ROYALTY AND LICENSE AGREEMENT
EXHIBIT 10.12
This License Agreement (this “AGREEMENT”) is entered into and effective upon the date of last
signature herein (the “EFFECTIVE DATE”), by and between Integral LifeSciences Corporation, a
corporation organized under the laws of the State of Delaware, having a business office at 000
Xxxxxx Xxxx, Xxxxxxxxxx, Xxx Xxxxxx 00000 (hereinafter referred to as “INTEGRA”), and XXXX Medical,
Inc., a corporation duly organized and existing under the laws of California, having its principal
office at 0000 Xxxxxx Xxxxx, Xxxxx X, Xxx Xxxxx, XX 00000 (hereinafter referred to as “XXXX”).
ARTICLE I — DEFINITIONS
All definitions below or elsewhere in this AGREEMENT apply to both their singular and plural
forms, as the context may require. “H/herein,” “hereunder,” and “hereof” and other similar
expressions refer to this AGREEMENT. “Article(s)” and “Section(s)” refer to a
section(s) in this AGREEMENT. “I/including” means “including without limitation.” “D/day(s)”
means “calendar day(s),” unless otherwise stated.
1.1 “CONFIDENTIAL INFORMATION” means the proprietary or confidential information of a party
(each, a “DISCLOSER”) which is disclosed to the other party (each, a “RECIPIENT”) before or after
the EFFECTIVE DATE and (i) is identified as “confidential” by DISCLOSER in writing prior to
disclosure and (ii) relates to products, plans, designs, costs, prices, finances, marketing plans,
business opportunities, personnel, research, development, know-how, trade secrets, inventions,
blueprints, techniques, chemical or biological materials, drugs, devices, specimens, apparatus,
processes, algorithms, software programs, schematics, designs, contracts, customer lists,
procedures, formulae, patent applications and other information relating to DISCLOSER’s business,
services, processes or technology. CONFIDENTIAL INFORMATION shall not include information that:
(i) was known by RECIPIENT, or was publicly available, prior to disclosure by DISCLOSER to
RECIPIENT; (ii) become publicly available after disclosure by DISCLOSER to RECIPIENT through no act
of RECIPIENT; (iii) is hereafter rightfully furnished to RECIPIENT by a third party without
confidentiality restriction; or (iv) is disclosed with the prior written consent of DISCLOSER or as
expressly authorized under this AGREEMENT.
1.2 “FIELD” shall mean the field of or related to products for blood vessels.
1.3 “FIRST GENERATION MATERIAL” shall mean the family of desaminotyrosyl-L-tyrosine derived
polycarbonates which include poly(DTE-carbonate), poly(DTB-carbonate), poly(DTH-carbonate),
poly(DTO-carbonate) and poly(X%DTR-co-Y%DT-carbonate)s and tyrosine derived polycarbonates
containing the chain-terminating groups derived from acetic anhydride or the ethyl, butyl, hexyl,
or octyl esters of L-tyrosine or desamino tyrosine. FIRST GENERATION MATERIAL are further
described in subsections (a) and (d) of the definition of “POLYCARBONATES,” as set forth in
Appendix C.
1.4 “INTELLECTUAL PROPERTY” means the PATENT RIGHTS and the TECHNICAL INFORMATION AND RIGHTS.
1.5 “LICENSED PRODUCT” shall mean (i) any product or part thereof in the FIELD which is
covered in whole or claimed in at least one unexpired claim of PATENT RIGHTS or RUTGERS PATENT
RIGHTS in the country in which any such product or part thereof is made, used, or sold by LICENSEE
or its sublicensees; and (ii) any product made by any process which is covered in whole or claimed
in at least one unexpired claim of PATENT RIGHTS or RUTGERS PATENT RIGHTS in the country in which
any such process is used to make such product or part thereof by XXXX.
1.6 “PATENT RIGHTS” shall mean all of the following patent applications and patents, but only
to the extent that such have not reverted or been assigned to RUTGERS pursuant to the LICENSE
TERMINATION AGREEMENT, including, without limitation:
(a) US Xxx # 6,359,102, issued on March 19, 2002, entitled, “Biphasic polymerization process,”
EP0991693A1, issued on April 12, 2000, entitled, “Biphasic
2
polymerization process,” EP0991693B1, issued on September 3, 2003, entitled, “Biphasic
polymerization process,” and US Xxx # 6620356, issued on September 16, 2003, entitled, “Porous
constructs fabricated by gas induced phase inversion,” together with all pending and issued foreign
counterparts of such application; and all pending and issued renewals, continuations,
continuations-in-part, divisions, patents of addition, reexaminations and/or reissues of such
application or foreign counterparts;
(b) United States Patent Application 10/350,202 and any other pending, issued or hereafter
filed, U.S. or foreign patents applications owned by INTEGRA covering the INTEGRA TECHNOLOGY; and
(c) Any United States or foreign patent application or patent that claims a right of priority
from any of the patents or patent applications in Section 1.6 (a) or (b).
1.7 “RUTGERS PATENT RIGHTS” shall: (i) have the meaning set forth in the LICENSE TERMINATION
AGREEMENT for the term “Rutgers’ Patent Rights” and shall also include any additional patents,
patent applications, all pending and issued foreign counterparts of such applications; and all
pending and issued renewals, continuations, continuations-in-part, divisions, patent of addition,
reexaminations and/or reissues of such applications or foreign counterparts, to which rights have
reverted from, or been assigned by, INTEGRA to RUTGERS pursuant to the LICENSE TERMINATION
AGREEMENT; and (ii) include the U.S. Patent Application Numbers and provisional patents listed on
Appendix C and patents issuing thereon, the U.S. Patent Number(s) listed on Appendix C, and shall
also include any additional patents, patent applications, all pending and issued foreign
counterparts of such applications; and all pending and issued renewals, continuations,
continuations-in-part, divisions, patent of addition, reexaminations and/or reissues of such
applications or foreign counterparts.
1.8 “SECOND GENERATION MATERIAL” shall mean FIRST GENERATION MATERIAL that are radiopaque
(using iodine and/or bromine) and/or PEGylated and are further described in subsections (a) and (d)
of the definition of “POLYCARBONATES,” as set forth in Appendix C.
1.9 “TECHNICAL INFORMATION AND RIGHTS” shall mean copyrights, moral rights, data rights,
business processes, trade secrets, all technical information, know-how, formulations,
specifications, processes, techniques and data, and any other proprietary right arising or
enforceable under the laws of the United States, any other country, or any bilateral or
multilateral treaty that arises from a laboratory of INTEGRA or exists at the time of this
AGREEMENT which are not readily available to others through public means, (i) other than the PATENT
RIGHTS, and (ii) only to the extent that such information and/or rights have not reverted or been
assigned to RUTGERS pursuant to the LICENSE TERMINATION AGREEMENT.
1.10 “TERRITORY” shall mean the entire world.
2.1 Subject to the terms and conditions of this AGREEMENT, INTEGRA hereby grants to XXXX and
XXXX hereby accepts from INTEGRA an exclusive right in the
3
TERRITORY in the FIELD, with right to sublicense as set forth in Section 2.2, (a) to the
INTELLECTUAL PROPERTY and (b) to make, have made, modify, distribute, lease, import, use, offer to
sell, have sold and otherwise dispose of LICENSED PRODUCTS and (c) to use, have used, perform and
have performed processes under the INTELLECTUAL PROPERTY.
2.2 XXXX shall have the exclusive right, at its sole discretion, to sublicense any of the
rights, privileges and licenses granted hereunder during the term of this AGREEMENT.
2.3 All sublicenses granted by XXXX of its rights hereunder shall be subject to terms
substantially similar to this AGREEMENT and if a sublicense allows sublicensee to sell the LICENSED
PRODUCTS, such sublicense shall provide for the payment of running royalties for the sale of such
LICENSED PRODUCTS hereunder at amounts equal to the amount specified for payments by XXXX to
INTEGRA in Section 5.2 hereof. XXXX agrees to forward to INTEGRA a copy of reports received by
XXXX from its sublicensees under the sublicenses as shall be pertinent to a royalty accounting
regarding the sale of LICENSED PRODUCTS, if any, under sublicense agreements.
2.4 The parties recognize and acknowledge that REVA’s reasonable efforts to develop and market
the LICENSED PRODUCTS may not result in the commercialization of the LICENSED PRODUCTS or profits
from such commercialization.
ARTICLE III - LICENSE RESTRICTIONS AND ENFORCEMENT
3.1 No license or other right is granted, by either party to the other, by implication,
estoppel or otherwise, under any intellectual property now or hereafter owned or controlled by such
party except for the licenses and rights expressly granted in this AGREEMENT.
3.2 At any time or from time to time on and after the EFFECTIVE DATE, each party shall, at the
request of the other party, (i) deliver to the requesting party such records, data or other
documents consistent with this AGREEMENT; (ii) execute, and deliver or cause to be delivered, all
such assignments or further instruments of transfer or license, and (iii) take or cause to be taken
all such other actions, as the requesting party may reasonably deem necessary or desirable in order
for the requesting party to obtain the full benefits of this AGREEMENT and the transactions
contemplated hereby.
