Development, Marketing and Supply Agreement by and between Arena Pharmaceuticals GmbH and Roivant Sciences Ltd. dated May 8, 2015
Exhibit 10.2
***Text Omitted and Filed Separately
with the Securities and Exchange Commission.
Confidential Treatment Requested
Under 17 C.F.R. Section 240.24b-2
Execution Copy
by and between
Arena Pharmaceuticals GmbH and
Roivant Sciences Ltd.
dated
May 8, 2015
1
This Development, Marketing and Supply Agreement (the “Agreement”) is made and entered into as of May 8, 2015 (the “Effective Date”) by and between Arena Pharmaceuticals GmbH, a company organized under the laws of Switzerland having a principal place of business at Xxxxxx Xxxxxxxxxxxx 0, 0000, Xxxxxxxx, Xxxxxxxxxxx (“Arena”), and Roivant Sciences Ltd., a Bermuda Exempted Limited Company having a principal place of business at 0 Xxxxxxxxx Xxxxx, Xxxxxxxx XX00, Xxxxxxx (“Roivant”). Each of Arena and Roivant may be referred to in this Agreement individually as a “Party” and collectively as the “Parties”.
In consideration of the mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Arena and Roivant, intending to be legally bound, hereby agree as follows:
ARTICLE 1
As used in this Agreement, the following capitalized terms have the meanings set out in this Article 1.
1.1 “Additional Product” means a particular pharmaceutical product (other than the Initial Product) with a [***], that contains a Compound, which product is added to this Agreement as provided in Section 3.2(b) for development and Commercialization in accordance with this Agreement. For clarity, an Additional Product may be a Combination Product.
1.2 “Additional Product Minimum Purchase Price” has the meaning set forth in Section 7.3(d)(ii).
1.3 “Affiliate” means, with respect to a Person, any other Person that, directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with such Person, as the case may be, but for only so long as such control exists. As used in this definition, the term “control” (with correlative meanings for the terms “controlled by” and “under common control with”) means (a) direct or indirect beneficial ownership of more than 50% of the voting share capital or other equity interest in such Person able to elect the directors or management of such Person or (b) the power to direct the management and policies of such Person by contract or otherwise.
1.4 “Agreement” has the meaning set forth in the opening paragraph hereto.
1.5 “Applicable Laws” means the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidance, ordinances, judgments, decrees, directives, injunctions, orders, permits (including Regulatory Approvals) of or from any court, arbitrator, Regulatory Authority or other governmental agency or authority having jurisdiction over or related to the subject activity or item as they may be in effect from time to time.
1.6 “Arena” has the meaning set forth in the opening paragraph hereto.
1.7 “Arena Indemnitees” has the meaning set forth in Section 11.1.
1.8 “Arena Know-How” means all Know-How that (a) is Controlled by Arena or any of its Affiliates as of the Effective Date, or at any time during the Term, and (b) is reasonably necessary for the development, manufacture or Commercialization of the Compounds or the Initial Product in the Field in accordance with this Agreement, but excluding [***].
1.9 “Arena Patent” means any Patent pending or issued that (a) is Controlled by Arena or any of its Affiliates as of the Effective Date, or at any time during the Term, and (b) is reasonably necessary for the development, manufacture or Commercialization of the Compounds or the Initial Product in the Field, including each Patent that is listed on Exhibit B or claims priority to a Patent listed on Exhibit B, but excluding [***].
1.10 “Auditor” has the meaning set forth in Section 7.7(a).
2
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.11 “Batch” means the total amount of a particular Finished Product or Compound resulting from one complete production run conducted by or on behalf of Arena using the applicable Master Batch Records and Manufacturing SOPs.
1.12 “Batch Records” means, with respect to a particular production run conducted by or on behalf of Arena for manufacturing one Batch of a particular Finished Product or Compound, the completed batch records, in the form of the Master Batch Records, for such production run containing all the relevant manufacturing details and information for the run, including any deviations.
1.13 “Calendar Quarter” means a period of three consecutive months during a Calendar Year beginning on and including January 1st, April 1st, July 1st or October 1st; provided, that the last Calendar Quarter shall end on the last day of the Term.
1.14 “Calendar Year” means a period of 12 consecutive months beginning on and including January 1st; provided, that the first Calendar Year of the Term shall commence on the Effective Date and end on December 31, 2015, and the last Calendar Year shall end on the last day of the Term.
1.15 “Certificate of Analysis” means a written certificate of analysis, in reasonable and customary form, which confirms (i) the purity of Compound manufactured by or on behalf of Arena for use in Finished Product, (ii) that the quantity of the Compound in the Finished Product manufactured by or on behalf of Arena meets the applicable Specifications, (iii) the purity of Finished Product manufactured by or on behalf of Arena, or (iv) that the quantity or lot number of the Finished Product manufactured by or on behalf of Arena meets the applicable Specifications. The Certificate of Analysis will include the results of all Product Acceptance Tests performed by or on behalf of Arena on the particular Batch of such Compound or Finished Product.
1.16 “cGMP” means the then-current good manufacturing practices required by the FDA, as set forth in the FFDCA for the manufacture and testing of pharmaceutical materials, including as set forth in 21 U.S.C. section 351 and 21 C.F.R. Parts 210 and 211. Good Manufacturing Practices shall include applicable quality guidelines promulgated under the ICH.
1.17 “Chairman” means the chairman of the Joint Steering Committee.
1.18 “Change of Control” means, with respect to either Party, the occurrence of any of the following:
(a) any “person” or “group” (as such terms are defined below) is or becomes the “beneficial owner” (as defined below), directly or indirectly, in a transaction or series of related transactions, of shares of capital stock or other interests (including partnership or LLC membership interests) of such Party (or any of its Controlling Affiliates) then-outstanding and normally entitled (without regard to the occurrence of any contingency) to vote in the election of the directors, managers or similar supervisory positions (“Voting Stock”) (or its Controlling Affiliate, as applicable) of such Party representing 50% or more of the total voting power of all outstanding classes of Voting Stock of such Party (or its Controlling Affiliate, as applicable); or
(b) such Party (or any of its Controlling Affiliates) enters into a merger, consolidation or other form of business combination, share exchange, reorganization, recapitalization or other similar extraordinary transaction with another Person (whether or not such Party (or its Controlling Affiliate, as applicable) is the surviving entity) and as a result of such merger, consolidation or other form of business combination, share exchange, reorganization, recapitalization or similar extraordinary transaction (i) the members of the board of directors or similar governing body (as the case may be, “Board of Directors”) of such Party (or its Controlling Affiliate, as applicable) immediately prior to such transaction constitute less than a majority of the members of the Board of Directors of such Party (or its Controlling Affiliate, as applicable) or, if not such Party (or its Controlling Affiliate, as applicable), such surviving Person immediately following such transaction or (ii) the Persons that beneficially owned, directly or indirectly, the shares of Voting Stock of such Party (or its Controlling Affiliate, as applicable) immediately prior to such transaction cease to beneficially own, directly or indirectly, shares of Voting Stock representing at least a majority of the total voting power of all outstanding classes of Voting Stock of the surviving Person immediately following such transaction; or
(c) such Party (or any of its Controlling Affiliates) sells or transfers to any Third Party, in one or more related transactions, properties or assets representing all or substantially all of the consolidated total assets of such Party and its Affiliates.
3
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
For the purpose of this definition: (x) “person” and “group” have the meanings given such terms under Section 13(d)(3) and 14(d)(2) of the Exchange Act and the term “group” includes any group acting for the purpose of acquiring, holding or disposing of securities within the meaning of Rule 13d-5(b)(1) under the Exchange Act; (y) “beneficial owner” shall be determined in accordance with Rule 13d-3 under the Exchange Act; and (z) the terms “beneficially owned” and “beneficially own” shall have meanings correlative to that of “beneficial ownership”.
1.19 “Combination Product” means any Finished Product that either (a) contains a Compound as an active agent formulated together with at least one other clinically active agent that is not a Compound; or (b) is a combination of a Product and at least one other therapeutic or prophylactic product (other than a Product) where such products are not formulated together but are sold together as a single product and invoiced as one product.
1.20 “Commercial Year” means each period of 12 consecutive months beginning on (a) the first day of the first full month that occurs after the date of the First Commercial Sale and (b) each anniversary of the date specified in the foregoing clause (a); provided, that the first Commercial Year of the Term shall also include the period beginning on and including the date of the First Commercial Sale and ending on the day immediately prior to the first day of the first full month that occurs after such date, and the last Commercial Year shall end on the last day of the Term.
1.21 “Commercialization” means marketing, promoting, detailing, offering for sale, selling, importing and distributing Product in the Field, and other similar activities related to the commercial sale of Product in the Field, but excluding for clarity all activities relating to research, development, preclinical and clinical testing (including post-approval clinical testing of any kind) or manufacturing of Compound, Product or Finished Product. When used as a verb, “Commercializing” means to engage in Commercialization and “Commercialize” and “Commercialized” have corresponding meanings.
1.22 “Commercially Reasonable Efforts” means, with respect to a particular Party’s specific obligations under this Agreement with respect to Product at the relevant point in time, that level of efforts and application of resources that is consistent with the usual practice followed by that Party in conducting similar activities with respect to other prescription pharmaceutical products owned or licensed by it or to which it has exclusive rights, in each case that have comparable market potential, probability of technical success, and technical and regulatory profile and patent protection to the Product, and are at a stage of development or product life comparable to, Product, but in no event less than the level of efforts and resources generally applied within the pharmaceutical industry in conducting such activities with respect to such comparable prescription pharmaceutical products.
1.23 “Competing Product” means a pharmaceutical product (whether an ethical pharmaceutical, over the counter drug, botanical product or other type of product) containing a compound that acts primarily by being a centrally acting (i.e. designed and thought to act primarily in the central nervous system) inverse agonist or antagonist of 5HT2A and 5HT2Ais the target for which such compound has the highest potency and selectivity (as determined reasonably and in good faith by the party Controlling the compound), other than any Compound or Reverted Compound.
1.24 “Compound” means the Initial Compound and the Related Compounds.
1.25 “Confidential Information” has the meaning set forth in Section 8.1.
1.26 “Control” (including any variations such as “Controlled” and “Controlling”), in the context of Materials, Patents, Know-How, Domain Names, Trademarks or regulatory filings (including specific Confidential Information), means that the applicable Party or its Affiliate owns or has a license (but excluding license rights granted to such Party by the other Party) to such Materials, Patents, Know-How, Domain Names, Trademarks or regulatory filings and has the ability to grant to the other Party the applicable license (or sublicense, as applicable) or right to access or use such Materials, Patents, Know-How, Domain Names, Trademarks or regulatory filings under this Agreement without violating the terms of an agreement with a Third Party.
1.27 “Development Data” means, with respect to clinical trials and other development work conducted on Product, all data, results, information and other Know-How generated from or related to such clinical trials and development work, including preclinical, non-clinical and clinical data, reports and information, protocols, statistical analysis plans, methods, and Batch Records for all Products used in such work.
1.28 “Development Plan” means, with respect to a specific Product, the plan for conducting the clinical trials and other development work (including any preclinical and non-clinical studies) pursuant to this Agreement to generate data for use in obtaining, maintaining or expanding Regulatory Approval of the Product.
4
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.29 “Disclosing Party” has the meaning set forth in Section 8.1.
1.30 “Dispute” has the meaning set forth in Section 12.7(a).
1.31 “Domain Name” means a combination of alpha-numeric characters with a top level domain.
1.32 “Effective Date” has the meaning set forth in the preamble of this Agreement.
1.33 “EMA” means the European Medicines Agency or any successor agency for the European Union.
1.34 “Excess Order” has the meaning set forth in Section 6.2(c).
1.35 “Exchange Act” means the Securities Exchange Act of 1934, as it may be amended from time to time.
1.36 “Excluded Claim” has the meaning set forth in Section 12.7(j).
1.37 “Excluded List” means any of the United States Department of Health and Human Service’s List of Excluded Individuals/Entities or the United States General Services Administration’s Lists of Parties Excluded from Federal Procurement and Non-Procurement Programs.
1.38 “Existing Arena Patents” has the meaning set forth in Section 10.2(a).
1.39 “Extended Term” has the meaning set forth in Section 12.1.
1.40 “Facility” has the meaning set forth in Section 6.12.
1.41 “Facility Licenses” has the meaning set forth in Section 6.12.
1.42 “FCPA” has the meaning set forth in Section 16.1.
1.43 “FDA” means the United States Food and Drug Administration or its successor.
1.44 “FFDCA” means the United States Federal Food, Drug, and Cosmetic Act, 21 U.S.C. 301, et. seq., as it may be amended from time to time, and the rules, regulations, guidances, guidelines, and requirements promulgated or issued thereunder.
1.45 “Field Infringement” has the meaning set forth in Section 9.3(b).
1.46 “Field” means the prevention or treatment of any disease, state or condition in humans.
1.47 “Finished Product” means, with respect to a particular Product: (a) if the Product is to be sold to end-users, Product in final form ready for sale to the end-user, (b) if Product is to be used for clinical trials or other development work, Product in final form ready for such clinical trials or other development work, (c) if the Product is to be used as a sample, Product in final form ready for distribution as a sample, or (d) if the Product is to be used as part of a compassionate use, named patient use or indigent patient program, Product in final form ready for such compassionate use, named patient use or indigent patient program, in each case ((a) - (d)), in appropriate final packaging and labeling.
1.48 “First Commercial Sale” means the first bona fide, arm’s length sale of Product by Roivant, its Sub-distributors, or any of its or their Affiliates to a Third Party (other than a Sub-distributor or its Affiliate). Sales of a Product for use as registration samples, compassionate use sales, named patient use, inter-company transfers to Affiliates of Roivant or Sub-distributors or their Affiliates and the like shall not constitute a First Commercial Sale.
1.49 “Fiscal Semester” means a period of six consecutive months during a Calendar Year beginning on and including July 1st or January 1st; provided, that the first Fiscal Semester of the Term shall commence on the Effective Date and end on December 31, 2015, and the last Fiscal Semester shall end on the last day of the Term.
5
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.50 “For Cause” means, with respect to an audit, that the audit is prompted by a regulatory agency critical finding or recall or a critical finding in an audit conducted by or on behalf of Roivant pursuant to Section 6.8, where “a critical finding” is a finding that would result in a regulatory action or is otherwise defined in the Quality Agreement.
1.51 “Force Majeure” has the meaning set forth in Section 15.1.
1.52 “Forecast” has the meaning set forth in Section 6.2(b).
1.53 “FTE” means the equivalent of the work of one full-time qualified employee of Arena for one year (constituting [***] working hours). An individual contributing work for less than [***] hours per year shall be deemed a fraction of an FTE on a pro-rata basis. Each FTE shall be placed in a category of FTEs based on such individual’s position.
1.54 “FTE Costs” means the sum, for each category of FTE, of the applicable FTE Rate times the number of FTEs in such category expended during the applicable financial period. The FTE Costs shall be determined based on time (as calculated in pro-rated FTEs) actually spent performing the applicable activities, unless another basis is expressly specified herein or otherwise agreed in advance by the Parties in writing.
1.55 “FTE Rate” means, with respect to a particular category of FTEs, the monetary rate at which such FTEs expended during the applicable financial reporting period accrue. As of the Effective Date, such annual rates are (a) US$[***] per FTE for department head, director or equivalent level or above, (b) US$[***] per FTE for team leader, scientist or equivalent level (up to director or equivalent level), and (c) US$[***] per FTE for below team leader, scientist or equivalent level. Commencing January 1, 2016, each FTE Rate shall adjust annually, effective January 1 of the applicable Calendar Year, to reflect any year-to-year percentage increase or decrease (as the case may be) in the U.S. Bureau of Labor Statistics Employee Cost Index (“ECI”) (based on the change in the ECI from the most recent index available as of the Effective Date to the most recent index available as of the date of the calculation of such adjusted FTE Rate).
1.56 “GAAP” means generally accepted accounting principles in the United States and means international financial reporting standards (“IFRS”) at such time as IFRS becomes the generally accepted accounting standard in the United States.
1.57 “Good Clinical Practices” or “GCP” means the then-current standards, practices and procedures promulgated or endorsed by the applicable Regulatory Authority (applying the standards, practices or procedures of the FDA for this definition where such standards, practices or procedures are more strict) for designing, conducting, recording, analyzing and reporting clinical trials that involve the participation of human subjects, including as set forth in 21 C.F.R. part 50, 54, 56 and 312 and in the ICH guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” and comparable regulatory standards, practices and procedures in other countries and jurisdictions, as they may be updated from time to time.
1.58 “Good Laboratory Practices” or “GLP” means the then-current good laboratory practice standards promulgated or endorsed by the applicable Regulatory Authority (applying the standards, practices or procedures of the FDA for this definition where such standards, practices or procedures are more strict) for nonclinical laboratory studies that support or are intended to support applications to conduct research on human subjects or to obtain regulatory approval, including as set forth in 21 C.F.R. Part 58, and comparable regulatory standards, practices and procedures in other countries and jurisdictions, as they may be updated from time to time.
1.59 “ICC” has the meaning set forth in Section 12.7(a).
1.60 “ICC Rules” has the meaning set forth in Section 12.6(a).
1.61 “ICH” means the International Conference on Harmonization (of Technical Requirements for Registration of Pharmaceuticals for Human Use).
1.62 “IND” means an Investigational New Drug Application (including any amendments thereto) filed with the FDA pursuant to 21 C.F.R. §312 before commencement of clinical trials of a pharmaceutical product and its equivalent in other countries or regulatory jurisdictions outside the United States.
1.63 “Indemnitee” has the meaning set forth in Section 11.3(a).
6
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.64 “Indemnitor” has the meaning set forth in Section 11.3(a).
1.65 “Indication” means a separate and distinct disease, disorder or medical condition that a Product is intended to treat, prevent, cure, or ameliorate, or that is the subject of a clinical trial on a Product and where it is intended that the data and results of such clinical trial (if successful) will be used to support a regulatory submission and approval that is intended to result in distinct labeling within the indications section of the label relevant to usage in the disease, disorder or medical condition that is separate and distinct from another disease, disorder or medical condition. Examples of three distinct Indications are Alzheimer’s Disease Psychosis, Dementia with Lewy Bodies Psychosis and Xxxxxxxxx’x Disease Psychosis.
1.66 “Initial Compound” means the compound known as 1-[3-(4-Bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-difluoro-phenyl)-urea, the structure of which is set forth in Exhibit A.
1.67 “Initial Compound Patents” has the meaning set forth in Section 9.2(b).
1.68 “Initial Product” means the pharmaceutical product that contains the Initial Compound, [***] with the formulation described in IND 73405, but in doses of up to 40 mg.
1.69 “Initial Product Minimum Purchase Price” has the meaning set forth in Section 7.3(d)(i).
1.70 “Initial Purchase Price Payment” has the meaning set forth in Section 7.3(b)(ii).
1.71 “Initial Term” has the meaning set forth in Section 12.1.
1.72 “Joint Manufacturing Committee” or “JMC” has the meaning set forth in Section 4.1(a).
1.73 “Joint Steering Committee” or “JSC” has the meaning set forth in Section 4.1(a).
1.74 “Know-How” means all tangible and intangible scientific, technical, trade, financial or business information and materials, including compounds, compositions of matter, formulations, techniques, processes, methods, trade secrets, formulae, procedures, tests, data, results, analyses, documentation, reports, information (including pharmacological, toxicological, non-clinical (including chemistry, manufacturing and control)), and clinical test design, methods, protocols, data, results, analyses, and conclusions, quality assurance and quality control information, regulatory documentation, information and submissions pertaining to, or made in association with, filings with any Regulatory Authority, product life cycle management strategies, knowledge, know‑how, skill, and experience, and all other discoveries, developments, inventions (whether or not confidential, proprietary, patented or patentable), and tangible embodiments of any of the foregoing.
1.75 “Knowledge” means, with respect to a particular statement to which such term is attributed, that none of the applicable Party’s respective officers or the officers of its Affiliates, are aware of any facts or information that make such statement untrue after performing a reasonable investigation with respect to such statement.
1.76 “Losses” has the meaning set forth in Section 11.1.
1.77 “MAA” means (a) a marketing authorization application filed with (i) the EMA under the centralized EMA filing procedure or (ii) a Regulatory Authority in any European country if the centralized EMA filing procedure is not used; or (b) any other equivalent or related regulatory submission, in either case to gain approval to market a Product in any country in the European Union, in each case including, for the avoidance of doubt, amendments thereto and supplemental applications.
1.78 “Major Market Country” has the meaning set forth in Section 5.2.
1.79 “Manufacturing Know-How” means any and all Know-How Controlled, discovered, identified, conceived, reduced to practice or otherwise made by or on behalf of Arena or its Affiliates prior to or during the term of this Agreement, in each case to the extent such Know-How relates to the manufacture of pharmaceutical products generally, but excluding Know-How that relates solely to, or to the extent it is specifically tailored for use in, the manufacture of any Compound or Product.
7
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.80 “Manufacturing Patent” means any Patent to the extent it claims or covers any invention within the Manufacturing Know-How.
1.81 “Manufacturing SOPs” means, with respect to a particular Finished Product being supplied by Arena to Roivant under Article 6 and the Compound contained therein, the specific methods, techniques, processes and standard operating procedures (including Quality Control Procedures) that are established in accordance with the Quality Agreement and are used by or on behalf of Arena in manufacturing such Finished Product or such Compound.
1.82 “Master Batch Records” means the master batch records for Arena’s (or its designee’s) manufacturing of a specific Finished Product or Compound, as established in accordance with the Quality Agreement, including the applicable Manufacturing SOPs, the in-process testing and QA/QC testing for such Finished Product or such Compound, which records are to be used in (a) the manufacture by or on behalf of Arena of such Finished Product pursuant to this Agreement or (b) the manufacture by or on behalf of Arena of such Compound for use in the manufacture of Finished Product pursuant to this Agreement.
1.83 “Materials” has the meaning set forth in Section 3.6.
1.84 “Minimum Product Purchase Price” for each Product means either the Initial Product Minimum Product Purchase Price or the applicable Additional Product Minimum Purchase Price.
1.85 “NDA” means a New Drug Application (including an Abbreviated New Drug Application) as described in 21 C.F.R. § 314.50, et seq., and all amendments and supplements thereto, that is filed with the FDA, and its equivalent in other countries and regulatory jurisdictions outside the United States, in each case including all documents, data, and other information concerning the applicable product filed therewith.
1.86 “Net Sales” means, with respect to a Finished Product during any period, the gross invoiced sales price in US Dollars (as converted into US Dollars for sales made in other currency) for all quantities of such Finished Product sold by Roivant, its Sub-distributors, or any of its or their Affiliates to a Third Party (other than a Sub-distributor or its Affiliates) during such period, less the following deductions to the extent actually incurred, allowed, or paid with respect to such sale by the selling party, using GAAP applied on a consistent basis:
(a) [***] included in the gross invoiced sales price;
(b) pharmaceutical excise taxes (such as those imposed by the United States Patient Protection and Affordable Care Act of 2010 (Pub. L. No. 111-48) and other comparable laws), in each [***];
(c) [***] with respect to such Finished Product;
(d) [***],
(e) [***], to the extent separately set forth in the applicable invoice;
(f) [***], in amounts not exceeding [***]; and
(g) [***], in amounts not exceeding usual and customary amounts and calculated in accordance with GAAP.
If Roivant proposes to develop or commercialize one or more Combination Products, Roivant will propose to Arena adjustments to Net Sales calculations for such Combination Products. Arena will reasonably consider such proposal and the Parties will negotiate in good faith to agree upon a reasonable adjustment. Any adjustments agreed by the Parties will be set forth in a writing signed by authorized officers of the Parties. If notwithstanding the Parties’ good faith discussions, the Parties are unable to agree on such adjustment [***] in the case of [***] will [***].
8
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
In no event shall any particular amount of deduction identified above, be deducted more than once in calculating Net Sales (i.e., no “double counting” of reductions). Each of the above deductions shall be substantially consistent with Roivant’s, its Sub-distributors’ and its and their Affiliates’ internal accounting policies as consistently applied by Roivant, its Sub-distributors or its or their Affiliates in the applicable country across its products at the time of sale. In no event shall the deductions with respect to [***] (set forth in part (c) above) for any Calendar Quarter exceed [***]% of the amount arrived at after deducting the items described in clauses (a), (b), (d) and (e) above from the gross invoiced sales price in US Dollars (as converted into US Dollars for sales made in other currency) for all quantities of such Product sold by Roivant, its Sub-distributors or its or their Affiliates to a Third Party during such Calendar Quarter; provided, that any deductions for [***] pursuant to this sentence shall be [***]. Roivant shall [***] of a Product [***] by Roivant, its Sub-distributors or its or their Affiliates. Sales of a Product between Roivant and any of its Sub-distributors or its or their Affiliates for resale shall be excluded from the computation of Net Sales, but the subsequent resale of such Product to a Third Party (other than a Sub-distributor or its Affiliates) shall be included within the computation of Net Sales.
