EX-10.15 17 vglife_ex1015.htm EXCLUSIVE LICENSE AGREEMENT WITH UC, DATED NOVEMBER 30, 2009 METABOLIC DISRUPTION EXCLUSIVE LICENSE General Terms and Conditions Appendix A - Patent Rights Appendix B - Royalties Appendix C - Due Diligence Appendix D -...
Exhibit 10.15

METABOLIC DISRUPTION EXCLUSIVE LICENSE
TABLE OF CONTENTS
General Terms and Conditions
Section 1 Novation
Section 2 - Definitions
Section 3 - Grant of Rights and Improvements
Section 4 - Financial Consideration
Section 5 - Sublicensing
Section 6 - U.S. Government Rights and Requirements
Section 7 - Reports, Records, and Audits
Section 8 - Proprietary Information and Materials
Section 9 - Export
Section 10 - Sponsored Research
Section 11 - Due Diligence
Section 12 - Patent Prosecution
Section 13 - Patent Enforcement
Section 14 - Warranties, Indemnifications, and Insurance
Section 15 - Duration, Termination, and Conversion
Section 16 - General
____
Appendix A - Patent Rights
Appendix B - Royalties
Appendix C - Due Diligence
Appendix D - Diligence Reports
Appendix E - Form of Royalty Report
Appendix F - Material Transfer Agreement Template
| 1 |
This Exclusive License Agreement (the "Agreement") between the Regents of the University of Colorado, a body corporate, having its principal office at 0000 Xxxxx Xxxxxx, 0xx Xxxxx, Xxxxxx, XX 00000 (hereinafter "University") and Viral Genetics, Inc., a Delaware corporation having its principal office at 0000 Xxxxxxxxxx Xxxxx, Xxxxx 000, Xxx Xxxxxx, Xxxxxxxxxx 00000 (hereinafter "Licensee") is effective on the 22nd day of November, 2009, (the "Effective Date").
WHEREAS, University and Licensee now wish to substitute this Agreement for the Prior Agreement; and
| 1. | NOVATION |
This Agreement is a novation of, and entirely replaces, the Prior Agreement.
| 2. | DEFINITIONS |
For the purposes of this Agreement, the following words and phrases shall have the following meanings:
2.1 "Active Program" shall mean a developmental program advancing a distinct diagnostic or therapeutic application covered in the Licensed Patents (a) which is the subject of a sublicense or (b) on which Licensee itself is actively pursuing development or on which an IND Phase I, II or Ill has been issued.
2.2 "Affiliate(s)" means any business entity that controls, is controlled by, or is under common control with Licensee. Control means the direct or indirect ownership of at least fifty percent (50%).
2.3 "Field of Use" means any use of the Metabolic Disruption Technologies.
2.4 "Inventor'' means Xx. Xxxxx Xxxxxx.
2.5 "Know-How" shall mean, and is limited to: (a) University's proprietary information that has been created, developed, and fixed in any tangible medium of expression by Inventor prior to or as of the Effective Date and which is directly related to the use of, or desirable for the practice of, the licensed Patent Rights; and (b) University's proprietary information that is necessary for the use of, or desirable for the practice of, the licensed Patent Rights developed by Inventor after the Effective Date while Inventor remains an employee of University and voluntarily involved as an employee, board member or consultant of Licensee.
| 2 |
2.6 "Licensed Patents means the United States patents and patent applications and invention disclosures listed in Appendix A together with any and all foreign patent applications and patents based thereon, divisionals, continuations of those applications and the patents issued therefrom, including any reissues or reexaminations or extensions of such patents, and claims of any continuations-in-part applications and resulting patents that are directed to subject matter specifically described in the patents and patent applications listed in Appendix A, and any invention made during the term of this Agreement by Xx. Xxxxx Xxxxxx, or by anybody working in a laboratory under the supervision or direction of Xx. Xxxxxx, that is in the Field of Use.
2.7 "Licensed Process(es)" means any method, procedure, service, or process, the practice of which, in the absence of a license, would infringe, or contribute to infringement of, a Valid Claim of a Licensed Patent.
2.8 "Licensed Product(s)" means any and all products the making, using, importing, exporting or selling of which, in the absence of a license, would infringe, or contribute to infringement of, a Valid Claim of a Licensed Patent.
2.9 "Metabolic Disruption Technologies" means the technologies described in the Licensed Patents as defined below.
2.10 "Net Sales" means the amount of gross receipts on sales of any Licensed Products or practice of any Licensed Processes by Licensee, Affiliates, or Sublicensees. Net Sales excludes the following items, but only to the extent they pertain to the sale of Licensed Products or the practice of Licensed Processes, are included in gross revenue, and are separately stated on purchase orders, invoices, or other documents of sale: (a) customary trade, quantity, or cash discounts and non-affiliated brokers' or agents' commissions actually allowed and taken; (b) amounts repaid or credited by reason of rejection or return; and (c) taxes levied on and other governmental charges made as to production, sale transportation, delivery or use and paid by or on behalf of Licensee.
2.11 "Sublicensee(s)" means any third party sublicensed by Licensee to make, have made, use, have used, sell, have sold, import, have imported, exported, or have exported Licensed Product or to practice or have practiced any Licensed Process.
2.12 "Sublicense Income" shall mean any and all consideration received by Licensee or an Affiliate from a third party as consideration for the grant of a sublicense, or an option for a sublicense, to the Licensed Patents. Such consideration shall include without limitation any upfront, license or option initiation or signing fees, license maintenance fees, milestone payments, equity, and research and development funding received by Licensee or Affiliate in excess of the actual costs of performing such research and development. Sublicense Income shall also include the fair market value of any non cash consideration paid to Licensee for sublicense rights. Sublicense Income shall not include sums received as royalties on Net Sales by Sublicensees, such Net Sales being subject to the royalty on Net Sales as set forth in Appendix B, Section 3.
2.13 "Territory" is worldwide
| 3 |
2.14 "Valid Claim" means a pending or issued and unexpired claim of a patent under the Intellectual Property so long as such claim has not been irrevocably abandoned or declared to be invalid in an unappealable decision of a court or other government agency of competent jurisdiction through no fault of Licensee.
3. GRANT OF RIGHTS AND IMPROVEMENTS
3.5.1 That may be created or developed at any time by any employee of Licensee, or by Inventor working or consulting at Licensee using Licensee's own facilities, for Licensee's own account and benefit as demonstrated by written records kept in the normal course of research and independent of her work performed or contemplated under funded research at the University or employment agreement at the University; or
| 4 |
3.5.2 That may be created or developed at any time by Licensee, using its own facilities, or under a contract with, or under a grant from, a third party without any contribution from Inventor while Inventoris at the time obligated to assign intellectual property to University and as demonstrated by written records kept in the normal course of research.
