DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
Exhibit 10.22
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
Execution Document 2/27/13
DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
THIS DEVELOPMENT AND COMMERCIALIZATION AGREEMENT (this “Agreement”) is effective as of February 28, 2013 (“Effective Date”), by and between ▇▇▇▇▇▇ Molecular Inc., a corporation organized under the laws of Delaware (“Abbott”), and Epizyme, Inc. (“Epizyme”), a corporation organized under the laws of Delaware.
RECITALS
A. Epizyme is developing a certain proprietary compound, a DOT1L inhibitor known as EPZ-5676, for the treatment of acute leukemias that result from MLL translocations.
▇. ▇▇▇▇▇▇ is in the business of developing and marketing in vitro diagnostic devices and has developed certain technology and possesses experience relating to the preparation and development of FISH assays performed on specimens.
C. Epizyme desires that a fluorescence in situ hybridization diagnostic test to detect MLL translocations (“Diagnostic Test,” as further defined herein) be developed, registered and commercialized in conjunction with Epizyme’s registration, marketing and sale of an Epizyme Product (as further defined below), and Abbott is willing to undertake such activities pursuant to the provisions of this Agreement.
NOW, THEREFORE, in consideration of these premises and the terms and conditions contained herein, the Parties hereto agree as follows.
ARTICLE 1
DEFINITIONS
As used in this Agreement, the following terms shall have the following meanings, whether used in the singular or plural:
“Abbott” shall have the meaning set forth in the Preamble.
“▇▇▇▇▇▇ Claim” shall have the meaning set forth in Section 10.1(a).
“Abbott Diagnostics” shall mean any and all existing FISH assay platforms/systems (including the VP 2000™ and ThermoBrite™) and FISH assay reagents, kits and protocols for use on existing FISH assay platforms/systems developed, Controlled, or sold by Abbott, and all related development, manufacturing and commercialization activities, including the Diagnostic Test.
“Abbott Inventions” shall have the meaning set forth in Section 7.8(b).
“Abbott Losses” shall have the meaning set forth in Section 10.1(a).
“Abbott Know-How” means all Know-How relating to or arising from the Abbott Diagnostics which is (i) existing now or at any time in the future, and (ii) Controlled by Abbott independent of this Agreement, and (iii) necessary for the development or commercialization of the Diagnostic Test.
“Abbott Party” shall have the meaning set forth in Section 10.1(a).
“Abbott Patent Rights” means all Patent Rights (a) covering ▇▇▇▇▇▇’▇ FISH Platform Technology or (b) covering the Diagnostic Test, which are Controlled now or at any time in the future by Abbott or its Affiliates independent of this Agreement.
“Abbott Technology” shall mean, collectively, Abbott Patent Rights and Abbott Know-How, to the extent such Abbott Know-How specifically relates to Abbott Diagnostics or the Diagnostic Test.
“Affiliate” shall mean any Person which controls, is controlled by, or is under common control with the applicable Person. For purposes of this definition, “control” shall mean: (a) in the case of corporate entities, direct or indirect ownership of fifty percent (50%) or more of the stock or shares (or such lesser percentage which is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) entitled to vote for the election of directors, or otherwise having the power to control or direct the affairs of such Person; and (b) in the case of non-corporate entities, direct or indirect ownership of at least 50% of the equity interest or the power to direct the management and policies of such noncorporate entities.
“Agreement” shall have the meaning set forth in the Preamble.
“Alliance Lead” shall have the meaning set forth in Section 4.7.
“Applicable Law” shall mean all applicable provisions of all statutes, laws, rules, regulations, administrative codes, ordinances, decrees, orders, decisions, injunctions, awards judgments, permits and licenses of or from governmental authorities, including those relating to or governing the use or regulation of the subject item and the listing standards or agreements of any national or international securities exchange.
“Breaching Party” shall have the meaning set forth in Section 9.2.
“Bridging and Clinical Trial Site Costs” shall include the costs incurred by Abbott in accordance with the budget estimate set forth in Exhibit E in performing clinical studies; validation studies; laboratory start-up costs; investigational review board costs; case report forms; shipping cost for supplies and slides; monitoring visits (including travel costs); and reagents and other consumable products, all as necessary in connection with conducting clinical trials to obtain required Regulatory Approvals for the Diagnostic Test for the Diagnostic Test Intended Use. Abbott shall not exceed the budget estimate for such costs set forth in Exhibit E unless the excess costs have been approved in advance by Epizyme.
“CDA” shall mean that Disclosure Agreement executed by the Parties as of January 11, 2012.
2
“Claim” shall have the meaning set forth in Section 10.3(a).
“Commercially Reasonable Efforts” shall mean, with respect to the research, development and sale of any Epizyme Product by Epizyme, or of the Diagnostic Test by Abbott, such efforts and resources substantially equivalent to those efforts and resources commonly used by Abbott for diagnostic products and by Epizyme for pharmaceutical products, in each case as such product is owned by such Party or to which such Party has rights, which product is at a similar stage in its development or product life and is of similar market potential taking into account efficacy, safety, approved labeling, the competitiveness of alternative Third Party products in the marketplace, the patent and other proprietary position of the product, the likelihood of regulatory approval given the Regulatory Authority involved, the profitability of the product including the amounts payable to licensors of patent or other intellectual property rights (it being understood that, because Epizyme is funding ▇▇▇▇▇▇’▇ development of the Diagnostic Test, Abbott shall at all times exercise at least reasonable efforts with respect to the Diagnostic Test notwithstanding profitability concerns, if any, and Abbott shall not reduce its efforts with respect to the Diagnostic Test pursuant to this Agreement based on opportunities to develop the Diagnostic Test or similar diagnostic products as companion diagnostics for Third Party therapeutic products that are competitive with the Epizyme Product) and other relevant factors. Commercially Reasonable Efforts shall be determined on a market-by-market basis for a particular product, and it is anticipated that the level of effort will be different for different markets, and will change over time, reflecting changes in the status of the product and the market(s) involved. For the avoidance of doubt, if an injunction issues based on any intellectual property infringement alleged in the Enzo Lawsuit, as between Abbott and Epizyme, Abbott shall be solely responsible for, and shall use its commercially reasonable best efforts toward, either having such injunction lifted or obtaining a license to the underlying intellectual property that is the basis for such injunction.
“Commercialization Lead” shall have the meaning set forth in Section 4.5.
“Commercialization Plan” shall have the meaning set forth in Article 3.
“Compound” shall mean Epizyme’s DOT1L inhibitor EPZ-5676 or, if Epizyme develops a successor compound in lieu of EPZ-5676, such successor compound, it being understood that if a successor compound is pursued by Epizyme, appropriate changes to the Development Plan and payments to Abbott hereunder will be made pursuant to Sections 2.1 and 5.2.
“Confidential Information” shall mean any and all non-public information, materials, data, samples, business plans, financial information, marketing plans, reports, forecasts, technical or commercial information that is provided by Disclosing Party to a Receiving Party hereunder and which is either disclosed in writing and marked as confidential at the time of disclosure or, if disclosed orally, referenced in writing and identified as confidential within a reasonable period of time following such oral disclosure; including any and all information regarding, related to, arising from or associated with this Agreement or the activities contemplated hereby, the Compound, any Epizyme Product, the Diagnostic Test, Inventions, and the existence, terms and conditions of this Agreement.
3
“Control” or “Controlled” means, with respect to a Party and with respect to an item of Abbott Technology or Epizyme Technology, as applicable, the possession, whether by ownership or license (other than pursuant to this Agreement), by such Party of the ability to grant to the other Party access and/or a license as provided herein under such item or right without violating the terms of any agreement with any Third Party.
“Data” shall mean any and all data, results, conclusions, reports, and other information generated by or for Epizyme resulting from the activities performed under the Development Plan.
“Development Lead” shall have the meaning as set forth in Section 4.5.
“Development Plan” shall have the meaning as set forth in Article 2.
“Diagnostic Test” shall mean the MLL FISH assay to detect MLL break apart status in acute leukemia patients, developed by Abbott for the Diagnostic Test Intended Use and developed and commercialized pursuant to the provisions of this Agreement.
“Diagnostic Test Intended Use” shall mean the identification of patients who are appropriate candidates for treatment with an Epizyme Product for the Epizyme Product Indication, based on the use of the Diagnostic Test to identify such patients as approved by a Regulatory Authority.
“Diagnostic Test Trademarks” shall mean the Trademarks used in conjunction with the Diagnostic Test in the Territory (excluding, however all Epizyme owned or Controlled Trademarks).
“Disclosing Party” shall have the meaning set forth in Section 6.1.
“Effective Date” shall have the meaning set forth in the Preamble.
“EU” shall mean all member states of the European Union.
“Enzo Lawsuit” means the lawsuit captioned Enzo Life Sciences Inc. ▇. ▇▇▇▇▇▇ Laboratories and ▇▇▇▇▇▇ Molecular Inc., filed in the United States District Court for the District of Delaware as case number 1:2013cv00225, and any lawsuit between or among Enzo Life Sciences Inc., ▇▇▇▇▇▇ Laboratories and/or Abbott based on the same or similar underlying subject matter filed in any other jurisdiction.
“Epizyme” shall have the meaning set forth in the Preamble.
“Epizyme Claim” shall have the meaning set forth in Section 10.2(a).
“Epizyme Inventions” shall have the meaning set forth in Section 7.8(c).
“Epizyme Know-How” means all Know-How (i) existing now or at any time in the future, and (ii) Controlled by Epizyme independent of this Agreement, and (iii) is necessary for the development or commercialization of the Diagnostic Test for the Diagnostic Test Intended Use.
4
“Epizyme Losses” shall have the meaning set forth in Section 10.2(a).
“Epizyme Materials” shall meaning set forth in Section 2.2(a).
“Epizyme Party” shall have the meaning set forth in Section 10.2(a).
“Epizyme Patent Rights” means all Patent Rights claiming or covering: (a) the use of Compound or Epizyme Product; and/or (b) the use of the Diagnostic Test for the Diagnostic Test Intended Use, in each case Controlled now or at any time in the future by Epizyme independent of this Agreement.
“Epizyme Product” means any pharmaceutical or biological preparation in final form containing the Compound (i) for sale by prescription, over-the-counter or any other method; or (ii) for administration to human patients in a clinical trial, including in each case any monotherapy containing the Compound, any combination product containing the Compound, or any concomitant administration of a preparation containing the Compound or any other Epizyme DOT1L inhibitor with a preparation containing a small molecule or biological.
“Epizyme Product Indication” means the treatment of acute leukemias resulting from MLL translocations, with a label claim for such Epizyme Product approved by a Regulatory Authority based on the therapeutic response of patients with acute leukemias. If Epizyme develops an Epizyme Product for one or more additional indications, Abbott shall not have any obligation hereunder to develop the Diagnostic Test for such additional indication(s) unless and until appropriate changes to the Development Plan and payments to Abbott hereunder are made pursuant to Sections 2.1 and 5.2.
“Epizyme Technology” shall mean, collectively, Epizyme Patent Rights and Epizyme Know-How, to the extent such Epizyme Know-How specifically relates to the Compound or any Epizyme Product.
“FDA” shall mean the United States Food and Drug Administration, or any successor agency thereto.
“First Commercial Sale” shall mean with respect to the Diagnostic Test and any country in the Territory, the first commercial sale by Abbott or its Affiliates, sublicensees or distributors of the Diagnostic Test in that country to a Third Party, after such Diagnostic Test has been granted final Regulatory Approval by the competent Regulatory Authorities in such country.
“FISH” shall mean a process known as fluorescence in situ hybridization.
“FISH Platform Technology” shall mean the FISH process technology and the FISH probe composition and manufacturing technology disclosed and claimed in the United States patents listed in Exhibit A(1).
5
“Invention” shall mean and include any and all inventions and discoveries which are, or may be, patentable or otherwise protectable under the patent or other intellectual property laws of any country, which are conceived or discovered by either Party, its Affiliates or sublicensees during its or their respective activities pursuant to the Development Plan, the Regulatory Plan and the Commercialization Plan during the term of this Agreement.
“Joint Inventions” shall have the meaning as defined in Section 7.8.
“Joint Patent Right” shall have the meaning as set forth in Section 7.8.
“JSC” shall mean the Joint Steering Committee established pursuant to Article 4.
“Know-How” shall mean technical and other information which is not in the public domain, including information comprising or relating to concepts, discoveries, data (including raw data), designs, formulae, ideas, inventions, materials, methods, models, research plans, procedures, designs for experiments and tests and results of experimentation and testing, processes, laboratory records, chemical, pharmacological, toxicological clinical, analytical and quality control data, pre-clinical, clinical and non-clinical trial data, case report forms, data analyses, reports, manufacturing data or summaries and information contained in submissions to an information from ethical committees and Regulatory Authorities. Know-How includes documents containing Know-How, including any rights including trade secrets, copyright, database or design rights protecting such Know-How.
“MLL” shall mean the human Mixed Linage Leukemia (MLL) gene.
“Non-Breaching Party” shall have the meaning set forth in Section 9.2.
“Orange Book” shall mean the publication entitled “Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book),” or any successor thereto, as published from time to time by the FDA.
“Party” or “Parties” shall mean Epizyme or Abbott, or Epizyme and Abbott, as the context admits.
