Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Double asterisks denote omissions. LICENSE AGREEMENT by and between ARGOS THERAPEUTICS, INC. and GREEN CROSS CORP.
Exhibit 10.17
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
by and between
ARGOS THERAPEUTICS, INC.
and
GREEN CROSS CORP.
THIS LICENSE AGREEMENT (the “Agreement”), effective as of July 31, 2013 (the “Effective Date”), is by and between Argos Therapeutics, Inc., a corporation organized and existing under the laws of Delaware (“Argos”) and Green Cross Corp., a corporation organized and existing under the laws of Republic of Korea (“Green Cross”).
WHEREAS, Argos controls a proprietary immunotherapy system referred to as “ArcelisTM” for the production of personalized therapeutic products for the treatment of cancer and infectious disease;
WHEREAS, Argos is developing a proprietary therapeutic product referred to as “AGS-003” based on the Arcelis TM system targeting the treatment of metastatic renal cell carcinoma (“mRCC”);
WHEREAS, Green Cross desires to develop and commercialize the AGS-003 product for the treatment of mRCC as set forth in this Agreement in humans in the Green Cross Territory (hereinafter defined); and
WHEREAS, Argos and Green Cross believe that a license for such purpose on the terms and conditions of this Agreement would be desirable.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, the Parties hereby agree as follows:
Unless specifically set forth to the contrary herein, the following terms, whether used in the singular or plural, shall have the respective meanings set forth below:
1.1 “Affiliate” means a corporation or non-corporate business entity that, directly or indirectly, controls, is controlled by, or is under common control with the Person specified, for so long as such control continues. An entity will be regarded as in control of another entity if: (a) it owns, directly or indirectly, at least 50% of the voting securities or capital stock of such entity, or has other comparable ownership interest with respect to any entity other than a corporation; or (b) it possesses, directly or indirectly, the power to direct or cause the direction of the management and policies of the corporation or non-corporate business entity, as applicable, whether through the ownership or control of voting securities, by contract or otherwise.
1.2 “Argos Data” means all clinical data that is produced or generated by Argos or its Related Parties during a Clinical Study for the Licensed Product.
1.3 “Argos Indemnitees” has the meaning set forth in Section 7.5.1.
1.4 “Argos In-License” means an agreement between Argos and a Third Party pursuant to which Argos has rights and obligations with respect to, or which otherwise Cover, the Licensed Product and is necessary to Develop, Commercialize and/or Manufacture the Licensed Product in the Field in the Green Cross Territory, including without limitation, the Existing Argos In-Licenses.
1
1.5 “Argos Know-How” means Know-How Controlled by Argos during the Term that is not in the public domain and is reasonably necessary or useful for Green Cross and its Related Parties to perform their obligations or exploit their rights under this Agreement with respect to products incorporating Argos’ Arcelis Personalized Immunotherapy Platform for the treatment of mRCC, as such platform is more particularly described on Schedule A attached hereto. For the avoidance of doubt, Argos Know-How shall include Argos Data, but shall not include any Know-How associated with or relating to Automated Systems, dendritic cell transfected with IL4 RNA for the treatment of unwanted autoimmune responses, anti-interferon alpha antibodies, soluble CD83 or regulatory T cells.
1.6 “Argos Patent Rights” means those Patent Rights Controlled by Argos during the Term that relate to Argos’ Arcelis Personalized Immunotherapy Platform for the treatment of mRCC and that are reasonably necessary or useful for Green Cross and its Related Parties to perform their obligations or exploit their rights under this Agreement with respect to the Licensed Product in the Field in the Green Cross Territory, including without limitation, any foreign counterparts in the Green Cross Territory to the Patent Rights set forth in Schedule B of this Agreement. For the avoidance of doubt, Argos Patent Rights shall not include patent rights associated with or relating to Automated Systems, dendritic cell transfected with IL4 RNA for the treatment of unwanted autoimmune responses, anti-interferon alpha antibodies, soluble CD83 or regulatory T cells.
1.7 “Argos Technology” means, collectively, Argos Know-How and Argos Patent Rights.
1.8 “Argos Territory” means all countries of the world other than the Green Cross Territory.
1.9 “Argos Trademark” has the meaning set forth in Section 8.8.2.
1.10 “Automated Systems” means the automated cellular and RNA systems used from time to time to Manufacture the Licensed Product, as such systems are generally described in Schedule C attached hereto.
1.11 “Bankrupt Party” has the meaning set forth in Section 9.2.3(c).
1.12 “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31; provided, that (a) the first Calendar Quarter of the Term shall begin on the Effective Date and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter and the last Calendar Quarter of the Term shall end on the last day of the Term and (b) the first Calendar Quarter of the Royalty Term shall begin on the First Commercial Sale of the Licensed Product and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter and the last Calendar Quarter of the Royalty Term shall end on the last day of such Royalty Term.
1.13 “Calendar Year” means each successive period of twelve (12) months commencing on January 1 and ending on December 31; provided, that (a) the first Calendar Year of the Term shall begin on the Effective Date and end on the first December 31 thereafter and the last Calendar Year of the Term shall end on the last day of the Term and (b) the first Calendar Year of the Royalty Term shall begin on the First Commercial Sale of the Licensed Product and end on the first December 31 thereafter and the last Calendar Year of the Term shall end on the last day of the Royalty Term.
2
1.14 “Clinical Data” means Argos Data and/or Green Cross Data, as the context requires.
1.15 “Clinical Study” means a Phase I Study, Phase II Study (including a Phase II(a) and Phase II(b) Study), Phase III Study or Post-Approval Studies, as applicable.
1.16 “Code” has the meaning set forth in Section 9.2.3(c).
1.17 “COGs” shall mean Green Cross’ cost of goods sold as determined in accordance with K-IFRS.
1.18 “Commercialization” or “Commercialize” means any and all activities directed to marketing, promoting, distributing, importing, exporting, offering to sell and/or selling the Licensed Product, including the conduct of Post-Approval Studies, and activities directed to obtaining pricing and reimbursement approvals, as applicable.
1.19 “Commercialization Plan” has the meaning set forth in Section 4.3.
1.20 “Commercially Reasonable Efforts” means the carrying out of obligations in a diligent and sustained manner using such effort and employing such resources as would normally be exerted or employed by a similarly situated pharmaceutical company for a product of similar market or profit potential or strategic value at a similar stage of its product life.
1.21 “Confidential Information” means any and all information and data, including without limitation all scientific, pre-clinical, clinical, regulatory, manufacturing, marketing, financial, trade secret and commercial information or data, whether communicated in writing or orally or by any other method, which is provided by one Party to the other Party in connection with this Agreement. Argos Technology is Confidential Information of Argos. Green Cross Improvements are Confidential Information of Green Cross. Joint IP is the Confidential Information of the Parties.
1.22 “Control”, “Controls” or “Controlled by” means, with respect to any (a) material, Know-How or other information or (b) intellectual property right, the possession of (whether by ownership or license, other than pursuant to this Agreement), or the ability of a Party or its Affiliates to assign, transfer, grant access to, or a license or sublicense of, such item or right as provided for herein without violating the terms of any agreement or other arrangement with any Third Party existing at the time such Party would be required hereunder to assign, transfer or grant the other Party such access or license or sublicense.
1.23 “Cover,” “Covering” or “Covers” means that in the absence of a license granted under a Valid Claim, the Development, Manufacture or Commercialization of the Licensed Product would or is reasonably likely to infringe such Valid Claim.
1.24 “Development,” “Developing” or “Develop” means the research and development activities related to the generation, characterization, optimization, construction, expression, use and production of the Licensed Product, any other research and development activities related to the pre-clinical testing and qualification of the Licensed Product for clinical testing, and such other tests,
3
studies and activities as may be required or recommended from time to time by any Regulatory Authority to obtain Regulatory Approval of the Licensed Product, including toxicology studies, statistical analysis and report writing, pre-clinical testing, Clinical Studies and regulatory affairs, product approval and registration activities.
1.25 “Dispute” has the meaning set forth in Section 10.11.1.
1.26 “Effective Date” has the meaning set forth in the preamble.
1.27 “Excluded Claim” has the meaning set forth in Section 10.11.1.
1.28 “Existing Argos In-Licenses” means the Argos In-Licenses set forth on Schedule D.
1.29 “Field” means the treatment of mRCC in humans.
1.30 “First Commercial Sale” means the first sale for end use or consumption of the Licensed Product in the Field in the Green Cross Territory after all required Regulatory Approvals have been granted by the Regulatory Authority.
1.31 “Follow-on Licensed Product” has the meaning set forth in Section 2.5.3.
1.32 “Green Cross Data” means all clinical data that is produced or generated by Green Cross or its Related Parties during a Clinical Study for the Licensed Product.
1.33 “Green Cross Improvements” mean any improvements, ideas, inventions, developments, derivatives, modifications, technologies, discoveries, know-how ant techniques, whether or not patentable, conceived or reduced to practice by Green Cross or Related Parties during the term of this Agreement that cover or relate to Argos Technology, the Automated System or the Licensed Product.
1.34 “Green Cross Indemnitees” has the meaning set forth in Section 7.5.2.
1.35 “Green Cross In-License” means an agreement between Green Cross and a Third Party pursuant to which Green Cross has rights and obligations with respect to, or which otherwise Cover, the Licensed Product and is necessary to Develop, Commercialize and/or Manufacture the Licensed Product in the Field.
1.36 “Green Cross Product Notice” has the meaning set forth in Section 2.5.4.
1.37 “Green Cross Territory” means the Republic of Korea.
1.38 “Green Cross Trademark” has the meaning set forth in Section 8.8.2.
1.39 “ICC” has the meaning set forth in Section 10.11.1.
1.40 “IND” means an Investigational New Drug application, Clinical Trial Application or similar application or submission for approval to conduct human clinical investigations filed with or submitted to a Regulatory Authority in conformance with the requirements of such Regulatory Authority. Without limiting the generality of the foregoing, a Clinical Trial Application of the Ministry of Food and Drug Safety in the Republic of Korea constitutes an IND.
4
1.41 “Indemnitee” has the meaning set forth in Section 7.5.4.
1.42 “Infringement Claim” has the meaning set forth in Section 8.5.1.
1.43 “Initiate”, “Initiated” or “Initiation” means, with respect to a Clinical Study, the administration of the first dose to the first subject in such study; provided, however, that in the case of a Clinical Study in which the protocol is a combination of a Phase I Study and a Phase II Study, the Phase II Study portion of such Clinical Study shall be deemed Initiated only upon commencement of the Phase II Study portion of such Clinical Study.
1.44 “In-Licenses” means, collectively, the Argos In-Licenses and the Green Cross In-Licenses.
1.45 “Joint IP” has the meaning set forth in Section 8.2.
1.46 “K-IFRS” means a set of international accounting standards stating how particular types of transactions and other events should be reported in financial statements, generally accepted in Korea.
1.47 “Know-How” means all biological materials and other tangible materials, inventions, practices, methods, protocols, formulas, knowledge, know-how, trade secrets, processes, assays, skills, experience, techniques and results of experimentation and testing, including without limitation pharmacological, toxicological and pre-clinical and clinical test data and stability, analytical and quality control data, patentable or otherwise.
1.48 “Knowledge,” with respect to a Party, means the actual knowledge of any of the executive officers of such Party.
1.49 “Licensed Product” means the product developed, manufactured and/or sold utilizing the Argos Technology in the Field.
1.50 “Licensed Product Trademarks” has the meaning set forth in Section 8.8.2.
1.51 “Losses” has the meaning set forth in Section 7.5.1.
1.52 “Manufacturing” or “Manufacture” means, as applicable, all activities associated with the production, manufacture, processing, filling, finishing, packaging, labeling, shipping, and storage of the Licensed Product, including process and formulation development, process validation, stability testing, manufacturing scale-up, pre-clinical, clinical and commercial manufacture and analytical development, product characterization, quality assurance and quality control development, testing and release.
1.53 “mRCC” has the meaning set forth in the recitals.
5
1.54 “Necessary Third Party IP” means Know-How or Patent Rights owned or controlled by a Third Party that Cover the Development, Manufacturing and/or Commercialization of the Licensed Product in the Green Cross Territory.
