LICENSE AGREEMENT BY AND BETWEEN CUBIST PHARMACEUTICALS, INC. AND CHIRON HEALTHCARE IRELAND LTD. October 2, 2003
Exhibit 10.1
BY AND BETWEEN
CUBIST PHARMACEUTICALS, INC.
AND
CHIRON HEALTHCARE IRELAND LTD.
October 2, 2003
TABLE OF CONTENTS
|
|
Page |
|
|
|
|
|
|
ARTICLE 1. DEFINITIONS |
|
2 |
|
1.1. “Additional Daptomycin Product” |
|
2 |
|
1.2. “Affiliate” |
|
2 |
|
1.3. “Approved New Trademark” |
|
2 |
|
1.4. “Chiron Data” |
|
2 |
|
1.5. “Chiron Development Plan” |
|
2 |
|
1.6. “Chiron Interest” |
|
2 |
|
1.7. “Chiron Inventions” |
|
2 |
|
1.8. “Chiron Joint Technology Rights” |
|
2 |
|
1.9. “Chiron Know-How” |
|
3 |
|
1.10. “Chiron Marketing Information” |
|
3 |
|
1.11. “Chiron Patent” |
|
3 |
|
1.12. “Chiron Related Know-How” |
|
3 |
|
1.13. “Chiron Related Patent” |
|
3 |
|
1.14. “Chiron Technology” |
|
3 |
|
1.15. “Commercial Launch” |
|
3 |
|
1.16. “Commercialize” |
|
4 |
|
1.17. “Commercially Important Indications” |
|
4 |
|
1.18. “Commercially Reasonable Efforts” |
|
4 |
|
1.19. “Confidential Information” |
|
4 |
|
1.20. “Contract Manufacturing Agreements” |
|
4 |
|
1.21. “Control” |
|
5 |
|
1.22. “cSSSI” |
|
5 |
|
1.23. “Cubist Data” |
|
5 |
|
1.24. “Cubist Development Plan” |
|
5 |
|
1.25. “Cubist Inventions” |
|
5 |
|
1.26. “Cubist Joint Technology Rights” |
|
5 |
|
1.27. “Cubist Know-How” |
|
5 |
|
1.28. “Cubist Marks” |
|
5 |
|
1.29. “Cubist Patent” |
|
5 |
|
1.30. “Cubist Technology” |
|
5 |
|
1.31. “Daptomycin” |
|
6 |
|
1.32. “Daptomycin IV Product” |
|
6 |
|
1.33. “Defective Manufactured Product” |
|
6 |
|
1.34. “Directly Competitive Product” |
|
6 |
|
1.35. “Dollar” |
|
6 |
|
1.36. “Drug Approval Application” |
|
6 |
|
1.37. “Drug Master File,” |
|
6 |
|
1.38. “Effective Date Third Party Licenses” |
|
6 |
|
1.39. “EMEA” |
|
6 |
|
1.40. “Fair Market Value” |
|
6 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
i
|
1.41. “FDA” |
|
6 |
|
1.42. “Global Harm” |
|
7 |
|
1.43. “IND” |
|
7 |
|
1.44. “Indemnify” |
|
7 |
|
1.45. “Information” |
|
7 |
|
1.46. “Infringement” |
|
7 |
|
1.47. “Injection” |
|
7 |
|
1.48. “Joint Coordination Team” |
|
7 |
|
1.49. “Joint Inventions” |
|
7 |
|
1.50. “Joint Know-How” |
|
7 |
|
1.51. “Joint Patents” |
|
7 |
|
1.52. “Joint Technology” |
|
7 |
|
1.53. “Key Development Studies” |
|
7 |
|
1.54. “Knowable Patent” |
|
7 |
|
1.55. “Launch Indication(s)” |
|
7 |
|
1.56. “Licensed Products” |
|
7 |
|
1.57. “Lilly” |
|
8 |
|
1.58. “Lilly License” |
|
8 |
|
1.59. “Lilly Patent” |
|
8 |
|
1.60. “Losses” |
|
8 |
|
1.61. “MAA” |
|
8 |
|
1.62. “Major Market Countries” |
|
8 |
|
1.63. “Manufacturing Information” |
|
8 |
|
1.64. “Manufacturing Plan” |
|
8 |
|
1.65. “Marketing Plan” |
|
8 |
|
1.66. “Marketing Plan Trigger Event” |
|
8 |
|
1.67. “Medical Affairs Studies” |
|
8 |
|
1.68. “NDA” |
|
8 |
|
1.69. “Net Sales” |
|
8 |
|
1.70. “Other Licensee” |
|
9 |
|
1.71. “Other Licensee Data” |
|
9 |
|
1.72. “Patent” |
|
10 |
|
1.73. “Price Approval” |
|
10 |
|
1.74. “Primary Endpoint” |
|
10 |
|
1.75. “Reasonable Buyer” |
|
10 |
|
1.76. “Reciprocating Licensee” |
|
11 |
|
1.77. “Regulatory Approval” |
|
11 |
|
1.78. “Regulatory Authority” |
|
11 |
|
1.79. “Regulatory Plan” |
|
11 |
|
1.80. “Replacement Indication” |
|
11 |
|
1.81. “Replacement Indication Study” |
|
11 |
|
1.82. “Replacement Study” |
|
11 |
|
1.83. “Required Study” |
|
11 |
|
1.84. “Restricted Period” |
|
11 |
|
1.85. “Royalty Rate” |
|
11 |
|
1.86. “Stock Purchase Agreement” |
|
11 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
ii
|
1.87. “Supply Agreement” |
|
11 |
|
1.88. “Term” |
|
11 |
|
1.89. “Territory” |
|
12 |
|
1.90. “Territory Specific Studies” |
|
12 |
|
1.91. “Third Party” |
|
12 |
|
1.92. “Third Party Infringement Claim” |
|
12 |
|
1.93. “Transfer Price” |
|
12 |
|
1.94. “Unlicensed Product” |
|
12 |
|
1.95. “Unlicensed Sales Threshold” |
|
12 |
|
1.96. “Valid Claim” |
|
12 |
|
|
|
|
|
ARTICLE 2. PRODUCT RIGHTS AND RIGHTS OF FIRST NEGOTIATION |
|
12 |
|
2.1. Product Rights |
|
12 |
|
2.2. Licenses to Chiron |
|
12 |
|
2.3. Licenses to Cubist |
|
13 |
|
2.4. Existing Related Intellectual Property of Chiron |
|
14 |
|
2.5. Field |
|
14 |
|
2.6. Use of Patents and Know-How |
|
14 |
|
2.7. Exclusivity |
|
14 |
|
(a) Chiron |
|
14 |
|
(b) Cubist |
|
15 |
|
2.8. Sublicenses and Transfers of Chiron Technology or Chiron Joint Technology Rights |
|
15 |
|
2.9. Rights of First Negotiation |
|
15 |
|
2.10. Right of Co-Negotiation |
|
15 |
|
2.11. Marketing a Directly Competitive Product |
|
16 |
|
2.12. Mechanism to Determine Fair Market Value |
|
17 |
|
2.13. Subcontracting |
|
17 |
|
2.14. Rights of Last of Refusal for Canada |
|
18 |
|
2.15. Applicable Supply Agreement Provisions |
|
18 |
|
|
|
|
|
ARTICLE 3. COORDINATION |
|
19 |
|
3.1. General |
|
19 |
|
3.2. Joint Coordination Team |
|
20 |
|
(a) Formation |
|
20 |
|
(b) Membership |
|
20 |
|
(c) Meetings |
|
20 |
|
(d) Specific Activities |
|
20 |
|
(e) Limited Authority; Not a Decision-Making Body |
|
21 |
|
(f) Meeting Agendas |
|
22 |
|
3.3. JCT Coordinators |
|
22 |
|
3.4. Independence |
|
22 |
|
|
|
|
|
ARTICLE 4. DEVELOPMENT |
|
22 |
|
4.1. Key Development Studies; Diligence Obligations |
|
22 |
|
4.2. Cubist Development Plan |
|
24 |
|
4.3. Development by Chiron |
|
25 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
iii
|
(a) Territory Specific Studies |
|
25 |
|
(b) Proposed Indications |
|
25 |
|
(c) Medical Affairs Studies |
|
25 |
|
(d) Chiron Development Plan |
|
26 |
|
(e) Limitation on Chiron’s Development Rights |
|
26 |
|
4.4. Access to Chiron Data |
|
27 |
|
(a) Information Sharing |
|
27 |
|
(b) Reciprocating Licensee |
|
27 |
|
(c) License Option |
|
27 |
|
4.5. No Debarred Personnel |
|
28 |
|
4.6. Chiron Compliance |
|
28 |
|
|
|
|
|
ARTICLE 5. REGULATORY |
|
28 |
|
5.1. Regulatory Plan; Diligence Obligation |
|
28 |
|
(a) Cubist’s and Other Licensees’ Activities |
|
28 |
|
(b) Regulatory Plan |
|
28 |
|
(c) Specific Regulatory Activities |
|
30 |
|
(d) Costs and Expenses |
|
32 |
|
(e) Limitation on Activities |
|
32 |
|
5.2. Ownership of Regulatory Approvals |
|
32 |
|
5.3. Chiron Access to Cubist and Other Licensee Data |
|
33 |
|
(a) Information Sharing |
|
33 |
|
(b) Chiron Use of Cubist Data |
|
33 |
|
(c) License Option |
|
33 |
|
(d) Manufacturing Information |
|
33 |
|
5.4. Free Sales Certificates |
|
34 |
|
5.5. Safety; Adverse Event Reporting |
|
34 |
|
(a) General |
|
34 |
|
(b) Reporting Outside the Territory |
|
35 |
|
(c) Reporting in the Territory |
|
35 |
|
(d) Global Database |
|
35 |
|
5.6. Communications |
|
35 |
|
(a) Communications Relating to Regulatory Approval |
|
35 |
|
(b) Communications Relating to Development |
|
35 |
|
5.7. Recalls and Voluntary Withdrawals |
|
36 |
|
5.8. Label |
|
37 |
|
5.9. Governmental Inspections |
|
37 |
|
|
|
|
|
ARTICLE 6. COMMERCIALIZATION; DILIGENCE |
|
37 |
|
6.1. Marketing Plan; Diligence Obligation |
|
37 |
|
(a) Marketing Plan |
|
37 |
|
(b) Marketing Activities |
|
38 |
|
(c) Costs and Expenses |
|
39 |
|
(d) Ownership of Marketing Information |
|
39 |
|
6.2. Prohibited Marketing and Sales Activities |
|
40 |
|
6.3. Discounting |
|
40 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
iv
|
6.4. Marketing and Promotional Literature |
|
40 |
|
|
|
|
|
ARTICLE 7. COMPENSATION |
|
40 |
|
7.1. Upfront Consideration |
|
40 |
|
7.2. Milestone Payments |
|
41 |
|
(a) Development Milestone Payments |
|
41 |
|
(b) Sales Milestone Payments |
|
42 |
|
7.3. Royalties |
|
42 |
|
(a) Royalty |
|
42 |
|
(b) Know-How Step-Down |
|
43 |
|
(c) Competition Step-Down |
|
43 |
|
(d) Competition Step-Down Procedures |
|
44 |
|
(e) Holdback of Royalties Upon Certain Cubist Enforcement Actions |
|
44 |
|
(f) Chiron Enforcement Action |
|
45 |
|
(g) Clinical Supplies |
|
45 |
|
7.4. Adjustments |
|
46 |
|
7.5. Third Party Royalties and Other Payments |
|
46 |
|
7.6. Royalty Payments and Reports |
|
46 |
|
7.7. No Reductions or Offsets |
|
46 |
|
7.8. Tax Matters |
|
47 |
|
7.9. Foreign Exchange |
|
47 |
|
7.10. Late Payments |
|
47 |
|
7.11. Exports of Licensed Product from the Territory; [*] |
|
47 |
|
|
|
|
|
ARTICLE 8. INTELLECTUAL PROPERTY |
|
47 |
|
8.1. Ownership of Inventions |
|
47 |
|
8.2. Prosecution of Patents |
|
48 |
|
(a) Cubist Patents |
|
48 |
|
(b) Chiron Patents |
|
48 |
|
(c) Joint Patents |
|
49 |
|
8.3. Patent Term Extensions |
|
49 |
|
8.4. Infringement of Patents by Third Parties |
|
50 |
|
(a) Notification |
|
50 |
|
(b) Infringement of Patents in the Territory |
|
50 |
|
(c) Other Infringement of Cubist Patents |
|
50 |
|
(d) Infringement of Chiron Patents and Joint Patents outside the Territory |
|
50 |
|
(e) Settlement; Allocation of Proceeds |
|
51 |
|
8.5. Infringement of Third Party Rights |
|
51 |
|
(a) Notice |
|
51 |
|
(b) Avoidance of Infringement |
|
52 |
|
(c) Licensing to Resolve Infringement by Cubist Technology |
|
52 |
|
(d) Determination of Relative Financial Benefits |
|
52 |
|
(e) Limitation of Obligation to Sublicense |
|
53 |
|
(f) Defense |
|
53 |
|
(g) Settlement |
|
53 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
v
|
8.6. Patent Oppositions. |
|
53 |
|
(a) Third Party Patent Rights |
|
53 |
|
(b) Parties’ Patent Rights |
|
54 |
|
(c) Noncontravention |
|
54 |
|
8.7. Sublicensed Technology |
|
54 |
|
(a) Generally |
|
54 |
|
(b) Lilly License |
|
55 |
|
(c) Decision to License Third Party Technology |
|
55 |
|
(d) Licensing of Third Party Technology |
|
55 |
|
(e) Limitation of Obligation to Sublicense |
|
56 |
|
(f) Determination of Relative Financial Benefits |
|
56 |
|
8.8. Patent Marking |
|
56 |
|
8.9. Applicability to Chiron Patents |
|
56 |
|
8.10. Trademarks |
|
56 |
|
(a) Trademark License |
|
56 |
|
(b) Selection and Registration of Product Trademarks |
|
57 |
|
(c) Infringement of Trademarks by Third Parties |
|
57 |
|
(d) Product Trademarks Infringe Third Party Rights |
|
58 |
|
8.11. Subordination to Lilly Rights |
|
58 |
|
|
|
|
|
|
58 |
|
|
9.1. Mutual Representations and Warranties |
|
58 |
|
(a) Corporate Existence and Power |
|
58 |
|
(b) Authority and Binding Agreement |
|
59 |
|
(c) No Conflict |
|
59 |
|
(d) Validity |
|
59 |
|
(e) Consents |
|
59 |
|
9.2. Cubist Representations and Warranties |
|
59 |
|
(a) Ownership of Intellectual Property |
|
59 |
|
(b) Claims Related to Use of Intellectual Property |
|
59 |
|
(c) Notice to Third Persons |
|
60 |
|
(d) Effective Date Third Party Licenses |
|
60 |
|
(e) No Misappropriation |
|
60 |
|
(f) Regulatory Filings |
|
60 |
|
(g) Regulatory Data and Affairs |
|
60 |
|
(h) Non-Infringement of Cubist Technology by Third Parties |
|
61 |
|
(i) Litigation |
|
61 |
|
(j) Restrictive Agreements |
|
61 |
|
(k) Patent Prosecution |
|
61 |
|
9.3. Chiron Representation and Warranty - No Intellectual Property |
|
61 |
|
9.4. Disclaimer |
|
61 |
|
9.5. No Other Representations |
|
61 |
|
|
|
|
|
ARTICLE 10. INDEMNIFICATION |
|
62 |
|
10.1. Indemnification by Cubist |
|
62 |
|
10.2. Indemnification by Chiron |
|
62 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
vi
|
10.3. Liability for Third Party Products Liability Claims |
|
63 |
|
10.4. Procedure |
|
66 |
|
10.5. Insurance |
|
66 |
|
10.6. Limitation of Liability |
|
67 |
|
|
|
|
|
ARTICLE 11. RECORDS; PUBLICATIONS |
|
68 |
|
11.1. Records |
|
68 |
|
11.2. Publications |
|
68 |
|
|
|
|
|
ARTICLE 12. CONFIDENTIALITY |
|
69 |
|
12.1. Treatment of Confidential Information |
|
69 |
|
12.2. Authorized Disclosure |
|
69 |
|
12.3. Publicity |
|
70 |
|
|
|
|
|
ARTICLE 13. TERM AND TERMINATION |
|
70 |
|
13.1. Term |
|
70 |
|
13.2. Termination For Convenience by Chiron |
|
71 |
|
13.3. Termination By Either Party Upon Bankruptcy or Insolvency |
|
71 |
|
13.4. Termination for Breach |
|
71 |
|
(a) Notice |
|
71 |
|
(b) Failure to Cure |
|
71 |
|
(c) Disputes |
|
71 |
|
(d) Termination as to Certain Licensed Products |
|
72 |
|
(e) Right to Sell |
|
72 |
|
13.5. INTENTIONALLY OMITTED |
|
72 |
|
13.6. Consequences of Termination |
|
72 |
|
13.7. Survival |
|
74 |
|
|
|
|
|
ARTICLE 14. DISPUTE RESOLUTION |
|
75 |
|
14.1. Disputes |
|
75 |
|
14.2. Arbitration |
|
75 |
|
14.3. Governing Law; Judicial Resolution |
|
76 |
|
14.4. Equitable Remedies; Injunctive Relief |
|
77 |
|
14.5. [*]. |
|
77 |
|
14.6. Interest |
|
77 |
|
|
|
|
|
ARTICLE 15. MISCELLANEOUS |
|
77 |
|
15.1. Entire Agreement; Amendment |
|
77 |
|
15.2. Force Majeure |
|
77 |
|
15.3. Notices |
|
78 |
|
15.4. Maintenance of Records |
|
79 |
|
15.5. No Strict Construction |
|
79 |
|
15.6. Assignment |
|
79 |
|
(a) Assignment by Cubist |
|
79 |
|
(b) Assignment by Chiron |
|
79 |
|
(c) Restrictions on Payments |
|
80 |
|
(d) Injunctive Relief |
|
80 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
vii
|
15.7. Performance by Affiliates |
|
81 |
|
15.8. Guaranty |
|
81 |
|
15.9. Counterparts |
|
81 |
|
15.10. Further Actions |
|
81 |
|
15.11. Severability |
|
81 |
|
15.12. Headings |
|
81 |
|
15.13. No Waiver |
|
81 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
viii
This License Agreement (the “Agreement”) is made effective as of the 2nd day of October, 2003 (the “Effective Date”) by and between Chiron Healthcare Ireland Ltd., a company organized under the laws of Ireland with offices at United Xxxx Xxxxx, Xxxxxxx Xxxx, Xxxxxxxx, Xxxxxx, Xxxxxxx (“Chiron”) and Cubist Pharmaceuticals, Inc., a Delaware corporation having its principal place of business at 00 Xxxxxx Xxxxxx, Xxxxxxxxx, Xxxxxxxxxxxxx 00000 (“Cubist”). Cubist and Chiron are sometimes referred to herein individually as a “Party” and collectively as the “Parties”. Chiron Corporation, a Delaware corporation having its principal place of business at 0000 Xxxxxx Xxxxxx, Xxxxxxxxxx, Xxxxxxxxxx 00000 (the “Chiron Parent Company”), is a party to this Agreement only with respect to certain selected provisions of this Agreement as specified herein.
Whereas, Cubist is developing a proprietary compound known under the generic name of daptomycin, and in particular a form of daptomycin which is administered by intravenous injection;
Whereas, Cubist has filed a New Drug Application with the United States Food and Drug Administration for an intravenous formulation of daptomycin for the treatment of complicated skin and skin structure bacterial infections;
Whereas, Chiron possesses capabilities in the promotion and marketing of anti-infective pharmaceutical products throughout Europe and certain other countries (as further defined below as the “Territory”) and desires to seek regulatory approval for and market an injectable form of daptomycin in the Territory;
Whereas, Chiron wishes to obtain (i) exclusive (even as to Cubist) rights to commercialize all injectable forms of daptomycin in the Territory, and (ii) a right of first negotiation for rights in the Territory to all other forms of daptomycin, including oral formulations and combination products, and Cubist wishes to grant such rights to Chiron as set forth herein;
Whereas, the parties have executed a Manufacturing and Supply Agreement (the “Supply Agreement”) contemporaneously with this Agreement pursuant to which Cubist has agreed to manufacture or have manufactured Licensed Products for use by Chiron in connection with Commercialization of Daptomycin Products;
Whereas, Chiron wishes for Cubist to complete the Required Studies (as defined below) in order to enable Chiron to obtain regulatory approval to market in the Territory an injectable form of daptomycin for complicated skin and skin structure bacterial infection and for a second
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
indication, and Cubist has agreed to use commercially reasonable efforts to complete the Required Studies; and
Whereas, the parties wish to establish a mechanism for exchanging information and providing one another an opportunity to discuss and comment upon activities relating to daptomycin products in their respective territories, it being understood that such mechanism is for transparency purposes only.
Now Therefore, based on the foregoing premises and the mutual covenants and obligations set forth below, the Parties agree as follows:
ARTICLE 1.
DEFINITIONS
The following terms shall have the following meanings as used in this Agreement:
1.1. “Additional Daptomycin Product” shall mean any pharmaceutical composition containing Daptomycin other than a Daptomycin IV Product. For the avoidance of doubt, “Additional Daptomycin Product” shall include, without limitation, any pharmaceutical composition containing Daptomycin formulated for oral delivery and any pharmaceutical composition containing Daptomycin and one or more active pharmaceutical ingredient other than Daptomycin.
1.2. “Affiliate” shall mean, with respect to any Person, (i) any other Person of which fifty percent (50%) or more of the securities or other ownership interests representing the equity, the voting stock or, if applicable, general partnership interest of such other Person are owned, controlled, or held, directly or indirectly by, or under common ownership or control with, such Person; or (ii) any other Person that, directly or indirectly, owns, controls, or holds fifty percent (50%) or more of the securities or other ownership interests representing the equity, the voting stock or, if applicable, the general partnership interest, of such Person.
1.3. “Approved New Trademark” shall have the meaning assigned to such term in Section 8.10(b) hereof.
1.4. “Chiron Data” shall have the meaning assigned to such term in Section 4.4(a) hereof.
1.5. “Chiron Development Plan” shall have the meaning assigned such term in Section 4.3(d).
1.6. “Chiron Interest” shall have the meaning assigned to such term in Section 15.6(b) hereof.
1.7. “Chiron Inventions” shall have the meaning assigned to such term in Section 8.1.
1.8. “Chiron Joint Technology Rights” shall mean all of Chiron’s right, title and interest in the Joint Patents, the Joint Know-How and the Joint Inventions.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
2
1.9. “Chiron Know-How” shall mean all Information (i) that (A) is owned or Controlled by Chiron after the Effective Date, (B) is not generally known and is not disclosed in any published Chiron Patents, (C) is necessary or useful in connection with the development, manufacture, use, sale, import or export of Licensed Products, including, without limitation, all data and information regarding the safety and efficacy of Licensed Products, and (D) is created, conceived, invented, developed or reduced to practice during the Term in the course of performing research and development activities undertaken or carried out for the specific purpose of developing Licensed Products or of developing any invention necessary or useful to the development, manufacture, use, sale, import or export of Licensed Products, or (ii) that is included in “Chiron Know-How” pursuant to the provisions of Section 2.4 hereof. Notwithstanding the foregoing, the term “Chiron Know-How” shall not include any Chiron Marketing Information, Chiron Data (other than data generated from Medical Affairs Studies), Joint Know-How or any interest of Chiron in any of the foregoing.
1.10. “Chiron Marketing Information” shall have the meaning assigned to such term in Section 6.1(d).
1.11. “Chiron Patent” shall mean (i) any Patent that is owned or Controlled by Chiron, that has a filing date after the Effective Date and that covers any invention owned or Controlled by Chiron that is created, conceived, invented, developed or reduced to practice during the Term in the course of performing research and development activities undertaken or carried out for the specific purpose of developing Licensed Products or of developing any invention useful to the development, manufacture, use, sale, import or export of Licensed Products, and (ii) any Patent that becomes a “Chiron Patent” pursuant to the provisions of Section 2.4 hereof. Notwithstanding the foregoing, the term “Chiron Patent” shall not include any Joint Patents or any interest of Chiron in any Joint Patents.
1.12. “Chiron Related Know-How” shall mean all Information that (i) is not Chiron Marketing Information, Chiron Data, Chiron Know-How or Joint Know-How, (ii) is owned or Controlled by Chiron on the Effective Date, (iii) is not generally known and is not disclosed in any published patents or patent applications of Chiron, (iv) is necessary or useful in connection with the development, manufacture, use, sale, import or export of Licensed Products, and (v) is created, conceived, invented, developed or reduced to practice prior to the Effective Date.
1.13. “Chiron Related Patent” shall mean any Patent (i) that is not a Chiron Patent or a Joint Patent, (ii) that is owned or Controlled by Chiron on or at any time after the Effective Date, and (iii) that covers any invention, compound, improvement, method, apparatus, material, method or technique of manufacture created, conceived, invented, developed or reduced to practice prior to the Effective Date that is necessary or useful to the development, manufacture, use, sale, import or export of Licensed Products.
1.14. “Chiron Technology” shall mean Chiron Patents and Chiron Know-How.
1.15. “Commercial Launch” shall mean the first commercial sale of a Licensed Product to a Third Party in a given country after obtaining Regulatory Approval to Commercialize such Licensed Product in such country. For the purposes of determining whether
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
3
or not Commercial Launch has occurred, sales of Licensed Product for use in clinical trials and named patient sales shall not be considered.
1.16. “Commercialize” shall mean all activities relating to the commercialization of a Licensed Product including, without limitation, promotion, marketing, sales, distribution, development for label extensions, and conducting Medical Affairs Studies, whether conducted by a Party or for such party by another, and “Commercialization” shall be interpreted accordingly.
1.17. “Commercially Important Indications” shall mean, with respect to a given Licensed Product on any given date of determination, those indications that are included in the label approved by a Regulatory Authority in the Territory for such Licensed Product and that each account for [*] of such Licensed Product for the four calendar quarters immediately preceding such date of determination, or, in the event that [*] in the Territory occurred within twelve months of such date of determination, then for the period from [*] through such date of determination, or if there is no indication that [*], then those three indications that account for the [*].
1.18. “Commercially Reasonable Efforts” shall mean, with respect to a Party’s obligation under this Agreement, the level of efforts required to carry out such obligation in sustained manner consistent with the efforts a similarly situated pharmaceutical company devotes to a product of similar market potential, profit potential and strategic value and similar scientific, technical, development and regulatory risks, based on conditions then prevailing.
1.19. “Confidential Information” shall mean all Information, and other information and materials, received by either Party from the other Party pursuant to this Agreement, other than that portion of such information or materials which:
(a) is publicly disclosed by the disclosing Party, either before or after it becomes known to the receiving Party;
(b) was known to the receiving Party, without obligation to keep it confidential, prior to when it was received from the disclosing Party;
(c) is subsequently disclosed to the receiving Party by a Third Party lawfully in possession thereof without obligation to keep it confidential;
(d) has been publicly disclosed other than by the disclosing Party and without breach of an obligation of confidentiality with respect thereto; or
(e) has been independently developed by the receiving Party without the aid, application or use of Confidential Information, as demonstrated by competent written proof.
1.20. “Contract Manufacturing Agreements” means any and all agreements pursuant to which Licensed Products are manufactured on behalf of Cubist (including any such agreements for any step in the manufacturing process), including without limitation, the Xxxxxx
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
4
Laboratories, ACS Dobfar S.p.A. and DSM Capua S.p.A. contracts, together with all amendments and extensions of such agreements.
1.21. “Control” shall mean possession of the ability to grant a license or sublicense as provided for herein without violating the terms of any agreement or other arrangement with any Third Party, and “Controlled” shall be interpreted accordingly.
1.22. “cSSSI” shall mean complicated skin and skin structure bacterial infection.
1.23. “Cubist Data” shall mean all Information (other than Other Licensee Data) owned or Controlled by Cubist pertaining to Licensed Products that is necessary or useful for making applications for Regulatory Approval or other regulatory filings for, or Commercializing, Licensed Products in the Territory.
1.24. “Cubist Development Plan” shall have the meaning assigned such term in Section 4.2(a).
1.25. “Cubist Inventions” shall have the meaning assigned to such term in Section 8.1.
1.26. “Cubist Joint Technology Rights” shall mean all of Cubist’s right, title and interest in the Joint Patents, the Joint Know-How and the Joint Inventions.
1.27. “Cubist Know-How” shall mean all Information (i) that (A) is owned or Controlled by Cubist on the Effective Date or at any time during the Term, (B) is not generally known and is not disclosed in any published Cubist Patents, and (C) is necessary or useful in connection with the development, manufacture, marketing, promotion, use, sale, import or export of Licensed Products, including, without limitation, all data and information regarding the safety and efficacy of Licensed Products. Notwithstanding the foregoing, the term “Cubist Know-How” shall not include any Joint Know-How or any interest of Cubist in any Joint Know-How.
1.28. “Cubist Marks” shall mean (i) all registered trademarks issued or that have issued on the application listed at Exhibit A; (ii) all pending trademark applications listed at Exhibit A, as amended by Cubist from time to time; (iii) the Cubist name and xxxx; and (iv) any and all Approved New Trademarks.
