DEBT SETTLEMENT AGREEMENT dated as of July 18, 2011 (this “Agreement”) among BIRMINGHAM ASSOCIATES LTD. a Cayman Islands company (“Lender”) and MEDICURE INTERNATIONAL INC. a corporation organized under the laws of Barbados (“Medicure-Barbados”) and...
DEBT SETTLEMENT AGREEMENT
dated as of July
18, 2011
(this “Agreement”) among
BIRMINGHAM ASSOCIATES LTD.
a Cayman Islands
company
(“Lender”)
and
MEDICURE INTERNATIONAL INC.
a corporation
organized under the laws of Barbados
(“Medicure-Barbados”)
and
MEDICURE PHARMA INC.
a corporation
organized under the laws of Delaware
(“Medicure-Pharma”)
and
MEDICURE INC.
a corporation organized under
the laws of Canada
(“Medicure-Manitoba”)
Medicure-Barbados, Medicure-Pharma and Medicure-Manitoba are collectively referred to herein as the “Medicure Group”. Each of the Lender and the Medicure Group is referred to herein as a “Party” and are together referred to herein as the “Parties”. Capitalized terms used herein but not otherwise defined herein shall have the meaning ascribed to such terms in the Debt Financing Agreement between the Parties, dated as of September 17, 2007 (the “Debt Financing Agreement”).
| A. |
Medicure-Barbados and Medicure-Pharma are wholly-owned subsidiaries of Medicure-Manitoba. | |
| B. |
Pursuant to the Debt Financing Agreement, among other things: | |
| (i) |
the Lender loaned to Medicure-Barbados the Principal Amount of twenty- five million US dollars (US$25,000,000) in cash; | |
| (ii) |
Medicure-Barbados covenanted and agreed to make certain Debt Payments to the Lender and granted Payment Rights to the Lender in respect thereof; | |
| (iii) |
the Medicure Group granted to the Lender certain rights including Payment Collateral Liens, to secure the Debt Payments and other obligations of the Medicure Group to the Lender, in respect of which the Lender was provided with specific security documentation including the security documentation described in the Debt Financing Agreement (the “Lender Security”) and made certain filings, recordations and registrations in respect of the Lender Security (the “Lender Registrations”), including without limitation, those filings, recordations and registrations described in Schedule “A” hereto; | |
| (iv) |
Medicure-Manitoba became a Guarantor as to any Medicure Obligations of Medicure-Barbados and Medicure-Pharma; and | |
| (v) |
Medicure-Manitoba made and delivered to the Lender a certain warrant to purchase Medicure Manitoba Common Shares dated September 17, 2007. | |
| C. |
Medicure-Barbados has not made certain required Debt Payments, under the Debt Financing Agreement, totalling as of the Closing Date the sum of USD $7,839,659 (for total outstanding indebtedness as at the date of this Agreement of USD $32,839,659) due on various payment dates beginning from and after July 15, 2009, and the Medicure Group is presently in default under the Debt Financing Agreement and the Lender Security documents, which default is continuing and pursuant to which the Lender, is entitled to exercise secured creditor and other rights available to it under the Debt Financing Agreement, the Lender Security and other documents. The full amount of the indebtedness to the Lender from time to time, including the Principal Amount, Debt Payments and all other payment and financial obligations of the Medicure Group to the Lender under or arising from the Debt Financing Agreement and related documents, is hereinafter referred to as the “Birmingham Debt”). | |
2
| D. |
The Government of Manitoba has approved a loan (the “Manitoba Loan”) to Medicure-Manitoba under the Manitoba Industrial Opportunities Program, to be provided by the Manitoba Development Corporation (“MDC”) to a maximum amount of five million Canadian dollars (CDN$5,000,000) to be used exclusively to fund the settlement, pay-out and discharge of the Birmingham Debt on a discounted basis and certain professional costs relating thereto. |
| E. |
The Parties wish to settle and discharge the Birmingham Debt and terminate the Debt Financing Agreement on the terms and conditions set out below in this Agreement. |
1.1 For the purposes of this Agreement, the following terms shall have the meanings ascribed to them below.
“Closing” means the completion of the Birmingham Debt settlement transactions contemplated by this Agreement;
“Closing Date” means July 18, 2011, or such other date as the Parties may agree to in writing;
“Discharges” means any and all discharges, authorizations and termination statements required to discharge in full the Lender Registrations.
“Fiscal Quarter” has the meaning ascribed to it in the Royalty and Guarantee Agreement;
“Follow-Along Period” means that period of time beginning on the day immediately following the Closing Date and ending on November 30, 2011;
“Follow-Along Securities” means securities of Medicure-Manitoba, in the aggregate maximum of 10,430,000 Medicure Manitoba Common Shares, to be duly authorized for issuance or grant (including but not limited to any securities to be issued or granted by Medicure-Manitoba that require shareholder approval) to be issued or granted to certain directors, management, consultants and employees of Medicure-Manitoba in the Follow-Along Period, comprising:
| (i) |
a maximum of 10,430,000 Medicure Manitoba Common Shares; or | |
| (ii) |
an option or options to purchase a maximum of 10,430,000 Medicure Manitoba Common Shares; or |
3
| (iii) |
a warrant or warrants to purchase a maximum of 10,430,000 Medicure Manitoba Common Shares; or | |
| (iv) |
any combination of the foregoing providing for an aggregate maximum of 10,430,000 Medicure Manitoba Common Shares. |
“Guarantee” means the guarantee of Medicure-Manitoba and Medicure-Pharma of the royalty payment obligations as described in the Royalty and Guarantee Agreement;
“Medicure-Manitoba Common Shares” means common shares of Medicure-Manitoba without voting restrictions, being of the same class and having the same rights as Medicure-Manitoba common shares traded as at the Closing Date on the NEX Board of the TSX Venture Exchange;
“Medicure-Manitoba Equity” means all of the issued and outstanding Medicure-Manitoba Common Shares and all other shares of Medicure-Manitoba of any class or series and all rights to acquire any of the foregoing, however arising, as at the Closing Date, together with the Follow-Along Securities, all on a fully diluted basis, including without limitation all options, warrants, contracts, arrangements (whether in writing or otherwise) and other rights to acquire shares, options or warrants, whenever exercisable;
“Net Sales” has the meaning ascribed to such term in the Royalty and Guarantee Agreement;
“NEX Approval” means the approval of the NEX Board of the TSX Venture Exchange to (i) the transactions and agreements contemplated by this Agreement, (ii) the transactions and agreements contemplated by or in relation to the Manitoba Loan, including the issuance of certain Medicure-Manitoba Common Shares and other consideration to Xx. Xxxxxx Xxxxxxx in consideration of the provision of guarantees required by the terms of the Manitoba Loan, and (iii) certain options to purchase Medicure-Manitoba Common Shares granted to directors, management, consultants and employees of Medicure-Manitoba;
“Royalty and Guarantee Agreement” means the agreement described in subparagraph 3.1(d) in the form attached to this Agreement as Schedule “B” hereto;
“Settlement Amount” means the amount to be paid by Medicure-Barbados to the Lender on or before the Closing Date pursuant to subparagraph 3.1(a) of this Agreement;
“Settlement Shares” means the Medicure-Manitoba Common Shares described in subparagraph 3.1(b) of this Agreement;
“Switch Option” has the meaning ascribed to such term in the Royalty and Guarantee Agreement;
4
“Transaction Documents” means this Agreement and the items and documents described in paragraph 5.1 of this Agreement; and
“Treasury Direction” means the irrevocable direction of Medicure–Manitoba to its transfer agent to deliver the Settlement Shares to the Lender (or as the Lender may direct in writing to Medicure-Manitoba not less than one (1) business day prior to the Closing Date) by electronic deposit through CDSX.
2.1 Subject to the terms and conditions of this Agreement, the Lender hereby agrees to accept the consideration described in paragraph 3.1 hereof in full and final settlement of the Birmingham Debt and each and every other related indebtedness, liability and obligation of any kind whatsoever (including without limitation obligations to make Debt Payments) (the “Medicure Obligations”) of the Medicure Group to the Lender or otherwise described in, under, related to or arising in any way from the Debt Financing Agreement, the Lender Security, and related documents and instruments, and to fully and finally release and discharge the Medicure Group from the Medicure Obligations pursuant to Section 2.2 hereof.
2.2 Each of the Parties acknowledges and agrees that this Debt Settlement Agreement and the consideration set out herein constitutes a full and final settlement of the Birmingham Debt, any and all other obligations relating thereto howsoever arising and all matters in dispute between the Parties in respect thereof and, upon:
| (i) |
receipt by the Lender (or as the Lender may direct in writing to Medicure- Manitoba not less than one (1) business day prior to the Closing Date) of the Settlement Amount; |
| (ii) |
receipt by the Lender (or as the Lender may direct in writing to Medicure- Manitoba not less than one (1) business day prior to the Closing Date) of the Settlement Shares; |
| (iii) |
execution and delivery by the Medicure Group of the Royalty and Guarantee Agreement; and |
| (v) |
execution and delivery by Birmingham of the Discharges; |
5
the Lender on the one hand and each member of the Medicure Group on the other hand hereby fully and finally release and discharge each other and each other’s respective affiliates, subsidiaries, shareholders, directors, officers, employees, agents, legal counsel and representatives from any right, manner of action, cause of action, suit, dues, sum of money, damages, demand, counterclaim, debt, account, covenant, judgment, expense, execution, charge and other recovery, claim for accounting, reconciliation, contribution or indemnity, restitution or otherwise, or other claim, whether at law or in equity and whether arising by reason of the commission of a tort (intentional or unintentional), any breach of contract or other agreement (oral or written), any breach of duty (including, without limitation, any legal, statutory, equitable or fiduciary duty) or otherwise, and whether by guarantee, surety or otherwise, existing or hereafter arising and whether or not any right or claim is executory or anticipatory in nature including, without limitation, any right or ability of any party to advance a claim for contribution or indemnity or otherwise with respect to any matter, action, cause or chose in action whether existing at present or commenced in the future, of the Lender or the Medicure Group or any member thereof, as applicable, that may be asserted or made in whole or in part against the Lender or any member of the Medicure Group, as applicable, whether or not asserted or made, in connection with any indebtedness, liability, obligation, demand or cause of action of whatever nature, or kind whatsoever, and any interest accrued thereon or costs payable in respect thereof, which Birmingham, on the one hand, or the Medicure Group or any member of the Medicure Group, on the other hand, may be entitled to assert against each other, of any nature or kind whatsoever or howsoever arising based in whole or in part on facts which existed prior to Closing, under or in connection with or in respect of or related in any manner whatsoever to or based in whole or in part on the Debt Financing Agreement, the Lender Security and all other agreements, obligations, documents and instruments of whatsoever nature and kind ancillary or made pursuant to the Debt Financing Agreement; provided that nothing herein shall release or discharge any Party from any liability, obligation, representation, warranty, covenant, claim or cause of action arising under or in connection with or with respect to this Debt Settlement Agreement, the Royalty and Guarantee Agreement or any other agreements, documents or instruments executed or provided in connection therewith; and provided further that any shares, options, warrants or other equity securities of any of the Medicure Group held by Birmingham or its affiliates pursuant to the Securities Purchase Agreement and Warrant dated September 17, 2007 or otherwise shall not be released or cancelled by this Agreement and Birmingham shall be entitled to keep and exercise its rights in respect of such shares, options, warrants or other equity securities in accordance with the terms thereof.
