AUTOGEN Research Pty Ltd and LIPHA S.A.
Exhibit 4.2(e)
“CONFIDENTIAL TREATMENT REQUESTED. CONFIDENTIAL PORTIONS OF THIS DOCUMENT HAVE BEEN OMITTED AND HAVE BEEN SEPARATELY FILED WITH THE COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.”
|
DATE |
|
February 11 2002 |
AUTOGEN Research Pty Ltd
and
LIPHA S.A.
RESEARCH AND LICENCE AGREEMENT
(Strategic Alliance in Human Gene)
1
TABLE OF CONTENTS
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
2
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
3
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
434343
4
|
THIS AGREEMENT is made on |
|
February 11 2002 |
|
|
|
|
|
|
|
|
|
BETWEEN |
|
|
|
|
|
|
|
|
|
|
|
Autogen Research Pty Ltd |
|
|
|
of 000 Xxxxx Xxx, Xxxxx Xxxxxxxxx, Xxxxxxxx, Xxxxxxxxx |
|
|
|
|
|
(“Autogen”) |
|
|
|
|
|
|
|
|
|
AND |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
of 00 xxx Xxxxx Xxxxxx, 00000 Xxxx, XXXXX 00, Xxxxxx |
|
|
|
|
|
(“Lipha”) |
RECITALS:
A. Lipha has for many years, been strongly involved in the research, development, manufacturing and commercialisation of pharmaceuticals addressing metabolic diseases and has invented, developed, manufactured and marketed pharmaceutical products containing metformin, one of the leading oral anti-diabetic products throughout the world.
B. Lipha has agreed to provide research support to the International Diabetes Institute (IDI) research program for the discovery of novel genes involved in obesity and diabetes, the product of these genes being able to be used as therapeutic agents or as a target for drug discovery as well as providing in house facilities including combinatorial chemistry to advance subsequent drug development in that field.
C. Lipha and Autogen have entered into a “Letter of Understanding” dated November 9th, 1999, under which Autogen has agreed to carry out research above mentioned.
D. Lipha and Autogen have agreed to enter into this research and license agreement related to human genome project.
5
1. INTERPRETATION
1.1 Definitions
In this agreement the following expressions have the following meanings:
“Affiliate” means with respect to a Party, any person which directly or indirectly Controls, or is Controlled by, or is under common Control with, such Party.
“Autogen Know-How” means Know-How owned by or licensed to Autogen in relation to the Autogen Patents, or of relevance or use in relation to the Licensed Field or the conduct of Stage 1 Research or Stage 2 Research but excludes Stage 1 Know-How.
“Autogen Patents” means the patents and patent applications owned by or licensed to Autogen related to the Licensed Field including but not limited to those referred to in Schedule 1 and including:
(i) any re-issue, renewal or extension of such a patent or patent application (whether in whole or in part) and any patent of addition thereto; and
(ii) any supplementary protection certificate or other form of extension based on or arising from such patents or patent applications.
“Base Rate” means EURIBOR rate applicable on January first (1st), 2000 and published as such by the European Federation of Banks (Federation Européenne de Banque (FEB)) plus in either case the amount of one point five per cent (1.5%) per annum.
“Business Day” means a day on which the trading banks are open for general banking business in both Melbourne, Australia and Lyon, France.
“Commencement Date” means the date of execution of this agreement by the Party last signing it.
“Commercialisation Licence” means a licence in respect of Patents and Know-How in the form of the agreement contained in Schedule 3.
“Complementary Agreement” means a detailed agreement, if any, entered into towards the end of Stage 2 Research, including all the terms of the Commercialisation Licence, unless the Parties agree to amend the same, and otherwise taking account of the terms of any Joint Venture Agreement.
“Control” means the possession, directly or indirectly, of the power to direct or cause the direction of the management of policies of a person.
6
“Direct Program” means a Stage 2 Research Program based on a Novel Gene Product to investigate its direct use as a drug or for other gene therapies.
“Dollars” or “USD” means US dollars.
“Europe” means those countries specified in Schedule 2.
“EUR” means the new European currency called EURO officially replacing FRF from December 31st 2001.
“FFR” means French Francs.
“Force Majeure” means any cause which is not within the reasonable control of the party affected by it including, but not limited to, acts of God, industrial disputes of any kind, war declared or undeclared, civil disturbance, acts or omissions of Government or other competent authority, fire, lightning, explosion or flood.
“Further Development” means the program of further research and development in relation to a Product or potential Product undertaken after completion of Stage 2 Research.
“IDI” means International Diabetes Institute, a company established in Victoria, Australia.
“Indirect Program” means a Stage 2 Research Program which uses the Novel Gene Product as the basis for the discovery of other chemicals or biological compounds or other gene therapies or otherwise in ways not contemplated by Direct Programs.
“Insolvency Event” means any event of insolvency, bankruptcy or liquidation of the relevant party, including any voluntary or involuntary judicial liquidation or re-organisation proceedings.
“Joint Venture Agreement” means a joint venture in respect of certain specified Patents and Know-How, containing the definitions and provisions contained in this agreement as specified in Schedule 4, and dealing with such other matters as are specified in that schedule.
“Know-How” means technical, commercial and other information, data, know-how, drawings, specifications and/or designs, animal or other models, methodologies and biological materials embodied in some Material Form, and without prejudice to the generality of the foregoing includes:
(i) all experimental, manufacturing, process, analytical, packaging, product, warehousing, quality control and quality assurance and marketing specifications, standards, procedures, processes, methods, instructions and techniques, samples, prototypes, formulae, writings of any kind, opinions or otherwise unwritten data or in the form of computer software or computer programs or any part thereof in any code; and
7
(ii) all other information and other material supplied to or received by a party or its Representatives from another party or Representatives on a confidential basis pursuant to this agreement.
“Licence” means the right and licence granted by Autogen to Lipha pursuant to this agreement.
“Licensed Field” means the field of human therapeutic applications for the treatment or prevention of:
(i) obesity;
(ii) diabetes and its complications; and
(iii) insulin resistance syndrome (excluding hypertension).
For clarity it is noted that this does not include diagnostics or veterinary applications.
“Lipha Research License” means the Research License granted to Lipha pursuant to Clause 2.9.
“Loss” means any loss, damage, cost, interest, expense, fee, penalty, fine, forfeiture, assessment, demand, action, suit, claim, proceeding, cause of action, liability or damages incurred by a person, and includes:
(i) the cost of any action taken by the person to protect itself against any loss or to preserve any right it has under this agreement; and
(ii) where applicable reasonable legal costs.
“Material Form” in relation to Information includes any form (whether visible or not) of storage from which Information can be reproduced, and any form in which Information is embodied or encoded.
“Month” means calendar month.
“Novel Gene Product” means any new gene, novel protein or novel antigen, and any product arising from the transcription of these genes.
“Option Period” means the Research Term and the 24 month period following the Research Term within which Lipha has the option to decide whether it wishes to progress some part of Stage 1 Research to Stage 2 Research.
“Party” means Autogen or Lipha and their respective successors and permitted assigns and “Parties” means both of them.
“Patent” means any patent or patent application as defined in the Patents Xxx 0000 (C’th) and any similar international, national or regional patent or patent application and includes any and all extensions, renewals, continuations, patent-of-addition and/or supplementary protection certificates to any of the foregoing and includes any corresponding patent or patent application taken out or applied for in any country in the Territory which is fairly based upon or derived from any of the aforesaid patents.
8
“Pre-Stage 2 Results” means the Autogen Patents, Autogen Know-How, Stage 1 Patents and Stage 1 Know-How.
“Products” means any products produced or arising out of use of the results of Stage 1 Research or Stage 2 Research or arising out of all and any further development by Lipha pursuant to Stage 1 Research or Stage 2 Research or both.
“Project Patents” means the Autogen Patents, the Stage 1 Patents and/or the Stage 2 Patents.
“Project Research” means the research in the area of obesity and diabetes of novel genes involved in obesity and diabetes and the research arising therefrom, consisting of the Stage 1 Research and the Stage 2 Research.
“Representatives” of a party means that party’s directors, officers, employees or agents.
“Required Insurance” means:
(i) public liability insurance in respect of Losses up to a limit of USD 5,000,000 covering property damage and personal injury as may be relevant to the performance of each party’s obligations under this agreement to the other, including (without limitation) liability, loss or damage due to negligent or malicious damage, data corruption or loss, fire, theft, electrical and water damage; and
(ii) if the relevant party sells a Product, product liability insurance in respect of Losses up to a limit of USD 20,000,000 covering any liability associated with use or misuse of the Product including, personal injury and consequential loss.
