CLINICAL SERVICES AGREEMENT This CLINICAL SERVICES AGREEMENT (this “ Agreement ”) is made effective as of the date of last signature below (the “ Effective Date ”) by and between Labcorp Drug Development Inc . with a place of business at 10 Moore...
Exhibit 4.25


Laboratory Improvement Act (CLIA) or other legally binding requirements or instructions of any Regulatory Authority applicable to the performance of a Study. 3. “ Audit ” in respect of any Services, means a review by or on behalf of Sponsor of Labcorp’s performance of the Services and related activities, including Third Party Provider, Investigator and Site, and Pass - Through Costs financial records . 4. “ Background IP ” means all Intellectual Property (a) owned or controlled by a Party prior to the Effective Date or (b) developed or acquired by or for a Party after the Effective Date independently of the Services . 5. “ CFR ” means the United States Code of Federal Regulations. 6. “ Claims ” means third party claims, demands, suits, actions, causes of action, proceedings, investigations, losses, damages, fines and liabilities, including reasonable attorneys’ fees, alleged to arise out of or in connection with or attributable to a condition giving rise to a Party’s indemnification obligations pursuant to Section 17 . 7. “ Confidential Information ” means any and all information, including financial, scientific, strategic or commercial information, the business, affairs, customers, clients, suppliers, plans or market opportunities of a Party or its Affiliates and the operations, processes, methods, product information, know - how, designs, Trade Secrets or software of a Party, its Affiliates, or third parties to whom a Party or its Affiliates has obligations of confidentiality (including pricing by Third Party Providers (howsoever recorded or preserved) disclosed by the Disclosing Party to the Receiving Party and is either : (i) identified by a suitable legend or other marking as being confidential (or similar designation) in a suitable prominent position ; (ii) described as confidential at the time of disclosure or which would reasonably be considered to be confidential given the nature of the information or the circumstances of disclosure ; (iii) obtained by examination, testing or analysis in any way from such confidential information ; (iv) any information that is observed or which a Party has access to at the other Party’s premises ; or (v) any derivative of such confidential information . Notwithstanding the foregoing, failure by a Party to mark documents or reduce oral disclosures to writing shall not alleviate the Receiving Party of its obligations under this Agreement if the disclosed information would reasonably be considered confidential based upon the nature of the information or the circumstances surrounding its disclosure . For avoidance of doubt, all Deliverables, Raw Data, Sponsor Background IP, Sponsor Test Materials, Sponsor Information, and the Final Report, shall be deemed the Confidential Information of Sponsor . 8. “ Deliverables ” means, as applicable to the Services, Results, Study Records or any other deliverable specified in this Agreement and any amendments made thereto (including physical products but excluding Software) . 2 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information

3 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 9. “ Disclosing Party” in relation to Confidential Information, means the Party or its Representatives disclosing Confidential Information in connection with this Agreement . 10. “Documentation” means, with respect to any Software product, all applicable documentation, including, for example, the technical specifications, documentation, and user guides and all descriptions of or about the Software product, or otherwise made available by or on behalf of Labcorp . 11. “ Essential Documents ” means those essential documents defined in ICH Guideline E 6 (R 2 ), Section 8 (including case report forms, Investigator files, regulatory files and any other core Study material agreed to in writing by the Parties) . 12. “ Force Majeure Event ” means any event, occurrence or condition which is beyond the reasonable control of a Party, such as the following : fire, flood, earthquake, epidemics, disasters, explosion, strike, accident, destruction or other casualty, supply chain issues, acts of terrorism, war, insurrection, embargo, changes in Applicable Laws, government requirement, civil or military authority, or acts of God . 13. “Good Clinical Practice ” or “ GCP ” means a standard for design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of Study participants are protected as implemented within the Applicable Laws where Services are performed . 14. “ HBS ” or “ Human Biological Samples ” means any human biological material, including human bodily parts and organs in whole or sub - samples, any tissue, skin, bone, muscle, connective tissue, blood, cerebrospinal fluid, cells, gametes or sub - cellular structures, such as DNA, or any derivative or product of such human biological materials including stem cells, cell lines, bodily fluids, blood derivatives and feces . 15. “ HBS Donor ” means an individual, living or deceased, from whom the HBS was obtained . 16. “ ICH GCP ” means the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/ 135 / 95 ) together with such other good clinical practice requirements as are specified in local national law where the Study is being performed including, by way of example, for studies in the European Union, Clinical Trial Regulation EU No 536 / 2014 and as applicable, Directive 2001 / 20 /EC of the European Parliament and the Council as amended relating to medicinal products for human use and in guidance published by the European Commission pursuant to such Directive, and ICH E 6 (R 2 ) guidelines .

4 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 17. “ Informed Consent ” means an informed consent form that is approved by an independent ethics committee or institutional review board and signed by the HBS Donor, their next of kin or legal representative authorizing the use of their HBS . 18. “Intellectual Property ” or “IP” means any and all right, title and interest in, arising from, or relating to inventions, ideas, discoveries, improvements, know - how, procedures, processes, formulations, software (including codes), data, designs, information, technology, works of authorship, including copyrights, patents and patent applications, Trade Secrets, and any other rights of a similar nature or character whether now existing or hereafter created, developed, arising or otherwise coming into being, and foreign equivalents of any of the foregoing . 19. “Invention” means any registerable Intellectual Property developed, discovered, conceived or made by Labcorp specifically as a result of performing the Services for the Sponsor pursuant to this Agreement and specifically relating to the Test Materials, Sponsor Background IP, and/or the Sponsor Information . For the avoidance of doubt, Inventions do not include Labcorp Property . For purposes of this Agreement, “Invention” shall not include any Software or any portion thereof (including all related Intellectual Property) or any activities or Intellectual Property associated with the development or delivery of Software . 20. “Investigator” means the person responsible for the performance of a clinical trial at a Site, except that if a trial is performed by a team of individuals at a Site, the Investigator is the responsible leader of the team and may be called the principal investigator . For purposes of this Agreement, an Investigator shall also be considered a Third Party Provider . 21. “Investigator Agreement ” means an agreement or group of agreements between an Investigator, hospital, or other parties and Labcorp and/or Sponsor for the performance of a clinical trial at a Site setting out the arrangements, tasks, and obligations, including financial arrangements, of the parties to such agreement or group of agreements . 22. “ISO 14155 ” means, with regard to device and diagnostic studies, the international standard 14155 of the International Organization for Standardization together with such other good clinical practice requirements as are specified in local national law where the Study is being performed . 23. “Pass - Through Costs” means costs and expenses incurred by Labcorp in providing the Services that are not direct fees for performance of Services, as set forth in this Agreement . For purposes of this Agreement, (a) Investigator grants shall be deemed Pass - Through - Costs and (b) payments made by Labcorp to Third Party Providers who are common carriers or couriers as part of performance of central laboratory services pursuant to this Agreement shall not be considered Pass - Through Costs .

5 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 24. “Protected Personal Data” has the meaning set forth in any applicable data protection laws . 25. “Protocol” means a document (and any amendments thereto) that describes the objectives, design, methodology, statistical considerations, and organization of the Study and, if applicable to the Services, includes a scientific plan, laboratory testing procedure or sample analysis outline . The Protocol shall, to the extent applicable to the Services, specify any Regulatory Authority and the country or countries to which Sponsor intends to submit the Results and other matters pertinent to the completion of the Study or Services . 26. “Quality Agreement” means any separate agreement entered into between the Parties specifically addressing quality assurance matters . 27. “Receiving Party” means, in relation to Confidential Information, the Party or its Representatives receiving or otherwise acquiring Confidential Information in connection with this Agreement . 28. “Regulatory Authority” means any national or state, or local agency or authority of any government of any country having jurisdiction over the respective activities contemplated by this Agreement or over the respective Parties . 29. “Representatives” in relation to a Party, means the Affiliates of that Party, and the directors, officers, employees, agents, contractors (such as freelance clinical research associates), and advisors (including attorneys, accountants, consultants, bankers, financial advisors and members of advisory boards) of that Party and its Affiliates who have a need to know the Confidential Information in connection with this Agreement and who have entered into agreements for the protection of the Confidential Information on terms substantially similar to the terms of this Agreement or are bound by professional obligations of confidentiality . 30. “Results” means (i) all materials, data, documents and information produced or developed by Labcorp specifically as a result of the Services and related to the Test Materials and/or the Sponsor Information ; and (ii) the Study Records (if applicable) . 31. “Serious Breach” means a breach which is likely to affect to a significant degree the safety, physical or mental integrity of the participants in the Study, or the scientific value of the Study . 32. “Services” means the tasks or services (excluding the provision of Software) described in this Agreement to be provided by Labcorp for Sponsor . 33. “Site” means the location where activities related to the Study or trial are performed by the Investigator or others associated with the Investigator or where records relating to the Study are stored .

6 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 34. “Software” means software products provided by Labcorp or otherwise made available under this Agreement, as detailed in this Agreement or other applicable agreement, together with all Documentation . The term “Software” shall be deemed to include any source code, object code, binaries, executables, configurations, enhancements, additions, derivative works, or other modifications of or to the Software (including descriptions thereof), whether made by Labcorp, by Sponsor, or by the Parties jointly, whether or not prepared in response to the Protocol or design of Sponsor Studies or other information provided by Sponsor . 35. “Specimen Kit” means specimen collection supplies and instructions that are necessary to collect and ship specimens to Labcorp for analysis in connection with the Services being provided . 36. “Sponsor Information” means Test Materials, data, specifications or other materials or information supplied by the Sponsor to Labcorp in connection with the Services . 37. “Study” means Sponsor’s protocol TT - 10 - 101. 38. “Study Records” in relation to Services, means all records, notes, reports (including case report forms ; monitoring logs ; data correction forms ; case histories ; medical images ; drug safety records ; records of receipt, use, processing and disposition of Test Materials and trial master file) and other observations, notations or data of activities or procedures (in each case whether in a written or electronic format) which Labcorp obtains from each Investigator or which Labcorp specifically generates or produces for the Study under Applicable Law, excluding the Study participant’s personal medical records . 39. “ System Data ” means control data from laboratory tests or transactional, volume and performance data related to the Services, which does not contain any : (i) data following treatment with any Test Materials ; or (ii) Protected Personal Data . 40. “Subcontractor” means third parties engaged by Labcorp for the performance of services customarily provided by Labcorp (e . g . freelance clinical research associates) in the ordinary course of its business . For purposes of this Agreement a Subcontractor is not considered a Third Party Provider and Labcorp is not considered a Subcontractor of Sponsor . 41. “Taxes” means value added tax (“VAT”) or goods and services tax (“GST”), local, national, state, federal sales or use taxes, excise taxes, duties, import/export fees, country specific business or professional services tax or similar tax on international services or foreign entities providing services or consumption taxes but shall not include income taxes of Labcorp as a result of fees paid by the Sponsor . 42. “Test Materials” in respect of the Study, means all compounds, devices, products, placebo, comparators, materials or other substances meeting relevant specifications that are necessary to perform the Study .

