CLINICAL TRIAL AGREEMENT
Exhibit 10.1
This Clinical Trial Agreement (“Agreement”), effective this 1st day of June 2017 (“Effective Date”), is made by and between Southern California Permanente Medical Group, with its offices at 000 Xxxxx Xxx Xxxxxx, 0xx Xxxxx, Xxxxxxxx, Xxxxxxxxxx 00000 (hereinafter “Institution”), and Enteromedics, Inc., with its offices at 0000 Xxxxxx Xxxx, Xx. Xxxx, XX 00000 (hereinafter “Sponsor”), to have the Institution conduct a clinical trial (the “Study”) at several sites.
Investigators. Xxxx X. Xxxxx, PhD, will serve as the principal investigator, and (hereinafter, collectively “Investigator”). Dr. Xiang, as the principal investigator shall work with Institution to ensure that she and the co-investigators comply with the obligations and terms of this Agreement as specified for the Investigator hereunder.
1.1 Study Product. Sponsor agrees to provide vBloc Maestro® Rechargeable System (the “Study Product”) in amounts necessary to conduct the Study. Sponsor will provide the Study Product free of charge to the Institution. Institution and Investigator agree that the Study Product will be used solely for purposes of performing the Protocol under this Agreement. At the completion of the Study, Institution shall return any remaining Study Product to Sponsor, unless otherwise instructed by Sponsor.
1.2 Protocol. The Protocol which was developed by the Institution includes the details of the Study known as “Xxxxxx Permanente So CA Partnership Study Design Outline for vBloc Therapy with the Maestro Rechargeable System,” (the “Protocol”), which is incorporated along with any Protocol Amendments by reference as part of this Agreement.
1.3 Compliance with Laws; IRB Approval; HIPAA. Institution and Investigator agree to comply with this Agreement, the Protocol, requirements of the IRB in connection with the Study, and all applicable federal, state and local laws and regulations, including but not limited to the privacy regulations issued pursuant to the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), 45 C.F.R. Parts 160 & 164. Institution and Investigator agree that it will appropriately safeguard protected health information (“PHI”). As used in this Agreement, PHI shall have the meaning of that term as defined in the HIPAA privacy regulations. The Investigator and Institution shall handle all Study data, including medical records of Study participants, in accordance with HIPAA requirements and all other applicable laws. The Investigator and Institution shall obtain from each Study participant a valid authorization that complies with HIPAA before the Investigator or Institution provide any Study data to Sponsor.
Sponsor also shall comply with this Agreement and all applicable laws and regulations. Sponsor shall comply with the regulations of HIPAA, governing the privacy and security of health information. To the extent required by applicable law, Sponsor will also require all personnel and any other third parties involved in the conduct of the Study to comply with applicable law. Sponsor shall treat all information regarding diagnosis, history or treatment that allows unique identification of an individual’s PHI, as confidential information.
2.1 Protocol Submission to IRB. Institution and Investigator will submit the Protocol for approval to the Institutional Review Board (“IRB”). Protocol will become final upon approval by the designated IRB. Investigator will ensure that the IRB will be responsible for the continuing review and approval of the Study.
2.2 IRB Required Documents. Institution and Investigator will submit to the IRB, any documents required by the IRB in connection with the Study, including Informed Consent and HIPAA Authorizations. These documents will become final upon approval, if applicable, by the IRB. Institution and Investigator will use the current IRB approved version of the Informed Consent to obtain consent from each Study participant.
2.3 Changes to Protocol. Institution and Investigator will submit to the IRB, any proposed amendment to, or deviation from, the Protocol, or such other documents as required by the IRB in connection with the Study. Prior to implementation of any amended Protocol, Institution and Investigator will submit such amendments to the IRB and obtain prior written approval, or a determination that written approval is not required.
2.4 Data Safety Monitoring Board. Sponsor understands that the Data Safety Monitoring is a requirement of this study and the Data Safety Monitoring Board (DSMB) services provided by Sponsor must meet the requirements of the FDA policy (reference: xxxxx://xxx.xxx.xxx/XXXXX/XXXXXXX/00xx/00x-0000-xxx0000.xxx). DSMB Members will not be Staff of the Sponsor or Institution. Additionally, Sponsor will allow Institution's Principal Investigator to review and approve DSMB Committee Members prior to their appointment to the Committee."