1.29. “Cubist Patent” shall mean any Patent that is owned or Controlled by Cubist on the Effective Date, including those set forth on Exhibit B, or at any time during the Term, and that covers any Licensed Product or covers any invention, compound, improvement, method, apparatus, material, method or technique of manufacture necessary or useful in the development, manufacture, use, sale, import or export of Licensed Products, including without limitation, any and all Cubist Inventions. Notwithstanding the foregoing, the term “Cubist Patent” shall not include any Joint Patents or any interest of Cubist in any Joint Patents.
1.30. “Cubist Technology” shall mean all Cubist Patents and Cubist Know-How.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
5
1.31. “Daptomycin” shall mean the molecule set forth and identified as the daptomycin molecule on Exhibit C, and all acids, salts and esters of such daptomycin molecule.
1.32. “Daptomycin IV Product” shall mean any pharmaceutical composition containing Daptomycin in all current and future formulations, but only if such pharmaceutical composition is formulated for delivery via Injection. The term “Daptomycin IV Product” shall not include (i) any pharmaceutical composition formulated for delivery by any means other than via Injection or (ii) any pharmaceutical composition that includes one or more active pharmaceutical ingredients other than Daptomycin.
1.33. “Defective Manufactured Product” shall have the meaning assigned to such term in the Supply Agreement.
1.34. “Directly Competitive Product” shall mean, with respect to a given Licensed Product, (A) [*] or (B) any other pharmaceutical composition (other than a Licensed Product) formulated for delivery [*] and that is marketed and sold (i) primarily for [*], and (ii) solely to treat [*], and which other pharmaceutical composition has a label approved by a Regulatory Authority in the Territory which includes as indications for such other pharmaceutical composition [*]. Commercially Important Indications that are included in the label approved by a Regulatory Authority in the Territory for such Licensed Product. Notwithstanding the foregoing, the term “Directly Competitive Product” shall not include [*.]
1.35. “Dollar” shall mean a United States dollar, and “$” shall be interpreted accordingly.
1.36. “Drug Approval Application” shall mean an application for Regulatory Approval required before commercial sale or use of a Licensed Product as a drug in a regulatory jurisdiction, including without limitation an NDA and a Biologics License Application (BLA) filed in the United States.
1.37. “Drug Master File,” or “DMF” shall mean a voluntary submission that may be used to provide confidential, detailed information about the active pharmaceutical ingredient, daptomycin, and facilities, processes or articles used during the manufacturing, processing, packaging and storing of daptomycin or one or more other drug products.
1.38. “Effective Date Third Party Licenses” shall have the meaning assigned to such term in Section 8.7(a).
1.39. “EMEA” shall mean the European Medicines Evaluation Agency, or any successor thereto, which coordinates the scientific review of human pharmaceutical products under the centralized licensing procedures of the European Community.
1.40. “Fair Market Value” shall have the meaning assigned to such term in Section 2.12 hereof.
1.41. “FDA” shall mean the United States Food and Drug Administration, or any successor thereto.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
6
1.42. “Global Harm” shall mean [*].
1.43. “IND” shall mean an Investigational New Drug application.
1.44. “Indemnify” shall have the meaning assigned to such term in Section 10.1 hereof.
1.45. “Information” shall mean (i) techniques, information and data specifically relating to development, manufacture, use, sale, import or export of Licensed Products, including, but not limited to, inventions, practices, methods, knowledge, know-how, skill, experience, test data including pharmacological, toxicological and clinical test data, analytical and quality control data, regulatory submissions, correspondence and communications, marketing, pricing, distribution, cost, sales, manufacturing, patent and legal data or descriptions and (ii) compositions of matter, assays and biological materials specifically relating to development, manufacture, use, sale, import or export of Licensed Products.
1.46. “Infringement” or “Infringe” shall have the meaning assigned to such term in Section 8.4(a) hereof.
1.47. “Injection” shall mean all means of delivering a pharmaceutical composition by injection and includes, without limitation, delivery by intravenous, intramuscular and subcutaneous injection.
1.48. “Joint Coordination Team” or “JCT” shall mean the committee formed as described in Section 3.2(a).
1.49. “Joint Inventions” shall have the meaning assigned in Section 8.1.
1.50. “Joint Know-How” shall mean all Information that (i) consists of Joint Inventions, (ii) is not generally known and is not disclosed in any published Joint Patents, and (iii) is necessary or useful in connection with the development, manufacture, marketing, promotion, use, sale, import or export of Licensed Products, including, without limitation, all data and information regarding the safety and efficacy of Licensed Products.
1.51. “Joint Patents” shall have the meaning assigned such term in Section 8.2(c).
1.52. “Joint Technology” shall mean all Joint Patents and Joint Know-How.
1.53. “Key Development Studies” means [*.]
1.54. “Knowable Patent” shall have the meaning assigned to such term in Section 8.5(c) hereof.
1.55. “Launch Indication(s)” shall mean cSSSI and [*], but if at the relevant time Cubist has [*,] then the term “Launch Indication(s)” shall at such time mean cSSSI only.
1.56. “Licensed Products” shall mean all Daptomycin IV Products, and all Additional Daptomycin Products that become Licensed Products pursuant to Section 2.9 hereof.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
7
1.57. “Lilly” shall mean Xxx Xxxxx and Company.
1.58. “Lilly License” shall mean that certain Licensing Agreement between Cubist and Lilly, dated October 6, 2000, as amended by the amending agreement dated July 1, 2003, and as further amended and in effect from time to time, which License Agreement replaced the prior agreement between such parties, dated November 7, 1997.
1.59. “Lilly Patent” shall have the meaning assigned to such term in Section 8.11.
1.60. “Losses” shall have the meaning assigned such term in Section 10.1.
1.61. “MAA” shall mean an application filed with the EMEA for regulatory approval to market and sell Licensed Products in the European Union, or an application filed through the mutual recognition procedures in the European Union having a similar purpose to the NDA in the United States.
1.62. “Major Market Countries” shall mean, collectively, France, Germany, Italy, Spain, and the United Kingdom. The term “Major Market Country” shall mean any of the Major Market Countries.
1.63. “Manufacturing Information” shall have the meaning assigned to such term in Section 5.3(d) hereof.
1.64. “Manufacturing Plan” shall have the meaning assigned to such term in Section 2.5 of the Supply Agreement.
1.65. “Marketing Plan” shall have the meaning assigned such term in Section 6.1(a).
1.66. “Marketing Plan Trigger Event” shall have the meaning assigned such term in Section 6.1(a).
1.67. “Medical Affairs Studies” shall mean those clinical studies conducted after Regulatory Approval of Licensed Product has been obtained which are neither intended nor designed to support an application for Regulatory Approval, including but not limited to, pharmaco-economic studies, pharmaco-epidemiology studies, and investigator-sponsored clinical studies.
1.68. “NDA” shall mean a New Drug Application for Regulatory Approval filed in the United States.
1.69. “Net Sales” shall mean, with respect to a particular time period, the aggregate gross sales invoiced by Chiron and its Affiliates for Licensed Products sold directly by Chiron and its Affiliates to a Third Party (including, without limitation, any Third Party that is a distributor of Chiron and its Affiliates in any country within the Territory) during such time period, less:
(i) discounts, including cash and quantity discounts, charge-back payments and rebates granted to managed health care organizations or to domestic and foreign
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
8
governments (including any subdivision thereof), their agencies, purchasers and reimbursers or to trade customers, in each case to the extent actually allowed in amounts customary in the trade;
(ii) credits or allowances actually granted upon returns of damaged, spoiled or Non-Conforming (as defined in the Supply Agreement) units of Licensed Products or upon recalls of units of Licensed Products, in each case in amounts customary in the trade;
(iii) freight, postage, shipping, transportation and insurance charges actually allowed or paid by Chiron or any of its Affiliates for delivery of Licensed Products sold by Chiron or any of its Affiliates to a Third Party; and
(iv) taxes, duties and other governmental charges levied on, absorbed or otherwise imposed on import, export, sale, distribution and use of such Licensed Products that are paid by Chiron (including, without limitation, any taxes paid by Chiron pursuant to Section 7.5 of the Supply Agreement), all as adjusted for rebates and refunds actually granted or reasonably anticipated to be granted if application therefor is made; provided, however, that in no event shall any of the following taxes or governmental charges paid or required to be paid by Chiron constitute a permitted deduction in calculating Net Sales: any taxes or governmental charges calculated based on, or levied or imposed on any profit or income earned by Chiron or any of its Affiliates or distributors.
Amounts received by Chiron and its Affiliates for the sale of Licensed Products among Chiron and its Affiliates for resale shall not be included in the computation of Net Sales hereunder.
[*] or otherwise in connection with any Licensed Products or in connection with any rights to any Licensed Products, then the amount of such payment to Chiron or its Affiliates shall be included in Net Sales in the calendar quarter in which Chiron or its Affiliates received such payment for purposes of calculating the royalty due to Cubist pursuant to Article 7. Without limiting the applicability of the provisions of the foregoing sentence and without limiting any remedy or choice of remedies that Cubist may have under applicable law in connection with any breach by Chiron of the provisions of [*] hereof, [*] makes any payment or payments to Chiron or its Affiliates [*] Chiron or its Affiliates of any of the [*] then such payment or payments made to Chiron or its Affiliates shall be included in Net Sales in the calendar quarter in which Chiron or its Affiliates performed such actions or activities.
For the avoidance of doubt, any payments made by [*] (other than Licensed Products and other than products that are subject to the provisions of [*], or for customary services provided for customary consideration, [*] shall not be included in Net Sales.
1.70. “Other Licensee” shall mean any Third Party to whom Cubist has granted or grants a license and/or sublicense to develop or Commercialize a Licensed Product outside the Territory.
1.71. “Other Licensee Data” shall mean all Information that results from any development activities conducted by any Other Licensee with respect to Licensed Products and
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
9
that is necessary or useful for making regulatory filings for, or Commercializing, Licensed Products in the Territory.
1.72. “Patent” shall mean (i) unexpired letters patent (including inventor’s certificates) which have not been held invalid or unenforceable by a court of competent jurisdiction from which no appeal can be taken or has been taken within the required time period, including without limitation any substitution, extension, registration, confirmation, reissue, re-examination, renewal or any like filing thereof and (ii) pending applications for letters patent, including without limitation any provisional, converted provisional, continued prosecution application, continuation, divisional or continuation-in-part thereof.
1.73. “Price Approval” shall mean, with respect to any country in which the price at which Chiron sells Licensed Product must be approved by a governmental authority for reimbursement or payment purposes, the receipt of approval by the applicable governmental authority with respect to such price.
1.74. “Primary Endpoint” shall mean, with respect to a clinical trial, the point at which the primary efficacy objective has been achieved with respect to a clinical or microbiological outcome as specified in the protocol for such trial, unless otherwise agreed by the Parties in writing.
1.75. “Reasonable Buyer” shall mean, with respect to the sale, assignment, sublicense or other transfer of the Chiron Interest in accordance with the provisions of Section 15.6(b) hereof, a Third Party that, both at the time a definitive agreement is entered into by Chiron and such Third Party in connection any such transfer and at the time of the closing of such transfer:
|
(i) |
[*]; |
|
|
|
|
|
|
(ii) |
[*]; |
|
|
|
|
|
|
(iii) |
[*] |
|
|
|
|
|
|
(iv) |
[*]: |
|
|
|
|
|
|
|
(a) |
[*] |
|
|
|
|
|
|
|
[*] |
|
|
|
|
|
|
(b) |
[*]*; |
|
|
|
|
|
(v) |
[*]; |
|
|
|
|
|
|
(vi) |
[*]; |
|
|
|
|
|
|
(vii) |
[*]; |
|
|
|
|
|
|
(viii) |
[*]; |
|
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
10
|
(ix) |
[*] |
|
|
|
|
|
|
(x) |
[*.] |
|
1.76. “Reciprocating Licensee” shall have the meaning assigned to such term in Section 4.4(b).
1.77. “Regulatory Approval” shall mean any approvals (including supplements, variations, amendments, pre- and post-approvals and Price Approvals), licenses, registrations or authorizations of any national, supra-national (e.g., the FDA, the European Commission or the Council of the European Union, or other similar body in any country), regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, necessary for the manufacture, distribution, use, sale, import or export of Licensed Products in a regulatory jurisdiction.
1.78. “Regulatory Authority” shall mean a foreign counterpart of the FDA.
1.79. “Regulatory Plan” shall have the meaning assigned to such term in Section 5.1(b) hereof.
1.80. “Replacement Indication” shall have the meaning assigned to such term in Section 4.1(d) hereof.
1.81. “Replacement Indication Study” shall have the meaning assigned to such term in Section 4.1(d) hereof.
1.82. “Replacement Study” shall have the meaning assigned to such term in Section 4.1(c).
1.83. “Required Study” shall have the meaning assigned to such term in Section 4.1.
1.84. “Restricted Period” shall mean, with respect to either Party, the period of time commencing on the Effective Date and ending on the earlier of (i) the expiration or termination of the Term and (ii) the date when such Party sells, assigns, sublicenses or otherwise transfers all of its rights in this Agreement pursuant to, and in accordance with, the provisions of Section 15.6.
1.85. “Royalty Rate” shall have the meaning assigned to such term in Section 7.3(a) hereof.
1.86. “Stock Purchase Agreement” shall mean that certain Stock Purchase Agreement, dated as of the Effective Date, by and between Cubist and the Chiron Parent Company.
1.87. “Supply Agreement” shall mean that certain Manufacturing and Supply Agreement, dated as of the Effective Date, by and between Cubist and Chiron.
1.88. “Term” shall mean the term of this Agreement.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
11
1.89. “Territory” shall mean the countries listed in Exhibit D, and the possessions and territories of each such country. Whenever, in accordance with the terms of this Agreement, Chiron’s rights under this Agreement shall terminate with respect to any country or countries initially included in the definition of Territory, any reference in this Agreement to the term Territory shall, from and after such termination, be deemed not to refer to any such country or countries as to which Chiron’s rights under this Agreement have so terminated.
1.90. “Territory Specific Studies” shall have the meaning mean assigned to such term in Section 4.3(a).
1.91. “Third Party” shall mean any entity other than Cubist or Chiron or an Affiliate of either of them.
1.92. “Third Party Infringement Claim” shall have the meaning assigned such term in Section 8.5(a).
1.93. “Transfer Price” shall have the meaning assigned to such term in Section 7.1 of the Supply Agreement.
1.94. “Unlicensed Product” shall mean, with respect to any given Licensed Product in any given country within the Territory, any pharmaceutical composition containing Daptomycin that (A) is commercially available in such country other than [*] and other than [*] (B) has the same [*], such Licensed Product, and (C) [*] as such Licensed Product. If Chiron’s rights under this Agreement are expanded to include rights in Additional Daptomycin Products, then the foregoing definition of “Unlicensed Product” shall be deemed to be expanded to include those formulations of pharmaceutical compositions containing Daptomycin which have been so added to the scope of Licensed Products and the marketing and other rights of Chiron under this Agreement.
1.95. “Unlicensed Sales Threshold” shall have the meaning assigned to such term in Section 7.3(e) hereof.
1.96. “Valid Claim” shall mean (i) an unexpired claim of an issued patent within the Cubist Patents or Joint Patents which has not been found to be unpatentable, invalid or unenforceable by a court or other authority in the subject country, from which decision no appeal is taken or can be taken; or (ii) a claim of a pending patent application within the Cubist Patents or Joint Patents.
ARTICLE 2.
PRODUCT RIGHTS AND RIGHTS OF FIRST NEGOTIATION
2.1. Product Rights. Subject to and upon the terms and conditions set forth in this Agreement, Chiron shall have exclusive rights (even as to Cubist) to Commercialize the Licensed Products in all countries of the Territory.
2.2. Licenses to Chiron. Subject to, and in accordance with, the terms and conditions of this Agreement:
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
12
(a) Cubist grants to Chiron the exclusive (even as to Cubist) license, under the Cubist Technology and the Cubist Joint Technology Rights, to sell, offer for sale, have sold, import, export and Commercialize Licensed Products in the Territory. Except to the extent otherwise provided in this Agreement, the rights granted to Chiron pursuant to this Section 2.2(a) may not be sublicensed or transferred.
(b) Cubist grants to Chiron the nonexclusive license, under the Cubist Technology, to practice and use the Cubist Technology and the Licensed Products anywhere in the world for the sole purpose of engaging in those research and development activities that Chiron is expressly required or permitted to perform pursuant to this Agreement. Except to the extent otherwise provided in this Agreement, the rights granted to Chiron pursuant to this Section 2.2(b) may not be sublicensed or transferred.
(c) Cubist grants to Chiron a nonexclusive license, under the Cubist Technology, to make, have made, manufacture and have manufactured anywhere in the world Licensed Products for sale in the Territory, provided that such right to make, have made, manufacture and have manufactured shall not be exercised by Chiron so long as the Supply Agreement remains in effect. Except to the extent otherwise provided in this Agreement, the rights granted to Chiron pursuant to this Section 2.2(c) may not be sublicensed or transferred.
(d) In addition to any rights expressly retained by Cubist in the foregoing provisions of this Section 2.2, Cubist shall retain any and all rights in and to the Cubist Technology and the Cubist Joint Technology Rights that are not expressly granted to Chiron pursuant to this Section 2.2.
2.3. Licenses to Cubist. Subject to the terms and conditions of this Agreement:
(a) Chiron grants to Cubist the exclusive (even as to Chiron) license, under the Chiron Technology and the Chiron Joint Technology Rights, to sell, offer for sale, have sold, import, export and Commercialize Licensed Products outside of the Territory. Except to the extent otherwise provided in this Agreement, the rights granted to Cubist pursuant to this Section 2.3(a) may not be sublicensed or transferred.
(b) Chiron grants to Cubist the nonexclusive license, under the Chiron Technology, to practice and use the Chiron Technology and the Licensed Products anywhere in the world for the sole purpose of engaging in research and development activities with respect to Licensed Products pursuant to this Agreement. Except to the extent otherwise provided in this Agreement, the rights granted to Cubist pursuant to this Section 2.3(b) may not be sublicensed or transferred.
(c) Chiron grants to Cubist a nonexclusive license, under the Chiron Technology, to make, have made, manufacture and have manufactured anywhere in the world Licensed Products. Except to the extent otherwise provided in this Agreement, the rights granted to Cubist pursuant to this Section 2.3(c) may not be sublicensed or transferred.
(d) In addition to any rights expressly retained by Chiron in the foregoing provisions of this Section 2.3, Chiron shall retain any and all rights in and to the Chiron
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
13
Technology and the Chiron Joint Technology Rights that are not expressly granted to Cubist pursuant to this Section 2.3.
(e) Notwithstanding any provision of this Agreement to the contrary, the provisions of this Section 2.3 shall apply to any Chiron Patent only to the extent that the Parties have not otherwise agreed on the scope of rights granted and reserved in respect of any such Chiron Patent.
2.4. Existing Related Intellectual Property of Chiron. If Chiron decides to use or apply any Chiron Related Patent or any Chiron Related Know-How, Chiron shall notify Cubist and provide any information concerning any such Chiron Related Patent or Chiron Related Know-How that Cubist reasonably requests. In the event that either Party sends a written request to the other Party requesting a discussion at a JCT meeting concerning the need, usefulness and advisability of using any Chiron Related Patent or Chiron Related Know-How in connection with the development, manufacture, use or Commercialization of Licensed Products within and/or outside the Territory, then a meeting of the JCT shall be convened as promptly as practicable and the Parties shall discuss same. If consensus is reached at the JCT that any such Chiron Related Patent and/or Chiron Related Know-How should be used for such purpose, then the Parties shall discuss the compensation that Chiron would require in order to license to Cubist any such Chiron Related Patent and/or Chiron Related Know-How. If Cubist and Chiron agree on the terms of any such compensation, then the Parties may enter into an appropriate technology license agreement which license agreement shall address the scope of the license granted, compensation and responsibilities of the Parties on matters such as further research and development, prosecution, maintenance and enforcement of relevant Patents, and other customary provisions contained in such agreements. If the Parties elect not to enter into a specific technology license agreement, any such Chiron Related Patent shall be included within the Chiron Patents and shall become subject to the licenses granted to Cubist pursuant to Section 2.3 hereof and/or any such Chiron Related Know-How shall be included within the Chiron Know-How and shall become subject to the licenses granted to Cubist pursuant to Section 2.3 hereof.
2.5. Field. Notwithstanding anything in this Agreement to the contrary, it is acknowledged and agreed by the Parties that the licenses granted by Cubist to Chiron under this Agreement with respect to the Cubist Patents and Cubist Know-How that are licensed to Cubist pursuant to the Lilly License shall be solely for application to the treatment of infectious diseases.
2.6. Use of Patents and Know-How. Each Party covenants to the other that it will not practice the Patents or Know-How of the other Party except as expressly permitted in the licenses granted to it in this Agreement.
2.7. Exclusivity.
(a) Chiron. Chiron shall not develop, promote, sell or offer for sale Licensed Products for use outside of the Territory. Chiron shall require its distributors who sell Licensed Products to make a covenant similar to that provided in this Section 2.7(a) with respect to Licensed Products.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
14
(b) Cubist. Cubist shall not promote, sell, or offer for sale a Licensed Product for use within the Territory (except for sales to Chiron pursuant to this Agreement or the Supply Agreement). Cubist shall require any of its Other Licensees and distributors of Licensed Products to make a covenant similar to that provided in this Section 2.7(b) with respect to Licensed Products.
2.8. Sublicenses and Transfers of Chiron Technology or Chiron Joint Technology Rights. Chiron shall not sublicense or transfer any interest in the Chiron Technology or the Chiron Joint Technology Rights to any Third Party unless such Third Party shall have acknowledged and agreed in writing that any such sublicense or transfer to such Third Party, and the rights acquired by any such Third Party in and to the Chiron Technology and the Chiron Joint Technology Rights as a result of such sublicense or transfer, shall be subject to any and all rights that Chiron may have granted to Cubist pursuant to this Agreement and the Supply Agreement.
2.9. Rights of First Negotiation. Cubist hereby grants Chiron (a) a right of first negotiation to obtain Commercialization rights in the Territory with respect to any and all Additional Daptomycin Products, and (b) a right of first negotiation to obtain Commercialization rights in the Territory to any and all Directly Competitive Products, in each case, on the following terms: In the event that Cubist proposes to grant, sell, assign or otherwise transfer to a Third Party all or any portion of any such Commercialization rights, regardless of whether Cubist or a Third party makes the initial proposal, then Cubist will promptly notify Chiron in writing thereof. As soon as practicable, Chiron will respond to Cubist in writing regarding its interest in entering into negotiations to obtain such rights and the Parties will promptly [*] following the date that Cubist gives such written notice to Chiron. Upon commencement of such negotiations, [*] to grant Commercialization rights with respect to such Additional Daptomycin Product or Directly Competitive Product. If Chiron and Cubist are unable to agree on material terms within [*] after receipt by Chiron of Cubist’s notice of its intent to transfer Commercialization rights, then Cubist will [*]. Chiron shall have an exclusive [*] period to present (but shall not be obligated to present) to Cubist a revised proposal. If Cubist does not accept Chiron’s revised proposal upon expiration of such exclusive period, in its sole discretion, Cubist will be free to enter into negotiations with any Third Party, provided, however, Chiron shall have the non-exclusive right to continue discussions with Cubist. If Cubist determines that it is likely to accept terms with a Third Party for such Commercialization rights, Cubist will offer Chiron an opportunity for [*], at Chiron’s headquarters or at any other location that the Parties may mutually agree upon, within [*] of Cubist’s notification to Chiron that it is affording Chiron such opportunity to meet, for the purpose of explaining in reasonable detail the reasons that Cubist is likely to accept such terms. Chiron shall have a period of [*] from the date of such meeting to present to Cubist [*] and Cubist hereby agrees that it will not accept the Third Party terms until the earlier of (a) the expiration of such [*] period, (b) the date that Chiron affirmatively declines to make an offer, and (c) the date that Chiron proposes, and Cubist rejects, such offer. For the avoidance of doubt, Cubist is not obligated to offer the foregoing opportunity for [*]. Cubist will select the party with which it wishes to enter into negotiations for a definitive agreement in its sole discretion, provided, however, in making its determination, [*]. Chiron hereby acknowledges and agrees that [*]
2.10. Right of Co-Negotiation. Chiron hereby grants Cubist a right of co-negotiation to obtain all or any portion of the Commercialization rights in the Territory with respect to any and
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
15
all Directly Competitive Products on the following terms: In the event that Chiron proposes to grant, sell, assign or otherwise transfer to a Third Party all or any portion of such Commercialization rights in the Territory for a Directly Competitive Product, regardless of whether Chiron or a Third Party makes the initial proposal, then Chiron will promptly notify Cubist in writing thereof and thereafter, Cubist, to the extent that it remains so interested, shall be included among the interested parties with whom Chiron holds discussions for the transfer of such rights until such time as Chiron selects the party with whom it wishes to enter into negotiations for a definitive agreement for such rights. Cubist acknowledges that [*]. Without limiting the generality of the foregoing, Cubist acknowledges that Chiron shall have [*] for the Territory as Chiron sees fit. Except as expressly provided in Section 2.11, Chiron shall have no obligation or liability to Cubist for selecting a Third Party as its Commercialization partner for Directly Competitive Products in the Territory.
2.11. Marketing a Directly Competitive Product. Subject to the right of first negotiation in favor of Chiron in Section 2.9, and the right of co-negotiation in favor of Cubist in Section 2.10, the Parties acknowledge that either Party may, at any time during the Restricted Period applicable to such Party, market a Directly Competitive Product in the Territory. In the event that either Party launches, either directly or indirectly, a Directly Competitive Product in the Territory at any time during the Restricted Period applicable to such launching Party, then the other Party (the “Aggrieved Party”) shall have recourse to the remedies provided herein. The Parties acknowledge and agree that the objective of the remedies provided in this Section 2.11 is to have the Party launching the Directly Competitive Product compensate the Aggrieved Party for the [*] [*] of its interest in the Licensed Products resulting from such action. If Cubist is the Aggrieved Party, Cubist may elect to:
(a) require Chiron to [*] to receive royalties for the sale of Licensed Products in the Territory (“Cubist Royalty Interest”) at the [*] as it existed immediately prior to Chiron’s launch of the Directly Competitive Product in the Territory, following which Chiron shall be entitled to continue to sell Licensed Products in the Territory without any further obligation to pay royalties to Cubist; or
(b) require Chiron to pay the [*] of the Cubist Royalty Interest attributable to Chiron’s launch of the Directly Competitive Product in the Territory but following which Chiron shall be entitled to continue to sell Licensed Products in the Territory subject to payment of the royalties as provided herein.
If Chiron is the Aggrieved Party, then Chiron may elect to:
(c) require Cubist to purchase the Chiron Interest at its [*] as it existed immediately prior to Cubist’s launch of the Directly Competitive Product in the Territory, following which Chiron shall have no further right to sell Licensed Products in the Territory; or
(d) require Cubist to pay to Chiron the [*] of the Chiron Interest attributable to Cubist’s launch of the Directly Competitive Product in the Territory but following which Chiron shall be entitled to continue to sell Licensed Products in the Territory subject to payment of royalties as provided herein.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
16
In each of the foregoing [*] shall be determined in accordance with the [*]. In each instance in which a Party is required to make payment of compensation to the Aggrieved Party pursuant to the provisions of this Section 2.11, the Party required to pay compensation shall have the option of paying such amount due as a one-time lump sum or by installments paid over a period of time not to exceed [*], provided that, in the event that a compensating Party elects to make payment over a period of time, such compensating Party shall pay interest on all amounts due at the rate stipulated in Section 7.10 for late payments.
2.12. Mechanism to Determine Fair Market Value. [*] of this Agreement “Fair Market Value” (including any diminution thereof) shall mean the fair market value of the Cubist Royalty Interest or the Chiron Interest, as the case may be, determined as follows: If the Parties cannot agree on the Fair Market Value within 30 days after the launch of the Directly Competitive Product, each Party shall promptly designate a reputable investment banking or appraisal firm of its choice (which in the case of an investment bank shall not be the regular banker of the Party) (the “Appraisers” or each an “Appraiser”), who will each be asked to provide its best, single number estimate of the applicable Fair Market Value (the “Estimate”), using a common set of assumptions provided by the Parties, or if the Parties cannot agree, determined by the Appraisers. Each Party shall use its best efforts to cause its designated Appraiser to provide the Estimate not later than 30 days after the Parties or the Appraiser, as the case may be, have determined the common set of assumptions to be used.
If the Estimate submitted by one of the Appraisers exceeds the Estimate submitted by the other Appraiser by 10% or less, the Fair Market Value shall be deemed to be the arithmetic mean of the two Estimates.
If the Estimate submitted by one of the Appraisers exceeds the Estimate submitted by the other Appraiser by more than 10%, the Parties, or if the Parties cannot agree, the Appraisers, shall designate a third appraiser (which in the case of an investment bank shall not be the regular banker of either Party) (the “Third Appraiser”) to prepare an estimate of Fair Market Value using the same common set of assumptions but without access to the earlier Estimates. The Third Appraiser shall be directed to provide its estimate of Fair Market Value within 30 days of its designation. If the estimate of Fair Market Value submitted by the Third Appraiser falls between the Estimates of the Appraisers, the Fair Market Value shall be deemed to be the arithmetic mean of all three estimates of Fair Market Value. If the estimate of Fair Market Value submitted by the Third Appraiser does not fall in between the Estimates of the Appraisers, the Estimate submitted by one of the two Appraisers that is closest to the estimate of Fair Market Value submitted by the Third Appraiser shall be deemed to be the Fair Market Value.
