THIS AGREEMENT dated 27th JULY 2006 is made BETWEEN: WHEREAS
Exhibit 10.32
THIS AGREEMENT dated 27th JULY 2006 is made BETWEEN:
| (1) | Imperial College of Science Technology and Medicine, whose address is at Xxxxxxxxxx Xxxx, Xxxxx Xxxxxxxxxx Xxxxxx, Xxxxxx XX0 0XX, Xxxxxxx (“Imperial”); |
| (2) | Somanta Pharmaceuticals Inc whose UK offices are at 00 Xxxxxx Xxxxxx, Xxxxxx, X0X 0XX (the “Sponsor”) and |
| (3) | Xxxx Xxxxx Xxxxxx of Division of Cell and Molecular Biology, Faculty of Natural Sciences, Xxxxxxx Biochemistry Building, Imperial College London, Xxxxxxxxxx Xx, Xxxxxx, XX0 0XX, XX (the “Student”) |
WHEREAS
| (A) | The Parties wish to enter into this Agreement in order to record their collaboration on a post-graduate studentship scheme under the rules laid down by the Biotechnology Biological Sciences Research Council (BBSRC) for the Council’s Co-operative Awards in Science and Engineering (“CASE” Awards). |
| (B) | The Parties acknowledge that the terms of this Agreement are to govern the funding and conduct of a studentship, to enable the Student to carry out a research project and submit a related thesis for examination in accordance with Imperial’s regulations governing post-graduate study in fulfilment of the requirements of a higher degree of Imperial. The Parties further acknowledge that the research is intended to lead to academic publications relating to the results of the studentship in furtherance of the Student’s career. |
| (C) | The Parties further acknowledge that in the course of the studentship the Parties may be exposed to proprietary and commercially valuable information or materials of the Sponsor and/or Imperial. All Parties recognise the importance of holding in confidence such information or materials. |
| 1. | DEFINITIONS |
| 1.1 | In this Agreement the following expressions shall have the following meanings: |
| 1.1.1 | “Academic Supervisor” means Xx Xxxxxxxx Xxxxxxxxx or his or her successor, appointed under Clause 9.2. |
| 1.1.2 | “Affiliate” means any company which directly or indirectly through one or more intermediaries controls, is controlled by or is under common control with the Sponsor. |
| 1.1.3 | “Arising Intellectual Property” means any inventions, designs, information, know-how, specifications, formulae, data, processes, methods, techniques, and other technology obtained or developed in the course of the Project and the Intellectual Property Rights therein. |
| 1.1.4 | “Background Intellectual Property” means any inventions, designs, information, know-how, specifications, formulae, data, processes, methods, techniques, and other technology, other than Arising Intellectual Property, used in, or disclosed in connection with the performance of, the Project and the Intellectual Property Rights therein. |
| 1.1.5 | “Biological Materials” means any material provided by the Sponsor for use within the Project that is biological or of biological origin. |
| 1.1.6 | “Compounds” means |
| (a) | Any characterised chemical entity that is the active component of a drug formulation currently or previously marketed by the Sponsor; |
| (b) | Any characterised chemical entity currently or previously under development by the Sponsor as a potential active drug substance; or |
| (c) | Any other characterised chemical entity, novel or otherwise, synthesised by or for the Sponsor. |
| 1.1.7 | “Industrial Supervisor” means Xxxxxxxxx X. Xxxxxxxx or his or her successor, as appointed from time to time by the Sponsor. |
| 1.1.8 | “Intellectual Property Right” means any patent, registered design, copyright, database right, design right, trade xxxx, application to register any of the aforementioned rights, trade secret, right in unpatented know-how, right of confidence and any other intellectual or industrial property right of any nature whatsoever in any part of the world. |
| 2. | THE PROJECT |
| 2.1 | The “Project” shall be the programme of work entitled “Pre-clinical evaluation of Angiolix (Mc-3) humanised monoclonal antibody and lactadherin target for anti-vasculature cancer therapy” which is undertaken by the Student and is described in the First Schedule to this Agreement; and any modifications, deletions or expansions approved in writing by all parties in accordance with the provisions of clause 7.2. |
| 2.2 | The PhD project shall run for the period (the “Project Period”) from and including 1st October 2005 for a period of three (3) years. |
| 2.3 | The Project will be conducted mainly in laboratories of Imperial, under the supervision of the Academic Supervisor, and partly under the supervision of the Industrial Supervisor. |
| 2.4 | Imperial will use its reasonable endeavours to provide adequate facilities; to obtain any requisite materials, equipment and personnel; and to carry out the Project diligently within the scope allowed by the funding provided by the Sponsor pursuant to Clause 3. Although Imperial will use its reasonable endeavours to perform the research described in the First Schedule, Imperial does not undertake that the work |
| carried out under or pursuant to this Agreement will lead to any particular result, nor is the success of such work guaranteed. |
| 2.5 | The Sponsor will allow the Student to attend one or more other research establishments for a minimum of three months during and for the purpose of the Project if appropriate. The periods of such attendance will be scheduled by mutual agreement. The Student undertakes to comply with all works rules and safety and other regulations communicated to him/her by the Sponsor and which the Sponsor may reasonably prescribe during those periods. For the avoidance of doubt, the Student will not be an employee of the Sponsor during such periods and the Sponsor will not require the Student to sign any contract of employment or other such legally binding agreement. However, the Sponsor may require the Student to sign a confidentiality agreement which does not conflict with the terms of this Agreement. |
| 2.6 | Imperial, through the Academic Supervisor and the Student, shall keep the Sponsor informed of the progress of the Project at meetings held at intervals of not more than six (6) months, or as otherwise agreed by Imperial and the Sponsor. At the reasonable request of the Sponsor, Imperial, through the Academic Supervisor and the Student, shall supplement the meetings with written reports, and the Student shall provide the Sponsor with a bound copy of any thesis prepared by the Student on the Project (the “Thesis”). |
| 3. | FUNDING BY THE SPONSOR |
| 3.1 | The Sponsor agrees to make payments to Imperial on the dates and in the amounts as set out in the Second Schedule to this Agreement. |
| 3.2 | Except as otherwise provided by agreement in writing, as between the Sponsor and Imperial the full and unencumbered title to all equipment purchased or constructed using funds provided by the Sponsor shall vest in Imperial. Imperial shall acknowledge the financial contribution by the Sponsor towards any such equipment in such reasonable way as may be mutually agreed between the Sponsor and Imperial. |
| 4. | INTELLECTUAL PROPERTY |
| 4.1 | All Background Intellectual Property belonging to one Party is and shall remain the exclusive property of the Party owning it (or, where applicable, the third party from whom its right to use the Background Intellectual Property has derived). |
