DEVELOPMENT AND CLINICAL SUPPLY AGREEMENT DPT Laboratories, Ltd. And Peplin, Inc.
Exhibit 10.23
DPT Laboratories, Ltd.
And
Xxxxxx, Inc.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
1
This Development And Manufacturing Agreement (this “Agreement”), effective as of this
October 23, 2007 (the “Effective Date”), is made by and between Xxxxxx, Inc., a Delaware
corporation with its principal place of business at 0000 Xxxxxxxx Xxxxxx, Xxxxxxxxxx,
Xxxxxxxxxx 00000 (“COMPANY”), and DPT Laboratories, Ltd., a Texas Limited Partnership with
its principal place of business at 000 X. Xxxxxxxxx, Xxx Xxxxxxx, Xxxxx 00000 (“DPT”).
1 — Definitions. For purposes of this Agreement, the following definitions shall
apply, and the terms defined herein in plural shall include the singular and vice-versa.
| 1.1 | “API” means the active pharmaceutical ingredient of the Product. | ||
| 1.2 | “cGMPs” means current Good Manufacturing Practices, regulations and guidelines applicable to the manufacture and testing of pharmaceutical products in the United States, including those in 21 CFR Parts 210 and 211. | ||
| 1.3 | “Claims” has the meaning given in Section 9.1. | ||
| 1.4 | “Company Confidential Information” has the meaning given in Section 5.1. | ||
| 1.5 | “Company Technology” has the meaning given in Section 4.2(a). | ||
| 1.6 | “Confidential Information” has the meaning given in Section 5.1. | ||
| 1.7 | “Data” has the meaning given in Section 6.3. | ||
| 1.8 | “Deliverables” “has the meaning given in Section 2.5. | ||
| 1.9 | “DPT Confidential Information” has the meaning given in Section 5.1. | ||
| 1.10 | “Equipment” has the meaning given in Section 2.4. | ||
| 1.11 | “Facility” means DPT’s manufacturing facility located in San Antonio, Texas. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
2
| 1.12 | “GMP Batch or Batches” means the final bulk Product produced in accordance with current Good Manufacturing Practices and resulting from completion of the compounding process and reconciliation prior to the packaging process. | ||
| 1.13 | “Inventions” has the meaning given in Section 6.2. | ||
| 1.14 | “Know-how” has the meaning given in Section 6.1. | ||
| 1.15 | “Materials” has the meaning given in Section 2.3(b). | ||
| 1.16 | “MSDS” means Material Safety Data Sheets prepared in accordance with regulations promulgated by the Occupational Safety & Health Administration. | ||
| 1.17 | “Process Inventions” has the meaning given in Section 6.2(b). | ||
| 1.18 | “Product” means PEP005 formulated into a topical gel at a 0.05% (or other concentration or formulations as may be required) for use in Phase III clinical trials. | ||
| 1.19 | “Product Inventions” has the meaning given in Section 6.2(a). | ||
| 1.20 | “Project Scope Document” or “PSD” means the document attached hereto as Exhibit A and incorporated herein by reference, as it may be modified from time to time upon written agreement of the parties. In the event of any conflict between the terms of this Agreement and the PSD, the terms of this Agreement shall govern. | ||
| 1.21 | “Protocol” has the meaning given in Section 2.2. | ||
| 1.22 | “Recipient” has the meaning given in Section 5.2. | ||
| 1.23 | “Services” has the meaning given in Section 2.1. |
2.1 Project Scope. DPT shall use commercially reasonable, good faith
efforts to perform the services outlined in the Project Scope Document and the
Protocols (collectively, the “Services”), in accordance with the schedule
attached hereto as Exhibit B and incorporated herein by reference, and to
accomplish the desired results. Such efforts shall include efforts to manufacture
all Product batches, other than the feasibility/placebo batch, in accordance with
all applicable cGMPs and, except as provided in Section 4.3(c), with respect to
the GMP Batches, DPT makes no warranties or representations that it will be
able to achieve the desired results.
2.2 Project Protocols. Prior to beginning each of the tasks outlined on
Exhibit B, (a) DPT shall prepare a written Protocol that details, specifically, the
work to be performed in connection with the task, the Deliverables to be
produced and/or delivered to COMPANY, and the itemized costs of the task (a
“Protocol”), all of which shall be consistent with the PSD and prior Protocols,
subject to Section 2.6. DPT shall not commence (or charge Company for) any
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
3
work described in a Protocol unless and until the Protocol has been signed by both
parties. For the avoidance of doubt, no batch of Product shall be produced (and no work on
such production shall begin) unless the parties have signed a Protocol authorizing such
production and work.
2.3 Materials
| (a) | COMPANY shall provide DPT with all API required for the performance of the Services. Prior to delivery of API to DPT, and as a condition precedent of any testing or formulation work by DPT pursuant to this Agreement, COMPANY shall provide DPT with the MSDS for the API. COMPANY also shall be responsible for any necessary or desired cGMP audits of the API supplier. All API shall be and shall remain the property of COMPANY and shall be treated as COMPANY’S Confidential Information pursuant to Sections 5.1 - 5.4. | ||
| (b) | DPT shall be responsible for the acquisition of all raw materials other than API, and all packaging components necessary to produce and supply the Product (collectively, the “Materials”), subject to Section 3.2, and for ensuring that Materials suppliers have been qualified in accordance with DPT’s supplier qualification procedures. All Materials shall be the sole and exclusive property of COMPANY. DPT agrees to handle and store the Materials in accordance with all applicable laws and regulations, and under conditions prescribed by the manufacturer, in order to maintain their quality and suitability for use. |
2.4 Equipment. DPT has purchased certain capital equipment, on
Company’s behalf, with which to manufacture and/or package Product, and it
may purchase additional such equipment, upon written agreement of the parties,
on Company’s behalf (the “Equipment”). Subject to Section 3.2, the Equipment
shall be the exclusive property of Company; provided, however, that DPT shall
have the right to use the Equipment, with Company’s prior written consent,
which shall not be unreasonably withheld, as long as (a) the Equipment is
located at the Facility and (b) such use does not interfere with scheduled
Product production. As soon as reasonably practicable, upon request by
Company (or at any later date requested by Company), or expiration or
termination of the Agreement, DPT shall have the option, subject to approval by
the Company in the form of written consent which shall not be unreasonably
withheld to either (i) purchase the Equipment, at its then depreciated value, or (ii)
transfer the Equipment to COMPANY, at COMPANY’S sole expense. In the
event that DPT exercises such option, and the Company does not accept the
option by providing its written consent, the Parties will jointly develop a transition
plan to ensure uninterrupted production of Company and other products utilizing
the Equipment.
