Distribution Agreement by and between Cardica, Inc. a Delaware Corporation and Century Medical, Inc. a Japanese Corporation Dated as of June 16, 2003
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
Exhibit 10.6
by and between
Cardica, Inc.
a Delaware Corporation
and
Cardica, Inc.
a Delaware Corporation
and
Century Medical, Inc.
a Japanese Corporation
Dated as of June 16, 2003
a Japanese Corporation
Dated as of June 16, 2003
Table of Contents
| Page | ||||||||
| 1. | DEFINITION OF TERMS | 1 | ||||||
| 1.1 | “Competing Products” | 1 | ||||||
| 1.2 | “Contract Year” | 1 | ||||||
| 1.3 | “First Commercial Sale” | 1 | ||||||
| 1.4 | “Initial Term” | 1 | ||||||
| 1.5 | “Party” or “Parties” | 1 | ||||||
| 1.6 | “Premarketing Term” | 2 | ||||||
| 1.7 | “Products” | 2 | ||||||
| 1.8 | “Territory” | 2 | ||||||
| 2. | APPOINTMENT OF DISTRIBUTOR | 2 | ||||||
| 2.1 | Appointment as DISTRIBUTOR by COMPANY | 2 | ||||||
| 2.2 | Subdistributors | 3 | ||||||
| 3. | TERM OF DISTRIBUTORSHIP | 3 | ||||||
| 4. | DUTIES OF DISTRIBUTOR | 3 | ||||||
| 4.1 | Duties of DISTRIBUTOR | 3 | ||||||
| 4.2 | Product Approvals | 4 | ||||||
| 5. | DUTIES OF COMPANY | 5 | ||||||
| 5.1 | Duties of COMPANY | 5 | ||||||
| 6. | EXPENSES | 6 | ||||||
| 6.1 | DISTRIBUTOR’s Expenses | 6 | ||||||
| 6.2 | COMPANY’s Expenses | 6 | ||||||
| 7. | RECORDS AND REPORTS | 6 | ||||||
| 7.1 | Records and Reports | 6 | ||||||
| 7.2 | Adverse Experience Reporting | 7 | ||||||
| 7.3 | Updating/Revising Agreement | 7 | ||||||
| 7.4 | Recall | 7 | ||||||
| 8. | SALES OF PRODUCT TO DISTRIBUTOR | 7 | ||||||
| 8.1 | Purchase Prices and Terms | 7 | ||||||
| 8.2 | Risk of Loss, Deliveries | 8 | ||||||
| 8.3 | Acceptance and Cancellation of Orders | 8 | ||||||
-i-
Table of Contents
(continued)
(continued)
| Page | ||||||||
| 8.4 | Product Specifications | 8 | ||||||
| 8.5 | Taxes | 9 | ||||||
| 8.6 | Purchase Levels | 9 | ||||||
| 9. | PRODUCT LIABILITY | 11 | ||||||
| 9.1 | Claim, Suit or Action | 11 | ||||||
| 9.2 | Product Liability Insurance | 11 | ||||||
| 10. | WARRANTY POLICY | 12 | ||||||
| 10.1 | Warranties | 12 | ||||||
| 10.2 | Rejection of Products | 13 | ||||||
| 11. | PATENTS, TRADEMARKS, COPYRIGHTS; PROPRIETARY AND CONFIDENTIAL INFORMATION | 13 | ||||||
| 11.1 | Trademark License | 13 | ||||||
| 11.2 | Duty to Preserve Confidentiality | 14 | ||||||
| 11.3 | Proprietary | 14 | ||||||
| 12. | INDEMNITIES | 14 | ||||||
| 12.1 | Indemnity | 14 | ||||||
| 12.2 | Infringing Products | 15 | ||||||
| 13. | TERMINATION | 15 | ||||||
| 13.1 | Cancellation for Cause | 15 | ||||||
| 13.2 | Obligations upon Cancellation or Termination | 16 | ||||||
| 14. | GENERAL PROVISIONS | 18 | ||||||
| 14.1 | Force Majeure | 18 | ||||||
| 14.2 | Relationship Between Parties | 18 | ||||||
| 14.3 | Successors, Nonassignability | 19 | ||||||
| 14.4 | Survival of Obligations | 19 | ||||||
| 14.5 | Remedies | 19 | ||||||
| 14.6 | Notices | 19 | ||||||
| 14.7 | Disputes | 20 | ||||||
| 14.8 | Unenforceable Terms | 20 | ||||||
| 14.9 | Waivers | 20 | ||||||
-ii-
Table of Contents
(continued)
(continued)
| Page | ||||||||
| 14.10 | Governing Law; Headings | 20 | ||||||
| 14.11 | Entire Agreement, Modification | 21 | ||||||
| 14.12 | Further Assurances | 21 | ||||||
| 14.13 | Schedules | 21 | ||||||
| 14.14 | Counterparts | 21 | ||||||
| SCHEDULE 1. | Products and Prices | 22 | ||||||
| SCHEDULE 2. | Memorandum of Compliance | 23 | ||||||
| SCHEDULE 3. | Reporting for Product Defects, Adverse Events, Overseas Corrective Action Reports and Research Reports | 25 | ||||||
-iii-
This DISTRIBUTION AGREEMENT (“Agreement”) is made this 16th day of June, 2003 (“Effective Date”),
by and between Cardica, Inc., a Delaware corporation with its principal place of business located
at 000 Xxxxxxxxx Xxxxx, Xxxxx Xxxx, XX 00000, XXX (hereinafter referred to as “COMPANY”) and
Century Medical, Inc., a Japanese Corporation with its principal place of business located at 0-0-0
Xxxxxx, Xxxxxxxxx-Xx, Xxxxx, 000-0000, Xxxxx (hereinafter referred to as “DISTRIBUTOR”) in
consideration of the mutual covenants and conditions hereinafter stated.
1. DEFINITION OF TERMS
1.1 “Competing Products”
“Competing Products” shall mean automated anastomosis products that seal coronary artery bypass
grafts to accomplish surgical anastomosis except for and excluding hemostasis products that, as of
the Effective Date, DISTRIBUTOR distributes in the Territory that are manufactured by CryoLife,
Inc. (BioGlue™), which shall not be considered Competing Products or other anastomosis devices that
create a surgical anastomosis outside the coronary area of the body.
1.2 “Contract Year”
“Contract Year” shall mean a twelve (12) month period commencing on, and thereafter beginning on
the anniversary of, the first day of the first full month following the date of First Commercial
Sale in the Territory of any Product.
1.3 “First Commercial Sale”
“First Commercial Sale” shall mean the first sale of any Product with the intended maximum shelf
life of twelve (12) months or more by DISTRIBUTOR to a third party in the Territory with all
medical device approvals required to market and sell such Product (“Xxxxxx” or “Lui Betsu Kyoka”)
from The Japanese Ministry of Health, Labour and Welfare (“MHLW”) and all import permits from the
appropriate government authorities (“Hinmoku Kyoka”). COMPANY hereby represents and warrants that,
as of the Effective Date, COMPANY has obtained conditional CE Xxxx approval for the “Proximal
Device” Product and the Proximal Device Product is a commercially available Product to DISTRIBUTOR
to begin the device approval process stated above.
1.4 “Initial Term”
“Initial Term” shall mean the five (5) year period beginning on the date of expiration of the
Premarketing Term.
1.5 “Party” or “Parties”
“Party” or “Parties” shall mean COMPANY or DISTRIBUTOR, individually and collectively.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
1.
1.6 “Premarketing Term”
“Premarketing Term” shall mean the period beginning on the Effective Date and ending on the first
day of the first full month following the date of First Commercial Sale of all Products.
1.7 “Products”
“Products” shall mean those Products specifically listed in Schedule 1, whether
manufactured by or for COMPANY or its affiliates, including any improvements or modifications
thereto, as such schedule may be amended from time to time. As used herein, “affiliate” means a
person or entity that directly, or indirectly through one or more intermediaries, controls, or is
controlled by, or is under common control with, a specified person or entity, where “control” means
(a) fifty percent (50%) or more common equity ownership, or (b) the ability to direct the
management or policies of a person or entity, whether by contract or otherwise.
1.8 “Territory”
“Territory” shall mean Japan.