ARTICLE IV - OWNERSHIP
4.1 XXXX shall retain all rights, title and interests in and to of all REVA’s intellectual
property, and any and all other tangible forms thereof.
4.2 Except for the license grants set forth in Article II, INTEGRA shall retain all rights,
title and interests in and to all of INTEGRA’S INTELLECTUAL PROPERTY.
ARTICLE V - PAYMENTS
5.1 In consideration of the license grants and for executing the LICENSE TERMINATION
AGREEMENT, among other good and valuable consideration, XXXX shall
4
pay INTEGRA a royalty of: (i) *** U.S. Dollars ($***) per unit in the
United States and Japanese markets, and *** U.S. Dollars ($***) per unit in all other
territories, only on coronary stents made from FIRST GENERATION MATERIALS sold by XXXX or a
sublicensee of XXXX, as appropriate (but in no instances both XXXX and sublicensee of XXXX so to
create a “double royalty” for INTEGRA); and (ii) *** U.S. Dollars and *** Cents ($***) per unit
only on coronary stents made from SECOND GENERATION MATERIALS sold by XXXX or a sublicensee of
XXXX, as appropriate (but in no instances both XXXX and sublicensee of XXXX so to create a “double
royalty” for INTEGRA). Such royalties shall be payable as provided in Section 5.3.
5.2 Payment of the royalties specified in Section 5.1, if any such royalty is due, for the
preceding calendar quarter shall be made by XXXX to INTEGRA within thirty (30) days after March 31,
June 30, September 30 and December 31 of each year during the term of this AGREEMENT. After
termination or expiration of this AGREEMENT, a final payment shall be made by XXXX covering the
last whole or partial calendar quarter. Each quarterly payment shall be accompanied by a written
statement of royalties due, as described in Section 6.2 hereunder.
5.3 All monetary payments due hereunder are expressed in and shall be paid by check payable in
United States dollars, without deduction of exchange, collection or other charges, to INTEGRA.
5.4 For converting into United States dollars any monetary payment accrued hereunder in the
currency of any other country, the rate of exchange for the purchase of United States dollars with
such currency quoted by The Chase Manhattan Bank, New York, New York, on the last business day of
the payment period in question shall be used.
5.5 No multiple royalties shall be payable because any LICENSED PRODUCTS, their manufacture,
use, lease or sale are or shall be covered by more than one patent application, patent or
certificate of registration licensed under this AGREEMENT. For avoidance of doubt, XXXX has no
obligations under this AGREEMENT to make any royalty, milestone or maintenance payments to INTEGRA,
except as explicitly set forth in Section 5.1.
ARTICLE VI - REPORTS AND RECORDS
6.1 XXXX shall keep and preserve, in accordance with generally accepted accounting principles
and procedures, complete and accurate books, records and accounts containing particulars that are
necessary for the purpose of showing the amounts payable to INTEGRA hereunder. Said books, records
and accounts shall be kept at REVA’s principle place of business or the principle place of business
of the appropriate division of XXXX to which this AGREEMENT relates. No more than once per annum
said books and supporting data shall be open, upon reasonable notice at all reasonable times and
places during business hours for two (2) years following the end of the calendar year to which they
pertain, to the inspection of an independent auditor engaged at INTEGRA’S cost (that has signed a
confidentiality agreement at least as protective of REVA’s CONFIDENTIAL INFORMATION as Article XII)
mutually
| *** | Portions of this page have been omitted pursuant to a request for Confidential Treatment filed separately with the Commission. |
5
agreeable to the parties for the purpose of verifying REVA’s royalty statement or compliance
in other respects with this AGREEMENT.
6.2 XXXX shall, within thirty (30) days after March, 31, June 30, September 30 and December
31, of each year, deliver to INTEGRA true and accurate reports, giving such particulars of the
business conducted by XXXX and its sublicensees during the preceding calendar quarter under this
AGREEMENT as shall be pertinent to a royalty accounting hereunder. These reports shall be duly
signed by an authorized signatory of XXXX on behalf of XXXX and shall include at least the
following:
(a) number of LICENSED PRODUCTS which are coronary stents made from FIRST GENERATION MATERIALS
and the number of LICENSED PRODUCTS which are coronary stents made from SECOND GENERATION MATERIALS
manufactured and sold by XXXX and its sublicensees;
(b) total royalties due; and
(c) names and addresses of all sublicensees of XXXX.
6.3 With each such report submitted, XXXX shall pay to INTEGRA the royalties due and payable
under this AGREEMENT. If no royalties are due, XXXX shall so report.
6.4 XXXX shall use the royalty reporting sheet attached hereto as Appendix D, or a substantial
equivalent, to fulfill the royalty and reporting requirements of this Article VI.
ARTICLE VII - PATENT PROSECUTION AND MAINTENANCE
7.1 Responsibility for Patent Prosecution. Within thirty (30) days after the
EFFECTIVE DATE, INTEGRA shall deliver to XXXX all relevant United States or foreign filings for any
PATENT RIGHTS which were filed on any date prior to thirty (30) days after the EFFECTIVE DATE.
Subject to this Article VII, XXXX shall file, prosecute, and maintain the PATENT RIGHTS during the
term of this AGREEMENT. XXXX shall keep INTEGRA advised of the continuing prosecution of the
PATENT RIGHTS. XXXX shall provide INTEGRA with a copy of each relevant proposed United States and
foreign filing for the PATENT RIGHTS at least fifteen (15) days prior to the date of such filing so
that INTEGRA may be informed of the continuing prosecution and have an opportunity to review and
comment on such filings and upon the breadth and scope of the related patent applications of the
PATENT RIGHTS.
7.2 Payments for Patent Filings. Payment of all fees and costs relating to the
filing, prosecution, maintenance and defense of PATENT RIGHTS shall be borne by XXXX. In the event
INTEGRA has entered, or enters, into licenses with third parties outside the FIELD, INTEGRA shall
require said parties to pay a pro-rata share of said prior expenses to XXXX.
7.3 Abandonment by XXXX. If XXXX decides not to continue payment for prosecution of a
United States or foreign patent application to issuance or grant, or decides not to continue
payment for maintenance of any United States or foreign patent application or patent within the
PATENT RIGHTS, then (a) XXXX shall timely notify INTEGRA in writing in order that INTEGRA may
continue said prosecution or maintenance of such intellectual property at
6
INTEGRA’S own expense, and (b) from and after the date of the written notice under (a), XXXX
shall have no further obligation to pay any fees or costs relating to the filing, prosecution, or
maintenance of such patent application or patent of the PATENT RIGHTS, and REVA’s license with
regard to such patent application or patent shall cease become non-exclusive (but only for the
applicable country or countries in which XXXX has decided not to continue prosecution or not to
maintain such patent application or patent of the PATENT RIGHTS).
ARTICLE VIII - INFRINGEMENT AND OTHER ACTIONS
8.1 Notice. Each of the parties, as a notifying party, shall promptly provide to the
other party (a) written notice of any alleged infringement by a third party of any patent licensed
hereunder under PATENT RIGHTS, and (b) any available evidence of any infringement referenced in (a)
that is in such notifying party’s possession or control.
8.2 Infringement. XXXX, at its expense, may enforce the PATENT RIGHTS licensed
hereunder against infringement by third parties and is entitled to retain recovery from such
enforcement. INTEGRA hereby agrees that XXXX may join INTEGRA as a party in any such action (with
INTEGRA having the right to participate in such action and to be represented, if it so desires and
at its own expense, by counsel of its own selection) and to give XXXX reasonable assistance and any
needed authority to control, file and prosecute such action, without expense to INTEGRA. XXXX
agrees to keep INTEGRA reasonably informed as to the status of any such action and to provide
copies to INTEGRA, upon request by INTEGRA, of any papers or information relevant to the
prosecution of any such action. XXXX shall timely inform INTEGRA of any offer for settlement
presented by a third party for any such action and XXXX shall consider INTEGRA’S input in deciding
whether or not to accept any such settlement offer. The total cost of any such action, commenced
or defended solely by XXXX, shall be borne by XXXX. After reimbursement of REVA’s reasonable legal
costs and expenses related to such recovery, XXXX agrees to pay INTEGRA the royalty detailed in
Section 5.1 for any monetary recovery that is for sales of coronary stents which are LICENSED
PRODUCTS lost due to the infringement and a pro-rata portion of any related punitive or
consequential damages awarded XXXX.
8.3 Right to Withhold. If XXXX undertakes to prosecute and/or defend any action
alleging infringement of, and/or any action challenging the validity of, any of the PATENT RIGHTS,
as provided in Section 8.2, XXXX may withhold up to fifty percent (50%) of the payments otherwise
thereafter due INTEGRA under Article V hereunder and apply the same toward reimbursement of up to
one-half (1/2) of REVA’s expenses, including attorneys’ fees, in connection therewith.