“Research Agreement” means the Research Agreement dated 14 January 1998 between Autogen and IDI to carry out research in the field of, amongst other things, the License Field.
“Research Term” means the period which commences on 1 January 2000 and expires on 31 December 2005, unless otherwise extended pursuant to clause 2.4.
“Scientific Board” means the board established under clause 4.1.
“Stage 1 Know-How” means the Know-How relating to or arising out of Stage 1 Research.
“Stage 1 Patents” means Patents relating to or arising out of Stage 1 Research.
“Stage 1 Research” means discovery of Novel Gene Products, characterisation and studies of such Novel Gene Products in animal models and human gene expression studies as resulting from such Stage 1 Research performed according to this agreement as described in Schedule 5.
“Stage 1 Results” means the Stage 1 Know-How and the Stage 1 Patents.
“Stage 2 Know-How” means in the context of a Stage 2 Research Program, the Know-How relating to or arising out of that Stage 2 Research Program.
9
“Stage 2 Patents” means in the context of a Stage 2 Research Program, Patents relating to or arising out of that Stage 2 Research Program.
“Stage 2 Research” means a program of research and development directed at developing relevant Novel Gene Products within the Licensed Field and discovering Target Compounds for use within the Licensed Field and includes antibody production, large scale protein production, structural analysis, screening of compounds affecting such Novel Gene Product, combinatorial chemistry, toxiological studies and first clinical studies in target patient groups and completion of dose efficiency relationship studies in those patients (clinical Phase 2), the undertaking of relevant human clinical trials on Target Compounds, but does not include the generation of relevant scientific and clinical data which is uniquely required by the regulatory authorities of Europe or the United States.
“Stage 2 Research Program” means a program of Stage 2 Research in respect of a Novel Gene Product.
“Stage 2 Results” means in the context of a Stage 2 Research Program, the Stage 2 Know-How and the Stage 2 Patents for that Stage 2 Research Program.
“Target Compound” means a compound or small molecule, or group of the same, identified in the course of Project Research, being a compound which it is expected will be Covered By a Project Patent.
“Term” means the period this agreement is in force pursuant to clause 12.
1.2 Construction
In this agreement unless the context otherwise requires:
(a) Business Day. If any day appointed or specified by this agreement for the payment of any money or the doing of any act or thing falls on a day that is not a Business Day, the day so appointed or specified is deemed to be the next day which is a Business Day.
(b) Collective references. Reference to any thing (including, without limitation, any amount) is a reference to the whole or any part of it and a reference to a group of things or persons is a reference to any one or more of them.
(c) Defined expressions. If a word or phrase is defined, cognate words and phrases have corresponding definitions.
(d) Gender. Words importing any gender include the other genders.
(e) Headings. Headings must be ignored in construing this document.
10
(f) Joint liability. An obligation of two or more Parties binds them jointly and severally.
(g) Joint obligations. An obligation incurred in favour of two or more Parties is enforceable by them jointly and severally.
(h) Month. Means a calendar month.
(i) Numbers. Words importing the singular include the plural and vice versa.
(j) Parts of agreement. References to this agreement include its recitals, schedules and annexures.
(k) Persons. References to persons include corporations and bodies politic.
(l) Reconstituted bodies. References to a body which has ceased to exist or has been reconstituted, amalgamated, reconstructed or merged, or the functions of which have become exercisable by any other person or body in its place, is taken to refer to the person or body established or constituted in its place or the person or body by which its functions have become exercisable.
(m) Representatives and assigns. References to a person include the legal personal representatives, successors and permitted assigns of that person.
(n) Statutory amendments. A reference to a statute, ordinance, code or other law includes regulations and other statutory instruments under it and consolidations, amendments, re-enactments or replacements of any of them (whether of the same or any other legislative authority having jurisdiction).
(o) Variation. References to this or any other document include the document as varied or replaced, and notwithstanding any change in the identity of the Parties.
(p) Writing. References to writing include any mode of representing or reproducing words in tangible and permanently visible form, and include telex and facsimile transmissions.
2. STAGE 1 RESEARCH
2.1 Undertaking of Stage 1 Research
Autogen has been carrying out and must continue to carry out through arrangement with IDI under the Research Agreement Stage 1 Research for the period of the Research Term. The currently agreed program for Stage 1 Research is as set out in Schedule 5. The general objective of Stage 1 Research is to identify Novel Gene Products that may be of utility in the Licensed Field.
11
2.2 Initial contribution
Lipha has already paid the sum of [*] US dollars (USD [*]) in respect of years 2000 and 2001 of the Research Term as referred herebelow.
2.3 Payment for Stage 1 Research
Taking into account initial contribution above mentioned Lipha will pay to Autogen in respect of Stage 1 Research for each year during the Research Term the sum of [*] US dollars (USD [*]) per annum, such amount to be paid in equal quarterly instalments in advance, the first such payment being due on January 1st, 2000.
2.4 Extension of Stage 1 Research
(a) If Stage 1 Research is not completed during the Research Term Lipha shall have the right to require Autogen to continue Stage 1 Research, and at the same time extend the Research Term, for successive periods of one (1) year, by giving written notice to Autogen not less than three (3) months prior to the expiry of the current Research Term.
(b) Where Lipha does extend the Research Term then:
(i) Lipha shall pay to Autogen for each renewal period the amount of [*] US dollars (USD [*]) in equal quarterly instalments as contribution to the research being funded by Autogen under the Research Agreement in the Licensed Field; and
(ii) Autogen will then continue to carry out through arrangement with IDI the Stage 1 Research for the extended period of the Research Term.
(c) Following the giving by Lipha of notice under clause 2.4(a), the Parties will liaise in relation to the content of the Stage 1 Research for the following year, which will, unless otherwise agreed, be conducted under the Research Agreement. Initially this liaison will take place through the Scientific Board, who will prepare a draft research plan which is to be finally agreed between the Parties.
(d) The research being undertaken under the Research Agreement may include more than just Stage 1 Research, and Autogen is solely responsible for funding this.
(e) If Lipha elects not to extend the Research Term, then without in any way limiting Lipha’s discretion in relation to such decision, Lipha will give Autogen written reasons why it has elected not to do so.
12
2.5 Transition to Stage 2 Research
From time to time throughout the Stage 1 Research including Option Period it is expected that Novel Gene Products may be found. When this occurs there may be a Direct Program and one or more Indirect Programs. The Parties will first determine whether to pursue a Direct Program in accordance with the provisions of clause 2.6 herebelow. The Parties will then investigate whether the Novel Gene Product could be used for the discovery of chemical or biological compounds or in other gene therapies (Indirect Program). This process will be covered by the provisions of clause 2.7 herebelow.
Lipha will make every endeavours to exercise and to conduct the Stage 2 Research.
2.6 Direct Programs
In respect of each Novel Gene Product and in accordance with elements given in schema as described in Schedule 6 (including the respective funding obligations of the parties) if:
(a) The Parties both agrees to pursue a Direct Program, then the provisions of clause 2.8 will apply,
(b) neither Party wishes to pursue a Direct Program, then there will be no Direct Program in respect of the Novel Gene Product unless the Parties otherwise later agree,
(c) Autogen wishes to pursue a Direct Program but Lipha does not, then the Direct Program may commence subject that:
(i) Autogen will be wholly responsible for the initial funding of the Direct Program,
(ii) Lipha will have a period of six (6) months to determine if it wishes to participate,
(iii) If it determines that it does, then it must reimburse Autogen for one third (1/3) of the costs incurred by Autogen up to that date and thereafter the provisions of clause 2.8 will apply,
(iv) If Lipha determines that it does not wish to participate, or makes no election within that period of six (6) months, then Lipha will have no further rights in the Direct Program, and Autogen must fully fund it. Further Autogen will be free to offer the Direct Program in respect of the Novel Gene Product to third parties on such terms as it considers appropriate. In doing so Autogen may grant to third parties a licence in respect of the Autogen Patents and the Stage 1 Patents to the extent that such a licence is necessary to conduct the Direct Program and exploit the Novel Gene Product and such licence will not be a breach of any licence granted by Autogen in the Licensed Field to Lipha, the scope of any licence in favour of Lipha being commensurately reduced, such reduction being limited to reduction in respect of such Novel Gene Product and its use as a Direct Program.