3.1 Compensation. In consideration of the work to be performed under this Agreement, Sponsor will provide payments to Institution in accordance with the budget, which is attached hereto as Exhibit A. Institution represents that the budget is based on fair market value of the work and services to be provided for Institutions of similar size and scope. Payment as set forth in this Section and Exhibit A constitutes full payment for the Study, and other than applicable Study participant injuries under Section 5.2, Sponsor shall have no other payment obligations either under this Agreement or in connection with the Study.
3.2 Schedule of Payments. All payments due hereunder shall be paid by Sponsor within forty-five (45) days from its receipt of an invoice for such payment from Institution. Invoices must be provided to Sponsor no more than six (6) months after the date of Study completion or termination. Final payment will be made after Sponsor receives any remaining Study Product.
3.3 Disclosure. Institution and Investigator understand and agree that, pursuant to local, state, and federal laws, Sponsor may have a legal obligation to disclose the financial relationship between Sponsor and the Investigator and Institution, including any compensation under this Agreement, and does not object to any such disclosure required by law.
Institution acknowledges that pursuant to the federal Physician Payments Sunshine Act (Section 6002 of the 2010 Patient Protection and Affordable Care Act) and certain State laws, Sponsor may be obligated to report all payments made to Institution in performance of this Agreement to Government agencies for further public disclosure. For clarity and avoidance of doubt, and consistent with the invoicing and payment provisions of this Agreement, Institution and Sponsor agree that Southern California Permanente Medical Group is the Institution contracting entity and recipient of all payments to be made by Sponsor under this Agreement. Sponsor will report such payments accordingly. Institution and Sponsor will cooperate to resolve any apparent errors or discrepancies discovered in publicly reported information about payments made in performance of this Agreement and obtain or make corrections in such information.
In the event that Sponsor requires Investigator or Institution personnel to attend meetings for the Study, Sponsor will arrange and pay for the expenses directly for travel, accommodation, and meals in connection with such attendance. Such covered expenses may be publicly reportable. No compensation will be paid in connection with attending the investigator meeting. Sponsor shall provide Institution documentation verifying the amount of anything of value provided to Investigator and Institution personnel including all travel accommodations and meals. Institution shall reimburse Investigator and Institution personnel for incidental expenses authorized by Sponsor and shall invoice and be reimbursed by Sponsor for those expenses.
4. INVESTIGATOR AND INSTITUTION REPRESENTATIONS
4.1 Investigator. Investigator is a Partner of Institution and will be responsible for performing the Study and supervising any individual performing portions of the Study. Investigator represents and certifies that he/she will conduct the Study in compliance with the Protocol, any other documents required by the IRB in connection with the Study, and all terms and conditions of this Agreement. Investigator or assigned qualified designee will: (a) maintain patient records and source documentation for each Study participant and monitor the Study for compliance to good clinical practices; and (b) assist Sponsor in clarification of clinical results and resolve and account for any data discrepancies, Study participant missed visits, and Protocol discrepancies.
4.2 Unavailability of Investigator. Investigator is essential to the Study. If Investigator becomes unable to complete the Study, Institution will consult with and obtain written approval from Sponsor prior to appointing a new Investigator. Sponsor reserves the right to terminate this Agreement if Institution and Sponsor cannot agree upon an acceptable substitute within sixty (60) days.
4.3 Independent Contractors. In undertaking to perform this Study for Sponsor, it is understood and agreed that Investigator and Institution are doing so as independent contractors and not as an employee, partner or joint venture of Sponsor.
4.4 Event of Debarment or Disqualification. If, during the term of this Agreement, Institution or Investigator or any employee or agent performing services for the Study (i) becomes debarred, suspended, excluded, or otherwise sanctioned, or (ii) receives notice of an action or threat of an action with respect to any such debarment, suspension, exclusion, or sanction, Institution and Investigator each agrees to immediately notify Sponsor. Institution and Investigator also each agrees that if it/he/she becomes debarred, suspended, excluded, or
otherwise sanctioned, Institution and Investigator will immediately notify Sponsor and cease all activities relating to this Agreement, to the extent medically permissible and otherwise possible.
4.5 Restrictions on Charging. Neither Institution nor Investigator will charge any participant in the Study or third party payer for the Study Product, or procedures associated with administering the Study Product, or any other research services covered by the budget as specified in Exhibit A.
4.6 Assignment and Subcontracting Restrictions. Institution and Investigator may not assign their rights, delegate their obligations, or otherwise transfer or subcontract under this Agreement without the prior written consent of Sponsor, which consent will not be unreasonably withheld.
