LUMERA CORPORATION SPONSORED RESEARCH AGREEMENT RESEARCH PLAN
Exhibit 10.7
LUMERA CORPORATION
RESEARCH PLAN
A. Start Date: March 1, 2001
B. University Personnel
| 1. | Principal Investigator: Dr. Xxxxx Xxxxxx |
| 2. | Other University Personnel: |
| a. | Dr. Xxxxx Xxxxxxxx, Group Leader—Theory |
| b. | Dr. Alex K. Y. Jen, Group Leader—Materials |
| c. | Postdoctoral Research Associates (to be named) |
| d. | Administrative Specialist (to be named) |
| e. | Graduate Research Assistants (to be named) |
It is well known that a number of parameters must be optimized if organic electro-optic (EO) materials are to be used to fabricate prototype EO modulator devices: These include large molecular hyperpolarizability for chromophores—the active component of such materials, good xxxxxx efficiency resulting in a high degree of acentric order for chromophores, high thermal stability of materials used for xxxxxx-induced EO activity, good photochemical stability, low optical loss from either absorption or scattering mechanisms, and compatibility with substrates and cladding materials. Over time, some structure/function relationships have been defined to the point of permitting systematic improvement of material performance. For example, chromophore hyperpolarizability values of 10 to 44 esu are now routinely obtained. Moreover, new statistical mechanical methods have resulted in a new paradigm for designing chromophores for improved xxxxxx efficiencies. The frequency response of certain organic EO materials now routinely surpasses lithium niobate devices at telecommunication wavelengths.
Although equilibrium and Monte Carlo statistical mechanical calculations permit a quantitative understanding of chromophore-chromophore intermolecular electrostatic interactions and the role that these interactions play in attenuating xxxxxx efficiency, very little insight currently exists into chromophore/solvent interactions. Slight variations in chromophore structure can lead to significant changes in solubility which can in turn effect the optical quality of spin cast films. For example, out of nearly 100 chromophores of the CLD type, only a handful led to optical- quality films appropriate for device fabrication. Chromophore aggregation and phase separation continues to be a problem at the high concentrations (e.g., 20 to 30 wt %) required for large EO activity. Although it is expected that a number of materials characterized by optical loss values of greater than 2 dB/cm will likely be produced, it will be a challenge to produce materials having the required values of 1 dB/cm or less. Chromophore/solvent and polymer (host)/solvent
Page 1 of 11 Pages
interactions have inhibited the use of fluorination to reduce optical absorption loss at wavelengths of 1.3 microns and 1.55 microns. In general, fluorinated materials do not exhibit good compatibility with traditional spin casting solvents. Optical quality films are obtained only when optimal spin casting conditions are achieved. Those conditions include comparable solubility of chromophores and host material in the spin casting solvent, high chromophore concentrations, appropriate viscosity, and optimal spin speed.
Achieving adequate thermal stability of xxxxxx-induced EO activity typically has required some form of lattice hardening—most commonly thermally-induced crosslinking has been employed to improve the thermal stability of EO activity by 40-70°C. Although lattice hardening has been accomplished, typically it has been accompanied by at least some reduction in EO activity. Recent research has shown that this reduction can vary between an insignificant reduction to 50% or more in xxxxxx-induced EO activity [1]. Moreover, the chemical reactivity of chromophores must be considered in choosing the type of lattice hardening chemistry employed [1].
Lattice hardening must also be accomplished without increasing optical loss due to increased scattering. This means that the lattice hardening process must be extremely homogeneous throughout the material. This typically requires that no significant shrinkage or lattice disruption occur during the hardening process. Side reactions that disrupt the stoichiometry of lattice hardening chemistry typically prove disastrous by promoting phase separation. Such side reactions include those with atmospheric moisture. Again, considerable trial and error is involved in defining a successful xxxxxx/lattice hardening protocol leading to materials appropriate for device fabrication. In general, the trial and error search for optimum conditions must be repeated for each new chromophore system being considered.
During the past year, photochemical stability has been a significant concern. A number of studies have shown that photochemical stability strongly depends on the presence of oxygen, depends on the offset of the operational wavelength from the interband charge transfer transition of the EO chromophores, can be reduced by the introduction of reactive species scavengers, depends on lattice hardness (diffusion of reactive species), and depends on chromophore structure. Studies of materials with simple packaging suggest that photochemical stability may be achieved by additional attention to material processing and packaging. However, we have noted that molecular oxygen forms a very strong Van der Waals complex with most high µ EO chromophores; thus, it is very difficult to pump off residual oxygen. Although many routes likely exist for improvement of photochemical stability to levels required for long-term operation of devices, it is not clear what the optimum route is at this point in time. This topic will clearly require more exploratory research, both theoretical and experimental.