1.87 “Non-Conforming Finished Product” has the meaning set forth in Section 6.9.
1.88 “Notice Date” has the meaning set forth in Section 12.7(b).
1.89 “Order Acceptance” has the meaning set forth in Section 6.2(c).
1.90 “Order Commitment” has the meaning set forth in Section 6.2(b).
1.91 “Panel” has the meaning set forth in Section 12.7(b).
1.92 “Paragraph IV Notice” has the meaning set forth in Section 9.3(c).
1.93 “Party” and “Parties” has the meaning set forth in the opening paragraph of this Agreement.
1.94 “Patent(s)” means (a) all patents, certificates of invention, applications for certificates of invention, priority patent filings and patent applications, including provisional patent applications, and (b) any renewal, division, continuation (in whole or in part), or request for continued examination of any of such patents, certificates of invention and patent applications, and any all patents or certificates of invention issuing thereon, and any and all reissues, reexaminations, extensions, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions, and additions of or to any of the foregoing.
1.95 “Patent Term Extension” means any term extensions, supplementary protection certificates, regulatory exclusivity and equivalents thereof offering patent protection beyond the initial term with respect to any issued Patents.
1.96 “Payee Party” has the meaning set forth in Section 7.5.
1.97 “Paying Party” has the meaning set forth in Section 7.5.
1.98 “Payment” has the meaning set forth in Section 7.5.
1.99 “Person” means any individual, corporation, partnership, limited liability company, trust, governmental entity, or other legal entity of any nature whatsoever.
1.100 “Phase 2 Trial” means a human clinical trial in any country that would satisfy the requirements of 21 CFR 312.21(b).
1.101 “Phase 3 Trial” means a human clinical trial in any country that would satisfy the requirements of 21 CFR 312.21(c).
1.102 “Pricing Approval” means such governmental approval, agreement, determination or decision establishing prices for a Product that can be charged and/or reimbursed in regulatory jurisdictions where the applicable governmental authorities approve or determine the price and/or reimbursement of pharmaceutical products and where such approval, agreement, determination or decision establishes prices for a Product that are acceptable to Roivant or its Sub-distributor or its or their Affiliate, as applicable.
9
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.103 “Product” means (a) the Initial Product or (b) an Additional Product.
1.104 “Product Acceptance Tests” means, with respect to a particular Compound or Finished Product being manufactured by or on behalf of Arena pursuant to this Agreement, the specific tests (including release tests) to be used to determine whether such Compound or Finished Product conforms to the terms of this Agreement and the Quality Agreement, including the Specifications for such Compound or Finished Product, which tests the Parties shall establish (and amend from time to time if required) in accordance with the terms of the Quality Agreement.
1.105 “Product Domain Names” means the Domain Names listed on Exhibit C, and any other Domain Names that are specific to one or more Compounds or Products, and that are Controlled by Roivant.
1.106 “Product Liability Claim” means any Third Party Claim brought against any Arena Indemnitee or Roivant Indemnitee arising from, based on or occurring as a result of personal injury, death or property damage (to the extent resulting from personal injury or death) caused by or resulting from the use of a Product sold, distributed, dispensed or otherwise administered, except to the extent caused by any failure of the Product manufactured by or on behalf of Arena or its Affiliates (including any Product manufactured by a Third Party contractor of Arena or its Affiliates) to meet the Product Warranty.
1.107 “Product Purchase Price” has the meaning set forth in Section 7.3(a) with respect to each Product.
1.108 “Product Purchase Price Adjustment Payment” has the meaning set forth in Section 7.3(g).
1.109 “Product Trademark” has the meaning set forth in Section 9.10.
1.110 “Product Warranty” has the meaning set forth in Section 6.11.
1.111 “Program Know-How” means any and all Know-How discovered, identified, conceived, reduced to practice or otherwise made in the course of or as a result of activities conducted under this Agreement, including (i) Development Data and (ii) pursuant to a Development Plan, or any Commercialization activities to the extent such Know-How relates to [***] and is not applicable to [***], either (a) solely by one or more employees of or subcontractors (including Sub-distributors) or consultants to Roivant, its Sub-distributors, Arena or any of their respective Affiliates, or (b) jointly by one or more employees of or subcontractors or consultants to Arena or any of its Affiliates, on the one hand, and one or more employees of or subcontractors (including Sub-distributors) or consultants to Roivant, its Sub-distributors, or any of their respective Affiliates, on the other hand; [***].
1.112 “Program Patent” means any Patent to the extent it claims or covers any invention within the Program Know-How, excluding any Patents to the extent related to Reverted Compounds.
1.113 “Prosecution” has the meaning set forth in Section 9.2(a)(i).
1.114 “Purchase Order” means a written order submitted by Roivant to Arena, in a form reasonably acceptable to Arena, for Arena to manufacture (or have manufactured) and deliver, and Roivant to purchase, a specific quantity of a particular Finished Product, as provided in Section 6.2(c).
1.115 “Quality Agreement(s)” means each agreement containing or referring to the agreed policies, procedures, and standards, which shall be customary and reasonable, by which the Parties will coordinate and implement the operational and quality assurance activities needed to efficiently achieve regulatory compliance objectives with respect to manufacturing and supply by Arena of the Finished Products.
1.116 “Quality Control Procedures” has the meaning set forth in Section 6.6.
1.117 “Receiving Party” has the meaning set forth in Section 8.1.
1.118 “Recipient” has the meaning set forth in Section 8.1.
1.119 “Regulatory Approval” means, with respect to a Product to be sold for use in a particular country: all approvals, registrations, authorizations, licenses and permits by the Regulatory Authorities in such country necessary to market or sell such Product, but specifically not including Pricing Approvals, or regulatory approvals relating to the manufacture of a Product.
10
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.120 “Regulatory Authority” means, as to a particular country, any national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity whose review, approval or authorization is necessary for the manufacture, packaging, use, storage, import, export, distribution, promotion, marketing, offer for sale or sale of a Product in such country.
1.121 “Regulatory Filings” means all applications, approvals, licenses, notifications, registrations, submissions and authorizations made to or received from a Regulatory Authority specific to and necessary for the development, manufacture or commercialization of a pharmaceutical product, including any INDs, NDAs and Regulatory Approvals.
1.122 “Regulatory Standards” has the meaning set forth in Section 6.6.
1.123 “Related Compound” means (a) any compound in the chemical scope of [***], or (b) any [***] described in (a); provided however that Related Compound shall not include the Initial Compound or any Reverted Compound.
1.124 “Reverted Compound” means a former Related Compound that Roivant has lost all rights to under this Agreement pursuant to Section 3.4 or a Compound for which Roivant has terminated its rights pursuant to Section 12.3.
1.125 “Roivant” has the meaning set forth in the opening paragraph hereto.
1.126 “Roivant Indemnitees” has the meaning set forth in Section 11.2.
1.127 “Roivant Know-How” means any and all Know-How that is Controlled by Roivant or its Affiliate as of the Effective Date.
1.128 “SEC” has the meaning set forth in Section 8.5(a).
1.129 “Secondary Purchase Price Payment” has the meaning set forth in Section 7.3(b)(ii).
1.130 “Senior Executives” means, with respect to Roivant, the Chief Executive Officer of Roivant and with respect to Arena, the Chairman of the Managing Directors of Arena.
1.131 “Specifications” means, with respect to a particular Finished Product to be sold or otherwise used in a particular country or with respect to a particular Compound to be used in the manufacture of Finished Product, the specifications, characteristics, qualities and labeling and packaging requirements established in writing for such Finished Product or Compound in the Quality Agreement and in conformance with the Regulatory Approval in such country for the applicable Finished Product or Compound and Applicable Laws in such country, with which such Finished Product must conform (including release criteria and associated analytical methods) when delivered pursuant to this Agreement or with which such Compound must conform (including release criteria and associated analytical methods) for use by or on behalf of Arena for manufacture into Finished Product, and as the same may be amended from time to time under the terms of the Quality Agreement.
1.132 “Sub-distributor” means any Person other than Roivant or its Affiliate that Roivant appoints or grants the right, for its own account (rather than on behalf of Roivant), to (i) develop Product, (ii) obtain and maintain Regulatory Approval for Product, and/or (iii) market, promote, sell, distribute or otherwise Commercialize one or more Products in a country (or countries), pursuant to the terms of Section 5.7.
1.133 “Taxes” has the meaning set forth in Section 7.5.
1.134 “Term” has the meaning set forth in Section 12.1.
1.135 “Testing Laboratory” has the meaning set forth in Section 6.10.
1.136 “Third Party” means any Person other than Arena, Roivant, and their respective Affiliates.
1.137 “Third Party Claim” has the meaning set forth in Section 11.1.
11
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
1.138 “Three Year Forecast” has the meaning set forth in Section 6.2(b).
1.139 “Trademark” means any word, name, symbol, color, designation or device or any combination thereof, including any trademark, trade dress, brand xxxx, service xxxx, trade name, brand name, logo or business symbol, whether or not registered.
1.140 “United States” means the United States of America and its territories and possessions, including Puerto Rico and the District of Columbia.
1.141 “United States Dollar” and “US$” means the official currency of the United States.
1.142 “Upfront Payment” has the meaning set forth in Section 7.1.
ARTICLE 2
2.1 Appointment of Roivant as Exclusive Distributor. Subject to the terms and conditions of this Agreement, Arena hereby appoints Roivant as the exclusive worldwide distributor during the Term of Products in the Field and grants to Roivant during the Term the exclusive rights to Commercialize Products worldwide. Pursuant to such appointment, subject to the terms and conditions of this Agreement, Roivant shall have the exclusive right during the Term to invoice and book all Product sales in the Field and may exercise such right, in its discretion, through its Sub-distributors and its and their Affiliates. For clarity, as between the Parties, Arena and its Affiliates shall retain exclusively all rights to Products other than the rights granted to Roivant in the foregoing appointment or otherwise in this Agreement.
2.2 Supply of Product for Distributorship. Arena shall supply (or have supplied) to Roivant, and Roivant shall purchase exclusively from Arena, Roivant’s, its Sub-distributors’ and its and their Affiliates’ requirements of Products for sale by Roivant its Sub-distributors and its and their Affiliates pursuant to Section 2.1, subject to and under the provisions of Article 6.
(a) Compounds. [***] hereby covenants and agrees that during the Term, neither [***] nor any of its Affiliates shall [***] any Third Party [***] (except to the extent reasonably necessary to perform [***] obligations, if any, under any [***] or to [***] obligations to [***] under this Agreement) [***] a (i) [***] (other than [***]) or (ii) any [***] of the [***] or any [***] for which [***] (other than [***]). [***] additionally hereby covenants and agrees that during the Term, neither [***] nor any of its Affiliates currently has as of the Effective Date, or shall [***] any Third Party [***] of a [***] (other than [***]). For the purpose of this Section 2.3(a), a “[***] means any [***] that (1) [***] in the [***] through a [***], such as [***] of an [***], and (2) [***] and is [***] to be [***] through such [***].
(b) Competing Products.
(i) Arena hereby covenants and agrees that neither Arena nor any of its Affiliates shall clinically develop, market, sell, distribute or commercialize, prior to the fifth anniversary of the Effective Date of this Agreement (the “Arena Non-Compete Period”), or [***] during the Non-Compete Period, any Competing Product for the treatment of behavioral disturbances in patients with dementia, including but not limited to agitation and psychosis; provided there are [***] (such prohibited activities, “Competing Arena Program”). “[***]” shall be [***] of such [***]. For clarity, [***] provided such [***] does not [***] during the Non-Compete Period. [***] (including any [***]) to a [***]. Notwithstanding anything to the contrary in this Agreement (including this subsection), Roivant agrees that (i) [***] (including [***] in the [***])) to [***], and/or other [***] and (ii) any Third Party [***] without any [***].
(ii) Notwithstanding Section 2.3(b)(i):
12
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(A) if any Person with a Competing Arena Program becomes an Affiliate of Arena or succeeds Arena through a Change of Control of Arena during the Arena Non-Compete Period (which Arena agrees to notify Roivant in writing promptly after the closing of such Change of Control of Arena), then such Person shall be permitted to continue such Competing Arena Program and the continuation of such Competing Arena Program shall not be a breach of Section 2.3(b)(i), provided that the level of efforts required by Commercially Reasonable Efforts shall not be take into account such Competing Arena Program; and
(B) if during the Arena Non-Compete Period (1) any [***] of such [***] or any of its Affiliates or [***] with such [***] (including [***] of such [***]) by [***] or any of its Affiliates, which [***] in a [***] or (2) [***] or any of its Affiliates [***] of the [***] of a [***], then in each case ((1) and (2)) [***] (whether by a [***] in a [***] in the [***], or otherwise) [***]; provided, that in any case [***], as the case may be, shall [***] during such [***]; and provided, further, that [***] under this Agreement shall [***], and the [***] of such [***] shall not be a breach of Section 2.3(b)(i) if it complies with the terms of this Section 2.3(b)(ii)(B).
(iii) Roivant hereby covenants and agrees that prior to the first expiration of an Initial Term for a Product under this Agreement (the “Roivant Non-Compete Period”), neither Roivant nor any of its Affiliates shall clinically develop, market, sell, distribute or commercialize, or license, authorize or appoint any Third Party to clinically develop, market, sell, distribute or commercialize any Competing Product for the treatment of any neurodegenerative or neuropsychiatric disorder (“Competing Roivant Program”). If a Sub-distributor or its Affiliates has a Competing Roivant Program then [***].
(iv) Notwithstanding Section 2.3(b)(iii):
(A) if any Person with a Competing Roivant Program becomes an Affiliate of Roivant or succeeds Roivant through a Change of Control of Roivant during the Roivant Non-Compete Period (which Roivant agrees to notify Arena of in writing promptly after the closing of such Change of Control of Roivant), then such Person shall be permitted to continue such Competing Roivant Program and the continuation of such Competing Roivant Program shall not be a breach of Section 2.3(b)(iii), provided that the level of efforts required by Commercially Reasonable Efforts shall not take into account such Competing Roivant Program; and
(B) if during the Roivant Non-Compete Period (1) any [***] of such [***] or any of its Affiliates or [***] with such [***] (including [***] of such [***]) by [***] or any of its Affiliates, which [***] in a [***] or (2) [***] or any of its Affiliates [***] of the [***] of a [***], then in each case ((1) and (2)) [***] (whether by a [***] in a [***] in the [***], or otherwise) [***]; provided, that in any case [***], as the case may be, shall [***] during such [***]; and provided, further, that [***] under this Agreement shall [***], and the [***] of such [***] shall not be a breach of Section 2.3(b)(iii) if it complies with the terms of this Section 2.3(b)(iv)(B).
(c) Purchasing Product. Roivant hereby covenants and agrees that during the Term it shall not, and it shall cause its Sub-distributors and its and their Affiliates not to, purchase any Compound or Product from any Third Party, or Commercialize, or conduct other similar activities related to the commercial sale of, any Product or any other product containing Compound during the Term other than Compound or Finished Product that was purchased and Commercialized in accordance with the terms of this Agreement.
2.4 Acknowledgement. Roivant acknowledges that Arena is engaged in research and development in psychosis and related areas, and Arena has the right to continue to engage in such research and development and to commercialize products in such areas, subject to [***] Sections 2.3(a) and 2.3(b) and further subject to the limitation with respect to [***] set forth in Section [***].
ARTICLE 3
3.1 Product Development. Roivant shall have the exclusive right and responsibility to conduct, or cause its Affiliates or a Third Party to conduct, or pay Arena to conduct, in accordance with the terms of this Agreement, all preclinical and clinical activities with respect to the Products, in accordance with this Agreement, in each case, as between the Parties, at Roivant’s sole expense.
13
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(a) All clinical trials and other development work for Products conducted by or on behalf of Roivant or its Affiliates will be conducted pursuant to a Development Plan. Each such Development Plan shall: (i) describe in appropriate detail the clinical trials or other development work to be conducted (including the protocol, the statistical analysis plan and related documents for any clinical trials); (ii) establish an anticipated timeline for such clinical trials or other development work; (iii) include sufficient details for Arena to determine the activities required for development of manufacturing processes and capabilities for Arena to manufacture clinical and commercial supply of the Product, and (iv) address any other material matter relating to such clinical trials or other development work.
(b) For purposes of this Section, “Arena Plan Obligations” shall mean obligations of Arena and its Affiliates under any proposed Development Plans and under any proposed material modifications to existing Development Plans to perform any manufacturing, process development or other work that is (i) [***] to a [***], to which Arena has not previously agreed under this Agreement, or (ii) not [***] a Compound or Product. Roivant shall provide to Arena each Development Plan that contains Arena Plan Obligations in draft form (with at least [***] the Development Plan provided to Arena prior to the Effective Date with respect to the Initial Product) at least [***] prior to the JSC meeting at which they will be discussed (or such shorter period of time as Arena agrees in writing with respect to a particular draft Development Plan). The JSC shall review and discuss any such proposed Development Plans. The consent of Arena, which shall not be unreasonably conditioned, withheld or delayed, is required solely with respect to all such proposed Arena Plan Obligations, provided, that it would be unreasonable for Arena to condition, withhold or delay consent if (A) it has [***], or can reasonably [***] to the [***] such other [***] in such [***] and (B) it has [***] with the [***] and other [***], or can reasonably [***] to the [***] and other [***] such [***]. If Arena conditions, withholds or delays its consent with respect to any Arena Plan Obligations, Arena shall provide [***] and shall work in good faith with [***] to achieve the [***]. For clarity, Arena’s consent is not needed with respect to those portions of any proposed Development Plans or [***] Development Plans that [***]. Roivant shall keep the JSC updated at its regularly scheduled meetings regarding the status of activities under each Development Plan and any material Development Plan modifications made by Roivant that do not require Arena’s consent. Upon [***] Development Plan concerning [***] (which shall include [***] as described in this Section 3.2(b)), the [***] such Development Plan shall [***] within the scope of this Agreement.
(a) Compliance with Development Plan and Applicable Laws. Roivant shall conduct its clinical trials and other development work with respect to Products (i) in accordance with the applicable Development Plan and (ii) in compliance with all Applicable Laws, including in accordance with GLP and GCP, of the country in which the activities are conducted. Arena shall conduct all development work allocated to it under any Development Plan (1) in accordance with the applicable Development Plan and (2) in compliance with all Applicable Laws, including in accordance with GLP and GCP, of the country in which the activities are conducted.
(b) Information Regarding Development Activities. Each Party shall maintain, or cause to be maintained, records of all the clinical trials and other development work conducted by or on behalf of such Party hereunder on Product, in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, which shall fully and properly reflect all work done and results achieved by or on behalf of such Party in the performance of such clinical trials and other development work under the Development Plans. Each Party shall retain such records for at least three (3) years after the Term, or for such longer period as may be required by Applicable Laws. Each Party shall keep the other Party appropriately informed of the status and results of the clinical trials and other development work with respect to Product under any Development Plan, including, if requested by the other Party, disclosing to such other Party all Development Data and other results of such development work. Roivant shall have the right to inspect and copy any such records and notebooks reflecting the work done and results achieved under a Development Plan by or on behalf of Arena in the performance of clinical trials and other development work with respect to Product under the Development Plans. It is understood and agreed that any and all research, development and Commercialization work conducted by or on behalf of Roivant, its Sub-distributors, Arena or their respective Affiliates, on Compound or Product in accordance with the Development Plan shall be deemed activities conducted under this Agreement.
14
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(i) Subject to the terms and conditions of this Agreement, Arena hereby grants to Roivant and its Affiliates a worldwide, non-exclusive, royalty-free, limited license under the applicable Arena Know-How and Arena Patents to conduct the clinical trials and other development work under the Development Plans to develop Product worldwide in the Field pursuant to this Agreement (all such development work to be conducted in accordance with the applicable Development Plan), and to perform the regulatory activities for Product worldwide. Roivant may grant sublicenses under the foregoing license to subcontractors and Sub-distributors solely for activities under a Development Plan. Promptly following the Effective Date, Arena will conduct a reasonable search of its records to locate the documentation and data set forth on Exhibit E, and provide Roivant with a copy of such documentation and data as is accessible to Arena following such search.
(ii) Subject to the terms and conditions of this Agreement, Roivant hereby grants to Arena and its Affiliates a worldwide, non-exclusive, royalty-free, limited license under the applicable Program Know-How and Program Patents to conduct the development work under the Development Plans to develop Product worldwide in the Field for Roivant, its Sub-distributors and its and their Affiliates pursuant to this Agreement (all such development work to be conducted in accordance with the applicable Development Plan). Arena may grant sublicenses under the foregoing license solely to subcontractors approved by Roivant in writing.
(d) IND Transfer. Within ten (10) days of the Effective Date, (a) Arena shall submit to the FDA letters (substantially set forth on Exhibit F) transferring sponsorship of IND No 73405 to Roivant and (b) Roivant shall submit to the FDA letters (substantially in the form set forth on Exhibit F) accepting transfer of sponsorship of IND No 73405 from Arena.
(a) Development Plans. Roivant will develop Products in accordance with Development Plans and will use Commercially Reasonable Efforts to do so according to the timelines in Development Plans. Without limiting the generality of the foregoing, Roivant or its Affiliate shall commence a Phase 2 Trial regarding the Initial Compound prior to January 1, 2017; provided, that such date shall automatically be extended to account for any delay in Arena’s provision of conforming Finished Product pursuant to Section 3.9(b) or Arena’s fulfillment of its obligations pursuant to Section 3.3(c)(i) and 3.3(d). Roivant may fulfill any of its obligations (which obligations do not include manufacturing of Products) under this Section 3.4 itself or with or through any of its Sub-distributors or its or their Affiliates.
(b) Related Compounds.
(i) Roivant will use Commercially Reasonable Efforts to develop, obtain Regulatory Approval of, and Commercialize at least one Related Compound.
(ii) Without limiting the foregoing, if neither Roivant nor any of its Sub-distributors or its or their Affiliates files an IND in the United States prior to the 4th anniversary of the Effective Date with respect to at least one Related Compound, then Roivant’s rights with respect to all Related Compounds, other than Related Compounds that are pharmaceutical acceptable salts, hydrates, solvates, bases, acids, enantiomers, diastereomers, tautomers, racemates or polymorphs of the Initial Compound, shall terminate and all rights granted by Arena to Roivant with respect to such terminated Related Compounds shall revert to Arena. Any termination of Related Compound rights under this Section 3.4(b) shall be made by written notice from Arena to Roivant. The Related Compounds for which Arena has terminated Roivant’s rights shall be “Reverted Compounds” hereunder. There are [***] with respect to Reverted Compounds. The Parties acknowledge and agree that neither Roivant nor its Affiliates have any obligation to file an [***] anniversary of the Effective Date with respect to at least one Related Compound. For clarity, any failure by Roivant and/or its Affiliates to file an IND in the United States prior to the 4th anniversary of the Effective Date with respect to at least one Related Compound, shall not be a breach of this Agreement and Arena’s sole right with respect to any such failure shall be the termination of the Related Compound rights. For further clarity, [***] under this Section 3.4(b)(ii) [***] shall not include, any [***] of the [***] and in the event of a termination of Related Compounds pursuant to this Section 3.4(b), Roivant shall not thereafter, notwithstanding Section 3.4(b)(i), have any obligation to use Commercially Reasonable Efforts to develop, obtain Regulatory Approval for or Commercialize any [***] of the [***].
15
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
3.5 Use of Subcontractors. Roivant may subcontract clinical trials and other development work under a Development Plan to one or more subcontractors; provided, that such subcontracting is in accordance with Section 15.4(b).
3.6 Materials Transfer. In order to facilitate the clinical trials and other development work contemplated by this Agreement, Arena may (but is not required to except as set forth herein) provide Roivant certain biological materials or chemical compounds (other than Compound or Product) (collectively, “Materials”) for use by Roivant in furtherance of such clinical trials or other development work; at Roivant’s reasonable request, Arena shall provide Roivant with reference standards and impurity samples. Except as otherwise provided for under this Agreement, all Materials delivered to Roivant will remain the sole property of Arena. Roivant shall: (a) only use such Materials in furtherance of the clinical trials and other development work under a Development Plan, (b) not use or deliver any Materials to or for the benefit of any Third Party, except for subcontractors pursuant to Section 3.5, without the prior written consent of Arena, and (c) use the Materials in compliance with all Applicable Laws. Roivant shall use the Materials supplied under this Agreement with prudence and appropriate caution in any experimental work because not all of their characteristics may be known. Except as otherwise expressly set forth in this Agreement, THE MATERIALS ARE PROVIDED “AS IS” AND WITHOUT ANY REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR ANY PARTICULAR PURPOSE OR ANY WARRANTY THAT THE USE OF THE MATERIALS WILL NOT INFRINGE OR VIOLATE ANY PATENT OR OTHER PROPRIETARY RIGHTS OF ANY THIRD PARTY.