4.7 Permitted Sites at Institution. Sponsor acknowledges and agrees that the Study will be conducted at the following sites within the Institution:
x. Xxxxxx Permanente
South Bay Medical Center
00000 X. Xxxxxxx Xxxxxx, Xxx 00
Xxxxx XXXX 0xx
Xxxxxx Xxxx, XX 00000
5.1 Clinical Study Participants. Institution and Investigator will include only participants in the Study who upon entrance into the Study meet all of the inclusion criteria and none of the exclusion criteria set forth in the Protocol, have executed a written informed consent form, and have executed a HIPAA Authorization.
5.2 Participant Injuries. Sponsor shall reimburse the Institution for the cost of reasonable and necessary medical care, including hospitalization, incurred by a Study participant in treating an injury that directly results from the use of the Study Product performed in accordance with the Protocol, provided such costs are in no way attributable to the Study participant not following instructions. In no case will Sponsor reimburse the Institution or any other party for (i) the treatment of medical complications that are a part of the natural course of the primary disease or for any medical treatment for injuries that are unrelated to the use of the Study Product, (ii) other injury-or illness-related costs (such as lost wages), or (iii) medical expenses that are incurred as a result of a material violation of the Protocol. It is understood and acknowledged that Sponsor’s obligations under this provision are primary to the obligations of Medicare or any other government provider. Sponsor will not reimburse for any reasonable and necessary medical expenses incurred by Study participants to the extent caused by the Institution’s or its personnel’s negligent acts or omissions or violations of this Agreement, intentional wrongdoing or failure to follow the Protocol or applicable law.
6.1 Sponsor Confidential Information. Institution and Investigator each agree to maintain in confidence and not disclose to any third party, or use for its benefit or the benefit of any third party, without the prior written consent of Sponsor any confidential or proprietary information, including the Study Product (“Sponsor Confidential Information”).
6.2 Institution Confidential Information. Sponsor, including its representatives or employees, shall not release, disclose or use Institution’s confidential information other than in connection with the Study unless required by law or regulation. “Institution Confidential Information” means the Protocol, all participant medical records and other data originating at Institution (including but not limited to any PHI) from which Study data is collected or generated (“Source Documents”), including without limitation of the foregoing any such information obtained by inspection or copying in connection with an audit or examination under this Agreement or any Study Addendum; any confidential or proprietary information (including all tangible and intangible embodiments thereof) concerning any Institution’s business practices (e.g., health care delivery practices, utilization data, membership or other health plan information).
Institution's electronic medical record system known as KP HealthConnect™ contains confidential and proprietary information of Institution and its software licensors. Accordingly, in the event Sponsor comes into contact with KP HealthConnect™, Sponsor agrees to treat the design, functionality, features and information available in KP HealthConnect as Institution's Confidential Information, which Sponsor will hold in confidence and will not use or disclose for any purpose other than performance of the Study.
6.3 Use of Confidential Information. Each Party agrees to use the Confidential Information of the other Party only for fulfilling their respective obligations under this Agreement. If requested, the Party receiving the Confidential Information (“Receiving Party”) will return such Confidential Information of the Party disclosing the Confidential Information (“Disclosing Party”) at the end of the Study, other than items required to be retained under Regulations.
6.4 Exceptions. The obligations of non‑disclosure and non‑use do not apply when:
1. The information is in the public domain or becomes publicly available through no fault of Receiving Party or any employee, agent or representative of Receiving Party;
2. Receiving Party knows the information before receipt from the Disclosing Party, as evidenced by written records maintained by Receiving Party prior to the disclosure of the information;
3. The information is lawfully received from a third party that has a right to make such disclosure, who did not obtain such information in violation of Disclosing Party’s or its affiliates’ rights or under obligation of confidentiality to Disclosing Party and/or its affiliates;
4. Applicable law, rule, regulation or order requires disclosure of Confidential Information. If such disclosure is required, Receiving Party will notify Disclosing Party immediately, will give Disclosing Party time and opportunity to file appropriate
motions to protect the confidentiality of such information, and, at Disclosing Party’s request will reasonably cooperate in any Disclosing Party’s efforts to obtain a protective order, confidential treatment or an exemption or limitation with respect to such required disclosure; or
