, ▇. ▇▇▇▇▇▇, ▇. ▇▇▇▇▇▇▇▇▇, S. Methott and ▇. ▇▇▇▇▇ (2016). Case series of deep repetitive transcranial magnetic stimulation to the medial prefrontal and anterior cingulate cortices after H1 failure. In: American Psychiatric Association Annual Meeting, Atlanta.
20. Rotem, A., Neef, A., Neef, N. E., ▇▇▇▇▇▇▇-▇▇▇▇, A., Rakhmilevitch, D., ▇▇▇▇▇▇, W., & Moses, E. (2014). Solving the orientation specific constraints in transcranial magnetic stimulation by rotating fields. PloS one, 9(2), e86794.
21. ▇▇▇▇▇▇▇▇▇▇, ▇. ▇., ▇▇▇, K., ▇▇▇▇▇▇▇▇▇▇, ▇. ▇., & Kulkarni, J. (2009). A randomized trial of the anti-depressant effects of low-and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depression and anxiety, 26(3), 229-234.
22. Sackeim, H. A. (2001). The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry.
23. Rossi, S., ▇▇▇▇▇▇▇, M., ▇▇▇▇▇▇▇, P. M., & ▇▇▇▇▇▇▇-▇▇▇▇▇, A., The Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology, 120(12), 2008-2039.
24. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC.
|
|
Annex C — Magneton Application State of Israel - Economics Innovation Authority — Generic Technological Studies Administration |
|
Application for support for a Magneton project — 2017
|
Date of application filing October 17, 2016 |
1. Details of the company and the application
|
Company name: Moach Research and Development Services Ltd. | ||
|
Plant/Division: |
| |
|
The study institution: Weizmann Institute |
Faculty: Physics | |
|
Company number in the Registrar of Companies/Partnerships 513443788 |
Company no. in the Office of the Chief Scientist (if known) 5175 | |
|
Planned duration |
|
R&D period for this plan |
|
Time scale for the | ||
|
▇▇ |
|
▇▇▇. ▇▇, ▇▇▇▇ |
|
▇▇▇. 17, 2016 |
|
3.5 years |
|
Is this a new plan? x |
Yes ¨ |
No, state number of previous plan: |
Ending on: |
2. Subject of the plan:
Development of a rotational field TMS system (rfTMS) for the treatment of cerebral disorders
3. Key personnel in the plan
|
Position |
|
First name and |
|
Telephone |
|
Cellular |
|
Fax |
|
|
|
Project manager in the company |
|
▇▇▇▇▇▇▇ ▇▇▇▇ |
|
▇▇-▇▇▇▇▇▇▇ |
|
5665875-052 |
|
5812517-02 |
|
▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇.▇▇▇ |
|
Company contact person with the Office of the Chief Scientist |
|
▇▇▇▇▇ ▇▇▇▇ |
|
▇▇-▇▇▇▇▇▇▇ |
|
054-5699133 |
|
5812517-02 |
|
hadarl@brainsway. com |
|
Principal investigator from academia |
|
▇▇▇▇▇▇ ▇▇▇▇▇ |
|
08-9343139 |
|
9420866-054 |
|
9344109-08 |
|
elisha.moses@weizmann. ▇▇.▇▇ |
|
Joint principal investigator |
|
|
|
|
|
|
|
|
|
|
|
Research institution’s contact person with the Office of the Chief Scientist (Not the investigator) |
|
▇▇▇▇ Pesach |
|
08-9346050 |
|
3872598-052 |
|
9315927-08 |
|
▇▇▇▇.▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇.▇▇ |
4. R&D budget for the plan (NIS thousands) (up to NIS 3,400,000 for two years)
|
|
|
Current year of this |
|
Year A (if there was one) |
|
Year B |
|
Total years |
|
In the company |
|
840 |
|
|
|
600 |
|
1440 |
|
In the research institute |
|
280 |
|
|
|
200 |
|
480 |
|
Total for both bodies |
|
1120 |
|
|
|
800 |
|
11800 |
5. For a continuation plan: Use of budget for year A (as a %)
|
Estimate of budget use in |
|
Industry: |
|
Academia: |
|
Total: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6. Description of the application
Notes:
· A full explanation must be given for each sub-item. The space allotted for each response may be exceeded.
· If the subject is not relevant to the application being considered, state explicitly “Not relevant” — do not leave any sub-item unanswered.
· Annexes may be attached to the application, but they are not a substitute for completing this section of the application.
6.1. Managers’ summary in Hebrew (this part should be copied word for word to the expert opinion form and presented to the members of the GTSA committee
6.1.1. Description of the project (max. 20 lines)
Description of the “Magneton” plan — The main idea, the need, and the technology (challenges and technological differences, uniqueness, patents, competition), division of activities between the company and academia, the R&D program and the planned achievements for each year (on filing an application for year A, relate also to the activities of year B. On filing an application for year B, summarize performance against planning and achievements of year A). Description of the expected achievement at the end of the “Magneton”.