As consideration for the license and option rights granted under this Agreement, Xxxxxxxx agrees to pay to University the economic consideration specified in Appendix B. In addition, University and License agree to the following:
| 5 |
4.7 Payments. All payments to University shall be in United States Dollars, made payable to "The Regents of the University of Colorado" and mailed to:
Office of Technology Transfer
University of Colorado
Suite 100,0000 Xxxxxx Xxxxxx
Campus Box 588
Boulder, CO 80309
ATTN: Accounts Receivable
5.1.1 Shall be subject to the termination of this Agreement;
5.1.2 Will provide that any Sublicensee will not further sublicense, unless such further sublicensee agrees to all relevant terms of this agreement mutatis mutandis;
5.1.3 Will expressly include the provisions of Section 4 Financial Consideration, Section 7 Reports, Records and Audits, and Section 14 Warranties, Indemnification, and Insurance for the benefit of the University and Vermont;
5.1.4 State that in the event this Agreement is terminated pursuant to Section 15.2, provide for assignment of the sublicense to University so long as the Sublicensee complies with Section 5.1 and the Sublicensee is not in breach; and
5.1.5 Provide only for cash or marketable securities consideration from Sublicensee(s) unless University has expressly consented in writing and in advance to other consideration
| 6 |
6. U.S. GOVERNMENT RIGHTS AND REQUIREMENTS
Licensee understands that this Agreement is subject to all of the terms and conditions of 35 U.S.C. §§ 200-212, ("The Xxxx-Xxxx Act") and 37 C.F.R. § 401. Licensee agrees to take all reasonable action necessary to enable University to satisfy its obligations thereunder. Licensee shall use commercially diligent efforts to cause any Licensed Products subject to the Xxxx-Xxxx Act to be manufactured substantially in the United States.
7. REPORTS, RECORDS, AND AUDITS
7.1.1 Quarterly and annual diligence reports describing Licensee's progress and plans for the development of Licensed Products. Quarterly reports may be an oral or written (at Licensee's discretion) summary, and an annual report in written form shall be delivered to the University. All written Diligence Reports, when used, shall conform to those as set forth in Appendix D, and Licensee shall endeavor to include Inventor whenever possible in any oral diligence report made to University. Should Licensee fall under the control of, be acquired by or merged with another company, all reports thereafter must be in written form; and
7.1.2 Royalty Reports as set forth in Appendix E for each calendar quarter beginning the first quarter in which Net Sales occurs or Sublicense Income is received and each quarter thereafter regardless of Net Sales or Sublicense Income.
7.2.1 Licensee shall keep accurate records and shall compel its Affiliates and Sublicensees to keep accurate records in sufficient detail to reflect its operations under this Agreement and to enable the royalties accrued and payable under this Agreement to be determined.
7.2.2 Such records shall be retained for at least three (3) years after the close of the period to which they pertain, or for such longer time as may be required to finally resolve any question or discrepancy raised by University.
7.3.1 Upon the request of University, with reasonable notice, but not more frequently than once a year, Licensee shall permit an independent public accountant selected and paid by University to have access during regular business hours to such records as may be necessary to verify the accuracy of royalty payments made or payable hereunder.
7.3.2 Said accountant shall disclose information acquired to University only to the extent that it should properly have been contained in the royalty reports required under this Agreement.
7.3.3 If an inspection shows an underreporting or underpayment in excess of five percent (5%) for any twelve (12) month period, then Licensee shall reimburse University for the cost of the inspection and pay the amount of the underpayment including any interest as required by this Agreement.
| 7 |
8. PROPRIETARY INFORMATION AND MATERIALS
8.4 Exceptions. Confidential Information shall not include:
8.4.1 information which at the time of disclosure had been previously published or was otherwise in the public domain through no fault of Recipient;
8.4.2 information which becomes public knowledge after disclosure unless such knowledge results from a breach of this Agreement;
8.4.3 information which was already in Recipient's possession prior to the time of disclosure as evidenced by written records kept in the ordinary course of business or by proof of actual use thereof;
8.4.4 information that is independently developed without use of the Confidential Information; and
8.4.5 information which is required to be disclosed by law, court order, or government regulation.
8.5 XXXX. Licensee acknowledges that University is subject to the Colorado Open Records Act (C.R.S. §§ 00-00-000, et seq.). All plans and reports marked "Confidential" shall be treated by University as confidential to the extent permitted under §00-00-000.
| 8 |
Licensee will not export or re-export Licensed Patents to any country, individual, or entity except when such export or re-export is in full compliance with the laws and regulations of the United States of America, as applicable. Applicable laws and regulations may include but are not limited to the Export Administration Regulations, the International Traffic in Arms Regulations, and the economic sanctions regulations administered by the U.S. Department of the Treasury.
10. SPONSORED RESEARCH
10.1 The Sponsored Research Agreement between the parties effective May 30, 2008 shall remain in full force and effect but with respect to the Metabolic Disruption Technologies.
10.2 In addition, Licensee shall, for the life of this Agreement, pay to University for sponsored research on Metabolic Disruption Technologies, to be governed by the terms of the May 30, 2008 Sponsored Research Agreement except for Article 3 thereof, an amount equal to the greater of (a) ten percent (10%) of all funds it receives from any source other than Net Sales, funds from or resulting in debt obligation, and payment for shares in Licensee, or (b) starting April 1, 2012, twenty-five thousand dollars ($25,000.00) per quarter.
10.3 Notwithstanding anything to the contrary in Section 10.1 or 10.2, any payment to University for sponsored research is subject to the requirement that Xx. Xxxxxx is employed by University at the time any such payment is due.
11.1 Licensee shall use its best efforts to bring Licensed Products and Licensed Processes to market through a thorough, vigorous and diligent program for exploitation of the Patent Rights, to develop manufacturing capabilities, to continue active, diligent marketing efforts, and to satisfy the needs of such market with the Licensed Products and Licensed Processes throughout the life of this Agreement. Licensee acknowledges and agrees to the performance milestones defined in Appendix C.
| 9 |
| 10 |
14. WARRANTIES, INDEMNIFICATIONS, AND INSURANCE
(1) A warranty or representation by University as to the validity or scope of any of the rights included in the Licensed Patents;
(2) A warranty or representation that the Licensed Patents or anything made, used, sold or otherwise disposed of under the License will or will not infringe patents, copyrights or other rights of third parties;
| 11 |
(3) An obligation to furnish any know-how or services not agreed to in this Agreement, or
(4) An obligation by the University to bring or prosecute actions or suits against third parties for infringement, or to provide any services other than those specified in this Agreement.
(1) the use by or on behalf of Licensee, its sublicensees, Affiliates, directors, officers, employees, or third parties of any Licensed Patents;
(2) the design, manufacture, production, distribution, advertisement, consumption, sale, lease, sublicense or use of any Licensed Product(s), Licensed Process(es) or materials by Licensee, or other products or processes developed in connection with or arising out of the Licensed Patents; or
(3) any right or obligation of Licensee under this Agreement.
15.DURATION, TERMINATION, AND CONVERSION
15.2 Termination by Licensee. Licensee may terminate this Agreement in its entirety, if Licensee:
(1) Pays all amounts due as well as all non-cancelable costs to University through the termination date;
(2) Submits final payments with interest as provided in Section 4.4 and a final report of the type described in Section 7;
(3) Returns any confidential materials provided to Licensee by University in connection with this Agreement;
(4) Suspends its use and sales of the Licensed Product(s) and Licensed Process(es); provided however, that subject to making the payments required by Section 4 and the reports required by Section 7, Licensee may, for a period of ninety (90) days after the effective date of such termination, sell all Licensed Products which may be in inventory; and
| 12 |
(5) Provides University the right to access any regulatory information filed with any U.S. or foreign government agency with respect to Licensed Products and Licensed Processes.
