JOINT VENTURE AGREEMENT
EXHIBIT 10.1
[***] Represents material information which has been redacted and filed separately with the Commission pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
THIS JOINT VENTURE AGREEMENT (this "Agreement") is made and entered into this 6th day of September, 2011 by and among Allied Moral Holdings, Ltd., a company incorporated under the laws of the British Virgin Islands with its principal place of business located at Huiheng Building, Gaoxin 7 Street South, Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057 (hereinafter “Allied”), Intact Medical Corporation, a Delaware corporation with its principal place of business at 000 Xxxxxxxxxx Xxxx, Xxxxx 00, Xxxx Wing, Floor 4, Xxxxxxxxxx XX 00000 (hereinafter "Intact"), and BMG Diamond Holdings Limited, a company incorporated
under the laws of the British Virgin Islands with its principal place of business at Xxxx X, 00/X, Xxxxxxx Commercial Building, No. 00-00 Xxxxxx Xxxxxx Xxxx, Xxxxxx Xxx, Xxxx Xxxx (hereinafter “BMG”). Allied, Intact and BMG are sometimes referred to singularly as a “Party” and collectively “Parties.
WHEREAS, Allied is wholly owned by Huiheng Medical, Inc., a Nevada corporation, and is engaged in the design, manufacture and sale of large-scale radiotherapy equipment and is in the process of manufacturing and distributing a breast brachytherapy system throughout Asia;
WHEREAS, Intact is engaged in the design, production and sale of a product known as the Intact Breast Lesion Excision System; and
WHEREAS, the parties wish to expand the sales of the Intact Breast Lesion Excision System in the Territory (as defined below) and possibly other markets, it will be useful to establish a manufacturing and development facility in China in order to gain a competitive advantage with regard to costs of developing, manufacturing, delivering and servicing the Breast Lesion Excision System, and intend to establish a new joint venture company to develop, manufacture and sell the Breast Lesion Excision System in order to achieve this goal;
NOW, THEREFORE, in consideration of the mutual premises and obligations set forth herein and the mutual benefits to be derived therefrom, the parties have agreed as follows:
1.1 “Articles of Association” shall mean the official articles of the New Company to be established pursuant to this Agreement.
1.2 “Board of Directors” shall mean the Board of Directors of the New Company.
1.3 “Confidential Information” means any information disclosed by one Party to the other Party, either directly or indirectly, in writing, orally, electronically or by inspection of tangible objects (including without limitation (a) intellectual property, such as, but not limited to, patents, patent applications, copyrights, copyright registrations, and trade secrets and/or (b) confidential information, including without limitation ideas, physical, chemical or biological materials, and techniques for their handling and use, assays, techniques, schematics, drawings, designs, inventions, preclinical
and clinical data, know-how, technical documentation, processes, equipment, algorithms, software programs, software source documents, formulae, information concerning research and development projects, design details and specifications, financial information, information relating to procurement requirements, purchasing, manufacturing, customer lists, product plans, product ideas, business strategies, marketing or business plans, financial or personnel matters, investors, employees, business and contractual relationships, business forecasts, sales and merchandising, and information regarding third parties, suppliers, customers, employees, investors or facilities). Confidential Information may also include information previously disclosed to the disclosing Party by third parties.
- 1 -
1.4 “Effective Tax Rate” shall mean all taxes (domestic and foreign) paid (or to be paid) by New Company on all taxable income for the applicable period divided by all taxable income before taxes during such applicable period, express as a percentage.
1.5 “BMG Affiliate” shall mean a legal entity (i) at least 50% of whose capital is directly or indirectly owned by BMG, (ii) at least 50% of whose capital is directly or indirectly owned by a company which owns, directly or indirectly, at least 50% of the capital of BMG, or (iii) that owns directly or indirectly at least 50% of the capital of BMG.
1.6 “Allied Affiliate” shall mean a legal entity (i) at least 50% of whose capital is directly or indirectly owned by Allied, (ii) at least 50% of whose capital is directly or indirectly owned by a company which owns, directly or indirectly, at least 50% of the capital of Allied, or (iii) that owns directly or indirectly at least 50% of the capital of Allied.
1.7 “Intact Affiliate” shall mean a legal entity (i) at least 50% of whose capital is directly or indirectly owned by Intact, (ii) at least 50% of whose capital is directly or indirectly owned by a company which owns, directly or indirectly, at least 50% of the capital of Intact, or (iii) that owns directly or indirectly at least 50% of the capital of Intact.
1.8 “Intact Intellectual Property” means all of Intact’s present and future rights in relation to copyright, trade marks, designs, patents, inventions and confidential information whether or not registrable, registered or patentable relating to or connected with the Product;
1.9 “License” shall mean the License, Supply Marketing and Distribution Agreement in substantially the form attached hereto as Exhibit A in which Intact grants an exclusive license to the New Company to manufacture, promote, distribute and sell the Product in the Territory.
1.10 “Product” shall mean Intact’s Breast Lesion Excision System and all improvements related thereto including, but not limited to, the Intact RF generator, Intact handles, and Intact wands.
1.11 “SFDA Approval” shall mean the approval of the Product by China State Food and Drug Administration for sale in China.
1.12 “Territory” shall mean China, including Hong Kong SAR and Macua SAR.
1.13 “Year 2” shall mean months 13th – 24th after receipt of SFDA Approval
1.14 “Year 3” shall mean months 25th – 36th after receipt of SFDA Approval
1.15 “Year 4” shall mean months 37th – 48th after receipt of SFDA Approval
- 2 -
2.1 As soon as practical following the effective date of this Agreement, the parties shall establish a New Company under the laws of British Virgin Islands in accordance with the Memorandum and Articles of Association (“New Company”) as approved by Intact and Allied in writing. Intact and Allied will have the right to terminate this Agreement by written notice to the other parties to this Agreement if they are unable to agree to the form of Memorandum and Articles of Association..
2.2 The corporate name of the New Company shall be H & I Medical China Limited.
2.3 The New Company shall be primarily engaged in developing, manufacturing, marketing, selling and supporting of the Product in the Territory pursuant to the License. New Company will also manufacture all components of the Product including the RF Generator, the reusable handle tool and the disposable wand. New Company, in its sole discretion, shall have the right to subcontract for the manufacturing of the Product and related components.
2.4 The New Company may establish a wholly-foreign owned enterprise in China for the purpose of facilitating the developing, manufacturing, marketing, selling and supporting of the Product in the Territory (the “Operating Subsidiary”). Subject to Intact approval, the Operating Subsidiary’s principal office shall be in China as determined by the New Company.
2.5 All costs with respect to the New Company and the Operating Subsidiary incurred after its establishment will be borne by the New Company. Costs incurred in connection with the organization of the New Corporation will be borne by Allied.
2.6 In the event there are any inconsistencies between the terms of New Company’s charter documents, including the Articles of Association, and this Agreement, Allied, as the majority shareholder of the New Company, agrees to vote its shares to amend, modify or change the inconsistent terms of such charter documents, including the Articles of Association, to conform to the terms of this Agreement.
3.1 The New Company will have an initial authorized capital of (1) two thousand (2,000) shares of voting common stock ; (2) 300 shares of non-voting Series A Preferred Stock; and (3) 700 shares of non-voting Series B Preferred Stock; each share having a par value of ($0.001).
3.2 The initial shareholders of the New Company, including the number of shares subscribed and paid for shall be as follows:
- 3 -
|
Name and Address
|
No. of
Common Shares
|
Consideration
|
Series A
|
Series B
|
Preferred
Stock
Consideration
|
|
Allied Moral Holdings, Ltd.
Huiheng Building,
Gaoxin 7 Street South, Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057
|
650
|
$650
|
0
|
650
|
$650
|
|
Intact Medical Corporation
000 Xxxxxxxxxx Xxxx, Xxxxx 00, Xxxx Wing, Floor 4,
Xxxxxxxxxx XX 00000
|
300
|
$300
|
300
|
0
|
$300
|
|
BMG Diamond Holdings Limited
Flat A, 15/F, Hillier Commercial Building
Xx. 00-00 Xxxxxx Xxxxxx Xxxx
Xxxxxx Xxx, Xxxx Xxxx
|
50
|
$50
|
0
|
50
|
$50
|
3.3 New Company may not change its registered capital and share structure without the prior written consent of Allied and Intact, and in accordance with respective shareholders' resolutions and applicable laws. In the case of any capital increases, each party shall have the right to subscribe to the amount of such additional capital in accordance with its respective proportional equity interest at that time. If the parties do not subscribe to its pro-rata share of any newly issued shares, the other parties may choose to subscribe to purchase such shares. Any capital increases shall be approved by both Allied and Intact as provided
in Section 6.1.3.4.
3.4 Each party shall timely subscribe and pay for its shares in accordance with applicable laws.
3.5 The holders of Series A preferred stock will be entitled to preference distributions when and as declared by the Board of Directors in an amount equal to (i) $1,100,000 less any proportional taxes accrued by New Company for Year 2 (which tax calculation shall be based on the Effective Tax Rate as determined by the Board of Directors) (“Year 2 Dividend”); (ii) $2,250,000 less any proportional taxes accrued by New Company for Year 3 (which tax calculation shall be based on the Effective Tax Rate as determined by the Board of Directors) (“Year 3 Dividend”); and (iii)$3,250,000 less any proportional taxes accrued by New Company
for Year 4 (which tax calculation shall be based on the Effective Tax Rate as determined by the Board of Directors) (“Year 4 Dividend”)(collectively the “Series A Accrued Dividend”).
- 4 -
3.6 Once the holders of the Series A preferred stock have received their preference distributions for the year indicated, then the holders of Series B preferred stock will be entitled to distributions out of available funds thereof if and when declared by the Board of Directors in amount equal to (i) $3,666,667 - $1,100,000 less any proportional taxes accrued by New Company for Year 2 (which tax calculation shall be based on the Effective Tax Rate as determined by the Board of Directors); (ii) $7,500,000 - $2,250,000 less any proportional taxes accrued by New Company for Year 3 (which tax calculation shall be based on the Effective Tax Rate as
determined by the Board of Directors); and (iii) $10,833,333 - $3,250,000 less any proportional taxes accrued by New Company for Year 4 (which tax calculation shall be based on the Effective Tax Rate as determined by the Board of Directors)(collectively the “Series B Accrued Dividend”). If New Company is unable to make a full distribution to the holders of Series B preferred stock in any given year, any such deficit in distribution will accumulate. In any given year, however, Intact shall receive its preference distribution regardless of any accumulation amount from prior periods owed to the holders of Series B preferred stock and any unpaid accumulated amounts for the account of holders of Series B preferred stock shall continue to accumulate and be paid in subsequent years out of available funds.
3.7 Once the holders of Series A and B preferred stock have received their preference distributions, including any accumulated distributions, any further distributions as determined by the Board of Directors of New Company will be made to the holders of common shares.
3.8 To the extent permitted under applicable law, the New Company shall pay, out of funds legally available therefor, all accrued and unpaid dividends on common shares and preferred stock, when and as declared by the Board of Directors. If and to the extent not paid, such dividends shall accrue, accumulate, and shall remain accumulated and unpaid dividends until paid.
3.9 Upon (i) payment in full of the Series A Accrued Dividend and Series B Accrued Dividend after Year 4, (ii) upon Intact’s election to terminate the License as a result of New Company not meeting the minimum targets as identified in the License, or (iii) upon New Company’s election to terminate the License pursuant to Section 7.B.(ii) of the License, all of the outstanding shares of Series A and Series B preferred stock will automatically be converted into common shares of the New Company on a one-for-one basis.
4.1 Upon execution of the License, Allied (or Allied’s Affiliate) will advance an amount equal to $150,000.00 (“Loan”) to Intact pursuant to the form of Promissory Note attached hereto as Exhibit B. The Loan will be unsecured, non interest bearing, and will be repaid out of Intact’s distributions of retained earning from the New Company. Intact will direct New Company to direct its portion of any and all cash distributions to Allied (or Allied’s Affiliate as the case may be) until the Loan is paid in
full.
5.1 The shares of the New Company shall not be transferred by any party without the prior written consent of the other parties.
5.2 The shares of the New Company shall not be encumbered or mortgaged by any party without the prior written consent of the other parties.
- 5 -
5.3 If any party wishes to transfer all or part of its shares, it shall first offer said shares to the other parties in accordance with this Agreement. It shall be a condition to any transfer of shares that the transferee shall agree in writing to be bound by the provisions of this Agreement. Any transfer not in accordance with the provisions of this Agreement shall be void.
5.4 Notwithstanding any provision of this Agreement to the contrary, Intact may transfer the Intact’s shares in the New Company to an Intact Affiliate, Allied may transfer the Allied’s shares in the New Company to a Allied Affiliate, and BMG may transfer the BMG’s shares in the New Company to a BMG Affiliate without the consent of the other Parties.
5.5 The board of directors of the New Company shall have the right to refuse to register in the New Company's shareholder register the shares belonging to any person or entity who owns such shares as a result of a transfer not in conformity with the provisions of this Agreement.