Consideration to the Lender
3.1 Subject to the terms and conditions of this Agreement, the Lender shall receive the following in full payment and satisfaction of the outstanding Birmingham Debt and full and final settlement and discharge of all the Medicure Obligations:
| (a) |
From the proceeds of the Manitoba Loan, Medicure-Manitoba shall enable, by means of a share subscription, and cause Medicure-Barbados to pay to the Lender (or as the Lender may direct in writing to Medicure-Manitoba not less than one (1) business day prior to the Closing Date) by means of wire transfer at Closing an amount equal to five million Canadian dollars (CDN$5,000,000) minus only the lesser of (i) the amounts of reasonable professional costs of the Province of Manitoba/MDC, the Medicure Group and Xx. Xxxxxx Xxxxxxx related to the Manitoba Loan and the Closing of the transaction contemplated by this Agreement, and (ii) two hundred fifty thousand Canadian dollars (CDN$250,000) in the aggregate; |
6
| (b) |
Medicure-Manitoba shall issue to the Lender (or as the Lender may direct in writing to Medicure-Manitoba not less than one (1) business day prior to the Closing Date) at Closing the Settlement Shares consisting of 32,640,043 Medicure-Manitoba Common Shares, which shall be at Closing equal to and represent fifteen percent (15%) of the aggregate of all of the Medicure-Manitoba Equity on a fully diluted basis as at Closing; |
| (c) |
Medicure-Manitoba agrees to use its best efforts to have the Medicure-Manitoba Common Shares listed for trading, at Medicure-Manitoba’s cost, on the Toronto Stock Exchange (TSX) or the TSX Venture Exchange (other than the NEX board) as soon as possible after Closing; and |
| (d) |
The Medicure Group shall execute and deliver to the Lender at Closing the Royalty and Guarantee Agreement, pursuant to which, inter alia, Medicure- Barbados shall pay to the Lender and the Lender shall be entitled to receive, commencing upon the Closing, on or before the 60th calendar day following the end of each Fiscal Quarter in which there occur Net Sales of AGGRASTAT, payments equal to the percentages of Net Sales of AGGRASTAT in the immediately preceding Fiscal Quarter, as set forth in the Royalty and Guarantee Agreement. |
| 3.2 |
The consideration set out in Section 3.1 above shall be in addition to any shares, options, warrants or other securities of any of the Medicure Group companies held by the Lender prior to the Closing Date pursuant to the Securities Purchase Agreement and Warrant dated September 17, 2007, or otherwise which shall be unaffected by this Agreement and which the Lender shall be entitled to keep and exercise in accordance with the terms thereof. |
| 3.3 |
The Lender may direct that any or all of the consideration set out in Section 3.1 above be paid by the Medicure Group to an affiliate or subsidiary of the Lender, or any other entity as directed by the Lender by providing not less than one (1) business day written notice to the Medicure Group. |
| 3.4 |
All payments and distributions to the Lender under or in connection with this Agreement shall be applied first to reduce the principal amount of the Birmingham Debt and second, to the extent that sufficient excess funds are received by the Lender after full repayment of the principal amount, such excess funds shall be applied to reduce any interest or other amounts due on the Birmingham Debt; provided that, for greater certainty, the Birmingham Debt shall be settled and released in accordance with section 2.2 hereof. |
| 3.5 |
Except as required by applicable law, the payment to the Lender of the Settlement Amount shall be made free and clear of and without withholding or deduction for any taxes; provided that if any person shall be required by law to withhold or deduct any taxes from such payments, then (i) such person shall make such withholdings or deductions, (ii) such person shall pay the full amount withheld or deducted to the relevant governmental authority in accordance with applicable law, and (iii) the amount payable by such person shall be increased as necessary so that, after all required withholdings and deductions for taxes have been made, the Lender receives the amount it would have received had no such withholdings or deductions been made. |
7
Representations and Warranties
4.1 Each of Medicure-Manitoba, Medicure-Pharma and Medicure-Barbados, as the case may be, make to the Lender the following representations and warranties and acknowledge that the Lender is relying upon such representations and warranties in connection with the execution of this Agreement and the consummation of the transactions contemplated by this Agreement:
| (a) |
each member of the Medicure Group is a corporation validly existing and in good standing under the laws of the jurisdiction set out on the first page of this Agreement; |
| (b) |
each member of the Medicure Group has entered into this Agreement in good faith, and at arm’s length with the Lender; |
| (c) |
each member of the Medicure Group has all necessary approvals of its directors and shareholders and all requisite power and authority to execute and deliver the Agreement, to perform all of its obligations hereunder, and to undertake all actions required of such member of the Medicure Group hereunder; |
| (d) |
this Agreement has been duly and validly authorized, executed and delivered by, and constitutes a legal, valid, binding and enforceable obligation of each member of the Medicure Group; |
| (e) |
except for the approval of the NEX Board of the TSX Venture Exchange, the execution, delivery and performance by each member of the Medicure Group of this Agreement and the completion of the transactions (including the issuance of the Settlement Shares by Medicure-Manitoba) contemplated hereby do not and will not result in a violation of, or require any consent, approval or other authorization under, any law, regulation, order or ruling, including securities laws, applicable to any member of the Medicure Group, and do not and will not constitute a breach of, default under, or require any consent, approval or other authorization under, any member of the Medicure Group’s constating documents, by-laws or resolutions or any agreement to which any member of the Medicure Group is a party or by which it is bound; |
8
| (f) |
the SEDAR database contains in a publicly available format, complete and correct copies of all reports, schedules, forms, statements and other documents filed with or furnished to the Canadian Securities Administrators (the “CSA”) by Medicure-Manitoba since January 1, 2008 (together with all exhibits and schedules thereto and documents and other information incorporated therein by reference, the “CSA Documents”). Medicure-Manitoba has filed with or furnished to the CSA each report, schedule, form, statement or other document or filing required by law to be filed or furnished since January 1, 2008, and none of the CSA Documents at the time it was filed or furnished contained any untrue statement of a material fact or omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Except to the extent that information contained in any CSA Document filed or furnished and publicly available (a “Filed CSA Document”) has been revised or superseded by a later filed or furnished Filed CSA Document, none of the CSA Documents contains any untrue statement of a material fact or omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. The comparative financial statements (including the related notes) of Medicure-Manitoba included in the CSA Documents complied, at the time the respective statements were filed, as to form in all material respects with the applicable accounting requirements and the rules of the CSA with respect thereto, have been prepared in accordance with generally accepted accounting principles in effect from time to time in Canada (“GAAP”) applied on a consistent basis during the periods involved (except as may be indicated in the notes thereto) and fairly present in all material respects the consolidated financial position of Medicure-Manitoba and its consolidated subsidiaries as of the dates thereof and their consolidated results of operations and cash flows for the periods then ended (subject, in the case of unaudited quarterly financial statements, to normal and recurring year-end audit adjustments and provided that such unaudited interim financial statements may omit notes that are not required in the unaudited financial statements); |
| (g) |
except for transactions in relation to the Medicure Group selling or providing access to AGGRASTAT active pharmaceutical ingredient to Iroko Cardio, LLC, since February 28, 2011, other than as disclosed in the CSA Documents, (a) there has been no change, event, circumstances or set of facts which has had, or would reasonably be expected to have, a material adverse effect on the condition (financial or otherwise), properties, capital, affairs, prospects, operations, assets or liabilities of the Medicure Group, taken as a whole, and (b) there have been no material transactions entered into by any member of the Medicure Group other than in the ordinary course of business consistent with past practice; |
| (h) |
upon the Closing, the Settlement Shares will be duly authorized and validly issued and outstanding common shares in the capital of Medicure-Manitoba registered in the name of the Lender (or as the Lender may direct in writing to Medicure-Manitoba not less than one (1) business day prior to the Closing Date) and will be fully paid and non-assessable and shall be free and clear of any liens; |
9
| (i) |
(A) Medicure-Manitoba is a "reporting issuer" or the equivalent under the laws of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Nova Scotia, Xxxxxx Xxxxxx Island and Newfoundland and is not in material default of any requirements under the securities legislation of such provinces, and (B) the Manitoba-Medicure Common Shares are listed on the TSX Venture Exchange (NEX Board) and Medicure-Manitoba is in compliance in all material respects with the applicable rules and regulations of the TSX Venture Exchange (NEX Board); |
| (j) |
the first trade in the Settlement Shares will not be (i) subject to any escrow imposed under Canadian provincial securities laws or by any stock exchange rules, or (ii) a distribution or a primary distribution to the public under Canadian provincial securities laws, unless the Lender is a "control person" as defined in applicable Canadian provincial securities laws or the first trade constitutes or forms part of a transaction or series of transactions involving a purchase or sale or repurchase or resale in the course of or incidental to a distribution or a primary distribution to the public of the Settlement Shares under Canadian provincial securities laws; and |
| (i) |
the authorized capital of Medicure-Manitoba consists only of an unlimited number of Medicure-Manitoba Common Shares, Class A Common Shares and Preferred Shares. As at the date of this Agreement there are no other classes or series of shares or any options, warrants, conversion privileges, calls or other rights, agreements, arrangements or commitments (pre-emptive, contingent or otherwise) obligating Medicure-Manitoba or any of the Medicure Group to issue or sell any securities of Medicure-Manitoba or securities or obligations of any kind convertible into or exchangeable for any securities of Medicure-Manitoba or any other person, nor are there any outstanding stock appreciation rights, phantom equity or similar rights, agreements, arrangements or commitments based upon the share price, book value, income or any other attribute of Medicure-Manitoba or any of the Medicure Group, except as set out as Schedule "C". |
4.2 The Lender makes to the Medicure Group the following representations and warranties and acknowledges that the Medicure Group is relying upon such representations and warranties in connection with the execution of this Agreement and the consummation of the transactions contemplated by this Agreement:
| (a) |
Lender is a company validly existing and in good standing under the Laws of the Cayman Islands; |
| (b) |
Lender is an “accredited investor” for purposes of National Instrument 45–106 – Prospectus and Registration Exemptions (Canada); |
10
| (c) |
Lender has all necessary approvals of its directors and shareholders and all requisite power and authority to execute and deliver the Agreement, to perform all of its obligations hereunder, and to undertake all actions required of the Lender hereunder; |
| (d) |
this Agreement has been duly and validly authorized, executed and delivered by, and constitutes a legal, valid, binding and enforceable obligation of the Lender; |
| (e) |
the execution, delivery and performance by the Lender of this Agreement and the completion of the transactions contemplated hereby do not and will not result in a violation of any law, regulation, order or ruling, including securities laws, applicable to the Lender, and do not and will not constitute a breach of or default under any member of the Lender’s constating documents, by-laws or resolutions or any agreement to which the Lender is a party or by which it is bound; |

| (g) |
the Lender represents that it is not a “U.S. person” as defined in Regulation S under the U.S. Securities Act of 1933, as amended (the “Act”), or acting for the account or benefit of a U.S. person, and that the Settlement Shares are being issued in an “offshore transaction” within the meaning of Regulation S under the Act. The Lender agrees to resell the Settlement Shares in the United States or to U.S. persons only pursuant to an effective registration statement under the Act, in accordance with the provisions of Regulation S, or pursuant to another available exemption from registration; and agrees not to engage in hedging transactions with regard to the Settlement Shares unless in compliance with the Act. The Lender will ensure that with respect to any transfer of Settlement Shares not effected through the TSX Venture Exchange (or other securities exchange on which the Manitoba Common Shares are traded), such transferee(s) agree to be bound by and comply with the resale restrictions set forth in the preceding sentence. |
5.1 At the Closing, the following shall be delivered or issued as contemplated by this Agreement:
| (a) |
by the Medicure Group to the Lender: | |
| (i) |
the Settlement Amount (to be paid by wire transfer to the Lender (or as the Lender may direct in writing to Medicure-Manitoba not less than one (1) business day prior to the Closing Date) at or prior to Closing); | |
11
| (ii) |
the Royalty and Guarantee Agreement; | |
| (iii) |
the Treasury Direction and evidence of the issuance thereof to the Transfer Agent; | |
| (iv) |
corporate incumbency certificates setting out the authorized signatories for each member of the Medicure Group; and | |
| (v) |
all such other documents and agreements as the Lender’s solicitors reasonably consider necessary or desirable to give effect to the transactions contemplated by this Agreement |
(b) by the Lender to the Medicure Group, after actual receipt of the Settlement Amount and Settlement Shares by the Lender:
| (i) |
the Discharges; | |
| (ii) |
the Royalty and Guarantee Agreement; | |
| (iii) |
a receipt in respect of the Settlement Amount and Settlement Shares; | |
| (iv) |
corporate incumbency certificates setting out the authorized signatories for the Lender; and | |
| (v) |
all such other documents and agreements as the Medicure Group’s solicitors reasonably consider necessary or desirable to give effect to the transactions contemplated by this Agreement. |
5.2 As soon as practicable following Closing, Medicure will register and file all of the Discharges and any other documents or filings required to discharge, remove and terminate all of the Lender Registrations.
6.1 The obligations of the Lender under this Agreement are subject to the following conditions to be fulfilled or performed, which conditions are for the exclusive benefit of the Lender and may be waived, in whole or in part, by the Lender in its sole discretion:
| (a) |
The representations and warranties of each member of the Medicure Group contained in this Agreement and in the Royalty and Guarantee Agreement shall be true and correct as of the Closing Date. |
| (b) |
Each member of the Medicure Group shall have fulfilled or complied with, in all material respects, all covenants contained in this Agreement to be fulfilled or complied with by the Medicure Group at or prior to the Closing. |
| (c) |
Medicure-Barbados shall have paid to the Lender the Settlement Amount. |
| (d) |
Medicure-Manitoba shall have issued an irrevocable direction to its treasury agent authorizing and directing the issuance of the Settlement Shares. |
12
| (e) |
Medicure-Manitoba shall have obtained the conditional approval of the TSX Venture Exchange (NEX Board) to issue the Settlement Shares to the Lender on terms satisfactory to the Lender and to list the Settlement Shares on the TSX Venture Exchange (NEX Board). |
| (f) |
The TSX Venture Exchange (NEX Board) shall have provided clearance with respect to all necessary Personal Information Forms with respect to the Lender. |
| (g) |
Each member of the Medicure Group shall have executed and delivered to the Lender the Royalty and Guarantee Agreement. |
| (h) |
The Lender shall have received the opinion letter of Xxxxxx, XxxXxxxx & Thorvaldson LLP dated as of the Closing Date in the form attached hereto as Schedule “D”. |
| (i) |
The Lender shall have received the opinion letter of Hampton Xxxxxxxx dated as of the Closing Date in the form attached hereto as Schedule “E”. |
| (j) |
The Lender shall have received the opinion letter of Xxxxxx LLP dated as of the Closing Date in the form attached hereto as Schedule “F”. |
| (k) |
The Lender shall have received such other documents, instruments and certificates as the Lender may reasonably request in connection with the transactions contemplated by this Agreement in form and substance satisfactory to the Lender acting reasonably. |
6.2 The obligations of the Medicure Group under this Agreement are subject to the following conditions to be fulfilled or performed, which conditions are for the exclusive benefit of the Medicure Group and may be waived, in whole or in part, by the Medicure Group in its sole discretion:
| (a) |
The representations and warranties of the Lender contained in this Agreement and in the Royalty and Guarantee Agreement shall be true and correct as of the Closing Date. |
| (b) |
The Lender shall have fulfilled or complied with, in all material respects, all covenants contained in this Agreement to be fulfilled or complied with by the Lender at or prior to the Closing. |
| (c) |
The Lender shall have executed and delivered to the Medicure Group the Royalty and Guarantee Agreement. |
| (d) |
The Medicure Group shall have received such other documents, instruments and certificates as the Medicure Group may reasonably request in connection with the transactions contemplated by this Agreement in form and substance satisfactory to the Medicure Group acting reasonably. |
13
6.3 The obligations of the Parties contained in this Agreement are subject to the following conditions for the benefit of all of the Parties, and which may not be waived, in whole or in part, except by mutual agreement of the Parties:
| (a) |
Medicure-Manitoba shall have received the NEX Approval. |
| (b) |
The Manitoba Loan transaction shall have closed and Medicure-Manitoba shall have received the Manitoba Loan proceeds, net only of deductions relating to the costs described in Section 3.1(a) hereof (not to exceed CDN$250,000 in the aggregate). |
| (c) |
No legal or regulatory action or proceeding shall be pending to enjoin, restrict or prohibit the Closing or any of the transactions contemplated by this Agreement. |
7.1 Each Party shall make such disclosures and file such forms, reports, undertakings and other documents as may be required under applicable Canadian provincial securities legislation, including, (a) in the case of Medicure-Manitoba, the issuance of a press release announcing the Closing and the filing of a material change report on SEDAR in respect of the Closing, and (ii) in the case of the Lender, the filing of an early warning report on SEDAR in respect of the acquisition of the Settlement Shares.
7.2 Subject to the transactions contemplated herein closing, Medicure-Manitoba agrees not to issue any shares or grant any options or warrants in the capital of Medicure-Manitoba during the Follow-Along Period, other than the Follow-Along Securities.
7.3 In the event that shareholder approval is required to issue or grant the Follow-Along Securities, the Lender irrevocably agrees to vote, and agrees to obtain and deliver on Closing the written agreement of any party directed by the Lender hereunder to receive the Settlement Shares (the “Lender’s Designee”) to vote, the Settlement Shares (if the Lender of the Lender’s Designee stills holds the Settlement Shares) and any other securities of Medicure-Manitoba that the Lender or such party may hold at the time of Medicure-Manitoba’s annual general meeting of shareholders for 2011 (the “AGM”) to be held on or before November 30, 2011, in favour of the issuance and/or granting of the Follow-Along Securities (which shall be structured by Medicure-Manitoba as a stand-alone resolution distinct from any other matter sought to be approved at the AGM, other than any correlating amendments to Medicure-Manitoba’s Stock Option Plan) and against any conflicting transaction or resolution, to the extent that the Lender or such party is entitled to vote on such matters at the AGM. Not less than fifteen (15) days prior to the AGM, the Lender or such party will appoint will appoint Xx. Xxxxxx Xxxxxxx as its proxy for that purpose and if Xx. Xxxxxx Xxxxxxx is unwilling or unable to act, the Lender or such party will appoint Xxxxxx Xxxxxx as its proxy for that purpose (such individual the “Lender’s Proxy”) and execute and deliver any other documentation reasonably requested by the Medicure Group to give effect to the appointment of the Lender’s Proxy provided however, that (i) the foregoing shall not prevent the Lender or the Lender’s Designee from transferring any Medicure-Manitoba Common Shares held by the Lender or the Lender’s Designee (including, but not limited to, the Settlement Shares) during the Follow-Along Period or thereafter, (ii) any transferee (other than a transferee that is an affiliate of the Lender) of such transferred Medicure-Manitoba Common Shares shall not be bound by the voting requirement in this Section 7.3 and shall be permitted to vote such transferred Medicure-Manitoba Common Shares at the AGM in its sole discretion, and (iii) the Lender shall be permitted to revoke any Lender's Proxy appointed prior to the transfer of any Medicure-Manitoba Common Shares immediately prior to the effective date of such transfer.
14
Survival of Representations, Warranties and Covenants
8.1 The representations, warranties and covenants contained in this Agreement shall forever survive the execution, deliver and performance of this Agreement and the Closing. Any investigation made by or on behalf of the Party entitled to the benefit thereof or any knowledge of such Party shall not mitigate, diminish or affect the representations and warranties of the other Party pursuant to this Agreement.
Further Assurances
9.1. From time to time after the Closing Date, each Party shall at the request of any other Party execute and deliver such additional documents and instruments and do any and all things, as may be reasonably required to give full effect to the transactions contemplated by this Agreement and carry out the intent of this Agreement. In addition, Medicure-Manitoba agrees to continue to make all filings and take all actions required to maintain its reporting issuer status under applicable Canadian and provincial securities legislation.