2. APPOINTMENT OF DISTRIBUTOR
2.1 Appointment as DISTRIBUTOR by COMPANY.
COMPANY hereby appoints DISTRIBUTOR as its exclusive importer and distributor of COMPANY’s Products
for the Territory in consideration of COMPANY’s issuance of a promissory note (the “Note”),
concurrent with the execution of that certain Subordinated Convertible Note Agreement in the amount
of three million U.S. dollars ($3,000,000.00), and DISTRIBUTOR hereby accepts such appointment on
the terms and conditions set forth in this Agreement. Under no circumstances shall DISTRIBUTOR have
authority to sell or distribute any Products outside the Territory. COMPANY shall also grant to
DISTRIBUTOR a right of first negotiation for the import and distribution in the Territory of all
new and future products with all line extensions, modifications and improvements thereto,
manufactured and sold by COMPANY or products acquired by COMPANY or its affiliates for distribution
by COMPANY. Such distribution shall be in accordance with the terms and conditions of this
Agreement, with a per unit purchase price and minimum purchase levels (“MPL”) mutually agreeable to
COMPANY and DISTRIBUTOR. If within thirty (30) days of COMPANY’s first written proposal to
DISTRIBUTOR, COMPANY and DISTRIBUTOR cannot agree upon a per unit purchase price and MPL for such
new products or if DISTRIBUTOR declines to distribute such products, then COMPANY will be permitted
to distribute or cause to distribute by alternate means only such products as were first offered to
DISTRIBUTOR for distribution in the Territory; provided, however, that COMPANY’s
distribution of said products by alternate means shall be upon terms and conditions (including the
per unit purchase price and MPL) to such alternate distributor no more favorable than the terms and
conditions under which such products were last offered to DISTRIBUTOR for distribution.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
2.
2.2 Subdistributors.
DISTRIBUTOR may appoint subdistributors to make sales of Products within the Territory on such
terms and conditions as DISTRIBUTOR determines to be necessary to fulfill its obligations under
this Agreement; provided that no such appointment or delegation shall relieve DISTRIBUTOR from any
obligations hereunder. COMPANY acknowledges and agrees that DISTRIBUTOR will use subdistributors in
the sale of Products, the use of said subdistributors being a normal business custom in the
Territory.
This Agreement and the rights conferred on DISTRIBUTOR hereunder shall come into effect on the
Effective Date and shall remain in effect until the expiration of the Initial Term. At the end of
the Initial Term, this Agreement shall automatically renew for an additional five (5) years (the
“Renewal Period”) subject to DISTRIBUTOR having met the MPL for each Contract Year during the
Initial Term as required under Section 8.6 below.
DISTRIBUTOR covenants and agrees to do each of the following:
(i) DISTRIBUTOR shall use commercially reasonable efforts to promote and sell the Products in
the Territory;
(ii) DISTRIBUTOR shall send one person from its sales and marketing organization to COMPANY
for training prior to the First Commercial Sale of the Products in the Territory for a period of
time mutually agreed upon by the Parties;
(iii) DISTRIBUTOR shall maintain a commercially reasonable stock of the Products in order to
promote the Products in the Territory;
(iv) DISTRIBUTOR shall exhibit Products at industry meetings in the Territory;
(v) DISTRIBUTOR shall create and develop a training program for end-user physician customers
in the Territory in cooperation with COMPANY; DISTRIBUTOR shall not sell Products to any end-users
who have not been trained in the use of the Products.
(vi) DISTRIBUTOR shall confer with COMPANY, from time to time, upon the written request of
COMPANY, on matters relating to the marketing and promotion of the Products in the Territory;
(vii) DISTRIBUTOR shall keep COMPANY informed regarding regulatory requirements in the
Territory and shall, from time to time, provide COMPANY with updated amendments to Schedule
2 attached hereto;
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
3.
(viii) DISTRIBUTOR shall not solicit the sale of, promote the sale of, sell, exhibit for sale,
distribute or manufacture any Competing Products in the Territory;
(ix) DISTRIBUTOR shall translate Product literature into the Japanese language when necessary;
and
(x) during the term of this Agreement, and for a period of [*] years from the expiration or
termination of this Agreement, DISTRIBUTOR, its affiliates, successors and assigns shall not
directly solicit or indirectly solicit for employment or hire in any capacity any personnel
employed by COMPANY or any affiliate of COMPANY.
DISTRIBUTOR shall use its commercially reasonable efforts to obtain, at its own expense (except as
otherwise provided herein), all “Lui Betsu Kyoka,” “Me-too” or “Kairyo Iryoyogu” Xxxxxx (as defined
below) from the MHLW needed to market the Products in the Territory. DISTRIBUTOR shall be under no
obligation to conduct or perform any clinical trial for purposes of obtaining any Xxxxxx or
marketing the Products in the Territory. For the purposes of this section, “Lui Betsu Kyoka,”
“Me-too” and “Kairyo Iryoyogu” Xxxxxx shall mean those Shonins approved without conducting any
clinical trials in the Territory. Lui Betsu Kyoka approval is not expected to exceed six (6) months
from the Effective Date, subject to DISTRIBUTOR’s receipt of all necessary information from COMPANY
required to prepare and file for the Lui Betsu Kyoka. COMPANY and DISTRIBUTOR recognize that
competitive devices already on the market in the Territory have been classified as Lui Betsu Kyoka
qualified devices; the easiest category to register a Product in the Territory. In the event that
the MHLW requires a more formal Xxxxxx application, either a “Me-too” or “Kairyo Iryoyogu” Xxxxxx
or “Shin Iryoyogu,” COMPANY and DISTRIBUTOR will diligently and in good faith apply for a Xxxxxx in
the appropriate category. “Shin Iryoyogu” shall mean the Xxxxxx approved based on clinical trials
or utilizing foreign clinical study data obtained for the purpose of seeking regulatory approval.
In the event that the Parties agree to conduct any clinical trials in the Territory with a
reasonable number of clinical cases for obtaining Xxxxxx approval, COMPANY shall, at no charge to
DISTRIBUTOR, supply DISTRIBUTOR with all necessary Products. All other costs associated with any
clinical trials conducted in the Territory shall be borne by DISTRIBUTOR. COMPANY shall support any
clinical trial activity in the Territory with all information available at its disposal. COMPANY
reserves the right to approve any study design (protocol) related to clinical trials in the
Territory, which approval shall not be unreasonably withheld.
In the event that DISTRIBUTOR is unable, within five (5) years of the Effective Date of this
Agreement, to obtain First Commercial Sale, COMPANY shall have the sole and exclusive right to
terminate this Agreement with immediate effect.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
4.
COMPANY covenants and agrees to do each of the following:
(i) COMPANY shall use its commercially reasonable efforts to research and respond to Product
improvement needs of end-users in the Territory;
(ii) COMPANY shall not, (a) appoint any other distributor or importer of the Products in the
Territory during the term of this Agreement or (b) make sales, directly or indirectly, of any of
the Products to any person in the Territory other than DISTRIBUTOR or to any customer outside the
Territory who is known to COMPANY, or who COMPANY should reasonably know, intends to introduce,
directly or indirectly, the Products into the Territory. Further, subject to Section 2.1, COMPANY
shall not, directly or indirectly, import, manufacture, sell, market or otherwise distribute in the
Territory (except pursuant to this Agreement) the Products or any products directly competitive
with the Products;
(iii) COMPANY shall provide DISTRIBUTOR with all materials necessary to obtain and maintain
Xxxxxx or Lui Betsu Kyoka for the import and sale of Products within the Territory by promptly
furnishing to DISTRIBUTOR, at COMPANY’s cost, such technical descriptions, specifications, data,
drawings, information, service manuals, quality control audits, facility inspection reports issued
by governmental regulators or international quality control auditors, and so forth regarding the
Products, in the English language, as DISTRIBUTOR may reasonably request;
(iv) COMPANY shall provide DISTRIBUTOR, at no cost, all Products necessary for DISTRIBUTOR to
fulfill its obligations under Section 4.2 however, the number of Products supplied at no charge to
DISTRIBUTOR shall not exceed ten (10) units of sterile Products per non Shin Iryoyogu Xxxxxx
application. The number of units of sterile Products necessary for a Shin Iryoyogu Xxxxxx shall be
determined by the number of patients required by the clinical protocol;
(v) COMPANY shall provide DISTRIBUTOR with the information, documentation, data and
certificates listed in Schedule 2, as amended from time to time, necessary for DISTRIBUTOR
to remain in compliance with the Good Manufacturing Practices for Importers laws and regulations of
the Territory;
(vi) COMPANY shall inform DISTRIBUTOR, from time to time, of technical and other developments
regarding the Products as they may occur;
(vii) COMPANY shall furnish to DISTRIBUTOR on an on-going basis, at COMPANY’s cost, with a
reasonable quantity of such technical, advertising and selling information and other promotional
literature in the English language regarding the Products;
(viii) COMPANY shall provide Product training to personnel of DISTRIBUTOR at times and places
mutually agreed upon by both Parties, with each Party bearing its own expenses for attending such
training;
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
5.