15.3 University may terminate the relevant portion of this Agreement if Licensee:
(1) Is delinquent on any report or payment that is not in dispute; is in breach of the diligence obligations described in Appendix B or C, including the milestone requirements and such missed milestone is not otherwise excused pursuant to the terms of this Agreement; provides any false report, as determined by Section 16.8 Dispute Resolution of this Agreement, or is in breach of any other material provision of this Agreement, and fails to cure any of these circumstances within 30 days of University's written notice to Licensee;
(2) Violates any laws or regulations of applicable governmental entities;
(3) Becomes insolvent, defined by the voluntary filing of a Chapter 7 proceeding under the Bankruptcy Law, or if Licensee shall cease to carry on its business or development activities pertaining to Licensed Patents; or
(4) Institutes a legal action challenging the validity of any Licensed Patent.
16.1.2 As licensee currently is exploiting the Metabolic Disruption Technology through MetaCytoLytics Inc., a wholly owned, but separately incorporated, corporation, Licensee may assign this Agreement to MetaCytoLytics, Inc. upon its spin-off or upon divestiture of more than 50% interest of MetaCytoLytfcs, Inc. to one or more third parties
16.1.3 Any other attempt to assign this Agreement by Licensee is null and void.
16.1.4 Prior to any assignment, the following conditions must be met: (i) Licensee must give University ten (10) days prior written notice of the assignment, including the new assignee's contact information; and (ii) the new assignee must agree in writing to University to be bound by this Agreement.
| 13 |
16.1.5 In the event of a bankruptcy, assignment is permitted only to a party that can provide adequate assurance of future performance, including diligent development and sales, of Licensed Patents.
16.2.1 Licensee will provide written notice to University at least ninety (90) days prior to bringing an action seeking to invalidate any Licensed Patent or a declaration of non-infringement. Licensee will include in such written notice an identification of all prior art it believes invalidates any claim of the patent.
16.2.2 Notice hereunder shall be deemed sufficient if given by registered mail, postage prepaid, and addressed to the Party to receive such notice at the address given below, or such other address as may hereafter be designated by notice in writing. All general notices to Licensee shall be mailed to:
Xxxx Xxxxxxxxx, President and CEO
Viral Genetics, Inc.:
0000 Xxxxxxxxxx Xxxxx, Xxxxx 000
San Marino, California 91108
16.2.3 All financial invoices to Licensee (i.e., accounting contact) shall be e-mailed to Xxxx Xxxxxxxxx at the above address.
16.2.4 All general notices to University shall bee-mailed or mailed to:
License Administrator, CU Case#- _
Office of Technology Transfer
University of Colorado, 588 SYS
0000 Xxxxxx Xxxxxx
Boulder, CO 80309
16.2.5 Either party may change its mailing or e-mail address with written notice to the other party.
| 14 |
16.7 Choice of Law. This Agreement shall be governed by and construed in accordance with the laws of the State of Colorado.
16.8.1 Any disputes not so resolved shall be referred to the Principal Technology Transfer Officer for the University and to Licensee's senior executives with settlement authority ("Senior Executives"), who shall meet at a mutually acceptable time and location within thirty (30) days of the Notice Date and shall attempt to negotiate a settlement.
16.8.2 If the Senior Executives fail to meet within thirty (30) days of the Notice Date, or if the matter remains unresolved for a period of sixty (60) days after the Notice Date, the Parties hereby irrevocably submit to the jurisdiction of a court of competent jurisdiction in the State of Colorado, and, by execution and delivery of this Agreement, each (i) accepts, generally and unconditionally, the jurisdiction of such court and any related appellate court, and (ii) irrevocably waives any objection it may now or hereafter have as to the venue of any such suit, action or proceeding brought in such court or that such court is an inconvenient forum.
| 15 |
16.12 Preservation of Immunity. The Parties agree that nothing in this Agreement is intended or shall be construed as a waiver, either express or implied, of any of the immunities, rights, benefits, defenses or protections provided to University under governmental or sovereign immunity Jaws from time to time applicable to University, including, without limitation, the Colorado Governmental immunity Act (C.R.S. § 00-00-000, et seq.) and the Eleventh Amendment to the United States Constitution.
| University: | Licensee: | |
| By: /s/ Xxxxx X. Xxxxx | By: /s/ Xxxx Xxxxxxxxx | |
| Xxxxx X. Xxxxx | Xxxx Xxxxxxxxx | |
| Title: Associate Vice President | Title: President | |
| Date: 30NOV09 | Date: 1-12-2009 | |
| Office of Technology Transfer | ____________________ | |
| University of Colorado, 588 SYS | ____________________ | |
| Suite 100, 0000 Xxxxxx Xxxxxx | ____________________ | |
| Boulder, CO 80309 | ____________________ | |
| 16 |
APPENDIX A
Invention Disclosures
10031C --Methods and Uses to Alter Neuronal Stem Cell Fate
10037C -- Use of soluble Fas (CD95) in cyanoacrylate to promote wound healing and as a wound sealant (no patents filed under this number)
10038C -- Promotion of diabetic wound healing by incorporation of insulin in a cyanoacrylate wound sealant (no patents filed under this number)
10039C -- Use of soluble FasL in cyanoacrylate to promote wound healing and as a wound sealant (no patents filed under this number)
10040C --Wound healing promotion by delivery of colloidal silver in a cyanoacrylate wound sealant
2002.012C --Use of Low Frequency Microwave for Repair of Central Nervous System Injury Including Stroke, Trauma, Alzheimers, Xxxxxxxxx'x Disease, Multiple Sclerosis
2002.013C -- Use of Low Frequency Microwaves to Alter Plasma Membrane Potential in Cells in Diseases (no patents filed under this number)