13
d) Lipha wishes to pursue a Direct Program but Autogen does not, then the Direct Program may commence and the provisions of clause 2.8 will apply subject that:
(i) Lipha will be wholly responsible for the initial funding of the Direct Program,
(ii) Autogen will have a period of six (6) months to determine if it wishes to participate,
(iii) If it determines that it does, then it must reimburse Lipha for two third (2/3) of the costs incurred by Lipha up to that date,
(iv) If Autogen determines that it does not wish to participate, or makes no election within that period of six (6) months, then Autogen will have no further rights in the Direct Program and Lipha must fully fund it. Further Lipha will be free to offer the Direct Program in respect of the Novel Gene Product to third parties on such terms as it considers appropriate
2.7 Indirect Programs
There may be multiple Indirect Programs based upon Novel Gene Products and there could be Indirect Programs where there may or may not exist a Direct Program. In respect of each Novel Gene Product and in accordance with elements given in schema as described in Schedule 6 the Parties will seek to identify Indirect Programs which they would wish to undertake in respect of that Novel Gene Product. These Indirect Programs may be generated jointly or independently. In respect of each of these it is agreed as follows:
(a) if Lipha wishes to pursue an Indirect Program, then the provisions of clause 2.8 will apply,
(b) if neither Party wishes to pursue an Indirect Program, then the proposed Indirect Program will not proceed,
(c) if only Autogen wishes to pursue an Indirect Program but Lipha does not, then the Indirect Program may not commence. In such a case Lipha will have a period during which it can preclude the commencement of the proposed Indirect Program and preserve its potential position in respect of that program. That period will be:
(i) in the event Lipha is funding and continuing to fund another Indirect Program based on Novel Gene Products, until the end of the Option Period as referred to in Article 1.1,
(ii) in any other case two (2) years from the date of identification of the Novel Gene Product or the cessation of funding of another Indirect Program based on the same Novel Gene Product, whichever is the later.
If Lipha does not by the end of the relevant period, agree to proceed under clause 2.7, then Lipha will have no further rights in the Indirect Program and Autogen may fully fund it. In doing so Autogen may grant to third parties a licence in respect of the Autogen Patents and the Stage 1 Patents to the extent that such a licence is necessary to conduct the Indirect Program and exploit the Novel Gene Product and such licence will not be a breach of any licence granted by Autogen in the Licensed Field to Lipha, the scope of any Licence in favour of Lipha being commensurately reduced and such reduction being limited to reduction in respect of such Novel Gene Product and its use as contemplated in the Indirect Program.
14
2.8 Stage 2 Research Payment
Referring to the first commencement of a Direct Program or Indirect Program in respect of a Novel Gene Product:
(a) Lipha must only pay to Autogen the sum of [*] french francs (FRF [*]) such amount to be paid within thirty (30) Business Days of the commencement of the first Direct Program or Indirect Program, it being acknowledged that Lipha will not have to pay such sums for transition of further Novel Gene Products to Stage 2 Research following such first transition; and
(b) the Parties will execute a Commercialisation Licence in respect of such Novel Gene Product which will reflect the respective funding contributions of the parties to the relevant Stage 2 Research. This right for Lipha may be separately exercised by Lipha in respect of separate Novel Gene Products, and each decision to enter into Stage 2 Research will lead to additional Commercialisation Licences and Stage 2 Research Programs.
2.9 Autogen and Stage 1 Research Rights
Subject to the terms of this agreement and any other licence granted by Autogen in favour of Lipha, Autogen will own and have an unfettered right to use and exploit all Stage 1 Patents and Stage 1 Know-How in any and all fields.
2.10 Lipha Research Licence
Whilst Lipha is continuing Stage 1 Research, Stage 2 Research, Further Development or paying royalties to Autogen under any Commercialisation Licence, Lipha has a non-exclusive royalty free right to use the Pre-Stage 2 Results for the purposes of its own internal research for human therapeutic purposes. This research licence extends to Affiliates of Lipha.
2.11 Stage 1 Patents
(a) Where either party in the course of Stage 1 Research makes or discovers any patentable invention then it must forthwith advise the other party in writing together with full details. Lipha will have the first option to patent such invention at its own cost, and if it elects not to do so then Autogen may do so at its cost. Any such patent application in respect of Stage 1 Research will be in the name of Autogen.
(b) If Autogen has patented an invention under clause 2.11(a) and paid the costs of such patenting, and Lipha elects to participate in Stage 2 Research in respect of the Stage 1 Research to which the invention relates, Lipha will then reimburse to Autogen the reasonable external costs incurred by Autogen in respect of each patent.
15
(c) In the case where Lipha patents an invention pursuant to clause 2.11(a) where the invention also has application in areas outside the Licensed Fields, Autogen may:
(i) require Lipha to patent the invention in such a manner as to include the application of the invention in those areas outside the Licensed Fields (and if this involves any additional external cost to Lipha, Autogen will reimburse to Lipha the reasonable additional external costs); or
(ii) patent the invention itself in so far as the invention has application outside the Licensed Fields and, in this case, Autogen can require Lipha to amend or limit the patent lodged by Lipha so that the patent only covers the Licensed Fields.
2.12 Continuing Research Agreement
Where Lipha has ceased to provide support for research being conducted under the Research Agreement as contemplated by clause 2.4, Lipha acknowledges it will have no rights in respect of any new or further Know-How or Patents arising under such Research Agreement.
3. STAGE 2 RESEARCH
3.1 Multiple Stage 2 Research Programs
The Parties acknowledge that there may be multiple Stage 2 Research Programs with varying obligations and rights in respect of funding, control and ownership of results as determined in relevant commercialisation licence, each of which will be based on or around different Novel Gene Products, and will generate their own Know-How and Patents.
3.2 Undertaking Stage 2 Research
(a) Where Lipha has exercised its rights regarding Indirect Programs as provided in clause 2.7 and Schedule 6 in respect of a Novel Gene Product, Lipha will have sole carriage of all research and development in respect of that Novel Gene Product until completion of the Stage 2 Research Program in respect of that Novel Gene Product. Each Stage 2 Research Program will be conducted and funded by Lipha until completion of that Stage 2 Research Program. Each Stage 2 Research Program must be directed at developing Novel Gene Products within the Licensed Field and discovering Target Compounds for use within the Licensed Field.
(b) Where Autogen is solely funding regarding Indirect Programs as provided in clause 2.7 and Schedule 6 in respect of a Novel Gene Product, Autogen will have sole carriage of all research and development in respect of that Novel Gene Product until completion of the Stage 2 Research Program in respect of that Novel Gene Product. Each Stage 2 Research Program will be conducted and solely funded by Autogen until completion of that Stage 2 Research Program. Each Stage 2 Research Program must be directed at developing Novel Gene Products within the Licensed Field and discovering Target Compounds for use within the Licensed Field.
16
(c) Where Lipha and Autogen desires to share in a Direct Program as provided in clause 2.6 and Schedule 6 in respect of a Novel Gene Product, Lipha and Autogen will share pursuant to provisions of clause 2.6 all research and development in respect of that Novel Gene Product until completion of the Stage 2 Research in respect of that Novel Gene Product.
(d) Where Autogen desires to develop and exploit Novel Gene Products outside of the Licensed Field through a third party, then Autogen will at first instance engage in discussions with Lipha with a view to looking at opportunities for the Parties to work together on such development and exploitation.
3.3 Licence to Lipha
Where Lipha is solely undertaking a Stage 2 Research Program (“Lipha Program”) then:
(a) all Stage 2 Know-How or Stage 2 Patents in respect of that Stage 2 Research Program will belong solely to Lipha, subject to the remainder of this clause and clauses 3.6, 3.8 and 3.9, and Lipha will be solely responsible for the cost of obtaining and maintaining any patent or other protection, provided that:
(i) Lipha may not assign any Stage 2 Results to a third party without the prior written consent of Autogen which consent will not be unreasonably withheld provided the assignee agrees to similar conditions in respect of such Stage 2 Results as are applicable under this agreement and the relevant Commercialisation Licence and in a manner which makes those conditions enforceable against the assignee by Autogen; and
(ii) if Lipha seeks to patent an invention from the Stage 2 Research which has application exclusively outside the Licensed Field, then Lipha must notify Autogen of such invention prior to lodgment of the application for the patent and Autogen can require Lipha to extend the patent in respect of claims or other matters outside of the Licensed Field;
(b) Autogen grants to Lipha a licence in respect of the Pre-Stage 2 Results within the Licensed Field to the extent necessary to undertake the Stage 2 Research Program, but not to undertake commercialisation (which must be subject to the Commercialisation Licence or a Complementary Agreement), such licence to be exclusive in the Licensed field, royalty free and extending to Affiliates of Lipha.