| 4.2 | Each Party grants the other Parties a royalty-free, non-transferable, non-exclusive, non-sublicensable licence to use its Background Intellectual Property for the sole purpose of the performance of the Project. |
| 4.3 | It is understood and agreed that a major objective of the Project is to validate, further characterise, extend and develop the Sponsor’s technology for commercial application in the Field (where “the Field” is defined as, and limited to, the specific use of the Angiolix™ antibody and its lactadherin target in cancer diagnosis and therapy), such technology being covered by patents, patent applications and licences included within Sponsor’s Background Intellectual Property. It is therefore envisaged that a |
| significant part of the Arising Intellectual Property will represent improvements to the Sponsor’s technology. |
| 4.4 | It is further understood and recognised that there may be Arising Intellectual Property that does not represent an improvement to Sponsor’s Background Intellectual Property, or which has application both as an improvement to Sponsor’s Background Intellectual Property and in other areas. |
| 4.5 | With respect to Arising Intellectual Property, the following shall apply: |
| 4.5.1 | To the extent that the Arising Intellectual Property is generated, developed, discovered or invented by the Sponsor alone, then it shall vest in and be owned absolutely by the Sponsor; |
| 4.5.2 | To the extent that the Arising Intellectual Property represents a non-severable improvement to Sponsor’s Background Intellectual Property (the “Sponsor Arising Intellectual Property”), whether such Sponsor Arising Intellectual Property is generated, discovered, developed or invented solely by Imperial or jointly by the Sponsor and Imperial, such Sponsor Arising Intellectual Property shall vest in and be owned absolutely by the Sponsor. Each of Imperial and the Student hereby assign to Sponsor all of such Party’s right, title and interest, if any, that such Party has or obtains in any Sponsor Arising Intellectual Property; provided that in the event that the foregoing assignments would be void or impermissible, then each of Imperial and the Student automatically shall be deemed to have granted to Sponsor the perpetual, irrevocable, fully paid-up, freely sub-licensable licence to use and practise such Sponsor Arising Intellectual Property for any and all purposes, which licences shall be exclusive to Sponsor, and which licences shall survive any expiration or termination of this Agreement. Imperial hereby agrees that any and all Imperial employees, students or any other persons who participate in the Project will have assigned to Imperial any and all rights such employee, student or any other person may obtain in any technology or other information by virtue of such participation. |
| 4.5.3 | In the case of Arising Intellectual Property that is not Sponsor Arising Intellectual Property and that is generated, discovered, developed or invented jointly by Imperial and the Sponsor (“Joint Arising Intellectual Property”), then it shall vest in and be owned jointly by the Sponsor and Imperial jointly, subject to the options set forth in Clauses 4.5.6.4 and 4.5.7 below. Student hereby assigns to Sponsor and Imperial an undivided interest in and to all of Student’s right, title and interest, if any, that Student has or obtains in any Joint Arising Intellectual Property; provided that in the event that the foregoing assignments would be void or impermissible, then the Student automatically shall be deemed to have granted to each of Sponsor and Imperial the perpetual, irrevocable, fully paid-up, freely sub-licensable licence to use and practice such Joint Arising Intellectual Property for any and all purposes, which licences shall be exclusive to Sponsor and Imperial, and which licences shall survive any expiration or termination of this Agreement. |
| 4.5.4 | In the case of Arising Intellectual Property that is not Sponsor Arising Intellectual Property and that is generated, discovered, developed or invented by Imperial alone (or jointly with the Student only), then it shall vest in and be owned absolutely by Imperial (“Imperial Arising Intellectual Property”), subject to the option in favour of Sponsor set forth in Section 4.5.8 below. Imperial shall promptly notify Sponsor at such time as any Imperial Arising Intellectual Property is generated, discovered, developed or invented. Student hereby assigns to Imperial all of Student’s right, title and interest, if any, that Student has or obtains in any Imperial Arising Intellectual Property; provided that in the event that the foregoing assignment would be void or impermissible, then the Student automatically shall be deemed to have granted to Imperial the perpetual, irrevocable, fully paid-up, freely sub-licensable licence to use and practice such Imperial Arising Intellectual Property for any and all purposes, which licence shall be exclusive to Imperial, and which licences shall survive any expiration or termination of this Agreement. |
| 4.5.5 | With respect to all Imperial Arising Intellectual Property, Imperial may take such steps as it may decide from time to time, and at its own expense, to register and maintain any protection for such Imperial Arising Intellectual Property as the case may be, including filing and prosecuting patent applications for any of Imperial Arising Intellectual Property in any jurisdiction worldwide. |
| 4.5.6 | In the event that it is or may be possible to obtain any registered Intellectual Property Rights in any Joint Arising Intellectual Property, the Sponsor agrees to be responsible for the filing and prosecution on behalf of the Parties and in their joint names of applications for registration, and the maintenance and renewal of any registrations, in such countries as the Parties agree in writing, subject to Imperial co-operating in the provision of all necessary assistance, information and instructions, with respect to the same, provided that: |
| 4.5.6.1 | if Imperial but not the Sponsor wishes to apply for registration in any country or countries, Imperial may do so at its sole cost and expense on behalf of both Parties and in their joint names, and the Sponsor shall provide Imperial with all necessary assistance, information, and instruction; |
| 4.5.6.2 | neither Party shall amend or abandon any registration in respect of which the Parties are jointly registered as owners unless, in the case of an amendment, the other Party shall have given its prior written consent or, in the case of abandonment, the other Party shall be given the opportunity to maintain the registration at its own cost. |
| 4.5.6.3 | the Party making an application for registration shall consult with the other Party at reasonable intervals concerning the application for and maintenance of such registration. |
| 4.5.6.4 | With respect to Joint Arising Intellectual Property that has application outside the Field, each of Sponsor and Imperial hereby grant to the other an option, exercisable for a period of twelve (12) months after the date on which the applicable Joint Arising Intellectual |