7 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 43. “Third Party Provider” means third parties other than Subcontractors and Labcorp Affiliates who are engaged by Labcorp in connection with the Services as an accommodation to Sponsor for the provision of goods or services ancillary to or outside of Labcorp’s core area of business, including investigator meeting planners, software and technology providers including electronic data capture (ED C ) and electronic patient - reported outcome (ePRO) providers, imaging vendors, translation companies, clinical trial material packagers, clinical trial material storage depots, common carriers, couriers, specimen kit material providers, laboratory supply and reagent providers, laboratory and laboratory management vendors, mobile nursing, mobile phlebotomy, and other scientific service providers and will also include clinical trial Investigators, Sites and Site related parties for the implementation of the Study at the Site where Labcorp contracts with the Investigator or Site . Specific Third Party Provides must be pre - approved by Sponsor . For purposes of this Agreement, a Third Party Provider is not considered a Subcontractor . 44. “Third Party Provider Agreement” means an agreement entered into between Labcorp and a Third Party Provider for the provision of goods or services relating to the Study . 45. “Trade Secret” means proprietary information, including a formula, pattern, compilation, program, device, method, technique, or process, which is marked in writing as a ‘trade secret’ at the time of disclosure, that : (i) gives its owner an opportunity to obtain an advantage over competitors who do not know or use it ; (ii) derives independent economic value, actual or potential, from not being generally known to, and not being readily ascertainable by proper means or by reverse engineering by other persons who can obtain economic value from its disclosure or use ; and (iii) is the subject of reasonable efforts, under the circumstances, by its owner to maintain its secrecy . 2. PROVISION OF SERVICES 2 . 1 Agreement Structure . This Agreement contains the terms and conditions under which Sponsor would engage Labcorp and under which Labcorp would provide Services as described in Appendix A, Description of Services and Estimated Budget . Labcorp shall not commence performing Services until this Agreement mutually acceptable to the Parties for the commencement of Services is executed . The relevant Protocol forms part of (and is incorporated into) this Agreement . In the event of a conflict between the Protocol and this Agreement, the terms of the Protocol shall prevail with respect to the scientific, medical, quality, technical and regulatory guidelines used in the conduct of the Study . This Agreement shall govern in all other instances . To the extent any Quality Agreement conflicts with the terms of this Agreement, this Agreement shall supersede the Quality Agreement . Further, this Agreement may not be amended by any such Quality Agreement .

8 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 2. Provision of Services . Labcorp agrees to perform the Services described in Appendix A, Description of Services and Estimated Budget attached to this Agreement, including any Protocol incorporated therein, in accordance with generally accepted industry standards and in compliance with Good Clinical Practices (where applicable), the Protocol, and any standard operating procedures specified in this Agreement . Labcorp shall use commercially reasonable efforts to perform the Services within the timeframe estimated in the Protocol or this Agreement . Labcorp reserves the right to refuse to perform any Services, including in relation to the Test Materials, that are deemed by Labcorp, in its sole discretion, as hazardous in nature . 3. Modification of this Agreement . Upon the request of either Party, a Work Order may be amended from time to time with the written agreement of both Parties . Modifications that may require an amendment to a Work Order include changes in recognized scientific and medical practice, Applicable Law, or to the contractual assumptions, responsibilities or timelines . Both Parties agree to act in good faith and promptly when considering a request for an amendment During the performance of central laboratory Services, Labcorp may be required to provide certain unbudgeted items or budgeted items in different amounts including but not limited to ancillary supplies, logistics, and minor modifications to database design (the “Adjustment Items”) . In the event Adjustment Items are incurred, Labcorp may invoice Adjustment Items as incurred ; provided however such uncontracted Adjustment Items may not exceed 2 % of the applicable Work Order’s central laboratory Services budget and will be reconciled in an amendment or at Work Order end . Labcorp reserves the right to postpone effecting changes to the Services, and Sponsor will not be obligated to pay for such changes to Services, until such time as the Parties agree to and execute an amendment . Labcorp will not deviate in any material way from a Work Order without Sponsor’s prior written approval ; provided that deviations from a Work Order may be made at Labcorp’s reasonable discretion in an emergency and/or in order to comply with Applicable Law, so long as Labcorp has first used commercially reasonable efforts to inform Sponsor of the issue . 4. Delays ; Extension of Timelines . Labcorp is not accountable for delays in its performance due to the actions, errors or omissions of persons or entities not under its direct control, including delays (i) in any governmental or regulatory agency ; (ii) resulting from regulatory or ethics committee actions or omissions ; (iii) in Study enrolment for clinical Studies at the Sites of third parties ; (iv) associated with the Test Material’s properties or its availability (v) resulting from competing studies not performed by Labcorp ; (vi) as a consequence of a Force Majeure Event ; or (vii) as a result of Sponsor, its Affiliates, agents or contractors, including delays in Protocol or case report form availability, changes in the Protocol or failure of Sponsor to provide the necessary clinical supplies, materials, licenses and documents required for the performance of the Services in accordance with the timelines described in this Agreement . The Parties agree that any such delay may result in discounted change in costs or fees which will be agreed in writing in the form of an amendment to this Agreement pursuant to Section 2 . 3 . If the Parties








16 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 9. HUMAN BIOLOGICAL SAMPLES 1. Labcorp’s Responsibilities . Where Labcorp is performing Services that include clinical site monitoring activities of the Study on behalf of Sponsor at Sites, Labcorp shall : (a) verify that the HBS Donor has given informed consent as specified in the Study Informed Consent ; (b) confirm that any HBS and associated data are managed in compliance with any Applicable Law relating to the use of HBS providing protection for human participants in the country of origin ; (c) use its reasonable efforts to confirm that any HBS shall be de - identified or coded according to Applicable Law to protect the identity and confidentiality of the HBS Donor ; and (d) in the event of a withdrawal of, or a material variation to the Informed Consent, promptly notify all relevant parties of such withdrawal or variation following Labcorp’s notification of such withdrawal or variation . 2. Sponsor’s Responsibilities . In all other circumstances where the Sponsor or third parties for which the Sponsor is responsible supply HBS to Labcorp in connection with the Services, the Sponsor represents and warrants that : (a) all HBS and associated data supplied in connection with the Services under this Agreement are or have been procured and supplied to Labcorp ethically in full compliance with any and all Applicable Laws (including any submissions, approvals and registrations to any applicable Regulatory Authority) relating to the use of HBS providing protection for human participants in the country of origin ; (b) the HBS Donor has given Informed Consent; (c) all HBS shall be de - identified or 'coded' using commercially reasonable methods according to applicable regulatory requirements and Data Protection Laws to protect the identity and confidentiality of the HBS Donor . (d) all HBS supplied to Labcorp : (i) may be used for the Services as set forth in this Agreement ; (ii) may be used to provide data in support of Sponsor’s commercial product development ; and (iii) were procured without inappropriate financial benefit to the HBS Donor ; and (e) in the event of a withdrawal of, or a material variation to the Informed Consent (including any material changes that may affect the Services), it shall promptly notify Labcorp and any other relevant parties of such changes or withdrawal .








24 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information Data . Labcorp shall only process such Protected Personal Data on behalf and upon the reasonable instructions of Sponsor for purposes notified to it by Sponsor for which consent, or other appropriate legal bases, from the relevant data subjects has been established by or for the benefit of Sponsor in accordance with all Applicable Law . Labcorp shall follow such procedures, policies and reasonable instructions as may be agreed by the Parties from time to time . Furthermore, Labcorp shall reasonably cooperate in entering into any additional agreements for the processing of Protected Personal Data, including any Data Processing Agreements . 3. Data Security . Labcorp shall take reasonable technical and organizational measures to protect against the unauthorized or unlawful processing of or the unauthorized or unlawful disclosure of such Protected Personal Data . Labcorp shall promptly notify Sponsor in the event of a security breach involving any Protected Personal Data which Labcorp is processing on behalf of Sponsor . 4. Sponsor Compliance with Data Protection Laws . Sponsor warrants that it has complied, and will comply, with any and all consent, notification and information requirements under Applicable Laws . 14. SOFTWARE RIGHTS If specified in this Agreement, Labcorp may make Software and/or Documentation available to Sponsor . If Labcorp makes available any Software and any Documentation to Sponsor, any access to or use of such Software and/or Documentation will be governed by Labcorp’s standard end user access terms and conditions contained in Appendix B of this Agreement . 15. DATA MANAGEMENT SERVICES Sponsor acknowledges and agrees that Labcorp is prohibited from sharing licensed data services, copyrighted or licensed scales and instruments, or medical dictionary terminology or data with any non - subscribing client . Sponsor represents and warrants that it has or shall obtain, prior to the commencement of the Study, a current subscription for using such copyrighted or licensed data services, scales, instruments or coding with the applicable licensing entity (for example, Xxxxxxxx Grumman/MSSO for MedDRA and Uppsala Monitoring Center for WHODRUG) . Labcorp shall have the right to verify Sponsor’s subscription to such scales, instruments, dictionary or data services before the commencement of the Study . If Labcorp determines that Sponsor does not have an appropriate subscription, Labcorp shall have the right to (a) inform the applicable licensing entity and (b) cease provision of any of licensed scales, instruments, dictionary terminology or data to Sponsor . Sponsor shall be responsible for all costs, expenses and damages incurred by Labcorp associated with Sponsor’s failure to properly obtain licenses to such licensed or copyrighted scales, instruments, dictionary or data services . 16. REPRESENTATIONS AND WARRANTIES; DISCLAIMER 1. Sponsor Representations and Warranties . Sponsor represents and warrants that:

25 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information (a) it owns all rights, title and interest in the Test Materials and Sponsor Information provided by it under this Agreement and related Intellectual Property ; (b) the use of any and all of such Test Materials or Sponsor Information in connection with the Services does not infringe any third party rights ; (c) all Sponsor Information delivered to Labcorp under this Agreement, including as related to the performance of data management, quality assurance or biostatistics Services, will be delivered to Labcorp in a form and condition reasonably calculated to allow Labcorp to perform the tasks described in this Agreement in a timely fashion ; (d) before and after entering into this Agreement, it will disclose to Labcorp all material facts discovered relating to the Study and the Study data that may affect Labcorp’s performance under this Agreement as well as any potential hazards related to the Test Materials including the emergence of information impacting the safety of Study participants or which otherwise impacts the toxicity assessment or risk profile of the Test Materials ; (e) it will comply with all Applicable Law and obligations of Sponsor in this Agreement ; (f) it will obtain and maintain during the term of the Study all approvals and licenses necessary for the performance of the Study and related Services, including national regulatory approvals, software licenses, Protocol required documentation licenses (e . g . , questionnaire licenses) and medical coding dictionary licenses ; (g) [All HBS provided to Labcorp is duly consented for the Services to be performed, including any retention period required by Applicable Law ; ] and (h) [For Studies conducted in the People’s Republic of China : (i) the collection, handling and Services for HBS provided to Labcorp has been authorized by the Human Genetics Resources Administration of China (“ HGRAC ”) ; (ii) no HBS, data resulting from performance of the Services or Sponsor Information is subject to heightened data security or exportation restrictions under Applicable Law, including but not limited to data subject to the Law of the People’s Republic of China on Guarding State Secrets, “important data” as classified by the Guidelines for Data Cross - Border Transfer Security Assessment, and other non - exportable data under Administrative Measures on Population Health Information or the Administrative Measures on Standards, Security and Services of National Healthcare Big Data . Any special requirements by the HGRAC or National Medical Products Administration (“ NMPA ”) for the Study shall be set forth in this Agreement . ]

26 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 2. Labcorp Representations and Warranties . Labcorp warrants that the Services performed by it will conform to the nature and scope of Services as agreed in this Agreement and all Applicable Law . 3. Labcorp Warranty Disclaimers . Except as set forth in Section 16 . 2 , all other representations or warranties, express or implied, including any implied warranties of merchantability or fitness for a particular purpose or for non - infringement of any Intellectual Property in respect of any Services to be provided by Labcorp are excluded to the fullest extent permitted by law . Labcorp does not warrant or represent that the results of the Study will be acceptable to any Regulatory Authority to which they are presented or that the results of the Study will enable Sponsor to further develop, market or otherwise exploit the Test Materials or any other product or service . 17. INDEMNIFICATION 1. Sponsor Indemnity . Sponsor will defend, indemnify, save and hold harmless Labcorp and its parent, subsidiaries and Affiliates and their respective directors, officers, employees, Subcontractors and agents (“ Labcorp Indemnitees ”) from and against any Claims to the extent that they arise out of or in connection with or attributable to : (a) personal injury to a participant in connection with the Study to which this Agreement relates or in connection with the Services, (b) the performance of the Services, (c) the harmful or otherwise unsafe effect of any Test Materials provided to Labcorp, including Claims based on the research, development, manufacture, distribution, use, sales or other disposition by Sponsor or any other person of the Test Materials and/or any other substances upon which Labcorp performed the Services, (d) the use by Sponsor of the Results or Deliverables, (e) any infringement, unauthorized use or misappropriation of any third party’s Intellectual Property in connection with the Study or the Services, (f) Sponsor’s failure to comply with this Agreement, any other agreements with Labcorp in relation to the Study or Applicable Law (including Trade Control Laws), or (g) Sponsor’s negligence or intentional misconduct in connection with the Test Materials, the Study or this Agreement; provided, however, that Sponsor’s obligation of indemnity hereunder is limited by the extent to which such Claims arose, from any indemnity even for which Labcorp is obligated to indemnify Sponsor pursuant to Section 17 . 2 below on the part of any

27 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information Labcorp Indemnitee . Notwithstanding the foregoing, Sponsor is not under any duty to defend, indemnify or hold Labcorp harmless with respect to any Claim which Labcorp settles without Sponsor’s prior written consent . 2. Labcorp Indemnity . Labcorp will defend, indemnify, save and hold harmless Sponsor and its parent, subsidiaries and Affiliates and their respective directors, officers, agents and employees (the “ Sponsor Indemnitees ”) from and against any Claims to the extent that they arise out of or in connection with or attributable to (i) Labcorp Indemnitee’s negligence or intentional misconduct in the performance of the Services or in connection with this Agreement or any Work Order, (ii) any material, and uncured, breach by Labcorp Indemnitees of its obligations under this Agreement or any Work Order ; or (iii) Sponsor’s use of any Labcorp processes owned or controlled by Labcorp (and not combined with any other Sponsor or third party information) which are used to perform the Services constituting an infringement or misappropriation of the intellectual property rights of a third party ; provided, however, that Labcorp shall have no obligation of indemnity hereunder with respect to any Claims to the extent that such Claims arise in whole, or in part, from the negligence, intentional misconduct, or breach of this Agreement (or any Work Order) on the part of any Sponsor Indemnitee . Notwithstanding the foregoing, Labcorp is not under any duty to defend, indemnify or hold Sponsor harmless with respect to any Claim which Sponsor settles without Labcorp’s prior written consent . 3. Indemnification of Third Party Service Providers or Investigators . In the event a Third Party Provider or Investigator requests a promise of indemnity with respect to the Test Materials, Sponsor’s products or actions, or services to be provided in connection with this Agreement, Labcorp shall notify Sponsor and Sponsor shall cooperate with Labcorp for the establishment of an indemnity agreement directly between Sponsor and the Third Party Provider . Labcorp shall have no other obligation with respect to the indemnity of a Third Party Provider Site, or Investigator . 4. Indemnification Procedure . The indemnifying Party’s obligations under Section 17 . 1 or Section 17 . 2 , as applicable, are subject to and conditional on (a) the Party seeking indemnification (the “ Indemnitee ”) providing prompt written notice of any Claims to the indemnifying Party (the “ Indemnitor ”), provided, however, failure to give such notification shall not affect the indemnification provided hereunder except to the extent the Indemnitor is actually prejudiced as a result of untimely notice, (b) the Indemnitee providing all information and reasonable assistance to the Indemnitor in respect of the Claims, and (c) the Indemnitor having sole authority to defend and/or settle the Claims, except that in the Indemnitor may not admit fault or liability on the part of Indemnitee without the Indemnitee’s prior written consent . Notwithstanding the foregoing, if the Indemnitor has not assumed the defence of any Claim within thirty ( 30 ) days of receiving notice of such Claim, the Indemnitee may assume the defence on behalf of, at the risk and expense of, the Indemnitor with all reasonable costs and expenses of such defence to be paid by the Indemnitor .




31 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information termination fees listed in this Agreement . For the avoidance of doubt, if payments are unit or milestone based, and this Agreement is terminated after costs have been incurred toward achieving portions of one or more incomplete units or toward achieving one or more incomplete milestones, Sponsor will pay for actual work performed toward those incomplete units or milestones up to the effective date of termination, in addition to paying for completed units or milestones . 21.6 Effects of Termination. (a) After receiving or providing notice of termination of this Agreement, Labcorp shall promptly act to mitigate and cancel, to the extent possible, all obligations that would incur expenses related to this Agreement, as applicable, and shall not, without Sponsor’s prior written approval, perform any additional Services, incur expenses, or enter into any further obligations related to this Agreement, as applicable . Labcorp shall use its commercially reasonable efforts to conclude or transfer the Services as expeditiously as practicable and in accordance with all Applicable Law . Further, Labcorp and Sponsor shall reasonably cooperate with each other during such Study termination to safeguard patient safety, continuity of patient treatment and to comply with Applicable Law . (b) In connection with the termination of this Agreement, the Parties agree to use commercially reasonable efforts to promptly agree on a wind - down plan and Labcorp shall cease performing all work not necessary for the orderly wind - down and/or transfer of the Services or required by Applicable Law . The wind - down plan will provide for the transfer of regulatory obligations, the assignment or termination of certain third party agreements, and the completion of Services, including any additional wind - down activities, by a mutually agreed termination date . The Parties further agree that Test Materials will be released by Labcorp once regulatory obligations have been transferred from Labcorp to Sponsor or Sponsor’s designee and the financial reconciliation under this Agreement has been agreed between the Parties . (c) On termination of this Agreement, Labcorp may terminate, or if possible under the Third Party Provider Agreements, may transfer to Sponsor, upon Sponsor’s request, any Third Party Provider Agreements or Investigator Agreements to which it is a party in respect of this Agreement . (d) On termination of this Agreement, unless previously destroyed or returned in accordance with Section 4 . 3 , and unless otherwise agreed in the Protocol, any remaining Test Materials shall, at Sponsor’s expense, be destroyed or, upon Sponsor’s request and expense, returned to Sponsor for retention in compliance with Applicable Law . (e) On termination of this Agreement, Labcorp shall retain, return or dispose of all HBS in accordance with Sponsor’s reasonable instructions, provided that




35 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information 22 . 14 Language of Agreement . The Parties acknowledge that it is their express wish that this Agreement and all notices and other documents to be given or executed under this Agreement be in English . [signature page follows]