5. Disclosing Party grants written permission for disclosure.
7.1 Rights to Study Data. All clinical data, including case report forms and other relevant information generated during the Study, will be promptly and fully disclosed to Sponsor and may be used by Sponsor for any purpose(s) stated in the informed consent form or otherwise in compliance with applicable law. All data generated during this Study (including without limitation all case report forms, safety information and other data reports) is and will be jointly owned by Institution and Sponsor. The Institution and the Investigator hereby assign to Sponsor any rights, title or interest they may have therein. “Original Source Documents” defined as individual patient records, investigators’ research and clinical records, hospital records, clinical and patient charts, laboratory notes, pharmacy dispensing records, recorded data from automated instruments, microfiches, photographic negatives, microfilm or magnetic media, X-rays and other diagnostic images, and other records generated and maintained by the pharmacy, laboratories and medico-technical departments of the Institution and any other information that the Institution maintains for regulatory, research and patient care purposes will remain the property of the Institution. The Institution will be free to use the results of the Study for its own teaching, research, educational, clinical and publication purposes, subject to Section 8 (Publication) and Section 6 (Confidentiality) of this Agreement. The Institution understands and agrees that (i) it will have no ownership, license or access rights in or to Sponsor’s regulatory filings based upon the inclusion of research results therein and (ii) they will not acquire any interest whatsoever in the Study Product as a result of performing the Study contemplated by this Agreement. This Section is subject to all applicable law, including any privacy legislation applicable to the performance of the Study by the Institution and the Institution personnel, and will survive the expiration and termination of this Agreement.
7.2 Biological Samples. Institution owns biological samples and grants Sponsor access to use biological samples for purposes of the Study and in accordance with the Study subjects’ Informed Consent Forms, HIPAA Authorizations, and Applicable Law. Upon completion or termination of the Study, or sooner at the request of Sponsor, all biological samples shall remain with Institution.
7.3 Existing Inventions. It is recognized and understood that certain existing inventions and technologies are the separate property of Sponsor or Institution and are not affected by this Agreement, and neither Party shall have any claims to or rights in such separate inventions and technologies.
7.3 Sponsor’s Rights. Any concepts, know-how, ideas, innovations, inventions, discoveries, data, designs, technology and improvements, whether or not protectable under patent, copyright, trade secrecy or similar laws (collectively, “Intellectual Property”), and conceived of and/or
reduced to practice by Investigator or any Institution personnel whether alone or jointly with others, including but not limited to Sponsor’s personnel, during the course of the Study or otherwise as a result of the services performed under this Agreement which constitutes a new use, modification, enhancement or improvement to the Study Product, shall be owned solely by Sponsor. The Intellectual Property described in the preceding sentence is defined herein collectively as “Sponsor’s Rights.” The Institution hereby assigns, and shall ensure that the Investigator and the other Institution personnel will assign, to Sponsor the entire right, title and interest they may have in and to any and all Sponsor’s Rights and they will execute and deliver all agreements or instruments deemed reasonably necessary to effectuate any assignment of right at the expense of Sponsor.
7.4 Joint Rights. Other than Sponsor’s Rights, Intellectual Property jointly conceived or developed by the Institution or other Institution personnel and Sponsor shall be owned jointly by the Institution and Sponsor (“Joint Rights”). The parties hereto shall not license or otherwise transfer any rights in or to any Joint Rights until the parties have entered into a joint cooperation agreement regarding licensing and patenting; provided, however,
Sponsor shall have the right to exploit the Joint Rights in any way, including but not limited to commercial sales or licensing to third parties, and shall not be required to provide an accounting or remit any revenues therefrom to the Institution.
Sponsor shall have the first option to negotiate an exclusive, worldwide, royalty-bearing license for the Institution’s interest in and to the Joint Rights or purchase such Joint Rights. Sponsor may exercise its option with regard to any Joint Rights at any time during the period of one hundred eighty (180) days after disclosure of such Joint Rights by providing the Institution with written notice of its desire to exercise its option. The aforesaid disclosure shall specify the terms upon which Sponsor may acquire such exclusive license. The Institution shall provide Sponsor with the information Sponsor reasonably requests to determine whether to exercise its option. If Sponsor does not exercise its option during the 180-day period, the Institution may license such Joint Rights to third parties, provided, however, that the Institution may not, for a period of one (1) year, license such Joint Rights to third parties on terms more favorable than those offered to the Sponsor.
