DISTRIBUTOR of products that compete with the Products covered hereby and that DISTRIBUTOR is not precluded by any contractual obligation or any other reason from entering into or performing under this Agreement. DISTRIBUTOR agrees that during the...
|
|
Exhibit 10.42 INTERNATIONAL DISTRIBUTORSHIP AGREEMENT THIS INTERNATIONAL DISTRIBUTORSHIP AGREEMENT (''Agreement") is entered into effective as of the Effective Date contained in Schedule A, between ReShape Medical Inc., having its principal place of business at 100l Calle Amanacer, San Clemente, USA ("RSM") and the company identified in Schedule A ("DISTRIBUTOR"). WITNESSETH WHEREAS, RSM is in the business of selling various medical devices primarily used to perform medical procedures; and WHEREAS, DISTRIBUTOR desires to actively and diligently promote the sale, on its own behalf and for its own account, of certain of RSM's products; and WHEREAS, RSM and DISTRIBUTOR desire to enter into an exclusive distributorship agreement covering certain ReShape Medical product lines under the terms and conditions set out below. NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein, the parties agree as follows: 1. DISTRIBUTION 1.1 Products. The products that are subject to this Agreement (the "Products") shall be those products identified on Schedule B hereto, together with such other products as may from time to time be included thereon by mutual written agreement of the parties. DISTRIBUTOR acknowledges that Schedule B will not necessarily include all products sold by RSM, and that Products are subject to modification or discontinuance by RSM upon notice to DISTRIBUTOR, and, upon DISTRIBUTOR's receipt of such notice, Schedule B will be deemed amended accordingly. 1.2 Appointment. 1.2.1 Effective as of the Effective Date of this Agreement, RSM hereby appoints DISTRIBUTOR, and DISTRIBUTOR accepts such appointment, as an exclusive distributor of Products in the geographical area described on Schedule C hereto (the "Territory"), subject to the terms and conditions set forth in this Agreement. 1.2.2 DISTRIBUTOR shall not directly or indirectly deliver or promote the sale of the Products outside the Territory or locate or utilize an office, branch, or distribution depot for the sale or distribution of the Products outside the Territory. DISTRIBUTOR shall immediately notify RSM if it becomes aware that any DISTRIBUTOR customer exports or sells or plans to export or sell any of the Products outside the Territory. 1.3 Noncompetition. DISTRIBUTOR represents that as of the Effective Date there are no agreements in effect providing for the marketing, sale or distribution by « Distributorname» «Territory» 478291Jvl |
|
|
2.4.1Prices for the Products as of the Effective Date of this Agreement shall be as set forth on Schedule B hereto
2.5 Payments. All payments due to RSM pursuant to this Agreement shall be paid according to the payment terms set fmth on Schedule E hereto. All payments to RSM pursuant to this Agreement shall be made in United States dollars, without set-off or counterclaim and without deduction for any other charges. RSM shall retain a security interest in the Products until full payment is made, and DISTRIBUTOR shall assist RSM in any local recording of such security interest. If DISTRIBUTOR fails to make any payment when due, RSM shall have the right to take whatever action it deems appropriate or necessary, including, but not limited to, requiring immediate return of unsold Products, refusal of further orders, requiring payment in full before shipment, or termination of this Agreement pursuant to Section 6.2 hereof.
2.6 Shipping. Except as set forth below, all fees for shipping to the DISTRIBUTOR will be paid by the DISTRIBUTOR. Products shall be shipped to DISTRIBUTOR at the address (es) specified by DISTRIBUTOR from time to time. Risk of loss and title to the Products will pass to DISTRIBUTOR at port of entry at the foreign airport destination in the Territory. DISTRIBUTOR shall bear the cost of any handling, shipping, and insurance, within the designated territory. DISTRIBUTOR shall be responsible for clearing the Products through customs unless RSM notifies DISTRIBUTOR otherwise. DISTRIBUTOR shall be responsible for paying any and all duties and taxes due in connection with the importation of the Products. DISTRIBUTOR will be responsible for inspecting Product upon receipt in the Territory. DISTRIBUTOR shall submit to RSM all claims for non-delivery, shortages in shipment or defects reasonably discoverable on careful inspection in writing within 10 days of receipt of such shipment by DISTRIBUTOR. If DISTRIBUTOR does not provide such written notice to RSM within the specified timeframe, RSM will be discharged from liability for any such non-delivery, short delivery or defect. RSM shall promptly file a notice of claim against the freight handler in the event that DISTRIBUTOR provides written notice to RSM that any of the Products arrive other than in external good order and condition.