5.6 If any party hereto (hereinafter "Offering Shareholder") receives a bona fide third party offer to purchase any or all of its shares in New Company (hereinafter "Offered Shares"), the Offering Shareholder shall, before selling the Offered Shares to said third party, provide the other parties hereto all such information relating to the third party and its principals as the other parties hereto may reasonably request to the extent legally permissible and consistent with any contractual obligations of the disclosing party) as well as with reliable written evidence setting forth the price and other material terms and conditions of the proposed sale
(the "Offer Price and Terms"), including but not limited to, written evidence that such offer is firm and irrevocable, provided that this Section 5.6 (including subsections 5.6.1 through 5.6.3) shall not apply to any offer to purchase all or substantially all of the assets of Intact, whether by merger, acquisition, change of control, stock purchase or otherwise. Upon receipt of all such requested information as well as the Offer Price and Terms in writing, the other parties hereto shall each have the option of purchasing said shares as follows:
5.6.1 If Intact is the Offering Shareholder, Allied shall have the right within thirty (30) days after its receipt of the Offer Price and Terms, (hereinafter "Option Period") to purchase all, but not less than all, of the Offered Shares at the Option Price and Terms by giving written notice to each other party within the Option Period specifying a date for the purchase being not more than thirty (30) days after the end of the Option Period. If Allied does not elect to purchase said shares and (a) the third party to which Intact intends to sell its shares is unacceptable to Allied, or (b) following the sale of such shares it would no longer be
possible, in Allied’s opinion, for the New Company to successfully pursue the goals for which it was established, Allied shall have the option of terminating this Agreement and dissolving the New Company following written notice to Intact dispatched during the Option Period. If Allied fails to purchase the Offered Shares pursuant to this Article 5.6.1 but does not exercise its right to dissolve the New Company, Intact shall have the right, within thirty (30) days following its receipt of written notice of Allied’s decision not to purchase said shares, or from the end of the Option Period, whichever is earlier, to sell all, but not less than all, of the Offered Shares at the Option Price and Terms to said third party offeror. This Section 5 shall not prevent or prohibit the Parties from selling its own shares or transferring substantially all of the assets provided
that such transfer does not impair the rights granted to the New Company under the License.
- 6 -
5.6.2 If Allied is the Offering Shareholder, Intact shall have the right within thirty (30) days after its receipt of the Offer Price and Terms, (hereinafter "Option Period") to purchase all, but not less than all, of the Offered Shares at the Option Price and Terms by giving written notice to Allied within the Option Period specifying a date for the purchase being not more than thirty (30) days after the end of the Option Period. If Intact does not elect to purchase said shares and (a) the third party to which Allied intends to sell its shares is unacceptable to Intact, or (b) following the sale of such shares it would no longer be possible, in
Intact’s opinion, for the New Company to successfully pursue the goals for which it was established, Intact shall have the option of terminating this Agreement and dissolving the New Company following written notice to Allied dispatched during the Option Period. If Intact fails to purchase the Offered Shares pursuant to this Article 5.6.2 above but Intact does not exercise its right to dissolve the New Company, Allied shall have the right, within thirty (30) days following its receipt of written notice of Intact’s decision not to purchase said shares or from the end of the Option Period, whichever is earlier, to sell all, but not less than all, of the Offered Shares at the Option Price and Terms to said third party offeror.
5.6.3 If BMG is the Offering Shareholder, both Allied and Intact (on a pro-rata basis unless the other parties elects to purchase less than its pro-rata share) shall have the right within thirty (30) days after its receipt of the Offer Price and Terms, (hereinafter "Option Period") to purchase all, but not less than all, of the Offered Shares at the Option Price and Terms by giving written notice to BMG within the Option Period specifying a date for the purchase being not more than thirty (30) days after the end of the Option Period. If Allied and/or Intact fails to purchase the Offered Shares
pursuant to this Article 5.6.3 above, BMG shall have the right, within thirty (30) days following its receipt of written notice of Allied’s and Intact’s decision not to purchase said shares or from the end of the Option Period, whichever is earlier, to sell all, but not less than all, of the Offered Shares at the Option Price and Terms to said third party offeror.
5.7 The certificates representing the shares of the New Company shall each bear an inscription notifying third parties that such shares are subject to the restrictions on transfer set forth in this Agreement and that the transferee will be bound to the obligations of this Agreement.
6.1 Shareholders Meetings.
6.1.1 Ordinary Meeting: An ordinary annual Shareholders Meeting for holders of common shares shall be held at the New Company's head office or at any other location designated by the Board of Directors on a date determined by the Board of Directors in accordance with laws of the place of incorporation; provided, however, that shareholder resolutions which are unanimously approved by the shareholders of common shares may be set forth in minutes signed by all shareholders
without the necessity of actually holding a meeting if permitted under the place of incorporation. The agenda of the meeting shall be established in accordance with the requirements of applicable law and of the Articles of Association.
6.1.2 Extraordinary Meetings: Extraordinary shareholders meeting(s) of common shares may be convened at any time by the Board of Directors or otherwise in accordance with the laws of the place of incorporation.
- 7 -
6.1.3 Quorum and Voting:
6.1.3.1 Only holders of common shares of the New Company shall be entitled to vote. Each shareholder may be represented by proxy at the shareholders' meeting and such proxy may exercise such shareholder's voting rights in common shares. It will constitute sufficient authorization if power of attorney is given to such proxy in writing and submitted at the meeting.
6.1.3.2 Except as otherwise mandatory under applicable law or under the Articles of Association, the quorum for shareholders meetings consisting of shareholders present and shareholders represented by proxies shall be a simple majority of the New Company's outstanding common shares.
6.1.3.3 Except as otherwise provided by laws of the place of incorporation or in the Articles of Association, the majority of the common shares at the shareholders meetings shall be the simple majority of those present and represented by proxy.
6.1.3.4 The decisions on the matters listed below shall be reached only by super-majority representing more than seventy-five percent (75%) of the total outstanding common shares:
(i) amendment of the Articles of Association of the New Company;
(ii) the creation of any new series or class of capital stock of New Company, or any securities convertible into or exchangeable for such capital stock, or any increase or decrease in the New Company's capital stock;
(iii) dissolution and liquidation of the New Company, for reasons other than those defined in law or by exercise of the rights set forth in Article 5 hereof;
(iv) decisions or agreements to merge or consolidate, or sell substantially all of New Company’s assets or intellectual property, including without limitation by way of the grant of an exclusive license to such assets or intellectual property, or otherwise reorganize or recapitalize;
(v) changes in the objectives of the New Company;
(vi) issue additional shares of the New Company (after the initial issuance of the New Company)
(vii) the raising of additional capital;
- 8 -
(viii) a material acquisition, joint venture or partnership;
(ix) authorization of any indebtedness, lease, guaranty, letter of credit or financing arrangement in an amount exceeding $100,000, except as otherwise authorized in a then current Board approved budget;
(x) declare dividend to holders of common stock; or
(xi) the creation of affiliates or subsidiaries;
(xii) the initiation or settlement of any lawsuit, administrative proceeding, tax matter or other legal claim involving in excess of $250,000;
(xiii) any amendment, or modification of, or waiver under, this Agreement; or
(xiv) any other decision that falls outside the ordinary course of business of the New Company.
6.1.4 Minutes. The minutes shall be drawn up in English, at the expense of the New Company and without undue delay, following any shareholders resolution and must be signed by all participants in the resolution. The minutes shall be kept in the New Company's minute books.
6.2 Board of Directors.
6.2.1 Board of Directors. The New Company shall be governed by a Board of Directors consisting of three (3) members which shall meet a minimum of once each calendar year. So long as Allied is a shareholder of the New Company, two (2) members of the Board of Directors shall be elected among persons nominated by Allied (or its permitted successors). So long as Intact is a shareholder of the New Company, one (1) member of the Board of Directors shall be elected among persons nominated by Intact (or
its permitted successors).
- 9 -
6.2.2 Meetings. Meetings of the Board of Directors shall be held at the New Company's head office or other location in or outside China which may be agreed upon by the Board of Directors. The proceedings of meetings of the Board of Directors will be in English and the official minutes of meetings and resolutions of the Board of Directors shall be drafted in English and shall be kept in the New Company's minutes book. Directors shall not be entitled to any compensation or reimbursement of costs
or expenses.
6.2.3 Quorum for Meeting: The quorum required for meetings of the Board of Directors shall be all three members of the Board of Directors, provided, however, if any director fails to attend two consecutive meetings after due notice, the quorum requirement for the following meeting shall be reduced to two members of the Board of Directors. Decisions may be taken by the Board of Directors without a meeting if a proposal for action is submitted in writing in turn to each of the members of the Board of Directors and each such member consents in writing to such action, or
to vote upon the proposal in writing. A director may by written notice appoint an alternate who need not be a director. The alternate can attend meetings in the absence of the appointing director and vote or consent in his place.
6.2.4 Decisions: All actions by consent (without a call meeting) of the Board of Directors shall be by unanimous written consent. All corporate actions during a called meeting requiring Board of Directors approval shall be approved by a majority of the members of the Board of Directors with the exception of the following actions which requires unanimous approval of all members of the Board of Directors.
6.2.4.1 Any corporate action which, in the reasonable opinion of the Intact designated Board member as communicated to the New Company's Chairman at or before the called meeting, is likely to affect adversely Intact's reputation, Intact Intellectual Property, or its financial performance outside of the Territory and separate from the New Company; provided, however, such Intact Board member shall provide the Board a written statement presenting the basis of his opinion within 15 days after said meeting;
6.2.4.2 Approval of the Company’s annual budget and/or business plan, if any, each year (such approval, to the extent that any member of the Board of Directors deems it necessary, shall be approved within a reasonable time (15 days after presentment of the proposed annual budget and/or business plan), and to the extent that the Board is unable to timely approve the annual budget and/or business plan, the New Company shall continue to operate its business consistent (within 15%) with the prior years excluding extraordinary expenses); and
6.2.4.3 Any proposal by New Company to appoint a sub-distributor of the Product pursuant to the License.
6.2.5 Authority; Delegation: The administration and representation of the New Company shall be the exclusive authority of the Board of Directors, which may delegate such authority to the officers of the New Company.
6.3. Delegation. The power to direct and represent the New Company is vested with the Board of Directors, which may delegate that power to the officers or a general manager.
- 10 -
7.1 Fiscal Year. The fiscal year of the New Company shall be the calendar year.
8.1 Audit. The New Company’s financial statement shall be audited by Huiheng Medical, Inc.’s then current auditors. The books and records of New Company shall be prepared in accordance with US GAAP.
8.2. Expenses of the New Company. All expenses, including any reasonable expenses incurred by Parties and approved by the Board, related to the developing, manufacturing, marketing and selling of the Product and related accessories will be charged to the New Company. Pursuant to the License, the initial manufacturing, marketing and sale of the Product shall be borne by Allied at its sole cost and expense until the New Company has adequate working capital from operations.
9.1 Payment of Capital Contributions. The Parties shall respectively be responsible for timely paying their contribution in full to the registered capital and any additional registered capital in accordance with the provisions herein.
9.2 Permits and Approvals. On behalf of the New Company, Allied will be responsible, at its sole cost and expense, for (i) obtaining the various permits and regulatory approvals required to market the Product in the Territory, including SFDA Approval, (ii) manufacturing the Product in China, including all of the costs associated with manufacturing the Product in
China, and (iii) marketing the Product in the Territory, including all of the costs associated with marketing the Product in the Territory. All of the costs associated with obtaining approvals, and developing, manufacturing and selling the Product will be undertaken by Allied at its sole cost and expense.
9.3 License. Intact will grant to the New Company an exclusive license and rights to develop, manufacture, promote, distribute and sell the Product, including any improvements made thereon, in the Territory. Intact will make its best efforts to provide Allied with all of the information, including marketing information, and other support necessary to train Allied, on behalf of the New Company, to manufacture the Product in China.
9.4 Sales to Intact; Cost of Components. At Intact’s request, New Company will sell the Product to Intact at New Company’s [***] for the Product that will be sold by Intact in the markets outside of the Territory. At Intact’s request at any time, New Company will provide Intact with a current component price list for the Product.
9.5 Additional Countries. Intact and New Company agree that they will have further discussions in good faith for the opportunity of New Company to market the Product in Vietnam, and Taiwan, or other countries, where Allied has sales channels.
- 11 -
10.1 Use, Nondisclosure. With respect to the Confidential Information of the other Party, each Party agrees, during the term of this Agreement and for seven (7) years after this Agreement is terminated: (a) to hold such Confidential Information in confidence and to take reasonable precautions to protect the same (including, without limitation, all precautions such Party employs with respect to its own confidential information), (b) not to divulge any such Confidential Information to any third party or to make
any use whatsoever at any time of such Confidential Information, except for the purpose of performing the Party’s obligations under this Agreement, (c) not to use any such Confidential Information as the basis for beginning any new research and development programs; and (d) not to copy or reverse engineer any such Confidential Information.
10.2 Termination or Expiry. At the conclusion of this Agreement, each party shall within thirty (30) days of such termination or expiry, either return the other’s Confidential Information in its possession (including all copies) or shall, at the disclosing party’s direction, destroy such Confidential Information (including all copies) and certify its destruction to the disclosing party.
10.3 Exclusions. The term “Confidential Information” shall not include any information which: (a) is in the public domain at the time of disclosure or enters the public domain following disclosure through no fault of the receiving party, (b) is already in the receiving party’s possession prior to disclosure hereunder (as reflected by such party’s written records) or is subsequently disclosed to the receiving party with no obligation of confidentiality
by a third party having the right to disclose it, (c) is independently developed by the receiving party without reference to the disclosing party’s Confidential Information, or (d) is required to be disclosed pursuant to an order of any competent court or government agency or rules of a securities exchange with prior notice to disclosing party, or as required by any regulatory authority or for any regulatory filing, but shall notify the other Party prior to disclosing such information, and shall cooperate with the other Party either to enable the disclosing Party to seek protective measures for the Confidential Information, or to seek confidential treatment of such Confidential Information, and will limit any disclosure required to be made to any such agency to the minimum required for to meet the requirements of such order or regulatory filing.