Upon Sponsor’s exercise of its option with regard to any Joint Rights, the Institution and Sponsor will negotiate in good faith in an attempt to reach a license or sale agreement satisfactory to both parties; provided however, that the negotiation period shall not exceed twelve (12) months. If both parties participate in good faith and in a timely manner in the negotiations but cannot reach an agreement, then upon the expiration of the twelve-month negotiation period the Institution shall have no further obligation to Sponsor under this Agreement with regard to the Institution’s interests under the Joint Rights; provided, however, Sponsor shall continue have the right to exploit the Joint Rights in any way.
7.5 Institution’s Rights. Other than Sponsor’s Rights and Joint Rights, any Intellectual Property that is conceived and/or reduced to practice solely by Institution employees or agents during the performance of services pursuant to this Agreement shall be owned by the Institution (collectively, “Institution’s Rights”).
7.6 Treatment. The Institution shall promptly disclose in writing to Sponsor all Intellectual Property conceived of and/or reduced to practice by the Investigator or any other Institution personnel as a result of performing services under this Agreement.
7.7 Use of Name/Logo. Except as required by law or regulation, no party to this Agreement will use the name or logo of any other party or of any employee, agent or representative of any other party in connection with any product, service, promotion, news release, report, or other publication, oral or written without the prior written approval of the party or individual whose name or logo is to be used.
8.1 Right to Publish. The Institution and Sponsor will each have a right to publish, present or use any final results arising out of the performance of the Study (individually, a “Publication”) for their publication objectives. With respect to Institution and Investigator Publication, such Publication shall occur only after completion of the Study, shall contain only final research data and analysis, and shall be subject to the regulations and the provisions of this Agreement relating to confidentiality and non‑disclosure. At least thirty (30) days prior to submission for publication, presentation or use, Institution or Investigator will submit to Sponsor for review any proposed oral or written Publication.
If Sponsor believes that any Publication contains confidential or proprietary information belonging to Sponsor, Sponsor will notify the Institution or Investigator, which will remove all references to such confidential or proprietary information prior to publication, presentation or use. Any comments regarding the deletion of materials shall not be editorial in nature or content, but shall constitute comments pertaining to matters that Sponsor considers confidential or proprietary in nature. Upon Sponsor’s notice to Institution or Investigator that Sponsor reasonably believes that one or more patent applications should be filed, which relate to inventions owned by Sponsor according to Section 7, prior to any Publication, such Publication will be delayed until such patent application(s) have been filed, provided that Investigator, Institution and Sponsor will cooperate in expeditiously filing any such patent application(s), and provided further that any such delay of a Publication will not exceed forty-five (45) days from the date of such notice to Institution or Investigator.
The foregoing notwithstanding, Sponsor will not make any public presentation of Study Data or reports provided to Sponsor by Institution or Investigator until the earlier of (i) such data or reports have been publicly disclosed; (ii) twenty four (24) months following completion or earlier termination of the Study; or (iii) Institution’s prior written consent, which shall not be unreasonably delayed or withheld. For the avoidance of doubt, Sponsor may use non-public Study Data or reports at any time for regulatory or risk management evaluation purposes. At least thirty (30) days prior to submission for publication, presentation or use, Sponsor will submit to Institution for review any proposed oral or written Publication; provided that such information has not previously been publicly disclosed by Institution.
9.1 Term. The terms of this Agreement will commence on the Effective Date and will continue in force until the Study has been completed or this Agreement has been terminated, whichever occurs sooner.
9.2 Termination. This Agreement may be terminated in accordance with the following provisions: (i) Either party may terminate this Agreement if the other party materially breaches any of its obligations or provisions of this Agreement, provided, however, that the breaching party shall be given not less than thirty (30) days’ prior written notice of such material breach and the opportunity to cure the breach during such period; (ii) Institution may terminate this Agreement immediately for safety reasons relating to the use of the Study Product; and (iii) Institution and/or Sponsor may terminate this Agreement for any reason upon thirty (30) days' prior written notice to the other party.
9.3 Effect of Receipt of Termination. Upon receipt of notice of termination, Institution shall immediately stop entering new Study participants into the Study and, to the extent medically permissible, cease use of the Study Product and conducting procedures on participants already entered into the Study. Institution and Investigator shall immediately deliver to Sponsor all unused Study Product, if any, unless otherwise instructed by Sponsor. In the event of termination, Institution and Investigator will provide notice of such termination to the IRB. Upon termination, Sponsor's sole obligation shall be to pay Institution a pro-rated amount for actual work performed and non-cancellable obligations incurred by Institution within forty (45) days of receipt of termination notice.
