AMENDMENT NO. 2 TO 2004 LICENSE AGREEMENT
Exhibit 10.1
AMENDMENT NO. 2 TO 2004 LICENSE AGREEMENT
Amendment No. 2, dated as of August 17, 2021 (this “Amendment”), to the Exclusive Patent License Agreement, dated as of July 15, 2004, as amended by the Amendment effective as of April 11, 2007 and as further amended by the Amendment No. 1 to 2004 License Agreement dated as of July 9, 2012 (as so amended, excluding this Amendment, the “License Agreement”), by and among the Board of Regents (the “BOARD”) of The University of Texas System (the “SYSTEM”), an agency of the State of Texas, whose address is 000 Xxxx 0xx Xxxxxx, Xxxxxx, Xxxxx 00000, on behalf of The University of Texas M. D. Xxxxxxxx Cancer Center (“UTMDACC”), a component institution of the SYSTEM, Trustees of Dartmouth College (“DARTMOUTH”), a non-profit educational and research institution existing under the laws of the State of New Hampshire, and being located at Hanover, New Hampshire (BOARD, UTMDACC and DARTMOUTH collectively “LICENSORS”), and Reata Pharmaceuticals, Inc., a Delaware corporation and also formerly known as Reata Discovery, Inc. (“REATA”), having a principal place of business located at 0000 Xxxxxx Xxxxx, Xxxxx, Xxxxx 00000.
WHEREAS, as part of a corporate reorganization (the “Transaction”), REATA anticipates assigning certain intellectual property rights and agreements, including the License Agreement (the “Assignment”), to its wholly-owned subsidiary, [***] (“Reata Sub”), pursuant to a Contribution Agreement (hereinafter defined). Upon the closing of the Transaction, Reata Sub will assume all obligations of REATA under the License Agreement arising from and after the closing of the Transaction. However, REATA will remain liable for and guarantee all obligations under the License Agreement assumed by Reata Sub.
WHEREAS, prior to the Assignment, REATA has sublicensed its rights under the License Agreement (pursuant to Section 4.2 thereof) to [***] (“[***] Sub”), a newly-formed indirect wholly-owned subsidiary of REATA (the agreement giving effect to such sublicense, the “Sublicense”). REATA has also licensed its rights to omaveloxolone to [***] Sub pursuant to an amended and restated license agreement (such license agreement, together with the Sublicense, being referred to herein collectively as the “Sublicenses”). The Sublicenses will be assigned from REATA to Reata Sub as part of the Assignment.
WHEREAS, under the License Agreement, DARTMOUTH’s consent and UTMDACC’s consent are required for REATA to assign the License Agreement to Reata Sub.
WHEREAS, DARTMOUTH desires to monetize (a “Monetization”) all or a portion of its rights to receive running royalties under Section 5.1(c) and Article 17 of the License Agreement and payments related thereto (including, without limitation, as distributed to DARTMOUTH pursuant to the Amended and Restated Commercialization Agreement between UTMDACC and DARTMOUTH, dated October 6, 2000) (collectively, the “Receivables”), including by means of an assignment of such Receivables, and such a Monetization may take the form of a direct sale, a loan or otherwise.
WHEREAS, each of DARTMOUTH and UTMDACC agrees to consent to the assignment of the License Agreement as amended by this Amendment (the “Amended License Agreement”)
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
1
by REATA to Reata Sub on the terms and conditions specified in this Amendment and in the Amended License Agreement.
WHEREAS, the parties now wish to amend the License Agreement to, among other things, (i) reflect the Assignment, (ii) include a guarantee granted by REATA of the obligations of Reata Sub under the Amended License Agreement, (iii) amend the definition of NET SALES, (iv) provide that all reports (including, without limitation, royalty reports) and correspondence to be delivered by the LICENSEE (as defined below) to UTMDACC in connection with the License Agreement are to also be concurrently delivered by the LICENSEE (as defined below) directly to DARTMOUTH, (iv) revise the confidentiality provisions of the License Agreement to permit the disclosure of certain information in connection with a Monetization, (v) specify the resolution procedure in respect of the Article 17 Matter (as defined below) and (vi) clarify the applicability of certain running royalty payment obligations with respect to certain compounds, in each case on the terms and conditions specified in this Amendment and in the Amended License Agreement.