Each party shall bear the costs and expenses of its own Appraiser as well as 50% of the costs and expenses of the Third Appraiser, if necessary.
2.13. Subcontracting. Each Party may exercise any of the rights or obligations that such Party may have under this Agreement or the Supply Agreement to engage in research and development activities, manufacturing activities or testing activities with respect to Licensed Products by subcontracting all or any portion of such research and development activities, manufacturing activities or testing activities, and, in connection with any such subcontracting, to grant such sublicenses as may be necessary to permit any Third Party subcontractor to perform
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
17
the activities subcontracted to such Third Party subcontractor. Each Party may also exercise any of its rights or obligations under this Agreement to Commercialize Licensed Products by subcontracting all or any portion of such rights or obligations to distributors, and, in connection with any such subcontracting, to grant such sublicenses as may be necessary to permit any such distributor to perform the activities subcontracted to such distributor, provided, however, that Chiron shall not grant to any such distributor any right other than the right to Commercialize in the Territory Licensed Products supplied to such distributor by or on behalf of Chiron, and, specifically, Chiron shall not grant to such distributor any right to engage in research or development activities with respect to, or to manufacture or have manufactured, Licensed Products anywhere in the world. Any sublicenses granted pursuant to this Section 2.13 shall be consistent with all of the terms and conditions of this Agreement and the Supply Agreement that are applicable. The scope of any such sublicenses shall be no greater than the scope of the rights under this Agreement or the Supply Agreement held by the Party granting such sublicenses and such sublicenses shall be subject to all of the limitations imposed on the rights under this Agreement and the Supply Agreement of the Party granting such sublicenses. Subcontracting as contemplated by this Section 2.13 by either Party of any of the rights or obligations that such Party may have under this Agreement or the Supply Agreement shall not relieve such Party from any of its obligations under this Agreement or the Supply Agreement.
2.14. Rights of Last of Refusal for Canada. Cubist hereby grants Chiron a right of last refusal to obtain rights to sell any and all Licensed Products in Canada (the “Canadian Marketing Rights”), on the following terms: In the event that Cubist proposes to grant, sell, assign or otherwise transfer all or any portion of the Canadian Marketing Rights to a Third Party (other than a distributor appointed by Cubist, directly or indirectly, who sells Licensed Products) who is not the party who has licensed or holds or will be licensed or hold the Commercialization rights with respect to all Licensed Products in the United States (the “U.S. Rights Holder”), then Cubist acknowledges and agrees that prior to entering into any agreement for the grant of the Canadian Marketing Rights with any Third Party (other than the U.S. Rights Holder or a distributor appointed by Cubist, directly or indirectly, who sells Licensed Products), Cubist will provide to Chiron a copy of the fully negotiated final draft of such proposed agreement with such Third Party and offer to Chiron the opportunity to enter into an agreement with Cubist for the same rights and on the same terms as set forth in such draft. Provided that Cubist has given Chiron [*] notice of the possibility of such transaction, Chiron shall have [*] to provide Cubist written notice of its decision. If and only if Chiron declines such offer, or fails to accept such offer within such [*], will Cubist then be free to enter into such agreement with such Third Party, it being understood and acknowledged by Cubist that any material modification of the terms of such proposed agreement with the Third Party after it had been declined by Chiron shall constitute a breach of Cubist’s obligations under this Section 2.14 and a violation of Chiron’s right of last refusal hereunder. The provsions of this Section 2.14 shall not apply if Chiron is in material breach of this Agreement or the Supply Agreement.
2.15. Applicable Supply Agreement Provisions. The following provisions of the Supply Agreement are incorporated herein by reference and shall apply to this Agreement in the same manner as which they apply to the Supply Agreement: Sections 2.4 (Second Manufacturing Source(s)), 2.5 (Global Manufacturing Plan), 7.1 (Transfer Pricing), 8.1 (Allocation in the Event of Shortages) and 11.6 (Back-up Supply Rights of Chiron).
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
18
ARTICLE 3.
COORDINATION
3.1. General. The Parties desire to establish a committee to review the Parties’ activities under this Agreement, to exchange information regarding, and to discuss the Parties’ development and commercialization of, Licensed Products within and outside of the Territory. It is the intent of the Parties that such committee would serve as a forum for discussion and exchange of ideas and that the Parties shall endeavor to reach consensus on matters discussed in this forum. Such committee shall operate as set forth in this Article 3 and in other provisions of this Agreement.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
19
3.2. Joint Coordination Team.
(a) Formation. Within thirty (30) days after the Effective Date, Cubist and Chiron shall establish the Joint Coordination Team (the “JCT”), which shall (i) serve as a forum for the review and discussion of the Parties’ efforts under this Agreement including the development and marketing and sales activities in the Territory and outside the Territory with respect to Licensed Products, and (ii) serve as a forum for the resolution of disputes between the Parties in accordance with the provisions of Article 15 of this Agreement.
(b) Membership. Cubist and Chiron each shall designate an equal number of representatives (but in no event more than four (4) representatives each) with appropriate expertise to serve as members of the JCT. Of the initial representatives to be designated by each Party, there shall be expertise in regulatory matters, clinical development matters, and sales and marketing matters. The Parties shall be free to change their representatives over time as the needs evolve over time. Each representative serving on the JCT shall have appropriate technical credentials, experience and knowledge, and ongoing familiarity in the specific area of such representative’s expertise. A Party may replace its representatives serving on the JCT from time to time by written notice to the other Party specifying the prior representative(s) to be replaced and the replacement(s) therefor. Each Party shall select one (1) person appointed by it to the JCT to serve as co-chair. The co-chairpersons shall be responsible for calling meetings, preparing and circulating an agenda in advance of each meeting, and preparing and issuing minutes of each meeting within thirty (30) days thereafter.
(c) Meetings. The JCT shall hold meetings at such times as it elects to do so, but in no event shall such meetings be held less frequently than once every three (3) months unless otherwise agreed by the Parties. The first meeting of the JCT shall be held no earlier than 60 days after the Effective Date and no later than 90 days after the Effective Date. The JCT shall meet alternately at Cubist’s facility and at Chiron’s facility, or at such location(s) as the Parties may otherwise agree. With the consent of the representatives of each Party serving on the JCT, other representatives of each Party or of Third Parties involved in the manufacture, development or commercialization of the Licensed Products may attend meetings of the JCT. Meetings of the JCT may be held by audio or video teleconference with the consent of each Party, provided that at least one (1) meeting per year shall be held in person. Each Party shall be responsible for all of its own expenses of participating in the JCT. Meetings of the JCT shall be effective only if at least two (2) representatives of each Party are present or participating. The co-chairpersons will alternate responsibility for preparing minutes of each meeting of the JCT, which minutes will not be finalized until the co-chairperson that did not prepare such minutes reviews and confirms the accuracy of such minutes in writing.
(d) Specific Activities. In addition to its functions described in Section 3.2(a), the JCT shall in particular review, consider, discuss and comment upon where appropriate:
(i) the overall strategy for the development of Licensed Products for sale within and outside the Territory, and the design of all pre-clinical trials and clinical trials in connection with the development of Licensed Products for sale within and outside the Territory;
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
20
(ii) the development activities of Cubist with respect to Licensed Products, and the development activities of Chiron with respect to Licensed Products;
(iii) the Cubist Development Plan and Chiron’s development plan (if any), each updated annually;
(iv) the strategies for obtaining and maintaining Regulatory Approvals within and outside the Territory;
(v) the regulatory activities of Cubist for obtaining and maintaining Regulatory Approvals of Licensed Products in the United States, and the regulatory activities of Chiron for obtaining and maintaining Regulatory Approvals of Licensed Products in the Territory;
(vi) the Regulatory Plan and the corresponding plan of Cubist for obtaining Regulatory Approval outside of the Territory updated annually;
(vii) the general strategy for the manufacturing and supply of Licensed Products and the corresponding Manufacturing Plan, updated annually;
(viii) the general strategy for the Commercialization of Licensed Products within and outside the Territory;
(ix) the Commercialization activities of Cubist with respect to Licensed Products in the United States, and the Commercialization activities of Chiron with respect to Licensed Products in the Territory;
(x) the Marketing Plan and the corresponding plan of Cubist for Commercializing the Licensed Products outside of the Territory updated annually;
(xi) the Manufacturing Plan for the manufacture and supply of Licensed Products worldwide updated annually;
(xii) the Parties’ scientific presentation and publication strategy relating to Licensed Products; and
(xiii) such other materials as are specifically provided for review at the JCT elsewhere in this Agreement or in the Supply Agreement.
(e) Limited Authority; Not a Decision-Making Body.
(i) Except for its function as a dispute resolution forum, the role of the JCT shall be facilitative. The JCT shall serve as a forum for the sharing of information. In addition, the JCT may serve the purpose of preventing, or informally resolving, disputes between the Parties. The rights and responsibilities of each Party shall be governed by this Agreement, including the exhibits hereto, and the JCT shall not have any power to amend, modify or waive compliance with this Agreement.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
21
(ii) Although it is not the purpose of the JCT to make decisions that control the Parties’ respective activities under this Agreement, the JCT shall at all appropriate times endeavor to operate by consensus. In the event that one Party elects to deviate from a course of action for which consensus was achieved in the JCT, as a courtesy to the other Party, the deviating Party will notify the other Party in writing of the intended deviation and the reasons therefor. In addition, when a Party brings a matter to the JCT for review and comment (either as required by the provisions of this Agreement or voluntarily), if the Party seeking comment elects not to incorporate the other Party’s comments, then, as a courtesy to the other Party, the Party that sought comments will notify the other Party in writing of the decision not to incorporate the comments and the reasons therefor. With respect to matters to be discussed by the JCT, the representatives of each Party shall endeavor to present a unified position on behalf of such Party.
(f) Meeting Agendas. Each Party will disclose to the other Party its final agenda items along with appropriate related information at least ten (10) business days in advance of each meeting of the JCT.
3.3. JCT Coordinators. Each Party shall appoint a designee (a “JCT Coordinator”) to coordinate its activities under this Agreement. The JCT Coordinators shall serve as primary contacts between the Parties with respect to this Agreement. Each Party shall notify the other Party within thirty (30) days of the date of this Agreement of the appointment of its JCT Coordinator and shall notify the other Party as soon as practicable upon changing such appointment. The JCT Coordinator appointed by each Party shall be responsible for (i) preparing such Party’s representatives serving on the JCT for meetings of the JCT, (ii) coordinating the distribution and exchange of information to, from and among such Party’s representatives serving on the JCT, and (iii) assisting in the coordination of the day-to-day activities of such Party’s representatives serving on the JCT so that the JCT can function effectively and such representatives can more effectively discharge their responsibilities as members of the JCT.
3.4. Independence. Subject to the terms of this Agreement, the activities and resources of each Party shall be managed by such Party, acting independently and in its individual capacity. The relationship between Cubist and Chiron is that of independent contractors, and neither Party shall have the power to bind or obligate the other Party in any manner, other than as is expressly set forth in this Agreement.
ARTICLE 4.
DEVELOPMENT
4.1. Key Development Studies; Diligence Obligations. Cubist shall use Commercially Reasonable Efforts to complete, subject to the provisions of this Section 4.1, the Key Development Studies. Cubist reserves the right to change or modify any of the Key Development Studies, or to abandon or discontinue any of the Key Development Studies, in accordance with the provisions set forth below:
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
22
(a) Cubist may change or modify any Key Development Study if such change or modification is designed to improve the probability of obtaining Regulatory Approval of the indication targeted by such Key Development Study.
(b) Cubist may change, modify, abandon or discontinue any Key Development Study in response to (i) any regulatory feedback, (ii) increases of [*] or more in the anticipated costs of clinical trials for any reason other than due to an increase in the size or scope of such clinical trials, or (iii) any significant adverse event or condition relating to the safety or efficacy of a Licensed Product.
(c) If Cubist abandons or discontinues any Key Development Study, Cubist will use Commercially Reasonable Efforts to replace such study with another clinical study that is designed to improve the probability of obtaining Regulatory Approval of the indication targeted by such abandoned or discontinued study (a “Replacement Study”). Following any such replacement, Cubist shall use Commercially Reasonable Efforts to complete the Replacement Study provided that Cubist may change, modify, abandon or discontinue such study to the same extent as provided in this Section 4.1 for the Key Development Studies.
Notwithstanding anything in this Section 4.1 to the contrary, prior to making any decision to abandon or discontinue any of the Key Development Studies, any Replacement Study or any Replacement Indication Study (each a “Required Study”), or to change or modify any Required Study so as to effectively abandon same, Cubist shall have first provided its reasons in support of such decision to Chiron via the JCT and the JCT shall have met to discuss the merits of such decision. Cubist shall bear all of the costs and expenses in connection with any and all development activities engaged in by Cubist and its Affiliates in connection with all Required Studies.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
23
4.2. Cubist Development Plan.
(a) Cubist shall prepare and update annually a development plan for Licensed Products (the “Cubist Development Plan”). The Cubist Development Plan may include, as appropriate and without limitation, the following elements:
(i) Cubist’s plan for conducting and completing the Required Studies;
(ii) Cubist’s general strategy for developing indications other than the indications pursued in the Required Studies for use of Licensed Products throughout the world;
(iii) design and execution of pre-clinical and clinical studies for indications other than the indications pursued in the Required Studies;
(iv) development of improvements in formulation, presentation and other features of Licensed Products considered desirable for life cycle management and maximizing sales of Licensed Products throughout the world; and
(v) an analysis of the total costs and expenses incurred or to be incurred by Cubist in connection with its development activities set forth in the Development Plan.
Cubist shall update the Cubist Development Plan annually and provide such updated Cubist Development Plan to Chiron prior to the JCT’s last scheduled meeting during the then current year. Chiron shall have the right to review and comment, via the JCT, on the Cubist Development Plan and any and all revisions and updates thereto, and Cubist shall, in good faith, consider all comments made by Chiron.
(b) Subject to the provisions of this Section 4.2(b), Cubist shall use Commercially Reasonable Efforts to carry out and perform during each calendar year the activities set forth in the Cubist Development Plan as may be in effect for such calendar year that are materially significant for obtaining Regulatory Approval. With the exception of the Required Studies (which Cubist may not change, modify, abandon or discontinue except in accordance with the provisions of Section 4.1 hereof), Cubist reserves the right at any time to change or modify the Cubist Development Plan or any of the preclinical studies or clinical trials described in the Cubist Development Plan, or to abandon any portion of the Cubist Development Plan or discontinue any such preclinical studies or clinical trials, in response to (i) changes in clinical or regulatory strategy, (ii) regulatory feedback, (iii) scientific feasibility, (iv) increases in the anticipated costs of clinical trials, (v) any significant adverse event or condition relating to the safety or efficacy of a Licensed Product, (vi) manufacturing feasibility, including, without limitation, changes in the anticipated costs of manufacturing, (vii) significant adverse changes in market conditions or in market potential of a drug candidate, or (viii) any other reason that Cubist determines in its reasonable discretion justifies such change, modification, abandonment or discontinuation; however, prior to making any decision to abandon any development effort (or change or modify any such effort so as to effectively abandon same), Cubist shall have first
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
24
provided its reasons in support of such decision to Chiron via the JCT and the JCT shall have met to discuss the merits of such decision.
(c) Except to the extent otherwise provided in Section 4.2(d) below, Cubist shall bear all of the costs and expenses in connection with any and all development activities engaged in by Cubist and its Affiliates pursuant to, and in accordance with, the provisions of this Section 4.2 and any Cubist Development Plan made hereunder.
(d) In the event that Cubist and Chiron consider it appropriate to consolidate their efforts in the Territory in the Cubist Development Plan and the Chiron Development Plan into common clinical studies, the Parties shall discuss at the JCT an appropriate sharing of resources, costs and expenses in connection with such studies. If the Parties cannot agree to a sharing of resources, costs and expenses, then neither Party shall be obligated to consolidate its respective clinical activities with those of the other Party. Except pursuant to any such agreement between Cubist and Chiron, Cubist shall have no obligation to undertake any clinical studies or to make material protocol changes that are not required by the FDA or any other Regulatory Authorities outside the Territory but are proposed to be undertaken for the sole purpose of complying with the requirements of any Regulatory Authority in the Territory.
4.3. Development by Chiron.
(a) Territory Specific Studies. The Parties acknowledge that Regulatory Authorities in the Territory may require that certain clinical studies be conducted in connection with Regulatory Approval sought in the Territory by Chiron, but which studies are not required by the FDA or any other Regulatory Authorities outside the Territory (the “Territory Specific Studies” and each a “Territory Specific Study”). Chiron shall use Commercially Reasonable Efforts to conduct the Territory Specific Studies provided that Chiron shall be entitled to decline to perform any Territory Specific Study if, in its sole discretion, it considers the study to be prohibitively expensive, uneconomical or not in its commercial interest. If Chiron elects not to conduct any particular Territory Specific Study, it will notify Cubist in writing of its reasons therefor and discuss same at a meeting of the JCT. If the Parties are unable to reach an agreement on cost sharing to conduct such Territory Specific Study, Chiron shall have no further obligation to conduct such Territory Specific Study.
(b) Proposed Indications. Chiron may, from time to time, propose to Cubist through the JCT that Cubist consider pursuing development for a new indication (the “Proposed Indication”). Such proposal shall be in writing and contain such information as may be readily gathered which will assist Cubist to evaluate the proposal on its merits. If after due consideration by Cubist and Chiron at the JCT, consensus is reached to pursue development for such new indication, Cubist shall add the Proposed Indication to the Cubist Development Plan. In the event that the Parties are unable to reach consensus on adding the Proposed Indication then Chiron shall be free to pursue, at its own expense, development of such Proposed Indication.
(c) Medical Affairs Studies. Chiron may conduct Medical Affairs Studies of the Licensed Products at its sole cost and expense. Chiron shall provide to Cubist via the JCT its proposal for any Medical Affairs Study it wishes to conduct, for review and discussion purposes only. Cubist shall supply Licensed Products to be used in Medical Affairs Studies (i) at a
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
25
purchase price equal to the Transfer Price, and (ii) in an amount not to exceed [*], during the first complete calendar year (and any part thereof) after Commercial Launch, and for any calendar year thereafter, [*] by Chiron for the corresponding period as reflected in the forecasts of purchase quantities delivered by Chiron to Cubist pursuant to the Supply Agreement.
(d) Chiron Development Plan. Chiron will prepare and update annually a development plan for Chiron’s development activities pursuant to this Section 4.3 (the “Chiron Development Plan”). Chiron shall update the Chiron Development Plan each year to reflect the activities that Chiron expects to conduct during the following calendar year and Chiron shall provide the updated Chiron Development Plan to Cubist prior to the JCT’s last scheduled meeting during the then current year. Cubist shall have the right to review and comment, via the JCT, on the Chiron Development Plan and any and all revisions or updates thereto and Chiron shall, in good faith, consider all comments made by Cubist. Subject to the provisions set forth below in this Section 4.3(d), Chiron shall use Commercially Reasonable Efforts to carry out and perform during each calendar year the activities set forth in the Chiron Development Plan, as may be in effect for such calendar year, that are materially significant for obtaining Regulatory Approval in the Territory. With the exception of the Territory Specific Studies (which Chiron may decline to perform in accordance with the provisions of Section 4.3(a) above), Chiron reserves the right at any time to change or modify the Chiron Development Plan or any of the preclinical studies or clinical trials described in the Chiron Development Plan, or to abandon any portion of the Chiron Development Plan or discontinue any such preclinical studies or clinical trials, in response to (i) changes in clinical or regulatory strategy of either Chiron or Cubist, (ii) regulatory feedback, (iii) scientific feasibility, (iv) increases in the anticipated costs of clinical trials, (v) any significant adverse event or condition relating to the safety or efficacy of a Licensed Product, (vi) manufacturing feasibility, including, without limitation, changes in the anticipated costs of manufacturing and/or procuring Licensed Product, (vii) significant adverse changes in market conditions or in market potential of a drug candidate, or (viii) any other reason that Chiron determines in its reasonable discretion justifies such change, modification, abandonment or discontinuation; however, prior to making any decision to abandon any development effort (or change or modify any such effort so as to effectively abandon same), Chiron shall have first provided its reasons in support of such decision to Cubist via the JCT and the JCT shall have met to discuss the merits of such decision. The exercise by Chiron of any right that it may have under this Section 4.3(d) to change, modify, abandon or discontinue all or any portion of the Chiron Development Plan or any of the preclinical studies or clinical trials described in the Chiron Development Plan shall not relieve Chiron from its obligations to use Commercially Reasonable Efforts under Section 5.1 or Section 6.1 hereof.
(e) Limitation on Chiron’s Development Rights. Notwithstanding anything in this Section 4.3 to the contrary, in the event that at any time Cubist reasonably believes, on the basis of medical, clinical, scientific and other data, facts and knowledge that have been published or are otherwise documented and available or are known to subject matter experts, and presented to Chiron, that any of the development activities being conducted or to be conducted by Chiron pursuant to this Section 4.3 is substantially likely to result in Global Harm, Cubist shall promptly convene a meeting of the JCT to discuss same. Prior to the meeting, Cubist will provide to Chiron all relevant documents, materials and information suggesting the likelihood of Global Harm as well as Cubist’s recommendations to Chiron on the course of action (e.g. modify the
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
26
study, terminate the study, etc.). At the JCT, the Parties will discuss and attempt to reach consensus on the course of action. Following such discussion, in the absence of consensus, Cubist shall have the right to require Chiron to modify or discontinue those development activities that Cubist reasonably and in good faith believes, based on the totality of the data, facts and knowledge presented to Chiron and the data, facts and knowledge presented by Chiron in response, are substantially likely to result in Global Harm. In the event that there is no person (including a successor-in-interest to Cubist) to carry out Cubist’s development obligations under Section 4.1, then (i) the provisions of this Section 4.3(e) shall not be applicable to the Licensed Product(s) as to which such development obligations are not being performed, and (ii) Chiron shall be free from its obligations to consult via the JCT under Sections 4.3(b) and 4.3(c) with respect to the Licensed Product(s) as to which such development obligations are not being performed. In the event that a successor-in-interest to Cubist shall be in material breach of such development obligations, then (i) the provisions of this Section 4.3(e) shall not be applicable to the Licensed Product(s) and the indication(s) as to which such development obligations are not being performed, and (ii) Chiron shall be free from its obligations to consult via the JCT under Sections 4.3(b) and 4.3(c) with respect to the Licensed Product(s) and the indications as to which such development obligations are not being performed.
4.4. Access to Chiron Data.
(a) Information Sharing. Chiron will provide to Cubist all Information that results from any development activities conducted pursuant to Section 4.3 above or the Chiron Development Plan (the “Chiron Data”). Subject to Section 4.4(c), any such Chiron Data so provided shall be strictly for information purposes only and Cubist shall have no right to use the Chiron Data for any purpose whatsoever, including without limitation, to access, reference, interpret, disclose, tabulate, analyze or otherwise use any such Chiron Data for the purpose of supporting any Drug Approval Application anywhere in the world.
(b) Reciprocating Licensee. Cubist may share the Chiron Data with any Other Licensee who has agreed in writing to permit reciprocal sharing by Cubist with Chiron of the results of such Other Licensee’s own development activities in accordance with the provisions of Section 5.3 hereof (each a “Reciprocating Licensee”), provided that Cubist has first communicated the restrictions set forth in this Section 4.4 on use of the Chiron Data and obtained the Reciprocating Licensee’s written agreement to abide by such restrictions.
(c) License Option. Chiron hereby grants to Cubist an option to acquire a nonexclusive license on commercially reasonable terms (as to which the Parties shall agree thereon at any time on or prior to the date of the exercise of the option) to access, reference, interpret, disclose, tabulate, analyze or otherwise use all Chiron Data for the purpose of supporting Drug Approval Applications in any country outside the Territory. The Parties contemplate that such license will include a right of Cubist to grant sublicenses to any Reciprocating Licensee who agrees in writing to grant a reciprocal option to Cubist to acquire a nonexclusive license on commercially reasonable terms (as to which such Reciprocating Licensee and Cubist shall agree thereon at any time on or prior to the date of the exercise of the option) to access, reference, interpret, disclose, tabulate, analyze or otherwise use all similar development data of such Reciprocating Licensee for the purpose of supporting Drug Approval
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
27
Applications in any country within the Territory, with a right of Cubist to grant sublicenses to Chiron.
4.5. No Debarred Personnel. In the course of the development of Licensed Products, neither Party shall use, during the term of this Agreement, the services of any employee or consultant that has been debarred by the FDA or Regulatory Authorities, or, to the best of such Party’s knowledge, is the subject of debarment proceedings by the FDA or Regulatory Authorities.
4.6. Chiron Compliance. In connection with any development activities undertaken by Chiron in connection with any Licensed Product, Chiron shall comply with all applicable laws and regulations regarding the care and use of experimental animals, as such laws and regulations are in effect where such development activities are undertaken. All animals used by Chiron to evaluate Daptomycin or any Licensed Product shall be provided humane care and treatment in accordance with the most acceptable veterinary practices.
ARTICLE 5.
REGULATORY
5.1. Regulatory Plan; Diligence Obligation.
(a) Cubist’s and Other Licensees’ Activities. Cubist shall prepare and present to Chiron via the JCT, and update annually, its regulatory plan for the Licensed Products outside of the Territory. Cubist shall keep Chiron apprised of the status of Cubist’s and the Other Licensees’ efforts to obtain Regulatory Approval in countries outside of the Territory. Prior to making any decision to abandon any efforts and activities set forth in such regulatory plan to obtain Regulatory Approval in the United States for a Licensed Product (or for an indication or formulation of same), Cubist shall have first provided its reasons in support of such decision to Chiron via the JCT and the JCT shall have met to discuss the reasons for such decision.
(b) Regulatory Plan. Within the time period set forth in Section 5.1(c)(i), Chiron shall prepare and deliver to Cubist one regulatory plan for the Territory, which regulatory plan shall set forth Chiron’s plan, strategy and proposed activities to obtain Regulatory Approval in the Territory for the Launch Indication(s) (the “Regulatory Plan”). The Regulatory Plan may include, as appropriate and without limitation, the following elements:
(i) Chiron’s general strategy for seeking Regulatory Approval for the Launch Indication(s) in the Major Market Countries;
(ii) Chiron’s plans for the preparation and filing of applications for Regulatory Approval for the Launch Indication(s) in the Major Market Countries;
(iii) Chiron’s general strategy for seeking Regulatory Approval for the Launch Indication(s) in the remaining countries of the Territory, however such outline may at Chiron’s sole discretion deal with such remaining countries individually or in the aggregate;
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
28
(iv) if known, a description of any clinical studies or clinical data that will be necessary in order to obtain Regulatory Approval in the countries referenced in the foregoing clauses (i)-(iii) of this Section 5.1(b), with respect to the Launch Indication(s), and a discussion of the impact of any such clinical studies or clinical data requirement on the timing of obtaining Regulatory Approval for the Launch Indication(s) in such country or countries; and
(v) the proposed timetable for obtaining anticipated Regulatory Approval for the Launch Indication(s) in the countries referenced in the foregoing clauses (i) and (ii) of this Section 5.1(b), and the countries referenced in the foregoing clause (iii) of this Section 5.1(b), individually or in the aggregate, and a discussion of the factors that could result in an acceleration or a delay of such proposed timetable.
Chiron shall update the Regulatory Plan annually and provide such updated Regulatory Plan to Cubist prior to the JCT’s last scheduled meeting during the then current year. Such updated Regulatory Plan shall, at such later time as and when considered appropriate by Chiron in its sole discretion, cover, among other things, additional Licensed Products, additional indications and additional countries, considered individually or in the aggregate. Cubist shall have the right to review and comment, via the JCT, on the Regulatory Plan and any and all revisions and updates thereto, and Chiron shall, in good faith, consider all comments made by Cubist. For the avoidance of doubt, the foregoing provisions of this Section 5.1(b) shall not impose any obligation on Chiron to have any plan, strategy or any proposed activities, or to conduct any particular activities, with respect to any specific country within the Territory (it being understood that Chiron may have no such plan, strategy or any proposed activities with respect to any specific country in the Territory) but shall only impose an obligation on Chiron to set forth in the Regulatory Plan any such plan, strategy or proposed activities with respect to any specific country within the Territory to the extent that Chiron has any such plan, strategy or proposed activities. The provisions of the foregoing sentence shall not relieve Chiron from its obligations to use Commercially Reasonable Efforts under Section 5.1(c) below.
