COLLABORATIVE RESEARCH AGREEMENT
Exhibit 6.3
COLLABORATIVE RESEARCH AGREEMENT
THIS COLLABORATIVE RESEARCH AGREEMENT (this “Agreement”) effective as of the 15th day of June, 2017 (the “Effective Date”), is entered into between EMERALD HEALTH PHARMACEUTICALS INC., a Delaware corporation (“Collaborator”), with a place of business at 0000 Xxxxx Xxxxx Xxxx, Xxx Xxxxx, Xxxxxxxxxx, Xxxxxx Xxxxxx and VIVACELL BIOTECHNOLOGY ESPANA, S.L. a limited liability company (Sociedad Limitada) incorporated under the laws of Spain (“VivaCell”), having a place of business at Parque Científico, Tecnologico de Cordoba, Xxxxxxxxx 21, c/ Astronoma Xxxxxxx Xxxxx, Xxxxxxx Xxxxxxxx, 00000, Xxxxxxx, Xxxxx.
A. VivaCell has discovered and developed certain new chemical entities and natural extracts that may be effective in the treatment of human and animal diseases, including two sets of cannabinoid derivatives known as “Series 3” and “Series 4”, as further described in Exhibit A and Exhibit B, respectively, attached hereto.
B. Collaborator has acquired the Series 3 and Series 4 technology from Vivacell pursuant to an Intellectual Property Transfer Agreement dated the 15 day of June, 2017 (the “Transfer Agreement”).
C. Collaborator desires to sponsor certain research by VivaCell as further described in one or more Project Plans (as defined below) and VivaCell desires to conduct such research in accordance with the terms and conditions of this Agreement.
D. Such research is of mutual interest to Collaborator and VivaCell and furthers research objectives of developing new drugs based on cannabinoids for the treatment of human and animal diseases.
NOW, THEREFORE, in consideration of the foregoing premises and he mutual covenants herein contained, the parties hereby agree as follows:
| 1. | Definitions. For the purposes of this Agreement, the following terms shall have the respective meanings set forth below: |
| 1.1 | “Confidential Information” means, with respect to a party (the “Discloser”), any and all proprietary or confidential data and information disclosed by the Discloser to the other party (the “Recipient”), whether in writing, or in oral, graphic, electronic or any other form, or obtained by or on behalf of the Recipient through inspection or observation of the foregoing. Confidential Information shall not include any information that |
| (a) | is or becomes publicly known through no act or omission of the Recipient; |
| (b) | was rightfully known by Recipient before receipt from the Discloser; |
| (c) | becomes known to Recipient by a third party without confidential or proprietary restriction from the Discloser; or |
| (d) | is independently developed by Recipient without the use of or reference to the Confidential Information of Discloser. The terms and conditions of this Agreement shall constitute Confidential Information of both parties. Without limiting the generality of the foregoing, Series 3 and Series 4 shall each be deemed to be the Confidential Information of the Collaborator. |
| 1.2 | “Collaborator Innovations” mean all discoveries, designs, developments, improvements, inventions and other technology (whether or not protectable under patent laws), all works of authorship or information fixed in any tangible medium of expression (whether or not protectable under copyright laws), and all trade secrets, know-how and ideas (whether or not protectable under trade secret laws), in each case of the Collaborator and which are not derived from (a) a Project (b) VivaCell Innovations, or (c) both. |
| 1.3 | “Collaborator Materials” mean, collectively, all information and materials provided by or on behalf of Collaborator to VivaCell in connection with a Project, including, without limitation, Series 3, Series 4 and any technology acquired pursuant to the Transfer Agreement or subsequent similar agreements between the parties, and any other Collaborator materials described in a Project Plan. |
| 1.4 | “Collaborator Technology” means, collectively, the Collaborator Materials, together with (a) all parts, compositions, formulations, modifications, derivatives, improvements and enhancements thereof and thereto, (b) all methods specific to the manufacture thereof, and (c) all uses thereof. |
| 1.5 | “Derived” or “derived” means obtained, developed, created, synthesized, designed, derived or resulting from, based upon or otherwise generated (whether directly or indirectly, or solely or jointly with others, or in whole or in part). |
| 1.6 | “Initial Project Plan” means the Project Plan set out in Exhibit C. |
| 1.7 | “Initial Project Plan Budget” means the Budget set out in Exhibit D to the Initial Project Plan. |
| 1.8 | “Intellectual Property Rights” mean: |
| (a) | any and all proprietary rights, in any jurisdiction in the world, provided under (i) patent law, (ii) copyright law, (iii) trade-xxxx law, (iv) design patent or industrial design law, (v) plant breeders’ rights or plant variety rights law; or (vi) any other applicable statutory provision or common law principle, including trade secret law, that may provide a right in ideas, formulae, algorithms, concepts, inventions, inventors’ notes, research data, works or know-how, or the expression or use thereof, and including all past, present and future causes of action, rights of recovery, and claims for damage, accounting of profits, royalties, or other relief relating, referring or pertaining to any of the foregoing; and |
- 2 -
| (b) | any and all applications, registrations, licenses, sublicenses, collaboration agreements, technology transfer agreements, and other agreements or other evidence of a right in any of the foregoing; |
| 1.9 | “JSC” means the joint steering committee; |
| 1.10 | “Principal Investigator” shall mean, with respect to a Project, the principal investigator identified in the Project Plan for such Project. |
| 1.11 | “Project” means the project described in a Project Plan. |
| 1.12 | “Project Plan” means, a written project plan mutually agreed in writing by the parties, and includes the Initial Project Plan. |
| 1.13 | “Project Plan Amendment” means any individual, mutually agreed, duly executed and delivered written description, in substantially the form of Exhibit E setting forth any amendment, change or other modification to a specific Project Plan, and expressly referencing the specific Project Plan to be amended, changed or otherwise modified. |
| 1.14 | “Project Technology” means, all discoveries, designs, developments, improvements, inventions and other technology (whether or not protectable under patent laws), all works of authorship or information fixed in any tangible medium of expression (whether or not protectable under copyright laws), and all trade secrets, know-how and ideas (whether or not protectable under trade secret laws), in each case generated in connection with the Project. |