NOW, THEREFORE, in consideration of the recitals above and the mutual promises contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, do hereby agree as follows:
1.Definitions. Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Amended License Agreement.
2.Consent to Assignment. Each of DARTMOUTH and UTMDACC hereby consents, under Article 10 of the Amended License Agreement, to the assignment of the Amended License Agreement by REATA to Reata Sub. REATA shall ensure that, on the effective date of the Contribution Agreement, Reata Sub assumes all obligations of REATA under the Amended License Agreement (other than those set forth in Article 22 of the Amended License Agreement) arising from and after the closing of the Transaction. REATA hereby agrees to (i) remain liable for and guarantee all obligations under the Amended License Agreement assumed by Reata Sub and (ii) on the effective date of the Contribution Agreement, deliver to DARTMOUTH and UTMDACC a contribution agreement duly executed by REATA and Reata Sub that is in the form of the contribution agreement attached hereto as Exhibit 1 (the “Contribution Agreement”).
3.Amendment to LICENSEE Definition. With effect from and after the effective date of the Contribution Agreement, the definition of the term “LICENSEE” in the preamble of the License Agreement is hereby amended and restated in its entirety as follows:
“[***] (“LICENSEE”), a Delaware limited liability company having a principal place of business located at 0000 Xxxxxx Xxxxx, Xxxxx, Xxxxx 00000.”
4.REATA Guaranty. With effect from and after the effective date of the Contribution Agreement, the following new Article 22 is hereby added to the License Agreement:
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
2
“22. GUARANTY
(a) Reata Pharmaceuticals, Inc. (“GUARANTOR”) hereby unconditionally guarantees the full and punctual payment (upon demand or otherwise) of all of the payment obligations of LICENSEE payable under this AGREEMENT (the “PAYMENT OBLIGATIONS”). Upon failure by LICENSEE to pay punctually any such PAYMENT OBLIGATION, GUARANTOR shall forthwith on demand pay the amount not so paid at the place and in the manner and the currency specified in this AGREEMENT (it being understood and agreed that any disputes with respect to the amount of any PAYMENT OBLIGATION owed under Section 5.1(c) or Article 17 will be addressed with the LICENSEE in accordance with Article 18 prior to any claim being made against the GUARANTOR pursuant to this Article 22 with respect to such amount).
(b) GUARANTOR hereby unconditionally guarantees the full and punctual performance (upon demand or otherwise) of all other obligations of LICENSEE under this AGREEMENT (the “PERFORMANCE OBLIGATIONS” and together with the PAYMENT OBLIGATIONS, the “LICENSEE OBLIGATIONS”). Upon failure by LICENSEE to perform punctually any such PERFORMANCE OBLIGATION, GUARANTOR shall forthwith on demand perform such PERFORMANCE OBLIGATION in the manner specified in this AGREEMENT.
22.2 Guaranty Unconditional. The obligations of GUARANTOR under this Article 22 shall be unconditional and absolute and, without limiting the generality of the foregoing, shall not be released, discharged or otherwise affected by:
(a) any extension, renewal, settlement, compromise, waiver or release in respect of any LICENSEE OBLIGATION, by operation of law or otherwise; provided, however, that the release by LICENSORS in writing of LICENSEE in respect of any LICENSEE OBLIGATION under this AGREEMENT shall also operate to release GUARANTOR from its obligations hereunder in respect of such LICENSEE OBLIGATION;
(b) any modification or amendment of or supplement to this AGREEMENT or any other document referred to herein; provided, however, that the release by LICENSORS in writing of LICENSEE in respect of any LICENSEE OBLIGATION under this AGREEMENT shall also operate to release GUARANTOR from its obligations hereunder in respect of such LICENSEE OBLIGATION;
(c) any change in the existence, structure or ownership of LICENSEE, or any insolvency, bankruptcy, reorganization or other similar proceeding affecting LICENSEE or its assets or any resulting release or discharge of any LICENSEE OBLIGATION;
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
3
(d) the existence of any claim, set-off or other rights which GUARANTOR may have at any time against LICENSEE, any LICENSOR or any other corporation, entity or person, whether in connection herewith or any unrelated transaction, provided that nothing herein shall prevent the assertion of any such claim by separate suit or compulsory counterclaim;
(e) any invalidity or unenforceability relating to or against LICENSEE for any reason of this AGREEMENT, or any provision of applicable law or regulation purporting to prohibit the payment by LICENSEE of any PAYMENT OBLIGATION or the performance by LICENSEE of any of its other LICENSEE OBLIGATIONS under this AGREEMENT; or
(f) any other act or omission to act or delay of any kind by LICENSEE, any LICENSOR or any other corporation, entity or person or any other circumstance whatsoever which might, but for the provisions of this paragraph, constitute a legal or equitable discharge of GUARANTOR’s obligations hereunder.