2002.014C --Use of Low Frequency/Intensity Microwaves in Combination with Metallic Particles to Enhance the Effect of Microwaves on Electron Transport and Mitochondrial Function (no patents filed under this number)
2002.015C --Use of 2-deoxyglucose to Promote the Effectiveness of Low Frequency/Intensity Microwaves to Alter Mitochondrial Function (no patents filed under this number)
2002.016C --Use of Low Frequency Microwaves to Alter Electron Transport in Mitochondria of Wounds (no patents filed under this number)
2002.017C -- Use of Low Intensity Microwaves to Modulate Expression and Transport of Costimulatory Molecules (no patents filed under this number)
2002.018C --Use of Low Frequency/Intensity Microwaves as a Method for the Detection of Breast Cancer Cells (no patents filed under this number)
2002.019C --Use of Low Intensity Microwaves to Treat Type I and Type II Diabetic Neuropathies, Nephropathies, Pathologies of the Eye, Muscle Wasting, and Wound Healing (no patents filed under this number)
2002.020C --Use of Low Frequency Microwaves to Treat Cancers in Concert with Immunological Therapeutics (no patents filed under this number)
2002.021C --Use of Low Frequency Microwaves as a Method of Augmenting Traditional Chemotherapeutic Techniques and/or Traditional Radiation Therapy (no patents filed under this number)
2002.022C --Methods and Uses of Radio Protection Using Small Acidic Spore Protein and Human and Animal Analogs of Small Acidic Spore Protein (no patents filed under this number)
| 17 |
2002.023C -- Method of Promoting, Preventing, Sustaining, or Shortening Neural Synapse by Manipulation of Mitochondrial, Liposomal, or Plasma Membrane Uncoupled (no patents filed under this number)
2002.030C --Methods and Products to Lower, Increase, or Remove Cell Surface Costimulatory Molecules by Altering Levels of Intracellular Reactive Oxygen Intermediates (no active patents)
2002.031C --Use of Inhibitors or Promoters of 2, 4 dienoyi-CoA-reductase to Promote or Prevent Reactive Oxygen Production
2002.032C --Use of Inhibitors or Promoters of 2,4 denoyi-CoA-isomerase to Promote or Prevent Reactive Oxygen Production (no patents filed under this number)
2002.033C -- Use of Probiotic Delivery of Self Antigens for Immunological therapy in Autoimmune Diseases/Use of Various Strains of Probiotics to Determine Cytokine Bias (no patents filed under this number)
2002.034C -- Use of Oral Administration ofT Cell Receptor Peptides Expressed in Probiotic Delivery System to Induce Therapeutic Immune Responses as Indicated (no patents filed under this number)
2002.035C --Use of Ligands for B7.1, B7.2, CD28, or CTLA4 in Cyanoacrylates to Promote Wound Healing (no patents filed under this number)
2002.036C -- Use of Microwaves and Cyanoacrylate to Promote Wound Healing (no patents filed under this number)
2002.081C --Methods and Uses to Alter Neuronal Stem Cell Fate by Manipulation of Uncoupling Protein(s)
2002.119C --A Means of Preserving Organs, Tissue, and Stem Cells for Transplant, and for Improving the Success of Transplantation (no patents filed under this number)
CU1008C --Methods for Lowering Costimulatory Molecule Expression on Embryonic Stem Cells through Modulations of Oxidant Levels in the Cell with or without TCR Peptide or Other Peptide Vaccination (no patents filed under this number)
CU1014C --Method to Determine Rates of Cellular Division, Apoptosis, Oncosis or Growth Arrest through Use of Flow Cytometry (no patents filed under this number)
CU1047C --The use of saturated and polyunsaturated fatty acids to promote the effectiveness of gamma interferon as an immunogenic agent (no patents filed under this number)
CU1048C --A Diagnostic Screen of Peripheral Blood as an Indicator of Metabolic Dysfunction and Consequent Altered Immunity Disease (no patents filed under this number)
CU1049C -- Use of polyphosphorylated nucleotides to change metabolic base states in cells (no patents filed under this number)
CU1050C --Methods and uses to induce or inhibit translocation of uncoupling proteins to and from cellular sites including plasma membrane organelles (no patents filed under this number)
| 18 |
CU1063C --Use of Fas Engagement and Selective Fatty Acids to Promote or Prevent Cell Viability Through Induction of Active Uncoupling Proteins Activity (no patents filed under this number)
CU1065C -- Microinjection of Mitochondria Into Stem Cells, Growing Cells, or Dysfunctional Cells as a Therapeutic Manipulation in Diseases (no patents filed under this number)
CU1081C --Biochemical Process for the Separation of Toxic Substances, Including Heavy Metals Contained in Circuit Boards (no active patents)
CU1098C --A Therapeutic Approach to Pathogenesis of Down Syndrome (no patents filed under this number)
CU1099C --Blood Test for Early Detection of Down Syndrome (no patents filed under this number)
CU1105C -- Micro/Nano Devices and Nanoparticles and Implants for Detecting and Altering Cellular and Tissue Metabolism (no patents filed under this number)
CU1125C --Methods and uses to protect or destroy nuclear DNA by manipulations of a newly discovered nuclear membrane uncoupling protein (UCP) or uncoupling-like protein/molecule (no patents filed under this number)
CU1128C --NUCLEI: Methods and Uses to Protect or Destroy Nuclear DNA by Manipulations of a Newly Discovered Nuclear Membrane Uncoupling Protein (UCP) or Uncoupling-Like Protein Molecule (no patents filed under this number)
CU1138C -- Novel Therapies for Tissue Repair, Remodeling, Reconstruction and Regeneration
CU1244C --Methods and Uses to Kill Tumor Cells In Vivo Using a Toxin Derived from the Black Widow Spider, Latrotoxin (no patents filed under this number)
CU1245C --The Use of Genistein to Supplement the Activity of Chemotherapeutic and Metabolic Modifying Agents (no patents filed under this number)
CU1246C --Use of Myxococcus Xanthus to Eliminate the Environmental Toxicity of Methane Production by Methanobacteria (no patents filed under this number)