1.55 “Net Sales” means the total amount actually received by Green Cross or its Related Parties in connection with sales of the Licensed Product to any Third Party, after deduction of all the following to the extent applicable to such sales:
(a) all trade, case and quantity credits, discounts, refunds or rebates, including without limitation rebates actually allowed or granted from the billed amount;
(b) allowances or credits for returns, including without limitation amounts received for sales which become the subject of a subsequent temporary or partial recall by a regulatory agency for safety or efficacy reasons outside the control of a Party, and retroactive price reductions (including Medicaid, managed care and similar types of rebates);
(c) any adjustments on account of price adjustments, billing errors, rejected goods, damaged goods, product recalls;
(d) credits, charge-backs and prime vendor rebates, fees, reimbursements, and similar payments actually granted or given to wholesalers and other distributors, buying groups, health care insurance carriers, pharmacy benefit management companies, health maintenance organizations, other institutions or health care organizations or other customers;
(e) cost of freight, postage, and freight insurance, (if paid by seller);
(f) sales taxes, value added taxes, excise taxes, and customs duties; and
(g) cost of export licenses and any taxes (excluding income taxes or similar taxes), fees or other charges associated with the exportation or importation of the Licensed Product.
Net Sales shall be calculated in accordance with K-IFRS.
A sale or transfer to a Related Party for re-sale by such Related Party shall not be considered a sale for the purpose of this provision but the resale by such Related Party to a Third Party shall be a sale for such purposes. Any amounts received by Green Cross or its Related Parties in exchange for Licensed Product transferred or provided to any person or entity for use in testing, clinical trials for obtaining Regulatory Approval, compassionate use, or as marketing samples to develop or promote the Licensed Product are expressly excluded from the definition of Net Sales. In the event that Licensed Product is sold in conjunction with a product or service (e.g., as a bundled or combination therapy) that is not a Licensed Product, “Net Sales” with respect to such conjoined sale shall be deemed to mean that portion of the total proceeds proportionate to the value attributable to the Argos Technology that Covers such bundled or combination therapy. In the event of a dispute with respect to the proper allocation of value, the provisions of Section 10.11 shall apply.
1.56 “Non-Bankrupt Party” has the meaning set forth in Section 9.2.3(c).
1.57 “Party” means Green Cross or Argos; “Parties” means Green Cross and Argos.
6
1.58 “Patent Expenses” has the meaning set forth in Section 8.3.6.
1.59 “Patent Rights” means all patents (including all reissues, extensions, substitutions, confirmations, re-registrations, re-examinations, invalidations, supplementary protection certificates and patents of addition) and patent applications (including all provisional applications, requests for continuation, continuations, continuations-in-part and divisions) and all foreign equivalents of the foregoing.
1.60 “Person” means any individual, corporation, company, partnership, trust, incorporated or unincorporated association, joint venture or other entity of any kind.
1.61 “Pharmacovigilance Agreement” has the meaning set forth in Section 4.9.2.
1.62 “Phase I Study” means a clinical study of Licensed Product in human volunteers or patients the purpose of which is preliminary determination of pharmacokinetics, safety and tolerability of a dosing regime and for which there are no primary endpoints (as understood by the applicable Regulatory Authorities) in the protocol relating to efficacy.
1.63 “Phase II Study” means (a) a dose exploration, dose response, duration of effect, kinetics, dynamic relationship or preliminary efficacy and safety study of the Licensed Product in the patient population (a “Phase II(a) Study”), or (b) a controlled dose ranging clinical study to evaluate further the efficacy and safety of the Licensed Product in the patient population and to define the optimal dosing regimen (a “Phase II(b) Study”).
1.64 “Phase III Study” means a controlled clinical study of the Licensed Product that is prospectively designed to demonstrate with statistical significance the efficacy and safety of the Licensed Product for use in a particular indication and that is sufficient to obtain Regulatory Approval to market the Licensed Product in such indication.
1.65 “Post-Approval Study” means a clinical study of Licensed Product Initiated after receipt of Regulatory Approval for the Licensed Product in the Green Cross Territory.
1.66 “Promotional Materials” has the meaning set forth in Section 4.6.
1.67 “Recoveries” has the meaning set forth in Section 8.4.4.
1.68 “Regulatory Approval” means any and all approvals (including pricing and reimbursement approvals), licenses, registrations or authorizations of any Regulatory Authority, necessary for the Development, Commercialization and Manufacture of the Licensed Product.
1.69 “Regulatory Authority” means any applicable government regulatory authority involved in granting approvals for the Development, Manufacturing, Commercialization, reimbursement and/or pricing of the Licensed Product.
1.70 “Related Party” means a Party’s Affiliates and Sublicensees.
1.71 “Royalty Term” has the meaning set forth in Section 3.1.2.
7
1.72 “Sublicense Agreement” means a written agreement between Green Cross (or its Affiliate) and a Third Party in which Green Cross grants a sublicense to such Third Party of rights licensed by Argos to Green Cross pursuant to this Agreement.
1.73 “Sublicensee” means a Third Party to whom Green Cross grants a sublicense under the rights granted to Green Cross by Argos hereunder.
1.74 “Technology Transfer Completion Target Date” means the date that is [**] months after the date of written request from Green Cross for the Technology Transfer, or such other date as the Parties may agree in writing.
1.75 “Term” has the meaning set forth in Section 9.1.
1.76 “Territory” means (a) with respect to Argos, the Argos Territory and (b) with respect to Green Cross, the Green Cross Territory.
1.77 “Third Party” means an entity other than a Party and its Affiliates.
1.78 “United States” means the United States of America and its territories, possessions and commonwealths.
1.79 “Valid Claim” means a claim of: (a) an issued and unexpired Argos Patent Right, which claim has not been revoked or held unenforceable, unpatentable or invalid by a decision of a court or other governmental agency of competent jurisdiction, which is not appealable or has not been appealed within the time allowed for appeal, and which has not been abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination or disclaimer or otherwise, or (b) a patent application for a patent included within the Argos Patent Rights which has not been cancelled, withdrawn or abandoned or finally rejected by an administrative agency action from which no appeal can be taken.
2.1 License Grants.
2.1.1 Development and Commercialization License.
(a) Subject to the terms and conditions of this Agreement, Argos hereby grants Green Cross a license under and to the Argos Technology to Develop and Commercialize and have Developed and Commercialized the Licensed Product in the Field in the Green Cross Territory.
(b) The license granted pursuant to this Section 2.1.1 is exclusive and royalty-bearing for the Royalty Term, and shall thereafter be a non-exclusive, irrevocable, perpetual, fully paid-up license to Develop and Commercialize the Licensed Product in the Field in the Green Cross Territory. Such license shall include the right for Green Cross and its Affiliates to grant sublicenses as provided in Section 2.2 below. Notwithstanding the foregoing, for clarity Argos retains the full right to import (and have imported) from the Green Cross
8
Territory, and export (and have exported) to the Argos Territory, Licensed Product (and components thereof) for Development, Commercialization and/or Manufacture of the Licensed Product in the Argos Territory.
2.1.2 Manufacturing License. Subject to the terms and conditions of this Agreement, Argos hereby grants Green Cross an exclusive license under and to Argos Technology to Manufacture and have Manufactured in the Green Cross Territory the Licensed Product solely for the purpose of Commercializing the Licensed Product in the Green Cross Territory in the Field. Such license is royalty-bearing for the Royalty Term as set forth in Section 3.1, and shall thereafter be a non-exclusive, irrevocable, perpetual, fully paid-up license to Manufacture the Licensed Product in the Field in the Green Cross Territory. Such license shall include the right to grant sublicenses as provided in Section 2.2 below. For the avoidance of doubt, the license granted pursuant to this Section 2.1.2 shall not include the right to Manufacture or have Manufactured Automated Systems or components thereof.
2.1.3 Argos License. Green Cross hereby grants to Argos (a) an exclusive, worldwide license under and to any and all Green Cross Improvements conceived or reduced to practice by Green Cross or its Related Parties and Green Cross Data to Develop and/or Commercialize products using the Argos Technology in the Argos Territory; and (b) a non-exclusive, worldwide, license under any Green Cross Improvements and Green Cross Data to Manufacture products using the Argos Technology anywhere in the world. Such license shall include the right to grant sublicenses. Unless otherwise agreed, the foregoing license shall be royalty free. However, in the event that Green Cross makes a significant financial investment in generating the Green Cross Improvements and such improvements generate significant commercial benefit in the Argos Territory, Argos will negotiate in good faith a reasonable royalty. For clarification, Argos shall have no obligation to pay any royalty with respect to process improvements or other similar incremental benefits but may have a royalty obligation if Green Cross independently develops a product based on the ArcelisTM system for an indication other than mRCC. Green Cross shall promptly notify Argos in writing after conceiving or reducing to practice a Green Cross Improvement.
2.2.1 Affiliates. The license grants in Section 2.1 shall apply to an entity that is an Affiliate only for so long as such entity remains an Affiliate of such Party and complies in all respects with the obligations of such Party under this Agreement. Each Party hereby guarantees the full payment and performance of its Affiliates under this Agreement.
2.2.2 Sublicense of Green Cross’ Rights. Subject to the terms of Section 2.2.3, with Argos’ prior written consent, not to be unreasonably withheld or delayed, Green Cross and its Affiliates are entitled to grant sublicenses of all or any portion of their rights under this Agreement; provided, however, that Green Cross may not grant a sublicense of Commercialization rights under this Agreement to more than one (1) Third Party in the Green Cross Territory. Consent shall be presumed and deemed given if Argos does not provide a written objection within [**] days of Argos’ receipt of a written request for consent.
2.2.3 Sublicensing Terms. Each sublicense granted by Green Cross pursuant to this Section 2.2 shall be subject and subordinate to the terms and conditions of this Agreement and shall contain
9
terms and conditions consistent with those in this Agreement. Green Cross shall promptly provide Argos with a copy of the fully executed Sublicense Agreement with any Sublicensee, and such Sublicense Agreement shall contain the following provisions: (a) a requirement that such Sublicensee submit applicable sales or other reports consistent with those required under this Agreement; (b) the audit requirement set forth in Section 3.4; (c) a requirement that such Sublicensee comply with the confidentiality and non-use provisions of Article 6 with respect to both Parties’ Confidential Information; and (d) any other provisions required under any Argos In-License. In the event Green Cross becomes aware of a material breach of any Sublicense Agreement by a Sublicensee, Green Cross shall promptly notify Argos of the particulars of same and shall enforce the terms of such Sublicense Agreement. If Green Cross does not cause the Sublicensee to comply with the terms of the Sublicense Agreement within [**] days of Argos’ request, Green Cross shall, upon Argos’ written direction, terminate the sublicense.
2.2.4 Liability. Green Cross shall at all times be responsible for the performance of its Sublicensees under this Agreement.
2.3 In-Licenses. All licenses and other rights granted to Green Cross under this Agreement are subject to the rights and obligations of Argos under the Argos In-Licenses. During the Term, Argos shall maintain the Existing Argos In-Licenses in full force and effect with respect to the rights granted to Green Cross under this Agreement. Green Cross shall comply with all applicable terms and conditions of the Argos In-Licenses, and shall perform and take such actions as may be required to allow Argos to comply with its obligations thereunder, including but not limited to, obligations relating to sublicensing, patent matters, confidentiality, reporting, audit rights, indemnification and diligence. Argos agrees to provide Green Cross with copies of any Argos In-Licenses that are relevant to the rights granted to Green Cross under this Agreement. Confidential Information of Argos or the counterparty may be redacted from such copies, except to the extent that such information is required in order to enable Green Cross to comply with its obligations under this Section 2.3 with respect to such Argos In-License.
2.4 Licenses of Necessary Third Party IP. During the Term, Argos shall be responsible for obtaining licenses of any Necessary Third Party IP for Argos Territory and Green Cross Territory; [**], Green Cross [**], and shall [**] and [**] Argos [**] after the Effective Date. If, [**], Argos [**] Green Cross [**] Green Cross or Argos, [**] Argos [**] Green Cross [**]. Green Cross will have [**] Argos [**]. If [**], Argos [**] Green Cross [**], which shall [**]. Green Cross [**] Argos [**] Green Cross [**] Green Cross [**] Argos [**], Argos’ [**], and Argos will [**] Green Cross [**].
2.5.1 During the Term, Argos shall share with Green Cross, on a [**] basis, its available, unblinded Clinical Data and available study updates (e.g., enrollment, subject demographics, IDMC interim reviews) with respect to the Licensed Product generated during any Clinical Studies (including without limitation Phase II(b) Studies, Phase III Studies and Post Approval Studies). Green Cross shall be entitled to reference such Clinical Data in any Regulatory Approval submissions for the Licensed Product by Green Cross and its Related Parties in the Green Cross Territory.
2.5.2 During the Term, Green Cross shall share with Argos, on a [**] basis, its available, unblinded Clinical Data with respect to the Licensed Product generated during any Clinical Studies
10
(including without limitation Phase II(b) Studies, Phase III Studies and Post Approval Studies). Argos shall be entitled to reference such Clinical Data in any Regulatory Approval submissions by Argos and its Related Parties in the Argos Territory.