15
10.1 This Agreement may be terminated and abandoned at any time prior to the Closing Date (i) by mutual written consent of the Parties; (ii) by the Lender, if any of the conditions set forth in subparagraph 6.1 have not been satisfied, complied with or performed and such non-satisfaction, noncompliance or nonperformance has not been cured or eliminated (or by its nature cannot be cured or eliminated) on or before the Closing Date, as applicable; (iii) by the Medicure Group, if the conditions set forth in subparagraph 6.2 have not been satisfied, complied with or performed and such non-satisfaction, noncompliance or nonperformance has not been cured or eliminated (or by its nature cannot be cured or eliminated) on or before the Closing Date; or (iv) by the Lender or the Medicure Group in the event the conditions set forth in subparagraph 6.3 have not been satisfied on or before the Closing Date, by prompt written notice of termination by the terminating party to the other party, in which case this Agreement shall become void and have no effect (other than those provisions which specifically survive the termination of this Agreement).
11.1 Any notice, direction or other communication given under this Agreement or any ancillary agreement shall be in writing and given by delivering it or sending it by facsimile or other similar form of recorded communication addressed:
| (a) |
to the Lender at: |
 |
With a copy to:
|
Xxxxxxx Xxxxx LLP | |
|
3400 One First Canadian Place | |
| (b) |
to the Medicure Group at: |
|
Medicure International, Inc. |
00
Xx. Xxxxx, Xxxxxxxx
XX00000
Attention: Treasurer
Facsimile No. (246) 432-4004
With a copy to:
Medicure Inc.
0-0000 Xxxxxxxx
Xxxxxx
Xxxxxxxx, Xxxxxxxx
Xxxxxx X0X 0X0
Attention:
President
Facsimile No. (000) 000-0000
And a copy to:
Xxxxxx, XxxXxxxx & Xxxxxxxxxxx
XXX
00xx Xxxxx – 000 Xxxx Xxxxxx
Xxxxxxxx, Xxxxxxxx
X0X
0X0
Attention: Xxxxx Xxxxxx
Facsimile No. (000) 000-0000
Any such communications shall be deemed to have been validly and effectively given (i) if personally delivered, on the date of such delivery if such date is a business day and such delivery was made prior to 4:00 p.m. (Winnipeg time) and otherwise on the next business day, or (ii) if transmitted by facsimile or similar means of recorded communication on the business day following the date of transmission. Any party may change its address for service from time to time by notice given in accordance with the foregoing and any subsequent notice shall be sent to such party at its changed address.
12.1 Time shall be of the essence of this Agreement.
13.1 The Parties intend that this Agreement shall not benefit or create any right or cause of action in, or on behalf of, any Person, other than the Parties to this Agreement, and no Person, other than the Parties to this Agreement, shall be entitled to rely on the provisions of this Agreement in any action, suit, proceeding, hearing or other forum.
17
14.1 This Agreement may only be amended or otherwise modified by written agreement executed by the Parties.
15.1 No waiver of any of the provisions of this Agreement shall be deemed to constitute a waiver of any other provision (whether or not similar), nor shall such waiver be binding unless executed in writing by the Party to be bound by the waiver. No failure on the part of any Party to exercise, and no delay in exercising, any right under this Agreement shall operate as a waiver of such right, nor shall any single or partial exercise of any such right preclude any other or further exercise of such right or the exercise of any other right.
16.1 Subject to Section 8.1 and except as otherwise expressly provided in this Agreement, the covenants, representations and warranties shall not merge on and shall survive the Closing. Closing shall not prejudice any right of one party against any other party in respect of anything done or omitted under this Agreement or in respect of any right to damages or other remedies.
Entire Agreement
17.1 This Agreement together with the Transaction Documents constitutes the entire agreement between the Parties with respect to the transactions contemplated in this Agreement and supersedes all prior agreements, understandings, negotiations and discussions, whether oral or written, of the Parties. There are no representations, warranties, covenants, conditions or other agreements, express or implied, collateral, or otherwise, between the Parties in connection with the subject matter of this Agreement, except as specifically set forth herein and therein and the Parties have not relied and are not relying on any other information, discussion or understanding in entering into and completing the transactions contemplated by this Agreement and the Transaction Documents. If there is any conflict or inconsistency between the provisions of this Agreement and the provisions of any other Transaction Document, the provisions of this Agreement shall govern.
18.1 This Agreement shall become effective when executed by the Parties and after that time shall be binding upon and enure to the benefit of the Parties and their respective successors and permitted assigns. Notwithstanding the foregoing, Section 2.2 hereof shall be binding upon and shall enure to the benefit of the heirs, administrators, executors, representatives, successors and assigns of any person named or referred to in, or subject to, Section 2.2, for all purposes. Neither this Agreement nor any of the rights or obligations under this Agreement shall be assignable or transferable by any Party without the prior written consent of the other Party.
18
19.1 If any provision of this Agreement shall be determined by any court of competent jurisdiction to be illegal, invalid or unenforceable, that provision will be severed from this Agreement and the remaining provisions shall remain in full force and effect.
20.1 This Agreement shall be governed by and interpreted and enforced in accordance with the laws of the Province of Manitoba and the federal laws of Canada applicable therein. Each of the Parties irrevocably attorn and submit to the non-exclusive jurisdiction of the Court of Queen's Bench of Manitoba with respect to any matter arising under or related to the Agreement or any Transaction Document.
21.1 This Agreement may be executed in any number of counterparts (including counterparts by facsimile and email) and all such counterparts taken together shall be deemed to constitute one and the same instrument.
22.1 Except as required by applicable laws or by any governmental authority or in accordance with the requirements of any stock exchange, none of the Medicure Group shall make any public announcement or statement with respect to this Agreement without the prior approval of the Lender. Moreover, each member of the Medicure Group agrees to consult with the Lender prior to making any public filing, summarizing any provisions of this Agreement in any disclosure documents or making any public announcement or statement with respect to this Agreement. The Lender agrees that any press release with respect to the transactions contemplated herein shall be acceptable to the Province of Manitoba and MDC. Notwithstanding any of the foregoing, the Medicure Group may make usual course disclosure in their financial statements respecting the settlement of the Medicure Obligations pursuant to this Agreement without the prior approval of the Lender.
[REMAINDER OF PAGE BLANK.
SIGNATURE PAGE TO FOLLOW.]
19
IN WITNESS WHEREOF the parties hereto have executed this Agreement.
| BIRMINGHAM ASSOCIATES LTD. | MEDICURE INTERNATIONAL INC. | |||
| Per: | /s/ Xxxxxx Xxxxxxxxx | Per: | /s/ Xxxx X. Cyrus | |
| Vice President | Treasurer | |||
| Per: | Per: | |||
| MEDICURE PHARMA INC. | MEDICURE INC. | |||
| Per: | /s/ Xxxxxx Xxxxxxx | Per: | /s/ Xxxxxx Xxxxxxx | |
| President | President & CEO | |||
| Per: | Per: | |||
SCHEDULE “A”
LENDER REGISTRATIONS
Manitoba
-
PPSA Registration 200717173804 made on September 15, 2007 against Medicure Inc. by Secured Party Birmingham
-
PPSA Registration 200717173707 made on September 15, 2007 against Medicure International Inc. by Secured Party Birmingham
-
PPSA Registration 200717173600 made on September 15, 2007 against Medicure Pharma Inc. by Secured Party Birmingham
Canadian Intellectual Property Office
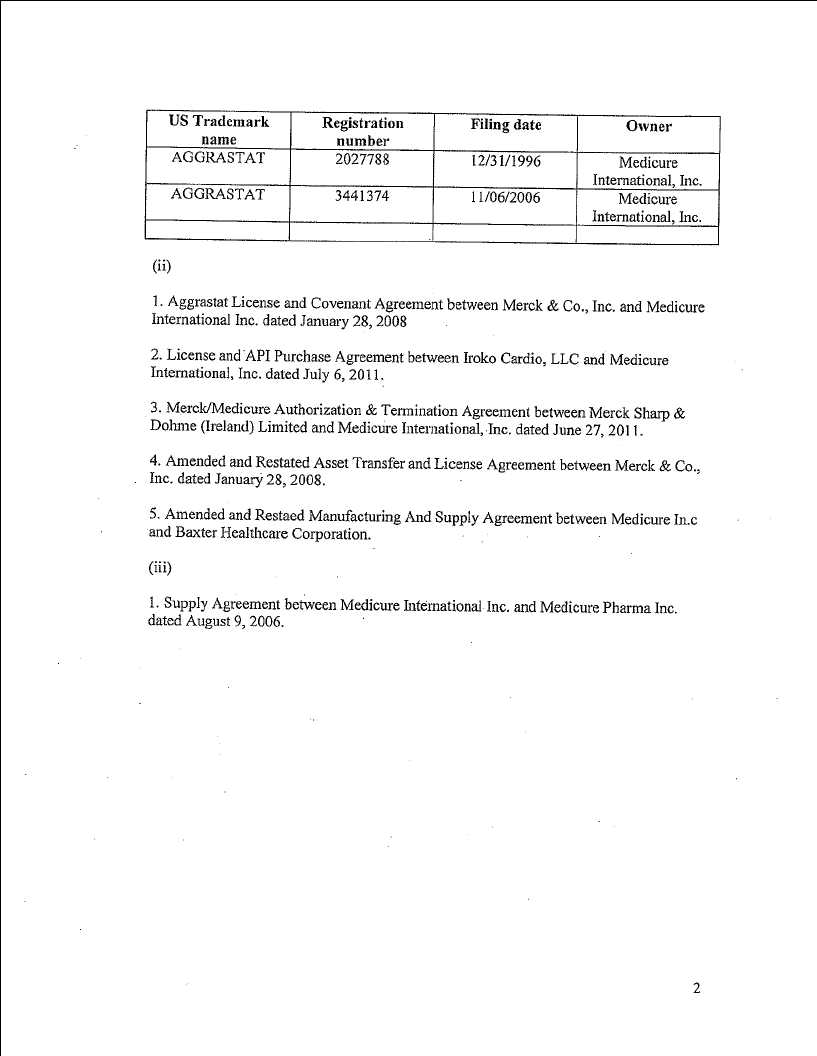
-
Security interest records relating to Birmingham Patent Security Agreement placed on patents and/or patent applications of Medicure International Inc. on May 2, 2008 as Registration No. 5471135 pursuant to CIPO File No. 2383252
-
Security interest records relating to Birmingham Patent Security Agreement placed on patents and/or patent applications of Medicure Inc. on May 2, 2008 as Registration No. 5471081 pursuant to CIPO File No. 2328730
-
Security interest records relating to Birmingham Trademark Security Agreement placed on the following trade-xxxx and/or trade-xxxx applications of Medicure Inc. on October 29, 2007 pursuant to CIPO File No. 1060417:
| Trade-xxxx Name | Registration No./Application No. |
| CARDOXAL | TMA585,514 |
| CARDOXAL | Appl. No. 1201333 |
| CARDOXAPRIL | Appl. No. 1205356 |
| MYOSALUS | Appl No. 1305051 |
| WINDOXAL | Appl. No. 1305052 |
| CARDAXION | Appl. No. 1305053 |
| PYRISAL | Appl. No. 1305054 |
| PYRIMAX | Appl. No. 1305056 |
| PRISALUS | Appl. No. 1305058 |
21
Delaware
- UCC Registration 73586525 made on September 21, 2007 against Medicure Pharma Inc. by Secured Party Birmingham
Washington, D.C.
-
UCC Registration 2007123812 made on September 21, 2007 against Medicure Inc. by Secured Party Birmingham
-
UCC Registration 2007123812 made on September 21, 2007 against Medicure International Inc. by Secured Party Birmingham
United States Patent and Trademark Office
-
USP&TO Patent Assignment No. 500359294 (Reel/Frame: 019864/0092) made on September 21, 2007 by Secured Party Birmingham
-
USP&TO Patent Assignment No. 500359393 (Reel/Frame: 019850/0887) made on September 21, 2007 by Secured Party Birmingham
-
USP&TO Trademark Assignment No. 900087535 (Reel/Frame: 003626/0056) made on September 21, 2007 by Secured Party Birmingham
-
USP&TO Trademark Assignment No. 900087531 (Reel/Frame: 003626/0038) made on September 21, 2007 by Secured Party Birmingham
Barbados
-
Debenture from Barbados International Inc. to Birmingham dated September 17, 2007 and registered on September 21, 2007 in volume 55 at page 2 for US$25,000,000.00
-
Security Agreement from Barbados International Inc. to Birmingham dated September 17, 2007 and registered on September 21, 2007 in volume 54 at page 250 for US$25,00,000.00
-
Trademark Security Agreement from Barbados International Inc. to Birmingham dated September 17, 2007 and registered on September 21, 2007 in volume 54 at page 251 for US$25,000,000.00
-
Charge over Shares dated 17th September 2007 from Medicure Inc. to Birmingham to secure US$ 25,000,000
22
SCHEDULE “B”
(ROYALTY AND GUARANTEE AGREEMENT)
Attached.
23
SCHEDULE “C”
(PROFORMA SHARE TABLE)
Redacted
24
SCHEDULE “D”
FORM OF OPINION
(MEDICURE-MANITOBA)
Redacted.
25
SCHEDULE “E”
FORM OF OPINION
(MEDICURE-BARBADOS)
Redacted.
26
SCHEDULE “F”
FORM OF OPINION
(MEDICURE-PHARMA)
Redacted.
27
ROYALTY AND GUARANTEE AGREEMENT
dated as of
July 18, 2011
(this “Agreement”) among
Birmingham Associates Ltd.
a Cayman Islands
company
(“Birmingham”)
and
Medicure International Inc.
a corporation
organized under the laws of Barbados
(“Medicure-Barbados”)
and
Medicure Pharma Inc.
a corporation
organized under the laws of Delaware
(“Medicure-Pharma”)
and
Medicure Inc.
a corporation organized under
the laws of Canada
(“Medicure-Manitoba”)
Medicure-Barbados, Medicure-Pharma and Medicure-Manitoba are collectively referred to herein as “Medicure”. Each of Birmingham and Medicure is referred to herein as a “Party” and are together referred to herein as the “Parties”.
A. Pursuant to that certain Debt Settlement Agreement made among the Parties dated July 18, 2011 (the “Debt Settlement Agreement”), Medicure-Barbados agreed to, among other things, resolve Medicure’s debt to Birmingham, and such resolution includes Medicure-Barbados’ agreement to pay certain royalties to Birmingham to be applied by Birmingham in accordance with the Debt Settlement Agreement, and the agreement of Medicure-Manitoba and Medicure-Pharma to guarantee performance by Medicure-Barbados of such royalty obligations to Birmingham, all as more particularly described in this Agreement.
B. In accordance with the Debt Settlement Agreement, the Parties wish to set out in this Agreement the full terms and conditions of the royalty arrangements among them.
NOW, THEREFORE, in consideration of the mutual covenants and agreements contained herein and in the Debt Settlement Agreement, the adequacy and sufficiency of which is hereby acknowledged by the Parties, and intending to be bound hereby, the Parties agree as follows:
1.0 Certain Definitions and Interpretation
1.1 For the purposes of this Agreement, the following terms shall have the meanings ascribed to them below.
“Affiliate” means, with respect to any Person, any other Person who directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person. The term "control" means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities by contract or otherwise, and the terms "controlled" and "controlling" have meanings correlative thereto.
“AGGRASTAT” means all products referred to as AGGRASTAT® (as currently sold by Medicure) and any other pharmaceutical product sold by Medicure containing Tirofiban as an active ingredient and any AGGRASTAT Combination Product.