| 1.15 | “Results” mean all data, information and other deliverables and results derived from a Project or use of the Collaborator Technology, but excluding the VivaCell Innovations and Collaborator Innovations. |
| 1.16 | “VivaCell Innovations” mean all discoveries, designs, developments, improvements, inventions and other technology (whether or not protectable under patent laws), all works of authorship or information fixed in any tangible medium of expression (whether or not protectable under copyright laws), and all trade secrets, know-how and ideas (whether or not protectable under trade secret laws), in each case of VivaCell, and which are not derived from (a) a Project (b) the Collaborator Technology, or (c) both. |
- 3 -
| 1.17 | “VivaCell Materials” mean, collectively, all information and materials provided by or on behalf of VivaCell to Collaborator in connection with a Project and any other VivaCell materials described in a Project Plan. |
| 1.18 | “VivaCell Technology” means, collectively, the VivaCell Materials, together with (a) all parts, compositions, formulations, modifications, derivatives, improvements and enhancements thereof and thereto, (b) all methods specific to the manufacture thereof, and (c) all uses thereof. |
| 2. | Conduct of Projects. |
| 2.1 | Formation of JSC. Prior to commencement of any Project Plan, Collaborator and VivaCell shall each appoint two representatives to the initial JSC and shall promptly thereafter notify the other party of such appointment. Either party may change its representatives to the JSC upon providing prior written notice to the other party. Each party shall ensure that its representatives on the JSC have the requisite experience and expertise to oversee the activities of the collaboration during the term of the applicable Project Plan. Each Project Plan must be reviewed and approved by the JSC prior to any work being commenced on such Project Plan, and the JSC shall oversee VivaCell’s activities with respect to such Project Plan. All decisions of the JSC will be determined by a majority vote of the members of the JSC. |
| 2.2 | Meetings. The JSC shall meet not less than quarterly on such dates and at such times and places as agreed to by the representatives. JSC meetings may be conducted in person, by teleconference, or by videoconference. Meetings shall be effective only if all four representatives are in attendance or participating in the meeting. Each party may permit observers to attend meetings of the JSC, provided that any such observers agree in writing to be bound by the confidentiality provisions set out in this Agreement. Each party shall be responsible for its own costs in connection with the meetings of the JSC. |
| 2.3 | Role of JSC. The JSC shall oversee and review VivaCell’s activities with respect to any Project Plan, and determine any necessary amendments or modifications to, or the termination of, any Project Plan from time to time. The JSC shall not have power to amend or modify this Agreement or to impose additional economic burdens on either party beyond those specifically contemplated by this Agreement without the prior written consent of the party on which such burden is imposed. |
| 2.4 | Projects. Collaborator engages VivaCell to conduct each Project on the terms and conditions of this Agreement, including, without limitation, the applicable Project Plan. VivaCell accepts such engagement, and shall conduct each Project and otherwise act in strict accordance with the terms and conditions of this Agreement, including, without limitation, the applicable Project Plan. No activities under a Project Plan shall be commenced until the JSC has had opportunity to review, propose modifications to and approve such Project Plan. |
- 4 -
| 2.5 | Initial Project Plan. Collaborator will engage VivaCell to conduct the activities contemplated under the Initial Project Plan after the JSC has reviewed, made modifications to and approved the Initial Project Plan. |
| 2.6 | Project Plan. It is contemplated that the parties shall from time to time enter into new Project Plans. Upon mutual agreement, due execution and delivery of a Project Plan for a Project, such Project Plan automatically shall be incorporated into this Agreement; provided, however, that to the extent any term or condition of such Project Plan conflicts with the terms and conditions of this Agreement, the terms and conditions of this Agreement shall control unless otherwise expressly stated in the applicable Project Plan that such conflicting term or condition is specifically intended to supersede the terms and conditions of this Agreement, and expressly referencing the specific terms and conditions to be superseded. |
| 2.7 | Amendments; Termination of Project Plan. A party’s representative(s) on the JSC may, from time to time, request that a Project Plan be amended, changed or otherwise modified, in whole or in part. Without limiting the generality of the foregoing, such representative may request amendments, changes or modifications to any part of a Project Plan, including provisions relating to the scope of work, the principal investigators, collaborators, types of studies, budget, deliverables, timelines and/or funding matters. Any amendment, change or modification will not be binding on the parties unless both Collaborator and VivaCell have evidenced their agreement to same by the due execution and delivery of a Project Plan Amendment for the applicable Project. Notwithstanding the foregoing, if the parties are unable to reach a mutual agreement on any requested amendment, change, or modification to a Project Plan, then Collaborator shall be entitled to terminate the Project Plan by giving the VivaCell thirty (30) days’ prior written notice. If any milestones or deliverables set out in the Initial Project Plan are not achieved in accordance with the Initial Project Plan (as amended and approved by the parties), then Collaborator may terminate the Initial Project Plan and reallocate any unused funds from the Initial Project Plan Budget to any current or future research. |
| 2.8 | Principal Investigator. VivaCell shall conduct each Project under the direction of the Principal Investigator identified in the applicable Project Plan. The Principal Investigator for a Project shall be responsible for the supervision and administration thereof. If the Principal Investigator for a Project becomes unable or unwilling to continue to direct such Project in accordance with this Agreement, for any reason whatsoever, the JSC shall select a replacement Principal Investigator. |