2.5.3 Argos will promptly notify Green Cross in writing (“Argos Product Notice”) prior to entering into bona fide negotiations with a Third Party for Development and Commercialization rights in the Green Cross Territory to any product utilizing the Argos Technology other than the Licensed Product (an “ Follow-on Licensed Product”). Such Argos Product Notice shall include material information relating to such Follow-on Licensed Product that Green Cross may reasonably need in order for Green Cross to evaluate the Follow-on Licensed Product. Green Cross shall have [**] days after receipt of the Argos Product Notice to notify Argos in writing of its interest in such Follow-on Licensed Product in the Green Cross Territory. If Green Cross notifies Argos in writing within such [**] day period that it is interested in such Follow-on Licensed Product in the Green Cross Territory, then the Parties shall promptly commence good faith negotiations for a period of up to [**] months after Green Cross receives the Argos Product Notice in an effort to reach a mutually acceptable definitive agreement (or amendment to this Agreement) for such Follow-on Licensed Product in the Green Cross Territory. If (x) Green Cross does not notify Argos in writing within such [**] day period that it is interested in such Follow-on Licensed Product, or (y) despite each Party’s good faith efforts, Argos and Green Cross are not able to reach agreement on and execute a definitive agreement within such [**] month period, then Argos may execute an agreement with any Third Party for Development, Manufacture and Commercialization rights to, or Develop, Manufacture and Commercialize on its own, such Follow-on Licensed Product in the Green Cross Territory and Green Cross’ rights under this Section 2.5.3 with respect to such Follow-on Licensed Product in the Green Cross Territory will terminate. Nothing in this Section 2.5.3 shall be construed as limiting Argos’ right to Develop, Manufacture and Commercialize a Follow-on Licensed Product in the Green Cross Territory on its own.
2.5.4 In the event Green Cross wishes to Develop a Follow-on Product for the Green Cross Territory that Argos is not then Developing or Commercializing in the Green Cross Territory, Green Cross may notify Argos in writing (“Green Cross Product Notice”). Such Green Cross Product Notice shall include material information relating to Green Cross’ plans to Develop a Follow-on Licensed Product that Argos may reasonably need in order for Argos to evaluate the Follow-on Licensed Product. The Parties shall promptly commence good faith negotiations for a period of up to [**] months after Argos receives the Green Cross Product Notice in an effort to reach a mutually acceptable definitive agreement (or amendment to this Agreement) under which Argos would license the Argos Technology to Green Cross for such Follow-on Licensed Product in the Green Cross Territory. If despite each Party’s good faith efforts, Argos and Green Cross are not able to reach agreement on and execute a definitive agreement within such [**] month period, then Argos may Develop, Manufacture and Commercialize on its own, such Follow-on Licensed Product in the Green Cross Territory and Green Cross’ rights under this Section 2.5.4 with respect to such Follow-on Licensed Product in the Green Cross Territory will terminate. Nothing shall in this Section 2.5.3 shall be construed as limiting Argos right to Develop, Manufacture and Commercialize a Follow-on Licensed Product in the Green Cross Territory on its own prior to receipt of an applicable Green Cross Product Notice.
2.6 No Other Rights. Except as otherwise expressly provided in this Agreement, under no circumstances shall a Party hereto, as a result of this Agreement, obtain any ownership interest or other
11
right in any Know-How or Patent Rights of the other Party, including items owned, controlled or developed by the other Party, or provided by the other Party to the receiving Party at any time pursuant to this Agreement.
3.1 Royalties.
3.1.1 Royalties Payable on the Licensed Product. Subject to the terms and conditions of this Agreement, as partial consideration for the licenses and other rights granted in this Agreement, Green Cross shall pay to Argos a royalty of [**] percent of Net Sales [**]. However, if Green Cross’ COGS for Licensed Product sold in any calendar year exceeds [**]% of Net Sales of Licensed Product sold in that year, the royalty rate payable during that year shall be reduced to [**]% of Net Sales [**].
3.1.2 Royalty Term. Royalties for Licensed Product for the treatment of mRCC shall continue to be payable until the fifteenth (15th) anniversary of the First Commercial Sale of Licensed Product for mRCC in Green Cross Territory (the “Royalty Term”). [**], the Royalty Term for a Licensed Product for [**] shall be for a period of fifteen years from the First Commercial Sale of Licensed Product [**]. No royalties shall be due upon the sale or other transfer among Green Cross and its Related Parties, but in such cases the royalty shall be due and calculated upon Green Cross’ or its Related Party’s Net Sales to the first independent Third Party.
3.1.3 Necessary Third Party IP. [**]. Any royalties and any fees, milestones or other payments under the Existing Argos In-Licenses shall be borne exclusively by Argos. [**].
3.1.4 Medical Tourism. Each Party and its Related Parties will use Commercially Reasonable Efforts, to the best of its knowledge, to monitor and prevent sales of Licensed Product in its Territory from being administered in the Territory to a Person whose primary domicile is outside that Party’s Territory, in each case using methods commonly used in the industry for such purpose, and shall promptly inform the other Party of any such activities, and the actions taken to prevent such activities. In any event, if Licensed Product made by or on behalf of a Party (or its Related Parties) is administered in its Territory to a Person whose primary domicile is outside that Party’s Territory for more than [**] individual patients in any Calendar Year, the other Party shall be entitled to reasonable compensation determined in accordance with Section 10.
3.2 Milestones. Subject to the terms and conditions of this Agreement, as partial consideration for the licenses and other rights granted in this Agreement, Green Cross shall make the non-refundable, non-creditable milestone payments to Argos set forth below no later than [**] days after the earliest date on which the corresponding milestone event has first been achieved with respect to the Licensed Product for mRCC.
| Milestone Payment | ||||
| (i) | First submission of a Regulatory Approval for the Licensed Product in the Green Cross Territory | $500,000 | ||
| (ii) | First approval of the Regulatory Approval by the Regulatory Authority for the Licensed Product in the Green Cross Territory | $500,000 | ||
Milestone payments, if any, for any Follow-on Licensed Product shall be as agreed pursuant to Section 2.5.3.
12
3.3 Reports; Payment of Royalty. Green Cross shall furnish to Argos a written report within [**] days after the end of [**] showing the quantity of Licensed Product sold in the Green Cross Territory, the gross sales of Licensed Product in the Green Cross Territory, the itemized deductions for Licensed Product for the Green Cross Territory included in the calculation of Net Sales, the Net Sales in the Green Cross Territory of the Licensed Product during the reporting period, the royalties payable under this Agreement, and milestones achieved. In addition, Green Cross shall prepare and deliver to Argos any additional reports as required under the Argos In-Licenses, in each case within a time period sufficiently in advance to enable Argos to comply with its obligations under such Argos In-Licenses. Royalties shown to have accrued by each report shall be due and payable on the date such report is due. Green Cross and its Related Parties shall keep complete and accurate records in sufficient detail to enable the royalties and other payments payable hereunder and to Third Parties under the Argos In-Licenses to be determined.
3.4 Audits.
3.4.1 Upon the written request of Argos delivered at least [**] days in advance and not more than [**], Green Cross and its Related Parties shall permit an independent certified public accounting firm of internationally-recognized standing selected by Argos and reasonably acceptable to Green Cross, at Argos’ expense except as set forth below, to have access during normal business hours to such of the records of Green Cross and its Related Parties as may be reasonably necessary to verify the accuracy of the royalty and other reports hereunder for any year ending not more than [**] years prior to the date of such request for the sole purpose of verifying the basis and accuracy of payments made under this Agreement and mRCC Licensed Product Development Expenses reported.
3.4.2 If such accounting firm identifies a discrepancy made during such period, the appropriate Party shall pay the other Party the amount of the discrepancy, together with interest calculated at the rate of [**] percent ([**]%) per month (or such higher rate as may be required pursuant to any applicable In-License) or the maximum amount permitted by applicable law, from the time of the over-payment or under-payment, within [**] business days of the date Argos delivers to Green Cross such accounting firm’s written report so concluding, or as otherwise agreed by the Parties in writing. Such written report shall be binding upon the Parties. The fees charged by such accounting firm shall be paid by Argos, unless such discrepancy represents an underpayment by Green Cross exceeding [**] percent ([**]%) of the total amounts due hereunder in the audited period, in which case such fees shall be paid by Green Cross.
3.4.3 Green Cross shall comply with all applicable audit requirements in the Argos In-Licenses and shall include in each Sublicense Agreement granted by it pursuant to this Agreement a provision requiring the Sublicensee to make reports to Argos, to keep and maintain records of sales made pursuant to such Sublicense Agreement and to grant access to such records by Argos’ independent accountant to the same extent required of Green Cross under this Agreement.
3.4.4 Argos shall treat all financial information subject to review under this Section 3.4 or under any Sublicense Agreement in accordance with the confidentiality and non-use provisions of this Agreement, and shall cause its accounting firm to enter into an acceptable confidentiality agreement with Green Cross and/or its Related Parties obligating it to retain all such information in confidence pursuant to such confidentiality agreement.
13
3.5 Payment Exchange Rate. All payments to be made under this Agreement shall be made in United States dollars and shall be paid by bank wire transfer in immediately available funds to such bank account in the United States as may be designated in writing by Argos from time to time. In the case of COGs and Net Sales, the rate of exchange to be used in computing the amount of currency equivalent in United States dollars shall be made at the rate of exchange utilized by such party in its worldwide accounting system and calculated in accordance with K-IFRS, prevailing on the [**] last business day of the month preceding the month in which such sales are recorded, but in any case consistent with the requirements of the Argos In-Licenses.
3.6 Registration. Green Cross will promptly make all filings with and submissions to all governmental or regulatory authorities and obtain and maintain all consents, permits, registrations and authorizations that are necessary or required in order for Green Cross to make timely payments under this Agreement, including, without limitation, any foreign exchange approvals or requirements. Green Cross will promptly provide Argos with evidence thereof upon Argos’ written request.
3.7 Income Tax Withholding. If laws, rules or regulations require withholding of income taxes or other taxes imposed upon payments set forth in this Article 3, Green Cross shall make such withholding payments as required and subtract such withholding payments from the payments set forth in this Article 3. Green Cross shall submit appropriate proof of payment of the withholding taxes to Argos within a reasonable period of time. At the request of Argos, Green Cross shall, at its cost, give Argos such reasonable assistance, which shall include the provision of appropriate certificates of such deductions made together with other supporting documentation as may be required by the relevant tax authority, to enable Argos to claim exemption from such withholding or other tax imposed or obtain a repayment, reduction or credit and shall upon request provide such additional documentation from time to time as is reasonably required to confirm the payment of tax.
4. DEVELOPMENT AND COMMERCIALIZATION RESPONSIBILITIES
4.1 Diligence. Green Cross will use Commercially Reasonable Efforts to Develop for Regulatory Approval and Commercialization in the Green Cross Territory the Licensed Product. In addition, Green Cross shall use Commercially Reasonable Efforts to Commercialize the Licensed Product in the Green Cross Territory.
4.2 Development Activities. Argos will continue its Licensed Product clinical development program in Argos Territory and will use commercially reasonable efforts to obtain regulatory approval to sell the Licensed Product in the United States of America. Green Cross, at its sole decision, cost and expense, may conduct any studies required by Ministry of Food and Drug Safety (“MFDS”) to obtain regulatory approval to sell Licensed Product and other Products it acquires rights to in Green Cross Territory and will use commercially reasonable efforts to obtain regulatory approval to sell Product in Green Cross Territory. Neither Party may conduct Clinical Studies or other Development activities with respect to the Licensed Product in the Field in the Territory of the other Party without the other Party’s prior written consent, which consent shall not be unreasonably withheld or delayed by the other Party.A Party may reasonably withhold consent if granting consent would conflict with a Party’s contractual obligations to a Sublicensee or other Third Party.
14
4.3 Commercialization Plan. Commencing with the Initiation of any Clinical Study of the Licensed Product, Green Cross shall prepare and deliver to Argos, (a) a Commercialization strategy plan for the following [**] year period that would include, among other things, a description of the planned Post-Approval Studies, if applicable, which plan would be updated at least [**], and (b) by no later than [**], a written plan that describes in detail the Commercialization activities to be undertaken with respect to the Licensed Product in the Green Cross Territory in the [**] and the dates by which such activities are targeted to be accomplished (each, a “Commercialization Plan”).