For EO materials to be practical, they must be capable of being prepared in large quantities at low cost. Syntheses must be high yield and virtually free of side reactions that would require extensive and time-consuming purification by chromatographic methods. Thus, although bridge structures based on fused ring thiophenes have shown exceptional optical nonlinearity and thermal stability, these structures have only recently been synthesized in quantities appropriate for device development.
The integration of active (core waveguide) EO materials with compatible cladding materials is another issue of growing concern. Cladding materials must in general possess many of the same
Page 2 of 11 Pages
characteristics as the active EO materials. In general, cladding materials must not contribute to optical loss, must not exhibit photoconductivity, must exhibit appropriate solubility and processability, and must otherwise be fully compatible with the active EO material. In addition, it is desirable for cladding materials to exhibit higher conductivity than the active core so that xxxxxx and drive voltages are dropped across the cladding layer enhancing the effective electric field experience by the active chromophores.
To date, off-the-shelf materials (UV or thermally-curable epoxies) have largely been used for cladding purposes. Some work on conductive cladding has been carried out by Xx. Xxxxx Xxxxx at AFRL and by Xx. Xxxxx Xxxxx and coworkers at Lockheed Xxxxxx Corporation; however, no generally acceptable cladding material exists at this time. Indeed, it is likely that increased attention will have to be given to the issue of cladding materials, and custom claddings (designed to match active core EO materials) will ultimately have to be identified or developed.
The foregoing discussion relates only to basic materials issues. There still remain substantial engineering issues to be addressed including the frequency limitations imposed by electrical connectors and the problem of optical loss associated with mode mismatch in coupling to silica fibers. Positioning passive and active waveguides continues to be an issue.
Thus, although dramatic advances in organic (polymer [12,17,18] and dendrimer [3] EO materials have been demonstrated in the past year (suggesting the superiority of these types of EO materials in terms of bandwidth and drive voltage), a substantial effort will be required to realize the potential of these types of materials in the near future. Theory can provide useful guidance but more sophisticated calculations (e.g., atomistic kinetic Monte Carlo calculations run on supercomputer clusters) will be required to understand macromolecular conformations and interdigitation. Chromophore/solvent interactions are not likely to be effectively modeled even by these more sophisticated calculations so considerable trial and error will have to be anticipated in producing EO materials amenable to the spin casting of optical quality films having the required optical loss values of ldB/cm or less.
It is now well appreciated that one approach to reduction of optical loss due to vibrational overtone absorption can be achieved by reducing the hydrogen content of EO materials; however, such modification usually affects solubility in traditional spin casting solvents and generally results in higher optical loss. Trial and error experimentation will likely be required to produce practical organic EO materials with optical loss values approaching that of lithium niobate EO devices. Thus, although waveguide optical loss values of 0.2 dB/cm have been realized for EO dendrimers, these results can be considered to be little more than proof of concept at this point in time. Thermal and photochemical stability will likely become issues of focus in the near future. Again, the issue currently appears to be not whether such materials can be obtained as isolated properties but whether they can be optimally realized in conjunction with all other desired properties in a single low cost material that can be produced in high volume.
It is important to realize that a great many properties must be simultaneously optimized in order to produce commercially useful EO materials. Given the limitations in available research resources and time and the inherent uncertainties in the outcome of this type of research, risks must be taken to achieve a significant advance in the state-of-the-art of organic EO materials. Inevitably, some materials that are produced or approaches that are takenwill be ultimately found to be technically or commercially unacceptable for one reason or another.
Page 3 of 11 Pages
| 1. | Project Summary |
The Project will focus on the development of new and/or improved organic EO materials supporting the Company’s commercialization of organic EO device technology within the “Field of Use” as defined under the Company’s Exclusive License Agreement effectively dated October 20, 2000 with the University. Specifically, the Project Work will consist of research directed in support of the Company’s development of a commercial Electro-Optic Modulator (as also defined in said Exclusive License Agreement); principally it will involve development of EO chromophore compounds for use in such a device. Project Work will consist primarily of efforts to (i) synthesize compounds and (ii) perform certain theoretical analysis in connection therewith. Theoretical analysis efforts will include developing condensed matter theoretical methods that permit guidance in the design of optimized materials and the exploitation of nanoscale engineering methods (including the utilization of custom-designed dendrimer materials). Compound synthesis efforts will include synthesis of new and/or improved EO chromophores, of host materials (including dendrimer macromolecular materials), and of compatible cladding materials. The Principal Investigator believes that the exploitation of dendrimer synthetic methods has a reasonable likelihood of resulting in improvement of EO material activity, transparency, processability, and stability.