22.3 Discharge Only Upon Payment in Full; Reinstatement In Certain Circumstances. GUARANTOR’s obligations under Section 22.1 shall remain in full force and effect until all LICENSEE OBLIGATIONS have been irrevocably and unconditionally satisfied and paid in full. If at any time any payment made of any LICENSEE OBLIGATION is rescinded or must be otherwise restored or returned upon the insolvency, bankruptcy or reorganization of LICENSEE or otherwise, GUARANTOR’s obligations hereunder with respect to such payment shall be reinstated as though such payment had been due but not made at such time.
22.4 Waiver by GUARANTOR. GUARANTOR irrevocably waives acceptance hereof, presentment, demand, protest and any notice not expressly provided for herein, as well as any requirement that at any time any action be taken by any LICENSOR or any other corporation, entity or person against LICENSEE or any other corporation, entity or person.
22.5 Subrogation. Upon making any payment hereunder with respect to LICENSEE, GUARANTOR shall be subrogated to the rights of LICENSORS against LICENSEE with respect to such payment; provided that GUARANTOR shall not enforce any payment right by way of subrogation until all LICENSEE OBLIGATIONS have been paid in full.
22.6 Confirmation by GUARANTOR. GUARANTOR confirms, acknowledges and agrees that the provisions of this Article 22 constitute obligations of GUARANTOR (and have not been, and will not be, assigned to, or assumed by, LICENSEE pursuant to the Contribution Agreement referred to in Section 2 of Amendment No. 2 to this AGREEMENT).”
5.Other Amendments. With effect from and after the date hereof, the License Agreement is hereby amended as follows:
(a)Section 2.10 of the License Agreement is hereby amended and restated in its entirety as follows:
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
4
“2.10 NET SALES means, with respect to a LICENSED PRODUCT for any period in any country, the total amount billed or invoiced on sales of such LICENSED PRODUCT during such period by LICENSEE or its AFFILIATES or its or their Sublicensees/Distributors in such country to third parties (including wholesalers or distributors who are not Sublicensees/Distributors) in bona fide arm’s length transactions, less the following deductions, in each case to the extent such deductions relate specifically to such LICENSED PRODUCT in such country and are actually allowed and taken by such third parties and are not otherwise recovered by or reimbursed to LICENSEE or its AFFILIATES or its or their Sublicensees/Distributors:
(a)trade, cash and quantity discounts;
(b)price reductions or rebates, retroactive or otherwise, imposed by, negotiated with or otherwise paid to governmental authorities;
(c)taxes on sales (such as sales, value added, or use taxes) to the extent added to the sale price and set forth separately as such in the total amount invoiced;
(d)freight, insurance, and other transportation charges to the extent added to the sale price and set forth separately as such in the total amount invoiced, as well as any fees for services provided by wholesalers and warehousing chains related to the distribution of such LICENSED PRODUCT;
(e)amounts repaid or credited by reason of rejections, defects, one percent (1%) return goods allowance, recalls or returns, or because of retroactive price reductions, including rebates or wholesaler charge backs; and
(f)any invoiced amounts from a prior period that are written off or reserved as not collectable by LICENSEE or its AFFILIATES or its or their Sublicensees/Distributors, including bad debts.
NET SALES shall include the amount or fair market value of all other consideration received by LICENSEE or its AFFILIATES or its or their Sublicensees/Distributors in respect of such LICENSED PRODUCT, whether such consideration is in cash, payment in kind, exchange, or other form. NET SALES shall not include transfers or dispositions for charitable, promotional, pre-clinical, clinical, regulatory, or governmental purposes so long as such transfer or disposition is made at or below cost. NET SALES shall not include sales between or among LICENSEE or its Affiliates or its or their Sublicensees/Distributors so long as such AFFILIATES or Sublicensees/Distributors are not end-users of such LICENSED PRODUCT. Subject to the above, NET SALES shall be calculated in accordance with the standard internal policies and procedures of LICENSEE or its AFFILIATES or its or their Sublicensees/Distributors, which must be in accordance with GAAP and consistently applied.
