License and Transfer Agreement by and between GlaxoSmithKline (China) R&D Co., Ltd and Zai Lab (Shanghai) Co., Ltd. October 18, 2016
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Exhibit 10.5
License and Transfer Agreement
by and between
GlaxoSmithKline (China) R&D Co., Ltd
and
Zai Lab (Shanghai) Co., Ltd.
October 18, 2016
| Confidential | Execution Copy |
License and Transfer Agreement
This License and Transfer Agreement (this “Agreement”), dated as of October 18, 2016 (the “Effective Date”), is made by and between GlaxoSmithKline (China) R&D Co., Ltd, a foreign invested enterprise duly established and validly existing under PRC law, whose registered office is at ▇▇▇▇▇▇▇▇ ▇, ▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇-▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇ New Area, Shanghai, PRC (“GSK”) and Zai Lab (Shanghai) Co., Ltd., a company duly organized and validly existing under PRC law, whose registered office is at 1043 Halei Road, ▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇-▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇, ▇▇▇ (“Zai Lab”). GSK and Zai Lab are each referred to herein individually as a “Party” and collectively as the “Parties.”
WHEREAS, GSK owns, has in-licensed and/or otherwise controls certain intellectual property rights, including patent rights and know-how, with respect to certain proprietary compounds known as “FUGAN” and “GRAPE”;
WHEREAS, Zai Lab is focused on the development of innovative drug candidates and is desirous of acquiring from GSK the right to develop and commercialize such proprietary compounds and funding all costs associated with all such activities.
NOW, THEREFORE, in consideration of the mutual covenants contained herein, and for other good and valuable consideration, the amount and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
| 1. | Definitions. |
The following capitalized terms as used in this Agreement, whether in the singular or plural, will have their respective meanings as set forth below:
1.1 “Affiliate” means with respect to a Party any entity which (directly or indirectly) is controlled by, controls, or is under common control with, such Party. For the purposes of this definition, the terms “control” and “controlled” mean the direct or indirect ownership of more than fifty percent (50%) of the outstanding voting securities of an entity, or such other relationship as results in actual control over the management, assets, business and affairs of such entity.
| 1.2 | “C.F.R” means the Code of Federal Regulations of the United States, as amended from time to time. |
| 1.3 | “CFDA” means the China Food and Drug Administration and any successor agency thereto. |
1.4 “Commercialize” or “Commercialization” means any and all activities related to the import, export, marketing, detailing, promotion, distribution and/or sale of a pharmaceutical product in a country or region in the Territory pursuant to and in accordance with the Marketing Authorizations for such product in such country or region.
1.5 “Commercially Reasonable Efforts” means that the level of efforts to be expended by a Party under this Agreement with respect to the research, discovery, Development, Manufacture and/or Commercialization of Compounds and Products will be consistent with the level of reasonable, diligent, good faith efforts and resources that would normally be used by such Party (whether acting alone or through its Affiliates) for a pharmaceutical product of similar commercial potential at a similar stage in its lifecycle, and taking into account issues of safety and efficacy, product profile, market and profit potential, the patent and other proprietary position of the product, the then current competitive environment for such product, the likely timing of such product’s entry into the market, the regulatory environment, and other relevant scientific,
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 2 of 36
| Confidential | Execution Copy |
technical and commercial factors. Commercially Reasonable Efforts shall be determined on a market-by-market and indication-by-indication basis for a particular Product, and it is acknowledged and understood that the level of efforts will be different for different markets and will change over time.
1.6 “Compound” means FUGAN and/or GRAPE.
1.7 “Confidential Information” means any and all proprietary and/or confidential data, information or Know-How, of whatever kind and in whatever form or medium, that is disclosed by or on behalf of a Party to the other Party during the Term and in connection with this Agreement, including, without limitation, the Transferred Know-How and Development Know-How.
1.8 “Control” or “Controlled” means, with respect to any Know-How, Materials, Patent Rights, or other intellectual property, the possession (whether by ownership or license, other than by a license granted pursuant to this Agreement) by a Party of the ability to grant (and/or to ensure that its Affiliates grant) to the other Party the licenses, sublicenses, and/or rights to access and use, such Know-How, Materials, Patent Rights, or other intellectual property, as provided for herein without violating the terms of any agreement or other arrangement with any Third Party in existence as of the time such Party or its Affiliates would be required hereunder to grant such license, sublicense, and/or rights of access and use.
1.9 “Covers” means, with reference to Patent Rights, that the performance of one or more activities related to the Development, Manufacture or Commercialization of a Compound or Product (or the use of any Materials in connection therewith) would infringe at least one claim of such Patent Right in the country(ies) in which such activities occur.
1.10 “Develop” or “Development” means to engage in research and development activities intended to research, discover or develop Compounds and/or to support INDs, NDAs or other Regulatory Approvals for Products, including, without limitation, (i) development of the applicable active drug substance(s), (ii) toxicology, pre-clinical and clinical drug development activities, (iii) clinical trials (except for Phase IV Studies), (iv) assay/test method development, validation and stability testing, (v) formulation development, (vi) manufacture of pre-clinical, clinical and commercial supplies, and manufacturing process development, scale-up and validation, (vii) quality assurance/quality control, statistical analysis, and regulatory affairs (including without limitation the preparation, submission and maintenance of all INDs and NDAs for the Products), and (viii) to have any of the activities described in (i)-(vii) performed.
1.11 “Development Costs” means any and all internal and out-of-pocket costs and expenses incurred by or on behalf of Zai Lab, its Affiliates licensees, and/or sublicensees in connection with the Development of the Products in the Territory pursuant to this Agreement. For clarity, Development Costs shall include, without limitation, the costs of manufacturing, any pre-clinical studies, Phase I Studies, Phase II Studies, Phase III Studies, Phase IV Studies, and any post-approval studies that are required by Regulatory Authorities as a condition to receiving Regulatory Approval for the Product.
1.12 “Development IP” means Development Know-How and Development Patents.
1.13 “Development Plan” means the development plan for the Compounds and Products attached hereto as Exhibit A.
1.14 “Development Program” means the program of Development activities to be undertaken by and on behalf of Zai Lab, its Affiliates, licensee and/or sublicensees to obtain and maintain Regulatory Approvals for one or more Products in the Territory, all as more fully described in the Development Plan. For clarity, all Development activities related to Compounds and Products undertaken by or on behalf of Zai Lab or any of its Affiliates, licensee or sublicensees will be considered as part of a Development Program.
1.15 “Development Know-How” means any and all Know-How generated as a result of activities performed pursuant to the Development Program.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 3 of 36
| Confidential | Execution Copy |
1.16 “Development Patents” means any and all Patent Rights filed by or on behalf of Zai Lab to Cover any Development Know-How.
1.17 “EMA” means the European Medicines Agency and any successor agency thereto.
1.18 “EU” means the organization of member states of the European Union, including as it may be constituted from time to time.
1.19 “FDA” means the United States Food and Drug Administration and any successor agency thereto.
1.20 “Field” means the treatment, prevention and diagnosis of any and all diseases in humans.
1.21 “First Commercial Sale” means the first sale for use or consumption of any Product in a country or region in the Territory after a Regulatory Approval, Marketing Authorization and/or Expanded Access/Compassionate Use authorization (as defined by 21 C.F.R. part 312 subpart 1 or any analogous laws or regulations in other countries in the Territory) for the Product has been obtained in such country or region.
1.22 “Generic Product” means, with respect to a particular Product being Commercialized in a country or region in the Territory, a pharmaceutical product that (i) contains the same active ingredient(s) as the Product; and (ii) is being sold in such country or region by a Third Party; provided that such product is not being sold pursuant to a license or sublicense granted by Zai Lab or any of its Affiliates for such country or region, and/or was not manufactured and supplied to such Third Party by or on behalf of Zai Lab or its Affiliates for resale in such country or region.
1.23 “GRAPE” means the formulation with the profile set forth in Exhibit B.
1.24 “FUGAN” means the formulation with the profile set forth in Exhibit B.
1.25 “IND” means an Investigational New Drug application, Clinical Study Application, Clinical Trial Exemption, or similar application or submission for approval to conduct human clinical investigations filed with or submitted to a Regulatory Authority in conformance with the requirements of such Regulatory Authority.
1.26 “Inventory” means all inventory of the Compounds and Products, including active pharmaceutical ingredient, finished product (if applicable), work in progress, raw materials, intermediates, retention samples, stability samples, that are in the possession of GSK or any of its Affiliates or being held on GSK’s or any of its Affiliates’ behalf as of the Effective Date.
1.27 “Know-How” means any and all proprietary commercial, technical, scientific and other data, information, materials, trade secrets, knowledge, technology, methods, processes, formulae, instructions, techniques, designs, drawings and specifications (including biological, chemical, pharmacological, toxicological, pharmaceutical, physical and analytical, pre-clinical, clinical, safety, manufacturing and quality control data and know-how, including study designs and protocols).
1.28 “License Agreements” means that certain License and Assignment Agreement between GSK and Chengdu Bater Pharmaceutical Co., Ltd (“Bater”), dated July 15, 2013 and that certain Development and License Agreement between GSK and Traditional Chinese Medical Hospital, Xinjiang Medical University, dated September 25, 2014 (“Xinjiang”, together with Bater the “Licensors”).
1.29 “Licensed Patents” means those Patent Rights listed in Exhibit D, which are the Patent Rights licensed by GSK under the License Agreement, registrations, supplementary protection certificates and renewals of such Patent Rights, together with foreign equivalents of any of the foregoing).
1.30 “Manufacture” or “Manufacturing” means any and all activities related to the manufacture, formulation and packaging of Compounds and/or Products, including, without limitation, related quality control and quality assurance activities. For clarity, the Manufacture of pre-clinical, clinical and commercial supplies and Manufacturing activities related to process development and scale up work will also be considered part of Manufacturing.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 4 of 36
| Confidential | Execution Copy |
1.31 “Marketing Authorization” means, with respect to a country or region in the Territory, all Regulatory Approvals and Pricing Approvals necessary to import, distribute, market and sell a pharmaceutical product in such country or region.
1.32 “NDA” means a New Drug Application or Supplemental New Drug Application filed with the FDA (including amendments and supplements thereto) to obtain Regulatory Approval in the U.S., or any corresponding applications or submissions filed with the relevant Regulatory Authorities to obtain Regulatory Approvals in any other country or region in the Territory.
1.33 “Net Sales” means the gross amount received for Product that is sold by Zai Lab or its Affiliates, licensees or sublicensees to the first Third Party (other than a licensee or sublicensee) after deducting, if not previously deducted, from such amount the following accrual basis deductions as applicable to such Products:
[*]
No deductions shall be made for commissions to any person on Zai Lab’s or any of its Affiliate, licensee or sublicensee’s payroll or for the cost of collection.