“Patent Right” shall mean any and all (i) patents, (ii) pending patent applications, including all provisional applications, substitutions, continuations, continuations-in-part, divisionals, renewals, and all patents granted thereon, (iii) all patents-of-addition, reissues, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms, including patent term extensions, supplementary protection certificates or the equivalent thereof, and (iv) all foreign counterparts of any of the foregoing.
“Payment Timetable” shall have the meaning set forth in Section 5.1.
“Person” shall mean any natural person, corporation, unincorporated organization, partnership, association, joint stock company, joint venture, limited liability company, trust or government, or any agency or political subdivision of any government, or any other entity.
6
“Project Timeline Leads” shall have the meaning set forth in Section 4.8.
“Receiving Party” shall have the meaning set forth in Section 6.1.
“Regulatory Approvals” shall mean and include all licenses, permits, authorizations and approvals of, and all registrations, filings and other notifications to any Regulatory Authority, governmental agency or department within the Territory (including applications therefore) necessary or appropriate for the development, testing, manufacture, production, distribution, marketing, sale and/or use, as applicable, of the Diagnostic Test or any Epizyme Product in a particular country or region of the Territory.
“Regulatory Authority(ies)” shall mean any national, supra-national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity in each country of the world involved in the reviewing, granting or revoking of Regulatory Approvals for the Diagnostic Test.
“Regulatory Plan” shall have the meaning set forth in Exhibit C.
“Specifications” shall mean the specifications applicable to the Diagnostic Test as set forth in Exhibit A(2). The Specifications may be amended, in writing, from time-to-time as deemed necessary by the JSC, provided both Parties consent in writing to such amendment.
“Territory” shall mean the entire world.
“ThermoBrite™” shall mean Abbott and ▇▇▇▇ Sample Processing, Inc.’s co-branded programmable temperature controlled slide processing system, including any similar new and/or successor system developed during the term of this Agreement.
“Third Party” means any Person other than Epizyme or Abbott (or their respective Affiliates).
“Trademarks” shall mean all registered and unregistered trademarks (including all common law rights thereto), service marks, trade names, brand names, logos, taglines, slogans, certification marks, Internet domain names, trade dress, corporate names, business names and other indicia of origin, together with the goodwill associated with any of the foregoing and all applications, registrations, extensions and renewals thereof throughout the world, and all rights therein provided by international treaties and conventions.
“VP 2000™” shall mean ▇▇▇▇▇▇’▇ proprietary consolidated workstation for automated front-end fluorescence in situ hybridization processing, including any similar new and/or successor system developed during the term of this Agreement.
7
ARTICLE 2
DEVELOPMENT
2.1 Development Plan. Abbott shall use Commercially Reasonable Efforts to develop the Diagnostic Test and, with Epizyme’s cooperation, shall develop the Diagnostic Test pursuant to the provisions of a development plan which shall set forth, inter alia:
(a) the activities to be performed by each Party under the Development Plan;
(b) the timelines for each activity;
(c) key assumptions underlying the Parties’ respective performance obligations under the Development Plan (the “Key Assumptions”); and
(d) the specifications of the Diagnostic Test.
(as amended from time to time, the “Development Plan”). The initial Development Plan is attached hereto as Exhibit B. The Parties acknowledge that the initial Development Plan does not address all of the items set forth above, but the Parties agree that, as soon as practicable following the Effective Date, the initial Development Plan will be modified and made more comprehensive pursuant to the provisions of this Agreement. Thereafter, as may be necessary from time-to-time, the Development Leads shall suggest appropriate revisions to the Development Plan to the JSC for its prior written review and approval. If the JSC approves such revisions, and the Parties consent in writing to such revisions, then the JSC shall revise the Development Plan accordingly without need for amending this Agreement. The Parties shall not unreasonably withhold their consent to appropriate Development Plan revisions. The revised Development Plan shall thereafter be the Development Plan for all purposes of this Agreement. The Parties shall each use their Commercially Reasonable Efforts to perform all of their obligations under the Development Plan in compliance with Applicable Law.
2.2 Epizyme Materials.
(a) Epizyme will furnish to Abbott certain quantities, and/or Abbott will procure certain quantities using Epizyme’s funds, of clinical trial specimens and other biological and pharmaceutical materials, as agreed upon and set forth in the Development Plan, the Regulatory Plan or the Commercialization Plan (“Epizyme Materials”). For avoidance of doubt, if Abbott procures specimens and other biological and pharmaceutical materials with its own funds, such materials shall not be Epizyme Materials, it being understood that, unless otherwise set forth in the Development Plan, specimens and other biological and pharmaceutical materials required for the performance of the Development Plan will be presumed to be, and shall be, purchased by Abbott using Epizyme’s funds paid to Abbott in accordance with Section 5.1. Abbott will comply with all Applicable Laws relating to the Epizyme Materials. Without limiting the foregoing, to the extent that the Epizyme Materials include human specimens, Epizyme represents and warrants to Abbott that either it has obtained all informed consents and Institutional Review Board (IRB)/Ethics Committee (EC) approval(s) required by Applicable
8
Law with respect to such Epizyme Materials procured by Epizyme or that it is not required under Applicable Law to obtain such informed consents and/or has received a waiver for consent from an IRB/EC.
(b) Abbott agrees to retain possession of the Epizyme Materials and not to provide the Epizyme Materials to any Third Party (except for Third Parties conducting clinical trials on ▇▇▇▇▇▇’▇ behalf pursuant to this Agreement and for whose performance and compliance with the terms of this Agreement Abbott remains primarily liable to Epizyme) or to use or permit the use of any of the Epizyme Materials for any purpose other than the development and commercialization of the Diagnostic Test for the Diagnostic Test Intended Use without Epizyme’s prior written consent.
(c) It is not contemplated that Epizyme will transmit any data that identifies or could be used to identify an individual (“Personal Data”). However, to the extent that Personal Data can be identified from Epizyme Materials, Abbott shall hold in confidence all Personal Data except as required or permitted under this Agreement, or to the extent necessary to be disclosed to regulatory agencies as part of the review process. In addition, Abbott shall comply with all Applicable Laws with respect to the collection, use, storage, and disclosure of any Personal Data, including without limitation, the U.S. Health Insurance Portability and Accountability Act (HIPAA) and the regulations promulgated thereunder. Abbott agrees to ensure that all appropriate technical and organization measures are taken to protect Personal Data against loss, misuse, and any unauthorized, accidental, or unlawful access, disclosure, alteration, or destruction, including without limitation, implementation and enforcement of administrative, technical, and physical security policies and procedures applicable to Personal Data.
2.3 Regulatory Approvals.
(a) In addition to the responsibilities set forth in the Development Plan, Abbott shall use Commercially Reasonable Efforts to seek Regulatory Approvals for the Diagnostic Test and, with Epizyme’s cooperation, shall obtain the necessary Regulatory Approvals in the countries agreed upon in the Regulatory Plan for the commercialization of the Diagnostic Test with the Diagnostic Test Intended Use; provided that, Epizyme may from time to time during the term of this Agreement request that Abbott seek Regulatory Approvals in additional countries or that Abbott refrain from seeking Regulatory Approvals in one or more of the agreed countries identified in the Regulatory Plan, and Abbott shall agree to amend the Regulatory Plan accordingly; provided further that, if Epizyme requests the addition of a country in which Abbott does not then have a presence with any fluorescence in situ hybridization diagnostic test and Abbott does not wish to directly establish such a presence in such country, Abbott shall not be required to add such country to the Regulatory Plan, but Abbott and Epizyme shall discuss in good faith alternative distribution arrangements that might facilitate obtaining Regulatory Approval for and the availability of the Diagnostic Test in such country. The Parties shall agree upon a priority list of countries, from among the countries agreed upon in the Regulatory Plan, for which Abbott shall attempt to first obtain Regulatory Approvals. Abbott shall be responsible for the responsibilities set forth in Exhibit C (the “Regulatory Plan”) with respect to obtaining and maintaining in good standing for the periods covered by the Development Plan and the Commercialization Plan all necessary Regulatory Approvals from the relevant Regulatory
9
Authorities that are required for the development, commercialization and sale of the Diagnostic Test pursuant to the Development Plan and the Commercialization Plan. The Parties acknowledge that the initial Regulatory Plan may not address all steps necessary to obtain all of the necessary Regulatory Approvals, but the Parties agree that, as soon as practicable following the Effective Date, the initial Regulatory Plan will be modified and made more comprehensive pursuant to the provisions of this Agreement. Thereafter, as may be necessary from time-to-time, the Regulatory lead representatives of each Party shall suggest appropriate revisions to the Regulatory Plan to the JSC for its written review and approval. If the JSC approves such revisions, and the Parties consent in writing to such revisions, then the JSC shall revise the Regulatory Plan accordingly without need for amending this Agreement. The Parties shall not unreasonably withhold their consent to appropriate Regulatory Plan revisions. The revised Regulatory Plan shall thereafter be the Regulatory Plan for all purposes of this Agreement.
(b) Epizyme shall be responsible for reimbursing Abbott for the costs and expenses incurred by Abbott in obtaining all Regulatory Approvals for the Diagnostic Test for the Diagnostic Test Intended Use that are approved by Epizyme; provided that, the budget for such costs (i) [**]. Abbott shall [**] Epizyme [**]. Epizyme will [**].
2.4 Rights of Reference. Epizyme hereby grants to Abbott a non-exclusive, non-transferable (except in connection with a permitted assignment, sublicense or subcontract) “right of reference” (as defined in 21 CFR 314.3(b) and comparable regulations outside the United States) with respect to clinical trial Data and results related to any Epizyme Product and Controlled by Epizyme, as necessary for Abbott and its permitted assigns and sublicensees to prepare and submit submissions to Regulatory Authorities and maintain Regulatory Approvals related to the Diagnostic Test and related matters. Abbott hereby grants to Epizyme a non-exclusive, non-transferable (except in connection with a permitted assignment, sublicense or subcontract) “right of reference” (as defined in 21 CFR 314.3(b) and comparable regulations outside the United States) with respect to ▇▇▇▇▇▇’▇ clinical sample analytical trial data and results related to the Diagnostic Test, as necessary for Epizyme and its permitted assigns and sublicensees to prepare and submit submissions to Regulatory Authorities, and to maintain Regulatory Approvals, related to any Epizyme Product and related matters.
2.5 [**]. In the event that [**], Epizyme shall [**]. If within [**] Abbott [**] [**], Epizyme shall [**] Abbott [**] Epizyme [**] Abbott [**] Epizyme; provided that, Epizyme shall have [**], if any, and Epizyme shall [**] Abbott [**] with Epizyme [**]Epizyme [**].
ARTICLE 3
COMMERCIALIZATION
3.1 Commercialization Plan. Abbott shall use Commercially Reasonable Efforts to make the Diagnostic Test commercially available in each country in which Regulatory Approval is obtained pursuant to the Regulatory Plan and, with Epizyme’s cooperation as appropriate and agreed by Epizyme in Epizyme’s reasonable discretion, shall commercialize the Diagnostic Test pursuant to the provisions of a joint commercialization plan (the “Commercialization Plan”), which shall include the following:
(a) the activities to be performed by each Party (and, in the case of Epizyme, if Epizyme has licensed to or is otherwise collaborating with a Third Party with respect to the development or commercialization of the Epizyme Product in any country or territory, the activities to be performed by such Third Party) and the deliverables related thereto;
10
(b) the timelines for each activity under the Commercialization Plan;
(c) the overarching commercialization strategy and commercial goals for the Diagnostic Test, including the availability and distribution of the Diagnostic Test in each country for which Regulatory Approval is sought pursuant to the Regulatory Plan;
(d) customer service;
(e) responsibilities for and restrictions on public relations activity/Direct-To-Consumer and other promotional advertising activity;
(f) coordination of the pre-launch/launch and post launch of Abbott/Epizyme (and Third Party, if applicable) sales teams and scientific teams;
(g) forecasting and measurement of sales and distribution data, including reporting of sales units of the Diagnostic Test to Epizyme and the Epizyme Product to Abbott;
(h) the establishment of “launch success factors” for each market. Such launch success factors shall be agreed upon by the Parties prior to any launch;
(i) activities to be performed post-launch to ensure ongoing alignment of the Diagnostic Test Intended Use and Epizyme Product Indication, including fulfilling any commitment(s) imposed by Regulatory Authorities;
(j) plans for manufacturing and supplying the Diagnostic Test; and
(k) plans for maintaining acceptable levels of regulatory and GMP/GLP compliance during development, manufacture and marketing of the Diagnostic Test.
(as amended from time to time, the “Commercialization Plan”). The initial Commercialization Plan is attached hereto as Exhibit D. The Parties acknowledge that the initial Commercialization Plan does not address all of the items set forth above, but the Parties agree that, as soon as practicable following the Effective Date, the initial Commercialization Plan will be modified and made more comprehensive pursuant to the provisions of this Agreement. Thereafter, as may be necessary from time-to-time, in particular as the Diagnostic Test gets closer to commercialization, the Commercialization Team Lead shall suggest appropriate revisions to the Commercialization Plan to the JSC for its prior written review and approval. If the JSC approves such revisions, and the Parties consent in writing to such revisions, then the JSC shall revise the Commercialization Plan accordingly without need for amending this Agreement. The Parties shall not unreasonably withhold their consent to appropriate Commercialization Plan revisions.
11
The revised Commercialization Plan shall thereafter be the Commercialization Plan for all purposes of this Agreement. The Parties shall each use their Commercially Reasonable Efforts to perform all of their obligations under the Commercialization Plan in accordance with generally accepted ethical, best sales and marketing practices and in compliance with Applicable Law.