Because a great diversity of device structures may be of interest and because each device structure may require modification of materials to fulfill specific requirements, a great deal of flexibility is required in the Project. It is the University’s intention that such flexibility be brought to the Project and that there be a recognition of the need for modifications during the course of the Project, after appropriate consultation with the Company.
| 2. | Research Strategy |
The University, under the direction of the Principal Investigator, intends to carry out a systematic research program to exploit and improve upon the theoretical guidance for preparing optimized nanostructured EO materials and to systematically explore the use of nanoscale dendrimers to achieve improvement in the fabrication and utilization of EO materials. Particular attention will be focused on control of aggregation and molecular relaxation events using nano-engineering methods and techniques. Specific attention will be given to phase phenomena and nanoscale phase separation defined by molecular shape-dependent intermolecular electrostatic interactions.
| 3. | Theoretical Analysis |
The primary focus of the theoretical effort will be upon Monte Carlo (MC) calculations aimed at gaining a detailed understanding of the role of chromophore (and dendrimer) shape upon ordering under the influence of an electric xxxxxx field. Simulations will be run using a to-be- acquired high-speed computer. Calculations will be carried out as functions of chromophore number density, chromophore shape and electronic structure (dipole moment, polarizability, etc.) of the chromophores.
The Principal Investigator believes that a particular advantage of MC methods is the opportunity for visualization of transient ordered states. One result of MC calculations appears to be the confirmation of the fundamental correctness of the Xxxxxxx picture of ordering due to long-range, spatially-anisotropic dipole-dipole interactions. MC methods appear to be appropriate for all
Page 4 of 11 Pages
concentration domains (from dilute solutions of chromophores to neat chromophore materials) and the pictures of transiently ordered phases may be useful in understanding light scattering as a function of chromophore concentration and also for understanding the results of pulsed xxxxxx experiments. Such subtle physical insight may prove a useful guide for the further modification of chromophore shape and electronic properties and for optimizing the protocols for inducing acentric chromophore order.
A secondary, but nevertheless important, theoretical objective will be to develop improved analytical expressions and computational algorithms that can be executed on personal computers. Such theoretical results have in the recent past been of value to industrial researchers under certain circumstances. Analytical results appear to have the potential of sufficiently approaching the results of sophisticated numerical calculations to be used to determine chromophore concentrations leading to optimum EO activity.
The Principal Investigator also believes that these analytical results may be extended to include repulsive as well as electrostatic interactions. The shape-dependent repulsive molecular interactions appear to be very important in defining macroscopic order, and control of these interactions by adjustment of molecular shape is an important paradigm for the optimization of EO activity. The Principal Investigator has experimentally and theoretically shown that spherical and oblate ellipsoidal (discotic) chromophores lead to optimum EO activity. Theory thus can show how the ordering (over nano and mesoscale dimensions) of nanoscale objects (chromophores and chromophore-containing dendrimers) is defined by the shape of the nanoscale objects as well as by electronic electrostatic interactions.
| 4. | Compound Synthesis |
In overview, it is intended that the Project start with the synthesis of new chromophores exploring new donor, bridge, and acceptor segments. The types of chromophores to be explored are numerous and the selection will depend upon the judgment of University Personnel and the success of the Project Work. Not only will structural possibilities be explored but also preliminary results will be used as a guide to which structural modifications will be explored in detail and which will be abandoned. Because of different application requirements, it is anticipated that several, rather than only a single class of chromophores will be developed, depending upon the success of the Project Work.
Certain types of nanoscale dendrimers (multi-chromophore dendrimers, dendrimers containing cyanurate and fluorinated dendrons, dendrimers based on the trifluorovinyl-ether moieties for crosslinking) may represent a means for control of intermolecular electrostatic interactions by systematic modification of chromophore shape. They currently appear to be a way of implementing the guidance suggested by theoretical calculations. Such structures may also provide a possible route to control of proton density (and hence optical absorption due to C-H vibrational overtones).
Cyanurate and fluorinated dendrons may also be used to control and systematically vary the index of refraction of an EO material. Such variation may be useful for matching to silica transmission fibers. Moreover, dendrimers appear to permit control over processability (e.g., solubility in spin casting solvents, molecular weight and hence solution viscosity, etc.). Certain chromophore-containing dendrimers can be spin cast without the use of a polymer host.
Page 5 of 11 Pages
Crosslinking functionalities may be able to be placed on the surface of dendrimers and preliminary results of work already performed suggest that significantly improved stability of xxxxxx-induced EO activity is obtained with such materials.
Preliminary results from earlier work suggest that EO materials based on chromophore-containing dendrimers can exceed the performance characteristics of lithium niobate materials in certain respects. Moreover, University personnel are only beginning to explore the range of performance characteristics that can be obtained with EO dendrimers and believe that there is potential for considerable future improvements.
As is evident from a consideration of existing compounds, dendrimer structures must be designed around specific EO chromophore structures. Project Work will include attempts to synthesize a number of new chromophores; paying particular attention to functionalizing such chromophores so that intermolecular interactions are inhibited (e.g., by incorporation into dendrimer structures).