[Signature Page to Clinical Services Agreement] IN WITNESS WHEREOF , duly authorized representatives of the Parties have executed and delivered this Agreement as of the Effective Date. Labcorp Drug Development Inc. Tarus Therapeutics LLC duly authorized By: duly authorized By: Xxx Xxxxxxx, MD Name: Xxxxxxxxxxx XxXxxxx Name: CEO Title: Manager, Contracts Management Title: 27 Feb 2023 Date: March 1, 2023 Date: 36 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information

37 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information APPENDIX A: DESCRIPTION OF SERVICES AND ESTIMATED BUDGET Attachment “A” - Scope of Work, Transfer of Obligations, Specifications, Assumptions and Estimated Timelines - Estimated Budget - Schedule of Payments and Terms Attachment “B” Attachment “C”

Attachment “ A ” Scope of Work, Transfer of Obligations, Specifications, Assumptions and Estimated Timelines PROJECT ASSUMPTIONS SUMMARY Key Cost Drivers 1 Number of Countries 10 Number of Active Sites 0 Number of Back - Up Sites 105 Number of Patients Screened 84 Number of Patients Enrolled 76 Number of Patients Completed 20.00% Estimated Screen Failure Rate 9.52% Estimated Drop - Out Rate 188 Total Number of eCRFs per Patient 36 Total Study Duration (Months) Assumptions Proposed Country and Site Distribution Number of Back - Up Sites Number of Active Sites Country 0 10 United States 0 10 Total Sites Timelines End Start Months Milestone 3/30/2023 8/25/2022 7 Startup 9/14/2024 3/30/2023 18 Enrollment - Primary 4/14/2025 9/14/2024 7 Treatment - Primary 4/14/2025 4/14/2025 0 Follow Up 5/14/2025 4/14/2025 1 Database Lock 8/14/2025 5/14/2025 3 Final Deliverable 8/14/2025 8/25/2022 36 Complete Study 38 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023

39 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Service Level Assumptions Sponsor Project Management Month 7 X Project Management - Startup Month 18 X Project Management - Enrollment Month 7 X Project Management - Treatment Month 4 X Project Management - Close Out Protocol 1 X RIM - Risk and Issue Management Unit of Measure Metric Labcorp Sponsor Vendor Management # of Vendors Contracted 4 X X Vendor Contracting Vendor Invoicing Months 143 X X Vendor Invoicing Vendor Management Months 178 X Vendor Management (Startup to Close Out) Unit of Measure Metric Labcorp Sponsor Regulatory Submissions (Not in Scope) US IND Submission 0 X US IND - Initial Project 0 X US IND - Maintenance Assumptions Project Management FTE for all study phases assumes the development of the Project Management Plan, which includes but is not limited to, the Communication plan, Training Matrix, Unblinding plan per Labcorp SOPs and review of other study plans per SOPs. Further planning and implementation of project delivery strategy, oversight of the study from Startup through recruitment to closure, client and internal communication, status reports of study/site metrics, financial and contractual oversight are assumed in these hours. Conduct of study risk analysis utilizing the Xcellerate RIM/RACT technology (set - up and usage free of charge). The study team will maintain the risk review process through the study with the RIM/RACT technology. Assumptions 1 standard complexity, 2 low complexity, 1 start up vendor(s) contracted. Assumes contract negotiation and finalization of contracts with preferred vendors. 1 standard complexity, 2 low complexity, 1 start up vendor(s) invoiced. Assumes invoicing of vendor payments throughout the study duration. 1 high complexity, 1 standard complexity, 2 low complexity, 1 start up vendor(s) managed. Assumes management of preferred vendors from Startup to Close Out of study include close of vendors. Assumptions

40 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Site Startup IRB - Central 1 X Central Ethics Submissions/IRB(s) IRB - Local 4 X Local Ethics Submissions/IRB(s) # of Sites Identified 30 X Site Identification Sites Total 10 X Site Contracts per Amendment 1 X Protocol Amendments ICF Core 1 X ICF - Develop Core ICF Additional/Supplemental 1 X ICF - Develop Additional/Supplemental Sites Total Requiring Adaptation 10 X ICF - Localizations Sites Total 10 X Regulatory Document Collection Month 9 X Startup Management and Coordination Unit of Measure Metric Labcorp Sponsor Clinical Ancillary Supplies Services (Not in Scope) Assumptions Initial submission for Central IRB/EC. Initial submission for Local IRB/EC. Process to identify a larger number of sites and determine which sites are appropriate to participate in Pre - Study visits . Development of site contracts based upon Labcorp templates with final approval of language by client; assumes two contracts per site average across all sites. Assumes all tasks to update the study for the most current version of the protocol to include, revision of the ICFs, site contracts/budgets and required documents, submission to ethics committees/IRBs, country HA and oversight of activities by team leadership. Assumes Labcorp will develop the core informed consent in English based on the final protocol provided by the client. Assumes Labcorp will develop special additional informed consents as required by country regulatory requirements. Assumes Labcorp will develop country specific informed consents that are then customized per site per investigator/institutional language. The collection, review and submission of all documents require from each site for the initial submission to the IRB/ethics committee per client specifications and country regulations. Also includes cost for annual updates per country regulations and protocol amendments as required per request for proposal. Management and oversight of the Startup process to include development of plans, oversight of essential document collection and investigator contract development and routine status reporting.

41 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Clinical Monitoring Site Months for Site Management 250 X Site Management Pre - Study Visit - Remote 12 X Pre - Study Visit - Remote Site Initiation Visit - Onsite 10 X Site Initiation Visit - Onsite Monitoring Visit - Onsite 253 X Monitoring Visit - Onsite Close Out Visit - Onsite 10 X Close Out Visit - Onsite Additional Day - Onsite 100 X Additional Days - Onsite Assumptions - CRA track eCRF completion for per investigator providing grant trackers for investigator payment teams - CRA routine contact with sites to ensure enrollment/recruitment goals are met, CRFs are completed for visits, protocol procedures are followed, and study updates are delivered to sites. - Update of study trackers, preparation of study documents, preparation/delivery of site correspondence, preparation for study meetings. - CRA review of CRFs to prepare for and follow - up monitoring visits resolving monitor queries. - Development of CTMS reports, maintenance of CTMS status reports /metrics. Remote visit to verify investigators qualifications for study participation. On - site visit to train site staff for study participation and reconfirm study qualifications. Frequency (ROW) - 4.3 weeks during Enrollment, 4 weeks during Treatment. On - site visit to source document verify (CRF Review) study data, review regulatory documents, conduct drug accountability, and ensure protocol/study procedures are followed to GCP standards. Onsite visit to ensure all patient data CRF Reviewed, all queries resolved, all regulatory documents collected for internal files, as required, drug accountability completed and drug returned or destroyed, as required. All close out documents should be signed and exit discussion with investigator completed by end of visit. Additional units of hours for CRF Review time onsite, also includes additional follow - up time for report writing.

