LICENSE AGREEMENT Between VG Life Sciences, Inc. and Scott & White Healthcare PATENT LICENSE AGREEMENT NO. SW11-003PLA
Exhibit 10.115
LICENSE AGREEMENT
Between
and
Xxxxx & White Healthcare
PATENT LICENSE AGREEMENT NO. SW11-003PLA
This agreement ("Agreement") is made between VG Life Sciences, Inc., a Delaware corporation with principal offices in San Marino, California, ("LICENSEE") and Xxxxx & White Healthcare, a Texas non profit corporation, with offices located at 0000 Xxxxx 00xx Xxxxxx, Xxxxxx, XX 00000, on behalf of itself and its Affiliates within its organizational structure ("S&W"), collectively referred to as "Parties" and individually as "Party.''
| 1 |
| 1.1 | "AFFILIATE" or "AFFILIATES" means any entity that directly or indirectly owns or controls, is owned or controlled by, or under common ownership or control with a Party, wherein "owns" as used in this definition means possession or the right to possession of at least 50% of the voting stock of a corporation; and "controls" as used in this definition means (i) the power to direct the management and policies of the entity or (ii) the power to appoint or remove a majority of the board of directors; or (iii) the right to receive 50% or more of the profits or earnings. |
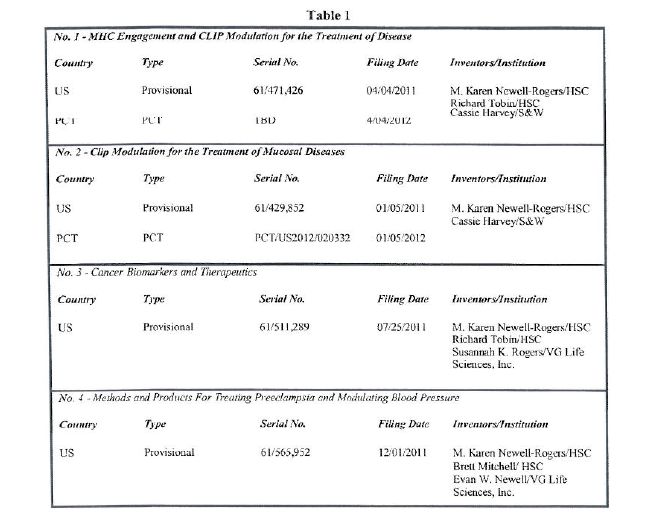
| 1.2 | "PATENT RIGHTS"··means S&W's and SYSTEM's rights in Table 1 in this Paragraph 1.2, all U.S., PCT and foreign patent applications claiming priority thereto, all patents issuing therefrom, reissues, reexaminations and any extensions of or supplementary protection certificates allowed on any of the foregoing, in each case only to the extent of subject matter claimed that is fully disclosed and enabled by the disclosures in the patent applications below to satisfy 35 U.S.C. §112: |
| 1.3 | "PROPRIETARY INFORMATION" means any know-how and materials disclosed to Parties by Xx. Xxxxxx between March 20, 2010 and the Effective Date regarding the Patent Rights. |
| 1.4 | "LICENSED PRODUCT" or "LICENSED PRODUCTS" means any product, process, or composition of matter that is within the scope of any VALID CLAIM of PATENT RIGHTS. |
| 2 |
| 1.5 | "VALID CLAIM" means and includes a claim of a patent application or an unexpired patent or a patent whose expiration date has been extended by law, so long as the claim has not been held invalid and/or unenforceable in an unappealable decision of a court or other authority of competent jurisdiction. |
| 1.6 | "EFFECTIVE DATE.. means the date this Agreement has been executed by the last Party. |
| 1.7 | '"NET SALES" means LICENSEE's and SUBLICENSEE's receipts for sales of LICENSED PRODUCTS or for services requiring the use of LICENSED PRODUCTS less the sum of the following: |
| (a) | sales taxes, tariffs, duties and/or use taxes directly imposed with reference to particular sales; |
| (b) | outbound transportation prepaid or allowed; and |
| (c) | amounts allowed or credited on returns. |
Commissions paid to individuals, whether independent sales agents or regularly employed by LICENSEE, and the cost of collections may not be deducted from NET SALES.