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
5
i.If a LICENSED PRODUCT is sold as a Combination Product for any period in any country, the NET SALES for such Combination Product will be calculated as follows: If LICENSEE, its AFFILIATES, or Sublicensees/Distributors separately sells in such country, (x) LICENSED PRODUCTS containing as its sole active ingredient the compound which is covered by or is produced using LICENSED SUBJECT MATTER contained in such Combination Product (the “Mono Product”) and (y) products containing as their sole active ingredients the other active ingredient(s) in such Combination Product, the NET SALES attributable to such Combination Product shall be calculated by multiplying actual NET SALES of such Combination Product by the fraction A/(A+B) where: A is LICENSEE’s (or its AFFILIATE’s or Sublicensees/Distributor’s, as applicable) average NET SALES price during the period to which the NET SALES calculation applies for the Mono Product(s) in such country and B is LICENSEE’s (or its AFFILIATE’s or Sublicensees/Distributor’s, as applicable) average NET SALES price during the period to which the NET SALES calculation applies in such country, for products that contain as their sole active ingredient(s) the other active ingredient(s) in such Combination Product.
ii.If LICENSEE, its AFFILIATES, or Sublicensees/Distributors separately sells in such country the Mono Product but does not separately sell in such country products containing as their sole active ingredient(s) the other active ingredient(s) in such Combination Product, the NET SALES attributable to such Combination Product shall be calculated by multiplying the NET SALES of such Combination Product by the fraction A/C where: A is LICENSEE’s (or its AFFILIATE’s or Sublicensees/Distributor’s, as applicable) average NET SALES price during the period to which the NET SALES calculation applies for the Mono Product in such country, and C is LICENSEE’s (or its AFFILIATE’s or Sublicensees/Distributor’s, as applicable) average NET SALES price in such country during the period to which the NET SALES calculation applies for such Combination Product.
iii.If LICENSEE, its AFFILIATES, or Sublicensees/Distributors do not separately sell in such country the Mono Product but do separately sell products containing as their sole active ingredient(s) the other active ingredient(s) contained in such Combination Product, the NET SALES attributable to such Combination Product shall be calculated by multiplying the NET SALES of such Combination Product by the fraction (D-E)/D where: D is the average NET SALES price during the period to which the NET SALES calculation applies for such Combination Product in such country and E is the average NET SALES price during the period to which the NET SALES calculation applies for products that contain as their sole active ingredient(s) the other active ingredient(s) in such Combination Product.
iv.If LICENSEE, its AFFILIATES, or Sublicensees/Distributors do not separately sell in such country both the Mono Product and the other active ingredient or ingredients in such Combination Product, the NET SALES attributable to such Combination Product shall be determined by the PARTIES in
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
6
good faith based on the relative fair market value of such Mono Product and such other active ingredient or ingredients.
As used herein, “Sublicensee/Distributor” means (a) a sublicensee or (b) a third party who is not a sublicensee, but to whom LICENSEE or any of its AFFILIATES has granted the right to distribute LICENSED PRODUCTS wherein such third party makes payments to LICENSEE or any of its AFFILIATES for the right to sell (or resell) LICENSED PRODUCTS, whether or not such payment is in the form of a royalty (or other amount) based upon the revenues received by such third party for the sale (or resale) of such LICENSED PRODUCTS. For clarity, the following entities are not Sublicensee/Distributors under the foregoing clause (b): (i) McKesson Corporation, AmerisourceBergen, Cardinal Health and Xxxxx Medical, in each case based on the activities performed by those entities as of the date of Amendment No. 2 to this AGREEMENT, and other entities performing like activities in other countries in the Territory; and (ii) any other third party that acts as a wholesaler or provides warehousing or logistical support with respect to the sale or distribution of LICENSED PRODUCTS, without more.
As used herein, “Combination Product” means a LICENSED PRODUCT that comprises or contains both (1) a compound which is covered by or is produced using LICENSED SUBJECT MATTER as an active pharmaceutical ingredient; plus (2) one or more other active pharmaceutical ingredients that is not described in the foregoing clause (1), and that is sold either as a fixed dose or as separate doses in a single package for a single price.”