42 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Clinical Monitoring Visits with Trip Report Review 285 X Trip Report Review CQC Visits 1 X Clinical Quality Control Visits Month 36 X Clinical Team Management Assumptions Labcorp uses standard trip report templates which are part of our Clinical Trial Management System (CTMS). Our CTMS allows our monitors to start writing their trip reports on a laptop during the visit or while traveling. Labcorp strives for a 10 - day turnaround time from date of visit to date of final approval and availability to Sponsor. CTMS has electronic signature functionality so there is no need for paper copies to be generated for wet ink signature. The Labcorp Clinical Quality Control Visit (CQC)is conducted to ensure that the Site and Monitor are following ICH GCP/ISO 14155 Guidelines, applicable SOPs, project - specific procedures, monitoring plan, to ensure subject safety and data integrity are well protected, all applicable regulations are being followed, and that the Site and Monitor are adequately performing their responsibilities. A CQC Visit is not the same as an audit. CQCs are conducted during on - site RMVs or COVs. A Site is selected for a CQC Visit based on the evaluation of potential risk associated with the Site and/or Monitor. Clinical Team Lead FTE assumes the management of all CRA activities for the duration of the study, the oversight of escalated site issues through the CRAs, training of CRAs, organization of CRA teleconferences/meetings, maintenance/distribution of status reports, development of study documents, newsletters/correspondence to sites and audit support.
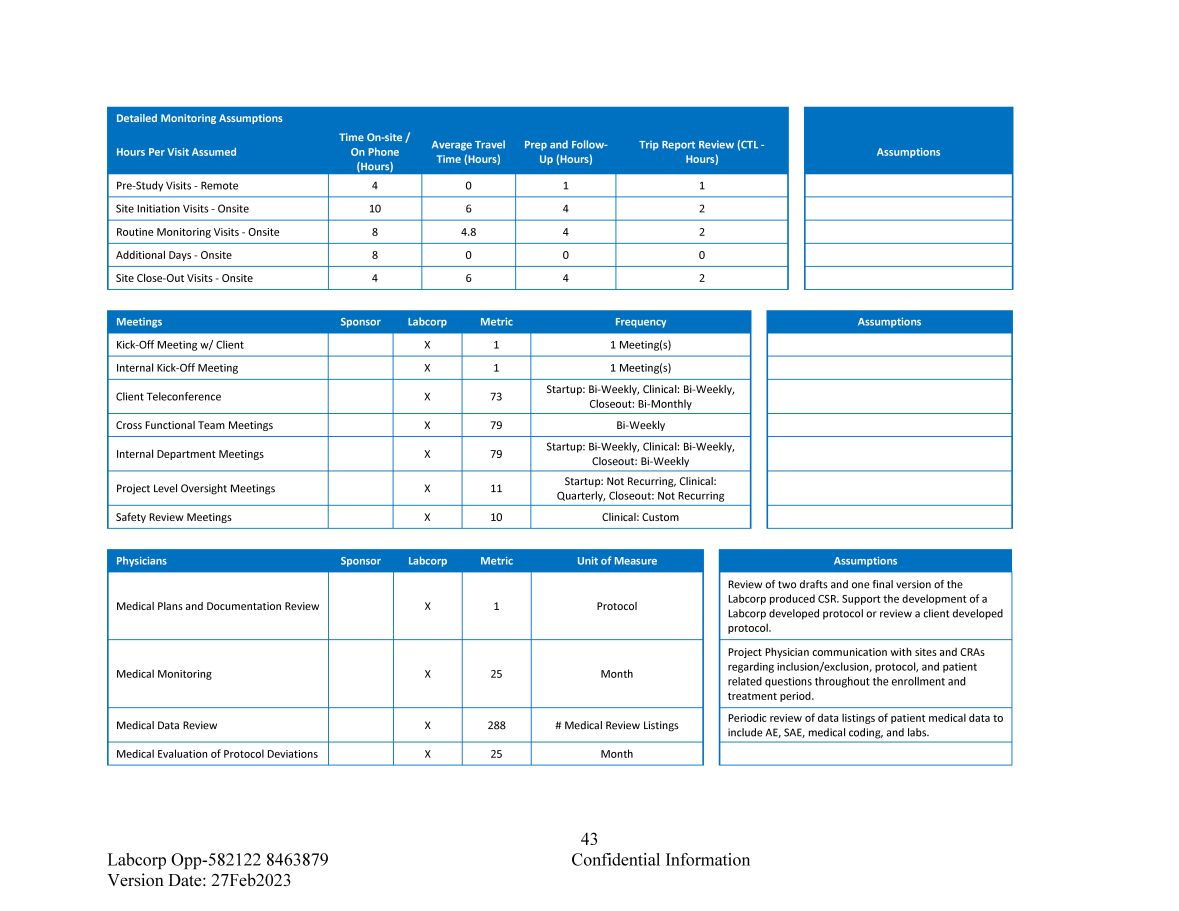
43 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Trip Report Review (CTL - Hours) Prep and Follow - Up (Hours) Average Travel Time (Hours) Time On - site / On Phone (Hours) Detailed Monitoring Assumptions Hours Per Visit Assumed 1 1 0 4 Pre - Study Visits - Remote 2 4 6 10 Site Initiation Visits - Onsite 2 4 4.8 8 Routine Monitoring Visits - Onsite 0 0 0 8 Additional Days - Onsite 2 4 6 4 Site Close - Out Visits - Onsite Assumptions Frequency Metric Labcorp Sponsor Meetings 1 Meeting(s) 1 X Kick - Off Meeting w/ Client 1 Meeting(s) 1 X Internal Kick - Off Meeting Startup: Bi - Weekly, Clinical: Bi - Weekly, Closeout: Bi - Monthly 73 X Client Teleconference Bi - Weekly 79 X Cross Functional Team Meetings Startup: Bi - Weekly, Clinical: Bi - Weekly, Closeout: Bi - Weekly 79 X Internal Department Meetings Startup: Not Recurring, Clinical: Quarterly, Closeout: Not Recurring 11 X Project Level Oversight Meetings Clinical: Custom 10 X Safety Review Meetings Assumptions Unit of Measure Metric Labcorp Sponsor Physicians Protocol 1 X Medical Plans and Documentation Review Month 25 X Medical Monitoring # Medical Review Listings 288 X Medical Data Review Month 25 X Medical Evaluation of Protocol Deviations Assumptions Review of two drafts and one final version of the Labcorp produced CSR. Support the development of a Labcorp developed protocol or review a client developed protocol. Project Physician communication with sites and CRAs regarding inclusion/exclusion, protocol, and patient related questions throughout the enrollment and treatment period. Periodic review of data listings of patient medical data to include AE, SAE, medical coding, and labs.
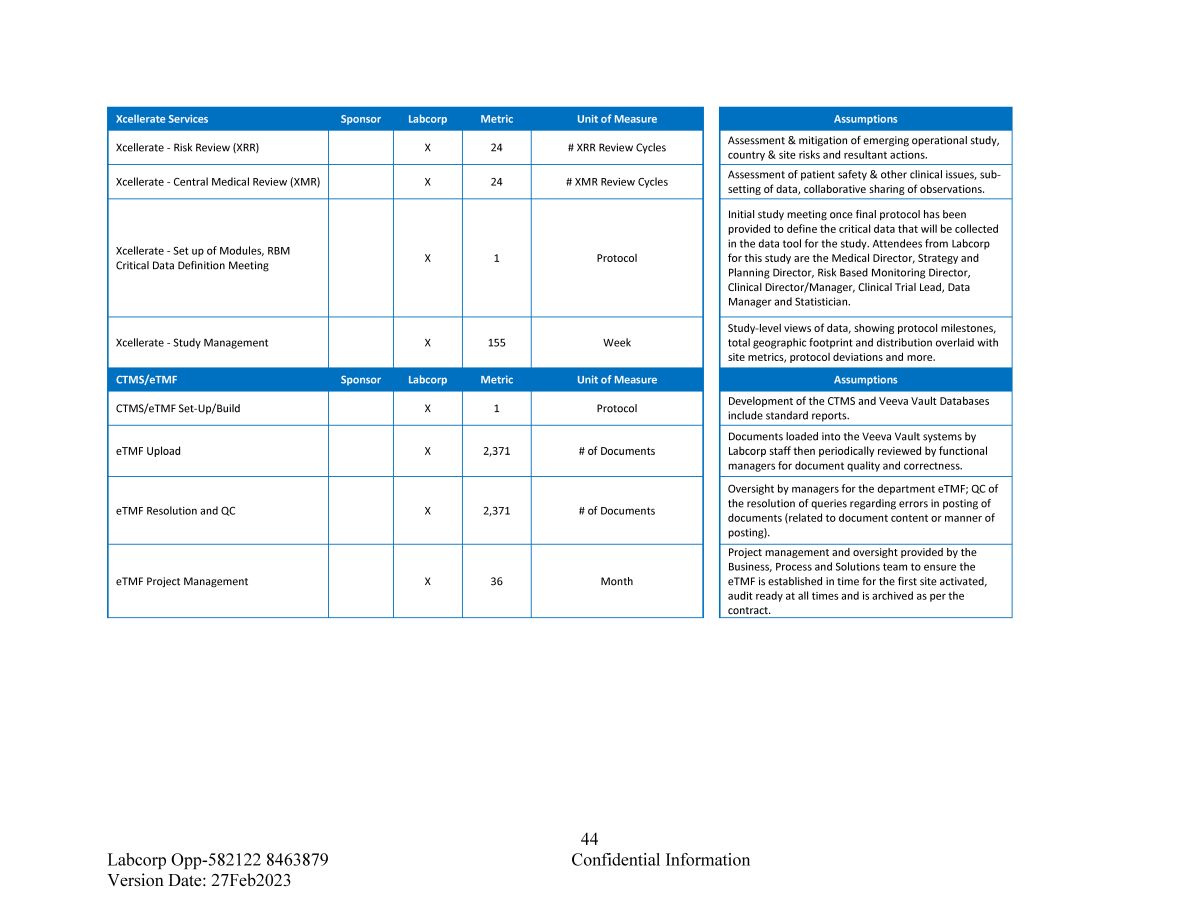
44 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Xcellerate Services # XRR Review Cycles 24 X Xcellerate - Risk Review (XRR) # XMR Review Cycles 24 X Xcellerate - Central Medical Review (XMR) Protocol 1 X Xcellerate - Set up of Modules, RBM Critical Data Definition Meeting Week 155 X Xcellerate - Study Management Unit of Measure Metric Labcorp Sponsor CTMS/eTMF Protocol 1 X CTMS/eTMF Set - Up/Build # of Documents 2,371 X eTMF Upload # of Documents 2,371 X eTMF Resolution and QC Month 36 X eTMF Project Management Assumptions Assessment & mitigation of emerging operational study, country & site risks and resultant actions. Assessment of patient safety & other clinical issues, sub - setting of data, collaborative sharing of observations. Initial study meeting once final protocol has been provided to define the critical data that will be collected in the data tool for the study. Attendees from Labcorp for this study are the Medical Director, Strategy and Planning Director, Risk Based Monitoring Director, Clinical Director/Manager, Clinical Trial Lead, Data Manager and Statistician. Study - level views of data, showing protocol milestones, total geographic footprint and distribution overlaid with site metrics, protocol deviations and more . Assumptions Development of the CTMS and Veeva Vault Databases include standard reports. Documents loaded into the Veeva Vault systems by Labcorp staff then periodically reviewed by functional managers for document quality and correctness. Oversight by managers for the department eTMF; QC of the resolution of queries regarding errors in posting of documents (related to document content or manner of posting). Project management and oversight provided by the Business, Process and Solutions team to ensure the eTMF is established in time for the first site activated, audit ready at all times and is archived as per the contract.

45 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Drug Safety Protocol 1 X Set - Up/Maintenance of Systems Year 2 X Safety Management Plan (SMP) - Initial and Maintenance Month 36 X Safety Oversight and Management # of SAE's 420 X SAE Processing/QC/Follow Ups/Archiving # of SAE's 420 X Medical Review of SAEs # of Total ESRs Expected in the Study 19 X SUSAR Notifications and Periodic Reports to Investigators # of Total ESRs Expected in the Study 19 X SUSAR Notifications and Periodic Reports to ECs # of SAE's 420 X Safety Narratives # of DSURs 2 X Drug Safety Update Report(s) (DSUR) Assumptions Initial set up and ongoing maintenance of Argus safety database if Labcorp is contracted to host the safety database. Includes set - up, maintenance, and licensing costs for SPEED portal if applicable. Initial development and ongoing updates of the safety management plan. Regular oversight of the project deliverables, contractual obligations, sponsor communications, KPI generation and reporting. Initial SAE(s): 84, Follow - Up SAE(s): 336 - Processing of SAE includes Argus database entry (if contracted), review of the SAE information for completeness, generation of queries, preparation of submission emails, query emails, etc. - Comprehensive review of the SAE data entry, narrative, client submission email, site query emails, etc. - Archival of SAE information from Argus in XML format, after CRF DBL . Each case in Argus is exported in XML format and written on CD or provided via secured email . - Resolution of follow - up queries on SAEs filed by the SAE team, Safety Physician or Client SAE team . Initial SAE(s): 84, Follow - Up SAE(s): 336 Review of SAE information by Drug Safety Physician, generation of medical queries, assessment of the events, etc. Preparation, distribution and submission of SUSAR/Expedited safety letters and distribution to investigator, in accordance with country specific/ study specific requirements. Preparation, distribution and submission of SUSAR / expedited safety report submission documents and distribution to central EC/ IRB, in accordance with country specific/ study specific requirements, and per EC/ IRB preference of submission method (email, fax, mail). Initial SAE(s): 84, Follow - Up SAE(s): 336 Writing of SAE narrative according to standard conventions and template specific to the study. Preparation or support preparation of DSURs and/or distribution of DSURs.

