FINANCING AGREEMENT Dated as of April 29, 2010 between NEUROGESX, INC. and COWEN HEALTHCARE ROYALTY PARTNERS, L.P.
Exhibit 10.2
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXECUTION COPY
Dated as of April 29, 2010
between
NEUROGESX, INC.
and
XXXXX HEALTHCARE ROYALTY PARTNERS, L.P.
TABLE OF CONTENTS
| Page | ||||||
| ARTICLE I DEFINITIONS |
1 | |||||
| Section 1.01 |
Definitions | 1 | ||||
| ARTICLE II ASSIGNMENT |
11 | |||||
| Section 2.01 |
Assignments | 11 | ||||
| Section 2.02 |
Payments to CHRP | 12 | ||||
| Section 2.03 |
Advances to NeurogesX | 13 | ||||
| Section 2.04 |
No Assumed Obligations | 13 | ||||
| Section 2.05 |
Excluded Assets | 14 | ||||
| ARTICLE III REPRESENTATIONS AND WARRANTIES OF THE ASSIGNOR |
14 | |||||
| Section 3.01 |
Organization | 14 | ||||
| Section 3.02 |
Corporate Authorization | 14 | ||||
| Section 3.03 |
Governmental Authorization | 14 | ||||
| Section 3.04 |
Ownership | 15 | ||||
| Section 3.05 |
Financial Statements | 15 | ||||
| Section 3.06 |
No Undisclosed Liabilities | 16 | ||||
| Section 3.07 |
Solvency | 16 | ||||
| Section 3.08 |
Litigation | 16 | ||||
| Section 3.09 |
Compliance with Laws | 16 | ||||
| Section 3.10 |
Conflicts | 16 | ||||
| Section 3.11 |
Subordination | 17 | ||||
| Section 3.12 |
Intellectual Property | 17 | ||||
| Section 3.13 |
Regulatory Approval | 20 | ||||
| Section 3.14 |
Material Contracts | 20 | ||||
| Section 3.15 |
Place of Business | 22 | ||||
| Section 3.16 |
Broker’s Fees | 22 | ||||
| Section 3.17 |
Information | 22 | ||||
| Section 3.18 |
Security Agreement | 22 | ||||
| Section 3.19 |
Bankruptcy Event | 23 | ||||
| Section 3.20 |
Material Adverse Effect | 23 | ||||
- i -
| Section 3.21 |
Field of Use | 23 | ||||
| Section 3.22 |
No Other Representations | 23 | ||||
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF CHRP |
23 | |||||
| Section 4.01 |
Organization | 23 | ||||
| Section 4.02 |
Authorization | 23 | ||||
| Section 4.03 |
Broker’s Fees | 24 | ||||
| Section 4.04 |
Conflicts | 24 | ||||
| Section 4.05 |
Financial Capacity | 24 | ||||
| ARTICLE V COVENANTS |
24 | |||||
| Section 5.01 |
Consents and Waivers | 24 | ||||
| Section 5.02 |
Access; Information | 24 | ||||
| Section 5.03 |
Material Contracts | 25 | ||||
| Section 5.04 |
Confidentiality; Public Announcement | 27 | ||||
| Section 5.05 |
Security Agreement | 28 | ||||
| Section 5.06 |
Further Assurance | 28 | ||||
| Section 5.07 |
Remittance to Initial Concentration Account | 29 | ||||
| Section 5.08 |
Intellectual Property | 31 | ||||
| Section 5.09 |
Negative Covenants | 32 | ||||
| Section 5.10 |
Competing Products | 32 | ||||
| Section 5.11 |
Notice | 33 | ||||
| ARTICLE VI THE CLOSING; CONDITIONS TO CLOSING |
33 | |||||
| Section 6.01 |
Closing | 33 | ||||
| Section 6.02 |
Conditions Applicable to CHRP to Effect the Closing | 33 | ||||
| Section 6.03 |
Conditions Applicable to NeurogesX | 35 | ||||
| ARTICLE VII TERMINATION |
35 | |||||
| Section 7.01 |
Term | 35 | ||||
| Section 7.02 |
Termination | 35 | ||||
| Section 7.03 |
Effects of Expiration or Termination | 37 | ||||
| ARTICLE VIII MISCELLANEOUS |
38 | |||||
| Section 8.01 |
Survival | 38 | ||||
| Section 8.02 |
Specific Performance | 38 | ||||
| Section 8.03 |
Notices | 38 | ||||
| Section 8.04 |
Successors and Assigns | 39 | ||||
- ii -
| Section 8.05 | Indemnification | 40 | ||||
| Section 8.06 | Independent Nature of Relationship | 41 | ||||
| Section 8.07 | Tax | 42 | ||||
| Section 8.08 | Entire Agreement | 42 | ||||
| Section 8.09 | Amendments; No Waivers | 42 | ||||
| Section 8.10 | Interpretation | 43 | ||||
| Section 8.11 | Headings and Captions | 43 | ||||
| Section 8.12 | Counterparts; Effectiveness | 43 | ||||
| Section 8.13 | Severability | 43 | ||||
| Section 8.14 | Expenses | 43 | ||||
| Section 8.15 | Governing Law; Jurisdiction | 43 | ||||
| Section 8.16 | Waiver of Jury Trial | 44 | ||||
- iii -
EXHIBITS
| Exhibit A |
– | Form of Assignment Agreement | ||
| Exhibit B |
– | CHRP Replacement License Agreement | ||
| Exhibit C |
– | Copy of Existing License Agreement | ||
| Exhibit D |
– | Form of Security Agreement | ||
| Exhibit E |
– | Legal Opinion of Xxxxxx Xxxxxxx Xxxxxxxx & Xxxxxx, PC | ||
| (Transaction Opinion – U.S.) | ||||
| Exhibit F |
– | Legal Opinion of Xxxxxxxx & Xxxxxxxx LLP (IP Opinion) | ||
| Exhibit G-1 |
– | Form of Astellas Consent | ||
| Exhibit G-2 |
– | Form of LTS Consent | ||
| Exhibit G-3 |
– | Form of The Regents Consent | ||
| Exhibit H |
– | Form of Licensee Direction | ||
| Exhibit I |
– | Form of Officer’s Certificate (NeurogesX) | ||
| Exhibit J |
– | Form of Officer’s Certificate (CHRP) | ||
| Exhibit K |
– | Form of Deposit Agreement | ||
| Exhibit L |
– | Amortization Schedule | ||
| Exhibit M |
– | Joint Press Release |
- iv -
This FINANCING AGREEMENT (as amended, supplemented or otherwise modified from time to time in accordance herewith, this “Agreement”) is made and entered into as of April 29, 2010 between NeurogesX, Inc., a corporation organized under the laws of the State of Delaware (“NeurogesX”), and Xxxxx Healthcare Royalty Partners, L.P., a limited partnership organized under the laws of the State of Delaware (“CHRP”).
WHEREAS, NeurogesX has previously entered into the Existing License Agreement (as hereinafter defined) with Astellas (as hereinafter defined) relating to the development and commercialization of the Licensed Product (as hereinafter defined); and
WHEREAS, under the terms of the Existing License Agreement, NeurogesX has the right, among other things, to receive Sales Milestones, Earned Royalties, the Option Retention Fee and the Option Exercise Fee (as such terms are defined in the Existing License Agreement); and
WHEREAS, NeurogesX wishes to borrow the Revenue Investment Advance from CHRP and CHRP is willing to lend the Revenue Investment Advance to NeurogesX; and
WHEREAS, NeurogesX is willing to assign, convey and transfer to CHRP, and CHRP wishes to accept the assignment, conveyance, and transfer from NeurogesX of the Assigned Rights (as hereinafter defined), upon and subject to the terms and conditions hereinafter set forth.
NOW, THEREFORE, in consideration of the mutual covenants, agreements representations and warranties set forth herein, the Parties hereto agree as follows:
ARTICLE I
Section 1.01 Definitions
The following terms, as used herein, shall have the following meanings:
“Account Instruction” shall have the meaning set forth in the Deposit Agreement.
“Actual Knowledge” shall mean the actual knowledge of a key employee (i.e., officer, general manager and internal counsel) of NeurogesX relating to a particular matter.
“Affiliate” shall mean, with respect to a particular Party, any corporation or non-corporate business entity that, directly or indirectly, controls, is controlled by, or is under common control with such Party. For purposes of this definition, an entity will be regarded as in control of another entity (with correlative meanings for “controlled by” and “under common control with”) if: (a) such entity owns, directly or indirectly, at least fifty percent (50%) of the securities or capital stock with the ability to vote to elect directors (or similar controlling management) of such entity, or has other comparable ownership and voting interest with respect to any entity other than a corporation; or (b) it possesses, directly or indirectly, the power to direct or cause the direction of the management and policies of the corporation or non-corporate
- 1 -
business entity, as applicable, whether through the ownership or control of voting securities, by contract or otherwise.
“Agreement” shall have the meaning set forth in the first paragraph hereof.
“Assigned Rights” shall mean all rights of NeurogesX and its Subsidiaries under the License Agreement, in and to the Revenue Interest.
“Assignment Agreement” shall mean that agreement substantially in the form of Exhibit A pursuant to which NeurogesX shall, assign, convey and transfer to CHRP the Assigned Rights, all on the terms and conditions as set forth therein.
“Astellas” shall mean Astellas Pharma Europe Ltd., a company organized under the laws of the United Kingdom, and its permitted successors and assigns under the Existing License Agreement.
“Bankruptcy Event” shall mean with respect to a Person, the occurrence of any of the following:
(a) commencement by such Person of any case, proceeding or other action (A) under any existing or future law of any jurisdiction, domestic or foreign, relating to bankruptcy, insolvency, reorganization, relief of debtors or the like, seeking to have an order for relief entered with respect to it, or seeking to adjudicate it bankrupt or insolvent, or seeking reorganization, arrangement, adjustment, winding-up, liquidation, dissolution, composition or other relief with respect to it or its respective debts, or (B) seeking appointment of a receiver, trustee, custodian or other similar official for it or for all or any portion of its assets, or such Person shall make a general assignment for the benefit of its creditors; or
(b) there shall be commenced against such Person any case, proceeding or other action of a nature referred to in subsection (a) above which remains undismissed, undischarged or unbonded for a period of sixty (60) calendar days; or
(c) there shall be commenced against such Person any case, proceeding or other action seeking issuance of a warrant of attachment, execution or distraint or similar process against (A) all or any substantial portion of its assets and/or (B) the Licensed Product or any substantial portion of the Intellectual Property related to the Licensed Product, which results in the entry of an order for any such relief which shall not have been vacated, discharged, stayed, satisfied or bonded pending appeal within sixty (60) calendar days from the entry thereof; or
(d) such Person shall take any action in furtherance of, or indicating its consent to, approval of, or acquiescence in, any case, proceeding or other action set forth in subsection (a), (b) or (c) above; or
(e) such Person shall generally not, or shall be unable to, or shall admit in writing its inability to, pay its debts as they become due, shall be insolvent under any applicable law, or
(f) such Person does not have liquid assets equal to at least such Person’s liabilities due and payable during the immediately following three (3) months.
- 2 -
“Business Day” shall mean any day other than a Saturday and Sunday, any day which is a legal holiday under the laws of the State of New York, or any day on which banking institutions located in the State of New York are required by law or other governmental action to close or are not otherwise open for banking business.
“CAP Amount” shall mean a rate of return at the Rate of Interest.
“CHRP” shall have the meaning set forth in the first paragraph hereof.
“CHRP Concentration Account” shall mean a segregated account established for the benefit of CHRP and maintained at the Depositary Bank pursuant to the terms of the Deposit Agreement and this Agreement. The CHRP Concentration Account shall be the account into which the funds first held in the Joint Concentration Account that are payable to CHRP pursuant to this Agreement are swept daily by the Depositary Bank in accordance with the terms of this Agreement and the Deposit Agreement.
“CHRP Indemnified Party” shall have the meaning set forth in Section 8.05(a).
“CHRP Replacement License Agreement” shall mean the License Agreement between NeurogesX and CHRP granting CHRP, subject to (i) the terms and conditions thereof, and (ii) the rights of Astellas under the Existing License Agreement, an irrevocable, royalty-free, assignable and sublicenseable license under the Intellectual Property with respect to the Licensed Products for the Territory, in the form of Exhibit B, annexed hereto, to protect CHRP’s interests in the event of a termination of the Existing License Agreement by enabling it to negotiate and enter into a Replacement License Agreement.
“Closing” shall have the meaning set forth in Section 6.01.
“Closing Date” shall mean the date on which the Revenue Interest Advance is received in the account designated by NeurogesX in accordance with Section 2.03(b).
“Collateral” shall have the meaning set forth in the Security Agreement.
“Competitive Product” shall have the meaning set forth in the Existing License Agreement.
“Confidential Information” shall mean, with respect to a Party, all secret, confidential or proprietary information or data, whether provided in writing, orally, graphically, electronically or other form, provided by such Party (the “Disclosing Party”) to the other Party (the “Receiving Party”), which may include without limitation information relating to the Disclosing Party’s existing or proposed research, development efforts, patent applications, business or products and any other materials that have not been made available by the Disclosing Party to the general public. Notwithstanding the foregoing, the term “Confidential Information” shall not include any information or materials that the Receiving Party can demonstrate by documentary evidence:
(a) were already known to the Receiving Party (other than under an obligation of confidentiality), at the time of disclosure by the Disclosing Party;
- 3 -
(b) were generally available to the public or otherwise part of the public domain at the time of their disclosure to the Receiving Party;
(c) became generally available to the public or otherwise part of the public domain after their disclosure, other than through any act or omission of the Receiving Party in breach of its confidentiality obligations under this Agreement;
(d) were subsequently lawfully disclosed to the Receiving Party by a Third Party who had no obligation to the Disclosing Party not to disclose such information to others;
(e) were independently discovered or developed by or on behalf of the Receiving Party by individuals who had no access to and without the use of the Confidential Information belonging to the Disclosing Party; or
(f) is approved for general release by the Disclosing Party in writing.
“Deposit Accounts” shall mean, collectively, the Initial Concentration Account, the Joint Concentration Account, the NeurogesX Concentration Account and the CHRP Concentration Account, each established and maintained pursuant to the Deposit Agreement.
“Deposit Agreement” shall mean any agreement (including initially that certain Deposit Account Control Agreement substantially in the form of Exhibit K attached hereto) entered into by the Depositary Bank, NeurogesX and CHRP, pursuant to which, among other things, the Deposit Accounts shall be established and maintained.
“Depository Bank” shall mean JPMorgan Chase Bank, N.A. or such other bank or financial institution approved by each of CHRP and NeurogesX in their respective sole discretions.
“Development Cost” shall mean the actual and direct costs incurred in connection with the development and testing of a product, including actual costs of services provided by Third Parties, efforts of employees of NeurogesX or its Affiliates in performing activities at the actual costs of such employees determined in accordance with GAAP, costs of raw materials, supplies and other resources directly consumed in the development of the product and other direct, out of pocket costs, and “fixed costs”. For purposes of this definition, “fixed costs” shall mean the costs of facilities, utilities, insurance, facility, and equipment depreciation, and other fixed costs allocable on a reasonable basis to the development of the product, without xxxx-ups of NeurogesX or its Affiliates. All costs determinations shall be made in accordance with GAAP.
“Dispute” shall have the meaning set forth in Section 3.12(j).
“Dollars” or “$” shall mean U.S. Dollars.
“Earned Royalties” shall have the meaning set forth in the Existing License Agreement.
“Existing License Agreement” shall have the meaning set forth in the definition of License Agreement herein.
- 4 -
“Excluded Liabilities and Obligations” shall have the meaning set forth in Section 2.04.
“Excluded Revenue Interests” shall have the meaning set forth in the definition of “Revenue Interest.”
“Exploit” shall mean, with respect to a product, the manufacture, sale, offer for sale (including marketing and promotion), importation, distribution or other commercialization or exploitation of such product; and “Exploitation” shall have the correlative meaning.
“FDA” shall mean the United States Food and Drug Administration or any successor federal agency thereto.
“Field” shall mean the [***] of any condition in humans.
“Financial Statements” shall mean the audited balance sheets of NeurogesX as of December 31, 2009 and 2008, and the related audited statements of operations, stockholders’ equity, and cash flows of NeurogesX for the year ended December 31, 2009 and 2008 and the accompanying footnotes thereto.
“First Commercial Sale” shall have the meaning set forth in the Existing License Agreement.
“First Stepdown” shall mean the sum of Revenue Interest Payments actually received by CHRP equals $90 million.
“GAAP” shall mean United States generally accepted accounting principles as interpreted and accepted by the Financial Accounting Standards Board, consistently applied through the applicable entity’s organization.
“Governmental Authority” shall mean any government, court, regulatory or administrative agency or commission, or other governmental authority, agency or instrumentality, whether foreign, federal, state or local (domestic or foreign), including any Patent Office, the FDA, the United States National Institutes of Health, or any other government authority in any country.
“Initial Concentration Account” shall mean the deposit account established and maintained at the Depositary Bank pursuant to the Deposit Agreement and this Agreement. The Initial Concentration Account shall be the account into which all payments made in respect of the Revenue Interest are to be remitted or deposited in the first instance in accordance with the terms of this Agreement and the Deposit Agreement.
“Intellectual Property” shall mean all proprietary information; trade secrets; Know-How; utility models; confidential information; inventions (whether patentable or unpatentable and whether or not reduced to practice or claimed in a pending patent application) and improvements thereto; Patents; registered or unregistered trademarks, trade names and service marks, including all goodwill associated therewith; registered and unregistered copyrights and all applications thereof; in each case that (i) are owned, controlled by, issued to, licensed to, licensed by or hereafter acquired by or licensed by NeurogesX or its Subsidiaries, including, without limitation,
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 5 -
any intellectual property subject to the agreements listed on Schedule 3.12(b) and (ii) relating to, embodied by, covering or involving or necessary or used to (a) manufacture or have manufactured, the Licensed Products for Exploitation in the Territory or (b) sell, have sold, market, or have marketed, the Licensed Products, in each case in the Territory. For the sake of clarity, nothing contained in any Transaction Document shall be deemed to limit or otherwise restrict the rights of NeurogesX to manufacture or have manufactured Licensed Products inside or outside the Territory for commercialization outside of the Territory.
“Joint Concentration Account” shall mean a segregated account for the benefit of NeurogesX and CHRP and maintained at the Depositary Bank pursuant to the terms of the Deposit Agreement and this Agreement. The Joint Concentration Account shall be the account into which the Depositary Bank sweeps on a daily basis the funds held in the Initial Concentration Account in accordance with the terms of this Agreement and the Deposit Agreement.
“Know-How” shall mean all non-public information, results and data of any type whatsoever, in any tangible or intangible form (and whether or not patentable), including databases, practices, methods, techniques, specifications, formulations, formulae, knowledge, skill, experience, data and results (including pharmacological, medicinal chemistry, biological, chemical, biochemical, toxicological and clinical study data and results), analytical and quality control data, stability data, studies and procedures, and manufacturing process and development information, results and data.
“Knowledge” shall mean the actual knowledge of a key employee (i.e., officer, general manager and internal counsel) of NeurogesX relating to a particular matter and the knowledge of facts of which any of such key employees are reasonably expected to be aware in the prudent exercise of their duties and responsibilities on behalf of NeurogesX or its Subsidiaries.
“License Agreement” shall mean (i) the Distribution, Marketing and License Agreement, dated as of June 19, 2009, between NeurogesX and Astellas (as amended as of the date hereof), and as may be further amended, supplemented or modified as permitted under this Agreement (the “Existing License Agreement”) and (ii) in the event of the termination thereof, any Replacement License Agreement including, without limitation, any sublicense and subdistribution agreements which survive and are assigned to NeurogesX. A copy of the Existing License Agreement existing as of the date hereof is attached hereto as Exhibit C.
“Licensed Product(s)” shall mean any “Product” licensed to Astellas and any “Licensing Option Product” licensed to Astellas or a Third Party, and with respect to Section 5.03(b)(i) shall also include any Liquid Formulation Product, as those terms are defined in the Existing License Agreement.
“Licensees” shall mean, collectively, (i) Astellas and (ii) all Replacement Licensees; each a “Licensee”.
“Licensee Direction” shall mean the irrevocable direction by NeurogesX to Astellas and/or any Licensee to pay the Revenue Interest directly into the Initial Concentration Account, in substantially the form set forth in Exhibit H.
- 6 -
“Lien” shall mean any lien, hypothecation, charge, security agreement, security interest, mortgage, pledge, or any encumbrance, right or claim of any other Person of any kind whatsoever whether xxxxxx or inchoate, filed or unfiled, noticed or unnoticed, recorded or unrecorded, contingent or non-contingent, material or non-material, known or unknown.
“Losses” shall mean, collectively, any and all claims, damages, losses, judgments, liabilities, costs and expenses (including reasonable expenses of investigation and reasonable attorneys’ fees and expenses in connection with any action, suit or proceeding).
“Material Adverse Effect” shall mean (i) a Bankruptcy Event; (ii) an adverse effect on the validity or enforceability of any material provision of the Transaction Documents; (iii) an adverse effect on the ability of NeurogesX to perform any of its material obligations under any of the Transaction Documents; (iv) an adverse effect on the material rights or remedies of CHRP under any of the Transaction Documents; (v) a material adverse effect on the right of CHRP to receive the Revenue Interest Payments or any other payment due to CHRP hereunder; or (vi) a material adverse effect on the Revenue Interest or any portion of the Collateral, including any a material adverse effect with respect to the Licensed Product or the ability of Licensee to distribute, market and/or sell the Licensed Product in the Territory in accordance with a License Agreement, (vii) a material breach by Licensee of a License Agreement (which is uncured within the time period therefor); or (viii) a right to terminate or receive damages inuring to the benefit of a Third Party arising from the material, uncured breach by NeurogesX under any Material Contract.
“Material Contract” shall mean any contract, agreement or other arrangement to which NeurogesX or any of its Subsidiaries is a party or any of NeurogesX’s or its Subsidiaries’ respective assets or properties are bound or committed (other than the Transaction Documents), in each case related to (i) the Exploitation of a Licensed Product in the Territory or (ii) the Intellectual Property (including the Existing License Agreement).
“Maturity Date” shall mean the latest to occur of the following: (i) ten (10) years from the last to occur of the First Commercial Sale in all countries in the Territory, or (ii) the expiration or abandonment of the last Valid Patent Claim covering any Licensed Product in all countries in the Territory or (iii) the expiration of all applicable periods of regulatory exclusivity covering all Licensed Products in all countries in the Territory.
“Net Sales” shall have the meaning set forth in the Existing License Agreement or any Replacement License Agreement, if applicable.
“NeurogesX” shall have the meaning set forth in the first paragraph hereof.
“NeurogesX Concentration Account” shall mean a segregated account established for the benefit of NeurogesX and maintained at the Depositary Bank pursuant to the terms of the Deposit Agreement and this Agreement. The NeurogesX Concentration Account shall be the account into which the funds remaining in the Joint Concentration Account after payment therefrom of the amounts payable to CHRP pursuant to this Agreement are swept daily in accordance with the terms of this Agreement and the Deposit Agreement.
- 7 -
“NeurogesX Indemnified Party” shall have the meaning set forth in Section 8.05(b).
“Option Exercise Fee” shall have the meaning set forth in the Existing License Agreement.
“Option Retention Fee” shall have the meaning set forth in the Existing License Agreement.
“Party” shall mean NeurogesX or CHRP; and “Parties” shall mean NeurogesX and CHRP.
“Patent Office” shall mean, with respect to the particular country or jurisdiction, the respective patent office responsible for reviewing and issuing Patents for such country or jurisdiction.
“Patents” shall mean any and all issued patents and pending patent applications, including without limitation, all provisional applications, substitutions, continuations, continuations-in-part, divisions, and renewals, all letters patent granted thereon, and all patents-of-addition, reissues, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms (including regulatory extensions), and all supplementary protection certificates, together with any foreign counterparts thereof anywhere in the Territory covering the Licensed Product, composition of matter, formulation, or methods of manufacture or use thereof that are issued or filed on or after the date hereof, including, without limitation, those identified in Schedule 3.12(a) in each case, which are owned, controlled by, issued to, licensed to or licensed by NeurogesX or any of its Affiliates.
“Permitted Liens” means (a) Liens for taxes not yet delinquent or Liens for taxes being contested in good faith and by appropriate proceedings for which adequate reserves have been established; (b) Liens in respect of property or assets imposed by law which were incurred in the ordinary course of business, such as carriers’, warehousemen’s, materialmen’s and mechanics’ Liens and other similar Liens arising in the ordinary course of business which are not delinquent or remain payable without penalty or which are being contested in good faith and by appropriate proceedings; (c) Liens incurred or deposits made in the ordinary course of business in connection with workers’ compensation, unemployment insurance and other types of social security, and other Liens to secure the performance of tenders, statutory obligations, contract bids, government contracts, performance and return of money bonds and other similar obligations, incurred in the ordinary course of business, whether pursuant to statutory requirements, common law or consensual arrangements; (d) Liens in favor of CHRP; (e) Liens upon any equipment acquired or held by NeurogesX or any of its Subsidiaries to secure the purchase price of such equipment or indebtedness incurred solely for the purpose of financing the acquisition of such equipment, so long as such Lien extends only to the equipment financed, and any accessions, replacements, substitutions and proceeds (including insurance proceeds) thereof or thereto; (f) Liens in favor of customs and revenue authorities arising as a matter of law to secure payments of customs duties in connection with the importation of goods, (g) Liens of depository banks which constitute rights of setoff of a customary nature, whether arising by law or contract, with respect to funds deposited in deposit accounts maintained with such depository banks; (h) the License Agreement; and (i) Liens existing on the date of this Agreement which are set forth on
- 8 -
Schedule 1. For avoidance of doubt a Permitted Lien may not affect the rights of CHRP to any Revenue Interest Payment.
“Person” shall mean an individual, corporation, partnership, limited liability company, association, trust or other entity or organization, but not including a government or political subdivision or any agency or instrumentality of such government or political subdivision.
“Rate of Interest” shall mean the rate of interest specified in the amortization schedule attached hereto as Exhibit L.
“Regulatory Agency” shall mean a Governmental Authority with responsibility for the approval of the marketing and sale of pharmaceuticals or other regulation of pharmaceuticals.
“Regulatory Approval” shall mean all approvals (including, without limitation, where applicable, pricing and reimbursement approval and schedule classifications), product and/or establishment licenses, registrations or authorizations of any Regulatory Agency necessary for the manufacture, use, storage, import, export, transport, offer for sale, or sale of the Licensed Product in the Territory in accordance with the License Agreement.
“[***]” shall mean any [***] after the date hereof pursuant to which (i) [***] or (ii) [***]
“[***]” shall have the meaning given in the definition of [***].
“Reports” shall mean, with respect to the relevant period, (i) the quarterly and year-end reports to be provided by Astellas pursuant to Section 4.6 of the Existing License Agreement, or, any such similar report under any Replacement License Agreement; (ii) quarterly and year-end statements of Revenue Interest paid and payable to CHRP prepared by NeurogesX for each calendar quarter and calendar year of the Term and, with respect to each quarterly report, for the twelve (12) consecutive months ending with the last month of such quarter report, together with information, including summaries of reports to the Joint Steering Committee (as that term is defined in the Existing License Agreement) regarding the status of development of the Liquid Formulation Product (as defined in the Existing License Agreement) and summaries of reports on the commercialization of the Licensed Products in the Territory, (iii) a reconciliation of such reports referred to in clause (ii) above to all information and data deliverable to NeurogesX, CHRP or their Affiliates by the Licensee, including, without limitation, the reports delivered pursuant to clause (i) above, together with relevant supporting documentation, and (iv) such additional information as CHRP may reasonably request.
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 9 -
“Revenue Investment Advance” shall have the meaning set forth in Section 2.03(a).
“Revenue Interest” shall mean, with respect to the Existing License Agreement, any and all of NeurogesX’s rights to receive (i) any payments under Section 3 or Section 4 (except Section 4.4) of the Existing License Agreement, including, without limitation, rights to receive Sales Milestones under Section 3.2 of the Existing License Agreement, the Option Retention Fee under Section 3.3.2 of the Existing License Agreement, the Option Exercise Fee under Section 3.3.3 of the Existing License Agreement, and Earned Royalties under Section 4 of the Existing License Agreement, in each case payable on and after Xxxxx 0, 0000, (xx) any payments under Section 11.5.2 of the Existing License Agreement in amounts in the aggregate in excess of out-of-pocket costs and expenses (including reasonable attorneys’ and professional fees) incurred in connection with any Enforcement Action (as such term is defined in the Existing License Agreement), (iii) any payments similar to those described in clauses (i) and (ii) from Astellas or its affiliates with respect to Licensing Option Products, regardless of whether such Licensing Option Products are included under the Existing License Agreement or licensed to Astellas pursuant to a separate agreement, (iv) any payments similar to those described in clauses (i), (ii) and (iii) under any Replacement License Agreement, (v) any payments similar to those described in clauses (i) – (iv) made by a Licensee after rejection of the Existing License Agreement or Replacement License Agreement by NeurogesX in bankruptcy, and (vi) any other payments for or derived from the sale, offer for sale (including marketing and promotion), distribution or other commercialization or exploitation of Licensed Products in the Territory.
For clarity the Revenue Interest shall exclude all rights under any License Agreement to receive (a) payments or amounts which are used to pay Third Parties for rights to intellectual property necessary for or incorporated in the Licensed Products (including amounts payable (I) under Section 4.4 of the Existing License Agreement, (II) to The Regents of the University of California (“The Regents”) pursuant to Section 3.2 or Section 19.4 of that certain Exclusive License Agreement between NeurogesX and The Regents effective November 1, 2000 or (III) to LTS Xxxxxxx Therapie Systeme AG (“LTS”) pursuant to Section 7.3 of that certain Commercial Supply and License Agreement between NeurogesX and LTS dated January 2007), (b) any payments under the Supply Agreement (as entered into pursuant to and defined in Section 9.1 of the Existing License Agreement) and (c) any payment intended to reimburse for any costs or expenses (whether internal or external reasonably allocated in accordance with GAAP) incurred or to be incurred by NeurogesX with respect to activities under such License Agreement or otherwise with respect to the Licensed Product in the Territory (collectively, the “Excluded Revenue Interests”).
“Revenue Interest Payments” shall have the meaning set forth in Section 2.02(a).
“Sales Milestones” shall mean those payments payable to NeurogesX pursuant to Section 3.2 of the Existing License Agreement.
“Second Stepdown” shall mean the date on which the sum of Revenue Interest Payments actually received by CHRP equals $106 million.
“Security Agreement” shall mean the security agreement, by and between NeurogesX and CHRP, providing for, among other things; the grant by NeurogesX in favor of CHRP of a
- 10 -
valid continuing, perfected lien on and security interest in, the Revenue Interest and the Collateral described therein, which Security Agreement shall be substantially in the form of Exhibit D.
“Security Documents” shall mean, collectively, the Assignment Agreement and the Security Agreement.
“Subsidiary” or “Subsidiaries” shall mean with respect to any Person (i) any corporation having at least a majority of the capital stock entitled to vote in the election of directors under ordinary circumstances owned, directly or indirectly, by such Person or (ii) any other Person of which at least a majority voting interest under ordinary circumstances is at the time, directly or indirectly, owned by such Person.
“Term” shall mean the term of this Agreement, as provided in Section 7.01 hereof.
“Territory” shall have the meaning set forth in the License Agreement.
“Third Party” shall mean any Person other than NeurogesX or CHRP or their respective Affiliates.
“Third Party Consents” shall mean the consents of Astellas, LTS and The Regents, substantially in the form of Exhibits G-1, G-2 and G-3, respectively.
“Transaction Documents” shall mean, collectively, this Agreement, the Assignment Agreement, the Security Agreement, the CHRP Replacement License Agreement, the Deposit Agreement, the Third Party Consents, and the Licensee Direction and all other documents delivered in connection therewith.
“UCC” shall mean the Uniform Commercial Code (or any similar or equivalent legislation) as in effect in any applicable jurisdiction.
“United States” shall mean the United States of America.
“Valid Patent Claim” shall have the meaning set forth in the License Agreement.
ARTICLE II
Section 2.01 Assignments
(a) Upon the terms and subject to the conditions set forth in this Agreement, on the Closing Date, NeurogesX agrees to assign, transfer and convey to CHRP, and CHRP agrees to accept the assignment, transfer and conveyance from NeurogesX, free and clear of all Liens (except the Permitted Liens and those Liens created in favor of CHRP pursuant to the Security Documents) and subject to the conditions set forth in ARTICLE VI, the Assigned Rights. CHRP’s ownership interest in the Assigned Rights so assigned and the Lien granted under the Security Documents shall vest simultaneously with and only upon NeurogesX’s receipt of the
- 11 -
full, irrevocable, non-refundable and non-deductible Revenue Investment Advance pursuant to Section 2.03(a) for such Assigned Rights.
(b) Upon the occurrence of the First Stepdown, CHRP agrees to assign, transfer and convey to NeurogesX, and NeurogesX agrees to accept the assignment, transfer and conveyance from CHRP an undivided joint interest in and to the Assigned Rights, free and clear of all Liens other than the Permitted Liens and Liens in favor of CHRP under the Security Agreement. Further upon the earlier of (i) occurrence of the Second Stepdown, (ii) the payment to CHRP of any amount due under Section 2.02(e), or (iii) NeurogesX’s exercise and payment of its buy-out right under Section 7.02(c), CHRP agrees to assign, transfer and convey to NeurogesX, and NeurogesX agrees to accept the assignment, transfer and conveyance from CHRP any and all rights in and to the Assigned Rights not previously assigned to NeurogesX, free and clear of all Liens.
Section 2.02 Payments to CHRP
(a) Subject to CHRP paying to NeurogesX the Revenue Investment Advance pursuant to Section 2.03(a), from and after April 1, 2010 throughout the Term, CHRP shall be entitled to receive the following percentages of Revenue Interest (collectively, the “Revenue Interest Payments”):
(i) until the occurrence of the First Stepdown, 100% of Revenue Interest; plus
(ii) after the occurrence of the First Stepdown and until the occurrence of the Second Stepdown, 5% of Revenue Interest.
For clarity, as between the Parties, NeurogesX shall have the right to receive and retain (A) any and all Excluded Revenue Interests and (B) from and after the occurrence of the Second Stepdown any and all Revenue Interest.
In the event there is a good faith dispute whether payments receivable under the Existing License Agreement or a Replacement License Agreement not explicitly set forth herein are included within the Revenue Interest or are Excluded Revenue Interests which is not resolved after submission first to the officers and then to the chief executive officer of NeurogesX and managing directors of CHRP, or their designees, in the manner provided in Section 8.15(a), [***] of such payments shall be [***] the Revenue Interest subject to the provisions of this Section 2.02(a). In the event pursuant to the Existing License Agreement or a Replacement License Agreement, NeurogesX or its Affiliate licenses the Exploitation of a [***] to a Third Party in the Territory other than Licensee, all payments receivable by NeurogesX or its Affiliates with respect to [***].
(b) NeurogesX shall instruct the Licensee to deposit the Revenue Interest into the Initial Concentration Account, pursuant and subject to Section 5.07. In the event NeurogesX receives any portion of the Revenue Interest due to CHRP hereunder, NeurogesX shall hold such amounts in trust for the benefit of CHRP, provide CHRP with an accounting thereof, and, within ten (10) Business Days after receipt thereof, deliver such amounts to CHRP by wire transfer of immediately available funds.
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 12 -
(c) In the event Licensee fails to pay any Revenue Interest Payment when due under the License Agreement (each such unpaid amount, a “Discrepancy”), then, provided such failure to pay is not due to an actual default by NeurogesX, NeurogesX shall not be obligated to pay to CHRP or otherwise compensate or make CHRP whole with respect to any such Discrepancy; provided, however, that NeurogesX shall use commercially reasonable efforts to enforce the terms of the License Agreement as if NeurogesX was the sole owner of the Revenue Interest (including taking actions reasonably requested by CHRP in furtherance of such enforcement) and upon any recovery of a Discrepancy and CHRP shall receive its portion of any such recovery as set out in Section 2.02(a). Accordingly, NeurogesX shall not be responsible with respect to any Revenue Interest receivable or payable under any License Agreement until actually received by or paid to NeurogesX thereunder unless Licensee’s failure to pay is due to an actual default by NeurogesX.
(d) NeurogesX agrees that CHRP is entitled to the Assigned Rights and may enforce such entitlement directly against NeurogesX, provided that unless due to the actions of NeurogesX, NeurogesX shall not be responsible for any Revenue Interest not paid to CHRP unless such amount has been received by NeurogesX. Notwithstanding any claim or set-off that NeurogesX may have against CHRP or that Astellas has against NeurogesX, NeurogesX agrees that, subject to the provisions of Section 5.07, it will require that Astellas remit all Revenue Interest that Astellas is required to pay to NeurogesX under the Existing License Agreement with respect to the Assigned Rights directly into the Initial Concentration Account, pursuant to the Licensee Direction.
(e) Unless this Agreement has been earlier terminated in accordance with the terms hereof, within ten (10) Business Days of the Maturity Date, NeurogesX shall pay to CHRP an amount equal to the Revenue Investment Advance less all Revenue Interest Payments received by CHRP. The foregoing shall be deemed a minimum repayment obligation of NeurogesX and shall not reduce or diminish the rights of CHRP to receipt of Revenue Interest Payments or as otherwise provided herein.
Section 2.03 Advances to NeurogesX.
(a) Revenue Investment Advance. Subject to the terms and conditions set forth herein, including, without limitation, Section 6.02, CHRP shall advance to NeurogesX the sum of Forty Million Dollars ($40,000,000.00) (the “Revenue Investment Advance”) no later than fifteen (15) Business Days following the date of this Agreement.
(b) Advance Procedure. The Revenue Investment Advance to be made by CHRP to NeurogesX under Section 2.03(a) shall be paid by wire transfer of immediately available funds to the account(s) designated by NeurogesX. If the conditions set forth in Section 6.02 for advancement of the Revenue Investment Advance are not satisfied within fifteen (15) Business Days following the date of this Agreement, then the provisions of Section 7.02 shall govern.
Section 2.04 No Assumed Obligations.
Notwithstanding any provision in this Agreement or any other Transaction Document or writing to the contrary, CHRP is accepting the assignment of only the Assigned Rights and is not
- 13 -
assuming any liability or obligation of NeurogesX or any of its Affiliates of whatever nature, whether presently in existence or arising or asserted hereafter, whether known or unknown, and whether under the License Agreement or any Transaction Document or otherwise. All such liabilities and obligations shall be retained by and remain obligations and liabilities solely of NeurogesX or its Affiliates (the “Excluded Liabilities and Obligations”).
Section 2.05 Excluded Assets.
CHRP does not, by assignment of the rights granted hereunder or otherwise pursuant to any of the Transaction Documents, acquire any assets of NeurogesX or any of its Affiliates, other than to the Revenue Interest and the Assigned Rights.
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF THE ASSIGNOR
Except with respect to any section of this ARTICLE III as set forth in the correspondingly identified section of the disclosure schedule delivered by NeurogesX to CHRP concurrently herewith (the “Disclosure Schedule”), NeurogesX hereby represents and warrants to CHRP, as of the date of this Agreement, the following:
Section 3.01 Organization.
NeurogesX is a corporation duly incorporated, validly existing and in good standing under the laws of the State of Delaware, and has all corporate powers and all licenses, authorizations, consents and approvals required to carry on its business as now conducted and as proposed to be conducted and in connection with the transactions contemplated by the Transaction Documents. NeurogesX is duly qualified to do business as a foreign corporation and is in good standing in each jurisdiction in which it is required to be so qualified, except where the failure to be so qualified would not have a Material Adverse Effect.
Section 3.02 Corporate Authorization.
NeurogesX has all necessary power and authority to enter into, execute and deliver the Transaction Documents and to perform all of the obligations to be performed by it hereunder and thereunder and to consummate the transactions contemplated hereunder. The Transaction Documents have been duly authorized, executed and delivered by NeurogesX and each Transaction Document constitutes the valid and binding obligation of NeurogesX, enforceable against it in accordance with such Transaction Document’s respective terms, subject, as to enforcement of remedies, to bankruptcy, insolvency, reorganization, moratorium or similar laws affecting creditors’ rights generally or general equitable principles (regardless of whether enforcement is sought in equity or at law).
Section 3.03 Governmental Authorization.
The execution and delivery by NeurogesX of the Transaction Documents, and the performance by NeurogesX of its obligations hereunder and thereunder, does not require any
- 14 -
notice to, action or consent by, or in respect of, or filing with, any Governmental Authority, except with respect to the Security Documents for the filing and registration of financing statements under the UCC, filings with any Patent Office, and such other notice, action, consent or filing as may be required to perfect the security interest granted in the Collateral.
Section 3.04 Ownership.
(a) Except as otherwise provided in or granted under the License Agreement and the Transaction Documents, NeurogesX owns, or holds a valid license under all of the Intellectual Property and the Regulatory Approvals, with respect to the Licensed Product free and clear of all Liens (other than Permitted Liens), and no license or covenant not to xxx under any Intellectual Property or Regulatory Approvals has been granted to any Third Party in the Territory.
(b) (i) NeurogesX, immediately prior to the assignment of the Assigned Rights, owns, and is the sole holder of, all rights to receive the Revenue Interest, free and clear of all Liens other than Permitted Liens; (ii) NeurogesX has not transferred, sold, conveyed, assigned, or otherwise disposed of, or agreed to transfer, sell, convey, assign, or otherwise dispose of any portion of the Revenue Interest or the Collateral other than as contemplated by this Agreement; (iii) no Person other than NeurogesX has any right to receive the payments payable under any License Agreement in the Territory, other than CHRP’s rights with respect to the Revenue Interest, from and after April 1, 2010; (iv) NeurogesX has the full right to transfer, convey and assign to CHRP the rights and interests in and to the Revenue Interest being transferred, conveyed and assigned to CHRP and to grant a security interest in the Collateral pursuant to this Agreement and the other Transaction Documents without any requirement to obtain the consent of any Person; (v) subject to the payment of the Revenue Investment Advance by CHRP to NeurogesX pursuant to Section 2.03(a), by the delivery to CHRP of the executed Assignment Agreement and the Licensee Direction, NeurogesX shall transfer, convey and assign to CHRP the rights and interests in and to the Revenue Interest being transferred, conveyed and assigned to CHRP pursuant to this Agreement and the Assignment Agreement, free and clear of any Liens, except the Permitted Liens and those Liens created in favor of CHRP pursuant to the Security Documents and any other Transaction Document; and (vi) subject to the payment of the Revenue Investment Advance by CHRP to NeurogesX pursuant to Section 2.03(a), at the Closing, and upon the delivery of the Assignment Agreement to CHRP by NeurogesX and delivery of the Licensee Direction by NeurogesX to the Licensee, CHRP shall have acquired good and valid rights and interests of NeurogesX in and to the Revenue Interest being transferred, conveyed and assigned to CHRP pursuant to this Agreement and the Assignment Agreement, free and clear of any and all Liens, except the Permitted Liens and those Liens created in favor of CHRP pursuant to the Security Documents and any other Transaction Document.
Section 3.05 Financial Statements.
The Financial Statements are complete and accurate in all material respects, were prepared in accordance with GAAP and present fairly in all material respects the financial position and the financial results of NeurogesX as of the dates and for the periods covered thereby.
- 15 -
Section 3.06 No Undisclosed Liabilities.
Except for those liabilities (i) specifically identified on the face of the Financial Statements and (ii) incurred by NeurogesX in the ordinary course of business since December 31, 2009, there are no material liabilities of NeurogesX taken as a whole, of any kind whatsoever, whether accrued, contingent, absolute, determined or determinable.
Section 3.07 Solvency.
No event has occurred which with the passage of time or the giving of notice would constitute a Bankruptcy Event with respect NeurogesX. Assuming consummation of the transactions contemplated by the Transaction Documents, (i) the present fair saleable value of NeurogesX’s assets is greater than the amount required to pay its debts as they become due, (ii) NeurogesX does not have unreasonably small capital with which to engage in its business, and (iii) NeurogesX has not incurred, nor does it have present plans to or intend to incur, debts or liabilities beyond its ability to pay such debts or liabilities as they become absolute and matured.
Section 3.08 Litigation.
There is no (i) action, suit, arbitration proceeding, claim, investigation or other proceeding pending or, to the Actual Knowledge of NeurogesX, threatened against NeurogesX, or its Subsidiaries or (ii) governmental inquiry pending or, to the Actual Knowledge of NeurogesX, threatened against NeurogesX or its Subsidiaries, in each case with respect to clauses (i) and (ii) above, which, if adversely determined, would question the validity of, or would reasonably be expected to adversely affect the transactions contemplated by any of the Transaction Documents or would reasonably be expected to have a Material Adverse Effect. There is no action, suit, claim, proceeding or investigation pending or, to the Actual Knowledge of NeurogesX, threatened against NeurogesX or its Subsidiaries or, to the Actual Knowledge of NeurogesX, any other Person relating to the Revenue Interest, which, if adversely determined, would question the validity of, or would affect the transactions contemplated by any of the Transaction Documents or would have a Material Adverse Effect.
Section 3.09 Compliance with Laws.
None of NeurogesX or any of its Subsidiaries (i) is in violation of, has violated, or to the Knowledge of NeurogesX is under investigation with respect to, and (ii) has been, or to the Knowledge of NeurogesX threatened to be, charged with or been given notice of any violation of any law, rule, ordinance or regulation of, or any judgment, order, writ, decree, permit or license entered by any Governmental Authority applicable to NeurogesX or the Revenue Interest which would reasonably be expected to have a Material Adverse Effect.
Section 3.10 Conflicts.
(a) Neither the execution and delivery of any of the Transaction Documents nor the performance or consummation of the transactions contemplated hereby and thereby will: (i) contravene, conflict with, result in a breach or violation of, constitute a default under, or accelerate the performance provided by, in any material respects any provisions of: (A) any law,
- 16 -
rule, ordinance or regulation of any Governmental Authority, or any judgment, order, writ, decree, permit or license of any Governmental Authority, to which NeurogesX or any of its Subsidiaries or any of their respective assets or properties related to the Exploitation of the Licensed Product in the Territory may be subject or bound; or (B) any contract, agreement, commitment or instrument to which NeurogesX or its Subsidiaries is a party or by which NeurogesX or any of its Subsidiaries or any of their respective assets or properties related to the Exploitation of the Licensed Product in the Territory is bound or committed; (ii) contravene, conflict with, result in a breach or violation of, constitute a default under, or accelerate the performance provided by, any provisions of the certificate of incorporation or by-laws (or other organizational or constitutional documents) of NeurogesX; (iii) except for the filing of the UCC-1 financing statements required hereunder and filings with the United States Patent and Trademark Office or any foreign equivalents thereof in the Territory, the Third Party Consents, and such other notifications, filings and consents as may be required to perfect the security interest in the Collateral, require any notification to, filing with, or consent of, any Person or Governmental Authority; (iv) give rise to any right of termination, cancellation or acceleration of any right or obligation of NeurogesX or any other Person or to a loss of any benefit, in each case relating to the Revenue Interest; or (v) result in the creation or imposition of any Lien on (y) the assets or properties of NeurogesX related to the Exploitation of the Licensed Product in the Territory, or (z) the Revenue Interest or any Collateral, other than Liens on behalf of CHRP.
(b) Neither NeurogesX nor its Subsidiaries have granted, nor does there exist, any Lien on the Revenue Interest or any Collateral other than pursuant to the Security Documents, the Permitted Liens and the License Agreement.
Section 3.11 Subordination.
The claims and rights of CHRP created by any Transaction Document in and to the Revenue Interest and other Assigned Rights and any other Collateral are not subordinated to any creditor of NeurogesX or any other Person.
Section 3.12 Intellectual Property.
(a) Schedule 3.12(a) sets forth an accurate, true and complete list (by category and family) of (1) Patents (including pending Patent applications) and utility models, (2) trade names, common law trademarks, common law service marks, registered trademarks, registered service marks, and applications for trademark registration or service xxxx registration, (3) registered and unregistered copyrights and (4) domain name registrations and websites owned by NeurogesX, in each case with respect to clauses (1), (2), (3) and (4) above in this subsection (a) that are owned by, or licensed to, NeurogesX and are necessary or used to make, have made, use, sell, have sold, offer for sale, import, develop, promote, market, distribute, manufacture, commercialize or otherwise Exploit the Licensed Product in the Territory by NeurogesX, its Affiliates or Licensees in accordance with the License Agreement. For each item of Intellectual Property listed on Schedule 3.12(a), NeurogesX has identified, as applicable, [***] Except as disclosed therein, (1) each item of Intellectual Property listed on Schedule 3.12(a) and owned by NeurogesX is valid and subsisting and no such listed Intellectual Property has lapsed, expired, been cancelled or become abandoned and (2) to the Knowledge of NeurogesX, each item of Intellectual Property listed on Schedule 3.12(a) which is licensed by NeurogesX from a Third
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 17 -
Party is, to the Knowledge of NeurogesX, valid and subsisting and no such listed Intellectual Property has lapsed, expired, been cancelled or become abandoned. To the Knowledge of NeurogesX the Patent applications listed in Schedule 3.12(a) have been and continue to be prosecuted by competent Patent counsel in a commercially reasonable manner consistent with standards in the biotechnology industry for similarly situated entities. Except for references identified to the applicable Patent Office as of December 31, 2009, during prosecution of the patent applications, to the Knowledge of NeurogesX, there are no published patents, patent applications, articles, prior art references or other factors or circumstances that would materially adversely affect the validity or enforceability of any of the Patents listed in Schedule 3.12(a)(1) or have a Material Adverse Effect. To the Knowledge of NeurogesX, each of the Patents and Patent applications listed in Schedule 3.12(a) correctly identifies each and every inventor of the claims thereof as determined in accordance with the laws of the jurisdiction in which such Patent is issued or such Patent application is pending. [***]
(b) Schedule 3.12(b) sets forth an accurate, true and complete list of all agreements, whether oral or written, express or implied, including, without limitation, assignments, licenses, options, franchise, distribution, marketing and manufacturing agreements, sponsorships, project agreements, collaboration agreements, joint development agreements, agreements not to enforce, consents, settlements, assignments, Liens or mortgages, and any amendments(s) renewal(s), novation(s) and termination(s) pertaining thereto, pursuant to which NeurogesX has the legal right to exploit intellectual property that is owned by a Third Party with respect to the Exploitation of the Licensed Product in the Territory or the manufacture or supply of Licensed Products for sale in the Territory. There are no unpaid fees or royalties under any agreement listed on Schedule 3.12(b) that have become due, or are expected to become overdue, as of the Closing Date.
(c) To the Knowledge of NeurogesX, each agreement listed in Schedule 3.12(b) is legal, valid, binding, enforceable, and in full force and effect. Each of NeurogesX or its Subsidiaries is not in breach of such listed agreements and, to the Knowledge of NeurogesX, no circumstances or grounds exist that would give rise to a claim of breach or right of rescission, termination, revision, or amendment of any of the agreements specified in Schedule 3.12(b), including, without limitation, the execution, delivery and performance of this Agreement and the other Transaction Documents.
(d) Except for Intellectual Property licensed to NeurogesX pursuant to any agreement listed on Schedule 3.12(b) and Intellectual Property owned by NeurogesX, to the Knowledge of NeurogesX, no other Intellectual Property is necessary to make or have made the Licensed Products in the Territory or for sale in the Territory and to Exploit the Licensed Product in the Territory. To the Knowledge of NeurogesX, the manufacture, use or sale of the Licensed Product by Licensee as contemplated in the Existing License Agreement will not infringe upon any valid and enforceable Third Party’s patents. NeurogesX has not received any written notice from a Third Party alleging that the manufacture, use or sale of the Licensed Product infringes on any rights of a Third Party.
(e) NeurogesX possesses sole, exclusive, valid, marketable and unencumbered title to the Intellectual Property for which it is listed as the owner on Schedule 3.12(a), and is a party to the agreements listed on Schedule 3.12(b); all assignments from each inventor, as the case may
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 18 -
be, to NeurogesX or to a predecessor in interest of NeurogesX, have been executed and recorded for each of the Patents for which NeurogesX is listed as the owner on Schedule 3.12(a)(1); there are no Liens on or to any Intellectual Property listed on Schedule 3.12(a) that it owns or agreement listed on Schedule 3.12(b), except the Permitted Liens and Liens created by the Existing License Agreement or the Transaction Documents.
(f) NeurogesX has the full right, power and authority to grant all of the rights and interests granted to CHRP in this Agreement and to Astellas under the Existing License Agreement.
(g) There are no maintenance, annuity or renewal fees that are currently overdue beyond their allotted grace period for any of the Intellectual Property listed on Schedule 3.12(a), nor have any applications or registrations therefor lapsed or become abandoned, been cancelled or expired.
(h) No payments by NeurogesX are due to any other Person in respect of the Licensed Product or the Intellectual Property, other than pursuant to those agreements listed on Schedule 3.12(b) and those fees payable to Patent Offices in connection with the prosecution and maintenance of such Intellectual Property and associated attorney fees. It being acknowledged by NeurogesX that CHRP shall have no obligation to make such payments.
(i) None of NeurogesX, or, to the Actual Knowledge of NeurogesX, any other Person, has undertaken or omitted to undertake any acts, and no circumstance or grounds exist, that would invalidate, reduce or eliminate, in whole or in part, the enforceability or scope of (i) any Intellectual Property or, in the case of Intellectual Property owned or licensed by NeurogesX, NeurogesX’s entitlement to exclusively exploit such Intellectual Property, or (ii) NeurogesX’s right to enjoy any Revenue Interest, in each case that would reasonably be expected to have a Material Adverse Effect.
(j) There is no pending, decided or settled opposition, interference proceeding, reexamination proceeding, cancellation proceeding, injunction, lawsuit, hearing, investigation, complaint, arbitration, mediation, demand, International Trade Commission investigation, decree, or any other dispute, disagreement, or claim, in each case alleged in writing to NeurogesX (collectively referred to hereinafter as “Disputes”), nor has any such Dispute to the Actual Knowledge of NeurogesX been threatened, in each case challenging the legality, validity, enforceability or ownership of any Intellectual Property or the Licensed Product or which would give rise to a credit or right of set off against any Revenue Interest.
(k) There is no pending or, to the Actual Knowledge of NeurogesX, threatened action, suit, or proceeding, or any investigation or claim by any Governmental Authority to which NeurogesX is a party (1) that would be the subject of a claim for indemnification by any Person or Third Party under any agreement, or (2) that alleges that the marketing, sale or distribution of the Licensed Product worldwide by NeurogesX or its Licensees pursuant to the related License Agreement, as applicable, does or will infringe on any patent or other intellectual property rights of any other Person, and, to the Knowledge of NeurogesX, there is no basis for any such action, suit, proceeding, investigation or claim of the type described in clause (1) or (2) above. There are no pending published, or to the Actual Knowledge of NeurogesX unpublished,
- 19 -
United States, international or foreign patent applications owned by any other Person, which, if issued, would limit or prohibit, in any material respect, the use of the Licensed Product in the Territory or the licensed Intellectual Property relating to the Licensed Product in the Territory.
(l) NeurogesX has previously made available to CHRP all documents related to the Intellectual Property specifically requested in writing by CHRP or its counsel.
Section 3.13 Regulatory Approval.
(a) NeurogesX has previously made available to CHRP the Regulatory Approvals, all material correspondence with Regulatory Agencies (including, without limitation, the EMEA and the FDA), and all adverse event reports with respect to the Licensed Product and all requested documents related to the Licensed Product in each case in the possession and control of NeurogesX and NeurogesX has not withheld any document or information with respect to the Licensed Product that would reasonably be considered to be material to CHRP’s decision to make the Revenue Investment Advance.
(b) NeurogesX and its Subsidiaries, and, to the Knowledge of NeurogesX, NeurogesX’s licensees (including Licensee), are in compliance with, and have materially complied with, all applicable federal, state, local and foreign laws, rules, regulations, standards, orders and decrees governing its business, including all regulations promulgated by each Regulatory Agency in the Territory, the failure of compliance with which would result in a Material Adverse Effect; NeurogesX has not received in the Territory any notice citing action or inaction by any of them that would constitute any non-compliance with any applicable federal, state, local and foreign laws, rules, regulations, or standards, which would result in a Material Adverse Effect.
(c) To the Knowledge of NeurogesX the studies, tests and preclinical and clinical trials conducted relating to the Licensed Product in the Territory were conducted in all material respects in accordance with experimental protocols, procedures and controls pursuant to, where applicable, accepted professional and scientific standards at the time when conducted; the descriptions of the results of such studies, tests and trials provided to CHRP are accurate in all material respects; and NeurogesX has not received any notices or correspondence from any Regulatory Agency in the Territory or comparable authority requiring the termination, suspension, or material modification or clinical hold of any such studies, tests or preclinical or clinical trials conducted by or on behalf of NeurogesX, which termination, suspension, material modification or clinical hold would reasonably be expected to result in a Material Adverse Effect.
Section 3.14 Material Contracts.
(a) There are no Material Contracts other than those set forth on Schedule 3.14(a). Neither NeurogesX nor any of its Subsidiaries is in breach of or in default under any Material Contract, which default, individually or in the aggregate, would reasonably be expected to result in a Material Adverse Effect. To the Actual Knowledge of NeurogesX, nothing has occurred and no condition exists that would permit any other party thereto to terminate any Material Contract. NeurogesX has not received any notice or, to the Actual Knowledge of NeurogesX, any threat of
- 20 -
termination of any such Material Contract. To the Actual Knowledge of NeurogesX, no other party to a Material Contract is in material breach of or in material default under such Material Contract. All Material Contracts are valid and binding on NeurogesX and, to the Knowledge of NeurogesX, on each other party thereto, and are in full force and effect.
(b) Without limiting the generality of the foregoing, with respect to the License Agreement:
i. Exhibit C contains a true, complete and correct copy of the Existing License Agreement. Neither NeurogesX nor, to the Knowledge of NeurogesX, Licensee, has impaired, waived, altered or modified in any respect, whether by way of any sublicense or consent or otherwise, the Existing License Agreement or any provisions thereof. To the Actual Knowledge of NeurogesX, Licensee has not granted any sublicense under or with respect to the Existing License Agreement.
ii. NeurogesX has not released Licensee, in whole or in part, from any of its material obligations under the Existing License Agreement.
iii. NeurogesX has not received any notice from Licensee requesting any amendment, alteration, modification or termination of the Existing License Agreement, nor has Licensee communicated in writing any desire or intent to do so.
iv. To the Knowledge of NeurogesX, no event has occurred and no condition exists that would adversely affect the right of NeurogesX to receive any Revenue Interest payable under the Existing License Agreement. Neither NeurogesX nor, to the Actual Knowledge of NeurogesX, Licensee, has taken any action or omitted to take any action, that would reasonably be expected to result in a Material Adverse Effect.
v. The Existing License Agreement is enforceable against NeurogesX and, to the Actual Knowledge of NeurogesX, Licensee, in accordance with its terms, subject, as to enforcement of remedies, to bankruptcy, insolvency, reorganization, moratorium or similar laws affecting creditors’ rights generally and by general equitable principles (regardless of whether enforcement is sought in equity or at law). The execution, delivery and performance of the Existing License Agreement was and is within the corporate powers of NeurogesX and, to the Knowledge of NeurogesX, Licensee. The Existing License Agreement was duly authorized by all necessary action on the part of, and validly executed and delivered by, NeurogesX and, to the Knowledge of NeurogesX, Licensee. There is no breach or default, or event which, upon notice or the passage of time, or both, could give rise to any breach or default, in the performance of the Existing License Agreement by NeurogesX or, to the Actual Knowledge of NeurogesX, Licensee.
vi. There has been no correspondence or other written communication sent by or on behalf of NeurogesX to, or received by or on behalf of NeurogesX from, Licensee, the subject matter of which would reasonably be expected to result in a Material Adverse Effect.
vii. Licensee has no right of set-off against any Revenue Interest or any other amount payable to NeurogesX under the Existing License Agreement.
- 21 -
viii. Licensee has not communicated in writing any desire or intent to replace the Licensed Product with an alternative product, nor has Licensee communicated in writing any desire or intent to reduce materially its efforts to develop and commercialize the Licensed Product.
ix. Schedule 3.14(b) sets forth to the Actual Knowledge of NeurogesX without further investigation a true and complete list of all Net Sales and Revenue Interest paid by Licensee pursuant to the Existing License Agreement through March 31, 2010.
Section 3.15 Place of Business.
NeurogesX’s principal place of business is set forth on Schedule 3.15.
Section 3.16 Broker’s Fees.
Neither NeurogesX nor any of its Affiliates have taken any action that would entitle any Person to any commission or broker’s fee in connection with the transactions contemplated by the Transaction Documents.
Section 3.17 Information.
No written statement, information, report or materials prepared by or on behalf of NeurogesX and its Subsidiaries and furnished to CHRP by or on behalf of NeurogesX in connection with any Transaction Document or any transaction contemplated hereby or thereby, no written representation, warranty or statement made by NeurogesX in any Transaction Document, and no Schedule or Exhibit hereto, in each case taken in the aggregate, contains any untrue statement of a material fact or omits any statement of material fact necessary in order to make the statements made therein in light of the circumstances under which they were made not materially misleading.
Section 3.18 Security Agreement.
The Security Agreement, when executed and delivered by NeurogesX and CHRP creates in favor of CHRP a legal, valid and enforceable (except as enforceability may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or similar laws affecting the enforcement of creditors’ rights generally and by general equitable principles (regardless of whether enforcement is sought in equity or at law)) security interest in the Collateral and proceeds thereof. In the case of the Collateral described in the Security Agreement, when financing statements and other filings specified on Schedule 3.18 in appropriate form are or have been filed in the offices specified on Schedule 3.18, the Security Agreement shall constitute a fully perfected Lien on, and first priority security interest, subject to the Permitted Liens, in, all right, title and interest of NeurogesX in the Collateral and the proceeds thereof to the extent a security interest therein can be perfected by such filings as security for the Obligations (as defined in the Security Agreement).
- 22 -
Section 3.19 Bankruptcy Event.
No Bankruptcy Event has occurred with respect to NeurogesX or, to the Actual Knowledge of NeurogesX, with respect to Licensee.
Section 3.20 Material Adverse Effect.
Since the filing of NeurogesX’s Annual Report, to the Knowledge of NeurogesX no event has occurred which with the passage of time and/or the giving of notice would constitute a Material Adverse Effect and no Material Adverse Effect has occurred with respect to NeurogesX or, to the Actual Knowledge of NeurogesX, with respect to Licensee.
Section 3.21 Field of Use.
[***]
Section 3.22 No Other Representations.
Except as expressly set forth in the Transaction Documents, NeurogesX and its Affiliates make no representation or warranty, express or implied, at law or in equity, in respect of NeurogesX or any of its Affiliates or any of their respective assets, liabilities or operations, including, without limitation, with respect to any License Agreement and the Assigned Rights, and any such other representations or warranties are hereby expressly disclaimed.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF CHRP
CHRP represents and warrants to NeurogesX the following:
Section 4.01 Organization.
CHRP is a limited partnership duly formed and validly existing under the laws of the State of Delaware.
Section 4.02 Authorization.
CHRP has all necessary power and authority to enter into, execute and deliver the Transaction Documents and to perform all of the obligations to be performed by it hereunder and thereunder and to consummate the transactions contemplated hereunder and thereunder. The Transaction Documents have been duly authorized, executed and delivered by CHRP and each Transaction Document constitutes the valid and binding obligation of CHRP, enforceable against CHRP in accordance with their respective terms, subject, as to enforcement of remedies, to bankruptcy, insolvency, reorganization, moratorium or similar laws affecting creditors’ rights generally or general equitable principles (regardless of whether enforcement is sought in equity or at law).
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 23 -
Section 4.03 Broker’s Fees.
CHRP has not taken any action that would entitle any Person to any commission or broker’s fee in connection with the transactions contemplated by the Transaction Documents.
Section 4.04 Conflicts.
Neither the execution and delivery of this Agreement or any other Transaction Document nor the performance or consummation of the transactions contemplated hereby or thereby will: (i) contravene, conflict with, result in a breach or violation of, constitute a default under, or accelerate the performance provided by, in any material respects any provisions of: (A) any law, rule or regulation of any Governmental Authority, or any judgment, order, writ, decree, permit or license of any Governmental Authority, to which CHRP or any of its assets or properties may be subject or bound; or (B) any contract, agreement, commitment or instrument to which CHRP is a party or by which CHRP or any of its assets or properties is bound or committed; (ii) contravene, conflict with, result in a breach or violation of, constitute a default under, or accelerate the performance provided by, any provisions of the organizational or constitutional documents of CHRP; or (iii) require any notification to, filing with, or consent of, any Person or Governmental Authority.
Section 4.05 Financial Capacity.
CHRP has and will have at the Closing sufficient funds, from cash and cash equivalents on hand and/or from proceeds of credit facilities available to it, for CHRP to make the Revenue Interest Advance.
ARTICLE V
During the Term, the following covenants shall apply:
Section 5.01 Consents and Waivers.
NeurogesX shall use its commercial reasonable efforts to obtain and maintain any required consents, acknowledgements, certificates or waivers so that the transactions contemplated by this Agreement or any other Transaction Document may be consummated and shall not result in any default or breach or termination of any of the Material Contracts.
Section 5.02 Access; Information.
(a) Promptly after receipt by NeurogesX or any of its Subsidiaries of notice of any action, claim, investigation, proceeding (commenced or threatened in writing), certificate, offer, proposal, material correspondence or other material written communication relating to the transactions contemplated by this Agreement, any other Transaction Document, the Revenue Interest, or any License Agreement relating to the sale of the Licensed Product in the Territory, then, NeurogesX shall inform CHRP of the receipt of such notice and the substance of such action, claim, investigation, proceeding, certificate, offer, proposal, correspondence or other
- 24 -
written communication and shall furnish CHRP with a copy of such notice and any related materials with respect to such action, claim, investigation, proceeding, certificate, offer, proposal, correspondence or other written communication.
(b) NeurogesX shall keep and maintain, or use commercially reasonable efforts (as if it were the sole owner of the Revenue Interest) to cause Astellas or any other Licensee to keep and maintain, at all times full and accurate books of account and records adequate to reflect correctly all payments paid and/or payable with respect to the Revenue Interest as required by the License Agreement. NeurogesX shall provide (or cause the Licensee to provide) to CHRP any Report required under the License Agreement. In the event that Astellas or any other Licensee does not provide copies of such Reports to CHRP, NeurogesX shall provide CHRP with copies of all such Reports received by NeurogesX pursuant to the License Agreement. Upon written request of CHRP, NeurogesX shall audit the books and records of Licensee as provided for and to the extent NeurogesX has the right to do so in Section 10.4.1 of the Existing License Agreement, at the sole cost and expense of NeurogesX; provided, however, that in the event any audit does not reveal the existence of a underpayment by Astellas or any other Licensee of at least five percent (5%) of amounts due for the period audited, the immediate following audit (if any) requested by CHRP pursuant to this Section 5.02(b), which does not reveal the existence of a underpayment by Astellas or any other Licensee of at least five percent (5%) of amounts due for the period audited shall be at CHRP’s sole cost and expense. In addition, within a reasonable time after completion of any audit of Astellas or any other Licensee by NeurogesX in accordance with the License Agreement, NeurogesX shall deliver to CHRP an audit report summarizing the results of such audit.
(c) CHRP and any of CHRP’s representatives shall have the right, from time to time upon prior written notice given in accordance with this Section 5.02(c), to visit NeurogesX’s and its Subsidiaries’ offices and properties where NeurogesX and its Subsidiaries keep and maintain books and records relating or pertaining to the Revenue Interest and the Collateral for purposes of conducting an audit of such books and records with respect thereto, and to inspect, copy and audit such books and records, during normal business hours. Upon ten (10) Business Days’ written notice given by CHRP to NeurogesX, NeurogesX will provide CHRP and any of CHRP’s representatives reasonable access to such books and records, and shall permit CHRP and any of CHRP’s representatives to discuss the business, operations, properties and financial and other condition of NeurogesX with respect to matters relating to the Revenue Interest and the Collateral with NeurogesX’s officers and with its independent certified public accountants (to the extent such independent certified accountants agree to discuss such matters with CHRP).
(d) NeurogesX shall maintain a system of accounting established and administered in accordance with sound business practices to permit preparation of financial statements in accordance with GAAP.
Section 5.03 Material Contracts.
(a) NeurogesX shall use commercially reasonable efforts (as if it was the sole owner of the Revenue Interest) to comply with all terms and conditions of and fulfill all of its obligations under all Material Contracts relating to the Revenue Interest (including any License Agreement). NeurogesX shall not amend any such Material Contract or issue any consents or
- 25 -
other approvals under any such Material Contract in a manner which would materially adversely affect CHRP’s rights hereunder (including, without limitation, the right to receive the Revenue Interest Payments) without the prior written consent of CHRP, which consent shall not be unreasonably withheld, conditioned or delayed. NeurogesX shall use commercially reasonable efforts (as if it was the sole owner of the Revenue Interest) to take all actions necessary to enforce its rights and the rights of CHRP under any such Material Contract (subject to consultation with and reasonable direction and approval of CHRP, which approval shall not be unreasonably withheld, conditioned or delayed) and perform all of its obligations under any such Material Contract. In the event NeurogesX fails to take such action, NeurogesX hereby appoints CHRP and any officer thereof, its agent-in-fact to take all such actions and exercise and enforce all such rights.
(b) In the event any License Agreement is terminated for any reason whatsoever, NeurogesX shall (in consultation and coordination with CHRP) take prompt and commercially reasonable action (as if it were the sole owner of the Revenue Interests) to preserve the market for the Licensed Products in the Territory and commercialize the Licensed Products. NeurogesX shall as promptly as practicable upon or following such termination notify CHRP in writing of all immediate and short term activities it proposes to conduct, and [***] of such termination advise CHRP [***] to (I) [***] provided, however, that NeurogesX has, [***], or (II) [***]; provided, that [***] NeurogesX shall consult with CHRP with respect to the appropriate alternative and the basis therefore, and shall respond to CHRP’s inquires, acting at all times in good faith to [***].
(i) [***] In such case, NeurogesX shall (A) provide to CHRP reports in form, substance and timing identical to the Reports required to be provided by Astellas to NeurogesX under Section 4.6 of the Existing License Agreement, (B) consult and meet regularly with CHRP through establishment of committees which operate similar to the JCC, JDC, JSC and JRC under the Existing License Agreement (except that, to the extent applicable, NeurogesX shall act in the same capacity as Astellas and CHRP shall act in the capacity that NeurogesX had acted under the Existing License Agreement with respect to such matters) and (C) [***]For clarity and without limiting the foregoing, [***] NeurogesX may in consultation with CHRP and with CHRP’s written consent, not to be unreasonably withheld, conditioned or delayed, [***]
(ii) [***] provided that (1) [***] and NeurogesX agrees to undertake in connection with [***]such obligations and liabilities as it has under the [***], as defined in the Existing License Agreement, has not expired prior to the termination of the Existing License Agreement, an option on terms and conditions materially similar to those set forth in Section 2.3 of the Existing License Agreement for any such remaining period of the Option Period had the Existing License Agreement not been terminated), (2) any [***] shall execute a letter [***], and (3) CHRP may, but without any obligation, also seek to locate and secure a [***], and NeurogesX shall in good faith consider candidates proposed by CHRP. If NeurogesX does not secure [***] of termination of the Licensee’s right to commercialize Licensed Product in the Territory (including any Wind-down Period (as defined in the Existing License Agreement)) under the Existing License Agreement (or termination of the right to commercialize Licensed Product in the Territory under any subsequent Replacement License Agreement), CHRP may
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 26 -
(but shall have no obligation to) upon written notice to NeurogesX (A) [***] itself or through an Affiliate on and subject to the provisions of the CHRP Replacement License Agreement or (B) [***]; provided that in such case CHRP shall consult with NeurogesX and keep NeurogesX reasonably informed as to the progress thereof and NeurogesX shall have the right to [***], such approval not to be unreasonably withheld, conditioned or delayed (taking into account the respective interests of the Parties in the Revenue Interest). NeurogesX shall, upon consultation with CHRP, and at CHRP’s request, request and accept assignment of [***] or pursuant to any [***]. If the event NeurogesX fails to take such action, NeurogesX hereby appoints CHRP and any officer thereof, its agent-in-fact to take all such actions and exercise and enforce all such rights.
Section 5.04 Confidentiality; Public Announcement.
(a) All Confidential Information furnished by the Disclosing Party, in connection with this Agreement and any other Transaction Document and the transactions contemplated hereby and thereby shall be kept confidential by the Receiving Party, and shall be used by the Receiving Party only in connection with this Agreement and any other Transaction Document and the transactions contemplated hereby and thereby. Notwithstanding the foregoing, the Receiving Party may disclose such Confidential Information to its existing or potential acquirers, partners, directors, employees, managers, officers, investors, bankers, lenders or other sources of financing, advisors (including, without limitation, financial advisors, attorneys and accountants), trustees, representatives, and other Persons on a need to know basis provided that such Persons shall be informed of the confidential nature of such information and shall be obligated to keep such information confidential pursuant to the terms of this Section 5.04(a) and the Receiving Party shall be responsible for such Person’s failure to comply with such obligations.
(b) Each Party agrees not to disclose to any Third Party the terms and conditions of this Agreement or any other Transaction Document or issue any press release with respect to this Agreement or any other Transaction Document without the prior approval of the other Party, except a Party may disclose the terms and conditions hereof (i) to its existing or potential acquirers, partners, directors, employees, managers, officers, investors, bankers, lenders or other sources of financing, advisors (including, without limitation, financial advisors, attorneys and accountants), trustees, representatives, and other Persons on a need to know basis, provided that such Persons shall be informed of the confidential nature of such information and shall be obligated to keep such information confidential pursuant to the terms of this Section 5.04(b) and the Party disclosing such terms and conditions shall be responsible for such Person’s failure to comply with such obligations. Notwithstanding the foregoing, the Parties agree upon a joint press release to announce the execution of this Agreement, which is attached hereto as Exhibit M; thereafter, either Party may each disclose to Third Parties the information contained in such press release without the need for further approval by the other Party.
(c) In addition, a Party may disclose the Confidential Information of the other Party and the terms and conditions of this Agreement or the other Transaction Documents (i) as necessary to enforce the terms of this Agreement or the other Transaction Documents and (ii) comply with applicable law or the rules of a recognized stock exchange or order of any court, administrative agency or other tribunal of competent jurisdiction, provided, however, that if a Party is required by applicable law, stock exchange or order to make any such disclosure it will
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 27 -
give reasonable advance notice to the other Party of such disclosure requirement and use its reasonable efforts to secure confidential treatment thereof and shall only disclose that portion thereof that, in the opinion of its legal counsel, is required to be disclosed. Further, with respect to any such disclosures made pursuant to applicable securities laws or made to investment or other analysts each Party shall consult with the other Party regarding the form, content and timing of such disclosures, provided that nothing in any Transaction Document shall prevent a Party from fully complying with applicable law or regulation.
(d) This Agreement supersedes the Confidentiality Agreement between the Parties dated August 12, 2009 (the “Prior CDA”) with respect to information disclosed thereunder. All information exchanged between the Parties under the Prior CDA shall be deemed Confidential Information of the Disclosing Party and shall be subject to the terms of this Section 5.04.
(e) Except with respect to CHRP’s internal communications or private communications with its representatives, CHRP shall not, and shall cause its representatives, its Affiliates and its Affiliates’ representatives not to make use of the name, nickname, trademark, logo, service xxxx, trade dress or other name, term, xxxx or symbol identifying or associated with NeurogesX without NeurogesX’s prior written consent to the specific use in question, provided that the consent of NeurogesX shall not be required with respect to publication of NeurogesX’s name and logos in CHRP’s promotional materials, including without limitation the websites for CHRP and its Affiliates consistent with its use of other similarly situated Third Parties’ names and logos.
Section 5.05 Security Agreement.
NeurogesX hereby grants, and shall maintain, at all times during the term of this Agreement, in favor of CHRP a valid, continuing, first priority perfected lien, subject to Permitted Liens, on and security interest in the Revenue Interest, the Intellectual Property and other Collateral described in the Security Agreement in accordance therewith, and NeurogesX shall perform all of its obligations under the Security Agreement. Furthermore, subject to any limitations set forth in the Security Agreement, NeurogesX agrees to perform such actions and execute, file, register, and deliver such documents as reasonably requested by CHRP to perfect the security interest of CHRP in the Revenue Interest, the Intellectual Property and other Collateral described in the Security Agreement.
Section 5.06 Further Assurance.
(a) Subject to the terms and conditions of this Agreement, each of CHRP and NeurogesX shall use its commercially reasonable efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary under applicable laws and regulations to consummate the transactions contemplated by this Agreement and any other Transaction Document. CHRP and NeurogesX agree to execute and deliver such other documents, certificates, agreements and other writings (including any financing statement filings requested by CHRP) and to take such other actions as may be reasonably necessary in order to consummate or implement expeditiously the transactions contemplated by this Agreement and any other Transaction Document and to vest in CHRP good, valid and marketable rights and interests in and to the Revenue Interest free and clear of all Liens, except the Permitted Liens and those
- 28 -
Liens created in favor of CHRP pursuant to the Security Documents and any other Transaction Document and NeurogesX retained interest therein, and to perform the covenants and obligations contained herein.
(b) CHRP and NeurogesX shall execute and deliver such additional documents, certificates and instruments, and to perform such additional acts, as may be reasonably requested and necessary or appropriate to carry out and effectuate all of the provisions of this Agreement and any other Transaction Document and to consummate all of the transactions contemplated by this Agreement and any other Transaction Document.
(c) Except for disputes between the Parties, CHRP and NeurogesX shall cooperate and provide assistance as reasonably requested by the other respective Party in connection with any litigation, arbitration or other proceeding (whether threatened, existing, initiated, or contemplated prior to, on or after the date hereof) to which either Party or any of its officers, directors, shareholders, agents or employees is or may become a Party or is or may become otherwise directly or indirectly affected or as to which any such Persons have a direct or indirect interests, in each case relating to this Agreement, any other Transaction Document, the Revenue Interest or any Collateral, or the transactions described herein or therein.
Section 5.07 Remittance to Initial Concentration Account.
(a) On or before the Closing Date, NeurogesX and CHRP shall enter into a Deposit Agreement, substantially in the form of Exhibit K attached hereto, which will provide for, among other things, the establishment and maintenance of an Initial Concentration Account, a Joint Concentration Account, a NeurogesX Concentration Account and a CHRP Concentration Account in accordance with the terms herein and therein. Any CHRP Concentration Account shall be held solely for the benefit of CHRP, but shall be subject to the terms and conditions of the Transaction Documents. Funds deposited into the Initial Concentration Account shall be treated as provided in the Deposit Agreement. CHRP shall have immediate and full access to and control of any funds held in the CHRP Concentration Account and such funds shall not be subject to any conditions or restrictions whatsoever. After all amounts payable to CHRP under Section 2.02 are swept into the CHRP Concentration Account, as provided in the Deposit Agreement, the amounts remaining in the Joint Concentration Account which are payable to NeurogesX shall then be swept into the NeurogesX Concentration Account on a daily basis. NeurogesX shall have immediate and full access to and control of any funds held in the NeurogesX Concentration Account and such funds shall not be subject to any conditions or restrictions whatsoever other than those of the Depositary Bank. The foregoing shall not (i) affect or reduce NeurogesX’s obligations to pay in full all amounts due to CHRP under this Agreement or (ii) in any manner limit or expand the recourse of CHRP to the assets of NeurogesX to satisfy NeurogesX’s payment obligations hereunder.
(b) NeurogesX shall pay all fees, expenses and charges of the Depository Bank in respect of the Deposit Accounts.
(c) At all times during the Term, NeurogesX shall instruct and use commercially reasonable efforts to cause Licensee to pay directly into the Initial Concentration Account all Revenue Interest, and on or before the Closing Date, NeurogesX shall send the letter attached
- 29 -
hereto as Exhibit H to Licensee. Without in any way limiting the foregoing, commencing on the Closing Date, subject to the provisions of Section 2.02, any and all payments in respect of Revenue Interest received by NeurogesX shall be held in trust for the benefit of CHRP and directed into the Initial Concentration Account within five (5) Business Days of NeurogesX’s receipt thereof and, any and all payments in respect of Excluded Revenue Interests received by CHRP shall be held in trust for the benefit of NeurogesX and remitted to NeurogesX within five (5) Business Days of CHRP’s receipt thereof. Accordingly, from and after the Closing Date NeurogesX shall not establish or maintain any account (as defined in the UCC) other then the Initial Concentration Account for the collection or deposit of any Revenue Interest.
(d) With respect to any License Agreement entered into by NeurogesX during the Term regarding the sale of a Licensed Product in the Territory, NeurogesX shall (i) at the time of the execution and delivery of such License Agreement, instruct the Licensee to remit to the Initial Concentration Account when due all payments of Revenue Interest that are due and payable to NeurogesX during the Term and (ii) in the case of any License Agreement, deliver to CHRP written evidence of such instruction and of such Licensee’s agreement thereto. For purposes of the Existing License Agreement, NeurogesX providing the Licensee the form of Licensee Direction attached hereto as Exhibit H in accordance with the provision of Section 20.5 of the Existing License Agreement and obtaining the consent and agreement of Licensee in accordance with Section 6.02(c) shall be deemed to fulfill the obligation set forth in subclause (i).
(e) The Parties acknowledge that for administrative and recordkeeping purposes, Astellas has requested that NeurogesX provide to Astellas invoice with respect to amounts payable by Astellas to NeurogesX under the Existing License Agreement. [***] All invoices shall reflect that Revenue Interest payments shall be made by Astellas to the Initial Concentration Account. In the event the Parties determine that an amount being paid by Astellas to the Initial Concentration Account is an Excluded Revenue Interest, the Parties shall promptly (and in any event within five (5) Business Days of the issuance of an invoice) mutually establish a written Account Instruction with respect to the Excluded Revenue Interest amount to be paid by Astellas to the Initial Concentration Account and swept into the Joint Concentration Account based on such invoice to implement the payment requirements of Section 2.02 which joint Account Instruction shall be issued to the Depository Bank by CHRP.
(f) Except as otherwise provided in the Deposit Agreement, neither Party hereto shall have any right to terminate the Depositary Bank during the Term with respect to its rights and obligations under the Deposit Agreement without the other Party’s prior written consent. Any such consent, which the other Party may grant or withhold in its discretion, shall be subject to the satisfaction of each of the following conditions to the satisfaction of the other Party:
(i) a successor Depositary Bank shall be appointed by mutual agreement of the Parties;
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 30 -
(ii) CHRP and NeurogesX and the successor Depositary Bank shall have entered into a deposit account control agreement substantially in the form of the Deposit Agreement attached hereto as Exhibit K;
(iii) all funds and items in the accounts subject to the Deposit Agreement to be terminated shall be transferred to the new accounts held at the successor Depositary Bank prior to the termination of the then existing Depositary Bank; and
(iv) CHRP shall have received written evidence that prior to termination Licensee has been instructed to remit all future payments of Revenue Interest to the new accounts held at the successor Depositary Bank.
Section 5.08 Intellectual Property.
