EXECUTION COPY -2- or to develop, manufacture, commercialize, use, import, offer to sell, or sell Licensed Products and expressly excluding the Conformetrix Technology. Section 1.5 “C4X Patents” means all patents controlled by C4X or its Affiliate(s)...

EXECUTION COPY -1- ASSET PURCHASE AGREEMENT THIS ASSET PURCHASE AGREEMENT (this "Agreement"), dated as of July 31, 2023 (the “Effective Date”), is made by and between Indivior UK Limited (Co.), a company incorporated in England and Wales under the company number 7183451, whose registered office is at The Chapleo Building Xxxxx Boot Way, Priory Park, Hull, HU4 7DY (the "Buyer"), and C4X Discovery Limited, a company incorporated in England and Wales under the company number 06324250, whose registered office is at Manchester Xxx, 00 Xxxxxxxx Xxxxxx, Xxxxxxxxxx, X0 0XX (the "Seller"), each a "Party" and together the "Parties". WHEREAS, the Seller owns the rights to the Licensed Technology with respect to the development, manufacture, marketing, sale and distribution of Licensed Compounds and Licensed Products; WHEREAS, the Seller and Buyer entered into a License Agreement dated 28 March 2018 (“License Agreement”) whereby Seller granted an exclusive, sublicensable, perpetual, transferable license under the Licensed Technology to develop, use, manufacture, have manufactured, import, export, obtain Regulatory Approval and commercialize the Licensed Compound(s) and/or Licensed Product(s); WHEREAS, the Seller now desires to sell to the Buyer, and the Buyer now desires to purchase from the Seller, the Purchased Assets (as defined herein); and WHEREAS, the Parties wish to as of Closing terminate the Licence Agreement, all upon the terms and subject to the conditions set forth in this Agreement NOW, THEREFORE, in consideration of the mutual covenants contained herein and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Buyer and the Seller agree as follows: ARTICLE I DEFINITIONS Section 1.1 Definitions. As used in this Agreement, the following terms shall have the meanings ascribed to them below. Section 1.2 "Affiliate" means, with respect to a Party, any person that, directly or indirectly, controls, is controlled by or is under common control with such Party; for the purposes of this definition, “control” shall refer to: (i) the possession, directly or indirectly, of the power to direct the management or policies of an entity whether through ownership of interests representing equity, securities, or partnership interests, by contract, or otherwise, or (ii) the ownership, directly or indirectly, of more than fifty per cent (50%) of the voting securities or capital stock or other ownership interest of an entity. Section 1.3 "Applicable Law(s)" means all federal, state, national and local laws, statutes, ordinances, rules, regulations, codes, guidelines as amended, re-enacted or in force from time to time applicable to the particular activities and jurisdictions hereunder, including, as applicable, bribery legislation, GCP, GDP, GMP, GLP and the rules and regulations of relevant governmental authority having jurisdiction over the development, manufacture and/or commercialization of the Licensed Products. Section 1.4 “C4X Know-How” means all know-how (including data) controlled by the Seller as of the Effective Date that is reasonably necessary or useful to develop, make, or use Licensed Compounds DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1 Exhibit 4.22 PORTIONS OF THIS EXHIBIT HAVE BEEN REDACTED. CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (i) NOT MATERIAL AND (ii) WOULD BE LIKELY TO CAUSE COMPETITIVE HARM IF PUBLICLY DISCLOSED. REDACTED MATERIAL IS MARKED WITH [***].

EXECUTION COPY -2- or to develop, manufacture, commercialize, use, import, offer to sell, or sell Licensed Products and expressly excluding the Conformetrix Technology. Section 1.5 “C4X Patents” means all patents controlled by C4X or its Affiliate(s) at the Effective Date that claim the composition, development, manufacture, commercialization, or use of Licensed Compounds and/or Licensed Products being those specifically listed in Exhibit A. Section 1.6 "C4X Patent Records” means the records of the C4X Patents in the possession of or controlled by the Seller and the Seller’s patent attorneys, or to which the Seller has a legal right to. Section 1.7 "Claim” means any right of action or claim (whether in contract, tort, misrepresentation or otherwise) which the Buyer may have or make in respect of any provision of this Agreement (including for breach of any of the warranties). Section 1.8 "Conformetrix Data” means three-dimensional structures of dynamic molecules in solution, including calculations or rationale used to derive the final ligand structures and conformational features and calculations based upon nuclear magnetic resonance (NMR) and other data used to generate the dynamic behaviour of molecules or ligands