Unit of Measure Metric Labcorp Sponsor Drug Safety # of Native ESRs - From This Protocol (Initials, Follow - Ups) 17 X Analysis of Similar Events Assumptions Generation Analysis of similar events with search results from the safety database, includes safety physician's review and assessment. Unit of Measure Metric Labcorp Sponsor Medical Writing Protocol 1 X Review of Client Developed Protocol # Protocol Amendments 1 X Protocol Amendment Production CSR 1 X Clinical Study Report - Final 46 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Assumptions Review of a client study protocol and provision of comments A substantial amendment requires changes to the study scope of work and may require revisions to the data collection tools such as the eCRF, diaries, lab samples, patient reported outcomes (PRO) tools or the addition of new sites and countries. It will also require all the changes of the administrative amendment as well. ICH E3 compliant Clinical Study Report, including client review of each draft. Assumes fewer than 10 tables and fewer than 10 paragraphs. Unit of Measure Metric Labcorp Sponsor Data Management Protocol 1 X Data Management Plan Development and Maintenance # of Unique Forms 41 X CRF Design - Unique Forms # of Non - Unique Forms 147 X CRF Design - Non - Unique Forms # of Unique Forms 41 X Database Set - Up and Build - Unique Forms # of Non - Unique Forms 147 X Database Set - Up and Build - Non - Unique Forms Protocol 1 X tSDV Module Set - Up and Build # of Users Entering Into DB 65 X User Access Administration # of Sites Enrolling - Yearly 20 X EDC User Support and Administration # of Edit Checks 824 X Edit Check Specifications, Programming and Testing Custom Functions 66 X Custom Functions Assumptions Preparation of DMP and ongoing updates over duration of the project timeline. Design of the eCRFs and submit for Sponsor approval (assumes 2 review cycles) - Unique Forms Design of the eCRFs and submit for Sponsor approval (assumes 2 review cycles) - Non - Unique Forms Design and set up the database to match the design of the eCRFs - Unique Forms Design and set up the database to match the design of the eCRFs - Non - Unique Forms Includes time to configure the TSDV module of Medidata RAVE to enable SDV to be targeted to the appropriate sites, data fields, forms and patients. Set - up of external EDC users and passwords. Set - up and maintenance of users for EDC. Production, Testing and Deployment of Edit Checks within the DB. Programming, Testing and Deployment of Custom Functions within the DB.

47 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Data Management # External Data Streams 5 X Vendor Data Streams - Set - Up # External Data Imports 78 X Vendor Data Streams - Import and Reconciliation Custom Status Reports 3 X Custom Status Reports # Custom Listings 2 X Custom EDC Listings # SAS Data Review Listings 15 X SAS Data Review Listings Programming and Production # Pages for Data Management Review 15,449 X Data Review Month 26 X Data Review - Database Maintenance # Custom Listing Production Runs 2 X Custom Listing Production Runs # SAS Data Review Listing Production Runs 50 X SAS Data Review Listing Production Runs # Local Lab Normal Ranges Entered 60 X Local Lab Management # of SAE's 420 X SAE Reconciliation # Terms Coded 3,780 X Coding including CTCAE Grading (If Applicable) # Database Locks 1 X Management of Database Locks Protocol 1 X End of Project Archiving Patient Profile Domains Programmed 25 X Patient Profile Programming Patient Profile Production Run 8 X Patient Profile Delivery Assumptions Integration of 3rd party vendor data with the DB. Program to receive external data (e.g., labs, IRT) to include the transfer of the clinical database. Including review of transfer specifications and timing and will verify the cleanliness of each vendor dataset. Programming and Production of Custom Reports within the EDC System. Programming and Production of Custom Listings within the EDC system. Production of listings in SAS to support Data Review. Data cleaning of EDC data entered by Sites. #N/A Includes the time taken to run and produce custom listings from the EDC system. Includes the time for running and producing listings in SAS to assist in Data Review. Range normals and ongoing Management of local lab data. Support SAE reconciliation between EDC and Safety database. Generate queries for discrepancies in Safety database. Address discrepancies in safety database. Coding of terms for Medical Hx, ConMeds ad AEs. We assume Sponsor has MedDRA and WHO Drug Dictionary licenses if these are required for Coding purposes. Management of both the Interim and final data base lock for the study. Archiving of all Data Management documentation within the TMF. Programming set up the delivery of patient profiles from the database. The number of Patient Profile Domains reflects the number of datasets, from the EDC system or other sources, that need to be combined to create the patient profiles. Includes costs for all production runs, including Interim, Draft, Dry Run and Final transfers. Transfer format will be agreed during development of the SAP.

48 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Data Management Protocol 1 X Protocol Deviation Management Setup # Programmable Deviation Production Runs 25 X Protocol Deviation Management Ongoing Production Month 36 X Data Management Project Management Assumptions Includes development of programs for detection of Protocol Deviations. Includes reporting of Protocol Deviations to the monitoring team throughout the life of the study. Management and oversight of all data management activities for the study from planning, development of the timeline, status and metrics reporting and meeting attendance. Unit of Measure Metric Labcorp Sponsor Biostatistics SAP 1 X Statistical Analysis Plan Development TFL Data Display Templates 86 X Data Display Template Development SDTM Domains Programmed 32 X SDTM Programming # Analysis Datasets Programmed 17 X Analysis Dataset Programming Unique Table Programmed 47 X Unique Table Programming Non - Unique Table Programmed 35 X Non - Unique Table Programming Unique Figure Programmed 10 X Unique Figure Programming Non - Unique Figure Programmed 3 X Non - Unique Figure Programming Unique Listing Programmed 29 X Unique Listing Programming Assumptions Authoring of the text of the Statistical Analysis Plan incorporating any client specifications. Authoring of the Mock - Up shells for data displays. The version of the SDTM Implementation Guide to be followed will be agreed at the start of the study. SUPP - - Domains are not counted as separate domains. The number of Domains will be re - assessed when the SDTM specifications are written. If applicable, costs for Define.xml and Reviewers Guide are included here. The number of Analysis Datasets will be reassessed when the SAP is finalized. The version of the XXxX Implementation Guide to be followed will be agreed to at the start of the study. Assumes the number of unique tables to be programmed/QC'd/produced. The number will be reassessed when the TFL shells are final. Assumes the number of non - unique tables to be programmed/QC'd/produced. The number will be reassessed when the TFL shells are final. Assumes the number of unique figures to be programmed/QC'd/produced. The number will be reassessed when the TFL shells are final. Assumes the number of non - unique figures to be programmed/QC'd/produced. The number will be reassessed when the TFL shells are final. Assumes the number of unique listings to be programmed/QC'd/produced. The number will be reassessed when the TFL shells are final.

49 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Unit of Measure Metric Labcorp Sponsor Biostatistics # SDTM Domains Transferred 96 X SDTM Delivery # Analysis Datasets Transferred 51 X Analysis Dataset Delivery # TFLs Transferred 342 X Table/Listing/Figure Delivery Month 36 X Biostatistics Project Management Assumptions SDTM will be transferred in . xpt format unless otherwise specified . Formal deliveries include validation using OpenCDiSC or Pinnacle 21 . Includes costs for all production runs, including Interim, Draft, Dry Run and Final transfers. Transfer format will be agreed during development of the SAP. Includes costs for all production runs, including Interim, Draft, Dry Run and Final transfers. Transfer format will be agreed during development of the SAP. All general project management activities carried out by the Biostatistics department. Assumptions Total # Total # Total # USAG USAG: Total # Deliveries Delivered Deliveries Delivered Biometrics Deliverables Transfer of SAS Programs 0 0 1 1 SAS Programs Top Line Results 0 0 15 1 Unique Table Final Analysis 0 0 1 1 Database Lock 0 0 90 3 SDTM Domain 0 0 45 3 Analysis Dataset 0 0 105 3 Unique Table 0 0 105 3 Non - Unique Table 0 0 60 3 Unique Listing 0 0 0 3 Non - Unique Listing 0 0 18 3 Unique Figure 0 0 9 3 Non - Unique Figure PK Analysis 0 0 6 3 SDTM Domain 0 0 6 3 Analysis Dataset 0 0 6 3 Unique Table 0 0 0 3 Non - Unique Table 0 0 12 3 Unique Listing 0 0 0 3 Non - Unique Listing 0 0 12 3 Unique Figure 0 0 0 3 Non - Unique Figure Safety Monitoring (Single Arm) Assumes programmed outputs for Safety Monitoring Committee; Overriding formulas to match the original budget. DM 0 0 0 0 SDTM Domain 1 Dry run plus one per meeting programmed off raw data; Overriding formulas to match the original budget. DM 0 0 0 0 Unique Table