3.7 Product Regulatory Activities. Roivant, its Sub-distributors and its and their Affiliates shall have the right to perform and have performed all regulatory activities for obtaining the Regulatory Approval(s) of Product. Roivant shall keep Arena reasonably informed, at JSC meetings, of the planning and conduct of such activities and the material decisions with respect thereto. Arena shall cooperate reasonably with Roivant with respect to activities for obtaining Regulatory Approval, including responding promptly to all of Roivant’s reasonable requests for information and comments as necessary or useful to conduct such regulatory activities, provided, that if such assistance requires more than a nominal amount of time of an Arena employee or consultant or requires Arena to incur out of pocket cost, Arena shall notify Roivant in advance and if Roivant maintains its request for such assistance, then Arena’s actual costs of providing such assistance shall be at Roivant’s expense. In no event shall Arena be required to undertake at its expense any specific activities with respect to any Regulatory Authority meetings or other such regulatory activities. Except as otherwise provided below, Roivant shall hold all Regulatory Filings made by Roivant and all Regulatory Approvals, and shall provide to Arena copies of all such filings and approvals if requested by Arena. Each Party shall conduct all of its regulatory activities with respect to Product in compliance with all Applicable Laws.
3.8 Pharmacovigilance. Roivant shall be responsible, at its own expense, for all safety reporting with respect to development and Commercialization of Product.
(a) Clinical Development and Process Development. Roivant is responsible for all manufacturing costs relating to clinical trials and development of the commercial manufacturing process. Such work includes, without limitation, active pharmaceutical ingredient development and acquisition, technical transfer costs, drug product formulation and development, stability testing, related analytical work, quality assurance review and batch release. Some (if not all) of these activities may be performed or overseen by Arena, at Roivant’s request and Roivant agrees that it will pay Arena for such requested activities an amount equal to Arena’s FTE Costs plus 37%, plus Arena’s out-of-pocket costs, without markup, or such other amount as the Parties agree, which payment shall be invoiced and paid in accordance with Section 3.11.
(b) Initial Clinical Supply Order. Using Initial Compound existing as of the Effective Date, Arena shall manufacture for the quantities and formats of Finished Product and placebo specified in Exhibit D (which Finished Product shall be Initial Product in final form ready for clinical trials) and deliver such Finished Product and placebo to Roivant no later than five (5) months after the Effective Date. Arena will invoice Roivant in accordance with Section 3.11 for (i) Arena’s FTE Costs; and (ii) Arena’s out-of-pocket costs without markup; in each of the foregoing sections (i) and (ii), to the extent incurred after the Effective Date, which amount is due within thirty (30) days of receipt of invoice.
16
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(c) Other Clinical Supply. Except as set forth in Section 3.9(b) above, details of the orders placed by Roivant with Arena for Finished Product and placebo for use in clinical trials will be agreed in writing in advance. The Parties will negotiate in good faith to reach agreement upon such details that will satisfy, in a timely manner, those quantities and formats of Finished Products and placebo that are reasonably requested by Roivant for use in clinical trials.
(d) Warranty. Arena warrants that at the time of delivery to Roivant all Finished Product and placebo delivered to Roivant under this Section 3.9: (i) will have been manufactured, tested, and packaged in accordance with cGMP, applicable Manufacturing SOPs and the Quality Agreement; and (ii) will meet the applicable Specifications [***] with respect to [***] of the [***] or, in the case [***] of the [***]. Each of the foregoing warranties is subject to the limitation that Arena shall have no liability or responsibility under the foregoing for any defects, damage or harm to the Finished Product or placebo to the extent resulting from improper storage, transportation, mishandling or any other cause occurring after delivery by or on behalf of Arena.
(e) Other Applicable Supply Terms. All Finished Product and placebo delivered to Roivant under this Section 3.9 shall be subject to the (i) title and risk of loss and export and import license provisions set forth in Section 6.3, (ii) the acceptance and rejection provisions set forth in Section 6.9, (iii) the rejection dispute provisions set forth in Section 6.10, and (iv) the supply problem provisions set forth in Section 6.15.
(f) Batch Records and Certificates. Prior to the first manufacture of Finished Product under this Section 3.9 by or on behalf of Arena, Arena shall provide Roivant with a copy of the Master Batch Record for such Finished Product for Roivant’s review and approval. Arena shall consider in good faith any reasonable comments Roivant provides regarding the Master Batch Record for such Finished Product. To the extent required for inclusion in a Regulatory Filing to be made by Roivant, its Sub-distributors or its or their Affiliates, Arena shall provide to Roivant: (a) a copy of the Batch Records for each Batch of Compound covered by the Regulatory Filing, (b) a completed and accurate Certificate of Analysis as to such Batch of Compound, and (c) copies of all other documentation required for Compound release as provided in the Quality Agreement. Arena shall provide to Roivant, accompanying each delivery of Finished Product under this Section 3.9 by Arena: (i) the Batch number of the delivered Finished Product, (ii) a copy of the Batch Records for such Finished Product, (iii) a completed and accurate Certificate of Analysis as to such Batch of Finished Product, and (iv) copies of all other documentation required for Finished Product release as provided in the Quality Agreement.
(g) Clinical Supply Costs. Except as set forth in Section 3.9(b) above, Arena will manufacture and supply Roivant, its Sub-distributors and its and their Affiliates with Finished Product and placebo for use in clinical trials at an amount equal to Arena’s FTE Costs plus 37%, plus Arena’s out-of-pocket costs without markup, or such other amount as the Parties agree, which amount shall be invoiced and paid in accordance with Section 3.11. If there [***] the Effective Date after Arena satisfies its obligations under Section 3.9(b), Arena shall not charge Roivant for [***] of any [***]. The [***] as of the Effective Date is [***] and [***].
(h) Development and Manufacture of Additional Products. If Roivant desires to develop any Additional Products, it shall first discuss the Additional Products with Arena. The Parties will discuss in good faith the terms (which shall include a commercially reasonable Additional Product Minimum Product Purchase Price) pursuant to which each Additional Product will be manufactured and sold to Roivant by or on behalf of Arena pursuant to this Agreement.
3.10 Engagement and Qualification of Third Party Manufacturers. For purposes of supplying Product to Roivant pursuant to Section 3.9 and Article 6, (i) the active pharmaceutical ingredients used in the manufacture of Products (Initial Compound and Related Compounds) and (ii) Finished Products, may be sourced by Arena from Third Party manufacturers.
17
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(a) Primary Manufacturer of Initial Compound. Subject to the oversight of the JMC, promptly after the Effective Date, Arena (in consultation with Roivant) shall perform diligence assessments of one or more Third Party manufacturers, which assessments shall include obtaining a bid, to identify a Third Party manufacturer to manufacture and supply to Arena the Initial Compound. Arena will consider Roivant’s comments and suggestions with respect to the selection of the Third Party manufacturer, and the selection of such Third Party manufacturer shall be subject to the prior consent of Roivant (which consent shall not be unreasonably withheld, delayed or conditioned). Arena shall seek to negotiate a supply agreement with such Third Party manufacturer for the supply of the Initial Compound to Arena for use in the manufacture of Finished Product pursuant to this Agreement. Arena shall use Commercially Reasonable Efforts to negotiate with such Third Party manufacturer an agreement that includes the right for Arena to perform quality audits on such Third Party manufacturer consistent with the terms of Section 6.8 of this Agreement. Arena shall keep Roivant apprised of the status of its negotiations, shall provide Roivant with copies of the draft agreement and shall use good faith efforts to implement Roivant’s reasonable comments with respect thereto. Arena shall provide Roivant with a copy of the final, executed supply agreement. At Roivant’s reasonable request, Arena will take action to enforce the supply agreement. After entry into such supply agreement, Arena shall use diligent efforts to qualify such Third Party manufacturer no later than a reasonable time after such entry. Arena shall keep Roivant apprised of the status of its efforts to get such Third Party manufacturer qualified and shall implement Roivant’s reasonable comments with respect thereto, including reasonable requests regarding validation batches. Roivant agrees that it will pay Arena for the diligence, engagement and qualification activities undertaken pursuant to this Section 3.10(a) in an amount equal to Arena’s FTE Costs plus 37%, plus Arena’s out-of-pocket costs without markup, or such other amount as the Parties agree, which payment shall be invoiced and paid in accordance with Section 3.11.
(b) Primary Manufacturer for Related Compounds. Subject to the oversight of the JMC, at Roivant’s reasonable request after the adoption of a Development Plan for a Related Compound, Arena (in consultation with Roivant) shall perform diligence assessments of one or more Third Party manufacturers, which assessments shall include obtaining a bid, to identify a Third Party manufacturer to manufacture and supply to Arena such Related Compound. Arena will consider Roivant’s comments and suggestions with respect to the selection of the Third Party manufacturer, and the selection of such Third Party manufacturer shall be subject to the prior consent of Roivant (which consent shall not be unreasonably withheld, delayed or conditioned). Arena shall seek to negotiate a supply agreement with such Third Party manufacturer for the supply of such Related Compound to Arena for use in the manufacture of Finished Product pursuant to this Agreement. Arena shall use Commercially Reasonable Efforts to negotiate with such Third Party manufacturer an agreement that includes the right for Arena to perform quality audits on such Third Party manufacturer consistent with the terms of Section 6.8 of this Agreement. Arena shall keep Roivant apprised of the status of its negotiations, shall provide Roivant with copies of the draft agreement and shall use good faith efforts to implement Roivant’s reasonable comments with respect thereto. Arena shall provide Roivant with a copy of the final, executed supply agreement. At Roivant’s reasonable request, Arena will enforce the supply agreement. After entry into such supply agreement, Arena shall use diligent efforts to qualify such Third Party manufacturer no later than a reasonable time after the establishment of a manufacturing process for the Related Compound. Arena shall keep Roivant apprised of the status of its efforts to get such Third Party manufacturer qualified and shall implement Roivant’s reasonable comments with respect thereto, including reasonable requests regarding validation batches. Roivant agrees that it will pay Arena for the diligence, engagement and qualification activities undertaken pursuant to this Section 3.10(b) in an amount equal to Arena’s FTE Costs plus 37%, plus Arena’s out-of-pocket costs without markup, or such other amount as the Parties agree, which payment shall be invoiced and paid in accordance with Section 3.11.
18
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(c) Additional Source Manufacturers of Compounds. Subject to the oversight of the JMC, at Roivant’s reasonable request after the primary Third Party manufacturer for a Compound has been qualified in accordance with Section 3.10(a) or Section 3.10(b), as applicable, Arena (in consultation with Roivant) shall perform diligence assessments of one or more additional source Third Party manufacturers, which assessments shall include obtaining a bid, to identify additional source Third Party manufacturers to manufacture and supply to Arena such Compound. Arena will consider Roivant’s comments and suggestions with respect to the selection of additional source Third Party manufacturers, and the selection of such Third Party manufacturers shall be subject to the prior consent of Roivant (which consent shall not be unreasonably withheld, delayed or conditioned). Arena shall seek to negotiate a supply agreement with such Third Party manufacturers for the supply of such Compound to Arena for use in the manufacture of Finished Product pursuant to this Agreement. Arena shall use Commercially Reasonable Efforts to negotiate with such Third Party manufacturer an agreement that includes the right for Arena to perform quality audits on such Third Party manufacturers consistent with the terms of Section 6.8 of this Agreement. Arena shall keep Roivant apprised of the status of its negotiations, shall provide Roivant with copies of the draft agreement and shall use good faith efforts to implement Roivant’s reasonable comments with respect thereto. Arena shall provide Roivant with a copy of the final, executed supply agreement. At Roivant’s reasonable request, Arena will enforce the supply agreement. After entry into such supply agreement, Arena shall use diligent efforts to qualify such Third Party manufacturer no later than a reasonable time after such entry. Arena shall keep Roivant apprised of the status of its efforts to get such Third Party manufacturer qualified and shall implement Roivant’s reasonable comments with respect thereto, including reasonable requests regarding validation batches. Roivant agrees that it will pay Arena for the diligence, engagement and qualification activities undertaken pursuant to this Section 3.10(c) and the costs of maintaining such Third Party manufacturer, in an amount equal to Arena’s FTE Costs plus 37%, plus Arena’s out-of-pocket costs without markup, or such other amount as the Parties agree, which payment shall be invoiced and paid in accordance with Section 3.11.
(d) Additional Source Manufacturer of Finished Product. Subject to the oversight of the JMC, at Roivant’s reasonable request after first Regulatory Approval of Finished Product, Arena (in consultation with Roivant) shall perform diligence assessments of one or more additional source Third Party manufacturers, which assessments shall include obtaining a bid, to identify additional source Third Party manufacturers to manufacture and supply to Arena such Finished Product. Arena will consider Roivant’s comments and suggestions with respect to the selection of additional source Third Party manufacturers, and the selection of such Third Party manufacturers shall be subject to the prior consent of Roivant (which consent shall not be unreasonably withheld, delayed or conditioned). Arena shall seek to negotiate a second source supply agreement with such Third Party manufacturer for the supply of such Finished Product to Arena. Arena shall use Commercially Reasonable Efforts to negotiate with such Third Party manufacturer an agreement that includes the right for Arena to perform quality audits on such second source Third Party manufacturer consistent with the terms of Section 6.8 of this Agreement. Arena shall keep Roivant apprised of the status of its negotiations, shall provide Roivant with copies of the draft agreement and shall use good faith efforts to implement Roivant’s reasonable comments with respect thereto. Arena shall provide Roivant with a copy of the final, executed supply agreement. At Roivant’s reasonable request, Arena will enforce the supply agreement. After entry into such supply agreement, Arena shall use diligent efforts to qualify such Third Party manufacturer no later than a reasonable time after such entry. Arena shall keep Roivant apprised of the status of its efforts to get such Third Party manufacturer qualified and shall implement Roivant’s reasonable comments with respect thereto, including reasonable requests regarding validation batches. Roivant agrees that it will pay Arena for the diligence, engagement and qualification activities undertaken pursuant to this Section 3.10(d) and the costs of maintaining such Third Party manufacturer, in an amount equal to Arena’s FTE Costs plus 37%, plus Arena’s out-of-pocket costs without markup, or such other amount as the Parties agree, which payment shall be invoiced and paid in accordance with Section 3.11; provided, however, that the Parties shall share equally such diligence, engagement, qualification and maintenance costs if Roivant requested the engagement of such Third Party manufacturer to address Arena’s capacity constraints occurring [***] after the First Commercial Sale based on Roivant’s good faith long term forecasts that exceed Arena’s capacity at the Facility.
19
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
3.11 Payment of FTE cost and Out-of-Pocket Cost.
(a) Invoice and Payment. Within [***] days after the end of each Calendar Quarter during which Arena has incurred any FTE Costs or out-of-pocket costs that Roivant is obligated to pay, Arena shall submit to Roivant a reasonably detailed invoice setting forth such FTE Costs (including markup where applicable) and out-of-pocket costs for such Calendar Quarter. Such invoice shall include reasonably detailed supporting documents, such as time entry for FTE Costs and receipts for out-of-pocket costs. Arena shall provide additional information and documentation reasonably requested by Roivant. Roivant shall pay the undisputed invoiced amount within thirty (30) days after the receipt of such invoice. In the event Roivant disputes one or more items in an invoice, Roivant will promptly notify Arena in writing and such notice shall contain a reasonably detailed description of the item(s) being disputed and the basis therefor. Arena will promptly respond to Roivant and the Parties will use good faith efforts to promptly resolve the dispute. Amounts determined to be owed following resolution will be paid to Arena within [***] days of resolution of the dispute.
(b) Records and Audit.
(i) Arena shall keep complete, true and accurate books of accounts and records for the purpose of determining the amounts of FTE Costs and out-of-pocket costs payable to Arena under this Section 3.11. Such books and records shall be kept for such period of time required by Applicable Laws, but no less than at least three (3) years following the end of the Calendar Quarter to which they pertain. Such records shall be subject to inspection in accordance with this Section 3.11(b).
(ii) Upon not less than [***] days’ prior written notice, Arena shall permit an independent, certified public accountant of international recognition selected by Roivant and reasonably acceptable to Arena, which acceptance shall not be unreasonably conditioned, withheld or delayed, to audit or inspect those books and records of Arena that relate to the amounts of FTE Costs and out-of-pocket costs payable to Arena under this Section 3.11 for the sole purpose of verifying such payments. Prior to any such audit, the auditor shall execute a confidentiality agreement that is reasonably acceptable to Arena.
(iii) The auditor shall send a copy of the report to Arena at the same time it is sent to Roivant. Such audits or inspections [***] (unless [***], in which case [***]), during normal business hours and upon reasonable advance notice. If such report shows that the amounts paid by Roivant for the period audited are more than the amounts actually payable by Roivant to Arena during the period audited, then (absent manifest error or fraud in such audit report) Arena shall refund to Roivant the amount of such overpayment plus interest under Section 7.8, from the date such amounts were originally paid until refund is made, Roivant shall deliver to Arena an invoice for such overpaid amount, and Arena shall pay such invoice within thirty (30) days of receipt of such invoice. If such report shows that the amounts paid by Roivant for the period audited are less than the amounts actually owed by Roivant to Arena for the period audited, then (absent manifest error or fraud in such audit report) Arena shall deliver to Roivant an invoice for such underpaid amount, and Roivant shall pay such invoiced underpaid amount within thirty (30) days of receipt of such invoice. Such [***] subject to [***] with respect to [***] such Calendar Quarter. Audits and inspections conducted under this Section 3.11(b) shall be at the expense of Roivant, unless such an audit or inspection demonstrates an overpayment in amounts paid by Roivant exceeding an amount equal to [***] of the amount actually due for a period covered by the audit or inspection, in which case all reasonable and verifiable costs relating to the audit or inspection for such period and any overpaid amounts that are discovered shall be paid by Arena, based on invoices delivered by Roivant. Roivant shall endeavor in any such audit not to unreasonably disrupt the normal business activities of Arena.
(a) Establishment. Promptly after the Effective Date, the Parties shall establish a joint steering committee (the “Joint Steering Committee” or “JSC”), under the terms of this Article 4.
(b) Duties. The JSC shall:
(i) generally oversee the relationship and activities of the Parties under the Agreement;
20
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(ii) serve as a forum for discussion and exchange of information regarding activities contemplated by the Agreement;
(iii) review, coordinate and discuss the overall development and regulatory strategies for obtaining Regulatory Approval, and supply chain and manufacturing strategies, for Products;
(iv) review and discuss each Development Plan proposed by Roivant and any material changes to each Development Plan;
(v) appoint other subcommittees as the JSC deems appropriate, which subcommittees shall consist of equal numbers of appropriately qualified representatives appointed by the respective Parties, and to oversee, and attempt to resolve disputes arising on, such subcommittees; and
(vi) perform such other duties as are specifically assigned by the Parties to the Joint Steering Committee pursuant to this Agreement or in a writing executed by each Party.
(c) Joint Steering Committee Membership; Procedure. The JSC shall be composed of four members, two of whom shall be appointed by Arena and two of whom shall be appointed by Roivant. Each Party may appoint employees of its Affiliates to serve as JSC members; provided, that at least one representative of such Party on the JSC shall be an employee of such Party (and not any of its Affiliates) and that each representative must be an employee of the applicable Party or one of its Affiliates. One of Roivant’s two JSC members shall serve as Chairman of the JSC. The Chairman shall act to lead the meetings of the JSC, to prepare the agenda for each meeting (based on the comments and suggestions of each Party, with each such agenda to contain all agenda items requested by a Party to be included) and to prepare the minutes of each meeting for review and approval by the JSC at the next meeting. The draft minutes shall be sent to all members of the JSC for comment promptly after each such meeting (but in no event more than [***] days after each such meeting). All actions noted in the minutes shall be reviewed and approved at the next meeting of the JSC; provided, that if the Parties cannot agree as to the content of the minutes by the time the JSC next meets, such minutes shall be finalized to reflect any areas of disagreement. Any member of the JSC may designate a substitute (who is an employee of the Party or any of its Affiliates) to attend and perform the functions of that member at any meeting of the JSC. Each Party may, with the consent of the other Party, such consent not to be unreasonably conditioned, withheld or delayed, invite non-member representatives of such Party (who must be employees of such Party or any of its Affiliates unless otherwise agreed in writing by the other Party, such agreement not to be unreasonably conditioned, withheld or delayed) to attend meetings of the JSC.
(d) Meetings. The JSC shall hold meetings as often as the members may determine, but in any event JSC meetings shall occur not less than twice per Calendar Year when [***] and no less than once per Calendar Year at other times. The Chairman shall provide the other JSC members at least 20 days prior written notice of the day, time and location of each JSC meeting. Such JSC meetings may be held in person, or by any means of telecommunications or video conference, as the members deem necessary or appropriate. A quorum for JSC meetings shall be all four members, with two members from each Party. Each Party shall bear its own costs to attend and participate in the JSC meetings, including expenses incurred by the members nominated by it in connection with their activities as members of the JSC.
(e) Joint Steering Committee Decisions. Actions to be taken by the JSC shall be taken only following unanimous vote, with each Party having one (1) vote. If the JSC fails to reach unanimous agreement on a matter before it for decision for a period in excess of [***] days, the JSC shall submit the respective positions of the Parties with respect to such matter for discussion in good faith by the Senior Executives in accordance with Section 14.1. If such individuals are not able to mutually agree upon the resolution to such matter within the timeframe set forth in such Section 14.1, then instead of resolution in accordance with either Section 12.7 or Section 14.2, as applicable:
(i) [***] shall have final decision making authority with respect to all matters relating to the [***], provided that such decision does not [***] under this Agreement; and
(ii) Matters not subject to Section 4.1(e)(i) shall be subject to the dispute resolution procedures set forth in Section 12.7 or Section 14.2, as applicable.
21
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(a) Establishment. Within thirty (30) days after the Effective Date, the Parties shall establish a joint manufacturing committee (the “Joint Manufacturing Committee” or “JMC”) as a subcommittee of the JSC. The JMC shall only have the powers expressly assigned to it pursuant to this Section 4.2.
(b) Duties. The Parties acknowledge and agree that one of the goals of this Agreement is to provide for the efficient ordering, manufacture and supply by or on behalf of Arena of the Finished Products ordered by Roivant on a timely basis and meeting all requirements of this Agreement, so that each Party benefits from such manufacturing and Roivant’s subsequent Commercialization. In support of achieving such goals, the role of the JMC shall be to discuss and coordinate the implementation of the global plans for the manufacturing of Compounds and Finished Products and the introduction of manufacturing process improvements for Compounds or Finished Products.
(c) Joint Manufacturing Committee Membership; Procedure. The JMC shall be composed of four members, two of whom shall be appointed by Arena and two of whom shall be appointed by Roivant. Each Party may appoint employees of its Affiliates to serve as JMC members; provided, that at least one representative of such Party on the JMC shall be an employee of such Party (and not any of its Affiliates) and that each representative must be an employee of the applicable Party or one of its Affiliates. One of Arena’s two JMC members shall serve as chairman of the JMC. The chairman of the JMC shall act to lead the meetings of the JMC, to prepare the agenda for each meeting (based on the comments and suggestions of each Party, with each such agenda to contain all agenda items requested by a Party to be included) and to prepare the minutes of each meeting for review and approval by the JMC at the next meeting. The draft minutes shall be sent to all members of the JSC for comment promptly after each such meeting (but in no event more than 15 days after each such meeting). All items noted in the minutes shall be reviewed and approved at the next meeting of the JMC; provided, that if the Parties cannot agree as to the content of the minutes by the time the JMC next meets, such minutes shall be finalized to reflect any areas of disagreement. Any member of the JMC may designate a substitute (who is an employee of the Party or any of its Affiliates) to attend and perform the functions of that member at any meeting of the JMC. Each Party may, with the consent of the other Party, such consent not to be unreasonably conditioned, withheld or delayed, invite non-member representatives of such Party (who must be employees of such Party or any of its Affiliates unless otherwise agreed in writing by the other Party, such agreement not to be unreasonably conditioned, withheld or delayed) to attend meetings of the JMC.
(d) Meetings. The JMC shall hold meetings as often as the members may determine, but in any event JMC meetings shall occur not less than two times per Calendar Year. The first meeting of the JMC shall occur within thirty (30) days of the Effective Date. The chairman of the JMC shall provide the other JMC members at least 20 days prior written notice of the day, time and location (New York or Switzerland) of each JMC meeting. Such JMC meetings may be held in person, or by any means of telecommunications or video conference, as the members deem necessary or appropriate. A quorum for JMC meetings shall be all four members, with two members from each Party. Each Party shall bear its own costs to attend and participate in the JMC meetings, including expenses incurred by the members nominated by it in connection with their activities as members of the JMC.