The brain rests in the hard and protected skull envelope that ▇▇▇▇▇▇▇ it from external interference and therefore medicine has difficulty performing non-invasive neurological activity. Transcranial Magnetic Stimulation (TMS) is a non-invasive technique that stimulates brain activity with short but powerful magnetic pulses. TMS overcomes the obstacle of the skull by defining a magnetic field outside the head which causes an electrical field in the brain. On the other hand, it is extremely sensitive to precise location on the head and because the electric field induced is created in a specific direction it stimulates only those nerve cells whose axon increases in the direction of the induced field.
There are many advantages of external, controlled stimulation, and it has been approved for clinical use. Clinical studies globally are currently examining this approach to a wide variety of brain disorders, including depression and bipolar disorder, schizophrenia, and autism, and implementation in conditions such as migraines or desire to stop smoking is extremely desirable. Therefore, in order to overcome the directionality limit of TMS and improve the effectiveness of brain stimulation a new type of coil has been developed in the Weizmann Institute with a cloverleaf configuration that overcomes the problem of directionality. The coil is adapted to the existing TMS system and more effectively activates the region to which it is directed, and this overcomes the problem of angular sensitivity of TMS. This improvement was made by rotating the magnetic field, an effect achieved by superposition of the fields of two coils in the common figure-of-eight configuration, located perpendicular to each other and operated with a phase time difference of 90 degrees. Rotating field TMS can be used at present to treat and to obtain the optimal placement in regions of the brain in which there is no preferred direction for the nerve cells, but their axons are spread out and distributed in all directions.
Brainsway has a special TMS coil that allows access to especially deep brain layers (deep TMS) and considerable experience in operating the coil in a fitted helmet with cooling for a large variety of brain and mental disorders. The purpose of this Magneton application is to produce a device that has all the advantages of Brainsway’s deep access and the Weizmann Institutes rotating field coil. To do this, special helmets will be built to contain double the coils and which will be operated by two separate power units with appropriate timing to move the phase though ninety degrees. The project includes building the helmet and the appropriate coils and characterizing the rotating field produced. This will be followed by a test of the effect of the field and the level of its effectiveness in reaching and
activating various regions in the brain. At an advanced stage, the efficacy of the treatment in a variety of disorders for which the efficacy of the Brainsway device (non-rotating) has already been proved.
6.1.2. The market, the commercial opportunity (max. 15 lines)
General description, size and rate of growth of the global market, competitive products, the business model, sales forecasts.
The global market for treatments of the central nervous system is estimated at 3 trillion dollars a year. Of this, the segment treating depression is approximately 150 million dollars a year. Approximately 240 million people in the world suffer from depression and another approximately 20% are at risk of developing severe depression during their lifetime. The depression segment is the main target market in the first stage in which the system can capture approximately 5% of the depression market, mainly due the limitations of existing medications (30% of patients are resistant to treatment using pharmaceuticals) and it is characterized by efficacy and speed of response to treatment, a low risk level, and fewer side effects.
Market size potential — There are estimated to be approximately 10 thousand mental health institutes in the target markets (United States, Europe and Japan) and another 100 thousand registered psychiatrists.
The market share / sales target for 2023 (million dollars): 2023 — 14, 2024 — 32, 2025 — 76
Competing products — the current edition of the rTMS system has limited ability to penetrate the magnetic field (up to 1-2 cm into the brain cortex) and the field induced has limited directionality and so mainly neurological structures with directionality parallel to the induced electrical field can be stimulated. This is in contrast with the innovative rfTMS technology which creates a rotating field so that neurological structures with different orientation will be stimulated. The rTMS systems cost 100 thousand dollars and a payment of 100-150 dollars for every treatment with the system. Business model — Research, development and technological innovation. Deep understanding of the neurological and psychological processes arising from irregular brain activities. The product will be produced and integrated by subcontractors. The product will be marketed in strategic cooperation with distribution and medical instrumentation manufacturing companies, and will focus on system sales, setting up treatment centers and sale of franchises.
The company obtained FDA approval in January 2013 to market the company’s product for depression in the United States. The company has also obtained Health Canada approval to market in Canada.
The company is marketing its products in the United States by itself and is in advanced negotiations with a number of bodies to market the product in other countries worldwide.
An exclusivity contract has been signed for a geographical region for a period of 10 years, subject to a minimum number of treatments, the device has been installed in treatment sites and payment during the initial period is based on a fixed monthly payment and thereafter a payment for the number of treatments conducted with the device or the fixed price whichever is higher. A remote monitoring system meters the treatments and the system makes variable charges for different applications. The company also allows a purchase model in certain circumstances.
The company has signed a contract with a high-tech company in Italy and Neuromagnetics in Chile as well as Brainsway Scandinavia AB in the Scandinavian countries and with CMI in Japan and Moksha8 in Mexico and Brazil. The company has received ANVISA approval to market in Brazil.