4.4 Reporting Obligations. Green Cross shall prepare and deliver to Argos, by no later than each [**] (for the period ending December 31 of the prior Calendar Year), written reports summarizing Green Cross’ Commercialization activities for the Licensed Product performed to date (or updating such report for activities performed since the last such report submitted hereunder, as applicable). In addition, Green Cross shall provide Argos with written notice of (a) all filings and submissions for Regulatory Approval regarding the Licensed Product in the Green Cross Territory in a timely manner; (b) all Regulatory Approvals obtained or denied, the filing of any IND for the Licensed Product, and the First Commercial Sale of the Licensed Product in the Green Cross Territory, within [**] days of such event; and (c) the Initiation of each Clinical Study of the Licensed Product by or on behalf of Green Cross within [**] business days of such event; provided, however, that in all circumstances, Green Cross shall inform Argos of such event at least [**] business days prior to public disclosure of such event by Green Cross. Moreover, Green Cross shall use Commercially Reasonable Efforts to prepare and deliver to Argos any additional reports reasonably requested by Argos to enable it to meet its obligations under the Argos In-Licenses, in each case sufficiently in advance to enable Argos to comply with its obligations under the Argos In-Licenses. Green Cross shall also provide such other information to Argos as Argos may reasonably request and shall keep Argos reasonably informed of Green Cross’ Commercialization activities with respect to the Licensed Product.
4.5 Sales and Distribution. Each Party and its Related Parties shall be responsible for booking sales and shall store and distribute the Licensed Product in its own Territory. If a Party receives any orders for the Licensed Product in the other Party’s Territory or if a Party has reason to believe that a Licensed Product is intended to be administered in the Territory to a Person whose primary domicile is outside the that Party’s Territory, it shall refer such orders to the other Party. Moreover, each Party and its Related Parties shall be solely responsible for handling all returns of a Licensed Product, as well as all aspects of a Licensed Product order processing, invoicing and collection, distribution, inventory and receivables, in its own Territory.
4.6 Advertising and Promotional Materials. Green Cross will be responsible for the creation, preparation, production, reproduction and filing with the applicable Regulatory Authorities, of relevant written sales, promotion and advertising materials relating to the Licensed Product (“Promotional Materials”) for use in the Green Cross Territory. All such Promotional Materials will be compliant with all applicable laws, rules and regulations, and consistent with the Commercialization Plan for the Green Cross Territory.
4.7 Export Monitoring. Each Party and its Related Parties will use Commercially Reasonable Efforts to monitor and prevent (i) exports of Licensed Product from its own Territory to the other Party’s Territory, and (ii) sales of Licensed Product in its Territory from being administered in the Territory to a Person whose primary domicile is outside that Party’s Territory, in each case using methods commonly used in the industry for such purpose, and shall promptly inform the other Party of
15
any such activities, and the actions taken to prevent such activities. Each Party agrees to take any actions reasonably requested in writing by the other Party that are consistent with applicable law and regulation to prevent such activities.
4.8 Records. Green Cross will maintain scientific records, in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, which will fully and properly reflect all work done and results achieved in the performance of the Development activities with respect to the Licensed Product.
4.9 Regulatory Matters.
4.9.1 Regulatory Filings and Interactions. As between the Parties, each Party will own any regulatory documents and applications submitted to the applicable Regulatory Authorities in its own Territory with respect to the Licensed Product, and each Party will, with respect to its own Territory and the Licensed Product, (i) oversee, monitor and coordinate all regulatory actions, communications and filings with, and submissions to, each Regulatory Authority, (ii) be responsible for interfacing, corresponding and meeting with each Regulatory Authority, (iii) be responsible for maintaining all regulatory filings, and (iv) apprise the other Party of all material communications from Regulatory Authorities as soon as reasonably possible but in any event within [**] business days. Each Party will have the right to reference the other Party’s (and the other Party’s Related Parties’ subject to any limitations set forth in a Party’s agreement with such Related Parties) INDs and other filings with and submissions to Regulatory Authorities with respect to the Licensed Product for the purpose of conducting its Development activities and to otherwise obtain Regulatory Approval of the Licensed Product in its own Territory.
4.9.2 Complaints; Adverse Event Reporting Procedures; Notice of Adverse Events Affecting the Licensed Product. Each Party will maintain a record of any and all complaints it or its Related Parties receive with respect to the Licensed Product. Each Party will notify the other Party in reasonable detail of any such complaints within sufficient time to allow the other Party and its Related Parties to comply with any and all regulatory and other requirements imposed upon them in any jurisdiction in which the Licensed Product is being marketed or tested in Clinical Studies and/or Post-Approval Studies. Each Party will maintain at its own expense an adverse event database for the Licensed Product, and the other Party will have access to all data in such adverse event database. Notwithstanding the foregoing, each Party will report to the other Party the details around any adverse events and serious adverse events relating to the Licensed Product in its Control within the time periods for such reporting as specified in the Pharmacovigilance Agreement (defined below). Each Party shall be responsible, at its own expense, for obtaining all adverse event information and safety data relating to the Licensed Product from its Related Parties in a timely manner, and for submitting adverse event reports with respect to the Licensed Product to the applicable Regulatory Authorities in its own Territory. Within [**] months after the Effective Date, the Parties will develop and agree in writing upon a pharmacovigilance agreement (“Pharmacovigilance Agreement”) that will include safety data exchange procedures governing the coordination of collection, investigation, reporting, and exchange of information concerning any adverse experiences, and any product quality and product complaints involving adverse experiences, related to the Licensed Product, sufficient to enable each Party to comply with its legal and regulatory obligations. In addition, each Party shall promptly notify the other if such Party becomes aware of any information or circumstance that is likely to have a material adverse effect on the Development, Manufacture or Commercialization of the Licensed Product in the other Party’s Territory.
16
4.9.3 Recalls, Market Withdrawals or Corrective Actions. In the event that any Regulatory Authority issues or requests a recall or takes a similar action in connection with the Licensed Product in a Territory, or in the event either Party determines that an event, incident or circumstance has occurred that may result in the need for a recall or market withdrawal in its own Territory, the Party notified of such recall or similar action, or the Party that desires such recall or similar action, shall within [**] hours advise the other Party thereof by telephone, or by email or facsimile together with telephone confirmation. Each Party, in consultation with the other Party, shall decide whether to conduct a recall in its own Territory and the manner in which any such recall shall be conducted (except in the case of a government mandated recall, when such Party may act without such advance notice but shall notify the other Party as soon as possible). [**]. Each Party will make available all of its pertinent records that may be reasonably requested in order to effecting a recall in the other Party’s Territory.
4.10 Third Parties. Green Cross shall be entitled to utilize the services of Third Party contract research and contract manufacturing organizations to perform its Development and Manufacturing activities under this Agreement; provided, that (a) Green Cross shall ensure that such Third Party operates in a manner consistent with the terms of this Agreement and (b) Green Cross shall remain at all times fully liable for its respective responsibilities. Green Cross shall ensure that any such Third Party agreement shall include confidentiality, non-disclosure and non-use provisions that are substantially similar to those set forth in Article 6 of this Agreement and shall obtain ownership of any Green Cross Improvements developed, conceived or reduced to practice by such Third Party in the performance of such agreement. Green Cross shall provide Argos with a copy of the fully executed agreement and any amendment thereto with any contract manufacturing organization together with a certified English translation, in each case within [**] days of effectiveness.
5. MANUFACTURE AND SUPPLY OF THE LICENSED PRODUCT
5.1 Green Cross Responsibilities. Green Cross will have responsibility, at its expense, to obtain all its requirements of Licensed Product for Development and Commercialization of the Licensed Product in the Green Cross Territory, and will use Commercially Reasonable Efforts to Manufacture Licensed Product, or have Licensed Product Manufactured, at the most efficient scale.
5.2 Technology Transfer Responsibilities. Argos shall use Commercially Reasonable Efforts to transfer to Green Cross, or to arrange to have transferred to Green Cross by a Third Party, the Argos Technology set forth on Schedule E, which transfer will commence as soon as practicable after the Effective Date but not later than [**] days after the date of written request by Green Cross for the Technology Transfer, and such that the Technology Transfer is completed no later than the Technology Transfer Completion Target Date. Green Cross shall provide such written request as soon as reasonably practicable following the Effective Date and in any event before submission for Regulatory Approval for the mRCC Licensed Product in the United States of America. [**]. Green Cross would not administer any Licensed Product to humans in Clinical Studies or otherwise until Argos has determined that the Argos Technology has been satisfactorily transferred.
17
5.3 Automated Systems. Notwithstanding the foregoing, in no event shall Argos be obligated to transfer any technology associated with the Automated System. Upon completion of the development of the Automated System and the approval of its use by applicable Regulatory Authorities in the Green Cross Territory, Argos shall supply Green Cross’ requirements for Automated Systems pursuant to a supply agreement to be negotiated in good faith by the Parties; provided, however, that the price for Automated Systems to be included in such supply agreement shall be Argos’ fully burdened cost of supplying the Automated Systems.
6. CONFIDENTIALITY AND PUBLICATION
6.1 Nondisclosure Obligation. (a) All Confidential Information disclosed by one Party to the other Party hereunder shall be maintained in confidence by the receiving Party and shall not be disclosed to a Third Party or used for any purpose except as set forth herein without the prior written consent of the disclosing Party, except to the extent that such Confidential Information:
| (i) | is known by the receiving Party at the time of its receipt, and not through a prior disclosure, directly or indirectly, by the disclosing Party, as documented by the receiving Party’s business records; |
| (ii) | is in the public domain by use and/or publication before its receipt from the disclosing Party, or thereafter enters the public domain through no fault of the receiving Party or its Related Parties; |
| (iii) | is subsequently disclosed to the receiving Party by a Third Party who may lawfully do so and is not under an obligation of confidentiality to the disclosing Party; or |
| (iv) | is developed by the receiving Party independently of Confidential Information received from the disclosing Party, as documented by the receiving Party’s business records. |
(b) Notwithstanding the obligations of confidentiality, non-disclosure and non-use set forth above and in Section 6.2 below, a receiving Party may provide Confidential Information disclosed to it, and disclose the existence and terms of this Agreement as may be reasonably required in order to perform its obligations and to exploit its rights under this Agreement, and specifically to (i) Related Parties, and their employees, directors, agents, consultants, advisors and/or other Third Parties for the performance of its obligations hereunder (or for such entities to determine their interest in performing such activities) in accordance with this Agreement in each case who are bound by confidentiality, non-disclosure and non-use obligations substantially similar to those set forth herein; (ii) governmental or other Regulatory Authorities in order to obtain patents or perform its obligations or exploit its rights under this Agreement; provided, that such Confidential Information shall be disclosed only to the extent reasonably necessary to do so, (iii) the extent required by applicable law, including without limitation by the rules or regulations of the United States Securities and Exchange Commission, Korean Financial Supervisory Commission or similar regulatory agency in a country other than the United States or of any stock exchange or listing entity, (iv) any bona fide actual or prospective underwriters, investors, lenders or other financing sources and any bona fide actual or prospective collaborators or strategic partners and to consultants and advisors of such Party, in each case who are bound by confidentiality, non-disclosure and non-use obligations substantially similar to those set forth herein, and (v) to Third Parties to the extent a Party is required to do so pursuant to the terms of an In-License.
18
If a Party is required by judicial or administrative process to disclose Confidential Information that is subject to the non-disclosure provisions of this Section 6.1 or Section 6.2, such Party shall promptly inform the other Party of the disclosure that is being sought in order to provide the other Party an opportunity to challenge or limit the disclosure obligations. Confidential Information that is disclosed by judicial or administrative process shall remain otherwise subject to the confidentiality, non-disclosure and non-use provisions of this Section 6.1 and Section 6.2, and the Party disclosing Confidential Information pursuant to law or court order shall, at the other Party’s expense, take all steps reasonably practical, including without limitation seeking an order of confidentiality, to ensure the continued confidential treatment of such Confidential Information. In addition to the foregoing restrictions on public disclosure, if either Party concludes that a copy of this Agreement must be filed with the United States Securities and Exchange Commission or similar regulatory agency in a country other than the United States, such Party shall provide the other Party with a copy of this Agreement showing any sections as to which the Party proposes to request confidential treatment, will provide the other Party with an opportunity to comment on any such proposal and to suggest additional portions of the Agreement for confidential treatment, and will take such Party’s reasonable comments into consideration before filing the Agreement.
6.2 Publicity. (a) Except as set forth in Section 6.1 above and clause (b) below, the terms of this Agreement may not be disclosed by either Party, and no Party shall use the name, trademark, trade name or logo of the other Party or its employees in any publicity, news release or disclosure relating to this Agreement or its subject matter, without the prior express written permission of the other Party, except as may be required by law or expressly permitted by the terms hereof.