1.34 “Patent Rights” means any and all patents and patent applications in the Territory (which for purposes of this Agreement shall include certificates of invention and applications for such certificates), including, without limitation, any divisionals, continuations, continuations-in-part, substitutions, reissues, re-examinations, revalidations, extensions (including, without limitation, U.S. pediatric exclusivity patent extensions), registrations, supplementary protection certificates and renewals of any such patents or patent applications, together with foreign equivalents of any of the foregoing including the right to claim priority.
1.35 “Phase I Study” means a human clinical trial in any country or region that would satisfy the requirements of 21 C.F.R. 312.21(a) or the counterpart in such country or region, but which is not a Phase II Study, Phase III Study or Phase IV Study.
1.36 “Phase II Study” means a human clinical trial in any country or region that would satisfy the requirements of 21 C.F.R. 312.21(b) or the counterpart of it in such country or region, but which is not a Phase III Study or Phase IV Study.
1.37 “Phase III Study” means a large scale human clinical trial in any country or region that would satisfy the requirements of 21 C.F.R. 312.21(c) or the counterpart of it in such country or region, but which is not a Phase IV Study.
1.38 “Phase IV Study” means a clinical study or data collection effort for a Product that is initiated in one or more countries after the receipt of Regulatory Approval in such country(ies) and is principally intended to support the Commercialization of such Product in such country/countries and not to support or maintain the same or any additional Regulatory Approvals or otherwise obtain any labeling change. Phase IV Studies shall include, without limitation, clinical experience trials, but shall exclude post-approval studies that are required by a Regulatory Authority as a condition to receiving Regulatory Approval.
1.39 “Pricing Approvals” means in those countries in the Territory where Regulatory Authorities approve or determine pricing or pricing reimbursement for pharmaceutical products, such approval or determination.
1.40 “Product” means any pharmaceutical composition or preparation containing, as an active pharmaceutical ingredient, a Compound.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 5 of 36
| Confidential | Execution Copy |
1.41 “Prosecute” or “Prosecution” means in relation to any Patent Rights, (a) to prepare and file patent applications, including, without limitation, re-examinations or re-issues thereof, and represent applicant(s) or assignee(s) before relevant patent offices or other relevant governmental authorities during examination, re-examination and re-issue thereof, in appeal processes and interferences, or any equivalent proceedings, (b) to defend all such applications against Third Party oppositions, (c) to secure the grant of any Patent Rights arising from such patent application, (d) to maintain in force any issued Patent Right (including, without limitation, through payment of any relevant maintenance fees and/or any patent term extension), and (e) to make all decisions with regard to any of the foregoing activities.
1.42 “Regulatory Approval” means, with respect to a country or region in the Territory, any and all approvals, licenses, registrations or authorizations from the relevant Regulatory Authority necessary in order to import, distribute, market and sell a pharmaceutical product in such country or region, but not including Pricing Approvals.
1.43 “Regulatory Authority” means the CFDA, FDA, the EMA, and any other analogous government regulatory authority or agency involved in granting approvals (including any required pricing and/or reimbursement approvals) for the Manufacture and/or Commercialization of pharmaceutical products in the Territory.
1.44 “Regulatory Exclusivity Period” means any period of data, market or other regulatory exclusivity (as distinct from and excluding any exclusivity arising under Patent Rights) for a Product in a country or region in the Territory under applicable laws, rules and regulations in such country or region which prevents any unlicensed Third Party from marketing, promoting or selling a Generic Product in such country or region, including, without limitation, any such exclusivity provided in countries in the EU under national laws and regulations implementation Section 10.1(a)(iii) of Directive 2001/EC/83 or any analogous laws or regulations in other countries in the Territory.
1.45 “Regulatory Materials” means the China, U.S. and foreign regulatory applications, submissions and approvals (including all INDs, NDAs, Regulatory Approval and all foreign counterparts thereof) for any Compound or Product, correspondence with the FDA and other Regulatory Authorities relating to any Compound or Product or any of the foregoing regulatory applications, submissions and approvals, in each case owned by GSK or any of its Affiliates or held on GSK’s or any of its Affiliates’ behalf as of the Effective Date.
1.46 “Renminbi” or “RMB” means the lawful currency of People’s Republic of China.
1.47 “Technology Transfer” means (i) delivery to Zai Lab of [*] either by direct shipment or by written transfer of ownership of samples located at a CRO and (ii) GSK’s granting access to its data room containing [*]. For avoidance of doubt, GSK may retain copies of all GSK documentation delivered to Zai Lab in its archives after the completion of Technology Transfer for archival, compliance and contract monitoring purposes.
1.48 “Territory” means worldwide.
1.49 “Third Party” means any person or entity other than GSK, Zai Lab and their respective Affiliates.
1.50 “Transferred Know-How” means the Know-How listed on Exhibit C Part II.
1.51 “United States” or “U.S.” means the United States of America, including its territories and possessions, and the District of Columbia.
1.52 “Valid Claim” means a composition-of-matter claim Covering a Compound or Product, and/or a method-of-use claim Covering the use of a Compound or Product for one or more indications at least one of which is the subject of a Regulatory Approval, of an issued and unexpired Licensed Patent which has not been revoked or held invalid or unenforceable by a final decision of a court or other governmental agency of competent jurisdiction with no further possibility of appeal.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 6 of 36
| Confidential | Execution Copy |
| 2. | Assignment. |
2.1 Assignment. GSK hereby transfers, assigns and sells to Zai Lab all of its right, title and interest in and to the Licensed Patents, Transferred Know-How, Inventory, and Regulatory Materials to research, develop, make, have made, manufacture, use and commercialize the Compounds and Products in any indications in the Field, and such transfer, assignment and sale of all GSK’s right, title and interest in and to the License Patents, Transferred Know-how, Inventory and Regulatory Materials shall be effective upon GSK’s receipt of the upfront fee under Section 4.1.
2.2 Assignment and Assumption Agreement. As of the Effective Date, GSK and Zai Lab shall execute the assignment and assumption agreement substantially in the form attached hereto as Exhibit F, under which GSK will assign the License Agreements to Zai Lab. GSK will cause each of Bater and Xinjiang to execute the assignment and assumption agreement applicable to their respective License Agreements with GSK no later than [*] days after the Effective Date. Each such assignment and assumption agreement shall become effective upon [*].
2.3 Transfer of Third Party Services. Within [*] days following the Effective Date [*].
2.4 Licensing/Sublicensing by Zai Lab. To the extent that Zai Lab licenses or sublicenses to its Affiliates or to any Third Party under any Transferred Know-How and Licensed Patents, Zai Lab shall remain responsible for ensuring (and liable to GSK with respect to) the performance of and compliance by such Affiliates and/or Third Parties under the terms and conditions of this Agreement. Zai Lab shall ensure that any such license or sublicense agreement is consistent with the terms and conditions of this Agreement (in the case of a sublicense under the Licensed Patents, also consistent with the terms and conditions of the License Agreement) and complies with applicable laws, rules and regulations, including, without limitation, import and export control regulations.
2.5 Technology Transfer. [*].
2.6 No Implied Licenses. Nothing herein shall be construed as creating, granting or otherwise conveying to either Party any license or other right (whether by implication, estoppel or otherwise) other than those expressly provided for in this Agreement.
| 3. | Development and Commercialization. |
3.1 Product Development Program. After the Effective Date, Zai Lab will, either by itself or through its Affiliates, licensees and/or sublicensees, be solely responsible for designing and performing all aspects of the Development Program in accordance with the Development Plan, provided that Zai Lab may undertake changes to its development plans from time to time as long as it continues to satisfy its diligence obligations under this Agreement. Zai Lab will have sole responsibility and control for the managing and the financing of the Development Plan and all Development Costs. The primary focus of the Development Program will be to Develop and obtain Regulatory Approvals for one or m`ore Products.
3.2 Regulatory, Manufacturing and Commercialization. After the Effective Date, (i) Zai Lab will be solely responsible for and control (at its own expense) all regulatory matters related to the Development and Commercialization of Compounds and/or Products in the Territory, including, without limitation, taking full responsibility for preparing and filing the relevant applications with the Regulatory Authorities for pre-clinical and clinical studies and for Regulatory Approval; and (ii) Zai Lab will be solely responsible for and control (at its own expense) all aspects of Commercialization of Products and the Manufacturing and supply of Products (including, without limitation, the Manufacture and supply of related Compounds being Developed by Zai Lab) in the Territory and will have sole responsibility for all costs arising therefrom.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 7 of 36
| Confidential | Execution Copy |
3.3 Diligence. During the Term of this Agreement, Zai Lab shall use Commercially Reasonable Efforts to implement the Development Plan to Develop at least one (1) Product. Without limiting the generality of the foregoing, Zai Lab will use, and will cause its Affiliates, licensees and/or sublicensees to use Commercially Reasonable Efforts to Develop, Manufacture, seek Regulatory Approval and Marketing Authorization for, and following Regulatory Approval or Marketing Authorization to Commercialize FUGAN in China. Zai Lab will also use, and will cause its Affiliates, licensees and/or sublicensees to use, Commercially Reasonable Efforts to assess the feasibility to Develop, Manufacture, seek Regulatory Approval or Marketing Authorization for, and following Regulatory Approval or Marketing Authorization, to Commercialize GRAPE in the Territory and FUGAN in countries and regions other than China, provided that the foregoing shall not be construed as requiring ZAI to conduct any Development program with respect to GRAPE. [*].
3.4 Record Keeping and Reports. Zai Lab will prepare and maintain, and will cause each of its Affiliates and any licensees or sublicensees to prepare and maintain, appropriate records (in accordance with its standard policies and procedures) regarding the Development and Commercialization of Compounds and/or Products. During the Term hereof, Zai Lab will provide GSK with annual reports setting forth (i) a summary of updated development progress and achievements of such Development and Commercialization tasks as set forth in the Development Plan and a listing of any Regulatory Approvals achieved for Products in the Territory, (ii), information relating to any Zai Lab’s sublicensing under or assignment of this Agreement. Upon GSK’s request at any time during the Term hereof, Zai Lab shall provide to GSK a complete list of its licensees and sublicensees of Transferred Know-How and Licensed Patents, as well as a true and complete copy of each license agreement and sublicense agreement (as the case may be) and each amendments thereto within three (3) days after the notification. Any and all such reports (and all data and information set forth therein), lists and agreements shall be considered Zai Lab’s Confidential Information and shall be subject to the confidentiality and use restrictions under this Agreement.
3.5 Compliance.
(a) Debarment. Each Party hereby certifies (on behalf of itself and its Affiliates) that it will not and has not employed or otherwise used in any capacity the services of any person debarred under Title ▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇ Code Section 335a in performing any activities under this Agreement. Each Party shall immediately notify the other Party in writing if any such debarment occurs or comes to its attention, and shall, with respect to any person or entity so debarred, promptly remove such person or entity from performing any activities related to or in connection with the Development Plan or this Agreement.