3.4 Autogen Stage 2 Research assistance
Autogen agrees upon Lipha’s request to cooperate with Lipha for Stage 2 Research and both Parties may agree on specific studies to be included in a Stage 2 Research Program which are to be performed by Autogen or through Autogen and paid by Lipha, provided Lipha has previously given Lipha’s written approval on the costs of such studies. Lipha agrees that it will not in respect of any Stage 2 Research contract directly with IDI without the prior written consent of Autogen, it being the expectation that any research conducted by those Parties will be contracted through Autogen.
17
3.5 Obligations of Lipha
Lipha will use its best endeavours to progress each Stage 2 Research Program in a timely and diligent fashion and with a substantial dedication of resources. Where activities forming part of a Stage 2 Research Program could properly and effectively be undertaken at Autogen, then Lipha will consider the possible performance of those activities at those institutions, subject to clause 3.4, and will not conduct such activities through other third Parties without first offering them to Autogen as appropriate.
3.6 Rights of Autogen
Where Lipha is undertaking a Lipha Program the following applies:
(a) Lipha grants to Autogen and its Affiliates, with a right to sublicencee to IDI, a non-exclusive royalty free right to use for the purposes of their own internal research the Stage 2 Results of all Stage 2 Research Programs.
(b) If such internal research leads to an invention which can be commercialised within the Licensed Fields, then Lipha will have the first option to commercialise such invention within the Licensed Fields, and the Parties will negotiate in good faith in this regard. If agreement cannot be reached within 90 days of the making of a formal offer by Autogen, then Autogen will be free to offer the commercialisation opportunity to third Parties, but on no better terms than those offered to Lipha, unless it first offers such better terms to Lipha, which will have 14 days to either accept or reject such terms.
(c) If the internal research conducted under clause 3.6(a) leads to the commercialisation of the results of such internal research outside the Licensed Fields by Autogen, either on its own account, or acting as licensee on behalf of IDI then the Parties agree to negotiate in good faith for the grant by Lipha of a royalty bearing licence in respect of the ongoing use of the Stage 2 Results in such commercialisation. The Parties acknowledge that the natural outcome of such discussions may be that Lipha takes on the role of commercialisation of the results of such internal research.
3.7 Rights of Parties
(a) Notwithstanding any other provision of this agreement, each of the Parties shall have control of a Stage 2 Research Program and associated matters where it is providing a majority of the funding in relation to that program.
(b) Notwithstanding any other provision to this agreement ownership of any intellectual property, know how or like right or interest resulting from a Stage 2 Research Program shall vest in or reside with the Parties in proportion to their funding contributions to that program.
18
3.8 Discontinuation of Stage 2 Research
In the case of a Lipha Program Lipha has the right at its own option and without any liability to Autogen to stop a Stage 2 Research Program if further development is not justifiable due to Lipha’s reasonable determination that for efficacy, safety or medical reasons, the validity of the Patents covering such Novel Gene Product, a substantial change of economic factors or otherwise, such development should be terminated. If Lipha does cease such development it must promptly advise Autogen and provide written reasons as to why Lipha has ceased such development, but Lipha’s determination will prevail. Lipha must endeavour to consult with the Scientific Board prior to ceasing development.
3.9 Unwanted rights Stage 2 Results
Where in accordance with clauses 3.7 or 6.2 Lipha ceases a Stage 2 Research Program, Lipha retains the exclusive ownership of the relevant Stage 2 Results generated up to the date of the cessation of that Stage 2 Research Program, provided that Autogen may request a commercial and research licence of such Stage 2 Results, in which case the Parties will negotiate in good faith the terms of a licence (which may be exclusive or non-exclusive) for development and commercialisation of such Stage 2 Results, and unless the negotiated licence otherwise provides, Autogen must not assign the rights licensed from Lipha to a third party without the prior written consent of Lipha, which consent must not be unreasonably withheld.
3.10 Commercialisation Licences required
In the event Lipha wishes to in any way use, commercialise, licence, or assign Stage 2 Results, then, unless it does so pursuant to a Complementary Agreement, it must enter into a Commercialisation Licence in respect of those Stage 2 Results, to the intent and with the effect that amongst other things Autogen will receive “royalties” in respect of such use, commercialisation, licensing or assignment.
4. RESEARCH GENERALLY
4.1 Scientific Board
The Parties agree to establish a scientific board comprised of both Parties’ representatives. The Scientific Board may consist of two (2) representatives of Autogen and two (2) representatives of Lipha.
4.2 Meetings of the Scientific Board
Scientific Board meetings will be held every three (3) months, or as otherwise agreed by the Parties and each party may have any of its employees participate in said meeting if the party considers it necessary. Such meeting shall take place alternating at each party’s place of business or at any other place subject to prior agreement by the Parties, and be chaired by a representative of the party who hosts the meeting. The chairman of the meeting must circulate the agenda for the meeting at least two (2) weeks prior to each meeting and will issue the formal minutes of the meeting.
19
The Parties agree that Scientific Board meetings are only a place to report and discuss:
(a) the progress of each party’s research and development work related to Stage 1 Research; and
(b) the progress of research and development work related to each Stage 2 Research Program,
but the Scientific Board does not have capacity to make decisions on important matters to bind either party, unless otherwise agreed.
4.3 Reports
(a) Autogen agrees that it will prepare and submit reports to Lipha every three (3) months during Stage 1 Research and any stage research in progress and it shall promptly give to Lipha at the end of Stage 1 Research and such stage research in progress a final and detailed report.
(b) Lipha agrees that it will prepare and submit reports to Autogen every three (3) months during each Stage 2 Research Program and it shall promptly give to Autogen at the end of each Stage 2 Research Program a final and detailed report on such completion of Stage 2 Research.
(c) The reports provided above must in each case set out in reasonable and informative detail the activities undertaken in the period to which the report relates, and the activities planned for the next reporting period, if any.
5. OPTION FOR FURTHER AGREEMENT
5.1 Multiple Complementary Agreements
If, and each time, Lipha participates in Direct Programs or Indirect Programs under clause 2.6 or 2.7 then the Parties agree, if it is necessary, to enter into a Complementary Agreement which will take effect no later than six (6) months prior to completion by Lipha of relevant Stage 2 Research Program. A Complementary Agreement may not be required if Lipha does not elect to proceed with a Joint Venture Agreement.
5.2 Licence milestones
Within 30 days after the commencement of Phase III clinical trials in respect of a Product the subject of a Stage 2 Research Program Lipha must pay to Autogen a milestone payment of:
(a) in case the protein could be directly useable as a drug or other gene therapies (Direct Program) if Lipha elects to proceed with a Joint Venture Agreement, FRF [*] for Lipha’s exclusive European rights (less any amount already paid under clause 2.8) which is non refundable and will not be credited against future royalty payments, if any, payable to Autogen,
20
Lipha will have exclusive further development and marketing rights throughout Europe Autogen having the rights to develop and market such Autogen invention in the rest of the world; or
(b) in case gene and/or protein could be used for discovery of chemical or biological compounds or other gene therapies (Indirect Program), FRF [*] for Lipha’s worldwild exclusive rights (less any amount paid in respect of the first transition of a Novel Gene Product to Stage 2 Research under clause 2.8) provided that FRF [*] of such milestone payment will be treated as an advance royalty and be credited against future royalty payments, if any, payable to Autogen.
Lipha will fund the whole further development.
(c) In case gene and/or protein could be used for discovery of chemical or biological compounds (Indirect Program), FRF [*] for Lipha’s exclusive European rights (less any amount paid in respect of the first transition of a Novel Gene Product to Stage 2 Research under clause 2.8).
Lipha will fund a third (1/3) of the further common development expenses, the J.V. funding the other two third (2/3).
6. COMMERCIALISATION LICENCE
6.1 Requirement for such a licence
Upon the first commencement of a Direct Program or Indirect Program, the Commercialisation Licence for the Stage 2 Research Program (in the case of Lipha Programs or a Stage 2 Research Program funded by both Autogen and Lipha) will be deemed to be in force, and Lipha and Autogen must promptly execute a Commercialisation Licence.