Subject to the provisions of this Section 5.1(b), Chiron shall use Commercially Reasonable Efforts to carry out and perform during each calendar year the plan, strategy and proposed activities set forth in the Regulatory Plan, as may be in effect for such calendar year, that are materially significant for obtaining Regulatory Approval in the Territory. Chiron reserves the right at any time to change or modify the Regulatory Plan in response to (i) changes in clinical strategy or regulatory strategy of either Chiron or Cubist, (ii) regulatory feedback, (iii) scientific feasibility, (iv) increases in anticipated costs of any Territory Specific Studies, (v) any significant adverse event or condition relating to the safety or efficacy of a Licensed Product, (vi) manufacturing feasibility, including, without limitation, changes in the anticipated costs of manufacturing and/or procuring Licensed Product, (v) significant adverse changes in market conditions or a market potential of a drug candidate, or (vi) any other reason that Chiron determines in its reasonable discretion justifies such change or modification; however, prior to making any decision to change or modify the Regulatory Plan (other than the annual updates), Chiron shall have first provided its reasons in support of such decision to Cubist via the JCT and the JCT shall have met to discuss the merits of such decision. The exercise by Chiron of any right that it may have under this Section 5.1(b) to change or modify all or any portion of the Regulatory Plan shall not relieve Chiron from its obligations to use Commercially Reasonable Efforts under Section 5.1(c) below or Section 6.1 hereof.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
29
(c) Specific Regulatory Activities. Chiron will use Commercially Reasonable Efforts to:
(i) inform Cubist whether Chiron will pursue a central filing strategy or a mutual recognition strategy in the European Union as soon as practicable, but in no event later than [*] after the date that Cubist provides Chiron with all information submitted by Cubist to the FDA in connection with its application for Regulatory Approval of Licensed Product in the United States;
(ii) if Chiron has determined to pursue a central filing strategy in the European Union, prepare and file an application to obtain Regulatory Approval of Licensed Products for the Launch Indication(s) with respect to all countries in the European Union within [*] after either (a) receiving from Cubist, or (b) collection on its own, as the case may be, all data and information required for such filing;
(iii) if Chiron has determined to pursue a mutual recognition strategy in the European Union, prepare and file an application for Regulatory Approval of Licensed Products for the Launch Indication(s) in the reference member state within [*] after either (a) receiving from Cubist, or (b) collecting on its own, as the case may be, all data and information required for such filings;
(iv) if Chiron has determined to pursue a mutual recognition strategy in the European Union, prepare and file applications for Regulatory Approval of Licensed Products for the Launch Indication(s) in all Major Market Countries within [*] of obtaining Regulatory Approval in the reference member state;
(v) if Chiron has determined to pursue a mutual recognition strategy in the European Union, then no later than [*] after obtaining Regulatory Approval in the reference member state, prepare and file applications for Regulatory Approval of Licensed Products for the Launch Indication(s) in at least [*] other countries to be selected by Chiron from the countries listed under the caption “EUROPE” on Exhibit D hereto that are not Major Market Countries;
(vi) no later than [*] after obtaining Regulatory Approval in the reference member state (if pursuing a mutual recognition strategy in the European Union) or in all countries of the European Union (if pursuing a central filing strategy in the European Union), prepare and file an application for Regulatory Approval of Licensed Products for the Launch Indication(s) in at least one country listed under the caption “LATIN AMERICA” on Exhibit D hereto, provided, however, that, in lieu of complying with the foregoing provisions of this clause (vii), Chiron may elect, instead, to have at least [*] with the Regulatory Authorities of at least [*] countries listed under the caption “LATIN AMERICA” on Exhibit D hereto no later than such [*] period, and, if Chiron makes such election, then the number of countries as to which Chiron must satisfy the requirements of clause (v) above in this Section 5.1(c) shall be increased from [*] other countries in the European Union to [*] other countries in the European Union;
(vii) respond in timely fashion to requests for data and information from Regulatory Authorities, provided that such data and information are in Chiron’s possession,
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
30
given to Chiron by Cubist or otherwise available to Chiron without having to incur any substantial cost in order to acquire such data and information;
(viii) meet with officials from Regulatory Authorities at such times as may be requested by such Regulatory Authorities;
(ix) subject to Chiron’s right under Section 4.3(a) to decline to perform any given Territory Specific Study for economic reasons (and subject to Cubist’s right under Section 4.3(e) to require Chiron to modify or discontinue a specific study for substantial likelihood of Global Harm), perform the following activities, as appropriate, with respect to any Territory Specific Study:
(1) prepare draft protocols for the Territory Specific Study after the Regulatory Authority has confirmed any such requirement for such study;
(2) take such actions to obtain approvals from appropriate Regulatory Authorities and other governmental authorities of any draft protocol and corresponding clinical trial application for the Territory Specific Study as soon as reasonably practicable after such draft protocol has been prepared;
(3) identify and engage suitable investigators and study sites for the Territory Specific Study as soon as reasonably practicable after the draft protocol for the Territory Specific Study has been prepared and such protocol and corresponding clinical trial application has been approved by the appropriate Regulatory Authorities and other governmental authorities; and
(4) commence enrollment for the Territory Specific Study as soon as reasonably practicable after all required approvals have been obtained, suitable investigators and study sites have been engaged, suitable quantities of Licensed Product for clinical use have been obtained by Chiron, and all other necessary or advisable steps have been taken to prepare investigators and study sites to enroll patients;
(x) conduct and complete any Territory Specific Study in accordance with the approved protocol, subject to (X) Chiron’s right under Section 4.3(a) to decline to perform any given Territory Specific Study for economic reasons, (Y) Cubist’s right under Section 4.3(e) to require Chiron to modify or discontinue a specific study for substantial likelihood of Global Harm, and (Z) completion of the steps described in Section 5.1(c)(ix), above.
Notwithstanding any provision of this Section 5.1(c) to the contrary, Chiron shall not be deemed to be in material breach of any of its obligations under this Section 5.1(c) to prepare and file applications for Regulatory Approval for each Launch Indication if and to the extent that Chiron cannot meet any of such obligations solely by virtue of the fact that one or more Regulatory Authorities do not permit Chiron to file such applications for Regulatory Approval for only a single indication. If a Regulatory Authority in the Territory informs Chiron that it may not file applications for Regulatory Approval for a single indication, then the Parties shall convene a meeting of the JCT to discuss alternative strategies that could be pursued by the
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
31
Parties. In the event that Chiron is not permitted to file applications for Regulatory Approval in any country within the Territory for a single indication, then the Parties agree that as soon thereafter as Chiron is permitted to file for Regulatory Approval in such country (whether because such filing will be for more than a single indication or because the regulatory environment in such country has changed so as to permit a filing for Regulatory Approval for a single indication), then Chiron shall at that time again be obligated to comply with the requirements, if any, imposed by this Section 5.1(c) with respect to preparing and making filings for Regulatory Approval in such country, and, for purposes of applying the timelines in this Section 5.1(c) at that time, the date on which Chiron is first permitted to make such filing in such country shall be deemed to be the end of the [*] period after Cubist has given to Chiron all of the information necessary to make such filing.
(d) Costs and Expenses. Chiron shall bear all of the costs and expenses in connection with any and all regulatory activities within the Territory engaged in by Chiron and its Affiliates pursuant to, and in accordance with, the provisions of this Section 5.1, it being understood that the foregoing regulatory activities shall not include any activities of Chiron to assist Cubist with its development obligations under this Agreement or outlined in the Cubist Development Plan. Cubist shall bear all of the costs and expenses in connection with any and all regulatory activities engaged in by Cubist and its Affiliates in connection with obtaining and maintaining Regulatory Approval for Licensed Products outside the Territory, regardless of whether such activities occur within the Territory or outside the Territory.
(e) Limitation on Activities. Notwithstanding anything in this Section 5.1 or elsewhere in this Agreement to the contrary, in the event that at any time Cubist reasonably believes on the basis of medical, clinical, scientific and other data, facts and knowledge that have been published or are otherwise documented and available or are known to subject matter experts, and presented to Chiron, that obtaining Regulatory Approval in the Territory for any Licensed Product for any particular indication either: (i) substantially increases the risk that an adverse safety event will occur; or (ii) would result in the use of a Licensed Product to treat an indication for which such Licensed Product is an ineffective or inferior therapeutic alternative and such use would materially adversely affect the development, marketing, distribution or Commercialization of such Licensed Product or any other Licensed Product within or outside the Territory for other indications; Cubist shall promptly convene a meeting of the JCT to discuss same. Prior to the meeting, Cubist will provide to Chiron all relevant documents, materials and information suggesting the likelihood of events described in clauses (i) and (ii) above, as well as Cubist’s recommendations to Chiron on the course of action (e.g., not seeking approval of a particular Licensed Product or not seeking approval of a particular indication, etc.). At the JCT, the Parties will discuss and attempt to reach consensus on the course of action. Following such discussion, in the absence of consensus, Cubist shall have the right to require Chiron to modify or discontinue those regulatory activities that Cubist reasonably and in good faith believes, based on the totality of the data, facts and knowledge presented to Chiron and the data, facts and knowledge presented by Chiron in response, are substantially likely to result in the events described in clause (i) or clause (ii) above in this Section 5.1(e).
5.2. Ownership of Regulatory Approvals. Except to the extent otherwise provided elsewhere in this Agreement, Chiron shall file in its own name and own all Drug Approval Applications and Regulatory Approvals for Licensed Products in the Territory, and shall be
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
32
solely responsible for all communications with regulatory authorities in such countries. All such filings shall be made in such manner as may be required under the laws of the applicable jurisdiction to allow for the expeditious transfer of such Drug Approval Applications and Regulatory Approvals for Licensed Products to Cubist if such transfer is required pursuant to Article 13 hereof upon termination (but not expiration) of this Agreement. Promptly following execution of this Agreement, Cubist shall transfer and assign to Chiron any and all such Drug Approval Applications or Regulatory Approvals held by Cubist as of the date hereof.
5.3. Chiron Access to Cubist and Other Licensee Data.
(a) Information Sharing. Subject to the provisions of Section 5.3(d) below, during the Term, Cubist will provide to Chiron any Cubist Data and Other Licensee Data necessary or useful for making regulatory filings for, or marketing of, Licensed Products in the Territory as such Cubist Data and Other Licensee Data are or become available. The Parties shall discuss, via their participation in the JCT, the form in which Cubist shall provide Chiron Cubist Data and Other Licensee Data pursuant to this Section 5.3(a). Subject to the provisions of Section 5.3(c) below, any such Other Licensee Data so provided shall be strictly for information purposes only and Chiron shall have no right to use the Other Licensee Data for any purpose whatsoever, including without limitation, to access, reference, interpret, disclose, tabulate, analyze or otherwise use any such Other Licensee Data for the purpose of supporting any Drug Approval Application anywhere in the world.
(b) Chiron Use of Cubist Data. Chiron shall have a right of access, a right of reference and the right to use and incorporate all Cubist Data provided to it pursuant to this Section 5.3 in its Drug Approval Applications for Regulatory Approvals of Licensed Products within the Territory.
(c) License Option. Cubist shall use Commercially Reasonable Efforts to obtain the written agreement of each Other Licensee granting to Cubist an option to acquire a nonexclusive license to access, reference, interpret, disclose, tabulate, analyze or otherwise use all Other Licensee Data for the purpose of supporting Drug Approval Applications in any country within the Territory. Cubist shall also use Commercially Reasonable Efforts to obtain the written agreement of such Other Licensee that Cubist shall have the right to grant to Chiron a sublicense to any such nonexclusive license such that Chiron can access, reference, interpret, disclose, tabulate, analyze or otherwise use all the Other Licensee Data of such Other Licensee for the purpose of supporting Drug Approval Applications in any country within the Territory. In the event that any such nonexclusive license may be sublicensed to Chiron and that Chiron requests that Cubist exercise any such option to acquire such nonexclusive license, then Chiron shall pay for any and all of the costs of exercising such option to the extent attributable to the grant of the sublicense to Chiron.
(d) Manufacturing Information. Cubist acknowledges and agrees that certain Cubist Know-How and other Information pertaining to the manufacture of the Licensed Products (the “Manufacturing Information”) will be required to be provided or made available to Chiron so as to permit Chiron to obtain Regulatory Approval in the Territory, conduct quality assurance testing and release of Licensed Products for use in the Territory and otherwise comply with the requirements of the Regulatory Authorities in the Territory. The right of Chiron to
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
33
receive and use Manufacturing Information shall be as set forth in this Section 5.3(d) and the Supply Agreement.
To the extent that Chiron needs to submit and make available any Manufacturing Information in connection with obtaining Regulatory Approval in the Territory, the Parties agree that, to the fullest extent permissible under applicable law, rules and regulations, such Manufacturing Information shall be made available to the applicable Regulatory Authorities in the Territory via a Drug Master File appropriately formatted for the country in which such DMF is filed and [*] (i.e. upon Chiron’s [*] under the Supply Agreement to manufacture or have manufactured Licensed Products). Drug Master Files will be maintained and kept up to date by Cubist or the Cubist Suppliers. Chiron will be notified of changes proposed to the DMF [*] in accordance with change control procedures set forth in Section 3.2 of the Supply Agreement. Chiron shall be responsible for notifying the Regulatory Authorities in the Territory, as required by applicable laws, of the updating of the DMF by Cubist or the Cubist Suppliers.
Notwithstanding the foregoing, the applicable Regulatory Authorities in the Territory shall have full access to all of the Manufacturing Information in such DMF [*], to the extent considered necessary by such applicable Regulatory Authorities to consider and act upon any applications for Regulatory Approvals in the Territory that are submitted by Chiron, and [*] to allow such Regulatory Authorities to refer to all of the Manufacturing Information in such DMF to the extent necessary to assess applications for Regulatory Approvals of Licensed Products in the Territory and to obtain such Regulatory Approvals.
If and to the extent that Chiron needs to submit and make available any Manufacturing Information in connection with any submission by Chiron of any application for Regulatory Approval of Licensed Product within the Territory and the applicable regulatory requirements do not permit the submission of such Manufacturing Information via a DMF, then the Parties agree that such Manufacturing Information shall be made available to Chiron for the sole purpose of filing applications for Regulatory Approvals of Licensed Products in the Territory and obtaining such Regulatory Approvals, and that Chiron shall not be entitled to use such Manufacturing Information for any other purpose whatsoever other than to manufacture or have manufactured Licensed Products and conduct quality assurance testing and release of Finished Products, but only if, when and to the extent that Chiron is expressly authorized under the Supply Agreement to use such Information for such manufacturing purposes.
5.4. Free Sales Certificates. The Parties agree to cooperate with each other to obtain free sale certificates where appropriate.
5.5. Safety; Adverse Event Reporting.
(a) General. The Parties shall promptly exchange any and all appropriate safety data, and the Parties shall report, and take other appropriate actions in relation to, adverse events with Licensed Products to each other, all in accordance with a reporting protocol that will be established by the JCT. In addition, each Party shall have the right to review the other Party’s internal processes and procedures for the collection and processing of safety data. Without limiting the generality of the foregoing, each Party shall maintain a record of all non-medical and medical product-related complaints and reports of adverse events that it receives with respect to
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
34
any Licensed Product and each Party shall notify the other Party of any complaint received by it and, within [*] of the initial receipt, provide the other Party with a copy of such complaint(s) and adverse event reports.
(b) Reporting Outside the Territory. Cubist shall be responsible for reporting to the FDA and Regulatory Authorities outside of the Territory any adverse experience and safety issues for Licensed Products in compliance with the requirements of the U.S. Food, Drug and Cosmetic Act, 21 U.S.C. §321 et seq., the regulations promulgated thereunder, and the equivalent laws, rules and regulations in countries other than the United States outside of the Territory, and shall promptly thereafter provide to Chiron a copy of such report.
(c) Reporting in the Territory. Chiron shall be responsible for reporting to Regulatory Authorities in the Territory any adverse experience and safety issues for Licensed Products in compliance with the requirements of the applicable laws, rules and regulations, and shall promptly thereafter provide to Cubist a copy of such report. Cubist shall require each of its Other Licensees to comply with obligations corresponding to those in this Section 5.5.
(d) Global Database. Cubist shall be responsible for compiling a validated global database that captures all adverse events reported to Cubist from any source and maintaining said database as defined in the Supply Agreement (or any associated Quality Agreement referenced in the Supply Agreement). Cubist shall also provide Chiron with [*] so as to support the applications for Regulatory Approval and the maintenance of marketing authorizations in the Territory, provided that it is technically feasible to do so and that Chiron makes payment of any incremental license fees that may be required in order add Chiron as an authorized user.
5.6. Communications.
(a) Communications Relating to Regulatory Approval. Except as may be required by law or as otherwise expressly provided in this Agreement or the Supply Agreement, during the Term, Cubist shall not communicate with any Regulatory Authority having jurisdiction in the Territory regarding Regulatory Approval for any Licensed Product in the Territory unless requested to do so by Chiron. If Cubist is required by applicable laws or regulations or a Regulatory Authority having jurisdiction in the Territory to disclose such information directly to such Regulatory Authority, Cubist shall notify Chiron in writing of the requirement and the particulars of the information required to be disclosed, and Cubist shall coordinate with Chiron in making any such disclosure. [*] between Cubist and such Regulatory Authority but Chiron shall lead any such face-to-face meetings or scheduled conference calls. Each party shall advise the other party of material developments and events relating to their respective regulatory responsibilities in writing within two (2) business days after notice of such material development event.
(b) Communications Relating to Development. Cubist shall have the right to communicate with Regulatory Authorities in the Territory regarding Licensed Products for the purpose of advancing its global development activities in connection with Licensed Products, including, those development activities as described in the Cubist Development Plan, provided that Cubist complies with the provisions of this Section 5.6. Cubist shall notify Chiron in
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
35
advance of any such communications and Chiron shall have the right to be present and to participate at any face-to-face meetings or scheduled conference calls between Cubist and such Regulatory Authority regarding such communications, but Cubist shall lead any such face-to-face meetings or scheduled conference calls at which Chiron is present and participating. Cubist shall consult with Chiron prior to any such face-to-face meeting or scheduled conference calls at which Chiron is present and participating to develop a strategy for the meeting.
5.7. Recalls and Voluntary Withdrawals.
(a) The Parties shall exchange their internal standard operating procedures (“SOPs”) as to product recalls and the treatment of product complaints and inquiries as to safety, quality or efficacy reasonably in advance of the date of Commercial Launch of any Licensed Product in the Territory. If either Party becomes aware of information about any Licensed Product indicating that it may not conform to the specifications for Licensed Product then in effect pursuant to the Supply Agreement, or that there are potential adulteration, misbranding and/or other issues regarding safety or effectiveness, it shall promptly so notify the other Party. The JCT shall meet to discuss such circumstances and to consider appropriate courses of action, which courses of action with respect to each recall shall be consistent with the internal SOP of the Party having the right to control such recall pursuant to this Section 5.7. Except to the extent otherwise provided in Section 5.7(b) below, Chiron shall control, at its sole expense, all recalls of Licensed Product within the Territory. Cubist shall control, at its sole expense, all recalls of Licensed Product outside the Territory. Chiron shall maintain complete and accurate records of any recall for such periods as may be required by legal requirements, but in any event for no less than three (3) years.
(b) In the event that any recall of Licensed Product in the Territory is required or necessary by virtue of such Licensed Product being Defective Manufactured Product, then both Parties shall work together to devise a reasonable and appropriate plan to implement such recall, it being understood that the objective of such plan shall be to effect such recall as quickly and as cost-effectively as reasonably possible under the circumstances. After the Parties shall have reached agreement on the terms of such plan (or, in the event that the Parties cannot reach agreement as to the terms of such plan, Chiron shall have the right, acting reasonably and in good faith, to adopt another plan), Chiron shall implement such recall in a manner consistent with the terms of such plan. Cubist shall cooperate with Chiron in any way that Chiron reasonably requests in order to support the implementation of such recall. Subject to the limitation of liability set forth in Section 10.6(a), Cubist shall reimburse Chiron for all Recall Expenses (as defined below) provided that [*]. For the avoidance of doubt, the limitation set forth above in this Section 5.7(b) (and in Section 6.6(b) of the Supply Agreement) on Cubist’s responsibility for Recall Expenses shall not apply to limit Chiron’s remedies and Cubist’s obligations or liabilities with respect to matters other than Recall Expenses, including, without limitation, (i) Cubist’s obligation to indemnify Chiron pursuant to Sections 10.1 and 10.3 of this Agreement with respect to costs and expenses other than Recall Expenses, and (ii) Chiron’s remedies under Sections 8.1, 9.1(d) and 11.6 of the Supply Agreement. “Recall Expenses” shall mean the costs and expenses incurred by Chiron in connection with a recall of Licensed Product that is implemented in accordance with this Section 5.7(b) (and/or Section 6.6(b) of the Supply Agreement), including, without limitation, the costs and expenses associated with notification and destruction and/or return of such recalled Licensed Product.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
36
5.8. Label. To the extent permitted by applicable law, regulation or the Regulatory Authority having jurisdiction, Chiron shall identify Cubist as the licensor of the Cubist Marks applied to Licensed Products on the vial label, package insert, outside of the packaging and advertising materials for such Licensed Product in each country of the Territory in a manner approved in advance in writing by Cubist, such consent not to be unreasonably withheld, conditioned or delayed. Chiron shall also comply with the patent marking provisions of Section 8.8 hereof.
5.9. Governmental Inspections. Each party shall advise the other party of any governmental visits to, or written or oral inquires about, any facilities or procedures for the manufacture, storage, or handling of the Licensed Product, or the marketing, selling, promotion or distribution of the Licensed Product, promptly (but in no event later than [*] after notice of such visit or inquiry. Each party shall, within [*] of receipt or submission, furnish to the other party any report or correspondence issued by or provided to the governmental authority in connection with such visit or inquiry.
ARTICLE 6.
COMMERCIALIZATION; DILIGENCE
6.1. Marketing Plan; Diligence Obligation.
(a) Marketing Plan. Not later than three (3) months after the acceptance by a Regulatory Authority of an application for Regulatory Approval for the Launch Indication(s) in any Major Market Country (the “Marketing Plan Trigger Event”), Chiron shall prepare and deliver to Cubist one marketing plan for the Territory, which marketing plan shall set forth Chiron’s plan, strategy and proposed activities to market the Licensed Products in the Territory with respect to the Launch Indication(s) (the “Marketing Plan”). The Marketing Plan may include, as appropriate and without limitation, the following elements:
(i) the anticipated date of Commercial Launch for the Launch Indication(s) in each Major Market Country;
(ii) a description of Chiron’s general strategy with respect to pre-launch and post-launch marketing, advertising and promotion activities (including, without limitation, sponsoring medical education events) for the Launch Indication(s) in each Major Market Country;
(iii) a description of the marketing, advertising and promotional activities (including, without limitation, sponsoring medical education events and product positioning of Chiron) for the Launch Indication(s) in each Major Market Country;
(iv) an estimated time schedule for the performance of marketing activities;
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
37
(v) a general description of the personnel resources of Chiron that will perform the marketing activities; and
(vi) a general description of Chiron’s pricing strategy in the Territory.
Effective upon the occurrence of the Marketing Plan Trigger Event, Chiron shall update annually and provide such updated Marketing Plan to Cubist prior to the JCT’s last scheduled meeting during the then current year. Such updated Marketing Plan shall, at such later time as Chiron shall determine in its sole discretion, cover, among other things, additional Licensed Products, additional indications and additional countries for which Regulatory Approval has been obtained, considered individually or in the aggregate. Cubist shall have the right to review and comment, via the JCT, on the Marketing Plan and any and all revisions and updates thereto, and Chiron shall, in good faith, consider all comments made by Cubist. For the avoidance of doubt, the foregoing provisions of this Section 6.1(a) shall not impose any obligation on Chiron to have any plan, strategy or any proposed activities, or to conduct any particular activities, with respect to any specific country within the Territory (it being understood that Chiron may have no such plan, strategy or any proposed activities with respect to any specific country in the Territory) but shall only impose an obligation on Chiron to set forth in the Marketing Plan any such plan, strategy or proposed activities with respect to any specific country within the Territory to the extent that Chiron has any such plan, strategy or proposed activities. The provisions of the foregoing sentence shall not relieve Chiron from its obligations to use Commercially Reasonable Efforts under Section 6.1(b) below.
Effective from and after the Marketing Plan Trigger Event, and subject to the provisions of this Section 6.1(a), Chiron shall use Commercially Reasonable Efforts to carry out and perform during each calendar year the plan, strategy and proposed activities set forth in the Marketing Plan, as may be in effect for such calendar year. Chiron reserves the right at any time to change or modify the Marketing Plan in response to (i) changes in clinical strategy or regulatory strategy of either Chiron or Cubist, (ii) regulatory feedback, (iii) scientific feasibility, (iv) increases in anticipated costs of any Territory Specific Studies, (v) any significant adverse event or condition relating to the safety or efficacy of a Licensed Product, (vi) manufacturing feasibility, including, without limitation, changes in the anticipated costs of manufacturing and/or procuring Licensed Product, (v) significant adverse changes in market conditions or a market potential of a drug candidate, or (vi) any other reason that Chiron determines in its reasonable discretion justifies such change or modification; however, prior to making any decision to change or modify the Marketing Plan (other than annual updates), Chiron shall have first provided its reasons in support of such decision to Cubist via the JCT and the JCT shall have met to discuss the merits of such decision. The exercise by Chiron of any right that it may have under this Section 6.1(a) to change or modify all or any portion of the Marketing Plan shall not relieve Chiron from its obligations to use Commercially Reasonable Efforts under Section 6.1(b) below.
(b) Marketing Activities. Chiron will use Commercially Reasonable Efforts to:
(i) effect the Commercial Launch of Licensed Product for the Launch Indication(s) within [*] after obtaining Regulatory Approval and acceptable Price Approval therefor; and
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
38
(ii) market, sell and distribute Licensed Products for the Launch Indication(s), upon receipt of Regulatory Approval and acceptable Price Approval.
In addition and without limiting the foregoing, during the period commencing [*] before the date of first Commercial Launch of Licensed Product in each of the Major Market Countries, and continuing through the [*] after such first Commercial Launch, Chiron shall:
(1) budget and spend on marketing, advertising and promotion activities with respect to Licensed Product (exclusive of fully loaded direct and indirect costs attributable to salespersons): (A) in each Major Market Country an amount equal to the greater of: (I) [*], or (II) [*] of the forecasted Net Sales of such Licensed Product in such Major Market Country; or (B) in all of the Major Market Countries collectively, an aggregate amount equal to the greater of: (III) [*] the amount in the foregoing clause (I) during the period commencing [*] before the date of first Commercial Launch of Licensed Product in the first such country and continuing through the [*] after such first Commercial Launch in the last such country, or (IV) [*] the amount in the foregoing clause (II) during the period commencing [*] before the date of first Commercial Launch of Licensed Product in the first such country and continuing through the [*] after such first Commercial Launch in the last such country; and
(2) employ or engage not less than [*] salespersons to sell Licensed Products in each Major Market Country or not less than [*] salespersons in the aggregate in all of the Major Market Countries.
For purposes of the foregoing provisions of this Section 6.1(b), the availability of supply of Licensed Products shall be considered in determining whether Chiron has used Commercially Reasonable Efforts to meet its diligence obligations. Further, any delay by Chiron in effecting Commercial Launch in any Major Market Country until Regulatory Approval in such country has been obtained for a second indication (to the extent that a single indication does not allow Chiron to obtain acceptable Price Approval in such country) shall be deemed not to be a material breach by Chiron of any of its obligations under this Agreement.
(c) Costs and Expenses. Chiron shall bear all of the costs and expenses in connection with any and all Commercialization activities engaged in by Chiron and its Affiliates pursuant to, and in accordance with, the provisions of this Section 6.1.
(d) Ownership of Marketing Information. Chiron shall be the sole owner of all rights, including intellectual property rights, in and to all sales, marketing, financial and business Information of Chiron, including without limitation, all customer and distributor lists of Chiron and all Information of Chiron regarding sales projections, distribution channels, market potential and competitive products analyses for the Licensed Products (the “Chiron Marketing Information”), and, except to the extent otherwise expressly provided elsewhere in this Agreement or the Supply Agreement, no right of access, use or other license is granted to Cubist or to any Other Licensee under this Agreement. Notwithstanding any provision in this Agreement to the contrary, no Chiron Marketing Information shall form any part of Chiron Data, Chiron Know-How, Chiron Related Know-How or any Joint Know-How.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
39
6.2. Prohibited Marketing and Sales Activities. Chiron shall not engage in any direct marketing activities for the Licensed Products outside of the Territory without Cubist approval. Cubist shall not engage in any direct marketing activities inside the Territory without Chiron approval. The foregoing shall not prohibit either party from participating in any conferences, symposia or other industry gatherings merely because the venue for such gathering happens to be outside of the Territory in the case of Chiron or inside the Territory in the case of Cubist. Chiron shall require that its distributors make all commercially reasonable efforts to prevent any units of Licensed Product sold by such distributors in any country within the Territory from being resold in, or transported to, any country outside of the Territory.
6.3. Discounting. Neither Chiron nor its Affiliates shall discount the price of Licensed Products or agree to reduce the unit volume of purchases or sales of any Licensed Product in consideration of any price increase on, the receipt of any payment in connection with, or the entering of an arrangement for the purchase or sale (including, without limitation, any arrangement guaranteeing volume increases or minimum volume) of, a product other than a Licensed Product, or shall enter into any agreement for such purpose.
6.4. Marketing and Promotional Literature. Marketing and promotional literature related to the Licensed Products and prepared for use in the Territory by Chiron shall be prepared in a manner consistent with relevant local statutes and regulations. In all marketing and promotional literature, Cubist shall be presented and described as the party who developed the Licensed Product. To the extent permitted by law, all marketing and promotional literature shall display the Cubist Marks, trade names, logos and trade dress of the Licensed Product in a manner that promotes the Product and each of the parties in an appropriate manner. To the extent practical, the Cubist name and logo shall appear the same in size and proximity as the Chiron name and logo on all marketing and promotional literature used in each country of the Territory unless prohibited by the law, statutes or regulations of such country. With the consent of Chiron, which consent shall not be unreasonably withheld or delayed, Cubist shall have the right to reproduce, distribute and otherwise use outside the Territory all Licensed Product related marketing and promotional literature prepared by Chiron. With the consent of Cubist, which consent shall not be unreasonably withheld or delayed, Chiron shall have the right to reproduce, distribute and otherwise use in the Territory all Licensed Product related marketing and promotional literature prepared by Cubist.