| 1.8 | "FIELD OF USE" means all uses. |
| 1.9 | "TERRITORY" means world-wide. |
| 1.10 | "LICENSEE" means VG Life Sciences, Inc. and any entity in which VG Life Sciences, Inc. has an equity ownership share at least seventy five percent (75%). |
| 1.11 | "SUBLICENSEE" means any third party sublicensed by LICENSEE to make, have made, use, have used, sell, have sold, import, have imported, exported, or have exported LICENSED TECHNOLOGY. |
| 1.12 | "LICENSED TECHNOLOGY" means technology that falls within the scope of any VALID CLAIM of PATENT RIGHTS or any PROPRIETARY INFORMATION. |
| 2.1 | Grant. S&W grants LICENSEE an exclusive license under PATENT RIGHTS to make, have made, use. and sell the LICENSED PRODUCTS in the FIELD OF USE in the TERRITORY, and to grant sublicenses of the same scope, to the end of the term of this Agreement as prescribed in Article VIII. |
| 2.2 | Reservation. S&W on behalf of itself and SYSTEM hereby reserves an irrevocable, nonexclusive, royalty-free right to practice the grant made in paragraph 2.1 for research, humanitarian, and educational purposes only, and to grant sublicenses of the same scope, and not for commercial purposes or for the commercial benefit of third parties. |
| 2.3 | Government Reservation. To the extent that rights under this Agreement are subject to rights required to be granted to the Government of the United States of America under 35 USC Sections 200-212, such rights are so granted including a nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for the United States the subject inventions throughout the world. |
| 3 |
| 3.1 | License Fee. In consideration for the license granted in this Agreement, LICENSEE must make an initial payment in the amount fifty thousand dollars ($50,000) to S&W. This payment is due no later than sixty (60) days after the EFFECTIVE DATE. Failure to make this payment within the specified period will cause this Agreement to immediately terminate. |
| 3.2 | Royalty Rate. As additional consideration for the license granted in this Agreement, LICENSEE must remit to S&W a royalty of three percent (3%) of NET SALES in developed countries and one half percent (0.5%) of NET SALES in underdeveloped countries. Underdeveloped countries shall mean any country which, at the time the sale occurs, meets the definition of a developing country as defined by the World Bank. Developed countries shall mean all countries other than underdeveloped countries as defined by the World Bank. LICENSEE may not accept anything of value in lieu of money payment without the express written permission of S&W. When calculating NET SALES, S&W may assign fair market value based upon comparable sales to receipts from transactions that are not made at fair market value. |
| 3.3 | No Multiple Royalties. No multiple royalties shall be payable because any LICENSED PRODUCT is covered by more than one of the PATENT RIGHTS. |
| 3.4 | Reduction in Royalty Rate. In the event that LICENSEE must enter into a license with a third party and agrees to pay a royalty thereunder in order to make, use, or sell a LICENSED PRODUCT or sublicense PATENT RIGHTS, then any such royalty shall be reduced by fifty percent (50%) of the royalty paid to said third party for the same reporting period. However, in no event shall any such royalty be less than one half the otherwise applicable royalties. |
| 3.5 | Minimum Annual Consideration. In order to maintain this license to PATENT RIGHTS, LICENSEE must pay S&W minimum annual consideration according to the following schedule: |
| (a) Calendar Year 2013, payable January 1,2014 | 20,000 | |
| (b) Calendar Year 2014, payable January 1, 2015 | 40,000 | |
| (c) Calendar Year 2015, payable January 1, 2016 | 70,000 | |
| (d) Calendar Year 2016, payable January 1, 2017 | 100,000 | |
| (e) Calendar Year 2017, payable January 1, 2018 | 150,000 | |
| (f) Calendar Year 2018 (payable January I , 2019) | ||
| and each year thereafter (payable each January 1st) | ||
| through the expiration of this Agreement | $200,000 |
In the event that LICENSEE's payment of royalties for the Calendar Year due under paragraph 3.2 do not meet or exceed the required minimum annual consideration, LICENSEE's royalty payment for the last quarter of the Calendar Year must include payment of the balance needed to achieve the required minimum. If this Agreement expires or is terminated before the end of a Calendar Year, the corresponding minimum annual consideration must be prorated for that year.