(ix) COMPANY shall provide DISTRIBUTOR a reasonable amount of non-sterile functional Products
for demonstration purposes at [*] percent ([*]%) of the sterile Products price. The total number of
non-sterile functional Products sold to DISTRIBUTOR at the discounted price shall be determined at
COMPANY’s sole discretion. The total number of non-sterile non-functional Products given to
DISTRIBUTOR at no charge shall initially be twenty (20) units and any additional quantities shall
be provided at COMPANY’s sole discretion. All such non-sterile Products shall be used for
demonstration purposes only and may not be used for any other commercial activity (e.g., sale;
lease; loaner; etc.) or implanted; and
(x) during the term of this Agreement and for a period of [*] ([*]) years from the expiration
or termination of this Agreement, COMPANY, its affiliates, successors and assigns shall not
directly solicit or indirectly solicit for employment or hire in any capacity any personnel
employed by DISTRIBUTOR or any affiliate of DISTRIBUTOR.
Except as otherwise specifically provided herein, DISTRIBUTOR shall be responsible for all expenses
incurred by it in connection with the implementation of this Agreement, including without
limitation salaries, office and travel expenses of its employees, advertising and trade shows
within the Territory and any and all taxes which may be imposed on DISTRIBUTOR within the
Territory. COMPANY shall bear only such of these expenses as to which it has given prior written
approval.
Except as otherwise specifically provided herein, COMPANY shall be responsible for payment of all
expenses incurred by it including any taxes imposed on it and shall also pay those expenses
incurred in connection with the implementation of this Agreement for which it has given prior
written approval.
Subject at all times to Section 11.2, DISTRIBUTOR shall maintain complete and accurate records of
aggregate purchases and resales of the Products. DISTRIBUTOR shall provide to COMPANY, by the
thirtieth (30th) day of the first month following the end of each quarter during the term of this
Agreement, a quarterly report summarizing DISTRIBUTOR’S sales activities under this Agreement for
the prior calendar quarter and containing such other information as COMPANY may reasonably request,
including without limitation a description of and the amount of all Products in DISTRIBUTOR’s
inventory as of the first day of each calendar month.
DISTRIBUTOR and COMPANY each shall, for tracking purposes, maintain accurate delivery, receiving
and shipping records including model and lot numbers of the Products.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
6.
COMPANY and DISTRIBUTOR shall follow the guidelines contained in Schedule 3 regarding
adverse events associated with the Products.
The Parties shall amend this Agreement from time to time to the extent necessary to incorporate
changes to Schedule 3 reflecting changes in the regulatory requirements applicable to
adverse events within their respective territories.
If either Party believes or is notified that a recall in the Territory of any Product is desirable
or required by law, it will notify the other Party within twenty-four (24) hours of any such
notice. The Parties will then discuss reasonably, expediently and in good faith whether such recall
is appropriate or required and the manner in which any mutually agreed recall shall be handled. The
Party whose mistake, negligence or gross negligence results in such recall shall bear the expenses
incurred in connection with such recall. In addition, if COMPANY is the responsible Party, COMPANY
shall reimburse DISTRIBUTOR for the price paid hereunder for such Products as may be recalled, plus
all freight and related travel costs incurred by DISTRIBUTOR in connection with such recalled
Products, including any costs incurred in disposing of or returning such recalled Products to
COMPANY at COMPANY’s instruction. The Parties will mutually agree upon the methods of disposal
consistent with applicable laws in the Territory.
COMPANY shall sell the Products to DISTRIBUTOR at the prices set forth in Schedule 1.
Payments on purchase orders shall be due at the end of the month immediately following the month of
shipment of the Products to DISTRIBUTOR. Payment shall be made by wire transfer in U.S. funds to an
account designated in writing by COMPANY. All shipments of Products shall be billed to DISTRIBUTOR
at the price in effect for each Product in accordance with this Section 8.1 and Schedule 1,
on the date of DISTRIBUTOR’s purchase order for such Products. COMPANY shall have the right to
change the prices of the Products no more than [*] each Contract Year consistent with prices
charged to third-party international distributors of the Products, taking into consideration such
factors as exchange rates, device-specific reimbursement rates for the Products in the Territory,
if any, competition, and the like, by notifying DISTRIBUTOR in writing of any such change at least
ninety (90) days prior to the effective date of any such change. Notwithstanding the foregoing, in
no event shall any price increase exceed [*]% of the then current price for such Product. Further,
DISTRIBUTOR shall have the right to request a change in price, taking into consideration such
factors as exchange rates, device-specific reimbursement rates for the Products in the Territory,
if any, competition, and the like, by notifying COMPANY in writing of any such request and the
reason for such request which request COMPANY shall consider in good faith.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
7.
DISTRIBUTOR shall purchase the Products from COMPANY FCA (as defined under Incoterms 2000 of the
International Chamber of Commerce) place of manufacture with risk of loss passing to DISTRIBUTOR
upon delivery of the Products to the carrier. DISTRIBUTOR shall be responsible for taxes (other
than any taxes on COMPANY’s income) and import duties imposed in the Territory and for shipping
fees. COMPANY shall deliver accepted orders within the acknowledged time of shipment stated in
COMPANY’s acceptance of the order. All Products shall be packed for shipment and storage in
accordance with COMPANY’s standard commercial practices, unless DISTRIBUTOR notifies COMPANY of
special packaging requirements, in which event COMPANY shall be entitled to charge DISTRIBUTOR for
any additional costs approved in advance by DISTRIBUTOR.
All orders for Products by DISTRIBUTOR shall be initiated by DISTRIBUTOR’s issuance of a written
purchase order sent via facsimile or mail to COMPANY or such other place as designated by COMPANY.
Such orders shall state unit quantities, unit descriptions, requested delivery dates, and shipping
instructions. The acceptance by COMPANY of an order shall be indicated by written acknowledgment
thereof by COMPANY within [*] business days following receipt of each order. This Agreement shall
control orders of Products by DISTRIBUTOR. Any conflicting or different or additional terms or
conditions contained in DISTRIBUTOR’s purchase order, COMPANY’s acknowledgment or other similar
document shall not add to or modify the terms of this Agreement. COMPANY shall have the right to
cancel any order placed by DISTRIBUTOR or to refuse or delay the shipment thereof to the extent
that DISTRIBUTOR is in default of any payment obligations hereunder. DISTRIBUTOR may cancel an
order, or any part thereof, for standard Products normally kept in COMPANY’s inventory which
COMPANY has accepted only by providing written notice to COMPANY prior to the shipment of such
Products and by paying such reasonable cancellation charge as requested by COMPANY. DISTRIBUTOR may
not cancel an order for non-inventory Products or custom made Products which COMPANY has accepted
unless confirmed in writing by COMPANY and by paying such reasonable cancellation charge as
requested by COMPANY, which cancellation charge may include, without limitation reasonable tooling
and works-in-progress expenses requested by COMPANY.
COMPANY shall be obligated to deliver Products of the specifications and quality standards in
effect at the time and made known to DISTRIBUTOR and which contain a minimum shelf life of the
greater of [*] months or [*] percent ([*]%) of the intended maximum shelf life for such Product at
the time COMPANY delivers an order. COMPANY shall use reasonable efforts to extend the intended
maximum shelf life of Products to [*] months or more by December 31, 2003 and [*] months or more
for such Products during 2004. COMPANY reserves the right to change the design or specifications of
any of the Products at any time with ninety (90) days prior written notice to DISTRIBUTOR. COMPANY
also reserves the right to discontinue the manufacture and distribution of any of the Products at
any time, with ninety (90) days prior written notice to DISTRIBUTOR and, without substitution, in
COMPANY’s sole discretion;
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
8.
provided that a discontinuation or cancellation of a Product or Product line for the purpose of
transfer to a third party or to an affiliate of the COMPANY shall be deemed an assignment of the
COMPANY’s rights and obligations of this Agreement with respect to such Products, and COMPANY shall
ensure that such transferee shall be bound by the terms and conditions of this Agreement to the
same extent as the COMPANY with respect to any such Products. COMPANY acknowledges and understands
that a substantial lead time is required to obtain Xxxxxx for Product specification changes and
COMPANY will use its commercially reasonable efforts to give DISTRIBUTOR as much advance
notification as possible in excess of ninety (90) days concerning Product specification changes. In
the event COMPANY discontinues the manufacture or distribution of any Product or in the event of a
Product specification change or in the event of COMPANY’s refusal to or failure to accept or fill
any bona fide purchase orders for Products, DISTRIBUTOR’s MPL under Section 8.6 herein shall be
amended and adjusted accordingly.
DISTRIBUTOR shall be responsible for all taxes levied and/or imposed by the Japanese government or
Japanese taxing authority (other than any tax on COMPANY’s income) related to this Agreement;
provided that DISTRIBUTOR may withhold from any payments to COMPANY any amounts required by
Japanese law to be withheld, and shall provide to COMPANY receipts of any amounts so withheld
issued by the proper authority, and such withholding taxes shall not be “grossed up”. COMPANY shall
be responsible for all taxes levied and/or imposed by the United States government or any taxing
authority in the United States related to this Agreement.