6.2. Abstract of a summarizing technical report (one page at most)
If the proposed plan is a continuation plan, give details of the development plan and the R&D period that has passed (abstract of a technical report), including:
* Achievements in the areas of development and marketing in the previous stages, including performance compared with planning
* Anomalies in performance in comparison with planning in R&D and marketing
* Changes in the development plan compared with the original plan [and] technological developments in the development plan
* Economic/marketing aspects that were clarified during the report period.
|
Hebrew |
English |
6.3. The product and the technology
6.3.1. The need and the product
* The need that the product fulfills and compatibility of the subject with the corporation’s operations.
* Describe the product from a functional aspect with reference to its performance, mode of use, and how the product integrates with other products (if required).
|
Hebrew |
English |
Most of the effort towards innovation in the operation of TMS focuses on achieving greater depth of penetration and a higher rate of operation, with the aim of more effectively activating deeper areas of the brain. This process is powered mainly by a hope of replacing the more invasive electroconvulsive method ECT but is a last resort in cases of depression that is unresponsive to medications. However, the strong directionality of the induced field has continued to be the main limitation of TMS at this stage.
The sensitivity to direction is the result of neurons in the target region in the brain only being activated if the induced electrical field is precisely aligned with their axons. Both the location and the direction of the stimulating magnetic coil must be adjusted with high resolution in order to activate the brain in the optimal location, and the magnet must be kept in position during the entire treatment. A stable and fixed location can be found using MRI in combination with a stereotactic device. However, it is completely impossible to determine the optimal orientation of the coil if stimulation of the focused brain region does not include axons lined up together in one bundle or if contrary to activating a muscle, there is no measurable response to activating the brain.
This is especially true of the prefrontal dorsolateral regions which are the main target in the treatment of depression. Moreover, in these regions of the brain there is no single unique direction that includes sufficient neurons, and so the ability of TMS to stimulate is limited. The entire region may also be less sensitive to stimulation using a directional coil. The ability of the rotating field to improve the directional sensitivity and to allow more effective TMS stimulation is therefore an important objective defined for the development of future magnets. The object of this Magneton application is to develop such a technology in conjunction with a Brainsway H-coil which has the ability deep to penetrate effectively into regions deep in the brain. The combined product will be able to rotate the magnetic field and apply stimulation to chains of neuron at a depth in the brain where the axons are oriented in a greater variety of different directions.
The final product is a system of a type routinely manufactured by Brainsway but with a helmet that includes a deep TMS type double coil with sufficient cooling for both coils so as to allow operation over a long period, and with two Magstim power supplies operated by moving a phase through 90 degrees relative to each other or 60 micro-seconds for a typical magnetic pulse.
6.3.2. Comparison with the current situation — in industry and academia (in Israel and worldwide), with the emphasis on state of the art.
|
Hebrew |
English |
This Magneton application provides a solution to a defined lack in the market in which there are currently no solutions to improve the effectiveness of TMS stimulation in regions of the brain that are important for therapy but do not have defined axon
directionality. The competition in the field comes mainly from companies employing different protocols with a standard device, for the most part a coil in a figure-of-eight configuration, which allows location of the activation in regions of the brain close to the skull, and which is effective for nerve cells with axons bundled together with defined directionality.
Another competitive field is of electric stimulation of the brain using electrodes implanted in it (DBS) or placed on the scalp (tDCS). The former has important clinical effects that have not yet been fully explained and mainly requires more invasive surgical intervention. The latter is problematic in that the physics of the transfer of currents and the results of the transfer are in dispute.
6.3.3. Detailed description of the R&D program
* The study plan and the division between participants (with the emphasis on the technology being transferred from academia to industry)
* A detailed explanation of the technological challenges and knowledge gaps
* The study group’s achievements in technological development up until the start of the project
* Adoption of the technology in an industrial corporation
* Continued development after the end of the “Magneton”
|
Hebrew |
English |
The R&D program is aimed at the development of rfTMS technology, to investigate the clinical repercussion of the innovative technology and to develop it for the product.
The study team in the Weizmann Institute under ▇▇▇▇. ▇▇▇▇▇▇ ▇▇▇▇▇ has developed the technology from an initial concept to feasibility testing, has conducted simulations, built a laboratory model, and performed tests to prove feasibility on nerve cell cultures, in animals and in preliminary trials in humans.
Objectives of the current plan:
a. Development of an advanced rfTMS system, including a dual-channel coil arrangement connected to a dual-channel stimulator with the possibility of precise timing.
b. Conduct of a clinical trial in depressive patients. The trial will include a number of components that will examine the capabilities of the new technology in comparison with existing rTMS technology. These components include:
1. Test of the efficacy of the treatment of depression
2. The effectiveness of motor stimulation of the arm and the leg
3. The effectiveness of inducing inhibition in the motor cortex using the LICI protocol
4. The effectiveness of creating neuroplastic changes in the motor cortex following high and low frequency treatment.
In Sections 2-4, measurements will be taken using an EMG system.