10.4 Necessary disclosures. Each Party may disclose the Confidential Information it receives under this Agreement to (a) its employees, contractors and permitted sublicensees, (b) to a potential or actual acquirer of, or an investor or potential investor in, such Party or the assets of such Party to which this Agreement relates, (c) for the purposes of Patent filing, prosecution and enforcement and (d) to its advisors, provided that in each case the disclosure is
limited to the extent required for the performance of either Party’s obligations under this Agreement, and provided that in the case of subsections (a), (b) and (d), the individuals are subject to obligations of confidentiality in relation to such information no less stringent than those contained in this Agreement.
10.5 Publicity. The Parties agree that no public disclosure, publicity release or announcement concerning the transactions contemplated hereby will be issued without the advance written consent of the other Parties except as such disclosure, release or announcement may be required (a) by applicable Laws, (b) for filings with governmental agencies, (c) for prosecuting or defending litigation, and (d) for complying with applicable governmental regulations, court
orders, and legal requirements, including filings with the U.S. Securities Exchange Commission and with regulatory authorities, in each of which cases the Party required to make such disclosure, release or announcement will, to the extent reasonably practicable before making any such disclosure, release or announcement, afford the other Party with a reasonable opportunity to review and comment upon such release or announcement and use reasonable efforts to seek confidential treatment of such information.
- 12 -
10.6 Allied Required Disclosure. Each party understands that Huiheng Medical, Inc. is a reporting company with the U.S. Securities and Exchange Commission and is required to make disclosure of certain information regarding the New Company. Each party understands that this Agreement and the contents thereto will be filed as a exhibit to Huiheng Medical, Inc. periodic filings.
Article 11 – IP Ownership and Licenses
11.1 Licenses. Upon incorporation of the New Company, Intact and the New Company will promptly (within 20 days from the date of incorporation) enter into the License which will grant the New Company, pursuant to terms of the License, a license to use the Intact Intellectual Property necessary for the New Company to undertake the activities set forth herein.
11.2 IP Ownership. Each of New Company and Allied acknowledge that Intact owns all right, title and interest in and to the Product, and the Intact Intellectual Property and agrees that it does not by this Agreement and will not otherwise have or acquire ownership in the Intact Intellectual Property and further warrants that it will not at any time, either during the term of the Agreement or after termination of this Agreement contest (or assist any third party in
contesting) the validity or ownership of the Intact Intellectual Property or any of its constituent elements, including any improvements to the Intact Intellectual Property or the Product.
12.1 Term. Unless earlier terminated or amended in accordance with any applicable provision hereto, this Agreement shall remain in force and binding on the parties for a term equal to the License, including any and all extensions including the Wind Down Period, as that term is defined in the License.
12.2 Material Breach. In the event of failure or neglect of any party hereto to fulfill any of the provisions hereof to be performed by it, and if any other party gives written notice of such default and such default is not cured within sixty (60) days after the giving of such notice, the party giving such notice shall have the right to terminate this Agreement at any time thereafter by giving a written notice of such termination to the defaulting
party.
12.3 Mutual Termination. This Agreement may be terminated by mutual written agreement by Allied and Intact.
12.4 Survival. Articles 10, 11, 12 and 13, and such other Sections as by their nature should survive, and such obligations shall survive any expiration or termination of this Agreement.
12.5 Effect of Termination. Upon the termination or expiration of this Agreement, each Party will immediately cease using all of the Confidential Information of the other Parties, including the Intact Intellectual Property, and return all such Confidential Information to the owning party, or at the owning party’s option, destroy all such Confidential Information. On request, the Party returning or destroying such Confidential Information will
promptly provide the owning party with a written certification of such return or destruction.
- 13 -
13.1 Governing Law. This Agreement shall be governed by and construed in accordance with the laws of Commonwealth of Massachusetts without regard to the conflicts of laws principles thereof.
13.2 Arbitration. All disputes, controversies or differences which may arise between the parties, and/or between a party and the New Company, out of or in relation to or in connection with this Agreement which cannot be settled by full discussion between appropriate senior representatives of the parties will be settled, by arbitration in Boston, Massachusetts, according to the commercial arbitration rules of the American Arbitration Association and applying the procedural laws of the State of
Massachusetts. The proceedings shall take place in the local language with provision for simultaneous interpretation at the expense of the party desiring it. The decision of the arbitrator(s) will be final and binding and enforceable in any court with appropriate jurisdiction.
13.3 Attorney Fees. If any action at law or equity or arbitration is necessary to enforce or interpret the terms of this Agreement, the prevailing party shall be entitled to reasonable attorneys' fees, costs, and necessary disbursements in addition to any other relief to which the party may be entitled.
13.4 Notices. Any notice or request which shall or may be given under this Agreement shall be made by airmail, registered mail with return receipt requested or facsimile and shall be directed to the intended recipient at its address set forth below or at such other address as such party may notify the other parties in accordance with this paragraph. The effective date of delivery of such notice shall, in the case of airmail or registered airmail, be seven (7) business days following
dispatch, or in the case of facsimile or e-mail, the date of successful transmission as evidenced by a transmission report.
Allied Moral Holdings, Ltd.
Huiheng Building,
Gaoxin 7 Street South,
Keyuannan Road, Nanshan District,
Shenzhen Guangdong, P.R. China 518057
Facsimile: 0000-0000 0000
Intact Medical Corporation
000 Xxxxxxxxxx Xxxx, Xxxxx 00, Xxxx Wing, Floor 4,
Xxxxxxxxxx XX 00000
Facsimile:(000) 000-0000
BMG Diamond Holdings Limited
Flat A, 15/F, Hillier Commercial Building
Xx. 00-00 Xxxxxx Xxxxxx Xxxx
Xxxxxx Xxx, Xxxx Xxxx
Facsimile: 000-000-0000
- 14 -
13.5 Waiver. The failure, delay or omission of any party hereto at any time to require due performance by any other party of any provision under this Agreement shall in no way affect the right of such party to require performance which may be due thereafter pursuant to such provision and shall constitute neither a future waiver of the same provision nor a waiver of any subsequent provision on the same matter. Any failure by any party hereto to protest a breach of any provision under
this Agreement shall not constitute waiver of its right to protest any subsequent breach of the same or any other provision.
13.6 Cumulative Remedies. No remedy conferred by any of the provisions of this Agreement is intended to be exclusive of any other remedy which is otherwise available at law, in equity, by statute or otherwise and each and every other remedy shall be cumulative and shall be in addition to every other remedy given hereunder or now or hereafter existing at law, in equity, by statute or otherwise. The election of any one or more of such remedies by either of
the Parties shall not constitute a waiver by such party of the right to pursue any other available remedy.
13.7 Assignment. Neither Party may assign or otherwise transfer this Agreement without the other Party’s prior, express written consent, except that no such consent shall be required for Intact to assign its rights or transfer its obligations (i) to its Affiliates or (ii) in connection with the sale or transfer of the majority of its stock or all or substantially all of its assets to which this Agreement relates, whether as part of a merger, acquisition, or
asset sale. Any assignment or transfer in violation of this Agreement will be null and void. This Agreement benefits and binds the parties and their respective successors and permitted assigns.
13.8 Language. This Agreement shall be executed both in the English language and the Chinese language, but in the event of inconsistency or difference between the English language version and the Chinese language version of this Agreement, the English language version shall prevail.
13.9 Severability. If any provision or part of this Agreement is rendered void, illegal or unenforceable in any respect under any enactment or rule of law, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
13.10 Construction. The headings to the clauses, sub-clause and parts of this Agreement are inserted for convenience of reference only and are not intended to be part of or to affect the meaning or interpretation of this Agreement. Any ambiguity in this Agreement shall be interpreted equitably without regard to which party drafted the Agreement or any provision thereof. The terms “this Agreement,” “hereof,”
“hereunder” and any similar expressions refer to this Agreement and not to any particular Section or other portion hereof. The parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting party will not be applied in the construction or interpretation of this Agreement. As used in this Agreement, the words “include” and “including,” and variations thereof, will be deemed to be followed by the words “without limitation” and “discretion” means sole discretion.
- 15 -
13.12 Relationships of the Parties. This Agreement shall not constitute any party the legal agent of any other party. No party shall have the right or authority to assume, create, or incur any liability of any kind, express or implied, against or on behalf of any other party.
13.13 Limitation of Liability. Except for breaches of confidentiality obligations, in no event shall either party have any liability to the other, or to any party claiming through or under the other, for any lost profits, or any indirect, incidental, special or consequential damages of any kind in any way arising out of or related to this Agreement and however caused and under any theory of liability, even if such party has been advised of the possibility of such
damages.
13.14 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed to be an original and all of which together shall be deemed to be one and the same instrument. Copies of executed counterparts transmitted by telecopy, or other electronic transmission service shall be considered original executed counterparts for purposes of this paragraph, provided that receipt of copies of such counterparts is confirmed.
13.15 Entire Agreement; Amendments. The terms and conditions of this Agreement constitute the entire agreement and understanding between the parties concerning its contents, and supersede all previous communications, whether oral or in writing, between the parties, including any previous agreement or understanding varying or extending the same, and may not be released, discharged, abandoned, changed or modified except by an instrument in writing signed personally or by the duly authorized
officers or representatives of the respective parties hereto.
- 16 -
Allied Moral Holdings, Ltd.
By: /s/ Xxx Xxxxxxxx
Name: Xxx Xxxxxxxx
Title: Chairman
Intact Medical Corporation
By: /s/ Xxxx X. Xxxxx
Name: Xxxx X. Xxxxx
Title: President and CEO
BMG Diamond Holdings Limited
By: /s/ Xxxxxxx
Xxxxx
Name: Xxxxxxx Xxxxx
Title: Principal
- 17 -
Exhibit A
LICENSE, SUPPLY, MARKETING AND DISTRIBUTION AGREEMENT
[***] Represents material information which has been redacted and filed separately with the Commission pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit A
LICENSE, SUPPLY, MARKETING AND DISTRIBUTION AGREEMENT
This License, Supply, Marketing and Distribution Agreement (“Agreement”) is entered into as of September___, 2011 (the “Effective Date”) by and between H & I Medical China Limited, a British Virgin Islands company with its principal place of business at Huiheng Building, Gaoxin 7 Street South, Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057 (hereinafter “Newco”), and Intact Medical Corporation, a Delaware corporation with its principal place of business at
000 Xxxxxxxxxx Xxxx, Xxxxx 00, Xxxx Wing, Floor 4, Xxxxxxxxxx XX 00000 (hereinafter "Intact"). Newco and Intact are sometimes referred to singularly as a “party” and collectively “parties”.
WHEREAS, Intact is engaged in the design, production and sale of a product known as the Intact Breast Lesion Excision System, as described in Exhibit A, attached hereto, and Intact owns certain Intellectual Property (as defined below) rights with respect to Product (as defined below);
WHEREAS, Newco has been organized and formed to engage in the development, manufacturing, promotion and distribution of the Product in the Territory (as defined below); and
WHEREAS, Intact desires to grant to Newco, and Newco desires to receive an exclusive license to manufacture, have manufactured, market, develop, distribute and sell the Product in the Territory, and a limited license thereafter pursuant to the terms of this Agreement.
NOW, THEREFORE, in consideration of the mutual covenants and agreements contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties, intending to be legally bound, hereby covenant and agree as follows:
1. Definitions.
For the purposes of this Agreement the following capitalized terms are defined in this Section 1 and shall have the meaning specified herein. Other terms that are capitalized but not specifically defined in this Section 1 or in the body of this Agreement shall have the meanings set forth in the Joint Venture Agreement.
“Affiliate” means, with respect to an entity, any Person that, directly or indirectly, through one or more intermediaries, Controls, is Controlled by, or is under common Control with, that entity.
“Acceptance Criteria” means the visual inspection criteria, performance test parameters and other criteria for each Product as set forth in Exhibit B-1, to be met by Newco prior to commercialization of Product in the Territory.
A - 1
“Confidential Information” means all proprietary, confidential and other non-public information, Know-How and data (oral, written, graphic, demonstrative, machine recognizable or otherwise) relating to the proprietary technology and/or business of (i) the Disclosing Party, and (ii) a third party who has disclosed such information, Know-How, and data to the Disclosing Party under obligations of confidentiality, and which in either case is disclosed by the Disclosing Party to the Receiving Party within the context of this Agreement; provided, that, it shall be explicitly understood that Confidential Information includes, information
specifically related to the Product, and “Disclosing Party” means the party disclosing Confidential Information to the other party pursuant to this Agreement and “Receiving Party” means the party receiving Confidential Information from the other party pursuant to this Agreement.
“Control” or “Controlled” shall mean with respect to Know-How or Patents, that the applicable Party has licensed (or otherwise obtained rights to or under) such Know-How or Patents from a third party and such Party has the right to grant sublicenses at no additional cost to such Know-How or Patents.
“Huiheng” means Allied Moral Holdings, Ltd., a British Virgin Islands company, with its principal place of business located at Huiheng Building, Gaoxin 7 Street South, Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057 and wholly owned by Huiheng Medical, Inc., a Nevada corporation, with its principal place of business located at Huiheng Building, Gaoxin 7 Street South, Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057.
“Improvement” means all improvements, variations, updates, modifications, and enhancements to the Product that is made by either Party in performing activities under this Agreement, in each case whether or not patentable or protectable,
“Intact Breast Lesion Excision System” means the system developed by Intact for the purposes of capture of breast tissue for histological review in a human patient using radiofrequency energy.
“Intact Handles” means the reusable handle tool known as Intact Handle that is an integral component of the Product.
“Intact’s Knowledge” means the actual knowledge of any of the executive officers and board of directors of Intact.