DISTRIBUTOR’s right to maintain its exclusive distributorship as set forth in Section 2.1, herein,
shall be subject to the following:
(i) The MPL for Contract Years 1 through 3 shall be the following sales goals that, if not
met, are non-breach, non-termination events:
| Proximal Device | Distal Device | |
Contract Year 1 : [*] units ([*]% market share)
|
Year 1: [*] ([*]% market share) | |
Contract Year 2 : [*] units ([*]% market share)
|
Year 2: [*] ([*]% market share) | |
Contract Year 3 : [*] units ([*]% market share)
|
Year 3: [*] ([*]% market share) |
Contract Year 1 for “Distal Device” shall commence when (1) DISTRIBUTOR has obtained all
necessary regulatory approvals for this Product in accordance with Section 4.2 of this Agreement
and (2) COMPANY has extended the intended maximum shelf life of this Product to twelve (12) months
or more in accordance with Section 8.4 of this Agreement.
(ii) Contract Year 4 and thereafter: DISTRIBUTOR and COMPANY shall prepare and agree upon an
MPL ninety (90) days prior to the anticipated beginning of the Contract Year 4 of the Initial Term
and ninety (90) days prior to the beginning of each
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
9.
subsequent Contract Year thereafter during the term of this Agreement and any subsequent
Renewal Periods. With respect to Product line extensions, the Parties shall make MPL adjustments as
mutually agreed upon, commensurate with the expanded total available market opportunity associated
with the expanded Product offerings. If after exhausting all reasonable efforts COMPANY and
DISTRIBUTOR are unable to mutually agree upon an MPL ten (10) days prior to the beginning of a
subsequent Contract Year, then the default MPL for the subsequent Contract Year shall be the
product of the actual purchases by DISTRIBUTOR during the Contract Year immediately preceding the
subsequent Contract Year times [*] for Contract Year 4 and [*] for any subsequent Contract Year
after Contract Year 4.
(iii) For thirty (30) days following the conclusion of any Contract Year of the Agreement in
which DISTRIBUTOR has not purchased the MPL for that Contract Year, DISTRIBUTOR shall have the
discretionary right but not the obligation to purchase additional Products from COMPANY at the then
applicable purchase prices in order to satisfy DISTRIBUTOR’s MPL for the prior Contract Year. Any
purchases credited towards the prior Contract Year’s MPL in accordance with the immediately
preceding sentence shall not be credited towards the then current Contract Year’s MPL.
(iv) Except as described in Section 8.6(iii) above, for purposes of this Section 8.6, a
Product shall be deemed purchased during a designated Contract Year when a firm purchase order has
been received and accepted by COMPANY during such Contract Year, and which order calls for delivery
of Products within that Contract Year.
(v) Notwithstanding any other provision of this Agreement to the contrary, any MPL then in
effect shall be adjusted accordingly to reflect the effect of any new Products and any Product line
extensions, any Product recall, any discontinuation of a Product or Product line, any change in
design or specifications of any Product which has a material adverse impact on DISTRIBUTOR’s
ability to market or sell such Product, any transfer to a third party or affiliate of COMPANY of
any Product as described in Section 8.4 that has a material adverse impact on DISTRIBUTOR’s ability
to market or sell such Product, any termination of this Agreement with respect to a Product as
described in Section 12.2, any refusal or failure by COMPANY to satisfy a bona fide purchase order
made in accordance with the terms of Section 8.3, or any relevant event of force majeure as set
forth in Section 14.1.
(vi) COMPANY shall have the right but not the obligation to terminate this Agreement in the
event that DISTRIBUTOR has not purchased the MPL for Contract Year 4 and any Contract Year
thereafter during the term of this Agreement.
(vii) Notwithstanding any provision of this Agreement to the contrary, the MPL has been and
will be established solely for the purpose of providing COMPANY with a contingent right to
terminate the exclusive distributorship granted to DISTRIBUTOR under Section 2.1, in Contract Year
4 and any Contract Year thereafter. COMPANY agrees that its sole remedy for any failure of
DISTRIBUTOR to achieve the MPL for Contract Year 4 and any Contract Year thereafter shall be the
termination of DISTRIBUTOR’s exclusive distributorship rights hereunder without any rights to claim
against DISTRIBUTOR for any alleged losses or damages of any kind, for reimbursement of any costs
of any kind, including attorneys’ fees, or for payment of any other kind; provided,
however, that the obligations between DISTRIBUTOR
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
10.
and COMPANY under Section 13.2, which may include payments, shall survive a termination for
failure of DISTRIBUTOR to fulfill the MPL. This Section 8.6(vii) is not intended to, and shall not
be construed to, create any obligation on the part of DISTRIBUTOR to purchase, or any right on the
part of COMPANY to cause DISTRIBUTOR to purchase, Products in quantities necessary to satisfy any
MPL for any Contract Year.
9.1 Claim, Suit or Action.
If any claim is made or any suit or action is instituted against DISTRIBUTOR arising out of or
otherwise in connection with any defect or alleged defect in the Products sold by COMPANY to
DISTRIBUTOR under this Agreement, COMPANY shall, without limiting the general indemnity provided by
Section 12.1 of this Agreement, at its own expense and upon request by DISTRIBUTOR:
(i) investigate or research the causes of accidents, occurrences, injuries or losses affecting
any person or property as a result of the manner in which the Products are designed, manufactured,
treated, packaged, labeled, delivered, sold or used, and use its best efforts to correct or
eliminate such causes within a reasonable period; and
(ii) provide to DISTRIBUTOR any and all assistance (including, without limitation, technical
and other information, documents, data, materials and witnesses) which are, in the opinion of
DISTRIBUTOR or its counsel, necessary or useful for DISTRIBUTOR’s defense to such claim, suit or
action in relation to the Products sold by COMPANY to DISTRIBUTOR hereunder.
9.2 Product Liability Insurance.
COMPANY shall, at its own expense, obtain and maintain product liability insurance underwritten by
a company or companies authorized to do business in the state, countries and Territory contemplated
by this Agreement, subject to DISTRIBUTOR’s prior written approval, to cover any and all losses,
damages (actual, consequential or indirect), liabilities, penalties, claims, demands, suits or
actions, and related costs and expenses of any kind (including without limitation, expenses of
investigation, counsel fees, judgments and settlements) for injury to or death of any person or
property damages or any other loss suffered or allegedly suffered by any person or entity arising
out of or otherwise in connection with the Products sold by COMPANY to DISTRIBUTOR pursuant to this
Agreement. COMPANY shall maintain such insurance in a minimum amount of [*] U.S. dollars ($[*]) per
occurrence in connection with such insurance. COMPANY shall furnish DISTRIBUTOR with copies of all
applicable insurance policies, which insurance policies shall not be canceled, modified or reduced
without the prior written consent of DISTRIBUTOR.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
11.
COMPANY warrants that the Products sold to DISTRIBUTOR shall be (1) free from any defects in
material, design, workmanship, manufacture, treatment, packing, instruction manuals, labeling,
warning or otherwise until the use before date (“UBD”) for sterile Products, (2) substantially in
conformance with the written specifications maintained by COMPANY at the date of delivery of such
Products, and (3) in compliance at all times with the requirements of and regulations adopted
pursuant to the U.S. Federal Food, Drug and Cosmetic Act and applicable Japanese law. COMPANY
further warrants that it will convey good title to all Products delivered to DISTRIBUTOR free from
any security interest, liens or other encumbrance. COMPANY will provide, when requested by
DISTRIBUTOR, certification that, to the best of its knowledge, it is in compliance with U.S. and
applicable Japanese laws, statutes, rules, and regulations and relevant orders relating to the
manufacture, use, distribution and sale of the Products. COMPANY’S SOLE OBLIGATION UNDER THE
FOREGOING WARRANTY SHALL BE, AT COMPANY’S SOLE ELECTION, TO EITHER REPLACE THE RELEVANT PRODUCT OR
REFUND DISTRIBUTOR’S FULLY-LANDED PURCHASE PRICE FOR SUCH PRODUCT. Such obligation shall be subject
to COMPANY being granted the reasonable opportunity to inspect, at COMPANY’s expense, the defective
Product at the location of its use or storage and, upon request in accordance with COMPANY’s
instruction, return of the Product to COMPANY at COMPANY’s cost. Any such replacement of Products
may be made by substitution of any similar Product meeting substantially identical quality
specifications and payment by the COMPANY of all freight, handling and duty charges or taxes
incident to the delivery of such replacement Products. Upon request by COMPANY, in accordance with
COMPANY’S instruction, DISTRIBUTOR shall return the Product to COMPANY at COMPANY’s cost;
provided, however, that IN THE EVENT THAT THE RETURN OF A PRODUCT POSES A HEALTH
RISK, DUE TO THE POSSIBILITY THAT SUCH PRODUCT HAS BEEN EXPOSED TO AN INFECTIOUS DISEASE OR
OTHERWISE, COMPANY, DISTRIBUTOR AND THE END-USER SHALL DETERMINE A MUTUALLY SATISFACTORY METHOD FOR
COMPANY TO INSPECT OR OTHERWISE OBTAIN ADDITIONAL INFORMATION ABOUT THE PRODUCT IN ORDER FOR
COMPANY TO DETERMINE ITS OBLIGATION UNDER THE FOREGOING WARRANTY. NOTWITHSTANDING THE FOREGOING,
COMPANY MAKES NO WARRANTY, NOR SHALL IT HAVE ANY OTHER OBLIGATION TO DISTRIBUTOR WITH RESPECT TO
ANY PRODUCT SOLD HEREUNDER, TO THE EXTENT THAT, PRIOR TO USE, SUCH PRODUCT HAS EXCEEDED ITS UBD
ACCORDING TO THE PRODUCT’S LABEL OR HAS NOT BEEN USED, HANDLED OR STORED IN ACCORDANCE WITH COMPANY
GUIDELINES AS COMMUNICATED BY COMPANY TO DISTRIBUTOR.