(e) Joint Manufacturing Committee Decisions. The JMC shall be forum for discussion and information exchange only and shall not have any decision-making authority.
4.3 Scope of Governance. Notwithstanding the creation of the JSC and the JMC, each Party shall retain the rights, powers and discretion granted to it under this Agreement, and neither the JSC nor the JMC shall be delegated or vested with any rights, powers or discretion unless such delegation or vesting is expressly provided in this Agreement, or the Parties expressly so agree in writing. Neither the JSC nor the JMC shall have any power to amend or modify this Agreement or to determine compliance or non-compliance with this Agreement, and no decision of the JSC shall be enforceable to the extent it is in contravention of any terms and conditions of this Agreement.
22
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
ARTICLE 5
5.1 Commercialization Rights and Responsibility. Roivant shall be solely responsible, and has the exclusive rights, for Commercializing Products worldwide in the Field, whether directly or with or through its Sub-distributors and its and their Affiliates, in each case such Commercialization to be in accordance with the terms and conditions of this Agreement. In connection with such Commercialization, Roivant shall purchase all of its and its Sub-distributors’ and its and their Affiliates’ requirements of Product from Arena pursuant to Article 6.
5.2 Commercialization Plans and Communication. Within a reasonable time (no shorter than [***] months) prior to the anticipated date of the First Commercial Sale of each Product in [***] (each, a “Major Market Country” ), Roivant shall prepare and deliver to Arena a plan setting forth the commercialization plan for the Commercialization of the Product in such country, which plan shall be in reasonable scope and detail, shall include the resources and timing therefor, and shall reflect the information such as [***]. Roivant shall provide such plans to Arena on an annual basis and shall provide any interim material updates thereto at the regularly scheduled JSC meetings.
5.3 Roivant Commercialization Commitments. Roivant shall use Commercially Reasonable Efforts to Commercialize Products in each country in which it has received Regulatory Approval for such Product. Without limiting the foregoing, after receiving Regulatory Approval for a Product in each country, Roivant shall (a) use Commercially Reasonable Efforts to [***] commercially reasonable Product Commercialization activities in such country, (b) [***] Products in such country [***] with respect to Product, (c) use Commercially Reasonable Efforts to conduct all activities necessary to [***]; and (d) use Commercially Reasonable Efforts to [***]. Roivant may fulfill any of its obligations under this Section 5.3 itself or with or through any of its Sub-distributors or its or their Affiliates. The Parties acknowledge and agree that a commercially reasonable Commercialization strategy might involve delaying launch of a Product until Pricing Approval is obtained in such country or not launching such Product if Pricing Approval is not obtained.
(a) Roivant shall, and shall cause its Sub-distributors and its and their Affiliates to, in all respects comply with all Applicable Laws in Commercializing Products, including to the extent applicable all federal, state and local “fraud and abuse,” consumer protection and false claims statutes and regulations.
(b) Roivant shall, and shall cause its Sub-distributors and its and their Affiliates to, ensure that all sales representatives promoting Products (i) have skills, training and experience generally consistent with industry standards in the applicable country applicable to the promotion, marketing and sale of a prescription pharmaceutical product in such country and (ii) have satisfactorily completed all Products-specific training and ethics and compliance training required by Roivant.
(c) Roivant shall not, and shall cause its Sub-distributors and its and their Affiliates and its and their respective sales representatives not to, (i) make any statement, representation or warranty, oral or written, concerning a Product in any country, or use any labeling, literature or promotional or marketing material for Product in any country that (A) is contrary to or inconsistent with Regulatory Approval in such country of such Product in a manner that violates any Applicable Laws or (B) otherwise violates any Applicable Laws in such country or (ii) make any arrangements with, make payments to or provide gifts or other incentives to any healthcare professionals in violation of Applicable Laws relating thereto in such country. Roivant shall, and shall cause its Sub-distributors and its and their Affiliates to, ensure that its and their sales representatives are familiar with the procedures, obligations, rights, and responsibilities imposed by the terms of this Agreement as applicable to the performance of promotional activities hereunder.
5.5 [***]. [***] hereby covenants and agrees that it and its Affiliates shall not, directly or indirectly, [***] the manufacture [***] Product [***] in accordance with the terms of this Agreement.
23
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
5.6 Recalls. In the event that any Regulatory Authority issues or requests a recall or takes similar action in connection with Product, or in the event either Party determines that an event, incident or circumstance has occurred that may result in the need for a recall or market withdrawal, the Party notified of or desiring such recall or similar action shall, within 24 hours, advise the other Party thereof by telephone (and confirmed by email or facsimile), email or facsimile. Roivant shall, to the extent practicable, endeavor to provide notice to Arena regarding any Roivant assessment whether to recall or withdraw Product; provided, that if providing such notice is not practicable within an appropriate time period (recognizing the exigencies of the situation), then Roivant shall decide whether to recall or withdraw Product without first providing notice of the situation to Arena. Roivant shall be responsible for conducting, at its sole expense, any such recall or withdrawal, and shall keep Arena fully informed of all actions taken in conducting such recall or withdrawal. Notwithstanding the foregoing, Arena shall be responsible for the expenses of such recall or withdrawal to the extent that such recall or withdrawal is due to Arena’s breach of the Product Warranty or the gross negligence or willful misconduct of Arena or its Affiliates.
5.7 Sub-distributors of Roivant. If Roivant grants a Sub-distributor the right to develop, market, promote, sell and/or distribute a Product in a country, Roivant shall promptly notify Arena in writing thereof. Roivant may grant a Sub-distributor such right to develop, market, promote, sell and/or distribute Product in a country subject to (a) Roivant’s agreement with the Sub-distributor meeting the requirements for subcontractors in Section 15.4(b) and (b) such Sub-distributor agreeing to provide timely reports to Roivant regarding Product sales data, and agreeing that Roivant is permitted to disclose such data to Arena. Roivant will disclose such Product sales data information to Arena. Any agreement between Roivant and a Sub-distributor shall be subject to and consistent with the terms of this Agreement.
5.8 Returned Product. Roivant shall have the sole responsibility under this Agreement to accept any returned Product. Arena shall be reimburse Roivant for the expenses of accepting such returned Product to the extent that such return is due to Arena’s breach of its obligations hereunder or the negligence or willful misconduct of Arena or its Affiliates.
ARTICLE 6
6.1 Manufacture and Supply Commitment. In accordance with the terms and conditions of this Agreement, Arena shall be solely responsible and has exclusive rights, for supplying, or causing to be supplied, to Roivant the Product ordered by Roivant in accordance with this Article 6. Roivant shall purchase all of its (and its Sub-distributors’ and its and their Affiliates’) requirements for Finished Products from Arena for Commercialization, pursuant to the terms of this Article 6. If either Party anticipates that demand for any Finished Product would exceed Arena’s actual manufacturing capacity, such Party shall promptly notify the other Party, and the Parties shall meet, discuss in good faith and agree upon a plan for addressing such demand.
(a) Safety Stock. At Roivant’s reasonable request, after the primary Third Party manufacturer for a Compound has been qualified in accordance with Section 3.10(a) or Section 3.10(b), Arena shall establish and maintain in inventory at the Facility (or such other appropriate storage facility as approved by Roivant, which approval shall not be unreasonably withheld, delayed or conditioned) at least such quantities of safety stock of such Compound as reasonably requested by Roivant and to which Arena does not reasonably object. At Roivant’s reasonable request, Arena shall establish and maintain in inventory at the Facility (or such other appropriate storage facility as approved by Roivant, which approval shall not be unreasonably withheld, delayed or conditioned) at least such quantities of safety stock of such Finished Product as reasonably requested by Roivant and to which Arena does not reasonably object; provided that, with respect to any such safety stock made by or on behalf of Arena prior to [***] such Finished Product, such Finished Product shall not be released until [***]. Arena will manufacture Finished Product using safety stock of Compound, will fulfill Purchase Orders for Finished Product out of safety stock of Finished Product on a “first to expire, first out” basis, and will accordingly replace the consumed inventory of safety stock on a timely basis as appropriate. Arena will be responsible for the inventory of Finished Products and Compounds described in this Section 6.2(a) until ownership of a particular quantity of Finished Product transfers to Roivant in accordance with Section 6.3. Upon release of Compound needed to establish or replenish safety stock, Arena will invoice Roivant, and Roivant will pay Arena within thirty days of receipt of invoice, for an amount equal to thirty-five percent (35%) of the Minimum Product Purchase Price for corresponding quantity of the applicable Finished Product comprising such Compound (at the specified concentration and dosage), which amount will be credited against [the invoiced price for such Finished
24
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
Product at the time of delivery. Upon release of Finished Product needed to establish or replenish safety stock [***], Arena will invoice Roivant, and Roivant will pay Arena within [***] days of receipt of invoice, for an amount equal to an [***] for such Finished Product, which aggregate amount will be credited against the invoiced price for the Finished Product at the time of delivery. To the extent Roivant does not place an order for which such Compound may be used to produce Finished Product so that such Finished Product may be delivered while it still has sufficient shelf life to meet the Product Warranty and Roivant does not waive such shelf life requirement or (unless the Parties otherwise mutually agree in writing) upon termination of this Agreement, Roivant will pay the costs associated with the destruction of the Compound. To the extent Roivant does not place an order for which such Finished Product may be delivered while it still has sufficient shelf life to meet the Product Warranty and Roivant does not waive such shelf life requirement or (unless the Parties otherwise mutually agree in writing) upon termination of this Agreement, Roivant will pay the costs associated with the destruction of the Finished Product. Risk of loss for all safety stock of Finished Product and Compound shall remain with Arena until such Finished Product is delivered to Roivant and risk of loss transfers pursuant to Section 6.3. Arena shall promptly replace, free of charge to Roivant, any safety stock of Finished Product and/or Compound that is lost or damaged while held by or on behalf of Arena as safety stock.
(b) Forecasts. At the time of application for Regulatory Approval of a Product in a country, and [***] thereafter, Roivant shall provide Arena a good faith 18-month rolling forecast of anticipated orders of such Product, in Finished Product form, to be placed during each month of such period (broken down (A) on a country-by-country and packaging configuration-by-packaging configuration basis and (B) by quantities to be sold commercially or distributed as samples or as part of a compassionate use, named patient use or indigent patient program) (each, a “Forecast” for such Product). Each Forecast will specify, [***] during the 18-month period covered by the particular Forecast, the amounts of Finished Product to be ordered in each month and the requested delivery dates for each such order of Finished Product anticipated to be placed. Prior to the due date for the first Forecast for each Product in each country, the Parties will negotiate in good faith and reasonably agree [***] upon [***] such Product in that country, and [***] the Product in such country. If Roivant provides the Three Year Forecast commencing at least [***] months prior to the anticipated launch date and [***] of such Three Year Forecast (the first month of which shall be the launch order), such [***] such Three Year Forecast (and [***]), subject to [***] therein. The requested delivery dates for each order covered by a Forecast shall not be sooner than [***] months, or later than [***] months, after the order date specified in the Forecast (such [***]month window, the “Delivery Window”); provided, that, if the Parties agree, Finished Product may be delivered sooner than [***] months after the order date. The first [***] months of each such Forecast shall be a binding commitment (the “Order Commitment” for the applicable Finished Product for such months) on Roivant to place Purchase Orders, in each such month, to order the applicable Finished Product in amounts [***] the amounts forecast to be ordered in such month in such Forecast (and with delivery dates within the applicable Delivery Window), which commitment [***]. Roivant shall not (a) increase or decrease the quantity estimated for the [***] of each Forecast from the quantity estimated for the [***] of the previous Forecast, or (b) increase or decrease the quantity estimated for the [***] of each Forecast by more than [***] of the quantity estimated for the [***] of the [***] Forecast, respectively, without the prior express written consent of Arena. Each such Forecast shall otherwise be non-binding, except as provided above, but shall reflect Roivant’s good faith expectation (at the time of submitting the Forecast) of the orders of Finished Product and projected delivery dates during the 18-month period. In addition to the Forecasts described above in this Section 6.2(b), Roivant shall provide Arena, at the time [***], with a good-faith three-year forecast of anticipated orders of such Product, which forecast shall be nonbinding and used by Arena for capacity planning purposes (each, a “Three Year Forecast”).
(c) Orders. To order Finished Product for supply by Arena under this Article 6, Roivant shall submit to Arena a Purchase Order (which is deemed binding on Roivant) complying with the other applicable terms of this Article 6 and specifying the amount of Finished Product ordered (broken down (A) on a country-by-country and packaging configuration-by-packaging configuration basis and (B) by quantities (separate quantities for each different packaging configuration ordered) to be sold commercially or distributed as samples or as part of a compassionate use, named patient use or indigent patient program) and the requested delivery date (which shall be within the applicable Delivery Window, unless otherwise agreed by Arena). Not later than [***] days after receipt of a Purchase Order, Arena shall confirm in writing its receipt and acceptance of the Purchase Order (“Order Acceptance”) and Arena’s proposed delivery date, which shall be within the applicable Delivery Window. Roivant shall notify Arena within [***] days after receipt of the Order Acceptance if such proposed delivery date is unworkable for Roivant, and in such event the Parties shall promptly discuss in good faith and seek to agree on an alternative delivery date, which if the Parties fail to agree shall be the half-way point between the requested delivery date and the proposed delivery date. If Roivant does not respond within such [***]-day period, the proposed date will be the confirmed delivery date. For any Purchase Order that contains an Excess Order, Arena shall notify Roivant in the Order Acceptance whether Arena will be able to fulfill such Excess Order (or part thereof) and the expected delivery date for fulfillment. For any such Purchase Order submitted by Roivant, Arena shall supply to Roivant the amount of Finished Product covered by such Purchase
25
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
Order by the confirmed delivery date in the aggregate up to [***] of the quantity forecasted for the applicable month of the most recent Forecast. In the event that such ordered amount, when combined with the total amounts of such Finished Product previously ordered by Roivant during the same month, exceeds [***] of the Order Commitment for such Finished Product in such month (such excess amount, the “Excess Order”), Arena shall not be obligated to fill any Purchase Orders to the extent of the Excess Orders therein, but Arena shall use good faith efforts to fill such Excess Orders (i.e., those order amounts that exceed [***] of the Order Commitment for such month). If there is any conflict between a Purchase Order or an Order Acceptance and the terms and conditions of this Agreement, this Agreement prevails and such conflicting terms are rejected and of no effect, unless the Parties mutually agree otherwise in writing.
6.3 Delivery and Purchase. Roivant shall engage a common carrier, at Roivant’s expense, to ship Finished Product to Roivant. Upon Roivant’s request, Arena shall assist Roivant in identifying a suitable common carrier. Title and risk of loss with respect to Finished Product shall pass to Roivant, and delivery to Roivant shall be made for purposes of this Agreement when Arena tenders such Finished Product to Roivant’s designated common carrier at [***] (or such other location [***] designated by Arena in writing [***] to the [***]). Arena, at its own expense, shall be responsible for [***]. Roivant, at its own expense, shall be responsible for [***]. Upon delivery to Roivant (but subject to Section 6.9), except as set forth in Section 7.3(e) Roivant shall have the obligation to pay Arena the Initial Purchase Price Payment pursuant to Section 7.3(c) for such delivered Finished Product.
6.4 Labeling and Packaging. Arena and Roivant shall discuss and reasonably agree on all packaging configurations, packaging and labeling (including package inserts) used with Finished Product in each country, with Roivant having the final decision with respect thereto provided that the configuration, packaging and labeling chosen by Roivant are consistent with all legal and regulatory requirements and within Arena’s capabilities using Arena’s existing equipment. Arena shall label and package (in appropriate primary, secondary and tertiary packaging) Finished Product to be supplied in accordance with the foregoing, the applicable Manufacturing SOPs, and Applicable Laws of each applicable country, for delivery in final form to Roivant under this Agreement. Roivant shall be responsible for providing to Arena (or its designees, including printed packaging material vendors utilized by Arena) all artwork for all such labeling and packaging on a timely basis, for each applicable packaging configuration for each country, as necessary for Arena to perform such labeling and packaging, and in formats as reasonably agreed by the Parties and reasonably acceptable to Arena. It is agreed that Arena’s obligations to manufacture and supply Finished Product shall be delayed to the extent Roivant does not timely decide on all packaging and labeling used with Finished Product (which must be compatible with Arena’s equipment) and deliver such necessary artwork. Arena shall have the right to subcontract the manufacture of all printed packaging materials, including labels, and Arena shall be responsible for all such subcontractors as provided in Section 15.4(b).
6.5 Quality Agreement. No later than 120 days after the Effective Date, the Parties shall enter into a Quality Agreement with respect to clinical trial supply of the Initial Product. Such Quality Agreement shall provide Roivant with the following rights with respect to Compound and Product [***]. The Parties shall amend such Quality Agreement (or enter into a new Quality Agreement) (i) [***], and (ii) to address commercial supply of each Finished Product, in each case within [***] days after the agreement of the Parties that all applicable information regarding the [***] Finished Product, necessary to reach an agreement with respect to all quality issues, has been obtained. Each Party shall duly and punctually perform all of its obligations under and pursuant to the Quality Agreement. Arena shall release all Finished Products in accordance with the terms of the Quality Agreement.
6.6 Quality Control. Arena shall maintain and follow a quality control and quality assurance testing program consistent with the Specifications, the Quality Agreement, and all other requirements of Applicable Laws and reasonably consistent with industry standards (the “Quality Control Procedures”), which shall include performing the applicable Product Acceptance Tests on each Batch of Compound before release and subsequent use to manufacture Finished Product and on each Batch of Finished Product prior to delivery to Roivant. Arena shall ensure that all Compound and Finished Product manufactured by or on behalf of Arena pursuant to this Agreement is manufactured in accordance with the applicable Manufacturing SOPs, the Quality Agreement, and all other Applicable Laws, and all other applicable requirements of Regulatory Authorities (collectively, “Regulatory Standards”) and conforms to the applicable Product Warranty. Arena shall ensure that the Third Party manufacturers engaged by Arena pursuant to Section 3.10 manufacture Finished Product and Compound (as applicable) in accordance with Regulatory Standards and, with respect to Finished Product, conforming to the applicable Product Warranty.
26
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
6.7 Batch Records and Certificates. Prior to the first manufacture of a Finished Product under Article 6 of this Agreement by or on behalf of Arena, Arena shall provide Roivant with a copy of the Master Batch Record for such Finished Product for Roivant’s review and approval. Arena shall consider any reasonable comments Roivant provides regarding the Master Batch Record for the Finished Product. Arena shall provide to Roivant, accompanying each delivery of Finished Product by or on behalf of Arena: (i) the Batch number and Purchase Order number (if included on the applicable Purchase Order) of the delivered Finished Product, and (ii) a completed and accurate Certificate of Analysis as to such Batch. [***], Arena shall also provide to Roivant, accompanying each such delivery: (A) a copy of the Batch Records for the Finished Product, and (B) copies of all other documentation required for release of the Finished Product as provided in the Quality Agreement.
6.8 Quality Audits. Arena shall maintain all quality control documentation and Product Acceptance Test results for each Batch of Finished Product for a period and in a manner consistent with Regulatory Standards and the Quality Agreement. Roivant may periodically (but no more frequently than [***], unless [***] review such documentation and results, and, as provided for in the Quality Agreement, audit and verify the adherence of Arena to the Quality Control Procedures and Regulatory Standards. Such review and audit shall be on reasonable prior notice and conducted during business hours and in a manner that does not unreasonably disrupt Arena’s business or operations.
6.9 Acceptance/Rejection. Roivant (or its authorized representative) shall perform a reasonable and customary visual inspection in accordance with its quality policies of a statistical sampling of Finished Product delivered by Arena and shall report to Arena any Finished Product that is reasonably discernible upon such visual inspection not to conform to the Product Warranty (“Non-Conforming Finished Product”) within [***] days of receipt by Roivant. Roivant shall report to Arena Non-Conforming Finished Product with hidden defects within [***] days of Roivant’s discovery of the same. A defect is hidden if it could not reasonably have been discovered by a reasonable and customary visual inspection upon receipt of the Finished Product. If any Finished Product is found to be Non-Conforming Finished Product and is reported by Roivant to Arena in the above time frame, then Arena shall, at Roivant’s request and option (to be exercised by Roivant promptly), either: (a) (i) if conforming safety stock of Finished Product is available, replace such Non-Conforming Finished Product within [***] days or (ii) if conforming safety stock of Finished Product is not available, use Commercially Reasonable Efforts to replace such Non-Conforming Finished Product as soon as reasonably practicable, and at no additional charge to Roivant; (b) refund within [***] days to Roivant Product Purchase Price paid (if already paid) to Arena for such Non-Conforming Finished Product or cancel the applicable Purchase Order if not paid; or (c) credit Roivant’s account within [***] days in an amount equal to Product Purchase Price paid for such Non-Conforming Finished Product, and in any case ((a), (b) or (c)) Arena shall reimburse all shipping, insurance, taxes, import licensure and customs charges (in each case that are non-refundable) for the Non-Conforming Finished Product from the point of delivery [***] to the destination of the original shipment, subject to receipt of invoice. Arena shall reimburse Roivant for the reasonable costs incurred by Roivant in properly disposing of or shipping to Arena (as instructed by Arena) such Non-Conforming Finished Product, subject to receipt of invoice. Any notice given under this Section 6.9 shall specify the reason why such Finished Product was found to be Non-Conforming Finished Product. If Roivant does not report any defect or non-conformity of any Finished Product that could reasonably have been discovered by a reasonable and customary visual inspection upon receipt as described above within [***] days of receipt by Roivant or any hidden defect within [***] days after discovery thereof, then Roivant shall be deemed to have accepted such Finished Product.
6.10 Dispute Regarding Rejection. If the Parties disagree as to whether a particular delivery of Finished Product contains Non-Conforming Finished Product, and cannot resolve such disagreement within [***] days, the Parties shall appoint an independent testing laboratory or other appropriate expert mutually acceptable to the Parties (the “Testing Laboratory”) to (a) review data that are in question or (b) to oversee the evaluation and testing of a sample of such Finished Product at the Testing Laboratory. The Testing Laboratory will conduct testing in accordance with the methods established for testing as set forth in the applicable Specifications. The Party whose position in the dispute was not supported by the Testing Laboratory’s findings shall bear the costs of the Testing Laboratory. Arena shall address all amounts of Non-Conforming Finished Product as determined by the Testing Laboratory as provided in Section 6.10.
27
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
6.11 Product Warranty. Arena warrants that at the time of delivery to Roivant, all Finished Product delivered to Roivant under this Article 6: (a) will have been manufactured, tested, and packaged in accordance with cGMP, applicable Manufacturing SOPs and the Quality Agreement; and (b) will meet the applicable Specifications and, solely with respect to the Initial Product, will have for a minimum shelf life period equal to [***] or, in the case the Initial Product has achieved Regulatory Approval, [***] or the shelf life for the Initial Product (such warranty, collectively the “Product Warranty”). Each of the foregoing warranties is subject to the limitation that Arena shall have no liability or responsibility under the foregoing for any defects, damage or harm to the Finished Product to the extent resulting from improper storage, transportation, mishandling or any other cause occurring after delivery by or on behalf of Arena to Roivant.
(a) Arena shall maintain for the facility(ies) at which Arena manufactures Finished Product for supply to Roivant (each, a “Facility”), at Arena’s sole cost, all permits, licenses and approvals (including facilities licenses) needed for Arena to be able to manufacture and supply Finished Product in compliance with the Product Warranty (the “Facility Licenses”), in a timely manner such that Arena is able to meet its manufacturing and supply obligations under this Agreement. Arena shall keep Roivant regularly informed about the status of Facility Licenses for each Facility and shall provide Roivant copies thereof upon reasonable request. Arena shall ensure that each Facility complies with Applicable Laws (including environmental laws) with regard to its manufacturing and supply of Finished Product. Arena shall use Commercially Reasonable Efforts to resolve as soon as possible any issues that arise in seeking or maintaining Facility Licenses for any Facility, including completely addressing and rectifying any deviations or other issues raised in any Warning Letter from the FDA or any similar warning or objection by any other Regulatory Authority. Arena shall have the right to subcontract with Third Parties for storage services and storage facilities for Finished Products manufactured for supply to Roivant hereunder, subject to Roivant’s approval of each such Third Party which approval will not be unreasonably withheld, delayed or conditioned and Arena shall be responsible for all such subcontractors as provided in Section 15.4(b).