8.4 Cooperation. In any action brought by XXXX under this Article VIII, INTEGRA
shall, at the request and expense of XXXX, cooperate in all respects and, to the extent possible,
have its employees testify when requested and make available relevant records, papers, information,
samples and specimens.
8.5 Licenses to Xxxxx Third Party Infringement. XXXX shall have the sole right in
accordance with the terms and conditions herein to sublicense any alleged infringer for the
7
FIELD to the INTELLECTUAL PROPERTY of no greater scope than set forth in Section 2.1. Any
royalties as part of such a sublicense shall be treated per Article V.
8.6 Licenses to Xxxxx Own Infringement. If a third party asserts that a patent or
other right owned by it is infringed by the manufacture, import, use, sale or offer for sale of any
LICENSED PRODUCT, the party first obtaining knowledge of such a claim shall immediately provide the
other party notice of such claim and the related facts in reasonable detail. Notwithstanding any
provision in this AGREEMENT to the contrary, except subject to the indemnification provisions of
Article XI, the defense of any such claim (including without limitation the institution and
prosecution of any counterclaims thereto) shall be controlled by XXXX, and XXXX shall have the
right to enter into settlements, stipulated judgments or other voluntary, final disposition
respecting such claim (and related counterclaims). If XXXX should be of the reasonable opinion
that it cannot commercially reasonably make, use, sell, offer for sale, or import any of the
LICENSED PRODUCTS because the INTELLECTUAL PROPERTY or RUTGERS PATENT RIGHTS (or such other
intellectual property right licensed by XXXX from RUTGERS) infringe a third party patent or other
intellectual property right, XXXX shall obtain the consent from INTEGRA, which shall not be
unreasonably withheld, conditioned or delayed, if such party is willing to grant such license, to
credit fifty percent (50%) of any payment payable to the above-mentioned third party for such a
license against the amounts payable to INTEGRA hereunder on a going-forward basis, but in no event
will INTEGRA’S total payments received hereunder each calendar year be reduced by more than fifty
percent (50%). INTEGRA’S consent hereunder shall only be withheld on the basis of a bona fide
independent legal opinion that no such infringement exists. INTEGRA shall be required to provide
or withhold its consent within twenty (20) days of the date of such notice. No response from
INTEGRA within twenty (20) days shall be deemed to be consent.
9.1 INTEGRA hereby represents and warrants as follows:
(a) INTEGRA has full power and authority to enter into and perform this AGREEMENT;
(b) Neither INTEGRA’S entering nor performing this AGREEMENT will violate any contractual
right of or breach any contractual obligation to any third party under any agreement or arrangement
between INTEGRA and such third party;
(c) INTEGRA has received and currently holds valid and effective assignments of all inventors’
rights in and to the INTEGRA TECHNOLOGY and the INTELLECTUAL PROPERTY, and no other person may
claim rights to the INTEGRA TECHNOLOGY or the INTELLECTUAL PROPERTY;
(d) There are no outstanding liens, encumbrances, third party rights, agreements or
understandings of any kind, either written, oral or implied, other than the LICENSE. TERMINATION
AGREEMENT, regarding the INTEGRA TECHNOLOGY or the INTELLECTUAL PROPERTY which are inconsistent or
in conflict with any provision of this AGREEMENT;
8
(e) INTEGRA is the owner of, and has not, other than as set forth in the LICENSE TERMINATION
AGREEMENT, assigned any of its rights, title or interests in or to, and is not aware that any other
person or entity has assigned or licensed, or agreed to grant an assignment or license to any third
party any rights, title or interests in or to, the INTEGRA TECHNOLOGY or the INTELLECTUAL PROPERTY;
(f) INTEGRA shall not assert against XXXX and its sublicensees any of INTEGRA’S rights under
any intellectual property of any kind owned or licensed by INTEGRA for any activities by XXXX or
its sublicensees related to the discovery, research, development, manufacture or commercialization
of LICENSED PRODUCTS or of any other products sold by XXXX or its sublicensees in the FIELD;
(g) INTEGRA has not received any notice of any potential or threatened claim of infringement
of third party patents and, (i) INTEGRA, is not aware of any facts which could give rise to any
claim of infringement of third party intellectual property relating to the use and practice of the
INTELLECTUAL PROPERTY or the INTEGRA TECHNOLOGY; and (ii) INTEGRA is not aware of any issued or
granted patent that would be infringed by the practice of the INTELLECTUAL PROPERTY as permitted
under the license rights granted in this AGREEMENT;
(h) There are no court orders, judgments or decrees that impair or restrict the use, validity
or enforceability of the INTELLECTUAL PROPERTY and no action, suit, inquiry, proceeding or
investigation is currently pending before any court, administrative agency or other governmental
body in which such use, validity or enforceability is being challenged or questioned in any way,
either directly or indirectly, by way of claim, counterclaim or affirmative defense, it being
clearly acknowledged by XXXX that such representation and warranty does not apply to any
proceedings before any patent office with respect to the prosecution and issuance of any patent;
(i) To INTEGRA’S knowledge there is no actual infringement by any third party in the FIELD of
the licensed INTELLECTUAL PROPERTY; and
(j) To INTEGRA’S knowledge, there are no publications, public uses or public sales or offers
for sale to any party other than XXXX which would render any of the PATENT RIGHTS invalid.
9.2 XXXX hereby represents and warrants as follows:
(a) XXXX has full power and authority to enter into and perform this AGREEMENT; and
(b) Neither REVA’s entering nor performing this AGREEMENT will violate any contractual right
of or breach any contractual obligation to any third party under any agreement or arrangement
between XXXX and such third party.
9.3 EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, INTEGRA AND XXXX AND THEIR
TRUSTEES, DIRECTORS, OFFICERS, EMPLOYEES, AND AFFILIATES MAKE NO REPRESENTATIONS AND EXTEND NO
9
WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTIES OF
MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, VALIDITY OF PATENT RIGHTS CLAMS, ISSUED OR
PENDING, AND THE ABSENCE OF LATENT OR OTHER DEFECTS, WHETHER OR NOT DISCOVERABLE.
ARTICLE X - LIMITATION OF LIABILITY
10.1 EXCEPT FOR EACH PARTY’S INDEMNIFICATION OBLIGATIONS WITH REGARD TO INTELLECTUAL PROPERTY
INFRINGEMENT SET FORTH IN ARTICLE XI, OR A BREACH OF ARTICLE XII, CONFIDENTIAL INFORMATION, OR
ARTICLE XVIII, TERMINATION, TO THE MAXIMUM EXTENT PERMITTED BY LAW,
(a) NEITHER PARTY WILL BE LIABLE FOR ANY DAMAGES WITH RESPECT TO ANY CLAIMS ARISING FROM OR
RELATING TO THIS AGREEMENT, WHETHER IN CONTRACT, TORT (INCLUDING NEGLIGENCE), STRICT PRODUCT
LIABILITY OR ANY OTHER THEORY OF LIABILITY, FOR LOST PROFIT, OR OTHER SPECIAL, INCIDENTAL OR
CONSEQUENTIAL DAMAGES OF ANY CHARACTER, INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF
GOODWILL, WORK STOPPAGE, COMPUTER FAILURE OR MALFUNCTION, LOSS OF DATA, OR OTHER COMMERCIAL DAMAGES
OR LOSSES, EVEN IF THAT PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
(b) NEITHER PARTY WILL BE LIABLE FOR ANY DAMAGES WITH RESPECT TO ANY CLAIMS ARISING FROM OR
RELATING TO THIS AGREEMENT, WHETHER IN CONTRACT (OTHER THAN FOR PAYMENT OF ROYALTIES), TORT
(INCLUDING NEGLIGENCE), STRICT PRODUCT LIABILITY OR ANY OTHER THEORY OF LIABILITY, THAT EXCEED THE
SUM OF THE AMOUNTS PAID BY XXXX TO INTEGRA AND THE AMOUNTS OUTSTANDING OWED BY XXXX TO INTEGRA
HEREUNDER PRIOR TO THE DATE OF THE EVENT GIVING RISE TO SUCH LIABILITY. THIS LIMITATION SHALL
APPLY NOTWITHSTANDING ANY FAILURE OF ESSENTIAL PURPOSE OF ANY LIMITED REMEDY PROVIDED HEREIN.
ARTICLE XI - INDEMNIFICATION
11.1 INTEGRA’S Indemnification. INTEGRA shall at all times during the term of this
AGREEMENT and thereafter, indemnify, defend and hold XXXX, its trustees, directors, officers,
employees and affiliates (“REVA’s INDEMNITEES”), harmless against all third party claims,
proceedings, demands and liabilities of any kind whatsoever, including legal expenses and
reasonable attorneys’ fees, arising out of what is judicially determined to be a breach of any
representation or warranty as set forth in Section 9.1, and to pay all damages and costs awarded
against XXXX or that arise out of settlement provided that REVA’s INDEMNITEES: (a) promptly notify
INTEGRA in writing of any such suit or proceeding, (b) provides INTEGRA with sole control over the
defense or settlement of such suit or proceeding, and (c) provide reasonable information and
assistance in the defense and/or settlement of any such claim or action. Notwithstanding the
foregoing, INTEGRA’S indemnification obligation with respect to claims arising from or relating to
breaches of representations or warranties that do not relate to INTEGRA’S infringement of a third
party’s intellectual property rights shall (x) exclude
10
damages of the type described in Section 10.1(a), and (y) be limited in amount to the sum of
the amounts paid or owed by XXXX to INTEGRA hereunder. XXXX will have the right to approve any
settlement or compromise that may adversely affect its rights. In the event that XXXX disapproves
such settlement and compromise, XXXX shall compensate INTEGRA for any additional damages,
liabilities, costs and expenses incurred by INTEGRA resulting from such disapproval.
