THIS DOCUMENT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST PURSUANT TO RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED. REDACTED MATERIAL IS MARKED WITH [XXXXXXXXX] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES...
EXHIBIT 10.6
THIS DOCUMENT HAS BEEN REDACTED AND IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST PURSUANT TO RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED. REDACTED MATERIAL IS MARKED WITH [XXXXXXXXX] AND HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
4 December 2012
Dated 4 December 2012
By and Between
DOMAIN RUSSIA INVESTMENTS LIMITED
And
MARINUS PHARMACEUTICALS, INC.
THIS TECHNOLOGY TRANSFER AGREEMENT (the “Agreement”) is dated as of 4 December 2012 (the “Effective Date”), by and between Domain Russia Investments Limited, a private limited company incorporated and existing under the laws of England and Wales with registration number 7899075, having its registered office at The Broadgate Tower, Third Floor, 20 Primrose Street, City of Xxxxxx, XX0X 0XX, Xxxxxx Xxxxxxx (“DRI”), and Marinus Pharmaceuticals, Inc., a corporation organized under the laws of the State of Delaware, USA, and having its place of business at 00 Xxxxxxxx Xxxx Xxxxx, Xxxxxxxx, Xxxxxxxxxxx 00000, XXX (“Company”). DRI and Company may each be referred to herein as a “Party” or, collectively, as “Parties.”
WHEREAS, Company is a development-stage company engaged in the discovery and development of certain Product(s) (defined below), including the Covered Products (defined below); and
WHEREAS, Company, on the one hand, and one or more funds affiliated with DRI, including RMI Investments S.á x.x. (“RMI”), a private limited liability company organized under the laws of Luxemburg, with an address at 00 Xxx Xxx Xxxxxxxxxx X-0000 Xxxxxxxxx and certain other investors (collectively the “Investors”), on the other hand, have entered into a Series C Preferred Stock Purchase Agreement dated as of 4 December 2012 (the “Stock Purchase Agreement”), pursuant to which Company will issue and sell, and the Investors will purchase, shares of capital stock of Company with an aggregate purchase price of up to approximately twenty-one million two hundred seventy-four thousand seven hundred ninety-three U.S. dollars (US$21,274,793) (the “Investment”); and
WHEREAS, in connection with the Investment and the other transactions relating thereto, DRI desires to acquire exclusive rights from Company in the Field in the Territory in order to transfer such exclusive rights to NovaMedica (defined below), and Company wishes to exclusively transfer to NovaMedica, through DRI, the right to Develop and Commercialize (defined below) Covered Products throughout the Territory (as defined below); and
WHEREAS, immediately following execution of this Agreement DRI shall enter into two Assignment and Assumption Agreements with NovaMedica, pursuant to which DRI shall assign all rights and obligations under this Agreement and the Sublicense Agreement (defined below), respectively, to NovaMedica, and NovaMedica shall accept such assignment of rights and agreed to assume such obligations. The term “Transferee,” as used in this Agreement (a) shall refer to DRI immediately upon execution and delivery of this Agreement, and (b) from and after DRI’s assignment to NovaMedica, pursuant to Assignment and Assumption Agreement, of the rights transferred to DRI by Company pursuant this Agreement, shall refer to DRI and/or NovaMedica, as applicable in the specific context.
NOW, THEREFORE, in consideration of the various promises and undertakings set forth herein, and other good and valuable consideration the receipt and sufficiency of which is hereby acknowledged, the Parties agree as follows:
Unless otherwise specifically provided herein, the following capitalized terms used in the Agreement its appended Schedules shall have the following meanings:
1.1 “Acquirer” means, in a Change of Control (as defined below) with respect to Company, NovaMedica or a Permitted Transferee, the acquiring entity in such Change of Control.
1.2 “Affiliate” shall mean, in relation to any Party, NovaMedica or any Permitted Transferee, any Person who directly or indirectly controls, is controlled by, or is under common control with, such Party, NovaMedica or Permitted Transferee. For purposes of this definition of Affiliate, “control” means: (a) ownership of more than fifty percent (50%) of the voting rights, shares or other equity interest of the applicable entity; and/or (b) the power to direct or cause direction of all aspects of the management and policies of the applicable entity (directly or indirectly, whether through ownership of securities or partnership or other ownership interests, by contract or otherwise).
1.3 “Ancillary Agreements” means the Assignment and Assumption Agreement, the Clinical Development and Collaboration Agreement, any Supply Agreement, and the Pharmacovigilance Agreement.
1.4 “Assigned IP” means Company Patents in the Territory assigned or to be assigned to the Transferee pursuant to this Agreement, including Company Improvement Patent Rights. The Assigned IP as of the date of this Agreement is listed in Schedule 1.1 attached hereto.
1.5 “Assignment and Assumption Agreements” means those certain agreements between DRI and NovaMedica referenced above in the “Recitals” section.
1.6 “Clinical Development and Collaboration Agreement” shall have the meaning set forth in Section 5.1, below.
1.7 “Clinical Trial” means an a adequate and well-controlled clinical trial, investigation, or study in human subjects (as defined in 21 CFR 314.126) that has been approved by a Regulatory Authority and is designed to measure the safety and/or efficacy of a Covered Product in humans.
1.8 “Change of Control” means (a) a transaction or series of related transactions that results in the sale, exclusive license in the US or other major country or other disposition of all or substantially all of a Party’s assets; or (b) a merger or consolidation in which a Party is not the surviving corporation or in which, if a Party is the surviving corporation, the shareholders of such Party immediately prior to the consummation of such merger or consolidation do not, immediately after consummation of such merger or consolidation, own stock or other securities of the Party that possess a majority of the voting power of all of the Party’s outstanding stock and other securities and the power to elect a majority of the members of the Party’s board of directors; or (c) a transaction or series of related transactions (which may include a tender offer for a Party’s stock or the issuance, sale, or exchange of stock of a Party) if the shareholders of such Party immediately prior to the initial such transaction do not, immediately after consummation of such transaction or any of such related transactions, own, directly or indirectly through a parent, stock or other securities of the Party that possess a majority of the voting power of all of the Party’s outstanding stock and other securities and the power to elect a majority of the members of the Party’s board of directors; provided, however, that a Change of Control excludes (A) any transaction (or series of related transactions) in which the pre-transaction shareholders of the applicable Party own more than 50% of the outstanding capital stock or equity interests of the surviving or acquiring entity or its parent, and (B) bona fide equity financings of Company by financial investors.
1.9 “Commercialization” or “Commercialize” means any and all activities that relate to the Manufacture, packaging, marketing, promoting, distributing, importing, offering for sale, selling, or
having sold Covered Products and interacting with Regulatory Authorities. Commercialization shall also include conducting Phase IV Studies.
1.10 “Commercially Reasonable Efforts” means, (a) with respect to the efforts to be expended by any Party with respect to any objective, such reasonable, diligent, and good faith efforts not less than a company similar to such Party would devote to accomplish a similar objective under similar circumstances, and (b) with respect to any objective relating to Development or Commercialization of a Covered Product by the Transferee (or a Permitted Transferee), the application by the Transferee (or a Permitted Transferee), consistent with the exercise of its prudent scientific and business judgment, of diligent and good faith efforts and resources to fulfill the obligation in issue, consistent with the level of efforts a company similar to the Transferee (or a Permitted Transferee), as the case may be, would devote to a product at a similar stage in its product life as the Covered Product and having profit potential and strategic value comparable to that of the Covered Product, taking into account the following factors: scientific, development, technical, commercial, and regulatory factors, target product profiles, product labeling, past performance, the regulatory environment and competitive market conditions in therapeutic area safety and efficacy of a subject product, and the strength of its proprietary position, all based on conditions then prevailing. Commercially Reasonable Efforts will not mean that the Transferee, alone or through one or more Permitted Transferees, commits that it will actually accomplish the applicable task.
1.11 Company Improvement Know-How” means an Improvement Controlled by Company other than Company Improvement Patent Rights, whether patentable or not, that is necessary or useful for the use, Development or Commercialization of a Covered Product, or otherwise for the practice of the Assigned IP or Company Patent Rights, or any of them, in the Field and in the Territory.
1.12 “Company Improvement Patent Rights” means any Company Patent Right(s) obtained in the Field and in the Territory during the Term that claims an Improvement.
1.13 “Company Indemnitees” shall have the meaning set forth in Section 10.1, below.
1.14 “Company IP” means Company Patents, Company Know-How, and Company Materials.
1.15 “Company Know-How” means all Know-How, including Company Improvement Know How, that is Controlled by the Company as of the Effective Date or thereafter during the Term of this Agreement that is reasonably necessary or useful in the Development or Commercialization of Covered Product(s) in the Field, but excluding Know-How Controlled by Company pursuant to the License Agreement or any other Third Party Agreement unless and until the Transferee elects to include in the Licensed IP pursuant to Section 2.1(f) of this Agreement.
1.16 “Company Materials” means all Materials Controlled by the Company that are necessary or useful in the Development or Commercialization of the Covered Product(s).
1.17 “Company Patents” or “Company Patent Rights” means any Patent Rights that are Controlled by the Company, including any Improvement Patent Rights, that are necessary or useful for the Development or Commercialization of Covered Products in the Territory, but excluding any Patent Right Controlled by the Company pursuant to the License Agreement or any other Third Party Agreement unless the Transferee elects to include in the Licensed IP pursuant to Section 2.1(f) of this Agreement.
1.18 “Compound” means ganaxolone (3a-hydroxy-3b-methyl-5a-pregnan-20-one), the chemical structure of which is described in Schedule 1.3, in any formulation, the use, Development or
Commercialization of which in the absence of this Agreement, a sublicense to a Permitted Transferee or other ownership, assignment or license of applicable rights, would infringe at least one Valid Claim of the Assigned IP or Company Patent Rights, or that is manufactured or otherwise produced, at least in part, using Company Know-How.
1.19 “Confidential Information” of a Party means such Party’s confidential information relating to its business, operations and products, including but not limited to any technical information, formulae, processes, techniques, preclinical information, toxicology information, clinical, non-clinical, or pre-clinical information, regulatory information, Manufacturing information, formulation information, packaging information, dosing information, dose regimen information, target patient information, marketing information, sales information, pricing information, reimbursement information, Know-How, trade secrets, or inventions (whether patentable or not) that is disclosed to or learned by the other Party in connection with this Agreement. All Company Know-How shall be deemed Confidential Information of the Company.
1.20 “Control” or “Controlled” means, with respect to (a) Patent Rights, (b) Know-How, or (c) Materials, in each case that Company, a Transferee, an Acquirer, or an Affiliate of a Party or an Acquirer owns or has a license or sublicense to such Patent Rights, Know-How, or Materials (and additionally, in the case of Materials, has the right to physical possession of such Materials) and has the ability to assign or grant a license or sublicense to such Patent Rights, Know-How, or Materials as provided for in this Agreement.
1.21 “Cover”, “Covering” or “Covered” means, with respect to a Covered Product, that the Development or Commercialization of such Covered Product would, but for ownership of, or assignment of rights under this Agreement to, the relevant Assigned IP or Company Patent Rights, infringe a Valid Claim of the relevant Company Patent Rights in the country in which the activity occurs.
1.22 “Covered Product” means any pharmaceutical product (including, without limitation, any diagnostic product or therapeutic product) that (a) contains or comprises the Compound in any formulation designed and intended for use in and for the Field, whether or not combined with other compounds, (b) in the absence of this Agreement, a sublicense to the Permitted Transferee or other ownership, assignment or license of applicable rights, would infringe at least one Valid Claim of the Assigned IP or Company Patent Rights, or (c) is manufactured or otherwise produced, at least in part, using Company Know-How.
1.23 “Cure Period” shall have the meaning set forth in Section 11.3(b), below.
1.24 “Development” or “Develop” means the performance of all development activities, including any pre-clinical and clinical development activities, including, without limitation, toxicology, pharmacology, test method development and stability testing, process development, formulation development, quality control development, statistical analysis, Clinical Trials, and manufacturing and regulatory activities or any similar activities that are useful or otherwise required to obtain Regulatory Approval of a Covered Product, including interacting with any Regulatory Authorities regarding the foregoing.
1.25 “Development Disputes” shall have the meaning set forth in Section 12.1, below.
1.26 “Development Support” shall have the meaning set forth in Section 3.4(b), below.
1.27 “EMA” means the European Medicines Agency or any successor agency.
1.28 “Excluded Field” means and includes the treatment of any unpleasant sensory or emotional experience associated with actual or potential tissue damage, or described in terms of such damage, including discomfort, either acute or chronic, caused by a primary lesion or dysfunction in the peripheral or central nervous system (e.g., neuropathic pain, post-herpetic neuralgia, trigeminal neuralgia), phantom pain, back pain, surgical pain, cancer pain, and pain associated with inflammation or damage of any tissues (e.g., muscle, bone, organs, tendons, nerves, and skin) by disease, injury, infection, surgery, or at birth. For avoidance of doubt, the Transferee (or any Permitted Transferee) shall not be permitted hereunder to pursue or obtain any Regulatory Approval or labeling for a Covered Product as an analgesic or anesthetic agent or for the treatment of pain as a result of contractual restrictions imposed on Company by the License Agreement.
1.29 “FDA” means the United States Food and Drug Administration, or a successor federal agency thereto.
1.30 “Field” means any and all prophylactic, palliative, therapeutic, and diagnostic uses for any human or animal disease or condition, other than diseases, disorders and conditions that are in the Excluded Field; provided, however, that during the Term should any Excluded Field limitations imposed on Company by the License Agreement expire or be expanded, the “Field” under this Agreement shall thereafter be expanded on an equivalent basis.
1.31 “First Commercial Sale” means, with respect to a Covered Product in any country in the Territory, the first commercial transfer, or disposition, for value of such Covered Product in such country to a Third Party after all Regulatory Approvals for an NDA (and/or other required approvals) have been obtained in such country.
1.32 “Fixed Payment” has the payment to be paid by DRI to Company pursuant to Section 6.1, below.
1.33 “Fundamental Breach” has the meaning provided in Section 11.3(e), below.
1.34 “Governmental Body” means any: (a) nation, principality, state, commonwealth, province, territory, county, municipality, district, or other jurisdiction of any nature; (b) federal, state, local, municipal, foreign, or other government; (c) governmental or quasi-governmental authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, board, instrumentality, officer, official, representative, organization, unit, body, or entity and any court or other tribunal); (d) multi-national or supranational organization or body; or (e) individual, entity, or body exercising, or entitled to exercise, any executive, legislative, judicial, administrative, regulatory, police, military, or taxing authority or power of any nature.
1.35 “Improvement” means any improvement, alteration, or modification to a Covered Product, Company IP, Assigned IP or Licensed IP or to any prior Improvement that is made, conceived, reduced to practice, discovered, or otherwise discovered after the Effective Date and during the Term, including methods of synthesis, Manufacture, or use, compositions, Know-How, statistically significant or repeatable observations, protocols, inactive ingredients, presentation, means of delivery, dosage, formulation, or analysis, whether or not patentable.
1.36 “IND” means an investigational new drug application filed with the FDA or the equivalent application or filing filed with any equivalent agency or Governmental Body outside the United States (including any supra-national entity such as in the European Union) for approval to commence a Clinical Trial in such jurisdiction.
1.37 “Indication” means a generally acknowledged disease or condition, a significant manifestation of a disease or condition, or symptoms associated with a disease or condition or a risk for a disease or condition. For the avoidance of doubt, all variants of a single disease or condition (whether classified by severity or otherwise) shall be treated as the same Indication.
1.38 “Initial Closing of the Investment Transaction” means Initial Closing under the Stock Purchase Agreement, which is referenced in the “Recitals,” above.
1.39 “Intellectual Property” or “Intellectual Property Rights” or “IP” means, collectively, all intellectual property and similar proprietary rights, including: (i) Patent Rights; (ii) Know-How and non-public business or technical information; (iii) copyrights and copyrightable works; (iv) software, including data files, source code, object code, application programming interfaces, databases and other software-related specifications and documentation; (v) designs; (vi) domain names; and (vii) claims, causes of action, and defenses relating to the enforcement of any of the foregoing; in each of the foregoing cases (i) to (vii), including any registrations or applications to register, and renewals and extensions of, and of the foregoing, with any Governmental Body in any jurisdiction.
1.40 “Joint Commercialization Committee” or “JCC” shall have the meaning as set forth in Section 5.3, below.