(b) Arena shall ensure that each Third Party manufacturer maintains for the facility(ies) at such Third Party manufactures Compounds or Finished Product hereunder, at Arena’s and/or such Third Party’s cost, all permits, licenses and approvals (including facilities licenses) needed for such Third Party to be able to manufacture and supply Compound or Finished Product, as applicable, in compliance with the Specifications or Product Warranty, in a timely manner such that Arena is able to meet its manufacturing and supply obligations under this Agreement. Arena shall keep Roivant regularly informed about the status of such permits, licenses and approvals (including facilities licenses) for each such Third Party facility. Arena shall ensure that each such Third Party facility complies with Applicable Laws (including environmental laws) with regard to its manufacturing and supply of Compound or Finished Product. Arena shall ensure that such Third Party manufacturers use Commercially Reasonable Efforts to resolve as soon as possible any issues that arise in seeking or maintaining such permits, licenses and approvals (including facilities licenses) for such Third Party facility, including completely addressing and rectifying any deviations or other issues raised in any Warning Letter from the FDA or any similar warning or objection by any other Regulatory Authority.
(c) If despite the Commercially Reasonable Efforts of Arena or the Third Party manufacturer, the issues that arise in seeking or maintaining such permits, licenses and approvals (including facilities licenses) for such Arena or Third Party facility, including completely addressing and rectifying any deviations or other issues raised in any Warning Letter from the FDA or any similar warning or objection by any other Regulatory Authority, are not resolved within a reasonable time, then at Roivant’s request, Arena will engage and qualify a Third Party manufacturer in accordance with Section 3.10 and [***]
28
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
6.13 Inspection by Roivant. Arena agrees that Roivant and its respective agents (but no more than a total of three persons per inspection) shall have the right, pursuant to a reasonable confidentiality agreement with Arena, no more than [***] (unless any such inspection reveals a material compliance issue or a compliance issue is raised by a Regulatory Authority, in which event Roivant and its respective agents shall have the right to conduct such additional inspections during [***] as necessary to verify that such issue has been remedied), upon reasonable prior notice to Arena and during business hours, and in a manner that does not unreasonably disrupt Arena’s manufacturing operations, to inspect the portion of the Facility where Finished Product is manufactured or stored as well as the manufacturing of the Finished Products, including inspection of (a) the raw materials used in the manufacture of the Finished Products, (b) the holding facilities for such raw materials, (c) the equipment used in the manufacture of the Finished Products, and (d) all material records reasonably relating to such manufacturing at the Facility, to the extent they relate to the Finished Products (which records may be copied by Roivant or its agent, at Roivant’s expense, but to the extent of the actual expense incurred only). Following such inspection, Roivant shall discuss its observations and conclusions with Arena and if Roivant believes that any corrective actions are necessary for Arena to comply with the terms and conditions of this Article 6, then within 15 days after such discussion, Roivant shall prepare a schedule that sets forth the corrective actions that Roivant reasonably believes in good faith are required, and Arena will consider such actions in good faith and use Commercially Reasonable Efforts to implement such corrective actions that Arena reasonably and in good faith determines to be required. Roivant’s agents participating in inspections shall execute a confidentiality agreement that is reasonable acceptable to Arena. Roivant shall endeavor in any such inspection not to unreasonably disrupt the normal business activities of Arena. Arena shall promptly provide Roivant with a copy of each report for its inspection of the facility of a Third Party manufacturer of Compound or Finished Product.
(i) Upon the request of the Regulatory Authority from whom a preapproval inspection or Regulatory Approval for a Product has been requested by Roivant, Arena shall provide such Regulatory Authority reasonable access to observe and inspect (including pre-approval inspections) the Facility and the procedures used for the manufacture, release and stability testing, or warehousing of Finished Product and to audit the Facility for compliance with applicable Regulatory Standards. Arena shall notify Roivant promptly of any inspection at the Facility by a Regulatory Authority that is scheduled in advance and shall provide Roivant with an opportunity to be present, but not participate, during such inspection. Arena specifically agrees to cooperate with any inspection by such a Regulatory Authority at the Facility, whether prior to or after Regulatory Approval of the applicable Product, and to provide Roivant a copy of any inspection or audit report resulting from any such inspection.
(ii) Arena shall ensure that any Third Party that manufactures Finished Product or any active pharmaceutical ingredient contained in Finished Product to provide each Regulatory Authority, from whom a preapproval inspection or Regulatory Approval for a Product has been requested by Roivant, reasonable access to observe and inspect (including pre-approval inspections) the facility at which such Third Party manufacturers the Finished Product or such active pharmaceutical ingredient and the procedures used for the manufacture, release and stability testing, or warehousing of such Finished Product or active pharmaceutical ingredient, and to audit such Third Party’s facility for compliance with all applicable Regulatory Standards. Promptly upon Arena’s receipt thereof, Arena shall provide to Roivant notice of inspections at such Third Party’s facility by a Regulatory Authority that is scheduled in advance. Arena shall ensure that such Third Party manufacturer cooperates with any inspection by such a Regulatory Authority at such Third Party’s facility, whether prior to or after Regulatory Approval of the applicable Product, and to provide Arena a copy of any inspection or audit report resulting from any such inspection.
29
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(b) Remedial Actions. Arena shall notify Roivant promptly in writing in the event Arena receives notification that any action is taken or threatened by a Regulatory Authority relating to the manufacture or storage of Compound or the manufacture or storage of Finished Product by Arena (or relating to the manufacture or storage of Compound or Finished Product by a Third Party manufacturer of Compound or Finished Product), or relating to the Facility or the facility of a Third Party manufacturer of Compound or Finished Product, as applicable, that would reasonably be expected to impair the ability of Arena or such Third Party manufacturer to manufacture and supply Finished Product to Roivant, or in the case of the Third Party Manufacturer, to supply Compound or Finished Product to Arena (including any impairment to Arena’s or such Third Party manufacturer’s ability to manufacture Finished Product conforming to the Product Warranty, or in the case of the Third Party manufacturer, to supply Compound according to the applicable Specifications), in each case in accordance with this Agreement. Notwithstanding the foregoing, Arena shall notify Roivant promptly in writing of any FDA Form 483 notices received by Arena (or such Third Party manufacturer, as applicable) with respect to the Facility (or facility of a Third Party manufacturer). In any event, Arena shall use Commercially Reasonable Efforts to address and resolve as soon as reasonably practicable any issues, concerns or warnings from the Regulatory Authority that would reasonably be expected to affect Arena’s (or such Third Party manufacturer’s, as applicable) ability to manufacture and supply to Roivant Compound and Finished Product in accordance with this Agreement. If despite the Commercially Reasonable Efforts of Arena or the Third Party manufacturer, such issues, concerns or warnings have not been resolved within a reasonable time, then at Roivant’s request, Arena will engage and qualify a Third Party manufacturer in accordance with Section 3.10 [***]
6.15 Supply Problems.
(a) If due to Arena not supplying to Roivant amounts of Finished Product by the applicable confirmed delivery date(s) under a Purchase Order complying with the terms of Section 6.2(c) (other than amounts that are Excess Orders), there is a back-order of more than twenty days under pending Purchase Orders of more than [***] of the amount of Finished Product ordered by Roivant pursuant to such Purchase Orders (without regard to whether a Force Majeure event has caused such supply delays), then the Parties shall meet as soon as practicable (but in any event within [***] hours) to discuss the situation and seek to find resolution, and in any event Arena shall continue to use good faith diligent efforts to deliver to Roivant such back-ordered amounts of Finished Product as soon as possible. Roivant will continue to order all its requirements for supply by Arena, in accordance with the supply commitments of this Agreement.
(b) For purposes of Section 6.15(a), delivery of any quantity of Non-Conforming Finished Product shall be deemed a failure to supply such quantity of Finished Product by the confirmed delivery date if Roivant has timely given Arena notice of such failure under the terms of Section 6.9.
7.1 Initial Payment. In partial consideration for the rights granted under this Agreement, Roivant shall pay to Arena within five (5) days of the Effective Date a one-time upfront payment in the amount of US$4,000,000 (the “Upfront Payment”). The Upfront Payment is not refundable or creditable against any other payments owed or payable by Roivant to Arena under this Agreement.
7.2 Milestone Payments. In further consideration for entering into this Agreement, Roivant shall pay to Arena each milestone payment set out below within thirty (30) days following the first achievement of the corresponding milestone event. The payments set forth in this Section 7.2 shall not be refundable or creditable against any other payments owed or payable by Roivant to Arena under this Agreement. Each milestone payment set forth in this Section 7.2 shall be payable only once. For clarity, the total amount payable under this Sections 7.2 shall not exceed US$41,500,000.
30
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(a) Initiation of dosing in the first Phase 3 trial involving a Product in a first Indication | US$2,000,000 |
(b) Initiation of dosing in the first Phase 3 trial involving a Product in a second Indication | US$2,000,000 |
(c) Upon the first submission of an NDA for a Product by the FDA | US$5,000,000 |
(d) Upon the first submission of a Marketing Authorization Application (MAA) for a Product by the EMA | US$2,500,000 |
(e) Upon the first NDA approval for a Product | US$20,000,000 |
(f) Upon the first MAA approval for a Product | US$10,000,000 |
(a) Product Purchase Price Calculations. In consideration of the commercial supply of a Product under this Agreement to Roivant by Arena, except as set forth in Section 7.3(e), Roivant shall pay to Arena a purchase price for Roivant’s purchase of each unit of Finished Product (the “Product Purchase Price”) equal to the greater of (i) the Minimum Product Purchase Price (set forth in Section 7.3(d) below) and (ii) (x) during the Initial Term for such Product in such `Product’s country of sale, 15% of the Net Sales of such Product and (y) during the Extended Term for such Product in such Product’s country of sale, 10% of the Net Sales of such Product. The Product Purchase Price shall be determined as provided in subclause (b) below.
(i) Initial Purchase Price Payment. For all amounts of Product purchased by Roivant in a Fiscal Semester for commercial sale, promptly after Arena delivers such Product to Roivant, Arena shall invoice Roivant an amount equal to the Minimum Product Purchase Price for all units of such Product delivered by Arena to Roivant (the “Initial Purchase Price Payment”). Roivant shall pay all undisputed amounts in such invoice no later than 30 days after receipt of the invoice. In the event Roivant disputes one or more items in an invoice, Roivant will promptly notify Arena in writing and such notice shall contain a reasonably detailed description of the item(s) being disputed and the basis therefor. Arena will promptly respond to Roivant and the Parties will use good faith efforts to promptly resolve the dispute. Amounts determined to be owed following resolution will be paid to Arena within thirty (30) days of resolution of the dispute.
31
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(ii) Secondary Purchase Price Reports and Payments. Within [***] days after the end of each Calendar Quarter after the First Commercial Sale of the first Product, Roivant shall prepare and send to Arena a report stating: (a) the total amount of Net Sales of each Product during such Calendar Quarter, and the detailed and total deductions from gross amounts invoiced (or otherwise charged) to arrive at such Net Sales; (b) the sales in units of each Product and gross amounts invoiced for such sales, on a Product-by-Product basis during such Calendar Quarter; (c) the total amount of Product used as samples or as part of compassionate use, named patient use or indigent patient program (and specifying the total amount of Product used in each such way); (d) the total amount of Initial Purchase Price Payments paid by Roivant to Arena upon invoice (as provided above under Section 7.3(b)(ii)) for the delivery of such Products to Roivant; (e) a detailed description of the inventory of Product being held at distributors at the end of such Calendar Quarter; and (f) the total amount of the Secondary Purchase Price Payments (as defined below) for such Calendar Quarter owed by Roivant to Arena, if any. The Parties agree that each of the quarterly reports to be provided by Roivant to Arena pursuant to this Section shall be broken down to report all gross invoiced sales and Net Sales on a Product-by-Product and country-by-country basis. Within [***] days after the end of the first and second months of each such Calendar Quarter, Roivant shall prepare and send to Arena a report containing the information described in clauses (a)-(f) above for the applicable month for sales by Roivant and its Affiliates and, to the extent available to Roivant for sales by each Sub-distributor and its Affiliates; Roivant shall use Commercially Reasonable Efforts to obtain such information from its Sub-distributors on a timely basis. Within [***] days after the end of each Calendar Quarter after the First Commercial Sale of the first Product, Roivant shall pay Arena an amount equal to the following (provided that such following amount is a positive number): the sum of the applicable Product Purchase Price for each unit of Product sold during such Calendar Quarter, minus the sum of the applicable Initial Purchase Price Payment made for each unit of Product sold during such Calendar Quarter (the portion of such payment attributable to each unit of Product, the “Secondary Purchase Price Payment”). For clarity, the Parties acknowledge and agree that in any event for each unit of Product delivered by Arena to Roivant under this Agreement and sold by Roivant, Sub-distributors or its or its Sub-distributors’ Affiliates, to generate Net Sales, the Initial Purchase Price Payment for such unit of Product plus the Secondary Purchase Price Payment for such unit of Product shall equal the Product Purchase Price for such Product. Roivant shall be responsible for payment of any sales or value added tax applicable to the sale of Finished Product by Arena to Roivant.
(c) Purchase Price for Products Not Sold. The Parties hereby agree that, for any particular Finished Product delivered to Roivant per Section 7.3(b), other than Non-Conforming Finished Product, that is not and cannot be subsequently resold by Roivant, its Sub-distributors, or its or their Affiliates, because it reaches the end of its shelf life prior to sale, or it is destroyed or is damaged, the Product Purchase Price that Roivant shall pay Arena for such Finished Product shall be [***] at the time of purchase.
(i) Initial Product. Notwithstanding anything to the contrary in this Agreement, in no event will Product Purchase Price for any Initial Product sold by Roivant, or its Sub-distributors or its or their Affiliates, and included in Net Sales, after applying the applicable purchase price calculations under this Agreement (including any applicable deductions), be less on a per tablet basis than US$0.50 (the “Initial Product Minimum Product Purchase Price”), which amount shall be (A) reasonably adjusted to account for any packaging specifications or packaging requirements different than standard (as of the Effective Date) packaging, labels (including package inserts), bottles and closures, and (B) annually adjusted to account for any increased costs (i) of raw materials and components (to the extent that such increase exceeds the CPI Adjustment made pursuant to Section 7.3(d)(iii)), packaging or regulatory compliance activities, or (ii) resulting from changes to the Specifications requested by Roivant.
(ii) Additional Products. The Parties will negotiate in good faith to agree upon a commercially reasonable minimum product purchase price on a per unit basis for each Additional Product sold by Roivant or its Sub-distributors, or its or their Affiliates (the “Additional Product Minimum Product Purchase Price”), which amount shall be [***] adjusted to account for any increased or decreased costs (i) of raw materials and components (to the extent that [***] pursuant to Section 7.3(d)(iii)]), [***], or [***]. The Parties shall also agree [***] applicable for such Additional Product.
(iii) CPI-U Adjustments. The Minimum Product Purchase Price for each Product shall be adjusted annually to reflect any year-to-year percentage increase or decrease (as the case may be) in the U.S. Bureau of Labor Statistics’ All Items Consumer Price Index for All Urban Consumers (CPI-U) for the U.S. City Average, 1982-84 = 100. The annual adjustment to the Minimum Product Purchase Price for CPI-U changes shall first occur as of [***] based on the percentage increase or decrease (as the case may be) in the CPI-U from the most recent index available as of [***].
32
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(e) Non-Commercial Product Purchase Price. With respect to any units of Finished Products delivered by Arena to Roivant that are used in commercially reasonable and customary quantities as samples or as part of a compassionate use, named patient use or indigent patient program and thus are disposed of by Roivant (or its Sub-distributor or its or their Affiliates) without charge, the Product Purchase Price therefor shall be [***], payable within 30 days of receipt of invoice.
(f) Product Purchase Price Adjustment Payments. The Product Purchase Prices owed by Roivant for its purchase of Product from Arena for commercial sale in a particular Commercial Year are subject to adjustment by the payment by Roivant of one-time purchase price adjustment payments, as provided below (each, a “Product Purchase Price Adjustment Payment”) for the first achievement of Net Sales in any Commercial Year above each threshold Net Sales amount set forth below:
Aggregate Products Net Sales for a Commercial Year | Product Purchase Price Adjustment Payment |
(1) at least US$300,000,000 | US$10,000,000 |
(2) at least US$600,000,000 | US$20,000,000 |
(2) at least US$900,000,000 | US$30,000,000 |
If Roivant (including its Sub-distributors and its and their Affiliates) has sold (for the first time) an amount of Product in excess of one or more threshold Net Sales amounts in the above table, then Roivant shall pay to Arena, as an adjustment to the Product Purchase Price paid by Roivant for its purchase of Products, a one-time Product Purchase Price Adjustment Payment in the amount listed above for each such threshold exceeded, such payment to be made within thirty (30) days of the end of the Calendar Quarter during such Commercial Year when such threshold amount is first reached. For clarity, the total amount payable under this Section 7.3(f) shall not exceed US$60,000,000.
7.4 Payment Method; Currency. Unless otherwise expressly stated in this Agreement, invoices shall be issued for all services and goods provided pursuant to this Agreement. All payments to the Payee Party under this Agreement shall be made by bank wire transfer in immediately available funds to an account in the name of the Payee Party designated in writing by the Payee Party. Payments hereunder shall be considered to be made as of the day on which they are received by the Payee Party’s designated bank. Unless otherwise expressly stated in this Agreement, all amounts specified to be payable under this Agreement are in United States Dollars and shall be paid in United States Dollars. For Net Sales outside the United States received in a currency other than United States Dollars, the rate of exchange to be used in computing the amount of currency equivalent in Dollars shall be made at the rate of exchange published in Reuters or Bloomberg on the last business day of the applicable Calendar Quarter.
33
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
7.5 Taxes. The Upfront Payment, milestones, Product Purchase Price, FTE Costs, reimbursements and other amounts payable by one Party (the “Paying Party”) to the other Party (the “Payee Party”) pursuant to this Agreement (each, a “Payment”) shall not be reduced by any deduction or withholding for any present or future taxes, levies, imposts, duties, fees, charges or liabilities imposed by any governmental authority, including any interest, additions to tax or penalties applicable to any of the foregoing (collectively, “Taxes”); provided, however, if it is required by Applicable Laws to impose an obligation on the Paying Party to deduct or withhold Taxes directly from any amount paid to the Payee Party, then the Paying Party will deduct or withhold the required amount and will timely pay the full amount deducted or withheld to the relevant governmental authority in accordance with the Applicable Laws, and the amount paid to the Payee Party will be decreased by the amount so deducted or withheld. Notwithstanding the foregoing, to the extent Roivant or its Affiliates (i) assigns or otherwise transfers this Agreement or its obligations hereunder to an Affiliate or Third Party, (ii) changes its location of incorporation from Bermuda to another location, or (iii) makes payments from an entity other than the Bermuda entity originally entering into this Agreement, in each case that results in a Tax being required to be withheld under Applicable Laws that would not have been required to be withheld if such action had not been taken (each, a “Tax Changing Decision”) such that as a result of a Tax Changing Decision, Roivant is required by Applicable Laws to deduct or withhold Taxes directly from any amount paid to Arena, then (A) Roivant will increase the amount paid to Arena by the required amount such that the net amount actually received by Arena equals the full amount originally invoiced or stated by Arena to be payable and (B) Roivant will timely pay the applicable Taxes to the relevant governmental authority in accordance with Applicable Laws. In the event Applicable Laws require Taxes be deducted or withheld, the Paying Party will provide reasonable assistance and documentation to allow the Payee Party to receive a refund or credit for Taxes paid.
7.6 Records. Roivant shall keep, and cause its Sub-distributors and its and their Affiliates to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable to Arena under this Agreement. Such books and records shall be kept for such period of time required by Applicable Laws, but no less than at least [***] years following the end of the Calendar Quarter to which they pertain. Such records shall be subject to inspection in accordance with Section 7.7.
7.7 Audits.
(a) Upon not less than [***] days’ prior written notice, Roivant shall permit an independent, certified public accountant of international recognition (for the purposes of this Section 7.7, the “Auditor”) selected by Arena and reasonably acceptable to Roivant, which acceptance shall not be unreasonably conditioned, withheld or delayed, to audit or inspect those books and records of Roivant and its Sub-distributors and its and their Affiliates that relate to Net Sales for the sole purpose of verifying Product Purchase Price payments. Prior to any such audit, the Auditor shall execute a confidentiality agreement that is reasonably acceptable to Roivant.
(b) The Auditor shall send a copy of the report to Roivant at the same time it is sent to Arena. Such audits or inspections may be made no more than [***] (unless an audit or inspection reveals a material inaccuracy in reports made or amounts invoiced under this Agreement, in which case [***]), during normal business hours and upon reasonable advance notice. If such report shows that the amounts paid by Roivant for the period audited are less than the amounts actually payable by Roivant to Arena during the period audited, then (absent manifest error or fraud in such audit report) Roivant shall pay to Arena the amount of such underpayment plus interest under Section 7.8, from the date such amounts were originally owed until payment is made, Arena shall deliver to Roivant an invoice for such underpaid amount, and Roivant shall pay such invoice within [***] days of receipt of such invoice. If such report shows that the amounts paid by Roivant for the period audited exceed the amounts actually owed by Roivant to Arena for the period audited, then (absent manifest error or fraud in such audit report) Roivant shall deliver to Arena an invoice for such excess amount, and Arena shall pay such invoiced excess amount, within thirty (30) days of receipt of such invoice. Such [***] subject to no more than [***] with respect to [***] such Calendar Quarter. Audits and inspections conducted under this Section 7.7 shall be at the expense of Arena, unless an audit or inspection pursuant to subsection (a) demonstrates an underpayment in amounts payable by Roivant exceeding an amount equal to [***] of the amount paid for a period covered by the audit or inspection, in which case all reasonable and verifiable costs relating to the audit or inspection for such period and any unpaid amounts that are discovered shall be paid by Roivant, based on invoices delivered by Arena. Arena shall endeavor in any such audit not to unreasonably disrupt the normal business activities of Roivant.
34
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
7.8 Payment Due Dates; Late Payments. Unless otherwise expressly stated in this Agreement, all Payments under this Agreement are due within thirty (30) days of receipt of invoice. If any Payment is due on a day when banks in New York, New York, USA, are generally closed, then such Payment shall not be considered late if made on the next day on which such banks are generally open. In the event that any Payment due under this Agreement is not made when due, such Payment shall accrue interest from the date due at a rate per annum equal to 2% above the U.S. Prime Rate (as set forth in The Wall Street Journal, Eastern Edition) for the date on which payment was originally due until the date such Payment plus accrued interest hereunder is actually made, calculated daily on the basis of a 365-day year, or similar reputable data source; provided, that in no event shall such rate exceed the maximum legal annual interest rate. The payment of such interest shall not limit the Party entitled to receive such payment from exercising any other rights it may have as a consequence of the lateness of any Payment.
ARTICLE 8
8.1 Confidential Information. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, and subject to the other applicable terms of this Article 8, the Parties agree that the receiving Party (the “Receiving Party”) shall keep confidential and not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any Know-How, information or materials, patentable or otherwise, in any form (written, oral, photographic, electronic, magnetic, or otherwise) that is disclosed to it by the other Party (the “Disclosing Party”) pursuant to this Agreement, including all information concerning any Product or Compound and any other technical or business information of whatever nature concerning the Disclosing Party or its technology or business (collectively, “Confidential Information” of the Disclosing Party), except that the Receiving Party may disclose Confidential Information of the Disclosing Party to its Affiliates and its and its Affiliates’ respective officers, directors, employees, agents, subcontractors and consultants (and in the case of Roivant as the Receiving Party, Sub-distributors) with a need to know such Confidential Information to assist the Receiving Party with (or in the case of Sub-distributors, to perform) the activities contemplated or required of it by this Agreement (and who shall be advised of the Receiving Party’s obligations hereunder and who are bound by confidentiality and non-use obligations with respect to such Confidential Information no less onerous than those set forth in this Agreement) (each, a “Recipient”). For clarity, all Program Know-How and all preclinical and clinical data provided by Arena to Roivant under this Agreement is deemed to be the Confidential Information of Roivant and shall be deemed to have been disclosed by Roivant to Arena for purposes of Sections 8.1 and 8.2, regardless of whether developed or made by Roivant or Arena. Notwithstanding the foregoing, the Parties acknowledge the practical difficulty of policing the use of information in the unaided memory of the Receiving Party or its Recipients, and as such each Party agrees that the Receiving Party shall not be liable for the use by any of its Recipients of specific Confidential Information of the Disclosing Party that is retained in the unaided memory of such Recipient; provided, that (a) such Recipient is not aware that such Confidential Information is the confidential information of Disclosing Party at the time of such use; (b) the foregoing is not intended to grant, and shall not be deemed to grant, the Receiving Party, its Affiliates, or its Recipients (i) a right to disclose the Disclosing Party’s Confidential Information or (ii) a license under any Patents or other intellectual property right of the Disclosing Party; and (c) such Recipient has not intentionally memorized such Confidential Information for use outside this Agreement. For the purpose of Article 8, the term “Disclosing Party” shall include each Party and its Affiliates and its and their respective officers, directors, employees, agents, subcontractors and consultants who are directed or authorized to disclose such Party’s and/or its Affiliates’ Confidential Information, and the term “Receiving Party” shall include each Party and its Affiliates.