6.2 Failure to sign Commercialisation Licence
In the event that the Commercialisation Licence in respect of a Stage 2 Research Program is not executed in accordance with clause 6.1, then:
(a) the relevant Stage 2 Research Program must cease; and
(b) the provisions of clause 3.8 will apply.
6.3 Commercialisation
The obligations of each of the Parties in respect of commercialisation will be as contained in the relevant Commercialisation Licence or Complementary Agreement (if any).
21
7. INDUSTRIAL AND INTELLECTUAL PROPERTY RIGHTS
7.1 Autogen Patents, Autogen Know-How and Stage 1 Results
The Autogen Patents, Autogen Know-How and Stage 1 Results (as per clause 2.9) are owned by Autogen, or exclusively licensed by it from IDI and are freely exploitable by it, subject to:
(a) a research licence in favour of Lipha and its Affiliates within the Licensed Field (as per clause 2.10);
(b) an exclusive licence in favour of Lipha within the Licensed Field for commercialisation in terms of the relevant Commercialisation Licence (if any); and
(c) if appropriate, the rights granted under the Joint Venture Agreement.
7.2 Scope of any exclusive licence
Any grant of an exclusive licence in a given limited field in respect of Patents or Know-How, does not preclude the licensing of the same Patents or Know-How for use or exploitation in some other field.
7.3 Stage 2 Results
As provided in clause 3.3(a) Stage 2 Results in respect of each Lipha Program are owned by Lipha and are freely exploitable by it, subject to:
(a) a research licence in favour of Autogen and its Affiliates and IDI for the purpose of their own internal research, as provided in clause 3.6;
(b) a right for Autogen to seek a licence in certain circumstances as provided in clause 3.8;
(c) the use of, including the licensing of such Stage 2 Results, being subject to the same restrictions as are applicable to Pre-Stage 2 Results, under a Commercialisation Licence, save for the Licensed Field restriction, but including, amongst other things:
(i) the obligation to pay “royalties” to Autogen for the use or exploitation of the Stage 2 Results; and
(ii) the requirements in relation to the grant of licences of the Stage 2 Results,
to the intent that Stage 2 Results will be accorded the same treatment and generate for Autogen the same benefits and entitlements as Pre-Stage 2 Results, even though their legal ownership is different.
22
7.4 Use outside of Licensed Field
While Stage 2 Results from a Lipha Program are freely exploitable by Lipha outside of the Licensed Field (subject to clause 7.3), this does not imply any licence to use Pre-Stage 2 Results outside of the Licensed Field, and Lipha will need to seek a licence from Autogen in relation to the same, if such exploitation uses Pre-Stage 2 Results, or would amount to an infringement of Stage 1 Patents or Autogen Patents.
8. CONFIDENTIAL INFORMATION
8.1 Obligations to Autogen
Subject to clauses 8.3 and 8.6, Lipha covenants with Autogen as follows:
(a) to keep all Know-How belonging to Autogen or supplied to it by Autogen, including the existence of such Know-How, strictly secret and confidential (including from all its employees, servants and agents), exercising at least the same degree of care as it uses to maintain its own Know-How;
(b) to provide proper and secure storage for such Know-How within its possession or control;
(c) to use such Know-How only for the purposes of this agreement and not for any other activity or purpose whatsoever without the prior written approval of Autogen; and
(d) to not copy or reduce to writing or any other medium any part of such Know-How except as may be reasonably necessary for the purposes of this agreement.
8.2 Obligations to Lipha
Subject to clauses 8.3 and 8.6, Autogen covenants with Lipha as follows:
(a) to keep all Know-How belonging to Lipha or supplied to it by Lipha, including the existence of such Know-How, strictly secret and confidential (including from all its employees, servants and agents), exercising at least the same degree of care as it uses to maintain its own Know-How;
(b) to provide proper and secure storage for such Know-How within its possession or control;
(c) to use such Know-How only for the purposes of this agreement and not for any other activity or purpose whatsoever without the prior written approval of Lipha; and
(d) to not copy or reduce to writing or any other medium any part of such Know-How except as may be reasonably necessary for the purposes of this agreement.
8.3 Exceptions to obligations
The obligations of confidence set out in clauses 8.1 and 8.2 do not extend to Know-How which:
23
(a) at the time of disclosure to a party is in the public domain;
(b) after disclosure to a party becomes part of the public domain otherwise than as a result of the wrongful act of that party or one of that party’s disclosees;
(c) a party can show was in its possession at the time of disclosure and was not acquired directly or indirectly from the other party; or
(d) is received from a third party provided that it was not acquired directly or indirectly by that third party from a party to this agreement or under an obligation of confidence;
(e) is required by compulsion of law to be disclosed,
provided that:
(f) the onus is on the party alleging the same to prove that one of the above exceptions has application; and
(g) in any case of uncertainty as to whether the obligations in clauses 8.1 or 8.2 have application to any information, such information must be treated as subject to the obligations until advised otherwise by the party to whom the obligations are owed.
8.4 Rights in Know-How
Each party acknowledges and agrees that each other party has made a substantial investment in that party’s Know-How and has a legitimate right to protect itself against wrongful disclosure or use of such Know-How.
8.5 Term of obligation
The obligations in this clause 8 survive the expiry or termination of this agreement for whatever reason and continue for a period of 10 years, subject always to the exceptions included in clause 8.3.
8.6 Permitted disclosures
Each party (“the first party”) is permitted to disclose Know-How belonging to another party or supplied to it by another party (“the other party”) to such of the first party’s Representatives as require access to such information for the purposes of this agreement, provided that:
(a) only such Know-How as needs to be disclosed to a person for the purposes of this agreement will be disclosed to that person; and
(b) the first party must:
(i) have obtained from each such person undertakings in favour of the other party substantially in the form of the relevant obligations and undertakings in this clause 8 (but not this clause 8.6);
(ii) be responsible for the performance of its Representatives’ undertakings referred to in clause 8.6(b)(i); and
24
(ii) take whatever steps are reasonably necessary, including the institution of legal proceedings, to ensure that each of its Representatives is bound by and observes the terms of the undertakings referred to in clause 8.6(b)(i).
8.7 Delivery-up of Know-How
All of a party’s Know-How and all materials containing or embodying such Know-How and all copies of or extracts from or notes on the same in the possession, power or control of another party or any of its employees, servants or agents together with all forms and other materials relating to practices and procedures in relation to the Know-How to the extent possible must:
(a) in the event of the expiration or sooner termination of this agreement, be delivered up by the other party to the first party at the expense of the other party; and
(b) in the event of any demand made by the first party be delivered up by the other party to the first party at the expense of the first party.
8.8 Terms of this Agreement
Lipha and Autogen shall not disclose any terms or conditions of this agreement to any third party without the prior consent of the other party, except as required by applicable law or local regulations or to a third party with whom Autogen or Lipha has entered into or proposes to enter into a business relationship, provided that such third party shall enter into a confidentiality agreement with, or otherwise owe a duty or confidentiality to Lipha or Autogen, as applicable.
8.9 Public Announcement
Except as required by law, order or regulation of a governmental agency or a court of competent jurisdiction, no other announcement, public release or notice of any kind may be issued without the express written consent of both Parties, which consent shall not be unreasonably withheld, provided, however, the Parties shall prepare a joint press release announcing the transaction set forth in this agreement to be issued promptly upon execution of this agreement.
8.10 Continuous disclosure obligations
Autogen is a subsidiary of Autogen Limited which is listed on the Australian Stock Exchange (“ASX”). As such Autogen is subject to the continuous disclosure requirements of the ASX. The obligations of Autogen under this agreement will not restrict it or AWI from making whatever disclosures are necessary for the purposes of fulfilling the requirements applicable to AWI as a company listed on the ASX, and neither Autogen or AWI is under any obligation to delay the public release of any required announcement pending the provision of any consent or approval from Lipha. The above provision will also apply with such adaptations as are necessary to disclosure obligations imposed upon Lipha as a subsidiary of its listed parent Merck XXxX.
00
0. LIABILITY
9.1 Responsibility for Products
Lipha must ensure at all times that Stage 2 Research is conducted and Products are developed, manufactured, tested and used strictly in accordance with all relevant applicable requirements and standards of relevant jurisdictions and Lipha will be responsible for conducting its own independent examination and verification of the accuracy and suitability of Pre-Stage 2 Results and for ensuring the same are suitable for the purposes for which the same are used.