ARTICLE 7.
COMPENSATION
7.1. Upfront Consideration.
(a) In consideration for the licenses granted to Chiron under this Agreement, Chiron shall, as soon as practicable after the Effective Date (but in no event later than the seventh (7th) business day following the Effective Date), make payment to Cubist of a license fee in the amount of eight million dollars ($8,000,000). Such license fee shall be in the form of a
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
40
cash payment made by wire transfer of immediately available funds to a bank account or bank accounts designated by Cubist. Such license fee shall be nonrefundable and noncreditable.
(b) Simultaneously with the execution and delivery of this Agreement, the Chiron Parent Company and Cubist are entering into the Stock Purchase Agreement pursuant to which, among other things, at the closing referred to below in this Section 7.1(b), Cubist shall agree to sell to the Chiron Parent Company, and the Chiron Parent Company shall agree to purchase from Cubist, [*] shares of Cubist common stock (subject to adjustment for any reclassification, stock split, stock dividend, reverse stock split merger, consolidation or similar transaction or event). The aggregate purchase price payable by the Chiron Parent Company for all of such shares of Cubist common stock shall be equal to ten million dollars ($10,000,000), payable in the form of a cash payment made by wire transfer of immediately available funds to a bank account or bank accounts designated by Cubist.
(c) The closing of the sale and purchase of such shares of Cubist Common Stock shall take place as soon as practicable after the Effective Date but in no event later than the seventh (7th) business day following the Effective Date.
7.2. Milestone Payments. Subject to Section 13.6(a)(v), in consideration for the licenses and exclusive rights of this Agreement, Chiron shall make a milestone payment to Cubist upon the achievement of each development milestone with respect to a Licensed Product as set forth in the table in Section 7.2(a) below, and Chiron shall also make a milestone payment to Cubist upon the achievement of each sales milestone with respect to a Licensed Product as set forth in the table in Section 7.2(b) below. Chiron shall make payment of the applicable milestone payment within [*] after Chiron’s receipt of notice from Cubist of the first achievement of each such development milestone and of each such sales milestone, as documented by appropriate written and/or other materials. Each such milestone payment by Chiron shall be in the form of a cash payment made by wire transfer of immediately available funds to a bank account or bank accounts designated by Cubist. Each milestone payment by Chiron to Cubist hereunder shall be paid only once, and shall be noncreditable and nonrefundable.
(a) Development Milestone Payments.
|
|
|
|||
|
|
|
(in millions) |
|
|
|
1. |
Regulatory Approval of a Licensed Product [*] |
|
[*] |
|
|
2. |
Price Approval of a Licensed Product [*] |
|
[*] |
|
|
3. |
Regulatory Approval of a Licensed Product for [*] by Regulatory Authorities [*] |
|
[*] |
|
|
Total Potential Development Milestone Payments |
|
[*] |
|
|
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
41
(b) Sales Milestone Payments.
|
Milestone Event |
|
Payment |
|
||
|
|
|
(in millions) |
|
||
|
1. |
Cumulative Net Sales in the Territory is equal to or greater than [*] |
|
[*] |
|
|
|
2. |
Cumulative Net Sales in the Territory is equal to or greater than [*] |
|
[*] |
|
|
|
3. |
Cumulative Net Sales in the Territory is equal to or greater than [*] |
|
[*] |
|
|
|
4. |
Cumulative Net Sales in the Territory is equal to or greater than [*] |
|
[*] |
|
|
|
5. |
Cumulative Net Sales in the Territory is equal to or greater than [*] |
|
[*] |
|
|
|
|
Total Potential Sales Milestone Payments |
|
[*] |
|
|
7.3. Royalties.
(a) Royalty. Subject to the other terms and conditions of this Agreement (and, in particular , the provisions of Section 7.3(b) and Section 7.3(c) below), Chiron shall pay Cubist a royalty with respect to each Licensed Product sold by Chiron or its Affiliates equal to a percentage determined in accordance with the table below or in accordance with Sections 7.3(b) or 7.3(c) below, as applicable, taking into consideration the protection afforded by Cubist Patents at the relevant time, (such applicable percentage, the “Royalty Rate”), of Net Sales in any country within the Territory of units of such Licensed Product during each calendar quarter from and after the date of Commercial Launch in such country, less the aggregate Transfer Price previously paid to Cubist for having supplied pursuant to the Supply Agreement such units of such Licensed Product that are sold by Chiron or its Affiliates during such calendar quarter:
|
Royalty Year |
|
Royalty Rate for the First [*] of |
|
Royalty Rate for Aggregate |
|
|
[*] |
|
[*] |
|
[*] |
|
|
[*] |
|
[*] |
|
[*] |
|
|
[*] |
|
[*] |
|
[*] |
|
For purposes of this Section 7.3, the first Royalty Year with respect to all Licensed Products shall commence on the date of Commercial Launch by Chiron or any of its Affiliates in any country within the Territory and end on December 31 of the year in which the Commercial Launch occurred. Each succeeding Royalty Year shall commence on January 1 of the ensuing
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
42
year and end on December 31 of such year. Accordingly, the determination of the Royalty Year for the purpose of determining the Royalty Rate which applies shall not be either on a Licensed Product-by-Licensed Product basis or on a country-by-country basis.
The determination of the Royalty Rate pursuant to the table in this Section 7.3(a) (i.e. whether the Royalty Rate in left hand column or the right hand column in the table above applies) shall also not be made on a Licensed Product-by-Licensed Product basis or on a country-by-country basis, but, instead, shall be made based on the aggregate amount of annual Net Sales for all Licensed Products in all countries in the Territory.
The determination of the amount of royalties due Cubist pursuant to this Section 7.3(a) shall be made on a Licensed Product-by-Licensed Product basis and on a country-by-country basis by multiplying the Royalty Rate that is applicable for any particular Royalty Year, as determined in accordance with the foregoing provisions of this Section 7.3(a), by the Net Sales arising from sales in each country within the Territory of units of each Licensed Product by Chiron or its Affiliates during each calendar quarter of the applicable Royalty Year.
(b) Know-How Step-Down. Notwithstanding Section 7.3(a) but subject to Section 7.3(c), for any given country in the Territory, if there is no Valid Claim that would be infringed by the sale by Chiron or its Affiliates of Licensed Product in such country but for the licenses granted by Cubist to Chiron hereunder (treating, for purposes of this Section 7.3(b) only, all Joint Patents as if Cubist owned all right, title and interest in and to the Joint Patents and Chiron had no interest in the Joint Patents other than the rights licensed to Chiron by Cubist), then the Royalty Rate applicable to Net Sales of such Licensed Product by Chiron or its Affiliates in such subject country shall be the rate as determined in accordance with the table in Section 7.3(a) above, but reduced by [*] (for example, the Royalty Rate for the second royalty year where aggregate annual Net Sales are less than [*] shall be [*] instead of [*]). The determination of the amount of royalties due Cubist pursuant to this Section 7.3(b) shall be made on a Licensed Product-by-Licensed Product basis and on a country-by-country basis by multiplying the Royalty Rate that is applicable for any particular Royalty Year, as determined in accordance with the foregoing provisions of this Section 7.3(b), by the Net Sales arising from sales in each country within the Territory of units of each Licensed Product by Chiron or its Affiliates during each calendar quarter of the applicable Royalty Year. Notwithstanding anything in this Agreement to the contrary, no royalty shall be payable by Chiron pursuant to this Section 7.3(b) after [*].
(c) Competition Step-Down. Notwithstanding anything in the foregoing provisions of this Section 7.3 to the contrary, for any given country within the Territory, if (i) the aggregate unit sales by all Third Parties in such country of Unlicensed Products constitute more than [*] of the market share on a per unit basis with respect to all unit sales of such Unlicensed Products and the affected Licensed Product in such country (i.e., including the unit sales by Chiron and its Affiliates and distributors of the Licensed Product whose market share has been affected by sales of such Unlicensed Product), and (ii) there is no Valid Claim which is being infringed by such Third Parties in connection with such Third Parties’ use or sale of such Unlicensed Products in such country or in connection with such Third Parties’ manufacture of such Unlicensed Products anywhere in the world, then, subject to the provisions set forth below
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
43
in this Section 7.3(c) and Sections 7.3(d), 7.3(e) and 7.3(f), the obligation of Chiron and its Affiliates to pay royalties to Cubist under this Section 7.3 with respect to sales of such Licensed Product in such country shall terminate and be of no further force or effect.
(d) Competition Step-Down Procedures. If Chiron believes that the provisions of Section 7.3(c) apply, Chiron shall notify Cubist in writing of such belief and shall provide, together with such notice, copies of the market research studies, market surveys or other marketing data that show that the aggregate unit sales in such subject country of Unlicensed Products exceeds the [*] market share threshold set forth in clause (i) of Section 7.3(c). Cubist shall have a period of [*] from the date that such notice and market data is delivered to Cubist to evaluate the issue and reach its own determination as to whether the criteria of Section 7.3(c) have been satisfied.
(i) If within such [*] period Cubist sends written notice to Chiron stating that Cubist agrees with Chiron’s position on the issue, then the provisions of Section 7.3(c) shall apply to sales of such Licensed Product by Chiron or its Affiliates in such country and Chiron shall be entitled to cease paying royalties under this Section 7.3 in connection with such sales.
(ii) If Cubist does not send such written notice to Chiron within the [*] period in clause (i) above, but within [*] after the end of such [*], Cubist commences an action against the allegedly infringing Third Parties to enforce the Valid Claims and prevent sales of Unlicensed Products in such country, then Chiron shall continue making payment of royalties in connection with sales of the affected Licensed Product by Chiron or its Affiliates in such country in the manner set forth in Section 7.3(e).
(iii) If Cubist does not send such written notice to Chiron within the [*] period in clause (i) above and if Cubist does not commence such action within the [*] period in clause (ii) above, then the provisions of Section 7.3(c) shall be deemed to apply to sales of such Licensed Product by Chiron or its Affiliates in such country and Chiron shall be entitled to cease paying royalties under this Section 7.3 in connection with such sales.
If Chiron becomes entitled to cease paying royalties under Section 7.3(c) (i.e. pursuant to clause (i) or (iii) of this Section 7.3(d)), then, within [*] after Chiron so becomes entitled to cease paying royalties, Cubist shall make payment to Chiron of all unearned royalties previously paid by Chiron to Cubist as of the earliest month when sales of Unlicensed Product exceeded the [*] market share threshold, together with interest thereon at the rate specified for late payments pursuant to Section 7.10 hereof.
(e) Holdback of Royalties Upon Certain Cubist Enforcement Actions. If after having received Chiron’s notice to Cubist pursuant to Section 7.3(d), Cubist has timely commenced an action to enforce the Valid Claims as required by Section 7.3(d)(ii), then, with respect to any sales of such Licensed Product in the country in question, from and after the date that Cubist commences such action, Chiron shall pay to Cubist [*] of the royalties that Chiron otherwise would be required to pay to Cubist in connection with such sales pursuant to Section 7.3(a) or Section 7.3(b), as applicable, and without giving effect for this purpose to Section 7.3(c). The obligation of Chiron to make the partial payment of royalties in accordance with the
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
44
foregoing provisions of this Section 7.3(e) shall continue with respect to such sales of such Licensed Product in such country for so long as Cubist shall continue the action to enforce the Valid Claims in order to prevent sales in such country of the applicable Unlicensed Products, subject however to the other terms of this Agreement governing the duration of the obligation of Chiron to pay royalties to Cubist. If the final adjudication (following the expiration of all rights of appeal) or final settlement of such action will result in aggregate unit sales by all Third Parties in such country of such applicable Unlicensed Products to be less than [*] of the market share on a per unit basis with respect to all unit sales of such applicable Unlicensed Products and the affected Licensed Product in such country (i.e., including the unit sales by Chiron and its Affiliates and distributors of the Licensed Product whose market share has been affected by sales of such applicable Unlicensed Product) (such [*] [*] market share on a per unit basis, the “Unlicensed Sales Threshold”), then, within [*] after written notice to Chiron that such final adjudication or final settlement has occurred, Chiron shall make payment to Cubist the remaining [*] of the royalties payable that Chiron withheld pursuant to this Section 7.3(e) together with interest thereon at the rate specified for late payments pursuant to Section 7.10 hereof. If, on the other hand, the final adjudication or final settlement of such action will not result in the aggregate unit sales by all Third Parties in such country of such applicable Unlicensed Products to be below the Unlicensed Sales Threshold in such country, then, within [*] of such final adjudication or final settlement, Cubist shall make payment to Chiron of all unearned royalties paid by Chiron to Cubist pursuant to this Section 7.3(e) together with interest thereon at the rate specified for late payments pursuant to Section 7.10 hereof.
(f) Chiron Enforcement Action. Notwithstanding anything expressed or implied in Sections 7.3(c), 7.3(d) or 7.3(e) to the contrary, if Chiron commences an action to enforce a Valid Claim against any sales of Unlicensed Products in any given country in the Territory, then Chiron shall continue to make payment to Cubist of royalties payable in connection with sales of Licensed Products in such country in accordance with the provisions of Section 7.3(a) or Section 7.3(b), as applicable, unless and until there is a final adjudication (following the expiration of all rights of appeal) or final settlement of such action that will result in aggregate unit sales by all Third Parties in such country of such Unlicensed Products to be greater than the Unlicensed Sales Threshold in such country. If the final adjudication or final settlement of such action will result in aggregate unit sales by all Third Parties in such country of such Unlicensed Products to be greater than the Unlicensed Sales Threshold in such country, then, within [*] after written notice to Cubist that such final adjudication or final settlement has occurred, Cubist shall make payment to Chiron of all unearned royalties paid by Chiron to Cubist pursuant to this Section 7.3(f) as of the earliest month when sales of Unlicensed Product exceeded the [*] market share threshold, together with interest thereon at the rate specified for late payments pursuant to Section 7.10 hereof. Chiron shall not enter into any such final settlement without the prior written consent of Cubist (which consent shall not be unreasonably withheld or delayed).
(g) Clinical Supplies. Notwithstanding any provision in this Agreement to the contrary, Chiron shall have no obligation to pay any royalty to Cubist for any Licensed Products distributed by Chiron for use in any clinical study conducted by Chiron under this Agreement, including without limitation, Territory Specific Studies, clinical studies for Proposed Indications and Medical Affairs Studies.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
45
7.4. Adjustments. In recognition and acknowledgement of the commercial advantage provided to Chiron under this Agreement even in the absence of a Valid Claim, including, but not limited to, advantages obtained by use of Cubist’s Know-How or regulatory exclusivity, the Parties have agreed to the royalty rates set forth in Section 7.3(b). In the event that a court of competent jurisdiction or arbitration panel determines that the downward adjustment on the applicable Royalty Rate effected by the provisions of Section 7.3(b) is insufficient to avoid violating the doctrine of patent misuse or otherwise to avoid rendering the obligation of Chiron to pay royalties to Cubist pursuant to Section 7.3 unenforceable, in either case in connection with sales of any Licensed Product in any country in the Territory that would be subject to the foregoing provisions of Section 7.3(b), then the Parties hereby agree (i) that any such court of competent jurisdiction or arbitration panel shall have the discretion and right to reduce the applicable Royalty Rate to a level where such court of competent jurisdiction or arbitration panel deems appropriate or necessary so that the doctrine of patent misuse or any other applicable law is not violated and (ii) that such court of competent jurisdiction or arbitration panel shall enforce the obligation of Chiron and its Affiliates to pay royalties to Cubist pursuant to Section 7.3 using such reduced applicable Royalty Rate to calculate Chiron’s royalty obligation under Section 7.3.
7.5. Third Party Royalties and Other Payments. The royalties payable by Chiron to Cubist under Section 7.3 shall be inclusive of (and Cubist shall be solely responsible for) any and all royalty obligations or other payment obligations to Third Parties arising from license or other agreements entered into by Cubist prior to the Effective Date relating to the manufacture, use, sale, offer for sale or importation of the Licensed Products, including without limitation, the Lilly License, or after the Effective Date with respect to such obligations incurred by Cubist pursuant to Section 8.5(c) relating to Patents of any Third Party that were published or generally available prior to the Effective Date.
7.6. Royalty Payments and Reports. All amounts payable to Cubist under this Agreement shall be paid by Chiron in Dollars within [*] of the end of each calendar quarter except as otherwise specifically provided herein. Each payment of royalties owing to Cubist shall be accompanied by a statement, on a country-by-country basis, of (i) the amount of gross sales of Licensed Product, (ii) an itemized calculation of Net Sales showing deductions during such calendar quarter provided for in the definition of Net Sales, (iii) the amount of aggregate gross sales of Licensed Product and Net Sales in all countries within the Territory during such calendar quarter and on a cumulative basis as of the end of such calendar quarter, (iv) the amount of royalty due on such sales, and (v) the currency exchange rates used in determining the amount of Dollars payable to Cubist. If any royalty reductions are claimed by Chiron under this Agreement from the full royalty rates set forth in Section 7.3, then the report shall set forth in detail the claimed reduction and the related facts.
7.7. No Reductions or Offsets. Chiron and its Affiliates shall make payment to Cubist of all amounts that Chiron is required to pay to Cubist pursuant to this Article 7 without any reduction, offset or setoff, except to the extent otherwise expressly provided in Sections 7.3(c), 7.3(d), 7.3(e) or 7.3(f). In addition, in the event that the amount of royalties due from Chiron to Cubist pursuant to this Agreement is less than the aggregate Transfer Price previously paid to Cubist for such units, then Chiron acknowledges and agrees that it shall not be entitled to any refund or credit of such previously paid Transfer Price, and that Cubist shall not be liable or responsible for, or owe Chiron any monies as a result of, such deficiency.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
46
7.8. Tax Matters.
(a) The Parties acknowledge and agree that under the current United States - Ireland Tax Treaty (the “Tax Treaty”), Chiron has no obligation to withhold any amounts on account of taxes from any payment by Chiron to Cubist under this Article 7.
(b) [*] to Cubist under this Article 7 [*] shall at its sole discretion either (i) [*], or (ii) [*] to the extent of any [*].
(c) [*] to Cubist [*] shall at its sole discretion either (i) [*], or (ii) [*] to the extent of any [*].
7.9. Foreign Exchange. For the purpose of computing the Net Sales for Licensed Products sold in a currency other than Dollars, such currency shall be converted into Dollars using the average monthly rate of exchange for the month in which such currencies are received, as at 12:00 P.M. New York time for the Federal Reserve Bank of New York (Bloomberg Page USCF Index).
7.10. Late Payments. Any amounts not paid by Chiron when due under this Agreement shall be subject to interest from and including the date payment is due through and including the date upon which Chiron has made a wire transfer of immediately available funds into an account designated by Cubist at a rate equal to [*] quoted in the Money Rates section of the Wall Street Journal (New York Edition) calculated daily on the basis of a 365-day year, or similar reputable data source, or, if lower, the highest rate permitted under applicable law.
7.11. Exports of Licensed Product from the Territory; [*] In the event that Cubist provides to Chiron written evidence that Licensed Products sold in any country in the Territory by Chiron are being exported from such country and imported into a country outside the Territory, and that such export of Licensed Product has had a material adverse effect on the sales by Cubist or an Other Licensee of Licensed Product in such country outside the Territory, then Chiron shall use Commercially Reasonable Efforts to employ such remedies as are available to Chiron under applicable laws in the Territory to prevent the continued export of Licensed Product from the country in the Territory to such country outside of the Territory. In the event that Chiron is in breach of its obligations under this Section 7.11, The [*] until such time as Chiron shall have provided to Cubist and implemented a plan to remedy the situation.
ARTICLE 8.
INTELLECTUAL PROPERTY
8.1. Ownership of Inventions. Subject to the licenses granted by either Party to the other Party pursuant to this Agreement, the entire right, title and interest in and to all discoveries, improvements, processes, formulas, data, inventions, enhancements, know-how and trade secrets, patentable or otherwise, that arise from activities performed under or pursuant to this Agreement or the Supply Agreement and that were or are developed or invented:
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
47
(a) solely by employees of Cubist (“Cubist Inventions”) shall be owned solely by Cubist;
(b) solely by employees of Chiron (“Chiron Inventions”) shall be owned solely by Chiron; and
(c) jointly by employees of Cubist and Chiron (“Joint Inventions”) shall be owned jointly by Cubist and Chiron.
Inventorship shall be determined in accordance with U.S. patent laws.
8.2. Prosecution of Patents.
(a) Cubist Patents. Cubist shall be responsible for the filing, prosecution and maintenance of the Cubist Patents at its sole expense; provided, however, that Cubist shall (i) provide Chiron with all material documentation and correspondence from, sent to or filed with patent offices in the Territory regarding the Cubist Patents, (ii) provide Chiron with a reasonable opportunity to review and comment upon all filings with such patent offices in advance of submissions to such patent offices, and (iii) shall consider, in good faith, incorporating any reasonable comments provided by Chiron. If Cubist determines in its sole discretion to abandon or not file or maintain any claim or patent application within the Cubist Patents anywhere in the Territory, then Cubist shall provide Chiron with thirty (30) days prior written notice of such determination, or reasonable notice if the period for determination is less than thirty (30) days, and shall provide Chiron with the opportunity to file, prosecute and maintain such claim or patent application in the Territory in the name of Chiron (or an Affiliate of Chiron) as assignee and Cubist shall assign to Chiron its entire right in such claim or patent application in the Territory, and thereafter Chiron shall be responsible for all costs and expenses in connection with the filing, prosecution or maintenance of any such claim or patent application assigned by Cubist to Chiron pursuant to this Section 8.2(a). Chiron shall also pay for all costs and expenses in connection with any assignment by Cubist to Chiron of any claim or patent application pursuant to this Section 8.2(a). Cubist shall inform Chiron of any patents, information or proceeding of which Cubist becomes aware that relate to Cubist Patents that may adversely impact the validity, title or enforceability of Cubist Patents in the Territory.
(b) Chiron Patents. Chiron shall be responsible for the filing, prosecution and maintenance of the Chiron Patents at its sole expense; provided, however, that Chiron shall (i) provide Cubist with all material documentation and correspondence from, sent to or filed with patent offices regarding the Chiron Patents, (ii) provide Cubist with a reasonable opportunity to review and comment upon all filings with such patent offices in advance of submissions to such patent offices, and (iii) shall consider, in good faith, incorporating any reasonable comments provided by Cubist. If Chiron determines in its sole discretion to abandon or not file or maintain any claim or patent application within the Chiron Patents, then Chiron shall provide Cubist with thirty (30) days prior written notice of such determination, or reasonable notice if the period for determination is less than thirty (30) days, and shall provide Cubist with the opportunity to file, prosecute and maintain such claim or patent application in the name of Cubist (or an Affiliate of Cubist) as assignee and Chiron shall assign to Cubist its entire right in such claim or patent application, and thereafter Cubist shall be responsible for all costs and expenses in connection
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
48
with the filing, prosecution or maintenance of any such claim or patent application assigned by Chiron to Cubist pursuant to this Section 8.2(b). Cubist shall also pay for all costs and expenses in connection with any assignment by Chiron to Cubist of any claim or patent application pursuant to this Section 8.2(b). Chiron shall inform Cubist of any patents, information or proceeding of which Chiron becomes aware that relate to Chiron Patents that may adversely impact the validity, title or enforceability of Chiron Patents.
(c) Joint Patents. Unless the Parties otherwise mutually agree in writing, Cubist shall have the first right to file, prosecute and maintain in the Territory and outside the Territory, upon appropriate consultation with Chiron, all patent applications and patents that claim any Joint Inventions (any such patent application and any patents issuing therefrom a “Joint Patent”), provided however, in the event that Cubist elects not to file any patent application in the Territory or outside the Territory with respect to any Joint Invention, Chiron shall have such right and upon exercise of such right, Chiron shall have the right to prosecute and maintain in the Territory and outside the Territory, upon appropriate consultation with Cubist, the Joint Patents to which such Joint Invention relates. In each case, the filing Party (A) shall give the non-filing Party a reasonable opportunity to review the text of the application or submission before filing, (B) shall consult with the non-filing Party with respect thereto, (C) shall, prior to filing any application or submission, incorporate any reasonable comments that the non-filing Party shall make on a timely basis to such application or submission and (D) shall supply the non-filing Party with a copy of the application or submission as filed, together with notice of its filing date and serial number and all substantive prosecution. Each Party shall keep the other advised of the status of the actual and prospective patent filings described above in this Section 8.2(c) and, upon the request of the other, provide advance copies of any papers related to the filing, prosecution and maintenance of such patent filings. Cubist shall promptly give notice to Chiron of the grant, lapse, revocation, surrender, invalidation or abandonment in the Territory or outside the Territory of any Joint Patent being prosecuted by Cubist. Chiron shall promptly give notice to Cubist of the grant, lapse, revocation, surrender, invalidation or abandonment in the Territory or outside the Territory of any Joint Patent being prosecuted by Chiron. With respect to all filings hereunder, the filing Party shall be responsible for payment of all costs and expenses related to such filings (subject to partial reimbursement to the extent provided in the next sentence). Where the filing Party is Chiron, it shall be entitled to reimbursement from Cubist for [*] of the expenses and costs incurred by the filing Party in connection with the prosecution and maintenance of Joint Patents. Except to the extent either Party is restricted by the licenses granted to the other Party, and covenants contained herein, and to the extent permitted by law, each Party shall be entitled to practice and sublicense Joint Patents and Joint Know-How without restriction or an obligation to account to the other Party. Either Party may disclaim its interest in any particular Patent covering a Joint Invention, in which case (X) the disclaiming Party shall assign its ownership interest in such Patent to the other Party for no additional consideration, (Y) the Party which is then the sole owner shall be solely responsible for all future costs of such Patent, and (Z) the disclaiming Party shall hold no further rights thereunder.
8.3. Patent Term Extensions. Cubist will, in its sole discretion, after discussing its strategy with Chiron and reasonably considering Chiron’s comments, in each country in the Territory, determine for which, if any, of the Patents within the Cubist Patents and Joint Patents, the Parties will apply to extend the patent term with respect to Licensed Products, as provided for
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
49
in patent term extension laws or regulations in the Territory similar to the Patent Term Restoration Act or other similar laws and regulations affording an extension or restoration of patent terms in the United States, which similar laws and regulations shall include without limitation any Supplementary Protection Certificates. Chiron shall not make any submissions, filings or other communications with any governmental agency with respect to patent term restoration (or other similar grant of a monopoly right with respect to any Licensed Product) for any Patents within the Cubist Patents or Joint Patents without Cubist’s express consent. Chiron will cooperate fully with Cubist in making such filings at Cubist’s sole expense which may include without limitation, making available regulatory data and information for such purpose. Notwithstanding anything in the foregoing provisions of this Section 8.3 to the contrary, in the event that Cubist, in its sole discretion, makes a determination not to seek an extension within the Territory of the patent term of any Cubist Patent or Joint Patent in the Territory with respect to Licensed Products, then Cubist shall provide Chiron with thirty (30) days prior written notice of such determination, or reasonable notice if the period for determination is less than thirty (30) days, and shall provide Chiron with the opportunity, at Chiron’s sole discretion and sole cost and expense, to make submissions and filings, and take such other actions as may be reasonably required, on behalf of Cubist to extend within the Territory the patent term of any Cubist Patent or Joint Patent in the Territory with respect to License Products.
8.4. Infringement of Patents by Third Parties.
(a) Notification. Each Party shall promptly notify the other Party in writing of any alleged or threatened infringement of the Cubist Patents, Chiron Patents and Joint Patents of which it becomes aware (such infringement, “Infringement”, and “Infringe” shall be interpreted accordingly).
(b) Infringement of Patents in the Territory. For all Infringement of Cubist Patents, Chiron Patents or Joint Patents in the Territory, Chiron shall have the right, but not the obligation, to bring, at its own expense and in its sole control, an appropriate action against the person or entity engaged in such Infringement directly or contributorily. If Chiron does not bring such action within ninety (90) days of notification thereof to or by Cubist pursuant to Section 8.4(a) or within ninety (90) days of the date upon which notification thereof to Cubist was given by Chiron pursuant to Section 8.4(a) hereof, Cubist shall have the right, but not the obligation, to bring at Cubist’s expense and in its sole control, such appropriate action. The Party not bringing an action under this paragraph (b) shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall cooperate reasonably with the Party bringing such action, including without limitation agreeing to be named as a party to such action if necessary.
(c) Other Infringement of Cubist Patents. For all Infringement of Cubist Patents other than Infringement described in Section 8.4(b) above, Cubist shall have the exclusive right, but not the obligation, to bring, at Cubist’s expense and in its sole control, an appropriate action against any person or entity engaged in such Infringement directly or contributorily.
(d) Infringement of Chiron Patents and Joint Patents outside the Territory. For all Infringement of Chiron Patents and Joint Patents outside of the Territory,
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
50
Cubist shall have the right, but not the obligation, to bring, at Cubist’s expense and in its sole control, an appropriate action against any person or entity engaged in such Infringement directly or contributorily. If Cubist does not bring such action within ninety (90) days of notification thereof to or by Chiron pursuant to Section 8.4(a) or within ninety (90) days of the date upon which notification thereof to Chiron was given by Cubist pursuant to Section 8.4(a) hereof, Chiron shall have the right, but not the obligation, to bring at Chiron’s expense and in its sole control, such appropriate action. The Party not bringing an action under this paragraph (d) shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall cooperate reasonably with the Party bringing such action, including without limitation agreeing to be named as a party if necessary for such action.