CU1248C --Computer Software for Improved Flow Cytometric Data Analysis (no active patents)
CU1249C -- Cybenetic Biovitiation (no patents filed under this number) CU1344C --Array for Metabolic Analysis (no active patents)
CU1518C -- Myxococcus xanthus derived oncolytic agent (no patents filed under this number)
CU1696H --Induction of apoptosis in tumor cells (no patents filed under this number)
CU1715C --Metabolic Modifications in Plants as a Mechanism for Increasing Plant derived Ethanol or Oil for Fuel Production
CU1968C --Inhibition of Fatty Acid Oxidation (FAO) to potentiate drug sensitivity in breast cancer
CU2407H --Systems and Methods for Treating Human Inflammatory and Proliferative Diseases and Wounds, with a Combination of Chloroquine and a Glycolytic Inhibitor
| 19 |
CU2421H --Proteins for Use in Diagnosing and Treating Infection and Disease
Patents
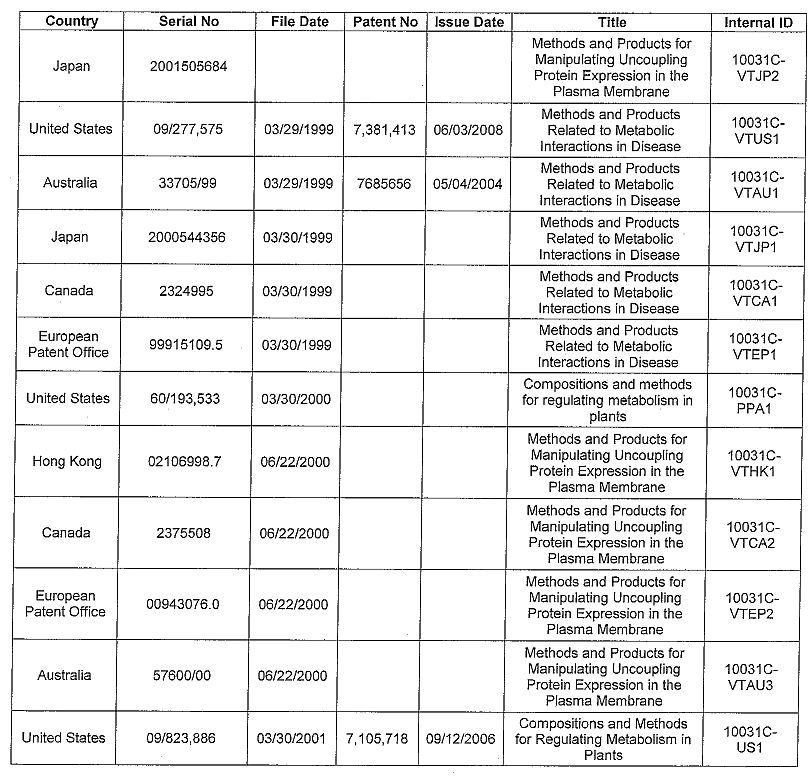
| 20 |
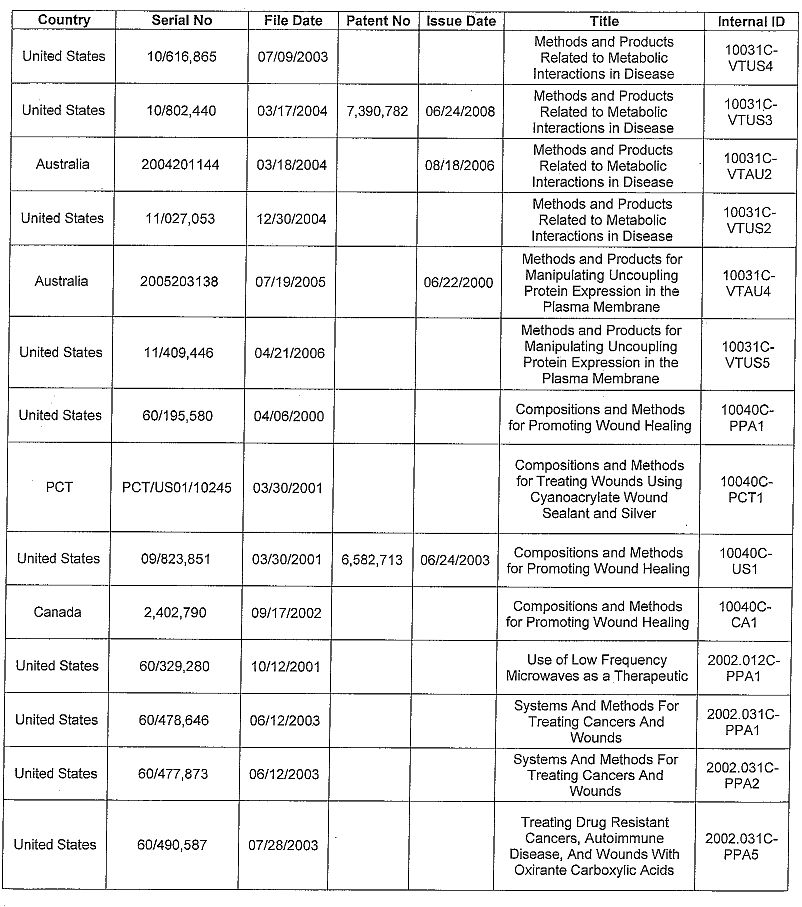
| 21 |

| 22 |

| 23 |

| 24 |

| 25 |
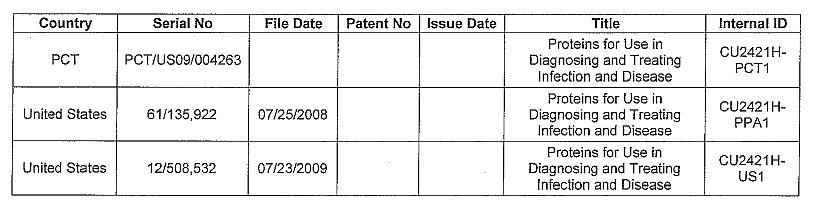
| 26 |
APPENDIX B
Royalties
1. License Fee
Licensee agrees to pay, within thirty (30) days of the Effective Date, a one time license fee of one-hundred fifty-thousand dollars ($150,000.00) which may be paid, at Licensee's discretion, as cash or as an equity grant. If Licensee chooses to grant University's duly appointed designee, University License Equity Holdings, Inc. (ULEHI) equity in consideration for the license fee, such equity consideration shall be paid as follows:
Fifty-five percent (55%) of such equity shall be issued to the University License Equity Holdings, Inc. (ULEHI) pursuant to a stock subscription or other agreement acceptable to ULEHI.
Forty-five percent (45%) of such equity shall be issued to the University of Vermont State and Agricultural College (Vermont) pursuant to a stock subscription or other agreement acceptable to Vermont.
Any such equity grant shall be equivalent in value to the license fee at the closing price of Viral Genetics on the date of this agreement.
2. Minimum annual royalty:
Twenty Five Thousand Dollars ($25,000.00) in each calendar year starting January 1, 2010 and annually thereafter until first commercial sales occur.
Seventy Five Thousand Dollars ($75,000.00) in each calendar year after first commercial sales occur.
3. Earned royalty:
Three Percent (3.0%) of Net Sales from sales in developed countries, and One Half Percent (0.5%) from sales in underdeveloped countries. Underdeveloped countries shall mean any country which, at the time the sale occurs, meets the definition of a developing country as defined by the World Bank. Developed countries shall mean all countries other than underdeveloped countries as defined by the World Bank.
4. Benchmark royalties as follows:
Provided that a patent has issued on the subject matter, the following amounts shall be paid to University within ninety (90) days of the following events, where "indication" refers to the use of a drug for treating a particular disease:
| 1. | Thirty Five Thousand Dollars ($35,000.00) upon acceptance of each Investigational New Drug Application (INDA) with the Food and Drug Administration (FDA) or with the European Agency for the Evaluation of Medicinal Products (EMEA). |
| 2. | One Hundred Thousand Dollars ($100,000.00) within ninety (90) days of each first indication at the initiation of Phase I. |
| 3. | Two Hundred Thousand Dollars ($200,000.00) within ninety (90) days of each first indication at the initiation of Phase II |
| 4. | Three Hundred Thousand Dollars ($300,000.00) within ninety (90) days of each first indication at the initiation of Phase III |
| 5. | Five Hundred Thousand Dollars ($500,000.00) within ninety (90) days of FDA approval of a first indication. |
| 27 |
| 6. | One-half of all aforementioned milestones for each second and subsequent indications. |
5. Payment of sublicensing royalties as follows:
Sublicensing royalties payable to the University shall be, with respect to each sublicense, in accordance with the following:
| 1. | For sublicense agreements executed within the first twelve (12) months, fifty percent (50%) of Sublicense Income. |
| 2. | For sublicense agreements executed in the second and third years after the Effective Date, thirty-five percent (35%) of Sublicense Income. |
| 3. | For sublicense agreements executed after the third year after the Effective Date, twenty percent (20%) of Sublicense Income. |
| 28 |
APPENDIX C
Due Diligence
Licensee shall use commercially reasonable efforts to develop, manufacture, market and sell the Licensed Products and Licensed Processes in the Field of Use and Territory in accordance with the below milestones.
a. Licensee shall show evidence of the initiation of at least two Active Programs in (2) types of cancers (e.g., Glioblastoma multiform, melanoma, non-small cell lung cancer, etc.) and at least one (1) plant or biofuels or non therapeutic field within three (3) years of the Effective Date.
b. On or before six months from the Effective Date of this Agreement, Licensee shall secure at least half time employment of an officer level employee, or engage a senior outside consultant, with drug development experience to oversee clinical trials in the Field of Use.
c.Licensee shall reach a pre-clinical stage by September 15, 2010.
d.Licensee shall file its first IND by September 15, 2011.
e. Licensee shall be at Phase I by September 15, 2012.
f. Licensee shall be at Phase Ill by September 15, 2015, but shall be afforded more time if such is due to the nature of the requirements met in Phase I or II.
| 29 |
APPENDIX D
Diligence Report
Licensee shall submit the Diligence Report and mark it as confidential as provided in Section 7 of the Agreement. The Diligence Report shall include the following:
| a. | Date development plan initiated and time period covered by this report. |
| b. | Development Report (4-8 paragraphs) |
1. Activities completed since last report including the object and parameters of the development, when initiated, when completed and the results.