(a) To the extent NeurogesX has the right to do so, it shall use commercially reasonable efforts (as if it were the sole owner of the Revenue Interest) to, at its sole expense, either directly or by causing any Licensee or licensor, as applicable to do so, take any and all actions (including taking legal action to specifically enforce the applicable terms of any License Agreement) and prepare, execute, deliver and file any and all agreements, documents or instruments which are necessary or desirable to (i) diligently prosecute and maintain the applicable Intellectual Property (including Patents therein) and (ii) diligently defend or assert such Intellectual Property and such Patents against commercially significant infringement or interference by any other Persons, and against any claims of invalidity or unenforceability, in any jurisdiction (including, without limitation, by bringing any legal action for infringement or defending any counterclaim of invalidity or action of a Third Party for declaratory judgment of non-infringement or non-interference) in the Territory. Consistent with NeurogesX’s rights under any License Agreement and the agreements listed on Schedule 3.12(b), [***] NeurogesX shall not, and shall use its commercially reasonable efforts (as if it were the sole owner of the Revenue Interest) to cause any Licensee or licensor, as applicable, not to, disclaim or abandon, or fail to take any action necessary or desirable to prevent the disclaimer or abandonment of Intellectual Property, in each case that would have a Material Adverse Effect. Subject to the rights of Licensee and parties to any Material Contract, if, in CHRP’s reasonable judgment, NeurogesX does not exercise such commercially reasonable efforts with respect to its rights under the License Agreement relating to the prosecution or maintenance of the Patents or enforcement or defense of the Patents (taking into account the Revenue Interest in the Territory), then CHRP may, in its sole discretion, upon written notice to NeurogesX, assume control of the prosecution and maintenance of the Patents or prosecution or defense of such action or proceeding, at NeurogesX’s sole cost and expense; notwithstanding the foregoing, NeurogesX shall not be required to prosecute and maintain a Patent or prosecute or defend an action or proceeding or reimburse CHRP for any such action or proceeding which (i) a de minimus or insubstantial benefit would be obtained if successful or (ii) the failure to undertake the same would not cause a Material Adverse Effect to occur.
(b) NeurogesX shall use its commercially reasonable efforts (as if it were the sole owner of the Revenue Interest) to prosecute all pending Patent applications within the Intellectual Property in the Territory as listed in Schedule 3.12(a)(1) consistent with standards in the biotechnology industry for similarly situated entities.
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 31 -
(c) NeurogesX shall directly take, or cause Licensee to take, any and all actions and prepare, execute, deliver and file any and all agreements, documents or instruments that are necessary or commercially reasonable or desirable to secure and maintain all Regulatory Approvals consistent with the License Agreement. To the extent that NeurogesX has the right to the control the same under the applicable License Agreement, NeurogesX shall not withdraw or abandon, or fail to take any action necessary to prevent the withdrawal or abandonment of, any Regulatory Approval once obtained without the prior written consent of CHRP, which consent shall not be unreasonably withheld, conditioned or delayed.
Section 5.09 Negative Covenants.
NeurogesX and its Subsidiaries shall not, without the prior written consent of CHRP:
(a) forgive, release, delay, postpone or compromise payment of any Revenue Interest;
(b) waive, amend, cancel or terminate, exercise or fail to exercise, any material rights constituting or relating to any Material Contract, the Revenue Interest or the Collateral, in each case which could have a material adverse effect on the rights of CHRP or otherwise would reasonably be expected to have a Material Adverse Effect;
(c) amend, modify, restate, cancel, supplement, terminate or waive any provision of any Material Contract (including the License Agreement) in the Territory, or grant any consent thereunder, or agree to do any of the foregoing, including, without limitation, entering into any agreement with any Licensee under the provisions of such License Agreement in the Territory, in each case which would have a Material Adverse Effect;
(d) create, incur, assume or suffer to exist any Lien, or exercise any right of rescission, offset, counterclaim or defense, upon or with respect to the Revenue Interest or Collateral, or agree to do or suffer to exist any of the foregoing, except for any Permitted Lien or Lien or agreements in favor of CHRP granted under or pursuant to this Agreement and the other Transaction Documents;
(e) sell, lease, license, transfer or assign (or attempt to do any of the foregoing) all or any portion of the Revenue Interest or, except as set forth in Section 5.03(b), any rights to the Licensed Product in the Territory; or
(f) default under, or take any action or fail to take any action which with the passage of time or the giving of notice or both would constitute a default or event of default under any Material Contract.
Section 5.10 Competing Products.
NeurogesX shall not violate any of its obligations under the Existing License Agreement, any Replacement License Agreement and the CHRP Replacement License Agreement, in each case with respect to Competing Products.
- 32 -
Section 5.11 Notice.
NeurogesX shall provide CHRP with written notice as promptly as practicable (and in any event within five (5) Business Days) after becoming aware of any of the following:
(a) any material breach or default by NeurogesX of any covenant, agreement or other provision of this Agreement or any other Transaction Document;
(b) any express representation or warranty made by NeurogesX in any of the Transaction Documents or in any certificate delivered to CHRP pursuant hereto shall prove to be untrue, inaccurate or incomplete in any material respect;
(c) any sublicense by a Licensee of any rights licensed pursuant to any License Agreement in the Territory;
(d) the occurrence of a Bankruptcy Event with respect to either NeurogesX or Licensee; and
(e) any material breach or default by Licensee under any License Agreement, or the termination of any License Agreement.
ARTICLE VI
THE CLOSING; CONDITIONS TO CLOSING
Section 6.01 Closing.
Subject to the closing conditions set forth in Sections 6.02 and 6.03, the payment of the Revenue Interest Advance and the assignment of the Revenue Interest (the “Closing”) shall take place at the offices of Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx P.C., 000 Xxxxxxxxx Xxxxxx, Xxxxx 0000, Xxx Xxxx, XX 00000 on the Closing Date.
Section 6.02 Conditions Applicable to CHRP to Effect the Closing.
The obligations of CHRP to effect the Closing shall be subject to the satisfaction of the following conditions, as of the Closing Date, any of which may be waived in writing by CHRP in its sole discretion. In the event the conditions set forth below have not been satisfied within fifteen (15) Business Days following the date of this Agreement, then the making of the Revenue Interest Advance shall be deferred until such time as all of such conditions have been satisfied (or waived in writing by CHRP) unless this Agreement is terminated in accordance with Section 7.02(a):
(a) Accuracy of Representations and Warranties. At Closing, the representations and warranties set forth in the Transaction Documents shall be true and correct in all material respects.
(b) No Adverse Circumstances. There shall not have occurred any event or circumstance (including, without limitation, any development with respect to the efficacy of the
- 33 -
Licensed Product or the validity or enforceability of the Intellectual Property) that would reasonably be expected to have a Material Adverse Effect.
(c) Third Party Consents. All notices to, consents, approvals, authorizations and waivers from Third Parties and Governmental Authorities that are required for the consummation of the transactions contemplated by this Agreement or any of the Transaction Documents shall have been obtained or provided for and shall remain in effect. CHRP shall have received the Third Party Consents, in the form of Exhibits G-1, G-2 and G-3 hereto.
(d) CHRP Replacement License Agreement. The CHRP Replacement License Agreement shall have been executed and delivered by NeurogesX, and CHRP shall have received the same and a copy of the CHRP Replacement License Agreement or a memorandum of such agreement or other applicable document shall have been filed with all applicable Patent Offices to the extent necessary to protect CHRP’s interests therein.
(e) Officer’s Certificate. CHRP shall have received a certificate of an executive officer of NeurogesX pursuant to which such officer certifies to such officer’s Knowledge that the conditions set forth in Sections 6.02(a), (b) and (j) have been satisfied in all respects.
(f) Assignment Agreement. The Assignment Agreement shall have been executed and delivered by NeurogesX to CHRP, and CHRP shall have received the same.
(g) Security Agreement. The Security Agreement shall have been duly executed and delivered by the Parties, together with proper financing statements or other documents for filing under the UCC and/or any other applicable law, rule, statute or regulation relating to the perfection of a security interest in filing offices in the jurisdictions listed on Schedule 6.02(g). The Security Agreement shall be in full force and effect. The UCC-1 Financing Statement shall have been duly filed and a first priority security interest, subject to Permitted Liens, in the collateral under the Security Agreement shall have been created and all requisite fees in connection with such filing shall have been paid.
(h) Legal Opinions.
(i) CHRP shall have received an opinion of Xxxxxx Xxxxxxx Xxxxxxxx & Xxxxxx, PC, transaction counsel to NeurogesX, in form and substance set forth in Exhibit E.
(ii) CHRP shall have received an opinion of Xxxxxxxx & Xxxxxxxx LLP, intellectual property counsel to NeurogesX, in form and substance set forth in Exhibit F.
(i) Corporate Documents of NeurogesX. CHRP shall have received a certificate of an executive officer of NeurogesX to the effect set forth in Exhibit I (the statements made in which shall be true and correct on and as of the Closing Date) together with a copy of the incorporation of NeurogesX certified by the Secretary of State of the State of Delaware and a certificate of good standing of NeurogesX issued by the Secretary of State of the State of Delaware, in each case dated not more than twenty (20) days prior to the Closing Date.
- 34 -
(j) Covenants. NeurogesX shall have complied in all material respects with its covenants set forth in the Transaction Documents.
(k) Licensee Direction. A copy of the Licensee Direction shall have been signed and delivered by NeurogesX to CHRP, and CHRP shall have received the same.
Section 6.03 Conditions Applicable to NeurogesX.
The obligations of NeurogesX to effect the Closing shall be subject to the satisfaction of the following conditions, as of the Closing Date, any of which may be waived in writing by NeurogesX in its sole discretion:
(a) Accuracy of Representations and Warranties. The representations and warranties of CHRP set forth in the Transaction Documents shall be true, correct and complete in all material respects.
(b) Officer’s Certificate. NeurogesX shall have received a certificate of an executive officer of CHRP pursuant to which such officer certifies to such officer’s knowledge that the conditions set forth in Section 6.03(a) and (d) have been satisfied in all respects.
(c) Corporate Documents of CHRP. NeurogesX shall have received a certificate of an executive officer of CHRP to the effect set forth in Exhibit J (the statements made in which shall be true and correct on and as of the Closing Date).
(d) Covenants. CHRP shall have complied in all material respects with its covenants set forth in the Transaction Documents.
ARTICLE VII
Section 7.01 Term.
Unless sooner terminated as provided in Section 7.02, the term of this Agreement shall commence on the date hereof and continue in effect until the earlier to occur of (i) the Maturity Date and (ii) the Second Stepdown (the “Term”).
Section 7.02 Termination.
(a) Non-occurrence of the Closing Date. In the event that the Closing does not occur:
(i) on or before [***] after the date of this Agreement and such failure is not a result of a breach of CHRP’s obligations under this Agreement or any of the other Transaction Documents, then CHRP shall have the right to terminate this Agreement upon written notice to NeurogesX referencing this Section 7.02(a), provided that the conditions set forth in Section 6.02 and Section 6.03 have not been fulfilled or waived in accordance therewith prior to the date of such notice;
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 35 -
(ii) on or before [***] after the date of this Agreement and such failure is not a result of a breach of NeurogesX’s obligations under this Agreement or any of the other Transaction Documents, then NeurogesX shall have the right to terminate this Agreement upon written notice to CHRP referencing this Section 7.02(a), provided that the conditions set forth in Section 6.02 and Section 6.03 have not been fulfilled or waived in accordance therewith prior to the date of such notice.
(b) Events of Default. Upon the occurrence of an Event of Default and so long as such Event of Default is continuing, CHRP may upon written notice to NeurogesX (or upon not less than ten (10) Business Days written notice if the Event of Default is the event described in clause (f) in the definition Bankruptcy Event and such event is not cured within [***]referencing this Section 7.02(b) terminate this Agreement and declare the an amount equal to (i) the Revenue Investment Advance to be immediately due and payable by NeurogesX together with interest accrued thereon from the Closing Date to the date of repayment at a rate equal to the lesser of (A) the Rate of Interest or (B) the maximum rate permitted by law, less (ii) Revenue Interest Payments received by CHRP, so that amounts paid to CHRP shall be in accordance with the Amortization Schedule annexed hereto as Exhibit L. For purposes of the foregoing, an “Event of Default” shall mean any of:
(i) A material misrepresentation by or breach by NeurogesX of any covenant or warranty contained herein or in any of the Transaction Documents that would have a Material Adverse Effect, which misrepresentation or breach is not remedied within [***] of NeurogesX’s receipt of notice from CHRP specifying such misrepresentation or breach and referencing this Section 7.02(b); or
(ii) The occurrence of a Bankruptcy Event with respect to NeurogesX.
(c) Buy-Out Rights. At anytime during the Term, NeurogesX shall have the right to terminate this Agreement upon thirty (30) days prior written notice to CHRP referencing this Section 7.02(c) and delivery to CHRP an amount equal to:
(i) If such notice is given in connection with or within [***] the close of a Change of Control, then the greater of (A) $68 million and (B) an amount that would generate the return of the Revenue Investment Advance together with interest accruing at an internal rate of return (utilizing the actual date of each payment and the same methodology utilized by the XIRR function in Microsoft Excel) to CHRP of [***] in respect of the Revenue Investment Advance from the date of the making of the Revenue Investment Advance through the date of payment of the Buy-Out amount, in each case less all Revenue Interest Payments previously received by CHRP; or
(ii) If such notice is not given in connection with or within [***] the close of a Change of Control, then the greater of (A) $76 million and (B) an amount that would generate the return of the Revenue Investment Advance together with interest accruing at an internal rate of return (utilizing the actual date of each payment and the same methodology utilized by the XIRR function in Microsoft Excel) to CHRP of [***] in respect of the Revenue Investment Advance from the date of the making of the Revenue Investment Advance through the date of
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 36 -
payment of the Buy-Out amount, in each case less all Revenue Interest Payments previously received by CHRP.
(iii) For purposes of the foregoing, “Change of Control” shall mean, with respect to NeurogesX, any transaction or series of related transactions that would occasion: (A) any consolidation, merger, share exchange, conversion or other form of corporate reorganization or business combination involving NeurogesX, other than any such consolidation merger, share exchange, conversion or other form of corporate reorganization or business combination which would result in the record and beneficial owners of the voting securities of NeurogesX outstanding immediately prior to such event continuing to own, in substantially the same proportions, voting securities representing (either by remaining outstanding or by being converted into voting securities of the surviving entity or any parent thereof) a majority of the voting power of the voting securities of the surviving or resulting person, (B) a sale, lease, or other transfer of all or substantially all of the assets of NeurogesX to a person or group; (C) any sale, transfer, tender offer or exchange offer for fifty percent (50%) or more of the outstanding voting securities of NeurogesX; or (D) any person or group acting in concert to control NeurogesX having acquired beneficial ownership or the right to acquire beneficial ownership of fifty percent (50%) or more of the outstanding voting securities of NeurogesX. For purposes of the foregoing, a person or group shall not be deemed to have “beneficial ownership” of any shares that any such person or group has the right to acquire, if such right has not been exercised and any required consideration therefore has not been paid and “person” and “group” shall have the meanings given to such terms when used in Sections 13(d) and 14(d) of the United States Securities Exchange Act of 1934.
Section 7.03 Effects of Expiration or Termination.
(a) Accrued Obligations. Expiration or termination of this Agreement for any reason shall not release either Party any obligation or liability which, at the time of such expiration or termination, has already accrued to the other Party or which is attributable to a period prior to such expiration or termination. Accordingly, if any payments are required to be made by a Party to the other Party hereunder after the expiration of the Term, this Agreement shall remain in full force and effect until any and all such payments have been made in full, and solely for that purpose.
(b) Non-exclusive Remedy. Notwithstanding anything herein to the contrary, termination of this Agreement by a Party shall be without prejudice to other remedies such Party may have at law or equity (including any enforcement of its rights under any of the Transaction Documents).
(c) General Survival. ARTICLE 1 and Sections 2.01(b) (last sentence), 2.05, 5.04, 5.06(c), 7.03, 8.03, 8.04, 8.05 (with respect to activities during the Term), 8.06, 8.10, 8.11, 8.13, 8.14, 8.15 and 8.16 shall survive expiration or termination of this Agreement for any reason. Except as otherwise provided in this Section 7.03, all rights and obligations of the Parties under this Agreement shall terminate upon expiration or termination of this Agreement for any reason.
- 37 -
ARTICLE VIII
Section 8.01 Survival.
(a) All representations and warranties made herein and in any other Transaction Document, any certificates or in any other writing delivered pursuant hereto or in connection herewith shall survive the execution and delivery of this Agreement and the Closing until the expiration or termination of this Agreement for any reason.
(b) Any investigation or other examination that may have been made or may be made at any time by or on behalf of the Party to whom representations and warranties are made shall not limit, diminish or in any way affect the representations and warranties in the Transaction Documents, and the Parties may rely on the representations and warranties in the Transaction Documents irrespective of any information obtained by them by any investigation, examination or otherwise.
Section 8.02 Specific Performance.
Each of the Parties hereto acknowledges that the other Party will have no adequate remedy at law if it fails to perform any of its obligations under any of the Transaction Documents. In such event, each Party agrees that the other Party shall have the right, in addition to any other rights it may have (whether at law or in equity), to specific performance of this Agreement.
Section 8.03 Notices.
All notices, consents, waivers and communications hereunder given by any Party to the other shall be in writing (including facsimile transmission and electronic mail) and delivered personally, by facsimile (receipt confirmed), by electronic mail (read receipt confirmed), by a recognized overnight courier, or by dispatching the same by certified or registered mail, return receipt requested, with postage prepaid, in each case addressed:
If to CHRP to:
Xxxxx Healthcare Royalty Partners, L.P.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Attention: [***]
Facsimile No.: [***]
Email: [***]
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 38 -
with a copy to:
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxx, XX 00000
Attention: [***]
Facsimile No.: [***]
Email: [***]
If to NeurogesX or its Subsidiaries, to:
NeurogesX, Inc.
0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000
Xxx Xxxxx, Xxxxxxxxxx 00000
Attention: [***]
Facsimile No.: [***]
Email: [***]
with a copy to:
Xxxxxx Xxxxxxx Xxxxxxxx & Xxxxxx, P.C.
000 Xxxx Xxxx Xxxx
Xxxx Xxxx, Xxxxxxxxxx 00000-0000
Attention: [***]
Facsimile No.: [***]
Email: [***]
or to such other address or addresses as CHRP or NeurogesX may from time to time designate by notice as provided herein, except that notices of changes of address shall be effective only upon receipt. All such notices, consents, waivers and communications shall: (a) when posted by certified or registered mail, postage prepaid, return receipt requested, be effective three (3) Business Days after dispatch, (b) when facsimiled or sent by electronic mail, be effective upon confirmation of receipt, or (c) when delivered by a recognized overnight courier or in person, be effective upon receipt when hand delivered.
Section 8.04 Successors and Assigns.
The provisions of this Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and assigns. NeurogesX shall not be entitled to assign any of its obligations and rights under the Transaction Documents without the prior written consent of CHRP, which consent shall not be unreasonably withheld, conditioned or delayed; provided, however that NeurogesX may, without the consent of CHRP, assign any of its obligations and rights under the Transaction Documents to any other Person with which it may merge or consolidate or to which it may sell all or substantially all of its assets; provided, further, however that no assignment by NeurogesX shall relieve NeurogesX of its obligations hereunder or under any Transaction Document even if such assignment has been consented to by CHRP and such assignee shall be jointly and severally liable with NeurogesX to CHRP. CHRP may
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 39 -
assign without consent of NeurogesX any of its rights under the Transaction Documents without restriction.
Section 8.05 Indemnification.
(a) NeurogesX hereby indemnifies and holds CHRP and its Affiliates and any of their respective partners, directors, managers, members, officers, employees and agents (each a “CHRP Indemnified Party”) harmless from and against any and all Losses (including all Losses in connection with any product liability claims or claims of infringement or misappropriation of any intellectual property rights of any Third Parties to the extent that such Losses are directly or indirectly incurred by a CHRP Indemnified Party) incurred or suffered by any CHRP Indemnified Party as a result of a claim by a Third Party arising out of any breach of any representation, warranty or certification made by NeurogesX in any of the Transaction Documents or certificates given by NeurogesX in writing pursuant hereto or thereto or any breach of or default under any covenant or agreement by NeurogesX pursuant to any Transaction Document, including any failure by NeurogesX to satisfy any of the Excluded Liabilities and Obligations to the extent that any such Losses are not caused by a CHRP Indemnified Party or otherwise subject to indemnification by CHRP pursuant to Section 8.05(b).
(b) CHRP hereby indemnifies and holds NeurogesX, its Affiliates and any of their respective partners, directors, managers, officers, employees and agents (each a “NeurogesX Indemnified Party”) harmless from and against any and all Losses incurred or suffered by a NeurogesX Indemnified Party as a result of a claim by a Third Party arising out of any breach of any representation, warranty or certification made by CHRP in any of the Transaction Documents or certificates given by CHRP in writing pursuant hereto or thereto or any breach of or default under any covenant or agreement by CHRP pursuant to any Transaction Document to the extent that any such Losses are not caused by a NeurogesX Indemnified Party or otherwise subject to indemnification by NeurogesX pursuant to Section 8.05(a).
(c) If any claim, demand, action or proceeding (including any investigation by any Governmental Authority) shall be brought or alleged against an indemnified Party in respect of which indemnity is to be sought against an indemnifying Party pursuant to the preceding paragraphs, the indemnified Party shall, promptly after receipt of notice of the commencement of any such claim, demand, action or proceeding, notify the indemnifying Party in writing of the commencement of such claim, demand, action or proceeding, enclosing a copy of all papers served, if any; provided, that the omission to so notify such indemnifying Party will not relieve the indemnifying Party from any liability that it may have to any indemnified Party under the foregoing provisions of this Section 8.05 unless, and only to the extent that, such omission results in the forfeiture of, or has a material adverse effect on the exercise or prosecution of, substantive rights or defenses by the indemnifying Party. In case any such action is brought against an indemnified Party and such indemnified Party notifies the indemnifying Party of the commencement thereof, the indemnifying Party will be entitled to participate therein and, to the extent that it may wish, jointly with any other indemnifying Party similarly notified, to assume the defense thereof, with counsel reasonably satisfactory to such indemnified Party, and after notice from the indemnifying Party to such indemnified Party of its election so to assume the defense thereof, the indemnifying Party will not be liable to such indemnified Party under this Section 8.05 for any legal or other expenses subsequently incurred by such indemnified Party in
- 40 -
connection with the defense thereof other than reasonable costs of investigation. In any such proceeding, an indemnified Party shall have the right to retain its own counsel, but the reasonable fees and expenses of such counsel shall be at the expense of such indemnified Party unless (i) the indemnifying Party and the indemnified Party shall have mutually agreed to the retention of such counsel, (ii) the indemnifying Party has assumed the defense of such proceeding and has failed within a reasonable time to retain counsel reasonably satisfactory to such indemnified Party or (iii) the named Parties to any such proceeding (including any impleaded Parties) include both the indemnifying Party and the indemnified Party and representation of both Parties by the same counsel would be inappropriate due to actual or potential conflicts of interests between them based on the advice of such counsel. It is agreed that the indemnifying Party shall not, in connection with any proceeding or related proceedings in the same jurisdiction, be liable for the reasonable fees and expenses of more than one separate law firm (in addition to local counsel where necessary) for all such indemnified Parties. The indemnifying Party shall not be liable for any settlement of any proceeding effected without its written consent, but if settled with such consent or if there be a final judgment for the plaintiff, the indemnifying Party agrees to indemnify the indemnified Party from and against any loss or liability by reason of such settlement or judgment. No indemnifying Party shall, without the prior written consent of the indemnified Party, effect any settlement of any pending or threatened proceeding in respect of which any indemnified Party is or could have been a Party and indemnity could have been sought hereunder by such indemnified Party, unless such settlement includes an unconditional release of such indemnified Party from all liability on claims that are the subject matter of such proceeding.
(d) UNLESS CAUSED BY THE BREACH OF Section 5.04(a), THE GROSS NEGLIGENCE, FRAUD, INTENTIONAL OR WILLFUL MISCONDUCT, OR BAD FAITH OF CHRP, IN NO EVENT SHALL CHRP BE LIABLE TO NEUROGESX FOR ANY CONSEQUENTIAL, INCIDENTAL, INDIRECT, SPECIAL OR PUNITIVE DAMAGES, INCLUDING LOSS OF FUTURE REVENUE, INCOME OR PROFITS, DIMINUTION OF VALUE OR LOSS OF BUSINESS REPUTATION OR OPPORTUNITY RELATING TO THE BREACH OR ALLEGED BREACH HEREOF.
(e) UNLESS CAUSED BY THE BREACH OF Section 5.04(a), THE GROSS NEGLIGENCE, FRAUD, INTENTIONAL OR WILLFUL MISCONDUCT, OR BAD FAITH OF NEUROGESX, IN NO EVENT SHALL NEUROGESX BE LIABLE TO CHRP FOR ANY CONSEQUENTIAL, INCIDENTAL, INDIRECT, SPECIAL OR PUNITIVE DAMAGES, INCLUDING LOSS OF FUTURE REVENUE, INCOME OR PROFITS, DIMINUTION OF VALUE OR LOSS OF BUSINESS REPUTATION OR OPPORTUNITY RELATING TO THE BREACH OR ALLEGED BREACH HEREOF WHICH EXCEEDS THE “CAP AMOUNT”.
Section 8.06 Independent Nature of Relationship.
(a) The relationship between NeurogesX and its Subsidiaries, on the one hand, and CHRP, on the other hand, is solely that of lender and a borrower and an assignor and assignee, and neither CHRP, on the one hand, nor NeurogesX or its Subsidiaries, on the other hand, has any fiduciary or other special relationship with the other or any of their respective Affiliates.
- 41 -
(b) No officer or employee of CHRP will be located at the premises of NeurogesX or any of its Affiliates, except in connection with an audit performed pursuant to Section 5.02. No officer, manager or employee of CHRP shall engage in any commercial activity with NeurogesX or any of its Affiliates other than as contemplated herein and in the other Transaction Documents.
Section 8.07 Tax.
(a) For United States federal, state and local tax purposes, NeurogesX and CHRP.[***] Each Party hereto agrees [***] unless (i) the other Party to this Agreement has consented to such actions, or (ii) as a result of a material change in applicable law following the date hereof, counsel for such Party has advised it in writing that it is more likely than not (x) [***] as amended.
(b) NeurogesX shall be entitled to [***]; provided however that if CHRP will not [***]. To the extent such [***].
(c) This Agreement is not intended to create a deemed partnership, association or joint venture between CHRP and NeurogesX. Each Party agrees not to refer to the other as a “partner” or the relationship as a “partnership” or “joint venture”.
Section 8.08 Entire Agreement.
This Agreement, together with the Exhibits and Schedules hereto (which are incorporated herein by reference), and the other Transaction Documents constitute the entire agreement between the Parties with respect to the subject matter hereof and supersede all prior agreements (including the Letter of Intent dated February 18, 2010, between CHRP and NeurogesX), understandings and negotiations, both written and oral, between the Parties with respect to the subject matter of this Agreement, including the Prior CDA. No representation, inducement, promise, understanding, condition or warranty not set forth herein (or in the Exhibits, Schedules or other Transaction Documents) has been made or relied upon by either Party hereto. None of this Agreement, nor any provision hereof, is intended to confer upon any Person other than the Parties hereto any rights or remedies hereunder.
Section 8.09 Amendments; No Waivers.
(a) This Agreement or any term or provision hereof may not be amended, changed or modified except with the written consent of the Parties hereto. No waiver of any right hereunder shall be effective unless such waiver is signed in writing by the Party against whom such waiver is sought to be enforced.
(b) No failure or delay by either Party in exercising any right, power or privilege hereunder shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude any other or further exercise thereof or the exercise of any other right, power or privilege. The rights and remedies herein provided shall be cumulative and not exclusive of any rights or remedies provided by law.
| [***] | Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 42 -
Section 8.10 Interpretation.
When a reference is made in this Agreement to Articles, Sections, Schedules or Exhibits, such reference shall be to an Article, Section, Schedule or Exhibit to this Agreement unless otherwise indicated. The words “include,” “includes” and “including” when used herein shall be deemed in each case to be followed by the words “without limitation”. Neither Party hereto shall be or be deemed to be the drafter of this Agreement for the purposes of construing this Agreement against one Party or the other.
Section 8.11 Headings and Captions.
The headings and captions in this Agreement are for convenience and reference purposes only and shall not be considered a part of or affect the construction or interpretation of any provision of this Agreement.
Section 8.12 Counterparts; Effectiveness.
This Agreement may be executed in two (2) or more counterparts, each of which shall be an original, but all of which together shall constitute one and the same instrument. This Agreement shall become effective when each Party hereto shall have received a counterpart hereof signed by the other Party hereto. Any counterpart may be executed by facsimile or pdf signature and such facsimile or pdf signature shall be deemed an original.
Section 8.13 Severability.
If any provision of this Agreement is held to be invalid or unenforceable, the remaining provisions shall nevertheless be given full force and effect.
Section 8.14 Expenses.
Each Party hereto will pay all of its own fees and expenses in connection with entering into and consummating the transactions contemplated by this Agreement; provided, that NeurogesX agrees to reimburse CHRP for CHRP’s actual, reasonable and documented out-of-pocket expenses to cover due diligence and other, including legal, expenses associated with the transactions contemplated hereby.
Section 8.15 Governing Law; Jurisdiction.
(a) This Agreement shall be governed and construed in accordance with the laws of the State of New York, USA, without giving effect to any choice of law provisions thereof. Each Party hereby submits itself for the purpose of this Agreement and any controversy arising hereunder to the exclusive jurisdiction of the state and federal courts located in the County of New York, State of New York, USA, and any courts of appeal therefrom, and waives any objection on the grounds of lack of jurisdiction (including, without limitation, venue) to the exercise of such jurisdiction over it by any such courts. Prior to bringing a legal action against the other Party (other than an action for injunctive relief, which may be brought at any time), such dispute shall be separately negotiated by the Parties hereto in good faith and all reasonable efforts undertaken to settle amicably such matters before resorting to further legal recourse, as
- 43 -
follows: upon the occurrence of a dispute between the Parties, including, without limitation, any breach of this Agreement or any obligation relating thereto, the matter shall be referred first to the officers of NeurogesX and CHRP having responsibility for the subject matter of the dispute, or their designees. The officers, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner for up to thirty (30) days. If such efforts do not result in mutually satisfactory resolution of the dispute, the matter shall be referred to the chief executive officer of NeurogesX and the managing director of CHRP, or their designees. The chief executive officer and managing director, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner for up to thirty (30) additional days, or such longer period of time to which the chief executive officer and managing director may agree. In the event the dispute has not been resolved at the end of such thirty (30) day period (or such longer period as agreed to by the chief executive officer and managing director of the Parties), either Party shall be entitled to bring an action in accordance with Section 8.15(a) and (b).
(b) Each Party hereto hereby irrevocably consents to the service of process out of any of the courts referred to in subsection (a) above of this Section 8.15 in any such suit, action or proceeding by the mailing of copies thereof by registered or certified mail, postage prepaid, to it at its address set forth in this Agreement. Each Party hereto hereby irrevocably waives any objection to such service of process and further irrevocably waives and agrees not to plead or claim in any suit, action or proceeding commenced hereunder or under any other Transaction Document that service of process was in any way invalid or ineffective. Nothing herein shall affect the right of a Party to serve process on the other Party in any other manner permitted by law. In the event of any litigation under this Section 8.15, the prevailing Party shall be entitled to reimbursement of any reasonable and documented out-of-pocket expenses (including reasonable fees and expenses of legal counsel) incurred by the prevailing Party in connection with asserting or enforcing such action hereunder, including, without limitation, in the case CHRP is the prevailing Party in connection with any Bankruptcy Event with respect to NeurogesX and the non-prevailing Party agrees to reimburse and indemnify the prevailing Party for such expenses.
Section 8.16 Waiver of Jury Trial.
Each Party hereto hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any action, proceeding, claim or counterclaim arising out of or relating to any Transaction Document or the transactions contemplated under any Transaction Document. This waiver shall apply to any subsequent amendments, renewals, supplements or modifications to any Transaction Document.
[SIGNATURE PAGE FOLLOWS]
- 44 -
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be duly executed by their respective authorized officers as of the date first above written.
| COMPANY: | NEUROGESX, INC. | |||
| By: | /s/ Xxxxxxx Xxxxxxxxx | |||
| Name: | Xxxxxxx Xxxxxxxxx | |||
| Title: | EVP, COO, CFO | |||
| CHRP: | XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. | |||
| By | Xxxxx Healthcare Royalty GP, LLC | |||
| Its General Partner | ||||
| By: | /s/ Xxxxxxx X. Xxxxx, M.D. | |||
| Name: | Xxxxxxx X. Xxxxx, M.D. | |||
| Title: | Managing Director | |||
[SIGNATURE PAGE TO FINANCING AGREEMENT]
- 45 -
EXHIBIT A
FORM OF ASSIGNMENT AGREEMENT
- 1 -
EXECUTION COPY
ASSIGNMENT
This ASSIGNMENT (this “Assignment”), dated as of April 29, 2010, is made and entered into by and between NeurogesX Inc., a Delaware corporation (the “Assignor”), and Xxxxx Healthcare Royalty Partners, L.P., a Delaware limited partnership (together with its Affiliates, the “Assignee”). All capitalized terms used and not defined herein shall have the meanings ascribed to them in the Financing Agreement referred to below.
WHEREAS, the Assignor and the Assignee are parties to that certain Financing Agreement, dated even herewith (the “Financing Agreement”), pursuant to which, among other things, the Assignor agrees to assign, transfer and convey to the Assignee, and the Assignee agrees to accept the assignment, transfer and conveyance from the Assignor, all of the Assignor’s right, title and interest in and to the Assigned Rights, as that term is defined in the Financing Agreement, for consideration in the amount and on the terms and conditions provided therein; and
WHEREAS, the parties now desire to carry out the purposes of the Financing Agreement by the execution and delivery of this instrument evidencing the Assignee’s purchase and acceptance of the Revenue Interest and the Assigned Rights.
NOW, THEREFORE, in consideration of the foregoing premises and of other valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto hereby agree as follows:
1. Assignment and Assumption of Assigned Rights. The Assignor hereby assigns, transfers and conveys to the Assignee free and clear of all Liens (except for the Permitted Liens and Liens created in favor of CHRP), and the Assignee hereby accepts, all of the Assignor’s right, title and interest in and to all of the Assigned Rights, subject to Section 2 below. The Assignor hereby represents and warrants to the Assignee that the assignment of the Assigned Rights effected hereby is sufficient to vest in the Assignee a valid ownership interest in all of the Assigned Rights, including, without limitation the Assigned Rights that are “payment intangibles” as defined in the UCC.
2. No Assumption of Obligations. The parties acknowledge that the Assignee is not assuming any debt, liability or obligation of the Assignor, known or unknown, fixed or contingent, in connection with the Assigned Rights, including, without limitation, the Excluded Liabilities and Obligations.
3. Further Assurances. Each party hereto shall execute, acknowledge and deliver to the other party any and all documents or instruments, and shall take any and all actions, reasonably required by such other party from time to time, to confirm or effect the matters set forth herein, or otherwise to carry out the purposes of the Financing Agreement and this Assignment and the transactions contemplated thereby and hereby.
4. Financing Agreement. This Assignment is entered into pursuant to and is subject in all respects to all of the terms, provisions and conditions of the Financing Agreement, and nothing herein shall be deemed to modify any of the representations, warranties, covenants and obligations of the parties thereunder. For clarity, the parties acknowledge that Assignee has
1
certain obligations to assign back rights in and to the Assigned Rights as set forth in Section 2.01(b) of the Financing Agreement.
5. Interpretation. In the event of any conflict or inconsistency between the terms, provisions and conditions of this Assignment and the Financing Agreement, the terms, provisions and conditions of the Financing Agreement shall govern.
6. Counterparts; Effectiveness. This Assignment may be executed in two (2) or more counterparts, each of which shall be an original, but all of which together shall constitute one and the same instrument. This Assignment shall become effective when each party hereto shall have received a counterpart hereof signed by the other party hereto. Any counterpart may be executed by facsimile or pdf signature and such facsimile or pdf signature shall be deemed an original.
7. Governing Law; Jurisdiction; Service of Process; Waiver of Jury Trial.
(a) This Assignment shall be governed and construed in accordance with the laws of the State of New York, USA, without giving effect to any choice of law provisions thereof. Each party hereby submits itself for the purpose of this Assignment and any controversy arising hereunder to the exclusive jurisdiction of the state and federal courts located in the County of New York, State of New York, USA, and any courts of appeal therefrom, and waives any objection on the grounds of lack of jurisdiction (including, without limitation, venue) to the exercise of such jurisdiction over it by any such courts. Prior to bringing a legal action against the other party (other than an action for injunctive relief, which may be brought at any time), such dispute shall be separately negotiated by the parties hereto in good faith and all reasonable efforts undertaken to settle amicably such matters before resorting to further legal recourse, as follows: upon the occurrence of a dispute between the parties, including, without limitation, any breach of this Assignment or any obligation relating thereto, the matter shall be referred first to the officers of NeurogesX and CHRP having responsibility for the subject matter of the dispute, or their designees. The officers, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner for up to thirty (30) days. If such efforts do not result in mutually satisfactory resolution of the dispute, the matter shall be referred to the chief executive officer of NeurogesX and the managing director of CHRP, or their designees. The chief executive officer and managing director, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner for up to thirty (30) additional days, or such longer period of time to which the chief executive officer and managing director may agree. In the event the dispute has not been resolved at the end of such thirty (30) day period (or such longer period as agreed to by the chief executive officer and managing director), either party shall be entitled to bring an action in accordance with Section 7(a) and (b).
(b) Each party hereto hereby irrevocably consents to the service of process out of any of the courts referred to in subsection (a) above of this Section 7 in any such suit, action or proceeding by the mailing of copies thereof by registered or certified mail, postage prepaid, to it at its address set forth in Section 8.03 of the Financing Agreement. Each Party hereto hereby irrevocably waives any objection to such service of process and further irrevocably waives and agrees not to plead or claim in any suit, action or proceeding commenced hereunder or under any
2
other Transaction Document that service of process was in any way invalid or ineffective. Nothing herein shall affect the right of a party to serve process on the other party in any other manner permitted by law. In the event of any litigation under this Section 7, the prevailing party shall be entitled to reimbursement of any reasonable and documented out-of-pocket expenses (including reasonable fees and expenses of legal counsel) incurred by the prevailing party in connection with asserting or enforcing such action hereunder, including, without limitation, in the case CHRP is the prevailing party in connection with any Bankruptcy Event with respect to NeurogesX and the non-prevailing party agrees to reimburse and indemnify the prevailing party for such expenses.
(c) Each party hereto hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any action, proceeding, claim or counterclaim arising out of or relating to this Assignment. This waiver shall apply to any subsequent amendments, renewals, supplements or modifications to this Assignment.
[SIGNATURE PAGE FOLLOWS]
3
IN WITNESS WHEREOF, the Assignor and the Assignee have caused this Assignment to be duly executed by their respective authorized officers as of the date first above written.
| ASSIGNOR:
| ||
| By: |
| |
| Name: | ||
| Title: | ||
| ASSIGNEE: | ||
| XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. | ||
| By Xxxxx Healthcare Royalty GP, LLC Its General Partner | ||
| By: |
| |
| Name: | ||
| Title: | ||
[SIGNATURE PAGE TO ASSIGNMENT]
4
EXHIBIT B
CHRP REPLACEMENT LICENSE AGREEMENT
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXECUTION COPY
DATED April 29, 2010
and
XXXXX HEALTHCARE ROYALTY PARTNERS, L.P.
CHRP
DISTRIBUTION, MARKETING AND LICENSE AGREEMENT
Confidential
DISTRIBUTION, MARKETING AND LICENSE AGREEMENT
This DISTRIBUTION, MARKETING AND LICENSE Agreement (hereinafter “Agreement”), made effective as of the 29th day of April, 2010 (the “Effective Date”), between NeurogesX Inc., a Delaware corporation having a place of business at 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000, Xxx Xxxxx, Xxxxxxxxxx 00000, Xxxxxx Xxxxxx of America (“NGX”) and Xxxxx Healthcare Royalty Partners, L.P., a Delaware limited partnership having a place of business at 000 Xxxxx Xxxxxx, Xxxxx 0000, Xxxxxxxx, XX 00000 (“CHRP”). Each of NGX and CHRP shall be a “Party,” and together the “Parties.”
A. NGX has entered into a Distribution, Marketing and License Agreement, dated June 19, 2009 (the “Astellas License Agreement”), with Astellas Pharma Europe Ltd. (“Astellas”), granting to Astellas (i) an exclusive right to commercialize and distribute the Existing Product during the term thereof, and (ii) an option to obtain the exclusive right to co-develop, commercialize and distribute the Liquid Formulation Product in the Territory, all on the terms and conditions set forth therein.
B. NGX is entering into a Financing Agreement with CHRP, dated the date hereof (the “Financing Agreement”), providing for, among other things, the collateral assignment of certain rights relating to the Patent Rights, NGX Know-How and the Astellas License Agreement, to secure the repayment of the amounts advanced by CHRP under the Financing Agreement and the obligations of NGX to CHRP thereunder (together, the “Financing Agreement Obligations”).
C. In connection with and as a condition to CHRP’s entry into the Financing Agreement, to further secure the payment due and performance of the Financing Agreement Obligations, CHRP desires to obtain from NGX, and NGX desires to grant to CHRP, exclusive rights in the Territory to the Patent Rights and NGX Know-How (as defined below) to manufacture and commercialize the Products, subject, however, to the rights of Astellas under the Astellas License Agreement, so that, in the event the exclusive rights granted to Astellas under the Astellas License Agreement are terminated for any reason or become non-exclusive, with respect to one or more Products in a country or region in the Territory or in their entirety, CHRP may secure in accordance with the provisions of Section 5.03(b) of the Financing Agreement a Sublicensee or otherwise commercialize the Products in the Territory, subject, however, to any and all remaining rights of Astellas.
1
| 1. | DEFINITIONS |
1.1 Capitalized terms used herein which are not otherwise defined herein shall have the meanings ascribed to such terms in the Astellas License Agreement. However, the definitions of the following terms used herein shall have the meanings given to them in the Astellas License Agreement with the following modifications:
1.1.1 The reference in the definition of “Commercially Reasonable Efforts” to “Astellas” shall mean a “Party” as defined in the preamble to this Agreement.
1.1.2 “Exhibit 2.2.1” shall mean the terms of provisions of Exhibit 2.2.1 to the Astellas License Agreement, except that all references to “Astellas” thereunder shall be deemed to refer to “CHRP or its Sublicensee”.
1.1.3 Each reference in the definition of “Liquid Formulation Product” to “Astellas” shall be deemed to refer to “Astellas, CHRP or any Sublicensee”.
1.1.4 “NGX Know-How” shall also include, subject to the provisions of Section 2.6, any rights to Third Party IP granted to Astellas pursuant to Section 2.5 of the Astellas License Agreement.
1.1.5 “Patent Rights” shall also include (i) subject to the provisions of Section 2.6, any rights to Third Party IP granted to Astellas pursuant to Section 2.5 of the Astellas License Agreement and (ii) patent rights within the Astellas Improvements in the Territory granted to NGX pursuant to Section 11.1.4 of the Astellas License Agreement and (iii) patent rights comprising the NGX Improvements.
1.1.6 “Product(s)” shall include (i) Existing Products, (ii) the Liquid Formulation Product if Astellas exercised its Option with respect thereto in accordance with Section 2.3 of the Astellas License Agreement or if CHRP exercises the LFP Option as described in Section 2.4 herein below and (iii) any Competitive Products licensed to Astellas.
1.2 “Deposit Agreement” shall have the meaning set forth in the Financing Agreement.
1.3 “Exploit” and “Exploitation” shall have the meaning set forth in the Financing Agreement.
1.4 “Replacement License Agreement” and “Replacement Licensee” shall have the meanings set forth in the Financing Agreement.
1.5 “Revenue Interest” shall have the meaning set forth in the Financing Agreement.
1.6 “Revenue Interest Advance” shall have the meaning set forth in the Financing Agreement.
2
1.7 “Revenue Interest Payments” shall have the meaning set forth in the Financing Agreement.
1.8 “Security Agreement” shall have the meaning set forth in the Financing Agreement.
1.9 “Subdistributor” shall mean a Third Party appointed by a Sublicensee as a distributor of a Product, in accordance with Section 2.5.2, which Third Party purchases Product in the Territory from such Sublicensee and resells the Product in its respective territory. Notwithstanding the foregoing, if a Sublicensee grants the rights to market and promote a Product in a country to one entity and sells the Product to another entity in the same country for distribution and resale then both entities shall be considered to be Subdistributors. For the avoidance of doubt, “Subdistributor” shall specifically exclude Sublicensees.
1.10 “Sublicensee” shall mean any Replacement Licensee to whom CHRP has granted, pursuant to Section 2.5, a sublicense under the Patent Rights and/or NGX Know-How to Manufacture, package and have packaged, develop, register, keep, import, offer for sale, sell, have sold, use, market, distribute and/or promote a Product in any country or countries in the Territory.
1.11 “Third Party” shall mean any entity other than CHRP, NGX and their Affiliates.
1.12 “Transaction Documents” shall have the meaning set forth in the Financing Agreement.
1.13 References. Unless otherwise specified herein references to any Section, Article or Exhibit shall mean the Sections, Articles and Exhibits to this Agreement.
| 2. | GRANT |
2.1 Certain Limitations. IT IS EXPRESSLY ACKNOWLEDGED AND AGREED BY CHRP THAT NOTWITHSTANDING ANYTHING HEREIN TO THE CONTRARY ALL OF THE RIGHTS AND LICENSES OF CHRP AND ANY SUBLICENSEE SHALL BE SUBJECT AND SUBORDINATE TO THE RIGHTS OF ASTELLAS UNDER THE ASTELLAS LICENSE AGREEMENT, AND CHRP ACKNOWLEDGES AND AGREES THAT TO THE EXTENT THE ASTELLAS LICENSE AGREEMENT IS IN EFFECT, CHRP SHALL NOT TAKE ANY ACTION WHICH IS INCONSISTENT WITH THE RIGHTS AND LICENSES OF ASTELLAS UNDER THE ASTELLAS LICENSE AGREEMENT. FOR FURTHER CLARITY, CHRP ACKNOWLEDGES THAT NGX HAS PREVIOUSLY GRANTED EXCLUSIVE RIGHTS AND LICENSES TO ASTELLAS WITH RESPECT TO THE TERRITORY AS SET FORTH IN THE ASTELLAS LICENSE AGREEMENT AND TO THE EXTENT THAT SUCH RIGHTS OR LICENSES ARE CONVERTED TO NON-EXCLUSIVE OR THAT ONE OR MORE COUNTRY(IES) IS REMOVED FROM THE TERRITORY IN ACCORDANCE WITH THE TERMS OF THE ASTELLAS LICENSE AGREEMENT, THEN THE RIGHTS AND LICENSES GRANTED TO CHRP SHALL BE LIMITED TO THE EXTENT THAT NGX HAS THE RIGHT TO GRANT THE SAME WITHOUT BEING IN BREACH OF THE RIGHTS AND LICENSES GRANTED TO
3
ASTELLAS PURSUANT TO THE ASTELLAS LICENSE AGREEMENT. CHRP FURTHER ACKNOWLEDGES AND AGREES IT SHALL ONLY EXERCISE THE RIGHTS AND LICENSES GRANTED TO IT HEREIN CONSISTENT WITH THE PROVISIONS OF SECTION 5.03(b) OF THE FINANCING AGREEMENT. CHRP FURTHER ACKNOWLEDGES AND AGREES THAT NOTWITHSTANDING ANYTHING HEREIN TO THE CONTRARY NGX’S OBLIGATIONS HEREUNDER ARE (A) SUBJECT TO AND SUBORDINATE TO NGX’S OBLIGATIONS UNDER THE ASTELLAS LICENSE AGREEMENT AND NOTHING HEREIN SHALL BE DEEMED TO REQUIRE NGX TO PERFORM OR FOREBEAR ANY ACT THAT IS IN CONTRAVENTION WITH OR WOULD OTHERWISE REQUIRE NGX TO BE IN BREACH OF THE ASTELLAS LICENSE AGREEMENT AND (B) SUBJECT TO THE TERMS OF SECTION 5.03(b) OF THE FINANCING AGREEMENT
2.2 Appointment. Subject to the terms of this Agreement and the rights of Astellas under the Astellas License Agreement, NGX hereby grants to CHRP the right (even as to NGX and its Affiliates) to enter into one or more Replacement License Agreements in accordance with the provisions of Section 5.03(b) of the Financing Agreement and to sublicense to the applicable Sublicensee the right to distribute and market the Products in the Field in the Territory as set forth in this Agreement below. CHRP shall be responsible for the acts and omissions of any its Affiliates hereunder as if such acts and omissions were those of CHRP.
2.3 Licenses
2.3.1 Subject to the terms and conditions of this Agreement and the rights of Astellas under the Astellas License Agreement, NGX grants to CHRP:
(a) an exclusive (even as to NGX and its Affiliates), irrevocable, royalty-free (except as otherwise provided in Sections 2.5 and 4), sublicensable (in accordance with Section 2.5) license during the term of this Agreement under the Patent Rights and NGX Know-How to: (i) register (including conducting such clinical trials as may be required to support such registrations) the Existing Product (ii) import, keep, package and have packaged the Components supplied by NGX, and (iii) promote, market, offer for sale, sell, have sold, import and otherwise distribute and use the Existing Product in the Territory for any and all indications in the Field;
(b) an irrevocable, royalty-free, sublicensable (in accordance with Section 2.5) license (co-exclusive with NGX and its designees) during the term of this Agreement under the Patent Rights and NGX Know-How to Manufacture Components for the exclusive use in the Territory and Field; and
(c) in the event (I) Astellas exercises the Option in accordance with the Astellas License Agreement or (II) CHRP exercises the LFP Option in accordance with this Agreement, (i) an exclusive (even as to NGX and its Affiliates), irrevocable, royalty-free (except as otherwise provided in Sections 2.5 and 4), sublicensable (in accordance with Section 2.5) license during the term of this Agreement under the Patent Rights and NGX Know-How to (A) co-develop (together with NGX), register (including conducting such clinical trials as may be required to support such registrations), promote, market, sell, have sold, keep, import and
4
otherwise distribute and use the Liquid Formulation Product in the Territory for any and all indications in the Field; (B) make, have made, package and have packaged the Liquid Formulation Product for the exclusive use in the Territory and Field provided that NGX and CHRP shall use (or CHRP shall cause its Sublicensees to use) commercially reasonable endeavors to use the same manufacturer(s) to supply the Liquid Formulation Product for both inside and outside the Territory; and (ii) the right to (X) carry out marketing activities outside the Territory, in cooperation with NGX, for the promotion of sales of Liquid Formulation Product (by CHRP or its Affiliates, any Sublicensee or Subdistributor) in the Territory strictly in accordance with the license and terms of Section 1.2 of Exhibit 2.2.1 and (Y) carry out clinical trials outside the Territory, in cooperation with NGX, for the development (by CHRP or its Affiliates, or any Sublicensee) of Liquid Formulation Product which is to be promoted, marketed and sold in the Territory, strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1. For clarity, NGX shall retain the right to (x) carry out marketing activities and clinical trials in the Territory, in cooperation with the applicable Sublicensee, for the promotion of sales of Liquid Formulation Product (by NGX, any NGX Affiliate or their licensees) outside of the Territory strictly in accordance with the license and terms set forth in Section 1.3 of Exhibit 2.2.1 and (y) carry out clinical trials inside the Territory, in cooperation with the applicable Sublicensee, for the development by NGX of Liquid Formulation Product which is to be promoted, marketed and sold outside the Territory, strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1.
Notwithstanding the foregoing, it is understood that the [***] has been obtained.
NGX reserves all rights not expressly granted herein. Additionally it is understood and agreed that, subject to the rights of Astellas under the Astellas License Agreement, CHRP shall have the right to: (x) carry out marketing activities outside the Territory, in cooperation with NGX, for the promotion (by CHRP, its Affiliates, Sublicensees or Subdistributors) of sales of Products in the Territory strictly in accordance with the license and terms set forth in Section 1.2 of Exhibit 2.2.1; and (y) carry out clinical trials outside the Territory, in cooperation with NGX, for the development (by CHRP, its Affiliates, or any Sublicensee) of Products which is to be promoted, marketed and sold in the Territory, strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1.
2.3.2 NGX shall not, and shall not license or authorize any Affiliate or Third Party to develop (save in accordance with this Agreement, Section 5.03(b) of the Financing Agreement and the Astellas License Agreement), register, promote, market, offer for sale, sell, have sold, import and otherwise distribute or sell:
(a) Components and/or Existing Product within the Field and Territory during the term of this Agreement; or
(b) Liquid Formulation Product within the Field and Territory during the LFP Option Period (if any) and thereafter during the remainder of the term of this Agreement only if Astellas exercises the Option in accordance with the Astellas License Agreement or CHRP exercises the LFP Option in accordance with this Agreement;
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
5
provided that notwithstanding the foregoing, NGX shall have the right to (x) carry out marketing activities in the Territory, in cooperation with CHRP, its Affiliate or Sublicensee, as applicable, for the promotion of sales of Existing Product and Liquid Formulation Product (by NGX, any NGX Affiliate or their licensees) outside of the Territory strictly in accordance with the license and terms set forth in Section 1.3 of Exhibit 2.2.1 and (y) carry out clinical trials inside the Territory, in cooperation with CHRP, its Affiliate or Sublicensee, as applicable, for the development by NGX of Existing Product which is to be promoted, marketed and sold outside the Territory, strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1. For clarity, nothing herein is intended to prevent NGX from performing or having others perform any preclinical or non-clinical tests or development with respect to any Product in the Field in the Territory.
2.4 LFP Option
2.4.1 Pursuant to the Astellas License Agreement, NGX has granted to Astellas an exclusive option to include the Liquid Formulation Products as a Product under the Astellas License Agreement on the terms and conditions as set forth therein. Subject to the rights of Astellas under the Astellas License Agreement, NGX hereby grants to CHRP an exclusive option, to include the Liquid Formulation Product as a Product under this Agreement (the “LFP Option”) on the terms and conditions set forth in this Section 2.4 below.
2.4.2 For clarity, in the event Astellas exercises the Option (as such term is defined in the Astellas License Agreement), and the Liquid Formulation Product becomes a “Product” (as term is defined in the Astellas License Agreement), the Liquid Formulation Product shall be licensed to CHRP hereunder in accordance with Section 2.3.1(c), subject to the rights of Astellas under the Astellas License Agreement.
2.4.3 In the event, prior to the termination of the Astellas License Agreement, the Option Period has expired or otherwise terminated without Astellas having exercised its rights, in accordance with the terms of the Astellas License Agreement, to include Liquid Formulation Products as a Product under the Astellas License Agreement, the LFP Option shall terminate and CHRP shall have no rights to any Liquid Formulation Product.
2.4.4 In the event, upon termination of the Astellas License Agreement, the Option Period shall be continuing (but for termination of the Astellas License Agreement), CHRP shall be entitled to exercise the LFP Option on the same terms and conditions of the Option granted to Astellas under the Astellas License Agreement (including Sections 2.3.4 – 2.3.16 and Sections 3.3.2 and 3.3.3 of the Astellas License Agreement) all of which terms are hereby incorporated by reference, and, in such case, NGX shall perform all of its obligations with respect to the Liquid Formulation Product so incorporated for the benefit of CHRP.
2.4.5 For clarity, CHRP shall have no right to exercise the LFP Option prior to termination of the Astellas License Agreement, it being acknowledged and agreed that so long as the Astellas License Agreement has not been terminated or expired on its terms, the rights of CHRP to exercise the LFP Option are expressly subordinated to the rights of Astellas.
6
2.5 Sublicensees. Subject to the rights of Astellas under the Astellas License Agreement, CHRP shall have the right, upon the approval of NGX to grant sublicenses to Sublicensees under the Patent Rights and/or NGX Know-How to Manufacture, package and have packaged, develop, register, keep, import, offer for sale, sell, have sold, use, market, distribute and/or promote any Product in any country or countries in the Territory, provided that NGX shall not withhold or delay any such approval for economic reasons (i) prior to such time as CHRP has received Revenue Interest Payments under the Financing Agreement in an amount equal to 1.75 times the Revenue Investment Advance, provided, however, that CHRP shall act in good faith to maximize the Revenue Interest (both during the term of this Agreement and thereafter) derived from such sublicense; (ii) after such time as CHRP has received Revenue Interest Payments under the Financing Agreement in an amount equal to [***] the Revenue Investment Advance, if the economics in consideration with such sublicense are reasonable taking into consideration all relevant factors including CHRP’s and NGX’s respective interest in the Revenue Interest generated therefrom; provided that in all events each Sublicensee shall be responsible for and reimburse NGX for any and all amounts due by NGX to Third Parties in consideration of any rights to the NGX Know-How or Patent Rights as a result of the grant to or exercise of by or under authority of such Sublicensee (including amounts due by NGX to LTS under the LTS Agreement and UC under the UC License Agreement) and (iii) any other obligations of NGX thereunder is no more onerous on NGX and materially similar to those set forth in the Astellas License Agreement with respect to Astellas. For clarity, unless NGX otherwise agrees (such agreement not to be unreasonably withheld or delayed) any such sublicense shall (a) be pursuant to a written agreement (each, a “Sublicense Agreement”) that includes the provisions materially similar to the provisions of Sections 2.6 (No Modifications to the Product), 2.7.1 – 2.7.3 (Competitive Products; Astellas, for the Exclusivity Period and scope described therein), 2.7.8 (Ex-Territory; No Exploitation except as Licensed), 4.3 (Extraterritorial DPR), 4.4 (Existing NGX Third Party Royalties), 4.5 (Discounting), 4.6 (Royalty Reports), 5.1 (with references to “JSC” meaning the JSC as established by NGX and CHRP hereunder and references to “Existing Product” replaced by “each Product”), 8 (Commercialization), 10 (Payments; Book and Records), 11 (Intellectual Property), 12 (Trademarks), 13 (Confidentiality), 15 (Liability), 16 (Indemnification), 17 (Term and Termination), 18 (Effect of Expiration or Termination), 19 (Dispute Resolution) and 20 (Miscellaneous) of the Astellas License Agreement with the applicable Sublicensee in place of Astellas in such provisions, (b) include a provision materially similar to Section 2.3.2 hereof with the applicable Sublicensee in place of CHRP, (c) otherwise consistent with the terms and conditions of this Agreement, including the assignment to NGX upon expiration hereof as provided in Section 2.5.3 below and (d) NGX shall be either a (I) signatory thereto or (II) a named and intended third party beneficiary thereof, in either case with the right to enforce the terms and conditions thereof in consultation with CHRP.
2.5.1 Economic Considerations. NGX and CHRP shall treat all amounts received by CHRP or NGX or their Affiliates in connection with any Sublicense Agreement in the same manner as payments received under the Astellas License Agreement, namely any Revenue Interest shall be shared by and paid to the Parties as set forth in Section 2.02 of the Financing Agreement and any Excluded Revenue Interests (as defined in the Financing Agreement) shall inure to the benefit of and paid to NGX.
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
7
2.5.2 Subdistributors. Subject to the rights of Astellas under the Astellas License Agreement, Sublicensees shall have the right, upon the approval of NGX, not to be unreasonably withheld or delayed, to appoint Third Parties as Subdistributors to distribute, sell, market and promote the Product in the Territory, provided that (i) each such appointment be made pursuant to a written agreement consistent with the terms and conditions hereof and the Sublicense Agreement (each, a “Subdistributor Agreement”) and (ii) the applicable Sublicensee shall promptly notify NGX of the execution of each Subdistributor Agreement and provide a copy thereof to NGX. The Sublicensee appointing any such Subdistributor shall be responsible for the failure by each such Subdistributor to comply with, and shall guarantee the compliance by each of its Subdistributor with, all relevant restrictions, limitations and obligations in this Agreement and the applicable Sublicense Agreement.
2.5.3 Upon Expiration. Upon the expiration of this Agreement, CHRP shall, without further consideration, assign its rights under any and all Sublicense Agreements to NGX.
2.6 NGX IP Acquired after the Effective Date. Section 2.5 of the Astellas License Agreement is hereby incorporated by reference as if set forth herein in full, with all references thereunder to Astellas being deemed to refer to CHRP, provided however, (i) CHRP’s rights thereunder are subject and subordinate to the rights of Astellas; (ii) except as provided in Section 4,[***]; (iii) to the extent [***]as described above; and (iv) except as provided in Section 4, CHRP shall have[***], its [***]being to include such an[***].
2.7 No Conflict
2.7.1 Competitive Products: NGX. Subject to Sections 2.7.2 - 2.7.4, NGX and its Affiliates shall not, by themselves or through any Third Party(ies) conduct any sales, marketing, promotion or distribution activities in the Territory[***].
2.7.2 NGX shall not be in breach of Section 2.7.1 if, during the Exclusivity Period, NGX is acquired by any Third Party (whether by purchase, merger or otherwise), which Third Party at the date of such acquisition was commercializing a Competitive Product or has a Competitive Product in development beyond the commencement of Phase III clinical trials and following such acquisition NGX and/or the new entity commercializes such Competitive Product during the Exclusivity Period. For the avoidance of doubt, if the Third Party has a Competitive Product in development at an earlier stage than Phase III clinical trials neither NGX nor the new entity can commercialize such Competitive Product during the Exclusivity Period.
2.7.3 NGX shall not be in breach of Section 2.7.1 if NGX acquires (whether by purchase, merger or otherwise) any Third Party which Third Party at the date of its acquisition by NGX was commercializing a Competitive Product provided that NGX disposes of such Competitive Product within a period of [***] following the date that such acquisition is completed, and the terms of such divestiture provides that NGX (or its Affiliates) no longer retain any rights to receive any future royalties or other payments based on the sales of such Competitive Product.
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
8
2.7.4 License in Respect of Competitive Products Licensed to Astellas. For clarity if, at any time during the term of this Agreement, [***], (i) such [***]shall be [***]and (ii) the[***], but in all cases shall be limited to the Territory.
2.7.5 License in Respect of Competitive Products After Termination of the Astellas License Agreement. If, at any time after the termination of the Astellas License Agreement and prior to the end of the Exclusivity Period, NGX wishes to out-license the development and commercialization of a[***]:
(a) when NGX decides that it wishes to[***], it will notify CHRP in writing and provide CHRP with a description of [***]of the general type and quantity that NGX would typically provide to its potential development partners, to enable CHRP to make an[***];
(b) within thirty (30) days (or such other longer period as the Parties may agree) of receipt of the notice and dossier in Section 2.7.5(a) above (the “[***]”), CHRP will inform NGX in writing whether it wishes to enter into an exclusive license for the[***];
(c) If CHRP notifies NGX that it wishes to exercise the [***]in relation to [***]that is the subject of the notice in Section 2.7.5(a), then the Parties will enter into negotiations to[***]. In such negotiations the Parties will act reasonably, diligently and in good faith to achieve an agreement which is acceptable to both Parties. Such diligence shall be consistent with the aim of achieving[***]; and
(d) If CHRP notifies NGX pursuant to Section 2.7.5(b) that it does not wish [***]that is the subject of the notice in Section 2.7.5(a), or CHRP does not respond within the period in Section 2.7.5(b) above, or a final agreement in accordance with Section 2.7.5(c) is not achieved in such four (4) month period then NGX will have no further obligations to CHRP under this Section 2.7.5 and shall be[***]. Without limiting the foregoing, the Parties understand that the revenues received by NGX from NGX’s license of such [***]respect to the Territory or such Third Party’s sale or other commercialization thereof in the Territory shall be shared by the Parties in accordance with Section 2.02 of the Financing Agreement.
2.7.6 Ex-Territory; No Exploitation Except as Licensed. Except as otherwise permitted under Sections 1.2 and 3 of Exhibit 2.2.1 or required by applicable law, neither CHRP nor its Affiliates will develop, file for marketing approval with respect to, use, market, import, export, distribute, promote or sell Product anywhere in the world, except in the Territory, and, within the Territory, only in accordance with and under this Agreement. CHRP agrees that it and its Affiliates shall not use nor otherwise exploit Patent Rights, Data and the Product Trademark, except as licensed in this Agreement.
| 3. | INTENTIONALLY LEFT BLANK |
| 4. | DIRECT COMMERCIALIZATION BY CHRP OR ITS AFFILIATES |
Notwithstanding anything herein to the contrary, in the event CHRP (itself or through one or more Affiliates) elects to directly markets, sells or otherwise commercializes any Product in the Territory hereunder as set forth in, and consistent with, Section 5.03(b) of the Financing Agreement, the following shall apply:
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
9
4.1 Incorporation by Reference. The following provisions of the Astellas License Agreement are hereby incorporated by reference as if set forth herein in full except, in each case “Astellas” being replaced by “CHRP”): Sections 2.5 (NGX IP Acquired after the Effective Date; with respect to payment that would have been due by Astellas thereunder had the Astellas License Agreement not been terminated), 2.6 (No Modifications to the Product), 2.7.1 – 2.7.3 (Competitive Products; Astellas, for the Exclusivity Period and scope described therein), 2.7.8 (Ex-Territory; No Exploitation except as Licensed), 4.3 (Extraterritorial DPR), 4.4 (Existing NGX Third Party Royalties), 4.5 (Discounting), 4.6 (Royalty Reports), 5.1 (with references to “JSC” meaning the JSC as established by NGX and CHRP hereunder and references to “Existing Product” replaced by “each Product”), and 10 (Payments; Book and Records) of the Astellas License Agreement.
4.2 Economics. During the term hereof, an amount equal to the royalty that would have been due to NGX under Section 4.1 of the Astellas License Agreement (had Astellas made the sale of such Product) with respect to all Net Sales of all Products by or under authority of CHRP and its Affiliates (the “Royalty Equivalent Amount”) shall be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement and paid into the Initial Concentration Account as required by Section 5.07 of the Financing Agreement and the Deposit Agreement.
| 5. | COOPERATION |
5.1 In the event the Astellas License Agreement is terminated for any reason, NGX and CHRP shall each use commercially reasonable efforts and cooperate with one another as set forth in Section 5.03(b) of the Financing Agreement. If NGX (i) [***] or (ii) [***] to (a) the rights of NGX to so develop and commercialize the Products in the Territory itself or (b) the rights and licenses of the applicable [***]in the same manner such rights, licenses and obligations are subject and subordinated to the rights of Astellas under and otherwise to the Astellas License Agreement as described in Section 2.1.
5.2 If (i) the Astellas License Agreement is terminated and (ii) NGX does not elect to develop and commercialize (including applicable promotion, marketing and sales) the Products in the Territory itself pursuant to the provisions of Section 5.03(b) of the Financing Agreement, the provisions of Sections 5.2, 5.3, 5.4, 5.6 and 5.7 of the Astellas License Agreement are incorporated herein by reference as if fully set forth herein, except that CHRP (or upon its selection, a Sublicensee) shall have all the rights which are granted to Astellas under such sections of the Astellas License Agreement.
| 6. | TRANSFER OF THE MARKETING AUTHORIZATION |
6.1 MAA Approval Transfer. To the extent that any MAA or MAA Approval with respect to a Product in any country in the Territory is acquired by NGX (whether pursuant to assignment with respect to a Terminated Product pursuant to Sections 18.3.2, 18.5.2 or 18.6 of the Astellas License Agreement or otherwise) NGX shall hold such MAA or MAA Approval in trust on behalf of CHRP, and use commercially reasonable efforts to maintain, renew or obtain final approval of such MAA or MAA Approval, as applicable, and each such MAA or MAA Approval free and clear of all liens, claims or encumbrances, in each case in coordination with CHRP and in accordance with CHRP’s instructions; provided, however, NGX shall not have any
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
10
obligation to perform any clinical development or otherwise incur any material costs or expenses (other than filing or maintenance fees) unless CHRP agrees to reimburse NGX with respect to all costs and expenses therefor. After the termination of the Astellas License Agreement and upon CHRP’s written request reasonably in connection with CHRP’s protection of its collateral interest in and to the XXXx or MAA Approvals under the Security Agreement, NGX shall transfer and assign, and provide all reasonable assistance requested by CHRP to receive, the benefit of and interest in any MAA or MAA Approval to CHRP free from all encumbrances (other than those encumbrances created by this Agreement) on the terms of and in accordance with this Section 6 and in such case the cost of such transfer shall be at the expense of NGX. From and after such time as CHRP has elected to (i) take the lead in locating and securing a Replacement Licensee or (ii) Exploit the Products itself or through an Affiliate, in either case in accordance with Section 5.03(b) of the Financing Agreement, then upon CHRP’s request NGX shall transfer and assign, and provide all reasonable assistance requested by CHRP or its designated Sublicensee to receive, the benefit of and interest in any MAA or MAA Approval to CHRP, its Affiliate or its designated Sublicensee, free from all encumbrances (other than those encumbrances created by this Agreement) on the terms of and in accordance with this Section 6 (and at all times until transfer, and to the extent not so transferable or assignable, NGX shall make all such regulatory filings, XXXx and MAA Approvals available to CHRP, its Affiliate or its Sublicensees) and in such case the cost of such transfer shall initially be borne by NGX, provided, however, (i) such amount shall be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement if paid on behalf of CHRP or its Affiliates, or (ii) if paid by NGX on behalf of a Sublicensee, such amount shall be reimbursed to NGX by the Sublicensee or CHRP.
6.2 Parties’ Obligations. CHRP shall be solely responsible for preparing and submitting all notices, applications, submissions, reports and other instruments, documents, correspondence or filings necessary to obtain a transfer to CHRP, its Affiliate or its designated Sublicensee of any such regulatory filings, XXXx and MAA Approvals in accordance with Section 6.1. In connection therewith, NGX shall or shall procure that any of its Affiliates will:
6.2.1 as soon as reasonably possible after the transfer of an MAA or MAA Approval sign any document presented to it by CHRP, its Affiliate or its designated Sublicensee that are necessary for: (i) the transfer to CHRP, its Affiliate or its designated Sublicensee of such MAA or MAA Approval, as applicable; or (ii) maintaining, renewing or varying the MAA or MAA Approval;
6.2.2 as soon as reasonably possible provide notice of its consent to the transfer of the MAA or MAA Approval, as applicable, to CHRP, its Affiliate or its designated Sublicensee if required by any applicable governmental or regulatory authority; and
6.2.3 as soon as reasonably possible conduct all communications with the EMEA relating to the transfer of the MAA or MAA Approval, as applicable, at the direction of CHRP, its Affiliate or its designated Sublicensee.
6.3 Prior to MAA Approval Transfer. So long as NGX is holding a MAA or MAA Approval in trust for CHRP as required in Section 6.2, NGX shall not encumber, amend, cancel or surrender the Existing Product MAA Approval and other MAA Approval, as applicable, or
11
take any step in relation thereto unless requested to do so by CHRP or any applicable governmental or regulatory authority (any such request of a governmental or regulatory authority a “Regulatory Request”). Before taking any such step pursuant to a Regulatory Request, NGX shall act in accordance with any reasonable instruction given by CHRP in relation to such Regulatory Request unless action is required to be taken urgently by the relevant governmental or regulatory authority, in which case NGX shall inform CHRP of such action as soon as possible thereafter.
6.4 After MAA Approval Transfer. Following transfer to CHRP, its Affiliate or its designated Sublicensee of an MAA Approval, CHRP, its Affiliate or its designated Sublicensee, as the case may be shall be responsible for the Maintenance of the such MAA Approval, as well as for the filing and Maintenance of all additional XXXx filed and MAA Approvals obtained in its name in the Territory, in each case at its own cost, provided that NGX shall, upon CHRP’s request, provide to CHRP reasonable assistance in relation with such Maintenance, as well as all electronic documents in its possession and under its Control that are reasonably necessary to perform such Maintenance, including without limitation the relevant dossier for the MAA or MAA Approval in the format of an electronic common technical document (eCTD). NGX shall also within thirty (30) days of the applicable transfer date thereof provide CHRP with all original documents comprising the MAA or MAA Approval and to the extent that such original documents are required by NGX for regulatory filings outside the Territory, NGX shall allow CHRP to have access to and to copy such original documents at all reasonable times on reasonable notice for the purpose of the Maintenance of the MAA Approvals and filing of XXXx in relation to developments of the Products in the Territory. As used in this Section 6.4, “Maintenance” with respect to MAA Approvals and/or MAA shall include, without limitation, payment of the annual fees, renewal of the MAA Approval, implementation of any variations with respect to such MAA Approval and/or MAA and responding to requests by the relevant regulatory authorities.
6.5 Communications with Regulatory Authorities. Subject to applicable laws, following transfer of any MAA Approval to CHRP, its Affiliate or its designated Sublicensee, CHRP or such designated Sublicensee, as the case may be, shall be the party responsible for communicating directly with and appearing before the EMEA or any other regulatory authority, provided that CHRP or its designated Sublicensee shall keep NGX reasonably informed in advance as to any meetings CHRP, its Affiliate or its designated Sublicensee may have with the EMEA or any other regulatory authority with respect to any Product and at NGX’s request, allow NGX to participate in such meetings and/or promptly provide NGX with any minutes of such meetings. NGX agrees, upon CHRP’s request, to provide CHRP with reasonable assistance in connection with any such communications and meetings.
6.6 The Parties acknowledge and agree that in the event NGX does not transfer the Existing Product MAA Approval and the other MAA Approvals to CHRP or its designees promptly following when it is obligated to do so, CHRP may suffer irreparable damage for which an adequate remedy at law may not exist, and, accordingly, NGX agrees that CHRP may, in addition to all remedies available to it at law, seek equitable relief without the posting of any bond, requiring NGX to transfer the Existing Product MAA Approval and the other MAA Approvals to CHRP or its designees.
12
| 7. | DEVELOPMENT |
7.1 Development of Existing Product
(a) Neither Party shall be under any obligation to perform any study, including, without limitation, any open label safety study, PDN Trial, post-marketing study or clinical trial with respect to the Products for the Territory in the Field, it being understood that any applicable Sublicense Agreement, may to the extent deemed necessary or desirable by CHRP and NGX, contain provisions obligating the corresponding Sublicensee to perform some or all of such studies and trials.
(b) In the event the Astellas License Agreement has been terminated and either Party wishes to develop the Existing Product for a new indication (i.e., an indication other than PNP and PDN) or to modify or improve the Existing Product, including but not limited to, developing an improved Gel, then it shall raise the issue at the JSC. If the JSC agrees to such further development then the JDC shall be responsible for drawing up a development plan which it shall present to the JSC for approval.
(1) If both Parties wish to participate in the development then the JDC shall oversee the performance of such development and shall allocate responsibility for the performance of the various tasks required in connection with such studies based upon which Party is better situated to perform such tasks, taking into account among other things, the estimated costs associated with having each Party perform such task. The aim of the development plan will be to generate clinical data which is intended for, and in the correct format for, submission to satisfy the requirements of both the EMEA and the FDA. To the extent that any activities under the development plan are necessary to satisfy the requirements of both the EMEA and FDA then the Development Expenses for such activities will be borne equally by the Parties. To the extent that any activity is necessary to satisfy the requirements of only the EMEA or only the FDA then CHRP and NGX respectively shall be solely responsible for the Development Expenses for such activity.
(2) If only one Party wishes to carry out any development under this Section 7.1.(b) (the “Developing Party”), and the JSC has approved the development plan then the Developing Party shall carry out the activities under such development plan and shall be solely responsible for all Development Expenses related to such activities. If the other Party subsequently wishes to include any of the Data that has been created as a result of such development in its regulatory filings for Product in its territory, then it can only do so if it reimburses the Developing Party fifty percent (50%) of all the Developing Party’s Development Expenses incurred in relation to all the activities under the development plan.
7.2 Exchange of Data. If CHRP elects to (i) take the lead in locating and securing a Replacement Licensee or (ii) Exploit the Licensed Products itself or through an Affiliate, in either case in accordance with Section 5.03(b) of the Financing Agreement, then NGX shall provide to CHRP or its designated Sublicensee and CHRP shall and shall cause each such Affiliate and Sublicensee to provide to NGX all Data for use for the purposes set forth in Section 7.2.1 in a timely fashion and as promptly as possible upon request of the other Party. In addition in such circumstances, (a) NGX shall provide CHRP with all Data submitted to the FDA
13
within fourteen (14) days of such submission, and (b) CHRP shall provide NGX with all Data submitted by CHRP or its Affiliates or Sublicensees to the EMEA or any other regulatory authority in the Territory within fourteen (14) days of such submission. Each Party shall have the right to withhold Data for a reasonable period to allow it to file for patent protection relating to such Data consistent with the provisions of Section 11. Further is such circumstances, NGX shall provide CHRP with copies of all Data submitted to or received by NGX from Astellas under Section 7.2 of the Astellas License Agreement or by any Replacement Licensee within thirty (30) days of request therefor with respect to any Product or country or region in which the rights of Astellas have been terminated or become non-exclusive, and thereafter shall promptly provide CHRP with copies of all Data submitted to or received from Astellas under such Section 7.2 or a Replacement Licensee on an ongoing basis.
7.2.1 Use; Disclosure.
(a) If CHRP or its Sublicensee shall desire to obtain and/or maintain MAA Approval for Products in the Territory including without limitation cross referencing drug master files or other regulatory filings outside the Territory in each case to the extent it has the right to do so hereunder and for commercialization of Products inside the Territory; provided, however, that in no event shall CHRP or its Sublicensee take such action to the extent such action would conflict with or violate any exclusive rights of Astellas. CHRP or its Sublicensee may and will only use and disclose Data to Third Parties as required to obtain and maintain any such MAA Approval and NGX gives CHRP and its Sublicensees the right to so cross-reference as may be necessary in performing its obligations and exercising its rights granted under this Agreement or pursuant to the applicable Sublicense Agreement, i.e. development activities, marketing activities, medical education activities, professional services activities and public relations activities; or for purposes of obtaining consultation services in the normal course of business (such as business consultants, advertising agencies, law firms, accounting firms, etc.) in each case solely to the extent necessary for development and commercialization of Products in the Territory in accordance herewith; or as may otherwise be agreed by NGX and CHRP. CHRP may not use any Data (or permit any Third Party (including any Affiliate or Sublicensee) to use Data) outside the Territory, or outside the Field or for any products other than the Products, except for the assessment and validation of the Data by Affiliates or Third Party consultants outside the Territory but solely for purposes of developing or commercializing Products within the Territory. NGX may not use any Data provided by CHRP or its Affiliates or Sublicensees (or permit any Third Party to use such Data) inside the Territory (except as required to fulfill any development obligations it may have under a development plan agreed with CHRP), or outside the Field or for any products other than the Products, except for the assessment and validation of the Data by Affiliates or Third Party consultants inside the Territory but solely for purposes of developing or commercializing products outside the Territory. Notwithstanding anything to the contrary in this Section 7.2.1(a) or Section 7.2.1(b) below, each Party’s right to use Data generated by the other Party under Section 7.1(b) shall be subject to the requirements set forth therein.
(b) CHRP hereby grants NGX a non-exclusive sublicensable right and license to use Data provided by CHRP (whether directly or through an Affiliate or Sublicensee), and to disclose such Data to Third Parties, in each case as is reasonably necessary or useful for commercialization of Products outside the Territory including without limitation for use by
14
NGX’s licensees of the Products and for cross referencing drug master files or other regulatory filings (and CHRP hereby gives NGX the right to so cross-reference), in each case for commercialization outside the Territory, provided that any disclosure of such Data to a non-governmental Third Party is made under reasonable and customary confidentiality restrictions. Notwithstanding the foregoing, upon expiration or termination of this Agreement, the scope of NGX’s license to Data provided by CHRP shall thereafter be worldwide.
7.2.2 Third Parties.
(a) After the termination of the Astellas License Agreement, in all agreements with Third Parties or Affiliates involving the development of Data, CHRP and NGX, respectively, shall require that such Third Parties and Affiliates provide the other Party access to all such Data, to the extent that such Data is required for preparation of XXXx and filing of XXXx with the applicable regulatory authorities in and outside the Territory in accordance with this Agreement.
(b) Notwithstanding Section 7.2.2(a) above, CHRP acknowledges that under NGX’s agreement(s) with LTS prior to and after the Effective Date, NGX may not have the right to obtain certain Data in the possession of LTS relating to its manufacturing or patch trade secrets, but LTS will file such Data directly with regulatory authorities upon the request of NGX, subject to acceptance of such direct filing by the regulatory authorities. If any regulatory authority does not accept such a direct filing by LTS then the Parties and LTS shall cooperate to find an alternative route by which such LTS Data shall be filed with such regulatory authority. Such arrangement with LTS shall not be deemed a breach by NGX of this Section 7.2.2. It is understood that NGX shall ensure that it obtains reference rights to such Data filed by LTS directly with regulatory authorities for the use by each of NGX and CHRP as set forth in this Section 7.2.
7.2.3 Subject to the rights of Astellas under the Astellas License Agreement, as between the Parties, all XXXx and MAA Approvals and any other regulatory filings relating to developments of the Product in the Territory shall be owned by CHRP, its Affiliate or its designated Sublicensee.
7.3 Development of Liquid Formulation Product. In the event that Astellas License Agreement is terminated and the Liquid Formulation Product is or becomes a Product under this Agreement and CHRP elects to (i) take the lead in locating and securing a Replacement Licensee or (ii) Exploit the Licensed Products itself or through an Affiliate, in either case in accordance with Section 5.03(b) of the Financing Agreement, then, CHRP (or its Affiliate or designated Sublicensee) shall have the right to file all XXXx, with respect to the Liquid Formulation Product in the Territory, in each case at its own cost, provided that NGX shall, upon CHRP’s request, provide reasonable assistance in relation to such activities.
7.4 Coordination. Upon the other Party’s reasonable request after the termination of the Astellas License Agreement, each Party shall keep the other reasonably informed as to the progress of the clinical development and regulatory activities relating to the Liquid Formulation Product in and outside the Territory, including its correspondence and meetings with regulatory authorities as reasonably requested by the other Party. If CHRP elects to (a) take the lead in
15
locating and securing a Replacement Licensee or (b) Exploit the Licensed Products itself or through an Affiliate, in either case in accordance with Section 5.03(b) of the Financing Agreement, then, each Party shall reasonably consider and promptly respond to any comments provided by the other Party with respect to such clinical development and regulatory activities. Subject to CHRP’s obligation to assign to NGX any and all regulatory filings, XXXx and MAA Approvals upon expiration of this Agreement, and the rights of Astellas under the Astellas License Agreement, all MAA filings and MAA Approvals in the Territory for the Liquid Formulation Product (including all variations and line extensions) shall be in the name of, and owned by, CHRP, its Affiliate or its designated Sublicensee, as the case may be, and in the event Astellas has obtained MAA Approvals in the Territory for the Liquid Formulation Product (including all variations and line extensions) and assigned such MAA Approvals to NGX pursuant to the Astellas License Agreement, NGX shall, upon CHRP’s request, assign such MAA Approvals to CHRP or its designated Sublicensee.
| 8. | COMMERCIALIZATION |
It the event the Astellas License Agreement has been terminated with respect to a Product in a country or region in the Territory, or the Astellas License Agreement has been terminated in its entirety, or the rights of Astellas with respect to a Product in a country or region in the Territory become non-exclusive, except as otherwise provided in Section 4, CHRP shall have no obligation to commercialize the Products; provided, however, that any Sublicense Agreement entered into by CHRP shall contain provisions obligating the Sublicensee to commercialize the Products substantially similar to manner in which Astellas is obligated to commercialize the Products pursuant to Section 8 of the Astellas License Agreement, and provided further, however, that NGX shall continue to perform its obligations contained in Section 8 of the Astellas License Agreement as if such agreement were still in full force and effect and the Sublicensee was the licensee thereunder.
| 9. | SUPPLY OF EXISTING PRODUCT |
If CHRP elects to (i) take the lead in locating and securing a Replacement Licensee or (ii) Exploit the Licensed Products itself or through an Affiliate, in either case in accordance with Section 5.03(b) of the Financing Agreement, then until such time as CHRP or its Sublicensee enters into a direct contractual relationship with the Third Party contractors for the supply of Products or Components, NGX shall be obligated to supply such Components or Products to CHRP or its Sublicensee or any Replacement Licensee to the same extent and on the same terms and conditions as it was obligated to supply Astellas under the Astellas License Agreement on the Effective Date thereof; provided, however, the reasonable and documented costs associated with the reestablishment of such supply, to the extent not reimbursed by CHRP or its Sublicensee or any Replacement Licensee, as the case may be, shall be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement.
| 10. | BOOKS AND RECORDS |
Each Party shall maintain records, in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, which shall accurately reflect all work done and results achieved in the performance of the development and regulatory program regarding
16
Products by or on behalf of such Party (or its Affiliate or Sublicensee) for the Territory in the Field.
| 11. | INTELLECTUAL PROPERTY |
11.1 General. As between the Parties, NGX shall be responsible for the prosecution, maintenance and enforcement of the Patent Rights in accordance with and without limiting Section 5.08(a) and (b) of the Financing Agreement.