1.41 “Joint Development Committee” or “JDC” shall have the meaning as set forth in Section 5.3, below.
1.42 “Joint Invention” means any discovery, invention, innovation, or other technological advance, whether or not patentable, jointly conceived or discovered by at least one Person who is an employee, officer, director, agent, or contractor of Company while acting for or on behalf of Company and at least one Person who is an employee, officer, director, agent, or contractor of the Transferee or a Permitted Transferee.
1.43 “Joint Patent” shall have the meaning as set forth in Section 1(e) of attached Schedule 4.
1.44 “Joint Steering Committee” or “JSC” shall have the meaning as set forth in Section 5.3, below.
1.45 “Know-How” means any scientific or technical information, results and data of any type whatsoever, in any tangible or intangible form whatsoever, that is not in the public domain or otherwise publicly known, including, without limitation, Improvements, discoveries, inventions (whether patentable or not), trade secrets, databases, practices, protocols, regulatory filings, methods, processes, techniques, information concerning reagents and biological and other materials, specifications, formulations, formulae, data (including pharmacological, biological, chemical, toxicological and clinical) analytical, quality control, and stability data), dosing and target patient information, studies and procedures, and Manufacturing process, and Covered Product Development information, results and data, whether or not patentable, in each of the foregoing cases to the extent not claimed or disclosed in any Patent Rights; provided, however, that “Know How” excludes Patent Rights and Materials.
1.46 “Knowledge” means, with respect to a matter that is the subject of a given representation or warranty, the knowledge, information or belief that any officer, director, or other employee of a Party who would reasonably be expected to have knowledge of the matter in question, has, or should reasonably be expected to have, with respect to the relevant subject matter. “Knowingly” means with Knowledge.
1.47 “Law” or “Laws” means all applicable laws, statutes, rules, regulations, ordinances and other pronouncements having the binding effect of law of any Governmental Body.
1.48 “License Agreement” means Amended and Restated Agreement dated May 23, 2008, by and between Purdue Neuroscience and Company, the true and accurate copy of which has been delivered to DRI and NovaMedica.
1.49 “Licensed IP” means all Company IP Controlled by Company other than by ownership or assignment and that is licensed or sublicensed or to be licensed or sublicensed by Company to Transferee, including Company Improvement Know-How, pursuant to this Agreement, other than IP subject to the License Agreement. The Licensed IP as of the date of this Agreement is as indicated in Schedule 1.2 attached to this Agreement.
1.50 “Losses” shall have the meaning as set forth in Section 10.1, below.
1.51 “Manufacture” or “Manufacturing” shall mean all activities related to the manufacturing of the Compound or a Covered Product, or any ingredient thereof, including but not limited to test method development and stability testing, formulation, process development, manufacturing scale-up, manufacturing quality assurance/quality control development, quality control testing (including in-process release and stability testing), packaging, release of product or any component or ingredient thereof, quality assurance activities related to manufacturing and release of product, and regulatory activities related to all of the foregoing.
1.52 “Materials” means chemical materials.
1.53 “NDA” means a New Drug Application filed pursuant to the requirements of the FDA, a Biologics License Application filed pursuant to the requirements of the FDA, and any equivalent application filed in any country outside of the Territory, together, in each case, with all additions, deletions, or supplements thereto.
1.54 “NovaMedica” refers to that certain entity incorporated in Russia as NovaMedica LLC, a limited liability company organized under the laws of the Russian Federation with an address 107113, xxxx. 00, Xxxxxxxxxxxxx Xxx Xxxxxx, Xxxxxx, Russian Federation.
1.55 “Out-of-Pocket Expenses” means documented reasonable expenses actually paid by Company to any Third Party that is either (i) not an Affiliate of Company, or (ii) is an Affiliate of Company where such payment is limited to reimbursing such Affiliate for expenses actually paid by such Affiliate to a Third Party that is not, in turn, an Affiliate.
1.56 “Patent Right” means: (a) an issued or granted patent, including any extension, supplemental protection certificate, registration, confirmation, reissue, reexamination, extension, or renewal thereof; (b) a pending patent application, including any continuation, request for continued examination, divisional, continuation-in-part, substitute, or provisional application thereof; (c) all pending or issued counterparts or foreign equivalents of any of the foregoing; or (d) a patent application in preparation.
1.57 “Permitted Transferee” means an assignee, licensee, or sublicensee of a Transferee (as permitted by Section 2.3, below) of any the rights assigned to or licensed by Company to the Transferee under Section 2.1 below, pursuant to a written instrument meeting the requirements of this Agreement, including but not limited to Sections 2.1(d), 2.3, 2.5 and 9.4 hereof.
1.58 “Person” means any natural person, corporation, limited liability company, firm, business trust, joint venture, association, organization, company, partnership, or other business entity, or any government or agency or political subdivision thereof.
1.59 “Pharmacovigilance Agreement” refers to that form of agreement set forth in Schedule 6 hereto.
1.60 “Prosecution and Maintenance” or “Prosecute and Maintain” means, with regard to a Patent Right, the preparing, filing, prosecuting, and maintenance of a patent or patent application, as well as re-examinations, reissues, requests for patent term extensions, and the like, together with the conduct of interferences, the initiation and defense of oppositions and other similar proceedings with respect to the particular patent or patent application.
1.61 “Purdue Neuroscience” means Purdue Neuroscience Company, a partnership formed under the laws of the State of Delaware.
1.62 “Regulatory Approval” means, with respect to a particular Covered Product, any and all approvals, licenses, registrations, or authorizations of a relevant Regulatory Authority (including Price Approvals) necessary for the Development, Manufacture and/or Commercialization of such Covered Product in a particular country or jurisdiction. For the avoidance of doubt, Regulatory Approval in the United States shall be deemed to occur upon approval of the applicable NDA (as defined above) in the United States, and shall not be construed to require a determination or approval of reimbursements of any type.
1.63 “Regulatory Authority” means (a) the FDA, (b) the EMA, (c) any successor or equivalent of the foregoing; or (d) any regulatory body with regulatory authority or oversight over pharmaceutical or biotechnology products in any other jurisdiction anywhere in the world, or whose review or approval is necessary for the Development, Manufacture and/or Commercialization of a Covered Product in a particular country or jurisdiction in the Territory.
1.64 “RMI” shall have the meaning set forth in the Recitals section.
1.65 “Rospatent” shall have the meaning set forth in Section 2.3(b), below.
1.66 “Second Closing of the Investment Transaction” means any subsequent Closing under the Stock Purchase Agreement, which is referenced in the “Recitals,” above.
1.67 “Sublicense Agreement” refers to that certain Sublicense Agreement in form and substance mutually satisfactory to the Parties and approved by Purdue Neuroscience to be entered into by and between Company and NovaMedica as promptly as practicable after the written request of NovaMedica to Company, pursuant to which Company will sublicense to NovaMedica rights to certain Purdue Neuroscience know-how licensed to Company under the License Agreement in accordance with the terms and provisions this Agreement.
1.68 “Supply Agreement” means an agreement to manufacture (or cause to be manufactured) and supply and sell the Compound and/or Covered Product(s) to NovaMedica or any Permitted Transferee for Development or Commercialization in the Territory, as set forth in Section 3.5, below.
1.69 “Term” shall have the meaning set forth in Section 11.1, below.
1.70 “Territory” means all countries listed in Schedule 2 hereto.
1.71 “Third Party” means any Person other than Company, Transferee a Permitted Transferee or an Affiliate of Company, Transferee or a Permitted Transferee.
1.72 “Third Party Agreements” means the License Agreement, together with all other future agreements, if any, between Company and any Third Party pursuant to which the Company in-licenses rights under Intellectual Property rights owned or Controlled by a Third Party that are necessary or useful for Development or Commercialization of a Covered Product in the Territory in the Field.
1.73 “Transfer” has the meaning defined in Section 2.3(a), below.
1.74 “Transferee” has the meaning defined in the Recitals section of this Agreement.
1.75 “Transfer Registration” has the meaning defined in Section 2.3(b), below.
1.76 “Transferee Improvement Know-How” means an Improvement Controlled by a Transferee or a Permitted Transferee other than Transferee Improvement Patent Rights, whether patentable or not, that is necessary or useful for the use, Development or Commercialization of a Covered Product, or otherwise for the practice of the Assigned IP or Company Patent Rights, or any of them, in the Field.
1.77 “Transferee Improvement Patent Rights” means any Patent Right(s) Controlled by a Transferee or a Permitted Transferee that claims an Improvement.
1.78 “Valid Claim” means (a) a claim in the Territory of an issued and unexpired patent that has not lapsed or been revoked, abandoned, or held unenforceable or invalid by a final decision of a court or governmental or supra-governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and which has not been disclaimed, denied or admitted to be invalid or unenforceable through reissue, reexamination or disclaimer or otherwise or (b) a claim in the Territory of a pending patent application that has not been abandoned or finally rejected without the possibility of appeal or refiling and that has been pending for less than seven (7) years from its priority date.
1.79 Interpretation. The captions and headings to this Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement. Unless specified to the contrary, references to Articles, Sections, or Schedules mean the particular Articles, Sections or Schedules to this Agreement and references to this Agreement include all Schedules hereto. Unless context otherwise clearly requires, whenever used in this Agreement: (i) “include” or “including” shall be construed as if followed by the words “but not limited to” or “without limitation” or words of similar import; (ii) the word “or” shall be construed as the inclusive meaning identified with the phrase “and/or;” (iii) provisions that require that a Party or the Parties to “agree,” “consent” or “approve” or the like shall require that such agreement, consent, or approval be specific and in writing, whether by written agreement, letter, written approval of minutes or otherwise; and (iv) references to any specific Law or article, section, or other division thereof shall be deemed to include then-current amendments thereto or any replacement Law thereof. This Agreement was prepared in the English language, which language shall govern the interpretation of, and any dispute regarding, the terms of this Agreement.
2.1 Transfer of Rights to Transferee.
(a) Licensed IP. Subject to the terms and conditions of this Agreement, Company hereby provides to Transferee an exclusive (even as to Company), fully paid up and royalty free license (except for the Fixed Payment), which shall be irrevocable except as set forth in Section 11 below, and including the right to grant and authorize sublicenses (subject to the provisions of the License Agreement) and other Third Party Agreement and Section 2.1(f), below under the Licensed IP to Develop and Commercialize Covered Products in and for the Field solely in the Territory.
(b) Assigned IP. Subject to the terms and conditions of this Agreement, Company hereby grants, assigns, and transfers to the Transferee, and the Transferee hereby accepts, all of Company’s right, title, and interest in and to the Assigned IP. For clarity, Company’s obligation to assign shall be limited to only Company IP in the Field in the Territory; Company shall have no obligation to grant, assign, or transfer any right, title, or interest in any Company IP outside of the Territory or outside the Field.
Company shall assign the Assigned IP to the Transferee in accordance with Section 2.2, below. Company shall assist the Transferee and any Permitted Transferee to evidence, record, and perfect the foregoing assignment in accordance with Section 2.2 of this Agreement below.
(c) Non-Commercial Research and Development. Unless expressly permitted by this Section 2.1(c), neither Transferee shall have (and neither Transferee shall transfer to any Permitted Transferee) any right or license to, and shall not, export, or assist with or participate in any way in the exportation of, any assigned, licensed, or sublicensed property, including the Compound or Covered Products, outside the Territory. Notwithstanding the foregoing, nothing in this Agreement shall preclude Transferee or a Permitted Transferee from importing or exporting the Compound or Covered Products to Third Parties outside the Territory solely for non-clinical Development (i.e., Development that is not a Clinical Trial or that otherwise involves administration of the Compound or Covered Product to a human subject, including preclinical studies), but in each case only with the prior written permission of Company, which permission shall not be unreasonably refused, conditioned, delayed or withheld. In the context of academic or university research supported by Transferee or a Permitted Transferee, it is agreed that such research shall be conducted pursuant to a written materials transfer agreement (“MTA”) (i) that specifically prescribes the non-clinical Development to be conducted; (ii) the Transferee or the Permitted Transferee provides only quantities of the Compound or Covered Product sufficient to conduct the research described in the corresponding MTA; (iii) the MTA provides that Company shall have an irrevocable, royalty-free, fully paid-up worldwide exclusive license, including the right to grant and authorize sublicenses, to any discovery, invention, or other technological advance that results from research using a Compound or a Covered Product, for any and all purposes outside the Territory; and (iv) Company (and the successors and assigns of Company) is designated in the MTA as an intended third party beneficiary having the right and authority to independently and without prior notice or permission, enforce all rights of Company pursuant to the applicable MTA.
i. Company Improvements. During the Term, Company shall promptly inform Transferee in writing of any Improvement Controlled by Company, and any such Improvement, Company Improvement Patent Rights, and Company Improvement Know-How directly related to such Improvement in the Field and in the Territory shall then, if the Company has the right to do so, automatically be included within the Company IP and, if Company holds Company Improvement Patent Rights to the respective Improvement, Company shall take the action required by Section 2.2(c) below; otherwise, any Company Improvement Know- How Controlled by Company shall be Licensed IP under this Agreement. Such transfer of Company Improvement Patent Rights and Company Improvement Know How shall require no additional payments by Transferee (or any Permitted Transferee) to Company beyond the payments set forth in Article 6 and the reimbursement of Out-of-Pocket Expenses pursuant to Section 2.2, below, except as expressly applicable under the Sublicense Agreement or any other Third Party Agreement to which the Transferee acquires a sublicense pursuant to Section 2.1(f) below, as to which, in each case, the Transferee shall be fully responsible for the payment of all fees, costs and royalties required thereunder with respect to the sublicense of rights and/or sale of Covered Products in the Territory.
ii. Improvements by Transferee or Permitted Transferee. During the Term the Transferee agrees to inform Company of any Improvement developed by Transferee and each Affiliate and Permitted Transferee, and, to the extent it has the right to do so, the Transferee hereby agrees to provide, or cause the respective Affiliate and/or Permitted Transferee to provide, to Company (or Company’s assignee or other successor) an exclusive, fully paid up, irrevocable, royalty-free license any and all Improvements Controlled by a Transferee and/or any Permitted Transferee solely for the purpose of Developing and/or Commercializing Covered Products anywhere outside the Territory. Furthermore, each Transferee agrees that an agreement with or between each Permitted Transferee, or between Permitted Transferees, shall require that each Permitted Transferee grant to Company (and Company’s assignee or other successor) a royalty-free, fully paid up, irrevocable, exclusive (even as to Transferee and any and all Permitted Transferees) license in and to any and all Improvements Controlled by a Transferee and/or any Permitted Transferee, Transferee Improvement Patent Rights, and Transferee Improvement Know-How anywhere outside of the Territory, with the exclusive right to sublicense or assign in any part of the world outside of the Territory.
(e) Non-competition Covenant. As this Agreement conveys exclusive rights to the Transferee to the Assigned IP and to use Licensed IP for the Development and Commercialization of Covered Products in the Field in the Territory, it is understood and agreed that during any unexpired or non-terminated term of the licenses and sublicenses granted under this Agreement only the Transferee and Permitted Transferees will be permitted to Develop and Commercialize Covered Products in the Field in the Territory, and that, during any unexpired or non-terminated term of the licenses and sublicenses granted under this Agreement, Company will not directly or indirectly use any of the Assigned IP or Licensed IP to Develop or Commercialize any Covered Products in the Field in the Territory, excepting only as is expressly authorized by a written authorization or agreement signed by the Transferee. The Company (its Affiliates, licensees or sublicenses outside the Territory, successors and assignees) will not prevent, directly or indirectly the Transferee and Permitted
Transferees from Development and Commercialization of the Covered Product or Compound in the Field and in the Territory. Further, to enable the Transferee to realize the intended benefits of this Agreement, Company will not engage, directly or indirectly, in any competition within the Territory with the Development or Commercialization of any Covered Product (“non-competition covenant”). After a Change of Control of Company, the Acquirer of Company and any of the Acquirer’s Affiliates shall be bound by this non-competition covenant; provided however that the foregoing non-competition covenant shall not prohibit, limit or restrict such Acquirer or any of the Acquirer’s Affiliates from using, developing, manufacturing, having manufactured, marketing, selling or otherwise commercializing in the Territory any product that is not a Covered Product.