2. Activities currently under investigation, i.e., ongoing activities including object and parameters of such activities, when initiated, and projected date of completion.
| c. | Future development activities (4-8 paragraphs) |
1. Activities to be undertaken before next report including, but not limited to, the type and object of any studies conducted and their projected starting and completion dates.
2. Estimated total development time remaining before a product will be commercialized.
| d. | Changes to initial development plan (2-4 paragraphs) |
1. Reasons for change.
2. Variables that may cause additional changes.
3. Rationale for delay in or termination of development plans or product lines, including financial, strategic, and legal reasons.
4. Rationale for non-termination of Agreement or retention of Field of Use
| e. | Items to be provided if applicable: |
1. If the Licensee receives funding from the State of Colorado,. including funds received through the University of Colorado, Licensee shall provide a summary of capital investments and an employee growth chart (# of FTEs, etc.)
2. Information relating to Product that has become publicly available, e.g., published articles, competing products, patents, etc.
3. Development work being performed by third parties other than Licensee to include name of third party, reasons for use of third party, planned future uses of third parties including reasons why and type of work.
4. Update of competitive information trends in industry, government compliance (if applicable) and market plan.
5. Information and copies of relevant materials evidencing the status of any patent applications or other protection relating to Licensed Products, Licensed Processes, or the Licensed Patents.
| 30 |
APPENDIX E
Form of Royalty Report

| 31 |
APPENDIX F
Material Transfer Agreement Template
THIS MATERIAL TRANSFER AGREEMENT, together with its Attachments, (the "Agreement") is made and entered into this 22nd day of November, 2009 (the "Effective Date") by and between the Regents of the University of Colorado, a body corporate, having its principal office at 0000 Xxxxx Xxxxxx, 0xx Xxxxx, Xxxxxx, XX 00000 (hereinafter "University") and the Institution receiving the Material, ___________________ the "Recipient").
SECTION 1. DEFINITIONS
| 1.1 | "Commercial Purposes" shall mean the sale, lease, license, or other transfer of the Material or Modifications to a for-profit organization. Commercial Purposes shall also include uses of the Material or Modifications by any organization, including Recipient, to perform contract research, to screen compound libraries, to produce or manufacture products for general sale, or to conduct research activities that result in any sale, lease, license, or transfer of the Material or Modifications to a for-profit organization. However, industrially sponsored academic research shall not be considered a use of the Material or Modifications for Commercial Purposes per se, unless any of the above conditions of this definition are met. |
| 1.2 | "Material" or "Materials" shall mean all material provided to Recipient, Progeny, Unmodified Derivatives, and any modification to Material, if such modified Material is substantially based on or incorporates a substantial element of original Material), or any modification that is not new or not obviously distinct from the original Material. |
| 1.3 | "Modifications" shall mean substances created by Recipient that do not contain/incorporate the Material. |
| 1.4 | "Progeny" shall mean unmodified descendant from the Material, such as virus from virus, cell from cell, organism from organism. |
| 1.5 | "Unmodified Derivatives" shall mean substances created by Recipient that constitute an important unmodified functional sub-unit of the Original Material. |
| 32 |
SECTION 2. OWNERSHIP, USE AND TRANSFER
| 2.1 | Ownership of Material: Legal title to the Material shall remain with University. Nothing in this Agreement grants any rights under any patents or in any know-how of University nor any rights in the Material or any product or process related thereto or derived therefrom other than those rights specifically set forth herein. Except as expressly provided in this Agreement, no rights are provided to Recipient under any patents, patent applications, copyrights, trade secrets or other proprietary rights of University. In particular, no rights are provided to use the Material or any related patents of University for any profit-making or Commercial Purposes. |
| 2.2 | Use of Material: University will use commercially reasonable efforts to provide Recipient with the quantity of the material described in Exhibit A. Recipient will use the Material exclusively for the non-commercial research described in Exhibit A and for no other purpose. The Research will be conducted solely by or under the direction of the designated investigator at Recipient's research facilities (the "Investigator"). In addition, Recipient shall only allow employees and agents underi ts direct control and supervision to have access to the Material. Recipient will not use the Material for testing in or treatment of human subjects. Recipient agrees to use the Material in compliance with all applicable laws, governmental regulations, and guidelines. The Material will not be distributed further to third parties for any purpose without the prior written consent of University. |
| 2.3 | Transfer of Material: |
| a. | Recipient shall have the right, without restriction, to distribute substances created by Recipient through the use of the Material only if those substances do not include the Material or Modifications that incorporate the Material. Recipient shall not attempt to reverse engineer, deconstruct, or in any way determine the structure or composition of the Material. |
| b. | Upon written permission from University, Recipient may distribute Modifications that incorporate Material for commercial use. It is recognized by Recipient that such commercial use may require a license from University and University has no obligation to grant such a license. Nothing in this paragraph, however, shall prevent Recipient from granting commercial licenses under Recipient's patent rights claiming Modifications as defined herein. |
SECTION 3. INVENTIONS
| 3.1 | Tangible Property: Ownership of tangible property between University and Recipient, as may or may not be applicable, is defined in Exhibit A. |
| 33 |
| 3.2 | Disclosure: Recipient will promptly and fully disclose in writing to University any and all Inventions (as defined herein), know-how and other rights (whether or not protectable under state, federal, or foreign intellectual property Jaws) related to the Material or its use, or developed using the Material, which are conceived and/or reduced to practice by Recipient, alone or jointly with others, in the course of its research (the "Inventions"). Recipient shall be free to file patent applications claiming Inventions made through the use of the Material but agrees to notify University in advance of such filing if it files patent applications claiming Modifications that incorporate or use the Material in any way. |
| 3.3 | lnventorship: lnventorship shall be determined according to U.S. patent law. |
| 3.4 | Joint Inventions: Any patent applications necessary to protect the proprietary positions of the parties in any Inventions made jointly by University and Recipient shall be prepared, filed and prosecuted by University, jointly in its and Recipient's names, with expenses shared equally by the parties. If University elects not to prepare, file, prosecute or maintain an application or patent arising from any joint Invention, University shall promptly notify Recipient, and Recipient shall have the right to prepare, file, prosecute and maintain such applications or patents, in Recipient's and University's names, and at Recipient's expense. Subject only to Recipient's grant of an option to University under Section 4, each of University and Recipient's shall have the right to license, transfer, and/or sell its rights in such joint inventions without the consent of the other. |
| 3.5 | Recipient's Sole Inventions: Any patent applications necessary to protect the proprietary positions of the parties in any Inventions made solely by Recipient shall be prepared, filed and prosecuted by Recipient, solely in Recipient's name, with the expenses paid by Recipient. If Recipient elects not to prepare, file, prosecute, or maintain an application or patent arising from any sole Inventions, Recipient shall promptly notify University, and University shall have the right to prepare, file, prosecute, and maintain such applications or patents, in Recipient's name and at University's expense. In return for University's support of patent prosecution and maintenance, the parties agree to good faith negotiation regarding University's share of any and all consideration received under any licenses resulting from such patentable subject matter. |