2.5 Deliverables. The deliverables to be produced (and, unless the parties
agree otherwise in writing, provided to Company by DPT) hereunder are (a) the
eight Product batches identified on page 2 of the PSD, (b) a pharmaceutical
development report, stability reports and validation reports, as provided at page
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
4
4 of the PSD and (c) any other deliverables specifically identified as such in a
Protocol (collectively, the “Deliverables”). Following completion of manufacture of each
Product-batch, DPT shall do one or more of the following, as directed by COMPANY in
writing: (i) store the batch on DPT’s premises, (ii) destroy it, and/or (iii) ship the
batch to a third party designated by COMPANY, according to COMPANY’S instructions, at
COMPANY’S sole cost and expense. (Charges for storage and destruction shall be at DPT’s
standard rates for such activities.)
2.6 Changed Services. Both parties understand that, during any development project,
unforeseen events may occur, including unacceptable results, leading to termination of the
project, or marginal results leading to significant re-evaluation and modification of the
project. DPT shall promptly notify COMPANY of any such unforeseen events before proceeding
further with the Services, at which time either COMPANY or DPT may terminate this
Agreement, upon written notice to the other party, or may agree to a change in Services,
pursuant to an amendment to this Agreement or a Protocol. In either case, COMPANY will be
obligated to pay for all tasks that have been completed through the date of DPT’s notice of
the unforeseen events, as described in a Protocol issued thereafter, and for all Materials
in inventory, other than those that DPT, using commercially reasonable efforts, can return
or use for other purposes, and Materials on order, if the order cannot be cancelled.
3.1 Costs of Services. Costs of Services are based on the assumptions
stated in the PSD. They include costs of labor for all reasonably foreseeable
development, testing, scale-up and stability-testing activities, as well as all other
reasonably foreseeable events and expenses (other than costs of Materials).
3.2 Costs of Materials and Equipment. Materials delivered to DPT, and
any Equipment acquired in accordance with Section 2.4, will be charged to
COMPANY at their actual cost, plus **** percent (****%), plus any applicable sales
taxes, use taxes and freight charges (and installation costs, where applicable to
Equipment). Costs will be invoiced to COMPANY in accordance with Section
3.3.
3.3 Invoicing and Payment. Costs of technical Services shall be invoiced
as indicated in the relevant Protocol. Any differences between such costs and
those provided in the PSD, and the changes in assumptions and/or
circumstances that led to such differences, shall be noted and explained in the
Protocol or otherwise provided to the Company in writing. Costs of Services and
Materials directly related to Product manufacture will be invoiced to COMPANY
at the time of shipment of the Deliverables to which they apply and shall be
consistent with the costs provided for in the PSD/Protocol. Payments shall be
due within thirty (30) days of COMPANY’S receipt of all relevant Deliverables and
applicable invoice. Except for those Services and Materials performed or
ordered in connection with replacement any GMP Batches described in Section
4.3(c), costs of completed Services and of Materials previously ordered that DPT
cannot, after using commercially reasonable efforts, return or use for other
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
5
purposes, shall be paid to DPT, regardless of whether DPT is able to accomplish the
results that COMPANY requested.
3.4 Collateral Security. As collateral security for COMPANY’S payment obligations contained
in this Agreement, COMPANY grants to DPT a security interest in all raw materials,
inventory, work-in-progress, and finished goods. Chapter 9 of the Texas Uniform Commercial
Code shall govern the rights and obligations of the parties relative to the security
interests granted herein.
4.1 Joint Responsibilities. The parties shall use commercially reasonable and good faith
efforts to agree in writing, on the following criteria, within the time-periods provided:
| (a) | Specifications and other criteria which have been mutually agreed by both parties to which cGMP Product must conform, including the test methods to be used in determining whether Product conforms thereto: at least two (2) weeks before the scheduled commencement of manufacture of the 1st GMP Batch. | ||
| (b) | Specifications for the containers in which Product will be filled: at least two (2) weeks before the scheduled commencement of manufacture of any Batch. | ||
| (c) | Specifications for bulk-packaging containers: at least two (2) weeks before the scheduled commencement of manufacture of the final GMP Batches. | ||
| (d) | The terms, conditions, roles and responsibilities of the parties with respect to quality matters, either in a separate quality agreement or in Protocols, as required by applicable regulations: as soon as reasonably practicable after the Effective Date, and in any event, at least two (2) weeks prior to the commencement of manufacture of any Batch. | ||
| (e) | The content of the Chemistry and Manufacturing Controls (CMC) portions of any submission for regulatory approval in connection with the manufacture or sale of Product (e.g., a document to be filed in support of a New Drug Application to the FDA or a new product application as specified by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use), and COMPANY shall use such agreed-upon information as the source of the CMC portion of any such submission. If additional information is requested or required by any regulatory agency or required for any regulatory filing, COMPANY shall provide DPT with a copy of the documents incorporating such information, and DPT will have ten (10) business days to review them, unless the parties agree otherwise, in writing, in order to verify the accuracy of (i) such documents as they relate to |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
6
the data generated by DPT hereunder, and (ii) the description of the
work performed and manufacturing processes used by DPT hereunder.