| Property is first generated, discovered, developed or invented, to take a licence under such Joint Arising Intellectual Property on reasonable commercial terms to be mutually agreed upon by the parties, taking into account any patent expenses incurred by the Parties with respect thereto. Each Party may exercise this option at any time during the option period by delivered written notice thereof to the other Party. Following the expiration of this option period, each Party shall be free to commercialise such Intellectual Property as it sees fit, including the grant of exclusive or non-exclusive licences (or the assignment of such Intellectual Property) to third parties on commercial terms to be agreed, subject in each case to the other Party’s rights with respect to any such Joint Arising Intellectual Property. |
| 4.5.7 | With respect to Joint Arising Intellectual Property that has application within the Field, Imperial hereby grants to Sponsor an option, exercisable for a period of twelve (12) months after the date on which the applicable Joint Arising Intellectual is first generated, discovered, developed or invented, to take a royalty-free, freely sub-licensable, exclusive licence under Imperial’s rights in such Joint Arising Intellectual Property for use solely within the Field; PROVIDED that the Sponsor undertakes to refund 75% (seventy five percent) of all external patent costs incurred by Imperial with respect to the applicable Joint Arising Intellectual prior to and following grant of the licence with respect thereto. Sponsor may exercise this option at any time during the option period by delivered written notice thereof to Imperial. Following the expiration of this option period, Imperial shall be free to commercialise such Intellectual Property as it sees fit, including the grant of exclusive or non-exclusive licences (or the assignment of such Intellectual Property) to the Sponsor and/or third parties on commercial terms to be agreed, subject in each case to Sponsor’s rights with respect to any Joint Arising Intellectual Property. |
| 4.5.8 | With respect to Imperial Arising Intellectual Property, Imperial hereby grants to Sponsor an option, exercisable for a period of twelve (12) months after the date on which the applicable Imperial Arising Intellectual is first generated, discovered, developed or invented, to take a freely sub-licensable, exclusive, worldwide licence to such Imperial Arising Intellectual Property for use solely within the Field on reasonable commercial terms subject to the provisions of Clause 4.7 below. Sponsor may exercise this option at any time during the option period by delivered written notice thereof to Imperial. Following the expiration of this option period, Imperial shall be free to commercialise such Intellectual Property as it sees fit, including the grant of exclusive or non-exclusive licences (or the assignment of such Intellectual Property) to the Sponsor and/or third parties on commercial terms to be agreed. |
| 4.6 | The Sponsor hereby grants to Imperial and the Student a royalty-free irrevocable, non-transferable, non-exclusive, non-sublicensable right and licence to use the Sponsor Arising Intellectual Property for their own non-commercial activities such as teaching and scientific research. |
| 4.7 | The terms of any licence agreements provided for in Clauses 4.5.6.4, 4.5.7 or 4.5.8 above shall be negotiated in good faith by the Sponsor and Imperial and shall contain all such |
| terms and conditions which are usual and customary in a licence agreement, including but not limited to liability, audit provisions, termination, governing law provisions. With respect to a licence agreement provided for in Clause 4.5.6.4, the financial terms of any such licence will be fair and reasonable in the circumstances and will be negotiated on a case-by-case basis taking into account the scientific and financial contributions of the Parties to the Joint Arising Intellectual Property being licensed and the subsequent scientific and financial contribution of the Parties that will be necessary to commercially exploit such Joint Arising Intellectual Property. |
| 5. | CONFIDENTIALITY |
| 5.1 | The Parties each undertake to use reasonable endeavours to keep confidential and not to disclose to any third party (other than an Affiliate) or to use themselves other than for the purposes of the Project or as permitted under Clauses 4, 6 and 7 of this Agreement any confidential or secret information in any form directly or indirectly belonging or relating to the other, its Affiliates, its or their business or affairs, disclosed by one and received by another pursuant to or in the course of the Project, including without limitation any Background Intellectual Property of the other or the Arising Intellectual Property (“Confidential Information”). |
| 5.2 | Each of the Parties undertakes to use reasonable endeavours to disclose Confidential Information of the other only to those of its officers, employees, students, agents and contractors, (and those of its Affiliates) to whom and to the extent to which, such disclosure is necessary for the purposes contemplated under this Agreement and to ensure that all such personnel are bound by terms of confidentiality equivalent to those contained herein. |
| 5.3 | The obligations contained in this Clause 5 shall survive the expiry or termination of this Agreement for any reason for a period of five (5) years (other than obligations with respect to trade secrets which shall survive for the life of such trade secret) but shall not apply to any Confidential Information which: |
| 5.3.1 | is publicly known at the time of disclosure to the receiving Party; |
| 5.3.2 | after disclosure becomes publicly known otherwise than through a breach of this Agreement by the receiving Party, its officers, employees, agents or contractors; |
| 5.3.3 | can be shown by reasonable proof by the receiving Party to have reached its hands otherwise than by being communicated by the other Party including being known to it prior to disclosure, or having been developed by or for it wholly independently of the other Party or having been obtained from a third party without any restriction on disclosure on such third party of which the recipient is aware, having made due enquiry; |
| 5.3.4 | is required by law, regulation or order of a competent authority (including any regulatory or governmental body or securities exchange) to be disclosed by the receiving Party, provided that, where practicable, the disclosing Party is given reasonable advance notice of the intended disclosure and provided that the relaxation of the obligations of confidentiality shall only last for as long as |
| necessary to comply with the relevant law, regulation or order and shall apply solely for the purposes of such compliance; or |
| 5.3.5 | is approved for release, in writing, by an authorised representative of the disclosing Party. |
| 6. | PUBLICATIONS |
| 6.1 | The Project will form part of the actual carrying out of a primary charitable purpose of Imperial; that is, the advancement of education through teaching and research. |