| 3.6 | Milestone Payments. In order to maintain this license to PATENT RIGHTS, LICENSEE must pay S&W milestone payments according to the following schedule: |
| (a) | Upon successful conclusion of each Phase I clinical trial for each Licensed Product/Indication: Milestone payment of one hundred thousand dollars ($100,000) or the amount paid by a SUBLICENSEE per Article IV, Paragraph 4.2, whichever is higher. |
| 4 |
| (b) | Upon successful conclusion of each Phase Ill clinical trial or any other clinical trial following a Phase II clinical trial for each Licensed Product/indication: Milestone payment of five hundred thousand dollars ($500,000) or the amount paid by a SUBLICENSEE per Article IV, Paragraph 4.2, whichever is higher. |
| (c) | Upon each regulatory/market approval on each Licensed Product/indication: Milestone payment of two million dollars ($2,000,000) or the amount paid by a SUBLICENSEE per Article IV, Paragraph 4.2, whichever is higher. |
| 4.1 | Sublicenses. LICENSEE may grant sublicenses to persons, firms, or corporations under conditions consistent with this Agreement as long as each sublicense is not repugnant to the mission of S&W or the public policies of SYSTEM, the State of Texas, or the United States. |
| 4.2 | SUBLICENSEE Consideration. Sales of LICENSED PRODUCTS by each SUBLICENSEE will be subject to the unit royalty due to S&W prescribed in paragraph 3.2. Further, LICENSEE must pay S&W for other considerations not in the form of royalty received by LICENSEE from each sublicense for a grant of rights in PATENT RIGHTS in accordance with the following Schedule 1: |
Schedule 1
| Effective Date of Sublicense | Percentage of Other Sublicense Consideration due to S&W |
| Within the first year of the Effective date of this Agreement | 50% |
| Within the second year of the Effective Date of this Agreement | 35% |
| Within the third year of the Effective Date of this Agreement | 35% |
| Any year following the third year of the Effective Date of this Agreement | 20% |
In the event that LICENSEE is required to pay a percentage of Sublicense Consideration to one or more third parties in order to produce and/or sell the LICENSED PRODUCTS, the running royalty rate prescribed above shall be reduced in accordance with the following Schedule 2:
Schedule 2
| Effective Date of Sublicense | Percentage of Other Sublicense Consideration due to third parties | Percentage of Other Sublicense Consideration due to SYSTEM | Percentage of Other Sublicense Consideration due to LICENSEE |
| Within the first year of the Effective date of this Agreement | 35% | 15% | 50% |
| Within the second and third year of the Effective Date of this Agreement | 20% | 20% | 60% |
| Any year following the third year of the Effective Date of this Agreement | 20% | 15% | 65% |
Notwithstanding the foregoing, and as the only exception, LICENSEE may not be required to remit to S&W any portion of funds it receives from any SUBLICENSEE(s) when the funds are documented in writing as payments for the following purposes: (i) research, development, or testing of LICENSED PRODUCTS, or (ii) patent expenses for protection of PATENT RIGHTS to which the SUBLICENSEE is contributing.
| 5 |
| 4.3 | Reporting. LICENSEE must notify S&W of the grant of sublicense to a third party and must provide S&W with copies of each sublicense and of each SUBLICENSEE's report as is pertinent to calculation of amounts due S&W under this Agreement. |
| 4.4 | Non-Cash Transactions. LICENSEE may not accept anything of value in lieu of money payment under a sublicense without the express written permission of S&W. |
ARTICLE V - LICENSEE RESPONSIBILITIES
| 5.1 | Patent Expenses: Past Expenses and Future Costs. Licensee is responsible for its documented expenses incurred prior to the EFFECTIVE DATE in the prosecution and maintenance of PATENT RIGHTS, and will be directly responsible for such future expenses beyond the EFFECTIVE DATE as further described in Article VI. |
| 5.2 | Milestones. In accomplishing the commercialization under this Agreement, LICENSEE must achieve the following milestones to the satisfaction of S&W: |
| (a) | LICENSEE shall submit a Business Plan to S&W on or before 9/18/13. The Business Plan must, at a minimum, include LICENSEE's plans for the development and commercialization of Licensed Products, including milestones, summary of personnel, expenditures, anticipated market potential and timeline. |
| (b) | LICENSEE shall submit annual diligence reports to S&W that describe progress made in the progress of the research and development, evaluation, testing. regulatory approvals, manufacturing. sublicensing, marketing, sales, and commercialization of any LICENSED PRODUCTS the most recent time period and plans for the forthcoming year per each application or patent of the Patent Rights. |
| (c) | LICENSEE must provide written notification to S&W within thirty (30) days of achieving each milestone. Failure to meet a milestone constitutes a breach that if uncured within 120 days, will result in removing the affected PATENT RIGHTS and PROPRIETARY INFORMATION, if any, from the Agreement. |
| (d) | LICENSEE by itself or through its AFFILIATES and SUBLICENSEES will use diligent efforts to make LICENSED PRODUCTS commercially available in accordance with the terms of the Agreement. Without limiting the foregoing, LICENSEE will, itself or through one or more SUBLICENSEEs: |
| (1) | maintain a bona fide, funded, ongoing and active research, development, manufacturing, regulatory, marketing and/or xxx es program (all as commercially reasonable) to make LICENSE PRODUCTS commercially available to the public as soon as commercially practicable, and |
| (2) | fulfill each of the following diligence milestones as set forth in Table 3 by the deadlines indicated in Table 3: |
| 6 |
Table 3
1 . LICENSEE or a SUBLICENSEE at any Phase 1 clinical trial for a Licensed Product/indication ·within 2 years of the effective date of the license agreement
2. A Phase II clinical trial for a Licensed Product/indication within two years following completion of a Phase clinical trial license agreement, but subject to approval by S&W shall be afforded more time if such is due to the nature of requirements met in Phase 1.