(b) FCPA Compliance. Each Party shall, and shall ensure that its Affiliates and any Third Party contractors shall, comply with the United Stated Foreign Corrupt Practices Act (including as it may be amended) (the “FCPA”), and any analogous laws or regulations existing in any other country or region in the Territory, in connection with its performance under this Agreement. Neither Party will make any payment, either directly or indirectly, of money or other assets, including but not limited to compensation derived from this Agreement, to government or political party officials, officials of international public organizations, candidates for public office, or representatives of other businesses or persons acting on behalf of any of the foregoing, that would constitute violation of any law, rule or regulation.
(c) Export Control. This Agreement and the obligations of the Parties hereunder are made subject to, and limited by, all applicable restrictions concerning the export of products or technical information from the United States of America which may be imposed upon or related to Zai Lab or GSK from time to time by the government of the United States of America. Furthermore, each Party agrees that it will not export, directly or indirectly, any technical information acquired from the other Party under this Agreement or any Products using such technical information to any country for which the United States government or any agency thereof at the time of export requires an export license or other governmental approval, without first obtaining the written consent to do so from the Department of Commerce or other agency of the United States government when required by an applicable statute or regulation.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 8 of 36
| Confidential | Execution Copy |
(d) Export and Other Restrictions. This Agreement and obligations of the Parties hereunder are made subject to, and limited by, all applicable restrictions concerning the export of products, resources, materials or technologies from the People’s Republic of China (“PRC” or “China”, for the purpose of this Agreement excluding Hong Kong, Macau and Taiwan) which may be imposed upon or related to Zai Lab or GSK from time to time by the government of PRC. Zai Lab acknowledges and agrees that the Transferred Know-How, Licensed Patents and/or Inventory contains traditional Chinese medicine substance and technologies which have been disclosed to Zai Lab prior to the Effective Date, and GSK will deliver all such Inventory and conduct Technology Transfer to the extent within the territory of PRC to Zai Lab. Zai Lab shall be solely responsible for the risk of export restrictions (if any) to any Compounds, Products, Inventory, Transferred Know-How and Licensed Patents, as well as the application and/or registration in respect of export of such products, resources, materials or technologies.
| 4. | Payments and Royalties. |
4.1 Up-Front Payment. Within [*] days after the Effective Date, Zai Lab will pay to GSK a one-time non-refundable, non-creditable up-front payment of four million five hundred thousand RMB (4,500,000 RMB).
4.2 Milestone Payments. In addition to the above, Zai Lab will pay to GSK each of the applicable milestone payments provided for in this Section 4.2 upon the occurrence of the indicated milestone event. Each such milestone payment will be due in RMB and payable only once to GSK within [*] days Zai Lab receives the applicable invoice from GSK, and Zai Lab shall notify GSK within [*] days after the achievement of the specified milestone event so that GSK may issue such invoice. Each such milestone payment shall be payable only once under this Agreement for each Compound (i.e., no more than twice under this Agreement, once for each Compound), and will be non-refundable, non-creditable and not subject to set-off. Following such payment, the subsequent repeated occurrence of the same milestone event by the same Compound or Product will not under any circumstances trigger any additional milestone payment as a result of such event.
| Milestone Event |
Milestone Payment (RMB) | |
| For Product(s) containing FUGAN: | ||
| [*] | [*] | |
| For Product(s) containing GRAPE | ||
| [*] | [*] | |
4.3 Royalties. Zai Lab will pay to GSK in RMB, on a Product-by-Product basis, running royalties on Net Sales of Products in the Territory at the applicable royalty rates, as set forth in the following table:
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 9 of 36
| Confidential | Execution Copy |
| Aggregate Annual Net Sales of a Product in the Territory |
Royalty Rate | |
| [*] | [*]% | |
| [*] | [*]% | |
| [*] | [*]% | |
(a) Duration of Royalty Obligations. Zai Lab’s obligation to pay royalties under Section 4.3 will be in effect during the “Royalty Period” which begins on the date of First Commercial Sale of a Product in the Territory and shall expire on a Product-by-Product and country-by-country basis upon the later of:
(i) the expiration of the last-to-expire Licensed Patent in China having a Valid Claim that Covers such Product;
(ii) the expiration of all Regulatory Exclusivity Periods that apply to such Product in such country; or
(iii) [*] after the First Commercial Sale of such Product in such country.
(b) Additional Provisions Regarding Royalties. For purposes of determining Zai Lab’s royalty payment obligations under Section 4.3, all Products [*] will be treated as the same Product. Royalties when owed or paid hereunder will be non-refundable and non-creditable and not subject to set-off. Notwithstanding the definition of Product, in the event that Zai Lab or its Affiliates sells Product [*] to a Third Party, the royalty obligations of this Section 4.3 shall be applicable to such sales of [*] Product in the Territory.
(c) Reports and Timing of Royalty Payments. Starting on the date of First Commercial Sale of a Product in the Territory, Zai Lab will furnish to GSK a quarterly written report for each subsequent calendar quarter showing the Net Sales of all Products sold by Zai Lab, its Affiliates, licensees and sublicensees for which royalties are payable hereunder, and the royalties due to GSK on such sales. Each such royalty report shall be due within [*] days after the end of the relevant calendar quarter. The royalty payments due under Section 4.3 for each calendar quarter will be due and payable to GSK on the same date that the royalty report for the calendar quarter is due. Each royalty report shall describe in reasonable detail (based upon the data then available to Zai Lab) the Net Sales of each Product (including, without limitation, the deductions specified in clauses (i) through (iii) of the Net Sales definition) and the calculation of royalty payments due for the relevant calendar quarter. The information contained in each report under this Section 4.3(c) shall be considered Confidential Information of Zai Lab. For clarity, GSK’s rights under this Section 4.3(c) is for monitoring purposes only. GSK’s exercise of any rights under this Section 4.3(c) or any other terms hereunder shall not be construed as GSK’s involvement in any Development, Manufacture, Commercialization, marketing, pricing, interactions with any healthcare professionals and/or governmental officials, or any other activities under the Development Plan and/or Development Program, and Zai Lab shall be solely responsible for all the activities as described under those reports.
4.4 Future Payment under the License Agreements. Zai Lab shall be solely responsible for any milestone payment due under the License Agreements for milestone events achieved after the Effective Date, including the milestone payment for [*].
4.5 Sublicense Revenue. If Zai Lab grants a sublicense, sells or otherwise divests the Licensed Patents and Transferred Know-How (other than a sublicense to its Affiliates and contractors) before [*] (provided that [*] sublicense, sale or divestment of the Licensed Patents and Transferred Know-how shall be
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 10 of 36
| Confidential | Execution Copy |
[*] and shall [*]), then Zai Lab shall pay to GSK [*] of all consideration received by it and its Affiliates from and attributed to the sublicense, sale or divesture of the Licensed Patents and Transferred Know-How, but excluding any payments [*]. Such payments shall be made to GSK within [*] days after receipt by Zai Lab and/or its Affiliates. If the contemplated transactions are on product-by-product basis, [*] shall [*] relating to the sublicensing, selling or divesting Product.
4.6 Payment Terms. This Section 4.6 will apply to all payments to be made by Zai Lab to GSK hereunder.
(a) Manner of Payment. All payments to be made by one Party to the other Party under this Agreement shall be made in RMB and by bank wire transfer set forth in Exhibit E in immediately available funds to such bank account as may be designated in writing by such Party from time to time. In the case of royalties due on sales of Product outside the China, the rate of exchange to be used in computing on a monthly basis the applicable royalty due GSK in RMB shall be made at the rate of exchange published by the People’s Bank of China, prevailing on to the last business day of the month preceding the month in which such sales are recorded.
(b) Records and Audits. Zai Lab will maintain (and will cause its Affiliates, licensees and/or sublicensees to maintain) accurate books and records of accounting to document the sales of Products and the calculation of royalties payable to GSK in the Territory. For a period of [*] following the end of the relevant calendar year, the relevant books and records will, upon written request by GSK, be made reasonably available for inspection by an internationally recognized firm of independent certified public accountants (to be selected by GSK and reasonably acceptable to Zai Lab) as reasonably necessary to verify the accuracy of royalty reports for the relevant period. Access to such books and records shall be during normal business hours and upon reasonable prior notice; provided that in no event will any such audits or inspections be conducted more frequently than [*]. The auditors will, upon request, enter into a confidentiality agreement as reasonably requested by Zai Lab. The auditors will be permitted to disclose to GSK whether the royalty reports are correct or incorrect, the details and amounts of any discrepancies, and the books and records as well as associated documentations that illustrate the discrepancies. The auditors will also provide to Zai Lab, upon request, a copy of any audit reports and findings that are provided to GSK as a result of such inspection. If the auditors correctly identify any underpayments or overpayments, the amount of any underpayments will be paid to GSK by Zai Lab within [*] days of notification of the results of such inspection, and any overpayments will be fully creditable against amounts payable to GSK in subsequent periods. GSK will be solely responsible for the costs and expenses of any such audit inspections, except that in the event of an underpayment of aggregate royalties due and payable to GSK for a calendar year of more than [*] of the total amount properly due, Zai Lab will reimburse GSK for all the reasonable and documented audit fees expenses charged by the auditors for such audit inspection within [*] days after receipt of auditor’s report, and pay to GSK within [*] days after receipt of such report the deficiency not previously paid plus the interests calculated based on Section 4.6(d).
(c) Taxes. GSK shall be liable for any applicable taxes under the PRC tax regulations, upon any payments made by Zai Lab to GSK pursuant to this Agreement. [*], Zai Lab agrees to [*] and GSK will [*] Furthermore, Zai Lab shall, upon request, provide GSK with reasonable assistance in order to assist GSK in seeking the benefit of any present or future tax exemptions which may apply to any payments due GSK under this Agreement.
(d) Interest Due. If any uncontested amount properly due and payable to a Party under this Agreement is overdue, then the paying Party will also pay interest on the unpaid amount accrued at the annual rate of RMB SHIBOR (Shanghai Interbank Offered Rate) 3 months plus [*] from the date of payment was due, prevailing on to the last business day of the month preceding the month in which such sales are recorded.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 11 of 36
| Confidential | Execution Copy |
5. Ownership of Patents and Know-How/Technology Transfer.
5.1 Ownership of Development IP. Zai Lab shall own all rights, title and interests in or to any Development Patents and Development Know-How.
5.2 Trademarks. Zai Lab and/or its Affiliates shall be responsible (at its/their own expense) for and control the selection, registration, maintenance, enforcement and defense of any and all trademarks for the Products in the Territory. Zai Lab and/or its Affiliates shall own all rights, title and interest in and to any such trademarks and any related domain names associated with the Products or which contain the trademarks.
6. Patent Provisions.
6.1 Prosecution of Patent Rights. After the Effective Date, Zai Lab shall be solely responsible (at its own expense) for and shall control the Prosecution of the Development Patents and the Licensed Patents in the Territory, including any patent term extension.