in 3D-space generated using Seller's proprietary Conformetrix Technology. Section 1.9 “Conformetrix Technology” means Seller’s proprietary analytical software, technology, and related scientific and other know-how and information, trade secrets, knowledge, technology, means, methods, processes, practices, formulae, instructions, skills, techniques, procedures, experiences, ideas, technical assistance, designs, drawings, assemble procedures, computer programs, apparatuses, specifications, data, results, laboratory notes and notebooks as such exists at the Effective Date and during the term used for the determination of three-dimensional structures of dynamic molecules in solution including calculations or rationale used to derive the final ligand structures and conformational features and calculations based upon nuclear magnetic resonance (NMR) and other data used to generate the dynamic behaviour of molecules or ligands in 3D-space and general learnings on molecular shape including but not limited to conformetrix and 4Sight (for clarity, this includes generalised shape learnings from the Licensed Compounds). Section 1.10 “Encumbrance" means any mortgage, charge, deed of trust, lien, security interest, easement, right of way, pledge, assessment, encumbrance or restriction of any nature whatsoever. Section 1.11 “Governmental Entity" means any court, administrative agency or commission or other governmental authority or instrumentality of applicable jurisdiction, whether domestic or foreign. Section 1.12 "Liabilities” means any and all debts, liabilities and obligations, whether accrued or fixed, known or unknown, absolute or contingent, matured or unmatured, due or to become due, or determined or determinable, including any liability for Taxes and those arising under any Applicable Law, contract or otherwise. Section 1.13 "Licensed Compound" means (i) C4X_3256; (ii) any other compound covered by the claims of the C4X Patents; (iii) as between the Parties the exclusive right to develop and commercialise any compound variant of (i) and/or (ii) that has the same mechanism of action as the molecules described in (i) and/or (ii); (iv) as between the Parties the exclusive right to develop and commercialise any molecules that are specifically derived from any of the molecules described in (i), (ii) and/or (iii) using the C4X Know-How and identified to have the same mechanism of action as the molecules described in (i), (ii) and/or (iii) above; and (v) as between the Parties, the exclusive right to develop and DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

EXECUTION COPY -3- commercialise any molecules created using the C4X Know-How or in connection with the development of any Licensed Products and identified to have the same mechanism of action as the molecules described in (i), (ii) and/or (iii) above. Section 1.14 "Licensed Product" means a pharmaceutical product in finished form (in all formulations, dosages and delivery systems) that incorporates a Licensed Compound. Section 1.15 "Licensed Technology" means the C4X Know-How and the C4X Patents. Section 1.16 “Material Adverse Effect" means a change, circumstance or effect that has had a materially adverse effect on the Licensed Products or any of the Purchased Assets but excluding any change, circumstance or effect not known to the Buyer and caused by or relating to: (a) changes in conditions generally affecting (i) the healthcare or biotechnology industry or (ii) the United States or world economy or securities markets, (b) changes in any Applicable Law, (c) the announcement of this transaction, or (d) the taking of any action required or contemplated by this Agreement. Section 1.17 "Records" means, to the extent such have not already been provided under the Licence Agreement, all files, documents, and records of the Seller related to the development by the Seller of the Licensed Compounds, Licensed Products, or the Purchased Assets together with the C4X Patent Records. Section 1.18 “Regulatory Applications" means any and all applications for approval to develop, test (including conducting clinical trials), manufacture, process, distribute, import, market, store, label, package, promote, sell, or offer to sell the Licensed Products in any jurisdiction, and all supplements, amendments and revisions thereto, whether approved or not owned or controlled by the Seller or any Affiliate of the Seller. Section 1.19 “Regulatory Approvals" means all licenses, permits, waivers, consents, certificates, registrations, approvals (including, without limitation, approvals of NDAs and ANDAs, pricing and third party reimbursement approvals, and labeling approvals) of, with or from any Governmental Entity pertaining to the Licensed Compounds or Licensed Products owned or controlled by the Seller or any Affiliate of the Seller. Section 1.20 “Tax" means all domestic and foreign taxes and assessments, including all interest, penalties and additions with respect thereto. Section 1.21 “Tax Return" means any report, return, election, notice, estimate, declaration, information statement and other forms and documents (including all schedules, exhibits and other attachments thereto) relating to and filed or required to be filed with a taxing authority in connection with any Taxes (including estimated Taxes). Section 1.22 TUPE: the Transfer of Undertakings (Protection of Employment) Regulations 2006, and any predecessor regulations including the Transfer of Undertakings (Protection of Employment) Regulations 1981. DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