- 5 -
| 2.9 | Research Procedures. VivaCell shall conduct the Project in good scientific manner, and in compliance with all applicable Collaborator policies and procedures as detailed in the applicable Project Plan, all requirements of applicable laws and regulations, the highest professional industry standards and good laboratory and/or clinical practices, including, without limitation, any quality assurance, quality control and other standards set forth in the applicable Project Plan. VivaCell shall proceed diligently with the work set out in the applicable Project Plan for such Project, including, without limitation, by allocating sufficient time and effort and using personnel with sufficient skills and experience, together with sufficient equipment and facilities, to conduct such Project and to accomplish the goals set out therein. |
| 2.10 | Records. VivaCell shall maintain complete and accurate records, appropriate for patent and Intellectual Property Rights protection purposes, of all work in connection with the Project and all Results, Collaborator Technology and Project Technology. Collaborator shall have the right, upon reasonable notice and during normal business hours, to make copies of such records, and to consult with the individuals conducting the Project. |
| 2.11 | Reports. |
| (a) | At such times as described in the applicable Project Plan for a Project and otherwise at Collaborator’s reasonable request, VivaCell shall prepare and deliver to Collaborator such reports (in such form as reasonably requested by Collaborator), including, without limitation, a report (in such form as reasonably requested by Collaborator) describing in reasonably sufficient detail the Results derived as of the date of such report, activities conducted under such Project and all resulting Project Technology and Collaborator Technology, and accounting for the use of the funding provided by Collaborator for such Project. |
| (b) | Unless otherwise set forth in the applicable Project Plan, within forty-five (45) days after completion or termination of such Project, VivaCell shall prepare and deliver to Collaborator a final report (in such form as reasonably requested by Collaborator) describing in reasonably sufficient detail all Results of such Project, all methodologies, techniques and other activities used in conducting such Project and all resulting Project Technology and Collaborator Technology, and accounting for the use of the funding provided by Collaborator for such Project. |
| 2.12 | Term. The term of each Project shall commence on Project Effective Date (as defined in the applicable Project Plan) and, unless terminated earlier in accordance with this Agreement or the parties otherwise mutually agree in writing, shall terminate upon delivery by VivaCell and acceptance by Collaborator of the final deliverable for such Project as described in the applicable Project Plan. |
| 2.13 | Insurance. VivaCell shall at all times maintain comprehensive personal injury and property damage insurance applicable to the operations of VivaCell that will include without limitation personal injury liability, product liability, and contractual liability, and any other forms of insurance, all of which must be in such reasonable amounts and with such reasonable deductions as would be obtained by a prudent person acting reasonably in similar circumstances. |
- 6 -
| 3. | Collaborator Materials; Collaborator Technology. |
| 3.1 | Collaborator Materials. Collaborator may provide VivaCell with the Collaborator Materials described in the applicable Project Plan for a Project on the terms and conditions of this Agreement. The transfer of physical possession of any Collaborator Materials and the physical possession and use of any Collaborator Materials by VivaCell shall not be (nor be construed as) a sale, lease, offer to sell or lease, or other transfer of title of such Collaborator Materials to VivaCell. |
| 3.2 | Ownership. VivaCell hereby acknowledges that, as between Collaborator and VivaCell, Collaborator is the sole owner or licensee of the Collaborator Technology, together with all Intellectual Property Rights therein and thereto, and hereby assigns to Collaborator, all right, title and interest in and to the foregoing. |
| 3.3 | Patent Rights. Collaborator shall control, at its sole cost, the filing, preparation, prosecution, maintenance and enforcement of all patent applications and patents claiming or covering the Collaborator Technology. Upon Collaborator’s reasonable request, VivaCell and its employees and consultants shall assist Collaborator, shall execute, acknowledge and deliver such further documents and instruments, and shall perform all such other acts as may be necessary or appropriate in preparing, filing, prosecuting, maintaining or enforcing all such patent applications and patents. |
| 3.4 | Restrictions. |
| (a) | VivaCell shall use the Collaborator Technology, and any information and other materials directly or indirectly derived therefrom solely to conduct the applicable Project in accordance with the terms and conditions of this Agreement, including, without limitation, the applicable Project Plan. VivaCell shall not use the Collaborator Technology, or any information or other materials directly or indirectly derived therefrom, for any other purpose. VivaCell shall not (and shall not attempt or purport to) file or prosecute or support in any country any patent application or Intellectual Property Rights registration or application which claims or uses or purports to claim or use the Collaborator Technology or its use, without the prior express written consent of Collaborator. VIVACELL UNDERSTANDS THAT THE COLLABORATOR MATERIALS ARE PROVIDED SOLELY FOR CONDUCTING THE PROJECT AND HAVE NOT BEEN APPROVED FOR HUMAN USE. VIVACELL SHALL NOT ADMINISTER THE COLLABORATOR MATERIALS TO HUMANS IN ANY MANNER OR FORM. |
- 7 -
| (b) | VivaCell shall not transfer the Collaborator Technology, or any information or other materials directly or indirectly derived therefrom, to any third party without the prior express written consent of Collaborator. VivaCell shall limit transfer and disclosure of the Collaborator Technology, and any information or other materials directly or indirectly derived therefrom, on a need to know basis, as reasonably necessary for conducting the Project in accordance with the terms and conditions of this Agreement, including, without limitation, the applicable Project Plan, to its employees who are bound by written agreements with VivaCell to hold in confidence and not make use of the Collaborator Technology, and such information and other materials, for any purpose other than those permitted by this Agreement. VivaCell shall notify Collaborator promptly upon discovery of any unauthorized use or disclosure of the Collaborator Technology. |