3. OBLIGATIONS OF DISTRIBUTOR
3.1 Distribution of Products. DISTRIBUTOR agrees to devote DISTRIBUTOR's best efforts to (i) develop and promote the use and sale of the Products in the Territory, and (ii) furnish such service of accounts as will enable DISTRIBUTOR adequately to develop and maintain the goodwill of customers and prospective customers and their acceptance of the Products. DISTRIBUTOR also agrees to abide by RSM's recommendations regarding the use of the Products, and plan orders adequately to meet customer delivery requirements.
3.2 Legal Requirements. Except as otherwise set forth in Section 3.4, DISTRIBUTOR will obtain and maintain, at its expense, all licenses, approvals, consents, and permits necessary for DISTRIBUTOR to perform its obligations under this Agreement. DISTRIBUTOR agrees to comply with all laws, statutes,
|
|
|
regulations and other legal requirements and not to place RSM in jeopardy of not complying with any such requirements. DISTRIBUTOR understands that the Reshape Medical Code of Conduct requires that the Products be sold only on the basis of quality, service, price and other legitimate marketing attributes, and that the payment of bribes for any purpose has no place in DISTRIBUTOR'S performance under this Agreement and is absolutely prohibited. Furthermore, DISTRIBUTOR agrees to use good judgment, high ethical standards and honesty in DISTRIBUTOR's dealings with customers, end-users and employees, recognizing that even the appearance of unethical actions is not acceptable. DISTRIBUTOR acknowledges and expressly agrees that certain laws of the United States of America and other countries, including, without limitation, the United States Export Control Regulations, the United States Anti-Money Laundering laws, the United States Anti-Terrorism laws and the Foreign Corrupt Practices Act, may result in the imposition of sanctions on RSM or its affiliated companies in the event that, directly or indirectly, (i) Products are exported to various countries, including without limitation Cuba, Iran, North Korea, Syria, Sudan, or any country embargoed by Executive order or otherwise, or (ii) offers, promises, or payments are made to government officials or others for the purpose of influencing decisions favorable to RSM. DISTRIBUTOR expressly agrees, therefore, that in performing its obligations under this Agreement it shall comply at all times with such laws or regulations and refrain from making or promising to make payment or transfer of anything of value that would have the purpose or effect of public or commercial bribery, or acceptance of or acquiescence in extortion, kickbacks, or other unlawful or improper means of obtaining business. DISTRIBUTOR also agrees to furnish to RSM by affidavit or other reasonable means from time to time at RSM's request, and to RSM's reasonable satisfaction, assurances that the appointment of DISTRIBUTOR and DISTRIBUTOR's activities under this Agreement, and the payment to DISTRIBUTOR of any commissions, discounts, or any monies or consideration contemplated in this Agreement, are proper and lawful under said laws and regulations. DISTRIBUTOR further acknowledges that no person employed by it is an official of any government agency or a corporation owned by a governmental unit within the Territory and that no part of any monies or consideration paid pursuant to the terms and conditions of this Agreement or any proceeds from the sale of the Products in the Territory shall accrue for the benefit of any such official. Breach of this provision, or reasonable grounds for RSM to believe it has been breached (in RSM's sole discretion), will result in immediate termination of this Agreement. DISTRIBUTOR will not make any performance or safety claims with respect to Product not contained in the label or otherwise approved by RSM consistent with applicable laws.