6.2 Enforcement and Defense of Patent Rights.
(a) Notice. During the Term, each Party will promptly notify the other Party in writing upon learning of (1) any actual or suspected infringement by a Third Party of any Licensed Patents or Development Patents that Cover the Compounds, Products and/or the manufacture or use thereof, (2) any claim of invalidity, unenforceability of any such Patent Rights, and/or (3) any misappropriation or unauthorized use by a Third Party of the Transferred Know-How or Development Know-How. Any such notice shall identify the Third Party in question and contain a brief description (based upon available information) of the relevant actions that are believed to constitute such infringement, misappropriation or unauthorized use or upon which such claims of invalidity or unenforceability are based.
(b) Enforcement.
(i) Right to Enforce. Zai Lab shall have the sole right, but not obligation, to enforce and defend worldwide under its control, at its own expense, the Licensed Patents (subject to the terms and conditions of the License Agreement) and any Development Patents with respect to such infringement. Zai Lab shall have the sole right, but not obligation, to undertake and control any legal proceedings or other actions to so enforce and/or defend such Patent Rights worldwide. Zai Lab will do so at its own expense, and may undertake such proceedings and actions in the name of Zai Lab, as appropriate.
(ii) Cooperation. With respect to any legal proceedings or actions initiated under this Section 6.2(b):
| (A) | GSK shall have the right to consult with Zai Lab to participate in decisions regarding the appropriate course of conduct for such action, and the additional right to join and participate in such action at its own cost and expense (GSK shall join such action at Zai Lab’s request if necessary for standing purposes); and |
| (B) | GSK shall have the right to be represented by legal counsel of its own choice and at its own cost and expense in connection with any legal proceedings or other actions undertaken pursuant to this Section 6.2 to defend or enforce the Licensed Patents and Development Patents. |
| (C) | Zai Lab shall keep GSK informed of any developments in the action. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 12 of 36
| Confidential | Execution Copy |
(c) Settlement. Zai Lab shall have the right to settle the relevant claim or actions; provided, however, that Zai Lab shall not, without the prior written consent of GSK, enter into any settlement, consent judgment or other voluntary final disposition of any claim or action that would: (i) subject GSK or its Affiliates to an injunction or otherwise adversely impact any of GSK or GSK Affiliates’ rights under this Agreement; (ii) impose any financial obligation upon GSK or its Affiliates; and/or (iii) constitute an admission of guilt or wrongdoing by GSK or its Affiliates.
6.3 Patent Marking. Zai Lab will comply, and will cause its Affiliates, licensees and sublicensees to comply with applicable laws, rules and regulations in governing the marking of pharmaceutical products in the Territory to identify the relevant issued patents.
7. Confidentiality.
7.1 Confidentiality.
(a) Confidentiality Obligations. One Party (the “Disclosing Party”) may disclose or otherwise make available to the other Party (the “Receiving Party”) certain of the Disclosing Party’s Confidential Information for use in connection with this Agreement. For clarity, all Transferred Know-How, Licensed Patents (to the extent unpublished) and Development IP shall, upon the effective date of the transfer or assignment of each such intellectual property to Zai Lab, be deemed Confidential Information of Zai Lab. During the Term and for [*] years thereafter, the Receiving Party will keep confidential, will not disclose to any Third Party, and shall not use for any purpose other than as expressly permitted hereunder, any Confidential Information of the Disclosing Party. The foregoing obligations shall not apply to the extent that such information:
(i) was known to Receiving Party or any of its Affiliates prior to the time of disclosure (and such prior knowledge can be properly documented);
(ii) is or becomes public knowledge through no fault or omission of Receiving Party or any of its Affiliates;
(iii) is obtained by the Receiving Party (or its Affiliates) without restrictions of confidentiality from a Third Party under no obligation of confidentiality to the Disclosing Party or its Affiliates;
(iv) is independently developed by employees or agents of Receiving Party (or its Affiliates) without the aid, application or use of the Disclosing Party’s Confidential Information (and such independent development can be properly documented); or
(v) is required by applicable law, rule, regulation, act or order of a governmental authority or agency, or a court of competent jurisdiction; provided, that the Receiving Party (1) promptly provides written notice of such requirement to the Disclosing Party so that the Disclosing Party can seek a protective order or other appropriate remedy to preserve the confidentiality of such information, (2) upon request, reasonably cooperates with the Disclosing Party in connection with such efforts, and (3) only discloses the minimum Confidential Information required to be disclosed in order to comply.
Any combination of features or disclosures shall not be deemed to fall within the foregoing exclusions merely because individual features are published or available to the general public or in the rightful possession of the Receiving Party unless the combination itself and principle of operation are published or available to the general public or in the rightful possession of the Receiving Party. In addition, to the extent that any Confidential Information is disclosed pursuant to legal requirement in accordance with Section 7.1(a)(v), it shall remain otherwise subject to the confidentiality and non-use provisions of this Section 7.1.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 13 of 36
| Confidential | Execution Copy |
(b) Other Permitted Disclosures. Each Party shall have the limited right to disclose the other Party’s Confidential Information if and solely to the extent reasonably necessary (as reasonably determined based upon the advice of such Party’s legal counsel) to be disclosed (1) to Third Parties and their respective legal counsel with whom such Party is negotiating a permitted assignment under Section 12.10, (2) to potential and actual licensees/sublicensees and other collaborators (and their legal counsel) of the Compounds or Products, and/or (3) to accredited investors, qualified institutional buyers, and qualified purchasers and their legal counsel (as such terms are defined in the U.S. Securities Act of 1933 and/or the U.S. Securities Exchange Act of 1934, as amended). Prior to making any such disclosure under this Section 7.1(b), such Party shall ensure that the recipient is subject to written obligations of confidentiality and non-use that are no less restrictive than those set forth in this Agreement, and such Party will limit the content and timing of any such disclosure as much as reasonably possible. Such Party shall remain responsible for and liable hereunder with respect to any breach caused by any of the foregoing.
7.2 Publications. Zai Lab shall have the sole right to make a publication (including without limitation abstracts, papers, or verbal public presentations) related to the discovery, Development, Manufacture or Commercialization of Compounds and/or Products. In the event such publication may disclose any GSK’s Confidential Information, Zai Lab shall first deliver to GSK a copy of the proposed publication (or an outline in the case of a planned verbal presentation) at least [*] days prior to submission for publication or presentation. GSK shall have the rights (1) to request modifications to the publication or presentation for patent reasons, trade secret reasons or business reasons and/or (2) to request a reasonable delay in publication or presentation in order to protect patentable information. If GSK requests modifications to the publication or presentation, Zai Lab shall edit such publication to prevent disclosure of trade secret or proprietary business information identified by GSK prior to submission of the proposed publication or presentation. If GSK requests a delay, Zai Lab shall delay submission or presentation for a period of [*] days to enable patent applications protecting GSK’s rights in such information.
7.3 Disclosure of Agreement Terms. Promptly after the Effective Date, Zai Lab may issue a press release in the form attached hereto as Exhibit G. No other public disclosure of the non-public terms and conditions of this Agreement may be made by either Party, without the prior written consent of the other Party. However, each Party shall have the limited right to disclose the non-public terms and conditions of this Agreement to its Affiliates and/or if and solely to the extent reasonably necessary (as reasonably determined based upon the advice of such Party’s legal counsel) to be disclosed (1) to Third Parties and their respective legal counsel with whom such Party is negotiating a permitted assignment under Section 12.10, (2) to potential and actual licensees/sublicensees and other collaborators (and their legal counsel) of the Compounds or Products, and/or (3) to accredited investors, qualified institutional buyers, and qualified purchasers and their legal counsel (as such terms are defined in the U.S. Securities Act of 1933 and/or the U.S. Securities Exchange Act of 1934, as amended). Prior to making any such disclosure under this Section 7.3, such Party shall ensure that the recipient is subject to written obligations of confidentiality and non-use that are no less restrictive than those set forth in this Agreement, and such Party will limit the content and timing of any such disclosure as much as reasonably possible to avoid and/or minimize the disclosure of competitively sensitive information. However, nothing in this Section 7.3 shall prohibit a Party from making such disclosures if and to the extent reasonably required to comply with applicable federal or state securities laws or any rule or regulation of any nationally recognized securities exchange; provided that in such event, the disclosing Party shall notify and consult with the other Party prior to such required disclosure and shall diligently seek confidential treatment to the fullest extent available.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 14 of 36
| Confidential | Execution Copy |
8. Warranties; Limitations of Liability; Indemnification.
8.1 Representations and Warranties. Each Party hereby represents and warrants to the other Party as of the Effective Date that: (i) it is a limited liability company duly organized, validly existing, and in good standing under applicable laws; (ii) it has obtained all necessary consents, approvals and authorizations of all governmental authorities and other persons or entities required to be obtained by it in connection with this Agreement; (iii) the execution, delivery and performance of this Agreement have been duly authorized by all necessary corporate action on its part; and (iv) it has the legal right and power to enter into this Agreement, to extend the rights and licenses granted or to be granted to the other in this Agreement, and to fully perform its obligations hereunder.
8.2 Additional Representations and Warranties of GSK. GSK hereby represents and warrants to Zai Lab as of the Effective Date that, except as otherwise disclosed in writing by GSK on or before the Effective Date:
[*]
8.3 Disclaimers. Without limiting the respective rights and obligations of the Parties expressly set forth herein, each Party specifically disclaims any guarantee that any Products will be successful, in whole or in part. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, GSK MAKES NO REPRESENTATIONS AND EXTEND NO WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, WITH RESPECT TO ANY TRANSFERRED KNOW-HOW, LICENSED PATENTS, DEVELOPMENT IP, COMPOUNDS, PRODUCTS, PATENT RIGHTS OR KNOW-HOW, INCLUDING WARRANTIES OF VALIDITY OR ENFORCEABILITY OF ANY RIGHTS, TITLE, QUALITY, MERCHANTABILITY, FITNESS FOR A PARTICULAR USE OR PURPOSE, PERFORMANCE, AND NONINFRINGEMENT OF ANY THIRD PARTY PATENTS OR OTHER INTELLECTUAL PROPERTY RIGHTS.
8.4 No Consequential Damages. IN NO EVENT WILL EITHER PARTY HAVE ANY CLAIMS AGAINST OR LIABILITY TO THE OTHER PARTY WITH RESPECT TO ANY INDIRECT, PUNITIVE, SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES (INCLUDING ANY CLAIMS FOR LOST PROFITS OR REVENUES) ARISING UNDER OR IN CONNECTION WITH THIS AGREEMENT UNDER ANY THEORY OF LIABILITY, EVEN IF SUCH PARTY HAS BEEN INFORMED OR SHOULD HAVE KNOWN OF THE POSSIBILITY OF SUCH DAMAGES; PROVIDED THAT THE FOREGOING LIMITATION SHALL NOT APPLY WITH RESPECT TO INDEMNITY FOR THIRD PARTY CLAIMS AS PROVIDED IN SECTION 8.5
| 8.5 | Indemnification. |