Asymmetric and chiral dendrimers may also be prepared which may aid in the promotion of acentric macroscopic order. Asymmetric crosslinking may also help to selectively stabilize overall acentric macroscopic order. Dendrimers potentially afford a means of minimizing problems arising from segmental flexibility of conventional chromophore-containing polymer systems. It is intended that this Project explore the use of a variety of dendron ligands to control dendrimer conformation and conformational flexibility.
The general prescription will be to prepare a chromophore-containing or multiple chromophore-containing dendrimer material. Both convergent and divergent dendrimer synthesis schemes will be explored. Electric field induced second harmonic (EFISH) generation and Hyper-Rayleigh scattering (HRS) measurements may be used to characterize certain properties. EFISH and HRS measurement capabilities are not currently operational at the University; an effort will be made to achieve operational capability. Thermal stability will be assessed by TGA and DSC methods. Other standard characterization such as NMR and mass spectroscopy may be carried out as required. Thin film samples will be prepared by spin casting with dendrimer structure modified to control processability (solubility and viscosity). Acentric chromophore order will be induced by electric field xxxxxx typically using stepped protocols where temperature and electric field are increased at fixed periods in time. Second harmonic generation may be used for in-situ monitoring of the induction of acentric order. EO coefficients may be measured by various standard methods and in prototype devices. However, no fixed set of measurements are guaranteed. Indeed, it is anticipated that measurements will be adapted as research proceeds and benefits from lessons learned.
Recently, the Principal Investigator has had considerable success developing new lattice hardening chemical processes. These in general appear to be more compatible with electric field xxxxxx requirements and to have less sensitivity to side reactions. An increased attention to lattice hardening will one of the themes of the Project Work. Such lattice hardening will be designed not only to improve thermal stability but also to improve photochemical stability by inhibiting the diffusion of chemically reactive species such as singlet oxygen.
Efforts to develop improved cladding, as well as EO materials, will be undertaken. The Principal Investigator has already published on conducting cladding materials; however, a number of
Page 6 of 11 Pages
concerns exist for such materials including increased optical loss (it has to date been difficult to design cladding materials that simultaneously exhibit greater electrical conductivity than the EO core material and low optical loss). Recently, a second problem has been observed with cladding materials; namely, photochemical charge injection from the EO core material. Such charge injection and the corresponding variation of effective voltage seen by the EO material gives rise to an optical power dependent operational halfwave voltage. Other issues can also arise with cladding materials including photochemical damage. At present, custom development of cladding materials has been relatively unknown. It is also intended that the Project attempt to synthesize cladding materials tailored to particularly EO materials and applications.
D. Project Work and Estimated Work Schedule
This program is currently scheduled to occur over a period of 36 months. At this time, it is planned that Project Work will involve the following research areas (shown are estimated time periods together with material development/synthesis and other results that the University will attempt to achieve):
Research Area 1
| • | Synthesize highly fluorinated PFCB monomers—see attached Exhibit 1. (3/01-8/01, gram quantity) |
| • | Practice with curing procedure for regular PFCB and establish database. (3/01-8/01) |
| • | Synthesize highly efficient NLO chromophores with suitable functional groups for the incorporation into PFCB—see attached Exhibit 1. (3/01-12/01, gram quantity) |
| • | Carry out statistical mechanical calculations in support of synthetic efforts. Initially, these will be executed on standard serial processor machines (e.g., IBM compatible PCs). An attempt will be made to implement Beowulf cluster type machines for more sophisticated computation and modeling. (3/01-12/01) |
Research Area 2
| • | Develop advanced PFCB passive materials. Establish structure/property relationships via monomer selection, for multi-layer device fabrication. (6/01-10/01) |
| • | Evaluate refractive index, optical loss, dielectric breakdown strengths, mechanical properties of PFCB cured at different conditions. (6/01-10/01) |
| • | Detailed evaluation of curing/xxxxxx conditions of PFCB-NLO chromophore candidates (Jen’s group, Xxxxxx’x group, and Lumera) by r33, relaxation, optical loss measurements and testing their mechanical and thermal properties. (8/01- 2/01) |
Page 7 of 11 Pages
Research Area 3
| • | Active PFCB process optimization by Lumera and UW. (12/01-3/02) |
| • | Process evaluation of best candidate by Lumera. (12/01-3/02). |
| • | Explore new material systems based on the triazole functionality as potential low loss materials. (12/01-6/02) |
| • | Explore crosslinkable NLO dendrimers. (7/02-7/03) |
| • | Explore new photo-crosslinking mechanisms for both passive and active materials. (1/02-7/02) |
Research Area 4
| • | Advanced chromophore development. (1/02-1/03) |
| • | Incorporate these chromophores into highly fluorinated polymer or dendrimer material systems. (12/02-12/03) |
| • | Exploration of mechanisms to “fine-tune” refractive index of single-mode channel waveguide devices by Lumera and UW. (9/02-9/03) |
| • | Develop rare-earth radio-frequency amplifier system in the waveguides. (12/02-9/03) |
| • | An effort will be made to develop ever more sophisticated modeling software including software based on Monte Carlo simulation techniques. (1/02-2/04) |
Research Area 5
| • | Assistance to Lumera in scale-up and optimization. (12/02-12/03). |
| • | Final report and wrap-up. (1/04-2/04) |
The EO material system that the University will attempt to develop in this program will include active and passive materials that have matching refractive indices for fabricating channel waveguides and balanced conductivities for efficient electric field xxxxxx. The University will also attempt to develop a general approach in this program that can serve as a prototype for future development of superior EO material systems.