| 3.6 | Patent Cooperation: Each party shall provide the other party with copies of all substantive communications from all patent offices regarding applications or patents on any joint Inventions and Recipient's sole Inventions promptly after the receipt thereof. Each party shall provide the other party with copies of all proposed substantive communications to such patent offices regarding applications or patents on any such Inventions in sufficient time before the due date in order to enable the other party an opportunity to comment on the content thereof. Each party shall make available to the other party or its authorized attorneys, agents, or representatives, such of its employees whom the other party in its reasonable judgment deems necessary in order to assist it in obtaining patent protection for such Inventions. Each party shall sign or use its best efforts to have signed all legal documents necessary to file and prosecute patent applications or to obtain or maintain patents at no cost to the other party. |
| 3.7 | Reimbursement of Patent Expenses: In the event University exercises its option and executes an exclusive license to Recipient's interest in an Invention under Section 4 herein, University shall reimburse Recipient for its reasonable patent expenses related to such Invention and thereafter, the prosecution and maintenance of such patent applications and patents shall be as provided in the license agreement. |
| 34 |
SECTION 4. COMMERCIAL USE
| 4.1 | Option for Exclusive License:· Recipient hereby grants to University an option to obtain an exclusive, royalty-bearing license to Recipient's interests in all Inventions. For each Invention, University's option must be exercised within ninety (90) days of the written disclosure of that Invention to University by Recipient. The royalty rates payable for the exclusive license to such Inventions, together with the other terms thereof, will be negotiated by the parties in good faith. If any prior agreements between Recipient and a third party would preclude Recipient from granting an exclusive license to University, then such license shall include the broadest possible rights licensable to University. |
| 4.2 | Commercial License to Material: If Recipient desires to use the Material for profit making or commercial purposes, Recipient agrees, in advance of such use, to negotiate in good faith with University to establish the terms of a commercial license. It is understood by Recipient that University shall have no obligations to grant such a license to Recipient, and may grant exclusive or non-exclusive commercial licenses to others. In consideration of University's supporting those costs and supplying the Material, Recipient hereby grants University a ninety (90) day period (after the filing of a US patent application claiming the Invention or Modification or after the supply of a sample of the Modification if no patent application is to be filed) to negotiate the terms of a worldwide commercial license. Such a license shall include a reasonable royalty based on the respective parties' contributions and relevant industry standards and, subject to University's policies, shall include such terms as are typical in licenses of similar technology from non profit organizations to for-profit organizations. |
SECTION 5. NOTIFICATION OF RISKS
| 5.1 | University shall inform Recipient of any toxicity, health risks, etc. associated with the Material that are reasonably known to University. Recipient's Investigator shall inform University of any toxicity, health risks, etc. discovered through the use of the Material. |
SECTION 6. WARRANTIES. INDEMNIFICATION, INSURANCE
| 6.1 | Any Material delivered pursuant to this Agreement is understood to be experimental in nature, and will be used with prudence and appropriate caution, since not all of its characteristics are known. |
| 6.2 | Negation of Warrantees: UNIVERSITY MAKES NO REPRESENTATIONS AND EXTENDS NO WARRANTIES, EITHER EXPRESS OR IMPLIED. THE MATERIAL IS PROVIDED WITHOUT WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED OR THAT THE USE OF THE BIOLOGICAL MATERIALS WILL NOT INFRINGE ANY PATENT, COPYRIGHT, TRADEMARK, OR ANY OTHER PROPRIETARY RIGHT. |
| 35 |
| 6.3 | Indemnification: Recipient agrees to indemnify University for liability, loss, or damage they may suffer as a result of claims, demands, costs or judgments against University arising out of the activities to be carried out pursuant to this Agreement. |
| 6.4 | Insurance: Recipient shall obtain general liability insurance, including product liability insurance, on such terms and in such amounts as are reasonable and customary within its industry. |
SECTION 7. RECIPIENT'S ASSUMPTION OF LIABILITY
| 7.1 | Assumption of Liability: To the extent permitted by applicable law, Recipient assumes all liability for damages that may arise from its use, storage, or disposal of the Material. University shall not be liable to Recipient for any loss, claim or demand made by the Recipient, or made against the Recipient by any other party, due to or arising from the use, storage or handling of the Material by Recipient, except to the extent caused by the gross negligence or willful misconduct of the University. |
SECTION 8. PUBLICATION RIGHTS
| 8.1 | This Agreement shall not be interpreted to prevent or delay publication of research resulting from the use of the Material or Modifications. Recipient shall have the right to publish and disclose the results of the Research. |
| 8.2 | In order to balance this right with the University's proprietary interests, Recipient shall submit any proposed disclosure or publication to University for its review at least thirty (30) days prior to the earlier of the date of submission to any journal for review or the date of publication or disclosure. University shall complete its review within thirty (30) days of receipt of the submitted documents. University may require that Recipient delete from its documents any reference to the University's confidential information. |
| 8.3 | If, during the thirty (30) day review period, University notifies Recipient that it desires to file a patent application on any Inventions disclosed in the documents, Recipient will defer publication/disclosure for up to sixty (60) additional days from the date of submission of the document to permit University to prepare and file a patent application.Recipient's Investigator agrees to provide copies of research results related to the Material and appropriate acknowledgment of University as the source of the Material in all publications. |
SECTION 9. TERM AND TERMINATION
| 9.1 | This Agreement shall terminate on the earliest of the following dates: |
| a. | when the Material becomes generally available from third parties, for example, through reagent catalogs or from a public repository; |
| 36 |
| b. | on completion of Recipient's current research with the Material; |
| c. | on thirty (30) days written notice by either party to the other; or |
| d. | one year from the last signature date. |
| 9.2 | If termination should occur as a result of 9.1(a), Recipient shall be bound to the University by the least restrictive terms applicable to Material obtained from the then-available sources. |
| 9.3 | Upon termination of this Agreement, Recipient shall discontinue its use of the Material and shall, upon direction of University, return or destroy any remaining Material. Recipient shall also either destroy Modifications or remain bound by the terms of this Agreement as they apply to Modifications. |
| 9.4 | In the event University terminates this Agreement for its convenience, University will defer the effective date of termination for a period of up to one (1) year, upon request from Recipient to complete research in progress. |
SECTION 10. REIMBURSEMENT
| 10.1 | The Material is provided for a fee, which is solely to reimburse University for its production and distribution costs. The amount of the fee is __________ U.S. dollars ($), due contemporaneously upon execution of this Agreement. At the request of the University, Recipient shall provide its courier name and account number to University in advance of distribution of Material. Courier Name: __________ Billing account number: _________________. |
SECTION 11. CONFIDENTIALITY
| 11.1 | Recipient agrees to use reasonable efforts (which shall be at least as great as the efforts to maintain the confidentiality of its own confidential information) to maintain the Material and information in confidence, and to use the same only in accordance with this Agreement. Such obligation of confidentiality shall not apply to information that Recipient can demonstrate: |
| a. | was at the time of disclosure in the public domain; |
| b. | has come into the public domain after disclosure through no fault of Recipient or its employees or agents; |
| c. | was known to Recipient or its employees prior to disclosure thereof by University; |