(a) Indemnification by Zai Lab. Zai Lab will indemnify, defend and hold harmless GSK, its Affiliates, and their respective directors, officers, employees and agents (collectively, “GSK Indemnitees”) from and against any and all claims, demands, judgments, losses, damages, liabilities, costs and expenses (including reasonable attorneys’ fees and expenses) (collectively, “Liabilities”) arising out of or in connection with any and all Third Party claims relating to: (i) any gross negligence, willful misconduct or breach of this Agreement (including its representations and warranties made under this Agreement) by Zai Lab or any of its Affiliates or sublicensees; or (ii) the Development, Manufacture or Commercialization by Zai Lab or any of its Affiliates, licensees or sublicensees of any Compounds or Products, except to the extent such Liabilities are subject to indemnification by GSK under Section 8.5(b) below.
(b) Indemnification by GSK. GSK will indemnify, defend and hold harmless Zai Lab, its Affiliates, and their respective directors, officers, employees and agents (collectively, “Zai Lab Indemnitees”) from and against any and all Liabilities arising out of or in connection with any and all
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 15 of 36
| Confidential | Execution Copy |
Third Party claims relating to any gross negligence, willful misconduct or any breach of this Agreement (including its representations and warranties in material aspects made under this Agreement) by GSK or any of its Affiliates, except to the extent such Liabilities are subject to indemnification by Zai Lab under Section 8.5(a) above. For the avoidance of doubt: (i) except as expressly set forth in Section 8.2, GSK makes no representation or warranty of any kind with respect to non-infringement of any Third Party patent rights; and (ii) GSK has no obligation to indemnify, defend or hold harmless Zai Lab Indemnitees, Zai Lab’s licensees or sublicensees against any allegation that the Development, Manufacture, use, sale, offer for sale or import of any Compounds or Products infringes Third Party intellectual property rights, except in the case of GSK’ breach of the representations and warranties expressly set forth in Section 8.2.
(c) Procedures. In the event that any Party intends to claim indemnification under this Section 8.5 with respect to a Liability, it shall promptly notify the other Party in writing of any such alleged Liability. The indemnifying Party shall have the right to control the defense thereof with counsel of its choice; provided, however, that the indemnified Party shall have the right to retain its own counsel, (with the fees and expenses to be paid by the indemnifying Party), if representation by the counsel retained by the indemnifying Party would be inappropriate due to actual or potential differing interests between the Parties in such proceeding. The affected Indemnitees shall, upon request, cooperate reasonably with the indemnifying Party and its legal representatives in the investigation and defense of any action, claim or liability covered by this Section 8.5. Neither Party may settle any claim or action related to a Liability without the consent of the other Party, if such settlement would (i) impose any monetary obligation on the other Party (unless the indemnifying Party agreed to be solely responsible for such monetary obligation), (ii) constitute an admission of guilt or wrong-doing by the other Party, or (iii) require the other Party to submit to an injunction or otherwise limit the other Party’s rights under this Agreement. Any payment made by the indemnified Party to settle any such claim or action without the indemnifying Party’s consent shall be at indemnified Party’s own cost and expense.
8.6 Insurance. Zai Lab will maintain at its sole cost and expense, an adequate liability insurance or self-insurance program (including product liability insurance) to protect against potential liabilities and risk arising out of activities to be performed under this Agreement and any agreement related hereto and upon such terms (including coverages, deductible limits and self-insured retentions) as are customary in the U.S. pharmaceutical industry for the activities to be conducted by such Party under this Agreement. The coverage limits set forth herein will not create any limitation on a Party’s liability to the other under this Agreement.
9. Term and Termination.
9.1 Term. This Agreement will commence as of the Effective Date and, unless sooner terminated in accordance with the terms hereof, will continue in effect until the expiration of Zai Lab’s royalty obligations to GSK under Section 4.3 in all countries in the Territory (the “Term”). However, effective upon the expiration of Zai Lab’s royalty obligations to GSK with respect to a given Product in a given country or in the Territory, Zai Lab and its Affiliates, licensee and sublicensees shall have the right to continue to Commercialize the relevant Product in such country without further obligation to GSK.
9.2 Termination for Causes.
(a) Termination by GSK. GSK shall have the unilateral right to terminate this Agreement on [*] day’s prior written notice if Zai Lab: (i) fails to reach the milestones scheduled in the Development Plan unless for reasons beyond the reasonable control of Zai Lab such as the requirements of competent Regulatory Authority, (ii) fails to make any payment owed to GSK for more than [*] days, or (iii) fails to use Commercially Reasonable Efforts in the Development and Commercialization of Products as provided for in this Agreement and does not cure such failure within [*] days after GSK’s notification of such failure. In the event of a good faith dispute with respect to the basis of any termination under Section 9.2(a)(iii), the cure period shall be tolled until such time as the dispute is resolved pursuant to Section 12.1 and GSK shall only have the right to terminate this Agreement if the dispute is resolved in its favor.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 16 of 36
| Confidential | Execution Copy |
(b) Termination by Zai Lab. Zai Lab may not terminate this Agreement before the completion of [*] unless for causes beyond the reasonable control of Zai Lab. Subject to the completion of [*], Zai Lab shall have the right to terminate this Agreement on [*] day’s prior written consent.
9.3 Termination for Uncured Material Breach. In addition to the separate termination rights set forth in Sections 9.2(a) and 9.2(b), each Party shall have the unilateral right to terminate this Agreement at any time during its Term by providing written notice to that effect if the other Party is in material breach of one or more of its obligations hereunder and has not cured such breach within [*] days after the date of such notice. In the event of a good faith dispute with respect to the existence of a material breach covered by this section, the cure period shall be tolled until such time as the dispute is resolved pursuant to Section 12.1 and the Party seeking to terminate shall only have the right to do so if the dispute is resolved in such Party’s favor.
9.4 Effects of Termination. The rights and obligations of the Parties upon termination of this Agreement shall be governed by the terms and conditions set forth in this Section 9.4 and in Section 9.5.
(a) Termination by GSK for Zai Lab’s Breach or Termination for Causes. In the event of termination of this Agreement by GSK under Section 9.2(a) or Section 9.3 or termination of this Agreement by Zai Lab under Section 9.2(b):
(i) Except as may otherwise be agreed in writing by the Parties, Zai Lab will be responsible at its own expense for an orderly wind-down, in accordance with accepted pharmaceutical industry norms and ethical practices, of any then on-going clinical studies of the Products for which it has responsibility.
(ii) Zai Lab shall, upon GSK’s request, [*] and/or [*] and, if requested by GSK, [*] and/or [*] and [*] and/or [*]. Zai Lab shall also [*].
(iii) [*] the Compounds and/or Products that are [*] and [*] or [*].
(iv) Zai Lab will [*] or [*].
(v) Should Zai Lab or any of its Affiliates have any remaining inventory of Compound and/or Product, Zai Lab shall [*].
(vi) If the agreement is terminated by Zai Lab under Section 9.2 (b) and at the time if such termination notice Zai Lab has completed [*], and if [*], then [*] Zai Lab [*], it being understood that [*], and [*].
(b) Termination by Zai Lab for GSK’s Material Breach. In the event of termination of this Agreement by Zai Lab under Section 9.3:
(i) Zai Lab’s further payment obligation under Sections 4.2 and 4.3 shall continue in full force and effect but [*], provided however that in the event of a dispute between the Parties as to whether grounds for termination pursuant to Section 9.3 have arisen, [*] unless and until the dispute is resolved [*] in accordance with Section 12.1, following which [*] (and GSK shall [*]).
9.5 Survival. Except as otherwise set forth in Section 9.4, the following provisions (as well as any other provision which by its terms is clearly intended to survive termination or expiration of this Agreement) will survive termination or expiration of this Agreement: Sections [*]. Termination or expiration of this Agreement will not relieve the Parties of any liability or obligation which accrued hereunder prior to the effective date of such termination or expiration nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement nor prejudice either Party’s right to obtain performance of any obligation. All other rights and obligations will terminate upon termination or expiration of this Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 17 of 36
| Confidential | Execution Copy |
10. Prevention of Corruption
(a) Zai Lab acknowledges that it has received and read the ‘Prevention of Corruption – Third Party Guidelines’ (either in hard copy in Appendix or at ▇▇▇▇://▇▇▇.▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇-▇▇-▇▇▇▇▇▇▇▇▇▇-▇▇▇▇▇-▇▇▇▇▇-▇▇▇▇▇▇▇▇▇▇.▇▇▇) and agrees to perform its obligations under the Agreement in accordance with the principles set out therein.
(b) Zai Lab shall comply fully at all time with all applicable laws and regulations, including but not limited to applicable anti-corruption laws, of the territory in which Supplier conducts business with GSK.
(c) Zai Lab agrees that it has not, and covenants that it will not, in connection with the performance of the Agreement, directly or indirectly, make, promise, authorise, ratify or offer to make, or take any act in furtherance of any payment or transfer of anything of value for the purpose of influencing, inducing or rewarding any act, omission or decision to secure an improper advantage; or improperly assisting it or GSK in obtaining or retaining business, or in any way with the purpose or effect of public or commercial bribery.
(d) Zai Lab shall not contact, or otherwise meet knowingly meet with any Government Official for the purpose of discussing activities arising out of or in connection with the Agreement, without the prior written approval of GSK and, when requested by GSK, only in the presence of a GSK designated representative.
(e) For the purpose of the Agreement, “Government Official” means: (a) any officer or employee of a government or any department, agency or instrument of a government; (b) any person acting in an official capacity for or on behalf of a government or any department, agency, or instrument of a government; (c) any officer or employee of a company or business owned in whole or part by a government; (d) any officer or employee of a public international organisation such as the World Bank or United Nations; (e) any officer or employee of a political party or any person acting in an official capacity on behalf of a political party; and/or (f) any candidate for political office; who, when such Government Official is acting in an official capacity, or in an official decision making role, has responsibility for performing regulatory inspections, government authorisations or licenses, or otherwise has the capacity to take decisions with the potential to affect GSK business.
(f) Zai Lab represents that except as disclosed to GSK in writing prior to the commencement of the Agreement, it has not been convicted of or pleaded guilty to a criminal offence, including one involving fraud or corruption, that it is not now, to the best of its knowledge, the subject of any government investigation for such offenses, and that it is not now listed by any government agency as debarred, suspended, proposed for suspension or debarment, or otherwise ineligible for government programs.
(g) Zai Lab represents and warrants that except as disclosed to GSK in writing prior to the commencement of the Agreement: (1) it does not have any interest which directly or indirectly conflicts with its proper and ethical performance of the Agreement; and (2) it shall maintain arms length relations with all third parties with which it deals for or on behalf of GSK in performance of the Agreement.
(h) GSK shall have the right during the terms of this Agreement to conduct an investigation and audit of Zai Lab, its Affiliates licensees and sublicensee’s activities under this Agreement to monitor compliance with the terms of this Section 10. Zai Lab shall cooperate fully with such investigation or
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 18 of 36
| Confidential | Execution Copy |
audit, the scope, method, nature and duration of which shall be at the sole reasonable discretion of GSK but shall be during normal business hours and with two (2) week advance written notice, and shall not adversely affect Zai Lab or its Affiliates licensees and sublicensee’s normal business operation.