11.2 Patent Marking. CHRP agrees to xxxx and have its Affiliates and Sublicensees and their Subdistributors xxxx all patented Products they sell or distribute pursuant to this Agreement in accordance with the applicable patent statutes or regulations in the country or countries of manufacture and sale thereof.
11.3 If CHRP elects to Exploit the Licensed Products itself or through an Affiliate, in accordance with Section 5.03(b) of the Financing Agreement, the following provisions shall apply:
11.3.1 The provisions of Section 11.1-11.2 of the Astellas License Agreement are hereby incorporated by reference as if set forth herein in full except that for purposes of this Agreement, all references in such Sections to “Astellas” shall be deemed references to “CHRP or its Affiliates.” As between the Parties, NGX shall be responsible for the prosecution, maintenance and enforcement of the Patent Rights in accordance with and without limiting Section 5.08(a) and (b) of the Financing Agreement; provided, however, all reasonable and documented costs associated with such prosecution, maintenance and enforcement shall be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement.
11.3.2 The provisions of Section 11.3 of the Astellas License Agreement are hereby incorporated by reference as if set forth herein in full except that for purposes of this Agreement, all references in such Sections to “Astellas” shall be deemed references to “CHRP or its Affiliates” and provided further, that (i) in the event Astellas shall at any time exercise the Option under Section 2.3.12 of the Astellas License Agreement with respect to the Liquid Formulation Product, [***] (ii) in the event[***]; (iii) in the event NGX fails to maintain or shall abandon any Patent Right, CHRP or its designee shall have the right, but not the obligation, at CHRP’s sole cost and expense (including, without limitation, reasonable attorney’s fees) to assume responsibility for the prosecution and maintenance of such Patent Right; provided, however, all reasonable and documented costs associated with such prosecution and maintenance shall be credited against the Royalty Equivalent Amount otherwise to be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement; and (iv) the cost of any agreed upon extensions of the terms of Patent Rights in the Territory shall be at the expense of NGX; provided, however, all reasonable and documented costs associated with such extensions shall be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement.
11.3.3 Third Party Patents and Trademarks.
(a) Each Party shall promptly notify the other Party hereto in writing of any Infringement Action brought against such Party. NGX, shall be obligated to defend such
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
17
Infringement Action using counsel of its own choice reasonably acceptable to CHRP, and the Infringement Action shall be at NGX’s own expense; provided, however, that CHRP may participate in the defense and/or settlement thereof at its own expense with counsel of its choice. NGX agrees to keep CHRP reasonably informed of all material developments in connection with any such Infringement Action. CHRP agrees not to (i) make admissions regarding infringement by the Product of any patents outside of the Patent Rights, or (ii) settle such Infringement Action, or make any admissions or assert any position in such Infringement Action, in a manner that would materially adversely affect the validity, enforceability or scope of the Patent Rights in or outside the Territory, in each case without obtaining the prior written consent of NGX which shall not be unreasonably withheld, conditioned or delayed. In respect of any infringement actions outside the Territory, NGX agrees not to settle such infringement action, or make any admissions or assert any position in such infringement action, in a manner that would materially adversely affect the validity, enforceability or scope of the Patent Rights inside the Territory, without obtaining the prior written consent of CHRP which shall not be unreasonably withheld, conditioned or delayed.
(b) Oppositions to Third Party Patents and Trademarks. NGX, at CHRP’s reasonable request and at NGX’s cost and expense, shall bring an Adverse Proceeding against any Third Party asserting a Third Party Right, using counsel of its own choice reasonably acceptable to CHRP; provided, however, all reasonable and documented costs associated with such Adverse Proceeding with respect to the Territory shall be treated as Revenue Interest Payments under Section 2.02 of the Financing Agreement. NGX shall keep CHRP reasonably informed of all material developments in connection with any such Adverse Proceedings and shall comply with CHRP’s advice and requests with respect to such Adverse Proceeding.
11.3.4 Enforcement of Patent Rights and Trademarks. The provisions [***]
11.3.5 Third Party Patent Rights.
(a) Subject to Section 11.3.5(b), the obligations of NGX and the rights of CHRP under this Section 11.3 shall be subject to, and limited by, any agreements pursuant to which NGX acquired or licensed any particular Patent Rights or Data, including without limitation the UC License Agreement and LTS Agreement. Without limiting the foregoing, with respect to the prosecution or enforcement of Patent Rights licensed by NGX from a Third Party, to the extent NGX has the right to do so, NGX shall cooperate with CHRP to prosecute and enforce such Patent Rights in the Territory in the same manner as set forth in Sections 11.3.3 and 11.3.4 above. As between NGX and CHRP, any recoveries from enforcement of such Patent Rights licensed from a Third Party (including any amounts that NGX receives from the Third Party licensor as a result of such enforcement) shall be shared in accordance with Section 11.5.2 of the Astellas License Agreement as incorporated herein by reference, after deducting from such recoveries any amounts owed to the Third Party licensor for such enforcement; provided that any Enforcement Actions initiated by the Third Party licensor shall be deemed initiated by NGX for purposes of such Section 11.5.2, and the costs and expenses incurred by NGX in such Enforcement Action shall include any costs and expenses reimbursed or required to be reimbursed by NGX to the Third Party licensor in such Enforcement Action.
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
18
(b) NGX shall not exercise its right to terminate any Patent Rights licensed under the UC License Agreement pursuant to Section 17.7 of the UC License Agreement.
(c) If NGX receives notification from LTS under Section 7.2(d) of the LTS Agreement that LTS has elected not to file, prosecute or maintain any patent or patent application licensed by NGX from LTS, then NGX shall so notify CHRP within five (5) days of NGX’s receipt of such notification. If CHRP wishes NGX to file, prosecute or maintain such patent or patent application it shall inform NGX within forty-five (45) days of receipt of the notice from NGX and NGX shall immediately (and in any event within the sixty (60) day period in Section 7.2(d) of the LTS Agreement) so inform LTS such that the provisions of Sections 7.2(d) and (e) of the LTS Agreement take effect.
| 12. | TRADEMARKS |
The provisions of Section 12 of the Astellas License Agreement are hereby incorporated by reference as if set forth herein in full, except that all references in said Section to “Astellas” shall be deemed to refer to “CHRP or its Affiliate”, provided, however, that provision of Section 12.9 thereof shall be deemed to apply if either Astellas exercises the Option pursuant to Section 2.3.12 of the Astellas License Agreement or CHRP exercises the LFP Option hereunder.
| 13. | CONFIDENTIALITY |
The provisions of Section 13 of the Astellas License Agreement are hereby incorporated by reference as if set forth herein in full, provided, however, that all references in such Section to “Parties” or a “Party” shall have the meanings set forth in the preamble to this Agreement.
| 14. | REPRESENTATIONS AND WARRANTIES |
14.1 Mutual Warranties. Each Party warrants and represents to the other that the Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms. The execution, delivery and performance of the Agreement by such Party does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor to such Party’s knowledge, violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
14.2 NGX Warranty. NGX warrants and represents to CHRP that it has the full right and authority to enter into this Agreement and grant the rights and licenses granted herein.
14.3 NGX Covenants. NGX covenants to CHRP that, subject to the rights of Astellas under the Astellas License Agreement and its right to enter into Replacement License Agreements in accordance with the terms of Section 5.03(b) of the Financing Agreement, during the term of this Agreement (a) it shall not grant any right, license or interest in or to the Patent Rights and NGX Know-How, or any portion thereof, that is in conflict with the rights or licenses granted under this Agreement, and (b) it shall not xxxxx x xxxx or other encumbrances on any of the subject matter of this Agreement or on any of NGX’s rights, benefits or obligations hereunder or on any of the Patent Rights, which would conflict with the rights or licenses of CHRP hereunder.
19
| 15. | LIABILITY |
15.1 DISCLAIMER. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT TO THE CONTRARY, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTIES OF ANY KIND EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT, OR VALIDITY OF ANY PATENTS ISSUED OR PENDING.
15.2 LIMITATION OF LIABILITY. NOTWITHSTANDING ANYTHING IN THIS AGREEMENT OR OTHERWISE, NEITHER PARTY SHALL BE LIABLE TO THE OTHER WITH RESPECT TO ANY SUBJECT MATTER OF THIS AGREEMENT, WHETHER UNDER ANY CONTRACT, NEGLIGENCE, STRICT LIABILITY OR OTHER LEGAL OR EQUITABLE THEORY, FOR ANY INCIDENTAL, INDIRECT, SPECIAL, EXEMPLARY, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES, LOST PROFITS, LOSS OF ROYALTIES AND MILESTONE PAYMENTS, LOSS OF USE, DAMAGE TO GOODWILL, OR LOSS OF BUSINESS.
15.3 Exceptions. Nothing in this Agreement shall limit any Party’s liability (a) caused by that Party’s willful misconduct, (b) for death or personal injury caused by that Party’s negligence, or (c) for fraudulent misrepresentation by that Party.
| 16. | INDEMNIFICATION |
16.1 Indemnification of NGX. Subject to Section 16.3, in the event the Astellas License Agreement is terminated and (i) CHRP elects to Exploit the Licensed Products itself or through an Affiliate or (ii) CHRP enters into any Sublicense Agreement, in either case in accordance with Section 5.03(b) of the Financing Agreement, CHRP hereby agrees to, or such Sublicense Agreement shall require any such Sublicensee and Subdistributor to, indemnify, defend and hold harmless the NGX Indemnitees from and against Liabilities from a Third Party Claim incurred by any NGX Indemnitee as a result of the use, marketing, distribution or sale of any Product by CHRP or its Affiliates or such Sublicensee or its Subdistributors, as applicable in the Territory including any Product Liability Claim (but not an Infringement Action, except to the extent arising from actions by CHRP or any Sublicensee or their Subdistributors occurring after the expiration of this Agreement).
16.2 Indemnification of CHRP. Subject to Section 16.3, NGX hereby agrees to indemnify, defend and hold harmless CHRP, its Affiliates, Sublicensees and their respective directors, officers, employees, agents and their respective successors, heirs and assigns (the “CHRP Indemnitees”) from and against any Liabilities from any Third Party Claims incurred by any CHRP Indemnitee as a result of: (a) the use, marketing, distribution or sale of any Product by NGX or its licensee outside the Territory, including, without limitation, any Products Liability Claims or Third Party infringement claims arising from NGX’s marketing of Products outside the Territory, or (b) any material breach of any representations, warranties or covenants by NGX in Section 14 above.
20
16.3 Procedure. Except with respect to Third Party infringement claims subject to Section 11.3.3 above, a Party that intends to claim indemnification under this Section 16 (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) in writing of any Third Party Claim, in respect of which the Indemnitee intends to claim such indemnification, and the Indemnitor shall have sole control of the defense and/or settlement thereof; provided, that the Indemnitee shall have the right to participate in the defense or settlement of such Third Party Claim with counsel of its own choosing at its expense. The Indemnitor shall keep the Indemnitee fully informed of the progress of any such Third Party Claim. The indemnity arrangement in this Section 16 shall not apply to amounts paid in settlement of any action with respect to a Third Party Claim, if such settlement is effected without the consent of the Indemnitor, which consent shall not be withheld, delayed or conditioned unreasonably. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim, if prejudicial to its ability to defend such action, shall relieve such Indemnitor of any liability to the Indemnitee under this Section 16 to the extent it is so prejudiced, but the omission to so deliver written notice to the Indemnitor shall not relieve the Indemnitor of any liability that it may have to any Indemnitee otherwise than under this Section 16. The Indemnitee under this Section 16 shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Third Party Claim covered by this indemnification.
16.4 [Intentionally left blank.]
16.5 Insurance. Section 16.5 of the Astellas License Agreement is hereby incorporated by reference as if set forth herein in full, except that for purposes of this Agreement the terms “Party” or “Parties” shall mean (i) NGX, and (ii) the event the Astellas License Agreement is terminated, CHRP (and its Affiliates), if it elects to directly sell and distribute Products in the Territory, or any Sublicensee of CHRP.
| 17. | TERM AND TERMINATION |
17.1 Term. This Agreement shall become effective as of the Effective Date and, unless earlier terminated pursuant to the other provisions of this Section 17, shall continue until the earlier of (1) the occurrence of the Second Stepdown (as defined in the Financing Agreement), (2) termination of the Financing Agreement in accordance with Section 7.02(a) thereof, (3) full payment has been made to CHRP pursuant to Section 2.02(e) or 7.02(c) or (4) on a country-by-country and Product-by-Product basis, until the later of (a) ten (10) years after the First Commercial Sale thereof in the respective country, (b) the expiration or abandonment of the last Valid Patent Claim covering the respective Product in the respective country and (c) the expiration of all applicable periods of regulatory exclusivity (e.g., regulatory data exclusivity) covering the respective Product in the respective country.
17.2 Termination By CHRP. CHRP may terminate this Agreement for any reason under this Section 17.2 without any penalty, consequence, termination compensation, loss of profits, goodwill indemnity or otherwise solely by reason of such termination (i.e., without prejudice to any remedies NGX may have for a breach of this Agreement by CHRP prior to such termination), as a whole, or with respect to the European Region, or on a country-by-country
21
basis with respect to countries in the Territory (other than those countries comprising the European Region) and a Product-by-Product basis, in each case upon written notice to NGX.
17.3 Termination for Material Breach. Without limiting either Party’s ability to terminate in accordance with the other provisions of this Agreement, in the event of a Party’s material breach of this Agreement, the non-breaching Party shall have the right to provide notice of its intention to terminate this Agreement. Such notice shall specify in reasonable detail the facts and circumstances constituting the material breach of this Agreement. Upon the expiration of ninety (90) days after receipt by the breaching Party of such notice, if the breaching Party has not cured such material breach, the non-breaching Party shall have the right to terminate this Agreement in whole by giving a notice of termination, which shall be effective on the date such notice is given.
| 18. | EFFECT OF EXPIRATION OR TERMINATION |
18.1 General. Termination of this Agreement for any reason shall not release NGX from any then-outstanding Financing Agreement Obligations then owing to CHRP or any Party hereto from any liability which, at the time of such termination, has already accrued to the other Party or which is attributable to a period prior to such termination nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement.
18.2 Sections 18.3-18.7 of the Astellas License Agreement are hereby incorporated by reference as if set forth herein in full except that all references to Astellas in such Sections shall be deemed to be references to CHRP.
18.3 Survival. The provisions of Sections 1, 7.2.1(b), 13, 15.1, 15.2, 16, 18, 19, 20.4 and 20.5 (and any provision of the Astellas License Agreement incorporated herein by reference that survives the expiration or termination thereof) shall survive the expiration or termination of this Agreement for any reason.
18.4 Rights in Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by NGX are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that CHRP, as licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against NGX under the U.S. Bankruptcy Code, which results in NGX’s rejection of this Agreement, CHRP shall be entitled to (a) retain ownership of all MAA Approvals and (b) a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in CHRP’ possession, shall be promptly delivered upon written request therefor by CHRP.
22
| 19. | DISPUTE RESOLUTION |
This Agreement shall be governed and construed in accordance with the laws of the State of New York, USA, without giving effect to any choice of law provisions thereof. Each Party hereby submits itself for the purpose of this Agreement and any controversy arising hereunder to the exclusive jurisdiction of the state and federal courts located in the County of New York, State of New York, USA, and any courts of appeal therefrom, and waives any objection on the grounds of lack of jurisdiction (including, without limitation, venue) to the exercise of such jurisdiction over it by any such courts. In the event of any dispute between the parties, such dispute shall be resolved in accordance with the provisions set forth in Section 8.15 of the Financing Agreement, the provisions of which are incorporated by reference as it set forth herein in full.
| 20. | MISCELLANEOUS |
20.1 Force Majeure. Nonperformance of any Party shall be excused to the extent that performance is rendered impossible by fire, strike (other than of their own workforce), earthquake, flood, acts of terrorism, governmental acts or orders or restrictions, failure of suppliers, or any other reason where failure to perform is beyond the reasonable control of the nonperforming Party. In such event CHRP or NGX, as the case may be, shall promptly notify the other Party of such inability and of the period for which such inability is anticipated to continue. Without limiting the foregoing, the Party subject to such inability shall use reasonable efforts to minimize the duration of the impact of any force majeure event on its performance hereunder.
20.2 No Implied Waivers; Rights Cumulative. No failure on the part of NGX or CHRP to exercise and no delay in exercising any right under this Agreement, or provided by statute or at law or in equity or otherwise, shall impair, prejudice or constitute a waiver of any such right, nor shall any partial exercise of any such right preclude any other or further exercise thereof or the exercise of any other right.
20.3 Independent Contractors. Nothing contained in this Agreement is intended implicitly, or is to be construed, to constitute NGX or CHRP as partners in the legal sense. No Party hereto shall have any express or implied right or authority to assume or create any obligations on behalf of or in the name of any other Party or to bind any other Party to any contract, agreement or undertaking with any Third Party. This Agreement does not create a partnership for USA federal income tax purposes (as defined in Section 761 of the USA Internal Revenue Code), for any USA state or local jurisdiction, or in any country other than the USA. Therefore there is no requirement to file Form 1065, USA Partnership Return of Income, any similar USA state or local income tax return, or any similar document with tax authorities in any country other than the USA.
20.4 Notices. Any notice required or permitted to be given hereunder shall be in writing and shall be delivered in person, by a nationally recognized overnight courier, or by facsimile (receipt confirmed), to the addresses given below or such other addresses as may be designated in writing by the Parties from time to time during the Term in accordance with the
23
provisions of Section 8.03 of the Financing Agreement, the provisions of which are incorporated herein by reference.
20.5 Assignment. This Agreement shall not be assignable by either Party to any Third Party without the written consent of the other Party; except CHRP may assign this Agreement without the consent of NGX to a Third Party to whom it assigns all of the other Transaction Documents and NGX may assign this Agreement without the consent of CHRP to a Third Party to whom it assigns all of the other Transaction Documents and which acquires all or substantially all of the assets of NGX; provided, however, that such assignment shall not relieve NGX of its obligations hereunder.
20.6 Modification. No amendment or modification of any provision of this Agreement shall be effective unless in writing signed by all Parties hereto. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance or any other matter not set forth in an agreement in writing and signed by all Parties.
20.7 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions hereof shall remain in full force and effect in such jurisdiction and shall be liberally construed in order to carry out the intentions of the Parties hereto as nearly as may be possible. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
20.8 Publicity Review. Any announcements regarding this Agreement or the activities of the Parties hereunder shall be governed by Section 5.04 of the Financing Agreement, the terms of which are incorporated by reference.
20.9 Counterparts. This Agreement may be executed in two counterparts, each of which shall be deemed an original, and all of which together, shall constitute one and the same instrument.
20.10 Headings. Headings used herein are for convenience only and shall not in any way affect the construction of or be taken into consideration in interpreting this Agreement.
20.11 Export Laws. Notwithstanding anything to the contrary contained herein, all obligations of NGX and CHRP are subject to prior compliance with United States and foreign export regulations and such other United States and foreign laws and regulations as may be applicable, and to obtaining all necessary approvals required by the applicable agencies of the governments of the United States and foreign jurisdictions. NGX and CHRP shall cooperate with each other and shall provide assistance to the other as reasonably necessary to obtain any required approvals.
20.12 Entire Agreement. This Agreement, together with all the Exhibits thereto, the Financing Agreement, and the other Transaction Documents constitute the entire agreement, both written or oral, with respect to the subject matter hereof, and supersede all prior or
24
contemporaneous understandings or agreements, whether written or oral, between NGX and CHRP with respect to such subject matter.
[The remainder of this page left intentionally blank; signature page follows.]
25
| NEUROGESX, INC. |
XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. | |||||||
| By: | /s/ Xxxxxxx Xxxxxxxxx |
By: | /s/ Xxxxxxx X. Browns | |||||
| Name: | Xxxxxxx Xxxxxxxxx |
Name: | Xxxxxxx X. Browns, M.D. | |||||
| Title: | EVP, COO, CFO |
Title: | Managing Director | |||||
[SIGNATURE PAGE TO DISTRIBUTION, MARKETING AND LICENSE AGREEMENT]
26
EXHIBIT C
COPY OF EXISTING LICENSE AGREEMENT
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
DATED 19th June 2009
- and -
ASTELLAS PHARMA EUROPE LIMITED
DISTRIBUTION, MARKETING AND LICENSE AGREEMENT
Confidential
TABLE OF CONTENTS
| Page | ||||||
| 1. |
DEFINITIONS | 1 | ||||
| 2. |
GRANT OF DISTRIBUTION RIGHTS | 12 | ||||
| 2.1 |
Appointment | 12 | ||||
| 2.2 |
License | 12 | ||||
| 2.3 |
Liquid Formulation Product Option | 13 | ||||
| 2.4 |
Subdistributors and Sublicensees | 16 | ||||
| 2.5 |
NGX IP Acquired after the Effective Date | 17 | ||||
| 2.6 |
No Modifications to the Product | 18 | ||||
| 2.7 |
No Conflict | 18 | ||||
| 3. |
INITIAL PAYMENTS AND MILESTONES | 19 | ||||
| 3.1 |
Initial Payment | 19 | ||||
| 3.2 |
Sales Milestones | 20 | ||||
| 3.3 |
Option Fee/Option Exercise Fee | 20 | ||||
| 4. |
ROYALTIES | 21 | ||||
| 4.1 |
Royalty | 21 | ||||
| 4.2 |
Certain Reductions to Royalties | 21 | ||||
| 4.3 |
[***] | 22 | ||||
| 4.4 |
Existing NGX Third Party Royalties | 23 | ||||
| 4.5 |
Discounting | 23 | ||||
| 4.6 |
Royalty Reports | 24 | ||||
| 5. |
COOPERATION | 25 | ||||
| 5.1 |
Commercialization Plan | 25 | ||||
| 5.2 |
Joint Steering Committee | 25 | ||||
| 5.3 |
Joint Development Committee | 26 | ||||
| 5.4 |
Joint Commercialisation Committee | 26 | ||||
| 5.5 |
Joint Regulatory Committee | 27 | ||||
| 5.6 |
General Provisions relating to Committees | 27 | ||||
| 5.7 |
Alliance Managers | 29 | ||||
| 5.8 |
Kick-off Meeting | 29 | ||||
| 5.9 |
Documents | 29 | ||||
| 5.10 |
Co-operation | 30 | ||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-i-
TABLE OF CONTENTS
(Continued)
| 6. |
TRANSFER OF THE MARKETING AUTHORISATION | 30 | ||||
| 6.1 |
MAA Approval Transfer | 30 | ||||
| 6.2 |
[***] | 30 | ||||
| 6.3 |
NGX’s Obligations | 30 | ||||
| 6.4 |
Prior to MAA Approval Transfer | 31 | ||||
| 6.5 |
After MAA Approval Transfer | 31 | ||||
| 6.6 |
Communications with Regulatory Authorities | 31 | ||||
| 7. |
DEVELOPMENT | 32 | ||||
| 7.1 |
Development of Existing Product | 32 | ||||
| 7.2 |
Exchange of Data | 34 | ||||
| 7.3 |
Development of Liquid Formulation Product | 35 | ||||
| 7.4 |
Coordination | 35 | ||||
| 8. |
COMMERCIALIZATION | 36 | ||||
| 8.1 |
Commercialization | 36 | ||||
| 8.2 |
Pricing and Reimbursement | 36 | ||||
| 8.3 |
Sales Materials | 36 | ||||
| 8.4 |
Reporting Adverse Drug Reactions/Experiences | 37 | ||||
| 8.5 |
Assistance for the Product | 37 | ||||
| 8.6 |
Medical Inquiries for the Product | 38 | ||||
| 8.7 |
Material Failure to Exploit | 38 | ||||
| 8.8 |
Commercialization of Liquid Formulation Product | 39 | ||||
| 9. |
SUPPLY OF EXISTING PRODUCT | 39 | ||||
| 9.1 |
General | 39 | ||||
| 9.2 |
Inventory | 39 | ||||
| 9.3 |
General Supply Terms | 39 | ||||
| 9.4 |
Tripartite Agreements | 40 | ||||
| 9.5 |
Quality Agreement | 40 | ||||
| 10. |
PAYMENTS; BOOKS AND RECORDS | 40 | ||||
| 10.1 |
Payment Method | 40 | ||||
| 10.2 |
Taxes | 40 | ||||
| 10.3 |
United States Dollars | 41 | ||||
| 10.4 |
Records; Inspection | 41 | ||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-ii-
TABLE OF CONTENTS
(Continued)
| 11. |
INTELLECTUAL PROPERTY | 42 | ||||
| 11.1 |
Ownership of Inventions | 42 | ||||
| 11.2 |
NGX Improvements | 43 | ||||
| 11.3 |
Maintenance of Patents | 44 | ||||
| 11.4 |
Third Party Patents and Trademarks | 45 | ||||
| 11.5 |
Enforcement of Patent Rights and Trademarks | 47 | ||||
| 11.6 |
Third Party Patent Rights | 48 | ||||
| 11.7 |
Patent Marking | 48 | ||||
| 12. |
TRADEMARKS | 49 | ||||
| 12.1 |
Product Trademark | 49 | ||||
| 12.2 |
Trade Dress | 49 | ||||
| 12.3 |
License | 49 | ||||
| 12.4 |
Quality Standards | 49 | ||||
| 12.5 |
Alternative Trademark | 50 | ||||
| 12.6 |
Registration & Ownership | 50 | ||||
| 12.7 |
Recordation | 50 | ||||
| 12.8 |
Domain Names | 50 | ||||
| 12.9 |
Trademark for Liquid Formulation Product | 51 | ||||
| 12.10 |
Promotional Materials | 51 | ||||
| 12.11 |
Termination | 51 | ||||
| 13. |
CONFIDENTIALITY | 51 | ||||
| 13.1 |
Confidential Information | 51 | ||||
| 13.2 |
Permitted Use and Disclosures | 52 | ||||
| 13.3 |
Terms of this Agreement | 52 | ||||
| 13.4 |
Prior Non-Disclosure Agreements | 53 | ||||
| 13.5 |
Publication of Product Information | 53 | ||||
| 14. |
REPRESENTATIONS AND WARRANTIES | 53 | ||||
| 14.1 |
Mutual Warranties | 53 | ||||
| 14.2 |
NGX Warranties | 53 | ||||
| 14.3 |
NGX Covenants | 58 | ||||
| 14.4 |
Astellas Warranties | 58 | ||||
| 15. |
LIABILITY | 58 | ||||
| 15.1 |
DISCLAIMER | 58 | ||||
| 15.2 |
LIMITATION OF LIABILITY | 58 | ||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-iii-
TABLE OF CONTENTS
(Continued)
| 15.3 | Exceptions | 59 | ||||
| 16. |
INDEMNIFICATION | 59 | ||||
| 16.1 |
Indemnification of NGX | 59 | ||||
| 16.2 |
Indemnification of Astellas | 59 | ||||
| 16.3 |
Procedure | 59 | ||||
| 16.4 |
Consequences of Failure to Transfer MA | 60 | ||||
| 16.5 |
Insurance | 60 | ||||
| 17. |
TERM AND TERMINATION | 61 | ||||
| 17.1 |
Term | 61 | ||||
| 17.2 |
Termination By Astellas | 61 | ||||
| 17.3 |
Termination for Material Breach | 61 | ||||
| 17.4 |
Termination for Insolvency | 61 | ||||
| 17.5 |
[***] | 61 | ||||
| 17.6 |
[***] | 62 | ||||
| 18. |
EFFECT OF EXPIRATION OR TERMINATION | 62 | ||||
| 18.1 |
General | 62 | ||||
| 18.2 |
Rights on Expiration | 62 | ||||
| 18.3 |
Rights on Termination of a Product | 63 | ||||
| 18.4 |
Sublicenses | 65 | ||||
| 18.5 |
Rights on Termination of a non-European Union country (under Section 7.1.1) or Region | 65 | ||||
| 18.6 |
Rights on Termination of this Agreement | 67 | ||||
| 18.7 |
No Renewal, Extension or Waiver | 67 | ||||
| 18.8 |
Survival | 67 | ||||
| 18.9 |
Rights in Bankruptcy | 68 | ||||
| 19. |
DISPUTE RESOLUTION | 68 | ||||
| 19.1 |
Disputes | 68 | ||||
| 19.2 |
Arbitration | 69 | ||||
| 20. |
MISCELLANEOUS | 70 | ||||
| 20.1 |
Governing Law | 70 | ||||
| 20.2 |
Force Majeure | 70 | ||||
| 20.3 |
No Implied Waivers; Rights Cumulative | 70 | ||||
| 20.4 |
Independent Contractors | 70 | ||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-iv-
TABLE OF CONTENTS
(Continued)
| 20.5 | Notices | 71 | ||||
| 20.6 | Assignment | 71 | ||||
| 20.7 | Modification | 71 | ||||
| 20.8 | Severability | 71 | ||||
| 20.9 | Publicity Review | 72 | ||||
| 20.10 | Counterparts | 72 | ||||
| 20.11 | Headings | 72 | ||||
| 20.12 | Export Laws | 73 | ||||
| 20.13 | Entire Agreement | 73 |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-v-
DISTRIBUTION, MARKETING AND LICENSE AGREEMENT
This DISTRIBUTION, MARKETING AND LICENSE Agreement (hereinafter “Agreement”), made as of the 19th day of June, 2009 (“Effective Date”), between NeurogesX Inc., a Delaware corporation having a place of business at 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000, Xxx Xxxxx, Xxxxxxxxxx 00000, Xxxxxx Xxxxxx of America (“NGX”) and Astellas Pharma Europe Ltd, a corporation established under the laws of England and Wales having a place of business at Xxxxxx Xxxxx, Xxxxxx Xxxx, Xxxxxxx, Xxxxxxxxx, XX00 0XX, Xxxxxx Xxxxxxx (“Astellas”). Each of NGX and Astellas shall be a “Party,” and together the “Parties.”
A. NGX has developed a patch product for the cutaneous delivery of capsaicin (as described below, the “Existing Product”).
B. NGX has obtained MAA Approval (as defined below) for the Existing Product in the European Union using the centralized procedure for treatment of PNP (as defined below) in non-diabetic adults.
C. Astellas desires to obtain (i) an exclusive right to commercialize and distribute the Existing Product, and (ii) an option to obtain the exclusive right to co-develop, commercialize and distribute the Liquid Formulation Product (as defined below), in each case in Europe and certain other countries (as described below, the “Territory”), and NGX desires to have the Existing Product and the Liquid Formulation Product commercialized in the Territory, in accordance with this Agreement.
1. DEFINITIONS
1.1 “Affiliate” shall mean, in the case of a subject entity, another entity that controls, is controlled by or is under common control with the subject entity, but only for so long as such control exists. For purposes of this definition only, “control” shall mean beneficial ownership (direct or indirect) of at least fifty percent (50%) of the shares of the subject entity entitled to vote in the election of directors (or, in the case of an entity that is not a corporation, in the election of the corresponding managing authority); provided that where the local law does not permit foreign equity ownership of at least fifty percent (50%), then “control” shall mean the beneficial ownership (direct or indirect) of the maximum percentage of outstanding stock or voting rights permitted by local law.
1.2 “Alternative Trademark” shall mean the [***] trademarks for the Existing Product, which shall be owned by NGX worldwide, including the applications and registrations for which are set out in Exhibit 1.44.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
1.3 “Brand Guiding Principles” shall mean the general framework for a common understanding of the Existing Product and the commercial focus it should have worldwide, such general framework to include at minimum the items set forth in Exhibit 1.3.
1.4 “cGCP” or “current Good Clinical Practices” means a set of internationally recognized ethical and scientific quality requirements which must be observed for designing, conducting, recording and reporting clinical trials that involve the participation of human subjects as set forth in European Union Commission Directive 2001/20/EC relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use, and brought into law by European Union Commission Directive 2005/28/EC laying down the principles and detailed guidelines for good clinical practice as regards investigational medicinal products, as well as the requirements for authorization of the manufacturing and importation of such products and any subsequent modifications or amendments thereto and any laws that apply in the location of performance of the clinical trial.
1.5 “cGLP” or “current Good Laboratory Practices” means the quality system concerned with the organizational process and the conditions under which laboratory studies are planned, performed, monitored, recorded and reported, as defined in European Commission Directive 87/18/EEC as amended and any subsequent modifications or amendments thereto and any laws that apply in the location of performance of the laboratory studies.
1.6 “cGMP” or “current Good Manufacturing Practices” means all applicable standards relating to manufacturing practices for fine chemicals, active pharmaceutical ingredients, intermediates, bulk products or finished pharmaceutical products, including current good manufacturing practices and standards as provided for (and as amended or superseded from time to time) in:
1.6.1 European Community Directive 2003/94/EC (Principles and guidelines of good manufacturing practice for medicinal products);
1.6.2 21 C.F.R. §§ 210, 211 and 600;
1.6.3 ICH Guidance for Industry Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients; and
1.6.4 Part II of Volume IV of the EU Guide to Good Manufacturing Practice.
1.7 “Commercially Reasonable Efforts” shall mean [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-2-
1.8 “Committee” shall mean the JSC, JDC, JRC and JCC or any other committee set up by the JSC, as the case may be.
1.9 “Competitive Product” shall mean [***].
1.10 “Component(s)” shall mean, individually or collectively, Patch and/or Gel.
1.11 “Consultation” shall mean, with respect to the representations, warranties and covenant of NGX, the diligent and informed consultation between any of the individuals identified in Section 1.28 and NGX’s external patent counsel who has meaningful responsibilities related to the prosecution, management and defense of NGX’s intellectual property rights, including the Patent Rights and the NGX Know-How, in each case, which consultation shall specifically concern the subject matter of the applicable representation, warranty or covenant, including any exceptions to the accuracy thereof.
1.12 “Control” shall mean possession of the ability to grant a license or sublicense, within the scope set forth in this Agreement, without violating the terms of any agreement or other arrangement with any Third Party.
1.13 “CPL” shall mean Contract Pharmaceuticals Limited Canada, 0000 Xxxxxx Xxxxxxxx, Xxxxxxxxxxx, Xxxxxxx, X0X 0X0, Xxxxxx, and its successors.
1.14 “Data” shall mean any and all scientific, technical or test data pertaining to Product(s) that is generated by or under authority of either Party, including research data, clinical pharmacology data, CMC data (including analytical and quality control data and stability data), preclinical data, clinical data and/or all submissions made in association with an IND or NDA or other MAA filed in or outside the Territory with respect to such Product(s), in each case to the extent such data either (a) is Controlled by a Party on the Effective Date or (b) comes within a Party’s Control during the term of this Agreement.
1.15 “Development Expenses” means the Direct Costs incurred by a Party in accordance with the approved budget included in the applicable development plan for the [***] (in accordance with Section 2.3.14) or the [***] (in accordance with Section 7.1.1). Development Expenses shall include the cost of any studies detailed in an agreed development plan as well as, in relation to the [***], the costs related to the development and testing of [***].
1.16 “Direct Costs” means the incurred variable costs and fixed costs including, without limitation (a) costs of services provided by contract research organizations and individuals , consultants and contractors, (b) the efforts of the employees of a Party or its Affiliates in performing
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-3-
its activities under [***], and (iii) may be different for each Party, and (c) any other direct costs including out of pocket external costs. In determining “Direct Costs” chargeable under this Agreement, each Party will use its respective project accounting systems consistent with GAAP, and will review its respective project accounting methodologies with the other Party upon the other Party’s reasonable request.
For the purpose of this definition:
(a) “variable costs” shall be deemed to be [***], including, but not limited to, the [***];
(b) “fixed costs” shall be deemed to be the costs of [***] and other fixed costs allocable on a reasonable basis to the development of the Product. All cost determinations shall be made in accordance with GAAP.
1.17 “Existing Product” shall mean a product containing a Patch and/or Gel, whether alone or in combination, together with any ancillary non-pharmacologically active components (e.g. gloves), if MAA Approval has been obtained for such a combination.
1.18 “First Commercial Sale” shall mean the first bona fide, arm’s length sale of a Product in a country following receipt of MAA Approval of such Product in such country; provided that where such a first sale has occurred in a country for which [***].
1.19 “Field” shall mean the [***].
1.20 “Fiscal Year” shall mean the twelve (12) month period beginning on 1 April and ending on 31 March each year.
1.21 “Formosa” shall mean Formosa Laboratories, Inc., Xx. 00-0 Xxxxxx Xxxxxx, Xxxxxx Xxxxxx, Xxxxxx, Xxxxxx 338, Republic of China, and its successors.
1.22 “GAAP” shall mean generally accepted accounting principles in the United States or International Financial Reporting Standards (IFRS) consistently applied.
1.23 “Gel” shall mean the packaged cleansing gel product, as further described in NGX’s EMEA MAA Approval No. EMEA/H/C/000909 and any supplements and amendments to such MAA Approval existing as of the Effective Date, together with any improvements or modifications (including but not limited to line extensions, enhanced or modified presentations and formulations) to such cleansing gel or any other cleansing gel [***], in each case developed by or on behalf of NGX during the term of this Agreement.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-4-
1.24 “Gross Sales” shall mean (i) the gross amounts invoiced for sales of Product by Astellas and its Affiliates and Sublicensees (but not Subdistributors) to Third Parties (including Subdistributors), and (ii) any royalties received by Astellas, its Affiliates or Sublicensees based on sales of Product by a Subdistributor, and (iii) any lump sums received by Astellas, its Affiliates or Sublicensees for the grant of subdistribution rights.
1.25 “IND” shall mean the approval granted by the FDA to conduct the first clinical trial of any Product in man in the United States or the equivalent approval in any other country.
1.26 “Key Metrics” shall mean those criteria set forth in Exhibit 1.26.
1.27 “Know-How” shall mean any information, results and data of any type whatsoever, in any tangible or intangible form whatsoever, including without limitation, databases, ideas, discoveries, inventions, trade secrets, practices, methods, tests, assays, techniques, specifications, processes, formulations, formulae, knowledge, know-how, skill, experience, materials, including pharmaceutical, chemical and biological materials, products and compositions, Data, studies and procedures, drawings, plans, designs, diagrams, sketches, technology, documentation or descriptions.
1.28 “Knowledge of NGX” or “Knowledge” shall mean, with respect to the existence or absence of a fact, the actual knowledge of:
(a) [***];
(b) [***];
(c) [***];
(d) [***];
(e) [***];
(f) [***];
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-5-
(g) [***];
(h) [***]; and
(i) [***].
1.29 “Launch” shall mean First Commercial Sale.
1.30 “Liquid Formulation Product” shall mean NGX-1998, the high concentration liquid formulation of capsaicin currently under development by NGX, together with [***] provided that [***] together with [***].
1.31 “LTS” shall mean LTS Therapie-Systeme AG with its head office at Xxxxxxxxxxxxx 0, 00000 Xxxxxxxxx, Xxxxxxx, and its successors.
1.32 “LTS Agreement” shall mean the Commercial Supply and License Agreement between NGX and LTS entered into on 28 January 2007 and attached as Exhibit 1.32.
1.33 “MAA” shall mean an application requesting regulatory approval, whether as a drug, device, or a combination thereof, for the commercialization of a Product for a particular indication in a country in or outside the Territory, including without limitation a New Drug Application (“NDA”) filed with the U.S. Food and Drug Administration (“FDA”) or a marketing authorization application filed with the FDA’s counterpart in a country or a Region in the Territory. It is understood that MAA does not include applications for pricing or reimbursement approval.
1.34 “MAA Approval” shall mean, with respect to each country in or outside the Territory for a particular Product, approval by the health regulatory authority in such country that is the counterpart of the FDA of the MAA for such Product filed in such country. It is understood that, as used herein, MAA Approval does not include pricing or reimbursement approval.
1.35 “Major Country” shall mean any of United Kingdom, Germany, France, Italy or Spain.
1.36 “Manufacture” shall mean:
(a) the purchase of Patches from (i) LTS under the LTS Tri-Partite Agreement (as defined in the Supply Agreement), (ii) an Alternative Patch Supplier (as defined in the Supply Agreement) in accordance with Section 13.4.4 of the Supply Agreement, or (iii) any other supplier of Patches under the back up manufacturing provisions in Section 13.7.1 of the Supply Agreement or the corresponding provision of the LTS Tri-Partite Agreement;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-6-
(b) the purchase of Gel from (i) CPL under the CPL Tri-Partite Agreement (as defined in the Supply Agreement), (ii) an Alternative Gel Supplier (as defined in the Supply Agreement) in accordance with Section 2.4(b) of the Supply Agreement, or (iii) any other supplier of Gel under the back up manufacturing provisions in Section 13.7.2 of the Supply Agreement or the corresponding provision of the CPL Tri-Partite Agreement;
(c) the right to manufacture Gel (i) in accordance with Section 2.4(b) of the Supply Agreement, or (ii) under the back up manufacturing provisions in Section 13.7.2 of the Supply Agreement or the corresponding provision of the CPL Tri-Partite Agreement;
(d) the purchase of capsaicin from (i) Formosa under the Formosa Tri-Partite Agreement (as defined in the Supply Agreement), (ii) an Alternative API Supplier (as defined in the Supply Agreement) in accordance with Section 2.4(a) of the Supply Agreement; and
(e) the right to manufacture capsaicin (i) in accordance with Section 2.4(a) of the Supply Agreement, or (ii) under the back up manufacturing provisions of the Formosa Tri-Partite Agreement;
in each of (a)-(e) above, either inside or outside the Territory but solely for the purpose of importing, keeping, packaging and having packaged, promoting, marketing, offering for sale, selling, having sold, importing and otherwise distributing and using the Existing Product in the Territory.
1.37 “Net Sales” shall mean Gross Sales less deductions for (a) customary trade, quantity and cash discounts allowed and actually taken; (b) credits to customers on account of rejection of Product; (c) sales and excise taxes and duties and any other similar governmental charges imposed upon the sale of Product (including VAT, but only to the extent such VAT taxes are not reimbursable or refundable; and (d) outbound transportation including freight shipping and insurance costs prepaid or allowed. Notwithstanding the foregoing, the amounts described in clauses (c) and (d) above shall be deducted only to the extent they are stated separately on the invoice and included within gross amounts received from sales of Product. If a Product is sold for consideration other than solely cash, the value of such other consideration shall be included in the calculation of Net Sales. In the case of any sale or disposal of Products among Astellas and its Affiliates and Sublicensees (but not Subdistributors) for resale, Net Sales shall include only Astellas’ or its Affiliates’ or Sublicensees’ (but not Subdistributors’) Gross Sales with respect to the resale of such Product to Third Parties (including Subdistributors), but not the amount invoiced among Astellas and its Affiliates and Sublicensees.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-7-
1.38 “NGX Know-How” shall mean any Know-How that is necessary for the development or commercialization of the Products in the Field in the Territory that either (a) is Controlled by NGX on the Effective Date or (b) comes within NGX’s Control during the term of this Agreement.
1.39 “Patch” shall mean the capsaicin-containing cutaneous patch described in NGX’s EMEA MA Approval No. EMEA/H/C/000909 and any supplements or amendments to such MAA Approval, together with any changes to formulation or other aspects of such patch (including but not limited to line extensions, enhanced or modified presentations and formulations) developed by or on behalf of NGX during the term of this Agreement, [***]. As used in this Agreement, a [***] means [***] such product comprising [***].
1.40 “Patent Rights” shall mean all issued, unexpired patents and all reissues, renewals, re examinations and extensions thereof, and patent applications therefor, and any divisions or continuations, in whole or in part, thereof, including those patents and applications set forth in Exhibit 1.40 which shall be updated from time to time, in each case which would, but for the license granted hereunder, be infringed by the Manufacture, packaging, importation, keeping, promoting, offering for sale, sale, marketing, distributing or use of a Product by Astellas, any of its Affiliates, Sublicensees or Subdistributors within the Field in the Territory, in each case to the extent Controlled by NGX during the term of this Agreement. Patent Rights also includes a Supplementary Certificate of Protection of a member state of the European Union or any similar protective rights in any country in the Territory.
1.41 “PDN” shall mean indication(s) for the Product relating to pain in connection with neuropathy or neuralgia associated with diabetes.
1.42 “PNP” shall mean indication(s) for the Product relating to the general treatment or mitigation of peripheral neuropathic pain.
1.43 “Product” shall mean (i) the Existing Product, and (ii) the Liquid Formulation Product to the extent Astellas has executed its Option under Section 2.3 below.
1.44 “Product Trademark” shall mean the QUTENZA trademark including those registered trademarks and applications for QUTENZA set forth in Exhibit 1.44, or another mutually agreed trademark for the Existing Product (including the Alternative Trademark), in either case which shall be owned by NGX worldwide.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-8-
1.45 “Region” shall mean a set of countries in the Territory, specified in Exhibit 1.48 as a single “region” for commercialization of the Products hereunder. It is understood and agreed that [***] constitute a single Region (“European Region”).
1.46 “Subdistributor” shall mean a Third Party appointed by Astellas or one of its Affiliates or Sublicensees as a distributor of a Product, in accordance with Section 2.4.1 and 2.4.3, which Third Party purchases Product in [***]. For the avoidance of doubt, “Subdistributor” shall specifically exclude Sublicensees.
1.47 “Sublicensee” shall mean a Third Party to whom Astellas has granted, in accordance with Section 2.4.2 and 2.4.3, a sublicense under the Patent Rights and/or NGX Know-How to Manufacture, package and have packaged, develop, register, keep, import, offer for sale, sell, have sold, use market, distribute and/or promote a Product in any country or countries in the Territory other than [***] and the countries comprising [***].
1.48 “Territory” shall mean the European Region (as defined in Exhibit 1.48), the countries of the SATR Region and the Rest of the Territory Region (as defined in Exhibit 1.48), and such other countries as may be added by written agreement between the Parties.
1.49 “Third Party” shall mean any party other than Astellas, NGX and their Affiliates.
1.50 “UC” shall mean The Regents of the University of California, 0000 Xxxxxxxx Xxxxxx, Xxxxxxx, Xxxxxxxxxx 00000.
1.51 “UC License Agreement” shall mean the Exclusive License Agreement between NGX and UC, effective as of November 1, 2000, together with any amendments in effect as of the Effective Date, as attached hereto as Exhibit 1.51.
1.52 “Valid Patent Claim” shall mean a claim of an issued and unexpired patent included within the Patent Rights which has not been abandoned, cancelled or held permanently revoked, unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction unappealed within the time allowed for appeal, or which has not been admitted to be invalid or unenforceable through reissue or disclaimer or otherwise.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-9-
In addition, the following terms shall have the meaning described in the corresponding section of this Agreement. Other terms may be defined throughout the Agreement and its Exhibits.
| Term |
Section Defined | |
| “Adverse Proceedings” |
11.4.2 | |
| “Agreement” |
Preamble | |
| “Alliance Manager” |
5.7 | |
| “Annual Net Sales” |
4.1.1 | |
| “Applicator” |
2.5.7 | |
| “Assumed Patent” |
11.3.5 | |
| “Astellas” |
Preamble | |
| “Astellas Improvements” |
11.1.4 | |
| “Astellas Indemnitees” |
16.2 | |
| “Clinical Supplies” |
4.2.1 | |
| “Commercialization Plan” |
5.1 | |
| “Confidential Information” |
13.1 | |
| “Costs” |
11.4.2 | |
| “Damages” |
11.4.1 | |
| “Developing Party” |
7.1.1(a)(ii)(2) | |
| “Document” |
6.3.1 | |
| “Dominating Patent Rights” |
4.2.3 | |
| “Earned Royalty” |
4.1 | |
| “Effective Date” |
Preamble | |
| “EMEA Requirements” |
7.1.1(a)(ii) | |
| “Enforcement Actions” |
11.5 | |
| “Exclusivity Period” |
2.7.1 | |
| “Existing Product MAA Approval” |
6.1 | |
| “Extraterritorial DPR” |
4.3 | |
| “Floor” |
4.2.3 | |
| “Generic Competition” |
4.2.2(a) | |
| “Generic Product” |
4.2.2(a) | |
| “Generic Sales” |
4.2.2(b)(i) | |
| “Indemnitee,” “Indemnitor” |
16.3 | |
| “Infringement Actions” |
11.4.1 | |
| “Infringing Product” |
11.5 | |
| “Initiating Party” |
11.5.2 | |
| “Joint IP” |
11.1.2 | |
| “Joint Commercialisation Committee”, “JCC” |
5.4 | |
| “Joint Development Committee”, “JDC” |
5.3 | |
| “Joint Patents” |
11.1.2 | |
| “Joint Steering Committee,” “JSC” |
5.2 | |
| “Joint Regulatory Committee”, “JRC” |
5.5 |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-10-
| Term |
Section Defined | |
| “LF Development Plan” |
2.3.4 | |
| “LF Trademark” |
12.9 | |
| “Liabilities” |
16.1 | |
| “Licensing Option” |
2.7.7 | |
| “Licensing Option Product” |
2.7.7 | |
| “Licensing Option Term” |
2.7.7(b) | |
| “LTS Royalties” |
4.4.2 | |
| “Maintenance” |
6.5 | |
| “Marketing” |
Exhibit 1.26 | |
| “Material Failure to Exploit” |
Exhibit 1.26 | |
| “NGX” |
Preamble | |
| “NGX Improvements” |
11.2.5 | |
| “NGX Indemnitees” |
16.1 | |
| “Non-Generic Sales” |
4.2.2(b)(i) | |
| “Option Fee” |
3.3.1 | |
| “Option Retention Fee” |
3.3.2 | |
| “Option Exercise Fee” |
3.3.3 | |
| “Option Period [***]” |
2.3.2 | |
| “Option Period [***] Deliverables” |
Exhibit 2.3.4 | |
| “Option Period [***]” |
2.3.2 | |
| “Option Period [***] Deliverables” |
Exhibit 2.3.7 | |
| “Party,” “Parties” |
Preamble | |
| “PDN Trial” |
7.1.1(a)(ii) | |
| “Post-Marketing Studies” |
7.1.1(a)(iii) | |
| “Product Liability Claim” |
16.1 | |
| “Promotional Samples” |
4.2.1 | |
| “Product Materials” |
18.3.4 | |
| “QA Samples” |
4.2.1 | |
| “QPPV” |
8.4 | |
| “Reference Product” |
4.2.2(a)(iii) | |
| “Regulatory Request” |
6.4 | |
| “Reimbursement Date” |
2.5.5; 4.3.4 | |
| “Royalty Adjustment” |
4.6.2(a) | |
| “Rejected IP” |
4.3.5 | |
| “Safety Study” |
7.1.1(a)(i) | |
| “Sales Materials” |
8.3.2 | |
| “Supply Agreement” |
9.1 | |
| “Terminated Product” |
18.3 | |
| “Terminated Region” |
18.4 | |
| “Third Party Claim” |
16.1 | |
| “Third Party IP” |
2.5 |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-11-
| Term |
Section Defined | |
| “Third Party Right” |
11.4.1 | |
| “Total UC Royalties” |
4.4.1 | |
| “UC Royalties” |
4.4.1 | |
| “UC Royalty Cap” |
4.4.1 | |
| “Wind-down Period” |
18.3, 18.4 | |
| “Withdrawal Notice” |
5.6.6 |
2.1 Appointment During the term of this Agreement, NGX appoints Astellas, and Astellas hereby accepts such appointment, as the exclusive (even as to NGX and its Affiliates) distributor and marketer of the Existing Product in the Field in the Territory.
2.2 License
2.2.1 Subject to the terms and conditions of this Agreement, NGX grants to Astellas:
(a) an exclusive (even as to NGX and its Affiliates) license during the term of this Agreement under the Patent Rights and NGX Know-How to: (i) register (including conducting such clinical trials as may be required to support such registrations) the Existing Product (ii) import, keep, package and have packaged the Components supplied by NGX, and (iii) promote, market, offer for sale, sell, have sold, import and otherwise distribute and use the Existing Product in the Territory for any and all indications in the Field; and
(b) a license (co-exclusive with NGX) during the term of this Agreement under the Patent Rights and NGX Know-How to Manufacture Components for the exclusive use by Astellas in the Territory and Field.
Notwithstanding the foregoing, it is understood that the licenses granted above shall exclude [***].
NGX reserves all rights not expressly granted herein. Additionally it is understood and agreed that Astellas shall have the right to: (x) carry out marketing activities outside the Territory, in co-operation with NGX, for the promotion of sales of Existing Product (by Astellas, any Astellas Affiliate, Sublicensee or Subdistributor) in the Territory strictly in accordance with the license and terms set forth in Section 1.2 of Exhibit 2.2.1; and (y) carry out clinical trials outside the Territory, in co-operation with NGX, for the development (by Astellas or any Astellas Affiliate) of Existing Product which is to be promoted, marketed and sold in the Territory, strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-12-
2.2.2 During the term of this Agreement, NGX shall not, and shall not license or authorise any Affiliate or Third Party to develop (save in accordance with this Agreement), register, promote, market, offer for sale, sell, have sold, import and otherwise distribute and use and sell
(a) Components and/or Existing Products within the Field and Territory; or
(b) [***], save that it may carry out pre-clinical development activities provided that notwithstanding the foregoing, NGX shall have the right to (x) carry out marketing activities in the Territory, in co-operation with Astellas, for the promotion of sales of Existing Product and Liquid Formulation Product (by NGX, any NGX Affiliate or their licensees) outside of the Territory strictly in accordance with the license and terms set forth in Section 1.3 of Exhibit 2.2.1. and (y) carry out clinical trials inside the Territory, in co-operation with Astellas, for the development by NGX of Existing Product which is to be promoted, marketed and sold outside the Territory, strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1.
2.2.3 Extension of License to Affiliates. Astellas shall have the right to exercise the licenses granted in Section 2.2 through one or more of its Affiliates, for as long as such entity remains an Affiliate of Astellas, provided that (a) Astellas hereby warrants and guarantees the performance of, and compliance with, the obligations set forth in this Agreement by its Affiliates, and (b) Astellas shall remain responsible for all other payments and other obligations under this Agreement arising from activities of its Affiliates.
2.3 Liquid Formulation Product Option
2.3.1 Subject to the receipt by NGX of the Option Fee specified in Section 3.3.1, NGX shall grant and does hereby grant to Astellas an exclusive option to include the Liquid Formulation Product as a Product under this Agreement (“Option”).
2.3.2 The Option shall commence upon receipt by NGX of the Option Fee and shall continue until the expiry of the [***] in Section 2.3.12 (“Option Period”). The Option Period shall be [***] (as defined below). Following the exercise of the Option by Astellas in accordance with Section 2.3.12 the Parties shall carry out Phase III trials of the Liquid Formulation Product in accordance with Section 2.3.14.
2.3.3 During the Option Period, NGX and its Affiliates shall not offer to or grant to any Third Party any right in or to the Liquid Formulation Product in the Field and Territory.
2.3.4 Within [***] of the payment of the Option Fee, NGX shall draw up a draft development plan for the Liquid Formulation Product (“LF Development Plan [***]”) which shall include [***] of the LF Development Plan [***] being described in Exhibit 2.3.4.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-13-
2.3.5 NGX shall present the LF Development Plan [***] to the JDC within the [***] period described in Section 2.3.4, following which the JDC will finalise the LF Development Plan [***], which shall be agreed by the JSC within a further [***] period, such that the LF Development Plan [***] shall be agreed by the JSC within [***]from the Effective Date.
2.3.6 Following the agreement of the LF Development Plan [***], NGX shall be responsible for performing the tasks allocated to it under the LF Development Plan [***] in respect of Option Period [***], subject to the oversight of the JDC and ultimately the JSC. NGX shall carry out such tasks in a [***]. [***].
2.3.7 Following completion of the activities set out in the LF Development Plan [***] that are to be carried out in [***], NGX shall provide Astellas with the [***] and a draft “LF Development Plan [***]” setting out the activities to be carried out in [***] which shall include at least [***] and the [***].
2.3.8 At any time up to [***] after the date of delivery to Astellas of the complete [***] and the draft LF Development Plan [***], Astellas may provide NGX with written notice of its intent to retain the Option. If it wishes to retain the Option then it shall pay the Option Retention Fee in accordance with Section 3.3.2.
2.3.9 If Astellas notifies NGX in accordance with Section 2.3.8 that it wishes to retain the Option and has paid the Option Retention Fee in accordance with Section 3.3.2, then within [***] of the payment of the Option Retention Fee the JDC shall finalise the LF Development Plan [***], which shall be agreed by the JSC within the same [***] period. For clarity, if Astellas does not provide NGX with written notice of its intent to retain the Option within the period set forth in Section 2.3.8 above, or fails to pay the Option Retention Fee in accordance with Section 3.3.2, then Astellas will be deemed to have declined to retain the Option, in which case the Option shall terminate and Sections 2.3.10 – 2.3.15 shall not apply.
2.3.10 Following the agreement of the LF Development Plan [***], NGX shall be responsible for performing the tasks allocated to it under the LF Development Plan [***] in respect of [***], subject to the oversight of the JDC and ultimately the JSC. NGX shall carry out such tasks in a diligent and sustained manner. All Development Expenses connected therewith shall be the responsibility of NGX.
2.3.11 Following completion of the activities set out in the LF Development Plan [***] that are to be carried out in [***], NGX shall provide Astellas with the [***] and a draft LF Development Plan [***] setting out the Phase III clinical trial activities that are to be carried out if Astellas exercises the Option.
2.3.12 At any time up to [***] after the date of delivery to Astellas of the complete [***] and the draft LF Development Plan [***], Astellas may provide NGX with written notice of its intent to exercise the Option. If it wishes to exercise the Option then it shall pay the Option Exercise Fee in accordance with Section 3.3.3.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-14-
2.3.13 If Astellas notifies NGX in accordance with Section 2.3.12 that it wishes to exercise the Option and has paid the Option Exercise Fee in accordance with Section 3.3.3, then within two months of the payment of the Option Exercise Fee the JDC shall finalise the LF Development Plan [***], which shall be agreed by the JSC within the same [***] period. If:
(a) Astellas does not provide NGX with written notice of its intent to exercise the Option within the period set forth in Section 2.3.12 above;
(b) Astellas fails to pay the Option Exercise Fee in accordance with Section 3.3.3; or
(c) the JSC (acting through the JDC) is unable to agree upon a final LF Development Plan [***] within the [***] period described above (or any extension of such period mutually agreed upon in writing by the Parties) due to deadlock;
then Astellas will be deemed to have declined to have exercised the Option, in which case the Option shall terminate and Sections 2.3.14 and 2.3.15 shall not apply. In the event of deadlock as described in Section 2.3.13(c), NGX shall refund Astellas the Option Exercise Fee within [***] of the end of the [***] period described above (or any extension of such period mutually agreed upon in writing by the Parties).
2.3.14 Following the agreement of the LF Development Plan [***], the JDC shall oversee the performance of the Phase III clinical trial activities and shall allocate responsibility for the performance of the various tasks required in connection with such activities based upon which Party is better situated to perform such tasks, taking into account among other things, the estimated costs associated with having each Party perform such task. The aim of the LF Development Plan [***] will be to generate clinical data which is intended for, and in the correct format for, submission to satisfy the requirements of both the EMEA and the FDA. The Development Expenses for such activities will be borne [***].
2.3.15 If Astellas has exercised its Option in accordance with Section 2.3.12 and upon NGX’s receipt of the appropriate fee in accordance with Section 3.3.3, the Liquid Formulation Product shall thereafter be considered a “Product” for the purposes of this Agreement and NGX shall:
(a) appoint Astellas as the exclusive (even as to NGX and its Affiliates) distributor and marketer of the Liquid Formulation Product in the Field in the Territory;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-15-
(b) grant to Astellas an exclusive (even as to NGX and its Affiliates) license under the Patent Rights and Know-How to co-develop, register, promote, market, sell, have sold, keep, import and otherwise distribute and use the Liquid Formulation Product in the Territory for any and all indications in the Field, and to appoint Subdistributors to do the same, in each case as further described in Section 2.4;
(c) grant to Astellas a license under the Patent Rights and Know-How to make, have made, package, and have packaged the Liquid Formulation Product for the exclusive use by Astellas in the Territory and Field providing that the Parties shall use [***] to supply the Liquid Formulation Product to each Party; and
(d) grant to Astellas the right to: (x) carry out marketing activities outside the Territory, in co-operation with NGX, for the promotion of sales of Liquid Formulation Product (by Astellas, any Astellas Affiliate, Sublicensee or their Subdistributor) in the Territory strictly in accordance with the license and terms of Section 1.2 of Exhibit 2.2.1 and (y) carry out [***], strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1, provided that Astellas shall grant to NGX the right to (x) carry out [***] strictly in accordance with the license and terms set forth in Section 1.3 of Exhibit 2.2.1. and (y) carry out [***], strictly in accordance with the license and terms set forth in Section 3 of Exhibit 2.2.1.
2.3.16 If Astellas does not retain the Option in accordance with Section 2.3.9 or does not exercise the Option in accordance with Section 2.3.13, for the term of the Exclusivity Period [***]. For clarity, it is acknowledged and agreed that the only rights and obligations of Astellas and NGX under this Section 2.3 are as expressly stated herein, and that there are no further implied obligations relating to the matters contemplated herein. As used herein, Regulatory Filing shall mean a marketing authorisation application in accordance with Title III, Chapter 1 of Directive 2001/83/EC, as amended (i.e., either a full marketing authorisation application or an abbreviated marketing authorisation application), or any equivalent filing made with a regulatory authority in the Territory.
2.4 Subdistributors and Sublicensees
2.4.1 Subdistributors. Astellas shall have the right, upon the approval of NGX, not to be unreasonably withheld or delayed, to appoint Third Parties as Subdistributors to distribute, sell, market and promote the Product in the Territory. For clarity, it is understood that the decision to treat sales to Subdistributors as Net Sales for royalty purposes under this Agreement (as opposed to basing Net Sales on such Subdistributors’ subsequent sale to Third Parties as is the case for sales by Affiliates and Sublicensees) was predicated on the understanding that Astellas’ use of Subdistributors would be limited to countries [***]. Consequently, it is understood and agreed that it shall not be unreasonable for NGX to withhold its approval with respect to the appointment of Subdistributors in [***] if such appointments would materially alter the economics for NGX under this Agreement with respect to the European Region.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-16-
2.4.2 Sublicensees. Astellas shall have the right, upon the approval of NGX, not to be unreasonably withheld or delayed, to grant sublicenses to Third Parties under the Patent Rights and/or NGX Know-How to Manufacture, package and have packaged, develop, register, keep, import, offer for sale, sell, have sold, use, market, distribute and/or promote a Product in any country or countries in the Territory other than [***].
2.4.3 Astellas shall ensure that all Subdistributors and Sublicensees are bound by written agreements which (a) shall not conflict with, and shall be subordinate to, the terms and conditions of this Agreement, and (b) shall contain provisions which are as protective of NGX and the Product(s) as those contained in this Agreement, including without limitation Sections 2.7.8, 7.2, 8.3 and 13. Astellas hereby warrants and guarantees the performance of, and compliance with, the obligations set forth in this Agreement by its Sublicensees and Subdistributors and shall remain responsible for all payments and other obligations under this Agreement arising from activities of its Sublicensees and Subdistributors. Without limiting the foregoing, in the event that Products supplied by Astellas, its Affiliates or Sublicensees to a Subdistributor are being directly or indirectly [***], Astellas agrees that, upon becoming aware of such sale or use or otherwise upon the request of NGX, it shall: [***].
2.5 NGX IP Acquired after the Effective Date
If, after the Effective Date, NGX acquires from a Third Party subject matter that would fall within the definition of Patent Rights and/or NGX Know-How in each case only to the extent that the Patent Rights or NGX Know How covers the Existing Product (“Third Party IP”), then the following shall apply:
2.5.1 Astellas at its sole discretion can decide that it wishes to obtain a license to such Third Party IP.
2.5.2 If Astellas decides that it wishes to obtain a license to the Third Party IP, the licenses granted under Section 2.2 above with respect to such Third Party IP shall be subject to [***] for the applicable Product outside the Territory to whom NGX has granted a license under such Third Party IP.
2.5.3 To the extent such Third Party IP constitutes [***] below.
2.5.4 Upon request by Astellas, NGX shall disclose to Astellas a true, complete and correct written description of such payment obligations including a copy of the license agreement between NGX and such Third Party, and Astellas’ obligation to reimburse such amounts following such request shall be limited to those payment obligations so disclosed by NGX.
2.5.5 If Astellas decides not to obtain a license to the Third Party IP or in the event [***], having notified NGX that it wishes to obtain a license to the Third Party IP, then such Third Party IP shall [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-17-
2.5.6 Should Astellas have declined to accept a license to such Third Party IP, then in the event that [***] then Astellas shall [***]. In the event of an [***] this Section 2.5, Section [***] shall apply to the defense and settlement of [***].
2.5.7 For clarity, any Third Party intellectual property that NGX may elect to in-license with respect to the [***] shall not constitute Third Party IP for purposes of this Section 2.5, and shall instead be subject to the following: [***] and (b) in the event Astellas exercises its Option under Section 2.3.12, [***] of the Liquid Formulation Product.
2.6 No Modifications to the Product
Except upon mutual agreement of the Parties in writing, Astellas covenants and warrants that neither Astellas nor its Affiliates or Sublicensee shall:
2.6.1 save for any modifications that [***], make any modifications to the Existing Product save to [***] and add [***] provided this is in accordance with the relevant MAA Approval and applicable laws and regulations; nor
2.6.2 use any Confidential Information of NGX for any purpose other than in connection with this Agreement.
2.7 No Conflict
2.7.1 [***]. Subject to Sections 2.7.2 and 2.7.3, Astellas and its Affiliates shall not, by themselves or through any Third Party(ies) [***] for a period starting on the Effective Date and ending [***] after the first First Commercial Sale of the Existing Product in a [***] unless NGX and Astellas have agreed in writing that such [***] are [***].
2.7.2 Astellas shall not be in breach of Section 2.7.1 of this Agreement if, during the Exclusivity Period, [***]. For the avoidance of doubt, [***].
2.7.3 Astellas shall not be in breach of Section 2.7.1 of this Agreement if Astellas [***] following the date that such [***] provides that Astellas (or its Affiliates) no longer [***] based on [***].
2.7.4 [***]. Subject to Sections 2.7.5 and 2.7.6, NGX and its Affiliates shall not, by themselves or through any Third Party(ies) [***].
2.7.5 NGX shall not be in breach of Section 2.7.4 of this Agreement if, [***]. For the avoidance of doubt, [***].
2.7.6 NGX shall not be in breach of Section 2.7.4 of this Agreement if NGX [***] provided that NGX [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-18-
2.7.7 [***]. If, at any time prior to the end of the Exclusivity Period, NGX wishes to [***] for the Territory (a “Licensing Option Product”), NGX grants Astellas [***] in respect of such Licensing Option Product. The [***] as follows:
(a) when NGX decides that it wishes to [***] for the Territory, it will [***] of the general type and quantity that NGX would typically [***]; and
(b) within [***] (or such other longer period as the Parties may agree) of receipt of the notice and [***] in Section 2.7.7(a) above (the “[***]”), Astellas will inform NGX in writing whether [***];
(c) If Astellas notifies NGX that it [***] in relation to the [***] that is the subject of the notice in Section 2.7.7(a), then the Parties will enter into negotiations to agree a licensing agreement between them. In such [***]. Such [***] shall be consistent with the [***] within [***], and shall include, but not be limited to, [***].
(d) If Astellas notifies NGX pursuant to Section 2.7.7(b) that it [***] that is the subject of the notice in Section 2.7.7(a), or Astellas [***] then NGX will have [***].
2.7.8 Ex-Territory; No Exploitation except as Licensed. Except as otherwise permitted under Sections 1.2 and 3 of Exhibit 2.2.1 or required by applicable law, neither Astellas nor its Affiliates or Sublicensees or their respective Subdistributors will [***], only in accordance with and under this Agreement. Astellas agrees that it and its Affiliates and Sublicensees shall not use nor otherwise exploit Patent Rights, Data and the Product Trademark, except as licensed in this Agreement.
3. INITIAL PAYMENTS AND MILESTONES
3.1 Initial Payment
In partial consideration of the costs incurred by NGX in connection with the research and development of the Existing Product and in exchange for the exclusive rights granted herein, and provided that NGX has provided to Astellas an invoice for the total sum (which may be sent by e-mail or facsimile), Astellas shall pay NGX Thirty Million Euros (€30,000,000) within [***] of the execution of this Agreement. For purposes of clarity, it is understood and agreed that this payment shall be non-refundable and non-creditable save as set out in Section 16.4. For further clarity, the term “non-refundable and non-creditable” as used in this Agreement shall not act as any limitation on any amounts obtainable by Astellas by way of damages resulting from NGX’s breach of this Agreement or any obligation under it.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-19-
3.2 Sales Milestones
In further consideration of the exclusive rights granted herein, Astellas shall pay NGX in accordance with the procedure set out in Section 4.6.1(a) and (b), the following milestone payments upon achievement of the corresponding sales milestones, which milestone payments shall be non-refundable and non-creditable:
3.2.1 Upon first achieving Annual Net Sales equal to or exceeding [***] in any Fiscal Year, Astellas shall pay NGX [***].
3.2.2 Upon first achieving Annual Net Sales equal to or exceeding [***] in any Fiscal Year, Astellas shall pay NGX [***].
3.2.3 Upon first achieving Annual Net Sales equal to or exceeding [***] in any Fiscal Year, Astellas shall pay NGX [***].
3.2.4 Upon first achieving Annual Net Sales equal to or exceeding [***] in any Fiscal Year, Astellas shall pay NGX [***].
The sales milestones are payable only once and are not on a Product-by-Product basis.
3.3 Option Fee/Option Exercise Fee
3.3.1 Option Fee. In consideration for the Option granted to Astellas with respect to the Liquid Formulation Product, and provided that NGX has provided to Astellas an invoice for the total sum (which may be sent by e-mail or facsimile), Astellas shall pay NGX Five Million Euros (€5,000,000) ) within [***] of the execution of this Agreement (“Option Fee”). For purposes of clarity, it is understood and agreed that this payment shall be non-refundable and non-creditable [***].
3.3.2 Option Retention Fee. In the event that Astellas elects to retain its Option with respect to the Liquid Formulation Product pursuant to Section 2.3.8 above, Astellas shall pay NGX [***] within [***] of such notice of retention provided that NGX has provided to Astellas an invoice for the total sum (which may be sent by e-mail or facsimile) (“Option Retention Fee”). For purposes of clarity, it is understood and agreed that this payment shall be non-refundable and non-creditable
3.3.3 Option Exercise Fee. In the event that Astellas elects to exercise its Option with respect to the Liquid Formulation Product pursuant to Section 2.3.12 above, Astellas shall pay NGX [***] within [***] of such exercise provided that NGX has provided to Astellas an invoice for the total sum (which may be sent by e-mail or facsimile) (“Option Exercise Fee”). For purposes of clarity, it is understood and agreed that this payment shall be non-refundable and non-creditable save as set out in Section 2.3.13.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-20-
4. ROYALTIES
4.1 Royalty In further consideration of the exclusive rights granted herein, Astellas shall, subject to the reduction of royalty rate pursuant to Section 4.2 below, pay to NGX the following running royalties on the Net Sales of the Product in the Territory (“Earned Royalty”):
| Annual Net Sales |
Percentage of Net Sales | |
| That portion of Annual Net Sales up to and including [***] | [***] of such Net Sales | |
| That portion of Annual Net Sales above [***] and up to and including [***] | [***] of such Net Sales | |
| That portion of Annual Net Sales above [***] and up to and including [***] | [***] of such Net Sales | |
| That portion of Annual Net Sales above [***] | [***] of such Net Sales | |
4.1.1 For purposes of this Agreement, “Annual Net Sales” shall mean total Net Sales of Products in each Fiscal Year occurring in those countries in the Territory in which Astellas’ royalty obligations have not yet expired. For clarity, it is understood that in the event that Astellas exercises its Option with respect to the Liquid Formulation Product, Annual Net Sales shall mean [***] in the Territory by or under authority of Astellas in each Fiscal Year. Units of Product shall be considered sold upon [***]. Notwithstanding the foregoing, to the extent that this Agreement would have expired with respect to a particular Product in a particular country but for the fact that [***], then it is understood that no Earned Royalties shall be payable in respect of such Joint Patents or Assumed Patents in accordance with Sections 11.1.3 and 11.3.5.
4.2 Certain Reductions to Royalties
4.2.1 Non-Commercial Sales. No Earned Royalty shall be due hereunder with respect to (a) [***] of Product used as samples for training programs, educational programs or customer demonstration (“Promotional Samples”) and (b) [***] of Product used for destructive testing, such as QA/QC or stability testing (“QA Samples”), and (c) [***] of Product used in clinical trials of Product (“Clinical Supplies”) conducted in accordance with Section 7.
4.2.2 Loss of Exclusivity. In the event of Generic Competition with respect to a Product in a country in the Territory, the Earned Royalty applicable to units of the Product in such country shall be reduced to [***] of the Net Sales from the Product from the beginning of the [***] after (a) [***] (b) [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-21-
(a) Certain Definitions
(i) “Generic Competition” in a country shall mean that one or more Generic Product(s) and/or Reference Product(s) is/are sold by an entity (including but not limited to NGX) who is not acting under the authority of Astellas, its Affiliates, Sublicensees or Subdistributors without infringing any Patent Rights and/or regulatory exclusivity in such country.
(ii) “Generic Product” shall mean:
(1) a product for [***], and
(2) which has been [***]; or
(3) [***].
(iii) “Reference Product” shall mean [***].
(b) Application of the Generic Royalty to the Tiered Earned Royalty Structure
(i) The Parties agree that the Net Sales of Product subject to a reduction of the Earned Royalties in accordance with this Section 4.2.2 (the “Generic Sales”) will be included in the Annual Net Sales for the relevant calendar year, for purposes of calculating the Earned Royalty for the rest of the Product sold in such calendar year (the “Non-Generic Sales”) as follows: the [***].
(ii) [***].
4.2.3 [***]. In the event Astellas or its Affiliates is required to pay to a Third Party [***] in order to obtain [***], Astellas may [***] to NGX under this Agreement, provided that in no event shall the [***] under Section 4.1 (the “[***]”). If the existence of the [***] results in Astellas not being able to [***] then Astellas can [***]. As used herein, “[***]” shall mean Third Party [***], including without limitation Third Party [***] under Section 11.4.1, without [***]. Notwithstanding the foregoing, it is agreed by the Parties that [***].
4.3 [***]
If Astellas’ license to [***] extends to the equivalent [***], then the following shall apply:
4.3.1 NGX at its sole discretion can decide that it wishes to [***].
4.3.2 If NGX decides that it wishes to obtain [***], then the grant of such [***] shall be subject to NGX [***] by reason of NGX’s [***] together with NGX’s [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-22-
4.3.3 Upon request by NGX, Astellas shall disclose to NGX a true, complete and correct written description of such [***], and NGX’s obligation to [***].
4.3.4 If NGX decides not to obtain a [***] or in the event NGX does not promptly [***] upon request, having notified Astellas that it wishes to obtain a [***], then such [***] to NGX, until NGX notifies Astellas that it wishes to obtain a [***].
4.3.5 Should NGX have declined to accept [***], then in the event that Astellas is subsequently subject to [***] as a result of NGX’s acts of [***], then NGX shall [***]. In the event of [***].
4.4 Existing NGX Third Party Royalties
4.4.1 UC License Agreement. [***] the royalties accruing on sales of Product by Astellas, its Affiliates, Sublicensees and Subdistributors in the Territory under Section 7.1 of the UC License Agreement, at the royalty rate of zero point five percent (0.5%) [***] until such time as the total amount of royalties that have been paid to UC in a given calendar year on world-wide sales of Products that would be considered “Licensed Product(s)” under the UC License Agreement (including amounts paid by NGX attributable to sales outside the Territory) (collectively, “Total UC Royalties”) equals One Million Dollars (US $1,000,000) (“UC Royalty Cap”). At such time as the total amount of Total UC Royalties that has been paid to UC in a given calendar year reaches the UC Royalty Cap, [***] under this Section 4.4.1 during the remainder of such calendar year. For the avoidance of doubt, the UC Royalties shall [***] such that they are [***] to the extent that NGX has an obligation to account to UC for such royalties under the UC Agreement.
4.4.2 LTS Agreement. [***] the royalties at the rate of one point sixty-seven percent (1.67 %) accruing on sales of the Existing Product by Astellas and its Affiliates and Sublicensees under Section 5.2(a) of the LTS Agreement, as of the Effective Date [***] after the end of each calendar half-year (i.e. after 30 June and 31 December of each calendar year). For the avoidance of doubt, the LTS Royalties shall [***] such that they are [***] to the extent that NGX has an obligation to account to LTS for such royalties under the LTS Agreement.
4.5 Discounting Astellas and its Affiliates shall set prices for the Product in the Territory in the best interest of the commercial success of the Product in the Territory. Without limiting the foregoing, if Astellas or its Affiliate, Sublicensee or Subdistributor sells any Product to a customer who also purchases other products or services from Astellas, its Affiliates, Sublicensees or Subdistributors and receives a specific discount for such “bundling” of products, Astellas shall ensure that for purposes of calculating the Earned Royalty due to NGX, [***]. Without limiting the foregoing obligations, it is understood and agreed that nothing in this Agreement is intended to dictate to Astellas, its Affiliates, Sublicensees and Subdistributors the resale prices for the Products in the Territory.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-23-
4.6 Royalty Reports
4.6.1 Quarterly Report
(a) Following the First Commercial Sale of a Product in the Territory, Astellas shall furnish to NGX a quarterly written report detailing on a country-by-country and product-by-product basis: (i) the Gross Sales of all Product(s) sold during such calendar quarter on a country-by-country and product-by-product basis, the calculation of Net Sales of such Product(s) from such Gross Sales (with all the Net Sales of Product subject to a reduction of the Earned Royalties in accordance with Section 4.2.2 (the “Generic Sales”) being clearly identified as such); (ii) the Earned Royalties, [***] (if applicable), payable in United States Dollars, which shall have accrued hereunder based upon such Net Sales of such Product(s) by Astellas and its Affiliates and/or Sublicensees; (iii) any adjustments to the Earned Royalties in accordance with Section 4.2; (iv) the date of the First Commercial Sale of each Product in each country in the Territory; (v) the exchange rates used in determining the amount of United States Dollars, as more specifically provided in Section 10.3 below, (vi) a statement as to whether any Sales Milestones in accordance with Section 3.2 have been reached in such quarter and (vii) any additional information reasonably required to support NGX’s reporting requirements as a public company. Reports shall be due [***] following the end of each calendar quarter.
(b) Following the delivery of each such report, within [***] following receipt of NGX’s invoice (which may be sent by e-mail or facsimile) Astellas shall, pay to NGX the total Earned Royalties, Sales Milestones, [***], if any, due to NGX for the period of such report. If no Earned Royalties, [***] are due, Astellas shall so report.
(c) Additionally, in any calendar year in which the [***], then upon NGX’s receipt of the final quarterly report for such calendar year from Astellas, NGX shall calculate each Party’s allocable share of the [***] based on each Party’s contribution to the total global annual sales of the Existing Product in such calendar year. NGX will provide Astellas with a report informing Astellas of the difference, if any, between each Party’s allocable share of such [***] in royalties (as calculated above) and the royalty amounts actually paid by the Parties in connection with [***] in such calendar year. The Party having paid higher royalties in connection with [***] than its calculated share shall provide the other Party with an invoice with respect to any amounts due. Payment of any such amounts owed to by either Party to the other shall be made within twenty (20) days after receipt of the other Party’s invoice.
4.6.2 Year End Report
(a) In any Fiscal Year in which (i) Astellas, its Affiliates and/or Sublicensees have incurred payment obligations under Section 4.1 above, and (ii) there are Generic Sales, Astellas shall provide to NGX a year end report detailing: (a) the total Generic Sales and Non-Generic Sales on a [***] for the just concluded calendar year; (b) the calculation of the
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-24-
total Earned Royalties due for such calendar year based on the application of Section 4.2.2(b) above; and (c) the difference, if any, between the amount calculated pursuant to subsection (b) of this Section 4.6.2 and the total Earned Royalties actually paid to NGX under Section 4.6.1 for such Fiscal Year (such difference, the “Royalty Adjustment”). Year end reports shall be due [***] following the end of the Fiscal Year to which they relate.
(b) Following the delivery of each such report, Astellas shall, within [***] following receipt of NGX’s invoice, pay to NGX any Royalty Adjustment owed to NGX. If a Royalty Adjustment is owed to Astellas by NGX, such Royalty Adjustments shall be [***].
(c) The year end report and the final quarterly report for a given Fiscal Year may be combined into a single document provided all required information are combined therein.
5. COOPERATION
JSC and Commercialization Plan for Existing Product
5.1 Commercialization Plan
The initial plan for Astellas’ development and commercialization of the Existing Product in the Territory (“Commercialization Plan”) will be provided by Astellas to the JSC [***] months, of the Effective Date. Astellas agrees to provide to NGX [***] updated versions of the Commercialization Plan at least [***] at the next JSC meeting [***], and any material modification or addition to the Commercialization Plan within a reasonable period of time prior to adoption and implementation thereof. It is understood and agreed that the Commercialization Plan shall be reasonably detailed including without limitation, [***]. The Parties agree that [***] shall be subject to NGX’s (and not the JSC’s) approval not to be unreasonably withheld, delayed or conditioned.
5.2 Joint Steering Committee
The Parties shall establish a committee (the “Joint Steering Committee” or “JSC”) to (a) oversee the development and commercialization activities relating to Products in the Territory during the term of this Agreement; (b) oversee the JDC, JRC and JCC (and any other committees set up under the control of the JSC) and [***] and (c) resolve matters on [***]. Each of such Committees shall have the responsibilities and authority allocated to it in this Section 5.2.
5.2.1 In addition to its overall responsibilities described in Section 5.2, subject to Section 5.6.2 and its subsections, the JSC shall, among other things:
(a) review the [***];
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-25-
(b) discuss [***];
(c) review [***], for Product in the Territory and outside the Territory;
(d) discuss [***];
(e) facilitate [***];
(f) coordinate [***];
(g) monitor and coordinate the [***]; and
(h) discuss, undertake and/or approve [***].
5.3 Joint Development Committee
The Parties shall establish a committee (the “Joint Development Committee” or “JDC”) to oversee the development activities relating to Products in the Territory during the term of this Agreement which shall, among other things:
(a) review and comment on [***];
(b) discuss [***] for Products in the Territory and outside the Territory;
(c) review [***];
(d) monitor [***];
(e) manage [***]; and
(f) coordinate [***].
5.4 Joint Commercialisation Committee
The Parties shall establish a committee (the “Joint Commercialisation Committee” or “JCC”) to oversee the commercialisation activities relating to Products in the Territory during the term of this Agreement which shall, among other things:
(a) review [***];
(b) discuss [***];
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-26-
(c) manage [***];
(d) cooperate [***];
(e) monitor [***]; and
(f) [***].
5.5 Joint Regulatory Committee
NGX and Astellas shall establish [***] a committee (the “Joint Regulatory Committee” or “JRC”) which shall co-ordinate [***].
5.6 General Provisions relating to Committees
For each Committee the Parties shall each designate [***] to serve as members of such Committee. The JSC will consist of [***] from each Party, [***] shall be a senior executive of each Party and the JSC shall determine the number of representatives of each Party for each other Committee; provided, however, that the JDC shall be composed of no less than [***] making representatives of each Party, [***]. Each representative may serve on more than one Committee as appropriate in view of the individual’s expertise. Each Party may replace its Committee representatives at any time upon written notice to the other Party, provided that the Parties will use reasonable endeavors to keep such replacements to a minimum. The chairperson of each Committee shall [***] from one of its representatives on such Committee and thereafter, the Parties shall rotate annually as to which Party selects such chairperson. The chairperson shall be responsible for calling meetings, preparing and circulating an agenda in advance of each meeting of such Committee, and preparing and issuing minutes of each meeting within [***] thereafter; [***]. Such minutes will not be finalized until a representative of the other Party on such Committee has reviewed and confirmed the accuracy of such minutes in writing.