|
|
|
7.6 Arbitration. Any and every dispute, controversy or claim between the parties and/or their valid and lawful assignees and successors, including, but not limited to (i) any and every dispute, controversy or claim arising out of or relating to this Agreement and/or its amendments, and (ii) any and every dispute, controversy or claim not arising out of or not relating to this Agreement and/or its amendments, shall be finally settled by arbitration in Orange County, U.S.A. in accordance with the International Arbitration Rules of the International Centre for Dispute Resolution ("ICDR"). Judgment on the award rendered by the arbitrator(s) may be entered in any court having jurisdiction thereof. A sole arbitrator shall be chosen at the mutual agreement of the parties from a list of ICDR proposed arbitrators under the ICDR's arbitration rules. If the parties fail to mutually agree on the choice of an arbitrator within 30 days of receipt of claimant's request for arbitration by the other party, the sole arbitrator shall be appointed by the ICDR in accordance with its International Arbitration Rules. The language of the arbitration proceedings shall be English and the law applied to the dispute shall be solely and exclusively the laws of the California. The award shall state with specificity the reasons upon which the Award is based, and shall contain the arbitrator's findings of fact. Except as required by law, neither party nor the arbitrator may disclose to a third party the existence, content, or results of any arbitration hereunder without the prior written consent of both parties. Notwithstanding the above, RSM shall, at its sole discretion, have the right to initiate in any court sitting in California, USA or in the Territory a non-jury collection lawsuit against DISTRIBUTOR in an effort to collect from DISTRIBUTOR any and all moneys charged by RSM to DISTRIBUTOR for the Products sold by RSM to DISTRIBUTOR or to obtain temporary injunctive relief. All other issues, without exception, must be arbitrated. 7.7 Entire Agreement. The terms and provisions contained in this Agreement and the attached Schedules constitute the entire Agreement between the parties and supersede all previous communications, representations, agreements, and understandings, whether oral or written, between the parties with respect to the subject matter hereof. Except as this Agreement specifically authorizes RSM to modify certain provisions of this Agreement or the attached Schedules upon written notice to DISTRIBUTOR, no agreement or understanding extending this Agreement or varying its terms (including any inconsistent terms in any purchase order, acknowledgment, or similar form) shall be binding upon either party unless it is in a writing specifically referring to this Agreement and signed by the duly authorized representatives of the respective parties. 7.8 Severability. Should any provision of this Agreement be determined to be unenforceable or prohibited by applicable law, such provision shall be ineffective only to the extent of such unenforceability or prohibition without invalidating the remainder of such provision or the remaining provisions of this Agreement. 7.9 Captions. The captions of provisions in this Agreement are for convenience only and shall not control or affect the meaning or construction of any of the provisions of this Agreement. ReShape Medical Inc. By Xxxxxxx X. Xxxxxxx Xxxxxxx X. Xxxxxxx President 5/26/2017 By Abdulrahman Ramadan Executive Director for and on behalf of Al Zahrawi Medical [SEAL] |
|
|