“AGGRASTAT Proprietary Rights" means all Proprietary Rights owned or co-owned or licensed or sub-licensed by Medicure, or under which Medicure has any actual rights to practice, whether existing on the date of this Agreement, in process or development, or created or acquired or licensed by Medicure after the date of this Agreement, that claim or cover or are appurtenant to AGGRASTAT or a method of manufacture, use or Commercialization of AGGRASTAT. Without limiting the generality of the foregoing, "AGGRASTAT Proprietary Rights" shall include, as of the date of this Agreement, all licenses and sublicenses, all Patents and all Trademarks listed on Schedule 4.7 (a) to this Agreement as of the date of this Agreement.
“AGGRASTAT Royalty Payments” means payments of the percentage amounts as described in paragraph 2 of this Agreement from Net Sales of AGGRASTAT or Infringement Payments related to the AGGRASTAT Proprietary Rights.
“AGGRASTAT Third Party Arrangement” means (i) AGGRASTAT Proprietary Rights owned or co-owned by a third party and which are licensed or sub-licensed to Medicure (other than off-the-shelf financial and accounting or other similar computer software); (ii) AGGRASTAT Proprietary Rights which Medicure licenses (or sub-licenses) to third parties; and (iii) all joint venture, co-promotion, participation, royalty, non-competition, market allocation and similar Contracts to which Medicure is a party that involve the creation, licensing (or sublicensing) or grant of any other rights in any AGGRASTAT Proprietary Rights or the use or exploitation thereof, or the Commercialization of AGGRASTAT.
2
“AGGRASTAT Inter-Company Arrangement” means any Contract between or among Medicure and any Affiliate relating to AGGRASTAT Proprietary Rights or the Commercialization of AGGRASTAT.
“Business Day” means a day, other than a Saturday or Sunday, on which commercial banks in the City of New York and the City of Winnipeg, Manitoba, Canada, are open for the general transaction of business.
“Combination Product” means, in relation to AGGRASTAT, any product that contains, in addition to Tirofiban, one or more other active pharmaceutical ingredients, and in relation to MC-1 means any product that contains, in addition to P5P, one or more other active pharmaceutical ingredients, except that any product containing P5P in combination with Tirofiban shall be deemed an AGGRASTAT Combination Product if the Switch Option has not been exercised (at the applicable time), and shall be deemed an MC-1 Combination Product if the Switch Option has been exercised.
“Commercialization” means the development, manufacture, marketing, offer to sell, sale, offer to import, import and export of AGGRASTAT or MC-1, as the case may be, including under the AGGRASTAT Proprietary Rights or MC-1 Proprietary Rights, as the case may be, by Medicure or its Affiliates or by third parties under Third Party Arrangements.
“Consent” means any consent, approval, authorization, license, approval, notice, registration or filing, including under any applicable Law, Order, Permit or any Contract to which Medicure is a party.
“Constituent Documents” means the articles or certificate of incorporation and by-laws, as applicable, of Medicure or Birmingham, and all amendments thereto or restatements thereof, as currently in effect.
“Contract” means all contracts, agreements, settlements, stipulations, leases, indenture, mortgage, lease, pledge, note, bond, orders, invoices, commitments, arrangements, understandings, instruments, permits or licenses, whether written or oral, to which Medicure is a party or is otherwise bound or which affects any of the property or assets of Medicure.
“Copyrights” means (i) all United States and foreign copyrights (including those in software and databases), whether or not registered or the underlying works of authorship have been published, and all copyright registrations and recordings and copyright applications, and any renewals or extensions thereof,, and (ii) all other rights of any kind whatsoever (including licenses) accruing thereunder or pertaining thereto.
3
“Defaulted Payment” means a Royalty Payment in respect of which there has been an Event of Default.
“Event of Default” means if Medicure shall fail to make any Royalty Payment in accordance with the terms hereof within five Business Days after such Royalty Payment becomes due and payable in accordance herewith.
“Fiscal Quarter” means a three-month period ending on August 31, November 30, February 28 or May 31 (as applicable) in any particular fiscal year of Medicure.
“GAAP” means generally accepted accounting principles as in effect in the Canada on the date of this Agreement, applied on a basis consistent with prior audited financial statements.
“Governmental Authority” means any international, European Union, supranational, national, federal, state, provincial, county, municipal or local government, foreign or domestic, or the government of any political subdivision of any of the foregoing, or any entity, authority, agency, ministry or other similar body exercising executive, legislative, judicial (including any court), regulatory, or administrative authority or functions of or pertaining to government, including any authority or other quasi-governmental entity established to perform any of such functions.
“Guarantee” means the guarantees of Medicure-Manitoba and Medicure-Pharma of the obligations of Medicure-Barbados hereunder.
“Guarantors” means Medicure-Manitoba, Medicure-Pharma and any Person that becomes an Additional Guarantor as provided in Section 6.4 of this Agreement.
“Infringement Payments” means all collections, recoveries, damages, awards, settlement payments or other payments, compensation or consideration of any kind which are received by Medicure as damages, compensation or consideration for or in respect of any lost component of Net Sales as a result of any Proceeding to enforce the AGGRASTAT Proprietary Rights or the MC-1 Proprietary Rights, as the case may be, against any infringement thereof due to development, manufacture, marketing, import, export, offer to sell or sale of any product containing Tirofiban or P5P as the case may be.
“Inter-Company Arrangement” means the AGGRASTAT Inter-Company Arrangement or MC-1 Inter-Company Arrangements.
“Knowledge” means, in relation to Birmingham, the knowledge of Birmingham or the directors, officers and other senior management of Birmingham, in each case after reasonable investigation by Birmingham and such individuals, and, in relation to Medicure, means the knowledge of Medicure or the directors, officers and other senior management of Medicure, in each case after reasonable investigation by Medicure and such individuals.
4
“Laws” means the following: statutes; laws; ordinances; regulations, rules, written policy and resolutions; judicial decisions and precedent; Orders; and the common law; applicable as to the foregoing to the specified Persons and to the businesses and assets thereof.
“Liability” means any Indebtedness, liability or obligation, whether known or unknown, asserted or unasserted, absolute or contingent, accrued or unaccrued, liquidated or unliquidated and whether due or to become due, regardless of when asserted. Without limiting the generality of the foregoing, in the case of any Contract, "Liability" shall mean also any obligation of Medicure to render any performance or to make any payment under such Contract, including any Liability arising out of or based upon any breach by Medicure, whether before or after the date of this Agreement, of any provision of such Contract.
“Material Adverse Change” means any circumstance, event, change in or effect on Medicure or its business or operations, or any restriction, limitation or impairment, that has a material adverse effect upon or material negative change in (whether such material adverse effect or material adverse change occurs before or after the date of this Agreement) (i) the ability of Medicure to Commercialize the Royalty Products, and (ii) the ability of Birmingham to receive on a timely basis the Royalty Payments contemplated under this Agreement.
“MC-1” means all pharmaceutical products that contain P5P as an active ingredient and any MC-1 Combination Product.
“MC-1 Proprietary Rights” means all Proprietary Rights owned or co-owned or licensed or sub-licensed by Medicure, or under which Medicure has any actual rights to practice, whether existing on the date of this Agreement, in process or development, or created or acquired or licensed by Medicure after the date of this Agreement, that claim or cover or are appurtenant to MC-1 or a method of manufacture, use or Commercialization of MC-1. Without limiting the generality of the foregoing, "MC-1 Proprietary Rights" shall include, as of the date of this Agreement, all licenses and sublicenses, all Patents and all Trademarks listed on Schedule 4.7 (b) to this Agreement as of the date of this Agreement.
“MC-1 Royalty Payments” means payments of the percentage amounts as described in paragraph 2 of this Agreement, after the exercise of the Switch Option, from Net Sales of MC-1 or Infringement Payments related to the MC-1 Proprietary Rights.
“MC-1 Third Party Arrangement” means (i) MC-1 Proprietary Rights owned or co-owned by a third party and which are licensed or sub licensed to Medicure (other than off-the-shelf financial and accounting or other similar computer software); (ii) MC-1 Proprietary Rights which Medicure licenses (or sublicenses) to third parties; and (iii) all joint venture, co-promotion, participation, royalty, non-competition, market allocation and similar Contracts to which Medicure is a party that involve the creation, licensing (or sublicensing) or grant of any other rights in any MC-1 Proprietary Rights or the use or exploitation thereof, or the Commercialization of MC-1.
5
“MC-1 Inter-Company Arrangement” means any Contract between or among Medicure and any Affiliate relating to MC-1 Proprietary Rights or the Commercialization of MC-1.
“Net Sales” means the total worldwide gross sales of AGGRASTAT, or MC-1, as the case may be, derived from the Commercialization of AGGRASTAT or MC-1, as the case may be, set forth on invoices for or any other document or contract evidencing such sales by Medicure or its Affiliates or by third parties under AGGRASTAT Third Party Arrangements or MC-1 Third Party Arrangements, as the case may be, in any given period, plus the fair market value of all other properties and services received in consideration of such sales by Medicure or its Affiliates or by third parties under AGGRASTAT Third Party Arrangements or MC-1 Third Party Arrangements, as the case may be, in any given period, less the following deductions directly paid or actually incurred or allowed by Medicure or its Affiliates or by third parties under AGGRASTAT Third Party Arrangements or MC-1 Third Party Arrangements, as the case may be, during such period with respect to the sales of AGGRASTAT or MC-1, as the case may be: (i) discounts off the invoiced price, and credits, rebates, allowances, adjustments, rejections, recalls and returns that relate to AGGRASTAT or MC-1, as the case may be; (ii) price reductions or rebates, retroactive or otherwise, imposed by any Governmental Authority that relate to AGGRASTAT or MC-1, as the case may be, sold (iii) sales and value-added taxes assessed on the sale of AGGRASTAT or MC-1, as the case may be; (iv) chargebacks and managed care fees paid to Governmental Authorities and managed care entities in respect of sales of AGGRASTAT or MC-1, as the case may be; and (v) amounts actually reserved or written off for bad debts related to the sale of AGGRASTAT or MC-1, as the case may be, (and provided that if the applicable amounts charged for such sales are actually later collected, such amounts shall be deemed Net Sales).
With respect to a Combination Product, the Net Sales (for the purpose of calculating royalty amounts owed as to sales of such Combination Product) shall be adjusted by multiplying the Net Sales (calculated as provided in the above paragraph) for such Combination Product by a fraction, the numerator of which shall be the established market price for contained in the Combination Product and the denominator of which shall be the sum of the established market prices for AGGRASTAT or MC-1, as the case may be, plus the established market prices for the other components contained in the Combination Product. If any of such separate market prices are not established in a country, then the Parties shall negotiate in good faith to determine a fair and equitable method of calculating Net Sales in that country for the Combination Product in question, which shall be based on the principle of allocating to the adjusted Net Sales the relative market value of the AGGRASTAT or MC-1, as the case may be, in such Combination Product, compared to the market value of the other components of such Combination Product.
6
"Order" means any judgment, award, determination, finding, writ, decree, claim, injunction, order, compliance agreement or settlement agreement by or before any Governmental Authority and any arbitrator.
“Ordinary Course of Business” means the ordinary course of business of Medicure in a manner materially consistent with past practices of Medicure and customary business operations of Medicure in compliance with applicable Laws
“Patents” means (i) all United States and foreign patents, patent applications and patentable inventions, (ii) all inventions claimed therein, and (iii) the right to xxx or otherwise recover for any and all past, present and future infringements thereof, and all reissues, divisions, continuations, continuations-in-part, substitutes, renewals, and extensions thereof, all improvements thereon and all other rights of any kind whatsoever (including licenses) accruing thereunder or pertaining thereto.
“Permit” means consents, permits, licenses, franchises, approvals, certificates, waivers, concessions, exemptions, variances, orders, registrations, filings, notices or other authorizations issued by a Governmental Authority, and all filings and registrations with any Governmental Authority or other Person.
“Person” means an individual, partnership, corporation, limited liability company, joint stock company, unincorporated organization or association, trust, joint venture, association or other organization, any division, segment or other unincorporated business, whether or not a legal entity, or a Governmental Authority.
“Proceeding” means any action, suit, proceeding (including any arbitration proceeding), complaint, claim, charge, hearing, inquiry, subpoena, audit or investigation before or by any Governmental Authority, including indictments, applications, petitions, complaints, investigations, notices of violations, letters of inquiry, notices of apparent Liability, forfeitures or other actions from or before any Governmental Authority relating to Medicure, the Royalty Proprietary Rights or the Royalty Rights.
“Proprietary Rights” means all rights, priorities and privileges relating to intellectual property, whether arising under United States, multinational or foreign laws or otherwise, including all Copyrights, Patents, Trademarks, Trade Secrets, mask works, rights of publicity and privacy, intangible rights in software and databases not otherwise included in the foregoing, technology, know-how and processes, and all rights to xxx at law or in equity for any past, present and future infringement or other impairment of any of the foregoing, including the right to receive all proceeds and damage awards therefrom.
"P5P" means pyridoxal-5-phosphate, also known as pyridoxal-5'-phosphate, or pyridoxal phosphate, or 3-hydroxy-2-methyl-5-((phosphonooxy)methyl)-4-pyridinecarboxaldehyde, and all salts, solvates, and/or hydrates thereof.
7
“Related Persons” of any other Person means the directors, officers, employees, and agents of such Person and any family member (any spouse, ancestor, direct descendant or sibling of the individual specified) of such Person or of such directors, officers, employees, and agents ..
“Royalty Products” means AGGRASTAT until Birmingham exercises the Switch Option, and thereafter means MC-1.
“Royalty Proprietary Rights” means the AGGRASTAT Proprietary Rights and the MC-1 Proprietary Rights.
“Royalty Rights” means the obligation of Medicure-Barbados to make, and the right of Birmingham to receive, Royalty Payments as provided in this Agreement.
“Subsidiary” means with respect to any Person, any corporation, partnership, association or other business entity of which (i) if a corporation, a majority of the total voting power of shares of stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or controlled, directly or indirectly, by that Person or one or more of the other Subsidiaries of that Person or a combination thereof, or (ii) if a partnership, association or other business entity, a majority of the partnership or other similar ownership interests thereof is at the time owned or controlled, directly or indirectly, by any Person or one or more Subsidiaries of that Person or a combination thereof. For purposes hereof, a Person or Persons shall be deemed to have a majority ownership interest in a partnership, association or other business entity if such Person or Persons shall be allocated a majority of partnership, association or other business entity gains or losses or shall be or control the managing director, managing member, general partner or other managing Person of such partnership, association or other business entity.
“Switch Option” means the option to elect to cease receiving AGGRASTAT Royalty Payments and instead receive MC-1 Royalty Payments.
“Tax” means any federal, state, local or foreign income, gross receipts, franchise, estimated, alternative minimum, add-on minimum, sales, use, transfer, real property gains, registration, value added, excise, natural resources, severance, stamp, occupation, windfall profits, environmental, customs, duties, real property, personal property, capital stock, social security (or similar), unemployment, disability, payroll, license, employee or other withholding, or other tax, of any kind whatsoever, including any interest, penalties or additions to tax or similar items in respect of the foregoing (whether disputed or not).
“Third Party Arrangements” means AGGRASAT Third Party Arrangements, or MC-1 Third Party Arrangements.
"Tirofiban" means N-(butylsulfonyl)-O-4-(4-piperidyl)butyl-L-tyrosine, also known as (2S)-2-(butylsulfonylamino)-3-4-(4-piperidin-4-ylbutoxy)phenylpropanoic acid, or tirofiban, and all salts, solvates, and/or hydrates thereof.
8
“Trade Secrets” means (i) all know-how and confidential or proprietary information having the legal protection as trade secrets under applicable Laws of the United States or any Governmental Authority of the United States or similar protection under non- United States Laws, (ii) the right to xxx or otherwise recover for any and all past, present and future misappropriations thereof, and (iii) all other rights of any kind whatsoever (including licenses) accruing thereunder or pertaining thereto.