5.6.1 Meetings. The JSC shall meet [***] until the [***], and thereafter [***] during the term of this Agreement, unless otherwise agreed by the Parties. The frequency of meetings of other Committees shall be decided by the JSC. All Committee meetings shall be in person at least [***] unless otherwise mutually agreed, and the other meetings may be through telephone or video conference or other mutually agreeable means. With the consent of the Committee members, other representatives of NGX or Astellas may attend Committee meetings as non-voting observers. [***].
5.6.2 Decisions.
(a) Decisions of the Committees shall be made [***]. If a Committee other than the JSC is unable to reach unanimous consent on a particular matter within [***] of its initial consideration of such matter, then such matter shall be referred to the JSC. In the event the JSC cannot reach agreement on a matter within [***] after it has met (whether in person or by telephone or video conference) and attempted to reach such agreement, the dispute [***]:
(i) by the [***] in good faith, giving appropriate consideration to the reasonable business and scientific concerns of [***], for all matters relating to [***]; and
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-27-
(ii) by the [***] in good faith, giving appropriate consideration to the reasonable business and scientific concerns of [***], for all matters relating to [***].
(b) Notwithstanding anything to the contrary in Section 5.6.2(a) above:
(i) With respect to disputes relating to [***] to the extent [***].
(ii) To the extent additional responsibilities are delegated to the JSC pursuant to Section 5.2(h)(ii), the Parties shall mutually agree at the time of such delegation [***] to decide any matter encompassed by that responsibility in the event that [***] an agreement regarding that dispute.
(iii) [***] over any dispute explicitly reserved for arbitration pursuant to Section 19.2.
(iv) Neither Party shall [***].
5.6.3 Authority. Notwithstanding the creation of the JSC or any other Committee, each Party shall [***] hereunder, and the JSC or any other Committee shall not [***]. No Committee shall have the power to amend or modify this Agreement, and its decisions shall not be in contravention of any terms and conditions of this Agreement. It is understood and agreed that issues to be formally decided by a Committee are only those specific issues that are expressly provided in this Agreement to be decided by such Committee.
5.6.4 From time to time, the JSC may establish further committees as it deems appropriate to oversee specific matters, activities and obligations of the Parties under this Agreement or the Supply Agreement as set forth herein, and ensure an optimal coordination between the Parties with respect to such matters, activities and obligations.
5.6.5 It is understood between the Parties that under no circumstances, the activities to be performed by the JSC (or any subcommittee) are intended or allowed to violate any applicable law (including but not limited to any competition and/or antitrust law).
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-28-
5.6.6 Upon the [***] the Effective Date the JSC and any other Committees then in existence shall be disbanded, provided that at any time following the [***] of the Effective Date, NGX shall have the right to withdrawal from participation in the JSC and all other Committees upon written notice to Astellas, which notice shall be effective [***] (“Withdrawal Notice”). Following the disbanding of the Committees (or if applicable, the issuance of a Withdrawal Notice), NGX shall no longer participate in any meetings of any Committees, nor shall NGX have any right to vote on decisions within the authority of the JSC or any other Committees. Following the disbanding of the Committees (or if applicable, NGX’s earlier withdrawal from the Committees), Astellas will have the sole decision with regard to matters that affect the commercialization and development of the Existing Product within the Territory and [***].
5.7 Alliance Managers
Promptly after the Effective Date, each Party shall appoint an individual to act as the alliance manager for such Party (the “Alliance Manager”). Each Alliance Manager who is not otherwise a member of the JSC shall thereafter be permitted to attend meetings of the JSC. The Alliance Managers shall be the primary contact for the Parties regarding the activities contemplated by this Agreement and shall facilitate all such activities hereunder. Each Party may replace its Alliance Manager with an alternative representative at any time with prior written notice to the other Party. The Alliance Managers shall not, in any manner, take over the role of the JSC and shall not have any rights, powers or discretion except as expressly granted to the Alliance Managers hereunder. In no event shall the Alliance Managers have any power to modify or amend this Agreement.
5.8 Kick-off Meeting
The Parties agree to hold a face to face meeting, within [***] after the Effective Date, with the aim of NGX sharing with Astellas (at no cost to Astellas) the existing NGX Know-How reasonably necessary for [***]. The Alliance Managers shall be responsible for co-ordinating and setting up the meeting. The agenda for the meeting will be mutually agreed by both Parties but will serve to exchange [***]. The Parties will also agree upon the subsequent transfer of the documents and files encompassing copies of NGX Know-How in existence prior to the Effective Date, which shall include establishing a key contact list to facilitate the transfer of such NGX Know-How and enable clear and effective communication thereafter.
5.9 Documents
Notwithstanding the above, NGX shall provide Astellas with all the documents set out in Exhibit 5.9 upon the later of (a) [***], or (b) [***] after the Effective Date although NGX shall use reasonable efforts to provide the documents within [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-29-
5.10 Co-operation
The Parties agree to the cooperation terms set out in Exhibit 2.2.1.
6. TRANSFER OF THE MARKETING AUTHORISATION
6.1 MAA Approval Transfer
At the Effective Date, NGX shall transfer the benefit of and interest in the NGX EMEA MAA Approval number EMEA/H/C/000909 (the “Existing Product MAA Approval”) to Astellas or its designated Affiliate free from all encumbrances on the terms of and in accordance with this Section 6.
6.2 [***]
[***] for preparing and submitting all notices, applications, submissions, reports and other instruments, documents, correspondence or filings necessary to obtain a transfer to Astellas or its designated Affiliate of the Existing Product MAA Approval [***].
6.3 NGX’s Obligations
NGX shall or shall procure that any of its Affiliates will:
6.3.1 [***] after the Effective Date sign any notices, applications, submissions, reports and other instruments, documents, correspondence or filings presented to it by Astellas or its designated Affiliate (any of these a “Document”) that are necessary for: (i) the transfer to Astellas or its designated Affiliate of the Existing Product MAA Approval; or (ii) maintaining, renewing or varying the Existing Product MAA Approval in the period from the Effective Date until the transfer of the Existing Product MAA Approval in accordance with Section 6.1;
6.3.2 [***] provide notice of its consent to the transfer of Existing Product MAA Approval to Astellas or its designated Affiliate if required by any applicable governmental or regulatory authority after the Effective Date;
6.3.3 [***] after the Effective Date where permitted and reasonably practicable conduct all communications with the EMEA relating to the transfer of the Existing Product MAA Approval at the direction of Astellas;
6.3.4 subject to Astellas’ or its designated Affiliate’s right in each case to review and approve all written material prepared by NGX prior to submission to the EMEA, NGX shall [***], and use [***]; and
6.3.5 [***] after the Effective Date provide such reasonable assistance as Astellas (or its designated Affiliate) may reasonably request, [***], that is necessary to facilitate the transfer of the Existing Product MAA Approval to Astellas (or its designated Affiliate).
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-30-
6.4 Prior to MAA Approval Transfer
After the Effective Date, and before the Existing Product MAA Approval is transferred to Astellas or its designated Affiliate, NGX shall hold the Existing Product MAA on trust for Astellas, and shall without prejudice to Sections 6.3.4 and 6.5 maintain in force the Existing Product MAA Approval ([***]), and shall not encumber, amend, cancel or surrender the Existing Product MAA Approval or take any step in relation thereto unless requested to do so by Astellas or any applicable governmental or regulatory authority (any such request of a governmental or regulatory authority a “Regulatory Request”). Before taking any such step pursuant to a Regulatory Request, NGX shall act in accordance with any reasonable instruction given by Astellas in relation to such Regulatory Request unless action is required to be taken urgently by the relevant governmental or regulatory authority, in which case NGX shall inform Astellas of such action as soon as possible thereafter.
6.5 After MAA Approval Transfer
Following transfer to Astellas of the Existing Product MAA Approval, Astellas shall be responsible for the Maintenance of the Existing Product MAA Approval, as well as for the filing and Maintenance of all additional XXXx filed and MAA Approvals obtained in its, its Affiliates’, Sublicensees’ or Subdistributors’ name in the Territory, [***], provided that NGX shall, upon Astellas ‘ request, provide to Astellas reasonable assistance in relation with such Maintenance, as well as all electronic documents in its possession or under its Control that are reasonably necessary to perform such Maintenance, including without limitation the relevant dossier for the Existing Product MAA Approval in the format of an electronic common technical document (eCTD). NGX shall also within [***] of the Effective Date provide Astellas with all original documents comprising the Existing Product MAA Approval and to the extent that such original documents are required by NGX for regulatory filings outside the Territory, NGX shall allow Astellas to have access and to copy such original documents at all reasonable times on reasonable notice for the purpose of the Maintenance of the MAA Approvals and filing of XXXx in relation to developments of the Products in the Territory. As used in this Section 6, “Maintenance” with respect to MAA Approvals and/or MAA shall include, without limitation, payment of the annual fees, renewal of the MAA Approval, implementation of any variations with respect to such MAA Approval and/or MAA and responding to requests by the relevant Regulatory Authorities.
6.6 Communications with Regulatory Authorities
Subject to applicable laws, following transfer of the Existing Product MAA Approval to Astellas, Astellas shall be the Party responsible for communicating directly with and appearing before the EMEA or any other regulatory authority, provided that Astellas shall [***]. NGX agrees, upon Astellas’ request, to provide Astellas with reasonable assistance in connection with any such communications and meetings.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-31-
7. DEVELOPMENT
7.1 Development of Existing Product
7.1.1 European Union.
(a) Additional Studies.
(i) Safety Study. Initial MAA Approval for the Existing Product in the European Union is accompanied by a post approval commitment requiring that an open label safety study in on label patient populations be carried out on the committed timelines with respect to the Existing Product (“Safety Study”), as further specified in Exhibit 7.1.1. Astellas shall be solely responsible for planning and overseeing the performance of the Safety Study and liaising with the regulatory authorities, provided that Astellas shall keep the JDC informed about all study activities and provided that Astellas shall give reasonable consideration to any comments of the JDC. [***].
(ii) [***] Trial. [***]. The [***] Trial shall be designed and carried out as follows:
(1) Astellas shall be responsible for drawing up a protocol that satisfies the EMEA’s conditions and requirements (“EMEA Requirements”) which may involve requesting scientific advice from the EMEA (the “Initial Protocol”);
(2) Astellas shall provide a copy of the Initial Protocol for the [***] Trial, to NGX, through the JDC, for NGX’s review and comment at which time NGX may request Astellas to add activities to the protocol to satisfy any requirements of the FDA. Astellas will reasonably consider any such request(s) and agrees to implement such request(s) to the extent they are commercially reasonable, it being understood that in no event shall Astellas be required to include any activities in the protocol which would conflict with EMEA Requirements for the [***] Trial, [***] and in all cases the final decision rests with Astellas;
(3) Astellas shall negotiate with the EMEA to obtain approval for the protocol for the [***] Trial and shall be responsible for the execution and delivery of the [***] Trial; and
(4) Astellas shall be responsible for [***] of the [***] Trial, provided that NGX shall be responsible for [***] relating to activities that have been added to the Initial Protocol at NGX’s request and to the extent that any communication or filings with the FDA are required in connection with such added activities, NGX shall be responsible for conducting such communications and making such filings [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-32-
(iii) Other Post-Marketing Studies. The Parties anticipate that it will be desirable to conduct certain additional post-marketing studies of the Existing Product in order to maximize the potential market for the Existing Product in or outside the Territory (“Post-Marketing Studies”). The Parties shall discuss all proposed Post-Marketing Studies at the JDC. In relation to the Territory, Astellas shall [***] for any Post-Marketing Study and shall present it to the JDC [***]. Astellas shall [***] any such Post-Marketing Studies in the Territory, [***], and shall update the JDC on the progress of such studies. Astellas shall [***] performing Post-Marketing Studies in the Territory and shall [***] on such studies within the ten (10) year period commencing upon the first First Commercial Sale of the Existing Product in the Territory, provided that in no event shall Astellas [***]. NGX shall be responsible for planning and overseeing the performance of any Post-Marketing Studies outside the Territory, subject to the oversight of the JDC, and shall update the JDC on the progress of such studies. NGX shall be responsible for [***] of performing Post-Marketing Studies outside the Territory.
(iv) Further Development of the Existing Product. If a Party wishes to develop the Existing Product for [***], then it shall raise the issue at the JSC. If the JSC agrees to such further development then the JDC shall be responsible for drawing up a development plan which it shall present to the JSC for approval.
(1) If both Parties wish to participate in the development then the JDC shall oversee the performance of such development and shall allocate responsibility for the performance of the various tasks required in connection with such studies based upon which Party is better situated to perform such tasks, taking into account among other things, the estimated costs associated with having each Party perform such task. The aim of the development plan will be to generate clinical data which is intended for, and in the correct format for, submission to [***]. To the extent that any activities under the development plan are necessary to satisfy the requirements of [***]. To the extent that any activity is necessary to satisfy the requirements [***].
(2) If only one Party wishes to carry out any development under this Section 7.1.1(iv) (the “Developing Party”), and the JSC has approved the development plan then the Developing Party shall carry out the activities under such development plan and shall be solely responsible for all Development Expenses related to such activities. If the other Party subsequently wishes to include any of the Data that has been created as a result of such development in its regulatory filings for Product in its territory, then it can only do so if it reimburses the Developing Party [***] of all the Developing Party’s Development Expenses incurred in relation to all the activities under the development plan.
7.1.2 Non-European Union Countries. Astellas shall be responsible, [***], for conducting such clinical trials as may be necessary to file and obtain MAA Approval for the Existing Product in each non-European Union country in the Territory as may be required to meet its obligations under the Commercialization Plan. Additionally, to the extent that compliance
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-33-
with the regulatory requirements for obtaining MAA Approval in one or more non-European Union countries in the Territory would result [***]. If a regulatory authority in a non-European Union country requires [***], then Astellas shall have the right to terminate this Agreement in respect of such non-European Union country by providing NGX at least ninety (90) days prior written notice.
7.1.3 Compliance with Laws. Each Party or its permitted Third Party contractors shall perform its responsibilities under this Section 7 in accordance with all applicable laws, including, without limitation, cGLPs, cGCPs and cGMPs to the extent applicable.
7.1.4 Diligence. Each Party shall use [***] to carry out its development obligations under Section 7.1 and such obligations are subject to the other Party providing all relevant support and Data reasonably necessary to perform such obligations.
7.2 Exchange of Data
NGX and Astellas, respectively, shall provide to each other [***] for use of each of Astellas and NGX for the purposes set forth in Section 7.2.1 in a timely fashion and as promptly as possible upon request of each of Astellas or NGX. In addition, (a) NGX shall provide Astellas with [***] within [***] of such submission, and (b) Astellas shall provide NGX with [***] within [***] of such submission. Astellas shall have the right to withhold Data for a reasonable period to allow it to file for patent protection relating to such [***].
7.2.1 Use; Disclosure.
(a) Astellas will only use and disclose Data to Third Parties as required to [***] including without limitation [***] in each case for commercialization of Products inside the Territory and NGX gives Astellas the right to [***], and as may be necessary in performing its obligations and exercising its rights under this Agreement, i.e. [***] in each case solely to the extent necessary for development and commercialization of Products in Territory; or as may otherwise be agreed by NGX and Astellas. Astellas may not use any Data (or permit any Third Party to use Data) outside the Territory, or outside the Field or for any products other than the Products, except for the assessment and validation of the Data by Affiliates or Third Party consultants outside the Territory but solely for purposes of [***]. NGX may not use any Data (or permit any Third Party to use Data) inside the Territory (except as required to fulfill any development obligations it may have under a development plan agreed with Astellas pursuant to Sections 7.1.1, 2.3.6, 2.3.10 or 2.3.14), or outside the Field or for any products other than the Products, except for the [***] but solely for purposes of developing or commercializing Products outside the Territory. Notwithstanding anything to the contrary in this Section 7.2.1(a) or Section 7.2.1(b) below, [***] shall be subject to the requirements set forth therein.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-34-
(b) Astellas hereby grants NGX a non-exclusive sublicensable right and license to use the Data provided by Astellas, and to disclose such Data to Third Parties, in each case as is reasonably necessary or useful for commercialization of Products outside the Territory including without limitation for use by NGX’s licensees of the Products and for [***] (and Astellas hereby gives NGX the right to so [***]), in each case for commercialization outside the Territory, provided that any disclosure of such Data to a non-governmental Third Party is made under reasonable and customary confidentiality restrictions. Notwithstanding the foregoing, upon termination of this Agreement by Astellas pursuant to Section 17.2 or by NGX pursuant to Sections 17.3-17.5, the scope of NGX’s license to Data provided by Astellas shall thereafter be worldwide.
7.2.2 Third Parties.
(a) In all agreements after the Effective Date with Third Parties or Affiliates involving the development of Data, Astellas and NGX, respectively, shall require that such Third Parties and Affiliates provide the other Party access to all such Data, to the extent that such Data is required for [***] in accordance with this Agreement.
(b) Notwithstanding Section 7.2.2(a) above, Astellas acknowledges that under NGX’s agreement(s) with LTS prior to and after the Effective Date, [***]. If any regulatory authority does not accept such a direct filing by LTS then the Parties and LTS shall cooperate to find an alternative route by which such LTS Data shall be filed with such regulatory authority. Such arrangement with LTS shall not be deemed a breach by NGX of this Section 7.2.2. It is understood that NGX shall ensure that it obtains [***] for the use by each of NGX and Astellas as set forth in this Section 7.2.
7.2.3 All XXXx and MAA Approvals and any other regulatory filings relating to developments of the Existing Product in the Territory including all variations and line extensions shall be owned by Astellas or its nominated Affiliate.
7.3 Development of Liquid Formulation Product
7.3.1 In the event that Astellas exercises the Option under Section 2.3.12 Astellas shall be responsible for [***] with respect to the Liquid Formulation Product in the Territory, in each case [***], provided that NGX shall, upon Astellas’ request, provide to Astellas reasonable assistance in relation to such activities.
7.4 Coordination Each Party agrees to keep the other [***] informed as to the progress of its clinical development and regulatory activities relating to the Liquid Formulation Product in and outside the Territory, including its correspondence and meetings with regulatory agencies, by way of updates to the JSC and JDC at its meetings and as otherwise [***] requested by the other Party. The Party responsible for conducting the clinical development of the Liquid Formulation Product shall reasonably consider and [***] respond to any comments provided by the other Party with respect to such clinical development and regulatory activities. Subject to
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-35-
Sections 18.3.2 and 18.5.2 below, all MAA filings and MAA Approvals in the Territory for the Liquid Formulation Product (including all variations and line extensions) shall be in the name of, and owned by, Astellas or its Affiliates.
8.1 Commercialization
Subject to oversight by the JCC and ultimately the JSC, Astellas shall [***] to launch and commercialize the Existing Product in the Territory, in accordance with the Commercialization Plan in order to maximize the Net Sales of such Product in the Territory. Astellas agrees to keep NGX [***] informed as to the progress of its launch and commercialization activities relating to Products in the Territory, by way of [***] at its meetings and as otherwise reasonably requested by NGX. Astellas shall [***] consider and [***] respond to any comments provided by NGX with respect to such launch and commercialization activities, provided that any final decision is in the discretion of Astellas to the extent that it is consistent with the Key Metrics. It is understood and agreed that all commercialization efforts for Products in the Territory shall, as between the Parties, [***]. Any failure of Astellas to comply with its obligations under the Commercialization Plan and the Key Metrics shall not be considered a breach of this Agreement to the extent [***].
8.2 Pricing and Reimbursement
[***].
8.2.1 Subject to [***] the Existing Product pursuant to Section 8.2.2, Astellas shall [***] to (i) obtain pricing and/or reimbursement approvals for the Existing Product [***] and make such regulatory filings as are necessary to [***] that are [***] to market the Existing Product in each country in the European Union, in each case in accordance with the Commercialization Plan and any development plans in accordance with Section 7.1, and (ii) subject to Section 7.1.2, obtain such regulatory approvals, including MAA Approvals and [***], as may be necessary to market the Existing Product in [***], in accordance with the Commercialization Plan.
8.2.2 Notwithstanding Astellas’ obligations under Section 8.2.1, Astellas shall always have the [***] the Existing Product in a country in the Territory if [***], provided that this Section 8.2.2 shall in no event excuse any [***].
8.3 Sales Materials
8.3.1 Brand Guiding Principles. Within [***] after the Effective Date, the Parties shall agree on Brand Guiding Principles, to be updated from time to time. Astellas agrees to adhere, and to cause its Affiliates, Sublicensees and Subdistributors to adhere, to the Brand Guiding Principles in connection with the commercialization of the Existing Product in the Territory. NGX agrees to adhere, and to cause its Affiliates and distributors and licensees to adhere, to the Brand Guiding Principles in connection with the commercialization of the Existing Product outside the Territory.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-36-
8.3.2 Sales Materials. Any marketing and promotional materials and packaging created or used by Astellas, its Affiliates, Sublicensees and Subdistributors for the Product shall be appropriate to the MAA Approval for the Product in the respective country, and, in the case of the Existing Product, consistent with the Brand Guiding Principles. Upon each Party’s reasonable request, the other Party shall [***]. It is understood and agreed that each Party shall have the right to [***] for purposes of commercializing Products in their respective territory provided that each Party shall be solely responsible for the compliance of such Sales Materials with laws and regulations in its own territory. Any claim, message or other material item in the Sales Materials, which is not consistent with the Brand Guiding Principles and [***], shall be subject to [***] provided that in the event of a dispute as to whether a claim complies with the laws and regulations in the Territory, Astellas’ view shall prevail. NGX shall use all reasonable efforts to complete any such review and provide Astellas with an answer within [***] from notification by Astellas to NGX of the relevant matter and in the absence of an answer within such [***] period such claim, message or other material item shall be deemed [***].
8.4 Reporting Adverse Drug Reactions/Experiences
Each Party shall observe and comply with its respective obligations regarding the exchange of data concerning adverse drug events, as set out in a safety agreement which the Parties shall enter into [***] after the Effective Date but in any event not later than [***] delivery of Product by NGX to Astellas and which will be attached to this Agreement as Exhibit 8.4 (hereinafter referred to as “Safety Agreement”). NGX will be responsible for managing and maintaining (itself or through its designee) the worldwide drug safety database, provided that Astellas [***] and receive appropriate information in order to fulfill the requirements specified in the EU Rules Governing Medicinal Products in the European Union, Volume 9A. The Parties agree that the management and maintenance of the worldwide drug safety database [***]. The costs associated with setting up the worldwide drug safety database shall be the sole responsibility of NGX. Any fees charged by such Third Party contractor to NGX for specific activities relating to the use of the worldwide drug safety database which exclusively relate to [***] shall [***].
8.5 Assistance for the Product
Each Party shall promptly inform the other Party of any notification of any action by, or notification or other information which it receives (directly or indirectly) from any governmental or regulatory authority (together with copies of correspondence related thereto), which (a) raises any material concerns regarding the safety or efficacy of a Product, (b) indicates or suggests a potential material liability for either Party to Third Parties arising in connection with a Product or (c) which indicates a reasonable potential for a recall or market withdrawal of a Product.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-37-
8.6 Medical Inquiries for the Product
Following the completion of the transfer of the Existing Product MAA Approval to Astellas in accordance with Section 6 above, Astellas shall be responsible for handling all medical questions or inquiries for the Field in the Territory with regard to the Product ([***]), but shall consider in good faith input from NGX in connection therewith. NGX shall immediately forward any and all medical questions or inquiries which it receives in relation to the Product for the Field in the Territory to Astellas in accordance with all applicable laws.
8.7 Material Failure to Exploit
This Section 8.7 shall not limit any other remedies in damages that NGX may have under this Agreement or applicable law but shall be NGX’s sole remedy in relation to Astellas’ obligations to commercialise the Products.
8.7.1 European Region. In the event Astellas commits a Material Failure to Exploit in the European Region [***]. Such notice shall specify in reasonable detail the facts and circumstances constituting the Material Failure to Exploit. Upon the expiration of [***].
8.7.2 Other Regions. In the event there is a Material Failure to Exploit with respect to Products in a Region (other than the European Region), then NGX shall have the right to [***].
8.7.3 In the event there are no Regions then in effect after such exclusion, then this Agreement shall be deemed terminated in whole.
8.7.4 In the event of such termination by NGX of a Region or in the event of non-exclusivity imposed by NGX for such Region, the Parties would adjust [***] set forth above in Section 4.1 as follows:
(a) For each Region (other than the European Region) excluded from the Territory (or in which Astellas’ rights are reduced to non-exclusive status) pursuant to Section 8.7.2, the [***] shall be reduced by [***].
(b) If the European Region is excluded from the Territory (Astellas’ rights in the European Region are reduced to non-exclusive status) pursuant to Section 8.7.1, the [***] shall be reduced by [***].
8.7.5 In the event Astellas commits a Material Failure to Exploit with respect to the required minimum Marketing spending, NGX shall have the right to provide notice of its intention to terminate this Agreement or make Astellas’ rights for the Territory non-exclusive. Such notice shall specify in [***] detail the facts and circumstances constituting the Material Failure to Exploit. Upon the expiration of [***] after receipt by Astellas of such notice, if Astellas (a) has not cured such Material Failure to Exploit, or (b) in the event that such Material Failure to Exploit is not capable of being cured in [***], is not [***] to cure such Material Failure to Exploit, NGX shall have the right to terminate this Agreement or make Astellas’ rights in the Territory non-exclusive by giving a notice, which shall be effective on the date such notice is given.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-38-
8.8 Commercialization of Liquid Formulation Product
In the event that Astellas elects to exercise its Option under Section 2.3.12 with respect to the Liquid Formulation Product, Astellas shall [***] to launch the Liquid Formulation Product in the Territory, and thereafter to market, promote and sell the Liquid Formulation Product so as to maximize the Net Sales of both the Existing Product and Liquid Formulation Product as a portfolio of products.
9.1 General
It is understood that NGX procures supplies of Components from Third Party contractors. However, the Parties anticipate that Astellas shall eventually enter into direct contractual relationships with such Third Party contractors for the supply of Components. Pending such direct agreements, on the Effective Date NGX enters into a separate written agreement with Astellas (“Supply Agreement”) pursuant to which NGX shall obtain from its Third Party contractors quantities of Components sufficient to supply Astellas’ reasonable requirements for Components in the Territory.
9.2 Inventory
In addition, Astellas shall purchase the Patch Inventory and Gel Inventory (both as defined in the Supply Agreement) in accordance with the Supply Agreement.
9.3 General Supply Terms
Until Astellas enters into direct contractual relationships with Third Party contractors for the supply of Components:
9.3.1 Astellas and its Affiliates shall exclusively purchase all of their requirements of Components from NGX;
9.3.2 NGX shall procure Components on behalf of and as reasonably requested in writing by Astellas, consistent with NGX’s arrangements with its suppliers;
9.3.3 Components ordered by NGX from such supplier on behalf of Astellas, shall be charged to Astellas at NGX’s cost of goods as defined in the Supply Agreement;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-39-
9.3.4 the Components supplied to Astellas under the Supply Agreement shall be supplied in the same form as like Components are supplied to NGX (save as to artwork and text), or as otherwise established by the JSC or by agreement of the Parties, it being understood that any differences in such form requested by Astellas shall be at Astellas’ expense; and
9.3.5 in the event that NGX would be required to pay to LTS any fees under Section 3.4(c) of the LTS Agreement in order to expand the “Territory” as defined in the LTS Agreement to be commensurate in scope with the “Territory” as defined in this Agreement, Astellas shall be responsible for such fees.
9.4 Tripartite Agreements
It is anticipated that NGX will also be a party to any direct agreements between Astellas and the Third Party suppliers so as to, among other things (a) [***]; (b) [***]; and (c) [***]. Irrespective of whether or not NGX is a party to any direct supply agreement that Astellas may enter into with NGX’s Third Party suppliers, it is understood that Astellas [***].
9.5 Quality Agreement
NGX and Astellas shall execute a quality agreement which allocates roles and responsibilities to each Party with respect to quality control and regulatory compliance with respect to Components and the Existing Product.
10. PAYMENTS; BOOKS AND RECORDS
10.1 Payment Method
All payments under this Agreement shall be made by bank wire transfer in immediately available funds to an account designated by the Party to which such payments are due. Any payments due under this Agreement which are not paid by the date such payments are due under this Agreement shall bear interest to the extent permitted by applicable law at the interest rate applied by the [***] to its most recent refinancing operations ([***] bid rate or fixed rate as published on [***]), plus [***], computed on the basis of the [***]. The applicable interest rate shall be adjusted each time there shall be a change in the aforementioned rate of the [***]. This Section 10.1 shall in no way limit any other remedies available to the Parties.
10.2 Taxes
Any tax (other than VAT) which Astellas is required to pay or withhold in respect of the payments to be made to NGX hereunder shall be deducted from the amount otherwise due provided that, in regard to any such deduction, Astellas shall give NGX reasonable assistance, which shall
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-40-
include the provision of such documentation as may be required by any revenue authority and other revenue services, as may be necessary to enable NGX to claim exemption therefrom or obtain a repayment thereof or a reduction thereof and shall upon request provide such additional documentation from time to time as is needed to confirm the payment of tax.
10.3 United States Dollars
All dollar amounts specified in this Agreement, and all payments made hereunder, are and shall be in U.S. dollars. For all payments other than the initial payment due under Section 3.1, the sales milestones due under Section 3.2, the Option Fee due under Section 3.3.1, the Option Retention Fee due under Section 3.3.2 and the Option Exercise Fee due under Section 3.3.3, monetary conversion from the currency of a foreign country, in which Product is sold, into United States currency shall be calculated for a calendar quarter in which such sales were made using an exchange rate equal to [***] prior to the date that the report detailed in Section 4.6.1(a) is delivered to NGX. With respect to the initial payment due under Section 3.1 and the Option Fee due under Section 3.3.1, monetary conversion from Euros into United States currency shall be calculated using an exchange rate equal to [***] days prior to the execution of this Agreement. With respect to the payments of the sales milestones due under Section 3.2, the Option Retention Fee due under Section 3.3.2 and the Option Exercise Fee due under Section 3.3.3, monetary conversion from Euros into United States currency shall be calculated using an exchange rate equal to [***] prior to achieving the relevant milestone, the election by Astellas to retain the Option or the election by Astellas to exercise the Option, as the case may be.
10.4 Records; Inspection
10.4.1 Astellas. Astellas shall keep, and require its Affiliates and Sublicensees to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable (or reimbursable) under this Agreement, including without limitation true and accurate records of Gross Sales, Net Sales and the costs incurred by Astellas in connection with the conduct of the clinical studies contemplated in Article 7. Such books and records shall be kept at the principal place of business of Astellas (or the place of business of its Affiliates or Sublicensees, as applicable) for at least [***] following the end of the calendar quarter to which they pertain. Such records will be open for inspection during such [***] period by an independent auditor chosen by NGX and reasonably acceptable to Astellas for the purpose of verifying the amounts payable by Astellas hereunder. Such inspections may be made no more than once each calendar year (it being understood that a single audit may require multiple visits) and no more than once in respect of any audited period, at reasonable times and on reasonable notice. The independent auditor shall be obligated to execute a reasonable confidentiality agreement prior to commencing any such inspection. Inspections conducted under this Section 10.4.1 shall be at the expense of NGX, unless a variation or error producing an underpayment in amounts payable exceeding [***] of the amount paid for any period covered by the inspection is established in the course of any such inspection, whereupon all costs relating to the inspection for such period and any
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-41-
unpaid amounts that are discovered shall be paid by Astellas, together with interest on such unpaid amounts at the rate set forth in Section 10.1 above. The Parties will endeavor to minimize disruption of Astellas’ normal business activities to the extent reasonably practicable. To the extent that Astellas does not have the right to grant NGX the right to audit its Sublicensees’ books and records hereunder, Astellas shall obtain for itself such right and, at the request of NGX, Astellas shall exercise such audit right with respect to Sublicensees and provide the results of such audit for inspection by NGX pursuant to this Section 10.4.1.
10.4.2 NGX. NGX shall keep complete, true and accurate books of accounts and records for the purpose of determining the amounts payable (or reimbursable) under this Agreement, including without limitation true and accurate records of the costs incurred by NGX in connection with the conduct of those development studies contemplated in Article 7 for which it is entitled to reimbursement from Astellas. Such books and records shall be kept open at the principal place of business of NGX and be for at least [***] following the end of the calendar quarter to which they pertain. Such records will be open for inspection during such [***] period by an independent auditor chosen by Astellas and reasonably acceptable to NGX for the purpose of verifying the amounts payable by NGX hereunder. Such inspections may be made no more than once each calendar year (it being understood that a single audit may require multiple visits) and no more than once in respect of any audited period, at reasonable times and on reasonable notice. Astellas’ independent auditor shall be obligated to execute a reasonable confidentiality agreement prior to commencing any such inspection. Inspections conducted under this Section 10.4.2 shall be at the expense of Astellas, unless a variation or error producing an overpayment in amounts payable exceeding [***] of the amount paid for any period covered by the inspection is established in the course of any such inspection, whereupon all costs relating to the inspection for such period shall be paid by NGX and Astellas shall receive a credit for any overpaid amounts that are discovered together with interest on such overpaid amounts at the rate set forth in Section 10.1 above. The Parties will endeavor to minimize disruption of NGX’s normal business activities to the extent reasonably practicable.
11.1 Ownership of Inventions
11.1.1 Title to all inventions and other intellectual property made solely by Astellas personnel in connection with this Agreement shall be owned by Astellas. Title to all inventions and other intellectual property made solely by NGX personnel in connection with this Agreement shall be owned by NGX.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-42-
11.1.2 Title to all inventions and other intellectual property made jointly by personnel of NGX and Astellas in connection with the performance of this Agreement (“Joint IP”) shall be jointly owned by Astellas and NGX. Prosecution of any patent applications and patents with respect to such jointly owned inventions and intellectual property (“Joint Patents”) shall be as mutually agreed.
11.1.3 It is understood that neither Party shall have any obligation to account to the other for profits, nor to obtain any approval of the other Party to exploit, license or assign any such Joint IP, by reason of joint ownership thereof, provided that to the extent that such Joint IP covers or directly relates to Products neither Party shall until expiry of this Agreement, without obtaining the approval of the other Party, (a) license such Joint IP to a Third Party, other than to a Sublicensee in the case of Astellas or a licensee of the Existing Product and/or the Liquid Formulation Product in the case of NGX, or (b) assign such Joint IP to a Third Party, other than to an assignee of all or substantially all of its assets or business in any country pertaining to the Components and/or the Products. Such Joint Patents shall be considered a Patent Right for the purposes of extending the term of this Agreement but no Earned Royalties shall be payable to NGX in respect of any Joint Patent.
11.1.4 Astellas Improvements. Astellas shall notify NGX of any Astellas Improvements in a [***]. Astellas shall have the right to file and maintain patents for Astellas Improvements as it, in its sole discretion, decides, provided that if Astellas elects not to maintain patent application(s) and patent(s) for an Astellas Improvement that Astellas has filed, Astellas shall notify NGX of such intention at least [***] prior to the date upon which such patent or patent application shall expire or be abandoned, and NGX shall thereupon have the right, but not the obligation, to assume responsibility for the prosecution and maintenance thereof at its own expense, provided that it is understood that ownership of such Patent Right shall remain with Astellas. Astellas hereby grants to NGX an [***] outside the Territory, with the right to sublicense, under any Astellas Improvements to make, have made, use, sell, offer for sale, import, practice and otherwise exploit the same for the Components and/or the Product outside the Territory (the “NGX License”). Upon the expiration of this Agreement the NGX License shall thereafter be non-exclusive but in all other respects shall remain unchanged. Upon the termination of this Agreement by Astellas pursuant to Section 17.2 or by NGX pursuant to Sections 17.3-17.5, the scope of the NGX License shall thereafter be worldwide but in all other respects shall remain unchanged. As used herein, “Astellas Improvements” means any patent rights or other intellectual property made by or under authority of Astellas or its Affiliates in connection with development and/or commercialization of a Product under this Agreement that is applicable to the Components or Product or the manufacture, use or formulation thereof and solely to the extent that such patent rights or other intellectual property cover a Product.
11.2 NGX Improvements
NGX shall notify Astellas of any NGX Improvements [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-43-
11.2.1 NGX shall license any Know-How comprised in NGX Improvements to Astellas under the terms of Section 2.2.
11.2.2 NGX shall notify Astellas of the grant of any patent for NGX Improvements within [***] of such grant and provide Astellas with a copy of the granted patent.
11.2.3 Within [***] of the date of the notice in Section 11.2.2, Astellas shall notify NGX whether it wishes such patent for NGX Improvements to be added to the Patent Rights and thereby be licensed to Astellas under Section 2.2 and extend the term of this Agreement.
11.2.4 If Astellas does not notify NGX within the [***] period in Section 11.2.3 or notifies NGX that it does not wish such patent for NGX Improvements to be added to the Patent Rights then Astellas shall not be licensed under such patent and the term of this Agreement shall not be extended. To the extent that such patent for an NGX Improvement is solely applicable to Products, NGX and its Affiliates shall not exploit or license it to a Third Party in the Territory until the expiry or earlier termination of this Agreement.
11.2.5 As used herein, “NGX Improvements” means any patent rights or other intellectual property made by or under authority of NGX or its Affiliates in connection with development and/or commercialization of a Product under this Agreement that is applicable to the Components or a Product or the manufacture, use or formulation thereof and solely to the extent that such patent rights or other intellectual property cover a Product.
11.3 Maintenance of Patents
11.3.1 Filings. As between Astellas and NGX, NGX shall have responsibility for and shall control the filing, prosecution and maintenance of all Patent Rights in the Territory. NGX agrees to keep Astellas informed of the course of patent prosecution or other proceedings with respect to the Patent Rights within the Territory (including those licensed under the UC Agreement and LTS Agreement), and Astellas shall have the right to review pending patent applications and make recommendations to NGX concerning the foregoing. NGX will consider in good faith all reasonable suggestions of Astellas with respect thereto. Astellas shall hold all information disclosed to it under this Section 11.3.1 as confidential.
11.3.2 Patent Costs. The costs of prosecuting and maintaining the Patent Rights ([***]) in the Territory will [***].
11.3.3 Maintenance of Existing Product Patent Portfolio. In respect of the Existing Product NGX [***].
11.3.4 Maintenance of Liquid Formulation Product Patent Portfolio. Following Astellas’ exercise of its Option under Section 2.3.12 with respect to the Liquid Formulation Product, the Parties shall agree upon those Patent Rights with respect to the Liquid Formulation Product that constitute “Core Patent Rights”. Such patents shall thereafter be added to Exhibit 1.40 and [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-44-
11.3.5 If, during the term of this Agreement, NGX intends to allow any [***] (as indicated in Exhibit 1.40) owned by NGX in the Territory to expire or otherwise be abandoned, NGX shall notify Astellas of such intention at least [***] prior to the date upon which such Patent Right shall expire or be abandoned, and Astellas shall thereupon have the right, but not the obligation, to assume responsibility for the prosecution and maintenance thereof at its own expense, such Patent Right being an “Assumed Patent”, provided that it is understood that ownership of such Patent Right shall remain with NGX. Although such Assumed Patent shall be licensed to Astellas under this Agreement and shall be considered a Patent Right for the purposes of extending the term of this Agreement no Earned Royalties shall be payable to NGX in respect of any Assumed Patent.
11.3.6 NGX shall not during the term of the Agreement assign or otherwise transfer any Patent Rights (owned by it) in the Territory for which it is the registered proprietor to any Third Party.
11.3.7 Extensions/Supplementary Protection Certificates. The Parties shall discuss and mutually agree upon the patent or patents within the Patent Rights to be extended in each country of the Territory and each Party shall cooperate with the other in seeking any such agreed upon extensions of the terms of Patent Rights in the Territory. Without limiting the foregoing, NGX shall, at Astellas’ request, either authorize Astellas to act as NGX’s agent for the purpose of making any application for any such agreed upon extensions or shall diligently seek to obtain such extensions, in either event, [***].
11.4 Third Party Patents and Trademarks
11.4.1 Third Party Infringement Claims. If the production, importation, keeping, sale, offering for sale, or use of any Product in the Territory pursuant to this Agreement results in a claim, suit or proceeding in the Territory by a Third Party alleging patent or trademark infringement against NGX or Astellas (or their respective Affiliates, licensees or Subdistributors) (collectively, “Infringement Actions”), such Party shall promptly notify the other Party hereto in writing. The Party [***]. The Party controlling the defense of such Infringement Action shall defend such Infringement Action using counsel of its own choice, and the Infringement Action shall be at such Party’s [***]; provided, however, that the other Party may participate in the defense and/or settlement thereof [***] with counsel of its choice. The Party controlling the Infringement Action agrees to keep the other Party hereto reasonably informed of all material developments in connection with any such Infringement Action. Astellas agrees not to (i) make admissions regarding infringement by the Product of any patents outside of the Patent Rights, or (ii) settle such Infringement Action, or make any admissions or assert any position in such Infringement Action, in a manner that would materially adversely affect the validity, enforceability or scope of the Patent
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-45-
Rights in or outside the Territory, in each case without obtaining the prior written consent of NGX which shall not be unreasonably withheld, conditioned or delayed. Subject to any right of Astellas to be indemnified by NGX under the Supply Agreement, [***]. As used herein, “Damages” shall mean out-of-pocket costs incurred by a Party, including reasonable attorneys’ fees and other legal costs, damages and other liabilities that are part of any final judgment awarded against such Party, and any amounts paid by such Party in a settlement of the action that is approved by the other Party, such approval not to be unreasonably withheld, delayed or conditioned. In respect of any infringement actions outside the Territory, NGX agrees not to settle such infringement action, or make any admissions or assert any position in such infringement action, in a manner that would materially adversely affect the validity, enforceability or scope of the Patent Rights inside the Territory, without obtaining the prior written consent of Astellas which shall not be unreasonably withheld, conditioned or delayed.
11.4.2 Oppositions to Third Party Patents and Trademarks. If NGX or Astellas becomes aware of a Third Party patent and/or trademark filed and/or registered in the Territory, which the production, sale or use of any Product in the Territory pursuant to this Agreement may infringe (a “Third Party Right”), such Party shall promptly notify the other Party hereto in writing. [***]. In the event that the Parties agree that it would be advisable to bring such revocation or opposition proceedings (“Adverse Proceedings”), Astellas shall have the right, [***], to bring and control the Adverse Proceedings using counsel of its own choice. Astellas agrees to keep NGX reasonably informed of all material developments in connection with any such Adverse Proceedings and shall reasonably consider NGX’s advice and requests with respect to such Adverse Proceeding. Astellas may treat its Costs from such Adverse Proceedings [***]. As used herein, “Costs” shall mean out-of-pocket costs incurred by Astellas, including reasonable attorneys’ fees and other legal costs, damages and other liabilities that are part of any final judgment awarded against Astellas, and any amounts paid by Astellas in a settlement of the action that is approved by the NGX, such approval not to be unreasonably withheld, delayed or conditioned. In the event that the Parties cannot agree on whether an Adverse Proceeding should be initiated, the Party wishing to bring the Adverse Proceeding shall have the right to do so using counsel of its own choice, provided that it agrees to [***] associated with such Adverse Proceeding and to keep the other Party reasonably informed of all material developments in connection with any such Adverse Proceedings. Notwithstanding the foregoing, in the event that the Parties cannot agree on whether an Adverse Proceeding should be initiated but the Party wishing to bring the Adverse Proceeding can reasonably demonstrate that (a) failure to bring an Adverse Proceeding with respect to a particular Third Party Right would have a material adverse effect on the commercialization of Product in the Territory, and (b) that the contemplated Adverse Proceeding has a reasonable chance of success, in each case through the reasoned legal opinion of such Party’s outside patent counsel, then the Party bringing such Adverse Proceeding [***] from such Adverse Proceedings [***], as applicable.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-46-
11.5 Enforcement of Patent Rights and Trademarks
Subject to the provisions of this Section 11.5, in the event that Astellas reasonably believes that the Product Trademark or any Patent Rights in the Territory are infringed or misappropriated by a Third Party or are subject to a declaratory judgment action in the Territory arising from such infringement, in each case with respect to the sale or use within the Field in the Territory of a [***] product comprising a [***] (each such product, an “Infringing Product”), Astellas shall promptly notify NGX. In such event, as between the Parties, NGX shall have the initial right (but not the obligation), to enforce the Product Trademark or Patent Rights (save in respect of any Assumed Patent), as appropriate, with respect to such infringement, or defend any declaratory judgment action with respect thereto (for purposes of this Section 11.5, an “Enforcement Action”). Astellas (or NGX in respect of any Assumed Patent) shall, [***], have the right to participate with counsel of its own choice, provided that it is understood that Astellas’ right to participate in any Enforcement Action with respect to the patents within the Patent Rights in-licensed to NGX under the LTS Agreement shall be subject to LTS’ consent. NGX shall keep Astellas reasonably informed of the progress of any such Enforcement Action.
11.5.1 Initiating Enforcement Actions. In the event that NGX, or the owner of the Patent Rights, as applicable, fails to initiate an Enforcement Action to enforce the Patent Rights or Product Trademark, as applicable, against an infringement by a Third Party in a country in the Territory, which infringement consists of the sale or use of an Infringing Product in the Field in such country, within [***] of a request by Astellas to initiate such Enforcement Action (or such shorter period required to allow obtaining an interim injunction in the relevant country), Astellas may initiate an Enforcement Action against such infringement. NGX shall cooperate in such Enforcement Action including by being joined as a party to such Enforcement Action. Astellas shall keep NGX reasonably informed of the progress of any such Enforcement Action. NGX shall, at its expense, have the right to join in as a party plaintiff and to give reasonable assistance to such Enforcement Action. Astellas agrees not to settle any Enforcement Action, or make any admissions or assert any position in such Enforcement Action, in a manner that would materially adversely affect the validity, enforceability or scope of the Patent Rights in or outside the Territory, without the prior written consent of NGX, which shall not be unreasonably withheld, delayed or conditioned. In respect of any proceedings to enforce any patents outside the Territory that are equivalent to the Patent Rights, NGX agrees not to settle any such proceedings, or make any admissions or assert any position in such proceedings, in a manner that would materially adversely affect the validity, enforceability or scope of the Patent Rights inside the Territory, without the prior written consent of Astellas, which shall not be unreasonably withheld, delayed or conditioned.
11.5.2 Recoveries. With respect to Enforcement Actions initiated by a Party (the “Initiating Party”) in a country, with the other Party’s prior written consent and upon mutual agreement not to be unreasonably withheld, delayed or conditioned, the other Party shall pay [***] of the costs and expenses (including attorneys’ and professional fees) incurred by the Initiating Party in such Enforcement Action. Any recovery received as a result of any Enforcement Action to enforce the Product Trademark or Patent Rights pursuant to this Section 11.5 by either Party shall, [***] incurred in connection with such Enforcement Action, and the [***] (to the extent the same
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-47-
represents damages from sales of products within the Field in the Territory) equally between the Parties; provided, however, that if the Initiating Party initiates the Enforcement Action and the other Party does not agree to pay [***] of the costs and expenses incurred by the Initiating Party therein, the other Party shall be entitled to any amount recovered in such Enforcement Action in a pro rata amount to its contribution to the costs and expenses incurred by the Initiating Party.
11.6 Third Party Patent Rights
11.6.1 Subject to Section 11.6.2, the obligations of NGX and the rights of Astellas under Sections 11.3 and 11.5 shall be subject to, and limited by, any agreements pursuant to which NGX acquired or licensed any particular Patent Rights or Data, including without limitation the UC License Agreement and LTS Agreement. Without limiting the foregoing, with respect to the prosecution or enforcement of Patent Rights licensed by NGX from a Third Party, to the extent NGX has the right to do so, NGX shall cooperate with Astellas to prosecute and enforce such Patent Rights in the Territory in the same manner as set forth in Sections 11.3 and 11.5 above. As between NGX and Astellas, any recoveries from enforcement of such Patent Rights licensed from a Third Party (including any amounts that NGX receives from the Third Party licensor as a result of such enforcement) [***], after deducting from such recoveries any amounts owed to the Third Party licensor for such enforcement; provided that any Enforcement Actions initiated by the [***].
11.6.2 NGX shall not [***].
11.6.3 If NGX receives notification from LTS under Section 7.2(d) of the LTS Agreement that LTS has elected not to file, prosecute or maintain any patent or patent application licensed by NGX from LTS, then NGX shall so notify Astellas within [***] of NGX’s receipt of such notification. If Astellas wishes NGX to file, prosecute or maintain such patent or patent application it shall inform NGX within [***] of receipt of the notice from NGX and NGX shall immediately (and in any event within the [***] period in Section 7.2(d) of the LTS Agreement) so inform LTS such that the provisions of Sections 7.2(d) and (e) of the LTS Agreement take effect.
11.7 Patent Marking
Astellas agrees to xxxx and have its Affiliates, Sublicensees and Subdistributors xxxx all patented Products they sell or distribute pursuant to this Agreement in accordance with the applicable patent statutes or regulations in the country or countries of manufacture and sale thereof.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-48-
12. TRADEMARKS
12.1 Product Trademark
The Existing Product shall be distributed, promoted, marketed and sold in the Territory by Astellas, its Affiliates, Sublicensees and Subdistributors exclusively under the Product Trademark. All packaging materials, labels and promotional materials for the Existing Product in the Territory shall display the Product Trademark to the extent such packaging materials, labels and promotional materials have enough space for such display. In addition, to the extent reasonably desired by NGX, all product information leaflets and promotional materials for the Product in the Territory shall include a statement acknowledging that the Product is sold under license from NGX.
12.2 Trade Dress
The Astellas trade dress and style of packaging with respect to the Existing Product may be determined by Astellas so as to be consistent with Astellas’ standard trade dress and style, provided that they conform to the Brand Guiding Principles. Any trade dress and style of packaging not consistent with the Brand Guiding Principles shall be subject to the prior written approval by NGX not to be unreasonably withheld, delayed or conditioned. Astellas shall own rights to any trade dress which is created by Astellas or on its behalf and used in the commercialization of the Existing Product in the Territory.
12.3 License
NGX hereby grants to Astellas an exclusive, royalty-free license, to use the Product Trademark solely in connection with marketing, promoting, distributing and selling the Existing Product in the Field and Territory in accordance with this Agreement. This license is sublicensable in accordance with Section 2.4.2 above. The ownership and all goodwill from the use of the Product Trademark shall vest in and inure to the benefit of NGX and will not create any right, title or interest for Astellas in the Product Trademark.
12.4 Quality Standards
12.4.1 The use of the Product Trademark by Astellas shall be in accordance with the Brand Guiding Principles. Astellas shall not take any action that impairs the validity or enforceability of the Product Trademark.
12.4.2 The quality of the Product associated with the Product Trademark, as well as the quality of all marketing and promotional materials and packaging that include the Product Trademark, shall be in accordance with the MAA Approval for the Product in the respective country or jurisdiction. Upon NGX’s reasonable and periodic request, Astellas shall provide NGX with a sample of the Product associated with the Product Trademark.
12.4.3 NGX shall have the right to [***], and the [***] that include the Product Trademark provided by Astellas, and Astellas shall assist NGX [***] in accordance with Section 8.3.2.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-49-
12.5 Alternative Trademark
If the Product Trademark cannot be used for legal, regulatory or other material reasons outside the Parties’ reasonable control, in one or more countries of the Territory, the Parties shall mutually select one of the then available Alternative Trademarks and NGX shall license such Alternative Trademark to Astellas during the term of this Agreement, at no additional cost to Astellas in accordance with Section 12.3 above. In such an event, the Alternative Trademark in such country or countries of the Territory shall become the Product Trademark.
12.6 Registration & Ownership
NGX agrees to use commercially reasonable efforts to file, register and maintain a registration for the Product Trademark (including, if appropriate pursuant to Section 12.5, the Alternative Trademark) in all countries of the European Union (and in countries of the Territory outside the European Union, upon notice from Astellas that an MAA for the Product is being filed in such country), in each case for use with the Existing Product during the term of this Agreement. The costs of filing and maintaining such registrations in the Territory shall [***]. Astellas hereby acknowledges NGX’s exclusive ownership rights in the Product Trademark, and accordingly agrees that at no time during or after the term of this Agreement to challenge or assist others to challenge the Product Trademark or the registration thereof or attempt to register any trademarks, marks or trade names confusingly similar to such Product Trademark. NGX shall not during the term of the Agreement assign or otherwise transfer any Product Trademark in the Territory for which it is the registered proprietor to any Third Party.
12.7 Recordation
In those countries where a trademark license must be recorded, NGX will provide and record a separate trademark license for the Product Trademark, at NGX’s sole expense. Astellas shall cooperate in the preparation and execution of such documents.
12.8 Domain Names
NGX shall own rights to any Internet domain names incorporating the Product Trademark or any variation or part of such Product Trademark as its URL address or any part of such address where the relevant Product Trademarks are owned by it. NGX agrees to grant, and hereby grants to Astellas a royalty-free, fully paid-up exclusive license to use the domain names listed on Exhibit 12.8 (“Domain Names”) in connection with Astellas’ commercialization of the Existing Product in the Territory in accordance with this Agreement, provided that [***]. If there is a requirement in any country in the Territory for the domain name holder to have a presence in that country and NGX does not have a presence in that country then NGX shall transfer the relevant Domain Name to Astellas provided that upon termination of this Agreement (but not expiration) Astellas shall promptly transfer such Domain Name back to NGX. The use rights granted to the Domain Names
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-50-
under this Section 12.8 are limited to the Territory, and Astellas shall not make or authorize any use, direct or otherwise, of the Domain Names outside the Territory. Astellas shall not sublicense the Domain Names without NGX’s prior written consent. For clarity, Astellas acknowledges and agrees that the Domain Names and the goodwill pertaining to such Domain Names shall belong to NGX. NGX agrees to take such additional actions as may be reasonably required to extend to Astellas the benefits of this license. The Parties agree that any use of the Product Trademark in any content describing or referring to the Existing Product in the Territory on any internet page or web site shall, subject to the terms of this Agreement, be in the sole control of Astellas.
12.9 Trademark for Liquid Formulation Product
If Astellas exercises the Option pursuant to Section 2.3.12 then the parties shall agree a trademark for use in relation to such Product (the “LF Trademark”). The terms of Sections 12.1 to 12.8 shall apply mutatis mutandis in relation to any such trademark such that references to Existing Product shall be read as references to the Liquid Formulation Product, references to Product Trademark shall be read as references to the LF Trademark and references to the Alternative Trademark shall be read as references to a mutually agreed alternative trademark for the Liquid Formulation Product.
12.10 Promotional Materials
All promotional materials and packaging for Product (whether of NGX, its Affiliates and licensees outside the Territory or Astellas, its Affiliates, Sublicensees and Subdistributors inside the Territory) using the Product Trademark shall conform to the Brand Guiding Principles.
12.11 Termination
Astellas’ right to use the Product Trademark shall terminate in each country of the Territory in which Astellas’ rights to distribute the Product are terminated in accordance with this Agreement. Astellas shall cooperate in the cancellation of any trademark licenses recorded or entered into in such countries. In the event that the Agreement expires then Astellas’ rights to the Product Trademark shall continue pursuant to Section 18.2(b).
13. CONFIDENTIALITY
13.1 Confidential Information Except as expressly provided herein, the Parties agree that the receiving Party shall not publish nor otherwise disclose, and shall not use for any purpose, any information furnished to it by the other Party hereto pursuant to this Agreement (collectively, “Confidential Information”). Notwithstanding the foregoing, Confidential Information shall not include information which, in each case as demonstrated by written documentation:
13.1.1 was already known to the receiving Party, other than under an obligation of confidentiality, at the time of disclosure;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-51-
13.1.2 was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
13.1.3 became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement;
13.1.4 was subsequently lawfully disclosed to the receiving Party by a person other than a Party, and who did not directly or indirectly receive such information from disclosing Party; or
13.1.5 was independently developed by the receiving Party without use of or reference to any information or materials disclosed by the disclosing Party.
13.2 Permitted Use and Disclosures
Notwithstanding the provisions of Section 13.1 above, each Party hereto may use and disclose the other Party’s Confidential Information to the extent such disclosure is reasonably necessary to exercise the rights granted to it, or reserved by it, under this Agreement (including the right to grant sublicenses, as applicable), prosecuting or defending litigation, complying with applicable governmental regulations, submitting information to tax or other governmental authorities (including without limitation the European Commission), or conducting clinical trials hereunder with respect to Products, provided that if a Party is required to make any such disclosure of the other Party’s Confidential Information, to the extent it may legally do so, it will give reasonable advance notice to the latter Party of such disclosure and, save to the extent inappropriate in the case of patent applications or otherwise, will use its reasonable efforts to secure confidential treatment of such information prior to its disclosure (whether through protective orders or otherwise). For any other disclosures of the other Party’s Confidential Information, including to Affiliates, licensees, Subdistributors and other Third Parties, a Party shall ensure that the recipient thereof is bound by a written confidentiality agreement as materially protective of such Confidential Information as this Section 13. If the Party whose Confidential Information is to be disclosed has not filed a patent application with respect to such Confidential Information, it may require the other Party to delay the proposed disclosure (to the extent the disclosing Party may legally do so), for up to [***], to allow for the filing of such an application. This Section 13 shall not limit either Party’s right under Section 7.2.1 to use and disclose Data.
13.3 Terms of this Agreement
Each Party agrees not to disclose to any Third Party the terms of this Agreement without the prior written consent of the other Party hereto, except each Party may disclose the terms of this Agreement (a) as required by law (as determined by the disclosing Party’s legal counsel) or in filings with governmental entities, e.g. the SEC, IRS or with national stock exchanges, or (b) under reasonable conditions of confidentiality, to advisors, auditors and actual or potential acquisition partners or private investors on a need to know basis.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-52-
13.4 Prior Non-Disclosure Agreements
Upon execution of this Agreement, the terms of this Section 13 shall supercede any prior non-disclosure, secrecy, or confidentiality agreement between the Parties. Any information disclosed under such prior agreements shall be deemed disclosed under this Agreement.
13.5 Publication of Product Information
Prior to publishing, publicly presenting and/or submitting for written or oral publication a manuscript, abstract or the like that includes Data or other information relating to Product that has not previously been published pursuant to this Section 13.5, a Party shall provide the other Party a copy thereof in English for its review for at least [***] (unless such Party is required by law to publish such information sooner). Such Party shall consider in good faith any comments provided by the other Party during such [***] period. In addition, such Party shall, at the request of the other Party: (a) remove any Confidential Information of the other Party therefrom, except each Party shall have the right to publicly disclose any information, including Confidential Information, pertaining to safety or efficacy of the Product that such Party believes in good faith it is obligated or appropriate to disclose; and (b) delay publication for a period not to exceed [***] in order to allow the other Party to file for patent protection in relation to its Data and Confidential Information. The contribution of each Party shall be noted in all publications or presentations by acknowledgment or coauthorship, whichever is appropriate.
14. REPRESENTATIONS AND WARRANTIES
14.1 Mutual Warranties
Each Party warrants and represents to the other that the Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms. The execution, delivery and performance of the Agreement by such Party does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor to such Party’s knowledge, violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
14.2 NGX Warranties
NGX warrants and represents to Astellas that as of the Effective Date:
14.2.1 it has the full right and authority to enter into this Agreement and grant the rights and licenses granted herein;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-53-
Intellectual Property
14.2.2 it has not previously granted any right, license or interest in or to the Patent Rights, or any portion thereof, that is in conflict with the rights or licenses granted to Astellas under this Agreement;
14.2.3 [***];
14.2.4 there are no pending proceedings in any court, arbitration, patent office, administrative or other tribunal which are concerned with the validity or ownership of any of the Patent Rights, NGX Know-How or the Product Trademark or the Alternative Trademark, [***] those that have been published in the following NGX SEC filings: (a) the Business and Risk Factors sections of (i) the Form S-1A dated 16 March 2007, (ii) the Form 10-K for the year ended 31 December 2007 and (iii) the Form 10-K for the year ended 31 December 2008 and (b) the Risk Factors Section of the Form 10Q for the quarter ended 31 March 2009 provided that in relation to Patent Rights which have been licensed to it and are not owned by it, the foregoing is given to the Knowledge of NGX having made reasonable enquiry of UC and LTS;
14.2.5 to the Knowledge of NGX, upon Consultation, [***];
14.2.6 the details of the Patent Rights, the Product Trademark and the Alternative Trademark that are set out in Exhibit 1.40 and Exhibit 1.44 are [***]. Without derogation from the generality of the foregoing, the Patents Rights, the Product Trademark and the Alternative Trademark (to the extent they are granted) are subsisting and all applications for Patents Rights, the Product Trademark or the Alternative Trademark indicated in Exhibit 1.40 and Exhibit 1.44 as pending are pending. The legal and beneficial owner or applicant for registration of each of the Patents Rights, the Product Trademark or the Alternative Trademark specified in Exhibit 1.40 and Exhibit 1.44 is [***];
14.2.7 [***] the Patent Rights and Product Trademarks set forth in Exhibit 1.40 and Exhibit 1.44;
14.2.8 the Patent Rights, the Product Trademark, the Alternative Trademark and the NGX Know-How are [***];
14.2.9 all actions required to be taken [***] in good faith ([***]) have been taken [***];
14.2.10 the Patent Rights owned by NGX are not and, during the term of this Agreement, will not become subject to any rights granted in favor of a Third Party, including a lien or other encumbrance, which would conflict with the rights or licenses of Astellas hereunder;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-54-
14.2.11 as of the Effective Date, [***] save for those that have been published in the following NGX SEC filings: (a) the Business and Risk Factors sections of (i) the Form S-1A dated 16 March 2007, (ii) the Form 10-K for the year ended 31 December 2007 and (iii) the Form 10-K for the year ended 31 December 2008 and (b) the Risk Factors Section of the Form 10Q for the quarter ended 31 March 2009.
14.2.12 NGX has disclosed to Astellas true and complete copies of the UC Agreement and the LTS Agreement and all actual amendments to these agreements and all contemplated amendments that have been discussed with, respectively, UC and LTS;
14.2.13 the UC Agreement and LTS Agreement are in full force and effect, each such agreement is binding on the parties and to the Knowledge of NGX no party is in material breach of any of its obligations under such agreements;
14.2.14 NGX (a) is not in breach and shall not breach its obligations under the UC Agreement or LTS Agreement or take any other actions, in each case which would result in the termination of such agreement(s) and the subsequent loss by Astellas of its rights with respect to the Patent Rights in-licensed under such agreement(s), provided that NGX will not be deemed to be in breach of this Section 14.2.14(a) in the event that Astellas’ rights under such agreement(s) would have survived the termination of such agreement(s) but for Astellas’ having failed to take the necessary steps to ensure the survival of its rights, (b) shall promptly inform Astellas in the event that it receives written notice from UC or LTS purporting to unilaterally terminate the UC Agreement and LTS Agreement and will use commercially reasonable efforts to dispute any allegation of material breach asserted by UC or LTS with respect to the UC Agreement and/or LTS Agreement as may be required to prevent termination of such licenses, and (c) shall not amend such agreements in a manner that would be to the detriment of the rights and obligations of Astellas under this Agreement without Astellas’ prior written consent;
14.2.15 NGX has provided UC with a copy of [***];
14.2.16 NGX has not been notified by [***] that [***] has commenced development [***];
Marketing Authorizations/Regulatory
14.2.17 EMEA MA Approval Number EMEA/H/C/000909 is the only MAA and MAA Approval held by NGX or its Affiliates in relation to the marketing and sale of the Existing Product in the Territory;
14.2.18 Astellas has been granted access to true and complete copies of all of NGX’s regulatory filings relating to the Product;
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-55-
14.2.19 all clinical trials for the Existing Product conducted by or on behalf of NGX and included in NGX’s regulatory filings with the EMEA [***];
14.2.20 [***] with respect to the Data used to support EMEA MA Approval Number EMEA/H/C/000909;
14.2.21 that as of the Effective Date, NGX has disclosed to Astellas all clinical data relating to SAEs relating to Products, such SAEs being set out in Exhibit 14.2.21;
14.2.22 NGX has not knowingly withheld from any regulatory authority any material information in the possession of NGX or its Affiliates related to the safety, toxicity, quality or efficacy of the Components and/or the Existing Product (a) that has been requested by a regulatory authority, or (b) that a biopharmaceutical company would reasonably consider to be material for the EMEA’s evaluation of the safety, toxicity, quality and/or efficacy of the Components and/or the Existing Product;
14.2.23 neither NGX nor its Affiliates or any of its or their officers, agents or employees (during the course of their duties) in relation to researching, and developing the Existing Product in the Territory has done or omitted to [***] adversely affect Astellas’ ability to commercialize the Existing Product as contemplated herein;
14.2.24 there has been no failure to [***] that would materially adversely affect Astellas’ ability to commercialize the Existing Product as contemplated herein;
14.2.25 NGX and its Affiliates have developed the Existing Product and the Liquid Formulation Product [***];
14.2.26 EMEA MA Approval Number EMEA/H/C/000909 was granted by the European Commission upon the submission by the NGX of a full dossier presented in accordance with Article 8.3 of EC Directive 2001/83 and does not refer to any other marketing approval;
14.2.27 the application for EMEA MA Approval Number EMEA/H/C/000909 includes the results of all clinical trials that have been started (i.e., in which patients have been enrolled) by or on behalf of NGX relating to the Existing Product whether discontinued or completed with the exception of [***] for which only a synopsis has been provided to the EMEA;
14.2.28 Astellas has the right to [***];
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-56-
General
14.2.29 neither NGX nor any NGX Affiliate is engaged in any litigation, opposition or arbitration proceedings affecting or relating to the Products anywhere in the world (including but not limited to claims relating to product liability) as plaintiff or defendant and [***] sections of (i) the Form S-1A dated 16 March 2007, (ii) the Form 10-K for the year ended 31 December 2007 and (iii) the Form 10-K for the year ended 31 December 2008 and (b) the Risk Factors Section of the Form 10Q for the quarter ended 31 March 2009;
14.2.30 NGX has not knowingly withheld from Astellas any material information in the possession of NGX related to the safety, toxicity, quality or efficacy of the Components and/or the Existing Product (a) that has been requested by Astellas, or (b) that a biopharmaceutical company would reasonably consider to be material to Astellas’ evaluation of the safety, toxicity, quality and/or efficacy of the Components and/or the Existing Product;
14.2.31 NGX has not knowingly withheld from Astellas any material CMC Information in the possession of NGX regarding the Existing Product (a) that has been requested by Astellas, or (b) that a biopharmaceutical company would reasonably consider to be material to Astellas’ evaluation of the quality of the Components and/or the Existing Product. As used herein, “CMC Information” means information of the type required to appear in Module 3 of EMEA MAA Approval No. EMEA/H/C/000909;
14.2.32 NGX has not knowingly withheld from Astellas any material information in the possession of NGX related to the market and sales potential of the Components and/or the Existing Product (a) that has been requested by Astellas, or (b) that a biopharmaceutical company would reasonably consider to be material to Astellas’ evaluation of the market and sales potential of the Components and/or the Existing Product in the Territory;
14.2.33 NGX and its Affiliates have no employees in the Territory;
14.2.34 all necessary consents, approvals and authorizations of all governmental authorities and other persons or entities required to be obtained by NGX in order to enter into this Agreement have been obtained;
14.2.35 other than with respect to [***], no US export control license is required (a) in order to enter into this Agreement, (b) transfer NGX Know-How and Data to Astellas, (c) for Astellas to purchase any Components or Existing Products, or (d) in order to enable Astellas to exercise its rights under this Agreement;
14.2.36 except as otherwise noted in Section 14.2.35, no approval from U.S. federal government or any U.S. state government (including any agency or division thereof) or to its Knowledge under any applicable law, is required (a) in order to enter into this Agreement, (b) transfer NGX Know-How and Data to Astellas, (c) for Components, Existing Products or the
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-57-
Liquid Formulation Product to be manufactured either inside or outside the US or (d) in order to enable Astellas to exercise its rights under this Agreement, in each case which lack of approval would materially adversely affect Astellas’ ability to commercialize the Existing Product as contemplated herein;
14.2.37 as of the Effective Date, [***]; and
14.2.38 except for the documents identified on Exhibit 14.2.38, all material information referred to in Sections 14.2.30, 14.2.31 and 14.2.32 has been made available to Astellas at least [***] prior to the Effective Date.
14.3 NGX Covenants
NGX covenants to Astellas that, during the term of this Agreement (a) it shall not grant any right, license or interest in or to the Patent Rights and NGX Know-How, or any portion thereof, that is in conflict with the rights or licenses granted under this Agreement, and (b) it shall not xxxxx x xxxx or other encumbrances on any of the subject matter of this Agreement or on any of NGX’s rights, benefits or obligations hereunder or on any of the Patent Rights, which would conflict with the rights or licenses of Astellas hereunder, provided that the Parties agree that this subsection (b) is not intended to, and shall not, restrict NGX from engaging in customary equity and debt financings.
14.4 Astellas Warranties
Astellas warrants and represents to NGX that, as of the Effective Date: (a) it has the full right and authority to enter into this Agreement and grant the rights and perform the obligations set forth herein; (b) [***] (c) all necessary consents, approvals and authorizations of all governmental authorities and other persons or entities required to be obtained by Astellas in order to enter into this Agreement have been obtained save in respect of any export control licenses.
15. LIABILITY
15.1 DISCLAIMER EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT TO THE CONTRARY, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTIES OF ANY KIND EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT, OR VALIDITY OF ANY PATENTS ISSUED OR PENDING.
15.2 LIMITATION OF LIABILITY NOTWITHSTANDING ANYTHING IN THIS AGREEMENT OR OTHERWISE, NEITHER PARTY SHALL BE LIABLE TO THE OTHER WITH RESPECT TO ANY SUBJECT MATTER OF THIS AGREEMENT, WHETHER UNDER ANY CONTRACT, NEGLIGENCE, STRICT LIABILITY OR OTHER LEGAL OR EQUITABLE THEORY, FOR ANY INCIDENTAL, INDIRECT, SPECIAL, EXEMPLARY, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES, LOST PROFITS, LOSS OF ROYALTIES AND MILESTONE PAYMENTS, LOSS OF USE, DAMAGE TO GOODWILL, OR LOSS OF BUSINESS.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-58-
15.3 Exceptions
Nothing in this Agreement shall limit any Party’s liability (a) caused by that Party’s willful misconduct, (b) for death or personal injury caused by that Party’s negligence, or (c) for fraudulent misrepresentation by that Party.
16. INDEMNIFICATION
16.1 Indemnification of NGX
Subject to Section 15.2, Astellas hereby agrees to indemnify, defend and hold harmless NGX, its Affiliates, and their respective directors, officers, employees, agents and their respective successors, heirs and assigns (the “NGX Indemnitees”) from and against any losses, costs, claims, damages, liabilities or expenses (including, without limitation, reasonable attorneys’ and professional fees and other expenses of litigation) (collectively, “Liabilities”) from any claims, suits, actions or proceedings brought by a Third Party (a “Third Party Claim”) incurred by any NGX Indemnitee as a result of: [***].
16.2 Indemnification of Astellas
Subject to Section 15.2, NGX hereby agrees to indemnify, defend and hold harmless Astellas, its Affiliates, and their respective directors, officers, employees, agents and their respective successors, heirs and assigns (the “Astellas Indemnitees”) from and against any Liabilities from any Third Party Claims incurred by any Astellas Indemnitee as a result of: [***].
16.3 Procedure
Except with respect to Third Party infringement claims subject to Section 11.4 above, a Party that intends to claim indemnification under this Section 16 (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) in writing of any Third Party Claim, in respect of which the Indemnitee intends to claim such indemnification, and the Indemnitor shall have sole control of the defense and/or settlement thereof; provided, that the Indemnitee shall have the right to participate in the defense or settlement of such Third Party Claim with counsel of its own choosing at its expense. The Indemnitor shall keep the Indemnitee fully informed of the progress of any such Third Party Claim. The indemnity arrangement in this Section 16 shall not apply to amounts paid in settlement of any action with respect to a Third Party Claim, if such settlement is effected without the consent of the Indemnitor, which consent shall not be withheld, delayed or conditioned unreasonably. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim, if prejudicial to its ability to defend such action,
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-59-
shall relieve such Indemnitor of any liability to the Indemnitee under this Section 16 to the extent it is so prejudiced, but the omission to so deliver written notice to the Indemnitor shall not relieve the Indemnitor of any liability that it may have to any Indemnitee otherwise than under this Section 16. The Indemnitee under this Section 16 shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Third Party Claim covered by this indemnification.
16.4 Consequences of Failure to Transfer MA
The Parties acknowledge and agree that the transfer of the Existing Product MAA Approval is fundamental to Astellas’ ability to commercialize the Existing Product, and accordingly it is agreed that in the event that NGX materially breaches its obligations under Sections 6.1, 6.3.1, 6.3.2 or 6.4, and as a result, the Existing Product MAA Approval is not transferred to Astellas or Astellas’ designated Affiliate within [***] of Astellas’ request for transfer, or is cancelled, withdrawn or substantially amended in a manner that is prevents the commercialisation of the Existing Product in the Territory (each of the foregoing a “Default”), then Astellas shall have the right under this Section 16.4 to terminate this Agreement upon written notice to NGX within thirty (30) days of the occurrence of a Default, in which case NGX shall refund to Astellas within thirty (30) days of the date of such notice, the Initial Payment of Thirty Million Euros (€30,000,000) together with the Option Fee of Five Million Euros (€5,000,000) and the terms of Section 18.6 shall apply. Save for willful misconduct by NGX, and its employees, the foregoing shall be Astellas’ sole remedy for a failure to transfer the Existing Product MAA Approval or the cancellation, withdrawal or amendment of the Existing Product MAA Approval, in each case which is the result of NGX’s breach of its obligations under Sections 6.1, 6.3.1, 6.3.2 or 6.4
16.5 Insurance
Each Party shall secure and maintain in effect during the term of this Agreement and for a period of [***] thereafter (a) commercial general liability insurance with a minimum coverage of [***] per occurrence and annual aggregate, (b) product liability insurance with a minimum coverage of [***] per occurrence and annual aggregate, and (c) clinical trial insurance with limits as required by local laws and regulations or, in the absence of such requirements, standard and customary in the relevant country, underwritten by a reputable insurance company in a form standard and customary for entities in the pharmaceutical industry for exposures related to this Agreement. Upon request by the other Party hereto, certificates of insurance evidencing the coverage required above shall be provided to the other Party.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-60-
17.1 Term
This Agreement shall become effective as of the Effective Date and, unless earlier terminated pursuant to the other provisions of this Section 17 or Section 8.7 above, shall continue on a country-by-country and Product-by-Product basis, until the later of (a) ten (10) years after the First Commercial Sale thereof in the respective country, (b) the expiration or abandonment of the last Valid Patent Claim covering the respective Product in the respective country and (c) the expiration of all applicable periods of regulatory exclusivity (e.g., regulatory data exclusivity) covering the respective Product in the respective country.
17.2 Termination By Astellas
Astellas may terminate this Agreement for any reason under this Section 17.2 without any penalty, consequence, termination compensation, loss of profits, goodwill indemnity or otherwise solely by reason of such termination (i.e., without prejudice to any remedies NGX may have for a breach of this Agreement by Astellas prior to such termination), as a whole, or with respect to the European Region, or on a country-by-country basis with respect to countries in the Territory (other than those countries comprising the European Region) and a Product-by-Product basis, in each case upon written notice to NGX.
17.3 Termination for Material Breach
Without limiting either Party’s ability to terminate in accordance with the other provisions of this Agreement, in the event of a Party’s material breach of this Agreement, the non-breaching Party shall have the right to provide notice of its intention to terminate this Agreement. Such notice shall specify in reasonable detail the facts and circumstances constituting the material breach of this Agreement. Upon the expiration of [***] after receipt by the breaching Party of such notice, if the breaching Party has not cured such material breach, the non-breaching Party shall have the right to terminate this Agreement in whole by giving a notice of termination, which shall be effective on the date such notice is given.
17.4 Termination for Insolvency
Either Party may terminate this Agreement upon written notice to other Party at any time, to the extent permitted by applicable law, if the other Party shall become insolvent, or shall make or seek to make or arrange an assignment for the benefit of creditors, or if proceedings in voluntary or involuntary bankruptcy shall be initiated by, on behalf of or against such Party (and, in the case of any such involuntary proceeding, not dismissed within [***]), or if a receiver or trustee of such Party’s property shall be appointed and not discharged within [***].
17.5 [***]
NGX may terminate this Agreement in whole or in part (on a country-by-country basis) at any time upon [***] prior written notice to Astellas in the event Astellas breaches Section 2.7.1 of this Agreement. Notwithstanding the foregoing, in the event that upon notice from NGX that Astellas is in breach of Section 2.7.1 (solely for the first such breach), Astellas ceases to [***], NGX’s right to terminate this Agreement shall cease.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-61-
17.6 [***]
Astellas may terminate this Agreement in whole or in part (on a country-by-country basis) at any time upon [***] prior written notice to NGX in the event NGX breaches Section 2.7.4 of this Agreement. Notwithstanding the foregoing, in the event that upon notice from Astellas that NGX is in breach of Section 2.7.4 (solely for the first such breach), NGX ceases to [***], Astellas’ right to terminate this Agreement shall cease.
18. EFFECT OF EXPIRATION OR TERMINATION
18.1 General
18.1.1 Accrued Obligations. Termination of this Agreement for any reason shall not release any Party hereto from any liability which, at the time of such termination, has already accrued to the other Party or which is attributable to a period prior to such termination nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement. For clarity, any sums owed to Astellas as a result of any overpayments (for example but not limited to Royalty Adjustments under Section 4.6.2(b) and payments under Section 10.4.2) that have been rolled over in the form of a credit shall be immediately due and payable on termination.
18.1.2 Disbanding of the Committees. Expiration or termination of this Agreement for any reason shall result in the immediate disbanding and termination of all Committees.
18.2 Rights on Expiration
(a) NGX Know-How. Upon the expiration, but not an earlier termination, of this Agreement with respect to a particular country in relation to a particular Product, if Astellas has not entered into direct agreements with Component suppliers in respect of the Existing Product then Astellas may request that NGX continue to supply Astellas with its requirements for Components in accordance with the Supply Agreement (or Liquid Formulation Products in the event that NGX is supplying Astellas with such Products), in which event the Parties shall revise the Supply Price (as defined in the Supply Agreement or Liquid Formulation Product supply agreement) to include a reasonable profit for NGX, and Astellas will have a fully paid-up, non-exclusive license, which includes the right to sublicense, under the NGX Know-How to use, promote, market, sell, offer for sale, import, export and otherwise commercialize the Product within the Field in such country. If the Parties are unable to agree upon a revised Supply Price or if Astellas has entered into direct agreements with Component suppliers or suppliers of the Liquid Formulation Product, Astellas shall have a paid-up, non-exclusive license, which includes the right
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-62-
to sublicense, under the NGX Know-How to make, have made, use, promote, market, sell, offer for sale, import, export and otherwise commercialize the Product within the Field in such country. For clarity, Astellas shall retain ownership of all MAA Approvals on expiration of this Agreement.
(b) Trademark Rights. Upon the expiration, but not an earlier termination, of this Agreement, Sections 12.1 (first sentence), 12.3, 12.4, 12.6 (provided that Astellas shall be responsible for all costs of filing and maintaining the registrations in the Territory), 12.7 (provided that Astellas shall be responsible for all costs of recordation), 12.8 and 12.9 above shall survive with respect to such Product and the Product Trademark in such country. Astellas shall have the right to terminate its license to the Product Trademark with respect to any particular Product in any country upon [***] notice to NGX, in which case this Section 18.2 shall have no further force or effect, with respect to such Product in such country, from and after the effective date of such termination.
(c) Surviving Terms. Upon expiration (but not the earlier termination) of this Agreement, all rights and obligations of the Parties under this Agreement shall terminate except: (i) Sections 1, 4.3.5 (to the extent the applicable Infringement Action arose from actions occurring prior to expiration), 7.2.1(b), 4.6 and 10.1-10.3 (with respect to payment obligations accruing prior to expiration and not included in a prior Quarterly Report and Year End Report), 10.4 (for a period of 6 months from the final required royalty report), 11.1.4 (in respect of the NGX License), 11.4.1 (with respect to alleged infringement that occurred prior to the date of expiration of this Agreement), with respect to Costs arising prior to expiration, Astellas’ rights under Section 11.4.2 to treat such Costs as Third Party Royalties, 11.5 (with respect to Enforcement Actions initiated prior to expiration), 13, 15, 16, 18, 19 and 20, and (ii) those provisions of this Agreement to the extent set forth above in Sections 18.2(a) and 18.2(b) as surviving expiration of this Agreement.
18.3 Rights on Termination of a Product
Upon any termination (but not the expiration) of this Agreement with respect to a Product (the “Terminated Product”) pursuant to Section 17:
18.3.1 Wind-down Period.
(a) Development. In the event there are any on-going clinical trials of a Terminated Product in the Territory, at NGX’s request, Astellas agrees to either transition such clinical trials to NGX, or to continue for a period not to exceed [***] after such termination to conduct such clinical trials and shall be responsible for the Development Expenses on the same basis as that in effect immediately prior to termination. In addition, to the extent NGX is then providing Astellas with any development support or services relating to the Terminated Product at the time of such termination, Astellas agrees to compensate NGX for the FTE’s actually used by Astellas in respect of such services at NGX’s rate set out in the relevant development plan.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-63-
(b) Commercialization. At NGX’s request, Astellas and its Affiliates, Sublicensees and Subdistributors shall continue to distribute in the same manner the Terminated Product in each country in the Territory for which MAA Approval therefor has been obtained, in accordance with the terms and conditions of this Agreement, for a period not to exceed [***] from such termination (for purposes of this Section 18.3, the “Wind-down Period”); provided that NGX may after such request terminate such Wind-down Period upon [***] written notice to Astellas. Notwithstanding any other provision of this Agreement, during the Wind-down Period, Astellas’, its Affiliates’, Sublicensees’ and Subdistributors’ rights with respect to the Terminated Product shall be non-exclusive, and NGX shall have the right to engage one or more other distributor(s) and/or Subdistributor(s) of Terminated Products in all or part of the Territory. Sections 3 and 4 shall apply with respect to all Terminated Products sold, used or disposed by Astellas, its Sublicensees or Affiliates during the Wind-down Period. All rights of Astellas with respect to Terminated Products shall be terminated after the Wind-down Period, and unless otherwise mutually agreed, any Terminated Products that are not sold after such Wind-down Period shall be destroyed.
18.3.2 Assignment of MAA and MAA Approvals. Astellas shall assign or cause to be assigned to NGX (or if not so assignable, Astellas shall take all reasonable actions to make available to NGX) all regulatory filings and registrations (including XXXx and MAA Approvals) for such Terminated Product in the Territory. In each case the foregoing assignment (or availability) shall be made within [***] after termination of this Agreement with respect to such Product. In addition, Astellas shall promptly provide to NGX a copy of all Data pertaining to the Terminated Product to the extent not previously provided to NGX, which together with the Data provided by Astellas during the term of this Agreement pertaining to the Terminated Product, NGX shall have the right to use and disclose for any purpose directly relating to the commercialization of the Terminated Product in the Territory during the Wind-down Period and thereafter.
18.3.3 Transition. Astellas shall cooperate with NGX or its designee(s) and use Commercially Reasonable Efforts to effect a smooth and orderly transition in the sale of Terminated Products in the Territory during the Wind-down Period. Without limiting the foregoing, subject to applicable laws and Third Party rights, Astellas shall provide NGX with copies of customer lists, customer data, Sales Materials and other customer information exclusively relating to the Terminated Product that are Controlled by Astellas to the extent not previously provided to NGX, which together with such materials provided to NGX during the term of this Agreement, NGX shall have the right to use, disclose, modify, reproduce and distribute for any purpose directly relating to the commercialization of the Terminated Product in the Territory during the Wind-down Period and thereafter. In addition, Astellas shall refer all inquiries regarding the Terminated Products after the Wind-down Period to NGX or any newly appointed distributors.