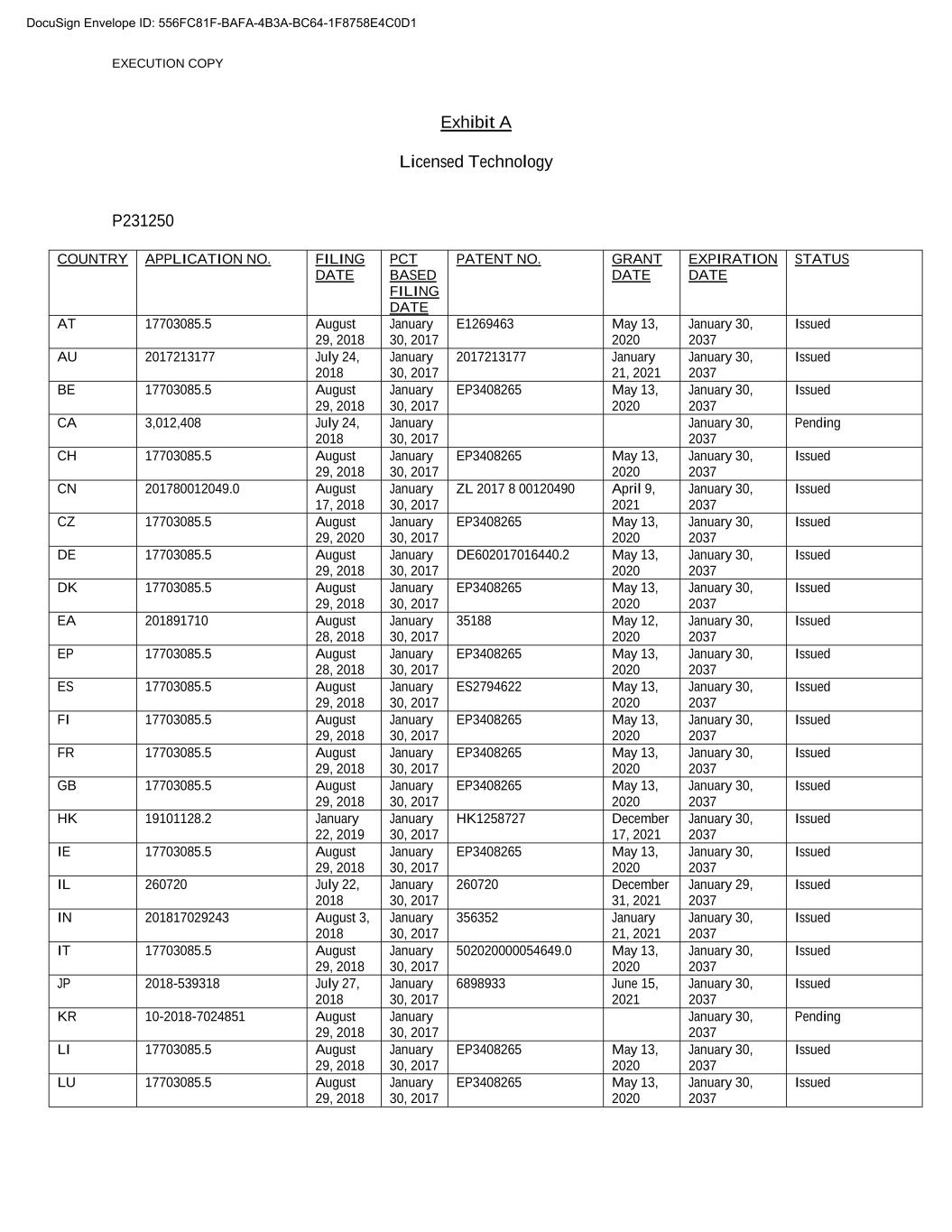
EXECUTION COPY Exhibit A Licensed Technology P231250 COUNTRY APPLICATION NO. FILING DATE PCT BASED FILING DATE PATENT NO. XXXXX DATE EXPIRATION DATE STATUS AT 17703085.5 August 29, 2018 January 30, 2017 E1269463 May 13, 2020 January 30, 2037 Issued AU 2017213177 July 24, 2018 January 30, 2017 2017213177 January 21, 2021 January 30, 2037 Issued BE 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued CA 3,012,408 July 24, 2018 January 30, 2017 January 30, 2037 Pending CH 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued CN 201780012049.0 August 17, 2018 January 30, 2017 ZL 2017 8 00120490 April 9, 2021 January 30, 2037 Issued CZ 17703085.5 August 29, 2020 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued DE 17703085.5 August 29, 2018 January 30, 2017 DE602017016440.2 May 13, 2020 January 30, 2037 Issued DK 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued EA 201891710 August 28, 2018 January 30, 2017 35188 May 12, 2020 January 30, 2037 Issued EP 17703085.5 August 28, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued ES 17703085.5 August 29, 2018 January 30, 2017 ES2794622 May 13, 2020 January 30, 2037 Issued FI 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued FR 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued GB 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued HK 19101128.2 January 22, 2019 January 30, 2017 HK1258727 December 17, 2021 January 30, 2037 Issued IE 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued IL 260720 July 22, 2018 January 30, 2017 260720 December 31, 2021 January 29, 2037 Issued IN 201817029243 August 3, 2018 January 30, 2017 356352 January 21, 2021 January 30, 2037 Issued IT 17703085.5 August 29, 2018 January 30, 2017 502020000054649.0 May 13, 2020 January 30, 2037 Issued JP 2018-539318 July 27, 2018 January 30, 2017 6898933 June 15, 2021 January 30, 2037 Issued KR 10-2018-7024851 August 29, 2018 January 30, 2017 January 30, 2037 Pending LI 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued LU 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
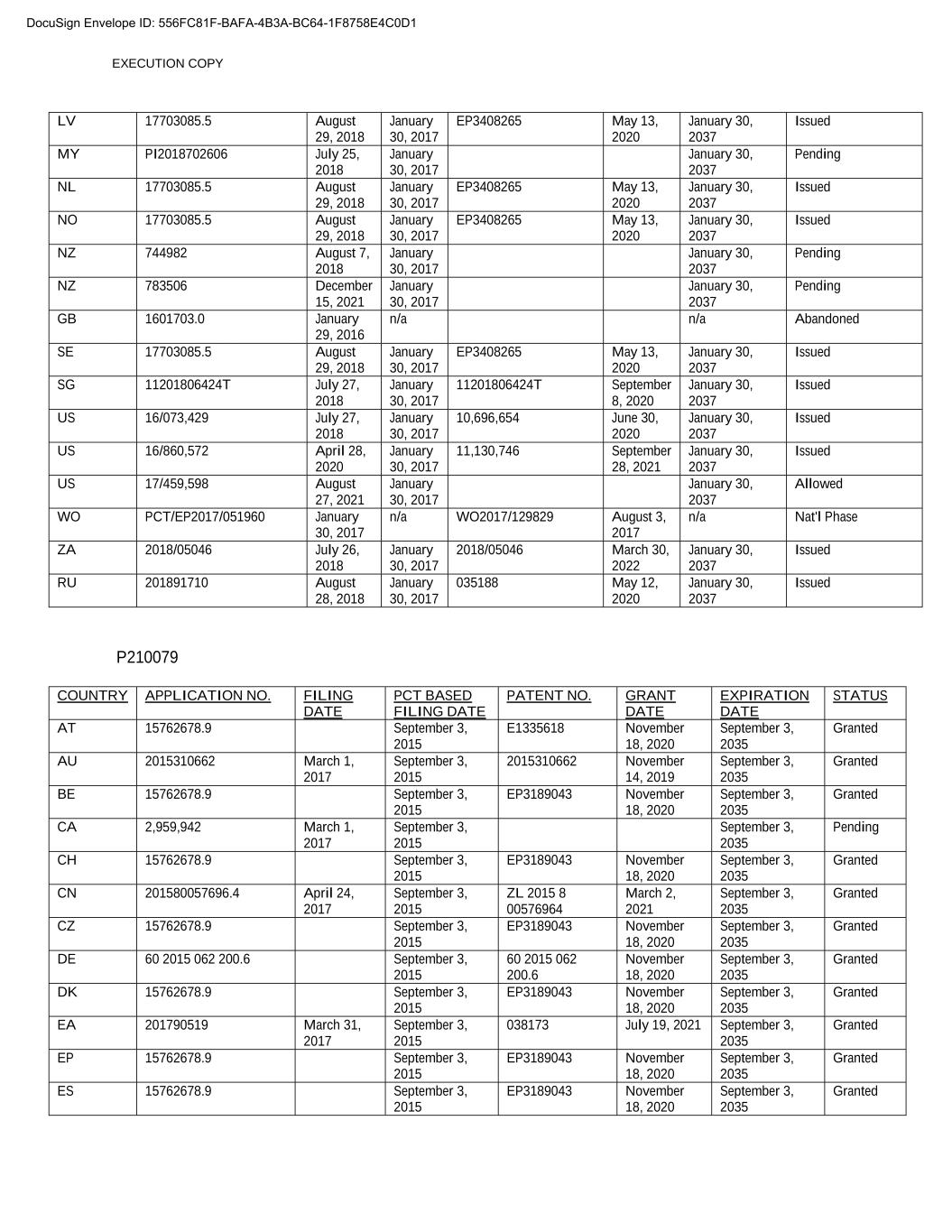
EXECUTION COPY LV 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued MY PI2018702606 July 25, 2018 January 30, 2017 January 30, 2037 Pending NL 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued NO 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued NZ 744982 August 7, 2018 January 30, 2017 January 30, 2037 Pending NZ 783506 December 15, 2021 January 30, 2017 January 30, 2037 Pending GB 1601703.0 January 29, 2016 n/a n/a Abandoned SE 17703085.5 August 29, 2018 January 30, 2017 EP3408265 May 13, 2020 January 30, 2037 Issued SG 11201806424T July 27, 2018 January 30, 2017 11201806424T September 8, 2020 January 30, 2037 Issued US 16/073,429 July 27, 2018 January 30, 2017 10,696,654 June 30, 2020 January 30, 2037 Issued US 16/860,572 April 28, 2020 January 30, 2017 11,130,746 September 28, 2021 January 30, 2037 Issued US 17/459,598 August 27, 2021 January 30, 2017 January 30, 2037 Allowed WO PCT/EP2017/051960 January 30, 2017 n/a WO2017/129829 August 3, 2017 n/a Nat'l Phase ZA 2018/05046 July 26, 2018 January 30, 2017 2018/05046 March 30, 2022 January 30, 2037 Issued RU 201891710 August 28, 2018 January 30, 2017 035188 May 12, 2020 January 30, 2037 Issued P210079 COUNTRY APPLICATION NO. FILING DATE PCT BASED FILING DATE PATENT NO. XXXXX DATE EXPIRATION DATE STATUS AT 15762678.9 September 3, 2015 E1335618 November 18, 2020 September 3, 2035 Granted AU 2015310662 March 1, 2017 September 3, 2015 2015310662 November 14, 2019 September 3, 2035 Granted BE 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted CA 2,959,942 March 1, 2017 September 3, 2015 September 3, 2035 Pending CH 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted CN 201580057696.4 April 24, 2017 September 3, 2015 ZL 2015 8 00576964 March 2, 2021 September 3, 2035 Granted CZ 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted DE 60 2015 062 200.6 September 3, 2015 60 2015 062 200.6 November 18, 2020 September 3, 2035 Granted DK 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted EA 201790519 March 31, 2017 September 3, 2015 038173 July 19, 2021 September 3, 2035 Granted EP 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted ES 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