Furthermore, for the avoidance of doubt and to fully realize the territorial restrictions intended by this Agreement, each Transferee agrees (and agrees on behalf of itself and all Permitted Transferees and Affiliates, which will also be bound by this non-competition covenant) that neither Transferee nor any Permitted Transferee(s) will engage, directly or indirectly, in any competition outside the Territory with any of the Covered Products. In the event NovaMedica is acquired by a Third Party, said Acquirer and its Affiliates shall be bound by this non-competition covenant.
i. If, any time during the Term, Company holds or acquires any Third Party IP under any agreement other than the Sublicense Agreement or under the License Agreement, and such IP includes Valid Claims or other IP rights in the Territory, the Company shall use Commercially Reasonable Efforts to obtain any consents or approvals necessary or required to transfer such IP rights to Transferee for use in the Territory. If Company obtains such required consents and approvals, Company shall promptly notify the Transferee to that effect, together with notification of any applicable pass-through fees, royalties and other payments, and the Transferee may, in its sole discretion, provide written confirmation to Company that the Transferee wishes such Third Party IP to be included in the Licensed IP. Effective upon such confirmation, such Licensed IP shall be sublicensed by Company to the Transferee pursuant to this Agreement and the Transferee shall be responsible for all pass-through fees, royalties and other pass-through consideration in connection with such Third Party IP. No sublicense shall be effective if Transferee does not provide such confirmation.
ii. It is understood and agreed that with respect to any Third Party Agreements under which Company has less than fully exclusive, worldwide rights (e.g., co-exclusive, non-exclusive, limited territorial, and/or otherwise restricted rights), the rights provided in Section 2.2(g) above, shall be limited to the scope of those rights in the Field and in the Territory that Company and/or its Affiliates Controls and has the right to provide to Transferee.
iii. In the event that Company is relieved in whole or in part of any obligation under any or all of the Third Party Agreements for any reason, the Transferee shall automatically be relieved of its obligation to Company under the applicable sublicense agreement in the Territory to the same extent as Company (or its successor or any affiliate of its successor under common control of such successor) is relieved of its obligation(s) under the corresponding Third Party Agreement(s). Company (or its successor or any affiliate of its successor under common control of
such successor) shall as soon as possible notify the Transferee in writing about such relief.
iv. Company shall promptly notify in writing the Transferee of any notice of default under, or termination or amendment of, the License Agreement or any other Third Party Agreement to which the Transferee obtains rights under Section 2.1(f). Company shall not take any action that would lead to the amendment of any Transferee obligations or rights or termination of the License Agreement or any other Third Party Agreements to which the Transferee obtains rights pursuant to Section 2.1(f), without first obtaining Transferee’s explicit prior written consent.
v. If after the Effective Date of this Agreement the Company agrees to any amendment of the Sublicense Agreement or any other Third Party Agreement that increases any obligations of the Transferee under the Sublicense Agreement and any other Third Party Agreement, the Transferee’s obligations under such amendment will apply only to the extent of Transferee obligation under the pre-amended Sublicense Agreement and other Third Party Agreement, and the Company will bear any increase in such obligations due to such amendment.
2.2 Registration of Transfers in the Territory.
(a) Each of DRI and NovaMedica shall take all actions required of it, and Company shall take all actions reasonably requested of it by the respective Transferee, to ensure that the assignment of the Assigned IP to DRI and from DRI to NovaMedica (collectively, the “Transfers”) are approved, registered, recorded, or noticed with the applicable Governmental Bodies in each country in the Territory where such approval, registration, recordation, or notice is required, and that all other actions required under applicable Laws are taken to ensure that such Transfers are fully effective and enforceable. The Transferee and Company shall each use Commercially Reasonable Efforts to ensure that such actions are completed as soon as practicable after the date of this Agreement. Specifically, DRI and Company shall use their respective Commercially Reasonable Efforts to complete the initial Transfers of the Assigned IP within ninety (90) calendar days of the Effective Date of this Agreement. Company as soon as practicable after the Effective Date of this Agreement or upon the Transferee’s reasonable request shall file applications to issue and register Company Patents with Government Bodies in the Territory and immediately thereafter assign such applications to Transferee.
(b) Without limiting the foregoing, Company and Transferee shall submit an application for registration of each Transfer to the Russian Federation with the Russian Federal Service for Intellectual Property, Patents and Trademarks (“Rospatent”) (with respect to any Company Patent Rights requiring such registration under applicable Russian Law) and similar Governmental Bodies in other countries in the Territory (with respect to any Company Patent Rights requiring such registration under applicable Laws of such countries) (collectively, the “Transfer Registrations”) as soon as practicable after the Effective Date of this Agreement and thereafter during the Term, and shall use all reasonable efforts to ensure that such registration is completed as soon as practicable. The Transferee shall provide such assistance as Company may reasonably require in completing such Transfer Registration.
(c) Company shall provide to the Transferee all such assistance as the Transferee shall reasonably request in connection with any subsequent Transfer of Company IP to the
Transferee as a Permitted Transferee, including but not limited to evidence, record, and perfect the foregoing assignment and to apply for and from time to time enforce, maintain and defend the assigned rights, including by entering into one or more assignment of Patent Rights agreements, to be registered with applicable Governmental Bodies in the Territory. Company shall, upon the Transferee’s request, promptly provide Transferee with all information and signs all documents reasonably requested in order to complete any such subsequent Transfer or Transfer Registration of any subsequent assignment, license, or sublicense of rights in Company IP.
(d) DRI or NovaMedica, as applicable, shall reimburse Company promptly for all Out-of-Pocket Expenses incurred by Company in performing its obligations under this Section 2.2.
(e) Survival of Sublicense. In the event that this Agreement is terminated by the Transferee for convenience pursuant to Section 11.2, below, subject to the Company’s prior written consent, which may be withheld by Company in its sole discretion, a sublicense of any Licensed IP granted by the Transferee to a Permitted Transferee shall, at the written election of such Permitted Transferee that is communicated to Company within thirty (30) days after the effective date of the termination of this Agreement, survive such termination, provided that such Permitted Transferee is at the time in full compliance with the terms of the applicable sublicense agreement. In such event, Company shall enter into a license agreement with such Permitted Transferee to grant a license under Company IP (a “Direct License”) directly to such Permitted Transferee. Subject in all respects to the prior written consent of Company, each Direct License shall be subject to the same terms and conditions as those in such sublicense agreement, including but not limited to scope, sublicense territory, duration of sublicense grant, financial and diligence obligations, in each case to the extent that such sublicense agreement provisions are not in conflict with the terms of this Agreement or applicable federal, state or local laws or regulations. In no event shall Company (a) be liable to Permitted Transferee for any actual or alleged breach of such sublicense agreement by the Transferee or (b) have any obligations to such Permitted Transferee other than Company’s obligations to the Transferee as set forth herein.
2.3 Transfers by the Transferee. The Transferee shall have the right to grant and authorize granting rights under this Agreement to Permitted Transferees, subject to the satisfaction in full of the following conditions:
(a) The Transferee shall, and shall require any Permitted Transferee to, promptly (and in any event no later than thirty (30) days) inform Company of the transfer or granting of any assignment, license, or sublicense of any portion of the Company IP in the Field in the Territory to any Permitted Transferee, and together with such information shall provide Company a true and complete copy of the applicable transfer agreement, provided that the Transferee (or a Permitted Transferee, as the case may be) shall have the right to redact from such copies portions thereof that are unrelated to the Company IP and the Covered Products or are not necessary for Company to verify the consistency of the particular transfer, license, or sublicense agreement with the terms and conditions of this Agreement.
(b) Each such transfer agreement shall be in writing and shall be expressly subject to, and require full compliance with, the material terms and provisions of, this Agreement.
(c) Each transfer, license or provision of rights by the Transferee or a Permitted Transferee with respect to the rights granted under the Agreement shall be subordinate to, consistent with, and subject to the terms and conditions of this Agreement (and, in the case of such rights
subject to any Third Party Agreements, the terms and conditions of such Third Party Agreements).
(d) Unless expressly stated in this Agreement, the transfer or other provision of rights to another Person by the Transferee of any of the rights provided to the Transferee by Company under this Agreement, the Sublicense Agreement or the sublicense of any Other Third Party Agreement shall not relieve the Transferee of any of its obligations hereunder, except for the assignment of such rights to the Third Party in whole or in part if such assignment to the Transferee’s assignee was approved in writing by the Company.
2.4 Retained Rights; No Implied Rights. Company expressly retains all rights with respect to the Development, Manufacture, and Commercialization of all Covered Products in all fields and for all uses and Indications in all countries and other jurisdictions and political subdivisions outside of the Territory, and under this Agreement does not grant, assign, or otherwise convey any right, title, or interest to the Transferee with respect to the foregoing except to the extent expressly provided in this Agreement or expressly agreed in writing by Company. Each Party acknowledges that the rights and licenses granted under this Article 2 and elsewhere in this Agreement are limited to the scope, including Field and Territory, expressly granted and all other requirements of this Agreement.
(a) For the avoidance of doubt, it is understood and agreed that neither a Transferee nor any Permitted Transferee shall have any right to, and shall not under any circumstances, to Develop or Commercialize the Compound or any Covered Product outside the Territory, and neither Company nor any assignees, licensees and sublicensees, other than the Transferee, (“Company’s Transferees”) shall have any right to, and shall not under any circumstances, to Develop or Commercialize the Compound and Covered Product in the Field and in the Territory.
(b) To ensure that neither the Compound or any Covered Product is exported from the Territory for Commercialization and Development, the Transferee shall use, and shall require each Permitted Transferee to use, its Commercially Reasonable Efforts to diligently monitor Development and Commercialization activities throughout the Territory in respect of the Covered Products. To ensure that neither the Compound nor any Covered Product is imported to the Territory for Commercialization and Development other than as contemplated by this Agreement, Company shall use Commercially Reasonable Efforts to diligently monitor Development and Commercialization activities outside the Territory in respect of the Covered Products. Such monitoring activities shall include the generation and maintenance of written records in sufficient detail so as to allow the production, distribution, and final disposition of units of Covered Products, and research, development and manufacture involving Covered Products and/or the Compound, to be accurately monitored, tracked, and logged. Each Transferee and Company shall keep and retain, and each Transferee shall require each Permitted Transferee to keep and retain, such written records and all supporting data during the Term.
(c) In the event Company, a Transferee, or a Permitted Transferee becomes aware of the export or import for Development or Commercialization from or into the Territory of the Covered Products, the Party becoming aware of such exportation or importation shall so inform the other Party in writing, including the provision of any supporting documentation or other information relating to such exportation. The Transferee shall require each Permitted Transferee by contract to promptly and concurrently inform Company and the Transferee in
the event the Permitted Transferee becomes aware of the export from the Territory or the import into the Territory of the Covered Products for Development or Commercialization, including the provision of any supporting documentation or other information relating to such exportation or importation. Promptly after receipt of such information, but in no event later than ten (10) business days, an executive officer of each of Company and the Transferee (and, if applicable, a Permitted Transferee) shall confer in person or by telephone with regard to such exportation or importation and how to xxxxx it to the reasonable satisfaction of Company and the Transferee. In the event Company reasonably suspects based on competent evidence that a Transferee or a Permitted Transferee has violated this Section 2.5, after the chief executive officers of Company and Transferee (and, if appropriate, a particular Permitted Transferee) have conferred, Company (or its successor) shall have the right, upon ten (10) business days after advance notice to the Transferee and/or the applicable Permitted Transferee(s), as the case may be, to request from the Transferee and/or the applicable Permitted Transferee(s), whereupon the Transferee and/or the applicable Permitted Transferee(s), as the case may be, shall promptly furnish to Company all information and documents that are useful for Company’s research and inspection, including those documents described in Section 2.5(b), above, for the purpose of verifying compliance with the territorial limitations on the rights granted under this Agreement. In the event Transferee reasonably suspects based on competent evidence that a Company or a Company’s Transferee has violated Section 2.5(a), above, after the chief executive officers of Company and Transferee (and, if appropriate, a particular Permitted Transferee or Company’s Transferee) have conferred, Transferee (or its successor) shall have the right, upon ten (10) Business days after advance notice to the Company and/or the applicable Company Transferee(s), as the case may be, to request from the Company and/or the applicable Company Transferee(s) all information and documents, whereupon the Company and/or the applicable Company Transferee(s), as the case may be, shall promptly furnish to Transferee and Permitted Transferee(s) all information and documents that are useful for Company’s research and inspection, including those documents described in Section 2.6(b), above, for the purpose of verifying compliance with the territorial limitations on the rights granted under this Agreement.
ARTICLE 3
PRODUCT DEVELOPMENT AND COMMERCIALIZATION
3.1 Ancillary Agreements. Company and Transferee shall enter into the Sublicense Agreement, Clinical Development and Collaboration Agreement, Supply Agreements for clinical and commercial supplies of Covered Products, and Pharmacovigilance Agreement, as provided herein. The Parties agree to enter into the Sublicense Agreement not later than sixty (60) days after the written request of the Transferee.
3.2 Development and Commercialization in the Territory. The Transferee, either itself or through one or more Permitted Transferees operating within the Territory, shall have the sole authority and the exclusive right to Develop and Commercialize any and all Covered Products in the Field in the Territory, including the sole and exclusive right to conduct all Clinical Trials and non-clinical studies appropriate to obtain Regulatory Approvals for the Covered Product(s) in the Territory in one or more Indications within the Field, and pursuant to the terms of the Clinical Development and Collaboration Agreement that shall be concluded by the Parties not later than 60 (sixty) calendar days from the Effective Date of this Agreement.
3.3 Materials Transfer Requests. The Transferee shall have the right, any time prior to the submission of the first NDA in the Territory in respect of a Covered Product, to request from Company in writing
(the “Material Transfer Request”) that Company transfer Company Materials to Transferee in the Territory for Transferee’s reasonable needs. Subject to reasonable availability of Company Materials, within thirty (30) calendar days of after the date of the Material Transfer Request, the Company shall transfer Company Materials requested by the Transferee subject to the payment to Company of all Out-of-Pocket Expenses incurred by Company in connection with the provision and transfer of the Company Materials to Transferee or information regarding obtaining such Material supply or source from a Third Party. Company shall thereafter deliver the Company Materials, or information regarding Material supply or source, in accordance the terms of the Technology Transfer Outline attached as Schedule 3 to this Agreement.
3.4 Knowledge Transfer Procedures.
(a) Company shall within thirty (30) Business Days from the Effective Date of this Agreement, transfer to the Transferee such Company Know-How, including any preclinical data, clinical data, assays and associated materials, protocols, procedures, and any other information in Company’s Control, as is reasonably necessary or useful to continue or initiate Development and Commercialization of a Covered Products in the Field in the Territory, including by providing electronic copies of any tangible embodiments thereof. The Transferee shall have a right from time to time request from the Company such additional information as is reasonably necessary or useful to continue or initiate Development and Commercialization of a Covered Products in the Field in the Territory and, in accordance with the terms and conditions of the Clinical Development and Collaboration Agreement, Company shall deliver such information and documents to Transferee as soon as reasonably practicable. Transferee shall reimburse Company for all Out-of-Pocket Expenses in connection with the foregoing.
(b) In the event that Transferee or a Permitted Transferee desires to obtain support for the Development or Commercialization of the Compound or a Covered Product, during the Transfer Period, Company agrees, upon reasonable notice and pre-arrangement, to make its employees that are knowledgeable of Covered Product (or the Compound), its properties, and functions, reasonably available via telephone and electronic communications to the Transferee and appropriate Permitted Transferees for scientific and technical explanations, advice, and support, that may reasonably be requested by the Transferee, relating to the Development and registration of a Covered Product (and/or such Compound) (the foregoing activities being referred to as “Development Support” or “Commercialization Support”). The Transferee shall reimburse Company for the reasonable costs of making its personnel available to provide such support as well as Company’s Out-of-Pocket Expenses incurred in providing the Development and Commercialization Support, subject to Company providing documented evidence of such Out-of-Pocket Expenses having been incurred and the time incurred by Company personnel in providing such support.
(c) In the event that Transferee or Permitted Transferee wishes to utilize the personnel of Company for support in connection with training, set-up, or other assistance in establishing Transferee or Permitted Transferee or an in-Territory manufacturer’s procedures, facility and capabilities for the Manufacture or supply of Covered Products for the Territory, the Company shall promptly upon Transferee or Permitted Transferee request shall assist and support the Transferee and Permitted Transferee in establishing Manufacturing in the Territory. Transferee or Permitted Transferee shall reimburse the Company for the reasonable costs of making its personnel available to provide such assistance as well as Company’s Out-of-Pocket Expenses, subject to Company providing documented evidence of
such Out-of-Pocket Expenses having been incurred and the time incurred by Company personnel in providing such assistance.