“Intact Wands” means the disposable wand known as Intact Wand that is an integral component of the Product.
“Intellectual Property” shall mean a Patent, industrial design, invention (whether patentable or unpatentable and whether or not reduced to practice), design, idea, work, methodology, innovation, creation, concept, development research, copyright, all confidential and/or proprietary assays, substances and materials, Know-How, and any registration, application, right or other grant analogous thereto with respect to any of the foregoing, but excluding trademarks and service xxxx.
“Joint Venture Agreement” means the agreement by and among Huiheng, Intact and BMG Diamond Holdings Limited, a company incorporated under the laws of British Virgin Islands, dated as of September 6, 2011.
A - 2
“Know-How” shall mean any and all confidential information, trade secrets, technology, technical information, genetic information (including information relating to any impairment, variance, or mutation thereof) discoveries, formulae, algorithms, developments, improvements, system, techniques, methods, test methods, procedures, processes, data (including development data), instructions, analysis, experiment, drawings and specifications, whether or not patentable or protectable.
“Licensed Technology” means (i) the Patents listed on Exhibit C, and other U.S. and international pending Patents Controlled by Intact or its Affiliates related to the Product, including any patents issuing from any of the patent applications included in such Patents and any reissues, re-examinations, confirmations, extensions, renewals, substitutions, continuations, divisions, patent term extensions, and continuation-in-part applications of the foregoing; (ii) Know-How regarding the manufacture and use of the Product; including the Process Technology and (iii) any and all
Improvements made or conceived during the term of this Agreement.
“Patents” means any and all original (priority-establishing) patents and patent applications filed anywhere in the world, including provisional and non-provisional applications, and all related applications thereafter filed or including a common priority right, including any continuations, continuations-in-part, divisional and substitute applications, any patents issued or granted from any such patent applications, and any reissues, renewals, reexaminations, extensions (including by virtue of any supplementary protection certificates) of any such patents, and any confirmation patents, inventor’s certificates or registration patents
or patents of addition based on any such patents, and all foreign counterparts or equivalents in any country or jurisdiction.
“Patent Offices” means the United States Patent and Trademark Office, and the equivalents in the Territory.
“Process Technology” means the confidential and proprietary process technology, including its family and derivatives, supplied by Intact to Newco hereunder to produce Product pursuant to this Agreement, which includes but is not limited to the instructional DVD videos which provide instructions on how to make the Product.
“Product” means Intact’s Breast Lesion Excision System, including all related components, and all Improvements related thereto including, but not limited to, the Intact RF generator, Intact Handles, and Intact Wands.
“Qualification” means Intact’s determination that the Product meets the Acceptance Criteria in accordance with the Qualification Plan.
“Qualification Plan” means the plan and process determined by Intact at its sole discretion to ascertain whether the Product as manufactured by Newco meets Intact’s reliability and quality specifications, as attached hereto as Exhibit B-2.
“SFDA” means China State Food and Drug Administration.
“SFDA Approval” means the approval of the Product by China State Food and Drug Administration for sale in China.
“Territory” means China, including Hong Kong SAR and Macau SAR.
A - 3
“Trademarks” means the trademarks currently used in relation to the Product as listed on Exhibit D attached hereto, including “Intact® ,”n-bloc®, and the trade name “Intact.”
“Year 2 Dividend” has the ascribed meaning set forth under the Joint Venture Agreement.
“Year 3 Dividend” has the ascribed meaning set forth under the Joint Venture Agreement.
“Year 4 Dividend” has the ascribed meaning set forth under the Joint Venture Agreement.
A. License. Subject to the terms and conditions of this Agreement, Intact hereby grants to Newco an exclusive (even as to Intact), fee-bearing, non-transferable license (with the right of sublicense pursuant to the terms of this Agreement) under and to the Licensed Technology to manufacture, have manufactured by subcontracted manufacturers pre-approved in writing by Intact, use, promote, market, have marketed, distribute, have distributed, service, sell, offer for sale, the
Product solely in the Territory to end users in the Territory, and to import and export the Product outside the Territory solely to Intact. Newco may appoint one or more sub-distributors and subcontractors to manufacture, market, distribute, offer to sell and sell the Product in the Territory without Intact’s consent, provided that such sub-distributor or subcontractor shall be required to comply with the terms of this Agreement, and Newco shall remain fully liable to Intact for the performance of, and compliance with the terms of this Agreement by, such third parties.
B. Trademark and Trade Names License. Subject to the terms and conditions of this Agreement, Intact hereby further grants to Newco an exclusive license to use the Trademarks in the Territory for the purposes of the manufacture, distribution, marketing, use and sale of the Product, subject to the terms of this Agreement, which license shall also include Newco’s right to use the name “Intact Medical Corporation.” “Intact” or other trade names associated with the Product in accordance with the terms of this Agreement,
provided that the license to use the names “Intact Medical Corporation” and “Intact” shall be granted solely for the purposes of identifying the Product as having been designed by Intact. In the event Newco uses such Trademarks in the Territory, Newco shall use such Trademarks at all times in accordance with the specifications and guidelines for use of such Trademarks notified to it in writing by Intact from time to time, and the license granted under this Section 2B may be revoked at any time at Intact’s sole discretion if, in Intact’s reasonable opinion, Newco does any act or omission that may damage the goodwill in, or reputation of, the Trademarks. Newco may not use the Trademarks on any packaging, or on any marketing, promotional, advertising or any other materials in relation to the Product (together the
“Branded Materials”) without Intact’s prior consent in writing, which may be withheld at Intact’s sole discretion; provided however, Intact agrees not to withhold consent on Newco’s use of Trademarks on packaging, marketing, promotional, advertising, or any other material relating to the Product currently used or used during the term of this Agreement by Intact so long as they have only been modified to reflect direct translation into Mandarin and to comply with the SFDA and China law. Intact understands that the Branded Materials will be primarily in Mandarin and Intact’s review of such materials will require Intact to have the resources to have such materials translated in English. Newco will provide Intact no less than fifteen (15) days in advance
with samples of the Branded Materials which
A - 4
will be primarily in Mandarin on which Newco proposes to use the Trademarks, and Intact will consider such Branded Materials within fifteen (15) days of receipt, and provide Newco with its consent, rejection, or its suggestions on modifications required to make the Branded Materials suitable for use. In the event Newco does not receive a written response from Intact within such fifteen (15) days of receipt, Newco may use the Branded Materials. Newco agrees to use its best efforts to maintain a high standard of quality for any marketing materials, packaging, facilities or goods with which the Product is associated, consistent with the specifications for use. In the event that Intact
determines that Newco’s method or manner of use of the Trademarks is inconsistent with Intact’s approved quality standards, Intact shall provide Newco with a written itemization of such defects. Newco shall use its best efforts to cure any such defects within fifteen (15) days of receipt of such notice, and immediately take all reasonable steps to correct such deficiency. If Newco does not cure such deficiency within fifteen (15) days, Intact shall have the right to suspend the Trademark license until such deficiency has been cured. For clarification, Intact retains the right to use the “Intact” trademark on its website and in promotional materials, such as brochures, case studies, white papers, and advertising related to the Product outside the Territory. As long as Newco maintains its exclusivity under Section 2A,
Intact agrees not to otherwise use the Trademarks in the Territory.
C. Right of First Offer. Intact hereby grants Newco a first right of first offer to have the sole and exclusive right to manufacture, market, distribute, license, promote, service and sell the Product in Vietnam, and Taiwan. Intact shall first negotiate or commence discussions with Newco regarding such new territories before commencing such discussion with any third parties. Intact and Newco shall engage in such negotiations in good faith and upon agreement by both parties not to pursue further discussions, or following thirty (30)
days good faith negotiation, Intact may then commence discussions with third parties.
A. Delivery of Materials. Intact will deliver and explain the Process Technology to Newco, or at the written request of Newco, Authorized Manufacturer (as defined in Section 4E) in accordance with an implementation plan to be agreed upon in writing by the Parties. In addition, Intact agrees to deliver to Newco labels, brochures, packaging, instructions materials, and all other marketing and promotional materials currently being used by Intact for the Product, to be translated and used by Newco in connection with the
commercialization of the Product in the Territory.
B. Commercialization Plan and Progress Reports. All manufacturing and sales and marketing activities for the Product in the Territory will be the responsibility of Newco. Newco agrees that it shall use commercially reasonable efforts to carry out the manufacturing, marketing and commercialization of the Product throughout the Territory.
C. Technical Support and Training. For no additional consideration, Intact shall, as reasonably requested by Newco, use its commercially reasonable efforts to render the technical support and training, and advice in connection with familiarizing Newco or its Authorized Manufacturer in the specifications, characteristics and use of the Product to enable Newco to manufacture and commercialize the Product in the Territory as contemplated by this Agreement (For the purpose of this Section 3C, the Parties
agree that commercially reasonable efforts shall, at a minimum, be substantially the same effort that Intact provided to other manufacturers and distributors of the Product). Unless otherwise agreed in writing by the Parties, any assistance and training provided by Intact at Intact’s site in the United States, and all ancillary expenses (including without limitation travel and accommodation) associated with the attendance of any personnel at such training, excluding Intact staff, will be the sole responsibility of
A - 5
Newco, or its Authorized Manufacturer if the Authorized Manufacturer receives the training. If initial training requires that Intact personnel visit the operations in China, then Intact shall cover those expenses, so long as those expenses are reasonable. Such expenses would exclude any expenses associated with the attendance of Huiheng or Newco personnel at such training in China. Both Parties agree that Newco shall establish a budget to cover reasonable travel expenses for Newco and Intact personnel relating to the business of Newco
once Newco is cash flow positive.
D. Expenses of Newco. Newco will assume and pay all costs incurred in manufacturing, marketing and commercializing the Product in the Territory, including without limitation all costs associated with set-up of any manufacturing facilities and tooling for production, unless otherwise specifically provided for herein.
A. Newco’s Manufacturing Obligation. Newco will have the exclusive right to manufacture or have manufactured and supply the Product, including the Intact RF Generator, the reusable handle tool known as Intact Handle and the disposable wand know as Intact Wand, in the Territory pursuant to the terms of this Agreement. Subject to prior written approval of Intact, which approval will not be unreasonably withheld pursuant to Xxxxxxx 0X, Xxxxx shall have the right to subcontract for the manufacturing of the Product.
B. Initial Product Manufacturing and Evaluation. Prior to any commercialization of the Product in the Territory, Newco will manufacture Product or have it manufactured using the Process Technology, and deliver an initial sample or batch of samples of the Product to Intact for testing. Upon fifteen (15) days of receipt of the sample(s), using the Qualification Plan, Intact will evaluate the Product and provide written notification to Newco informing Newco of results of its evaluation which will either (i) confirm satisfaction
of Acceptance Criteria resulting in approval of Product, or (ii) identify any material deficiencies, by itemizing and describing such deficiencies and corrective measures. Once the Product receives Qualification, the manufacturing process established by Newco, whether at its own manufacturing facility or at the manufacturing facility of an Authorized Manufacturer, will be deemed qualified. Newco may not market or sell any Product until Intact has given its confirmation that the Product meets the Acceptance Criteria following evaluation of the Product in accordance with the Qualification Plan provided by Intact and agreed upon by Newco.
C. Changes to Manufacturing Process or Product. Newco may not make any material changes to the Product or to the manufacturing process after it has been qualified pursuant to Section 4B, without the prior written consent of Intact on a case-by-case basis which will not be unreasonably withheld
D. General Manufacturing. Upon Qualification, Newco will manufacture the Product to conform to the Acceptance Criteria. Newco shall not (i) have any third party manufacture the Product, or (ii) source from any third party any component of the Product, for any purpose without Intact’s express prior consent in writing, such consent not to be unreasonably withheld. Any third party to which Intact gives consent, which consent will not be unreasonable withheld by Intact if such third party meets current
U.S., European and Chinese commonly accepted manufacturing standards, that is appointed to manufacture the Product or a component of the Product in the Territory (including pursuant to Section 4E) shall be an “Authorized Manufacturer” for the purposes of this Agreement.
A - 6
E. Authorized Manufacturers. Intact acknowledges that as of the Effective Date, Newco does not currently have the capability to manufacture the Product in the Territory, and that Newco intends to appoint Huiheng as an Authorized Manufacturer. Intact hereby consents to the appointment of Huiheng as an Authorized Manufacturer, provided that (i) Huiheng agrees to be bound by the terms of this Agreement, and (ii) Huiheng may not subcontract or otherwise delegate any of its manufacturing obligations to any third party without the
express consent in writing of Intact, and (iii) Intact shall be an express intended third party beneficiary for any agreement between Huiheng and Newco to enforce the applicable provisions under this Agreement regarding Authorized Manufacturers, including without limitation any provisions regarding protection of Intact’s Intellectual Property, Intact’s inspection rights, and the Qualification of Product.
F. Core Components. Newco acknowledges that the quality of components utilized in the manufacture and assembly of the Product is of critical importance to Intact, and that certain components may not be able to be manufactured or supplied by Newco or an Authorized Manufacturer at the time of commencement of manufacturing operations under this Agreement (the “Core Components”). A list of such Core Components is attached to this Agreement as
Exhibit E. Newco agrees that it will use its commercially reasonable efforts to purchase, and ensure that its Authorized Manufacturer purchase, the Core Components from third party suppliers identified by Intact in writing, until such time as Newco evidences that such Core Components meeting the applicable Acceptance Criteria can be manufactured by Newco or an Authorized Manufacturer in the Territory.