18.3.4 Return of Materials. Within [***] after the end of the Wind-down Period, Astellas shall destroy all tangible items comprising, bearing or containing trademarks, marks, trade names, patents, copyrights, designs, drawings, formulas or other data, photographs, samples,
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-64-
literature, sales and promotional aids (“Product Materials”) and all Confidential Information of NGX, in each case pertaining to the Terminated Products, that is in Astellas’ possession, and provide written certification of such destruction, or prepare such tangible items of Promotional Materials and Confidential Information for shipment to NGX, as NGX may direct, at NGX’s expense; provided that Astellas may retain and use copies of any such Promotional Materials and Confidential Information that also relate to products (including Products) other than Terminated Products, as reasonably necessary for its continued promotion, marketing, sale and distribution of such products and non-terminated Products under this Agreement. Effective upon the end of the Wind-down Period, Astellas shall cease to use all trademarks and trade names of NGX with respect to the Terminated Product in the Territory.
18.3.5 Subdistributors. Any Subdistributors or subdistributors of Terminated Product in the Territory engaged by Astellas shall, at the request of NGX in its discretion, be assigned to NGX to the furthest extent possible. In the event NGX does not request assignment of such Subdistributors or subdistributors, then the rights of such Subdistributors or subdistributors with respect to Terminated Product in the Territory shall terminate upon termination of Astellas’ rights under this Agreement with respect to the Terminated Product.
18.4 Sublicenses
If NGX terminates this Agreement and the Agreement has been sublicensed by Astellas prior to such termination, then NGX shall enter into an agreement directly with such Sublicensee on the terms of this Agreement.
18.5 Rights on Termination of a non-European Union country (under Section 7.1.1) or Region Upon any termination (but not the expiration) of this Agreement with respect to a particular Region (each, a “Terminated Country” or “Terminated Region”) including pursuant to Section 8.7 or Section 17:
18.5.1 Wind-down Period.
(a) Development. In the event there are any on-going clinical trials of Products in a Terminated Country or Terminated Region, at NGX’s request, Astellas agrees to either transition such clinical trials to NGX, or to continue for a period not to exceed [***] after such termination to conduct such clinical trials and shall bear the Development Expenses on the same basis as that in effect immediately prior to termination. In addition, to the extent NGX is then providing Astellas any development support or services relating to Product in the Terminated Country or Terminated Region at the time of such termination, Astellas agrees to compensate NGX for the FTEs actually used by NGX in respect of such services at NGX’s rate set out in the relevant development plan.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-65-
(b) Commercialization. At NGX’s request, Astellas and its Affiliates, Sublicensees and Subdistributors shall continue to distribute Products in the Terminated Country or all countries of a Terminated Region for which MAA Approval therefor has been obtained, in accordance with the terms and conditions of this Agreement, for a period not to exceed [***] from such termination (for purposes of this Section 18.4, the “Wind-down Period”); provided that NGX may after such request terminate such Wind-down Period upon [***] written notice to Astellas. Notwithstanding any other provision of this Agreement, during the Wind-down Period, Astellas, its Affiliates’, Sublicensees’ and Subdistributors’ rights with respect to Products in the Terminated Country or Terminated Region shall be non-exclusive, and NGX shall have the right to engage one or more other distributor(s) and/or Subdistributor(s) of Products in all or part of the Terminated Country or Terminated Region. Sections 3 and 4 shall apply with respect to all Products sold, used or disposed in the Terminated Country or Terminated Region by Astellas, its Sublicensees or Affiliates during the Wind-down Period. All rights of Astellas with respect to Products in the Terminated Country or Terminated Region shall be terminated after the Wind-down Period.
18.5.2 Assignment of MAA and MAA Approvals. Astellas shall assign or cause to be assigned to NGX (or if not so assignable, Astellas shall take all reasonable actions to make available to NGX) all regulatory filings and registrations (including XXXx and MAA Approvals) for Products in the Terminated Country or Terminated Region. In each case the foregoing assignment (or availability) shall be made within [***] after termination of this Agreement with respect to such country or Region. In addition, Astellas shall promptly provide to NGX a copy of all Data pertaining to Products in the Terminated Country or Terminated Region to the extent not previously provided to NGX, which together with the Data provided by Astellas during the term of this Agreement pertaining to the Terminated Country or Terminated Region, NGX shall have the right to use and disclose for any purpose directly relating to the commercialization of the Terminated Product in the Territory during the Wind-down Period and thereafter.
18.5.3 Transition. Astellas shall cooperate with NGX or its designee(s) and use [***] to effect a smooth and orderly transition in the sale of Products in the Terminated Country or Terminated Region during the Wind-down Period. Without limiting the foregoing, subject to applicable laws and Third Party rights, Astellas shall provide NGX copies of customer lists, customer data, Sales Materials and other customer and marketing information that are Controlled by Astellas and exclusively relate to Products in the Terminated Country or Terminated Region to the extent not previously provided to NGX, which together with such materials provided to NGX during the term of this Agreement, NGX shall have the right to use, disclose, modify, reproduce and distribute for any purpose directly relating to the commercialization of the Terminated Product in the Territory during the Wind-down Period and thereafter. In addition, Astellas shall refer all inquiries regarding the Products from the Terminated Country or Terminated Region after the Wind-down Period to NGX or any newly appointed distributors.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-66-
18.5.4 Return of Materials. Within [***] after the end of the Wind-down Period, Astellas shall destroy all Product Materials and Confidential Information of NGX, in each case pertaining to the Terminated Country or Terminated Region, that is in Astellas’ possession, and provide written certification of such destruction, or prepare such tangible items of Promotional Materials and Confidential Information for shipment to NGX, as NGX may direct, at NGX’s expense; provided that Astellas may retain and use copies of any such Promotional Materials and Confidential Information that also relate to countries or Regions other than the Terminated Country or Terminated Region, as reasonably necessary for its continued promotion, marketing, sale and distribution of products (including Products) in such non-terminated countries and Regions under this Agreement. Effective upon the end of the Wind-down Period, Astellas shall cease to use all trademarks and trade names of NGX in the Terminated Country or Terminated Region.
18.5.5 Subdistributors. Any Subdistributors of Products in the Terminated Country or Terminated Region engaged by Astellas shall, at the request of NGX in its discretion, be assigned to NGX to the furthest extent possible. In the event NGX does not request assignment of such Subdistributors, then the rights of such Subdistributors with respect to Products in the Terminated Country or Terminated Region shall terminate upon termination of Astellas’ rights with respect to such Terminated Country or Terminated Region.
18.6 Rights on Termination of this Agreement
The termination of this Agreement in whole shall be treated a termination of this Agreement with respect to all Products and all Regions, and Sections 18.3 and 18.5 above shall apply to all Products and all Regions, each of which shall be deemed Terminated Products and Terminated Regions, respectively.
18.7 No Renewal, Extension or Waiver
Acceptance of any order from, or sale or license of, any Product to Astellas after the effective date of termination or expiration of this Agreement shall not be construed as a renewal or extension hereof, or as a waiver of termination of this Agreement.
18.8 Survival
Upon termination (but not expiration) of this Agreement in whole, all rights and obligations of the Parties under this Agreement shall terminate except: (a) Sections 1, 2.5.6 and 4.3.5 to the extent the applicable Infringement Action arose from actions occurring prior to termination, 7.2.1(b), 4.6 and 10.1-10.3 (with respect to payment obligations accruing prior to termination and not included in a prior Quarterly Report and Year End Report), 10.4 (for a period of 6 months from the final required royalty report), 11.1.4 (in respect of the NGX License), 11.4.1 (with respect to alleged infringement that occurred prior to the later of the date of termination of this Agreement or the end
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-67-
of the Wind-down Period), with respect to Costs arising prior to termination, Astellas’ rights under Section 11.4.2 to treat such Costs as Third Party Royalties, 11.5 (with respect to Enforcement Actions initiated prior to termination), 12.8 (with respect to Astellas’ obligations to assign back to NGX any Domain Names assigned to Astellas), 12.11, 13, 15, 16.1 and 16.2 (with respect to actions occurring prior to the later of termination of this Agreement or the end of the Wind-down Period), 16.3 -16.5, 18, 19 and 20, and (b) those provisions of this Agreement to the extent set forth above in this Section 18 as surviving during the Wind-down Period or after termination of this Agreement. For clarity, in the event this Agreement is terminated with respect to one or more countries or Regions, but not the entire Territory, then the affected country or Region shall thereafter cease to be within the Territory for all purposes of this Agreement, but Sections 11.4 (with respect to alleged infringement occurring prior to the later of such termination or the end of the Wind-down Period in such country or Region), 11.5 (with respect to Enforcement Actions for infringement if such infringement occurred prior to such termination), shall survive such termination with respect to such affected country or Region.
18.9 Rights in Bankruptcy
All rights and licenses granted under or pursuant to this Agreement by NGX are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that Astellas, as licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against NGX under the U.S. Bankruptcy Code, which results in NGX’s rejection of this Agreement, Astellas shall be entitled to (a) retain ownership of all MAA Approvals and (b) a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in Astellas’ possession, shall be promptly delivered upon written request therefor by Astellas.
19.1 Disputes
In the event of any dispute between the Parties arising out of or in connection with this Agreement, either Party may, by written notice to the other, have such dispute referred to the [***] for attempted resolution by good faith negotiations within [***] after such notice is received, and, in such event, each Party shall cause its representative to meet and be available to attempt to resolve such issue. Notwithstanding the foregoing, neither Party shall be obligated to negotiate for more than [***]. If the Parties should resolve such dispute or claim, a memorandum setting forth their agreement will be prepared and signed by both Parties if requested by either Party.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-68-
19.2 Arbitration
Any dispute, controversy or claim with respect to the breach, interpretation or enforcement of this Agreement, including disputes relating to termination of this Agreement that cannot be resolved pursuant to Section 19.1 (or which this Agreement directs shall be resolved pursuant to this Section 19.2) shall be settled by binding arbitration in the manner described in this Section 19. The arbitration shall be conducted by the [***] under its rules of arbitration then in effect. Notwithstanding those rules, the following provisions shall apply to the arbitration hereunder:
19.2.1 Arbitrators. The arbitration shall be conducted by a single [***] arbitrator; provided that at the request of either Party, the arbitration shall be conducted by a panel of three (3) arbitrators, with one (1) [***] arbitrator chosen by each of Astellas and NGX and the third appointed by the other two (2) arbitrators. If the Parties are unable to agree upon a single arbitrator, or the other two arbitrators are unable to agree upon the third arbitrator in case of a panel of three (3), such single or third arbitrator (as the case may be) shall be appointed in accordance with the rules of [***]. In any event, the arbitrator or arbitrators selected in accordance with this Section 19.2.1 are referred to herein as the “Panel” and shall be comprised of arbitrators who are familiar with worldwide research and business development in the pharmaceutical industry, unless otherwise agreed.
19.2.2 Proceedings. Except as otherwise provided herein, the Parties and the arbitrators shall use their best efforts to complete the arbitration within [***] after the appointment of the Panel under Section 19.2.1 above, unless a Party can demonstrate to the Panel that the complexity of the issues or other reasons warrant the extension of one or more of the time tables. In such case, the Panel may extend such time table as reasonably required. The Panel shall, in rendering its decision, apply the substantive law of the [***], without regard to its conflicts of laws provisions, except that the interpretation and enforcement of this Section 19 shall be governed by the [***]. The proceeding shall take place in [***]. The arbitral proceedings and all pleadings, responses and evidence shall be in the English language. If so requested by the arbitrator(s), any evidence originally in a language other than English shall be submitted with an English translation accompanied by an original or true copy thereof. The decision and/or award rendered by the arbitrator(s) shall be written, final and non-appealable, and judgment on such decision and/or award may be entered in any court of competent jurisdiction. If the Panel determines that it is reasonable to do so, the fees of the Panel shall be paid by the losing Party, which Party shall be designated by the Panel. Otherwise, the fees of the Panel shall be split equally between the Parties. Each Party shall bear the costs of its own attorneys’ and experts’ fees; provided that the Panel may in its discretion award the prevailing Party all or part of the costs and expenses incurred by the prevailing Party in connection with the arbitration proceedings.
19.2.3 Interim Relief. Notwithstanding anything in this Section 19.2 to the contrary, Astellas and NGX shall each have the right to apply to any court of competent jurisdiction for a temporary restraining order, preliminary injunction or other similar interim or
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-69-
conservatory relief, as necessary, pending resolution under the above described arbitration procedures. Nothing in the preceding sentence shall be interpreted as limiting the powers of the arbitrators with respect to any dispute subject to arbitration under this Agreement. The Panel may award injunctive relief.
20. MISCELLANEOUS
20.1 Governing Law
This Agreement and any dispute arising from the performance or breach hereof shall be governed by and construed and enforced in accordance with, the laws of the [***], without reference to conflicts of laws principles. [***] shall not apply to this Agreement.
20.2 Force Majeure
Nonperformance of any Party, except for failure to pay amounts due hereunder, shall be excused to the extent that performance is rendered impossible by fire, strike (other than of their own workforce), earthquake, flood, acts of terrorism, governmental acts or orders or restrictions, failure of suppliers, or any other reason where failure to perform is beyond the reasonable control of the nonperforming Party. In such event Astellas or NGX, as the case may be, shall promptly notify the other Party of such inability and of the period for which such inability is anticipated to continue. Without limiting the foregoing, the Party subject to such inability shall use reasonable efforts to minimize the duration of the impact of any force majeure event on its performance hereunder.
20.3 No Implied Waivers; Rights Cumulative
No failure on the part of NGX or Astellas to exercise and no delay in exercising any right under this Agreement, or provided by statute or at law or in equity or otherwise, shall impair, prejudice or constitute a waiver of any such right, nor shall any partial exercise of any such right preclude any other or further exercise thereof or the exercise of any other right.
20.4 Independent Contractors
Nothing contained in this Agreement is intended implicitly, or is to be construed, to constitute NGX or Astellas as partners in the legal sense. No Party hereto shall have any express or implied right or authority to assume or create any obligations on behalf of or in the name of any other Party or to bind any other Party to any contract, agreement or undertaking with any Third Party. This Agreement does not create a partnership for USA federal income tax purposes (as defined in Section 761 of the USA Internal Revenue Code), for any USA state or local jurisdiction, or in any country other than the USA. Therefore there is no requirement to file Form 1065, USA Partnership Return of Income, any similar USA state or local income tax return, or any similar document with tax authorities in any country other than the USA.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-70-
20.5 Notices
Any notice required or permitted to be given hereunder shall be in writing and shall be delivered in person, by a nationally recognized overnight courier, or by facsimile (receipt confirmed), to the addresses given below or such other addresses as may be designated in writing by the Parties from time to time during the Term, and shall be deemed to have been given when received unless otherwise specified herein as when sent.
| Astellas: | Astellas Pharma Europe Limited | |
| [***] | ||
| [***] | ||
| NGX: | NeurogesX Inc. | |
| [***] | ||
| with a copy to: | [***] | |
20.6 Assignment
This Agreement shall not be assignable by either Party to any Third Party without the written consent of the other Party hereto; except either Party may assign this Agreement without the other Party’s consent to an entity that acquires substantially all of the business or assets of the assigning Party, in each case whether by merger, acquisition, or otherwise, provided the acquiring Party assumes this Agreement in writing or by operation of law. In addition, either Party shall have the right to assign this Agreement to an Affiliate upon notice to the non-assigning Party; provided that the assigning Party guarantees the performance of this Agreement by such Affiliate, and further provided that if the non-assigning Party reasonably believes such assignment could result in material adverse tax consequences to the non-assigning Party, such assignment shall not be made without the non assigning Party’s consent.
20.7 Modification
No amendment or modification of any provision of this Agreement shall be effective unless in writing signed by all Parties hereto. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance or any other matter not set forth in an agreement in writing and signed by all Parties.
20.8 Severability
If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions hereof shall remain in full force and effect in such jurisdiction and shall be liberally construed in order to carry out the intentions of the Parties hereto as nearly as may be possible. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-71-
20.9 Publicity Review
Neither Party shall originate any written publicity, news release or other announcement or statement relating to the announcement or terms of this Agreement (collectively, a “Written Disclosure”), without the prior review and written approval of the other Party, which approval shall not be unreasonably withheld, delayed or conditioned. Notwithstanding the foregoing, either Party may make any public Written Disclosure it believes in good faith based upon the advice of counsel is required by applicable law, rule or regulation or any listing or trading agreement concerning its or its Affiliates’ publicly traded securities; provided, however, that such Written Disclosure shall minimize to the extent possible the financial information disclosed, and that prior to making such Written Disclosure, the disclosing Party shall provide to the other Party a copy of the materials proposed to be disclosed and provide the receiving Party with an opportunity to promptly review the Written Disclosure. Notwithstanding the foregoing, the Parties have agreed upon a joint press release to announce the execution of this Agreement, together with a corresponding Question & Answer outline for use in responding to inquiries about the Agreement which is at Exhibit 20.9; thereafter, Astellas and NGX may each disclose the information contained in such press release and Question & Answer outline without the need for further approval by the other. In addition, notwithstanding anything to the contrary, each Party shall have the right to disclose the existence and terms of this Agreement as required by law; or as advisable or required in connection with any government or regulatory filings, including without limitation filings with the U.S. Security and Exchange Commission provided that NGX provides Astellas with a reasonable period to review the redactions of any confidential information prior to submission to the U.S. Security and Exchange Commission; or under reasonable obligations of confidentiality to its financial, legal and other advisors, auditors, potential or actual investors, acquisition partners, and others on a need to know basis.
20.10 Counterparts
This Agreement may be executed in two counterparts, each of which shall be deemed an original, and all of which together, shall constitute one and the same instrument.
20.11 Headings
Headings used herein are for convenience only and shall not in any way affect the construction of or be taken into consideration in interpreting this Agreement.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-72-
20.12 Export Laws
Notwithstanding anything to the contrary contained herein, all obligations of NGX and Astellas are subject to prior compliance with United States and foreign export regulations and such other United States and foreign laws and regulations as may be applicable, and to obtaining all necessary approvals required by the applicable agencies of the governments of the United States and foreign jurisdictions. NGX and Astellas shall cooperate with each other and shall provide assistance to the other as reasonably necessary to obtain any required approvals.
20.13 Entire Agreement
This Agreement, together with all the Exhibits thereto, constitute the entire agreement, both written or oral, with respect to the subject matter hereof, and supersede all prior or contemporaneous understandings or agreements, whether written or oral, between NGX and Astellas with respect to such subject matter.
[signatures on next page]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-73-
| NEUROGESX, INC. | ASTELLAS PHARMA EUROPE LIMITED | |||||||
| By: | /s/ Xxxxxxx X. XxXxxxx | By: | /s/ Xxxxx Xxxxxxx | |||||
| Name: | Xxxxxxx X. XxXxxxx | Name: | Xxxxx Xxxxxxx | |||||
| Title: | President and Chief Executive Officer | Title: | President and Chief Executive Officer | |||||
EXHIBIT 1.3
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 1.26
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 1.32
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
COMMERCIAL SUPPLY AND LICENSE AGREEMENT
This COMMERCIAL SUPPLY AND LICENSE AGREEMENT (this “Agreement”), is entered into as of day of January, 2007 (“Effective Date”) by and between
NeurogesX Inc., a California corporation with its principal place of business at San Xxxxxx Business Park, 000X Xxxxxxxxxx Xxxx, Xxx Xxxxxx, XX 00000, XXX (“NeurogesX”)
and
LTS Xxxxxxx Therapie-Systeme AG, a company existing under the laws of Germany and having its head office at Xxxxxxxxxxxxx 0, X-00000 Xxxxxxxxx, Xxxxxxx (“LTS”).
Each of NeurogesX and LTS shall be a “Party,” and together shall be referred to as the “Parties.”
ARTICLE 1
DEFINITIONS
As used herein, the following terms will have the meanings set forth below:
1.1 “Active Ingredient” means the active pharmaceutical ingredient capsaicin meeting the Specifications therefor. The specifications for the Active Ingredient as of the Effective Date are attached as Exhibit D hereto.
1.2 “Affiliates” of an entity means the other entities that control, are under common control or are controlled by the subject entity. For purposes of this definition, an entity shall be regarded as in control of another entity if it owns or controls fifty percent (50%) or more of the shares of the subject entity entitled to vote in the election of directors (or, in the case of an entity that is not a corporation, for the election of the corresponding managing authority).
1.3 “Batch Size” means the quantity of Patches produced from a [***] production run as established in accordance with Section 2.4(a).
1.4 “Blocking Patent” means an issued patent or similar intellectual property right (e.g., utility model) of a third party, not licensed to either Party, which would in the reasonable opinion of a Party’s counsel be infringed by (a) the manufacture or sale of the Patch by the Parties in accordance with the terms of this Agreement, or (b) any or all uses of the Patch, in a particular jurisdiction.
1.5 “Calendar Quarter” shall mean the calendar quarters of the year beginning first of January, April, July and October.
1.6 “Commercialization Patents” means patents anywhere in the world owned or Controlled by LTS covering or claiming any subject matter which is incorporated into, or utilized as part of the operation of, the Patches, any method of use of the Patches, or otherwise which is necessary for the sale, distribution, marketing and/or commercialization of the Patches supplied to NeurogesX hereunder, including but not limited to the patents listed in Exhibit H.
1.7 “Control” means, with respect to any patent or other intellectual property right, the possession at any time during the term of this Agreement of the right or power of a Party to grant licenses under such patent or other intellectual property right within the scope set forth herein without violating the terms of any of such Party’s agreements with non-Affiliate third parties.
1.8 “Current GMP,” or “cGMP” means then current Good Manufacturing Practices promulgated by the United States Food & Drug Administration (FDA) and its counterpart governmental agencies in the Territory outside the United States, in the form of laws, regulations or guidance documents, including those practices and standards set forth in Current Good Manufacturing Practice Regulations of the US Code of Federal Regulations Title 21 (21 CFR §§210 and 211) in relation to the production of pharmaceutical products and those practices and standards provided for (as amended from time to time) in the European Community Directive 91/356/EEC, as interpreted by the ICH Harmonized Tripartite Guideline, and any arrangements, additions or clarifications agreed from time to time between the Parties.
1.9 “Defect” or “Defective” when applied to Patches means the failure of such Patches to meet the warranty set forth in Section 8.2.
1.10 “Ex Works” shall have the meaning as set forth in the Incoterms 2000.
1.11 “Facility” means LTS’ cGMP manufacturing facility for the Patches located at [***]. In the event the Parties qualify a second source for the Patches pursuant to Section 4.5 below, then the term “Facility” shall also mean LTS’ cGMP manufacturing facility for such second source.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-79-
1.12 “Field” has the meaning as set forth in Section 6.3.
1.13 “Kit” means the commercial product form sold by NeurogesX, its Affiliates or Sublicensees of which the Patch(es) is a component.
1.14 “Launch Team” has the meaning as set forth in Section 4.1.
1.15 “Manufacturing Cost” shall mean the [***], in each case incurred by LTS during and allocable to the manufacture of Patches hereunder, and [***] incurred by LTS in implementing new GMP requirements requested by NeurogesX hereunder, which are not otherwise paid for or reimbursed by NeurogesX, all calculated in accordance with Generally Accepted Accounting Principles in the United States (“GAAP”) or its equivalent in Germany.
1.16 “Marketing Approval” means all approvals, registrations or authorizations of any federal, state or local regulatory agency, department, bureau or other governmental entity, necessary for the manufacturing, use, storage, import, transport and sale of Patches in a regulatory jurisdiction.
1.17 “Maximum Capacity” means NeurogesX’s forecast of the maximum quantities of Patches that NeurogesX may require be supplied from LTS by NeurogesX, its Affiliates or Sublicensees, per year of the term of this Agreement, as set forth on Exhibit G hereto. Such forecast may be amended from time to time in accordance with Section 3.4(d). It is understood that the Maximum Capacity is not a commitment by NeurogesX to purchase such amounts from LTS hereunder
1.18 “Net Sales” means the [***] sold by NeurogesX, its Affiliates or Sublicensees to a non-Affiliate third party in bona-fide, arms-length transactions, after deducting (to the extent actually incurred and to the extent not already deducted in the amount invoiced) (a) [***] determined in accordance with GAAP (as defined in Section 1.15), consistently applied.
If mutually agreed in writing by the Parties, some or all such items may be [***] and subsequently adjusted. The Parties agree that items (b) and (f) above may be [***] and subsequently adjusted as part of customary practice in accordance with GAAP. If a Kit is sold for consideration other than solely cash, the value of such other consideration attributable to the sale of the Kit shall be included in calculating Net Sales. In the event Kit is sold among NeurogesX and its Affiliates or Sublicensees for resale, Net Sales shall include the amounts invoiced by such entities to third parties on the resale, but not the amounts invoiced among such entities prior to the resale. Net Sales shall not include any sales of Kits as [***] NeurogesX, its Affiliates or Sublicensees.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-80-
1.19 “Patch” or “Patches” means the TTS containing the Active Ingredient developed by the Parties under the MOU and the Clinical Supply Agreement.
1.20 “Patch Regulatory Information” means any and all information in the possession of LTS relating to the Patches, as reasonably necessary for NeurogesX’s, its Affiliates’ and Sublicensees’ efforts to file for, obtain or maintain Marketing Approvals for the Patch in any regulatory jurisdiction, including without limitation (a) such information as is required for the Chemistry, Manufacturing and Controls (CMC) section of an Investigational New Drug application (IND) or New Drug Application (NDA) for the Patches, (b) all data regarding stability, storage conditions and shelf life of Patches, and (c) all data reasonably required to qualify a manufacturing facility for the Patches under Regulatory Requirements or demonstrate compliance with cGMP.
1.21 “Quality Assurance Agreement” means the then-current quality assurance procedures as mutually agreed upon by the Parties in writing. The template of Quality Assurance Agreement as of the Effective Date is attached as Exhibit E. The Parties agree that the commercial supply of Patches by LTS hereunder is subject to the Parties entering into a Quality Assurance Agreement, which the Parties shall use good faith efforts to conclude at least three (3) months prior to the first delivery of Patches by LTS hereunder. Thereafter, the Parties shall update the Quality Assurance Agreement from time to time to reflect best practice at such time.
1.22 “Raw Materials” means the Active Ingredient, excipients, components, labels, primary packaging material and shipping containers, necessary for the manufacturing, processing and primary packaging of the Patch as set forth in the master batch record for the Patch.
1.23 “Reasonable Commercial Efforts” of a Party means [***].
1.24 “Regulatory Requirements” means all laws, regulations and other legal requirements applicable to the manufacture of Patches or components thereof, including without limitation cGMP, FDA regulations, ICH guidelines, any applicable local laws and regulations in the place of manufacture, storage and handling, and any requirements set forth in any IND, NDA, applications for Marketing Approval and other regulatory filings or approvals for the Patches in the Territory.
1.25 “Specifications” means the specifications for the Patch (including the components thereof) as set forth in the Marketing Approval for the Patch, as amended, clarified or supplemented from time to time upon mutual agreement of the Parties in writing. The Specifications for the Patch as of the Effective Date is attached as Exhibit C. For clarity, in the event there are different Specifications set forth in the Marketing Approvals for different countries, the applicable Specifications shall be as set forth in the Marketing Approval for the country identified by NeurogesX or its designee on the purchase order for the Patch.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-81-
1.26 “Sublicensees” means a non-Affiliate third party to whom NeurogesX has granted (i) the right to market and sell Patches purchased from LTS under this Agreement, provided that such third party has [***] responsibility for and has the right to [***] the marketing and promotion of such Patches in its distribution territory and the right to [***] of such Patches for its own account, or (ii) the right to make (to the extent NeurogesX has the right to do so pursuant to Section 6.4 below) and sell a Patch, with respect to Patches that are manufactured and then sold by such third party pursuant to the license set forth in Section 6.4 below. For the [***], wholesalers, distributors or specialty distributors, but shall include [***] of NeurogesX for the Patches
1.27 “Territory” means the [***]. In addition, the Territory may be extended by NeurogesX to include additional countries and/or regulatory jurisdictions in accordance with Section 3.4(c) below.
1.28 “Transfer Price” has the meaning as set forth in Section 5.1 below.
1.29 “TTS” means transdermal therapeutic patches, including but not limited to liquid reservoir patches, microreservoir patches, monolithic layer patches, or other patch products for delivering drugs topically.
1.30 “VR1-Ligand” means any compound that bind to the vanilloid receptor subtype 1, as further described in Exhibit B. The Parties may amend Exhibit B from time to time upon mutual written agreement.
ARTICLE 2
SUPPLY OF PATCHES
2.1 Supply of NeurogesX’s Requirements.
(a) Commercial Requirements. Subject to the terms and conditions of this Agreement, LTS shall manufacture and supply NeurogesX with all of NeurogesX’s commercial requirements for Patches worldwide up to the Maximum Capacity for each year, and shall use [***] to manufacture and supply NeurogesX with any of NeurogesX’s commercial requirements exceeding the Maximum Capacity for each year. Subject to the terms and conditions of this Agreement, NeurogesX shall purchase all of its commercial requirements for Patches worldwide from LTS.
(b) Sublicensees. To the extent NeurogesX authorizes a Sublicensee to purchase directly from LTS, LTS agrees to supply the commercial requirements for Patches of such Sublicensee subject to the Maximum Capacity in accordance with this Agreement, in the manner authorized by NeurogesX.
(c) Clinical Supplies. NeurogesX and its designees may continue to purchase units of Patches for clinical trial purposes under the Clinical Supply Agreement, provided that such units shall be designated as clinical supplies at the time of order. Unless otherwise designated, all
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-82-
orders for Patches submitted by NeurogesX and its designees after the First Forecast provided under Section 2.2 below shall be deemed orders for Patches under this Agreement. To the extent LTS becomes aware that any Raw Materials or works-in-progress procured or made under this Agreement may be used to reduce the cost or price of units of Patches ordered under the Clinical Supply Agreement, LTS shall notify NeurogesX and the Parties shall negotiate in good faith to so use such Raw Materials and works-in-progress and correspondingly reduce the price to NeurogesX of such units under the Clinical Supply Agreement. It is understood that this Section 2.1(c) is not intended to prevent NeurogesX from using any supplies of Patches in its inventory that may have been purchased under this Agreement, for clinical trial purposes, provided however, that NeurogesX shall act in accordance with all applicable laws and regulations.
2.2 First Forecast NeurogesX will provide LTS with a written forecast of the number of Patches which will be required to be delivered during the first year following the first projected delivery hereunder (the “First Forecast”), at least [***] prior to the beginning of the Calendar Quarter in which the first delivery of Patches by LTS is projected to occur. This First Forecast shall contain an estimate of the number of Patches required to be delivered, on a monthly basis, for such Calendar Quarter and the [***] three (3) Calendar Quarters. It is understood that only the first Calendar Quarter of the First Forecast shall be binding on the Parties, and the subsequent Calendar Quarters in the First Forecast shall be non-binding for both Parties. NeurogesX agrees that the first Calendar Quarter in the first Rolling Forecast (as defined below) shall specify the same quantities as specified in the first Calendar Quarter in the First Forecast.
2.3 Rolling Forecast. Each subsequent written forecast (each a “Rolling Forecast”) shall update the prior forecast, and will be provided by NeurogesX [***] prior to the beginning of each Calendar Quarter in which Patches are required to be delivered. Each Rolling Forecast shall include an estimate of requirements on a monthly basis for such Calendar Quarter and the [***] three (3) Calendar Quarters, so that estimates for a rolling one (1) year period are always provided. The Parties agree that only the first Calendar Quarter in each Rolling Forecast shall be binding on the Parties, as set forth in Section 2.4 below (quantities set forth in such first Calendar Quarter, the “Binding Quantities”). The quantities forecasted for subsequent Calendar Quarters in such forecasts shall be non-binding for both Parties
2.4 Purchase Orders.
(a) Orders. NeurogesX shall place orders for quantities of Patches, on a [***] basis, [***] days before the month in which delivery of such Patches is requested (the “Delivery Month”). For example, [***]. Each order shall specify the requested delivery date(s) within the Delivery Month, the shipping destination(s) and any shipping instructions. Unless otherwise agreed, the quantities specified in each order shall be a [***] of the Batch Size. NeurogesX shall ensure that such orders, aggregated on a Calendar Quarter basis, specify a quantity of Patches to be delivered in such Calendar Quarter that is at least [***] of the Binding Quantities for such Calendar Quarter. The Parties agree to use good faith efforts to establish the Batch Size by mutual written agreement after manufacture of the first [***] batches of Product based on actual batch sizes for production hereunder; provided unfit such time the Batch Size for purposes of this Agreement shall be deemed to be the Theoretical Batch Size set forth in Exhibit A.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-83-
(b) Acceptance. LTS shall accept and fill all orders from NeurogesX for quantities of Product, subject to the terms and conditions of this Agreement, provided that LTS shall not be obligated to accept orders to the extent the quantity for delivery in a particular Calendar Quarter exceeds [***] of the Binding Quantities for such Calendar Quarter. In addition, the exact delivery dates within the Delivery Month shall be subject to acceptance by LTS, which acceptance shall not be unreasonably withheld. In the event LTS does not accept a delivery date requested by NeurogesX, the Parties shall mutually agree to a delivery date which is as close as reasonable to the delivery date requested by NeurogesX (but not more than [***] weeks before or after the requested delivery date). Any objection to the delivery date or the quantities or other aspects of an order must be submitted by LTS within [***] days of receipt of the order from NeurogesX, or such order shall be deemed fully accepted. After acceptance of an order, such order shall be deemed a “Firm Order,” and the accepted or agreed delivery date, the “Scheduled Delivery Date”.
(c) Excess Quantities. LTS shall use [***] to accept and fill orders for any quantities of Patches for delivery in a Calendar Quarter exceeding [***] of the Binding Quantities for such Calendar Quarter, on the requested delivery dates (“Excess Orders”). In the event LTS is unable to accept or fill any Excess Orders despite using [***], LTS shall notify NeurogesX as soon as practicable, and such inability shall not be deemed a material breach of this Agreement entitling NeurogesX to terminate this Agreement nor a Failure Event under Section 6.4 below. LTS shall provide NeurogesX a Scheduled Delivery Date for Excess Orders within [***] days after receiving an Excess Order.
2.5 Packaging. Patches shall be shipped packaged in containers in accordance with the Quality Assurance Agreement, Exhibit E or as otherwise agreed by the Parties hereto in writing. Each such container shall be individually labeled with a description of its contents, including the manufacturer lot number, quantity of Patches, and the date of manufacture.
2.6 Delivery. Subject to the terms and conditions of this Agreement, including Section 2.8 below, LTS shall deliver quantities of Patches [***] on the Scheduled Delivery Dates. All Patches for delivery will be placed at the disposal of NeurogesX or its designee at the relevant LTS manufacturing Facility. Title and risk of loss, delay or damage to the Patches in transit shall pass to NeurogesX [***]. Unless NeurogesX requests otherwise, all Patches shall be packed for shipment and storage fit for the respective way of transportation and in compliance with any requirements set forth in the Quality Assurance Agreement. In the event NeurogesX has any special freight packaging or shipping instructions, it shall notify LTS and LTS will use [***] to comply with any such instructions. All costs associated with such instructions shall be borne by [***]. [***] shall bear all costs of freight, shipping and insurance as well as indirect taxes, including import, customs, excise and sales taxes but not income taxes related to the sale and purchase of Patches hereunder.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-84-
2.7 Refusal to Supply. LTS, after good faith consultation with NeurogesX for at least [***] days, may refuse to supply Patches in a particular country, if LTS can reasonably demonstrate by evidence prepared or corroborated by an expert (recognized in the industry and having substantial experience in matters of public safety and toxicollogy) that the proper sale, distribution and use of the Patch hereunder is likely to unreasonably endanger the public health.
2.8 Invoicing; Payment. LTS shall submit an invoice to NeurogesX upon shipment of the Patches ordered by NeurogesX hereunder. All invoices shall be sent to the address specified in the purchase order, and each such invoice shall state the aggregate and unit Transfer Price for Patches in a given shipment, plus any insurance, taxes or other costs incident to the purchase or shipment initially paid by LTS but to be borne by NeurogesX hereunder.
2.9 Late delivery. For purpose of this Agreement, delivery within [***] business days before or [***] business days after the Scheduled Delivery Date shall be deemed meeting such delivery date; provided that it is understood LTS is only obligated to employ Reasonable Commercial Efforts to meet the requested delivery dates for Excess Orders. In the event that LTS does not deliver the ordered Patches within such a seven (7) business day window (i.e. [***] days before or [***] days after), then NeurogesX shall, as its sole remedy for LTS being late with its delivery of Patches within the time periods set forth in clause (a) and (b) below, have the right to reduce its payment of the Transfer Price for such Patches by: (a) [***] if NeurogesX receives the Patches between [***] days and [***] days after the Scheduled Delivery Date; or (b) [***] if NeurogesX receives the Patches later than [***] days after the Scheduled Delivery Date. In the event NeurogesX does not receive the Patches within [***] days after the Scheduled Delivery Date, then in addition to reduction of payment of the Transfer Price set forth in clause (b) above, NeurogesX shall be entitled to deem such delivery as having been ordered but never made, for purposes of determining a Failure to Supply under Section 6.4(a) below. For the purpose of this Section 2.9, a “business day” shall exclude a weekend day or a public holiday in Germany or regional legal holiday at the Facility where the Patch is manufactured and those days where LTS is closed down in the regular course of business, e.g. between Christmas and New Year.
2.10 Correct Quantities. In the event LTS delivers at least [***] of the order and not more than [***], LTS shall be deemed to have delivered the ordered quantities of Patches. It is understood that NeurogesX shall however only pay for the Patches it actually received, but not more than [***] of the applicable order, and only the actual number of Patches ordered and received shall be used to determine whether or not LTS has supplied [***] as the case may be, of the Binding Quantities under Section 6.4(a)(i)(1) below.
2.11 Letter of Credit. In the event NeurogesX has not paid the Transfer Price for [***] shipment of Patches, which payment is not disputed by NeurogesX and is overdue for [***] or more days, and such failure to pay adds to a total amount of [***] or more, then upon LTS’ reasonable request, NeurogesX shall cause to be delivered to LTS a confirmed letter of credit from an international bank (reasonably acceptable to LTS) at that point in time to secure its obligations hereunder to pay the Transfer Price for any subsequent order of Patches, on terms and conditions reasonable and customary for such arrangements. NeurogesX shall maintain such letter of credit in effect until it has paid such outstanding overdue amount for the Transfer Prices.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-85-
2.12 Cancellation. Subject to the reimbursement set forth in Sections 2.14(a) and 2.14(b) below, it is understood and agreed that NeurogesX may cancel an outstanding order by so notifying LTS in writing, provided LTS receives such notification at least [***] days prior to the Scheduled Delivery Date, and provided further that no more than one Firm Order shall be cancelled by NeurogesX during a consecutive [***] month period unless otherwise mutually agreed.
2.13 Artwork and Text. The artwork and text required to be printed on the primary Patch packaging and reasonably approved by LTS as to technical feasibility shall be furnished to LTS by NeurogesX or its licensees as soon as reasonably practicable within [***] months prior to the first delivery of Patches hereunder, it being understood that LTS shall not be liable (including but not limited to the remedies foreseen pursuant to Section 6.4 hereof) for any complications or delivery failures resulting from delays in the delivery of artwork and text by NeurogesX (or its designee).
Any change to the artwork and text of the primary Patch packaging reasonably requested by NeurogesX or its Sublicensees or required by any regulatory authority shall be communicated by NeurogesX to LTS in writing together with suitable samples of the revised artwork or text no later than [***] months prior to the first delivery of Patches with such new primary Patch packaging. NeurogesX shall reimburse LTS for any packaging materials that can no longer be used as a result of any such change up to a maximum of [***] months inventory of such materials. Provided that NeurogesX shall communicate such changes in the artwork and LTS has given its reasonable consent to such changes with a lead time of not less than [***] days, such changes shall not affect or modify the lead times for the supplies pursuant to Section 2.4.
2.14 Raw Materials. All Raw Materials, other than the Active Ingredient, to be used in the manufacture of the Patch shall be purchased by [***] at its own expense. LTS shall be responsible for testing and releasing all such Raw Materials in accordance with the Specifications, the Quality Assurance Agreement and all Regulatory Requirements.
In the event [***] orders or purchases appropriate quantities of Raw Materials in order to be able to supply the Patches as forecasted in the most current [***] month period in the Rolling Forecast, as defined in Section 2.3, and has paid or has incurred a non-cancelable commitment to pay for such Raw Materials, and (A) [***] (i) cancels such orders pursuant to Section 2.12; (ii) changes the forecasted quantities subject to the terms of this Agreement; or (iii) does not place orders in the amounts forecasted in such [***] month forecast; or (B) the Specifications or the artwork has changed after the Raw Materials have been purchased or ordered, then:
(a) such Raw Materials and any works-in-progress may, in a manner to be reasonably agreed between LTS and NeurogesX, be kept in storage by LTS for future production of the Patches hereunder, provided that NeurogesX shall reimburse LTS for all costs and expenses associated with such storage; or
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-86-
(b) in the event such storage is not practicable or such Raw Materials become obsolete or unusable due to one or more of the events specified under (i) – (iv) above or the Parties do not agree on the manner or compensation for such storage, NeurogesX shall reimburse LTS for all documented out-of-pocket costs and expenses incurred by LTS for such Raw Materials (other than the Active Ingredient) and those reasonable costs and expenses incurred by LTS to produce the works-in-progress, which in each case, cannot be used by LTS for manufacturing Patches and cannot be used by LTS in the manufacture of products for its other clients. NeurogesX may then, at its option upon prior written notice to LTS, take ownership of such Raw Materials and works-in-progress, it being understood that any costs and expenses for transport shall be borne by [***]. If NeurogesX does not exercise such option, LTS will destroy such Raw Materials and works-in-progress, it being understood that NeurogesX shall pay any reasonable costs and expenses for destroying such Raw Materials and works-in- progress.
2.15 Active Ingredient. Notwithstanding anything herein to the contrary, LTS’ obligations to supply Patches hereunder is subject to and conditioned upon NeurogesX making available to LTS appropriate quantities of the Active Ingredient. Accordingly, NeurogesX shall provide LTS with the amounts of the Active Ingredient that LTS reasonably (on the basis of the then current First Forecast or Rolling Forecast) notifies NeurogesX (with a lead time of at least [***] months) that LTS will require to fulfill its supply obligations hereunder. LTS shall be responsible for conducting only identity testing of the Active Ingredient. LTS shall use all quantities of Active Ingredient provided hereunder for the [***] purpose of producing Patches ordered by NeurogesX hereunder.
(a) Wastage. LTS agrees to minimize the wastage of the Active Ingredient involved in the production of Patch hereunder and to maximize Patch yields, and will regularly report to and consult with NeurogesX regarding its Active Ingredient / Patch yields, and its efforts to improve such yields. Without limiting the foregoing, promptly after [***] months from the [***] month in which Patches are delivered hereunder, or after manufacture of at least * full batches of Patches, [***], the Parties agree to establish a minimum yield level for Active Ingredient / Patch based on the yield levels during such [***] month period, or after manufacture of [***] full batches of Patches, which ever comes later, (the “Minimum Yield Level”).
(b) Safety Stock. As requested by NeurogesX from time to time and at NeurogesX’s cost (in accordance with a budget agreed upon by the Parties in advance), LTS shall procure and maintain an additional quantity of Active Ingredient and other Raw Materials as a safety stock for its production of Patches hereunder (“Safety Stock”) subject to LTS’ available storage capacity. LTS shall [***] report to NeurogesX as requested, regarding the expenses incurred in procuring and maintaining the Safety Stock (documented and itemized in accordance with the budget), and the levels of inventory and use of such safety stock. LTS agrees to manage such levels of inventory and use of the Safety Stock in accordance with the reasonable requests of NeurogesX, and in any event LTS shall use the Safety Stock for the sole purpose of producing Patches for NeurogesX hereunder and shall use [***] to minimize expenses and wastage. When any unit of Safety Stock purchased at the expense of NeurogesX (“NeurogesX Stock”) is used in the production of Patches hereunder, NeurogesX shall receive a credit, in the amount of the price paid by NeurogesX for such unit of Safety Stock excluding the Active Ingredient and excluding any storage costs incurred by LTS with respect thereto, which may be applied against (i) the Transfer Price for the Patch or (ii) at NeurogesX’s option, the costs of procuring replacement NeurogesX Stock.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-87-
(c) Consignment. NeurogesX shall retain all right, title and interest in the Active Ingredients provided hereunder and the NeurogesX Stock. The Active Ingredients and NeurogesX Stock and shall be (i) clearly marked as property of NeurogesX, (ii) stored under conditions specified in the Specifications at the Facility, (iii) kept free of any liens and encumbrances, and (iv) subject to inspection by NeurogesX at any time during LTS’ business hours upon reasonable notice to LTS. LTS shall maintain an accurate inventory of the Active Ingredients and the NeurogesX Stock. NeurogesX shall keep the Active Ingredient and NeurogesX Safety Stock adequately insured during storage at LTS. Upon termination or expiration of this Agreement (or an earlier request of NeurogesX), LTS will promptly deliver to NeurogesX or NeurogesX’s designee all quantities of Active Ingredient and NeurogesX Stock [***] LTS Facility.
2.16 Conflicting Terms and Conditions. The supply of Patches by LTS to NeurogesX and of Active Ingredient by NeurogesX to LTS shall be solely in accordance with the terms and conditions of this Agreement. ANY TERMS OR CONDITIONS OF ANY PURCHASE ORDER OR ACKNOWLEDGMENT GIVEN OR RECEIVED WHICH ARE ADDITIONAL TO OR INCONSISTENT WITH THIS AGREEMENT SHALL HAVE NO EFFECT AND SUCH TERMS AND CONDITIONS ARE HEREBY EXCLUDED AND REJECTED BY EACH PARTY.
2.17 Delegation to LTS Affiliates. LTS shall have the right to delegate all or any portion of its obligations under this Agreement to its Affiliates, provided that such delegation does not affect any Marketing Approvals filed or obtained by NeurogesX, its Affiliates or Sublicensees or in case any Marketing Approval would be affected, NeurogesX has given its prior written consent. Any such Affiliates shall be bound by all of the terms and conditions set forth herein as if named as a party hereto, and LTS shall remain responsible for the performance of such Affiliates under this Agreement.
ARTICLE 3
QUALITY; REGULATORY ISSUES
3.1 Quality Assurances. LTS shall comply with each requirement set forth in the Quality Assurance Agreement with respect to manufacturing and associated support functions including storage, handling and delivery of Patches to NeurogesX and its designees hereunder; provided, however, the provisions concerning LTS responsibility and liability as set forth in this Agreement shall [***] any provisions that in the event of a conflict between the terms in the Quality Assurance Agreement or any amendment thereof and this Agreement, the terms of this Agreement shall [***].
3.2 Patch Regulatory Information. LTS shall provide NeurogesX with Patch Regulatory Information upon NeurogesX’s request. To the extent that portions of the Patch Regulatory Information constitute specific LTS’ manufacturing or patch trade secrets not previously disclosed to NeurogesX and involving confidential information of LTS’ other customers, LTS may notify NeurogesX and provide a general description thereof, and if
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-88-
requested by NeurogesX, LTS may, instead of providing such parts of the Patch Regulatory Information to NeurogesX, elect to directly provide such parts of the Patch Regulatory Information to the applicable regulatory authority in support of the regulatory filings of NeurogesX and its designees (and if so elected, will diligently do so), and will grant NeurogesX and its designees rights to reference such filings made by LTS. Except as set forth in this Section 3.2 or otherwise required by law, LTS shall not correspond directly with the FDA or other health regulatory agencies relating to the process of obtaining Marketing Approvals or any obtained Marketing Approvals for the Patch, without NeurogesX’s permission. It is understood and agreed that the license of Section 7.1 of the Clinical Supply Agreement shall apply with respect to Patch Regulatory Information disclosed under this Agreement, and that NeurogesX may disclose any and all Patch Regulatory Information to regulatory authorities in connection with obtaining and maintaining Marketing Approval for the Patches worldwide.
3.3 Support of Registration. If NeurogesX reasonably requests LTS to undertake such activities LTS shall use its [***] to support NeurogesX’s efforts to obtain and maintain Marketing Approvals for the Patch, provided, however, that NeurogesX shall pay for all labor costs of such activities at the then current man-hour rates of LTS and reimburse LTS for third party costs incurred with respect thereto, in each case as requested by NeurogesX.
3.4 Changes.
(a) Specifications. Neither Party shall make any changes to the Specifications except as mutually agreed. If such changes result in a material change in the Manufacturing Cost, the Parties shall agree on an appropriate adjustment to the Transfer Price of the Product hereunder covering at least the increased costs]. If such modifications result in a delay in delivery, the Parties shall negotiate a reasonable extension of the affected lead times. Notwithstanding the foregoing, the Parties agree that they shall not withhold approval for any changes to the Specifications (i) requested by NeurogesX which are necessary to make them comply with Regulatory Requirements in any country in which the Patches are being commercialized by NeurogesX, its Affiliates and/or Sublicensees or manufactured by LTS, or to address concerns regarding the toxicity, safety and/or efficacy of the Patch, provided that NeurogesX agrees to pay LTS for the reasonable, documented, incremental labor and material costs incurred in connection with implementing such change, to the extent such costs are not covered by the mechanism for adjusting the Transfer Price in Section 5.6, or (ii) requested by LTS as a result of events beyond its reasonable control (e.g. bankruptcy of its supplier), and such changes to Specifications as are necessary so that LTS can meet the Marketing Approval.
(b) Manufacturing Process. Unless otherwise required by applicable laws or regulations, LTS shall not, without the prior written consent of NeurogesX, change any aspect of manufacture, including the facilities, equipment, processes, vendors, sub-contractors or record keeping procedures, in any manner (i) which to LTS’ [***] knowledge has the potential to [***] affect the Patch, including any changes which may impact its safety or effectiveness, or (ii) which would delay or otherwise impact any filings for Marketing Approval for the Patch or would require an affirmative approval of the FDA, or any other health regulatory authority in the Territory in which the Patch is being marketed, prior to its implementation. In the event NeurogesX requests a change or adjustment of any aspect of manufacture, including
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-89-
the facilities, equipment, processes, vendors, sub-contractors or record keeping procedures and LTS agrees to implement such change or adjustment, or if such change or adjustment is necessary, in case the annual requirements of the Patch can no longer be supplied according the current manufacturing process (including but not limited to facilities and equipment), however subject to the prior written consent of NeurogesX as foreseen in the preceding sentence, NeurogesX shall pay LTS for the reasonable, documented, incremental labor and material costs incurred in connection with implementing such change, to the extent such costs are not covered by the mechanism for adjusting the Transfer Price in Section 5.6.
(c) Territory. NeurogesX shall have the right to elect from time to time to commercialize Patches in countries or regulatory jurisdictions outside the Territory upon written notice to LTS, with a reasonable lead time. In the event NeurogesX’s decision to add any country or regulatory jurisdiction to the Territory will cause LTS to (i) incur additional costs, as a result of specific Regulatory Requirements of such country or regulatory jurisdiction (“Territory-specific Costs”), and/or (ii) impose requirements beyond LTS’ standard manufacturing practice, LTS shall in due time notify and discuss with NeurogesX such costs and such additional requirements. Upon such mutual agreement of the Territory-specific Costs and ways of accommodating such additional requirements, NeurogesX shall have the option to either (1) modify or limit such addition to the Territory, or (2) include such countries or regulatory jurisdiction within the Territory and reimburse LTS for the Territory-specific Costs. It is understood that LTS shall use [***] to mitigate any Territory-specific Costs and accommodate such reasonable requirements.
(d) Maximum Capacity. NeurogesX shall update the Maximum Capacity - if necessary - to cover the whole term of the Agreement and shall have the right to elect from time to time to [***] the Maximum Capacity under this Agreement, upon written notice to LTS with a reasonable lead time, provided, however, that any increase of the Maximum Capacity exceeding [***] Patches per year is subject to LTS prior written agreement, not to be unreasonably withheld.
3.5 Facility. All Patches supplied by LTS hereunder shall be manufactured at the Facility. The Facility (and any other facility that is involved in the manufacture of Patches by or under authority of LTS) is and shall continue to be in compliance with cGMP and Regulatory Requirements and shall be available for governmental inspection if any competent governmental authority or governmental organization so requests. LTS shall promptly provide to NeurogesX any cGMP certificate issued by any such competent governmental authority or organization.
3.6 Regulatory Issues. LTS will notify NeurogesX in due time (and no later than [***] days after LTS obtaining notice thereof) of any inspections, written notice of findings and/or actions by regulatory agencies or other enforcement bodies of LTS facilities and/or processes which will directly affect the Patch or the manufacture thereof. Where reasonably possible, LTS shall afford NeurogesX the opportunity to be present at any such inspections. LTS shall consult with NeurogesX in responding to any such inspections, written notice of findings and/or actions that directly affect the manufacture of the Patch, including by providing NeurogesX copies of any responses thereto for NeurogesX’s review and comment in advance of their submission to the regulatory agency, and using [***] to incorporate therein NeurogesX’s comments as appropriate.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-90-
3.7 Audits. NeurogesX, its Affiliates and its Sublicensees shall have the right to audit LTS, its Affiliates and any sub-contractors and vendors of LTS with respect to the Patches, as shall other third parties designated by NeurogesX and approved by LTS in written form (which approval shall not be unreasonably withheld) for compliance with this Agreement, at [***]; provided that to the extent that a particular vendor does not allow audits by LTS customers, LTS shall use [***] to (i) receive the consent of its vendor to have such audit conducted by a third party or (ii) to the extent it has the right to do so, perform such audit on NeurogesX’s behalf at NeurogesX’s expense. To the extent LTS is required under the “Arzneimittelgesetz” (German pharmaceutical law) to audit the other sub-contractors or vendors of NeurogesX with respect to the Patches LTS shall have the right to do so at [***], subject to audit procedures to be reasonably agreed between the Parties and the particular sub-contractor or vendor.
3.8 Reporting Safety and Toxicity Problems. Each Party shall promptly advise the other Party of any safety or toxicity problems of which it becomes aware regarding the Patches or Raw Materials used in the manufacture of the Patches.
3.9 Recalls. To the extent (i) any governmental or regulatory authority issues a request, directive or order that the Patches be recalled or withdrawn, or (ii) a court of competent jurisdiction orders a recall or withdrawal of Patches, or (iii) either Party determines after consultation with the other Party, that the Patches should be recalled or withdrawn due to safety issues, the Parties shall recall or withdraw the Patches as set forth in this Section 3.9.
(a) Control of Recall Activities. As between the Parties, NeurogesX shall control and coordinate all activities, including making all contacts with regulatory authorities, it deems necessary in connection with such recall or withdrawal.
(b) Initial Allocation of Recall Expenses. The Parties shall initially allocate all expenses (including out-of-pocket expenses) [***] related to the execution of any recall or withdrawal of the Patches (“Logistic Costs”) [***] between the Parties, provided that in the case of Section 3.9(iii) above, if the Parties do not mutually agree on having the Patches recalled or withdrawn, then the Party requesting the recall or withdrawal [***], in each case subject to the final allocation between the Parties as set forth in Section 3.9(c).
(c) Final Allocation of Recall Expenses. All Logistic Costs shall be borne by the Party which is [***]. The responsibility of a Party shall be determined in accordance with the principles of Article 9. For example, [***]. Notwithstanding the foregoing, LTS’ total liability under this Section 3.9(c) for Logistic Costs shall be limited to an amount equal to [***] plus any additional amounts covered by LTS’ insurance coverage, except in the case of LTS’ gross negligence or willful misconduct. In case of LTS’ [***], LTS’ liability for Logistic Costs shall not be so limited.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-91-
3.10 Stability Testing. LTS shall use [***] to perform the stability testing of the Patches as set forth in Exhibit I; provided that NeurogesX may terminate the activities under this Section 3.10 at any time by providing at least [***] days’ prior written notice referencing this Section 3.10. NeurogesX shall pay for those activities initiated prior to any termination pursuant to this Section 3.10, the compensation as set forth in Exhibit I with respect thereto.
ARTICLE 4
COORDINATION
4.1 Launch Team. Shortly before NeurogesX provides the First Forecast under Section 2.2 above, the Parties will form a team (the “Launch Team”) to facilitate communications and decision making by the Parties regarding the activities to be carried out pursuant to this Agreement during the initial phase of commercial supply starting [***] months prior to first manufacturing of Patches and ending one year after launch of the Patch. The Launch Team’s responsibilities shall include, but not be limited to: overseeing the ramp up to commercial production and addressing manufacturing or quality problems.
4.2 Composition. The Launch Team shall consist of [***] representatives from each Party, one of which shall be a designated project leader for such Party (the “Project Leader”). Each Party shall provide the other Party written notice of, and contact information for, its representatives on the Launch Team. In the event that a member of the Launch Team resigns, or a Party desires to replace one of its members, such Party will provide the other Party written notice of such event and the name of the member’s replacement
4.3 Meetings. The Launch Team will conduct formal meetings via teleconference or in person on at least a quarterly basis until [***] after the first Calendar Quarter in which Patches were delivered hereunder, and thereafter from time to time as mutually agreed between the Parties and will issue minutes of those meetings within [***] days to the management of each respective company.
4.4 Decisions. All decisions made by the Launch Team shall be based on the [***] agreement of the Project Leaders. Each Project Leader shall have the authority to fully represent the position of his company and the decision of his company management, except with respect to any aspects of [***]. In case the Project Leaders do not agree, the [***] of NeurogesX and LTS or their designees shall try to resolve the dispute. Notwithstanding the foregoing, no action, inaction, decision or inability to reach a decision by or of the Launch Team shall vary the terms and conditions of this Agreement, or the rights and obligations of the Parties hereunder.
4.5 Qualifying a Second Site. LTS shall use Reasonable Commercial Efforts to implement NeurogesX’s requests, if any, to qualify LTS’ GMP facility in [***] as a second site for the manufacture of Patches under this Agreement. It is understood that NeurogesX shall pay for the cost of such qualification; provided that the total cost, along with any other relevant terms and conditions such as a project plan, timeline, budget, etc., shall be mutually agreed to in writing by the Parties in advance. Upon the request of NeurogesX, the Parties shall negotiate in good faith the total cost and any other relevant terms and conditions based on the proposal provided by LTS in its letter to NeurogesX dated [***].
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-92-
ARTICLE 5
PAYMENT
5.1 Transfer Price. NeurogesX shall pay LTS the price set forth in Exhibit A (the “Transfer Price”) for each Patch ordered by NeurogesX and delivered by LTS hereunder. All payments hereunder shall be made in [***], by direct bank transfer to an account as designated in LTS’ invoice. Such payments shall be made by NeurogesX within [***] days after the later of (a) receipt of an invoice, or (b) the end of the Acceptance Period (as defined in Section 8.2(a) below) for the respective Patches. In addition, in the event NeurogesX has rejected, in accordance with Section 8.2(a), the Patches prior to the time payment of the Transfer Price is due and LTS is required to deliver replacement Patches in accordance with Section 8.2(b), then the Transfer Price shall not be due until replacements therefore have been received in accordance with Section 8.2(b). In the event LTS disagrees that Patches rejected were Defective, then NeurogesX shall pay [***] of the Transfer Price until a laboratory has determined whether such Patches were Defective in accordance with Section 8.2(c). In case the laboratory determines that Patches were Defective, then such payment shall be [***]. In case the laboratory determines that Patches were not Defective, NeurogesX shall immediately pay the remaining [***] of the Transfer Price. NeurogesX shall pay interest at an annual rate of [***] above [***] for any payments of Transfer Price which LTS did not receive on the date payment is due under this Section 5.1.
5.2 Royalties.
(a) As further consideration for the supply of Patches and the other rights and licenses granted hereunder, NeurogesX shall pay LTS royalties at the rate of [***] on the Net Sales of Kits sold by NeurogesX, its Affiliates or Sublicensees, containing Patch(es) purchased from LTS under this Agreement.
(b) Notwithstanding Section 5.2(a) above, NeurogesX has the option to pay LTS royalties at the rate of [***] rather than [***] on the Net Sales of Kits sold by NeurogesX, its Affiliates or Sublicensees, containing Patch(es) purchased from LTS under this Agreement, in certain jurisdictions selected by NeurogesX as follows. NeurogesX shall exercise such option with respect to a jurisdiction within [***] days after the first commercial sale of a Kit in such jurisdiction. In the event NeurogesX exercises such option with respect to a jurisdiction, NeurogesX shall be entitled to a [***] share of recoveries from an Infringement as set forth in Sections 7.3(a)(ii) and 7.3(b)(ii) below.
(c) For the avoidance of doubt, if NeurogesX elects to exercise the option set forth in Section 5.2 (b), the royalty set forth in Section 5.2 (b) shall not be additional to those in Section 5.2 (a), NeurogesX shall in this case only pay the royalty set forth in Section 5.2 (b) with respect to the selected jurisdiction.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-93-
5.3 Reporting and Records.
(a) Reporting. The royalties set forth in Section 5.2 and Section 6.4 shall be computed for each applicable [***] ([***]), and shall be due and payable within [***] days after such [***] period. For purposes of computing royalties, each Kit will be considered sold when paid for. NeurogesX shall report to LTS, within [***] days after the end of each such [***] period during the term of this Agreement, and thereafter until all dispositions made pursuant to this Agreement have been accounted for, any and all royalties under Section 5.2 and Section 6.4 below accruing during such six months period.
(b) Records. NeurogesX shall keep records in sufficient detail to enable the royalties payable under Section 5.2 and Section 6.4 below to be determined. The records of NeurogesX shall be available during reasonable business hours for inspection not more than [***] period, by an independent Certified Public Accountant reasonably acceptable to NeurogesX acting in confidence and retained by LTS at LTS’ expense, for the purpose of verifying royalty reports and payments due hereunder. In the event of an underpayment by NeurogesX of more than [***] of the total is found, NeurogesX shall bear the reasonable costs connected with the verification.
5.4 Marketing Approval Milestone. As further consideration for the supply of Patches and the other rights and licenses granted hereunder, NeurogesX shall pay LTS an amount equal to [***] within [***] days after receipt by NeurogesX of the [***] Marketing Approval to sell the Patch in the Territory.
5.5 No Setoff nor Suspension of Performance.
(a) NeurogesX. NeurogesX shall not exercise any right of setoff, net-out or deduction, take any credit, or otherwise reduce the balance owed to LTS, in each case with respect to payments under Sections 5.1 of this Agreement, unless otherwise expressly provided herein, the Parties otherwise agree or until NeurogesX has obtained a final determination under Section 12.1(b) against LTS in the amount asserted by NeurogesX.
(b) LTS. LTS shall not have the right to suspend performance (including without limitation refusing or withholding delivery of Patches) under this Agreement (i) unless and until LTS has obtained a [***] determination under Section 12.1(b) that NeurogesX has [***] failed to perform its obligations under this Agreement relating thereto, (ii) in the case where NeurogesX has failed to pay the Transfer Price for [***] shipments of Patches, which payment is not disputed by NeurogesX, is outstanding for longer than [***] or more days and, in the aggregate, exceeds a total amount of [***] unless NeurogesX fails to provide a letter of credit upon the request of LTS as set forth in Section 2.11; (iii) except as set forth in Section 7.6 below, or (iv) unless otherwise mandated by applicable laws, regulations or orders.
(c) No Other Charges. Except as otherwise set forth in this Agreement, LTS shall perform all of its obligations hereunder at its [***], and shall not [***] NeurogesX any amounts for the manufacture and supply of Patches hereunder except as expressly set forth in this Article 5 or otherwise mutually agreed in writing.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-94-
5.6 Adjustment to the Transfer Price.
(a) Prior to first launch of Patch the Parties shall annually review the Transfer Price but starting not earlier than [***] Thereafter, beginning [***] years after the first calendar month in which Patches are delivered hereunder, the Parties shall annually review the Transfer Price and negotiate in good faith an adjustment for the Transfer Price taking into account all relevant factors, including but not limited to Manufacturing Costs, provided, that any increase will be commensurate with actual changes in the relevant factors relating to the manufacture of the Patch.
(b) LTS shall have the right to adjust the Transfer Price up to [***] over the Transfer Price set forth in Exhibit A for increases in costs for raw materials, labor or waste. Any further increases are subject to the mutual agreement of the Parties.
(c) If LTS requests an increase of the Transfer Price by more than [***] then NeurogesX shall have the right to have the Manufacturing Cost audited by an independent Certified Public Accountant (CPA), reasonably acceptable to LTS acting in confidence and retained by NeurogesX at NeurogesX’s expense, for purposes of confirming such increase in Manufacturing Costs. Such CPA shall however not disclose details of such Manufacturing Costs.
(d) LTS shall use [***] to avoid substantial increases in its Manufacturing Costs. In addition, at the request of NeurogesX from [***] LTS shall use [***] to suggest, and the Parties shall discuss, methods to reduce Manufacturing Costs and to provide a commensurate reduction in Transfer Price hereunder; provided it is agreed that any such methods shall be implemented only upon mutual agreement.
(e) In case the Transfer Price is adjusted according to the foregoing, Exhibit A shall be amended accordingly.
ARTICLE 6
LICENSES
6.1 Manufacturing License to LTS. NeurogesX hereby grants to LTS a non-exclusive, non-transferable license under NeurogesX Patents to manufacture the Patches ordered by NeurogesX hereunder and deliver such Patches to NeurogesX or its designee as specified in this Agreement. As used herein, “NeurogesX Patents” means any and all patents owned or Controlled by NeurogesX which cover, is incorporated in or is used as part of the Patch, or which relate to a method of use or manufacture of the Patch.
6.2 Commercialization License to NeurogesX. LTS hereby grants NeurogesX a worldwide, royalty-free, sublicensable right and license under the Commercialization Patents to use, sell, offer to sell, import, market, promote, distribute and otherwise commercialize the Patches purchased hereunder. The license set forth in this Section 6.2 shall be exclusive in the Field.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-95-
6.3 Limited Exclusivity of LTS.
(a) LTS shall for the duration of this Agreement not develop for, sell or license to, any third parties any TTS with capsaicin (1) with an area based content above [***], or (2) that is covered by LTS’ U.S. patent application no. [***], continuations, continuations-in-part, divisions and foreign counterparts thereof, patents issuing thereon, including renewals, reissues and extensions (such TTS with capsaicin as described in clauses (1) or (2), the “Field”). For clarity, during the term of this Agreement LTS shall not exploit on its own nor directly or indirectly license, assist or enable any third party under any patent rights, know-how, data or other intellectual property owned or Controlled by LTS to make, use, offer for sale, sell or import any TTS with capsaicin in the Field, provided, however, that LTS shall at all times have the right to toll manufacture (including performing manufacturing scale-up of) any products containing capsaicin the design and technical aspects solely developed by a third party and not falling within the scope of the Commercialization Patents.
(b) LTS shall not use, manufacture, sell, distribute or license for or to any third party any Patches, except as authorized in writing by NeurogesX.
(c) Except as set forth in this Sections 6.3, and subject to Section 7.1 below, LTS shall at all times have the right to develop and/or manufacture any other TTS’s or products developed by LTS and/or a third party, even if it contains any VRI-Ligand.
(d) During the term of the Agreement and except as otherwise provided herein, NeurogesX shall not purchase or otherwise procure Patches from any supplier other than LTS or its Affiliates.
6.4 Backup Manufacturing License.
(a) Definitions. For the purpose of this Section 6.4, the following terms will have the meanings set forth below.
(i) “Failure Event” means any of the following events: a Force Majeure Event, Failure to Supply, an Anticipated Failure to Supply, receipt of a Notice of Suspension (as defined in Section 7.6 below), termination by either Party under Section 11.3 below or termination by NeurogesX under Section 11.2 below.
(ii) “Force Majeure Event” means a failure of LTS to perform its obligations to supply the Patches to NeurogesX in accordance with the terms and conditions set forth herein due to Force Majeure (as defined in Section 12.6) causing an interruption of supply of Patches to the customer, and then a failure to remove any such cause by employing [***] and resume supply hereunder within [***] days thereafter.
(iii) “Failure to Supply” means either: (A) not including cases of Force Majeure, an inability of LTS to supply in accordance with this Agreement at least [***] in any one Calendar Quarter and at least [***] in the subsequent Calendar Quarter of the Binding Quantities of Patches ordered by NeurogesX for delivery in such quarters, provided that such failure is not due to failure of NeurogesX to supply Active Ingredient in accordance with Section 2.5 or otherwise caused by NeurogesX; or (B) a material breach of this Agreement by LTS causing an interruption of supply of Patches to the customer, which is not cured within [***] days after notice specifying such breach.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-96-
(iv) “Anticipated Failure to Supply” means either: (A) a written notice by LTS that it anticipates that it will be unable to supply NeurogesX’s requirements of the Patch in accordance with this Agreement for at least [***] consecutive orders, when it can be foreseen by LTS that the following [***] consecutive orders cannot be fulfilled even though such orders were placed in accordance with Section 2.4 (a); or (B) a failure of LTS to maintain compliance with all Regulatory Requirements relating to the Facility and its manufacturing activities hereunder, which non-compliance cannot (or LTS anticipates will not) be or is not cured by using [***] and such non-compliance causes interruption of supply of Patches by LTS for [***] or more. LTS shall promptly notify NeurogesX in the event of clauses (A) or (B) above, and in the case of clause (B), shall keep NeurogesX updated as to its efforts to cure any such non-compliance.
(v) “LTS Know-how” means instructions, specifications, know-how, technology, materials and intellectual property describing the composition and manufacture of the Patches, including a description of the suppliers, raw materials, processes, equipment and instruments used for such manufacture and all Patch Regulatory Information. It is understood that LTS Know-how shall include all information or materials disclosed or provided under this Agreement, the Clinical Supply Agreement, the MOU and the Secrecy Agreement.
(vi) “LTS Technology” means LTS Know-how, the Commercialization Patents, and any and all other patents or intellectual property worldwide owned or Controlled by LTS which cover, is incorporated in or is used as part of any of the Patches, or which relate to a method of use or manufacture of any of the Patches (or equipment used in such manufacturing).
(b) Delivery of Technology. Upon any Failure Event, in order to permit NeurogesX to manufacture or have manufactured and to commercialize the Patch, LTS shall, upon NeurogesX’s request, deliver to NeurogesX at no additional cost, information regarding the then existing manufacturing process and quality procedures relating to the Patch, and all LTS Know-how, which would be reasonably required for a third party manufacturer of ordinary skill in the art of manufacturing TTS, to manufacture for NeurogesX Patches conforming to the Specifications. EXCEPT AS SET FORTH IN THIS SECTION 6.4(b), LTS SHALL IN NO CASE BE OBLIGATED TO PROVIDE ITS PROPRIETARY KNOW-HOW WITH REGARD TO GENERAL TTS MANUFACTURING METHODS, BUT SHALL IF REQUESTED BY NEUROGESX AND AT NEUROGESX’S COST AND EXPENSE ASSIST NEUROGESX OR ITS THIRD PARTY MANUFACTURER IN THE PRODUCTION OF THE FIRST THREE (3) COMMERCIAL BATCHES OF THE PATCHES. Upon a Failure Event, NeurogesX, its Affiliates and Sublicensee shall thereafter be relieved of any obligations under this Agreement, including under Section 2.1 above, to purchase all or any of their commercial, clinical or other requirements of Patches from LTS, and NeurogesX, its Affiliates and Sublicensees shall be free to purchase some or all of its Patches from third party(ies).
(c) Other LTS Facilities. Upon any Failure Event LTS shall have the option, exercisable upon written notice to NeurogesX given within [***] days of the notice of the Failure Event to qualify LTS’ facility at LTS Xxxxxxx Therapy Systems Corp., [***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-97-
and to have the Patches manufactured at this facility or, in the event the facility has already been qualified according to Section 4.5, to have the Patches manufactured at the LTS facility at LTS Xxxxxxx Therapy Systems Corp. or any other Facility; provided that the Parties enter into an agreement on bearing of the costs, the timelines for qualification and the manufacturing capacity within [***] days after execution of the option (such [***] day period, the “Negotiation Period”).
(d) Backup License. LTS hereby grants NeurogesX a world-wide right and license, with the right to grant and authorize sublicenses, under LTS Technology, to use, make, have made, sell, offer for sale, import, promote, market, develop, obtain regulatory approval for and otherwise commercialize Patches and any derivatives or improvements thereof made by or for NeurogesX, its Affiliates or Sublicensees, and to practice any methods, processes or procedures described or claimed therein in connection with the foregoing; provided that NeurogesX, its Affiliates or Sublicensees shall not exercise any rights set forth in this Section 6.4(d) except and until after a Failure Event and after LTS has informed NeurogesX in writing that LTS will not exercise the option set forth in Section 6.4(c), or such option or the Negotiation Period have expired and the Parties have not come to an agreement as contemplated under Section 6.4(c) above. NeurogesX shall not disclose any LTS Know-how to any third party except in connection with exercising the rights set forth in this Section 6.4(d), and subject to reasonable confidentiality obligations on the part of such third party. The license set forth in this Section 6.4(d) (the “Backup License”) shall be exclusive for any LTS patent in the Field as defined in Section 6.3 in case of a Failure Event for a period of five years, unless such LTS patent expires or is abandoned earlier. The period shall be referred to as the “Maximum Backup License Exclusivity Period.” Notwithstanding Section 6.3, this Section and subject to Section 6.4(e) below, beginning [***] months after a Force Majeure Event or a Notice of Suspension, LTS shall have the right to develop, by itself or with third parties, manufacture and supply products to [***] in the Field; provided that it does not utilize any [***] of the patent application referred to in Section 6.3(a)(2) and / or the patent possibly resulting therefrom for a period of [***] years, unless such patent expires or is abandoned earlier, it being understood that this exemption shall only pertain to the patent and the claims granted, if any. In the event NeurogesX desires at any time to have the license set forth in this Section 6.4(d) become non-exclusive, NeurogesX shall notify LTS in writing. This Section 6.4 shall be NeurogesX’s [***] for the breach of LTS’ general obligation under this Agreement to supply Patches ordered by NeurogesX, provided that LTS has employed its [***] to do so; but the foregoing shall not limit any remedies set forth in this Agreement NeurogesX may have for the breach of any specific obligations of LTS.
(e) LTS Supply after Failure Event. In case of any Failure Event, if after NeurogesX has exercised its License set forth under Section 6.4(d) and NeurogesX has qualified a third party manufacturer for the Patches, LTS is able to demonstrate by a statement of an independent expert ([***]) that it is able to resume manufacturing and supply of Patches, NeurogesX shall offer to have LTS supply [***] of its requirements of Patches from LTS. In the event LTS accepts such offer, its supply of Patches shall be subject to all of the terms and conditions of this Agreement; provided that if NeurogesX offers to have LTS supply less than [***] of its requirements for Patches, then notwithstanding Section 6.3 and Section 6.4(d), LTS shall have the right to develop by itself or with third parties, manufacture and supply products to third parties in the Field.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-98-
(f) Royalties.
(i) In consideration of the license set forth in this Section 6.4, NeurogesX shall pay LTS a [***] on the [***] by NeurogesX, its Affiliates or Sublicensees of Kits made under the license set forth in this Section 6.4 after a Failure Event, calculated as follows. The royalty shall be [***] for the exclusive Backup License, and [***] in case of a non exclusive Backup-License.
(ii) Notwithstanding Section 6.4(f)(i) above, NeurogesX shall have the option to pay LTS a royalty at the rate of [***] in case of a non-exclusive Backup License and [***] in case of an exclusive Backup License, rather than the percentage calculated in accordance with Section 6.4(f)(i), on the Net Sales by NeurogesX, its Affiliates or Sublicensees of Kits made under the license set forth in this Section 6.4 after a Failure Event, in certain jurisdictions selected by NeurogesX as follows. NeurogesX shall exercise such option with respect to a jurisdiction within [***] days after the first commercial sale of a Kit in such jurisdiction after the Failure Event. In the event NeurogesX exercises such option with respect to a jurisdiction, NeurogesX shall be entitled to a [***] share of recoveries from Infringement as set forth in Sections 7.3(a)(ii) and 7.3(b)(ii) below.
(iii) For the avoidance of doubt, if NeurogesX elects to exercise the option set forth in Section 6.4(f)(ii), the royalty set forth in Section 6.4(f)(ii) shall not be additional to those in Section 6.4(f)(i), NeurogesX shall in this case only pay the royalty set forth in Section 6.4(f)(ii) with respect to the selected jurisdictions. In addition, for the avoidance of doubt, the royalty set forth in Section 5.2 above shall not apply to the Patches made under the license set forth in this Section 6.4, and the royalty set forth in this Section 6.4(f) shall not apply to the Patches purchased from LTS.
6.5 Sublicensing. In the event NeurogesX desires to grant a sublicense to any third party under the licenses set forth in Section 6.2 and/or 6.4, NeurogesX is free to do so, provided that
(a) LTS is informed of the identity of the Sublicensee and the scope of rights that are being granted;
(b) those Sublicensees shall be obligated to indemnify LTS regarding IP Claims and product liability to the extent NeurogesX is so obligated under Sections 9.2 and 9.3(b); and
(c) those Sublicensees shall be obligated to maintain a customary and reasonable level of product liability insurance which covers the Patch, for those countries in which such Sublicensee is authorized by NeurogesX to distribute the Patch.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-99-
ARTICLE 7
INTELLECTUAL PROPERTY
7.1 Inventions relating to the Patch.
(a) LTS Owned. All inventions developed by the Parties or by either Party under this Agreement or the Clinical Supply Agreement solely relating to LTS’ existing TTS technology, including but not limited to design, formulation, manufacturing, testing and packaging of TTS, shall be the property of LTS (“LTS IP”). LTS shall not use any LTS IP for the development of TTS containing VR1-Ligand until [***], other than for NeurogesX. Thereafter, until [***], NeurogesX shall have a right of first refusal in case LTS commences development of TTS which contain any VR1-Ligand using LTS IP. NeurogesX hereby irrevocably assigns, and agrees to assign to LTS any right, title and interest it may have in and to the LTS IP, and shall assist LTS, upon reasonable request and at LTS’[s] sole expense, to secure or perfect any and all such rights.
(b) NeurogesX Owned. All inventions developed by the Parties or by either Party under this Agreement or the Clinical Supply Agreement solely relating to medical uses of any VR1-Ligand shall be the property of NeurogesX (“NeurogesX IP”). LTS hereby irrevocably assigns, and agrees to assign to NeurogesX all of its right, title and interest in and to NeurogesX IP, and shall assist NeurogesX, upon reasonable request and at NeurogesX’s sole expense, to secure or perfect any and all such rights.
(c) Jointly Owned. All other inventions developed by the Parties or by either Party under this Agreement or the Clinical Supply Agreement shall be the joint property of the Parties (“Joint IP”), provided that it does not belong under Section 7.1(a) or (b) to either LTS IP or NeurogesX IP. Neither Party shall have any obligation to account to the other Party for profits with respect to, or to obtain any approval of the other Party to license or exploit, any Joint IP by reason of their joint ownership, and each Party waives any such right it might have under the applicable laws in any country; provided, that NeurogesX shall only have the right to exploit and license the Joint IP for VR1-Ligand applications and products, and LTS shall only have the right to exploit or license the Joint IP for any applications and products other than VR1-Ligand applications and products.
(d) Exclusive License. Accordingly, the Parties grant to each other licenses under the Joint IP (which to avoid any doubt for purposes of these licenses includes all patent applications and patents claiming the inventions included therein) as follows:
(i) NeurogesX hereby grants to LTS an exclusive (even as to NeurogesX), royalty-free, fully-paid, freely sublicensable (though one or more layers of sublicensees without consent), perpetual, irrevocable, worldwide license under the Joint IP to [***] Notwithstanding Section 7.3 below, LTS shall have the [***] to enforce the Joint IP against infringement within the scope of the license set forth in this Section 7.1(d)(i) and to retain [***] recoveries therefrom, and NeurogesX shall cooperate with LTS, at LTS’ reasonable request and expense; however, nothing herein shall require NeurogesX to join any such action as a party to any such action. For clarity, the Parties intend that LTS shall have sufficient rights to enforce such Joint IP as set forth in this Section 7.1(d)(i) without NeurogesX joining as a co-plaintiff.
(ii) LTS hereby grants to NeurogesX an exclusive (even as to LTS), royalty-free, fully-paid, freely sublicensable (though one or more layers of sublicensees without consent),
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-100-
perpetual, irrevocable, worldwide license under the Joint IP to make, have made, use, sell, offer to sell, and import product candidates and products that contain any VR-1 Ligand(s) and/or are VR-1 Ligand applications and products. Notwithstanding Section 7.3 below, NeurogesX shall have the sole right to enforce the Joint IP against infringement within the scope of the license set forth in this Section 7.1(d)(ii) and to retain all recoveries therefrom, and LTS shall cooperate with NeurogesX, at NeurogesX’s reasonable request and expense; however, nothing herein shall require LTS to join any such action as a party to any such action. For clarity, the Parties intend that NeurogesX shall have sufficient rights to enforce such Joint IP as set forth in this Section 7.1(d)(ii) without LTS joining as a co-plaintiff.
7.2 Prosecution of Patents Covering the Patch.
(a) As used in Sections 7.2 and 7.4, “prosecute” means the procedure(s) involved in securing patent rights in patent offices worldwide.
(b) LTS shall have the right, [***] to file, prosecute and/or maintain any patent or patent application solely owned by LTS covering the Patches. With respect to such LTS patents and patent applications that are specific to the Patch, including [***] and foreign counterparts thereof, NeurogesX shall have the right to review and consult with LTS regarding the prosecution and maintenance thereof; provided however that in the event NeurogesX does not provide comments within a period of [***] days after receipt of a respective draft brief or office action by LTS, NeurogesX shall be deemed to have waived its right to review and consult.
(c) If LTS elects to file, prosecute and maintain any such patent or patent application, the expenses of such action shall be borne by LTS.
(d) If LTS elects not to file, prosecute or maintain any such patent or patent application in any jurisdiction, it shall notify NeurogesX at least [***] days prior to the due date for any action or payment. NeurogesX shall then have the right, to file, prosecute and/or maintain any such patent or patent application.
(e) If NeurogesX elects to file, prosecute and maintain any such patent or patent application in such jurisdiction, the expenses of such action shall be borne by NeurogesX, and NeurogesX shall have a [***] (except as set forth in this Section 7.2(e[***]. The foregoing license shall be exclusive for the Field. NeurogesX shall have the first right to enforce such patents and patent applications in the Field and retain all revenues therefrom, notwithstanding Section 7.3 below.
7.3 Third Party Infringement in the Field. Each Party agrees to [***] notify the other Party if it becomes aware of any third party’s TTS in the Field that infringes LTS’ patents covering the Patch (an “Infringement”). LTS shall have the [***], but not the obligation, to enforce its patents against any Infringement. LTS shall [***] control any such enforcement action. LTS shall keep NeurogesX informed of the proceeding of such enforcement. In case NeurogesX believes LTS is not [***] pursuing the enforcement, NeurogesX shall have the
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-101-
[***] with LTS. LTS agrees not to settle or otherwise terminate or delay its enforcement against any Infringement in a manner which would permit the third party to continue manufacturing and/or selling infringing TTS in the Field in contravention of the exclusivity granted to NeurogesX under Article 6 of this Agreement. LTS shall not settle any Infringement action without the prior written approval of NeurogesX. In the event LTS notifies NeurogesX within [***] days after notice of an alleged Infringement by either Party that it intends to file suit against any alleged Infringement, or does not give any notification whether it intends to file suit within [***] days after notice of an alleged Infringement by either Party or drops any action against an alleged Infringement, then NeurogesX shall have the right, but not the obligation, to enforce LTS’ patents covering the Patch against such alleged Infringement. LTS agrees to use Reasonable Commercial Efforts to assist and cooperate with NeurogesX in any enforcement by NeurogesX under this Section 7.3, at NeurogesX’s cost and expense.