(a) During the Term and thereafter, Company shall have no right to Manufacture the Covered Products (or the Compound) in the Territory.
(b) Transferee shall have the exclusive right within the Territory to Manufacture the Covered Products (and any corresponding Compound) solely for Development and Commercialization in the Territory within the Field, itself or through one or more Affiliates or Permitted Transferees, or other Third Parties located in the Territory selected by Transferee.
(c) Until the First Commercial Sale of a Covered Product within the Territory, Transferee and any Permitted Transferee shall have the right to purchase supplies of Compound and/or Covered Product from Company as are reasonably available to Company and as are reasonable and necessary to conduct Clinical Trials of Covered Product in the Territory within the Field, provided that any such purchase does not reasonably interfere with Company having sufficient supplies of Compound and/or Covered Products on hand for use in Development (including the conduct of Clinical Trials) or Commercialization outside of the Territory. Such purchases will be made on a cost-plus basis, not to exceed [XXXXXXXXX] above Company’s reasonable documented cost, to be negotiated in good faith by the Parties. The Parties shall enter into the Supply Agreement to supply Compound and/or Covered Product for Development in the Territory within sixty (60) calendar days from the Transferee’s request. Delivery terms specified in the Supply Agreement shall be construed in accordance with Incoterms 2010.
(d) At least thirty six (36) months prior to the planned First Commercial Sale by the Transferee of a Covered Product in the Territory, the Parties shall negotiate in good faith a Supply Agreement pursuant to which Company, itself or through a Third Party contract manufacturer authorized by Company to manufacture and supply the Covered Products, shall supply needed quantities of Covered Product to Transferee and Permitted Transferees solely for Commercialization of Covered Products in the Field in the Territory, on commercially fair and reasonable terms. Such Supply Agreement negotiations shall be in good faith and on an arm’s length basis. Such purchases will be made on a cost-plus basis, not to exceed [XXXXXXXXX] above Company’s reasonable documented cost, to be negotiated in good faith by the Parties. In the event the Parties are unable to agree on pricing under the Supply Agreement, they shall engage an internationally recognized consulting firm reasonably acceptable to both Parties to perform an analysis to determine final pricing under the Supply Agreement, which decision shall be binding upon the Parties. Delivery terms specified in the Supply Agreement shall be construed in accordance with Incoterms 2010.
(e) In the event that (i) the parties are unable to reach a reasonably acceptable Supply Agreement pursuant to Section 3.5(c) or 3.5(d) of this Agreement, or (ii) Company is unable to supply
|
[XXXXXXXXX] |
|
REPRESENTS CONFIDENTIAL MATERIAL WHICH HAS BEEN REDACTED AND FILED SEPARATELY WITH THE COMMISSION PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT IN ACCORDANCE WITH RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED. A COMPLETE VERSION OF THIS SCHEDULE HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. |
the Compound or Covered Products to the Transferee under a Supply Agreement entered into pursuant to Section 3.5(c) or 3.5(d) of this Agreement for a period of at least sixty (60) calendar days after the specified delivery date and Company thereafter fails to cure such failure within sixty (60) days after written notice from the Transferee, Company shall cooperate with the Transferee to identify a mutually acceptable alternative source of supply and shall provide the necessary consents to allow such alternative source of supply to provide the needed quantities of the Compound and/or the Covered Products to NovaMedica. The terms of the alternative source of supply would be negotiated directly by the Transferee with the supplier.
4.1 Regulatory Filings. The Transferee shall solely own and maintain all regulatory filings and Regulatory Approvals for the Covered Products in the Territory. It is understood and agreed that nothing herein shall preclude the Transferee or any Permitted Transferee, from referencing any IND or NDA Controlled by Company (or a Company Affiliate, successor, or licensee outside of the Territory) in respect of a Covered Product Developed or Commercialized outside of the Territory, or using data therein in support of regulatory filings for Covered Products in the Territory, or from authorizing Third Parties to do so. Company shall provide reasonable assistance to Transferee, and its Permitted Transferees, in the preparation of and filing of any IND, IND amendment, or NDA with respect to any Covered Product for use in any Indication in the Territory. Such assistance shall include, in particular, Company providing the Transferee with a complete electronic copies (including serial submissions) of all relevant documentation submitted to the FDA or EMA in respect of any Clinical Trial for any Covered Product that would be necessary or useful to the Transferee in preparing its own IND for a particular Covered Product for use in the Territory, and, to the extent necessary or useful, to allow the Transferee to cross-reference any such IND, IND amendment, or NDA held by Company (or its Affiliate, successor, or licensee outside of the Territory). The Transferee shall provide, and shall require any Permitted Transferee to provide, Company with complete electronic copies of filings (including serial submissions) submitted by the Transferee and all Permitted Transferees to a Regulatory Authority in the Territory in respect of Development or Commercialization of any Covered Product, and Company shall have the right to use the data generated by the Transferee (and its and Permitted Transferees) in support of Development and Commercialization (including use in regulatory filings) of Covered Products for any Indication outside of the Territory. The Transferee shall reimburse Company for Company’s Out-of-Pocket Expenses providing such documents and information.
4.2 Communications with Authorities. The Transferee (or a Permitted Transferee) shall be responsible for and act as the sole point of contact for communications with Regulatory Authorities in the Territory in connection with the Development and Commercialization of Covered Products in the Territory. To the extent Company or the Transferee (or a Permitted Transferee) receives any written or oral communication from any Regulatory Authority relating to a Covered Product in the Field, as soon as reasonably practicable the Party receiving such communication shall notify the other Party thereof, which notice shall include a copy of any written communication received or, if applicable, complete and accurate minutes of such oral communication.
4.3 English Language Requirement. Subject to the payment by Company to the Transferee or a Permitted Transferee, as applicable, of its reasonable Out-of-Pocket Expenses with respect to the English language translation hereunder that may be provided by a contract research organization, the Transferee shall provide to Company, and shall require any Permitted Transferee to provide to Company, English language translations of any and all documents delivered in accordance with
Section 4.1 or 4.2, above, that are not otherwise provided in English for use by some or all members of the Joint Steering Committee, Joint Development Committee, and/or Joint Development Committee, or either of the Parties pursuant to the Clinical Development and Collaboration Agreement. In the event that the Transferee or a Permitted Transferee uses a third party clinical research organization to provide the documents delivered in accordance with Section 4.1 and 4.2, above, and to provide the English translation required by this Section 4.3, Company shall reimburse Transferee or a Permitted Transferee, as the case may be, for the reasonable costs of such translation billed on an hourly basis and not on a cost-per-page or cost-per-word basis.
4.4 Pharmacovigilance. The Parties agree, within four (4) weeks after the date of the first Material Transfer Request or such other time-frame as shall be approved by the JDC, to conclude a detailed Pharmacovigilance Agreement in a form substantially as contemplated by Schedule 6 hereof, or such other form as is mutually satisfactory to the Parties. Such Pharmacovigilance Agreement shall provide for the exchange by the Parties of any information of which a Party becomes aware in the Territory concerning any side effect, injury, toxicity, or sensitivity reaction, or any unexpected incident, in or involving a research patient or subject or, in the case of non-clinical studies, an animal in a toxicology study, and the seriousness thereof, whether or not determined to be attributable to any Covered Product or Compound, including any such information received by either Party from a Third Party. It is understood that each of Company and its Affiliates and the Transferee and its Permitted Transferees shall have the right to disclose such information as reasonably necessary to comply with Regulatory Authorities within its respective territory with respect to its filings and activities related to Compounds and Covered Products.
ARTICLE 5
GOVERNANCE AND SUPPORT ENGAGEMENTS
5.1 Clinical Development and Collaboration Agreement. The Parties understand that assistance from Company will be needed and accepted in connection with the Development and Commercialization of the Compound and Covered Products in the Field in the Territory. With regard to Development, the Parties agree that within ninety (90) calendar days after the Effective Date (or such longer period as the Parties may agree in writing), the Transferee and Company will enter into a mutually acceptable “Clinical Development and Collaboration Agreement” regarding Development of the Compound and Covered Product(s) in the Territory.
5.2 Clinical Trials and Non-Clinical Studies. The Clinical Development and Collaboration Agreement shall, among other things, provide that all Clinical Trials necessary to register a Covered Product for any Indication in the Field in any country or jurisdiction within the Territory shall be the sole obligation and responsibility of the Transferee. Transferee and any Permitted Transferee shall be entitled at no cost to access, use, and reference the data and results from any Company Clinical Trial of a Covered Product in any Indication outside of the Territory for their own Regulatory Approval, Development, and Commercialization purposes in the Field in the Territory. Company shall be entitled at no cost to access, use, and reference the data and results from any Transferee or Permitted Transferee Clinical Trial of a Covered Product in any Indication in the Territory or otherwise under the Clinical Development and Collaboration Agreement for the Company’s own regulatory approval, development, and commercialization purposes outside of the Territory.
5.3 Development and Commercialization. The Clinical Development and Collaboration Agreement shall, among other things, require that Company and the Transferee establish a Joint Steering Committee (“Joint Steering Committee” or “JSC”) to oversee Development of Covered Products until the First Commercial Sale of a Covered Product in the Territory, unless otherwise agreed in writing by Company and the Transferee. The JSC will be comprised of an equal number of members
appointed by the Transferee and by Company. The JSC shall oversee the Development of Covered Products in the Field in the Territory, and shall plan, implement, and oversee activities relating thereto, including the preparation and implementation of a development plan. All JSC decisions will be made by unanimous vote, with the JSC representatives of Company collectively having one vote and the JSC representatives of Transferee collectively having one vote. If the JSC is unable to decide or resolve unanimously any matter properly presented to it for action, then such matter shall be resolved as provided in the definitive Clinical Development and Collaboration Agreement.
The Clinical Development and Collaboration Agreement shall, among other things, require that for so long as the Transferee (and/or one or more Permitted Transferees) is Developing a Covered Product(s) for use in the Field in the Territory, the day-to-day Development work shall be conducted under the direction of the Joint Development Committee (“Joint Development Committee” or “JDC”) comprised of an equal number of representatives from Transferee and Company. All JDC decisions will be made by unanimous vote. If the JDC is unable to decide or resolve any matter properly presented to it for action, then the decision of Company shall be final and shall be in compliance with the terms and conditions of this Agreement, the Clinical Development and Collaboration Agreement and Law. The JDC will be responsible for coordinating amendments to any plan for Development in respect of a Covered Product for use in the Field in the Territory for review and approval by the JSC, for overseeing such Development work, and for making operational decisions related to such Development work. Unless otherwise agreed in writing by the Transferee and Company, until the First Commercial Sale of a Covered Product in the Territory, the JDC will meet on a regular basis, at such times and in such manner as provided in Clinical Development and Collaboration Agreement.
The Clinical Development and Collaboration Agreement shall, among other things, require that for so long as the Transferee (or a Permitted Transferee) is preparing to Commercialize a Covered Product(s) in the Territory, the day-to-day Commercialization preparation work shall be conducted under the direction of a Joint Commercialization Committee (“Joint Commercialization Committee” or “JCC”) and supply chain audit procedures. The JCC shall be comprised of an equal number of representatives from the Transferee and Company. All JCC decisions will be made by unanimous vote. If the JCC is unable to decide or resolve any matter properly presented to it for action, then the decision of the Transferee shall be final and in compliance with the terms and conditions of this Agreement and Law. Prior to the First Commercial Sale of a Covered Product (or such longer period as the Parties may agree in writing), the JCC will be responsible for coordinating any amendments to the plan for Commercialization of Covered Product(s) in the Territory, for overseeing performance of the Commercialization program, and for making operational decisions related to that program. Periodically, a member of the JCC for each party shall provide to the other party a reasonably detailed summary of the Commercialization activities conducted in the Territory. The JCC will jointly prepare and provide to each Party on at least a Calendar Quarter basis a report, via e-mail, regarding the status of Commercialization activities hereunder.
ARTICLE 6
FINANCIAL PROVISIONS
6.1 Consideration Payable by Transferee. DRI shall pay Company the Fixed Payment, in the amount of One Hundred Thousand U.S. Dollars (US$100,000), in full consideration for all rights and licenses granted by Company pursuant hereto, and Company’s performance of all its obligations hereunder.
6.2 Payment of Fixed Payment. The Fixed Payment shall be paid by DRI to Company by wire transfer concurrently with or prior to the Initial Closing of the Investment Transaction.
6.3 Third Party Agreements. Company shall maintain each Third Party Agreement at its own cost and expense, and shall continue to be responsible for making payments, if any, to each licensor in a timely manner and to the extent set forth in each of the respective Third Party Agreement, subject to the Transferee meeting its obligations to pay all license fees, royalties and other payments under each Third Party Agreement arising from or relating to the sublicense or transfer of rights thereunder to the Transferee and/or the Development or Commercialization of Covered Products in the Territory. Any payment due by Company to a Third Party under a Third Party License Agreement shall be paid by the Transferee in a timely manner. In the event that Company shall fail to make any payment when due under the Third Party License Agreement with respect to the activities of the Transferee or the Permitted Transferee and does not do so promptly (and in any event within fifteen (15) days) after receiving notice of such failure, Company shall so notify the Transferee and the Transferee shall thereafter have the right to make such payment directly to such Third Party on behalf of Company. The Transferee shall promptly notify Company in writing of any such payment, and notwithstanding anything to the contrary in this Agreement.
6.4 Taxes. Company shall be responsible for the payment of any and all income taxes levied on account of royalties and other payments paid to Company by a Transferee or any Permitted Transferee, but not for any value added tax or similar tax. If applicable Law requires that taxes be deducted and withheld from royalties or other payments paid pursuant to this Agreement, Transferee shall (a) deduct those taxes from the payment; (b) pay the taxes to the proper Governmental Body; (c) send evidence of the obligation together with proof of payment to Company within ninety (90) days following such payment; (d) remit to Company the net amount, after deductions or withholding made under this Section 6.4; and (e) cooperate with Company in any way reasonably requested by Company, to obtain available reductions, credits, or refunds of such taxes.
ARTICLE 7
INVENTIONS AND PATENTS
7.1 Title to Inventions. Except for the Company Patent Rights and the Company Improvement Patent Rights that shall be assigned by Company to the Transferee pursuant to Section 2.1(b), above, Company retains ownership of all Company Know-How, Company Materials, Company Patents, and other Company IP and Company Confidential Information in and outside of the Territory of or after the Effective Date, and Transferee retains ownership of all Patent Rights, Know-How, Materials, and Confidential Information owned by Transferee as of or after the Effective Date.
7.2 Patent Prosecution and Maintenance. Each Party shall have the right to control the Prosecution and Maintenance of Patent Rights such Party Controls at such Party’s expense in accordance with the provisions of appended Schedule 4.
7.3 Patent Enforcement and Defense. Enforcement and defense of Company Patents and Joint Patents shall be governed by the provisions of appended Schedule 5.
8.1 Confidentiality; Exceptions. Except to the extent authorized by this Agreement or otherwise agreed in writing, the Parties agree that the receiving Party (the “Receiving Party”) shall keep confidential and shall not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement Confidential Information in any form (written, oral, photographic, electronic, magnetic, or otherwise) which is disclosed to it by the other Party (the “Disclosing Party”) or otherwise received or accessed by a Receiving Party in the course of performing its obligations or exercising its
rights under this Agreement, except to the extent that it can be established by the Receiving Party that such Confidential Information:
(a) was in the lawful knowledge and possession of the Receiving Party prior to the time it was disclosed to, or learned by, the Receiving Party, or was otherwise developed independently by the Receiving Party, as evidenced by written records kept in the ordinary course of business, or other documentary proof of actual use by the Receiving Party;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the Receiving Party in breach of this Agreement; or
(d) was disclosed to the Receiving Party, other than under an obligation of confidentiality, by a Third Party who had no obligation to the Disclosing Party not to disclose such information to others.