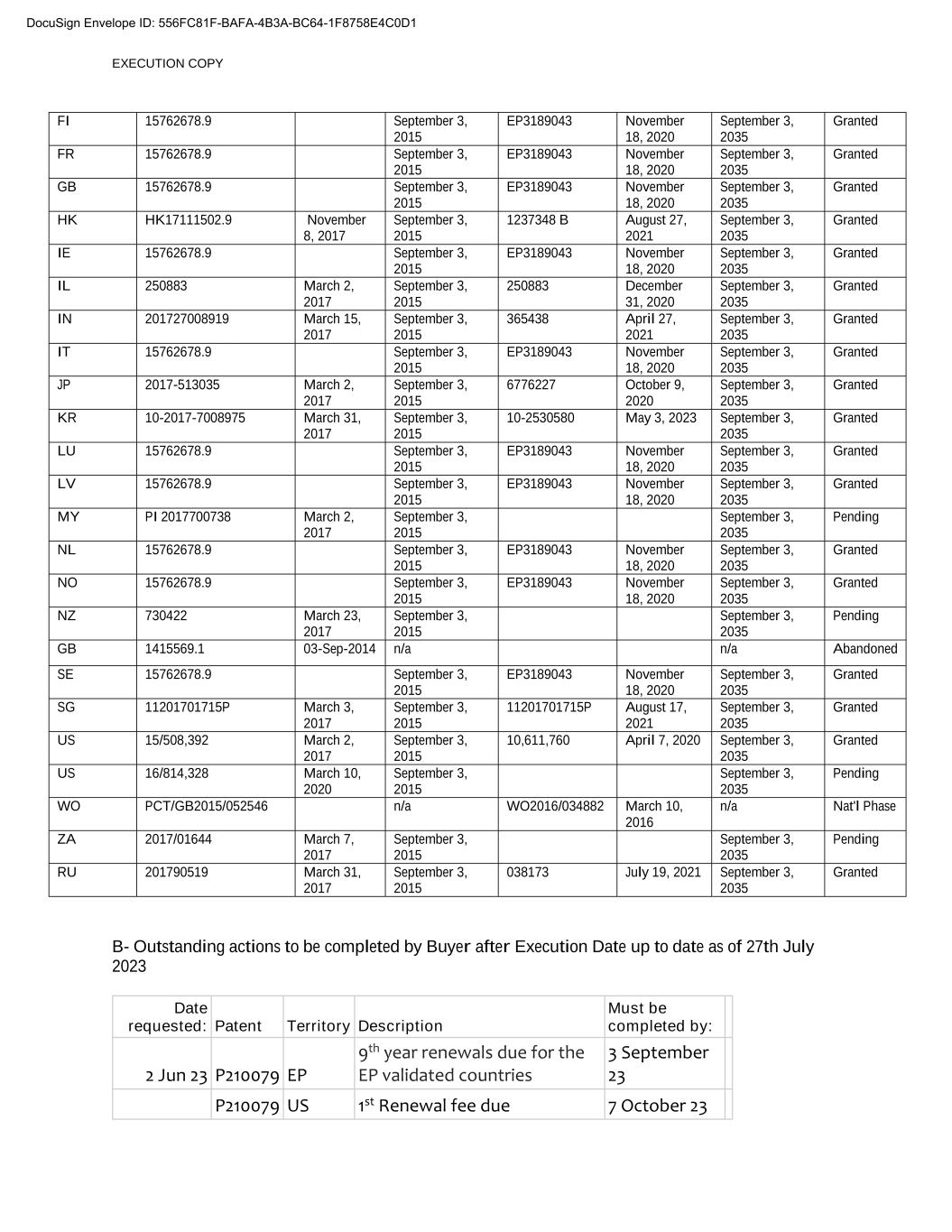
EXECUTION COPY FI 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted FR 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted GB 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted HK HK17111502.9 November 8, 2017 September 3, 2015 1237348 B August 27, 2021 September 3, 2035 Granted IE 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted IL 250883 March 2, 2017 September 3, 2015 250883 December 31, 2020 September 3, 2035 Granted IN 201727008919 March 15, 2017 September 3, 2015 365438 April 27, 2021 September 3, 2035 Granted IT 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted JP 2017-513035 March 2, 2017 September 3, 2015 6776227 October 9, 2020 September 3, 2035 Granted KR 10-2017-7008975 March 31, 2017 September 3, 2015 00-0000000 May 3, 2023 September 3, 2035 Granted LU 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted LV 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted MY PI 2017700738 March 2, 2017 September 3, 2015 September 3, 2035 Pending NL 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted NO 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted NZ 730422 March 23, 2017 September 3, 2015 September 3, 2035 Pending GB 1415569.1 03-Sep-2014 n/a n/a Abandoned SE 15762678.9 September 3, 2015 EP3189043 November 18, 2020 September 3, 2035 Granted SG 11201701715P March 3, 2017 September 3, 2015 11201701715P August 17, 2021 September 3, 2035 Granted US 15/508,392 March 2, 2017 September 3, 2015 10,611,760 April 7, 2020 September 3, 2035 Granted US 16/814,328 March 10, 2020 September 3, 2015 September 3, 2035 Pending WO PCT/GB2015/052546 n/a WO2016/034882 March 10, 2016 n/a Nat'l Phase ZA 2017/01644 March 7, 2017 September 3, 2015 September 3, 2035 Pending RU 201790519 March 31, 2017 September 3, 2015 038173 July 19, 2021 September 3, 2035 Granted B- Outstanding actions to be completed by Buyer after Execution Date up to date as of 27th July 2023 Date requested: Patent Territory Description Must be completed by: 2 Jun 23 P210079 EP 9th year renewals due for the EP validated countries 3 September 23 P210079 US 1st Renewal fee due 7 October 23 DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
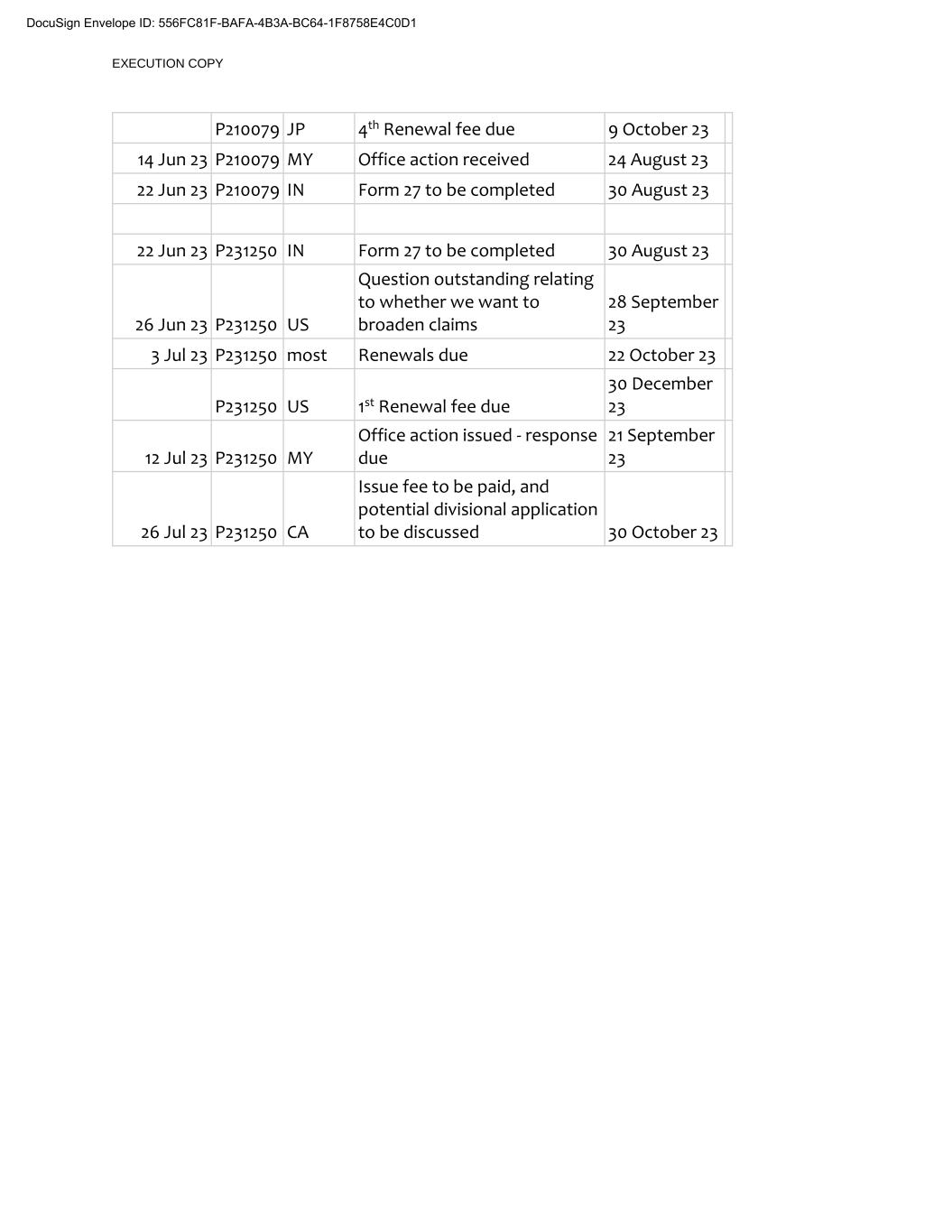
EXECUTION COPY P210079 JP 4th Renewal fee due 9 October 23 14 Jun 23 P210079 MY Office action received 24 August 23 22 Jun 23 P210079 IN Form 27 to be completed 30 August 23 22 Jun 23 P231250 IN Form 27 to be completed 30 August 23 26 Jun 23 P231250 US Question outstanding relating to whether we want to broaden claims 28 September 23 3 Jul 23 P231250 most Renewals due 22 October 23 P231250 US 1st Renewal fee due 30 December 23 12 Jul 23 P231250 MY Office action issued - response due 21 September 23 26 Jul 23 P231250 CA Issue fee to be paid, and potential divisional application to be discussed 30 October 23 DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

NY: 1242891-3 Exhibit B Assignment of Patent Rights THIS ASSIGNMENT OF PATENT RIGHTS (the “Assignment”) dated as of 04 August, 2023 (the “Effective Date”), is made in accordance with, and pursuant to the terms and conditions of the Asset Purchase Agreement having an effective date of 31 July, 2023 (the “Agreement”) by and between Indivior UK Limited (Co.), a company incorporated in England and Wales under the company number 7183451, whose registered office is 000-000 Xxxx Xxxx, Xxxxxx, Xxxxxxxxx, XX0 0XX (the "Assignee") as Buyer, and C4X Discovery Limited, a company incorporated in England and Wales under the company number 06324250, whose registered office is at Manchester Xxx, 00 Xxxxxxxx Xxxxxx, Xxxxxxxxxx, X0 0XX (the "Assignor") as Seller. “Patents” means the patents identified in the Patent Annex attached hereto. NOW, THEREFORE, TO ALL WHOM IT MAY CONCERN: For good and valuable consideration, the receipt of which is hereby acknowledged, Assignor agrees to and does hereby irrevocably sell, assign, transfer and convey unto said Assignee, and Assignee hereby accepts, all of Assignor’s right, title, and interest (i) in and to the Patents, the same to be held and enjoyed by said Assignee for its own use, and for the use of its successors, assigns, or other legal representatives to the end of the term or terms for which said Patents may be granted as fully and entirely as the same would have been held and enjoyed by Assignor if this Assignment had not been made; and (ii) in and to causes of action and enforcement rights for the Patents including all rights to pursue damages, injunctive relief and other remedies for past and future infringement of the Patents. Notwithstanding anything to the contrary herein, Assignor is executing and delivering this Assignment in accordance with and subject to all of the terms and provisions of the Agreement. In the event of