“Trademark” means (i) all United States, state and foreign trademarks, service marks, trade names, domain names, corporate names, company names, business names, fictitious business names, trade dress, trade styles, logos, or other indicia of origin or source identification, and all registrations of and applications to register the foregoing and any renewals thereof, (ii) the right to xxx or otherwise recover for any and all past, present and future infringements and dilutions thereof, (iii) all income, royalties, damages and other payments now and hereafter due and/or payable with respect thereto (including payments under all licenses entered into in connection therewith, and damages and payments for past, present or future infringements and dilutions thereof), and (iv) all other rights of any kind whatsoever (including licenses) accruing thereunder or pertaining thereto, together in each case with the goodwill of the business connected with the use of and symbolized by, each of the above
“Transfer” means any transfer or assignment of any Royalty Proprietary Rights or any interest or participation therein or in any Commercialization by any third party of Royalty Proprietary Rights.
1.2 Interpretation
Unless otherwise indicated to the contrary herein by the context or use thereof: (i) the words, "herein," "hereto," "hereof" and words of similar import refer to this Agreement as a whole and not to any particular Section or paragraph hereof; (ii) the word "including" means "including, but not limited to" and "including without limitation"; (iii) masculine gender shall also include the feminine and neutral genders, and vice versa; (iv) words importing the singular shall also include the plural, and vice versa; and (v) unless otherwise expressly indicated in the context, all references herein to any "Section", "Article", "clause", "Schedule", "Disclosure Schedule" or "Exhibit" refer to Sections, Articles, clauses, Schedules, Disclosure Schedules and Exhibits contained in, or attached to, this Agreement.
1.3 Currency
Unless otherwise specified, all references in this Agreement to monetary amounts are references to the lawful currency of the United States of America.
9
2.1 Royalty Payments. Subject to the terms and conditions of this Agreement, Medicure-Barbados) shall make payments (“Royalty Payments”) to Birmingham and Birmingham shall be entitled to receive, effective upon the date of this Agreement and for and in respect of the period commencing on the date of this Agreement and ending on May 1, 2023 (the “Royalty End Date”), on or before the 60th calendar day following the end of each Fiscal Quarter or part thereof in which there occur Net Sales of AGGRASTAT or Infringement Payments related to the AGGRASTAT Proprietary Rights, payments equal to the percentages (the “Royalty Percentages”) of Net Sales of AGGRASTAT and Infringement Payments related to the AGGRASTAT Proprietary Rights in the immediately preceding Fiscal Quarter, as follows:
(a) Four percent (4%) of Net Sales and Infringement Payments related to the AGGRASTAT Proprietary Rights up to and including two million dollars ($2,000,000) during such Fiscal Quarter;
(b) Six percent (6%) of Net Sales and Infringement Payments related to the AGGRASTAT Proprietary Rights exceeding two million dollars ($2,000,000) but less than four million dollars ($4,000,000) during such Fiscal Quarter; and
(c) Eight percent (8%) of Net Sales and Infringement Payments related to the AGGRASTAT Proprietary Rights equal to or exceeding four million dollars ($4,000,000) during such Fiscal Quarter.
2.2 Calculation. For clarity, the above Royalty Percentages are applicable to the incremental amounts of Net Sales and Infringement Payments occurring in any particular Fiscal Quarter. For example, if there were a total of $10 million in Net Sales of AGGRASTAT or Infringement Payments related to AGGRASTAT in a Fiscal Quarter, the total Royalty Payment owed under this Section for such Fiscal Quarter would equal:
| (a) |
4% x $2 million, which is $80,000, plus |
| (b) |
6% x $2 million, which is $120,000, plus |
| (c) |
8% x $6 million, which is $480,000, |
for a total Royalty Payment of $680,000.
3.1 Switch Option. Birmingham shall have the one-time option to exchange the Royalty Payments described in sections 2.1 and 2.2 hereof from AGGRASTAT to MC-1, by exercising the Switch Option.
3.2 Exercise of Switch Option. Birmingham shall exercise the Switch Option in its sole discretion, by giving Medicure written notice of such exercise.
10
3.3 Royalty Payments after Switch Option. If Birmingham exercises the Switch Option, then commencing from and after the first day (the “Switch Date”) of the next succeeding Fiscal Quarter after the exercise of the Switch Option and ending on the Royalty End Date, the Royalty Percentages shall be applied to, and Royalty Payments shall thereafter accordingly be made by Medicure-Barbados to Birmingham, in respect of Net Sales of MC-1 and Infringement Payments related to MC-1 Proprietary Rights and not in relation to Net Sales of AGGRASTAT and Infringement Payments related to AGGRASTAT Proprietary Rights.
3.4 Greater Clarity. For greater clarity, Birmingham shall be entitled to receive Royalty Payments in relation to only one of AGGRASTAT and MC-1 at a time, and, having exercised the Switch Option, Birmingham shall have no further right to switch Royalty Payments and shall have no further right or interest of any kind whatsoever in AGGRASTAT or Infringement Payments related to AGGRASTAT Proprietary Rights provided that Medicure-Barbados will remain obligated to pay all Royalty Payments accrued and owing up to the Switch Date. This Agreement shall continue in full force and effect after the Switch Date.
4.0 Representations and Warranties of Medicure
As of the date of this Agreement, each Medicure company, jointly and severally, hereby makes to Birmingham the representations and warranties set forth in this Article 4, and Medicure acknowledges that Birmingham is relying upon such representations and warranties in connection with the execution of this Agreement, notwithstanding any investigation made or conducted by Birmingham or on its behalf.
4.1 Organization and Qualification; Subsidiaries. Schedule 4.1 sets forth the full corporate names, the jurisdictions of incorporation or organization, the principal place of business and addresses, and the ownership of the capital stock of all classes and series, of each of the Medicure companies. Each Medicure company is a corporation duly organized, validly existing and in good standing under the Laws of the jurisdictions indicated on Schedule 4.1 and has the power and authority and all material Permits necessary to own or lease its property and assets and to carry on its business as presently conducted, and as presently proposed to be conducted, and is duly qualified to do business as a foreign corporation or other entity and is in good standing in each jurisdiction wherein the nature of its business or the ownership of its assets makes such qualification necessary. Medicure has previously provided to Birmingham true and complete copies of its Constituent Documents. Except as set forth on Schedule 4.1, Medicure has no, and has never had any, Subsidiaries.
4.2. Authorization; Enforceability. Medicure has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder, all of which have been, or will be, duly authorized by all requisite corporate action. This Agreement has been duly authorized, executed and delivered by Medicure and, assuming that this Agreement has been duly authorized, executed and delivered by Birmingham, constitutes a valid and binding agreement of Medicure, enforceable against Medicure in accordance with its terms, except as such enforcement may be limited by applicable bankruptcy, insolvency or similar Laws affecting creditors' rights generally or the application of general principles of equity (regardless of whether such enforcement is considered in a Proceeding in equity or at law).
11
4.3. Non-Contravention. The execution, delivery and performance of this Agreement will not (i) contravene any provision contained in the Constituent Documents of Medicure, (ii) conflict with, violate or result in a breach (with or without the lapse of time, the giving of notice, or both) of or constitute a default (with or without the lapse of time, the giving of notice, or both) under any Contract, or any Consent, Permit and Order, in each case to which Medicure is a party or by which it is bound or to which any of its assets or properties are subject, (iii) result in the creation or imposition of any Lien on any of the Royalty Products, or (iv) result in the acceleration of, or permit any Person to terminate, modify, cancel, accelerate or declare due and payable prior to its stated maturity, any obligation of Medicure related to the Royalty Products.
4.4. No Consents, Etc. No Consent, Permit or Order is necessary for the execution, delivery or performance of this Agreement by Medicure.
4.5. Governmental Authorizations: Licenses; Etc. The business of Medicure relating to the Royalty Products has been operated in compliance, in all material respects, with all applicable Laws, Consents, Permits and Orders and, without limiting the generality of the foregoing, neither Medicure nor, to the Knowledge of Medicure, any of its officers, directors, employees or agents or other Persons acting on behalf of any of them have used any corporate or other funds for unlawful contributions, payments, gifts or entertainment, or made any unlawful expenditures relating to political activity to government officials or others. Medicure has, and after giving effect to this Agreement, will continue to have, all Consents, Permits and Orders, and has made all notifications, registrations, certifications and filings with all Governmental Authorities, necessary or advisable for the operation of its business as currently conducted relating to the Royalty Products other than in all cases where the failure to have any Consent, Permit and Order, or to make any notification, registration, certification or filing, would not result in a Material Adverse Change. There is no Proceeding pending or, to the Knowledge of Medicure, threatened by any Governmental Authority with respect to (i) any alleged violation by Medicure or its Affiliates of any Law, Consent, Permit or Order relating to the AGGRASTAT Proprietary Rights or the MC-1 Proprietary Rights, or (ii) any alleged failure by Medicure or its Affiliates to have any Consent, Permit or Order required in connection with the operation of the business of Medicure relating to the Royalty Products other than in all cases where the violation of any Law, or the failure to have any Consent, Permit or Order, would not result in a Material Adverse Change.
12
4.6. Orders and Proceedings. Except as set forth on Schedule 4.6, there are no Orders or Proceedings pending or, to the Knowledge of Medicure, threatened against Medicure, (i) relating to Medicure, its businesses or properties relating to the AGGRASTAT Proprietary Rights, MC-1 Proprietary Rights, or Royalty Products or (ii) seeking to enjoin the transaction contemplated by this Agreement. None of the Proceedings listed on Schedule 4.6 would have, if adversely determined against Medicure, result in a Material Adverse Change.
4.7. Royalty Proprietary Rights.
(a) Schedule 4.7 (a) contains a complete and accurate list of the following: (i) AGGRASTAT Proprietary Rights owned or co-owned by, or registered in the name of, Medicure or any of its Affiliates; (ii) AGGRASTAT Third Party Arrangements except for any research agreements, feasibility agreements, product development agreements, promotional agreements, product support service arrangements, clinical safety arrangements, and other Contracts in the nature of service contracts, in each case, under which there are no sales of AGGRASTAT and no sales or exclusive license or transfer of Royalty Proprietary Rights; and (iii) AGGRASTAT Inter-Company Arrangements. Each registration scheduled above should include the registered owner, registration/filing number, registration/filing date and jurisdiction of registration/filing, and each Contract scheduled above should include the name of the agreement, the date and the parties thereto.
(b) Schedule 4.7 (b) contains a complete and accurate list of the following: (i) MC-1 Proprietary Rights owned or co-owned by, or registered in the name of, Medicure or any of their Affiliates; (ii) MC-1 Third Party Arrangements, except for any research agreements, feasibility agreements, product development agreements, promotional agreements, product support service arrangements, clinical safety arrangements, and other Contracts in the nature of service contracts, in each case, under which there are no sales of MC-1 and no sales or exclusive license or transfer of Royalty Proprietary Rights; and (iii) MC-1 Inter-Company Arrangements. Each registration scheduled above should include the registered owner, registration/filing number, registration/filing date and jurisdiction of registration/filing, and each Contract scheduled above should include the name of the agreement, the date and the parties thereto.
(c) MC-1 and AGGRASTAT are the only products currently undergoing research and development at Medicure and Medicure does not anticipate any other product other than MC-1 and AGGRASTAT undergoing research and development in the three month period following the date hereof.
(d) The expiration date of all of Medicure’s patents relating to AGGRASTAT is May 1, 2023.
(e) Medicure is not a party to any past, pending or, to the Knowledge of Medicure, threatened action, lawsuit, or other judicial, arbitral or administrative proceeding relating to any Royalty Proprietary Rights or the Commercialization of the Royalty Products. None of the Royalty Proprietary Rights owned or co-owned by Medicure or any Affiliate is or has been involved in any opposition, cancellation, interference, reissue or reexamination proceeding; or is the subject of any Order. To the Knowledge of Medicure, the Royalty Proprietary Rights owned or co-owned by Medicure or any Affiliate are valid and enforceable. Medicure has not received any written (or to Knowledge of Medicure, oral) notice alleging that any Royalty Proprietary Rights are invalid or unenforceable, or challenging Medicure's ownership of or right to use any such rights; and each of the registrations and recordations of Royalty Proprietary Rights identified in Schedules 4.7(a) and 4.7(b) is held and/or recorded in the name of Medicure, has been duly applied for and registered in accordance with applicable law, and all past or outstanding maintenance obligations have been satisfied. Medicure has taken all reasonable and appropriate steps to protect and maintain all Royalty Proprietary Rights material to the business as currently conducted and as proposed to be conducted relating to Royalty Products, including to preserve the confidentiality of any Trade Secrets necessary for the Commercialization of Royalty Products.
13
(f) To the Knowledge of Medicure, the business and operations of Medicure as conducted with respect to the use or exploitation of the Royalty Proprietary Rights and the Commercialization of the Royalty Products, will not infringe, dilute, misappropriate or otherwise violate the Proprietary Rights of any third party under the Laws of any jurisdiction. Medicure has not received any written (or to the Knowledge of Medicure, oral) notice alleging that the use or exploitation of the Royalty Proprietary Rights or the Commercialization of the Royalty Products infringes, misappropriates, violates or dilutes any of the Proprietary Rights of any third party under the Laws of any jurisdiction. Medicure is not subject to any Order barring or limiting Medicure's use or exploitation of any Royalty Proprietary Rights. Medicure has a policy to secure and has secured valid written assignments, from all consultants, contractors and employees who contribute or have contributed to the creation or development of any of the Royalty Proprietary Rights, of the rights to such contributions that Medicure does not already own by operation of law. None of the Royalty Proprietary Rights is currently owned or co-owned in whole or in part by any current or past director, officer or employee of Medicure or any Affiliate or Related Person of any such director, officer or employee, and no director, officer or employee of, or any other contractor or consultant that performed work on behalf of, Medicure or any Affiliate or Related Person of any such director, officer, employee or contractor or consultant that performed work on behalf of Medicure has any interest in any of such Royalty Proprietary Rights. To the Knowledge of Medicure, no third party is infringing, misappropriating or violating the Royalty Proprietary Rights owned or co-owned by Medicure, and in the last six years, Medicure has not sent any notice to or asserted or threatened in writing any action or claim against any Person alleging infringement, misappropriation or violation of any Royalty Proprietary Rights.
14
(g) No liens, claims, or encumbrances of any kind or nature exist in respect of the AGGRASTAT Proprietary Rights or the MC-1 Proprietary Rights other than those of (i) Birmingham under the Debt Financing Agreement and documents related thereto, to be discharged in connection with the closing of the transactions contemplated by the Debt Settlement Agreement, and (ii) registrations in favour of Xxxxxxx Xxxxx Capital Canada Inc., to be discharged.
(h) No other party to any AGGRASTAT Third Party Arrangement or MC-1 Third Party Arrangement has given notice to Medicure that it intends to terminate or materially alter the provisions of such arrangement either as a result of the transaction contemplated by this Agreement or otherwise, and Medicure has not received notice from any other party to any such arrangement that it intends to terminate or materially alter the provisions of any such arrangement. Except as described in Schedule 4.7 (h) of this Agreement, Medicure is not in material default, and has not given notice of any default or claimed, purported or alleged default, and, to the Knowledge of Medicure, there are no facts that, with notice or lapse of time, or both, would constitute a material default (or give rise to a termination right) on the part of any party in the performance of any obligation to be performed under any of the AGGRASTAT Third Party Arrangements or MC-1 Third Party Arrangements and each constitutes legal, valid and binding obligations of Medicure, and, to the Knowledge of Medicure, of the other parties thereto, except as such enforcement may be limited by applicable bankruptcy, insolvency or similar Laws affecting creditors' rights generally or the application of general principles of equity (regardless of whether such enforcement is considered in a Proceeding in equity or at law).