8.2 Authorized Disclosure. Except as expressly provided otherwise in this Agreement, including but not limited to the restrictions set forth in Section 2.5 above, a Receiving Party may use and disclose Confidential Information of the Disclosing Party as follows: (a) in connection with the performance of its obligations or exercise of rights granted or reserved in this Agreement (including the rights to Develop and Commercialize the Covered Products); or (b) to the extent such disclosure is reasonably necessary in filing or prosecuting patent, copyright and trademark applications, prosecuting or defending litigation, complying with applicable governmental regulations, obtaining regulatory approval, conducting Clinical Trials, Developing and/or Commercializing Covered Products, or otherwise required by Law; provided, however, that if a Receiving Party is required in litigation or by Law or regulation to make any such disclosure of a Disclosing Party’s Confidential Information it shall give reasonable advance notice to the Disclosing Party of such disclosure requirement and, except to the extent inappropriate in the case of patent applications, shall use its reasonable efforts to secure confidential treatment of such Confidential Information required to be disclosed; or (c) to the extent mutually agreed to in writing by the Parties; provided, however, that, in each of the above situations, the Receiving Party shall remain responsible for any failure by any Person who receives the Confidential Information from the Receiving Party pursuant to this Section 8.2 to treat such Confidential Information as required under this Section 8.2.
In addition, a Receiving Party may disclose Confidential Information of the Disclosing Party to (i) any of its Affiliates and Permitted Transferees, or in connection with due diligence investigations by or on behalf of a Third Party in connection with a potential license, collaboration, investment, merger, or acquisition with or by such Third Party, and (ii) in the case of Company, to Third Parties in connection with due diligence investigations by or on behalf of a Third Party in connection with a potential license, collaboration, investment or other financing, merger, or acquisition with or by such Third Party, provided in each of the foregoing cases that such Third Party that reasonably needs to have access to such Confidential Information agrees to be bound by reasonable terms of confidentiality and non-use at least as stringent as those set forth in this Article 8, to limit such disclosure to only personnel having a need to know such information, and to return or certify to the Receiving Party as to the destruction of such Confidential Information promptly after completing the due diligence investigation, negotiation, or transaction, as the case may be.
8.3 Disclosure of Agreement. No Party shall issue any press release or other public disclosure regarding this Agreement or the Parties’ activities hereunder, or any results or data arising hereunder, except with all the other Party’s prior written consent. Except to the extent required by Law or as otherwise permitted in accordance with this Section 8.3, neither Party shall make any public announcements concerning this Agreement or the subject matter hereof without the prior written consent of the other, which shall not be unreasonably withheld, conditioned, or delayed. Notwithstanding the foregoing, to the extent information regarding this Agreement has already been publicly disclosed other than through any act or omission of a Party in breach of this Agreement, a Party may subsequently disclose the same information to the public without the consent of the other Party. Each Party shall be permitted to disclose the terms of this Agreement, in each case under appropriate confidentiality provisions no less stringent than those of this Agreement, to any actual or potential acquirers, merger partners, financing sources, and professional advisors.
8.4 Remedies. Each Party shall be entitled to seek, in addition to any other right or remedy it may have, at law or in equity, a temporary or preliminary injunction, without the posting of any bond or other security, enjoining or restraining the other Party from any violation or threatened violation of this Article 8.
ARTICLE 9
REPRESENTATIONS, WARRANTIES, AND COVENANTS
9.1 Representations and Warranties. Each Party represents and warrants to the other Party that, as of the Effective Date:
(a) such Party is duly organized and validly existing under the Laws of the jurisdiction of its incorporation or organization;
(b) such Party has taken all actions necessary to authorize the execution and delivery of this Agreement and the performance of its obligations under this Agreement;
(c) this Agreement is a legal and valid obligation of such Party, binding upon such Party, and enforceable against such Party in accordance with the terms of this Agreement;
(d) the execution, delivery, and performance of this Agreement by such Party does not conflict with, breach, or create in any Third Party the right to accelerate, terminate, or modify any agreement or instrument to which such Party is a party or by which such Party is bound, and does not violate any Law of any Governmental Body having authority over such Party;
(e) no consent by any Third Party or Governmental Body is required with respect to the execution and delivery of this Agreement by either Party or the consummation by either Party of the transactions contemplated hereby; and
(f) such Party has all right, power, and authority to enter into and to perform its obligations under this Agreement.
9.2 Additional Representations and Warranties of Company. Company represents, warrants and covenants to Transferee that:
(a) to Company’s knowledge, all of the Licensed IP and Assigned IP is valid, subsisting, and enforceable;
(b) the Assigned IP is not encumbered, with liens, mortgages, security interests or otherwise, and Company will not encumber, with liens, mortgages, security interest or otherwise, any Assigned IP;
(c) the Licensed IP is not encumbered, with liens, mortgages, security interests or otherwise, and Company will not encumber, with liens, mortgages or security interests, any Licensed IP without prior written notification to the Transferee;
(d) Company has not entered, and will not enter, into any contract or agreement that is inconsistent with or conflicts with the terms of this Agreement, nor has the Company taken or failed to take any action that might reasonably be expected to have the effect of waiving any rights granted or licensed to the Transferee under this Agreement;
(e) Company has not entered into any agreement other than this Agreement that grants any interest in, or any right to use or exploit, any of the Assigned IP in the Territory otherwise than in favor of Transferee;
(f) except for the Assigned IP and the Licensed IP, neither Company nor its Affiliates own or control any Intellectual Property that is necessary or useful for the Development and Commercialization of the Covered Products in the Field in the Territory;
(g) except as contemplated by this Agreement and the Sublicense Agreement, the Company has not entered into any agreement that grants any interest in, or any right to use or exploit, any of the Assigned IP and Licensed IP, nor is Company is under any obligation to enter into such agreement or grant (whether or not contingent on any future event or state of affairs) any such rights;
(h) the Company will not entered into any agreement other than this Agreement or the Assignment and Assumption Agreement that grants any interest in, or any right to use or exploit, any of Licensed IP in the Territory, nor is Company is under any obligation to enter into such agreement or grant (whether or not contingent on any future event or state of affairs) any such rights;
(i) no claims have been asserted or threatened against the Company in writing by any Person challenging the validity, effectiveness, enforceability, or ownership of the Licensed IP or the Assigned IP, and, to Company’s Knowledge, there is no reasonable basis for any such challenge;
(j) to Company’s Knowledge, Company’s activities prior to the Effective Date, and Company’s expected activities subsequent to the Effective Date, in connection with the Development and contemplated Commercialization of Covered Products in the Field and in the Territory do not and will not infringe or misappropriate any Patent Right, Know-How, or Xxxx of any Person or than Company, and to Company’s Knowledge, no claims have been asserted or threatened against the Company in writing by any Person with respect to the foregoing; and, to Company’s Knowledge, there is no reasonable basis for any such challenge;
(k) to Company’s Knowledge, there is no material unauthorized use, infringement, or misappropriation of any Company IP related to Development and Commercialization of any Covered Products in the Field in the Territory by any employee or former employee, or by any other Third Party;
(l) to Company’s Knowledge, none of the Licensed IP or Assigned IP is the subject of any litigation or dispute resolution procedure, discovery process, interference, reissue, reexamination, opposition, appeal proceedings, or any other legal dispute;
(m) Company has no Knowledge of any Patent Rights Controlled by Company as of the Effective Date, other than Company Patents, that relate to, and are necessary or useful for, the Development and/or Commercialization of Covered Products in the Field in the Territory;
(n) Company has no Knowledge of any Know-How Controlled by Company as of the Effective Date, other than Company Know-How, that relates to, and is necessary or useful for, the Development and/or Commercialization of Covered Products in the Field in the Territory;
(o) Company has the right to provide Company Materials to Transferee and to grant to Transferee the right and license to use Company Materials for Development and/or Commercialization of Covered Products in the Field in the Territory;
(p) Company has no Knowledge that any Covered Product or any Company Materials infringe, or may infringe, any intellectual property right of any Third Party;
(q) all employees of Company who have performed any activities on its behalf in connection with research regarding any Company IP, Covered Product (or the Compound) have assigned to Company the whole of their rights in any discovery, invention, improvement, or other technical advance, whether or not patentable, and any corresponding intellectual property, conceived, made, discovered, or otherwise developed by them as a result of such research, and, to Company’s Knowledge, no Third Party has any rights to any such intellectual property;
(r) prior to any assignment to DRI of any Patent Right within the Assigned IP in contemplation of or pursuant to this Agreement, Company owns (or owned) all right, title, and interest in and to such Patent Right;
(s) Company has sufficient right, title, and interest in and to Company IP, by ownership or license, to convey to Transferee the licenses and rights as set forth herein;
(t) licenses and rights of the Company IP under this Agreement do not conflict with any other agreement between the Company and any Third Party, subject, however, to the applicable provisions of each Third Party Agreement;
(u) subject to the approval of Purdue Neuroscience, Company has the right, power, and authority to grant to Transferee the rights granted to Transferee with respect to the License Agreement;
(v) the License Agreement is in full force and effect and Company is not in breach or default in the performance of its obligations under the License Agreement, and Company has not received any notice from Purdue Neuroscience of any breach, default, or non-compliance of Company under the terms of the License Agreement;
(w) Company has provided to Transferee an accurate, true, and complete copy of the License Agreement. There have been no amendments or other modification to the License Agreement, except as have been disclosed to Transferee in writing;
(x) the Company is not under obligation to obtain consent of any Third Party to convey to Transferee the licenses and rights as set forth in this Agreement, except as under License Agreement and as may be required by a future Third Party Agreement;
(y) Company has provided to Transferee or made available for Transferee’s review all material information and data in Company’s Control relating to Company Materials and Covered Products and the transactions contemplated under this Agreement that might reasonably be expected to be relevant to Transferee’s decision to enter into this Agreement;
(z) with respect to any Patent Rights Controlled by Company, to Company’s Knowledge Company has complied with all applicable Laws, rules, and regulations, including any information disclosure requirements, in connection with the filing, prosecution, and maintenance of such Company Patents in the Territory;
(aa) unless specifically indicated in a patent application or patent within Patent Rights Controlled by Company, to Company’s Knowledge none of the inventions claimed in such Patent Rights were conceived or first reduced to practice using funding from the United States government or any other Governmental Body;
(bb) with respect to any Patent Rights Controlled by Company, to Company’s knowledge, assignments of all inventorship rights from all Persons listed as inventors on such Patent Rights, and all such assignments of inventorship rights relating to such Patent Rights, have been obtained and, for those patent applications within the Patent Rights that have been filed in the United States, such assignments have been recorded at the United States Patent and Trademark Office and, to the knowledge of Company, are valid and enforceable;
(cc) with respect to any Patent Rights Controlled by Company, to Company’s Knowledge, all provisions of any Laws, foreign or domestic, related to the rights as inventors or Persons that are named as inventors on such Patent Rights have been complied with;
(dd) Company has disclosed or made available to Transferee all material scientific and technical information and all information relating to safety and efficacy known to Company or its Affiliates with respect to the Covered Product(s) and Compound(s), and Company undertakes to promptly update Transferee during the Term with any additional material scientific or technical information or any other information relating to safety and efficacy of the Covered Product(s) and Compound(s) in the Field that becomes known to Company or its Affiliates; and
(ee) Company has disclosed to the Transferee all material correspondence and contact information between Company and the FDA, EMA, or other Governmental Body regarding the Covered Product(s) and Compound(s), and, until such time as a Change of Control of the Company shall occur, Company undertakes to promptly update Transferee during the Term with any additional material scientific or technical information or any other information relating to safety and efficacy of the Covered Product(s) and Compound that becomes known to Company or its Affiliates.
9.3 Company Covenants. Company covenants to the Transferee that:
(a) Company shall fulfill all of its obligations, including but not limited to its payment obligations, under any Third Party Agreement, throughout the term of this Agreement provided that the Transferee and any Permitted Transferee shall make all payments and
perform all obligations required on the party of the Transferee or Permitted Transferee under the Sublicense Agreement or any other Third Party Agreement;
(b) Company shall not amend, waive, or otherwise alter any of Company’s rights under any Third Party Agreement in any manner without prior written consent of the Transferee that adversely affects the rights transferred to the Transferee pursuant to this Agreement that flow from such Third Party Agreement;
(c) Company shall promptly notify Transferee of any notice of default under, or termination or amendment of, the License Agreement or any Third Party Agreement;
(d) Company shall not, for as long as Transferee enjoys rights with respect to Company IP and Covered Products under this Agreement, grant rights within the Territory in or with respect to any Company IP or Covered Product to any Third Party, in a manner inconsistent with the grants and other rights assigned to Transferee under this Agreement; and
(e) For so long as Transferee retains its license under this Agreement, Company shall not provide Covered Products or the Compound to any Third Party in, or for importation into, the Territory for any purpose without the prior consent of, or pursuant to a written agreement with, the Transferee.
9.4 Transferee Covenants. Transferee covenants to Company that:
(a) Transferee shall not, and shall require in all agreements with or including as a party Permitted Transferees that the Permitted Transferee shall not, directly or indirectly, alone or in conjunction with one or more Third Parties, distribute, export, or facilitate or assist in exportation of the Compound or any Covered Products outside of the Territory or outside the Field; and
(b) Transferee shall, and shall require in the all other agreements with or including as a party Transferee or any Permitted Transferees, that Transferee and any Permitted Transferee conduct all activities in connection with Development, Manufacture, and Commercialization of the Compound and/or Covered Products in compliance with applicable Laws.
9.5 Disclaimer. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, OR ANY OTHER AGREEMENT CONTEMPLATED HEREUNDER, NEITHER PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AND EACH PARTY EXPRESSLY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY AND OF FITNESS FOR A PARTICULAR PURPOSE, THE PROSPECTS OR LIKELIHOOD OF DEVELOPMENT OR COMMERCIAL SUCCESS OF ANY COVERED PRODUCT, AND ASSUMES NO LIABILITIES WHATSOEVER WITH RESPECT TO THE COMPOUND, ANY COVERED PRODUCT, OR COMPANY IP.
ARTICLE 10
INDEMNIFICATION AND INSURANCE
10.1 Indemnification by Transferee. Each Transferee hereby does, and shall cause each Permitted Transferee to, indemnify, defend, and hold Company and its Affiliates, and each of their respective employees, officers, directors, agents, successors and assigns (individually or collectively, the “Company Indemnitees”) harmless from and against any and all liability, damage, loss, cost, or expense (including reasonable attorneys’ fees) (collectively, “Losses”) arising out of Third Party
claims and all other Losses arising out of or related to (a) a breach of a representation, warranty, covenant or other obligation of such Transferee or any of its Affiliates, Permitted Transferees or subcontractors under this Agreement or the Assignment and Assumption Agreement; (b) any negligent or reckless act or omission to act or willful misconduct by such Transferee or any of its Affiliates, Permitted Transferees or subcontractors; and (c) any act or omission of such Transferee or any of its Affiliates, Permitted Transferees or their respective employees, Affiliates, agents or subcontractors in connection with the Development, Manufacturing, Commercialization, use, consumption, sale, lease, license, sublicense or other disposition of the Compound or a Covered Product, including but not limited to product liability claims, except in cases where, and to the extent that, such Losses result directly from the breach of this Agreement, negligence or willful misconduct by or on the part of any of the Company Indemnitees and/or any misrepresentation by Company under this Agreement. For clarity, it is understood and agreed that the provisions of Section 10.1(c), above, shall not apply to DRI for so long as DRI does not hold any rights under the Assigned IP or the Licensed IP.
10.2 Indemnification by Company. Company shall indemnify, defend, and hold Transferee, and any Permitted Transferee, Affiliates, and each of their respective agents, employees, officers, and directors, agents, successors and assigns (individually or collectively, the “Transferee Indemnitees”) harmless from and against any and all Losses arising out of Third Party claims and all other Losses to the extent arising out of or related to (a) a breach of a representation, warranty, covenant or other obligation of Company or any of its Affiliates, licensees (other than a Transferee) or subcontractors under this Agreement; (b) any negligent or reckless act or omission to act or willful misconduct by Company or any of its Affiliates, licensees (other than a Transferee) or subcontractors; and (c) any act or omission of Company or any of its Affiliates, licensees (other than a Transferee) or their respective employees, Affiliates, agents or subcontractors in connection with the Development, Manufacturing, Commercialization, use, consumption, sale, lease, license, sublicense or other disposition of the Compound or a Covered Product, including but not limited to product liability claims, except in cases where, and to the extent that, such Losses result directly from the breach of this Agreement, negligence or willful misconduct by or on the part of any of the Transferee Indemnitees and/or any misrepresentation by a Transferee under this Agreement.