Page 8 of 11 Pages
| E. | Sponsored Research Agreement Budget (subject to revisions and adjustment by University based on actual expenditures and actual research activities) |
Year 1 (3/1/01 to 2/28/02)
| Personnel |
|||
| Senior Personnel |
|||
| Xxxxx X. Xxxxxx, Principal Investigator, 12 mo appt, 75%* |
$ | — | |
| Xxxxx X. Xxxxxxxx, co-PI, 9 mo appt, 3 mo 100% |
21,060 | ||
| Xxxxx X. Xxxxxxxx, co-PI, summer, 1 mo 100% |
7,300 | ||
| Xxxx X.-Y. Xxx, co-PI, summer, 1 mo 100% |
12,931 | ||
| Other Personnel |
|||
| Postdoctoral Research Associates, tbn, 6 @ 2,189/mo, 12 mo 100% |
157,608 | ||
| Administrative Specialist, tbn, 1 @ 3,500/mo, 12 mo 100% |
42,000 | ||
| Graduate Student Research Associates, tbn, 7 @ 3,170/mo, 12 mo 50% |
133,140 | ||
| Total Salary and Wages |
374,039 | ||
| Total Fringe Benefits (21.80% Senior and PRA; 24.20% AS; 10.20% GRA) |
67,104 | ||
| Total Salary and Wages and Benefits |
441,143 | ||
| Operating Fee Portion of Graduate Assistant Tuition |
53,445 | ||
| Permanent Equipment |
300,000 | ||
| Travel |
50,000 | ||
| Alterations and Renovations |
1,000,000 | ||
| Materials and Supplies |
592,117 | ||
| Subtotal Direct Costs |
2,436,705 | ||
| Indirect Costs (52% of MTDC of 1,083,260)** |
563,295 | ||
| Total Direct and Indirect Costs |
$ | 3,000,000 | |
Year 2 (3/1/02 to 2/28/03)
| Personnel |
|||
| Senior Personnel |
|||
| Xxxxx X. Xxxxxx, Principal Investigator, 12 mo appt, 75%* |
$ | — | |
| Xxxxx X. Xxxxxxxx, co-PI, 9 mo appt, 3 mo. 100% |
21,902 | ||
| Xxxxx X. Xxxxxxxx, co-PI, summer, 1 mo 100% |
7,592 | ||
| Xxxx X.-Y. Xxx, co-PI, summer, 1 mo 100% |
13,448 | ||
| Other Personnel |
|||
| Postdoctoral Research Associates, tbn, 12 @ 2,277/mo, 12 mo. 100% |
327,825 | ||
| Administrative Specialist, tbn, 1 @ 3,640/mo., 12 mo 100% |
43,680 | ||
| Graduate Student Research Associates, tbn, 12 @ 3,281/mo., 12 mo. 50% |
236,228 | ||
| Total Salary and Wages |
650,675 | ||
| Total Fringe Benefits (21.80% Senior and PRA; 24.20% AS; 10.20% GRA) |
115,494 | ||
| Total Salary and Wages and Benefits |
766,169 | ||
| Operating Fee Portion of Graduate Assistant Tuition |
95,304 | ||
| Other Misc. |
50,000 | ||
| Travel |
50,000 | ||
| Materials and Supplies |
1,044,815 | ||
| Subtotal Direct Costs |
2,006,288 | ||
| Indirect Costs (52% of MTDC of 1,910,984)** |
993,712 | ||
| Total Direct and Indirect Costs |
$ | 3,000,000 | |
Page 9 of 11 Pages
Year 3 (3/1/03 to 2/28/04)
| Personnel |
|||
| Senior Personnel |
|||
| Xxxxx X. Xxxxxx, Principal Investigator, 12mo appt, 75%* |
$ | — | |
| Xxxxx X. Xxxxxxxx, co-PI, 9 mo appt, 3 mo 100% |
22,778 | ||
| Xxxxx X. Xxxxxxxx, co-PI, summer, 1 mo 100% |
7,896 | ||
| Xxxx X.-Y. Xxx, co-PI, summer, 1 mo 100% |
13,986 | ||
| Other Personnel |
|||
| Postdoctoral Research Associates, tbn, 12 @ 2,368/mo, 12 mo 100% |
340,937 | ||
| Administrative Specialist, tbn, 1@ 3,786/mo, 12 mo 100% |
45,427 | ||