(i) Zai Lab shall ensure that all transactions under the Agreement are properly and accurately recorded in all material respects on its books and records and each document upon which entries such books and records are based is complete and accurate in all material respects. Supplier must maintain a system of internal accounting controls reasonably designed to ensure that it maintains no off-the-books accounts.
(j) Zai Lab agrees that in the event that GSK believes that there has been a possible violation of the terms of the Agreement, GSK may make full disclosure of such belief and related information at any time and for any reason to any competent government bodies and its agencies, and to whomsoever GSK determines in good faith has a legitimate need to know.
(k) GSK shall be entitled to terminate this Agreement immediately on written notice to Zai Lab, if Zai Lab fails to perform its obligations in accordance with this Section 10. Zai Lab shall have no claim against GSK for compensation for any loss of whatever nature by virtue of the termination of this Agreement in accordance with Section 9.2 and this Section 10. To the extent (and only to the extent) that the laws of the territory provide for any such compensation to be paid to Zai Lab upon the termination of this Agreement, Zai Lab hereby expressly agrees to waive (to the extent possible under the laws of the territory) or to repay to GSK any such compensation or indemnity resulting from termination of this Agreement under Section 9.2 and this Section 10.
11. Human Rights
(a) Zai Lab represents that, with respect to employment and conducting the activities under this Agreement, Institution will:
(i) not use child labor in circumstances that could cause physical or emotional impairment to the child;
(ii) not use forced labor (prison, indentured, bonded or otherwise);
(iii) provide a safe and healthy workplace; safe housing (if housing is provided by Zai Lab to its employees); and access to clean water, food, and emergency healthcare in the event of accidents in the workplace;
(iv) not discriminate against employees on any grounds (including race, religion, disability or gender);
(v) not use corporal punishment or cruel or abusive disciplinary practices;
(vi) pay at least the minimum wage and provide any legally mandated benefits;
(vii) comply with laws on working hours and employment rights;
(viii) respect employees’ right to join and form independent trade unions;
(ix) encourage subcontractors under this Master Agreement to comply with these standards;
(x) maintain a complaints process to address any breach of these standards.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 19 of 36
| Confidential | Execution Copy |
12. General Provisions.
12.1 Dispute Resolution. The Parties shall negotiate in good faith and use reasonable efforts to resolve or settle any dispute, controversy or claim arising from or related to this Agreement or the breach thereof. In the event that such dispute, controversy or claim is not resolved on an informal basis within twenty (20) days, any Party may, by written notice to the other, have such dispute referred to senior executives having decision-making authority on behalf of such Party, who shall attempt in good faith to resolve such dispute for a thirty (30) day period following receipt of such written notice. If the Parties do not fully settle by the foregoing process, and a Party then wishes to pursue the matter, each such dispute, controversy or claim that is not an Excluded Claim (as defined below) shall be finally resolved by binding arbitration administered by [*] in accordance with its arbitration rules and the procedures set forth in Exhibit H, attached hereto. Judgment on the arbitration award may be entered in any court having jurisdiction thereof. As used in this Section 12.1, the term “Excluded Claim” means a dispute, controversy or claim that concerns (i) the validity or infringement of a patent, trademark or copyright; or (ii) any antitrust, anti-monopoly or competition law or regulation, whether or not statutory.
12.2 Relationship of Parties. The relationship of the Parties hereto is that of independent contractors. Nothing in this Agreement is intended or will be deemed to constitute a partnership, agency, employer-employee or joint venture relationship between the Parties. No Party will incur any debts or make any commitments for the other, except to the extent, if at all, specifically provided therein. There are no express or implied third party beneficiaries hereunder.
12.3 Compliance with Law. Each Party will perform or cause to be performed any and all of its obligations or the exercise of any and all of its rights hereunder in good scientific manner and in compliance with all applicable law.
12.4 Governing Law. This Agreement and any dispute regarding the performance or breach hereof will be governed, interpreted and construed in accordance with the laws of [*], without respect to its conflict of laws rules.
12.5 Counterparts; Facsimiles. This Agreement may be executed in two or more counterparts, each of which will be deemed an original, and all of which together will be deemed to be one and the same instrument.
12.6 Headings. All headings in this Agreement are for convenience only and will not affect the meaning of any provision hereof.
12.7 Waiver of Rule of Construction. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement will be construed against the drafting party will not apply.
12.8 Interpretation. “Herein,” “hereby,” “hereunder,” “hereof” and other equivalent words refer to this Agreement as an entirety and not solely to the particular portion of this Agreement in which any such word is used. References to any Articles or Sections include Articles, Sections and subsections that are part of the related Article or Section (e.g., a section numbered “Section 2.1” would be part of “Article 2”, and references to “Section 2.1” would also refer to material contained in the subsection described as “Section 2.1(a)”).
12.9 Binding Effect. This Agreement will inure to the benefit of and be binding upon the Parties and their respective lawful successors and assigns.
12.10 Assignment. This Agreement may not be assigned by either Party, except as expressly permitted hereunder or otherwise without the prior written consent of the other Party, which consent will not be unreasonably withheld, delayed or conditioned; provided that, without any requirement for consent, (i) Zai Lab may assign this Agreement to an Affiliate or to its successor in connection with the merger,
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 20 of 36
| Confidential | Execution Copy |
consolidation, or sale of all or substantially all of its stock or assets, and (ii) GSK may assign this Agreement to an Affiliate or to its successor in connection with the merger, consolidation, or sale of all or substantially all of its stock or assets to which this Agreement relates.
12.11 Notices. All notices, requests, demands and other communications required or permitted to be given pursuant to this Agreement will be in writing and will be deemed to have been duly given upon the date of receipt if delivered by hand, recognized international overnight courier, confirmed facsimile transmission, or registered or certified mail, return receipt requested, postage prepaid to the following addresses or facsimile numbers:
| If to GSK: | GlaxoSmithKline (China) R&D Company Limited Building 3, 898 Halei Road, Zhangjiang High-Tech Park, Shanghai 201203, China Attention: Director, Business Development | |
| With a copy to: | ▇▇▇ ▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Attention: BDTT Legal | |
| If to Zai Lab: | Zai Lab (Shanghai) Co., Ltd. ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇, Zhangjiang High-Tech Park, Shanghai 201203, China Attention: Samantha Du, CEO | |
Either Party may change its designated address and facsimile number by notice to the other Party in the manner provided in this Section 12.11.
12.12 Amendment and Waiver. This Agreement may be amended or modified only by means of a written instrument signed by both Parties. The waiver by either Party hereto of any right hereunder, or of any failure of the other Party to perform, or of any breach by the other Party, shall only be effective if expressly made in writing. Any waiver of any rights or failure to act in a specific instance will relate only to such instance and will not be construed as an agreement to waive any rights or fail to act in any other instance, whether or not similar.
12.13 Severability. In the event that any provision of this Agreement will, for any reason, be held to be invalid or unenforceable in any respect, such invalidity or unenforceability will not affect any other provision hereof, and the Parties will negotiate in good faith to modify this Agreement to preserve (to the extent possible) their original intent.
12.14 Entire Agreement. This Agreement is the sole agreement with respect to the subject matter and supersedes all other agreements and understandings between the Parties with respect to the same subject matter. The Exhibits to this Agreement are expressly incorporated herein by reference and shall be deemed a part of this Agreement.
12.15 Force Majeure. Failure of any Party to perform its obligations under this Agreement (except the obligation to make payments when properly due) shall not subject such Party to any liability or place them in breach of any term or condition of this Agreement to the other Party to the extent (and only to the extent) that such failure is due to fire, explosion, flood, drought, war, terrorism, riot, sabotage, embargo, strikes or other labor trouble, failure of suppliers, a national health emergency, compliance with any order or regulation of any government entity acting with color of right, or any other cause beyond the reasonable control of such non-performing Party and which is not caused by the negligence, intentional conduct or misconduct of the non-performing Party (each such event or cause referred to as “force majeure”). The Party affected shall
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 21 of 36
| Confidential | Execution Copy |
promptly notify the other Party of the condition constituting force majeure as defined herein and shall exert reasonable diligent efforts to eliminate, cure or overcome any such event of force majeure and to resume performance of its obligations with all possible speed. If a condition constituting force majeure as defined herein exists for more than ninety (90) consecutive days, the Parties shall meet to negotiate a mutually satisfactory resolution to the problem, if practicable. The foregoing notwithstanding, nothing herein shall require any Party to settle on terms unsatisfactory to such Party any strike, lock-out or other labor difficulty, any investigation or proceeding by any public authority or any litigation by any Third Party.
12.16 Further Actions. Each Party hereby agrees to execute, acknowledge and deliver such further instruments, and to do all other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement including, without limitation, any filings with any government antitrust agency which may be required.
{Remainder of this Page Intentionally Left Blank}
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 22 of 36
| Confidential | Execution Copy |
IN WITNESS WHEREOF, the Parties have caused this License and Transfer Agreement to be executed by their respective duly authorized officers as of the Effective Date.
GlaxoSmithKline (China) R&D Co., Ltd
| By: | /s/ ▇▇▇ ▇▇ |
(Signature)
Name: ▇▇▇ ▇▇
Title: SVP, Global Head of Neuroscience TAU and GM of R&D China
Date: October 18, 2016
Zai Lab (Shanghai) Co., Ltd.
| By: | /s/ ▇▇▇▇ ▇▇ |
(Signature)
Name: ▇▇▇▇ ▇▇
Title: CEO
Date: Oct 18, 2016
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 23 of 36
| Confidential | Execution Copy |
Exhibit A
Development Plan
| Task |
Estimated Start Date |
Estimated End Date | ||||
| FUGAN: |
||||||
| [*] | [*] | [*] | ||||
| GRAPE: |
||||||
| [*] | [*] | [*] | ||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 24 of 36
| Confidential | Execution Copy |
Exhibit B
Structure of Compounds:
FUGAN:
The formulation comprising extracts from two traditional Chinese herbs, [*]
GRAPE:
the formulation comprising extracts from two traditional Chinese herbs, [*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 25 of 36
| Confidential | Execution Copy |
Exhibit C
Transferred Material, Data, Files and Documents
[*] (2 pages omitted)
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 26 of 36
| Confidential | Execution Copy |
Exhibit D
Licensed Patents
| Patent Number |
Patent title |
Filing Date |
Grant Date |
Country | ||||
| [*] |
[*] | [*] | [*] | [*] |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 27 of 36
| Confidential | Execution Copy |
Exhibit E
Manner of the Payment
GSK will provide Zai Lab an invoice for each Payment and Royalty. The Payments and Royalties will be paid by wire transfer to GSK’s account provided herein.
Wire transfer from Chinese external party:
[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 28 of 36
| Confidential | Execution Copy |
Exhibit F
Form of Assignment and Assumption Agreement
ASSIGNMENT AND ASSUMPTION AGREEMENT