G. Supply Prices. At Intact’s option, Intact shall have the right to purchase the Product from Newco to sell outside of the Territory. At Intact’s request and sole discretion, Newco agrees to sell the Product to Intact. The cost of the Product to Intact shall be [***]. As soon as available, Newco will provide Intact with a current component price list for the Product and a detailed accounting of the parts and labor costs
[***] In addition at Intact’s request, Newco will provide Intact with a current component price list for the Product. Notwithstanding the generality of the foregoing, Newco will provide Intact at the end of each calendar year and upon a material changes in price list defined as a change in a component price greater than [***], with an updated price list report of its costs for parts and labor, including a breakdown of how those costs have been calculated. Intact shall have the right, upon reasonable written notice to Newco, to request that Newco provides additional validation of the costs charged or notified to Intact for any component or ancillary costs or combination thereof. [***]. The
parties agree that the foregoing costs are estimates only, and are non-binding upon the parties. Newco agrees to provide Intact with reasonable notice in writing of any material changes in the estimated cost of such components.
H. Inspection of Manufacturing Facility. Upon Intact’s reasonable advance request and at Intact’s sole expense, Intact shall have the right to perform a reasonable technical audit and inspection of any Newco manufacturing facilities or Authorized Manufacturer manufacturing facilities, as reasonably necessary for Intact to verify Newco’s and its Authorized Manufacturers’ compliance with this Agreement and the Qualification Plan, including an audit and inspection of yields, delivery, performance, and
financial and operational records. In the event an inspection of any manufacturing facility for the Product or any components of the Product reveals any deficiency, Newco shall promptly take action to remedy such deficiency and may not sell any Product affected by such deficiency or manufacture any further Product or Product components at the deficient facility until such Product or Product components have passed Qualification. Intact’s right to audit and inspect any Product manufacturing facility shall continue for a period of five(5) years after the expiration or termination of this Agreement for the sole purpose of allowing Intact to verify that neither Newco or any of its
Authorized Manufacturers possess, or continue to use, Intact’s Confidential Information and Process Technology.
A - 7
I. Financial Audits. Intact will also have the right to engage at its own expense an internationally recognized independent auditor reasonably acceptable to Newco to examine Newco’s records from time to time to the extent necessary to verify the costs and financial information to justify the pricing under Section 4G; provided, however, that (i)Intact will provide Newco reasonable prior written notice of such audit and may conduct audits no more than once every twelve (12) months, (ii) no such examination may be of a period previously examined and (iii) such results are provided to Newco. If any such audit reveals (a) an over-charge of more than five percent (5%) invoiced for the audited period, such audit will be at the expense of Newco and all such over-charges will be, at Intact’s option, either
refunded or credited to Intact, or (b) an under-charge of more than five percent (5%), such audit will be at the expense of Intact, and all such under charges will be, at Newco's option, charged to Intact for immediate payment or credited.
A. Orders. Any such orders placed by Intact shall be evidenced by a written purchase order identifying the number, requested delivery date(s) and any shipping instructions, including preferred carrier and shipping destination. Upon receipt of an order, Newco shall advise Intact within three (3) days of the acceptance of an order and of the lead time required for the manufacture (not to exceed the standard lead time) and delivery of such order in its entirety. Unless the Parties otherwise agree in writing, the standard lead time for the Product will be
60-90 days. Once such delivery date is identified and agreed to by the parties, Newco shall fully comply therewith.
C. Risk of Loss and Title. Delivery of all Product shall be made F.C.A.. (INCOTERMS 2000) Intact delivery point in China. Risk of loss for the Product shall pass to Intact at the Intacts delivery point in China. Title to the Product will pass to Intact at the Intact delivery destination in China.
A - 8
E. Payment. Upon shipment of Product ordered, Newco will submit to Intact an invoice showing invoice number and date, remit to address, the purchase order number, part number and revision, quantity of each Product, unit prices, each applicable tax and totals. Intact shall pay each invoice by the later of thirty (30) days after the receipt of invoice, or (ii) thirty (30) days after receipt of the shipment by wire transfer.
A. Year 1 Minimum Milestone Requirements. Within the first twelve (12) months after receipt of SFDA approval (“Term Year 1”), Newco agrees that it shall achieve the following (“Year 1 Milestone”):
i. Install Intact RF generators in no less than 20 different hospitals in the Territory;
ii. Sell a minimum of 100 Intact Handles in the Territory; and
iii. Sell a minimum of 4,000 Intact Wands in Territory.
In the event Newco fails to achieve Year 1 Milestone, this Agreement will automatically terminate within forty-five (45) days after Term Year 1 after Intact gives Newco written notice of termination pursuant to Section 7B, unless Huiheng, in its sole option and discretion, pays Intact $150,000 to maintain Newco's rights under this Agreement.
B. Year 2-4 Minimum Targets. In each of the following respective Term Years (referred to as the “Target Milestones”): Intact shall have the right to terminate this Agreement pursuant to 7, if Newco fails to satisfy the following milestones for the respective year:
i. For Term Year 2 (a period of twelve (12) months commencing at end of Term Year 1 (“Term Year 2”)) if Newco fails to pay out the Year 2 Dividend on or before 90 calendar days following the end of Term Year 2 (“Year 2 Dividend Date”), provided. however that Huiheng or an affiliate of Huiheng will have the right, at its sole option, to purchase the balance of the Year 2 Dividend owed to Intact from Intact for cash on a dollar-for-dollar basis on or before the Year 2 Dividend Date to maintain Newco’s rights under this Agreement. Newco shall make an effort in good faith to declare some portion of the Year 2 Dividend after the end of each quarter, provided that Newco has accumulated available, free cash and
provided that distributing that cash will not have a negative effect on Newco’s ability to execute its business plan. The cumulative Year 2 Dividend as ascribed in the Joint Venture Agreement will be the total of the four quarterly dividends in Year 2;
ii. For Term Year 3 (a period of twelve (12) months commencing at end of Term Year 2 (“Term Year 2”) if Newco fails to pay out the Year 3 Dividend on or before 90 calendar days following the end of Term Year 3 (“Year 3 Dividend Date”), provided however that Huiheng or its affiliate of Huiheng will have the right, at its sole option, to purchase the balance of the Year 3 Dividend owed to Intact from Intact for cash on a dollar-for-dollar basis on or before the Year 3 Dividend Date to maintain Newco’s rights under this Agreement. Newco shall make an effort in good faith to declare some portion of the Year 3 Dividend after the end of each quarter, provided that Newco has accumulated available, free cash and provided that distributing that cash will not have a negative effect on
Newco’s ability to execute its business plan. The cumulative Year 3 Dividend as ascribed in the Joint Venture Agreement will be the total of the four quarterly dividends in Year 3;
A - 9
iii. For Term Year 4 (a period of twelve (12) months commencing at end of Term Year 3 (“Term Year 4”) if Newco fails to pay out the Year 4 Dividend on or before 90 calendar days following the end of Term Year 4 (“Year 4 Dividend Date”), provided however that Huiheng or its affiliate of Huiheng will have the right, at its sole option, to purchase the balance of the Year 4 Dividend owed to Intact from Intact for cash on a dollar-for-dollar basis on or before the Year 4 Dividend Date to maintain Newco’s rights under this Agreement. Newco shall make an effort in good faith to declare some portion of the Year 4 Dividend after the end of each quarter, provided that Newco has accumulated available, free cash and
provided that distributing that cash will not have a negative effect on Newco’s ability to execute its business plan. The cumulative Year 4 Dividend as ascribed in the Joint Venture Agreement will be the total of the four quarterly dividends in Year 4.
C. Subsequent to Term Year 4. Following the end of Term Year 4 (and if renewed, each year thereafter), this Agreement will be automatically renewed on its terms for successive one (1) year terms without further conditions until terminated by Newco, or by the terms of this Agreement, provided however, that if in any subsequent Renewal Term Newco’s revenues from Wand sales fall below 60% of the average of the two years revenue from Wand sales (“Wand Revenue
Milestone”), then Intact shall have the right to terminate this Agreement.
A. Term. This Agreement shall commence on the Effective Date and, unless earlier terminated as provided for herein, shall continue in effect for four (4) years from the date of SFDA Approval (the “Initial Term”). Subject to Section 6C, upon expiration of the Initial Term, this Agreement shall automatically extended for additional successive one (1) year terms (each, a “Renewal Term”) until
terminated pursuant to Section 7B. The Initial Term and the Renewal Term will be collectively referred to as the “Term”.
B. Termination Rights. The parties may terminate this Agreement at any time by mutually agreeing in writing to do so. In addition, each party shall have the right to terminate as set forth below:
i. Intact shall have the right to terminate the Agreement upon forty-five (45) days prior written notice if any of the following occurs:
|
|
(a)
|
Newco materially breaches its obligations, representations or warranties under this Agreement and fails to cure such breach within such forty-five (45) day period;
|
|
|
(b)
|
Newco fails to achieve the Target Milestones or Wand Revenue Milestone as set out in Section 6;
|
|
|
(c)
|
Newco is or becomes bankrupt or insolvent, enters into an arrangement, readjustment of debt, general assignment for the benefit of creditors, dissolution or liquidation proceeding; or
|
|
|
(d)
|
if the Joint Venture Agreement is terminated
|
ii. Newco shall have the right to terminate this Agreement upon forty-five (45) days prior written notice under the following:
A - 10
|
|
(a)
|
Intact materially breaches its obligations, representation or warranties under this Agreement and fails to cure such breach within such forty-five (45) day period;
|
|
|
(b)
|
in its sole discretion in any Renewal Term; or
|
|
|
(c)
|
The Product ceases to have valid and enforceable patent protection in the United States.
|
|
|
(d)
|
if the Joint Venture Agreement is terminated.
|
C. Effects of Termination. Subject to the remainder of this Section 7D, upon termination or expiration of this Agreement, Newco will have (i) the right to sell the RF generators remaining in its inventory (“Residual Inventory”), (ii) for a period of five (5) years commencing on the expiration or termination of this Agreement (“Wind-Down Period”), Newco will retain and have the non-exclusive right to continue to manufacture the Intact Wands and Intact Handles and the exclusive right to market, distribute, sell and supply to customers who purchased the Residual Inventory in the Territory, or the RF Generators units sold by Newco prior to the termination or expiration of this
Agreement (“Installed Base”), and (iii) a continuing license to the Licensed Technology and Trademarks granted hereunder to supply the Intact Wands and Intact Handles during the Wind-Down Period; provided that if this Agreement has been terminated by Intact for material breach by Newco, Newco shall not have any rights with respect to the Product following such termination during the Wind-Down Period, including the rights set out in (ii) and (iii) above; provided, however, that if this Agreement has been terminated as a result of the termination of the Joint Venture Agreement by Huiheng for material breach by Intact, Newco (y) will also retain and have the non-exclusive right to continue to manufacture the Product and the Product components, and the exclusive right to market, distribute, and sell the Product and the
Product components in the Territory during the Wind Down Period, and (z) will have a continuing license to the Licensed Technology and Trademarks granted hereunder to supply and market the Product and Product components during the Wind-Down Period. For the purpose of the rights granted to Newco during the Wind-Down Period under this Section 7C, failure to meet the Target Milestone or Wand Revenue Milestone as provided in Section 6 does not constitute as a material breach by Newco. Notwithstanding anything to the contrary in this Agreement, upon termination or expiration of this Agreement all licenses granted by Intact to Newco hereunder, express or implied, shall automatically terminate except to the extent necessary for Newco to sell Product, Intact Wands and Intact Handles after termination as permitted by this Section 7C.
D. Survival. Expiration or termination of this Agreement shall not affect the rights or obligations of either party hereto which shall have accrued hereunder prior to such expiration or termination, or the rights and obligations of the parties intended hereunder to survive the expiration or termination of this Agreement. Without limiting the generality of the foregoing, no expiration or termination of this Agreement, whether by lapse of time or otherwise, shall serve to terminate the obligations of the Parties hereto under Sections 1,
3D, 4H, 4I, 7C, 7D, and 8 through 22.
E. Representations and Warranties of Each Party. Each party represents and warrants that:
A - 11
(i) this Agreement has been duly executed and delivered by such party and constitutes a valid and binding obligation of such party, enforceable against such party in accordance with its terms, except as enforceability may be limited by bankruptcy, fraudulent conveyance, insolvency, reorganization, moratorium and other laws relating to or affecting creditors’ rights generally and by general equitable principles;
(ii) as of the Effective Date, the execution, delivery and performance of this Agreement have been duly authorized by all necessary action on the part of such party, its officers and directors and does not conflict with any agreement, instrument or understanding, oral or written, to which such party is a party or by which it may be bound, and, to the best of its knowledge, does not violate any material law or regulation of any court, governmental body or administrative or other agency having authority over it;
(iii) this Agreement and the performance hereunder does not and shall not contravene with any agreement by which such party is bound by; and the consummation of the transactions contemplated hereby and the fulfillment of the terms hereof do not and will not conflict with or result in a violation of or default under the charter or under any agreement, lease, contract, loan agreement, indenture or other instrument or obligation to which such party is a party;
(iv) such party has full power and authority to perform the obligations set forth herein;
(v) such party is not subject to any order, decree or injunction by a court of competent jurisdiction which may prevent or materially delay the consummation of the transactions contemplated by this Agreement; and
(vi) such party is duly organized, validly existing and in good standing under the laws of the jurisdiction where it is organized.