The technological challenges and the knowledge gaps: Planning, development and building the system, while treating with interaction between the coils in which a current is passed in different directions at the same time, collection of the neurophysiological indexes, identification of the indexes in which the innovative technology has a significant advantage.
At the end of the current program, the object will be to incorporate rfTMS technology in Brainsway’s products. The company is developing a deep TMS system, including a dual-channel stimulator. As part of this development, coil systems will be integrated to enable rfTMS in various regions of the brain.
After the end of the Magneton, wide scale clinical studies will be conducted to examine the safety and efficacy of a multi-channel system, including rfTMS, in their effect on various brain disorders.
6.3.4. The uniqueness and innovation of the product that will be developed (after the Magneton), including reference to the technological entry barriers to competitors who might seek to develop a competing product.
|
Hebrew |
English |
Brainsway is currently the world leader in the field of improved coils. The addition of the ability to rotate the coil, with the resultant increased stimulation will be a product that has no equal in the market.
It can be supposed that following the success that Brainsway has demonstrated in the use of the rfTMS rotating field, competitors will have a great interest in developing a similar product. The first obstacle will be legal, based on a patent that protects the use of the rfTMS rotating field in any configuration.
The second barrier is technological and is supported by the knowledge accumulated by the Weizmann Institute, for example with regard to the creation of a correlation between the coils and the prevention of mutual inductions that might cause the coils to collapse. Brainsway’s know-how in building a suitable helmet that will include two coils and will enable cooling and effective operation are also a technological barrier. It should be noted that these technological barriers can be overcome given enough time and with considerable engineering capability. There is therefore an advantage to first and early entry into the market, and to protecting the patents granted to the Weizmann Institute.
6.3.5. The characteristics of the product — a description of the product that will later be developed (after the “Magneton” and its characteristics and its incorporation in the target product in the company.
* Does the process / product come under any regulations with regard to environmental protection either in Israel or in the countries for which the product in development is intended. (Give details of regulations). If the question is not relevant to the product / the process being developed, it must be marked: Not relevant.
* What steps are being taken to ensure that the product / process will meet all the environmental protection standards? If the question is not relevant to the product / process being developed, it must be marked: Not relevant.
|
Hebrew |
English |
The system is intended for the treatment of patients with various brain disorders, such as depression, stroke, ▇▇▇▇▇▇▇▇▇’▇, Alzheimer’s, etc.
The rfTMs system will be integrated into a Brainsway multi-channel deep TMS system. The system will include coil systems intended to affect various regions of the brain. The system will combine the advantages of Brainsway deep TMs that enables timing of an effect on various regions of the brain and of rfTMS technology that enables the effect in defined regions of the brain to be dramatically increased by stimulation in multiple directions in the same region, with other areas being less affected, by limiting the stimulation there to a single direction. We expect that the aforementioned technological combination will provide enormous advantages from the aspect of the flexibility and efficacy of rTMS in their combined effect on various regions of the brain.
Aspect of environmental protection — not relevant.
6.3.6. Patents and intellectual property
* Is the technology being used protected by patents and/or other intellectual property rights? If so, give details.
* Give details of the distribution of the ownership of intellectual property rights between academia and industry.
* Has a patent review been conducted? Have the partners verified that the development does not infringe the intellectual property of others? How?
|
Hebrew |
English |
The Rotational Field TMS technology on which this project is based is protected by a family of patents discovered by the principal investigator, ▇▇▇▇▇▇ ▇▇▇▇▇, and the rights to them belong to Yeda, the Weizmann Institute’s trading company.
Brainsway and Yeda have reached agreements that will soon be anchored in a signed agreement to grant an exclusive license for the use of the IP in question.
The status of the family of patents is described below:
Title: Magnetic Configuration and Timing Scheme for Transcranial Magnetic Stimulation
Inventors: ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇▇
|
Country |
|
Application |
|
Publication |
|
Grant |
|
Status |
|
U.S.A |
|
02/03/2009 - 61/156.835 |
|
— |
|
— |
|
Expired |
|
PCT |
|
02/03/2010 - PCT/IL2010/000171 |
|
10/09/2010 - WO 2010/100643 |
|
— |
|
Expired |
|
European Patent Office |
|
— |
|
— |
|
— |
|
Pre-filing |
|
European Patent Office |
|
02/03/2010 - 10710911,8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Allowed |
|
France |
|
02/03/2010 - 10710911,8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Germany |
|
02/03/2010 - 10710911,8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Italy |
|
02/03/2010 - 10710911,8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Spain |
|
02/03/2010 - 10710911,8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
United Kingdom |
|
02/03/2010 - 10710911,8 |
|
18/01/2012 - 2 405 970 |
|
— |
|
Pre-filing |
|
Israel |
|
02/03/2010 - 214905 |
|
— |
|
214905 - 30/03/2017 |
|
Granted |
|
Israel |
|
02/03/2010 - 230414 |
|
— |
|
230414 - 30/03/2017 |
|
Granted |
|
Japan |
|
02/03/2010 - 2011-552573 |
|
23/08/2012 - 2012-519050 |
|
5688380 - 30/01/2015 |
|
Granted |
|
U.S.A |
|
02/03/2010 - 13/254.361 |
|
01/03/2012 - 2012-0053449 |
|
9.067.052 - 30/06/2015 |
|
Granted |
|
U.S.A |
|
02/03/2010 - 14/714.368 |
|
03/09/2015 - US-2015-0246238-A1 |
|
— |
|
Allowed |
6.3.7. Describe the points of technological uncertainty that are preventing the company from making a decision to enter immediately into a product development process.