| 6.2 | In accordance with normal academic practice, all employees, students, agents or appointees of Imperial (including the Student and any others who work on the Project) shall be permitted, following the procedures laid down in Clause 6.3, to publish Arising Intellectual Property or discuss Arising Intellectual Property in internal seminars, and to give instructions within Imperial on questions related to such work. |
| 6.3 | All proposed publications (including, but not limited to, scientific publications, patent applications and non-confidential presentations), shall be submitted in writing to the other of the Sponsor and Imperial for review at least thirty (30) days before submission for publication or before presentation, as the case may be. The reviewing Party may require the deletion from the publication of any Background Intellectual Property of the reviewing Party, or an amendment to the publication through which commercially sensitive Background Intellectual Property is disguised to the satisfaction of the reviewing Party. The reviewing Party may also request the delay of the publication if in the reviewing Party’s opinion the delay is necessary in order to seek patent or similar protection to Arising Intellectual Property owned by the reviewing Party. Any delay imposed on publication shall not last longer than is reasonably necessary for the reviewing Party to obtain the required protection; and shall not exceed six (6) months from the date of receipt of the proposed publication by the reviewing Party. Notification of the requirement for delay in publication must be received by the publishing Party within thirty (30) days after receipt of the proposed publication by the reviewing Party, failing which the publishing Party shall be free to assume that the reviewing Party has no objection to the proposed publication. |
| 6.4 | Each Party agrees that any publication in a scientific/academic journal shall give due acknowledgement to the financial and/or intellectual contribution of the others in accordance with standard scientific practice. |
| 6.5 | Clause 6 does not apply to the submission of the Thesis, which is governed by Clause 7. |
| 7. | THESIS |
| 7.1 | This Agreement shall not prevent or hinder the Student from submitting for degrees of Imperial Theses based on results generated within the scope of the Project, as outlined in the First Schedule to this Agreement, as amended from time to time in accordance with clause 7.2; or from following Imperial’s procedures for examination and for admission to postgraduate degree status (such procedures to include provisions to place the thesis on restricted access within Imperial’s library where applicable). |
| 7.2 | During the Project Period, the Academic Supervisor, the Industrial Supervisor and the Student shall identify at the progress meetings any Background Intellectual Property of the Sponsor which the Student may wish to incorporate into the Project. The Sponsor shall decide whether or not to allow the identified Background Intellectual Property to be used in the Project. If this decision gives rise to a requirement to amend the description of the Project, as outlined in the First Schedule, such amendment shall be mutually agreed in principle between the Academic Supervisor, the Industrial Supervisor and the Student and forwarded to the contracting authorities of Imperial and the Sponsor for authorisation. |
| 7.3 | The Student shall follow Imperial’s regulations for the submission of the Thesis or Theses for examination. In any event the Student shall submit a draft Thesis to the Academic Supervisor and Industrial Supervisor at least thirty (30) days prior to the date for submission for examination. |
| 7.4 | The Student may not, without the Sponsor’s express written consent, include in any Thesis any Background Intellectual Property or Arising Intellectual Property belonging solely to the Sponsor, which is not directly related to the Project, as outlined in the First Schedule, as amended. |
| 8. | MATERIALS |
During the term of this Agreement it may be necessary for the Sponsor to provide Imperial with various proprietary Biological Materials and/or Compounds for which the following terms will apply:
| 8.1 | Biological Materials and/or Compounds will be provided solely for use in the Project, in Imperial’s laboratories only. Imperial undertakes that any Biological Materials and/or Compounds provided will be used only by the Academic Supervisor, the Student and such persons under the direct supervision of the Academic Supervisor as are required to perform the Project. The Biological Materials and/or Compounds will not be provided to any other scientist or institution (public or private) without prior written permission from the Sponsor. |
| 8.2 | Biological Materials and/or Compounds are experimental in nature and are provided without warranties of any kind expressed or implied. Except in cases of the Sponsor’s negligence or wilful misconduct, the Sponsor, its employees and its Affiliates, accept no liability for damages which might arise in connection with the use, storage or disposal by Imperial of Biological Materials and/or Compounds. Furthermore, the Sponsor makes no representation that the use of the Biological Materials and/or Compounds provided by it will not infringe any patent, copyright, trademark or other proprietary rights. |
| 8.3 | On termination of the Agreement Imperial will discontinue use of the Biological Materials and/or Compounds and at the direction of the Sponsor any remaining Biological Materials and/or Compounds will be returned to the Sponsor at the Sponsor’s expense or destroyed, and destruction certified by Imperial. |
| 8.4 | All experimental work within the Project and any destruction of Biological Materials or Compounds pursuant to Clause 8.3 above will be carried out in accordance with all |
| applicable local, national and international legislation relating to the safe handling, use and disposal of potentially hazardous materials |
| 8.5 | Imperial maintains and will continue to maintain a policy or policies of insurance at levels sufficient to cover any liability that Imperial or the Student may incur pursuant to the performance of its obligations under this agreement, including, without limitation, a policy covering damage or destruction to any property, including, without limitation, the Biological Materials or Compounds and a policy covering the injury of the Student or any employee of Imperial or other person participating in the Project. |
| 9. | TERMINATION |
| 9.1 | This Agreement may be terminated by either the Sponsor or Imperial for any breach of the obligations set out in this Agreement, by giving ninety (90) days’ written notice to the other Parties of its intention to terminate. The notice shall include a detailed statement describing the nature of the breach. If the breach is capable of being remedied and is remedied within the ninety-day notice period, then the termination shall not take effect. If the breach is of a nature such that it can be fully remedied but not within the ninety-day notice period, then termination shall also not be effected if the Party involved begins to remedy the breach within that period, and then continues diligently to remedy the breach until it is remedied fully. If the breach is incapable of remedy, then the termination shall take effect at the end of the ninety-day notice period in any event. Except in the case of termination due to a breach by the Student involving gross negligence, wilful misconduct or withdrawal from the Project, the Sponsor will continue to pay Imperial the Student’s stipend fees in accordance with the payment schedule in the Second Schedule. |