9.2 Autogen not liable
Except as provided in clauses 10.2 and 16.2, Autogen is not liable (in contract or tort or otherwise) to compensate Lipha for any loss howsoever arising suffered by Lipha arising directly or indirectly from the use by Lipha of Pre-Stage 2 Results or the sale of Products.
10. INDEMNITIES
10.1 Indemnity by Lipha
Lipha agrees to indemnify Autogen against and hold Autogen harmless from any and all Losses arising from or in connection with:
(a) a breach by Lipha of any of its warranties or obligations under this agreement;
(b) the conduct of a Lipha Program,
provided that Lipha is not required to indemnify Autogen for any Losses to the extent they result from Autogen’s or its Representatives negligence or breach of any of Autogen’s warranties or obligations under this agreement.
10.2 Indemnity by Autogen
Autogen agrees to indemnify Lipha and hold Lipha harmless from any and all Losses, arising from or in connection with:
(a) a breach by Autogen of any of its warranties or obligations under this agreement;
(b) the negligent performance by Autogen or IDI of the Stage 1 Research; and
(c) the termination of the Research Agreement as a result of the failure of Autogen to provide research funding under this agreement (other than as a result of any act or omission of Lipha or its Representatives),
provided that Autogen is not required to indemnify Lipha for any Losses to the extent they result from Lipha’s or its Representatives’ negligence or breach of any of Lipha’s warranties or obligations under this agreement.
26
10.3 Notification regarding indemnity
Each party must promptly notify the other of any claims or suits for which the first party may assert indemnification from the other party and the first party will permit the other party and its insurer at the other party’s expense to assume or participate in the defence of any such claims or suits and the first party will co-operate with the other party or its insurers in such defence when reasonably requested to do so.
11. INSURANCE
Each party must have and must maintain for the Term the relevant Required Insurance. The Required Insurance must be with a reputable insurer and name the other party as an additional insured. Each party will at the others request provide to that party a certificate from a reputable insurance broker confirming that the insurance required by this clause in currently in effect.
12. TERM AND TERMINATION
12.1 Term
Subject to making of the payment contemplated by clause 2.4(b), or earlier termination pursuant to the further provisions of this clause 12, the initial term of this agreement will expire on 31st December 2005, subject to extension pursuant to clause 2.4. In the event that Lipha exercises its option in relation to undertaking Stage 2 Research, then this agreement will be extended and continue to apply whilst and so long as any Stage 2 Research Program continues, it being acknowledged that in such circumstances Lipha is not obliged to extend the Research Term (and comply with clause 2.4(b)) unless it wishes to extend Stage 1 Research in respect of some other Novel Gene Product.
12.2 Termination for breach
If one party breaches any term, provision or obligation of this agreement (the “Defaulting Party”) and the Defaulting Party fails to:
(a) remedy such breach within 60 days after receipt of notice from the other party requiring remedy of the breach; or
(b) if the breach cannot be remedied within the said 60 day period, commence action within the said 60 day period to remedy the breach and undertake in writing to the other party to complete remedy of the breach as soon as practicable thereafter,
the other party has the right to terminate this agreement immediately upon the expiration of the said period of 60 days by written notice to the Defaulting Party.
12.3 Termination in default of payment
This agreement may be forthwith terminated by a party by giving notice to the other party if that other party defaults in the payment of any money due by that other party to the first party under this agreement and such default continues for a period of 30 days after notice has been given to the other party demanding the payment of such money.
27
12.4 Grounds for immediate Termination
Subject to clause 12.5, this agreement may be terminated by a party giving notice to the other party upon the happening of any of the following events in respect of that other party:
(a) an Insolvency Event; or
(b) if the other party is in breach of an undertaking given pursuant to clause 12.2(b).
12.5 Reconstruction exception
A winding up or liquidation for the purposes of reconstruction or amalgamation by the other party is not an event permitting or giving rise to termination if after that reconstruction or amalgamation the resulting corporation becomes bound by the terms of this agreement by way of assignment or novation.
12.6 Termination by Autogen
Autogen may terminate this agreement upon notice to Lipha if:
(a) there is a change of greater than 50% in the control of the issued voting capital of Lipha or a holding company (if any) of Lipha other than for the purpose of internal re-construction;
(b) a person or persons not previously in Control of Lipha obtain Control of Lipha or a holding company (if any) of Lipha; or
(c) if Lipha or any of its affiliates challenges or seeks or causes to be challenged the validity of the Project Patents.
12.7 Termination by Lipha
Lipha may terminate this agreement upon notice to Autogen if there is a change of greater than 50% in the control of the issued voting capital of Autogen or a holding company (if any) of Autogen other than for the purpose of internal re-construction.
12.8 Termination to be without prejudice
Any termination of this agreement is without prejudice to the rights which a party has against the other in respect of anything done or omitted to be done hereunder prior to such termination or in respect of any sums or other claims outstanding at the time of termination.
12.9 Survival of provisions
The provisions of clauses 1, 2.9, 2.10, 3.3, 3.6, 3.8, 3.9, 3.10, 7.3, 7.4, 8, 9, 10, 11, 12.8, 12.9, 14.1, 16, 18, 19, 20 will survive termination of this agreement.
28
13. OBLIGATIONS AND RIGHTS ON TERMINATION
Immediately upon termination or expiration of this agreement, each party must return or destroy all copies of Know-How owned or supplied by the other party, except for the retention of a copy as necessary for that party to be able to exploit any continuing licenses.
14. WARRANTIES
14.1 General warranties
Each party represents and warrants to the other that:
(a) it has all necessary powers and authorisations necessary to enter into this agreement and observe its obligations hereunder and allow this agreement to be enforced against it; and
(b) all necessary consents, approvals and authorisations of all governmental authorities required to be obtained by that party in connection with this agreement have been obtained; and
(c) the execution and delivery of this agreement does not contravene any law, regulation or official directive or any obligations or undertakings, contractual or otherwise, by which it or any of its assets are bound or cause a limitation on its powers to be exceeded; and
(d) the performance of the party’s obligations under this agreement:
(i) do not and will not conflict with or violate any requirement of applicable laws or regulations; and
(ii) do not and will not conflict with, or constitute a default under any contractual obligation of that party; and
(e) there does not presently exist any event which would either now or with the effluxion of time entitle the other party to terminate this agreement pursuant to clause 12; and
(f) it is not a party to any pending or threatened action or proceeding affecting it or any of its assets before a court, governmental agency, commission or arbitrator where an adverse outcome could reasonably be expected to adversely impact upon the performance of its obligations under this agreement; and
(g) it has no immunity from the jurisdiction of a court or from legal process (whether through service of notice, attachment prior to judgment, attachment in aid of execution, execution or otherwise).
29
14.2 Specific warranty for Autogen Patents
Autogen warrants to Lipha that:
(a) as at the date of this agreement it has disclosed to Lipha details of all prior art sighted by Autogen by way of international search in relation to the Autogen Patents, but does not warrant that it has undertaken a thorough or exhaustive search of prior art; and
(b) at the date of this agreement it is unaware of any other Patent which has not been disclosed to Lipha and which may be infringed by Lipha using the Autogen Patents in accordance with the terms of this agreement.
14.3 Warranties by Lipha
Lipha warrants to Autogen that:
(a) it will obtain or has obtained from its Representatives who have access to the Autogen Know-How and Stage 1 Know-How written obligations to treat such information as confidential strictly in accordance with this agreement.
(b) that it has made its own enquiries into the validity and enforceability of the Autogen Patents and satisfied itself that the usage by Lipha, its Affiliates or sub-licensees of the Autogen Patents and the Confidential Information do not and will not infringe the intellectual property rights of any third party.
15. PROTECTION OF PATENTS
15.1 Maintenance of Patents
Autogen is responsible for the cost of preparing filing prosecuting and maintaining the Autogen Patents but nothing expressed or implied herein necessarily obligates Autogen to institute legal proceedings to protect same. The party responsible for the cost of preparing, filing, prosecuting and maintaining the Stage 1 Patents will initially be determined in accordance with clause 2.11, provided that as from the date that a Commercialisation Licence in respect of Stage 1 Patents is entered into between the Parties, Lipha will assume full responsibility for the full cost of preparing, filing, prosecuting and maintaining such Stage 1 Patents. Lipha is responsible for the cost of preparing, filing, prosecuting and maintaining the Stage 2 Patents. Nothing expressed or implied herein necessarily obligates Lipha to institute legal proceedings to protect Stage 1 Patents or Stage 2 Patents.