7.10 Counterparts. This Agreement may be executed in any manner of counterparts, each of which shall be deemed to be an original as against any party whose signature appears thereon, and all of which shall together constitute one and the same instrument. This Agreement shall become binding when one or more counterparts hereof, individually or taken together, shall bear the signature of all of the parties reflected hereon as the signatories. 7.11 Governing Law. This Agreement shall be governed and construed in accordance with the laws of the California, United States of America, to the exclusion of both its rules or conflicts of laws and the provisions of the United Nations Convention on Contracts for the International Sale of Goods. 7.12 Release. In exchange for the agreement by RSM to enter into this Agreement with DISTRIBUTOR, DISTRIBUTOR hereby releases RSM and its affiliated companies from and waives any claims it may have had against RSM and its affiliated companies related to any previous distribution agreement or business dealings between DISTRIBUTOR and RSM or its affiliated companies. IN WITNESS WHEREOF, the parties have caused this Agreement to be executed as of the Effective Date. |
|
|
Schedule A Distributor and Term Distributor Information Corporate Name: Al Zahrawi Medical a Company incorporated under the laws of United Arab Emirates ("UAE"), having its registered office at Caterpillar building, Salahudinne St, Diera, Dubai, hereinafter referred to as the "Distributor". 4782913vl Xxxxxxx X. Xxxxxxx |
|
|
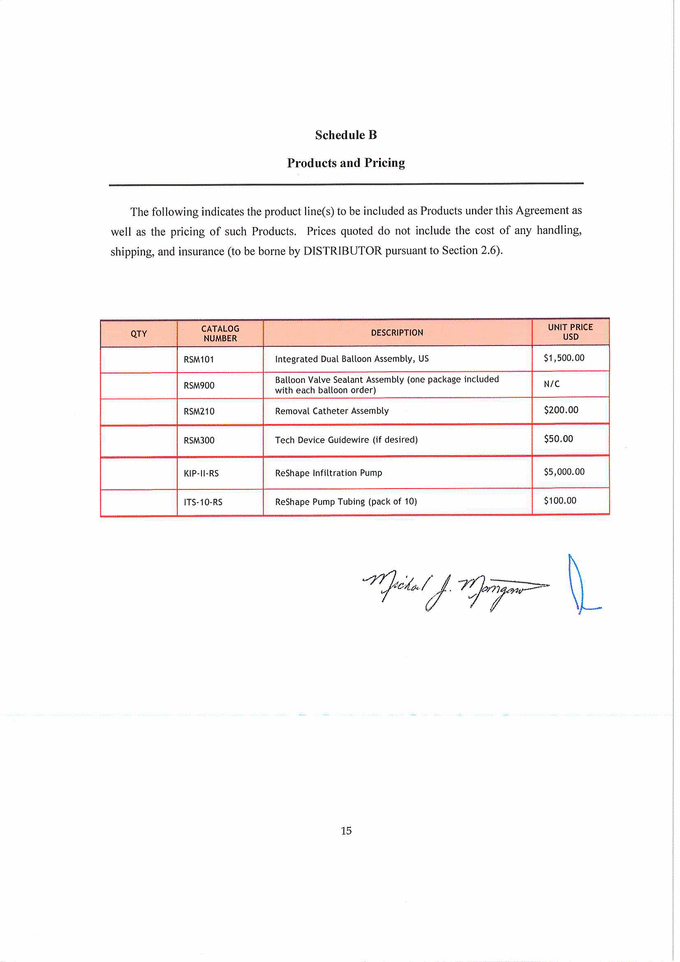
Schedule B Products and Pricing The following indicates the product line(s) to be included as Products under this Agreement as well as the pricing of such Products. Prices quoted do not include the cost of any handling, shipping, and insurance (to be borne by DISTRIBUTOR pursuant to Section 2.6). QTY CATALOG NUMBER DESCRIPTION UNIT PRICE USD RSM101 Integrated Dual Balloon Assembly, US $1, 500.00 RSM900 Balloon Valve Sealant Assembly (one package included with each balloon order) N/C RSM210 Removal Catheter Assembly $200.00 RSM300 Tech Device Guidewire (if desired) $50.00 XXX-II-RS ReShape Infiltration Pump $5,000.00 ITS-10-RS ReShape Pump Tubing (pack of 10) $100.00 15 |
|
|
Schedule C Territory The customers and or geographical area subject to this Agreement shall be specifically and exclusively limited to the United Arab Emirates. 16 |
|
|
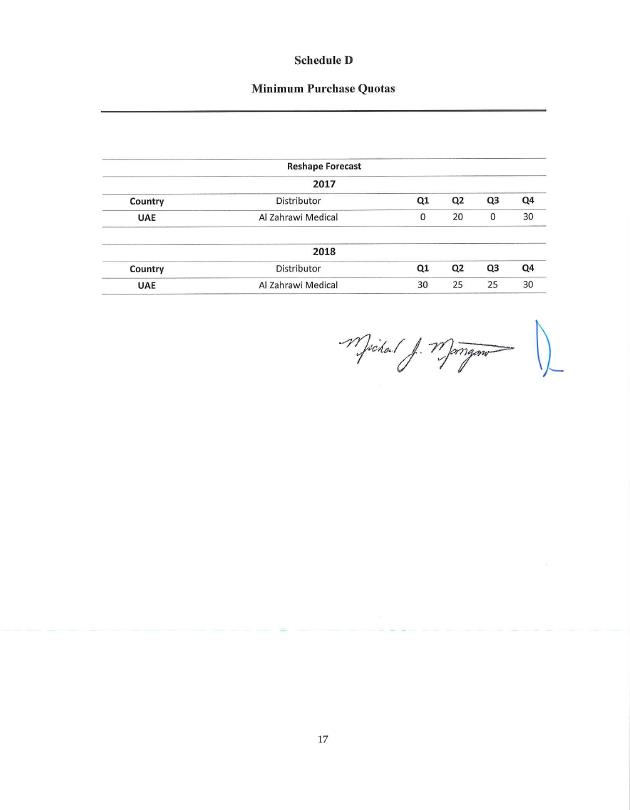
Schedule D Minimum Purchase Quotas Reshape Forecast 2017 Country Distributor Ql Q2 Q3 Q4 UAE Al Zahrawi Medical 0 20 0 30 2018 Country Distributor Ql Q2 Q3 Q4 UAE Al Zahrawi Medical 30 25 25 30 |
|
|
ANNEX A— Quality Requirements A) Storage of Products: If applicable Distributor is required to store products in accordance with product labeling statements and within an environment that prevents any of their characteristics from being altered until delivered to the customer. The minimum storage requirements for RSM products include the following: • If applicable, Distributor must establish a secure storage location and limit access to this location to only those personnel authorized by Distributor. Products must be stored within this location to prevent Products from being contaminated or tampered with in any way. Additionally, Products must be kept in a clean area, free of insects, rodents and any pests. • Distributor shall have a process to prevent expired, rejected and/or quarantined Products from being sent to final customers. ReShape Medical • RSM reserves the right to provide other instructions to Distributor regarding such Product, and Distributor agrees to comply with such instructions. B) Traceability: Distributor is required to maintain records to ensure the traceability of ReShape Medical products in accordance with applicable regulatory requirements, and to provide ReShape Medical or its authorized agents or representatives with reasonable access to such records. The minimum traceability requirements for ReShape Medical Products include the following: • Distributor is required to maintain a complete and current list of all customers who have purchased ReShape Medical products from Distributor, the dates of such purchases, the quantity, and the lot numbers, UPNs, serial numbers