| (a) | COMPANY will transfer to DPT the Product formulation, tests and other technology and information (collectively, the “Company Technology”). All Company Technology shall be and shall remain the sole property of COMPANY and shall be deemed Company Confidential Information, subject to Sections 5.1 — 5.4. COMPANY hereby grants to DPT a non-exclusive, nontransferable, royalty-free license to use the Company Technology solely for purposes of performing the Services. | ||
| (b) | The API provided to DPT for use in manufacturing the GMP Batches shall, at the time of delivery to DPT, conform to the specifications therefor. | ||
| (c) | COMPANY shall bear sole responsibility for the validity of all test methods to be used for Product development and manufacture, and the appropriateness for use of all Product and Product packaging. | ||
| (d) | Except as otherwise provided in Section 4.1(e), COMPANY shall bear sole responsibility for all regulatory approvals, filings, and registrations, including the adequacy of all validation, stability, and preservative efficacy studies required therefor. |
| (a) | DPT warrants and agrees that (i) it shall perform all of its obligations hereunder in accordance with all applicable laws, regulations and rules, (ii) it has and shall maintain all licenses and permits required in order to manufacture Product at the Facility, (iii) it has not been debarred and has not and will not knowingly use in any capacity the services of any person debarred under subsections 306(a) or of the United States Generic Drug Enforcement of 1992 or any comparable law of any foreign jurisdiction. | ||
| (b) | DPT shall not subcontract any Services without COMPANY’S prior written consent. | ||
| (c) | DPT agrees that each GMP Batch produced hereunder shall be manufactured at the Facility, in accordance with cGMPs, at the time of delivery to Company, provided that the API intended for use in the GMP Batches conforms, at the time of delivery to DPT, to the specifications therefor. In the event that any GMP Batch fails to meet the foregoing requirements, DPT, at its sole cost and expense, shall replace the failing batch(es) with conforming batch(es), |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
7
| provided that Company supplies the API needed for such replacement batches. | |||
| (d) | DPT shall maintain all original documents relating to the development, manufacture and control of the Product, the Materials and the API, including inventory records, testing procedures and specifications, master and lot manufacturing instructions, data from testing and inspections, and original records of experimental work performed to establish capability to manufacture and test the Product. DPT shall store these original documents in a safe and organized manner so that they may be provided upon request to COMPANY or to the U.S. Food and Drug Administration or other federal or state regulatory agencies within two (2) business days of their request. In the event that COMPANY elects not to pursue marketing, sale, license, or transfer of the Product, DPT shall surrender all original documents to COMPANY within ten (10) business days of receipt of a written request for such. |
4.4 Safety. DPT shall be responsible for maintaining safety procedures and
required training documentation for DPT’s handling and manufacture of the
Product, the Materials and the API. DPT acknowledges that it has been advised
that the API is a hazardous or potentially hazardous substance, and DPT shall
ensure that all of its employees and contractors providing Services have been
(a) duly advised, in advance, of the hazardous nature of the API and of all
handling instructions contained in the MSDS therefor, and (b) trained in the
proper handling of the API and the Product.
4.5 Observation of Product Manufacture. During the term of this
Agreement, upon reasonable advance written notice to DPT, and at mutually
agreed dates and times, COMPANY may have up to three (3) representatives
present at the Facility to observe any and/or all steps relating thereto.
4.6 Compliance Audits. Once per year during the term of this Agreement
and for one year thereafter, on dates and at times agreed upon by the parties,
COMPANY shall have the right to have up to two (2) representatives present at
the Facility, for up to two (2) days, in order to conduct a compliance audit of
DPT’s facilities pertaining to the manufacture, laboratory work, packaging,
storage, testing, shipping or receiving of the Product and its components.
4.7 Waste Handling. DPT shall be responsible for maintaining procedures
for the generation, treatment, storage and disposal of wastes resulting from the
Services (including any rejected or obsolete Product in its possession), all of
which shall comply with all federal, state and local environmental and
occupational safety and health requirements (including those applicable to
hazardous substances, as defined by the U.S. Occupational Safety and Health
Administration and/or the U.S. Environmental Protection Agency).
5.1 Definitions; Ownership. During the term of this Agreement, (a) DPT
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
8
may generate for the benefit of COMPANY, and (b) each party may provide
to the other information, including proprietary materials, proprietary
technologies, economic information, business or research strategies, trade
secrets and material embodiments thereof. Except as set forth in the
following sentence, all such written information shall be marked
“confidential,” and all such oral information shall be confirmed in a writing
marked “confidential” within thirty (30) days of the disclosure
(collectively, “Confidential Information”). Confidential Information also
shall include any manufacturing operations or processes or technologies of
either party or their affiliates that are learned by the other party or its
representatives in connection with this Agreement. For purposes of this
Agreement, all Confidential Information provided by COMPANY to DPT hereunder
and all Data shall be deemed “Company Confidential Information,” which shall
be solely owned by Company. All other information provided by DPT to COMPANY,
including Know-how and information pertaining directly to Process Inventions,
hereunder shall be deemed “DPT Confidential Information,” which shall be
solely owned by DPT.
5.2 Obligations. During the term of this Agreement and for a period of five (5) years thereafter, the party
receiving or in possession of Confidential
Information belonging to the other party (the “Recipient”) (a) shall maintain such
Confidential Information in confidence, (b) shall use such information solely to
exercise its rights and perform its obligations under this Agreement, unless
otherwise mutually agreed in writing, and (c) shall at all times protect such
information from misuse or disclosure with at least the same degree of care it
uses to protect its own Confidential Information, such care to be of the type and
degree that would be used by a reasonable and prudent business person in the
biopharmaceutical industry.
5.3 Exclusions. Confidential Information (other than Company Confidential
Information generated by DPT) shall not include information that (a) the Recipient
can demonstrate, through contemporaneous documentation, (i) was in its
possession prior to receipt from the party that owns the Confidential Information
(the “Owner”) or (ii) was furnished to the Recipient by a third party without
breach of a duty of confidentiality, (b) was or became, through no fault of the
Recipient, publicly known, or (c) is required by law, regulation or rule to be
disclosed, provided that the Recipient notifies the Owner of such requirement as
soon as reasonably practicable and cooperates in any action that the Owner
reasonably elects to take in order to protect the confidentiality of such
Confidential Information.
5.4 Return. Upon termination or expiration of this Agreement, the Recipient
shall return or destroy (at the election of the Owner) all Confidential
Information
of the Owner in its possession (including but not limited to all copies,
abstracts or
summaries thereof), other than that information it is required by law or
regulation
to retain (which the Recipient shall destroy when such requirement no longer
applies), and shall provide the Owner with a written verification of such
return or
destruction. Thereafter, the Recipient shall make no further use and shall
not
allow any of its officers, employees or agents to make any use of the Owner’s
Confidential Information for any purpose, other than as expressly allowed
under
this Agreement. Notwithstanding the preceding sentence, the Recipient’s legal
counsel may retain one copy of the other party’s Confidential Information in
a
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
9
secure location solely for purposes of identifying such party’s obligations under this
Agreement.