|
# |
|
Technological uncertainty |
|
Actions to remove/reduce uncertainty |
|
1 |
|
Quantification of the ability of rfTMS to stimulate significantly more neurological structures in comparison with current rMTS with defined directionality and to create more effective motor stimulation |
|
A comparative study of the threshold of motor stimulation of the leg using rfTMS compared with the threshold with a single coil in a posterior-anterior direction and in a right-left direction |
|
2 |
|
Quantification of the ability of rfTMS to create significantly greater inhibition in comparison with current rMTS with defined directionality |
|
A comparative study of the effects on the motor cortex of the LICI protocol using rfTMS compared with a single coil |
|
3 |
|
Quantification of the ability of rfTMS to create significant neurological effects in comparison with current rMTS with defined directionality |
|
A comparative study of the effects on the motor cortex of 20-minute treatments at low frequency (that creates inhibition) and at high frequency (that creates facilitation) using rfTMS compared with a single coil |
|
4 |
|
Quantification of the effectiveness of rfTMS in a clinical improvement in patients with depression |
|
A comparative study of treatment of depressive patients for 4 weeks using rfTMS compared with a single coil |
6.4. The R&D program
6.4.1. Describe the abilities of the academic group, the capabilities of the company and of the development team relevant to this program — including previous R&D between the bodies, previous experience of academia-industry cooperation, the conduct of similar projects, the relevant personnel in the company for introducing the technology
|
Hebrew |
English |
The company’s direct workers include inventors of deep TMS technology who registered the patent while they were working in the American National Institute of Health (NIH), ▇▇▇▇. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇, the company’s neurobiology consultant, and ▇▇. ▇▇▇▇▇▇▇ ▇▇▇▇, the Chief Scientist. Our company has been granted exclusive use of said patent by the American National Institute of Health.
The leading team also includes the medical director, ▇▇. ▇▇▇▇ ▇▇▇▇▇▇▇, and Ahava ▇▇▇▇▇, Regulatory Affairs Consultant.
Brainsway has considerable experience in the development of TMS products. The company’s R&D department includes physicists, electronic engineers, mechanical engineers, software engineers, and biomedical engineers with vast experience in the development of such products. rfTMS technology intersects with the company’s developments and is at the core of its sector of operations.
The company has considerable experience of cooperation with academia, including partnership in a Magnet BSMT consortium and in international cooperation projects.
The laboratory in the Weizmann Institute has been engaged for 15 years in the study of the effect of electrical and magnetic fields on the activities of the nerve centers in various contexts, from cultures and neural networks growing in a test tube, by way of responses to stimulation in laboratory animals, to a study of stimulation in healthy and unhealthy humans. The laboratory is well equipped and experienced in the technological aspects of development and testing instrumentation and in trials of biological samples from humans through animals to tissue cultures. The combination of high-level experimental physics and innovative neurobiology characteristics of the laboratory is indeed unique. The laboratory is equipped to deal with tissues and care for animals, as well as for the development of new power supplies, optical microscopy techniques, and coil design.
6.4.2. Are there any differences in the aforesaid ability compared with the ability needed to develop the plan?
|
No |
No |
|
If yes, what are they and how does the company intend to eliminate these differences (such as subcontractors, acquisition of know-how), give details. |
|
Hebrew |
English |
6.4.3. Give details of the specific tasks that make up the work plan for the entire Magneton period and the resources required to carry them out (the tasks should be activities that end with deliverables. Avoid general descriptions such as: planning, execution, etc.). Give details of the activities (recommended up to 10 activities) for each year of R&D activities.