10.1 Sponsor Indemnity. Except as set forth below, Sponsor agrees to defend, indemnify and hold harmless Investigator and Institution, its trustees, officers, affiliates, agents and employees (collectively, the “Institutional Indemnitees”) from any and all liabilities, claims, actions, suits, or proceedings from third-parties (collectively “Claims”), for any injury arising out of Sponsor’s performance of the activities under this Agreement to the extent caused by the use of the Study Product in accordance with the Protocol. Indemnification shall not extend, however, to that portion of any Claims resulting from Institutional Indemnitees (a) negligence or malfeasance (b) failure of Institution to comply with the terms of this Agreement, and/or any written instructions provided by Sponsor regarding the contents of this Agreement. Notwithstanding the foregoing, Sponsor shall not settle, or admit liability with respect to, any such Claims, which would result in liability to Institution without prior written consent of Institution.
10.2 Institution Indemnity. Institution agrees to defend, indemnify, and hold harmless Sponsor, its agents and employees (collectively, the “Sponsor Indemnitees”) from any and all liabilities, claims, actions, suits, or proceedings (collectively “Claims”), resulting from the negligent or intentional misconduct of Investigator and Institution, and their agents or employees arising out of the performance of the activities under this Agreement. Indemnification shall not extend, however, to that portion of any Claims resulting from Sponsor Indemnitees (a) negligence or malfeasance (b) failure of Sponsor to comply with the terms of this Agreement, and/or any written instructions provided by Institution regarding the contents of this Agreement.
Notwithstanding the foregoing, Institution shall not settle, or admit liability with respect to, any such Claims, which would result in liability to Sponsor without prior written consent of Sponsor.
11.1 Insurance. Institution represents that it is self-insured. Institution will at Sponsor’s request provide a certificate of self-insurance coverage to Sponsor to evidence dedicated financial capacity to cover losses in amounts specified in the certificate.
12.1 Records. Institution and Investigator will maintain adequate records and documents pertaining to the conduct of the Study, including, but not limited to, Study participant identifications, clinical observations, informed consents, HIPAA authorizations, laboratory tests and other records required by the regulations to be maintained, and will maintain those records during the Study and as long as required by applicable law.
12.2 Case Report Forms. The Investigator will complete any forms or reports requested by Sponsor and/or described in the Protocol and will do so in an accurate, complete and timely manner, in accordance with applicable law and as permitted under the consent forms. If a referring physician is following the Study participant, the Investigator will coordinate data collection with the referring physician. All case report forms will be completed by the Investigator and submitted to Sponsor at Study completion or termination, whichever is earlier.
12.3 Reports. The Investigator will provide in a timely manner annual and final reports to Sponsor, as described in the Protocol.
12.4 Access. Institution shall allow Sponsor’s authorized representatives to visit Institution’s facilities where the Study is conducted at reasonable times and with reasonable advance notice to observe and verify Institution’s compliance with this Agreement and to review the work being performed for the Study, to inspect the facilities which are being utilized in the Study, including having access to all relevant records, including the Study Data, as needed. Institution will provide Sponsor’s Representatives the necessary access to the Institution’s electronic medical record system and other Study Data only if Sponsor’s Representatives provide the required information and complete the necessary training.
13.1 Audit and Review Rights. Institution will review and audit records for data quality purposes. Except for the rights granted in Section 12, Sponsor or its authorized representatives shall not have the right to review, audit or monitor site records.
14.1 Notices. Notices under this Agreement will be given by personal delivery, first class mail, recognized overnight courier service or by FAX to the person designated below:
If to Sponsor:
Enteromedics, Inc.