(b)In Sections 4.3, 5.1(c), 5.4, 5.5 and 12.3 of the License Agreement, (i) each reference to “sublicensee” is hereby replaced with a reference to “Sublicensee/Distributor”, (ii) each reference to “sublicensees” is hereby replaced with a reference to “Sublicensees/Distributors”, (iii) each reference to “sublicensee’s” is hereby replaced with a reference to “Sublicensee’s/Distributor’s, (iv) each reference to “ sublicensees’ ” is hereby replaced with a reference to “ Sublicensees’/Distributors’ ” and (v) each reference to “sublicensee(s)” is hereby replaced with a reference to “Sublicensees/Distributors”.
(c)In Exhibit 2 to the License Agreement, the reference to “Sublicensees” is hereby replaced with a reference to “Sublicensees/Distributors”.
(d)Section 5.5 of the License Agreement is hereby amended by deleting the reference to “30 days” in the first sentence of such Section 5.5 and replacing it with a reference to “60 days”.
(e)Article 10 of the License Agreement is hereby amended by adding the following to the end of such Article 10:
“Notwithstanding any provision of this AGREEMENT to the contrary, each LICENSOR shall have the right to assign, convey, sell or otherwise transfer all or any portion of its rights, interests and obligations under this AGREEMENT to any other LICENSOR at any time, including, without limitation, any PATENT RIGHTS and the rights to receive payments pursuant to Articles 5, 9, 17, 18 and 22
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
7
of this AGREEMENT. In the event of any such assignment, conveyance or other transfer of such rights, interests or obligations, each LICENSOR agrees to provide notice thereof to LICENSEE promptly following the execution of any agreements reflecting the same, as well as a description of each right, interest or obligation thereby assigned, conveyed, sold or otherwise transferred.”
(f)Article 14 of the License Agreement is hereby amended by adding the following new Section 14.5 to the end of such Article 14:
“14.5Notwithstanding anything in this AGREEMENT to the contrary, the PARTIES acknowledge and agree that:
(a)(i) DARTMOUTH may monetize (a “Monetization”) all or a portion of its rights to receive running royalties under Section 5.1(c) and Article 17 hereof and payments related thereto (including, without limitation, as distributed to DARTMOUTH pursuant to the Commercialization Agreement) (collectively, the “Receivables”), including by means of an assignment of such Receivables, and (ii) such a Monetization may take the form of a direct sale (through an auction process or otherwise) or a financing (through a borrowing of loans or otherwise), it being understood and agreed, for the avoidance of doubt, that nothing in this Section 14.5 shall be construed to require UTMDACC or LICENSEE to incur any obligations in connection with a Monetization other than as otherwise set forth in this AGREEMENT or the Commercialization Agreement; and
(b)in connection with a Monetization, DARTMOUTH may provide interested parties and the actual purchaser in such Monetization on an ongoing basis with copies of (i) the Relevant Agreements (as defined below), (ii) the royalty reports provided under the Relevant Agreements, and (iii) notices, reports and correspondence given or received under the Relevant Agreements; provided, however, that prior to disclosing any of the foregoing, each such interested party and actual purchaser shall execute a customary confidentiality agreement with DARTMOUTH covering such information. “Relevant Agreements” means, collectively, (A) this AGREEMENT, the Commercialization Agreement, the Settlement Agreement, the Contribution Agreement (as defined in Section 2 of Amendment No. 2 to this AGREEMENT) and the Sublicenses (as defined in the second Whereas clause of Amendment No. 2 to this AGREEMENT), (B) any agreements required to be provided by UTMDACC to DARTMOUTH, or by LICENSEE to UTMDACC or DARTMOUTH, under any of the agreements referenced in the immediately preceding clause (A), and (C) all amendments and other modifications to the agreements referenced in the immediately preceding clauses (A) and (B).”