▇▇▇▇▇ chart: Prepare a detailed ▇▇▇▇▇ Chart for each “Magneton” period and submit it to the professional examiner during the working meeting on the plan.
|
# |
|
Activity/Task |
|
Responsible |
|
Task |
|
End date |
|
Human |
|
Total cost |
|
|
Year A |
|
|
|
|
|
|
|
|
|
|
| ||
|
1. |
|
Development of a dual-channel coil arrangement for rfTMS in the motor cortex and in the prefrontal cortex |
|
Brainsway |
|
8 |
|
May 15, 2018 |
|
0,85 |
|
680 |
|
|
2. |
|
Development of a synchronization system between two stimulators and adjustment between two coils for rfTMS study purposes |
|
Weizmann Institute |
|
8 |
|
May 15, 2018 |
|
|
|
120 |
|
|
3. |
|
Completion of preparations and beginning of a clinical study of rfTMS in depressive patients |
|
Weizmann Institute and Brainsway |
|
8 |
|
May 15, 2018 |
|
0,15 |
|
140 |
|
|
4. |
|
Clinical study of rfTMS in depressive patients |
|
Weizmann Institute and Brainsway |
|
4 |
|
October 15, 2018 |
|
0,1 |
|
180 |
|
|
5. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year B |
|
|
|
|
|
|
|
|
|
|
| ||
|
1. |
|
Conduct and completion of clinical study of rfTMS in depressive patients |
|
Weizmann Institute and Brainsway |
|
12 |
|
October 15, 2019 |
|
|
|
800 |
|
|
2. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. |
|
|
|
|
|
|
|
|
|
|
|
|
|
6.4.4. For each of the tasks in the above list describe the activities required to achieve them at a level of detail that will allow a professional examiner to judge the reasonableness of the amount of resources required: human years and overall cost of a task. Give details of all elements of the activities in the company and in academia.
|
Year A |
|
|
|
|
|
|
|
|
|
|
| ||
|
1. |
|
The task includes planning the system, mechanical planning, thermal planning, constructing a prototype, integration, testing and validation, regulation and standardization, running-in and implementation. The required objective is to build a dual-channel coil setup to operate rfTMS protocols. The main challenges: 1. The current pulses in both coils overlap at some time during the pulse. The current in one pulse creates forces on the other coil and so meticulous mechanical planning is required to ensure the strength necessary to carry out the protocols. Extensive operating tests are also required to ensure that the mechanical strength meets the requirements. 2. Current in one coil induces a current in the other coil. This will need to be taken into consideration when planning the coils. We will measure the current in various states and at various strengths and also take motor threshold measurements, and we will make adjustments so that the effect will be the required effect for each protocol. 3. Cooling The coils will be positioned one above the other. Both coils, including the lower one, must be kept at a normal temperature while the protocol is being conducted. To this end we will need detailed thermal planning, planning and constructing of the casings and the airflow directions so as to ensure efficient cooling, and thorough thermic testing while operating the various protocols. 4. Harnessing: The coil setup will need to be applied to the prefrontal cortex for the treatment of depression. It will also need to be applied to the leg motor cortex and to the arm motor cortex to [measure] the neurophysiological indexes aimed at examining the technological advantages. | |||||||||||
|
2. |
|
Planning the system, planning hardware, organizing software, mechanical planning, software writing, | |||||||||||
|
|
|
building a prototype, integration, testing, validation, regulation and standardization, running in an implementation. This task is being performed by the Weizmann Institute. |
|
3. |
|
The task includes regulatory activities, installation, preliminary training, training treatment providers, recruiting patients, monitoring and technical support. Helsinki Committee approval will need to be obtained, there will need to be involvement in writing the protocol, the system will need to be installed at the site, and the site operators will need to be trained. This task is being undertaken jointly by Brainsway and the Weizmann Institute. |
|
4. |
|
The task includes recruiting patients, conducting the trial, monitoring, technical support and advising the trial, while ensuring that the procedures are being followed as required. This task is being undertaken jointly by Brainsway and the Weizmann Institute. |
|
5. |
|
|
|
Year B | ||
|
1. |
|
Recruiting patients, monitoring, regulatory activities, technical support, analyzing the results |
|
2. |
|
|
|
3. |
|
|
|
4. |
|
|
|
5. |
|
|
|
|
|
End of the period of the R&D plan (in “Magneton”) |
6.4.5. Give details of measurable milestones for the entire plan (such as: outcomes, interim products, or clear engineering achievements). Include at least two milestones in each year of operations.
|
# |
|
Activities/Milestones |
|
End date |
|
Description of the achievement at the milestone |
|
Year A |
|
|
|
| ||
|
1. |
|
Completing the rfTMS system, including the array of coils and the dual-channel system |
|
May 15, 2018 |
|
Completing the system that will allow rfTMS to be created in the motor cortex and the prefrontal cortex |
|
2. |
|
Beginning of the rfTMS study in depressive patients |
|
May 15, 2018 |
|
Start of a clinical study of rfTMS technology |
|
3. |
|
|
|
|
|
|
|
Year B |
|
|
|
| ||
|
4. |
|
Completion of the rfTMS study in depressive patients |
|
October 15, 2019 |
|
End of the clinical study |
|
5. |
|
Analysis of the study results |
|
October 15, 2019 |
|
An analysis of the results and quantification of the technological advantages compared with existing technology |
|
6. |
|
|
|
|
|
|
6.5. The market
6.5.1. Define the target market for the future product in Israel and abroad, the existing market segments (customers with similar characteristics), the market distribution on a geographical basis, and the market dynamics.
|
Hebrew |
English |
Large market potential in the target markets (United States, Europe and Japan) estimated at 10 thousand mental health institutions and another 100 thousand registered psychiatrists. 240 million people out of the entire world population suffer from depression and 20% are at risk of developing severe depression at some stage in their lives. The depressive segment is the largest of all CNS sufferers and that is the main target market in the first stage of launching the project, although the device’s ability to stimulate deep regions of the brain opens an extremely large variety of additional applications.