any conflict between the terms of this Assignment and those of the Agreement, the terms of the Agreement shall be controlling. This Assignment shall be binding upon and shall inure to the benefit of the parties and their respective successors and assigns. This Assignment shall be governed by, and construed in accordance with, the laws of England and Wales, without giving effect to the conflict of laws rules thereof. IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be signed by their respective representatives thereunto duly authorized, all as of the date hereof. ASSIGNEE: ___________________________ By: Indivior UK Ltd Name: Xxxxxxx Xxxxxxxx Title: Finance Director, EUCAN ASSIGNOR: ________________________ DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

2 By: ____________________________________ Name: Title: DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
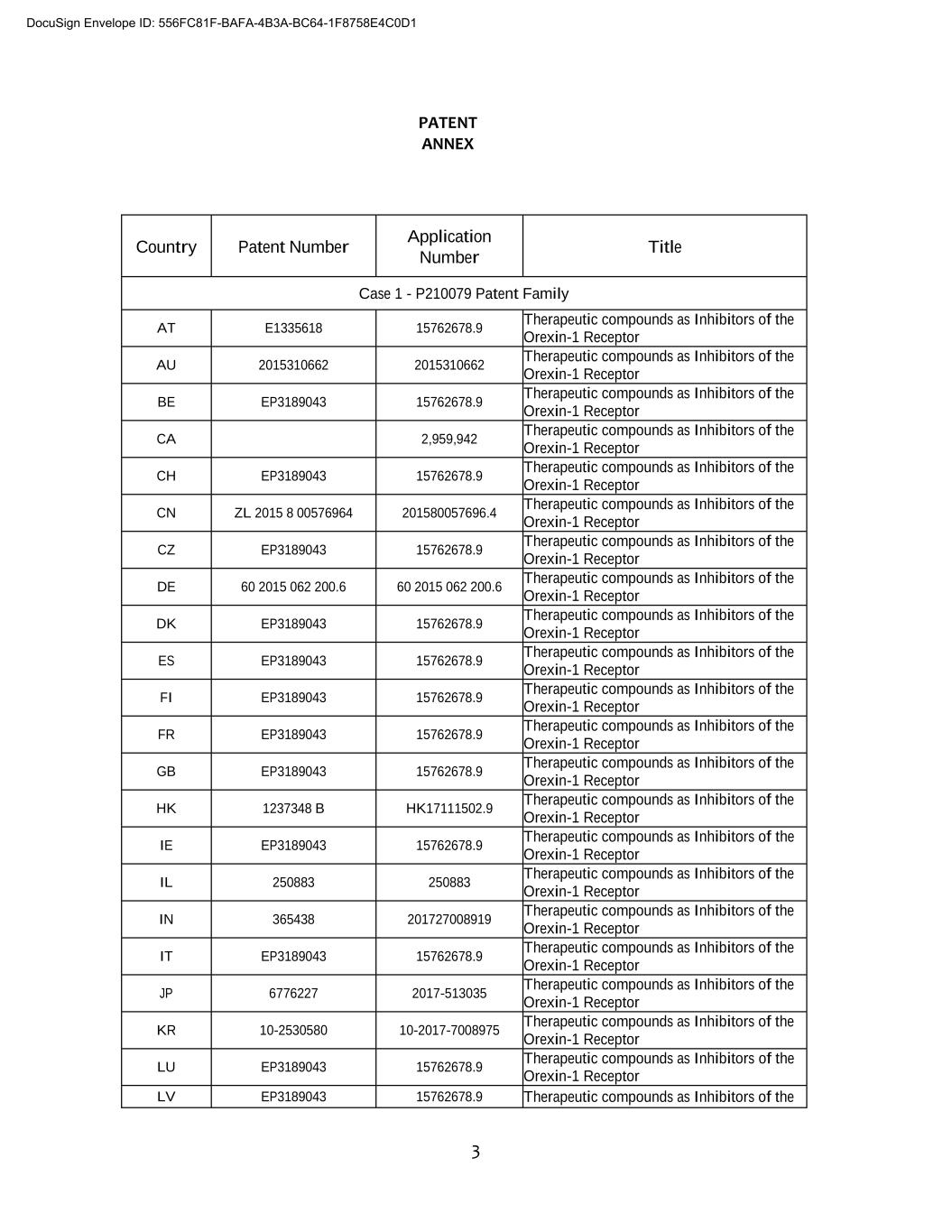
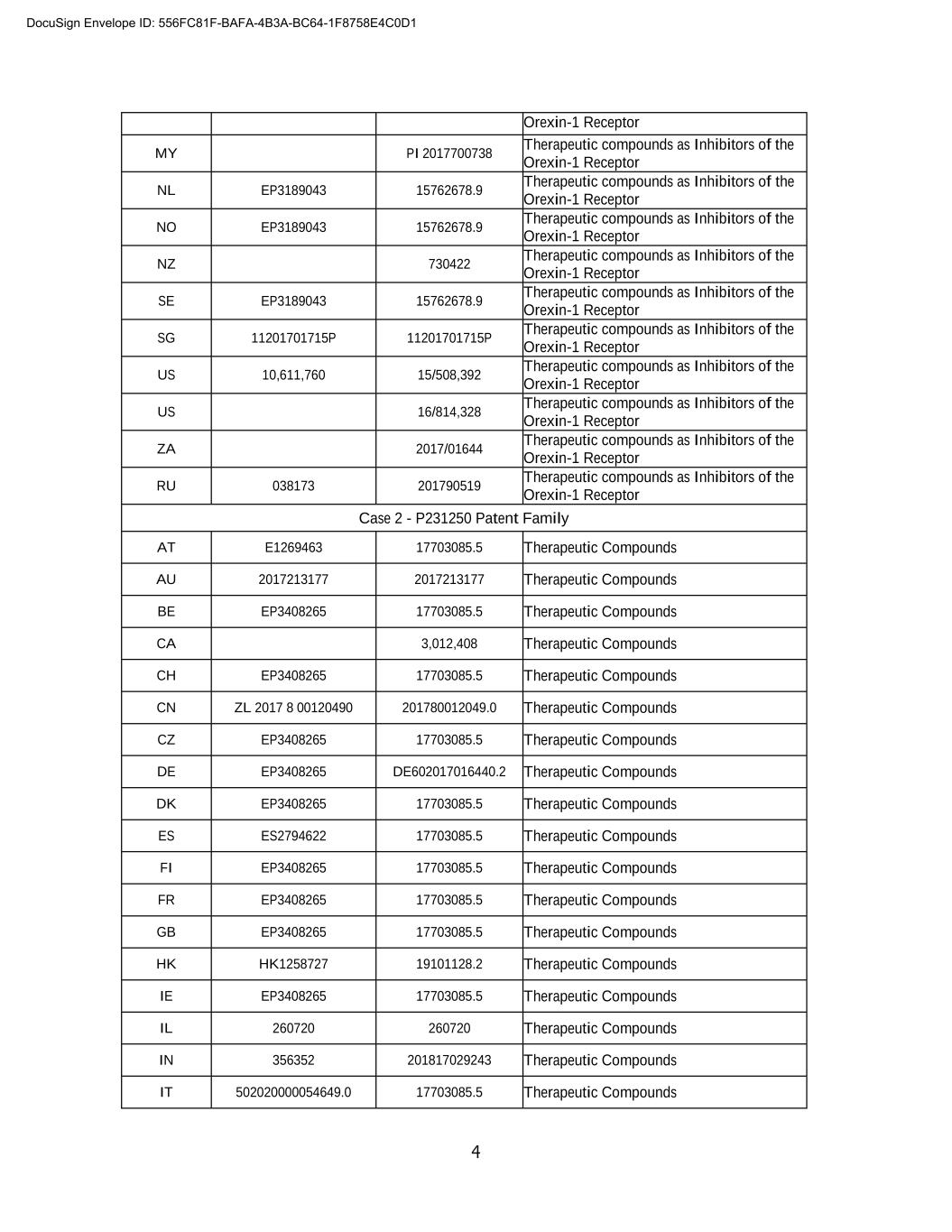
3 PATENT ANNEX Country Patent Number Application Number Title Case 1 - P210079 Patent Family AT E1335618 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor AU 2015310662 2015310662 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor BE EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CA 2,959,942 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CH EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CN ZL 2015 8 00576964 201580057696.4 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CZ EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor DE 60 2015 062 200.6 60 2015 062 200.6 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor DK EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor ES EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor FI EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor FR EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor GB EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor HK 1237348 B HK17111502.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IE EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IL 250883 250883 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IN 365438 201727008919 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IT EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor JP 6776227 2017-513035 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor KR 00-0000000 00-0000-0000000 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor LU EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor LV EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
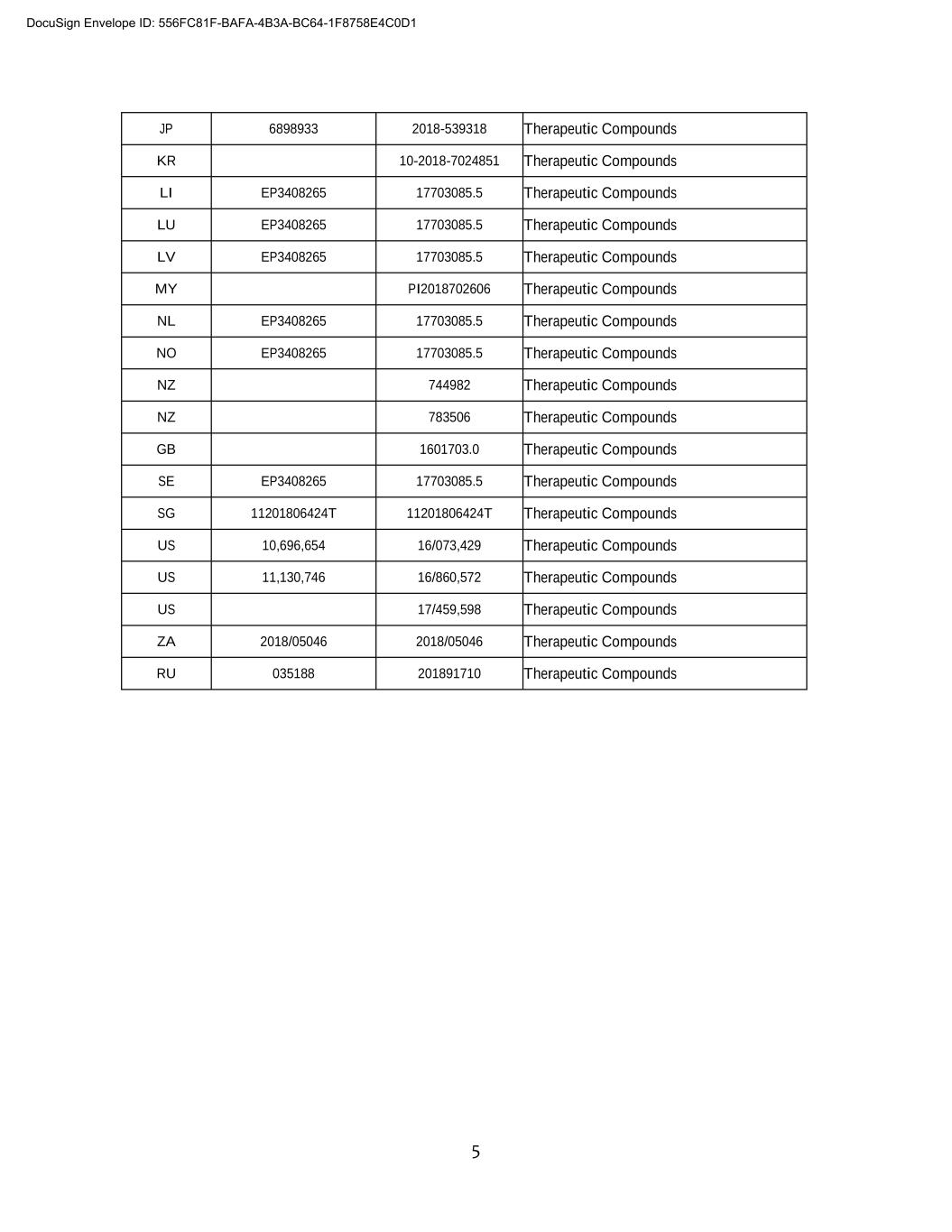
5 JP 6898933 2018-539318 Therapeutic Compounds KR 00-0000-0000000 Therapeutic Compounds LI EP3408265 17703085.5 Therapeutic Compounds LU EP3408265 17703085.5 Therapeutic Compounds LV EP3408265 17703085.5 Therapeutic Compounds MY PI2018702606 Therapeutic Compounds NL EP3408265 17703085.5 Therapeutic Compounds NO EP3408265 17703085.5 Therapeutic Compounds NZ 744982 Therapeutic Compounds NZ 783506 Therapeutic Compounds GB 1601703.0 Therapeutic Compounds SE EP3408265 17703085.5 Therapeutic Compounds SG 11201806424T 11201806424T Therapeutic Compounds US 10,696,654 16/073,429 Therapeutic Compounds US 11,130,746 16/860,572 Therapeutic Compounds US 17/459,598 Therapeutic Compounds ZA 2018/05046 2018/05046 Therapeutic Compounds RU 035188 201891710 Therapeutic Compounds DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

6 Exhibit C This announcement contains inside information C4X Discovery Holdings plc (“C4XD”, “C4X Discovery” or the “Company”) Strategic Divestment of C4XD’s Orexin-1 Receptor Antagonist Programme to Indivior for £15.95 Million Sale encompasses all rights and enables immediate revenue recognition Non-dilutive proceeds to accelerate progress of immuno-inflammatory portfolio 1 August 2023 – C4X Discovery Holdings plc (AIM: C4XD), a pioneering Drug Discovery company, today announces the execution of an asset purchase agreement for Indivior PLC (LSE: INDV) (“Indivior”) to acquire the proprietary rights to C4XD’s oral Orexin-1 receptor antagonist, C4X_3256 (INDV-2000) for substance use disorder, for £15.95 million. The completion of this non-dilutive strategic divestment forms part of C4XD’s evolution towards becoming an immuno-inflammatory therapeutics company. As indicated in C4XD’s April 2023 interim results, the Company’s portfolio will focus on treatments for immuno-inflammatory diseases, including its lead small molecule α4β7 integrin inhibitor programme that aims to expand access to disease modifying treatments for patients with inflammatory bowel disease. The Company believes it can deliver greater value for shareholders by progressing these programmes further and intends to advance the α4β7 programme towards the clinic and move two further immuno-inflammatory programmes into Lead Optimisation. Under the terms of the agreement, the previous license agreement announced on 