(a) If LTS decides to enforce its patents against Infringement and NeurogesX permits LTS to claim NeurogesX damages, then any proceeds from such enforcement of its Patents recovered from any such Infringement action shall be divided (i) [***] between NeurogesX and LTS unless clause (ii) applies, or (ii) [***] to NeurogesX and [***] to LTS with respect to recoveries for Infringement in a jurisdiction for which NeurogesX has elected to pay a higher royalty under Section 5.2(b) or 6.4(f)(ii) above, in each case after first subtracting [***].
(b) In the event NeurogesX is given the right to enforce LTS’ patents against Infringement and LTS permits NeurogesX to claim LTS’ damages, then any proceeds from such enforcement of LTS’ patents recovered from any such Infringement action shall be divided (i) [***] between NeurogesX and LTS unless clause (ii) applies, or (ii) [***] to NeurogesX and [***] to LTS with respect to recoveries for Infringement in a jurisdiction for which NeurogesX has elected to pay a higher royalty under Section 5.2(b) above or 6.4(f)(ii), in each case after first subtracting [***].
7.4 Cooperation. Each Party shall cooperate with the other Party at the other Party’s expense to execute all lawful papers and instruments, to make all rightful oaths and declarations, and to otherwise assist, including without limitation participating (but not joining as a plaintiff) in enforcement actions, the other Party as may be necessary and reasonably requested in the preparation and prosecution of any or all patents and patents applications regarding the Patches, and the enforcement thereof.
7.5 IP Review by LTS. LTS shall review on a [***] basis the intellectual property rights relevant for LTS’ obligations hereunder. LTS shall give NeurogesX a confirmation in writing [***] a year (on or about [***] of each year) that, to the best of its actual knowledge, LTS does not infringe third parties’ intellectual property rights with respect to the Patches.
(a) In the event a Party becomes aware of a Blocking Patent, such Party shall [***] notify the other Party giving such [***] details as such Party may be aware, and
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-102-
the Parties shall discuss the situation in [***] and agree on a plan to address the situation, with the mutual objective of removing the risk posed by such Blocking Patent so that the Parties may continue performance under this Agreement, including if appropriate and agreed by the Parties, by licensing and/or challenging such Blocking Patent. In the event the Parties agree on a plan to address such Blocking Patent, the Parties shall designate a lead Party to use Reasonable Commercial Efforts to perform such plan. It is understood that the costs of any license obtained with respect to a Blocking Patent (to the extent allocable to the manufacture, use or sale of Patches hereunder) will be shared by the Parties in proportion to their indemnification obligations for IP Claims under Section 9.2. It is understood and agreed that notices, communications and discussions under this Section 7.6 shall not be construed as [***] by either Party. In addition, all notices, communications and discussions under this Section 7.6 shall be deemed protected by the [***].
(b) After discussing the matter in [***] as described in Section 7.6(a) above, LTS and NeurogesX shall each have the right to [***] under this Agreement with respect to the affected country upon written notice (“Notice of Suspension”), until the risk of infringing such Blocking Patent has been removed, including if a Party or a Sublicensee has obtained a license with respect to the Blocking Patent for such country, or otherwise if in the [***] opinion of the Suspending Party’s counsel, such risk is removed. In the event LTS elects to provide a Notice of Suspension, then such notice shall be deemed a “Failure Event” under Section 6.4(a) above, effective upon receipt of the Notice of Suspension, provided however that the Parties shall discuss resuming their obligations as foreseen in case of termination under Section 11.3.
(c) Notwithstanding the foregoing, in the event that upon receiving a Notice of Suspension, a Party disputes in good faith such notice, then the Party providing the Notice of Suspension may [***] its performance as set forth in this Section 7.6 if it can show by an opinion of an independent counsel, approved by the other Party (whose approval shall not be unreasonably withheld), that the determination of its counsel that a third party’s intellectual property right would be infringed is [***]. Such opinion shall also summarize the underlying facts. The Parties shall cooperate to ensure that all attorney-client privilege and work product privilege shall be protected with respect to the opinion. If the Party providing the Notice of Suspension does not propose an independent counsel, which is [***] to the other Party, then an independent counsel shall be appointed by the ICC International Centre for Expertise in accordance with the rules for Expertise of the International Chamber of Commerce. Such independent counsel shall be a [***].
ARTICLE 8
REPRESENTATIONS AND WARRANTIES; DISCLAIMER; LIMITATION OF LIABILITY
8.1 General Warranties. Each Party represents and warrants to the other Party that it is a corporation duly organized and validly existing under the laws of the state or country of its incorporation, the execution, delivery and performance of this Agreement by such Party has been duly authorized by all requisite corporate action, and it has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder. Each Party warrants and represents to the other Party that it has not previously granted and will not grant any rights in conflict with the rights granted herein.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-103-
8.2 Patches. Subject to the limited liability provisions set forth in this Agreement, LTS represents and warrants that the Patches delivered to NeurogesX (a) shall (i) be in conformity with the Specifications, (ii) be manufactured in accordance with Current GMP and the Quality Assurance Agreement, and (iii) comply with all Regulatory Requirements, and (b) no later than [***] days after initiation of the manufacture thereof, be released for shipment by LTS and made available for ex works delivery to NeurogesX.
(a) Rejection of Defective Patches. NeurogesX may reject Defective Patches, subject to NeurogesX providing sufficient evidence of appropriate and correct storage at all times according to the storage conditions set forth in the applicable Specifications (which evidence is subject to confirmation under Section 8.2(c) below), by delivering to LTS written notice of rejection, and if practical, specifying the nature of the Defect and the Patches lot number, within [***] days after actual receipt by NeurogesX or its designee of the applicable shipment (the “Acceptance Period”). In the case of Patches with Defects that are not reasonably discoverable upon NeurogesX’s [***] incoming quality control testing (“Latent Defects”), NeurogesX may reject such Patches within [***] days after discovery of the Latent Defects. After said [***] days period or after the Acceptance Period, whichever is applicable, the Patches shall be deemed accepted.
(b) Replacement of Defective Patches. During the [***] month period commencing on the date the Patches are delivered to NeurogesX, LTS shall promptly (and in any event within [***] days of receipt of notice of rejection) deliver to NeurogesX, at LTS’ own cost and risk, including shipping costs and Active Ingredient costs, replacements for any rejected Defective Patches, up to the [***] contained in the applicable lot. The foregoing shall be NeurogesX’s [***] for the rejection of Defective Patches, but does not limit any remedies NeurogesX may have under other sections of this Agreement.
(c) Confirmation of Cause of Defects. LTS shall have the right to examine any Patches rejected by NeurogesX, provided such examination shall not delay the shipping of replacement Patches. In the case LTS disagrees that the rejected Patches are subject to this Section 8.2, the claim may be submitted for tests and a decision by a mutually agreed upon, independent and reputable third party laboratory in the United States, which appointment shall not be unreasonably withheld or delayed by either Party. In making its determination, such laboratory shall take into consideration any evidence provided by LTS that the Patches complied with the Specifications at the time of shipment, and also any evidence provided by NeurogesX that the Patches were correctly handled and stored (i.e. in accordance with the Specifications) by NeurogesX and third parties under authority of NeurogesX. The determination of such laboratory shall be [***] upon the Parties. The Party against whom the determination is made shall be responsible for the [***], as well as the costs of the replacements for the rejected Patches at issue, including [***].
8.3 Disclaimer of Warranties. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY WARRANTIES EITHER EXPRESS OR IMPLIED, STATUTORY OR OTHERWISE, WITH RESPECT TO THE PATCHES AND
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-104-
ACTIVE INGREDIENT SUPPLIED HEREUNDER, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. THE WARRANTIES GIVEN UNDER THIS AGREEMENT ARE EXPRESSLY IN LIEU OF ALL OTHER WARRANTIES EXPRESS OR IMPLIED.
8.4 Limitation of Liability. EXCEPT WITH RESPECT TO ARTICLES 9 AS RELATED TO THIRD PARTIES, AND ARTICLE 10, NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR ANY SPECIAL, CONSEQUENTIAL, PUNITIVE, EXEMPLARY OR INCIDENTAL DAMAGES (INCLUDING LOST OR ANTICIPATED REVENUES OR PROFITS RELATING TO THE SAME), ARISING FROM ANY CLAIM RELATING TO THIS AGREEMENT, WHETHER SUCH CLAIM IS BASED ON CONTRACT, TORT (INCLUDING NEGLIGENCE) OR OTHERWISE, EVEN IF ADVISED OF THE POSSIBILITY OR LIKELIHOOD OF SAME.
ARTICLE 9
INDEMNIFICATION
9.1 Product Claims. LTS shall indemnify NeurogesX from any third party claims, damages, losses, costs, expenses arising from the development, manufacturing and supply under this Agreement of Patches which, at the time of delivery, do not conform to the Specifications (“Product Claims”). NOTWITHSTANDING THE FOREGOING, LTS’ AGGREGATE LIABILITY UNDER THIS SECTION 9.1 IN EACH CALENDAR YEAR FOR PRODUCT CLAIMS THAT DO NOT RESULT FROM LTS’ GROSS NEGLIGENCE OR WILLFUL MISCONDUCT, SHALL NOT EXCEED THE LIMITS OF LTS’ INSURANCE COVERAGE FOR SUCH INDEMNIFIABLE CLAIMS IN SUCH YEAR PLUS [***]
9.2 Intellectual Property Claims.
(a) NeurogesX Caused. NeurogesX shall indemnify and hold harmless LTS from any third party claims, actions, proceedings alleging the infringement of any patent, trade secrets or other intellectual property of a third party (“IP Claims”), arising from LTS’ development, manufacturing, sale and using the Patches for or to NeurogesX, which IP Claims are caused by LTS’ use of any technology or intellectual property owned or supplied by NeurogesX.
(b) LTS Caused. With respect to any IP Claims arising from the development, manufacture, sale and use of the Patches under this Agreement by or for NeurogesX, which IP Claims are caused by use of any technology or intellectual property owned or supplied by LTS, including without limitation the design of the Patch and any LTS Technology (as defined in Section 6.4(a)(iii)) (an “LTS Infringement”), the following shall apply:
(i) If either Party is sued for an alleged LTS Infringement, LTS is liable up to an amount of [***] incurred by NeurogesX in connection with the defense of such IP Claims, provided that this does not apply in the event that NeurogesX is sued for an alleged LTS Infringement in case the Patches being subject of such IP Claims were manufactured by a third party in accordance with Section 6.4. For all costs and expenses
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-105-
incurred by NeurogesX in defense of such IP Claims in excess of [***], NeurogesX is liable and shall indemnify and hold harmless LTS. In the event LTS is the Party sued for an alleged LTS Infringement, and LTS desires indemnification from NeurogesX, LTS shall [***] notify NeurogesX of the alleged LTS Infringement, permit NeurogesX [***] of the defense and/or settlement of the alleged LTS Infringement as requested (but LTS may participate in such defense and/or settlement with counsel of its choice and its own expense), and cooperate with NeurogesX with respect to such defense and settlement.
(ii) LTS agrees to automatically reduce the royalty rates under this Agreement by the royalty rate or other amounts a court may require NeurogesX to pay to a third party claiming a LTS Infringement in satisfaction of a judgment against NeurogesX, or by the royalty rate or other amounts owed by NeurogesX in any settlement of the LTS Infringement; provided, that in no event shall the royalty rate under this Agreement fall below [***]
(iii) In case of a final and non-appealable court judgment which prohibits NeurogesX from using or selling Patches as a result of an LTS Infringement, LTS shall reimburse NeurogesX for [***] of the Development Costs (as defined in the Clinical Supply Agreement). This obligation shall only be valid during the term of this Agreement.
(c) Sole Remedy for IP Claims. Notwithstanding any other provision of this Agreement, this Section 9.2 and Sections 3.9 and 7.6 and 11.3 state the entirety of each Party’s obligations to indemnify and/or hold harmless the other Party and the entirety of each Party’s remedy from the other Party, with respect to IP Claims arising from the development, manufacturing, sale and/or use of the Patches.
9.3 Other Indemnification.
(a) Employee Claims. Each Party shall indemnify the other Party against any claims against such other Party by the indemnifying Party’s employees, directors, officers and other persons involved from the indemnifying Party’s side in the performance of obligations under this Agreement (“Employees”) to the extent such claims relate to such Employees’ inventorship of, rights in or right to receive proceeds from any inventions conceived or reduced to practice under this Agreement, and are inconsistent with Section 7.1.
(b) LTS. Without regard to LTS insurance, LTS shall indemnify and hold NeurogesX harmless from any third party claims, damages, losses, costs and expenses (including attorney’s fees) (“Claims”) that arise from LTS’ gross negligence or willful misconduct in connection with the use, development, manufacturing, sale, distribution or application of the Patch.
(c) NeurogesX. Other than (i) to the extent that LTS is obligated to indemnify NeurogesX pursuant to this Article 9, to the extent arising from LTS’ gross negligence or willful misconduct, and (iii) to the extent in excess of LTS’ limitation on liability hereunder, NeurogesX shall indemnify and hold LTS harmless from any Claims that arise from the use, development, manufacturing, sale, distribution or application of the Patch by NeurogesX, its Sublicensees, or its distributors, including claims under Sections 9.1 and 9.2; provided that (i) LTS promptly
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-106-
notifies NeurogesX of the Claim, (ii) NeurogesX has sole control of the defense and/or settlement of the Claim (but LTS may participate in such defense and/or settlement with counsel of its choice and its own expense), and (iii) LTS fully cooperates with NeurogesX with respect to such defense and settlement.
9.4 Insurance.
(a) NeurogesX. Prior to [***] of the Patches, NeurogesX shall obtain and maintain during the term of this Agreement sufficient insurance coverage of an amount reasonable and customary in the pharmaceutical industry considering the nature of the Patch, which insurance coverage can also be self insurance.
(b) LTS. LTS shall obtain and maintain during the term of this Agreement and thereafter liability insurance covering its activities under this Agreement at a level no lower than that set forth in Exhibit F, to the extent such coverage is available at terms and conditions not substantially more unfavorable than LTS’ present insurance coverage. LTS shall certify to NeurogesX each year of the extent of its insurance coverage, and shall promptly notify NeurogesX in the event its liability insurance covering its activities under this Agreement falls below the level set forth in Exhibit F.
ARTICLE 10
CONFIDENTIALITY
10.1 Confidential Information. Except as expressly provided in this Article 10, each Party shall, during the term of this Agreement plus [***] years thereafter, keep completely confidential and shall not use, put into production, publish nor otherwise disclose the Confidential Information received from the other Party under this Agreement, the MOU or the Clinical Supply Agreement. As used herein, “Confidential Information” means all information, including but not limited to data, know-how, formulas, processes, specifications, organizational information, mechanical equipment and/or trade secrets which have been or may hereafter be disclosed, directly or indirectly by a Party to the other Party, either orally or in writing, or through inspection as well as samples; except that Confidential Information shall not include information and know-how which the receiving Party can show by competent proof:
(a) was, at the time of disclosure, in the public domain,
(b) has subsequent to disclosure, become part of the public domain through no fault, act, omission, or violation by the receiving Party of the confidentiality obligations hereunder or under the MOU, the Secrecy Agreement dated of January 23, 2001 or the Clinical Supply Agreement,
(c) was, at the time of disclosure, in the possession of the receiving Party and not otherwise acquired, directly or indirectly, from the disclosing Party,
(d) has been developed independently by the receiving Party without access to the Confidential Information of the disclosing Party, or
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-107-
(e) has subsequent to disclosure been obtained by the receiving Party from any other third party, provided however, that it was not obtained by said third party, directly or indirectly, from the disclosing Party under any obligation of confidentiality.
10.2 It is understood that a specific item of the Confidential Information shall not be deemed to be within the exceptions set forth in Section 10.1 above, if it is merely embraced by more general Confidential Information within one of such exceptions.
10.3 Each receiving Party shall limit access to the Confidential Information from the disclosing Party to those of its officers, employees and consultants who need to know such Confidential Information for the purpose of this Agreement and who will be advised of the conditions of this Agreement. In case a receiving Party wishes to disclose Confidential Information to one of its consultants, it may only do so on a need to know basis and provided that such consultant agrees in writing to be bound to the terms of this Article 10 and gives LTS the right to enforce any breach of such an agreement.
10.4 Each receiving Party shall not use Confidential Information of the disclosing Party to contest or challenge any protected rights of, or applications for protection of rights by, the disclosing Party concerning such Confidential Information.
10.5 Permitted Uses and Disclosures.
(a) Performance of this Agreement. Each Party hereto may use Confidential Information of the other Party as is [***] necessary or to perform obligations hereunder or to exercise rights granted to it hereunder.
(b) Disclosure to certain Third Parties. NeurogesX may disclose Confidential Information of LTS to third parties in connection with sublicenses, strategic collaborations, equity or debt financing, IPO, merger, acquisition, changes of control or other similar transactions (“Transactions”), for the sole purposes of enabling such third parties to conduct such Transactions and/or any diligence in connection with such Transactions. In the case of Confidential Information comprising batch records, manufacturing instructions, details of the manufacturing process, specifications for materials, Transfer Price, quality audit reports and regulatory filings detailing manufacturing know-how and LTS patent applications, in each case relating to the Patch, prior to disclosing such Confidential Information of LTS to a third party as described in this Section 10.5(b), NeurogesX shall first obtain a written confidentiality agreement with such third party that is reasonable and customary for such Transaction or diligence, which specifies LTS as a third party beneficiary with respect to the Confidential Information of LTS disclosed to such third party.
(c) Legal Requirements or Governmental Filings. In addition, each Party may use and disclose Confidential Information of the other Party to the extent such use or disclosure is necessary in prosecuting or defending litigation in accordance with this Agreement, complying with applicable governmental laws, regulations, such as SEC regulations, or court order or otherwise submitting information to tax or other governmental authorities, in submissions to regulatory authorities, as a part of patent applications filed on inventions made under this Agreement, or as a part of health registration applications; provided that if a Party is required by
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-108-
law to make any such disclosure, other than pursuant to a confidentiality agreement, it will give reasonable advance notice to the other Party of such disclosure and, save to the extent inappropriate in the case of patent applications or the like, will use Reasonable Commercial Efforts to secure confidential treatment of such information.
10.6 Confidential Terms/Publicity. Each Party agrees not to disclose to any third party the terms of this Agreement without the prior written consent of the other Party hereto, except each Party may disclose the terms of this Agreement (a) as required by securities or other applicable laws or regulations, such as SEC regulations or (b) to advisors, actual or potential investors, acquisition partners, Sublicensees, and others on a need to know basis; provided, however, that in the case of clause (b), prior to disclosing the Transfer Price for the Patches to a third party, NeurogesX shall first obtain a written confidentiality agreement with such third party that complies with Section 10.5(b) above.
10.7 Expiration or Termination of this Agreement. Upon any expiration or termination of this Agreement, each Party shall promptly return all Confidential Information of the other Party, provided however, that each Party may retain such Confidential Information as reasonably necessary to exercise rights hereunder or perform obligations hereunder that survive such expiration or termination, and each Party may retain one copy of the Confidential Information thereof in its legal files solely for archival purposes.
ARTICLE 11
TERM AND TERMINATION
11.1 Term. Unless terminated in accordance with this Article 11, this Agreement shall be in effect until ten (10) years after the first Calendar Quarter of delivery of Patches hereunder. The term shall be automatically renewed for additional two (2) year terms, unless terminated by either Party with two (2) years prior written notice.
11.2 Termination for Breach. Each Party shall have the right to terminate this Agreement upon written notice, in the event of the other Party’s (the “Breaching Party”) material breach hereof which is not cured within sixty (60) after notice specifying such breach. Notwithstanding the foregoing, in the event that within the sixty (60) day period after receiving a notice under this Section 11.2, the Breaching Party disputes in good faith that there has been a material breach, then such dispute shall be resolved in accordance with Section 12.1 below, and this Agreement shall not be terminated under this Section 11.2 until it has been finally determined in accordance with Section 12.1(b) below that the Breaching Party has materially breached this Agreement, and such Breaching Party fails to comply with this Agreement within sixty (60) days thereafter.
11.3 Termination due to Blocking Patents in a Country. Either Party shall have the right to terminate this Agreement with respect to a particular country upon written notice, in the event that a court of competent jurisdiction determines in a final non-appealable judgment, in a case involving one of the Parties or a Sublicensee of NeurogesX, that the manufacture or sale of the Patch in such country pursuant to this Agreement infringes a Blocking Patent. In the event a Party or a Sublicensee thereafter obtains a license with respect to such Blocking Patent in such country, or otherwise removes in the reasonable opinion of the other Party’s counsel the risk of
-109-
infringing a Blocking Patent in such jurisdiction, the Parties shall discuss in good faith to resume their obligations (including the terms and conditions thereof) under this Agreement with respect to such country.
11.4 Other Termination.
(a) Failure Event. NeurogesX shall have the right to terminate this Agreement at any time in the case of a Failure Event (as defined in Section 6.4), provided that such Failure Event is due to LTS not employing [***] to supply Patches. In case NeurogesX purchases all its commercial requirements of Patches from a third party after a Failure Event, then LTS shall have the right to terminate the Agreement upon written notice. In case LTS declines the offer to supply NeurogesX’s requirements set forth in Section 6.4(e), either Party shall have the right to terminate the Agreement upon written notice.
(b) Termination upon Abandonment of the Patch. NeurogesX shall have the right to terminate this Agreement upon [***] months written notice in the event NeurogesX and its Sublicensees have abandoned development and commercialization of the Patch worldwide. In this case LTS may discuss with NeurogesX obtaining a license for its development and commercialization of the Patches on terms (financial and otherwise) to be mutually agreed; provided that neither Party shall be obligated to discuss such license for more than [***] and neither Party shall be obligated to agree to granting or accepting such license, nor any particular terms and conditions for such license.
11.5 Survival. Sections 3.2, 3.8 (to the extent necessary to comply with the other Party’s legal obligations after expiration or termination), 3.9 (with respect to Patches supplied by LTS to NeurogesX prior to such expiration or termination), 6.2, 6.4, 6.5, 7.1, 7.2, 7.4 and Articles 1, 8 (with respect to Patches delivered by LTS under this Agreement), 9 (with respect to Patches delivered by LTS under this Agreement), 10, 11 and 12 shall survive any expiration or termination of this Agreement. Except as set forth in this Section 11.5, all other sections of this Agreement shall terminate upon any expiration or termination of this Agreement.
(a) Accrued Liabilities. Termination of this Agreement for any reason shall not release either Party hereto from any liability which at the time of such termination has already accrued to the other Party.
(b) Backup Manufacturing License. In the event of termination of this Agreement by NeurogesX under Section 11.4(a) or 11.2 or by LTS under Section 11.4(a) or 11.3 any time after NeurogesX has the right to exercise its Backup License the following Sections shall survive (in addition to those described in Section 11.5 above), Sections 5.3 (as it relates to Section 6.4), 6.4, and 7.3 shall survive such termination. For clarity, an election to have this Agreement expire after a Failure Event shall be treated as a termination of this Agreement under Section 11.4(a) by either or both Parties. In any other termination or expiration of this Agreement, Section 7.3 shall survive only with respect to infringement occurring prior to such termination or expiration.
(c) Pending Orders. In the event of any termination or expiration of this Agreement other than termination under Section 11.2, 11.3 or 11.4(a), unless otherwise mutually agreed, all pending orders for the Patches shall be cancelled, and NeurogesX shall reimburse LTS for the
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-110-
cost of Raw Materials and works-in-progress of Patches that cannot be used by LTS in the manufacture of products for its other clients, and such Raw Materials and works-in-progress shall be disposed of or transferred in accordance with NeurogesX’s instruction at NeurogesX’s expense. In the event of termination of this Agreement under Section 11.3 with respect to a country, unless otherwise mutually agreed, all pending orders for Patches specifically for such country shall be cancelled.
ARTICLE 12
MISCELLANEOUS
12.1 Dispute Resolution.
(a) Disputes. The Parties shall attempt in good faith to resolve promptly any dispute arising out of or relating to this Agreement by negotiation. If the matter cannot be resolved in the normal course of business, any interested Party shall give to the other Party written notice of any such dispute not resolved, after which the dispute shall be referred to the principal executive officers of both Parties or their designees who shall likewise attempt to resolve the dispute. If the dispute has not been resolved by negotiations within [***] days of such written notice, each Party may have the dispute arbitrated in accordance with Section 12.1(b) below.
(b) Arbitration. Any dispute or claim arising out of or in connection with this Agreement or the performance, breach or termination thereof, shall be finally settled by binding arbitration under the rules of arbitration of the International Chamber of Commerce by [***] appointed in accordance with said rules, unless the Parties have agreed to have only [***]. The decision and/or award rendered by the arbitrator(s) shall be written, final and non-appealable, and judgment on such decision and/or award may be entered in any court of competent jurisdiction. The arbitral proceedings and all pleadings and evidence shall be in the English language. Any evidence originally in a language other than English shall be submitted with an English translation accompanied by an original or true copy thereof. The place of arbitration shall be in [***]. The costs of any arbitration, including administrative fees and fees of the arbitrator(s), shall be shared equally by the Parties, unless otherwise determined by the arbitrator(s). Each Party shall bear the cost of its own attorneys’ and expert fees.
(c) THE UNDERSIGNED PARTIES ACKNOWLEDGE THAT THE RIGHT TO TRIAL BY JURY IS A CONSTITUTIONAL ONE, BUT THAT IT MAY BE WAIVED AND AFTER CONSULTING WITH COUNSEL, KNOWINGLY AND VOLUNTARILY WAIVE ANY RIGHT TO TRIAL BY JURY IN THE EVENT OF LITIGATION REGARDING THE PERFORMANCE OR ENFORCEMENT OF, OR IN ANY WAY RELATED TO, THIS AGREEMENT AND ANY AGREEMENT CONNECTED THERETO.
12.2 Governing Law. This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the [***], United States of America (including U.S. federal law), without regard to conflict of laws principles.
12.3 Assignment. Neither Party shall have the right to assign this Agreement or its rights hereunder without a prior written consent of the other Party; provided, however, that no
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-111-
consent is required in connection with a sale or transfer of all or substantially all of the assets, securities or business of the assigning Party whether by sale, merger, operation of law, reincorporation or otherwise.
12.4 Independent Contractors. The relationship of LTS and NeurogesX established by this Agreement is that of independent contractors. Nothing in this Agreement shall be construed to create any other relationship between LTS and NeurogesX. Neither Party shall have any right, power or authority to assume, create or incur any expense, liability or obligation, express or implied, on behalf of the other Parties.
12.5 English Language. This Agreement is in the English language only, which language shall be controlling in all respects, and all versions of this Agreement in any other language shall not be binding on the Parties hereto. All communications and notices to be made or given pursuant to this Agreement shall be in the English language.
12.6 Force Majeure. Neither Party shall be responsible or liable to the other Party for, nor shall this Agreement be terminated as a result of, any failure to perform any of its obligations hereunder, if such failure results from circumstances beyond the control of such Party, including, without limitation, requisition by any government authority, the effect of any statute, ordinance or governmental order or regulation, wars, strikes, lockouts, riots, epidemic, disease, an act of God, civil commotion, fire, earthquake, storm, failure of public utilities, common carriers or supplies, or any other circumstances, whether or not similar to the above causes and whether or not foreseeable (each, a “Force Majeure”). The Parties shall use their Reasonable Commercial Efforts to avoid or remove any such cause and shall resume performance under this Agreement as soon as feasible whenever such cause is removed; provided, however, that the foregoing shall not be construed to require either Party to settle any dispute with any third party, to commence, continue or settle any litigation, or to incur any unusual or extraordinary expenses.
12.7 Notices. Any notice or other communication required by this Agreement shall be made in writing and given by prepaid, first class, certified mail, return receipt requested, and shall be deemed to have been given on the date received by the addressee at the following address or such other address as may from time to time be designated to the other Party in writing:
| If to NeurogesX: | NeurogesX, Inc. | |
| San Xxxxxx Business Park, | ||
| 000X Xxxxxxxxxx Xxxx, | ||
| Xxx Xxxxxx, XX 00000 | ||
| Xxxxxx Xxxxxx of America | ||
| Attn: President | ||
| If to LTS: | LTS Xxxxxxx Therapie-Systeme XX, | |
| Xxxxxxxxxxxxx 0, | ||
| X-00000 Xxxxxxxxx, | ||
| Xxxxxxx | ||
| Attn: General Counsel | ||
-112-
12.8 Compliance with Law. Each Party shall comply with all applicable national and local laws and regulations in connection with its activities pursuant to this Agreement.
12.9 No Waiver. A waiver, express or implied, by either LTS or NeurogesX of any right under this Agreement or of any failure to perform or breach hereof by the other Party hereto shall not constitute or be deemed to be a waiver of any other right hereunder or of any other failure to perform or breach hereof by such other Party, whether of a similar or dissimilar nature thereto.
12.10 Severability. If any provision of this Agreement shall be found by a court to be void, invalid or unenforceable, the same shall be reformed to comply with applicable law or stricken if not so conformable, so as not to affect the validity or enforceability of the remainder of this Agreement, and the remainder of this Agreement shall remain in full force and effect.
12.11 Entire Agreement. This Agreement and the Clinical Supply Agreement, together with their Exhibits, constitute the entire understanding and agreement between the Parties as of the Effective Date with respect to the subject matter hereof and supersedes any and all prior and contemporaneous negotiations, representations, agreements, and understandings, written or oral, that the Parties may have reached with respect to the subject matter hereof. No agreements altering or supplementing the terms hereof may be made except by means of a written document signed by the duly authorized representatives of each of the Parties hereto. Upon execution of this Agreement, the Secrecy Agreement dated January 23, 2001 executed between LTS Xxxxxxx Therapy-Systems Corp. and NeurogesX and Sections 6.2, 7.3, 8.3, and 8.4 and Article 11 of the Clinical Supply Agreement shall be terminated and shall be of no further force and effect. Except as amended or qualified in this Agreement, and except as inconsistent with the provisions of this Agreement, the Clinical Supply Agreement shall remain in full force and effect in accordance with its terms.
12.12 Article and Section Headings, Language and Construction. The article and section headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. All references in this Agreement to “Articles,” “Sections” and “Exhibits” refer to the articles, sections and exhibits of this Agreement. The words “hereof,” “herein” and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any subdivision contained in this Agreement. The words “include” and “including” when used herein are not exclusive and mean “include, without limitation” and “including, without limitation,” respectively. This Agreement has been negotiated by the Parties and their respective counsel. Accordingly, this Agreement will be interpreted fairly in accordance with its terms and without any strict construction in favor of or against either Party.
12.13 Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but both of which together shall constitute one and the same instrument.
IN WITNESS WHEREOF, the Parties have caused their duly authorized representatives to execute this Agreement on the dates set forth below.
-113-
| NeurogesX, Inc. | LTS Xxxxxxx Therapie-Systeme AG | |||||||
| By: | /s/ Xxxxxxx XxXxxxx |
By: | /s/ Xxxx Xxxxxx | |||||
| Name: | Xxxxxxx XxXxxxx | Name: | Xxxx Xxxxxx | |||||
| Title: | President and Chief Executive Officer | Title: | Head of Sales and Marketing | |||||
| Date: 1-28-2207 | Date: January 19th, 2007 | |||||||
-114-
Exhibits
| Exhibit A: | Transfer Price |
| Exhibit B: | VR1-Ligands |
| Exhibit C: | Patch Specifications |
| Exhibit D: | Active Ingredient Specifications |
| Exhibit E: | Quality Assurance Agreement |
| Exhibit F: | LTS Liability Insurance |
| Exhibit G: | Maximum Capacity |
| Exhibit H: | List of Patents/Patent Applications |
| Exhibit I: | Stability Testing |
-115-
EXHIBIT A
TRANSFER PRICE
The prices represent delivery in bulk, printing on front side of the pouch in [***] colours and lacquer and backside as random print in one colour.
Above [***] patches [***] : [***] / Patch
Above [***] patches [***] : [***] / Patch
Above [***] patches [***] : [***] / Patch
Above [***] patches [***] : [***] / Patch
Above [***] patches [***] : [***] / Patch
All prices are Ex Works Andernach plus prospective legal taxes.
The minimum quantity per label version is [***] pieces each.
Annual minimum volume: [***] Patches
Product description:
Patch Size: [***]
Consisting of: [***]
Coated with: [***]
Covered by: [***]
Punched: [***]
Packed in: [***]
Theoretical Batch size: [***] Patches based on mass preparation of [***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-116-
EXHIBIT B
VR1-LIGANDS
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-117-
EXHIBIT C
PATCH SPECIFICATIONS
[Information in the Patch Specifications will be updated with information from final
marketing approval including packaging information. Specifications will also include
compliance with GMP and all applicable laws and regulations]
-118-
EXHIBIT D
ACTIVE INGREDIENT SPECIFICATIONS
[to be attached]
-119-
EXHIBIT E
QUALITY ASSURANCE AGREEMENT
[to be attached]
-120-
EXHIBIT F
LTS LIABILITY INSURANCE
-121-
| Versicherungsbestätigung | Confirmation of Insurance | |
| Art der Versicherung
Betriebs-Haftpflichtversicherung inkl. Produkthaftpflicht (Grundvertrag)
|
Type of Cover
General Liability, including products liability (Primary) | |
| Summen-Anschlußversicherungen | Excess-Layers | |
| Versicherungsschein Nr.
[***] |
Policy No.
[***] | |
| Versicherungenehmer | Named insured | |
| LTS Xxxxxxx Therapie-Systeme XXXxxxxxxxxx. 0 00000 Xxxxxxxxx |
||
| Versicherungsbeginn
[***] |
Inception
[***] | |
| Versicherungsablauf
[***] Uhr MEZ Der Verträge verlängern sich still-schweigend um ein Jahr, sofem diese nicht unter Einhaltung einer drei-monatigen Kon-digungsfrist vor dem Ablauf von einem der Vertragspartelen schriftlich gekündigt werden. |
Expiry
[***] The policies will be automatically renewed for a further year, unless one of the contracting parties gives written notice three months before the date of expiration | |
| Örtlicher Geltungsbereich
[***] |
Policy Territory
[***] | |
| Deckungssummen
[***] |
Limits of Indemnity
[***] | |
| Betriebshaftpflicht EUR 10.000.000,00
[***] |
General Liability
[***] | |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-122-
| Rechts - und Gerichtsstand
[***] |
Governing Law and Jurisdiction
. [***] | |
| Hinweis
[***] |
Annotation
[***] | |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-123-
| Bestätigung der Versicherer:
[***] |
Confirmation of Insurer:
[***] | |
| XL Insurance Company Ltd. Direction of Germany München |
||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-124-
| Bestätigung der Versicherer:
[***] |
Confirmation of Insurer:
[***] | |
| Xxxxxxx Allgemeine Versicherungs-AG Düsseldorf |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-125-
EXHIBIT G
MAXIMUM CAPACITY
The maximum capacity is [***] Patches per year.
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-126-
EXHIBIT H
LIST OF PATENTS/PATENT APPLICATIONS
Mikroreservoirsystem
Familie: 19930340
| Country | EP | WO | File-No | Priority date | Patent-No | granted | Status | |||||||
| AR P | [***] | [***] | [***] | applied | ||||||||||
| AT P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| AU P | WO | [***] | [***] | [***] | 11.11.04 | granted | ||||||||
| BE P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| BR P | WO | [***] | [***] | [***] | applied | |||||||||
| CA P | WO | [***] | [***] | [***] | applied | |||||||||
| CH P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| CN P | WO | [***] | [***] | [***] | applied | |||||||||
| CZ P | WO | [***] | [***] | [***] | applied | |||||||||
| DE P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| DK P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| EP P | WO | [***] | [***] | [***] | 12.03.03 | granted | ||||||||
| ES P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| FI P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| FR P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| GB P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| GR P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| HK P | WO | [***] | [***] | [***] | applied | |||||||||
| HU P | WO | [***] | [***] | [***] | applied | |||||||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-127-
| IE P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| IL P | WO | [***] | [***] | [***] | applied | |||||||||
| IN P | WO | [***] | [***] | [***] | applied | |||||||||
| IT P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| JP P | WO | [***] | [***] | [***] | applied | |||||||||
| KR P | WO | [***] | [***] | [***] | applied | |||||||||
| LU P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| MX P | WO | [***] | [***] | [***] | applied | |||||||||
| NL P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| NZ P | WO | [***] | [***] | [***] | 06.10.03 | granted | ||||||||
| PH P | [***] | [***] | [***] | applied | ||||||||||
| PL P | WO | [***] | [***] | [***] | applied | |||||||||
| PT P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| RU P | WO | [***] | [***] | [***] | applied | |||||||||
| SE P | EP | WO | [***] | [***] | [***] | 12.03.03 | granted | |||||||
| TR P | WO | [***] | [***] | [***] | 22.07.02 | granted | ||||||||
| TW P | [***] | [***] | [***] | applied | ||||||||||
| ZA P | WO | [***] | [***] | [***] | 24.12.02 | granted | ||||||||
| DE P | [***] | [***] | [***] | 13.06.02 | granted | |||||||||
| TW P | [***] | [***] | [***] | applied | ||||||||||
| US P | WO | [***] | [***] | [***] | applied | |||||||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-128-
Capsaicinpflaster
Familie: 2003/103
| Country | EP | WO | File-No | Priority date | Patent-No | granted | Status | |||||||
| WO P |
[***] | [***] | [***] | applied | ||||||||||
| US P |
[***] | [***] | [***] | applied | ||||||||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-129-
EXHIBIT I
STABILITY TESTING PROGRAM
[To be completed and attached]
-130-
CONFIDENTIAL
June 5, 2009
LTS XXXXXXX Therapie-Systeme XX
Xxxxxxxxxxxxxx 0
X-00000, Xxxxxxxxx, Xxxxxxx
Attn: Xxxxxxx Xxxxxxxxxxx
| Re: | Amendment 1 to the Commercial Supply and License Agreement |
Dear Klaudia:
The following sets forth certain amendments to the Commercial Supply and License Agreement entered into by LTS XXXXXXX Therapie-Systeme AG (“LTS”) and NeurogesX, Inc. (“NeurogesX”) of January 28, 2007 (the “Agreement”), as agreed to by the Parties as of the date set forth in the signature line below. Unless otherwise defined in this letter, all terms shall have the meaning given to such terms in the Agreement.
| • | LTS and NeurogesX hereby agree to extend the term of the Agreement until the expiration of the last patent within the Commercialization Patents covering the Patch. Accordingly, Section 11.1 of the Agreement shall be amended to read as follows: |
“11.1 Unless terminated in accordance with this Article 11, this Agreement shall continue in effect on a country-by-country basis until the expiration, revocation or invalidation of the last patent within the Commercialization Patents that covers the Patch in such country. The term shall be automatically renewed for additional two (2) year terms, unless terminated by either Party with two (2) years prior written notice.”
| • | LTS and NeurogesX hereby agree to amend the Agreement to permit the survival of sublicenses granted by NeurogesX to its Sublicensees. Accordingly, The following shall be added to the Agreement as Section 11.5(d): |
“11.5(d) Survival of Sublicenses. Upon termination of this Agreement for any reason, any sublicense granted by NeurogesX to a Sublicensee in accordance with Section 6.5 shall survive, and such Sublicensee shall be entitled to continue to purchase Patches from LTS under the terms of the Agreement, provided that upon request by LTS, each Sublicensee shall promptly agree in writing to be bound by the applicable terms of this Agreement.
Unless otherwise specifically amended herein, all terms of the Agreement shall remain as set forth in the Agreement. Please sign below to indicate LTS’ agreement with the foregoing.
| Very truly yours, |
| /s/ Xxxxxxx X. Xxxxxxxxx |
| Xxxxxxx X. Xxxxxxxxx Chief Financial Officer |
| LTS XXXXXXX THERAPIE-SYSTEME AG | ||||||||
| By: | /s/ X. Xxxxxxx | By: | /s/ X. Xxxxxxxxxxx | |||||
| X. Xxxxxxx, General Counsel | K. Kaczkiewicz, Head of Business Development | |||||||
| Date: | June 05, 2009 | Date: | June 05, 2009 | |||||
EXHIBIT 1.40
PATENT RIGHTS
[***]:
| Title |
Region/ Country |
Status | Application Number |
File Date |
Patent Number |
Grant Date |
Designated Countries |
Inventors | Owner | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
| Title |
Region/ Country |
Status | Application Number |
File Date |
Patent Number |
Grant Date |
Designated Countries |
Inventors | Owner | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
|
[***]:
|
||||||||||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-134-
EXHIBIT 1.44
PRODUCT TRADEMARK AND ALTERNATIVE TRADEMARK
|
Xxxx |
Country |
Status | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| [***] |
[***] | [***] | ||
| * | European Community Trademark Countries: [***]. |
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 1.48
TERRITORY
| A. | European Region |
European Union (present and future member states):
Austria
Belgium
Bulgaria
Cyprus
Czech Republic
Denmark
Estonia
Finland
France
Germany
Greece
Hungary
Ireland
Italy
Latvia
Lithuania
Luxembourg
Malta
Netherlands
Poland
Portugal
Romania
Slovakia
Slovenia
Spain
Sweden
United Kingdom
Norway
Iceland
Liechtenstein
Switzerland
Andorra
Monaco
San Marino
Vatican City
| B. | SATR Region |
South Africa
Turkey
Russia
| C. | Rest of Territory Region |
Armenia
Azerbaijan
Belarus
Georgia
Kazakhstan
Kyrgyzstan
Moldova
Tajikistan
Turkmenistan
Ukraine
Uzbekistan
Albania
Bosnia & Herzegovina
Croatia
Kosovo
Macedonia
Montenegro
Serbia
Republic Srpska
Bahrain
Gaza Strip
[***]
Iraq
Xxxxxx
Xxxxxx
Xxxxxx
Xxxxxxx
Xxxx
Xxxxx
Xxxxx Xxxxxx
[***]
United Arab Emirates
West Bank (Palestine)
Yemen
Algeria
Egypt
Libya
Morocco
Tunisia
Benin
Cameroon
Xxxx
Xxxxx Coast
Guinea
Mali
Mauritania
Niger
Senegal
Togo
Angola
Burkina Faso
Mozambique
Botswana
Central African Republic
Congo
Democratic Republic of Congo
Ethiopia
Gabon
Ghana
Kenya
Lesotho
Liberia
Madagascar
Mauritius
Namibia
Nigeria
Somalia
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-137-
[***]
Swaziland
Tanzania
Zambia
Zimbabwe
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-138-
EXHIBIT 1.51
UC LICENSE AGREEMENT
EXCLUSIVE LICENSE AGREEMENT
between
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA
and
NEUROGESX, INC.
for
HIGH DOSE CAPSAICIN FOR NEUROPATHIC PAIN
UCSF Case No. SF00-056
UC Case No. SF00-056
EXCLUSIVE LICENSE AGREEMENT
For
HIGH DOSE CAPSAICIN FOR NEUROPATHIC PAIN
This license agreement (the “Agreement”) is made effective November 1, 2000 (the “Effective Date”) between THE REGENTS OF THE UNIVERSITY OF CALIFORNIA, a California corporation having its statewide administrative offices at 0000 Xxxxxxxx Xxxxxx, Xxxxxxx, Xxxxxxxxxx 00000-0000, (“The Regents”), and acting through its Office of Technology Management, University of California San Francisco, 1294 Ninth Avenue – Xxxxx 0, Xxx 0000, Xxx Xxxxxxxxx, XX 00000-0000 (“UCSF”), and NEUROGESX, INC., a Delaware corporation having a principal place of business at 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000, Xxx Xxxxx, Xxxxxxxxxx, (the “Licensee”).
BACKGROUND
A. Certain inventions, generally characterized as “High Dose Capsaicin Relieves Neuropathic Pain” (collectively the “Invention”), were made in the course of research at the University of California San Francisco by Xxxxxx X. Xxxxxxx as described in UCSF Case Number SF00-056, and are covered by Regents’ Patent Rights as defined below;
B. The Licensee has evaluated the Invention under a Secrecy Agreement with The Regents effective August 20, 1999 (U.C. Control No. 2000-20-0104);
E. The Licensee and The Regents have executed a Letter of Intent dated June 1, 2000 (U.C. Control No. 2000-30-0050) for the purpose of negotiating a license to Regents’ Patent Rights;
F. The Licensee wishes to obtain rights from The Regents for the commercial development, use, and sale of products from the Invention, and The Regents is willing to grant those rights so that the Invention may be developed to its fullest and the benefits enjoyed by the general public; and
G. The Licensee is a “small business firm” as defined in 15 U.S.C. §632;
H. Licensee recognizes and agrees that royalties due under this Agreement will be paid on both pending patent applications and issued patents;
In view of the foregoing, the parties agree:
1. DEFINITIONS
1.1 “Regents’ Patent Rights” means the Regents’ interest in any subject matter claimed in or covered by any of the following: Pending U.S. Patent Application Serial No. 08/746,207 entitled “Therapeutic Method with Capsaicin and Capsaicin Analogs” filed November 6, 1996, the
continuation-in-part U.S. Patent Application Serial No. 08/990,633 entitled “Transdermal Therapeutic Device and Method with Capsaicin and Capsaicin Analogs” filed December 15, 1997 and assigned to The Regents; and continuing applications thereof including divisions, substitutions, continuation-in-part applications (but only to the extent however, that claims in the continuation-in-part applications are entitled to the priority filing date of the foregoing applications); any patents issuing on said applications including reissues, reexaminations and extensions; and any corresponding foreign applications or patents based on said applications.
1.2 “Licensed Product” means any material for which the manufacture, use, sale, or import would constitute an infringement of a Valid Claim within the Regents’ Patent Rights if not for the license granted to the Licensee under this Agreement.
1.3 “Licensed Method” means any method for which the use or sale would constitute an infringement of a Valid Claim within the Regents’ Patent Rights if not for the license granted to the Licensee under this Agreement.
1.4 “Net Sales” means the total of the gross invoice prices of Licensed Products sold or Licensed Methods performed by the Licensee, an Affiliate, or a Sublicensee, less the sum of the following actual and customary deductions where applicable: cash, trade, or quantity discounts; sales, use, tariff, import/export duties or other excise taxes imposed on particular sales; transportation charges and allowances (including insurance); or credits to customers because of rejections, returns, or expired goods. For purposes of calculating Net Sales, transfers to an Affiliate or Sublicensee for end use by the Affiliate or Sublicensee will be treated as sales to an independent third party for purposes of calculating earned royalties (as specified in Article 7)
1.5 “Affiliate” means any corporation or other business entity in which the Licensee owns or controls, directly or indirectly, at least fifty percent (50%) of the outstanding stock or other voting rights entitled to elect directors, or in which the Licensee is owned or controlled directly or indirectly by at least fifty percent (50%) of the outstanding stock or other voting rights entitled to elect directors; but in any country where the local law does not permit foreign equity participation of at least fifty percent (50%), then an “Affiliate” includes any company in which the Licensee owns or controls or is owned or controlled by, directly or indirectly, the maximum percentage of outstanding stock or voting rights permitted by local law.
1.6 “Field of Use” means all fields and uses.
1.7 “Commercial Sale” means with respect to each Licensed Product in each country, a bona fide commercial sale of such Licensed Product following marketing approval by the regulatory authority in such country; provided that where such a first commercial sale has occurred in a country for which government pricing or government reimbursement approval is needed for widespread commercial sale (for clarification, the parties acknowledge that no such approval is required in the United States) then a sale shall not be deemed a Commercial Sale until such pricing or reimbursement approval has been obtained.
-142-
1.8 “IND” means an Investigational New Drug Application, as defined in the U.S. Federal Food, Drug and Cosmetic Act and the regulations promulgated thereunder, or comparable filing in a foreign jurisdiction, in each case with respect to a Licensed Product.
1.9 “NDA” means a New Drug Application, as defined in the U.S. Food, Drug and Cosmetic Act and the regulations promulgated thereunder, and all subsequent supplements to that NDA, as well as any equivalent foreign application, registration or certification in the relevant country, such as a Marketing Approval Application (“MAA”) in Europe, in each case with respect to a Licensed Product.
1.10 “Phase I” means a clinical trial involving the initial introduction of a Licensed Product into humans and which is designed to determine the metabolism and pharmacologic actions of the Licensed Product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness.
1.11 “Phase III” means human clinical trials of a Licensed Product performed after obtaining preliminary evidence suggesting effectiveness of the drug, and are intended to gather the additional information about effectiveness and safety that is needed to evaluate the overall benefit-risk relationship of the drug, to provide an adequate basis for physician labeling, and to form the basis for approval to market such Licensed Product.
1.12 “Valid Claim” means a claim of an issued and unexpired patent or a claim of a pending patent application within the Regents’ Patent Rights which has not been held invalid or unenforceable by a court or other government agency of competent jurisdiction from which no appeal can be or has been taken, and has not been admitted to be invalid or unenforceable through re-examination, disclaimer or otherwise; provided that if a claim of a pending application has not issued as a claim of an issued patent within the Regents’ Patent Rights within ten (10) years after the filing date from which such claim takes priority, such pending claim shall not be a Valid Claim for purposes of this Agreement until such time as such claim issues.
1.13 “Sublicensee” means, with respect to a particular Licensed Product, a third party to whom Licensee has granted a right or license to both make and sell such Licensed Product or practice the Licensed Method. As used in this Agreement, “Sublicensee” shall also include a non-Affiliate third party to whom Licensee has granted a right to distribute such Licensed Product, provided that such third party is responsible for some or all of the marketing and promotion of such Licensed Product within the territorial region in which it has such sublicensed rights.
2. LIFE OF PATENT EXCLUSIVE GRANT
2.1 Subject to the limitations set forth in this Agreement, The Regents grants to the Licensee a world-wide license under Regents’ Patent Rights to make, have made, use, sell, offer to sell, import and have imported Licensed Products, to practice Licensed Methods, and to otherwise exploit the Regents’ Patent Rights, and to have each of the foregoing performed by a third party on Licensee’s behalf.
-143-
2.2 Except as otherwise provided in this Agreement, the license granted in Paragraph 2.1 is exclusive for the life of this Agreement.
2.3 The license granted in Paragraphs 2.1 and 2.2 is limited to methods and products that are within the Field of Use. For other methods and products, the Licensee has no license under this Agreement.
2.4 To the extent it is legally able, The Regents also grants Licensee a non-exclusive license to any proprietary know-how relating to Regents’ Patent Rights that The Regents has an interest in and is reasonably needed by Licensee to practice and/or commercially develop Licensed Products and/or Licensed Methods.
2.5 The Regents reserves the nontransferable right to use the Invention and associated technology for its own bona fide non-commercial research and education purposes.
3. SUBLICENSES
3.1 The Regents also grants to the Licensee the worldwide right to issue sublicenses to third parties to make, have made, use, sell, offer to sell, import and have imported Licensed Products, to practice Licensed Methods, and to otherwise exploit the Regents’ Patent Rights, in each case as long as the Licensee has current exclusive rights thereto under this Agreement. Each sublicense shall be subordinate to the terms and conditions of this Agreement (including, if applicable, those regarding the U.S. Government’s and other sponsors’ rights and the rights reserved by The Regents).
3.2 In addition, Licensee shall pay The Regents a percentage of all sublicense fees (as defined below).
3.2.1 For purposes of this Agreement, “sublicense fees” shall consist of amounts received in the form of up-front fees and milestone payments as direct consideration for the grant of a sublicense for Licensed Products under the Regents’ Patent Rights, but shall not include any amounts received as royalties on sales of Licensed Products, as support for research and development activities, as a loan, as income derived from debt financing, for the purchase of an equity interest in the Licensee, as reimbursement for patent and patent related expenses, as earned royalties on net sales, or as consideration for the grant of intellectual property rights and materials other than those claimed under Regents’ Patent Rights. Licensee shall pay The Regents the following percentages of sublicense fees:
| Percentage |
Aggregate Sublicense Fees | |
| 25% |
< $250,000 | |
| 10% |
$250,000 — $2,000,000 | |
| 5% |
> $2,000,000 |
-144-
3.2.2 For purposes of calculating the applicable percentage for the foregoing, “Aggregate Sublicense Fees” shall be the cumulative amount of sublicense fees stated in each sublicense of Regents’ Patent Rights entered into by Licensee and assuming that all milestone payments therein are made to Licensee. However, the actual sublicensee fee payments due The Regents from Licensee shall be determined based on the actual amounts of sublicensee fees received by Licensee in each calendar year during the term of this Agreement.
3.3 The Licensee shall promptly provide The Regents with a copy of each sublicense issued; collect all payments due The Regents from Sublicensees; and summarize and deliver all reports due The Regents from Sublicensees, provided that the Licensee may redact from such copy any terms which are not necessary to determine whether Licensee has complied with its obligations under this Agreement.
3.4 Upon termination of this Agreement for any reason, any sublicense granted by Licensee hereunder shall survive, provided that upon request by The Regents, each Sublicensee promptly agrees in writing to be bound by the applicable terms of this Agreement..
4. PAYMENT TERMS
4.1 Licensee shall pay to The Regents earned royalties (as specified in Section 7) on Net Sales of Licensed Products on a Licensed Product-by-Licensed Product and country-by-country basis. The earned royalty due pursuant to this Section will continue on such basis until the manufacture, use or sale of a Licensed Product in such country does not infringe a Valid Claim within the Regents’ Patent Rights.
4.2 Licensee shall pay earned royalties quarterly on or before February 28, May 31, August 31 and November 30 of each calendar year. Each payment will be for earned royalties accrued within the Licensee’s most recently completed calendar quarter.
4.3 Licensee shall pay the applicable percentage of sublicense fees to The Regents on or before February 28 of each calendar year. Each payment will be for sublicensee fees received by Licensee during the most recently completed calendar year.
4.4 [Intentionally left blank]
4.5 All monies due The Regents are payable in United States dollars. When Licensed Products are sold for monies other than United States dollars, the Licensee shall first determine the applicable earned royalty in the currency of the country in which Licensed Products were sold and then convert the amount into equivalent United States funds, using the exchange rate quoted in the Wall Street Journal on the last business day of the reporting period. In no event shall the Licensee be required to pay The Regents more than twenty-five (25%) of its total annual revenue derived from Licensed Products.
4.6 Earned royalties earned on sales occurring in any country outside the United States may not be reduced by any taxes, fees, or other charges imposed by the government of such country on the payment of royalty income. The Licensee is also responsible for all bank transfer charges. Notwithstanding this, all payments made by the Licensee in fulfillment of The Regents’ tax liability in any particular country will be credited against earned royalties or fees due The Regents for sales of Licensed Products in that country.
-145-
4.7 If at any time legal restrictions prevent the prompt remittance of earned royalties by the Licensee from any country where a Licensed Product is sold, the Licensee shall deposit the amount owed in an interest-bearing account within that country until such time as the restrictions are lifted, at which time the Licensee shall promptly convert the current balance of said account into United States funds and pay such amount to The Regents.
4.8 If any patent or Valid Claim within Regents’ Patent Rights is held invalid in a final decision by a court of competent jurisdiction and last resort and from which no appeal has or can be taken, all obligation to pay earned royalties based on that patent or Valid Claim or any Valid Claim patentably indistinct therefrom will cease as of the date of final decision. The Licensee will not, however, be relieved from paying any earned royalties that accrued before the final decision or that are based on another patent within Regents’ Patent Right or Valid Claim not involved in the final decision.
4.9 If payments, rebillings or fees are not received by The Regents when due, the Licensee shall pay to The Regents interest charges on the unpaid amount at a rate often percent (10%) per annum or the maximum permitted by law, whichever is less. Interest is calculated from the date due until actually received by The Regents.
5. LICENSE-ISSUE FEE
5.1 Within sixty (60) days after the Effective Date, the Licensee shall pay to The Regents a license-issue fee equal to the total amount of out-of-pocket costs and expenses of patent prosecution of the Regents’ Patent Rights incurred by The Regents through the Effective Date. This fee is non-refundable, non-cancelable, and is not an advance against earned royalties.
6. LICENSE MAINTENANCE FEE
6.1 [Intentionally left blank]
7. EARNED ROYALTIES, MINIMUM ANNUAL ROYALTIES AND MILESTONE PAYMENTS
7.1 The Licensee shall also pay to The Regents an earned royalty of one-half percent (0.5%) of the Net Sales of Licensed Products worldwide, up to a maximum of one million dollars ($1,000,000) per year.
7.2 If it becomes necessary for Licensee to license intellectual property rights from an unaffiliated third party, and Licensee is required to pay a royalty to that unaffiliated third party in order to practice Licensed Methods and make, use or sell Licensed Products, and the combined earned royalty due The Regents and unaffiliated third parties exceeds eleven percent (11%), then the earned royalties to be paid to The Regents by Licensee shall be reduced by an amount equal to one-half (1/2) the excess over eleven percent (11%) of the royalty rate(s) due to the unaffiliated third party(ies). However, in no event shall the amount paid to The Regents be reduced below fifty percent (50%) of the original earned royalty otherwise due The Regents.
-146-
7.3 No cumulation of earned royalties shall be made in the event a Licensed Product is covered by Valid Claims of more than one patent within Regents’ Patent Rights.
7.4 No earned royalty shall be payable under this Article 7 with respect to transfers of Licensed Product to Affiliates, Sublicensees, customers or prospective customers for use in research and/or development, in clinical trials or other regulatory purposes, as samples, or in any other transfer that is not a Commercial Sale.
7.5 In the event that a Licensed Product is sold in combination with other products, components or services (other than another Licensed Product having therapeutic activity) for which no amounts would be payable to The Regents if such other products, components or services having therapeutic activity were sold or performed separately, amounts invoiced for such combination sales for purposes of calculating Net Sales of the Licensed Product in such combination shall be the Net Sales of the Licensed Product calculated by multiplying the Net Sales of the combination product by the fraction A/B where A is the average price on a unit basis of Licensed Product containing a certain amount (by weight) of active ingredient, and B is the average unit price of the combination product containing the same amount of that active ingredient. In the event that such average sale price cannot be determined for both the Licensed Product and the other product(s) in combination, Net Sales for purposes of determining royalty payment shall be mutually agreed by the parties based on relative value contributed by each component, and such agreement shall not be unreasonably withheld.
7.6 [Intentionally left blank]
7.7 Licensee shall pay The Regents non-creditable milestone payments should either of the following occur while Commercial Sales or commercialization of a Licensed Product by Licensee are continuing: (i) the closing of an Initial Public Offering (“IPO”) of the Licensee’s common stock pursuant to a registration statement on Form S-1 filed with the U.S. Securities and Exchange Commission; or (ii) the closing of (x) a consolidation or merger of the Licensee with any other entity, (y) sale of all or substantially all of the assets of Licensee, or (z) sale of all or substantially all of the outstanding stock of the Licensee, pursuant to which the shareholders of the Licensee receive cash or publicly traded securities. Upon the occurrence of such event, the Licensee shall pay to The Regents total cash payments equal to X times P, where X is equal to 16,400 stock equivalents (which number of stock equivalents shall be subject to adjustment from time to time for any stock split, stock dividends, recapitalizations a the like) and P is equal to either the IPO offering price per share of common stock, or in the case of a merger, acquisition, or sale, the average price per share actually received by shareholders of the Licensee’s common stock from the acquirer. Such payments shall be paid in three (3) installments, with one third (1/3) of the total to be paid twelve (12) months after the closing date of such transaction, one third (1/3) to be paid twenty four (24) months after such closing date, and the remaining one third (1/3) to be paid thirty six (36) months after such closing date. In the event that the transaction contemplated hereunder involves more than one closing date, the due date for payments owed to The Regents shall be measured from the date of the last closing.
-147-
7.8 Notwithstanding the foregoing Section 7.7, no milestone payments shall be due from Licensee under Section 7.7 in the event that: (i) a patent with a claim covering a Licensed Product has not issued under the Regents’ Patent Rights at the time such milestone payment is due; and (ii) The Regents is no longer funding active prosecution of the Regents’ Patent rights in the United States patent office or a foreign equivalent; and (iii) the first Commercial Sale of a Licensed Product has already occurred.
8. DUE DILIGENCE
8.1 The Licensee, on execution of this Agreement, shall use commercially reasonable efforts to develop, manufacture and sell Licensed Products, or cause a Sublicensee to do so, and shall use the same level of effort to market the same within a reasonable time after execution of this Agreement.
8.2 The Licensee shall use commercially reasonable efforts to obtain all governmental approvals necessary for the manufacture, use and sale of Licensed Products.
8.3 The Licensee shall commit to use commercially reasonable efforts (either itself or through a Sublicensee) to develop and commercialize at least one Licensed Product, including achievement of the following milestones: (i) dosing of the first human patient with a Licensed Product in support of a Phase I clinical trials by the end of the third year after the Effective Date; (ii) filing at least one NDA by November 1, 2009; and (iii) commencing the marketing of at least one Licensed Product in one country by November 1, 2011.
8.4 If the Licensee fails to complete any of the above provisions, and The Regents does not otherwise extend or waive such period of performance, then The Regents has the right and option to terminate this Agreement pursuant to Paragraph 12.1 or, upon consent of Licensee, reduce the Licensee’s exclusive license to a nonexclusive license. This right, if exercised by The Regents, supersedes the rights granted in Article 2 (GRANT).
9. PROGRESS AND ROYALTY REPORTS
9.1 Beginning February 28, 2002 and semi-annually thereafter, the Licensee shall submit to The Regents a progress report covering the Licensee’s (and any Affiliate or Sublicensee’s) activities regarding the development and testing of all Licensed Products and the obtaining of any governmental approvals necessary for marketing. Progress reports are required until the first Commercial Sale of the first Licensed Product occurs in the United States, at which time the Licensee may discontinue such reports; provided that such reports may again be required by The Regents if Commercial Sales of such Licensed Product are suspended or discontinued.
9.2 Progress reports submitted under Paragraph 9.1 shall include, but are not limited to, the following topics, as they specifically refer to Licensed Products:
| • | summary of work completed |
| • | key scientific discoveries |
| • | summary of work in progress |
| • | current schedule of anticipated events or milestones |
-148-
| • | market plans for introduction of Licensed Products, and |
| • | a summary of resources (dollar value) spent in the reporting period. |
9.3 The Licensee has a continuing obligation to keep The Regents’ informed of the large/small business entity status (as defined by the United States Patent and Trademark Office) of itself and its Sublicensees and Affiliates.
9.4 The Licensee shall report to The Regents the date of first Commercial Sale of a Licensed Product in each country in its immediately subsequent royalty report (as described below).
9.5 After the first Commercial Sale of a Licensed Product anywhere in the world, the Licensee shall make quarterly royalty reports to The Regents on or before each February 28, May 31, August 31 and November 30 of each year. Each royalty report will cover the Licensee’s most recently completed calendar quarter and will show (a) the gross sales and Net Sales of Licensed Products sold during the most recently completed calendar quarter; (b) the number of each type of Licensed Product sold; (c) the earned royalties, in U.S. dollars, payable with respect to sales of Licensed Products; (d) the method used to calculate the earned royalties; and (e) the exchange rates used (if applicable).
9.6 If no sales of Licensed Products have been made during any reporting period, Licensee shall provide a statement to this effect.
10. BOOKS AND RECORDS
10.1 The Licensee shall keep accurate books and records showing all Licensed Products manufactured, used, and/or sold under the terms of this Agreement. Books and records must be preserved for at least five (5) years from the date of the royalty payment to which they pertain.
10.2 The Regents is entitled to have a mutually acceptable independent auditor under a duty of confidentiality inspect these books and records during regular office hours for the purpose of verifying the payment of earned royalties and sublicense payments due hereunder with respect to Net Sales and sublicense fees received by Licensee not more than five (5) years prior to the date of The Regents’ request. The Regents may exercise its right to review Licensee’s books and records not more than once per calendar year and must provide Licensee at least fourteen (14) days advance written notice. The auditor shall only report to The Regents the amount of under or over payment of earned royalties and sublicense fees, if any. The Regents shall bear the fees and expenses of examination but if an error in royalties of more than five percent (5%) of the total royalties due for any year is discovered in any examination then the Licensee shall bear the fees and expenses of that examination. The information learned by The Regents pursuant to this Paragraph 10.2 or contained in reports provided to The Regents under Article 9 above shall be deemed Data of Licensee for purposes of Article 29 below.
11. LIFE OF THE AGREEMENT
11.1 Unless otherwise terminated by operation of law or by acts of the parties in accordance with the terms of this Agreement, this Agreement will be in force from the Effective Date and shall expire on a country-by-country basis as to each Licensed Product on the date that
-149-
neither the sale nor use of such Licensed Product would be covered by a Valid Claim in such country. This Agreement shall expire in its entirety, unless otherwise terminated or otherwise provided herein, upon the last expiration date of a patent licensed under this Agreement; or until the last patent application licensed under this Agreement is abandoned and no patent in Regents’ Patent Rights ever issues.
11.2 Any termination of this Agreement will not affect the rights and obligations set forth in the following Articles:
| Article 1 | Definitions | |
| Paragraph 3.4 | Survival of Sublicenses | |
| Article 10 | Books and Records | |
| Article 14 | Disposition of Licensed Products on Hand on Termination | |
| Article 15 | Use of Names and Trademarks | |
| Article 20 | Indemnification | |
| Article 24 | Failure to Perform | |
| Article 29 | Secrecy | |
| Article 30 | Miscellaneous |
12. TERMINATION BY THE REGENTS
12.1 If the Licensee fails to materially perform or materially violates any term of this Agreement, then The Regents may give written notice of default (“Notice of Default”) to the Licensee. If the Licensee fails to repair the default within sixty (60) days of the effective date of Notice of Default, The Regents may terminate this Agreement and its licenses by a second written notice (Notice of Termination). However, if Licensee disputes the alleged breach within the cure period, then The Regents shall not have the right to terminate this Agreement unless it has been determined by an arbitrator or court proceeding that this Agreement was materially breached, and Licensee fails to comply with its obligations hereunder within sixty (60) days after that determination. If a properly given Notice of Termination is sent to the Licensee, this Agreement will automatically terminate on the effective date of such Notice. Termination will not relieve the Licensee of its obligation to pay any fees owing at the time of termination and will not impair any accrued right of The Regents. These notices are subject to Article 21 (Notices).
13. TERMINATION BY LICENSEE
13.1 The Licensee has the right at any time to terminate this Agreement in whole or as to any portion of Regents’ Patent Rights by giving notice in writing to The Regents. Notice of termination will be subject to Article 21 (Notices) and termination of this Agreement will be effective sixty (60) days from the effective date of notice.
13.2 Any termination under the above paragraph does not relieve the Licensee of any obligation or liability accrued under this Agreement prior to termination or rescind any payment made to The Regents or anything done by Licensee prior to the time termination becomes effective. Termination does not affect in any manner any rights of The Regents arising under this Agreement prior to termination.
-150-
14. DISPOSITION OF LICENSED PRODUCTS ON HAND UPON TERMINATION
14.1 Upon termination of this Agreement the Licensee is entitled to dispose of all previously made or partially made Licensed Products, but no more, within a period of one hundred and eighty (180) days provided that the sale of those Licensed Products is subject to the terms of this Agreement, including but not limited to the rendering of reports and payment of royalties required under this Agreement
15. USE OF NAMES AND TRADEMARKS
15.1 Nothing contained in this Agreement confers any right to use in advertising, publicity, or other promotional activities any name, trade name, trademark, or other designation of either party hereto (including contraction, abbreviation or simulation of any of the foregoing). Unless required by law, the use by the Licensee of the name “The Regents of the University of California” or the name of any campus of the University of California is prohibited.
16. LIMITED WARRANTY
16.1 The Regents, as represented by the actual knowledge of the undersigned on behalf of the Regents as of the Effective Date, warrants to the Licensee that that it owns all right, title, and interest in and to the Regents’ Patent Rights, and that it has the lawful right to grant this license.
16.2 This license and the associated Invention are provided WITHOUT WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED. THE REGENTS MAKES NO REPRESENTATION OR WARRANTY THAT THE LICENSED PRODUCTS OR LICENSED METHODS WILL NOT INFRINGE ANY PATENT OR OTHER PROPRIETARY RIGHT OF A THIRD PARTY.
16.3 IN NO EVENT WELL EITHER PARTY BE LIABLE TO THE OTHER FOR ANY INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES RESULTING FROM EXERCISE OF THIS LICENSE OR THE USE OF THE INVENTION OR LICENSED PRODUCTS.
16.4 This Agreement does not:
16.4.1 express or imply a warranty or representation as to the validity or scope of any of Regents’ Patent Rights;
16.4.2 express or imply a warranty or representation that anything made, used, sold, offered for sale or imported or otherwise disposed of under any license granted in this Agreement is or will be free from infringement of patents of third parties;
16.4.3 obligate The Regents to bring or prosecute actions or suits against third parties for patent infringement except as provided in Article 19;
16.4.4 confer by implication, estoppel or otherwise any license or rights under any patents of The Regents other than Regents’ Patent Rights as defined in this Agreement, regardless of whether those patents are dominant or subordinate to Regent’s Patent Rights; or
-151-
16.4.5 obligate The Regents to furnish any know-how not provided in Regents’ Patent Rights.
17. PATENT PROSECUTION AND MAINTENANCE
17.1 As long as the Licensee has paid patent costs as provided for in this Article, The Regents shall diligently prosecute and maintain the United States and foreign patents comprising Regents’ Patent Rights using counsel of its choice, reasonably acceptable to Licensee. If Licensee objects to the choice of patent counsel, then The Regents shall appoint an alternative choice of patent counsel with Licensee’s approval. The Regents shall provide the Licensee with copies of all relevant documentation and correspondence so that the Licensee may be informed of the continuing prosecution and submit timely comments and guidance for consideration by patent counsel. The Licensee agrees to keep such correspondence and documentation confidential. The Regents agrees to allow Licensee to submit advice, counsel, and guidance regarding patent prosecution to The Regents’ counsel, although such counsel will take instructions only from The Regents. All patents and patent applications under this Agreement which are based solely on research performed at UCSF and filed prior to the Execution Date will be assigned solely to The Regents, subject to the grant of exclusive rights hereunder.
17.2 The Regents shall use best efforts to amend any patent application to include claims reasonably requested by the Licensee to protect the products contemplated to be sold under this Agreement and shall ensure that the Licensee is kept apprised of patent-related activities so that it may provide such requests in a timely manner.
17.3 The Licensee shall, if appropriate in Licensee’s business judgment, apply for an extension of the term of any patent included within Regents’ Patent Rights under the Drug Price Competition and Patent Term Restoration Act of 1984 and/or European, Japanese and other foreign counterparts of this Law. If Licensee elects to apply for such extension, Licensee shall prepare all documents, and The Regents agrees to execute the documents and to take additional action as the Licensee reasonably requests in connection therewith.
17.4 If either party (in the case of the Regents, the actual knowledge of the Licensing Officer responsible for administration of this Agreement) receives notice from a third party pursuant to 21 CFR §314.95(a) stating that one or more patents within the Regents’ Patent Rights is invalid, unenforceable or not infringed by the manufacture, use or sale of a drug product for which such third party has submitted an ANDA under 21 CFR §§314.92–314.99 (or an equivalent notice with respect to a drug product for which such third party has submitted a marketing application under Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act), then that party shall notify the other party within ten (10) days after receipt of notice of infringement.
17.5 The Regents shall bear the costs of preparing, filing, prosecuting and maintaining all United States and foreign patent applications contemplated by this Agreement incurred after the Effective Date, and as required under Article 5 and Section 17.6.
17.6 The Licensee may request The Regents to obtain patent protection on the Invention in foreign countries if available and if it so desires. The Licensee shall notify The Regents of its
-152-
decision to obtain or maintain foreign patents not less than sixty (60) days prior to the deadline for any payment, filing, or action to be taken in connection therewith. This notice concerning foreign filing must be in writing and must identify the countries desired. The absence of such a notice from the Licensee to The Regents will be considered an election not to obtain or maintain foreign rights.
17.7 Licensee may terminate its obligations with respect to any given patent application or patent upon three (3) months written notice to The Regents. If such notice is received from the Licensee, The Regents may continue to prosecute and maintain such application(s) or patent(s) at its sole discretion and expense, but the Licensee will have no further right or licenses thereunder. If Licensee does not provide such a notice of termination as to any given patent application or patent within The Regents’ patent Rights, then The Regents’ obligation to underwrite and to pay patent prosecution costs will continue for so long as this Agreement remains in effect.
17.8 [Intentionally left blank]
18. PATENT MARKING
18.1 The Licensee shall xxxx all Licensed Products made, used or sold under the terms of this Agreement, or their containers, in accordance with the applicable patent marking laws.
19. PATENT INFRINGEMENT
19.1 If The Regents (to the extent of the actual knowledge of the licensing professional responsible for the administration of this Agreement) or the Licensee learns of the substantial infringement of any patent licensed under this Agreement, the knowledgeable party will provide the other (i) with written notice of such infringement and (ii) with any evidence of such infringement available to it (the “Infringement Notice”). Neither party will notify a third party of the infringement of any of Regents’ Patent Rights without first obtaining consent of the other party, which consent will not be unreasonably denied or delayed. Both parties shall use their best efforts in cooperation with each other to terminate infringement without litigation.
19.2 If the infringing activity has not been abated within ninety (90) days following the date the Infringement Notice takes effect, then the Licensee or its designee may institute suit for patent infringement against the infringer. The Regents may voluntarily join such suit at its own expense, but may not thereafter commence suit against the infringer for the acts of infringement that are the subject of the Licensee’s suit or any judgment rendered in the suit. The Licensee may not join The Regents in a suit initiated by Licensee without The Regents’ prior written consent. If, in a suit initiated by the Licensee, The Regents is involuntarily joined other than by the Licensee, then the Licensee will pay any out-of-pocket costs incurred by The Regents arising out of such suit, including but not limited to, legal fees of counsel that The Regents selects and retains to represent it in the suit. The Regents will join as a party to any suit initiated by Licensee if found to be a necessary party by a court of law.
19.3 If, within a hundred and eighty (180) days following a request by The Regents that Licensee take action to xxxxx the infringing activity, the infringing activity of potential commercial significance by the infringer has not been abated and the Licensee or it designee has not brought suit
-153-
against the infringer or begun negotiations regarding the terms under which Licensee would grant a sublicense to the infringer, then The Regents may institute such suit for patent infringement against the infringer. If The Regents institutes such suit, then the Licensee may not join such suit without The Regents’ consent and may not thereafter commence suit against the infringer for acts of infringement that are subject to The Regents’ suit or any judgment rendered in that suit. The Regents may not join Licensee in a suit initiated by The Regents without Licensee’s prior written consent.
19.4 Legal action as is decided on will be at the expense of the party bringing suit, but legal action brought jointly by The Regents and the Licensee and fully participated in by both will be at the joint expense of the parties. Any recovery or settlement received in connection with any suit will be applied as follows: (i) first to reimburse the controlling party for its out-of-pocket costs and expenses (including attorneys’ and court costs and expenses and amounts reimbursed to the other party); (ii) next to reimburse the out-of-pocket costs and expenses of the other party; and (iii) any remainder after reimbursing the amounts set forth in clause (i) and (ii) shall be retained by the controlling party; provided that if Licensee is the controlling party (or in the event that the action is jointly controlled), Licensee, while being entitled to retain any remainder of such recovery, agrees to pay The Regents fifteen percent (15%) of such remainder, or to the extent of enhanced damages twenty five percent (25%) of such remainder.
19.5 Each party shall cooperate with the other in litigation proceedings instituted hereunder but at the expense of the party bringing suit. Litigation will be controlled by the party bringing the suit.
20. INDEMNIFICATION
20.1 The Licensee shall indemnify, hold harmless and defend The Regents, its officers, employees, and agents; the sponsors of the research that led to the invention; and the inventors of the patents and patent applications in Regents’ Patent Rights and their employers against any and all claims, suits, losses, liabilities, damages, costs, fees, and expenses asserted by third parties (“Claims”) resulting from or arising out of exercise of this license or any voluntary sublicense, except to the extent resulting from a breach of this Agreement by The Regents; provided that Licensee shall have no obligations with respect to any Claims unless the person or entity claiming under this Paragraph 20.1 (i) promptly notifies Licensee in writing of such Claims, (ii) gives Licensee sole control of the defense and settlement thereof (provided that Licensee shall not admit to wrongdoing or liability on the part of The Regents without the prior written consent of The Regents), and (iii) provides Licensee, at Licensee’s expense, with reasonable assistance and full information with respect to such Claims. This indemnification includes, but is not limited to, any product liability. Notwithstanding the foregoing, the Licensee shall have no obligations for any claim if the person (as listed above) seeking indemnification makes any admission or settlement regarding such claim without the prior written consent of the Licensee, which consent shall not be unreasonably withheld.
20.2 The Licensee, at its sole cost and expense, shall insure its activities in connection with the work under this Agreement and obtain (not later than the start of Phase I clinical trials of a product intended for Commercial Sale), keep in force and maintain insurance as follows, or an equivalent program of self insurance.
-154-
20.3 Comprehensive or commercial form general liability insurance (contractual liability included) with limits as follows:
| • | Each Occurrence $1,000,000 |
| • | Products/Completed Operations Aggregate $5,000,000 |
| • | Personal and Advertising Injury $1,000,000 |
| • | General Aggregate (commercial form only) $5,000,000 |
The coverage and limits referred to under the above do not in any way limit the liability of the Licensee. The Licensee shall furnish The Regents with certificates of insurance showing compliance with all requirements. Certificates must:
| • | Provide for thirty (30) days’ advance written notice to The Regents of any modification. |
| • | Indicate that The Regents has been endorsed as an additional Insured under the coverage referred to under the above. |
| • | Include a provision that the coverage will be primary and will not participate with nor will be excess over any valid and collectable insurance or program of self-insurance carried or maintained by The Regents. |
20.4 The Regents shall notify the Licensee in writing of any claim or suit brought against The Regents in respect of which The Regents intends to invoke the provisions of this Article. The Licensee shall keep The Regents informed on a current basis of its defense of any claims under this Article.
21. NOTICES
21.1 Any notice or payment required to be given to either party is properly given and effective (a) on the date of delivery if delivered in person or (b) five (5) days after mailing if mailed by first-class certified mail, postage paid, to the respective addresses given below, or to another address as is designated by written notice given to the other party.
| In the case of the Licensee: | NeurogesX, Inc. 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000 Xxx Xxxxx, XX 00000 Attn: CEO | |||
| In the case of The Regents: | Office of Technology Management University of California San Francisco 0000 Xxxxx Xxxxxx - Xxx 0000 Xxx Xxxxxxxxx, XX 00000-0000 Attention: Director Referring to: XXXX Xxxx Xx. XX00-000 | |||
-155-
22. ASSIGNABILITY
22.1 This Agreement may be assigned by The Regents, but is personal to the Licensee and assignable by the Licensee only with the written consent of The Regents, which consent will not be unreasonably withheld; provided that the Licensee may assign this Agreement without The Regents’ consent to an entity that acquires substantially all of the business or assets of the Licensee (or that portion thereof to which this Agreement relates), in each case whether by merger, acquisition, or otherwise the acquiring party assumes this Agreement in writing or by operation of law.
23. NO WAIVER
23.1 No waiver by either party of any default of this Agreement may be deemed a waiver of any subsequent or similar default.
24. FAILURE TO PERFORM
24.1 If either party finds it necessary to undertake legal action against the other on account of failure of performance due under this Agreement, then the prevailing party is entitled to reasonable attorney’s fees in addition to costs and necessary disbursements.
25. GOVERNING LAWS
25.1 THIS AGREEMENT WILL BE INTERPRETED AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF CALIFORNIA, but the scope and validity of any patent or patent application will be governed by the applicable laws of the country of the patent or patent application.
26. PREFERENCE FOR UNITED STATES INDUSTRY
26.1 [Intentionally left blank]
27. GOVERNMENT APPROVAL OR REGISTRATION
27.1 Licensee shall notify The Regents if it becomes aware that this Agreement is subject to any U.S. or foreign government reporting or approval requirement. Licensee shall make all necessary filings and pay all costs including fees, penalties, and all other out-of-pocket costs associated with such reporting or approval process.
28. EXPORT CONTROL LAWS
28.1 The Licensee shall observe all applicable United States and foreign laws with respect to the transfer of Licensed Products and related technical data to foreign countries, including, without limitation, the International Traffic in Arms Regulations (ITAR) and the Export Administration Regulations.
-156-
29. SECRECY
29.1 If either party discloses confidential information (“Data”) to the other party, the disclosing party will designate this information as confidential by appropriate legend or instruction, e.g. “Confidential” or “Proprietary”, and the receiving party will:
29.1.1 not to use the Data except for the sole purpose of accomplishing the objectives of this Agreement;
29.1.2 to safeguard Data against disclosure to others with the same degree of care as it exercises with its own data of a similar nature, but in no event less than reasonable care;
29.1.3 not to disclose Data to others (except to its employees, agents, consultants, distributors, sublicensees, potential and actual investors and others who have a need to know and who are bound to the Licensee by a like obligation of confidentiality) without the express written permission of The Regents,
29.1.4 Neither party will have any confidentiality obligation with respect to Data belonging to or disclosed by the other party that:
(i) the receiving party can demonstrate by written records was previously known to it;
(ii) is now, or becomes in the future, public knowledge other than through acts or omissions of the receiving party;
(iii) is lawfully obtained by the receiving party from sources independent of the disclosing party;
(iv) is independently developed by the receiving party without use of the Data of the disclosing party; and
29.1.5 that the secrecy obligations of the parties with respect to Data will continue for a period ending five (5) years from the termination date of this Agreement.
29.1.6 The non-disclosure obligations contained in Article 29 shall not apply to particular Data that a receiving party received from the other party to the extent that such receiving party is required to disclose such Data by law, order or regulation of a governmental agency (including the California Public Records Act or governmental audit requirement) or a court of competent jurisdiction, provided that such receiving party first gives reasonable notice of such requirement to the disclosing party, removes the then actual trade secrets of the disclosing party upon request and as directed by the disclosing party to the extent permitted by law, and cooperates with the disclosing party in the event the disclosing party challenges the requirement and/or scope of such disclosure.
-157-
30. MISCELLANEOUS
30.1 The headings of the several sections are inserted for convenience of reference only and are not intended to be a part of or to affect the meaning or interpretation of this Agreement.
30.2 This Agreement is not binding on the parties until it has been signed below on behalf of each party. It is then effective as of the Effective Date.
30.3 No amendment or modification of this Agreement is valid or binding on the parties unless made in writing and signed on behalf of each party.
30.4 For the purposes of this Agreement and all services to be provided hereunder, the parties shall be, and shall be deemed to be, independent contractors and not agents or employees of either of the other parties. No party shall have authority to make any statements, representations or commitments of any kind, or to take any action which shall be binding on either of the other parties, except as may be expressly provided for herein or authorized in writing.
30.5 This Agreement embodies the entire understanding of the parties and supersedes all previous communications, representations or understandings, either oral or written, between the parties relating to the subject matter hereof. The Secrecy Agreement effective August 20, 1999 (U.C. Control No. 2000-20-0104) is hereby terminated.
30.6 In case any of the provisions contained in this Agreement is held to be invalid, illegal, or unenforceable in any respect, that invalidity, illegality or unenforceability will not affect any other provisions of this Agreement, and this Agreement will be construed as if the invalid, illegal, or unenforceable provisions had never been contained in it.
30.7 In the event that, within one (1) year after the Amendment Date, Licensee agrees, at its sole discretion, to accept an assignment or license of rights to US Patent Application Serial No. 08/746,207 from Messrs. Xxxxxx and Xxxxxxxxxx (or their executors, successors, or assigns), named inventors on such Application, such that Licensee has exclusive rights under such Application, The Regents and Licensee agree that the paragraphs 1-8, 10-12 of [Amendment Number One] shall be rescinded as of the date such assignment or license is perfected, and the original terms of the Agreement shall apply thereafter. In addition, Licensee shall be entitled to deduct its costs and expenses incurred with respect to such assignment or license, as well as any on-going royalty obligations, from any amounts due The Regents hereunder after the date such assignment or license is perfected.