8.2 Exceptions. Notwithstanding Section 8.1, the obligations of Section 8.1 shall not apply to any specific Confidential Information that the Receiving Party thereof can demonstrate, in each case by competent evidence:
(a) was already known to the Receiving Party or any of its Recipients, other than under an obligation of confidentiality, at the time of disclosure;
(b) was generally available to the public or was otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure by the Disclosing Party and other than through any act or omission of the Receiving Party or any of its Recipients in breach of this Agreement;
35
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(d) was subsequently lawfully disclosed to the Receiving Party or any of its Recipients without any obligation of confidentiality or non-use by a Person other than the Disclosing Party, and who, to the knowledge of the Receiving Party or such Recipient, did not directly or indirectly receive such information from the Disclosing Party or any of its Affiliates under an obligation of confidence; or
(e) was independently developed by the Receiving Party or any of its Recipients without use of or reference to any information or materials disclosed by the Disclosing Party.
Information specific to the use of certain compounds, methods, conditions or features shall not be deemed to be within the foregoing exceptions merely because such information is embraced by general disclosures in the public domain or in the possession of the Receiving Party or its Recipients. In addition, a combination of information will not be deemed to fall within the foregoing exceptions, even if all of the components fall within an exception, unless the combination itself and its significance are in the public domain or in the possession of the Receiving Party prior to the disclosures hereunder. Notwithstanding anything to the contrary herein, neither the act of using information in a clinical trial nor the filing of information with a governmental authority shall, for the purpose of this Article 8, in and of itself be deemed to place such information in the public domain.
8.3 Permitted Disclosures. Notwithstanding the provisions of Section 8.1 or Section 8.2, the Receiving Party may disclose Confidential Information of the Disclosing Party as expressly permitted by this Agreement or if and to the extent such disclosure is reasonably necessary or useful in the following instances:
(a) the performance by the Receiving Party of its obligations or exercise of its rights as contemplated by this Agreement; provided, that wherever reasonable and practicable in the circumstances the recipient of any such Confidential Information shall be subject to obligations of confidentiality and non-use with respect to such Confidential Information substantially similar to the obligations of confidentiality and non-use of the Receiving Party pursuant to this Article 8;
(b) filing or prosecuting Patents as permitted by this Agreement;
(c) seeking, obtaining or maintaining any Regulatory Approval as permitted by this Agreement; provided, that the Receiving Party shall take reasonable measures to assure confidential treatment of such Confidential Information, to the extent such treatment is available;
(d) prosecuting or defending litigation with respect to a Party or its Affiliates as permitted by this Agreement, provided, that a protective order or any similar measures are sought by such Party with respect to the information to be disclosed, to the extent reasonably possible;
(e) complying with Applicable Laws;
(f) disclosure to Third Parties in connection with due diligence or similar investigations by or on behalf of a Third Party in connection with an actual or potential marketing, distribution or supply agreement with, or license to, or collaboration with such Third Party or an actual or potential merger or acquisition or investment by such Third Party, or in connection with performance of any such license, collaboration or merger agreement, and disclosure to actual or potential Third Party investors in confidential financing documents, provided, in each case, that any such Third Party agrees to be bound by obligations of confidentiality and non-use substantially similar to the obligations of confidentiality and non-use of the Receiving Party pursuant to this Article 8, but of shorter duration if customary under the circumstances;
provided that wherever reasonable and practicable in the circumstances the recipient of any such Confidential Information shall be subject to reasonable and customary obligations of confidentiality with respect to such Confidential Information.
Notwithstanding the foregoing, in the event the Receiving Party or a Recipient is required to make a disclosure of the Disclosing Party’s Confidential Information pursuant to Section 8.3(d) or Section 8.3(e) to comply with a subpoena or other legal order, it shall, except where impracticable, give reasonable advance notice to the Disclosing Party of such disclosure and give the Disclosing Party a reasonable opportunity to quash such subpoena or order and to obtain a protective order requiring that the Confidential Information and documents that are the subject of such subpoena or order be held in confidence by such court or agency or, if disclosed, be used only for the purposes for which such subpoena or order was issued; and provided, further, that if such subpoena or order is not quashed or a protective order
36
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
is not obtained, the Confidential Information disclosed in response to such subpoena or order shall be limited to the Disclosing Party’s Confidential Information that is legally required to be disclosed in response to such subpoena or order and shall still be subject to the restrictions on use set forth in this Article 8.
8.4 Confidentiality of this Agreement and its Terms. Except as otherwise provided in this Article 8, each Party agrees not to disclose to any Third Party the existence of this Agreement or the terms and conditions of this Agreement without the prior written consent of the other Party, except that each Party may disclose the terms and conditions of this Agreement that are not otherwise made public as contemplated by Section 8.5 as permitted under Section 8.3.
8.5 Public Announcements.
(a) As soon as practicable following the Effective Date, each Party may issue a mutually agreed to press release announcing the entry into this Agreement. The Parties may make disclosures as required by Applicable Laws (including disclosure requirements of the U.S. Securities and Exchange Commission (“SEC”) (including disclosure requirements of a Party’s Affiliate), the NASDAQ stock exchange or any other stock exchange on which securities issued by a Party or any of its Affiliates are or are proposed to be traded). In the event of a public announcement required under Applicable Laws, to the extent practicable under the circumstances, the Party making such announcement shall provide the other Party with a copy of the proposed text of such announcement at least [***] business days in advance of the scheduled release to afford such other Party a reasonable opportunity to review and comment upon the proposed text.
(b) The Parties shall reasonably coordinate in advance with each other in connection with the filing of this Agreement (including, if applicable, redaction of certain provisions of this Agreement) with the SEC or any other governmental agency, the NASDAQ stock exchange or any other stock exchange on which securities issued by a Party or any of its Affiliates are traded, and each Party shall reasonably consider seeking confidential treatment for the terms reasonably requested by the other Party to be redacted; provided that such request is made within [***] business days after the other Party’s receipt of a draft redacted agreement and further provided, that each Party shall ultimately retain control over what information to seek confidential treatment for and what information to disclose to the SEC or any other governmental agency, the NASDAQ stock exchange or any other stock exchange, as the case may be, and nothing in this Agreement shall prevent a Party from taking all actions it reasonably considers necessary to comply with Applicable Laws with respect to any such filings or disclosures; and provided, further, that the Parties shall use their reasonable efforts to file redacted versions with any governing bodies that are consistent with redacted versions previously filed with any other governing bodies. Except as provided in the preceding sentence, neither Party nor any of their respective Affiliates shall be obligated to consult with the other Party with respect to any filings to the SEC, the NASDAQ stock exchange or any other stock exchange or governmental agency.
8.6 Use of Name. Neither Party shall use the name, insignia, symbol, Trademark, trade name or logotype of the other Party (or any abbreviation or adaptation thereof) in any publication, press release or marketing and promotional material or other form of publicity without the prior written approval of such other Party in each instance, which approval shall not be unreasonably conditioned, withheld or delayed, or except as expressly permitted in this Agreement. The restrictions imposed by this Section 8.6 shall not prohibit either Party from making any disclosure (a) identifying the other Party as a counterparty to this Agreement to its actual or potential subcontractors, licensees, collaborators, merger partners, or investors (or in the case of Roivant, Sub-distributors), (b) that is required by Applicable Laws or the requirements of a national securities exchange or another similar regulatory body (provided, that any such disclosure shall be governed by this Article 8), (c) that is necessary for the performance by Roivant or Arena of its obligations or exercise of its rights as contemplated by this Agreement or (d) with respect to which written consent has previously been obtained. Further, the restrictions imposed on each Party under this Section 8.6 are not intended, and shall not be construed, to prohibit a Party from identifying the other Party in its internal business communications; provided, that any Confidential Information in such communications remains subject to this Article 8.
8.7 Publication of Product Information. At least [***] days prior to Roivant publicly presenting at a scientific conference or submitting for written publication in a scientific journal a manuscript, abstract or the like that includes technical information relating to any Compound or any Product that has not been previously published, Roivant shall provide Arena a draft copy thereof for its review. In addition, if Roivant materially changes, prior to submission, the version of the manuscript, abstract or the like provided to Arena for review, Roivant shall provide Arena with such updated version and Arena shall have [***] business days to review and comment on such updated version.
37
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
8.8 Financial Information. From and after the Effective Date, Arena shall reasonably cooperate with Roivant and/or its Affiliates and their respective accountants and auditors, including by providing access to information, books and records related to the Products as Roivant may reasonably request in connection with the preparation by Roivant and/or its Affiliates of historical and pro forma financial statements related to the Products as may be required to be included in any filing made by Roivant and/or any of its Affiliates under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, and the regulations promulgated thereunder, including Regulation S-X. Roivant will be responsible for all costs incurred by Arena or its Affiliates in connection therewith.
(a) Arena Intellectual Property Rights. Arena and its Affiliates have, and shall retain, all right, title and interest in and to the Arena Know-How and Arena Patents and any other intellectual property rights owned by Arena or its Affiliates as of the Effective Date or developed by Arena or its Affiliates during the Term, excluding the Program Patents and the Program Know-How.
(b) Manufacturing Intellectual Property Rights. Arena and its Affiliates have, and shall retain, all right, title and interest in and to the Manufacturing Know-How and Manufacturing Patents.
(c) Roivant Intellectual Property Rights. Roivant and its Affiliates have, and shall retain, all right, title and interest in and to the Roivant Know-How and any other intellectual property rights owned by Roivant or its Affiliates as of the Effective Date.
(d) Program Intellectual Property Rights. Roivant shall have and own the entire right, title and interest in and to all Program Know-How and Program Patents and shall have and retain the right to use, disclose and exploit the Program Know-How and Program Patents for any and all purposes, including the right to disclose the Program Know-How to its Affiliates and Sub-distributors and to use and grant to its Sub-distributors and its and their Affiliates the right to use, disclose and exploit the Program Know-How for developing and commercializing Products. Arena shall promptly disclose to Roivant in writing the discovery, identification, conception, reduction to practice or other making of any Program Know-How or Program Patents by or on behalf of Arena. Arena shall acquire from its employees, officers, directors, consultants and subcontractors any and all rights in the Program Know-How and Program Patents in accordance with any applicable work-for-hire laws and rules, and hereby does, assign, and shall cause its Affiliates to so assign, to Roivant or an Affiliate of Roivant designated by Roivant in writing, without additional compensation (beyond the payments made to Arena under this Agreement), all its right, title and interest in and to the Program Know-How and Program Patents as well as any intellectual property rights with respect thereto to fully effect the ownership by Roivant provided for in this Section 9.1(d). If applicable, Arena shall cause all subcontractors and consultants of Arena to assign all of their right, title and interest in and to the Program Know-How and Program Patents as well as any intellectual property rights with respect thereto to Roivant or its designee. Arena and its Affiliates, as well as their respective subcontractors and consultants, shall execute all documents and take all actions reasonably requested by Roivant to fully effect the ownership by Roivant provided for in this Section 9.1(d).
(a) Subject to the provisions of this Section 9.2, [***] shall have the first right, but not the obligation, for the preparation, filing, prosecution and maintenance (“Prosecution”) of all [***] Patents and all [***] Patents, at its discretion and expense. If [***] chooses to exercise such right, it shall do so in good faith.
(b) Provided that [***] on a [***], and with [***] Patents and [***] Patents, or [***] regarding a Product, [***] shall have the first right, but not the obligation, for the Prosecution of all [***] Patents related to the Initial Compound (the “Initial Compound Patents”) and all [***] Patents, at its discretion and expense. If [***] chooses to exercise such right, it shall do so in good faith.
(c) Provided that [***] on a [***], and with [***] Patents and [***] Patents, or [***] regarding a Product, [***] shall have the first right, but not the obligation, for the Prosecution of all [***] Patents related to the Related Compounds [***] covered or claimed by such [***] Patent, at its discretion and expense. If [***] chooses to exercise such right, it shall do so in good faith.
38
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(d) The Party controlling the Prosecution of any Patents within the [***] Patents or the [***] Patents shall keep the other Party reasonably informed of progress with regard to the Prosecution of such Patents in a timely manner, but not less frequently than once per Calendar Quarter. To that end, the controlling Party shall: (i) provide the other Party with a copy of the final draft of any proposed application for such Patents at least [***] days prior to filing the same, unless otherwise agreed by patent counsel for each Party, and shall consider in good faith any reasonable comments or revisions suggested by the other Party or its counsel; (ii) promptly provide the other Party with a copy of each such patent application, as filed, together with a notice of its filing date and serial number; (iii) provide the other Party with a copy of any action, communication, letter, or other correspondence issued by the applicable patent office within at least [***] days of receipt thereof, and shall consult with the other Party regarding responding to the same and shall consider in good faith any reasonable comments, strategies, and the like proposed by the other Party or its counsel; (iv) provide the other Party with a copy of any response, amendment, paper, or other correspondence filed with the applicable patent office within [***] days of the controlling Party’s receipt of the as-filed document; and (v) promptly notify the other Party of the allowance, grant, or issuance of such Patents. If the controlling Party elects to abandon or cease Prosecution of any specific Patents described above, the controlling Party shall provide reasonable prior written notice to the other Party of such intention to abandon (which notice shall, to the extent possible, be given no later than [***] days prior to the next deadline for any action that must be taken with respect to any such Patent in the applicable patent office). In such case, at the other Party’s sole discretion, upon written notice to the controlling Party, the other Party may elect to continue Prosecution of such Patent, and the controlling Party shall execute such documents and take such actions (or shall cause its Affiliates, as applicable, to execute such documents and take such actions) as may be reasonably necessary to enable the other Party to do so. If the other Party elects to continue Prosecution of any such Patent, then it shall do so in good faith.
(e) [***] shall have the sole right, but not the obligation, to control the Prosecution and enforcement of all [***] Patents and all [***] Patents that claim or cover Reverted Compounds (but not any other Compounds) or the manufacture or use thereof, at its sole discretion and expense. Upon the written request of [***] promptly shall transfer to [***] all files (including all outside counsel files) relating thereto, and shall execute and deliver all documents and instruments reasonably requested by [***] to effectuate the foregoing.
(a) Notice. In the event that either Arena or Roivant becomes aware of any infringement or threatened infringement by a Third Party of any Arena Patents or Program Patents, it shall notify the other Party in writing to that effect. Any such notice shall include any evidence that such notifying Party has in its possession and is legally able to disclose that supports such allegation of infringement or threatened infringement by such Third Party.
(b) Enforcement Procedures. Roivant shall have the first right, but not the obligation, (subject to the following) to bring and maintain any action or proceeding with respect to infringement of any Arena Patent or Program Patent by a Third Party (a “Field Infringement”), using counsel of its choosing. Roivant shall keep Arena reasonably informed of any actions or proceedings it takes with respect to any Field Infringement, and the Parties shall cooperate and consult with each other in strategizing regarding any such action or proceeding, provided that Roivant shall control and have the final decisions (regardless of whether or not Roivant is a party to such action or proceeding) regarding all matters in the preparation and conduct of any action or proceeding as to a Field Infringement; Roivant shall make such decisions in good faith. Arena shall cooperate fully with Roivant with respect to such actions or proceedings, including being joined as a party plaintiff in any such action or proceeding against a Third Party with respect to such Field Infringement (or, to the extent required under Applicable Laws and at the request and direction of Roivant, bringing such action or proceeding directly against a Third Party with respect to such Field Infringement) and providing access to relevant documents and other evidence and making its employees available at reasonable business hours. The Parties’ reasonable and documented out-of-pocket costs and expenses of conducting any such action or proceeding against a Field Infringement, shall be entirely borne by Roivant. Roivant shall promptly inform Arena if it elects not to exercise such first right and Arena shall thereafter have the right to initiate any action or proceeding with respect to such Field Infringement in its name and Roivant shall cooperate fully with Arena with respect to such action or proceeding. Each Party shall have the right to be represented by counsel of its own choice. Any monetary recovery resulting from such actions or proceedings will be allocated as follows: each of Roivant and Arena first will be reimbursed, out of such recovery, for its reasonable and verifiable costs and expenses with respect to such action or proceeding (such reimbursement to be pro-rata based on the Parties’ relative costs and expenses if the recovery is not sufficient to reimburse both Parties fully) with any remainder being shared by the Parties as follows: [***].
(c) Paragraph IV Notices. If either Party receives a notice under 21 U.S.C. §355(b)(2)(A)(iv) or 355(j)(2)(A)(vii)(IV) concerning an Arena Patent or a Program Patent, or any similar notice under the Applicable
39
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
Laws of a particular country (each, a “Paragraph IV Notice”), then it shall provide a copy of such notice to the other Party within [***] days after its receipt thereof. Patent infringement litigation based on a Paragraph IV Notice concerning an Arena Patent or Program Patent shall be brought as provided in Section 9.3(b), with such Paragraph IV Notice being deemed a Field Infringement.
9.4 Third Party Intellectual Property Rights. If either Party becomes aware of a Patent owned by a Third Party that it believes will, or may, be infringed by the manufacture, importation, development or Commercialization of any Product as contemplated by this Agreement, such Party shall promptly notify the other Party of such Patent. The Parties then shall discuss the matter, consider any design-arounds or alternative technologies, and if a license under such Patent is necessary, [***] obtain such license under such Patent. With respect to any such license, [***] the Third Party, [***] such that [***] under this Agreement. The [***]. If a reasonable design-around or alternative technology is identified, then [***], Arena and its Affiliates shall and shall ensure that its Third Party manufacturers cease use of the allegedly infringing technology and implement the design around or alternative technology instead, in each case as soon as practicable. In any event Arena and its Affiliates shall and shall ensure that its Third Party manufacturers cease use of the allegedly infringing technology as soon as practicable [***].
(a) Third Party Defense or Counterclaim. If a Third Party asserts, as a defense or as a counterclaim in any action or proceeding with respect to a Field Infringement under Section 9.3, that any Arena Patent or Program Patent is invalid or unenforceable, then Roivant, through the counsel engaged by Roivant pursuant to Section 9.3, shall respond to such defense or defend against such counterclaim (as applicable); provided, that the Parties shall fully discuss and seek to agree on the strategy of such response, considering and accommodating Arena’s and Roivant’s global intellectual property litigation positions in all such decisions that may impact such global positions. The Parties’ reasonable and documented out-of-pocket costs and expenses of conducting any such action or proceeding shall be entirely borne by Roivant.
(b) Third Party Declaratory Judgment or Similar Action. If a Third Party asserts, in a declaratory judgment action or similar action or claim filed by such Third Party, that any Arena Patent or Program Patent is invalid or unenforceable, then the Party first becoming aware of such action or claim shall promptly give written notice to the other Party. With respect to such Arena Patent and Program Patent, Roivant shall engage counsel to defend against such action or claim. The Parties shall consult fully with each other in strategizing, preparing, presenting and conducting any such defense, with Roivant having the final decision (regardless of whether or not Roivant is a party to such defense), which it shall exercise in good faith. Each Party shall cooperate fully with the other Party with respect to such defense, including being joined as a party defendant or joining the other Party as a party defendant in such defense and providing access to relevant documents and other evidence and making its employees available at reasonable business hours. The Parties’ reasonable and documented out-of-pocket costs and expenses of defending any such action or proceeding shall be [***].
9.6 Consent for Settlement. Except as set forth in this Section 9.6, neither Party shall enter into any settlement or consent judgment or other voluntary final disposition of an action or proceeding under Section 9.3 or Section 9.5 without the prior written consent of the other Party, which consent shall not be unreasonably conditioned, withheld or delayed. A settlement or consent judgment or other voluntary final disposition of an action or proceeding brought by a Party under Section 9.3 or Section 9.5 may be entered into without the consent of the other Party if (a) such settlement, consent judgment, or other final disposition does not admit the invalidity or unenforceability of any Arena Patent or Program Patent, and (b) any rights granted to a Third Party to continue any activity upon which such action or proceeding was based in such settlement, consent judgment, or other final disposition is limited to the Third Party’s product or activity that was the subject of such action.
9.7 Patent Term Extensions. The Parties shall discuss and recommend for which, if any, of the Patents within the Arena Patents and the Program Patents the Parties should seek Patent Term Extensions. Roivant shall have the final decision-making authority with respect to applying for any such Patent Term Extensions for the Arena Patents and the Program Patents, and shall act with reasonable promptness in light of the development stage of each Product to apply for any such Patent Term Extensions, where it so elects; provided, that (a) Roivant shall consult with Arena in good faith to determine which such Arena Patent or Program Patent should be the subject of efforts to obtain a Patent Term Extension, and (b) with respect to seeking exclusivity for a Product under FFDCA Section 505A(b) or (c) or any similar laws or regulations of a country, Roivant shall be responsible for seeking and using Commercially Reasonably Efforts to obtain such exclusivity and have final decision-making with respect thereto. Arena shall cooperate fully with Roivant in making such filings or actions, for example and without limitation, by making available all required regulatory
40
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
data and information and executing any required authorizations to apply for such Patent Term Extension. All expenses incurred in connection with activities of each Party with respect to the Arena Patents and Program Patents for which Roivant seeks Patent Term Extensions pursuant to this Section 9.7 shall be [***].
9.8 Patent Listings. Roivant shall consult with Arena in the planning and decisions regarding listing the applicable Arena Patents and the applicable Program Patents with the applicable Regulatory Authorities for Product and shall consider in good faith comments from Arena regarding such listing, including all so-called “Orange Book” listings required under the Xxxxx-Xxxxxx Act in the United States and equivalent listings in other countries; provided, that Roivant shall have final decision-making authority regarding which Arena Patents and Program Patents to list and shall maintain such listings. The costs of all “Orange Book” (and any equivalent) filings with respect to any Product shall be [***].
9.9 Product Domain Names. Within sixty (60) days following the Effective Date, Arena shall transfer the Product Domain Names to Roivant. Roivant shall have the right, but not the obligation, at its sole cost to maintain the registration of the Product Domain Names, and to register and maintain any additional domain names related to Compound or Product in its sole discretion. Roivant shall notify Arena prior to abandoning any of the Product Domain Names and Arena shall have the right, at its own cost, to continue maintaining such Product Domain Names; provided, that if Roivant does abandon any of the Product Domain Names, Roivant shall no longer have the right to use such Product Domain Names under this Agreement.
9.10 Product Trademarks. Roivant shall have the responsibility to select Trademarks for the Commercialization of Product (each, a “Product Trademark”). Roivant or its Affiliates shall own all right, title, and interest in and to Product Trademarks, all corresponding trademark applications and registrations thereof, and all common law rights thereto. All goodwill of the business associated with or symbolized by Product Trademarks shall inure to the benefit of Roivant. Roivant shall, control the registration, prosecution, maintenance and enforcement of Product Trademarks with respect to each Product at Roivant’s expense. Arena may display Product Trademarks on Arena’s and its Affiliates’ websites, press releases, presentations and SEC filings in a form consistent with Roivant’s brand style guidelines provided that each such web site content, press release, presentation or SEC filing is made in accordance with the applicable provisions of Sections 8.3, 8.5 and 8.6.
10.1 Mutual Representations, Warranties and Covenants. Each Party hereby represents and warrants to the other Party, as of the Effective Date, and covenants as follows:
(a) Duly Organized. Such Party (i) is a corporation or limited liability company, with restricted liability, duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization and (ii) is qualified to do business and is in good standing as a foreign corporation or organization in each jurisdiction in which the conduct of its business or the ownership of its properties requires such qualification and failure to have such qualification would prevent such Party from performing its obligations under this Agreement.
(b) Due Authorization; Binding Agreement. The execution, delivery and performance of this Agreement by such Party have been duly authorized by all necessary corporate or organizational action. This Agreement is a legal and valid obligation binding on such Party and enforceable in accordance with its terms subject to the effects of bankruptcy, insolvency or other laws of general application affecting the enforcement of creditor rights and judicial principles affecting the availability of specific performance and general principles of equity, whether enforceability is considered in the proceeding at law or equity. The execution, delivery and performance of this Agreement by such Party does not: (i) violate any law, rule, regulation, order, writ, judgment, decree, determination or award of any court, governmental body or administrative or other agency having jurisdiction over such Party; or (ii) conflict with, or constitute a default under, any agreement, instrument or understanding a court or administrative order, oral or written, to which such Party is a party or by which it is bound.
(c) Consents. Such Party has obtained, or is not required to obtain, or prior to performance will obtain, the consent, approval, order or authorization of any Third Party, or has completed, or is not required to complete, or prior to performance will complete, any registration, qualification, designation, declaration, or filing with, any Regulatory Authority or other governmental authority, in connection with the execution and delivery of this Agreement and the performance by such Party of its obligations under this Agreement.