and/or model numbers of the units purchased (as applicable) as identified on the product label. • Distributor must ensure the traceability of all ReShape Medical products at UPN, lot level including the model and serial number where applicable. The final users for all products must be identified (units sold directly to hospitals, units sold directly to doctors, units sold directly to patients). If ReShape Medical products are consigned, the batches consumed at the account must be reconciled. • The traceability y of multi-p ack boxes must be maintained and single units originally from multi-packs must never be re-boxed. C) Complaint Reporting and Handling: Distributor is required to promptly forward all complaints concerning the products, cooperate fully with ReShape Medical in dealing with customer complaints, and take such action to resolve such complaints as may be reasonably requested by ReShape Medical. The complaint reporting requirements for ReShape Medical products include the following: |
|
|
• All customer complaints involving or contributing to serious adverse events (including patient death or serious injury) must be reported to ReShape Medical within 24 hours of Distributor 's becoming aware. Complaints involving nonserious events including comments regarding product dissatisfaction, potential malfunctions, non-serious patient injury, or unanticipated medical or surgical intervention shall be reported to ReShape Medical within 48 hours. • A Complaint Notification Form will be provided to Distributor by ReShape Medical and must be completed in full to document each complaint. Additionally, any ancillary documentation that may facilitate the complaint investigation process should also be attached, particularly if the product is not available for return. In cases where additional information is required from the customer, at least three (3) due diligent attempts must be performed by Distributor to try to collect this additional complaint information, if requested by ReShape Medical. Should requested information not be available, Distributor shall document the reason(s) it is not available and/or the 3 attempts. • Products subject to complaints should be returned to ReShape Medical. Returned products must either, as directed by ReShape Medical, be • accompanied by a disinfection certificate, even if the products have not been used; • or be returned in biohazard controlled packaging and under safe handling controls. In cases where the customer has indicated that the complaint product is available to be returned but it has not been received, at least three (3) due diligent attempts must be made by Distributor to retrieve the product. |
|
|
Date: 23/05/2017 Xxxxxxx X. Xxxxxxx President Reshape Medical Inc. 1001Calle Amanecer Xxx Xxxxxxxx, XX 00000 Tel.: 000-000-0000, ext. 102 Mob.: 000-000-0000 Email: xxxxxxxx@xxxxxxxxxxxxxx.xxx Website: www.ReShapeReady .com Sub: International Distributorship Agreement Dear Xxxxx, Please find the enclosed two original copies of International Distributorship Agreement between Al Zahrawi Medical Supplies and Reshape Medical Inc., for initializing, signature and stamping from your side. Kindly arrange to send us back one duly signed and stamped copy on the below address: Al Zahrawi Medical Supplies LLC. P.O. Box: 5973, Dubai, UAE Tel: x000 000 0000 Fax: x000 000 0000 Thanking you and looking forward to continue our successful business cooperation. Best Regards, Xxxx Xxxxxx Senior Supervisor Administration [SEAL] Al Zahrawi Medical Supplies L LC X.X.Xxx: 0000 Xxxxx, Xxxxxx Xxxx Xxxxxxxx Tel: x000 0 0000000. Fax: x000 0 0000000 Email: xxxx@xxxxxxxxxxxxxx.xxx xxx.xxxxxxxxxxxxxx.xxx f-U fU. .tfa Qµhjlu l)_µi\..:;illl 0 xxxX,xXX 00XX" ,x,X.xX'x ol'.:>.loll Uµµ,JICJIJL,,}Jl ,y-J +9VI U ')( O,O l ,l)ll..'.:,li>,+9VI u ir r vr ,u'ulm.JI info @zahr awim ed ica x.xxx , gµ.'.,J).11.)..JµJI xxx.xxxxxxxxxxxxxx.xxx,Q;jll).11&n <,o.JI |