| 9.2 | Imperial agrees to notify the Sponsor promptly if at any time the Academic Supervisor is unable or unwilling to continue the supervision of the Project. Within sixty (60) days after such incapacity or expression of unwillingness Imperial shall nominate a successor to the Academic Supervisor. The Sponsor will not decline unreasonably to accept the nominated successor. However, if the successor is not acceptable to the Sponsor on reasonable grounds, then the Sponsor may terminate this Agreement by giving ninety (90) days’ written notice to Imperial. Nevertheless, the Sponsor will continue to reimburse the Student in accordance with the payment schedule in the Second Schedule. |
| 9.3 | Except as set out in this clause the Sponsor may not terminate this Agreement before the expiry of the Project Period. |
| 9.4 | Clauses 4 to 8 inclusive, 10 and 12 shall survive termination, for whatever reason, of this Agreement. |
| 10. | LIMITATION OF LIABILITY |
| 10.1 | Neither Imperial nor the Student makes any representation or warranty that advice or information given by the Student, the Academic Supervisor or any other of Imperial’s employees, students, agents or appointees who works on the Project, or the content or |
| use of any materials, works or information provided in connection with the Project, will not constitute or result in infringement of third-party rights. |
| 10.2 | Imperial and the Student accept no responsibility for any use which may be made of any work carried out under or pursuant to this Agreement, or of the results of the Project, nor for any reliance which may be placed on such work or results, nor for advice or information given in connection with them. |
| 10.3 | The Sponsor undertakes to make no claim in connection with this Agreement or its subject matter against the Student, the Academic Supervisor or any other employee, student, agent or appointee of Imperial (apart from claims based on fraud or wilful misconduct). This undertaking is intended to give protection to individual researchers: it does not prejudice any right which the Sponsor might have to claim against Imperial. |
| 10.4 | The liability of any Party for any breach of this Agreement, or arising in any other way out of the subject matter of this Agreement, will not extend to loss of business or profit, or to any indirect or consequential damages or losses. |
| 10.5 | The maximum liability of Imperial to the Sponsor under or otherwise in connection with this Agreement shall not exceed four times the value of the payments received under clause 3.1. For the avoidance of doubt, nothing in this clause 10 shall be deemed to exclude or limit in any way Imperial’s liability for intentional wrongdoing or Imperial’s statutory liability in respect of death or personal injury caused to any person as a result of Imperial’s negligence. |
| 11. | NOTICES |
Imperial’s representative for the purpose of receiving Project-related notices shall until further notice be:
Xx X. X. Xxxxxxxxx
with a copy to:
The Administrator
Division of Cell & Molecular Biology
Faculty of Life Sciences
Xxx Xxxxxxxxx Xxxxxxx Building
Imperial College of Sciences, Technology and Medicine
Xxxxx Xxxxxxxxxx Xxxxxx
Xxxxxx, XX0 0XX
Xxxxxx Xxxxxxx
Imperial’s representative for the purpose of receiving legal notices shall be:
Research Services
Faculty of Life Sciences
Xxxxx 0, Xxxxxxx Xxxxxxxx
Xxxxxxxxxx Xxxx
Xxxxx Xxxxxxxxxx Campus
Xxxxxx, XX0 0XX
Xxxxxx Xxxxxxx
with a copy to:
Xx X. X. Xxxxxxxxx
Senior Lecturer & Group Leader
Recombinant Antibody Therapeutics Lab
Dept of Biological Sciences
Xxxxxxx Biochemistry Building
Imperial College London
Xxxxxxxxxx Xx
Xxxxxx, XX0 0XX
Xxxxxx Xxxxxxx
The Sponsor’s representative for the purpose of receiving invoices, reports and other notices shall until further notice be:
Xxxxxxxxx X. Xxxxxxxx
Somanta Pharmaceuticals Inc
00 Xxxxxx Xxxxxx
Xxxxxx, X0X 0XX
Xxxxxx Xxxxxxx
Tel: 000 0000 0000
Fax: 000 0000 0000
with a copy to:
Xxxx Xxxxxx,
Xxxxx & Xxxxxxx LLP,
000 X. Xxxxxxxx, 00xx Xxxxx,
Xxx Xxxxx,
XX 00000
Tel: 000-000-0000
| 12. | GENERAL |
| 12.1 | No Party shall be liable for delay in performing or for failure to perform obligations hereunder if the delay or failure results from any cause or circumstance whatsoever beyond its reasonable control, including any breach or non-performance of this Agreement by the other party (hereinafter “Event of Force Majeure”), provided the same arises without the fault or negligence of such party. If an Event of Force Majeure occurs, the date(s) for performance of the obligation affected shall be postponed for as long as is made necessary by the Event of Force Majeure, provided that if any Event of Force Majeure continues for a period of three (3) months or more, Imperial and the Sponsor shall have the right to terminate this Agreement forthwith |
| by written notice to the other parties. All Parties shall use their reasonable endeavours to minimise the effects of any Event of Force Majeure. |
| 12.2 | Clause headings are inserted into this Agreement for convenience only, and they shall not be taken into account in the interpretation of this Agreement. |
| 12.3 | Nothing in this Agreement shall create, imply or evidence any partnership or joint venture between Imperial or the Student and the Sponsor or the relationship between them of principal and agent or employers and employee. |
| 12.4 | Neither Imperial nor the Sponsor shall use the name, crest, logo or registered image of the other or the other’s Affiliates in a press release or promotional materials, without the prior written consent of the other; provided, however, that publication of the sums received from the Sponsor in Imperial’s Annual Report and similar publications shall not be regarded as breach of this clause. |
| 12.5 | Except as expressly provided in this Agreement, nothing in this Agreement shall confer or purport to confer on a third party any benefit or any right to enforce any term of this Agreement. |
| 12.6 | This Agreement and its two Schedules (which are incorporated into and made a part of this Agreement) constitute the entire agreement between the parties for the Project. Any variation shall be in writing and signed by authorised signatories for both Parties. |
| 12.7 | This Agreement shall be governed by English Law. The English Courts shall have exclusive jurisdiction to deal with any dispute which may arise out of or in connection with this Agreement. |
| 12.8 | If any one or more clauses or sub-clauses of this Agreement would result in this Agreement being prohibited pursuant to any applicable competition law then it or they shall be deemed to be omitted. The Parties shall uphold the remainder of this Agreement, and shall negotiate an amendment which, as far as legally feasible, maintains the economic balance between the Parties. |
IN WITNESS WHEREOF this Agreement has been signed by the duly authorised representatives of the Sponsor and Imperial, and by the Academic Supervisor and the Student to the extent stated below.