Without limiting the generality of the foregoing, and except as provided in Section 12.1,
DISTRIBUTOR shall not purport to give, or assume on behalf of COMPANY, any other or different
guarantee, warranty, obligation or liability whatsoever, including without limitation liability for
loss or damage to person or property resulting from default or defect in design, workmanship or
material or goods of any kind, other than stipulated in such warranties as COMPANY may specify from
time to time. Furthermore, DISTRIBUTOR shall only give such warranties as specified in this Section
10.1 or as specified by COMPANY from time to time on
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
12.
its own behalf and shall not give such other or different warranties or guarantees on its own
behalf unless DISTRIBUTOR obtains the prior written consent of COMPANY on each such occasion.
EXCEPT AS EXPRESSLY PROVIDED ABOVE AND IN SECTION 12.1 BELOW, COMPANY GRANTS DISTRIBUTOR NO OTHER
WARRANTIES, WHETHER EXPRESS, IMPLIED OR BY STATUTE REGARDING THE PRODUCTS, THEIR FITNESS FOR ANY
PARTICULAR PURPOSE, THEIR QUALITY, THEIR MERCHANTABILITY OR OTHERWISE.
(i) DISTRIBUTOR shall inspect all Products promptly upon receipt thereof and may reject any
Product that fails in any material way to meet the then-current specifications for such Product.
Any Product not properly rejected within [*] days of receipt of such Product by DISTRIBUTOR (the
“Rejection Period”) shall be deemed accepted. To reject a Product, DISTRIBUTOR shall, within the
Rejection Period, notify COMPANY of its rejection and request a Material Return Authorization
(“MRA”) number. COMPANY shall provide the MRA number to DISTRIBUTOR within seven (7) days of
receipt of the request. Within seven (7) days of receipt of the MRA number, DISTRIBUTOR shall
return to COMPANY the rejected Product, freight collect, with the MRA number displayed on the
outside of the carton. COMPANY reserves the right to refuse to accept any rejected Products that do
not bear an MRA number on the outside of the carton. As promptly as possible but no later than [*]
working days after receipt of properly rejected Products, COMPANY shall, at its option and expense,
either replace the Products or refund DISTRIBUTOR’s original fully landed purchase price for the
Products. COMPANY shall pay the cost of shipping charges incurred by DISTRIBUTOR for properly
rejected products.
(ii) Notwithstanding the foregoing, Products which are found to be defective for failure to
conform to COMPANY’s specifications at an end-user’s site shall be initially replaced by
DISTRIBUTOR. COMPANY shall then replace such defective Products with Products meeting
specifications within [*] days of (1) receipt of the defective Products, or (2) confirmation by
DISTRIBUTOR that such defective Products have been disposed of by an end-user and receipt of a
completed customer complaint form. The final good faith determination concerning non-conformance of
any Product shall rest solely with COMPANY.
PROPRIETARY AND CONFIDENTIAL INFORMATION
COMPANY hereby grants to DISTRIBUTOR a non-exclusive, royalty-free right and license (with right of
sub-license to sub-distributors appointed under Section 2.2) to use the trademarks, trade names,
copyrights, and other intellectual property (except patents which are expressly excluded from this
Agreement) of COMPANY as communicated to DISTRIBUTOR from time to time (hereinafter referred to as
the “Trademarks”) in connection with the sale or other distribution, promotion, advertising and
maintenance of the Products under this Agreement.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
13.
DISTRIBUTOR may indicate in its advertising and promotion and on its stationery that it is an
“authorized exclusive distributor” of the Products for the Territory. DISTRIBUTOR has no permission
to and will not adopt, use or register as a trademark, trade name, business name, or corporate name
or part thereof, whether during the term of this Agreement or after its termination, any word, or
symbol confusingly similar to any Trademarks. Furthermore, upon request and at COMPANY’s expense,
DISTRIBUTOR shall discontinue or cancel the registration of any and all Trademarks utilized and
registered by DISTRIBUTOR in connection with the Products prior to or after the execution of this
Agreement, except as provided in Section 13.2(iii).
Without the prior written consent of the supplying Party, no receiving Party, its officers, agents,
or employees shall, in any manner whatsoever for use in any way for its own account or for any
third-party disclose or communicate to a third-party, any technical, engineering, manufacturing,
business, financial, or other information or know-how (hereinafter referred to as the “Confidential
Information”) generated by any Party hereto and acquired directly or indirectly by the other Party.
Nothing in this Section 11.2 shall prevent disclosure or use of information: (i) previously known
to the receiving Party; (ii) which is or later becomes public knowledge, by publication or
otherwise, through no breach of this Agreement by the receiving Party; (iii) which is properly
acquired by the receiving Party from a third party having the legal right to disclose such
information; (iv) is required to be disclosed by a governmental or judicial authority; or (v) which
the receiving Party can demonstrate in writing was independently developed without reference to or
reliance upon the other Party’s Confidential Information. No receiving Party shall, in any manner
whatever for use in any way for its own account or for the account of any third-party, disclose or
communicate to a third-party, any Confidential Information for any purpose except for the purpose
for which such Confidential Information was supplied, and such receiving Party shall take every
reasonable precaution to protect the confidentiality of such information. Each Party acknowledges
that any breach of any obligation under this Section 11.2 is likely to cause or threaten
irreparable harm to the other Party, and accordingly, each Party agrees that in such event the
non-breaching Party shall be entitled to equitable relief to protect its interests, including, but
not limited to, preliminary and permanent injunctive relief.
DISTRIBUTOR acknowledges that the Products are proprietary to COMPANY and may not be copied and
that all rights of design and invention are reserved by COMPANY.
COMPANY shall, at its own expense, defend, indemnify and hold harmless DISTRIBUTOR, its officers,
directors, employees, agents, successors and assigns (the “Indemnified Parties”) against any and
all liabilities, claims, actions, suits, fines, penalties, losses, settlements, costs and expenses
(including, without limitation, reasonable attorneys’ fees and expenses) (collectively “Losses”)
relating to or arising out of any suit, claim or proceeding instituted against any of the
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
14.
Indemnified Parties by any third party or incurred in connection therewith, alleging that: (i) any
patent, trademark or any other intellectual property right of COMPANY infringes the intellectual
property rights of such third party; or (ii) any defect in the Products or their design or
manufacture caused bodily injury or death to any person or damage to any property. Notwithstanding
the foregoing, COMPANY shall not be responsible for such Losses as set forth in this section to the
extent that: (a) such Product has been altered, modified or tampered with by DISTRIBUTOR directly
resulting in such Product defect; or (b) such Product has been misused as a result of DISTRIBUTOR’s
unauthorized representation about the Product directly resulting in such Losses; or (c) such Losses
arise directly out of any negligence, recklessness or willful misconduct by DISTRIBUTOR or any of
its employees; or (d) DISTRIBUTOR fails to give COMPANY reasonable written notice of any such claim
as soon as is reasonably practicable. COMPANY shall have the sole control of the defense and/or
settlement of any claim subject to indemnification under this Section 12.1; provided, however, that
COMPANY shall not control or settle any such claim without prior consultation with DISTRIBUTOR.