(g)The following new Article 20 is hereby added to the License Agreement:
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
8
|
|
“20. |
ROYALTY PAYMENTS RELATED TO Bardoxolone and Omaveloxolone |
20.1“BARDOXOLONE” means that compound having the chemical structure set forth below:
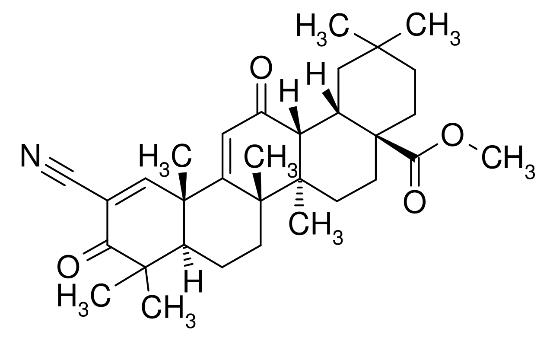
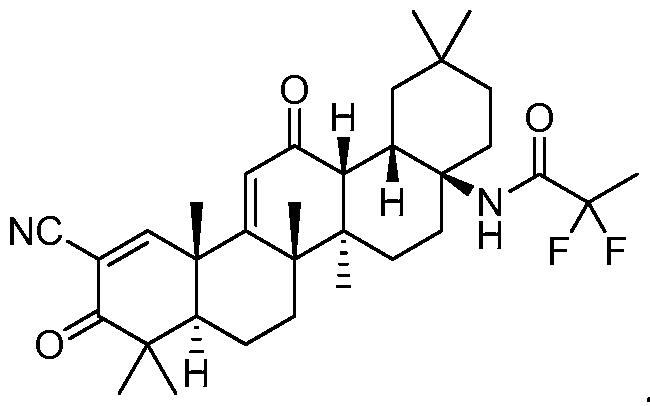
20.2“OMAVELOXOLONE” means that compound having the chemical structure set forth below:
20.3Notwithstanding anything to the contrary in this AGREEMENT, LICENSEE, DARTMOUTH and UTMDACC acknowledge and agree that:
(a)BARDOXOLONE is LICENSED SUBJECT MATTER (as defined in Section 2.8) because it is an invention, discovery or process covered by PATENT RIGHTS and/or TECHNOLOGY RIGHTS within LICENSED FIELD. As such, a world-wide running royalty of [***] percent ([***]%) of NET SALES of any product, process or service which is covered by or is produced using BARDOXOLONE (“BARDOXOLONE PRODUCT”) shall, on a country-by-country and product-by-product basis, be payable by LICENSEE pursuant to Section 5.1(c) hereof on NET SALES of any BARDOXOLONE PRODUCT in any particular country until the expiration of the last-to-expire of the PATENT RIGHTS that Covers such BARDOXOLONE PRODUCT in such country. The dispute (which remains unresolved as of the date of Amendment No. 2 to this AGREEMENT) between the LICENSEE and the LICENSORS regarding whether BARDOXOLONE PRODUCTS are also products on which the [***] percent ([***]%) world-wide running royalty under Article 17 is payable (such dispute, the “Article 17 Matter”) shall be resolved in accordance with the provisions of Section 16.6. The PARTIES hereby agree to initiate mediation under Section 16.6 regarding the Article 17 Matter within [***] ([***]) days after the first date on
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
9
which the FDA approves the sale and marketing of a BARDOXOLONE PRODUCT. Nothing in Amendment No. 2 to this AGREEMENT shall be considered, referenced or introduced into evidence in connection with the Article 17 Matter or the resolution thereof. The PARTIES’ agreement and understanding that BARDOXOLONE is LICENSED SUBJECT MATTER as defined in Section 2.8 and that a [***] percent ([***]%) world-wide running royalty under Section 5.1(c) is payable with respect to NET SALES of BARDOXOLONE PRODUCTS (as further described in the first and second sentences of this clause (a)) shall not in any way be affected by (i) the existence of the Article 17 Matter, (ii) the manner in which the Article 17 Matter is resolved (or in which it remains unresolved) or (iii) the terms and conditions of any resolution of the Article 17 Matter.
(b)OMAVELOXOLONE is not LICENSED SUBJECT MATTER as defined in Section 2.8, and the OMAVELOXOLONE PATENTS (as defined in Schedule A to Amendment No. 2 to this AGREEMENT) Cover any products that contain or embody OMAVELOXOLONE (such products, “OMAVELOXOLONE PRODUCTS”). OMAVELOXOLONE PRODUCTS are products on which the [***] percent ([***]%) world-wide running royalty under Article 17 is payable. As such, a world-wide running royalty of [***] percent ([***]%) of NET SALES of each OMAVELOXOLONE PRODUCT shall, on a country-by-country and product-by-product basis, be payable by LICENSEE pursuant to Article 17 hereof on NET SALES of any OMAVELOXOLONE PRODUCT in any particular country until the expiration of the last-to-expire of the OMAVELOXOLONE PATENTS (as defined in Schedule A to Amendment No. 2 to this AGREEMENT) that Covers such OMAVELOXOLONE PRODUCT in such country.