11.2 REVA’s Indemnification. XXXX shall at all times during the term of this
AGREEMENT and thereafter, indemnify, defend and hold University and INTEGRA, its trustees,
directors, officers, employees and affiliates (“INTEGRA’s INDEMNITEES”), harmless against all third
party claims, proceedings, demands and liabilities of any kind whatsoever, including legal expenses
and reasonable attorneys’ fees, directly arising out of the death of or injury to any person or
persons or out of any damage to property, or directly resulting from the production, manufacture,
sale, use, lease, consumption or advertisement of the LICENSED PRODUCTS by XXXX or sublicensees or
arising from any breach of any contractual obligation of XXXX hereunder, and to pay all damages and
costs awarded against INTEGRA or that arise out of settlement provided that INTEGRA’S INDEMNITEES:
(a) promptly notify XXXX in writing of any such suit or proceeding, (b) provide XXXX with sole
control over the defense or settlement of such suit or proceeding, and (c) provide reasonable
information and assistance in the defense and/or settlement of any such claim or action. INTEGRA
will have the right to approve any settlement or compromise that may adversely affect its rights.
In the event that INTEGRA disapproves such settlement and compromise, INTEGRA shall compensate XXXX
for any additional damages, liabilities, costs and expenses incurred by XXXX resulting from such
disapproval.
ARTICLE XII - CONFIDENTIALITY
12.1 Nondisclosure. RECIPIENT shall not, except as otherwise expressly provided
herein, disclose, disseminate or otherwise allow access to the CONFIDENTIAL INFORMATION of
DISCLOSER to anyone other than RECIPIENT’S employees that have a need to know such CONFIDENTIAL
INFORMATION to implement this AGREEMENT and who are bound by written confidentiality obligations
with provisions no less stringent than those contained in this ARTICLE XII. RECIPIENT shall
prevent unauthorized disclosure or use of the CONFIDENTIAL INFORMATION of DISCLOSER. DISCLOSER and
RECIPIENT shall execute any documents and otherwise shall take all necessary steps to ensure that
DISCLOSER and RECIPIENT shall each be able to enforce DISCLOSER’s rights hereunder against
RECIPIENT, its employees and all other third parties to whom RECIPIENT discloses DISCLOSER’s
CONFIDENTIAL INFORMATION, under the laws of each jurisdiction in which DISCLOSER’s CONFIDENTIAL
INFORMATION is disclosed by RECIPIENT. RECIPIENT shall be responsible for any breach of this
ARTICLE XII by RECIPIENT’S employees, contractors or agents.
12.2 Ownership. Except as set forth in ARTICLE TV, RECIPIENT acknowledges and agrees
that DISCLOSER (or its licensors) owns all rights, title and interests, including intellectual
property rights in and to DISCLOSER’s CONFIDENTIAL INFORMATION.
11
12.3 Notification. If RECIPIENT learns or believes that any person who has had access
to the CONFIDENTIAL INFORMATION of DISCLOSER has violated or intends to violate this AGREEMENT,
RECIPIENT shall immediately notify DISCLOSER and shall cooperate with DISCLOSER in seeking
injunctive or other equitable relief against any such person.
12.4 Exceptions. RECIPIENT may disclose the CONFIDENTIAL INFORMATION of DISCLOSER, if
such disclosure is required by law, provided that RECIPIENT promptly notifies DISCLOSER to allow
intervention by DISCLOSER (prior to the disclosure), cooperates with DISCLOSER to contest or
minimize the disclosure (including application for a protective order) at DISCLOSER’s expense and
limits such disclosure to the party entitled to receive the CONFIDENTIAL INFORMATION and to the
scope of the legal requirement. Notwithstanding the foregoing, any CONFIDENTIAL INFORMATION
disclosed pursuant to this Section 12.4 shall otherwise continue to be treated as CONFIDENTIAL
INFORMATION hereunder.
12.5 Confidentiality of AGREEMENT. Neither party will publicly disclose any term of
this AGREEMENT or announce the existence of this AGREEMENT without the prior written consent of the
other party, except that each party may reveal the terms of this AGREEMENT (a) to its accountants,
banks, financing sources, lawyers and other professional advisors, provided that such parties
undertake in writing to keep such information confidential, or (b) as required by applicable laws
and regulations including those of the U.S. Securities and Exchange Commission on the notification.
Each party may also disclose the tax treatment and tax structure of the transactions contemplated
by this AGREEMENT and all materials of any kind (including opinions or other tax analyses) that are
provided to it relating to such tax treatment and tax structure.
12.6 Reproduction of CONFIDENTIAL INFORMATION. CONFIDENTIAL INFORMATION shall not be
reproduced except as reasonably required to implement this AGREEMENT. Any reproduction or
derivative of any CONFIDENTIAL INFORMATION of DISCLOSER by RECIPIENT shall remain the property of
DISCLOSER and shall contain all confidential or proprietary notices or legends which appear on the
original.
ARTICLE XIII - EXPORT CONTROLS
13.1 It is understood that INTEGRA is subject to United States laws and regulations
controlling the export of technical data, computer software, laboratory prototypes and other
commodities (including the Arms Export Control Act, as amended and the Export Administration Act of
1979). The transfer of certain technical data and commodities may require a license from the
cognizant agency of the United States Government and/or written assurances by XXXX that XXXX shall
not export data or commodities to certain foreign countries without prior approval of such agency.
INTEGRA shall assist XXXX to comply with the United States export laws and regulations set forth in
this Section 13.1.
ARTICLE XIV - NON-USE OF NAMES
14.1 XXXX shall not use the names or trademarks of INTEGRA, or any of its employees, or any
adaptation thereof, in any advertising, promotional or sales literature without
12
prior written consent obtained from INTEGRA, in each case, except that XXXX may, without prior
written consent, state that it is licensed by INTEGRA, under one or more of the patents and/or
applications comprising the PATENT RIGHTS.
ARTICLE XV - PAYMENTS. NOTICES AND OTHER COMMUNICATIONS
15.1 Any payment, notice or other communication pursuant to this AGREEMENT shall be
sufficiently made or given on the date of mailing if sent to such party by certified or registered
first class mail, postage prepaid, addressed to it at its address below or as it shall designate by
written notice given to the other party as follows:
In the case of INTEGRA:
To the Attention of: General Counsel
Integra LifeSciences Corporation
000 Xxxxxx Xxxx
Xxxxxxxxxx, Xxx Xxxxxx 00000
Integra LifeSciences Corporation
000 Xxxxxx Xxxx
Xxxxxxxxxx, Xxx Xxxxxx 00000
In the case of XXXX:
To the Attention of: Xxxxxx Xxxxxxx, President
XXXX Medical, Inc.
0000 Xxxxxx Xxxxx, Xxxxx X
Xxx Xxxxx, XX 00000
XXXX Medical, Inc.
0000 Xxxxxx Xxxxx, Xxxxx X
Xxx Xxxxx, XX 00000
ARTICLE XVI - ASSIGNMENT
16.1 Neither this AGREEMENT nor any right or obligation hereunder is assignable in whole or in
part by any party without the prior written consent of the other party. Notwithstanding the
foregoing, either party may assign this AGREEMENT, without such consent to a third party in
connection with any merger, acquisition, consolidation, reorganization (in which a change of
control occurs), CHANGE OF CONTROL of or by the assigning party, or sale of all, substantially all
or a majority of such parties’ assets or voting securities. A “CHANGE OF CONTROL” occurs when over
fifty percent (50%) of a party’s then outstanding securities are acquired by a third party. This
AGREEMENT shall inure to the benefit of each of the party’s successors and assignees provided that
such successors or assignees assume the party’s obligations under this AGREEMENT.
ARTICLE XVII - DISPUTE RESOLUTION
17.1 This AGREEMENT is entered into in and shall be governed, construed and enforced in all
respects solely and exclusively under the laws of the State of California, USA without giving
effect to any law which would result in the application of a different body of law.
17.2 Any and all claims, disputes or controversies arising under, out of, or in connection
with this AGREEMENT, including any dispute relating to patent validity or
13
infringement, which the parties shall be unable to resolve within sixty (60) days, shall be
mediated in good faith. The party raising such dispute shall promptly advise the other party of
such claim, dispute or controversy in a writing which describes in reasonable detail the nature of
such dispute. By not later than five (5) business days after the recipient has received such
notice of dispute, each party shall have selected for itself a representative who shall have the
authority to bind such party, and shall additionally have advised the other party in writing of the
name and title of such representative. By not later than ten (10) business days after the date of
such notice of dispute, the party against whom the dispute shall be raised shall select a mediation
firm in San Diego, California and such representatives shall schedule a date with such firm for a
mediation hearing. The parties shall enter into good faith mediation and shall share the costs
equally.