4.8. Complete Disclosure; Accuracy of Disclosure on Schedules. Medicure has delivered to Birmingham complete copies of (including all amendments, modifications, supplements and waiver of or under) all Third Party Arrangements and all Inter-Company Arrangements. The Schedules to this Agreement do not contain any untrue statement of a material fact or, to the Knowledge of Medicure, omit to state a material fact necessary to make the statements contained in the Schedules not misleading.
4.9. Taxes. Medicure has paid when due all material Taxes relating to the Royalty Products. None of the Royalty Products are subject to any liens, claims, or encumbrances relating to Taxes, other than for Taxes not yet due and payable. No Medicure entity is treated for income or similar tax purposes as engaged in business in any jurisdiction other than the jurisdiction in which it is incorporated.
4.10. Financial Statements
(a) The unaudited consolidated financial statements of Medicure for the three and nine months ended February 28, 2011 (the "Financial Statements”) are attached hereto as Schedule 4.10(a) .
15
(b) Except as set forth on Schedule 4.10(b) or as explicitly indicated in the notes to the Financial Statements, the Financial Statements have been prepared from, and are in accordance with, the books and records of Medicure, and fairly present in all material respects, the transactions, assets, Liabilities, financial position, results of operations and cash flows of Medicure as of the dates and for the periods indicated, in each case in accordance with GAAP.
(c) Medicure has devised and maintains a system of internal accounting controls for Medicure sufficient to provide reasonable assurances that: (i) transactions are executed in accordance with management's general or specific authorization; and (ii) transactions are recorded as necessary to permit preparation of the Financial Statements in accordance with GAAP. The accounting books and records of Medicure during the periods covered by Financial Statements fairly reflect in all material respects the items of income and expense and the assets and Liabilities of Medicure, including the nature thereof and the transactions giving rise thereto, and provide (or will provide) a fair basis for the preparation of the Financial Statements in accordance with GAAP.
4.11. Undisclosed Liabilities. Other than those specifically reflected in the balance sheet included in the Financial Statements (the "Balance Sheet"), specifically identified and described in the notes thereto, or disclosed on Schedule 4.11, to the Knowledge of Medicure, there are no material Liabilities of Medicure, absolute, accrued, contingent or otherwise, whether due or to become due, other than Liabilities incurred in the Ordinary Course of Business since the date of the Balance Sheet (none of which Ordinary Course of Business Liabilities are material Liabilities for breach of contract, breach of warranty, tort, infringement or violation of Law).
4.12. Brokers. No Person is or will be entitled to a broker's, finder's, investment banker's, financial adviser's or similar fee from Medicure in connection with the execution of this Agreement.
5.0 Representations and Warranties of Birmingham
Birmingham represents and warrants to Medicure, and acknowledges that Medicure is relying upon such representations and warranties in connection with the execution of this Agreement, notwithstanding any investigation made or conducted by any Medicure or on its behalf, as follows:
5.1. Organization. Birmingham is a company validly existing and in good standing under the Laws of the Cayman Islands.
5.2. Authorization. Birmingham has the corporate power and authority to execute and deliver this Agreement and to perform its obligations thereunder, all of which have been duly authorized by all requisite corporate action. This Agreement has been duly authorized, executed and delivered by Birmingham and constitutes a valid and binding agreement of Birmingham, enforceable against Birmingham in accordance with its terms, except as such enforcement may be limited by applicable bankruptcy, insolvency or similar Laws affecting creditors' rights generally or the application of general principles of equity (regardless of whether such enforcement is considered in a Proceeding in equity or at law).
16
5.3. Non-Contravention. The execution, delivery and performance by Birmingham of this Agreement will not (i) contravene any provision contained in its Constituent Documents, (ii) conflict with, violate or result in a material breach (with or without the lapse of time, the giving of notice or both) of or constitute a material default (with or without the lapse of time, the giving of notice or both) under any Contract, Consent or Permit, or any Order, Law or Permit, in each case to which it is a party or by which it is bound or to which any of its material assets or properties are subject, or (iii) result in the acceleration of, or permit any Person to terminate, modify, cancel, accelerate or declare due and payable prior to its stated maturity any obligation of Birmingham except, in the cases of clauses (ii) and (iii), for such violations, conflicts, defaults, terminations or accelerations which would not result in a Material Adverse Change to Birmingham or its ability to consummate the transaction contemplated by this Agreement.
5.4. No Consents: Etc. No Consent, Permit or Order is necessary for the execution, delivery or performance of this Agreement.
5.5. Orders and Proceedings. There are no Orders or Proceedings by or before any Governmental Authority pending or, to the Knowledge of Birmingham, threatened against Birmingham (i) seeking to enjoin the transaction contemplated by this Agreement or (ii) asserting any infringement, dilution, misappropriation or other violation by Medicure of the Proprietary Rights of any other Person.
6.0 Certain Covenants and Agreements
6.1. Reports and Other Information.
From the date hereof, Medicure shall provide to Birmingham the following (the "Required Reports and Information"):
(a) upon written request of Birmingham, provide unaudited consolidated and consolidating financial statements of Medicure; however, such obligation shall be deemed satisfied if and so long as Medicure files on a timely basis quarterly unaudited consolidated and consolidating financial statements of Medicure with the United States Securities and Exchange Commission or the Toronto Stock Exchange.
(b) within 60 calendar days following the end of each Fiscal Quarter, a report (a "Quarterly Royalty Payment Report") of the Net Sales of Medicure (with respect to the applicable Royalty Product for which Royalty Payments are due under the terms of this Agreement) for such Fiscal Quarter on which a Royalty Payment is due under this Agreement and Medicure's calculation of the Royalty Payments due with respect thereto; such Quarterly Royalty Payment Report to be in reasonable detail and showing the calculations made to derive Net Sales of Medicure and the amount of the Royalty Payment due, and any adjustments to prior Quarterly Royalty Payment Reports;
17
(c) within 120 calendar days following the end of each fiscal year of Medicure, an annual report for such year (an "Annual Royalty Payment Report"), which shall include any adjustments to the Quarterly Royalty Payment Reports for such preceding fiscal year;
(d) each Annual Royalty Payment Report shall be accompanied by a report of the regular independent auditors of Medicure, which report shall indicate the concurrence of such auditors with the Annual Royalty Payment Report and the calculation by Medicure of the Royalty Payments for the applicable fiscal year, provided that such obligation shall be deemed satisfied if and so long as the notes to the annual audited financial statements by the regular independent auditors of Medicure indicate the specific amounts of net revenues for the AGGRASTAT and (if applicable) the MC-1 separately (if and to the extent that either such net revenues are material);
(e) promptly upon discovery by Medicure or the receipt of any notice or communication by Medicure, of a reasonably detailed report of any Proceeding, Order or claim pending, threatened, commenced, entered or imposed against Medicure with respect to either (i) the Royalty Proprietary Rights, or (ii) any infringement, dilution, misappropriation or other violation of any Proprietary Rights of any third party by the Commercialization of Royalty Products, or (iii) any AGGRASTAT Third Party Arrangement or MC-1 Third Party Arrangement, which, if adversely determined against Medicure or Birmingham, might result in a Material Adverse Change, and such other documents and information in Medicure's possession and control which Birmingham may reasonably request from time to time relating directly to the matters described in clauses (i) through (iii) above, provided that Medicure need not provide specific documents or information if doing so would breach a pre-existing (prior to the date of the request by Birmingham) confidentiality obligation to a third party or destroy a legal privilege;
(f) upon reasonable written request of Birmingham, any additional financial information relating to the Commercialization of the Royalty Products as necessary for Birmingham to protect its rights under this Agreement;
18
6.2. Royalty Payments.
(a) On or before the 60th calendar day following the end of each Fiscal quarter in which there occur Net Sales for which Royalty Payments are due, Medicure-Barbados shall make, or cause to be made, to Birmingham or its assigns the Royalty Payment applicable to such Fiscal Quarter, together with any additional Royalty Payment which may be due based on any adjustments reflected in the most recent Quarterly Royalty Payment Report or the most recent Annual Royalty Payment Report. Absent fraud or manifest error, Birmingham shall not be entitled to dispute the calculation of any Royalty Payment or any Quarterly Royalty Payment Report or any Annual Royalty Payment Report for any fiscal year or Fiscal Quarter within such fiscal year subsequent to the 180th calendar day following the date on which an Annual Royalty Payment Report for such fiscal year satisfying the requirements of this Agreement has been delivered to Birmingham (the “Review Period”). Medicure shall keep, and shall cause (through any new AGGRASTAT Third Party Arrangement and, if the Switch Option has been exercised, any new MC-1 Third Party Arrangement) any third party that Commercializes any Royalty Products to keep, for three (3) years complete and accurate records pertaining to the sale of Royalty Products, for which Royalty Payments are due, by itself or such third party, in sufficient detail to permit Birmingham to confirm the accuracy of all Royalty Payments due under this Agreement. Birmingham shall have the right to cause an independent, public accountant or other expert with the necessary expertise in calculating royalty payments of this nature reasonably acceptable to Medicure to audit such records to confirm such Net Sales and Royalty Payments in respect of the preceding fiscal year. Such audits may be exercised during normal business hours upon reasonable prior written notice to Medicure. Prompt adjustments shall be made by the Parties to reflect the results of such audit. Birmingham shall bear the full cost of such audit unless such audit discloses an underpayment by Medicure of at least four (4%) of the amount of Royalty Payments due under this Agreement for the period audited, in which case Medicure shall pay the full cost of such audit. Any period audited pursuant to this Section 6.2 may be audited only once, and the report of the auditing accountant shall be binding on the Parties absent manifest mistake or fraud.
(b) In the event of any disagreement by Birmingham with respect to any Quarterly Royalty Payment Report or Annual Royalty Payment Report, or Medicure's calculation of any Royalty Payment, such disagreement shall be referred to the audit procedure of Section 6.2 by independent auditors, the determination of which shall be final and binding on the Parties absent fraud or manifest error.
(c) The fiscal year of Medicure shall end on May 31st of each year.
(d) All Royalty Payments shall be made by wire transfer to the following account, unless and until Birmingham shall have given Medicure-Barbados different instructions at least three Business Days prior to the date on which any Royalty Payment is required to be made:

19

6.3. Medicure Obligations in Respect of Proprietary Rights. So long as Birmingham shall have Royalty Rights, Medicure shall comply with the following covenants:
(a) Medicure (either itself or through any AGGRASTAT Third Party Arrangement or MC-1 Third Party Arrangement) shall not knowingly do any act, or knowingly omit to do any act, whereby any Royalty Proprietary Right owned or co-owned by Medicure is, to Medicure’s knowledge, likely to become forfeited, abandoned or dedicated to the public in a manner that materially negatively affects the Royalty Rights.
(b) Medicure shall not knowingly infringe the Proprietary Rights of any other Person in Commercializing any Royalty Products.
(c) To the extent that it is commercially practicable to do so, Medicure shall (and shall use commercially reasonable efforts to ensure that the applicable third party under any Third Party Arrangement shall) label the Royalty Products with proper statutory patent notices.
(d) Medicure shall notify Birmingham promptly if it knows, or becomes aware of facts that indicate, that any application or registration within or comprising any of the Royalty Proprietary Rights owned or co-owned by Medicure likely will become forfeited, abandoned or dedicated to the public, or of any material adverse determination or development (including due to the institution of or any such determination or development in, any applicable Proceeding in the United States Patent and Trademark Office, the United States Copyright Office or the similar offices of any other Governmental Authority) regarding Medicure's ownership of or the validity or enforceability of, the Royalty Proprietary Rights or Medicure's right to register, own, maintain and exploit the same.
(e) Medicure shall take all commercially reasonable and necessary steps, including in any Proceeding before the United States Patent and Trademark Office or the similar offices of any other Governmental Authority, to maintain and pursue each application (and to obtain the relevant registration) and to maintain each registration of the Royalty Proprietary Rights owned or co-owned by Medicure or any Affiliate, or licensed, sub-licensed by Medicure or any Affiliate (to the extent Medicure has the ability to maintain such registrations), including the payment of required fees and taxes, the filing of responses to office actions issued by the United States Patent and Trademark Office and the United States Copyright Office or any other Governmental Authority, the filing of applications for renewal or extension, the filing of affidavits of use and affidavits of incontestability, the filing of divisional, continuation, continuation-in-part, reissue, and renewal applications or extensions, the payment of maintenance fees, and the participation in interference, reexamination, opposition, cancellation, infringement and misappropriation Proceedings, to the extent in Medicure's reasonable judgment such actions are reasonably necessary to Commercialize the Royalty Products in a commercially reasonable manner. Provided that Medicure’s obligation hereunder in respect of MC-1 Proprietary Rights shall not extend to MC-1 Proprietary Rights that are applicable only in relation to the treatment of cardiovascular disorders.
20
(f) Medicure (either itself or through licensees) shall not, without the prior written consent of Birmingham, such consent not to be unreasonably withheld, abandon any Royalty Proprietary Rights, or abandon any application or any right to file an application for or cancel or allow to lapse any registration for any Royalty Proprietary Rights, unless Medicure shall have previously determined that such pursuit or maintenance of such Royalty Proprietary Rights is no longer necessary or desirable in the conduct of Medicure's business and that the loss thereof could not reasonably be expected to result in a Material Adverse Change with respect to Commercialization of Royalty Products, in which case, Medicure shall give prompt notice of any such discontinuance, abandonment or cancellation to Birmingham in accordance herewith and the reasons therefore.
(g) In the event that Medicure becomes aware that any third party asserts that the Commercialization of the Royalty Products by Medicure or any of its licensees infringes, violates, or misappropriates the Proprietary Rights of any third party, Medicure shall promptly provide written notice to Birmingham of same, and shall take actions as are commercially reasonable under the circumstances to defend against such assertion and any related claim or proceeding, and/or to design-around, obtain a license under such Proprietary Rights and/or to avoid or cease any such infringement or misappropriation.
(h) In the event that any Royalty Proprietary Rights are infringed, misappropriated, violated or diluted by a third party in a manner that will result in a Material Adverse Change to the Commercialization of Royalty Products, Medicure promptly shall provide written notice to Birmingham of same, and shall take such actions as are commercially reasonable under the circumstances to protect such Royalty Proprietary Rights, which may include suing for infringement, misappropriation or dilution, seeking injunctive relief where appropriate and seeking to recover any and all damages for such infringement, misappropriation or dilution, and provided that if Medicure would receive any material benefit, compensation or other consideration from any such infringer or its Affiliates for forbearance by Medicure from taking such action, Medicure shall take all necessary action against such infringement to protect such Royalty Proprietary Rights.
(i) Medicure shall take all steps reasonably necessary to protect the secrecy of all Trade Secrets of Medicure that are necessary for the Commercialization of Royalty Products.
21
(j) Whenever Medicure shall acquire any material Royalty Proprietary Rights, or (either by itself or through any agent, employee, licensee or designee) file an application for the registration of any Royalty Proprietary Rights with the United States Patent and Trademark Office, the United States Copyright Office or the similar offices of any other Governmental Authority as Medicure may consider appropriate, Medicure promptly shall notify Birmingham in writing of such acquisition or filing.
(k) Without limiting Section 6.6(b) or (c), Medicure shall not assign, grant any exclusive license or otherwise transfer to any Person (including any Affiliates) all or substantially all of the Royalty Proprietary Rights (including through any assignment or transfer of Third Party Arrangements) unless (a) such Person agrees in writing to be bound by and perform all the obligations of Medicure herein (as if Medicure) and (b) such successor in interest to Medicure or its assets executes a joinder or novation agreement reasonably satisfactory to Birmingham whereby such successor in interest agrees to be bound by this Agreement as if an original party thereto.