| x. | was lawfully disclosed to Recipient without prior obligation of confidence by a third party that was not under an obligation of confidence to Recipient with respect thereto; |
| e. | was independently developed without use of or reference to the confidential material or |
| f. | is required to be disclosed by law, government regulation, or court order, and is disclosed only to the extent it satisfies that requirement. |
| 37 |
| 11.2 | Recipient acknowledges that University is subject to the Colorado Open Records Act (C.R.S. § 00-00-000, et seq.). All documents marked "Confidential" shall be treated by University as confidential to the extent permitted under§ 00-00-000. |
SECTION 12. GENERAL
| 12.1 | Assignment: This Agreement is not assignable, whether by operation of law or otherwise, and sets forth the entire agreement and understanding of the parties and cannot be changed or amended except by written agreement executed by both parties. |
| 12.2 | Notice: Notice hereunder shall be deemed sufficient if given by registered mail, postage prepaid, and addressed to the Party to receive such notice at the address given below, or such other address as may hereafter be designated by notice in writing. |
| University: | Recipient: | ||
| License Administrator | __________________ | ||
| Office of Technology Transfer | __________________ | ||
| University of Colorado, 588 SYS | __________________ | ||
| Suite 100, 0000 Xxxxxx Xxxxxx | __________________ | ||
| Boulder, CO 80309 | __________________ |
| 12.3 | Use of Names and Marks: Recipient agrees not to identify University in any promotional advertising, press releases, sales literature or other promotional materials to be disseminated to the public or any portion thereof without University prior written consent in each case, except that Recipient may state that it has an agreement with University. Recipient further agrees not to use the name of University or any University faculty member, inventor, employee or student or any trademark, service mark, trade name, copyright or symbol of University, without the prior written consent of the University, entity or person whose name is sought to be used. |
| 12.4 | Marking: Recipient agrees to: |
| a. | Cause any products which may be sold as a result of this MTA to be marked with the notice of the patent numbers or patent pending, as may be appropriate. |
| b. | Comply with all laws and regulations of the United States and any other country as appropriate concerning or controlling the import or export of the Licensed Products, data, software, laboratory prototypes or other commodities. University makes no representation that a license or consent for export will not be required by applicable governmental agencies, or if required, that it will be issued. |
| 38 |
| c. | Comply with all applicable statutes, regulations, and guidelines, including applicable governmental regulations, policies, and guidelines in its use of any University-supplied materials. Recipient agrees not to use the materials for research involving human subjects or clinical trials in the United States without complying with 21 C.F.R. Part 50 and 45 C.F.R. Part 46 (as those regulations may be amended from time to time). Recipient agrees not to use the materials for research involving human subjects or clinical trials outside of the United States without notifying University in writing, of such research or trials and complying with the applicable regulations of the appropriate national control authorities. Written notification to University of research involving human subjects or clinical trials outside of the United States shall be given no later than sixty (60) days prior to commencement of such research or trials. |
| 12.5 | Compliance with the Law: Recipient shall comply with all commercially material local, state, federal, and international laws and regulations relating to its obligations under this Agreement regarding the development, manufacture, use, and sale of any products incorporating the Materials. |
| 12.6 | Choice of Law: This Agreement shall be governed by and construed in accordance with the laws of the State of Colorado. |
| 12.7 | Dispute Resolution: In the event of any dispute arising out of or relating to this Agreement, the affected Party shall promptly notify the other Party ("Notice Date"), and the Parties shall attempt in good faith to resolve the matter. |
| a. | Any disputes not so resolved shall be referred to senior executives, who shall meet at a mutually acceptable time and location within thirty (30) days of the Notice Date and shall attempt to negotiate a settlement. |
| b. | If the senior executives fail to meet within thirty (30) days of the Notice Date, or if the matter remains unresolved for a period of sixty (60) days after the Notice Date, the Parties hereby irrevocably submit to the jurisdiction of a court of competent jurisdiction in the State of Colorado, and, by execution and delivery of this Agreement, each (i) accepts, generally and unconditionally, the jurisdiction of such court and any related appellate court, and (ii) irrevocably waives any objection it may now or hereafter have as to the venue of any such suit, action or proceeding brought in such court or that such court is an inconvenient forum. |
| 12.8 | Merger and Modification of Agreement: The terms and provisions contained in this Agreement constitute the entire Agreement between the Parties and shall supersedeall previous communications, representations, agreements or understandings, either oral or written, between the Parties hereto with respect to the subject matter hereof, and no agreement or understanding varying or extending this Agreement will be binding upon either Party hereto, unless in writing which specifically refers to this Agreement, signed by duly authorized officers or representatives of the respective Parties, and the provisions of this Agreement not specifically amended thereby shall remain in full force and effect according to their terms. |
| 12.9 | Severability: The provisions and clauses of this Agreement are severable, and in the event that any provision or clause is determined to be invalid or unenforceable under any controlling body of the law, such invalidity or unenforceability will not in any way affect the validity or enforceability of the remaining provisions and clauses hereof. |
| 39 |
| 12.10 | Scope: This Agreement does not establish a joint venture, agency, or partnership between the Parties, nor create an employer- employee relationship. |
| 12.11 | Preservation of Immunity: The Parties agree that nothing in this Agreement is intended or shall be construed as a waiver, either express or implied, of any of the immunities, rights, benefits, defenses or protections provided to University under governmental or sovereign immunity laws from time to time applicable to University, including, without limitation, the Colorado Governmental Immunity Act (C.R.S. § 00-00-000, et seq.) and the Eleventh Amendment to the United States Constitution. |
| 12.12 | Headings: Headings are included herein for convenience only and shall not be used to construe this Agreement. |
| 12.13 | Survival. The provisions of Sections 3 Inventions, 4 Commercial Use, 6 Warranties, Indemnifications and Insurance, 7 Recipient's Assumption of Liability, 8 Publication Rights, 11 Confidentiality, 12.11 Preservation of Immunity, 12.13 Survival, , and any other provision of this Agreement that by its nature is intended to survive, shall survive any termination or expiration of this Agreement. |
| University: | Recipient: | |
| By: ____________________ | By: ____________________ | |
| Title: ____________________ | Title: ____________________ | |
| Date: ___________________ | Date: ___________________ | |
| Office of Technology Transfer | ________________________ | |
| University of Colorado, 588 SYS | ________________________ | |
| Suite 100, 0000 Xxxxxx Xxxxxx | ________________________ | |
| Boulder, CO 80309 | ________________________ |
Recipient’s Investigator:
___________________________________
| 40 |
EXHIBIT A
Materials, Contact Information and Research Plan
Materials:
Amount of Materials to Be Provided:
Facilities Address:
_____________________________________
_____________________________________
_____________________________________
Investigator:
Name: ______________________________
Title: _______________________________
Address: _____________________________
____________________________
Phone: ______________________________
Fax: ________________________________
Email: _______________________________
Account Number of FedEx or DHL: ___________________________
Research Plan:
Title:
Aims:
Research Plan:
Anticipated Research Completion Date: _______________, 2000_
| 41 |
EXHIBIT B
University describes any preexisting obligations that University has to third parties (other than the federal government or non-profit foundations) that would affect Recipient.
| 42 |