0000 Xxxxxx Xxxx
Xx. Xxxx, XX 00000
If to Institution:
Southern California Permanente Medical Group
000 Xxxxx Xxx Xxxxxx, 0xx Xxxxx
Xxxxxxxx, Xxxxxxxxxx 00000
Attn: ***************, Manager, Sponsored Projects Administration
Payment remit to:
Xxxxxx Foundation Hospitals Inc
P. O. Xxx ******
Xxx Xxxxxxx, XX 00000-0000
Overnight Mail:
Bank of America Lockbox Services
Xxxxxx Foundation Hospitals Inc
Lockbox ******
0000 Xxxxx Xxxxxx Xxxxx
Xxx Xxxxxxx, XX 00000-0000
Phone: 000-000-0000 X 00000
If to Investigator:
Xxxx X. Xxxxx, PhD
Director, Biostatistics Research
Southern California Permanente Medical Group
Department of Research & Evaluation
000 X Xxx Xxxxxx, 0xx Xxxxx, Xxxxxxxx, XX 00000
15.1 Non-Referral/Anti-Corruption Language. The parties agree that it is not their intent under this Agreement to induce or encourage the unlawful referral of Study participants or business between the parties, and there shall not be any requirement under this Agreement that either party, its employees or affiliates, including its medical staff, engage in any unlawful referral of subjects to, or order or purchase products or services from, the other party. Each party shall require that their employees, who are involved in the conduct of the Study, will not offer, pay, request or accept any bribe, inducement, kickback or facilitation payment, and shall not make or cause another to make any offer or payment to any individual or entity for the purpose of influencing a decision for the benefit of the other party.
15.2 Entire Agreement. This Agreement sets forth the entire Agreement and understanding between the parties hereto as to the subject matter hereof and has priority over all documents, verbal consents or understandings made between Sponsor, Institution and Investigator with respect to the subject matter hereto. None of the terms of this Agreement may be amended or modified except in writing and signed by the parties hereto.
15.3 Amendment or Modification. Any amendment to or modification of this Agreement must be in writing and signed by authorized representatives of each party.
15.4 Severability. The invalidity or unenforceability of any provision of this Agreement will in no way affect the validity or enforceability of any other provision of this Agreement.
15.5 Governing Law. This Agreement, and all disputes and claims arising under this Agreement, will be interpreted and governed by the laws of the State of California, without regard to conflicts of laws principles.
15.6 Event of Inconsistency Between This Agreement and Protocol. In the event of inconsistency between this Agreement and the Protocol, this Agreement shall govern and control as to any legal issue, and the Protocol shall govern and control as to any issue regarding treatment of Study participants.
15.7 Construction. The headings of this Agreement are for convenience and ease of reference only and do not define, describe, extend or limit the scope or intent of this Agreement of the scope or intent of any provision contained in the Agreement.
15.8 Counterparts. This Agreement may be executed in two or more counterpart copies, each of which shall be deemed an original and all of which, taken together, shall be deemed to constitute one and the same instrument.
15.9 Force Majeure. No party will be liable or deemed to be in default for any delay or failure in performance under this Agreement or other interruption of service resulting directly or indirectly from Acts of God, civil or military authority, acts of public enemy, war, accident, fire, explosion, earthquake, flood, failure of transportation, strike, or other work interruption by a party’s employees or any similar or dissimilar cause beyond the reasonable control of the party.
|
Sponsor: |
By: |
/s/ Xxx X. Xxxxxxx |
|
Date: |
4/21/2017 |
|
|
|
|
|
|
|
|
|
Name: |
Xxx Xxxxxxx |
|
|
|
|
|
|
|
|
|
|
|
|
Title: |
Chief Executive Officer, Enteromedics, Inc. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Institution: |
By: |
/s/ Xxxxxxx Xxxxxx |
|
Date: |
4/26/2017 |
|
|
|
|
|
|
|
|
|
Name: |
Xxxxxxx Xxxxxx |
|
|
|
|
|
|
|
|
|
|
|
|
Title: |
Chief Financial Officer, SCPMG |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although not a party to this Agreement, the Principal Investigator’s signature below indicates that she understands the terms of this Agreement and her obligations hereunder |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investigator: |
By: |
/s/ Xxxx X. Xxxxx |
|
Date: |
4/24/2017 |
|
|
|
|
|
|
|
|
|
Name: |
Xxxx X. Xxxxx, PhD |
|
|
|
|
|
|
|
|
|
|
|
|
Title: |
Principal Investigator |
|
|
|
Page 13 of 16
EXHIBIT A
Page 1 of 2
|
|
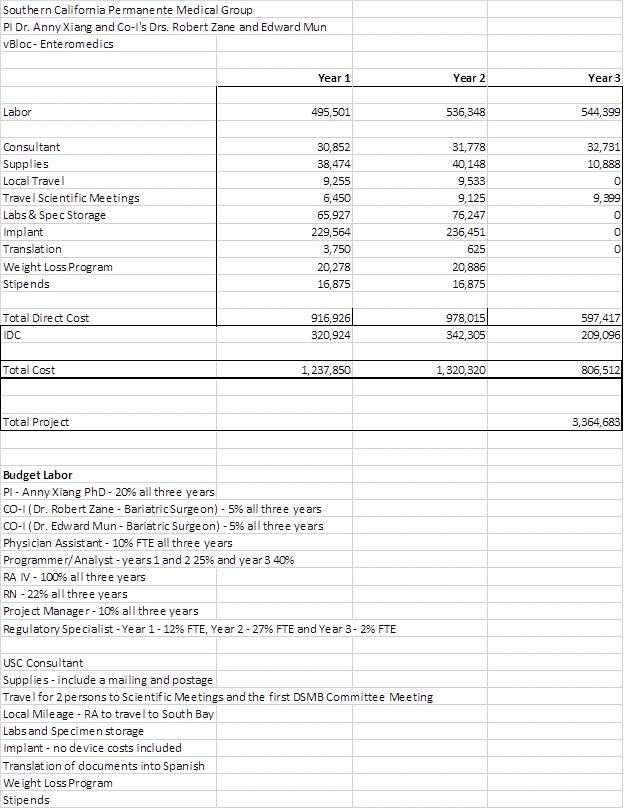
Southern California Permanente Medical Group PI Xx. Xxxx Xxxxx and Co-I's Drs. Xxxxxx Xxxx and Xxxxxx Xxx vBloc - Enteromedics Year 1 Year 2 Year 3 Labor 495,501 536,348 544,399 Consultant 30,852 31,778 32,731 Supplies 38,474 40,148 10,888 Local Travel 9,255 9,533 0 Travel Scientific Meetings 6,450 9,125 9,399 Labs & Spec Storage 65,927 76,247 0 Implant 229,564 236,451 0 Translation 3,750 625 0 Weight Loss Program 20,278 20,886 Stipends 16,875 16,875 Total Direct Cost 916,926 978,015 597,417 IDC 320,924 342,305 209,096 Total Cost 1,237,850 1,320,320 806,512 Total Project 3,364,683 Budget Labor PI - Xxxx Xxxxx PhD - 20% all three years CO-I (Xx. Xxxxxx Xxxx - Bariatric Surgeon) - 5% all three years CO-I (Xx. Xxxxxx Xxx - Bariatric Surgeon) - 5% all three years Physician Assistant - 10% FTE all three years Programmer/Analyst - years 1 and 2 25% and year 3 40% XX XX - 100% all three years RN - 22% all three years Project Manager - 10% all three years Regulatory Specialist - Year 1 - 12% FTE, Year 2 - 27% FTE and Year 3 - 2% FTE USC Consultant Supplies - include a mailing and postage Travel for 2 persons to Scientific Meetings and the first DSMB Committee Meeting Local Mileage - RA to travel to South Bay Labs and Specimen storage Implant - no device costs included Translation of documents into Spanish Weight Loss Program Stipends |
EXHIBIT A
Page 2 of 2
|
Milestone Payments |
||||||
|
Year 1 |
$148,051 |
Quarterly or 4 payments |
||||
|
$21,522 |
*per patient @ 30 patients |
|||||
|
Total budget year 1 $592,202 in fixed payments and $645,648 in per patient costs |
||||||
|
Year 2 |
$168,669 |
Quarterly or 4 payments |
||||
|
$21,522 |
*per patient @ 30 patients |
|||||
|
Total budget year 2 $674,675 in fixed payments and $645,648 in per patient costs |
||||||
|
Year 3 |
$201,628 |
Quarterly or 4 payments |
||||
|
Total budget in year 3 $806,512 in fixed payments |
||||||
|
*In years 1 and 2, the enrollment goal is 60 patients and we reserve the right to re-evaluate per patient costs based on study progression. |
||||||
Lockbox payment info
Post Office Remittance Address:
Xxxxxx Foundation Hospitals Inc
P. O. Xxx ******
Xxx Xxxxxxx, XX 00000-0000
Overnight Mail:
Bank of America Lockbox Services
Xxxxxx Foundation Hospitals Inc
Lockbox ******
0000 Xxxxx Xxxxxx Xxxxx
Xxx Xxxxxxx, XX 00000-0000
Phone: 000-000-0000 X 00000
SCPMG Finance Contact
*****************, Director of Research Finance & Sponsored Projects
000 X. Xxx Xxxxxx, 0xx xxxxx
Xxxxxxxx, XX 00000
Phone: ************
email: ***********************