F. Additional Representations of Intact. Intact represents, warrants and covenants to Newco that (i) it is the sole owner of the Licensed Technology; (ii) Exhibits C and D lists each Patent, that is included among the Intellectual Property owned by Intact and Trademarks related to the Product; (iii) Intact has all right, title and interest in and to the Intellectual Property it Controls that is related to the Product (iv) the clinical studies done on the Product prior to the date hereof were conducted in good faith and not
in contravention of any applicable laws, rules, regulations or guidelines concerning the performance of such studies and the interpretation of data collected, (iv) to Intact’s Knowledge as of the Effective Date, Intact has not received any written claim or notice of infringement of the Intellectual Property rights of any other person pending or threatened in writing against Intact relating to the Product or operation of Intact’s business, (v) as of the Effective Date, no payments are due to others by reason of the sale of the Product or licensing of the Licensed Technology as contemplated hereunder (vi) to Intact’s Knowledge as of the Effective Date, the granting of the Licensed Technology hereunder does not infringe upon the rights of any other third parties, vii) to Intact’s Knowledge as of the Effective Date, Intact has taken
reasonable precautions to protect trade secrets constituting material Intellectual Property owned or used by Intact in connection with Product, including the execution of appropriate agreements, and (xii)to Intact’s Knowledge as of the Effective Date, Intact’s current use of the Licensed Technology does not not infringe, misappropriate or violate any intellectual property rights of any person.
A - 12
i. Performance; Defective Product. Newco warrants that all Product delivered hereunder shall meet all applicable specifications for the Product provided by Intact and shall be free from defects in material and workmanship under normal use and service for a period of 12 months from the date of shipment from Newco. Intact shall not be obligated to pay for any Product delivered to it of such Product does not conform to approved sample under Section 4B (“Defective
Product”). Intact may at its sole discretion request that Newco replace or repair such Defective Product, or Intact may return such Defective Product to Newco, at Newco’s sole cost
ii. Service Levels; Performance of Third Parties. Newco will use its best efforts to perform, and as necessary cause its authorized sub-distributors or authorized subcontractors to perform, all manufacturing and other services pursuant to this Agreement in a timely and professional manner and in accordance with industry standards for manufacturing services.
iii. Compliance with Laws. Newco will comply with all applicable federal, state, and local laws and regulations, and will obtain all applicable permits and licenses, necessary to manufacture and sell the Product, provided, however, that Intact shall solely be responsible for all permits and licenses required by any US, state or foreign country or international agency that Intact needs for it’s sales of the Product outside of the Territory.
iv. Anticorruption Matters. Newco represents and warrants that it has not taken and covenants that it will not take during the Term any action in violation of any applicable anticorruption law, including the U.S. Foreign Corrupt Practices Act (“FCPA”) (15 U.S.C. § 78 dd-1 et seq.); or (ii) has corruptly, offered, paid, given, promised to pay or give, or authorized the payment or gift of anything of value, directly or indirectly, to any “Public
Official,” as defined in this Section 8C, for purposes of (A) influencing any act or decision of any Public Official in his official capacity; (B) inducing such Public Official to do or omit to do any act in violation of his lawful duty; (C) securing any improper advantage; or (D) inducing such Public Official to use his or her influence with a government, governmental entity, or commercial enterprise owned or controlled by any government (including state-owned or controlled veterinary or medical facilities), in order to assist the business of Newco or any party related in any way to the business of Newco. For purposes of this Section 8C, “Public Official” means: (i) any officer, employee or representative of any regional, federal, state, provincial, county or municipal government or government department, agency, or other division; (ii) any
officer, employee or representative of any commercial enterprise that is owned or controlled by a government, including any state-owned or controlled medical facility; (iii) any officer, employee or representative of any public international organization, such as the African Union, the International Monetary Fund, the United Nations or the World Bank; (iv) any person acting in an official capacity for any government or government entity, enterprise, or organization identified above; and (v) any political party, party official or candidate for political office. Newco does not have knowledge of any actual or potential issues under the FCPA or any other applicable anticorruption law involving any of its directors or officers, employee, acting on behalf of Newco in any way relating to its business.
A - 13
v. Export Controls and Sanctions Matters. Neither Newco nor any of its directors, or officers, employees, nor, to Newco's knowledge, any distributor, agent, representative, sales intermediary or other third party acting on behalf of Newco, has taken or will take during the Term any action in violation of any applicable export control law, trade or economic sanctions law, or anti-boycott law, in the United States or any other jurisdiction, including: the Arms Export Control Act (22
U.S.C.A. § 2278), the Export Administration Act (50 U.S.C. App. §§ 2401-2420), the International Traffic in Arms Regulations (22 C.F.R. 120-130), the Export Administration Regulations (15 C.F.R. 730 et seq.), the Office of Foreign Assets Control Regulations (31 C.F.R. Chapter V), the Customs Laws of the United States (19 U.S.C. § 1 et seq.), the International Emergency Economic Powers Act (50 U.S .C. § 1701-1706), the U.S. Commerce Department anti-boycott regulations (15 C.F.R. 560), the U.S. Treasury Department anti-boycott requirements (26 U.S.C. § 999), any other export control regulations issued by the agencies listed in Part 730 of the Export Administration Regulations, or any applicable non-U.S. Laws of a similar nature.
vi. Neither Newco nor, to Newco's knowledge any of its director or officers, employee, distributor, agent, representative, sales intermediary or other third party acting on behalf of Newco, is listed on the U.S. Office of Foreign Assets Control “Specially Designated Nationals and Blocked Persons” (“SDN List”) or any other similar list.
vii. All export licenses, license exceptions and other consents, notices, waivers, approvals, orders, authorizations, registrations, declarations, classifications and filings required for the export, import and re-export of the Product (“Export Approvals”) will be obtained; and to the Knowledge of Newco, no Export Approvals are required by the laws identified in Section 8C(v) for continued export, re-export or import of the Product.
viii. Newco has taken and will take during the Term, all reasonable measures, to ensure that its directors, employees, agents, distributors, representatives and sales intermediaries take all reasonable measures, to protect the Confidential Information and any trade secrets included in the Licensed Technology.
ix. No Warranty Pass Through Except for the limited warranty expressly set forth in Section 8C(i) above, Newco shall not be entitled to make or pass through any warranties to any third parties regarding the Product unless specifically authorized in writing by Intact on a case-by-case basis. Newco shall be responsible for all representations and warranties it makes to customers.
9. Indemnification.
A. Indemnification by Newco. Newco agrees to indemnify and hold Intact, its officers, directors, employees, successors and assigns (the “Intact Indemnities”) harmless from and against all losses, damages, action, suit, claim, demand, liability, penalty, expense (including, without limitation, reasonable attorneys’ fees and expenses), bodily injury, death, or property damage (collectively, “Losses”), finally
awarded against Intact Indemnitees by a court of competent jurisdiction or agreed upon by Newco in settlement which the Intact Indemnitees, or any of them, resulting from any claims, actions or suits (including a governmental investigation) by third parties to the extent arising from; (i) any gross negligent or wrongful act or omission of Newco or its directors, officers, Affiliates, employees or agents; (ii) violation by Newco (or any of its directors, officers, Affiliates, employees or agents) of any applicable law, rule, regulation or order), or (iii) defects in manufacturing of the Product.
A - 14
B. Indemnification by Intact. Intact agrees to indemnify and hold Newco, its officers, directors, employees, successors and assigns (the “Newco Indemnitees”) harmless from and against all Losses, finally awarded against Newco Indemnitees by a court of competent jurisdiction or agreed upon by Intact in settlement which the Newco Indemnitees, or any of them, resulting from, any claims, actions or suits (including a governmental investigation) by third parties to the extent arising from; (i) any gross negligent or wrongful act or omission of Intact or its directors, officers, Affiliates, employees or agents; (ii) violation by Intact (or any of its directors, officers, Affiliates, employees or agents) of any
applicable law, rule, regulation or order; (iii) an inherent defect in the design or functionality of the Product; or (iv) the infringement of intellectual property rights in the Territory by the Product as designed by Intact, but not as a result of any modifications to design or manufacturing by or on behalf of Newco, and not for combinations of the Product with any other products or use of the Product for any other purpose than the use to which the Product is put by Intact as of the Effective Date .
C. Indemnification Procedure. If indemnification is sought pursuant to Sections 9A or B above, the indemnified party shall: (i) give written notice to the indemnifying party within fifteen (15) days after receipt by the indemnified party of such claim, suit or demand; provided, however, that the failure to give notice within such time period shall not relieve the indemnifying party of its obligation to indemnify, unless it shall be materially prejudiced by
such failure; (ii) permit the indemnifying party to assume direction and control of the defense of claims resulting therefrom; and (iii) at its own cost and expense, cooperate fully as requested by the indemnifying party in the defense of the claims. No offer of settlement, settlement or compromise by the indemnifying party shall be binding on an indemnified party without its prior written consent (which consent shall not be unreasonably withheld or delayed), unless such settlement fully releases the indemnified party without any liability, loss, cost or obligation incurred by such indemnified party. No offer of settlement, settlement or compromise by the indemnified party shall be binding on an indemnifying party without its prior written consent (which consent shall not be unreasonably withheld or delayed).
D. Disclaimer of Warranties. EXCEPT AS EXPRESSLY PROVIDED IN THIS AGREEMENT, EACH PARTY MAKES NO REPRESENTATIONS OR WARRANTIES AS TO ANY MATTER, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, AND EACH PARTY SPECIFICALLY DISCLAIMS ANY AND ALL IMPLIED OR STATUTORY WARRANTIES, INCLUDING, ALL IMPLIED WARRANTIES OF, MERCHANTABILITY, AND FITNESS FOR A PARTICULAR PURPOSE.
E. Limitation of Liability. IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER PARTY FOR LOSS OF PROFITS OR OTHER COMMERCIAL OR ECONOMIC LOSS, OR LOSS OR INTERRUPTION OF BUSINESS, OR ANY INDIRECT, SPECIAL, CONSEQUENTIAL OR EXEMPLARY DAMAGES OF ANY KIND ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES NOTWITHSTANDING THE FOREGOING, NOTHING IN THIS SECTION 9E IS INTENDED TO LIMIT OR RESTRICT THE AMOUNTS PAYABLE TO THIRD PARTIES TO FULFILL INDEMNITY OBLIGATIONS SECTIONS 9A OR 9B, A BREACH OF LICENSE
RESTRICTIONS, OR DAMAGES AVAILABLE FOR A BREACH OF CONFIDENTIALITY OBLIGATIONS IN SECTION 11. The foregoing provision shall not be construed to limit a party’s indemnification obligation under this Agreement for third party claims which may include consequential, punitive or other types of damages.
A - 15
10. Dispute Resolution.
A. Negotiation of Parties. In the event of any dispute, claim or controversy arising out of or relating to the interpretation of any provision of this Agreement, to the performance of either party under this Agreement or to any other matter under this Agreement, including any action in tort, contract or otherwise, at equity or at law, and any claims of fraud in the inducement (a “Dispute”), either party may at any time provide the other party written notice specifying the terms of such
Dispute in reasonable detail. As soon as practicable after receipt of such notice, the Chief Executive Officers of both Newco and Intact shall meet (in person or otherwise) at a mutually agreed upon time and location for the purpose of resolving such Dispute. They shall engage in good faith discussions and/or negotiations for a period of up to thirty (30) days to resolve the Dispute or negotiate an interpretation or revision of the applicable portion of this Agreement which is mutually agreeable to both parties, without the necessity of formal procedures relating thereto. During the course of such discussion and/or negotiation, the parties shall reasonably cooperate and provide information that is not materially confidential in order so that each of the parties may be fully informed with respect to the issues in Dispute.
B. Arbitration. All disputes, controversies or differences which may arise between the parties, and/or between a party and the Newco, out of or in relation to or in connection with this Agreement which cannot be settled by full discussion between appropriate senior representatives of the parties will be settled, by arbitration in Boston, Massachusetts, according to the commercial arbitration rules of the American Arbitration Association and applying the procedural laws of the State of Massachusetts. The proceedings shall take place in the local language with
provision for simultaneous interpretation at the expense of the party desiring it. The decision of the arbitrator(s) will be final and binding and enforceable in any court with appropriate jurisdiction. Notwithstanding the generality of the foregoing, nothing in this Section 10B shall be construed to limit either Party’s right to apply for injunctive or other equitable relief to which it may be entitled to preserve any of its rights under this Agreement.
A. Confidentiality. It has been contemplated that in the course of the negotiation and the performance of this Agreement each party may have access to, or may disclose, Confidential Information of or to the other, as the case may be. Each party agrees that for the Term and for ten (10) years thereafter, the Receiving Party shall keep confidential and shall not publish or otherwise disclose, and will not use any Confidential Information of the Disclosing Party except for the limited purposes set forth in this Agreement;
provided, however that if any disclosure of Confidential Information to a third party is authorized by this Agreement, the Receiving Party shall obtain from such third party an agreement to hold such information in strictest confidence, and not to make use of such information for any purpose other than as permitted by this Agreement. No provision of this Agreement shall be construed to preclude such disclosure of Confidential Information as may be necessary or appropriate (i) to obtain from any governmental agency any necessary approval contemplated by this Agreement, (ii) solely in the case of Intact, to file patent applications or obtain patents that are
included in the Licensed Technology consistent with the terms of this Agreement. If any disclosure of Confidential Information is required under the foregoing subsection (ii), the Receiving Party shall notify as soon as possible the Disclosing Party of such disclosure and the Receiving Party shall, if requested by the Disclosing Party, use reasonable good faith efforts, at the expense of the Disclosing Party, to assist in seeking a protective order (or equivalent) with respect to such disclosure or otherwise avoid making such disclosure, and in each case of (i) or (ii) above, the disclosure by the Receiving Party shall be limited to the minimum required to comply with the requirement to disclose the Confidential Information. The Receiving Party will take all precautions as are reasonably necessary to prevent unauthorized access to, reproduction,
A - 16
duplication, disclosure or use of the other party’s Confidential Information and shall only disclose the Confidential Information of the other party to those of its officers, directors and employees, or to officers, directors and employees of its Affiliates, on a “need to know basis” provided each such officer, director, contractor or employees agrees in writing in favor of the Disclosing Party to be bound by the same obligations of secrecy and confidentiality that the Receiving Party is bound to under this Agreement and provided, further, that the Receiving Party shall be strictly responsible to the Disclosing Party for any losses or damages suffered as a result of the breach of such obligations
by the Receiving Party’s directors, officers, contractors or employees.