[Signature Page Follows]
-158-
IN WITNESS WHEREOF, both The Regents and the Licensee have executed this Agreement, in duplicate originals, by their respective and duly authorized officers on the day and year written.
| LICENSEE | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | |||||||
| By: | /s/ Xxxx XxXxxxx |
By: | /s/ Xxxx X. Xxxxxxxxxx | |||||
| (Signature) | (Signature) | |||||||
| Name: | Xxxx XxXxxxx |
Name: | Xxxx X. Xxxxxxxxxx | |||||
| (Please Print) | (Please Print) | |||||||
| Title: | CEO | Title: | Director - OTM | |||||
| Date: | 10/6/08 | Date: | 10/6/08 | |||||
-159-
EXHIBIT 2.2.1
COOPERATION TERMS
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 2.3.4
[***] AND PRINCIPLES OF
LF DEVELOPMENT PLAN [***]
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 2.3.7
[***] AND PRINCIPLES OF
LF DEVELOPMENT PLAN [***]
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 5.9
DOCUMENTATION TO BE PROVIDED BY NGX
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 6.3.4
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 7.1.1
CHMP COMMITMENT

|
NeurogesX UK Limited Xxxxxx Xxxx Xxxxx Xxxxxxxx, Xxxxxxxxx XX0 0XX, XX Artr: Xxxxx Xxxx | |
| Date: 09 March 2009 | ||
Xx. Xxxx Xxxxxx
European Medicines Agency
0 Xxxxxxxxx Xxxxxx
Xxxxxx Xxxxx
Xxxxxx
X00 0XX
United Kingdom
Dear Xx. Xxxxxx,
| RE: | EMEA/H/C/000909 |
(Qutenza 179 mg cutaneous patch, NeurogesX UK Ltd)
NeurogesX UK Ltd agrees to undertake the following Post-Authorisation Commitments requested by the CHMP and commit to submit the data listed below within the specified timeframe.
We also agree to submit any variation application resulting from the assessment of the below mentioned data.
Follow-up Measures:
[***]
| Your Sincerely, |
| /s/ Xxxxxxx X. XxXxxxx |
| Xxxxxxx X. XxXxxxx |
| President and Chief Executive Officer |
NeurogesX UK Ltd
000 Xxxxxx
Xxxxxx
XX0X 0XX
Xxxxxx Xxxxxxx
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
-166-
EXHIBIT 8.4
SAFETY AGREEMENT
EXHIBIT 12.8
DOMAIN NAMES
XXXXXXXXXXXXXXXX.XXX
| Domain Name |
Expiration Date |
WHOIS Administrative Contact |
WHOIS Technical Contact |
Account Holder | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
XXXXXXXXXXXXXX.XXX
| Domain Name |
Registered For |
WHOIS Administrative Contact |
WHOIS Technical Contact |
Account Holder | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
| [***] |
[***] | [***] | [***] | [***] | ||||
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 14.2.21
SAEs
[***]
EXHIBIT 14.2.38
[***]
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index of QA Change Controls (Approved) Since
May 21, 2009 to Present
| Date |
Material/Project | CMO/CLO Name |
Document Category |
CMO/CLO Doc && Rev No |
Document Title |
Change Type |
Change Requested |
Doc Effective Date |
NGX QA Approval Date | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | |||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index of QA Change Controls (Pending) Since
May 21, 2009 to Present
| Date |
Material/Project | CMO/CLO Name |
Document Category |
CMO/CLO Doc && Rev No |
Document Title | Change Type |
Change Requested |
Doc Effective Date | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index of QA Change Controls (Pending) Since
May 21, 2009 to Present
| Date |
Material/Project | CMO/CLO Name |
Document Category |
CMO/CLO Doc && Rev No |
Document Title |
Change Type |
Change Requested |
Doc Effective Date | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index/History of FDA Interactions for NDA 22-395
May 21, 2009 to Present
| Date |
Type of Contact |
Serial No. |
To/From | Tracking Information |
Contents of Submission | |||||||
| [***] |
[***] | [***] | [***] | [***] | ||||||||
| [***] |
[***] | [***] | [***] | [***] | ||||||||
| [***] [***] |
[***] [***] |
[***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] [***] |
[***] [***] |
[***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] [***] |
[***] [***] |
[***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] [***] |
[***] [***] |
[***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index/History of FDA Interactions for NDA 22-395
May 21, 2009 to Present
| Date |
Type of Contact |
Serial No. |
To/From | Tracking Information |
Contents of Submission | |||||||
| [***] | [***] | |||||||||||
| [***] [***] |
[***] [***] |
[***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index/History of FDA Interactions for IND 63,354
Capsaicin Dermal Patch since May 21, 2009
| Date |
Type of Contact |
Serial No. |
To/From | Tracking Information |
Contents of Submission | |||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | |||||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index/History of EMEA - MAA Interactions
QUTENZA – Since May 21, 2009
| Date |
Type of Contact |
Serial No. |
To/From | Tracking Information |
Contents of Submission | |||||||
| [***] |
[***] | [***] | [***] | |||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | [***] | |||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
Index/History of FDA Interactions for NDA 22-395
May 21, 2009 to Present
| Date |
Type of Contact |
Serial No. |
To/From | Tracking Information |
Contents of Submission | |||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | |||||||||||
| [***] |
[***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | |||||||||||

Confidential & Proprietary
***Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT 20.9
PRESS RELEASE

|

|
| NeurogesX, Inc. Xxxxxxx Xxxxxxxxx Chief Financial Officer (000) 000-0000 |
The Xxxx Group Xxxx Xxxxxxxxxx (investors) (000) 000-0000 xxxxxxxxxxx@xxxxxxxxxxxx.xxx | |
| Astellas Pharma Europe Xxxxx Dooa Corporate Communications Director x00 (0)0000 000 000 xxxxx.xxxx@xx.xxxxxxxx.xxx |
Xxxxx Xxxxx (media)/Xxxxxx XxXxxxx (000) 000-0000/7033 xxxxxx@xxxxxxxxxxxx.xxx xxxxxxxx@xxxxxxxxxxxx.xxx | |
NeurogesX and Astellas Enter Commercialization
Agreement for Qutenza™
Conference Call Today at 9:00am ET
Covers Europe, Middle East and Africa
Includes Licensing Option and Development Funding for NGX-1998
San Mateo, Calif. and Staines, U.K. (June 22, 2009) – NeurogesX, Inc. (NASDAQ: NGSX) and Astellas Pharma Europe Ltd., (Astellas), the European subsidiary of Tokyo based Astellas Pharma Inc. announced today that the companies have entered into an exclusive Distribution, Marketing and License agreement for the commercialization of Qutenza™ in the European Economic Area (EEA) including the 27 countries of the European Union, Iceland, Norway, and Liechtenstein as well as Switzerland, certain countries in Eastern Europe, the Middle East and Africa. The agreement closely follows the European Commission’s approval received in May 2009, of Qutenza (capsaicin 179 mg) cutaneous patch for the treatment of peripheral neuropathic pain in non-diabetic adults, either alone or in combination with other medicinal pain products.
Under terms of the agreement, Astellas will commercialize Qutenza in the above-mentioned territories and perform certain development of Qutenza including post-marketing commitments, to support Qutenza in the EU market. NeurogesX will receive EUR 30 million (approximately $42 million) for Qutenza commercialization rights, and EUR 5 million (approximately $7 million) for a license option of NGX-1998, the next-generation liquid formulation which uses the same active ingredient as Qutenza.
Astellas has an established and strong product portfolio with franchises in the therapeutic areas of urology, transplantation, dermatology and infectious disease. Commenting on

the agreement, Xxxxx Xxxxxxx, President and CEO of Astellas Pharma Europe Ltd., said, “We are very pleased to enter into this agreement with NeurogesX. Astellas is dedicated to developing a specialty focused franchise and Qutenza’s launch in the European Union fits with our strategy. We are confident in the market potential for Qutenza given its safety and efficacy to provide site-specific pain management for up to 12 weeks. Leveraging our foothold in these territories with our strong sales and marketing teams and Qutenza’s product profile, we are positioning for a successful commercial launch that will introduce an important new product to patients with neuropathic pain.”
Xxxxxxx XxXxxxx, CEO of NeurogesX, commented, “Securing an agreement with Astellas for the commercialization of Qutenza in Europe and additional territories is a significant milestone achievement for NeurogesX. Selecting the right partner was especially important to us since the European Union represents the first approval and the initial launch market for Qutenza. Astellas brings a tremendous amount of sales experience and presence across Europe and we are confident in our partner’s ability and commitment to launch Qutenza with the motivation required for commercial success. Having secured a commercial partnership in Europe, we are now turning our full attention to the remaining steps involved for the potential U.S. approval and commercialization of Qutenza.”
Collaboration Arrangements
Under the Distribution, Marketing and License Agreement, NeurogesX will receive two upfront payments from Astellas, EUR 30 million (approximately $42 million) for Qutenza commercialization rights and EUR 5 million (approximately $7 million) for a co-development and commercialization option of NGX-1998.
NeurogesX is eligible for additional sales-based milestone payments and additional option payments related to the liquid formulation totaling approximately EUR 70 million ($97 million) and royalties based on a double-digit percentage of net sales for Qutenza.
Astellas has committed investment for additional studies to support the marketing and promotion of Qutenza and to fulfill post-marketing commitments outlined in the European Commission’s approval. These post-marketing commitments include a long-term safety study of Qutenza in on-label indications.
The option payment to license NGX-1998 includes an initial upfront payment as mentioned previously as well as further option payments as development progresses. These payments are intended, in part, to accelerate NGX-1998 into Phase 2 clinical evaluation. In the event that Astellas exercises its option for NGX-1998, the companies expect to collaborate in Phase 3 development.
All payments to NeurogesX are expressed in euros and the dollar equivalents expressed in this press release are stated at current exchange rates. Actual conversion rates will vary depending on exchange rates in effect at the time payments are due.

Conference Call Details
NeurogesX will hold a teleconference today at 9:00 a.m. ET (6:00 a.m. PT) to discuss the distribution and licensing agreement with Astellas Pharma Europe Ltd.
To participate, please dial 0-000-000-0000 (USA) or 0-000-000-0000 (International). To access the live web cast please visit the Investor Relations section on the corporate web site at xxxx://xxx.xxxxxxxxx.xxx.
A replay of the conference call will be available beginning June 22, 2009 at 12:00 p.m. ET (9:00 a.m. PT) and ending on July 2, 2009 by dialing 0-000-000-0000 (USA) or 0-000-000-0000 (International), Account Number: 3055, Conference ID Number: 326456. A replay of the webcast will also be available on the corporate website for one month, through July 22, 2009.
About NeurogesX, Inc.
NeurogesX (NASDAQ: NGSX) is a biopharmaceutical company focused on developing and commercializing novel pain management therapies. Its initial focus is on chronic peripheral neuropathic pain, including postherpetic neuralgia (PHN), painful HIV-distal sensory polyneuropathy (HIV-DSP) and painful diabetic neuropathy (PDN). NeurogesX’ late stage product portfolio is led by its product candidate Qutenza, a dermal patch designed to manage pain associated with peripheral neuropathic pain conditions. Qutenza is currently approved in the European Union for the treatment of neuropathic pain in non-diabetic adults, either alone or in combination with other medicinal products for pain. NeurogesX submitted a new drug application (NDA) for Qutenza to the U.S. Food and Drug Administration (FDA) which was accepted for filing by the FDA in December 2008 and was given a Prescription Drug User Fee Act (PDUFA) date of August 16, 2009.
NeurogesX’ second most advanced product candidate, NGX-1998, is a topically applied, liquid formulation containing a high concentration of capsaicin designed to treat pain associated with neuropathic pain conditions. NGX-1998 has completed three Phase 1 studies and NeurogesX is currently evaluating the timing of entering Phase 2 development.
NeurogesX’ early stage product pipeline includes pre-clinical compounds, which are prodrugs of acetaminophen and various opioids. The company has evaluated these compounds in vitro and in vivo and is currently seeking development partners for these programs.
About Astellas Pharma Europe Ltd.,
Astellas Pharma Europe Ltd., located in the UK, is a European subsidiary of Tokyo-based Astellas Pharma Inc. Astellas is a pharmaceutical company dedicated to improving the health of people around the world through the provision of innovative and reliable pharmaceutical products. The organization is committed to becoming a global company by combining outstanding R&D and marketing capabilities and continuing to grow in the

world pharmaceutical market. Astellas Pharma Europe is responsible for 20 affiliate offices located across Europe, the Middle East and Africa, an R&D site and three manufacturing plants with approximately 3,400 staff. For more information about Astellas Pharma Europe Ltd., please visit our website at xxx.xxxxxxxx.xxx.
Safe Harbor Statement
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). NeurogesX disclaims any intent or obligation to update these forward-looking statements, and claims the protection of the Safe Harbor for forward-looking statements contained in the Act. Examples of such statements include, but are not limited to, expectations with respect to the successful commercial launch and market potential of Qutenza; expectations with respect to the activities of NeurogesX and Astellas under the Distribution, Marketing and License Agreement (the Agreement); the potential receipt of post-execution payments under the Agreement; potential uses of proceeds from the Agreement; expectations regarding additional studies, including a safety study to be carried out by Astellas under the Agreement; the timing of regulatory decisions with respect to the NDA for Qutenza with the FDA, including the PDUFA date for the NDA; plans for entry into a U.S. commercialization partnership for NeurogesX pre-clinical compounds; and plans for clinical development of NGX-1998.
Such statements are based on management’s current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to; NeurogesX’ product candidates may have unexpected adverse side effects or inadequate therapeutic efficacy; Astellas may not devote sufficient resources or personnel to the commercialization of Qutenza; adoption of Qutenza by physicians may be longer than anticipated; discussions with governmental or administrative entities may not result in adequate reimbursement or pricing to support commercialization efforts for Qutenza; Astellas may not elect to make option related payments or co-develop NGX-1998; positive results in Qutenza clinical trials may not be sufficient to obtain FDA approval; the FDA may request additional clinical trials or other information prior to granting approval for Qutenza; and other difficulties or delays in the successful commercialization of Qutenza, carrying out activities or obtaining payments under the Agreement and in clinical development of, and obtaining regulatory approval for, NeurogesX’ product candidates. For further information regarding these and other risks related to NeurogesX’ business, investors should consult NeurogesX’ filings with the Securities and Exchange Commission.
EXHIBIT D
FORM OF SECURITY AGREEMENT
- 1 -
EXECUTION COPY
SECURITY AGREEMENT
This SECURITY AGREEMENT (as amended, supplemented or otherwise modified from time to time in accordance herewith, this “Agreement”) is made and entered into as of April 29, 2010 by and between NeurogesX, Inc., a Delaware corporation (together with its successors and assigns, “NeurogesX”), and Xxxxx Healthcare Royalty Partners, L.P., a Delaware limited partnership (together with its successors and assigns, “CHRP”).
RECITALS:
A. NeurogesX and CHRP are parties to that certain Financing Agreement by and between CHRP and NeurogesX of even date herewith (as amended, modified or otherwise supplemented from time to time, the “Financing Agreement”).
B. NeurogesX has agreed pursuant to the terms of the Financing Agreement to enter into this Agreement, under which NeurogesX grants to CHRP a first priority security interest in and to the Collateral (as hereinafter defined) as general and continuing security for the due performance and payment of all of NeurogesX’s obligations to CHRP under the Financing Agreement.
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
SECTION 1. Definitions.
For purposes of this Agreement, capitalized terms used herein shall have the meanings set forth in Attachment 1 attached hereto. Capitalized terms used herein and not otherwise defined shall have the meaning given such terms in the UCC, as applicable.
SECTION 2. Grant of Security.
NeurogesX hereby grants to CHRP a continuing first priority security interest, subject to Permitted Liens, in, and collateral assignment of, all of NeurogesX’s right, title and interest in, to and under the following property, whether now or hereafter existing or acquired, whether tangible or intangible and wherever the same may be located (collectively, the “Collateral”):
(a) the Revenue Interest;
(b) (i) the Intellectual Property and (ii) all agreements pursuant to which NeurogesX obtained rights thereunder including (A) the Commercial Supply and License Agreement, dated January 2007 by and between NeurogesX and LTS Xxxxxxx Therapie-Systeme AG (the “LTS Agreement”) and (B) the Exclusive License Agreement, effective November 1, 2000, by and between The Regents of the University of California and NeurogesX (the “UC Agreement”) (together with the LTS Agreement, the “Related Rights Agreements”), each agreement solely to the extent related to the Exploitation of, or the preservations of rights and property necessary for the Exploitation of, the Licensed Products in the Territory;
1
(c) all License Agreements, including, without limitation, (i) all rights to receive moneys due or to become due under or pursuant to the License Agreements, (ii) all rights to receive proceeds of any insurance, indemnity, warranty or guaranty claim with respect to the License Agreements, (iii) all claims for damages arising out of any breach of or default under the License Agreements and (iv) all rights to terminate, amend, supplement, modify or exercise rights or options under the License Agreements, to perform thereunder and to compel performance and otherwise exercise all remedies thereunder;
(d) the Regulatory Approvals;
(e) any agreement with respect to the manufacture of Licensed Product for Exploitation in the Territory including the LTS Agreement (including manufacture of such Licensed Products outside of the Territory for Exploitation in the Territory), but solely to the extent related to the Exploitation of the Products in the Territory;
(f) the Deposit Agreement, the Joint Concentration Account and the Initial Concentration Account and all funds on deposit in the Joint Concentration Account and the Initial Concentration Account, and all monies and credit balances from time to time held in the Joint Concentration Account and the Initial Concentration Account; and all interest, cash, instruments and other property at any time and from time to time received, receivable or otherwise distributed in respect of such accounts, such funds or received in exchange for any or all of the items described in this subsection (f);
(g) all money now or at any time in the possession or under the control of, or in transit or payable to, NeurogesX or CHRP directly relating to any of the foregoing Collateral;
(h) all Accounts, contract rights, Commercial Tort Claims, Payment Intangibles, Letter-of-Credit Rights, Promissory Notes, Software, Instruments, Chattel Paper, Electronic Chattel Paper, Tangible Chattel Paper and General Intangibles, cash monies and other rights to payment, in each case, with directly relating to, constituting, comprising or evidencing any of the foregoing;
(i) all present and future books, Documents, invoices, records, statements, in each case, in any form whatsoever and directly relating to any of the foregoing; and
(j) all Proceeds, rents and profits of or from any and all of the foregoing, all proceeds that constitute property, and, to the extent not otherwise included, all payments under insurance (whether or not CHRP is the loss payee or beneficiary thereof), or any indemnity, warranty or guaranty, payable by reason of loss or damage to or otherwise with respect to any of the foregoing, in each case, in any form whatsoever.
Each item of Collateral in this Section 2 that is defined in Article 8 or Article 9 of the UCC shall have the meaning set forth in the UCC.
Notwithstanding anything herein to the contrary, in no event shall the security interest granted hereunder attach to any Collateral comprising (I) subject matter acquired by NeurogesX or its Affiliates after the date hereof to the extent relating to any Competitive Product to the extent the consent of a Third Party is required for such the attachment of such security interest or
2
(II) any Intellectual Property licensed after the date hereof from a Third Party to the extent relating to the manufacture, use, sale, offer for sale, importation, other commercialization or other Exploitation of any Competitive Product, or any agreement entered into hereafter for the license of such Intellectual Property to NeurogesX or its Affiliates; provided, however, that NeurogesX shall use commercially reasonable efforts to obtain the consent of all Third Parties whose consent is necessary for the grant of a security agreement in such subject matter or Intellectual Property, and such security interest shall automatically attach at such time as all such necessary consents of Third Parties are obtained.
SECTION 3. Security for Obligations.
This Agreement secures, and the Collateral is collateral security for, the due and punctual payment or performance in full (including without limitation the payment of amounts that would become due but for the operation of the automatic stay under Subsection 362(a) of the Bankruptcy Code) of all Secured Obligations.
SECTION 4. NeurogesX to Remain Liable.
Anything contained herein to the contrary notwithstanding, (a) NeurogesX shall remain liable under any contracts and agreements included in the Collateral to perform all of its duties and obligations thereunder to the same extent as if this Agreement had not been executed, (b) the exercise by CHRP of any of its rights hereunder shall not release NeurogesX from any of its duties or obligations under the contracts and agreements included in the Collateral, and (c) CHRP shall not have any obligation or liability under any contracts, licenses, and agreements included in the Collateral by reason of this Agreement, nor shall CHRP be obligated (i) to perform any of the obligations or duties of NeurogesX thereunder, (ii) to take any action to collect or enforce any claim for payment assigned hereunder, or (iii) to make any inquiry as to the nature or sufficiency of any payment NeurogesX may be entitled to receive thereunder.
SECTION 5. Representations and Warranties.
Except with respect to any section of this Section 5 as identified in the Disclosure Schedule with respect to ARTICLE III of the Financing Agreement, NeurogesX represents and warrants to CHRP, as of the date of this Agreement, as follows:
(a) Ownership of Collateral. Except for the security interest created by this Agreement, NeurogesX owns or has exclusive rights to the Collateral free and clear of any Liens other than Permitted Liens and as otherwise provided in the License Agreement and the Related Rights Agreements. NeurogesX has the power to transfer and grant the Liens and security interests granted hereunder. Except as such may have been filed in favor of CHRP relating to this Agreement, no security agreement, financing statement, assignment, equivalent security, lien or other instrument similar in effect covering all or any part of the Collateral is on file in any filing or recording office.
(b) Validity. This Agreement creates a valid continuing first priority security interest, subject to Permitted Liens, in the Collateral securing the payment and performance in full of the Secured Obligations. Upon the filing of appropriate UCC financing statements in the filing offices listed on Attachment 5(b) and any required filings with Patent Offices, the security
3
interest granted to CHRP in the Collateral will be a perfected security interest to the extent that perfection may occur by such filings.
(c) Authorization, Approval. No authorization, approval, or other action by, and no notice to or filing with, any Governmental Authority or other Person is required either (i) for the grant by NeurogesX of the security interest granted hereby or for the execution, delivery and performance of this Agreement by NeurogesX; or (ii) for the grant of the security interest created hereby other than (A) any such authorizations, approvals, actions, notices or filings that have been obtained or made, (B) the filing of financing statements in the offices listed on Attachment 5(b) and (C) any required filings with any Patent Office.
(d) Enforceability. This Agreement is the legally valid and binding obligation of NeurogesX, enforceable against NeurogesX in accordance with its terms, subject, as to enforcement of remedies, to bankruptcy, insolvency, reorganization, moratorium or similar Applicable Laws affecting creditors’ rights generally or general equitable principles (regardless of whether enforcement is sought in equity or at law).
(e) Office Locations; Type and Jurisdiction of Organization; Good Standing. The chief executive office of NeurogesX and each office where NeurogesX keeps its records regarding the Collateral are, as of the date hereof, located at the locations set forth on Attachment 5(e); its type of organization (e.g., corporation), jurisdiction of organization and organization number provided by the applicable government authority of its jurisdiction of organization are listed on Attachment 5(e).
(f) Names. NeurogesX (or any predecessor by merger or otherwise) has not, within the four (4) month period preceding the date hereof, had a different name from the name listed on the signature pages hereof.
(g) Event of Default. No Event of Default has occurred and is continuing and no event has occurred and is continuing and no condition exists that would, with notice or the lapse of time, or both, constitute an Event of Default.
SECTION 6. Further Assurances.
From time to time, at its sole expense, NeurogesX will promptly execute and deliver and will cause to be executed and delivered all further instruments and documents, and will take all further action, that may be necessary or desirable, or that CHRP may reasonably request, in order to perfect and protect any security interest granted or purported to be granted hereby or to enable CHRP to exercise and enforce its rights and remedies hereunder with respect to any Collateral. Without limiting the generality of the foregoing, NeurogesX will: (i) (A) execute and file, record or register such financing or continuation statements, or amendments thereto, (B) execute and deliver, and cause to be executed and delivered, agreements establishing that CHRP has “control” within the meaning of Article 9 of the UCC of any applicable items of Collateral, (C) execute and deliver such Intellectual Property Security Agreements as are requested by CHRP within ten (10) Business Days of any such request and (D) deliver such other instruments or notices, in each case, as may be necessary or desirable, or as CHRP may reasonably request, in order to perfect and preserve the security interests granted or purported to be granted hereby,
4
(ii) furnish to CHRP from time to time statements and schedules further identifying and describing the Collateral and such other reports in connection with the Collateral as CHRP may reasonably request, all in reasonable detail, (iii) at CHRP’s reasonable request, appear in and defend any action or proceeding that may affect the title of NeurogesX to or CHRP’s security interest in all or any part of the Collateral, and (iv) use commercially reasonable efforts to obtain any necessary consents of Third Parties to the perfection of a security interest to CHRP with respect to any Collateral or the exercise of any right hereunder. NeurogesX hereby authorizes CHRP to file one or more financing or continuation statements, and amendments thereto, relative to all or any part of the Collateral without the signature of NeurogesX. In addition to and notwithstanding the foregoing, NeurogesX hereby irrevocably constitutes and appoints CHRP, with full power of substitution, as its true and lawful attorney-in-fact, with full irrevocable power and authority in its place and stead and in its name or otherwise, from time to time in CHRP’s sole discretion, at NeurogesX’s sole cost and expense, to take any and all appropriate action and to execute and deliver any and all documents and instruments which CHRP may deem reasonably necessary or advisable to accomplish the purposes of this Agreement and to create and perfect, and to continue and preserve, an indefeasible continuing first priority security interest, subject to Permitted Liens, in any and all of the Collateral in favor of CHRP.
NeurogesX agrees that a carbon, photographic or other reproduction of this Agreement shall be sufficient as a financing statement and may be filed as a financing statement in any and all jurisdictions. NeurogesX agrees to furnish CHRP promptly upon request by CHRP, with any information that is requested by CHRP in order to complete such financing statements, continuation statements, or amendments thereto.
SECTION 7. Certain Covenants of NeurogesX.
NeurogesX shall:
(a) not use or permit any Collateral to be used in violation of any provision of the Transaction Documents or in violation in any material respect of any Applicable Law or any policy of insurance covering the Collateral;
(b) give CHRP contemporaneous written notice of any change in its name or form of organization, or taking of any other action that results in a change of the jurisdiction of organization of NeurogesX;
(c) give CHRP contemporaneous written notice of any change in its chief executive office or the office where NeurogesX keeps its records regarding the Collateral; and
(d) pay promptly when due all taxes, assessments and governmental charges or levies imposed upon, and all claims against, the Collateral, except to the extent the validity thereof is being contested in good faith and NeurogesX maintains reserves appropriate therefor under GAAP; provided that NeurogesX shall in any event pay such taxes, assessments, charges, levies or claims not later than three (3) Business Days prior to the date of any proposed sale under any judgment, writ or warrant of attachment entered or filed against NeurogesX or any of the Collateral as a result of the failure to make such payment.
SECTION 8. Special Covenants With Respect to the Collateral.
5
(a) NeurogesX shall:
(i) diligently keep records respecting the Collateral and at all times keep at least one (1) complete set of its records concerning such Collateral at its chief executive office or principal place of business;
(ii) not create, incur, assume, allow or cause to exist any Lien on any property included within the definition of Collateral except as may be otherwise permitted under the Financing Agreement and except for Permitted Liens;
(iii) not Transfer any Collateral except as otherwise permitted under the Financing Agreement including among others pursuant to an assignment of the Financing Agreement as permitted under Section 8.04 thereof;
(iv) not terminate any License Agreement or either of the Related Rights Agreements without the consent of CHRP; and
(v) faithfully perform in all material respects all of its obligations under the License Agreement and the Related Rights Agreements.
(b) NeurogesX will use commercially reasonable efforts to process, prosecute and maintain any Patents as required by Section 5.08 of the Financing Agreement.
(c) NeurogesX shall, concurrently with the execution and delivery of this Agreement, execute and deliver to CHRP five (5) originals of a Special Power of Attorney in the form of Exhibit I annexed hereto for execution of an assignment of the Collateral to CHRP, or the implementation of the sale or other disposition of the Collateral pursuant to CHRP’s good faith exercise of the rights and remedies granted hereunder; provided, however, CHRP agrees that it will not exercise its rights under such Special Power of Attorney unless an Event of Default has occurred and is continuing.
(d) NeurogesX further agrees that a breach of any of the covenants contained in this Section 8 will cause irreparable injury to CHRP, that CHRP has no adequate remedy at law in respect of such breach and, as a consequence, that each and every covenant contained in this Section 8 shall be specifically enforceable against NeurogesX, and NeurogesX hereby waives and agrees not to assert any defenses against an action for specific performance of such covenants.
SECTION 9. Protection of Intellectual Property.
If either Party becomes aware that any Person shall do or perform any acts which NeurogesX or CHRP reasonably believes constitute an infringement of any Patents, or violate or infringe any right of NeurogesX or CHRP in any Intellectual Property or if any Person shall do or perform any acts which constitute an unauthorized or unlawful use thereof, then such Party shall promptly notify the other thereof and the Parties shall enforce such rights as provided in Section 5.08 of the Financing Agreement.
SECTION 10. Standard of Care.
6
The powers conferred on CHRP hereunder are solely to protect its interest in the Collateral and shall not impose any duty upon it to exercise any such powers. Except for the exercise of good faith and of reasonable care in the accounting for moneys actually received by CHRP hereunder or the exercise of reasonable care with respect to any Collateral in its possession, CHRP shall have no duty as to any Collateral or as to the taking of any necessary steps to preserve rights against prior parties or any other rights pertaining to any Collateral. CHRP shall be deemed to have exercised reasonable care in the custody and preservation of Collateral in its possession if such Collateral is accorded treatment substantially equal to that which CHRP accords its own property.
SECTION 11. Remedies Upon Event of Default.
(a) If, and only if, any Event of Default shall have occurred and be continuing, CHRP may exercise in respect of the Collateral (I) all rights and remedies provided for herein, under the Financing Agreement or otherwise available to it, (II) all the rights and remedies of a secured party on default under the UCC, in all relevant jurisdictions, and (III) the right to:
(i) accelerate the payment of all Secured Obligations and demand immediate payment thereof to CHRP;
(ii) require NeurogesX to, and NeurogesX hereby agrees that it will at its expense and upon request of CHRP forthwith, assemble all or part of the Collateral as directed by CHRP and make it available to CHRP at a place and time to be designated by CHRP;
(iii) personally or by agents or attorneys, with or without judicial process or the aid or assistance of others, to the extent permitted by Applicable Law, enter upon any premises on or in which any of the Collateral may be located and immediately take possession of the Collateral or any part thereof, from NeurogesX or any other person who has possession of any part thereof, and complete or have completed any activities with respect to all or any portion of the Collateral;
(iv) collect, foreclose, receive, appropriate, setoff or otherwise enforce or realize upon any and all Collateral, and exercise any and all rights of NeurogesX under any of the Collateral, including, without limitation, any License Agreement or Related Rights Agreement;
(v) extend the time of payment of, compromise or settle for cash, credit and return, upon any terms or conditions, any and all Collateral which includes a monetary obligation and discharge or release the account debtor or other obligor, without affecting any of the Secured Obligations;
(vi) exercise all rights of NeurogesX, as assignee of NeurogesX, under (i) the LTS Agreement and the UC Agreement to the extent related to the Exploitation of, or the preservations of rights and property necessary for the Exploitation of, or the manufacture and supply of License Products in any country or Territory for Exploitation of, the Licensed Products in the Territory and (ii) any and all License Agreements;
7
(vii) sell, lease, transfer, assign, deliver or otherwise dispose of any and all Collateral at such prices or terms as CHRP may deem reasonable (taking into consideration the price paid by Third Parties for similar property or rights), for cash or credit or any other means of payment, with CHRP having the right to purchase the whole or any part of the Collateral at any public sale, all of the foregoing being free from any right or equity of redemption of NeurogesX, which right or equity of redemption is hereby expressly waived and released by NeurogesX. If any of the Collateral is sold or otherwise disposed of by CHRP upon credit terms or for future payment, the Secured Obligations shall not be reduced as a result thereof until payment therefore is finally collected by CHRP. If notice of disposition of Collateral is required by Applicable Law, ten (10) days’ prior notice by CHRP to NeurogesX designating the time and place of any public sale or the time after which any private sale or other intended disposition of Collateral is to be made, shall be deemed to be reasonable notice thereof and NeurogesX waives any other notice. In the event CHRP institutes an action to recover any Collateral or seeks recovery of any Collateral by way of prejudgment remedy, NeurogesX waives the posting of any bond which might otherwise be required.
(b) In the event that CHRP sells, leases, transfer, assigns, delivers or otherwise disposes of any Collateral, all Proceeds therefrom actually received by CHRP shall first be applied against those documented expenses incurred by CHRP in connection therewith (including reasonable attorneys’ fees and legal expenses incurred by CHRP with respect thereto or otherwise chargeable to NeurogesX) and any remainder shall be applied against the Secured Obligations. For clarity, NeurogesX shall remain liable to CHRP for the payment on demand of any deficiency together with all costs and expenses of collection or enforcement, including reasonable attorneys’ fees and legal expenses as provided herein.
(c) Anything contained herein to the contrary notwithstanding, upon the occurrence and during the continuation of an Event of Default, CHRP shall have the right (but not the obligation) to bring suit, in the name of NeurogesX, CHRP or otherwise, to enforce any Collateral, in which event NeurogesX shall, at the reasonable request of CHRP, do any and all lawful acts and execute any and all documents required by CHRP in aid of such enforcement and NeurogesX shall promptly, upon demand, reimburse and indemnify CHRP as provided in Section 13 hereof in connection with the exercise of its rights under this Section 11.
(d) The Parties acknowledge that NeurogesX and CHRP have entered into that certain CHRP Replacement License Agreement that provides certain rights and licenses to, among other things, the Intellectual Property, all on the terms and conditions set forth therein and elsewhere in the Transaction Documents.
(e) CHRP shall be under no obligation whatsoever to proceed first against any of the Collateral or other property which is security for the Secured Obligations before proceeding against any other of the Collateral. It is expressly understood and agreed that all of the Collateral or other property which is security for the Secured Obligations stands as equal security for all Secured Obligations, and that CHRP shall have the right to proceed against, sell or dispose of any or all of the Collateral or other property which is security for the Secured Obligations in any order, or simultaneously, as in its sole and absolute discretion it shall determine.
8
(f) From time to time as reasonably requested by CHRP, at the sole expense of NeurogesX, CHRP or its designee shall have access, prior to an Event of Default upon reasonable prior notice and during reasonable business hours, and on or after an Event of Default, at any time, to all of the premises where Collateral is located for the purposes of inspecting (and upon the occurrence and during the continuation of an Event of Default, disposing and realizing upon) the Collateral, and all NeurogesX’s books and records relating to the Collateral, and NeurogesX shall permit CHRP or its designee to make such copies of such books and records or extracts therefrom as CHRP may reasonably request. Without expense to CHRP, CHRP may use such of NeurogesX’s personnel, equipment, including computer equipment, programs, printed output and computer readable media, supplies and premises for the inspection of and, as applicable, realization on Collateral as CHRP, in its sole discretion, deems appropriate.
SECTION 12. Release of CHRP.
NeurogesX hereby releases and exculpates CHRP, its officers, partners, directors, employees, agents, representatives and designees, from any liability arising from any acts under this Agreement or in furtherance thereof, whether as attorney-in-fact or otherwise, whether of omission or commission, and whether based upon any error of judgment or mistake of law or fact, in each case taken in good faith and except for any liability arising from the gross negligence or willful misconduct of CHRP or any such Person as determined by a final and non-appealable order from a court of competent jurisdiction. In no event will CHRP have any liability to NeurogesX for lost profits or other special or consequential damages. This Section 12 shall survive the termination of this Agreement and the discharge of NeurogesX’s other obligations under this Agreement and the Financing Agreement.
SECTION 13. Expenses.
(a) NeurogesX agrees to pay to CHRP upon demand the amount of any and all costs and expenses, including the reasonable fees and expenses of its counsel and of any experts and agents, that CHRP may incur in connection with (i) the custody, preservation, use or operation of, or the sale of, collection from, or other realization upon, any of the Collateral, (ii) the exercise or enforcement of any of the rights of CHRP hereunder, or (iii) the failure by NeurogesX to perform or observe any of the provisions hereof. If NeurogesX defaults in the performance of any of the provisions of this Agreement, CHRP may (but without any obligation to do so) perform same for NeurogesX’s account. Any costs and expenses expended by CHRP under this Section 13, if not timely paid by NeurogesX, shall be added to the Secured Obligations and chargeable with interest to NeurogesX.
(b) The obligations of NeurogesX in this Section 13 shall survive the termination of this Agreement and the discharge of NeurogesX’s other obligations under this Agreement and the Financing Agreement.
SECTION 14. Continuing Security Interest; Termination and Release.
(a) This Agreement shall create a continuing security interest in the Collateral and shall (i) remain in full force and effect until the payment and performance in full of the Secured
9
Obligations, (ii) be binding upon NeurogesX and its respective successors and assigns, and (iii) inure, together with the rights and remedies of CHRP hereunder, to the benefit of CHRP and its successors, transferees and assigns.
(b) Upon the occurrence of payment and performance in full of all Secured Obligations, the security interest granted hereby shall terminate and all rights to the Collateral shall be returned to NeurogesX. Upon any such termination CHRP will, at NeurogesX’s expense, execute and deliver to NeurogesX and authorize NeurogesX to file or record such documents as NeurogesX shall reasonably request or deem appropriate to evidence such termination.
SECTION 15. Amendments.
No amendment, modification, termination or waiver of any provision of this Agreement, and no consent to any departure by NeurogesX therefrom, shall in any event be effective unless the same shall be in writing and signed by CHRP and, in the case of any such amendment or modification, by NeurogesX. Any such waiver or consent shall be effective only in the specific instance and for the specific purpose for which it was given.
SECTION 16. Notices.
All notices, consents, waivers and other communications hereunder shall be in writing and shall be delivered in accordance with Section 8.03 of the Financing Agreement.
SECTION 17. Failure or Indulgence Not Waiver; Remedies Cumulative.
No failure or delay on the part of CHRP in the exercise of any power, right remedy or privilege hereunder shall impair, prejudice or constitute a waiver of any such power, right, remedy or privilege or be construed as a waiver of any Event of Default or acquiescence therein, nor shall any single or partial exercise of any such power, right or privilege preclude any other or further exercise thereof or of any other power, right or privilege. All rights and remedies existing under this Agreement are cumulative to, and not exclusive of, any rights or remedies otherwise available.
SECTION 18. Severability.
If any provision of this Agreement is held to be invalid or unenforceable, the remaining provisions shall nevertheless be given full force and effect.
SECTION 19. Headings and Captions.
The headings and captions in this Agreement are for convenience and reference purposes only and shall not be considered a part of or affect the construction or interpretation of any provision of this Agreement.
SECTION 20. Governing Law; Jurisdiction.
10
(a) This Agreement shall be governed and construed in accordance with the laws of the State of New York, USA, without giving effect to any choice of law provisions thereof. Each Party hereby submits itself for the purpose of this Agreement and any controversy arising hereunder to the exclusive jurisdiction of the state and federal courts located in the County of New York, State of New York, USA, and any courts of appeal therefrom, and waives any objection on the grounds of lack of jurisdiction (including, without limitation, venue) to the exercise of such jurisdiction over it by any such courts. Prior to bringing a legal action against the other Party (other than an action for injunctive relief, which may be brought at any time), such dispute shall be separately negotiated by the Parties hereto in good faith and all reasonable efforts undertaken to settle amicably such matters before resorting to further legal recourse, as follows: upon the occurrence of a dispute between the Parties, including, without limitation, any breach of this Agreement or any obligation relating thereto, the matter shall be referred first to the officers of NeurogesX and CHRP having responsibility for the subject matter of the dispute, or their designees. The officers, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner for up to thirty (30) days. If such efforts do not result in mutually satisfactory resolution of the dispute, the matter shall be referred to the chief executive officer of NeurogesX and the managing director of CHRP, or their designees. The chief executive officer and the managing director, or their designees, as the case may be, shall negotiate in good faith to resolve such dispute in a mutually satisfactory manner for up to thirty (30) additional days, or such longer period of time to which the chief executive officer and managing director may agree. In the event the dispute has not been resolved at the end of such thirty (30) day period (or such longer period as agreed to by the chief executive officer and managing director), either Party shall be entitled to bring an action in accordance with Section 20(a) and (b).
(b) Each Party hereto hereby irrevocably consents to the service of process out of any of the courts referred to in subsection (a) above of this Section 20 in any such suit, action or proceeding by the mailing of copies thereof by registered or certified mail, postage prepaid, to it at its address set forth in this Agreement. Each Party hereto hereby irrevocably waives any objection to such service of process and further irrevocably waives and agrees not to plead or claim in any suit, action or proceeding commenced hereunder or under any other Transaction Document that service of process was in any way invalid or ineffective. Nothing herein shall affect the right of a Party to serve process on the other Party in any other manner permitted by Applicable Law. In the event of any litigation under this Section 20, the prevailing Party shall be entitled to reimbursement of any reasonable and documented out-of-pocket expenses (including reasonable fees and expenses of legal counsel) incurred by the prevailing Party in connection with asserting or enforcing such action hereunder, including, without limitation, in the case CHRP is the prevailing Party in connection with any Bankruptcy Event with respect to NeurogesX and the non-prevailing Party agrees to reimburse and indemnify the prevailing Party for such expenses.
SECTION 21. Waiver of Jury Trial.
Each Party hereto hereby irrevocably waives, to the fullest extent permitted by Applicable Law, any and all right to trial by jury in any action, proceeding, claim or counterclaim arising out of or relating to any Transaction Document or the transactions
11
contemplated under any Transaction Document. This waiver shall apply to any subsequent amendments, renewals, supplements or modifications to any Transaction Document.
SECTION 22. Counterparts; Effectiveness.
This Agreement may be executed in two (2) or more counterparts, each of which shall be an original, but all of which together shall constitute one and the same instrument. This Agreement shall become effective when each Party hereto shall have received a counterpart hereof signed by the other Party hereto. Any counterpart may be executed by facsimile or pdf signature and such facsimile or pdf signature shall be deemed an original.
SECTION 23. Successors and Assigns.
The provisions of this Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and assigns. NeurogesX shall not be entitled to assign any of its obligations and rights under the Transaction Documents without the prior written consent of CHRP, which consent shall not be unreasonably withheld, conditioned or delayed; provided, however that NeurogesX may, without the consent of CHRP, assign any of its obligations and rights under the Transaction Documents to any other Person with which it may merge or consolidate or to which it may sell all or substantially all of its assets; provided, further, however, that no assignment by NeurogesX shall relieve NeurogesX of its obligations hereunder even if such assignment has been consented to by CHRP and such assignee shall be jointly and severally liable with NeurogesX to CHRP. CHRP may assign without consent of NeurogesX any of its rights under the Transaction Documents without restriction.
[SIGNATURE PAGE FOLLOWS]
12
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be duly executed by their respective authorized officers as of the date first above written.
| NEUROGESX, INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
| XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. | ||
| By Xxxxx Healthcare Royalty GP, LLC Its General Partner | ||
| By: |
| |
| Name: | ||
| Title: | ||
[SIGNATURE PAGE TO SECURITY AGREEMENT]
13
ATTACHMENT 1 TO
SECURITY AGREEMENT
DEFINITIONS
Capitalized terms used in this Agreement and not defined in this Attachment 1 shall have the meanings attributed thereto in the UCC.
“Affiliate” has the meaning set forth in the Financing Agreement.
“Agreement” has the meaning set forth in the preamble to this Agreement.
“Applicable Law” means, with respect to any Person, individually and collectively, any statute, law, treaty, rule, regulation, ordinance, order, decree, determination, writ, injunction, rule, ruling, directive and regulation of any kind whatsoever of any arbitrator or Governmental Authority, in each case applicable to or binding upon such Person or any of its property or to which such Person or any of its property is subject.
“Bankruptcy Code” means Title 11 of the United States Code entitled “Bankruptcy”.
“Bankruptcy Event” has the meaning as set forth in the Financing Agreement.
“Business Day” has the meaning set forth in the Financing Agreement.
“CHRP” has the meaning set forth in the preamble to this Agreement.
“CHRP Replacement License Agreement” has the meaning as set forth in the Financing Agreement.
“Collateral” has the meaning set forth in Section 2 of this Agreement.
“Competitive Product” has the meaning as set forth in the Financing Agreement.
“Deposit Agreement” has the meaning as set forth in the Financing Agreement.
“Event of Default” has the meaning as set forth in the Financing Agreement.
“Exploitation” has the meaning as set forth in the Financing Agreement.
“Financing Agreement” has the meaning set forth in the recitals of this Agreement.
“Governmental Authority” has the meaning as set forth in the Financing Agreement.
“Initial Concentration Account” has the meaning as set forth in the Financing Agreement.
“Intellectual Property” has the meaning as set forth in the Financing Agreement.
- 1 -
“Intellectual Property Security Agreements” means any short-form security agreement that CHRP reasonably requests be executed and delivered by NeurogesX for filing in any jurisdiction in the Territory in which NeurogesX owns any Intellectual Property in order to perfect CHRP’s security interest in the Collateral in such jurisdiction.
“Joint Concentration Account” has the meaning as set forth in the Financing Agreement.
“License Agreement” has the meaning as set forth in the Financing Agreement.
“Licensed Products” has the meaning as set forth in the Financing Agreement.
“Lien” has the meaning as set forth for “Liens” in the Financing Agreement.
“NeurogesX” has the meaning set forth in the preamble of this Agreement.
“Patents” has the meaning as set forth in the Financing Agreement.
“Patent Office” has the meaning as set forth in the Financing Agreement.
“Permitted Liens” has the meaning as set forth in the Financing Agreement.
“Person” has the meaning as set forth in the Financing Agreement.
“Regulatory Approval” has the meaning as set forth in the Financing Agreement.
“Revenue Interest” has the meaning set forth in the Financing Agreement.
“Revenue Interest Payments” has the meaning set forth in the Financing Agreement.
“Secured Obligations” means all obligations, indebtedness and liabilities of every nature of NeurogesX now or hereafter existing under or arising out of or in connection with the Financing Agreement, whether for fees, charges, damages, principal, interest (including without limitation interest that, but for the filing of a petition in bankruptcy with respect to NeurogesX, would accrue on such obligations, whether or not a claim is allowed against NeurogesX for such interest in the related bankruptcy proceeding), reimbursement of fees, expenses, indemnities or otherwise, whether voluntary or involuntary, due or not due, primary or secondary, secured or unsecured, direct or indirect, absolute or contingent, liquidated or unliquidated, whether or not jointly owed with others, and whether or not from time to time decreased or extinguished and later increased, created or incurred, and all or any portion of such obligations or liabilities that are paid, to the extent all or any part of such payment is avoided or recovered directly or indirectly from CHRP as a preference, fraudulent transfer or otherwise, and all obligations of every nature of NeurogesX now or hereafter existing under this Agreement.
“Territory” has the meaning as set forth in the Financing Agreement.
“Third Party” has the meaning as set forth in the Financing Agreement.
“Transaction Document” has the meaning as set forth in the Financing Agreement.
- 2 -
“Transfer” means any sale, conveyance, assignment, disposition, pledge, hypothecation or transfer.
“UCC” has the meaning as set forth in the Financing Agreement.
- 3 -
ATTACHMENT 5(b)
TO
SECURITY AGREEMENT
Filing Offices
Delaware Secretary of State
- 1 -
ATTACHMENT 5(e)
TO
SECURITY AGREEMENT
Office Locations, Type and Jurisdiction of Organization
Chief Executive Office of Grantor: NeurogesX, Inc., 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000, Xxx Xxxxx, Xxxxxxxxxx 00000
Addresses of the Properties at which Grantor Maintains Records Relating to the Collateral: NeurogesX, Inc., 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000, Xxx Xxxxx, Xxxxxxxxxx 00000
Jurisdiction of Organization: State of Delaware
Type of Organization: Corporation
Organizational Number: 4271074
- 1 -
EXHIBIT I
TO SECURITY AGREEMENT
SPECIAL POWER OF ATTORNEY
| STATE OF | ) | |||||
| ) | ss.: | |||||
| COUNTY OF | ) |
KNOW ALL MEN BY THESE PRESENTS, that NEUROGESX, INC. (“Grantor”), hereby appoints and constitutes XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. (“CHRP”) and each of its officers, managers, members and directors of its General Partner, Xxxxx Healthcare Royalty GP, LLC, its true and lawful attorney, with full power of substitution and with full power and authority to perform the following acts on behalf of Grantor:
(a) upon the occurrence of an event which, but for the giving of notice or lapse of time or both, would give rise to an Event of Default (an “Unmatured Default”), and during the continuance of an Event of Default, to ask for, demand, collect, xxx for, recover, compound, receive and give acquittance and receipts for moneys due and to become due under or in respect of any of the Collateral;
(b) upon the occurrence of an Unmatured Default, and during the continuance of an Event of Default, to receive, endorse and collect any drafts or other instruments, documents and chattel paper in connection with clause (a) above;
(c) upon the occurrence of an Unmatured Default, and during the continuance of an Event of Default, to file any claims or take any action or institute any proceedings that CHRP may in its good faith sole discretion deem necessary or desirable for the collection of any of the Collateral or otherwise to enforce the rights of CHRP with respect to any of the Collateral;
(d) to pay or discharge taxes or liens levied or placed upon or threatened against the Collateral, the legality or validity thereof and the amounts necessary to discharge the same to be determined by CHRP in its reasonable commercial judgment, any such payments made by CHRP to become obligations of NeurogesX to CHRP, due and payable immediately without demand;
(e) upon the occurrence of an Unmatured Default, and during the continuance of an Event of Default, to sign and endorse any invoices, drafts against debtors, assignments, verifications, notices and other documents relating to the Collateral; and
(f) upon the occurrence of an Unmatured Default, and during the continuance of an Event of Default, to prepare, file and sign NeurogesX’s name on an assignment document in form acceptable to CHRP in its sole discretion necessary or desirable to transfer ownership of the Collateral to CHRP or an assignee or transferee of CHRP.
- 1 -
This Power of Attorney is made pursuant to a Security Agreement, dated as of April 29, 2010 between Grantor and CHRP (the “Security Agreement”) and is subject to the terms and provisions thereof. This Power of Attorney, being coupled with an interest, is irrevocable until all “Secured Obligations”, as such term is defined in the Security Agreement, are indefeasibly paid in full.
Date: April 29, 2010
| NEUROGESX, INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
- 2 -
EXHIBIT E
LEGAL OPINION OF XXXXXX XXXXXXX XXXXXXXX & XXXXXX, P.C. (TRANSACTION OPINION – U.S.)
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
[WSGR Letterhead]
Opinion Letter of Xxxxxx Xxxxxxx Xxxxxxxx & Xxxxxx, P.C.
April 29, 2010
Xxxxx Healthcare Royalty Partners, L.P.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Ladies and Gentlemen:
Reference is made to the Financing Agreement dated as of April 29, 2010 (the “Agreement”), by and among NeurogesX, Inc., a Delaware Corporation (the “Company”), and Xxxxx Healthcare Royalty Partners, L.P., a limited partnership organized under the laws of the State of Delaware (“CHRP”). This opinion letter is delivered to CHRP pursuant to Section 6.02(h)(i) of the Agreement, and all terms used herein have the meanings defined for them in the Agreement unless otherwise defined herein. Reference in this opinion letter to the Agreement excludes any schedule or substantive agreement attached as an exhibit to the Agreement, unless otherwise indicated herein.
We have acted as counsel for the Company in connection with the negotiation of the Agreement, the Assignment Agreement, the Security Agreement, the CHRP Replacement License and the Deposit Agreement (collectively, the “Transaction Documents”). As such counsel, we have made such legal and factual examinations and inquiries as we have deemed advisable or necessary for the purpose of rendering the opinions and statements set forth below.
In rendering the opinions expressed below, we have examined executed originals or copies of the following documents:
| (a) | the Transaction Documents; |
| (b) | the Amended and Restated Certificate of Incorporation of the Company, certified by the Delaware Secretary of State (the “Certificate of Incorporation”), and the Bylaws of Company, as amended to date; |
| (c) | records of proceedings of the Board of Directors of the Company during or by which resolutions were adopted relating to matters covered by this opinion; |
| (d) | (i) a certificate of the Secretary of State of the State of Delaware, dated April 29, 2010, with respect to the standing of the Company as a corporation incorporated under the laws of the State of Delaware; and (ii) a Certificate of the Secretary of State of the State of California dated April 5, 2010, with |
| respect to the standing of the Company as a foreign corporation qualified to do business in the State of California; |
| (e) | the certificates of the Secretary and certain officers of the Company as to certain factual matters (the “Officer’s Certificate”); |
| (f) | the copy of the UCC-1 financing statement attached as Schedule A hereto (the “UCC-1 Financing Statement”); |
| (g) | each of the documents expressly identified on Schedule B hereto, if any (the “Reviewed Agreements”); and |
| (h) | each of the judgments and decrees that are expressly identified on Schedule C hereto, if any (the “Reviewed Judgments”). |
In addition, we have reviewed originals or copies of such corporate records of the Company, certificates of public officials and such other documents that we consider necessary or advisable for the purpose of rendering the opinions and statements set forth below. We have not independently established the facts stated therein. In such examination we have assumed the genuineness of all signatures, the authenticity and completeness of all documents submitted to us as originals and the conformity to original documents of all copies submitted to us. In making our examination of documents, we have assumed that each party to any such document (other than the Company in connection with the Transaction Documents) has satisfied those requirements that are applicable to it to the extent necessary to make such document a valid and binding obligation of such party, enforceable against such party in accordance with its terms.
As used in the opinions or statements set forth below, the expressions “to our knowledge,” “known to us” or similar language with reference to matters of fact refer to the current actual knowledge of the attorneys of this firm who have rendered legal services in connection with the representation described in the second paragraph of this opinion letter. Except to the extent expressly set forth herein, we have not undertaken any independent investigation to determine the existence or absence of any fact, and no inference as to our knowledge of the existence or absence of any fact should be drawn from our representation of the Company or the rendering of the opinions or statements set forth below.
We have assumed that (i) the representations and warranties as to factual matters made by the parties to the Transaction Documents and pursuant thereto are correct, (ii) the representations and warranties made by officers of the Company as to factual matters made in the Officer’s Certificate and other certificates delivered in connection with the Transaction Documents are correct, (iii) the parties to the Agreement have complied and will comply with their obligations under the Transaction Documents, and (iv) that CHRP is exempt from the restrictions of Section 1 of Article XV of the California Constitution and related statutes with respect to usury.
We are members of the bar of the State of California and we express no opinion as to any matter relating to laws of any jurisdiction other than the federal laws of the United States of America, the General Corporation Law of the State of Delaware, the Delaware Uniform Commercial Code and the laws of the State of California, as such are in effect on the date hereof, and we have made no inquiry into, and we express no opinion as to, the statutes, regulations, treaties, common laws or other laws of any other nation, state or jurisdiction or whether the laws of any particular jurisdiction govern any aspect of the Transaction Documents. We note that the parties to the Transaction Documents have designated the laws of the State of New York as the laws governing the Transaction Documents. Our opinion in paragraph 1 as to the validity, binding effect and enforceability of the Transaction Documents is premised upon the result that would be obtained if a California court were to apply the internal laws of the State of California (excluding conflicts of laws principles) to the interpretation and enforcement of the Transaction Documents. As you know, we are not licensed to practice law in the State of Delaware and, accordingly, our opinions as to General Corporation Law of the State of Delaware and the Delaware Uniform Commercial Code are based solely on a review of the official statutes of the State of Delaware.
In rendering the opinion set forth in paragraph 2 below concerning violations of and defaults under Reviewed Agreements and violations of Reviewed Judgments, we have relied solely upon an examination of the Reviewed Agreements and Reviewed Judgments in the forms provided to us by the Company. The opinion in paragraph 2 below concerning violations of and defaults under Reviewed Agreements is based on the result that would be obtained if a California court were to apply the internal laws of the State of California (excluding conflicts of law principles) to the interpretation and enforcement of the Reviewed Agreements.
The opinions hereinafter expressed are subject to the following additional qualifications:
(a) We express no opinion as to the effect of applicable bankruptcy, insolvency, reorganization, arrangement, fraudulent transfer, moratorium or other similar federal or state laws relating to or affecting the rights of creditors generally;
(b) We express no opinion as to the effect of general principles of equity, including without limitation concepts of materiality, reasonableness, good faith and fair dealing, and the possible unavailability of specific performance or injunctive relief, regardless of whether considered in a proceeding in equity or at law;
(c) We express no opinion as to the enforceability of any provision of the Transaction Documents (i) purporting to waive broadly or vaguely stated rights, unknown future defenses, rights to damages, rights to jury trials, any statute of limitations for a period in excess of four years beyond the statutory period, or the benefits of other statutory, regulatory or constitutional rights that cannot be waived or, if they can be waived, cannot be waived prospectively; (ii) providing for a choice of venue or for exclusive venue with respect to disputes arising under the Transaction Documents; (iii) imposing penalties, forfeitures, liquidated damages, late charges, acceleration of future amounts due (other than principal) without appropriate discount to present value, prepayment charges, or increased interest rates upon default; or (iv) prescribing or varying rules of evidence,
method or quantum of proof or other legal standards in a manner contrary to applicable statutes and rules of law;
(d) We express no opinion as to the enforceability of any provisions in the Transaction Documents (i) giving rights or remedies, or permitting the exercise of rights or remedies, in a manner not in compliance with applicable statutes and rules of law, (ii) providing that rights or remedies are not exclusive, that every right or remedy is cumulative and may be exercised in addition to any other right or remedy, or that the election of some particular remedy does not preclude recourse to one or more other remedies, or (iii) providing for rights of set-off;
(e) We express no opinion as to the enforceability of indemnification and contribution provisions to the extent they may be subject to limitations of public policy and the effect of applicable statutes and rules of law;
(f) We call your attention to the limitations imposed by Section 1717 of the California Civil Code as to the legality, validity, binding nature or enforceability of any provision of the Transaction Documents regarding any party’s ability to collect attorney’s fees and costs incurred to enforce rights under the Transaction Documents;
(g) We express no opinion as to the applicability or effect of compliance or non-compliance by any lender (or its agent) with any state, federal or other laws applicable to such lender (or its agent) or to the transactions contemplated by the Transaction Documents because of the nature of its business, including its legal or regulatory status;
(h) Except as set forth in paragraphs 4 and 5 below, we express no opinion as to the creation, attachment, validity, perfection or priority of a security interest in any item of collateral or the necessity of making any filings or taking any other action in connection therewith;
(i) We express no opinion as to the enforceability or legal effect of any provision of any Transaction Document purporting to reinstate, as against any obligor or guarantor, obligations or liabilities of such obligor which have been avoided or which have arisen from transactions which have been rescinded or the payment of which has been required to be returned by any court of competent jurisdiction; and
(j) We express no opinion regarding compliance or noncompliance (or the effect thereof) with any federal or state securities laws.
Based upon and subject to the foregoing, and except as set forth in the Disclosure Schedule to the Agreement, we are of the opinion that:
1. Each of the Transaction Documents has been duly and validly executed and delivered by the Company and constitutes a valid and binding obligation of the Company, enforceable against the Company in accordance with its terms.
2. The execution and delivery by the Company of the Transaction Documents and the performance by the Company of its obligations under the Transaction Documents do not violate any provision of the Amended and Restated Certificate of Incorporation or Amended and Restated Bylaws of the Company, or any provision of any applicable federal or state law, rule or regulation known to us to be customarily applicable to transactions of this nature. The execution and delivery by the Company of the Transaction Documents and the performance by the Company of its obligations under the Transaction Documents do not violate, or constitute a default under, the Reviewed Agreements, and do not violate any Reviewed Judgment.
3. No consent, approval or authorization of or designation, declaration or filing with any governmental authority on the part of the Company is required for the valid execution and delivery of the Transaction Documents, except as contemplated by the Transaction Documents.
4. The Security Agreement is sufficient to create a valid security interest in favor of CHRP in the collateral described therein to the extent a security interest in such collateral may be created under Division 9 of the California Uniform Commercial Code.
5. If a financing statement in the form of the UCC-1 Financing Statement is communicated to the Delaware Secretary of State by an authorized method of communication and an amount equal to the applicable filing fee is tendered to such filing office, such filing office will have an obligation to accept such financing statement. Upon acceptance of the UCC-1 Financing Statement by such filing office, the security interest in the collateral described in both the UCC-1 Financing Statement and the Security Agreement, and for which perfection under Article 9 of the Delaware Uniform Commercial Code may occur by the filing of a UCC-1 financing statement with the Delaware Secretary of State, will be perfected.
* * *
We advise you that, except as identified in the Disclosure Schedule to the Agreement, to our knowledge, there are no lawsuits pending against the Company nor, to our knowledge, has the Company received any written threat thereof. We note that we have not conducted a docket search in any jurisdiction with respect to lawsuits that may be pending against the Company.
* * *
This opinion letter is furnished to CHRP solely for its benefit in connection with the Agreement, and may not be relied upon by any other person or for any other purpose without our prior written consent. We assume no obligation to inform you of any facts, circumstances, events or changes in the law that may arise or be brought to our attention after the date of this opinion letter that may alter, affect or modify the opinions or statements expressed herein.
| Very truly yours, |
| XXXXXX XXXXXXX XXXXXXXX & XXXXXX |
| Professional Corporation |
Schedule A
UCC-1 Financing Statement
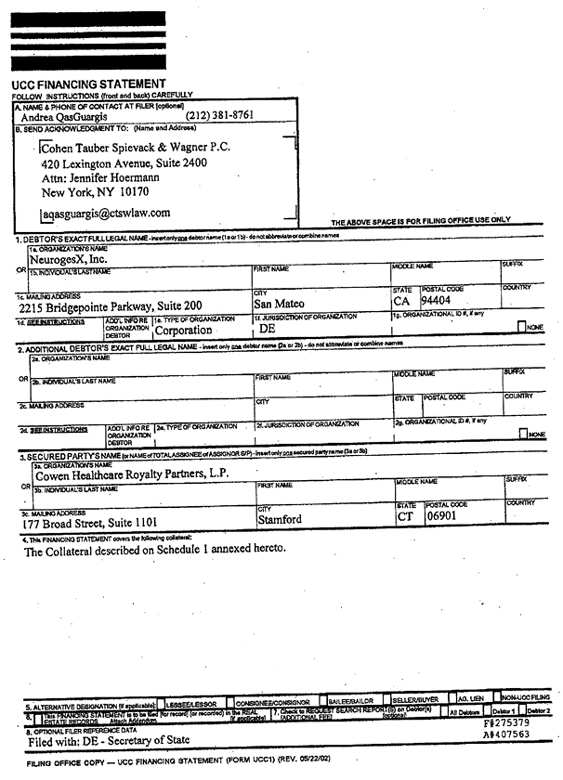
UCC FINANCING STATEMENT
FOLLOW INSTRUCTIONS (front and back) CAREFULLY
A. NAME & PHONE OF CONTACT AT FILER [optional]
Xxxxxx XxxXxxxxxx (000) 000-0000
B. SEND ACKNOWLEDGMENT TO: (Name & Address)
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Attn: Xxxxxxxx Xxxxxxxx
Xxx Xxxx, XX 00000
xxxxxxxxxxx@xxxxxxx.xxx
THE ABOVE SPACE IS FOR FILING OFFICE USE ONLY
1. DEBTOR’S EXACT FULL LEGAL NAME - insert only one debtor name (1a or 1b) – do not abbreviate or combine names
1a. ORGANIZATION’S NAME
NeurogesX, Inc.
--
OR
1b. INDIVIDUAL’S LAST NAME
FIRST NAME
MIDDLE NAME
SUFFIX
1c. MAILING ADDRESS
0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000
XXXX
Xxx Xxxxx
XXXXX
XX
POSTAL CODE
94404
COUNTRY
1d. SEE INSTRUCTIONS
ADD’L INFO RE ORGANIZATION DEBTOR
1e. TYPE OF ORGANIZATION
Corporation
1f. JURISDICTION OF ORGANIZATION
DE
1g. ORGANIZATIONAL ID #, if any
NONE
2. ADDITIONAL DEBTOR’S EXACT FULL LEGAL NAME - insert only one debtor name (2a or 2b) – do not abbreviate or combine names
2a. ORGANIZATION’S NAME
OR
2b. INDIVIDUAL’S LAST NAME
FIRST NAME
MIDDLE NAME
SUFFIX
2c. MAILING ADDRESS
CITY
STATE
POSTAL CODE
COUNTRY
2d. SEE INSTRUCTIONS
ADD’L INFO RE ORGANIZATION DEBTOR
2e. TYPE OF ORGANIZATION
2f. JURISDICTION OF ORGANIZATION
2g. ORGANIZATIONAL ID #, if any
NONE
3. SECURED PARTY’S NAME (or NAME of TOTAL ASSIGNEE of ASSIGNOR S/P) - insert only one secured party name (3a or 3b)
3a. ORGANIZATION’S NAME
Xxxxx Healthcare Royalty Partners, L.P.
OR
3b. INDIVIDUAL’S LAST NAME
FIRST NAME
MIDDLE NAME
SUFFIX
3c. MAILING ADDRESS
000 Xxxxx Xxxxxx, Xxxxx 0000
XXXX
Xxxxxxxx
XXXXX
CT
POSTAL CODE
06901
COUNTRY
4. This FINANCING STATEMENT covers the following collateral:
The Collateral described on Schedule 1 annexed hereto.
5. ALTERNATIVE DESIGNATION (if applicable):
LESSEE/LESSOR
CONSIGNEE/CONSIGNOR
BAILEE/XXXXXX
SELLER/BUYER
AG. LIEN
NON-UCCFILING
6. This FINANCING STATEMENT is to be filed [for record] (or recorded) in the REAL ESTATE RECORDS
Attach Addendum [if applicable]
7. Check to REQUEST SEARCH REPORT(S) on Debtor(s)
[ADDITIONAL FEE]
[optional]
All Debtors
Xxxxxx 0
Xxxxxx 0
8.OPTIONAL FILER REFERENCE DATA
F#275379
Filed with: DE - Secretary of State
A#407563
FILING OFFICE COPY — UCC FINANCING STATEMENT (FORM UCC1) (REV. 05/22/02)
SCHEDULE 1
All of the right, title and interest of NeurogesX, Inc. (“NGX”) in, to and under the following property, whether now or hereafter existing or acquired, whether tangible or intangible and wherever the same may be located (collectively, the “Collateral”):
(k) the Revenue Interest with respect to the Distribution, Marketing and License Agreement, dated as of June 19, 2009 (as amended, restated, supplemented or modified, the “Existing License Agreement”), between NGX and Astellas Pharma Europe Ltd. (“Astellas”);
(l) (i)the Intellectual Property and (ii) all agreements pursuant to which NGX obtained rights thereunder including (A) the Commercial Supply and License Agreement, dated January 2007 (the “LTS Agreement”), by and between NGX and LTS Xxxxxxx Therapie-Systeme AG (“LTS”) and (B) the Exclusive License Agreement, effective November 1, 2000 (the “UC Agreement”), by and between The Regents of the University of California (“The Regents”) and NGX, each agreement solely to the extent related to the Exploitation of, or the preservation of rights and property necessary for the Exploitation of, the Licensed Products in the Territory;
(m) all License Agreements, including, without limitation, (i) all rights to receive moneys due or to become due under or pursuant to the License Agreements, (ii) all rights to receive proceeds of any insurance, indemnity, warranty or guaranty claim with respect to the License Agreements, (iii) all claims for damages arising out of any breach of or default under the License Agreements and (iv) all rights to terminate, amend, supplement, modify or exercise rights or options under the License Agreements, to perform thereunder and to compel performance and otherwise exercise all remedies thereunder;
(n) the Regulatory Approvals;
(o) any agreement with respect to the manufacture of Licensed Product for Exploitation in the Territory including the LTS Agreement (including manufacture of such Licensed Products outside of the Territory for Exploitation in the Territory), but solely to the extent related to the Exploitation of the Products in the Territory;
(p) the Deposit Agreement, dated April 29, 2010 (the “Deposit Agreement”), by and among NGX, CHRP and JPMorgan Chase Bank, N.A., Joint Concentration Account and Initial Concentration Account and all funds on deposit in the Joint Concentration Account and the Initial Concentration Account, and all monies and credit balances from time to time held in the Joint Concentration Account and the Initial Concentration Account; and all interest, cash, instruments and other property at any time and from time to time received, receivable or otherwise distributed in respect of such accounts, such funds or received in exchange for any or all of the items described in this subsection (f);
(q) all money now or at any time in the possession or under the control of, or in transit or payable to, NGX or Xxxxx Healthcare Royalty Partners, L.P. (“CHRP”) directly relating to any of the foregoing Collateral;
(r) all Accounts, contract rights, Commercial Tort Claims, Payment Intangibles, Letter-of-Credit Rights, Promissory Notes, Software, Instruments, Chattel Paper, Electronic Chattel Paper, Tangible Chattel Paper and General Intangibles, cash monies and other rights to payment, in each case, with directly relating to, constituting, comprising or evidencing any of the foregoing;
(s) all present and future books, Documents, invoices, records, statements, in each case, in any form whatsoever and directly relating to any of the foregoing; and
(t) all Proceeds, rents and profits of or from any and all of the foregoing, all proceeds that constitute property, and, to the extent not otherwise included, all payments under insurance (whether or not CHRP is the loss payee or beneficiary thereof), or any indemnity, warranty or guaranty, payable by reason of loss or damage to or otherwise with respect to any of the foregoing, in each case, in any form whatsoever.
Each item of Collateral that is defined in Article 8 or Article 9 of the Uniform Commercial Code shall have the meaning set forth in the Uniform Commercial Code.
Capitalized terms used and not otherwise defined herein have the meanings given to them in the Financing Agreement.
In addition, the following capitalized terms used herein have the following meanings:
“Exploit” means, with respect to a product, the manufacture, sale, offer for sale (including marketing and promotion), importation, distribution or other commercialization or exploitation of such product; and “Exploitation” shall have the correlative meaning.
“Gel” has the meaning set forth in the Existing License Agreement, and includes, among other things, the packaged gel cleansing product, as further described in NGX’s EMEA MAA Approval No. EMEA/H/C/000909 and any supplements or amendments to such approval existing as of June 19, 2009, together with any improvements or modifications (including but not limited to line extensions, enhanced or modified presentations and formulations) to such cleansing gel, in each case developed by or on behalf of NGX during the term of the Existing License Agreement.
“Initial Concentration Account” means the deposit account established and maintained at the Depositary Bank pursuant to the Deposit Agreement and the Financing Agreement, dated April 29, 2010 (the “Financing Agreement”), between NGX and CHRP. The Initial Concentration Account shall be the account into which all payments made in respect of the Revenue Interest are to be remitted or deposited in the first instance in accordance with the terms of the Financing Agreement and the Deposit Agreement.
“Intellectual Property” means all proprietary information; trade secrets; Know-How; utility models; confidential information; inventions (whether patentable or unpatentable and whether or not reduced to practice or claimed in a pending patent application) and improvements thereto; Patents; registered or unregistered trademarks, trade names and service marks, including all goodwill associated therewith; registered and unregistered copyrights and all applications thereof; in each case that (i) are owned, controlled by, issued to, licensed to, licensed by or hereafter acquired by or licensed by NGX or its Subsidiaries, including, without limitation, any intellectual property subject to the agreements listed on Schedule 3.12(b) of the Financing Agreement, and (ii) relating to, embodied by, covering or involving or necessary or used to (a) manufacture or have manufactured, the Licensed Products for Exploitation in the Territory or (b) sell, have sold, market, or have marketed, the Licensed Products, in each case in the Territory.
“Joint Concentration Account” means a segregated account for the benefit of NGX and CHRP and maintained at the Depositary Bank pursuant to the terms of the Deposit Agreement and the Financing Agreement. The Joint Concentration Account shall be the account into which the Depositary Bank sweeps on a daily basis the funds held in the Initial Concentration Account in accordance with the terms of the Financing Agreement and the Deposit Agreement.
“Know-How” means all non-public information, results and data of any type whatsoever, in any tangible or intangible form (and whether or not patentable), including databases, practices, methods, techniques, specifications, formulations, formulae, knowledge, skill, experience, data and results (including pharmacological, medicinal chemistry, biological, chemical, biochemical, toxicological and clinical study data and results), analytical and quality control data, stability data, studies and procedures, and manufacturing process and development information, results and data.
“License Agreement” means (i) the Existing License Agreement, and (ii) in the event of the termination thereof, any Replacement License Agreement including, without limitation, any sublicense and subdistribution agreements which survive and are assigned to NGX.
“Licensed Product(s)” means any Product licensed to Astellas and any Licensing Option Product (as defined in the Existing License Agreement) licensed to Astellas or a Third Party.
“Patch” has the meaning set forth in the Existing License Agreement and includes the capsaicin-containing cutaneous patch described in NGX’s EMEA MAA Approval No. EMEA/H/C/000909 and any supplements or amendments to such approval, together with any changes to formulation or other aspects of such patch (including but not limited to line extensions, enhanced or modified presentations and formulations) developed by or on behalf of NGX during the term of the Existing License Agreement.
“Patents” means any and all issued patents and pending patent applications, including without limitation, all provisional applications, substitutions, continuations, continuations-in-part, divisions, and renewals, all letters patent granted thereon, and all patents-of-addition, reissues, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms (including regulatory extensions), and all supplementary protection certificates,
together with any foreign counterparts thereof anywhere in the Territory covering the Licensed Product, composition of matter, formulation, or methods of manufacture or use thereof that are issued or filed on or after April 29, 2010, in each case, which are owned, controlled by, issued to, licensed to or licensed by NGX or any of its Affiliates.
“Product” means (1) a product containing a Patch and/or Gel (as defined in the Existing License Agreement), whether alone or in combination, together with any ancillary non-pharmacologically active components (e.g. gloves), if the MAA Approval (as defined in the Existing License Agreement) has been obtained for such a combination and (ii), to the extent Astellas has executed its Option (as defined in the Existing License Agreement) under Section 2.3 of the Existing License Agreement, NGX-1998, the high concentration liquid formulation of capsaicin currently under development by NGX, together with such other items identified in the Existing License Agreement.
“Regulatory Approval” means all approvals (including, without limitation, where applicable, pricing and reimbursement approval and schedule classifications), product and/or establishment licenses, registrations or authorizations of any Regulatory Agency necessary for the manufacture, use, storage, import, export, transport, offer for sale, or sale of the Licensed Product in the Territory in accordance with the License Agreement.
“[***]” means any [***] pursuant to which (i) [***] or (ii) [***].
“Revenue Interest” means, with respect to the Existing License Agreement, any and all of NGX’s rights to receive (i) any payments under Section 3 or Section 4 (except Section 4.4) of the Existing License Agreement, including, without limitation, rights to receive Sales Milestones (as defined in the Existing License Agreement) under Section 3.2 of the Existing License Agreement, the Option Retention Fee (as defined in the Existing License Agreement) under Section 3.3.2 of the Existing License Agreement, the Option Exercise Fee (as defined in the Existing License Agreement) under Section 3.3.3 of the Existing License Agreement, and Earned Royalties (as defined in the Existing License Agreement) under Section 4 of the Existing License Agreement, in each case payable on and after Xxxxx 0, 0000, (xx) any payments under Section 11.5.2 of the Existing License Agreement in amounts in the aggregate in excess of out-of-pocket costs and expenses (including reasonable attorneys’ and professional fees) incurred in connection with any Enforcement Action (as defined in the Existing License Agreement), (iii) any payments similar to those described in clauses (i) and (ii) from Astellas or its affiliates with respect to Licensing Option Products (as defined in the Existing License Agreement), regardless of whether such Licensing Option Products
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
are included under the Existing License Agreement or licensed to Astellas pursuant to a separate agreement, (iv) any payments similar to those described in clauses (i), (ii) and (iii) under any Replacement License Agreement, (v) any payments similar to those described in clauses (i) - (iv) made by a Licensee after rejection of the Existing License Agreement or Replacement License Agreement by NGX in bankruptcy, and (vi) any other payments for or derived from the sale, offer for sale (including marketing and promotion), distribution or other commercialization or exploitation of Licensed Products in the Territory.
The Revenue Interest excludes all rights under any License Agreement to receive (a) payments or amounts which are used to pay Third Parties for rights to intellectual property necessary for or incorporated in the Licensed Products (including amounts payable (I) under Section 4.4 of the Existing License Agreement, (II) to The Regents pursuant to Section 3.2 or Section 19.4 of the UC Agreement or (III) to LTS pursuant to Section 7.3 of the LTS Agreement, (b) any payments under the Supply Agreement (as entered into pursuant to and defined in Section 9.1 of the Existing License Agreement) and (c) any payment intended to reimburse for any costs or expenses (whether internal or external reasonably allocated in accordance with GAAP) incurred or to be incurred by NGX with respect to activities under such License Agreement or otherwise with respect to the Licensed Product in the Territory.
“Territory” means the countries and regions set forth in the Existing License Agreement, including those set forth on Exhibit A attached hereto, and such other countries as are agreed to or as may be added by written agreement between the parties to the Existing License Agreement.
EXHIBIT A
TO
SCHEDULE I
TERRITORY
| A. | European Region [***] |
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
Schedule B
List of Reviewed Agreements
| 1. | Distribution, Marketing and License Agreement between NeurogesX and Astellas Pharma Europe Ltd dated June 19, 2009 |
| 2. | Exclusive License Agreement between NeurogesX and The Regents of the University of California dated November 1, 2000 |
| 3. | Commercial Supply and License Agreement between NeurogesX and LTS Xxxxxxx Therapie-Systems AG dated January 28, 2007, as amended June 2009 |
| 4. | Manufacturing and Supply Agreement between NeurogesX and Contract Pharmaceuticals Limited Canada. dated December 22, 2005 |
| 5. | Manufacturing and Supply Agreement between NeurogesX and Formosa Laboratories, Inc. dated August 19, 2008 |
| 6. | Tri-partite Agreement between Astellas Pharma Europe Ltd., LTS Xxxxxxx Therapie-Systems AG and NeurogesX dated June 4, 2009 |
Schedule C
List of Reviewed Judgments
None.
EXHIBIT F
LEGAL OPINION OF XXXXXXXX & XXXXXXXX (IP OPINION)
- 1 -
|
000 XXXX XXXX XXXX XXXX XXXX XXXXXXXXXX 00000-0000
TELEPHONE: 000.000.0000 FACSIMILE: 650.494.0792
XXX.XXXX.XXX |
XXXXXXXX & XXXXXXXX LLP
NEW YORK, SAN FRANCISCO, LOS ANGELES, PALO ALTO, SAN DIEGO, WASHINGTON, D.C.
DENVER, NORTHERN VIRGINIA, ORANGE COUNTY, SACRAMENTO, WALNUT CREEK, CENTURY CITY
TOKYO, LONDON, BEIJING, SHANGHAI, HONG KONG, SINGAPORE, BRUSSELS |
April 29, 2010
Xxxxx Healthcare Royalty Partners, L.P.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Attention: Xxxxxxx X. Xxxxx, M.D.
| Re: | NeurogesX, Inc. |
Ladies and Gentlemen:
We have acted as patent counsel to NeurogesX, Inc., a Delaware corporation (the “Company”), in the preparation, filing and/or prosecution of the patents and patent applications listed on Exhibit A attached hereto (collectively, the “Patent Rights”). This opinion is being delivered to you pursuant to section 6.02(h)(ii) of that certain Financing Agreement, dated as of April 29, 2010, by and between the Company and you (the “Financing Agreement”:)
In connection with this opinion, we have examined the representations made by the Company in section 3.12 of the Financing Agreement, with respect to the Intellectual Property of the Company (the “Representations”).
We have assumed the genuineness of all signatures, the authenticity of all items submitted to us as originals and the conformity with originals of all items submitted to us as copies.
Whenever our opinions or views herein with respect to the existence or absence of facts are indicated to be based on our knowledge or belief, it is intended to signify that, in the course of our representation of the Company in connection with the matters referred to in the first sentence of the first paragraph hereof, no information has come to the attention of Xxxxxx X. Xxxxxx or Xxxx Xxxxx (who are the only attorneys in this firm who have devoted significant attention to such matters) that would give them actual knowledge of the existence or absence of such facts. We have not made any independent verification of any corporate records or other documents of the Company, nor undertaken any independent investigation of the Company or any third party to determine the existence or absence of such facts, and no inference as to our knowledge of the existence or absence of such facts should be drawn from the fact of our representation of the Company as described above.
Other than those opinions or views stated herein, we express no opinions or views (a) with respect to any patent rights or other intellectual property rights of the Company other than the Patent Rights, (b) as to whether the Company is infringing any patents or other rights of others or whether others are infringing any patents or other rights of the Company, (c) as to whether the Company owns or possesses sufficient licenses or other rights to use all patents or other rights
Page 2
necessary for the conduct of the Company’s business, or (d) with respect to the Company’s license agreements or similar agreements. In addition, we express no opinions or views as to (i) whether any patent applications within the Patent Rights will issue as patents, (ii) whether any patents within the Patent Rights or any patents that may issue from any patent applications within the Patent Rights, if challenged, will be held valid and enforceable, (iii) the scope of claim coverage of any patents within the Patent Rights or any patents that may issue from any patent applications within the Patent Rights, (iv) whether the Company will be able to conduct its business without infringing the patents or other intellectual property rights of third parties, (v) whether the Company will be able to prevent third parties from competing with the Company or developing products or business methods similar to those of the Company, or (vi) any other Representation made by the Company in Section 3.12 of the Financing Agreement.
Based upon and subject to the foregoing, and based upon the fact that we are expressing no opinion as to matters governed by the laws of any jurisdiction other than the substantive laws of the State of California and the federal laws of the United States, we are of the opinion:
| 1. | To our knowledge, the patent applications within the Patent Rights that are owned by the Company are owned free and clear of all liens, encumbrances, defects of title or other restrictions on ownership. |
| 2. | To our knowledge, the issued patents within the Patent Rights have been duly maintained and, to our knowledge, are in full force and effect. |
| 3. | To our knowledge, the views expressed in our 2003 opinion to the Company were accurate as of the date thereof. We have performed no further diligence in that regard, however, other than and except for reference(s) identified by the Company and disclosed in connection with the Financing Agreement, nothing has specifically come to our attention that would change the views expressed in that 2003 opinion. |
In addition, although we have not independently verified the accuracy, completeness or fairness of the Representations, based upon and subject to the foregoing, nothing has come to our attention that leads us to believe that at the Closing the Representations contained an untrue statement of a material fact with respect to the Patent Rights or omitted to state a material fact with respect to the Patent Rights required to be stated therein or necessary to make the statements therein not misleading.
This letter is solely for your benefit, and neither this letter nor any opinion expressed herein may be relied upon, nor may copies be delivered or disclosed to, any other person or entity without our prior written consent.
Very truly yours,
Xxxxxxxx & Xxxxxxxx LLP
EXHIBIT G-1
FORM OF ASTELLAS CONSENT
- 1 -
[NGX Letterhead]
April 29, 2010
Astellas Pharma Europe Limited
Xxxxxx Xxxxx,
Xxxxxx Xxxx,
Xxxxxxx,
Xxxxxxxxx,
XX00 0XX
Xxxxxx Xxxxxxx
Attention: Xx. Xxxxx Xxx
RE: Distribution, Marketing and License Agreement, dated 19 June 2009 between NeurogesX, Inc. (“NeurogesX”) and Astellas Pharma Europe Ltd. (“Astellas”) (the “Distribution Agreement”)
Dear Xx. Xxx,
As you know NeurogesX is entering into a financing transaction with Xxxxx Healthcare Royalty Partners, L.P. (“CHRP”) pursuant to which NeurogesX will finance certain of the payments receivable by NeurogesX under the Distribution Agreement (the “Financing Transaction”). As part of the Financing Transaction, NeurogesX will be (i) assigning all of its rights with respect to such payments to CHRP until such time as such financed amounts together with appropriate returns to CHRP have been paid (after which such payments will revert to NeurogesX), (ii) granting a first-priority security interest to CHRP in the Distribution Agreement and related collateral assignment thereof to secure NeurogesX’s obligations thereunder and (iii) subject to Astellas’s rights under the Distribution Agreement, granting certain rights and licenses to CHRP under the Know-How, Patents and regulatory filings and approvals with respect to the Products in the Territory (as such capitalized terms are defined in the Distribution Agreement) to further secure NeurogesX’s obligations thereunder in certain circumstances if the rights of Astellas under the Distribution Agreement are terminated, in whole or in part.