THIS ASSIGNMENT AND ASSUMPTION AGREEMENT (“Assignment Agreement”) is made and entered into as of [date], 2016 by and among GlaxoSmithKline (China) R&D Co., Ltd, whose registered office is at ▇▇▇▇▇▇▇▇ ▇, ▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇-▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇ New Area, Shanghai, PRC (“Former Licensee”), and [Chengdu Bater Pharmaceutical Co., Ltd./ Traditional Chinese Medical Hospital, Xinjiang Medical University], which has its registered office at [ ] (“Licensor”), and Zai Lab (Shanghai) Co., Ltd., whose registered office is at 1043 Halei Road, ▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇-▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇ New Area, Shanghai 201203, PRC (“New Licensee”).

RECITALS

WHEREAS, Former Licensee and Licensor are the parties to the [License and Assignment Agreement/Development and License Agreement] attached hereto as Exhibit I, which dated [July 15, 2013/ September 25, 2014] (“Existing Agreement”),

WHEREAS, Former Licensee wishes to assign and transfer, and New Licensee wishes to accept and assume, all of Former Licensee’s rights and obligations, respectively, under the Existing Agreement,
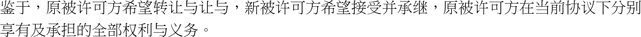
WHEREAS, Former Licensee and New Licensee have executed the License and Transfer Agreement (“License and Transfer Agreement”) on the same date hereof.
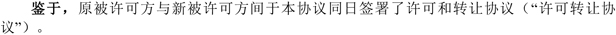
WHEREAS, Licensor has agreed to consent to the assignment according to the terms set forth herein,

[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 29 of 36
| Confidential | Execution Copy |
NOW THEREFORE, in consideration of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, the parties hereby agree as follows,

| 1. | Assignment. Former Licensee hereby conveys, assigns and transfers to New Licensee all its rights, title, interest and any and all liabilities and obligations in and to the Existing Agreement, and New Licensee hereby accepts and assumes the assignment of Former Licensee’s right, title, interest, and any and all liabilities and obligations of Former Licensee under the Existing Agreements, and shall be bound by all of the terms of the Existing Agreements in Former Licensee’s place and stead in every way as if New Licensee were a party to the Existing Agreements in lieu of Former Licensee (“Assignment”). |

| 2. | Consent of Licensor. Licensor hereby consents to the Assignment, and, with effect from the Effective Date, Licensor also undertakes to perform the Existing Agreement and to be bound by its terms in every way as if New Licensee were a party to the Existing Agreement in lieu of Former Licensee. |

| 3. | Change of Obligations and Notice. |

| 3.1 | Licensor hereby agrees and acknowledges that: |

| (i) | as of the date hereof, Former Licensee has made the following payments to Licensor under the Existing Agreement in an aggregate amount of [*], including the settled payments as listed below, and any other payables by Former Licensee under the Existing Agreement shall be paid by New Licensee upon the Effective Date; |