29th March 2018 will be terminated and Indivior will assume all rights related to the development and use of C4X_3256 (INDV-2000) and related compounds1. In 2019, Indivior received a $10.6 million grant for the development of C4X_3256 (INDV-2000) from the National Institutes of Health (NIH) HEAL (Helping to End Addiction Long-term) Initiative, which aims to improve prevention and treatment strategies for opioid misuse and addiction. Previously, C4X Discovery was eligible to receive potential milestone payments from Indivior that in aggregate could have reached a maximum of $284 million over time if all clinical development, regulatory and commercial goals were achieved, as well as royalties on net sales of C4X_3256 (INDV-2000). The Phase 1 multiple ascending dose (MAD) study is currently ongoing. DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

7 Xxxxxxxxx Xxxxxxxxxx, Ph.D., CSO of Indivior PLC, said: “The acquisition of full rights to INDV- 2000 is aligned with our goal to build a strong and balanced pipeline focused on addiction treatments. Importantly, we know the asset well, having worked closely with C4X Discovery for more than five years. We recognize its exciting potential and will continue to progress it as a novel approach to the treatment of Opioid Use Disorder, as well as more broadly in substance use disorders.” Xxxxxx Xxxxxx, CBO of C4X Discovery, added: “Indivior has made excellent progress with our Orexin-1 candidate, and we are proud that they now wish to take this programme fully in-house. Their decision highlights the confidence they have in the programme and further underlines the power of our drug discovery expertise to produce valuable, commercially relevant, small-molecule drug candidates. The agreement also provides an opportunity for us to crystallise value for the programme early and underpins and accelerates our new strategy. This non-dilutive funding, alongside potential preclinical milestone payments from our licensing deals for C4XD immuno-inflammatory assets with Sanofi and AstraZeneca, will allow us to further advance our newly focused portfolio towards and into the clinic. With our internal portfolio progressing well and partnering on our MALT-1 programme initiated, we look to a strong future as an immuno-inflammation therapeutics Company.” 1. C4XD will not receive any further milestone or royalty payments from Indivior for the development or commercialisation of C4X_3256 (INDV- 2000) - Ends - DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

8 Contacts C4X Discovery Holdings Mo Xxxxxx, Communications x00 (0)000 0000000 Xxxxxxx Xxxxxx (UK) Limited (NOMAD and Broker) Xxxxxx Xxxxxxxx, Xxxx Xxxx (Corporate Finance) x00 (0)00 0000 0000 Xxxxxx Xxxxxxx (Corporate Broking) C4X Discovery Media – Consilium Strategic Communications Xxxx-Xxxx Xxxxxxx, Xxxxx Xxxxxxx, Xxxxxxx Xxxx x00 (0)000 000 0000 Notes to Editors: About C4X Discovery C4X Discovery (“C4XD”) is a pioneering Drug Discovery company, combining scientific expertise with cutting-edge Drug Discovery technologies to efficiently deliver world-leading medicines. We have a highly valuable and differentiated approach to Drug Discovery through our enhanced candidate molecule design and patient stratification capabilities, generating small molecule drug candidates across multiple disease areas focused on immuno-inflammation. Our commercially attractive portfolio ranges from early-stage target opportunities to late-stage Drug Discovery programmes and we have three commercially partnered programmes with one candidate in clinical development. For more information visit us at xxx.x0xxxxxxxxxx.xxx or follow us on twitter @C4XDiscovery. DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

9 Exhibit D Power of Attorney Letter This power of attorney letter is made on 04 August 2023 by C4X Discovery Limited, a company incorporated in England and Wales under the company number 06324250, whose registered office is at Manchester Xxx, 00 Xxxxxxxx Xxxxxx, Xxxxxxxxxx, X0 0XX (C4X) following the transfer of its C4X Patents to Indivior UK Limited (Co.), a company incorporated in England and Wales under the company number 7183451, whose registered office is 000-000 Xxxx Xxxx, Xxxxxx, Xxxxxxxxx, XX0 0XX (Indivior) to confirm and appoint, HGF Limited a company incorporated in England and Wales under the company number 08998652, whose registered office is 0 Xxxx Xxxx , Xxxxx, XX00 0XX, Xxxxxx Xxxxxxx as its attorney (Attorney) to attend to, sign, file, execute all documents and undertake such actions as required and/or directed by Indivior for the management, maintenance and prosecution of the C4X Patents, in accordance with the terms set out below. 