3.2 Forecasts. Prior to the anticipated commercial launch of the Diagnostic Test and periodically thereafter, Epizyme shall provide Abbott with nonbinding epidemiologic data and/or market demand forecast information in Epizyme’s possession as may be reasonably agreed by the Parties via the JSC. Epizyme shall have no liability for the accuracy of any such data or information that Epizyme provides.
3.3 Price of Diagnostic Test. The price charged for the Diagnostic Test will be determined by Abbott in its reasonable discretion; provided that, Abbott shall use Commercially Reasonable Efforts to obtain appropriate pricing and reimbursement approvals for the Diagnostic Test in countries in which the Diagnostic Test is commercialized and to price the Diagnostic Test in a manner consistent with market norms for the pricing of companion diagnostic products. If requested by ▇▇▇▇▇▇, Epizyme shall reasonably cooperate with ▇▇▇▇▇▇’▇ efforts to obtain reimbursement approvals for the Diagnostic Test.
ARTICLE 4
GOVERNANCE OF AGREEMENT
4.1 Governance of Agreement. As set forth in this Article 4, the Parties agree to govern the Agreement by way of the following five collaborative groups: (1) Joint Steering Committee; (2) Development and Commercialization Leads; (3) Team Leads; (4) Alliance Leads; (5) Project Timeline Leads. Charts showing such governance and roles and responsibilities of each group are set forth in Exhibits F, G and H.
4.2 Joint Steering Committee. The Parties shall form a Joint Steering Committee (the “JSC”), which shall have the primary role in monitoring and ensuring the overall success of the development and commercialization of the Diagnostic Test. The JSC shall be comprised of no more than [**] professionally and technically qualified representatives, with [**] representatives from each Party and comprised of representatives with sufficient qualifications to make decisions regarding the Development Plan, Regulatory Plan and Commercialization Plan, considering the stage of development or commercialization of the Diagnostic Test. The members of the JSC shall represent the following functional areas of each Party: (1) Research & Development; (2) Commercial; (3) Regulatory/Clinical; and (4) Business Development. The JSC shall meet for the first time within [**] days after the Effective Date and thereafter at least [**] during the term of this Agreement, unless the Parties or the JSC decide that more or less frequent meetings are required; provided, however, that in the event of an emergent situation, including a situation in which a decision by the JSC is required, a meeting shall be held within [**] business days after written request for such meeting by either Party. Each Party shall appoint co-chairpersons of the JSC from among the members of the JSC, who shall be responsible for setting the agenda for and chairing JSC meetings. Each Party’s representatives shall collectively have one vote on each matter that is decided by the JSC. Both votes of the Parties’ representatives shall be required for approval of all actions required of the JSC. In the event of an impasse, the matter shall be
12
resolved pursuant to Section 4.4. The organization of the meeting and the meeting place shall alternate between the offices of Epizyme in Cambridge, Massachusetts and the offices of Abbott in Des Plaines, Illinois or as otherwise decided by the JSC. JSC meetings may be conducted in person, by telephone or videoconference as agreed by the JSC. Each Party shall provide the other Party with written notice of its representatives for the JSC promptly after the Effective Date of this Agreement. Each Party may substitute or replace any of its representatives on the JSC at any time and for any reason upon written notice to the other Party. Additionally, Alliance Leads shall attend JSC meetings as non-voting members, and each Party may invite a reasonable number of other guests to the meetings, in order to discuss special technical or commercial topics relevant to the applicable agenda; provided, that any guests are subject to the confidentiality provisions set forth in Article 6.
4.3 JSC Responsibilities. The JSC’s activities shall include the following responsibilities with regard to the Diagnostic Test:
(a) review, confirmation, modification and/or update of the Development Plan, the Regulatory Plan and the Commercialization Plan, subject to final approval by the Parties;
(b) monitoring of the development, regulatory and commercialization activities under the Development Plan, the Regulatory Plan and Commercialization Plan, respectively;
(c) resolution of issues raised by the Alliance Leads;
(d) exchange of development and commercialization information; and
(e) alignment of the regulatory submissions and Regulatory Approvals between any Epizyme Product and the Diagnostic Test.
The JSC shall keep accurate and complete confidential minutes of its meetings. The Alliance Leads shall be responsible for taking such minutes and distributing them to the JSC members for their review and comment within [**] business days after the date of each meeting, and within [**] business days after the receipt thereof, the JSC members shall remit such minutes back to the Alliance Leads with their comments, if any. The JSC members shall in good faith attempt as quickly as is reasonably possible to resolve any disputes as to the content of such minutes so as to have a final agreed version as quickly as is reasonably possible. Each Party shall be responsible for all expenses incurred by its representatives on the JSC in connection with performing their duties hereunder, including all costs of travel, lodging and meals. For the avoidance of doubt, the JSC shall not have the authority to amend this Agreement, and shall have authority to amend the Development Plan, the Regulatory Plan and/or the Commercialization Plan only as expressly set forth herein, with the written consent of the Parties.
4.4 JSC Decisions. All decisions of the JSC shall be made in good faith in the interest of furthering the purposes of this Agreement and the JSC members shall use their Commercially Reasonable Efforts to take decisions unanimously (but Section 4.1 shall apply if unanimity is not
13
achieved). If the JSC is unable to agree on any matter, then either Party’s co-chairperson of the JSC may refer the disagreement to a meeting between the Chief Executive Officer of Epizyme (currently ▇▇▇▇▇▇ ▇▇▇▇▇) and the President of Abbott (currently ▇▇▇▇ ▇▇▇▇▇▇▇) which meeting shall take place as soon as practicable, but in no event later than [**] days after the date of the relevant referral. Notwithstanding the foregoing, except as otherwise provided in this Agreement, following the foregoing referral and meeting, if such officers are unable to resolve the issue by mutual agreement: (a) Epizyme will have the unilateral right to make final decisions that solely impact the development, manufacture or marketing of any Epizyme Product; and (b) Abbott will have the unilateral right to make final decisions that solely impact the development, manufacture or marketing of the Diagnostic Test; provided that neither Party shall be entitled to make a unilateral decision which imposes an obligation on the other Party to take on a financial obligation or deviates from the previously approved Development Plan, Regulatory Plan or Commercialization Plan or which is inconsistent with such Party’s obligations under this Agreement. A description of the escalation process is set forth in Exhibit G. Matters that remain unresolved after the escalation process set forth in Exhibit G and that are not subject to resolution under either Party’s final decisionmaking authority may be submitted for resolution by either Party as set forth in Exhibit I.
4.5 Development and Commercial Leads.
(a) Each Party shall designate a single individual to serve as its “Development Lead.” The Development Leads shall be the principal point of contact for each Party for matters relating to the Development Plan and shall be responsible for implementing and coordinating, on a day-to-day basis, all development activities and facilitating the exchange of information between the Parties regarding the Development Plan.
(b) Each Party shall designate a single individual to serve as its “Commercialization Lead.” The Commercialization Leads shall be the principal point of contact for each Party for matters relating to the Commercialization Plan and shall be responsible for implementing and coordinating, on a day-to-day basis, all commercialization activities and facilitating the exchange of information between the Parties regarding the Commercialization Plan.
(c) Within [**] days after the Effective Date, each Party shall provide the other Party with the names of its Commercialization Lead and Development Lead. Each Party may replace its Commercialization Lead and Development Lead at any time and for any reason upon written notice to the other Party.
(d) The Development Leads from each Party and the Commercialization Leads from each Party, respectively, shall meet as soon as practicable after the Effective Date and thereafter during the performance of the Development Plan, the Regulatory Plan and the Commercialization Plan, as applicable, at least [**] and at such additional times as the Parties reasonably deem appropriate. Meetings of the Leads may be conducted in person, by telephone or videoconference as agreed by the Development Leads or the Parties. Additionally, the Development Leads (or their designees) shall maintain close regular communications with each other as to the status of the ongoing and planned activities under the Development Plan, the Regulatory Plan and Commercialization Plan, as applicable.
14
4.6 Team Leads
The Parties shall form smaller teams of employees who will work together on the following teams: Clinical, Regulatory, Quality, Supply Chain, Reimbursement/Market Access, Business Development & Licensing, and others as defined by the project scope and as the Parties may agree to form (“Team”). Each Party shall designate a single individual to serve as Team Leads for each Team. Each Team shall meet as frequently as the Team may decide.
4.7 Alliance Leads
Each Party shall designate a single individual to serve as its “Alliance Lead.” The Alliance Leads shall be the principal point of contact for each Party for matters relating to the overall governance of the collaboration, including leadership of team collaboration and interactions between the Parties, which includes scheduling, coordinating and attending JSC meetings, drafting agendas, preparing and circulating minutes of JSC meetings, and assuring that there is clear and smooth communications between the Parties and coordination among the Development Leads, Commercialization Leads, Team Leads, Project Timeline Leads and JSC.
4.8 Project Timeline Leads
Each party shall designate an individual to serve as its “Project Timeline Lead.” The Project Timeline Leads will develop, track and update the Project timelines for the development and commercialization of the Diagnostic Test and the Compound. The Project Timeline Leads will coordinate the timelines for the Diagnostic Test and the Compound to enable both Parties to meet the contract deliverables.
4.9 Escalation.
Any matter that cannot be received by unanimous consent of the relevant teams or team members, as the case may be, shall be submitted to the relevant Alliance Leads for resolution and thereafter for further resolution in accordance with Sections 4.3 and 4.4 of the Agreement. The escalation structure described is attached in Exhibit G.
4.10 Other Governance Matters
(a) Neither the Development Leads, Commercialization Leads, Team Leads, Alliance Leads, Project Timeline Leads nor the JSC shall have authority to amend this Agreement. None of the foregoing shall have authority to amend the Development Plan, the Regulatory Plan or the Commercialization Plan, which may be modified only with the approval of the Parties as permitted pursuant to Sections 2.1, 2.3(a) or 3.1, as applicable.
(b) Unless otherwise provided for herein, each Party shall be responsible for all expenses incurred by its employees in connection with performing their duties hereunder, including all costs of travel, lodging and meals.
15
ARTICLE 5
PAYMENTS
5.1 Payments. As consideration for ▇▇▇▇▇▇’▇ activities under the Development Plan, the Clinical Plan and the Commercialization Plan, Epizyme shall remit to ▇▇▇▇▇▇ payments aggregating up to a maximum of [**] U.S. Dollars ($[**]) (unless increased pursuant to Section 5.2) in accordance with the Payment Timetable. In addition, Epizyme shall pay to Abbott a non-refundable, irrevocable license fee of [**] U.S. Dollars ($[**]) within [**] days after the Effective Date. All other payments shall be made in accordance with the Payment Timetable set forth in Exhibit E (the “Payment Timetable”) and subject to the following:
(a) The aggregate payments for each Stage in the Payment Timetable shall be the Total amount set forth for such Stage in the Payment Timetable, and such amounts shall be paid in [**].
(b) Once [**], Abbott shall invoice Epizyme [**], with subsequent payments invoiced thereafter [**]; provided that, [**] Section 5.1(d) below. For example, [**].
(c) Payments for [**]. If ▇▇▇▇▇▇ [**], ▇▇▇▇▇▇ shall [**], and Epizyme shall [**] Section 5.3.
(d) Abbott shall give Epizyme at least [**] days prior written notice before commencement of each Stage in the Payment Timetable subsequent to Stage 1.
5.2 Additional Payments. If circumstances arise that materially deviate from the Key Assumptions, and such circumstances result from factors outside the reasonable control of Abbott and are not a result of any failure by Abbott to perform in accordance with this Agreement, then the Parties shall amend existing payments(s), to cover the additional costs, if any, resulting from such material deviation from the Key Assumptions; provided, however, that Abbott shall not be required to incur such additional costs and Epizyme shall not be responsible for paying such additional costs until the Parties reach agreement as to an additional appropriate payments(s), or amendment to existing payments(s), to cover such costs.
5.3 Invoices. Abbott shall invoice Epizyme for Payment Timetable payments in accordance with Section 5.1. Epizyme shall pay all such invoices within [**] days of receipt in the manner described in Section 5.5.
5.4 Bridging, Clinical Trial Site Cost and Regulatory Cost Payments. In addition to the payments set forth in Section 5.1 and 5.2, Epizyme shall pay, or reimburse Abbott for, one hundred percent (100%) of (a) all Bridging and Clinical Trial Site Costs for the first Epizyme Product and (b) costs and expenses incurred by Abbott in obtaining Regulatory Approvals in accordance with Sections 2.3(a) and 2.3(b). Epizyme shall pay all such invoices for such costs and expenses within [**] days of receipt thereof in the manner described in Section 5.5.
16
5.5 Payment Terms. The payment terms for all payments shall be as follows:
(a) Payments are payable within [**] days after Epizyme’s receipt of an invoice from Abbott.
(b) All payments by Epizyme to Abbott under this Agreement shall be made in U.S. Dollars to the following account:
Northern Trust Company
Chicago, Illinois, USA
ABA: ▇▇▇▇▇▇▇▇▇
SWIFT Code: ▇▇▇▇▇▇▇▇
Account Name: ▇▇▇▇▇▇ Laboratories Inc.