| 5.3 | Failure to Accomplish Milestones. Should LICENSEE fail to achieve any milestone specified in paragraph 5.2, or should LICENSEE fail to record NET SALES for two (2) consecutive Calendar Years once sales begin, S&W, at its sole option, may waive the requirement to achieve the milestone, reduce the license granted to a nonexclusive license, renegotiate the missed milestone, or terminate this Agreement under paragraph 8.3. |
| 5.3 | Legal Compliance. LICENSEE must comply with all applicable federal, state and local laws and regulations in its exercise of all rights granted by S&W under this Agreement. |
| 5.4 | U.S. Manufacture. To the extent that rights under this Agreement are subject to rights required to be granted to the Government of the United States of America under 35 USC §§ 200-212, LICENSED PRODUCTS must be manufactured substantially in the United States of America. |
ARTICLE VI- PROTECTION OF INTELLECTUAL PROPERTY
| 6.1 | Authorization. As to prosecution, registration, and/or protection of PATENT RIGHTS, S&W hereby authorizes LICENSEE to: 1) direct the preparation and filing of patent applications, 2) direct the prosecution of broad patent claims for the mutual benefit of LICENSEE, S&W, and SYSTEM, 3) maintain U.S. and non-U.S. issued and granted patents, and 4) be invoiced directly by LICENSEE's outside patent counsel (as approved by S&W under paragraph 6.2 herein) and/or annuity service providers for patent prosecution and associated maintenance fees and costs. S&W may revoke this authorization at any time by giving ten (10) days written notice to LICENSEE. |
| 6.2 | Selection of Counsel. LICENSEE may select an outside patent counsel (Counsel) law firm staffed by experienced, reputable, and licensed intellectual property attorneys for the prosecution, registration, protection, and maintenance of PATENT RIGHTS. LICENSEE will notify S&W of its selection of Counsel and S&W will have final approval on such selection, and such approval shall not be unreasonably withheld. |
| 6.3 | Contract with Counsel. LICENSEE shall execute a written agreement with Counsel establishing that: 1) the attorney/client relationship relative to the prosecution, registration, or protection of PATENT RIGHTS will be with S&W and LlCENSEE jointly; 2) Counsel will not take any actions adverse to the interests of S&W in relation to PATENT RIGHTS including, for example and without limitation, any future invalidity or adverse litigation actions; 3) costs for prosecution, registration, or protection of PATENT RIGHTS will be invoiced directly to LICENSEE with a courtesy copy of the invoice lo S&W; and 4) S&W will not be responsible for payment of invoices relating to prosecution, registration, or protection of PATENT RIGHTS conducted under this Agreement. including, without limitation, attorneys· fees, costs, official filing fees, and foreign associates' fees and costs. LICENSEE shall provide a copy of such written agreement to S&W. Additionally, LICENSEE shall ensure that Counsel promptly signs the standard Outside Counsel Agreement, which Counsel can obtain from S&W's Office of General Counsel. |
| 7 |
| 6.4 | Approvals. LICENSEE shall notify S&W before any substantive actions are taken in prosecuting, continuing, or abandoning any patents or patent applications or otherwise affecting PATENT RIGHTS and LICENSEE will instruct Counsel to so notify S&W. In addition to other substantive actions, LICENSEE agrees that S&W will have final approval on the filing of any action or application that seeks to, or effects, changes in inventorship related to PATENT RIGHTS, and will so instruct Counsel. LICENSEE agrees that S&W will have final approval on how to proceed with any substantive actions relating to and/or affecting PATENT RIGHTS. |
| 6.5 | Patent Maintenance. During the term of this Agreement, LICENSEE shall be directly responsible for annual or periodic annuity payments to maintain the pendency of non-U.S. patent applications in countries that require such annual or periodic annuities. Furthermore, during the term of this Agreement, LICENSEE agrees to continue any required annual and periodic payments for maintenance of U.S. and non-U.S. issued and granted patents. |
| 6.6 | Patent Maintenance Contract. LICENSEE shall engage Counsel or a reputable annuity service to be responsible for docketing and payment of annual or periodic annuities and maintenance fees for both U.S. and non-U.S. pending applications and U.S. and non-U.S. issued and granted patents during the term of this Agreement. |