41
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(d) No Conflicting Grant of Rights. Such Party has the right to grant (or cause its Affiliates to grant) the rights granted by such Party to the other Party under this Agreement and has not granted any rights to any Person that are in conflict with the rights granted by such Party to the other Party under this Agreement.
(e) Debarment. Such Party is not debarred under the FFDCA or listed on either Excluded List and it does not, and shall not during the Term, employ or use the services of any Person who is debarred or listed on either Excluded List, in connection with the development, manufacture or Commercialization of Product. In the event that either Party becomes aware of the debarment or threatened debarment of, or listing or threatened listing on either Excluded List of, any Person providing services to such Party, including the Party itself and its Affiliates, contractors, licensees, or distributors, that directly or indirectly relate to activities under this Agreement, the other Party shall be immediately notified in writing.
10.2 Representations, Warranties and Covenants of Arena. Arena represents and warrants to Roivant, as of the Effective Date, and hereby (where applicable) covenants to Roivant:
(a) Patent Rights. The Arena Patents existing as of the Effective Date are set forth on Exhibit B attached hereto (“Existing Arena Patents”). Arena’s Affiliate, Arena Pharmaceuticals, Inc., is the sole and exclusive owner of the Existing Arena Patents. Arena and its Affiliates have the exclusive right under the Arena Patents to develop, manufacture and Commercialize Compounds and Products in the Field, subject to the rights granted to Roivant pursuant to this Agreement. The Existing Arena Patents are not subject to any liens, mortgages, encumbrances, pledges or security interests, or, to the Knowledge of Arena, claims of ownership, by any Third Party. To the Knowledge of Arena, the Existing Arena Patents have been and are being diligently prosecuted before the patent office in each applicable country in accordance with Applicable Laws. To the Knowledge of Arena, the Existing Arena Patents have been filed and maintained in accordance with Applicable Laws. All applicable fees owed with respect to the prosecution and maintenance of the Existing Arena Patents have been paid on or before the due date for payment to the extent necessary to prevent the irrevocable abandonment of the Existing Arena Patents. To the Knowledge of Arena, there is no infringement or threatened infringement of the Existing Arena Patents by any Person.
(b) Patent Status. As of the Effective Date, (i) all issued Arena Patents are in full force and effect and subsisting, and have not been declared or adjudicated to be invalid or unenforceable; (ii) to the Knowledge of Arena, none of the Arena Patents is currently involved in any interference, reissue, reexamination, or opposition proceeding; and (iii) neither Arena nor any of its Affiliates has received any written notice from any Person, or has Knowledge, of any such actual or threatened proceeding.
(c) Non-Action or Claim. As of the Effective Date, there are no pending, or threatened in writing, adverse actions, suits, claims, or formal governmental investigations by or against Arena or any of its Affiliates in or before any court, Regulatory Authority or other governmental authority with respect to a Compound, including in connection with the conduct of any clinical trials or manufacturing activities with respect thereto. As of the Effective Date, there are no material unsatisfied judgments or outstanding orders, injunctions, decrees, stipulations or awards (whether rendered by a court, an administrative agency or by an arbitrator) against Arena (or any of its Affiliates) with respect to a Compound.
(d) No Conflicting Agreement. Neither Arena nor any of its Affiliates has entered into any contract, whether written or oral, that granted any Third Party the right to develop, promote, market or sell a Compound or otherwise assigned, transferred, licensed, conveyed or otherwise encumbered in a manner that would prevent Roivant from exercising its rights under this Agreement.
(e) Authority. Arena and/or its Affiliates have the full and legal right and authority to grant Roivant the license under Section 3.3(c) and to appoint Roivant as the exclusive distributor for the Product under Section 2.1.
(f) Prior Development and Manufacture. To the Knowledge of Arena, the preclinical and clinical development of the Initial Compound and Initial Product prior to the Effective Date and the manufacture of the Initial Compound and Initial Product used in such development was performed in compliance in all material respects with Applicable Laws.
42
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(g) No Blocking Patents. To the actual knowledge of Arena’s and its Affiliates’ in-house patent attorneys and patent agents, no issued Patent owned by a Third Party as of the Effective Date would be infringed by the development, manufacturing or commercialization activities of the Initial Product contemplated to be conducted under this Agreement as of the Effective Date.
(h) Government Funding. None of the Arena Patents or Arena Know-How existing as of the Effective Date is subject to any funding agreement with any government or governmental agency.
(i) Additional Legal Compliance. No governmental authority has commenced or, to the Knowledge of Arena, threatened to initiate any action to enjoin production of a Compound at any Facility, nor has Arena or any of its Affiliates or, to the Knowledge of Arena, any of its contractors, received any written notice thereof.
10.3 Representations, Warranties and Covenants of Roivant. Roivant represents and warrants to Arena that, as of the Effective Date, and hereby (where applicable) covenants to Arena:
(a) No Conflicting Agreement. Neither Roivant nor any of its Affiliates has entered into any contract that would, and shall not enter into during the Term any such agreement that would, conflict with its obligations, or prevent or impair its performance, under this Agreement.
(b) Non-Action or Claim. As of the Effective Date, there are no pending, or threatened in writing, adverse actions, suits, claims, or formal governmental investigations by or against Roivant or any of its Affiliates in or before any court, Regulatory Authority or other governmental authority with respect to Roivant’s marketing, promotion or sale of pharmaceutical products that would materially negatively affect Roivant’s ability to perform its obligations under this Agreement.
(c) No Blocking Patents. To Roivant’s Knowledge, Roivant does not own or control any Patents as of the Effective Date that would be infringed by Arena’s conduct of the development or manufacturing activities contemplated to be conducted under this Agreement as of the Effective Date.
10.4 Disclaimer. EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AND EACH PARTY EXPRESSLY DISCLAIMS ALL SUCH OTHER WARRANTIES, INCLUDING THE IMPLIED WARRANTIES OF MERCHANTABILITY AND OF FITNESS FOR A PARTICULAR PURPOSE OR USE, NON-INFRINGEMENT, VALIDITY AND ENFORCEABILITY OF PATENTS, OR THE PROSPECTS OR LIKELIHOOD OF DEVELOPMENT OR COMMERCIAL SUCCESS OF THE PRODUCT.
43
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
11.1 Indemnification of Arena. Roivant shall defend, indemnify and hold harmless each of Arena, its Affiliates, and its and their respective directors, officers, stockholders and employees (collectively, the “Arena Indemnitees”) from and against any and all losses, liabilities, damages, penalties, fines, costs and expenses (including reasonable attorneys’ fees and other expenses of litigation) (“Losses”) from any claims, actions, suits or proceedings brought by a Third Party, including investigation by a Regulatory Authority, (each, a “Third Party Claim”) against any Arena Indemnitee to the extent arising from, based upon or occurring as a result of: (a) the (i) negligence or willful misconduct of or (ii) violation of Applicable Laws by, in each case ((i) and (ii)), Roivant or any of its Affiliates or its or their respective subcontractors in performing any activity contemplated or permitted by this Agreement; (b) any breach or default by Roivant (or any of its Sub-distributors or its or their Affiliates) of this Agreement or the Quality Agreement; (c) any Product Liability Claim, (d) Roivant’s or any of its Sub-distributors’ or its or their Affiliates’ Commercialization, development, use, handling, storage, administration or other exploitation of Product; (e) subject to Section 9.4, infringement or violation of any Patents or other intellectual property rights of any Third Party relating to any Compound or Product by or behalf of Arena or its Affiliates and occurring after the Effective Date with respect to the performance of Arena’s obligations under this Agreement (other than by the manufacturing activities undertaken by or on behalf of Arena that (i) are generally applicable to pharmaceutical products and not specific to a Compound or Product or (ii) are not requested or approved by Roivant); or (f) Product manufactured according to the Product Warranty by or on behalf of Arena or its Affiliates (including by a Third Party contractor of Arena or its Affiliates); except, in each case, that the foregoing indemnification obligations shall not apply to the extent any such Third Party Claim falls within the scope of the indemnification obligations of Arena set forth in Section 11.2, as to which Third Party Claim each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such Third Party Claim.
11.2 Indemnification of Roivant. Arena shall defend, indemnify and hold harmless each of Roivant, its Affiliates, and its and their respective directors, officers, stockholders and employees (collectively, the “Roivant Indemnitees”) from and against any and all Losses from any Third Party Claims against any Roivant Indemnitee to the extent arising from, based on or occurring as a result of: (a) the (i) negligence or willful misconduct of or (ii) violation of Applicable Laws by, in each case ((i) and (ii)), Arena or any of its Affiliates or its or their respective subcontractors in performing any activity contemplated or permitted by this Agreement (for clarity, other than related to Product Liability); or (b) any breach or default by Arena (or any of its Affiliates) of this Agreement or the Quality Agreement, including failure of any Product manufactured by or behalf of Arena or its Affiliates (including by a Third Party contractor of Arena or its Affiliates) to comply with the Product Warranty; except, in each case, that the foregoing indemnification obligations shall not apply to the extent any such Third Party Claim falls within the scope of the indemnification obligations of Roivant set forth in Section 11.1, as to which Third Party Claim each Party shall indemnify the other Party to the extent of its liability with respect to the Losses applicable to such Third Party Claim.
11.3 Procedure.
(a) A Party that intends to exercise its rights to defense, indemnity or hold harmless under this Article 11 (the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of any Third Party Claim in respect of which the Indemnitee intends to exercise such rights. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim shall only relieve the Indemnitor of its obligations under this Article 11 if and to the extent the Indemnitor is actually prejudiced thereby. The Indemnitee shall provide the Indemnitor with reasonable assistance, at the Indemnitor’s expense, in connection with the defense of the Third Party Claim. The Indemnitor shall have the right to assume and conduct the defense of the Third Party Claim with counsel of its choice. The Indemnitee may participate in and monitor such defense with counsel of its choice, which shall be at its own expense. The Indemnitor shall not settle any Third Party Claim without the prior written consent of the Indemnitee, not to be unreasonably conditioned, withheld or delayed, unless the settlement involves only the payment of money by the Indemnitor and does not involve any admission of liability or wrongdoing on the part of any Arena Indemnitees or Roivant Indemnitees, as applicable. So long as the Indemnitor is defending the Third Party Claim, the Indemnitee shall not settle such Third Party Claim without the prior written consent of the Indemnitor, unless Indemnitee releases Indemnitor for all liability for such settlement.
(b) The assumption of a defense by the Indemnitor shall not be deemed an admission that the Indemnitor has an obligation to defend, indemnify or hold harmless an Arena Indemnitee or Roivant Indemnitee, as applicable, from and against any Loss from a Third Party Claim. If the Indemnitor assumes and conducts the defense
44
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
of a Third Party Claim as provided above, and if it is ultimately determined that the Indemnitor was not obligated to indemnify, defend, or hold harmless an Arena Indemnitee or Roivant Indemnitee, as applicable, from and against any Loss from such Third Party Claim, the Indemnitee shall reimburse the Indemnitor for any and all reasonable and verifiable costs and expenses (including attorneys’ fees and costs of suit) and all other Losses incurred by the Indemnitor in connection with such Third Party Claim.
(c) If the Indemnitor does not assume and conduct the defense of a Third Party Claim as provided above, (i) the Indemnitee may defend against such Third Party Claim; provided, that the Indemnitee shall not settle any Third Party Claim without the prior written consent of the Indemnitor, not to be unreasonably conditioned, withheld or delayed and (ii) if it is ultimately determined that the Indemnitor was obligated to indemnify, defend, or hold harmless an Arena Indemnitee or Roivant Indemnitee, as applicable, from and against any Loss from such Third Party Claim, the Indemnitor shall reimburse the Indemnitee for any and all reasonable and verifiable costs and expenses (including attorneys’ fees and costs of suit) and all other Losses incurred by the Indemnitee in connection with such Third Party Claim.
11.4 Insurance. Each Party, at its own expense, shall maintain insurance required by this section with an insurance carrier(s) that has a minimum rating of A.M. Best’s rating of A-7. Each party shall maintain general liability insurance in the minimum amount of [***] per occurrence and [***] in the aggregate, and [***] umbrella coverage. In addition, commencing no later than the first enrollment of a patient in a clinical trial sponsored by Roivant for a Product, Roivant shall maintain clinical and product liability insurance in the minimum amount of [***] per occurrence and in the aggregate: Clinical trial insurance shall be required to be maintained at the same level for [***] years after the last clinical trial for a Product is conducted; product liability insurance shall be maintained at the same level for not less than [***] years after termination of this Agreement. Each Party shall provide the other Party with written notice at least [***] days prior to any cancellation, nonrenewal or material change in the insurance and, commencing no later than the first enrollment of a patient in a clinical trial sponsored by Roivant for a Product, shall name the other Party as an additional insured with respect to such insurance. Each Party shall provide a certificate of insurance evidencing such coverage to the other Party upon request. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Article 11.
12.1 Term. Unless earlier terminated pursuant to this Article 12, this Agreement shall commence on the Effective Date and shall continue in full force and effect on a Product-by-Product and country-by-country basis until the later of (i) expiration of all issued Arena Patents and Program Patents covering such Product in such country and (ii) 12 years after the First Commercial Sale of such Product in such country (such period, the “Initial Term”). Upon expiration of the Initial Term, this Agreement shall continue in full force and effect on a Product-by-Product and country-by-country basis until the Agreement is terminated pursuant to this Article 12 (such period, the “Extended Term” and the Initial Term together with the Extended Term, the “Term”).
12.2 Termination by Mutual Agreement. This Agreement may be terminated by mutual written agreement of the Parties.
12.3 Termination for Convenience. Roivant may terminate this Agreement on a Compound-by-Compound basis or in its entirety upon 90 days’ prior written notice to Arena.
12.4 Termination for Material Breach. This Agreement may be terminated by a Party upon written notice by such Party to the other Party if the other Party is in material breach of this Agreement and has not cured such breach within [***] days (or [***] days with respect to any payment breach) after notice from the terminating Party detailing the specific material breach that is alleged. Any such termination shall become effective at the end of such [***] day (or [***]-day with respect to any payment breach) period unless the breaching Party has cured any such breach prior to the end of such period.
12.5 Termination for Bankruptcy. This Agreement may be terminated by a Party upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other Party; provided, however, in the case of any involuntary bankruptcy proceeding such right to terminate shall only become effective if the Party consents to the involuntary bankruptcy or such proceeding is not dismissed within [***] days after the filing thereof.
45
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
12.6 Termination for Patent Challenge. Arena shall have the right to terminate this Agreement immediately upon written notice to Roivant if Roivant or any of its Affiliates directly or indirectly commences, or knowingly and materially assists or encourages any Third Party to commence or conduct, any interference, observation, inter-partes review, re-examination, opposition or other proceeding with respect to, challenges the validity, enforceability or claim construction (other than disputes under this Agreement between the Parties solely with respect claim coverage for Compounds and Products) of, or opposes any extension of or the grant of a market exclusivity or supplementary protection certificate with respect to, any Arena Patent. Arena shall also have the right to terminate this Agreement immediately upon written notice to Roivant if (a) a Sub-distributor of Roivant directly or indirectly commences, or knowingly and materially assists or encourages any Third Party to commence or conduct, any interference, observation, inter-partes review, re-examination, opposition or other proceeding with respect to, challenges the validity, enforceability or claim construction of, or opposes any extension of or the grant of a market exclusivity or supplementary protection certificate with respect to, any Arena Patent and (b) Roivant does not, promptly after becoming aware of such circumstances, terminate the Sub-distributor’s rights with respect to Compounds and Products if such challenge or other proceeding has not been withdrawn or terminated within [***] days thereafter.
(a) In the event of any dispute, controversy or claim arising from or related to a material breach of this Agreement or termination pursuant to Section 12.4 (in each case, a “Dispute”), the Parties shall attempt to resolve such Dispute in accordance with Section 14.1. If such Dispute is not resolved in accordance with Section 14.1 and a Party wishes to pursue the matter, each such Dispute that is not an Excluded Claim shall be resolved by binding arbitration in accordance with the Rules of Arbitration of the International Chamber of Commerce (“ICC”) as then in effect (the “ICC Rules”) as such rules may be modified by this Section 12.7 or agreement of the Parties, and judgment on the arbitration award may be entered in any court having jurisdiction thereof. The decision rendered in any such arbitration will be final and not appealable, absent manifest error. If either Party intends to commence binding arbitration of such Dispute, such Party shall file a request for arbitration with the ICC and provide written notice to the other Party informing the other Party of such intention and the issues to be resolved, including the amount of damages that the non-breaching Party is entitled to receive if it elects to terminate this Agreement or the amount of damages that the non-breaching Party is entitled to receive if it does not elect to terminate this Agreement. Within [***] days after the receipt of such notice, the other Party may, by written notice to the Party initiating binding arbitration, add any related issues to be resolved.
(b) The arbitration shall be conducted by a panel of three arbitrators experienced in the pharmaceutical business, each of whom shall not be a current or former employee, consultant or director, or a then-current stockholder, of either Party or any of its Affiliates (the “Panel”). Within [***] days after receipt of the original notice of binding arbitration (the “Notice Date”), each Party shall nominate one arbitrator for the ICC’s confirmation (with the right to nominate a replacement arbitrator until an arbitrator nominated by such Party is confirmed by the ICC) and such two arbitrators shall jointly nominate the third arbitrator for the ICC’s confirmation; provided that if the two arbitrators nominated by the Parties are unable or fail to agree upon the third arbitrator within such period, the third arbitrator shall be appointed by the ICC. The place of arbitration shall be New York, New York, USA. The language of the arbitration shall be English.
(c) Within [***] days after the appointment and selection of the Panel, the Parties shall reach an agreement upon and thereafter shall follow the arbitration procedures, including limits on discovery, ensuring that the arbitration will be concluded and the award rendered as expeditiously as possible, but in any event within [***] months from appointment and selection of the Panel. In the event the Parties fail to reach an agreement on procedures, procedures meeting such time limits shall be determined by the Panel and adhered to by the Parties.
(d) All rulings of the Panel shall be in writing and shall be delivered to the Parties within [***] days of the conclusion of the arbitration.
(e) The Panel shall, in rendering its decision, apply the substantive law of the laws of the State of New York, United States, without reference to its conflicts of law principles with the exception of sections 5-1401 and 5-1402 of New York General Obligations Law, and without giving effect to any rules or laws relating to arbitration.
(f) The Panel, in rendering its decision, shall not modify or amend the terms and conditions of this Agreement or determine any issue in a manner that would conflict with the express terms and conditions of this Agreement.
46
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(g) Either Party may apply to the Panel for interim injunctive relief until the arbitration award is rendered or the controversy is otherwise resolved. Either Party also may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any injunctive or provisional relief necessary to protect the rights or property of that Party pending the arbitration award. Subject to Section 11.3 each Party shall bear its own costs and expenses and attorneys’ fees, and the non-prevailing Party shall pay the full costs of the Panel’s fees and any administrative fees of arbitration.
(h) All proceedings and decisions of the Panel shall be deemed Confidential Information of each of the Parties, and shall be subject to Article 8. Except to the extent necessary to confirm or enforce an award or as may be required by Applicable Laws, neither a Party nor any member of the Panel may disclose the existence, content, or results of an arbitration without the prior written consent of both Parties.
(i) In no event shall an arbitration be initiated after the date when commencement of a legal or equitable proceeding based on the Dispute would be barred by the applicable New York statute of limitations.
(j) As used in this Section, the term “Excluded Claim” means a Dispute that concerns (i) the validity, enforceability, claim construction or infringement of a Patent; (ii) any antitrust, anti-monopoly or competition law or regulation, whether or not statutory; or (iii) whether a Party is obligated to indemnify, defend or hold harmless an Arena Indemnitee or Roivant Indemnitee, as applicable, from and against a Third Party Claim under Section 11.1 or Section 11.2, as applicable.
(k) Any relevant time period under this Agreement related to any Dispute, including any cure period with respect thereto, shall be tolled during any dispute resolution proceeding pursuant to this Section 12.7.
13.1 Accrued Obligations. The termination of this Agreement, in whole or part, for any reason shall not release either Party from any liability or obligation that, at the time of such termination, has already accrued to such Party or that is attributable to a period prior to such termination, nor will any termination of this Agreement preclude either Party from pursuing all rights and remedies it may have under this Agreement, at law or in equity, with respect to breach of this Agreement.
13.2 Effects of Termination. If this Agreement is terminated by a Party pursuant to 12.2 (mutual agreement), 12.3 (convenience), 12.4 (material breach), 12.5 (bankruptcy), or 12.6 (patent challenge) the following shall apply to the extent set forth below (in addition to any other rights and obligations under this Agreement with respect to such termination):
(a) Winding-Down of Development Activities. In the event there are any on-going clinical trials or other development work with respect to a Product, Roivant shall wind-down such clinical trials or other development work in an orderly fashion or, at Arena’s election and subject to Section 13.2(c) (and any agreement required under Section 13.2(c)(ii), if applicable), promptly transition such clinical trials or other development work activities to Arena or its designee, including the transfer to Arena of any Development Data then in Roivant’s or its Affiliate’s possession that has not previously been transferred (or developed) by Arena, with due regard for patient safety and the rights of any subjects that are participants in any clinical trials of a Product, and take any actions it deems reasonably necessary or appropriate to avoid any human health or safety problems and in compliance with all Applicable Laws. All expenses incurred from the winding-down or transitioning of the clinical trials or other development work shall be borne by (i) [***] in the event this Agreement is terminated [***]; (ii) [***] in the event this Agreement is terminated [***]; (iii) [***] in the event this Agreement is terminated [***].
47
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(b) Inventory.
(i) If Roivant terminates this Agreement pursuant to Section 12.4 (material breach) or 12.5 (bankruptcy), at Roivant’s election Roivant and its Sub-distributors, and its and their Affiliates, shall have the right, subject to Section 13.2(b)(ii), to sell off any inventory of Product in its or their possession as of the termination date during a sell-off period of [***] days after the effective date of such termination. Any sales of Product by Roivant and its Sub-distributors, and its and their Affiliates, under this Section 13.2(b)(i) shall be taken into account in calculating Net Sales for purposes of calculating Product Purchase Price under Section 7.3. For clarity, Roivant and its Sub-distributors, and its and their Affiliates, shall not be the sole distributors to sell Product upon the effective date of any termination of this Agreement.
(ii) Upon termination of this Agreement, except in the case of termination by Roivant pursuant to Section 12.4 (material breach) or 12.5 (bankruptcy), Arena shall have the right, but not obligation, on written notice to Roivant, to repurchase from Roivant and its Sub-distributors, and its and their Affiliates, quantities of Product remaining in inventory as of the effective date of such termination of this Agreement at the applicable Initial Product Purchase Price paid by Roivant for such Product.
(iii) If Roivant elects not to sell off its remaining inventory of Product in accordance with clause (i) above and provides written notice thereof to Arena within [***] days after the effective date of such termination, Arena shall have the right, but not the obligation, on written notice to Roivant, to repurchase from Roivant and its Sub-distributors, and its and their Affiliates, quantities of Product remaining in inventory as of the effective date of such termination of this Agreement (or, if later, as of the date of receipt by Arena of such notice from Roivant that it elects not to sell of its remaining inventory) at the applicable Initial Product Purchase Price paid by Roivant for such Product.
(i) In the event this Agreement is terminated by Roivant pursuant to Section 12.4 (material breach) or 12.5 (bankruptcy), then Roivant shall maintain [***] any right, title or interest in or to any Program Patents, Program Know-How, Product Trademarks or any Regulatory Filings for Products.
(ii) In the event this Agreement is terminated, except in the case of termination by Roivant pursuant to Section 12.4 (material breach) or 12.5 (bankruptcy), then upon Arena’s written request (which request may be given upon notice of termination but in any event prior to the effective date of such termination), promptly after the effective date of such termination, Roivant grants to Arena [***]:
(A) [***] license to Arena (with the right to sublicense [***] to the extent pertaining to Products) under all Program Patents and Program Know-How solely to develop, make, use, sell and commercialize Products. Promptly after Arena’s written request, Roivant shall [***].
(B) an assignment and transfer to Arena or its designees (or to the extent not so assignable, Roivant shall take all reasonable actions to make available to Arena or its designee the benefits) of (i) all Regulatory Filings (including INDs, NDAs and Regulatory Approvals) for Products, including any such Regulatory Filings made or owned by Roivant or any of its Sub-distributors, and its and their Affiliates, and (ii) all Product Domain Names. Promptly after Arena’s written request, Roivant shall provide Arena with a complete copy of all Regulatory Filings assigned (or made available), as well as copies of all correspondence with Regulatory Authorities not already provided to Arena, pertaining to Products.