Account Number: [**]
(c) If applicable laws, rules or regulations require the withholding of taxes, Epizyme shall make such withholding payments and shall subtract the amount thereof from the applicable payments hereunder. Epizyme shall submit to Abbott appropriate proof of payment of the withheld taxes as well as the official receipts within a reasonable period of time. Epizyme shall provide Abbott reasonable assistance in order to allow Abbott to obtain the benefit of any present or future treaty against double taxation which may apply to the applicable payments hereunder.
ARTICLE 6
CONFIDENTIALITY, PUBLICITY AND PUBLICATIONS
6.1 Confidentiality. As of and after the Effective Date, all Confidential Information disclosed, revealed or otherwise made available by one Party (“Disclosing Party”) to the other Party (“Receiving Party”) under, or as a result of, this Agreement is furnished to the Receiving Party solely to permit the Receiving Party to exercise its rights, and perform its obligations, under this Agreement. The Receiving Party shall not use any of the Disclosing Party’s Confidential Information for any other purpose, and shall not disclose, reveal or otherwise make any of the Disclosing Party’s Confidential Information available to any other person, firm, corporation or other entity, without the prior written authorization of the Disclosing Party. As of and after the Effective Date all exchanges of Confidential Information shall be governed by the provisions of this Agreement and no longer by the CDA (which shall remain in effect pursuant to the provisions thereof with respect to all exchanges of Confidential Information prior to the Effective Date).
6.2 Safeguarding of Confidential Information. In furtherance of the Receiving Party’s obligations under Section 6.1, the Receiving Party shall protect the Disclosing Party’s Confidential Information to the same extent it protects its own confidential information of like kind and sensitivity. Without limiting the generality of this Section 6.2, the Receiving Party shall disclose any of the Disclosing Party’s Confidential Information only to those of its officers,
17
employees, licensees, sublicensees, consultants, and attorneys that have a need to know the Disclosing Party’s Confidential Information, in order for the Receiving Party to exercise its rights and perform its obligations under this Agreement, and only if such officers, employees, licensees, sublicensees, consultants, attorneys and financial advisors have executed appropriate non-disclosure agreements containing substantially similar terms regarding confidentiality as those set out in this Agreement or are otherwise bound by obligations of confidentiality effectively prohibiting the unauthorized use or disclosure of the Disclosing Party’s Confidential Information. The Receiving Party shall promptly furnish the Disclosing Party with written notice of any known unauthorized use or disclosure of any of the Disclosing Party’s Confidential Information by any officer, employee, licensee, sublicensees, consultants, attorneys or financial advisors of the Receiving Party, and shall take all actions that the Disclosing Party reasonably requests in order to prevent any further unauthorized use or disclosure of the Disclosing Party’s Confidential Information.
6.3 Exceptions. Confidential Information shall not include information that:
(a) is or becomes publicly available through no breach of this Agreement by the Receiving Party;
(b) was known to the Receiving Party prior to disclosure hereunder by the Disclosing Party (as evidenced by the Receiving Party’s written records);
(c) is disclosed, revealed or otherwise made available to the Receiving Party by a Third Party that, to the Receiving Party’s knowledge, is under no obligation of confidentiality relating to such information; or
(d) Notwithstanding the provisions of Section 6.2, a Receiving Party may disclose Confidential Information if such Receiving Party is required to disclose such Confidential Information under Applicable Law, or in connection with any application by the Receiving Party for any Regulatory Approvals; provided, however, that the Receiving Party shall furnish the Disclosing Party with as much prior written notice of such disclosure requirement as reasonably practicable, so as to permit the Disclosing Party, in its sole discretion, and at its sole expense, to take appropriate action, including seeking a protective order, in order to prevent the Disclosing Party’s Confidential Information from passing into the public domain or becoming generally available to the public. Such Confidential Information disclosed pursuant to this paragraph shall remain Confidential Information under this Agreement despite such required disclosure, unless the exceptions set forth in Section 6.3(a) through (c) apply.
6.4 Term of Confidentiality Obligations. All of the Receiving Party’s obligations under Sections 6.1 and 6.2 with respect to the protection of the Disclosing Party’s Confidential Information shall survive for a period of [**] years following the expiration or termination of this Agreement for any reason.
6.5 Publicity. No public announcement concerning the existence or terms of this Agreement shall be made, either directly or indirectly, by either Party, without first obtaining the prior written approval of the other Party and agreement upon the nature and text of such public
18
announcement. Notwithstanding the foregoing, if, in the opinion of legal counsel for the Party desiring to make such public announcement, such disclosure is required under Applicable Law, subject to Section 6.3(c) above, the Party required to make such public announcement shall inform the other Party of the proposed announcement or disclosure in reasonably sufficient time prior to public release, which shall be not less than [**] business days (or such shorter period as may be required under Applicable Law) prior to release of such proposed public announcement, and shall provide the other Party with a written copy thereof in order to allow such other Party to comment upon such public announcement. The Receiving Party shall reasonably cooperate with the Disclosing Party (at the Disclosing Party’s expense) with respect to all disclosures regarding this Agreement required under Applicable Law, including requests for confidential treatment of proprietary information of the Disclosing Party included in any such disclosure. Notwithstanding the foregoing, the Parties shall mutually agree upon the form and substance of, and release, separate press release(s) announcing their development relationship regarding the Diagnostic Test pursuant to this Agreement upon the initial use of the Diagnostic Test in a phase II registrational trial of the Epizyme Product. Any ▇▇▇▇▇▇ ▇▇▇▇▇ releases will remain subject to this Section 6.5.
6.6 Applicable Law. Nothing in this Agreement shall be construed as preventing or in any way inhibiting either Party from complying with Applicable Law governing activities and obligations undertaken pursuant to this Agreement, in any manner which it reasonably deems appropriate, including, for example, by disclosing to Regulatory Authorities confidential or other information received from the other Party, subject to Section 6.3(d) and 6.5.
6.7 Non-Use of Names. Except as otherwise provided in this Agreement, neither Party (or its Affiliates) shall use, either directly or indirectly, the names of any of their officers, employees or board members in any publicity, marketing advertising or other documents (or other disclosures) unless a copy or transcript of the proposed disclosure is submitted to and approved in advance in writing by the other Party (in its sole discretion), except in the case in which a governmental authority requires the use in the sale or distribution of the Diagnostic Test or any Epizyme Product. Each Party will use Commercially Reasonable Efforts to review and approve any proposed disclosure within [**] business days of its receipt from the other Party of a copy or transcript of the proposed disclosure. If a Party approves the other Party’s usage of the names of any of their officers, employees or board members in accordance with this Section 6.7, the other Party shall comply with any usage guidelines or requirements imposed by the approving Party.
6.8 Publications. Epizyme and Abbott each acknowledge the other Party’s interest in publishing the results of its research in order to obtain recognition within the scientific community and to advance the state of scientific knowledge. Each Party also recognizes the mutual interest in obtaining valid patent protection and in protecting business interests and trade secret information. Consequently, except for disclosures permitted pursuant to Section 6.3, either Party, its employee(s) or consultant(s) wishing to make a publication in a journal, paper, magazine or presentation relating to the Diagnostic Test shall deliver to the other Party a copy of the proposed written publication or an outline of an oral disclosure at least [**] days prior to submission for publication (or in the case of an abstract or presentation at least [**] days prior to submission of such abstract for publication or the making of such presentation). The reviewing
19
Party shall have the right (a) to propose modifications to the publication or presentation for patent reasons, trade secret reasons or business reasons or (b) to request a reasonable delay in publication or presentation in order to protect patentable information. If the reviewing Party requests a delay, the publishing Party shall delay submission or presentation for a period of [**] days to enable patent applications protecting each Party’s rights in such information to be filed in accordance with the laws governing intellectual property protection. Upon expiration of such [**] days, the publishing Party shall be free to proceed with the publication or presentation. If the non-publishing Party requests modifications to the publication or presentation, the publishing Party shall edit such publication or presentation to prevent disclosure of trade secret or proprietary business information Controlled by the non-publishing Party prior to submission of the publication or presentation. Epizyme’s and/or ▇▇▇▇▇▇’▇ contribution shall be acknowledged in any publication by co-authorship or acknowledgment, whichever is appropriate.
ARTICLE 7
INTELLECTUAL PROPERTY
7.1 License Grants.
(a) Abbott grants to Epizyme, as of the Effective Date a perpetual, royalty-free non-exclusive license in the Territory under the Abbott Patent Rights and Abbott Know-How to research, develop, make, have made, use, sell, offer for sale and import any Epizyme Product. Subject to Section 7.6, Abbott further grants to Epizyme, as of the Effective Date, a royalty-free, non-exclusive, perpetual license to refer to or recommend the use of the Diagnostic Test in Epizyme’s label, package insert, promotional or regulatory material for any Epizyme Product in each country of the Territory in which Epizyme has obtained Regulatory Approval for any Epizyme Product.
(b) Epizyme grants to Abbott, as of the Effective Date, a perpetual royalty-free non-exclusive license in the Territory under the Epizyme Patent Rights, Epizyme Know-How and Epizyme Inventions to research, develop, make, have made, use, sell, offer for sale and import the Diagnostic Test for the Diagnostic Test Intended Use. Subject to Section 7.6, Epizyme further grants to Abbott, as of the Effective Date, a royalty-free, non-exclusive, perpetual license in the Territory to refer to the Compound and any Epizyme Product, as may be required pursuant to Applicable Law, in ▇▇▇▇▇▇’▇ label, package insert, promotional or regulatory material for the Diagnostic Test in each country of the Territory in which ▇▇▇▇▇▇ has obtained Regulatory Approval for the Diagnostic Test.
(c) Epizyme and Abbott each grant to the other, a perpetual, royalty-free non-exclusive license (without the right to grant sublicenses except to Affiliates) in the Territory under the Epizyme Technology and ▇▇▇▇▇▇ Technology, respectively, to perform such other Party’s obligations under this Agreement.
7.2 Right to Sublicense. Both Parties shall be entitled to sublicense all or any of their rights under Section 2.4, Section 7.1(a) and Section 7.1(b) (subject to any applicable restrictions on any intellectual property in-licensed from Third Parties) (i) to their respective Affiliates, and (ii) to Third Party contractors used in the development, manufacturing and commercialization of
20
the Diagnostic Test or any Epizyme Product, as applicable, (iii) in the case of Epizyme, to a Third Party that acquires the exclusive rights in a country to develop or commercialize an Epizyme Product or is a party to a collaboration with Epizyme or another Third Party with respect to the development or commercialization of an Epizyme Product in a country, and (iv) in the case of Abbott, to a Third Party that acquires the exclusive, rights in a country to develop or commercialize the Diagnostic Test.
7.3 License to Data. Epizyme hereby grants to Abbott a perpetual, non-exclusive, non-transferable (except in connection with a permitted assignment, sublicense or subcontract) royalty-free license under the Data as necessary for Abbott to research, develop and commercialize the Diagnostic Test for the Diagnostic Test Intended Use and to use Data as necessary in patent filings with respect to the Diagnostic Test for the Diagnostic Test Intended Use.
7.4 Further Actions. Each Party shall execute all documents and give all declarations regarding the licenses granted in Section 7.1 and reasonably cooperate with the other Party, to the extent such documents, declarations and/or cooperation are reasonably required for the recording or registration of the licenses granted hereunder at the various intellectual property offices in the Territory for the benefit of the licensee Party.
7.5 No Other Licenses. Nothing in this Agreement shall be deemed or implied to be, and the Parties disclaim all implied rights to, the grant by either Party to the other Party of any license, right, title or interest in either Party’s product, intellectual property rights (including Patent Rights, Know-How, trade secrets, copyrights, and Trademarks), any formulation technology or know-how, manufacturing technology or know-how, operating procedures, marketing materials or strategies, intangibles, material or proprietary rights or any other tangible or intangible property, except as are expressly set forth in this Agreement.
7.6 Trademarks.
(a) Abbott shall own all right, title and interest in and to any Trademarks developed by or for ▇▇▇▇▇▇ for use in connection with the Diagnostic Test (“▇▇▇▇▇▇’▇ Trademarks”). ▇▇▇▇▇▇ hereby grants to Epizyme a royalty-free non-exclusive license to use ▇▇▇▇▇▇’▇ Trademarks for the purpose of referring to the Diagnostic Test in connection with advertising and marketing any Epizyme Product. Any and all goodwill derived from the use of ▇▇▇▇▇▇’▇ Trademarks shall inure solely to the benefit of ▇▇▇▇▇▇.
(b) Epizyme shall own all right, title and interest in and to all Trademarks developed by or for Epizyme for use in connection with any Epizyme Product (“Epizyme’s Trademarks”). Epizyme hereby grants to Abbott a royalty-free non-exclusive license to use Epizyme’s Trademarks for the purpose of referring to Epizyme Products in connection with advertising and marketing the Diagnostic Test. Any and all goodwill derived from the use of Epizyme’s Trademarks shall inure solely to the benefit of Epizyme.
(c) Each party agrees that the use of the other Party’s Trademarks in connection with advertising and marketing the Diagnostic Test or any Epizyme Product shall be in accordance with the terms of this Agreement and shall comply with all federal, state and local laws and regulations.
21
1. Approval of Trademark Use.
(a) Each party agrees to provide artwork for any packaging, labeling and promotional materials that display the other Party’s Trademarks for approval prior to any use or distribution of such materials. Each party shall respond in writing with its approval or requested changes, such approval not to be unreasonably withheld, within [**] business days after receipt of such artwork from the other Party.