(e) Settlement; Allocation of Proceeds. Cubist shall not settle a claim brought under this Section 8.4 involving Cubist Patents, Chiron Patents or Joint Patents in a manner that would limit or restrict the ability of Chiron to sell, or Chiron’s sales of, Licensed Products in the Territory, without the prior written consent of Chiron (which consent shall not be unreasonably withheld or delayed). Chiron shall not settle a claim brought under this Section 8.4 involving Cubist Patents, Chiron Patents or Joint Patents in a manner that would limit or restrict the ability of Cubist to sell, or Cubist’s sales of, Licensed Products outside the Territory, without the prior written consent of Cubist (which consent shall not be unreasonably withheld or delayed). In the event of any recovery of monetary damages from the Third Party in an action brought under Section 8.4(b), Section 8.4(c) or Section 8.4(d), whether such damages result from the infringement of Cubist Patents, Chiron Patents or Joint Patents, such recovery shall be allocated first to the reimbursement of any reasonable expenses incurred by the Parties in the litigation under this Section 8.4 (including, for this purpose, a reasonable allocation of internal counsel), and any remaining amounts (“Remainder”) shall be allocated as follows:
(i) the portion of the Remainder that represents recovery for Infringement in the Territory shall be treated as Net Sales of Licensed Products in accordance with Section 7.3 with Chiron retaining such amounts and paying to Cubist the applicable royalty thereon unless the Remainder consists of a recovery in any action brought by Cubist pursuant to Section 8.4(b) hereof in which case the portion of the Remainder that represents recovery for Infringement in the Territory shall be [*] Cubist and [*] Chiron; and
(ii) the portion of the Remainder that represents recovery for Infringement outside the Territory shall be [*] for Cubist unless the Remainder consists of a recovery in an action brought by Chiron pursuant to Section 8.4(d) hereof in which case the portion of the Remainder that represents recovery for Infringement outside the Territory shall be [*] Cubist and [*] Chiron.
8.5. Infringement of Third Party Rights.
(a) Notice. If any Licensed Product developed, manufactured, used or sold by either Party, its Affiliates, licensees, sublicensees or distributors becomes the subject of a Third Party claim of patent infringement relating to the development, manufacture, use, sale, offer for sale or importation of such Licensed Product, or if either Party reasonably believes, based on facts that such Party has become aware of, that a reasonable potential for any such claim exists, the Party first having notice of such claim or having such reasonable belief shall promptly notify
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
51
the other Party in writing, and the Parties shall promptly meet to evaluate the claim and discuss the appropriate course of action. Any claim or potential claim referred to in this Section 8.5(a) is hereinafter referred to as a “Third Party Infringement Claim”.
(b) Avoidance of Infringement. In the event that there is a Third Party Infringement Claim that arises from the use or practice of any Cubist Technology, Chiron Technology or any Joint Technology in connection with the research, development, manufacture, use, sale, offer for sale or importation of Licensed Products in the Territory and/or outside the Territory, the Parties shall confer in good faith as promptly as practicable after both parties become aware of such Third Party Infringement Claim as to whether it is feasible to alter their approach to the infringing activities with respect to the Licensed Product so as to avoid such infringement without adversely affecting their rights under this Agreement. In the event the Parties determine in good faith that it is feasible to alter their approach to such infringing activities without adversely affecting their rights under this Agreement, the Parties shall implement such alternative approach to such infringing activities.
(c) Licensing to Resolve Infringement by Cubist Technology. If the Parties determine in good faith that it is not feasible to alter their approach to any infringing activities referred to in Section 8.5(b) so as to avoid such infringement without adversely affecting their rights under this Agreement, then, if such infringement arises from the use or practice of any Cubist Technology or the research, development, manufacture, use, sale, offer for sale or importation of Licensed Products in the Territory, Cubist shall use commercially reasonable efforts to obtain a license on commercially reasonable terms from the Third Party whose intellectual property rights are being infringed so as to avoid, eliminate, resolve or settle such infringement. In the event that Cubist shall obtain a license from a Third Party pursuant to this Section 8.5(c) in order to avoid, eliminate, resolve or settle any Third Party Infringement Claim of such Third Party and such license consists of or provides rights relating to the development, manufacture, use, sale, offer for sale or import of Licensed Products in the Territory, then Cubist shall sublicense such rights to Chiron for use in the Territory to the same extent that Cubist Technology is licensed by Cubist to Chiron for use in the Territory pursuant to Article 2 hereof, provided that Chiron agrees in writing to make payment, and makes payment when due, to Cubist of the portion of the costs or other amounts paid and required to be paid by Cubist to obtain and maintain such license that are reasonably appropriate for Chiron to bear in light of the relative financial benefits that could reasonably be expected to be derived from such license by Chiron in the Territory, on the one hand, and by Cubist and, if applicable, Third Party licensees of Cubist, outside the Territory, on the other hand. Notwithstanding any provision of this Agreement to the contrary, for purposes of this Section 8.5(c), the Parties hereby agree that Chiron shall not be obligated to bear any portion of the costs or other amounts paid and required to be paid by Cubist to obtain and maintain such license if the intellectual property rights of such Third Party that are infringed or allegedly infringed by the use or practice of any Cubist Technology, or the research, development, manufacture, use, sale, offer for sale or importation of Licensed Products in the Territory, consists of Patents of such Third Party that had published or were generally available to the public as of the Effective Date (a “Knowable Patent”).
(d) Determination of Relative Financial Benefits. In connection with any determination of the relative financial benefits that could reasonably be expected to be derived by Chiron, Cubist and, if applicable, Third Party licensees of Cubist in connection with any
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
52
license obtained by Cubist or Chiron pursuant to Section 8.5(c), such relative financial benefits shall be determined by considering actual and projected net sales and actual or projected royalties from sales of the infringing Licensed Product by Chiron, Cubist and, if applicable, Third Party licensees of Cubist in the territory or territories that is or are covered by any such license.
(e) Limitation of Obligation to Sublicense. Notwithstanding anything in this Agreement expressed or implied to the contrary, Cubist shall have no obligation to sublicense to Chiron any rights licensed by Cubist pursuant to Section 8.5(c) if Chiron has not agreed in writing to pay, and does not make payment when due, of the portion of the cost and expense incurred or to be incurred by Cubist in connection with seeking and maintaining such license that Chiron is required to pay pursuant to Section 8.5(c).
(f) Defense. The Party against which a Third Party Infringement Claim is brought shall defend against such claim at its sole expense and the other Party shall have the right, but not the obligation, to participate in any such suit, at its sole option and at its own expense. Such other Party shall reasonably cooperate with the Party conducting the defense of the claim, including if required to conduct such defense, furnishing a power of attorney.
(g) Settlement. Neither Party shall enter into any settlement of a claim brought under this Section 8.5 that materially adversely affects the other Party’s rights or interests without such other Party’s written consent, which consent shall not be unreasonably withheld or delayed.
8.6. Patent Oppositions.
(a) Third Party Patent Rights. If either Party desires to bring an opposition, action for declaratory judgment, nullity action, interference, reexamination or other attack upon the validity, title or enforceability of a Patent owned or Controlled by a Third Party that covers or may cover the manufacture, use, sale, offer for sale or importation of any Licensed Product, such Party shall so notify the other Party and the Parties shall promptly confer in good faith to determine whether to bring such action or the manner in which to settle such action. Cubist shall have the first right, but not the obligation, to bring at its own expense and in its sole control such action outside the Territory. If Cubist does not bring such action within ninety (90) days of notification thereof pursuant to this Section 8.6(a) (or earlier, if required by the nature of the proceeding), Chiron shall have the right, but not the obligation, to bring at Chiron’s expense and in its sole control, such action. Chiron shall have the first right, but not the obligation, to bring at its own expense and in its sole control such action in the Territory. If Chiron does not bring such action within ninety (90) days of notification thereof pursuant to this Section 8.6(a) (or earlier, if required by the nature of the proceeding), Cubist shall have the right, but not the obligation, to bring at Cubist’s expense and in its sole control, such action. The Party not bringing an action under this Section 8.6(a) shall be entitled to separate representation in such proceeding by counsel of its own choice and at its own expense, and shall cooperate reasonably with the Party bringing such action. Any awards or amounts received in bringing any such action shall be first allocated to reimburse the Parties’ reasonable expenses incurred in such action, and any remaining amounts shall be retained by the Party bringing such action.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
53
(b) Parties’ Patent Rights. If a Cubist Patent, a Chiron patent or a Joint Patent becomes the subject of any proceeding commenced by a Third Party in connection with an opposition, reexamination request, action for declaratory judgment, nullity action, interference or other attack upon the validity, title or enforceability thereof, then the Party responsible for the prosecution of such Cubist Patent, such Chiron Patent or such Joint Patent pursuant to Section 8.2 hereof shall control such action at the sole cost or expense of the controlling Party unless the Parties otherwise agree in writing. The controlling Party will permit the non-controlling Party to participate in the proceeding to the extent permissible under law, and to be represented by its own counsel in such proceeding, at the non-controlling Party’s expense. If either Party decides that it does not wish to defend against such action, then the other Party shall have a backup right to assume defense of such Third Party action at its own expense. Any awards or amounts received in defending any such Third Party action shall be allocated between the Parties as provided in Section 8.4(e) as if the Party conducting such opposition were the Party that brought an action against an alleged infringer.
(c) Noncontravention. Nothing in this Section 8.6 shall be construed to relieve either Party of its obligations under Section 8.5 hereof.
8.7. Sublicensed Technology.
(a) Generally. The licenses granted under Article 2, to the extent they include sublicenses of Third-Party technology licensed by Cubist as of the Effective Date, a complete list of which is set forth in Exhibit E, and including without limitation the Lilly License (“Effective Date Third Party Licenses”), shall be subject to the terms and conditions of such Effective Date Third Party Licenses. Cubist shall faithfully and timely perform and discharge its obligations under the Effective Date Third Party Licenses (including without limitation the payment of all royalties and other financial compensation due to such Third Parties thereunder in connection with the sale of Licensed Products) and shall not permit any action to be taken or event to occur, in each case, within Cubist’s reasonable control, which would give such Third Party the right to terminate such Effective Date Third Party License. Further, Cubist shall not amend any Effective Date Third Party License without Chiron’s consent, such consent not to be unreasonably withheld. Notwithstanding the provisions of the foregoing two sentences, Cubist shall not be liable to Chiron for breach of the obligations set forth in the foregoing two sentences unless such breach shall materially adversely affect Chiron’s ability to develop or Commercialize Licensed Products in accordance with the provisions of this Agreement. If Cubist is notified or otherwise becomes aware of its material breach of any such Effective Date Third Party License, it shall promptly notify Chiron in writing, reasonably detailing such breach. Cubist shall also notify Chiron of any material breach by such Third Parties of any of their obligations under the Effective Date Third Party Licenses. The Parties shall promptly confer in good faith regarding an appropriate manner for curing any such breach within the time allotted by such Effective Date Third Party License, or if the Third Party is in breach, monitoring the ability and adequacy of the Third Party’s efforts to cure such breach. If Cubist does not perform the agreed-upon remedy of such breach within the designated time, then Chiron may itself perform the agreed-upon remedy for such breach for the benefit of Cubist and any amounts that Chiron pays to cure such breach shall be either promptly reimbursed by Cubist or Chiron may withhold an equal amount from any payments due to Cubist under this Agreement. If a good faith dispute between a Third Party and Cubist arises about the interpretation of any provision within such Effective Date Third Party
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
54
License, Chiron shall use its Commercially Reasonable Efforts to ensure that its actions, if any, under this Section 8.7(a) do not detrimentally affect the ability of Cubist to contest the interpretation advanced by such Third Party.
(b) Lilly License. It is agreed and acknowledged that in the event of termination of the Lilly License, any sublicense granted by Cubist to Chiron under this Agreement of any rights granted by Lilly to Cubist under the Lilly License shall automatically be assigned by Cubist to Lilly in accordance with the provisions of Section 10.06 of the Lilly License.
(c) Decision to License Third Party Technology. In the event that either Party believes that certain Third Party intellectual property may be useful to the development, manufacture, use, sale, offer for sale or import of Licensed Products in the Territory and/or outside the Territory, then such Party shall request through the JCT that the Parties consider (i) whether the Parties should seek a license to such Third Party intellectual property to allow either or both Parties to use or practice such Third Party intellectual property in connection with the development, manufacture, use, sale, offer for sale or import of Licensed Products, (ii) how to allocate the costs of obtaining and maintaining such license between the Parties, (iii) how the Parties should proceed to seek such license, and (iv) whether, instead of seeking such license, the Parties can develop, manufacture, use, sell, offer for sale or import Licensed Product in a way that would eliminate or reduce the usefulness or desirability of licensing such Third Party intellectual property.
(d) Licensing of Third Party Technology. If the Parties reach a consensus that the Parties should seek a license to any Third Party intellectual property referred to in Section 8.7(c), then the Parties shall seek such license in a manner and on terms consistent with the determinations made by the Parties with respect to the matters set forth in clauses (i), (ii) and (iii) of Section 8.7(c). If the Parties are unable to reach a consensus that the Parties should seek such license and if the Parties are unable to reach a consensus that the Parties can develop, manufacture, use, sell, offer for sale or import Licensed Products in a way that would eliminate or reduce the usefulness and desirability of obtaining such license, then either Party shall be free to itself seek such license at its sole cost and expense. If Cubist is the Party that seeks such license and such license consists of or provides rights useful to the development, manufacture, use, sale, offer for sale or import of Licensed Products in the Territory, then, at Chiron’s request made at any time during the Term, Cubist shall sublicense such rights to Chiron for use in the Territory provided that Chiron agrees in writing to make payment, and makes payment when due, to Cubist of the portion of the costs paid and required to be paid by Cubist to obtain and maintain such license that are reasonably appropriate for Chiron to bear in light of the relative financial benefits that could reasonably be expected to be derived from such license by Chiron in the Territory, on the one hand, and by Cubist and, if applicable, Third Party licensees of Cubist, outside the Territory, on the other hand. If Chiron is the Party that seeks such license and such license consists of or provides rights useful to the development, manufacture, use, sale, offer for sale or import of Licensed Products outside the Territory, then, at Cubist’s request made at any time during the Term, Chiron shall sublicense such rights to Cubist outside the Territory provided that Cubist agrees in writing to make payment, and makes payment when due, to Chiron of the portion of the costs paid and required to be paid by Chiron to obtain and maintain such license that are reasonably appropriate for Cubist to bear in light of the relative financial benefits that could reasonably be expected to be derived from such license by Cubist and, if
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
55
applicable, Third Party licensees of Cubist outside the Territory, on the one hand, and by Chiron in the Territory, on the other hand.
(e) Limitation of Obligation to Sublicense. Notwithstanding anything in this Agreement expressed or implied to the contrary, the Party that obtains a license pursuant to Section 8.7(d) above shall have no obligation to make the licensed rights obtained pursuant to such license available to the other Party if such other Party is not bearing a reasonably appropriate portion of the cost and expense of seeking and maintaining such license in light of the relative financial benefits that could reasonably be expected to be derived from such license by Chiron in the Territory, on the one hand, and by Cubist and, if applicable, Third Party licensees of Cubist outside the Territory, on the other hand.
(f) Determination of Relative Financial Benefits. In connection with any determination of the relative financial benefits that could reasonably be expected to be derived by Chiron, Cubist and, if applicable, Third Party licensees of Cubist in connection with any license obtained by Cubist or Chiron pursuant to this Section 8.7, such relative financial benefits shall be determined by considering actual and projected net sales and actual or projected royalties from sales of the applicable Licensed Product by Chiron, Cubist and, if applicable, Third Party licensees of Cubist in the territory or territories that is or are covered by any such license.
8.8. Patent Marking. Licensed Products marketed and sold by the Parties hereunder shall be marked with appropriate patent numbers or indicia at Cubist’s request, to the extent permitted by law, in those countries in which such markings have notice value as against infringers of patents.
8.9. Applicability to Chiron Patents. Notwithstanding any provision of this Agreement to the contrary, the provisions of this Article 8 shall apply to any Chiron Patent only to the extent that the Parties have not otherwise agreed on responsibilities for prosecution, maintenance, enforcement, defense and other management of such Chiron Patent.
8.10. Trademarks.
(a) Trademark License. Subject to the provisions set forth in this Section 8.10, Cubist hereby grants Chiron an exclusive, royalty-free license under its entire right, title and interest in and to the Cubist Marks to use and display the Cubist Marks in connection with the Commercialization of Licensed Products within the Territory. Chiron shall not use Cubist’s trade names and/or marks in a way which would be confusing or otherwise adversely affect their value. Chiron agrees that it will not reproduce or use the Cubist Marks in any manner whatsoever other than as authorized by this Agreement, and that its uses of the Cubist Marks will comply with the current trademark guidelines that Cubist may provide from time to time. Chiron shall provide Cubist with copies of any materials containing any Cubist Marks prior to using or disseminating such materials, and shall reasonably consider all comments made by Cubist regarding the use of the Cubist Marks. Cubist will have the right to monitor Chiron’s use of the Cubist Marks and to request that Chiron correct any failure to comply with this Section 8.10 adversely affecting the strength or value of such trademark.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
56
(b) Selection and Registration of Product Trademarks. Subject to the provisions set forth below in this Section 8.10, Chiron shall use in connection with the Commercialization of any Licensed Product in the Territory those trademarks used by Cubist in connection with the Commercialization of such Licensed Product outside the Territory. In the event that (i) any trademark or trademarks used by Cubist in connection with the Commercialization of any Licensed Product outside the Territory may not be registered for use in the Territory with such Licensed Product or (ii) Chiron believes that it would be a significant advantage for the Commercialization of any Licensed Product in the Territory to select and use a trademark or trademarks in connection therewith that is or are different from the trademark or trademarks being utilized by Cubist in connection with the Commercialization of such Licensed Product outside the Territory, then Chiron shall notify Cubist about the matter and shall submit to Cubist several other trademarks that Chiron desires to use, and believes can be used, with the Licensed Product in question in the Territory. Cubist and Chiron shall jointly consider, discuss and decide the matter. Chiron shall not use any trademark in connection with the Commercialization of any Licensed Product within the Territory that is different from the trademark or trademarks being utilized by Cubist in connection with the Commercialization of such Licensed Product outside the Territory without Cubist’s written consent. Any trademark used by Chiron in connection with the Commercialization of any Licensed Product in the Territory that is different than the trademark or trademarks utilized by Cubist in connection with the Commercialization of such Licensed Product outside the Territory and that has been selected and approved in accordance with the provisions of this Section 8.10(b) is hereinafter referred to as an “Approved New Trademark”. Subject to Chiron’s right to use each Approved New Trademark in accordance with the provisions of this Agreement, Cubist shall own all right, title and interest to each Approved New Trademark, and each Approved New Trademark shall become one of, and be included among, the Cubist Marks, and, to the extent permitted by applicable law, Cubist shall be responsible for registering and maintaining such Approved New Trademark within and outside the Territory, at Cubist’s own expense. Chiron hereby assigns to Cubist any and all right, title, interest, and goodwill that Chiron may have in any such Approved New Trademark (other than the right, if applicable, set forth below in this Section 8.10 to register and maintain such Approved New Trademark and Chiron’s license and right to use such Approved New Trademark pursuant to the provisions of Section 8.10(a) hereof) and hereby agrees to execute such further documents as Cubist shall reasonably request to further evidence and perfect such assignment. In the event that the laws of any applicable country within the Territory shall not permit Cubist to register any Cubist Xxxx, then Chiron shall be responsible for registering and maintaining such Cubist Xxxx in such applicable country, notwithstanding the fact that Cubist shall own such Cubist Xxxx, and any use or goodwill arising from use of the xxxx shall inure to the benefit of Cubist.
(c) Infringement of Trademarks by Third Parties. Each Party shall notify the other Party in writing promptly upon learning of any actual, alleged or threatened infringement in the Territory or outside the Territory of any Cubist Xxxx or of any unfair trade practices, trade dress imitation, passing off of counterfeit goods, or like offenses, in any case involving or affecting Licensed Products in the Territory or outside the Territory (hereinafter “TM Infringement”). In the case of any TM Infringement in the Territory, after any such written notice has been sent and received with respect to such TM Infringement, the Parties shall confer in good faith as to the reasonable response to such TM Infringement. In the absence of other
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
57
agreement between the Parties, (i) Cubist shall have the exclusive right, in its own discretion and at its own expense, to bring an action to address TM Infringement outside the Territory, (ii) Chiron shall have the first right, in its own discretion and at its own expense, to bring an action to address TM Infringement in the Territory, and (iii) if Chiron does not exercise its rights under the foregoing clause (ii) within ninety (90) days after the Parties shall have conferred in good faith as to the reasonable response to such TM Infringement in the Territory in accordance with the above provisions of this Section 8.10(c), Cubist shall have the right, in its own discretion and its own expense, to bring an action to address such TM Infringement in the Territory. Any recovery by the Party pursuing the Third Party for the alleged TM Infringement shall be applied first to reimburse the costs and expenses incurred by the Party pursuing the infringer, and the portion of any remainder that is attributable to TM Infringement outside the Territory shall be allocated [*] to Cubist, and the portion of such remainder that is attributable to TM Infringement in the Territory shall be allocated as follows: (i) if the pursuing Party is Chiron, [*]; and (ii) if the pursuing Party is Cubist, [*].
(d) Product Trademarks Infringe Third Party Rights. Each Party shall notify the other Party in writing promptly upon being notified that any Cubist Xxxx or trade dress associated with the sale of License Products in the Territory or outside the Territory is alleged to infringe any Third Party trademark or other related intellectual property rights. In the case of any such alleged infringement in the Territory, after any such written notice has been sent and received with respect to such alleged infringement, the Parties shall confer in good faith as to the reasonable response to such alleged infringement.
8.11. Subordination to Lilly Rights. Notwithstanding anything in this Article 8 expressed or implied to the contrary, the Parties hereby agree that the respective rights and obligations of the Parties under this Article 8 with respect to the Cubist Patents licensed by Cubist from Lilly pursuant to the Lilly Agreement and set forth on Exhibit F hereto (in each case, a “Lilly Patent”) shall, to the extent applicable, be subject to the respective rights and obligations of Lilly and Cubist with respect to such Lilly Patent as set forth in the Lilly Agreement. In the event that the rights and obligations of Lilly and Cubist under the Lilly Agreement with respect to any Lilly Patent shall be in conflict with the respective rights and obligations of Cubist and Chiron under this Article 8 with respect to such Lilly Patent, the respective rights and obligations of Lilly and Cubist under the Lilly Agreement with respect to such Lilly Patent shall control, take precedence and supersede the respective rights and obligations of Cubist and Chiron under this Article 8 with respect to such Lilly Patent to the extent of any such conflict.
ARTICLE 9.
REPRESENTATIONS AND WARRANTIES
9.1. Mutual Representations and Warranties. Each Party hereby represents and warrants to the other Party as follows:
(a) Corporate Existence and Power. It is a corporation duly organized, validly existing and in good standing under the laws of the state in which it is incorporated, and has full corporate power and authority and the legal right to own and operate its property and assets and
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
58
to carry on its business as it is now being conducted and as contemplated in this Agreement, including, without limitation, the right to grant the licenses granted hereunder.
(b) Authority and Binding Agreement. As of the Effective Date, (i) it has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; (ii) it has taken all necessary corporate action on its part required to authorize the execution and delivery of the Agreement and the performance of its obligations hereunder; and (iii) the Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid and binding obligation of such Party that is enforceable against it in accordance with its terms.
(c) No Conflict. It has not entered into any agreement with any Third Party that is in conflict with the rights granted to the other Party under this Agreement, and has not taken any action that would in any way prevent it from granting the rights granted to the other Party under this Agreement, or that would otherwise materially conflict with or adversely affect the rights granted to the other Party under this Agreement. Its performance and execution of this Agreement will not result in a breach of any other contract to which it is a Party.
(d) Validity. It is aware of no action, suit, inquiry or investigation instituted by any Third Party which questions or threatens the validity of this Agreement.
(e) Consents. All necessary consents, approvals and authorizations of all governmental authorities and other persons or entities required to be obtained by such Party in connection with the execution and delivery of this Agreement have been obtained.
9.2. Cubist Representations and Warranties. Cubist hereby represents and warrants to Chiron as follows:
(a) Ownership of Intellectual Property. Except for the rights licensed to Cubist pursuant to the Effective Date Third Party Licenses, except for any Third Party intellectual property rights that would be infringed or misappropriated by the use or practice of the Cubist Patents, Cubist Know-How and the Cubist Marks (as to which infringement or misappropriation Cubist has no knowledge as of the Effective Date), [*] Cubist is the owner of the Cubist Patents, the Cubist Know-How and Cubist Marks that exist as of the Effective Date, free and clear (as of the Effective Date) of all liens, encumbrances, security interests, licenses, and options to acquire or license in favor of Third Parties. Except for any Third Party intellectual property rights that would be infringed or misappropriated by the use or practice of the Cubist Patents, Cubist Know-How and the Cubist Marks (as to which infringement or misappropriation Cubist has no knowledge as of the Effective Date), as of the Effective Date, (1) Cubist has the requisite right to grant to Chiron the licenses granted herein to Chiron, and (2) no right or license of any Third Party is required to permit Cubist to perform its obligations under this Agreement in accordance with the terms of this Agreement and to permit Chiron to exercise its rights hereunder in accordance with the terms of this Agreement.
(b) Claims Related to Use of Intellectual Property. As of the Effective Date, Cubist is not aware of any pending or threatened claims against Cubist asserting that any of the activities of Cubist relating to the Licensed Products or the conduct by the Parties of any of the
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
59
activities contemplated by this Agreement relating to the Licensed Products infringes the rights of any Third Party. As of the Effective Date, Cubist is not aware of any published or generally available Patents of any Third Party that would be infringed by the use or practice of the Cubist Patents, Cubist Know-How or the Cubist Marks.
(c) Notice to Third Persons. As of the Effective Date, Cubist has not given any notice to any Third Party asserting infringement, misappropriation or violation by such Third Party upon any of the Cubist Technology.
(d) Effective Date Third Party Licenses. Exhibit E is an accurate and complete list of all Effective Date Third Party Licenses, including without limitation, the Lilly License. As of the Effective Date, all Effective Date Third Party Licenses remain in effect and Cubist is not in breach or default in the performance of its obligations under the Effective Date Third Party Licenses. Cubist has not received any notice from any of the other parties to any of the Effective Date Third Party Licenses, including without limitation, Lilly, of any breach, default or non-compliance of Cubist under the terms of any of the Effective Date Third Party Licenses, including without limitation, the Lilly License. There have been no amendments or other modification to the Effective Date Third Party Licenses, including without limitation, the Lilly License, except as have been disclosed to Chiron in writing. Cubist has the requisite right under the Lilly License to grant to Chiron a sublicense of Cubist’s rights under the Lilly License. Lilly’s right of first negotiation under the Lilly License to acquire the rights licensed by Cubist to Chiron as of the Effective Date pursuant to this Agreement has expired or been exhausted or waived.
(e) No Misappropriation. To the best knowledge of Cubist as of the Effective Date (after having made due inquiry of its employees on or prior to the Effective Date), it has not misappropriated the trade secret of any Third Party in its activities to develop and Commercialize Licensed Products.
(f) Regulatory Filings. Exhibit G is an accurate and complete list of all INDs, NDAs and other Drug Approval Applications for Licensed Products filed by Cubist anywhere in the world as of the Effective Date. Except for the rights granted to Chiron pursuant to this Agreement and the Supply Agreement, Cubist is as of the Effective Date the owner of all INDs, NDAs and other Drug Approval Applications set forth on Exhibit G attached hereto, free and clear (as of the Effective Date) of all liens, encumbrances, security interests, licenses, and options to acquire or license in favor of Third Parties. As of the Effective Date, Cubist has complied in all material respects with all laws applicable to all INDs, NDAs and other Drug Approval Applications set forth on Exhibit G attached hereto.
(g) Regulatory Data and Affairs. Cubist has disclosed to Chiron all material information and data that is known to Cubist and that is in the possession of, or otherwise available to, Cubist relating to (i) the results of pre-clinical and clinical studies of Licensed Products conducted by or on behalf of Cubist or Lilly, and (ii) Cubist’s pending Drug Approval Application in the United States for the Licensed Product and its status. All such material information and data disclosed by Cubist to Chiron are complete and accurate in all material respects.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
60
(h) Non-Infringement of Cubist Technology by Third Parties. As of the Effective Date, Cubist is unaware of any activities by Third Parties that would constitute infringement or misappropriation of any Cubist Technology.
(i) Litigation. [*,] Cubist is not aware of any litigation against Cubist that has been pending or threatened with respect to the Cubist Technology, Licensed Products or Cubist’s rights in either of the foregoing.
(j) Restrictive Agreements. As of the Effective Date, Cubist has not entered into any agreement with a Third Party pursuant to which Cubist shall have agreed not to enforce any right of Cubist to preclude such Third Party from using or practicing any or all of the Cubist Technology or Commercialize Licensed Products in the Territory.
(k) Patent Prosecution. To the best of Cubist’s knowledge as of the Effective Date, (i) Cubist has complied in all material respects with all laws applicable to the prosecution and maintenance of the Cubist Patents, and (ii) those employees of Cubist having a duty to disclose to the patent office of any country in the Territory information material to the patentability of the subject matter of any or all of the Cubist Patents in such country have disclosed all such information.