(C) an assignment and transfer to Arena or its designees (or to the extent not so assignable, Roivant shall take all reasonable actions to make available to Arena or its designee the benefits) of all Product Trademarks in connection with Arena’s commercialization of the Product (including all registrations thereof and the associated good will). Promptly after Arena’s written request, Roivant shall [***] and, if requested by Arena, Roivant will [***].
(iii) Upon any termination of this Agreement, Roivant promptly shall transfer to Arena all files [***] relating to the Arena Patents, and shall execute and deliver all documents and instruments reasonably requested by Arena to effectuate the foregoing.
48
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(d) Commercialization Transition. In the event this Agreement is terminated, except in the case of termination by Roivant pursuant to Section 12.4 (material breach) or 12.5 (bankruptcy), Roivant shall, at Arena’s written request, and subject to any agreement required under Section 13.2(c)(ii), if applicable, use Commercially Reasonable Efforts to cooperate with Arena or its designee to effect a smooth and orderly transition of the Commercialization of Products following the effective date of any other termination of this Agreement.
(e) Customer Agreements. Upon the completion of the rights and obligations defined in this Section 13.2, at the written request of Arena, Roivant shall assign to Arena or its designee any Third Party distribution agreements that solely relate to Products, to the extent permitted under each such distribution agreement. In the event such assignment is not requested by Arena or is not permitted under any distribution agreement, then the rights of such Third Party with respect to each Product shall terminate upon termination of Roivant’s rights with respect thereto. Roivant shall use its good faith efforts to include provisions requiring compliance with the foregoing provision in the agreements with applicable Third Parties. Notwithstanding the foregoing, in the event that Roivant terminates this Agreement pursuant to Section 12.4 (material breach) or 12.5 (bankruptcy), Roivant shall have no obligations under this Section 13.2(e) unless and until the Parties agree upon commercially reasonable terms with respect to Roivant’s assignment to Arena or its designee of the Third Party distribution agreements that solely relate to Products.
13.3 Return of Confidential Information. Upon termination of this Agreement, each Party shall promptly return to the other Party, or delete or destroy, all relevant records and materials in such Party’s possession or control containing Confidential Information of the other Party; provided, that such Party may keep one copy of such materials for archival purposes only subject to continuing confidentiality and non-use obligations, and provided further all preclinical and clinical data provided by Arena to Roivant under this Agreement will be returned to Arena and deemed to be the Confidential Information of Arena (notwithstanding Section 8.1).
13.4 Purchase of Binding Order. Upon the termination of this Agreement, the Parties will discuss and negotiate in good faith and agree on the equitable division between the Parties of costs associated with any Product that is the subject of any then-pending Order Commitment that has not been otherwise fulfilled by Arena.
13.5 Survival. Upon termination of this Agreement, all rights and obligations of the Parties under this Agreement shall terminate, except those described in the following Articles and Sections: Sections 3.3(b), 3.6 (last sentence only), 3.7 (penultimate sentence only), 3.11(b), 5.4, 5.5 (for Sections 5.4 and 5.5, solely with respect to any inventory sold by Roivant, its Sub-distributors or its or their Affiliates, after termination of this Agreement pursuant to Section 13.2(b)(i)), 5.6, 5.8 (for 5.6 and 5.8, solely to the extent related to Products sold by Roivant its Sub-distributors or its or their Affiliates), 6.9, 6.10, 6.11 (for 6.9, 6.10, and 6.11, solely with respect to Finished Product delivered prior to termination), 7.3 (with respect to Product delivered prior to termination only), 7.4, 7.5, 7.6, 7.7, 7.8, Article 8, 9.1, 10.4, 11.1, 11.2, 11.3, 11.4 (to the extent provided therein), 12.7, Article 13, Articles 14, 15.2, 15.3, 15.4(b) (last sentence only), 15.5, 15.6, 15.7, 15.8, 15.9, 15.10, 15.11, 15.12, 15.13, 15.14, 15.15, 15.16, 15.17 and 15.18.
49
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
14.1 Dispute Resolution Process. The Parties recognize that disputes as to certain matters may from time to time arise during the Term that relate to interpretation of a Party’s rights or obligations hereunder or any alleged breach of this Agreement. If the Parties cannot resolve any such dispute within [***] days after written notice of a dispute from one Party to the other, either Party may, by written notice to the other Party, have such dispute referred to the Senior Executives. The Senior Executives shall negotiate in good faith to resolve the dispute within [***] days. During such period of negotiations, any applicable time periods under this Agreement shall be tolled. If the Senior Executives are unable to resolve the dispute within such time period, except any Dispute required to be arbitrated pursuant to Section 12.7, either Party may pursue any remedy available to such Party at law or in equity, subject to the terms and conditions of this Agreement. Notwithstanding anything in this Article 14 to the contrary, Arena and Roivant shall each have the right to apply to any court of competent jurisdiction for appropriate injunctive or provisional relief, as necessary to protect its rights or property.
14.2 Governing Law; Litigation; Exclusive Venue and Service. This Agreement and all questions regarding its existence, validity, interpretation, breach or performance, shall be governed by, and construed and enforced in accordance with, the laws of the State of New York, United States, without reference to its conflicts of law principles with the exception of sections 5-1401 and 5-1402 of New York General Obligations Law. Subject to Section 12.7, the Parties hereby irrevocably and unconditionally consent to the exclusive jurisdiction of the courts of the State of New York and the United States District Court for the Southern District of New York for any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement, and agree not to commence any action, suit or proceeding (other than appeals therefrom) related thereto except in such courts. Subject to Section 12.7, the Parties further hereby irrevocably and unconditionally waive any objection to the laying of venue of any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement in the courts of the State of New York or in the United States District Court for the Southern District of New York, and hereby further irrevocably and unconditionally waive and agree not to plead or claim in any such court that any such action, suit or proceeding brought in any such court has been brought in an inconvenient forum. Each Party further agrees that service of any process, summons, notice or document by registered mail to its address set forth in Section 15.9 shall be effective service of process for any action, suit or proceeding brought against it under this Agreement in any such court. Application of the United Nations Convention on Contracts for the International Sale of Goods is specifically excluded from this Agreement.
15.1 Force Majeure. If the performance of any part of this Agreement by a Party (other than making payment when due) is prevented, restricted, interfered with or delayed by any reason or cause beyond the reasonable control of such Party (including: fire, flood, volcano, embargo, power shortage or failure, acts of war, insurrection, riot, terrorism, strike, lockout or other labor disturbance, shortage of raw materials, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, or storm or like catastrophe, acts of God or any acts, omissions or delays in acting of the other Party or the other Party’s Affiliates) or by compliance with any injunction, law, order, proclamation, regulation, ordinance, demand or requirement of any government or of any subdivision, authority or representative of any such government (including changes in the requirements of a Regulatory Authority), whether or not it is later held to be invalid, except to the extent any such injunction, law, order, proclamation, regulation, ordinance, demand or requirement operates to delay or prevent the non-performing Party’s performance as a result of any breach by such Party or any of its Affiliates of any term or condition of this Agreement or the Quality Agreement (an event of “Force Majeure”), the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such Force Majeure event; provided, that the affected Party shall use good faith diligent efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed or it is otherwise able to perform its obligations.
(a) Notification. If either Party becomes aware that such an event of Force Majeure has occurred, is imminent or likely, it shall immediately notify the other Party.
50
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
(b) Keeping the Other Informed. The Party subject to an event of Force Majeure shall keep the other Party informed as to the progress of overcoming or avoiding the effects of such an event of Force Majeure and of recommending performing the affected obligation.
15.2 Waiver of Breach. Any condition or term of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof. No such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the waiving Party. No delay or waiver by either Party of any condition or term of this Agreement in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term of this Agreement. Notwithstanding anything to the contrary in this Section, where Roivant’s approval is required pursuant to this Agreement with respect to [***], and such approval is provided by Roivant, then to the extent that [***] of this Agreement, Roivant shall [***] in this Agreement [***].
15.3 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to perform all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
(a) To the extent that this Agreement imposes obligations on Affiliates of a Party, such Party agrees to cause its Affiliates to perform such obligations. Either Party may contract with one or more of its Affiliates to perform its obligations hereunder; provided, that the Parties shall remain liable hereunder for the prompt payment and performance of all of their respective obligations hereunder.
(b) Each Party may subcontract its obligations under this Agreement subject to the provisions of this Agreement; provided, that: (i) none of the other Party’s rights hereunder are materially diminished or otherwise materially adversely affected as a result of such subcontracting; (ii) the subcontractor undertakes in writing reasonable and customary obligations of confidentiality and non-use no less stringent than those set forth in this Agreement; (iii) the subcontractor does not have the right to further subcontract such obligation unless agreed by the other Party; (iv) the applicable agreement with such subcontractor assigns all Know-How developed by such subcontractor under the applicable agreement to Roivant or its designee, and requires that all employees of such subcontractor be under written obligation to assign without any additional compensation all such Know-How to Roivant or its designee, (v) the subcontracting Party shall remain responsible and liable for the performance by any subcontractor of its obligations under this Agreement; and (vi) such permitted subcontracting shall not relieve the subcontracting Party of any liability or obligation under this Agreement, except to the extent satisfactorily performed by such subcontractor. In the event a Party performs any of its obligations under this Agreement through a subcontractor, then such Party shall at all times be fully responsible for the performance and payment of such subcontractor.
15.5 Modification. No amendment or modification of any provision of this Agreement shall be effective unless in a prior writing signed by authorized officers of both Parties. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance, or any other matter not set forth in an agreement in writing and signed by authorized officers of both Parties.
15.6 Severability. In the event any provision of this Agreement is held invalid, illegal or unenforceable in any jurisdiction, to the fullest extent permitted by Applicable Laws, (a) the Parties shall negotiate, in good faith and enter into a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and (b) if the rights and obligations of either Party will not be materially and adversely affected, all other provisions of this Agreement shall remain in full force and effect in such jurisdiction. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
15.7 Entire Agreement. This Agreement (including the Exhibits attached hereto) constitutes the entire agreement between the Parties relating to the subject matter hereof and supersedes and cancels all previous express or implied agreements and understandings, negotiations, writings and commitments, either oral or written, in respect to the subject matter hereof. Each of the Parties acknowledges and agrees that in entering into this Agreement, and the documents referred to in it, it does not rely on, and shall have no remedy in respect of, any statement, representation, warranty or understanding (whether negligently or innocently made) of any Person (whether party to this Agreement or not) other than as expressly set out in this Agreement. Nothing in this clause shall, however, operate to limit or exclude any liability for fraud.
51
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
15.8 Language. The language of this Agreement is English. Any translation of this Agreement in another language shall be deemed for convenience only and shall never prevail over the original English version. All reporting and provision to Arena of documents, including Regulatory Filings and the like, by Roivant shall include English translations of the applicable documents and writings, but provided that source data (e.g., case report forms, hospital records, clinical and office charts, laboratory notes, etc.) need not be translated and may be provided in the original language of the document.
15.9 Notices. Any notice or communication required or permitted under this Agreement shall be in writing in the English language, delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), or sent by internationally-recognized overnight courier to the following addresses of the Parties (or such other address for a Party as may be at any time thereafter specified by like notice):
To Arena: Arena Pharmaceuticals GmbH Xxxxxx Xxxxxxxxxxxx 0 0000 Zofingen Switzerland Facsimile: 41 62 746 7505 Attention: General Manager | To Roivant: Roivant Sciences Ltd. 0 Xxxxxxxxx Xxxxx Xxxxxxxx XX00 Xxxxxxx Facsimile: x0 (000) 000-0000 Attention: Senior Vice President, Finance and Operations |
with a copy to: Arena Pharmaceuticals, Inc. 0000 Xxxxx Xxxxx Xxxxx Xxx Xxxxx, XX 92121 USA Facsimile: (858) 677-0065 Attention: General Counsel | |
Any such notice shall be deemed to have been given: (a) when delivered if personally delivered, (b) on the third day after dispatch if sent by confirmed facsimile, or (c) on the sixth day after dispatch if sent by internationally-recognized overnight courier. Notices hereunder will not be deemed sufficient if provided only between or among each Party’s representatives on the Joint Steering Committee. This Section 15.9 is not intended to govern the day-to-day business communications necessary between the Parties in performing their obligations under this Agreement.
15.10 Assignment. This Agreement shall not be assignable or otherwise transferred, nor may any right or obligation hereunder be assigned or transferred, by either Party to any Third Party without the prior written consent of the other Party, except that either Party may assign or otherwise transfer this Agreement without the consent of the other Party to an Affiliate or to a successor in interest that acquires all or substantially all of the business or assets of the assigning Party to which this Agreement relates, whether by merger, acquisition, sale of stock, sale of assets or otherwise; provided that the successor in interest assumes this Agreement in writing or by operation of law. Subject to the foregoing, this Agreement shall inure to the benefit of each Party, its successors and permitted assigns. Any assignment of this Agreement in contravention of this Section 15.10 shall be null and void.
15.11 No Partnership or Joint Venture. Each Party is an independent contractor under this Agreement. Nothing contained herein shall be deemed to create an employment, agency, joint venture or partnership relationship between the Parties or any of their agents or employees, or any other legal arrangement that would impose liability upon one Party for the act or failure to act of the other Party. The Parties shall operate their own businesses separately and independently and they shall hold themselves out as, act as, and constitute independent contractors in all respects and not as principal and agent, partners or joint venturers. The Parties shall each be responsible for fulfilling their own obligations under this Agreement, and they shall not have control or responsibility over the actions of the other Party. The Parties shall make and receive only such payments as are required under this Agreement, and shall not share in, or participate in, the business operations of the other Party. Neither Party shall have any express or implied power to enter into any contracts or commitments nor to incur any liabilities in the name of, or on behalf of, the other Party, nor to bind the other Party in any respect whatsoever.
52
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
15.12 Interpretation. The captions to the several Articles and Sections of this Agreement are not a part of this Agreement but are included for convenience of reference and shall not affect its meaning or interpretation. In this Agreement: (a) the word “including” shall be deemed to be followed by the phrase “without limitation” or like expression; (b) the singular shall include the plural and vice versa; (c) masculine, feminine and neuter pronouns and expressions shall be interchangeable; (d) except where the context requires otherwise, “or” has the inclusive meaning represented by the phrase “and/or”; and (e) a reference to any agreement includes any supplements and amendments to such agreement. Each accounting term used herein that is not specifically defined herein has the meaning given to it under GAAP consistently applied, but only to the extent consistent with its usage and the other definitions in this Agreement. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction shall be applied against either Party. When this Agreement refers to actions performed or Know-How or intellectual property made, created, or discovered by a Party or its Affiliate under this Agreement, such reference is understood to apply also to actions performed or Know-How or intellectual property made, created, or discovered by one or more employees, consultants, agents or subcontractors (including, in the case of Roivant and its Sub-distributors, and its and their Affiliates) of such Party or its Affiliate.
15.13 References. Unless otherwise specified, (a) references in this Agreement to any Article, Section or Exhibit means references to such Article, Section or Exhibit of this Agreement and (b) references in any section to any clause are references to such clause of such section.
15.14 Counterparts; Electronic Signature Pages. This Agreement may be executed in any number of counterparts each of which shall be deemed an original, and all of which together shall constitute one and the same instrument. This Agreement may be executed by facsimile or other electronic signatures and such signatures shall be deemed to bind each Party as if they were original signatures.
15.15 Limitation of Liability. EXCEPT FOR LIABILITY FOR BREACH OF ARTICLE 8, NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THIS AGREEMENT OR ANY LICENSE GRANTED HEREUNDER; PROVIDED HOWEVER, THAT THIS SECTION 15.15 SHALL NOT BE CONSTRUED TO LIMIT EITHER PARTY’S INDEMNIFICATION OBLIGATIONS UNDER ARTICLE 11. NOTWITHSTANDING ANYTHING TO THE CONTRARY IN THIS AGREEMENT, THE AGGREGATE LIABILITY OF ARENA TO ROIVANT FOR ANY AND ALL CLAIMS AND LOSSES UNDER OR IN CONNECTION WITH THIS AGREEMENT (EXCEPT TO THE EXTENT CAUSED BY [***] SHALL NOT EXCEED THE AGGREGATE AMOUNT PAID BY ROIVANT TO ARENA UNDER THIS AGREEMENT [***].
(a) The Parties acknowledge and agree that the obligations and restrictions set forth in Article 8 are reasonable and necessary to protect the legitimate interests of the other Party and that such other Party would not have entered into this Agreement in the absence of such obligations and restrictions, and that any breach or threatened breach of any provision of Article 8 will result in irreparable injury to such other Party for which there will be no adequate remedy at law. In the event of a breach or threatened breach of any provision of Article 8 the non-breaching Party shall be authorized and entitled to obtain from any court of competent jurisdiction injunctive relief, whether preliminary or permanent, and an equitable accounting of all earnings, profits and other benefits arising from such breach, which rights shall be cumulative and in addition to any other rights or remedies to which such non-breaching Party may be entitled in law or equity. Each Party hereby waives any requirement that the other Party post a bond or other security as a condition for obtaining any such relief. Nothing in this Section 15.16 is intended, or should be construed, to limit either Party’s right to equitable relief or any other remedy for a breach of any other provision of this Agreement.
(b) Arena acknowledges and agrees that Arena’s obligations under Sections 3.9 and 3.10 and Article 6 are each unique and that Roivant would not have entered into this Agreement in the absence of such obligations, and that any breach or threatened breach of Section 3.9 or 3.10 or Article will result in irreparable injury to Roivant for which damages will not be an adequate remedy. Accordingly, Roivant shall be entitled to specific performance of Section 3.9 or 3.10 and Article 6.
15.17 No Benefit to Third Parties. The representations, warranties, covenants and agreements set forth in this Agreement are for the sole benefit of the Parties and their successors and permitted assigns, and they shall not be construed as conferring any rights on any other Persons.
53
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
15.18 Cumulative Rights. Except as expressly provided herein, the Parties’ respective rights under the various provisions of this Agreement shall be construed as cumulative, and no one of them is exclusive of the other or exclusive of any rights allowed by Applicable Laws.
ARTICLE 16
16.1 Generally. Each Party covenants that it shall, and shall cause its Affiliates to, comply with Applicable Laws with respect to performing its obligations or exercising its rights under this Agreement. Each Party shall, and shall cause its Affiliates (and, in the case of Roivant, Sub-distributors), to comply with the Foreign Corrupt Practices Act of 1977, as amended (“FCPA”), and all other applicable anti-corruption laws and regulations, and each Party represents and warrants that it and its Affiliates have (and, in the case of Roivant, that it will insure that its Sub-distributors have) adequate procedures in place to support their compliance with the FCPA and all other applicable anti-corruption laws and regulations.
16.2 Securities Laws. Each of the Parties acknowledges that it is aware that the securities laws of the United States and other countries prohibit any Person who has material non-public information about a publicly listed company from purchasing or selling securities of such company or from communicating such information to any person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities.
16.3 Certain Payments. Each of the Parties acknowledges that it is aware that the United States and other countries have stringent laws that prohibit persons directly or indirectly to make unlawful payments to, and for the benefit of, government officials and related parties to secure approvals or permission for their activities.
[Signature Page Follows]
54
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
ARENA PHARMACEUTICALS GMBH | ROIVANT SCIENCES LTD. | ||||
By: | /s/ Xxxxxx Xxxxxx | By: | /s/ Xxxxxxxx X. Xxxxx | ||
Name: | Xxxxxx Xxxxxx | Name: | Xxxxxxxx X. Xxxxx | ||
Title: | General Manager & Managing Director | Title: | Head, Global Transactions & Risk Management | ||
By: | /s/ Xxxxx Xxxxxx | ||||
Name: | Xxxxx Xxxxxx | ||||
Title: | Head Human Resources | ||||
[Signature Page to Marketing and Supply Agreement]
55
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
EXHIBIT A
Compound Structure
Compound Structure
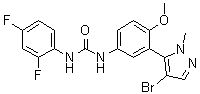
1-[3-(4-Bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-difluoro-phenyl)-urea
56
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
EXHIBIT B
Existing Arena Patents
Existing Arena Patents
[***] PATENT FAMILY
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
57
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
58
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
59
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
]
[***]PATENT FAMILY
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
]
[***] PATENT FAMILY
60
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
61
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] PATENT FAMILY
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
]
[***] PATENT FAMILY
62
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | [***] | [***] |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] PATENT FAMILY
REF. NO. | COUNTRY | SERIAL NO. | PATENT NO. | ISSUE DATE | STATUS |
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
[***] | [***] | [***] | [***] | ||
63
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
EXHIBIT C
Product Domain Names
Domain Names for nelotanserin existing as of the Effective Date:
[***]
64
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
EXHIBIT D
Product for Phase 2 Trials Under Section 3.9(b)
Finished Product: [***]
Placebo: [***]
65
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
EXHIBIT E
All of the following to the extent not previously provided to Roivant:
[***]
66
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
EXHIBIT F
IND Transfer Letters
Arena Letterhead
May [__], 2015
[ADDRESS]
Re: IND No. 73405 ([***]);
• | Notice of IND Transfer of Ownership |
Dear Dr. [__],
Arena Pharmaceuticals, Inc. at 6100 Xxxxx Xxxxx Xxxxx, Xxx Xxxxx, XX 00000 xereby transfers ownership of IND No. 73405, including any and all rights and responsibilities thereunder, to Roivant Sciences Ltd. (Clarendon House, 2 Xxxxxx Xxxxxx, Xxxxxxxx XX00, Xxxxxxx) effective as of 12 Noon on May [__], 2015. Roivant Sciences Ltd., agrees to accept the transfer of ownership of all rights to this application and accepts all Sponsor responsibilities attendant to IND No. 73405 as described in 21 CRF 312 Part D.
Effective as of May [__], 2015, Roivant Sciences Ltd.’s US Agent and the regulatory contact for IND No. 73405 is provided below:
Roivant Sciences, Inc.
Attn: Xxxxxxx Xxxxxxx, PharmD
SVP, Clinical Research
1400 Xxxxxxxx, 0xx Xxxxx
Xxx Xxxx, XX 00000 XXX
(000) 000-0000
xxxx.xxxxxxx@xxxxxxx.xxx
This is an electronic submission and has been scanned for computer viruses using Symantec Anti-virus software. If you have technical questions on the electronic submission, please contact [Name], [Title] at [Telephone] ([email]).
Sincerely,
[Name]
[Title]
[Arena Pharmaceuticals, Inc.]
[Address and contact info]]
67
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.
[ROIVANT LETTERHEAD]
[May [__], 2015
[ADDRESS]
Re: IND No. 73405 ([***]);
• | Notice of Acceptance of IND Transfer of Ownership |
Dear Dr. [__]:
Please reference IND No. 73405 ([***]), submitted on May [__], 2015. In that submission, Arena Pharmaceuticals, Inc. provided notice to the FDA that ownership of the IND was being transferred from Arena Pharmaceuticals, Inc. to Roivant Sciences Ltd.
This submission confirms the transfer of ownership of IND No. 73405 from Arena Pharmaceuticals, Inc. to Roivant Sciences Ltd., effective as of 12 Noon on May [__], 2015. Roivant Sciences Ltd., hereby accepts all rights to this application and accepts all Sponsor responsibilities attendant to IND No. 73405 as described in 21 CRF 312 Part D. We further state that we have received a complete copy of the IND application, including records that are required to be reported and kept under 21 CFR 312.33
Roivant Sciences Ltd. company information is given below:
Roivant Sciences Ltd.
Clxxxxxxx Xxxxx
0 Xxxxxx Xxxxxx
Xxxxxxxx XX00
Xxxxxxx
Effective May [__], 2015, Roivant Sciences Ltd.’s US Agent and regulatory contact for IND No. 73405 is given below:
Roivant Sciences, Inc.
Attn: Xxxxxxx Xxxxxxx, PharmD
SVP, Clinical Research
1400 Xxxxxxxx, 0xx Xxxxx
Xxx Xxxx, XX 00000 XXX
(000) 000-0000
xxxx.xxxxxxx@xxxxxxx.xxx
[If you have questions or require additional information regarding this submission, please do not hesitate to contact the Roivant Sciences Ltd.’s US agent and regulatory contact listed above using their phone number and e-mail address.
This submission is being provided electronically via the Electronic Submission Gateway (ESG). Please see the “Guide to FDA Reviewer” for complete details regarding the electronic submission. If you have technical questions on the electronic submission, please contact the Roivant Sciences Ltd.’s regulatory contact listed above using their phone number and e-mail address.
Xxxxxxxx Romeo Authorized Signatory For and On Behalf of Roivant Sciences Ltd. | ||
Trade secret and/or confidential commercial information contained in this submission is exempt from public disclosure to the full extent provided under law.
68
*** This portion has been redacted pursuant to a confidential treatment request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. A complete copy of this document has been filed separately with the Securities and Exchange Commission.