(b) Once a proposed use of the Trademarks has been approved by the Party that is the licensor, and provided that the Party that is the licensee does not make any changes to the presentation of the Trademarks in such approved product packaging, labeling or promotional materials, the Party using the Trademarks under license may utilize the same approved presentation in other product packaging, labeling or promotional materials.
(d) Each party will refrain from any use of the other’s Trademarks in a manner that threatens to damage the goodwill associated with the respective Trademarks or which threatens to tarnish the reputation or otherwise reflect unfavorably upon the owner of the Trademarks.
(e) Epizyme shall not, during or after the term of the Agreement, anywhere in the world, take any action that in ▇▇▇▇▇▇’▇ sole and absolute discretion impairs or contests or tends to impair or contest the validity of ▇▇▇▇▇▇’▇ right, title and interest in and to ▇▇▇▇▇▇’▇ Trademarks, including, but not limited to, using, or filing an application to register, any word, ▇▇▇▇, symbol or device, or any combination thereof, that is confusingly similar to or dilutes the distinctiveness of any of ▇▇▇▇▇▇’▇ Trademarks.
(f) ▇▇▇▇▇▇ shall not, during or after the term of the Agreement, anywhere in the world, take any action that in Epizyme’s sole and absolute discretion impairs or contests or tends to impair or contest the validity of Epizyme’s right, title and interest in and to Epizyme’s Trademarks, including, but not limited to, using, or filing an application to register, any word, ▇▇▇▇, symbol or device, or any combination thereof, that is confusingly similar to or dilutes the distinctiveness of any of Epizyme’s Trademarks.
(g) If either Epizyme or ▇▇▇▇▇▇ becomes aware of any infringement of the other’s Trademarks, anywhere in the world, it will promptly notify the other Party in writing. Each Party agrees to fully cooperate with the Party that is the owner of the Trademarks regarding any action the owner may take with respect to such infringement or violation. The Party that is the owner of the Trademarks shall have the exclusive right, exercisable in its sole and unlimited discretion, to institute in its own name and to control all actions against Third Parties relating to such Trademarks, at the owner’s expense. The Party that is the owner of the Trademarks shall be entitled to receive and retain all amounts awarded, if any, as damages, profits or otherwise in connection with such actions.
22
7.7 Ownership of Existing Technology. As between the Parties, ▇▇▇▇▇▇ shall retain sole and exclusive ownership of the ▇▇▇▇▇▇ Technology. As between the Parties, Epizyme shall retain sole and exclusive ownership of the Epizyme Technology.
7.8 Inventions.
(a) All Inventions shall be promptly disclosed to the other Party hereto in confidence and in writing.
(b) ▇▇▇▇▇▇ shall have exclusive ownership of and title to any and all Inventions solely related to the ▇▇▇▇▇▇ Technology, the Diagnostic Test, and the ▇▇▇▇▇▇ Diagnostics and to all intellectual property rights solely related thereto or arising therefrom (“▇▇▇▇▇▇ Inventions”), that are discovered or reduced to practice solely by employees or agents of either Party, or jointly by employees or agents of either Party, during the term of this Agreement. ▇▇▇▇▇▇ shall have the sole right, but not the obligation, to file for, prosecute, maintain, enforce and defend any and all Patent Rights claiming the ▇▇▇▇▇▇ Inventions, and shall have the right, in its sole discretion, whether or not, or where, to file a patent application, to abandon the prosecution of any patent or patent application, to discontinue the maintenance of, or enforce any such patent or patent application within the ▇▇▇▇▇▇ Patent Rights.
(c) Epizyme shall have exclusive ownership of and title to the Data and all Inventions solely related to the Epizyme Technology, the Compound and any Epizyme Product, and to all intellectual property rights related thereto or arising therefrom (“Epizyme Inventions”), that are discovered or reduced to practice solely by employees or agents of either Party, or jointly by employees or agents of either Party, during the term of this Agreement. Epizyme shall, at its own cost, have the sole right, but not the obligation, to file for, prosecute, maintain, enforce and defend any and all patents claiming the Epizyme Inventions, and shall have the right, in its sole discretion, whether or not, or where, to file a patent application, to abandon the prosecution of any patent or patent application, to discontinue the maintenance of, or to enforce any such patent or patent application.
(d) As for all Inventions not covered by Section 7.8(b) or Section 7.8(c), the ownership and title thereto (including the ownership and title to all intellectual property rights related thereto or arising there from) shall be based on inventorship, as determined pursuant to the inventorship principles arising under the patent laws of the United States of America, and any such Inventions having an employee and/or collaborator of each Party as co-inventors shall be “Joint Inventions.” [**]:
(i) [**].
(ii) [**]. Epizyme [**] Epizyme [**] Epizyme [**]Epizyme [**] and [**] Epizyme; and [**] Epizyme [**]. Epizyme shall [**] ▇▇▇▇▇▇ [**], and shall [**] ▇▇▇▇▇▇ [**]; provided such [**].
23
(e) Neither Party shall be entitled to use or exploit any Joint Invention without the prior written consent of the other Party except that: (i) each Party shall be entitled to use in the Territory, without consideration to the other Party, any Joint Inventions in its internal research programs, including research performed under confidentiality agreements with Third Party collaborators, without the right to sublicense or commercialize such Joint Invention without the other Party’s prior written consent, and with the other Party receiving a non-exclusive, royalty-free license to use any subsequent inventions directly resulting from the use of such Joint Invention in its internal research programs; (ii) each Party shall be entitled to use in the Territory any Joint Inventions for the purpose of performing its obligations under this Agreement, without consideration to the other Party; (iii) Epizyme and its Affiliates shall be entitled to use in the Territory any Joint Inventions without the prior written consent of ▇▇▇▇▇▇, without consideration to ▇▇▇▇▇▇, and without accounting to ▇▇▇▇▇▇, to make, have made, use, have used, sell, have sold, offer for sale, and import the Compound and any Epizyme Product and to exercise the rights and licenses set forth in Section 7.8(d), including the right to sublicense Third Parties under such rights and licenses as set forth in Section 7.8(d); and (iv) ▇▇▇▇▇▇ and its Affiliates shall be entitled to use in the Territory any Joint Inventions without the prior written consent of Epizyme, without consideration to Epizyme, and without accounting to Epizyme, to make, have made, use, have used, sell, have sold, offer for sale, and import diagnostic tests.
(f) If the Parties create a Joint Invention, the Parties shall promptly meet to discuss and decide whether to seek patent protection therefore (“Joint Patent Right”). If the Parties decide to seek patent protection on a Joint Invention, Epizyme has the first right, at its own expense, to prepare, file, prosecute and maintain any Joint Patent Right throughout the world on behalf of both Parties. Epizyme shall give ▇▇▇▇▇▇ an opportunity to review and comment on any patent application with respect to such Joint Patent Right before filing, shall consult with ▇▇▇▇▇▇ with respect thereto, and shall supply ▇▇▇▇▇▇ with a copy of the patent application as filed, together with notice of its filing date and serial number. Epizyme shall keep ▇▇▇▇▇▇ advised of the status of the actual and prospective patent filings. Preparation, filing, prosecution and maintenance of such Joint Patent Rights will be handled by patent counsel acceptable to both Parties. If Epizyme elects not to file a patent application on a Joint Invention or to cease the prosecution and/or maintenance of any Joint Patent Right in any country of the Territory, Epizyme shall provide ▇▇▇▇▇▇ with written notice upon the decision to not file or continue the prosecution of such application or maintenance of such Joint Patent Right, in any event not later than [**] days before any relevant deadline relating to or any public disclosure or loss of the relevant Joint Patent Rights. In such event, Epizyme shall permit ▇▇▇▇▇▇ to file and/or continue prosecution and/or maintenance of such Joint Patent Rights in the names of both Parties but at ▇▇▇▇▇▇’▇ own expense. Epizyme agrees to provide documents and perform such acts, at Epizyme’s expense, as may be reasonably necessary to permit ▇▇▇▇▇▇ to file, prosecute and/or maintain such Joint Patent Rights.
(g) If either Epizyme or ▇▇▇▇▇▇ becomes aware of any infringement, anywhere in the world, of any issued patent within the Joint Patent Rights, it will promptly notify the other Party in writing to that effect.
(h) Epizyme shall have the first right, but not the obligation, to take action to obtain a discontinuance of infringement or bring suit against a Third Party infringer of any Joint
24
Patent Rights within [**] days from the date of notice and to join ▇▇▇▇▇▇ as a party plaintiff. Epizyme shall bear all the expenses of any suit brought by it claiming infringement of any Joint Patent Right. ▇▇▇▇▇▇ will reasonably cooperate with Epizyme, at ▇▇▇▇▇▇’▇ expense, in any such suit and shall have the right to consult with Epizyme and to participate in and be represented by independent counsel in such litigation at its own expense. If, after the expiration of such [**]-day period (or, if earlier, the date upon which Epizyme provides written notice that it does not plan to bring suit), Epizyme has not obtained a discontinuance of infringement of the Joint Patent Right or filed suit against any such Third Party infringer of the Joint Patent Right, then ▇▇▇▇▇▇ shall have the right, but not the obligation, to bring suit against such Third Party infringer of the Joint Patent Right, provided that ▇▇▇▇▇▇ shall bear all the expenses of such suit, and further provided that such infringement is not the result of a notice to Epizyme from such Third Party infringer under 21 U.S.C. §355(b)(2)(A)(iv) or 355(j)(2)(A)(vii)(IV) (“Paragraph IV Notice”) with respect to any Joint Patent Right and any Epizyme product for which Epizyme holds an approved New Drug Application. Epizyme will reasonably cooperate with ▇▇▇▇▇▇, at Epizyme’s expense, in any such suit for infringement of a Joint Patent Right brought by ▇▇▇▇▇▇ against a Third Party, and shall have the right to consult with ▇▇▇▇▇▇ and to participate in and be represented by independent counsel in such litigation at its own expense. Any recoveries obtained by Epizyme or ▇▇▇▇▇▇, as applicable, as a result of any proceeding against such a Third Party infringer shall be allocated as follows: (A) such recovery shall first be used to reimburse each Party for all reasonable attorney fees and other litigation costs actually incurred in connection with such litigation by that Party, and (B) any remainder shall be allocated [**] percent ([**]%) to the enforcing Party and [**] percent ([**]%) to the non-enforcing Party; provided, however, that Epizyme shall be entitled to retain all such recoveries if the proceeding against a Third Party infringer is a result of the Third Party’s filing of a Paragraph IV Notice with respect to a Epizyme product for which Epizyme holds an approved New Drug Application.
(i) ▇▇▇▇▇▇ and Epizyme agree to cooperate with respect to filing for and prosecuting any application for patent term extension of any Joint Patent Right to the extent such patent term extension is not based on the approval of any New Drug Application or any other regulatory approval for an Epizyme Product (“Term Extension”). Epizyme shall have the first right, but not the obligation, to file and prosecute a Term Extension, at its sole cost and expense. Where Epizyme decides not to file for a Term Extension, it shall provide ▇▇▇▇▇▇ written notice thereof at least [**] days before the deadline for filing a Term Extension for the particular Joint Patent Right. After such notice, ▇▇▇▇▇▇ shall have the right, but not the obligation, to file and prosecute a Term Extension, at its sole cost and expense. The party prosecuting a Term Extension will keep the other party apprised of the prosecution. For the avoidance of doubt, Epizyme shall have no obligation to seek or permit ▇▇▇▇▇▇ to seek any patent term extension relating to any regulatory approval of an Epizyme Product.
(j) ▇▇▇▇▇▇ agrees that Epizyme shall have the right to list any Joint Patent Right in an Orange Book filing for the Compound and any Epizyme Product, in its sole discretion, where Epizyme reasonably determines the particular Joint Patent Right is eligible for listing in the Orange Book as applicable to the Compound or any Epizyme Product.
7.9 Additional Documents. Each Party agrees to cooperate reasonably and in good faith in the execution and filing of any necessary applications, assignments, powers of attorney,
25
or other filings or documents as may be reasonably deemed necessary by the other Party to vest in and ensure the proper ownership, Control, and maintenance of the Inventions pursuant to this Article 7, and each Party shall obtain the cooperation of its employees or agents to execute the same.
7.10 Notification of Infringement; Third Party License. If the making, having made, importing, exporting, using, distributing, marketing, promoting, offering for sale or selling of the Diagnostic Test is alleged by a Third Party to infringe a Third Party’s intellectual property rights, the Party becoming aware of such allegation shall promptly notify the other Party. Additionally, if either Party determines that, based upon the review of a Third Party’s intellectual property rights, it may be desirable to obtain a license from such Third Party with respect thereto; such Party shall promptly notify the other Party of such determination. The Parties shall work together toward a resolution of any Third Party claim or potential claim.
ARTICLE 8
REPRESENTATIONS, WARRANTIES AND COVENANTS
8.1 General. Each of ▇▇▇▇▇▇ and Epizyme hereby represents, warrants and covenants to the other Party as follows as of the Effective Date:
(a) it is a corporation or entity duly organized and validly existing under the laws of its state of incorporation;
(b) the execution, delivery and performance of this Agreement by such Party does not conflict with any other agreement by which it is bound, and has been duly authorized by all requisite corporate action and does not require any shareholder action or approval;
(c) it has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder; and
(d) it shall at all times comply with all Applicable Laws relating to its activities under this Agreement.