1. Appointment and powers Each of C4X and Indivior appoints HGF Limited a company incorporated in England and Wales under the company number 08998652, whose registered office is 0 Xxxx Xxxx , Xxxxx, XX00 0XX, Xxxxxx Xxxxxxx as its attorney (Attorney) and in either C4X or Indivior's name and behalf to: a) consider, file, submit, approve, sign, execute, deliver and issue all agreements, documents, certificates and instruments (all whether as a deed or not) which the Attorney in its absolute discretion considers desirable or is required in connection with and for the purpose of the filing, prosecution, maintenance and management of the C4X Patents (as defined in the Asset Purchase Agreement between Indivior UK Limited and C4X Discovery Limited dated 31 July 2023 (the “Main Agreement”)) (Patent Maintenance and Prosecution) in accordance with the terms of 9.1(a) of the Main Agreement from 17.01 BST on Friday 04 August 2023 ; and b) take any steps or do anything which the Attorney in its absolute discretion considers desirable in connection with the implementation of the Patent Maintenance and Prosecution. 2. Delegation by corporate attorney Any Attorney which is a corporation aggregate may delegate one or more of the powers conferred on the Attorney by this power of attorney to an officer or officers appointed for that purpose. 3. Validity Each of C4X and Xxxxxxxx declares that a person who deals with the Attorney in good faith may accept a written statement signed by that Attorney to the effect that this power of attorney has not been revoked as conclusive evidence of that fact. DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

10 4. Governing law and jurisdiction This power of attorney and any dispute or claim (including non-contractual disputes or claims) arising out of or in connection with it, its subject matter or its formation shall be governed by and construed in accordance with the law of England and Wales. Each party irrevocably agrees that the courts of England and Wales shall have non-exclusive jurisdiction to settle any dispute or claim (including non-contractual disputes or claims) arising out of or in connection with this power of attorney or its subject matter or formation. This document has been executed as a deed and is delivered and takes effect on the date stated at the beginning of it. DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

12 Exhibit E BILL OF SALE (C4X Discovery Limited – Indivior UK Limited) THIS BILL OF SALE, ASSIGNMENT AND ASSUMPTION AGREEMENT (this “Agreement”) is made as of 17.01 British Summer Time on Friday 04 August 2023 (the “Effective Date”), by and between by and between C4X Discovery Limited, a company incorporated in England and Wales under the company number 06324250, whose registered office is at Manchester Xxx, 00 Xxxxxxxx Xxxxxx, Xxxxxxxxxx, X0 0XX (“Assignor”), and INDIVIOR UK LIMITED, a company incorporated in England and Wales under the company number 7183451, whose registered office is 000-000 Xxxx Xxxx, Xxxxxx, Xxxxxxxxx, XX0 0XX (“Assignee”). W I T N E S E T H : WHEREAS, Assignor and Assignee are parties to that certain Asset Purchase Agreement dated as of 31 July 2023 (the “Purchase Agreement”), pursuant to which Assignor has agreed to sell to Assignee all right, title and interest in, to and under the Assigned Assets (as defined in the Purchase Agreement), upon the terms and subject to the conditions set forth in the Purchase Agreement. NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and in accordance with the terms and conditions of the Purchase Agreement, Assignee and Assignor hereby agree as follows: 1. Defined Terms. Capitalized terms used but not defined herein shall have the meanings given to them in the Purchase Agreement. 2. Sale and Assignment of Assets. Pursuant to the terms and subject to the terms and conditions of the Purchase Agreement, effective as of the date hereof, Assignor does hereby sell, convey, deliver, transfer and assign to Assignee and its successors and assigns, and Assignee does hereby take delivery of, accept and acquire from Assignor, all of Assignor’s right, title and interest in, to and under the Intellectual Property Rights described on Schedule A to this Agreement. 3. Assumption of Liabilities. Pursuant to the terms and subject to the terms and conditions of the Purchase Agreement, effective as of the date hereof, Assignee hereby assumes, and shall pay and perform when due, all liabilities and obligations to the extent arising out of the Intellectual Property Rights described on Schedule A to this Agreement, in each case from and after the Effective Date. 4. Interpretation; Successors. Nothing contained in this Agreement shall in any way supersede, modify, replace, amend, change, rescind, waive, exceed, expand, enlarge or in any way affect the provisions set forth in the Purchase Agreement nor shall this Agreement reduce, expand or enlarge any remedies under the Purchase Agreement. This Agreement is intended only to effect the sale, assignment, transfer, conveyance and delivery of the Assigned Assets specified herein by Assignor to Assignee, and the assumption by Assignee of the Assumed Liabilities, in each case as DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1