(b) As soon as practicable after the execution of this Agreement by both Parties, the Parties shall use good faith efforts to agree in writing upon a press release to be issued jointly by the Parties publicizing the execution of this Agreement. After such initial press release, neither Party shall issue a press release or public announcement relating to this Agreement without the prior written approval of the other Party, which approval shall not be unreasonably withheld or delayed, except that a Party may (i) once a press release or other written statement is approved in writing by both Parties, make subsequent public disclosure of the information contained in such press release or other written statement without the further approval of the other Party, and (ii) issue a press release or public announcement as required, in the reasonable judgment of such Party, by applicable law, including without limitation by the rules or regulations of the United States Securities and Exchange Commission or similar regulatory agency in a country other than the United States or of any stock exchange or listing entity.
7. REPRESENTATIONS, WARRANTIES AND COVENANTS; INDEMNIFICATION
7.1 Mutual Representations and Warranties. Each Party represents and warrants to the other Party that as of the Effective Date of this Agreement:
7.1.1 It is duly organized and validly existing under the laws of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Agreement, and to carry out the provisions hereof.
19
7.1.2 It is duly authorized to execute and deliver this Agreement, and to perform its obligations hereunder, and the person or persons executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate action.
7.1.3 This Agreement is legally binding upon it and enforceable in accordance with its terms. The execution, delivery and performance of this Agreement by it does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party and by which it may be bound, or with its charter or by-laws.
7.1.4 It has not granted, and will not grant, during the Term, any right to any Third Party that would conflict with the rights granted to the other Party hereunder.
7.1.5 Neither Party nor any of its Affiliates has been debarred or is subject to debarment and neither Party nor any of its Affiliates will use in any capacity, in connection with the exercise of its rights and the performance of its obligations under this Agreement, any person or entity that has been debarred pursuant to Section 306 of the United States Federal Food, Drug, and Cosmetic Act or any similar law in any foreign jurisdiction, or that is the subject of a conviction described in such section or similar law in any foreign jurisdiction. Each Party agrees to inform the other Party in writing immediately if it or any person or entity that is performing activities under this Agreement, is debarred or is the subject of a conviction described in Section 306 or similar law in any foreign jurisdiction, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to the best of the notifying Party’s knowledge, is threatened, relating to the debarment or conviction of the notifying Party or any person or entity used in any capacity by such Party or any of its Affiliates in connection with the performance of its obligations under this Agreement.
7.2 Additional Representations and Warranties of the Parties.
7.2.1 Additional Representations and Warranties of Argos. Argos represents and warrants to Green Cross that:
(a) As of the Effective Date, except for any Argos Know-How Controlled by Argos under an Argos In-License and sublicensed to Green Cross, Argos is the sole and exclusive owner of all right, title and interest in and to the Argos Know-How. As of the Effective Date, Argos has no Knowledge of any claim made against it in the Green Cross Territory challenging Argos’ Control of the Argos Technology or making any adverse claim of ownership of the Argos Technology.
(b) Listed on Schedule D are all the Argos In-Licenses applicable to the Green Cross Territory existing as of the Effective Date.
(c) As of the Effective Date, (i) each Existing Argos In-License is valid, binding and in full force and effect, (ii) Argos is in compliance in all material respects with its material obligations under each Existing Argos In-License, (iii) to Argos’ Knowledge, each Third Party is in compliance in all materials respects with its material obligations under each Existing Argos In-License and (iv) no party has claimed a breach of, or initiated any dispute resolution proceedings under, any Existing Argos In-License.
20
(d) As of the Effective Date and to Argos’ Knowledge, Argos has not received any written notice from any Third Party asserting or alleging that any Development or Manufacture of the Licensed Product by Argos prior to the Effective Date infringed or misappropriated the Patent Rights or other intellectual property rights of such Third Party.
(e) As of the Effective Date, Argos has made available to Green Cross all material information that Argos Controls relating to the Development or Manufacture of the Licensed Product as conducted to such date.
(f) All Clinical Data delivered by Argos pursuant to Section 2.5.1 will have been collected in compliance with all applicable laws in the country in which the applicable Clinical Study(s) were conducted, and, to Argos’ Knowledge, will be true and accurate in all material respects.
(g) NOTWITHSTANDING ANYTHING IN THIS AGREEMENT TO THE CONTRARY, ARGOS MAKES NO REPRESENTATIONS OR WARRANTIES THAT ANY PATENT RIGHTS (OTHER THAN ARGOS PATENT RIGHTS TO KOREAN PATENTS ISSUED AS OF THE EFFECTIVE DATE OF THIS AGREEMENT) THAT COVER THE DEVELOPMENT, MANUFACTURE OR COMMERCIALIZATION OF LICENSED PRODUCT IN THE GREEN CROSS TERRITORY WILL ISSUE IN THE GREEN CROSS TERRITORY.
7.2.2 Additional Representations and Warranties of Green Cross. Green Cross represents, warrants and covenants to Argos that:
(a) It has or has the ability to obtain and will maintain as and when necessary the financial and other capabilities reasonably necessary to discharge its obligations under this Agreement and will pay in a timely manner all costs and expenses associated therewith.
(b) All Clinical Data delivered by Green Cross pursuant to Section 2.5.2 will have been collected in compliance with all applicable laws in the country in which the applicable Clinical Study(s) were conducted, and, to Green Cross’ Knowledge, will be true and accurate in all material respects.
(c) It will comply with all laws in the Green Cross Territory applicable to the exercise of its rights and performance of its obligations hereunder.
7.3 Warranty Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, TO THE OTHER PARTY AND HEREBY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, TITLE AND NONINFRINGEMENT. EACH PARTY HEREBY DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE DEVELOPMENT, MANUFACTURE OR COMMERCIALIZATION OF THE LICENSED PRODUCT PURSUANT TO THIS AGREEMENT WILL BE SUCCESSFUL OR THAT ANY PARTICULAR SALES LEVEL WITH RESPECT TO THE LICENSED PRODUCT WILL BE ACHIEVED.
21
7.4 Certain Covenants.
7.4.1 Exclusivity. Except as expressly provided in this Agreement, neither Green Cross nor its Related Parties will, alone or with or through a Third Party, during the Term, research, develop, manufacture or commercialize any cell therapy outside of the scope of this Agreement for the treatment of mRCC in humans for use or sale in the Green Cross Territory [**]. Green Cross [**] of this Agreement, Green Cross [**] Argos [**]. Upon request by Argos, Green Cross shall promptly provide Argos with reasonable assurance that Green Cross is continuing to devote substantial resources to the continued development and commercialization of Arcelis for the treatment of mRCC in humans in the Green Cross Territory.
7.4.2 Compliance. Green Cross and its Related Parties shall conduct the Development, Manufacture and Commercialization of the Licensed Product in accordance with all applicable laws, rules and regulations, including without limitation current governmental regulations concerning good laboratory practices, good clinical practices and good manufacturing practices (including but not limited the guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)).
7.4.3 Employee Inventions. Prior to performing any activities in connection with this Agreement, Green Cross shall ensure that its and its Affiliates’ employees, agents and consultants have executed valid and binding agreements with it that assign and otherwise effectively vest in Green Cross any and all rights that such employees, agents and/or consultants might otherwise have in any Green Cross Improvements made by such employees, agents and/or consultants without any obligation to pay any royalties or other consideration to such employees, agents and/or consultants. Should any royalties or other consideration become payable to such employees, agents and/or consultants, Green Cross shall remain solely responsible for making such payments.
7.5 Indemnification.
7.5.1 General Indemnification by Green Cross. Green Cross shall indemnify, hold harmless, and defend Argos, its Related Parties and the other parties to the Argos In-Licenses, and their respective directors, officers, employees and agents (“Argos Indemnitees”) from and against any and all Third Party claims, suits, losses, liabilities, damages, costs, fees and expenses (including reasonable attorneys’ fees) (collectively, “Losses”) to the extent arising out of or resulting from, directly or indirectly, (a) any breach of this Agreement by Green Cross, or (b) the negligence or willful misconduct by or of Green Cross, its Related Parties, and their respective directors, officers, employees and agents.
7.5.2 General Indemnification by Argos. Argos shall indemnify, hold harmless, and defend Green Cross, its Related Parties and their respective directors, officers, employees and agents (“Green Cross Indemnitees”) from and against any and all Losses to the extent arising out of or resulting from, directly or indirectly, (a) any breach of this Agreement by Argos, or (b) the negligence or willful misconduct by or of Argos, its Related Parties, and their respective directors, officers, employees and agents.
7.5.3 Product Liability.
(a) Green Cross shall indemnify and hold harmless the Argos Indemnitees from, against and in respect of any and all Losses arising out of Third Party product liability claims incurred or suffered by the Argos Indemnitees, or any of them, directly or indirectly relating to Licensed Product Developed, Manufactured or Commercialized by Green Cross or its Related Parties.
22
(b) Argos shall indemnify, defend and hold harmless the Green Cross Indemnitees from, against and in respect of any and all Losses arising out of Third Party product liability claims incurred or suffered by the Green Cross Indemnitees, or any of them, directly or indirectly relating to the Licensed Product Developed, Manufactured or Commercialized by Argos or its Related Parties.
7.5.4 Indemnification Procedure. In the event of any such claim against any Green Cross Indemnitee or Argos Indemnitee (individually, an “Indemnitee”), the indemnified Party shall promptly notify the other Party in writing of the claim and the indemnifying Party shall manage and control, at its sole expense, the defense of the claim and its settlement. The Indemnitee shall cooperate with the indemnifying Party and may, at its option and expense, be represented in any such action or proceeding. The indemnifying Party shall not be liable for any settlements, litigation costs or expenses incurred by any Indemnitee without the indemnifying Party’s written authorization. Notwithstanding the foregoing, if the indemnifying Party believes that any of the exceptions to its obligation of indemnification of the Indemnitees set forth in Sections 7.5.1, 7.5.2 or 7.5.3 may apply, the indemnifying Party shall promptly notify the Indemnitees, which shall then have the right to be represented in any such action or proceeding by separate counsel at their expense; provided, that the indemnifying Party shall be responsible for payment of such expenses if the Indemnitees are ultimately determined to be entitled to indemnification from the indemnifying Party.
7.6 Limitation of Liability. NEITHER PARTY HERETO WILL BE LIABLE FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES ARISING OUT OF THIS AGREEMENT OR THE EXERCISE OF ITS RIGHTS HEREUNDER, INCLUDING LOST PROFITS, REGARDLESS OF ANY NOTICE OF SUCH DAMAGES, EXCEPT AS A RESULT OF A PARTY’S WILLFUL MISCONDUCT OR A MATERIAL BREACH OF THE CONFIDENTIALITY AND NON-USE OBLIGATIONS IN ARTICLE 6. NOTHING IN THIS SECTION 7.6 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY.
7.7 Insurance. Each Party shall obtain and/or maintain insurance during the Term and for a period of at least [**] years after the last commercial sale of the Licensed Product under this Agreement, with a reputable, solvent insurer in an amount appropriate for its business and products of the type that are the subject of this Agreement, and for its obligations under this Agreement. Without limiting the foregoing, such insurance coverage shall include amounts, respectively, which are reasonable and customary in pharmaceutical and cell therapy industry for companies of comparable size and activities at the respective Territory of each Party. Each Party shall name the other as an additional insured under such policies, and, upon request, each Party shall provide the other Party with evidence of the existence and maintenance of such insurance coverage. Such insurance coverage shall include, without limitation, general commercial liability insurance and/or clinical trials insurance to provide insurance coverage with respect to each Clinical Study conducted by or on behalf of Green Cross involving Licensed Product.
23
8. INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION AND RELATED MATTERS
8.1 Inventorship. Inventorship for patentable inventions conceived or reduced to practice during the course of the performance of activities pursuant to this Agreement shall be determined in accordance with the principles that are used to determine inventorship under the United States patent laws.
8.2 Ownership. Subject to the licenses granted by Argos pursuant to this Agreement, Argos shall own the entire right, title and interest in and to all inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered solely by employees or consultants of Argos or acquired solely by Argos. Subject to the licenses granted by Green Cross pursuant to this Agreement, Green Cross shall own the entire right, title and interest in and to all inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered solely by employees or consultants of Green Cross or acquired solely by Green Cross. The Parties shall jointly own any inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered jointly during the Term (“Joint IP”).
8.3 Prosecution and Maintenance of Patent Rights.
8.3.1 Argos Technology. Argos has the sole right to, at Argos’s discretion, file, conduct prosecution, and maintain (including without limitation the defense of any interference or opposition proceedings), all Argos Patent Rights. Argos agrees to use Commercially Reasonable Efforts to prosecute and maintain such Argos Patent Rights in the Green Cross Territory.
8.3.2 Green Cross Technology. Green Cross has the sole right to, at Green Cross’ discretion, file, conduct prosecution, and maintain (including without limitation the defense of any interference or opposition proceedings), all Patent Rights comprising Green Cross Improvements. Green Cross agrees to use Commercially Reasonable Efforts to prosecute and maintain the Green Cross Improvements in the Argos Territory, and will notify Argos in writing if Green Cross elects not to continue to seek or maintain any such Patent Rights in the Argos Territory.