| 6.7 | Advance Payment of Maintenance Fees. Should LICENSEE request in writing that S&W direct payment of annuities and/or maintenance fees relating to PATENT RIGHTS, LICENSEE agrees to provide payment of estimates for such annuities/fees ninety (90) days in advance of the due date. LICENSEE agrees that failure to make such timely advance payments to S&\V shall be reasonably construed to be a decision to abandon such patent application or patent and that S&W has full rights to determine whether or not to pay the annuity or maintenance fees with no further obligation to LICENSEE. Furthermore, should S&W decide to continue maintenance of the patent application or patent at its own expense, LICENSEE hereby agrees that any such application or patent will be excluded from PATENT RIGHTS. |
| 6.8 | Confidential Communications. S&W and LICENSEE have a community of interest with regard to work conducted in relation to PATENT RIGHTS due to their common interest in the generation of enforceable Intellectual Property rights relating to LICENSED PRODUCTS and/or LICENSED SERVICES. Any communications between LICENSEE and Counsel shall not be confidential vis a-vis S&W, but shall be otherwise confidential and protected by attorney client privilege. |
| 6.9 | Correspondence. LICENSEE shall contemporaneously copy SYSTEM and S&W on all correspondence to and from any patent office, U.S. or non-U.S., including all periodic annuities and maintenance fees correspondence, and LICENSEE agrees to so instruct Counsel to provide copies of such correspondence to SYSTEM and S&W. LICENSEE further agrees that LICENSEE's failure to timely provide such correspondence will be considered a breach of this Agreement in accordance with Paragraph 8.3 below. |
| 6.10 | Information. To aid LICENSEE in the prosecution, registration, protection, and maintenance of PATENT RIGHTS, S&W will provide information, execute and deliver documents, and perform other acts as LICENSEE reasonably requests from time to time. LICENSEE will reimburse S&W for S&W's reasonable costs incurred in complying with such requests. |
| 6.11 | Abandonment. Should LICENSEE decide to abandon any U.S. or non-U.S. parent application or issued or granted patent for commercial reasons or by declining to make an annuity or maintenance payment. LICENSEE shall immediately notify S&W in writing, and S&W shall have the right to continue patent prosecution or maintenance at its own expense and any such patent application and granted or issued patent there from will be excluded from PATENT RIGHTS in this License Agreement. |
| 8 |
| 6.12 | Assignee. All patent applications and patents related to this Agreement shall have named as the assignee(s): ·"The Texas A&M University System" and/or "Xxxxx & White Healthcare", as appropriate. |
| 6.13 | Obligation to File or Maintain. Should LICENSEE decide not to file or maintain patent protection, S&W may. at its own expense, without reimbursement from LICENSEE, file, prosecute, or maintain the patent protection and LICENSEE hereby agrees that any such patent will be excluded from the rights granted herein. |
ARTICLE VII- PAYMENTS AND REPORTS
| 7.1 | When Payments are Due. Unless otherwise specified, LICENSEE must make payments to Xxxxx & White Healthcare, in Temple, Texas, not later than sixty (60) days after the last day of the calendar quarter in which they accrue. |
| 7.2 | Royalty Reports. Beginning the first quarter in which Net Sales occurs or sublicensing income is received, LICENSEE must provide a sales report to &W each calendar quarter, providing information sufficient to allow S&W to calculate amounts due S&W for the reporting period. |
| 7.3 | Currency. Payment due to S&W must be paid in U.S. dollars. Royalty payments requiring conversion must use the exchange rate as reported in The Wall Street Journal on the last business day of the royalty reporting period. |