In connection with the foregoing and in order to induce CHRP to enter into the Financing Transaction, we ask Astellas agree to the following, without further consideration:
| • | to hereby consent to the grant by NeurogesX of security interests in, and related collateral assignment of, the rights of and licenses granted to NeurogesX and the Distribution Agreement with respect thereto to CHRP as described in the provisions of (i) – (iii) above; |
| • | to hereby acknowledge that the Distribution Agreement is in full force and effect as of the date hereof, that to Astellas’s actual knowledge no default exists, or with the passage of time or the giving of notice will exist by either Astellas or NeurogesX and that no breach of the Distribution Agreement has been threatened by either Astellas or NeurogesX and that Astellas has not sent any notice thereunder of termination or default to NeurogesX; |
| • | to hereby acknowledge that, as between NeurogesX and CHRP, NeurogesX shall remain solely obligated under the Distribution Agreement; however, CHRP may (but has no obligation to) exercise the rights and perform the duties of NeurogesX under the Distribution Agreement as agent of and on |
| behalf of NeurogesX in the event NeurogesX fails to exercise such rights or perform such duties and obligations; |
| • | to provide copies to CHRP (at the addresses set forth on Attachment A hereto) of all notices, reports and documents required to be or otherwise delivered to NeurogesX under the Distribution Agreement with respect to (i) amounts payable to NeurogesX under Articles 3, 4 or 11 thereof (including, without limitation, the reports required under Section 4.6 thereof) or (ii) any default or termination of the Distribution Agreement, in each case at the time of delivering the same to NeurogesX; provided, however, that the failure by Astellas to deliver any such notices, reports or documents shall not affect the validity of the delivery of such notice, report or document to NeurogesX under the Distribution Agreement. CHRP acknowledges that such notices, reports and documents contain Confidential Information (as defined in the Distribution Agreement) and agrees to be bound by the obligations under the Distribution Agreement of a receiving Party (as defined in the Distribution Agreement) with respect to such Confidential Information. |
| • | to pay all amounts due to NeurogesX under the Distribution Agreement in the manner described in the Distribution Agreement to one or more accounts as set forth in the joint instruction letter of CHRP and NeurogesX to be provided by such parties or any subsequent joint instruction of such parties; |
Each of NeurogesX and Astellas acknowledges that CHRP is a beneficiary of the terms and conditions of this letter; and accordingly, CHRP shall have the right to enforce the terms and conditions of this letter for its own benefit and no term or condition hereof may be amended or waived without the prior written consent of CHRP.
NeurogesX and CHRP acknowledge and agree that (i) nothing contained in any document executed in connection with the Financing Transaction (the “Financing Documents”) or this letter will diminish, prejudice or limit in any way the rights of Astellas under the Distribution Agreement or result in the subordination of any such rights to those granted by NeurogesX to Xxxxx under the Financing Documents, (ii) nothing in the Financing Documents is inconsistent with the Distribution Agreement or this letter, and (iii) in the event of any inconsistency between this letter and any Financing Document, the provisions of this letter shall govern.
Astellas acknowledges that LTS Xxxxxx Therapie-Systems AG (“LTS”) is also entering into a consent agreement with NeurogesX and CHRP and consents to such agreement provided such agreement specifically provides that it shall not limit the rights of Astellas to obtain Patches directly from LTS. NeurogesX and CHRP acknowledge and agree that (i) nothing contained in such agreement with LTS will diminish, prejudice or limit in any way the rights of Astellas under the Distribution Agreement or any agreements with LTS or result in the subordination of any such rights to those granted by LTS to Xxxxx under such agreement and (ii) nothing in such agreement is inconsistent with any agreement between Astellas and LTS.
Each of NeurogesX and Astellas represents that it has the full right, power and authority to enter into this letter and to perform hereunder, and as such this letter shall be a legal and valid obligation binding upon it. This Agreement shall be construed and enforced in accordance with the laws of the United States of America and the State of New York without regard to principles of conflicts of law.
If the foregoing is agreeable please have the appropriate corporate officer acknowledge below.
Sincerely,
NEUROGESX, INC.
Xxxxxxx Xxxxxxxxx Executive Vice President, Chief Operating Officer and Chief Financial Officer
Intending to be bound by the terms and conditions of this letter from and after the date below ASTELLAS PHARMA EUROPE LTD. executes below:
| By: |
| |
| Print Name: |
| |
| Title: |
| |
| Date: |
| |
Intending to be bound by the terms and conditions of this letter from and after the date below XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. executes below:
| By: |
| |
| Print Name: |
| |
| Title: |
| |
| Date: |
| |
ATTACHMENT A
CONTACT INFORMATION FOR CHRP
Xxxxx Healthcare Royalty, L.P.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Attention: Xxxxxxx X. Xxxxx, M.D.
Facsimile No.: x(000) 000-0000
Email: Xxxx.Xxxxx@xxxxx.xxx
with a copy to:
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx, P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxx, XX 00000
Attention: Y. Xxxxx Xxxxx, Esq.
Facsimile No.: x(000) 000-0000
Email: xxxxxx@xxxxxxx.xxx
or such other contact information as CHRP may notify Astellas from time to time in writing.
EXHIBIT G-2
FORM OF LTS CONSENT
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
[NGX Letterhead]
April 29, 2010
LTS XXXXXXX Therapie-Systeme AG
Xxxxxxxxxx. 0
Xxxxxxxxx, X-00000
XXXXXXX
Attention: Xxxxxxx Xxxxxxxxxxx
RE: Commercial Supply and Supply Agreement between NeurogesX, Inc. (“NeurogesX”) and LTS Xxxxxxx Therapie-Systems AG (“LTS”) (the “Supply Agreement”)
Dear Klaudia,
As you have been informed NeurogesX is entering into a financing transaction with Xxxxx Healthcare Royalty Partners, L.P. (“CHRP”). NeurogesX intends to secure the repayment of certain parts of the CHRP payments with monies receivable by NeurogesX under its Distribution, Marketing and Supply Agreement with Astellas Pharma Europe, Ltd. (“Astellas”) (the “Financing Transaction”). As part of the Financing Transaction, NeurogesX and CHRP are entering into certain arrangements to, among other things (i) secure performance and payment of NeurogesX obligations to CHRP, (ii) permit CHRP to ensure performance of NeurogesX’s obligations to LTS and other parties, in the event of a default by NeurogesX or a bankruptcy of NeurogesX, and (iii) assist in securing the continued commercialization of products incorporating or comprising Patches (as defined in the Supply Agreement, “Products”) in the “Territory” (i.e., only those countries listed on Attachment A hereto) in the event Astellas is no longer commercializing Products. In furtherance of these objectives, NeurogesX will be granting a first-priority security interest to CHRP in the Supply Agreement with respect to the Territory and related collateral assignment thereof to secure NeurogesX’s obligations thereunder. The rights granted to CHRP, however, will at all times be subject and subordinate to the rights of Astellas, it being the intention of both NeurogesX and CHRP for Astellas to continue commercializing Products in the Territory.
In connection with the foregoing and to induce CHRP to enter into the Financing Transaction, LTS confirms and agrees:
| • | to hereby consent to the grant of security interests in and related collateral assignment of the Supply Agreement in the Territory; |
| • | to hereby acknowledge that the Supply Agreement is in full force and effect and that it is not aware of any default or occurrence which may constitute a default thereunder, in each case as of the date hereof; the parties agree, however, that this acknowledgment does not constitute a waiver of any rights of LTS. |
| • | In the event NeurogesX fails to perform any of its obligations under the Supply Agreement in a timely manner, in accordance with Section 12.7 of the Supply Agreement LTS agrees to provide copies to CHRP (at the addresses set forth on Attachment B hereto) of all notices, required to be delivered to NeurogesX under Article 11 under the Supply Agreement. For |
| clarity, NeurogesX hereby authorizes such disclosure by LTS to CHRP notwithstanding Article 10 of the Supply Agreement; NeurogesX shall provide copies to CHRP of all notices, reports and documents exchanged between NeurogesX and LTS under the Supply Agreement that shall be made available to CHRP upon request. LTS hereby authorizes such disclosure by NeurogesX to CHRP notwithstanding Article 10 of the Supply Agreement. |
| • |
|
| • | If NeurogesX defaults under the Finance Agreement with CHRP or becomes bankrupt, and CHRP notifies LTS that CHRP has foreclosed on its security, CHRP may elect to continue to perform NeurogesX’s obligations under the Supply Agreement with respect to the Territory so that commercialization of Products in the Territory may continue without interruption, in which event LTS agrees to continue to perform under the Supply Agreement with respect to the Territory, subject to all of the terms and conditions of the Supply Agreement (including CHRP’s performance of NeurogesX’s obligations). CHRP agrees to declare such continuation of performance within 30 days of notice by NeurogesX. This shall not limit any rights of Astellas to obtain Patches directly from LTS. |
This letter shall be construed and enforced in accordance with the laws of the State of New Jersey, U.S.A., without regard to principles of conflicts of law and any dispute related to this letter will be resolved in the same manner as disputes are resolved under the Supply Agreement (i.e., in accordance with Section 12.1 of the Supply Agreement, with “Parties” referring to NeurogesX, LTS and CHRP for such purposes). This letter will terminate upon complete performance by NeurogesX (including payment of amounts due thereunder) of its obligations under the Finance Agreement. NeurogesX shall inform LTS in a timely manner thereof.
The parties agree that this agreement will only become effective upon the conditions of LTS’ receipt of a written consent by Astellas to this Agreement.
If the foregoing is agreeable please have the appropriate corporate officer acknowledge below.
Sincerely,
NEUROGESX, INC.
Xxxxxxx Xxxxxxxxx
Executive Vice President, Chief Operating Officer and Chief Financial Officer
CHRP and LTS intending to be bound by the terms and conditions of this letter:
XXXXX HEALTHCARE ROYALTY PARTNERS, L.P.
By Xxxxx Healthcare Royalty GP, LLC, Its General Partner
| By: | ||
| Name: | Xxxxxxx X. Xxxxx, M.D | |
| Title: | Managing Director |
| XXXXXXX THERAPIE-SYSTEMS AG: | ||
| By: | ||
| Print Name: | ||
| Title: |
ATTACHMENT A
TERRITORY
[***]
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
ATTACHMENT B
CONTACT INFORMATION FOR CHRP
Xxxxx Healthcare Royalty, L.P.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Attention: Xxxxxxx X. Xxxxx, M.D.
Facsimile No.: x(000) 000-0000
Email: Xxxx.Xxxxx@xxxxx.xxx
with a copy to:
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx, P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxx, XX 00000
Attention: Y. Xxxxx Xxxxx, Esq.
Facsimile No.: x(000) 000-0000
Email: xxxxxx@xxxxxxx.xxx
or such other contact information as CHRP may notify LTS from time to time in writing.
EXHIBIT G-3
FORM OF THE REGENTS CONSENT
- 1 -
[***] CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
[NGX Letterhead]
April 28, 2010
UCSF Office of Technology Management (OTM)
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxxxxxxx, XX 00000
Attention: Xxxx X. Xxxxxxxxxx, Ph.D.
RE: Exclusive License Agreement dated 1 November, 200 between NeurogesX, Inc. (“NeurogesX”) and The Regents of the University of California (“UC”) (the “License Agreement”)
Dear Xxxx,
As you know NeurogesX is entering into a financing transaction with Xxxxx Healthcare Royalty Partners, L.P. (“CHRP”) pursuant to which NeurogesX will finance certain of the payments receivable by NeurogesX under its Distribution, Marketing and License Agreement with Astellas Pharma Europe, Ltd. (the “Financing Transaction”). As part of the Financing Transaction, NeurogesX will be granting a first-priority security interest to CHRP in the License Agreement with respect to the “Territory” (i.e., those countries listed on Attachment A hereto) and related collateral assignment thereof to secure NeurogesX’s obligations thereunder.
In connection with the foregoing and to induce CHRP to enter into the Financing Transaction, we ask UC to agree to the following without further consideration:
| • | to hereby consent to the grant of security interests in and to related collateral assignment of the License Agreement in the Territory; |
| • | to hereby acknowledge that the License Agreement is in full force and effect as of the date hereof, and that no default exists, or with the passage of time or the giving of notice will exist by either UC or NeurogesX and that no breach of the License Agreement has been threatened by either UC or NeurogesX and that UC has not sent any notice thereunder of termination or default to NeurogesX; |
| • | to hereby agree not to amend or waive any term, condition or provision of the License Agreement in a manner that would diminish NeurogesX rights or UC’s obligations with respect to Regent Patent Rights (as defined in the License Agreement) in the Territory or otherwise in a manner adverse to the interests of CHRP without the prior written consent of CHRP; |
| • | to hereby acknowledge that, as between NeurogesX and CHRP, NeurogesX shall remain solely obligated under the License Agreement; however, CHRP may (but has no obligation to) exercise the rights and perform the duties of NeurogesX under the License Agreement as agent of and on behalf of NeurogesX in the event NeurogesX fails to exercise such rights or perform such duties and obligations; |
| • | to provide copies to CHRP (at the addresses set forth on Attachment B hereto) of all notices, reports and documents required to be or otherwise delivered to NeurogesX under the License Agreement with respect to (i) Regent Patent Rights in the Territory or (ii) any default or termination of the License Agreement, in each case at the time of delivering the same to NeurogesX; |
| • | to provide CHRP, in the event NeurogesX fails to perform any of its obligations under the License Agreement in a timely manner, an additional thirty (30) days from the date CHRP has received written notice of such failure to perform such obligations or if the same cannot be performed within such thirty (30) day period, such additional time as is necessary to perform such obligations, provided CHRP is at all times diligently acting to complete such performance and the period for performance does not exceed sixty (60) days total from the date CHRP received written notice of such failure; and |
| • | to continue to perform under the License Agreement with respect to the Territory subject to the terms and conditions thereof, if CHRP notifies UC that CHRP has foreclosed on the License Agreement with respect to the Territory as a result of NeurogesX’s default under the Financing Transaction. |
Each of NeurogesX and UC acknowledges that CHRP is an intended third party beneficiary of the terms and conditions of this letter; and accordingly, CHRP shall have the right to enforce the terms and conditions of this letter for its own benefit and no term or condition hereof may be amended or waived without the prior written consent of CHRP.
Each of NeurogesX and UC represents that it has the full right, power and authority to enter into this letter and to perform hereunder, and as such this letter shall be a legal and valid obligation binding upon it. This Agreement shall be construed and enforced in accordance with the laws of the United States of America and the State of California without regard to principles of conflicts of law.
If the foregoing is agreeable please have the appropriate corporate officer acknowledge below.
Sincerely,
NEUROGESX, INC.
Xxxxxxx Xxxxxxxxx
Executive Vice President, Chief Operating Officer and Chief Financial Officer
Intending to be bound by the terms and conditions of this letter from and after the date below THE REGENTS OF THE UNIVERSITY OF CALIFORNIA executes below:
| By: |
|
| Print Name: |
| |
| Title: |
| |
| Date: |
| |
ATTACHMENT A
TERRITORY
[***]
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
ATTACHMENT B
CONTACT INFORMATION FOR CHRP
Xxxxx Healthcare Royalty, L.P.
000 Xxxxx Xxxxxx, Xxxxx 0000
Xxxxxxxx, XX 00000
Attention: Xxxxxxx X. Xxxxx, M.D.
Facsimile No.: x(000) 000-0000
Email: Xxxx.Xxxxx@xxxxx.xxx
with a copy to:
Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx, P.C.
000 Xxxxxxxxx Xxxxxx, Xxxxx 0000
Xxx Xxxx, XX 00000
Attention: Y. Xxxxx Xxxxx, Esq.
Facsimile No.: x(000) 000-0000
Email: xxxxxx@xxxxxxx.xxx
or such other contact information as CHRP may notify UC from time to time in writing.
EXHIBIT H
FORM OF LICENSEE DIRECTION
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
NEUROGESX, INC.
0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000
Xxx Xxxxx, Xxxxxxxxxx 00000
May 6, 2010
Astellas Pharma Europe Ltd
Xxxxxx Xxxxx
Xxxxxx Xxxx
Xxxxxxx, Xxxxxxxxx, XX00 0XX
Xxxxxx Xxxxxxx
Attn: Xxxxx Xxxxx, Senior Vice President and General Counsel
Xxxxx:
Reference is made to (i) that letter between NeurogesX Inc. (“NGX”), Xxxxx Healthcare Royalty Partners, L.P. (“CHRP”) and Astellas Pharma Europe Ltd (“Astellas”) as of April 29, 2010 pursuant to which Astellas agreed among other things to pay amounts due to NGX under the Distribution, Marketing and License Agreement between NGX and Astellas dated 19 June 2009 (the “Distribution Agreement”) to one or more account jointly designated by NGX and CHRP and (ii) Sections 10.1 and 20.5 of the Distribution Agreement. NGX and CHRP hereby requests effective as of the date hereof that Astellas make all payments due to NGX under the Distribution Agreement (with the exception of payments due under Sections 2.5, 4.4, 7.1 and 8.4 thereof (the “Excepted Payments”)) by wire transfer in immediately available funds to the following account:
| Bank: | [***] | |||
| ABA Number: | [***] | |||
| Account Name: | [***] [***] | |||
| Account Number: | [***] |
For clarity, this letter does not change the payment instructions previously provided by NGX to Astellas for the Excepted Payments and any and all payments due pursuant to the Supply Agreement between NGX and Astellas dated 19 June 2009 (the “Supply Agreement”) and CHRP acknowledges that NGX shall have the sole right to specify the account(s) into which all such payments are due pursuant to Sections 10.1 of the Distribution Agreement and 10.1 of the Supply Agreement.
| Very truly yours, | ||
| NEUROGESX, INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
[ASTELLAS DIRECTION LETTER - SIGNATURES FOLLOW]
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
| XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. | ||
| By: |
| |
| Name: | ||
| Title: | ||
ACKNOWLEDGED AND
AGREED TO:
ASTELLAS PHARMA EUROPE LTD.
| By: |
| |
| Name: |
| |
| Title: |
[SIGNATURES TO ASTELLAS DIRECTION LETTER]
2
EXHIBIT I
FORM OF OFFICER’S CERTIFICATE (NEUROGESX)
- 1 -
[***] CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Dated as of April 29, 2010
OFFICER’S CERTIFICATE
This Officer’s Certificate is delivered pursuant to Section 6.02(i) of the Financing Agreement, dated as of April 29, 2010, (as amended, supplemented or otherwise modified from time to time, the “Financing Agreement”), between NeurogesX, Inc., a Delaware corporation (“NeurogesX”), and Xxxxx Healthcare Royalty Partners, L.P., a limited partnership organized under the laws of the State of Delaware (“CHRP”). Capitalized terms used but not defined herein have the meanings given to them in the Financing Agreement.
The undersigned, Xxxxxxx Xxxxxxxxx, a duly authorized officer of NeurogesX, hereby certifies to CHRP as follows:
(a) Attached hereto as Annex 1 are a true and complete copies of resolutions duly adopted by the Board of Directors of NeurogesX on February 16, 2010 and April 29, 2010 authorizing the execution and delivery of the Transaction Documents. Such resolutions have not in any way been amended, modified, revoked or rescinded, have been in full force and effect since their adoption through and including the date hereof, are now in full force and effect, and are the only corporate proceedings of NeurogesX now in force relating to or affecting the matters referred to therein.
(b) Attached hereto as Annex 2 is a true and complete copy of the Certificate of Incorporation and By-Laws of NeurogesX, together with all amendments, modifications and restatements thereof. Such Certificate of Incorporation and By-Laws are in full force and effect as of the date hereof, have not been repealed or supplemented except as may be specifically set forth in Annex 2, and are not subject to pending proceedings to amend, repeal, supplement, surrender or cancel same, or to any agreement(s) among directors or officers of NeurogesX, except as may be specifically disclosed in Annex 2.
[NGX OFFICER’S CERTIFICATE (CORPORATE MATTERS) - CONTINUED ON NEXT PAGE]
(c) Each of the following persons is a duly elected or appointed, qualified and acting officer of NeurogesX who holds the office or position set forth opposite such individual’s name, and is duly appointed by the resolutions attached hereto to execute and deliver documents on behalf of NeurogesX. The specimen signature written opposite each such officer’s name is such officer’s genuine signature:
| Name |
Title |
Signature | ||
| Xxxxxxx XxXxxxx | President & Chief Executive Officer |
| ||
| Xxxxxxx Xxxxxxxxx | Executive Vice President, Chief Operations Officer, Chief Financial Officer and Secretary |
|
IN WITNESS WHEREOF, the undersigned has executed this certificate as of the date first set forth above.
|
| ||
| Name: |
Xxxxxxx Xxxxxxxxx | |
| Title: |
Executive Vice President, Chief Operations Officer, Chief Financial Officer and Secretary | |
[SIGNATURE PAGE – NGX OFFICER’S CERTIFICATE (CORPORATE MATTERS)]
ANNEX 1
| 1. | The following resolutions were approved at a meeting of the Board of Directors in [***] |
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT A
Financing Agreement
EXHIBIT B
Assignment
EXHIBIT C
Distribution, Marketing and License Agreement
EXHIBIT D
Security Agreement
EXHIBIT E
Deposit Account Control Agreement
ANNEX 2
EXHIBIT J
FORM OF OFFICERS’ CERTIFICATE (CHRP)
- 1 -
Dated as of April 29, 2010
OFFICER’S CERTIFICATE
This Officer’s Certificate is delivered pursuant to Section 6.03(c) of the Financing Agreement, dated as of April 29, 2010, (as amended, supplemented or otherwise modified from time to time, the “Financing Agreement”), between NeurogesX, Inc., a Delaware corporation (“NeurogesX”), and Xxxxx Healthcare Royalty Partners, L.P., a limited partnership organized under the laws of the State of Delaware (“CHRP”). Capitalized terms used but not defined herein have the meanings given to them in the Financing Agreement.
The undersigned, Xxxxxxx Xxxxx, being a Managing Director of Cowen Healthcare Royalty GP, LLC (“CHRGP”), the general partner of CHRP, hereby certifies to NeurogesX, as follows:
(a) CHRGP is the general partner of CHRP pursuant to the Limited Partnership Agreement of CHRP, as amended through the date hereof, and has full power and authority to execute the Transaction Documents on behalf of CHRP.
(b) Attached hereto as Annex 1 is a true and complete copy of resolutions duly adopted by the Managing Directors of CHRGP as if April 29, 2010, authorizing the execution and delivery of the Transaction Documents. Such resolutions have not in any way been amended, modified, revoked or rescinded, have been in full force and effect since their adoption through and including the date hereof, are now in full force and effect and are the only proceedings of CHRP or CHRGP now in force relating to or affecting the matters referred to therein.
(c) The following person holds the office or position of CHRGP set forth opposite such individual’s name, and is duly appointed by the resolutions attached hereto to execute and deliver documents on behalf of CHRP and CHRGP. The specimen signature written opposite such person’s name is such person’s genuine signature:
| Name |
Title |
Signature |
||||||
| Xxxxxxx Xxxxx |
Managing Director |
IN WITNESS WHEREOF, the undersigned has executed this certificate as of the date first set forth above.
|
| ||
| Name: Xxxxxxx Xxxxx | ||
| Title: Managing Director | ||
[CHRP OFFICER’S CERTIFICATE (CORPORATE MATTERS)]
ANNEX 1
RESOLVED, that Xxxxx Healthcare Royalty Partners, L.P. (“CHRP”) enter into the transactions described in that certain Financing Agreement (the “Financing Agreement”), with NeurogesX, Inc., a Delaware corporation (“NeurogesX”), substantially in the form presented to the Investment Committee of Cowen Healthcare Royalty GP, LLC (“CHRGP”); and it is further
RESOLVED, that CHRGP, on behalf of CHRP, execute and deliver the Financing Agreement, substantially in the form of the agreement presented to the Investment Committee of CHRGP, and all Transaction Documents described in the Financing Agreement; and it is further
RESOLVED, that Xxxxxxx Xxxxx as a Managing Director of CHRGP, the general partner of CHRP, is hereby authorized and directed to execute all Transaction Documents with such terms and in such form as he, in his sole discretion, may deem necessary, appropriate or desirable to effectuate the foregoing resolutions; and it is further
RESOLVED, that, without further action by the Managing Directors of CHRGP, each of them is hereby authorized and empowered, in the name of and on behalf of CHRGP and CHRP, to execute and deliver all such further instruments and documents and take all such further actions as they may deem necessary, appropriate or desirable in order to implement and give effect to the foregoing resolutions and the intent and purposes thereof.
EXHIBIT K
FORM OF DEPOSIT AGREEMENT
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXECUTION COPY
DEPOSIT ACCOUNT CONTROL AGREEMENT
This DEPOSIT ACCOUNT CONTROL AGREEMENT (as amended, supplemented or otherwise modified from time to time, this “Deposit Agreement”) is dated as of April 29, 2010, and entered into by and among NeurogesX, Inc., a Delaware corporation (“NeurogesX”), Xxxxx Healthcare Royalty Partners, L.P., a Delaware limited partnership (“CHRP”), and JPMorgan Chase Bank, N.A., a national association, in its capacity as a depositary bank and in its capacity as a “bank” (as defined in Section 9-102(a)(8) of the UCC) (in such capacities, the “Financial Institution”).
RECITALS
WHEREAS, NeurogesX and CHRP are parties to that certain Financing Agreement, dated as of April 29, 2010 (as amended, supplemented or otherwise modified from time to time, the “Financing Agreement”), pursuant to which NeurogesX has among other things, agreed to assign, transfer and convey to CHRP certain rights in the Revenue Interest;
WHEREAS, in the Financing Agreement, NeurogesX agreed to instruct and use commercially reasonable efforts to cause Astellas Pharma Europe Ltd. (“Astellas”) to send payments in respect of the Licensed Products to the Initial Concentration Account (as such term is defined below);
WHEREAS, NeurogesX is a party to the Security Agreement, dated as of April 29, 2010 (as the same may be amended, supplemented or otherwise modified from time to time, the “Security Agreement”), pursuant to which, among other things, NeurogesX granted in favor of CHRP a security interest in, among other property, this Deposit Agreement, the Initial Concentration Account and Joint Concentration Account and all funds on deposit in such accounts;
WHEREAS, the Financial Institution acknowledges that NeurogesX has granted in favor of CHRP a security interest in the Joint Concentration Account and the Initial Concentration Account and all Deposit Funds held therein or credited thereto; and
WHEREAS, NeurogesX and CHRP contemplate that Accounts will be maintained by the parties hereto at the Financial Institution will be established as of the date hereof; and
WHEREAS, each of the parties hereto desires to enter into this Deposit Agreement in order (i) to perfect CHRP’s security interest in the Joint Concentration Account and the Initial Concentration Account by “control” pursuant to Section 9-104 of the UCC (as such term is defined below), and (ii) to set forth their respective rights and obligations with respect to the Accounts.
NOW, THEREFORE, in consideration of the foregoing and of the mutual covenants hereinafter set forth and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto hereby agree as follows:
Section 1. Certain Terms. Capitalized terms when used in this Deposit Agreement, including its preamble, recitals and schedules, shall have the meanings set forth in Annex A attached hereto.
Section 2. Accounts.
(a) NeurogesX and CHRP have caused the Financial Institution to establish, and the Financial Institution hereby confirms that the Financial Institution has established, the following accounts at the Financial Institution (collectively, the “Accounts”; each, an “Account”) designated as indicated below:
(i) a deposit account in the name of NeurogesX and CHRP bearing account number [***] (the “Initial Concentration Account”), which account shall be interest-bearing;
(ii) a deposit account in the name of NeurogesX and CHRP bearing account number [***] (the “Joint Concentration Account”), which account shall be interest-bearing;
(iii) a deposit account in the name of NeurogesX bearing account number [***] (the “NeurogesX Concentration Account”), which account shall be interest-bearing; and
(iv) a deposit account in the name of CHRP, bearing account number [***] (the “CHRP Concentration Account”), which account shall be interest-bearing.
(b) The Financial Institution shall not change the name or account number of any Account (other than the NeurogesX Concentration Account) without the prior written consent of CHRP. Similarly, the Financial Institution shall not change the name or account number of any Account (other than the CHRP Concentration Account) without the prior written consent of NeurogesX.
(c) The parties hereto acknowledge and agree that each Account is a “deposit account” within the meaning of Section 9-102(a)(29) of the UCC and that the Financial Institution’s jurisdiction for purposes of the Accounts under the UCC shall be New York.
(d) The Deposit Funds in the Accounts shall be invested in Permitted Investments.
Section 3. Control of the Joint Concentration Account and the Initial Concentration Account. In order to perfect CHRP’s security interest in the Joint Concentration Account and the Initial Concentration Account and any and all Deposit Funds held in the Joint Concentration Account or in the Initial Concentration Account or credited to the Joint
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
2
Concentration Account or to the Initial Concentration Account by “control” pursuant to Section 9-104 of the UCC, NeurogesX, CHRP and the Financial Institution agree as follows:
(a) Notwithstanding any separate agreement NeurogesX may have with the Financial Institution but without limiting Section 5.07(e) of the Financing Agreement, CHRP shall be entitled at any time to give the Financial Institution an Account Instruction pertaining to or concerning the Initial Concentration Account, the Joint Concentration Account and/or any Deposit Funds in or credited to the Initial Concentration Account or the Joint Concentration Account. If at any time the Financial Institution shall receive an Account Instruction from CHRP pertaining to or concerning the Initial Concentration Account or Joint Concentration Account, and/or any Deposit Funds in or credited to the Initial Concentration Account or the Joint Concentration Account, the Financial Institution shall comply with such Account Instruction without prior or further consent by NeurogesX or any other Person. The Financial Institution shall comply with, and is fully entitled to rely upon, any Account Instruction from CHRP pertaining to or concerning the Initial Concentration Account, the Joint Concentration Account and/or any Deposit Funds in or credited to the Joint Concentration Account or the Initial Concentration Account, even if such Account Instruction is contrary to any Account Instruction that NeurogesX may give or may have given to the Financial Institution pertaining to or concerning the Initial Concentration Account, the Joint Concentration Account, or any Deposit Funds in or credited to the Initial Concentration Account or Joint Concentration Account. Without limiting or expanding the foregoing, Financial Institution agrees to notify NeurogesX of any and all Account Instructions given by CHRP with respect to the Initial Concentration Account, Joint Concentration Account or any Deposit Funds in or credited to the Initial Concentration Account or Joint Concentration Account.
(b) NeurogesX shall not be entitled to give to the Financial Institution any Account Instruction pertaining to or concerning the Initial Concentration Account or Joint Concentration Account or any Deposit Funds in or credited to the Joint Concentration Account or the Initial Concentration Account, except with the prior written consent of CHRP. The Financial Institution shall not comply with any Account Instruction given by NeurogesX pertaining to or concerning the Initial Concentration Account, the Joint Concentration Account or any Deposit Funds in or credited to the Initial Concentration Account or the Joint Concentration Account, except with the prior written consent of CHRP.
(c) The Financial Institution has not agreed and will not agree with any third party to comply with any Account Instruction or other instruction, order or direction from such third party pertaining to or concerning the Joint Concentration Account, the Initial Concentration Account or any Deposit Funds in the Joint Concentration Account or in the Initial Concentration Account or credited to the Joint Concentration Account or to the Initial Concentration Account without the prior written consent of CHRP.
Section 4. Financial Institution’s Obligations with respect to the Accounts.
(a) The Initial Concentration Account, upon its establishment pursuant hereto, shall be used for remittances which are to be deposited on a daily basis pursuant to the terms of this Deposit Agreement and the procedures set forth in Schedule 1. The Financial Institution
3
shall have exclusive and unrestricted access to, and use of, the Initial Concentration Account for the purpose of handling such remittances in accordance with this Deposit Agreement.
(b) The Financial Institution agrees to maintain the Joint Concentration Account and the Initial Concentration Account as contemplated by this Deposit Agreement and agrees not to commingle the Deposit Funds in or credited to, or designated for deposit in, the Joint Concentration Account or the Initial Concentration Account with any other Deposit Funds held on behalf of NeurogesX, CHRP or any other Person or entity. The Financial Institution acknowledges that the Joint Concentration Account, the Initial Concentration Account and any Deposit Funds in or credited to the Joint Concentration Account or the Initial Concentration Account are subject to the first priority security interest of CHRP therein. The Financial Institution agrees not to apply any Deposit Funds received in the Joint Concentration Account or the Initial Concentration Account and not to make disbursements from or debits to the Joint Concentration Account or the Initial Concentration Account in each case other than in accordance with this Deposit Agreement.
(c) The parties hereto agree that items deposited in the Initial Concentration Account shall be deemed to bear the valid and legally binding endorsement of the payee and to comply with all of the Financial Institution’s requirements for the supplying of missing endorsements, now or hereafter in effect. Any deposit made into the Initial Concentration Account shall be deemed deposited and credited into the Initial Concentration Account only when the funds in respect of such deposit shall become cleared funds.
(d) The Financial Institution shall redeposit with advice any item returned for any reason. If any item is returned a second time, NeurogesX agrees to pay the Financial Institution its then-current standard charges for returned items promptly after notification thereof. In the event NeurogesX does not pay the Financial Institution its charge for returned items, the Financial Institution may charge the amount of such items and charges to any account of NeurogesX (other than the Joint Concentration Account described herein) held at the Financial Institution to the extent funds therefor are available in such account. The Financial Institution shall return the item along with the debit advice to NeurogesX with a copy to CHRP. The Financial Institution is granted the further right to debit from the Initial Concentration Account any amounts deposited therein in error or as necessary to correct processing errors.
(e) On each Business Day that the Financial Institution receives any new Deposit Funds in any Account, the Financial Institution shall notify NeurogesX and CHRP in writing that it has received such Deposit Funds into such Account, and the Financial Institution shall set forth in such writing (i) the name of the Account into which such Deposit Funds were received, if such payments have been received, (ii) the date of receipt of such Deposit Funds, (iii) the amount of such receipt and (iv) the name of the payor.
(f) Within five (5) Business Days following the end of each month, the Financial Institution shall cause to be made available to NeurogesX and CHRP by means of online computer access in a format or formats reasonably satisfactory to NeurogesX and CHRP, and within ten (10) Business Days following the end of each month shall send or cause to be sent a statement for such month to NeurogesX and CHRP outlining a list of, (i) the amounts, if any, transferred from the Initial Concentration Account and the Joint Concentration Account, the
4
dates of each such transfer and the accounts into which such transfers were made, (ii) the balance, if any, in the Initial Concentration Account and the Joint Concentration Account as of the end of such month, and (iii) any other debits or credits made during the month to the Initial Concentration Account and the Joint Concentration Account together with a description thereof.
(g) The Financial Institution will notify CHRP and NeurogesX promptly if any other Person claims a property interest in any of the Accounts.
Section 5. Disposition of Deposit Funds in the Initial Concentration Account.
(a) All Deposit Funds received by the Financial Institution in the Initial Concentration Account will be deposited in the Joint Concentration Account, in accordance with the terms of this Deposit Agreement and the procedures and allocations set forth in Schedule 1; and those Deposit Funds will, in turn, be withdrawn from the Joint Concentration Account and distributed to the CHRP Concentration Account and the NeurogesX Concentration Account, in accordance with the terms of this Deposit Agreement and the procedures and allocations set forth in Schedule 1.
(b) Any transfer of funds from the Joint Concentration Account to the NeurogesX Concentration Account shall be made by wire transfer or similar method of transfer of immediately available funds, unless otherwise agreed to in writing by NeurogesX. Any transfer of funds from the Joint Concentration Account to the CHRP Concentration Account shall be made by wire transfer or similar method of transfer of immediately available funds, unless otherwise agreed to in writing by CHRP.
(c) Notwithstanding anything to the contrary as set forth in Section 15, any instructions setting forth, claiming, containing, objecting to, or in any way related to the transfer or distribution of funds, including but not limited to any such funds transfer instructions that may otherwise be set forth in a written instruction permitted pursuant to Section 3 of this Agreement, may be given to the Financial Institution only by confirmed facsimile and no instruction for or related to the transfer or distribution of the Deposit Funds, or any portion thereof, shall be deemed delivered and effective unless the Financial Institution actually shall have received such instruction by facsimile at the number provided to the parties by the Financial Institution in accordance with Section 15 and as further evidenced by a confirmed transmittal to that number.
(d) In the event funds transfer instructions are so received by the Financial Institution by facsimile, the Financial Institution is authorized to seek confirmation of such instructions by telephone call-back to the person or persons designated on Schedules 3 or 4 hereto, and the Financial Institution may rely upon the confirmation of anyone purporting to be the person or persons so designated. The persons and telephone numbers for call-backs may be changed only in a writing actually received and acknowledged by the Financial Institution. If the Financial Institution is unable to contact any of the authorized representatives identified in Schedules 3 or 4, the Financial Institution is hereby authorized both to receive written instructions from and seek confirmation of such instructions by telephone call-back to any one or more of NeurogesX or CHRP’s executive officers, (“Executive Officers”), as the case may be, which shall include the titles of President, Chief Executive Officer, Vice-President, Managing Director, as the Financial Institution may select. Such “Executive Officer” shall deliver to the
5
Financial Institution a fully executed incumbency certificate, and the Financial Institution may rely upon the confirmation of anyone purporting to be any such officer. The Financial Institution and the beneficiary’s bank in any funds transfer may rely solely upon any account numbers or similar identifying numbers provided by NeurogesX or CHRP to identify (i) the beneficiary, (ii) the beneficiary’s bank, or (iii) an intermediary bank. The Financial Institution may apply any of the Deposit Funds for any payment order it executes using any such identifying number, even when its use may result in a person other than the beneficiary being paid, or the transfer of funds to a bank other than the beneficiary’s bank or an intermediary bank designated.
(e) NeurogesX acknowledges that the Financial Institution is authorized to use the following funds transfer instructions to disburse any funds due to NeurogesX under this Deposit Agreement without a verifying call-back as set forth in Section 5(d) above:
NeurogesX’s Bank account information: [***]
CHRP acknowledges that the Financial Institution is authorized to use the following funds transfer instructions to disburse any funds due to CHRP under this Deposit Agreement without a verifying call-back as set forth in Section 5(d) above:
CHRP’s Bank account information: [***]
(f) The parties acknowledge that the security procedures set forth in this Section 5d are commercially reasonable.
Section 6. Subordination of Lien; Waiver of Set-Off. In the event that the Financial Institution has obtained or subsequently obtains by agreement, by operation of law or otherwise a security interest in, lien on, or encumbrance, claim or (except as provided in the next sentence) right of set-off against, the Initial Concentration Account, the Joint Concentration Account or any security entitlement or any Deposit Funds therein or credited thereto, the Financial Institution hereby agrees that such security interest, lien, encumbrance, claim, and right of set-off shall be subordinate to the security interest or other interest of CHRP in the Initial Concentration Account, the Joint Concentration Account and the security entitlement or any Deposit Funds therein or credited thereto. The Financial Institution agrees not to exercise any present or future right of recoupment or set-off against the Initial Concentration Account or Joint Concentration Account or to assert against the Initial Concentration Account or Joint Concentration Account any present or future security interest, banker’s lien or any other lien or claim (including claim for penalties) that the Financial Institution may at any time have against or in any of the Initial Concentration Account, Joint Concentration Account or any security entitlement or any Deposit Funds therein or credited thereto; provided, however, that, the Financial Institution may set-off the face amount of any checks which have been credited to the Initial Concentration Account but are subsequently returned unpaid because of uncollected or insufficient funds which are not repaid by NeurogesX in accordance with Section 4(d).
Section 7. Certain Matters Affecting the Financial Institution.
(a) The Financial Institution undertakes to perform only such duties and obligations as are expressly set forth herein and no implied duties and obligations shall be read into this Deposit Agreement against the Financial Institution. The Financial Institution shall
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
6
have no liability under and no duty to inquire as to the provisions of any agreement other than this Deposit Agreement. The Financial Institution shall neither be responsible for, nor chargeable with knowledge of, nor have any requirements to comply with, the terms and conditions of any other agreement, instrument or document between NeurogesX and CHRP in connection herewith, if any, including without limitation the Financing Agreement and/or the Security Agreement (the “Underlying Agreements”), nor shall the Financial Institution be required to determine if any person or entity has complied with any Underlying Agreement, nor shall any additional obligations of the Financial Institution be inferred from the terms of any Underlying Agreements, even though reference thereto may be made in this Deposit Agreement. In the event of any conflict between the terms and provisions of this Deposit Agreement, those of any Underlying Agreement, any schedule or exhibit attached to the Deposit Agreement, or any other agreement among the parties, the terms and conditions of this Deposit Agreement shall control.
(b) The Financial Institution may rely upon and shall not be liable for acting in accordance with this Deposit Agreement upon any written notice, instruction or request furnished to it hereunder and reasonably believed by it to be genuine and to have been signed or presented by the proper party or parties, or for refraining from acting if and to the extent that such written notice, instruction or request requires it to refrain from acting. The Financial Institution shall not be liable to any party, any beneficiary or other person for refraining from acting upon any instruction setting forth, claiming, containing, objecting to, or related to the transfer or distribution of the Deposit Funds, or any portion thereof, unless such instruction shall have been delivered to the Financial Institution in accordance with Section 5(c) above and the Financial Institution has been able to satisfy any applicable security procedures as may be required thereunder. The Financial Institution shall be under no duty to inquire into or investigate the validity, accuracy or content of any such document. The Financial Institution shall have no duty to solicit any payments which may be due it of the Accounts, including, without limitation, the Deposit Funds nor shall the Financial Institution have any duty of obligation to confirm or verify the accuracy or correctness of any amounts deposited with it hereunder.
(c) The Financial Institution shall not be liable for any action taken or omitted by it in good faith except to the extent that a final adjudication of a court of competent jurisdiction determines that the Financial Institution’s gross negligence or willful misconduct was the cause of any loss, expense, cost, damage or liability to either or both of NeurogesX or CHRP.
(d) The Financial Institution (i) may execute any of its powers and perform any of its duties hereunder directly or through agents or affiliates and (ii) may consult with counsel, accountants and other skilled persons to be selected and retained by it. The Financial Institution shall not be liable for any actions taken, suffered or omitted to be taken by it in accordance with, or in reliance upon, the advice or opinion or such counsel, accountants or other skilled persons.
(e) In the event that (i) the Financial Institution is in doubt as to its duties or rights hereunder or as to the action it should take hereunder or (ii) the Financial Institution shall receive any Account Instruction or other instructions, claims or demands from NeurogesX or
7
CHRP which, in its opinion, conflict with any of the provisions of this Deposit Agreement, then the Financial Institution, in each case, shall be entitled to refrain from taking any action and its sole obligation shall be to keep safely all property held in the Initial Concentration Account and the Joint Concentration Account and the Financial Institution shall be entitled to place a hold on the Deposit Funds in the Initial Concentration Account and the Joint Concentration Account, in each case until the Financial Institution shall be directed otherwise in writing by another party hereto in accordance with this Deposit Agreement, by an order of an arbitrator, or by a final order or judgment of a court of competent jurisdiction. The parties agree to use their reasonable efforts to pursue any redress or recourse in connection with any dispute without making the Financial Institution a party to the same.
(f) The Financial Institution may resign and be discharged from its duties or Obligations hereunder by giving thirty (30) days advance writing of such resignation to NeurogesX and CHRP specifying a date when such resignation shall take effect. If NeurogesX and CHRP have failed to appoint a successor financial institution prior to the expiration of thirty (30) days following receipt of the notice of resignation, the Financial Institution may petition any court of competent jurisdiction for the appointment of a successor financial institution or for other appropriate relief, and any such resulting appointment shall be binding upon all of the parties hereto. The Financial Institution’s sole responsibility after such thirty (30) day notice period expires shall be to hold the Deposit Funds (without any obligation to reinvest the same) and to deliver the same to a designated substitute financial institution, if any, or in accordance with the directions of a final order or judgment of a court of competent jurisdiction, at which time of delivery the Financial Institution’s obligations hereunder shall cease and terminate.
(g) Any legal entity into which the Financial Institution in its individual capacity may be merged or converted or with which it may be consolidated, or any legal entity resulting from any merger, conversion or consolidation to which the Financial Institution in its individual capacity shall be a party, or any legal entity to which substantially all the escrow business of the Financial Institution in its individual capacity may be transferred, shall be the Financial Institution under this Deposit Agreement without further act or notice.
(h) In the event that the Financial Institution is unable to perform its obligations under the terms of this Deposit Agreement (i) because of acts of God, strikes, equipment or transmission failure or damage reasonably beyond its control, or any other cause reasonably beyond its control, or (ii) because, upon advice of the Financial Institution’s counsel, performance would violate any applicable guideline, rule or regulation of any governmental authority having jurisdiction over the Financial Institution, then, in each case with respect to the foregoing clauses (i) and (ii) in this subsection (h), the Financial Institution shall promptly notify NeurogesX and CHRP thereof in writing and shall not be liable for damages to the other parties for any unforeseeable damages resulting from such failure to perform or otherwise from such causes. Performance under this Deposit Agreement by the Financial Institution shall resume when the Financial Institution is able to perform substantially its duties hereunder.
(i) Anything in this Deposit Agreement to the contrary notwithstanding, in no event shall the Financial Institution be liable for any special, indirect, exemplary or consequential loss or damage of any kind whatsoever (including, but not limited to, lost profits),
8
even if the Financial Institution has been advised of the likelihood of such loss or damage and regardless of the form of action.
Section 8. Conflict with Other Agreements; Adverse Claims.
(a) In the event of any conflict between this Deposit Agreement (or any portion hereof) and any other agreement now existing or hereafter entered into by any of the parties hereto, the terms of this Deposit Agreement shall prevail; provided, that NeurogesX and CHRP confirm to each other that nothing herein is intended to expand, modify or limit the rights and/or obligations of NeurogesX and CHRP under the Financing Agreement and/or the Security Agreement; and provided, further, that in the event of any inconsistency between this Deposit Agreement and the terms of the Financing Agreement and/or the Security Agreement, the terms and provisions of the Financing Agreement and the Security Agreement shall control as between NeurogesX, on the one hand, and CHRP, on the other hand.
(b) The Financial Institution hereby confirms and agrees that:
(i) there are no agreements entered into between the Financial Institution and any other Person with respect to any Account except for this Deposit Agreement;
(ii) it has not entered into, and until the termination of this Deposit Agreement will not enter into, any agreement with any Person (other than NeurogesX and CHRP) relating to the Accounts and/or any Deposit Funds therein or credited thereto pursuant to which it has agreed to comply with any instructions of such Person concerning any of the Accounts.
Section 9. Authorized Representatives. Each individual designated as an authorized representative of NeurogesX or of CHRP, respectively (an “Authorized Representative”), is authorized to give and receive notices, requests and instructions and to deliver certificates and documents in connection with this Deposit Agreement on behalf of NeurogesX or CHRP, as the case may be. The specimen signature for each Authorized Representative of NeurogesX initially authorized hereunder is set forth on Schedule 3. The specimen signature for each Authorized Representative of CHRP initially authorized hereunder is set forth on Schedule 4. From time to time, NeurogesX or CHRP may, by delivering to each other and the Financial Institution a revised Schedule 3 or 4 (as applicable), change the information previously given pursuant to this Section 9, but each of the parties hereto shall be entitled to rely conclusively on the then current Schedule until receipt of a superseding Schedule.
Section 10. Representations, Warranties and Covenants.
(a) Representations, Warranties and Covenants of the Financial Institution. The Financial Institution hereby represents and warrants to NeurogesX and CHRP that:
(i) the Accounts have each been established as set forth in Section 2, and the Financial Institution covenants that the Accounts will be maintained in the manner set forth herein until termination of this Deposit Agreement;
9
(ii) it has not assigned, pledged or granted a security interest in any of the Accounts or any Deposit Funds in or credited in such Accounts;
(iii) this Deposit Agreement constitutes the legal, valid and binding obligation of the Financial Institution, enforceable against the Financial Institution in accordance with its terms, except as enforcement may be limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance or other similar laws affecting the enforcement of creditors’ rights generally or by general equitable principles (regardless of whether enforcement is sought in a proceeding in equity or at law); and
(iv) Bank has marked its books and records to indicate that CHRP has a first priority security interest in the Initial Concentration Account and Joint Concentration Account and the right to control such accounts as set forth herein.
(b) Representations and Warranties of NeurogesX. NeurogesX hereby represents and warrants to the Financial Institution and CHRP that:
(i) the execution, delivery and performance by NeurogesX of this Deposit Agreement have been duly authorized by all necessary action on the part of NeurogesX;
(ii) this Deposit Agreement has been duly executed and delivered by NeurogesX;
(iii) this Deposit Agreement constitutes the legal, valid and binding obligation of NeurogesX, enforceable against NeurogesX in accordance with its terms, except as enforcement may be limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance or other similar laws affecting the enforcement of creditors’ rights generally or by general equitable principles (regardless of whether enforcement is sought in a proceeding in equity or at law);
(iv) the performance by NeurogesX of its obligations under this Deposit Agreement will not constitute or result in a breach of (A) NeurogesX’s certificate of incorporation or bylaws or (B) the provisions of any material contract to which NeurogesX is a party or by which it is bound; and
(v) all approvals and authorizations required to permit the execution, delivery, performance by NeurogesX of this Deposit Agreement have been obtained.
(c) Representations and Warranties of CHRP. CHRP hereby represents and warrants to the Financial Institution and NeurogesX that:
(i) the execution, delivery and performance by CHRP of this Deposit Agreement have been duly authorized by all necessary action on the part of CHRP;
10
(ii) this Deposit Agreement has been duly executed and delivered by CHRP;
(iii) this Deposit Agreement constitutes the legal, valid and binding obligation of CHRP, enforceable against CHRP in accordance with its terms, except as enforcement may be limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance or other similar laws affecting the enforcement of creditors’ rights generally or by general equitable principles (regardless of whether enforcement is sought in a proceeding in equity or at law);
(iv) the performance by CHRP of its obligations under this Deposit Agreement will not constitute or result in a breach of (A) CHRP’s partnership agreement or other organizational documents, or (B) the provisions of any material contract to which CHRP is a party or by which CHRP is bound; and
(v) all approvals and authorizations required to permit the execution, delivery, performance by CHRP of this Deposit Agreement have been obtained.
Section 11. Termination of this Deposit Agreement.
(a) Except as otherwise provided in this Section 11, this Deposit Agreement shall continue in effect until CHRP has notified the Financial Institution in writing that the security interest of CHRP in the Initial Concentration Account and Joint Concentration Account have been terminated pursuant to the terms of the Security Agreement.
(b) CHRP may terminate this Deposit Agreement at any time upon its delivery of written notice of such termination to the Financial Institution and NeurogesX and shall terminate this Deposit Agreement by notification thereof to the Financial Institution as soon as practicable upon any early termination (but not expiration) of the Security Agreement or the Financing Agreement.
(c) NeurogesX may not terminate this Deposit Agreement for any reason without the prior written consent of CHRP, provided, however, that upon the occurrence of any one of the following conditions (each, a “Termination Condition”): (i) the Second Stepdown, (ii) termination of the Financing Agreement in accordance with 7.02(a) thereof, or (iii) full payment has been made to CHRP pursuant to Sections 7.02(c) or 2.02(e) of the Financing Agreement, NeurogesX may terminate this Deposit Agreement upon five (5) Business Days’ prior written notice to Financial Institution copying CHRP, which notice shall certify the Termination Condition that has been met; provided that CHRP does not provide notice objecting to such termination within such five (5) Business Day-period by written notice disputing that such Termination Condition has been met.
(d) The Financial Institution, acting alone, may terminate this Deposit Agreement at any time and for any reason by written notice delivered to NeurogesX and CHRP not less than forty-five (45) Business Days prior to the effective termination date.
(e) Prior to any termination of this Deposit Agreement, the Financial Institution hereby agrees that it shall promptly take, at the expense of NeurogesX and CHRP, all reasonable
11
actions necessary to facilitate the transfer of any Deposit Funds in or credited to the Accounts as follows: (i) in the case of a termination of this Deposit Agreement under Section 11(b), to the depository institution designated in writing by CHRP copying NeurogesX; (ii) in the case of a termination of this Deposit Agreement under Section 11(c), to the depository institution designated in writing by NeurogesX copying CHRP; (iii) in the case of a termination of this Deposit Agreement under Section 11(d), to the depository institution jointly designated in writing by NeurogesX and CHRP; (iv) in all other cases, in accordance with CHRP’s instructions if an Account Instruction has been given by CHRP in accordance with the terms and conditions of this Deposit Agreement.
(f) No termination of this Deposit Agreement shall impair the rights of any party hereto with respect to checks processed prior to the effective date of termination.
Section 12. Fees and Expenses. The Financial Institution agrees to look solely to NeurogesX for payment of its Annual Fee (as defined below) and other fees in connection with its maintenance of the Accounts and services hereunder along with any fees or charges for the Accounts, including those levied by any governmental authority which the Financial Institution may impose, charge or pass-through; provided, however, that the fees which the Financial Institution may charge NeurogesX shall, in the aggregate, not exceed the fees and charges customarily charged by the Financial Institution to its customers with respect to the customary and standard maintenance of a clearing account. Annual fees for the maintenance of the Accounts and the services to be provided by the Financial Institution hereunder are $5,000 for the first twelve (12) months following the date hereof, and $5,000 annually thereafter (the “Annual Fee”), without proration for partial calendar years. The obligations contained in this Section 12 shall survive the termination of this Deposit Agreement and the resignation, replacement, or removal of the Financial Institution.
Section 13. Indemnity.
(a) NeurogesX and CHRP shall jointly and severally indemnify, defend and hold harmless the Financial Institution and its affiliates and their respective successors, assigns, directors, officers, managers, agents and employees (collectively, the “Indemnitees”) from and against any and all Liabilities incurred by the Financial Institution arising out of or in connection with (i) this Deposit Agreement or (ii) the Financial Institution’s performance of its obligations and services under this Deposit Agreement, the enforcement of any rights and remedies under or in connection with this Deposit Agreement, or may arise by reason of any act, omission or error of the Indemnitee in connection with Financial Institution’s performance of its obligations and services under this Deposit Agreement, except in the case of any Indemnitee to the extent that such Liabilities are finally adjudicated by a court of competent jurisdiction to have been caused by the gross negligence or willful misconduct of such Indemnitee, or (iii) its following any instructions or other directions given under this Deposit Agreement, whether joint or singular, from NeurogesX and CHRP, except to the extent that its following any such instruction or direction is expressly forbidden by the terms hereof.
(b) The parties hereto acknowledge that the foregoing indemnities shall survive the resignation, replacement or removal of the Financial Institution hereunder or the termination of this Deposit Agreement until the expiration of the applicable statute of limitations.
12
Section 14. TINs and Account Opening Information.
(a) NeurogesX and CHRP each represent to the Financial Institution and to each other that its respective correct taxpayer identification number (“TIN”) assigned by the Internal Revenue Service is set forth in Schedule 2. Upon execution of this Deposit Agreement, each of NeurogesX and CHRP shall deliver to the Financial Institution a W-8 or W-9 Internal Revenue Service form duly executed by it.
(b) All interest or other income earned on amounts deposited in the Initial Concentration Account and the Joint Concentration Account (i) shall be allocated to NeurogesX unless otherwise instructed by CHRP after delivery by CHRP of an Account Instruction pertaining to or concerning the Joint Concentration Account in accordance with Section 3(a) of this Deposit Agreement and (ii) shall be reported to the Internal Revenue Service or any other applicable taxing authority by the recipient or other party hereto as directed by CHRP after delivery by CHRP of such an Account Instruction. Notwithstanding such written directions, the Financial Institution shall report and, as required withhold any taxes as it reasonably determines may be required by any law or regulation in effect at the time of the distribution. In addition, the Financial Institution shall withhold from the Initial Concentration Account and Joint Concentration Account any taxes required by law to be withheld and shall remit such taxes to the appropriate authorities. Any taxes withheld by the Financial Institution shall be included in the statement required by Section 4(f).
(c) All items of income, gain, expense and loss recognized in the NeurogesX Concentration Account shall be reported to the Internal Revenue Service and all state and local taxing authorities under the name and TIN of NeurogesX. All items of income, gain, expense and loss recognized in the CHRP Concentration Account shall be reported to the Internal Revenue Service and all state and local taxing authorities under the name and TIN of CHRP.
(d) The parties hereto agree that if NeurogesX and CHRP deliver to the Financial Institution an appropriately completed Form W-8 or W-9 in accordance with Section 14(a) of this Deposit Agreement, the Financial Institution shall not deduct or withhold any taxes on any Deposit Funds in any Account or interest or other income earned on such Deposit Funds.
(e) Section 326 of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (“USA PATRIOT Act”) requires the Financial Institution to implement reasonable procedures to verify the identity of any person that opens a new account with it. Accordingly, the Parties acknowledge that Section 326 of the USA PATRIOT Act and the Financial Institution’s identity verification procedures require the Financial Institution to obtain information which may be used to confirm the NeurogesX and CHRP’s identity including without limitation name, address and organizational documents (“identifying information”). The NeurogesX and CHRP agree to provide the Financial Institution with and consent to the Financial Institution obtaining from third parties any such identifying information required as a condition of opening an account with or using any service provided by the Financial Institution.
Section 15. Notices. All communications hereunder shall be given in writing and except for communications from NeurogesX and CHRP setting forth, claiming, objecting to, or
13
in any way related to the transfer of distribution of funds, including but not limited to funds transfer instructions (all of which shall be specifically governed by Xxxxxxx 0x xxx 0x xxxxx), xxxxx xx deemed to be duly given after it has been received and the receiving party has had a reasonable time to act upon such communication if it is sent or served:
(a) if by facsimile;
(b) by a recognized overnight courier; or
(c) by prepaid registered mail, return receipt requested;
to the appropriate notice address set forth on Schedule 2 or to such other address or addresses as the parties may from time to time designate by notice as provided. Notwithstanding the above, in the case of communications delivered to the Financial Institution, such communications shall be deemed to have been given on the date received by an officer of the Financial Institution or any employee of the Financial Institution who reports directly to any such officer at the address set forth on Schedule 2. In the event that the Financial Institution, in its sole discretion, shall determine an emergency exists, the Financial Institution may use such other means of communication as the Financial Institution deems appropriate. Any such communication received after 5 pm ET shall be deemed to have been received on the next Business Day.
Section 16. Entire Agreement. This Deposit Agreement, together with the Annexes and Schedules hereto (which are incorporated herein by reference), constitutes the entire agreement among all the parties with respect to the subject matter hereof and supersedes all prior agreements, understandings and negotiations, both written and oral, among the parties with respect to the subject matter of this Deposit Agreement. No representation, inducement, promise, understanding, condition or warranty not set forth herein (or in the Annexes or Schedules hereto) has been made or relied upon by any party hereto. None of this Deposit Agreement, nor any provision hereof, is intended to confer upon any Person other than the parties hereto any rights or remedies hereunder.
Section 17. Amendments; No Waivers. This Deposit Agreement or any term or provision hereof may not be amended, changed or modified except with the written consent of the parties hereto. No waiver of any right hereunder shall be effective unless such waiver is signed in writing by the party against whom such waiver is sought to be enforced. No failure or delay by any party in exercising any right, power or privilege hereunder shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude any other or further exercise thereof or the exercise of any other right, power or privilege. The rights and remedies herein provided shall be cumulative and not exclusive of any rights or remedies provided by law.
Section 18. Successors and Assigns. The provisions of this Deposit Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and assigns. NeurogesX shall not be entitled to assign any of its obligations and rights under the Deposit Agreement without the prior written consent of CHRP and the Financial Institution, which consent shall not be unreasonably withheld, conditioned or delayed; provided, however that NeurogesX may, without the consent of CHRP, assign any of its obligations and rights under the Deposit Agreement to any third party with which it may merge or consolidate or to which it
14
may sell all or substantially all of its assets; provided, further, however, that no assignment by NeurogesX shall relieve NeurogesX of its obligations hereunder even if such assignment has been consented to by CHRP and such assignee shall be jointly and severally liable with NeurogesX to CHRP. CHRP may assign without consent of NeurogesX any of its rights under the Deposit Agreement without restriction, but not without prior consent from the Financial Institution.
Section 19. Counterparts. This Deposit Agreement may be executed in two or more counterparts, each of which shall be an original, but all of which together shall constitute one and the same instrument. This Deposit Agreement shall become effective when each party hereto shall have received a counterpart hereof signed by the other parties hereto. Any counterpart may be executed by facsimile or pdf signature and such facsimile or pdf signature shall be deemed an original.
Section 20. Severability. If any provision of this Deposit Agreement is held to be invalid or unenforceable, the remaining provisions shall nevertheless be given full force and effect.
Section 21. Interpretation. When a reference is made in this Deposit Agreement to Sections, subsections, Annexes or Schedules, such reference shall be to a Section, subsection, Annex or Schedule to this Deposit Agreement unless otherwise indicated. The words “include”, “includes” and “including” when used herein shall be deemed in each case to be followed by the words “without limitation”. No party hereto shall be or be deemed to be the drafter of this Deposit Agreement for the purposes of construing this Deposit Agreement against any other party.
Section 22. Governing Law; Jurisdiction.
(a) This Deposit Agreement shall be governed by, and construed, interpreted and enforced in accordance with, the laws of the State of New York, without giving effect to the principles of conflicts of law thereof.
(b) Any legal action or proceeding with respect to this Deposit Agreement may be brought in any state or federal court of competent jurisdiction in the state, county and city of New York. By execution and delivery of this Deposit Agreement, each party hereto hereby irrevocably consents to and accepts, for itself and in respect of its property, generally and unconditionally the non-exclusive jurisdiction of such courts. Each party hereto hereby further irrevocably waives any objection, including any objection to the laying of venue or based on the grounds of forum non conveniens, which it may now or hereafter have to the bringing of any action or proceeding in such jurisdiction in respect of this Deposit Agreement.
(c) Each party hereto hereby irrevocably consents to the service of process out of any of the courts referred to in subsection (b) above of this Section in any such suit, action or proceeding by the mailing of copies thereof by registered or certified mail, postage prepaid, to it at its address set forth in this Deposit Agreement. Each party hereto hereby irrevocably waives any objection to such service of process and further irrevocably waives and agrees not to plead or claim in any suit, action or proceeding commenced hereunder that service of process was in any
15
way invalid or ineffective. Nothing herein shall affect the right of a party to serve process on the other party in any other manner permitted by law.
(d) To the extent that in any jurisdiction either party may now or hereafter be entitled to claim for itself or its assets, immunity from suit, execution attachment (before or after judgment), or other legal process, such party shall not claim, and it hereby irrevocably waives, such immunity.
Section 23. Waiver of Jury Trial. Each party hereto hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any action, proceeding, claim or counterclaim arising out of or relating to this Deposit Agreement or the transactions contemplated under this Deposit Agreement. This waiver shall apply to any subsequent amendments, renewals, supplements or modifications to this Deposit Agreement.
[SIGNATURE PAGE FOLLOWS]
16
IN WITNESS WHEREOF, the parties hereto have caused this Deposit Agreement to be duly executed by their respective authorized officers as of the date first above written.
| JPMORGAN CHASE BANK, N.A. | ||
| By: | /s/ | |
| Name: Xxxx Xxxxx-Xxxxxxxxxx | ||
| Title: Vice President | ||
| NEUROGESX, INC. | ||
| By: | /s/ | |
| Name: Xxxxxxx Xxxxxxxxx | ||
| Title: EVP, COO, CFO | ||
| XXXXX HEALTHCARE ROYALTY PARTNERS, L.P. | ||
| By Xxxxx Healthcare Royalty GP, LLC | ||
| Its General Partner | ||
| By: | /s/ | |
| Name: Xxxxxxx X. Xxxxx, M.D. | ||
| Title: Managing Director | ||
ANNEX A
TO
DEPOSIT AGREEMENT
DEFINITIONS
“Account” and “Accounts” shall have the meaning set forth in Section 2(a).
“Account Instruction” shall mean, with respect to the Initial Concentration Account or the Joint Concentration Account, any entitlement order, order, direction or instruction concerning or directing the disposition, transfer, withdrawal, disbursement or redemption of any Deposit Funds in or credited to the Initial Concentration Account or the Joint Concentration Account, respectively, or otherwise relating to any matters pertaining to or concerning the Initial Concentration Account or the Joint Concentration Account, respectively, and/or any Deposit Funds therein or credited thereto, that in each case is in accordance with the terms and conditions of the Financing Agreement and this Deposit Agreement.
“Annual Fee” shall have the meaning set forth in Section 12.
“Astellas” shall mean Astellas Pharma Europe Ltd., a company organized under the laws of the United Kingdom, and its permitted successors and assigns under the Existing License Agreement.
“Authorized Representative” shall have the meaning set forth in Section 9.
“Business Day” shall mean any day other than (a) a Saturday (b) a Sunday or (c) any other day on which the Financial Institution located at the address set forth on Schedule 2 is authorized or required by law or executive order to remain closed.
“CHRP” shall have the meaning set forth in the preamble (first paragraph) of this Deposit Agreement.
“CHRP Concentration Account” shall have the meaning set forth in Section 2(a)(iv).
“Deposit Agreement” shall have the meaning set forth in the preamble (first paragraph) of this Deposit Agreement.
“Deposit Funds” shall mean any and all financial assets, funds, notes, monies, checks or other items or properties, and any claims to the foregoing.
“Dollars” or “$” shall mean the lawful money of the United States of America.
“Existing License Agreement” shall mean the Distribution, Marketing and License Agreement, dated as of June 19, 2009, between NeurogesX and Astellas (as amended as of the date hereof), and as may be further amended, supplemented or modified as permitted under the Financing Agreement.
A-1
“Financial Institution” shall have the meaning set forth in the preamble (first paragraph) of this Deposit Agreement.
“Financing Agreement” shall have the meaning set forth in the Recitals to this Deposit Agreement.
“Indemnitees” shall have the meaning set forth in Section 13(a).
“Initial Concentration Account” shall have the meaning set forth in Section 2(a)(i).
“Joint Concentration Account” shall have the meaning set forth in Section 2(a)(ii).
“Liabilities” shall mean any and all losses, liabilities, penalties, judgments, settlements, proceedings, litigation, investigations, damages, claims, suits, actions, costs or expenses (including the reasonable fees and expenses of in-house counsel and of outside counsel), but in each case excluding any special, indirect, exemplary or consequential loss or damage of any kind (including lost profits) (each a “Liability”).
“NeurogesX Concentration Account” shall have the meaning set forth in Section 2(a)(iii).
“Permitted Investments” shall mean the Cash Compensation Account at JPMorgan Chase Bank, N.A. Cash Compensation Accounts have rates of compensation that may vary from time to time based upon market conditions.
“Person” shall mean an individual, corporation, partnership, limited liability company, association, trust or other entity or organization, but not including a government or political subdivision or any agency or instrumentality of such government or political subdivision.
“Revenue Interest” shall mean, with respect to the Existing License Agreement, any and all of NeurogesX’s rights to receive (i) any payments under Section 3 or Section 4 (except Section 4.4) of the Existing License Agreement, including, without limitation, rights to receive Sales Milestones under Section 3.2 of the Existing License Agreement, the Option Retention Fee under Section 3.3.2 of the Existing License Agreement, the Option Exercise Fee under Section 3.3.3 of the Existing License Agreement, and Earned Royalties under Section 4 of the Existing License Agreement, in each case payable on and after Xxxxx 0, 0000, (xx) any payments under Section 11.5.2 of the Existing License Agreement in amounts in the aggregate in excess of out-of-pocket costs and expenses (including reasonable attorneys’ and professional fees) incurred in connection with any Enforcement Action (as such term is defined in the Existing License Agreement), (iii) any payments similar to those described in clauses (i) and (ii) from Astellas or its affiliates with respect to Licensing Option Products, regardless of whether such Licensing Option Products are included under the Existing License Agreement or licensed to Astellas pursuant to a separate agreement, (iv) any payments similar to those described in clauses (i), (ii) and (iii) under any Replacement License Agreement, (v) any payments similar to those described in clauses (i) — (iv) made by a Licensee after rejection of the Existing License Agreement or Replacement License Agreement by NeurogesX in bankruptcy, and (vi) any other payments for or derived from the sale, offer for sale (including marketing and promotion), distribution or other commercialization or exploitation of Licensed Products in the Territory.
A-2
For clarity the Revenue Interest shall exclude all rights under any License Agreement to receive (a) payments or amounts which are used to pay Third Parties for rights to intellectual property necessary for or incorporated in the Licensed Products (including amounts payable (I) under Section 4.4 of the Existing License Agreement, (II) to The Regents of the University of California (“The Regents”) pursuant to Section 3.2 or Section 19.4 of that certain Exclusive License Agreement between NeurogesX and The Regents effective November 1, 2000 or (III) to LTS Xxxxxxx Therapie Systeme AG (“LTS”) pursuant to Section 7.3 of that certain Commercial Supply and License Agreement between NeurogesX and LTS dated January 2007), (b) any payments under the Supply Agreement (as entered into pursuant to and defined in Section 9.1 of the Existing License Agreement) and (c) any payment intended to reimburse for any costs or expenses (whether internal or external reasonably allocated in accordance with GAAP) incurred or to be incurred by NeurogesX with respect to activities under such License Agreement or otherwise with respect to the Licensed Product in the Territory (collectively, the “Excluded Revenue Interests”).
“Security Agreement” shall have the meaning set forth in the Recitals to this Deposit Agreement.
“Second Stepdown Date” shall mean the date on which the total of amounts deposited into the CHRP Concentration Account from the Joint Concentration Account, in accordance with Section 3 of Schedule 1 to this Agreement, equals $106 million.
“TIN” shall have the meaning set forth in Section 14(a).
“UCC” shall mean the Uniform Commercial Code as in effect from time to time in the State of New York.
A-3
SCHEDULE 1
TO
DEPOSIT AGREEMENT
FINANCIAL INSTITUTION PROCEDURES OF ACCOUNT MANAGEMENT
| 1. | Inspection of Items. Items contained in the envelopes received in respect of the Initial Concentration Account will be inspected and handled as follows: |
| a. | Payees. An item not bearing an acceptable payee designation, as set forth in the specifications, or a reasonable variation thereof, will not be deposited in the Initial Concentration Account. If a necessary endorsement of a payee other than NeurogesX is missing, the item will not be deposited into the Initial Concentration Account. |
| b. | Dates. An item will be deposited into the Initial Concentration Account whether it is stale-dated, post-dated or does not bear a date. |
| c. | Amounts. If the written and numeric amounts of an item differ, the written amount shall control over the numeric amount unless the written amount is ambiguous. If the amount of an item cannot be determined from application of the preceding sentence, or if the amount is missing altogether, the item will not be deposited into the Initial Concentration Account, and the Financial Institution will notify NeurogesX and CHRP thereof and return the item to NeurogesX for ultimate disposition. |
| d. | Drawer’s Signatures. For an item in which the drawer’s signature is missing, the Financial Institution will deposit it into the Initial Concentration Account and affix a stamp requesting the drawee bank or other payor to contact the drawer for authority to pay the item. |
| e. | Alterations. An item which appears to the Financial Institution to have been materially altered will not be deposited into the Initial Concentration Account. |
| f. | Other Language. The Financial Institution will not examine the front and backsides of items to detect handwritten or typed “paid in full” or similar language. Such items will be deposited into the Initial Concentration Account and the Financial Institution shall have no liability to NeurogesX or CHRP for depositing such items. |
| g. | International Payments. An item denominated in foreign currency and drawn on a foreign bank will not be deposited into the Initial Concentration Account but will be submitted for collection only. An appropriate advice will be forwarded to NeurogesX with a copy to CHRP. The Financial Institution shall not be responsible for fluctuation in exchange rates. If any |
1-1
| amounts are received by the Financial Institution in foreign currency, the Financial Institution shall convert all such funds into Dollars at the then applicable currency conversion rates utilized by the Financial Institution and such amounts of Dollars shall be deposited in the Initial Concentration Account. |
| 2. | Processing Procedures. Items found acceptable for deposit under paragraph 1 above will be encoded, photocopied, endorsed and deposited into the Initial Concentration Account. The endorsement will be the standard endorsement for checks as used by the Financial Institution from time to time, and such endorsement will function as the endorsement of the payee of the item. In order to maximize daily receipts and funds availability, the Financial Institution will make deposits throughout the day in anticipation of major check clearing deadlines. |
| 3. | Distribution Provisions. |
(a) Payments deposited in the Initial Concentration Account shall be allocated 100% to the Joint Concentration Account.
(b) Payments deposited in the Joint Concentration Account shall be allocated in the manner and priority as follows:
(i) 100% of the first $90,000,000 deposited into the Joint Concentration Account shall be deposited into the CHRP Concentration Account;
(ii) 5% of all amounts in excess of $90,000,000 that are deposited into the Joint Concentration Account shall be deposited into the CHRP Concentration Account, until the Second Stepdown Date;
(iii) 95% of all amounts in excess of $90,000,000 that are deposited into the Joint Concentration Account shall be deposited into the NeurogesX Concentration Account, until the Second Stepdown Date; and
(iv) on and after the Second Stepdown Date, 100% of all amounts deposited into the Joint Concentration Account shall be deposited into the NeurogesX Concentration Account.
The transfer of funds in clauses (i) - (iv) above shall be swept from the Joint Concentration Account and into CHRP Concentration Account or NeurogesX Concentration Account, as applicable, on a daily basis.
1-2
SCHEDULE 2
TO
DEPOSIT AGREEMENT
NOTICES AND TIN
NEUROGESX
| Notice Address: | NeurogesX, Inc. | |||||||
| 0000 Xxxxxxxxxxxx Xxxxxxx, Xxxxx 000 | ||||||||
| Xxx Xxxxx, Xxxxxxxxxx 00000 | ||||||||
| Attention: Xxxxxxx XxXxxxx, | ||||||||
| President and CEO | ||||||||
| Facsimile No.: (000) 000-0000 | ||||||||
| Email: xxxxxxxx@xxxxxxxxx.xxx | ||||||||
| with a copy to: | ||||||||
| Xxxxxx Xxxxxxx Xxxxxxxx & Xxxxxx, P.C. | ||||||||
| 000 Xxxx Xxxx Xxxx | ||||||||
| Xxxx Xxxx, Xxxxxxxxxx 00000-0000 | ||||||||
| Attention: Xxxxxxx X’Xxxxxxx, Esq. | ||||||||
| Facsimile No.: (000) 000-0000 | ||||||||
| Email: xxxxxxxxx@xxxx.xxx | ||||||||
| NeurogesX’s TIN: | [***] | |||||||
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
2-1
| CHRP | ||||||||
| Notice Address: | Cowen Healthcare Royalty, L.P. | |||||||
| 000 Xxxxx Xxxxxx, Xxxxx 0000 | ||||||||
| Xxxxxxxx, XX 00000 | ||||||||
| Attention: Xxxxxxx X. Xxxxx, M.D. – | ||||||||
| Facsimile No.: (000) 000-0000 | ||||||||
| Email: Xxxx.Xxxxx@xxxxx.xxx | ||||||||
| with a copy to: | ||||||||
| Xxxxx Xxxxxx Xxxxxxxx & Xxxxxx P.C. | ||||||||
| 000 Xxxxxxxxx Xxxxxx, Xxxxx 0000 | ||||||||
| Xxx Xxxx, XX 00000 | ||||||||
| Attention: Y. Xxxxx Xxxxx, Esq. | ||||||||
| Facsimile No.: (000) 000-0000 | ||||||||
| Email: xxxxxx@xxxxxxx.xxx | ||||||||
| CHRP’s TIN: | [***] | |||||||
FINANCIAL INSTITUTION
| Notice Address: | JPMorgan Chase Bank, N.A. | |||||||
| 0 Xxx Xxxx Xxxxx — 00xx Xxxxx | ||||||||
| Xxx Xxxx, XX 00000 | ||||||||
| Attention: Xxxx Xxxx-Francois/Xxxx Xxxxx-Xxxxxxxxxx | ||||||||
| Facsimile No.: (000) 000-0000 |
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
2-2
SCHEDULE 3
TO
DEPOSIT AGREEMENT
NeurogesX’s
Authorized Representatives and Telephone Number(s) for Call-Backs
| Authorized Representative Name
|
Specimen Signature
|
Telephone Number
| ||
| Xxxxxxx Xxxxxxxxx
|
/s/ | [***] | ||
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
3-1
SCHEDULE 4
TO
DEPOSIT AGREEMENT
CHRP’s
Authorized Representatives and Telephone Number(s) for Call-Backs
| Authorized Representative Name
|
Specimen Signature | Telephone Number | ||
| Xxxxxxx X. Xxxxx, M.D.
|
/s/ | [***] | ||
| Xxxx X. Xxxxx
|
/s/ | [***] | ||
| Xxxxxx X. Xxxxx
|
/s/ | [***] | ||
| Xxxxxx X. Xxxxxxxx
|
/s/ | [***] |
[***] Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
4-1
EXHIBIT L
AMORTIZATION SCHEDULE
- 1 -
[***]CERTAIN INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT L
AMORTIZATION SCHEDULE
This Amortization Schedule is intended as sample calculation for illustration purposes only and shall not be construed as a representation, guarantee or binding commitment of any kind by NeurogesX as to the timing, amount or other details concerning potential Revenue Interest.
| Revenue Interest |
Beginning Balance |
Interest At [***] |
Payment | Ending Balance | ||||||
| [***] |
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
| [***] |
[***]Certain information on this page has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions.
EXHIBIT M
JOINT PRESS RELEASE
- 1 -

| NeurogesX, Inc. Xxxxxxx Xxxxxxxxx Executive Vice President, COO and CFO (000) 000-0000 |
The Xxxx Group Xxxx Xxxxxxxxxx (investors) (000) 000-0000 xxxxxxxxxxx@xxxxxxxxxxxx.xxx
Xxxxx Xxxxx (media) (000) 000-0000 xxxxxx@xxxxxxxxxxxx.xxx |
NeurogesX Enters $40 Million Royalty Financing Agreement
with Cowen Healthcare Royalty Partners
San Mateo, Calif., (April 30, 2010) – NeurogesX, Inc. (NASDAQ: NGSX), a biopharmaceutical company focused on developing and commercializing novel pain management therapies, announced today that it has entered into a $40 million royalty financing agreement with Cowen Healthcare Royalty Partners, L.P. (“Xxxxx Royalty”). The agreement creates a debt obligation that will be repaid through and secured by royalties and future milestone payments payable to NeurogesX under its Distribution, Marketing and License Agreement (the Astellas Agreement) with Astellas Pharma Europe Ltd. (Astellas) for NeurogesX’ Qutenza® (capsaicin) 8% patch, a dermal delivery system containing prescription strength capsaicin.
Qutenza is marketed in the U.S. by NeurogesX for patients with postherpetic neuralgia (PHN). NeurogesX retains all economics resulting from the U.S. market, and Xxxxx Royalty is not entitled to any payment on U.S. sales.
Under the terms of the royalty financing agreement, Xxxxx Royalty will be entitled to receive up to 100% of all royalties and sales milestones, as well as certain other payments due to NeurogesX under the Astellas Agreement. After the debt obligation has been fulfilled, payments under the Astellas Agreement will revert to NeurogesX. At any point NeurogesX can retire the Cowen financing and regain access to 100% of its European royalty interest in Qutenza®.
The Astellas Agreement provides for royalties on net sales of Qutenza in the Astellas Territory: the European Economic Area, including all 27 EU-member states, Iceland, Norway, and Liechtenstein; Switzerland; and certain countries in Eastern Europe, the Middle East and Africa. The Astellas Agreement also provides for total sales milestones and license option payments of up to € 70 million related to Qutenza and the company’s product candidate, NGX-1998.

Xxxxxxx Xxxxxxxxx, Chief Operating Officer and Chief Financial Officer commented, “We are very pleased to announce this royalty financing with Cowen Healthcare Royalty Partners. We believe this structure provides us with capital to continue executing our operating plan through at least 2011. Importantly, this royalty financing agreement only encumbers revenues generated from the territory currently licensed to Astellas, and does not involve revenues arising from our U.S. operations or other world markets. This desirable feature allows for future financing flexibility as we move forward.”
Xxxxxxx X. Xxxxx, M.D., Co-Founder and Managing Director of Xxxxx Royalty, stated, “Qutenza is a unique locally acting therapy, offering a significant benefit for patients. We believe that Astellas is well positioned to capitalize on the European neuropathic pain market. Our team consistently seeks out differentiated products with strong marketing partners, and we believe this transaction with NeurogesX affords a significant and attractive market opportunity for our investors, while providing a non-dilutive source of financing to maximize both the Qutenza opportunity and the potential value creation for NeurogesX shareholders.”
Additional details on the loan agreement can be found in the Current Report on Form 8-K that will be filed by NeurogesX with the Securities and Exchange Commission on or about the date hereof.
About NeurogesX, Inc.
NeurogesX, Inc. (Nasdaq: NGSX) is a San Francisco Bay Area-based biopharmaceutical company focused on developing and commercializing novel pain management therapies. NeurogesX was founded on the concept that use of prescription-strength capsaicin could help manage the pain associated with neuropathic pain conditions. Since its inception, NeurogesX has leveraged its passion to help people with pain to efficiently develop this concept and is now poised to bring its lead product to patients and physicians. In addition, we continue to apply our knowledge and expertise in the development of other novel treatments for pain.
The Company’s lead product, Qutenza® (capsaicin) 8% patch, is a localized dermal delivery system containing a prescription strength of capsaicin that is currently approved in the United States and the European Union. Qutenza is now available in the United States for patients with postherpetic neuralgia (PHN). In Europe, Qutenza will be marketed by Astellas Pharma Europe Ltd. (Astellas), the European subsidiary of Tokyo-based Astellas Pharma Inc.
The Company’s most advanced product candidate, NGX-1998, is a topically applied liquid formulation containing a high concentration of capsaicin designed to treat pain associated with neuropathic pain conditions such as PHN. NGX-1998 has completed three Phase 1 studies.
The Company’s early-stage product pipeline includes pre-clinical compounds which are prodrugs of acetaminophen and various opioids. The Company has evaluated these compounds in vitro and in vivo.

About Cowen Healthcare Royalty Partners
Cowen Healthcare Royalty Partners is a global healthcare private equity firm, with more than $900 million under management, that invests principally in commercial-stage biopharmaceutical and medical device companies and products through the purchase of royalty interests. The investment team has over 100 years of healthcare related experience including principal investing, structured finance, healthcare industry senior management, Wall Street research and consulting, and scientific and clinical experience. For more information, visit xxx.xxxxxxxxxxxx.xxx.
Safe Harbor Statement
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). NeurogesX disclaims any intent or obligation to update these forward-looking statements, and claims the protection of the Safe Harbor for forward-looking statements contained in the Act. Examples of such statements include but are not limited to: statements about the ability of NeurogesX to fund its operations through at least 2011, the ability of Astellas to capitalize on the European pain market with Qutenza, the utility and potential benefits of the royalty financing arrangement to NeurogesX and its shareholders, and the benefits of Qutenza. Such statements are based on management’s current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to: difficulties or delays in the commercialization of Qutenza, including with respect to manufacture and supply of Qutenza; Qutenza and NeurogesX’s other product candidates may have unexpected adverse side effects; physician or patient reluctance to use Qutenza; and other difficulties or delays in the launch of Qutenza in the United States and, by Astellas in the European Union. For further information regarding these and other risks related to NeurogesX’s business, investors should consult NeurogesX’s filings with the Securities and Exchange Commission.