8.3.3 Joint IP. Subject to Green Cross’ continuing right to the timely prior review of and comment on material documents, Argos has the sole right to, at Argos’s discretion, incorporate reasonable and timely presented comments, file, conduct prosecution, and maintain (including without limitation the defense of any interference or opposition proceedings), all Patent Rights comprising Joint IP, in the names of both Argos and Green Cross. Green Cross shall use Commercially Reasonable Efforts to make available to Argos or its authorized attorneys, agents or representatives, such of its employees, consultants or representatives as Argos in its reasonable judgment deems necessary in order to assist it in obtaining patent protection for Joint IP Each Party shall sign, or use Commercially Reasonable Efforts to have signed, all legal documents necessary to file and prosecute patent applications or to obtain or maintain patents in respect of Joint IP, at its own cost.
8.3.4 Contingent Rights. (a) In the event Argos has been granted a license under such Green Cross Improvements and Green Cross elects not to seek or continue to seek or maintain patent protection on any Green Cross Improvements in the Argos Territory, Argos shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain in any country patent protection on such Green Cross Improvements in the name of Green Cross. Green Cross shall use Commercially Reasonable Efforts to make available to Argos its authorized attorneys, agents or representatives, and such of its employees as are reasonably necessary to assist Argos in obtaining and maintaining the
24
patent protection described under this Section 8.3.4(a). Green Cross shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary to file and prosecute such patent applications or to obtain or maintain such patents.
(b) In the event that Argos elects not to seek or continue to seek or maintain patent protection on Joint IP in the Green Cross Territory, Green Cross shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain in any country in the Green Cross Territory patent protection on Joint IP in the names of Green Cross with respect to Joint IP. Argos shall use Commercially Reasonable Efforts to make available to Green Cross its authorized attorneys, agents or representatives, and such of its employees as are reasonably necessary to assist Green Cross in obtaining and maintaining the patent protection described under this Section 8.3.4(b). Argos shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary to file and prosecute such patent applications or to obtain or maintain such patents.
8.3.5 Cooperation; Patent Challenges. Each Party hereby agrees: (a) to make its employees, agents and consultants reasonably available to the other Party (or to the other Party’s authorized attorneys, agents or representatives), to the extent reasonably necessary to enable such Party to undertake patent prosecution; (b) to provide the other Party with copies of all material correspondence pertaining to prosecution with the patent offices in the Green Cross Territory; (c) to cooperate, if necessary and appropriate, with the other Party in gaining patent term extensions wherever applicable to Patent Rights; and (d) to endeavor in good faith to coordinate its efforts with the other Party to minimize or avoid interference with the prosecution and maintenance of the other Party’s patent applications. Without limiting the foregoing, the Party prosecuting and maintaining the Patent Right shall furnish to the other Party copies of substantive documents (e.g., applications, office actions and responses) relevant to any such efforts in advance with sufficient time for such other Party to review and provide comments on such documents, and shall in good faith take such comments into account.
8.3.6 Patent Expenses. The patent filing, prosecution and maintenance expenses incurred after the Effective Date with respect to Patent Rights (“Patent Expenses”) shall be borne by each Party filing, prosecuting and maintaining such Patent Rights under this Section 8.3; [**].
8.4.1 Notices. Each Party shall promptly report in writing to the other Party during the Term any (a) known or suspected infringement of any Argos Technology, Green Cross Improvements or Joint IP or (b) unauthorized use or misappropriation of any Confidential Information, Argos Technology, Green Cross Improvements or Joint IP by a Third Party of which it becomes aware, and shall provide the other Party with all available evidence supporting such infringement, or unauthorized use or misappropriation.
8.4.2 Rights to Enforce.
(a) Green Cross’ First Right. Green Cross shall have the sole and exclusive right (but not obligation) to initiate an infringement or other appropriate suit anywhere in the world against any Third Party who at any time has infringed, or is suspected of infringing, any Green Cross Improvements, or of using without proper authorization any Green Cross Know-How incorporated into
25
the Green Cross Improvements. Notwithstanding the foregoing, in the event such infringement, suspected infringement, or unauthorized use is by an Argos Related Party, the Parties shall discuss in good faith a resolution to the foregoing prior to engaging in litigation.
(b) Argos’s First Right. Argos shall have the sole and exclusive right (but not obligation) to initiate an infringement or other appropriate suit anywhere in the world against any Third Party who at any time has infringed, or is suspected of infringing, any Argos Patent Rights, Argos Know-How, or Joint IP. Notwithstanding the foregoing, in the event such infringement, suspected infringement, or unauthorized use in the Green Cross Territory is by a Green Cross Related Party, the Parties shall discuss in good faith for up to [**] days a resolution to the foregoing prior to engaging in litigation.
8.4.3 Step-In Rights. (a) Argos will consider in good faith any request from Green Cross to initiate an infringement or other appropriate suit against any Third Party with respect to matters described in Section 8.4.2(b) occurring in the Green Cross Territory; provided, however, that Argos shall not be required to initiate any such suit. In the event that Argos does not promptly initiate and diligently prosecute such a suit reasonably requested by Green Cross, then Green Cross shall have the right, at its expense, to initiate and conduct such suit in the Green Cross Territory and Argos shall reasonably cooperate with Green Cross in any such suit.
(b) To the extent such suit is related to Green Cross Improvement in the Argos Territory, Green Cross will consider in good faith any request from Argos to initiate an infringement or other appropriate suit against any Third Party with respect to matters described in Section 8.4.2(a) occurring in the Argos Territory, however Green Cross shall not be required to initiate any such suit. In the event that Green Cross does not promptly initiate and diligently prosecute such a suit reasonably requested by Argos, then Argos shall have the right, at its expense, to initiate and conduct such suit in the Argos Territory and Green Cross shall reasonably cooperate with Argos in any such suit.
8.4.4 Procedures; Expenses and Recoveries. The Party having the right to initiate any infringement suit under Section 8.4.2 or 8.4.3 above shall have the sole and exclusive right to select counsel for any such suit and shall pay all expenses of the suit, including but not limited to attorneys’ fees and court costs and reimbursement of the other Party’s reasonable out-of-pocket expense in rendering assistance requested by the initiating Party. If required under applicable law in order for the initiating Party to initiate and/or maintain such suit, or if either Party is unable to initiate or prosecute such suit solely in its own name or it is otherwise advisable to obtain an effective legal remedy, in each case, the other Party shall join as a party to the suit and will execute and cause its Affiliates to execute all documents necessary for the initiating Party to initiate litigation to prosecute and maintain such action. In addition, at the initiating Party’s request, the other Party shall provide reasonable assistance to the initiating Party in connection with an infringement suit at no charge to the initiating Party except for reimbursement by the initiating Party of reasonable out-of-pocket expenses incurred in rendering such assistance. The non-initiating Party shall have the right to participate and be represented in any such suit by its own counsel at its own expense. If the Parties obtain from a Third Party, in connection with such suit, any damages, license fees, royalties or other compensation (including but not limited to any amount received in settlement of such litigation) (“Recoveries”), such amounts shall be allocated in all cases as follows regardless of which Party brings the enforcement action:
| (a) | first, to reimburse each Party for all expenses of the suit incurred by such Party, including but not limited to attorneys’ fees and disbursements, travel costs, court costs and other litigation expenses; |
26
| (b) | second, (i) if such suit is related to the Argos Technology in the Green Cross Territory, then Green Cross shall be entitled to receive that portion of the remaining Recoveries reasonably attributable to Net Sales of the Licensed Product in the Green Cross Territory (as determined by a court of competent jurisdiction in a final, non-appealable decision); provided, that the Recoveries reasonably attributable to Net Sales of Licensed Product to which Green Cross is entitled after reimbursement of expenses shall be treated as Net Sales for purposes of this Agreement and Argos shall be entitled to receive royalties on such constructive Net Sales pursuant to the terms of Section 3.1 as if such Net Sales had occurred during the time period of the infringement, and (ii) if such suit is related to Green Cross Improvements in the Argos Territory, then Argos shall be entitled to receive that portion of the remaining Recoveries reasonably attributable to Net Sales of the Licensed Product in the Argos Territory (as determined by a court of competent jurisdiction in a final, non-appealable decision); and |
| (c) | [the Party initiating the suit shall be entitled to [**] percent ([**]%), and the non-initiating Party shall be entitled to [**] percent ([**]%), of the balance of the Recoveries]. |
8.5 Claimed Infringement.
8.5.1 Notice. In the event that after the Effective Date a Third Party at any time provides written notice of a claim to, or brings an action, suit or proceeding against, any Party, or any of their respective Affiliates or Sublicensees, claiming infringement of its patent rights or unauthorized use or misappropriation of its Know-How, based upon an assertion or claim arising out of the Development, Manufacture or Commercialization of the Licensed Product in the Field (“Infringement Claim”), such Party shall promptly notify the other Party of the claim or the commencement of such action, suit or proceeding, enclosing a copy of the claim and all papers served. Each Party agrees to make available to the other Party its advice and counsel regarding the technical merits of any such claim at no cost to the other Party and to offer reasonable assistance to the other Party at no cost to the other Party.
8.5.2 Responsibility. Green Cross shall assume full responsibility for any Infringement Claims brought against Green Cross or its Affiliates or Sublicensees arising out of the Development or Commercialization of the Licensed Product in, or Manufacture of Licensed Product for, the Green Cross Territory by Green Cross or its Related Parties in breach of this Agreement, and all liabilities, damages, costs and expenses arising out of such Third Party Infringement Claims shall be borne by Green Cross. Argos shall assume full responsibility for any Infringement Claims brought against either Party or its Affiliates or Sublicensees arising out of the Commercialization of the Licensed Product in, or Manufacture of Licensed for, the Argos Territory by Argos in or its Related Parties or in or for the Green Cross Territory by Argos or its Related Parties in breach of this Agreement. All liabilities, damages, costs and expenses arising out of such Third Party Infringement Claims shall be borne by Argos.
27
8.5.3 Procedure. Each Party shall have the sole and exclusive right to select counsel for any Infringement Claim that it defends; provided, that it shall consult with the other Party with respect to selection of counsel for such defense. Each Party will keep the other Party informed, and shall from time to time consult with the other Party regarding the status of any such claims and shall provide the other Party with copies of all documents filed in any suit brought in connection with such claims. The other Party shall also have the right to participate and be represented in any such claim or related suit, at its own expense. Argos shall have the sole and exclusive right (but not the obligation) to control the defense of an Infringement Claim for which Green Cross has the responsibility in the event Green Cross fails to assume such defense within [**] days following written notice from Argos. No Party shall settle any claims or suits involving rights of another Party without obtaining the prior written consent of such other Party, which consent shall not be unreasonably withheld or delayed.
8.6 Other Infringement Resolutions. In the event of a dispute or potential dispute that has not ripened into a demand, claim or suit of the types described in Sections 8.4 and 8.5 of this Agreement (e.g., actions seeking declaratory judgments and revocation proceedings), the same principles governing control of the resolution of the dispute, consent to settlements of the dispute, and implementation of the settlement of the dispute (including but not limited to the sharing in and allocating the payment or receipt of damages, license fees, royalties and other compensation) shall apply.
8.7 Patent Certification. To the extent required by law or permitted by law, the Parties shall use Commercially Reasonable Efforts to maintain with the applicable Regulatory Authorities during the Term correct and complete listings of applicable Patent Rights for the Licensed Product being commercialized.
8.8 Trademarks.
8.8.1 Each Party and its Affiliates shall retain all right, title and interest in and to its and their respective corporate names and logos. To the extent permitted by local law, upon Argos’ request, Green Cross and its Related Parties shall include Argos’ (or its designee’s) name with equal prominence, or as close thereto as permitted by local law, on all Licensed Product promotional materials related to the Licensed Product in the Green Cross Territory.
8.8.2 Green Cross will develop and propose, and Argos shall review and comment on for approval by Green Cross, one or more trademarks for the Licensed Product (the “Licensed Product Trademarks”) for use by Green Cross and its Related Parties throughout the Green Cross Territory. Any Licensed Product Trademark(s) (other than the Argos Trademarks) that are used by Green Cross to promote and sell the Licensed Product in the Green Cross Territory are hereinafter referred to as the “Green Cross Trademarks”. Argos (or its Related Parties, as appropriate) shall own all rights to the trademarks developed and/or used by Argos with respect to the Commercialization of the Licensed Product in the Argos Territory (the “Argos Trademarks”), and all goodwill associated therewith. Green Cross (or its Related Parties, as appropriate) shall own all rights to Green Cross Trademarks and all goodwill associated therewith. Argos shall also own rights to any Internet domain names incorporating the applicable Argos Trademarks or any variation or part of such Argos Trademarks used as its URL address or any part of such address; and Green Cross shall also own rights to any Internet domain names incorporating the applicable Green Cross Trademarks or any variation or part of such Green Cross Trademarks used as its URL address or any part of such address.