50 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Assumptions Total # Total # Total # USAG USAG: Total # Deliveries Delivered Deliveries Delivered Biometrics Deliverables Overriding formulas to match the original budget. DM 0 0 0 0 Unique Listing Overriding formulas to match the original budget. DM 0 0 0 0 Unique Figure Patient Profiles 0 0 225 9 Patient Profile Assumptions # Data Streams Import Frequency Total # Imports External Data Streams 0 0 Total 0 IVRS - Header Variables 3 3 Total 3 IVRS - Randomization Codes 26 Monthly, 26 Deliveries 1 Labcorp Central Lab 26 Monthly, 26 Deliveries 1 Imaging Vendor Needed for dose escalations, etc. 26 Monthly, 26 Deliveries 1 PK Assumptions Unit of Measure Metric Labcorp Sponsor Data Monitoring Committee (DMC) Services Identify Qualified Members for DMC participation, submit them to the client for approval (up to 2 CV's per identified member). # DMC Members to Identify 0 NA DMC - Identify Members Provide NDAs and CDAs to potential members, and obtain contracts for members. # DMC Members to Arrange Contracts With 0 NA DMC - Establish CDA's and Contracts Manage member stipends for contracted members # DMC Stipends to Pay 0 NA DMC - Manage Member Stipends Prepare an Interim Analysis Communication Plan for unblinded analyses. # Interim Analysis Communication Plans 0 NA DMC - Interim Analysis Communication Plan Development Author DMC Charter # DMC Charters Authored 1 X DMC - Charter Development Review a Client - authored DMC Charter. # DMC Charters Reviewed 0 NA DMC - Review Sponsor or Vendor Charter Author a DMC Statistical Analysis Plan separate from the main study SAP # DMC Statistical Analysis Plans Authored 0 NA DMC - Statistical Analysis Plan Development Review a Client - authored DMC Statistical Analysis Plan # DMC Statistical Analysis Plans Reviewed 0 NA DMC - Review Sponsor or Vendor Statistical Analysis Plan Prepare amendments of the DMC Statistical Analysis Plan # DMC Statistical Analysis Plans Amended 0 NA DMC - Statistical Analysis Plan Amendments

51 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Assumptions Unit of Measure Metric Labcorp Sponsor Data Monitoring Committee (DMC) Services Develop, or amend, DMC Mock - Up shells # Mock Shells in DMC Analysis Plans Authored 0 NA DMC - Mock Shell Development Perform statistical review of client authored Mock - Up Shells. # Mock Shells in DMC Analysis Plans Reviewed 0 NA DMC - Review Sponsor or Vendor Mock Shell Establish a secure portal for transfer of analyses and documentation to the DMC Protocol 0 NA DMC - Establish DMC Data Sharing System Portal Organize, attend, and prepare minutes for the DMC organizational meeting(s). Total # DMC Organizational Meetings 0 NA DMC - Organizational Meeting Logistics, Attendance and Minutes Prepare a blinded overview of the study status for the DMC Data Review Meetings DMC: Total # Open Presentations 0 NA DMC - Author Open Presentation Prepare a blinded report for the DMC Data Review Meeting open session(s). DMC: Total # Open Executive Summaries 0 NA DMC - Author Open Executive Summary for DMC Data Review Package Prepare an unblinded statistical summary and report for the DMC Data Review Meeting Closed Session(s) DMC: Total # Closed Executive Summaries 0 NA DMC - Author Closed Executive Summary for DMC Data Review Package Total # DMC Data Review Meetings 0 NA DMC - Data Review Meeting Logistics, Attendance, Minutes Archive and file all DMC specific material. Protocol 0 NA DMC - Close - Out activities Establish an unblinded Statistical Analysis Team (USAG) and secure analysis area. USAG: # of Data Areas to Set Up 0 NA USAG: Establish Unblinded Team and Analysis Area Obtain randomization codes for unblinded DMC Data Review Meetings USAG: # Times Randomization Code Obtained 0 NA USAG: Obtain Randomization codes USAG team: Apply randomization codes to either SDTM and/or XXXX datasets (as applicable) for Data Review Meetings USAG: # Unblinded Datasets Produced 0 NA USAG: Unblinded Analysis Dataset Production USAG: Create unblinded Tables, Listings and Figures. USAG: # Unblinded TFLs Produced 0 NA USAG: Unblinded Data Display production Provide SAE reports to the DMC for safety reviews # DMC SAE Reports 0 NA DMC - SAE Listing Provision

52 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Assumptions Unit of Measure Unit of Measure Metric Metric Labcorp Labcorp Sponsor Sponsor Quality Assurance (Not in Scope) Pharmacokinetics PK methodology sections incorporated into main SAP. Protocol 1 X Pharmacokinetic Analysis Plan (PKAP) PK Non - compartmental analysis (NCA) using validated Phoenix WinNonlin. One PK analysis will be performed on final data. Any additional PK analyses may incur additional costs. # of NCA Profiles - PK 168 X PK Analysis Pharmacokineticist input and review of clinical protocol Protocol 1 X PK Protocol, CSR, and TFL Review PLEASE SPECIFY Protocol 1 X PK Project Oversight Unit of Measure Unit of Measure Unit of Measure Unit of Measure Unit of Measure Unit of Measure Unit of Measure Metric Metric Metric Metric Metric Metric Metric Labcorp Labcorp Labcorp Labcorp Labcorp Labcorp Labcorp Sponsor Sponsor Sponsor Sponsor Sponsor Sponsor Sponsor Events Adjudication Committee (EAC) (Not in Scope) Patient Recruitment (Not in Scope) Regulatory Strategy (Not in Scope) eCOA Services (Not in Scope) IRT User Acceptance Testing (Not in Scope) Post - Marketing Services (Not in Scope) Mobile Clinical Services (Not in Scope)

Attachment “B” Estimated Budget 27 - Jan - 23 Tarus Therapeutics - 8463879 - Phase I/II FIH Study of TT - 10 as a Single Agent in Participants with Advanced Solid Tumors PROJECT ESTIMATE SUMMARY USD Total Price in Contract Currency Unit Price Units Unit of Measure Department 745,969.64 Project Management 190,731.79 26,633.72 7 Month Project Management - Startup 341,679.03 19,524.52 18 Month Project Management - Enrollment 137,376.36 19,625.19 7 Month Project Management - Treatment 72,784.82 18,196.21 4 Month Project Management - Close Out 3,397.64 3,397.64 1 Protocol RIM - Risk and Issue Management 86,944.22 Vendor Management 3,514.36 878.59 4 # of Vendors Contracted Vendor Contracting 7,034.85 49.34 143 Vendor Invoicing Months Vendor Invoicing 76,395.01 428.64 178 Vendor Management Months Vendor Management (Startup to Close Out) 299,320.28 Site Startup 5,953.25 5,953.25 1 IRB - Central Central Ethics Submissions/IRB(s) 3,242.51 810.63 4 IRB - Local Local Ethics Submissions/IRB(s) 63,959.05 2,131.97 30 # of Sites Identified Site Identification 46,481.59 4,648.16 10 Sites Total Site Contracts 52,393.40 52,393.40 1 per Amendment Protocol Amendments 4,440.38 4,440.38 1 ICF Core ICF - Develop Core 1,758.29 1,758.29 1 ICF Additional/Supplemental ICF - Develop Additional/Supplemental 7,867.92 786.79 10 Sites Total Requiring Adaptation ICF - Localizations 28,479.32 2,847.93 10 Sites Total Regulatory Document Collection 84,744.57 9,416.06 9 Month Startup Management and Coordination 1,996,591.68 Clinical Monitoring 53 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023

54 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Total Price in Contract Currency Unit Price Units Unit of Measure Department 244,712.59 978.85 250 Site Months for Site Management Site Management 13,639.48 1,136.62 12 Pre - Study Visit - Remote Pre - Study Visit - Remote 45,347.03 4,534.70 10 Site Initiation Visit - Onsite Site Initiation Visit - Onsite 978,660.43 3,868.22 253 Monitoring Visit - Onsite Monitoring Visit - Onsite 32,905.11 3,290.51 10 Close Out Visit - Onsite Close Out Visit - Onsite 188,010.91 1,880.11 100 Additional Day - Onsite Additional Days - Onsite 50,038.42 175.57 285 Visits with Trip Report Review Trip Report Review 7,520.44 7,520.44 1 CQC Visits Clinical Quality Control Visits 435,757.27 12,224.86 36 Month Clinical Team Management 380,598.22 Meetings 27,768.00 9,256.00 3 Kick Off Meeting Kick Off Meeting(s) 126,336.66 1,730.64 73 Teleconference Teleconference Meetings 208,768.46 1,235.32 169 Internal Meeting Internal Team Meetings 17,725.10 1,772.51 10 Safety Review Meeting Safety Review Meetings 190,038.04 Physicians 31,337.82 31,337.82 1 Protocol Medical Plans and Documentation Review 73,085.34 2,983.08 25 Month Medical Monitoring 66,317.03 230.27 288 # Medical Review Listings Medical Data Review 19,297.85 787.67 25 Month Medical Evaluation of Protocol Deviations 201,865.22 Xcellerate Services 41,204.06 1,716.84 24 # XRR Review Cycles Xcellerate - Risk Review (XRR) 131,829.43 5,492.89 24 # XMR Review Cycles Xcellerate - Central Medical Review (XMR) 17,735.84 17,735.84 1 Protocol Xcellerate - Set up of Modules, RBM Critical Data Definition Meeting 11,095.89 71.59 155 Week Xcellerate - Study Management 66,386.76 CTMS/eTMF 10,387.34 10,387.34 1 Protocol CTMS/eTMF Set - Up/Build 48,695.35 20.54 2371 # of Documents eTMF Upload 5,562.81 2.35 2371 # of Documents eTMF Resolution and QC

55 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Total Price in Contract Currency Unit Price Units Unit of Measure Department 1,741.26 48.85 36 Month eTMF Project Management 403,145.04 Drug Safety 44,901.36 44,901.36 1 Protocol Set - Up/Maintenance of Systems 14,447.86 7,223.93 2 Year Safety Management Plan (SMP) - Initial and Maintenance 52,973.16 1,486.12 36 Month Safety Oversight and Management 134,285.27 319.73 420 # of SAE's SAE Processing/QC/Follow Ups/Archiving 75,993.70 180.94 420 # of SAE's Medical Review of SAEs 3,581.13 188.48 19 # of Total ESRs Expected in the Study SUSAR Notifications and Periodic Reports to Investigators 3,853.96 202.84 19 # of Total ESRs Expected in the Study SUSAR Notifications and Periodic Reports to ECs 14,310.98 34.07 420 # of SAE's Safety Narratives 51,504.51 25,752.26 2 # of DSURs Drug Safety Update Report(s) (DSUR) 7,293.11 429.01 17 # of Native ESRs - From This Protocol (Initials, Follow - Ups) Analysis of Similar Events 142,332.73 Medical Writing 6,427.72 6,427.72 1 Protocol Review of Client Developed Protocol 7,608.24 7,608.24 1 # Protocol Amendments Protocol Amendment Production 128,296.77 128,296.77 1 CSR Clinical Study Report - Final 736,422.83 Data Management 12,344.12 12,344.12 1 Protocol Data Management Plan Development and Maintenance 20,537.79 500.92 41 # of Unique Forms CRF Design - Unique Forms 8,556.02 58.20 147 # of Non - Unique Forms CRF Design - Non - Unique Forms 75,108.37 1,831.91 41 # of Unique Forms Database Set - Up and Build - Unique Forms 22,492.42 153.01 147 # of Non - Unique Forms Database Set - Up and Build - Non - Unique Forms 36,104.53 36,104.53 1 Protocol tSDV Module Set - Up and Build 4,657.72 71.66 65 # of Users Entering Into DB User Access Administration 679.21 33.96 20 # of Sites Enrolling - Yearly EDC User Support and Administration 156,650.81 190.11 824 # of Edit Checks Edit Check Specifications, Programming and Testing 61,760.98 935.77 66 Custom Functions Custom Functions 10,878.25 2,175.65 5 # External Data Streams Vendor Data Streams - Set - Up