Various addictions, including drugs, smoking, alcohol — more than 48 million Americans suffer from various addictions. The annual cost of treatment is estimated at 360 billion dollars.
Obesity — 80 million Americans suffer from obesity. The annual cost of treatment is estimated at 220 billion dollars.
Autism — More than a million Americans suffer from autism. The annual cost of treatment is estimated at 100 billion dollars.
Alzheimer’s and MCI more than 17 million Americans suffer from Alzheimer’s or MCI. The annual cost of treatment is estimated at 180 billion dollars.
PTSD — More than five million Americans suffer from PTSD. The annual cost of treatment is estimated at 10 billion dollars.
OCD — The fourth most common mental illness. One in every 50 adults in the United States suffers from OCD. The market in the treatment of OCD was estimated at 700 million dollars in 2010.
ADHD — 3% to 5% of children worldwide suffer from the syndrome. The market in treatments of ADHD is estimated at 3.85 billion dollars.
As previously stated, the product we are developing is an effective solution that is currently unavailable for the treatment of brain disorders and especially the problem of depression. The total world market is ready to adopt this innovative product with the main customers being: medical institutions and hospitals also treating brain disorders, neurology clinics, psychiatric centers, independent psychiatrists (private clinics), etc.
6.5.2. What is the current annual size of the market for the product in Israel and worldwide in units and dollars? What is potential global annual size for the future product? (State the source of the data). What is the growth potential for the entire market and for the product under developed? (State the source of the data).
|
Hebrew |
English |
The global market for Central Nervous System (CNS) treatments is estimated at 3 trillion dollars a year. Of this the depression treatment segment in 150 billion dollars a year. There is currently no identical product on the market.
Our business plan indicates rentals and sales of the systems for the following sums (million dollars)
From 2023: 2023 — 14, 2024 — 32, 2025 — 76
Data on that subject is published in various professional journals and the sources on which they rely are:
Espicom Business Intelligence
US National Mental Information Center, European Board of Psychiatry
6.5.3. Competition — describe the competing products and competing companies. Include the website addresses of those companies, if known. Give details of the relative advantages of the company compared with the competitors and its basis.
|
Hebrew |
English |
|
Manufacturer’s |
|
Name of the |
|
Price in $ |
|
Market |
|
Capabilities, performance, advantages and |
|
Magstim |
|
Rapid2 |
|
|
|
|
|
The main products are stimulators, as well as standard coils, and the system is inferior in comparison to ours. They received FDA approval for depression in 2015. |
|
Neuronetics |
|
Neurostar |
|
|
|
|
|
Self-production of stimulators and standard coil, including ferromagnetic core. They received FDA approval for depression in 2018. They market in the USA. |
|
MagVenture |
|
MagPro |
|
|
|
|
|
Manufactures stimulators and standard TMS coils, as well as production of stimulators and coils for magnetic seizure therapy (MST). They received FDA approval for depression in 2015. |
|
Neurosoft |
|
|
|
|
|
|
|
Manufactures stimulators and standard TMS coils. They received FDA approval for depression in 2016 |
|
Nexstim |
|
|
|
|
|
|
|
Manufactures stimulators and standard TMS coils, as well as systems for neuronavigation of the head. |
|
Cyberonics |
|
|
|
|
|
|
|
Manufacture of vagus nerve (VNS) stimulation for the treatment of epilepsy and depression. The treatment is invasive and involves surgery under general anesthetic. |
|
Medtronic |
|
|
|
|
|
|
|
Manufacture of deep brain stimulation (DBS) systems for the treatment of ▇▇▇▇▇▇▇▇▇’▇, depression and OCD. The treatment is invasive and involves brain surgery, implantation of electrodes deep in the brain, and anesthesia). |
|
St. Jude |
|
|
|
|
|
|
|
Manufacture of deep brain stimulation (DBS) systems for the treatment of ▇▇▇▇▇▇▇▇▇’▇ and depression. The treatment is invasive and involves brain surgery, implantation of electrodes deep in the brain, and anesthesia). |
6.5.4. Who are the potential customers for the product(s) at the subject of the plan (if there are any, state who they are)? Are these new or existing customers?
|
Hebrew |
English |
The main potential customers worldwide are:
a. Hospitals with departments treating brain problems, particularly in the area of depression
b. Psychiatric centers
c. Independent psychiatrists (private clinics)
d. Other medical institutions treating various kinds of brain problems, such as severe cigarette and drug addiction
Schizophrenia, Alzheimer’s, CVA, autism, PTSD, stroke, and ▇▇▇▇▇▇▇▇▇’▇
e. Universities and research institutes
f. Research laboratories
g. Local, regional and global distributors of medical devices
The products that are the subject of the plan will be marketed to both existing and new customers.