28
8.8.3 If Green Cross Trademarks are used to promote and sell the Licensed Product in the Green Cross Territory then Green Cross will use Commercially Reasonable Efforts to establish, maintain and enforce the Green Cross Trademarks in the Green Cross Territory during the Term, at its expense. If Green Cross requests a license to Argos Trademarks in writing to promote and sell the Licensed Product in the Green Cross Territory, then Argos shall xxxxx Xxxxx Cross an exclusive license to use such Argos Trademarks to Commercialize the Licensed Product in the Green Cross Territory on terms and conditions to be negotiated by the Parties in good faith. Argos shall be entitled to no additional compensation for the grant of such license other than the reimbursement in full of Argos’ costs and expenses of establishing, maintaining and enforcing such Argos Trademarks in the Green Cross Territory. If Green Cross Trademarks are used to promote and sell the Licensed Product in the Argos Territory, then Green Cross shall grant Argos an exclusive license to use such Green Cross Trademarks to Commercialize the Licensed Product in the Argos Territory on terms and conditions to be negotiated by the Parties in good faith. Green Cross shall be entitled to no additional compensation for the grant of such license other than the reimbursement in full of Green Cross’ costs and expenses of establishing, maintaining and enforcing such Green Cross Trademarks in the Argos Territory.
8.8.4 In the event either Party becomes aware of any infringement of any Licensed Product Trademark or Argos Trademark by a Third Party, such Party shall promptly notify the other Party and the Parties shall consult with each other and jointly determine the best way to prevent such infringement, including, without limitation, by the institution of legal proceedings against such Third Party.
9.1 Term. This Agreement shall be effective as of the Effective Date and, unless terminated earlier pursuant to Section 9.2 below, this Agreement shall continue in effect until expiration of the Royalty Term (“Term”). Upon expiration of the Term, all licenses of the Parties under Article 2 then in effect shall become fully paid-up, perpetual, non-exclusive licenses.
9.2 Termination Rights.
9.2.1 Termination for Cause. This Agreement may be terminated at any time during the Term:
(a) upon written notice by either Party if the other Party is in breach of its material obligations hereunder and has not cured such breach within [**] business days in the case of a payment breach, or [**] days in the case of all other breaches, after written notice requesting cure of the breach; or
(b) by either Party upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings of the other Party, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other Party; provided, however, that in the event of any involuntary bankruptcy or receivership proceeding such right to terminate shall only become effective if the Party consents to the involuntary bankruptcy or receivership or such proceeding is not dismissed within sixty (60) days after the filing thereof.
29
9.2.2 Challenges of Patent Rights. In the event that Green Cross or any of its Related Parties (a) commences or participates in any action or proceeding (including, without limitation, any patent opposition or re-examination proceeding), or otherwise asserts any claim, challenging or denying the validity or enforceability of any of the Argos Patent Rights or any claim thereof or (b) actively assists any other person or entity in bringing or prosecuting any action or proceeding (including, without limitation, any patent opposition or re-examination proceeding) challenging or denying the validity or enforceability of any of such Argos Patent Rights or any claim thereof, then (i) Green Cross shall give notice thereof to Argos within [**] days of taking such action and (ii) Argos will have the right, in its sole discretion to give notice to Green Cross that either (A) the licenses granted to Green Cross with respect to all or any portion of the Argos Technology under this Agreement will terminate in [**] days following such notice (or such longer period as Argos may designate in such notice), and, unless Green Cross ceases all participation with respect to all such challenge(s) (including withdrawing any challenge within its control) within such [**]day period, such licenses will so terminate, or [**]. In the event that Argos elects to terminate the licenses but is not permitted to do so under applicable law, then the Parties agree to construe this provision as to permit Argos to terminate the licenses to that portion of such Argos Technology with respect to which Argos has the legal right to do so.
9.2.3 Effect of Termination.
(a) Termination by Argos. Without limiting any other legal or equitable remedies that Argos may have, if Argos terminates this Agreement in accordance with Section 9.2.1 or 9.2.2, then (i)notwithstanding anything in Section 7.4.1 to the contrary, Green Cross’ obligations under Section 7.4.1 shall survive for a period of [**] years after the effective date of termination, (ii) [**], (iii) [**] relating to the Development, Manufacture or Commercialization of the Licensed Product and all Licensed Product Trademarks and execute any and all documents and carry out any other actions as may be requested by Argos to assist Argos with all regulatory filings with the applicable Regulatory Authorities required in connection with the termination of this Agreement to ensure that all Regulatory Approvals in the Green Cross Territory can be transferred or issued to Argos(B) copies of all data, reports, records and materials in Green Cross’ possession or Control relating to the Development, Manufacture or Commercialization of the Licensed Product, including without limitation all non-clinical and clinical data relating to the Licensed Product, including without limitation customer lists and customer contact information and all adverse event data in Green Cross’ possession or Control, and (C) all records and materials in Green Cross’ possession or Control containing Confidential Information of Argos, (iv) [**], (vi) if Green Cross or its Related Parties are Manufacturing Licensed Product, at Argos’ option, supply the Licensed Product to Argos in the Green Cross Territory on commercially reasonable terms (but any event, no less favorable than those on which Green Cross supplied the Licensed Product prior to such termination to the applicable distributor(s) in the Green Cross Territory) [**], Argos has obtained all necessary manufacturing approvals and Argos has procured or developed its own source of Licensed Product supply, (vii) if Argos so requests, Green Cross shall transfer to Argos any Third Party agreements relating to the Development, Manufacture or Commercialization of the Licensed Product to which Green Cross is a party, subject to any required consents of such Third Party, which Green Cross shall use Commercially Reasonable Efforts to obtain promptly, and (viii) unless otherwise agreed by Argos in writing, all Sublicense Agreements shall automatically terminate. The license granted and other transfers to be effected pursuant to this Section 9.2.3(a) shall be royalty-free, fully paid and perpetual. Green Cross shall execute all documents and take all such further actions as may be reasonably requested by Argos in order to give effect to the foregoing clauses (i) through (viii).
30
(b) Termination by Green Cross for Cause. Without limiting any other legal or equitable remedies that Green Cross may have, if Green Cross terminates this Agreement in accordance with Section 9.2.1(a) or (b), then the licenses granted to Argos under this Agreement shall terminate and the licenses granted to Green Cross under this Agreement shall continue in full force and effect; provided, that Green Cross may continue to use Commercially Reasonable Efforts to Develop and Commercialize the Licensed Product and Green Cross shall (a) withhold and retain [**]% of the royalties and milestones otherwise payable to Argos under this Agreement until Green Cross has recouped by way of such retention an amount equal to any milestone payments previously paid to Argos hereunder; and (b) thereafter, pay Argos [**]% of the royalties that would otherwise be payable hereunder. However, if royalties owed by Argos to Third Parties under the Argos In-Licenses attributable to such sales of Licensed Product by or on behalf of Green Cross are greater than the amount specified in (a) or (b), Green Cross shall pay royalties in an amount equal to the royalty obligations under the Argos In-Licenses and Green Cross shall continue to comply with the terms of the Argos In-Licenses applicable in the Green Cross Territory. Notwithstanding anything to the contrary, Argos shall continue to provide Green Cross, for at least [**] months, technical support for the production of the Licensed Product as contemplated by Section 5.2.
(c) Termination upon Bankruptcy of a Party. If this Agreement is terminated by either Party (the “Non-Bankrupt Party”) pursuant to Section 9.2.1(b) due to the rejection of this Agreement by or on behalf of the other Party (the “Bankrupt Party”) under Xxxxxxx 000 xx xxx Xxxxxx Xxxxxx Bankruptcy Code (the “Code”), all licenses and rights to licenses granted under or pursuant to this Agreement by the Bankrupt Party to the Non-Bankrupt Party are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the Code, licenses of rights to “intellectual property” as defined under Section 101(35A) of the Code. The Parties agree that the Non-Bankrupt Party, as a licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the Code, and that upon commencement of a bankruptcy proceeding by or against the Bankrupt Party under the Code, the Non-Bankrupt Party shall be entitled to a complete duplicate of, or complete access to (as the Non-Bankrupt Party deems appropriate), any such intellectual property and all embodiments of such intellectual property. Such intellectual property and all embodiments thereof shall be promptly delivered to the Non-Bankrupt Party (i) upon any such commencement of a bankruptcy proceeding upon written request therefor by the Non-Bankrupt Party, unless the Bankrupt Party elects to continue to perform all of its obligations under this Agreement or (ii) if not delivered under (i) above, upon the rejection of this Agreement by or on behalf of the Bankrupt Party upon written request therefor by the Non-Bankrupt Party. The foregoing provisions are without prejudice to any rights the Non-Bankrupt Party may have arising under the Code or other applicable law. The parties intend for the substance of this Section 9.2(c) to apply worldwide, even if the Code does not expressly apply to the Bankrupt Party or to the Non-Bankrupt Party.
9.3 Effect of Expiration or Termination; Survival. Expiration or termination of this Agreement shall not relieve the Parties of any obligation accruing prior to such expiration or termination. Any expiration or termination of this Agreement shall be without prejudice to the rights of either Party against the other accrued or accruing under this Agreement prior to expiration or termination, including without limitation the obligation to pay royalties for the Licensed Product sold prior to such expiration or termination. The provisions of Articles 6 and 10, and Sections 3.3, 4.4,
31
4.10.2, 4.10.3, 7.2.1(f), 7.3, 7.5, 7.6, 7.7, 8.3.1, 8.3.2, 8.3.3, 8.4.1, 8.4.2, 9.2.3 and 9.3 shall survive any expiration or termination of this Agreement. Except as set forth in this Article 9, upon termination or expiration of this Agreement all other rights and obligations of the Parties under this Agreement cease.
10. MISCELLANEOUS
10.1 Assignment. Except as provided in this Section 10.1, this Agreement may not be assigned or otherwise transferred, nor may any right or obligation hereunder be assigned or transferred, by either Party without the consent of the other Party. However, either Party may, without the other Party’s consent, assign this Agreement and its rights and obligations hereunder in whole or in part to an Affiliate or to a party that acquires, by merger, sale of assets or otherwise, all or substantially all of the business of the assigning Party to which the subject matter of this Agreement relates. The assigning Party shall remain responsible for the performance by its assignee of this Agreement or any obligations hereunder so assigned. An assignment to an Affiliate shall terminate, and all rights so assigned shall revert to the assigning Party, if and when such Affiliate ceases to be an Affiliate of the assigning Party.
10.2 Governing Law. This Agreement shall be construed and the respective rights of the Parties determined in accordance with the substantive laws of the State of New York, notwithstanding any provisions of New York law governing conflicts of laws to the contrary, and the patent laws of the relevant jurisdiction without reference to any rules of conflict of laws.
10.3 Entire Agreement; Amendments. This Agreement contains the entire understanding of the Parties with respect to the subject matter hereof, and supersedes all previous arrangements with respect to the subject matter hereof, whether written or oral. This Agreement (including the Schedules hereto) may be amended, or any term hereof modified, only by a written instrument duly-executed by authorized representatives of both Parties hereto.
10.4 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any respect in any jurisdiction, the Parties hereto shall substitute, by mutual consent, valid provisions for such invalid, illegal or unenforceable provisions, which valid provisions in their economic effect are sufficiently similar to the invalid, illegal or unenforceable provisions that it can be reasonably assumed that the Parties would have entered into this Agreement with such valid provisions. In case such valid provisions cannot be agreed upon, the invalid, illegal or unenforceable of one or several provisions of this Agreement shall not affect the validity of this Agreement as a whole, unless the invalid, illegal or unenforceable provisions are of such essential importance to this Agreement that it is to be reasonably assumed that the Parties would not have entered into this Agreement without the invalid, illegal or unenforceable provisions.
10.5 Headings. The captions to the Articles and Sections hereof are not a part of this Agreement, but are merely for convenience to assist in locating and reading the several Articles and Sections hereof.
10.6 Waiver of Rule of Construction. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement shall be construed against the drafting Party shall not apply.
32
10.7 No Implied Waivers; Rights Cumulative. No failure on the part of Argos or Green Cross to exercise, and no delay in exercising, any right, power, remedy or privilege under this Agreement, or provided by statute or at law or in equity or otherwise, shall impair, prejudice or constitute a waiver of any such right, power, remedy or privilege or be construed as a waiver of any breach of this Agreement or as an acquiescence therein, nor shall any single or partial exercise of any such right, power, remedy or privilege preclude any other or further exercise thereof or the exercise of any other right, power, remedy or privilege.