17.3 If the representatives of the parties have not been able to resolve a dispute within
fifteen (15) business days after a mediation hearing, as set forth in Section 17.2, the parties
shall have the right to pursue any other remedies legally available to resolve such dispute solely
and exclusively in, and the parties hereby irrevocably consent to the exclusive jurisdiction and
proper venue of, the state and federal courts located in the County of San Diego, State of
California, USA, and waive any objections thereto based on any ground including improper venue or
Forum Non-Conveniens. The parties agree that service of process may be effected in accordance with
Article XV. Any decision rendered by such court shall be binding, final and conclusive upon the
parties, and a judgment thereon may be entered in, and enforced by, any court having jurisdiction
over the party against which an award is entered or the location of such party’s assets.
17.4 Notwithstanding anything to the contrary herein, each party shall be entitled to seek
injunctive or other equitable relief, wherever such party deems appropriate in any jurisdiction, in
order to preserve or enforce such party’s rights for any breach or threatened breach of the other
party of Articles II, IV or XII. Each party agrees that: (i) Articles II, IV and XII are
necessary and reasonable to protect the other party and its business, (ii) any violation of these
provisions could cause irreparable injury to the other party for which money damages would be
inadequate, and (iii) as a result, the other party will be entitled to seek and obtain injunctive
relief against the breach or threatened breach of the provisions of Articles II, IV or XII without
the necessity of posting bond or proving actual damages. The parties agree that the remedies set
forth in this Section 17.4 are in addition to and in no way preclude any other remedies or actions
that may be available at law or under this AGREEMENT.
18.1 Unless earlier terminated as hereinafter provided, this AGREEMENT shall continue until
the later of the end of the life of the last to expire patent of the PATENT RIGHTS and the end of
the life of the last to expire patent of the RUTGERS PATENT RIGHTS. The term of the license for
TECHNICAL INFORMATION AND RIGHTS set forth in Section 2.1 shall last until the later of the end of
the life of the last to expire patent of the PATENT RIGHTS, the end of the life of the last to
expire patent of the RUTGERS PATENT RIGHTS and fifteen (15) years from the EFFECTIVE DATE.
18.2 If XXXX shall cease to carry on its business, this AGREEMENT shall terminate upon notice
by INTEGRA.
14
18.3 Upon any default by XXXX of its obligation to pay INTEGRA the royalties set forth in
ARTICLE V, INTEGRA shall have the right to terminate this AGREEMENT and the rights, privileges and
license granted hereunder effective on three (3) months notice to XXXX. Such termination shall
become automatically effective unless XXXX shall have cured any such default prior to the
expiration of the three (3) month period.
18.4 Upon termination or expiration of this AGREEMENT for any reason, nothing herein shall be
construed to release either party from any obligation that matured prior to the effective date of
such termination; and Articles I, IV, VI, IX — XV, XVII and XIX and Section 18.4 shall survive any
such termination by their terms. XXXX and any sublicensee thereof may, however, after the
effective date of such termination, sell all LICENSED PRODUCTS, and complete LICENSED PRODUCTS in
the process of manufacture at the time of such termination and sell the same, provided that XXXX
shall make the payments to INTEGRA as required by Article V and shall submit the reports required
by Article VI hereof. Except as necessary to carry out the terms of this Section 18.4, upon
termination each party shall immediately return the other party’s CONFIDENTIAL INFORMATION, and any
copies thereof in any form, or certify the destruction of the same.
ARTICLE XIX - MISCELLANEOUS PROVISIONS
19.1 Entire Agreement. This AGREEMENT, including Appendices A, B, C and D embodies
the sole, final and entire understanding of the parties and shall supersede all previous
communications, representations, or undertakings, either verbal or written, between the parties
relating to the subject matter hereof.
19.2 Amendment. This AGREEMENT may be amended only by a written agreement embodying
the full terms of the amendment signed by authorized representatives of both parties.
19.3 Severability. If one or more provisions in this AGREEMENT are ruled entirely or
partly invalid or unenforceable by any court or governmental authority of competent jurisdiction,
then: (i) the validity and enforceability of all provisions not ruled to be invalid or
unenforceable shall remain unaffected; (ii) the effect of such ruling shall be limited to the body
making the ruling; (iii) the provision(s) held wholly or partly invalid or unenforceable shall be
deemed amended, and the parties shall reform the provision(s) to the minimum extent necessary to
render them valid and enforceable in conformity with the parties’ intent as manifested herein; and
(iv) if the ruling, or the controlling principle of law or equity leading to the ruling, is
subsequently overruled, modified, or amended, then the provision(s) in question, as originally set
forth in this AGREEMENT, shall be deemed valid and enforceable to the maximum extent permitted by
the new controlling principle of law or equity.
19.4 No Strict Construction. The language used in this AGREEMENT shall be deemed to
be the language chosen by both parties hereto to express their mutual intent and no rule of strict
construction against either party shall apply to any term or condition of this AGREEMENT.
15
19.5 Relationship of Parties. The parties are independent contractors. Nothing
contained in this AGREEMENT shall be construed as creating a partnership, joint venture, agency or
an association of any kind.
19.6 No Waiver. The failure of one party hereto to enforce at any time any of the
provisions of this AGREEMENT, or any rights in respect thereto, or to exercise any election herein
provided, shall in no way be considered to be a waiver of such provision, rights or elections or in
any way to affect the validity of this AGREEMENT, or excuse a similar subsequent failure to perform
any such term or condition by the other party. Any waiver must be in writing.
19.7 Interpretation. The headings of sections contained herein are inserted for
convenience of reference only, and are not intended to be a part of or to affect the meaning or
interpretation of this AGREEMENT.
19.8 Counterparts. This AGREEMENT may be executed in two counterparts, each of which
will be deemed an original, but together will constitute one and the same instrument. A party may
deliver this AGREEMENT by transmitting a facsimile of this AGREEMENT signed by such party to the
other party, which facsimile signature shall be deemed an original for all purposes.
[REMAINDER OF THIS PAGE INTENTIONALLY LEFT BLANK]
16
| XXXX MEDICAL, INC. (XXXX) |
||||
| By: | /s/ Xxxxxx X. Xxxxxxx | |||
| Name:Xxxxxx X. Xxxxxxx | ||||
| Title: President | ||||
| Date: 1/30/04 | ||||
| INTEGRA LIFESCIENCES CORPORATION (INTEGRA) |
||||
| By: | /s/ Xxxxxx Xxxxx | |||
| Name:Xxxxxx Xxxxx | ||||
| Title: CEO | ||||
| Date: 1/25/04 | ||||
17
Appendix A
LICENSE TERMINATION AGREEMENT
Appendix A - 1
AGREEMENT
This
Agreement (the “Agreement”) is made as of the 27 day of
January, 2004, by and
between Rutgers, The State University of New Jersey, having its statewide administrative offices at
New Brunswick, New Jersey (hereafter referred to as “Rutgers”), and Integra LifeSciences
Corporation, a corporation organized under the laws of the State of Delaware, having a business
office at 000 Xxxxxxxxxx Xxxxx, Xxxxxxxxxx, Xxx Xxxxxx 00000 (hereinafter referred to as
“Integra”).
1. Definitions.
1.1 The defined terms in the License Agreement shall have the same meanings when they are used
in this Agreement.
1.2 “First Generation Polycarbonates” shall mean Licensed Products in the “Licensed Field,” as
those terms are defined in the License Agreement, covered by the patents or patent applications
identified in Schedule B attached hereto. First Generation Polycarbonates shall not include Second
Generation Polycarbonates.
1.3 “Integra Process Patents” shall mean United States Patent Numbers 6,120,491, 6,359,102 and
6,620,356 and patents issuing on United States Patent Application Number 10/350,202, together with
all issued foreign counterparts of such patents and patent application, and all issued renewals,
continuations, divisions, patents of addition, reexaminations and/or reissues of such patents or
foreign counterparts.
1.4 “Second Generation Polycarbonates” shall mean polycarbonates containing sulfur,
phosphorus, chlorine, bromine, boron or iodine atoms or repeat units other than a
desaminotyrosyl-tyrosine alkyl ester and desaminotyrosyl-tyrosine.
1.5 “Master File” shall mean United States Food and Drug Administration (“FDA”) designated
file MAF-1140 Tyrosine-Derived Polycarbonates, and all amendments and addendums to date, and all
FDA communications of any kind relevant to the same.
1.6 “End Product” shall mean a product incorporating (i) the First Generation Polycarbonates
in finished product form or (ii) any material whose manufacture would, but for
the license granted Rutgers under this Agreement, violate one or more claims of the Integra
Process Patents.