B. Exceptions. The obligations of confidentiality contained in Section 11 will not apply to the extent that such Confidential Information: (i) was already known to the Receiving Party, other than under an obligation of confidentiality, at the time of disclosure by the Disclosing Party; (ii) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party; (iii) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or
omission of the Receiving Party in breach of this Agreement; (iv) was disclosed to the Receiving Party, other than under an obligation of confidentiality, by a third party who had no obligation not to disclose such information to others; (v) was developed independently by the Receiving Party without any use of Confidential Information; or (vi) is required to be disclosed publicly by applicable law.
12. Relationship of Parties. Nothing herein shall be deemed to create an agency, joint venture, amalgamation, partnership, franchise or similar relationship between Newco and Intact. Notwithstanding any of the provisions of this Agreement, neither party shall at any time enter into, incur, or hold itself out to third parties as having authority to enter into or incur, on behalf of the other party, any commitment, expense, or liability whatsoever, and all such commitments, expenses and liabilities undertaken or incurred by one party in connection with or relating to the
development, manufacture or sale of Product or components thereof shall be undertaken, incurred or paid exclusively by that party, and not as an agent or representative of the other party.
A. IP Ownership. The parties agree that Intact is the sole owner of all legal and beneficial right, title and interest in and to the Licensed Technology and any Improvements thereto, and nothing in this Agreement shall be interpreted as granting any rights of ownership to Newco in any Licensed Technology. Ownership of all inventions and all intellectual property rights therein, relating to the Licensed Technology and, arising out of the activities conducted under this Agreement, including Improvements and other inventions relating to methods of making or using, configuration, performance or other attributes of the Product, that are made by employees, independent contractors, agents or
Affiliates of one or both of the Parties (“Inventions”) shall be owned solely by Intact, regardless of creator. All such Inventions and intellectual property rights shall be included within the scope of the Licensed Technology and are licensed to Newco pursuant to Section 2 of this Agreement. Notwithstanding the generality of the foregoing, the parties agree that any inventions conceived and reduced to practice solely by or on behalf of Newco that are not derived from the Licensed Technology, or which relate to the manufacturing process (the “Process Improvements”) shall be owned by Newco.
Newco hereby grants to Intact, and agrees to cause to be granted by any Authorized Manufacturer to Intact, a worldwide, perpetual, non-exclusive, royalty free, fully paid, transferable license in and to the Process Improvement.
A - 17
B. Further Assurances. Except as otherwise mutually agreed upon, Intact and Newco agree that without additional consideration, Newco hereby assigns all of its right, title and interest in and to Inventions, excluding Process Improvements, and related intellectual property rights (including enforcement rights) to Intact. Newco shall promptly notify Intact in writing of all Inventions and if Intact desires to secure protection on such Inventions, Newco will cooperate with Intact for the purpose of filing and prosecuting patent applications, including the execution of any and all legal papers which may be deemed necessary or desirable by Intact to record such assignment or for the filing and prosecution of patent applications and for assignment of
such applications to Intact. Newco hereby designates Intact as its agent for, and grants to Intact a power of attorney, which power of attorney shall be deemed coupled with an interest, solely for the purpose of effecting the foregoing assignment from Newco to Intact.
C. Patents and Related Expenses. Intact shall, at its own expense, apply for, prosecute before the Patent Offices and maintain in the Patent Offices all Patents and all trademark applications and trademarks listed on Exhibit D. Upon request of Newco, Intact may at its own discretion, agree to undertake to apply for, prosecute and maintain all Patents in the Territory at Intact’s sole expense.
D. Infringement of Patents. Intact shall have the first right, at its sole discretion, but is not obligated, to take any and all action that it deems appropriate, at its expense, to assert any or all of the Licensed Technology against any party(s) deemed by Intact or Newco (by written notice to Intact) to infringe said Licensed Technology (the “Infringing Party”) anywhere in the world. If Intact elects to enforce or assert its Licensed Technology against an Infringing Party, Newco agrees to cooperate with Intact and shall provide all reasonable assistance reasonably requested by Intact in pursuit of such action(s), at Intact’s sole expense. If Intact elects not to
assert or otherwise enforce the Licensed Technology against any Infringing Party, it shall notify Newco in writing within thirty (30) days of making that decision, and Newco shall be entitled, but shall have no obligation to take any and all action that it deems appropriate, at its expense, to assert any or all of the Licensed Technology against any Infringing Party in the Territory only, and Intact hereby agrees to cooperate with Newco as reasonably requested and reasonably necessary in the pursuit of said action(s), at Newco’s sole expense. Newco shall promptly keep Intact informed of and provide copies of all material correspondence and documents relating to any such actions, and shall consult with Intact on all material decisions regarding such actions, and Intact shall have the right to consent to material decisions which are reasonably likely to affect Intact, which consent
shall not be unreasonably withheld, conditioned or delayed.
E. Infringement by Third Party Product. In the event that an infringement suit is filed in the Territory naming Intact and/or Newco as defendants and alleging that the Product infringes one or more patent(s) held by a third party, then Intact shall be obligated to take any and all action that it deems reasonably appropriate, at its expense, to defend the Licensed Technology against the third party alleging infringement, and Newco hereby agrees to cooperate with Intact as reasonably necessary in the pursuit of said action(s).
F. Force Majeure. Neither party shall be liable for any delay in or failure of performance hereunder due to any contingency beyond its reasonable control. Should the delay or failure continue for more than twelve (12) months, then the other party shall have the right to immediately terminate this Agreement upon written notice to the other party. However, nothing contained herein shall relieve either party from its obligation to make all payments due hereunder when such payments are due.
A - 18
G. Notices. Any notice or request which shall or may be given under this Agreement shall be made by airmail, registered mail with return receipt requested or facsimile and shall be directed to the intended recipient at its address set forth below or at such other address as such party may notify the other parties in accordance with this paragraph. The effective date of delivery of such notice shall, in the case of airmail or registered airmail, be seven (7) business days following dispatch, or in the case of facsimile or e-mail, the date of successful transmission as evidenced by a
transmission report.
H & I Medical China Limited
Gaoxin 7 Street South,
Keyuannan Road, Nanshan District,
Shenzhen Guangdong, P.R. China 518057
Facsimile: 0000-0000 0000
Intact Medical Corporation
000 Xxxxxxxxxx Xxxx, Xxxxx 00, Xxxx Wing, Floor 4,
Xxxxxxxxxx XX 00000
Facsimile: (000) 000-0000
H. Assignment; Successor and Assigns. Neither party may assign or otherwise transfer this Agreement without the prior written consent of the other party; provided, however, that Intact may assign this Agreement to a successor in interest to Intact without Newco’s consent upon a merger, acquisition, reorganization, change of control, or sale of all or substantially all of the assets of Intact Subject to the foregoing, this Agreement is binding upon, and shall inure to the benefit of the parties hereto and
their respective permitted successors and assigns. Any purported assignment not in compliance with this Section 16 shall be null and void from the beginning.
14. Governing Law. This Agreement shall be governed by the laws of the State of Massachusetts, without regard to any rules on conflicts of laws.
15. Entire Agreement; Modifications; Waiver. This Agreement, together with all Exhibits hereto, fully expresses the entire understanding between the parties, and supersedes any prior agreements, understandings, or discussions between the parties. It may not be hereafter added to, altered, or modified except by written instrument signed by both parties. No delay, or omission in the exercise of any right, power, or remedy hereunder by either party shall impair such right, power, or remedy or be considered to be a waiver of any default or acquiescence therein by
such party.
16. Headings. The section headings contained herein are not part of this Agreement and are included solely for the convenience of the parties.
17. Language. This Agreement shall be executed both in the English language and the Chinese language, but in the event of inconsistency or difference between the English language version and the Chinese language version of this Agreement, the English language version shall prevail.
18. Further Assurances. Each of the parties agrees to take such actions, including but not limited to the execution and/or delivery of additional agreements and other written documents, as may be required to assure that each party to this Agreement enjoys all rights accorded to such party under this Agreement.
A - 19
19. Execution in Counterpart. This Agreement may be executed in counterpart by the parties, either through original copies or by facsimile or electronic copies. An executed copy of this Agreement delivered by facsimile or electronically will constitute valid execution and delivery of this Agreement.
H & I Medical China Limited
By: ________________________________
Name:______________________________
Title:_______________________________
Intact Medical Corporation
By: ________________________________
Name: ______________________________
Title: _______________________________
A - 20
Exhibit A
Description of Intact Breast Lesion Excision System
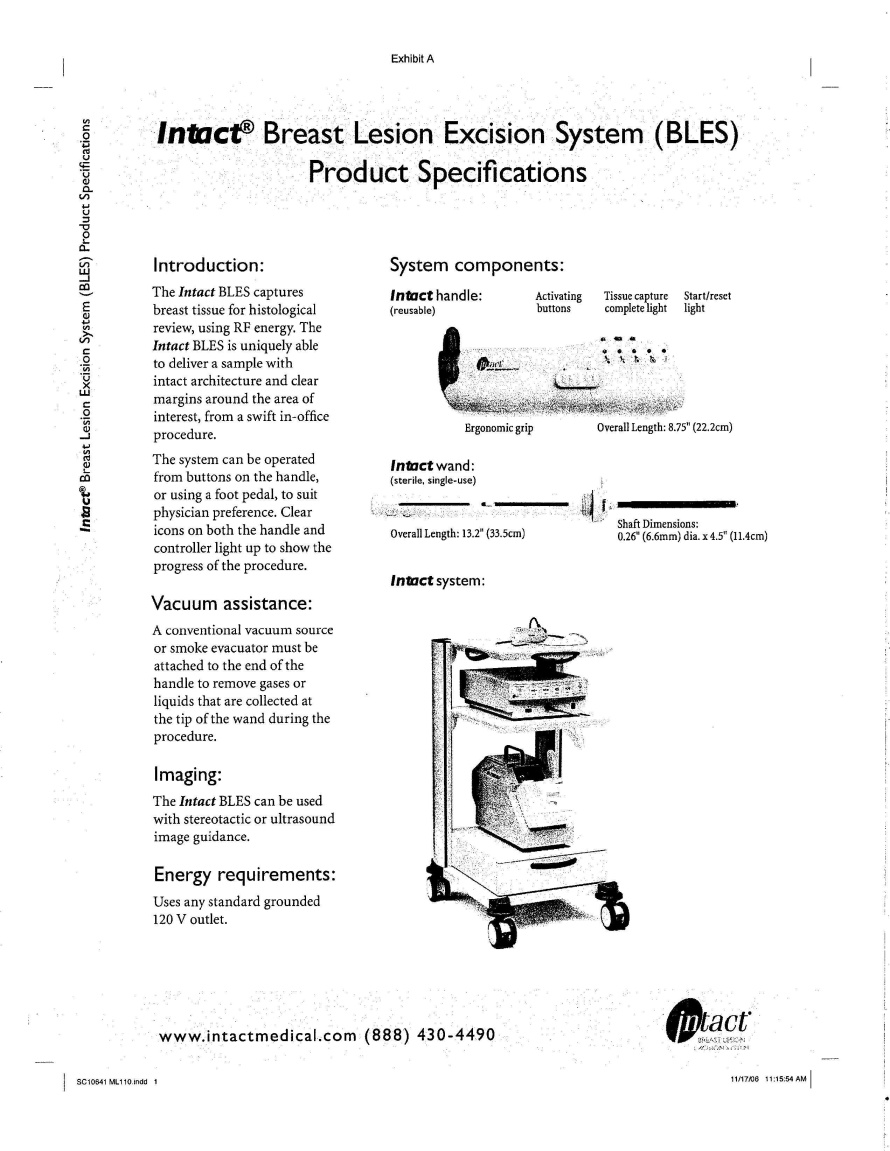
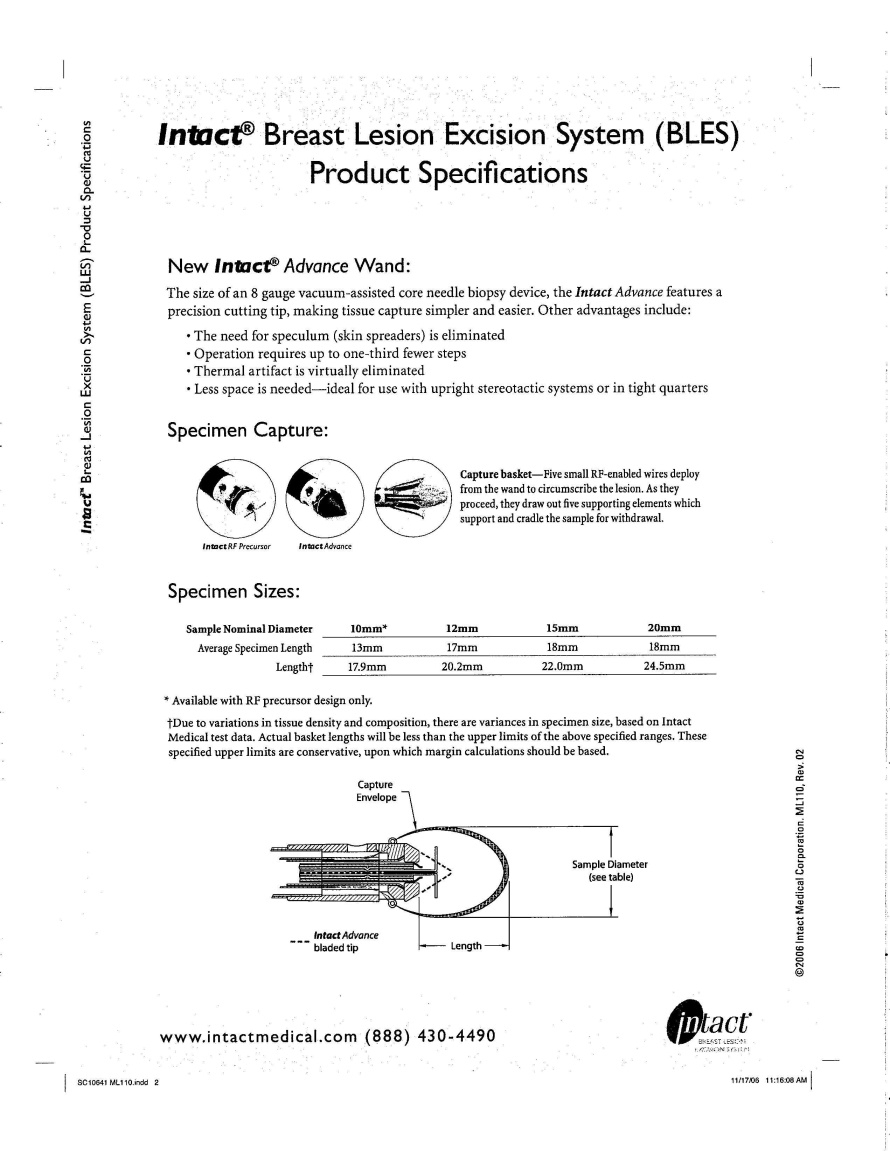
Exhibit B-1
Acceptance Criteria
[***]
[***] Represents material information which has been redacted and filed separately with the Commission pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit B-2
The Qualification Plan for product produced by NEWCO Intact's reliability and quality specifications will be the same as the current reliability and quality standards required by Intact's current manufacturers of its products. These standards are being obtained and will be provided. Intact ensures that the standards will not change.