10.3 No Consequential Damages. EXCEPT FOR LIABILITY FOR BREACH OF ARTICLE 8 and SECTION 2.5, IN NO EVENT SHALL EITHER PARTY (OR ANY OF ITS AFFILIATES, ASSIGNEES, LICENSEES, OR SUCCESSORS) BE LIABLE TO THE OTHER PARTY (OR ANY OF THE OTHER PARTY’S AFFILIATES, ASSIGNEES, LICENSEES, OR SUCCESSORS) FOR SPECIAL, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR PUNITIVE DAMAGES, WHETHER IN CONTRACT, WARRANTY, TORT, NEGLIGENCE, STRICT LIABILITY, OR OTHERWISE, ARISING OUT OF OR RELATING TO THIS AGREEMENT OR ANY BREACH THEREOF; PROVIDED, HOWEVER, THAT THIS SECTION 10.3 SHALL NOT BE CONSTRUED TO LIMIT EITHER PARTY’S INDEMNIFICATION OBLIGATIONS WITH RESPECT TO THIRD PARTY CLAIMS UNDER SECTION 10.1 OR 10.2, ABOVE.
10.4 Notification of Claims; Conditions to Indemnification Obligations. As a condition to a Company Indemnitee’s or a Transferee Indemnitee’s right to receive indemnification under this Article 10, such Indemnitee shall (a) promptly notify the other party as soon as it becomes aware of any Third Party claim or suit for which indemnification may be sought hereunder, (b) reasonably cooperate, and cause the individual indemnitees to cooperate, with the indemnifying party in the defense, settlement, or compromise of such claim or suit, and (c) permit the indemnifying party to control the defense, settlement, or compromise of such claim or suit, including the right to select defense counsel; provided, however, the indemnified party shall have the right to join any defense with its own counsel at its own expense, or if the indemnifying party declines or fails to assert its intention to
defend such action within sixty (60) days of receipt/sending of notice under this Section 10.4, then the indemnified party shall have the right, but not the obligation, to defend such action. In no event, however, may the indemnifying party compromise or settle any claim or suit in a manner that admits fault or negligence on the part of the indemnified party (or any indemnitee) without the prior written consent of the indemnified party. The indemnifying party shall have no liability under this Article 10 with respect to claims or suits settled or compromised by the indemnified party without the indemnifying party’s its prior written consent.
ARTICLE 11
TERM AND TERMINATION
11.1 Term and Expiration. The term of this Agreement (the “Term”) shall commence on the Effective Date and, unless earlier terminated as expressly provided in this Article 11, shall continue in full force and effect until the later of (i) expiration of the last to expire of a Company Patent in the Territory assigned to the Transferee under this Agreement or (ii) ten (10) years after the date of the First Commercial Sale in the Territory, at which time this Agreement shall expire. After expiration of this Agreement, Transferee’s license to Licensed IP shall become exclusive, fully paid-up, irrevocable, non-terminable, and perpetual, subject to the territorial limitations set forth in Section 2.5 of this Agreement.
11.2 Revocation of the Agreement for Convenience. NovaMedica shall have the right, any time during the Term and at its convenience, to terminate this Agreement in its entirety upon thirty (30) days’ notice to Company. Company shall have no right during the Term to unilaterally terminate the Agreement, in whole or in part, for convenience.
11.3 Termination upon Fundamental Breach.
(a) Company shall have the right to give to a Transferee a notice of termination in the event of Fundamental Breach by the Transferee.
(b) Any notice of termination given pursuant to Section 11.3(a), above, shall specify the nature of the Fundamental Breach, require that the Fundamental Breach be cured, and state Company’s intention to terminate this Agreement if such Fundamental Breach is not cured or disputed with competent evidence reasonably acceptable to Company within six (6) months from the date such notice of termination is given (the “Cure Period”).
(c) In order to constitute a notice of termination under Section 11.3(a), above, such notice shall identify the nature of the alleged breach and shall also include documentary information to substantiate the breach allegation. The Transferee shall have twenty (20) business days following the date of such notice to request in writing such additional supporting information from Company as the Transferee reasonably believes is necessary to allow evaluation of the breach allegation, in response to which Company shall have up to thirty (30) days to provide such additional supporting information in writing.
(d) During the Cure Period, Transferee shall have the right to attempt to cure such Fundamental Breach. If Transferee does not use at least Commercially Reasonable Efforts to cure such Fundamental Breach and such Fundamental Breach is not cured or disputed during the Cure Period, Company shall have the right, at its sole discretion, to terminate this Agreement. Notwithstanding anything to the contrary set forth in this Agreement, upon the occurrence of a Fundamental Breach, Company shall have the right, in its sole discretion and at any time, to seek injunctive relief (including permanent, preliminary and temporary injunctive relief)
against the Transferee and/or any Permitted Transferee. In any request by Company for injunctive relief (whether permanent, preliminary, or temporary injunctive relief) pursuant to this Section 11.3(d), Transferee agrees (for itself and on behalf of any and all Permitted Transferees) that it (they) shall only be entitled to contest likelihood of success on the merits, and shall not be entitled to the contest the propriety of an injunction by arguing a balance of hardships in its favor or the lack of irreparable injury to Company. The balance of hardships in favor of Company and irreparable injury to Company shall be conclusively presumed if the court or other tribunal was not to grant the injunction (whether by a permanent injunction, preliminary injunction, or temporary restraining order).
(e) For the avoidance of doubt, only a breach as described below by or on behalf of the Transferee (including any conduct by or on behalf of a Permitted Transferee that, if committed by Transferee, would constitute such a breach) of this Agreement shall constitute a “Fundamental Breach”: (i) knowing exportation of any Covered Product or the Compound outside the Territory, including any willful transfers of Company Know-How for manufacturing or commercialization of Covered Product outside the Territory, or any other violation of Section 2.5 of this Agreement that resulted in the exportation of any Covered Product or the Compound outside the Territory, (ii) a breach by Transferee or any Permitted Transferee or any of their respective Affiliates of the provisions of Section 8 of this Agreement that resulted in unauthorized use of the Assigned IP, Company Patent Rights and/or Company Know-How for manufacture or otherwise produce the Compound or Covered Products outside the Territory by the Third Parties, or (iii) unless authorized in writing in advance by Company, initiation anywhere in the world of any judicial and administrative action or proceeding that challenges the validity or enforceability of any Company Patent, or any claim of a Company Patent, inside of the Territory, which action or proceeding is not expressly abandoned or withdrawn, dismissed with prejudice, or otherwise terminated with prejudice within the Cure Period.
(f) Any dispute regarding an alleged Fundamental Breach of this Agreement shall be resolved in accordance with Article 12, below. In the event that the Party that has allegedly materially breached this Agreement disputes such breach, then any consequences of termination described in this Article 11 shall apply only from and after such time as such termination has been upheld in a final judgment or arbitral decision from which no appeal can be taken, or that is unappealed with the time allowed for appeal, or such time as the Party allegedly in material breach is no longer disputing such termination.
11.4 Termination by Company with Immediate Effect. Company shall have the right to terminate this Agreement immediately upon written notice to the Transferee if RMI shall breach its obligations to invest the full amount required to be invested by it at the Second Closing of the Investment Transaction in a timely manner, provided that all conditions to and covenants relating to the Second Closing have been satisfied by Company and the other investors.
(a) Termination of Rights. Upon any termination of this Agreement pursuant to Section 11.2, 11.3 or 11.4, above, (i) all Company Improvement Know-How shall automatically revert to Company, and the Transferee shall promptly execute (and shall require each Permitted Transferee to execute) whatever documents Company reasonably requests be executed in order to convey and reconvey and vest all right, title, and interest in and to the Company Improvement Know How, (ii) all sublicenses and license rights granted by Company under this Agreement (and any and all licenses and sublicenses granted by Transferees and/or any
Permitted Transferee(s)) to Company shall terminate immediately, and all rights in and to the Licensed IP under this Agreement (and any related licenses and sublicenses) shall revert automatically to Company, and (iii) immediately and without further action on the party of Company, the Transferee or any Third Party, Company is hereby granted an exclusive, irrevocable, fully paid up, royalty-free license to all Assigned IP. Additionally, (i) Transferee and any Permitted Transferee(s) shall thereafter be without any rights to Develop, Commercialize and/or if applicable, Manufacture, the Compound or Covered Products, and (ii) Transferee and any Permitted Transferee(s) shall promptly cease and desist all such activities and all activities related thereto.
(b) Return of Confidential Information. Upon termination, but not expiration, of this Agreement, each Party shall promptly return to the other Party, or delete or destroy, all relevant records and materials in such Party’s possession or control containing Confidential Information of the other Party; provided, however, that each Party shall be entitled to retain one (1) copy of the other Party’s Confidential Information (subject to a continuing obligation of confidentiality) for the sole purpose of monitoring compliance with the terms of this Agreement, and all Confidential Information received by Company may continue to be used by it insofar as it relates to the Company, any Covered Product or any Improvement.
(c) Accrued Obligations. Expiration or termination of this Agreement for any reason shall not release either Party from any obligation or liability which, at the time of such expiration or termination, has already accrued to the other Party or which is attributable to a period prior to such expiration or termination.
(d) Non-Exclusive Remedy. Termination of this Agreement by a Party shall be without prejudice to other remedies such Party may have at law or equity.
(e) General Survival. The provisions of Articles 1, 8, 10, and 12, and Sections 2.1(c) 2.1(e), 2.5, 6.4, 7.1, 11.5(a), 11.5(b), 11.5(c), 11.5(d), 11.5(e), 13.4, 13.8, 13.10, 13.11, 13.12, 13.13, and 13.15, shall survive expiration or termination of this Agreement. Furthermore, any other provision that by its nature is intended to survive expiration and/or termination of this Agreement shall survive such expiration or termination, as the case may be.
12.1 Disputes. The Parties recognize that disputes as to certain matters may from time to time arise under this Agreement that relate to either Party’s rights and/or obligations hereunder. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes relating to or arising under this Agreement in an expedient manner by mutual cooperation and without resort to litigation. In the event that the Parties are unable to resolve such dispute within thirty (30) days from the day that one Party had designated to the other an issue as a dispute, then either Party shall have the right to escalate such issue to senior management as set forth in Section 12.2, below. Notwithstanding the foregoing, disputes between the Parties with respect to the subject matter of the Clinical Development and Collaboration Agreement (“Development Disputes”) shall be resolved in accordance with dispute resolution procedures in the Clinical Development and Collaboration Agreement for Development Disputes.
12.2 Escalation to Senior Representatives. Either Company or the Transferee may, by written notice to the other, request that a dispute that remained unresolved for a period of thirty (30) days as set forth in Section 12.1, above, be resolved by the chief executive officer of the Transferee (or such person’s designee) (the “Transferee Representative”) and the chief executive officer of Company (or such person’s
designee) (the “Company Representative”) (collectively, the “Representatives”) within sixty (60) days of the Representatives’ first consideration of such dispute, but in all cases within ninety (90) days after a Party’s written request for resolution by the Representatives. If the Representatives cannot resolve such dispute within such ninety (90) day period, either Party may proceed to enforce any and all of its rights with respect to such dispute in accordance with Section 12.3, 12.4, or 12.5, below, as applicable.
12.3 Arbitration.
(a) Any dispute, controversy or claim arising out of, relating to or in connection with, this Agreement, including any dispute regarding the interpretation, validity or termination or the performance or breach of this Agreement, as well as any non-contractual obligation arising out of or in connection with this Agreement, which is not resolved by mutual agreement, above, shall be finally settled by binding arbitration under the Rules of the London Court of International Arbitration (the “LCIA”) then in effect.
(b) The arbitration shall be conducted by arbitrators, of whom one shall be nominated by the Transferee and one shall be nominated by Company. The two arbitrators so appointed shall nominate the third arbitrator, who shall act as chairman of the Arbitral Tribunal. In the event that an arbitrator is not appointed pursuant to the foregoing provisions within the time period prescribed under the Rules of the LCIA or within the time-period agreed upon by the parties to the arbitration, upon request of either party to the arbitration, such arbitrator shall instead be appointed by the Court of the LCIA.
(c) The Parties shall use good faith efforts to complete arbitration under this Section 12.3 within one hundred eighty (180) days following the initiation of such arbitration. The Arbitral Tribunal shall permit full and complete discovery, both written and oral by deposition and shall establish reasonable additional procedures to facilitate and complete such arbitration within such one hundred eighty (180) day period. The place of arbitration will be London, England. The language of the arbitration, and all proceedings thereunder, including all discovery (both written and oral) will be English.
(d) By agreeing to arbitration, the Parties do not intend to deprive any court of competent jurisdiction of its ability to issue any form of provisional remedy, including but not limited to a preliminary injunction or attachment in aid of the arbitration, or order any interim or conservatory measure. A request for such provisional remedy or interim or conservatory measure by a Party to a court or other government entity shall not be deemed a waiver of this agreement to arbitrate.
(e) The award rendered by the Arbitral Tribunal, which shall cover which party shall bear the costs of the arbitration in accordance with subsection (f) below, shall be final and binding on the parties. Judgment on the award may be entered in any court of competent jurisdiction.
(f) The costs of such arbitration, including administrative and fees of the arbitrators comprising the arbitration panel, shall be shared equally by the Transferee and Company, and each Party shall bear its own expenses and attorney’s fees incurred in connection with the arbitration; provided, however, that the arbitration panel may direct that the costs and attorney fees paid by one party be reimbursed by the other party. The arbitration panel shall consider the following factors in determining whether to award costs and attorney fees to be paid by one party to another party: (i) the conduct of the Parties in the transactions or occurrences that
gave rise to the dispute or claim, including any conduct of a party that was reckless, willful, malicious, in bad faith or illegal; (ii) the objective reasonableness of the claims and defenses asserted by a Party; (iii) the extent to which an award of costs and attorney fees would deter future bad faith claims or defenses; (iv) the objective reasonableness of the Parties and the diligence of the Parties and their attorneys during the proceedings; (v) the objective reasonableness of the Parties and the diligence of the parties in pursuing settlement of the dispute; and (vi) such other factors as the arbitration panel may consider appropriate under the circumstances.
12.4 Provisional Remedies. Nothing in this Agreement shall limit the right of either Party to seek to obtain in any court of competent jurisdiction any equitable or interim relief or provisional remedy, including injunctive relief, pending resolution under Section 12.1, 12.2, or 12.3, above, as applicable, that may be necessary to protect the rights or property of that Party. Seeking or obtaining such equitable or interim relief or provisional remedy in a court shall not be deemed a waiver of the agreement to arbitrate. For clarity, any such equitable remedies shall be cumulative and not exclusive and are in addition to any other remedies that either Party may have under this Agreement or applicable Law.
12.5 Remedies. In the event a Party is in breach of its obligations under this Agreement, and the breach is not remedied in accordance with the terms of this Agreement, the other Party shall be entitled to all remedies available in law or equity, consistent with the terms of this Agreement, including without limitation, collection of damages, injunctive relief, and specific performance.
ARTICLE 13
MISCELLANEOUS PROVISIONS
13.1 Relationship of the Parties. Nothing in this Agreement is intended or shall be deemed to constitute a partnership, agency, joint venture, or other relationship between the Parties.
13.2 Third Party Beneficiaries. NovaMedica is hereby designated as an intended third party beneficiary under this Agreement, and may independently and without prior notice or permission, enforce all rights and obligations of Transferee pursuant to this Agreement. Further, Company shall be deemed a third party beneficiary of any agreement between DRI and NovaMedica and/or between NovaMedica and any Permitted Transferee relating to this Agreement, the Assigned IP, the Licensed IP and/or any Improvements thereto, and Company may enforce the provisions of this Agreement and all such other agreements against NovaMedica and/or any such Permitted Transferee to the same extent that the Company may enforce the provisions of this Agreement against Transferee. Except as provided in the preceding sentence and as expressly provided elsewhere in this Agreement with respect to Company Indemnitees and Transferee Indemnitees, this Agreement is neither expressly nor impliedly made for the benefit of any Person other than Company or NovaMedica, or Transferee.
13.3 Assignment.
(a) Except as expressly provided herein, neither this Agreement nor any interest hereunder shall be assignable, nor any other obligation delegable, by either Party without the prior written consent of the other Party (not to be unreasonably withheld or delayed). Notwithstanding the foregoing, each Party shall have the right to assign this Agreement in whole without the consent of the other Party to (X) any Affiliate or (Y) an Acquirer, whether by merger, sale of stock, sale of assets or similar transaction, operation of law, or otherwise; provided, however, that such Affiliate must accept all rights and obligations under this Agreement as well as
those in any Ancillary Agreements, including the Clinical Development and Collaboration Agreement and any Supply Agreement, or (Z), in the case of DRI, to NovaMedica.