8.2 Intellectual Property. Each Party hereby represents and warrants that as of the Effective Date it currently owns and/or has (other than the Trademarks for use in connection with the Diagnostic Test, which will be developed by or for ▇▇▇▇▇▇ after the Effective Date and the Trademarks for use in connection with the Epizyme Product, which will be developed by or for Epizyme after the Effective Date), and will maintain throughout the term of this Agreement, the right and ability to grant the other Party the licenses under its intellectual property that are set forth in this Agreement. Nothing in this Agreement shall be construed as a warranty by either Party that their respective intellectual property rights will be valid or enforceable, provided, however, that each Party hereby represents and warrants to the other Party that it has no present actual knowledge that: (a) its intellectual property rights are or will be invalid or unenforceable; (b) any Third Party intellectual property rights are infringed (or alleged to be infringed, except as alleged in the Enzo Lawuit) by the practice of such Party’s intellectual property rights; or (c) any Third Party is infringing such Party’s intellectual property rights.
26
8.3 Disclaimer. EXCEPT FOR THE EXPRESS REPRESENTATIONS AND WARRANTIES CONTAINED IN THIS AGREEMENT, NEITHER ▇▇▇▇▇▇ NOR EPIZYME MAKES, AND EACH HEREBY EXPRESSLY DISCLAIMS, ANY REPRESENTATIONS OR WARRANTIES, EITHER EXPRESS OR IMPLIED, WHETHER IN FACT OR IN LAW, INCLUDING ANY IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT.
8.4 No Representations Regarding Approval or Commercial Success. Neither Party makes any representations or warranties as to: (a) whether the any Epizyme Product or the Diagnostic Test will be approved for commercial sale by the applicable Regulatory Authorities; or (b) the commercial potential or success of any Epizyme Product or the Diagnostic Test.
8.5 Other Indications. If Epizyme develops an indication for the Compound or any Epizyme Product other than an acute leukemia, the Parties shall each in good faith consider either amending this Agreement to include such indication or entering into another similar agreement related to such indication. The Parties agree that any such amendment or other agreement remains subject to the Parties reaching a mutually acceptable agreement on such terms and conditions and the receipt of all necessary management approvals by each of the Parties.
ARTICLE 9
TERM AND TERMINATION
9.1 Term. This Agreement shall become effective as of the Effective Date. Unless earlier terminated pursuant to the provisions of this Article 9, this Agreement shall remain in full force and effect on a country-by-country basis until the date on which Epizyme ceases to market an Epizyme Product in such country of the Territory.
9.2 Breach. If either Party (the “Breaching Party”) commits a material breach or default of any of its obligations hereunder, the other Party hereto (the “Non-Breaching Party”) may give the Breaching Party written notice of such material breach or default. If the Breaching Party fails to cure such breach or default within [**] days after the date of the Non-Breaching Party’s notice thereof, the Non-Breaching Party may terminate this Agreement upon written notice to the Breaching Party. If the Breaching Party indicates in writing that it will be unable or is unwilling to cure the breach, this Agreement may be terminated by the Non-Breaching Party with immediate effect.
9.3 Bankruptcy or Insolvency. Each Party shall have the right to terminate this Agreement, immediately by giving written notice of termination to the other Party, if the other Party files a voluntary petition, or if an involuntary petition is granted in respect of the other Party and appeal proceedings are not commenced within ten (10) business days from the date of such petition under the bankruptcy provisions of Applicable Law, or the other Party is declared insolvent, undergoes voluntary or involuntary dissolution, or makes an assignment for the benefit of its creditors, or is unable to pay its debts as they come due, or suffers the appointment of a
27
receiver or trustee over all, or substantially all, of its assets or properties. All rights and licenses granted under or pursuant to this Agreement by ▇▇▇▇▇▇ to Epizyme, and by Epizyme to ▇▇▇▇▇▇, are, and shall otherwise be deemed to be, for purposes of Article 365(n) of the U.S. Bankruptcy Code, licenses of rights to “intellectual property” as defined under Article 101(52) of the U.S. Bankruptcy Code. The Parties agree that each Party, as a licensee of such intellectual property rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code.
9.4 Injunction.
(a) ▇▇▇▇▇▇ may terminate this Agreement upon providing Epizyme ninety (90) days prior written notice if: (i) ▇▇▇▇▇▇’▇ making, having made, importing, exporting, using, distributing, marketing, promoting, offering for sale or selling the Diagnostic Test for the Diagnostic Intended Use; or (ii) Epizyme’s offering for sale or selling the Epizyme Product, in either case ((i) or (ii)) is (x) enjoined by a court of competent jurisdiction because such enjoined activity actually or allegedly infringes or misappropriates a Third Party’s intellectual property rights, (y) such actual or alleged infringement or misappropriation is not infringement or misappropriation for which ▇▇▇▇▇▇ is obligated to indemnify Epizyme pursuant to Section 10.2(b), and (z) during such ninety (90) day period, Epizyme is unable or unwilling to obtain a license to the applicable Third Party intellectual property rights; provided that such termination shall be limited to the countries affected by such injunction.
(b) Epizyme may terminate this Agreement upon providing ▇▇▇▇▇▇ ninety (90) days prior written notice if ▇▇▇▇▇▇’▇ making, having made, importing, exporting, using, distributing, marketing, promoting, offering for sale or selling the Diagnostic Test is enjoined by a court of competent jurisdiction because such enjoined activity actually or allegedly infringes or misappropriates a Third Party’s intellectual property rights and, during such ninety (90) day period, ▇▇▇▇▇▇ is unable or unwilling to obtain a license to the applicable Third Party intellectual property rights; provided that such termination shall be limited to the countries affected by such injunction. In the event that Epizyme terminates this Agreement pursuant to this Section 9.4(b), and the injunction that is the basis for such termination is based on actual or alleged infringement or misappropriation for which ▇▇▇▇▇▇ is obligated to indemnify Epizyme pursuant to Section 10.2(b), ▇▇▇▇▇▇ shall refund to Epizyme in full (i) all Payment Timetable payment amounts made by Epizyme to ▇▇▇▇▇▇ under Sections 5.1 and 5.2 prior to such termination and (ii) all amounts paid by Epizyme to ▇▇▇▇▇▇ or to any Third Party under Sections 2.3(b) and 5.4 prior to such termination, and in addition to the refund amounts described in the foregoing clauses (i) and (ii) ▇▇▇▇▇▇ shall pay to Epizyme a termination payment equal to [**] percent of the initially budgeted aggregate Payment Timetable payment amounts under Section 5.1 (i.e., [**]U.S. Dollars ($[**]).
9.5 Elective Termination by Epizyme. Epizyme may terminate this Agreement upon sixty (60) days written notice to ▇▇▇▇▇▇; provided that, in the event that Epizyme terminates this Agreement pursuant to this Section 9.5 effective after the date eighteen (18) months following the Effective Date but prior to the completion of the Development Plan, Epizyme shall pay to ▇▇▇▇▇▇ a termination fee of [**] U.S. Dollars ($[**]), but shall not pay any other amounts with respect to committed or uncancelable out-of-pocket Development Plan costs or otherwise.
28
9.6 Survival. Termination of the Agreement for whatever reason in accordance with the provisions hereof or expiration of this Agreement shall not affect the accrued rights of the Parties, and shall not limit remedies that may be otherwise available in law or equity. Articles 6, 8, 9, 10 and 11 shall survive expiration or termination of this Agreement for any reason. Survival or termination of the licenses granted in Article 7 shall be governed by the terms set forth in Article 9.7.
9.7 Licenses Survive. If ▇▇▇▇▇▇ terminates this Agreement for any reason, the licenses and rights granted by ▇▇▇▇▇▇ to Epizyme in Section 2.4 and Article 7 shall continue on a fully paid and perpetual basis. If Epizyme terminates this Agreement for any reason, then (a) the licenses and rights granted by Epizyme to ▇▇▇▇▇▇ in Section 2.4 and Article 7 shall continue on a fully paid and perpetual basis, (b) if such termination occurs prior to the regulatory registration of the Diagnostic Test in any country, the licenses and rights granted by ▇▇▇▇▇▇ to Epizyme in Section 7.8 shall continue on a fully paid and perpetual basis, and (c) if such termination occurs after the regulatory registration of the Diagnostic Test in any country, the licenses and rights granted by ▇▇▇▇▇▇ to Epizyme in Section 2.4 and Article 7 shall continue on a fully paid and perpetual basis, provided that any regulatory or promotional materials used by Epizyme and its permitted assigns and sublicensees in relation to the Diagnostic Test shall be consistent with Epizyme’s and its permitted assigns’ and sublicensees’ package insert for the Epizyme Product as approved by the applicable Regulatory Authority.
9.8 Return of Confidential Information. Upon expiration or termination of this Agreement for any reason, the Receiving Party shall use reasonable efforts to either return to the Disclosing Party or destroy Confidential Information of the Disclosing Party (including hard and electronic copies thereof) that is not required by the Receiving Party to exercise surviving license rights as set forth in Section 9.7; provided, however, that the Receiving Party is not required to destroy electronic copies of the Confidential Information stored in its electronic archive systems, and may retain one (1) copy of such Confidential Information for purpose of complying with its obligations under this Agreement or under Applicable Law. The Receiving Party shall provide written confirmation that the Receiving Party has complied with its obligations under this Section 9.8 as to such return or destruction of Confidential Information reasonably promptly after termination of the Agreement.
ARTICLE 10
INDEMNIFICATION, LIABILITY LIMITATION AND INSURANCE
10.1 Indemnification by Epizyme.
(a) Subject to Sections 10.3 and 10.4, Epizyme shall indemnify, defend and hold ▇▇▇▇▇▇, its Affiliates, and its and their officers, directors, agents and employees (individually and/or collectively referred to herein as an “▇▇▇▇▇▇ Party”) harmless from and against any and all losses, liabilities, damages, expenses (including reasonable attorney fees) paid or payable by ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party to a Third Party (collectively, “▇▇▇▇▇▇ Losses”) to the extent that such ▇▇▇▇▇▇ Losses result from or arise in connection with a claim, suit or other proceeding made or brought by a Third Party against ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party (an “▇▇▇▇▇▇ Claim”) based on, resulting from, or arising in connection with:
(i) the breach of any obligation, covenant, agreement, representation or warranty of Epizyme or an Epizyme Party contained in this Agreement; or
29
(ii) any act or omission by Epizyme, or an Epizyme Party, which constitutes gross negligence or willful misconduct on the part of Epizyme, or an Epizyme Party; or
(iii) any violation of Applicable Law by Epizyme or an Epizyme Party in connection with the performance of Epizyme’s or its Affiliates’ obligations under this Agreement; or
(iv) the development, commercialization or sale of any Epizyme Product;
provided, however, that Epizyme shall not be obligated to indemnify, defend or hold harmless ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party under this Section from any ▇▇▇▇▇▇ Claim or for any ▇▇▇▇▇▇ Losses incurred by ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party to the extent arising out of or attributable to: (A) a breach by ▇▇▇▇▇▇, or any ▇▇▇▇▇▇ Party of any obligation, covenant, agreement, representation or warranty of ▇▇▇▇▇▇, or any ▇▇▇▇▇▇ Party contained in this Agreement; (B) any violation of Applicable Law by ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party in connection with its obligations under this Agreement; or (C) any act or omission by ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party, which constitutes negligence or willful misconduct on the part of ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party.
(b) Epizyme shall indemnify, defend and hold harmless ▇▇▇▇▇▇ and each ▇▇▇▇▇▇ Party from and against all ▇▇▇▇▇▇ Losses arising out of any ▇▇▇▇▇▇ Claim alleging infringement by ▇▇▇▇▇▇ or Epizyme or misappropriation by Epizyme of a Third Party Patent Right or other intellectual property right by either Party’s importing, exporting, using, distributing, marketing, promoting, offering for sale or selling of the Epizyme Product or the Diagnostic Test for the Diagnostic Test Intended Use; provided that ▇▇▇▇▇▇ shall indemnify, defend and hold harmless Epizyme and each Epizyme Party (and Epizyme shall not indemnify, defend or hold harmless ▇▇▇▇▇▇ or any ▇▇▇▇▇▇ Party) as to any infringement or misappropriation that is covered by Section 10.2(b). For the avoidance of doubt, the indemnification, defense and hold harmless obligations of Epizyme pursuant to this Section 10.1(b) shall not apply to infringement or misappropriation by ▇▇▇▇▇▇’▇ importing, exporting, using, distributing, marketing, promoting, offering for sale or selling of the Diagnotic Test for any purpose other than the Diagnostic Test Intended Use.