If a claim of patent or other proprietary right infringement is made by a third party with respect
to a Product, then COMPANY, at its option and expense, shall (i) obtain for DISTRIBUTOR the right
to continue to market and distribute the Product, (ii) replace the Product with a
functionally-equivalent non-infringing Product, (iii) modify the Product so that it becomes
non-infringing, so long as the functionality of the Product is not thereby adversely affected, and
replace the infringing Product with such modified Product or (iv) have dismissed, settle or
otherwise cause such claim to be withdrawn. If COMPANY is unable to accomplish any of the foregoing
within [*] days of the initial infringement claim and the ability of DISTRIBUTOR to market such
Product is effectively prevented by a court of relevant jurisdiction in the Territory, then COMPANY
shall grant DISTRIBUTOR a full refund of DISTRIBUTOR’S fully-landed cost for all affected Products
and accept return of such Products at COMPANY’s expense, the Parties shall remove all such affected
Products from then current and future MPL and adjust DISTRIBUTOR’s MPL accordingly and this
Agreement shall be terminated with respect to such affected Product. If partial termination of this
Agreement with respect to one or more Products pursuant to this Section 12.2 results in a greater
than [*] percent ([*]%) decrease in DISTRIBUTOR’s total sales of Products in the [*]-month period
following any such partial termination as compared to the average quarterly sales over the
[*]-month period immediately preceding the third party claim which precluded DISTRIBUTOR from
marketing and distributing any Product, then DISTRIBUTOR shall have the option to terminate this
Agreement in its entirety, subject to Section 13.2.
COMPANY or DISTRIBUTOR, as the non-defaulting Party, may cancel this Agreement, immediately by
providing written notice to the other Party, upon the occurrence of any of the following events:
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
15.
(i) The other Party becomes insolvent or is unable to pay its debts as they mature or ceases
to pay in the ordinary course of business its debts as they mature; or the other Party makes an
assignment for the benefit of its creditors; or a receiver, liquidator, custodian, trustee or the
like is appointed for the other Party or its property; or the other Party commences a voluntary
case under any applicable bankruptcy or insolvency law or consents to the entry of an order for
relief in any involuntary case, or a court with jurisdiction enters a decree for relief in any
involuntary case involving the other Party.
(ii) The other Party defaults in the material performance of any of its obligations under this
Agreement and fails to cure such default within sixty (60) days after written notice thereof from
the non-defaulting Party.
Upon the expiration of this Agreement or its termination in accordance with Section 8.6(vi),
Section 12.2 or Section 13.1 above, and after the allowance for any applicable cure periods,
DISTRIBUTOR and COMPANY each promise to do the following immediately:
(i) DISTRIBUTOR shall pay to COMPANY all amounts which are then due and payable by DISTRIBUTOR
to COMPANY under this Agreement less any such amounts which reasonably may be set-off by
DISTRIBUTOR based on a dispute or otherwise arising out of or related to this Agreement.
(ii) Each Party shall return to the other Party all Confidential Information of the other
Party in its possession or under its control, together with a statement signed by an officer or
duly authorized representative of the Party to the effect that all of the Confidential Information
has been returned to the other Party.
(iii) Except to the extent that DISTRIBUTOR requires use of the Trademarks in order to
exercise DISTRIBUTOR’s right to sell any existing inventories of Products upon the expiration or
termination of this Agreement, as provided for in Section 13.2(iv), DISTRIBUTOR shall cease to use
any of the Trademarks and return to COMPANY all materials supplied to DISTRIBUTOR by COMPANY which
contain any of the Trademarks. Furthermore, upon receipt of written notice from COMPANY,
DISTRIBUTOR shall dispose of all packaging, labels, brochures, lists, and other similar materials
containing any of the Trademarks in accordance with COMPANY’s instructions, except for those
materials necessary for DISTRIBUTOR’s continuing sale of existing inventories of Products provided
for in Section 13.2(iv). COMPANY shall reimburse DISTRIBUTOR for the direct costs (exclusive of
overhead) and disposal costs of such materials.
(iv) COMPANY or its successor (A) shall repurchase all sterile Products with a UBD at the date
of termination or expiration of this Agreement of [*]% of the intended maximum shelf life remaining
or greater at DISTRIBUTOR’s original landed cost plus any consumption tax applicable in the
Territory to sales or deliveries of Products to a third party in the Territory; provided,
however, that any Products with [*]% or more of the UBD remaining but less than [*]% of the
UBD remaining shall be subject to a [*] percent ([*]%) restocking fee to be charged by COMPANY, and
(B) shall pay DISTRIBUTOR for all documented out of pocket
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
16.
expenses directly related to all Xxxxxx then held by DISTRIBUTOR; provided,
however, that DISTRIBUTOR shall have the absolute right to continue to sell any such
Products not repurchased by COMPANY, in accordance with this Section 13.2(iv). All payments by
COMPANY under this Section 13.2(iv) shall be made by wire transfer to an account to be specified by
DISTRIBUTOR on or prior to thirty (30) days after DISTRIBUTOR has delivered the Products and
provided COMPANY with an invoice.
For purposes of this Section 13.2(iv), “out of pocket expenses” shall include any Product costs,
documentation, Product testing fees, and any clinical trial related expenses, etc. Specifically
excluded from “out of pocket expenses” are DISTRIBUTOR overhead, salary, travel expenses and other
expenses, which derive from the operation of DISTRIBUTOR’s business as an ongoing business concern
in the Territory.
(v) Subject to payment by COMPANY to DISTRIBUTOR of all out of pocket expenses, as detailed in
Section 13.2(iv) above, DISTRIBUTOR shall diligently and expediently take the necessary steps to
transfer any Xxxxxx held by DISTRIBUTOR for the Products to a third-party affiliated with COMPANY,
and located and organized under the law of the Territory, that is authorized and legally entitled
to hold the Xxxxxx.
(vi) During the period that the Xxxxxx are in the process of being transferred, DISTRIBUTOR
shall otherwise cooperate with COMPANY by importing and reselling the Products to COMPANY’S next
authorized distributor at DISTRIBUTOR’s fully landed cost for the Products plus a xxxx-up of [*]
percent ([*]%) and any applicable consumption tax. The general purchase and sales terms of this
Agreement will govern the sale of Products during this transfer period. COMPANY expressly agrees to
indemnify DISTRIBUTOR for any non-payment by COMPANY’s next distributor for Products so resold by
DISTRIBUTOR or for any other non-performance of DISTRIBUTOR out of DISTRIBUTOR’s immediate control
during such transfer period.
(vii) In the event that COMPANY or its successor exercises its right to terminate this
Agreement pursuant to Section 14.3(ii) below, COMPANY shall be obligated to pay DISTRIBUTOR a
termination fee which shall be calculated in accordance with the following table (the “Change of
Control Termination Fee”):
| If Change of Control | Then the Change of Control | |||
| termination occurs during: | Termination Fee shall be equal to: | Multiplied by a factor of | ||
[*]
|
[*] | [*] | ||
[*]
|
[*] | [*] | ||
[*]
|
[*] | [*] | ||
[*]
|
[*] | [*] |
Gross profit shall mean net sales in YEN of the Products less the price of the Products paid by
DISTRIBUTOR to COMPANY in YEN.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
17.
In the event that COMPANY challenges the applicability or efficacy of COMPANY’s Change of Control
Termination Fee, or if this provision is held to be void or unenforceable for any reason,
DISTRIBUTOR shall be entitled to all remedies provided at law, including attorney’s fees. The
Parties expressly acknowledge that substantial injury will result to DISTRIBUTOR upon a termination
of this Agreement by COMPANY or its successor pursuant to Section 14.3(ii) below. The Parties
further expressly acknowledge that it may be difficult or impossible to determine with precision
the amount of monetary damages that would be required to compensate DISTRIBUTOR for such injury.
Accordingly, the Parties have made a good-faith effort to accurately determine what those damages
might be and the amount agreed to as reasonable by the Parties is COMPANY’s Change of Control
Termination Fee.
(viii) If any portion of the Note is still outstanding, COMPANY shall immediately repay to
DISTRIBUTOR the principal balance of the Note and any accrued interest owed at the time of such
repayment.
Save in respect of payments due under this Agreement, neither Party to this Agreement is
responsible to the other Party for nonperformance or delay in performance of the terms and
conditions herein due to any event of force majeure, including without limitation acts of god, acts
of government, wars, civil disturbances, strikes, and other labor unrest, accidents in
transportation or other cause beyond the control of the Parties. The Party whose performance is
prevented under this paragraph shall immediately inform the other Party of the state of affairs.
Notwithstanding the foregoing, should any Party be prevented from materially performing its
obligations under this Agreement due to any such event of force majeure for a period in excess of
four (4) months, then the other Party may elect to terminate this Agreement upon delivery of thirty
(30) days prior written notice to such effect.
DISTRIBUTOR’s relationship to the COMPANY shall be that of an independent contractor. Nothing
contained in this Agreement shall make DISTRIBUTOR a partner, joint venturer, employee, or agent of
COMPANY for any purpose whatsoever. DISTRIBUTOR shall not sign any contract in the name of the
COMPANY, shall not purport to bind COMPANY in any way to any obligation, and shall not hold itself
out or purport to act as COMPANY’s legal partner or legally empowered agent or representative for
any purpose whatsoever.
(i) This Agreement and each and every covenant, term and condition hereof is binding upon and
inures to the benefit of the Parties hereto and their respective successors and permitted assigns.