15.2 Reporting infringements
Other than in the case of a Direct Program or Indirect Program solely funded by Autogen, a party must promptly report to the other in writing particulars of any action or activity of which the first party becomes aware which might reasonably amount to infringement of or challenge to any of the Pre-Stage 2 Results or Stage 2 Results.
30
15.3 Conduct of proceedings regarding Pre-Stage 2 Results
Lipha has the first right to take control of and conduct any infringement proceedings in respect of the Pre-Stage 2 Results from a Lipha Program where such infringement is within the Licensed Field. Should Lipha undertake such proceedings Autogen must fully co-operate with Lipha in relation to such action including the lending of Autogen’s name to the action and assistance by personnel of Autogen. The costs and expenses of any such action will be borne by Lipha, and the proceeds of such action after payment of Lipha’s reasonable external costs will be divided between Autogen and Lipha on the basis that Autogen gets 50% and Lipha the balance. In the event Lipha does not wish to undertake the conduct of such proceedings on its own, Autogen and Lipha must discuss whether they will undertake joint proceedings and if so on what basis. If no such agreement can be reached Autogen is entitled to bring an action in its own name, and Lipha must provide Autogen with all reasonable assistance including lending Lipha’s name to such action. Autogen must bear all the costs of such an action, and the proceeds of any such action belong to Autogen absolutely.
16. INFRINGEMENT OF OTHERS RIGHTS
16.1 Notification of action
In the event that legal action is threatened or commenced against Lipha arising out of Lipha’s use of Autogen Patents or Autogen Know-How, Lipha must not make any admissions or enter into any substantive steps in connection therewith but must promptly notify Autogen.
16.2 Autogen action
If such legal action against Lipha arises out of the use of the Autogen Patents or Autogen Know-How in accordance with the terms and conditions of this agreement in relation to Products, then Autogen must defend and/or assist in the defence of such litigation, and must bear the reasonable costs and expenses of such defence. If as a result of such proceedings any damages or awards are assessed against Lipha, then provided that Lipha has at all times followed the instructions of, or otherwise obtained the consent of, Autogen in respect of the defence of such claims, then such damages or awards, as well as any and all reasonable costs incurred by Lipha, must be satisfied and paid by Autogen.
16.3 Lipha action
If such legal action against Lipha related to the use by Lipha, Lipha Affiliates or sub-licensees of the Autogen Patents or Autogen Know-How or use of Stage 1 Patents, Stage 1 Know-How or Stage 2 Patents or Stage 2 Know-How other than in accordance with the terms and conditions of this agreement Lipha must promptly notify Autogen of the commencement of legal action. Autogen must (at the expense of Lipha) assist Lipha’s efforts to settle and/or defend such claims. Lipha must bear all its own costs and expenses and must be responsible for awards against it and Autogen, as well as any and all reasonable costs incurred by Autogen, to the extent that indemnification, warranties and other claims may not be available against Autogen.
31
If any amounts are recovered by or awarded or paid to Lipha from or by a third party as a result of any such action or litigation, Lipha must from such amounts reimburse Autogen for all costs and amounts paid by Autogen in connection with such action or litigation and must, after deducting the legal costs incurred by it in taking such legal or other action, pay to Autogen from any compensation recovered thereby, Autogen’s part thereof determined in accordance with the respective interests of the Parties in such compensation.
17. FORCE MAJEURE
17.1 Party not liable
Where a party is required under this agreement to perform an obligation or do any act or thing by a designated time or date (other than an obligation to make payment) (“Obligation”), the party is not to be liable for any delay in performing or for failure to perform an Obligation where the delay or failure arises from Force Majeure and that party has complied with this clause.
17.2 Notice of Force Majeure
A party who claims Force Majeure must:
(a) give the other party prompt notice of the Force Majeure with reasonably full particulars and an estimate of the extent and duration of its delay in performance, or inability to perform; and
(b) use all possible diligence to remove the Force Majeure as quickly as possible provided that this will not oblige the party to settle on terms unsatisfactory to that party any strike, lockout or other labour difficulty, or any investigation or proceeding by any governmental authority or any litigation by any third party.
17.3 Termination in case of Force Majeure
If the delay continues beyond 30 Business Days, after the notice given under clause 17.2, the Parties must meet to discuss in good faith a mutually satisfactory resolution of the problem and, if unable to achieve such a resolution within a further 30 Business Days, either party may elect to terminate this agreement by five (5) Business Days prior written notice to the other.
18. ARBITRATION
In case of any disputes arising between the Parties or arising out of the performance or non-performance of the obligations of either party hereunder, or the termination of this agreement, the Parties will endeavour to settle such disputes amicably between themselves. If the Parties fail to resolve such disputes, then such disputes shall be finally resolved by arbitration in accordance with the Rules of Conciliation and Arbitration of the International Chamber of Commerce (ICC) by one or more arbitrators appointed in accordance with the said rules.
Any such arbitration will be held in English language and take place in Paris - FRANCE.
32
19. NOTICES
19.1 Form of Notice
Any notice, approval, consent or other communication (“notice”) from one party to another (“Recipient”) must be in writing and be signed by a person duly authorised by the person giving the notice.
19.2 Manner of Service
A notice must be served by:
(a) leaving it at the Recipient’s address;
(b) sending it by ordinary pre-paid post (airmail if being sent from or to a place outside of Australia) to the Recipient’s address; or
(c) sending it by facsimile to the facsimile number of the Recipient.
19.3 Address for Service
Until other details are specified by a Party as its address or facsimile number for service the following apply:
|
Autogen |
|
|
|
|
|
|
|
Address : |
000 Xxxxx Xxx, Xxxxx Xxxxxxxxx, Xxxxxxxx, Xxxxxxxxx |
|
|
|
|
|
|
Facsimile : |
61 3 9234 1190 |
|
|
|
|
|
|
Attention : |
Company Secretary |
|
|
|
|
|
|
Lipha |
|
|
|
|
|
|
|
Address : |
00 xxx Xxxxx Xxxxxx, 00000 Xxxx, XXXXX 00, Xxxxxx |
|
|
|
|
|
|
Facsimile : |
33-4-78-75-39-05 |
|
|
|
|
|
|
Attention : |
Head of Legal Department |
|
19.4 Time of Service
A letter or facsimile will be taken to be served:
(a) in the case of a delivered letter, on the day of delivery, unless delivery is made on a non Business Day or after 4:30 p.m. (local time in the place of receipt) on a Business Day, in which case it will be taken to be served on the next Business Day;
(b) in the case of a posted letter, on the third (or seventh in the case of airmail) Business Day after posting; and
33
(c) in the case of a facsimile, on receipt by the party giving the notice of a transmission confirmation report, unless within one Business Day of receipt the Recipient has informed the party giving the notice that the transmission was incomplete or garbled, provided that in any case if transmission is completed after 4:30 p.m. (local time in the place of receipt) or is received on a non Business Day, the notice will be taken to be served on the next Business Day.
20. MISCELLANEOUS
20.1 Severance
If any provision is held invalid or unenforceable, in whole or in part in any jurisdiction, then such invalidity or unenforceability will only affect such provision or part thereof in such jurisdiction, and will not in any manner affect the provision in this agreement in any other jurisdiction. To the extent legally permissible an arrangement which reflects the original intent of the Parties must be substituted for such invalid or unenforceable provision.
20.2 Waiver
The failure, delay, relaxation or indulgence by any party in exercising any power or right given to that party under this agreement will not operate as a waiver of that power or right, nor will any single exercise of a power or right preclude any other or further exercise of it or the exercise of any other power or right under this agreement. A power or right may only be waived in writing, signed by the party to be bound by the waiver.
20.3 Relationship of Parties
Nothing contained in this agreement is to be construed so as to place any Party in the relationship of principal, employee, agent, partner, joint venturer or legal representative of any other Party. The Parties expressly agree and acknowledge that each of the Parties is an independent contracting Party and does not, unless expressly provided, have the authority or power for or on behalf of any other Party to enter into any contract, to incur debts, to accept money, to assume any obligations or to make any warranties or representations.