10.2 Indemnification by ▇▇▇▇▇▇.
(a) Subject to Sections 10.3 and 10.4, ▇▇▇▇▇▇ shall indemnify, defend and hold Epizyme, its Affiliates, and Celgene Corporation and its Affiliates (as licensees or sublicensees of Epizyme with respect to the Epizyme Product under a certain Collaboration and License Agreement dated as of April 2, 2012, as such agreement may be amended from time to
30
time), and its and their officers, directors, agents and employees (individually and/or collectively referred to herein as a “Epizyme Party”) harmless from and against any and all losses, liabilities, damages, expenses (including reasonable attorney fees) paid or payable by Epizyme or an Epizyme Party to a Third Party (collectively, “Epizyme Losses”) to the extent that such Epizyme Losses result from or arise in connection with a claim, suit or other proceeding made or brought by a Third Party against Epizyme or an Epizyme Party (a “Epizyme Claim”) based on, resulting from, or arising in connection with:
(i) the breach of any obligation, covenant, agreement, representation or warranty of ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party contained in this Agreement;
(ii) any act or omission by ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party, which constitutes gross negligence or willful misconduct on the part of ▇▇▇▇▇▇ or an ▇▇▇▇▇▇ Party; or
(iii) any violation of Applicable Law by ▇▇▇▇▇▇, or an ▇▇▇▇▇▇ Party in connection with the performance of ▇▇▇▇▇▇’▇ or its Affiliates’ obligations under this Agreement; or
provided, however, that ▇▇▇▇▇▇ shall not be obligated to indemnify, defend or hold harmless Epizyme or an Epizyme Party from any Epizyme Claim or for any Epizyme Loss incurred by Epizyme or an Epizyme Party to the extent arising out of, or attributable to: (A) a breach by Epizyme or any Epizyme Party of any obligation, covenant, agreement, representation or warranty of Epizyme or an Epizyme Party contained in this Agreement; (B) any violation of Applicable Law by Epizyme, or an Epizyme Party in connection with its obligations under this Agreement; or (C) any act or omission by Epizyme, or an Epizyme Party, which constitutes negligence, or willful misconduct on the part of Epizyme, or an Epizyme Party.
(b) [**].
10.3 Indemnification Procedures.
(a) Each indemnified Party shall notify the indemnifying Party in writing (and in reasonable detail) of the Claim within [**] business days after receipt by such indemnified Party of notice of the Epizyme Claim or ▇▇▇▇▇▇ Claim, as the case may be, or otherwise becoming aware of the existence or threatened existence thereof (such Epizyme Claim or ▇▇▇▇▇▇ Claim being referred to as a “Claim”). Failure to give such notice shall not constitute a defense, in whole or in part, to any claim by an indemnified Party hereunder except to the extent the rights of the indemnifying Party are materially prejudiced by such failure to give notice. An indemnifying Party shall have no obligation or liability under this Article 10 as to any Claim for which settlement or compromise of such Claim, or an offer of settlement or compromise of such Claim, is made by an indemnified Party without the prior written consent of the indemnifying Party, which consent shall not be unreasonably withheld.
31
(b) The indemnifying Party shall assume exclusive control of the defense and settlement (including all decisions relating to litigation, defense and appeal) of any such Claim; provided, however, that, without the indemnified Party’s prior written consent, which shall not be unreasonably withheld, the indemnifying Party may not settle such Claim in any manner that would: (i) require payment (unless fully indemnified hereunder) or admission of liability by the indemnified Party; (ii) materially adversely affect the rights granted to the indemnified Party under this Agreement; (iii) materially conflict with the terms of this Agreement; or (iv) adversely affect other products or services of the indemnified Party or its Affiliates.
(c) The indemnified Party shall reasonably cooperate with the indemnifying Party, at the Indemnifying Party’s expense, in its defense of the Claim (including making documents and records available for review and copying and making persons within its control available for pertinent testimony in accordance with the confidentiality provisions of Article 6, and neither Party shall be required to divulge privileged material to the other). The indemnified Party may participate in, but not control, the defense of such Claim using attorneys of its choice and at its sole cost and expense, with such cost and expense not being covered by the indemnifying Party. If an indemnifying Party does not assume the defense of the Claim asserted against the indemnified Party, or if the indemnifying Party assumes the defense of the Claim in accordance with Section 10.3 yet fails to defend or take other reasonable, timely action, in response to such Claim asserted against the indemnified Party, the indemnified Party shall have the right to defend or take other reasonable action to defend its interests in such proceedings, and shall have the right to litigate, settle or otherwise dispose of any such Claim, without in any way limiting the indemnified Party’s right to be fully indemnified under this Section 10.1 for all Abbott Losses or Epizyme Losses, as applicable; provided, however, that, without the indemnifying Party’s prior written consent, which shall not be unreasonably withheld, the indemnified Party may not settle such Claim in any manner that would: (i) require payment by the indemnifying Party; (ii) materially adversely affect the rights granted to the indemnifying Party under this Agreement; (iii) materially conflict with the terms of this Agreement; or (iv) adversely affect other products or services of the indemnifying Party or its Affiliates.
(d) Neither Party will assert any Claim for indemnification under this Agreement more than two (2) years after the Claim arises.
10.4 Liability Limitation. EXCEPT WITH RESPECT TO A BREACH OF A PARTY’S CONFIDENTIALITY OBLIGATIONS UNDER ARTICLE 6, OR FOR SUCH DAMAGES THAT MAY BE PAYABLE TO THIRD PARTIES AND SUBJECT TO INDEMNIFICATION UNDER THIS ARTICLE 10, IN NO EVENT WILL EITHER PARTY BE LIABLE TO THE OTHER FOR CONSEQUENTIAL, INDIRECT, SPECIAL, EXEMPLARY OR PUNITIVE DAMAGES FOR ANY CAUSE OF ACTION, WHETHER IN CONTRACT, TORT OR OTHERWISE, INCLUDING, WITHOUT LIMITATION, LOST REVENUES, PROFITS OR BUSINESS OPPORTUNITIES ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT, WHETHER OR NOT THE OTHER PARTY WAS OR SHOULD HAVE BEEN AWARE OF THE POSSIBILITY OF THESE DAMAGES.
32
10.5 Insurance.
(a) Abbott hereby represents that it (through its parent company Affiliate, ▇▇▇▇▇▇ Laboratories) is self-insured for product liability and general liability, and that it has and will maintain such coverage for the Term and for a period of [**] years after the expiration of this Agreement or the earlier termination thereof. Such self-insurance is in an amount which is reasonable and customary in the global medical device and diagnostics industry for companies of comparable size and activities. Abbott further represents that it has and will matintain additional coverage for the term of this Agreement and for a period of [**] years after the expiration of this Agrement or the earlier termination thereof, in the amounts and types set forth in a certificate of insurance which Abbott has previously provided to Epizyme.
(b) Epizyme hereby represents that it has and will maintain product liability and general liability coverage for the term of this Agreement and for a period of [**] years after the expiration of this Agreement or the earlier termination thereof. Such insurance is in an amount which is reasonable and customary in the global pharmaceutical industry for companies of comparable size and activities to those of Epizyme.
ARTICLE 11
MISCELLANEOUS
11.1 Assignment. Neither Party shall assign, or transfer to any Third Party this Agreement or its rights and obligations hereunder without the prior written consent of the other Party, which consent shall not be unreasonably withheld or delayed, except that Epizyme may assign this Agreement (i) to an Affiliate, or (ii) to a Third Party that acquires the exclusive worldwide rights to develop, market or sell any Epizyme Product and Abbott may assign this Agreement (i) to an Affiliate or (ii) to a Third Party that acquires the exclusive worldwide rights to develop, market or sell the Diagnostic Test. This Agreement shall be binding upon the successors and permitted assigns of the Parties.
11.2 Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument.
11.3 Force Majeure. Neither Party shall lose any rights hereunder or be liable to the other Party for damages or losses on account of failure of performance by the defaulting Party, other than a failure to make payment, if the failure is occasioned by government action, war, terrorism, fire, explosion, flood, strike, lockout, embargo, shortage of materials or utilities, vendor failure to supply, act of God, or any other cause beyond the control and without the fault or negligence of the defaulting Party, provided that the Party claiming force majeure has exerted all Commercially Reasonable Efforts to avoid or remedy such force majeure; provided, however, that in no event shall a Party be required to settle any labor dispute or disturbance. Such excuse shall continue as long as the condition preventing the performance continues. Upon cessation of such condition, the affected Party shall promptly resume performance hereunder. Each Party agrees to give the other Party prompt written notice of the occurrence of any such condition, the nature thereof, and the extent to which the affected Party will be unable to perform its
33
obligations hereunder. Each Party further agrees to use all Commercially Reasonable Efforts to correct the condition as quickly as possible and to give the other Party prompt written notice when it is again fully able to perform its obligations hereunder.
11.4 Further Assurances. Each Party hereto agrees to execute, acknowledge and deliver such further instruments and do all such further acts as may be necessary or appropriate to carry out the purposes and intent of this Agreement.
11.5 Modification. No waiver, alteration or modification of any of the provisions hereof shall be binding unless made in writing and signed by the Parties by their respective officers thereunto duly authorized.
11.6 Independent Contractors. The Parties are independent contractors and this Agreement shall not constitute or give rise to an employer-employee, agency, partnership or joint venture relationship among the Parties and each Party’s performance hereunder is that of a separate, independent entity.
11.7 Governing Law; Alternative Dispute Resolution. This Agreement shall be governed by and construed in accordance with the laws of the State of New York, United States of America, without regard to its conflicts of laws principles. The Parties hereby disclaim the application to this Agreement of the United Nations Convention on the International Sale of Goods. Any controversy or claim arising from or related to this Agreement or the breach thereof shall be decided pursuant to the Alternative Dispute Resolution set forth in Exhibit I.
11.8 Headings. Article and section headings in this Agreement are included for convenience of reference and shall not affect the construction or interpretation of any of the provisions of this Agreement.
11.9 Notices. Notices required or permitted under this Agreement shall be in writing and sent by prepaid registered or certified air mail or by overnight express courier providing evidence of receipt (e.g., Federal Express), and shall be deemed to have been properly served to the addressee upon receipt of such written communication, to the following addresses of the Parties:
If to Abbott:
▇▇▇▇▇▇ Molecular Inc.
Attn: Senior Director, Business Development and Licensing
Dept. 09JR
▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
34
With a copy to:
▇▇▇▇▇▇ Laboratories
Attn: Division Vice President, Commercial Legal Operations
Dept. 32MP, Bldg. AP6A-2
▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
If to Epizyme:
Attn: Chief Business Officer
▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
With a copy to:
WilmerHale
Attn: ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇, Esq.
▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
11.11 Third Parties. None of the provisions of this Agreement shall be for the benefit of or enforceable by any Third Party.
11.12 Waiver. The waiver by either Party of a breach or a default of any provision of this Agreement by the other Party shall not be construed as a waiver of any succeeding breach of the same or any other provision, nor shall any delay or omission on the part of either Party to exercise or avail itself of any right, power or privilege that it has or may have hereunder operate as a waiver of any right, power or privilege by such Party.
11.13 Severability. If any part of this Agreement is declared invalid by any legally governing authority having jurisdiction over either Party, then such declaration shall not affect the remainder of the Agreement and the invalidated provision shall be revised in a manner that will render such provision valid while preserving the Parties’ original intent to the maximum extent possible.
11.14 Entire Agreement. This Agreement (including the exhibits attached hereto) constitutes the entire agreement between the Parties relating to the subject matter hereof and supersedes all previous writings and understandings including the CDA (as modified pursuant to Section 6.1).
35
* * *
SIGNATURES FOLLOW.
36
IN WITNESS WHEREOF, the Parties by their respective authorized officers, have executed this Development and Commercialization Agreement on the date first above written.
| ▇▇▇▇▇▇ MOLECULAR INC. | ||
| By: | /s/ ▇▇▇▇ ▇▇▇▇▇▇▇ | |
| ▇▇▇▇ ▇▇▇▇▇▇▇, President | ||
| EPIZYME, INC. | ||
| By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇ | |
| ▇▇▇▇▇ ▇▇▇▇▇▇ | ||
| Its: | EVP & CBO | |
EXHIBIT A(1)
FISH PLATFORM TECHNOLOGY
| United States Patent Number |
Filing or Issue Date | |
| [**] | ||
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | ||
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | [**] | |
| [**] | ||
| [**] | [**] | |
EXHIBIT A(2)
SPECIFICATIONS
EXHIBIT “SPECIFICATIONS”
Technical Specifications of MLL Break Apart FISH Assay
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of one page was omitted. [**]
EXHIBIT B
DEVELOPMENT PLAN
| 1. | Objective |
| • | Develop the Diagnostic Test for clinical studies and launch for commercial sale. |
| 2. | Responsibilities |
[**]
| 3. | Development Timelines |
The development timeline will be developed and agreed to by the Abbott and Epizyme Project Timeline Leads.
| 4. | Interactions with US FDA |
Epizyme and Abbott will work together to prepare for and participate in regulatory discussions with FDA. It is likely that several interactions with US FDA during the development of the test will be required.
| 5. | Reagent/Component Manufacturing |
[**].
| 6. | Reagent/Assay Development – Cost Breakdown by Activity |
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of three pages were omitted. [**].
Key Assumptions
| • | As of the Effective Date, the following items shall be deemed Key Assumptions which were used to prepare the initial Development Plan, the Regulatory Plan and the Commercialization Plan and associated Payment Schedule agreed upon by the Parties: |
| • | The budget and associated Payment Schedule are estimates based on activities that are currently planned in the long term for support clinical trials has been provided and are subject to change based on the assumptions listed below and may be modified in the future as the scope of the project becomes more fully defined. Abbott and Epizyme will agree upon modification of the scope and budget of long-term activities according to the terms of this agreement. |
Overall Program Assumptions Included in Current Proposal
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of three pages were omitted. [**].