1.7 “Net Sales” means the total of the gross invoice prices of End Product sold or transferred
by Rutgers, its Affiliates, and its licensees and sublicensees plus rebates and allowances received
by Rutgers, its Affiliates or licensees or sublicensees of amounts that have been deducted from
gross sales less the sum of the following actual and customary deductions (net of rebates or
allowances of such deductions received) included on the invoice and actually paid: cash, trade, or
quantity discounts; sales, use, tariff, or other excise taxes imposed upon particular sales;
import/export duties; and transportation charges. Sales among Rutgers, its Affiliates and its
licensees or sublicensees for ultimate third party use shall be disregarded for purposes of
computing payments under clause 3.1. Payments shall be made only upon sales or transfers between
unrelated third parties and shall be based on arms-length consideration.
2. Effective Date.
2.1 This Agreement shall become effective (the “Effective Date”) upon the execution of this
Agreement and both of the following agreements:
(a) A license from Rutgers to XXXX Medical, Inc., a corporation organized under the laws of
the State of California, having a business office at 0000 Xxxxxx Xxxxx, Xxx Xxxxx, Xxxxxxxxxx
(hereinafter “XXXX”) in respect of First Generation Polycarbonates and Second Generation
Polycarbonates for the manufacture, use and sale of vascular devices; and
(b) An agreement between Integra and XXXX providing for payments by XXXX to Integra of a fee
per coronary stent sold.
2.2 Rutgers shall promptly notify Integra in writing when the license described in clause
2.1(a) is executed and Integra shall promptly notify Rutgers in writing when the agreement
described in clause 2.1(b) is executed.
(a) Promptly following the Effective Date, Integra shall notify in writing, with a copy to
Rutgers, the current manufacturer of tyrosine polymers and advise the manufacturer (1) that
Rutgers, its licensees and its sublicensees have any and all rights necessary to have manufactured
any tyrosine polymers, and (2) to deal directly and exclusively with Rutgers, its licensees and its
sublicensees on a going forward basis, except for winding down Integra’s relationships with the
manufacturer relating to the subject matter of this agreement.
2.3 The License Agreement shall be terminated and of no further force or effect as of the
Effective Date. As of the Effective Date, Integra irrevocably grants to Rutgers a worldwide
license, with the right to grant sublicenses, to make, have made, use, sell, lease and otherwise
dispose of products employing the Integra Process Patents. The termination of the License
Agreement shall not relieve either party of any obligation or liability accrued thereunder for
royalties,/fees/sublicensing payments or patent costs.
2.4 Within thirty (30) days following the Effective Date, Integra shall deliver to Rutgers all
tangible documents, information, data and materials described in clause 12.1 of the
2
License Agreement, including the Master File and any research data relating to the
biocompatibility of tyrosine polymers, as well as all other materials it has relating to First
Generation Polycarbonates or Second Generation Polycarbonates. Notwithstanding the foregoing,
Integra may retain a copy of such documents and materials for use in rendering such advice as
Rutgers may request pursuant to Section 2.5 hereof. Integra hereby irrevocably assigns to Rutgers
all of its right, title and interest in and to all such tangible documents, information, data and
materials as of the Effective Date.
2.5 Throughout the term of this Agreement, Integra will provide reasonable assistance,
including, without limitation, the prompt execution of all documents to effect the aforementioned
assignments, to Rutgers in Rutgers’ efforts to effect the transfer of intellectual property,
information, documents, and materials to Rutgers. In addition, Integra will, upon the reasonable
request of Rutgers, provide technical advice to Rutgers on the commercialization of the First
Generation Polycarbonates and the Integra Process Patents and on the Master File during the
three-year period following the date hereof; provided that Integra personnel may render such advice
at times reasonably convenient to Integra and to Rutgers and that Integra shall not be obligated to
devote more than 5 man days to the provision of such advice in the first year following the
execution of this Agreement and no more than two man days in any subsequent year.
3. Payments.
3.1 Rutgers shall pay to Integra a portion of any milestone payments and royalties received by
Rutgers from third parties for licensing arrangements made in respect of First Generation
Polycarbonates . The payments shall comprise (i) ***% of milestone payments and of royalties
received by Rutgers until the total amount paid to Integra hereunder exceeds
$*** and (ii) ***% of milestone payments and royalties received by Rutgers
thereafter; but in no case shall the royalties paid to Integra be less than (a) ***% of Net Sales
if End Products contain material whose manufacture would, but for a sublicense granted by Rutgers
or one of its licensees, violate one or more claims of the Integra Process Patents, or (b) ***% of
Net Sales of all other End Products. The agreement between XXXX and Rutgers entered into on the
date hereof, and payments received by Rutgers thereunder for use of tyrosines or other materials in
stent, coating and embolotherapy products, shall not be subject to any payments by Rutgers to
Integra under this paragraph 3.1.
3.2 Rutgers shall deliver to Integra a semi-annual report within thirty (30) days following
the close of any calendar 6 month period in which Rutgers receives a milestone payment or a royalty
under clause 3.1. The report shall set forth the amounts of milestone payments and royalties
received by Rutgers and it shall be accompanied by a check payable to Integra for the amount due.
3.3 Rutgers shall keep good and accurate books of account sufficient to permit determination
of the payments due hereunder and shall make such books of account available for inspection by an
independent accountant designated by Integra and reasonably acceptable to Rutgers provided that
such accountant executes and delivers to Rutgers a Confidentiality
| *** | Portions of this page have been omitted pursuant to a request for Confidential Treatment filed separately with the Commission. |
3
Agreement in the form appended as Attachment A hereto. Such inspections shall be no more
frequent than twice each calendar year during the term hereof and once within six months after
termination of this Agreement. The designated accountant shall retain in confidence the
information in the books of account and shall report to Integra only the accuracy or inaccuracy of
the reports rendered pursuant to clause 3.2 hereof. Such inspections will be at Integra’s expense
unless the designated accountant identifies underpayment of royalties due by five percent (5%) or
more in which event Rutgers shall pay for such inspection. Integra’s failure to inspect shall not
constitute a waiver of Integra’s right to object to the accuracy of the reports rendered or
payments made under this Agreement.
3.4 All underpayments discovered pursuant to clause 3.3, plus interest at the then prevailing
prime interest rate published by Citicorp, New York, New York, U.S.A., on the amount underpaid
shall be promptly paid to Integra; provided, however, that no more than one year’s interest shall
be due on such underpayment.
4.1 This Agreement shall be effective on the Effective Date and shall expire on expiration of
the last to expire of the patents listed on Schedule B hereto unless extended or earlier terminated
pursuant to the terms hereof.
4.2 This Agreement shall automatically terminate in the event (1) substantially all of the
assets of Integra are seized or attached in conjunction with any action against it by a third
party, or (2) Integra is dissolved or the sale of all or substantially all of its assets is made
other than as part of a merger, or (3) an attempt to assign this Agreement is made without the
prior written consent of Rutgers. Notwithstanding the foregoing this agreement shall be assigned
to any entity into which Integra shall combine or merge.
4.3 This Agreement may be terminated by either party for a material breach by the other party
of the provisions hereof. Such termination shall be effective thirty (30) days after written
notice to the other party of the breach if the breach has not been remedied.
4.4 The right of either party to terminate under the provisions of this Article shall not be
an exclusive remedy, and either party shall be entitled, if the circumstances warrant,
alternatively or cumulatively, to damages for breach of this Agreement, to an order requiring
performance of the obligations of this Agreement, or to any other legally available remedy.
4.5 The following Articles shall survive termination or expiration of this Agreement by their
terms: Articles 1, 2, 3.3, 4.4, 4.5 and 5 through 7.
5.1 Each party represents, warrants and covenants to the other that:
(a) It has the full power and authority to execute and deliver this Agreement and perform its
covenants, duties and obligations described in this Agreement; and
4
(b) This Agreement is a valid, legal and binding obligation upon such party, enforceable in
accordance with its terms, except as enforceability may be limited by applicable insolvency and
other laws affecting creditors’ rights generally or by the availability of equitable remedies.
5.2 Integra represents, warrants and covenants to Rutgers that:
(a) It is validly existing and in good standing;
(b) The execution of this Agreement and the full and timely performance of the covenants,
duties and obligations described herein have been duly authorized by all necessary corporate action
in accordance with all applicable laws.
(c) If Integra becomes aware that on the date hereof it possessed intellectual property or
information in tangible form that (i) is necessary or useful to the commercialization of First
Generation Polycarbonates or Second Generation Polycarbonates or the Integra Process Patents and
(ii) was not assigned or licensed to Rutgers hereby, it will promptly disclose to Rutgers the
existence of such intellectual property or information and either assign without cost or grant a
paid up license with right to sublicense of such intellectual property or information to Rutgers.
(d) It has good title to the documents, information, data and materials (“Materials”) it is
assigning to Rutgers under Article 2.4, and the Integra Process Patents it is licensing under
Article 2.3, free of all liens and encumbrances. Integra believes that, taken as a whole, the
Materials accurately reflect the findings of the scientists engaged in the development of the
materials. Integra represents that to the best of its knowledge and belief, the Integra Process
Patents do not infringe the intellectual property rights of third parties.