| 3.5 | No Warranties. VivaCell hereby acknowledges that the Collaborator Materials are experimental in nature and that they are provided on an “AS IS” basis. COLLABORATOR MAKES NO REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, WITH RESPECT TO THE COLLABORATOR MATERIALS OR THE USE THEREOF. COLLABORATOR DISCLAIMS ALL IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NON-INFRINGEMENT. |
| 4. | Results and Project Technology. |
| 4.1 | Ownership and Assignment. |
| (a) | Collaborator shall be the exclusive owner of all rights, title and interests, including without limitation the Intellectual Property Rights, in and to the Results and Project Technology, regardless of whether they were made or conceived (a) solely by employees or others acting on behalf of VivaCell, (b) solely by employees or others acting on behalf of Collaborator or (c) jointly by employees or others acting on behalf of Collaborator and by employees or others acting on behalf of VivaCell. Collaborator shall have the right, subject to the provisions of this Agreement, to freely exploit, transfer, license or encumber its rights in any Results and Project Technology, and the Intellectual Property Rights therein, without the consent of, or payment or accounting to, VivaCell. |
| (b) | VivaCell hereby assigns to Collaborator all right, title and interest in and to all Results and Project Technology, together with all Intellectual Property Rights therein and thereto, effective at the time each is created. |
| 4.2 | Disclosure. VivaCell promptly shall disclose to Collaborator any Results and Project Technology made or conceived by or on behalf of VivaCell, and provide Collaborator with copies of all information available to VivaCell regarding such Results and Project Technology. Furthermore, VivaCell shall promptly disclose to Collaborator in writing any VivaCell Innovations as they arise or come into existence. |
- 8 -
| 4.3 | VivaCell Innovations. If VivaCell incorporates or permits to be incorporated any VivaCell Innovations into the Results or Project Technology, or any VivaCell Innovations is reasonably necessary or useful for the exploitation of the Results or Project Technology, then VivaCell hereby grants to Collaborator and Collaborator's designees a non-exclusive, royalty free, irrevocable, worldwide, fully paid-up license (with rights to sublicense through multiple tiers) to practice such VivaCell Innovations and all Intellectual Property Rights therein and thereto to the extent reasonably necessary or useful for the exploitation of the Results or Project Technology |
| 5. | Further Assistance; Power of Attorney. |
| 5.1 | Further Assistance. VivaCell shall perform, during and after the term of this Agreement, all acts that Collaborator deems necessary or desirable to permit and assist Collaborator in obtaining, perfecting and enforcing the full benefits, enjoyment, rights and title throughout the world in the Collaborator Technology, the Results, the Project Technology and other rights under this Agreement. |
| 5.2 | Power of Attorney. If Collaborator is unable for any reason to secure VivaCell’s signature to any document required to file, prosecute, register or memorialize the assignment of any rights under any Collaborator Technology or Result, VivaCell hereby irrevocably designates and appoints any officer or director of Collaborator as VivaCell' s agent and attorney-in-fact to act for and on VivaCell' s behalf and instead of VivaCell to take all lawfully permitted acts to further the filing, prosecution, registration, memorialization of assignment, issuance and enforcement of rights under such Innovation or Result, all with the same legal force and effect as if executed by VivaCell. The foregoing is deemed a power coupled with an interest and is irrevocable. |
| 6. | Options and Licenses. |
| 6.1 | Option. With respect to each discovery or invention comprising VivaCell Innovations, VivaCell hereby grants to Collaborator an exclusive option (the “Option”) to: |
| (a) | obtain an exclusive, worldwide, royalty-bearing license (with the exclusive right to sublicense) any Intellectual Property Rights owned by VivaCell relating to such discovery or invention; or |
| (b) | acquire ownership of any Intellectual Property Rights owned by VivaCell relating to such discovery or invention. |
- 9 -
| 6.2 | Exercise of Option. The Option with respect to each such discovery or invention shall be exercisable for the six-month period after disclosure to Collaborator of all information available to VivaCell regarding such discovery or invention. The license or acquisition of ownership shall be on mutually acceptable terms and conditions, which may include a license fee, purchase price, royalty rate, or combination thereof. The terms and conditions of such license or acquisition shall be negotiated in good faith based on reasonable factors including without limitation the parties' respective contributions, the stage of development of the discovery or invention, relevant industry standards and additional royalty obligations owing to third parties. Collaborator shall have the right to control the filing, prosecution, maintenance and enforcement of all registrations, filings, and applications in respect of the Intellectual Property Right(s) so licensed or acquired. |
| 6.3 | Right of First Refusal - Sale or License. VivaCell may not sell or license any Intellectual Property Rights with respect to a discovery or invention comprising any VivaCell Innovations to a third-party unless VivaCell first offers in writing such Intellectual Property Right to Collaborator (the “IP Offer”). Collaborator shall have six (6) months after receiving the IP Offer to accept or reject the IP Offer. If Collaborator accepts the IP Offer, a binding agreement will be deemed to have formed between VivaCell and Collaborator. If Collaborator does not accept the IP Offer, VivaCell may for three (3) months offer the Intellectual Property Right, which is the subject of the IP Offer to any third-party on the same terms and conditions as the IP Offer. |