EXHIBIT C
REGULATORY PLAN
Epizyme and Abbott will actively cooperate in the regulatory process for the development and global regulatory registration of the Diagnostic Test. The regulatory process includes the development of regulatory strategy, the sharing of regulatory information and data and the submission and liaison with global Regulatory Authorities. Abbott will serve as the formal sponsor of any submissions to global Regulatory Authorities that pertain to the Diagnostic Test, and therefore will be ultimately responsible for the submission of such required documentation per the jointly agreed timelines; provided, however, that the preparation, including strategy, for such submissions will be done in cooperation and consultation with Epizyme and Epizyme shall have the right to attend and participate in all meetings between Abbott and Regulatory Authorities relating to the co-development of the Diagnostic Test. Activities included in the regulatory process that are subject to this regulatory plan include the following:
| • | Routine interactions of Abbott and Epizyme regulatory representatives to share regulatory information relevant to the agreed strategy for development and registration of the Diagnostic Test. |
| • | Abbott will inform Epizyme about any inspections from Regulatory Authorities related to the Diagnostic Test and will provide reports of any findings to Epizyme. |
| • | [**]. |
| • | [**]. |
| • | [**]. |
| • | Epizyme and Abbott will jointly develop a timeline and priority list for regulatory submissions. The timeline will include submission dates and anticipated approval dates for both the Diagnostic Test and the Epizyme Product. |
As a part of the regulatory plan regarding the timelines for regulatory submissions and target timelines for approval, [**]:
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of one page was omitted. [**].
EXHIBIT D
COMMERCIALIZATION PLAN
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of six pages were omitted. [**].
EXHIBIT E
PAYMENT SCHEDULE
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of one page was omitted. [**].
EXHIBIT F
GOVERNANCE OF AGREEMENT
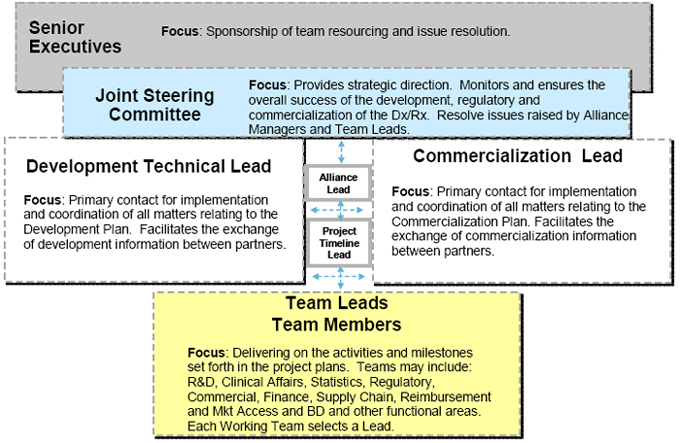
EXHIBIT G
GOVERNANCE ESCALATION PROCESS
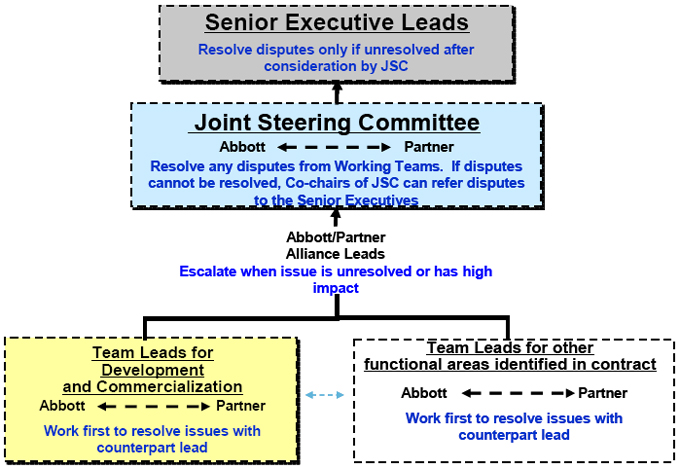
EXHIBIT H
GOVERNANCE ROLES AND RESPONSIBILITIES
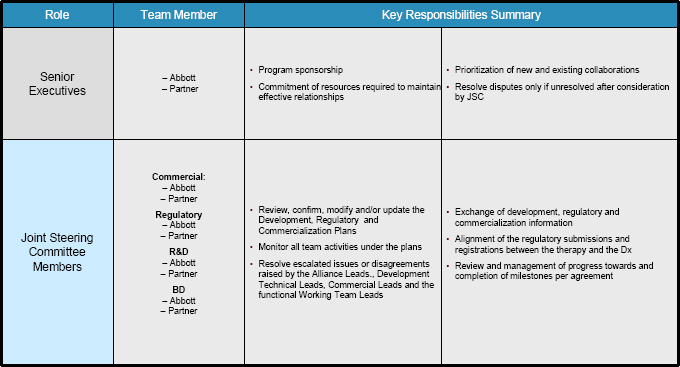
EXHIBIT H
GOVERNANCE ROLES AND RESPONSIBILITIES CON’T
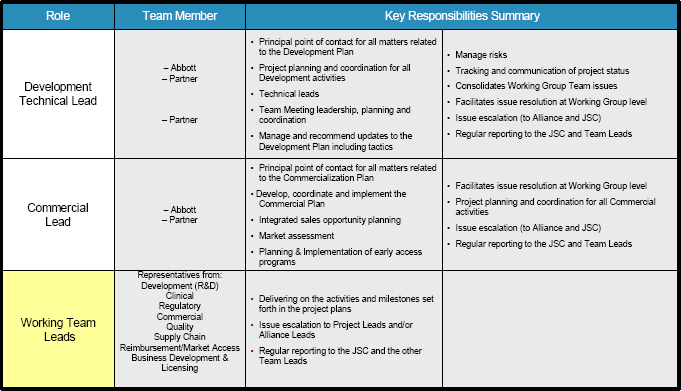
EXHIBIT H
GOVERNANCE ROLES AND RESPONSIBILITIES CON’T
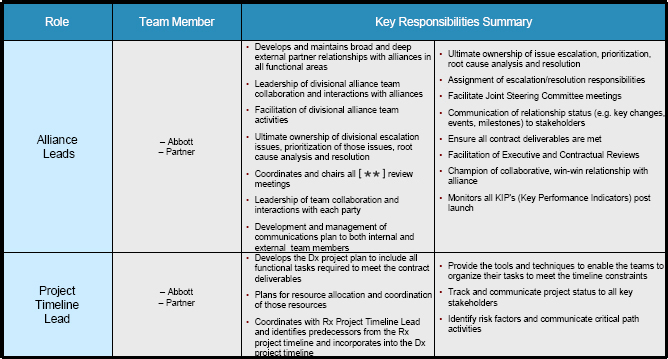
EXHIBIT I
ALTERNATIVE DISPUTE RESOLUTION
The parties recognize that a bona fide dispute as to certain matters may arise from time to time during the term of this Agreement which relates to either party’s rights and/or obligations. To have such a dispute resolved by this Alternative Dispute Resolution (“ADR”) provision, a party first must send written notice of the dispute to the other party for attempted resolution by good faith negotiations between their respective representatives of the affected subsidiaries, divisions, or business units within [**] days after such notice is received (all references to “days” in this ADR provision are to calendar days).
If the matter has not been resolved within [**] days of the notice of dispute, or if the parties fail to meet within such [**] days, either party may initiate an ADR proceeding as provided herein. The parties shall have the right to be represented by counsel in such a proceeding.
1. To begin an ADR proceeding, a party shall provide written notice to the other party of the issues to be resolved by ADR. Within [**] days after its receipt of such notice, the other party may, by written notice to the party initiating the ADR, add additional issues to be resolved within the same ADR.
2. Within [**] days following receipt of the original ADR notice, the parties shall select a mutually acceptable neutral to preside in the resolution of any disputes in this ADR proceeding. If the parties are unable to agree on a mutually acceptable neutral within such period, the parties shall request the President of the Center for Public Resources (“CPR”), ▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇, ▇▇▇ ▇▇▇▇ ▇▇▇▇▇ to select a neutral pursuant to the following procedures:
(a) The CPR shall submit to the parties a list of not less than five (5) candidates within [**] days after receipt of the request from the parties, along with a Curriculum Vitae for each candidate. No candidate shall be an employee, director, or shareholder of either party or any of their subsidiaries or affiliates.
(b) Such list shall include a statement of disclosure by each candidate of any circumstances likely to affect his or her impartiality.
(c) Each party shall number the candidates in order of preference (with the number one (1) signifying the greatest preference) and shall deliver the list to the CPR within [**] days following receipt of the list of candidates. If a party believes a conflict of interest exists regarding any of the candidates that party shall provide a written explanation of the conflict to the CPR along with its list showing its order of preference for the candidates. Any party failing to return a list of preferences on time shall be deemed to have no order of preference.
(d) If the parties collectively have identified fewer than three (3) candidates deemed to have conflicts, the CPR immediately shall designate as the neutral the candidate for whom the parties collectively have indicated the greatest preference. If a tie should result
between two candidates, the CPR may designate either candidate. If the parties collectively have identified three (3) or more candidates deemed to have conflicts, the CPR shall review the explanations regarding conflicts and, in its sole discretion, may either (i) immediately designate as the neutral the candidate for whom the parties collectively have indicated the greatest preference, or (ii) issue a new list of not less than five (5) candidates, in which case the procedures set for in subparagraphs 2(a) - 2(d) above shall be repeated.
3. No earlier than [**] days or later than [**] days after selection, the neutral shall hold a hearing to resolve each of the issues identified by the parties. The ADR proceeding shall take place at a location agreed upon by the parties. If the parties cannot agree, the neutral shall designate a location other than the principal place of business of either party or any of their subsidiaries or affiliates.
4. At least [**] days prior to the hearing, each party shall submit the following to the other party and the neutral:
(a) a copy of all exhibits on which such party intends to rely in any oral or written presentation to the neutral;
(b) a list of any witnesses such party intends to call at the hearing, and a short summary of the anticipated testimony of each witness;
(c) a proposed ruling on each issue to be resolved, together with a request for a specific damage award or other remedy for each issue. The proposed rulings and remedies shall not contain any recitation of the facts or any legal arguments and shall not exceed [**] per issue.
(d) a brief in support of such party’s proposed rulings and remedies, provided that the brief shall not exceed [**] pages. This page limitation shall apply regardless of the number of issues raised in the ADR proceeding.
Except as expressly set forth in subparagraphs 4(a) - 4(d) above, no discovery shall be required or permitted by any means, including depositions, interrogatories, requests for admissions, or production of documents.
5. The hearing shall be conducted on [**] consecutive days and shall be governed by the following rules:
(a) Each party shall be entitled to [**] hours of hearing time to present its case. The neutral shall determine whether each party has had the [**] hours to which it is entitled.
(b) Each party shall be entitled, but not required, to make an opening statement, to present regular and rebuttal testimony, documents or other evidence, to cross-examine witnesses, and to make a closing argument. Cross-examination of witnesses shall occur immediately after their direct testimony, and cross-examination time shall be charged against the party conducting the cross-examination.
(c) The party initiating the ADR shall begin the hearing and, if it chooses to make an opening statement, shall address not only issues it has raised but also any issues raised by the responding party. The responding party, if it chooses to make an opening statement, also shall address all issues raised in the ADR. Thereafter, the presentation of regular and rebuttal testimony and documents, other evidence, and closing arguments shall proceed in the same sequence.
(d) Witnesses shall be excluded from the hearing until closing arguments.
(e) Neither affidavits nor settlement negotiations shall be admissible under any circumstances. As to all other matters, the neutral shall have sole discretion regarding the admissibility of any evidence.
6. Within [**] days following completion of the hearing, each party may submit to the other party and the neutral a post-hearing brief in support of its proposed rulings and remedies, provided that such brief shall not contain or discuss any new evidence and shall not exceed [**] pages. This page limitation shall apply regardless of the number of issues raised in the ADR proceeding.
7. The neutral shall rule on each disputed issue within [**] days following completion of the hearing. Such ruling shall adopt in its entirety the proposed ruling and remedy of one of the parties on each disputed issue but may adopt one party’s proposed rulings and remedies on some issues and the other party’s proposed rulings and remedies on other issues. The neutral shall not issue any written opinion or otherwise explain the basis of the ruling.
8. The neutral shall be paid a reasonable fee plus expenses. These fees and expenses, along with the reasonable legal fees and expenses of the prevailing party (including all expert witness fees and expenses), the fees and expenses of a court reporter, and any expenses for a hearing room, shall be paid as follows:
(a) If the neutral rules in favor of one party on all disputed issues in the ADR, the losing party shall pay 100% of such fees and expenses.
(b) If the neutral rules in favor of one party on some issues and the other party on other issues, the neutral shall issue with the rulings a written determination as to how such fees and expenses shall be allocated between the parties. The neutral shall allocate fees and expenses in a way that bears a reasonable relationship to the outcome of the ADR, with the party prevailing on more issues, or on issues of greater value or gravity, recovering a relatively larger share of its legal fees and expenses.
9. The rulings of the neutral and the allocation of fees and expenses shall be binding, non-reviewable, and non-appealable, and may be entered as a final judgment in any court having jurisdiction.
10. Except as provided in paragraph 9 of this Exhibit or as required by law, the existence of the dispute, any settlement negotiations, the ADR hearing, any submissions (including exhibits, testimony, proposed rulings, and briefs), and the rulings shall be deemed Confidential Information. The neutral shall have the authority to impose sanctions for unauthorized disclosure of Confidential Information.
11. The neutral may not award punitive damages. The parties hereby waive the right to punitive damages.
12. The hearings shall be conducted in the English language in Boston, Massachusetts.