9.3. Chiron Representation and Warranty - No Intellectual Property. Chiron hereby represents and warrants to Cubist that as of the Effective Date, Chiron is not aware (without having conducted any review of its existing intellectual property portfolio) that it has any patents, trade secrets or know-how that would be useful in connection with the development, manufacture, marketing, promotion, use, sale, import or export of Licensed Products.
9.4. Disclaimer. Chiron understands that Licensed Products are the subjects of ongoing clinical research and development and that Cubist cannot assure the safety or usefulness of Licensed Products, however, the foregoing shall not be construed to affect the allocation of risks agreed upon by the Parties under to Article 10.
9.5. No Other Representations. THE EXPRESS REPRESENTATIONS AND WARRANTIES STATED IN THIS ARTICLE 9 ARE IN LIEU OF ALL OTHER REPRESENTATIONS AND WARRANTIES, EXPRESS, IMPLIED, OR STATUTORY, INCLUDING WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT OR NON-MISAPPROPRIATION OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS.
* CONFIDENTIAL TREATMENT
REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED WITH THE COMMISSION
61
ARTICLE 10.
INDEMNIFICATION
10.1. Indemnification by Cubist. Subject to all of the provisions of this Article 10, Cubist hereby agrees to defend, hold harmless and indemnify (collectively “Indemnify”) Chiron and its Affiliates, and their respective agents, directors, officers and employees (the “Chiron Indemnitees”) from and against any and all suits, claims, actions, demands, liabilities, expenses and/or losses, including without limitation reasonable legal expenses and attorneys’ fees (collectively “Losses”) suffered or incurred by any of such Chiron Indemnitees resulting from (i) a material breach of any of Cubist’s representations and warranties pursuant to Article 9 of this Agreement or Article 9 of the Supply Agreement; (ii) a material breach of any of Cubist’s covenants and agreements made pursuant to this Agreement or the Supply Agreement; (iii) any Third Party claim made against any such Chiron Indemnitee for damages suffered by such Third Party arising from any failure of Cubist or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, to comply in any material respect with applicable laws or regulations; (iv) any Third Party claim made against any such Chiron Indemnitees for death, bodily injury or property damage suffered by such Third Party arising from the negligence, recklessness or willful misconduct of Cubist or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, in the course of carrying out any of the actions or activities of Cubist under or in connection with this Agreement or the Supply Agreement; or (v) any Third Party claim made against any such Chiron Indemnitees for death, bodily injury or property damage suffered by such Third Party arising from any activities of Cubist or any of its Affiliates, licensees (other than Chiron), contractors or distributors, or any of their respective agents or employees, anywhere in the world prior to, on, or after the Effective Date in connection with their respective development, manufacturing or Commercialization activities with respect to Licensed Products; provided, however, that Cubist shall not be required to indemnify the Chiron Indemnitees for any Losses pursuant to this Section 10.1 to the extent that (1) such Losses arise from matters or claims for which Chiron is required to indemnify any of the Cubist Indemnities pursuant to Section 10.2 hereof, (2) such Losses arise from Chiron’s breach or non-compliance with any of the provisions of this Agreement or the Supply Agreement, (3) such Losses arise or result from the negligence, recklessness or willful misconduct of Chiron or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, (4) such Losses arise or result from any Third Party products liability claim, (5) such Losses consist of Recall Expenses that are in excess of the maximum amount of such Recall Expenses for which Cubist is liable under Section 5.7(b) of this Agreement and Section 6.6(b) of the Supply Agreement or (6) Cubist’s liability for such Losses is limited pursuant to Section 10.6. Cubist’s liability to any of the Chiron Indemnitees with respect to Third Party products liability claim is set forth in Section 10.3 below.
10.2. Indemnification by Chiron. Subject to all of the provisions of this Article 10, Chiron hereby agrees to Indemnify Cubist and its Affiliates, and their respective agents, directors, officers and employees (the “Cubist Indemnitees”) from and against any and all Losses suffered or incurred by any of such Cubist Indemnitees resulting from (i) a material breach of any of Chiron’s representations and warranties pursuant to Article 9 of this Agreement or Article 9 of the Supply Agreement; (ii) a material breach of any of Chiron’s covenants and
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
62
agreements made pursuant to this Agreement or the Supply Agreement; (iii) any Third Party claim made against any such Cubist Indemnitee for damages suffered by such Third Party arising from any failure of Chiron or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, to comply in any material respect with applicable laws and regulations; (iv) any Third Party claim made against any such Cubist Indemnitee for death, bodily injury or property damage suffered by such Third Party arising from the negligence, recklessness or willful misconduct of Chiron or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, in the course of carrying out any of the actions or activities of Chiron under or in connection with this Agreement or the Supply Agreement; or (v) any Third Party claim made against any such Cubist Indemnitees for death, bodily injury or property damage suffered by such Third Party arising from any activities of Chiron or any of its Affiliates, licensees (other than Cubist), contractors or distributors, or any of their respective agents or employees, anywhere in the world on or after the Effective Date in connection with their respective development, manufacturing or Commercialization activities with respect to Licensed Products; provided, however, that Chiron shall not be required to indemnify the Cubist Indemnitees for any Losses pursuant to this Section 10.2 to the extent that (1) such Losses arise from matters or claims for which Cubist is required to indemnify any of the Chiron Indemnities pursuant to Section 10.1 hereof, (2) such Losses arise from Cubist’s breach or non-compliance with any of the provisions of this Agreement or the Supply Agreement, (3) such Losses arise or result from the negligence, recklessness or willful misconduct of Cubist or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, (4) such Losses arise or result from any Third Party products liability claim or (5) Chiron’s liability for such Losses is limited pursuant to Section 10.6. Chiron’s liability to any of the Cubist Indemnitees with respect to Third Party products liability claim is set forth in Section 10.3 below.
10.3. Liability for Third Party Products Liability Claims.
(a) Subject to all of the provisions of this Article 10, Cubist hereby agrees to Indemnify the Chiron Indemnitees from and against any and all Losses suffered or incurred by any of such Chiron Indemnitees resulting from any Third Party product liability claim made against any such Chiron Indemnitee for death, bodily injury or property damage suffered by such Third Party from or in connection with any Licensed Product sold by Chiron or its Affiliates or distributors for use in the Territory but only if and to the extent that such death, bodily injury or property damage was or is caused by reason of such Licensed Product being a Defective Manufactured Product; provided, however, that Cubist shall not be required to indemnify the Chiron Indemnitees for any Losses pursuant to this Section 10.3(a) to the extent that (1) such Losses arise from matters or claims for which Chiron is required to indemnify any of the Cubist Indemnities pursuant to Section 10.3(b) hereof or (2) such Losses arise from Chiron’s breach or non-compliance with any of the provisions of this Agreement or the Supply Agreement.
(b) Subject to all of the provisions of this Article 10, Chiron hereby agrees to Indemnify the Cubist Indemnitees from and against any and all Losses suffered or incurred by any of such Cubist Indemnitees resulting from any Third Party product liability claim made against any such Cubist Indemnitee for death, bodily injury or property damage suffered by such Third Party from or in connection with any Licensed Product sold or used by Chiron or its Affiliates or distributors but only if and to the extent that such death, bodily injury or property damage arises or results from (A) the improper use, transportation, packaging, storage or
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
63
handling of such Licensed Product by Chiron or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, including, without limitation, any such use, transportation, packaging, storage or handling of such Licensed Products that does not conform with any of the applicable specifications of such Licensed Product or with the requirements of any Regulatory Authorities or any applicable laws within the Territory, (B) the failure of such Licensed Product to have an appropriate shelf-life at the time such Licensed Product was sold or used by Chiron or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, provided that such Licensed Product is not a Defective Manufactured Product, (C) any defect or deficiency in the label or package insert of, or the marketing or promotional materials with respect to, such Licensed Product, including, without limitation, the failure of the label or package insert of, or the marketing or promotional materials with respect to, such Licensed Product to meet the requirements of any Regulatory Authority or any applicable laws within the Territory, except to the extent that such defect or deficiency resulted from incorrect information received by Chiron from Cubist or from the failure by Cubist to disclose to Chiron information that is in the possession of Cubist, (D) the promotion (including the establishment of any program to provide treatment on a named patient basis or other similar basis) by Chiron or any of its Affiliates, contractors or distributors, or any of their respective agents or employees, of such Licensed Product for use in connection with an indication for which such Licensed Product has not received Regulatory Approval if data in Chiron’s possession establishes that it would not be safe or effective to use such License Product for the treatment of such indication, (E) a defect in such Licensed Product due to improper manufacture by Chiron or by a Third Party manufacturer, (F) any failure by Chiron to develop, use, market, promote or sell such License Product in compliance with all of the applicable requirements of the Regulatory Authorities in the Territory and all of the applicable laws within the Territory, (G) any of the activities or responsibilities that Chiron performs or is required to perform pursuant to the Supply Agreement, or (H) any breach by Chiron of any of its obligations under this Agreement or the Supply Agreement; provided, however, that Chiron shall not be required to indemnify the Cubist Indemnitees for any Losses pursuant to this Section 10.3(b) to the extent that (1) such Losses arise from matters or claims for which Cubist is required to indemnify any of the Chiron Indemnities pursuant to Section 10.3(a) hereof or (2) such Losses arise from Cubist’s breach or non-compliance with any of the provisions of this Agreement or the Supply Agreement.
(c) Subject to the provisions of Section 10.3(d) and 10.4(b) below, in the event that there is a Third Party products liability claim for death, bodily injury or property damage suffered by such Third Party from or in connection with any Licensed Product sold or used by Chiron or its Affiliates or distributors and neither Party is required to indemnify the other Party pursuant to Section 10.3(a) or 10.3(b) above in connection with such Third Party products liability claim, then the Parties agree to [*] amounts that are paid or are required to be paid to such Third Party in connection with such Third Party products liability claim (regardless of whether such amounts are determined by a court of competent jurisdiction, arbitration proceeding or negotiated settlement), with [*] of this Agreement in connection with [*] in connection with the [*]. The Parties hereby agree that this clause (c) is not intended to cover Third Party products liability claims arising from sales of Licensed Product outside of the Territory by Cubist, its Affiliates or Other Licensees, or any of their respective distributors.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
64
(d) Notwithstanding anything expressed or implied in Section 10.3(c) to the contrary, in no event shall Cubist have any obligation under Section 10.3(c) with respect to any Third Party products liability claim if and to the extent that, prior to the time that such Third Party products liability claim arises, (i) Cubist has taken steps to eliminate or mitigate the risk of any such Third Party products liability claim arising outside the Territory, the adequacy of such steps to be judged in accordance with the applicable laws and regulatory requirements outside the Territory, (ii) Cubist has communicated to Chiron the potential risk of any such Third Party products liability claim could arise in the future and the steps that Cubist has taken, is taking or will be taking to eliminate or mitigate such risk outside the Territory and (iii) Chiron has not taken substantially the same steps in the Territory as Cubist has taken outside the Territory. Cubist shall also not have any obligation under Section 10.3(c) with respect to any Third Party products liability claim if and to the extent that, prior to the time that such Third Party products liability claim arises, (x) Chiron has knowledge of facts and circumstances that establish that there is a risk of any such Third Party products liability claim arising in the Territory and fails to disclose such knowledge to Cubist, (y) Chiron fails to implement any plan previously discussed and agreed to by the Parties to eliminate or mitigate the risk of any such Third Party products liability claim arising in the Territory or (z) if the Parties have not discussed any such plan or cannot agree on any such plan, and Chiron fails to take steps to eliminate or mitigate the risk of any such Third Party products liability claim arising in the Territory, the adequacy of such steps to be judged in accordance with the applicable laws and regulatory requirements in the Territory.
(e) EXCEPT TO THE EXTENT EITHER PARTY HAS ANY LIABILITY TO THE OTHER PARTY PURSUANT TO THIS SECTION 10.3, NEITHER PARTY SHALL HAVE ANY LIABILITY TO THE OTHER PARTY UNDER THIS AGREEMENT, THE SUPPLY AGREEMENT OR OTHERWISE UNDER ANY THEORY OF LIABILITY FOR ANY THIRD PARTY PRODUCTS LIABILITY CLAIM IN CONNECTION WITH LICENSED PRODUCTS.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
65
10.4. Procedure
(a) If either Party is seeking indemnification under Section 10.1, 10.2, 10.3(a) or 10.3(b) in connection with a Third Party claim, it shall inform the indemnifying Party of such Third Party claim giving rise to the obligation to indemnify pursuant to such section as soon as reasonably practicable after receiving notice of the claim. The indemnifying Party shall have the right to assume the defense of any such Third Party claim for which it is obligated to indemnify the indemnified Party under Section 10.1, 10.2, 10.3(a) or 10.3(b). The indemnified Party shall cooperate with the indemnifying Party (and its insurer) as the indemnifying Party may reasonably request, and at the indemnifying Party’s sole cost and expense. The indemnified Party shall have the right to participate, at its own expense and with counsel of its choice, in the defense of any claim or suit that has been assumed by the indemnifying Party. Neither Party shall have any obligation to indemnify the other Party in connection with any settlement made without the indemnifying Party’s written consent, provided that the indemnifying Party does not unreasonably withhold or delay any such written consent. If the Parties cannot agree as to the application of Sections 10.1, 10.2, 10.3(a) or 10.3(b) to any Third Party claim, the Parties may conduct separate defenses of such claims, with each Party retaining the right to claim indemnification from the other in accordance with Section 10.1, 10.2, 10.3(a) or 10.3(b) upon resolution of the underlying claim.
(b) If either Party becomes subject to a Third Party products liability claim for which such Party believes that it has the right to require the other Party to share, in accordance with the provisions of Section 10.3(c) above, in any payments to be made to such Third Party in connection with such claim, such Party shall inform the other of such Third Party products liability claim as soon as reasonably practicable after receiving notice of the claim. The Party that is subject to the Third Party products liability claim shall have the right to control the defense of such claim, but the other Party shall have the right to participate, at its own expense and with counsel of its choice, in the defense of such Third Party products liability claim. Neither Party shall have any obligation to share in the cost of any settlement of any such Third Party products liability claim if such settlement is made without such Party’s written consent, provided that such Party does not unreasonably withhold or delay any such written consent.
10.5. Insurance. Each Party shall procure and maintain insurance or self-insurance, including product liability insurance, adequate to cover its obligations hereunder and which are consistent with normal business practices of prudent companies similarly situated at all times during which any Licensed Product is being clinically tested with human subjects or commercially distributed or sold by Chiron, Cubist or Other Licensees. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Article 10. Each Party shall provide the other with written evidence of such insurance (or financial information that describes the amounts available under any self-insurance facility) upon request.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
66
10.6. Limitation of Liability
(a) NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR LOST PROFITS OR FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, PUNITIVE OR EXEMPLARY DAMAGES OF THE OTHER PARTY IN CONNECTION WITH THIS AGREEMENT OR THE SUPPLY AGREEMENT OR THE TRANSACTIONS CONTEMPLATED BY THIS AGREEMENT OR THE SUPPLY AGREEMENT, HOWEVER CAUSED, UNDER ANY THEORY OF LIABILITY. THE FOREGOING LIMITATION SHALL NOT APPLY SO AS TO LIMIT THE LIABILITY OF EITHER PARTY FOR LOST PROFITS OR FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, PUNITIVE OR EXEMPLARY DAMAGES THAT MAY BE IMPOSED UPON SUCH PARTY UNDER ANY THEORY OF LIABILITY (OTHER THAN BREACH OF CONTRACT), WHETHER BY STATUTE OR COMMON LAW, AS A RESULT OF ANY INFRINGEMENT BY SUCH PARTY OF THE INTELLECTUAL PROPERTY RIGHTS OF THE OTHER PARTY OR AS A RESULT OF THE FAILURE OF SUCH PARTY TO PERFORM AND OBSERVE ITS CONFIDENTIALITY OBLIGATIONS TO THE OTHER PARTY.
(b) EXCEPT AS A RESULT OF A MATERIAL BREACH BY EITHER PARTY OF ITS REPRESENTATIONS AND WARRANTIES PURSUANT TO ARTICLE 9 OF THIS AGREEMENT OR OF A MATERIAL BREACH BY SUCH PARTY OF ITS COVENANTS AND AGREEMENTS IN SECTION 8.5 OR SECTION 8.10 OF THIS AGREEMENT, AND EXCEPT FOR ANY INDEMNIFICATION OBLIGATION THAT EITHER PARTY MAY HAVE UNDER CLAUSE (i) OR (ii) OF SECTION 10.1 OR 10.2 (AS APPLICABLE) OF THIS AGREEMENT WITH RESPECT TO ANY MATERIAL BREACH BY SUCH PARTY OF ITS REPRESENTATIONS, WARRANTIES, COVENANTS AND AGREEMENTS UNDER ARTICLE 9, SECTION 8.5 OR SECTION 8.10 OF THIS AGREEMENT, SUCH PARTY SHALL NOT HAVE ANY LIABILITY TO THE OTHER PARTY UNDER THIS AGREEMENT OR THE SUPPLY AGREEMENT FOR ANY THIRD PARTY CLAIM MADE AGAINST SUCH OTHER PARTY FOR ACTUAL OR ALLEGED INFRINGEMENT OR MISAPPROPRIATION OF THE INTELLECTUAL PROPERTY RIGHTS OF SUCH THIRD PARTY TO THE EXTENT THAT SUCH INFRINGEMENT OR MISAPPROPRIATION ARISES FROM ACTIVITIES UNDER OR PURSUANT TO THIS AGREEMENT OR THE SUPPLY AGREEMENT, INCLUDING, WITHOUT LIMITATION, ANY ACTUAL OR ALLEGED INFRINGEMENT OR MISAPPROPRIATION OF THE INTELLECTUAL PROPERTY RIGHTS OF SUCH THIRD PARTY CAUSED AS A RESULT OF THE USE OR PRACTICE BY SUCH OTHER PARTY OF THE INTELLECTUAL PROPERTY RIGHTS OF SUCH PARTY.
(c) CUBIST’S LIABILITY UNDER THIS AGREEMENT OR THE SUPPLY AGREEMENT OR UNDER TORT PRINCIPLES OF NEGLIGENCE FOR FAILURE TO SUPPLY LICENSED PRODUCT TO CHIRON OR THE SUPPLY OF DEFECTIVE MANUFACTURED PRODUCTS [*] ANY FAILURE TO SUPPLY, OR ANY SUPPLY OF DEFECTIVE MANUFACTURED PRODUCT, WHICH ARISES OUT OF A PARTICULAR CIRCUMSTANCE (EVEN IF SUCH CIRCUMSTANCE AFFECTS MULTIPLE LOTS OF LICENSED PRODUCT) CONSTITUTES A SINGLE “BREACH” FOR THIS PURPOSE. [*] SHALL NOT APPLY IN THE CASE OF FRAUD OR A WILLFUL BREACH BY CUBIST OF ITS SUPPLY OBLIGATIONS OR BREACH OF CUBIST’S OBLIGATION TO ALLOCATE
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
67
LIMITED SUPPLIES IN ACCORDANCE WITH SECTION 8.1 OF THE SUPPLY AGREEMENT, OR TO CUBIST’S OBLIGATIONS UNDER SECTION 5.7 (RECALLS AND VOLUNTARY WITHDRAWALS) AND SECTION 10.3 (LIABILITY FOR THIRD PARTY PRODUCT LIABILITY CLAIMS) OF THIS AGREEMENT AND SECTION 9.1(d) (CUBIST’S PRODUCT WARRANTIES – EXCLUSIVE REMEDY) OF THE SUPPLY AGREEMENT.
ARTICLE 11.
RECORDS; PUBLICATIONS
11.1. Records. Each Party shall keep or cause to be kept full and accurate books of account and records containing all particulars that may be necessary to determine, in a manner consistent with generally accepted accounting principles in the United States, the sums or credits due under this Agreement, including, but not limited to Transfer Prices, Manufacturing Costs and Net Sales. At the written request (and expense) of either Party, the other Party and its Affiliates and licensees and sublicensees shall permit an independent certified public accountant appointed by such Party and reasonably acceptable to the other Party, accompanied by representatives of the financial department of the audited Party at reasonable times, upon reasonable notice and no more frequently than once per calendar year, to examine only (i) those records as may be necessary to determine the correctness or completeness of any report or payment made under this Agreement and the Supply Agreement, including but not limited to Transfer Prices, Manufacturing Costs, and Net Sales, with respect to any calendar year ending not more than three (3) years prior to such Party’s request and (ii) those records as may be necessary to comply with the requesting Party’s obligations under applicable law or with a request made by any governmental authority, in either case with respect to any calendar year ending not more than seven (7) years prior to such Party’s request. Results of any such examination shall be (i) made available to both Parties, (ii) limited to information relating to the Licensed Products and (iii) subject to Article 12. The Party requesting the audit shall bear the full cost of the performance of any such audit, unless such audit discloses a variance of more than [*] from the amount of the original report, royalty or payment calculation. In such case, the Party being audited shall bear the full cost of the performance of such audit.
11.2. Publications. Neither Party shall publish or present the results of studies carried out under this Agreement without the opportunity for prior review by the other Party in accordance with the provisions set forth in this Section 11.2. Subject to Section 12.2, each Party agrees to provide the other Party the opportunity to review any proposed abstracts, manuscripts or presentations (including verbal presentations) which relate to any Licensed Product at least sixty (60) days prior to their intended submission for publication and agrees, upon request, not to submit any such abstract or manuscript for publication, or to make such presentation, until the other Party is given a reasonable period of time to secure patent protection for any material in such publication or presentation that is owned by the requesting Party (either individually or jointly with the non-requesting Party) and which the requesting Party believes to be patentable. In the event that the nature of the content of any proposed publication or presentation is such that a Party is entitled to request that submission of such publication or delivery of such presentation be delayed pursuant to the foregoing provisions of this Section 11.2, then both Parties understand that a reasonable commercial strategy may require delay of publication or presentation of
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
68
information or filing of patent applications. The Parties agree to review and consider delay of publication or presentation and filing of patent applications under certain circumstances. Neither Party shall have the right to publish or present Confidential Information of the other Party, and each Party shall remove the Confidential Information of the other Party from any proposed publication or presentation upon request by such other Party. Nothing contained in this Section 11.2 shall prohibit the inclusion of information necessary to file a patent application with a government authority, except for Confidential Information of the non-filing Party, provided the non-filing Party is given a reasonable opportunity to review the information to be included prior to submission of such patent application. Notwithstanding the foregoing, the Parties recognize that independent investigators have been engaged, and will be engaged in the future, to conduct clinical trials of Licensed Products. Independent investigators that have been engaged by a Party or both Parties prior to or on the Effective Date may release information regarding such studies in a manner consistent with academic standards within the scope of such investigator’s agreement with the relevant Party. Independent investigators that are engaged by a Party or both Parties after the Effective Date are understood to operate in an academic environment and shall be allowed to release information regarding such studies in a manner consistent with academic standards and within the scope of such investigator’s agreement with the relevant Party. With respect to any agreement entered by either Party with any independent investigator after the Effective Date to conduct clinical trials of Licensed Products, such Party shall use Commercially Reasonable Efforts to include in such agreements provisions that would give such Party the right to limit the publication rights of such independent investigator with respect to any results of such clinical trials to the same extent as such Party would have under this Section 11.2 if such independent investigator were the other Party to this Agreement; provided, however, that in no event shall such Party be required or obligated to make any payment to such independent investigator or incur any financial cost or penalty for the benefit of such independent investigator in order to limit the publication rights of such independent investigator in the manner contemplated under this Section 11.2.
ARTICLE 12.
CONFIDENTIALITY
12.1. Treatment of Confidential Information. The Parties agree that during the Term, and after this Agreement expires or terminates, a Party receiving Confidential Information of the other Party shall (i) maintain in confidence such Confidential Information to the same extent such Party maintains its own proprietary industrial information of similar kind and value (but at a minimum each Party shall use commercially reasonable efforts to maintain Confidential Information in confidence); (ii) not disclose such Confidential Information to any Third Party without prior written consent of the disclosing Party, except for disclosures made in confidence to any Third Party pursuant to a plan approved by the JCT or to its licensees or sublicensees who agree to be bound by obligations of non-disclosure and non-use at least as stringent as those contained in this Article 12; and (iii) not use such Confidential Information for any purpose except those purposes expressly permitted by this Agreement.
12.2. Authorized Disclosure. Notwithstanding any other provision of this Agreement, each Party may disclose Confidential Information of the other Party:
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
69
(a) to the extent and to the persons and entities required by an applicable governmental law, rule or regulation or court order; provided, however, that the Party required to disclose Confidential Information shall first have given prompt notice to the other Party hereto to enable it to seek any available exemptions from or limitations on such disclosure requirement and shall reasonably cooperate in such efforts by the other Party (in particular, the Parties acknowledge that Cubist and/or Chiron may be obligated to file a copy of this Agreement with the U.S. Securities and Exchange Commission with its next quarterly report on Form 10-Q, annual report on Form 10-K or current report on Form 8-K or with any registration statement filed with the U.S. Securities and Exchange Commission (the “SEC”) pursuant to the Securities Act of 1933, as amended; in the event of any such filing, the Parties agree to cooperate and work together to request confidential treatment pursuant to, and in accordance with, the rules and regulations of the SEC);
(b) to the extent and to the persons and entities required by rules of the National Association of Securities Dealers;
(c) as necessary to file or prosecute patent applications, prosecute or defend litigation or otherwise establish rights or enforce obligations under this Agreement, but only to the extent that any such disclosure is necessary;
(d) as required by the Lilly License;
(e) to investigators, institutions, contract research organizations, clinical research associates and Regulatory Authorities and the like in connection with conducting clinical trials and obtaining authorizations for same; or
(f) to Regulatory Authorities in connection with Drug Approval Applications.
12.3. Publicity. Subject to Section 11.2, any other publication, news release or other public announcement relating to this Agreement or to the performance hereunder, shall first be reviewed and approved by both Parties, which approval shall not be unreasonably withheld.
ARTICLE 13.
TERM AND TERMINATION
13.1. Term. This Agreement shall become effective on the Effective Date and shall remain in effect until the earlier of (i) the effective date of the termination of this Agreement pursuant to Section 13.2, 13.3 or 13.4 below, or (ii) the expiration of the term of this Agreement on the date on which Chiron is no longer obligated, pursuant to this Agreement, to make payment to Cubist of any royalties in connection with sales of Licensed Products in the Territory. In the event that the term of this Agreement expires pursuant to clause (ii) of this Section 13.1, then the licenses granted by Cubist to Chiron, and any licenses granted by Chiron to Cubist, pursuant to Article 2 and Section 8.10 shall survive such expiration and shall be fully paid-up, royalty-free, perpetual and irrevocable licenses.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
70
13.2. Termination For Convenience by Chiron. Chiron shall have, at any time, the right to terminate this Agreement in its entirety upon [*] prior written notice to Cubist. Chiron shall have, at any time, the right to terminate this Agreement with respect only to one or more countries within the Territory (but not more than [*] of all countries within the Territory in any [*] upon [*] prior written notice to Cubist; provided, however, that the foregoing right of Chiron to terminate this Agreement on a country-by-country basis shall not apply to any Major Market Country so as to permit Chiron to terminate this Agreement with respect to any Major Market Country. Chiron’s termination rights under this Section 13.2 shall not apply on a Licensed Product-by-Licensed Product basis.
13.3. Termination By Either Party Upon Bankruptcy or Insolvency. This Agreement may be terminated in its entirety by either Party by giving written notice of termination to the other Party in the event that such other Party files or institutes any bankruptcy, liquidation or receivership proceedings, or in the event that such other Party makes an assignment of a substantial portion of the assets of such other Party for the benefit of its creditors; provided, however, that, in the case of any involuntary bankruptcy proceeding such right to terminate shall only become effective if such other Party consents to the involuntary bankruptcy or such proceeding is not dismissed within [*] after the filing thereof.
13.4. Termination for Breach.
(a) Notice. If either Party believes that the other is in material breach of this Agreement with respect to one or more Licensed Products or if Cubist believes that Chiron is in material breach of the Supply Agreement with respect to one or more Licensed Products, then the Party holding such belief (the “Non-breaching Party”) may deliver notice of such breach to the other Party (the “Notified Party”). A breach by Chiron of its diligence obligations under Section 5.1 or under Section 6.1 hereof and a breach by Cubist of its obligation under Section 4.1 hereof, except as specified otherwise, shall be deemed to be a material breach of this Agreement by Chiron or Cubist, as the case may be. The Notified Party shall have ninety (90) days to cure such breach, provided that, if cure cannot be reasonably effected within such ninety (90) day period, the Notified Party may elect to deliver to the Non-breaching Party within such ninety (90) day period a plan to cure such breach within a timeframe that is reasonably prompt in light of the circumstances then prevailing, and the Non-breaching Party shall have the right to approve or reject in writing such proposed plan in its absolute discretion. If the Non-breaching Party approves in writing such proposed plan, then the cure period will be extended in accordance with the terms of such plan and the Notified Party shall use Commercially Reasonable Efforts to carry out such plan and cure the breach in accordance with the provisions of such plan.
(b) Failure to Cure. If the Notified Party fails to cure such breach as provided for in Section 13.4(a), the Non-breaching Party may terminate this Agreement either in its entirety or with respect to one or more Licensed Products upon written notice to the Notified Party, provided that, the Non-breaching Party gives such written notice of termination within [*] after the Notified Party has failed to cure such breach as provided for in Section 13.4(a).