6.5.5. Marketing obstacles (such as: licensing, standards etc.) and how the company intends to deal with them.
|
Hebrew |
English |
We are aware of possible obstacles that may arise in the medical systems market, the main ones being:
a. Delay in obtaining regulatory approvals that may delay the development process
b. Changes in regulation, in permits, and in international standardization
c. Breach of rights to registered payments requiring legal action or market waiver
d. Delays in completing the clinical trials necessary for the receipt of regulatory approvals
e. Uncertainty of developing additional applications
f. Unexpected competition from other technologies
We have been careful in our business plan to build in a reasonable reserve both in terms of timetables and performance of the necessary tasks, and unexpected costs resulting from these obstacles or others.
6.6. Summary: Risks and opportunities
Analyze the risks and opportunities facing the future product from both the technological and marketing aspects.
|
Hebrew |
English |
|
The risk |
|
The risk management |
|
In the area of technology — a risk of competition from similar products worldwide |
|
Low risks since the technology we have is protected by a patent registered by the Weizmann Institute. The incorporated products will also be protected by the Brainsway deep TMS patents. The company is the only one in the world developing, manufacturing and marketing deep TMS products. |
|
In the area of regulation — the company estimates that there are risks in this area and most of them will lead to delays in the timetables |
|
It does not appear that there will be any risks of demands that the company will not be able to meet at this time. The company is operating according to all the standards and ensures that all the processes meet the regulatory demands. |
|
In the area of marketing |
|
The area of professional medicine dealing with brain disorders needs the product we are developing. As we have previously demonstrated, the existing alternatives are far from the efficacy and effectiveness that our product will provide. |
We estimate that Brainsway’s integrated product including the rfTMS technology will open new horizons in the area of brain stimulations and the treatment of brain disorders. Brainsway will become a main world player in the area of a medical device for the treatment of brain disorders and depression in the first stage and later of various psychiatric and neurological disorders. The product that is the subject of the plan will become a main tool in the treatment of various brain disorders and in brain research.
6.7. Concomitant activities:
|
6.1 Has the project been previously submitted for support of any kind from the Office of the Chief Scientist? |
No |
|
6.2 Is the project part of another, funded project |
No |
|
6.3 Is the prior academic research being funded by the company |
No |
|
6.4 Is the prior academic research being funded by any other government source |
Yes |
|
If so, give details: ▇▇▇▇▇ |
|
7. Declaration for industry
I hereby declare that the information in this application, apart from academia’s information, is to the best of our knowledge the correct, most up-to-date and complete information the company has and known to me personally and that I must notify the Office of the Chief Scientist of any new information the company may subsequently have and known to me personally and which could affect the product being developed from any aspect whatsoever.
8. Declaration for academia
|
Date |
|
Signatory’s |
|
Signatory’s |
|
I.D. No. |
|
Signature and |
|
October 16, 2017 |
|
Company CEO / Vice CEO |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇▇ |
|
014404677 |
|
|
|
October 16, 2017 |
|
One of the plan’s leaders in the company |
|
▇▇▇▇▇▇▇ ▇▇▇▇ |
|
024698631 |
|
|
I hereby declare that the information in this application relating to the activities of academia is to the best of my knowledge the correct, most up-to-date and complete information the implementation company has and known to me personally and that I must notify the Office of the Chief Scientist of any new information the research institute may subsequently have and known to me personally and which could affect the product being developed from any aspect whatsoever.
|
Date |
|
Signatory’s |
|
Signatory’s |
|
I.D. No. |
|
Signature and |
|
October 16, 2017 |
|
CEO of the implementation company (obligatory) |
|
▇▇▇ ▇▇▇▇▇▇-▇▇▇▇ |
|
012263893 |
|
[signature] |
|
October 16, 2017 |
|
The principal investigator (obligatory) |
|
▇▇▇▇▇▇ ▇▇▇▇▇ |
|
079790812 |
|
[signature] |
Annex D: rfTMS Development Program (including Milestones)
Development program for rotational field TMS (rfTMS)
|
Milestone |
|
Details |
|
Completion by |
|
Integrate rfTMS in a Multi-channel TMS system for clinical research |
|
Development of Multi-channel TMS system of up to 5 channels including rfTMS for clinical trial. |
|
Dec 2019 |
|
Safety and feasibility clinical trial in humans |
|
Perform a clinical trial using multi-channel TMS system including rfTMS. The study will test safety of operation with parameters defined based on previous stages. |
|
Mar 2021 |
|
Advanced safety and efficacy clinical trial in humans |
|
Advanced clinical trial using multi-channel TMS system including rfTMS. The study will test safety and efficacy of improving features of neural effects in various measures. |
|
Oct 2022 |
|
First Commercial Sale of an rfTMS Product |
|
— |
|
Apr 2023 |