5.5 Inapplicability of Prior Agreement. For the avoidance of doubt, the
confidentiality of all information generated pursuant to the Agreement or
disclosed by one party to the other hereunder shall be governed solely by this
Agreement; that certain Confidentiality Agreement between the parties dated
August 14, 2006 shall not apply.
5.6 Confidentiality of Agreement Terms. Either party may disclose the
existence and the general subject matter, but not the financial terms of this
Agreement, except that COMPANY may disclose this Agreement and the terms
thereof to its potential or actual investors, collaborators and licensees, provided
that provided that such parties are bound by a duty of confidentiality at least as
restrictive as those provided in Sections 5.1 — 5.4.
6.1 Know-how. During the term of this Agreement, DPT may use and adapt
its proprietary knowledge, information and know-how and develop new
proprietary knowledge, information and know-how (“Know-how”) in an attempt to
perform the Services to COMPANY’S satisfaction. Except to the extent required
by subpart 6.2 of this Section, the Know-how shall be solely owned by DPT,
which hereby grants to COMPANY a non-exclusive, perpetual, paid-up, royalty-free, irrevocable license (including the right to sub-license) to use the Know-how
solely for the manufacture, use and/or commercialization of the Product.
COMPANY acknowledges that the business of DPT, as a contract manufacturing
organization, involves the application of its expertise, technology and know-how
to numerous pharmaceutical and other products, and that DPT retains the right,
subject to its obligations expressly provided herein, to apply such expertise,
technology and know-how to a variety of products or services.
6.2 Inventions. The party making any patentable invention, development,
improvement, process or the like, that is conceived or first reduced to practice in
the course of performance of the Services (an “Invention”) shall promptly
disclose the Invention, in writing, to the other party. Inventions shall be owned as
follows:
| (a) | COMPANY shall own all right, title, and interest in and to any Inventions that constitute modifications or improvements to the physical structure, composition, activity, or potency of the API, or that are otherwise solely related to the Product and not to pharmaceutical products in general (“Product Inventions”). DPT hereby irrevocably assigns and transfers to COMPANY DPT’s entire right, title, and interest in and to Product Inventions. DPT shall cooperate with COMPANY, at COMPANY’S sole expense, as may be necessary for the perfection, enforcement or defense of COMPANY’S rights in any Product Inventions. DPT is hereby granted a non-exclusive, perpetual, paid-up, royalty-free license, without the right to sub-license, to use Product Inventions solely in the performance of the Services. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
10
| (b) | DPT shall own all right, title and interest in and to any Inventions that constitute new or modified methods or processes that apply generally to the manufacture of pharmaceutical products and not specifically to the Product (“Process Inventions”). COMPANY hereby irrevocably assigns and transfers to DPT COMPANY’S entire right, title, and interest in and to Process Inventions. COMPANY shall cooperate with DPT, at DPT’s sole expense, as may be necessary for the perfection, enforcement, or defense of DPT’s rights in any Process Inventions. COMPANY is hereby granted a non-exclusive, perpetual, paid-up, royalty-free license, with the right to sub-license, to use Process Inventions solely for the formulation, development and/or manufacture of COMPANY’S Product. |
6.3 Data. All data and information (other than Know-how or Inventions) developed,
generated or derived by or on behalf of DPT for COMPANY during the term of this Agreement
(collectively, “Data”) is the property of COMPANY, subject to any regulations requiring
that DPT retain the data at the Facility. Reports containing such Data shall be provided
by DPT to COMPANY as mutually agreed by the parties.
7.1 Disclaimer of Warranties. EXCEPT AS SPECIFICALLY PROVIDED HEREIN, DPT AND COMPANY MAKE NO WARRANTIES, EXPRESS OR
IMPLIED, WITH RESPECT TO THE PRODUCT, API, LABELING OR PACKAGING. ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, ARE HEREBY DISCLAIMED.
7.2 Limitation on Damages. IN NO EVENT SHALL EITHER PARTY BE
LIABLE TO THE OTHER FOR INDIRECT, INCIDENTAL OR CONSEQUENTIAL
DAMAGES RESULTING FROM BREACH OF THIS AGREEMENT.
Each party shall be excused from the performance of its obligations hereunder to the
extent such performance is rendered impossible by an act of God, regulations or laws
enacted by any competent government authority, civil commotion, strike, shortage of raw
materials, unavailability of necessary equipment, substantial damage to or destruction of
production facilities or material by fire, earthquake or storm, epidemic, failure of public
utilities or common carriers, or other cause that was unavoidable or beyond the control of
such party, provided that (a) such event was not due to any legal violation, breach,
default, negligence or willful misconduct of the party seeking to be excused, (b) written
notice of the event and its expected duration is promptly given to the other party and (c)
the notifying party uses its best efforts to resume performance hereunder as soon as
practicable, and provided further, that if such force majeure event prevents performance
beyond three (3) months, the unaffected party may terminate this Agreement upon notice to
the affected party.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
11
9.1 Indemnification by DPT. DPT agrees to defend, indemnify and hold
harmless COMPANY, its employees, officers, directors and representatives from
and against any third-party claims, losses, damages or expenses, including
reasonable attorneys’ fees paid or incurred by any of them (collectively,
“Claims”) up to the extent of insurance coverage as required under Section 9.2
and arising out of:
| (a) | DPT’s failure to comply with its obligations under this Agreement, which were within its control; except to the extent that such Claims arise out of the gross negligence or willful misconduct of COMPANY, | ||
| (b) | the infringement of any third-party rights in the course of performing the Services, other than infringement by the use of any formula, component, art work or process provided by COMPANY to DPT. |
9.2 Insurance of DPT. DPT warrants that it currently has, and shall maintain
insurance covering its risks hereunder, including the following:
| • | Products liability Insurance coverage with minimum amounts of $**** per occurrence and $**** annual aggregate, | ||
| • | Contractual liability insurance with minimum amounts of with minimum amounts of $**** per occurrence and $**** annual aggregate, and | ||
| • | Worker’s Compensation insurance in compliance with all applicable laws, including employer’s liability insurance with respect to DPT’s employees, with minimum limits of One Million Dollars ($****) per accident, per disease, and per employee, and | ||
| • | Business personal property insurance covering all Materials, Product, intermediates and Equipment at the Facility that have been paid for and/or are owned by COMPANY, with coverage of at least full replacement value. |
9.3 Indemnification by COMPANY. COMPANY agrees to defend,
indemnify and hold harmless DPT, its employees, officers, directors and
representatives for any Claims arising out of (a) any ownership, testing, use
(including in clinical trials), application, consumption, distribution, marketing
or
sale of Product, following its delivery by DPT to COMPANY, except to the
extent that such Claims arise out of the gross negligence or willful
misconduct of DPT, and (b) any infringement of third-party rights or violation of
patent, copyright or trademark laws by any COMPANY-designated formulas,
components or artwork related to the Product, and (c) COMPANY’S failure to
comply with its obligations under this Agreement.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
12
9.4 Insurance of COMPANY. COMPANY warrants that it currently has, and shall maintain
insurance covering its risks hereunder, including (a) products liability insurance, (b)
contractual liability insurance and (c) clinical trials insurance (including coverage for
any negligence on the part of COMPANY in the conduct of any clinical trials, as well as for
any defects or negligence in the design of the clinical trial process) coverage. Insurance
coverage for each of the foregoing subsections shall be in the minimum amounts of Ten
Million Australian Dollars (AUD $10,000,000) per occurrence with an annual aggregate amount
of Ten Million Australian Dollars (AUD $10,000,000). COMPANY’S product liability coverage
shall be primary and non-contributing.
10.1 Term of Agreement. The term of this Agreement shall be four (4) years
from the Effective Date, unless earlier terminated pursuant to Section 10.2.
10.2 Termination. This Agreement may be terminated early, as follows:
| (a) | By either party, as provided in Section 2.6. | ||
| (b) | By either party if the other party defaults or breaches any material provision of this Agreement, upon thirty (30) days’ written notice to the defaulting party (which notice shall cite this § 10.2(c) and shall explain, in reasonable detail, the nature of the breach), unless the breach is cured within such 30-day period. | ||
| (c) | By either party, upon any of the following events: (i) the other party makes an assignment for the benefit of its creditors or files a voluntary petition under applicable bankruptcy or insolvency laws, (ii) a receiver or custodian is appointed for all or substantially all of the other party’s business, (iii) proceedings are instituted against the other party applicable bankruptcy or insolvency laws that have not been stayed or dismissed within sixty (60) days, (iv) all or substantially all of the other party’s business or assets become subject to attachment, garnishment or other process or (v) a court or other governmental authority of competent jurisdiction determines that the other party is insolvent. | ||
| (d) | By COMPANY at any time, with or without cause, on sixty (60) days’ written notice to DPT, provided that COMPANY pays for all Services rendered through the date of the notice of termination, all other work reasonably necessary to terminate the Services, and all Materials. | ||
| (e) | By either party, as provided in Article 8. |
10.3 Obligations upon Termination or Expiration. Upon termination or
expiration of this Agreement, (a) each party shall comply with the provisions of
Section 5.4, and (b) DPT shall (i) deliver to COMPANY all finished Product in
DPTs possession that COMPANY has paid for, (ii) return to COMPANY or
COMPANY’S designee, as requested by COMPANY in writing, all Materials and
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
13
Product intermediates in its possession and (iii) arrange for the delivery to COMPANY
or its designee of any Equipment in its possession. Costs of freight and insurance for
those activities described in subsections (b)(ii) and (b)(iii) shall be borne by COMPANY.
10.4 Survival. Expiration or termination of this Agreement for any reason shall not
release either party from any liability, obligation or agreement that has already accrued.
The provisions of Articles 5, 6, 9 and 11 and Sections 3.3, 4.1(e), 4.3(d), 4.4, 4.6, 4.7,
7.2, 10.3, 10.4, 12.4 and 12.5 shall survive any expiration or termination of this
Agreement.
Upon request by COMPANY, DPT shall provide COMPANY or its designee with reasonable
assistance in transferring any and all of COMPANY’S rights in Know-how, Inventions, Data
and any other technology, processes, methodologies and information (except for any that is
proprietary to DPT and to which COMPANY has no rights hereunder) that would be necessary or
useful in the scale-up, processing, manufacture, filling, packaging or shipment of Product,
to such third parties as COMPANY may designate, subject to COMPANY’S payment of DPT’s
customary charges for such assistance.
12.1 Assignment. This Agreement shall be binding upon and shall inure to
the benefit of the successors or permitted assigns of each of the parties and may
not be assigned or transferred by either party without the prior written consent of
the other, except that (a) such consent will not be required for any assignment to
a third party that acquires (i) substantially all of the assignor’s business or assets,
or (ii) acquires the assignor pursuant to a merger or consolidation, and (b) DPT’s
consent will not be required for an assignment by COMPANY to any third party
acquiring rights in the Product. No assignment, including sale, transfer, or
license of brand or Products, shall release an original party hereto from its
obligations under this Agreement.
12.2 Notices. Any notice required hereunder shall be effective upon receipt
and may be served by either party on the other by personal delivery, or by
sending same, post prepaid, by registered or by certified mail to the address first
set forth above.
12.3 Independent Contractors. The relationship between the parties under
this Agreement shall be strictly that of independent contractors. Neither party is
hereby constituted an agent or legal representative of the other party for any
purpose whatsoever, and neither party is granted any right or authority hereunder
to assume or create any obligation, express or implied, or to make any
representation, warranties or guarantees, except as are expressly granted or
made in this Agreement.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
14
12.4 Governing Law. The validity, interpretation and effect of this Agreement
shall be governed by and construed under the laws of the State of New Jersey.
12.5 Dispute Resolution. Any dispute arising under this Agreement shall be
resolved in the following manner:
| (a) | Either party may initiate resolution of the dispute by providing to the other party a brief and concise statement of the initiating party’s claims, together with relevant facts supporting them, and referring to this Section 12.5(a). For a period of thirty (30) days from the date of such statement, or such longer period as the parties may agree upon in writing, the parties shall make good faith efforts to settle the dispute. Such efforts shall include full presentation of the parties’ respective positions to executive officers of their respective companies. | ||
| (b) | Except as otherwise provided in Section 12.5(c), in the event the parties are unable to resolve the dispute pursuant to Section 12.5(a), the dispute shall be finally settled without recourse to the courts, in accordance with the Commercial Arbitration Rules of the American Arbitration Association then in effect, by a single arbitrator designated in accordance with those rules. The arbitration shall be held in San Francisco, California, if initiated by DPT, and in San Antonio, Texas, if initiated by COMPANY. All decisions of the arbitrator shall be final and binding on the parties, and may be enforced in any court of competent jurisdiction. The arbitrator may allocate the fees and expenses of the arbitration between the parties in any manner that the arbitrator deems equitable. | ||
| (c) | Notwithstanding anything stated in above in this Section 12.5, each party shall have the right, before or during the arbitration, to seek equitable relief from any court of competent jurisdiction. |
12.6 Waiver. No waiver of any term or condition of this Agreement shall be
valid or binding on either party unless made in writing by the party to be charged.
The failure of either party at any time to enforce, or to require performance by the
other party of any provisions of this Agreement shall in no way be construed as a
present or future waiver of such provisions, nor shall it in any way affect the
validity of either party to enforce each and every such provision thereafter.
12.7 Severability. If any provision of this Agreement is held to be void or
unenforceable, such provision shall be replaced by a valid and enforceable
provision that will achieve, as far as possible, the economic and business
intentions of the parties. Those provisions not held to be void or unenforceable
shall remain in full force and effect, to the extent consistent with the parties’
economic and business intentions.
12.8 Counterparts. This Agreement may be executed in two or more
counterparts (including by means of telecopied signature pages), each of which
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
15
shall be deemed an original and all of which together shall constitute
one instrument.
12.9 Entire Agreement. This Agreement sets forth the entire agreement and
understanding of the parties and supersedes all prior written or oral agreements or
understandings with respect to the subject matter hereof, and shall supersede any
conflicting portions of DPT’s quotation and acknowledgment forms
and COMPANY’s
Purchase Order and other written forms. No modification of any of the terms of this
Agreement, or any amendments thereto, shall be deemed to be valid unless in a
writing signed by both parties.
| XXXXXX, INC. | DPT LABORATORIES, LTD. | |||||
By:
|
/s/ Xxxxxx X. Xxxxx | By: | /s/ Xxxx Xxxxxxx | |||
Title:
|
CFO, VP Finance & Operations | Title: | VP, Sales & Commercial Operations | |||
Reviewed by Legal: /s/ Date: 10/24/07 |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
16
EXHIBIT A
Project Scope Document
between
DPT Laboratories, Ltd.
and
Xxxxxx Operations USA, Inc.
for
PEP005 Topical Gel, 0.05%
DPT Laboratories, Ltd.
and
Xxxxxx Operations USA, Inc.
for
PEP005 Topical Gel, 0.05%
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 1 of 7
General Introduction
This Project Scope Document (PSD) is between Xxxxxx Operations USA, Inc. (Client) and DPT
Laboratories, Ltd., 000 X. Xxxxxxxxx Xx., Xxx Xxxxxxx Xxxxx 00000 (DPT) and provides a cost
proposal based on the assumptions contained herein for the services to be performed by DPT for
PEP005 Topical Gel, 0.05%. The effective date of the PSD shall be the latest approval signature
date in the “APPROVALS” section. Information provided to DPT subsequent to the effective date may
change the scope or nature of this project and result in mutually agreed changes to the proposal
provided herein.
By signing this PSD, Client authorizes DPT to begin ordering project materials and performing one
or more of the activities detailed herein. The levels of effort and costs reflected in the PSD are
estimates only and are, therefore, subject to change upon the receipt and review of additional
information. Supplemental documentation to drive the various requirements of the services to be
provided will be prepared by DPT and signed by DPT and Client as required. This supplemental
documentation will contain requisite detail to implement the technical aspects of each particular
stage of the project.
This document will be an attachment to the R&D Agreement.
This PSD shall only be effective if the Client signs the PSD within sixty (60) days of the date
signed by DPT.
Project Introduction
This PSD describes in broad terms with assumptions the tasks involved in the site transfer,
process development, and validation of PEP005 Topical Gel, 0.05%. This IND Phase 3 product is for
used for the treatment of non-melanoma skin cancer and actinic keratosis and will be packaged into
two configurations. The following manufacturing activities are described:
| • | One (1) 8-kg feasibility/placebo batch followed by | ||
| • | One (1) 8-kg process development, clinical supply, registration, and stability study batch followed by | ||
| • | One (1) 8-kg scale-up, process development, and clinical supply placebo batch followed by | ||
| • | Two (2) 8-kg process development, registration, and stability study batches followed by | ||
| • | Three (3) 8-kg process validation batches |
Note: Projection of batch sizes is based on preliminary review of processing requirements. Batch
size adjustments may be required based on scaleable path identified. The third 8-kg registration
batch can be made at 50-kg if material is available for batch manufacture. Estimates provided in
this PSD are based upon the 8-kg batch size.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 2 of 7
Assumptions
The following assumptions, separated into project phases, have been made for this PSD:
Research & Development Phase:
| 1. | The client will provide the active ingredient. | ||
| 2. | DPT will fully test the active ingredient and introduce it into the DPT system. | ||
| 3. | All raw materials will be tested to USP compendial specifications where they exist. | ||
| 4. | The client will provide DPT with a fully developed formula for the product. | ||
| 5. | DPT will perform forced degradation and solubility studies on the client’s formula. | ||
| 6. | DPT will provide package engineering support to arrive at two (2) suitable container configurations for the product. | ||
| 7. | Costs related to the purchase of specialized equipment and/or tooling are not included in the cost proposal for this PSD. | ||
| 8. | The client will supply the Material Safety Data Sheet (MSDS) for the product. | ||
| 9. | DPT will transfer a single stability-indicating product assay method for the active, related substances, and the preservative, benzyl alcohol. | ||
| 10. | DPT will validate the cleaning methods to be used. | ||
| 11. | DPT will validate Microbial Limits Assay and Antimicrobial Effectiveness Testing for the product to meet USP requirements. | ||
| 12. | Except for the feasibility batch which will be completely destroyed after compounding and testing, all remaining batches will be packaged to satisfy all testing requirements and to provide the client with 1,500 X 0.25gm container for clinical supplies for just one (1) of the container types. | ||
| 13. | Testing of bulk product includes description, identification, pH, viscosity, and a single HPLC assay for active and the preservative, benzyl alcohol. | ||
| 14. | Testing of finished-product includes description, identification, pH, viscosity, package integrity, and a single HPLC assay for active, related substances, and the preservative, benzyl alcohol. | ||
| 15. | Testing of and stability samples for each of the two (2) 0.25gm container types includes description, identification, pH, viscosity, package integrity, weight loss, and a single HPLC assay for active, related substances, and the preservative, benzyl alcohol. | ||
| 16. | Microbial Limits Testing will be performed upon release and on stability as per the following schedule: |
;
Ix8-kg Scale-up Placebo + 3x8-kg Registration Batches
;
2-8°C – 1/2/3/6/9/12/18/24/30 months
;
25°C @ 60% RH – 1/2/3/6 months
| 17. | Antimicrobial Effectiveness Testing will be performed upon release and annually through to the end of the product’s shelf-life: |
;
Ix8-kg Scale-up Placebo + 3x8-kg Registration Batches
;
2-8°C – 12/24/30 months
;
25°C @ 60% RH – 6 months only
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 3 of 7
Assumptions (continued)
| 18. | Chemistry stability testing will be performed as follows: |
; 1x8-kg Scale-up Placebo Batch
; 25°C
@ 60% RH – 3/6 months
; Freeze/Thaw
; 3x8-kg Registration Batches
; 2-8°C
– 1/2/3/6/9/12/18/24/30 months
; 25°C
@ 60% RH – 1/2/3/6 months
; Freeze/Thaw
; Photo Stability
| 19. | Samples stored in the environmental xxxxxxxx will be stored in the upright, inverted, and horizontal orientations |
Validations Phase:
| 20. | DPT will provide Regulatory review of the CMC section of the client’s drug product dossier prior to submission to the FDA. | ||
| 21. | Packaging validation will take place on a minimum of three lot codes. | ||
| 22. | Process validation will take place on the first three commercial production batches. | ||
| 23. | For the three process validation batches, the costs associated with the commercial purchase of the units produced from these batches is not included the in the Cost Proposal section. | ||
| 24. | A 30-day bulk hold study will be conducted on product obtained from the first validation batch. | ||
| 25. | Microbial Limits Testing will be performed on the process validation batches upon release only. | ||
| 26. | Antimicrobial effectiveness testing will be performed on the process validation batches upon release and at the end of shelf-life. | ||
| 27. | Chemistry stability testing will be performed on the process validation batches as follows: |
; 2-8°C
– 1/2/3/6/9/12/18/24/30 months
; 25°C
@ 60% RH – 1/2/3/6 months
| 28. | DPT will provide the following reports: |
| • | Pharmaceutical Development Report (CTD-ready Q8 format (Word or pdf) sections C-F (2.3 – 2.6) | ||
| • | Stability reports | ||
| • | Validation reports |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 4 of 7
Cost Proposal
The summary below provides a cost proposal of the overall project scope based on assumptions
in this PSD. During the project, this cost proposal may increase or decrease due to changes in the
assumptions or if the scope of the project changes. Any changes in cost will be detailed in
subsequent protocols and agreed to by both parties.
| Allocation | Subtotals | Cost | ||||||||||
Research & Development |
||||||||||||
Formulations |
$ | **** | ||||||||||
Product Technical Services |
$ | **** | ||||||||||
MPM Planning and Organization |
$ | **** | ||||||||||
Analytical Sciences |
$ | **** | ||||||||||
Total Research & Development |
$ | **** | ||||||||||
Total Packaging Technical Support |
$ | **** | ||||||||||
Manufacturing |
||||||||||||
Weighing |
$ | **** | ||||||||||
Compounding |
$ | **** | ||||||||||
Packaging |
$ | **** | ||||||||||
Product Disposal |
$ | **** | ||||||||||
Filters & Hoses |
$ | **** | ||||||||||
Total Manufacturing |
$ | **** | ||||||||||
QE Plant Technical Services |
||||||||||||
Cleaning Validation |
$ | **** | ||||||||||
Cleaning Detection Limits |
$ | **** | ||||||||||
Total QE Plant Technical Services |
$ | **** | ||||||||||
Chemistry |
||||||||||||
QE Analytical Method Validation |
$ | **** | ||||||||||
QE AMV New Component Testing |
$ | **** | ||||||||||
QC Component Testing |
$ | **** | ||||||||||
Product Testing (Preliminary, Bulk-hold, & Finished Product) |
$ | **** | ||||||||||
Stability Testing |
$ | **** | ||||||||||
Laboratory Supplies |
$ | **** | ||||||||||
Stability Storage Fees |
$ | **** | ||||||||||
Total Chemistry |
$ | **** | ||||||||||
Microbiology |
||||||||||||
Microbial Limits Assay and Testing |
$ | **** | ||||||||||
Antimicrobial Effectiveness Assay and Testing |
$ | **** | ||||||||||
Total Microbiology |
$ | **** | ||||||||||
Total Line Inspections |
$ | **** | ||||||||||
Total Quality Assurance |
$ | **** | ||||||||||
Regulatory (CMC) |
$ | **** | ||||||||||
Regulatory — Post-submission |
$ | **** | ||||||||||
Subtotal (Process Development) |
$ | **** | ||||||||||
Validations |
||||||||||||
Packaging Validation |
$ | **** | ||||||||||
Process Validation |
$ | **** | ||||||||||
Stability in Conjunction with Process Validation |
$ | **** | ||||||||||
Total Validations |
$ | **** | ||||||||||
Total |
$ | **** | ||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 5 of 7
Payment Schedule
Upon Client signature of this PSD, the client shall provide:
Ø Project Deposit of $**** (1/3 of professional fees in the Proposed Project Total)
Ø Materials Deposit of
$**** to acquire materials in conjunction with this project.
The Client will pay the Project Deposit and the Materials Deposit upon
receipt of the invoice.
The Project Deposit will offset invoices sent by DPT for sequential, future milestones from
agreed-upon protocols associated with this project. Once the Project Deposit is consumed, DPT shall
continue to invoice the Client as protocol milestones are met, and the Client shall pay these
invoices within 30 days.
The Materials Deposit will offset the ordering and use of materials in conjunction with this
project. Any remaining Materials Deposit shall be credited back to the Client upon conclusion of
the project. In the event the Materials Deposit is insufficient, DPT may seek additional Materials
Deposit in future, agreed-upon protocol(s).
NOTE: In the event that this project is cancelled or a request is made that DPT cease working on
this project (placed on hold) by the Client, the Client shall be responsible for the standard value
of the work performed and materials sourced by DPT through the date of DPT receiving formal
notification of cancellation or work stoppage. The Client will also be financially responsible for
all materials ordered in anticipation of the completion of this project including all fees
associated with the disposition of materials in the event of a cancellation or request for work
stoppage.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 6 of 7
Approvals
IN WITNESS WHEREOF, the parties hereto approve the Project Scope Document and have
each caused this Project Scope Document to be signed by their duly authorized persons.
| Xxxxxx Operations USA, Inc. | DPT Laboratories, Ltd | |||||||||
By: |
/s/ Xxxx Xxxxxxx | 1/15/07 | ||||||||
| Research & Development | ||||||||||
By: |
/s/ Xxxxxx Xxxxxxxx | 1/18/07 | ||||||||
| Business Development Manager Marketing & Project Management |
||||||||||
Subsequent to execution of this PSD, DPT will forward to Client a Research & Development
Services Agreement and/or a Letter of Responsibility (LOR) for review and execution prior to any
compounding and/or packaging of any product under cGMP guidelines.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 7 of 7
EXHIBIT B
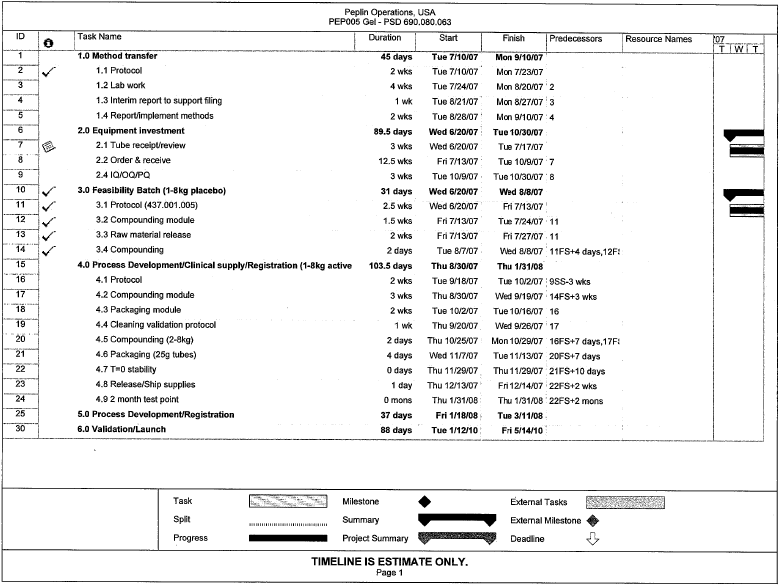
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Xxxxxx Operations, USA PEP005 Gel — PSD 690.080.063 |
| Start Duration Finish I Predecessors Resource Names Task Name |
| 1 I 1.0 Method transfer 45 days Tue 7/10/07 Mon 9/10/07 T |
| 2 1.1 Protocol 2 wks Tue 7/10/07’ Mon 7/23/07 |
| 3 1 2 Lab work 4wks Tue 7/24/07 Mon 8/20/07: 2 |
| interim report to support filing 1 wk Tue 8/21/07’ Mon 8/27/07 3 |
| 5 1.4 Report/implement methods 2 wks Tue 8/28/07’ Mon 9/10/07 4 |
| 6 2.0 Equipment investment 89.5 days Wed 6/20/07 Tue 10/30/07 7 2.1 Tube receipt/review 3 wks Wed 6/20/07 Tue 7/17/07 |
| 8 2.2 Order & receive 12.5 wks Fri 7/13/07: Tue 10/9/07 7 |
| 2 4 Q/OQ/pQ 3 wks Tue 10/9/07 Tue 10/30/07 8 |
| 10 3.0 Feasibility Batch (1-8kg placebo) 31 days Wed 6/20/07 Wed 8/8/07 |
| Ti 3.1 Protocol (437.001.005) 2.5 wks Wed 6/20/07 Fri 7/13/07; |
| 12 j 3.2 Compounding module 1.5 wks Fri 7/13/07 Tue 7/24/07 11 |
| 13 3.3 Raw material release 2 wks Fri 7/13/07 Fri 7/27/07 11 |
| 14 3.4 Compounding 2 days Tue 8/7/07 Wed 8/8/07 11FS+4 days,12F |
| 15 4.0 Process Development/Clinical supply/Registration (1-8kg active 103.5 days Thu 8/30/07 Thu 1/31/08 |
| 16 4.1 Protocol 2 wks Tue 9/18/07 Tue 10/2/07 ; 9SS-3 wks |
| 17 4.2 Compounding module 3 wks Thu 8/30/07 Wed 9/19/07 14FS+3 wks |
| 18 ] 4.3 Packaging module 2 wks Tue 10/2/07 Tue 10/16/07 16 |
| 19 4.4 Cleaning validation protocol 1 wk Thu 9/20/07 Wed 9/26/07 17 |
| 20 4.5 Compounding (2-8kg) 2 days Thu 10/25/07 Mon 10/29/07 16FS+7 days,17FJ |
| 21 4.6 Packaging (25g tubes) 4 days Wed 11/7/07 Tue 11 /13/07 20FS+7 days |
| 22 4.7 T=0 stability Odays Thu 11/29/07, Thu 11/29/07 21FS+10 days |
| 23 4.8 Release/Ship supplies 1day Thu 12/13/07 Fri 12/14/07 22FS+2 wks 24 4.9 2 month test point 0 mons Thu 1/31/08 Thu 1/31/08 : 22FS+2 mons 25 5.0 Process Development/Registration 37 days Fri 1/18/08 i Tue 3/11/08 |
| 6.0 Validation/Launch 88 days Tue 1/12/10; Fri 5/14/10’ '' |
| Task Progress Milestone Summary Project Summary External Tasks External Milestone Deadline TIMELINE IS ESTIMATE ONLY. |