10.8 Notices. All notices which are required or permitted hereunder shall be in writing and sufficient if delivered personally, sent by facsimile, sent by email, sent by nationally-recognized overnight courier or sent by registered or certified mail, postage prepaid, return receipt requested, addressed as follows:
| If to Argos, to: | Argos Therapeutics, Inc. | |
| 0000 Xxxxxxxxxx Xxxxx | ||
| Xxxxxx, XX 00000 | ||
| Attention: President | ||
| Facsimile: 000 000-0000 | ||
| Email:xxxxxx@xxxxxxxxxxxxxxxxx.xxx | ||
| With a copy to: |
Xxxxxxxxx PLLC | |
| 0000 Xxxxxxx Xxxx Xxxx, Xxxxx 000 | ||
| Xxxxxxx, XX 00000 | ||
| Attention: Xxxxxxx X. Xxxxxxx | ||
| Facsimile No.: (000) 000-0000 | ||
| Email: xxxxxxxx@xxxxxxxx.xxx | ||
| If to Green Cross, to: | Green Cross Corp. | |
| With a copy to: |
||
or to such other address as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such notice shall be deemed to have been given: (a) when delivered if personally delivered or sent by facsimile or email on a business day (or if delivered or sent on a non-business day, then on the next business day); (b) on receipt if sent by overnight courier; and/or (c) on receipt if sent by mail.
10.9 Compliance with Export Regulations. Neither Party shall export any technology licensed to it by the other Party under this Agreement except in compliance with U.S. and all other applicable export laws and regulations.
10.10 Force Majeure. Neither Party shall be held liable to the other Party nor be deemed to have defaulted under or breached this Agreement for failure or delay in performing any obligation
33
under this Agreement to the extent that such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party, potentially including without limitation embargoes, war, acts of war (whether war be declared or not), insurrections, terrorism, riots, civil commotions, strikes, lockouts or other labor disturbances, fire, floods, or other acts of God, or acts, omissions or delays in acting by any governmental authority or the other Party. The affected Party shall notify the other Party of such force majeure circumstances as soon as reasonably practical, and shall promptly undertake all reasonable efforts necessary to cure such force majeure circumstances.
10.11 Dispute Resolution.
10.11.1 Disputes. The Parties shall negotiate in good faith and use reasonable efforts to settle any dispute, controversy or claim arising from, or related to, this Agreement or to the breach hereof (collectively, “Dispute”). In particular, the Chief Executive Officers of the Parties shall attempt to resolve all Disputes. In the event that the Chief Executive Officers cannot reach an agreement regarding a Dispute, and a Party wishes to pursue the matter, each such Dispute that is not an “Excluded Claim” shall be finally resolved by binding arbitration under the then-current Rules of Arbitration of the International Chamber of Commerce (“ICC”) by three (3) arbitrators appointed in accordance with the said Rules and Section 10.11.2 below, and judgment on the arbitration award may be entered in any court having jurisdiction thereof. As used in this Section 10.11, the term “Excluded Claim” shall mean a dispute that concerns the validity or infringement of a patent, trademark or copyright.
10.11.2 Arbitration. The arbitration shall be conducted by a panel of three (3) persons experienced in the pharmaceutical business who are independent of both Parties and neutral with respect to the Dispute presented for arbitration. Within [**] days after initiation of arbitration, each Party shall select one person to act as arbitrator and the two Party-selected arbitrators shall select a third arbitrator within [**] days of their appointment. If the arbitrators selected by the Parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be appointed by the ICC International Court of Arbitration. The place of arbitration shall be in Singapore, and all proceedings and communications shall be in English.
Either Party may apply to the arbitrators for interim injunctive relief until the arbitration award is rendered or the controversy is otherwise resolved. Either Party also may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any injunctive or provisional relief necessary to protect the rights or property of that Party pending the arbitration award. The arbitrators shall have no authority to award punitive or any other type of damages not measured by a Party’s compensatory damages. Each Party shall bear its own costs and expenses and attorneys’ fees, and the Party that does not prevail in the arbitration proceeding shall pay the arbitrators’ and any administrative fees of arbitration. Except to the extent necessary to confirm an award or as may be required by law, neither a Party nor an arbitrator may disclose the existence, content, or results of an arbitration without the prior written consent of both Parties. In no event shall an arbitration be initiated after the date when commencement of a legal or equitable proceeding based on the dispute, controversy or claim would be barred by the applicable New York statute of limitations.
(a) The Parties agree that, in the event of a Dispute over the nature or quality of performance under this Agreement, neither Party may terminate this Agreement until final resolution of the Dispute through arbitration or other judicial determination. The Parties further agree that any payments made pursuant to this Agreement pending resolution of the Dispute shall be refunded promptly if an arbitrator or court determines that such payments are not due.
34
(b) The Parties hereby agree that any disputed performance or suspended performances pending the resolution of the arbitration that the arbitrators determine to be required to be performed by a Party must be completed within a reasonable time period following the final decision of the arbitrator.
(c) The Parties hereby agree that any monetary payment to be made by a Party pursuant to a decision of the arbitrators shall be made in United States dollars, free of any tax or other deduction. The Parties further agree that the decision of the arbitrators shall be the sole, exclusive and binding remedy between them regarding determination of the matters presented to the arbitrator.
10.12 Independent Contractors. It is expressly agreed that Argos and Green Cross shall be independent contractors and that the relationship between Argos and Green Cross shall not constitute a partnership, joint venture or agency. Argos shall not have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on Green Cross, without the prior written consent of Green Cross, and Green Cross shall not have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on Argos without the prior written consent of Argos.
10.13 Counterparts. The Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
10.14 Binding Effect; No Third Party Beneficiaries. As of the Effective Date, this Agreement shall be binding upon and inure to the benefit of the Parties and their respective permitted successors and permitted assigns. Except as expressly set forth in this Agreement, no person or entity other than the Parties and their respective Affiliates and permitted assignees hereunder shall be deemed an intended beneficiary hereunder or have any right to enforce any obligation of this Agreement.
[THE REMAINDER OF THIS PAGE HAS BEEN LEFT INTENTIONALLY BLANK]
35
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the date first set forth above.
| GREEN CROSS CORP. | ARGOS THERAPEUTICS, INC. | |||||||||
| BY: | /s/ XX Xxxx |
BY: | /s/ Xxxx Xxxxxxxxx | |||||||
| NAME: | XX Xxxx |
NAME: | Xxxx Xxxxxxxxx | |||||||
| TITLE: | President |
TITLE: | Vice President | |||||||
36
SCHEDULE A
ARCELIS PERSONALIZED IMMUNOTHERAPY PLATFORM
Arcelis is Argos’ proprietary active immunotherapy technology platform for generating fully personalized RNA-loaded dendritic cell immunotherapies. Argos uses the Arcelis platform to manufacture AGS-003, which is initially being developed for the treatment of mRCC, and AGS-004, which is being developed for the treatment of HIV.
The Arcelis platform is focused on dendritic cells that present antigens to the attention of the immune system and are critical to the human immune system’s recognition of the presence of proteins derived from cancer cells or virus-infected cells. Dendritic cells are capable of internalizing cancer protein antigens or virus protein antigens and displaying fragments of these protein antigens on their surface as small peptides. The dendritic cells then present these peptide antigens to T-cells capable of binding to these peptide antigens and producing a large complement of molecular factors that, in the case of cancer, lead to direct cancer cell death and, in the case of infectious disease, kill virus-infected cells to control the spread of infectious pathogens.
The following graphic illustrates the processes comprising our Arcelis platform:
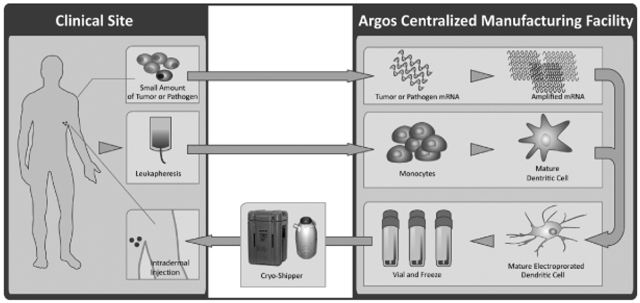
As shown in the graphic above, the Arcelis platform requires two components derived from the particular patient to be treated, specifically:
| • | a disease sample from the patient — tumor cells in the case of cancer or a blood sample containing virus in the case of infectious disease — which is generally collected at the time of diagnosis or initial treatment, and |
| • | dendritic cells derived from the patient’s monocytes, a particular type of white blood cell, which are obtained from the patient through a laboratory procedure called leukapheresis that occurs after diagnosis and at least four weeks prior to the initiation of our immunotherapy. |
The tumor cells, or the blood sample containing the virus, and the leukapheresis product are shipped separately following collection from the clinical site to a centralized manufacturing facility where we use standard methods to isolate the patient’s mRNA, which is a key component of the genetic code, from the disease sample and amplify the mRNA. In parallel, we take the monocytes from the leukapheresis product and culture them using a proprietary process to create matured dendritic cells. Argos then immerses the matured dendritic cells in a solution of the patient’s isolated mRNA and a synthetic RNA that encodes a protein known as CD40 ligand, or CD40L, and apply a brief electric pulse to the solution, in a process referred to as electroporation. This process enables the patient’s isolated mRNA and the CD40L protein to pass into, or load, the dendritic cells. Argos then further cultures the mRNA-loaded dendritic cells so that these cells allow for antigen expression from the patient’s mRNA and presentation in the form of peptides on the surface of the dendritic cells.
37
These mature, loaded dendritic cells are formulated into the patient’s plasma that was collected during the leukapheresis to become the Arcelis-based drug product. Argos then vials, freezes and ships the drug product to the clinic, which thaws the drug product and administers it to the patient by intradermal injection.
Upon injection into the skin of the patient, the antigen-loaded dendritic cells in the drug product migrate to the lymph nodes near the site of the injection. It is at these lymph nodes that the drug product comes into contact with T-cells. Argos believes that through this interaction the loaded dendritic cells orchestrate the differentiation, expansion and education, of antigen-specific T-cells. A unique property of the dendritic cells is that they result in the generation of CD8+ central and effector memory T-cells. Once activated and expanded, these T-cells are able to seek out and kill cancer or virus-infected cells that express the identical antigens as those displayed on the surface of the dendritic cells. Because the generation of these T-cells is dependent on secretion of IL-12 from the dendritic cells, measurement of IL-12 is a marker for potency of AGS-003 and potentially other Arcelis-based products.
38
SCHEDULE B
EXISTING ARGOS PATENT RIGHTS WITH COUNTERPARTS IN THE GREEN CROSS TERRITORY
[**]
39
SCHEDULE C
AUTOMATED SYSTEMS
Argos Automated Systems were designed as works for hire by Invetech in collaboration with Argos.
The Automated Nucleic Acid Processing System includes systems, devices and components thereof, as well as related methods for automated processing of samples in a closed container, including automated isolation, purification, amplification, processing and packaging of nucleic acids. Examples of the Argos Automated Nucleic Acid Processing System are described in PCT Publication [**]. Uses of this System include isolation of RNA from tumor lysates, RT-PCR, in vitro transcription and related nucleic acid purification and packaging steps.
The Automated Cell Processing Systems are held as trade secret, with the exception of a centrifuge bowl described in International Patent Application [**] and medicament devices described in International Patent Application [**]. These System and components thereof automate many aspects of cell processing, differentiation, electroporation, and packaging. Uses of these System include automated differentiation of monocytes into mature RNA-loaded dendritic cells.
40
SCHEDULE D
| 1. | Collaboration Termination Agreement between Argos and Kyowa Hakko Kirin Co., Ltd dated December 31, 2009. |
41
SCHEDULE E
TECHNOLOGY TRANSFER ACTIVITIES
| • | Transfer of all GMP documentation related to the manufacture, quality control assays and quality assurance of the Arcelis technology. |
| • | This would include MPs, STMs and SOPs for GMP processing |
| • | Training of Green Cross technical personnel at Argos Therapeutics in the RNA and cellular process |
| • | Multiple visits required to perform RNA and cellular training runs |
| • | Training of Green Cross technical personnel at Argos on the quality control assays |
| • | Multiple visits required to perform quality control assay training |
| • | Completion of all documentation, acquisition of reagents and provide information and expertise for upfitting of GMP labs by Green Cross |
| • | Perform initial feasibility runs at Green Cross with Argos personnel oversight |
| • | Perform [**] successful GMP engineering runs at Green Cross |
42