(b) Any permitted assignment under this Section 13.3 shall relieve the assigning Party of any and all of its responsibilities or obligations hereunder, provided that, as a condition of such assignment, the assignee agrees in writing to be bound by all obligations of the assigning Party hereunder.
(c) This Agreement shall be binding upon the successors and permitted assigns of the Parties.
(d) Any assignment not in accordance with this Section 13.3 shall be void.
13.4 Performance by Transferee and Permitted Transferees. Notwithstanding anything to the contrary in this Agreement, the Transferee shall have the right to have any of its obligations hereunder performed, or its rights hereunder exercised, by one or more Permitted Transferees, and the performance of such obligations by the Transferee or a Permitted Transferee shall be deemed to be performance by Transferee and, provided that (a) the Transferee or any Permitted Transferee that agrees to perform or exercise on the Transferee’s behalf any of the Transferee’s obligations or rights under this Agreement agrees that Company is an intended third party beneficiary of any such performance or exercise, (b) the Transferee provides Company with a copy of such agreement between the Transferee or a Permitted Transferee, and (c) all other provisions of this Agreement relating to such transfer shall apply.
13.5 Protection under Section 365(n) US Bankruptcy Code. All rights granted under or pursuant to this Agreement by Company are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, if applicable, grants of rights in and to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Transferee, as grantee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against Company under the U.S. Bankruptcy Code, the Transferee shall be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in the Transferee’s possession, shall be promptly delivered to it (or its designee) (a) upon any such commencement of a bankruptcy proceeding upon the Transferee’s written request thereof, or (b) if not delivered under clause (a), following the rejection of this Agreement by Company upon written request thereof by the Transferee.
13.6 Further Actions. Each Party agrees to execute, acknowledge, and deliver such further instruments and to do all such other acts as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
13.7 Force Majeure. Neither Party shall be liable to the other for failure or delay in the performance of any of its obligations under this Agreement for the time and to the extent such failure or delay is caused by acts of God, earthquake, riot, civil commotion, terrorism, war, strikes or other labor disputes, fire, flood, failure or delay of transportation, default by suppliers or unavailability of raw materials, governmental acts or restrictions or any other reason which is beyond the control of the respective Party.
13.8 Entire Agreement of the Parties; Amendments. This Agreement and the schedules hereto constitute and contain the entire understanding and agreement of the Parties respecting the subject matter hereof and cancel and supersede any and all prior negotiations, correspondence,
understandings, and agreements between the Parties, whether oral or written, regarding such subject matter. No waiver, modification, or amendment of any provision of this Agreement shall be valid or effective unless made in a writing referencing this Agreement and signed by a duly authorized officer of each Party.
13.9 Captions. The captions to this Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement.
13.10 Governing Law. This Agreement shall be governed by and interpreted in accordance with the Laws of the State of New York, USA, excluding the application of any conflict of laws principles that would require application of the Law of another jurisdiction; provided, however, that matters of intellectual property law shall be determined in accordance with the national intellectual property laws relevant to the intellectual property at issue.
13.11 Notices and Deliveries. Any notice, request, approval, or consent required or permitted to be given under this Agreement shall be in writing and shall be deemed to have been sufficiently given if delivered in person, transmitted, by facsimile (receipt verified), or by express courier service (signature required) to the Party to which it is directed at its address or facsimile number shown below or such other address or facsimile number as such Party shall have last given by notice to the other Party.
If to DRI, addressed to:
Domain Russia Investments Limited
c/o Xxxx Xxxxx LLP
The Broadgate Tower, Third Floor
20 Primrose Street
City of Xxxxxx, XX0X 0XX
Xxxxxx Xxxxxxx
Attention: President & CEO
Facsimile: x00-00-0000-0000
With a copy to:
Xxxx Xxxxx, LLP
0000 Xxxxxx xx xxx Xxxxx, Xxxxx 000
Xxx Xxxxxxx, XX 00000
Fax: 000-000-0000
Attention: Xxxxxxx Xxxxxxx/Xxxxxx Xxxxx
If to Company, addressed to:
Marinus Pharmaceuticals, Inc.
00 Xxxxxxxx Xxxx Xxxxx
Xxxxxxxx, Xxxxxxxxxxx 00000 XXX
Attention: President and CEO
Facsimile: 203-3150565
With a copy to:
Xxxxx Xxxxxx LLP
00 Xxxxx 00xx Xxxxxx
Xxxxxxxxxxxx, XX 00000-0000
Attention: Xxxxxxxx X. Xxxx
Facsimile: (000) 000-0000
13.12 Waiver. A waiver by either Party of any of the terms and conditions of this Agreement in any instance shall not be deemed or construed to be a waiver of such term or condition for the future, or of any other term or condition hereof. All rights, remedies, undertakings, obligations, and agreements contained in this Agreement shall be cumulative and none of them shall be in limitation of any other remedy, right, undertaking, obligation, or agreement of either Party.
13.13 Severability. When possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable Law, but if any provision of this Agreement is held to be prohibited by or invalid under applicable Law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Agreement. The Parties shall make a good faith effort to replace the invalid or unenforceable provision with a valid one that in its economic effect is most consistent with the invalid or unenforceable provision.
13.14 Assumptions. The terms and provisions in this Agreement have been negotiated and drafted on the assumption by the Parties that there are no laws or regulations in the Territory that will prevent or significantly hinder Transferee (or Permitted Transferees) or Company from performing their obligations and realizing their benefits as set forth in this Agreement. If this assumption ultimately proves to be untrue, the Parties will use good faith efforts to make such revisions as are reasonable and equitable to the Parties and are in compliance with the laws and regulations of the Territory.
13.15 English as the Controlling Language for all Agreements. All notices and other communications under this Agreement and any related agreements, including assignments, licenses, and/or sublicenses with Transferee and/or a Permitted Transferee, shall be in the English language. Transferee shall, furthermore, require that any assignment, license, sublicense, or other agreement (i) having Transferee or a Permitted Transferee as a party and (ii) a copy of which is required by this Agreement to be provided a Party, shall be prepared in the English language, which language shall govern the interpretation of, and any dispute regarding, the terms of that agreement.
13.16 Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed an original, and all of which together will be deemed to be one and the same instrument. A facsimile copy of this Agreement, including the signature pages, will be deemed an original.
(Signature page follows.)
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed and delivered by their respective duly authorized officers as of the day and year first above written, each copy of which shall for all purposes be deemed to be an original.
|
MARINUS PHARMACEUTICALS, INC. |
|
DOMAIN RUSSIA INVESTMENTS LIMITED | ||
|
|
|
| ||
|
|
|
| ||
|
By: |
/s/ Xxxxxxxxxxx X. Xxxxxxx |
|
By: |
/s/ Xxxxxxx Xxxxxxxxxx |
|
|
|
|
|
|
|
Name: |
Xxxxxxxxxxx X. Xxxxxxx |
|
Name: |
Xxxxxxx Xxxxxxxxxx |
|
|
|
|
|
|
|
Title: |
CEO |
|
Title: |
CEO |
Schedule 1.1 — Assigned IP
CIS/EA/RU Patent Rights
|
Patent/App |
|
Title |
|
Description |
|
Status |
|
Filing |
|
Expiration |
|
Assignee |
|
EA200801468 |
|
|
|
|
|
Pending |
|
28 Nov 2006 |
|
28 Nov 2026 |
|
Marinus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EA201270241 |
|
Method for Making Ganaxolone |
|
efficient ganaxolone synthesis methods |
|
pending |
|
11 Aug 2010 |
|
11 Aug 2030 |
|
Marinus |
Schedule 1.2 — Licensed IP
All Company Know-How existing on the Effective Date.
ganaxolone (3a-hydroxy-3b-methyl-5a-pregnan-20-one), having the chemical structure:
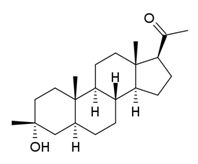
Schedule 2 — Territory
CIS states:
1. Armenia
2. Azerbaijan
3. Belarus
4. Kazakhstan
5. Kyrgyzstan
6. Xxxxxxx
0. Xxxxxx
8. Tajikistan
9. Ukraine
10. Uzbekistan
Non-CIS states:
11. Georgia
12. Turkmenistan
Schedule 3 — Technology Transfer Outline
The following database has been organized to support the transfer of Confidential data and information to NovaMedica.
1. Non-clinical and Clinical Data
2. CMC and Manufacturing Know How
a. Drug substance API
b. Drug product
3. Ganaxolone Patents and Applications
4. Regulatory correspondence
After the Effective Date of the Assignment and Assumption Agreement, NovaMedica will be granted access to the information above in a “Dropbox” folder, with the right to download copies of the documents subject to the provisions of Article 8 of this Agreement. After review the appropriate members of the Marinus Team will be available by phone to address any questions.
Schedule 4 — Prosecution and Maintenance of Patent Rights
1. Patent Prosecution and Maintenance. The provisions of this Schedule 4 concern the Prosecution and Maintenance of Patent Rights subject to this Agreement, in accordance with the terms of its Section 7.2.
(a) Each Party’s Patents. Subject to Sections 1(b) and 1(c), below, each Party shall Prosecute and Maintain the Patent Rights such Party Controls in the Territory (including in the case of the Transferee, the Patents included in the Assigned IP), at its expense before the date of registration of assignment of such Patent Rights with the Government Bodies on the name of the Transferee; and the Transferee shall Prosecute and Maintain the Company Patent Rights assigned to Transferee after such registration The Prosecuting Party shall provide the other Party a reasonable opportunity prior to a priority filing, but in no event less than thirty (30) days, to review and comment upon the content and text of any new application within such Party’s Patent Rights that contains at least one claim covering a Compound or a Covered Product, or a method of making or using the Compound or a Covered Product. The Prosecuting Party shall provide the other Party with an electronic copy of each patent application within such Party’s Patent Rights in the Territory. The Prosecuting Party shall keep the other Party advised of the status of all material communications to and from applicable patent offices, actual, and prospective filings or submissions regarding the Prosecuting Party’s Patent Rights in the Territory, and shall give the other Party an opportunity to review and comment in advance on any such communications, filings, and submissions proposed to be sent to any patent office in the Territory. Each Party agrees to provide the other Party with all information necessary or desirable to enable the other Party to comply with the duty of candor/duty of disclosure requirements of any patent authority in the Territory. Each Party shall provide to the other with an annual update of the patent status of the updating Party’s Patent Rights in the Territory relating to the Compound or any Covered Product.
(b) Transferee Patents. In addition to the provisions of Section 1(a), above, Company and the Transferee agree that Company shall have a reasonable opportunity to review and comment, either directly or indirectly through outside patent counsel selected by Company, on the design and implementation of strategy regarding all Patent Rights of the Transferee and all Permitted Transferees relating to the Compound or any Covered Product, and Transferee shall reasonably consider such comments.
(c) Election Not To Prosecute or Maintain Patents Rights. If any Party elects not to Prosecute or Maintain any Patent Right that such Party Controls in any country or jurisdiction within the Territory, then it shall notify the other Party in writing at least ninety (90) days before any final deadline applicable to the filing, prosecution, or maintenance of such the Patent Right, as the case may be, or any other date by which an action must be taken to establish or preserve such the Patent Right in such country or possession. In such case, by no later than thirty (30) days before any final deadline applicable to the filing, prosecution, or maintenance of such the Patent Right, or any other date by which an action must be taken to establish or preserve such Patent Right in such country or possession, the other Party (or its assignee) shall have the right, but not the obligation, to pursue the filing or support the continued Prosecution and Maintenance of such the Patent Right, at such other
Party’s (or its assignee’s) expense, and the Party shall use Commercially Reasonable Efforts assist and perform all actions to ensure that the other Party has all powers, authority and ability that are necessary to Prosecute and Maintain the in accordance with this Agreements.
(d) Joint Patents. With respect to any potentially patentable Joint Invention, the Transferee (or its designee(s)) shall have the first right to be responsible for, and control the Prosecution and Maintenance of Patents covering such Joint Invention (“Joint Patent”) throughout the Territory, and Company shall have the first right to be responsible for, and control the Prosecution and Maintenance of Joint Patent throughout the rest of the world outside the Territory. If the Transferee (or its designee(s)) elects in its sole discretion not to Prosecute and Maintain a Joint Patent in any country or jurisdiction in the Territory, then it shall notify Company in writing at least ninety (90) days before any deadline applicable to the filing, prosecution, or maintenance of such Joint Patent, as the case may be, or any other date by which an action must be taken to establish or preserve such Joint Patent in such country or jurisdiction, and in such case Company shall have the right, but not the obligation, to pursue the filing or support the continued Prosecution or Maintenance of such Joint Patent in the Territory. The prosecuting party for any Joint Patent shall provide the other an opportunity to review and comment upon the content and text of the applications within the Joint Patents filed after the Effective Date at least thirty (30) days before filing with any patent office. The prosecuting party shall provide the other Party with an electronic copy of each patent application within the Joint Patents as filed, together with notice of its filing date and serial number. The prosecuting party of any Joint Patent shall keep the other Party advised of the status of all material communications to and from applicable patent offices, actual, and prospective filings or submissions regarding Joint Patents, and shall give the other Party an opportunity to review and comment in advance on any such communications, filing, and submissions proposed to be sent to any patent office. Each Party agrees to provide the other Party with all information reasonably necessary or desirable to enable the prosecuting party for any Joint Patent to comply with the duty of candor/duty of disclosure requirements of any patent authority.
(e) Patent Term Restoration. Company and Transferee (or, as the case may be, Permitted Transferee) will cooperate with each other in obtaining patent term restoration or supplemental protection certificates or their equivalents in any country in the Territory where applicable to Company Patents assigned to the Transferee hereunder and Joint Patents.
(f) Expenses. Notwithstanding anything to the contrary set forth in this Agreement, the Transferee shall reimburse Company for all Out-of-Pocket Expenses incurred by Company in connection with the Prosecution and Maintenance of Assigned IP in the Territory.
Schedule 5 — Enforcement and Defense of Patent Rights
1. Patent Enforcement and Defense. The provisions of this Schedule 5 concern the Enforcement and Defense of Patent Rights subject to this Agreement, in accordance with the terms of its Section 7.3.
2. Enforcement of Patent Rights.
(a) Notice. If either Party believes that a Company Patent assigned to the Transferee hereunder or a Joint Patent is being infringed in the Territory by a Third Party or if a Third Party claims that any Company Patent assigned to the Transferee hereunder or a Joint Patent within the Territory is invalid or unenforceable (collectively, “Infringing Activities”), the Party possessing such knowledge or belief shall notify the other Party and provide it with details of such infringement or claim that are known by such Party. As between the Parties, the right to enforce such Company Patent or Joint Patent with respect to such infringement, or to defend any declaratory judgment action with respect thereto, or to compromise or settle such infringement claim or declaratory judgment action, in each case to the extent the same pertains to Infringing Activities (each, an “Enforcement Action”) shall be as set forth in this Section 2. For purposes of this Agreement, “Initiating” or to “Initiate”, with respect to an Enforcement Action, refers to bringing an infringement claim or defending a declaratory judgment action, or compromising or settling such infringement claim or allegation or declaratory judgment action, within any country or jurisdiction in the Territory.
(b) Right to bring an Action. The Transferee, or a Transferee Assignee or Permitted Transferee, as applicable, shall have the first right to attempt to xxxxx Infringing Activities within the Territory, including by taking and controlling an Enforcement Action with respect to Company Patents assigned to the Transferee hereunder and Joint Patents. If the Transferee (or a Transferee Assignee or Permitted Transferee) does not intend to prosecute or defend an Enforcement Action, the Transferee shall promptly inform Company. If the Transferee (or a Transferee Assignee or Permitted Transferee) does not take an Enforcement Action with respect to such an infringement or claim within one hundred twenty (120) days following notice thereof or a request by Company to do so, Company shall have the right, but not the obligation, to take and control an Enforcement Action in the name of either or both Parties. The Party taking such Enforcement Action shall have the sole and exclusive right to select counsel for any suit it initiates pursuant to this Section 2 of Schedule 5.