56 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Total Price in Contract Currency Unit Price Units Unit of Measure Department 12,940.22 165.90 78 # External Data Imports Vendor Data Streams - Import and Reconciliation 5,215.46 1,738.49 3 Custom Status Reports Custom Status Reports 3,579.45 1,789.73 2 # Custom Listings Custom EDC Listings 12,643.19 842.88 15 # SAS Data Review Listings SAS Data Review Listings Programming and Production 95,953.50 6.21 15449 # Pages for Data Management Review Data Review 99,835.38 3,915.11 26 Month Data Review - Database Maintenance 1,308.81 654.41 2 # Custom Listing Production Runs Custom Listing Production Runs 6,544.05 130.88 50 # SAS Data Review Listing Production Runs SAS Data Review Listing Production Runs 11,170.01 186.17 60 # Local Lab Normal Ranges Entered Local Lab Management 5,229.93 12.45 420 # of SAE's SAE Reconciliation 28,357.45 7.50 3780 # Terms Coded Coding including CTCAE Grading (If Applicable) 4,480.18 4,480.18 1 # Database Locks Management of Database Locks 2,870.11 2,870.11 1 Protocol End of Project Archiving 2,237.16 89.49 25 Patient Profile Domains Programmed Patient Profile Programming 525.26 65.66 8 Patient Profile Production Run Patient Profile Delivery 12,462.33 12,462.33 1 Protocol Protocol Deviation Management Setup 6,204.07 248.16 25 # Programmable Deviation Production Runs Protocol Deviation Management Ongoing Production 15,096.05 423.51 36 Month Data Management Project Management 577,514.70 Biostatistics 40,505.85 40,505.85 1 SAP Statistical Analysis Plan Development 27,510.15 319.89 86 TFL Data Display Templates Data Display Template Development 95,572.64 2,986.65 32 SDTM Domains Programmed SDTM Programming 107,255.35 6,309.14 17 # Analysis Datasets Programmed Analysis Dataset Programming 81,096.34 1,725.45 47 Unique Table Programmed Unique Table Programming 21,885.08 625.29 35 Non - Unique Table Programmed Non - Unique Table Programming 12,791.46 1,279.15 10 Unique Figure Programmed Unique Figure Programming 1,875.86 625.29 3 Non - Unique Figure Programmed Non - Unique Figure Programming 20,785.67 716.75 29 Unique Listing Programmed Unique Listing Programming

57 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Total Price in Contract Currency Unit Price Units Unit of Measure Department 11,422.20 118.98 96 # SDTM Domains Transferred SDTM Delivery 17,782.98 348.69 51 # Analysis Datasets Transferred Analysis Dataset Delivery 56,496.93 165.20 342 # TFLs Transferred Table/Listing/Figure Delivery 82,534.19 2,315.44 36 Month Biostatistics Project Management 7,651.14 Data Monitoring Committee (DMC) Services 7,651.14 7,651.14 1 # DMC Charters Authored DMC - Charter Development 116,492.51 Pharmacokinetics 4,259.65 4,259.65 1 Protocol Pharmacokinetic Analysis Plan (PKAP) 89,010.77 529.83 168 # of NCA Profiles - PK PK Analysis 7,414.00 7,414.00 1 Protocol PK Protocol, CSR, and TFL Review 15,808.09 15,808.09 1 Protocol PK Project Oversight 5,951,273.01 TOTAL SERVICE FEES (297,563.65) 5% - Bottom Line Discount (178,538.19) 3% - Non - Compete Discount (238,050.92) 4% - Integrated/Bundled Discount (If Awarded Partnership Volume Discount Services) 5,237,120.25 SERVICE FEES INCLUSIVE OF DISCOUNT 918,385.08 TOTAL INDIRECT FEES 5,812,827.25 PASS - THROUGH EXPENSES 146,112.15 VENDOR BID EXPENSES 12,114,444.73 TOTAL FEES INCLUDING PASS - THROUGHS / VENDOR BIDS 62,346.67 COST PER PATIENT (CPP) (SERVICE FEES ONLY) Please Note: The total prices of individual tasks in the budget grid are derived based on efforts required to deliver several sub - tasks, the duration of the sub - tasks to be delivered, and charge out rates for resources used, inclusive of inflation and applicable discounts. Labcorp price is not derived from the average unit price displayed in the budget grids, rather one listed task may consist of sub - tasks using multiple different units of measure. Therefore, unit prices in the grid are a calculation of total price divided by units, utilizing the unit of measure displayed in the grid. Average unit prices are provided to assist in the proposal evaluation process and may be subject to change based on the factors listed above. 918,385.08 Expense Description Estimated Indirect Fees – Labcorp Integrated Services 918,385.08 Central Labs (Labcorp)

58 Confidential Information Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Total Price in Contract Currency Unit Price Units Unit of Measure Department 5,812,827.25 Expense Description Estimated Pass - Through Expenses 20,606.25 IRB/EC expenses Clinical Fees 251,635.00 Onsite visit expenses Clinical - Travel & Subsistence 4,400.00 Internal Printing, Communication, Courier, etc. Communication and Miscellaneous Costs 5,535,186.00 Site expenses Clinical - Investigator Grants 1,000.00 Other Miscellaneous Expenses 146,112.15 Vendor Service Vendor Name 94,407.04 EDC Medidata 32,971.00 Institutional Review Board (IRB) Advarra 3,774.11 Printing Greens 14,960.00 Translations Welocalize

59 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information Attachment “C” Schedule of Payments and Terms Payment is subject to all payment terms as set forth in Section 10 of this Agreement . All invoices are due and payable by Sponsor within thirty ( 30 ) days of the date of invoice . I. Direct Fees Payment Schedule: Upon execution of this Agreement, Labcorp will invoice Sponsor for $ 523 , 712 . 02 representing ten percent ( 10 % ) (“Fees Advance”) of the total study budget labor fees exclusive of any upfront fees received during the startup agreement period . The Fees Advance will be reduced based on actual labor fees billed on a monthly basis . Every six ( 6 ) months a replenishment invoice will be issued to replenish the Fees Advance to 10 % of the total study budget labor remaining fees . This Work Order is subject to all payment terms set forth in Section 10 of this Agreement . All undisputed invoiced amounts are due and payable by Sponsor within thirty ( 30 ) days of the date of invoice . II. Pass - Through Costs Payment Schedule: A payment in the amount of $ 100 , 000 . 00 representing approximately seven percent ( 7 % ) of the pass - through expenses in the Work Order will be invoiced by Labcorp upon execution of this Agreement and will be paid by the Sponsor . Sponsor agrees to replenish the deposit held by Labcorp in increments of $ 50 , 000 . 00 once the total deposits of $ 100 , 000 . 00 drops below the agreed upon threshold of $ 50 , 000 . 00 . Labcorp will invoice and Sponsor will pay for Pass - Through - Costs actually incurred by Labcorp, as estimated in the Study budget . Sponsor is additionally responsible for any Third Party Provider non - refundable costs, cancellation fees as well as any additional fees associated with any Study holds, scope changes or premature terminations . III. Investigator Grants Payment Schedule: In order to facilitate timely processing of Investigator grant payments, Labcorp requires Sponsor to provide $ 400 , 000 . 00 broken up into two payments of $ 200 , 000 . 00 each respectively . An upfront initial payment of $ 200 , 000 . 00 , is due upon execution of this Agreement . The second payment of $ 200 , 000 . 00 is due upon screening of first patient . The $ 400 , 000 . 00 represents approximately 8 % of the total Study grant estimate . Xxxxxxx agrees to replenish the deposit held by Labcorp in increments of $ 200 , 000 . 00 once the total deposit of $ 400 , 000 . 00 drops below the agreed upon threshold of $ 200 , 000 . 00 . Site start up payments and fees are based on an estimate of the Study specific contractual obligations to be negotiated with the Sites and may include : advances, funds for advertising, pharmacy set up charges, local IRB fees and any other Site related administrative start up fees . Any remaining funds will be returned to Sponsor after the termination of the Study, following a financial reconciliation and all contracted obligations to the Investigators have been satisfied . In the event payments from Sponsor are insufficient to cover the payments to Investigators, Sponsor will promptly advance funds to Labcorp for the amount of grant payments required .

60 Labcorp Opp - 582122 8463879 Version Date: 27Feb2023 Confidential Information All invoices shall be sent to Sponsor at the following address: TARUS THERAPEUTICS LLC c/o Portage Development Services 00 Xxxxxx Xx, 0 xx Xxxxx Xxxxxxxx, XX 00000 Email: xxxx@xxxxxxxxxxxxxx.xxx with xxxxx@xxxxxxxxxxxxxx.xxx in copy Attention: Desi Xxxxxxx - Xxxxxxx, MS Payments should be sent via wire to: Labcorp Drug Development Inc. ABA #000000000 Account #4244842175 Swift Code (international) XXXXXX0X Or alternately, mailed to: Labcorp Drug Development Inc. P.O. Box 2445 Burlington, NC 27216 Taxpayer ID Number 22 - 3265977