| Graduate Student Research Associates, tbn, 12 @ 3,396/mo, 12 mo 50% |
244,496 | ||
| Total Salary and Wages |
675,520 | ||
| Total Fringe Benefits (21.80% Senior and PRA; 24.20% AS; 10.20% GRA) |
119,992 | ||
| Total Salary and Wages and Benefits |
795,512 | ||
| Operating Fee Portion of Graduate Assistant Tuition |
99,132 | ||
| Other Misc. |
30,000 | ||
| Travel |
50,000 | ||
| Materials and Supplies |
1,032,954 | ||
| Subtotal Direct Costs |
2,007,598 | ||
| Indirect Costs (52% of MTDC of 1,908,466)** |
992,402 | ||
| Total Direct and Indirect Costs |
$ | 3,000,000 | |
3-Year Summary (3/1/01 to 2/28/04)
| Personnel |
|||
| Senior Personnel |
|||
| Xxxxx X Xxxxxx, Principal Investigator, 12 mo appt, 75%* |
$ | — | |
| Xxxxx X. Xxxxxxxx,co-PI, 9-mo |
65,740 | ||
| Xxxxx X. Xxxxxxxx, co-PI, summer |
22,788 | ||
| Xxxx X.-Y. Xxx, co-PI, summer |
40,365 | ||
| Other Personnel |
|||
| Postdoctoral Research Associates, tbn |
826,370 | ||
| Administrative Specialist, tbn, 12 mo |
131,107 | ||
| Graduate Student Research Associates, tbn |
613,864 | ||
| Total Salary and Wages |
1,571,341 | ||
| Total Fringe Benefits (21.80% Senior and PRA; 24.20% AS; 10.20% GRA) |
302,590 | ||
| Total Salary and Wages and Benefits |
2,002,824 | ||
| Operating Fee Portion of Graduate Assistant Tuition |
247,881 | ||
| Other Misc. |
80,000 | ||
| Permanent Equipment |
300,000 | ||
| Travel |
150,000 | ||
| Alterations and Renovations |
1,000,000 | ||
| Materials and Supplies |
2,669,886 | ||
| Subtotal Direct Costs |
6,450,591 | ||
| Indirect Costs (52% of MTDC of 4,902,710)** |
2,549,409 | ||
| Total Direct and Indirect Costs |
$ | 9,000,000 | |
*Based on and assuming financial support by Company to University Chemistry Department of $300,000 per year independent of Sponsored Research Agreement.
**Estimate only. Indirect costs will be determined in accordance with University policies and practices in existence at the time of the expenditure and applicable to privately sponsored on-campus research agreements. Indirect costs will be calculated as a percentage of modified total direct costs (MTDC), as defined by such policies and practices. Until June 30, 2002, the indirect cost rate will be 52% and is subject to change thereafter.
Page 10 of 11 Pages
| F. | Extension Agreement. |
University and Company agree that this Research Plan is entered into pursuant to Section 2.1 of the Sponsored Research Agreement effectively dated October 20, 2000 between University and Company. University and Company further agree that the period of time for entering into the Research Plan as described in said Section 2.1 is hereby extended to the last date upon which either the University or the Company executes this Research Plan. Unless the context clearly requires otherwise, all capitalized terms as used in this Research Plan shall have the same meanings as used in said Sponsored Research Agreement.