| 7.4 | Inspection of Books and Records. At its own expense, S&W may annually inspect LICENSEE's books and records as needed to determine royalties payable. LICENSEE must maintain those books and records for at least three years following the dates of the underlying transactions. Any inspections will be in confidence and conducted during ordinary business hours, and S&W will provide LICENSEE advance notice two weeks before making an inspection. S&W may employ a Certified Public Accountant for this purpose. lf S&W's audit identities a shortage of five percent (5%) or more of amounts due to S&W, then LICENSEE must pay the costs of S&W's audit. LICENSEE must pay all amounts due as a consequence of an audit to S&W promptly, with interest. |
| 7.5 | Interest Charges. S&W may, in its sole discretion, charge daily interest on overdue payments commencing on the 31st day after the payment is due, compounded monthly, at the lower of either one and a half percent (1.5%) per month or the highest legal interest rate. The payment of interest will not foreclose S&W from exercising any other rights it may have due to the lateness of any payment. |
ARTICLE VIII - TERM AND TERMINATION
| 8.1 | Expiration. This Agreement, unless sooner terminated as provided below, will remain in effect until (a) expiration or the last to expire patent under PATENT RIGHTS, or (b) final and unappealable determination by a court of competent jurisdiction that PATENT RIGHTS are invalid. |
| 8.2 | Termination by LICENSEE. LICENSEE may terminate this Agreement by providing written notice to S&W at least ninety (90) days before the termination is to take effect. |
| 9 |
| 8.3 | Termination by S&W. lf LICENSEE materially breaches this Agreement, S&W may give LICENSEE written notice of the breach. LICENSEE will have sixty (60) days from receipt of the notice to cure the breach. lf LICENSEE does not cure the breach within this period, S&W may terminate this Agreement without further notice. |
| 8.4 | Matters Surviving Termination. All accrued obligations and claims, including license fee obligations, royalty obligations. minimum annual consideration obligations, interest charge obligations, and all other financial obligations, and claims or causes of action for breach of this Agreement, will survive termination of this Agreement. Obligations of confidentiality will survive termination of this Agreement This section controls in the case of a conflict with any other section of this Agreement. |
ARTICLE IX - INDEMNIFICATION AND REPRESENTATION
| 9.1 | Indemnification. LICENSEE MUST AT ALL TIMES DURING AND AFTER THE TERM OF THIS AGREEMENT INDEMNIFY, DEFEND, AND HOLD HARMLESS SYSTEM AND S&W, ITS REGENTS, OFFICERS, BOARD MEMBERS, AND CURRENT AND FORMER EMPLOYEES AGAINST ANY CLAIM, PROCEEDING, DEMAND, LIABILITY OR EXPENSE (INCLUDING LEGAL EXPENSE AND REASONABLE ATTORNEY'S FEES) WHICH RELATES TO INJURY TO PERSONS OR PROPERTY, ANY ACTION BROUGHT BY A THIRD PARTY ALLEGING INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS, OR AGAINST ANY OTHER CLAIM, PROCEEDING, DEMAND, EXPENSE, OR LIABILITY OF ANY KIND RESULTING FROM TH PRODUCTION, MANUFACTURE, SALE, COMMERCIAL USE, LEASE, CONSUMPTION, OR ADVERTISEMENT OF LICENSED PRODUCTS OR ARISING FROM ANY OBLIGATION OF LICENSEE OR SUBLICENSEE(S) UNDER THIS AGREEMENT. |
| 9.2 | Representation. S&W represents that it owns and has title to, either solely, jointly with SYSTEM, or jointly with SYSTEM and LICENSEE, PATENT RIGHTS and has the full right and power to grant the license in paragraph 2.1, and that there are no outstanding agreements, assignments, or encumbrances inconsistent with the provisions of this Agreement. S&W MAKES NO OTHER REPRESENTATIONS AND EXTENDS NO OTHER WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTIES OF MERCANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, NOR DOES S&W ASSUME ANY OBLIGATION REGARDING INFRINGEMENT OF PATENT RIGHTS OR OTHER RIGHTS OF THIRD PARTIES DUE TO LICENSEE'S ACTIVITIES UNDER THIS AGREEMENT. |
| 10.1 | Notices. Payments, notices, or other communications required by this Agreement will be sufficiently made or given if mailed by certified First Class United States mail, postage pre-paid, or by commercial carrier (e.g.. FedEx, UPS, etc.) when the carrier maintains receipt or record of delivery, addressed 10 the address stated below, or to the last address specified in writing by the intended recipient. |
| 10 |
If to S&W:
Contact for Payments/Accounting:
Attn: Xxxxx X. Xxxxx
AVP - Academic Finance
Xxxxx & White Healthcare
0000 Xxxxx 0000 Xxxxxx
XX- AR-M113
Xxxxxx, XX 00000
Phone: 000-000-0000
Fax: 000-000-0000
(Please reference Patent License Agreement Number SW11l-003PLA
Contact for all other communications:
Xxxxxx X. De La Xxx, M.D.