(c) Disputes. If a Party gives notice of termination under this Section 13.4 and the other Party disputes whether such termination is proper under this Section 13.4, then the issue of whether this Agreement may properly be terminated upon expiration of the notice period
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
71
(unless such breach is cured as provided in Section 13.4(a)) shall be resolved in accordance with Article 14 (Dispute Resolution). If as a result of such dispute resolution process it is determined that the notice of termination was proper, then such termination shall be deemed to have been effective ninety (90) days following the date of the notice of termination. If as a result of such dispute resolution process it is determined that the notice of termination was improper, then no termination shall have occurred and this Agreement shall remain in effect.
(d) Termination as to Certain Licensed Products. Notwithstanding the foregoing provisions of this Section 13.4, Cubist may exercise its right to terminate the Agreement pursuant to this Section 13.4 with respect to one or more particular Licensed Products as to which Chiron has breached its obligations under this Agreement or the Supply Agreement, rather than with respect to the entire Agreement, in which case the notice provided by Cubist pursuant to Section 13.4(a) shall specify that the Agreement is being terminated pursuant to this Section 13.4 only with respect to certain Licensed Products listed in such notice. If Cubist makes such an election under this Section 13.4(d), then subsections (a) through (c) shall be deemed to refer to termination of the Agreement only with respect to those Licensed Products set forth in the notice provided pursuant to Section 13.4(a).
(e) Right to Sell. Notwithstanding anything expressed or implied in this Agreement to the contrary, in the event that Cubist shall terminate this Agreement in accordance with the provisions of this Section 13.4, Chiron shall, notwithstanding any such termination, have the opportunity and right to sell, assign, sublicense or otherwise transfer this Agreement and the Chiron Interest to any Reasonable Buyer pursuant to, and in accordance with, the provisions of Section 15.6 of this Agreement, provided that any such sale, assignment, sublicense or other transfer is effected [*] after the expiration of the cure period referred to in Section 13.4(a) or, in the event that there is a dispute between the Parties as to whether Cubist’s termination of this Agreement has been proper, [*] after the resolution of such dispute pursuant to the provisions of Article 14 hereof. Any such Reasonable Buyer shall acquire the Chiron Interest free and clear of any breach by Chiron that led to the termination of this Agreement and such Reasonable Buyer shall following such acquisition have all of the rights and obligations that Chiron would have had under this Agreement as if Chiron had not breached this Agreement and Cubist had not terminated the Agreement. Any such sale, assignment, sublicense or other transfer of the Chiron Interest to a Reasonable Buyer pursuant to this Section 13.4(e) shall not relieve or release Chiron from any liability that Chiron may have to Cubist in connection with any breach that resulted in the exercise by Cubist of its right to terminate this Agreement. Until the expiration of the [*] referred to above in this Section 13.4(e), Chiron shall continue to perform in accordance with the provisions of this Agreement and the Supply Agreement, and shall be liable for, any and all obligations that accrue during such [*] under this Agreement and the Supply Agreement. In the event that Chiron is unable to consummate a sale, assignment, sublicense or other transfer of this Agreement and the Chiron Interest within such [*], then all of Chiron’s rights under this Section 13.4(e) shall terminate and be of no further force or effect whatsoever
13.5. INTENTIONALLY OMITTED.
13.6. Consequences of Termination.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
72
(a) If either Cubist or Chiron terminates this Agreement in its entirety, or with respect to one or more Licensed Product(s), or with respect to one or more countries, in each case pursuant to any provisions of this Article 13, then, subject to the right of Chiron to sell, assign, license or otherwise transfer its rights, obligations and interests under this Agreement pursuant to Section 13.4(e):
(i) The licenses granted by Cubist and Chiron under Article 2 shall terminate (1) with respect to all Licensed Products and all countries within the Territory if the Agreement has been terminated in its entirety, or (2) with respect to those Licensed Products as to which Chiron or Cubist has exercised termination rights, in which case the licenses granted by Cubist and Chiron under Article 2 shall terminate with respect to such Licensed Products for all countries in the Territory if Chiron or Cubist exercised termination rights with respect to such Licensed Products in all countries in the Territory or the licenses granted by Cubist and Chiron under Article 2 shall terminate with respect to such Licensed Products only in certain countries within the Territory if Chiron or Cubist exercised termination rights with respect to such Licensed Products only in such countries within the Territory but not the other countries within the Territory. For purposes of this Agreement, the term “Reverted Products” shall mean those Licensed Products as to which Cubist or Chiron exercise termination rights pursuant to this Article 13 (and could mean, in any particular context, all of the Licensed Products if this Agreement is terminated in its entirety), and the term “Reverted Country” shall mean any country within the Territory as to which Cubist or Chiron exercise termination rights pursuant to this Article 13 (and could mean, in any particular context, all of the countries within the Territory if this Agreement were terminated in its entirety or, with respect to any particular Licensed Product, if Chiron’s rights to such Licensed Product are terminated as to all countries within the Territory).
(ii) Chiron shall discontinue making any representation regarding its status as a licensee of or distributor for Cubist in all Reverted Countries for all applicable Reverted Products, and shall cease conducting any Commercialization activities in all Reverted Countries with respect to all applicable Reverted Products.
(iii) As promptly as possible, Chiron shall transfer to Cubist all Drug Approval Applications and Regulatory Approvals that Chiron holds as of the time of any such termination for Reverted Products in all applicable Reverted Countries. In such event, Chiron shall take all actions necessary to effect such transfer of such Drug Approval Applications and Regulatory Approvals to Cubist.
(iv) Promptly after any such termination, Chiron shall execute any documents required in the reasonable opinion of Cubist (1) for Cubist to be entered as a “registered user” or owner of the Cubist Marks in connection with the Commercialization of the Reverted Products in those applicable Reverted Countries in which Chiron was previously entered into as the registered user, owner or registered licensee of the Cubist Marks pursuant to, and in accordance with, the provisions of Section 8.10 hereof, or (2) for Chiron to be removed as registered user or licensee of the Cubist Marks in connection with the Commercialization of the Reverted Products in such applicable Reverted Countries.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
73
(v) Chiron shall be responsible for payment of any milestone payments that accrue to Cubist for any milestones achieved for any Reverted Product(s) prior to the effective date of any such termination, but not for any milestones achieved for any Reverted Product(s) after the effective date of any such termination.
(b) In the event that this Agreement is terminated due to the rejection of this Agreement by or on behalf of a Party under Xxxxxxx 000 xx xxx Xxxxxx Xxxxxx Bankruptcy Code (the “Code”), all licenses and rights to licenses granted under or pursuant to this Agreement by one Party to the other are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the Code, licenses of rights to “intellectual property” as defined under Section 101(35A)of the Code. The Parties agree that the licensed Party, as a licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the Code.
13.7. Survival.
(a) Expiration or termination of this Agreement shall not relieve the Parties of any liability hereunder which accrued, or which arose during or relates to, any period prior to the effective date of such expiration or termination, nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement prior to the effective date of such expiration or termination, nor prejudice either Party’s right to obtain performance of any obligation hereunder which accrued, or which arose during or relates to, any period prior to the effective date of such expiration or termination. The remedies provided in this Article 13 are not exclusive of any other remedies a Party may have in law or equity (it being understood that nothing in this sentence shall limit the scope or application of any limitations on Chiron’s remedies that may be expressly set forth elsewhere in this Agreement, including Section 10.6 of this Agreement). Upon any expiration of the term of this Agreement pursuant to clause (ii) of Section 13.1 hereof, the following provisions of this Agreement shall survive any such expiration: Articles 1, 10, 12, 14 and 15 and Sections 2.2, 2.3, 2.8, 4.6, 5.5, 8.2(c), 8.4, 8.7(a), 8.7(b), 8.8, 8.9, 8.10, 8.11, 11.1 and this 13.7, and Sections 9.1, 9.2 and 9.3 (but Sections 9.1, 9.2 and 9.3 shall survive only for purposes of making an indemnification claim in accordance with the provisions of Article 10 with respect to a Third Party claim that arises from any breach of Section 9.1, 9.2 or 9.3 and that relates to any period prior to any such expiration of the term of this Agreement). In addition, the provisions of Section 8.5 hereof shall survive the expiration of the term of this Agreement pursuant to clause (ii) of Section 13.1 hereof so as to cover and apply to any Third Party Infringement Claim that arises after such expiration and that is based on infringement of a Knowable Patent.
(b) Notwithstanding expiration of this Agreement,
(i) Chiron shall not promote, sell or offer for sale Licensed Products in any Cubist Patent Country;
(ii) Chiron shall require its distributors who sell Chiron’s Licensed Products to make a similar covenant;
(iii) Chiron shall require its sublicensees to make a similar covenant; and
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
74
(iv) Chiron shall not grant to any subcontractor or sublicensee any rights that are broader in scope than the rights that Chiron has at the time of such expiration.
“Cubist Patent Country” means any country in which there is an unexpired Cubist Patent or Joint Patent which includes a valid and enforceable claim that covers the Licensed Product sold by Chiron and in which Cubist or its licensees are selling their own Licensed Products.
(c) Upon any termination of this Agreement pursuant to Sections 13.2, 13.3 or 13.4, the following provisions of this Agreement shall survive any such termination: Articles 1, 10, 12, 14 and 15, and Sections 11.1, 13.4, 13.6 and this Section 13.7, and Sections 9.1, 9.2 and 9.3 (but Sections 9.1, 9.2 and 9.3 shall survive only for purposes of making an indemnification claim in accordance with the provisions of Article 10 with respect to a Third Party claim that arises from any breach of Section 9.1, 9.2 or 9.3 and that relates to any period prior to any such termination of this Agreement).
ARTICLE 14.
DISPUTE RESOLUTION
14.1. Disputes. The Parties recognize that disputes as to certain matters may from time to time arise during the term of this Agreement which relate to either Party’s rights and/or obligations hereunder. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation and without resort to litigation. To accomplish this objective, the Parties agree to follow the procedures set forth in this Section 14.1 if and when a dispute arises under this Agreement. Business issues that are not contractual disputes arising under this Agreement shall be discussed by the JCT at the request of either party. Any contractual dispute arising under this Agreement shall be discussed first by the JCT. In the event that the JCT is unable to resolve any such contractual dispute within thirty (30) days after such contractual dispute is submitted to the JCT, then any such contractual dispute shall, by either Party providing written notice to the other Party, be referred to the respective chief executive officers of the Parties for attempted resolution by good faith negotiations within thirty (30) days after such notice is received. In the event that the designated officers are not able to resolve such dispute within such thirty (30) day period, and do not agree to extend the time period for resolving the dispute, or if the terms and conditions of the resolution or settlement of the dispute are breached, the dispute shall be submitted for mediation by a mutually acceptable Third Party within thirty (30) days after expiration of the previous thirty (30) day period, unless the Parties agree to extend the period for submitting the contractual dispute for mediation. In the event that such contractual dispute is not resolved within thirty (30) days after such contractual dispute is submitted for mediation, unless the parties otherwise agree to extend the time period for resolving the dispute, then such contractual dispute shall be resolved by arbitration pursuant to the provisions of Section 14.2. Pending resolution of any dispute covered by this Section 14.1, both Parties will continue their performance under this Agreement and the Supply Agreement of any obligations (including, without limitation, payment obligations) that are not the subject of such dispute.
14.2. Arbitration. If the dispute is not resolved pursuant to Section 14.1 above, then the Parties shall follow the procedures set forth below.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
75
(a) Any claim, dispute, or controversy arising out of or relating to this Agreement that is not resolved in accordance with the provisions of Section 14.1 and that the Parties agree to submit to binding arbitration pursuant to this Section 14.2 will be submitted by the parties to arbitration under rules then in effect (“ICC Rules”) of the International Chamber of Commerce (“ICC”) in New York, New York U.S.A. as modified herein or by agreement of the parties. Any such arbitration shall be conducted in New York, New York by three (3) arbitrators. Each Party shall select one (1) arbitrator and such arbitrators shall jointly appoint the third arbitrator who shall act as the chairman. If either Party fails to appoint an arbitrator within thirty (30) days of a request by the other Party, or if the arbitrators selected by the parties cannot agree on a chairman within thirty (30) days after they have been selected, then either Party may request the ICC to appoint such co-arbitrator (for the non-responsive Party) or the chairman. Such appointment shall be binding on the Parties. Each Party irrevocably and unconditionally (i) consents to the jurisdiction of any such proceeding and waives any objection that it may have to personal jurisdiction or the laying of venue of any such proceeding; and (ii) knowingly and voluntarily waives its rights to have disputes tried and adjudicated by a judge and jury except as otherwise expressly provided herein. The Parties hereby agree to exercise their respective rights under the ICC Rules to cause any arbitration proceeding under this Section 14.2(a) to be finalized and a decision rendered by the arbitrators as soon as reasonably practicable but in no event more than six (6) months after the commencement of such arbitration proceeding. Without limiting the provisions of the preceding sentence, the Parties will cooperate with each other in causing the arbitration to be held in as efficient and expeditious a manner as practicable. Unless the Parties agree otherwise, they shall be limited in their discovery to directly relevant documents. Responses or objections to a document request shall be served twenty (20) days after receipt of the request. The arbitrators shall resolve any discovery disputes. Nothing herein shall prevent the Parties from settling any dispute by mutual agreement at any time.
(b) Except as otherwise required by law, the Parties and the arbitrator(s) shall maintain as confidential all information or documents obtained during the arbitration process, including the resolution of the dispute. The arbitration shall be conducted in English language.
(c) The arbitrator(s) shall not have the authority to award any injunctive relief or to award exemplary or punitive damages, and the Parties expressly waive any right to such damages. The arbitrator(s) shall have the authority to award actual money damages (including interest on unpaid amounts from the date due). The costs and expenses of the arbitration, but not the costs and expenses of the Parties, shall be shared equally by the Parties, provided that the non-prevailing Party in any arbitration shall pay the other Party’s costs and expenses (including travel expenses) and reimburse such Party for its portion of the arbitration costs. In the event that neither Party wins totally, reimbursement shall be made proportionally in accordance with the ICC Rules. Any award rendered by the arbitrator(s) shall be final and binding upon the Parties. Judgment upon the award may be entered in any court of competent jurisdiction. If a Party fails to proceed with arbitration, unsuccessfully challenges the arbitration award, or fails to comply with the arbitration award, the other Party is entitled to costs, including reasonable attorneys’ fees, for having to compel arbitration or defend or enforce the award.
14.3. Governing Law; Judicial Resolution. Resolution of all disputes arising out of or related to this Agreement or the performance, enforcement, breach or termination of this Agreement and any remedies relating thereto, shall be governed by and construed under the
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
76
substantive laws of the United States of America and the State of New York, as applied to Agreements executed and performed entirely in the State of New York by residents of the State of New York, without regard to conflicts of law rules. Any dispute arising under this Agreement shall be submitted to a state or federal court of competent jurisdiction, except as otherwise expressly provided in this Agreement.
14.4. Equitable Remedies; Injunctive Relief. Nothing in this Article 14 shall prohibit either Party from seeking or obtaining any equitable remedy, including, but not limited to, injunctive relief, from a court or government agency of competent jurisdiction.
14.5. [*] If a dispute is submitted to arbitration in accordance with Section 14.2 in connection with any alleged breach of this Agreement by [*], provided however, [*] pursuant to this Agreement [*] that relates to such dispute. [*] after such dispute is submitted to arbitration [*] final determination of the arbitration or upon mutual agreement of Cubist and Chiron.
14.6. Interest. All amounts owed by either Party to the other Party in connection with any breach of this Agreement or the Supply Agreement shall bear interest at the rate stipulated in Section 7.10 hereof for late payments.
ARTICLE 15.
MISCELLANEOUS
15.1. Entire Agreement; Amendment. This Agreement, including the Exhibits hereto, sets forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto and supersedes and terminates all prior agreements and understandings between the Parties. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties other than as are set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party.
15.2. Force Majeure. Both Parties shall be excused from the performance of their obligations under this Agreement to the extent that such performance is prevented by force majeure and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party uses reasonable efforts to remove the condition. For purposes of this Agreement, force majeure shall include conditions beyond the control of the parties, such as an act of God, voluntary or involuntary compliance with any regulation, law or order of any government, war, an act of terrorism, civil commotion, labor strike or lock-out, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe; provided, however, the payment of invoices due and owing hereunder shall not be delayed by the payer because of a force majeure affecting the payer.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
77
15.3. Notices. Any notice required or permitted to be given under this Agreement shall be in writing, shall specifically refer to this Agreement and shall be deemed to have been sufficiently given for all purposes if mailed by first class certified or registered mail, postage prepaid, express delivery service or personally delivered, or if sent by facsimile, electronic transmission confirmed. Unless otherwise specified in writing, the mailing addresses of the Parties shall be as described below.
|
|
For Chiron: |
Chiron Healthcare Ireland Ltd. |
|
|
|
United Xxxx Xxxxx, |
|
|
|
Xxxxxxx Xxxx, |
|
|
|
Xxxxxxxx, Xxxxxx, Xxxxxxx |
|
|
|
Fax: x000 (0) 000-0000 |
|
|
|
|
|
|
With a Copy to: |
Chiron Corporation |
|
|
|
000 Xxxxxx Xxxxxx |
|
|
|
Xxxxxxxxxx, XX 00000 |
|
|
|
Fax: (000) 000-0000 |
|
|
|
Attention: General Counsel |
|
|
|
|
|
|
For Chiron Corporation: |
Chiron Corporation |
|
|
|
000 Xxxxxx Xxxxxx |
|
|
|
Xxxxxxxxxx, XX 00000 |
|
|
|
Fax: (000) 000-0000 |
|
|
|
Attention: President, BioPharmaceuticals |
|
|
|
|
|
|
With a Copy to: |
Chiron Corporation |
|
|
|
000 Xxxxxx Xxxxxx |
|
|
|
Xxxxxxxxxx, XX 00000 |
|
|
|
Fax: (000) 000-0000 |
|
|
|
Attention: General Counsel |
|
|
|
|
|
|
For Cubist: |
Cubist Pharmaceuticals, Inc. |
|
|
|
00 Xxxxxx Xxxxxx |
|
|
|
Xxxxxxxxx, Xxxxxxxxxxxxx 00000 |
|
|
|
Attention: General Counsel |
|
|
|
Fax: 000-000-0000 |
|
|
|
|
|
|
With a Copy to: |
Xxxxxxx XxXxxxxxx LLP |
|
|
|
000 Xxxxxxx Xxxxxx |
|
|
|
Xxxxxx, Xxxxxxxxxxxxx 00000 |
|
|
|
Attention: Xxxxx X. Xxxx, Esq. |
|
|
|
Fax: (000) 000-0000 |
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
78
15.4. Maintenance of Records. Each Party shall keep and maintain all records required by law or regulation with respect to Licensed Products and shall make copies of such records available to the other Party upon request.
15.5. No Strict Construction. This Agreement has been prepared jointly and shall not be strictly construed against either Party.
15.6. Assignment.
(a) Assignment by Cubist. Cubist may sell, assign, sublicense or otherwise transfer this Agreement, the Supply Agreement, all (or any portion) of its rights under this Agreement or the Supply Agreement and/or all (or any portion) of its obligations under this Agreement or the Supply Agreement to a Third Party, without any requirement or condition that Chiron consent to any such sale, assignment, sublicense or other transfer, provided that:
(i) (A) if and to the extent that Cubist sells, assigns, sublicenses or transfers any of its obligations under this Agreement to such Third Party, then Cubist also sells, assigns, sublicenses or transfers to such Third Party any obligations of Cubist under the Supply Agreement that correspond, without limitation, on a Licensed Product by Licensed Product basis, or on a country by country basis, to the obligations of Cubist under this Agreement that are being sold, assigned, sublicensed or transferred to such Third Party by Cubist, and (B) if and to the extent that Cubist sells, assigns, sublicenses or transfers any of its obligations under the Supply Agreement to such Third Party, then Cubist also sells, assigns, sublicenses or transfers to such Third Party any obligations of Cubist under this Agreement that correspond, without limitation, on a Licensed Product by Licensed Product basis, or on a country by country basis, to the obligations of Cubist under the Supply Agreement that are being sold, assigned, sublicensed or transferred to such Third Party by Cubist; and
(ii) such Third Party has expressly agreed in writing to assume the performance of any and all obligations under this Agreement and the Supply Agreement that Cubist sells, assigns, sublicenses or transfers to such Third Party or is required to sell, assign, sublicense or transfer to such Third Party pursuant to the provisions of this Section 15.6(a).
(b) Assignment by Chiron. Notwithstanding any other provision in this Agreement to the contrary, Chiron may not sell, assign, sublicense or otherwise transfer this Agreement, the Supply Agreement or any of its rights or obligations under this Agreement or the Supply Agreement without the prior written consent of Cubist (which consent may be withheld or delayed by Cubist in its absolute discretion), except for any sale, assignment, sublicense or other transfer that is effected in strict compliance with the provisions set forth below in this Section 15.6(b) and Section 15.6(c) which shall not require or be conditioned upon any consent by Cubist to any such sale, assignment, sublicense or other transfer. At any time from and after [*], Chiron may sell, assign, sublicense or otherwise transfer this Agreement and the Supply Agreement and all (but not less than all) of its rights and obligations under this Agreement and the Supply Agreement (collectively, the “Chiron Interest”) to any Reasonable Buyer without having to obtain the consent of Cubist to any such sale, assignment, sublicense or other transfer; provided, however, that (i) prior to any such sale, assignment, sublicense or other transfer of the Chiron Interest, such Reasonable Buyer has expressly agreed in writing to assume the
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
79
performance of any and all obligations of Chiron under this Agreement and the Supply Agreement, and (ii) Chiron shall have given Cubist at least [*] advance notice of any such sale, assignment, sublicense or other transfer; and, provided, further, that, if and to the extent applicable, all of the conditions set forth in Section 15.6(c) below are satisfied. Notwithstanding the foregoing provisions of this Section 15.6(b), in the event that Cubist enters into a definitive agreement with any Third Party granting to such Third Party any Commercialization rights with respect to any Licensed Product in the United States, then at any time on or prior to [*] any such definitive agreement between Cubist and such Third Party, Chiron may sell, assign, sublicense or otherwise transfer the Chiron Interest to any Third Party without having to obtain the consent of Cubist; provided, however, that as a part of the consummation of such sale, assignment, sublicense or other transfer of the Chiron Interest, such Third Party has expressly agreed in writing to assume the performance of any and all obligations of Chiron under this Agreement and the Supply Agreement; and, provided, further, that, if and to the extent applicable, all of the conditions set forth in Section 15.6(c) below are satisfied.
(c) Restrictions on Payments. Notwithstanding any other provision in this Section 15.6 to the contrary, Chiron’s right to sell, assign, sublicense or otherwise transfer its rights to the Chiron Interest to a Reasonable Buyer or any other Third Party subject to, and in accordance with, Section 15.6(b) without obtaining the consent of Cubist shall also be subject to the requirements and conditions that (i) such sale, assignment, sublicense or other transfer results in the transfer by Chiron of all of its then prospective economic rights in and to the Chiron Interest to such Reasonable Buyer or other Third Party, and (ii) in the event that any such sale, assignment, sublicense or other transfer calls for royalty payments, earn-out payments, milestone-based or performance-based payments or other payments of any kind to be made over time by such Reasonable Buyer or other Third Party to Chiron, the agreement between Chiron and such Reasonable Buyer or other Third Party shall stipulate a maximum aggregate dollar amount of royalty payments, earn-out payments, milestone-based or performance-based payments and/or other payments that such Reasonable Buyer or other Third Party shall pay to Chiron in connection with such sale, assignment, sublicense or other transfer of the Chiron Interest and shall also stipulate that from and after the sixth anniversary of the closing of any such sale, assignment, sublicense or other transfer such Reasonable Buyer or other Third Party shall have no further obligation to make any payments of any kind or pay any kind of consideration to Chiron in connection with such sale, assignment, sublicense or other transfer of the Chiron Interest.
(d) Injunctive Relief. The Parties hereby acknowledge that Cubist would suffer irreparable damage and injury in the event of any sale, assignment, sublicense or other transfer by Chiron of the Chiron Interest to any Reasonable Buyer or other Third Party if any such sale, assignment, sublicense or other transfer would not be effected in compliance with the provisions of Section 15.6(b) and Section 15.6(c). Accordingly, it is the intent of the Parties that in the event that Chiron attempts or is attempting to sell, assign, sublicense or otherwise transfer the Chiron Interest without complying with all of the provisions of Section 15.6(b) and Section 15.6(c), Cubist may, in addition to any other remedies that Cubist may have at law, seek injunctive relief against Chiron (and/or any proposed transferee to the extent Cubist may be so entitled under applicable law) for the sole purpose of enforcing all of the provisions of Section 15.6(b) and Section 15.6(c). In the event that Cubist brings any such action for injunctive relief
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
80
to enforce the provisions of Section 15.6(b) and Section 15.6(c), Chiron hereby agrees to waive any defenses that Cubist has an adequate remedy at law and that Cubist will not suffer irreparable harm, and also any requirement that Cubist post any bond in connection with any such action for injunctive relief. For the avoidance of doubt, other than the defenses and the requirement specifically waived in the foregoing two sentences, nothing in this Section 15.6(d) shall be construed to be a waiver by Chiron of any of its remaining defenses at law or in equity.
15.7. Performance by Affiliates. Each of Cubist and Chiron acknowledge that obligations under this Agreement may be performed by Affiliates of Cubist and Chiron. Each of Cubist and Chiron guarantee performance of this Agreement by its Affiliates. Wherever in this Agreement the Parties delegate responsibility to Affiliates or local operating entities, the Parties agree that such entities may not make decisions inconsistent with this Agreement, amend the terms of this Agreement or act contrary to its terms in any way.
15.8. Guaranty. Chiron Parent Company hereby unconditionally and irrevocably guarantees, as primary obligor, to Cubist, the due and punctual payment and performance as and when due of the obligations, responsibilities, undertakings, representations, warranties, payment covenants, obligations and agreements of Chiron and its Affiliates under this Agreement, the Supply Agreement and any other agreement regarding Licensed Products.
15.9. Counterparts. This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
15.10. Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
15.11. Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable by any court of competent jurisdiction from which no appeal can be or is taken, the provision shall be considered severed from this Agreement and shall not serve to invalidate any remaining provisions hereof. The Parties shall make a good faith effort to replace any invalid or unenforceable provision with a valid and enforceable one such that the objectives contemplated by the Parties when entering this Agreement may be realized.
15.12. Headings. The headings for each article and section in this Agreement have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular article or section.
15.13. No Waiver. Any delay in enforcing a Party’s rights under this Agreement or any waiver as to a particular default or other matter shall not constitute a waiver of such Party’s rights to the future enforcement of its rights under this Agreement, except with respect to an express written and signed waiver relating to a particular matter for a particular period of time.
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN OMITTED AND FILED
WITH THE COMMISSION
81
In Witness Whereof, the Parties have executed this License Agreement in duplicate originals by their proper officers as of the Effective Date.
|
Cubist Pharmaceuticals, Inc. |
Chiron Healthcare Ireland Ltd. |
||||||
|
|
|
||||||
|
|
|
||||||
|
By: |
/s/ Xxxxxx X. Xxxxxx |
|
By: |
/s/ Xxxxx X. Xxxxxxx |
|
||
|
Title: |
Senior Vice President and Chief Business Officer |
|
Title: |
President, Chiron Pharmaceuticals |
|
||
|
Date: |
October 2, 2003 |
|
Date: |
October 2, 2003 |
|
||
|
Agreed only as to Section 7.1(b) and (c) |
|||
|
|
and Section 15.8 by: |
||
|
|
|
||
|
|
Chiron Corporation |
||
|
|
|
||
|
|
|
||
|
|
By: |
/s/ Xxxxx X. Xxxxxxx |
|
|
|
Title: |
President, Chiron Biopharmaceuticals |
|
|
|
Date: |
October 2, 2003 |
|
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT A
CUBIST XXXXX
|
Xxxx |
|
Country |
|
Filing Date |
|
Application |
|
|
|
|
|
|
|
|
|
[*] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT B
CUBIST PATENTS
|
Country |
|
Docket |
|
App Date |
|
App No |
|
Pub Date |
|
Pub No |
|
Sub Stat. |
|
Xxx No. |
|
Xxxxx Xx |
|
Exp Dt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[*] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT C
PRIMARY DAPTOMYCIN MOLECULE
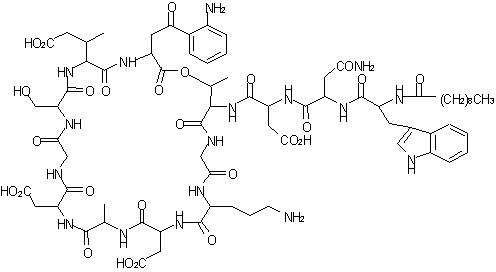
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT D
REGIONS AND COUNTRIES OF THE TERRITORY
|
[*] |
r
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT E
CUBIST TECHNOLOGY LICENSED FROM THIRD PARTIES
[*].
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT F
LILLY PATENTS
|
Country |
|
Docket |
|
App Date |
|
App No |
|
Pub Date |
|
Pub No |
|
Sub Stat. |
|
Xxx No. |
|
Xxxxx Xx |
|
Exp Dt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[*] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
EXHIBIT G
INDS, NDAS AND DRUG APPROVAL APPLICATIONS
IND # 57,693
CTX # 20364/0001/A
CTX # 17658/0001/A (Expired)
NDA # 21-572
* CONFIDENTIAL TREATMENT REQUESTED: MATERIAL HAS BEEN
OMITTED AND FILED WITH THE COMMISSION