| 6.4 | Right of First Refusal - Sponsorship. VivaCell may not enter into any new research collaboration with or undertake any research project for any third-party with respect to new research collaborations or sponsorship pursued by VivaCell unless VivaCell first offers such collaboration or sponsorship opportunity to Collaborator (the “Research Offer”). Collaborator shall have two (2) months after receiving the Research Offer to accept or reject the Research Offer. If Collaborator accepts the Research Offer, a binding agreement will be deemed to have formed between VivaCell and Collaborator with respect to the matters set out in the Research Offer. If Collaborator does not accept the Research Offer, VivaCell may, within one (1) month, enter into the research collaboration or undertake the research project subject of the Research Offer with any third-party on the same terms and conditions as the Research Offer made to Collaborator. |
| 6.5 | Patent Rights. |
| (a) | VivaCell shall have the right to control, at its sole expense, the preparation, filing, prosecution and maintenance of all Intellectual Property Rights to the extent they claim VivaCell Innovations. |
| (b) | Collaborator shall have the right to control, at its sole expense, the preparation, filing, prosecution and maintenance of all Intellectual Property Rights to the extent they claim Collaborator Innovations, Project Technology, Series 3 or Series 4. |
| (c) | Each party shall cooperate with the other party, execute all lawful papers and instruments and make all rightful oaths and declarations as may be necessary in the preparation, filing, prosecution, maintenance and enforcement of all Intellectual Property Rights described in this Section 6.5. |
- 10 -
| 7. | Financial Terms. With respect to each Project Plan entered into between the parties on mutual agreement and approval of the JSC, Collaborator shall pay to VivaCell such amounts as set forth in the budget of the applicable Project Plan, at such times an in accordance with such other terms and conditions as set forth in such Project Plan. VivaCell will provide services to Collaborator at reasonable market rates. Collaborator will have the right to review the rates in any Project Plan and request revisions to reflect reasonable market rates. If a third-party is able to offer Collaborator rates that are better than those offered by VivaCell for any part of a Project, then Collaborator shall be entitled to contract such third-party to conduct the research for such Project. VivaCell shall use funds received from Collaborator solely to conduct the applicable Project and for no other purpose. |
| 8. | Confidential Information. |
| 8.1 | Confidential Information. The Recipient shall hold all Confidential Information of the Discloser in confidence and shall not disclose such Confidential Information to any third party. The Recipient shall disclose Confidential Information of the Discloser only to its employees, in each case who need to know such Confidential Information, to the extent such disclosure is reasonably necessary performance of its obligations or the exercise of its rights under this Agreement, and who are bound by restrictions regarding disclosure and use of such Confidential Information no less restrictive than those set forth herein. The Recipient shall not use, or grant the use of, any Confidential Information of the Discloser for the benefit of itself or any third party or for any purpose other than the performance of its obligations or the exercise of its rights under this Agreement. The Recipient shall not make any copies of the Confidential Information of the Discloser except to the extent reasonably necessary for such purpose, or unless otherwise approved in writing in advance by the Discloser. The Recipient shall notify the Discloser promptly upon discovery of any unauthorized use or disclosure of the Confidential Information of the Discloser. The obligations of this Section 8 with respect to any item of Confidential Information or with respect to any discussions or agreements between the parties shall survive for five (5) years following the termination or expiration of this Agreement. |
| 8.2 | Permitted Disclosures. The Recipient may use or disclose Confidential Information to the extent (a) approved in writing in advance by the Discloser, (b) to investment bankers, investors, potential investors and acquirers, each of whom prior to disclosure must be bound by obligations of confidentiality and non-use not less restrictive than those set out in this Agreement, or (c) required by applicable law, regulation, court or administrative order or rules of a stock exchange or automated quotation system to disclose such Confidential Information, provided that prior to any such required disclosure, the Recipient shall give the Discloser reasonable advance notice of any such disclosure and shall reasonably cooperate with the Discloser in objecting to or narrowing the scope of such disclosure, and/or obtaining a protective order or confidential treatment of such disclosure. |
- 11 -
| 8.3 | Ownership. All Confidential Information (including, without limitation, all copies, extracts and portions thereof) is and shall remain the sole property of the Discloser. The Recipient does not acquire (by license or otherwise, whether express or implied) any Intellectual Property Rights or other rights under this Agreement or any disclosure hereunder, except the limited right to use such Confidential Information in accordance with the express provisions of this Agreement. All rights relating to the Confidential Information that are not expressly granted hereunder to the Recipient are reserved and retained by the Discloser. |
| 8.4 | Publication. Collaborator acknowledges VivaCell’s interest in publishing certain results of a Project to obtain recognition within the scientific community and to advance the state of scientific knowledge. Each party also recognizes their mutual interest in protecting all Intellectual Property Rights and business interests. Consequently, if VivaCell desires to make a publication (including any oral disclosure made without obligation of confidentiality) of any results of a Project, VivaCell shall provide Collaborator with a copy of the proposed written publication at least forty-five (45) days prior to submission for publication, or an outline of such oral disclosure at least fifteen (15) days prior to presentation. Collaborator shall have the 1ight (a) to propose modifications to the publication for reasons concerning the protection of Intellectual Property Rights, and (b) to request a reasonable delay in publication in order to protect information that involve Intellectual Property Rights. If Collaborator requests such a delay, VivaCell shall delay submission or presentation of the publication for a period of ninety (90) days to enable Collaborator to prepare and file applicable registrations or applications to protect Intellectual Property Rights which may otherwise be lost or released into the public domain following VivaCell's proposed disclosure. Upon the expiration of such forty-five (45) day period (in the case of proposed written disclosures) or fifteen (15) day period (in the case of proposed written disclosures) from receipt by Collaborator, VivaCell shall be free to proceed with the written publication or the presentation, respectively, unless Collaborator has requested the delay described above. |