For and on behalf of Somanta Pharmaceuticals Inc
| Signature | /s/ X. X. Xxxxxxxx | |||||||
| Name: | X X Xxxxxxxx | Title: | CEO, Somanta Pharmaceuticals Inc | |||||
| Date: | 27/7/06 | |||||||
For and on behalf of Imperial College of Science Technology and Medicine
| Signature: | /s/ Xxxxx Xxxxxxx | |||||||
| Name: | Xxxxx Xxxxxxx | Title: | Head of Research Operation | |||||
| Date: | 17/8/06 | |||||||
By the Student
| Signature: | /s/ Xxxxx Xxxxxx | |||||||
| Name: | Xxxxx Xxxxxx | |||||||
| Date: | 31/07/06 | |||||||
I, the Academic Supervisor, acknowledge that I have read and understood the terms and conditions of this Agreement and accept to be bound personally by Clauses 2.6, 4, 5, 6 and 7. I also agree to use all reasonable endeavours to enable Imperial to comply with its obligations under this Agreement.
| Signature: | /s/ Xx. Xxxxxxxx Xxxxxxxxx |
Date: | 31/07/06 | |||||
FIRST SCHEDULE
Research proposal: Pre-clinical evaluation of Angiolix (MC-3) humanised monoclonal antibody and lactadherin target for anti-vasculature cancer therapy
Xxxxx Xxxxxx, Xxxxxxxx Xxxxxxxxx and Xxxxxxxx Xxxxxxxxx
Division of Cell and Molecular Biology, Faculty of Natural Sciences, Imperial College London.
Aims
To characterise Angiolix in vitro and in vivo and assess its anti-tumour properties as a free antibody and in combination with chemotherapy agents. To investigate the target (lactadherin) and discover new information which will help improve Angiolix or its derivatives as a therapeutic.
Background
Lactadherin is a 46kDa extracellular matrix glycoprotein, with two functional domains; an N-terminal RGD sequence containing EGF domain, required for integrin binding and a C-terminal phosphatidylserine (PS) binding domain which associates directly with membrane phospholipids [1] and facilitates mobility within membranes [Unpublished work]. Lactadherin is thought to have several functions including roles in angiogenesis [2] and tissue remodelling post-embryogenesis [unpublished work]. The angiogenic functions are attributed to association with integrins avb3 and avb5, expressed on proliferating vascular endothelial cells [2]. The tissue remodelling functions of Lactadherin are thought to be a result of its ability to act as a linker between apoptotic cells, which display PS on their outer membranes and macrophages and dendritic cells, which display surface integrins [unpublished work]. Lactadherin is also known to be a milk fat globule protein and is expressed in mammary epithelial cells during pregnancy, lactation and gland involution following weaning. Lactadherin is thought to be involved in angiogenesis and tissue remodelling during these processes. The tissue remodelling and angiogenesis promoting functions of lactadherin are also though to be important for tumour development [unpublished work].
The mouse monoclonal antibody muMc3 and its humanised counterpart huMc3 (Angiolix) have been shown in vitro to bind to the integrin binding domain of lactadherin and inhibit association between lactadherin and its integrin receptors [3]. In both unconjugated and radioisotope conjugated forms these antibodies have been shown to preferentially localise in breast tumour tissues and reduce tumour size. One of the radioimmunoconjugates caused complete destruction of human breast epithelial tumour xenografts in mice [unpublished work, 4]. The success of these monoclonal antibodies in inhibiting tumour growth and destroying pre-existing cancer tissues are attributed partially to the blockage of association between Lactadherin and vascular endothelium integrins, precluding angiogenesis and new vessel survival [2]. Lactadherin was originally chosen as a target for breast cancer therapy due to overexpression in breast carcinomas. This may provide a second mechanism for tumour tissue destruction, by targeting antibody mediated cellular cytotoxicity (ADCC) to the tumour tissue and if conjugated, targeting ionising radiation. These in vivo studies indicated Angiolix as being a potentially highly effective breast cancer therapy.
A more general role of Lactadherin in angiogenesis was demonstrated by a recent paper, showing Lactadherin to be an essential component of both VEGF dependant and VEGF independent signalling pathways [2]. The potential of Lactadherin as a target for antiangiogenic therapy for all cancers and some chronic diseases may therefore be investigated.