Except as provided in Section 2.2 above, neither this Agreement nor any rights hereunder may be
assigned by DISTRIBUTOR directly, indirectly, voluntarily or by operation of law, without first
receiving the prior written consent of COMPANY. In the event of (A) any consolidation of COMPANY
with or merger of COMPANY with or into another entity, or (B) any sale, transfer or lease of all or
substantially all the Product related assets of
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
18.
COMPANY, or (C) any event in which any person or group of persons acting in concert acquire
more than 50% of the voting stock of COMPANY (each, a “Change in Control”), COMPANY shall to such
extent assign its rights and obligations under this Agreement to such acquirer of COMPANY’s stock
or assets. COMPANY shall further provide DISTRIBUTOR with prior written notice of such assignment
accompanied by a written undertaking of the assignee that the assignee shall be bound by this
Agreement and assume all obligations of COMPANY under this Agreement.
(ii) Provided that such Change in Control occurs, COMPANY or COMPANY’s successor shall have
ninety (90) days from the date of such a Change in Control to notify DISTRIBUTOR in writing of its
intention either to terminate this Agreement or to accept the assignment of and assume all rights
and obligations of COMPANY under the terms and conditions of this Agreement. In the event that the
COMPANY or COMPANY’s successor notifies DISTRIBUTOR within ninety (90) days of the Change of
Control that it has chosen to terminate this Agreement (the “Change of Control Termination Event”),
then COMPANY shall promptly pay to DISTRIBUTOR by wire transfer to an account to be specified by
DISTRIBUTOR the Change of Control Termination Fee set forth in Section 13.2(vii).
Both Parties agree that the obligations described in Sections 4.1(x), 5.1(x), 7.2, 7.3, 7.4, 8.5,
10.1, 13.2 and Articles 9, 11, 12 and 14 of this Agreement shall survive any termination,
cancellation, or expiration of this Agreement.
The rights and remedies of each Party under this Agreement are not exclusive but shall be in
addition to all of the rights and remedies to which a Party is entitled against the other Party
under the law governing this Agreement.
Unless otherwise specified, any notice required by this Agreement shall be made in a writing sent
by prepaid certified mail, overnight courier or any means of electronic communications with
confirmation copy sent by certified mail to the addresses first listed above, until notice of
another address shall be given in the manner provided herein. All notices, consents or requests
shall be effective from the date of transmission if sent by facsimile, seven days if sent by
certified mail and when received if sent by international courier.
In the event there arises a dispute between the Parties as to the performance or interpretation of
any of the provisions of this Agreement, or as to matters related to but not covered by this
Agreement, the Parties shall first attempt to find a mutually agreeable solution by consultation in
good faith. If the matter has not been resolved within thirty (30) days of their first meeting to
resolve a dispute, then any such dispute shall be determined finally by final and binding
arbitration in accordance with the International Arbitration Rules of the American Arbitration
Association. The place of arbitration shall be either (a) Menlo Park, California, U.S.A. if
initiated
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
19.
and brought by DISTRIBUTOR or (b) Tokyo, Japan if initiated and brought by COMPANY and the language
of the arbitration shall be English. The arbitral tribunal shall consist of a single arbitrator. If
the Parties shall not have agreed upon an arbitrator within thirty (30) days of the notice of
arbitration, then the Administrator of the American Arbitration Association shall appoint one. At
minimum, the arbitral tribunal shall be experienced in cross-border transactions in the area of
medical devices. The unsuccessful Party in an arbitration shall pay and discharge all reasonable
costs and expenses (including reasonable attorneys’ fees) which are incurred by the other Party in
enforcing this Agreement.
Judgment upon the award of the arbitrator may be entered in any court having jurisdiction thereof.
The Parties acknowledge that this Agreement and any award rendered pursuant to it shall be governed
by the 1958 United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Award.
Pending the submission to arbitrators and thereafter until the single arbitrator renders the award,
the Parties shall, except in the event of termination, continue to perform all their obligations
under this Agreement without prejudice to a final adjustment in accordance with the award.
Nothing herein shall prevent any party from seeking injunctive relief from any court of competent
jurisdiction, in order to preserve assets, prevent irreparable harm or as otherwise appropriate.
In the event any term or provision of this Agreement shall for any reason be invalid, illegal or
unenforceable in any respect, it shall be deemed separate and shall not affect any other provisions
hereof or the validity hereof. The Parties agree to re-negotiate in good faith any term or
provision held invalid and to be bound by the mutually agreed substitute term or provision.
No waiver of any of the terms and conditions of this Agreement shall be effective for any purpose,
unless expressed in a writing and signed by the Party thereto giving the same, and any such waiver
shall be effective only in the specific instance and for the purpose given.
This Agreement shall be governed by and construed in accordance with the substantive law of the
State of California, U.S.A. excluding that body of law applicable to choice or conflicts of law.
The headings to the paragraphs of this Agreement are for convenience of reference only, do not form
a part of this Agreement, and shall not in any way affect the interpretation hereof.
This Agreement constitutes the entire and final agreement between the Parties on the subject matter
hereof and supersedes any and all prior oral or written agreements or discussions on the subject
matter hereof. This Agreement may not be modified in any respect except in a writing which states
the modification and is signed by both Parties hereto.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
20.
The parties agree to execute any and all such further agreements, instruments or documents, and to
take any and all such further action as may be necessary or desirable to carry out the provisions
hereof and to effectuate the purposes of this Agreement.
The schedules attached hereto are incorporated herein by this reference and expressly made a part
hereof as fully as though completely set forth herein.
This Agreement may be executed in two or more counterparts, each of which shall be deemed an
original and all of which together shall constitute one instrument.
| CARDICA, INC. | |||||
By:
|
/s/ Xxxxxxx Xxxxxx | ||||
Name: Title: |
Xxxxxxx Xxxxxx, MD President & CEO |
||||
| CENTURY MEDICAL, INC. | |||||
By:
|
/s/ Xxxxx Xxxxxxx | ||||
Name:
|
Xxxxx Xxxxxxx | ||||
Title:
|
President and CEO | ||||
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
21.
Schedule 1. Products and Prices
$[*] per Proximal Device FCA.
$[*] per Distal Device FCA.
If the selling price to Distributor exceeds [*]% of COMPANY’s average U.S. selling price, then
DISTRIBUTOR shall have the right to discuss pricing matters with COMPANY. COMPANY shall reasonably
disclose its average U.S. selling price to Distributor upon written request by DISTRIBUTOR.
COMPANY shall on a case by case basis prepare price quotations for custom made Products based upon
DISTRIBUTOR’s specific inquiry, taking into consideration expected purchase volumes.
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
22.
In order to comply with the request from Century Medical, Inc. (“CMI”), Cardica, Inc (“We” or “we”)
shall implement and undertake the following:
We shall include a Quality Control Certificate in every shipment to CMI. Such Quality Control
Certificate shall be specific as to lots or Serial Number and the date of manufacture, and indicate
that the Product shipped is within Product specifications (inspection & test record). In case the
Products are sterilized by ETO, we shall also include an ethylene oxide residual analysis report.
We shall provide CMI with the latest Sterilization Validation Data at least once a year and when
validated.
We shall provide CMI with the up-dated “Certificate to Foreign Government” issued by the FDA
relating to our manufacturing plant or a copy of the certificate certifying compliance to ISO13485,
EN 46001 or successor regulations.
We shall provide CMI the following information at least once every two years for CMI to confirm
that the Product specifications conform to the contents of the Japanese Regulatory Approvals
document: copies of the (i) up-dated version of the Product specification sheet; (ii) production
flow chart; and (iii) SOP of quality control testing protocol; and (iv) other necessary documents
in order to maintain GQP, GVP and GMP, and keep CMI updated when these documents are amended or
modified. In addition, we shall allow CMI personnel to visit our facility, with reasonable notice,
to actually confirm these points at the manufacturing plant.
We shall keep CMI informed in writing regarding any and all modifications and/or improvements of
the Products, change in method of sterilization, change in packaging, change in facility or factory
location, changes in test standards and change in quality control method for transportation and
delivery of the Products. Product modifications that might reasonably be expected to affect the
validity of the regulatory status of the Products will be discussed with CMI prior to
implementation of such changes.
We shall promptly notify CMI of any actions taken with respect to our business or the Products by
regulators in other jurisdictions, including (i) any facility inspection resulting in any notice of
infraction, warning or other action, (ii) voluntary or mandatory recalls or withdrawal of Products,
(iii) administrative or court proceedings regarding the Products, (iv) MDR, (v) information on
proceedings taken to avoid occurrence or enlargement of health/hygiene danger and (vi) any
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
23.
similar matters. We will promptly provide CMI with copies of any correspondence with regulators
regarding any of the foregoing.