| 9. | Remedies. |
| 9.1 | Confidential Information. The Recipient acknowledges and agrees that, due to the unique nature of the Confidential Information of the Discloser, the unauthorized disclosure or use of such Confidential Information may cause irreparable harm and significant injury to the Discloser, the extent of which may be difficult to ascertain and for which there may be no adequate remedy at law. Accordingly, the Recipient agrees that the Discloser, in addition to any other available remedies, shall have the right to seek and obtain an immediate injunction and other equitable relief enjoining any breach or threatened breach of this Agreement, without the necessity of posting any bond or other security. The Recipient shall notify the Discloser in writing immediately upon the Recipient's becoming aware of any such breach or threatened breach. |
- 12 -
| 9.2 | Collaborator Technology. VivaCell acknowledges that, due to the unique nature of the Collaborator Technology, the unauthorized use, transfer or disclosure of the Collaborator Technology, Results or Project Technology will cause irreparable harm to Collaborator, the extent of which will be difficult to ascertain and for which there will be no adequate remedy at law. Accordingly, VivaCell agrees that Collaborator, in addition to any other available remedies, shall have the right to an immediate injunction and other equitable relief enjoining any breach or threatened breach of this Agreement, without the necessity of posting any bond or other security. VivaCell shall notify Collaborator in writing immediately upon VivaCell's becoming aware of any such breach or threatened breach |
| 9.3 | VivaCell Technology. Collaborator acknowledges that, due to the unique nature of the VivaCell Technology, the unauthorized use, transfer or disclosure of the VivaCell Technology, other than in accordance with this Agreement, will cause irreparable harm to VivaCell, the extent of which will be difficult to ascertain and for which there will be no adequate remedy at law. Accordingly, Collaborator agrees that VivaCell, in addition to any other available remedies, shall have the right to an immediate injunction and other equitable relief enjoining any breach or threatened breach of this Agreement, without the necessity of posting any bond or other security. Collaborator shall notify VivaCell in writing immediately upon Collaborator becoming aware of any such breach or threatened breach. |
| 10. | Indemnities. VivaCell hereby agrees to indemnify, hold harmless and defend Collaborator and the Collaborator’s directors, officers, employees, consultants and agents against any and all claims (including all legal fees and disbursements incurred in association therewith) arising out of VivaCell's performance of the Project or any receipt or use by VivaCell of any Collaborator Technology, Collaborator Innovations, Project Technology, VivaCell Innovations, Confidential Information, Results or any data or other results arising from the Projects including, without limitation, any damages or losses, consequential or otherwise, arising from or out of same. Collaborator hereby agrees to indemnify, hold harmless and defend VivaCell and VivaCell’s directors, officers, employees, consultants and agents against any and all claims (including all legal fees and disbursements incurred in association therewith) arising out of Collaborator's performance of the Project or any receipt or use by Collaborator of any VivaCell Technology, VivaCell Innovations, Project Technology, Collaborator Innovations, Confidential Information, Results or any data or other results arising from the Projects including, without limitation, any damages or losses, consequential or otherwise, arising from or out of same. |
- 13 -
| 11. | No Implied Rights. Except as expressly set forth in this Agreement, this Agreement shall not be construed to grant any license or other rights to either party in or to any patent or other intellectual property rights of the other party. |
| 12. | Term and Termination. |
| 12.1 | Term. This Agreement will commence on the Effective Date and, unless sooner terminated as provided in this Agreement, will remain in full force and effect for a term of five (5) years (the “Initial Term”). Thereafter, this Agreement will automatically renew for successive one (1) year terms (each, a “Renewal Term”), provided, however, that any party may terminate this Agreement on the expiration of the Initial Term or any Renewal Term by delivering written notice of termination to the other party not less than ninety (90) days before the expiration of such Initial Term or Renewal Term. |
| 12.2 | Termination. Any party shall have the right to terminate the Agreement in its entirety upon or after the material breach of this Agreement by Collaborator or VivaCell if the breaching party has not cured such breach within thirty (30) days after notice thereof by the other party. |
| 12.3 | Effects of Termination. |
| (a) | Promptly upon the completion or termination of any individual Project, unless the parties otherwise mutually agree in writing, the Recipient shall destroy or return to the Discloser (as requested by the Discloser) all tangible items regarding the Confidential Information of the Discloser relating to such Project and all copies thereof, except that (i) the Recipient shall have the right to retain one (1) copy for its legal files for the sole purpose of determining its obligations hereunder, and (ii) VivaCell shall return to Collaborator all remaining Collaborator Technology relating to such Project. |
| (b) | Promptly upon the expiration or termination of this Agreement in its entirety, unless the parties otherwise mutually agree in writing, (i) the Recipient shall destroy or return to the Discloser (as requested by the Discloser) all tangible items regarding the Confidential Information of the Discloser and all copies thereof; provided, however, that the Recipient shall have the right to retain one (1) copy for its legal files for the sole purpose of determining its obligations hereunder, and (ii) VivaCell shall return to Collaborator all remaining Collaborator Technology. |