The role of Lactadherin in angiogenesis indicates that it should be expressed by cancer tissues for them to grow beyond a critical size. This was demonstrated by a paper published in 1991 [5] which investigated the expression of BA46 mRNA in human cancer cell lines. A high level of expression was observed in A459 (lung), ELL-G (breast) tissue, XXXX (ovary) and HS578T (breast) carcinoma cells. Some expression was also demonstrated in BT20 (breast), XXX-XX-000 (xxxxxx), XXX-XX-000 (xxxxxx), XX00 (xxxxx) and MCF-7 (breast) carcinoma tissues. This expression profile provides sufficient rationale for testing the application of Angiolix as an antiangiogenic therapy for cancers other than mammary tumours. A paper published in 1987 [6] however raised some concerns by demonstrating a lack of efficacy of Mc3 (in a cocktail with two other milk fat globule proteins) against non-mammary human tumour tissues. As however only two tumour cell lines were investigated, (lung LX-1 and colon CX-1) this dos not necessarily eliminate Angiolix as an effective treatment for non-breast cancers
As Angiolix is expected to act as an angiogenesis inhibitor, it may have parallel effects with Avastin (Bevacizumab). Avastin is a monoclonal antibody which binds to VEGF, preventing association with and activation of the VEGF-R2 receptor, so inhibiting the signalling cascade that leads to vascular endothelial cell proliferation and ultimately new vessel formation [7]. Like VEGF, Lactadherin is expressed by tumour cells and released locally, where it acts on a receptor in the vascular endothelium. [7, unpublished work]. Whereas VEGF associates with the VEGF-R2 receptor and activates it in an integrin dependant manner, Lactadherin associates with the integrin and this association is required for integrin function [2]. Avastin and Angiolix may therefore inhibit different components of the same pathway. It is for this reason that the preclinical trials planned for Angiolix will follow along similar lines of those used for Avastin and the efficacy of both antibodies will be compared.
It is ultimately hoped that Angiolix, like Avastin therapy will be successful in increasing life expectancy and quality of life in cancer patients [8].
References
[1] Shi J., Xxxxxxx X.X. (2003) Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood. 101: 2628-36.
[2] Xxxxxxxxx X. X., Thery C., Hamard G., Boddaert J., Aguilar B., Delcayre A., Houbron C., Tamarat R., Blanc-Brude O., Heeneman S., Clergue M., Duriez M., Xxxxxx X., Xxxx B., Xxxxxx A., Amigorena S., Mallat Z. (2005) Lactadherin promotes VEGF-dependent neovascularisation. Nat Med. 11: 499-506.
[3] Xxxxxx M.R., Xxxxx J.R., Xxxxxxx X.X., Xxxxxxx X.X., Xxxxxxxx X.X. (1997). Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 16: 861-9.
[4] Xxxxxxx X.X., Blank X.X. Xxxxx X.X., Xxxxxxxx. (1995) Biological activity of two humanised antibodies against two different breast cancer antigens and comparison to their original murine forms. Cancer Res. (suppl) 55: 5852-56.
[5] Xxxxxxx D., Xxxxxxxx X.X., Xxxxx R., Xxxxxxxxx J., Xxxxxxxx A.M., Xxxxxxx X.X. (1991) A Mr 42,000 human milk fat globule protein that is highly expressed in human breast tumours contains factor VIII-like domains. Cancer Res. 51: 4994-8
[6] Xxxxxxx X.X., Blank E.W., Xxxxxxxx X.X. (1987) Experimental immunotherapy of human breast carcinomas implanted in nude mice with a mixture of monoclonal antibodies against human milk fat globule components. Cancer Res. 47: 532-40.
[7] Gerber H.P., Xxxxxxx N. (2005) Pharmacology and pharmacodynamics of Bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 65: 671-80.
[8] Xxxxxxx H., Xxxxxxxxxxxx L., Xxxxxxx W., Xxxxxxxxxx T., Xxxxxxxxxx J., Xxxx W., Berlin J., Baron A., Xxxxxxxx S., Xxxxxxxx E., Xxxxxxx N., Xxxx G., Xxxxxx B., Xxxx X., Kabbinavar F. (2004). Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 359: 2335-42.