Vice President, Intellectual Properties
Office of General Counsel
Xxxxx & White Healthcare
MS-20-D642
0000 Xxxxx 00xx Xxxxxx
Xxxxxx, Xxxxx 00000
Phone: 000-000-0000
Fax: 000.000.0000
Email:xxxxxxxx@xx.xxx
(Please reference Patent License Agreement Number SW11-003PLA
If to LICENSEE
Contact for notice:
Xxxxxx Xxxxxxxx
Xxxxxxxx & Associates
000 Xxxx Xxxxx Xxxxxx
00xx Xxxxx
Xxx Xxxxxxx, XX 00000
tel: 000-000-0000
fax:000-000-0000
xxxxxxxxx@xxxxxxxx-xx.xxx
Contact for all other matters:
Xxxx Xxxxxxxxx, President
0000 Xxxxxxxxxx Xxxxx Xxxxx 000
Xxx Xxxxxx, Xxxxxxxxxx, XXX 00000
Phone: 000-000-0000
FAX: 000-000-0000
Email: xxxx@xxxxxxxxxxxxx.xxx
| If to SYSTEM: | Associate Vice Chancellor |
| Office of Technology Commercialization | |
| 000 Xxxxxxx Xxxxxxx Xxxxxxx; Suite 0000 | |
| Xxxxxxx Xxxxxxx, Xxxxx, XXX 00000 |
| 11 |
ARTICLE XI- PATENT INFRINGEMENT
| 11.1 | Notice of Infringement. Each Party must promptly notify the other in writing of any alleged infringement of PATENT RIGHTS that comes to its attention. Within sixty (60) days after receipt of such notice, S&W and LICENSEE will formulate a strategy for resolving the alleged infringement. |
| 11.2 | LICENSEE Right to Bring Suit. Following the initial sixty (60) day strategy period pursuant to Paragraph 11.1, LICENSEE may institute suit for patent infringement against the infringer. SYSTEM and/or S&W may voluntarily join such suit at their own expense. but may not thereafter commence suit against the infringer for the acts of infringement that are the subject of the LICENSEE's suit or any judgment rendered in that suit. LICENSEE may join S&W as a party in a suit initiated by LICENSEE if S&W is a necessary party to the law suit under the procedural rules of the applicable jurisdiction. If S&W is involuntarily joined other than by the voluntary action of S&W or is joined as a necessary party as a matter of law, then LICENSEE will pay any costs incurred by S&W arising out of such suit, including but not limited to, any legal fees of the counsel that S&W selects and retains to represent it in the suit; provided, however, that S&W shall endeavor to retain the same counsel as LICENSEE i n the same lawsuit subject to potential conflict of interests. |
| 11.3 | S&W Right to Bring Suit. If, within a hundred and ninety (90) days following the notice of infringement, LICENSEE has not brought suit against the infringer pursuant to Paragraph 11.2, then SYSTEM and/or S&W may institute suit for patent infringement against the infringer. If SYSTEM and/or S&W institutes such suit, then LICENSEE may not join such suit without SYSTEM's or S&W's consent and may not thereafter commence suit against the infringer for the acts of infringement that are the subject of SYSTEM's and/or S&W's suit or any judgment rendered in that suit. |
| 11.4 | Control of Litigation. Any litigation proceedings will be controlled by the party bringing the suit. |
| 11.5 | Recovery. Any recovery or settlement received in connection with any suit will first be shared by S&W and LICENSEE to cover any litigation costs each incurred, in proportion to their respective costs. In any suit initiated by LICENSEE pursuant to Paragraph 11.2, any recovery in excess of litigation costs will be shared between LICENSEE and S&W as follows: (a) for any recovery other than amounts paid for willful infringement or as punitive damages LICENSEE shall receive an amount equal to its lost profits or a reasonable royalty on the infringing sales, or whichever measure of damages the court shall have applied, and LICENSEE shall pay to S&W a portion of its recovery a reasonable approximation of the royalties and other amounts that LICENSEE would have paid to S&W if LICENSEE bad sold the infringing products, processes and services rather than the infringer, and (b) as to amounts paid for willful infringement or as punitive damages, SYSTEM will receive thirty percent (30%) of the recovery if S&W was not a party in the litigation and did nor incur any litigation costs that were not reimbursed by LICENSEE or fifty percent (50%) of the recovery if SYSTEM was a party in the litigation whether joined as a party under the provisions of Paragraph 11.2 or voluntarily. In any suit initiated by SYSTEM and/or S&W pursuant to Paragraph 11.4, any recovery in excess of litigation costs will belong to SYSTEM and/or S&W. |
| 12 |
ARTICLE XI - MISCELLANEOUS PROVISIONS
| 12.1 | Export Controls. S&W and SYSTEM are subject to United States laws and regulations controlling the export of technical data, computer software laboratory prototypes and other commodities, and its obligations under this Agreement arc contingent on compliance with applicable laws and regulations. The transfer of certain technical data and commodities may require a license from the cognizant agency of the United States Government or written assurances by LICENSEE that LICENSEE will not export data or commodities to certain countries without advance approval of such agency. S&W neither represents that a license will not be required nor that, if required, it will be issued. |