| (c) | Sections 2.10, 2.11(b), 2.13, 3.2, 3.3, 4, 5, 6.5, 8, 9, 10, 12.3, 13, 14, 15, 16, 17, 18, and 19 shall survive expiration or termination of this Agreement or any Project Plan. |
| 13. | Independent Contractors. The relationship of the parties hereto is that of independent contractors. The parties hereto are not deemed to be agents, partners or joint venturers of the others for any purpose as a result of this Agreement or the transactions contemplated thereby. |
- 14 -
| 14. | LIMITATION OF LIABILITY. IN NO EVENT SHALL THE LIABILITY OF COLLABORATOR FOR A BREACH OR DEFAULT UNDER THIS AGREEMENT RELATING TO A PROJECT EXCEED THE AMOUNT OF THE FEES PAID OR PAYABLE BY COLLABORATOR TO VIVACELL FOR SUCH PROJECT. |
| 15. | Governing Law. This Agreement shall be governed by, interpreted and construed in accordance with the laws of the Province of British Columbia, without regard to the conflicts of law principles thereof. Any legal action or other proceeding to resolve any dispute arising from or relating to this Agreement shall be brought only in the courts of the Province of British Columbia, located in Vancouver, British Columbia, or the Federal Court of Canada, located in Vancouver, British Columbia. All parties agree that by executing this Agreement they have attorned to the jurisdiction of the courts of the Province of British Columbia and that such courts have the exclusive jurisdiction over this Agreement. |
| 16. | Notices. All reports, notices and other documents that a party is required or may want to deliver to any other party shall be delivered in writing and either by personal delivery or by registered or certified mail at the address for the receiving party set out on page 1 of this Agreement, or as varied by any notice. |
| 17. | No Waiver. No condoning, excusing or overlooking by any party of any default, breach or non-observance by any other party at any time or times regarding any terms of this Agreement (including any Project Plans) shall operate as a waiver of that party's rights under this Agreement. A waiver of any term or right under this Agreement shall be made in writing signed by the party entitled to the benefit of that term or right, and is effective only to the extent set out in the written waiver. |
| 18. | Severability. Any part of this Agreement that is indefinite, invalid, illegal or otherwise void or unenforceable may be severed from this Agreement and the balance of this Agreement will continue in full force and effect. |
| 19. | Miscellaneous. This Agreement embodies the entire agreement between the parties and supersedes any prior representations, communications, understandings and agreements between the parties regarding the subject matter hereof. There are no representations, communications, understandings or agreements, oral or written, between the parties regarding the subject matter hereof that are not fully expressed herein. This Agreement and the rights and obligations hereunder may not be assigned or delegated by VivaCell thereto, in whole or part, whether voluntarily, by operation of law, change of control or otherwise, without the prior written consent of Collaborator. Collaborator may assign this Agreement, provided that Collaborator must give VivaCell 15 days' prior written notice of such assignment and the assignee must agree in writing with VivaCell to be bound by this Agreement. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and permitted assigns. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. |
(Remainder of page left intentionally blank. Signature page follows.)
- 15 -
VIVACELL BIOTECHNOLGY ESPANA, S.L. |
||
By: |
/s/ Xxxxxx Xxxxx | |
| Authorized Signatory | ||
By: |
/s/ Xxxxx Xxxxxxx | |
| Authorized Signatory | ||
- 16 -
EXHIBIT A
Series 3
VCE-003 is a cannabigerol-hydroxy-quinone derivative according to the following formula:

Series 3: Compounds are derivatives of cannabigerol-hydroxy-quinone derivatives (CBG-Q derivatives) of Formula (I):

(I)
wherein R is the carbon atom of a linear or branched group, represented by: aryl, alkenyl, alkynyl or alcoxycarbonil groups; or wherein R is the nitrogen atom of a linear or branched group, represented by: alkylamino, arylamino, alkenylamino or alkynylamino groups; or, alternatively, R represents a bond between 2 molecules of formula (I) forming a dimer. In a preferred embodiment, the compounds of the invention are those of Formula (II), (III), (IV), (V), (VI), (VII), (VIII), (X), (XI) and (XII).
- 17 -

(II)
6-(3,7-dimethyl-octa-2, 6-dienyl)-5-hydroxy-3-pentyl-2-metoxycarbonil -[1,4]benzoquinone.

(III)
6-(3,7-dimethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl-2-ethylamino-[1,4]benzoquinone.

(IV)
6-(3,7-dimethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl-2-pentylamino-[1,4]benzoquinone.

(V)
6-(3,7-dimethyl-octa-2, 6-dienyl)-5-hydroxy- 3-pentyl-2-isobutylamino [1,4]benzoquinone.
- 18 -

(VI)
6-(3,7-dimethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl-2-butylamino [1,4]benzoquinone.
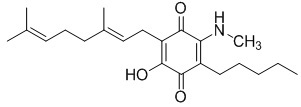
(VII)
6-(3,7-dimethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl-2-methylamino- [1,4]benzoquinone.

(VIII)
6-(3,7-imethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl-2-isopropylamino- [1,4]benzoquinone.

(IX)
6-(3,7-dimethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl- 2-benzylamino [1,4]benzoquinone
- 19 -

(X)
6-(3,7-dimethyl-octa-2,6-dienyl)- 5-hydroxy-3-pentyl-2-(2,2-dimethyl-propylamino)- [1,4]benzoquinone.
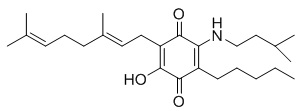
(XI)
6-(3,7-dimethyl-octa-2,6-dienyl)-5-hydroxy-3-pentyl-2-(3-methyl-butylamino)- [1,4]benzoquinone.

(XII)
3,3'-bis((E)-3,7-dimethyl-octa-2,6-dienyl)-4,4'-dihydroxy-6,6'-dipentyl-1,1'-bi(cyclohexa-3,6-diene)-2,2',5,5'-tetraone
- 20 -
EXHIBIT B
Series 4
VCE-004: All new uses of cannabinoid derivatives of the Series 4 that would be technical subject matter that is not excluded from patentability based on the chemical backbone described below, and including the embodiments described below.
Novel cannabidiol-quinone derivatives (CBD-Q derivatives) with exhibits activity in modulating PPARg without inducing Nrf2 activation and cytotoxicity have been discovered. More specifically, the novel compounds are derivatives of cannabidiol-quinone derivatives (CBD-Q derivatives) shown in Figure 1, wherein R is the carbon atom of a linear or branched group, represented by: aryl, alkenyl, alkynyl, acyl, or alkoxycarbonyl groups; or wherein R is the nitrogen atom of a linear or branched group, represented by: alkylamine, arylamine, alkenylamine or alkynylamine groups. In addition, the term “alkoxycarbonyl” may be equated with an ester moiety [i.e. -C(=O)-O-Alkyl].