Objectives
Closely following the pre-clinical research of the highly successful anti-vasculature monoclonal antibody, Avastin (Bevacizumab), experiments will be carried out to evaluate the potential of Angiolix. Short-term objectives include rapid in vivo targeting and tumour eradication and longer term goals include the cloning of the antibody genes towards the development of improved derivatives. The experiment planned are:
| 1. | Take delivery of the cell-line expressing Angiolix and make a cell bank. |
| 2. | Take delivery of the recombinant antigen (lactadherin) in the form of a cell line |
| 3. | Express Angiolix and purify by protein A chromatography, determining yields. |
| 4. | Modify Angiolix expression conditions to optimise protein yields. |
| 5. | Express lactadherin and purify, determining yields. |
| 6. | Obtain some Avastin antibody to use as a comparative control. |
| 7. | Carry out in vitro antigen-binding assays to confirm function and antibody and antigen. Develop biotinylated antigen system. Determine affinity value (ELISA, BIACore). |
| 8. | Identify at least 4 human tumour cell lines suitable for in vitro and in vivo models. |
| 9. | Evaluate the use of HUVEC and HSVEC endothelial lines as targets. |
| 10. | Carry out in vitro cell-binding assays to validate cell targets. Confirm with competition studies with free antigen. Use cell ELISA, FACS and confocal microscopy. |
| 11. | Establish human tumour xenografts in vivo on BALB/C nude or SCID mice with tumour cell lines. |
| 12. | Carry out immunohistochemical analysis of fresh frozen or paraffin-embedded tumours to confirm Angiolix binding to tumours. |
| 13. | Perform Reverse-transcriptase PCR on tumour samples to confirm lactadherin antigen expression. |
| 14. | Develop in vitro and in vivo models of vasculature proliferation with VEGF to test Angiolix binding. Compare with growth factors which do not stimulate the VEGF pathways such as bFGF. |
| 15. | Carry out in vitro cell growth inhibition studies with Angiolix. Assess effects with free antibody, free antibody and exogenous PBLs, free antibody and chemotherapy, all three components. Determine mechanism of cell death using kits which detect cell proliferation, necrosis and apoptosis. Use iotype-matched control monoclonal antibody as a negative control. Use Avastin (if available) as a positive control. |
| 16. | Establish human tumour xenografts and study the in vivo biodistribution and pharmacokinetics of radiolabelled (125-Iodine) Angiolix. |
| 17. | Carry out in vivo tumour regression/growth delay studies along similar lines to (15). |
| 18. | Develop more elaborate in vivo imaging techniques to visualise Angiolix binding. |
| 19. | Assess the ability of Angiolix to reduce the instances of metastases using a liver or lung metastatic tumour model in vivo. |
| 20. | PCR clone the variable and first constant domains and determine DNA sequence. |
| 21. | Construct an E.coli expression vector expressing the Fab and/or single-chain Fv forms of the Angiolix antibody. Compare binding to a proteolytically-derived Fab. |
| 22. | If results so far indicate Angiolix works by signalling, perform a series of in vivo experiments as described for the whole IgG with the Fab/scFv derivatives. Determine if faster targeting and improved tumour perfusion results in effective anti-tumour therapy. |
| 23. | Using site-directed mutagenesis and/or display technologies, identify higher affinity derivatives of Angiolix. |
| 24. | Depending on results so far, build back the higher affinity fragments into a whole immunoglobulin and characterise as already described for whole, unmodified Angiolix. Alternatively, assess in vitro and in vivo efficacy of higher affinity fragments of Angiolix. |
| 25. | Using the recombinant lactadherin antigen, select for new lactadherin binders using either traditional monoclonal immunization strategies or phage library selection. |
| 26. | Compare new lactadherin-binding antibodies with Angiolix using the approaches described above. |
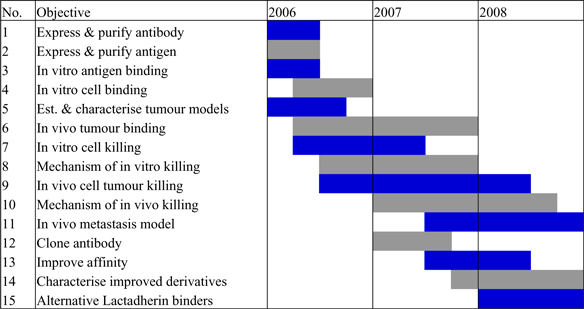
| Milestones | ||||
| No | Milestone |
Expected date | ||
| 1 | Expression & purification of Angiolix | Q2, 2006 | ||
| 2 | Expression & purification of Lactadherin | Q2, 2006 | ||
| 3 | In vitro binding | Q2, 2006 | ||
| 4 | In vivo binding | Q3, 2006 | ||
| 5 | In vivo tumour regression | Q4, 2006 | ||
| 6 | Mechanism of tumour cell killing | Q2, 2007 | ||
| 7 | High affinity derivatives | Q4, 2007 | ||
| 8 | New lactadherin binders | Q3, 2008 | ||
Finance
Per year for three years
| Student bursary- | Paid by BBSRC-CASE/Imperial College | |
| Student PhD Fees | Paid by BBSRC/Imperial College | |
| Student bursary top-up | ||
| (A condition of the BBSRC/CASE award)- | £2500 | |
| Laboratory consumable expenses- | £10,000 | |
| (Animal research- | £8,000 | |
| 20x£60per mouse=£1200 | ||
| Maintenance, technical help=£800) | ||
| Grand Total= | £20,500 pa | |
SECOND SCHEDULE (FUNDING)
Studentship Agreement between Somanta Pharmaceuticals Inc
and Imperial College of Science, Technology and Medicine
Student: Xxxx Xxxxx Xxxxxx;
REF:
The student will be co-funded by the BBSRC (Industrial CASE award/CASE Quota award) and Somanta Pharmaceuticals Inc
A fixed sum of £20,500 will be paid by the Sponsor annually to support the Studentship as indicated below.
| a) | Students Stipend: |
The BBSRC stipend rates may be adjusted each year. The CASE industrial bonus however shall for the term of the Project be £2,500 per annum.
| b) | Cost of Travel to Scientific Meetings: £0 per annum for the term of the Project. |
| c) | Consumables: |
All remaining funds are to be used for consumables, equipment or other direct costs required for the Programme of Research. The industrial contribution shall for the term of the Project be £18,000 per annum.
Payments will be made by BACs on receipt of the executed Agreement and invoice on the following dates:
| Payment # |
Date of Invoice |
Amount (£ GBP) | ||
| 1 |
28th July 2006 | 20500 | ||
| 2 |
1st October 2006 | 5125 | ||
| 3 |
1st January 2007 | 5125 | ||
| 4 |
1st April 2007 | 5125 | ||
| 5 |
1st July 2007 | 5125 | ||
| 6 |
1st October 2007 | 5125 | ||
| 7 |
1st January 2008 | 5125 | ||
| 8 |
1st April 2008 | 5125 | ||
| 9 |
1st July 2008 | 5125 |
Approved by the Institution Finance Department:
| Name: | Xxxxx Xxxxxxx | Signature: | /s/ Xxxxx Xxxxxxx | |||||
| Telephone: | 000-0000-0000 | Fax: | 000-0000-0000 | |||||
No VAT is due since Somanta Pharmaceuticals Inc is a US company.
All invoices should be sent on or the above dates directly to:
Xxxx. X. X. Xxxxxxxx
Somanta Pharmaceuticals Inc,
00 Xxxxxx Xxxxxx
Xxxxxx, X0X 0XX
Xxxxxx Xxxxxxx