Exhibit C
Status of Intact Medical Patents and Applications—December 2010
DRAFT
|
MSO Docket No.
|
Patent/Appln
No.
|
Title
|
Issue/Filing
Date
|
Status
|
|
NET 2-019-2
|
5,928,159
|
Apparatus and Method for Characterization and Treatment of Tumors
|
07/27/1999
|
Patented
$01/27/2011
|
|
NET 2-019-2-3A
|
5,947,964
|
Method and Apparatus for the Therapeutic Cauterization of Predetermined Volumes of Tissue
|
07/27/1999
|
Patented
$03/09/2011
|
|
NET 2-019-2-3C
|
6,106,524
|
Method and Apparatus for the Therapeutic Cauterization of Predetermined Volumes of Tissue
|
08/22/2000
|
Patented
$02/22/2012
|
|
NET 2-019-2-3NL
|
Dutch
1,006,903
|
Method and Apparatus for the Therapeutic Cauterization of Predetermined Volumes of Tissue
|
10/27/2004
|
Patented
|
|
NET 2-019-3NL
|
Dutch
1,440,665
|
Method and Apparatus for the Therapeutic Cauterization of Predetermined Volumes of Tissue
|
05/04/2005
|
Patented
|
|
NET 2-019AU
|
Australian
696,729
|
Apparatus and Method for Characterization and Treatment of Tumors
|
01/07/1999
|
Patented
|
|
NET 2-035
|
6,287,304
|
Interstitial Cauterization of Tissue Volumes with Electrosurgically Deployed Electrodes
|
09/11/2001
|
(Petition?)
Expired
9/11/2009
|
|
NET 2-035IL
|
Israeli
148,990
|
Interstitial Cauterization of Tissue Volumes with Electrosurgically Deployed Electrodes
|
11/20/2007
|
Patented
|
|
NET 2-039
|
6,277,083
|
Minimally Invasive Intact Recovery of Tissue
|
08/21/2001
|
Patented
$02/21/2013
|
|
NET 2-039AU
|
Australian
780,936
|
Minimally Invasive Intact Recovery of Tissue
|
09/18/2005
|
Patented
|
Intact Medical Patent Portfolio
December 15, 2010
Page 2 of 5
|
MSO Docket No.
|
Patent/Appln
No.
|
Title
|
Issue/Appl.
Date
|
Status
|
|
NET 2-039CA
|
Canadian
2,394,682
|
Minimally Invasive Intact Recovery of Tissue
|
11/29/2005
|
Patented
|
|
NET 2-039EP
|
EPO
00959852.5
|
Minimally Invasive Intact Recovery of Tissue
|
09/01/2000
|
Pending?
|
|
NET 2-039IL
|
Israeli
150,159
|
Minimally Invasive Intact Recovery of Tissue
|
09/04/2007
|
Patented
|
|
NET 2-039-31L
|
Israeli
159,830
|
Minimally Invasive Intact Recovery of Tissue
|
06/13/2006
|
Patented
|
|
NET 2-040
|
6,740,079
|
Electrosurgical Generator
|
05/25/2004
|
Patented
$11/25/2011
|
|
NET 2-040-3
|
6,923,804
|
Electrosurgical Generator
|
08/02/2005
|
Patented
$02/02/2013
|
|
NET 2-040-3CA
|
Canadian
2,529,758
|
Electrosurgical Generator
|
07/10/2007
|
Pending
|
|
NET 2-040-3IL
|
Israeli
173,247
|
Electrosurgical Generator
|
07/12/2004
|
Pending
|
|
NET 2-053
|
6,514,248
|
Accurate Cutting About and into Tissue Volumes with Electro-surgically Deployed Electrodes
|
02/04/2003
|
Patented
Fee due now
|
|
NET 2-053-2
|
7,335,198
|
Electrosurgical Generator
|
02/26/2008
|
Patented
$10/26/2011
|
|
NET 2-053-2B
|
12/371,740
|
Electrosurgical Generator
|
01/16/2009
|
Pending
|
|
NET 2-053AU
|
Australian
781,381
|
Electrosurgical Generator
|
09/01/2005
|
Patented
|
Intact Medical Patent Portfolio
December 15, 2010
Page 3 of 5
|
MSO Docket No.
|
Patent/Appln
No.
|
Title | Issue/Appl.
Date
|
Status
|
|
|
NET 2-053IL
|
Israeli
148,989
|
Electrosurgical Generator
|
10/25/2007
|
Patented
|
|
|
NET 2-055
|
D457,960
|
Electrosurgical Instrument Handle
|
05/28/2002
|
Patented
|
|
|
NET 2-056
|
D457,628
|
Electrosurgical Instrument Handle
|
05/21/2002
|
Patented
|
|
|
NET 2-057
|
D461,479
|
Electrosurgical Instrument Icon Array
|
08/13/2002
|
Patented
|
|
|
NET 2-058
|
D461,192
|
Electrosurgical Instrument Icon Array
|
08/06/2002
|
Patented
|
|
|
NET 2-059
|
D461,194
|
Electrosurgical Instrument Icon
|
07/06/2002
|
Patented
|
|
|
NET 2-060
|
D462,697
|
Electrosurgical Instrument Icon
|
09/10/2002
|
Patented
|
|
|
NET 2-061
|
D465,280
|
Electrosurgical Instrument Icon
|
04/05/2002
|
Patented
|
|
|
NET 2-062
|
D461,193
|
Electrosurgical Instrument Icon
|
08/06/2002
|
Patented
|
|
|
NET 2-069
|
10/235,131
|
Method & Apparatus for Positioning a Tissue Recovery Instrument
|
09/05/2002
|
Pending
|
|
|
NET 2-082
|
7,004,174
|
Electrosurgery with Infiltration Anesthesia
|
08/28/2006
|
Patented
$08/28/2013
|
|
|
NET 2-082A
|
11/194,800
|
Electrosurgery with Infiltration Anesthesia
|
08/11/2005
|
(Petition?)
Allowed,
fee not paid
|
|
|
NET 2-082EP
|
EPO
03716195.5
|
Electrosurgery with Infiltration Anesthesia
|
02/27/2003
|
Pending
|
|
|
NET 2-082HK
|
Hong Kong
05101897.7
|
Electrosurgery with Infiltration Anesthesia
|
03/04/2005
|
Pending
|
|
|
NET 2-097
|
6,955,653
|
Electrosurgical method and apparatus with dense tissue recovery capability
[Spring pre-tensioning of capture component]
|
10/18/2005
|
Patented
$04/20/2013
|
|
Intact Medical Patent Portfolio
December 15, 2010
Page 4 of 5
|
MSO Docket No.
|
Patent/Appln
No.
|
Title
|
Issue/Appl.
Date
|
Status
|
|
|
NET 2-097CA
|
Canadian
2,524,250
|
Electrosurgical method and apparatus with dense tissue recovery capability
[Spring pre-tensioning of capture component]
|
07/12/2004
|
Pending
(Request
Exam
07/12/09)
|
|
|
NET 2-097JP
|
Japanese
2006-521864
|
Electrosurgical method and apparatus with dense tissue recovery capability
[Spring pre-tensioning of capture component]
|
07/12/2004
|
Pending
|
|
|
NET 2-098
|
7,041,101
|
Electrosurgical accessing of tissue with controlled collateral thermal phenomena
[Evacuation system]
|
05/09/2006
|
(Petition?)
Expired
05/09/2010
$11/09/2013
|
|
|
NET 2-098A
|
11/265,582
|
Electrosurgical accessing of tissue with controlled collateral thermal phenomena
[Evacuation system]
|
11/02/2005
|
Pending
|
|
|
NET 2-098CA
|
Canadian
2,529,552
|
Electrosurgical accessing of tissue with controlled collateral thermal phenomena
[Evacuation system]
|
11/30/2006
|
Pending
(Request
Exam
07/12/09)
|
|
|
NET 2-098IL
|
Israeli
173,248
|
Electrosurgical accessing of tissue with controlled collateral thermal phenomena
[Evacuation system]
|
07/12/2004
|
Pending
|
|
|
NET 2-099
|
6,923,809
|
Minimally Invasive Instrumentation for Recovering Tissue
[improved eyelet structure and improved cable composition]
|
08/02/2005
|
Patented
$02/02/2013
|
|
Intact Medical Patent Portfolio
December 15, 2010
Page 5 of 5
|
MSO Docket No.
|
Patent/Appln
No.
|
Title
|
Issue/Appl.
Date
|
Status
|
|
NET 2-100
|
7,494,473
|
Electrosurgery Apparatus & System with Improved Tissue Capture Component
|
02/24/2009
|
Patented
$10/24/2012
|
|
NET 2-123
|
7,569,053
|
Bladed Ceramic Precursor
|
08/04/2009
|
Patented
$02/05/2013
|
|
NET 2-123A
|
12/487,307
|
Bladed Ceramic Precursor
|
06/18/2009
|
Pending
|
Exhibit D
Trademarks
USA
Word Xxxx: Intact
Filing Date: _9/12/2005
Published for Opposition: 8/1/2006
Serial Number: 3220764
Owner: (ApplicantIntact Medical Corporation
Exhibit E
Core Components
Parylene Coated Stew Assembly
Parylene Coated Trocar Tube
L605 Cable
Tungsten Cable ( Excise XL only)
Teflon Coated Trocar Tube ( Excise XL only)
Xxxxxxx Connector Pair ( Handle Cable and Controller)
Exhibit B
Form of Promissory Note
UNSECURED PROMISSORY NOTE
|
$150,000.00
|
Made as of __________, 2011
|
For value received, the undersigned, Intact Medical Corporation, a Delaware corporation (the “Maker”), promises to pay to the order of Allied Moral Holdings, Ltd., a company incorporated under the law of British Virgin Islands (the “Holder”) or its assign, the principal sum of one hundred and fifty thousand dollars ($150,000), without interest. The principal balance of the Note, which is outstanding and unpaid from time to time, is referred to as the “Principal Amount.”
Pursuant to the Joint Venture Agreement, dated _________, 2011, by and between the Maker, the Holder and BMG Diamond Holdings Limited, Maker hereby assign its rights to any and all cash distributions from H&I Medical China Limited (“Newco”), an entity incorporated under the laws of British Virgin Islands, up to the Principal Amount. Holder hereby agrees to take payment(s) up to the Principal Amount from Newco from time to time as declared by Newco’s board of directors. Maker may prepay this Note in whole or in part at any time without penalty.
Maker shall waive presentment by Holder for payment, demand, notice of dishonor and nonpayment of this Note, and consent to any and all extensions of time, renewals, waivers or modifications that may be granted by Holder with respect to the payment or other provisions of this Note, with or without substitution.
If for any reason one or more of the provisions of this Note or their application to any person or circumstances shall be held to be invalid, illegal or unenforceable in any respect or to any extent, such provisions shall nevertheless remain valid, legal and enforceable in all such other respects and to such extent as may be permissible. In addition, any such invalidity, illegality or unenforceability shall not affect any other provisions of this Note, but this Note shall be construed as if such invalid, illegal or unenforceable provision had never been contained therein.
MAKER:
Intact Medical Corporation
a Delaware corporation
Name:
Title:
Agreed to and accepted by:
HOLDER:
Allied Moral Holdings, Ltd.,
Name:
Title:

