COLLABORATION AND LICENSE AGREEMENT
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.29
CONFIDENTIAL
EXECUTION COPY
COLLABORATION AND LICENSE AGREEMENT
This COLLABORATION AND LICENSE AGREEMENT (“Agreement”) is entered into as of 16 December 2013 (the “Effective Date”) between ACELRX PHARMACEUTICALS, INC., a company organized under the laws of the State of Delaware, United States (“AcelRx”), and having a principal place of business at 000 Xxxxxxxxxx Xxxxx, Xxxxxxx Xxxx, XX 00000, Xxxxxx Xxxxxx, and GRÜNENTHAL GMBH, a company organized under the laws of Germany (“Grünenthal”), having its registered office at Xxxxxxxxxxxxxx 0, 00000 Xxxxxx, Xxxxxxx.
A. WHEREAS, AcelRx is a specialty pharmaceutical company focused on the development and commercialization of innovative therapies for the treatment of acute and breakthrough pain, and is developing ZalvisoTM (formerly known as ARX-01), the Sufentanil NanoTab PCA System, AcelRx’s novel sublingual patient-controlled analgesia (PCA) system. AcelRx owns or controls certain patents, know-how and other intellectual property relating to the ZalvisoTM product; and
B. WHEREAS, Grünenthal desires to obtain from AcelRx certain exclusive rights and licenses to commercialize, use, sell, offer for sale and import the Licensed Product (as defined hereinafter) in the Field (as defined hereinafter) in the Territory (as defined hereinafter), and AcelRx is willing to grant to Grünenthal such rights and licenses and to exclusively supply Grünenthal with the Licensed Product for the Territory, all on the terms and conditions set forth in this Agreement and the Supply Agreement (as defined hereinafter).
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, AcelRx and Grünenthal hereby agree as follows:
ARTICLE 1
As used in this Agreement, the following terms shall have the meanings set out in this Article 1 unless the context clearly and unambiguously dictates otherwise.
1.1 “Accessories” shall mean additional hardware accessories or components for use with the Licensed Product set forth on Exhibit 1.1 which are not included in the Reusables Kit or Dispenser Kit (for example, and not by way of limitation, an RFID reader).
1.2 “Accounting Standards” shall mean, with respect to AcelRx, US GAAP (United States generally accepted accounting principles as in effect from time to time), and with respect
CONFIDENTIAL
to Grünenthal, the IFRS (International Financial Reporting Standards as in effect from time to time), in each case, as consistently applied throughout the period involved. Each Party shall promptly notify the other in the event that it changes the Accounting Standards pursuant to which its records are maintained, it being understood that each Party may only use internationally recognized accounting principles (e.g. IFRS, US GAAP, etc.).
1.3 “Affiliate” of a Party shall mean any Person that, directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with such Party, as the case may be, but for only so long as such control exists. As used in this Section 1.1, “control” shall mean (i) direct or indirect beneficial ownership of at least 50% (or such lesser percentage which is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) of the voting share capital or other equity interest in such Person or (ii) the power to direct the management of such Person by contract or otherwise.
1.4 “AcelRx Copyrights” shall mean all copyrights (including registrations and applications therefor), copyrightable works which are necessary or reasonably useful for the commercialization, use, sale, offering for sale and import of the Licensed Product in the Field in the Territory, respectively.
1.5 “AcelRx Indemnitees” shall have the meaning set forth in Section 12.1.
1.6 “AcelRx Know-How” shall mean all Know-How that is necessary or reasonably useful for the research, development, registration, Manufacture, commercialization, use, sale, offering for sale and import of the Licensed Product in the Field in the Territory, which Know-How is Controlled by AcelRx or any of its Affiliates as of the Effective Date or during the Term. For the avoidance of doubt, AcelRx Know-How shall not include any Joint Know-How.
1.7 “AcelRx Patents” shall mean all Patents that are necessary or reasonably useful for the research, development, registration, Manufacture, commercialization, use, sale, offering for sale and import of the Licensed Product in the Field in the Territory, which Patents are Controlled by AcelRx or any of its Affiliates as of the Effective Date or during the Term. For the avoidance of doubt, AcelRx Patents shall not include any Joint Patents or Assigned Patents. A list of AcelRx Patents as of the Effective Date, which are owned by AcelRx is set forth on Exhibit 1.7, which list shall be updated from time to time upon written agreement between the Parties.
1.8 “AcelRx Technology” shall mean all AcelRx Know-How, AcelRx Patents and AcelRx’s interest in Joint Patents and Joint Know-How.
1.9 “AcelRx Trademarks” shall mean Trademarks of AcelRx related to the Licensed Product in the Territory as set forth on Exhibit 1.9 and any Alternative AcelRx Trademark.
1.10 “Alternative AcelRx Trademark” shall have the meaning set forth in Section 10.7.
2
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.11 “Anti-Corruption Laws” shall mean the U.S. Foreign Corrupt Practices Act, as amended, the UK Xxxxxxx Xxx 0000, as amended, and any other applicable anti-corruption laws and laws for the prevention of fraud, racketeering, money laundering or terrorism in the Territory.
1.12 “API” shall mean an active pharmaceutical ingredient.
1.13 “Applicable Laws” shall mean the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidance, ordinances, judgments, decrees, directives, injunctions, orders, permits (including Marketing Approvals) of or from any court, arbitrator, Regulatory Authority or governmental agency or authority having jurisdiction over or related to the subject item.
1.14 “Assigned Patents” shall mean [ * ] as set forth in Exhibit 1.14, if and when assigned to Grünenthal pursuant to the terms of this Agreement.
1.15 “Assigned Trademarks” shall mean the AcelRx Trademarks that are approved by the EMA and by any other Regulatory Authority in the Territory for use with the Licensed Product upon the grant of the respective Marketing Approval in the Territory, if and when assigned to Grünenthal pursuant to the terms of this Agreement.
1.16 “Auditor” shall have the meaning set forth in Section 8.5.
1.17 “Authorized Representative” shall mean Grünenthal who shall be designated by AcelRx for the Licensed Product to act towards and may be addressed by authorities and bodies in the Territory instead of AcelRx according to the applicable EU directives and guidelines and based upon a written agreement between AcelRx and Grünenthal in accordance with Section 3.3 of the Supply Agreement.
1.18 “Bankruptcy Laws” shall have the meaning set forth in Section 14.6.
1.19 “Budget” shall mean the budget included within the applicable Development Plan for conducting the clinical or non-clinical studies, regulatory activities (including making regulatory filings) and other activities under such Development Plan.
1.20 “Business Day” shall mean a day other than a Saturday or Sunday or any public holiday in San Francisco, California or Aachen, Germany, but excluding the nine (9) consecutive calendar days beginning on December 24th and continuing through January 1st of each calendar year during the Term. For the avoidance of doubt, references in this Agreement to “days” shall mean calendar days.
1.21 “Calendar Quarter” shall mean a period of three consecutive months during a Calendar Year beginning on and including January 1st, April 1st, July 1st or October 1st.
1.22 “Calendar Year” shall mean a period of twelve consecutive months beginning on and including January 1st.
3
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.23 “Candidate EU Member” shall have the meaning set forth in Section 2.5.
1.24 “CE Xxxx” shall mean a marking obtained and maintained by AcelRx for the Licensed Product that identifies conformity with medical device conformity requirements for use, sale and importation in the EU.
1.25 “Centralized Procedure” shall mean the procedures of the EU for obtaining marketing authorisation for a medicinal product as set forth in Regulation (EC) No 726/2004 of 31 March 2004, as amended from time to time during the Term.
1.26 “Certificate of Analysis” or “COA” shall mean a document identified as such and provided by AcelRx to Grünenthal that states: (a) the results of analytical tests required by the specifications to be performed with respect to the Licensed Product, (b) the quantity of the Licensed Product, and (c) the batch from which such the Licensed Product was produced.
1.27 “Change of Control” shall mean, with respect to a party (a) the acquisition of beneficial ownership, directly or indirectly, by any Person of securities or other voting interest of such party representing 50% or more of the combined voting power of such party’s then outstanding securities or other voting interests, (b) any merger, reorganization, consolidation or business combination involving such party that results in the holders of beneficial ownership of the voting securities or other voting interests of such party (or, if applicable, the ultimate parent of such party) immediately prior to such merger, reorganization, consolidation or business combination ceasing to hold beneficial ownership of 50% or more of the combined voting power of the surviving entity immediately after such merger, reorganization, consolidation or business combination, or (c) any sale, lease, exchange, contribution or other transfer (in one transaction or a series of related transactions) of all or substantially all of the assets of such party to which this Agreement relates. For clarity, Change of Control shall not include financing transactions, through public offering, private equity financing, debt financing or otherwise.
1.28 “Clinical Price” shall have the meaning set forth in Section 6.3.
1.29 “CMC” shall mean chemistry, manufacturing and controls.
1.30 “cGMP” shall mean the then-current good manufacturing practices required by the FDA, as set forth in the United States Federal Food, Drug and Cosmetic Act, as amended, and the regulations promulgated thereunder, for the Manufacture of APIs, intermediates, medical devices and combination products, and the then current good manufacturing practices required by the Regulatory Authorities in the EU, as may be updated from time to time and other Applicable Laws of the EU relating to the Manufacture of APIs, intermediates, medical devices and combination products.
1.31 “Commercial Strategy” shall have the meaning set forth in Section 5.1(a).
1.32 “Commercially Reasonable Efforts” shall mean that level of efforts and resources, with respect to a particular Party, at the relevant point in time, that is consistent with the usual practice followed by that Party, in the exercise of its reasonable scientific and business
4
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
judgment relating to other prescription pharmaceutical products owned or licensed by it or to which it has exclusive rights, which have market potential and are at a stage of development or product life similar to the applicable Licensed Product, taking into account: [ * ].
1.33 “Commercial Milestone” shall mean the applicable milestone set forth in Section 7.2(b).
1.34 “Commercialization Plan” shall have the meaning set forth in Section 5.1.
1.35 “Contractors” shall have the meaning set forth in Section 11.2(h).
1.36 “Confidential Information” shall have the meaning set forth in Section 9.1. “Confidentiality Agreement” shall mean that certain Bilateral Secrecy Agreement between AcelRx and Grünenthal dated 18 January 2013, as amended by the 1st Amendment dated July 23, 2013.
1.37 “Control” (including any variations such as “Controlled” and “Controlling”), in the context of intellectual property rights, Know-How and Confidential Information, shall mean possession (whether by ownership or license, other than pursuant to this Agreement) by a Party of the ability to grant access to, or a license or sublicense of, such rights, Know-How and Confidential Information, without violating the terms of an agreement with a Third Party.
1.38 “Development Plan” shall mean the initial Development Plan as attached hereto as Exhibit 1.38 and any subsequent amendments or updates to such Development Plan during the Term pursuant to Section 4.1.
1.39 “Device” shall mean any current or future device portion of the Licensed Product, or any part thereof.
1.40 “Device Failure” means a Licensed Product that is [ * ].
1.41 “Disclosing Party” shall have the meaning set forth in Section 9.1.
1.42 “Dispenser Kit” shall mean a complete kit consisting of a dispenser tip, fastening cap and thumbtag for use with or as part of the Device.
1.43 “Distributor” shall mean a Third Party or an Affiliate of Grünenthal to whom Grünenthal or an Affiliate of Grünenthal has granted the right to market, promote, co-promote, advertise, detail, sell and/or distribute the Licensed Product in the Field in the Territory without the control of MAA Approval for the Licensed Product in the Field in the Territory.
1.44 “Drug” shall mean the sufentanil drug cartridge for use with the Device.
1.45 “Effective Date” shall have the meaning set forth in the opening paragraph of this Agreement.
1.46 “EMA” shall mean the European Medicines Agency.
5
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.47 “EU” or “European Union” shall mean the supra national community consisting of as of the Effective Date, Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom.
1.48 “Expenses” shall mean the costs and expenses paid to Third Parties (or payable to Third Parties and accrued in accordance with Accounting Standards) incurred by a Party or any of its Affiliates in conducting the clinical or non-clinical studies, regulatory activities (including making regulatory filings) and other activities in accordance with the applicable Development Plan.
1.49 “FDA” shall mean the U.S. Food and Drug Administration or similar federal, state or local Regulatory Authorities.
1.50 “Field” shall mean human use in treatment of pain for (a) use within or dispensed by a hospital; or (b) use within a hospice, nursing home or other medically supervised setting, [ * ].
1.51 “First Commercial Sale” shall mean, on a country-by-country basis, the first bona fide, arm’s length sale of the Licensed Product in a country following receipt of Marketing Approval of such the Licensed Product in such country for use of such the Licensed Product in such country. Sales of the Licensed Product for registration samples, compassionate use sales, named patient use, transfers to, by or among Grünenthal, its Affiliates and/or Sublicensees shall not constitute a First Commercial Sale.
1.52 “Fully Burdened Manufacturing Cost” shall mean the fully burdened Manufacturing cost of the Licensed Product (including packaging for shipment) calculated in conformity with Accounting Standards and expressed on a per unit Manufactured basis, including the cost of: [ * ]
For clarity, the calculation of the cost of Manufacturing set forth above shall be based upon all Licensed Product manufactured by AcelRx over a specified period of time and shall in any event not be based on a disproportionate allocation of those costs incurred in the manufacture of the Licensed Product to Grünenthal’s units of Licensed Product relative to the costs allocated to units of Licensed Product for AcelRx and its other licensees. For further clarity, costs that are specific to the units of Licensed Product supplied to Grünenthal (including subsection (d) costs) shall be limited to Licensed Product supplied to Grünenthal unless those costs apply to the other units of Licensed Product manufactured in any particular runs or campaigns and allocated accordingly. For further clarity, costs that are specific to the units of Licensed Product supplied to Grünenthal (e.g., certain elements of subsection (a) and subsection (d) of Fully Burdened Manufacturing costs) shall be limited to Licensed Product supplied to Grünenthal unless those costs apply to the other units of Licensed Product manufactured in any particular runs or campaigns and allocated accordingly
6
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.53 “Further Development Studies” shall mean clinical trials that are not Post Approval Commitments or Post Marketing Studies, including clinical trials for Label Expansion.
1.54 “Generic Entry” shall mean [ * ].
1.55 “Generic Product” shall mean any pharmaceutical product [ * ].
1.56 “Good Clinical Practices” or “GCP” shall mean the then-current standards, practices and procedures promulgated or endorsed by the EU as set forth in the guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” including related regulatory requirements imposed by the FDA and comparable regulatory standards, practices and procedures in jurisdictions outside the United States, as they may be updated from time to time.
1.57 “Good Laboratory Practices” or “GLP” shall mean the then-current good laboratory practice standards promulgated or endorsed by the EU, and comparable regulatory standards in jurisdictions outside the EU, as they may be updated from time to time.
1.58 “Good Manufacturing Practices” or “GMP” shall mean the then-current good manufacturing practices required by the EU for the manufacture and testing of pharmaceutical materials, and comparable laws or regulations applicable to the manufacture and testing of pharmaceutical materials in jurisdictions outside the EU, as they may be updated from time to time. Good Manufacturing Practices shall include applicable quality guidelines promulgated under the ICH.
1.59 “Governmental Authority” shall mean any court, agency, department, authority or other instrumentality of any national, supranational, state, county, city or other political subdivision.
1.60 “Grünenthal Background IP” shall mean all Patents and Know-How Controlled by Grünenthal or its Affiliates prior to the Effective Date of this Agreement.
1.61 “Grünenthal Indemnitees” shall have the meaning set forth in Section 12.2.
1.62 “Grünenthal Know-How” shall mean all Know-How with respect to the Device or the Licensed Product that is generated by or on behalf of Grünenthal or any of its Affiliates during the Term pursuant to this Agreement in connection with the research, development, importation, use, manufacture, sale, having sold and offering for sale of the Licensed Product.
1.63 “Grünenthal Patents” shall mean all Patents that claim Inventions with respect to the Device or the Licensed Product generated by or on behalf of Grünenthal or any of its Affiliates during the Term pursuant to this Agreement in connection with the research, development, importation, use, manufacture, sale, having sold and offering for sale of the Licensed Product.
7
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.64 “Grünenthal Technology” shall mean all Grünenthal Know-How and Grünenthal Patents, including Grünenthal’s interest in Joint Patents and Joint Know-How. For clarity, Grünenthal Technology does not include Grünenthal Background IP.
1.65 “Grünenthal Trademark” shall have the meaning set forth in Section 10.7.
1.66 “Harmonized Standards” shall mean technical specifications meeting the essential requirements of the EU directives, compliance with which will provide a presumption of conformity with the essential requirements for the Licensed Product.
1.67 “ICH” shall mean the International Conference on Harmonization (of Technical Requirements for Registration of Pharmaceuticals for Human Use).
1.68 “IND” shall mean an Investigational New Drug Application (including any amendments thereto) filed with the FDA pursuant to 21 C.F.R. §312 before commencement of clinical trials of a pharmaceutical product, or any comparable filings with Health Canada in Canada, including clinical trial applications.
1.69 “Initial Label” shall mean human use in moderate to severe post operative pain in adults, for use within a hospital.
1.70 “Intervening Event” shall have the meaning set forth in Section 16.1.
1.71 “Inventions” shall mean any and all inventions, discoveries, improvements, processes and techniques discovered, conceived or reduced to practice in the course of or as a result of activities under this Agreement, whether or not patentable or included in any claim of patents and patent applications, together with all intellectual property rights therein.
1.72 “Joint Inventions” shall mean any and all Inventions discovered, conceived or reduced to practice jointly by or on behalf Grünenthal or its Affiliates, on the one hand, and by or on behalf of AcelRx or its Affiliates, on the other hand during the Term pursuant to this Agreement.
1.73 “Joint Know-How” shall mean all Know-How included in Joint Inventions, other than any Joint Patent.
1.74 “Joint Patents” shall mean all Patents claiming any Joint Invention.
1.75 “Joint Steering Committee” or “JSC” shall have the meaning set forth in Section 3.1(a).
1.76 “Know-How” shall mean all tangible and intangible scientific, technical, clinical, regulatory, trade, marketing, commercial, financial or business information and materials, including compounds, solid state forms, compositions of matter, formulations, devices, techniques, processes, methods, trade secrets, formulae, procedures, tests, data, results, analyses, documentation, reports, information (including pharmacological, toxicological, non-clinical
8
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(including chemistry, manufacturing and control)), and clinical test design, methods, protocols, data, results, analyses, and conclusions, quality assurance and quality control information, regulatory documentation, information and submissions pertaining to, or made in association with, filings with any Regulatory Authority, knowledge, know-how, skill, and experience.
1.77 “Label Expansion” shall mean any expansion of the label beyond the Initial Label.
1.78 “Licensed Product” shall mean (a) AcelRx’s ARX-01 (any and all components thereof, and the system, which as existing as of the Effective Date is described in Exhibit 1.78), and (b) any and all improvements and/or modifications thereof.
1.79 “Losses” shall have the meaning set forth in Section 12.1.
1.80 “MAA” shall mean an application with the EMA or other competent Regulatory Authority in the Territory seeking Marketing Approval of the Licensed Product.
1.81 “MAA Approval” shall mean the approval of a MAA to sell the Licensed Product in a country or region in the Territory. “Manufacture” shall mean to manufacture, generate, process, prepare, make, assemble, test, label, package, store, hold, handle, receive, release, transport, and deliver the Licensed Product (or any component thereof).
1.82 “Marketing Approval” of the Licensed Product shall mean all technical, medical and scientific approvals, licenses, registrations or authorizations from Regulatory Authorities in a country of the Territory necessary for the manufacture, commercialization, use, storage, promotion, marketing, sale, offering for sale and import of the Licensed Product in the Field in such country.
1.83 “Material Agreements” shall have the meaning set forth in Section 11.2(j). The Material Agreements existent on the Effective Date are listed in Exhibit 1.83
1.84 “MEDDEV Guidelines” shall mean those guidelines published by the European Commission promoting a common approach by manufacturers and notified bodies involved in the conformity assessment procedures according to the relevant annexes of the directives, and by the competent authorities charged with safeguarding public health.
1.85 “Medical Device Directive” shall mean the directive 93/42/EEC published by the European Commission and any successors thereof.
1.86 “Multi-Site Trials” means Post Marketing Studies that are conducted at multiple sites.
1.87 “Non-Interventional Studies” means studies in which results from the treatment of patients with pharmaceutical products are analysed with epidemiological methods.
9
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.88 “NDA” of the Licensed Product shall mean a New Drug Application as defined in Title 21 of the U.S. Code of Federal Regulations, §314.80 et seq., and all amendments and supplements thereto, which is filed with the FDA including all documents, data, and other information concerning the Licensed Product thus filed that are necessary for gaining Marketing Approval for the Licensed Product.
1.89 “Net Sales” shall mean the gross amounts invoiced by or on behalf of Grünenthal, its Affiliates and/or Sublicensees (the “Selling Party”) for sales of the Licensed Product to Third Parties (other than Sublicensees), less deductions actually incurred, allowed, paid, accrued or otherwise specifically identified as related to, and specifically allocated to, the Licensed Product by Grünenthal, its Affiliates and/or Sublicensees using Accounting Standards applied on a consistent basis for:
(a) sales allowances actually paid, granted, allowed, accrued or taken, including trade, cash, quantity discounts, chargeback rebates, reimbursements, buying groups, health care insurance carriers or other institutions, adjustments arising from consumer discount programs or other similar programs;
(b) credits or allowances given or made for rejection of or return of previously sold the Licensed Product (whether as a result of recalls, market withdrawals, other corrective actions, damaged, defective goods or otherwise), for retroactive price reductions and billing errors, or other allowances specifically identifiable as relating to the Licensed Product, including allowances and credits related to inventory management;
(c) governmental and other rebates (or equivalents thereof) granted to managed health care organizations, pharmacy benefit managers (or equivalents thereof), national, state/provincial, local, and other governments, their agencies and purchasers, and reimbursers, or to trade customers;
(d) costs of freight, insurance, and other transportation charges directly related to the distribution of such the Licensed Product;
(e) customs or excise duties, sales tax, consumption tax, value added tax, and other taxes (except income taxes) or duties relating to sales, any payment in respect of sales to any Governmental Authority, or with respect to any government-subsidized program or managed care organization; and
(f) amounts previously included in Net Sales that are written-off by the Selling Party as uncollectible in accordance with the standard practices of such Selling Party for writing of uncollectible amounts, consistent applied; provided that if any such written-off amounts are subsequently collected, such collected amounts shall be included in Net Sales in the period in which they are subsequently collected.
In no event shall any particular amount identified above be deducted more than once in calculating Net Sales (i.e., no “double counting” of reductions). Sales of the Licensed Product between Grünenthal and its Affiliates or Sublicensees for resale shall be
10
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
excluded from the computation of Net Sales, but the subsequent resale of such the Licensed Product by an Affiliate or Sublicensee, as applicable, to a Third Party shall be included within the computation of Net Sales. For clarity, for purposes of this Section 1.80, “the Licensed Product” shall include the Drug, Device, Reusables Kit, Dispenser Kit and Accessories, whether sold together or separately.
If the Licensed Product is sold as a Bundled Product (as hereafter defined) in a country in the Territory, then the Net Sales for the Licensed Product attributable to such Bundled Product shall be the average price of Licensed Product sold in such country in the Territory during the applicable period, provided that in any event any discount applied to such Bundled Product shall in any event be applied at the same discount across all products sold with the Bundled Product (i.e., no disproportionate discount shall apply to the Licensed Product). “Bundled Product” means products in which either (a) a non-Licensed Product is sold or discounted together with a Licensed Product for purchase by or for resale to a customer, or (b) a non-Licensed Product is sold together with Licensed Product in a kit at a single price. In any event, Grünenthal, Sublicensees and their respective Affiliates shall conduct pricing and discounting activities in a good faith, consistent manner without disadvantaging the Licensed Product relative to the other products priced or sold as Bundled Product.
Notwithstanding anything to the contrary herein, sale, disposal or use of the Licensed Product for marketing, regulatory, development or charitable purposes, such as clinical trials, preclinical trials, compassionate use, named patient use, or indigent patient programs, without consideration, shall not be deemed a sale hereunder.
1.90 “Party” shall mean AcelRx or Grünenthal individually, and “Parties” shall mean AcelRx and Grünenthal collectively.
1.91 “Patent(s)” shall mean (a) all patents, certificates of invention, applications for certificates of invention, priority patent filings and patent applications, and (b) any renewal, division, continuation (in whole or in part), or request for continued examination of any of such patents, certificates of invention and patent applications, and any and all patents or certificates of invention issuing thereon, and any and all reissues, reexaminations, extensions, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions, and additions of or to any of the foregoing.
1.92 “Patent Term Extension” shall mean any term extensions, supplementary protection certificates and equivalents thereof offering patent protection beyond the initial term with respect to any issued Patents.
1.93 “Person” shall mean any individual, corporation, partnership, limited liability company, trust, governmental entity, or other legal entity of any nature whatsoever.
1.94 “Pharmacovigilance Agreement” shall having the meaning set forth in Section 4.5(c).
11
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.95 “Post Approval Commitments” shall mean all clinical studies (including pediatric studies) conducted after receipt of a Marketing Approval for the Licensed Product that are requested by Regulatory Authorities or that are necessary to fulfill commitments made to the Regulatory Authority as a condition for the receipt and/or maintenance of such Marketing Approval in any country.
1.96 “Post Marketing Studies” shall mean all interventional studies of Licensed Product with the main objective to collect data to increase product knowledge or for marketing and market access purposes (e.g. post-marketing surveillance studies, patient outcome studies, patient preference studies) other than Non-Interventional Studies and Post Approval Commitments.
1.97 “Price Approval” shall mean, in any country where a Regulatory Authority authorizes reimbursement for, or approves or determines pricing for, pharmaceutical or medical device products, receipt (or, if required to make such authorization, approval or determination effective, publication) of such reimbursement authorization or pricing approval or determination (as the case may be).
1.98 “R&D Milestone” shall mean the applicable milestone set forth in Section 7.2(a).
1.99 “Receiving Party” shall have the meaning set forth in Section 9.1.
1.100 “Regulatory Authority” shall mean any national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity (a) whose review and/or approval is necessary (i) for the Manufacture, packaging, use, storage, import, export, distribution, promotion, marketing, offer for sale and sale of the Licensed Product, and/or (ii) for reviewing Regulatory Filings for the Licensed Product (or a component thereof); and/or (b) having authority to review and enforce cGMP and/or other Applicable Laws relating to the Licensed Product or the Manufacture, development, commercialization, use or sale thereof. For clarity, Regulatory Authority shall, as applicable, include any notified body with respect to the Device.
1.101 “Regulatory Filings” shall mean all applications, approvals, licenses, registrations, notifications, registrations, submissions and authorizations made to or received from a Regulatory Authority in a country necessary for the Manufacture, development, commercialization, use, storage, promotion, marketing, sale, offering for sale and import of the Licensed Product in such country, including any INDs, NDAs, the MAA Approval, any other Marketing Approvals, Price Approvals as well as the CE Xxxx.
1.102 “Reusables Kit” shall mean a complete kit consisting of [ * ].
1.103 “Royalty Term” shall have the meaning set forth in Section 7.3.
1.104 “SEC” shall have the meaning set forth in Section 8.5(a).
1.105 “Senior Executives” shall have the meaning set forth in Section 15.1.
12
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
1.106 “sNDA” shall mean a supplemental NDA.
1.107 “Specifications” shall mean the specifications for the Licensed Product, as established by inclusion in the Marketing Approval application filed for the Licensed Product and as required by a Regulatory Authority in the Territory for approval and such other specifications, such as specifications for packaging, storage conditions and labeling of the Licensed Product, as agreed by the Parties pursuant to the Supply Agreement.
1.108 “Sublicensee” shall mean a Third Party or an Affiliate of Grünenthal, other than a Distributor, to whom Grünenthal or an Affiliate of Grünenthal has granted a sublicense under the AcelRx Technology as permitted under Section 2.2(a) of this Agreement. For clarity, the term “Sublicensee” shall not include (i) any Distributors, wholesalers or importers that are not granted any sublicense under the AcelRx Technology under Section 2.2(a) or (ii) any contract manufacturers that are granted only the right to manufacture the Licensed Product in accordance with the terms and conditions of the Supply Agreement and herein for Grünenthal or its Affiliates or Sublicensees for commercialization in the Field in the Territory.
1.109 “Supply Agreement” shall have the meaning set forth in Section 6.1.
1.110 “Term” shall have the meaning set forth in Section 13.1.
1.111 “Territory” shall mean the European Union, Switzerland, Liechtenstein, Iceland, Norway and Australia.
1.112 “Territory Specific Trials” shall mean (a) all Post Approval Commitments that are requested only by the Regulatory Authorities in the Territory and (b) Post Marketing Studies that are conducted solely by Grünenthal for the Territory.
1.113 “Third Party” shall mean any Person other than AcelRx, Grünenthal and their respective Affiliates.
1.114 “Third Party Claim” shall have the meaning set forth in Section 12.1.
1.115 “Trademarks” shall mean trademarks, trade names, trade dresses, domain names, logos and brandings.
1.116 “United States” or “U.S.” shall mean the United States of America, including its territories and possessions and the District of Columbia.
1.117 “U.S. Specific Trials” shall mean all trials necessary to support premarket approval(s) that may be issued by the FDA.
1.118 “Valid Claim” shall mean: (a) an unexpired claim of an issued AcelRx Patent, Assigned Patent or Joint Patent which has not been rejected or revoked or found to be unpatentable, invalid or unenforceable by a court or other authority in the subject country, from which decision no appeal is taken or which is unappealed within the time allowable for appeal,
13
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
and that has not been explicitly disclaimed, or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise; or (b) a bona fide claim of a pending patent application within the AcelRx Patents or Joint Patents, which claim has not been cancelled, abandoned, withdrawn or finally rejected or expired without the possibility of appeal or refiling and has been pending [ * ].
ARTICLE 2
(a) Technology and AcelRx Trademark Licenses to Grünenthal. Subject to the terms and conditions of this Agreement, including the payment of royalties hereunder, AcelRx hereby grants and causes its Affiliates to grant to Grünenthal under the AcelRx Technology, the Assigned Patent(s) and the AcelRx Trademarks (i) an exclusive (even as to AcelRx, its Affiliates and Third Parties) license to develop (subject to Sections 2.1(c) and 2.3 hereunder), commercialize, import, sell, offer for sale the Licensed Product in the Field in the Territory, and (ii) a co-exclusive (with AcelRx or its Affiliates only) license to research, develop, Manufacture, have Manufactured, use and import the Licensed Product solely for use, commercialization, importation, sale or offer for sale in the Field in the Territory; provided, that the foregoing licenses to Grünenthal under the AcelRx Trademarks shall end upon assignment of the Assigned Trademark as provided under Section 10.1(c) and the foregoing licenses to Grünenthal under the Assigned Patents shall end upon assignment of the Assigned Patent as provided under Section 10.1(b) (subject to reinstatement in the case of Section 10.2(c)). For the avoidance of doubt, “Licensed Product” as used in this Section 2.1(a), and as applicable in other provisions of this Agreement, refers to and includes all the components of the Licensed Product (e.g. Device, Drug, Dispenser Kit, Reusables Kit and Accessories) as well as the system as a whole.
(b) Copyright License to Grünenthal. Subject to the terms and conditions of this Agreement, AcelRx hereby grants and causes its Affiliates to grant to Grünenthal a non-exclusive, royalty-free, fully-paid license under the AcelRx Copyrights solely to research, develop, commercialize, use, sell, offer for sale and import the Licensed Product in the Field in the Territory.
(c) License to AcelRx. Subject to the terms and conditions of this Agreement, including the licenses set forth in Sections 2.1(a) and 2.1(b), Grünenthal hereby grants and causes its Affiliates to grant to AcelRx (A) a royalty-free, fully-paid, exclusive license, with the right to grant sublicenses, under the Grünenthal Technology for AcelRx to Manufacture, have Manufactured, commercialize, use, sell, offer for sale and import the Licensed Product for any purpose outside the Territory and to perform AcelRx’s obligations under this Agreement and the Supply Agreement, and (B) a royalty-free, fully-paid, non-exclusive license, with the right to grant sublicenses, under the Grünenthal Technology for AcelRx to conduct clinical and non-clinical development activities with respect to the Licensed
14
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Product worldwide and to perform AcelRx’s obligations under this Agreement and the Supply Agreement but, during the Term, subject to the terms of Article 4 with respect to the Territory, including the limitations imposed by Section 4.3(d).
2.2 Sublicensees; Distributors.
(a) Right to Sublicense and Sub-Contract. Grünenthal shall have the right to sublicense any of its rights granted to it under this Agreement to its Affiliates as and when elected by Grünenthal. Grünenthal shall also have the right to sublicense its rights granted under this Agreement to any Third Parties (who may further sublicense to a Distributor), [ * ]. All such sublicense and sub-contract agreements with a Third Party or any Distributor shall be consistent with the terms and conditions of this Agreement and shall provide that further sublicensing or sub-contracting other than to a Distributor is prohibited. Grünenthal shall provide a copy of any such sublicense agreement with a Third Party Sublicensee promptly after execution, subject to prior redaction by Grünenthal with regard to provisions, according to Grünenthal’s reasonable assessment, [ * ]. Grünenthal shall remain responsible for the performance of its Affiliates, Sublicensees and sub-contractors hereunder. For clarity, Affiliates of Grünenthal to which Grünenthal has sublicensed its rights hereunder may further sublicense consistent with this Section 2.2(a) the same as Grünenthal itself may grant sublicenses consistent with this Section 2.2(a).
(b) Right to Engage Distributors. Grünenthal and its Sublicensees shall have the right to engage Distributors under this Agreement, provided that Grünenthal shall remain responsible for the performance of its Distributors hereunder, including the restrictions on further sublicensing (including to sub-Distributors) and compliance of Applicable Laws by such Distributors in connection with the distribution of the Licensed Product hereunder. In the event of termination of this Agreement pursuant to Section 13.2(b) for breach by Grünenthal, upon Grünenthal’s request, [ * ].
2.3 Rights Reserved. Except for the rights and licenses expressly granted in this Agreement, AcelRx retains all rights under its intellectual property, including the AcelRx Technology, and Grünenthal retains all rights under its intellectual property, including Grünenthal Technology, and no rights shall be deemed granted by one Party to the other Party by implication, estoppel or otherwise. Further, notwithstanding the grants of exclusive rights in Section 2.1, AcelRx retains the rights, without limitation, to: (a) perform or have performed all of its obligations under this Agreement, whether within or outside the Territory, including, but not limited to, to conducting development activities as contemplated by Article 4 including the limitations imposed by Section 4.3(d) and manufacturing or having manufactured the Licensed Product for supply to Grünenthal as contemplated by Article 5, this Agreement and the Supply Agreement, and (b) to make, have made, manufacture and have manufactured the Licensed Product worldwide. For the avoidance of doubt, however subject to Section 2.7, the license granted to Grünenthal under Section 2.1 does not confer any rights to Grünenthal with respect to (x) any product other than the Licensed Product, (y) any product comprising any individual component(s) of the Licensed Product, or (z) any product comprising the Licensed Product or any individual component of the Licensed Product, in each case, together with one or more additional products, devices or APIs.
15
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
2.4 Technology Transfer. Grünenthal shall have the right to [ * ] to facilitate the transfer of analytical methods for the market release of the Licensed Product and AcelRx Know-How including AcelRx manufacturing Know-How for use in Regulatory Filings or submissions to Regulatory Authorities or other similar purposes related to Regulatory Approval of the Licensed Product in the Territory. In that respect, as soon as reasonably practicable following [ * ], AcelRx shall, subject to reimbursement by Grünenthal for reasonable out-of-pocket costs, make available to Grünenthal personnel of AcelRx who are appropriately knowledgeable or experienced in the Manufacture of the Licensed Product for use in the Field to facilitate the transfer of the analytical methods for the market release of the Licensed Products existing as of the time of such request with a goal to effect transfer as soon as practicable, but in any event [ * ], subject to extension for any period in which the relevant personnel of Grünenthal are unavailable. In any event, Grünenthal shall cooperate with any such transfer and shall promptly undertake to complete the transfer. During the Term, AcelRx and Grünenthal shall agree upon a process to provide to Grünenthal, at Grünenthal’s reasonable request, any updates required to complete the transfer of analytical methods for the market release of the Licensed Product and AcelRx Know-How including AcelRx manufacturing Know-How for use in Regulatory Filings or submissions to Regulatory Authorities or other similar purposes related to Regulatory Approval of the Licensed Product in the Territory. Further, during the Term, AcelRx and Grünenthal shall agree upon a process to provide to each other, at the other Party’s reasonable request, any scientific product information or data reasonably required by such Party’s medical affairs to complete any Licensed Product-related tasks within the responsibility of medical affairs.
2.5 EU Country Additions to the Territory. If during the Term any additional countries (“Candidate EU Member”) apply to or become part of the European Union [ * ].
2.6 Covenants of AcelRx. During the Term, AcelRx covenants that it and its Affiliates will not directly or indirectly, [ * ].
2.7 [ * ]
2.8 Australia Termination Right. Either Party shall have the right to remove the country of Australia from the Territory upon prior written notice to the other Party if [ * ]. For clarity, effective upon such written notice from either Party, Australia shall no longer be included in the Territory either of this Agreement or the Supply Agreement, and such notice shall relieve both Parties of obligations with respect to one another relating to Australia.
16
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
ARTICLE 3
(a) Establishment. Within [ * ] following the Effective Date, AcelRx and Grünenthal shall establish a committee (the “Joint Steering Committee” or “JSC”) to oversee, review and/or coordinate development, Manufacture, regulatory strategy and commercialization of the Licensed Product in the Field in the Territory.
(b) Duties. The Joint Steering Committee shall:
(i) review, coordinate, and approve the overall development and regulatory strategies for obtaining Marketing Approval of the Licensed Product in the Field in the Territory;
(ii) provide a forum for the Parties to present, discuss and approve the Development Plan and material changes to the Development Plan, including Budgets contained therein;
(iii) provide a forum for the Parties to present any proposals regarding material development, and regulatory and manufacturing matters pertaining to the Licensed Product in the Territory and seek input from the Parties on the foregoing matters;
(iv) provide a forum for the Parties to present and discuss the Commercialization Plan;
(v) exchange information with respect to prelaunch, launch and subsequent commercialization activities with respect to the Licensed Product in the Territory;
(vi) provide a forum for the Parties to exchange information and coordinate their respective activities with respect to development, regulatory and manufacturing matters pertaining to the Licensed Product in the Field in the Territory and outside the Field or Territory;
(vii) discuss and review any opportunities for global brand synergies;
(viii) discuss and provide a forum for the Parties to seek mutual agreement on the design of a second generation Device, it being understood that no such agreement shall be required for the development of a second generation Device by AcelRx; and
(ix) perform such other duties as are specifically assigned by the Parties to the Joint Steering Committee pursuant to this Agreement.
17
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(c) Decision-Making. Subject to Section 3.1(d), the JSC shall act by consensus. The representatives from each Party will have, collectively, one (1) vote on behalf of that Party. If the JSC cannot reach consensus on an issue that comes before the JSC and over which the JSC has oversight, then the dispute resolution provisions as provided under Article 15 shall apply.
(d) Clarification to Decision-Making. Notwithstanding Sections 3.1(b) or (c) or Section 3.2, the Parties shall maintain final decision making authority over specific areas related to the Licensed Product that are set forth in Articles 4, 5 and 6.
(a) Establishment. From time to time, the Joint Steering Committee may establish additional subcommittees to oversee particular projects or activities within the scope of authority of the Joint Steering Committee, as it deems necessary or advisable. Each subcommittee shall consist of such number of representatives of each Party as the Joint Steering Committee determines is appropriate from time to time and shall meet with such frequency as the Joint Steering Committee shall determine.
(b) Development Subcommittee. One such subcommittee the JSC may form may be the Development Subcommittee, which to the extent agreed by the JSC may have one or more of the following responsibilities:
(i) discuss regular reports regarding the development of the Product, and discuss, prepare and consider for approval annual and interim amendments to the Development Plan (and the development budget) for the Licensed Product;
(ii) discuss and manage the implementation of the Development Plan;
(iii) oversee the conduct of development;
(iv) create, implement and review the development strategy for development and Regulatory Approval in the Territory and the design of all clinical trials,;
(v) oversee any CMC related development activities, e.g. stability studies or packaging development, as well as other activities to prepare for supply of drug substance and finished form of the Licensed Product;
(vi) allocate budgeted resources and determine priorities for each clinical trial and under the Development Plan other than Territory Specific Trials;
(vii) review Third Party contractors proposed to conduct clinical trials of the Licensed Product;
(viii) facilitate the flow of information between the Parties with respect to the potential development of the Licensed Product outside of the Field and Territory;
18
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(ix) discuss whether to develop the Licensed Product for other indications in the Field in the Territory;
(x) allocate primary responsibility as between the Parties for tasks relating to development of the Licensed Product where not already specified in the Development Plan;
(xi) discuss the requirements for Regulatory Approval in the Territory and oversee and coordinate regulatory matters with respect to the Licensed Product in the Territory, including to review material regulatory filings prior to submission thereof;
(xii) establish a publication strategy for publications and presentations related to the Licensed Product in the Territory;
(xiii) facilitate the flow of Information between the Parties with respect to obtaining Regulatory Approval for the Licensed Product; and
(xiv) review global harmonization of the Product, including annual review of the Development Plan and the Commercialization Plan, identify opportunities for brand synergy, discuss, and agree areas for shared investment.
3.3 Alliance Managers. Each of AcelRx and Grünenthal shall appoint one appropriately qualified representative who possesses a general understanding of clinical, regulatory, manufacturing, quality assurance and marketing issues to act as its respective alliance manager for this relationship (each, an “Alliance Manager”). The Alliance Managers of each Party as of the Effective Date are named in Exhibit 3.3. Each party may appoint and replace its respective Alliance Manager at any time upon written notice to the other party. Any Alliance Manager may designate a substitute to temporarily perform the functions of that Alliance Manager. Each Alliance Manager shall be charged with creating and maintaining a collaborative work environment between the parties. Each Alliance Manager will also be responsible for:
(a) Coordinating the relevant functional representatives of the parties, in developing and executing strategies and plans for the Licensed Product;
(b) Providing a single point of communication for seeking consensus both internally within the respective party’s organizations and together regarding key strategy and plan issues, including where all questions coming up will be channeled, where joint timelines, budget and capacity requirement are aligned; and
(c) Planning and coordinating: (i) cooperative efforts, (ii) helping to establish new work streams proactively at each party; and (iii) internal and external communications.
The Alliance Managers shall be entitled to attend meetings of the JSC and of any subcommittee, but shall not have, or be deemed to have, any rights or responsibilities of a member of the JSC or subcommittee unless formally appointed to such committees. Each
19
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Alliance Manager may bring any matter to the attention of the JSC or subcommittee when such Alliance Manager reasonably believes that such matter requires such attention.
3.4 Joint Steering Committee Membership. The Joint Steering Committee shall consist of individuals appropriately qualified and of appropriate seniority to discuss the development, manufacturing, regulatory and commercialization activities of the Parties and shall be responsible for coordinating communications, managing the roles, responsibilities and timelines for such activities based on the Development Plan. The Joint Steering Committee shall be composed of four members, two of whom shall be nominated by AcelRx and two of whom shall be nominated by Grünenthal. Any member of the Joint Steering Committee may designate an appropriately qualified substitute to attend and perform the functions of that member at any meeting of the Joint Steering Committee. Each Party may, with the consent of the other Party, such consent not to be unreasonably withheld or delayed, invite non-member representatives of such Party to attend meetings of the Joint Steering Committee.
3.5 Meetings. All Joint Steering Committee meetings shall be held as often as the members may determine, but in any event Joint Steering Committee meetings shall occur not less than once per Calendar Quarter. Such meetings may be held in person, or by any means of telecommunications or video conference, as the members deem necessary or appropriate; provided, that at least one meeting of the Joint Steering Committee per Calendar Year shall be held in person.
3.6 Minutes. Minutes for each of the Joint Steering Committee meetings shall be prepared by a Grünenthal or an AcelRx member of the Joint Steering Committee on an alternating basis. The draft minutes shall be sent to all members of the Joint Steering Committee for comment promptly after each such meeting (but in no event more than 15 days after each such meeting). All actions noted in the minutes shall be reviewed and approved at subsequent meetings of the Joint Steering Committee; provided, that if the Parties cannot agree as to the content of the minutes by the time the Joint Steering Committee next meets, such minutes shall be finalized to reflect any areas of disagreement.
3.7 Expenses. Each Party shall bear its own costs, including expenses incurred by the members nominated by it in connection with their activities as members of the Joint Steering Committee.
3.8 Dispute Resolution. If any subcommittee is unable to resolve a dispute within such subcommittee within [ * ] after written notice of a dispute from one Party to another, then such dispute shall be referred to the Joint Steering Committee for resolution. If the Joint Steering Committee is unable to resolve any dispute within [ * ] after written notice of a dispute at the level of the Joint Steering Committee from one Party to another, then either Party may, by written notice to the other Party, have such dispute referred to the Senior Executives in accordance with Section 15.1, and such dispute shall thereafter be handled in accordance with Section 15.1.
3.9 Discontinuation of Participation. The Joint Steering Committee (and any subcommittee established under this Article 3) shall continue to exist until the first to occur of:
20
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(a) the Parties mutually agreeing to disband the committee; or (b) AcelRx providing to Grünenthal written notice of its intention to disband and no longer participate in such committee at any time during the Term however not to be issued by AcelRx earlier than [ * ]. Once AcelRx has provided such written notice, AcelRx shall have no further obligations under this Agreement with respect to any such committee or subcommittee, and (x) any matters that would previously have been addressed by a subcommittee will be handled by the JSC, and (y) any matters that would previously have been addressed by the JSC will be handled by the Parties in accordance with the terms of this Agreement; provided, that in such event the consent of AcelRx (when and where required) for further clinical development of the Licensed Product in Field in the Territory shall be promptly given and not unreasonably withheld.
ARTICLE 4
DEVELOPMENT AND REGULATORY ACTIVITIES
4.1 Development Plan. The Parties will negotiate in good faith and enter into a Development Plan (including a Budget contained therein) with respect to the Licensed Product, which sets forth a comprehensive development program to support Regulatory Filings for the Licensed Product in the Territory and will be attached hereto as Exhibit 1.38, provided that, AcelRx shall be the controlling Party with regard to clinical development of the Licensed Product unless provided for otherwise in this Agreement. Grünenthal shall be responsible for the costs and conduct of Regulatory Filings in the Territory, including MAA Approval, and/or other regulatory approvals at a country-specific level. The JSC shall be responsible for initial review and discussion of the Development Plan as well as reviewing and approving the Development Plan and any changes to the Development Plan on an ongoing basis, and in no event less frequently than once annually. Notwithstanding Section 3.6, in the event that the JSC cannot agree on content of or changes to the Development Plan with respect to clinical development, the representatives of AcelRx to the JSC shall have the deciding vote, provided however that (i) AcelRx shall not exercise such right if it may result in increased costs for Grünenthal unless AcelRx is willing to bear such costs, and (ii) Grünenthal shall have the deciding vote for certain decision as set forth in Section 4.2. below. All material changes to the Development Plan shall be agreed to by the Parties in writing. Both Parties shall exchange safety relevant information obtained from any kind of investigation for reporting purposes such as, for non-limiting example, periodic safety reports. Further details shall be stipulated in the Pharmacovigilance Agreement.
4.2 Development Responsibilities. The Development Plan shall provide that:
(a) Post Approval Commitments.
(i) Grünenthal shall use Commercially Reasonable Efforts to conduct (at its cost) development activities for Territory-specific Post Approval Commitments for the Licensed Product in the Field that are not also required by the FDA in the U.S. for the Licensed Product in the Field. Grünenthal shall not be required to obtain AcelRx’s consent to conduct such development activities but shall provide, through the JSC, reasonable detail regarding these Post Approval Commitments.
21
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(ii) AcelRx shall use Commercially Reasonable Efforts to conduct development activities for all Post Approval Commitments in the U.S., including such Post Approval Commitments required by the FDA and any Regulatory Authorities in the Territory that overlap in whole or in parts. AcelRx shall bear all costs for Post Approval Commitments required by the FDA [ * ]. In the event that AcelRx is required to expand its development activities for Post Approval Commitments beyond FDA requirements and in order to meet additional requirements by Regulatory Authorities in the Territory, then Grünenthal shall bear any incremental costs beyond those costs for such FDA-required Post Approval Commitments, provided that the development activities applicable to the Territory and allocation of related costs shall be discussed and agreed by the JSC prior to their initiation.
(b) Non-Interventional Studies and Post Marketing Studies.
(i) Grünenthal shall be free to conduct (at its cost) Non-Interventional Studies for the Licensed Product in the Field in the Territory without AcelRx’s consent.
(ii) The Parties may also mutually agree to jointly conduct Post Marketing Studies for the Licensed Product in the Territory. In such case, responsibilities for the conduct of such Post Marketing Studies for the Licensed Product and sharing of related costs shall be mutually agreed by the JSC.
(c) Further Development Studies. Should a Party desire to conduct Further Development Studies for the Licensed Product, the Parties shall discuss the possible conduct of such development activities, including any sharing of costs. Grünenthal shall be required to obtain AcelRx’s consent before conducting such Further Development Studies for the Licensed Product, such consent not to be unreasonably withheld. In the event that Grünenthal shares in the cost of any such Further Development Study, [ * ].
4.3 Conduct of Development Activities.
(a) Compliance with Development Plan and Applicable Laws. All development and regulatory activities in connection with the clinical development program to support Regulatory Filings for the Licensed Product shall be conducted by and on behalf of the Parties in accordance with the Development Plan and the other provisions of this Agreement. Each Party shall conduct the development activities for which it is the responsible Party under the Development Plan in accordance with the Development Plan (including the applicable Budgets contained therein) and this Agreement. Each Party shall conduct those activities for which it is the responsible Party under the Development Plan in compliance in all material respects with all Applicable Laws and in accordance with GLP and GCP (when applicable) under the Applicable Laws of the country in which such activities are conducted.
(b) Diligence. The responsible Party shall use Commercially Reasonable Efforts to conduct and complete the studies and activities assigned to it in the Development Plan in order to achieve the goals of the Development Plan in accordance with the timelines specified therein. Without limiting the foregoing, each Party shall use Commercially Reasonable Efforts to conduct the studies and activities for which it is the responsible Party under the Development
22
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Plan by using its good faith efforts to allocate sufficient time, effort, equipment and facilities to such development activities and to use personnel with sufficient skills and experience as are required to accomplish such studies and activities in accordance with the Development Plan and the terms of this Agreement.
(c) Information Regarding Development Activities. Each Party shall maintain records, in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, which shall fully and properly reflect all work done and results achieved by or on behalf of such Party in the performance of its development activities under this Agreement. Each Party shall keep the Joint Steering Committee appropriately informed of the status of the clinical development program and other activities with respect the Licensed Product in the Field conducted under the Development Plan and other development activities under or pursuant to Sections 4.2 and 4.3. Upon request by the Joint Steering Committee, without limiting the foregoing, each Party shall promptly provide the Joint Steering Committee with summaries of data and results and, if requested by the Joint Steering Committee, all supporting data and results generated or obtained in the course of such Party’s performance of studies and activities under the Development Plan. Upon reasonable prior written notice, each Party shall have the right to inspect records and notebooks reflecting the work done and results achieved by or on behalf of the other Party or its Affiliates in the performance of its development activities with respect to the Licensed Product in the Field pursuant to the Development Plan.
(d) AcelRx Development Activities within the Territory. During the Term, [ * ] without obtaining the prior written consent of Grünenthal, such consent not to be unreasonably withheld or delayed.
4.4 Regulatory Responsibilities for the Licensed Product.
(a) Responsibilities for Obtaining and Maintaining Drug Regulatory Approvals for the Licensed Product. During the Term, Grünenthal shall have the exclusive (even as to AcelRx, its Affiliates and Third Parties) right and shall use Commercially Reasonable Efforts to obtain and maintain the MAA Approval and any other Drug-related Regulatory Filings for the Licensed Product in the Field in the Territory at its sole cost and expense, and AcelRx shall use Commercially Reasonable Efforts to obtain and maintain the CE Xxxx and any other Device-related Regulatory Filings at its sole cost and expense. At Grünenthal’s request, AcelRx shall use Commercially Reasonable Efforts to assist Grünenthal at AcelRx cost, with the preparation and filing of such Regulatory Filings. AcelRx shall provide Grünenthal with an updated dossier according to EU requirements and in EU compliant eCTD format as soon as possible however not later than [ * ] of the MAA in the EU as agreed upon in the Development Plan. AcelRx shall transfer or shall cause its consultants or subcontractors to transfer all responsibilities and activities related to the MAA Approval and any other Drug-related Regulatory Filings to Grünenthal as soon as reasonably practicable after the Effective Date.
(b) Responsibilities for Obtaining and Maintaining Device Regulatory Approvals for the Licensed Product. AcelRx shall use Commercially Reasonable Efforts to obtain and maintain a CE Xxxx and any other device-related Regulatory Filings including
23
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
compliance with all Harmonized Standards for the Licensed Product in the Territory at its sole cost and expense. Grünenthal shall use Commercially Reasonable Efforts to assist AcelRx with the preparation and filing of such Regulatory Filings. AcelRx shall designate Grünenthal as Authorized Representative for the Licensed Product in the Territory as provided in the Supply Agreement. In case AcelRx has previously designated another party as Authorized Representative for the Licensed Product in the Territory, AcelRx shall promptly take all necessary actions to transfer such responsibility from such party to Grünenthal in accordance with the Supply Agreement.
(c) CMC Information. AcelRx shall be, at its cost, solely responsible for preparing and providing Grünenthal with the necessary pre-clinical, clinical and chemistry, manufacturing and control (“CMC”) data to support and maintain MAA Approval for the Licensed Product in the Field in the Territory. Such documentation shall be compliant with EU requirements for eCTD format.
(a) Conduct of Regulatory Activities. Before the Effective Date of this Agreement, AcelRx has obtained clearance from the EMA that the Licensed Product can (but need not) be submitted through a Centralized Procedure and has submitted a respective letter of intent to the EMA and has requested a corresponding pre-submission meeting with the EMA and Grünenthal has received a copy of such documentation. Furthermore, AcelRx has initiated the clearance procedure(s) regarding the AcelRx Trademarks to be used for the commercialization of the Licensed Product under the EMA in accordance with the Centralized Procedure. Each Party shall conduct all of those regulatory activities for which it is the responsible Party as set forth in Section 4.4 or Section 4.5 as the case may be, and shall fund all regulatory activities for obtaining Marketing Approval in the Territory in accordance with Section 4.4 above. Each Party shall conduct such regulatory activities for which it is the responsible Party in compliance with this Agreement and in accordance with the Development Plan (including the Budget set forth therein) and shall use Commercially Reasonable Efforts to obtain Marketing Approval in the Field in the Territory.
(b) Regulatory Communications. During the period that a Party is the responsible Party for certain regulatory activities under the Development Plan, such responsible Party shall timely inform the other Party of all of its scheduled meetings with the Regulatory Authorities, invite such other Party to attend in such meetings as observers, and, if such other Party elects not to attend such meetings, provide such other Party with summaries of its meeting with the Regulatory Authority promptly after each meeting. In addition, each Party shall promptly provide the other Party with copies of all written communications and summary of material oral discussions with the Regulatory Authority with respect to the Licensed Product in the Field in the Territory. In addition to the information required to be provided to the other Party in other provisions of this Agreement, Grünenthal shall timely provide AcelRx with summaries of any of its communications and correspondence with the Regulatory Authorities in the Territory with respect to safety and manufacturing issues with respect to the Licensed Product for use in the Field in the Territory and AcelRx shall timely provide Grünenthal with
24
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
summaries of any of its communications and correspondence with the Regulatory Authorities with respect to safety and manufacturing issues with respect to the Licensed Product for use outside the Field in the Territory or for use outside the Territory. The Parties shall, upon the request of a Party, discuss in good faith whether a regulatory services agreement would be necessary to facilitate the disclosures and information sharing as well as to specify responsibilities of each Party as required pursuant to this Article 4.
(c) Pharmacovigilance. AcelRx shall be responsible for the maintenance of the global safety database for the Licensed Product, including being solely responsible for the costs for such maintenance. Grünenthal shall be responsible for the maintenance of its own safety database for the Licensed Product with respect to the Territory, including being solely responsible for the costs for such maintenance of its database; provided that Grünenthal shall in any event ensure that all safety database information is provided to AcelRx in a timely manner and in an electronic format requested by AcelRx in order to maintain the Grünenthal safety information as part of the global safety database. Both Parties shall in any event ensure that all safety information is exchanged in both directions in a timely manner. The Parties shall enter into a pharmacovigilance agreement (the “Pharmacovigilance Agreement”) as soon as practicable with a goal of entering into such Pharmacovigilance Agreement [ * ], setting forth the specific details and processes pursuant to which the Parties will share adverse event, device incidents and other safety data. The Pharmacovigilance Agreement will include those terms required by ICH guidelines, EU Medical Device Directive and applicable MEDDEV Guidelines, such as (i) providing detailed procedures regarding the maintenance of core safety information and the exchange of safety data relating to the Licensed Product worldwide within appropriate timeframes and in an appropriate format to enable each party to meet both expedited and periodic regulatory reporting requirements; and (ii) ensuring compliance with the reporting requirements of all applicable Regulatory Authorities on a worldwide basis for the reporting of safety data in accordance with standards stipulated in the ICH guidelines and the Medical Device Directive , and all applicable regulatory and legal requirements regarding the management of safety data.
4.6 Expenses Report and Audit Right. In order to demonstrate the share of the aggregate amount of costs and expenses for which it is responsible pursuant to Section 4.2, each Party shall provide to the other Party a written report of all costs and expenses which it has paid or committed to pay, together with documented supporting evidence. The other Party shall have the right to cause an independent, certified public accounting firm reasonably acceptable to the other Party to audit the other Party’s records relating to the allocated costs and expenses to confirm the amount of the costs and expenses reflected in such report. The auditing Party shall bear the full cost of such audit unless such audit discloses an over-charging by the audited Party [ * ] of the total amount of costs and expenses invoiced, in which case, the audited Party shall bear the full cost of such audit.
4.7 Language Translation for Device. AcelRx shall be responsible for and shall complete, at its cost, the preparation of the Device (including any modifications of the Device that may be necessary or useful) to receive and utilize languages other than American English for its intended operation as part of the Licensed Product, including all necessary technical
25
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
development and implementation of hardware and firmware. Grünenthal shall determine the languages that are necessary for the Device to be implemented in for the Territory. AcelRx shall, consulting with Grünenthal, competitively bid subcontracts with Third Parties providing for the implementation of the software for the Device in the languages specified by Grünenthal for review and approval by the JSC, provided that any subcontracts and the costs for the implementation of the software for the Device in the languages specified by Grünenthal thereunder must be approved in advance by Grünenthal prior to AcelRx entering into such agreements. Grünenthal shall be responsible for the costs of software development, verification, validation and implementation that are necessary to enable specifically the translation and presentation into languages selected by Grünenthal pursuant to contracts approved by Grünenthal. For clarity, Grünenthal shall not be responsible for any Device modification or preparation costs such as those described in the first sentence of this Section 4.7.
4.8 Second Generation Device. The JSC shall review market or other research that is presented to it by a Party regarding potential modifications that could be made to the Device to improve or add to its functionality in desirable ways to become a second generation Device for the Territory. The JSC shall seek to agree upon a joint, mutually agreed recommendation for such a second generation Device. Neither Party shall have final decision-making control regarding the selection of such a second generation Device for the Territory, but in any event AcelRx shall not be limited in proceeding with a second generation Device with the understanding that Grünenthal may determine (through the JSC) that such Device will not be used in the Territory.
ARTICLE 5
COMMERCIALIZATION AND MARKETING
5.1 Commercialization of the Licensed Product.
(a) Grünenthal’s Commercialization Rights. Pursuant to the license rights granted to Grünenthal under Sections 2.1 and 2.2, Grünenthal through its own efforts and that of its sublicensed Affiliates and permitted Third Party sublicensees, and its and their respective Distributors, shall have the exclusive (even as to AcelRx, its Affiliates and Third Parties) right to sell and otherwise commercialize any and all Licensed Products in the Field in the Territory, including the components thereof (e.g. Device, Drug, Dispenser Kit, Reusables Kit and Accessories) and the system as a whole. Grünenthal shall use Commercially Reasonable Efforts to: (i) establish the strategy for the commercialization of the Licensed Product in the Field in the Territory (the “Commercial Strategy”) and (ii) commercialize the Licensed Product in the Field in the Territory. It is anticipated that Grünenthal may enter into distribution and alternative supply agreement(s) with its Affiliate(s) or Third Party(ies) for the commercialization of the Licensed Product in the Field in the Territory in accordance with this Agreement.
(b) Commercialization Coordination. No later than [ * ] before the first anticipated launch of the Licensed Product in the Territory Grünenthal shall prepare and submit to the Joint Steering Committee for review and discussion a commercialization plan setting forth
26
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
the goals, Commercialization Strategies and plans for Grünenthal’s prelaunch activities, launch and subsequent commercialization of the Licensed Product in the Field in the Territory and the level of anticipated sales force and promotion efforts dedicated to the Licensed Product, together with the budget in connection therewith (the “Commercialization Plan”). Grünenthal shall use Commercially Reasonable Efforts to conduct the commercialization activities in accordance with such Commercialization Plan; provided, that for purposes of this Section 5.1(b), Commercially Reasonable Efforts means that Grünenthal shall have [ * ]. Grünenthal shall consult with and provide regular updates to AcelRx through the Joint Steering Committee regarding the Commercial Strategy and shall discuss coordination of commercial activities in the Field in the Territory with activities in the rest of the world. In the event that the JSC cannot agree as to the content of the Commercialization Plan, Grünenthal shall have the decisive vote with respect to the commercialization activities concerned. Nothing contained in this Section or Agreement shall be construed to interfere with Grünenthal’s right to set any resale prices.
(c) Prompt Assertion of Claims. If either Party is or becomes aware of facts that would constitute a reasonable basis to allege that the other Party is in material breach of this Agreement pursuant to Section 13.2(b), then the Party becoming aware of such facts will promptly notify the other Party in writing of the facts constituting such potential material breach. Promptly upon a Party’s receipt of any notice from the other Party of a such potential breach by such Party receiving such notice (a “Receiving Party”) pursuant to this Section 5.1(c), the Parties will discuss the specific nature of such potential breach and seek to identify an appropriate corrective course of action. If, no later than [ * ] after the Receiving Party’s receipt of such a notice, (i) the Parties have not reached consensus regarding whether Receiving Party has failed to satisfy its obligations pursuant to this Agreement, and (ii) the Parties’ have not agreed upon an appropriate corrective course of action for such failure, then such potential breach may be escalated by either Party and resolved pursuant to the provisions set forth in Article 15. If a Party fails to notify the other Party of a potential claim pursuant to this Section 5.1(c) within [ * ] after the date such first Party first becomes aware of sufficient facts that would reasonably constitute a material breach, then other Party will be deemed to have satisfied its obligations under this Agreement with respect to such potential material breach.
5.2 [ * ].
ARTICLE 6
6.1 Clinical Supply and Purchase of the Licensed Product. Subject to the terms of this Agreement, during the Term and until AcelRx supplies Licensed Product under the Supply Agreement (as defined below), AcelRx shall Manufacture in accordance with cGMP and supply the Licensed Product for use by the Parties in connection with the research and development of the Licensed Product in the Field in the Territory in accordance with the Development Plan. All other supply, including commercial supply, of the Licensed Product shall be as set forth in a separate commercial supply agreement (the “Supply Agreement”).
27
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
6.2 Samples. AcelRx shall Manufacture and have Manufactured the Device or other samples of components of the Licensed Product (“Samples”) for use in training in accordance with applicable Regulatory Requirements in the Territory, as then in effect for use by Grünenthal (and its Affiliates, distributors or licensees) for sampling or demonstration purposes. For clarity, the Parties expressly contemplate that Samples shall consist of the Device and placebo cartridges Manufactured by AcelRx, and the Samples will be supplied to Grünenthal [ * ]. In any event, AcelRx will discuss the nature and quality of the Samples, planned quantities and cost with Grünenthal in advance of any Manufacture of Samples.
6.3 Price. All Licensed Product supplied under this Agreement to Grünenthal for research and development purposes shall be provided at AcelRx’s Fully Burdened Manufacturing Cost for such items, as soon as practicable following written order from Grünenthal specifying the quantity and specifications for such Licensed Product.
6.4 Clinical Supply Terms. Grünenthal may submit purchase orders from time to time for clinical supply of Licensed Product and AcelRx shall use Commercially Reasonable Efforts to promptly fill such order. The provisions of Sections 3.1, 3.2, 3.7 and 4.2 of the Supply Agreement are hereby incorporated by reference with respect to such orders for clinical supplies.
ARTICLE 7
7.1 Initial Payment. In consideration for the licenses and rights granted to Grünenthal hereunder, Grünenthal shall pay to AcelRx a non-refundable, non-creditable payment in the amount of U.S. $30,000,000 (thirty million US dollars) by wire transfer of immediately available funds into an account designated by AcelRx no later than December 30, 2013.
7.2 Milestone Payments. In further consideration for the licenses and rights granted to Grünenthal hereunder, Grünenthal shall pay to AcelRx the following non-refundable, non-creditable milestone payments set out below following the first achievement of the corresponding milestone in the Territory during the Term. A Party shall notify the other Party in writing within [ * ] after the achievement of each milestone event with respect to the Licensed Product during the Term, and AcelRx shall invoice Grünenthal at the time of or following such notice for the applicable milestone payment. Grünenthal shall pay to AcelRx the amounts set forth below within [ * ] after its receipt of AcelRx’s invoice.
(a) R&D Milestones:
| R&D Milestones | ||
| Milestone |
Milestone Payment Amount | |
| 1. Upon first MAA submission for a Licensed Product in the Territory during the Term. |
$5,000,000 (five million US Dollars) | |
| [*] | [*] | |
28
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
[ * ]
| Commercial Milestones | ||
| Aggregate Annual Net Sales |
Milestone Payment | |
| [*] | [*] | |
Any milestone payment payable by Grünenthal pursuant to this Section 7.2 shall be made no more than once with respect to the achievement of each such milestone event.
7.3 Royalty Payments During Royalty Term.
(a) Royalty Rate. Subject to this Section 7.3 and the other terms and conditions of this Agreement, in further consideration for the licenses and rights granted to Grünenthal under this Agreement, Grünenthal shall pay to AcelRx royalties on a quarterly basis at the applicable royalty rate set forth below with the royalty based on aggregate annual Net Sales of such the Licensed Product in the Territory[*]:
| Aggregate Annual Net Sales of the Licensed Product in the Territory |
Applicable Royalty Rate | |
| [*] | [*] |
(b) Third Party Licenses and Disputes. If, during the Term,
(i) A license from any Third Party to any Patent(s) that [ * ] is required in order to research, develop, import, use, sell, have sold and offer for sale the Licensed Product sold by AcelRx to Grünenthal for use in the Field in the Territory, then AcelRx shall be solely responsible, including bearing all related costs, for either (A) obtaining a license for the Licensed Product for the importation, use, having sold or sale of the Licensed Product by Grünenthal, or (B) defending and indemnifying Grünenthal with respect to any action for such alleged infringement; and/or
(ii) Grünenthal is alleged to infringe any Third Party Patents or Third Party Trademarks with respect to the development, importation, use, having sold or sale of the Licensed Product as delivered and Manufactured by AcelRx for use in the Field in the Territory [ * ], then Grünenthal shall promptly notify AcelRx of the notice of alleged infringement. AcelRx shall have the first right to obtain a license from such Third Party or to defend any action with respect to such alleged infringement. If AcelRx obtains any license in any jurisdiction for such Patent(s) or Trademarks, AcelRx shall also obtain the same rights with respect to the Territory for the benefit of Grünenthal. If AcelRx takes action in any jurisdiction against such
29
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Third Party Patent(s), AcelRx shall also discuss with Grünenthal whether to undertake any similar action with respect to the Licensed Product in the Territory. AcelRx shall keep Grünenthal reasonably and promptly informed with regard to such licenses or actions taken by AcelRx in relation to same. To the extent that AcelRx determines not to obtain a license from such Third Party (and does nothing more) or AcelRx determines to bring an action against such Patent or Trademark and does not obtain rights to enable Grünenthal’s right to use, sell, have sold and offer for sale the Licensed Product as delivered by AcelRx in the Field in the Territory under such Patent or Trademark, then Grünenthal shall have the right to obtain a license from such Third Party directly [ * ] and any excess shall be applied towards future Calendar Quarters, as applicable. If AcelRx fails to undertake action against such alleged infringement following notification of alleged infringement of Third Party Patent(s) or Third Party Trademarks and Grünenthal thereafter takes action in any jurisdiction against such Third Party Patent(s) or Third Party Trademarks, then Grünenthal shall be allowed to deduct all reasonable out-of-pocket expenses as well as any damage payments incurred in such action from royalties and/or milestone payments otherwise due to AcelRx under this Agreement.
(c) Generic Competition. Upon Generic Entry in a country in the Territory, Grünenthal shall have the right upon thirty (30) days written notice to AcelRx, either (i) to terminate the Agreement with respect to such country, in which case the provisions of Section 14.5 shall apply; or (ii) to retain its licenses in such country, but reduce the royalties payable to AcelRx [ * ] until such time as the Generic Products are not being sold in such country, in which event the royalties on Net Sales shall be reinstated in the first Calendar Quarter following the removal of the Generic Products. For the avoidance of doubt, the [ * ] deduction set forth in this Section 7.3(c) shall apply only to the royalties on Net Sales for such country in the Territory in which [ * ] reduction in Net Sales has occurred. Calculation of the adjustments to royalties in any country in the Territory pursuant to this Section 7.3(c) shall be undertaken at the end of the Calendar Year based on the final then-applicable royalty rate on Net Sales in the Territory and Grünenthal shall include in the Royalty Report the Net Sales of Licensed Product in the country in the Territory with respect to which Generic Product reductions apply and the calculation of the applicable royalty in such country as a separate calculation from the unaffected countries in the Territory.
(d) Device Failure Royalty Reduction. In the event that (i) a Regulatory Authority in the Territory determines that as a result of a Device Failure the Licensed Product constitutes a potentially serious health risk and requests that a Recall of the Licensed Product should be initiated, or (ii) after the first anniversary of First Commercial Sale of the Licensed Product in the Territory, total Device Failures in any [ * ] then the royalty set forth in Section 7.3(a) shall be reduced [ * ] after the Calendar Quarter in which the Device Failure is finally determined.
For such purpose, Grünenthal shall, within [ * ] of return of a Licensed Product to Grünenthal from the end user customer, notify AcelRx in writing of any suspected Device Failure, including the details of use and return by the end user customer of Grünenthal, and AcelRx shall have [ * ] to review the documentation provided to dispute such claim of Device Failure. After timely notice of Device Failure is received by AcelRx, Grünenthal shall cooperate with AcelRx in
30
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
determining whether return is appropriate or justified. If AcelRx disagrees with Grünenthal’s determination that a certain Licensed Product is a Device Failure, the returned Licensed Product shall be submitted to a mutually acceptable Third Party laboratory, which shall determine whether such Licensed Product constitutes a Device Failure. The Parties agree that such Third Party laboratory’s determination shall be final and binding on the Parties. The costs for such Third Party’s laboratory’s determination shall be borne by (i) AcelRx if a Device Failure is confirmed, and (ii) by Grünenthal if no Device Failure is determined. Each Party is responsible for its own internal costs related to such procedures.
(e) Royalty Term. On a country-by-country basis, Grünenthal’s obligation to make royalty payments pursuant to this Section 7.3 will commence upon the First Commercial Sale of the Licensed Product in a country in the Territory and shall continue until the later of (i) expiration of the last-to-expire Valid Claim within the AcelRx Patents and/or Joint Patents that covers the making, use, sale, offer for sale or importation of the Licensed Product in the Field in such country, or (ii) [ * ] after the First Commercial Sale of the Licensed Product in such country (the “Royalty Term”). Following the expiration of the Royalty Term, subject to the payment obligations set forth in Section 7.4, the license grants set forth in Section 2.1(a) and Section 2.1(c) will convert to royalty-free, fully-paid licenses.
7.4 Trademark and Supply Fee. In addition to the royalty payable pursuant to Section 7.3, in further consideration of the rights, licenses, commitments to supply after the Royalty Term as set forth in Section 10.3(c) of the Supply Agreement and assignment of the Assigned Trademark hereunder, on a country-by-country basis for so long as AcelRx continues to supply Licensed Product pursuant to the Supply Agreement, Grünenthal shall pay to AcelRx (i) beginning on a country-by-country basis on the [ * ] of the Net Sales of Licensed Product in such country, and (ii) [ * ] for such annual period ((a) and (b) collectively, the “Trademark and Supply Fee”) until such time as, following Generic Entry in any country, the Net Sales of the Drug and Dispenser Kit in such country is reduced by [ * ]or more in the corresponding Calendar Quarter from the Net Sales in the preceding Calendar Year, in which event Grünenthal may elect within thirty (30) days to either (i) terminate the Trademark and Supply Fee in the Territory and AcelRx shall no longer have any obligation to supply under the Supply Agreement, or (ii) the Trademark and Supply Fee shall be reduced [ * ] in such country effective as of the end of the Calendar Quarter in which the Net Sales have been so reduced.
7.5 Royalty Reports; One Royalty. During the term of this Agreement following the First Commercial Sale of the Licensed Product, Grünenthal shall furnish to AcelRx a quarterly written report (“Royalty Report”) for the calendar quarter showing the gross amounts invoiced, permitted deductions and Net Sales of all the Licensed Product subject to royalty payments and the Trademark and Supply Fee sold by Grünenthal and its Affiliates, Sublicensees and Distributors on a country by country basis in the Territory during the reporting period and the amounts payable under this Agreement. Royalty Reports shall be due on the thirtieth (30th) day following the close of each calendar quarter. Royalties shown to have accrued by each Royalty Report shall be payable within three Business Days from the date such Royalty Report is
31
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
due. Grünenthal shall keep complete and accurate records in sufficient detail to enable the amounts payable hereunder to be determined. Only one royalty and Trademark and Supply Fee, as applicable, shall be due by Grünenthal to AcelRx with respect to the sale of the same unit of the Licensed Product.
7.6 Obligations under Existing Third Party Agreements. AcelRx shall be solely responsible for any and all payments, fees or other costs payable under its agreements with Third Parties existing as of the Effective Date.
7.7 No Measure of Damages. Grünenthal and AcelRx acknowledge and agree that nothing in this Agreement (including milestone payment amounts set forth in Section 7.2) will be construed as representing any estimate or projection of the damages, if any, that may be payable if this Agreement or the Supply Agreement is terminated for any reason and that each Party acknowledges its right and burden to prove the amount of damages to which it may be entitled in any successful action brought for uncured material breach of this Agreement by the other Party. Neither Party makes any representation, warranty or covenant that is not expressly set forth in this Agreement or the Supply Agreement.
ARTICLE 8
8.1 Payment Method. All payments to AcelRx under this Agreement shall be made by bank wire transfer in immediately available funds to an account in the name of AcelRx designated in writing by AcelRx. Payments hereunder shall be considered to be made as of the day on which they are dispatched by Grünenthal’s designated bank.
8.2 Payment Currency; Currency Conversion.
(a) United States Dollars. Unless otherwise expressly stated in this Agreement, all amounts specified to be payable under this Agreement are in United States Dollars and shall be paid in United States Dollars.
(b) Currency Conversion. Net Sales invoiced in currency other than United States Dollars, shall be converted to United States Dollars using an average exchange rate for the calendar quarter with respect to which such royalty is accrued based on published in public sources, e.g., Bloomberg or Reuters.
(a) Cooperation and Coordination. The Parties acknowledge and agree that it is their mutual objective and intent to minimize, to the extent feasible, income and other taxes payable with respect to their collaborative efforts under this Agreement and that they shall use their reasonable efforts to cooperate and coordinate with each other to achieve such objective.
32
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(b) Payment of Tax. A Party receiving a payment shall pay any and all taxes levied on such payment. If the fiscal or taxing authorities of any relevant jurisdiction assert that amounts are required to be withheld from the payments due to a Party hereunder, or the tax laws in one or more jurisdictions have changed so as to explicitly require such treatment, the Party made aware of such assertion or change in law shall inform the other Party within thirty (30) days and shall consult with the other Party regarding the consequences of such assertion or change. If Applicable Laws require that taxes be deducted and withheld from a payment, the remitting Party shall (i) deduct those taxes from the payment, (ii) pay the taxes to the proper taxing authority, (iii) send evidence of the obligation together with proof of payment to the other Party within sixty (60) days following that payment and (iv) provide such assistance as the other Party may reasonably require in obtaining any refund of such amounts to which the other Party may be entitled, to the extent that such assistance does not cause the remitting Party to incur any liability in respect of the taxes asserted to be due. All payments made under this Agreement are net prices and shall be free and clear of any and all taxes (like sales- and use taxes , consumption taxes, goods- and sale taxes or value added taxes or comparable taxes), duties, levies, fees or other charges, except for required withholding taxes.
8.4 Records. Grünenthal shall keep, and require its Affiliates and Sublicensees to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable to AcelRx pursuant to this Agreement. Such books and records shall be kept for such period of time required by law, but no less than at least [ * ] following the end of the Calendar Quarter to which they pertain. Such records shall be subject to inspection in accordance with Section 8.5.
8.5 Audits. Upon not less than [ * ] prior written notice, Grünenthal shall permit an independent, certified public accountant selected by AcelRx and reasonably acceptable to Grünenthal, which acceptance will not be unreasonably withheld or delayed (for the purposes of this Section 8.5, the “Auditor”), to audit or inspect those books or records of Grünenthal, its Affiliates and Sublicensees that relate to Net Sales and Royalty Reports for the sole purpose of verifying (a) the royalties payable hereunder in respect of Net Sales, (b) the withholding taxes, if any, required by Applicable Law to be deducted as a payment by Grünenthal in respect of such Net Sales and (c) the exchange rates used in determining the amount of United States dollars. The Auditor shall disclose to AcelRx only the amount and accuracy of payments reported and actually paid or otherwise payable under this Agreement. The Auditor shall send a copy of the report to Grünenthal at the same time it is sent to AcelRx. Such inspections may be made no more than once each Calendar Year and during normal business hours. Such records for any particular Calendar Quarter shall be subject to no more than one inspection. Inspections conducted under this Section 8.5 shall be at the expense of AcelRx, unless a variation or error producing an underpayment in amounts payable exceeding an amount equal to [ * ] for a period covered by the inspection is established, in which case all reasonable costs relating to the inspection for such period and any unpaid amounts that are discovered shall be paid by Grünenthal. AcelRx shall endeavor in such inspection not to disrupt the normal business activities of Grünenthal, or its Affiliates or Sublicensees. Promptly after receiving the audit report, Grünenthal shall submit to AcelRx any underpayment discovered in such audit, together with interest accrued in accordance with Section 8.7.
33
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
8.6 Financial Reporting Cooperation. In the event that Grünenthal and/or any of its Affiliates determine, based on its analysis and subsequent discussions with their auditors, that Grünenthal or any one of its Affiliate is required to consolidate AcelRx under Grünenthal’s Accounting Standards, AcelRx shall, for so long as Grünenthal or its Affiliate is required to so consolidate, collaborate in good faith with Grünenthal and its Affiliate to provide information as reasonably necessary under such consolidation requirement, provided that in no event shall any such other accommodation restrict AcelRx’s ability to conduct its operations in the normal course of business and provided further that Grünenthal shall engage in good faith negotiations with its auditors to exempt and waive compliance with such requirement.
8.7 Late Payment. Any amounts not paid within [ * ] after the date due under this Agreement shall be subject to interest from the foregoing date through and including the date upon which payment is received, calculated at the interest rate equal to three percentage points (3%) over the rate of interest according to the average six-month rate(s) of the London Inter-Bank Offering Rate (“LIBOR”) for U.S. dollars, as quoted on the British Banker’s Association’s website currently located at xxx.xxx.xxx.xx (or such other source as may be mutually agreed by the Parties) from time to time, effective for the applicable days of the period of default, on the last business day of the applicable Calendar Quarter prior to the date on which such payment is due, calculated daily on the basis of a 365-day year, or, if lower, the highest rate permitted under Applicable Law.
ARTICLE 9
9.1 Confidential Information. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, the Parties agree that the receiving Party (the “Receiving Party”) shall keep confidential and shall not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any confidential or proprietary information and materials, patentable or otherwise, in any form (written, oral, photographic, electronic, visual or otherwise) which is disclosed to it by the other Party (the “Disclosing Party”) including, but not limited to, all information concerning the Device and/or the Licensed Product, information disclosed by one Party to the other pursuant to the Confidentiality Agreement and any other technical or business information of whatever nature (collectively, “Confidential Information”).
9.2 Exceptions. Notwithstanding Section 9.1 above, the obligations of confidentiality and non-use shall not apply to Confidential Information that, in each case as demonstrated by competent evidence:
(a) was already known to the Receiving Party or any of its Affiliates, other than under an obligation of confidentiality, at the time of disclosure;
(b) was generally available to the public or was otherwise part of the public domain at the time of its disclosure to the Receiving Party;
34
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(c) became generally available to the public or otherwise part of the public domain after its disclosure by the Disclosing Party and other than through any act or omission of the Receiving Party or any of its Affiliates in breach of this Agreement;
(d) was subsequently lawfully disclosed to the Receiving Party or any of its Affiliates by a Person other than the Disclosing Party, and who, to the best knowledge of the Receiving Party, did not directly or indirectly receive such information directly or indirectly from the Disclosing Party under an obligation of confidence; or
(e) was developed by the Receiving Party or its Affiliate without use of or reference to any proprietary information or materials disclosed by the Disclosing Party.
9.3 Permitted Disclosures. Notwithstanding the provisions of Section 9.1, each Party may disclose Confidential Information belonging to the other Party as expressly permitted by this Agreement or if and to the extent such disclosure is reasonably necessary in the following instances:
(a) filing or prosecuting Patents as permitted by this Agreement;
(b) prosecuting or defending litigation in the Territory as permitted by this Agreement;
(c) complying with applicable court orders or governmental regulations; and
(d) disclosure to Third Parties in connection with a potential license to, distribution agreement with or collaboration with such Third Party (including entry into any such agreement), or a potential merger or acquisition by such Third Party, and disclosure to potential Third Party investors in connection with a potential financing, provided, in each case, that any such Third Party agrees to be bound by similar terms of confidentiality and non-use at least as stringent as those set forth in this Article 9.
Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to Section 9.3(b) or (c), it shall, except where impracticable, give reasonable advance notice to the other Party of such disclosure and use efforts to secure confidential treatment of such information at least as diligent as such Party would use to protect its own confidential information, but in no event less than reasonable efforts; provided that any Confidential Information so disclosed shall still be subject to the restrictions on use set forth in this Article 9. In any event, the Parties agree to take all reasonable action to avoid disclosure of Confidential Information hereunder.
9.4 Confidentiality of this Agreement and its Terms. Except as otherwise provided in this Article 9, each Party agrees not to disclose to any Third Party the existence of this Agreement or the terms of this Agreement without the prior written consent of the other Party hereto, except that each Party may disclose the terms of this Agreement that are not otherwise made public as contemplated by Section 9.5 and as permitted under Section 9.3.
35
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(a) As soon as practicable following the Effective Date hereof, the Parties shall each issue a mutually agreed to press release announcing the existence of this Agreement substantially in the applicable form attached hereto as Exhibit 9.5(a) Except as required by law (including disclosure requirements of the U.S. Securities and Exchange Commission (“SEC”), the NASDAQ stock market or any other stock exchange on which securities issued by a Party or its Affiliates are traded), neither Party shall make any other public announcement concerning this Agreement or the subject matter hereof without the prior written consent of the other, which shall not be unreasonably withheld or delayed; provided that it shall not be unreasonable for a Party to withhold consent with respect to any public announcement containing any of such Party’s Confidential Information. In the event of a required public announcement, to the extent practicable under the circumstances, the Party making such announcement shall provide the other Party with a copy of the proposed text of such announcement sufficiently in advance of the scheduled release to afford such other Party a reasonable opportunity to review and comment upon the proposed text.
(b) The Parties shall coordinate in advance with each other in connection with the filing of this Agreement (including redaction of certain provisions of this Agreement) with the SEC, the NASDAQ stock market or any other stock exchange or governmental agency on which securities issued by a Party or its Affiliate are traded, and each Party shall use reasonable efforts to seek confidential treatment for the terms proposed to be redacted; provided that each Party shall ultimately retain control over what information to disclose to the SEC, the NASDAQ stock exchange or any other stock exchange or governmental agency, as the case may be, and provided further that the Parties shall use their reasonable efforts to file redacted versions with any governing bodies which are consistent with redacted versions previously filed with any other governing bodies. Other than such obligation, neither Party (nor its Affiliates) shall be obligated to consult with or obtain approval from the other Party with respect to any filings to the SEC, the NASDAQ stock market or any other stock exchange or governmental agency.
9.6 Publication of the Licensed Product Information. Publication of any non-public scientific or technical information with respect to the Licensed Product shall be subject to prior review as follows: (a) at least thirty (30) days prior to submission of an original manuscript for publication, (b) at least seven (7) days prior to abstract submission for poster or podium presentation, or (c) at least seven (7) days prior to an oral or poster presentation, as the case may be, each Party shall provide to the other Party a draft copy thereof for such other Party’s review (unless such Party is required by law to publish such information sooner, in which case such Party shall provide such draft copy to the other Party as much in advance of such publication as possible). The publishing Party shall consider in good faith any comments provided by the other Party during such time period. In addition, the publishing Party shall, at the other Party’s reasonable request, remove therefrom any Confidential Information of such other Party.
9.7 Prior Non-Disclosure Agreements. As of the Effective Date, the terms of this Article 9 shall supersede any prior non-disclosure, secrecy or confidentiality agreement between the Parties (or their Affiliates) dealing with the subject of this Agreement, including the Confidentiality Agreement. Any information disclosed under such prior agreements shall be deemed disclosed under this Agreement.
36
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
ARTICLE 10
PATENT PROSECUTION AND ENFORCEMENT
10.1 Ownership of Intellectual Property. Inventorship shall be determined in accordance with Applicable Law. Ownership of intellectual property shall be as follows:
(a) Retained Rights. Subject to Section 10.1(b), AcelRx has, and shall retain all right, title and interest in and to, the AcelRx Technology. Grünenthal has, and shall retain all right, title and interest in and to, the Grünenthal Background IP and Grünenthal Know-How.
(b) Assignment of Assigned Patents. Within thirty (30) days following the Effective Date, AcelRx shall execute such documents and perform such acts, at Grünenthal’s expense, as may be reasonably necessary to effect an assignment of AcelRx’s entire right, title, and interest in and to the Assigned Patents in the Territory to Grünenthal.
(c) Assignment of Assigned Trademarks. Within thirty (30) days following the payment of R&D Milestone [*] set forth in Section 7.2 (i.e., [*]) and within thirty (30) days following Marketing Approval for the Licensed Product in any other country of the Territory, AcelRx shall execute such documents and perform such acts, at Grünenthal’s expense, as may be reasonably necessary to (i) in the case of the MAA Approval, effect an assignment to Grünenthal of AcelRx’s entire right, title, and interest in and to the Assigned Trademarks in the EU and (ii) in the case of another Marketing Approval, effect an assignment to Grünenthal of AcelRx’s entire right, title, and interest in and to the relevant Assigned Trademark, solely in such country in the Territory in which the Marketing Approval was obtained.
(d) Disclosure of Inventions. Grünenthal shall notify AcelRx in writing of any and all Inventions, generated by, resulting from or reduced to practice by or on behalf of Grünenthal, promptly after each such Invention is made or generated.
(i) Joint Inventions in the Territory. Subject to the license grants set forth in Section 2.1(a), Section 2.1(c) and Section 2.1(d), each Party can use, and grant licenses to use, any Joint Invention, Joint Patent or Joint Know-How in the Territory without the other Party’s consent and has no duty to account to the other Party for such use or license, and each Party hereby waives any right it may have under the laws of any country to require any such consent or accounting .
(ii) Joint Inventions outside the Territory. Grünenthal hereby assigns to AcelRx its entire right, title and interest in and to any Joint Invention, Joint Patent or Joint Know-How developed during the Term for use outside the Territory.
37
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(f) No Implied License. It is agreed that neither Grünenthal nor AcelRx transfers to the other by operation of this Agreement any patent right, copyright, trademark right, or other proprietary right of any party, except as expressly set forth herein.
10.2 Patent Prosecution and Maintenance.
(a) Initial Responsibility. AcelRx shall be responsible for the preparation, filing, prosecution and maintenance of all AcelRx Patents and Joint Patents, at AcelRx’s sole expense, using counsel of its choice which are reasonably acceptable to Grünenthal. Grünenthal shall be responsible for the preparation, filing, prosecution and maintenance of all Assigned Patents and Grünenthal Patents, at Grünenthal’s sole expense, using counsel of its choice which are reasonably acceptable to AcelRx. Each Party shall keep the other Party fully and promptly informed of progress with regard to the preparation, filing, prosecution and maintenance of the each of the Patents for which it has responsibility as provided under this Section 10.2(a) AcelRx shall consider and adopt in good faith the requests and suggestions of Grünenthal with respect to strategies for filing and prosecuting AcelRx Patents in the Territory. Further, AcelRx shall notify Grünenthal without undue delay of any patent applications that are specific to the Licensed Product and the Parties shall discuss in good faith assignment of such patent applications to Grünenthal for the Territory. Upon assignment such patent applications shall become “Assigned Patents”. Grünenthal shall consider and adopt in good faith the requests and suggestions of AcelRx with respect to strategies for filing and prosecuting Assigned Patents in the Territory.
(b) Option of Grünenthal to Prosecute, Maintain and Enforce. In the event that AcelRx desires to abandon or cease prosecution and maintenance of any AcelRx Patent or Joint Patent in the Territory, AcelRx shall provide reasonable prior written notice to Grünenthal of such intention to abandon (which notice shall, to the extent practicable, be given no later than 60 calendar days prior to the next deadline for any action that must be taken with respect to any such AcelRx Patent and Joint Patent in the relevant patent office). In such case, at Grünenthal’s sole discretion, upon written notice from Grünenthal, Grünenthal may elect to continue prosecution and maintenance of any such AcelRx Patent or Joint Patent at its own expense, and AcelRx shall execute such documents and perform such acts, at Grünenthal’s expense, as may be reasonably necessary to effect an assignment of AcelRx’s entire right, title, and interest in and to such AcelRx Patents and/or Joint Patents in the Territory to Grünenthal. Any such assignment shall be completed in a timely manner to allow Grünenthal to continue prosecution and maintenance of any such AcelRx Patent and Joint Patent in the Territory. Any AcelRx Patents and Joint Patent with respect to which Grünenthal so elects to continue prosecution and maintenance of shall no longer be considered AcelRx Patents and Joint Patents under this Agreement with respect to which royalties are to be paid under this Agreement and shall be solely owned by Grünenthal without further obligation or accounting to AcelRx.
(c) Option of AcelRx to Prosecute, Maintain and Enforce. In the event that Grünenthal desires to abandon or cease prosecution and maintenance of any Assigned Patent, Grünenthal shall provide reasonable prior written notice to AcelRx of such intention to abandon (which notice shall, to the extent practicable, be given no later than 60 calendar days prior to the next deadline for any action that must be taken with respect to any such Assigned
38
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Patent in the relevant patent office). In such case, at AcelRx’s sole discretion, upon written notice from AcelRx, AcelRx may elect to continue prosecution and maintenance of any such Assigned Patent at its own expense, and Grünenthal shall execute such documents and perform such acts, at AcelRx’s expense, as may be reasonably necessary to effect an assignment of Grünenthal’s entire right, title, and interest in and to such Assigned Patents in the Territory to AcelRx. Any such assignment shall be completed in a timely manner to allow AcelRx to continue prosecution and maintenance of any such Assigned Patent in the Territory. Any Assigned Patent with respect to which AcelRx so elects to continue prosecution and maintenance of shall no longer be considered Assigned Patents under this Agreement.
10.3 Infringement by Third Parties.
(a) Notice. In the event that either AcelRx or Grünenthal becomes aware of any infringement or threatened infringement by a Third Party of any AcelRx Patents, Joint Patents and/or Assigned Patents it will notify the other Party in writing to that effect. Any such notice shall include evidence to support an allegation of infringement or threatened infringement by such Third Party.
(b) Defense of Patents. Subject to this Section 10.3(b), AcelRx shall have the first right, as between AcelRx and Grünenthal, to bring and control any action or proceeding with respect to infringement of any AcelRx Patent and Joint Patent in the Territory, at its own expense and by counsel of its own choice and Grünenthal shall have the first right, as between AcelRx and Grünenthal, to bring and control any action or proceeding with respect to infringement of any Assigned Patent, at its own expense and by counsel of its own choice. The respective other Party shall have the right, at its own expense, to be represented in the Territory in any such action by counsel of its own choice, and the controlling Party and its counsel shall reasonably cooperate with the other Party and its counsel in strategizing, preparing and presenting any such action or proceeding in the Territory. If the Party having first right as set forth above fails to bring an action or proceeding with respect to infringement of any Patent in the Territory described in the preceding sentences within (i) ninety (90) days following the notice of alleged infringement or (ii) thirty (30) days before the time limit, if any, set forth in the appropriate laws and regulations for the filing of such actions, whichever comes first, the other Party shall have the right, but not the obligation (i.e., it has the right to indulge such infringement), to bring and control any such action in the Territory at its own expense and by counsel of its own choice. Upon the other Party’s request, the Party having first right as set forth above shall timely join as party-plaintiff in any such litigation and to cooperate with the other Party in connection with such infringement action, including timely filing such action in the name of the Party having first right as set forth above if required. The requesting Party shall reimburse the other Party for its reasonable costs and expenses related to such explicitly requested activities. Except as otherwise agreed to by the Parties as part of a separate cost-sharing arrangement, any recovery or damages realized as a result of such action or proceeding shall be used first to reimburse the Parties’ documented out-of-pocket legal expenses relating to the action or proceeding, and any remaining damages relating to the Licensed Product (including lost sales or lost profits with respect to the Licensed Product) shall be (a) shared 70%/30% for
39
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Grünenthal/AcelRx, respectively, in case AcelRx is the Party bringing suit; or (b) treated as Net Sales and subject to the payment obligations of Article 7, if Grünenthal is the Party bringing suit.
10.4 Infringement of Third Party Rights. Each Party shall promptly notify the other in writing of any allegation by a Third Party that the activity of either of the Parties pursuant to this Agreement infringes or may infringe the intellectual property rights of such Third Party in the Territory.
(a) AcelRx shall, at its own expense and by counsel of its own choice, have the sole right to control any defense of, and shall be solely responsible for, any and all such claims involving alleged infringement of Third Party rights in the Territory by any of (i) AcelRx’s activities, (ii) the Manufacture of the Licensed Products by, on behalf of or under license from AcelRx, (iii) sale of Licensed Products by AcelRx to any licensee of AcelRx including Grünenthal, and/or (iv) the importation for, sale or offering for sale of such Licensed Products as supplied by AcelRx. Grünenthal shall have the right, at its own expense, to be represented in any such action by counsel of its own choice.
(b) To the extent not covered by Section 10.4(a), Grünenthal shall, at its own expense and by counsel of its own choice, have the sole right to control any defense of, and shall be solely responsible for, any and all such claims involving alleged infringement of Third Party rights in the Territory by Grünenthal’s activities. AcelRx shall have the right, at its own expense, to be represented in any such action by counsel of its own choice.
10.5 Consent for Settlement. Neither Party shall enter into any settlement or compromise of any action or proceeding under this Article 10 which would materially alter, diminish, or be in derogation of the other Party’s rights under this Agreement without the prior written consent of such other Party, which consent shall not be unreasonably withheld.
10.6 Patent Term Extensions. AcelRx shall have the right to file any Patent Term Extensions for AcelRx Patents and Joint Patents in the Territory, and AcelRx shall act with reasonable promptness in light of the development stage of the Licensed Product to apply for any such Patent Term Extensions, where it is agreed upon between the Parties. Grünenthal shall cooperate fully with AcelRx in making such filings or actions, for example and without limitation, making available all required regulatory data and information and executing any required authorizations to apply for such Patent Term Extension. Grünenthal shall have the right to file any Patent Term Extensions for Assigned Patents in the Territory. All expenses incurred in connection with activities of each Party pursuant to this Section 10.6 shall be entirely borne by such Party.
10.7 Trademarks: General. The Licensed Product shall be sold in each country of the Territory under the AcelRx Trademark “Zalviso” unless such AcelRx Trademark is determined to be unacceptable to the respective competent Regulatory Authority for the country/countries of the Territory concerned, in which event AcelRx and Grünenthal will agree on another AcelRx Trademark (the “Alternative AcelRx Trademark”). If all AcelRx Trademarks are determined to be unacceptable to the respective competent Regulatory Authority in the Territory, then Grünenthal shall have the right to select a trademark owned by Grünenthal
40
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(the “Grünenthal Trademark”) for use with the Licensed Product in the Territory. Upon request of AcelRx, the Parties shall discuss whether Grünenthal may grant license rights under the Grünenthal Trademark to commercialize, sell, offer for sale the Licensed Product outside the Territory and under what terms. Grünenthal shall own the Assigned Trademark(s) in the relevant countries in the Territory, subject to prosecution and maintenance of such AcelRx Trademark with AcelRx’s consent, which consent shall not be unreasonably withheld, and subject to Sections 14.2, 14.3, 14.4 and 14.5, as applicable. AcelRx shall provide all reasonable assistance required by Grünenthal in connection therewith. AcelRx will have the right to use the AcelRx Trademarks used with the Licensed Product in connection with the supply of Licensed Product to Grünenthal. Grünenthal shall not use the Assigned Trademark(s) or the Grünenthal Trademark in connection with (i) the using, promotion, marketing, importing, distributing, selling or offering for sale of any product other than the Licensed Product nor (ii) in connection with the using, promotion, marketing, importing, distributing, selling or offering for sale of any product outside the Territory. The Assigned Trademarks shall be used in accordance with the quality guidelines of AcelRx to ensure that the use of such Assigned Trademarks in the Territory are maintained in a manner consistent with the quality standards of AcelRx applicable outside of the Territory.
10.8 Trademark Enforcement. The Party that owns (the “Trademark Owner”) the applicable Trademark (whether AcelRx Trademarks, Assigned Trademarks or Grünenthal Trademarks), shall have the right to take appropriate steps to protect its Trademark from all harmful or wrongful activities of Third Parties in the Territory. The steps the Trademark Owner may take include, but are not limited to, the filing and prosecution of: (a) litigation against infringement or unfair competition by Third Parties; (b) opposition proceedings against applications for trademark or service xxxx registration for marks that are confusingly similar to any one or more of such Trademarks; (c) cancellation proceedings against registration of marks that are confusingly similar to any one or more of such Trademarks; and (d) other appropriate administrative actions. The Trademark Owner shall have the right to include the other Party, at the Trademark Owner’s cost, in such litigation, opposition, cancellation or other proceedings when necessary or appropriate. Such other Party shall cooperate with the Trademark Owner in any such proceeding by providing oral testimony and documentary and other relevant evidence at reasonable cost to the Trademark Owner. Any amounts obtained in connection with any such proceeding (whether awarded by a court, received in settlement or otherwise) shall be paid to the Trademark Owner. If the Trademark Owner and the other Party mutually agree to jointly participate in any litigation or other proceeding with respect to such any Trademark, their respective responsibilities, contributions to the costs and their participation in any recoveries will be agreed upon in writing before undertaking such action. If the other Party desires to file litigation or other proceeding against a Third Party, and the Trademark Owner declines to commence such litigation or proceeding, the other Party shall be entitled to commence and prosecute the litigation or proceeding at its own expense, and shall be entitled to all monetary damages and other benefits received as a result, and in such event, at the other Party’s expense, the Trademark Owner shall cooperate with such other Party in the prosecution of such litigation or proceeding.
41
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
10.9 Trademarks: Defense of Claims of Infringement. The Trademark Owner shall at its cost defend claims that the use of its Trademarks in the Territory infringes the rights of a Third Party, and indemnify and hold the other Party harmless with respect to any such claims (except to the extent that such claims are indemnified by such other Party under Article 12, or relate to a breach of representation or warranty made by such other Party under Article 11). Such other Party shall have the right to participate in such defense at its own expense to protect its rights under this Agreement relating to such Trademarks. If such other Party is named as a party to such a claim and the Trademark Owner is not so named, such other Party shall tender such defense to the Trademark Owner and the Trademark Owner shall defend such action at its expense.
10.10 Trademark Settlements. The Trademark Owner shall be authorized to enter into an agreement, consent order or other resolution of any claim by or against such a Third Party with respect to its Trademarks, provided however that with regard to the Assigned Trademark only after consulting with the other Party, such consent not to be unreasonably withheld. In no event shall such other Party be authorized to enter into any agreement, consent order or other resolution of any claim by or against such a Third Party with respect to such Assigned Trademarks without the other Party’s prior written approval, which approval shall not be unreasonably withheld or delayed.
ARTICLE 11
REPRESENTATIONS, WARRANTIES AND COVENANTS
11.1 Mutual Representations, Warranties and Covenants. Each Party hereby represents and warrants to the other Party, as of the Effective Date, as follows:
(a) Duly Organized. Such Party is a corporation with restricted liability, duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization, is qualified to do business and is in good standing as a foreign corporation in each jurisdiction in which the conduct of its business or the ownership of its properties requires such qualification and failure to have such would prevent such Party from performing its obligations under this Agreement.
(b) Due Authorization; Binding Agreement. The execution, delivery and performance of this Agreement by such Party have been duly authorized by all necessary corporate or organizational action. This Agreement is a legal and valid obligation binding on such Party and enforceable in accordance with its terms and does not (i) to such Party’s knowledge and belief, violate any law, rule, regulation, order, writ, judgment, decree, determination or award of any court, governmental body or administrative or other agency having jurisdiction over such Party or (ii) conflict with, or constitute a default under, any agreement, instrument or understanding, oral or written, to which such Party is a party or by which it is bound.
(c) Consents. Such Party has obtained, or is not required to obtain, the consent, approval, order or authorization of any Third Party, or has completed, or is not required
42
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
to complete any registration, qualification, designation, declaration, or filing with, any Regulatory Authority or governmental authority, in connection with the execution and delivery of this Agreement and the performance by such Party of its obligations under this Agreement.
11.2 Representations, Warranties and Covenants of AcelRx. As used in this Section 11.2, “Best Knowledge” shall mean, as applied to AcelRx, that any of AcelRx’s executive officers knows of a particular fact or other matter. AcelRx represents and warrants to Grünenthal that as of the Effective Date:
(a) Right to Grant License. (i) AcelRx owns all right, title and interest in and to, or has a license, sublicense or otherwise permission to use and license, all of the AcelRx Technology free and clear of all encumbrances; (ii) AcelRx has not previously assigned, transferred, conveyed or otherwise encumbered or granted, and will not during the Term assign, transfer, convey or otherwise encumber its right, title and interest in any of the AcelRx Technology or any rights granted to Grünenthal hereunder for the development or commercialization of the Licensed Product in the Field in the Territory; (iii) specifically, there are no existing agreements, options, commitments, or rights, with, of or to any person to acquire or obtain any rights to, any of the AcelRx Technology for the development or commercialization of the Licensed Product in the Field in the Territory and (iv) no royalties, license fees or other payments are required to be paid to any Third Party in connection with the execution, delivery and performance of this Agreement, or in connection with the research, development, importation, use, sale, and offer for sale of the Device or the Licensed Product.
(b) Scope of License. Exhibit 1.7 and Exhibit 1.9 set forth true and complete lists of all AcelRx Patents and Trademarks included in AcelRx Trademarks and AcelRx Copyrights Controlled by AcelRx or its Affiliates as of the Effective Date. Exhibit 1.7 and Exhibit 1.9 also indicate the current status, date and country of filing and issuance. The AcelRx Patents and AcelRx Know-How constitute all intellectual property Controlled by AcelRx and its Affiliates that is necessary or reasonably useful for the research, development, importation, use, sale and offer for sale of the Licensed Product(s) in the Field in the Territory and to the Best Knowledge of AcelRx there is not any other Patent necessary for such purposes that is not Controlled by AcelRx (including any intellectual property Controlled by any Third Party supplier of the Device, Drug or the Licensed Product). All official fees, maintenance fees and annuities for the AcelRx Patents, AcelRx Trademarks and AcelRx Copyrights have been paid through the Effective Date.
(c) Patent Status and Trademark Status. To AcelRx’s Best Knowledge (i) all issued Patents listed on Exhibit 1.7 are in full force and effect, valid, subsisting and enforceable, and inventorship of each Patent is properly identified on such Patents; (ii) none of the Patents listed on Exhibit 1.7 is currently involved in any interference, reissue, reexamination, or opposition proceeding and (iii) neither AcelRx nor any of its Affiliates has received any written notice from any person, or has knowledge, of such actual or threatened proceeding and (bb) AcelRx has filed all trademark applications for the Trademark “Zalviso” and at least two alternative AcelRx Trademarks in all countries of the Territory. To AcelRx Best Knowledge none of the AcelRx Trademarks is subject to any opposition proceeding
43
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(d) Non-Infringement by Third Parties. to AcelRx’s Best Knowledge, there are no activities by Third Parties that would constitute infringement of the AcelRx Patents or misappropriation of the AcelRx Know-How.
(e) Non-Infringement of Third Party Rights. the commercialization, Manufacture, use, sale or importation of the Device or the Licensed Product(s) in the Field in the Territory does not infringe or misappropriate any Patent or other intellectual property Controlled by a Third Party. Neither AcelRx nor any of its Affiliates has received any written notice from any Person, or has knowledge of, any actual or threatened claim or assertion that the use or practice of the AcelRx Patents or AcelRx Know-How infringes or misappropriates the intellectual property rights of a Third Party.
(f) Non-Action or Claim. to AcelRx’s Best Knowledge, there are no actual, pending, alleged or threatened adverse actions, suits, claims, interferences or formal governmental investigations (i) involving the Device or the Licensed Product, including in connection with the conduct of any clinical trials or manufacturing activities, or (ii) questioning the validity of this Agreement or any action taken by AcelRx in connection with the execution of this Agreement, in each case, by or against AcelRx or any of its Affiliates in or before any court, Regulatory Authority or other governmental authority. There are no material unsatisfied judgments or outstanding orders, injunctions, decrees, stipulations or awards (whether rendered by a court, an administrative agency or an arbitrator) against AcelRx with respect to any AcelRx Technology, the Device or the Licensed Product.
(g) Employee Agreements. to AcelRx’s Best Knowledge, all current and former employees and consultants of AcelRx and its Affiliates who are or have been substantively involved in the design, review, evaluation or development of the AcelRx Know-How or AcelRx Patents have executed written contracts or are otherwise obligated to protect the confidential status and value thereof and to vest in AcelRx or its Affiliates exclusive ownership of the AcelRx Know-How or AcelRx Patents.
(h) Additional Legal Compliance.
(i) to AcelRx’s Best Knowledge, AcelRx and its Affiliates and any outsourcing company and contract research organization to which AcelRx or its Affiliates have subcontracted activities in connection with Device and the Licensed Product (the “Contractors”) have complied with all Applicable Laws, permits, governmental licenses, registrations, approvals, concessions, franchises, authorizations, orders, injunctions and decrees in the research, development, Manufacture and use of the Licensed Product and Device, and neither AcelRx nor any of its Affiliates or its Contractors has received any written notice from any governmental authority claiming that any such activities as conducted by them are not in such compliance.
(ii) no governmental authority (including the FDA) has commenced or, to AcelRx’s Best Knowledge, threatened to initiate any action to enjoin production of the Device or the Licensed Product at any facility, nor has AcelRx or any of its Affiliates or, to the Best Knowledge of AcelRx, any of its Contractors, received any notice to such effect.
44
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(iii) all development activities conducted by AcelRx and its Affiliates and Contractors relating to the Licensed Product and/or Device have been conducted in compliance with all Applicable Laws, including all GCPs, GLPs and GMPs when applicable.
(iv) to AcelRx’s Best Knowledge, no employee or agent of AcelRx or any of its Affiliates or Contractors has made an untrue statement of a material fact to any governmental authority with respect to the Licensed Product and/or Device (whether in any Regulatory Filings or otherwise), or failed to disclose a material fact to any governmental authority required to be disclosed with respect to the Licensed Product and/or Device.
(v) To AcelRx’s Best Knowledge, AcelRx has disclosed or otherwise provided Grünenthal with all information that would have, or would be reasonably likely to have, a material effect on the ability of Grünenthal to develop or commercialize the Licensed Product in the Field in the Territory under the terms and conditions of this Agreement and that relates to (A) the AcelRx Technology, (B) any Third Party intellectual property rights or claims that relate to the commercialization or development of the Licensed Product in the Territory, and (C) the safety or efficacy of the Device or the Licensed Product.
(i) Debarment. AcelRx is not debarred under the United States Federal Food, Drug and Cosmetic Act and it does not, and will not during the Term, employ or use the services of any Person who is debarred, in connection with the development, Manufacture or commercialization of the Device or the Licensed Product(s). In the event that AcelRx becomes aware of the debarment or threatened debarment of any Person providing services to AcelRx, including the Party itself and its Affiliates, Contractors, licensees or Sublicensees, which directly or indirectly relate to activities under this Agreement, Grünenthal shall be immediately notified in writing.
(j) Material Agreements. To AcelRx’s Best Knowledge, AcelRx is not in breach or default of any material agreement with a Third Party that is necessary or reasonably useful for the Manufacture, use, sale or importation of the Device and the Licensed Product(s) in the Field in the Territory (the “Material Agreements”) and will use its Commercially Reasonable Efforts to keep such Material Agreements in full force for the Term of this Agreement and in accordance with Section 14.5. AcelRx has not waived or allowed to lapse or terminate any of its rights under such Material Agreements.
(k) Disclaimer. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, OR ANY OTHER AGREEMENT CONTEMPLATED HEREUNDER, NEITHER PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AND EACH PARTY EXPRESSLY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY AND OF FITNESS FOR A PARTICULAR PURPOSE OR USE, NON-INFRINGEMENT, VALIDITY AND ENFORCEABILITY OF PATENTS, OR THE PROSPECTS OR LIKELIHOOD OF DEVELOPMENT OR COMMERCIAL SUCCESS OF THE PRODUCT.
45
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
ARTICLE 12
12.1 Indemnification of AcelRx. Grünenthal shall indemnify and hold harmless each of AcelRx and its Affiliates, and the directors, officers, shareholders and employees of such entities and the successors and assigns of any of the foregoing (the “AcelRx Indemnitees”), from and against any and all losses, liabilities, damages, penalties, fines, costs and expenses (including reasonable attorneys’ fees and other costs and expenses of litigation and settlements) (“Losses”) from any claims, actions, suits or proceedings brought by a Third Party (a “Third Party Claim”) incurred by any AcelRx Indemnitee, arising from, or occurring as a result of (a) gross negligence or willful misconduct of Grünenthal, its Affiliates, Sublicensees, Distributors or other subcontractors; (b) the research, development and regulatory activities relating to the Licensed Product conducted by or on behalf of Grünenthal, its Affiliates or Sublicensees (other than AcelRx and its Affiliates and licensees); and (c) any material breach of any representations, warranties or covenants by Grünenthal under this Agreement or the Supply Agreement; except to the extent such Third Party Claims fall within the scope of the indemnification obligations of AcelRx set forth in Section 12.2.
12.2 Indemnification of Grünenthal. AcelRx shall indemnify and hold harmless each of Grünenthal and its Affiliates and the directors, officers, shareholders, employees and agents of such entities and the successors and assigns of any of the foregoing (the “Grünenthal Indemnitees”), from and against any and all Losses from any Third Party Claims incurred by any Grünenthal Indemnitee, arising from, or occurring as a result of (a) gross negligence or willful misconduct of AcelRx, its Affiliates and or its subcontractors; (b) the research, development and regulatory activities, Manufacture, supply relating to the Licensed Product conducted by or on behalf of AcelRx, its Affiliates or Sublicensees (other than Grünenthal and its Affiliates and Sublicensees); and (c) any material breach of any representations, warranties or covenants by AcelRx under this Agreement or the Supply Agreement, except to the extent such Third Party Claims fall within the scope of the indemnification obligations of Grünenthal set forth in Section 12.1.
12.3 Procedure. A Party that intends to claim indemnification under this Article 12 or under Section 7.3(b)(i)(B) shall promptly notify the indemnifying Party in writing of any Third Party Claim, in respect of which the Indemnitee intends to claim such indemnification. The Indemnified Party shall provide the Indemnifying Party with reasonable assistance, at the Indemnifying Party’s expense, in connection with the defense of the Third Party Claim for which indemnity is being sought. The Indemnitee may participate in and monitor such defense with counsel of its own choosing at its sole expense; provided, however, the Indemnitor shall have the right to assume and conduct the defense of the Third Party Claim with counsel of its choice. The Indemnitor shall not settle any Third Party Claim without the prior written consent of the Indemnified Party, not to be unreasonably withheld, unless the settlement involves only the payment of money. So long as the Indemnitor is actively defending the Claim in good faith, the Indemnitee shall not settle any such Third Party Claim without the prior written consent of the Indemnifying Party. If the Indemnitor does not assume and conduct the defense of the Third Party Claim as provided above, (a) the Indemnitee may defend against, and consent to the entry of any judgment or enter into any settlement with respect to the Third
46
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Party Claim in any manner the Indemnitee may deem reasonably appropriate (and the Indemnitee need not consult with, or obtain any consent from, the Indemnitor in connection therewith), and (b) the Indemnitor will remain responsible to indemnify the Indemnitee as provided in this Article 12. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim shall only relieve the Indemnitor of its indemnification obligations under this Article 12 or under Section 7.3(b)(i)(B) if and to the extent the Indemnitor is actually prejudiced thereby.
12.4 Insurance. Each Party, at its own expense, shall maintain product liability and other appropriate insurance with an insurance carrier in an amount consistent with industry standards, for a company in a similar position to such Party, during the Term, which shall include, but not be limited to, (i) product liability insurance, which may include a self-insured retention, and (ii) general liability insurance in the minimum amount of $2 million in the aggregate and $10 million umbrella coverage, which may include a self-insured retention. Each Party shall provide a certificate of insurance or other reasonably satisfactory documentation evidencing such coverage to the other Party upon request. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Article 12 or under Section 7.3(b)(i)(B).
12.5 Set-Off. If and to the extent either Party is in material breach of this Agreement or the Supply Agreement for which notification has been provided and such Party fails to pay, reimburse, or credit the other Party for any amount owed when due under this Agreement or the Supply Agreement, whether under this Article 12 or under Section 7.3(b)(i)(B) or otherwise due under or in connection with this Agreement or the Supply Agreement, then the Party to whom such amount is owed may, at its election, without notice of its election and without demand, charge and setoff such amount against amounts otherwise due from it or its related entities to the other Party, including under this Agreement or the Supply Agreement, and the owing Party hereby authorizes all such charges and setoffs until such time as the material breach has been cured in accordance with the terms of this Agreement.
ARTICLE 13
13.1 Term. This Agreement shall commence on the Effective Date, and unless terminated earlier as provided in this Article 13 or 2.8, shall continue in full force and effect on a country-by-country basis until the later of (i) expiration of the Royalty Term, and (ii) expiration of Grünenthal’s obligation to pay the Trademark and Supply Fee to AcelRx as described in Section 7.4 (the “Term”).
13.2 Early Termination. Each Party shall have the right to terminate this Agreement before the end of the Term:
(a) in its entirety or on a country-by-country basis by mutual written agreement of the Parties;
47
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(b) with regard to the country/countries concerned upon written notice by either Party if the other Party is in material breach of this Agreement and has not cured such breach within ninety (90) days (30 days with respect to any payment breach) after notice from the terminating Party requesting cure of the breach. Any such termination shall become effective at the end of such ninety (90) day (30 day with respect to any payment breach) period unless the breaching Party has cured any such breach or default prior to the end of such period;
(c) in its entirety upon the bankruptcy or insolvency of, or the filing of an action to commence insolvency proceedings against the other Party, or the making or seeking to make or arrange an assignment for the benefit of creditors of the other Party, or the initiation of proceedings in voluntary or involuntary bankruptcy, or the appointment of a receiver or trustee of such Party’s property, in each case that is not discharged within one hundred twenty (120) days.
13.3 Additional Grünenthal Termination Right. Grünenthal shall have the right to terminate this Agreement in its entirety, for any or no reason upon one hundred eighty (180) days written notice.
ARTICLE 14
Effect of Expiration or Termination
14.1 Accrued Obligations. The expiration or termination of this Agreement, in whole or part, for any reason shall not release either Party from any liability which, at the time of such expiration or termination, has already accrued to such Party or which is attributable to a period prior to such expiration or termination, nor will any expiration or termination of this Agreement preclude either Party from pursuing all rights and remedies it may have under this Agreement, at law or in equity, with respect to breach of this Agreement. In particular, in the event this Agreement is terminated for any reason after the achievement of a particular milestone event, then Grünenthal shall have the obligation to make the milestone payment corresponding to such milestone event to AcelRx, regardless of whether the payment date of such accrued milestone payment occurs prior to, on or after the effective date of such termination.
48
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
14.2 Effects of Expiration. Upon expiration of this Agreement in a country, Grünenthal shall continue to have under the Assigned Trademarks (i) an exclusive, royalty-free, fully-paid license to commercialize, sell, offer for sale the Licensed Product in the Field in the Territory, and (ii) a co-exclusive (with AcelRx only), royalty-free, fully-paid license to research, develop, register, make, have made, use and import the Licensed Product in the Field in the Territory; provided, that in consideration for the continuing licenses and rights granted in this Section 14.2, Grünenthal shall pay to AcelRx the Trademark and Supply Fee; provided, further, that Section 2.6 shall not apply to AcelRx in such expired country.
14.3 Effects of Termination for Cause by AcelRx or Termination by Grünenthal under Section 13.3 or on a Country-Specific Basis under Section 13.2(a), 13.2(b) or 13.4. Upon the early termination of this Agreement by Grünenthal under Section 13.3 or termination by AcelRx under Section 13.2(b) or 2.8 or termination by mutual agreement under Section 13.2(a), the following shall apply (and references to Territory shall be deemed references to the country or countries only that are terminated under Sections 13.2(a), 13.2(b) or 2.8, as applicable):
(a) Winding Down of Development Activities. In the event there are any on-going clinical trials of the Licensed Product in the Field in the Territory,
(i) The Parties shall work together in good faith to adopt, and AcelRx shall have the final decisional power with respect to, a plan to wind down the development activities in an orderly fashion, with due regard for patient safety and the rights of any subjects that are participants in any clinical trials of the Licensed Product and take any actions it deems reasonably necessary or appropriate to avoid any human health or safety problems and in compliance with all Applicable Laws, provided that AcelRx shall not use its decision power to increase Grünenthal’s costs in winding down such development activities;
(ii) Each Party shall perform its outstanding non-cancellable obligations under the Development Plan that existed or accrued prior to the notice date of termination; and
(iii) All costs and expenses incurred from the effective date of the termination notice in winding down the development activities of Territory Specific Trials with respect to the applicable the Licensed Product(s) shall be borne solely by Grünenthal, of US-specific trials by AcelRx and for all other trials the costs shall be split equally between the Parties; provided, however, that in no case shall Grünenthal be obligated to pursue or support such activities for a period exceeding twelve (12) months after the date of notice of such termination.
(b) Inventory. To the extent ethical to do so given the then views of the Parties regarding the safety and efficacy of the Licensed Product, Grünenthal, its Affiliates, Distributors and Sublicensees shall continue, to the extent that Grünenthal, its Affiliates, Distributors and Sublicensees continue to have stocks of usable the Licensed Product, to fulfill orders received from customers for the Licensed Product in the Field in the Territory for more than six (6) months after the date of notice of termination. For the Licensed Product sold by
49
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Grünenthal after the effective date of a termination (i.e., after the expiration of the applicable termination notice period), Grünenthal shall continue to pay royalties on the amount of Net Sales of such the Licensed Product.
(c) Assignment to AcelRx. At AcelRx’s option, which shall be exercised by written notice to Grünenthal, to the extent permitted under Applicable Laws, Grünenthal shall assign or cause to be assigned to AcelRx or its designee (or to the extent not so assignable, Grünenthal shall take all reasonable actions to make available to AcelRx or its designee the benefits of): (i) all Regulatory Filings (including INDs, NDAs and Marketing Approvals) for the Licensed Product in the Territory, including any such Regulatory Filings made or owned by its Affiliates or Sublicensees, (ii) the Assigned Patents, and (iii) the Assigned Trademarks. AcelRx shall notify Grünenthal before the effective date of termination, whether the foregoing should be assigned to AcelRx or its designee, and if the latter, identify the designee, and provide Grünenthal with all necessary details to enable Grünenthal to effect the assignment (or availability). If AcelRx fails to provide such notification prior to the effective date of termination, Grünenthal shall have no obligation to assign such subject matter to AcelRx and Grünenthal shall be free to utilize, abandon or transfer to Third Parties any such subject matter.
(d) Grants by Grünenthal to AcelRx. Grünenthal hereby grants AcelRx, effective upon the effective date of such termination, a fully paid, royalty free, non-exclusive license, with the right to grant sublicenses, under any and all Patents and Know-How Controlled by Grünenthal or its Affiliates and used or incorporated into the Licensed Product at the time of such termination for AcelRx to make, have made, use, sell, offer for sale and import the Licensed Product in the Field in the Territory. Upon termination of this Agreement in a country, Grünenthal shall assign or cause to be assigned to AcelRx or its designee the Assigned Trademarks in such country and Section 2.6 shall not apply to AcelRx in such terminated country.
(e) Supply Agreement. In addition, Grünenthal may terminate the Supply Agreement, effective upon the effective date of the termination of this Agreement. To the extent of any transfer of Manufacturing of the Licensed Product prior to such termination to Grünenthal or Third Parties under contract with Grünenthal, the Parties shall discuss and cooperate with the termination, unwinding and/or transfer of such Manufacturing of the Licensed Product back to AcelRx and/or Third Parties designated by it.
(f) Transition. Both Parties shall use Commercially Reasonable Efforts to cooperate with the other Party to effect a smooth and orderly transition in the development, sale and marketing, promotion and commercialization of the Licensed Product in the Territory during the notice and the wind-down periods. AcelRx may use, identify and finalize an agreement or other arrangement with a Third Party in relation to the Licensed Product and/or, to the extent AcelRx is able to take over such activities under Applicable Laws, take over, directly or through an Affiliate, all activities related to the Licensed Product, and in particular development activities on-going at the time of the effective date of the termination and the transfer of the Regulatory Filings (including INDs, XXXx and Marketing Approvals) into the name of AcelRx or AcelRx’s designee so that the wind-down period will be as limited as possible; provided that in no event
50
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
shall Grünenthal be obligated to assist or provided cooperation under this subsection (f) after one hundred eighty (180) days following any such termination of this Agreement.
(g) Survival of Sublicense. Upon Grünenthal’s request, AcelRx shall allow Grünenthal’s Sublicensees the continuation of their sublicense agreements directly with AcelRx if such Sublicensee is not in breach of its Grünenthal sublicense agreement, and Grünenthal shall either assign or cooperate in the transfer of such sublicense to AcelRx. For clarity, this Section 14.3(g) shall not apply to sublicensees that are Affiliates of Grünenthal.
14.4 Effects of Termination for Cause by Grünenthal. Upon the early termination of this Agreement by Grünenthal under Section 7.3(c), 13.2(b) or 13.2(c) the following shall apply (in addition to any other rights and obligations under this Agreement with respect to such termination):
(a) Winding Down of Development Activities. In the event there are any on-going clinical trials of the applicable Licensed Product(s) in the Field in the Territory,
(i) The Parties shall work together in good faith to adopt, and Grünenthal shall have the final decisional power with respect to, a plan to wind down the development activities in an orderly fashion, with due regard for patient safety and the rights of any subjects that are participants in any clinical trials of the Licensed Products and take any actions it deems reasonably necessary or appropriate to avoid any human health or safety problems and in compliance with all Applicable Laws;
(ii) Each Party shall perform its outstanding non-cancellable obligations under the Development Plan that existed or accrued prior to the notice date of termination; and
(iii) All costs and expenses incurred from the effective date of the termination notice in winding down the development activities with respect to the applicable Licensed Product shall be borne solely by AcelRx; provided, however, that in no case shall AcelRx be obligated to pursue or support such activities for a period exceeding twelve (12) months after the date of notice of such termination.
(b) License under AcelRx Technology. Grünenthal may elect to have all or any portion of the licenses granted to Grünenthal pursuant to Section 2.1 continue in effect, in which case Grünenthal’s obligations to AcelRx under Article 7 of this Agreement and AcelRx’s rights under Article 7 shall continue to the extent that Grünenthal has not terminated its rights in an applicable country(ies).
(c) Assignment of AcelRx Regulatory Filings (including Marketing Approvals). If Grünenthal elects to continue the license under Section 2.1, at Grünenthal’s option, which shall be exercised by written notice to AcelRx, to the extent permitted under Applicable Laws, AcelRx shall assign or cause to be assigned to Grünenthal or its designee (or to the extent not so assignable, AcelRx shall take all reasonable actions to make available to Grünenthal or its designee the benefits of) all Regulatory Filings (including INDs, XXXx and
51
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Marketing Approvals) for the Licensed Product(s) in the applicable country(ies) in the Territory, including any such Regulatory Filings made or owned by its Affiliates and/or Distributors or licensees. Grünenthal shall notify AcelRx before the effective date of termination, whether the Regulatory Filings should be assigned to Grünenthal or its designee and, if the latter, identify the designee, and provide AcelRx with all necessary details to enable AcelRx to effect the assignment (or availability). If Grünenthal fails to provide such notification prior to the effective date of termination, AcelRx shall have no obligation to assign the Regulatory Filings to Grünenthal.
(d) Transition Assistance. AcelRx shall provide such assistance, at no cost to Grünenthal, as may be reasonably necessary or useful for Grünenthal to commence or continue developing or commercializing the applicable Licensed Products in the applicable countries of the Territory, to the extent AcelRx is then performing or having performed such activities, including without limitation transferring or amending as appropriate, upon request of Grünenthal, any agreements or arrangements with Third Party suppliers or vendors to supply or sell the Device and/or applicable Licensed Products.
(e) Grünenthal Regulatory Filings (including Marketing Approvals) In the event Grünenthal elects not to pursue the development or commercialization of the applicable Licensed Product(s) in the applicable country(ies), upon Grünenthal’s request and to the extent permitted by Applicable Laws, AcelRx shall purchase all Regulatory Filings (including Marketing Approvals) that are owned by Grünenthal for the applicable Licensed Product for the applicable countries at an amount equal to the actual direct costs incurred by Grünenthal in obtaining, maintaining and transferring such Regulatory Filings.
(f) Grants by Grünenthal to AcelRx. Grünenthal hereby grants AcelRx, effective upon the effective date of such termination in the event Grünenthal elects not to continue the development and commercialization of the Licensed Product after such termination, a royalty free and exclusive license, with the right to grant sublicensees, under any and all Patents (to the extent not previously assigned) and Know-How Controlled by Grünenthal or its Affiliates and incorporated into the Licensed Product at the time of such termination for AcelRx to make, have made, use, sell, offer for sale and import Licensed Products in the Field in the Territory. Upon termination of this Agreement in a country, Grünenthal shall assign or cause to be assigned to AcelRx or its designee the Assigned Trademarks in such country. and Section 2.6 shall not apply to AcelRx in such terminated country.
14.5 Licensed Product Supply and Technology Transfer. At any time prior to the expiration of this Agreement or effective date of any termination or partial termination of this Agreement by Grünenthal under Section 13.2(b) or (c), upon request of Grünenthal, the Parties shall agree upon a transition plan of Manufacturing of the Licensed Product in the Territory to minimize any disruption to the research, development, importation, Manufacture, having Manufactured, use, sale, having sold and offering for sale of the Licensed Product in the Territory. The transition plan shall include a mutually agreed-upon schedule for transition activities, under which the transfer of manufacturing-related AcelRx Know-How shall occur at AcelRx’s cost and expense. The Parties shall conduct transition activities pursuant to the
52
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
transition plan. Grünenthal shall cooperate with AcelRx on such transfer, shall promptly undertake to complete the transfer and shall be responsible for additional costs that may be incurred for failure by Grünenthal to timely cooperate in accordance with the transition plan. In addition, upon Grünenthal’s request, following the expiration of this Agreement or any termination or partial termination of this Agreement by Grünenthal under Section 13.2(b) or (c), AcelRx shall continue to supply Grünenthal and its Affiliates and Sublicensees with their requirements of the Licensed Product, pursuant to the Supply Agreement then in effect between the Parties [ * ], which Supply Agreement shall remain in effect until the earlier of (i) the [ * ] of the effective date of expiration or termination, or (ii) such time as Grünenthal notifies AcelRx that Grünenthal or a Third Party manufacturer engaged by Grünenthal is capable of supplying the Licensed Product.
14.6 Rights Upon Bankruptcy. All rights and licenses granted under or pursuant to this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of Title 11 of the United States Code and other similar laws in any jurisdiction in the Territory or where a Party is situated (collectively, the “Bankruptcy Laws”), licenses of rights to “intellectual property” as defined under the Bankruptcy Laws. If a case is commenced during the Term by or against a Party under Bankruptcy Laws then, unless and until this Agreement is rejected as provided in such Bankruptcy Laws, such Party (in any capacity, including debtor-in-possession) and its successors and assigns (including a trustee) shall perform all of the obligations provided in this Agreement to be performed by such Party. If a case is commenced during the Term by or against a Party under the Bankruptcy Laws, this Agreement is rejected as provided in the Bankruptcy Laws and the other Party elects to retain its rights hereunder as provided in the Bankruptcy Laws, then the Party subject to such case under the Bankruptcy Laws (in any capacity, including debtor-in-possession) and its successors and assigns (including a Title 11 trustee), shall provide to the other Party copies of all information necessary for such other Party to prosecute, maintain and enjoy its rights under the terms of this Agreement promptly upon such other Party’s written request therefor. All rights, powers and remedies of the non-bankrupt Party as provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Laws) in the event of the commencement of a case by or against a Party under the Bankruptcy Laws. In particular, it is the intention and understanding of the Parties to this Agreement that the rights granted to the Parties under this Section 14.6 are essential to the Parties’ respective businesses and the Parties acknowledge that damages are not an adequate remedy.
14.7 Return of Confidential Information. Upon termination or expiration of this Agreement, except to the extent necessary or reasonably useful for a Party to exercise its rights under any license surviving such termination or expiration, each Party shall promptly return to the other Party, or delete or destroy, all relevant records and materials in such Party’s possession or control containing Confidential Information of the other Party; provided that such Party may keep one copy of such materials for archival purposes only.
14.8 Survival. Expiration or termination of this Agreement shall not relieve the Parties of any rights or obligation accruing prior to such expiration or termination. In addition, upon expiration or termination of this Agreement, all rights and obligations of the Parties under
53
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
this Agreement shall terminate, except those described in the following Articles and Sections: 7.3(b)(i)(B), 9, 12 (to the extent any Third Party Claims (X) arose prior to the effective date of termination, (Y) relate to Licensed Products sold to Third Parties prior to effective date of termination, or (Z) relate to Licensed Products Manufactured and supplied to Grünenthal prior to effective date of termination), 14, 15 and 16.
ARTICLE 15
DISPUTE RESOLUTION AND GOVERNING LAW
15.1 Dispute Resolution Process. The Parties recognize that disputes as to certain matters may from time to time arise during the Term that relate to interpretation of a Party’s rights and/or obligations hereunder or any alleged breach of this Agreement. If the Parties cannot resolve any such dispute within thirty (30) days after written notice of a dispute from one Party to another, either Party may, by written notice to the other Party, have such dispute referred to the Chief Executive Officer of AcelRx and a Member of the Executive Board of Grünenthal (collectively, the “Senior Executives”). The Senior Executives shall negotiate in good faith to resolve the dispute within thirty (30) days. During such period of negotiations, any applicable time periods under this Agreement shall be tolled. If the Senior Executives are unable to resolve the dispute within such time period, either Party may pursue any remedy available to such Party at law or in equity, subject to the terms and conditions of this Agreement and the other agreements expressly contemplated hereunder. Notwithstanding anything in this Article 15 to the contrary, AcelRx and Grünenthal shall each have the right to apply to any court of competent jurisdiction for appropriate interim or provisional relief, as necessary to protect the rights or property of that Party.
15.2 Governing Law. This Agreement and all questions regarding the existence, validity, interpretation, breach or performance of this Agreement, shall be exclusively governed by, and construed and enforced in accordance with, the laws of [*], without reference to its conflicts of law principles.
(a) Any disputes arising in connection with this Agreement shall be finally settled under the Rules of Arbitration of the International Chamber of Commerce (“ICC”) by three arbitrators appointed in accordance with the said Rules.
(b) Each of the Parties shall nominate an arbitrator and these two arbitrators shall endeavor to agree on the third arbitrator, who shall act as chairman of the arbitral tribunal, within 30 days from the date when both Parties have received from the ICC confirmation of the second arbitrator by the ICC Court. The place of arbitration shall be Geneva, Switzerland. The language of the arbitration proceedings shall be English. The decision and award of the arbitral tribunal shall be final and binding on the parties to the arbitration proceedings.
(c) Either Party also may, without waiving any remedy under this Agreement, seek from any court having jurisdiction any injunctive or provisional relief necessary to protect
54
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
its rights hereunder. The arbitrators shall have no authority to award punitive or any other type of damages not measured by a Party’s compensatory damages. Each Party shall bear its own costs and expenses and attorneys’ fees and an equal share of the arbitrators’ fees and any administrative fees of arbitration.
(d) The Parties agree that, in the event of a dispute over the nature or quality of performance under this Agreement, neither Party may terminate this Agreement until final resolution of the dispute through arbitration determination. The Parties further agree that any payments made pursuant to this Agreement pending resolution of the dispute shall be refunded if an arbitrator determines that such payments are not due.
ARTICLE 16
16.1 Intervening Events. If the performance of any part of this Agreement by either Party is prevented, restricted, interfered with or delayed by any reason or cause beyond the reasonable control of such Party (including fire, flood, embargo, power shortage or failure, acts of war, insurrection, riot, terrorism, strike, lockout or other labor disturbance, shortage of raw materials, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, or storm or like catastrophe, acts of God or any acts, omissions or delays in acting of the other Party) (an “Intervening Event”), the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such Intervening Event, provided that the affected Party shall use its substantial efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed.
(a) Notification. If either Party becomes aware that such an Intervening Event has occurred or is imminent or likely, it shall immediately notify the other.
(b) Efforts to Overcome. The Party which is subject to such Intervening Event shall exert all reasonable efforts to overcome it.
(c) Keeping the Other Informed. Such Party shall keep the other informed as to the progress of overcoming such Intervening Event.
16.2 Waiver of Breach. No delay or waiver by either Party of any condition or term in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term.
16.3 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to perform all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
16.4 Affiliates; Continuing Responsibility. Either Party shall have the right to assign, sublicense, subcontract or delegate this Agreement or any or all of its obligations or
55
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
rights hereunder to an Affiliate upon written notice to the other Party; provided, however, the assigning, sublicensing, subcontracting or delegating Party hereby guarantees and shall remain fully and unconditionally obligated and responsible for the full and complete performance of this Agreement by such Affiliate and in no event shall such assignment, sublicensing, subcontracting or delegation be deemed to relieve such Party’s liabilities or obligations to the other Party under this Agreement. The other Party shall, at the request of the assigning, sublicensing, subcontracting or delegating Party, enter into such supplemental agreements with the applicable Affiliates as may be necessary or advisable to permit such Affiliates to avail itself of any rights or perform any obligations of the assigning, sublicensing, subcontracting or delegating Party hereunder.
16.5 Modification. No amendment or modification of any provision of this Agreement shall be effective unless in a prior writing signed by both Parties hereto. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance or any other matter not set forth in an agreement in writing and signed by both Parties hereto.
16.6 Severability. In the event any provision of this Agreement should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith and enter into a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties. All other provisions of this Agreement shall remain in full force and effect in such jurisdiction. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
16.7 Entire Agreement. This Agreement (including the Exhibits attached hereto and any letter delivering information referenced herein), the Supply Agreement, the Pharmacovigilance Agreement and the Quality Agreement constitute the entire agreement between the Parties relating to the subject matter hereof and supersede and cancel all previous express or implied agreements and understandings, negotiations, writings and commitments, either oral or written, in respect of the subject matter hereof. Each of the Parties acknowledges and agrees that in entering into this Agreement, and the documents referred to in it, it does not rely on, and shall have no remedy in respect of, any statement, representation, warranty or understanding (whether negligently or innocently made) of any person (whether party to this Agreement or not) other than as expressly set out in this Agreement and nothing in this clause shall, however, operate to limit or exclude any liability for fraud. In the event of a conflict or inconsistency between the provisions of this Agreement and the provisions of the Supply Agreementthis Agreement will prevail. In the event of a conflict or inconsistency between the provisions of this Agreement and any legal or regulatory requirements applicable for the Territory, Amendments to this Agreement shall be considered promptly in good faith in order to meet such requirements.
16.8 Language. The language of this Agreement and all activities to be pursued under this Agreement is English. Any and all documents proffered by one Party to the other in fulfillment of any provision of this Agreement shall only be in compliance if in English. Any translation of this Agreement in another language shall be deemed for convenience only and shall never prevail over the original English version. This Agreement is established in the English language.
56
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
16.9 Notices. Any notice or communication required or permitted under this Agreement shall be in writing in the English language, delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), sent by internationally-recognized courier or sent by registered or certified mail, postage prepaid to the following addresses of the Parties (or such other address for a Party as may be at any time thereafter specified by like notice):
Any such notice shall be deemed to have been given (a) when delivered if personally delivered; (b) on the next Business Day after dispatch if sent by confirmed facsimile or by internationally-recognized overnight courier; and/or (c) on the fifth (5th) Business Day following the date of mailing if sent by mail or other internationally-recognized courier. Notices hereunder will not be deemed sufficient if provided only between or among each Party’s representatives on the Joint Steering Committee.
16.10 Assignment; Change of Control of AcelRx.
(a) Subject to Section 16.4, this Agreement shall not be assignable or otherwise transferred, nor may any rights or obligations hereunder be assigned or transferred, by either Party to any Third Party without the prior written consent of the other Party; except that either Party may assign or otherwise transfer this Agreement without the consent of the other Party to an entity that acquires all or substantially all of the business or assets of the assigning Party relating to the subject matter of this Agreement, whether by merger, acquisition or otherwise. Subject to the foregoing, this Agreement shall inure to the benefit of each Party, its successors and permitted assigns. Any assignment of this Agreement in contravention of this Section 16.10 shall be null and void.
57
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(b) If AcelRx is subject to a Change of Control in which the party effecting the Change of Control of AcelRx is a competitor of Grünenthal, the following provisions shall apply:
(i) Grünenthal shall notify AcelRx of its belief that such party effecting the Change of Control of AcelRx is a competitor of Grünenthal and the Parties shall discuss such view and protective measures to ensure that Grünenthal Confidential Information is not accessible to such competitor. If the Parties are unable to agree upon a procedure within thirty (30) days of notification from Grünenthal, AcelRx will, and it will cause its Affiliates to, ensure that (A) no Grünenthal Confidential Information is disclosed to any Affiliate of AcelRx that becomes a AcelRx Affiliate as a result of such Change of Control or any representatives of such Affiliates, unless, in each case, such Affiliate or representatives, as applicable, have signed individual confidentiality agreements which include equivalent obligations to those set out in Article 9, and (B) no Grünenthal Confidential Information is disclosed whatsoever to any representatives of the acquiror or its Affiliates who are actively engaged in, or have direct supervisory responsibilities with respect to, the development or commercialization of any products for the treatment of pain.
(ii) If AcelRx is subject to a Change of Control, then, at Grünenthal’s discretion, effective as of the date of such Change of Control, the JSC shall be deemed to be automatically terminated with no further duties, rights or obligations under this Agreement, and to the extent of any authority granted to the JSC hereunder.
(iii) If AcelRx disputes that the Change of Control involves a competitor, then the determination shall be subject to dispute resolution in accordance with Article 15.
16.11 No Partnership or Joint Venture. Nothing in this Agreement or any action which may be taken pursuant to its terms is intended, or shall be deemed, to establish a joint venture or partnership between Grünenthal and AcelRx. Neither Party to this Agreement shall have any express or implied right or authority to assume or create any obligations on behalf of, or in the name of, the other Party, or to bind the other Party to any contract, agreement or undertaking with any Third Party.
16.12 Interpretation. The captions to the several Articles and Sections of this Agreement are not a part of this Agreement but are included for convenience of reference and shall not affect its meaning or interpretation. In this Agreement (a) “include”, “includes” and “including” are not limiting and shall be deemed to be followed by the phrase “without limitation” or like expression; (b) the singular shall include the plural and vice versa; (c) references to an agreement, statute or instrument mean such agreement, statute or instrument as from time to time amended, modified or supplemented; (d) references to a Person are also to its permitted successors and assigns; (e) the word “will” shall be construed to have the same meaning and effect as the word “shall”; and (f) the word “any” shall mean “any and all” unless otherwise indicated by context; and (g) masculine, feminine and neuter pronouns and expressions shall be interchangeable. Each accounting term used herein that is not specifically
58
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
defined herein shall have the meaning given to it under Accounting Standards consistently applied, but only to the extent consistent with its usage and the other definitions in this Agreement.
16.13 Counterparts. This Agreement may be executed in any number of counterparts each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
16.14 Limitation of Liability. EXCEPT FOR LIABILITY FOR BREACH OF ARTICLE 9, NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THIS AGREEMENT OR ANY LICENSE GRANTED HEREUNDER; PROVIDED, HOWEVER, THAT THIS SECTION 16.14 SHALL NOT BE CONSTRUED TO LIMIT EITHER PARTY’S INDEMNIFICATION OBLIGATIONS UNDER SECTION 7.3(b)(i)(B) AND ARTICLE 12.
ARTICLE 17
17.1 Export Laws. Notwithstanding anything to the contrary contained herein, all obligations of AcelRx and Grünenthal are subject to prior compliance with export and import regulations and such other laws and regulations in effect in such jurisdictions or any other relevant country as may be applicable, and to obtaining all necessary approvals required by the applicable agencies of the governments of any relevant countries. AcelRx and Grünenthal shall cooperate with each other and shall provide assistance to the other as reasonably necessary to obtain any required approvals.
17.2 Securities Laws. Each of the Parties acknowledges that it is aware that the securities laws of the United States and other countries prohibit any person who has material non-public information about a publicly listed company from purchasing or selling securities of such company or from communicating such information to any person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities. Each Party agrees to comply with such securities laws and make its Affiliates, licensees, Distributors, Sublicensees, employees, Contractors and agents aware of the existence of such securities laws and their need to comply with such laws.
17.3 Certain Payments. Each of the Parties acknowledges that it is aware that the United States and other countries have stringent laws which prohibit persons directly or indirectly from making unlawful payments to, and for the benefit of, government officials and related parties to secure approvals or permission for their activities. Each Party agrees that it will make no such prohibited payments, it will not indirectly make or have made such payments and it will make its Affiliates, employees and agents aware of the existence of such laws and their need to comply with such laws.
59
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
17.4 Anti-Bribery and Anti-Corruption Compliance.
(a) Each Party agrees, on behalf of itself, its officers, directors and employees and shall cause its Affiliates, agents, representatives, consultants and subcontractors hired in connection with the subject matter of this Agreement (together with such Party, the “Representatives”) to agree that for the performance of its obligations hereunder:
(i) The Representatives shall not directly or indirectly pay, offer or promise to pay, authorize the payment of any money or give, offer or promise to give, or authorize the giving of anything else of value, to: (a) any government official in order to influence official action; (b) any individual or entity (whether or not a government official) (1) to influence such individual or entity to act in breach of a duty of good faith, impartiality or trust (“acting improperly”), (2) to reward such individual or entity for acting improperly or (3) where such individual or entity would be acting improperly by receiving the money or other thing of value; (c) any individual or entity (whether or not a government official) while knowing or having reason to know that all or any portion of the money or other thing of value will be paid, offered, promised or given to, or will otherwise benefit, a government official in order to influence official action for or against either Party in connection with the matters that are the subject of this Agreement; or (d) any individual or entity (whether or not a government official) to reward that individual or entity for acting improperly or to induce that individual or entity to act improperly.
(ii) The Representatives shall not, directly or indirectly, solicit, receive or agree to accept any payment of money or anything else of value in violation of the Anti-Corruption Laws.
(b) The Representatives shall comply with the Anti-Corruption Laws and shall not take any action that will, or would reasonably be expected to, cause either Party or its Affiliates to be in violation of any such laws or policies.
(c) Each Party, on behalf of itself and its other Representatives, represents and warrants to the other Party that to the best of such Party’s and its Affiliates’ knowledge, no Representative will participate or support its performance of its obligations hereunder has, directly or indirectly, (i) paid, offered or promised to pay or authorized the payment of any money, (ii) given, offered or promised to give or authorized the giving of anything else of value or (iii) solicited, received or agreed to accept any payment of money or anything else of value, in each case ((i), (ii) and (iii)), in violation of the Anti-Corruption Laws during the three (3) years preceding the date of this Agreement.
(d) Each Party shall promptly provide the other Party with written notice of the following events: (i) upon becoming aware of any breach or violation by such Party or its Representative of any representation, warranty or undertaking set forth in Sections 17.4(a)-(c); or (ii) upon receiving a formal notification that it is the target of a formal investigation by a governmental authority for a material Anti-Corruption Law violation or upon receipt of information from any of the Representatives connected with this Agreement that any of them is the target of a formal investigation by a governmental authority for a material Anti-Corruption Law violation.
60
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(e) Without prejudice to any auditing or inspection rights set forth elsewhere in this Agreement, each Party shall for the term of this Agreement and six (6) years thereafter, for the purpose of allowing the other Party to audit and monitor the performance of its compliance with this Agreement and particularly this Section 17.4 permit the other Party, its Affiliates, any auditors of any of them and any governmental authority to have reasonable access to any premises of such Party or other Representatives used in connection with this Agreement, together with a right to reasonably access personnel and records that relate to this Agreement. The results of any such audit shall constitute Confidential Information of the audited Party, in respect of which the other Party shall comply with the provisions contained in Article 9 (subject to the terms and exceptions set forth therein or in this Section 17.4).
(i) To the extent that any audit by a Party requires access and review of any commercially or strategically sensitive information of the other Party or any of its other Representatives relating to the business of such Party or any other Representatives (including information about prices and pricing policies, cost structures and business strategies), such activity shall be carried out by a Third Party professional advisor appointed by the other Party and such professional advisors shall only report back to the other Party such information as is directly relevant to informing the other Party on such Party’s compliance with the particular provisions of the Agreement being Audited.
(ii) Each Party shall, and shall cause its Representatives to, provide all cooperation and assistance during normal working hours as reasonably requested by the other Party for the purposes of an Audit. Such other Party shall ensure that any Third Party auditor enters into a confidentiality agreement consistent with applicable requirements of Article 12 hereof in all material respects. Such other Party shall instruct any Third Party auditor or other Person given access in respect of an Audit to cause the minimum amount of disruption to the business of the audited Party and its Affiliates and to comply with relevant building and security regulations.
(iii) The costs and fees of any Audit shall be paid by the auditing Party, except that if an inspection or Audit reveals any breach or violation by the audited Party (including through its other Representatives) of any representation, warranty or undertaking set forth in Sections 17.4(a)-(c), the costs of such inspection or Audit shall be paid by the audited Party. The audited Party shall bear its own costs of rendering assistance to the Audit.
(f) On the occurrence of any of the following events: (A) A Party becomes aware of, whether or not through an Audit, that the other Party (or any other Representative) is in breach or violation of any representation, warranty or undertaking in Sections 17.4(a)-(c) or of the Anti-Corruption Laws; or (B) notification is received under Section 17.4(d) relating to any suspected or actual material Anti-Corruption Law violation by a Party or its Representative, in either case ((A) or (B)), the other Party shall have the right, in addition to any other rights or remedies under this Agreement or to which such other Party may be entitled in law or equity, to
61
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
(x) take such steps as are reasonably necessary in order to avoid a potential violation or continuing violation by such other Party or any of its Affiliates of the Anti-Corruption Laws, including by requiring that the Party agrees to such additional measures, representations, warranties, undertakings and other provisions as such other Party believes in good faith are reasonably necessary and (y) terminate any or all of the activities conducted by the Party pursuant to this Agreement or this Agreement in its entirety, immediately in the event that a Party reasonably concludes that there is no Provision available that would enable such Party or its Affiliates to avoid a potential violation or continuing violation of applicable Anti-Corruption Laws.
(g) Any termination of this Agreement pursuant to Section 17.4(f) shall be treated as a termination for breach.
(h) Each Party shall be responsible for any breach of any representation, warranty or undertaking in this Section 17.4 or of the Anti-Corruption Laws by any of its Representatives.
(i) Each Party may disclose the terms of this Agreement or any action taken under this Section 17.4 to prevent a potential violation or continuing violation of applicable Anti-Corruption Laws, including the identity of the other Party and the payment terms, to any Governmental Authority if such Party determines, upon advice of counsel, that such disclosure is necessary.
(SIGNATURE PAGE FOLLOWS)
62
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
IN WITNESS WHEREOF, each Party hereto has executed or caused this Agreement to be executed on its behalf as of the Effective Date.
ACELRX PHARMACEUTICALS, INC.
By: /s/ Xxxxxxx Xxxx
Name: Xxxxxxx Xxxx
Title: President & CEO
GRÜNENTHAL GMBH
By: /s/ Xxxx Xxxx Paques
Name: Prof. Xx. Xxxx Xxxx Paques
Title: Chairman of the Corporate Executive Board
By: /s/ Xxxxxxx Xxxx
Name: DoH. Xxxxxxx Xxxx
Title: Chief Commercial Officer EV, Australia and North America
[Signature Page to Collaboration and License Agreement]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Exhibit 1.1
[ * ]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 1.7
AcelRx Patents
| Docket No. | Patent Xxx.Xx./Pub No/Patent No. |
Location | Status/Validation | |||
| DEVICE-SIDE PATENTS | ||||||
| [ * ] | [ * ] | [ * ] | [ * ] | |||
| [ * ] | [ * ] | [ * ] | [ * ] | |||
| [ * ] | ||||||
| [ * ] | [ * ] | [ * ] | [ * ] | |||
| [ * ] | [ * ] | [ * ] | [ * ] | |||
| [ * ] | [ * ] | [ * ] | [ * ] | |||
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Exhibit 1.9
AcelRx Trademarks
ZalvisoTM
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
CONFIDENTIAL
Exhibit 1.14
| Docket No. | Patent Xxx.Xx./Pub No/Patent No. |
Location | Status/Validation | |||
| [ * ] | [ * ] | [ * ] | [ * ] |
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 1.38
[ * ]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 1.78
Licensed Product
The sufentanil sublingual microtablet system is comprised of the components shown below.
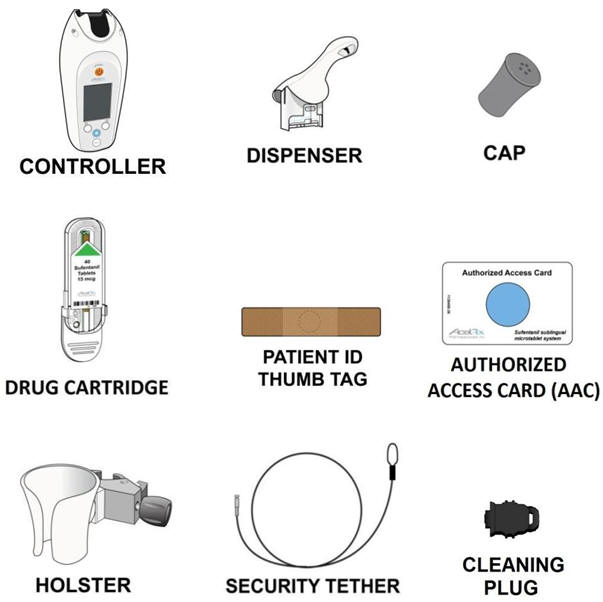
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
[ * ]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 1.83
[ * ]
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 3.3
For Grünenthal:
[ * ] VP Portfolio Development
For AcelRx:
[ * ] VP Clinical Operations
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 9.5(a)
Form of Press Release(s)
AcelRx Press Release

FOR IMMEDIATE RELEASE
AcelRx and Grünenthal Announce Collaboration
for EU Commercialization of ZALVISOTM
- FDA establishes the PDUFA action date of July 27, 2014 for Zalviso -
- Conference Call Scheduled Monday, December 16th 2013 for 8:30 a.m. Eastern Time –
Redwood City, California and Aachen, Germany – December 16, 2013 - AcelRx Pharmaceuticals, Inc. (Nasdaq: ACRX) and Grünenthal GmbH announced today that they have entered into a commercial collaboration, covering the territory of the European Union, certain other European countries and Australia for ZALVISO™ (previously known as ARX-01) for potential use in pain treatment within or dispensed by a hospital, hospice, nursing home or other medically supervised setting. ZALVISO, a drug-device combination product utilizing the opioid agonist sufentanil formulated in a proprietary sublingual tablet formulation and delivered through a pre-programmed, non-invasive proprietary delivery device is AcelRx’s lead program. AcelRx retains all rights in remaining countries, including the U.S. and Asia.
Under the terms of the agreement, AcelRx will receive an upfront cash payment of $30 million. AcelRx is eligible to receive approximately $220 million in additional milestone payments, based upon successful regulatory and product development efforts and net sales target achievements. Grünenthal will also make tiered royalty, supply and trademark fee payments in the mid-teens up to the mid-twenties percent range, on net sales of ZALVISO in the Grünenthal territory.
“As an established leader in providing pain management solutions to patients throughout Europe, Grünenthal is an excellent partner for AcelRx and for ZALVISO,” said Xxxxxxx Xxxx, President and CEO. “Grünenthal’s commercial track record across Europe demonstrates their ability to achieve commercial success in this large market, and will, following regulatory approval, enable patients in Europe suffering with moderate-to-severe pain in a medically supervised setting to receive the benefits of our innovative, patient-centric product ZALVISO.”
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
“We are extremely pleased to enter into this collaboration with AcelRx and its proven concept of a patient-controlled analgesia system to address a significant unmet medical need, thereby allowing hospitals to avoid the challenges of intravenous line-related infections, as well as freeing hospital personnel from the need to program intravenous infusion pump systems. With ZALVISO Grünenthal is building on its presence in the hospital market, an area that provides us with significant growth opportunities in the mid- and long-term,” said Xxxx. Xxxx-Xxxx Xxxxxx, Grünenthal’s Chief Executive Officer.
Grünenthal will be responsible for all commercial activities for ZALVISO, including obtaining and maintaining pharmaceutical product regulatory approval in the Grünenthal territory. AcelRx will be responsible for maintaining device regulatory approval in the Grünenthal territory and manufacturing and supply of ZALVISO to Grünenthal for commercial sales and clinical trials.
ZALVISO PDUFA Date
In addition, AcelRx announced today that the U.S. Food and Drug Administration (FDA) has established a Prescription Drug User Fee Act (PDUFA) action date of July 27, 2014, for AcelRx’s New Drug Application (NDA) for Zalviso. AcelRx announced on December 2, 2013 that FDA accepted for filing the Zalviso NDA.
Conference Call at 8:30 a.m. Eastern time on Monday, December 16, 2013
AcelRx will conduct a conference call and webcast today, December 16, 2013 at 8:30 a.m. Eastern time (5:30 a.m. Pacific time) to discuss the Grünenthal partnership. To listen to the conference call, dial in approximately ten minutes before the scheduled call to (000) 000-0000 for domestic callers, (000) 000-0000 for Canadian callers, or (000) 000-0000 for international callers. Those interested in listening to the conference call live via the Internet may do so by visiting the Investors section of the company’s website at xxx.xxxxxx.xxx and selecting the webcast link for Grünenthal collaboration conference call. A webcast replay will be available on the AcelRx website for 90 days following the call by visiting the Investors section of the company’s website at xxx.xxxxxx.xxx
About ZALVISO
ZALVISO is an investigational pre-programmed, non-invasive, handheld system that allows hospital patients with moderate-to-severe acute pain to self-dose with sublingual sufentanil microtablets to manage their pain. ZALVISO is designed to address the limitations of IV PCA by offering:
| • | A high therapeutic index opioid – ZALVISO uses the high therapeutic index, highly lipophilic opioid sufentanil, enabling delivery via a non-intravenous route, and also supporting fast onset of effect. |
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| • | A non-invasive route of delivery – The sublingual route of delivery used by ZALVISO eliminates the risk of IV-related analgesic gaps and IV complications, such as catheter-related infections in IV PCA treated patients. In addition, because ZALVISO patients do not require direct connection to an IV PCA infusion pump through IV tubing, ZALVISO allows for ease of patient mobility. |
| • | A simple, pre-programmed PCA solution – ZALVISO is a pre-programmed PCA system designed to eliminate the risk of programming errors. |
About AcelRx Pharmaceuticals, Inc.
AcelRx Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on the development and commercialization of innovative therapies for the treatment of acute and breakthrough pain. AcelRx’s lead product candidate, ZALVISO, is designed to solve the problems associated with post-operative intravenous patient-controlled analgesia which has been shown to cause harm to patients following surgery because of the side effects of morphine, the invasive IV route of delivery and the complexity of infusion pumps. AcelRx has announced positive results from each of the three Phase 3 clinical trials for ZALVISO and has submitted an NDA to the FDA seeking its approval. AcelRx has also announced positive top-line results for a Phase 2 trial for ARX-04, a sufentanil formulation for the treatment of moderate-to-severe acute pain, funded through a grant from the U.S. Army Medical Research and Materiel Command. The company has two additional pain treatment product candidates, ARX-02 and ARX-03, which have completed Phase 2 clinical development. For additional information about AcelRx’s clinical programs, please visit xxx.xxxxxx.xxx.
The Grünenthal Group is an independent, family-owned, international research-based pharmaceutical company headquartered in Aachen, Germany. Building on its unique position in pain treatment, its objective is to become the most patient-centric company and thus to be a leader in therapy innovation. Grünenthal is one of the last five remaining research-oriented pharmaceutical companies with headquarters in Germany which sustainably invests in research and development. Research and development costs amounted to about 26 percent of revenues in 2012. Grünenthal’s research and development strategy concentrates on selected fields of therapy and state-of-the-art technologies. We are intensely focused on discovering new ways to treat pain better and more effectively, with fewer side-effects than current therapies. Altogether, the Grünenthal Group has affiliates in 26 countries worldwide. Grünenthal products are sold in more than 155 countries. Today, approx. 4,400 employees are working for the Grünenthal Group worldwide. In 2012, Grünenthal achieved revenues of USD 1,251 mn.
More information: xxx.xxxxxxxxxx.xxx.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Forward Looking Statements
This press release contains forward-looking statements, including, but not limited to, statements related to potential approval of the NDA for Zalviso in the U.S. and the timing thereof, the potential of approval of the MAA for Zalviso in the EU and the timing thereof, the ability to successfully manufacture Zalviso to meet the requirements of Grünenthal and the therapeutic and commercial potential of Zalviso in the Grünenthal territory. These forward-looking statements are based on AcelRx’s current expectations and inherently involve significant risks and uncertainties. AcelRx’s actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks related to: AcelRx’s ability to receive regulatory approval for Zalviso; any delays or inability to obtain and maintain regulatory approval of AcelRx’s product candidates, including Zalviso, in the United States, Europe, Australia and other countries; the ability to attract additional funding partners or collaborators with development, regulatory and commercialization expertise; the ability to obtain sufficient financing to commercialize Zalviso; the market potential for AcelRx’s other product candidates; the accuracy of AcelRx’s estimates regarding expenses, capital requirements and needs for financing; and other risks detailed in the “Risk Factors” and elsewhere in AcelRx’s U.S. Securities and Exchange Commission filings and reports, including its Quarterly Report on Form 10-Q filed with the SEC on November 5, 2013. AcelRx undertakes no duty or obligation to update any forward-looking statements contained in this release as a result of new information, future events or changes in its expectations
Contact:
Xxx Xxxxx
Chief Financial Officer
650.216.3511
xxxxxx@xxxxxx.xxx
###
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| GRÜNENTHAL GROUP Press Release |

|
Grünenthal and AcelRx enter into partnership for
EU commercialization of ZALVISO®
Aachen, Germany / Redwood City (California), USA – December 16, 2013 – Grünenthal GmbH today announced that the company entered into a commercial partnership with AcelRx Pharmaceuticals, Inc. for ZALVISO, a patient-controlled analgesia device for self-administration of sufentanil nanotablets by patients following surgery. The agreement covers the countries of the European Union, EEA and Australia. AcelRx will supply Grünenthal with the product and retains all rights in remaining countries including the US and Asia. With this partnership Grünenthal, a family-owned company headquartered in Aachen, Germany, significantly strengthens its hospital franchise and underlines its strong market position as a pain specialist in the pharmaceutical market.
Strategic portfolio expansion for the benefit of more than 20 million patients
According to market research there are more than 20 million patients in Europe who could potentially benefit from this innovative alternative to current treatment options in acute post-operative pain management. Additionally, this device is an excellent expansion of Grünenthal’s existing portfolio of innovative drugs for the treatment of moderate to severe chronic pain.
“We are extremely pleased to enter into this collaboration with AcelRx and its proven concept of a patient-controlled analgesia system to address a significant unmet medical need, thereby allowing hospitals to avoid the challenges of intravenous line-related infections, as well as freeing hospital personnel from the need to program intravenous infusion pump systems. With ZALVISO Grünenthal is building on its presence in the hospital market, an area that provides us with significant growth opportunities in the mid- and long-term,” said Xxxx. Xxxx-Xxxx Xxxxxx, Grünenthal’s Chief Executive Officer.
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
“As an established leader in providing pain management solutions to patients throughout Europe, Grünenthal is an excellent partner for AcelRx and for ZALVISO” said Xxxxxxx Xxxx, President and CEO. “Grünenthal’s commercial track record across Europe demonstrates their ability to achieve commercial success in this large market, and will enable patients in Europe suffering with moderate-to-severe pain in a medically supervised setting to receive the benefits of our highly innovative, patient-centric product ZALVISO.”
Market launch of ZALVISO in Europe planned for 2015
An NDA for ZALVISO was submitted to the FDA on September 27 and accepted for filing by the FDA on December 02. An approval for the US-market could be expected in the third quarter of 2014. Grünenthal and AcelRx are planning to submit a MAA to EMA around mid-2014 with an anticipated approval and go to market in Europe by the end of 2015. Under the terms of the agreement, Grünenthal pays an upfront cash payment of $30 million to AcelRx and up to $220 million in additional milestone payments, based upon successful regulatory and product development efforts and net sales target achievements as well as royalty payments on net sales of ZALVISO in the Grünenthal territory.
The Grünenthal Group is an independent, family-owned, international research-based pharmaceutical company headquartered in Aachen, Germany. Building on its unique position in pain treatment, its objective is to become the most patient-centric company and thus to be a leader in therapy innovation. Grünenthal is one of the last five remaining research-oriented pharmaceutical companies with headquarters in Germany which sustainably invests in research and development. Research and development costs amounted to about 26 percent of revenues in 2012. Grünenthal’s research and development strategy concentrates on selected fields of therapy and state-of-the-art technologies. We are intensely focused on discovering new ways to treat pain better and more effectively, with fewer side-effects than current therapies. Altogether, the Grünenthal Group has affiliates in 26 countries worldwide. Grünenthal products are sold in more than 155 countries. Today, approx. 4,400 employees are working for the Grünenthal Group worldwide. In 2012, Grünenthal achieved revenues of USD 1,251 mn.
More information: xxx.xxxxxxxxxx.xxx.
About AcelRx Pharmaceuticals, Inc.
AcelRx Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on the development and commercialization of innovative therapies for the treatment of acute and breakthrough pain. AcelRx’s lead product candidate, ZALVISO, is designed to solve the problems associated with post-operative intravenous patient-controlled analgesia which has been shown to cause harm to patients following surgery because of the side effects of morphine, the invasive IV route of delivery and the complexity of infusion pumps. AcelRx has announced positive results from each of the three Phase 3 clinical trials for ZALVISO and has
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
submitted an NDA to the FDA seeking its approval. AcelRx has also announced positive top-line results for a Phase 2 trial for ARX-04, a sufentanil formulation for the treatment of moderate-to-severe acute pain, funded through a grant from the U.S. Army Medical Research and Materiel Command. The company has two additional pain treatment product candidates, ARX-02 and ARX-03, which have completed Phase 2 clinical development. For additional information about AcelRx’s clinical programs, please visit xxx.xxxxxx.xxx.
Contact: Xxxxx Xxxxxxxxx, Vice President Public Engagement
Tel.: x00 000 000-0000, Fax: x00 000 000-0000, xxxxx.xxxxxxxxxx@xxxxxxxxxx.xxx
Xxxxxxxxxx XxxX, 00000 Xxxxxx, Xxxxxxx, xxx.xxxxxxxxxx.xxx
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