Figure 1. VCE-004 Backbone. The quinone ring has been numbered arbitrarily in order to show in which position of the ring the substituents replacement is made for rendering the CBD-Q derivatives
- 21 -
In a first embodiment, the compounds of the invention are the VCE-004 derivatives shown in Fig. 2
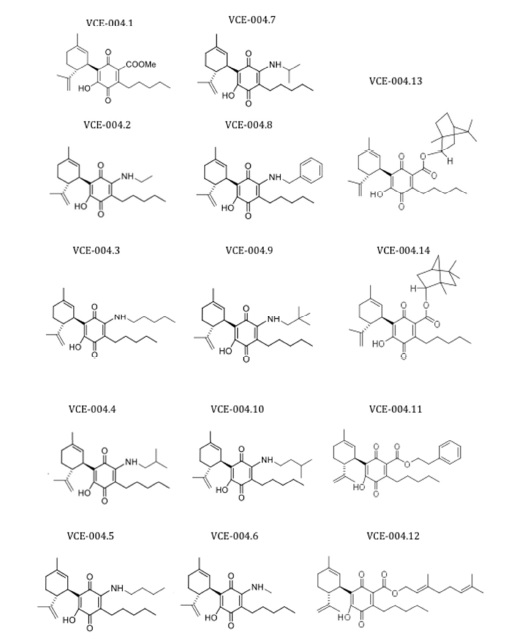
Figure 2. VCE-004 derivatives
- 22 -
Confidential Treatment Requested. Omitted Portions are Marked with [*****] and have been Filed Separately with the Securities and Exchange Commission.
EXHIBIT C
The overall objectives of the Initial Project Plan are:
1. IND(CTA)-enabling studies of VCE004.8 in Multiple Sclerosis [*****].
2. R&D and regulatory affairs to prepare VCE-004.8 [*****].
3. R&D of Series 3 for Huntington and Xxxxxxxxx’ Diseases
4. R&D of Series 4 for new applications
5. R&D of Series 3 for new application
Vivacell has been working during the last year to reach objectives 1 to 3. Accordingly the following on force contracts have been executed by VivaCell. They have pending payments after the closing date. So all costs related to the following contracts that are invoiced after the closing date will be part of the Initial Project Plan:
| - | Annex 1 |
Drug Development Plan for IND(CTA)-enabling studies of VCE004.8 in MS.
| - | Annex 2 |
4-2017-EHS101/SSc- UCO for the Efficacy of VCE004.8 (EHS101) in mice models of Scleroderma.
| - | Annex 3 |
Cote Orphan Statement of Work #1. Orphan Drug Designation of VCE004.8 in Scleroderma (FDA and EMEA). Pre-IND meetings at FDA for Multiple Sclerosis and Scleroderma.
| - | Annex 4 |
3-2017-EHS101/EAE- CAJAL for the Efficacy of VCE004.8 (EHS101) in mice models of Multiple Sclerosis.
| - | Annex 5 |
GALCHIMIA OF17-0011 for the upscale of VCE003.2
| - | Annex 6 |
CONTRATO-OPI-UCO-BIOMAP-2016 for the efficacy of VCE003.2 derivatives in mice models of inflammatory diseases and cancer.
| - | Annex 7 |
BIOMAP project for VCE003.2 derivatives. Other costs not described in previous annexes and which has not been already executed.
| - | Annex 8 |
2-2017-EHS102/PD- CIBERNED for the efficacy of VCE003.2 in Xxxxxxxxx’x Disease mice models
To reach the objectives 4 and 5, new projects will be executed immediately after the closing date. They are described in Annex 9.
As also described in Annex 9, the Initial Project Plan requires the direct work of an experienced Project Manager. Additionally, the company is setting up a Quality System for GMP production for VCE-004.8 and VCE-003.2 at short term, including a Quality Manager. Finally, the Project requires the work of a Production Manager. The laboratory work performed at VivaCell requires the work of Scientific Personnel, and Consumables.
Additionally, the cost of the patents that protect Series 3 and Series 4 are already included in the Initial Project Plan until the effective transfer of IP is done administrative. The references of those current patents are: PCT/ES: 2010/070156, PCT/2014/057767, EP14156954.1 / PCT/EP2015/053032, PCT/EP2017/057389.
- 23 -
Confidential Treatment Requested. Omitted Portions are Marked with [*****] and have been Filed Separately with the Securities and Exchange Commission.
EXHIBIT D
The estimated cost of the Initial Project Plan is:
| - | [*****] € in 2017 | |
| - | [*****] € in 2018 | |
| - | [*****] € as of 2019 |
EXHIBIT E
PROJECT PLAN AMENDMENT
EMERALD HEALTH PHARMACEUTICALS INC., a Delaware corporation (“Collaborator”), with a place of business at 0000 Xxxxx Xxxxx Xxxx, Xxx Xxxxx, Xxxxxxxxxx, Xxxxxx Xxxxxx and VIVACELL BIOTECHNOLOGY ESPANA, S.L. a limited liability company (Sociedad Limitada) incorporated under the laws of Spain (“VivaCell”), having a place of business at Parque Científico, Tecnologico de Cordoba, Xxxxxxxxx 21, c/ Astronoma Xxxxxxx Xxxxx, Xxxxxxx Xxxxxxxx, 00000, Xxxxxxx, Xxxxx,
WITH REFERENCE to [PROJECT PLAN REFERENCE], agree as follows
[SPECIFIC CONTENTS OF THE AMENDMENTS]
The contents of this Project Plan Amendment modify, substitute and supersede to the relevant extent the contents of the [AMENDED PROJECT PLAN].
In the sense above, this Project Plan Amendment shall be deemed a part of the [AMENDED PROJECT PLAN] to all relevant purposes.