6.4. Additional Guarantors. Medicure shall notify Birmingham at the time that any Person (an “Additional Guarantor”) becomes a Subsidiary entitled to any AGGRASTAT Proprietary Rights or to receive revenues from the Commercialization of AGGRASTAT Proprietary Rights (or if the Switch Option has been exercised, entitled to any MC-1 Proprietary Rights or to receive revenues from the Commercialization of MC-1 Proprietary Rights), and promptly thereafter (and in any event within 30 days), cause such Person to become a Guarantor by executing and delivering to Birmingham a "Joinder Agreement" in form, content and scope reasonably satisfactory to Birmingham.
6.5. Conduct of Business by Medicure. So long as the Royalty Rights are outstanding or any Royalty Payments are owed by Medicure under this Agreement, Medicure shall: (i) use its commercially reasonable efforts to keep in full force and effect its corporate existence, Royalty Proprietary Rights reasonably needed to Commercialize the Royalty Products, and the goodwill relating or pertaining thereto; and (ii) use its commercially reasonable efforts to obtain and keep in effect all Consents and Permits currently held or acquired from time to time by Medicure with respect to the Royalty Rights and the Commercialization of the Royalty Products.
6.6. Certain Negative Covenants of Medicure. So long as the Royalty Rights are outstanding or any Royalty Payments are owed by Medicure under this Agreement, Medicure shall not, directly or indirectly:
(a) amend, modify or waive any existing AGGRASTAT Third Party Arrangement, or if the Switch Option has been exercised, any MC-1 Third Party Arrangement in a manner whereby Medicure knows, or ought reasonably to have known, that such amendment, modification or waiver could materially negatively affect the Commercialization of the Royalty Products or the Royalty Rights;
22
(b) enter into any transaction (including, but not limited to, any Third Party Arrangement) or knowingly take any action affecting Medicure or the Royalty Proprietary Rights that will prohibit or interfere with or materially impair the Royalty Rights or the ability of Medicure or its permitted assignee, licensee or transferee under Section 6.3(k) to perform its obligations to make the Royalty Payments;
(c) grant or suffer to exist or become effective any consensual lien, claim, encumbrance or restriction on Medicure of whatever nature or kind that will prohibit or interfere with or materially impair the Royalty Rights or the ability of Medicure to perform its obligations to make the Royalty Payments, other than as may arise or become effective in the ordinary course of Medicure’s capitalization and financing activities;
(d) effect any change (i) in Medicure's legal name, (ii) in the location of Medicure's chief executive office, (iii) in Medicure's identity or organizational structure, (iv) in Medicure's Federal Taxpayer Identification Number or organizational identification number, if any, or (v) in Medicure's jurisdictions of organization (in each case, including by merging with or into any other entity, dissolving, liquidating, reorganizing or organizing in any other jurisdiction), unless Medicure shall have made all necessary arrangements such that such change does not materially impact in any negative manner the Royalty Rights.
6.7. Commercially Reasonable Efforts; Further Assurances. Subject to the terms and conditions of this Agreement, each of the Parties shall use its commercially reasonable efforts to take, or cause to be taken, all reasonable action, and to do, or cause to be done, all things reasonably necessary, proper or advisable under applicable Laws to give effect to consummate and make effective the transactions contemplated by this Agreement.
6.8. Public Announcements. The timing and content of all announcements regarding any aspect of this Agreement to the financial community, government agencies, employees or the general public shall be mutually agreed upon in advance by Medicure and Birmingham, such agreement not to be unreasonably withheld or delayed; provided, that each Party hereto may make any such announcement which it in good faith believes, based on advice of counsel, is necessary or advisable in connection with any requirement of Law, it being understood and agreed that each Party shall promptly provide the other Parties with copies of any such announcement prior to such announcement to the extent reasonably possible.
6.9. Disclosure Generally. The inclusion of any information in a schedule hereto shall not be deemed to be adequate unless full and complete in all material respects for the purpose of identifying or describing an exception or the requested information, and no knowledge of the materiality of any information shall be imputed to Birmingham.
23
6.10. Indemnification for Third Party Claims by Medicure.
(a) Medicure shall indemnify Birmingham, and save and hold Birmingham harmless against and pay on behalf of or reimburse Birmingham as and when incurred for any loss, Liability, cost, damage or expense (including (x) interest, penalties, reasonable attorneys', consultants' and experts' fees and expenses, and (y) all amounts paid in investigation, defense or settlement of any of the foregoing) (collectively, "Losses"), which Birmingham may suffer, sustain or become subject to, or asserted or sought to be imposed on Birmingham, based upon any third party claim, action, suit, or cause of action arising out of, as a result of, in connection with, relating or incidental to or by virtue of: (i) any breach of any representation or warranty of Medicure under this Agreement; (ii) the failure of Medicure to comply with its obligations under this Agreement; and (iii) the Commercialization of the Royalty Products by Medicure or its Affiliate or by a third party under authority of an AGGRASTAT Third Party Arrangement or MC-1 Third Party Arrangement. Excluded from the foregoing are any Losses resulting from claims for Taxes.
(b) Medicure shall be entitled to participate in the defense of any third party claim asserted against Birmingham giving rise to a claim for indemnification under subclause (a) above, at Medicure's expense, and at its option (subject to the limitations set forth below) shall be entitled to assume and control the defense thereof by appointing a reputable counsel reasonably acceptable to Birmingham to be the lead counsel in connection with such defense; provided that:
(i) Birmingham shall be entitled to participate in the defense of such third party claim and to employ counsel of its choice for such purpose; provided that the fees and expenses of such separate counsel shall be borne by Birmingham (other than any fees and expenses of such separate counsel that are incurred prior to the date Medicure effectively assumes control of such defense which, notwithstanding the foregoing, shall be borne by Medicure), and for the avoidance of doubt, any right of Medicure otherwise to control the defense of the third party claim is not affected by Birmingham's participation in accordance with this clause (i);
(ii) Medicure shall not be entitled to assume control, or to continue the control, of such defense and shall pay the reasonable fees and expenses of counsel retained by Birmingham if: (A) the claim for indemnification relates to or arises in connection with any criminal Proceeding, action, indictment, allegation or investigation; (B) the third party claim seeks an injunction or equitable relief against Birmingham; (C) a conflict of interest exists between Medicure and Birmingham; (D) Medicure failed or is failing to vigorously defend against such third party claim; or (E) the third party claim, if adversely determined against Birmingham, would result in a Material Adverse Change; but provided that if, due to the foregoing, Birmingham assumes and controls the defense of any such claim, then (1) Birmingham shall vigorously defend against any such claims for which Medicure would have an obligation to indemnify or hold harmless Birmingham or any other indemnified party for any Losses resulting from the claims , and (2) Birmingham shall obtain the prior written consent of Medicure before entering into any settlement of the Proceeding or ceasing to defend such Proceeding if such settlement does not expressly and unconditionally release Birmingham and Medicure from all Liabilities and obligations with respect to such claim, without prejudice; and
24
(iii) if Medicure shall control the defense of any such claim, Medicure shall obtain the prior written consent of Birmingham before entering into any settlement of a Proceeding or ceasing to defend such Proceeding if, pursuant to or as a result of such settlement or cessation, injunctive or other equitable relief will be imposed against Birmingham or if such settlement does not expressly and unconditionally release Birmingham from all Liabilities and obligations with respect to such claim, without prejudice.
6.11. Indemnification for Litigation Costs; Other Remedies.
(a) If a Party (the "Asserting Party") brings any claim, counterclaim, suit or action against another Party (the "Defending Party") based on any breach of any representation, warranty, covenant or obligation of the Defending Party under this Agreement, then: (i) with respect to each claim or counterclaim brought by such Asserting Party for which the Asserting Party prevailed and was awarded damages against the Defending Party, the Defending Party shall indemnify and hold harmless the Asserting Party from, and pay to the Asserting Party, in addition to any damages or other remedies awarded in the Proceeding with respect to such successful claim or counterclaim, the actual out-of-pocket costs and expenses in litigating such claim or counterclaim (such as reasonable attorneys', consultants' and experts' fees and expenses and court costs directly related to litigating such claim or counterclaim); and (ii) with respect to each claim or counterclaim brought by such Asserting Party for which the Defending Party prevailed against the Asserting Party, the Asserting Party shall indemnify and hold harmless the Defending Party from, and pay to the Defending Party, the actual out-of-pocket costs and expenses of the Defending Party in litigating and defending against such claim or counterclaim (such as reasonable attorneys', consultants' and experts' fees and expenses and court costs directly related to litigating such claim or counterclaim)
(b) An Asserting Party shall be entitled to assert and recover from any a Defending Party any and all Losses which Asserting Party may suffer, sustain or become subject to, or asserted or sought to be imposed on Asserting Party, based upon, arising out of, as a result of, in connection with, relating or incidental to or by virtue of any breach of any representation, warranty, covenant or obligation of a Defending Party under this Agreement.
6.12. Joint and Several Obligations of Medicure. The representations, warranties, covenants, obligations, and indemnities of Medicure (including all Guarantors) contained in this Agreement, and the Liabilities of Medicure and all Guarantors in respect thereof, are joint and several.
25
6.13. Confidential Information.
(a) Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, each Party agrees that, during the term of the Agreement and for three years thereafter, such Party (the "Receiving Party") shall keep confidential and shall not publish or otherwise disclose and shall not use for any purpose other than as expressly provided for in this Agreement any information furnished to it by another Party (the "Disclosing Party") in writing pursuant to this Agreement, or the discussions and negotiations preceding this Agreement, that is marked "confidential" or the equivalent or is of such a nature as to be reasonably understood to be confidential in the ordinary course of business (the "Confidential Information" of the Disclosing Party). Each Receiving Party may use the Confidential Information of the Disclosing Party only to the extent required to accomplish the purposes of this Agreement. Each Party will use at least the same standard of care (but in any case not less than reasonable care) as it uses to protect proprietary or confidential information of its own to ensure that its employees, agents, consultants and other representatives do not disclose or make any unauthorized use of the Confidential Information of the other Parties.
(b) Each Receiving Party will promptly notify the Disclosing Party upon discovery of any unauthorized use or disclosure of the Confidential Information of Disclosing Party.
(c) The obligations of the Receiving Party under this Section 6.13 with respect to specific Confidential Information of the Disclosing Party shall not apply to any specific information that the Receiving Party can demonstrate by competent evidence: (i) is, at the time of disclosure by the Disclosing Party to the Receiving Party, or thereafter becomes, through no act or failure to act on the part of the Receiving Party (or its agents or representatives), generally known or available; (ii) is known by the Receiving Party at the time of receiving such information, as evidenced by its written records; or (iii) is, after the time of disclosure by the Disclosing Party to the Receiving Party, furnished to the Receiving Party by a third party, as a matter of right and without restriction on disclosure.
(d) Notwithstanding the other provisions of this Section 6.13, a Receiving Party may disclose specific Confidential Information of the Disclosing Party:
(i) on a need-to-know basis and in connection with a Party's performance of its obligations and/or exercise of its rights under this Agreement, to its Affiliates, sub-licensees under any AGGRASTAT Third Party Arrangement or MC-1 Third Party Arrangement, employees, consultants, or agents; provided that such individuals or entities are bound by confidentiality and non-use obligations at least equivalent in scope to those set forth in this Section 6.13;
26
(ii) in connection with any Proceeding to enforce the rights of a Party under this Agreement or to defend any such Proceeding; and
(iii) to the extent that such disclosure is required by an Order of any Governmental Authority, or in order to comply with applicable Laws, but provided that such Party required to make the disclosure will, except where impracticable, give reasonable advance notice to the other Party of such required disclosure and use reasonable efforts to secure, or to assist the other Party in securing, a protective order relating to, or confidential treatment of, such information.
(e) The terms of this Agreement shall be deemed to be the Confidential Information of each Party; provided, however, that each Party may disclose the terms of this Agreement in confidence to its investors, directors and professional advisors, and in confidence to its prospective investors, acquirors, joint venture partners and merger partners and their respective professional advisors and, in the case of Birmingham, or other Person providing financing to Birmingham or any assignee or transferee of Birmingham with respect to Royalty Rights; and provided that each Party may disclose the terms of this Agreement as required by Law or regulation. The Parties will consult with each other reasonably on the provisions of this Agreement to be redacted in any filings made by either Party with the U.S. Securities and Exchange Commission or as otherwise required by law, including applicable securities Laws.
Upon the occurrence and continuation of an Event of Default, Birmingham shall have all of the remedies available to it at law and equity, which shall include, but not be limited to, the following rights (which shall be cumulative in nature):
(a) the right to declare the Royalty Payments then outstanding to be forthwith due and payable in whole or in part, without presentment, demand, protest or any other notice of any kind, all of which are hereby expressly waived by Medicure;
(b) the right to charge default interest on the Royalty Payments then outstanding at the rate of the U.S. Prime Rate (as indicated in the Wall Street Journal from time to time) plus 5% per annum from the date of the Event of Default to the date on which the Defaulted Payment is made (the “Cure Date”). All such interest shall accrue from day to day and shall be payable in arrears, calculated on the actual number of days elapsed from and including the date of the Event of Default, to but excluding the Cure Date. Interest shall be calculated on the basis of a calendar year unless otherwise specified;
(c) the right to exercise and enforce any and all remedies under this Agreement under applicable law; and
(d) the right to all of its fees and costs, including, but not limited to, its legal counsel’s fees and costs, incurred in connection with enforcing Birmingham’s rights under this Section 7.0.
27
For greater certainty, no Event of Default, or the exercise of Birmingham’s rights in respect of any Event of Default shall result in the termination of this Agreement or any of Medicure’s obligations hereunder, including, but not limited to, the obligation to make the Royalty Payments hereunder.
8.1 Guarantee Generally
(a) Guarantors, jointly and severally, hereby unconditionally and irrevocably guarantee to Birmingham the prompt and complete payment and performance by Medicure when due of each and all of the obligations of Medicure (the “Medicure Obligations”) herein. For greater clarity, Guarantors shall have the benefit of the limitations on review set forth in section 6.2 of this Agreement.
(b) Each Guarantor shall be liable under its guarantee set forth in Section 8.1(a), without any limitation as to amount, for all present and future Medicure Obligations; provided, that (i) enforcement of such guarantee against such Guarantor will be limited as necessary to limit the recovery under such guarantee to the maximum amount which may be recovered without causing such enforcement or recovery to constitute a fraudulent transfer or fraudulent conveyance under any applicable Law, including any applicable federal or state fraudulent transfer or fraudulent conveyance law (after giving effect, to the fullest extent permitted by law, to the reimbursement and contribution rights set forth in Section 8.2), and (ii) to the fullest extent permitted by applicable Law, the foregoing clause (i) shall be for the benefit solely of creditors and representatives of creditors of each Guarantor and not for the benefit of such Guarantor or the holders of any equity interest in such Guarantor.
(c) The guarantee contained in this Section 8.1 (i) shall remain in full force and effect until all Medicure Obligations and the obligations of each Guarantor under the guarantee contained in this Section 8.1 have been paid in full, notwithstanding that from time to time during the term of this Agreement Medicure may be free from any obligations herein, and (ii) shall survive the payment in full of the Royalty Payments and remain enforceable as to all Medicure Obligations that survive such payment and release.
(d) No payment made by Medicure or any of the other Guarantors, any other guarantor or any other Person or received or collected by Birmingham from Medicure, any of Guarantors, any other guarantor or any other Person by virtue of any action or Proceeding or any set-off or appropriation or application at any time or from time to time in reduction of or in payment of Medicure Obligations shall be deemed to modify, reduce, release or otherwise affect the liability of any Guarantor hereunder in respect of any other Medicure Obligations then outstanding or thereafter incurred.