[Settled Payments to Licensor for FUGAN]
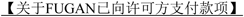
[*]
[Outstanding payments to be made upon achievement of milestone events for FUGAN:]

[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 30 of 36
| Confidential | Execution Copy |
[Settled Payments to Licensor for GRAPE]
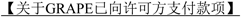
[*]
| (ii) | as of the date hereof, Former Licensee is in compliance with all of the terms and conditions under the Existing Agreement and no default by Former Licensee under the Existing Agreement has occurred or is continuing; |

| (iii) | unless otherwise provided herein, all the terms and conditions of the Existing Agreement and any exhibits or schedules thereof are in full force and effect and is enforceable in accordance with its terms. |

| 3.2 | Licensor hereby releases, acquits and forever discharges Former Licensee from and of each covenant and condition of, and each liability or other obligation arising under, the Existing Agreement to be observed or performed by Former Licensee pursuant to the terms thereof and Former Licensee shall no longer be bound by, or have any obligation or liability in respect of, the Existing Agreement. [*][*] |

| 3.3 | Any notice or other communication between New Licensee and Licensor required or permitted hereunder under the Existing Agreement or any other documents in connection herewith shall be directed as follows: |

If to New Licensee:
Attn: Samantha Du, CEO

Address: 1043 Halei Road, ▇▇▇▇ ▇, ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇-▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇

[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 31 of 36
| Confidential | Execution Copy |
If to Licensor:
Attn:
Address:

| 4. | Continued Effectiveness. This Assignment Agreement shall take effect from the date that New Licensee fulfils its payment obligations as per section 2.2 of the License and Transfer Agreement (“Effective Date”). Except as otherwise provided herein, all terms and conditions of the Existing Agreements shall remain in effect and unchanged. |

| 5. | Governing Law. This Assignment Agreement shall be governed by and construed in accordance with the laws of the People’s Republic of China. |

| 6. | Dispute Resolution. Any claim, controversy or dispute among the parties hereto arising out of, relating to, or in connection with this Assignment Agreement, including the interpretation, validity, termination or breach hereof, that cannot be settled amicably, shall be resolved in accordance with the dispute resolution provisions set forth in the Existing Agreement. |

| 7. | Counterparts. This Assignment Agreement may be executed in [three/five] counterparts each of which shall be deemed an original and all of which shall be deemed one and the same instrument. |

[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 32 of 36
| Confidential | Execution Copy |
IN WITNESS WHEREOF, the parties have caused this Assignment Agreement to be duly executed under seal on the date first written above.
GlaxoSmithKline (China) R&D Co., Ltd

By/
 :
: |
|
| (Signature) | ||
Name/
 :
: |
| |
Title/
 :
: |
| |
Date/
 :
: |
| |
| Zai Lab (Shanghai) Co., Ltd. | ||

| ||
By/
 :
: |
| |
| (Signature) | ||
Name/
 :
: |
| |
Title/
 :
: |
| |
Date/
 :
: |
| |
| [LICENSOR NAME] | ||
| ||
By/
 :
: |
| |
| (Signature) | ||
Name/
 :
: |
| |
Title/
 :
: |
| |
Date/
 :
: |
| |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 33 of 36
| Confidential | Execution Copy |
Exhibit G
Press Release

ZAI Lab announces a global agreement with GlaxoSmithKline in two anti-inflammatory assets
Shanghai, China – 18 October 2016 – Zai Lab Ltd. announces today a global agreement with GlaxoSmithKline (GSK) to develop and commercialize one phase 2 clinical and one pre-clinical anti-inflammatory assets. Both assets are products targeting multiple anti-inflammatory indications.
Specific details have not been released, but this agreement gives Zai Lab’s veteran team exclusive rights to lead future development of the products through clinical development, regulatory activities, and commercialization globally.
About Zai Lab
ZAI Lab is a leading biotech company based in China focused on discovering and developing innovative medicines for unmet medical needs globally. The company is building a strong portfolio of therapeutic programs aimed at transforming patients’ lives. Zai Lab has a world class leadership team with deep experience at global pharmaceutical and biotech organizations. The team has a strong track record of success – successfully taken five novel drug candidates into clinical trials in China, pioneered new regulatory channels, secured regulatory approvals in record times, conducted multiple IND trials in the US, and brought the first China discovered drug into Global Phase III trials. Zai Lab is committed to build a globally leading drug research and development powerhouse with a culture of excellence and teamwork and a strong focus on fostering innovation and creativity. For more information, please visit ▇▇▇.▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 34 of 36
| Confidential | Execution Copy |
Exhibit H
Arbitration Proceedings
| 1. | The arbitration shall be conducted by a panel of three (3) persons experienced in the pharmaceutical business. Within thirty (30) days after initiation of an arbitration, each Party shall select one person to act as arbitrator and the two Party-selected arbitrators shall select a third arbitrator within thirty (30) days of their appointment. If the arbitrators selected by the Parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be appointed by the [*]. The place of arbitration shall be [*], and all proceedings and communications shall be in English. |
| 2. | Either Party may apply to the arbitrators for interim injunctive relief until the arbitration award is rendered or the controversy is otherwise resolved. Either Party also may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any injunctive or provisional relief necessary to protect the rights or property of that Party pending the arbitration award. The arbitrators shall have no authority to award any damages excluded by Section 8.4. Each Party shall bear its own costs and expenses and attorneys’ fees and an equal share of the arbitrators’ fees and any administrative fees of arbitration. |
| 3. | Except to the extent necessary to confirm an award or as may be required by law, neither a Party nor an arbitrator may disclose the existence, content, or results of an arbitration without the prior written consent of both Parties. In no event shall an arbitration be initiated after the date when commencement of a legal or equitable proceeding based on the dispute, controversy or claim would be barred by the applicable [*] statute of limitations. |
| 4. | The Parties agree that, in the event of a good faith dispute over the nature or quality of performance under this Agreement, neither Party may unilaterally terminate this Agreement until final resolution of the dispute through arbitration or other judicial determination. The Parties further agree that any payments made pursuant to this Agreement pending resolution of the dispute shall be refunded if an arbitrator or court determines that such payments are not due. |
| 5. | During the pendency of any arbitration the Parties shall continue to perform their respective obligations under this Agreement. To the extent that such performance involves any matter which is the subject of the dispute, claim or controversy being arbitrated, the Parties shall continue performance of such matter under this Agreement in such a manner as to the fullest extent possible maintain the status quo of the Parties with respect to the disputed matter. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 35 of 36
| Confidential | Execution Copy |
Exhibit I
Prevention of Corruption – Third Party Guidelines
The GSK Anti-Bribery and Corruption Policy (POL-GSK-007) requires compliance with the highest ethical standards and all anti-corruption laws applicable in the countries in which GSK (whether through a third party or otherwise) conducts business. POL-GSK-007 requires all GSK employees and any third party acting for or on behalf of GSK to ensure that all dealings with third parties, both in the private and government sectors, are carried out in compliance with all relevant laws and regulations and with the standards of integrity required for all GSK business. GSK values integrity and transparency and has zero tolerance for corrupt activities of any kind, whether committed by GSK employees, officers, or third-parties acting for or on behalf of the GSK.
Corrupt Payments – GSK employees and any third party acting for or on behalf of GSK, shall not, directly or indirectly, promise, authorise, ratify or offer to make or make any “payments” of “anything of value” (as defined in the glossary section) to any individual (or at the request of any individual) including a “government official” (as defined in the glossary section) for the improper purpose of influencing or inducing or as a reward for any act, omission or decision to secure an improper advantage or to improperly assist the company in obtaining or retaining business.
Government Officials – Although GSK´s policy prohibits payments by GSK or third parties acting for or on its behalf to any individual, private or public, as a “quid pro quo” for business, due to the existence of specific anticorruption laws in the countries where we operate, this policy is particularly applicable to “payments” of “anything of value” (as defined in the glossary section), or at the request of, “government officials” (as defined in the glossary section).
Facilitating Payments – For the avoidance of doubt, facilitating payments (otherwise known as “greasing payments” and defined as payments to an individual to secure or expedite the performance of a routine government action by government officials) are no exception to the general rule and therefore prohibited.
GLOSSARY
The terms defined herein should be construed broadly to give effect to the letter and spirit of the ABAC Policy. GSK is committed to the highest ethical standards of business dealings and any acts that create the appearance of promising, offering, giving or authorising payments prohibited by this policy will not be tolerated.
Anything of Value: this term includes cash or cash equivalents, gifts, services, employment offers, loans, travel expenses, entertainment, political contributions, charitable donations, subsidies, per diem payments, sponsorships, honoraria or provision of any other asset, even if nominal in value.
Payments: this term refers to and includes any direct or indirect offers to pay, promises to pay, authorisations of or payments of anything of value.
Government Official shall mean:
| • | Any officer or employee of a government or any department, agency or instrument of a government; |
| • | Any person acting in an official capacity for or on behalf of a government or any department, agency, or |
| • | instrument of a government; |
| • | Any officer or employee of a company or business owned in whole or part by a government; |
| • | Any officer or employee of a public international organisation such as the World Bank or United Nations; |
| • | Any officer or employee of a political party or any person acting in an official capacity on behalf of a |
| • | political party; and/or |
| • | Any candidate for political office |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 36 of 36