| 12.2 | Confidential Information. Sales reports submitted by LICENSEE under ARTICLE VII will be considered Confidential Information under this Agreement and not be disclosed by S&W to any third party except as may be required by law. If the Parties contemplate exchanging other information of a confidential nature, they should enter into a separate confidentiality agreement. |
| 12.3 | Non-Use of Names. LICENSEE may not use the names or any adaptation of the names of Xxxxx & White Healthcare or SYSTEM, nor of any of its employees or members, in any advertising, promotional, or sales literature without the advance written consent of S&W and/or SYSTEM in each case, except that LICENSEE may state that it is licensed by S&W under PATENT RIGHTS. |
| 12.4 | Trademarks. LICENSEE may select, own and use its own trademark on LICENSED PRODUCTS. However, S&W does not grant LICENSEE any license or other right under any trade name, trademark or service xxxx owned or licensed by S&W or SYSTEM. Conversely, S&W has no rights to trade names, trademarks, or service marks owned by LICENSEE. |
| 12.5 | Assignment of this Agreement. This Agreement, with the rights and privileges it creates, 1s assignable only with the written consent of both Parties. |
| 12.6 | Force Majeure. Other than an obligation for the payment of money, S&W, upon receipt of documentation from LICENSEE which it deems appropriate, must excuse any breach of this Agreement which is proximately caused by war, strike, act of God, or other similar circumstance normally deemed outside the control of well-managed businesses. |
| 12.7 | Entire Agreement. This Agreement contains the entire understanding of the Parties regarding PATENT RIGHTS, and supersedes all other written and oral agreements between the Parties regarding PATENT RIGHTS. It may be modified only by a written amendment signed by the Parties. |
| 12.8 | Governing Law. The substantive Laws of the State of Texas (and not its conflicts of law principles), USA, govern all matters arising out of or relating to this Agreement and all of the transactions it contemplates. Venue for any suit brought against S&W in Texas state court must be in Xxxx County, Texas, and venue for any suit brought against S&W in federal court must be in Texas. |
| 12.9 | Headings. Headings are solely for convenience of reference and are not part of, and may not be used to construe, this Agreement. |
| 12.10 | No Waiver; Severability. If any provision of this Agreement is invalid, illegal, or unenforceable, the validity, legality and enforceability of the remaining provisions will not not in any way be affected or impaired. A waiver of any breach of this Agreement does not waive any other breach of the same or other provision of this Agreement. A waiver is not effective unless made in writing. |
| 13 |
| 12.11 | Counterparts. This agreement may be executed in any number of counterparts, including facsimile or scanned PDF documents. Each such counterpart, facsimile, or scanned PDF document shall be deemed an original instrument, and all of which, together, shall constitute one and the same executed Agreement. |
| 12.12 | No Other Promises and Agreements; Representation by Counsel. Licensee expressly certifies and represents that no promise or agreement which is not herein expressed has been made to Licensee in executing this Agreement except those explicitly set forth herein and that Licensee is not relying upon any statement or representation of Licensor or its representatives. Licensee is relying on Licensee's own judgment and has had the opportunity to be represented by legal counsel. Licensee hereby warrants and represents that Licensee understand and agrees to all terms and conditions set forth in this Agreement. |
| 12.13 | Deadline for Execution by Licensee. If this Agreement is executed first by the Licensor and is not executed by the Licensee and received by the Licensor at the address and in the manner set forth in paragraph 10.1 of the Agreement within 30 days of the date of signature set forth under the Licensor's signature below, then this Agreement shall be null and void and of no further effect. |
The Parties have caused this Agreement to become effective as of the date last executed below.
| LICENSEE: VG LIFE SCIENCES, INC. | LICENSOR: XXXXX & WHITE HEALTHCARE | |
| /s/ Xxxx Xxxxxxxxx | /s/ Xxxxxx X. Xxxxx | |
| By: Xxxx Xxxxxxxxx | Xxxxxx X. Xxxxx, M.D. | |
| Title: President | President/CEO | |
| Date: 7/6/2013 | Date: 7/18/2013 |
| 14 |