[Signature Page to Bill of Sale, Assignment and Assumption Agreement] IN WITNESS WHEREOF, the undersigned have, by their respective duly authorized representatives, executed this Bill of Sale, Assignment and Assumption Agreement as of the day and year first above written. C4X DISCOVERY LIMITED By: Name: Title: INDIVIOR UK LIMITED By: Name: Xxxxxxx Xxxxxxxx Title: Finance Director, EUCAN DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
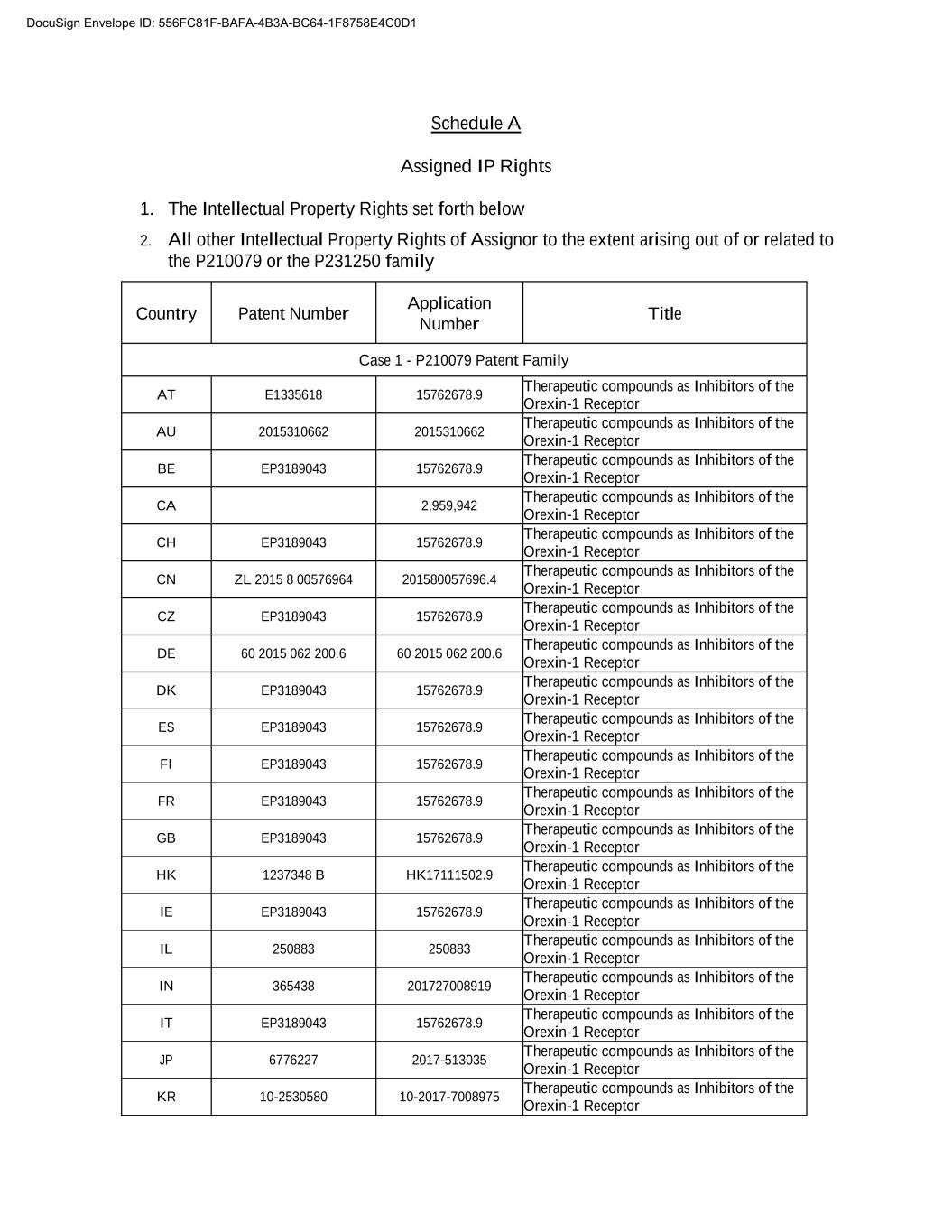
Schedule A Assigned IP Rights 1. The Intellectual Property Rights set forth below 2. All other Intellectual Property Rights of Assignor to the extent arising out of or related to the P210079 or the P231250 family Country Patent Number Application Number Title Case 1 - P210079 Patent Family AT E1335618 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor AU 2015310662 2015310662 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor BE EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CA 2,959,942 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CH EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CN ZL 2015 8 00576964 201580057696.4 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor CZ EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor DE 60 2015 062 200.6 60 2015 062 200.6 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor DK EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor ES EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor FI EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor FR EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor GB EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor HK 1237348 B HK17111502.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IE EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IL 250883 250883 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IN 365438 201727008919 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor IT EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor JP 6776227 2017-513035 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor KR 00-0000000 00-0000-0000000 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
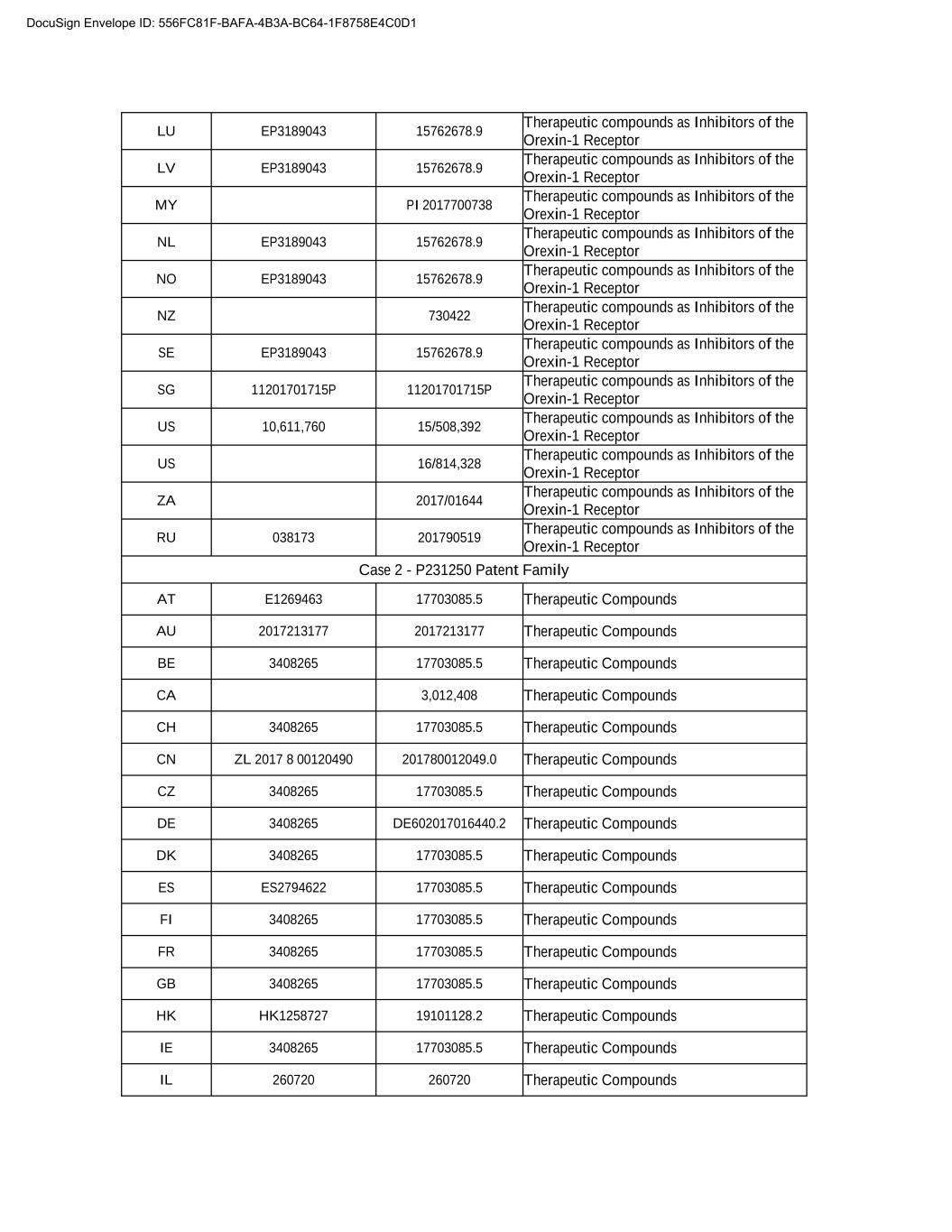
LU EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor LV EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor MY PI 2017700738 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor NL EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor NO EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor NZ 730422 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor SE EP3189043 15762678.9 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor SG 11201701715P 11201701715P Therapeutic compounds as Inhibitors of the Orexin-1 Receptor US 10,611,760 15/508,392 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor US 16/814,328 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor ZA 2017/01644 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor RU 038173 201790519 Therapeutic compounds as Inhibitors of the Orexin-1 Receptor Case 2 - P231250 Patent Family AT E1269463 17703085.5 Therapeutic Compounds AU 2017213177 2017213177 Therapeutic Compounds BE 3408265 17703085.5 Therapeutic Compounds CA 3,012,408 Therapeutic Compounds CH 3408265 17703085.5 Therapeutic Compounds CN ZL 2017 8 00120490 201780012049.0 Therapeutic Compounds CZ 3408265 17703085.5 Therapeutic Compounds DE 3408265 DE602017016440.2 Therapeutic Compounds DK 3408265 17703085.5 Therapeutic Compounds ES ES2794622 17703085.5 Therapeutic Compounds FI 3408265 17703085.5 Therapeutic Compounds FR 3408265 17703085.5 Therapeutic Compounds GB 3408265 17703085.5 Therapeutic Compounds HK XX0000000 19101128.2 Therapeutic Compounds IE 3408265 17703085.5 Therapeutic Compounds IL 260720 260720 Therapeutic Compounds DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1
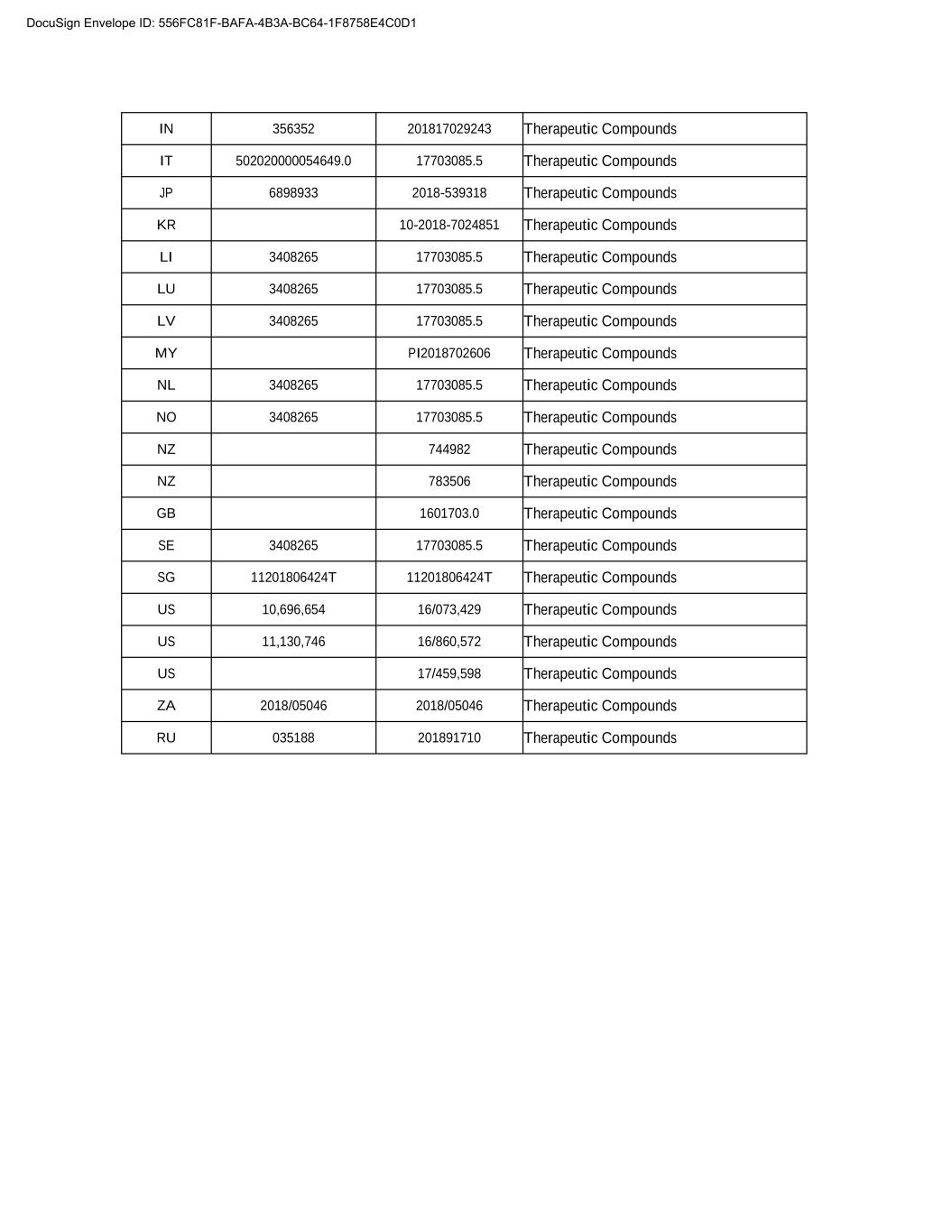
IN 356352 201817029243 Therapeutic Compounds IT 502020000054649.0 17703085.5 Therapeutic Compounds JP 6898933 2018-539318 Therapeutic Compounds KR 00-0000-0000000 Therapeutic Compounds LI 3408265 17703085.5 Therapeutic Compounds LU 3408265 17703085.5 Therapeutic Compounds LV 3408265 17703085.5 Therapeutic Compounds MY PI2018702606 Therapeutic Compounds NL 3408265 17703085.5 Therapeutic Compounds NO 3408265 17703085.5 Therapeutic Compounds NZ 744982 Therapeutic Compounds NZ 783506 Therapeutic Compounds GB 1601703.0 Therapeutic Compounds SE 3408265 17703085.5 Therapeutic Compounds SG 11201806424T 11201806424T Therapeutic Compounds US 10,696,654 16/073,429 Therapeutic Compounds US 11,130,746 16/860,572 Therapeutic Compounds US 17/459,598 Therapeutic Compounds ZA 2018/05046 2018/05046 Therapeutic Compounds RU 035188 201891710 Therapeutic Compounds DocuSign Envelope ID: 556FC81F-BAFA-4B3A-BC64-1F8758E4C0D1