Concerning the events specified in items 4 and 5 above, both Parties will notify the other of the
primary (preferred) method of transmitting information and the name and position title of the
person responsible for sending and/or receiving these transmissions. Both parties will settle the
detail of information transmission method, including mailing address, phone and fax numbers and
e-mail address of person in charge, and promptly inform the other Party in case of change.
We shall provide for the Products to be suitably packaged and packed for export.
We shall provide CM with a written report of our findings, in a reasonably timely manner, in
response to any Product complaint report submitted by CMI. Where a Product complaint involves a
Product failure that resulted in bodily injury or death, we shall expedite and place the highest
priority possible to investigating and reporting in writing our findings to CMI.
9. On-site investigation of Compliance with XXX (XXX 00000;0000) by regulator
In order to register each manufacturing site(s) related to compliance with XXX (XXX 00000;0000), we
shall allow regulator’s personnel, with reasonable notice, (i) to visit our manufacturing site(s)
and (ii) to investigate such compliance.
10. Addition or amendment to this Memorandum
When any addition or amendment to this Memorandum is required in compliance with GQP, GVP and GMP,
we agree to discuss them with CMI in good faith.
| Cardica, Inc. | Century Medical, Inc. | |||||||||||||
| Date: | 2-16-05 | Date: | February 22, 2005 | |||||||||||
| Signature: | /s/ Xxxxxxx Xxxxxx | Signature: | /s/ Xxxxx Xxxxxxx | |||||||||||
| Name: | Xxxxxxx Xxxxxx, MD, PhD | Name: | Xxxxx Xxxxxxx | |||||||||||
| Title: | President and CEO | Title: | President and CEO | |||||||||||
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
24.
Schedule 3 Reporting for Product Defects, Adverse Events, Overseas Corrective Action Reports and Research
Reports.
The language used for all communications under this Schedule 3 shall be English. The attached
Decision Trees delineate (i) what Product defects or Adverse Events are reportable or
non-reportable to MHLW under the Japanese Regulatory Affairs Law along with the time limit for
DISTRIBUTOR to report such matters after DISTRIBUTOR becomes aware of those and (ii) definitions of
the technical terms used in this Schedule 3. In any event, DISTRIBUTOR is obligated to report
Overseas Corrective Action Reports and Research Reports.
FDA Reportable Events by COMPANY means the following (see 21 C.F.R. §803 for further details
regarding reporting requirements and time frames):
| a. | Medical Device Report (MDR) is required whenever COMPANY becomes aware of information suggesting that one of its marketed devices (i) may have caused or contributed to a death or serious injury, or (ii) has malfunctioned and this malfunction is likely to cause or contribute to a death or serious injury if it recurs. | ||
| b. | Adverse Event or Serious injury/(Serious Illness) is an injury or illness that: | ||
| Is life threatening, even if temporary in nature; or | |||
| Results in permanent impairment of a body function or permanent damage to a body structure; or | |||
| Necessitates medical or surgical intervention to preclude permanent impairment of a body function or permanent damage to a body structure. | |||
| (i) | Regulatory Reporting Requirements. COMPANY and DISTRIBUTOR each will be responsible for regulatory reporting and for responding to regulatory inquiries within their respective territories. DISTRIBUTOR and COMPANY each shall use their best efforts to notify the other, by telephone, facsimile or email within forty-eight (48) hours (but in any event such notification shall occur within seventy-two (72) hours) after either Party becomes aware of any matter which must be reported to the MHLW or FDA. The Party which becomes aware of any such matter will use its best efforts to provide to the other Party within five (5) calendar days of first becoming aware of such matter (but in any event no later than within five (5) business days), with a detailed written report, by facsimile regarding the event. COMPANY shall supply DISTRIBUTOR with a copy of its medical device report filed with the FDA in accordance with the Medical Device Reporting Regulation (21 C.F.R. § 803 (2001) within twenty-four (24) hours of supplying such a report to FDA. DISTRIBUTOR shall supply COMPANY with a copy of its report filed with the MHLW within twenty-four (24) hours of supplying such a report to the MHLW. Follow-up reports to the initial report will be submitted using the same procedures and timelines. | ||
| (ii) | Non-reportable Product Defects or Non-Reportable Adverse Events. DISTRIBUTOR will notify COMPANY of non-reportable Product defects or non-reportable Adverse Events within ten (10) calendar days after it becomes aware of any such Product defects or Adverse Events. |
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
25.
Decision Trees
First check “Device Defect Report” decision tree, as attached hereto. After checking the
“Device Defect Report” decision tree, as attached hereto, cheek also the “Adverse Events Reports”
decision tree, as attached hereto, in case a patient’s death or injury has occurred.
Definition of the technical terms:
| 1. | Serious |
| (1) | Death. | ||
| (2) | Disability. | ||
| (3) | Cases which might be related to death or disability. | ||
| (4) | Cases which require admission or prolongation of the period of admission to a hospital or clinic for treatment. (excluding cases in (3)) | ||
| (5) | Severe cases which might be related to death or injury. | ||
| (6) | Congenital diseases or abnormalities in subsequent generations. |
| 2. | Moderate | ||
| Incidents neither Serious nor Slight. | |||
| 3. | Slight | ||
| Symptoms caused by incidents are slight and easily cured. | |||
| 4. | Level of seriousness of a case which might have occurred. | ||
| This is to be determined by judging how serious (in the worst case) a health hazard caused by the case has been, assuming the case occurs again; even if the patient or user was not deceased or injured, or a serious health hazard was avoided due to the intervention of a doctor, in spite of a device defect. | |||
| 5. | Prediction of the tendency of occurrence | ||
| This is to be determined by judging whether number, frequency, conditions, etc. relating to occurrence of an incident is clearly written in the device’s Instructions for Use (“IFU”) package insert. In order to be judged as predictable, it must be assured that not only manufacturer (importer) but also users such as doctors and nurses can predict the tendency of occurrence of the incident. Such case is categorized as a “known-incident.” | |||
| 6. | Prediction of occurrence | ||
| This is to be determined by judging whether occurrence of an incident is clearly written in the device’s IFU package insert. In order to be judged as predictable, it must be assured that not only manufacturer (importer) but also users such as doctors and nurses can predict the occurrence of the incident. Such case is categorized as a “known-incident.” For purposes of clarity, it is not to be determined as a “known-incident” where an incident has occurred under conditions other than those written in the IFU package insert. |
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
26.
Device Defect Reports
(in case Patient’s death or injury simultaneously occurs, check “Adverse Event Report” flowchart also.)
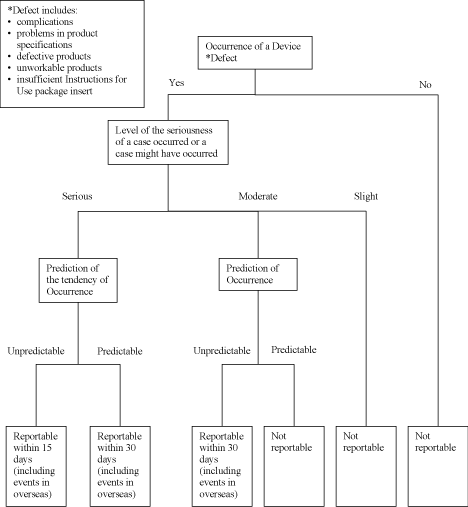
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
27.
Adverse Events Reports (with Patient’s death or injury)
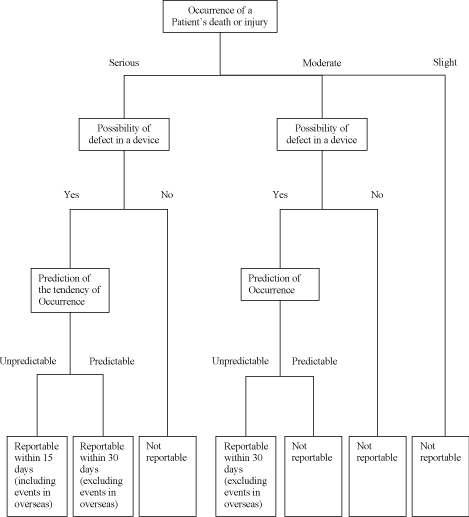
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
28.
Overseas Corrective Action Reports and Resarch Reports
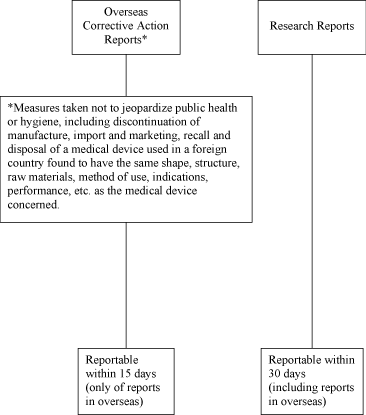
[*] = CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE
COMMISSION. CONFIDENTIAL TREATMENT HAS BEEN REQUESTED WITH RESPECT TO THE OMITTED PORTIONS.
29.