Agreed to:
| The University of Washington |
Lume ACorporation | |||||||
| By: |
/s/ Xxxxx Xxxxxxx |
By: |
/s/ Xxxx X. XxXxxxxx | |||||
| Name: |
Xxxxx Xxxxxxx, Director | Name: |
Xxxx X. XxXxxxxx | |||||
| Title: |
Grant and Contact Services | Title: |
Vice President | |||||
| Date: |
February 26, 2001 |
Date: |
February 26, 2001 | |||||
REVIEWED:
| By: | /s/ Xxxxx X. Xxxxxx | |
| Name: | Dr. Xxxxx Xxxxxx | |
| Title: | Principal Investigator | |
| Date: | February 26, 2001 | |
Page 11 of 11 Pages
EXHIBIT 1
PFCB-Perfluorocyclobutanes
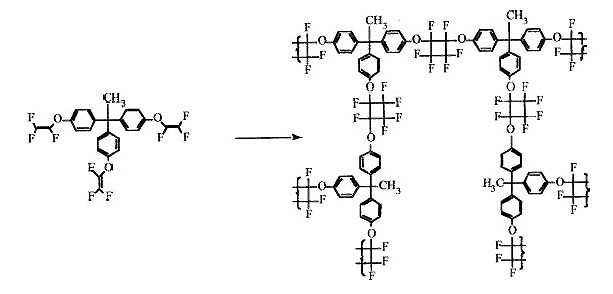
Page 1 of 2 Pages
EXHIBIT 1 (Continued)
Improved Highly Fluorinated NLO Dendrimer
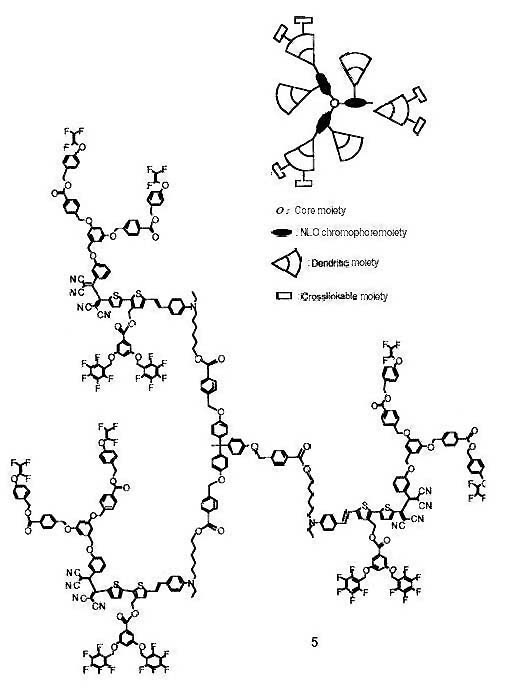
Page 2 of 2 Pages
EXHIBIT 2
Recent Xxxxxx Group Publications*
| 2. | X. Xxxxx, X. Xxxx, X. Xxxx, X. X. Xxxxxx, G. Sun, X. Xxxxx, and X. X. Xxxxxx, “Electric-Xxxxxx and Relaxation of Thermoset Polyurethane Second-Order Nonlinear Optical Materials: The Role of Cross-Linking and Monomer Rigidity,” Macromolecules, 34, 235-43 (2001). |
| 3. | H. Ma, X. Xxxx, S.Takafumi, X. X. Xxxxxx, and A. K. Y. Jen, “Highly Efficient and Thermally Stable Nonlinear Optical Dendrimer for Electro-Optics,” J. Am. Chem. Soc,123, in press (2001); available on-line as an ACS ASAP article. |
| 4. | Chen, V. Chuyanov, F. I. Xxxxx-Xxxxxxx. X. X. Xxxxxx, X. X. Xxxxxx, X. Xxxx, S. S. Sun, and X. X. Xxxxxx, ‘Vertically Tapered Polymer Waveguide Mode Size Transformer for Improved Fiber Coupling,” Opt. Eng., 39, 1507-16 (2000). |
| 5. | L. Sun, J.-X. Xxx, C.-X. Xxxx, X. X. Xxxx, D. An, X. Xxxx, X. Lu, X. X. Xxxxxxx, X. X. Xxxx, X. Xxxx, X. Xxxxx, X. X. Xxxxxx, A. S. Ren, and X. X. Xxxxxx, “Beam Deflection With Electronic-Optic Polymer Waveguide Prism Array,” Proc. SPIE, 3950, 98- 107 (2000). |
| 6. | X. X. Xxxxx, X. X. Xxxxx, X. X. Xxxxxxxx, X. X. Xxxxxx, X. X. Xxxxxxx, X. X. Xxxxx, X. X. Xxxxxx, and X. X. Xxxxxx, “Effect of Dielectric Constant on Modulation Voltage for Nonlinear Optic Polymer-Based Optoelectronic Devices,” Proc. SPIE, 3950, 108-116 (2000). |
| 7. | X. Xxxx and X. X. Xxxxxx, “A Facile Synthesis of Thienylmethylphosphonates: Direct Conversion From Thiophenes,” Tetrahedron Lett., 41, 617-20 (2000). |
| 8. | Liakatas, X. Xxx, X. Xxxxx, X. Xxxxx, Ch. Xxxxxxxx, X. Xxxxxx, X. Xxxxx, and X. X. Xxxxxx, “Importance of Intermolecular Interactions on the Nonlinear Optical Properties of Poled Polymers,” Appl. Phys. Lett., 76, 1368-70 (2000). |
| 9. | D. An, Z. Shi, L. Sun, X. X. Xxxxxxx, X. Xxxx, X. Xx, X. X. Xxxx, X. Xxxx, X. Xxxxx, X. X. Xxxxxx, A. Ren, and X. X. Xxxxxx, “Polymeric Electro-Optic Modulator Based on 1x2 Y-Fed Directional Coupler,” Appl. Phys. Lett., 76, 1972-4 (2000). |
| 10. | X. Xxxxx, X. X. Xxxxxx, D. S. Spells, and X. X. Xxxxxx, “A Facile Synthesis of 5-N,N-Bis(2- Hydroxyethyl) amino-2-Thiophenecarboxaldehyde,” Synth. Commun., 30( 8), 13 59-64 (2000). |
| 11. | X. Xxxx, X. Xxxxx, X. Xxxx, X. Xxx, X. Xx, and X. X. Xxxxxx, “High Tg Donor-Embedded Polyimides for Second-Order Nonlinear Optical Applications,” Polymer, 41, 2583-90 (2000). |