6. No Assignment.
6.1 Neither this Agreement, nor any rights or obligations hereunder, may be assigned, pledged
or encumbered by either party without the express prior written approval of the other party.
7. Miscellaneous.
7.1 A waiver of any breach of any provision of this Agreement shall not be construed as a
continuing waiver of other breaches of the same or other provisions of this Agreement.
7.2 Nothing herein shall be deemed to create an agency, joint venture or partnership relation
between the parties hereto.
7.3 This Agreement and Attachments A and B constitute the sole, entire and final agreement and
understanding of the parties with regard to the subject matter hereof and merges and supersedes all
prior discussions, negotiations, understandings and agreements between the parties concerning the
subject matter hereof. Neither party shall be bound by any definition, condition, warranty, right,
duty or covenant other than as expressly stated in this
5
Agreement or as subsequently set forth in a written document signed by both parties. Each
party expressly waives any implied right or obligation regarding the subject matter hereof. A
breach or termination of the Xxxx/Integra agreement referred to in Article 2.1(b) shall not affect
this Agreement.
7.4 This Agreement shall be interpreted and construed, and the legal relations created herein
shall be determined, in accordance with the laws of the State of New Jersey (excluding conflict of
laws) and the United States.
7.5 This Agreement may be amended only by a written document signed by authorized
representatives of both parties.
7.6 Each party hereto agrees to execute, acknowledge and deliver all such further instruments,
and to do all such further acts, as may be necessary or appropriate to carry out the intent and
purposes of this Agreement.
7.7 The headings contained in this Agreement are for reference purposes only and shall not
affect in any way the meaning or interpretation of this Agreement.
7.8 Should any part or provision of this Agreement be held unenforceable or in conflict with
the law of any jurisdiction, the validity of the remaining parts or provisions shall not be
affected by such holding.
7.9 Any and all notices or other communications required or permitted by this Agreement or by
law to be served on or given to either party hereto by the other party shall be in writing and
delivered or sent to:
Vice President, Technology
Integra LifeSciences Corporation
X.X. Xxx 000
000 Xxxxxx Xxxx
Xxxxxxxxxx, XX 00000
Integra LifeSciences Corporation
X.X. Xxx 000
000 Xxxxxx Xxxx
Xxxxxxxxxx, XX 00000
Rutgers, The State University of New Jersey
Office of Corporate Liaison and Technology Transfer
ASB III, 0 Xxxxxxx Xxxxx
Xxx Xxxxxxxxx, Xxx Xxxxxx 00000
Office of Corporate Liaison and Technology Transfer
ASB III, 0 Xxxxxxx Xxxxx
Xxx Xxxxxxxxx, Xxx Xxxxxx 00000
Att: Director
Each party may change its address for purposes of this Agreement by written notice to the
other party.
(a) Each notice or other communication shall be deemed duly served and given on the date when
personally delivered to the party to whom it is directed, when submitted to a nationally recognized
overnight delivery service, prepaid and addressed to the party at the address in paragraph 7.9, or
when deposited in the United States mail, postage
6
prepaid, certified-return receipt requested and addressed tot he party at the address in
paragraph 7.9. When transmitted electronically by telex, facsimile, or e-mail, such notice shall
be deemed duly served and given on the date when the party to whom it is directed confirms receipt
in writing.
7.10 Upon execution of this agreement, the parties will issue a joint press release announcing
the termination of Integra LifeSciences’ license to Rutgers’ polycarbonates, in form and substance
reasonably acceptable to both parties. Integra will refer current and future inquiries regarding
1st Generation Polycarbonates to Rutgers University.
7.11 The parties acknowledge and agree that XXXX shall be a third party beneficiary to this
Agreement with full rights to enforce its terms.
7
7.12 This Agreement may be executed by each party in duplicate originals, each of which shall be
deemed an original, but both originals together shall constitute only one and the same instrument.
| “Rutgers” | ||||||
| Rutgers, The State University | ||||||
| By: | /s/ Xxxxxxx X. Xxxxx | |||||
| Name: | Xxxxxxx X. Xxxxx | |||||
| Title: | Director - OCCTT | |||||
| Date: | 2-2-04 | |||||
| “Integra” | ||||||
| Integra LifeSciences Corporation | ||||||
| By: | /s/ Xxxx X. Xxxxxxxx, III | |||||
| Name: | Xxxx X. Xxxxxxxx, III | |||||
| Title: | SVP & CAO | |||||
| Date: | 1/26/04 | |||||
8
ATTACHMENT A
CONFIDENTIALITY AGREEMENT
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY, having its statewide administrative offices at
(hereafter referred to as “Rutgers”),
, having a
place of business at
(hereinafter “Accountant”), and Integra
LifeSciences Corporation, a corporation organized under the laws of the State of Delaware, having a
business office at 000 Xxxxxx Xxxx, Xxxxxxxxxx, Xxx Xxxxxx 00000 (hereinafter referred to as
“Integra”).
According to the terms of an Agreement dated , between Rutgers and Integra
(the “Agreement”), Rutgers is making available to Accountant its books of account as required by
paragraph 3.3 of the Agreement (“CONFIDENTIAL MATERIAL”) for Accountant’s use in preparing a report
to Integra according to the terms of said paragraph 3.3 (the “Report”).
1. Accountant will not use CONFIDENTIAL MATERIAL except to prepare the Report.
2. Accountant will not disclose CONFIDENTIAL MATERIAL to Integra or any third party and
Accountant shall provide only the Report to Integra and no other party.
3. Integra shall treat the Report as confidential under the same terms as required for the
treatment of CONFIDENTIAL MATERIAL hereunder and Integra shall not provide the Report or disclose
its contents to any third party.
4. Integra will limit access to the Report to those employees of Integra who need to know
about or participate in the review of the Report.
5. CONFIDENTIAL MATERIAL shall remain the property of Rutgers. Sixty (60) days following
completion of the Report, Accountant will return to Rutgers all CONFIDENTIAL MATERIAL in its
possession and destroy all computer entries relating thereto.
6. The obligations in paragraphs 1 and 2 shall not apply to CONFIDENTIAL MATERIAL:
(a) which was known to Accountant and was contained in a writing in Accountant’s possession
before its receipt from Rutgers;
(b) which at the time of its disclosure to Accountant is, or thereafter becomes through no act
or failure to act on the part of Accountant, part of the public domain; or
Attachment A - 1
(c) which has been rightfully furnished to Accountant by a third party without restriction on
disclosure or use and not in violation of any rights of, or obligations to, Rutgers.
An individual feature of the information shall not be considered within the above exceptions
merely because the feature is embraced by more general information within the exceptions. A
combination of features of the information shall not be considered within the above exceptions
unless the combination itself and its principle of operation are within the exceptions. The
obligation to maintain the secrecy of CONFIDENTIAL MATERIAL and limit its use shall terminate five
(5) years from the date of each disclosure of CONFIDENTIAL MATERIAL hereunder.
7. This Confidentiality Agreement shall be governed by the laws of the State of New Jersey
without giving effect to the principles of conflict of laws thereof.
8. This Confidentiality Agreement constitutes the entire agreement among the parties with
respect to the subject matter hereof, and all prior understandings or agreements between the
parties relating to such subject matter, except for the Confidentiality Agreement, whether oral or
written, are automatically cancelled by the execution of this Agreement. The terms and conditions
set forth herein may only be modified I subsequent writing signed by the parties.
All notices and other communications shall be considered given and effective when personally
delivered or when mailed, postage prepaid, to the addresses set forth on the first page of this
Agreement or to such other addresses as the parties may designate in writing.
| AGREED: | AGREED: | |||||||||||
| Rutgers, The State University of New Jersey | Integra LifeSciences Corporation | |||||||||||
By:
|
By: | |||||||||||
| Name: | Name: | |||||||||||
| Title: | Title: | |||||||||||
| AGREED: | ||||||||||||
| Accountant | ||||||||||||
By: |
||||||||||||
| Name: | ||||||||||||
| Title: | ||||||||||||
Attachment A - 2
ATTACHMENT B: SCHEDULE OF PATENTS
COVERING FIRST GENERATION POLYCARBONATES:
COVERING FIRST GENERATION POLYCARBONATES:
US Patent 5,198,507
US Patent 6,120,491 and its international filings listed below:
| 98957728 3 | European PCT | |||||
| 2.309,278 | Canadian PCT | |||||
| 730549 | Australian PCT | |||||
| 2000-520191 | Xxxxx XXX | |||||
| XX00/00000 | Xxxxxx |
Attachment B - 1
Appendix B
INTEGRA TECHNOLOGY
For purposes of clarity, “INTEGRA TECHNOLOGY” does not include inventions, technology, and
intellectual property thereunder, that have reverted or been assigned to RUTGERS pursuant to the
LICENSE TERMINATION AGREEMENT.