(c) Costs of an Action. Subject to the respective indemnity obligations of the Parties set forth in Article 10 of the Agreement, the Party (or its Affiliate or Permitted Transferee or successor) taking an Enforcement Action under Section 2(b) of this Schedule 5 shall pay all reasonable and incurred costs associated with such Enforcement Action, other than (subject to Section 2(e), below) the expenses of the other Party if the other Party elects to join such Enforcement Action. Each Party (or an Affiliate or Permitted Transferee or successor of such Party) shall, at its own expense, have the right to join an Enforcement Action taken by the other Party.
(d) Settlement. Neither Party (nor an Affiliate or Permitted Transferee or successor of a Party) shall settle or consent to any judgment or otherwise compromise any
Enforcement Action by admitting that any Company Patent assigned to the Transferee hereunder or Joint Patent is invalid or unenforceable without the other Party’s prior written consent, which consent shall not be unreasonably conditioned, refused, withheld or delayed, and, (i) in the case of Company, Company may not settle or otherwise compromise an Enforcement Action in a way that adversely affects or would be reasonably expected to adversely affect the Transferee’s rights or benefits hereunder in the Territory with respect to the Covered Product in the Field, without the Transferee’s) prior written consent, which consent shall not be unreasonably withheld or delayed, and (ii) in the case of the Transferee, the Transferee may not settle or otherwise compromise an Enforcement Action in a way that adversely affects or would be reasonably expected to adversely affect Company’s rights or benefits outside of the Territory without Company’s prior written consent, which consent shall not be unreasonably withheld or delayed.
(e) Reasonable Assistance. The Party not taking an Enforcement Action shall provide reasonable assistance to the other Party, including providing access to relevant documents and other evidence and making its employees available, subject to the other Party’s (or the other Party’s Affiliate’s) reimbursement of any Out-of-Pocket Expenses incurred by the non-enforcing or non-defending Party in providing such assistance.
(f) Distribution of Amounts Recovered. Any amounts recovered by the Party (or its Affiliate or Permitted Transferee or successor) taking an Enforcement Action pursuant to this Section 2, whether by settlement or judgment, shall first be applied reimbursement of all reasonable costs and expenses of the Parties that conducted an Enforcement Action, including to pro-rata reimbursement of the unreimbursed reasonable legal fees and expenses incurred by the Parties in such Enforcement Action, and any remainder shall be received by the Party initiating and conducting the Enforcement Action.
3. Defense of Patent Rights.
(a) Notice. If a Party becomes aware of any claim or action by a Third Party against either Party that claims that the Covered Product, or its use, Development, Manufacture, Commercialization or sale, in the Territory infringes such Third Party’s intellectual property rights in the Territory (each, a “Third Party Action”), such Party shall promptly notify the other Party of all details regarding such claim or action that is reasonably available to such Party.
(b) Right to Defend. The Transferee, or a Transferee Assignee or Permitted Transferee, shall have the first right, at its sole expense, but not the obligation, to defend a Third Party Action through counsel of its choosing, subject to Section 3(c), below. Company shall have the right to join any defense brought by the Transferee (or a Transferee Assignee or Permitted Transferee), with its own counsel at its own expense, or in the event that the Transferee (or a Transferee Assignee or Permitted Transferee) declines or fails to assert its intention to defend such Third Party Action within sixty (60) days of receipt/sending of notice under Section 3(a), above, then Company shall have the right, but not the obligation, to defend such Third Party Action to the extent legally permissible. The Party defending such Third Party Action shall have the sole and exclusive right to select its counsel for such Third Party Action.
(c) Consultation. The Party (including a Permitted Transferee) defending a Third Party Action pursuant to Section 3(b) shall be the “Controlling Party.” The Controlling Party shall consult with the non-Controlling Party on all material aspects of the defense. The non-Controlling Party shall have a reasonable opportunity for meaningful participation in decision-making and formulation of defense strategy. The Parties shall reasonably cooperate with each other in all such actions or proceedings. The non-Controlling Party will be entitled to be represented by independent counsel of its own choice at its own expense.
(d) Appeal. In the event that a judgment in a Third Party Action is entered against the Controlling Party and an appeal is available, the Controlling Party shall have the first right, but not the obligation, to file such appeal. In the event the Controlling Party does not desire to file such an appeal, it will promptly, within a reasonable time period (i.e., with sufficient time for the non-Controlling Party to take whatever action may be necessary) prior to the date on which such right to appeal will lapse or otherwise diminish, permit the non-Controlling Party to pursue such appeal at such non-Controlling Party’s own cost and expense. If applicable Law requires the other Party’s involvement in an appeal, the other Party shall be a nominal party of the appeal and shall provide reasonable cooperation to the appealing Party at the appealing Party’s expense.
(e) Costs of an Action. Subject to the respective indemnity obligations of the Parties set forth in Article 10 of this Agreement, the Controlling Party shall pay all costs associated with a Third Party Action, other than the expenses of the other Party if the other Party elects to join such Action. Each Party shall have the right to join a Third Party Action defended by the other Party, at its own expense.
(f) No Settlement Without Consent. A Controlling Party shall not settle or otherwise compromise any Third Party Action by admitting that any Company Patent assigned to the Transferee hereunder or Joint Patent is invalid or unenforceable without the non-Controlling Party’s prior written consent, and, (i) in the case of Company, Company may not settle or otherwise compromise a Third Party Action in a way that adversely affects or would reasonably be expected to adversely affect the Transferee’s rights and benefits hereunder with respect to Development and Commercialization of Covered Products within the Territory without the Transferee’s prior written consent, which consent shall not be unreasonably withheld, refused, conditioned or delayed and (ii) in the case of the Transferee, the Transferee may not settle or otherwise compromise an Enforcement Action in a way that adversely affects or would reasonably be expected to adversely affect Company’s rights or benefits outside of the Territory without Company’s prior written consent, which consent shall not be unreasonably withheld, conditioned, refused or delayed.
Schedule 6 — Form Pharmacovigilance Agreement
This Pharmacovigilance Agreement (this “PV Agreement”) is dated as of 201 (the “Effective Date”), by and between NovaMedica LLC, a limited liability company organized under the laws of the Russian Federation with an address 10113, xxxx. 00, Xxxxxxxxxxxxx Xxx Xxxxxx, Xxxxxx, Russian Federation (“NovaMedica”), and Marinus Pharmaceuticals, Inc., a corporation organized under the laws of the State of Delaware and having its place of business at 00 Xxxxxxxx Xxxx Xxxxx, Xxxxxxxx, Xxxxxxxxxxx 00000, XXX (“Marinus”). NovaMedica and Marinus may each be referred to herein as a “Party” or, collectively, as “Parties.”
WHEREAS, Domain Russia Investments Limited, a limited company organized under the laws of England and Wales with registration number 7899075, having its registered office at The Broadgate Tower, Third Floor, 20 Primrose Street, City of Xxxxxx, XX0X 0XX, Xxxxxx Xxxxxxx (“DRI”), and Marinus entered into a Technology Transfer Agreement (“TTA”) on December 4, 2012, whereby Marinus licensed to DRI the sole and exclusive right to itself or through Permitted Transferees (as defined in the TTA) to Develop and Commercialize in the Territory certain products in exchange for the consideration specified in the TTA;
WHEREAS, pursuant to Section 4.3 of the TTA, NovaMedica and Marinus wish to conclude the terms governing pharmacovigilance in respect of the Covered Products subject to the TTA and NovaMedica-DRI Assignment and Assumption Agreement.
NOW, THEREFORE, in consideration of the various promises and undertakings set forth herein, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
Unless otherwise specifically provided herein or in the TTA, the following terms shall have the following meanings, and other capitalized terms used herein shall have the meanings as set forth in the TTA:
1.1 “Adverse Event” (also referred to in the ICH guidance as an “Adverse Experience”) means any untoward medical occurrence in a patient or clinical investigation subject administered a Covered Product and which does not necessarily have to have a causal relationship with this treatment.
1.2 “Serious Adverse Event” means any Adverse Event that is (a) associated with the use of a Covered Product or has occurrence in a blinded study that included Covered Product and (b) serious, including death, a life-threatening event (at immediate risk of death from the event as it occurs), an event that requires inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant disability/incapacity, a congenital anomaly/birth defect, or is an important medical event that may not result in any of the above outcomes, but, based upon appropriate medical judgment, may jeopardize the patient or subject and may require medical or surgical intervention to prevent any such the outcome.
1.3 Interpretation. The captions and headings to this PV Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement. Unless context otherwise clearly requires, whenever used in this PV Agreement:
(i) “include” or “including” shall be construed as if followed by the words “but not limited to” or “without limitation” or words of similar import; (ii) the word “or” shall be construed as the inclusive meaning identified with the phrase “and/or;” (iii) provisions that require that a Party, the Parties, or any committee or team hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent, or approval be specific and in writing, whether by written agreement, letter, written approval of minutes, or otherwise; and (iv) references to any specific Law or article, section, or other division thereof shall be deemed to include the then-current amendments thereto or any replacement Law thereof. This Agreement was prepared in the English language, which language shall govern the interpretation of, and any dispute regarding, the terms of this PV Agreement.
ARTICLE 2
POLICIES
2.1 Existing Procedures. Each Party shall ensure that its existing procedures for intake, review, and reporting of Adverse Events and Serious Adverse Events comply with ICH Guidance E2A on Clinical Data Safety Data Management.
2.2 Document Maintenance. Each Party shall maintain the original source documents with regard to any Adverse Event, and Serious Adverse Event in accordance with current Law and regulatory requirements of the Regulatory Authority(ies) in its respective territory.
2.3 Information Exchange. The Parties shall exchange Adverse Event and Serious Adverse Event information as set forth in Article 3 and exchange any other safety information as appropriate.
2.4 Review. The Parties shall review this PV Agreement periodically, but in no event less than annually, and revise and/or update it as necessary in order to ensure the complete and timely exchange of safety information.
2.5 Central Database. Marinus shall maintain a central international Adverse Event database of Adverse Event and Serious Adverse Event reports associated with the Compound or a Covered Product (the “Central Database”). Not later thirty (30) days after the first clinical site initiation of a clinical study of Covered Product, Marinus shall provide NovaMedica with a copy of Marinus’s standard operating procedure (“SOP”) for safety-related reporting, including a detailed technical description of any data or information intended for inclusion and storage in, and retrieval from, the Central Database. Marinus shall periodically, but less than annually, review its SOP to ensure adequacy and compliance with then-applicable United States FDA and EMEA regulatory reporting requirements.
2.6 Data Use. NovaMedica shall have the right to obtain data from the central database within a reasonable time frame to meet requirements for providing such data and information to any Regulatory Authority in the Territory as may be required by Law or as otherwise may be reasonably necessary to comply with requirements of a Regulatory Authority within the Territory.
ARTICLE 3
ADVERSE EXPERIENCES
3.1 Adverse Event Reporting. With respect to Adverse Events, in the event either Party becomes aware through its own Development or Commercialization activities or receives a
report of an Adverse Event from a Third Party, the Party receiving such report shall enter the adverse report information according to SOPs into the Central Database. Marinus will run periodic safety reports at intervals to be determined and to be shared with NovaMedica. With respect to Serious Adverse Events, in the event either Party becomes aware of through its own Development or Commercialization activities or receives a report of a Serious Adverse Event from a Third Party, the Party receiving such report shall provide initial case notification to the other Party via electronic communication within one (1) calendar day for death and life threatening, and five (5) calendar days for all other, Serious Adverse Event reports. Marinus shall have the right to review all applicable data, records and reports regarding all Adverse Events and Serious Adverse Events reported by NovaMedica and to fully investigate such Adverse Events and Serious Adverse Events at the sites and locations thereof including conducting such personnel interviews as needed so that Marinus may comply with applicable regulations by regulatory agencies outside the Territory.
3.2 Reporting Procedures. Procedures regarding initial exchange safety data and frequency thereafter will be determined via the Joint Development Committee in accordance with requirements to support development activities.
3.3 Regulatory Filings in the Territory. With respect to regulatory filings filed by or on behalf of Marinus (or NovaMedica, as the case may be) in the Territory, the party designated by Marinus and NovaMedica to report to Regulatory Authorities in the Territory shall be responsible for reporting to such Regulatory Authorities any Adverse Event required to be reported, whether in non-clinical or clinical studies for or during Development or Commercialization of any Covered Product. The filing party shall promptly provide the other party with a complete and true copy of any Adverse Event report filed with a Regulatory Authority within the Territory.
3.4 Communication. Information exchanged by the Parties pursuant to this PV Agreement can be transmitted by a secure internet-based interface, e-mail, facsimile, overnight courier, or any other means the Parties agree. Communications hereunder shall be directed as follows:
If to NovaMedica:
[insert point of contact name and contact information]
If to Marinus:
[insert point of contact name and contact information]
ARTICLE 4
MISCELLANEOUS
4.1 Term. Following execution, this PV Agreement shall remain in full force and effect until expiration or termination of the TTA, unless terminated earlier by mutual written agreement between the Parties.
4.2 No Consequential Damages. IN NO EVENT SHALL EITHER PARTY OR ANY OF ITS AFFILIATES OR SUBLICENSEES BE LIABLE TO THE OTHER PARTY OR ANY OF ITS AFFILIATES OR SUBLICENSEES FOR SPECIAL, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR PUNITIVE DAMAGES, WHETHER IN CONTRACT, WARRANTY, TORT, NEGLIGENCE, STRICT LIABILITY, OR OTHERWISE ARISING OUT OF OR RELATING TO THIS AGREEMENT OR ANY BREACH THEREOF.
4.3 Additional Signatories. In the event that NovaMedica shall transfer any rights to Covered Products to a Permitted Transferee, then NovaMedica shall require such Permitted Transferee to become a signatory hereto and subject to the same rights and obligations as NovaMedica hereunder.
4.4 Entire Agreement; Amendments. This PV Agreement, the TTA, and any other agreement appended thereto as a schedule or exhibit, constitute and contain the entire understanding and agreement of the Parties respecting the subject matter hereof and cancel and supersede any and all prior negotiations, correspondence, understandings, and agreements between the Parties, whether oral or written, regarding such subject matter. No waiver, modification, or amendment of any provision of this PV Agreement shall be valid or effective unless made in a writing referencing this PV Agreement and signed by a duly authorized officer of each Party.
4.5 Captions. The captions to this PV Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions hereof.
4.6 Force Majeure. Neither Party shall be liable to the other for failure or delay in the performance of any of its obligations under this PV Agreement for the time and to the extent such failure or delay is caused by acts of God, earthquake, riot, civil commotion, terrorism, war, strikes or other labor disputes, fire, flood, failure or delay of transportation, default by suppliers or unavailability of raw materials, governmental acts or restrictions, or any other reason which is beyond the control of the respective Party
4.7 Governing Law. This PV Agreement shall be governed by and interpreted in accordance with the Laws of the State of Delaware, U.S.A., excluding the application of any conflict of laws principles that would require application of the Law of another jurisdiction.
4.8 Waiver. A waiver by either Party of any of the terms and conditions of this PV Agreement in any instance shall not be deemed or construed to be a waiver of such term or condition for the future, or of any other term or condition hereof. All rights, remedies, undertakings, obligations, and agreements contained in this PV Agreement shall be cumulative and none of them shall be in limitation of any other remedy, right, undertaking, obligation, or agreement of either Party.
4.9 Severability. When possible, each provision of this PV Agreement will be interpreted in such manner as to be effective and valid under applicable Law, but if any provision of this PV Agreement is held to be prohibited by or invalid under applicable Law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this PV Agreement. The Parties shall make a good faith effort to replace the invalid or unenforceable provision with a valid one that in its economic effect is most consistent with the invalid or unenforceable provision.
4.10 Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed an original, and all of which together will be deemed to be one and the same instrument. A facsimile copy of this Agreement, including the signature pages, will be deemed an original.
IN WITNESS WHEREOF, the Parties have caused this PV Agreement to be executed and delivered by their respective duly authorized officers as of the day and year first above written, each copy of which shall for all purposes be deemed to be an original.
|
Marinus Pharmaceuticals, Inc. |
|
NovaMedica LLC | ||
|
|
|
| ||
|
|
|
| ||
|
By: |
|
|
By: |
|
|
|
|
|
|
|
|
Name: |
|
|
Name: |
|
|
|
|
|
|
|
|
Title: |
|
|
Title: |
|