| 12. | Y. Shi, X. Xxxxx, X. Xxxxx, X. X. Xxxxxxx, X. X. Xxxxxx, X. X. Xxxxxxxx, and X. X. Xxxxxx, “Low (Sub-1 Volt) Halfwave Voltage Polymeric Electrooptic Modulators Achieved by Control of Chromophore Shape,” Science, 288, 119-122 (2000). |
| 13. | X. X. Xxx, S. M. Gamer, V. Chuyanov, X. Xxxxx, X. X. Xxxxxx, X. Xxxx, X. X. Xxxxxx, X.X. Xxxxx, and H, X. Xxxxxxxxx, “Optical Intensity Modulator Based on a Novel Electrooptic Polymer Incorporating a High µß Chromophore,” IEEE Journal of Quantum Electronics, 36, 527-32 (2000). |
Page 1 of 2 Pages
EXHIBIT 2 (Continued)
Recent Xxxxxx Group Publications*
| 14. | X. X. Xxxxxxxx and X. X. Xxxxxx, “Xxxxx Carlo Statistical Mechanical Simulations of the Competition of Intermolecular Electrostatic and Xxxxxx Field Interactions in Defining Macroscopic Electro-Optic Activity for Organic Chromophore/Polymer Materials,” J. Phys. Chem., 104, 4785-4795 (2000). |
| 15. | X. X. Xxxxx, X. Xxxxx, M. C. Oh, X. Xxxxx, X. X. Xxxxxx, X. X. Xxxxxx, and X. X. Xxxxxxxxx, “Time Stretching of 102 GHz Millimeter Waves Using a Novel 1.55 µm Polymer Electrooptic Modulator, IEEE Photonics Technology Letters, 12, 537-9 (2000). |
| 16. | D. An, X. Xxxx, Z. Shi, L. Sun, X. X. Xxxxxxx, X. Xxxx, X. Xx, X. X. Xxxx, X. Xxxxx, X. X. Xxxxxx, A. Ren, and X. X. Xxxxxx, “1x2 Y-Fed Directional Coupler Modulator Based on Electro-Optic Polymer,” Proc. SPIE, 3950, 90-7 (2000). |
| 17. | Y. Shi, X. Xxx, X. X. Xxxxx, X. X. Xxxxxxx, X. Xxxxx, X. X. Xxxxxx, X. Xxxxx, and X. X. Xxxxxx, “Electro-Optic Polymer Modulators with 0.8 V Half- Wave Voltage,” Appl. Phys. Lett., 77, 1-3 (2000). |
| 18. | M.-C. Oh, X. Xxxxx, X. Xxxx, V. Chuyanov, X. X. Xxxxxx, X. Xxxxx, X. X. Xxxxxx, X. Xxxxx, X. Xxxx, and X. X. Xxxxxxxxx, “Practical Electro-Optic Polymer Modulators for 1.55 µm Wavelength Using Phenyltetraene Bridged Chromophores in Polycarbonate,” Appl. Phys. Lett., 76, 3525-7 (2000). |
| 20. | K. Y. Jen, H. Ma, X. Xx, X. Xx, and X. X. Xxxxxx, “High Performance Side-Chain Aromatic Polyquinones for Electro-Optic (E-O) Devices,” Materials Research Society Symposium Proceedings, Vol. 598, Electrical, Optical and Magnetic Properties of Organic Solid State Materials (Materials Research Society, Pittsburgh, 2000) pp.BB4.4.1-6. |
| 21. | X. Xxxxx, X. X. Xxxxxxxx, X. X. Xxxxx, X. X. Xxxxxx, X. X. Xxxxxxx, X. Xxxxx, X. X. Xxxxxx, and X. X. Xxxxxx, “Enhanced Electrooptic Activity of NLO Polymers Via the Use of Conductive Polymers,” Materials Research Society Symposium Proceedings, Vol. 597, Thin Films for Optical Waveguide Devices (Materials Research Society, Pittsburgh, 2000) pp. 109-115. |
| 22. | Yacoubian, V. Chuyanov, X. X. Xxxxxx, X. X. Xxxxxx, A. S. Ren, and X. X. Xxxxxx, “EO Polymer-Based Integrated-Optical Acoustic Spectrum Analyzer,” IEEE J. Sel. Topics in Quantum Electronics, 6, 810-6 (2000). |
| *Note: | Provided as background information only. These references shall not be deemed to be covenants, representations or warranties by University or otherwise be made part of this Research Plan or the Sponsored Research Agreement. (Only references 1, 3, 12, 17 and 18 are referred to in text.) |
Page 2 of 2 Pages