INTEGRA TECHNOLOGY related to “FIRST GENERATION MATERIAL” is defined as the family of
desaminotyrosyl-L-tyrosine derived polycarbonates which include poly(DTE-carbonate),
poly(DTB-carbonate), poly(DTH-carbonate), poly(DTO-carbonate) and po!y(X%DTR-co-Y%DT-carbonate)s
and tyrosine derived polycarbonates containing the chain-terminating groups derived from acetic
anhydride or the ethyl, butyl, hexyl, or octyl esters of L-tyrosine or desamino tyrosine.
Manufacturing
| 1. | Specific current research, manufacturing and production information for GMP productions of (poly(DTE carbonate)s not limited to manufacturing standard operating procedures related to, but not limited to, equipment, purchased raw materials and material controls, manufacture processes, quality control procedures and characterization of materials. |
| 2. | Specific current research, manufacturing and production information for non-GMP productions of poly(X%DTE-co-Y%DT carbonate)s) not limited to manufacturing standard operating procedures related to, but not limited to, equipment, purchased raw materials and material controls, manufacture processes, quality control procedures and characterization of materials. |
| 3. | Current laboratory methods for: |
| (a) | Conversion of poly(DTE carbonate) to poly(DTE-co-DT carbonate); | ||
| (b) | Conversion of high to lower molecular weight; | ||
| (c) | Confirmation of percent DT achieved; | ||
| (d) | GPC analysis developed by Integra for first generation materials. | ||
| (d) | And, access to other non-published studies, reports, or methods related to material characterization of tyrosine polycarbonates. |
MAF and Polycarbonate Biocompatibility
| 4. | Non-published studies and reports represented in summary form in the Master File, FDA designated file MAF-1140 Tyrosine-Derived Polycarbonates. |
Appendix B - 1
| 5. | Non-published studies and reports related to biocompatibility studies for polycarbonates other than poly(DTE carbonate)s, e.g., poly(DTB carbonate)s, poly(DTH carbonate)s and poly(DTO carbonate)s. |
| 6. | Integra LifeSciences patent status and access to current, pending and new disclosures related to developments of the polymers and products related thereto, and new intellectual property related to FIRST GENERATION MATERIALS, including but not limited to the following patents: |
| Patent # | Title | Issuance Date | ||
US Xxx #6,359,102
|
Biphasic polymerization process | 03/19/02 | ||
EP0991693A1
|
Biphasic polymerization process | 04/12/00 | ||
EP0991693B1
|
Biphasic polymerization process | 09/03/03 | ||
US Xxx # 6620356
|
Porous constructs fabricated by gas induced phase inversion | 09/16/03 |
Appendix B - 2
APPENDIX C
RUTGERS PATENT RIGHTS
The following rights are included in the definition of RUTGERS PATENT RIGHTS:
THESE PATENTS ARE INCLUDED IN THE DEFINITION OF RUTGERS PATENT RIGHTS ONLY TO THE EXTENT THEY APPLY
TO “POLYCARBONATES”, AS DEFINED AT THE END OF THIS APPENDIX:
A. U.S. Patent 6,475,477B1 “Radio-opaque Polymer Biomaterials.”
B. US Patent 5,099,060 “Synthesis of amino acid-derived bioerodible polymers.” This patent is
included only to the extent that the patented monomers are used in the fabrication of
POLYCARBONATES.
C. US Patent 5,587,507 “Synthesis of tyrosine derived diphenol monomers.” This patent is
included only to the extent that the monomers produced thereby are used in the fabrication of
POLYCARBONATES.
D. US Patent 5,658,995 “Copolymers of tyrosine-based polycarbonate and poly(alkylene oxide).”
E. US Patent 6,048,521 “Copolymers of tyrosine-based polyarylates and poly(alkylene oxide).”
F. US Patent 6,319,492 B1 “Copolymers of tyrosine-based polyarylates and poly(alkylene
oxide)”.
G. US Patent 5,198,507 “Synthesis of amino acid-derived bioerodible polymers”.
H. US Patent 6,120,491 “Biodegradable, anionic polymers derived from the amino acid
L-tyrosine.”
I. US Patent 6,359,102 issued on March 19, 2002, “Biphasic polymerization process, EP0991693A1
issued on April 12, 2000, “Biphasic polymerization process,” and EP0991693B issued on September
3,2003, “Biphasic polymerization process.”
J. US Patent 6,620,356 issued on September 16, 2003, “Porous constructs fabricated by gas
induced phase inversion.”
K. Rutgers Docket 03-175.
“POLYCARBONATES” means tyrosine-derived polymers containing desaminotyrosyl-tyrosine or
desaminotyrosyl-tyrosine alkyl ester repeat units linked together by carbonate backbone linkages as
described in subsections (a) through (e) below and which comprise a subgroup of the polymers
defined by the comprehensive chemical Formula VIII listed under CLAIM 1 in US Patent 6,475,477B1.
POLYCARBONATES exclude all polymers commonly referred to as
Appendix C - 1
polyarylates. With reference to said Formula VIII, for the purposes of this License Agreement, a
POLYCARBONATE is a polymer that meets all the following conditions:
a. “A” described in said Formula VIII is limited to being a simple carbonyl group, e.g., carbon
double bonded to oxygen as indicated by Structure 1; AND
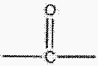
(Structure 1)
b. XI and X2 in said Formula VIII are independently Iodine or Bromine atoms; Y1 and Y2 in said
Formula VIII are independently 0, 1, or 2. Non-radio-opaque polymers are obtained if both Y1 and
Y2 are equal to zero (0); AND
c. R7 in said Formula VIII is independently an alkylene group containing up to 4 carbon atoms
(e.g., methylene, ethylene, propylene, or butylene); AND
d. R9 and R12 in said Formula VIII are limited by the requirements that
(i) R4 equals (-CH2-) with subscript “a” equals 2 as defined in Claim 3 of said US patent
(ii) R0 equals (-CH2-) with subscript “m” equals 1 as defined in Claim 3 of said US patent
(iii) Z in Claim 3 is limited to a free carboxylic acid group or carboxylic acid esters or amides having straight or branched alkyl and alkyl aryl groups containing up to 18 carbon atoms, or esters and amides of biologically and pharmaceutically active compounds; AND
(i) R4 equals (-CH2-) with subscript “a” equals 2 as defined in Claim 3 of said US patent
(ii) R0 equals (-CH2-) with subscript “m” equals 1 as defined in Claim 3 of said US patent
(iii) Z in Claim 3 is limited to a free carboxylic acid group or carboxylic acid esters or amides having straight or branched alkyl and alkyl aryl groups containing up to 18 carbon atoms, or esters and amides of biologically and pharmaceutically active compounds; AND
e. The parameter “f” in said Formula VIII can vary from 0 to 0.99 (including the values of “0” and
“0.99”). The parameter “g” in said Formula VIII can vary from 0 to 1 (including the values of “0”
and “1”). The parameter “k” in said Formula VIII is between 5 and 3,000.
Said definition of POLYCARBONATE contains as a subgroup, but is not limited to the polymers
exemplified by Formula 1 which is shown here for illustrative purposes only. In Formula 1, the I
stands for iodine, DTR stands for desaminotyrosyl-tyrosine alkyl ester, DT Stands for
desaminotyrosyl-tyrosine, and PEG stands for poly(ethylene glycol);
Formula 1. poly(IxDTR-co-f%PEGmw-co-g%DT carbonate)
One potential embodiment of Formula 1. further exemplified is:
poly(I2DTE-co-5%PEG1000-co-30%DT carbonate).
poly(I2DTE-co-5%PEG1000-co-30%DT carbonate).
Appendix C - 2
APPENDIX D
Xxxx/Integra License Agreement Royalty Report for the Period Through
Xxxx/Integra License Agreement Royalty Report for the Period Through
Instructions: Please fill in all boxes (write “none” if not applicable), and sign and date at
bottom. All section numbers refer to the License Agreement.
Answer questions 1 and 2 (AND SIGN AND DATE AT BOTTOM) EVEN IF THERE HAS BEEN NO ACTIVITY in
reporting period.
| 1. | Number of transactions: by REV A o by Sublicensees o | |
| 2. | Any new sublicenses entered into during the period? Yes o no o |
If yes, attach separate sheet listing names and addresses of sublicensees, and attach sublicense
agreements.
Total
amount enclosed $
XXXX Medical, Inc.
| Date: |
| Name and Title: |
Xxxxxxxx X - 0
XXXXXXXX X (Continued)
Continuation sheet number to XXXX/INTEGRA License Agreement Royalty Report for the Period through
Continuation sheet number to XXXX/INTEGRA License Agreement Royalty Report for the Period through
Use this sheet if there are additional transactions to report.
| By ____ or | Product or Process | |||||||||||||||||||||||||||||
| Date of | Sublicense? (if | Type (name and id | Deductions per § __ | |||||||||||||||||||||||||||
| Transaction | Type of Transaction | latter, identify) | number) | Total Xxxxxxxx | (specify type) | Royalties Due | Customer Name and Address | |||||||||||||||||||||||
Appendix D - 2