(c) As used herein, “Covers” means, with respect to a patent and a product, that such patent would (absent a license thereunder and/or ownership thereof) be infringed by the making, manufacture, use, importation, offer for sale or sale of such product in the applicable country.”
(h)The following new Article 21 is hereby added to the License Agreement:
|
|
“21. |
DOCUMENT DELIVERY TO DARTMOUTH |
Notwithstanding any provisions of this Agreement to the contrary, LICENSEE shall deliver all notices, reports, statements and other documents under this Agreement (including, without limitation, all royalty reports under Section 5.5 and Article 17 hereof) concurrently to both UTMDACC and DARTMOUTH by email (with PDF attachment) to (i) in the case of UTMDACC, xxxxx@xxxxxxxxxx.xxx (or such other email address as may be designated from time to time by UTMDACC to LICENSEE) and (ii) in the case of DARTMOUTH, xxx.x.xxxxxxxxxx@xxxxxxxxx.xxx (or such other email address as may be designated from time to time by DARTMOUTH to LICENSEE).”
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
10
(i)Section 8.4(b) of the License Agreement is hereby amended and restated in its entirety as follows:
“b.Without limiting the provisions of this Section 8.4 and notwithstanding anything herein to the contrary, each PARTY, as applicable, agrees and covenants to be bound by the provisions of Section 8.4, Article 12 (Indemnification), Article 13 (Use of Name), Article 14 (Confidential Information), Article 16 (General), Article 17 (Collaboration Agreement Fee), Article 18 (Royalty Dispute Resolution), Article 20 (Royalty Payments Related to Bardoxolone and Omaveloxolone) and Article 22 (Guaranty) and all definitions contained, and other Sections and all Exhibits cross-referenced, therein;”
6.Effect of Amendment. Except as amended by this Amendment, the License Agreement shall remain in full force and effect pursuant to its terms. By signing this Amendment, each of the parties hereto hereby agrees that the Amended License Agreement is hereby ratified and affirmed in all respects. Each reference in the Amended License Agreement to “this Agreement”, “herein”, “hereunder” or words of similar import shall mean and be a reference to (a) from and after the date hereof, the License Agreement as amended by Section 5 of this Amendment and (b) from and after the effective date of the Contribution Agreement, the License Agreement as amended by Sections 3, 4 and 5 of this Amendment.
7.Governing Law. This Amendment will be construed and enforced in accordance with the laws of the United States of America and of the State of Texas, without regard to its conflict of law provisions.
8.Counterparts. This Amendment may be executed in one or more counterparts all of which together shall constitute one and the same agreement. The delivery by any party of an executed counterpart hereof by facsimile transmission or email of .pdf copies shall be effective as an original executed counterpart of this Amendment by such party and shall constitute an original enforceable document.
[signatures set forth on the following page]
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
11
IN WITNESS WHEREOF, the parties have duly executed this Amendment as of the date first written above.
REATA PHARMACEUTICALS, INC.
By: /s/ Xxxxxxx X. Xxxx _______________
Name: Xxxxxxx X. Xxxx
Title: Chief Operating Officer, Chief Financial Officer and Executive Vice President
TRUSTEES OF DARTMOUTH COLLEGE
By: /s/_ Xxx X. Xxxxxxxxxx _____________
Name: Xxx X. Xxxxxxxxxx
Title: Director, Technology Transfer
BOARD OF REGENTS OF THE UNIVERSITY OF TEXAS SYSTEM ON BEHALF OF
THE UNIVERSITY OF TEXAS M.D. XXXXXXXX CANCER CENTER
By: /s/_ Ben Melson________________
Name: Xxx Xxxxxx
Title: SVP, CFO
[Signature Page to Amendment No. 2 to 2004 License Agreement]
Schedule A
Omaveloxolone Patents
[***]
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[*** |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***] |
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
|
[***] |
[***]
|
[***] |
[***] |
[***] |
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].
Exhibit 1
Contribution Agreement
[***]
Specific terms in this Exhibit have been redacted because such terms are both not material and are of the type that the Company treats as private or confidential. These redacted terms have been marked in this Exhibit with three asterisks [***].