28
8.2. Reimbursement, Contribution and Subrogation. In case any payment is made on account of Medicure Obligations by Medicure or any other Guarantor or is received or collected on account of thereof from Medicure or any other Guarantor or its property:
(a) The obligations of Guarantors under this Agreement are not contingent upon the validity, legality, enforceability, collectability or sufficiency of any right of reimbursement, contribution or subrogation. To the fullest extent permitted under applicable Laws, the invalidity, insufficiency, unenforceability or uncollectability of any such right shall not in any respect diminish, affect or impair any such obligation or any other claim, interest, right or remedy at any time held by Birmingham against any Guarantor or its property. Birmingham makes no representations or warranties in respect of any such right and shall, to the fullest extent permitted under applicable Laws, have no duty to assure, protect, enforce or ensure any such right or otherwise relating to any such right.
8.3. Amendments, etc. With Respect to the Obligations of Medicure under this Agreement. To the fullest extent permitted by applicable Laws, each Guarantor shall remain obligated hereunder notwithstanding that, without any reservation of rights against any Guarantor and without notice to or further assent by any Guarantor, any demand for payment of any payment obligation made by Birmingham may be rescinded by Birmingham and any of the Medicure Obligations continued, and such obligations, or the liability of any other Person upon or for any part thereof, or any collateral security or guarantee therefor or right of offset with respect thereto, may, from time to time, in whole or in part, be renewed, extended, amended, modified, accelerated, compromised, waived, surrendered or released by Birmingham, and this Agreement and any other documents executed and delivered in connection therewith may be amended, amended and restated, supplemented, replaced, refinanced, otherwise modified or terminated, in whole or in part, as Birmingham may deem advisable from time to time, and any collateral security, guarantee or right of offset at any time held by Birmingham for the payment of Medicure Obligations may be sold, exchanged, waived, surrendered or released.
29
8.4. Guarantee Absolute and Unconditional. To the fullest extent permitted by applicable Laws, each Guarantor waives any and all notice of the creation, renewal, extension or accrual of any of the obligations of Medicure herein and notice of or proof of reliance by Birmingham upon the guarantee contained in this Article 8 or acceptance of the guarantee contained in this Article 8. The Medicure Obligations shall conclusively be deemed to have been created, contracted or incurred, or renewed, extended, amended or waived, in reliance upon the guarantee contained in this Article 8. All dealings between Medicure and any of Guarantors, on the one hand, and Birmingham, on the other hand, likewise shall be conclusively presumed to have been had or consummated in reliance upon the guarantee contained in this Article 8. To the fullest extent permitted by applicable Laws, each Guarantor waives diligence, presentment, protest, demand for payment and notice of default or nonpayment to or upon Medicure or any of Guarantors with respect to the Medicure Obligations. Each Guarantor understands and agrees that the guarantee contained in this Article 8 shall be construed, to the fullest extent permitted by applicable Laws, as a continuing, absolute and unconditional guarantee of payment without regard to (i) the validity or enforceability of any of this Agreement, any of the Medicure Obligations herein or any other guarantee or right of offset with respect thereto at any time or from time to time held by Birmingham, (ii) any defense, set-off or counterclaim (other than a defense of payment or performance) which may at any time be available to or be asserted by Medicure or any other Person against Birmingham, or (iii) any other circumstance whatsoever (with or without notice to or knowledge of Medicure or such Guarantor) which constitutes, or might be construed to constitute, an equitable or legal discharge of Medicure for the Medicure Obligations or of such Guarantor under the guarantee contained in this Article 8, in bankruptcy or in any other instance. When making any demand hereunder or otherwise pursuing its rights and remedies hereunder against any Guarantor, Birmingham may, but shall be under no obligation to, make a similar demand on or otherwise pursue such rights and remedies as it may have against Medicure, any other Guarantor or any other Person or against any collateral security or guarantee for the Medicure Obligations or any right of offset with respect thereto, and any failure by Birmingham to make any such demand, to pursue such other rights or remedies or to collect any payments from Medicure, any other Guarantor or any other Person or to realize upon any such collateral security or guarantee or to exercise any such right of offset, or any release of Medicure, any other Guarantor or any other Person or any such collateral security, guarantee or right of offset, shall not relieve any Guarantor of any obligation or liability hereunder, and shall not impair or affect the rights and remedies, whether express, implied or available as a matter of law, of Birmingham against any Guarantor. For the purposes hereof "demand" shall include the commencement and continuance of any Proceedings.
8.5. Reinstatement. The guarantee contained in this Article 8 shall be reinstated and shall remain in all respects enforceable to the extent that, at any time, any payment of any of the Medicure Obligations is set aside, avoided or rescinded or must otherwise be restored or returned by Birmingham upon the insolvency, bankruptcy, dissolution, liquidation or reorganization of Medicure or any Guarantor, or upon or as a result of the appointment of a receiver, intervenor or conservator of, or trustee or similar officer for, Medicure or any Guarantor or any substantial part of its property, or otherwise, in whole or in part, and such reinstatement and enforceability shall, to the fullest extent permitted by applicable Laws, be effective as fully as if such payment had not been made.
8.6. Payments. Each Guarantor hereby agrees to pay all amounts payable by it under this Article 8 to Birmingham without set-off or counterclaim in immediately available funds
30
9.1. Mandatory Withholding Taxes If at any time any applicable Law requires Medicure Barbados to deduct or withhold any Tax (a “Withholding Tax”) from any Royalty Payment made hereunder, within 30 days after the date of any payment of Withholding Taxes, Medicure Barbados shall furnish to Birmingham on behalf of whom the Withholding Taxes were paid the original or a certified copy of a receipt evidencing such payment, or if such a receipt is not available at such time, the best available evidence of such payment.
9.2. Indemnity regarding Withholding Tax Medicure Barbados shall indemnify Birmingham for any Taxes imposed by withholding if Medicure Barbados has failed to withhold the proper amount required by law to be withheld or has failed to timely remit such Taxes to the appropriate Governmental Authority and such Governmental Authority has issued a notice or other communication to Birmingham that Birmingham is liable for such Tax. Medicure Barbados will be required to indemnify Birmingham under this section 9.2 only if Birmingham provides (A) Medicure prompt notice of the claim (in the case of a claim by a Governmental Authority received by Birmingham from such Governmental Authority) for payment of such Tax by the relevant Governmental Authority, and (B) the right, at Medicure's own expense, to conduct the defense against such claim in any and all Proceedings (including an audit by the relevant Governmental Authority or a Proceeding in a court of competent jurisdiction), in which the Liability for such Tax be at issue; provided, however, that Medicure shall not be permitted to contest any claim and shall promptly make any required indemnification payments hereunder if Birmingham shall have reasonably determined that such contest could reasonably result in any material danger of a Lien for Taxes being placed on the property of Birmingham and informed Medicure of the basis for such determination.
9.3 Cooperation. The Parties agree to take reasonable steps to cooperate in the preparation, execution and filing of any necessary tax forms, certificates or other documents as may be necessary to allow the Royalty Payments to be made without Withholding Tax (or minimizing the amount of Withholding Tax) to the extent permissible by applicable Law.
10.1. Notices. Any notice, request, demand or other communication which is required or permitted under this Agreement shall be in writing and shall be deemed to have been duly given (a) when received if personally delivered, (b) when transmitted if transmitted by telex or telecopy transmission only during the recipient's normal business hours unless arrangements have otherwise been made to receive such notice by telex or telecopy outside of normal business hours, with confirmation of successful transmission received by the sender, (c) the day after it is sent, if sent for next day delivery to a domestic address by recognized overnight delivery service (e.g., DHL, UPS or Federal Express); and
31
(d) upon receipt, if sent by certified or registered mail, return receipt requested. In each case notice shall be sent as indicated below:
| (a) |
to Birmingham at: |
 |
With a copy to:
Xxxxxxx Xxxxx LLP
3400 One First
Canadian Place
P.O. Box 130
Toronto, Ontario M5X 1A4
Attention: Xxx
Xxxxx and Xxx Xxxxxx
Facsimile No. (000) 000-0000
| (b) |
to Medicure (including a Guarantor) at: |
Medicure International, Inc.
Xx.
Xxxxx Xxxxx
Xxxxxx Xxxxxx, Xxxxxxxx
Xx. Xxxxx, Xxxxxxxx
BB24016
Attention: Treasurer
Facsimile No. (246) 432-4004
With a copy to:
Medicure Inc.
0-0000 Xxxxxxxx
Xxxxxx
Xxxxxxxx, Xxxxxxxx
Xxxxxx X0X 0X0
Attention:
President
Facsimile No. (000) 000-0000
And a copy to:
Xxxxxx, XxxXxxxx & Xxxxxxxxxxx XXX
00xx Xxxxx – 000 Xxxx Xxxxxx
Xxxxxxxx, Xxxxxxxx
X0X 0X0
Attention: Xxxxx Xxxxxx
Facsimile No. (000) 000-0000
or to such other address as any Party shall notify the other Parties (as provided above) from time to time.
32
10.2. Exhibits and Schedules. All schedules hereto, or documents expressly incorporated into this Agreement, are hereby incorporated into this Agreement and are hereby made a part hereof as if set out in full in this Agreement.
10.3. Time of the Essence; Computation of Time. Time is of the essence for each and every provision of this Agreement. Whenever the last day for the exercise of any privilege or the discharge of any duty hereunder shall fall other than on a Business Day, the Party having such privilege or duty may exercise such privilege or discharge such duty on the next succeeding day which is a Business Day.
10.4. Specific Performance. Each Party acknowledges that the rights of the Parties under this Agreement are unique and recognize and affirm that in the event of a breach hereof by any Party, money damages may be inadequate and the non-breaching Party may have no adequate remedy at law. Accordingly, the Parties agree that such non-breaching Party shall have the right, in addition to any other rights and remedies existing in its or their favor at law or in equity, to enforce its or their rights and the other Party's obligations herein not only by an action or actions for damages but also by an action or actions for specific performance, injunctive and/or other equitable relief (without posting of bond or other security).
10.5. Governing Law: Consent to Jurisdiction. This Agreement and the rights and obligations of the Parties hereunder shall be governed by, and construed and interpreted in accordance with, the internal Laws of the State of New York, without giving effect to the conflict of laws principles thereof. Each of the Parties hereby irrevocably submits to the exclusive jurisdiction of any Federal or state court sitting in the City of New York over any Proceeding arising out of or relating to this Agreement: provided, that equitable relief sought by any Party may be sought in any court having appropriate jurisdiction. Each of the Parties hereby irrevocably waives, to the fullest extent permitted or not prohibited by applicable Laws, any objection which such Party may now or hereafter have to the laying of the venue of any such Proceeding brought in such a court and any claim that any such Proceeding brought in such a court has been brought in an inconvenient forum. Each of the Parties hereby irrevocably consents to the service of process in any Proceeding by sending the same by certified mail, return receipt requested or by overnight courier service, to the address of such Party set forth in Section 10.1. EACH PARTY WAIVES ANY RIGHT IT MAY HAVE TO TRIAL BY JURY IN ANY ACTION BROUGHT HEREUNDER OR ARISING OUT OF THIS AGREEMENT.
33
10.6. Assignment; Successors and Assigns; No Third Party Rights, Except as otherwise provided herein, this Agreement may not be assigned by operation of law or otherwise, and any attempted assignment shall be null and void; except that (i) Birmingham may assign the Royalty Rights to any other Person who is not a direct competitor to Medicure in whole or in part from time to time (provided that such Person executes a joinder to this Agreement agreeing to be legally bound by this Agreement as if an original Party hereto), and (ii) Medicure may assign this Agreement to its successor in interest in connection with the merger or acquisition of Medicure or sale of all or substantially all of the assets of Medicure or transfer, sale, assignment or other disposition of all or substantially all of Medicure’s right, title and interest in and to the AGGRASTAT Proprietary Rights (or, if the Switch Option has been exercised, the MC-1 Proprietary Rights) provided that (a) such successor agrees in writing to be bound by and perform all the obligations of Medicure herein (as if Medicure) and (b) such successor in interest to Medicure or its assets executes a joinder or novation agreement reasonably satisfactory to Birmingham whereby such successor in interest agrees to be bound by this Agreement as if an original party thereto. For greater clarity, if the Switch Option has been exercised, Medicure may transfer, sell, assign or otherwise dispose of AGGRASTAT Proprietary Rights without restriction or limitation. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the Parties and their respective heirs, successors, permitted assigns and legal representatives. This Agreement shall be for the sole benefit of the Parties and their respective heirs, successors, permitted assigns and legal representatives and is not intended, nor shall be construed, to give any Person, other than the Parties and their respective heirs, successors, assigns and legal representatives, any legal or equitable right, remedy or claim hereunder.
10.7. Counterparts. This Agreement may be executed in counterparts, any one of which may be by facsimile or other electronic transmission, including email, each of which shall be deemed an original agreement, but all of which together shall constitute one and the same instrument.
10.8. Titles and Heading. The titles, captions and table of contents in this Agreement are for reference purposes only, and shall not in any way define, limit, extend or describe the scope of this Agreement or otherwise affect the meaning or interpretation of this Agreement.
10.9. Entire Agreement, Amendments and Waivers. This Agreement, including the Schedules attached hereto, constitutes the entire agreement among the Parties with respect to the matters covered hereby and supersedes all previous written, oral or implied understandings among them with respect to such matters. No amendment, modification or supplement of or to this Agreement, and no waiver of any obligation of any Party under this Agreement, shall be effective unless in writing and executed by all of the Parties.
34
10.10. Severability. The invalidity of any portion hereof shall not affect the validity, force or effect of the remaining portions hereof. If it is ever held that any restriction hereunder is too broad to permit enforcement of such restriction to its fullest extent, such restriction shall be enforced to the maximum extent permitted by applicable Laws.
10.11. No Strict Construction. Each of the Parties acknowledges that this Agreement has been prepared jointly by the Parties, and shall not be strictly construed against any Party.
10.12. Termination. This Agreement and the obligations of the Parties herein shall terminate upon full performance of the Parties of all obligations hereunder.
10.13. No Partnership, Joint Venture, Etc. Nothing contained in this Agreement shall be deemed to (i) create a partnership or joint venture among the Parties or any of their Affiliates or Related Persons, (ii) constitute an appointment of any Party as the agent or attorney-in-fact of any other Party, or (iii) except as expressly provided herein, authorize any Party to execute any document, agreement, instrument or filing with any Governmental Authority, return or report, or to take any other action, which would impose upon any other Party any Liability. Each Party shall indemnify and hold harmless each other Party and its Affiliates and Related Persons against and from any Liability sought to be imposed by any third party upon such other Party or its Affiliates or Related Persons based upon any act by the first Party or its Affiliates or Related Persons where such act was intended by such party to be on behalf of or in furtherance of any such partnership, joint venture, agency, or power-of-attorney.
[Signature Pages Follow]
35
IN WITNESS WHEREOF the parties hereto have executed this Agreement.
| BIRMINGHAM ASSOCIATES LTD. | MEDICURE INTERNATIONAL INC. | |||
| Per: | /s/ Xxxxxx Xxxxxxxxx | Per: | /s/ Xxxx X. Cyrus | |
| Vice President | Treasurer | |||
| Per: | Per: | |||
| MEDICURE PHARMA INC. | MEDICURE INC. | |||
| Per: | /s/ Xxxxxx Xxxxxxx | Per: | /s/ Xxxxxx Xxxxxxx | |
| President | President & CEO | |||
| Per: | Per: | |||