20.4 Injunctive relief
If there is any conduct or threatened conduct which is or may be a breach of this agreement, both Parties acknowledge that damages may be inadequate compensation for such a breach and that the other Party is entitled to apply to any court of competent jurisdiction for interim or permanent injunctive relief or both restraining the other Party from committing any breach or threatened breach of this agreement without showing or proving any actual damage sustained by it. Such rights and remedies will be cumulative and in addition to any other rights or remedies which that party may be entitled to at law or in equity.
34
20.5 Proper law
This agreement must be construed in accordance with and governed by the laws of the United Kingdom and its form, execution, validity, construction and effect is to be determined in accordance with the laws of the United Kingdom.
20.6 Rule of construction
No rule of construction applies to this agreement to the disadvantage of a Party because that Party was responsible for the preparation of this agreement or any part of it.
20.7 Variation
Any modification, alteration, change or variation of any term or condition of this agreement must be in writing, executed by all Parties.
20.8 Assignment
This agreement is personal to the respective Parties and neither is entitled to assign in whole or in part without the prior written consent of the other Party.
20.9 Further documents
Each Party agrees that it will forthwith upon the request of the other Party execute and deliver all such instruments and agreements and will take all such other actions as the other Party may reasonably request from time to time in order to give effect to the provisions and purposes of this agreement.
20.10 Affiliates’ actions
Each Party will ensure that none of its Affiliates takes any action which is inconsistent with that Party’s obligations under this agreement, or which if it was done or not done under this agreement by that Party would amount to a breach of this agreement by that Party.
20.11 Costs and Taxes
Each party must pay its own costs and expenses in relation to the negotiation, preparation, execution, delivery, stamping and registration, completion, variation and discharge of this agreement and Lipha must pay any Tax in respect of the execution, delivery, performance, release, discharge, amendment, enforcement or attempted enforcement or otherwise of any of the following:
(a) this agreement;
(b) any agreement or document entered into or signed under this agreement;
(c) any transaction contemplated under this agreement; and
(d) any payment made or received in respect of this agreement,
except in any case to the extent to which such Tax is payable in Australia or on or in respect of the income of Autogen.
35
20.12 Interest on overdue amounts
If any amount due under this agreement is not paid when due, then the Party obliged to make payment must pay to the other Party interest at the Base Rate on the amount due and payable, accruing from day to day and to be computed from the date for payment of the amount until payment of the amount in full.
20.13 Counterparts
This agreement may be executed in counterparts, and by the Parties on separate or the same counterparts, each of which is deemed an original, but all of which constitute one and the same instrument.
20.14 Registration of agreement
If this agreement or any associated transaction is required by the law of any country, other than Australia, to be either approved or registered in any country or with any governmental agency, Lipha is responsible for obtaining such approval or registration including without limiting the generality of the foregoing, causing this agreement to be stamped, recorded and registered at its cost in such country. Autogen agrees to co-operate in any such application or registration procedure. Lipha must furnish proof of compliance with the foregoing to Autogen when and if Autogen so requires.
20.15 Not obliged to act contrary to law
No Party is obliged to carry out or perform any of the terms of this agreement where doing so would constitute a violation of any treaty, law, code or regulation of any governmental authority whether local, national or supranational. In any event the other terms of this agreement nevertheless continue and the Parties must use all reasonable endeavours to re-negotiate and amend this agreement so that the performance of this agreement as so amended will not involve any such violation.
20.16 Statutory rights not limited
The powers, remedies and rights conferred upon the Parties by or under any statute are (except to the extent inconsistent with the terms and provisions expressed in this agreement) be in addition to the powers, remedies and rights conferred by this agreement.
20.17 Consents
Unless this agreement provides otherwise and to the extent permitted by law, a Party may, in its absolute discretion, conditionally or unconditionally give or withhold any approval or consent permitted or required to be given by it pursuant to this agreement.
20.18 Entire agreement
This agreement constitutes the entire agreement between the Parties in relation to the subject matter of this agreement. Any prior arrangements, agreements, representations or undertakings are superseded and, except as expressly provided, each party acknowledges that it has not relied on any arrangement, agreement, representation or understanding which is not expressly set out in this agreement.
36
Europe
|
Albania |
|
Macedonia |
|
Austria |
|
Malta |
|
Belgium |
|
Moldavia |
|
Belarus |
|
Netherlands |
|
Bosnia-Herzegovina |
|
Norway |
|
Bulgaria |
|
Poland |
|
Croatia |
|
Portugal |
|
Cyprus |
|
Romania |
|
Czech Republic |
|
Russia |
|
Denmark |
|
San Marino |
|
Estonia |
|
Slovak Republic |
|
Finland |
|
Slovenia |
|
France |
|
Spain |
|
Germany |
|
Sweden |
|
Greece |
|
Switzerland |
|
Hungary |
|
Turkey |
|
Iceland |
|
Ukraine |
|
Ireland |
|
United Kingdom |
|
Italy |
|
Yugoslav Federal Republic (Serbia-Montenegro) |
|
Latvia |
|
|
|
Liechtenstein |
|
|
|
Lithunia |
|
|
|
Luxembourg |
|
|
38
Joint Venture Agreement
1. Suggested Equity of Autogen [*]%, Lipha [*]%
2. Joint Venture would be conducted through a jointly owned company (“JVCO”) to be located in an agreed country.
3. JVCO would hold, either directly, or by way of exclusive “free” licence all relevant intellectual property rights, including Pre-Stage 2 Results and Stage 2 Results. the licence would be restricted to the Licensed Field and only apply to countries outside Europe.
4. An exclusive research and development and commercialization agreement (“Joint Venture”) on the Research relating to all countries outside Europe provided that each Party equally shares all costs and revenues of such research and development costs. Any sales made outside Europe by this joint venture shall not be subject to royalty payments.
5. In case any study is necessary for development both in Europe and outside Europe, both Parties shall agree on which of Lipha or JVCO will conduct such study, and the cost of such study shall be shared by Lipha and JVCO according to the part of the world in which such study may be used, considering of the world for Europe, for North and Latin America and for Japan, the other parts of the world not being considered in such sharing.
6. Both Parties are to contribute all relevant patents and technology (including core registration data and other material relevant to registration and approval) necessary or useful to commercialise products or processes in the relevant Licensed Field, which they already have, or subsequently separately create or generate at or prior to the date of commencement of JVCO at the end of Stage 2, such contribution to be without cost or royalty entitlements, unless the same are due to non-Affiliates.
7. This free contribution does not extend to manufactured product which, subject to the price being cost competitive with third Parties on a contract or toll manufacturing basis, will be supplied exclusively by Lipha at a price to be agreed.
40
STAGE 1
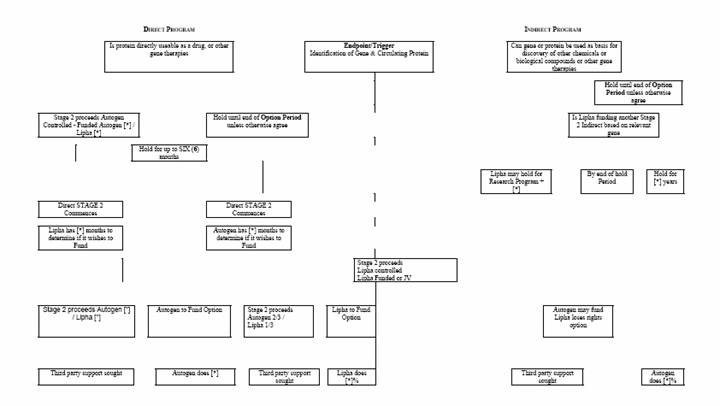
• Please note references to “[*]” and “[*]” mean the funding proportions to be borne by the parties.
42
EXECUTED as an agreement
|
The common seal of Autogen Research |
|
) |
|
Pty Ltd is affixed in accordance with its |
|
) |
|
articles of association in the presence of: |
|
) |
|
|
|
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Director |
|
(name printed) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Director/Secretary |
|
(name printed) |
|
|
|
|
|
|
|
|
|
The common seal of Lipha S.A. is affixed |
|
) |
|
in accordance with its articles of association |
|
) |
|
in the presence of: |
|
) |
|
|
|
) |
|
|
|
|
|
|
|
Xxxx-Xxxx TREILLES |
|
CHAIRMAN OF THE BOARD |
|
(name printed) |
|
|
|
|
|
|
|
|
|
|
|
Xxxx XXXXXXXX |
|
MANAGING DIRECTOR |
|
(name printed) |
43
